Abstract
Chronic pain has become a global health problem contributing to years lived with disability and reduced quality of life. Advances in the clinical management of chronic pain have been limited due to incomplete understanding of the multiple risk factors and molecular mechanisms that contribute to the development of chronic pain. The Acute to Chronic Pain Signatures (A2CPS) Program aims to characterize the predictive nature of biomarkers (brain imaging, high-throughput molecular screening techniques, or “omics,” quantitative sensory testing, patient-reported outcome assessments and functional assessments) to identify individuals who will develop chronic pain following surgical intervention. The A2CPS is a multisite observational study investigating biomarkers and collective biosignatures (a combination of several individual biomarkers) that predict susceptibility or resilience to the development of chronic pain following knee arthroplasty and thoracic surgery. This manuscript provides an overview of data collection methods and procedures designed to standardize data collection across multiple clinical sites and institutions. Pain-related biomarkers are evaluated before surgery and up to 3 months after surgery for use as predictors of patient reported outcomes 6 months after surgery. The dataset from this prospective observational study will be available for researchers internal and external to the A2CPS Consortium to advance understanding of the transition from acute to chronic postsurgical pain.
Keywords: postsurgical pain, thoracic surgery, pain, biomarker, risk factors, protocol, knee arthroplasty
Introduction
Chronic pain is a significant health problem with 20% of Americans reporting moderate to severe pain and 25 million Americans reporting daily pain (1, 2). Further, chronic pain produces the largest non-fatal burden of disease as many individuals experience moderate-to-severe chronic pain that contributes to years lived with disability (3–5). While concerted efforts to better manage pain have occurred over the past two decades, the adverse sequelae of increased use and misuse of opioid treatment has brought this important issue to the forefront. Yet, our understanding of factors that contribute to the transition from an acute pain event to chronic pain are poorly understood, likely due to its complex nature and the inherent individual variability. Factors associated with the transition to chronic pain have been identified in animal and human studies, providing potential candidates for biomarkers, but few large-scale prospective studies have been completed to explore the predictive power of these biomarkers. Factors across biopsychosocial domains have been implicated including psychosocial factors such as anxiety, depression, resilience, social support, childhood and adult trauma (6–27), neuroimaging signals (28–32), quantitative sensory testing (33–39), molecular changes in genes, proteins, extracellular RNA, lipids, and metabolites (40–50), and multiple patient reported outcome measures such as pain intensity, sleep dysfunction, and disability (6, 13–15, 19, 20, 51–63). To that end, the Acute to Chronic Pain Signatures (A2CPS) Program was funded by the National Institutes of Health (NIH) Common Fund to identify biomarkers and their collective biosignatures (a combination of several individual biomarkers) that predict susceptibility or resilience to the development of chronic pain after an acute pain event. This goal will be accomplished by characterizing the predictive nature of multiple targeted primary, secondary, and exploratory biomarkers for identifying those who develop chronic pain 6 months after a surgical intervention with the following objectives.
Objective 1 will use a candidate approach to examine whether individual biomarkers with prior evidence predict susceptibility or resilience to the development of chronic pain.
Objective 2 will develop biosignature(s) using the candidate biomarkers to determine if combinations of biomarkers improve the prediction from acute to chronic pain.
Objective 3 is exploratory and will use a discovery-validation approach to define novel putative biomarkers without sufficient preliminary data and biosignatures with combinations of novel and candidate biomarkers that predict the susceptibility and resilience to development of chronic pain.
When selecting biomarkers, the Consortium considered several factors including scalability, clinical usefulness, and the current evidence. While the FDA-NIH Biomarker Working Group defines biomarkers as molecular, histologic, radiographic, or physiologic characteristics that provide an indication of biological or pathogenic processes (64), use of the term “biomarker” and “biosignature” in this manuscript incorporates markers across biological, psychosocial, and clinical domains for the indication of chronic post-surgical pain. Because pain is a multidimensional construct, A2CPS will incorporate a comprehensive list of biomarkers across the biopsychosocial spectrum to follow a biopsychosocial approach to pain (16, 65). Content experts within and outside the consortium reviewed candidate biopsychosocial constructs and selected markers with the strongest prior evidence for inclusion as primary and secondary biomarkers for A2CPS. The A2CPS program will capitalize on recent scientific advances and current knowledge in brain imaging, high-throughput screening techniques (omics), quantitative sensory testing (QST), patient-reported outcome (PRO) assessments, and functional assessments. Biomarkers and biosignatures, collected before the transition to chronic pain, have the potential to allow providers to identify high- vs. low-risk individuals, which could be a game changer in the prevention and treatment of chronic pain. The investigation of multiple candidate markers simultaneously in a relatively large cohort has the greatest potential to identify key predictors. The dataset will also serve as a resource for the research community to explore additional scientific inquiries.
Two study populations were selected for inclusion in A2CPS: individuals undergoing knee arthroplasty or thoracic surgery. These two clinical populations are exposed to an acute painful event (surgery) and will be followed to identify who transitions to chronic pain at 6 months following surgery. However, they differ in the type of and underlying causes requiring surgery as well as in baseline pain status: knee osteoarthritis typically presents with pre-surgical pain, whereas thoracic surgery typically does not. This design will allow for investigation of baseline biomarkers that predict susceptibility or resilience to chronic post-surgical pain in people already experiencing persistent pain (knee arthroplasty) and another without (thoracotomy). Chronic post-surgical pain (CPSP) is a major cause of suffering and disability, occurring at rates between 10 and 40% after common surgical procedures (66–70). More specifically, transition rates for CPSP following knee replacement have been reported to range from 10 to 30% (71–74), whereas roughly 30-47% of patients following thoracic surgery procedures develop new chronic pain after 6 months (75–80). Thus, these two populations provide the opportunity to identify unique and shared biomarkers and biomarker signatures that predict the development of or resilience to CPSP. This report provides an overview of the A2CPS prospective observational study protocol and summary of the general data collection procedures.
Methods and Analysis
Study Design
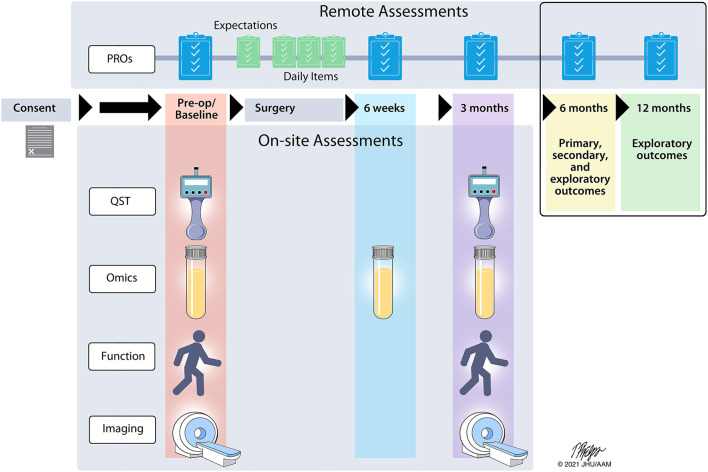
This is a longitudinal, multi-site, prospective observational study to identify candidate and novel biomarkers and biosignatures that predict development of or resilience to chronic pain 6 months after surgery. Multiple assessments of targeted PROs, QST, functional assessments, brain imaging, and biospecimen omics biomarkers will be assessed pre-surgery (baseline), across a 6-week post-operative period (psychosocial assessments and biospecimen only), and at 3 months (Figure 1) using a combination of on-site and remote assessments. All subjects will be asked to complete the baseline, acute post-operative, and 3-month assessments. The primary, secondary, and exploratory study endpoints will be assessed remotely at 6 months. In addition, subjects will be contacted at 12 months to collect exploratory follow-up endpoints. The selection and implementation of study assessments was completed with feedback from an advisory panel of content experts in each domain and two patients who previously underwent knee arthroplasty and thoracic surgery.
Figure 1.
A2CPS study timeline. The flow of assessments is indicated across the 12-month study timeline. Remote assessments consist of patient reported outcomes (PRO) while onsite assessments involve quantitative sensory testing (QST), a blood draw for high-throughput screening techniques (omics), physical function, and brain imaging.
Study Organization and Settings
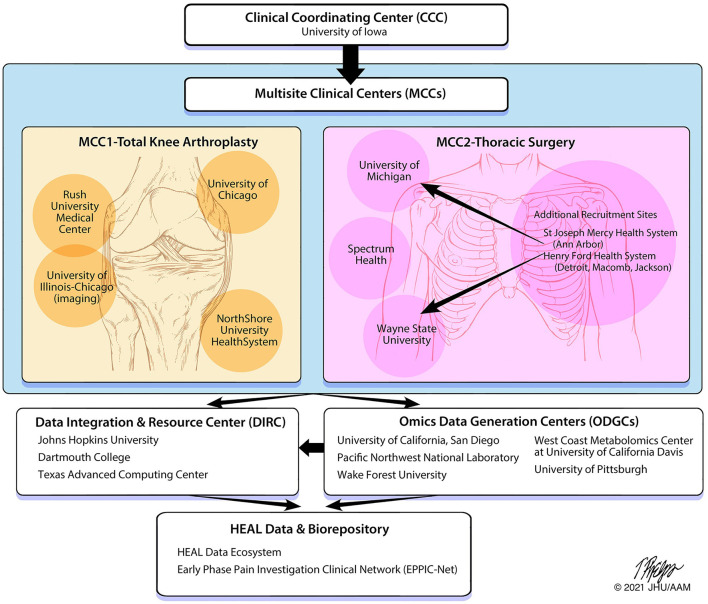
The A2CPS Consortium consists of multiple sites/institutions with specified roles to fulfill study objectives (Figure 2), which includes two Multisite Clinical Centers (MCCs), a Clinical Coordinating Center (CCC), three Omics Data Generating Centers (ODGCs), and a Data Integration and Resource Center (DIRC). In addition to sites/institutions, four NIH-appointed external Program Consultants and a patient representative have been incorporated on study committees and provide feedback on study design and ongoing review of the Consortium. A Steering Committee is the main governing board for the Consortium and has leadership representation from each institution and the NIH. A2CPS is a longitudinal study with anticipated recruitment of 3600 individuals divided between two cohorts scheduled for knee arthroplasty or thoracic surgery. Each Multisite Clinical Center (MCC) is set up to successfully recruit the target sample size with enrolment of knee arthroplasty patients initiated at MCC1 and the thoracic surgery cohort at MCC2. Both MCCs have the capacity to enroll both patient cohorts if needed to achieve enrolment goals. Additionally, clinical sites which serve various socioeconomic, racial, and ethnic patient populations were selected for inclusion in both MCCs to increase the diversity of patient enrolment.
Figure 2.
A2CPS structure and data flow. The organization of the A2CPS Consortium includes oversight from the Clinical Coordinating Center, data collection from the Multisite Clinical Centers, data generation from the Omics Data Generation Centers and Data Integration and Resource Center and storing of data and biospecimens within a repository.
MCC1 is located in the Chicago metropolitan area with recruitment sites at Rush University, the University of Chicago and NorthShore University HealthSystem. These sites are all part of the Institute of Translational Medicine (81), funded by the University of Chicago/Rush University Clinical and Translational Science Award of the National Center for Advancing Translational Sciences (NCATS) which is one of the 27 institutes and centers of the National Institutes of Health. The University of Illinois at Chicago is also a partner in this Consortium bringing expertise in brain imaging research (82). MCC1 initiated recruitment of the knee arthroplasty population, where collectively, their sites perform more than 4,700 knee arthroplasty procedures annually. MCC1 also serves a diverse population of patients with more than 30% African American and 15% of Hispanic origin. Potential participants will be recruited from the practices of more than 15 adult reconstructive orthopedic surgeons, and the MCC1 site has the advantage of recruiting from multiple high-volume joint replacement centers.
MCC2 consists of recruitment sites from across the State of Michigan, performing all assessments at 3 testing sites: University of Michigan in Ann Arbor, Wayne State University in Detroit, and Spectrum Health in Grand Rapids. Additional health centers will fill in as recruitment pools with follow up testing at the 3 centers. MCC2 initiated recruitment of the thoracic surgery population. Potential participants will be recruited from 10 clinical sites who partner in the Michigan Society of Thoracic and Cardiovascular Surgeons (MSTCVS) Quality Collaborative (83).
