Abstract
Objectives
To identify any associations between in utero exposure to five over-the-counter (non-prescription) analgesics (paracetamol, ibuprofen, aspirin, diclofenac, naproxen) and adverse neonatal outcomes.
Design
Retrospective cohort study using the Aberdeen Maternity and Neonatal Databank.
Participants
151 141 singleton pregnancies between 1985 and 2015.
Main outcome measures
Premature delivery (<37 weeks), stillbirth, neonatal death, birth weight, standardised birthweight score, neonatal unit admission, APGAR score at 1 and 5 min, neural tube and amniotic band defects, gastroschisis and, in males, cryptorchidism and hypospadias.
Results
83.7% of women taking over-the-counter analgesics reported first trimester use when specifically asked about use at their first antenatal clinic visit. Pregnancies exposed to at least one of the five analgesics were significantly independently associated with increased risks for premature delivery <37 weeks (adjusted OR (aOR)=1.50, 95% CI 1.43 to 1.58), stillbirth (aOR=1.33, 95% CI 1.15 to 1.54), neonatal death (aOR=1.56, 95% CI 1.27 to 1.93), birth weight <2500 g (aOR=1.28, 95% CI 1.20 to 1.37), birth weight >4000 g (aOR=1.09, 95% CI 1.05 to 1.13), admission to neonatal unit (aOR=1.57, 95% CI 1.51 to 1.64), APGAR score <7 at 1 min (aOR=1.18, 95% CI 1.13 to 1.23) and 5 min (aOR=1.48, 95% CI 1.35 to 1.62), neural tube defects (aOR=1.64, 95% CI 1.08 to 2.47) and hypospadias (aOR=1.27, 95% CI 1.05 to 1.54 males only). The overall prevalence of over-the-counter analgesics use during pregnancy was 29.1%, however it rapidly increased over the 30-year study period, to include over 60% of women in the last 7 years of the study. This makes our findings highly relevant to the wider pregnant population.
Conclusions
Over-the-counter (non-prescription) analgesics consumption during pregnancy was associated with a substantially higher risk for adverse perinatal health outcomes in the offspring. The use of paracetamol in combination with other non-steroidal anti-inflammatory drugs conferred the highest risk. The increased risks of adverse neonatal outcomes associated with non-prescribed, over-the-counter, analgesics use during pregnancy indicate that healthcare guidance for pregnant women regarding analgesic use need urgent updating.
Keywords: obstetrics, clinical pharmacology, epidemiology
Strengths and limitations of this study.
This is one of the largest and most comprehensive studies of this type.
It includes consumption of five different analgesics during pregnancy in a large cohort of singleton pregnancies.
It examines associations with extensive range of offspring perinatal outcomes, while adjusting for important confounding factors.
Analgesic consumption was analysed both as use of a single compound and in combinations of the five drugs considered in this study.
Details of the exact dose and timing of consumption during pregnancy were not available within our dataset.
Follow-up of the offspring health later in life was not available at this time.
Introduction
Globally, 23%–85% of women use one or more types of prescribed medications during pregnancy.1 2 A similarly high proportion of expectant mothers self-medicate using non-prescription, ‘over-the-counter’ (OTC) medicines3 4 and use during pregnancy is becoming increasingly prevalent, especially in Western countries.5 While some analgesics, for example, paracetamol (acetaminophen) are considered safe to consume throughout pregnancy, use of non-steroidal anti-inflammatory drugs (NSAIDs) is not recommended in pregnancy unless on the advice of a medical specialist and should be avoided beyond gestational week 30 because of the risk of premature closure of the ductus arteriosus. However, current evidence is largely conflicting regarding the safety of gestational analgesic use both for the pregnancy and offspring health.6 Several studies have reported increased risks for multiple adverse outcomes including hypospadias, cryptorchidism, amniotic band defects and neural tube defects,7–11 while others have not found significant associations.12–17 Taken overall, this has led to significant concern that postnatal health is adversely affected by maternal analgesic use during pregnancy.18
The use of small cohorts in the current epidemiological studies makes it difficult to draw firm conclusions and definite recommendations.12 17 19 20 There are other aspects of analgesic use that must be considered. First, due to their abundance, it is not always feasible to determine exact consumption rates and dosage. Second, even though the mechanisms of action for most of these compounds is not fully understood, most over-the-counter analgesics can diffuse through the placenta and reach the developing fetus.21 Third, maternal pharmacokinetics during pregnancy are altered and there are limited pregnancy safety data for these compounds.
Given the diversity in study population, methodology, sample size and findings in the published studies, we conclude that more extensive data from larger cohorts are essential in order to understand the risks over-the-counter analgesic use during pregnancy pose to neonatal health and function. Here, we address many limitations—however, not all22—of previous studies by analysing one of the largest cohorts, widest range of health data and pregnancy use of five over-the-counter analgesics consumed in combination or separately. We report on the prevalence of maternal consumption of five different over-the-counter analgesics during pregnancy and their associations with offspring neonatal outcomes using a large cohort of 151 141 singleton pregnancies spanning three decades of population-based data from a single maternity hospital serving the entire population of Aberdeenshire in the Northeast of Scotland.
Materials and methods
This retrospective cohort study used data collected in the Aberdeen Maternity and Neonatal Databank (AMND) in Aberdeen, UK on 151 141 pregnancies over a 30-year period (1985–2015). Details about AMND have been previously published.23 Data were collected from medical notes of women retrospectively after delivery. Women were specifically asked about their use of over-the-counter (non-prescription) analgesics at their first antenatal clinic. Data were entered by dedicated coding staff into a computerised database. Data validity was ensured via checking completeness of data entry against NHS (UK National Health Service) returns monthly and constant data cleaning and validation against case notes reported quarterly by the data management team to the AMND steering committee.
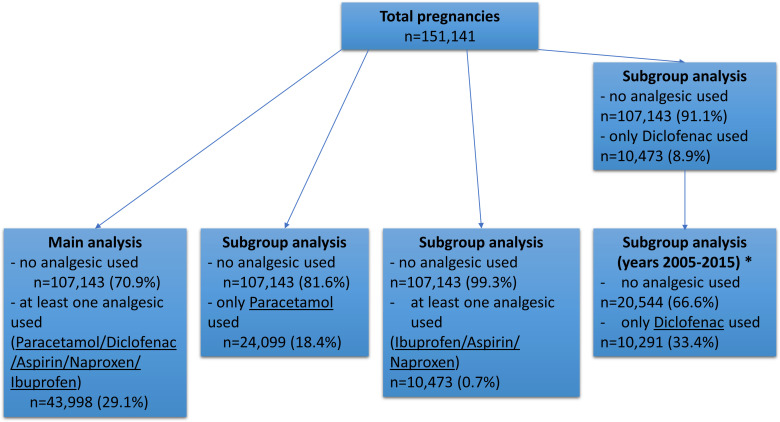
The main analysis considered consumption during pregnancy of at least one out of five different analgesics: paracetamol (no; yes), ibuprofen (no; yes), naproxen (no; yes), diclofenac (no; yes) or aspirin (no; yes) as the exposure group against no analgesic consumption as the unexposed group. Then, three subgroup analyses against the control group were performed using only paracetamol, only diclofenac, or at least one analgesic from aspirin/naproxen/ibuprofen as exposure groups, excluding pregnancies exposed to multiple analgesics at the same time (figure 1). As 98.3% of pregnancies using diclofenac were between 2005 and 2015, diclofenac subgroup analysis only considered pregnancies during that time frame in order to rule out any temporal effect. Analgesic consumption was not further assessed analytically.
Figure 1.
Flow chart of cohort selection and subgroup analyses. n=number of pregnancies in each analysis.*98.3% of pregnancies using only diclofenac occurred during 2005–2015, therefore analysis was performed only on data from that decade to rule out any temporal effect.
The offspring outcomes compared between control and exposed groups were: gestation at delivery (preterm <37 gestation weeks, term ≥37 gestation weeks), pregnancy outcome (live birth, stillbirth, neonatal death), baby weight (low birth weight (LBW) ≤2499 g, high birth weight (HBW) ≥4000 g, normal birth weight (NBW) 2500–3999 g), standardised birthweight score was considered as a continuous variable as previously described by Campbell et al,24 baby admission to neonatal unit (no; yes), APGAR score at 1 and 5 min (<7, >7), cryptorchidism (no; yes) (International Classification of Diseases, 10th revision (ICD-10) code Q53), neural tube defects (no; yes) (ICD-10 code Q00-07), amniotic band defects (no; yes) (ICD-10 codes Q70-74), hypospadias (no; yes) (ICD-10 code Q54), gastroschisis (no; yes) (ICD-10 code Q79.3). A composite outcome (presence of at least one congenital anomaly (no; yes)) was created using the variables neural tube defects, amniotic band defects and gastroschisis and, in males, cryptorchidism and hypospadias.
