Abstract
STUDY QUESTION
Does hysterosalpingo-foam sonography (HyFoSy) lead to similar pregnancy outcomes, compared with hysterosalpingography (HSG), as first-choice tubal patency test in infertile couples?
SUMMARY ANSWER
HyFoSy and HSG produce similar findings in a majority of patients and clinical management based on the results of either HyFoSy or HSG, leads to comparable pregnancy outcomes. HyFoSy is experienced as significantly less painful.
WHAT IS KNOWN ALREADY
Traditionally, tubal patency testing during fertility work-up is performed by HSG. HyFoSy is an alternative imaging technique lacking ionizing radiation and iodinated contrast medium exposure which is less expensive than HSG. Globally, there is a shift towards the use of office-based diagnostic methods, such as HyFoSy.
STUDY DESIGN, SIZE, DURATION
This multicentre, prospective, comparative study with a randomized design was conducted in 26 hospitals in The Netherlands. Participating women underwent both HyFoSy and HSG in randomized order. In case of discordant results, women were randomly allocated to either a management strategy based on HyFoSy or one based on HSG.
PARTICIPANTS/MATERIALS, SETTING, METHODS
We included infertile women between 18 and 41 years old who were scheduled for tubal patency testing during their fertility work-up. Women with anovulatory cycles not responding to ovulation induction, endometriosis, severe male infertility or a known iodine contrast allergy were excluded. The primary outcome for the comparison of the HyFoSy- and HSG-based strategies was ongoing pregnancy leading to live birth within 12 months after inclusion in an intention-to-treat analysis.
MAIN RESULTS AND THE ROLE OF CHANCE
Between May 2015 and January 2019, 1026 women underwent HyFoSy and HSG. HyFoSy was inconclusive in 97 of them (9.5%), HSG was inconclusive in 30 (2.9%) and both were inconclusive in 9 (0.9%). In 747 women (73%) conclusive tests results were concordant. Of the 143/1026 (14%) with discordant results, 105 were randomized to clinical management based on the results of either HyFoSy or HSG. In this group, 22 of the 54 women (41%) allocated to management based on HyFoSy and 25 of 51 women (49%) allocated to management based on HSG had an ongoing pregnancy leading to live birth (Difference −8%; 95% CI: −27% to 10%). In total, clinical management based on the results of HyFoSy was estimated to lead to a live birth in 474 of 1026 women (46%) versus 486 of 1026 (47%) for management based on HSG (Difference −1.2%; 95% CI: −3.4% to 1.5%). Given the pre-defined margin of −2%, statistically significant non-inferiority of HyFoSy relative to HSG could not be demonstrated (P = 0.27). The mean pain score for HyFoSy on the 1–10 Visual Analogue Scale (VAS) was 3.1 (SD 2.2) and the mean VAS pain score for HSG was 5.4 (SD 2.5; P for difference < 0.001).
LIMITATIONS, REASONS FOR CAUTION
Since all women underwent both tubal patency tests, no conclusions on a direct therapeutic effect of tubal flushing could be drawn.
WIDER IMPLICATIONS OF THE FINDINGS
HyFoSy or HSG produce similar tubal pathology findings in a majority of infertile couples and, where they differ, a difference in findings does not lead to substantial difference in pregnancy outcome, while HyFoSy is associated with significantly less pain.
STUDY FUNDING/COMPETING INTEREST(S)
The FOAM study was an investigator-initiated study funded by ZonMw, The Netherlands organization for Health Research and Development (project number 837001504). ZonMw funded the whole project. IQ Medical Ventures provided the ExEm-foam® kits free of charge. The funders had no role in study design, collection, analysis and interpretation of the data. K.D. reports travel and speaker fees from Guerbet. F.J.M.B. reports personal fees as a member of the external advisory board for Merck Serono, The Netherlands, and a research support grant from Merck Serono, outside the submitted work. C.B.L. reports speakers’ fee from Ferring in the past, and his department receives research grants from Ferring, Merck and Guerbet. J.S. reports a research agreement with Takeda on MR of motility outside the submitted work. M.V.W. reports leading The Netherlands Satellite of the Cochrane Gynaecology and Fertility Group. B.W.J.M. is supported by an NHMRC Investigator grant (GNT1176437). B.W.J.M. reports consultancy for Guerbet and research funding from Merck and Guerbet. V.M. reports non-financial support from IQ medicals ventures, during the conduct of the study; grants and personal fees from Guerbet, outside the submitted work. The other authors do not report conflicts of interest.
TRIAL REGISTRATION NUMBER
NTR4746/NL4587 (https://www.trialregister.nl)
TRIAL REGISTRATION DATE
19 August 2014
DATE OF FIRST PATIENT’S ENROLMENT
7 May 2015
Keywords: hysterosalpingo-foam sonography, hysterosalpingography, tubal patency test, fertility work-up, tubal pathology, effectiveness, ongoing pregnancy, live birth
Introduction
Tubal pathology is one of the main causes of female infertility with a prevalence between 11% and 30%, resulting from infections (often transmitted sexually, such as chlamydia and gonorrhoea), previous surgery or endometriosis (Hull et al., 1985; Collins et al., 1995; Snick et al., 1997; Farquhar et al., 2019). Considering that unilateral tubal pathology does not necessarily reduce pregnancy chances in comparison to no tubal pathology, the aim is to detect bilateral tubal pathology (Verhoeve et al., 2011; Tan et al., 2019). Therefore, evaluation of the fallopian tubes is a standard part of the fertility work-up.
Diagnostic laparoscopy with chromopertubation is considered the reference standard to assess tubal patency with direct visualization of the fallopian tubes and their surrounding pelvic structures (Mol et al., 1999; Saunders et al., 2011; NICE, 2013). As laparoscopy is invasive and expensive, it is deemed inappropriate for screening purposes in unselected infertile women (Jansen et al., 1997; NICE, 2013; ACOG, 2019).
Hysterosalpingography (HSG) is currently still considered as the first-choice tubal patency test during fertility work-up (NICE, 2013; ACOG, 2019). During HSG an iodinated contrast medium is flushed through the uterus and fallopian tubes, while radiographs are performed. Although HSG is less invasive than laparoscopy, it is often experienced as painful and it results in exposure to ionising radiation and iodinated contrast medium (Saunders et al., 2011; Chauhan et al., 2013).
