Significance Statement
Vascular calcification is associated with cardiovascular morbidity and mortality in people with CKD. In this systematic review, we summarized evidence from randomized and nonrandomized clinical trials investigating effects of interventions that might attenuate progression of vascular calcification in CKD; interventions were compared with placebo, other comparators, or standard of care. We reviewed 77 heterogeneous clinical trials (63 randomized) involving 6898 participants. Therapy involving magnesium or sodium thiosulfate appears the most promising, with consistent findings of attenuation of vascular calcification progression, but evaluable studies were small and of short duration. Many other studies had inconclusive or conflicting outcomes. This study highlights the need for more definitive trials to evaluate interventions targeting vascular calcification in people with CKD, preferably in association with patient-centered outcomes.
Keywords: vascular calcification, cardiovascular disease, CKD-MBD, phosphate binders
Abstract
Background
Vascular calcification is associated with cardiovascular morbidity and mortality in people with CKD. Evidence-based interventions that may attenuate its progression in CKD remain uncertain.
Methods
We conducted a systematic review of prospective clinical trials of interventions to attenuate vascular calcification in people with CKD, compared with placebo, another comparator, or standard of care. We included prospective clinical trials (randomized and nonrandomized) involving participants with stage 3–5D CKD or kidney transplant recipients; the outcome was vascular calcification measured using radiologic methods. Quality of evidence was determined by the Cochrane risk of bias assessment tool and the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) method.
Results
There were 77 trials (63 randomized) involving 6898 participants eligible for inclusion (median sample size, 50; median duration, 12 months); 58 involved participants on dialysis, 15 involved individuals with nondialysis CKD, and 4 involved kidney transplant recipients. Risk of bias was moderate over all. Trials involving magnesium and sodium thiosulfate consistently showed attenuation of vascular calcification. Trials involving intestinal phosphate binders, alterations in dialysate calcium concentration, vitamin K therapy, calcimimetics, and antiresorptive agents had conflicting or inconclusive outcomes. Trials involving vitamin D therapy and HMG-CoA reductase inhibitors did not demonstrate attenuation of vascular calcification. Mixed results were reported for single studies of exercise, vitamin E–coated or high-flux hemodialysis membranes, interdialytic sodium bicarbonate, SNF472, spironolactone, sotatercept, nicotinamide, and oral activated charcoal.
Conclusions
Currently, there are insufficient or conflicting data regarding interventions evaluated in clinical trials for mitigation of vascular calcification in people with CKD. Therapy involving magnesium or sodium thiosulfate appears most promising, but evaluable studies were small and of short duration.
Cardiovascular disease (CVD) is the leading cause of mortality in people with CKD. Although the excess cardiovascular burden is, in part, related to an over-representation of conventional cardiovascular risk factors, there is also a significant contribution from nontraditional, CKD-related risk factors, which manifests as accelerated vascular calcification (VC) and valvular calcification.1 Ectopic extraosseous calcification is accompanied by reduced bone mineral density2,3 and associated with accelerated vascular stiffening and significantly increased risk of cardiovascular morbidity and mortality. In CKD, VC is driven to a large extent by impaired mineral homeostasis which constitutes part of the condition termed by Kidney Disease Improving Global Outcomes (KDIGO) as CKD mineral and bone disorder (CKD-MBD).4,5
VC represents an aggregate end point of diverse vascular disease processes and considerable heterogeneity exists with respect to the anatomic site, the layer of the arterial wall affected, and the size of the calcifications present. Two major types of VC can be distinguished histologically, affecting either the intimal or medial arterial layer. Whereas intimal lesions are typically focal and associated with atheromatous lipid-rich plaques and inflammatory infiltrate, medial VC is usually more diffuse and occurs in the absence of lipid deposition or immune cell infiltration. Although considered distinct entities, with intimal VC showing a predilection for large- and medium-sized conduit arteries and medial VC for the same vessels as well as smaller muscular arteries of the periphery, both can coexist, even within the same arterial segment6 and with potentially amplificatory deleterious effects on the cardiovascular system.7 Cascades initiating and propagating mineralization at the cellular and biochemical levels are complex and not yet fully understood but thought to involve active cell-mediated mechanisms.8–11 This heterogeneity leads to potentially divergent pathobiologic associations and cardiovascular outcomes for the patient, with atherosclerotic intimal VC relating to luminal narrowing and medial VC being linked to arterial stiffening and increased cardiac afterload. Despite this heterogeneity with respect to anatomy, natural history, molecular pathogenesis, and clinical sequelae, the common end point for VC is the formation and deposition of hydroxyapatite within the extracellular matrix of the arterial wall. CKD represents a perfect storm for VC with dysregulated mineral metabolism leading not only to the accumulation of promoters (such as hyperphosphatemia) but also to the simultaneous suppression and/or consumption of systemic and local inhibitors (e.g., fetuin-A, matrix Gla protein [MGP], pyrophosphate).12 Consequently, both intimal and medial VC are highly prevalent and typically more severe in patients with CKD compared with age-matched individuals without kidney dysfunction.
VC has not been regarded as a direct therapeutic target in clinical practice as no intervention to date has been shown to reverse VC and there are few interventions to consistently reduce progression of VC.13 There is also a lack of evidence that slowing VC progression improves clinical outcomes, although associative data support this notion.14,15 Thus, VC is considered a surrogate marker of CVD rather than an actionable target. The majority of candidate drug therapies do not target VC specifically and instead rely on addressing the imbalance of mineralization promoters and inhibitors that may engender ectopic mineralization through direct (e.g., phosphate lowering) or indirect (e.g., MGP activation with vitamin K) means. Management of hyperphosphatemia in CKD may address VC and involves dietary phosphate restriction, phosphate-lowering medication (such as intestinal phosphate binders), and dialysis. Vitamin D deficiency is related to endothelial dysfunction and development of VC and use of vitamin D therapy may have a role in influencing VC. For associated secondary hyperparathyroidism (SHPT) in CKD, active vitamin D analogs and calcimimetics can be initiated to reduce the numerous consequences of SHPT including VC. Use of antiresorptive agents (such as bisphosphonates and denosumab) for bone demineralization accompanying ectopic calcification may also help to mitigate VC. Although there is no comparable evidence for treating VC in CKD alone, newer trials have evaluated effects of targeting VC directly with SNF472 (a novel myo-inositol hexaphosphate derivative that inhibits hydroxyapatite formation) and magnesium, or indirectly with sodium thiosulfate and vitamin K.
For future studies to elucidate whether reduction in VC affects clinical outcomes, it is important to determine which therapies have been shown to effectively attenuate VC progression. This systematic review looks at evidence-based strategies to attenuate the progression of VC in people with CKD as reported in prospective clinical trials.
Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The protocol is registered in the International Prospective Register of Systematic Reviews (PROSPERO 2020 CRD42021240400) (available at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=240400).
Inclusion Criteria
This systematic review included randomized controlled trials (RCTs) and prospective nonrandomized clinical trials evaluating interventions to attenuate VC progression in the CKD population. Included studies (1) had participants with stage 3–5D CKD or kidney transplant recipients; (2) compared an intervention with placebo, another comparator, or standard of care; and (3) involved VC as an outcome measured using radiologic methods (computed tomography [CT] or plain x-ray). Interventions involved pharmacological agents, exercise regimes, and changes in dialysis prescription. Observational studies in patients with kidney failure evaluating VC related to changes in dialysis modality or kidney transplantation were excluded.
Preprints, review articles, retrospective studies, cross-sectional observational studies, protocol papers, and conference abstracts were excluded. Only prospective clinical trials in humans were reviewed, with exclusion of in vitro or experimental studies in animals. Surrogate measures of VC including serum calcification propensity (T50) and calciprotein particles (despite being outlined in the registered protocol) were excluded as outcome measures, as well as other surrogate cardiovascular markers such as carotid intima media thickness and pulse wave velocity.
Search Strategy
MEDLINE and EMBASE (up to October 8, 2021) were searched with no restrictions on date of publication, number of trial participants, nor language. The complete search strategy is shown in Supplemental Table 1. Two authors (C.X. and N.D.T.) independently screened the title and abstracts of each study, discarding studies that clearly did not meet inclusion criteria. The full texts for all remaining studies were retrieved and assessed for eligibility for inclusion. Any disagreement about eligibility of a study was reviewed by a third author (I.R.).
Data Extraction and Quality Assessment
Two authors (C.X. and N.D.T.) assessed each trial, and extracted data on participant characteristics, interventions, comparators, and measurement of VC. Non-English language studies were translated before assessment. Where more than one publication of a study existed, reports were grouped together and the publication with the most complete data was used in the analysis.
Quality of evidence for intervention groups was assessed by the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach, with each being estimated as high, moderate, low, or very low level of evidence.16 Methodological quality of each RCT was also assessed using the risk of bias assessment tool developed by the Cochrane Bias Methods Group.17 The following items were assessed: (1) random sequence generation; (2) allocation concealment; (3) blinding of participants, investigators, and outcome assessors; (4) incomplete outcome data; (5) selective outcome reporting; and (6) any other bias (e.g., insufficient rationale and study design). Study selection, data extraction, risk of bias, and GRADE assessments were undertaken independently by two authors (C.X. and N.D.T.).
Results
Search Results
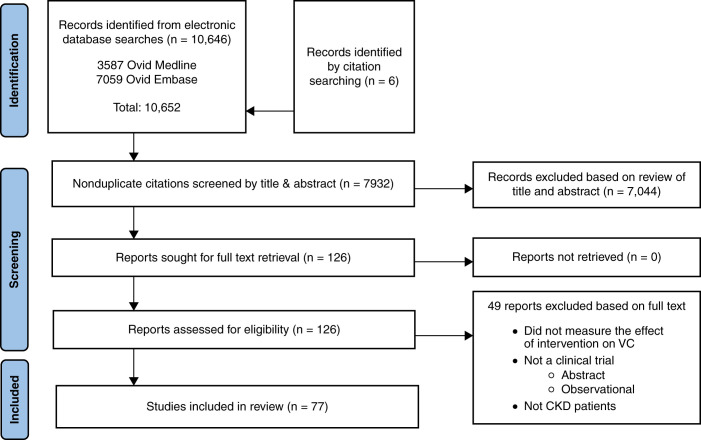
A PRISMA flow diagram of the search results is shown in Figure 1. A total of 10,646 potentially relevant records were identified through database searches. An additional six records were identified through citation searching. After duplicates were removed, 7932 records were screened by title and abstract and a remaining 126 records were identified for full text review. A total of 77 studies met the inclusion criteria and were included in this review.
Figure 1.
PRISMA flow diagram.
Study Characteristics
Tables 1–9 display the main participant, study characteristics, and outcome of included studies. Included studies involved a total of 6898 participants with CKD (median participants 50, range 7–508; median study duration 12 months, range 3–60 months). Of the 77 studies, 63 were RCTs and 14 were nonrandomized interventional trials. VC featured as a primary end point in 53 studies. Fifteen studies involved participants with nondialysis CKD, 58 involved participants on dialysis and 4 studies involved kidney transplant recipients. A significant proportion of studies included phosphate binder therapy as the intervention (n=25). Other interventions with multiple trials included calcimimetics, vitamin D therapy, β-hydroxy-β-methylglutaryl-CoA (HMG-CoA) reductase inhibitors, antiresorptive therapy, sodium thiosulfate, vitamin K, magnesium, and changes in dialysis prescription. There were single trials for each of exercise training, SNF472, sotatercept (a ligand trap for members of the TGF-β superfamily including bone morphogenetic proteins), oral activated charcoal, spironolactone, and nicotinamide.
Table 1.
