Abstract
Minority and underresourced communities experience disproportionately high rates of fatal cancer and cardiovascular disease. The intersection of these disparities within the multidisciplinary field of cardio‐oncology is in critical need of examination, given the risk of perpetuating health inequities in the growing vulnerable population of patients with cancer and cardiovascular disease. This review identifies 13 cohort studies and 2 meta‐analyses investigating disparate outcomes in treatment‐associated cardiotoxicity and situates these data within the context of oncologic disparities, preexisting cardiovascular disparities, and potential system‐level inequities. Black survivors of breast cancer have elevated risks of cardiotoxicity morbidity and mortality compared with White counterparts. Adolescent and young adult survivors of cancer with lower socioeconomic status experience worsened cardiovascular outcomes compared with those of higher socioeconomic status. Female patients treated with anthracyclines or radiation have higher risks of cardiotoxicity compared with male patients. Given the paucity of data, our understanding of these racial and ethnic, socioeconomic, and sex and gender disparities remains limited and large‐scale studies are needed for elucidation. Prioritizing this research while addressing clinical trial inclusion and access to specialist care is paramount to reducing health inequity.
Keywords: cardiotoxicity, cardio‐oncology, disparities, social determinants of health
Subject Categories: Cardio-Oncology, Cardiotoxicity, Disparities
Nonstandard Abbreviations and Acronyms
- AIAN
American Indian and Alaska Native
- API
Asian and Pacific Islander
- AYA
adolescent and young adult
Cardiovascular disease (CVD) and cancer are the leading causes of death in the United States, with a disproportionate burden of illness among Black patients, lower income communities, and other minority groups. 1 , 2 , 3 , 4 , 5 Incidence and mortality disparities in cancer and CVD remain pervasive because of complex interactions of social determinants of health including social, economic, geographic, and cultural factors that affect underlying biology and health‐related behaviors. 1 , 2 , 3 , 6 , 7 , 8 , 9 , 10 , 11 These social determinants of health are thought to be modulated by sex and gender 12 and structural racism 13 , 14 —defined as economic, institutional, cultural, and historical forces that systematically advantage White populations and disadvantage racial and ethnic minority populations. Such disparities have been magnified by the COVID‐19 pandemic, which unevenly has affected underresourced communities and racial and ethnic minority patients in the United States. 15 , 16 , 17 Cancer health disparities may be intertwined with cardiac outcomes, particularly given the rising overall incidence of cancer (owing to improved cancer screening and survival among common cancers) 1 , 2 , 4 that coincides with the increasing prevalence of CVD among patients with cancer.
Metabolic effects of malignancy, direct adverse effects of cancer therapeutics, and indirect effects of treatment (eg, physical deconditioning) have profound impacts on the cardiovascular health of patients with cancer. 18 , 19 , 20 , 21 Indeed, CVD has become a significant cause of morbidity and mortality among survivors of cancer. 22 , 23 For instance, among postmenopausal women with hormone receptor positive breast cancer, CVD has been shown to rival or exceed recurrent malignancy as the most common cause of death. 24 Now with an estimated 16.9 million people in the United States living with a history of cancer, 4 the prevalence of CVD among survivors of cancer continues to increase.
The multidisciplinary field of cardio‐oncology has emerged to balance cancer treatment with the prevention and management of associated cardiac disease. Collaborations across oncology and cardiology have developed rapidly to address this imperative, with a growing understanding of the cardiotoxicity of cancer treatment in context of other cardiovascular risk factors. Preexisting cardiovascular risk factors and CVD are now known to be associated strongly with posttreatment cardiac dysfunction. 25 , 26 , 27 , 28 , 29
Given the cross‐talk between cancer and cardiac disease, it is critical that we elucidate disparities in cardio‐oncology in order to improve health outcomes for the most vulnerable patients including Black populations and other minority populations with cancer. Unfortunately, our current understanding of intersectional disparities within cardio‐oncology is limited by a marked paucity of data. Two recently published reviews focus on racial and ethnic disparities in cardio‐oncology, 30 , 31 but our narrative review summarizes the available literature on disparities in cardiotoxicity outcomes across race and ethnicity, sex and gender, and socioeconomic status (SES) among patients with cancer in the United States. We situate these data in the context of disparities in cancer and preexisting CVD, as these bodies of knowledge provide important context for data interpretation. Studies to date on disparities in cardiotoxicity are limited in number and scope, but the available data presented here merit discussion to inform future investigations on disparities in cardio‐oncology and help create actionable strategies for equitable care.
Overview of Cancer Disparities
It is important for even the general cardiologist to understand basic cancer disparities together with cardiovascular ones given that most patients with cancer are more likely to have access to a general cardiologist than a cardio‐oncology specialist. Disparities exist across nearly all aspects of oncologic care from screening to survivorship. 1 , 8 On average, patients of lower SES have increased cancer incidence and decreased survival compared with their higher SES counterparts. 2 Individual SES is shaped by a multitude of factors including education, employment status and occupation, income, wealth, and health insurance. Unfortunately, the socioeconomic gap in cancer appears to be widening: a 2017 longitudinal study of cancer mortality demonstrated that inequalities based on SES increased from 1979 to 2011, primarily because of disproportionally improved mortality for high SES patients. 32 The impact of this widening gap is likely to be felt broadly given the rising overall incidence of cancer.
Even after controlling for income, significant disparities persist for racial and ethnic minority patients with cancer in the United States. 33 Within this review, we refer to “racial and ethnic” populations with the understanding that race and ethnicity are social constructs that reference diverse groups of people. Racial and ethnic minority populations are categorized by the US Office of Management and Budget into Black populations, Hispanic/Latino populations (referred to as Hispanic/Latinx in this review), Asian populations and Native Hawaiian/other Pacific Islander populations (referred to as Asian and Pacific Islander in this review), and American Indian and Alaska Native (AIAN) populations, 6 though it bears noting that this grouping does not address ancestry, genetic admixture, immigration status, or regional communities. For most cancers, White patients are more likely than patients of other races and ethnicities to be diagnosed at earlier stages, receive aggressive care, and have improved chances of survival even when accounting for staging. 1 , 4 , 7 , 34 Survival rates are lower for Black patients and AIAN patients in comparison to White patients for each major type of cancer except for renal and pancreatic cancer, for which mortality is approximately the same. 1 Although there are some instances of genetic differences in tumor biology (eg, the increased incidence of triple hormone receptor negative breast cancer in Black women), tumor genetics do not equate germline genetics and differential treatment responses alone do not account for racial and ethnic disparities in cancer outcomes. 7 , 35 Similarly, SES alone does not account for racial and ethnic cancer disparities. 33 Various other social determinants of health including environmental pollution exposures, neighborhood safety‐related concerns, and perceived racial discrimination have been implicated in these disparities, 2 , 7 , 8 influenced by ongoing structural racism. 36 , 37
Sex and gender disparities as well as geographic disparities also persist among patients with cancer. Male patients across different races/ethnicities and ages continue to have increased rates of cancer and worsened prognosis in comparison to female patients. 1 The reasons for this are not understood completely but are thought to reflect differences in health‐related behaviors, comorbid risk factors, and sex hormones. 1 , 38 Geographic disparities are most prominent among highly preventable cancers including lung and cervical cancer, reflecting the influences of existing risk factors, health‐related behaviors, and regional screening practices. 1
Disparities in Preexisting Cardiovascular Disease
Disparities within preexisting cardiovascular risk factors and CVD must be addressed given their strong association with posttreatment cardiac dysfunction. 25 , 26 , 27 , 28 , 29 Additionally, understanding cardiovascular disparities may provide valuable insight for discussions on understudied disparities in cardio‐oncology. Despite a decline in cardiovascular mortality nationwide, the burden of CVD remains disparately distributed among underresourced communities and racial and ethnic minority populations. 9 , 10 , 11 These disparities are discussed comprehensively in recent scientific statements and advisories from the American Heart Association, 9 , 10 , 39 , 40 , 41 , 42 in a recent workshop from the National Heart, Lung, and Blood Institute, 11 and most recently in a comprehensive narrative review. 43 Race and ethnicity, SES, neighborhood resources and environmental features, sex and gender, and cultural factors including psychosocial stress (eg, from racial discrimination), acculturation among immigrant communities, and social cohesion have been associated with disparate incidence and mortality from CVD. 9 , 10 , 11 , 39 , 40 , 41 , 42 , 44 , 45 As described in a recent American Heart Association presidential advisory, 13 these factors are in turn influenced by structural racism. Forces of structural racism as well as other forms of racism, including interpersonal, are known to have downstream effects that modulate social determinants of health and perpetuate social, economic, and health inequities in general as well as cardiovascular health. 13 , 14 , 46 , 47 However, racism is infrequently named in published literature as a contributor to health disparities, and little research has been done to evaluate in detail its effects on health. 13 , 48 Some genome‐wide association studies have identified genetic loci differentially associated with CVD in Black populations 9 and AIAN populations 41 as well as some polymorphisms associated with CVD across racial and ethnic groups, 40 but these data must be considered in context of the variability of results from replication studies, complex interaction between genes and environment, and risks of overattributing importance to genetic risk alleles at the expense of addressing significant contributions from social and structural determinants of health. 9 , 40 , 41
Black populations bear a disproportionately high burden of CVD and associated cardiovascular risk factors when compared with White counterparts. 9 , 43 Black patients have been shown to have earlier ages of onset as well as higher incidence of hypertension, dyslipidemia, diabetes, and obesity when compared with White patients. They also have been shown to experience earlier onset as well as higher incidence and mortality of heart failure, sudden cardiac arrest, cerebrovascular disease, and peripheral arterial disease. 9 Additionally, they have experienced smaller decreases in the incidence of coronary artery disease in comparison to White populations. 9 These disparities have been associated with a multitude of factors including SES and other social determinants of health. 9 , 10 However, persistence of these disparities among Black patients across the socioeconomic spectrum also implicates social epigenetic factors including psychosocial stress from racial discrimination and structural racism itself, 13 although the scope of its impact requires further investigation as mentioned previously. In addition to explicit forms of racism, implicit bias continues to contribute to disparities. 10 For example, Black patients are less likely than White counterparts to receive cardiac catheterization for suspected angina 49 and, together with other racial and ethnic minority patients, are less likely than White patients to receive cardiopulmonary resuscitation for out‐of‐hospital arrest. 50 Genetic differences alone do not explain these differences for the reasons mentioned previously, as well as the clear genetic heterogeneity and admixture among populations of African descent. 51 , 52
Disparities among Hispanic/Latinx as well as Asian and Pacific Islander (API) populations are masked by the reporting of disparities data in aggregate. Although some studies have shown a lower incidence of CVD among Hispanic/Latinx populations, recent discussions have emphasized the heterogeneity among this population and the variability of cardiac risk profiles among groups of different national origin or ancestral heritage. 39 , 43 , 53 Preliminary investigations suggest contributions from social determinants of health including language discordance, health literacy, cultural traditions, and psychosocial stress from perceived discrimination. 39 Heterogeneity among people of API descent also continues to be masked by surveys and studies that gather data on API patients in aggregate. 40 Among immigrant Hispanic/Latinx populations as well as immigrant API populations born outside the United States, acculturation and duration of US residence have been associated with worsened cardiovascular risk factors and CVD. 39 , 40 However, the direct relationship between social determinants of health, perceived discrimination, acculturation, and structural racism has not been explored for any of these groups. 13 , 39 , 40
AIAN populations experience the highest morbidity and mortality from CVD of any subgroup in the United States. 41 , 54 These populations share the same traditional cardiovascular risk factors as other groups, but with some distinctions: among AIAN populations, diabetes is the single‐most important risk factor for CVD, with a 3‐fold elevated risk of diabetes in comparison to White populations. 41 Additionally, renal impairment is a unique independent risk factor for predicting CVD. 41 Common social determinants of health including poverty, education, unemployment, and housing also affect CVD in this population, as well as distinct environmental factors including toxic metal exposure and groundwater contamination. 41 The influence of governmental exploitation and structural discrimination has been referenced in relation to these CVD disparities but has not been investigated comprehensively.