The baseline characteristics compared between exposed and unexposed pregnancies were (reference category first): year of delivery (1985–1994, 1995–2004, 2005–2015), maternal age at delivery (20–25, <20, 26–35, >35 years), previous pregnancy (no; yes), maternal body mass index (BMI) (normal weight 18.5–24.9 kg/m2, underweight <18.5 kg/m2, overweight 25–29.9 kg/m2, obese >30 kg/m2), maternal first antenatal visit (first, second, third trimester), maternal smoking status (non-smoker, smoker, ex-smoker), Scottish Index of Multiple Deprivation (SIMD) decile (1–6, 7–10, decreasing deprivation with increasing score), maternal hypertensive disorders (no disorder, gestational hypertension, pre-eclampsia, eclampsia), maternal antepartum haemorrhage (no haemorrhage, abruption, placental previa), type of labour (spontaneous, elective caesarean section, induced), type of delivery (spontaneous vaginal delivery, instrumental, caesarean section), analgesia during labour (no; yes), baby presentation at delivery (occiput anterior, occiput posterior), baby sex (female; male).
Patient and public involvement
This was a retrospective analysis of data on singleton pregnancies over a 30-year period. Therefore, there was no involvement of patients or the public in the design, conduct, reporting or any other aspect of the study.
Statistical analysis
Baseline characteristics were compared between exposed and unexposed pregnancies to any analgesic using χ2 test for categorical variables and t-test for normally distributed continuous variables as appropriate. Relationships between exposures and outcomes were examined by binary logistic regression for binary outcome variables, multinomial logistic regression for nominal categorical outcome variables and multiple linear regression for continuous variables. The strength of association was reported as ORs with 95% CIs. The sociodemographic characteristics that were likely to confound our exposure-to-outcome path were identified using directed acyclic graphs (online supplemental figures S1–S11).25 Factors that were associated with consumption of over-the-counter analgesics during pregnancy at 10% level of significance and deemed clinically relevant were included in the model as confounders. All outcomes were adjusted for year of delivery, maternal age at delivery, SIMD and maternal first antenatal visit. In addition to these confounders, individual outcomes were adjusted for relevant cofactors. Gestation at delivery and pregnancy outcome were both additionally adjusted for maternal hypertensive disorders and antepartum haemorrhage. Weight of the baby, neonatal unit admission, cryptorchidism, neural tube defects, amniotic band defects, hypospadias and gastroschisis variables were also adjusted for gestation at delivery. APGAR score at 1 and 5 min were adjusted for type of delivery. A p value of <0.05 was considered statistically significant. All statistical analyses were carried out using IBM SPSS Statistics V.25.0 (released 2017, IBM, Armonk, New York, USA). R V.3.6.2 was used to generate figure 2. Numbers needed to harm (NNH) were also calculated for each outcome and are provided in online supplemental tables 1 and 2.
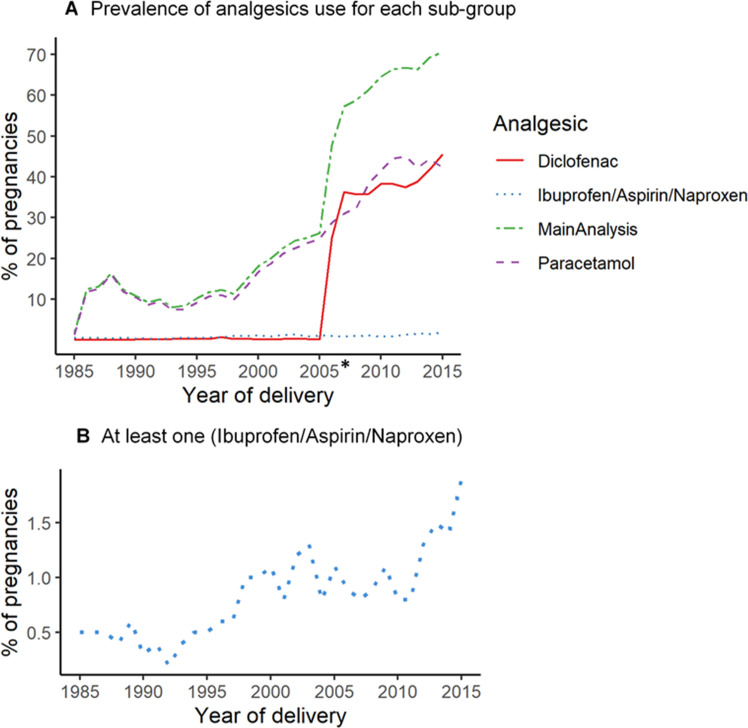
Figure 2.
Prevalence of use during pregnancy for each analgesic subgroup over our 30-year study period. (A) Merge graph showing percentage of pregnancies using each analgesic group during pregnancy. (B) Percentage of use for at least one analgesic out of ibuprofen, aspirin, naproxen. *In 2005, there was a change in legislation making diclofenac available without prescription.
bmjopen-2020-048092supp001.pdf (77.7KB, pdf)
bmjopen-2020-048092supp002.pdf (81.7KB, pdf)
bmjopen-2020-048092supp003.pdf (67.9KB, pdf)
bmjopen-2020-048092supp006.pdf (67.6KB, pdf)
bmjopen-2020-048092supp005.pdf (64.1KB, pdf)
bmjopen-2020-048092supp004.pdf (77.5KB, pdf)
bmjopen-2020-048092supp007.pdf (81.9KB, pdf)
bmjopen-2020-048092supp008.pdf (78.4KB, pdf)
bmjopen-2020-048092supp009.pdf (61.9KB, pdf)
bmjopen-2020-048092supp010.pdf (64.2KB, pdf)
bmjopen-2020-048092supp011.pdf (65.5KB, pdf)
bmjopen-2020-048092supp012.pdf (82.6KB, pdf)
bmjopen-2020-048092supp013.pdf (55.4KB, pdf)
bmjopen-2020-048092supp014.pdf (51.3KB, pdf)
Results
Overall, from the total 151 141 pregnancies across 30 years in 107 143 (70.9%) pregnancies, no over-the-counter analgesic consumption was reported. At least one over-the-counter analgesic was consumed in 43 998 (29.1%) pregnancies, whereas paracetamol use alone was reported in 24 099 (18.4%) pregnancies. Diclofenac use was observed in 20.0% of pregnancies in the 10-year period when diclofenac was available over-the-counter (without prescription). Finally, at least one out of three analgesics (naproxen, ibuprofen, aspirin) was consumed in 762 (0.7%) pregnancies (figure 1). At their first antenatal clinic visit, 83.7% of women taking over-the-counter analgesics reported use in the first trimester of pregnancy.
Prevalence of use for all five analgesics increased dramatically over the 30-year study period (1985–2015) (figure 2). Pregnancies with consumption of at least one analgesic increased from 1.8% in 1985 to 70.6% in 2015. Pregnancies reporting paracetamol use were 1.3% in 1985 and it continuously increased reaching 42.2% in 2015. Naproxen, ibuprofen or aspirin consumption during pregnancy was less prevalent (figure 2A), however it also increased during the 30-year study period, starting at 0.5% in 1985 and reaching 1.9% in 2015 (figure 2B). Diclofenac was consumed in very few pregnancies between 1985 (<0.01%) and 2005 (0.2%). Percentage of consumption, however, dramatically increased during the next decade following deregulation of diclofenac, reaching 25.0% in just 1 year (2006) and 45.6% of all pregnancies in 2015.
Table 1 compares the baseline characteristics between the unexposed group of pregnancies where no analgesic was consumed and each of the exposure groups. In most, but not all, comparisons across all four analyses, there was a statistically significant difference (p<0.001) for most variables. In the paracetamol subgroup analysis, baby presentation at delivery (p=0.525) and sex of the baby (p=0.861) were not significantly different between the groups. In the analysis considering consumption of at least one analgesic from aspirin/naproxen/ibuprofen, again the variables for baby presentation at delivery (p=0.093) and sex of the baby (p=0.732), together with maternal smoking status (p=0.132) and maternal antepartum haemorrhage (p=0.434) were not statistically different compared with the unexposed group. All variables were statistically different between unexposed and exposed groups for the main analysis and diclofenac subgroup analysis.
Table 1.