Summary of phosphate binder study characteristics and VC outcome
| Author and Year | Type of Study | Study Duration (months) | Participant | Intervention (Participants at Baseline) | Comparator (Participants at Baseline) | VC as Primary or Secondary End Point | VC Imaging | VC Site | VC Outcomea |
|---|---|---|---|---|---|---|---|---|---|
| Sperschneider 199318 | Non-RCT | 36 | HD | 3 months: calcium carbonate 4.5 g + aluminum hydroxide, n=9 | Calcium carbonate 9 g, n=8 | Secondary | X-ray | Hand | Linear increase in calcification with calcium carbonate |
| 6 months: calcium carbonate 9 g 24 months: calcium carbonate 4.5–9 g, n=22 | |||||||||
| Chertow 200219b (Treat to Goal) | RCT | 12 | HD | Sevelamer, n=99 | Calcium-based phosphate binder (acetate or carbonate), n=101 | Secondary | EB-CT | Coronary artery, thoracic aorta, mitral valve, aortic valve | Less progression at coronary arteries and aorta with sevelamer |
| Braun 200420cAsmus 200521d | RCT | 12 | HD | Sevelamer, n=55 | Calcium carbonate, n=59 | Secondary | EB-CT | Coronary artery, thoracic aorta, mitral valve, aortic valve | Less progression at all sites with sevelamer |
| 24 | |||||||||
| Block 200522 | RCT | 18 | Incident HD | Sevelamer, n=73 | Calcium-based phosphate binder (acetate or carbonate), n=75 | Primary | EB-CT | Coronary artery | Less progression with sevelamer |
| Ferramosca 200523 | RCT | 12 | HD | Sevelamer, n=54 | Calcium acetate, n=54 | Primary | EB-CT | Coronary artery | Less progression with sevelamer |
| Russo 200724 | RCT | 24 | CKD stage 3–4 | Sevelamer + low phosphate diet, n=30 | Calcium carbonate + low phosphate diet, n=30 | Low phosphate diet, n=30 | Primary | CT | Coronary artery | Less progression with sevelamer than calcium and diet |
| Barreto 200825 (BRiC) | RCT | 12 | HD | Sevelamer, n=52 | Calcium acetate, n=49 | Primary | CT | Coronary artery | No significant VC attenuation |
| Qunibi 200826 (CARE-2) | RCT | 12 | HD | Sevelamer, n=100 (+ atorvastatin, n=79) | Calcium acetate, n=103 (+ atorvastatin, n=100) | Primary | EB-CT | Coronary artery | No significant VC attenuation |
| Takei 200827 | Non-RCT | 6 | HD | Sevelamer, n=22 | Calcium carbonate, n=20 | Primary | CT | Abdominal aorta | Less progression with sevelamer (lower serum phosphate with sevelamer) |
| Kakuta 201128 | RCT | 12 | HD | Sevelamer, n=91 | Calcium carbonate, n=92 | Primary | CT | Coronary artery | Less progression with sevelamer |
| Toussaint 201129 | RCT | 18 | HD | Lanthanum carbonate, n=22 | Calcium carbonate, n=23 | Primary: aorta, secondary: femoral | CT | Abdominal aorta, SFA | Less progression at aorta (significant) and SFA (not significant) with lanthanum |
| Block 201230 (PNT study) | RCT | 9 | CKD stage 3b–4 | Sevelamer, n=30 | Lanthanum carbonate , n=30 | Calcium acetate, n=30 | Placebo, n=58 | Secondary | EB-CT | Coronary artery, thoracic aorta, abdominal aorta | No significant VC attenuation at all sites (lower serum phosphate with sevelamer) |
| Di Iorio 201231 | RCT | 24 | CKD stage 3–4 | Sevelamer, n=121 | Calcium carbonate, n=118 | Primary | CT | Coronary artery | Less progression with sevelamer (lower serum phosphate with sevelamer) |
| Kalil 201232 | RCT | 12 | HD | Lanthanum carbonate, n=7 | Remain on same non-lanthanum phosphate binder (calcium-based +/− sevelamer), n=6 | Primary | CT | Coronary artery | Less progression with lanthanum |
| Lemos 201333 | RCT | 24 | CKD stage 3–5 | Sevelamer, n=38 | Rosuvastatin, n=38 | Control condition undefined, n=41 | Primary | CT | Coronary artery | No significant VC attenuation |
| Ohtake 201334 | RCT | 12 | HD | 6 months of calcium carbonate then 6 months of lanthanum carbonate, n=26 | 6 months of calcium carbonate then another 6 months of calcium carbonate, n=26 | Primary | CT | Coronary artery | Less progression with lanthanum |
| Seifert 201335 | RCT | 12 | CKD stage 3 | Lanthanum carbonate, n=19 | Placebo, n=19 | Secondary | CT | Carotid artery, coronary artery, aortic arch, thoracic aorta | No significant VC attenuation at all sites |
| Wada 201436 | RCT | 12 | HD and diabetes | Lanthanum carbonate, n=21 | Calcium carbonate, n=22 | Primary | CT | Abdominal aorta | Less progression with lanthanum, only when baseline VC is not advanced |
| Russo 201537 | RCT | 36 | CKD stage 3–4 | Sevelamer + phosphate restricted diet, n=66 | Calcium carbonate + phosphate restricted diet, n=47 | Primary | CT | Coronary artery | Less progression with sevelamer |
| Wang 201538 | RCT | 3 | HD | Lanthanum carbonate, n=28 | Standard of care, n=26 | Primary | Lateral abdominal x-ray | Abdominal aorta | Less progression with lanthanum |
| Zhang 201739 | RCT | 12 | HD | Lanthanum carbonate, n=46 | Calcium carbonate, n=46 | Primary | CT | Coronary artery | Less progression with lanthanum |
| Fujii 201840e | RCT | 18 | Incident HD | Lanthanum carbonate, n=53 | Calcium carbonate, n=55 | Primary | CT | Coronary artery | Less progression with lanthanum |
| Toussaint 202041 (IMPROVE-CKD) | RCT | 22 (96 weeks) | CKD stage 3b–4 | Lanthanum carbonate, n=138 | Placebo, n=140 | Secondary | CT | Abdominal aorta | No significant VC attenuation |
| Isaka 202142 | RCT 2 × 2 factorial | 12 | HD | Factor 1: lanthanum carbonate, n=78 | sucroferric oxyhydroxide, n=78Factor 2: strict serum phosphate <4.5 mg/dl, or standard serum phosphate 5 to <6 md/dl | Primary | CT | Coronary artery | Less progression with strict phosphate control; no difference between binders | |
There was no difference in serum phosphate levels between groups in the majority of studies (apart from those outlined in the table). Most studies had similar reduction in serum phosphate in both groups, and some had no significant reduction in serum phosphate.
Raggi 2004139 was not included as this study was a post hoc analysis of Chertow 200219 and assessed valvular calcification in this cohort.
Braun 200420 had 114 participants; 93 of these participants were in Chertow 200219 study. The extra 21 participants in Braun are included in median and range calculations.
Asmus 200521 followed patients in Braun 200420 for an additional 12 months. None of these participants are included in median and range calculations (as same as Braun 2004 participants).
Watanabe 2020140 was not included as this study was a post hoc analysis of Fujii 201840 and assessed valvular calcification in this cohort.
EB, electron beam; HD, hemodialysis; SFA, superficial femoral artery.
Table 9.
Summary of other singleton therapy study characteristics and VC outcome
| Author and Year | Type of Study | Study Duration (months) | Participant | Intervention (Participants at Baseline) | Comparator (Participants at Baseline) | VC as Primary or Secondary End Point | VC Imaging | VC Site | VC Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Raggi 2020134 (CaLIPSO) | RCT | 12 (52 weeks) | HD | SNF472 600 mg, n=91 | SNF472 300 mg, n=92 | Placebo, n=91 | Primary | CT | Coronary artery | Less progression with SNF472 |
| Gueiros 2019135 | RCT | 12 | PD | Spironolactone daily, n=16 | Treatment as usual, n=17 | Primary | CT | Coronary artery | No significant VC attenuation |
| Coyne 2019136 | RCT | 7 (225 days) | HD, anemia | Sotatercept 0.3 mg/kg, n=9 | 0.5 mg/kg, n=8 | 0.7 mg/kg, n=9 | 0.7/0.4 mg/kg, n=6 | Placebo, n=11 | Secondary | CT | Abdominal aorta | Less progression with sotatercept |
| Liu 2020137 | RCT | 12 | HD | Nicotinamide + calcium-based phosphate binder, n=49 | Placebo + calcium-based phosphate binder, n=49 | Secondary | X-ray lateral | Abdominal aorta | No significant VC attenuation |
| Gao 2019138 | RCT | 24 | CKD stage 3–4 | Phase 2: oral activated charcoal, n=17 | Lanthanum carbonate, n=17 Calcium carbonate, n=16 | Primary during the second phase | CT | Coronary artery | VC progressed least with lanthanum followed by charcoal then calcium |
HD, hemodialysis; PD, peritoneal dialysis.
Study Interventions
Phosphate Binders
Twenty-five trials (18 dialysis, 7 nondialysis) evaluated effects of phosphate binders on VC and involved 2571 participants (median participants 97, range 13–278; median duration 12 months, range 3–36 months) (Table 1).18–42 VC was reported as the primary end point in 19 trials, with the coronary artery the most common site evaluated (n=15). There were only three placebo-controlled RCTs of phosphate binders, all in nondialysis-dependent CKD patients, and only one included a significant proportion of patients with hyperphosphatemia, where a modest but significant reduction in serum phosphate and urinary phosphate excretion was seen with active therapy compared with placebo.30 None of the placebo-controlled trials reported any attenuation of progression of VC with noncalcium-based phosphate binders.
The majority of phosphate binder trials (n=17) evaluated noncalcium-based binders compared with calcium-based binders and although results were conflicting, of studies with >100 participants, five of seven RCTs evaluating sevelamer in hemodialysis cohorts reported less coronary artery calcification (CAC) progression with sevelamer. Overall, trials predominantly demonstrated that noncalcium-based phosphate binders (sevelamer and lanthanum carbonate) attenuated VC progression when compared with calcium-based binders (likely as a result of exogenous calcium loading promoting VC with calcium-based binders), but not when compared with placebo or standard of care.
Of trials involving dialysis participants, the largest two trials (≥200 participants each) both evaluated sevelamer compared with calcium-based binders and showed conflicting results. Qunibi et al. (n=203) reported no difference in VC between sevelamer and calcium-based binders (both arms were prescribed concurrent atorvastatin), whereas Chertow et al. (n=200) reported less progression of VC with sevelamer.19,26 Of note, the study by Qunibi et al. had several limitations which included a greater preexisting cardiovascular risk in participants (higher frequencies of diabetes and smokers at baseline) and a high dropout rate.
Of the seven phosphate binder trials that enrolled nondialysis patients, four of these reported no attenuation of VC progression with noncalcium-based binders (three were placebo-controlled). In the largest two trials in nondialysis CKD (≥200 participants each), Di Iorio et al. (n=239) reported less progression of VC with sevelamer compared with calcium binders, but Toussaint et al. (n=278) showed no difference in VC with lanthanum in a placebo-controlled trial.31,41
Changes in Dialysis Prescription
Eight trials involved alterations in dialysis prescription and involved 1304 participants (median participants 70, range 50–508; median duration 12 months, range 12–24 months) (Table 2).43–50 Five trials evaluated effects of changes in dialysate calcium concentration44,46,47,49,50 with all but one reporting a lower serum calcium concentration in those randomized to lower dialysate calcium.49 Two trials reported that lower dialysate calcium concentration (1.25 mmol/L) attenuated VC progression compared with higher dialysate calcium,46,47 with the other three reporting no effect on VC.44,49,50 Two of the larger and longer dialysate calcium studies reported conflicting findings on effects of higher dialysate calcium (1.75 mmol/L) on VC.44,47 Other solitary studies that involved alterations to dialysis prescription reported attenuation of VC progression with interventions which included vitamin E–coated or high-flux hemodialysis membranes and interdialytic sodium bicarbonate,43,45,48 but all with <100 participants in each study.
Table 2.