The absolute numbers of women living with and dying from CVD are greater than those for men, and coronary artery disease mortality rates are rising for women aged 35 to 44 years. 12 Although women and men share the same cardiovascular risk factors, the prevalence of some risk factors differ: women older than 65 years old and women older than 20 years old have a higher prevalence than men for hypertension and diabetes, respectively. 12 Additionally, rates of physical inactivity are higher for women than men. 12 These differences may be influenced by gender‐based health behaviors. 12 Additionally, psychosocial stress from marital tension and the stress of traditional caregiving gender roles may contribute to cardiovascular risk factors. 12 Upstream factors of sexism also have been associated with increased risk of posttraumatic stress disorder, psychosocial stress, and health‐related behaviors like alcohol and tobacco use. 12 , 55 In the medical domain, inadequate treatment by providers in the management of acute and chronic cardiac disease may also be contributing to sex and gender disparities. Women with known coronary artery disease are less likely to be on appropriate lipid‐lowering therapy and aspirin for secondary prevention. 56 , 57 Although women are more likely to be treated for hypertension than men, they have achieved poorer levels of blood pressure control when matched for age, ethnicity, and comorbidities. 56 Women are also less likely to achieve a hemoglobin A1c target of <7% for appropriate diabetes control and cardiac risk mitigation. 56 It is well‐established that the clinical presentation and inpatient treatment of acute coronary syndrome differs between men and women, and several studies have demonstrated that fewer women are started on evidence‐based secondary prevention medications on discharge. 57 The reasons for these examples of substandard treatment need to be explored further, but implicit bias may play a contributory role. Implicit bias has been shown to influence gender‐based decision‐making in coronary artery disease, 49 , 58 but additional research is needed.
The persistence of race and ethnicity, socioeconomic, and sex and gender disparities among patients with CVD is deeply concerning, and raises the alarm for parallel disparities in interdisciplinary areas of medical care including cardio‐oncology.
Cardio‐Oncology
Although many studies on treatment‐associated cardiotoxicity primarily define this entity as clinical heart failure and/or radiographic measurements of left ventricular systolic dysfunction, possible adverse short‐ and long‐term cardiovascular effects encompass systolic and/or diastolic dysfunction, valvular disease, conduction abnormalities, coronary artery disease, impaired cardiac remodeling, disrupted mechanisms of cardiac homeostasis, vascular disease, and thrombosis. 59 , 60 Such adverse effects have been associated with nearly all categories of cancer therapy.
To inform our discussion of disparities in treatment‐associated cardiotoxicity, the most common and clinically relevant cardiotoxicity manifestations are summarized here (Table 1). 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 Comprehensively documenting the heterogeneous spectrum of short‐ and long‐term cardiotoxicities seen in both historical and novel cancer therapeutics is beyond the scope of this review; these cardiotoxicities are described elsewhere. 59 , 60 , 62 , 63 , 69 , 70 , 71 , 72
Table 1.
Cardiotoxicities Associated With Common Cancer Therapeutics
| Chemotherapy | Cardiotoxicity (relative incidence*) |
| Anthracyclines | |
| Doxorubicin/Daunorubicin | Heart failure/left ventricular dysfunction (common), arrhythmias |
| Epirubicin | Heart failure/left ventricular dysfunction (common) |
| Antimetabolites | |
| 5‐Fluorouracil | Cardiac ischemia (intermediate), arrhythmias, angina, heart failure |
| Methotrexate | Pericardial effusion |
| Capecitabine | Cardiac ischemia (intermediate) |
| Platinum‐based | |
| Cisplatin | Arrhythmias (rare), angina (rare) |
| Microtubule inhibitors | |
| Paclitaxel | Heart failure/left ventricular dysfunction (intermediate), cardiac ischemia (rare), QTc prolongation (rare), arrhythmia (rare) |
| Docetaxel | Heart failure/left ventricular dysfunction (intermediate), cardiac ischemia (rare) |
| Alkylating agents | |
| Cyclophosphamide | Heart failure/left ventricular dysfunction (intermediate), angina |
| Ifosfamide | Heart failure/left ventricular dysfunction (rare), arrhythmias |
| Mitomycin | Heart failure (common) |
| Immunotherapy | |
| Monoclonal antibodies | |
| Trastuzumab (HER‐2) | Heart failure (intermediate‐common), hypertension (intermediate) |
| Bevacizumab (vascular endothelial growth factor) | Hypertension (common), myocardial ischemia (intermediate‐common), heart failure (intermediate) |
| Rituximab (CD‐20) | Arrhythmia, heart failure, myocardial ischemia |
| Immune checkpoint inhibitors | |
| Pembrolizumab/Nivolumab (PD‐1) | Myocarditis/pericarditis (intermediate, more common with combined immune checkpoint inhibitor therapy), heart failure (rare), arrhythmias (rare), pericardial disease (rare), atherosclerotic disease |
| Ipilimumab (CTLA‐4) | |
| Chimeric antigen T‐cell therapies | |
| Tisagenlecleucel | Arrhythmia† (common), ventricular dysfunction† (common) |
| Axicabtagene ciloleucel | |
| Tyrosine kinase inhibitors | |
| Imatinib | Heart failure (rare), arrhythmias (rare) |
| Dasatinib | Heart failure (common), pulmonary hypertension (rare) |
| Nilotinib | Heart failure, myocardial ischemia (common), QT prolongation (intermediate) |
| Sunitinib | Hypertension (common), heart failure (intermediate‐common) |
| Sorafenib | Hypertension (common), heart failure (intermediate‐common) |
| Ponatinib | Myocardial ischemia (common), hypertension (common), heart failure (intermediate‐common), arrhythmias (intermediate), peripheral vascular disease |
| Proteasome inhibitors | |
| Bortezomib | Heart failure (intermediate‐common), arrhythmia, myocardial ischemia |
| Thoracic radiation‡ | Myocardial ischemia (common), conduction abnormalities (common), peripheral vascular disease (common), heart failure (common), valvular disease (intermediate‐common), pericardial disease (intermediate) |
*When possible, the incidence of each reported cardiotoxicity is categorized as rare <1%, intermediate 1% to 5%, or common >5%. However, the incidence of many toxicities cannot be determined reliably owing to insufficient data.
†Occur in setting of cytokine release syndrome.
‡The overall incidence of radiation‐induced cardiotoxicity varies depending on dose and historical era. Incidence of fatal radiation‐associated cardiotoxicity is estimated at 1% to 7%.
In the available guidelines for the prevention, screening, surveillance, and attenuation of cancer treatment‐associated cardiotoxicity from the American Society of Clinical Oncology, 25 European Society of Medical Oncology, 73 and the American Heart Association, 74 the identification of patients at elevated risk is viewed as the first step in reducing cardiotoxicity. In identifying these patients, it is imperative to recognize which patients have predisposing cardiovascular risk factors and preexisting cardiac disease. Age as well as preexisting diabetes, hypertension, hyperlipidemia, and tobacco smoking have been associated with an increased risk of cardiac dysfunction among patients with cancer treated with anthracyclines or trastuzumab. 26 , 27 , 28 , 29 A large‐scale retrospective study found that among patients treated for breast cancer, lung cancer, multiple myeloma, and non‐Hodgkin lymphoma, the presence of 2 or more cardiovascular risk factors conferred an even higher risk of post‐treatment CVD. 75 The presence of preexisting reduced systolic function and coronary artery disease has also been linked to increased rates of treatment‐associated cardiotoxicity. 27 , 28 , 29 The shared risk factors for cancer and CVD are thought to influence the pathogenesis of both disease entities as well as treatment‐associated cardiotoxicity. 76 , 77
Risks of System‐Level Inequity in Cardio‐Oncology: Access to Specialty Care
We suspect that the screening, surveillance, and management of treatment‐associated cardiotoxicity is influenced by social and structural determinants of health including health insurance status, geographic distance from specialty care, and transportation barriers.
Many people with cancer still do not have access to health insurance: among states that did not pursue Affordable Care Act Medicaid expansions, 1 in 5 survivors of cancer remains uninsured. 78 Even among those with insurance, cardio‐oncology specialists are not readily accessible to most people. Despite the rapid growth of the field, 79 specialized cardio‐oncology training and patient access to cardio‐oncology care is still limited world‐ and nationwide. Indeed, few cardiology fellowships offer formal training in cardio‐oncology 80 and institutions with cardio‐oncology services are clustered geographically in the Northeast and California. 80 Even in communities with access to cardio‐oncology providers, some patients may face local barriers to transportation that can impede regular access to specialist care. Among the general population in the United States, transportation barriers to medical care have been shown to have a disproportionately powerful influence on patients of lower SES and those with chronic conditions. 81 Ultimately, patients with cancer may find it difficult to access cardio‐oncology care because of inadequate insurance coverage, distance from cardio‐oncology providers, and barriers to transportation. The degree to which these barriers mediate disparities in cardiotoxicity screening and management must be explored further.
Risks of System‐Level Inequity in Cardio‐Oncology: Cost Differentials for Treatment With Reduced Cardiotoxicity
Among strategies for cardiotoxicity prevention, additional socioeconomic factors may contribute to cardiotoxicity disparities. Liposomal doxorubicin (used for multiple myeloma, Kaposi’s sarcoma, ovarian cancer, and breast cancer) has been associated with lower rates of reduced left ventricular ejection fraction and clinical heart failure 82 , 83 but is substantially more expensive than standard formulations. Continuous infusion of anthracycline therapy has been associated with lower risks of subclinical and clinical cardiotoxicity 84 but comes with elevated costs. The degree to which these cost differentials for less cardiotoxic therapies contribute to disparate cardiac outcomes among patients with cancer is not yet known.
Risks of System‐Level Inequity in Cardio‐Oncology: Inadequate Representation in Clinical Trials
We suspect that inadequate representation of diverse study participants in clinical studies on cardioprotective strategies for prevention of treatment‐associated cardiotoxicity may also contribute to outcome disparities. It is well‐established that racial and ethnic minority patients have been underrepresented in seminal clinical trials that resulted in Food and Drug Administration drug approvals for cancer therapeutics. 85 Cardioprotection studies have investigated neurohormonal agents (including angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers, and beta blockers), statins, exercise, and dexrazoxane. Only 21% of identified cohort studies and clinical trials on cardioprotective therapies provide either demographic information on the race and ethnicity of study participants or demographic information on participants’ socioeconomic status or other social determinants of health, though it does bear noting that some of the included studies were conducted in European countries with universal health care (Table S1). 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 In accordance with the National Institutes of Health Revitalization Act, 115 achieving diverse representation in clinical trials on cardioprotective therapies must be made a priority. Doing so will require direct targeting of known barriers to clinical trial enrollment (including patient trust, healthcare access, education, and communication) 116 as well as explorations of factors specific to cardioprotection studies.