The CCC is housed at the University of Iowa and includes a partnership between pain scientists from the Department of Physical Therapy and Rehabilitation Science in the Carver College of Medicine and clinical trialists from the Clinical Trials Statistical and Data Management Center (CTSDMC) in the College of Public Health. The CCC leads development and maintenance of the Master Protocol, the Manual of Procedures (MOP), and Standard Operating Procedures (SOPs); and is responsible for the central Institutional Review Board (cIRB) application and associated reliance agreements. To ensure consistent study design, progress and quality, the CCC works closely with the MCCs to provide training and monitoring of study procedures and assist with recruitment and retention procedures. A2CPS training is provided by the CCC to all sites to facilitate standardization of all study procedures and to improve data quality. Research assistants must complete a comprehensive training and certification process in administering and recording of data with inter-rater reliability checks prior to and every 6 months following site activation. Thus, the CCC ensures that processes are in place to allow the MCCs to collect high-quality data.
There are three ODGCs that each focus on different omics analyses. These ODGCs are responsible for preparing biospecimen-collection kits, analyzing samples collected by MCCs, and sending data to the DIRC for storage and analysis. The ODGC—Genetic Variants and exRNA is housed at the University of California, San Diego. ODGC—Proteomics is a collaboration between Pacific Northwest National Laboratories (PNNL) and the University of Pittsburgh and will analyze samples for proteins and cytokines. ODGC—Lipidomics and Metabolomics is a collaboration between the Center for Precision Medicine at the Wake Forest School of Medicine and the West Coast Metabolomics Center at the University of California, Davis.
The DIRC, primarily housed at Johns Hopkins University with collaborating sites at Dartmouth College and the Texas Advanced Computing Center (TACC), combines biostatisticians, informaticians, and database experts with pain scientists into a single integrated team. The DIRC integrates efforts of all funded components of the Consortium and serves as a community-wide nexus for protocol, assay and data standards. The DIRC set up data-processing pipelines for the imaging and omics data sets and a data-collection system (REDCap/My Data Helps) for data collected at the MCCs. The DIRC will perform statistical analysis of data to test the aims of the Consortium. The DIRC also leads an outreach component which includes a public website (www.a2cps.org), a portal for Consortium members, and will provide user-friendly, publicly accessible data to the scientific community for novel discovery approaches.
Cohorts and Recruitment
Two different surgical cohorts are being recruited for A2CPS. The first cohort consists of individuals scheduled for unilateral knee replacement. The second cohort consists of individuals scheduled for thoracic surgery [thoracotomy, video-assisted thoracic surgery (VATS), and robotic thoracic surgery]. Patients are recruited by study research assistants at each clinical/recruitment site with assistance from clinic and study personnel. Collaborating surgeons and clinical staff provide information pertaining to study participation, but all screening and written informed consent is performed by dedicated A2CPS research assistants. The established cohorts have shared and cohort-specific eligibility criteria (Table 1). Patients are not excluded for prior chronic pain conditions, including fibromyalgia, neuropathic pain, peripheral neuropathy, low back pain, mental health conditions, or prior surgery > 3 months previously; or based on prior or baseline pain medication use, including opioids, to maximize generalizability. A comprehensive list of co-morbidities are documented in the preoperative (“baseline”) period. Study enrollment commenced with English-speaking patients, and Spanish-speaking patients are now eligible for participation. Recruited subjects who do not undergo surgery and/or discontinue prior to completion of the baseline visit will not be included in the targeted enrollment. The A2CPS Recruitment and Retention committee and the DIRC are continually monitoring participant enrollment and retention and data completion throughout the course of the study. The recruitment and retention committee has developed materials to facilitate recruitment including brochures, letters, physician letters, and physician videos describing the study. They have also implemented strategies to facilitate study completion and data completeness: training of research assistants in reviewing all assessments for completeness, developing rapport, expressing appreciation, and routine communication to address any challenges and obtain participant feedback, automated follow-up email reminders, assistance with transportation to data collection sites, and relationship building with physicians. The DIRC will continually monitor data completeness across measures at all sites and will notify research assistants at clinical sites of missing data. Weekly reports of missing data will be aggregated from each site and concerning patterns in missing data will trigger follow-up with the CCC training team for the research staff.
Table 1.
A2CPS eligibility criteria.
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| A2CPS Common Criteria | 1. Provision of signed and dated informed consent form 2. Stated willingness to comply with all study procedures and availability for the duration of the study 3. Age 18–85 years |
1. Patients unable to provide informed consent or unable to read/speak English or Spanish 2. Patients with known contra-indications to magnetic resonance imaging (MRI) |
| Knee Arthroplasty Cohort | 1. Individuals diagnosed with knee osteoarthritis scheduled to undergo a single primary partial or total knee replacement; conversion of a partial to total knee replacement for mechanical failure (aseptic loosening, implant fracture, instability), wear-related complications (osteolysis, synovitis) or component malposition; or a revision of a knee replacement for mechanical failure (aseptic loosening, implant fracture, instability), wear-related complications (osteolysis, synovitis) or component malposition. All surgical approaches including robotic-controlled and muscle-sparing techniques will be included for the study. | 1. Patients undergoing unilateral primary partial or total knee replacement for an inflammatory arthritic condition such as rheumatoid arthritis or osteonecrosis 2. Patients undergoing revision surgery with an infectious diagnosis involving the joint to be replaced (as this will be a 2-stage procedure) 3. Patients undergoing bilateral knee replacements, planned staged bilateral knee replacements within 3 months of each other, or are within 3 months of a prior contralateral knee replacement |
| Thoracic Surgery Cohort | 1. Individuals who are scheduled for surgery using a thoracic approach (including thoracotomy, VATS, and robotic surgery) at any of the participating hospitals | 1. Patients who have undergone prior thoracic surgery within 3 months 2. Patients undergoing a bilateral thoracic procedure 3. Patients undergoing another planned major surgery in the 6-month follow-up period |
Data Collection Procedures
Protocol Overview
Longitudinal assessment of the broad range of identified biomarkers (Table 2; Supplementary Table S2) will occur through a combination of in-person visits and remote assessments in all study participants (Figure 1). The full Schedule of Activities is also provided in Supplementary Table S3. Self-reported assessments are collected electronically through a variety of surveys using Research Electronic Data Capture (REDCap™) (84, 85) and/or MyDataHelps™ (RKStudio™, CareEvolution, LLC, Ann Arbor, MI) (see below for more details) (86). A2CPS includes the core psychosocial assessments and associated Helping End Addiction Long-term (HEAL) Common Data Elements (CDE) for harmonization (87). In-person visits incorporate assessment of function, pain sensitivity testing (QST), brain magnetic resonance imaging (MRI), and biospecimen collection of blood samples (omics). Additional medical data are extracted from the electronic medical record. In-person and remote assessments have been implemented for English-speaking participants, and implementation of Spanish translation and interpreter services is currently in progress.
Table 2.
Primary biomarkers and assessments.
| Primary biomarkers | Assessment |
|---|---|
| Patient reported outcomes and behavior | |
| 1. General pain intensity | Brief pain intensity -whole body pain |
| 2. Local pain intensity | Modified BPI – surgical site pain |
| 3. Widespread body pain | Michigan Body Map |
| 4. Acute pain trajectory following surgery | Single item assessments of daily pain and/or pain interference |
| 5. Disability | Knee Injury and Osteoarthritis Outcome Score – 12 (Knee arthroplasty only), Danish Thoracic Surgery Questionnaire (Thoracic surgery only) |
| 6. Perceived physical function | PROMIS Short Form v2.0 Physical Function 8b |
| 7. Performance physical function | Five Times Sit-to-Stand, 10 Meter Walk Test (Knee arthroplasty only) |
| 8. Movement-evoked pain | Five Times Sit-to-Stand, 10 Meter Walk Test (Knee arthroplasty only), Coughing and deep breathing (Thoracic surgery only) |
| 9. Anxiety | General Anxiety Disorder – 7 |
| 10. Depressive symptoms | Patient Health Questionnaire – 8 |
| 11. Pain catastrophizing | Pain Catastrophizing Scale – 6 |
| 12. Fear of movement | Fear Avoidance Beliefs Questionnaire – Physical Activity |
| 13. Sleep | PROMIS Short Form v1.0 Sleep Disturbance 6a, Sleep duration = time of sleep obtained over the past month |
| 14. Trauma history | Adverse Childhood Experience questionnaire |
| 15. Resilience | Pain Resilience Scale |
| 16. Social support | PROMIS SFv2.0 Instrumental Support 6a PROMIS SFv2.0 Emotional Support 6a |
| 17. Cognitive dysfunction | Multidimensional Inventory of Subjective Cognitive Impairment |
| Omics | |
| 18. C-reactive protein | Proteomics/Luminex |
| 19. Inflammatory markers – Tumor necrosis factor-alpha | Proteomics/Luminex |
| 20. Inflammatory markers – Interleukin-6 | Proteomics/Luminex |
| 21. Inflammatory markers – Interleukin-12 | Proteomics/Luminex |
| 22. Soluble glycoprotein 130 | Proteomics/Luminex |
| 23. Catechol-O-methyltransferase haplotype (rs4680) | Genotyping Array |
| 24. Mu-opioid receptor (rs1799971) | Genotyping Array |
| 25. ATP binding cassette subfamily B1 (rs1045642) | Genotyping Array |
| 26. Brain derived neurotrophic factor (rs6265) | Genotyping Array |
| 27. Brain derived neurotrophic factor (rs1491850) | Genotyping Array |
| Quantitative sensory testing (QST) | |
| 28. Pressure pain threshold (PPT) | Pressure algometer at surgical site |
| 29. Temporal summation (TS) | Punctate stimulus (Neuropen) at surgical site |
| 30. Conditioned pain modulation (CPM) | Change in shoulder PPT following noxious cold water submersion |
| 31. Dynamic mechanical allodynia | Standardized brush (Thoracic surgery only) |
| Brain imaging | |
| 32. Gray matter volume of medial prefrontal cortex | T1 |
| 33. Structural integrity in Am-NAc-mPFC network | Diffusion weighted imaging, white matter tractography |
| 34. Core DMN vmPFC/CCC–NAc/ventral striatum | rsfMRI |
| 35. Core DMN: vmPFC/CCC–somatosensory (dplNS/S1) | rsfMRI |
| 36. Core DMN: vmPFC/CCC-anterior/middle insula | rsfMRI |
| 37. Hub disruption | rsfMRI |
| 38. Evoked response in neurologic pain signature | Task fMRI (pressure cuff) |
| 39. Evoked response in fronto-striatal systems related to descending / central pain modulation and self-regulation (vmPFC, NAc) | Optimized markers (SIIPS) |
List of identified primary biomarkers and designated assessments.
Am, amygdala; NAc, nucleus accumbens; mPFC, medial prefrontal cortex; vmPFC, ventromedial prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; CC, cingulate cortex; dpINS, dorsoposterior insula; S1 somatosensory cortex 1.
Patient-Reported Outcomes
A comprehensive list of questionnaires (Table 2; Supplementary Table S2) are used to remotely collect a range of self-reported outcomes at various time-points across the study (Supplementary Table S3).
Pain and Fatigue
Pain is assessed using the Brief Pain Inventory (BPI) (88) and the Michigan Body Map (89). In particular, we modified the BPI to assess the worst pain intensity at the surgical site over the past 24-h with anchors of 0 (no pain) to 10 (worst pain imaginable), which was chosen as the primary study outcome. The BPI also includes the pain interference subscale (90), again specifically querying how surgical site pain has interfered with daily activity over the past 24 h with item responses ranging from 0 (does not interfere) to 10 (completely interferes). The BPI body map was replaced with the Michigan Body Map to record the number of painful body regions with persistent pain over the past 3 months (at baseline) and current pain experienced at each in-person visit. Self-reported characteristics of neuropathic pain at the surgical site is assessed with the PainDETECT Questionnaire (PD-Q) (91). The Symptom Severity Index (SSI) from the 2016 Fibromyalgia Diagnostic Criteria survey (92) is used to assess common symptoms related to chronic pain including severity of fatigue, cognitive dysfunction, and unrefreshed sleep over the past week from 0 (no problem) to 3 (severe) and incidence of lower abdomen pain, depression, and headache for at least 3 months (yes/no). In addition, fatigue and fatigue interference is assessed using the Patient Reported Outcomes Measurement Information System (PROMIS) Short Form v1.0 – Fatigue 7a (93). This seven-item measure has Likert-scale responses ranging from 1 (never) to 5 (always) with higher scores indicating greater fatigue.
Other Treatments and Substance Use
To screen for use of tobacco, alcohol, or illicit substances, and prescription medication misuse the Tobacco, Alcohol, Prescription medication, and other Substance use (TAPS) Part 1 and Part 2 tool (94) is used. TAPS Part 1 (4 items) screens for use over the past 12 months with item responses ranging from “never” to “daily or almost daily” while TAPS Part 2 consists of 9 additional items inquiring about use over the past 3 months with yes/no item responses. Additional items regarding smoking duration and packs per day were added.