Comparison of baseline characteristics between exposed (use of analgesics) and unexposed (no analgesic use) groups of pregnancies (p values <0.05 shown in bold)
| Baseline characteristics | No analgesic (n=1 07 143) n (%) |
At least one analgesic (n=43 998) n (%) |
P value* | Paracetamol only (n=24 099) n (%) |
P value* | Ibuprofen/Aspirin/Naproxen (n=762) n (%) |
P value* | No analgesic 2005–2015 (n=20 544) n (%) |
Diclofenac only 2005–2015 (n=10 291) n (%) |
P value† |
| Year of delivery | ||||||||||
| 1985–1994 | 50 152 (46.8) | 5737 (13.0) | <0.001 | 5390 (22.4) | <0.001 | 213 (28.0) | <0.001 | n/a | n/a | <0.001 |
| 1995–2004 | 36 447 (34.0) | 7263 (16.5) | 6571 (27.3) | 321 (42.1) | n/a | n/a | ||||
|
2005–2015/ 2005–2009‡ |
20 544 (19.2) n/a |
30 998 (70.5) n/a |
12 138 (50.4) n/a | 228 (29.9) n/a |
n/a 11 105 (54.1) |
n/a 4021 (39.1) |
||||
| 2010–2015‡ | n/a | n/a | n/a | n/a | 9439 (45.9) | 6270 (60.9) | ||||
| Maternal age (years) at delivery | ||||||||||
| <20 | 9236 (8.6) | 3834 (8.7) | <0.001 | 2936 (12.2) | <0.001 | 34 (4.5) | <0.001 | 1286 (6.3) | 311 (3.0) | <0.001 |
| 20–25 | 24 249 (22.6) | 8700 (19.8) | 5932 (24.6) | 113 (14.8) | 3436 (16.7) | 1152 (11.2) | ||||
| 26–35 | 63 499 (59.3) | 25 367 (57.7) | 12 896 (53.5) | 464 (60.9) | 12 664 (61.1) | 6628 (64.4) | ||||
| >35 | 10 159 (9.5) | 6097 (13.9) | 2335 (9.7) | 151 (19.8) | 3158 (15.4) | 2200 (21.4) | ||||
| Previous parity | ||||||||||
| Nulliparity (0) | 48 684 (45.4) | 23 353 (53.1) | <0.001 | 12 510 (51.9) | <0.001 | 300 (39.4) | 0.004 | 8336 (40.6) | 5004 (48.6) | <0.001 |
| Multiparity (1–11) | 58 457 (54.6) | 20 639 (46.9) | 11 587 (48.1) | 462 (60.6) | 12 206 (59.4) | 5284 (51.4) | ||||
| Missing | 2 (<0.1)§ | 6 (<0.1)§ | 2 (<0.1)§ | 0 (0.0)§ | 2 (<0.1)§§ | 3 (<0.1)§§ | ||||
| Maternal BMI | ||||||||||
| Underweight (<18.5) | 1998 (2.4) | 869 (2.2) | <0.001 | 545 (2.6) | <0.001 | 10 (1.5) | 0.007 | 492 (2.7) | 174 (1.9) | <0.001 |
|
Normal weight (18.5–24.9) |
50 127 (60.8) | 18 958 (48.8) | 10 486 (50.5) | 361 (55.0) | 10 239 (55.2) | 4671 (50.0) | ||||
| Overweight (25.0–29.9) | 20 500 (24.9) | 10 960 (28.2) | 5733 (27.6) | 192 (29.5) | 4930 (26.6) | 2630 (28.1) | ||||
| Obese (≥30.0) | 9773 (11.9) | 8046 (20.7) | 3995 (19.2) | 88 (13.5) | 2881 (15.5) | 1871 (20.0) | ||||
| Missing data | 24 745 (23.1)§ | 5165 (11.7)§ | 3340 (13.9)§ | 111 (14.6)§ | 2002 (9.7)§ | 945 (9.2)§ | ||||
| Gestation weeks at earliest antenatal visit | ||||||||||
| First trimester | 69 896 (65.4) | 36 789 (83.7) | <0.001 | 19 075 (79.2) | <0.001 | 569 (75.0) | <0.001 | 18 155 (88.4) | 9185 (89.4) | 0.036 |
| Second trimester | 29 269 (27.4) | 5791 (13.2) | 4117 (17.1) | 166 (21.9) | 1770 (8.6) | 829 (8.1) | ||||
| Third trimester | 7741 (7.2) | 1376 (3.1) | 890 (3.7) | 24 (3.2) | 605 (2.9) | 264 (2.6) | ||||
| Missing | 237 (0.2)§ | 42 (0.1)§ | 17 (0.1)§§ | 3 (0.4)§ | 14 (0.1)§ | 13 (0.1)§ | ||||
| Maternal smoking status | ||||||||||
| Unknown | 6505 (6.1)§ | 819 (1.9)§ | <0.001 | 500 (2.1)§ | <0.001 | 32 (4.2)§ | 0.132 | 448 (2.2)§ | 155 (1.5)§ | <0.001 |
| Ex-smoker | 5952 (5.6) | 3363 (7.6) | 1923 (8.1) | 35 (4.8) | 1427 (7.1) | 660 (6.5) | ||||
| Non-smoker | 70 319 (69.9) | 31 421 (72.8) | 15 755 (66.8) | 534 (73.2) | 15 525 (77.3) | 8368 (82.6) | ||||
| Smoker | 24 367 (24.2) | 8395 (19.4) | 5921 (25.1) | 161 (22.2) | 3144 (15.6) | 1108 (10.9) | ||||
| Maternal SIMD decile | ||||||||||
| Least deprived (7–10) | 65 227 (61.8) | 25 192 (57.9) | <0.001 | 12 807 (53.8) | <0.001 | 501 (66.3) | 0.012 | 12 806 (62.9) | 6714 (66.1) | <0.001 |
| Most deprived (1–6) | 40 321 (38.2) | 18 289 (42.1) | 11 017 (46.2) | 255 (33.7) | 7564 (37.1) | 3442 (33.9) | ||||
| Missing | 1595 (1.5)§ | 517 (1.2)§ | 275 (1.1)§ | 6 (0.8)§ | 174 (0.8)§ | 135 (1.3)§ | ||||
| Maternal hypertensive disorders | ||||||||||
| None | 91 276 (85.2) | 35 529 (80.8) | <0.001 | 18 635 (77.3) | <0.001 | 636 (83.5) | 0.001 | 18 851 (91.8) | 9273 (90.1) | <0.001 |
| Gestational hypertension | 13 029 (12.2) | 5501 (12.5) | 3584 (14.9) | 88 (11.5) | 1165 (5.7) | 690 (6.7) | ||||
| Pre-eclampsia | 2780 (2.6) | 2941 (6.7) | 1861 (7.7) | 38 (5.0) | 523 (2.5) | 324 (3.1) | ||||
| Eclampsia | 58 (0.1) | 27 (0.1) | 19 (0.1) | 0 (0.0) | 5 (<0.1) | 4 (<0.1) | ||||
| Maternal antepartum haemorrhage | ||||||||||
| No haemorrhage | 97 527 (91.0) | 37 673 (85.6) | <0.001 | 20 306 (84.3) | <0.001 | 684 (89.8) | 0.434 | 18 549 (90.3) | 9244 (89.8) | <0.001 |
| Abruption | 697 (0.7) | 468 (1.1) | 221 (0.9) | 8 (1.0) | 103 (0.5) | 106 (1.0) | ||||
| Placenta previa | 308 (0.3) | 368 (0.8) | 152 (0.6) | 2 (0.3) | 23 (0.1) | 114 (1.1) | ||||
| Unspecified | 8611 (8.0) | 5489 (12.5) | 3420 (14.2) | 68 (8.9) | 1869 (9.1) | 827 (8.0) | ||||
| Type of labour | ||||||||||
| Elective caesarean section | 5967 (5.6) | 6925 (15.7) | <0.001 | 1384 (5.7) | <0.001 | 67 (8.8) | <0.001 | 616 (3.0) | 3843 (37.3) | <0.001 |
| Induced | 24 120 (22.5) | 16 276 (37.0) | 10 067 (41.8) | 228 (29.9) | 3895 (19.0) | 1998 (19.4) | ||||
| Spontaneous | 77 056 (71.9) | 20 797 (47.3) | 12 648 (52.5) | 467 (61.3) | 16 033(78.0) | 4450 (43.2) | ||||
| Type of delivery | ||||||||||
| Spontaneous vaginal delivery | 75 027 (70.1) | 19 287 (43.8) | <0.001 | 15 983 (66.3) | <0.001 | 496 (65.2) | 0.003 | 16 398 (79.8) | 1403 (13.6) | <0.001 |
| Instrumental | 15 409 (14.4) | 8107 (18.4) | 4043 (16.8) | 120 (15.8) | 2546 (12.4) | 1927 (18.7) | ||||
| Caesarean section | 15 566 (14.5) | 16 351 (37.2) | 3879 (16.1) | 141 (18.5) | 1509 (7.3) | 6937 (67.4) | ||||
| Other | 1096 (1.0) | 247 (0.6) | 191 (0.8) | 4 (0.5) | 89 (0.4) | 24 (0.2) | ||||
| Missing | 45 (<0.1)§ | 6 (<0.1)§ | 3 (<0.1)§§ | 1 (0.1)§ | 2 (<0.1)§ | 0 (0.0)§ | ||||
| Analgesia during labour | ||||||||||
| No | 105 176 (98.2) | 36 117 (82.1) | <0.001 | 20 974 (87.0) | <0.001 | 729 (95.7) | <0.001 | 19 915 (96.9) | 8235 (80.0) | <0.001 |
| Yes | 1967 (1.8) | 7881 (17.9) | 3125 (13.0) | 33 (4.3) | 629 (3.1) | 2056 (20.0) | ||||
| Baby presentation at delivery | ||||||||||
| Occiput posterior | 11 571 (10.8) | 8152 (18.6) | <0.001 | 2636 (11.0) | 0.525 | 68 (8.9) | 0.093 | 1401 (6.8) | 2967 (28.9) | <0.001 |
| Occiput anterior | 95 352 (89.2) | 35 745 (81.4) | 21 409 (89.0) | 694 (91.1) | 19 100 (93.2) | 7306 (71.1) | ||||
| Missing | 220 (0.2)§ | 101 (0.2)§ | 54 (0.2)§ | 0 (0.0)§ | 43 (0.2)§ | 18 (0.2)§ | ||||
| Sex of baby | ||||||||||
| Female | 52 265 (48.8) | 21 139 (48.0) | 0.010 | 11 739 (48.7) | 0.861 | 367 (48.2) | 0.732 | 10 124 (49.3) | 4907 (47.7) | 0.008 |
| Male | 54 866 (51.2) | 22 852 (51.9) | 12 354 (51.3) | 395 (51.8) | 10 417 (50.7) | 5384 (52.3) | ||||
| Missing | 12 (<0.1)§ | 7 (<0.1)§ | 6 (<0.1)§ | 0 (0.0)§ | 3 (<0.1)§ | 0 (0.0)§ | ||||
*P value in comparison with the first (‘no analgesic’) column.