Risks of System‐Level Inequity in Cardio‐Oncology: Patient Distrust
Additional investigation is needed on the impact of healthcare system distrust on cardio‐oncology care delivery. Many racial and ethnic minority populations in the United States have developed distrust of institutionalized healthcare settings, 117 influenced by the history of slavery, living legacy of oppression, and exploitation of Black and brown bodies in instances such as the Tuskegee syphilis experiment 118 and government‐sanctioned coerced sterilization of women of color, immigrants, and people with disabilities during the eugenics movement in the United States. 119 Medical distrust has been associated with decreased participation in clinical research and decreased use of general healthcare services as well as oncologic care. 120 , 121 , 122 The unstudied impact of medical distrust on participations in cardio‐oncology care is of great concern. Specifically, we must investigate how patient distrust shapes participation in preventative cardiotoxicity screening as well as treatment for detected cardiac dysfunction in the context of noxious medical and radiation treatments for cancer.
Disparities in Cardio‐Oncology
There are limited data on outcome disparities in treatment‐related cardiotoxicity. Our literature review identified 13 observational cohort studies and 2 systematic reviews with meta‐analyses. Most of the studies identified in our review addressed disparate outcomes according to race and ethnicity among patients with breast cancer. Few investigations have examined disparities in other types of cancer or among other underrepresented groups. The major findings on outcomes disparities in treatment‐associated cardiotoxicity are presented in the following sections and summarized visually (Figure 1).
Figure 1. Disparities in cardio‐oncology.
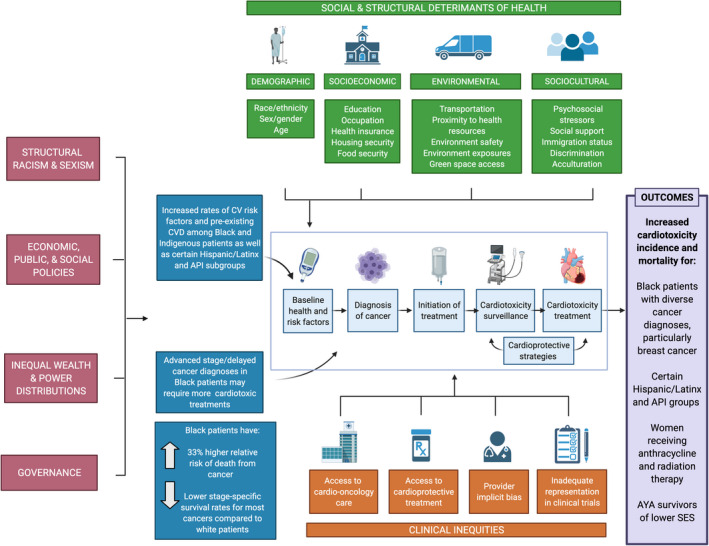
A visual summary of disparities in cardio‐oncology with a depiction of contributing etiologies. This figure demonstrates the influence of upstream factors (including structural racism and sexism) and intermediary factors (including social and structural determinants of health as well as clinical inequities in cardio‐oncology) on preexisting CVD and cancer disparities in the context of cardio‐oncologic care and summarizes the major outcome disparities in cardiotoxicity from studies presented in this review. API indicates Asian and Pacific Islander; CV, cardiovascular; CVD, cardiovascular disease; and SES, socioeconomic status. Created by biorender.com.
Race and Ethnicity Disparities Among Black Patients With Cancer and Asian and Pacific Islander Patients With Cancer
Most studies to date explore differences in cardiovascular outcomes between Black and White patients with breast cancer. Black women diagnosed with breast cancer are 40% more likely to die than their White counterparts. 123 Differences in cardiotoxicity are hypothesized to contribute to this disparity, particularly as Black women are more likely to have underlying CVD and more frequently have high‐risk cancer requiring cardiotoxic treatment. 124 Available research on this topic collectively supports this hypothesis (Table 2, 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 and summarized in Figure 1). Black patients with HER2+ breast cancer treated with trastuzumab are more likely to develop cardiotoxicity as defined by declines in left ventricular ejection fraction. 128 , 131 In a retrospective cohort study at Johns Hopkins University, this risk persisted even after controlling for age, disease state, and cardiovascular risk factors, 131 although it is not clear that the degree of risk factor control was evaluated. Importantly, the development of cardiotoxicity correlated strongly with treatment cessation. An earlier retrospective cohort study at Howard University also found that Black patients treated with doxorubicin were more likely than non‐Black patients to develop early cardiotoxicity, 125 although these patients were compared with non‐Black control subjects in a separate cohort study. A national multicenter prospective cohort study of patients with metastatic HER2+ breast cancer found that Black patients with diabetes or hypertension were more likely to experience cardiotoxicity after trastuzumab compared with White counterparts with the same conditions, although levels of statistical significance were not reported. 127 In a meta‐analysis of 18 studies across North America and Europe in which patients were treated with anthracycline therapy for a variety of cancers, Black race as well as cardiovascular risk factors were independent predictors of clinical and subclinical cardiotoxicity 134 ; this analysis is not shown in Table 2.
Table 2.
Cardiotoxicity Disparities by Race and Ethnicity Among Patients With Breast Cancer
| Author, setting, sample*, follow‐up | Diagnosis/treatment | Study design | Results | Conclusions |
|---|---|---|---|---|
|
Hasan, 2004 125 Howard University Black n=100 Non‐Black n=399 (from separate study) Median follow‐up: 1.3 y |
Breast cancer treated with doxorubicin | Retrospective cohort study measuring development of congestive heart failure or LVEF ≤45% | Higher incidence of cardiotoxicity among Black patients in registry in comparison to control population of non‐Black patients (7/100 in comparison to 10/399, P<0.027 with OR, 2.93; 95% CI, 0.98–8.6) | Black patients with breast cancer developed early cardiotoxicity after treatment with doxorubicin more frequently than non‐Black controls |
|
Braithwaite, 2009 126 Kaiser Permanente Northern California White n=838 Black n=416 Mean follow‐up: 9 y |
Invasive breast cancer, any treatment | Retrospective cohort study comparing overall crude mortality and breast cancer mortality | Higher all‐cause mortality in Black patients compared with White patients (165 [39.7%] vs 279 [33.3%], P=0.03). Hypertension was associated with lower all‐cause survival after controlling for race, age, tumor characteristics, and treatment (HR, 1.33; 95% CI, 1.07–1.67). Hypertension alone explained 30.3% of racial disparity in all‐cause survival | Hypertension is an independent predictor of the survival disparity between Black and White survivors of invasive breast cancer |
|
Rugo, 2013 127 Multicenter (United States) White n=793 Black n=126 Median follow‐up: 27 mo |
Metastatic HER2+ breast cancer treated with trastuzumab | Prospective cohort study measuring cardiac safety events as well as progression‐free and overall survival | Higher incidence† of cardiac safety events among Black patients with diabetes, hypertension, or CVD who were treated with trastuzumab in comparison to White patients with the same conditions: 3/15 (20%) Black vs 4/48 (8.3%) White patients with diabetes; 2/14 (14.2%) Black vs 2/29 (6.9%) White patients with hypertension; 5/36 (13.9%) Black/African American vs 12/117 (10.3%) White patients with any CVD | More Black patients with HER2+ breast cancer and cardiovascular risk factors (diabetes, hypertension) or CVD experienced cardiotoxicity following treatment with trastuzumab compared with White counterparts with the same conditions, although statistical significance was not reported |
|
Baron, 2014 128 University of Maryland Black n=48 White n=25 Hispanic/Latinx or API n=3 Follow‐up: every 3 mo up to 12 mo after treatment (mean not reported) |
Stage I to IV HER2+ breast cancer treated with trastuzumab | Retrospective cohort study measuring decline in LVEF over first year after treatment | More Black patients than White patients experienced decreased LVEF (17 vs 4 patients of total 21, P<0.05). No significant difference in cardiovascular risk factors or adjunctive treatment with anthracycline or radiation between patients with and without LVEF decline | Black patients with HER2+ breast cancer developed reduced ejection fraction following treatment with trastuzumab more frequently than White patients |
|
Berkman, 2014 129 Multicenter (United States), SEER White n=54 518 Other‡ n=6487 Black n=6113 Unknown n=401 Follow up: 1, 5, 10, 20 y post‐diagnosis (mean not reported) |
DCIS, any treatment | Retrospective cohort study comparing cardiovascular, breast cancer, and all‐cause mortalities | Higher hazard of cardiovascular death in Black survivors of breast cancer compared with White survivors breast cancer with DCIS at ages 40–49, 50–59, and 60–69 (HR, 14.99; 95% CI, 5.39–41.67; HR, 6.43; 95% CI, 3.61–11.46; HR, 2.26; 95% CI, 1.63–3.14). No significant difference in hazard of cardiovascular death between Black and White patients 70 y and older | Black survivors of DCIS had a higher risk of mortality from CVD, with a more pronounced effect in younger patients |
|
Solanki, 2016 130 Multicenter (United States), SEER White n=462 005 API n=44 531 Follow‐up: Through 2012 for diagnoses between 1991 and 2001 (mean not reported) |
Stage I to III invasive breast cancer, any treatment | Retrospective cohort study comparing cardiovascular, breast cancer, and all‐cause mortalities | Mortality rates varied by national origin. Filipino, Asian Indian and Pakistani, and Pacific Islander groups had a risk of cardiovascular mortality similar to White women. Hawaiian women had a higher risk of cardiovascular mortality (HR, 1.43; 95% CI, 1.17–1.75) compared with White women. US‐born API survivors of breast cancer had higher risk of cardiovascular mortality (HR, 1.29; 95% CI, 1.08–1.54) compared with immigrant API survivors of breast cancer | Despite reports of lower mortality risks among API patients in aggregate, risks of cardiovascular mortality among API survivors of breast cancer vary according to national origin and immigration status |
|
Litvak, 2018 131 Sidney Kimmel Cancer Center, Johns Hopkins White n=157 Black n=59 Median follow‐up: 5.2 y |
Stage I to III HER2+ breast cancer treated with trastuzumab | Retrospective cohort study measuring decline in LVEF and rates of incomplete therapy | Higher 1‐y probability of LVEF decline among Black women (24%, 95% CI, 12–34%) in comparison to White women (7%, 95% CI, 3–11%). Race was significant predictor of cardiotoxicity even after controlling for patient age, disease state, receipt of anthracycline, and presence of other cardiovascular risk factors (HR, 2.73; 95% CI, 1.24–6.01, P=0.012). Black patients had greater probability of incomplete therapy compared with White patients (OR, 4.61; 95% CI, 1.70–13.07; P=0.002). High correlation was observed between cardiotoxicity and incomplete therapy (96% concordance) | Black survivors of HER2+ breast cancer had higher risk of cardiotoxicity following treatment with trastuzumab even after controlling for age, disease state, and cardiovascular risk factors, with cardiotoxicity correlating with more frequent treatment cessation |
|
Troeschel, 2019 132 Multicenter (United States), SEER White n=364 025 Black n=43 562 Median follow‐up: 6.7 y |
Invasive breast cancer, any treatment | Retrospective cohort study comparing incidence and hazard of CVD mortality | 20‐y cumulative incidence of CVD mortality was higher in Black patients compared with White patients in groups age <69 (13.3% vs 8.9%). Hazard of CVD mortality was 173% higher in Black patients age <55 (HR, 2.73; 95% CI, 2.42–3.08) and 72% higher in age 55–68 compared with White patients (HR, 1.72; 95% CI, 1.60–1.85). Similar hazard detected among women 69 y and older | Black survivors of breast cancer had a higher risk of mortality from CVD, with a more pronounced effect in younger patients |
|
Collin, 2020 133 Georgia Cancer Registry White n=4923 Black n=3580 Follow‐up: Until 2018 for diagnoses between 2010 and 2014 (mean not reported) |
Invasive breast cancer, any treatment | Prospective cohort study comparing hazards of CVD mortality following treatment with hormone therapy or chemotherapy | Hormone therapy was associated with a nonsignificant higher hazard of CVD mortality among Black women (HR, 2.18; 95% CI, 0.78–6.12) but not in White women (HR, 0.66; 95% CI, 0.39–1.13). Chemotherapy was associated with a nonsignificant higher hazard among Black (HR, 1.45; 95% CI, 0.60–3.51) but not among White women (HR, 0.86; 95% CI, 0.40–1.88). Neither result reached statistical significance | Treatment with hormone therapy or chemotherapy may contribute to the CVD mortality disparities between Black and White survivors of breast cancer, but results in this study did not reach statistical significance |
API indicates Asian and Pacific Islander; CVD, cardiovascular disease; DCIS, ductal carcinoma in situ; HR, hazard ratio; LVEF, left ventricle ejection fraction; OR, odds ratio, and SEER, Surveillance, Epidemiology and End Results (national cancer registry).