Hysterosalpingo-contrast sonography (HyCoSy) has been introduced as a more patient-friendly alternative. It relies on transvaginal ultrasound while flushing the uterus and fallopian tubes with echogenic contrast medium, during which the ovaries can be visualized as well. Its accuracy was shown to be comparable to that of HSG in predicting tubal patency (Randolph et al., 1986; Reis et al., 1998; Dijkman et al., 2000; Saunders et al., 2011; Lim et al., 2015). However, the commonly used echogenic contrast medium for HyCoSy, Echovist® (Bayer Schering Pharma AG, Berlin, Germany), was found to potentially cause allergic reactions and is no longer licensed for gynaecological use (Luciano et al., 2014). An alternative medium is a combination of air and saline, which requires a very quick evaluation of the fallopian tubes, as the air bubbles rapidly disappear from the saline (Heikkinen et al., 1995). SonoVue® (sulphur hexafluoride; Bracco International BV, Amsterdam, The Netherlands) is another contrast medium for sonographic tubal patency testing. This second-generation microbubble contrast agent with a gas core generates useful patterns in 2- and 3-dimensional ultrasound, and it is a safe agent regarding consequences of its intravasation (Lanzani et al., 2009; Zhou et al., 2012; Exacoustos et al., 2013; He et al., 2013). Although SonoVue® is not registered for tubal patency testing, it is still used in studies and clinical practice (Wang and Qian, 2016).
Given the instable patterns of air and saline, a more stable echogenic medium was introduced in 2011: ExEm-foam® (IQ Medical Ventures BV, Rotterdam, The Netherlands) which is currently the only registered commercial contrast for tubal patency testing in ultrasound. Like HyCoSy, hysterosalpingo-foam sonography (HyFoSy) appears to be as accurate in diagnosing tubal patency as HSG (Maheux-Lacroix et al., 2014), although recent studies found HyFoSy to have a higher diagnostic accuracy than HyCoSy (Lim et al., 2015; Ludwin et al., 2017; Piccioni et al., 2017). HyFoSy is also considered to be less painful and less time-consuming than HSG (Dreyer et al., 2014; Van Schoubroeck et al., 2015a; Tanaka et al., 2018).
The effectiveness of any medical test should ultimately be judged by its ability to affect patient-important outcomes (AHRQ, 2008; Guyatt et al., 2008; Schünemann et al., 2016). In couples suffering from infertility, pregnancy outcomes are the most important goal and should guide the choice of tests, while treatment burden and inconvenience can be a second criterion.
So far, the effects of HyFoSy on pregnancy outcomes have only been studied in relatively small or observational studies (Emanuel et al., 2012; Exacoustos et al., 2015; Van Schoubroeck et al., 2015b; Tanaka et al., 2018). We hypothesized that HyFoSy and HSG have similar effectiveness in terms of pregnancy outcomes. Randomized trials are well-suited to evaluate the comparative effectiveness of medical tests and testing strategies, even though straightforward allocation to two testing strategies is not always the most efficient design (Bossuyt et al., 2000; Gazelle et al., 2011). We conducted a multicentre randomized trial with a discordancy design to compare HyFoSy to HSG in guiding clinical management in infertile couples, with ongoing pregnancy leading to live birth as the primary outcome.
Materials and methods
Study design and participants
The FOAM study was a multicentre prospective comparative non-inferiority trial of HyFoSy versus HSG, with randomization of couples with discordant test results. The study was performed in 26 hospitals in The Netherlands (4 academic hospitals, 15 teaching and 7 nonteaching hospitals) within the infrastructure of the Dutch Consortium for Healthcare Evaluation and Research in Obstetrics and Gynaecology (NVOG Consortium; https://www.zorgevaluatienederland.nl).
Infertile women between 18 and 41 years of age who were scheduled for tubal patency testing as part of the fertility work-up were eligible to participate. Women with anovulatory cycles not responding to ovulation induction, endometriosis, severe male factor (total motile sperm count <1 × 106/ml) or a known iodine contrast allergy could not participate.
The study was approved by the National Central Committee on Research involving Human Subjects (CCMO, The Netherlands; ref. no. NL50484.029.14) and by the ethics committee and institutional review board of the Amsterdam UMC, location VU University Medical centre (ref. no. 2014.454). The board of directors of all participating hospitals approved local execution of the study. The study was registered prospectively (original no. NTR4746; new no. NL4587; https://www.trialregister.nl), and the study protocol has been published previously (van Rijswijk et al., 2018). Trial oversight was provided by the ethics committee of the Amsterdam UMC, location VU University Medical centre. All participants provided written informed consent. Data monitoring was performed in accordance with the Good Clinical Practice guidelines by dedicated research nurses of the Dutch consortium for Healthcare Evaluation and Research in Obstetrics and Gynaecology (NVOG Consortium; https://www.zorgevaluatienederland.nl) in each of the participating centres. All gynaecologists, fertility doctors or ultrasound technicians were trained in their centre in the performance of HyFoSy by V.M., K.D., J.R. or N.W. The first, second, third and last authors vouch for accuracy and completeness of the data and analyses and for the fidelity of the trial to the protocol.
Randomization and blinding
Eligible women were informed about the study during a regular outpatient visit by their gynaecologist or fertility doctor. After providing written informed consent, participating women underwent both HyFoSy and HSG, in a randomly assigned order. This randomization was performed using ALEA 2.2, a web-based interface displaying the allocated order from a computer-generated randomization sequence (FormsVision BV, Abcoude, The Netherlands), stratified for centre with randomly permuted blocks, with block size varying between two and four. The physician who performed the second assigned test was blinded from the results of the first performed test. Women in whom the results of HyFoSy and HSG were discordant were randomized in a 1:1 ratio to either a clinical management strategy guided by HyFoSy or one guided by HSG using ALEA 2.2, non-centre-stratified with randomly permuted blocks, with block size varying between two and four.
Procedures
Both HyFoSy and HSG were performed within the two weeks of the follicular phase of the cycle after complete cessation of menstrual bleeding. Women were allowed to take pain medication (e.g. paracetamol or naproxen) before both tubal patency tests.
During HyFoSy 5–10 cc of echogenic foam was infused in the uterine cavity through a small cervical balloon-less GIS® catheter (IQ Medical Ventures BV, Rotterdam, The Netherlands). The foam was created by rigorously mixing 5 cc ExEm-gel® (IQ Medical Ventures BV, Rotterdam, The Netherlands) with 5 cc sterile purified water (IQ Medical Ventures BV, Rotterdam, The Netherlands). This foam is stable to show echogenicity for at least 5 min (Emanuel et al., 2012). The created foam was slowly infused into the uterine cavity during 2-dimensional transvaginal sonography, and subsequent into the fallopian tubes to assess patency (Fig. 1). Type of ultrasound system and machine settings depended on the local situation. Training before performing HyFoSy was mandatory.
Figure 1.
