Abstract
Neuropeptides, functioning as peptide neurotransmitters and hormones, are generated from proneuropeptide precursors by proteolytic processing at dibasic residue sites (i.e., KR, RK, KK, RR). The cysteine proteases cathepsin L and cathepsin V, combined with the serine proteases proprotein convertases 1 and 2 (PC1/3 and PC2), participate in proneuropeptide processing to generate active neuropeptides. To compare the dibasic cleavage properties of these proteases, this study conducted global, unbiased substrate profiling of these processing proteases using a diverse peptide library in multiplex substrate profiling by mass spectrometry (MSP-MS) assays. MSP-MS utilizes a library of 228 14-mer peptides designed to contain all possible protease cleavage sites, including the dibasic residue sites of KR, RK, KK, and RR. The comprehensive MSP-MS analyses demonstrated that cathepsin L and cathepsin V cleave at the N-terminal side and between the dibasic residues (e.g., ↓K↓R, ↓R↓K, and K↓K), with a preference for hydrophobic residues at the P2 position of the cleavage site. In contrast, the serine proteases PC1/3 and PC2 displayed cleavage at the C-terminal side of dibasic residues of a few peptide substrates. Further analyses with a series of dipeptide-AMC and tripeptide-AMC substrates containing variant dibasic sites with hydrophobic P2 residues indicated the preferences of cathepsin L and cathepsin V to cleave between dibasic residue sites with preferences for flanking hydrophobic residues at the P2 position consisting of Leu, Trp, Phe, and Tyr. Such hydrophobic amino acids reside in numerous proneuropeptides such as pro-NPY and proenkephalin that are known to be processed by cathepsin L. Notably, cathepsin L displayed the highest specific activity that was 10-, 64-, and 1268-fold greater than cathepsin V, PC1/3, and PC2, respectively. Peptide-AMC substrates with dibasic residues confirmed that PC1/3 and P2 cleaved almost exclusively at the C-terminal side of dibasic residues. These data demonstrate distinct dibasic cleavage site properties and a broad range of proteolytic activities of cathepsin L and cathepsin V, compared to PC1/3 and PC2, which participate in producing neuropeptides for cell–cell communication.
Keywords: cathepsin, proprotein convertase, protease, peptidomics, neuropeptide, mass spectrometry
Introduction
Neuropeptides are short peptides, typically 3–40 residues in length, that are essential mediators of cell–cell signaling. They function as neurotransmitters in the nervous system and as peptide hormones in the endocrine system.1−4 Distinct amino acid sequences of neuropeptides are key to their selective regulation of target cell receptors and neuroendocrine functions. For example, the brain neuropeptides enkephalin, β-endorphin, and dynorphin function as endogenous opioid regulators of pain relief.5−7 Galanin regulates cognitive brain functions.8,9 Corticotropin-releasing hormone (CRF) in the brain regulates stress and controls pituitary ACTH production involved in adrenal glucocorticoid production.10,11 The endocrine hormones insulin and glucagon regulate glucose metabolism.12,13 Neuropeptides are dynamic regulators of cell–cell communication for normal neuroendocrine functions, and neuropeptide dysregulation participates in human diseases.14
Neuropeptides are synthesized as inactive proneuropeptide precursor proteins (also known as prohormones in the endocrine system) within secretory vesicles which release active neuropeptides for cell–cell communication.1−4,15 The inactive proneuropeptide precursors undergo proteolytic processing to generate active peptides. Within precursor proteins, neuropeptide sequences are typically flanked by the dibasic residues Lys-Arg, Arg-Lys, Lys-Lys, and Arg-Arg (K-R, R-K, K-K, and R-R) and several processing proteases have been demonstrated to cleave at such dibasic sites for neuropeptide production. These enzymes consist of cathepsin L and cathepsin V cysteine proteases1,2 and the proprotein convertases 1/3 (PC1/3) and PC2 serine proteases.1−3 Evidence for participation of these proteases in neuropeptide production has been shown by gene knockout of these enzymes that results in reduced production of many neuropeptides examined by biochemical and molecular cellular studies of neuropeptide biosynthesis.1−3 It is of interest that the cysteine protease cathepsin V is a human-specific protease16−19 and, thus, represents a unique human-specific protease mechanism for neuropeptide production.19
Studies with several neuropeptide precursor-related substrates have suggested differential cleavage specificities of cathepsin L compared to PC1/3 and PC2 for dibasic residues.1−3 Cathepsin L cleaves enkephalin-containing peptide intermediates at the N-terminal side and between dibasic residues for the production of enkephalin20 and cleaves pro-NPY at the N-terminal side of dibasic residues for NPY production.21 In contrast, PC1/3 and PC2 proteases cleave at the C-terminal side of the dibasic residues of proneuropeptides including proinsulin,22−24 proenkephalin (PE),25,26 and others.27,28 These data suggest differential cleavage specificities at dibasic residues for these proneuropeptide processing proteases.
To evaluate the hypothesis that distinct cleavage specificities at dibasic residues may exist for cathepsin L and cathepsin V compared to PC1/3 and PC2, this study investigated the cleavage properties of these proteases by the strategy of global, unbiased multiplex substrate profiling by mass spectrometry (MSP-MS).29,30 Furthermore, MSP-MS analyses allow evaluation of the overall protease cleavage preferences using a peptide library as a model for diverse cleavage sites. MSP-MS utilizes a library of 228 14-mer peptides designed to have maximum neighbor and near-neighbor amino acid diversity. Cleavage of any of the 2964 peptide bonds within this library can be detected and quantified by mass spectrometry. Cathepsin L and V were found to cleave at 241 and 163 sites within this peptide library, respectively, while PC1/3 and PC2 cleaved only 4 and 1, respectively. These data revealed that cathepsin L and cathepsin V cleave between and at the N-terminal side of dibasic residues, whereas PC1/3 and PC2 cleave at the C-terminal side of dibasic residues. Analyses with a series of dipeptide-AMC and tripeptide-AMC fluorogenic substrates further demonstrated the distinct dibasic cleavage properties of these proteases. Furthermore, cathepsins L and V displayed high preferences for peptide-AMC substrates with hydrophobic residues in the P2 position adjacent to the dibasic sites. The distinct dibasic cleavage sites are important for determining the subsequent exopeptidases, aminopeptidase, and carboxypeptidases, needed for completion of neuropeptide production. Overall, the findings of this study demonstrate the different dibasic residue cleavage properties of cathepsin L and cathepsin V compared to the PC1/3 and PC2 proteases, which participate in neuropeptide production.
Results and Discussion
Results
MSP-MS Strategy for the Substrate Cleavage Profiling of Proneuropeptide Processing Enzymes
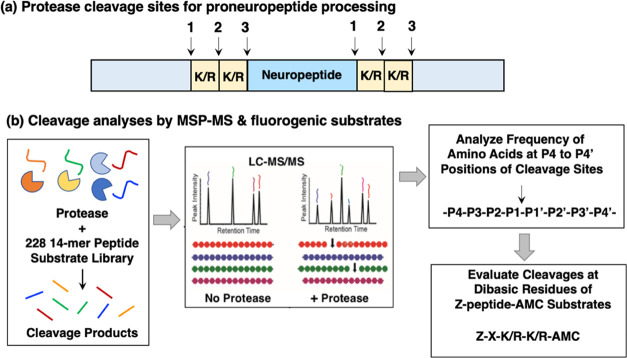
This study compared the dibasic cleavages of cathepsin L and cathepsin V with that of PC1/3 and PC2 that are utilized for proneuropeptide processing (Figure 1a). MSP-MS was conducted by incubating each of these proteases with the 228 14-mer peptide library at pH 5.5, the intravesicular pH within secretory vesicles where proneuropeptide processing occurs,31−33 followed by nano liquid chromatography tandem mass spectrometry (nano-LC−MS/MS) to identify and quantify peptide cleavage products (Figure 1b). The peptide library contains numerous substrates with the dibasic residues of KR, RK, RR, and KK. Peptide cleavage sites were analyzed for the frequency of residues at the P4 to P4′ positions of the P1-↓P1′ cleavage sites. This data was used to design peptide-AMC substrates for further evaluation of cleavage sites at dibasic residue peptide sequences (Figure 1b).
Figure 1.
Scheme for the cleavage profiling of the proneuropeptide processing proteases cathepsin L, cathepsin V, PC1/3, ad PC2, by MSP-MS and analyses by fluorogenic peptide substrates. (a) Proneuropeptides undergo proteolytic processing at dibasic residue sites. Neuropeptides are generated from proneuropeptide precursors that require proteolytic processing at dibasic sites (K/R-K/R) to generate active neuropeptides. (b) Strategy for the cleavage profiling of cathepsin L, cathepsin V, PC1/3, and PC2 processing enzymes by MSP-MS and fluorogenic substrates. The cleavage profile properties of cathepsin L and cathepsin V cysteine proteases, combined with PC1/3 and PC2 serine proteases, were evaluated by global, unbiased multiplex substrate profiling by mass spectrometry (MSP-MS) and fluorogenic peptide-AMC substrates containing variant dibasic residue sequences. For MSP-MS, the 228 peptide library was incubated with each of the processing proteases (as described in the Methods section), and peptide cleavage products were subjected to nano-LC–MS/MS tandem mass spectrometry for identification and quantification. Peptide cleavage products were analyzed for the frequency of each of the different amino acid residues at positions P4–P4′ and at the cleaved P1↓P1′ cleavage site. Based on MSP-MS results, peptide-AMC substrates were designed to further assess the dibasic cleavage site preferences of these proteases.