*For brevity, patients described in the referenced studies as Black, African American, African‐American, or Non‐Hispanic Black are referred to as Black. Patients described as non‐Hispanic White in the referenced studies are referred to as White.
†Measures of statistical significance not reported.
‡Study authors included a category of “Other” but did not provide additional details about the participant races or ethnicities.
Mortality from CVD among Black patients with breast cancer is strikingly elevated (summarized in Figure 1). One of the largest studies on this topic used SEER (Surveillance, Epidemiology, and End Results) data to compare death from breast cancer and CVD retrospectively between 43 562 Black and 364 025 White patients with invasive breast cancer. 132 Note that these groups were characterized as non‐Hispanic Black and White patients. The results showed that the cumulative incidence of CVD‐related mortality was significantly higher among Black patients in comparison to White patients in age groups under 69, with cumulative incidences reported up to time points of 25 years. There was no difference in CVD‐related mortality between Black and White patients 69 years and older. After accounting for the influence of other causes of death on CVD‐related mortality, Black patients were observed to have 173% higher hazard of CVD‐related mortality for ages <55 years and 72% higher hazard of CVD‐related mortality for ages 55 to 68. This difference in mortality between White and Black patients with breast cancer is greater than the difference in CVD‐related mortality between Black and White patients in the general population. Similar results were obtained in a different retrospective cohort study using SEER data from patients with ductal carcinoma in situ. 129 This survival disparity may be due partially to differences in preexisting cardiovascular risk factors: in a regional retrospective cohort study among 1254 patients with invasive breast cancer, hypertension was an independent predictor of the survival disparity between Black American and White survivors. 126
Preliminary research suggests that cardiovascular risk among API patients with breast cancer varies by national origin and immigration status (included in Table 2 and summarized in Figure 1). A retrospective cohort study used SEER registry data to investigate mortality differences among 44 531 API patients with invasive breast cancer in comparison to 462 005 White counterparts. 130 Although API patients in aggregate had lower risks of CVD‐related mortality, breast cancer mortality, and all‐cause mortality, Hawaiian women had a higher hazard of cardiovascular mortality compared with White patients. Pacific Islander women had elevated risk for breast cancer‐specific and all‐cause mortalities in comparison to White women. The grouping of API patients together in clinical research may mask such underlying disparities, and groups at higher risk such as Hawaiian and Pacific Islander women may need additional screening. Interestingly, US‐born API survivors of breast cancer were found to have a higher hazard of cardiovascular mortality when compared with immigrant API survivors of breast cancer. The effects of acculturation and associated lifestyle changes likely contribute to the higher risk of CVD among API women born in the United States.
Socioeconomic Disparities Among Patients With Cancer
There are even fewer published studies on socioeconomic disparities in treatment‐associated cardiotoxicity, although preliminary data demonstrate the impact of health insurance status, poverty, and geographic region (summarized in Figure 1). Among adolescent and young adult (AYA) survivors of various types of cancer, Black race as well as lower SES have been associated independently with increased risks of CVD and CVD‐associated mortality. In a retrospective cohort study of 79 167 survivors of AYA cancer ages 15 to 39 years old, Black patients had an elevated risk of CVD in comparison to White patients (hazard ratio [HR], 1.55; 95% CI, 1.33–1.81). 135 Cancer types included breast cancer, thyroid cancer, melanoma, testicular cancer, Hodgkin lymphoma, non‐Hodgkin lymphoma, acute lymphoid leukemia, acute myeloid leukemia, soft tissue sarcoma, bone sarcoma, colorectal cancer, central nervous system cancer, cervical cancer, and ovarian cancer. Additionally, uninsured or publicly insured patients had an elevated risk of CVD in comparison to patients with private or military insurance (HR, 1.78; 95% CI, 1.61–1.96), and those who resided in lower SES neighborhoods had increased risks of CVD in comparison to patients living in the highest SES neighborhoods (HR, 1.66; 95% CI, 1.42–1.93). 135 An even larger retrospective cohort study from SEER registry data among 242 940 AYA women and 158 347 AYA men with a broad spectrum of primary malignancies found that increasingly severe degrees of poverty were associated with increased risks of CVD mortality even after adjusting for race and ethnicity (HR, 1.18; 95% CI, 1.01–1.38 for quartile 2; HR, 1.37; 95% CI, 1.18–1.60 for quartile 3; HR, 1.41; 95% CI, 1.17–1.70 for quartile 4 below poverty line for women; HR, 1.11; 95% CI, 0.96–1.29 for quartile 2; HR, 1.35; 95% CI, 1.16–1.57 for quartile 3; and HR, 1.31; 95% CI, 1.09–1.58 for quartile 4 below the poverty line for men). 136 Additionally, education below high school levels was associated with increased risks of CVD mortality among both AYA women and men with cancer even after adjusting for race and ethnicity. 136 Geographic region was also found to have a significant impact: AYA patients residing in the South had increased risks of CVD mortality in comparison to patients living in the Northeast (HR, 1.40; 95% CI, 1.15–1.71 for AYA women and HR, 1.42; 95% CI, 1.16–1.73 for AYA men); no other regions demonstrated significant differences. 136
Sex and Gender Disparities Among Patients With Cancer
There is a notable paucity of published studies on cardiotoxicity outcome disparities according to sex and gender (Table 3, 137 , 138 , 139 and summarized in Figure 1). A retrospective cohort study of 6493 survivors of various childhood cancers treated with anthracycline therapy found that risk factors for early cardiotoxicity included female sex (relative risk, 1.89; 95% CI, 1.28–2.78, P<0.01) and Black race and ethnicity (relative risk, 1.68; 95% CI, 1.06–2.66, P=0.03). 138 Notably, White patients made up 75% of this study. A smaller retrospective cohort study of pediatric survivors of cancer treated with doxorubicin showed that 45% of female patients developed depressed contractility compared with 12% of male patients. 137 This disparate cardiotoxicity incidence widened with higher cumulative doses of doxorubicin. Similarly, a recent meta‐analysis of 10 observational studies among patients receiving radiation for Hodgkin lymphoma discovered sex and gender disparities for cardiovascular mortality. 139 Women had an aggregate incidence of life‐threatening cardiovascular events and mortality almost 4 times higher than men. However, this study did not match male and female patients by radiation therapy dose, age of cancer diagnosis, or method of cardiotoxicity diagnosis. Additionally, none of the 3 mentioned studies presented data on preexisting CVD or risk factors, nor did they control for other covariates such as SES or healthcare access.
Table 3.
Cardiotoxicity Disparities by Sex/Gender Among Patients Treated With Anthracyclines or Radiation
| Author, setting, sample, follow‐up | Diagnosis/treatment | Study design | Results | Conclusions |
|---|---|---|---|---|
|
Lipshultz, 1995 137 Boston Female sex, n=62 Male sex, n=58 Mean time since therapy completion: 8.1 y |
Childhood acute lymphoblastic leukemia or osteogenic sarcoma treated with doxorubicin | Retrospective cohort study measuring cardiac stress velocity in comparison to data from 296 normal subjects | 45% of female patients (28 of 62) had depressed contractility more than 2 SDs below normal as compared with 12% of male patients (7 of 58; P<0.001). Interactive relation between sex and cumulative dose with the higher the cumulative dose, the greater the difference in contractility between female and male patients | Female patients were more likely to have depressed left ventricular contractility after doxorubicin than male patients, with more disparate frequencies observed with higher cumulative doses |
|
Krischer, 1997 138 Multicenter (United States) Pediatric patients n=6493 Mean follow‐up: not specified |
Leukemia, lymphoma (Hodgkin and non‐Hodgkin), sarcoma, neuroblastoma, brain tumors treated with anthracyclines | Retrospective cohort study measuring event‐free survival until cardiotoxic event | Increased risk of early cardiotoxicity among female pediatric patients (relative risk, 1.89; 95% CI, 1.28–2.78, P<0.01) in multivariate analysis. | Female patients treated with anthracyclines had a higher risk of early cardiotoxicity in comparison to male patients. |
|
Khalid, 2020 139 Multicenter (North America, Europe) |
Hodgkin lymphoma treated with radiation | Systematic review evaluating incidence of cardiovascular events and cardiovasculamortality among 10 observational studies | Higher aggregate incidence of cardiovascula events and cardiovasculamortality in women compared with men (OR, 3.74; 95% CI, 2.44–5.72, P<0.001) with higher all‐cause mortality in women compared with men (OR, 1.94; 95%, CI, 1.10–3.44, P<0.023) | Women with Hodgkin lymphoma who received radiation had high risk of cardiovascula events or mortality in comparison to men |
Strategies and Future Directions
We provide a visual summary of our findings on outcome disparities according to race and ethnicity, socioeconomic status, and sex and gender in treatment‐associated cardiotoxicity with a depiction of contributing etiologies (Figure 1). The studies reviewed here have prominent limitations—most notably, size and the lack of controlling for relevant covariates. Many of these studies did not account for variables such as disease stage, preexisting cardiovascular risk factors, or CVD. Most studies did not control for socioeconomic factors, geographic factors, or other social variables.
Further research is necessary to determine to what degree the observed disparities among Black women with breast cancer exist within other cancer types, as well as among other racial and ethnic groups including diverse API, Hispanic/Latinx, and AIAN communities. Additional research is needed to examine the interactions of individual and neighborhood SES with other social determinants of health as well as broader dynamics of structural racism within the context of CVD among patients with cancer. Further research is required to elucidate the conflicting difference in sex and gender disparities among women described here in comparison to prior literature that cites worsened outcomes in men. Additionally, future investigations on sex and gender disparities should be broadened to include other groups subject to sex‐ and gender‐based discrimination, including lesbian, gay, bisexual, transgender, queer (or questioning), asexual (or allied), and intersex patients.
The intersection of these different disparities is deeply worrisome for potential compounding effects on disparities in cardiotoxicity and extreme vulnerability to inequity. This should be explored further not only for Black women with cancer but also for Hispanic/Latinx and API women (with attention paid to acculturation effects) as well AIAN women. It is important to note that although AIAN communities have the highest risk for CVD in the general population, there are no available studies on cardiotoxicity among this population. In addition, younger women who receive oncologic therapy with elevated risk of atherosclerotic disease 70 should be considered in the context of the increasing rates of coronary artery disease among the general population of young women ages 35 to 44 years old. 12 Given that the culprit contributing variables to general cardiovascular disparities extend beyond socioeconomic factors alone and include other social determinants of health as well as upstream structural racism and sexism 10 , 13 , 43 , 55 (Figure 1), future research in cardio‐oncology must also investigate the relationship of these factors to cardiotoxicity outcomes.
