Abstract
The directed migration of leukocytes to sites of damage or infection is necessary for a productive immune response. There is substantial evidence supporting a key role for chemoattractants in directed migration, however less is known about how cell-cell contacts affect the migratory behavior of leukocytes in innate immunity. Here, we explore how cell-cell contacts can affect the directed migration of innate immune cells including their role in attracting, repelling or stopping cell motility. Further investigation of cell contact dynamics as guidance cues may yield new insights into the regulation of innate immunity.
Keywords: cell-cell contact, migration, neutrophils, macrophages
Migratory contact responses in innate immunity
Directed cell migration guided by soluble or immobilized chemoattractants (see Glossary) is fundamental for leukocyte recruitment during an immune response [1]. However, individual moving cells do not exist in isolation, and neighboring cells may provide spatial and directional cues during cell migration. The best studied form of cell-cell interaction that may influence the direction of migrating cells is contact inhibition of locomotion (CIL), first characterized in the 1950s [2]. In addition to CIL, other forms of migratory contact behaviors exist, that have been described in recent years (see Box 1). Although the understanding of the biological roles and basic mechanisms of these migratory contact behaviors is still developing, most of the current knowledge is based on studies of slow-moving fibroblasts and neural crest cells (see Glossary), while other types of migratory cells, such as leukocytes (see Glossary), remain largely unexplored. Fibroblasts and other slow-moving cells mainly use a mesenchymal mode of migration that can be associated with strong adhesions and proteolysis of the extracellular matrix, while many leukocytes exhibit faster motility with minimal adhesive and proteolytic activity, with neutrophils demonstrating classic amoeboid migration [1, 3]. Leukocytes are key mediators of the inflammatory response during host defense and wound healing, and their motility is integral and uniquely adapted for serving their effector functions in the immune system [4, 5]. addition to being more migratory, it is likely that a productive immune response requires leukocytes to be more social compared to fibroblasts. Their primary job is to patrol interstitial environments for microbes and tissue damage, which involves frequent interactions with other cells. How does cell-cell contact affect the migratory behavior of fast-moving leukocytes? This is currently largely unknown. Here, we explore instances of cell-cell contacts during immune responses that affect the directional migration of the interacting cells, with a focus on innate immunity (see Glossary). We propose that cell-cell contacts provide important migratory cues during an immune response and further investigation of this area may yield important insights into the regulation of innate immunity. The directional cues discussed here are those that depend on cell-cell interactions and are distinct from contact guidance, a term often used in the literature to refer to the effects of extracellular physical cues on directed cell migration [6].
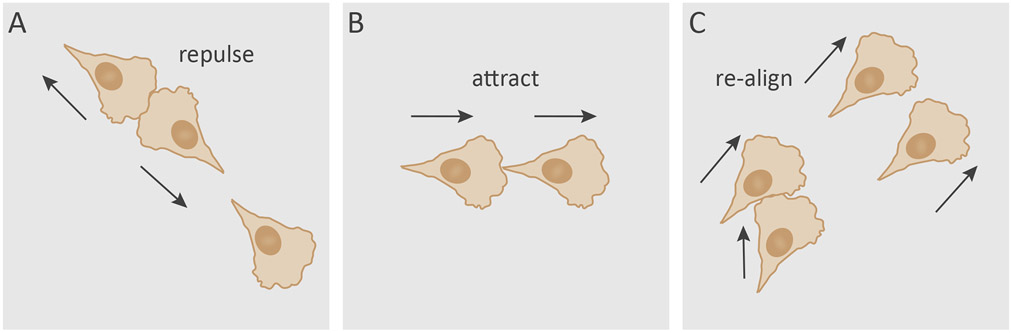
Box 1. Migratory contact responses in slow-moving cells.
The study of how cellular interactions modulate migratory behavior was pioneered by Michael Abercrombie and Joan Heaysman in the 1950s [7]. Their studies involved careful analyses of the interactions between fibroblasts derived from heart explants of chick embryos, which resulted in a series of reports describing the “social behaviour of cells in tissue culture”. One of these reports highlighted the tendency of fibroblasts to avoid clumping on top of each other, and attributed this behavior to “mutual restriction of movement” that only occurred “after contact has been established” between cells [8]. They named this phenomenon contact inhibition of locomotion (CIL) [8]. Abercrombie developed his definition of CIL over the years, and defined it as “the phenomenon of a cell ceasing to continue moving in the same direction after contact with another cell” [2, 9]. A range of CIL behaviors are recognized with variable migratory outcome, in which a head-to-head collision may either terminate cell movement or involve repulsion between the colliding partners, leading to the cells moving away from each other (Figure IA) [2]. A significant hurdle in the study of CIL was demonstrating its relevance in whole organisms. Renewed interest in recent years led to showing that CIL does occur in vivo [10], and plays important roles in cell dispersion and collective cell migration during embryogenesis [2, 7]. This field continues to develop, as other forms of migratory contact behaviors have emerged in recent years. Analysis of cell-cell interactions of fibroblasts and epithelial cells using micropatterned substrates showed that head-to-tail collisions are more likely to cause cells to follow each other (Figure IB) [11, 12]. This attracting behavior resembles contact following, first described in Dictyostelium discoideum [13], which has been proposed to be driven by contact activation of locomotion (CAL) [14]. During CAL, cell-cell contact promotes the assembly of leading-edge F-actin machinery to form a protrusion (see Glossary), thereby mediating forward migration [14]. This is in contrast to CIL, where cell-cell contact inhibits protrusions by suppressing leading-edge and promoting rear-end machinery instead [2]. A recent study reported that fibroblasts can also exhibit a more subtle social behavior the authors termed cell collision guidance [15]. Fibroblasts were observed to re-align following lateral collisions to resemble the orientation of the neighboring cell (Figure IC), which had important consequences for matrix patterning and overall tissue function [15]. These studies indicate that the site of contact is a contributing factor in determining the migratory outcome. It is yet to be determined if contact following and cell collision guidance are conserved behaviors in other cells types. While CIL may work in concert with contact following, cell collision guidance and CIL operate in a mutually exclusive manner [11, 12, 15].