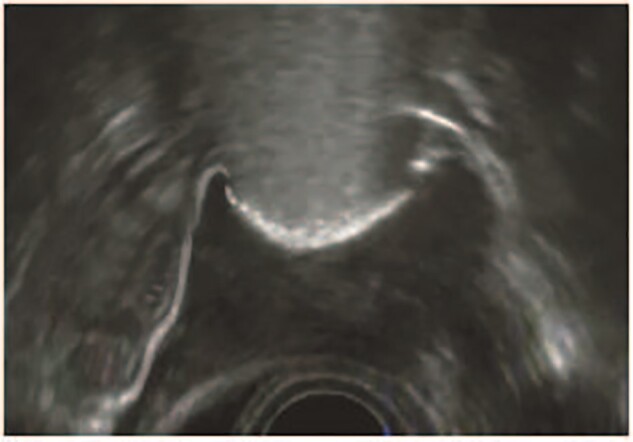
A typical 2D-hysterosalpingo-foam sonography image. The uterus is seen in transversal dimension with two patent fallopian tubes. Source: IQ Medical Ventures BV, Delft, the Netherlands.
HSG was performed according to the local protocol. A vacuum cervical cup, metal cannula (hysterophore) or a balloon catheter was used to infuse 5–10 cc contrast medium into the uterine cavity and fallopian tubes. The type of contrast medium, oil- or water-based, depended on local protocols. During instillation of the contrast medium, six to eight radiographs were made to assess the uterine cavity and patency of the fallopian tubes.
Test results of HyFoSy and HSG were categorized as: normal, one- or double-sided tubal pathology. The results of the two tests were then compared with decide whether they were concordant or discordant. Concordant test results were those leading to the same classification: normal/normal, one-sided tubal pathology/one-sided tubal pathology or double-sided tubal pathology/double-sided tubal pathology. No distinction was made between the side of the one-sided tubal pathology, as this has no consequences for the subsequent fertility management. Discordant was defined as conflicting test results. Test results were defined as inconclusive test results when the procedure was not completed successfully or was interrupted by technical or medical complications.
Subsequent fertility management was either based on the results of both concordant tests or, in case of discordant results, based on the results of the randomly assigned test. In case of bilateral or unilateral tubal patency, planned fertility treatment was initiated according to the prognosis for natural conception (Hunault et al., 2005) and the Dutch guideline (NVOG, 2015). If the chances of natural conception within 12 months exceeded 30%, expectant management for at least 6 months was advised before starting intrauterine insemination (IUI). In case the chances of natural conception were < 30%, women were advised to start IUI eventually followed by IVF. In case of bilateral occlusion, diagnostic laparoscopy with chromopertubation was performed to evaluate tubal pathology. If bilateral occlusion was confirmed, IVF was initiated. When at least one tube was patent during laparoscopy fertility treatment was based on the Hunault prognosis for natural conception (Hunault et al., 2005). Women with polycystic ovary syndrome continued with ovulation induction, once bilateral or unilateral tubal patency was confirmed.
Data were collected until 12 months after randomization in the study in a structured electronic case report form. If a pregnancy had occurred within 12 months, the outcome of that pregnancy was followed even if it exceeded 12 months. If the necessary information could not be extracted from the medical record, women received a questionnaire about pregnancy outcomes.
Outcomes
The primary outcome for the comparison of the two strategies was ongoing pregnancy leading to live birth within 12 months after inclusion. Ongoing pregnancy was defined as an intrauterine pregnancy with a heartbeat during ultrasound examination between 10 and 12 weeks of pregnancy. Live birth was defined as a live birth after 24 weeks of gestation.
Secondary outcomes reported here were: concordance between HyFoSy and HSG, pain score (measured by Visual Analogue Scale (VAS); ranging from 0.0 to 10.0 cm), time to ongoing pregnancy leading to live birth, biochemical pregnancy (defined as a positive pregnancy test or an increase in human chorionic gonadotropin combined with menstrual bleeding and absence of ultrasound visible pregnancy), miscarriage (defined as the presence of non-vitality on ultrasound or spontaneous loss of pregnancy), ectopic pregnancy (defined as an embryo implanted outside the uterine cavity), multiple pregnancy (defined as a pregnancy of two or more foetuses) and preterm birth rate (defined as a delivery before 37 weeks of pregnancy). Costs and cost-effectiveness were a prespecified secondary outcome and will be reported elsewhere. CORE outcomes (Duffy et al., 2020) are presented in Supplementary Table SI.
Statistical analysis
The effect on pregnancy outcomes of clinical management based on HyFoSy versus clinical management based on HSG was expressed as a difference, with 95% confidence interval, in the proportion of live births within 12 months in an intention-to-treat analysis.
To evaluate the pregnancy outcomes in a strategy in which management would be guided by HyFoSy, we studied the live birth rates in the subgroup with inconclusive results, in the subgroup with concordant results, and—for the subgroup with discordant test results—in the women randomly allocated to management guided by HyFoSy. To estimate the total number of live births, we added the results in each of the three subgroups, weighted by the corresponding fraction of the total study group.
We similarly estimated the pregnancy outcomes for a strategy in which management would be guided by HSG, by studying the live birth rates in the inconclusive group, the concordant group, and in the subgroup with discordant test results randomly allocated to management guided by HSG. Here also, weighted each of these three subgroups by the corresponding fractions in the total study group. The subgroup with inconclusive results was included in both strategies, since we reasoned that the alternative procedure would be invoked in case one of the procedures was inconclusive, and, optionally, a diagnostic laparoscopy to guide clinical management. In these cases, a choice for the initial test, HSG or HyFoSy, would not lead to a difference in clinical management and, hence, not in outcomes either.
Since the difference in live births between the two strategies is driven by the difference in live birth rates in the discordant subgroup, randomly allocated to either HyFoSy or HSG, we could estimate this difference as the difference between the randomized groups, multiplied by the fraction of discordant test results in the total study group. Similarly, the 95% CI in the randomized subgroup was multiplied by the same fraction of women with discordant test results (Lu and Gatsonis, 2013).
The sample size calculation was guided by a non-inferiority hypothesis, in which we wanted to exclude a decrease of 2% in ongoing pregnancy leading to live birth among women with discordant results with clinical management relying on the HyFoSy results instead of on the HSG results. A narrow non-inferiority margin was chosen, as we anticipated that a difference of more than 2% would be clinically relevant to infertile couples. We assumed a 50% ongoing pregnancy rate within 12 months after tubal testing, with no difference between management guided by either HyFoSy or HSG. The total sample size was guided by the anticipated fraction ƒ of women with discordant results (Lu and Gatsonis, 2013). Assuming that this fraction with discordant results was 7% (Emanuel et al., 2012), the non-inferiority margin in the discordant results would be 29% (2% divided by 7%). To achieve at least 80% power to reject inferiority at a 5% significance level, we needed to randomize 74 women with discordant results; the total number of included women would then have to be 1057 (74 divided by 7%). To account for 10% lost to follow-up, our goal was to include 1163 women, resulting in 82 women with discordant results.