†P value in comparison with ‘no analgesic 2005–2015’ control column.
‡Only applicable to diclofenac 2005–2015 analysis.
§Percentage of missing data on total, not included in the analysis.
BMI, body mass index; n, number of pregnancies; n/a, not applicable; SIMD, Scottish Index of Multiple Deprivation.
Table 2 summarises the comparison of neonatal outcomes between the unexposed group (no analgesic at all) and the exposed groups of at least one analgesic, only paracetamol and at least one out of aspirin/naproxen/ibuprofen. Comparison of outcomes for the diclofenac subgroup analysis is shown in table 3.
Table 2.
Regression analysis of offspring outcomes between control (no analgesic) and groups exposed to at least one analgesic, only paracetamol, and at least one from ibuprofen (Ibu), aspirin (Asp), naproxen (Napr)
| Outcomes | No analgesic (n=107 143) n (%) |
At least one analgesic (n=43 998) n (%) |
Crude OR (95% CI) |
Adjusted OR (95% CI) |
Paracetamol only (n=24 099) n (%) |
Crude OR (95% CI) |
Adjusted OR (95% CI) |
Ibu/Asp/Napr (n=762) n (%) |
Crude OR (95% CI) |
Adjusted OR (95% CI) |
| Gestation at delivery (weeks) | ||||||||||
| −37 | 100 879 (94.2) | 39 838 (90.5) | 1.00 | 1.00 | 21 589 (89.6) | 1.00 | 1.00 | 697 (91.5) | 1.00 | 1.00 |
| <37 | 6264 (5.8) | 4160 (9.5) | 1.68 (1.61 to 1.75) | 1.50 (1.43 to 1.58)* | 2510 (10.4) | 1.87 (1.78 to 1.97) | 1.56 (1.48 to 1.65)* | 65 (8.5) | 1.50 (1.16 to 1.94) | 1.42 (1.08 to 1.86)* |
| Pregnancy outcome | ||||||||||
| Live birth | 105 949 (98.9) | 43 407 (98.7) | 1.00 | 1.00 | 23 704 (98.4) | 1.00 | 1.00 | 747 (98.0) | 1.00 | 1.00 |
| Stillbirth | 803 (0.7) | 405 (0.9) | 1.23 (1.09 to 1.39) | 1.33 (1.15 to 1.54)* | 275 (1.1) | 1.53 (1.33 to 1.76) | 1.52 (1.30 to 1.77)* | 13 (1.7) | 2.30 (1.32 to 3.99) | 2.34 (1.29 to 4.25)* |
| Neonatal death | 373 (0.3) | 182 (0.4) | 1.19 (0.99 to 1.42) | 1.56 (1.27 to 1.93)* | 117 (0.5) | 1.40 (1.14 to 1.73) | 1.56 (1.24 to 1.96)* | 2 (0.3) | 0.76 (0.19 to 3.06) | 0.93 (0.23 to 3.74)* |
| Missing | 18 (<0.1) | 4 (<0.1) | n/a | n/a | 3 (<0.1) | n/a | n/a | 0 (0.0) | n/a | n/a |
| Weight of baby (g) | ||||||||||
| NBW | 87 966 (82.1) | 34 555 (78.6) | 1.00 | 1.00 | 19 163 (79.5) | 1.00 | 1.00 | 605 (79.5) | 1.00 | 1.00 |
| LBW | 5910 (5.5) | 3571 (8.1) | 1.54 (1.47 to 1.61) | 1.28 (1.20 to 1.37)† | 2213 (9.2) | 1.72 (1.63 to 1.81) | 1.60 (1.51 to 1.69)† | 59 (7.7) | 1.45 (1.11 to 1.90) | 1.29 (0.91 to 1.83)† |
| HBW | 13 233 (12.4) | 5863 (13.3) | 1.13 (1.09 to 1.17) | 1.09 (1.05 to 1.13)† | 2720 (11.3) | 0.94 (0.90 to 0.99) | 0.98 (0.93 to 1.02)† | 97 (12.7) | 1.07 (0.86 to 1.32) | 0.99 (0.80 to 1.24)† |
| Missing | 34 (<0.1) | 9 (<0.1) | n/a | n/a | 3 (<0.1) | n/a | n/a | 1 (0.1) | n/a | n/a |
| Standardised birthweight score | ||||||||||
| Mean (SD) | 0.001 (0.003) | −0.002 (0.065) | 0.03 (0.02 to 0.04) | 0.046 (0.032 to 0.059) ‡ | 0.001 (0.991) | −0.04 (−0.058 to -0.029) | −0.014 (−0.029 to 0.001) ‡ | 0.046 (0.038) | 0.045 (−0.029 to 0.119) | 0.049 (−0.025 to 0.123) ‡ |
| Admitted to neonatal unit | ||||||||||
| No | 62 378 (58.2) | 32 391 (73.6) | 1.00 | 1.00 | 16 342 (67.8) | 1.00 | 1.00 | 480 (63.0) | 1.00 | 1.00 |
| Yes | 11 011 (10.3) | 7448 (16.9) | 1.30 (1.26 to 1.35) | 1.57 (1.51 to 1.64)† | 3956 (16.4) | 1.37 (1.32 to 1.43) | 1.45 (1.38 to 1.53)† | 117 (15.4) | 1.38 (1.13 to 1.69) | 1.54 (1.22 to 1.94)† |
| Missing | 33 754 (31.5) | 4159 (9.5) | n/a | n/a | 3801 (15.8) | n/a | n/a | 762 (21.7) | n/a | n/a |
| APGAR score at 1 min | ||||||||||
| Normal | 92 217 (86.1) | 38 224 (86.9) | 1.00 | 1.00 | 20 593 (85.5) | 1.00 | 1.00 | 659 (86.5) | 1.00 | 1.00 |
| <7 | 14 335 (13.4) | 5674 (12.9) | 0.96 (0.92 to 0.99) | 1.18 (1.13 to 1.23)§ | 3437 (14.3) | 1.07 (1.03 to 1.12) | 1.23 (1.18 to 1.28)§ | 101 (13.3) | 0.99 (0.80 to 1.22) | 1.07 (0.86 to 1.32)§ |
| Missing | 591 (0.6) | 100 (0.2) | n/a | 69 (0.3) | n/a | n/a | 2 (0.3) | n/a | n/a | |
| APGAR score at 5 min | ||||||||||
| Normal | 104 292 (97.3) | 42 730 (97.1) | 1.00 | 1.00 | 23 334 (96.8) | 1.00 | 1.00 | 738 (96.9) | 1.00 | 1.00 |
| <7 | 2216 (2.1) | 1163 (2.6) | 1.28 (1.19 to 1.38) | 1.48 (1.35 to 1.62)§ | 690 (2.9) | 1.39 (1.28 to 1.52) | 1.53 (1.40 to 1.68)§ | 21 (2.8) | 1.34 (0.87 to 2.07) | 1.52 (0.97 to 2.36)§ |
| Missing | 635 (0.6) | 105 (0.2) | n/a | n/a | 75 (0.3) | n/a | n/a | 3 (0.4) | n/a | n/a |
| Cryptorchidism (only males included) | ||||||||||
| No | 54 509 (99.3) | 22 616 (99.0) | 1.00 | 1.00 | 12 247 (99.1) | 1.00 | 1.00 | 394 (99.4) | 1.00 | 1.00 |
| Yes | 357 (0.7) | 236 (1.0) | 1.59 (1.35 to 1.88) | 0.92 (0.77 to 1.11)† | 107 (0.9) | 1.33 (1.07 to 1.66) | 0.87 (0.69 to 1.09)† | 1 (0.3) | 0.39 (0.05 to 2.77) | 0.28 (0.04 to 1.98)† |
| Neural tube defects | ||||||||||
| No | 107 093 (99.9) | 43 928 (99.8) | 1.00 | 1.00 | 24 077 (99.9) | 1.00 | 1.00 | 762 (100) | 1.00 | 1.00 |
| Yes | 50 (0.1) | 70 (0.2) | 3.41 (2.37 to 4.91) | 1.64 (1.08 to 2.47)† | 22 (0.1) | 1.96 (1.19 to 3.23) | 1.21 (0.71 to 2.06)† | 0 (0.0) | n/a | n/a |
| Amniotic band defects | ||||||||||
| No | 107 053 (99.9) | 43 936 (99.9) | 1.00 | 1.00 | 24 070 (99.9) | 1.00 | 1.00 | 760 (99.7) | 1.00 | 1.00 |
| Yes | 90 (0.1) | 62 (0.1) | 1.68 (1.21 to 2.32) | 1.02 (0.71 to 1.47)† | 29 (0.1) | 1.43 (0.94 to 2.18) | 0.98 (0.63 to 1.52)† | 2 (0.3) | 3.13 (0.77 to 12.73) | 2.29 (0.56 to 9.37)† |
| Hypospadias (only males included) | ||||||||||
| No | 54 607 (99.5) | 22 600 (98.9) | 1.00 | 1.00 | 12 258 (99.2) | 1.00 | 1.00 | 390 (98.7) | 1.00 | 1.00 |
| Yes | 259 (0.3) | 252 (1.1) | 2.35 (1.98 to 2.80) | 1.27 (1.05 to 1.54)† | 96 (0.8) | 1.65 (1.31 to 2.09) | 1.07 (0.84 to 1.37)† | 5 (1.3) | 2.70 (1.11 to 6.59) | 1.91 (0.78 to 4.68)† |
| Gastroschisis | ||||||||||
| No | 107 120 (99.9) | 43 979 (99.9) | 1.00 | 1.00 | 24 089 (99.9) | 1.00 | 1.00 | 762 (100) | 1.00 | 1.00 |
| Yes | 23 (0.1) | 19 (0.1) | 2.01 (1.10 to 3.70) | 1.10 (0.56 to 2.20)† | 10 (0.1) | 1.93 (0.92 to 4.06) | 0.99 (0.45 to 2.21)† | 0 (0.0) | n/a | n/a |
| At least one outcome¶ | ||||||||||
| No | 106 367 (99.3%) | 43 363 (98.6%) | 1.00 | 1.00 | 23 835 (98.9%) | 1.00 | 1.00 | 754 (99.0%) | 1.00 | 1.00 |
| Yes | 776 (0.7%) | 635 (1.4%) | 2.01 (1.81 to 2.23) | 1.12 (0.99 to 1.26)† | 264 (1.1%) | 1.52 (1.32 to 1.75) | 0.97 (0.84 to 1.13)† | 8 (1.0%) | 1.45 (0.72 to 2.93) | 1.11 (0.55 to 2.23)† |
Bold values show a significant OR (95% CI) as reported in the table.