Based on our review of the literature and current practices, we propose actionable strategies within clinical practice, investigative research, and community engagement to reduce disparities in cardio‐oncology (Figure 2). In addition to developing standardized protocols for cardiovascular screening, risk factor optimization, and cardiotoxicity treatment per American Society of Clinical Oncology and American Heart Association guidelines, we propose developing effective tools to assess patient social and structural vulnerabilities at the time of care establishment in order to identify patients in need of additional interdisciplinary resources. Leveraging telehealth services from the COVID‐19 era may improve healthcare access for some patients without immediate access to cardio‐oncology providers or with personal or structural circumstances that challenge regular care. However, creative and informed approaches will be needed for populations with limited resources, as preliminary research on telehealth use during the COVID‐19 pandemic demonstrates that patients of color, older patients, patients who do not speak English, and patients from disadvantaged backgrounds have participated less in telemedicine and virtual video visits. 140 , 141 Additionally, we recommend longitudinal implicit bias training for all healthcare workers to decrease the interference of implicit bias with equitable care and recommend peer review of practice patterns to reduce substandard or inappropriate treatment of under‐represented minorities and women.
Figure 2. Strategies to reduce inequity in cardio‐oncology.
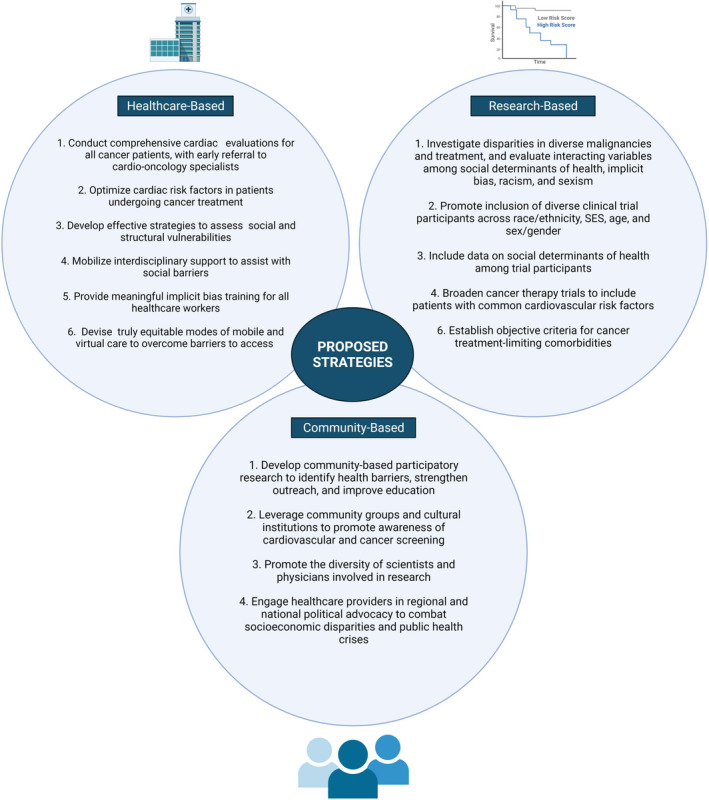
Strategies that can be taken by individual clinicians, scientists, and provider groups to reduce outcome disparities and improve cardiovascular and cancer‐associated health equity in the cardio‐oncology population. SES indicates socioeconomic status. Created by biorender.com.
Among research‐based strategies, we call for an expansion of health disparities research as discussed previously to the level of rigorous large‐scale studies investigating different types of disparities among diverse types of cancer and cardiotoxic treatment. These disparities should be evaluated in the context of comprehensive social determinants of health and forces of systemic discrimination. Encouragingly, the American Heart Association recently solicited research on cardio‐oncology disparities, with the aim to devise a disparities research network focused on collaborative basic, clinical, and population‐based investigations. 142 In addition to research expansion, we urge the robust recruitment of underrepresented groups to clinical trials for cancer therapeutics and cardioprotective agents, inclusive of patients with known cardiovascular comorbidities. In both health disparities studies and therapy‐based clinical trials, we recommend that data on social determinants of health be collected among all study participants. To inform these investigations, cultural and structural competency training should be pursued to improve clinician and scientist understanding of health determinants.
These strategies should be partnered with community initiatives to improve care access and patient outcomes. Initiatives to consider include community‐based participatory research to explore barriers to cardio‐oncologic care as well as community group collaborations to improve outreach to community members. Ongoing efforts should also be made to promote the diverse representation of clinicians and scientists involved in cardio‐oncology clinical care and research. Although addressing broader system‐level policies requires collaboration among many political, legal, and social groups, there is also a critical need for physician political advocacy in conversations on public health crises including poverty, housing insecurity, insufficient health insurance, and structural racism.
The general cardiologist has an important role in the collaborative work of reducing cardio‐oncology disparities. General cardiology practitioners are needed to help screen for CVD, aggressively manage cardiovascular risk factors, and refer early to cardio‐oncologists when possible. In areas where cardio‐oncology providers are not available, general cardiologists should assume the collaborative role with a patient’s oncology team. This requires diligent screening practices as well as an accurate understanding of which patients are most at risk for CVD and treatment‐associated cardiotoxicity. Individual cardiology and oncology providers are not able to single‐handedly change social or structural determinants of health, but they can commit to some of the clinical, research, and community strategies offered here to help reduce disparities.
Disparities in CVD and CVD‐related mortality will be an important area of study as the landscape of cancer treatment modalities continues to evolve. By further elucidating disparate cardiotoxicity outcomes among patients with cancer, we may improve our understanding of the relationship between cancer and cardiovascular disparities while guiding future research and equity‐focused care practices within cardio‐oncology. Eradicating cardio‐oncologic disparities among underresourced communities and minority groups including Black populations, who carry the brunt of both cancer mortality and cardiac disease, is a matter of compelling urgency—and ultimately, a matter of social justice. The improvement of cardiovascular health for all patients with cancer is critical for patient survival, quality of life, and health equity.
Sources of Funding
None.
Disclosures
Dr Yang receives research funding from CSL Behring (nonrelevant). The remaining authors have no disclosures to report.
Supporting information
Table S1
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023852
For Sources of Funding and Disclosures, see page 14.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 3. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics‐2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 4. Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69:363–385. doi: 10.3322/caac.21565 [DOI] [PubMed] [Google Scholar]
- 5. Sengupta R, Honey K. AACR Cancer Disparities Progress Report 2020: achieving the bold vision of health equity for racial and ethnic minorities and other underserved populations. Cancer Epidemiol Biomarkers Prev. 2020;29:1843. doi: 10.1158/1055-9965.EPI-20-0269 [DOI] [PubMed] [Google Scholar]
- 6. Alvidrez J, Castille D, Laude‐Sharp M, Rosario A, Tabor D. The National Institute on Minority Health and Health Disparities Research framework. Am J Public Health. 2019;109:S16–S20. doi: 10.2105/AJPH.2018.304883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J Clin Oncol. 2018;36:25–33. doi: 10.1200/JCO.2017.74.2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zavala VA, Bracci PM, Carethers JM, Carvajal‐Carmona L, Coggins NB, Cruz‐Correa MR, Davis M, de Smith AJ, Dutil J, Figueiredo JC, et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer. 2021;124:315–332. doi: 10.1038/s41416-020-01038-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA Jr, Willis M, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136:e393–e423. doi: 10.1161/CIR.0000000000000534 [DOI] [PubMed] [Google Scholar]
- 10. Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz‐Flores S, Davey‐Smith G, Dennison‐Himmelfarb CR, Lauer MS, Lockwood DW, et al. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132:873–898. doi: 10.1161/CIR.0000000000000228 [DOI] [PubMed] [Google Scholar]
- 11. Mensah GA, Cooper RS, Siega‐Riz AM, Cooper LA, Smith JD, Brown CH, Westfall JM, Ofili EO, Price LN, Arteaga S, et al. Reducing cardiovascular disparities through community‐engaged implementation research: a National Heart, Lung, and Blood Institute Workshop Report. Circ Res. 2018;122:213–230. doi: 10.1161/CIRCRESAHA.117.312243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mosca L, Barrett‐Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124:2145–2154. doi: 10.1161/CIRCULATIONAHA.110.968792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Churchwell K, Elkind MSV, Benjamin RM, Carson AP, Chang EK, Lawrence W, Mills A, Odom TM, Rodriguez CJ, Rodriguez F, et al. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation. 2020;142:e454–e468. doi: 10.1161/CIR.0000000000000936 [DOI] [PubMed] [Google Scholar]
- 14. Bailey ZD, Feldman JM, Bassett MT. How structural racism works—racist policies as a root cause of U.S. racial health inequities. N Engl J Med. 2021;384:768–773. doi: 10.1056/NEJMms2025396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Webb Hooper M, Napoles AM, Perez‐Stable EJ. COVID‐19 and racial/ethnic disparities. JAMA. 2020;323:2466–2467. doi: 10.1001/jama.2020.8598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bibbins‐Domingo K. This time must be different: disparities during the COVID‐19 pandemic. Ann Intern Med. 2020;173:233–234. doi: 10.7326/M20-2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Q, Berger NA, Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID‐19 infection. JAMA Oncol. 2021;7:220–227. doi: 10.1001/jamaoncol.2020.6178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Campia U, Moslehi JJ, Amiri‐Kordestani L, Barac A, Beckman JA, Chism DD, Cohen P, Groarke JD, Herrmann J, Reilly CM, et al. Cardio‐oncology: vascular and metabolic perspectives: a scientific statement from the American Heart Association. Circulation. 2019;139:e579–e602. doi: 10.1161/CIR.0000000000000641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blaes AH, Thavendiranathan P, Moslehi J. Cardiac toxicities in the era of precision medicine: underlying risk factors, targeted therapies, and cardiac biomarkers. Am Soc Clin Oncol Educ Book. 2018;38:764–774. doi: 10.1200/EDBK_208509 [DOI] [PubMed] [Google Scholar]
- 20. Blaes A, Prizment A, Koene RJ, Konety S. Cardio‐oncology related to heart failure: common risk factors between cancer and cardiovascular disease. Heart Fail Clin. 2017;13:367–380. doi: 10.1016/j.hfc.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Haas EC, Oosting SF, Lefrandt JD, Wolffenbuttel BH, Sleijfer DT, Gietema JA. The metabolic syndrome in cancer survivors. Lancet Oncol. 2010;11:193–203. doi: 10.1016/S1470-2045(09)70287-6 [DOI] [PubMed] [Google Scholar]
- 22. Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM. Cardiotoxicity of anticancer drugs: the need for cardio‐oncology and cardio‐oncological prevention. J Natl Cancer Inst. 2010;102:14–25. doi: 10.1093/jnci/djp440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–2247. doi: 10.1016/j.jacc.2009.02.050 [DOI] [PubMed] [Google Scholar]
- 24. Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13:R64. doi: 10.1186/bcr2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, Dent S, Douglas PS, Durand J‐B, Ewer M, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400 [DOI] [PubMed] [Google Scholar]