Additional sensitivity analyses were performed excluding women who were not eligible after reassessment, who did not receive assigned tests, or had a different order of tests than allocated. No interim analyses were performed. Missing data were assumed to be missing at random.
Mean pain scores for HyFoSy and HSG were compared using a paired samples t-test. To assess whether the mean pain scores were affected by the order (HyFoSy first or HSG first), an independent samples t-test of the difference in scores was performed. Time to pregnancy was compared between the two groups of women with discordant results using the log-rank test. The cumulative ongoing pregnancy leading to live birth rates over time is visualized as Kaplan–Meier curves. Statistical analysis was performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY, USA).
Results
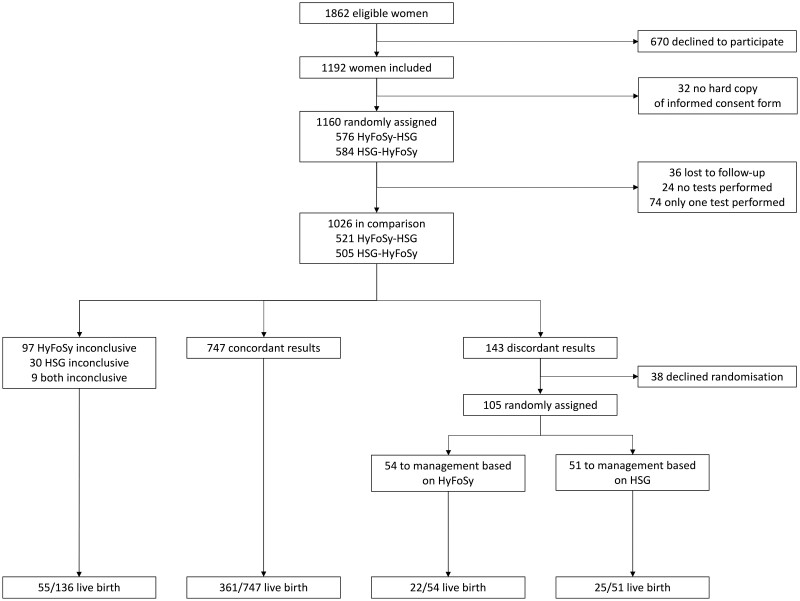
Between 7 May 2015 and 25 January 2019, 1862 women were registered as eligible (Fig. 2). A total of 1160 consenting women were assigned to undergo the two tubal patency tests, in random order (HyFoSy–HSG, n = 576, and HSG–HyFoSy, n = 584), of which 1026 received both tests and were included in the analysis (36 lost to follow-up, 24 no tests performed and 74 only one test performed). Table I presents the baseline characteristics of the study group.
Figure 2.
Study flow chart (based on intention-to-treat analysis). Trial screening, randomization and follow-up.
Table I.
Baseline characteristics.
| Characteristic | All women (N = 1026) |
Discordant results
management based on HyFoSy (n = 54) |
Discordant results
management based on HSG (n = 51) |
|---|---|---|---|
| Age (years) | 33.0 (30.0–36.0) | 33.0 (29.0–36.3) | 32.0 (29.0–36.0) |
| BMI (kg/m2) | 23.4 (21.0–26.6)a | 24.2 (21.4–27.9)b | 24.3 (21.3–27.5)b |
| Current smoker | 128/1008 (13)c | 13 (24) | 6 (12) |
| Ethnicityd | |||
| Caucasian | 830 (81) | 42 (78) | 42 (82) |
| Other | 139 (14) | 12 (22) | 8 (16) |
| Unknown | 57 (5) | 0 (0) | 1 (2) |
| Duration infertility (months) | 19.0 (15.0–26.2) | 20.0 (14.8–24.0) | 21.0 (16.7–36.3) |
| Primary infertility | 683 (67) | 34 (63) | 30 (59) |
| Duration of menstrual cycle (days) | 28.0 (28.0–30.0)e | 28.0 (28.0–30.0) | 30.0 (28.0–31.0) |
| High risk of tubal pathologyf | 135/888 (15)g | 11/50 (22)h | 10/47 (21)h |
| Total motile sperm count in male partner | 54.8 (22.0–122.0)i | 47.5 (12.0–89.8)j | 49.0 (13.3–112.3)j |
Data are medians (IQRs) or n (%), unless otherwise indicated; N is equal to the total number of women, unless otherwise indicated.
Data on BMI were available for 999 women.
Data on BMI were available for 53 versus 49 women.
Data on maternal smoking were available for 1008 women.
Reported by clinicians.
Data on duration of menstrual cycle were available for 1022 women.
Defined as positive Chlamydia Antibody titre, symptomatic Chlamydia infection (pelvic inflammatory disease) in the past, ectopic pregnancy or unilateral tubectomy in the past, ruptured appendicitis or peritonitis in the past, or pelvic surgery in the past.
Data on the risk of tubal pathology were available for 888 women.
Data on the risk of tubal pathology were available for 50 versus 47 women.
Data on total motile sperm count were available for 995 men.
Data on total motile sperm count were available for 54 versus 48 men.
HyFoSy, hysterosalpingo-foam sonography; HSG, hysterosalpingography.
Table II shows the results of HyFoSy versus HSG. In 97 (9.5%) of the 1026 women, HyFoSy was inconclusive, 30 (2.9%) had an inconclusive HSG, and in 9 (0.9%), both tests were inconclusive. In 747 women (73%) conclusive tests results were concordant. HyFoSy was more often inconclusive (10% vs. 4%) and less often normal (77% vs. 83%), whereas the proportion of women with one- or dual-sided pathology was comparable (13% vs. 13%).
Table II.
Comparison between hysterosalpingo-foam sonography (HyFoSy) result and hysterosalpingography (HSG) result (n = 1026).
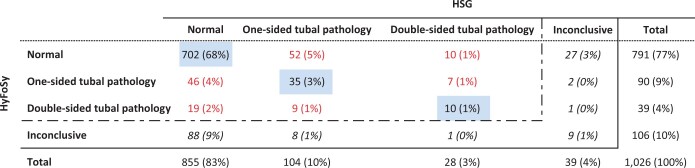
|
The completed tests are indicated by the dashed line. Concordance between HyFoSy and HSG is shown in the diagonal blue boxes; discordance between HyFoSy and HSG is illustrated in red; inconclusive is illustrated in italic.
In 38 of the 143 women with discordant test results, randomization was either declined by the participating woman or the study protocol was not fully adhered by the local investigator. The 105 other participants were randomly assigned to clinical management guided by either the results of HyFoSy (n = 54) or HSG (n = 51; Fig. 2). Table I also presents the baseline characteristics of the women with discordant test results randomly allocated to management based on HyFoSy or HSG. The baseline characteristics were similar in the two groups.