MSP-MS Analyses of Human Cathepsin L and Cathepsin V Reveal Cleavage Sites between Dibasic Residues and at the N-Terminal Side of Dibasic Residues within Peptide Substrates
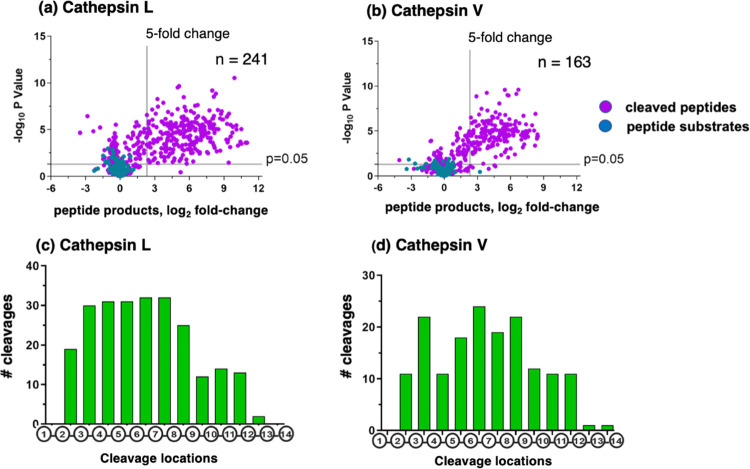
Cathepsin L and cathepsin V cleavage products were generated from the peptide library during 60 min incubation, illustrated by volcano plots showing the log2-fold change of the quantities of peptide products compared to a control assay that contained no enzyme activity (Figure 2a,b). Cathepsins L and V cleaved at 241 and 163 sites within the peptide library, respectively. The endopeptidase cleavage properties of cathepsin L and cathepsin V were illustrated by the cleavages that occurred at peptide bonds of 14-mer substrates (Figure 2c,d). In general, cleavage sites were located between residues 2 and 12 with few peptides being cleaved between residues 1 and 2 or between residues 12 and 14 (Figure 2c,d). This cleavage profile indicates that cathepsins L and V have endopeptidase specificity, without exopeptidase activity for removal of N- or C-terminal residues.
Figure 2.
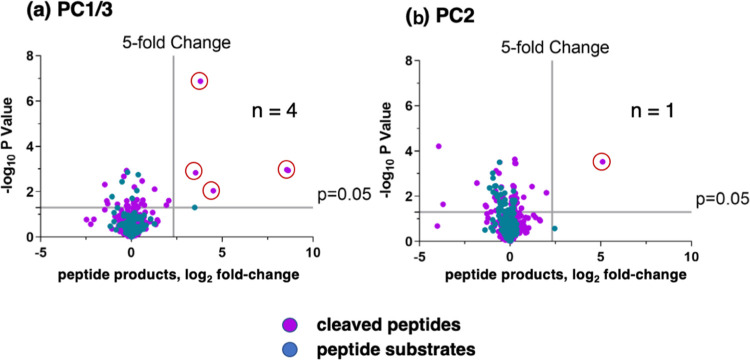
Cathepsin L and cathepsin V peptide cleavage profiling analyzed by multiplex substrate profiling by mass spectrometry (MSP-MS). (a,b) Volcano plots of cleaved peptides generated by cathepsin L (a) and cathepsin V (b). The log2 ratios of relative quantities of peptide products generated by cathepsin L or cathepsin V (60 min incubation at pH 5.5) compared to no enzyme activity controls are illustrated with −log10p values. Peptide products generated with at least a 5-fold change above no enzyme activity controls and with p < 0.05 numbered 241 and 163 peptides in panels “a” and “b”, respectively, representing 8.1 and 7.9%, respectively, of the entire number of 2964 cleavage sites among the 228 peptides of the library. Peptide sequences were analyzed for the frequencies of amino acid residues at the P4–P4′ positions of the P1–↓P1′cleavage site. (c,d) Cleavage positions of 14-mer peptide substrates for cathepsin L (c) and cathepsin V (d). The number of cleavages by cathepsin L and cathepsin V at each of the peptide bonds of the 14-mer peptide substrates are illustrated.
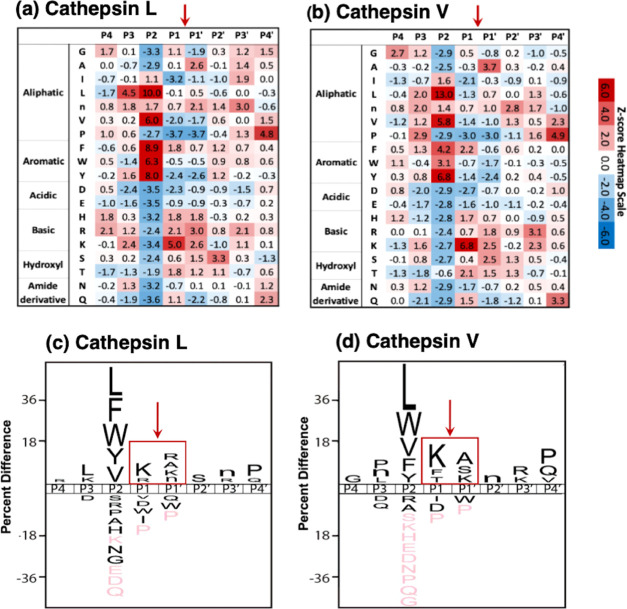
The frequencies of amino acid residues at P4–P4′ positions adjacent to P1–↓P1′ cleavage sites were represented by heat map and IceLogo analyses (Figure 3). The heat maps display the relative frequencies of residues at each of the cleavage positions as Z-scores, comparing protease cleavages with that of the reference peptide library (Figure 3a,b). Cathepsin L and cathepsin V displayed preferences for basic residues, Lys and Arg, at P1 and P1′ positions. Cathepsin L displayed a preference for Lys and Arg at the P1 position (Z-scores of 5.0 and 2.1, respectively) and the P1′ position (Z-scores of 2.6 and 3.0, respectively). Cathepsin V showed a preference for Lys at the P1 position (Z-score of 6.8) and some preference for Arg at the P1 position (Z-score of 0.7); at the P1′ position, cathepsin V showed preferences for Lys and Arg (Z-scores of 2.5 and 1.8, respectively). At the P2 position, both cathepsin L and cathepsin V showed strong preferences for the hydrophobic residues of Leu, Val, Phe, Trp, and Tyr.
Figure 3.
Cathepsin L and cathepsin V preferences for P4–P4′ residues of peptide cleavage sites in MSP-MS analyses. (a, b) Heat maps of amino acids preferred at cleavages sites. The peptide library cleavage data for cathepsin L (panel a) and cathepsin V (panel b) shows the frequencies of amino acid residues at each of the P4 to the P4′ positions of cleaved peptides, shown as the heat maps of Z-scores (explained in the Methods section) that compare protease cleavages with that of the reference peptide library. (c, d) IceLogo of cathepsin L and cathepsin V for preferred cleavages at P4–P4′ residues. Cathepsin L (panel c) and cathepsin V (panel d) cleavage data is illustrated by iceLogo. IceLogo shows the relative frequency of the preferred residues at the P1–↓P1′ cleavage site and at the P4–P4′ residues. Black letters above the line of P4–P4′ positions indicate preferred amino acid residues of the protease with p < 0.05, compared to the reference (negative data) of all possible residues at each position. Pink letters indicate residues that were never found at the indicated cleavage position.
IceLogo analyses illustrated the relative frequency of individual amino acids at P4–P4′ positions (p < 0.05) (Figure 3c,d). The cleavage of peptides by cathepsins L and V was largely driven by the P2 residue, where 62 and 63% of all cleavage sites have either Leu, Phe, Trp, Tyr, or Val at this position, respectively. Outside of the P2 position, cathepsin L frequently cleaved peptides with Lys and Arg at P1, and Lys, Arg, Ala, and norleucine (n) at P1′ while cathepsin V cleaved peptides with Lys, Phe, and Thr at P1 and Lys, Ser, and Ala at P1′. In general, these data show that cathepsin L and cathepsin V possess similar cleavage specificities, consistent with their high homology.16−19
Among the dibasic peptides of the library, cathepsin L cleaved between the dibasic residues of K↓R and R↓K, and at the N-terminal side of ↓KR and ↓RK dibasic sites, and the hydrophobic residues of Tyr and Trp and norleucine were present in the P2 positions of the cathepsin L dibasic cleavage sites (Table 1). Cathepsin V cleaved the peptides of the library at dibasic residues between K↓R, R↓K, and K↓K; furthermore, the preferred P2 residues of these cleaved peptides consisted of Tyr and Phe hydrophobic residues, as well as Ile (Table 1).
Table 1. Dibasic Residue Cleavages of Peptide Substrates by Cathepsin L and Cathepsin V Cysteine Proteases and PC1/3 and PC2 Serine Proteasesa.
| protease | peptide | fold change (60 min) |
|---|---|---|
| cathepsin L | GnYYK↓RFnAHWVGI | 142 |
| TPHHVNWYK↓RAPNQ | 46 | |
| EGADIWYR↓KHSHQL | 5 | |
| TPHHVNWY↓KRAPNQ | 5 | |
| LGWHAnF↓RKYPInA | 124 | |
| cathepsin V | GnYYK↓RFnAHWVGI | 14 |
| TPHHVNWYK↓RAPNQ | 6 | |
| LGWHAnFR↓KYPInA | 12 | |
| DAWAPnVIK↓KESSI | 32 | |
| PC1/3 | GnYYKR↓FnAHWVGI | 395 |
| IEPPWVDSHAKR↓Nn | 23 | |
| VDYIEHKDQVRR↓nN | 14 | |
| PC2 | YWnSTHLAGKR↓RDW | 34 |
Dibasic peptide cleavage sites of peptide substrates are illustrated for cathepsin L, cathepsin V, PC1/3, and PC2. Cleavage sites were determined by the identification and quantification of cleaved peptide products. Cleaved peptide products analyzed were the NH2-terminal peptide fragments, except for EGADIWR↓KHSHQL, where its COOH-terminal peptide product was identified and quantified. The fold changes of the cleaved peptide product generated at 60 min compared to 0 min controls are shown. The non-natural amino acid norleucine is indicated as lowercase “n.”