Attracting behaviors.
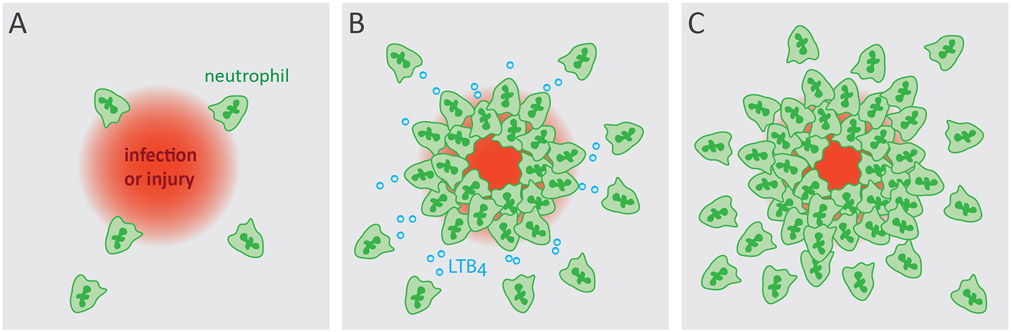
Infection or acute injury can elicit the coordinated recruitment of neutrophils, called swarming, resulting in a cluster of cells at the inflammatory site. Neutrophil swarming (Figure 1) has been described in vivo in two landmark studies. In a mouse model of Toxoplasma gondii infection small and transient, or large and persistent swarms were observed to form in the lymph nodes in a multi-step manner that were initiated by early ‘pioneer’ neutrophils which then recruited more neutrophils in a self-amplifying process [16]. Similar neutrophil behavior was independently reported in response to sites of Leishmania major-laden sand fly bites in mice [17]. Since then, neutrophil swarming has also been observed in mouse models of sterile, laser-induced injury [18-20]. These studies established the stages of neutrophil swarming which comprise of 1) swarm initiation by pioneer neutrophils, 2) cell death-mediated amplification, 3) signal relay-mediated amplification, 4) cluster aggregation and tissue remodeling, and finally 5) dispersal and resolution [21, 22]. Leukotriene B4 (LTB4) has been identified as a signal relay molecule that orchestrates neutrophil chemotaxis over long distances [23]. Subsequent work established that LTB4 signaling is required during neutrophil swarming, and found that CXCR2 and FPR2 also contributed to neutrophil aggregation [19]. Recently, connexins and calcium signaling have been shown to be important for this LTB4-mediated swarming response [24].
Figure 1. Neutrophil swarming at inflammatory sites.
Neutrophils are recruited, aided by relay signals such as LTB4, and aggregate at the sites of damage or infection [19].
Similarly to neutrophils, macrophages have also been observed to congregate and form clusters at sites of tissue damage or infection, as seen in Drosophila laser wound model [25], and larval zebrafish (Danio rerio) models of Mycobacterium marinum and Aspergillus fumigatus infections [26, 27]. The role of CIL or other migratory contact responses has not been examined in the context of inflammatory cell recruitment and aggregation. The absence of repelling behavior by leukocytes at swarming sites may be due to a lack of capacity for CIL or they may actively turn it off to allow clustering. There is evidence that neutrophils and monocytes have the capacity to undergo CIL [28]. In a more recent work an in silico simulation was performed to analyze CIL activity during macrophage accumulation at wound sites in Drosophila, which found that if CIL was operating during recruitment the number of cells at the wound site would be far less than what they normally observe in vivo [29]. The nature of neutrophil clusters are different from that of macrophages, as neutrophil swarms normally last minutes to hours [21], whereas macrophage aggregates are more stable structures that can persist for days before resolving [26, 27], Macrophages form tightly organized aggregates called granulomas during Mycobacterium tuberculosis infection that is recapitulated in the zebrafish-M. marinum model [27, 30]. Interestingly, macrophages were shown to undergo epithelial reprogramming during granuloma formation, which includes the upregulation of E-cadherin (see Glossary) expression that mediate the intercellular adhesion between macrophages [31]. E-cadherin at neural crest cell junctions is known to suppress CIL [32], suggesting that E-cadherin expression could be potential mechanism by which CIL is turned off to minimize repelling behavior and allow formation of immune cell clusters. Collectively, these studies suggest that leukocytes may actively turn off CIL to aggregate during inflammatory responses. The exact signals and mechanisms that drive swarm resolution also remain undetermined. It is possible that cluster dissociation could be a function of leukocytes’ ability to regain CIL and migrate away from the site of injury. This is an exciting area that stands to advance our understanding of immune cell behavior and resolution of inflammation (see Glossary).