Supplementary Tables SII and SIII show the results of HyFoSy vs. HSG allocated by the order of the tests (HyFoSy–HSG and HSG–HyFoSy).
Supplementary Table SIV shows the management decisions taken in the subgroup with discordant results who agreed to be randomized. The prognosis for natural conception was comparable between the HyFoSy group and the HSG group. In addition, a comparable percentage underwent ovulation induction, ovulation induction followed by IUI or IVF, IUI (with or without mild ovarian hyper stimulation) alone, IUI followed by IVF/ICSI or IVF/ISCI alone.
Outcomes
Of the 136 women with an inconclusive test result on one or both tests, 55 (40%) experienced an ongoing pregnancy leading to live birth within 12 months (Fig. 2). In the group of 747 with concordant test results, 361 (48%) experienced a live birth (Fig. 2).
Table III shows the ongoing pregnancy leading to live birth rates with clinical management based on the results of HyFoSy versus HSG. An ongoing pregnancy leading to live birth was observed in 22 of the 54 women (41%) randomly assigned to HyFoSy and in 25 of the 51 women (49%) randomly assigned to HSG (Difference −8%; 95% CI: −27% to 10%). No ectopic pregnancies or stillbirths were reported among the women with discordant results.
Table III.
Ongoing pregnancy leading to live birth with clinical management based on the results of hysterosalpingo-foam sonography (HyFoSy) versus hysterosalpingography (HSG) based on intention-to-treat analysis.
| Findings | n | Management based on HyFoSy | Management based on HSG | Difference (95% CI) |
|---|---|---|---|---|
| Inconclusive | 136 (13%) | 55 (40%) | 55 (40%) | 0® |
| Concordant | 747 (73%) | 361 (48%) | 361 (48%) | 0® |
| Discordant | 143 (14%) | 22/54 (41%)° | 25/51 (49%)° | −8% (−27% to 10%) |
| Total | 1,026 (100%) | 474 (46%)+ | 486 (47%)+ | −1.2% (−3.4% to 1.5%) |
In participants with inconclusive or concordant results, management would not differ depending on whether the strategy had been based on HyFoSy or on HSG, and the difference is 0 by definition.
As observed in the randomized trial.
Estimated, based on the number of live births observed in the group with concordant results, the group with inconclusive results, and the randomized subgroups, each weighted by their corresponding fraction of the total group. Intention-to-treat analysis.
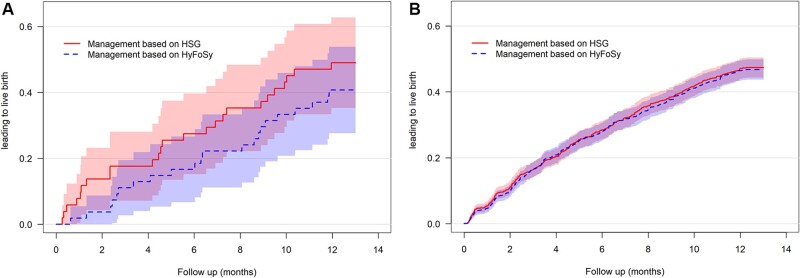
In total, clinical management based on the results of HyFoSy was estimated to lead to a live birth in 474 of 1026 women (46%) versus 486 of 1026 (47%) for management based on HSG (Difference −1.2%; 95% CI: −3.4% to 1.5%) (Table III). Given the 2% pre-defined margin, statistically significant non-inferiority of HyFoSy relative to HSG in terms of the effect of live births could not be demonstrated (P = 0.27). Time to ongoing pregnancy leading to live birth for the women with discordant results randomized for either management based on HyFoSy or HSG was comparable (Fig. 3).
Figure 3.
Time to ongoing pregnancy leading to live birth for management based on hysterosalpingo-foam sonography (HyFoSy) compared to hysterosalpingography (HSG). (A) Among discordant women (n = 105). (B) Among all women (N = 1026).
Sensitivity analyses revealed no substantial differences compared with the primary analysis (results not shown).
VAS pain scores for HyFoSy were reported by 1003 of the 1026 women (98%) and VAS scores for HSG were reported by 953 of the 1026 women (93%). HyFoSy was experienced as significantly less painful than HSG (P < 0.001). The mean VAS pain score for HyFoSy was 3.1 (SD 2.2) and the mean VAS pain score for HSG was 5.4 (SD 2.5). Although the mean VAS pain score of HyFoSy was not affected by the order of the tests (P = 0.57), the mean VAS pain score of HSG was higher when it was administered first, before HyFoSy (P = 0.01). There was no significant difference in the mean VAS pain scores for HSG when either oil-based contrast medium was used (n = 697; mean VAS pain score 5.5, SD 2.5) or water-based contrast medium (n = 281, mean VAS pain score 5.3, SD 2.7; P = 0.23).
Discussion
In infertile women scheduled for tubal patency testing during their fertility work-up, management based on the results of either HyFoSy or HSG leads to similar pregnancy outcomes, while HyFoSy is associated with significantly less pain. Though the estimated difference in proportions of women with a live birth within 12 months was only 1.2%, we could not demonstrate statistically significant non-inferiority of HyFoSy relative to HSG; the pre-defined 2% margin is included in the 95% CI. The direct therapeutic effect of tubal flushing was not taken into account.
Our study has several strengths and limitations. Major strengths of our study are the large sample size and the efficient paired design with randomization of women with discordant results and subsequent fertility management. An advantage of our study design is the efficiency, as every woman acts as her own control in comparing test results, which reduced our sample size compared with a traditional randomized trial. Our sample size was guided by the expected fraction of discordant results based on available literature (Emanuel et al., 2012). Additionally, we precluded observer bias by blinding the physician that performed the second test from the results of the first test. Other strengths are the execution of our trial according to a previously published protocol, the mandatory training before performing HyFoSy, and women with various causes of infertility included.
Our study also has some limitations. A number of eligible women declined participation, most often because of the burden of an additional test. The number of inconclusive test results on one or both tests was higher than anticipated. This might be explained by the operator-dependency and learning-curve of performing HyFoSy, although training was mandatory. Another possible explanation is that most of the women underwent both tubal patency tests on the same day in a relatively small time window, which might have resulted in interference of contrasts for either test. Even though Supplementary Tables SII and SIII show no clear evidence for interference. Thirty-eight women with discordant results could not be included in the randomized comparison; they either declined to be randomized or the local investigator decided not to follow the study protocol. A hard copy informed consent form was missing in 32 women and could not be traced, and these women were excluded from analysis. Finally, current guidelines advise to perform a tubal patency test only in selected women with a high risk for tubal pathology (NVOG, 2015; ACOG, 2019). Before onset of our study, international guidelines advised to perform a tubal patency test in all women during fertility work-up. Therefore, in our study majority of the women (85%) had a low risk for tubal pathology. Whether or not to perform a diagnostic tubal patency test during fertility work-up is still subject of debate, but this does not take the direct therapeutic effect of tubal flushing into account.