Overall, the unbiased, global substrate cleavage profiling by MSP-MS demonstrated that cathepsin L and cathepsin V cleave between dibasic residues or at the N-terminal side of dibasic residues. In both scenarios, there is a strong preference for hydrophobic residues at the P2 position adjacent to the dibasic sites.
MSP-MS Analyses of PC1/3 and PC2 Indicates Cleavage at the C-Terminal Side of Dibasic Residues
MSP-MS analyses of human PC1/3 and PC2 proteases showed that PC1/3 cleaved four peptide substrates, while PC2 cleaved only one peptide, shown by Volcano plots (Figure 4). PC1/3 cleaved at the C-terminal side of KR↓ and RR↓ of three peptide substrates (Table 1). PC2 cleaved one peptide of the library, YWnSTHLAGKR↓RDW, at the C-terminal side of KR↓ residues, present within the tribasic Lys-Arg-Arg site (Table 1). The Gly residue at the P3 position of the KR↓ cleavage site is typical of neuropeptides that undergo C-terminal amidation.34 The low number of peptides within the library cleaved by PC1/3 and PC2 suggests their low activity. Overall, PC1/3 and PC2 demonstrated their selectivity for cleaving at the C-terminal side of KR↓ and RR↓ dibasic residues of several substrates.
Figure 4.
PC1/3 and PC2 cleavage profiling analyzed by MSP-MS. Volcano plots of PC1/3 (panel a) and PC2 (panel b) peptide cleavages from MSP-MS data show the log2 ratios of relative quantities of peptide products generated by PC1/3 and PC2 (60 min incubation at pH 5.5) compared to no enzyme activity controls, illustrated by −log10p values. Peptide products generated with at least a 5-fold change above controls and with p < 0.05 were analyzed for the frequencies of amino acid residues at the P4–P4′ positions of the P1–↓P1′ cleavage site.
Protease Cleavage Sites Assessed with Peptide-AMC Substrates Containing Variant Dibasic Residue Sequences
The dipeptide substrates Z-K-R-AMC, Z-R-K-AMC, Z-K-K-AMC, and Z-R-R-AMC were utilized to compare cleavage at these variant dibasic residue sites by cathepsin L, cathepsin V, PC1/3, and PC2. Furthermore, tripeptide substrates were designed with Leu, Trp, Phe, Tyr, and Val adjacent to the N-terminal side of K-R sites to assess the influence of these hydrophobic residues on proteolysis at dibasic residues by cathepsin L and cathepsin V. These hydrophobic residues were frequently present at the P2 position of cathepsin L and cathepsin V cleavages of the peptide library in the MSP-MS experiments (Figure 3).
Cleavage at the C-terminal side of dibasic residues generates free AMC (7-amino-4-methylcoumarin) fluorescence that is quantitatively monitored (Supporting Information, Figure S1). However, the cleavage between the dibasic residues of the dipeptide and tripeptide substrates, or cleavage at the N-terminal side of dibasic residues, generates nonfluorescent products. To detect these cleaved products, we used the aminopeptidase enzyme, cathepsin H, in a coupled assay to convert nonfluorescent products to fluorescent AMC (Supporting Information, Figure S1). Cathepsin H hydrolyzes free N-terminal Arg and Lys residues and therefore releases the fluorescent AMC reporter. Cathepsin H does not cleave Z-peptide-AMC substrates since N-terminal residues are blocked with the Z group (benzyloxycarbonyl).
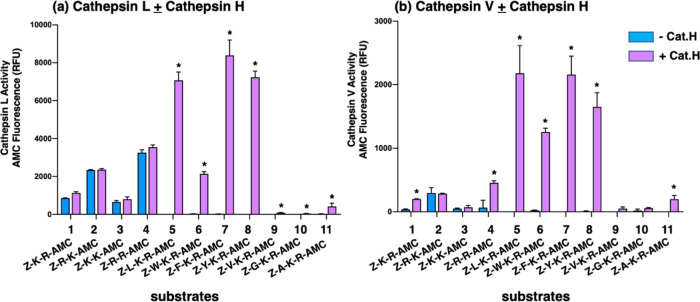
Cathepsin L cleavages of the tripeptide-AMC substrates Z-L-K-R-AMC, Z-W-K-R-AMC, Z-F-K-R-AMC, and Z-Y-K-R-AMC were readily detected following the addition of cathepsin H, but not in its absence (Figure 5a and Table 2). These findings show that cathepsin L cleaved between K-R sites, or at the N-terminal side of K-R, but not at the C-terminal side of the K-R. In contrast to tripeptide-AMC substrates, cathepsin L cleaved the dipeptide substrates Z-K-R-AMC, Z-R-K-AMC, Z-K-K-AMC, and Z-R-R-AMC at the C-terminal side of dibasic residues, since fluorescent measurements were similar in the absence and presence of cathepsin H. These data show that cathepsin L cleavage of tripeptide substrates is controlled by the presence of the hydrophobic residues Leu, Trp, Phe, and Tyr in the P2 position, which results in the cleavage between the adjacent dibasic residues yielding an R-AMC product that can be subsequently cleaved by cathepsin H to generate fluorescent AMC. Furthermore, cathepsin L displayed the highest activity with the Z-L-K-R-AMC, Z-F-K-R-AMC, and Z-Y-K-R-AMC tripeptide substrates compared to dibasic peptides.
Figure 5.
Cathepsin L and cathepsin V cleavage specificities at dibasic residue sites assessed with variant dipeptide-AMC and tripeptide-AMC substrates. Cathepsin L (panel a) and cathepsin V (panel b) were evaluated for the cleavage of dipeptide-AMC substrates containing the four dibasic variant cleavage sites KR, RK, KK, and RR and compared to the cleavage of tripeptide-AMC substrates containing the K-R with adjacent hydrophobic residues (Leu, Trp, Phe, Tyr, Val) or nonpolar residues (Gly, Ala) at the N-terminal side of the dibasic K-R site. Cathepsin L and cathepsin V were incubated with each of these substrates at 37 °C for 60 min. Then, the aminopeptidase cathepsin H or control buffer was added and incubation continued at 37 °C for another 30 min to allow conversion of basic residue-extended AMC products to free AMC for fluorometric measurement (conducted as described in the Methods section, with example shown in the Supporting Information, Figure S1). Controls included incubation of cathepsin H alone with each of the substrates, which resulted in no fluorescence, indicating that cathepsin H does not remove the blocked N-terminal residues of Z-peptide-AMC substrates. Comparison of fluorescence observed in the absence and presence of cathepsin H is illustrated, with significant differences with p < 0.05 (student’s t-test, n = 3) indicated by asterisks.
Table 2. Cathepsin L, Cathepsin V, PC1/3, and PC2 Cleavage of Peptide-AMC Substrates with Variant Dibasic Residues, without and with Cathepsin H Aminopeptidase Activity to Assess Processing Sitesa.
| ratio
of protease activity ± cathepsin H (RFU fluorescence) |
||||
|---|---|---|---|---|
| substrate | cathepsin L | cathepsin V | PC1/3 | PC2 |
| Z-K-R-AMC | 1.5 | 202.4 | infinity, CH only | 2.6 |
| Z-R-K-AMC | 1.0 | 1.0 | 1.0 | 0.9 |
| Z-K-K-AMC | 1.1 | 1.4 | 3.0 | 0.8 |
| Z-R-R-AMC | 1.1 | 6.8 | infinity, CH only | 1.5 |
| Z-L-K-R-AMC | infinity, CH only | infinity, CH only | 0.9 | 0.1 |
| Z-W-K-R-AMC | 73.9 | 48.3 | 0.9 | 1.3 |
| Z-F-K-R-AMC | infinity, CH only | infinity, CH only | 1.1 | 1.4 |
| Z-Y-K-R-AMC | infinity, CH only | 269 | 0.9 | 1.0 |
| Z-V-K-R-AMC | 29.7 | infinity, CH only | 2.9 | 0.7 |
| Z-G-K-R-AMC | infinity, CH only | 2.8 | infinity, CH only | 0.3 |
| Z-A-K-R-AMC | 26.1 | infinity, CH only | 1.9 | 1.2 |
The ratio of each protease activity was assessed in the absence and presence of cathepsin H in coupled assays to monitor cleavages at the C-terminal side of dibasic residues compared to cleavages occurring between or at the N-terminal side of dibasic residues. The ratio of activity, indicated by the relative fluorescence of free AMC, in the presence and absence of cathepsin H is shown in this table. When AMC fluorescence was generated only in the presence of cathepsin H (CH), the table indicates “infinity, with CH only.” The ratios of proteolytic activity ± cathepsin H represent data from Figures 5 and 6.