*Adjusted for year of delivery, maternal age at delivery, SIMD, first gestational booking, maternal hypertensive disorders, maternal antepartum haemorrhage.
†Adjusted for year of delivery, maternal age at delivery, SIMD, first gestational booking, gestation at delivery.
‡Adjusted for year of delivery, maternal age at delivery, SIMD, first gestational booking.
§Adjusted for year of delivery, maternal age at delivery, SIMD, first gestational booking, type of delivery.
¶Including cryptorchidism, neural tube defects, amniotic band defects, hypospadias, gastroschisis.
n, number of pregnancies; n/a, not applicable; SIMD, Scottish Index of Multiple Deprivation.
Table 3.
Subgroup regression analysis between control pregnancies and exposed to diclofenac
| Outcomes | No analgesic (n=20 544) n (%) |
Diclofenac only 2005–2015 (n=10 291) n (%) |
Crude OR (95% CI) |
Adjusted OR (95% CI) |
| Gestation at delivery (weeks) | ||||
| ≥37 | 19 407 (94.5%) | 9640 (93.7%) | 1.00 | 1.00 |
| <37 | 1137 (5.5%) | 651 (6.3%) | 1.15 (1.04 to 1.27) | 1.10 (0.99 to 1.22)* |
| Pregnancy outcome | ||||
| Live birth | 20 393 (99.3%) | 10 227 (99.4%) | 1.00 | 1.00 |
| Stillbirth | 116 (0.5%) | 39 (0.4%) | 0.67 (0.47 to 0.96) | 0.59 (0.41 to 0.87)* |
| Neonatal death | 35 (0.2%) | 25 (0.2%) | 1.42 (0.85 to 2.38) | 1.26 (0.73 to 2.15)* |
| Weight of baby (g) | ||||
| NBW | 16 869 (82.1%) | 8116 (78.9%) | 1.00 | 1.00 |
| LBW | 965 (4.7%) | 572 (5.6%) | 1.23 (1.11 to 1.37) | 1.22 (1.07 to 1.40)† |
| HBW | 2707 (13.2%) | 1600 (15.5%) | 1.23 (1.15 to 1.31) | 1.21 (1.13 to 1.29)† |
| Missing | 3 (0.0%) | 3 (0.0%) | ||
| Standardised birthweight score | ||||
| −0.039 (0.959) | 0.132 (1.036) | 0.171 (0.145 to 0.197) | 0.167 (0.141 to 0.193)‡ | |
| Admitted to neonatal unit | ||||
| No | 18 224 (88.7%) | 8747 (85.0%) | 1.00 | 1.00 |
| Yes | 2175 (10.6%) | 1492 (14.5%) | 1.43 (1.33 to 1.53) | 1.46 (1.35 to 1.58)† |
| Missing | 145 (0.7%) | 52 (0.5%) | ||
| APGAR score at 1 min | ||||
| Normal | 18 709 (91.1%) | 9350 (90.9%) | 1.00 | 1.00 |
| <7 | 1658 (8.1%) | 924 (9.0%) | 1.12 (1.03 to 1.21) | 0.93 (0.83 to 1.04)§ |
| Missing | 177 (0.9%) | 17 (0.2%) | ||
| APGAR score at 5 min | ||||
| Normal | 20 065 (97.7%) | 10 096 (98.1%) | 1.00 | 1.00 |
| <7 | 302 (1.5%) | 177 (1.7%) | 0.86 (0.71 to 1.04) | 0.94 (0.72 to 1.23)§ |
| Missing | 177 (0.9%) | 18 (0.2%) | ||
| Cryptorchidism (only males included) | ||||
| No | 10 284 (98.7%) | 5314 (98.7%) | 1.00 | 1.00 |
| Yes | 133 (1.3%) | 70 (1.3%) | 1.02 (0.76 to 1.36) | 1.05 (0.78 to 1.42)† |
| Neural tube defects | ||||
| No | 20 527 (99.9%) | 10 263 (99.7%) | 1.00 | 1.00 |
| Yes | 17 (0.1%) | 28 (0.3%) | 3.29 (1.80 to 6.02) | 3.62 (1.95 to 6.74)† |
| Amniotic band defects | ||||
| No | 20 514 (99.9%) | 10 277 (99.9%) | 1.00 | 1.00 |
| Yes | 30 (0.1%) | 14 (0.1%) | 0.93 (0.49 to 1.76) | 0.81 (0.41 to 1.58)† |
| Hypospadias (only males included) | ||||
| No | 10 317 (99.0%) | 5308 (98.6%) | 1.00 | 1.00 |
| Yes | 100 (1.0%) | 76 (1.4%) | 1.48 (1.09 to 1.99) | 1.49 (1.09 to 2.03)† |
| Gastroschisis | ||||
| No | 20 538 (99.9%) | 10 284 (99.9%) | 1.00 | 1.00 |
| Yes | 6 (0.1%) | 7 (0.1%) | 2.33 (0.78 to 6.94) | 2.93 (0.97 to 8.88)† |
| At least one outcome¶ | ||||
| No | 20 258 (98.6%) | 10 097 (98.1%) | 1.00 | 1.00 |
| Yes | 286 (1.4%) | 194 (1.9%) | 1.36 (1.13 to 1.64) | 1.38 (1.15 to 1.67)† |
Bold values show a significant OR (95% CI) as reported in the table.
*Adjusted for year of delivery, maternal age at delivery, SIMD, first gestational booking, maternal hypertensive disorders, maternal antepartum haemorrhage.
†Adjusted for year of delivery, maternal age at delivery, SIMD, first gestational booking, gestation at delivery.
‡Adjusted for year of delivery, maternal age at delivery, SIMD, first gestational booking.
§Adjusted for year of delivery, maternal age at delivery, SIMD, first gestational booking, type of delivery.
¶Including cryptorchidism, neural tube defects, amniotic band defects, hypospadias, gastroschisis.
HBW, high birth weight; LBW, low birth weight; NBW, normal birth weight; SIMD, Scottish Index of Multiple Deprivation.