Repelling behaviors.
CIL by leukocytes in the context of an immune response is poorly studied. However, studies of Drosophila macrophage (hemocyte) migration during embryonic development has become a useful system to gain insights into the role and mechanism of contact repulsion in vivo. At early stages of development embryonic hemocytes undertake a characteristic migratory route to populate the growing embryo. They spread from the anterior head region towards the posterior end along the ventral nerve cord at the midline, and afterwards migrate out laterally from the midline in both directions to create an evenly distributed three-lined pattern [33]. This dispersion pattern was shown to arise from contact repulsion; hemocytes along the ventral midline stop migrating upon contact, after which they repolarize and move away from each other [34]. Although secreted factors are believed to be important in orchestrating hemocyte dispersal, mathematical modeling has shown that CIL alone is sufficient to drive this cellular distribution in the Drosophila embryo [33, 35]. Owing to its capabilities for high-resolution in vivo imaging, this system has provided valuable insights into the importance of cytoskeletal dynamics in the regulation of CIL. Microtubules (see Glossary) were observed to bundle within the leading-edge protrusion of migrating hemocytes to form a microtubule arm that transiently interact between colliding cells during contact repulsion. Hemocytes with abnormal microtubule cytoskeleton not only lose their polarity, but also fail to repel each other and remain clumped together [34]. The actin network (see Glossary) also undergoes dynamic changes in the lamellae (see Glossary) of colliding cells that includes the formation of an actin fiber that physically links the cells, similarly as the microtubule arm [36]. Furthermore, the actin network and the microtubule arm in the lamellae couple to a transient cell-cell adhesion punctum at the site of contact that forms upon collision. Ultimately, the coordinated changes and coupling of actin dynamics with the adhesion punctum generates lamellar tension that drives the repulsive behavior between the interacting cells [36]. This detailed analysis of cytoskeletal changes also revealed that cell movement and actin remodeling appears to be synchronized between the colliding cells, suggesting that CIL is not a stochastic event in the context of embryonic development [36].
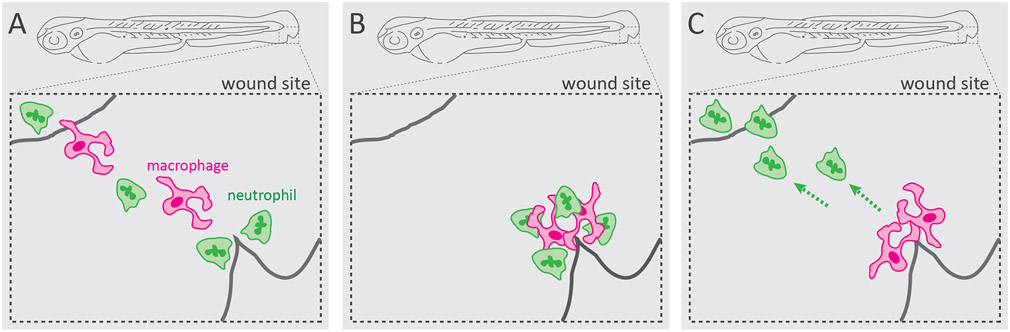
Clearing neutrophils is a critical phase in an inflammatory response to minimize tissue damage and prevent chronic inflammation. The predominant mechanism by which neutrophilic inflammation resolves is neutrophil apoptosis and their subsequent removal via macrophage efferocytosis [37]. However, studies using zebrafish tailfin wound model found that neutrophils can also resolve by migrating away from the inflammatory site and returning to the vasculature, known as reverse migration [38]. Since its discovery in zebrafish, neutrophil reverse migration has been shown to be relevant in mammalian wound models as well [39]. While the molecular mechanisms underlying neutrophil reverse migration are actively being investigated [40], detailed analysis of neutrophil and macrophage interaction at zebrafish tailfin wound revealed that there are cell-mediated mechanisms involved as well [41]. Neutrophils were observed to contact macrophages at the wound site before reverse migrating, suggesting that macrophages have an active role in this process. Indeed, temporary depletion of macrophages led to reduced resolution of neutrophils from the wound site [41], suggesting that macrophages may play a contact-dependent neutrophil-repelling role during wound response (Figure 2). Macrophage-mediated neutrophil resolution remains unclear, as other studies found no evidence that macrophages may alter the migratory behavior and reverse migration of neutrophils [39, 42]. The mechanisms by which physical interaction with macrophages may promote neutrophil reverse migration in unknown. The repelling behavior suggests that CIL may be at play, however this remains to be studied. As opposed to an active regulated process, such as CIL, neutrophil repelling by macrophages away from the wound site may be a stochastic process. Furthermore, neutrophils have to integrate contact-mediated cues on top of chemotactic and chemokinetic signals [43]. These incoming signals may operate in a hierarchy that influences the final migratory outcome [44]. Strong chemotactic gradients have the potential to override contact-mediated cues by enhancing the leading-edge machinery [45]. In addition, differential receptor trafficking and desensitization may also contribute to signal integration and final migratory outcome [46].
Figure 2. Neutrophil-macrophage interaction during wound response.