- 26. Armenian SH, Sun CL, Shannon T, Mills G, Francisco L, Venkataraman K, Wong FL, Forman SJ, Bhatia S. Incidence and predictors of congestive heart failure after autologous hematopoietic cell transplantation. Blood. 2011;118:6023–6029. doi: 10.1182/blood-2011-06-358226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Romond EH, Jeong J‐H, Rastogi P, Swain SM, Geyer CE Jr, Ewer MS, Rathi V, Fehrenbacher L, Brufsky A, Azar CA, et al. Seven‐year follow‐up assessment of cardiac function in NSABP B‐31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node‐positive, human epidermal growth factor receptor 2‐positive breast cancer. J Clin Oncol. 2012;30:3792–3799. doi: 10.1200/JCO.2011.40.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pinder MC, Duan Z, Goodwin JS, Hortobagyi GN, Giordano SH. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. 2007;25:3808–3815. doi: 10.1200/JCO.2006.10.4976 [DOI] [PubMed] [Google Scholar]
- 29. Advani PP, Ballman KV, Dockter TJ, Colon‐Otero G, Perez EA. Long‐term cardiac safety analysis of NCCTG N9831 (Alliance) adjuvant trastuzumab trial. J Clin Oncol. 2016;34:581–587. doi: 10.1200/JCO.2015.61.8413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prasad P, Branch M, Asemota D, Elsayed R, Addison D, Brown S‐A. Cardio‐oncology preventive care: racial and ethnic disparities. Curr Cardiovasc Risk Rep. 2020;14:18. doi: 10.1007/s12170-020-00650-8 [DOI] [Google Scholar]
- 31. Fazal M, Malisa J, Rhee J‐W, Witteles RM, Rodriguez F. Racial and ethnic disparities in cardio‐oncology. JACC CardioOncol. 2021;3:201–204. doi: 10.1016/j.jaccao.2021.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950–2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, Thun M. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78 [DOI] [PubMed] [Google Scholar]
- 34. Singh GK, Miller BA, Hankey BF, Edwards BK. Area Socioeconomic Variations in U.S. Cancer Incidence, Mortality, Stage, Treatment, and Survival, 1975–1999. Bethesda, Maryland: National Cancer Institute; 2003. [Google Scholar]
- 35. Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–357. doi: 10.1093/jnci/94.5.334 [DOI] [PubMed] [Google Scholar]
- 36. Chabner BA. Racism and cancer care: a call for recognition and reform. Oncologist. 2020;25:729. doi: 10.1634/theoncologist.2020-0752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Speaking up against inequity and racism. Nat Cancer. 2020;1(6):563–564. doi: 10.1038/s43018-020-0091-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mauvais‐Jarvis F, Bairey Merz N, Barnes PJ, Brinton RD, Carrero J‐J, DeMeo DL, De Vries GJ, Epperson CN, Govindan R, Klein SL, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet. 2020;396:565–582. doi: 10.1016/S0140-6736(20)31561-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rodriguez CJ, Allison M, Daviglus ML, Isasi CR, Keller C, Leira EC, Palaniappan L, Piña IL, Ramirez SM, Rodriguez B, et al. Status of cardiovascular disease and stroke in Hispanics/Latinos in the United States: a science advisory from the American Heart Association. Circulation. 2014;130:593–625. doi: 10.1161/CIR.0000000000000071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Palaniappan LP, Araneta MRG, Assimes TL, Barrett‐Connor EL, Carnethon MR, Criqui MH, Fung GL, Narayan KMV, Patel H, Taylor‐Piliae RE, et al. Call to action: cardiovascular disease in Asian Americans: a science advisory from the American Heart Association. Circulation. 2010;122:1242–1252. doi: 10.1161/CIR.0b013e3181f22af4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Breathett K, Sims M, Gross M, Jackson EA, Jones EJ, Navas‐Acien A, Taylor H, Thomas KL, Howard BV; American Heart Association Council on E . Cardiovascular health in American Indians and Alaska Natives: a scientific statement from the American Heart Association. Circulation. 2020;141:e948–e959. doi: 10.1161/CIR.0000000000000773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sims M, Kershaw KN, Breathett K, Jackson EA, Lewis LM, Mujahid MS, Suglia SF; American Heart Association Council on E, Prevention, Council on Quality of C . Importance of housing and cardiovascular health and well‐being: a scientific statement from the American Heart Association. Circ Cardiovasc Qual Outcomes. 2020;13:e000089. doi: 10.1161/HCQ.0000000000000089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Diaz CL, Shah NS, Lloyd‐Jones DM, Khan SS. State of the nation's cardiovascular health and targeting health equity in the United States: a narrative review. JAMA Cardiol. 2021;6:963. doi: 10.1001/jamacardio.2021.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mensah GA, Goff DC, Gibbons GH. Cardiovascular mortality differences‐place matters. JAMA. 2017;317:1955–1957. doi: 10.1001/jama.2017.4168 [DOI] [PubMed] [Google Scholar]
- 45. Mujahid MS, Diez Roux AV, Shen M, Gowda D, Sanchez B, Shea S, Jacobs DR Jr, Jackson SA. Relation between neighborhood environments and obesity in the Multi‐Ethnic Study of Atherosclerosis. Am J Epidemiol. 2008;167:1349–1357. doi: 10.1093/aje/kwn047 [DOI] [PubMed] [Google Scholar]
- 46. Williams DR, Lawrence JA, Davis BA. Racism and health: evidence and needed research. Annu Rev Public Health. 2019;40:105–125. doi: 10.1146/annurev-publhealth-040218-043750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Phelan JC, Link BG. Is racism a fundamental cause of inequalities in health? Annu Rev Sociol. 2015;41:311–330. doi: 10.1146/annurev-soc-073014-112305 [DOI] [Google Scholar]
- 48. Hardeman RR, Murphy KA, Karbeah J, Kozhimannil KB. Naming Institutionalized racism in the public health literature: a systematic literature review. Public Health Rep. 2018;133:240–249. doi: 10.1177/0033354918760574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schulman KA, Berlin JA, Harless W, Kerner JF, Sistrunk S, Gersh BJ, Dubé R, Taleghani CK, Burke JE, Williams S, et al. The effect of race and sex on physicians' recommendations for cardiac catheterization. N Engl J Med. 1999;340:618–626. doi: 10.1056/NEJM199902253400806 [DOI] [PubMed] [Google Scholar]
- 50. McNally B, Robb R, Mehta M, Vellano K, Valderrama AL, Yoon PW, Sasson C, Crouch A, Perez AB, Merritt R, et al. Out‐of‐hospital cardiac arrest surveillance—cardiac arrest registry to enhance survival (CARES), United States, October 1, 2005–December 31, 2010. MMWR Surveill Summ. 2011;60:1–19. [PubMed] [Google Scholar]
- 51. Campbell MC, Tishkoff SA. African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. Annu Rev Genomics Hum Genet. 2008;9:403–433. doi: 10.1146/annurev.genom.9.081307.164258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tishkoff SA, Reed FA, Friedlaender FR, Ehret C, Ranciaro A, Froment A, Hirbo JB, Awomoyi AA, Bodo J‐M, Doumbo O, et al. The genetic structure and history of Africans and African Americans. Science. 2009;324:1035–1044. doi: 10.1126/science.1172257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Daviglus ML, Talavera GA, Avilés‐Santa ML, Allison M, Cai J, Criqui MH, Gellman M, Giachello AL, Gouskova N, Kaplan RC, et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308:1775–1784. doi: 10.1001/jama.2012.14517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Howard BV, Lee ET, Cowan LD, Devereux RB, Galloway JM, Go OT, Howard WJ, Rhoades ER, Robbins DC, Sievers ML, et al. Rising tide of cardiovascular disease in American Indians. The Strong Heart Study. Circulation. 1999;99:2389–2395. doi: 10.1161/01.CIR.99.18.2389 [DOI] [PubMed] [Google Scholar]
- 55. Molix L. Sex differences in cardiovascular health: does sexism influence women's health? Am J Med Sci. 2014;348:153–155. doi: 10.1097/MAJ.0000000000000300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jarvie JL, Foody JM. Recognizing and improving health care disparities in the prevention of cardiovascular disease in women. Curr Cardiol Rep. 2010;12:488–496. doi: 10.1007/s11886-010-0135-4 [DOI] [PubMed] [Google Scholar]
- 57. Redfors B. Women are less likely to get secondary prevention medications and cardiac rehabilitation. Expert Analysis, American College of Cardiology. Available at: https://www.acc.org/latest‐in‐cardiology/articles/2017/10/30/15/02/women‐are‐less‐likely‐to‐get‐secondary‐prevention‐medications‐and‐cr. Accessed March 31, 2021.
- 58. Daugherty SL, Blair IV, Havranek EP, Furniss A, Dickinson LM, Karimkhani E, Main DS, Masoudi FA. Implicit gender bias and the use of cardiovascular tests among cardiologists. J Am Heart Assoc. 2017;6:e006872. doi: 10.1161/JAHA.117.006872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lenneman CG, Sawyer DB. Cardio‐oncology: an update on cardiotoxicity of cancer‐related treatment. Circ Res. 2016;118:1008–1020. doi: 10.1161/CIRCRESAHA.115.303633 [DOI] [PubMed] [Google Scholar]
- 60. Babiker HM, McBride A, Newton M, Boehmer LM, Drucker AG, Gowan M, Cassagnol M, Camenisch TD, Anwer F, Hollands JM. Cardiotoxic effects of chemotherapy: a review of both cytotoxic and molecular targeted oncology therapies and their effect on the cardiovascular system. Crit Rev Oncol Hematol. 2018;126:186–200. doi: 10.1016/j.critrevonc.2018.03.014 [DOI] [PubMed] [Google Scholar]
- 61. Shah CP, Moreb JS. Cardiotoxicity due to targeted anticancer agents: a growing challenge. Ther Adv Cardiovasc Dis. 2019;13:1753944719843435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D, Cipolla CM. Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J Clin. 2016;66:309–325. doi: 10.3322/caac.21341 [DOI] [PubMed] [Google Scholar]
- 63. Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf. 2000;22:263–302. doi: 10.2165/00002018-200022040-00002 [DOI] [PubMed] [Google Scholar]
- 64. Trent JC, Patel SS, Zhang J, Araujo DM, Plana JC, Lenihan DJ, Fan D, Patel SR, Benjamin RS, Khakoo AY. Rare incidence of congestive heart failure in gastrointestinal stromal tumor and other sarcoma patients receiving imatinib mesylate. Cancer. 2010;116:184–192. doi: 10.1002/cncr.24683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Adams MJ, Hardenbergh PH, Constine LS, Lipshultz SE. Radiation‐associated cardiovascular disease. Crit Rev Oncol Hematol. 2003;45:55–75. doi: 10.1016/S1040-8428(01)00227-X [DOI] [PubMed] [Google Scholar]
- 66. Raghunathan D, Khilji MI, Hassan SA, Yusuf SW. Radiation‐induced cardiovascular disease. Curr Atheroscler Rep. 2017;19:22. doi: 10.1007/s11883-017-0658-x [DOI] [PubMed] [Google Scholar]
- 67. Upadhrasta S, Elias H, Patel K, Zheng L. Managing cardiotoxicity associated with immune checkpoint inhibitors. Chronic Dis Transl Med. 2019;5:6–14. doi: 10.1016/j.cdtm.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jamal FA, Khaled SK. The cardiovascular complications of chimeric antigen receptor T cell therapy. Curr Hematol Malig Rep. 2020;15:130–132. doi: 10.1007/s11899-020-00567-4 [DOI] [PubMed] [Google Scholar]
