Abstract
Blockchain is a novel data architecture characterized by a chronological sequence of blocks in a decentralized manner. We aimed to evaluate the real‐world feasibility of a blockchain‐based dynamic consent platform (METORY) in a decentralized and multicenter trial. The study consisted of three visits (i.e., screening and 2 follow‐up visits) with a 2‐week interval. Each subject was required to report the self‐measured body temperatures and take a virtual investigational drug by entering the unique drug code on the application. To simulate real‐world study settings, two major (i.e., changes in the schedule of body temperature measurement) and three minor protocol amendments (i.e., nonsignificant changes without any changes in the procedures) were set. Overall study completion rates, proportion of consent, and response time to each protocol amendment and adherence were evaluated. A total of 60 subjects (30 in each center) were enrolled in two study centers. All subjects completed the study, and the overall proportion of consent to each protocol amendment was 95.7 ± 13.7% (mean ± SD), with a median response time of 0.2 h. Overall, subjects took 90.8% ± 19.2% of the total drug, whereas compliance with the schedule was 69.1% ± 27.0%. Subjects reported 96.7% ± 4.2% of the total body temperature measurements whereas the adherence to the schedule was 59.0% ± 25.0%, which remarkably decreased after major protocol amendments. In conclusion, we evaluated a blockchain‐based dynamic consent platform in real clinical trial settings. The results suggested that major changes should be avoided unless subjects’ proper understanding is warranted.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Blockchain technology has recently drawn attention in ensuring data integrity in clinical trials due to its immutability and traceability of data. Dynamic consent is a feasible field where blockchain could be adopted. Decentralized clinical trials after coronavirus disease 2019 (COVID‐19) accelerated the adoption of blockchain in clinical trials.
WHAT QUESTION DID THIS STUDY ADDRESS?
The study evaluated the real‐world feasibility of METORY by conducting a decentralized and multicenter clinical trial using virtual drugs.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
The results of our study revealed that the information given on the online platform could often be ignored or misunderstood, despite the prompt consent of the subjects. A system to verify the accurate understanding of the subjects should be incorporated in the platform.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Blockchain‐based dynamic consent could facilitate clinical trials in decentralized settings. The virtual drug approach could be used to evaluate drug behavior prior to the trials.
INTRODUCTION
Blockchain, a data architecture characterized by a chronological sequence of “blocks,” 1 has gained attention as a tool to ensure data integrity. 2 Especially for clinical trials, considerable efforts have been made to guarantee data integrity, which is a key objective mandated in Good Clinical Practice. 3 As blockchain does not allow data to be modified or deleted, data recorded on blockchain are not only immutable but also traceable. 2 , 4 These characteristics are reasons why blockchain can be used in the management of clinical trial data. 2 , 4 In addition, a decentralized data structure is another key feature of blockchain, providing reliable protection from malicious attempts to modify data. 5
A recent advent of the dynamic consent concept is an important factor that justifies the use of blockchain in clinical trials. Dynamic consent is a novel approach to allow granular choice of engagement in response to changes in the study over time via interactive and personalized online interfaces. 6 , 7 As dynamic consent requires a time‐intensive and transparent process, the immutability and traceability of blockchain are highly suitable for the concept. 7 , 8
The introduction of decentralized clinical trials also supports using blockchain in clinical trials. Decentralized clinical trials are characterized by data collection in an extensive environment with little engagement of study intermediaries. 9 Despite the low‐trust environment where the data were collected, the concept remarkably gained attention after the coronavirus disease 2019 (COVID‐19) pandemic. 10 Considering the robust data integrity blockchain provides, blockchain can be a plausible systematic solution for data integrity in decentralized clinical trials.