Cathepsin V cleavage of the tripeptide substrates Z-L-K-R-AMC, Z-W-K-R-AMC, Z-F-K-R-AMC, and Z-Y-K-R-AMC was prominently detected in the presence of cathepsin H, but not in the absence of cathepsin H (Figure 5b and Table 2). These data show that cathepsin V cleaved at the K-R dibasic site with a hydrophobic residue adjacent to the N-terminal side of the P1-↓P1′ cleavage site, with cleavage occurring between or at the N-terminal side of K-R residues. Among the dipeptide substrates, cathepsin V displayed lower activity. Cathepsin V cleaved Z-R-K-AMC and Z-K-K-AMC substrates at the C-terminal side of dibasic residues but cleaved between and at the C-terminal side of the dibasic residues of Z-K-R-AMC and Z-R-R-AMC.
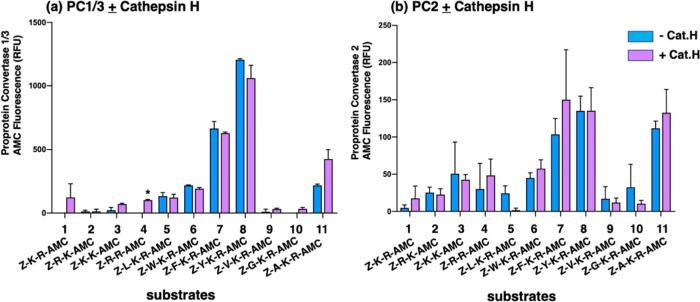
In contrast to cathepsin L and cathepsin V, PC1/3 and PC2 both displayed a preference for cleaving at the C-terminal side of dibasic residues within the peptide-AMC substrates (Figure 6 and Table 2). PC1/3 and PC2 displayed greater proteolysis of Z-F-K-R-AMC and Z-Y-K-R-AMC compared to the other substrates evaluated. These data illustrate differences in dibasic residue cleavage sites utilized by PC1/3 and PC2 compared to cathepsins L and V.
Figure 6.
PC1/3 and PC2 cleavage properties examined with variant dipeptide-AMC and tripeptide-AMC substrates containing dibasic residue sites. PC1/3 (panel a) and PC2 (panel b) were evaluated for the cleavage of dipeptide-AMC substrates containing the four dibasic variant cleavage sites KR, RK, KK, and RR and compared to the cleavage of tripeptide-AMC substrates containing the K-R with hydrophobic residues (Leu, Trp, Phe, Tyr, Val) or nonpolar residues (Gly, Ala) at the N-terminal side of the dibasic K-R site. After incubation of PC1/3 or PC2 with each of these substrates at 37 °C for 120 min, control buffer or the aminopeptidase cathepsin H was added and incubation at 37 °C continued for another 30 min (37 °C) to allow conversion of basic residue-extended AMC products to free AMC for fluorometric measurement. Comparison of fluorescence observed in the absence and presence of cathepsin H is illustrated and included evaluation of significant differences with p < 0.05 (Student’s t-test, n = 3).
Broad Range of Proteolytic Activities of Cathepsin L, Cathepsin V, PC1/3, and PC2
A broad range of proteolytic activities for these proteases was observed (Table 3). Cathepsin L was the most active protease, shown by its high specific activity compared to the lower specific activities of cathepsin V, PC1/3, and PC2 that were 10, 1.5, and 0.07%, respectively, compared to cathepsin L (100%) when assayed with their standard substrates of Z-Phe-Arg-AMC for cathepsins L and V,35,36 and substrate pGlu-Arg-Thr-Lys-Arg-AMC for PC1/3 and PC2.37 This rank order of high to low specific activity of cathepsin L to cathepsin V, PC1/3, and PC2 is consistent with the high to low number of cleavages by each of these proteases in MSP-MS assays (Figures 2 and 4). In addition, the same rank order of high to lower specific activities of these four proteases was observed by assay of these proteases with the Z-Tyr-Lys-Arg-AMC substrate (Table 3). The wide range of specific activities may represent the differing activity of each of these proteases for peptide processing.
Table 3. Specific Activities of Cathepsin L, Cathepsin V, PC1/3, and PC2a.
| protease | substrate | specific activity (pmol AMC/ng/min) |
|---|---|---|
| cathepsin L | Z-Phe-Arg-AMC | 8.5 |
| Z-Tyr-Lys-Arg-AMC | 3.2 | |
| cathepsin V | Z-Phe-Arg-AMC | 0.88 |
| Z-Tyr-Lys-Arg-AMC | 0.14 | |
| PC1/3 | pGlu-Arg-Thr-Lys-Arg-AMC | 0.132 |
| Z-Tyr-Lys-Arg-AMC | 0.463 | |
| PC2 | pGlu-Arg-Thr-Lys-Arg-AMC | 0.0067 |
| Z-Tyr-Lys-Arg-AMC | 0.0023 |
Specific activities were measured using Z-Phe-Arg-AMC for human cathepsin L (0.04 ng/μL) and human cathepsin V (0.2 ng/μL), and using pGlu-Arg-Thr-Lys-Arg-AMC for PC1/3 (0.9 ng/μL) and PC2 (4 ng/μL) as standard fluorogenic peptide substrates commonly used to assay for these proteases.35−37 Also, specific activities with the substrate Z-Tyr-Lys-Arg-AMC of this study were used to compare these four enzymes. Cathepsin L and cathepsin V activity with Z-Tyr-Lys-Arg-AMC required the use of cathepsin H in a coupled assay to generate AMC fluorescence (from Figure 5). PC1/3 and PC2 activities were the same when conducted without or without cathepsin H (from Figure 6) and, therefore, their activities without cathepsin H is provided for this table.
Discussion
The cysteine proteases cathepsin L and cathepsin V, combined with the serine proteases PC1/3 and PC2, participate in processing proneuropeptides at dibasic residue sites to generate active neuropeptides. In this study, the distinct cleavage specificities of cathepsin L and cathepsin V compared to PC1/3 and PC2 were demonstrated by multiplex substrate profiling mass spectrometry (MSP-MS) combined with analysis of peptide-AMC substrates with variant dibasic residue sequences. Cleavage profiling by MSP-MS showed that cathepsin L cleaved between the dibasic residues of K↓R and R↓K, and at the N-terminal side of ↓KR and ↓RK (Table 1). Cathepsin V cleaved between the dibasic residues of K↓R, R↓K, and K↓K. Cathepsin L and cathepsin V did not cleave at the C-terminal side of dibasic residue sites among the peptides of the library. Cathepsins L and V displayed strong preferences for the hydrophobic P2 residues of Leu, Val, Phe, and Trp adjacent to P1↓P1′ cleavage sites. PC1/3 cleaved at the C-terminal side of KR and RR sites, while PC2 only cleaved one peptide at the C-terminal side of KR within a tribasic KRR site. The differing cleavage specificities of these four proteases were confirmed with dipeptide-AMC with variant dibasic residues and with tripeptide-AMC substrates with hydrophobic residues adjacent to the N-terminal side of the K-R dibasic residue pair. Notably, a broad range of high to low levels of proteolytic activities were found for cathepsin L > cathepsin V > PC1/3 > PC2 (Table 3). These findings demonstrate the distinct preferences of the proneuropeptide processing enzymes cathepsin L and cathepsin V to cleave between or at the N-terminal side of dibasic residues, whereas PC1/3 and PC2 cleave at the C-terminal side of dibasic sites, with substantial differences in the levels of proteolysis (Figure 7).
Figure 7.
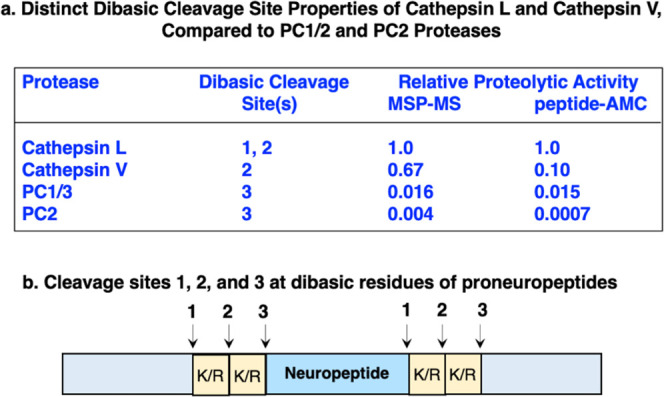
Distinct dibasic cleavage properties of cathepsin L and cathepsin V cysteine proteases compared to PC1/3 and PC2 serine proteases involved in proneuropeptide processing. (a) Information is shown for the relative proteolytic activity by the MSP-MS analyses of peptide library substrates and relative proteolytic activity observed in peptide-AMC assays using standard substrates for cathepsin L and cathepsin V (Z-F-R-AMC), and PC1/3 and PC2 (pERTKR-AMC). (b) Locations of dibasic cleavage sites (#1, 2, and 3) for each of the proneuropeptide processing proteases cathepsin L, cathepsin V, PC1/3, and PC2 are indicated.
Prior studies of cathepsin L cleavage of several proneuropeptides illustrate the presence of hydrophobic residues at the P2 position. For example, cathepsin L cleaved pro-NPY at the N-terminal side of KR within YG↓KR, with Tyr as the P2 residue (Supporting Information, Figure S2).21 With respect to enkephalin production, cathepsin L cleaved BAM-22P and peptide F proenkephalin-derived intermediates at the N-terminal side of dibasic residues of YGGFM↓RR and YGGFM↓KK with Phe at the P2 position, respectively, to generate enkephalin (YGGFM).20 Such dibasic residue cleavages with hydrophobic P2 residues by cathepsin L and cathepsin V are predicted for processing proenkephalin, pro-CCK (cholecystokinin), and prodynorphin.38−42
Subsequent to cathepsin L and cathepsin V processing of proneuropeptides, intermediate peptide products will then require exopeptidase steps to remove N-terminal and C-terminal basic residue extensions by aminopeptidase and carboxypeptidase enzymes (Supporting Information, Figure S4). Aminopeptidase B and cathepsin H participate as aminopeptidases for neuropeptide production.43−45 Carboxypeptidase E (CPE) participates as an exopeptidase for the removal of C-terminal basic residues of neuropeptide intermediates in neuroendocrine tissues.1,2
It is notable that cathepsin V is a human-specific cysteine protease, since orthologues of human cathepsin V have not been found in mouse and many other mammalian species.16−19 The human genome possesses the two highly homologous cathepsin V and cathepsin L cysteine proteases that are clan CA/family C1 cysteine proteases. The mouse cathepsin L possesses greater homology with human cathepsin V (74.5% protein sequence identity) compared to human cathepsin L (71.5% identify in protein sequence19), which supports the predicted role of cathepsin V in proneuropeptide processing as demonstrated by its processing of proenkephalin.19 Both cathepsin V and cathepsin L are present in secretory vesicles, where neuropeptides are generated from their precursors.