- 69. Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med. 2016;375:1457–1467. doi: 10.1056/NEJMra1100265 [DOI] [PubMed] [Google Scholar]
- 70. Herrmann J. Vascular toxic effects of cancer therapies. Nat Rev Cardiol. 2020;17:503–522. doi: 10.1038/s41569-020-0347-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Herrmann J, Yang EH, Iliescu CA, Cilingiroglu M, Charitakis K, Hakeem A, Toutouzas K, Leesar MA, Grines CL, Marmagkiolis K. Vascular toxicities of cancer therapies: the old and the new–an evolving avenue. Circulation. 2016;133:1272–1289. doi: 10.1161/CIRCULATIONAHA.115.018347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol. 2020;17:474–502. doi: 10.1038/s41569-020-0348-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, Criscitiello C, Goldhirsch A, Cipolla C, Roila F, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23(suppl 7):vii155–vii166. doi: 10.1093/annonc/mds293 [DOI] [PubMed] [Google Scholar]
- 74. Mehta LS, Watson KE, Barac A, Beckie TM, Bittner V, Cruz‐Flores S, Dent S, Kondapalli L, Ky B, Okwuosa T, et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation. 2018;137:e30–e66. doi: 10.1161/CIR.0000000000000556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Armenian SH, Xu L, Ky B, Sun C, Farol LT, Pal SK, Douglas PS, Bhatia S, Chao C. Cardiovascular disease among survivors of adult‐onset cancer: a community‐based retrospective cohort study. J Clin Oncol. 2016;34:1122–1130. doi: 10.1200/JCO.2015.64.0409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Meijers WC, de Boer RA. Common risk factors for heart failure and cancer. Cardiovasc Res. 2019;115:844–853. doi: 10.1093/cvr/cvz035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bertero E, Ameri P, Maack C. Bidirectional relationship between cancer and heart failure: old and new issues in cardio‐oncology. Card Fail Rev. 2019;5:106–111. doi: 10.15420/cfr.2019.1.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Angier HE, Marino M, Springer RJ, Schmidt TD, Huguet N, DeVoe JE. The Affordable Care Act improved health insurance coverage and cardiovascular‐related screening rates for cancer survivors seen in community health centers. Cancer. 2020;126:3303–3311. doi: 10.1002/cncr.32900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Alvarez‐Cardona JA, Ray J, Carver J, Zaha V, Cheng R, Yang E, Mitchell JD, Stockerl‐Goldstein K, Kondapalli L, Dent S, et al. Cardio‐oncology education and training: JACC council perspectives. J Am Coll Cardiol. 2020;76:2267–2281. doi: 10.1016/j.jacc.2020.08.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hayek SS, Ganatra S, Lenneman C, Scherrer‐Crosbie M, Leja M, Lenihan DJ, Yang E, Ryan TD, Liu J, Carver J, et al. Preparing the cardiovascular workforce to care for oncology patients: JACC review topic of the week. J Am Coll Cardiol. 2019;73:2226–2235. doi: 10.1016/j.jacc.2019.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wolfe MK, McDonald NC, Holmes GM. Transportation barriers to health care in the United States: findings from the National Health Interview Survey, 1997–2017. Am J Public Health. 2020;110:815–822. doi: 10.2105/AJPH.2020.305579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Harris L, Batist G, Belt R, Rovira D, Navari R, Azarnia N, Welles L, Winer E; Group TDS . Liposome‐encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first‐line therapy of metastatic breast carcinoma. Cancer. 2002;94:25–36. doi: 10.1002/cncr.10201 [DOI] [PubMed] [Google Scholar]
- 83. van Dalen EC, Michiels EM, Caron HN, Kremer LC. Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database Syst Rev. 2010;5:CD005006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Smith LA, Cornelius VR, Plummer CJ, Levitt G, Verrill M, Canney P, Jones A. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta‐analysis of randomised controlled trials. BMC Cancer. 2010;10:337. doi: 10.1186/1471-2407-10-337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Loree JM, Anand S, Dasari A, Unger JM, Gothwal A, Ellis LM, Varadhachary G, Kopetz S, Overman MJ, Raghav K. Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncol. 2019;5:e191870. doi: 10.1001/jamaoncol.2019.1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kirkham AA, Eves ND, Shave RE, Bland KA, Bovard J, Gelmon KA, Virani SA, McKenzie DC, Stohr EJ, Waburton DER, et al. The effect of an aerobic exercise bout 24 h prior to each doxorubicin treatment for breast cancer on markers of cardiotoxicity and treatment symptoms: a RCT. Breast Cancer Res Treat. 2018;167:719–729. [DOI] [PubMed] [Google Scholar]
- 87. Nakamae H, Tsumura K, Terada Y, Nakane T, Nakamae M, Ohta K, Yamane T, Hino M. Notable effects of angiotensin II receptor blocker, valsartan, on acute cardiotoxic changes after standard chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisolone. Cancer. 2005;104:2492–2498. doi: 10.1002/cncr.21478 [DOI] [PubMed] [Google Scholar]
- 88. Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, Rubino M, Veglia F, Fiorentini C, Cipolla CM. Anthracycline‐induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55:213–220. doi: 10.1016/j.jacc.2009.03.095 [DOI] [PubMed] [Google Scholar]
- 89. Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, Martinelli G, Veglia F, Fiorentini C, Cipolla CM. Prevention of high‐dose chemotherapy‐induced cardiotoxicity in high‐risk patients by angiotensin‐converting enzyme inhibition. Circulation. 2006;114:2474–2481. doi: 10.1161/CIRCULATIONAHA.106.635144 [DOI] [PubMed] [Google Scholar]
- 90. Georgakopoulos P, Roussou P, Matsakas E, Karavidas A, Anagnostopoulos N, Marinakis T, Galanopoulos A, Georgiakodis F, Zimeras S, Kyriakidis M, et al. Cardioprotective effect of metoprolol and enalapril in doxorubicin‐treated lymphoma patients: a prospective, parallel‐group, randomized, controlled study with 36‐month follow‐up. Am J Hematol. 2010;85:894–896. doi: 10.1002/ajh.21840 [DOI] [PubMed] [Google Scholar]
- 91. Bosch X, Rovira M, Sitges M, Domenech A, Ortiz‐Perez JT, de Caralt TM, Morales‐Ruiz M, Perea RJ, Monzo M, Esteve J. Enalapril and carvedilol for preventing chemotherapy‐induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J Am Coll Cardiol. 2013;61:2355–2362. doi: 10.1016/j.jacc.2013.02.072 [DOI] [PubMed] [Google Scholar]
- 92. Kaya MG, Ozkan M, Gunebakmaz O, Akkaya H, Kaya EG, Akpek M, Kalay N, Dikilitas M, Yarlioglues M, Karaca H, et al. Protective effects of nebivolol against anthracycline‐induced cardiomyopathy: a randomized control study. Int J Cardiol. 2013;167:2306–2310. doi: 10.1016/j.ijcard.2012.06.023 [DOI] [PubMed] [Google Scholar]
- 93. Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz‐Menger J, Fagerland MW, Gravdehaug B, von Knobelsdorff‐Brenkenhoff F, Bratland A, Storas TH, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 x 2 factorial, randomized, placebo‐controlled, double‐blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37:1671–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kalay N, Basar E, Ozdogru I, Er O, Cetinkaya Y, Dogan A, Oguzhan A, Eryol NK, Topsakal R, Ergin A, et al. Protective effects of carvedilol against anthracycline‐induced cardiomyopathy. J Am Coll Cardiol. 2006;48:2258–2262. doi: 10.1016/j.jacc.2006.07.052 [DOI] [PubMed] [Google Scholar]
- 95. Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777 [DOI] [PubMed] [Google Scholar]
- 96. Pituskin E, Mackey JR, Koshman S, Jassal D, Pitz M, Haykowsky MJ, Pagano JJ, Chow K, Thompson RB, Vos LJ, et al. Multidisciplinary approach to novel therapies in cardio‐oncology research (MANTICORE 101‐Breast): a randomized trial for the prevention of trastuzumab‐associated cardiotoxicity. J Clin Oncol. 2017;35:870–877. doi: 10.1200/JCO.2016.68.7830 [DOI] [PubMed] [Google Scholar]
- 97. Avila MS, Ayub‐Ferreira SM, de Barros Wanderley MR Jr, das Dores Cruz F, Gonçalves Brandão SM, Rigaud VOC, Higuchi‐dos‐Santos MH, Hajjar LA, Kalil Filho R, Hoff PM, et al. Carvedilol for prevention of chemotherapy‐related cardiotoxicity: the CECCY trial. J Am Coll Cardiol. 2018;71:2281–2290. doi: 10.1016/j.jacc.2018.02.049 [DOI] [PubMed] [Google Scholar]
- 98. Cardinale D, Ciceri F, Latini R, Franzosi MG, Sandri MT, Civelli M, Cucchi G, Menatti E, Mangiavacchi M, Cavina R, et al. Anthracycline‐induced cardiotoxicity: a multicenter randomised trial comparing two strategies for guiding prevention with enalapril: the International CardioOncology Society‐one trial. Eur J Cancer. 2018;94:126–137. [DOI] [PubMed] [Google Scholar]
- 99. Guglin M, Krischer J, Tamura R, Fink A, Bello‐Matricaria L, McCaskill‐Stevens W, Munster PN. Randomized trial of lisinopril versus carvedilol to prevent trastuzumab cardiotoxicity in patients with breast cancer. J Am Coll Cardiol. 2019;73:2859–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Acar Z, Kale A, Turgut M, Demircan S, Durna K, Demir S, Meric M, Agac MT. Efficiency of atorvastatin in the protection of anthracycline‐induced cardiomyopathy. J Am Coll Cardiol. 2011;58:988–989. doi: 10.1016/j.jacc.2011.05.025 [DOI] [PubMed] [Google Scholar]
- 101. Seicean S, Seicean A, Plana JC, Budd GT, Marwick TH. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: an observational clinical cohort study. J Am Coll Cardiol. 2012;60:2384–2390. doi: 10.1016/j.jacc.2012.07.067 [DOI] [PubMed] [Google Scholar]
- 102. Calvillo‐Arguelles O, Abdel‐Qadir H, Michalowska M, Billia F, Suntheralingam S, Amir E, Thavendiranathan P. Cardioprotective effect of statins in patients with HER2‐positive breast cancer receiving trastuzumab therapy. Can J Cardiol. 2019;35:153–159. [DOI] [PubMed] [Google Scholar]
- 103. Speyer JL, Green MD, Zeleniuch‐Jacquotte A, Wernz JC, Rey M, Sanger J, Kramer E, Ferrans V, Hochster H, Meyers M, et al. ICRF‐187 permits longer treatment with doxorubicin in women with breast cancer. J Clin Oncol. 1992;10:117–127. doi: 10.1200/JCO.1992.10.1.117 [DOI] [PubMed] [Google Scholar]
- 104. Venturini M, Michelotti A, Del Mastro L, Gallo L, Carnino F, Garrone O, Tibaldi C, Molea N, Bellina RC, Pronzato P, et al. Multicenter randomized controlled clinical trial to evaluate cardioprotection of dexrazoxane versus no cardioprotection in women receiving epirubicin chemotherapy for advanced breast cancer. J Clin Oncol. 1996;14:3112–3120. doi: 10.1200/JCO.1996.14.12.3112 [DOI] [PubMed] [Google Scholar]
- 105. Wexler LH, Andrich MP, Venzon D, Berg SL, Weaver‐McClure L, Chen CC, Dilsizian V, Avila N, Jarosinski P, Balis FM, et al. Randomized trial of the cardioprotective agent ICRF‐187 in pediatric sarcoma patients treated with doxorubicin. J Clin Oncol. 1996;14:362–372. doi: 10.1200/JCO.1996.14.2.362 [DOI] [PubMed] [Google Scholar]