Summary of dialysis intervention study characteristics and VC outcome
| Author and Year | Type of Study | Study Duration (months) | Participant | Intervention (Participants at Baseline) | Comparator (Participants at Baseline) | VC as Primary or Secondary End Point | VC Imaging | VC Site | VC Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Mune 199943 | RCT | 24 | HD | Vitamin E–coated cellulose membrane dialyser (CL-E), n=25 | Unmodified cellulose membrane dialyser, n=25 | Secondary | CT | Aorta | Less progression with intervention |
| Zhang 201444 | Non-RCT | 24 | HD | High DCa 1.75 mmol/L, n=285 | Standard DCa 1.5 mmol/L, n=223 | Secondary | X-ray, echo | Aortic arch, abdominal aorta, cardiac valve | No VC attenuation. Only aortic arch is reported (increased serum Ca with DCa 1.75) |
| Fu 201545 | RCT | 12 | HD | High-flux HD, n=25 | Low flux HD, n=25 | Primary | X-ray lateral | Abdominal aorta | Less progression with intervention |
| Lu 201646 | RCT | 12 | HD | Low DCa 1.25 mmol/L, n=41 | Standard DCa 1.5 mmol/L, n=41 | Secondary | X-ray lateral | Abdominal aorta | Less progression with intervention (lower serum Ca with DCa 1.25) |
| Ok 201647 | RCT | 24 | HD | Low DCa 1.25 mmol/L, n=212 | High DCa 1.75 mmol/L, n=213 | Primary | CT | Coronary | Less progression with intervention (lower serum Ca with DCa 1.25) |
| Voiculet 201648 | RCT | 12 | HD | Interdialytic NaHCO3 5 g, n=29. (Reduce HCO3− fluctuations) | No interdialytic NaHCO3, higher intradialytic NaHCO3, n=34 | Secondary | X-ray | Coronary | Less progression with intervention |
| Kim 201749 | RCT | 12 | HD | Low DCa 1.25 mmol/L, n=36 | Standard DCa 1.5 mmol/L, n=40 | Primary | CT | Coronary | No significant VC attenuation (no difference in serum Ca between groups) |
| Masterson 201750 | RCT | 12 | Nocturnal HD | Low DCa 1.3 mmol/L, n=24 | High DCa 1.6 or 1.75 mmol/L, n=26 | Secondary | CT | Abdominal aorta, superficial femoral artery | No significant VC attenuation at all sites (lower serum Ca in DCa 1.3) |
DCa, dialysate calcium; HD, hemodialysis.
Calcimimetics
Six trials involved calcimimetics with 562 participants (median participants 41, range 23–360; median duration 12 months, range 8.3–36 months) (Table 3).51–56 All trials assessed the effect of the calcimimetic cinacalcet on VC and predominantly demonstrated reduction in VC progression; however, studies were small and some were potentially confounded by disparate vitamin D administration between intervention and comparator groups. The largest study, conducted by Raggi et al. (n=360), reported a nonsignificant reduction in progression of CAC determined by the Agatston score with cinacalcet plus low-dose vitamin D analogs compared with vitamin D analogs alone,52 but reported a significant attenuation when CAC was measured as a volume score or when aortic valve calcification was assessed. Flexible use of vitamin D in the control group in this study resulted in controls receiving substantially higher doses, making it difficult to isolate effects of cinacalcet on VC. One RCT compared cinacalcet to parathyroidectomy in kidney transplant recipients reporting no difference in VC after 12 months.54
Table 3.
Summary of calcimimetic study characteristics and VC outcome
| Author and Year | Type of Study | Study Duration (months) | Participant | Intervention (Participants at Baseline) | Comparator (Participants at Baseline) | VC as Primary or Secondary End Point | VC Imaging | VC Site | VC Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Tsurata 200851a | Non-RCT | 7–13 | HD, SHPT | Cinacalcet, n=8 | Control condition undefined, n=60 | Primary | CT | Coronary artery | Less progression with cinacalcet |
| Raggi 201152 (ADVANCE) | RCT | 12 | HD, SHPT | Cinacalcet + low-dose vitamin D, n=180 | Flexible doses of vitamin D, n=180 | Primary: % change in Agatston CAC score, secondary: % & absolute change for other sites | CT | Coronary arteries, thoracic aorta, aortic valve, mitral valve | Less progression at aortic valve with cinacalcet. Primary outcome no significant difference |
| Nakayama 201453 | Non-RCT | 36 | HD, SHPT | Cinacalcet, n=23 | No control group | Primary | CT | Abdominal aorta | Less progression with cinacalcet |
| Cruzado 201654 | RCT | 12 | Kidney transplant recipients | Cinacalcet, n=15 | Subtotal parathyroidectomy, n=15 | Secondary | CT | Thoracic/abdominal aorta; iliac arteries | No significant VC attenuation at all sites |
| Susantitaphong 201955 | Non-RCT | 8.3 (36 weeks) | HD, SHPT | Phase 2: 12 weeks of cinacalcet, n=45 | Phase 2: no control group | Secondary | X-ray lateral | Not reported | Less progression with cinacalcet |
| Eddington 202156 | RCT | 12 | HD, SHPT | Cinacalcet, n=15 | Standard treatment, n=21 | Primary | CT | Coronary artery, abdominal aorta | No significant difference at both sites |
Tsurata 200851 was included in the study participants median and range, but excluded from the study duration median and range calculations due to the duration provided being a range from 7 to 13.
HD, hemodialysis.
Vitamin D Therapy
Six trials involved vitamin D therapy with 289 participants (median participants 44, range 36–76; median duration 15 months, range 6–60 months) (Table 4).57–62 Half of these studies involved nutritional vitamin D (cholecalciferol) and half evaluated effects on VC of active vitamin D analogs (calcitriol and paricalcitol). All studies showed no difference in VC between groups, regardless of whether the vitamin D intervention was compared with placebo, standard of care, or another vitamin D comparator.
Table 4.
Summary of vitamin D study characteristics and VC outcome
| Author and Year | Type of Study | Study Duration (months) | Participant | Intervention (Participants at Baseline) | Comparator (Participants at Baseline) | VC as Primary or Secondary End Point | VC Imaging | VC Site | VC Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Baker 198657 | RCT | 60 | HD | Calcitriol, n=38 | Placebo, n=38 | Secondary | X-ray | Hands, feet, pelvis, coronary artery | No significant VC attenuation at all sites |
| Morosetti 200858 | Non-RCT | 24 | HD | High-dose calcitriol, n=18 | Low-dose calcitriol + sevelamer, n=18 | Primary | CT | Coronary artery | No significant VC attenuation |
| Delanaye 201359 | RCT | 12 | HD, serum 25(OH)D <30 ng/ml | Cholecalciferol, n=22 | Placebo, n=21 | Secondary | X-ray lateral | Abdominal aorta | No significant VC attenuation |
| Kidir 201560 | Non-RCT | 6 | HD or PD | Cholecalciferol, n=26 | Standard treatment, n=17 | Primary | X-ray hand & pelvis | Radial arteries, digital arteries, iliac arteries, femoral arteries | No significant VC attenuation at all sites |
| Samaan 201961 | RCT | 18 | CKD stage 3–4, serum 25(OH)D <30 ng/ml | Cholecalciferol, n=23 | Placebo, n=24 | Primary | CT | Coronary artery | No significant VC attenuation |
| Anis 202062 | RCT | 11 (48 weeks) | CKD stage 3–4 with SHPT | Calcitriol, n=22 | Paricalcitol, n=22 | Primary | CT | Coronary artery, aortic valve, mitral valve | No significant VC attenuation at all sites |
HD, hemodialysis; PD, peritoneal dialysis.
HMG-CoA Reductase Therapy
Two trials involved HMG-CoA reductase therapy with 199 participants and involved different CKD populations (nondialysis CKD and kidney transplant recipients) but with similar size and measurement of VC (Table 5).33,63 Both studies reported that statins did not reduce progression of VC despite benefits on dyslipidemia.
Table 5.
Summary of exercise and statin study characteristics and VC outcome
| Author and Year | Type of Study | Study Duration (months) | Participant | Intervention (Participants at Baseline) | Comparator (Participants at Baseline) | VC as Primary or Secondary End Point | VC Imaging | VC Site | VC Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Zhou 2020132 (RENEXC) | RCT | 12 | CKD stage 3–5 | Exercise: endurance training plus: Balance train, n=75 | Exercise: endurance training plus: Strength train, n=76 | Secondary | X-ray lateral | Abdominal aorta | No significant VC attenuation |
| Lemos 201333 | RCT | 24 | CKD stage 3–5 | Sevelamer, n=38 | Rosuvastatin, n=38 | Control condition undefined, n=41 | Primary | CT | Coronary artery | No significant VC attenuation |
| Yazbek 201663 | RCT | 12 | Kidney transplant recipients | Rosuvastatin, n=45 | Atorvastatin, n=16 (due to supply issue) | Control condition undefined, n=59 | Primary | CT | Coronary artery | No significant VC attenuation |
Antiresorptive Agents
Nine trials involved antiresorptive agents with 346 participants (median participants 31, range 12–101; median duration 12 months, range 6–23 months) (Table 6).64–72 Of these, seven reported reduced progression of VC, and one study which compared two antiresorptive agents (alendronate versus denosumab) did not show reduced progression of VC.72 Of seven trials involving bisphosphonates, four studies examined etidronate in hemodialysis participants and all reported attenuation of VC progression.64–67 The other three bisphosphonate studies had different study designs, populations, and interventions, with conflicting findings.68–70 Of two trials involving denosumab, one nonrandomized trial reported attenuation of VC compared with standard of care, and the other, an RCT, showed no difference in VC when compared with alendronate.71,72
Table 6.
Summary of antiresorptive study characteristics and VC outcome
| Author and Year | Type of Study | Study Duration (months) | Participant | Intervention (Participants at Baseline) | Comparator (Participants at Baseline) | VC as Primary or Secondary End Point | VC Imaging | VC Site | VC Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Hashiba 200464a | RCT | 12 | HD | Etidronate for 6 months, n=8 | Control group, n=10 | Primary | CT | Abdominal aorta | Less progression with etidronate |
| Nitta 200465 | Non-RCT | Approximately 12 | HD | Etidronate, n=35 | Control group, n=21 | Primary | CT | Coronary artery | Less progression with etidronate |
| Ariyoshi 200666 | RCT | 12 | HD | Etidronate, n=8 | No intervention, n=6 | Primary | CT | Coronary artery, thoracic and abdominal aorta | Less progression with etidronate at coronary (not significant) and aorta (significant) |
| Hashiba 200667 | RCT | 23 | HD | Etidronate for 23 months, n=12 | No intervention, n=12 | Primary | CT | Aorta | Less progression with etidronate |
| Torregrosa 201068 | RCT | 12 | Kidney transplant recipients | Risedronate, vitamin D, and calcium, n=52 | Vitamin D and calcium, n=49 | Secondary | X-ray | Iliac arteries, femoral arteries, radial arteries, digital arteries | Less progression with risedronate. No detail on each site |
| Toussaint 201069 | RCT | 18 | CKD stage 3–4 | Alendronate, n=25 | Placebo, n=26 | Primary: aortic VC, secondary: femoral VC | CT | Abdominal aorta, superficial femoral artery | No significant VC attenuation at both sites |
| Okamoto 201470 | RCT | 24 | Kidney transplant recipients | Alendronate, n=5 | No intervention, n=7 | Secondary | CT | Abdominal aorta | Less progression with alendronate |
| Iseri 201971 | RCT | 12 | HD | Denosumab sc, n=24 (+ calcitriol & calcium for first 2 weeks) | Alendronate iv, n=24 (+ calcitriol & calcium for first 2 weeks) | Secondary | CT | Coronary artery | No significant VC attenuation |
| Chen 202072 | Non-RCT | 6 | HD, SHPT | Denosumab, n=21 | Standard of care, n=21 | Primary | CT | Coronary artery | Less progression with denosumab |
Magnesium
Three trials involved magnesium with 202 participants and all reported reduction in progression of VC (Table 7).73–75 These trials, however, were limited by small sample size, and two of the studies, both involving dialysis patients, were not completed. One trial by Sakaguchi et al. was prematurely terminated based on significant efficacy of magnesium on CAC at interim analysis, and one study by Spiegel et al. was discontinued due to concerns about rapid VC progression in the calcium binder control arm.73,75 Of note, these two studies were conducted using magnesium as a phosphate binder (one with a calcium binder comparator), rather than systemic magnesium supplementation per se. Two studies reporting less progression of VC demonstrated an increase in serum magnesium concentration with magnesium therapy.74,75
Table 7.