In a zebrafish model of inflammation, macrophages physically interact with neutrophils at the wound site and exhibit neutrophil-repelling behavior. This interaction promotes neutrophil reverse migration away from the wound and thereby the resolution of inflammation [41].
Behaviors promoting the metastatic cascade.
Abercrombie postulated that CIL plays a role in cancer metastasis (see Glossary). Specifically, he proposed that cancer cells lose their heterotypic (see Glossary) CIL responses, but maintain homotypic (see Glossary) CIL to promote dissemination [47-49]. The mechanisms behind this are still unknown, but cell-cell interactions appear to be an important component. Neutrophils have recently garnered growing interest in the context of cancer [50, 51]. In addition to various roles that neutrophils play in metastasis [52]. heterotypic interactions between neutrophils and cancer cells also play important roles in promoting tumor progression. Neutrophils were shown to interact with cancer cells over four decades ago [53-56] and can make up a substantial amount of the tumor’s cellular content [57]. The neutrophil β2 integrins Mac-1 and LFA-1 are critical mediators of neutrophil-cancer cell adhesion [58], which bind cancer surface ligands such as αvβ3 and intercellular adhesion molecule-1 (ICAM-1) [59-63]. In addition, cancer cell hyaluronan activates neutrophils by binding Toll-like receptor 4 (TLR4) and inducing PI3K/Akt signaling. The activation of TLR4/PI3K/Akt in neutrophils promotes neutrophil-cancer cell interaction and increases cancer cell motility [64]. In combination with neutrophil-derived soluble factors, this direct interaction promotes tumor cell intravasation into the vasculature [52, 64]. Tumor-derived signals, such as IL-8, can activate circulating neutrophils which increases expression of Mac-1 and LFA-1 [61]. In concert with neutrophil L-selectin, LFA-1 and Mac-1 promote neutrophil-cancer cell clustering in the circulation. This is followed by sequential tethering to endothelial ICAM-1 and tight adhesion after which the cancer cells can migrate across the endothelium and continue metastasis [58-60, 62, 63]. Once adhered to the vasculature, neutrophils in heterotypic tumor cell clusters activate the endothelium, which promotes vascular permeability, via IL-1β secretion [65]. Neutrophils also secrete matrix metallopeptidase 9 (MMP-9) which loosens endothelial cell junctions [65, 66]. Cancer cells in these heterotypic clusters form invasive protrusions that facilitate their transmigration across the weakened vascular cell junctions [65]. The coordinated effects of these various neutrophil-cancer cell interactions promote the systemic spread of cancer cells.
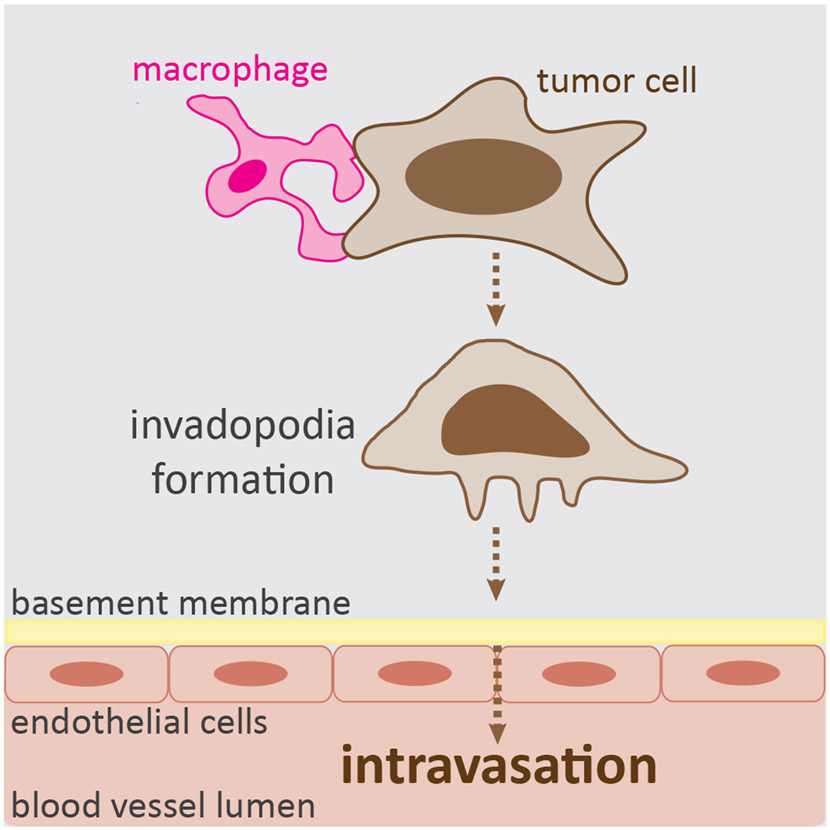
Macrophage-tumor cell interaction in the tumor microenvironment also contribute to the ability of tumor cells to disseminate from the primary site [67]. Macrophages employ multiple ways to facilitate the metastatic progression. Multiphoton intravital imaging of breast tumors in mice has shown that as tumor cells leave the primary site, they migrate in the stroma either in a random fashion or by streaming with macrophages, a coordinated movement where they align in a single line [68]. This directional streaming behavior resembles contact following [12, 14], however the role of cell-cell contact-mediated guidance during this process has not been studied. An EGF/CSF1 paracrine signaling loop is known to generate soluble chemotactic gradients and serve as a relay mechanism for the directed co-migration of macrophages and tumor cells towards the blood vessel, where macrophages express CSF1R and secrete EGF, and tumor cells express EGFR and secret CSF1 [69, 70]. However, a recent work hypothesizes that tunneling nanotubes may physically connect macrophages and tumor cells [71], suggesting that there may be cell contact-dependent mechanisms that contribute to the directional co-migration of these cells. Once the tumor cells reach the blood vessel, macrophages continue to assist during transmigration across the endothelial barrier into the bloodstream [72]. Direct contact by macrophages stimulate the tumor cells to generate invadopodia (see Glossary), which then enable tumor cells to breach the basement membrane and intravasate (Figure 3). This occurs both in vitro and mouse in vivo models of breast tumor [72]. Biosensor imaging revealed that physical contact by macrophages induces global upregulation of RhoA activity in tumor cells that fails to occur in the absence of macrophage contact [72]. This RhoA signaling is believed to drive the activation of invadopodia formation, however it remains to be directly demonstrated. Subsequent work found that Notch1, a pathway known to be involved in cell contact-mediated communication [73], is required in tumor cells for the formation of invadopodia induced by macrophage contact [74]. Collectively, these complex interactions with macrophages promote the forward migration of tumor cells towards the blood vessel and aid their systemic spread.