Several cases that adopted blockchain in clinical trials have been reported. An early attempt to incorporate blockchain into clinical trials obtained study consent via a Bitcoin‐based blockchain network. 11 In this prototype model, investigators and study participants interacted on a web‐based consent module that triggered blockchain transactions. 11 However, as Bitcoin was a public blockchain mainly aimed at cryptocurrency, 12 , 13 the prototype model confronted criticisms in the aspect of scalability and efficiency. 11 Recent attempts engaged next‐generation blockchains, such as Ethereum 14 or Hyperledger fabric, 15 which provided improved functionalities other than cryptocurrency. Private blockchains (e.g., Hyperledger fabric) are recognized as more suitable for clinical trials, as they can grant access by study centers or personnel. 5 , 16
In contrast to the current literature given, the actual clinical trials that incorporated blockchain in the dynamic consent process are relatively few. In addition, several concerns that were raised for dynamic consent are notable. One concern is that the information conveyed by investigators is not always appropriately understood by the study participants. 17 Another concern is the accessibility of the digital interface, particularly for vulnerable populations (e.g., elderly individuals). 17 , 18 The patients’ burden of making decisions over time is also a significant concern. 17 For the successful establishment of blockchain‐based consent frameworks, real‐world evaluation needs to be conducted.
To implement a blockchain‐based dynamic consent framework, we developed a platform named METORY. In this study, we evaluated the real‐world feasibility of METORY by conducting a decentralized and multicenter clinical trial using virtual drugs.
METHODS
Study subjects
Adults who were able to use web and smart applications were eligible for the study. Subjects with cognitive impairment that could hamper the use of the application were excluded. Subjects should also be able to report the self‐measured body temperatures via the application and understand the instructions from the investigators delivered via the application.
Study design
The study was conducted for 4 weeks. The study consisted of three visits (i.e., screening and 2 follow‐up visits) with a 2‐week interval (Figure 1a). Subjects installed the application and were given instructions on using the application at the screening visit. At each follow‐up visit, subjects completed the questionnaires on their user experience with the application. All other study procedures were self‐conducted in home‐based settings.
FIGURE 1.
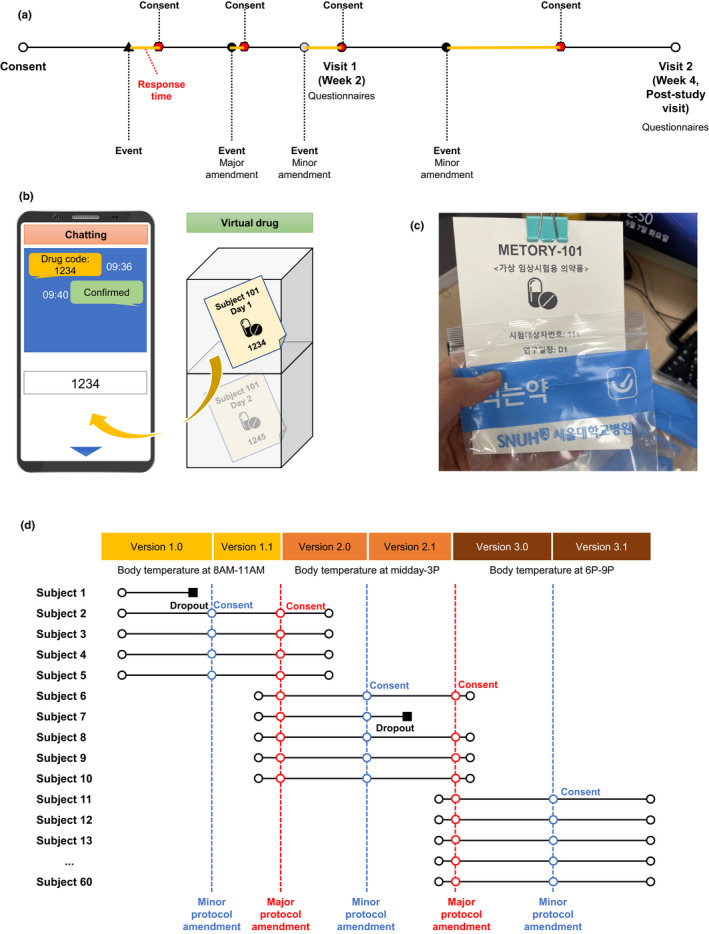
Study design. Schematic representation of the study schedule (a), administration of virtual drug (b), actual virtual drug used (c), and protocol amendments (d)
Each subject was required to report the self‐measured body temperatures and take a virtual investigational drug for COVID‐19 daily on the application. Each virtual investigational drug consisted of a subject number, study schedule, and a drug code with a unique four‐digit figure (Figure 1b). Entering the drug code on the application was regarded as taking the drug, and the time of administration was recorded on the application. Subjects were scheduled to take the investigational drug in the morning with a 4‐hour window period (i.e., 7 a.m. to 11 a.m.). Subjects and investigators interacted using the chatting system in the application during the study period (Figure 1c and Table S1).