Summary of magnesium and vitamin K study characteristics and VC outcome
| Author and Year | Type of Study | Study Duration (months) | Participant | Intervention (Participants at Baseline) | Comparator (Participants at Baseline) | VC as Primary or Secondary End Point | VC Imaging | VC Site | VC Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Spiegel 200973 | Non-RCT | 18 | HD | Mg/CaCO3, n=7 | No control group | Primary | EB-CT | Coronary artery | Less progression with magnesium (P=0.07) |
| Tzanakis 201474 | RCT | 12 | HD | MgCO3 + calcium acetate, n=36 | Calcium acetate, n=36 | Primary | X-ray | Hands, abdomen, pelvis, femur | Less progression with magnesium. No detail on each site (increased serum Mg with MgCO3) |
| Sakaguchi 201975 Prematurely terminated due to significant efficacy of MgO | RCT 2 × 2 factorial | 24 | CKD stage 3–4 | MgO + AST-120, n=35 MgO + control, n=28 | Control + AST-120, n=35 Control + control, n=25 | Primary | CT | Coronary artery, thoracic aorta | MgO but not AST-120 appeared to slow VC progression in coronary artery but not thoracic aorta (increased serum Mg with MgO) |
| Kurnatowska 201576 | RCT | 9 (270 days) | CKD stage 3–5 | Vitamin K2 + cholecalciferol, n=29 | Cholecalciferol 10 μg, n=13 | Primary | CT | Coronary artery | No significant VC attenuation |
| Li 201777 | RCT | 3 | HD | Vitamin K dialysis buffer, n=50 | No vitamin K dialysis buffer, n=50 | Primary | CT | Coronary artery | Less progression with vitamin K dialysate |
| Oikonomaki 201978 | RCT | 12 | HD | Vitamin K2 daily, n=44 | No vitamin K2, n=58 | Primary | CT | Abdominal aorta | No significant VC attenuation |
| De Vriese 202079 (Valkyrie) | RCT | 18 | HD with atrial fibrillation | Rivaroxaban + vitamin K2 3×/week postdialysis, n=42 | Rivaroxaban daily alone, n=46 | VKA continued or initiated, n=44 | Primary | CT | Coronary artery, thoracic aorta, cardiac valve | No significant VC attenuation at all sites |
| Witham 202080 (K4Kidneys) | RCT | 12 | CKD stage 3b–4 | Vitamin K2 daily, n=80 | Placebo, n=79 | Secondary | X-ray lateral | Abdominal aorta | No significant VC attenuation |
| Levy-Schousboe 202181 (RenaKvit) | RCT | 24 | HD | Vitamin K2, n=24 | Placebo, n=24 | Secondary | CT for heart, x-ray for aorta | Coronary, aortic valve, mitral valve, abdominal aorta | No significant VC attenuation at all sites |
HD, hemodialysis; VKA, vitamin K antagonist.
Vitamin K
Six trials involved vitamin K therapy with 583 participants (median participants 101, range 42–159; median duration 12 months, range 3–24 months) (Table 7).76–81 One study reported a benefit on CAC of vitamin K in dialysate over a 3-month period in an RCT of 100 hemodialysis participants.77 The remaining five studies reported no benefit on attenuation of VC progression with oral vitamin K2 therapy.
Sodium Thiosulfate
Seven trials involved sodium thiosulfate with 251 participants (median participants 32, range 12–60; median duration 6 months, range 3–6 months) (Table 8).82–88 All trials involved participants on hemodialysis and all reported reduction in progression of VC with sodium thiosulfate. The largest study by Djuric et al. randomized 60 participants to either sodium thiosulfate or saline over 6 months87 and reported attenuation of VC progression in iliac arteries and cardiac valves, but not the abdominal aorta which was the prespecified primary outcome.
Table 8.
Summary of sodium thiosulfate study characteristics and VC outcome
| Author and Year | Type of Study | Study Duration (months) | CKD Status | Intervention (Participants at Baseline) | Comparator (Participants at Baseline) | VC as Primary or Secondary End Point | VC Imaging | VC Site | VC Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Adirekkiat 201082 | Non-RCT | 4 | HD | STS post-HD twice weekly, n=16 | Dialysis as usual, n=16 | Primary | CT | Coronary artery | Less progression with STS |
| Mathews 201183 | Non-RCT | 5 | HD | STS post-HD three times per week, n=22 | No control group | Primary | CT | Carotid artery, coronary artery, thoracic arch | Less progression with STS |
| Yonova 201484 | Non-RCT | 6 | HD | STS intradialytic, n=6 | Controls, n=6 | Primary | CT | Coronary artery | Less progression with STS |
| Yu 201685 | RCT | 3 | HD | STS post-HD + calcium carbonate, n=15 | Calcium carbonate, n=10 | Primary | CT | Coronary artery | Less progression with STS |
| Saengpanit 201886 | RCT | 6 | HD | STS intradialytic twice weekly, n=24 | Standard of care, n=26 | Secondary | CT | Coronary artery | Less progression with STS |
| Djuric 202087 | RCT | 6 | HD | STS post-HD, n=30 | 0.9% NaCl during dialysis, n=30 | Primary: abdominal aorta, secondary: iliac | CT | Abdominal aorta, iliac arteries, cardiac valves | Less progression with STS at iliac arteries |
| Bian 202188 | RCT | 6 | HD | STS, n=25 | Usual treatment, n=25 | Primary | CT | Coronary artery | Less progression with STS |
HD, hemodialysis; STS, sodium thiosulfate.
Risk of Bias and GRADE Assessment
Supplemental Table 2 summarizes the risk of bias assessment for RCTs. Random sequence generation and allocation concealment were reported with low risks of bias in 45% and 47% of trials, respectively. Blinding of participants and investigators to the allocated intervention and blinding to outcomes were reported with low risk of bias in 22% and 77% of trials, respectively.
Table 10 outlines the GRADE assessment for quality of evidence of studies for each intervention. The majority of analyses were based on low certainty of evidence, primarily due to risk of bias, inconsistency of results, imprecision of reporting, and publication bias. Inconsistency grading was based on heterogeneity of study design and study outcome. Imprecision grading was based on small event numbers.
Table 10.
Summary of GRADE findings
| Intervention | Risk of Bias | Consistency | Imprecision | Indirectness | Publication Bias (No. of Studies) | Quality of Evidence (GRADE) | Comments |
|---|---|---|---|---|---|---|---|
| Phosphate binders | Moderate | Not all RCTs, inconsistent results | None | CKD | 25 | ⊕⊕OO Due to risk of bias and inconsistency | Many studies did not blind participants and investigators |
| Alterations to dialysis prescription | Moderate | Not all RCTs, inconsistent results | None, x-ray | CKD | 8 | ⊕OOO Due to risk of bias, inconsistency, and imprecision | Half used x-ray to assess VC |
| Calcimimetics | High | Not all RCTs, consistent results | None | CKD | 6 | ⊕⊕OO Due to risk of bias and inconsistency | Major study was industry sponsored. Half were non-RCTs |
| Vitamin D | Moderate | Not all RCTs, consistent results | Few participants, x-ray | CKD | 6 | ⊕OOO Due to risk of bias, inconsistency & imprecision | 2 out of 6 were non-RCTs. Half used x-ray to assess VC |
| Exercise | Moderate | RCT | Few participants, x-ray | CKD | 1 | ⊕OOO Due to risk of bias, imprecision, and publication bias | Only 1 study |
| Statins | Moderate | All RCTs, consistent results | Few participants | CKD | 2 | ⊕OOO Due to risk of bias, imprecision, and publication bias | |
| Antiresorptive agents | Moderate | Not all RCTs, inconsistent results | Few participants | CKD | 9 | ⊕ΟΟΟ Due to risk of bias, inconsistency, and imprecision | 4 out of 9 studies were missing information on randomization method and blinding |
| Magnesium | Moderate | Not all RCTs, consistent results | Few participants, x-ray | CKD | 3 | ⊕ΟΟΟ Due to risk of bias, inconsistency, imprecision, and publication bias | |
| Vitamin K | Moderate | All RCTs | None | CKD | 6 | ⊕⊕⊕O Due to risk of bias | |
| Sodium thiosulfate | Moderate | Not all RCTs, consistent results | Few participants | CKD | 7 | ⊕ΟΟΟ Due to risk of bias, inconsistency, imprecision | 3 out of 7 were non-RCTs |
| SNF472 | High | RCT | Few participants | CKD | 1 | ⊕OOO Due to imprecision and publication bias | Only 1 study, which was industry sponsored |
| Spironolactone | Low | RCT | Few participants | CKD | 1 | ⊕⊕OO Due to imprecision and publication bias | Only 1 study |
| Sotatercept | High | RCT | Few participants | CKD | 1 | ⊕ΟΟΟ Due to risk of bias, imprecision, and publication bias | Only 1 study |
| Nicotinamide | Low | RCT | Few participants, x-ray | CKD | 1 | ⊕⊕OO Due to imprecision and publication bias | Only 1 study |
| Oral activated charcoal | Moderate | RCT | Few participants | CKD | 1 | ⊕ΟΟΟ Due to risk of bias, imprecision, and publication bias | Only 1 study |
All interventions were compared with placebo, control, or another comparator, with VC as the outcome. High quality ⊕⊕⊕⊕; moderate quality ⊕⊕⊕O; low quality ⊕⊕OO; very low quality ⊕ΟΟΟ.
Discussion
We present the first systematic review of prospective clinical trials evaluating effects of all reported interventions on VC in people with CKD. Previous systematic reviews published have only appraised the effect of single interventions on VC in the CKD population.89–92 Our review identified a large number of heterogeneous prospective clinical trials (mostly RCTs) of numerous interventions studied to evaluate their potential effect on VC in people with CKD. Beneficial effects were more consistently seen with magnesium, sodium thiosulfate, and etidronate in hemodialysis participants. Overall, however, trials were of small sample size (median 50 participants) and generally short in study duration (median 12 months).
Heterogeneity in clinical trials was seen with participant characteristics including CKD stage, with most studies involving patients on dialysis. In the nondialysis CKD population, only four studies included in this systematic review reported attenuation of VC progression. Furthermore, the effect on VC of certain interventions has not been evaluated in clinical trials involving specific CKD cohorts, such as HMG-CoA reductase inhibitors and sodium thiosulfate on dialysis patients and nondialysis patients, respectively. Further studies are required to specifically target the nondialysis CKD population, as VC response to therapy may be different in this population compared with patients on dialysis. For example, the safety and efficacy of phosphate binders in nondialysis CKD has been recently called into question,93 in contrast to the potential benefit of noncalcium-based compared with calcium-based phosphate binders in dialysis patients.94
Heterogeneity was also seen in trials with regard to baseline VC, study design (randomized and nonrandomized), study duration, VC imaging modality, and VC anatomic site. No major differences were seen in our systematic review of randomized and nonrandomized studies on the effect of various interventions on VC, with similar proportions reporting no change. Five studies in our review were <6 months in duration (three sodium thiosulfate, one phosphate binder, and one vitamin K), and all demonstrated attenuation of VC progression. This suggests changes in VC can be demonstrated over a short duration and studies may not require long-term follow-up to determine the effects of interventions on VC in CKD, unlike hard cardiovascular end points.
A major limitation of the clinical trials included in this systematic review is that they all rely on interventions that not only lack specificity for VC but also undoubtedly target both intimal and medial VC, possibly obscuring a potential effect of these therapies and contributing to the inconsistent and inconclusive findings. This is an important issue as these two types of VC are pathobiologically distinct and disproportionately affected by CKD, with the burden of medial VC cumulatively much greater with declining kidney function. The consequences of targeting intimal and medial VC may also be divergent, because there is evidence that accelerated calcification of atheromatous lesions, as seen with high-intensity statin therapy in non-CKD populations,95,96 might be beneficial due to stabilization of the plaque and reduced risk of rupture. Of note, 48 of the 77 trials reviewed here assessed VC of the coronary arteries, which even in patients on dialysis remains predominantly intimal in nature.97,98 This issue is further compounded by the fact that conventional imaging techniques lack sufficient spatial resolution to reliably distinguish medial and intimal VC, and have insufficient sensitivity to detect smaller calcifications that are thought to be indicative of active mineral deposition.99
The most common intervention evaluated with respect to VC attenuation was oral phosphate binder therapy (34% of studies reviewed), with trials predominantly exploring the potential superiority of noncalcium-based compared with calcium-based binders. It was also notable that a considerable number of these studies involved pharmaceutical industry sponsorship. Our results are consistent with a previous systematic review on phosphate binders that also reported that reliable conclusions cannot be drawn on mortality and cardiovascular end points.90 Recently, the efficacy and safety of phosphate binders has been questioned by the updated KDIGO CKD-MBD guidelines,15 highlighting a lack of trial data for phosphate binder use especially in the nondialysis CKD population. Despite their widespread use for hyperphosphatemia in CKD, the effect of phosphate binders and phosphate lowering per se on VC, cardiovascular risk, or indeed any patient-centered outcome remains uncertain. To be informative, future trials of phosphate binders should only test effects of active therapy compared with no treatment or placebo.