Figure 3. Macrophage-tumor cell interaction during tumor cell intravasation.
In vitro and mouse model of breast cancer revealed that physical contact between macrophages and tumor cells induces invadopodia formation in tumor cells that in turn enables tumor cells to breach the basement membrane and intravasate [72].
Stop signals.
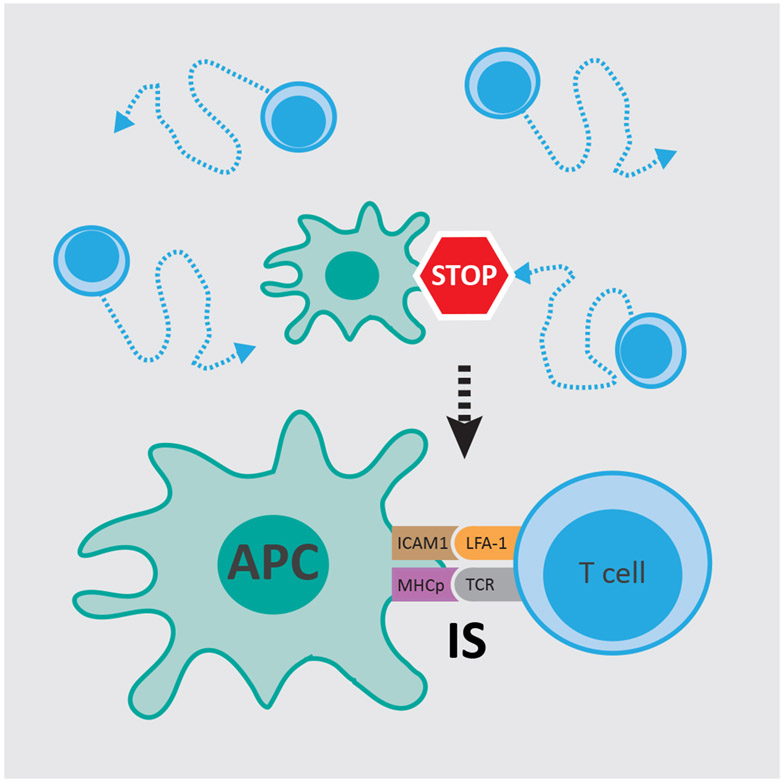
In addition to displaying attracting or repelling behaviors to influence direction of cell migration, cell-cell contact in innate immunity can also deliver a stop signal. This is most prevalent in the interaction of antigen-presenting cells (APC), such as dendritic cells, with T cells. In a seminal work a stop signal was discovered as an essential component in T cell activation (Figure 4) [75]. Analysis of T cell migration using an in vitro system of mouse T cells revealed that randomly migrating T cells on a surface of purified ICAM-1 stopped upon T cell antigen receptor engagement by purified antigenic major histocompatibility complex (MHC)-peptide complexes or interaction with LFA-1 locked in a high-affinity conformation. The authors proposed that this stop signal, mediated via adhesion-strengthening mechanisms, serves as a checkpoint in the coordination of T cell migration and activation [75]. This stopping event may be a type of CIL behavior, however this remains to be studied.
Figure 4. Stop signal delivered by antigen presenting cells.
Antigen presenting cells (APC), such as dendritic cells, provide a stop signal that halt randomly migrating T cells to allow stable interaction for efficient T cell activation at the immunological synapse (IS) [75].
Concluding remarks
There are numerous examples in the literature to suggest that cell-cell interactions can act as guidance cues during directional migration in innate immunity, however this is still an open question in the field (see Outstanding Questions Box). They resemble the attracting and repelling behaviors observed in slow-moving cells, however it is unclear if they are similar in nature to CIL and other forms of migratory contact responses of slow-moving cells. Cell adhesion and hierarchical signaling are likely to be involved in determining the migratory outcome during these cell-cell contact events. Nevertheless, the examples of contact events we have explored here provide compelling reason to speculate that these events have a significant impact during innate immune responses. Better understanding of the role and mechanisms of cell contact-dependent directional migration in innate immunity may yield insights into important physiological processes, such as resolution of inflammation.