Before the start of our study, there were no RCTs directly comparing HyFoSy with HSG in terms of management strategies and subsequent pregnancy outcomes. Only small observational studies reported on pregnancy rates after HyFoSy, which varied from 19% within 3 months till 43% within 6 months (Emanuel et al., 2012; Exacoustos et al., 2015; Tanaka et al., 2018). Another observational study reported 55% pregnancies after HyFoSy, although follow-up duration varied largely (3–42 months; Van Schoubroeck et al., 2015a,b). So far, no detrimental effect of HyFoSy on fecundity was found in previous studies. Our study found overall pregnancy rates of 46–47% within 12 months after HyFoSy and HSG. HyFoSy with the use of ExEm-foam® seems safe, although the number of studies on possible complications is limited. Recently, one case of cutaneous small-vessel vasculitis developed after HyFoSy was reported (Ludwin et al., 2019). More research is needed. In our trial, eight infants had congenital anomalies (Supplementary Table SV). Contrary to HyFoSy, the fertility-enhancing effect and potential complications of HSG have been evaluated to a greater extent (Fang et al., 2018; Wang et al., 2019, 2020; Roest et al., 2021). Especially the use of oil-based contrast during HSG compared with water-based contrast results in higher ongoing pregnancy and live birth rates in couples with unexplained infertility and a low risk of tubal pathology (Dreyer et al., 2017). Since all women underwent both tubal patency tests in our study, no conclusions about therapeutic effects of HyFoSy could be drawn from our results.
HyFoSy was performed with 2-dimensional-transvaginal sonography; however, the use of 3-dimensional or Doppler imaging might increase the accuracy of HyFoSy and may add information on the ovum pick-up mechanism of the tubes. Even though evidence is limited, one could argue that these adjuvant ultrasound techniques make HyFoSy less operator-dependent and possibly less time consuming (Exacoustos et al., 2009; Maheux-Lacroix et al., 2014; Ludwin et al., 2017). Furthermore, reassessment of the images would be possible if storage of HyFoSy images is standardized. The comparison of diagnostic accuracy of HyFoSy to HSG and laparoscopy with dye was not included in this article but needs to be studied further.
In summary, this study showed that relying on either HyFoSy or HSG in infertile women leads to similar pregnancy outcomes, while HyFoSy is associated with significantly less pain. Although we could not exclude a slight decrease in pregnancy outcomes with management based on HyFoSy instead of HSG, we propose a two-step policy. Given the similar outcomes and lower pain scores, on these arguments HyFoSy can be preferred as first-choice tubal patency test during fertility work-up. In case of suspected tubal pathology or inconclusive results, further testing can be done. Before final conclusions on clinical management can be drawn, a head-to-head comparison between HyFoSy and HSG with oil-based contrast may be needed.
Data availability
De-identified individual participant data collected during the FOAM trial will be shared 1 year after publication of the results on request (mijatovic@amsterdamumc.nl). Approval of a proposal will be necessary before data will be shared. To gain access, requesters will need to sign an agreement form and confirm that the data will be used for the purpose for which access was granted.
Supplementary Material
Acknowledgements
We thank the women who participated in the trial, the hospitals and their staff (particularly the research nurses and other recruiting staff) for their contributions to this trial. We thank Prof. Dr Fulco van der Veen for his contribution in designing this study. Furthermore, we thank the staff of the Dutch Consortium for Healthcare Evaluation and Research in Obstetrics and Gynaecology (NVOG Consortium; https://www.zorgevaluatienederland.nl) for logistic support, and Marijke M.C. van Nispen (database development) and the staff of the Clinical Research Unit of the Amsterdam UMC, location Academic Medical Centre for their help with the randomization program and the online database during this trial.
Authors’ roles
K.D., M.v.W., P.M.M.B., J.S., B.W.J.M. and V.M. designed the trial. N.v.W. and J.v.R. were the trial coordinators and were in charge of collecting the data. N.v.W., M.v.W. and P.M.M.B. performed the statistical analyses. N.v.W. was in charge of drafting the article. J.v.R., K.D., C.B.L., M.v.W., P.M.M.B., J.S., B.W.J.M. and V.M. participated in the analysis, article drafting and supervision of the work. M.H.A.v.H., J.P.d.B., H.R.V., F.M., W.M.v.B., M.A.F.T., A.M.v.P., A.P.M., J.G., C.H.d.K., A.M.H.K., N.B., D.P.v.d.H., F.P.J.M.V., M.K., B.I.G.v.d.L., J.K., A.F.L., W.J.M., F.J.M.B., O.V., L.F.v.d.V., J.v.D., M.J.L. and R.T. recruited and counselled participants of this study as local investigators. All authors read, edited and approved the final article.
Funding
The FOAM study was an investigator-initiated study funded by ZonMw, The Netherlands organization for Health Research and Development (project number 837001504). ZonMw funded the whole project. IQ Medical Ventures provided the ExEm-foam® kits free of charge. The funders had no role in study design, collection, analysis and interpretation of the data. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflict of interest
K.D. reports travel and speaker fees from Guerbet. F.J.M.B. reports personal fees as a member of the external advisory board for Merck Serono, The Netherlands, and a research support grant from Merck Serono, outside the submitted work. C.B.L. reports speakers’ fee from Ferring in the past, and his department receives research grants from Ferring, Merck and Guerbet. J.S. reports a research agreement with Takeda on MR of motility outside the submitted work. M.v.W. reports leading the Netherlands Satellite of the Cochrane Gynaecology and Fertility Group. B.W.J.M. is supported by an NHMRC Investigator grant (GNT1176437). B.W.J.M. reports consultancy for Guerbet and research funding from Merck and Guerbet. V.M. reports non-financial support from IQ medicals ventures, during the conduct of the study; grants and personal fees from Guerbet, outside the submitted work. The other authors do not report conflicts of interest.