Various alterations in dialysis prescription have been studied to assess the effect on VC, but with contradictory outcomes or insufficient evidence. Some studies suggest that low dialysate calcium concentrations may attenuate VC progression compared with high dialysate calcium concentrations,46,47 but others report no difference between different dialysate calcium concentrations on VC.49,50 Potential adverse effects of higher dialysate calcium on VC may result from calcium loading, similar to data assessing calcium-based phosphate binders with excess exogenous calcium. Two of the larger and longer dialysate calcium studies concluded with conflicting findings on the effects of high dialysate calcium,44,47 and therefore there is no consistent evidence for attenuation of VC progression with low dialysate calcium. These conflicting results may relate to the fact that serum calcium levels in general are likely maintained too high in patients on dialysis, with correction of hypocalcemia perhaps leading to promotion of VC.100 There is also minimal evidence for any other dialysis-specific interventions, with a paucity of trials evaluating effects on VC of other changes in dialysis prescription.
Clinical trials involving cinacalcet in dialysis patients consistently demonstrated potential reduction in progression of VC; however, studies were small and were potentially confounded by concomitant vitamin D administration. The other confounder with trials involving cinacalcet is that serum calcium levels were not allowed to decrease considerably and therefore interventions to maintain serum calcium in these trials may have limited the ability to see a more definitive benefit of calcimimetics on reducing VC progression.
Trials of vitamin D treatment alone consistently found no benefit on mitigation of VC. Currently, it is recommended that vitamin D therapy be prescribed in people with CKD due to reduced activation of vitamin D precursors by the kidneys, leading to hypocalcemia, SHPT, and bone resorption.101 Studies examining the effect of various forms of vitamin D treatment on VC, across the stages of CKD, do not indicate benefit for reducing progression of VC. Interestingly, even trials involving CKD participants with documented vitamin D deficiency did not demonstrate any beneficial effect of vitamin D supplementation on VC.59–61 Studies have demonstrated that calcitriol and other vitamin D receptor activators in nondialysis CKD participants may increase the risk of hypercalcemia, without cardiovascular benefit.102,103 The updated KDIGO CKD-MBD guidelines have consequently recommended reserving the use of calcitriol and other vitamin D receptor analogs for people with CKD who have severe and progressive SHPT.15
Lipid-lowering and other potential antiatherosclerotic effects of HMG-CoA reductase inhibitors appear to be insufficient in addressing the multifactorial pathogenesis of VC in CKD. Two studies in our systematic review evaluated the effect of statins in different CKD populations but without any benefit with respect to VC.33,63 These findings were consistent with a recent meta-analysis of statin studies in the non-CKD population, proposing that a multitarget drug approach is required to target both intimal and medial pathways for VC development.104 Interestingly, in secondary prevention studies of predominantly non-CKD populations, CAC has been shown to increase in those receiving high-intensity statin therapy despite improvement in clinical outcomes.95,105
Although the majority of trials involving antiresorptive agents in this systematic review reported possible attenuation of VC progression, use of these agents in people with CKD remains contentious. The effect of bisphosphonates on attenuation of VC in CKD is still unclear despite several studies in hemodialysis patients suggesting benefits on VC. Although the initial hypothesis underlying these studies was that increased bone resorption may lead to increased calcium deposition in the vasculature, the potential beneficial mechanism of action of bisphosphonates is that they are nonhydrolyzable analogs of pyrophosphate, which is a potent endogenous and direct inhibitor of hydroxyapatite formation.106 This is an important consideration when interpreting clinical trials of bisphosphonates, as etidronate, the first bisphosphonate, is a very good analog of pyrophosphate (which also may explain its side effect of osteomalacia) and studies of etidronate show consistent reduction in progression of VC. Subsequent generations of bisphosphonates were developed with more potency in inhibiting osteoclasts and are used at much lower concentrations, thus resulting in less pyrophosphate mimicry. Studies of bisphosphonates were generally small and of short duration, and there are potential concerns about bisphosphonate accumulation in dialysis patients due to its renal excretion and an increased risk of low bone turnover.106 Denosumab is not renally excreted although numerous studies have reported an increased risk of hypocalcemia,71 especially during the first 2 weeks of treatment in CKD cohorts,107 and evidence for a true benefit on attenuation of VC is lacking.
Magnesium supplementation has been proposed to exert beneficial effects on multiple phases of the calcification pathway, including downregulation of promoters of vascular smooth muscle cell osteogenic differentiation (Wnt/β-catenin, osteocalcin), upregulation of calcification inhibitors (osteopontin, MGP, BMP-7), and restoration of activity of TRPM7 channels.108 Magnesium is also a direct inhibitor of hydroxyapatite crystal formation and this may be the most likely mechanism of action to reduce VC.109 Magnesium has also been used as an intestinal phosphate binder, mentioned in two studies in our systematic review. Both RCTs involving treatment with magnesium reported reduction in progression of VC.74,75 Interestingly, Sakaguchi et al. also reported that progression of CAC, but not thoracic aorta VC, was reduced (greater reduction in those with higher CAC scores), prompting speculation that different pathophysiology between these sites requires a different therapeutic target.75 Although there is still a paucity of evidence for attenuation of VC progression by magnesium, results from these studies suggest magnesium may be a promising intervention that is relatively cheap and widely available. Concerns about magnesium supplementation contributing to hypermagnesemia in people with advanced CKD due to its renal excretion have been addressed by small clinical trials.110 Data about the possible risk of reduced bone mineral density with magnesium supplementation will be provided from ongoing trials.111
Despite the adverse effect of warfarin, a vitamin K antagonist, promoting VC and experimental studies reporting benefits of vitamin K on attenuating VC, clinical trials involving vitamin K (in the form of vitamin K2) predominantly reported no effect on VC progression. Vitamin K is an essential cofactor for the carboxylase enzyme that activates uncarboxylated MGP (uc-MGP) into carboxylated MGP, one of the most potent systemic VC inhibitors, and a decreased level of dephosphorylated uc-MGP (dp-uc-MGP) has been proposed as a marker of vascular vitamin K status.112,113 Patients with advanced CKD are often vitamin K deficient, in part due to dietary restrictions but also due to possible impaired endogenous recycling of vitamin K. Only one of six trials evaluating effects of vitamin K on VC in CKD reported reduction in progression of VC. Interestingly, VC change in this study occurred after only 3 months of a vitamin K dialysis buffer, and dp-uc-MGP was not measured. The other five trials reported no effect on VC, but did show reduction in dp-uc-MGP to assess the adequacy of oral supplementation.76,78–81 Notably, the Valkyrie trial by De Vriese et al. reported dp-uc-MGP reduction was not normalized,79 with the median level remaining elevated compared with the general population. The authors speculated that uremia might inactivate the carboxylase enzyme, such that supplementation with vitamin K2 does not enable MGP levels to normalize and does not inhibit VC due to a functional deficiency. Thus, it is unclear whether vitamin K2 supplementation can restore MGP levels let alone inhibit VC in CKD. Unless such a functional deficiency can be reversed therapeutically, the relatively high quality of evidence of null efficacy of vitamin K on VC compared with other therapies suggests that there is little virtue in conducting further RCTs of vitamin K in this patient population. In contrast, in the non-CKD population there is evidence that vitamin K1 does slow CAC progression.114
Intravenous sodium thiosulfate may be a promising intervention to reduce progression of VC, although its exact mechanism of action remains uncertain. Sodium thiosulfate was initially thought to reduce VC by forming a soluble complex with calcium from tissue deposits, with more recent evidence pointing toward thiosulfate acting as an antioxidant H2S-donor in vivo; however, these hypotheses were disproved as major effects.11,84,115–117 Seven clinical trials demonstrated reduction in VC progression. Notably, all were in participants on hemodialysis and all were 6 months or less in duration.82–88 Studies have shown that conventional CVD risk factors are more strongly associated with abdominal aortic VC, whereas CKD-related calcium and phosphate disturbances are more strongly associated with CAC.118,119 This is debatable, however, as CAC is generally considered intimal in nature and in the CKD population it may be that CAC lesions are more calcified (i.e., of greater severity). Sodium thiosulfate may have a greater effect on smaller peripheral arteries compared with larger vessels but further RCTs evaluating effects of sodium thiosulfate on VC should be longer, adequately powered, and ideally placebo-controlled.
Multiple interventions included in our systematic review only had a single clinical trial assessing effects on VC, which was insufficient to draw conclusions. Studies of SNF472, sotatercept, and oral activated charcoal each reported attenuation of VC progression, whereas studies of exercise training, spironolactone, and nicotinamide did not. We did not include in our review other studies which reported effects on VC after changes in kidney replacement therapies, such as several observational studies assessing the effect of kidney transplantation on VC120–125 and several evaluating changes in VC after commencement of more frequent or longer hours of hemodialysis (such as nocturnal dialysis).123,126,127 These studies have predominantly reported that VC progression is attenuated by both kidney transplantation and nocturnal hemodialysis when compared with continuation of conventional hemodialysis, but not all studies have consistently shown this.119,124,125
The strengths of this review include a systematic search of medical databases, with data extraction, analysis, and quality assessment involving two independent reviewers. Inclusion of both randomized and nonrandomized clinical trials across all stages of CKD was comprehensive but heterogeneity in participant populations and methods of quantifying VC prevented meta-analysis. Our systematic review was limited by studies of small sample size. Further, many studies were not adequately designed with sufficient sample size and statistical power to determine differences in VC as this outcome measure was a secondary end point in some trials. VC is also only a surrogate measure of CVD and it is unclear what magnitude of changes in VC (if any) may translate into a clinically meaningful effect on cardiovascular health. Indeed, if it transpires that VC is a secondary phenomenon to antecedent inflammatory processes as suggested by numerous preclinical studies,128 then targeting VC per se is unlikely to produce any clinical improvement. Moreover, with the exception of SNF472 (and perhaps etidronate and magnesium) whose effects are specific to mineral growth inhibition, the broad physiologic effects of the other interventions reviewed here imply that, even if effective, these therapies could not provide definitive proof that VC has a causal role in CVD outcomes in patients with CKD.
This systematic review found a large number of heterogeneous clinical trials for all interventions that have been shown to effect VC progression in people with CKD, many with conflicting results and providing a low quality of evidence (Table 11). The studies highlight that there are multiple therapeutic targets to address the complex and multifactorial VC pathogenesis in CKD, and further trials need to be more adequately powered to determine whether any intervention is beneficial to attenuate VC progression in this population. Choice of an effective intervention may need to be better tailored to baseline patient characteristics, such as presence of conventional cardiovascular risk factors, hyperphosphatemia, vitamin D deficiency, bone mineral density, and uremia. Appropriate selection of patients on the basis of more objective functional assessments of systemic calcification propensity such as the T50 test,129,130 could be considered in future trial protocols. Translation of promising research imaging tools with improved spatial resolution and sensitivity to better distinguish medial and intimal VC and detect earlier microcalcifications (e.g., fused positron emission tomography-CT using the hydroxyapatite-seeking positron-emitting isotope 18F-fluoride) may also provide more insight into the efficacy of existing and emerging drug candidates.
Table 11.
Summary table of evidence-based interventions reported to attenuate VC progression in CKD
| Probably Reduce VC Progression | Possibly Reduce VC Progressiona | Unlikely To Reduce VC Progression |
|---|---|---|
| Magnesium | Antiresorptive therapy | Vitamin D therapy |
| Sodium thiosulfate | Calcimimetics | Vitamin K2 |
| Kidney transplantation | SNF472 | HMG-CoA reductase inhibitors |
| Increased hours hemodialysis (nocturnal dialysis) | Low dialysate calcium concentration | Exercise |
| Noncalcium-based phosphate binders (when compared with calcium-based binders) | Strict control of phosphate balance | Phosphate binders (compared with placebo/standard of care) |
| Sotatercept | Spironolactone | |
| Oral activated charcoal | Nicotinamide |
There is a paucity of evidence or conflicting results.
Based on this systematic review, the most effective evidence-based strategies to reduce progression of VC in CKD appear to be treatment with magnesium, sodium thiosulfate, and etidronate, and reducing exogenous calcium loading, but these findings require confirmation in larger cohorts. Future clinical trials of single interventions to slow VC progression should prioritize inhibitors of calcification (such as sodium thiosulfate, magnesium, SNF472, or etidronate), or administered in conjunction with efforts to restrict calcium and phosphate, as multiple interventions simultaneously tackling both insufficient inhibition and promoter excess may be required to redress the propensity to calcify. However, augmentation of calcification inhibition with either endogenous or synthetic analogs will require prudent evaluation of potentially deleterious effects on bone health given the common mineral target.131 Osteomalacia is a known side effect of etidronate and reduced bone mineral density has recently been reported with SNF472.131 Because interventions aimed at modifying advanced VC lesions are unlikely to produce tangible health benefits, future trials should ideally focus on the nondialysis CKD population where long-term patient gains may be cumulatively greater. Critically, however, we await definitive evidence that targeting VC yields the anticipated improvement in patient-centered outcomes and this needs to be embraced as a research priority by public funding entities to lessen the potential risk of bias incurred by pharmaceutical company involvement as has undoubtedly been the case with phosphate binders.