Text Box Figure I.
Migratory contact responses by slow-moving cells. A) A type of contact inhibition of locomotion behavior where head-to-head collision may result in interacting cells repelling each other [2]. B) Contact following of locomotion typically occurs during head-to-tail collision and promotes forward migration [12]. C) Cell collision guidance occurs upon lateral collision with a neighboring cell that leads to re-alignment of the colliding cell [15].
Highlights.
Cell-cell contacts in innate immunity may repel, attract, or stop cell migration.
Contact-dependent migration in innate immunity contributes to the pathophysiology of inflammation and tumor progression.
The exact role and mechanisms of cell-cell contacts during migratory behavior remains poorly understood in the context of innate immunity.
Outstanding Questions .
Do cell-cell contacts influence the directional migration or migratory behavior of leukocytes during an immune response?
Are contact-dependent migratory behaviors similar in nature to contact responses observed in slow-moving cells, such as CIL?
Are migratory contact responses dependent on mode of migration (amoeboid versus mesenchymal)?
Are migratory contact responses stochastic or regulated events during an immune response?
Acknowledgement:
This work was supported by National Institutes of Health Grants R35 GM118027 to AH, Molecular and Cellular Pharmacology Training Grant (5T32 GM 8688-17) to LCK, and individual postdoctoral fellowship from American Heart Association to VM (17POST33410970).
Glossary
- Actin
cytoskeletal protein that forms contractile microfilaments and is important in cell motility
- Chemoattractant
a molecule capable of eliciting directed cell migration (chemotaxis)
- Fibroblasts
most common cell type found in connective tissue; they synthesize and secrete extracellular collagen
- E-cadherin
calcium-dependent cell adhesion molecule, mediating the formation of adherens junctions between neighboring cells
- Heterotypic
occurring between different cell types
- Homotypic
occurring between same cell types
- Inflammation
immune response to infection or tissue damage that triggers the recruitment of leukocytes
- Innate immunity
non-specific immune response serving as the first line of defense against invading pathogens, mediated by monocytes, macrophages, neutrophils, eosinophils, basophils, mast cells, natural killer cells and dendritic cells
- Invadopodia
actin-rich plasma membrane protrusions capable of degrading the extracellular matrix
- Lamellae
it is the cell region localized behind a lamellipodium (actin-rich, dynamic leading-edge protrusion of motile cells); this region has more stable actin network compared to lamellipodium
- Leukocytes
white blood cells that are part of the immune system, consisting of granulocytes (neutrophils, eosinophils and basophils), monocytes, macrophages and lymphocytes (B and T cells)
- Macrophage
also called big eaters; they are motile and phagocytic white blood cells that can respond to infection or tissue damage. They patrol almost every tissue in the body for pathogens and dead cells
- Metastasis
malignant growth at a distant location from the primary site of tumor
- Microtubule
polymers of tubulin that forms one of the components of the cell cytoskeleton
- Neutrophil
most abundant white blood cell of myeloid cell lineage; they have multi-lobed nucleus and their cytoplasm has several types of granules containing pathogen-killing agents; they are highly motile cells and capable of phagocytosis
- Neural crest cells
highly motile multipotent stem cells that can give rise to various cell types during embryonic development
- Protrusions
dynamic, actin-based plasma membrane extensions, important in cell migration and invasion
References
- 1.Friedl P and Weigelin B (2008) Interstitial leukocyte migration and immune function. Nat Immunol 9 (9), 960–9. [DOI] [PubMed] [Google Scholar]
- 2.Stramer B and Mayor R (2017) Mechanisms and in vivo functions of contact inhibition of locomotion. Nat Rev Mol Cell Biol 18 (1), 43–55. [DOI] [PubMed] [Google Scholar]
- 3.Friedl P and Wolf K (2010) Plasticity of cell migration: a multiscale tuning model. J Cell Biol 188 (1), 11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam PY and Huttenlocher A (2013) Interstitial leukocyte migration in vivo. Curr Opin Cell Biol 25 (5), 650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lammermann T and Germain RN (2014) The multiple faces of leukocyte interstitial migration. Semin Immunopathol 36 (2), 227–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charras G and Sahai E (2014) Physical influences of the extracellular environment on cell migration. Nat Rev Mol Cell Biol 15 (12), 813–24. [DOI] [PubMed] [Google Scholar]
- 7.Roycroft A and Mayor R (2018) Michael Abercrombie: contact inhibition of locomotion and more. Int J Dev Biol 62 (1-2-3), 5–13. [DOI] [PubMed] [Google Scholar]
- 8.Abercrombie M and Heaysman JE (1954) Observations on the social behaviour of cells in tissue culture. II. Monolayering of fibroblasts. Exp Cell Res 6 (2), 293–306. [DOI] [PubMed] [Google Scholar]
- 9.Mayor R and Carmona-Fontaine C (2010) Keeping in touch with contact inhibition of locomotion. Trends Cell Biol 20 (6), 319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carmona-Fontaine C et al. (2008) Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature 456 (7224), 957–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desai RA et al. (2013) Contact inhibition of locomotion probabilities drive solitary versus collective cell migration. J R Soc Interface 10 (88), 20130717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li D and Wang YL (2018) Coordination of cell migration mediated by site-dependent cell-cell contact. Proc Natl Acad Sci U S A 115 (42), 10678–10683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaffer BM (1965) Mechanical control of the manufacture and resorption of cell surface in collective amoebae. Journal of Theoretical Biology 8 (1), 27–40. [DOI] [PubMed] [Google Scholar]