Use of other treatments, including opioids, are assessed using items following the TAPS 1 format asking about use of common pharmacologic, rehabilitation, and integrative interventions for their pain (“never” to “daily or almost daily”). Amount of opioid use specifically is monitored at multiple time points (Supplementary Table S2) assessing specific opioids used and dosages. In addition, the Current Opioid Misuse Measure (COMM) (95, 96) is used to screen for potential risk for opioid misuse over the past month among patients reporting opioid use. The 11-item questionnaire asks patients about opioid related behaviors on a scale of 0 (never) to 5 (very often). During the immediate post-operative period (first 28 days) weekly assessments of opioid use, likeability, and side-effects are assessed. Satisfaction with pain control is assessed 6 weeks post-operatively.
Disability, Function, and Activity Levels
Disability is assessed using patient-specific and general measures. For the knee arthroplasty cohort, the Knee Injury and Osteoarthritis Outcome Score - 12 (KOOS-12) is a 12-item scale that assesses knee pain (4 items, never to always and none to extreme), difficulty with functional daily activities (4 items, none to extreme), and knee-related quality of life (97). For the thoracic surgery cohort, the modified Danish Thoracic Surgery Questionnaire is a 17-item scale which assesses functional impairment following thoracic surgery with item responses of 0 (“pain impairs me not at all”) to 4 (“I never do this activity due to pain”) (98, 99). General assessment of physical function for both cohorts is obtained using the PROMIS Short Form v2.0 Physical Function 8b, which contains eight questions assessing limitations in daily physical activities (response ranges: “without any difficulty” to “unable to do” and “not at all” to “cannot do”) (100, 101). Self-reported physical activity is assessed using the Rapid Assessment of Physical Activity (RAPA) consisting of 9 (yes/no) items that categorizes individuals into five physical activity levels ranging from sedentary to active (102).
Pain-Related Psychosocial Constructs
Several measures of pain-relevant psychosocial constructs are assessed including pain resilience, catastrophizing, kinesiophobia, and multisensory sensitivity. Pain-specific measure of resilience is assessed using the Pain Resilience Scale (PRS) (103). The PRS consists of 14 cognitive, affective and behavioral items with 0 (“not at all”) to 4 (“all the time”) responses for each question (104) where higher scores represent more resilience. Pain-related catastrophizing (highly negative appraisal of pain) is assessed using the Pain Catastrophizing Scale-6 (PCS-6) (105, 106). The PCS-6 includes 6 questions where each item is scored using 0 (“not at all”) to 4 (“all the time”) scale. In addition to the total score, three sub-scales assessing rumination, magnification and helplessness can be calculated (105, 106), where lower scores reflect less catastrophizing. Fear of movement is measured using the Fear Avoidance Beliefs Questionnaire—Physical Activity subscale (FABQ-PA) (107). The FABQ-PA subscale consists of 4-items with responses ranging from 0 (completely disagree) to 6 (completely agree) with higher scores indicating greater fear avoidance behavior. The FABQ-PA was modified replacing the term “back” pain to “knee” or “chest” pain for each respective cohort, consistent with prior studies (108–110). An 8-item General Sensory Sensitivity (GSS) scale is used to assess multisensory sensitivity to varied sensory stimuli including five external sensory stimuli (light, sound, odor, flavor, touch) and interoception (balance, nausea, heart rate) using dichotomous (yes or no) responses (111, 112) with higher scores indicating greater sensory sensitivity.
Depression and Anxiety
Depression and anxiety are assessed using the 8-item Patient Health Questionnaire-8 (PHQ-8) (113) and the 7-item General Anxiety Disorder (GAD-7) (114) instruments. Both the PHQ-8 and GAD-7 include questions about how often the patient experienced each item during the past 2 weeks, scored from 0 (“not at all”) to 3 (“nearly every day”). A summed score of ≥10 on PHQ-8 is considered a major depressive episode (113). A higher total score on GAD-7 represents elevated levels of anxiety (114).
Social Support
Two instruments are used to assess social support constructs. The quality of the supportive relationships received by the patient are assessed with the PROMIS SFv2.0 Instrumental Support 6a questionnaire (115). The patient's perceived feeling of being cared for and valued as a person is assessed using the PROMIS SFv2.0 Emotional Support 6a questionnaire (116). As in the other PROMIS questionnaires, a 5-point Likert scale ranges from “never” to “always”, and the raw scores for each patient for these two instruments will be standardized to T-scores with mean of 50 and standard deviation of 10.
Cognitive Function and Sleep
Cognitive function is assessed using the Multidimensional Inventory of Subjective Cognitive Impairment (MISCI), a 10-item scale that assesses varying domains of cognitive function including mental clarity, memory, attention/concentration, executive functioning, and language (117). Item responses vary from “not at all” to “very much” and “never” to “very often” with higher scores indicate a higher level of cognitive functioning. Sleep disturbance is assessed using the PROMIS Short Form v1.0 Sleep Disturbance 6a measure (118). Subjects rate their sleep quality and difficulty falling asleep over the past 14 days on a scale of 1 (not at all) to 5 (very much) with higher scores indicating greater sleep disturbance. In addition, sleep duration is assessed as the number of hours and minutes of actual sleep obtained per night during the past month (119).
Expectations and Perceived Change
Expectations of outcomes related to functional recovery, pain relief, and pain or complications following surgery are queried pre-operatively with 3 questions using a 0 to 10 scale. Patient's perception of pain relief following surgical intervention is assessed with the 7-item Patient's Global Impression of Change (PGIC) scale (88, 120, 121) ranging from 0 (very much improved) to 6 (very much worse) beginning 6 weeks after surgery.
Trajectory Items
In the acute post-surgical period from days 3 through 28, single item assessments of pain, pain interference, sleep, physical activity, medication use, and feelings of sadness, anger, and nervousness over the past 24 h are rated by participants using a 0-10 numerical rating scale. Similarly, at the 6-month follow up assessment participants complete these same items, referencing the past 7 days rather than past 24 h.
Other Assessments Performed at Baseline
Demographic information captured at the baseline visit includes age, sex, gender, ethnicity, race, level of education, employment status, relationship status, household income, and disability status. A modified Self-Administered Comorbidity Questionnaire (SCQ) (122) queries patients about common health problems and whether treatment is received for the related problem. Also assessed only at the baseline visit are childhood trauma and personality. The Adverse Childhood Experience questionnaire (ACE) (123) is used to retrospectively assess childhood (age 0–18 years) abuse, neglect, and household dysfunction. It has 10 items that are grouped according to adversity type. Personality is assessed using the Big Five Inventory−2 short form (BFI-2-S), a 30-item assessment used for assessing personality domains of extraversion, agreeableness, conscientiousness, negative emotionality, and open-mindedness (124). Participants rate each statement according to level of agreement from 1 (disagree strongly) to 5 (agree strongly).
Primary and Secondary Endpoints
Participants are sent electronic survey links to respond to a set of 6 single-item questions for 7 separate days. Participants rate their worst pain intensity (primary outcome), average pain, pain interference, sleep quality, physical activity, and pain medication intake over the past 24 h. The 7-day average of the worst pain intensity responses at the 6-month post-operative period was selected as the primary endpoint. The A2CPS biostatistics group performed a simulation based on pain ratings from other datasets to determine how to best use pain ratings from 7 sequential days. The mean daily response was chosen as the primary endpoint. Use of a continuous scale will allow for the identification of varying magnitude/severity of chronic pain which may exist post-surgically, while still allowing for secondary analyses using a dichotomous cut-point in the future. Further detail of the A2CPS statistical modeling and sample size calculations detailing the simulation study is beyond the scope of the current paper and will described elsewhere.
Biological Sample Collection and Processing (Omics)
Blood samples are collected from all study participants prior to surgery (baseline visit, range: 1 day−6 weeks), at the early post-operative visit (range: 3–7 weeks after surgery), and at the 3-month post-operative visit (range: ±2 weeks). Medication intake within the 24 h prior to the visit, and time of last food and stimulant intake are recorded prior to each blood draw. Two tubes, a 10 mL BD K2EDTA vacutainer (Becton-Dickinson) and a 2.5 mL BD PAXgene® Blood DNA Tube, are drawn at each visit. The samples in K2EDTA tubes are centrifuged within 30 min, processed and temporarily stored at −80 degrees within 1 h locally at the clinic sites. The PAXgene samples are placed directly at−80 degrees. All samples are barcoded to link participant ID to the sample and are scanned into REDCap. Samples are shipped on dry ice from the clinical site to a central omics center (UC San Diego) for long-term storage and distribution. Sample aliquots are provided to each of the ODGC sites for genetic variant, exRNA, proteomic, lipidomic, and metabolomic analyses.
Genetic Variants
DNA will be extracted from 1 mL of whole blood using the QIAsymphony DSP DNA Midi Kit (Qiagen), and quantity assessed using the Qubit™ dsDNA HS Assay Kit (Thermo Fisher Scientific). Gene variants will be assessed using the Infinium Global Screening Array (GSA) BeadChip (Illumina) which combines > 650,000 multi-ethnic genome-wide content, curated clinical research variants, and quality control (QC) markers (125). In addition, the arrays will feature focused content of particular interest including ~30,000 markers associated with common psychiatric disorders (Illumina Psych Booster), ~500 additional pharmacogenomics (PGx) markers, and 5,000 custom markers selected by the A2CPS Consortium to be of particular interest in the study of chronic pain. Quality Assurance/Control will include variant call rate and sample tracking metrics included comparisons to reported sex and ancestry (126).
Extracellular RNAs
Extracellular RNA will be extracted from 0.5 mL of plasma using the Plasma/Serum Circulating and Exosomal RNA Purification Kit, Slurry Format (Norgen Biotek), and quality assessed using the BioAnalyzer RNA Pico Chip (Agilent). Small RNA sequencing libraries will be constructed using the NEBNext® Multiplex Small RNA Library Prep Kit (NEB), pooled, size-selected using a Pippen Prep, and sequenced on an Illumina MiSeq (Nano run). The libraries will then be rebalanced and size-selected and sequenced on an Illumina NovaSeq instrument. Small RNA sequencing data preprocessing, mapping, and quality control will be performed using the ExceRpt pipeline (127). Selected miRNAs will be quantified by RT-qPCR using TaqMan Advanced miRNA Assays (Thermo Fisher Scientific).
Metabolomics
Metabolomics assays will be performed by teams at Wake Forest University School of Medicine and the West Coast Metabolomics Center at the University of California, Davis. Briefly, primary metabolism intermediates, particularly volatiles, non-polar and (derivatized) polar metabolites, will be targeted by the Wake Forest team using gas chromatography with high-resolution electron ionization mass spectrometry (128, 129), including targets that have been previously associated with pain phenotypes. Metabolites will be sequentially extracted and derivatized using trimethylsilylation prior to gas chromatography mass spectrometry (GCMS) analysis. Untargeted metabolomics data will be generated by high-resolution GCMS using electron impact ionization, and isotope-labeled added metabolites will be used for high confidence quantification of targets potentially related to chronic pain. Data will be processed using MS-DIAL (130) and metabolites will be identified using both in-house and public spectral libraries. Loess normalization using at least 3 external plasma QCs samples per batch will be used to normalize data to correct for batch effects. For quality assurance and system performance check, common metabolites in a quality control standard mix will be monitored to ensure confident data generation.
For biogenic metabolites such as acylcarnitines, one-carbon donor compounds, nucleotides and nucleosides, methylated and acetylated amines, di- and oligopeptides, UC Davis will use hydrophilic interaction chromatography with high-resolution tandem mass spectrometry (131). Data will be processed by MS-DIAL software (130), and compounds will be identified by MassBank.us (132) and constrained by Retip retention time software (133). Isotope labeled internal standards will ensure high confidence compound quantifications for specific targets involved in pain modulation.
Lipidomics
Lipidomic analyses will be completed at the West Coast Metabolomics Center at UC Davis (134) using both untargeted and targeted assays. After biphasic extraction and fractionation of polar and lipophilic compounds (135), complex lipids such as ceramides, sphingomyelins, cholesteryl esters, lyso- and diacylphospholipids, free fatty acids, and mono-, di- and triacylglycerols are separated and quantified by liquid chromatography and high-resolution mass spectrometer. Lipids are identified by our mass spectral library of 690,000 MS/MS spectra (136), in addition to accurate masses and retention time matching. Seventy-six stable isotope labeled internal standards are used for quantification, and Systematic Error Removal by Random Forest (SERRF) software is used for drift- and batch corrections from quality control pool samples (137). Specific low abundant target lipids are quantified in high-throughput LC-QTRAP 6500+ mass spectrometry. Evaluation of combined metabolomic and lipidomic profiles will be performed by chemical set enrichment statistics (138) to complement classic univariate statistics performed by the A2CPS Consortium.