- 106. Swain SM, Whaley FS, Gerber MC, Weisberg S, York M, Spicer D, Jones SE, Wadler S, Desai A, Vogel C, et al. Cardioprotection with dexrazoxane for doxorubicin‐containing therapy in advanced breast cancer. J Clin Oncol. 1997;15:1318–1332. doi: 10.1200/JCO.1997.15.4.1318 [DOI] [PubMed] [Google Scholar]
- 107. Lopez M, Vici P, Di Lauro K, Conti F, Paoletti G, Ferraironi A, Sciuto R, Giannarelli D, Maini CL. Randomized prospective clinical trial of high‐dose epirubicin and dexrazoxane in patients with advanced breast cancer and soft tissue sarcomas. J Clin Oncol. 1998;16:86–92. doi: 10.1200/JCO.1998.16.1.86 [DOI] [PubMed] [Google Scholar]
- 108. Lipshultz SE, Rifai N, Dalton VM, Levy DE, Silverman LB, Lipsitz SR, Colan SD, Asselin BL, Barr RD, Clavell LA, et al. The effect of dexrazoxane on myocardial injury in doxorubicin‐treated children with acute lymphoblastic leukemia. N Engl J Med. 2004;351:145–153. doi: 10.1056/NEJMoa035153 [DOI] [PubMed] [Google Scholar]
- 109. Galetta F, Franzoni F, Cervetti G, Cecconi N, Carpi A, Petrini M, Santoro G. Effect of epirubicin‐based chemotherapy and dexrazoxane supplementation on QT dispersion in non‐Hodgkin lymphoma patients. Biomed Pharmacother. 2005;59:541–544. [DOI] [PubMed] [Google Scholar]
- 110. Marty M, Espie M, Llombart A, Monnier A, Rapoport BL, Stahalova V; Dexrazoxane Study G . Multicenter randomized phase III study of the cardioprotective effect of dexrazoxane (Cardioxane) in advanced/metastatic breast cancer patients treated with anthracycline‐based chemotherapy. Ann Oncol. 2006;17:614–622. doi: 10.1093/annonc/mdj134 [DOI] [PubMed] [Google Scholar]
- 111. Sun F, Qi X, Geng C, Li X. Dexrazoxane protects breast cancer patients with diabetes from chemotherapy‐induced cardiotoxicity. Am J Med Sci. 2015;349:406–412. doi: 10.1097/MAJ.0000000000000432 [DOI] [PubMed] [Google Scholar]
- 112. Tahover E, Segal A, Isacson R, Rosengarten O, Grenader T, Gips M, Cherny N, Heching NI, Mesika L, Catane R, et al. Dexrazoxane added to doxorubicin‐based adjuvant chemotherapy of breast cancer: a retrospective cohort study with a comparative analysis of toxicity and survival. Anticancer Drugs. 2017;28:787–794. doi: 10.1097/CAD.0000000000000514 [DOI] [PubMed] [Google Scholar]
- 113. Kim IH, Lee JE, Youn HJ, Song BJ, Chae BJ. cardioprotective effect of dexrazoxane in patients with HER2‐positive breast cancer who receive anthracycline based adjuvant chemotherapy followed by trastuzumab. J Breast Cancer. 2017;20:82–90. doi: 10.4048/jbc.2017.20.1.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ganatra S, Nohria A, Shah S, Groarke JD, Sharma A, Venesy D, Patten R, Gunturu K, Zarwan C, Neilan TG, et al. Upfront dexrazoxane for the reduction of anthracycline‐induced cardiotoxicity in adults with preexisting cardiomyopathy and cancer: a consecutive case series. Cardiooncology. 2019;5:1. doi: 10.1186/s40959-019-0036-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. United States. Congress. House. Committee on Energy and Commerce. Subcommittee on Health and the Environment . NIH Revitalization Act: hearing before the Subcommittee on Health and the Environment of the Committee on Energy and Commerce, House of Representatives, One Hundred Third Congress, first session, on H.R. 4, a bill to amend the Public Health Service Act. Washington: U.S. G.P.O.: For sale by the U.S. G.P.O., Supt. of Docs., Congressional Sales Office; 1993. [Google Scholar]
- 116. Woods‐Burnham L, Johnson JR, Hooker SE Jr, Bedell FW, Dorff TB, Kittles RA. The role of diverse populations in US clinical trials. Med. 2021;2:21–24. doi: 10.1016/j.medj.2020.12.009 [DOI] [PubMed] [Google Scholar]
- 117. Jaiswal J, Halkitis PN. Towards a more inclusive and dynamic understanding of medical mistrust informed by science. Behav Med. 2019;45:79–85. doi: 10.1080/08964289.2019.1619511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Thomas SB, Quinn SC. The Tuskegee Syphilis Study, 1932 to 1972: implications for HIV education and AIDS risk education programs in the black community. Am J Public Health. 1991;81:1498–1505. doi: 10.2105/AJPH.81.11.1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Reilly PR. Eugenics and involuntary sterilization: 1907–2015. Annu Rev Genomics Hum Genet. 2015;16(1):351–368. doi: 10.1146/annurev-genom-090314-024930 [DOI] [PubMed] [Google Scholar]
- 120. LaVeist TA, Isaac LA, Williams KP. Mistrust of health care organizations is associated with underutilization of health services. Health Serv Res. 2009;44:2093–2105. doi: 10.1111/j.1475-6773.2009.01017.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Mouslim MC, Johnson RM, Dean LT. Healthcare system distrust and the breast cancer continuum of care. Breast Cancer Res Treat. 2020;180:33–44. doi: 10.1007/s10549-020-05538-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Dean LT, Moss SL, McCarthy AM, Armstrong K. Healthcare system distrust, physician trust, and patient discordance with adjuvant breast cancer treatment recommendations. Cancer Epidemiol Biomarkers Prev. 2017;26:1745–1752. doi: 10.1158/1055-9965.EPI-17-0479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto AB, Lewis DR, et al. SEER cancer statistics review, 1975–2017. Bethesda, Maryland: National Cancer Institute. Available at: https://seer.cancer.gov/csr/1975_2017/. Based on November 2019 SEER data submission. Posted to the SEER web site, April 2020. [Google Scholar]
- 124. Agurs‐Collins T, Dunn BK, Browne D, Johnson KA, Lubet R. Epidemiology of health disparities in relation to the biology of estrogen receptor‐negative breast cancer. Semin Oncol. 2010;37:384–401. doi: 10.1053/j.seminoncol.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 125. Hasan S, Dinh K, Lombardo F, Kark J. Doxorubicin cardiotoxicity in African Americans. J Natl Med Assoc. 2004;96:196–199. [PMC free article] [PubMed] [Google Scholar]
- 126. Braithwaite D, Tammemagi CM, Moore DH, Ozanne EM, Hiatt RA, Belkora J, West DW, Satariano WA, Liebman M, Esserman L. Hypertension is an independent predictor of survival disparity between African‐American and white breast cancer patients. Int J Cancer. 2009;124:1213–1219. doi: 10.1002/ijc.24054 [DOI] [PubMed] [Google Scholar]
- 127. Rugo HS, Brufsky AM, Ulcickas Yood M, Tripathy D, Kaufman PA, Mayer M, Yoo B, Abidoye OO, Yardley DA. Racial disparities in treatment patterns and clinical outcomes in patients with HER2‐positive metastatic breast cancer. Breast Cancer Res Treat. 2013;141:461–470. doi: 10.1007/s10549-013-2697-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Baron KB, Brown JR, Heiss BL, Marshall J, Tait N, Tkaczuk KH, Gottlieb SS. Trastuzumab‐induced cardiomyopathy: incidence and associated risk factors in an inner‐city population. J Card Fail. 2014;20:555–559. doi: 10.1016/j.cardfail.2014.05.012 [DOI] [PubMed] [Google Scholar]
- 129. Berkman A, F. Cole B, Ades PA, Dickey S, Higgins ST, Trentham‐Dietz A, Sprague BL, Lakoski SG. Racial differences in breast cancer, cardiovascular disease, and all‐cause mortality among women with ductal carcinoma in situ of the breast. Breast Cancer Res Treat. 2014;148:407–413. doi: 10.1007/s10549-014-3168-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Solanki PA, Ko NY, Qato DM, Calip GS. Risk of cancer‐specific, cardiovascular, and all‐cause mortality among Asian and Pacific Islander breast cancer survivors in the United States, 1991–2011. SpringerPlus. 2016;5:82. doi: 10.1186/s40064-016-1726-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Litvak A, Batukbhai B, Russell SD, Tsai H‐L, Rosner GL, Jeter SC, Armstrong D, Emens LA, Fetting J, Wolff AC, et al. Racial disparities in the rate of cardiotoxicity of HER2‐targeted therapies among women with early breast cancer. Cancer. 2018;124:1904–1911. doi: 10.1002/cncr.31260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Troeschel AN, Liu Y, Collin LJ, Bradshaw PT, Ward KC, Gogineni K, McCullough LE. Race differences in cardiovascular disease and breast cancer mortality among US women diagnosed with invasive breast cancer. Int J Epidemiol. 2019;48:1897–1905. doi: 10.1093/ije/dyz108 [DOI] [PubMed] [Google Scholar]
- 133. Collin LJ, Troeschel AN, Liu Y, Gogineni K, Borger K, Ward KC, McCullough LE. A balancing act: racial disparities in cardiovascular disease mortality among women diagnosed with breast cancer. Ann Cancer Epidemiol. 2020;4:4. doi: 10.21037/ace.2020.01.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lotrionte M, Biondi‐Zoccai G, Abbate A, Lanzetta G, D'Ascenzo F, Malavasi V, Peruzzi M, Frati G, Palazzoni G. Review and meta‐analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am J Cardiol. 2013;112:1980–1984. doi: 10.1016/j.amjcard.2013.08.026 [DOI] [PubMed] [Google Scholar]
- 135. Keegan THM, Kushi LH, Li Q, Brunson A, Chawla X, Chew HK, Malogolowkin M, Wun T. Cardiovascular disease incidence in adolescent and young adult cancer survivors: a retrospective cohort study. J Cancer Surviv. 2018;12:388–397. doi: 10.1007/s11764-018-0678-8 [DOI] [PubMed] [Google Scholar]
- 136. Anderson C, Lund JL, Weaver MA, Wood WA, Olshan AF, Nichols HB. Disparities in mortality from noncancer causes among adolescents and young adults with cancer. Cancer Epidemiol Biomarkers Prev. 2019;28:1417–1426. doi: 10.1158/1055-9965.EPI-18-1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lipshultz SE, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, Orav EJ, Gelber RD, Colan SD. Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332:1738–1743. doi: 10.1056/NEJM199506293322602 [DOI] [PubMed] [Google Scholar]
- 138. Krischer JP, Epstein S, Cuthbertson DD, Goorin AM, Epstein ML, Lipshultz SE. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J Clin Oncol. 1997;15:1544–1552. doi: 10.1200/JCO.1997.15.4.1544 [DOI] [PubMed] [Google Scholar]
- 139. Khalid Y, Fradley M, Dasu N, Dasu K, Shah A, Levine A. Gender disparity in cardiovascular mortality following radiation therapy for Hodgkin's lymphoma: a systematic review. Cardiooncology. 2020;6:12. doi: 10.1186/s40959-020-00067-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Eberly LA, Kallan MJ, Julien HM, Haynes N, Khatana SAM, Nathan AS, Snider C, Chokshi NP, Eneanya ND, Takvorian SU, et al. Patient characteristics associated with telemedicine access for primary and specialty ambulatory care during the COVID‐19 pandemic. JAMA Netw Open. 2020;3:e2031640. doi: 10.1001/jamanetworkopen.2020.31640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Eberly LA, Khatana SAM, Nathan AS, Snider C, Julien HM, Deleener ME, Adusumalli S. Telemedicine outpatient cardiovascular care during the COVID‐19 pandemic: bridging or opening the digital divide? Circulation. 2020;142:510–512. doi: 10.1161/CIRCULATIONAHA.120.048185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. American Heart Association . Request for Applications for the Disparities in Cardio‐Oncology Strategically Focused Research Network 2020. 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