Disclosures
E.R. Smith holds stock in Calciscon; has consultancy agreements with Vifor Pharma; has received research funding from Amgen, Baxter, Sanofi, and Vifor Pharma; has received honoraria from Shire; and is a recipient of a Viertel Charitable Foundation Clinical Investigator award. M. Tiong is a member of the Australia and New Zealand Dialysis and Transplant Registry and is a member of the Aboriginal and Torres Strait Islander Health Working Group. N.D. Toussaint has received honoraria, travel support, and research funding from Amgen, Sanofi, Shire, and Takeda. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
We thank Jim Berryman for assistance with the search strategy and the Royal Melbourne Hospital library for assistance in retrieving full text articles.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Author Contributions
N. Toussaint and C. Xu conceptualized the study, were responsible for data curation, formal analysis, investigation, methodology, and validation, and wrote the original draft; I. Ruderman, E. Smith, M. Tiong, and N. Toussaint were responsible for providing supervision; and all authors reviewed and edited the manuscript.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021101327/-/DCSupplemental.
Supplemental Table 1. Search strategy.
Supplemental Table 2. Risk of bias assessment for RCTs.
References
- 1.Braun J, Oldendorf M, Moshage W, Heidler R, Zeitler E, Luft FC: Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients. Am J Kidney Dis 27: 394–401, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Banks LM, Lees B, MacSweeney JE, Stevenson JC: Effect of degenerative spinal and aortic calcification on bone density measurements in post-menopausal women: Links between osteoporosis and cardiovascular disease? Eur J Clin Invest 24: 813–817, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Rix M, Andreassen H, Eskildsen P, Langdahl B, Olgaard K: Bone mineral density and biochemical markers of bone turnover in patients with predialysis chronic renal failure. Kidney Int 56: 1084–1093, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, Olgaard K, et al. ; Kidney Disease: Improving Global Outcomes (KDIGO) : Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69: 1945–1953, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Coen G, Pierantozzi A, Spizzichino D, Sardella D, Mantella D, Manni M, et al. : Risk factors of one year increment of coronary calcifications and survival in hemodialysis patients. BMC Nephrol 11: 10, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCullough PA, Agrawal V, Danielewicz E, Abela GS: Accelerated atherosclerotic calcification and Monckeberg’s sclerosis: A continuum of advanced vascular pathology in chronic kidney disease. Clin J Am Soc Nephrol 3: 1585–1598, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Lanzer P, Boehm M, Sorribas V, Thiriet M, Janzen J, Zeller T, et al. : Medial vascular calcification revisited: Review and perspectives. Eur Heart J 35: 1515–1525, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, Weissberg PL: Apoptosis regulates human vascular calcification in vitro: Evidence for initiation of vascular calcification by apoptotic bodies. Circ Res 87: 1055–1062, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, et al. : Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: A potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol 15: 2857–2867, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Doehring LC, Heeger C, Aherrahrou Z, Kaczmarek PM, Erdmann J, Schunkert H, et al. : Myeloid CD34+CD13+ precursor cells transdifferentiate into chondrocyte-like cells in atherosclerotic intimal calcification. Am J Pathol 177: 473–480, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, et al. : Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res 104: 733–741, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moe SM, Reslerova M, Ketteler M, O’neill K, Duan D, Koczman J, et al. : Role of calcification inhibitors in the pathogenesis of vascular calcification in chronic kidney disease (CKD). Kidney Int 67: 2295–2304, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Ruderman I, Holt SG, Hewitson TD, Smith ER, Toussaint ND: Current and potential therapeutic strategies for the management of vascular calcification in patients with chronic kidney disease including those on dialysis. Semin Dial 31: 487–499, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM: Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int 71: 438–441, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, et al. : Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: What’s changed and why it matters. Kidney Int 92: 26–36, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. ; GRADE Working Group : GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336: 924–926, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC: Assessing risk of bias in a randomized trial. In: Cochrane Handbook for Systematic Reviews of Interventions, version 6.2, edited by Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, Wiley-Blackwell, 2021 [Google Scholar]
- 18.Sperschneider H, Günther K, Marzoll I, Kirchner E, Stein G: Calcium carbonate (CaCO3): An efficient and safe phosphate binder in haemodialysis patients? A 3-year study. Nephrol Dial Transplant 8: 530–534, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Chertow GM, Burke SK, Raggi P; Treat to Goal Working Group : Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 62: 245–252, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Braun J, Asmus HG, Holzer H, Brunkhorst R, Krause R, Schulz W, et al. : Long-term comparison of a calcium-free phosphate binder and calcium carbonate--phosphorus metabolism and cardiovascular calcification. Clin Nephrol 62: 104–115, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Asmus HG, Braun J, Krause R, Brunkhorst R, Holzer H, Schulz W, et al. : Two year comparison of sevelamer and calcium carbonate effects on cardiovascular calcification and bone density. Nephrol Dial Transplant 20: 1653–1661, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, et al. : Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 68: 1815–1824, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Ferramosca E, Burke S, Chasan-Taber S, Ratti C, Chertow GM, Raggi P: Potential antiatherogenic and anti-inflammatory properties of sevelamer in maintenance hemodialysis patients. Am Heart J 149: 820–825, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Russo D, Miranda I, Ruocco C, Battaglia Y, Buonanno E, Manzi S, et al. : The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int 72: 1255–1261, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Barreto DV, Barreto FC, de Carvalho AB, Cuppari L, Draibe SA, Dalboni MA, et al. : Phosphate binder impact on bone remodeling and coronary calcification--results from the BRiC study. Nephron Clin Pract 110: c273–c283, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Qunibi W, Moustafa M, Muenz LR, He DY, Kessler PD, Diaz-Buxo JA, et al. ; CARE-2 Investigators : A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: The Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis 51: 952–965, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Takei T, Otsubo S, Uchida K, Matsugami K, Mimuro T, Kabaya T, et al. : Effects of sevelamer on the progression of vascular calcification in patients on chronic haemodialysis. Nephron Clin Pract 108: c278–c283, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Kakuta T, Tanaka R, Hyodo T, Suzuki H, Kanai G, Nagaoka M, et al. : Effect of sevelamer and calcium-based phosphate binders on coronary artery calcification and accumulation of circulating advanced glycation end products in hemodialysis patients. Am J Kidney Dis 57: 422–431, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Toussaint ND, Lau KK, Polkinghorne KR, Kerr PG: Attenuation of aortic calcification with lanthanum carbonate versus calcium-based phosphate binders in haemodialysis: A pilot randomized controlled trial. Nephrology (Carlton) 16: 290–298, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, et al. : Effects of phosphate binders in moderate CKD. J Am Soc Nephrol 23: 1407–1415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Iorio B, Bellasi A, Russo D; INDEPENDENT Study Investigators : Mortality in kidney disease patients treated with phosphate binders: A randomized study. Clin J Am Soc Nephrol 7: 487–493, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Kalil RS, Flanigan M, Stanford W, Haynes WG: Dissociation between progression of coronary artery calcification and endothelial function in hemodialysis patients: A prospective pilot study. Clin Nephrol 78: 1–9, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemos MM, Watanabe R, Carvalho AB, Jancikic AD, Sanches FM, Christofalo DM, et al. : Effect of rosuvastatin and sevelamer on the progression of coronary artery calcification in chronic kidney disease: A pilot study. Clin Nephrol 80: 1–8, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Ohtake T, Kobayashi S, Oka M, Furuya R, Iwagami M, Tsutsumi D, et al. : Lanthanum carbonate delays progression of coronary artery calcification compared with calcium-based phosphate binders in patients on hemodialysis: A pilot study. J Cardiovasc Pharmacol Ther 18: 439–446, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Seifert ME, de las Fuentes L, Rothstein M, Dietzen DJ, Bierhals AJ, Cheng SC, et al. : Effects of phosphate binder therapy on vascular stiffness in early-stage chronic kidney disease. Am J Nephrol 38: 158–167, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wada K, Wada Y: Evaluation of aortic calcification with lanthanum carbonate vs. calcium-based phosphate binders in maintenance hemodialysis patients with type 2 diabetes mellitus: An open-label randomized controlled trial. Ther Apher Dial 18: 353–360, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Russo D, Bellasi A, Pota A, Russo L, Di Iorio B: Effects of phosphorus-restricted diet and phosphate-binding therapy on outcomes in patients with chronic kidney disease. J Nephrol 28: 73–80, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Wang XH, Zhang X, Mu CJ, He Y, Peng QP, Yang GS, et al. : Effects of lanthanum carbonate on vascular calcification in elderly maintenance hemodialysis patients. J Huazhong Univ Sci Technolog Med Sci 35: 508–513, 2015 [DOI] [PubMed] [Google Scholar]
- 39.Zhang C, Wang S, Zhao S, Zhang X: Effect of lanthanum carbonate on coronary artery calcification and bone mineral density in maintenance hemodialysis patients with diabetes complicated with adynamic bone disease: A prospective pilot study. Medicine (Baltimore) 96: e8664, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujii H, Kono K, Nakai K, Goto S, Nishii T, Kono A, et al. : Effects of lanthanum carbonate on coronary artery calcification and cardiac abnormalities after initiating hemodialysis. Calcif Tissue Int 102: 310–320, 2018 [DOI] [PubMed] [Google Scholar]
- 41.Toussaint ND, Pedagogos E, Lioufas NM, Elder GJ, Pascoe EM, Badve SV, et al. ; IMPROVE-CKD Trial Investigators : A randomized trial on the effect of phosphate reduction on vascular end points in CKD (IMPROVE-CKD). J Am Soc Nephrol 31: 2653–2666, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isaka Y, Hamano T, Fujii H, Tsujimoto Y, Koiwa F, Sakaguchi Y, et al. : Optimal phosphate control related to coronary artery calcification in dialysis patients. J Am Soc Nephrol 32: 723–735, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mune M, Yukawa S, Kishino M, Otani H, Kimura K, Nishikawa O, et al. : Effect of vitamin E on lipid metabolism and atherosclerosis in ESRD patients. Kidney Int Suppl 71: S126–S129, 1999 [PubMed] [Google Scholar]
- 44.Zhang DL, Wang LY, Sun F, Zhou YL, Duan XF, Liu S, et al. : Is the dialysate calcium concentration of 1.75 mmol/L suitable for Chinese patients on maintenance hemodialysis? Calcif Tissue Int 94: 301–310, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Fu X, Cui QQ, Ning JP, Fu SS, Liao XH: High-flux hemodialysis benefits hemodialysis patients by reducing serum FGF-23 levels and reducing vascular calcification. Med Sci Monit 21: 3467–3473, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu JR, Yi Y, Xiong ZX, Cheng XF, Hu J, Hang HY, et al. : The study of low calcium dialysate on elderly hemodialysis patients with secondary hypoparathyroidism. Blood Purif 42: 3–8, 2016 [DOI] [PubMed] [Google Scholar]