Proteomics
Proteomic analyses will be performed in 2 phases as previously described by the team at PNNL (139–142). A discovery phase using comprehensive coverage (global) proteomics will be performed to validate previously annotated biomarker targets and to identify novel biomarker proteins and cytokines of interest. A second targeted phase will accurately quantify previously identified and novel biomarker targets using selected/multiple reaction monitoring (LC-SRM/MRM). Total protein from plasma will be isolated, enzymatically digested into peptides, and then analyzed by liquid chromatography with tandem mass-spectrometry (LC-MS/MS). For global proteomics, digested samples will be multiplexed using an isobaric labeling strategy (e.g., TMT) and analyzed in a data-dependent acquisition (DDA) mode. Targeted proteomics will be performed by optimized LC-SRM/MRM. Peptides uniquely belonging to protein biomarker targets, known as surrogate peptides, will be carefully selected to meet proteotypic and quantotypic criteria (143). Heavy isotope-labeled version of the surrogate peptides, used as standards with known concentrations, will be spiked into the digested samples and analyzed using a triple quadrupole mass spectrometer. Quantification will be based on the relative spectral intensities of endogenous peptides to heavy isotope-labeled internal standards using Skyline software (144). Quality assurance/control will include performance metrics for LC-MS/MS systems and external pooled reference plasma controls.
Performance-Based Function and Movement-Evoked Pain
In the knee arthroplasty cohort, physical function and movement-evoked pain (MEP) are assessed with the Five-Times Sit-to-Stand (5TSTS) Test (145, 146) and the 10-meter Walk Test (10MWT) (147, 148) following standard protocols. Time to completion is recorded for both tasks as the primary measure of function. Pain is assessed prior to and immediately following each test to assess MEP using an 11-point numerical pain rating scale (NPRS) with anchors of 0 (no pain) to 10 (worst pain imaginable). In the thoracic surgery cohort, participants are asked to take 3 deep breaths and perform 3 forceful coughs. Pain is rated prior to and immediately following each task. MEP is defined as the difference in pain with activity minus the initial resting pain.
Quantitative Sensory Testing
Pressure Pain Thresholds
Pressure Pain Thresholds (PPT) are assessed with a pressure algometer (Wagner Pain Test FPX25, Wagner Instruments, USA) applying a 1-cm2 rubber tip at a rate of 0.5 kgf/s. Three repetitions are performed at the surgical site (knee or chest) and three at a standard remote site (mid-deltoid of the shoulder contralateral to surgical site) (149). The average of the repetitions at each site are used as the PPT values, where higher PPTs indicate less pressure sensitivity.
Mechanical Temporal Summation
Temporal Summation (TS) is thought to reflect ascending pain facilitation (150); it is assessed using a punctate stimulus (Neuropen®, Owen Mumford, United Kingdom) applied to the skin. A single repetition is applied, followed by 10 repetitions at a rate of 1 per second, at the surgical site (knee or chest) and a standard remote site (mid-deltoid of the arm contralateral to surgical site). Pain ratings from the single stimuli and maximal pain ratings following the 10 stimuli are recorded. TS is indicated as an increase in pain ratings from repetitive noxious stimuli compared to the single stimulus (150–152).
Conditioned Pain Modulation
Conditioned Pain Modulation (CPM) is a psychophysical test paradigm which indirectly measures an individual's endogenous analgesia capacity (153–155). CPM assessment involves a test stimulus (PPT at the deltoid-3 repetitions) before and immediately after application of a noxious conditioning stimulus (hand immersion in a 10°C cold water bath) for 60 s (or to tolerance). CPM is indicated as an increase in PPT (less pain sensitivity) following the conditioning stimulus (154, 155).
Dynamic Mechanical Allodynia
Allodynia is assessed in the thoracic cohort only using a standardized brush (Somedic SENSELab Brush-05, Somedic, Sweden or equivalent) (152). Five, 4-cm brush strokes are applied at the surgical site (chest wall) and the contralateral chest wall with pain ratings recorded after each stimulation. The brush is applied with sufficient force to slightly bend the bristles 45 degrees (~200 ± 100 mN) to standardize the stimulus. The average pain ratings of the 5 repetitions at each site are used, where pain > 0 indicates allodynia.
Brain Imaging
Brain-imaging data will be collected using multiple sequences on 3 Tesla magnetic resonance imaging (MRI) scanners with a multi-channel (32- or 64-channel) brain coil, harmonized among 6 different imaging sites. A more detailed description of imaging methods will be provided elsewhere. Standard imaging screening will be performed at each site to ensure subject safety. Medication use over the prior 24-h and recent caffeine intake is assessed for potential influences on brain imaging. All enrolled participants are screened at each imaging site to ensure subject safety using guidelines established by the American College of Radiology (156).
Additional mood and pain items will be assessed prior to commencing scanning. The MRI protocol consists of structural MRI with T1-weighted contrast, resting-state functional MRI (rs-fMRI), task-fMRI using tonic cuff pain, and advanced diffusion imaging with multi-shell, multi-directional acquisitions. For the task-fMRI using tonic cuff pain, continuous cuff pain will be applied to the calf at (1) a subject-specific level designed to invoke a pain intensity rating of ~4/10 for 6 min and (2) at a pre-identified common pressure for all participants also for 6 min. The subject-specific cuff pressure is first identified earlier in the visit and re-checked once the participant is positioned in the scanner. The cuff is applied to the non-dominant leg for the thoracic surgery cohort and on the contralateral leg in the knee arthroplasty cohort. Two 6-min rs-fMRI runs will be performed, one before the task-fMRI runs and another immediately following them. The MRI protocol is built upon the protocol used for the Adolescent Brain Cognitive Development (ABCD) study (157, 158) but has been adapted to diverse scanner platforms across the 6 imaging sites. Before, during, and following the functional MRI runs the patient will be asked to rate any pain during the scanning procedure within their whole body, at their surgical site (knee or chest), and associated with the cuff pressure.
In addition to the above sequences, several calibration scans are performed to enable simultaneous multi-slice (SMS) imaging for faster acquisition and to reduce image distortion for better image quality. This robust MRI acquisition protocol has been harmonized on multiple scanner platforms and validated on healthy human volunteers across multiple imaging sites. To ensure the quality of the imaging data, the MRI physicists involved in the study have developed a time-efficient (~8 min) quality assurance protocol on a standard phantom. This protocol will be executed weekly throughout the study to assess both intra- and inter-site consistency in structural, diffusion-weighted, and functional imaging datasets.
Data Extraction From Electronic Health Record
The A2CPS Consortium will use EHR to obtain clinical and case characteristics (e.g., age, sex, height, weight, BMI, diagnosis, and surgical data). Information about the surgical procedure (e.g., procedure name, Current Procedural Terminology code, surgeon, laterality, duration, and anesthesia) will be extracted. Procedural anesthesia information will include American Society of Anesthesiology physical status, the primary anesthesia type, duration of anesthesia and medications administered during anesthesia care. Post-procedural information will be extracted including pain assessments, medications, and length of stay. Opioid medications will be converted to milligram morphine equivalents. For the knee arthroplasty cohort, the implant name, manufacturer, and number will be obtained. In the thoracic surgery cohort, data will be obtained from the Multicenter Perioperative Outcomes Group (MPOG) (159) and Michigan Society of Thoracic and Cardiovascular Surgeons (MSTCVS) databases; these sources allow additional structured information on medical history and 30-day postoperative outcomes to be included. Use of EHR derived data is included in study participant informed consent. Health Insurance Portability and Accountability Act (HIPAA) rules, other relevant federal or state laws, and local institutional requirements will be followed, as applicable.
Study Endpoints
Our primary endpoint, chronic pain at 6 months following surgery, will be operationally defined as worst pain in the last 24 h. This will be assessed on a 0–10 numeric rating scale (NRS) for 7 days, with the average value used as the endpoint. Additional secondary endpoints include measures of dysfunction (disability) and opioid use at the 6-month follow-up. Exploratory endpoints assessed at the 6-month follow-up include: pain interference; worst pain intensity at a single time at 6 months; use and misuse of opioids; perceived global impact of change; depressive symptoms; anxiety; and sleep quality. Similar items will be assessed at 12 months through remote electronic query as an optional follow-up.
Data Management and Analyses
Data Management
Data collected for this study will be analyzed and stored by the DIRC at the Texas Advanced Computing Center (TACC) based at the University of Texas. A data collection system using REDCap has been set up to collect patient-reported outcomes and researcher-driven data from the MCCs. For MCC1, patient surveys are sent out via REDCap at regularly scheduled time periods dictated by the study protocol. MCC2 has made the surveys available within the MyDataHelps app, allowing participants to complete the surveys directly using a smartphone app. The app data is automatically transferred daily into REDCap. Both MCCs use REDCap to fill out comprehensive information about the functional testing, QST, blood draws, and brain-imaging sessions. In addition, any protocol deviations are recorded in REDCap. All study participant research data is transmitted to and stored by the DIRC. This includes imaging data, electronic health records, processed omics data, and information collected in REDCap. Individual participants and their research data are identified by a unique study identification number and a universally unique identifier (UUID). To facilitate generalizability and consistency among studies, the HEAL CDE are used to code all data. Any missing data or data anomalies will be communicated to the center(s) for clarification/resolution.
Sample Size Determination
The appropriate sample size was determined both considering hypothesis testing and prediction as a primary analysis. For hypothesis testing, power was estimated using logistic regression models for binary biomarkers and t-tests for continuous biomarkers with effect sizes derived from existing literature under each of the following conditions: Type I Error (α) = 0.01, 0.05; Sample Size (N) = 1,000, 1,400, 1,800; and Transition Rate (to chronic pain) = 15%, 25%. For each combination of alpha, sample size, and transition rate we created a summary of which biomarkers would meet a threshold of at least 80% power. For prediction, sample size was determined based on controlling the prediction error bound at 0.025 assuming a transition rate of 25%. The initial sample size recommendation was 1,800 participants per cohort, allowing for a total number of up to 40 primary biomarkers. However, preliminary feasibility analyses after the first 100 participants have led to adjustments to our priori sample size calculations from a more conservative sample of 1,800 to 1,400, with minimal sample size estimates of 1,000. Thus, we are targeting ~1,400 participants per cohort to cover attrition and missing data.
Planned Statistical Analyses
Primary Analyses
The primary analysis involves the use of a candidate approach to examine whether the putative biomarkers across multiple domains (i.e., clinical, biospecimen, psychosocial, and brain structure/function) individually predict susceptibility or resilience to the development of chronic pain at 6 months after an acute painful event. The data will be analyzed using a predictive modeling approach with k-fold cross validation. Multiple regression will be used to evaluate how each of the pre-selected biomarkers of interest individually account for unique variance in predicting the transition from acute to chronic pain. The model will include potential confounders [e.g., differences across sites/batch effects in training sample size and head movement (for brain imaging)] and baseline characteristics (e.g., sex, age, socio-economic status, baseline pain). Significance will be assessed using the Wald test for the regression coefficient and effect size using a 0.05 significance level. All tests will be two-sided, unless a one-sided test is specifically called for by the biomarker (e.g., reduced volume of medial prefrontal cortex). Given the focus on prediction, multiplicity adjustment is not necessary in this phase. As each cohort corresponds to a different type of surgery, we will perform analyses both separately and combined.
Secondary Analyses
Secondary analyses involve the development of a biosignature(s) using the candidate primary biomarkers evaluated individually in the primary approach, to determine if combinations of biomarkers improve the prediction of transition from acute to chronic pain after an acute painful event. We will analyze the data using a predictive modeling approach. We will use a machine learning approach (e.g., random forest) to select the most predictive variables. Models will also investigate whether the relationships between biomarkers and our outcome is mediated by baseline characteristics. Methods such as random forest are ideal in this setting as they are particularly designed to find interactions between variables. Significance will be assessed using a Bootstrap procedure at the 0.05 significance level. Surgical cohort will be included as a variable to evaluate its interaction with all the predictors. We will assess the variability accounted for on the primary outcome by each combination of biomarkers. Within each type (e.g., genomics, brain imaging, behavioral) we can conduct a complete search and enumeration of all models. Variables can then be combined across types. We will further focus on finding combinations that require as few data collection modalities as possible.