All analgesics and neonatal outcomes
As shown in table 2, compared with unexposed pregnancies in which women did not use any analgesic, pregnancies with consumption of at least one analgesic (paracetamol, diclofenac, aspirin, naproxen, ibuprofen) were independently associated with significantly higher odds for premature delivery (adjusted OR (aOR)=1.50, 95% CI 1.43 to 1.58), stillbirth (aOR=1.33, 95% CI 1.15 to 1.54), LBW (aOR=1.28, 95% CI 1.20 to 1.37), HBW (aOR=1.09, 95% CI 1.05 to 1.13), baby admission to neonatal unit (aOR=1.57, 95% CI 1.51 to 1.64), APGAR score <7 at 5 min (aOR=1.48, 95% CI 1.35 to 1.62), neural tube defects (aOR=1.64, 95% CI 1.08 to 2.47) and hypospadias (aOR=1.27, 95% CI 1.05 to 1.54) in adjusted analyses. Significantly decreased odds for APGAR score <7 at 1 min were found in the crude analysis (cOR=0.96, 95% CI 0.92 to 0.99), however when adjusted for year of delivery, maternal age at delivery, SIMD, first gestational booking and type of delivery, the significance changed direction showing significantly increased odds (aOR=1.18, 95% CI 1.13 to 1.23). A significantly lower standardised birthweight score (p=0.046, 95% CI 0.032 to 0.059) was found for the exposure group compared with no analgesic at all. Cryptorchidism (aOR=0.92, 95% CI 0.77 to 1.11), amniotic band defects (aOR=1.02, 95% CI 0.71 to 1.47), gastroschisis (aOR=1.10, 95% CI 0.56 to 2.20) and the composite outcome variable (aOR=1.12, 95% CI 0.99 to 1.26), were all associated with increased odds in the exposure group compared with not exposed, however the association was not significant in the adjusted model. There was no significant association between neonatal death and exposure to at least one analgesic in the crude analysis (cOR=1.19, 95% CI 0.99 to 1.42), however there were significantly higher odds of neonatal death in the adjusted analysis (aOR=1.56, 95% CI 1.27 to 1.93) in the exposed group compared with control.
Paracetamol and neonatal outcomes
In the subgroup analysis considering only paracetamol consumption during pregnancy as our exposure group, most of the associations reported in the main analysis remained significant with the same direction of significance (table 2). The differences were: maternal paracetamol consumption during pregnancy was associated with significantly decreased odds for offspring HBW (cOR=0.94, 95% CI 0.90 to 0.99) in the crude analysis however significance was lost in the adjusted model (aOR=0.98, 95% CI 0.93 to 1.02), and there were no significant associations in the adjusted models for neural tube defects (aOR=1.21, 95% CI 0.71 to 2.06) and hypospadias (aOR=1.07, 95% CI 0.84 to 1.37).
Aspirin/Naproxen/Ibuprofen and neonatal outcomes
Consumption of at least one analgesic from aspirin, naproxen or ibuprofen during pregnancy was compared against the same control group of pregnancies where no analgesic was used (table 2). Again, when comparing associations between groups in this subgroup analysis and main analysis, fewer outcome variants showed similar significance pattern. The only shared significant associations were for increased odds for premature delivery (aOR=1.42, 95% CI 1.08 to 1.86), stillbirth (aOR=2.34, 95% CI 1.29 to 4.25) and baby admission to neonatal unit (aOR=1.54, 95% CI 1.22 to 1.94) in the adjusted regression analyses.
Diclofenac and neonatal outcomes
In the subgroup analysis of pregnancies coinciding with non-prescription, over-the-counter, availability of diclofenac (years 2005–2015) were considered, and outcomes compared between the diclofenac group and no analgesic consumption group (table 3). Compared with the main analysis, diclofenac consumption during pregnancy was not significantly associated with premature delivery (aOR=1.10, 95% CI 0.99 to 1.22), neonatal death (aOR=1.26, 95% CI 0.73 to 2.15) and APGAR score <7 in 1 min (aOR=0.93, 95% CI 0.83 to 1.04) in the adjusted models. Associations with APGAR score <7 in 5 min (aOR=0.94, 95% CI 0.72 to 1.23), cryptorchidism (aOR=1.05, 95% CI 0.78 to 1.42), amniotic band defects (aOR=0.81, 95% CI 0.41 to 1.58) and gastroschisis (aOR=2.93, 95% CI 0.97 to 8.88) were no longer significant in both crude and adjusted analyses. Maternal consumption of diclofenac was independently associated with a significant decrease in stillbirth (aOR=0.59, 95% CI 0.41 to 0.87). It is also interesting to note that diclofenac was the only subgroup analysis agreeing with the main analysis (exposure to at least one analgesic) on the significance of exposure association with increased incidence of neural tube defects (aOR=3.62, 95% CI 1.95 to 6.74) and hypospadias (aOR=1.49, 95% CI 1.09 to 2.03) compared with unexposed pregnancies in adjusted models. As most of the outcomes studied were relatively rare, the NNH were mostly >100. Preterm birth, low birth weight and admission to the neonatal unit were exceptions with NNH ranging from 15 to 38 (online supplemental tables S1 and S2).
Discussion
Main findings
Consumption of paracetamol, ibuprofen, aspirin and naproxen during pregnancy, either in combination or separately, was significantly associated with increased premature delivery, stillbirth, neonatal death, LBW, abnormal standardised birthweight score and more frequent admission to neonatal unit. Consumption of paracetamol alone was further associated with higher odds for APGAR score <7 at 1 and 5 min both in crude and adjusted analyses. There was a dramatic increase in the frequency of over-the-counter (non-prescription) analgesic use in pregnancies between 1985 and 2015, starting from only 10.3% of women using one or more of the compounds between 1985 and 1994, climbing to 60.1% of women in the final decade of our study. This means that our findings are applicable far beyond the percentage (between 14% and 38%)26 of pregnant women with underlying health deficits related to the adverse outcomes we report here.
Diclofenac use increased steeply from 2005 (figure 2A), which reflects the change in Scottish legislation, leading to diclofenac becoming available without prescription in that year. Diclofenac use was associated with fewer adverse outcomes but showed increased risk of neural tube defects and hypospadias in male neonates. Furthermore, and surprisingly, exposure to diclofenac only was associated with significant decrease in the incidence of stillbirth. The reasons for such differences between the changes in neonatal outcomes following diclofenac consumption compared with those following use of the other NSAIDs are not clear. The proportion of women using diclofenac, especially in the last 7 years of our study makes it highly unlikely to be due to an underlying maternal condition and/or other compounds used in combination (eg, prescriptions) by women taking diclofenac. It is possible that the drug could act directly on fetal development then this difference could also be due to structural and/or mechanistic differences of the compound compared with the other drugs. However, not enough is known about the specific mechanisms of action of the different analgesics studied to conclude further. Overall, comparing our main analysis with all three subanalyses, it is evident that the most significant differences were observed when paracetamol was taken with at least one other analgesic. This is mostly due to the high number of pregnancies where paracetamol was used, comprising almost 55% of the exposed cases in the main analysis. Most numbers needed to harm for our outcomes (online supplemental tables S1 and S2) ranged between 1000 and 100, apart from preterm birth, LBW and baby admission to neonatal unit, which were 27, 38 and 15, respectively for our main analysis further strengthening observed associations.
Strengths and limitations
A major strength of the present study is the large cohort of 151 141 pregnancies over a 30-year study period from 1985 until 2015, using a robust data source AMND. This is one of the largest cohorts used in studies examining the effects of analgesic use during pregnancy. The dataset contains high quality and consistent data from the geographically defined area of Aberdeen and surrounding district, in the North East of Scotland, UK. In addition, as Aberdeen Maternity Hospital is the only maternity hospital serving the area, over 95% of pregnancies in the area are included in the dataset, considerably minimising the risk for selection bias. We were able to analyse maternal consumption data of the five most commonly used analgesics available over-the-counter in the UK and most countries, which is not matched in the current literature. The nature of our data allowed for the analysis of analgesics consumed alone or in combination, unlike most existing studies, and this gives our study the added strength of better reflecting real-life consumption patterns.27 28 We were able to adjust for important confounding factors, relevant to each analysed outcome. Adjustment for maternal deprivation also allowed us to further account for potential unmeasured factors that can influence maternal and neonatal health, which is a major strength of our analysis compared with most studies.
A potential concern was that women were probably using analgesics to treat some inherent medical condition which in turn could have been the mediating factor for adverse outcomes. Data on indication for use were not available in the database. However, since these medications are widely available without prescription, this is unlikely to be a factor that affects the findings of this study. This is especially the case during the ‘diclofenac analysis’ covering 2005–2015, where this study presents results on multiple neonatal outcomes for the given cohort. In this way, we offer a comprehensive approach to the exploration of associations with in utero analgesic exposure rather than only focusing on a single outcome of interest. Our data were based on medical notes; however, over-the-counter consumption is self-reported, and details on the timing, duration, dosage, product type (single-ingredient vs combination) and administration type were not available in the database. In addition, the group of pregnancies with aspirin consumption might include use of low-dose aspirin which is recommended to help reduce risk of some pregnancy complications and outcomes related to placental function. Genetic factors potentially relating to the emergence of offspring health outcomes was an unmeasured variable in our analysis. This study does not include a quantitative bias analysis to identify potential distort of results presented here. Most women had their first antenatal clinic visit during the first pregnancy trimester, which might imply our results were affected by primarily first trimester exposure, although analgesic use in first trimester is most likely replicated in the rest of pregnancy. Complete case analyses were performed ignoring pregnancies with missing data in the covariates, however due to the low number of missing data there is little chance that this might have affected the validity of our results. Compared with our cohort size, there were, overall, very few cases of cryptorchidism, neural tube defects, amniotic band defects, hypospadias and gastroschisis, resulting in potentially underpowered statistical analyses to detect a difference for these outcomes. Our study only considered neonatal health outcomes and follow-up of the offspring was not available at this time.