The MSP-MS cleavage data of PC1/3 and PC2 parallels that of prior studies on processing proinsulin, proenkephalin, and proopiomelanocortin (POMC) proneuropeptides. PC1/3 cleaved proinsulin at the C-terminal side of Arg-Arg↓, and PC2 cleaved proinsulin at the C-terminal side of Lys-Arg↓22−24 (Supporting Information, Figure S3). PC1/3 and PC2 cleaved proenkephalin (PE) at the C-terminal sides of multiple dibasic residues of KR↓, RR↓, and KK↓ based on mass spectrometry of peptide products26 (Supporting Information, Figure S3). In addition, PC2 cleavage at the tribasic KR↓R site shown by the MSP-MS data is consistent with evidence for PC2 processing of the KKRR tetrabasic site of POMC to generate α-MSH.27,46,47 It is noted that PC1/3 does not cleave RK and KK dibasic sites of POMC.27,46,47 These data show that PC1/3 and PC2 cleave at the C-terminal side of dibasic residues of several proneuropeptides.
Subsequent to PC1/3 and PC2 processing, peptide intermediate products then require removal of C-terminal basic residues by exopeptidase carboxypeptidase E (CPE) to generate active neuropeptides1,2 (Supporting Information, Figure S4). In contrast, subsequent to cathepsin L and cathepsin V processing, aminopeptidases are needed for removal of N-terminal basic residues, and CPE is needed for removal of C-terminal basic residues to generate the mature neuropeptide.
These cysteine and serine proteases participate jointly in proneuropeptide processing, demonstrated by protease gene knockout studies. Enkephalin production utilizes cathepsin L as shown by cathepsin L gene knockout that results in the reduction of enkephalin levels in mouse brain;20 enkephalin is also generated by PC2 as shown by reduced enkephalin brain levels in PC2 gene knockout mice.49 POMC processing to generate ACTH, α-MSH, and β-endorphin peptide hormones utilizes cathepsin L, PC1/3, and PC2 proteases.27,46−50 Cathepsin L gene knockout results in substantial decreases in ACTH, β-endorphin, and α-MSH derived from POMC, indicated by accumulation of POMC in the cathepsin L knockout mice.48 PC2 gene knockout results in obliteration of α-MSH in pituitary and the brain and is accompanied by increases in ACTH and β-endorphin1−31 consistent with these intermediates as possible substrates of PC2;49 PC1/3 gene disruption reduces POMC processing.50 Cathepsin L gene knockout also results in decreased production of NPY, CCK, and dynorphin neuropeptides;21,51,52 production of these neuropeptides also involve PC2.53−55 Numerous neuropeptides utilized PC1/3, as shown in PC1/3 null mice.56
Our recent study of endogenous neuropeptide production in dense-core secretory vesicles (DCSV) assessed by neuropeptidomics and MSP-MS showed that MSP-MS represented endogenous proteases involved in the production of chromaffin granule neuropeptides.57 Thus, MSP-MS peptide library analysis of protease properties represents their neuropeptide-producing functions.
Cathepsin L had the highest specific activity compared to cathepsin V, PC1/3, and PC2 (Table 3). In vivo, endogenous inhibitors of cathepsin L exist in DCSV that regulate its activity. Cathepsin L is inhibited by the endogenous cystatin C protease inhibitor,58 which is present in dense-core secretory vesicles (DCSV).57 Cathepsin L is also inhibited by endogenous serpin type protease inhibitors present in DCSV.59−61
It will be of interest in future studies to gain an understanding of the coordinate roles of these cysteine and serine proneuropeptide processing proteases with the secretory vesicle proteome57 for the production of diverse neuropeptides utilized for neuroendocrine cell–cell communication.
Experimental Methods
Human Recombinant-Purified Cathepsin L, Cathepsin V, PC1/3, and PC2, with Peptide Library, Peptide-AMC Substrates, and Reagents
Human recombinant proteases (with catalogue numbers indicated) were obtained from R&D Systems (Minneapolis, MN), consisting of human recombinant cathepsin L (#952-CY-010), human recombinant cathepsin V (#1080-CY-010), human recombinant PC1/3 (#2810-SE-010), and human recombinant PC2 (#6018-SE-010). Protease MSP-MS assays utilized the library of 228 14-mer peptides designed to contain all possible protease cleavage sites, as previously described29,30 (peptides synthesized by Anaspec, Fremont, CA). Assays utilized octyl-β-glucopyranoside (Sigma-Aldrich, Darmstadt, Germany), dithiothreitol (DTT, Promega, Madison, WI), BEH C18 packing material (Waters Corporation, Milford, MA), acetonitrile (ACN, Fisher Chemical, Pittsburgh, PA), trifluoroacetic acid (TFA, Fisher Chemical, Pittsburgh, PA), urea (Teknova, Hollister, CA), and C18 for solid-phase extraction (SPE) stage-tips (3M, Maplewood, MN). Fluorogenic proteolytic assays used the substrates Z-K-R-AMC, Z-R-K-AMC, Z-K-K-AMC, Z-R-R-AMC, Z-L-K-R-AMC, Z-W-K-R-AMC, Z-F-K-R-AMC, Z-Y-K-R-AMC, Z-V-K-R-AMC, Z-G-K-R-AMC, and Z-A-K-R-AMC from Genscript (Piscataway, NH); Z-F-R-AMC was from Bachem (Vista, CA) and pERTKR-AMC was from R&D Systems.
Multiplex Substrate Profiling by Mass Spectrometry (MSP-MS)
MSP-MS utilized a peptide library of 228 14-mers with diverse sequences representing all possible protease cleavage sites, synthesized as described.29,30 The peptides of the library used for MSP-MS were designed to contain all neighbor and near-neighbor (+1 and +2) dipeptide pairs as described by O’Donoghue et al.29 This peptide library is a universal substrate library for all proteases as well as proteases with dibasic specificity. The length of 14 residues for the peptides and cleavage products are readily detected and quantified by LC–MS/MS tandem mass spectrometry. Among the 228 peptide library, 19 peptides have neighboring dibasic amino acids, while one of these peptides has a neighboring tribasic sequence. The peptides containing KR, RK, RR, and KK sequences are shown in the Supporting Information, Table S1.
Prior to incubation with the peptide library, cathepsin V was activated by incubation in 5 mM DTT, 20 mM Na-acetate pH 5.5, 1 mM ethylenediaminetetraacetic acid (EDTA), and 100 mM NaCl at 37 °C for 30 min. Cathepsin L, PC1/3, and PC2 did not require preactivation. The concentrations of each protease used for MSP-MS were determined by the incubation of cathepsin L and cathepsin V with the Z-Phe-Arg-AMC fluorogenic substrate and the incubation of PC1/3 and PC2 with pGlu-Arg-Thr-Lys-Arg-AMC, in the absence and presence of the peptide library. Diminished fluorogenic proteolytic activity in the presence of the peptide library indicated protease concentrations for peptide library incubations in MSP-MS.
Cathepsin L (0.04 ng/μL) or cathepsin V (0.16 ng/μL) was each incubated with the peptide library (0.5 μM final concentration for each of the 228 peptides) in 50 mM citrate phosphate, pH 5.5, 1 mM EDTA, 100 mM NaCl, and 5 mM DTT in a total volume of 22 μL at 37 °C. After 30 and 60 min incubation, 10 μL of aliquots were removed and added to 60 μL of 8 M urea for quenching and stored at −70 °C.
PC1/3 (3.6 ng/μL) and PC2 (5.4 ng/μL) were each incubated with the peptide library (0.5 μM final concentration for each of the 228 peptides) in 50 mM Na-acetate, pH 5.5, 5 mM CaCl2, and 0.1% octyl-β-glucopyranoside (bOG); the incubation for PC2 also included 100 mM NaCl. After 30 and 60 min incubation at 37 °C, 10 μL of aliquots were removed and added to 60 μL of 8 M urea, with vortexing, for quenching; samples were stored at −70 °C.
The control “0” time condition was conducted by denaturation of each protease in 8 M urea prior to the addition of the inactive enzyme to the peptide library. Each protease MSP-MS assay condition was conducted in quadruplicate.
Samples were then prepared for LC–MS/MS by solid-phase extraction (SPE) as we have described previously.29,30 Briefly, the samples were acidified by the addition of 40 μL 1% TFA and desalted using C18 LTS tips with peptides eluted sequentially by 50 and 80% ACN in 0.1% TFA, followed by evaporation to dryness in a Speedvac and stored at −70 °C. Prior to LC–MS/MS, samples were resuspended in 40 μL 0.1% TFA.