Exploratory Analyses
For the exploratory analysis, we will use a discovery-validation approach to define novel putative biomarkers and biosignatures across multiple domains that predict the susceptibility or resilience to development of chronic pain at 6 months. We will use multivariate predictive modeling to identify latent variables within sets of measures with multi-objective machine learning-based optimization of predictive accuracy, specificity to pain, generalizability across cohorts, and model reproducibility. Techniques include feature selection and feature engineering combined with penalized regression/classification, ensemble classifiers, and deep learning/convolutional neural networks where applicable. Outcomes will go beyond pain ratings alone to include latent trajectories of pain intensity and interference, chronic opioid use, physical and emotional function, and other domains. We will adopt a model-building practice of comparing simpler, more interpretable models with more complex ones, adding complexity only when essential and warranted by the data, and developing models to be comprehensible and usable by a broad audience. Prospective validation datasets will be separated and sequestered in advance. Training samples will be balanced on covariates using propensity score matching, ensuring that models are not driven by confounds. A systematic evaluation of processing, scaling, algorithms, and tuning parameters will be performed within k-fold cross-validation loops, leading to rigorous comparisons across methods and options, with stochastic optimization for intractably large model sets. Validation datasets will be tested only on final, optimized models with “locked down” parameter estimates, and evaluated for performance on multiple objective functions: sensitivity, specificity, and model complexity.
Interim Analyses
An initial feasibility assessment is planned after approximately the first 100 enrolled participants (1) to assess for any data collection concerns (baseline thru 3-month missing data analyses) and (2) to assess preliminary results following completion of the study (6 months after surgery/acute pain assessment). This will be done to better inform the risk prediction sample size calculations. In addition, a futility analysis will be performed for primary biomarkers and the primary study outcome after 50% of participants are completed. This futility analysis will assess whether the transition rate to chronic pain is adequate for the final analysis of the data.
Data Sharing
At the end of the study, all study databases will be archived by the DIRC. The de-identified datasets will be transmitted to and stored at the NIH HEAL Data Ecosystem, for use by other researchers including those outside of the study. With the participant's approval and as approved by the central internal review board (cIRB), de-identified biological samples will be stored at the HEAL Biorepository [Early Phase Pain Investigation Clinical Network (EPPIC-Net) at New York University] to be used to further research the causes of chronic pain and to improve treatment. The HEAL Data and Biorepositories will also be provided with a code-link that will allow linking the biological specimens with the phenotypic data from each participant, maintaining the blinding of the identity of the participant. In addition, links between the unique study identification number and the globally unique identifier will be maintained in the form of a registry by the DIRC for an undetermined time pending long-term resources. Permission to transmit data to the data repository is included in the informed consent. This dataset will serve as a resource for the investigators to evaluate a wide range of scientific inquiries, a priority for the A2CPS program.
Regulatory and Quality Control
Quality Assurance and Control
The A2CPS CCC and DIRC collaboratively provide quality oversight for all Consortium activities conducted at the MCCs and ODGCs. To ensure collection of high-quality data, we standardized data-collection procedures across sites, documented in our manual of procedures. All study personnel involved in data collection completed training and certification for relevant study procedures, including recruitment and consent, PROs, function, biospecimen collection, QST, and brain imaging. All Consortium personnel establish proficiency on standard operating procedures (SOPs), good clinical practice (GCP), the protection of human subjects, and standardized data collection and entry procedures. Adherence to established study protocols is necessary to obtain rigorous and reliable data collection longitudinally and across clinical sites.
The DIRC is responsible for developing, testing, and managing clinical data management activities, as required. All study data is collected by systems that comply with all applicable guidelines regarding patient confidentiality and data integrity (REDCap and MyDataHelps). The study data entry and study management systems used by clinical sites and by the DIRC research staff is secure and password protected.
Central Internal Review Board and Safety Monitoring
The A2CPS utilizes the University of Iowa as the cIRB for all study review. Documented approval from the A2CPS cIRB (IRB ID#: 201905783) and all necessary reliance agreements were obtained for all participating centers prior to commencing subject recruitment and data collection. The date of the initial cIRB approval to recruit subjects at the first site was October 5, 2020, with subsequent amendment approvals to add the additional sites. All participants are provided detailed descriptions of the study purpose, procedures and risks provided, and confirmation their decision to participate or withdrawal from the study in no way alters their medical care by clinical research staff at each site. Documentation of informed consent is completed electronically, in person or virtually prior to starting data collection. During the course of the study, an individual participant can choose to withdraw from the study at any time or withdraw consent to have biological specimens stored for future research. However, withdrawal of consent regarding biosample storage may not be possible after the study is completed and de-identified data has been transferred to the HEAL repositories.
Data Safety Monitoring Plan
Safety oversight for the A2CPS follows a DSMP with regular study review by external medical monitor. We assess and monitor safety in relation to participation in research study procedures. Adverse events are defined as those related to study procedures only, i.e., infection of blood draw site or claustrophobia during MRI, not new or worsened medical conditions, as this is an observational study and not a clinical trial. However, any other participant issues will be reported as unanticipated problems (UAPs). The CCC will monitor clinical sites to ensure compliance with study data collection procedures and data management requirements using both on-site and remote monitoring procedures.
Discussion
The A2CPS is a large multisite project that involves a diverse group of scientists. Our collaborative approach included group discussions with representation from all centers and the NIH staff to make decisions by consensus. Efforts were made to balance competing concerns of obtaining the most complete participant phenotyping while minimizing participant burden. The Consortium launched in September 2019 with the CCC, DIRC, ODGCs and only one MCC. The second MCC was brought into the Consortium in August 2020, resulting in unique challenges to harmonize data-collection procedures and consider inclusion of additional biomarkers. The integration of MCCs at different timepoints ultimately delayed the start of data collection for MCC1 and placed the MCCs on different study timelines.
The A2CPS has further experienced challenges associated with COVID-19, leading to adaptations in processes required to complete study design, implement protocols, and initiate recruitment. The Consortium has adapted to having all-virtual meetings to facilitate collaboration and continued study development. Training in study procedures were transitioned from onsite training to virtual training. There were unanticipated delays in regulatory approvals due to increased staff workloads due to COVID-19 protocol changes and increased administrative burden. Additionally, elective surgeries and recruitment were paused at times due to pandemic mitigation strategies. The Consortium also experienced delays in obtaining blood sample collection materials.
Despite these challenges, preliminary checks at both MCCs show high data completion rates for surveys and assessments, with neuroimaging, omics, and psychophysical assessments satisfying established quality control metrics. Once completed, the data generated from the A2CPS program will help identify risk and resilience biomarkers and biosignatures of the transition from acute to chronic pain. These results will be available to the scientific community for further data mining, and provide the potential to inform future clinical trials, identify novel therapeutic targets, advance personalized acute pain treatment strategies, thereby transforming the management of acute pain events.
In summary, the A2CPS brings together a consortium of experts in pain science, imaging, QST, omics, and the management of large clinical studies from multiple sites across the United States. This multisite observational study provides the ability to comprehensively phenotype a large cohort of individuals across multiple biopsychosocial domains in a relatively short time. The ultimate goal is to identify and validate novel biomarker candidates that can serve as a part of a final biosignature for predicting the susceptibility or resilience to the development of chronic post-surgical pain. Investigation of previously identified and novel biomarkers in a large cohort will help identify which biomarkers provide greatest prognostic value in a larger cohort and may help direct the focus of clinically relevant biomarkers that can be implemented in daily practice. Results from this study may not be generalizable to all surgical and chronic pain cohorts as contributing biopsychosocial factors may vary by diagnosis. Additionally, recruitment of a diverse population is inherently challenging, and the sample recruited for this observational study may not be representative of individuals from all races, ethnicities, and sociodemographic backgrounds. Beyond stated Consortium aims, A2CPS will provide the broad research community with a valuable dataset to drive future hypothesis-driven research.
Study Status and Progress
Recruitment started in 2021 and is ongoing at both MCCs according to established procedures in A2CPS Protocol #6.0 (February 14, 2022). As of January 2022, a total of 337 individuals (276 knee replacement, 61 thoracic surgery) have been enrolled in the study with 288 completing surgery (238 knee replacement, 50 thoracic surgery), 56 reaching the 6-month assessment (54 knee replacement, 2 thoracic surgery) and no participants at the 12-month assessment. A brief summary of completion rates by biomarker domains is provided in Supplementary Table 4. This study is estimated to be completed 48 months from the time of initial participant enrollment to completion of the final participant.
Ethics Statement
This study was approved by the University of Iowa Institutional Review Board, serving as the central IRB. All patients/participants provided their written informed consent prior to study participation.
Author Contributions
The manuscript was drafted by GB, LF-L, EB, DCl, CB, JB, RM, XZ, ML, DCo, LL, KF, KJ, MO, CL, AS, and PD, and all authors reviewed and provided edits to the manuscript. All authors contributed to the study conception and design and approved the final manuscript.
Funding
The A2CPS Consortium was supported by the National Institutes of Health Common Fund, which was managed by the OD/Office of Strategic Coordination (OSC). Consortium components include: Clinical Coordinating Center (UO1NS077179), Data Integration and Resource Center (UO1NS077352), Omics Data Generation Centers (U54DA049116, U54DA049115, U54DA09113), and Multisite Clinical Centers: MCC 1 (UM1NS112874) and MCC 2 (UM1NS118922). Postdoctoral support for GB provided by the National Institutes of Neurological Disease and Stroke (NINDS) of the NIH under Award Number: U24NS112873-03S2. Training and research support for DCo provided by the NHLBI under Award Number: K08 HL159327.
Author Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge the University of Iowa for coordination and implementation of the single IRB model, the External Program Consultants and the Patient Consultants for their contributions to the design and implementation of the projects.