Interpretation
Previous literature has considered fewer outcomes with fewer analgesic combinations compared with our study. Consistent with our results, increased risk of preterm birth and miscarriage has been associated with analgesic consumption during pregnancy,29–32 while others reported no associations with miscarriage, stillbirth or preterm delivery.20 29 30 33 Similarly, increased risk for offspring cryptorchidism, hypospadias, neural tube defects, amniotic band defects and gastroschisis have been shown by many studies,7–9 34–41 although, again, a lack of associations with major birth defects have been reported.13–17 42 43 Compared with our analysis, all these studies used a smaller cohort, considered a shorter study time and there was frequent disagreement with respect to the choices of adjusted confounding factors. Another difference is that maternal questionnaires/interviews were frequently the method of choice to evaluate maternal consumption. Some of the studies reported increased risks for specific pregnancy trimesters which is something our study could not evaluate. Differences in study design and adjustment for different confounders might also account for the disagreement of our results that provide a more accurate assessment. Our study is one of the largest in terms of cohort size, duration, number of analgesics and range of outcomes included which might also contribute to differences compared with other studies. Another study with a large sample size (98 190 pregnancies) and a 7-year study time from Rebordosa et al,29 also reported an increased risk of preterm birth following paracetamol use during pregnancy, which was increased in mothers with pre-eclampsia. Our results showed a significant association of the adjusted ORs following adjustment for maternal hypertensive disorders. In addition, they did not find a significant association with stillbirth, or LBW as we report here. This disagreement could be due to dataset differences including the information about use in each pregnancy trimester, and methodological differences such as the use of questionnaires versus medical notes or adjustment for different confounders.
The literature currently reports conflicting evidence, limiting our ability for definite decision-making. Over-the-counter analgesics are recommended to women by healthcare professionals in order to deal with pregnancy symptoms and other conditions. Policy-makers have taken a stand on the topic, either being reassuring about over-the-counter use during pregnancy or recommending caution when consumption is necessary.44–47 Different compounds can affect the mother and the fetus in a different way, and their combined use might worsen the risk for offspring ill health. This study demonstrates the need for additional research before the field can be confidently directed towards one direction or the other.
Whether the associations we report result from influenza, fever, rheumatological or inflammatory conditions, and/or combination with other prescribed medications or solely related to over-the-counter analgesics consumption is a matter of further research. Underlying health conditions could well influence the outcomes we see in this study, however, as these could be very different conditions it is biologically unlikely that they are responsible for the effects we observe here. Our study demonstrates an association of maternal over-the-counter analgesic consumption during pregnancy with adverse neonatal offspring outcomes. Future collaborative approaches such as an individual patient data meta-analysis that includes follow-up data on long-term outcomes during childhood and adulthood would significantly inform decision making. Going forward, uncovering the mechanisms of action and off target effects will also provide a solid foundation for the development of pregnancy-safe compounds. Finally, the findings present here suggest that diclofenac is associated with fewer changes in risk for the more frequent adverse outcomes, although it is associated more with rarer, but severe, negative outcomes, including neural tube defects. Diclofenac may have a lower risk for the main adverse neonatal outcomes reported for paracetamol. However, it should be noted that our study is not designed to specifically test differences in level of risk between the analgesics included. Therefore, it should be emphasised that this does not mean that the authors are stating that diclofenac is preferable to paracetamol.
Conclusions
Pain control is currently a therapeutic priority during pregnancy. Our findings of increased risk of adverse health outcomes for the offspring following at least first trimester maternal use of readily available over-the-counter analgesics are crucial to information for the management of pain during pregnancy.
Supplementary Material
Footnotes
Contributors: AZ, SB and PAF contributed to the conception, design and coordination of the research. EAR provided critical input in the design and planning of statistical analysis. AZ conducted the statistical analysis and prepared the manuscript, figures and tables and is the guarantor. AZ, SB, PAF, RTM and DCH substantially contributed to the analysis and interpretation of the work. All authors contributed to critical discussion of intellectual content, development and review/approval of the final manuscript version.
Funding: This work is supported by the Biotechnology and Biological Sciences Research council (BBSRC) funding the lead author under the EASTBIO doctoral training programme and to PAF, the EU Horizon 2020 project FREIA (grant number 825100). RTM is supported by MRC Centre for Reproductive Health Grant MR/N022556/1.
Disclaimer: The funders had no role in study design, data collection, data analysis, decision to publish or manuscript preparation.
Competing interests: DCH is a founder, director and shareholder in Stemnovate Limited. The remaining authors have no interests to disclose.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
A research protocol was submitted and approved by the AMND steering committee before data extraction. Approval was received on 6 June 2018. The dataset was fully anonymised, therefore there was no requirement for NHS ethics committee approval. The North of Scotland Research Ethics Service has devolved Caldicott approval to the Chair of the AMND steering committee. Approval to access and analyse data was obtained from the AMND steering committee (AMND 004/2018).
References
- 1.Gendron M-P, Martin B, Oraichi D, et al. Health care providers’ requests to teratogen information services on medication use during pregnancy and lactation. Eur J Clin Pharmacol 2009;65:523–31. 10.1007/s00228-008-0611-6 [DOI] [PubMed] [Google Scholar]
- 2.Mitchell AA, Gilboa SM, Werler MM, et al. Medication use during pregnancy, with particular focus on prescription drugs: 1976-2008. Am J Obstet Gynecol 2011;205:51.e1–8. 10.1016/j.ajog.2011.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Werler MM, Mitchell AA, Hernandez-Diaz S, et al. Use of over-the-counter medications during pregnancy. Am J Obstet Gynecol 2005;193:771–7. 10.1016/j.ajog.2005.02.100 [DOI] [PubMed] [Google Scholar]
- 4.Glover DD, Amonkar M, Rybeck BF, et al. Prescription, over-the-counter, and herbal medicine use in a rural, obstetric population. Am J Obstet Gynecol 2003;188:1039–45. 10.1067/mob.2003.223 [DOI] [PubMed] [Google Scholar]
- 5.Lupattelli A, Spigset O, Twigg MJ, et al. Medication use in pregnancy: a cross-sectional, multinational web-based study. BMJ Open 2014;4:e004365. 10.1136/bmjopen-2013-004365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zafeiri A, Mitchell RT, Hay DC. Over-the-counter analgesics during pregnancy: a comprehensive review of global prevalence and offspring safety. Hum Reprod Update 2020. [DOI] [PubMed] [Google Scholar]
- 7.Lynberg MC, Khoury MJ, Lu X, et al. Maternal flu, fever, and the risk of neural tube defects: a population-based case-control study. Am J Epidemiol 1994;140:244–55. 10.1093/oxfordjournals.aje.a117243 [DOI] [PubMed] [Google Scholar]
- 8.Werler MM, Louik C, Mitchell AA. Epidemiologic analysis of maternal factors and amniotic band defects. Birth Defects Res A Clin Mol Teratol 2003;67:68–72. 10.1002/bdra.10001 [DOI] [PubMed] [Google Scholar]
- 9.Snijder CA, Kortenkamp A, Steegers EAP, et al. Intrauterine exposure to mild analgesics during pregnancy and the occurrence of cryptorchidism and hypospadia in the offspring: the generation R study. Hum Reprod 2012;27:1191–201. 10.1093/humrep/der474 [DOI] [PubMed] [Google Scholar]
- 10.Lind JN, Tinker SC, Broussard CS, et al. Maternal medication and herbal use and risk for hypospadias: data from the National birth defects prevention study, 1997-2007. Pharmacoepidemiol Drug Saf 2013;22:783–93. 10.1002/pds.3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Interrante JD, Ailes EC, Lind JN, et al. Risk comparison for prenatal use of analgesics and selected birth defects, National birth defects prevention study 1997-2011. Ann Epidemiol 2017;27:645–53. 10.1016/j.annepidem.2017.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Philippat C, Giorgis-Allemand L, Chevrier C, et al. Analgesics during pregnancy and undescended testis. Epidemiology 2011;22:747–9. 10.1097/EDE.0b013e318225bf33 [DOI] [PubMed] [Google Scholar]
- 13.Hernandez RK, Werler MM, Romitti P, et al. Nonsteroidal antiinflammatory drug use among women and the risk of birth defects. Am J Obstet Gynecol 2012;206:228.e1 10.1016/j.ajog.2011.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cassina M, De Santis M, Cesari E, et al. First trimester diclofenac exposure and pregnancy outcome. Reprod Toxicol 2010;30:401–4. 10.1016/j.reprotox.2010.04.010 [DOI] [PubMed] [Google Scholar]
- 15.Padberg S, Tissen-Diabaté T, Dathe K, et al. Safety of diclofenac use during early pregnancy: a prospective observational cohort study. Reprod Toxicol 2018;77:122–9. 10.1016/j.reprotox.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 16.van Gelder MMHJ, Roeleveld N, Nordeng H. Exposure to non-steroidal anti-inflammatory drugs during pregnancy and the risk of selected birth defects: a prospective cohort study. PLoS One 2011;6:e22174. 10.1371/journal.pone.0022174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dathe K, Fietz A-K, Pritchard LW, et al. No evidence of adverse pregnancy outcome after exposure to ibuprofen in the first trimester - Evaluation of the national Embryotox cohort. Reprod Toxicol 2018;79:32–8. 10.1016/j.reprotox.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 18.ed. Pariente G, Leibson T, Carls A, et al. Pregnancy-Associated changes in pharmacokinetics: a systematic review. PLoS Med 2016;13:e1002160. 10.1371/journal.pmed.1002160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McElhatton PR, Sullivan FM, Volans GN. Paracetamol overdose in pregnancy analysis of the outcomes of 300 cases referred to the teratology information service. Reprod Toxicol 1997;11:85–94. 10.1016/S0890-6238(96)00200-6 [DOI] [PubMed] [Google Scholar]
- 20.Kozer E, Costei AM, Boskovic R, et al. Effects of aspirin consumption during pregnancy on pregnancy outcomes: meta-analysis. Birth Defects Res B Dev Reprod Toxicol 2003;68:70–84. 10.1002/bdrb.10002 [DOI] [PubMed] [Google Scholar]
- 21.Nitsche JF, Patil AS, Langman LJ, et al. Transplacental passage of acetaminophen in term pregnancy. Am J Perinatol 2017;34:541–3. 10.1055/s-0036-1593845 [DOI] [PubMed] [Google Scholar]
- 22.Bauer AZ, Swan SH, Kriebel D, et al. Paracetamol use during pregnancy - a call for precautionary action. Nat Rev Endocrinol 2021;17:757–66. 10.1038/s41574-021-00553-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayorinde AA, Wilde K, Lemon J, et al. Data resource profile: the Aberdeen maternity and neonatal Databank (AMND). Int J Epidemiol 2016;45:389–94. 10.1093/ije/dyv356 [DOI] [PubMed] [Google Scholar]
- 24.Campbell D, Hall M, Lemon J, et al. Clinical birthweight standards for a total population in the 1980s. Br J Obstet Gynaecol 1993;100:436–45. 10.1111/j.1471-0528.1993.tb15268.x [DOI] [PubMed] [Google Scholar]
- 25.Textor J, van der Zander B, Gilthorpe MS, et al. Robust causal inference using directed acyclic graphs: the R package “dagitty.”. Int J Epidemiol 2016;45:1887–94. 10.1093/ije/dyw341 [DOI] [PubMed] [Google Scholar]
- 26.Royal College of Obstetricians and Gynaecologists . RCOG review clarifies pain relief options for women during pregnancy and breastfeeding, 2018. Available: https://www.rcog.org.uk/en/news/new-rcog-review-clarifies-pain-relief-options-for-women-during-pregnancy-and-breastfeeding/ [Accessed 20 Nov 2020].