LC–MS/MS was performed on a Dionex UltiMate 3000 nano LC and Orbitrap Q-Exactive mass spectrometer (Thermo Fisher). Rehydration of dried samples in 0.1% trifluoracetic acid resulted in a peptide concentration of 57 μM with respect to the 14-mer substrates of the library. For each LC–MS/MS analysis, 4 μL of the sample (equivalent to 228 pmol of starting 14-mer peptide substrates) was injected into a nano LC column (75 μm ID, 360 μm OD, 25 cm length) packed with BEH C18 (1.7 μm diameter) solid-phase material and heated to 65 °C for LC. LC was conducted with a flow rate of 0.3 μL/min using a 60 min linear gradient of 5–30% solvent B, and 15 min linear gradient of 30–85% B (solvent B = 100% ACN in 0.1% TFA) with solvent A (0.1% TFA in water). MS1 was acquired in profile mode with a 1e5 AGC target, 50 ms max injection time, 70 000 resolution (at m/z 200), and a 250–1500 m/z window. MS2 was acquired in centroid mode with a 1e5 AGC target, 50 ms max injection time, 3e2 minimum AGC target, 20 s/10 ppm dynamic exclusion, 17,500 resolution (at m/z 200), a first mass of m/z 150, and normalized HCD collision energy set to 30. Each sample was injected once.
Peptides were identified by PEAKS (v 8.5) bioinformatics software. MS/MS data files were searched against the peptide library database containing all 228 peptides. Label-free peptide quantification was performed by PEAKS; peptide identifications were assigned intensities based on the area of the precursor ion LC peak. Match-between-runs mass and retention time shift tolerances were set to 8.0 ppm and 3 min, respectively. To minimize low-quality features in the data set, data were filtered by the following criteria: (1) identified peptides with amino acid sequences that did not match to a peptide in the FDR-curated identification list were discarded and (2) PEAKS scores above 0.3 were utilized for high-quality MS/MS data. Further, to account for instrumental drift, the filtered feature list was normalized with the Loess-G algorithm by Normalyzer (http://normalyzer.immunoprot.lth.se/).
After LC–MS and bioinformatics analyses, cleavage sites were compiled for each enzyme condition and the intensities of cleaved peptides detected were compared for cathepsin L, cathepsin V, PC1/3, and PC2 proteases. Cleaved peptide products which displayed intensity scores at least 5-fold above the 0 time control, with p < 0.05, were utilized for analyses of protease cleavage site frequencies and preferences.
Heat Map and IceLogo Analyses of Protease Cleavage Sites
The preferences of each of the proteases for amino acid residues at P4–P4′ positions of the cleavage site P1–↓P1′ of the cleaved peptides were assessed as Z-scores to evaluate the frequencies of each amino acid at each position. Z-scores were calculated by the equation X – μ/σ, where X is the frequency of the amino acid in the experimental data set, μ is the frequency of a particular amino acid at a specific position in the reference set (control 0 time), and σ is the standard deviation. Heat maps were generated to illustrate the magnitude of the positive or negative Z-scores are depicted as color-coded gradients of blue (negative Z-score), white (zero Z-score), and red (positive Z-score).
IceLogo was used to illustrate the relative frequencies of amino acid residues at each of the P4–P4′ positions of cleaved peptides.62 IceLogo utilizes different heights of single-letter amino acids to represent “percent difference,” defined as the frequency for an amino acid appearing in the experimental data minus the frequency for an amino acid appearing in the reference peptide library MSP-MS data. Positive differences are shown above the midline, and negative differences are represented below the midline. Residues below the line shown in pink are those that never occurred at the indicated position.
Fluorogenic Proteolytic Assays with Variant Dibasic Residue-Containing Peptide-AMC Substrates
Cathepsin L, cathepsin V, PC1/3, and PC2 were assayed with fluorogenic dipeptide-AMC and tripeptide-AMC substrates designed to contain variant dibasic residue sequences consisting of Z-K-R-AMC, Z-R-K-AMC, Z-K-K-AMC, Z-R-R-AMC, Z-L-K-R-AMC, Z-W-K-R-AMC, Z-F-K-R-AMC, Z-Y-K-R-AMC, Z-V-K-R-AMC, Z-G-K-R-AMC, and Z-A-K-R-AMC. After incubation of each protease with the substrates, incubation continued without and with the addition of cathepsin H to monitor cleavages within dibasic residue sites compared to cleavages at the C-termini of dibasic residues of peptide-AMC substrates (illustrated in Supporting Information, Figure S1).
Cathepsin L (0.04 ng/μL) and cathepsin V (0.2 ng/μL) assays contained 5 mM citrate-phosphate buffer pH 5.5, 4 mM DTT, 1 mM EDTA, 50 mM NaCl, and 1% dimethyl sulfoxide (DMSO) with peptide-AMC substrates at 60 μM; additionally, cathepsin L assays included 0.001% bovine serum albumin (BSA). Assays were incubated at 37 °C for 60 min and AMC was monitored at excitation/emission of 360/460 nm. Cathepsin H (0.5 ng/μL, activated by the protocol provided by the manufacturer R&D Systems) was then added with 50 mM 2-(N-morpholino)ethanesulfonic acid (MES) buffer to adjust the pH to 6.5; incubation continued at 37 °C for 30 min, and AMC fluorescence was measured.
PC1/3 (0.9 ng/μL) and PC2 (4 ng/μL) assays contained 5 mM Na-acetate pH 5.5, 5 mM CaCl2, 50 mM NaCl, 0.5% Brij-35, and 1% DMSO. Assays were incubated at 37 °C for 120 min and AMC was monitored at excitation/emission of 360/460 nm. Cathepsin H (0.5 ng/μL, activated by the protocol provided by the manufacturer R&D Systems) was then added with 50 mm MES buffer to adjust the pH to 6.5; incubation continued at 37 °C for 30 min, and AMC fluorescence was measured.
The specific activities of cathepsin L and cathepsin V were determined with the substrate Z-F-R-AMC, which is routinely used in the field for assay of these proteases.35,36 For PC1/3 and PC2, their specific activities were assessed with the substrate pERTKR-AMC that is typically used to assay these proteases.37
Control assays included the incubation of substrates with cathepsin H alone which resulted in no fluorescence, indicating that cathepsin H does not remove the blocked N-terminal residues of Z-peptide-AMC substrates.
AMC standards were used to calculate enzyme-specific activities (pmol AMC/ng/min). All assays were conducted in triplicate. Mean and SD values were calculated with a comparison of conditions in t-tests with a significance of p < 0.05.
Acknowledgments
This research was supported by NIH grant R01NS094597 (awarded to V. Hook). M.Y. was supported by NIH grant T32 GM067550 (awarded to Dr. William Gerwick, Univ. of Calif., San Diego). Assistance for mass spectrometry by C. Lietz is appreciated.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acschemneuro.1c00653.
Coupled protease assay with cathepsin H, cathepsin L cleavage of proneuropeptides at dibasic residues, PC1/3 and PC2 cleavage of proinsulin and proenkephalin at dibasic residues, and hypothesis for differential dibasic cleavage specificities of cathepsin L and cathepsin V compared to PC1/3 and PC2 for neuropeptide biosynthesis (PDF)
Dibasic residue-containing peptides in the library used for MSP-MS, LCL-MS/MS report, and data workbook (XLSX)
Author Contributions
V.H. and A.J.O. conceived the project design. M.Y., J.A., and C.M. conducted the experiments. Z.J. and S.P. provided input on mass spectrometry and bioinformatics analyses of data. V.H., A.J.O., M.Y., and J.A. wrote the manuscript, with editing by V.H., A.J.O., M.Y., J.A., C.M., and S.P.
The authors declare no competing financial interest.
Notes
LC–MS/MS files can be accessed through www.massive.ucsd.edu under the data set identifier numbers MSV000088091 (Cathepsin L and Cathepsin V) and MSV000088092 (PC1 and PC2).