The study was approved by the institutional review board of Seoul National University Hospital and Jeonbuk National University Hospital and was conducted in accordance with the Declaration of Helsinki (ClinicalTrials.gov registration no. NCT05047016).
Study scenarios
To simulate the real‐world study settings, two major and three minor protocol amendments were scheduled during the entire study period. Subjects could be enrolled during the entire study period and followed the study protocol at the time of enrollment (Figure 1d and Table S1). The major protocol amendments involved changes in the procedure schedule and were noted as changes in the integer part of the protocol version (e.g., versions 2.1 to 3.0). The minor protocol amendments were changes not related to study design or procedures (e.g., correction of typos).
In addition, to simulate real‐world dropout events due to serious adverse events, four out of 30 subjects in each study center were randomly notified of study discontinuation. When dropout was determined, the subject discontinued taking virtual drugs (entering the drug code on the application) from the time of dropout. Other study schedules were maintained as originally planned (i.e., self‐measurement of body temperature and follow‐up visits).
Blockchain‐based dynamic consent platform
The platform incorporated a web and application‐based user interface where subjects could give consent. We developed a separate user interface for the subjects and the investigators to maximize the convenience of the consent process. Each subject accessed the web or application and made a user account after the authentication process. The verified user was then given access to the platform.
When a subject and an investigator signed on an informed consent form, consent‐related information was integrated in the study management part of the system. The information was then sent to the decentralized application (dAPP), which was the only component that mediated the study management part and the blockchain part. The dAPP could write the consent‐related information onto the blockchain part after the validation process in the blockchain part. The dAPP could also could fetch the consent‐related information from the blockchain part requested by the user.
The blockchain part was developed using Hyperledger Fabric, an enterprise‐grade private blockchain framework. 19 Hyperledger Fabric was selected as it could not only ensure integrity of data but also enable an efficient center‐level authorization management. Newly added data in the blockchain architecture could be only appended to form a “chain” structure, which guaranteed immutability. Data must be validated by the participating peers in the blockchain prior to appendage, which was called the “consensus” process. The consensus process was mediated by the “platform orderer nodes.” The nodes also performed certification and access management process for each user. Comprehensively, restricted access of the credible accounts and immutability endorsed the implementation of the private blockchain architecture (Figure S1).
Assessment of the response to study consent and adherence
The count and proportion of consent to the total encountered protocol amendments were calculated. Study completion rates were evaluated excluding scheduled dropout due to serious adverse events. Response time was calculated as the difference between protocol amendment and when subjects signed the amended consent form.
Adherence evaluation comprised drug adherence (adherence related to drug administration) and procedural adherence (adherence related to body temperature measurement). Drug adherence consisted of administration of the right drug and adherence to the drug administration schedule. Procedural adherence comprised whether the body temperature was measured and adherence to the procedural schedule.
Statistical analysis
The sample size was not formally estimated given the exploratory nature of the study. Continuous variables are summarized as the mean, standard deviation, median, minimum, and maximum. Count data were summarized as the mean and standard deviation of the proportions in each subject and overall count of a specific event to the total counts. R version 4.1.0 (R Core Team, Vienna, Austria) was used for visualization and statistical analyses.