- 27.Sarganas G, Buttery AK, Zhuang W, et al. Prevalence, trends, patterns and associations of analgesic use in Germany. BMC Pharmacol Toxicol 2015;16:28. 10.1186/s40360-015-0028-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hider-Mlynarz K, Cavalié P, Maison P. Trends in analgesic consumption in France over the last 10 years and comparison of patterns across Europe. Br J Clin Pharmacol 2018;84:1324–34. 10.1111/bcp.13564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rebordosa C, Kogevinas M, Bech BH, et al. Use of acetaminophen during pregnancy and risk of adverse pregnancy outcomes. Int J Epidemiol 2009;38:706–14. 10.1093/ije/dyp151 [DOI] [PubMed] [Google Scholar]
- 30.Nielsen GL, Sørensen HT, Larsen H, et al. Risk of adverse birth outcome and miscarriage in pregnant users of non-steroidal anti-inflammatory drugs: population based observational study and case-control study. BMJ 2001;322:266–70. 10.1136/bmj.322.7281.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Czeizel AE, Dudás I, Puhó E. Short-term paracetamol therapy during pregnancy and a lower rate of preterm birth. Paediatr Perinat Epidemiol 2005;19:106–11. 10.1111/j.1365-3016.2005.00631.x [DOI] [PubMed] [Google Scholar]
- 32.Li Z, Ren A, Liu J, et al. Maternal flu or fever, medication use, and neural tube defects: a population-based case-control study in northern China. Birth Defect Res A 2007;79:295–300. 10.1002/bdra.20342 [DOI] [PubMed] [Google Scholar]
- 33.Pastore LM, Hertz-Picciotto I, Beaumont JJ. Risk of stillbirth from medications, illnesses and medical procedures. Paediatr Perinat Epidemiol 1999;13:421–30. 10.1046/j.1365-3016.1999.00196.x [DOI] [PubMed] [Google Scholar]
- 34.Kristensen DM, Hass U, Lesné L, et al. Intrauterine exposure to mild analgesics is a risk factor for development of male reproductive disorders in human and rat. Hum Reprod 2011;26:235–44. 10.1093/humrep/deq323 [DOI] [PubMed] [Google Scholar]
- 35.Jensen MS, Rebordosa C, Thulstrup AM, et al. Maternal use of acetaminophen, ibuprofen, and acetylsalicylic acid during pregnancy and risk of cryptorchidism. Epidemiology 2010;21:779–85. 10.1097/EDE.0b013e3181f20bed [DOI] [PubMed] [Google Scholar]
- 36.Correy JF, Newman NM, Collins JA, et al. Use of prescription drugs in the first trimester and congenital malformations. Aust N Z J Obstet Gynaecol 1991;31:340–4. 10.1111/j.1479-828X.1991.tb02816.x [DOI] [PubMed] [Google Scholar]
- 37.Lind DV, Main KM, Kyhl HB, et al. Maternal use of mild analgesics during pregnancy associated with reduced anogenital distance in sons: a cohort study of 1027 mother-child pairs. Hum Reprod 2017;32:223–31. 10.1093/humrep/dew285 [DOI] [PubMed] [Google Scholar]
- 38.Werler MM, Mitchell AA, Shapiro S. First trimester maternal medication use in relation to gastroschisis. Teratology 1992;45:361–7. 10.1002/tera.1420450407 [DOI] [PubMed] [Google Scholar]
- 39.Torfs CP, Katz EA, Bateson TF, et al. Maternal medications and environmental exposures as risk factors for gastroschisis. Teratology 1996;54:84–92. [DOI] [PubMed] [Google Scholar]
- 40.Werler MM, Sheehan JE, Mitchell AA. Maternal medication use and risks of gastroschisis and small intestinal atresia. Am J Epidemiol 2002;155:26–31. 10.1093/aje/155.1.26 [DOI] [PubMed] [Google Scholar]
- 41.Kozer E, Nikfar S, Costei A, et al. Aspirin consumption during the first trimester of pregnancy and congenital anomalies: a meta-analysis. Am J Obstet Gynecol 2002;187:1623–30. 10.1067/mob.2002.127376 [DOI] [PubMed] [Google Scholar]
- 42.Slone D, Siskind V, Heinonen OP, et al. Aspirin and congenital malformations. Lancet 1976;1:1373–5. 10.1016/S0140-6736(76)93025-7 [DOI] [PubMed] [Google Scholar]
- 43.Shaw GM, Todoroff K, Velie EM, et al. Maternal illness, including fever and medication use as risk factors for neural tube defects. Teratology 1998;57:1–7. [DOI] [PubMed] [Google Scholar]
- 44.Mosley JF, Smith LL, Dezan MD. An overview of upcoming changes in pregnancy and lactation labeling information. Pharm Pract 2015;13:605. 10.18549/PharmPract.2015.02.605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Society for Maternal-Fetal Medicine Publications Committee (SMFM) . Prenatal acetaminophen use and outcomes in children. Am J Obstet Gynecol 2017;216:B14–15. 10.1016/j.ajog.2017.01.021 [DOI] [PubMed] [Google Scholar]
- 46.Bisson DL, Newell SD, Laxton C, et al. Antenatal and postnatal analgesia: scientific impact paper No. 59. BJOG 2019;126:e114–24. 10.1111/1471-0528.15510 [DOI] [PubMed] [Google Scholar]
- 47.RCOG . RCOG review clarifies pain relief options for women during pregnancy and breastfeeding, 2018. Available: https://www.rcog.org.uk/en/news/new-rcog-review-clarifies-pain-relief-options-for-women-during-pregnancy-and-breastfeeding/ [Accessed 1 Nov 2019].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-048092supp001.pdf (77.7KB, pdf)
bmjopen-2020-048092supp002.pdf (81.7KB, pdf)
bmjopen-2020-048092supp003.pdf (67.9KB, pdf)
bmjopen-2020-048092supp006.pdf (67.6KB, pdf)
bmjopen-2020-048092supp005.pdf (64.1KB, pdf)
bmjopen-2020-048092supp004.pdf (77.5KB, pdf)
bmjopen-2020-048092supp007.pdf (81.9KB, pdf)
bmjopen-2020-048092supp008.pdf (78.4KB, pdf)
bmjopen-2020-048092supp009.pdf (61.9KB, pdf)
bmjopen-2020-048092supp010.pdf (64.2KB, pdf)
bmjopen-2020-048092supp011.pdf (65.5KB, pdf)
bmjopen-2020-048092supp012.pdf (82.6KB, pdf)
bmjopen-2020-048092supp013.pdf (55.4KB, pdf)
bmjopen-2020-048092supp014.pdf (51.3KB, pdf)
Data Availability Statement
No data are available.