Glossary
Abbreviations
- 5TSTS
Five-Times Sit-to-Stand
- 10MWT
10-meter Walk Test
- A2CPS
Acute to Chronic Pain Signatures
- ABCD
Adolescent Brain Cognitive Development
- ACE
Adverse Childhood Experience
- BFI-2-S
Big Five Inventory-2 Short Form
- BPI
Brief Pain Inventory
- CCC
Clinical Coordinating Center
- CDE
Common Data Elements
- cIRB
Central Institutional Review Board
- CPM
Conditioned pain modulation
- CPSP
Chronic post-surgical pain
- COMM
Current Opioid Misuse Measure
- DIRC
Data Integration and Resource Center
- DSMP
Data Safety Monitoring Plan
- EPPIC-Net
Early Phase Pain Investigation Clinical Network
- FABQ-PA
Fear Avoidance Beliefs Questionnaire –
- Physical Activity
- fMRI
Functional magnetic resonance imaging
- GAD-7
General Anxiety Disorder –
- 7
- GCMS
Gas chromatography mass spectrometry
- GCP
Good clinical practice
- GLP
Good laboratory practice
- GMP
Good manufacturing practices
- GSA
Global Screening Array
- GSS
General Sensory Sensitivity
- HEAL
Helping to End Addiction Long-term
- KOOS-12
Knee Injury and Osteoarthritis Outcome Score - 12
- LC-MS/MS
Liquid Chromatography with tandem mass spectrometry
- MCC
Multisite Clinical Center
- MISCI
Multidimensional Inventory of Subjective Cognitive Impairment
- MRI
Magnetic resonance imaging
- NCATS
National Center for Advancing Translational Sciences
- NIH
National Institutes of Health
- NPRS
Numerical Pain Rating Scale
- NRS
Numerical Rating Scale
- ODGC
Omics Data Generation Center
- PCS-6
Pain Catastrophizing Scale –
- 6
- PD-Q
PainDETECT Questionnaire
- PGIC
Patient's Global Impression of Change
- PGx
Pharmacogenomics
- PHQ-8
Patient Health Questionnaire –
- 8
- PPT
Pressure Pain Threshold
- PROMIS
Patient-Reported Outcomes Measurement Information System
- PRS
Pain Resilience Scale
- QC
Quality control
- QST
Quantitative sensory testing
- RAPA
Rapid Assessment of Physical Activity
- REDCap
Research Electronic Data Capture
- rs-fMRI
Resting-state fMRI
- SCQ
Self-Administered Comorbidity Questionnaire
- SOP
Standard operating procedure
- SSI
Symptom Severity Index
- TACC
Texas Advanced Computing Center
- TAPS, Tobacco, Alcohol, Prescription medication
and other Substance use
- TS
Temporal summation
- UAP
Unanticipated problem
- UUID
Universally unique identifier
- VATS
Video-assisted thoracic surgery.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.849214/full#supplementary-material
References
- 1.Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, et al. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. (2018) 67:1001–6. 10.15585/mmwr.mm6736a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. (2012) 13:715–24. 10.1016/j.jpain.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 3.Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2017) 390:1211–59. 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. (2021) 397:2082–97. 10.1016/S0140-6736(21)00393-7 [DOI] [PubMed] [Google Scholar]
- 5.Murray CJ, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, et al. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. (2013) 310:591–608. 10.1001/jama.2013.13805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epping-Jordan JE, Wahlgren DR, Williams RA, Pruitt SD, Slater MA, Patterson TL, et al. Transition to chronic pain in men with low back pain: predictive relationships among pain intensity, disability, and depressive symptoms. Health Psychol. (1998) 17:421–7. 10.1037/0278-6133.17.5.421 [DOI] [PubMed] [Google Scholar]
- 7.Iversen MD, Daltroy LH, Fossel AH, Katz JN. The prognostic importance of patient pre-operative expectations of surgery for lumbar spinal stenosis. Patient Educ Couns. (1998) 34:169–78. 10.1016/S0738-3991(97)00109-2 [DOI] [PubMed] [Google Scholar]
- 8.Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. (2007) 30:77–94. 10.1007/s10865-006-9085-0 [DOI] [PubMed] [Google Scholar]
- 9.Sullivan MJL, Thibault P, Simmonds MJ, Milioto M, Cantin AP, Velly AM. Pain, perceived injustice and the persistence of post-traumatic stress symptoms during the course of rehabilitation for whiplash injuries. Pain. (2009) 145:325–31. 10.1016/j.pain.2009.06.031 [DOI] [PubMed] [Google Scholar]
- 10.Shaw WS, Means-Christensen AJ, Slater MA, Webster JS, Patterson TL, Grant I, et al. Psychiatric disorders and risk of transition to chronicity in men with first onset low back pain. Pain Med. (2010) 11:1391–400. 10.1111/j.1526-4637.2010.00934.x [DOI] [PubMed] [Google Scholar]
- 11.Rakel BA, Blodgett NP, Zimmerman BM, Logsden-Sackett N, Clark C, Noiseux N, et al. Predictors of postoperative movement and resting pain following total knee replacement. Pain. (2012) 153:2192–203. 10.1016/j.pain.2012.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Theunissen M, Peters ML, Bruce J, Gramke HF, Marcus MA. Preoperative anxiety and catastrophizing: a systematic review and meta-analysis of the association with chronic postsurgical pain. Clin J Pain. (2012) 28:819–41. 10.1097/AJP.0b013e31824549d6 [DOI] [PubMed] [Google Scholar]
- 13.Althaus A, Arranz Becker O, Neugebauer E. Distinguishing between pain intensity and pain resolution: using acute post-surgical pain trajectories to predict chronic post-surgical pain. Eur J Pain. (2014) 18:513–21. 10.1002/j.1532-2149.2013.00385.x [DOI] [PubMed] [Google Scholar]
- 14.Lewis GN, Rice DA, McNair PJ, Kluger M. Predictors of persistent pain after total knee arthroplasty: a systematic review and meta-analysis. Br J Anaesth. (2015) 114:551–61. 10.1093/bja/aeu441 [DOI] [PubMed] [Google Scholar]
- 15.Noiseux NO, Callaghan JJ, Clark CR, Zimmerman MB, Sluka KA, Rakel BA. Preoperative predictors of pain following total knee arthroplasty. J Arthroplasty. (2014) 29:1383–7. 10.1016/j.arth.2014.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD. The role of psychosocial processes in the development and maintenance of chronic pain. J Pain. (2016) 17 (9 Suppl.):T70–92. 10.1016/j.jpain.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wirth B, Humphreys BK, Peterson C. Importance of psychological factors for the recovery from a first episode of acute non-specific neck pain - a longitudinal observational study. Chiropr Man Therap. (2016) 24:9. 10.1186/s12998-016-0090-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berube M, Choiniere M, Laflamme YG, Gelinas C. Acute to chronic pain transition in extremity trauma: a narrative review for future preventive interventions (part 1). Int J Orthop Trauma Nurs. (2016) 23:47–59. 10.1016/j.ijotn.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 19.Dave AJ, Selzer F, Losina E, Usiskin I, Collins JE, Lee YC, et al. The association of pre-operative body pain diagram scores with pain outcomes following total knee arthroplasty. Osteoarthritis Cartilage. (2017) 25:667–75. 10.1016/j.joca.2016.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang MMH, Hartley RL, Leung AA, Ronksley PE, Jette N, Casha S, et al. Preoperative predictors of poor acute postoperative pain control: a systematic review and meta-analysis. BMJ Open. (2019) 9:e025091. 10.1136/bmjopen-2018-025091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schade V, Semmer N, Main CJ, Hora J, Boos N. The impact of clinical, morphological, psychosocial and work-related factors on the outcome of lumbar discectomy. Pain. (1999) 80:239–49. 10.1016/S0304-3959(98)00210-3 [DOI] [PubMed] [Google Scholar]
- 22.Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother. (2009) 9:723–44. 10.1586/ern.09.20 [DOI] [PubMed] [Google Scholar]
- 23.Afari N, Ahumada SM, Wright LJ, Mostoufi S, Golnari G, Reis V, et al. Psychological trauma and functional somatic syndromes: a systematic review and meta-analysis. Psychosom Med. (2014) 76:2–11. 10.1097/PSY.0000000000000010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stickley A, Koyanagi A, Kawakami N, Group WHOWMHJS. Childhood adversities and adult-onset chronic pain: results from the World Mental Health Survey, Japan. Eur J Pain. (2015) 19:1418–27. 10.1002/ejp.672 [DOI] [PubMed] [Google Scholar]
- 25.Steine IM, Winje D, Krystal JH, Bjorvatn B, Milde AM, Gronli J, et al. Cumulative childhood maltreatment and its dose-response relation with adult symptomatology: findings in a sample of adult survivors of sexual abuse. Child Abuse Negl. (2017) 65:99–111. 10.1016/j.chiabu.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 26.Brown RC, Plener PL, Braehler E, Fegert JM, Huber-Lang M. Associations of adverse childhood experiences and bullying on physical pain in the general population of Germany. J Pain Res. (2018) 11:3099–108. 10.2147/JPR.S169135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schrepf A, Naliboff B, Williams DA, Stephens-Shields AJ, Landis JR, Gupta A, et al. Adverse childhood experiences and symptoms of urologic chronic pelvic pain syndrome: a multidisciplinary approach to the study of chronic pelvic pain research network study. Ann Behav Med. (2018) 52:865–77. 10.1093/abm/kax060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. (2010) 66:149–60. 10.1016/j.neuron.2010.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. (2012) 15:1117–9. 10.1038/nn.3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Apkarian AV, Baliki MN, Farmer MA. Predicting transition to chronic pain. Curr Opin Neurol. (2013) 26:360–7. 10.1097/WCO.0b013e32836336ad [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mansour AR, Baliki MN, Huang L, Torbey S, Herrmann KM, Schnitzer TJ, et al. Brain white matter structural properties predict transition to chronic pain. Pain. (2013) 154:2160–8. 10.1016/j.pain.2013.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vachon-Presseau E, Tetreault P, Petre B, Huang L, Berger SE, Torbey S, et al. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain. (2016) 139:1958–70. 10.1093/brain/aww100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, et al. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain. (2008) 138:22–8. 10.1016/j.pain.2007.10.033 [DOI] [PubMed] [Google Scholar]
- 34.Neogi T, Frey-Law L, Scholz J, Niu J, Arendt-Nielsen L, Woolf C, et al. Sensitivity and sensitisation in relation to pain severity in knee osteoarthritis: trait or state? Ann Rheum Dis. (2015) 74:682–8. 10.1136/annrheumdis-2013-204191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valencia C, Fillingim RB, Bishop M, Wu SS, Wright TW, Moser M, et al. Investigation of central pain processing in postoperative shoulder pain and disability. Clin J Pain. (2014) 30:775–86. 10.1097/AJP.0000000000000029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Georgopoulos V, Akin-Akinyosoye K, Zhang W, McWilliams DF, Hendrick P, Walsh DA. Quantitative sensory testing and predicting outcomes for musculoskeletal pain, disability, and negative affect: a systematic review and meta-analysis. Pain. (2019) 160:1920–32. 10.1097/j.pain.0000000000001590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petersen KK, Arendt-Nielsen L, Simonsen O, Wilder-Smith O, Laursen MB. Presurgical assessment of temporal summation of pain predicts the development of chronic postoperative pain 12 months after total knee replacement. Pain. (2015) 156:55–61. 10.1016/j.pain.0000000000000022 [DOI] [PubMed] [Google Scholar]
- 38.Izumi M, Petersen KK, Laursen MB, Arendt-Nielsen L, Graven-Nielsen T. Facilitated temporal summation of pain correlates with clinical pain intensity after hip arthroplasty. Pain. (2017) 158:323–32. 10.1097/j.pain.0000000000000764 [DOI] [PubMed] [Google Scholar]
- 39.Petersen KK, Simonsen O, Laursen MB, Arendt-Nielsen L. The role of preoperative radiologic severity, sensory testing, and temporal summation on chronic postoperative pain following total knee arthroplasty. Clin J Pain. (2018) 34:193–7. 10.1097/AJP.0000000000000528 [DOI] [PubMed] [Google Scholar]
- 40.Sturmer T, Raum E, Buchner M, Gebhardt K, Schiltenwolf M, Richter W, et al. Pain and high sensitivity C reactive protein in patients with chronic low back pain and acute sciatic pain. Ann Rheum Dis. (2005) 64:921–5. 10.1136/ard.2004.027045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schrepf A, O'Donnell M, Luo Y, Bradley CS, Kreder K, Lutgendorf S, et al. Inflammation and inflammatory control in interstitial cystitis/bladder pain syndrome: associations with painful symptoms. Pain. (2014) 155:1755–61. 10.1016/j.pain.2014.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schrepf A, Bradley CS, O'Donnell M, Luo Y, Harte SE, Kreder K, et al. Toll-like receptor 4 and comorbid pain in interstitial cystitis/bladder pain syndrome: a multidisciplinary approach to the study of chronic pelvic pain research network study. Brain Behav Immun. (2015) 49:66–74. 10.1016/j.bbi.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nielsen CS, Knudsen GP, Steingrimsdottir OA. Twin studies of pain. Clin Genet. (2012) 82:331–40. 10.1111/j.1399-0004.2012.01938.x [DOI] [PubMed] [Google Scholar]