Supplementary Material
References
- Hook V.; Funkelstein L.; Lu D.; Bark S.; Wegrzyn J.; Hwang S. R. Proteases for processing proneuropeptides into peptide neurotransmitters and hormones. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 393–423. 10.1146/annurev.pharmtox.48.113006.094812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook V.; Lietz C. B.; Podvin S.; Cajka T.; Fiehn O. Diversity of neuropeptide cell-cell signaling molecules generated by proteolytic processing revealed by neuropeptidomics mass spectrometry. J. Am. Soc. Mass Spectrom. 2018, 29, 807–816. 10.1007/s13361-018-1914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah N. G.; Sadr M. S.; Chrétien M.; Mbikay M. The multifaceted proprotein convertases: their unique, redundant, complementary, and opposite functions. J. Biol. Chem. 2013, 288, 21473–21481. 10.1074/jbc.R113.481549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P.; Baxter J. D.; Frohman L. A.. Endocrinology and Metabolism, 3rd ed.; McGraw Hill, Inc.: New York, 1981. [Google Scholar]
- Inturrisi C. E. Clinical pharmacology of opioids for pain. Clin. J. Pain 2002, 18, S3–S13. 10.1097/00002508-200207001-00002. [DOI] [PubMed] [Google Scholar]
- Charbogne P.; Kieffer B. L.; Befort K. 15 years of genetic approaches in vivo for addiction research: Opioid receptor and peptide gene knockout in mouse models of drug abuse. Neuropharmacology 2014, 76, 204–217. 10.1016/j.neuropharm.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podvin S.; Yaksh T.; Hook V. The Emerging Role of Spinal Dynorphin in Chronic Pain: A Therapeutic Perspective. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 511–533. 10.1146/annurev-pharmtox-010715-103042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner R. A.; Hohmann J. G.; Holmes A.; Wrenn C. C.; Cadd G.; Juréus A.; Clifton D. K.; Luo M.; Gutshall M.; Ma S. Y.; Mufson E. J.; Crawley J. N. Galanin transgenic mice display cognitive and neurochemical deficits characteristic of Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 2001, 98, 4184–4189. 10.1073/pnas.061445598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts S. E.; Perez S. E.; Kahl U.; Bartfai T.; Bowser R. P.; Deecher D. C.; Mash D. C.; Crawley J. N.; Mufson E. J. Galanin: neurobiologic mechanisms and therapeutic potential for Alzheimer’s disease. CNS Drug Rev. 2001, 7, 445–370. 10.1111/j.1527-3458.2001.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedic N.; Chen A.; Deussing J. M. The CRF family of neuropeptides and their receptors—mediators of the central stress response. Curr. Mol. Pharmacol. 2018, 11, 4–31. 10.2174/1874467210666170302104053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale T. L.; Vale W. W. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 525–557. 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Holst J. J.; Holland W.; Gromada J.; Lee Y.; Unger R. H.; Yan H.; Sloop K. W.; Kieffer T. J.; Damond N.; Herrera P. L. Insulin and Glucagon: Partners for Life. Endocrinology 2017, 158, 696–701. 10.1210/en.2016-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiband H. K.; Schmidt S.; Ranjan A.; Holst J. J.; Madsbad S.; Nørgaard K. Dual-hormone treatment with insulin and glucagon in patients with type 1 diabetes. Diabetes/Metab. Res. Rev. 2015, 31, 672–679. 10.1002/dmrr.2632. [DOI] [PubMed] [Google Scholar]
- Krieger D. T.; Martin J.; Brownstein M. J.. Brain Peptides; John Wiley & Sons., Inc., 1983. [Google Scholar]
- Kim T.; Gondré-Lewis M. C.; Arnaoutova I.; Loh Y. P. Dense-core secretory granule biogenesis. Physiology 2006, 21, 124–133. 10.1152/physiol.00043.2005. [DOI] [PubMed] [Google Scholar]
- Adachi W.; Kawamoto S.; Ohno I.; Nishida K.; Kinoshita S.; Matsubara K.; Okubo K. Isolation and characterization of human cathepsin V: a major proteinase in corneal epithelium. Invest. Ophthalmol. Visual Sci. 1998, 39, 1789–1796. [PubMed] [Google Scholar]
- Brömme D.; Li Z.; Barnes M.; Mehler E. Human cathepsin V functional expression, tissue distribution, electrostatic surface potential, enzymatic characterization, and chromosomal localization. Biochemistry 1999, 38, 2377–2385. 10.1021/bi982175f. [DOI] [PubMed] [Google Scholar]
- Reiser J.; Adair B.; Reinheckel T. Specialized roles for cysteine cathepsins in health and disease. J. Clin. Invest. 2010, 120, 3421–3431. 10.1172/JCI42918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funkelstein L.; Lu W. D.; Koch B.; Mosier C.; Toneff T.; Taupenot L.; O’Connor D. T.; Reinheckel T.; Peters C.; Hook V. Human cathepsin V protease participates in production of enkephalin and NPY neuropeptide neurotransmitters. J. Biol. Chem. 2012, 287, 15232–15241. 10.1074/jbc.M111.310607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasothornsrikul S.; Greenbaum D.; Medzihradszky K. F.; Toneff T.; Bundey R.; Miller R.; Schilling B.; Petermann I.; Dehnert J.; Logvinova A.; Goldsmith P.; Neveu J. M.; Lane W. S.; Gibson B.; Reinheckel T.; Peters C.; Bogyo M.; Hook V. Cathepsin L in secretory vesicles functions as a prohormone-processing enzyme for production of the enkephalin peptide neurotransmitter. Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 9590–9595. 10.1073/pnas.1531542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funkelstein L.; Toneff T.; Hwang S. R.; Reinheckel T.; Peters C.; Hook V. Cathepsin L participates in the production of neuropeptide Y in secretory vesicles, demonstrated by protease gene knockout and expression. J. Neurochem. 2008, 106, 384–391. 10.1111/j.1471-4159.2008.05408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson H. W.; Rhodes C. J.; Hutton J. C. Intraorganellar calcium and pH control proinsulin cleavage in the pancreatic beta cell via two distinct site-specific endopeptidases. Nature 1988, 333, 93–96. 10.1038/333093a0. [DOI] [PubMed] [Google Scholar]
- Bailyes E. M.; Shennan K. I.; Seal A. J.; Smeekens S. P.; Steiner D. F.; Hutton J. C.; Docherty K. A member of the eukaryotic subtilisin family (PC3) has the enzymic properties of the type 1 proinsulin-converting endopeptidase. Biochem. J. 1992, 285, 391–394. 10.1042/bj2850391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta M.; Carroll R.; Martin S.; Swift H. H.; Ravazzola M.; Orci L.; Steiner D. F. Incomplete processing of proinsulin to insulin accompanied by elevation of Des-31,32 proinsulin intermediates in islets of mice lacking active PC2. J. Biol. Chem. 1998, 273, 3431–3437. 10.1074/jbc.273.6.3431. [DOI] [PubMed] [Google Scholar]
- Johanning K.; Juliano M. A.; Juliano L.; Lazure C.; Lamango N. S.; Steiner D. F.; Lindberg I. Specificity of prohormone convertase 2 on proenkephalin and proenkephalin-related substrates. J. Biol. Chem. 1998, 273, 22672–22680. 10.1074/jbc.273.35.22672. [DOI] [PubMed] [Google Scholar]
- Peinado J. R.; Li H.; Johanning K.; Lindberg I. Cleavage of recombinant proenkephalin and blockade mutants by prohormone convertases 1 and 2: an in vitro specificity study. J. Neurochem. 2003, 87, 868–878. 10.1046/j.1471-4159.2003.02043.x. [DOI] [PubMed] [Google Scholar]
- Benjannet S.; Rondeau N.; Day R.; Chrétien M.; Seidah N. G. PC1 and PC2 are proprotein convertases capable of cleaving proopiomelanocortin at distinct pairs of basic residues. Proc Natl Acad Sci U.S.A. 1991, 88, 3564–3568. 10.1073/pnas.88.9.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanvantari S.; Seidah N. G.; Brubaker P. L. Role of prohormone convertases in the tissue-specific processing of proglucagon. Mol. Endocrinol. 1996, 10, 342–355. 10.1210/mend.10.4.8721980. [DOI] [PubMed] [Google Scholar]
- O’Donoghue A. J.; Eroy-Reveles A. A.; Knudsen G. M.; Ingram J.; Zhou M.; Statnekov J. B.; Greninger A. L.; Hostetter D. R.; Qu G.; Maltby D. A.; Anderson M. O.; Derisi J. L.; McKerrow J. H.; Burlingame A. L.; Craik C. S. Global identification of peptidase specificity by multiplex substrate profiling. Nat. Methods 2012, 9, 1095–1100. 10.1038/nmeth.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter M. B.; La Greca F.; Arastu-Kapur S.; Caiazza F.; Cimermancic P.; Buchholz T. J.; Anderl J. L.; Ravalin M.; Bohn M. F.; Sali A.; O’Donoghue A. J.; Craik C. S. Immunoproteasome functions explained by divergence in cleavage specificity and regulation. eLife 2017, 18, 968–981. 10.7554/eLife.27364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore C. G.; Varro A.; Dimaline R.; Bishop L.; Gallacher D. V.; Dockray G. J. Measurement of secretory vesicle pH reveals intravesicular alkalinization by vesicular monoamine transporter type 2 resulting in inhibition of prohormone cleavage. J. Physiol. 2001, 531, 605–617. 10.1111/j.1469-7793.2001.0605h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paroutis P.; Touret N.; Grinstein S. The pH of the secretory pathway: measurement, determinants, and regulation. Physiology 2004, 19, 207–215. 10.1152/physiol.00005.2004. [DOI] [PubMed] [Google Scholar]
- Estévez-Herrera J.; Domínguez N.; Pardo M. R.; González-Santana A.; Westhead E. W.; Borges R.; Machado J. D. ATP: The crucial component of secretory vesicles. Proc. Natl. Acad. Sci. U.S.A. 2016, 113, E4098–E4106. 10.1073/pnas.1600690113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper B. A.; Stoffers D. A.; Mains R. E. The biosynthesis of neuropeptides: peptide alpha amidation. Annu. Rev. Neurosci. 1992, 15, 57–85. 10.1146/annurev.ne.15.030192.000421. [DOI] [PubMed] [Google Scholar]