- 47.Ok E, Asci G, Bayraktaroglu S, Toz H, Ozkahya M, Yilmaz M, et al. : Reduction of dialysate calcium level reduces progression of coronary artery calcification and improves low bone turnover in patients on hemodialysis. J Am Soc Nephrol 27: 2475–2486, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voiculeț C, Zară O, Bogeanu C, Văcăroiu I, Aron G: The role of oral sodium bicarbonate supplementation in maintaining acid-base balance and its influence on the cardiovascular system in chronic hemodialysis patients - results of a prospective study. J Med Life 9: 449–454, 2016 [PMC free article] [PubMed] [Google Scholar]
- 49.Kim SJ, Lee YK, Oh J, Cho A, Noh JW: Effects of low calcium dialysate on the progression of coronary artery calcification in hemodialysis patients: An open-label 12-month randomized clinical trial. Int J Cardiol 243: 431–436, 2017 [DOI] [PubMed] [Google Scholar]
- 50.Masterson R, Blair S, Polkinghorne KR, Lau KK, Lian M, Strauss BJ, et al. : Low versus high dialysate calcium concentration in alternate night nocturnal hemodialysis: A randomized controlled trial. Hemodial Int 21: 19–28, 2017 [DOI] [PubMed] [Google Scholar]
- 51.Tsuruta Y, Ohbayashi T, Fujii M, Myochin H, Mizutani R, Narita M, et al. : Change in coronary artery calcification score due to cinacalcet hydrochloride administration. Ther Apher Dial 12[Suppl 1]: S34–S37, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Raggi P, Chertow GM, Torres PU, Csiky B, Naso A, Nossuli K, et al. ; ADVANCE Study Group : The ADVANCE study: A randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant 26: 1327–1339, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Nakayama K, Nakao K, Takatori Y, Inoue J, Kojo S, Akagi S, et al. : Long-term effect of cinacalcet hydrochloride on abdominal aortic calcification in patients on hemodialysis with secondary hyperparathyroidism. Int J Nephrol Renovasc Dis 7: 25–33, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cruzado JM, Moreno P, Torregrosa JV, Taco O, Mast R, Gómez-Vaquero C, et al. : A randomized study comparing parathyroidectomy with cinacalcet for treating hypercalcemia in kidney allograft recipients with hyperparathyroidism. J Am Soc Nephrol 27: 2487–2494, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Susantitaphong P, Vadcharavivad S, Susomboon T, Singhan W, Dumrongpisutikul N, Jakchairoongruang K, et al. : The effectiveness of cinacalcet: A randomized, open label study in chronic hemodialysis patients with severe secondary hyperparathyroidism. Ren Fail 41: 326–333, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eddington H, Chinnadurai R, Alderson H, Ibrahim ST, Chrysochou C, Green D, et al. : A randomised controlled trial to examine the effects of cinacalcet on bone and cardiovascular parameters in haemodialysis patients with advanced secondary hyperparathyroidism. BMC Nephrol 22: 106, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baker LR, Muir JW, Sharman VL, Abrams SM, Greenwood RN, Cattell WR, et al. : Controlled trial of calcitriol in hemodialysis patients. Clin Nephrol 26: 185–191, 1986 [PubMed] [Google Scholar]
- 58.Morosetti M, Jankovic L, Palombo G, Cipriani S, Dominijanni S, Balducci A, et al. : High-dose calcitriol therapy and progression of cardiac vascular calcifications. J Nephrol 21: 603–608, 2008 [PubMed] [Google Scholar]
- 59.Delanaye P, Weekers L, Warling X, Moonen M, Smelten N, Médart L, et al. : Cholecalciferol in haemodialysis patients: A randomized, double-blind, proof-of-concept and safety study. Nephrol Dial Transplant 28: 1779–1786, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Kidir V, Ersoy I, Altuntas A, Gultekin F, Inal S, Dagdeviren BH, et al. : Effect of cholecalciferol replacement on vascular calcification and left ventricular mass index in dialysis patients. Ren Fail 37: 635–639, 2015 [DOI] [PubMed] [Google Scholar]
- 61.Samaan F, Carvalho AB, Pillar R, Rocha LA, Cassiolato JL, Cuppari L, et al. : The effect of long-term cholecalciferol supplementation on vascular calcification in chronic kidney disease patients with hypovitaminosis D. J Ren Nutr 29: 407–415, 2019 [DOI] [PubMed] [Google Scholar]
- 62.Anis KH, Pober D, Rosas SE: Vitamin D analogues and coronary calcification in CKD stages 3 and 4: A randomized controlled trial of calcitriol versus paricalcitol. Kidney Med 2: 450–458, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yazbek DC, de Carvalho AB, Barros CS, Medina Pestana JO, Canziani ME: Effect of statins on the progression of coronary calcification in kidney transplant recipients. PLoS One 11: e0151797, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hashiba H, Aizawa S, Tamura K, Shigematsu T, Kogo H: Inhibitory effects of etidronate on the progression of vascular calcification in hemodialysis patients. Ther Apher Dial 8: 241–247, 2004 [DOI] [PubMed] [Google Scholar]
- 65.Nitta K, Akiba T, Suzuki K, Uchida K, Watanabe R, Majima K, et al. : Effects of cyclic intermittent etidronate therapy on coronary artery calcification in patients receiving long-term hemodialysis. Am J Kidney Dis 44: 680–688, 2004 [PubMed] [Google Scholar]
- 66.Ariyoshi T, Eishi K, Sakamoto I, Matsukuma S, Odate T: Effect of etidronic acid on arterial calcification in dialysis patients. Clin Drug Investig 26: 215–222, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Hashiba H, Aizawa S, Tamura K, Kogo H: Inhibition of the progression of aortic calcification by etidronate treatment in hemodialysis patients: Long-term effects. Ther Apher Dial 10: 59–64, 2006 [DOI] [PubMed] [Google Scholar]
- 68.Torregrosa J-V, Fuster D, Gentil M-Á, Marcen R, Guirado L, Zarraga S, et al. : Open-label trial: Effect of weekly risedronate immediately after transplantation in kidney recipients. Transplantation 89: 1476–1481, 2010 [DOI] [PubMed] [Google Scholar]
- 69.Toussaint ND, Lau KK, Strauss BJ, Polkinghorne KR, Kerr PG: Effect of alendronate on vascular calcification in CKD stages 3 and 4: A pilot randomized controlled trial. Am J Kidney Dis 56: 57–68, 2010 [DOI] [PubMed] [Google Scholar]
- 70.Okamoto M, Yamanaka S, Yoshimoto W, Shigematsu T: Alendronate as an effective treatment for bone loss and vascular calcification in kidney transplant recipients. J Transplant 2014: 269613, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iseri K, Watanabe M, Yoshikawa H, Mitsui H, Endo T, Yamamoto Y, et al. : Effects of denosumab and alendronate on bone health and vascular function in hemodialysis patients: A randomized, controlled trial. J Bone Miner Res 34: 1014–1024, 2019 [DOI] [PubMed] [Google Scholar]
- 72.Chen CL, Chen NC, Wu FZ, Wu MT: Impact of denosumab on cardiovascular calcification in patients with secondary hyperparathyroidism undergoing dialysis: A pilot study. Osteoporos Int 31: 1507–1516, 2020 [DOI] [PubMed] [Google Scholar]
- 73.Spiegel DM, Farmer B: Long-term effects of magnesium carbonate on coronary artery calcification and bone mineral density in hemodialysis patients: A pilot study. Hemodial Int 13: 453–459, 2009 [DOI] [PubMed] [Google Scholar]
- 74.Tzanakis IP, Stamataki EE, Papadaki AN, Giannakis N, Damianakis NE, Oreopoulos DG: Magnesium retards the progress of the arterial calcifications in hemodialysis patients: A pilot study. Int Urol Nephrol 46: 2199–2205, 2014 [DOI] [PubMed] [Google Scholar]
- 75.Sakaguchi Y, Hamano T, Obi Y, Monden C, Oka T, Yamaguchi S, et al. : A randomized trial of magnesium oxide and oral carbon adsorbent for coronary artery calcification in predialysis CKD. J Am Soc Nephrol 30: 1073–1085, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kurnatowska I, Grzelak P, Masajtis-Zagajewska A, Kaczmarska M, Stefańczyk L, Vermeer C, et al. : Effect of vitamin K2 on progression of atherosclerosis and vascular calcification in nondialyzed patients with chronic kidney disease stages 3-5. Pol Arch Med Wewn 125: 631–640, 2015 [DOI] [PubMed] [Google Scholar]
- 77.Li Y, Xie Z, Xu D: Inhibition of maintenance hemodialysis related vascular calcification by vitamin K in chronic kidney disease. Int J Clin Exp Med 10: 15309–15315, 2017 [Google Scholar]
- 78.Oikonomaki T, Papasotiriou M, Ntrinias T, Kalogeropoulou C, Zabakis P, Kalavrizioti D, et al. : The effect of vitamin K2 supplementation on vascular calcification in haemodialysis patients: A 1-year follow-up randomized trial. Int Urol Nephrol 51: 2037–2044, 2019 [DOI] [PubMed] [Google Scholar]
- 79.De Vriese AS, Caluwé R, Pyfferoen L, De Bacquer D, De Boeck K, Delanote J, et al. : Multicenter randomized controlled trial of vitamin K antagonist replacement by rivaroxaban with or without vitamin K2 in hemodialysis patients with atrial fibrillation: The Valkyrie study. J Am Soc Nephrol 31: 186–196, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Witham MD, Lees JS, White M, Band M, Bell S, Chantler DJ, et al. : Vitamin K supplementation to improve vascular stiffness in CKD: The K4Kidneys randomized controlled trial. J Am Soc Nephrol 31: 2434–2445, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Levy-Schousboe K, Frimodt-Møller M, Hansen D, Peters CD, Kjærgaard KD, Jensen JD, et al. : Vitamin K supplementation and arterial calcification in dialysis: Results of the double-blind, randomized, placebo-controlled RenaKvit trial. Clin Kidney J 14: 2114–2123, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Adirekkiat S, Sumethkul V, Ingsathit A, Domrongkitchaiporn S, Phakdeekitcharoen B, Kantachuvesiri S, et al. : Sodium thiosulfate delays the progression of coronary artery calcification in haemodialysis patients. Nephrol Dial Transplant 25: 1923–1929, 2010 [DOI] [PubMed] [Google Scholar]
- 83.Mathews SJ, de Las Fuentes L, Podaralla P, Cabellon A, Zheng S, Bierhals A, et al. : Effects of sodium thiosulfate on vascular calcification in end-stage renal disease: A pilot study of feasibility, safety and efficacy. Am J Nephrol 33: 131–138, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yonova DH, Vazelov ES, Trendafilov II, Stoinova VV, Nedevska MT, Antonov SA: First impressions of cardiovascular calcification treatment in hemodialysis patients with a new dialysis fluid containing sodium thiosulphate (STS). Int J Artif Organs 37: 308–314, 2014 [DOI] [PubMed] [Google Scholar]
- 85.Yu Y, Bi ZM, Wang Y, Chen ZQ, Xu SW: [Effect of sodium thiosulfate on coronary artery calcification in maintenance hemodialysis patients]. [Chinese] Zhonghua Yi Xue Za Zhi 96: 3724–3728, 2016 [DOI] [PubMed] [Google Scholar]
- 86.Saengpanit D, Chattranukulchai P, Tumkosit M, Siribumrungwong M, Katavetin P, Sitprija V, et al. : Effect of sodium thiosulfate on arterial stiffness in end-stage renal disease patients undergoing chronic hemodialysis (sodium thiosulfate-hemodialysis study): A randomized controlled trial. Nephron 139: 219–227, 2018 [DOI] [PubMed] [Google Scholar]
- 87.Djuric P, Dimkovic N, Schlieper G, Djuric Z, Pantelic M, Mitrovic M, et al. : Sodium thiosulphate and progression of vascular calcification in end-stage renal disease patients: A double-blind, randomized, placebo-controlled study. Nephrol Dial Transplant 35: 162–169, 2020 [DOI] [PubMed] [Google Scholar]
- 88.Bian Z, Zhang Q, Shen L, Feng Y, Chen S: The effect of sodium thiosulfate on coronary artery calcification in hemodialysis patients. ASAIO J 68: 402–406, 2021 [DOI] [PubMed] [Google Scholar]
- 89.Lees JS, Chapman FA, Witham MD, Jardine AG, Mark PB: Vitamin K status, supplementation and vascular disease: A systematic review and meta-analysis. Heart 105: 938–945, 2019 [DOI] [PubMed] [Google Scholar]
- 90.Navaneethan SD, Palmer SC, Craig JC, Elder GJ, Strippoli GF: Benefits and harms of phosphate binders in CKD: A systematic review of randomized controlled trials. Am J Kidney Dis 54: 619–637, 2009 [DOI] [PubMed] [Google Scholar]