- 44.Vargas-Alarcon G, Fragoso JM, Cruz-Robles D, Vargas A, Vargas A, Lao-Villadoniga JI, et al. Catechol-O-methyltransferase gene haplotypes in Mexican and Spanish patients with fibromyalgia. Arthritis Res Ther. (2007) 9:R110. 10.1186/ar2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cargnin S, Magnani F, Viana M, Tassorelli C, Mittino D, Cantello R, et al. An opposite-direction modulation of the COMT Val158Met polymorphism on the clinical response to intrathecal morphine and triptans. J Pain. (2013) 14:1097–106. 10.1016/j.jpain.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 46.Martinez-Jauand M, Sitges C, Rodriguez V, Picornell A, Ramon M, Buskila D, et al. Pain sensitivity in fibromyalgia is associated with catechol-O-methyltransferase (COMT) gene. Eur J Pain. (2013) 17:16–27. 10.1002/j.1532-2149.2012.00153.x [DOI] [PubMed] [Google Scholar]
- 47.Rut M, Machoy-Mokrzynska A, Reclawowicz D, Sloniewski P, Kurzawski M, Drozdzik M, et al. Influence of variation in the catechol-O-methyltransferase gene on the clinical outcome after lumbar spine surgery for one-level symptomatic disc disease: a report on 176 cases. Acta Neurochir. (2014) 156:245–52. 10.1007/s00701-013-1895-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Omair A, Mannion AF, Holden M, Fairbank J, Lie BA, Hagg O, et al. Catechol-O-methyltransferase (COMT) gene polymorphisms are associated with baseline disability but not long-term treatment outcome in patients with chronic low back pain. Eur Spine J. (2015) 24:2425–31. 10.1007/s00586-015-3866-5 [DOI] [PubMed] [Google Scholar]
- 49.Kolesnikov Y, Gabovits B, Levin A, Veske A, Qin L, Dai F, et al. Chronic pain after lower abdominal surgery: do catechol-O-methyl transferase/opioid receptor mu-1 polymorphisms contribute? Mol Pain. (2013) 9:19. 10.1186/1744-8069-9-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Linnstaedt SD, Hu J, Bortsov AV, Soward AC, Swor R, Jones J, et al. mu-Opioid receptor gene A118 G variants and persistent pain symptoms among men and women experiencing motor vehicle collision. J Pain. (2015) 16:637–44. 10.1016/j.jpain.2015.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh JA, Gabriel S, Lewallen D. The impact of gender, age, and preoperative pain severity on pain after TKA. Clin Orthop Relat Res. (2008) 466:2717–23. 10.1007/s11999-008-0399-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Liu Z, Chen S, Ye X, Xie W, Hu C, et al. Pre-surgery beliefs about pain and surgery as predictors of acute and chronic post-surgical pain: a prospective cohort study. Int J Surg. (2018) 52:50–5. 10.1016/j.ijsu.2018.02.032 [DOI] [PubMed] [Google Scholar]
- 53.Buvanendran A, Della Valle CJ, Kroin JS, Shah M, Moric M, Tuman KJ, et al. Acute postoperative pain is an independent predictor of chronic postsurgical pain following total knee arthroplasty at 6 months: a prospective cohort study. Reg Anesth Pain Med. (2019) 44:e100036. 10.1136/rapm-2018-100036 [DOI] [PubMed] [Google Scholar]
- 54.Hetmann F, Kongsgaard UE, Sandvik L, Schou-Bredal I. Prevalence and predictors of persistent post-surgical pain 12 months after thoracotomy. Acta Anaesthesiol Scand. (2015) 59:740–8. 10.1111/aas.12532 [DOI] [PubMed] [Google Scholar]
- 55.Bruce J, Thornton AJ, Scott NW, Marfizo S, Powell R, Johnston M, et al. Chronic preoperative pain and psychological robustness predict acute postoperative pain outcomes after surgery for breast cancer. Br J Cancer. (2012) 107:937–46. 10.1038/bjc.2012.341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams NM, Johnstone JM, Everson NW. The diagnostic value of symptoms and signs in childhood abdominal pain. J R Coll Surg Edinb. (1998) 43:390–2. [PubMed] [Google Scholar]
- 57.Yang XH, Zhang BL, Gu YH, Zhan XL, Guo LL, Jin HM. Association of sleep disorders, chronic pain, and fatigue with survival in patients with chronic kidney disease: a meta-analysis of clinical trials. Sleep Med. (2018) 51:59–65. 10.1016/j.sleep.2018.06.020 [DOI] [PubMed] [Google Scholar]
- 58.Luo ZY, Li LL, Wang D, Wang HY, Pei FX, Zhou ZK. Preoperative sleep quality affects postoperative pain and function after total joint arthroplasty: a prospective cohort study. J Orthop Surg Res. (2019) 14:378. 10.1186/s13018-019-1446-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Young Casey C, Greenberg MA, Nicassio PM, Harpin RE, Hubbard D. Transition from acute to chronic pain and disability: a model including cognitive, affective, and trauma factors. Pain. (2008) 134:69–79. 10.1016/j.pain.2007.03.032 [DOI] [PubMed] [Google Scholar]
- 60.Rice DA, McNair PJ, Lewis GN, Mannion J. Experimental knee pain impairs submaximal force steadiness in isometric, eccentric, and concentric muscle actions. Arthritis Res Ther. (2015) 17:259. 10.1186/s13075-015-0768-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomazeau J, Rouquette A, Martinez V, Rabuel C, Prince N, Laplanche JL, et al. Acute pain factors predictive of post-operative pain and opioid requirement in multimodal analgesia following knee replacement. Eur J Pain. (2016) 20:822–32. 10.1002/ejp.808 [DOI] [PubMed] [Google Scholar]
- 62.Hadlandsmyth K, Sabic E, Zimmerman MB, Sluka KA, Herr KA, Clark CR, et al. Relationships among pain intensity, pain-related distress, and psychological distress in pre-surgical total knee arthroplasty patients: a secondary analysis. Psychol Health Med. (2017) 22:552–63. 10.1080/13548506.2016.1189581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thomazeau J, Rouquette A, Martinez V, Rabuel C, Prince N, Laplanche JL, et al. Predictive Factors of Chronic Post-Surgical Pain at 6 Months Following Knee Replacement: Influence of Postoperative Pain Trajectory and Genetics. Pain Physician. (2016) 19:E729–41. 10.36076/ppj/2019.19.E729 [DOI] [PubMed] [Google Scholar]
- 64.FDA-NIH Biomarker Working Group . BEST (Biomarkers, EndpointS, and other Tools) Resource. Silver Spring, MD; Bethesda, MD: Food and Drug Administration (US); National Institutes of Health (US) (2016). [PubMed] [Google Scholar]
- 65.Turk DC, Monarch ES. Biopsychosocial Perspective on Chronic Pain. Psychological Approaches to Pain Management: A Practitioner's Handbook, 2nd ed. New York, NY: Guilford Press; (2002). p. 3–29. [Google Scholar]
- 66.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. (2006) 367:1618–25. 10.1016/S0140-6736(06)68700-X [DOI] [PubMed] [Google Scholar]
- 67.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. (2011) 152 (3 Suppl.):S2–S15. 10.1016/j.pain.2010.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khelemsky Y, Noto CJ. Preventing post-thoracotomy pain syndrome. Mt Sinai J Med. (2012) 79:133–9. 10.1002/msj.21286 [DOI] [PubMed] [Google Scholar]
- 69.Borsook D, Kussman BD, George E, Becerra LR, Burke DW. Surgically induced neuropathic pain: understanding the perioperative process. Ann Surg. (2013) 257:403–12. 10.1097/SLA.0b013e3182701a7b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weinstein EJ, Levene JL, Cohen MS, Andreae DA, Chao JY, Johnson M, et al. Local anaesthetics and regional anaesthesia versus conventional analgesia for preventing persistent postoperative pain in adults and children. Cochrane Database Syst Rev. (2018) 6:CD007105. 10.1002/14651858.CD007105.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open. (2012) 2:e000435. 10.1136/bmjopen-2011-000435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rice DA, Kluger MT, McNair PJ, Lewis GN, Somogyi AA, Borotkanics R, et al. Persistent postoperative pain after total knee arthroplasty: a prospective cohort study of potential risk factors. Br J Anaesth. (2018) 121:804–12. 10.1016/j.bja.2018.05.070 [DOI] [PubMed] [Google Scholar]
- 73.Gunaratne R, Pratt DN, Banda J, Fick DP, Khan RJK, Robertson BW. Patient dissatisfaction following total knee arthroplasty: a systematic review of the literature. J Arthroplasty. (2017) 32:3854–60. 10.1016/j.arth.2017.07.021 [DOI] [PubMed] [Google Scholar]
- 74.Canovas F, Dagneaux L. Quality of life after total knee arthroplasty. Orthop Traumatol Surg Res. (2018) 104:S41–S6. 10.1016/j.otsr.2017.04.017 [DOI] [PubMed] [Google Scholar]
- 75.Kalso E, Perttunen K, Kaasinen S. Pain after thoracic surgery. Acta Anaesthesiol Scand. (1992) 36:96–100. 10.1111/j.1399-6576.1992.tb03430.x [DOI] [PubMed] [Google Scholar]
- 76.Perttunen K, Tasmuth T, Kalso E. Chronic pain after thoracic surgery: a follow-up study. Acta Anaesthesiol Scand. (1999) 43:563–7. 10.1034/j.1399-6576.1999.430513.x [DOI] [PubMed] [Google Scholar]
- 77.Gotoda Y, Kambara N, Sakai T, Kishi Y, Kodama K, Koyama T. The morbidity, time course and predictive factors for persistent post-thoracotomy pain. Eur J Pain. (2001) 5:89–96. 10.1053/eujp.2001.0225 [DOI] [PubMed] [Google Scholar]
- 78.Gottschalk A, Ochroch EA. Clinical and demographic characteristics of patients with chronic pain after major thoracotomy. Clin J Pain. (2008) 24:708–16. 10.1097/AJP.0b013e318174badd [DOI] [PubMed] [Google Scholar]
- 79.Steegers MA, Snik DM, Verhagen AF, van der Drift MA, Wilder-Smith OH. Only half of the chronic pain after thoracic surgery shows a neuropathic component. J Pain. (2008) 9:955–61. 10.1016/j.jpain.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 80.Bayman EO, Brennan TJ. Incidence and severity of chronic pain at 3 and 6 months after thoracotomy: meta-analysis. J Pain. (2014) 15:887–97. 10.1016/j.jpain.2014.06.005 [DOI] [PubMed] [Google Scholar]
- 81.Institute for Translational Medicine . Available online at: https://chicagoitm.org/ (accessed December 2, 2021).
- 82.University of Illinois at Chicago Center for Magneti Resonance Research . Available online at: https://chicago.medicine.uic.edu/departments/centers/center-magnetic-resonance-research/ (accessed December 2, 2021).
- 83.The Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative (2021) . Available online at: https://mstcvs.org/ (accessed December 2, 2021).
- 84.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. (2019) 95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.CareEvolution RKStudio (2021). Available online at: https://careevolution.com/rkstudio/ (accessed December 2, 2021).
- 87.Wandner LD, Domenichiello AF, Beierlein J, Pogorzala L, Aquino G, Siddons A, et al. NIH's helping to end addiction long-term(SM) initiative (NIH HEAL Initiative) clinical pain management common data element program. J Pain. (2021) 23:370–8. 10.1016/j.jpain.2021.08.005 [DOI] [PubMed] [Google Scholar]
- 88.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. (2008) 9:105–21. 10.1016/j.jpain.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 89.Brummett CM, Bakshi RR, Goesling J, Leung D, Moser SE, Zollars JW, et al. Preliminary validation of the Michigan Body Map. Pain. (2016) 157:1205–12. 10.1097/j.pain.0000000000000506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singap. (1994) 23:129–38. [PubMed] [Google Scholar]
- 91.Freynhagen R, Baron R, Gockel U, Tolle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. (2006) 22:1911–20. 10.1185/030079906X132488 [DOI] [PubMed] [Google Scholar]
- 92.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RL, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. (2016) 46:319–29. 10.1016/j.semarthrit.2016.08.012 [DOI] [PubMed] [Google Scholar]
- 93.Ameringer S, Elswick RK, Jr., Menzies V, Robins JL, Starkweather A, et al. Psychometric evaluation of the patient-reported outcomes measurement information system fatigue-short form across diverse populations. Nurs Res. (2016) 65:279–89. 10.1097/NNR.0000000000000162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McNeely J, Wu LT, Subramaniam G, Sharma G, Cathers LA, Svikis D, et al. Performance of the tobacco, alcohol, prescription medication, and other substance use (TAPS) tool for substance use screening in primary care patients. Ann Intern Med. (2016) 165:690–9. 10.7326/M16-0317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Butler SF, Budman SH, Fernandez KC, Houle B, Benoit C, Katz N, et al. Development and validation of the current opioid misuse measure. Pain. (2007) 130:144–56. 10.1016/j.pain.2007.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Butler SF, Budman SH, Fanciullo GJ, Jamison RN. Cross validation of the current opioid misuse measure to monitor chronic pain patients on opioid therapy. Clin J Pain. (2010) 26:770–6. 10.1097/AJP.0b013e3181f195ba [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee injury and osteoarthritis outcome score (KOOS)–development of a self-administered outcome measure. J Orthop Sports Phys Ther. (1998) 28:88–96. 10.2519/jospt.1998.28.2.88 [DOI] [PubMed] [Google Scholar]
- 98.Ringsted TK, Wildgaard K, Kreiner S, Kehlet H. Pain-related impairment of daily activities after thoracic surgery: a questionnaire validation. Clin J Pain. (2013) 29:791–9. 10.1097/AJP.0b013e318278d4e2 [DOI] [PubMed] [Google Scholar]
- 99.Bayman EO, Lennertz R, Brennan TJ. Pain-related limitations in daily activities following thoracic surgery in a United States population. Pain Physician. (2017) 20:E367–E78. 10.36076/ppj.2017.E378 [DOI] [PubMed] [Google Scholar]
- 100.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. (2010) 63:1179–94. 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rose M, Bjorner JB, Becker J, Fries JF, Ware JE. Evaluation of a preliminary physical function item bank supported the expected advantages of the patient-reported outcomes measurement information system (PROMIS). J Clin Epidemiol. (2008) 61:17–33. 10.1016/j.jclinepi.2006.06.025 [DOI] [PubMed] [Google Scholar]