References
- ACOG. Infertility Workup for the Women’s Health Specialist: ACOG Committee Opinion, Number 781. Obstet Gynecol 2019;133:e377–e384. [DOI] [PubMed] [Google Scholar]
- AHRQ. AHRQ Methods for Effective Health Care Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US), 2008. [PubMed] [Google Scholar]
- Bossuyt PM, Lijmer JG, Mol BW.. Randomised comparisons of medical tests: sometimes invalid, not always efficient. Lancet 2000;356:1844–1847. [DOI] [PubMed] [Google Scholar]
- Chauhan MB, Lakra P, Jyotsna D, Nanda S, Malhotra V.. Pain relief during hysterosalpingography: role of intracervical block. Arch Gynecol Obstet 2013;287:155–159. [DOI] [PubMed] [Google Scholar]
- Collins JA, Burrows EA, Wilan AR.. The prognosis for live birth among untreated infertile couples. Fertil Steril 1995;64:22–28. [PubMed] [Google Scholar]
- Dijkman AB, Mol BW, van der Veen F, Bossuyt PM, Hogerzeil HV.. Can hysterosalpingocontrast-sonography replace hysterosalpingography in the assessment of tubal subfertility? Eur J Radiol 2000;35:44–48. [DOI] [PubMed] [Google Scholar]
- Dreyer K, Out R, Hompes PG, Mijatovic V.. Hysterosalpingo-foam sonography, a less painful procedure for tubal patency testing during fertility workup compared with (serial) hysterosalpingography: a randomized controlled trial. Fertil Steril 2014;102:821–825. [DOI] [PubMed] [Google Scholar]
- Dreyer K, van Rijswijk J, Mijatovic V, Goddijn M, Verhoeve HR, van Rooij IAJ, Hoek A, Bourdrez P, Nap AW, Rijnsaardt-Lukassen HGM. et al. Oil-Based or water-based contrast for hysterosalpingography in infertile women. N Engl J Med 2017;376:2043–2052. [DOI] [PubMed] [Google Scholar]
- Duffy JMN, Bhattacharya S, Bhattacharya S, Bofill M, Collura B, Curtis C, Evers JLH, Giudice LC, Farquharson RG, Franik S. et al. ; Core Outcome Measure for Infertility Trials (COMMIT) Initiative. Standardizing definitions and reporting guidelines for the infertility core outcome set: an international consensus development study. Hum Reprod 2020;35:2735–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel MH, van Vliet M, Weber M, Exalto N.. First experiences with hysterosalpingo-foam sonography (HyFoSy) for office tubal patency testing. Hum Reprod 2012;27:114–117. [DOI] [PubMed] [Google Scholar]
- Exacoustos C, Di Giovanni A, Szabolcs B, Binder-Reisinger H, Gabardi C, Arduini D.. Automated sonographic tubal patency evaluation with three-dimensional coded contrast imaging (CCI) during hysterosalpingo-contrast sonography (HyCoSy). Ultrasound Obstet Gynecol 2009;34:609–612. [DOI] [PubMed] [Google Scholar]
- Exacoustos C, Di Giovanni A, Szabolcs B, Romeo V, Romanini ME, Luciano D, Zupi E, Arduini D.. Automated three-dimensional coded contrast imaging hysterosalpingo-contrast sonography: feasibility in office tubal patency testing. Ultrasound Obstet Gynecol 2013;41:328–335. [DOI] [PubMed] [Google Scholar]
- Exacoustos C, Tiberio F, Szabolcs B, Romeo V, Romanini E, Zupi E.. Can tubal flushing with hysterosalpingo-foam sonography (HyFoSy) media increase women’s chances of pregnancy? J Minim Invasive Gynecol 2015;22:S238. [DOI] [PubMed] [Google Scholar]
- Fang F, Bai Y, Zhang Y, Faramand A.. Oil-based versus water-based contrast for hysterosalpingography in infertile women: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril 2018;110:153–160. [DOI] [PubMed] [Google Scholar]
- Farquhar CM, Bhattacharya S, Repping S, Mastenbroek S, Kamath MS, Marjoribanks J, Boivin J.. Female subfertility. Nat Rev Dis Primers 2019;5:7. [DOI] [PubMed] [Google Scholar]
- Gazelle GS, Kessler L, Lee DW, McGinn T, Menzin J, Neumann PJ, van Amerongen D, White LA; Working Group on Comparative Effectiveness Research for Imaging. A framework for assessing the value of diagnostic imaging in the era of comparative effectiveness research. Radiology 2011;261:692–698. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Geng Q, Liu H, Han X.. First experience using 4-dimensional hysterosalpingo-contrast sonography with SonoVue for assessing fallopian tube patency. J Ultrasound Med 2013;32:1233–1243. [DOI] [PubMed] [Google Scholar]
- Heikkinen H, Tekay A, Volpi E, Martikainen H, Jouppila P.. Transvaginal salpingosonography for the assessment of tubal patency in infertile women: methodological and clinical experiences. Fertil Steril 1995;64:293–298. [DOI] [PubMed] [Google Scholar]
- Hull MG, Glazener CM, Kelly NJ, Conway DI, Foster PA, Hinton RA, Coulson C, Lambert PA, Watt EM, Desai KM.. Population study of causes, treatment, and outcome of infertility. Br Med J (Clin Res Ed) 1985;291:1693–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunault CC, Laven JS, van Rooij IA, Eijkemans MJ, Te Velde ER, Habbema JD.. Prospective validation of two models predicting pregnancy leading to live birth among untreated subfertile couples. Hum Reprod 2005;20:1636–1641. [DOI] [PubMed] [Google Scholar]
- Jansen FW, Kapiteyn K, Trimbos‐Kemper T, Hermans J, Trimbos JB.. Complications of laparoscopy: a prospective multicentre observational study. Br J Obstet Gynaecol 1997;104:595–600. [DOI] [PubMed] [Google Scholar]
- Lanzani C, Savasi V, Leone FP, Ratti M, Ferrazzi E.. Two-dimensional HyCoSy with contrast tuned imaging technology and a second-generation contrast media for the assessment of tubal patency in an infertility program. Fertil Steril 2009;92:1158–1161. [DOI] [PubMed] [Google Scholar]
- Lim SL, Jung JJ, Yu SL, Rajesh H.. A comparison of hysterosalpingo-foam sonography (HyFoSy) and hysterosalpingo-contrast sonography with saline medium (HyCoSy) in the assessment of tubal patency. Eur J Obstet Gynecol Reprod Biol 2015;195:168–172. [DOI] [PubMed] [Google Scholar]
- Lu B, Gatsonis C.. Efficiency of study designs in diagnostic randomized clinical trials. Stat Med 2013;32: 1451–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano DE, Exacoustos C, Luciano AA.. Contrast ultrasonography for tubal patency. J Minim Invasive Gynecol 2014;21:994–998. [DOI] [PubMed] [Google Scholar]
- Ludwin A, Ludwin I, Szczeklik W, Martins WP.. Cutaneous small-vessel vasculitis following hysterosalpingo-foam sonography (HyFoSy). Ultrasound Obstet Gynecol 2019;54:831–834. [DOI] [PubMed] [Google Scholar]
- Ludwin I, Ludwin A, Wiechec M, Nocun A, Banas T, Basta P, Pitynski K.. Accuracy of hysterosalpingo-foam sonography in comparison to hysterosalpingo-contrast sonography with air/saline and to laparoscopy with dye. Hum Reprod 2017;32:1–769. [DOI] [PubMed] [Google Scholar]
- Maheux-Lacroix S, Boutin A, Moore L, Bergeron ME, Bujold E, Laberge P, Lemyre M, Dodin S.. Hysterosalpingosonography for diagnosing tubal occlusion in subfertile women: a systematic review with meta-analysis. Hum Reprod 2014;29:953–963. [DOI] [PubMed] [Google Scholar]
- Mol BW, Collins JA, Burrows EA, Van der Veen F, Bossuyt PM.. Comparison of hysterosalpingography and laparoscopy in predicting fertility outcome. Hum Reprod 1999;14:1237–1242. [DOI] [PubMed] [Google Scholar]
- NICE. Clinical Guideline—Fertility Problems: Assessment and Treatment. 2013. https://www.nice.org.uk/guidance/cg156.