- 91.Ruospo M, Palmer SC, Natale P, Craig JC, Vecchio M, Elder GJ, et al. : Phosphate binders for preventing and treating chronic kidney disease-mineral and bone disorder (CKD-MBD). Cochrane Database Syst Rev 8: CD006023, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Caffarelli C, Montagnani A, Nuti R, Gonnelli S: Bisphosphonates, atherosclerosis and vascular calcification: Update and systematic review of clinical studies. Clin Interv Aging 12: 1819–1828, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lioufas N, Pascoe EM, Hawley CM, Elder GJ, Badve SV, Block GA, et al. : Systematic review and meta-analyses of the effects of phosphate-lowering agents in nondialysis CKD. J Am Soc Nephrol 33: 59–76, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jamal SA, Vandermeer B, Raggi P, Mendelssohn DC, Chatterley T, Dorgan M, et al. : Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: An updated systematic review and meta-analysis. Lancet 382: 1268–1277, 2013 [DOI] [PubMed] [Google Scholar]
- 95.Puri R, Nicholls SJ, Shao M, Kataoka Y, Uno K, Kapadia SR, et al. : Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol 65: 1273–1282, 2015 [DOI] [PubMed] [Google Scholar]
- 96.Lee S-E, Chang H-J, Sung JM, Park H-B, Heo R, Rizvi A, et al. : Effects of statins on coronary atherosclerotic plaques: The PARADIGM study. JACC Cardiovasc Imaging 11: 1475–1484, 2018 [DOI] [PubMed] [Google Scholar]
- 97.Gross ML, Meyer HP, Ziebart H, Rieger P, Wenzel U, Amann K, et al. : Calcification of coronary intima and media: Immunohistochemistry, backscatter imaging, and x-ray analysis in renal and nonrenal patients. Clin J Am Soc Nephrol 2: 121–134, 2007 [DOI] [PubMed] [Google Scholar]
- 98.Nakamura S, Ishibashi-Ueda H, Niizuma S, Yoshihara F, Horio T, Kawano Y: Coronary calcification in patients with chronic kidney disease and coronary artery disease. Clin J Am Soc Nephrol 4: 1892–1900, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jinnouchi H, Sato Y, Sakamoto A, Cornelissen A, Mori M, Kawakami R, et al. : Calcium deposition within coronary atherosclerotic lesion: Implications for plaque stability. Atherosclerosis 306: 85–95, 2020 [DOI] [PubMed] [Google Scholar]
- 100.O’Neill WC: Targeting serum calcium in chronic kidney disease and end-stage renal disease: Is normal too high? Kidney Int 89: 40–45, 2016 [DOI] [PubMed] [Google Scholar]
- 101.Cunningham J, Locatelli F, Rodriguez M: Secondary hyperparathyroidism: Pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol 6: 913–921, 2011 [DOI] [PubMed] [Google Scholar]
- 102.Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, et al. : Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: The PRIMO randomized controlled trial. JAMA 307: 674–684, 2012 [DOI] [PubMed] [Google Scholar]
- 103.Wang AY, Fang F, Chan J, Wen YY, Qing S, Chan IH, et al. : Effect of paricalcitol on left ventricular mass and function in CKD--the OPERA trial. J Am Soc Nephrol 25: 175–186, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Henein MY, Owen A: Statins moderate coronary stenoses but not coronary calcification: Results from meta-analyses. Int J Cardiol 153: 31–35, 2011 [DOI] [PubMed] [Google Scholar]
- 105.Saremi A, Bahn G, Reaven PD; VADT Investigators : Progression of vascular calcification is increased with statin use in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care 35: 2390–2392, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Toussaint ND, Elder GJ, Kerr PG: Bisphosphonates in chronic kidney disease; balancing potential benefits and adverse effects on bone and soft tissue. Clin J Am Soc Nephrol 4: 221–233, 2009 [DOI] [PubMed] [Google Scholar]
- 107.Chen CL, Chen NC, Liang HL, Hsu CY, Chou KJ, Fang HC, et al. : Effects of denosumab and calcitriol on severe secondary hyperparathyroidism in dialysis patients with low bone mass. J Clin Endocrinol Metab 100: 2784–2792, 2015 [DOI] [PubMed] [Google Scholar]
- 108.Montezano AC, Zimmerman D, Yusuf H, Burger D, Chignalia AZ, Wadhera V, et al. : Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension 56: 453–462, 2010 [DOI] [PubMed] [Google Scholar]
- 109.Ter Braake AD, Tinnemans PT, Shanahan CM, Hoenderop JGJ, de Baaij JHF: Magnesium prevents vascular calcification in vitro by inhibition of hydroxyapatite crystal formation. Sci Rep 8: 2069, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bressendorff I, Hansen D, Schou M, Silver B, Pasch A, Bouchelouche P, et al. : Oral magnesium supplementation in chronic kidney disease stages 3 and 4: Efficacy, safety, and effect on serum calcification propensity-A prospective randomized double-blinded placebo-controlled clinical trial. Kidney Int Rep 2: 380–389, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bressendorff I, Hansen D, Schou M, Kragelund C, Brandi L. The effect of magnesium supplementation on vascular calcification in chronic kidney disease-a randomised clinical trial (MAGiCAL-CKD): Essential study design and rationale. BMJ Open 7: e016795, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kurnatowska I, Grzelak P, Masajtis-Zagajewska A, Kaczmarska M, Stefańczyk L, Vermeer C, et al. : Plasma desphospho-uncarboxylated matrix Gla protein as a marker of kidney damage and cardiovascular risk in advanced stage of chronic kidney disease. Kidney Blood Press Res 41: 231–239, 2016 [DOI] [PubMed] [Google Scholar]
- 113.Caluwé R, Verbeke F, De Vriese AS: Evaluation of vitamin K status and rationale for vitamin K supplementation in dialysis patients. Nephrol Dial Transplant 35: 23–33, 2020 [DOI] [PubMed] [Google Scholar]
- 114.Shea MK, O’Donnell CJ, Hoffmann U, Dallal GE, Dawson-Hughes B, Ordovas JM, et al. : Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr 89: 1799–1807, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.O’Neill WC, Hardcastle KI: The chemistry of thiosulfate and vascular calcification. Nephrol Dial Transplant 27: 521–526, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Snijder PM, Frenay AR, de Boer RA, Pasch A, Hillebrands JL, Leuvenink HGD, et al. : Exogenous administration of thiosulfate, a donor of hydrogen sulfide, attenuates angiotensin II-induced hypertensive heart disease in rats. Br J Pharmacol 172: 1494–1504, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nasi S, Ea H-K, Lioté F, So A, Busso N: Sodium thiosulfate prevents chondrocyte mineralization and reduces the severity of murine osteoarthritis. PLoS One 11: e0158196, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Criqui MH, Kamineni A, Allison MA, Ix JH, Carr JJ, Cushman M, et al. : Risk factor differences for aortic versus coronary calcified atherosclerosis: The multiethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol 30: 2289–2296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kimani C, Kadota A, Miura K, Fujiyoshi A, Zaid M, Kadowaki S, et al. ; SESSA Research Group : Differences between coronary artery calcification and aortic artery calcification in relation to cardiovascular disease risk factors in Japanese men. J Atheroscler Thromb 26: 452–464, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moe SM, O’Neill KD, Reslerova M, Fineberg N, Persohn S, Meyer CA: Natural history of vascular calcification in dialysis and transplant patients. Nephrol Dial Transplant 19: 2387–2393, 2004 [DOI] [PubMed] [Google Scholar]
- 121.Oschatz E, Benesch T, Kodras K, Hoffmann U, Haas M: Changes of coronary calcification after kidney transplantation. Am J Kidney Dis 48: 307–313, 2006 [DOI] [PubMed] [Google Scholar]
- 122.Bubenicek P, Kautznerova D, Sotornik I, Adamec M, Lanska V, Teplan V: Coronary calcium score in renal transplant recipients. Nephron Clin Pract 112: c1–c8, 2009 [DOI] [PubMed] [Google Scholar]
- 123.Jansz TT, Özyilmaz A, van Reekum FE, Boereboom FTJ, de Jong PA, Verhaar MC, et al. : Progression of coronary artery calcification in conventional hemodialysis, nocturnal hemodialysis, and kidney transplantation. PLoS One 15: e0244639, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maréchal C, Coche E, Goffin E, Dragean A, Schlieper G, Nguyen P, et al. : Progression of coronary artery calcification and thoracic aorta calcification in kidney transplant recipients. Am J Kidney Dis 59: 258–269, 2012 [DOI] [PubMed] [Google Scholar]
- 125.Seyahi N, Cebi D, Altiparmak MR, Akman C, Ataman R, Pekmezci S, et al. : Progression of coronary artery calcification in renal transplant recipients. Nephrol Dial Transplant 27: 2101–2107, 2012 [DOI] [PubMed] [Google Scholar]
- 126.Yuen D, Pierratos A, Richardson RM, Chan CT: The natural history of coronary calcification progression in a cohort of nocturnal haemodialysis patients. Nephrol Dial Transplant 21: 1407–1412, 2006 [DOI] [PubMed] [Google Scholar]
- 127.Van Eps CL, Jeffries JK, Anderson JA, Bergin PT, Johnson DW, Campbell SB, et al. : Mineral metabolism, bone histomorphometry and vascular calcification in alternate night nocturnal haemodialysis. Nephrology (Carlton) 12: 224–233, 2007 [DOI] [PubMed] [Google Scholar]
- 128.Voelkl J, Cejka D, Alesutan I: An overview of the mechanisms in vascular calcification during chronic kidney disease. Curr Opin Nephrol Hypertens 28: 289–296, 2019 [DOI] [PubMed] [Google Scholar]
- 129.Pasch A, Farese S, Gräber S, Wald J, Richtering W, Floege J, et al. : Nanoparticle-based test measures overall propensity for calcification in serum. J Am Soc Nephrol 23: 1744–1752, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Smith ER, Hewitson TD, Holt SG: Diagnostic tests for vascular calcification. Adv Chronic Kidney Dis 26: 445–463, 2019 [DOI] [PubMed] [Google Scholar]
- 131.Bushinsky DA, Raggi P, Bover J, Ketteler M, Bellasi A, Rodriguez M, et al. ; CaLIPSO Investigators*; CaLIPSO Study Group Clinipace GmbH, Intrinsic Imaging, LLC : Effects of myo-inositol hexaphosphate (SNF472) on bone mineral density in patients receiving hemodialysis: An analysis of the randomized, placebo-controlled CaLIPSO study. Clin J Am Soc Nephrol 16: 736–745, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhou Y, Hellberg M, Hellmark T, Höglund P, Clyne N: Twelve months of exercise training did not halt abdominal aortic calcification in patients with CKD - a sub-study of RENEXC-a randomized controlled trial. BMC Nephrol 21: 233, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tamura K, Hashiba H, Kogo H, Aizawa S, Shigematsu T: [Suppressive effects of bisphosphonates on the vascular calcification in ESRD patients]. Clin Calcium 12: 1129–1135, 2002 [PubMed] [Google Scholar]
- 134.Raggi P, Bellasi A, Bushinsky D, Bover J, Rodriguez M, Ketteler M, et al. : Slowing progression of cardiovascular calcification with SNF472 in patients on hemodialysis: Results of a randomized phase 2b study. Circulation 141: 728–739, 2020 [DOI] [PubMed] [Google Scholar]
- 135.Gueiros APS, Gueiros JEB, Nóbrega KT, Calado EB, Matta MCD, Torres LC, et al. : Effect of spironolactone on the progression of coronary calcification in peritoneal dialysis patients: A pilot study. J Bras Nefrol 41: 345–355, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Coyne DW, Singh HN, Smith WT, Giuseppi AC, Connarn JN, Sherman ML, et al. : Sotatercept safety and effects on hemoglobin, bone, and vascular calcification. Kidney Int Rep 4: 1585–1597, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu XY, Yao JR, Xu R, Xu LX, Zhang YF, Lu S, et al. : Investigation of nicotinamide as more than an anti-phosphorus drug in chronic hemodialysis patients: A single-center, double-blind, randomized, placebo-controlled trial. Ann Transl Med 8: 530, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gao Y, Wang G, Li Y, Lv C, Wang Z: Effects of oral activated charcoal on hyperphosphatemia and vascular calcification in Chinese patients with stage 3-4 chronic kidney disease. J Nephrol 32: 265–272, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Raggi P, Bommer J, Chertow GM: Valvular calcification in hemodialysis patients randomized to calcium-based phosphorus binders or sevelamer. J Heart Valve Dis 13: 134–141, 2004 [PubMed] [Google Scholar]
- 140.Watanabe K, Fujii H, Kono K, Goto S, Nishi S: Comparison of the effects of lanthanum carbonate and calcium carbonate on the progression of cardiac valvular calcification after initiation of hemodialysis. BMC Cardiovasc Disord 20: 39, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.