- 14.Fujimori T et al. (2019) Tissue self-organization based on collective cell migration by contact activation of locomotion and chemotaxis. Proc Natl Acad Sci U S A 116 (10), 4291–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park D et al. (2019) Extracellular matrix anisotropy is determined by TFAP2C-dependent regulation of cell collisions. Nat Mater. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chtanova T et al. (2008) Dynamics of neutrophil migration in lymph nodes during infection. Immunity 29 (3), 487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters NC et al. (2008) In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science 321 (5891), 970–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng LG et al. (2011) Visualizing the neutrophil response to sterile tissue injury in mouse dermis reveals a three-phase cascade of events. J Invest Dermatol 131 (10), 2058–68. [DOI] [PubMed] [Google Scholar]
- 19.Lammermann T et al. (2013) Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498 (7454), 371–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park SA et al. (2018) Real-time dynamics of neutrophil clustering in response to phototoxicity-induced cell death and tissue damage in mouse ear dermis. Cell Adh Migr 12 (5), 424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kienle K and Lammermann T (2016) Neutrophil swarming: an essential process of the neutrophil tissue response. Immunol Rev 273 (1), 76–93. [DOI] [PubMed] [Google Scholar]
- 22.Lammermann T (2016) In the eye of the neutrophil swarm-navigation signals that bring neutrophils together in inflamed and infected tissues. J Leukoc Biol 100 (1), 55–63. [DOI] [PubMed] [Google Scholar]
- 23.Afonso PV et al. (2012) LTB4 is a signal-relay molecule during neutrophil chemotaxis. Dev Cell 22 (5), 1079–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poplimont H et al. (2020) Neutrophil Swarming in Damaged Tissue Is Orchestrated by Connexins and Cooperative Calcium Alarm Signals. Curr Biol 30 (14), 2761–2776.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stramer B et al. (2005) Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J Cell Biol 168 (4), 567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosowski EE et al. (2018) Macrophages inhibit Aspergillus fumigatus germination and neutrophil-mediated fungal killing. PLoS Pathog 14 (8), e1007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis JM et al. (2002) Real-time visualization of mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos. Immunity 17 (6), 693–702. [DOI] [PubMed] [Google Scholar]
- 28.Oldfield FE (1963) Orientation behavior of chick leucocytes in tissue culture and their interactions with fibroblasts. Exp Cell Res 30, 125–38. [DOI] [PubMed] [Google Scholar]
- 29.Weavers H et al. (2016) Systems Analysis of the Dynamic Inflammatory Response to Tissue Damage Reveals Spatiotemporal Properties of the Wound Attractant Gradient. Curr Biol 26 (15), 1975–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramakrishnan L (2012) Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol 12 (5), 352–66. [DOI] [PubMed] [Google Scholar]
- 31.Cronan MR et al. (2016) Macrophage Epithelial Reprogramming Underlies Mycobacterial Granuloma Formation and Promotes Infection. Immunity 45 (4), 861–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scarpa E et al. (2015) Cadherin Switch during EMT in Neural Crest Cells Leads to Contact Inhibition of Locomotion via Repolarization of Forces. Dev Cell 34 (4), 421–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wood W and Jacinto A (2007) Drosophila melanogaster embryonic haemocytes: masters of multitasking. Nat Rev Mol Cell Biol 8 (7), 542–51. [DOI] [PubMed] [Google Scholar]
- 34.Stramer B et al. (2010) Clasp-mediated microtubule bundling regulates persistent motility and contact repulsion in Drosophila macrophages in vivo. J Cell Biol 189 (4), 681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis JR et al. (2012) Emergence of embryonic pattern through contact inhibition of locomotion. Development 139 (24), 4555–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis JR et al. (2015) Inter-cellular forces orchestrate contact inhibition of locomotion. Cell 161 (2), 361–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bratton DL and Henson PM (2011) Neutrophil clearance: when the party is over, clean-up begins. Trends Immunol 32 (8), 350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathias JR et al. (2006) Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J Leukoc Biol 80 (6), 1281–8. [DOI] [PubMed] [Google Scholar]
- 39.Wang J et al. (2017) Visualizing the function and fate of neutrophils in sterile injury and repair. Science 358 (6359), 111–116. [DOI] [PubMed] [Google Scholar]
- 40.de Oliveira S et al. (2016) Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol 16 (6), 378–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tauzin S et al. (2014) Redox and Src family kinase signaling control leukocyte wound attraction and neutrophil reverse migration. J Cell Biol 207 (5), 589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loynes CA et al. (2018) PGE2 production at sites of tissue injury promotes an anti-inflammatory neutrophil phenotype and determines the outcome of inflammation resolution in vivo. Sci Adv 4 (9), eaar8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Powell D et al. (2017) Chemokine Signaling and the Regulation of Bidirectional Leukocyte Migration in Interstitial Tissues. Cell Rep 19 (8), 1572–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bromley SK et al. (2000) Cutting edge: hierarchy of chemokine receptor and TCR signals regulating T cell migration and proliferation. J Immunol 165 (1), 15–9. [DOI] [PubMed] [Google Scholar]