- NVOG. National Guideline Basic Fertility Work Up. Dutch guidelines for Obstetrics and Gynaecology2015. https://www.nvog.nl/wp-content/uploads/2018/02/Ori%C3%ABnterend-Fertiliteitsonderzoek-OFO-3.0-12-11-2015.pdf.
- Piccioni MG, Riganelli L, Filippi V, Fuggetta E, Colagiovanni V, Imperiale L, Caccetta J, Panici PB, Porpora MG.. Sonohysterosalpingography: comparison of foam and saline solution. J Clin Ultrasound 2017;45:67–71. [DOI] [PubMed] [Google Scholar]
- Randolph JF, Ying YK, Maier DB, Schmidt CL, Riddick DH, Randolph JR.. Comparison of real-time ultrasonography, hysterosalpingography, and laparoscopy/hysteroscopy in the evaluation of uterine abnormalities and tubal patency. Fertil Steril 1986;46:828–832. [DOI] [PubMed] [Google Scholar]
- Reis MM, Soares SR, Cancado ML, Camargos AF.. Hysterosalpingo contrast sonography (HyCoSy) with SH U 454 (Echovist) for the assessment of tubal patency. Hum Reprod 1998;13:3049–3052. [DOI] [PubMed] [Google Scholar]
- Roest I, Rosielle K, van Welie N, Dreyer K, Bongers M, Mijatovic V, Mol BW, Koks C.. Safety of oil-based contrast medium for hysterosalpingography: a systematic review. Reprod Biomed Online 2021;42:1119–1129. [DOI] [PubMed] [Google Scholar]
- Saunders RD, Shwayder JM, Nakajima ST.. Current methods of tubal patency assessment. Fertil Steril 2011;95:2171–2179. [DOI] [PubMed] [Google Scholar]
- Schünemann HJ, Mustafa R, Brozek J, Santesso N, Alonso-Coello P, Guyatt G, Scholten R, Langendam M, Leeflang MM, Akl EA. et al. ; GRADE Working Group. GRADE Guidelines: 16. GRADE evidence to decision frameworks for tests in clinical practice and public health. J Clin Epidemiol 2016;76:89–98. [DOI] [PubMed] [Google Scholar]
- Snick HK, Snick TS, Evers JL, Collins JA.. The spontaneous pregnancy prognosis in untreated subfertile couples: the Walcheren primary care study. Hum Reprod 1997;12:1582–1588. [DOI] [PubMed] [Google Scholar]
- Tan J, Tannus S, Taskin O, Kan A, Albert AY, Bedaiwy MA.. The effect of unilateral tubal block diagnosed by hysterosalpingogram on clinical pregnancy rate in intrauterine insemination cycles: systematic review and meta-analysis. BJOG 2019;126:227–235. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Chua J, Cincotta R, Ballard EL, Duncombe G.. Hysterosalpingo-foam sonography (HyFoSy): tolerability, safety and the occurrence of pregnancy post-procedure. Aust N Z J Obstet Gynaecol 2018;58:114–118. [DOI] [PubMed] [Google Scholar]
- van Rijswijk J, van Welie N, Dreyer K, van Hooff MHA, de Bruin JP, Verhoeve HR, Mol F, Kleiman-Broeze KA, Traas MAF, Muijsers G. et al. The FOAM study: is Hysterosalpingo foam sonography (HyFoSy) a cost-effective alternative for hysterosalpingography (HSG) in assessing tubal patency in subfertile women? Study protocol for a randomized controlled trial. BMC Womens Health 2018;18:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schoubroeck D, Van den Bosch T, Ameye L, Boes AS, D’Hooghe T, Timmerman D.. Pain during Fallopian-tube patency testing by hysterosalpingo-foam sonography. Ultrasound Obstet Gynecol 2015a;45:346–350. [DOI] [PubMed] [Google Scholar]
- Van Schoubroeck D, den Bosch TV, Van Tornout K, D’Hooghe T, Timmerman D.. OC24.01: Spontaneous conception after hysterosalpingo-foam sonography (HyFoSy). Ultrasound Obstet Gynecol 2015b;46:51–51. [DOI] [PubMed] [Google Scholar]
- Verhoeve HR, Coppus SF, van der Steeg JW, Steures P, Hompes PG, Bourdrez P, Bossuyt PM, van der Veen F, Mol BW;. Collaborative Effort on the Clinical Evaluation in Reproductive Medicine. The capacity of hysterosalpingography and laparoscopy to predict natural conception. Hum Reprod 2011;26:134–142. [DOI] [PubMed] [Google Scholar]
- Wang R, van Welie N, van Rijswijk J, Johnson NP, Norman RJ, Dreyer K, Mijatovic V, Mol BW.. Effectiveness on fertility outcome of tubal flushing with different contrast media: systematic review and network meta-analysis. Ultrasound Obstet Gynecol 2019;54:172–181. [DOI] [PubMed] [Google Scholar]
- Wang R, Watson A, Johnson N, Cheung K, Fitzgerald C, Mol BWJ, Mohiyiddeen L.. Tubal flushing for subfertility. Cochrane Database Syst Rev 2020;10:Cd003718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Qian L.. Three- or four-dimensional hysterosalpingo contrast sonography for diagnosing tubal patency in infertile females: a systematic review with meta-analysis. Br J Radiol 2016;89:20151013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Zhang X, Chen X, Liao L, Pan R, Zhou N, Di N.. Value of three-dimensional hysterosalpingo-contrast sonography with SonoVue in the assessment of tubal patency. Ultrasound Obstet Gynecol 2012;40:93–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified individual participant data collected during the FOAM trial will be shared 1 year after publication of the results on request (mijatovic@amsterdamumc.nl). Approval of a proposal will be necessary before data will be shared. To gain access, requesters will need to sign an agreement form and confirm that the data will be used for the purpose for which access was granted.