- Boudreau P. D.; Miller B. W.; McCall L. I.; Almaliti J.; Reher R.; Hirata K.; Le T.; Siqueira-Neto J. L.; Hook V.; Gerwick W. H. Design of Gallinamide A Analogs as Potent Inhibitors of the Cysteine Proteases Human Cathepsin L and Trypanosoma cruzi Cruzain. J. Med. Chem. 2019, 62, 9026–9044. 10.1021/acs.jmedchem.9b00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P. P.; Myers M. C.; Beavers M. P.; Purvis J. E.; Jing H.; Grieser H. J.; Sharlow E. R.; Napper A. D.; Huryn D. M.; Cooperman B. S.; Smith A. B. 3rd; Diamond S. L. Kinetic characterization and molecular docking of a novel, potent, and selective slow-binding inhibitor of human cathepsin L. Mol. Pharmacol. 2008, 74, 34–41. 10.1124/mol.108.046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azaryan A. V.; Krieger T. J.; Hook V. Y. Purification and characteristics of the candidate prohormone processing proteases PC2 and PC1/3 from bovine adrenal medulla chromaffin granules. J. Biol. Chem. 1995, 270, 8201–8208. 10.1074/jbc.270.14.8201. [DOI] [PubMed] [Google Scholar]
- Horikawa S.; Takai T.; Toyosato M.; Takahashi H.; Noda M.; Kakidani H.; Kubo T.; Hirose T.; Inayama S.; Hayashida H.; et al. Isolation and structural organization of the human preproenkephalin B gene. Nature 1983, 306, 611–614. 10.1038/306611a0. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K.; Williams C.; Sabol S. L. Rat brain preproenkephalin mRNA. cDNA cloning, primary structure, and distribution in the central nervous system. J. Biol. Chem. 1984, 259, 14301–14308. 10.1016/S0021-9258(18)89893-3. [DOI] [PubMed] [Google Scholar]
- Eberlein G. A.; Eysselein V. E.; Davis M. T.; Lee T. D.; Shively J. E.; Grandt D.; Niebel W.; Williams R.; Moessner J.; Zeeh J.; et al. Patterns of prohormone processing. Order revealed by a new procholecystokinin-derived peptide. J. Biol. Chem. 1992, 267, 1517–1521. 10.1016/S0021-9258(18)45976-5. [DOI] [PubMed] [Google Scholar]
- Goetze J. P.; Hunter I.; Zois N. E.; Terzic D.; Valeur N.; Olsen L. H.; Smith J.; Plomgaard P.; Hansen L. H.; Rehfeld J. F.; Balling L.; Gustafsson F. Cardiac procholecystokinin expression during haemodynamic changes in the mammalian heart. Peptides 2018, 108, 7–13. 10.1016/j.peptides.2018.08.004. [DOI] [PubMed] [Google Scholar]
- Geijer T.; Telkov M.; Terenius L. Characterization of human prodynorphin gene transcripts. Biochem. Biophys. Res. Commun. 1995, 215, 881–888. 10.1006/bbrc.1995.2546. [DOI] [PubMed] [Google Scholar]
- Hwang S. R.; O’Neill A.; Bark S.; Foulon T.; Hook V. Secretory vesicle aminopeptidase B related to neuropeptide processing: molecular identification and subcellular localization to enkephalin- and NPY-containing chromaffin granules. J. Neurochem. 2007, 100, 1340–1350. 10.1111/j.1471-4159.2006.04325.x. [DOI] [PubMed] [Google Scholar]
- Yasothornsrikul S.; Toneff T.; Hwang S. R.; Hook V. Y. Arginine and lysine aminopeptidase activities in chromaffin granules of bovine adrenal medulla: relevance to prohormone processing. J. Neurochem. 1998, 70, 153–163. 10.1046/j.1471-4159.1998.70010153.x. [DOI] [PubMed] [Google Scholar]
- Douglas Lu W.; Funkelstein L.; Toneff T.; Reinheckel T.; Peters C.; Hook V. Cathepsin H functions as an aminopeptidase in secretory vesicles for production of enkephalin and galanin peptide neurotransmitters. J. Neurochem. 2012, 122, 512–522. 10.1111/j.1471-4159.2012.07788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A.; Bloomquist B. T.; Mains R. E. The prohormone convertases PC1 and PC2 mediate distinct endoproteolytic cleavages in a strict temporal order during proopiomelanocortin biosynthetic processing. J. Biol. Chem. 1993, 268, 1763–1769. 10.1016/S0021-9258(18)53918-1. [DOI] [PubMed] [Google Scholar]
- Friedman T. C.; Loh Y. P.; Birch N. P. In vitro processing of proopiomelanocortin by recombinant PC1 (SPC3). Endocrinology 1994, 135, 854–862. 10.1210/endo.135.3.8070378. [DOI] [PubMed] [Google Scholar]
- Funkelstein L.; Toneff T.; Mosier C.; Hwang S. R.; Beuschlein F.; Lichtenauer U. D.; Reinheckel T.; Peters C.; Hook V. Major role of cathepsin L for producing the peptide hormones ACTH, beta-endorphin, and alpha-MSH, illustrated by protease gene knockout and expression. J. Biol. Chem. 2008, 283, 35652–35659. 10.1074/jbc.M709010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R.; Aaron W.; Toneff T.; Vishnuvardhan D.; Beinfeld M. C.; Hook V. Y. Obliteration of alpha-melanocyte-stimulating hormone derived from POMC in pituitary and brains of PC2-deficient mice. J. Neurochem. 2003, 86, 556–563. 10.1046/j.1471-4159.2003.01856.x. [DOI] [PubMed] [Google Scholar]
- Wang L.; Sui L.; Panigrahi S. K.; Meece K.; Xin Y.; Kim J.; Gromada J.; Doege C. A.; Wardlaw S. L.; Egli D.; Leibel R. L. PC1/3 Deficiency Impacts Pro-opiomelanocortin Processing in Human Embryonic Stem Cell-Derived Hypothalamic Neurons. Stem Cell Rep. 2017, 8, 264–277. 10.1016/j.stemcr.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinfeld M. C.; Funkelstein L.; Foulon T.; Cadel S.; Kitagawa K.; Toneff T.; Reinheckel T.; Peters C.; Hook V. Cathepsin L plays a major role in cholecystokinin production in mouse brain cortex and in pituitary AtT-20 cells: protease gene knockout and inhibitor studies. Peptides 2009, 30, 1882–1891. 10.1016/j.peptides.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokadeh A.; Funkelstein L.; Toneff T.; Hwang S. R.; Beinfeld M.; Reinheckel T.; Peters C.; Zadina J.; Hook V. Cathepsin L participates in dynorphin production in brain cortex, illustrated by protease gene knockout and expression. Mol. Cell. Neurosci. 2010, 43, 98–107. 10.1016/j.mcn.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Miller R.; Toneff T.; Vishnuvardhan D.; Beinfeld M.; Hook V. Y. Selective roles for the PC2 processing enzyme in the regulation of peptide neurotransmitter levels in brain and peripheral neuroendocrine tissues of PC2 deficient mice. Neuropeptides 2003, 37, 140–148. 10.1016/S0143-4179(03)00027-1. [DOI] [PubMed] [Google Scholar]
- Berman Y.; Mzhavia N.; Polonskaia A.; Furuta M.; Steiner D. F.; Pintar J. E.; Devi L. A. Defective prodynorphin processing in mice lacking prohormone convertase PC2. J. Neurochem. 2000, 75, 1763–1770. 10.1046/j.1471-4159.2000.0751763.x. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Pan H.; Peng B.; Steiner D. F.; Pintar J. E.; Fricker L. D. Neuropeptidomic analysis establishes a major role for prohormone convertase-2 in neuropeptide biosynthesis. J. Neurochem. 2010, 112, 1168–1179. 10.1111/j.1471-4159.2009.06530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett L. C.; LeDuc C. A.; Sulsona C. R.; Paull D.; Rausch R.; Eddiry S.; Carli J. F.; Morabito M. V.; Skowronski A. A.; Hubner G.; Zimmer M.; Wang L.; Day R.; Levy B.; Fennoy I.; Dubern B.; Poitou C.; Clement K.; Butler M. G.; Rosenbaum M.; Salles J. P.; Tauber M.; Driscoll D. J.; Egli D.; Leibel R. L. Deficiency in prohormone convertase PC1 impairs prohormone processing in Prader-Willi syndrome. J. Clin. Invest. 2017, 127, 293–305. 10.1172/JCI88648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z.; Lietz C. B.; Podvin S.; Yoon M. C.; Toneff T.; Hook V.; O’Donoghue A. J. Differential Neuropeptidomes of Dense Core Secretory Vesicles (DCSV) Produced at Intravesicular and Extracellular pH Conditions by Proteolytic Processing. ACS Chem. Neurosci. 2021, 12, 2385–2398. 10.1021/acschemneuro.1c00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nycander M.; Estrada S.; Mort J. S.; Abrahamson M.; Björk I. Two-step mechanism of inhibition of cathepsin B by cystatin C due to displacement of the proteinase occluding loop. FEBS Lett. 1998, 422, 61–64. 10.1016/S0014-5793(97)01604-9. [DOI] [PubMed] [Google Scholar]
- Hwang S. R.; Steineckert B.; Yasothornsrikul S.; Sei C. A.; Toneff T.; Rattan J.; Hook V. Y. Molecular cloning of endopin 1, a novel serpin localized to neurosecretory vesicles of chromaffin cells. Inhibition of basic residue-cleaving proteases by endopin 1. J. Biol. Chem. 1999, 274, 34164–34173. 10.1074/jbc.274.48.34164. [DOI] [PubMed] [Google Scholar]
- Hwang S. R.; Steineckert B.; Toneff T.; Bundey R.; Logvinova A. V.; Goldsmith P.; Hook V. Y. The novel serpin endopin 2 demonstrates cross-class inhibition of papain and elastase: localization of endopin 2 to regulated secretory vesicles of neuroendocrine chromaffin cells. Biochemistry 2002, 41, 10397–10405. 10.1021/bi020088o. [DOI] [PubMed] [Google Scholar]
- Hwang S. R.; Bundey R.; Toneff T.; Hook V. Endopin serpin protease inhibitors localize with neuropeptides in secretory vesicles and neuroendocrine tissues. Neuroendocrinology 2009, 89, 210–216. 10.1159/000162916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaert N.; Helsens K.; Martens L.; Vandekerckhove J.; Gevaert K. Improved visualization of protein consensus sequences by iceLogo. Nat. Methods 2009, 6, 786–787. 10.1038/nmeth1109-786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.