- 45.Lin B et al. (2015) Interplay between chemotaxis and contact inhibition of locomotion determines exploratory cell migration. Nat Commun 6, 6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coombs C et al. (2019) Chemokine receptor trafficking coordinates neutrophil clustering and dispersal at wounds in zebrafish. Nat Commun 10 (1), 5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abercrombie M and Ambrose EJ (1962) The surface properties of cancer cells: a review. Cancer Res 22, 525–48. [PubMed] [Google Scholar]
- 48.Abercrombie M (1970) Contact inhibition in tissue culture. In Vitro 6 (2), 128–42. [DOI] [PubMed] [Google Scholar]
- 49.Abercrombie M (1979) Contact inhibition and malignancy. Nature 281 (5729), 259–62. [DOI] [PubMed] [Google Scholar]
- 50.Giese MA et al. (2019) Neutrophil plasticity in the tumor microenvironment. Blood 133 (20), 2159–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mukaida N et al. (2020) Two-Faced Roles of Tumor-Associated Neutrophils in Cancer Development and Progression. Int J Mol Sci 21 (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leach J et al. (2019) Neutrophils: Homing in on the myeloid mechanisms of metastasis. Mol Immunol 110, 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pickaver AH et al. (1972) Cytotoxic effects of peritoneal neutrophils on a syngeneic rat tumour. Nat New Biol 235 (58), 186–7. [DOI] [PubMed] [Google Scholar]
- 54.Gale RP and Zighelboim J (1975) Polymorphonuclear leukocytes in antibody-dependent cellular cytotoxicity. J Immunol 114 (3), 1047–51. [PubMed] [Google Scholar]
- 55.Chee DO et al. (1978) Selective reduction of human tumor cell populations by human granulocytes in vitro. Cancer Res 38 (12), 4534–9. [PubMed] [Google Scholar]
- 56.Gerrard TL et al. (1981) Human neutrophil-mediated cytotoxicity to tumor cells. J Natl Cancer Inst 66 (3), 483–8. [PubMed] [Google Scholar]
- 57.Eruslanov EB et al. (2014) Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest 124 (12), 5466–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jadhav S et al. (2001) Hydrodynamic shear regulates the kinetics and receptor specificity of polymorphonuclear leukocyte-colon carcinoma cell adhesive interactions. J Immunol 167 (10), 5986–93. [DOI] [PubMed] [Google Scholar]
- 59.Wu QD et al. (2001) Human neutrophils facilitate tumor cell transendothelial migration. Am J Physiol Cell Physiol 280 (4), C814–22. [DOI] [PubMed] [Google Scholar]
- 60.Liang S et al. (2008) Hydrodynamic shear rate regulates melanoma-leukocyte aggregation, melanoma adhesion to the endothelium, and subsequent extravasation. Ann Biomed Eng 36 (4), 661–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huh SJ et al. (2010) Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res 70 (14), 6071–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang P et al. (2011) Sequential binding of alphaVbeta3 and ICAM-1 determines fibrin-mediated melanoma capture and stable adhesion to CD11b/CD18 on neutrophils. J Immunol 186 (1), 242–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spicer JD et al. (2012) Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res 72 (16), 3919–27. [DOI] [PubMed] [Google Scholar]
- 64.Wu Y et al. (2011) Neutrophils promote motility of cancer cells via a hyaluronan-mediated TLR4/PI3K activation loop. J Pathol 225 (3), 438–47. [DOI] [PubMed] [Google Scholar]
- 65.Spiegel A et al. (2016) Neutrophils Suppress Intraluminal NK Cell-Mediated Tumor Cell Clearance and Enhance Extravasation of Disseminated Carcinoma Cells. Cancer Discov 6 (6), 630–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yan HH et al. (2010) Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res 70 (15), 6139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanchez LR et al. (2019) The emerging roles of macrophages in cancer metastasis and response to chemotherapy. J Leukoc Biol 106 (2), 259–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roussos ET et al. (2011) Mena invasive (MenaINV) promotes multicellular streaming motility and transendothelial migration in a mouse model of breast cancer. J Cell Sci 124 (Pt 13), 2120–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wyckoff J et al. (2004) A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res 64 (19), 7022–9. [DOI] [PubMed] [Google Scholar]
- 70.Goswami S et al. (2005) Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res 65 (12), 5278–83. [DOI] [PubMed] [Google Scholar]
- 71.Hanna SJ et al. (2019) Tunneling nanotubes, a novel mode of tumor cell-macrophage communication in tumor cell invasion. J Cell Sci 132 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roh-Johnson M et al. (2014) Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene 33, 4203–4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lai EC (2004) Notch signaling: control of cell communication and cell fate. Development 131 (5), 965–73. [DOI] [PubMed] [Google Scholar]
- 74.Pignatelli J et al. (2016) Macrophage-dependent tumor cell transendothelial migration is mediated by Notch1/MenaINV-initiated invadopodium formation. Sci Rep 6, 37874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dustin ML et al. (1997) Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc Natl Acad Sci U S A 94 (8), 3909–13. [DOI] [PMC free article] [PubMed] [Google Scholar]