- 102.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The rapid assessment of physical activity (RAPA) among older adults. Prev Chronic Dis. (2006) 3:A118. Available online at: http://www.cdc.gov/pcd/issues/2006/oct/06_0001.htm [PMC free article] [PubMed] [Google Scholar]
- 103.Slepian PM, Ankawi B, Himawan LK, France CR. Development and initial validation of the pain resilience scale. J Pain. (2016) 17:462–72. 10.1016/j.jpain.2015.12.010 [DOI] [PubMed] [Google Scholar]
- 104.Ankawi B, Slepian PM, Himawan LK, France CR. Validation of the pain resilience scale in a chronic pain sample. J Pain. (2017) 18:984–93. 10.1016/j.jpain.2017.03.013 [DOI] [PubMed] [Google Scholar]
- 105.Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. (1995) 7:524–32. 10.1037/1040-3590.7.4.52428616005 [DOI] [Google Scholar]
- 106.McWilliams LA, Kowal J, Wilson KG. Development and evaluation of short forms of the pain catastrophizing scale and the pain self-efficacy questionnaire. Eur J Pain. (2015) 19:1342–9. 10.1002/ejp.665 [DOI] [PubMed] [Google Scholar]
- 107.Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A fear-avoidance beliefs questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. (1993) 52:157–68. 10.1016/0304-3959(93)90127-B [DOI] [PubMed] [Google Scholar]
- 108.Piva SR, Fitzgerald GK, Irrgang JJ, Fritz JM, Wisniewski S, McGinty GT, et al. Associates of physical function and pain in patients with patellofemoral pain syndrome. Arch Phys Med Rehabil. (2009) 90:285–95. 10.1016/j.apmr.2008.08.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van Baar ME, Dekker J, Oostendorp RA, Bijl D, Voorn TB, Lemmens JA, et al. The effectiveness of exercise therapy in patients with osteoarthritis of the hip or knee: a randomized clinical trial. J Rheumatol. (1998) 25:2432–9. [PubMed] [Google Scholar]
- 110.Simon CB, Stryker SE, George SZ. Comparison of work-related fear-avoidance beliefs across different anatomical locations with musculoskeletal pain. J Pain Res. (2011) 4:253–62. 10.2147/JPR.S22457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Geisser ME, Strader Donnell C, Petzke F, Gracely RH, Clauw DJ, Williams DA. Comorbid somatic symptoms and functional status in patients with fibromyalgia and chronic fatigue syndrome: sensory amplification as a common mechanism. Psychosomatics. (2008) 49:235–42. 10.1176/appi.psy.49.3.235 [DOI] [PubMed] [Google Scholar]
- 112.Schrepf A, Williams DA, Gallop R, Naliboff BD, Basu N, Kaplan C, et al. Sensory sensitivity and symptom severity represent unique dimensions of chronic pain: a MAPP research network study. Pain. (2018) 159:2002–11. 10.1097/j.pain.0000000000001299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. (2009) 114:163–73. 10.1016/j.jad.2008.06.026 [DOI] [PubMed] [Google Scholar]
- 114.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. (2006) 166:1092–7. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 115.Hahn EA, DeWalt DA, Bode RK, Garcia SF, DeVellis RF, Correia H, et al. New English and Spanish social health measures will facilitate evaluating health determinants. Health Psychol. (2014) 33:490–9. 10.1037/hea0000055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hahn EA, Devellis RF, Bode RK, Garcia SF, Castel LD, Eisen SV, et al. Measuring social health in the patient-reported outcomes measurement information system (PROMIS): item bank development and testing. Qual Life Res. (2010) 19:1035–44. 10.1007/s11136-010-9654-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kratz AL, Schilling S, Goesling J, Williams DA. The PROMIS FatigueFM profile: a self-report measure of fatigue for use in fibromyalgia. Qual Life Res. (2016) 25:1803–13. 10.1007/s11136-016-1230-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yu L, Buysse DJ, Germain A, Moul DE, Stover A, Dodds NE, et al. Development of short forms from the PROMIS sleep disturbance and sleep-related impairment item banks. Behav Sleep Med. (2011) 10:6–24. 10.1080/15402002.2012.636266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 120.Perrot S, Lanteri-Minet M. Patients' global impression of change in the management of peripheral neuropathic pain: clinical relevance and correlations in daily practice. Eur J Pain. (2019) 23:1117–28. 10.1002/ejp.1378 [DOI] [PubMed] [Google Scholar]
- 121.Farrar JT, Young JP, Jr., LaMoreaux L, Werth JL, Poole MR. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. (2001) 94:149–58. 10.1016/S0304-3959(01)00349-9 [DOI] [PubMed] [Google Scholar]
- 122.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The self-administered comorbidity questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. (2003) 49:156–63. 10.1002/art.10993 [DOI] [PubMed] [Google Scholar]
- 123.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prevent Med. (1998) 14:245–58. 10.1016/S0749-3797(98)00017-8 [DOI] [PubMed] [Google Scholar]
- 124.Soto CJ, John OP. Short and extra-short forms of the Big Five Inventory-2: The BFI-2-S and BFI-2-XS. J Res in Person. (2017) 68:69–81. 10.1016/j.jrp.2017.02.004 [DOI] [Google Scholar]
- 125.Kaur RP, Shafi G, Benipal RPS, Munshi A. Frequency of pathogenic germline mutations in cancer susceptibility genes in breast cancer patients. Med Oncol. (2018) 35:81. 10.1007/s12032-018-1143-2 [DOI] [PubMed] [Google Scholar]
- 126.Smith EN, Jepsen K, Arias AD, Shepard PJ, Chambers CD, Frazer KA. Genetic ancestry of participants in the National Children's Study. Genome Biol. (2014) 15:R22. 10.1186/gb-2014-15-2-r22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rozowsky J, Kitchen RR, Park JJ, Galeev TR, Diao J, Warrell J, et al. exceRpt: a comprehensive analytic platform for extracellular RNA profiling. Cell Syst. (2019) 8:352–7 e3. 10.1016/j.cels.2019.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Misra BB, Bassey E, Bishop AC, Kusel DT, Cox LA, Olivier M. High-resolution gas chromatography/mass spectrometry metabolomics of non-human primate serum. Rapid Commun Mass Spectrom. (2018) 32:1497–506. 10.1002/rcm.8197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Misra BB, Olivier M. High resolution GC-Orbitrap-MS metabolomics using both electron ionization and chemical ionization for analysis of human plasma. J Proteome Res. (2020) 19:2717–31. 10.1021/acs.jproteome.9b00774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tsugawa H, Cajka T, Kind T, Ma Y, Higgins B, Ikeda K, et al. MS-DIAL: data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat Methods. (2015) 12:523–6. 10.1038/nmeth.3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Blazenovic I, Kind T, Sa MR, Ji J, Vaniya A, Wancewicz B, et al. Structure Annotation of all mass spectra in untargeted metabolomics. Anal Chem. (2019) 91:2155–62. 10.1021/acs.analchem.8b04698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.MassBank of North America . Available online at: https://massbank.us/ (accessed December 2, 2021).
- 133.Bonini P, Kind T, Tsugawa H, Barupal DK, Fiehn O. Retip: retention time prediction for compound annotation in untargeted metabolomics. Anal Chem. (2020) 92:7515–22. 10.1021/acs.analchem.9b05765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cajka T, Smilowitz JT, Fiehn O. Validating quantitative untargeted lipidomics across nine liquid chromatography-high-resolution mass spectrometry platforms. Anal Chem. (2017) 89:12360–8. 10.1021/acs.analchem.7b03404 [DOI] [PubMed] [Google Scholar]
- 135.Matyash V, Liebisch G, Kurzchalia TV, Shevchenko A, Schwudke D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J Lipid Res. (2008) 49:1137–46. 10.1194/jlr.D700041-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tsugawa H, Ikeda K, Takahashi M, Satoh A, Mori Y, Uchino H, et al. A lipidome atlas in MS-DIAL 4. Nat Biotechnol. (2020) 38:1159–63. 10.1038/s41587-020-0531-2 [DOI] [PubMed] [Google Scholar]
- 137.Fan S, Kind T, Cajka T, Hazen SL, Tang WHW, Kaddurah-Daouk R, et al. Systematic error removal using random forest for normalizing large-scale untargeted lipidomics data. Anal Chem. (2019) 91:3590–6. 10.1021/acs.analchem.8b05592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Barupal DK, Fiehn O. Chemical similarity enrichment analysis (ChemRICH) as alternative to biochemical pathway mapping for metabolomic datasets. Sci Rep. (2017) 7:14567. 10.1038/s41598-017-15231-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Qian WJ, Petritis BO, Kaushal A, Finnerty CC, Jeschke MG, Monroe ME, et al. Plasma proteome response to severe burn injury revealed by 18O-labeled “universal” reference-based quantitative proteomics. J Proteome Res. (2010) 9:4779–89. 10.1021/pr1005026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Qian WJ, Monroe ME, Liu T, Jacobs JM, Anderson GA, Shen Y, et al. Quantitative proteome analysis of human plasma following in vivo lipopolysaccharide administration using 16O/18O labeling and the accurate mass and time tag approach. Mol Cell Proteomics. (2005) 4:700–9. 10.1074/mcp.M500045-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu T, Qian WJ, Gritsenko MA, Xiao W, Moldawer LL, Kaushal A, et al. High dynamic range characterization of the trauma patient plasma proteome. Mol Cell Proteomics. (2006) 5:1899–913. 10.1074/mcp.M600068-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mertins P, Yang F, Liu T, Mani DR, Petyuk VA, Gillette MA, et al. Ischemia in tumors induces early and sustained phosphorylation changes in stress kinase pathways but does not affect global protein levels. Mol Cell Proteomics. (2014) 13:1690–704. 10.1074/mcp.M113.036392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hoofnagle AN, Whiteaker JR, Carr SA, Kuhn E, Liu T, Massoni SA, et al. Recommendations for the generation, quantification, storage, and handling of peptides used for mass spectrometry-based assays. Clin Chem. (2016) 62:48–69. 10.1373/clinchem.2015.250563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, et al. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. (2010) 26:966–8. 10.1093/bioinformatics/btq054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Newcomer KL, Krug HE, Mahowald ML. Validity and reliability of the timed-stands test for patients with rheumatoid arthritis and other chronic diseases. J Rheumatol. (1993) 20:21–7. [PubMed] [Google Scholar]
- 146.Bohannon RW. Reference values for the five-repetition sit-to-stand test: a descriptive meta-analysis of data from elders. Percept Mot Skills. (2006) 103:215–22. 10.2466/pms.103.1.215-222 [DOI] [PubMed] [Google Scholar]
- 147.Bohannon RW. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. (1997) 26:15–9. 10.1093/ageing/26.1.15 [DOI] [PubMed] [Google Scholar]
- 148.Bohannon RW, Williams Andrews A. Normal walking speed: a descriptive meta-analysis. Physiotherapy. (2011) 97:182–9. 10.1016/j.physio.2010.12.004 [DOI] [PubMed] [Google Scholar]
- 149.Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain. (2009) 10:556–72. 10.1016/j.jpain.2009.02.002 [DOI] [PubMed] [Google Scholar]
- 150.Granot M, Granovsky Y, Sprecher E, Nir RR, Yarnitsky D. Contact heat-evoked temporal summation: tonic versus repetitive-phasic stimulation. Pain. (2006) 122:295–305. 10.1016/j.pain.2006.02.003 [DOI] [PubMed] [Google Scholar]
- 151.Erpelding N, Davis KD. Neural underpinnings of behavioural strategies that prioritize either cognitive task performance or pain. Pain. (2013) 154:2060–71. 10.1016/j.pain.2013.06.030 [DOI] [PubMed] [Google Scholar]
- 152.Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, et al. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. (2006) 10:77–88. 10.1016/j.ejpain.2005.02.003 [DOI] [PubMed] [Google Scholar]
- 153.Nir RR, Granovsky Y, Yarnitsky D, Sprecher E, Granot M. A psychophysical study of endogenous analgesia: the role of the conditioning pain in the induction and magnitude of conditioned pain modulation. Eur J Pain. (2011) 15:491–7. 10.1016/j.ejpain.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 154.Nir RR, Yarnitsky D. Conditioned pain modulation. Curr Opin Support Palliat Care. (2015) 9:131–7. 10.1097/SPC.0000000000000126 [DOI] [PubMed] [Google Scholar]
- 155.Yarnitsky D, Bouhassira D, Drewes AM, Fillingim RB, Granot M, Hansson P, et al. Recommendations on practice of conditioned pain modulation (CPM) testing. Eur J Pain. (2015) 19:805–6. 10.1002/ejp.605 [DOI] [PubMed] [Google Scholar]
- 156.American College of Radiology Manual on MR Safety . Reston, VA: American College of Radiology; (2020). [Google Scholar]
- 157.Adolescent Brain Cognitive Development (ABCD) Study . (2021). Available online at: https://abcdstudy.org/ (accessed December 2, 2021).
- 158.Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, et al. The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Dev Cogn Neurosci. (2018) 32:43–54. 10.1016/j.dcn.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Colquhoun DA, Shanks AM, Kapeles SR, Shah N, Saager L, Vaughn MT, et al. Considerations for integration of perioperative electronic health records across institutions for research and quality improvement: the approach taken by the multicenter perioperative outcomes group. Anesth Analg. (2020) 130:1133–46. 10.1213/ANE.0000000000004489 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.