RESULTS
Subject disposition and response to consent
A total of 60 subjects (30 subjects for each center) were enrolled, and all subjects completed the study. The overall proportion of consent to each protocol amendment was 95.7 ± 13.2% (presented as the mean ± standard deviation). The median response time to each amendment was 0.2 h (Table 1). The entire response to consent of each subject is presented in Figure 2.
TABLE 1.
Summary of response to study consent and adherence
| Center 1 (n = 30) | Center 2 (n = 30) | Overall (n = 60) | |
|---|---|---|---|
| Response to study consent | |||
| Proportion of consents (%) | 93.3 ± 17.3 (129/139) | 98.3 ± 6.3% (118/120) | 95.7 ± 13.2 (247/259) |
| Study completion rate (%) | 100.0 (30/30) | 100.0 (30/30) | 100.0 (60/60) |
| Response time, h | 0.3 [0.0–91.6] | 0.2 [0.0–43.3] | 0.2 [0.0–91.6] |
| Drug adherence | |||
| Administration of right drug (%) | 89.6 ± 20.6 (753/840) | 92.0 ± 17.9 (773/840) | 90.8 ± 19.2% (1526/1680) |
| Adherence to the drug administration schedule (%) | 75.7 ± 27.8 (636/840) | 62.5 ± 24.9 (525/840) | 69.1 ± 27.0 (1161/1680) |
| Procedural adherence | |||
| Whether the body temperature was measured (%) | 96.7 ± 4.2 (812/840) | 98.5 ± 5.4 (827/840) | 97.6 ± 4.9 (1639/1680) |
| Adherence to the procedural schedule (%) | 50.5 ± 24.7 (424/840) | 67.5 ± 22.6 (567/840) | 59.0 ± 25.0 (991/1680) |
Data are presented as mean ± standard deviation of the proportions in each subject (only overall values for study completion rate) and overall counts to total except for response time. Response time is presented as median [minimum‐maximum].
FIGURE 2.
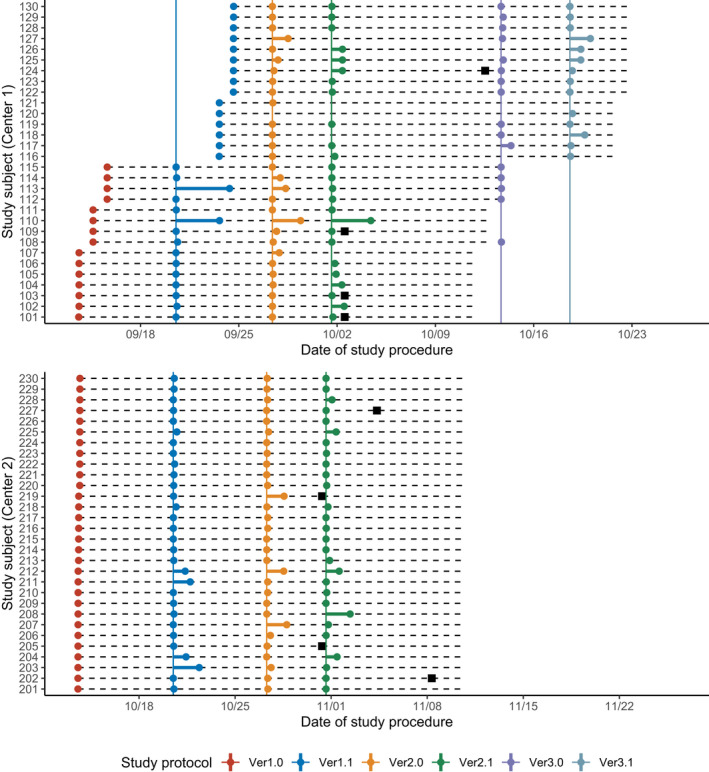
Summary of the responses to protocol amendments. Dots (●) represent each subject’s consent and each protocol amendment is denoted as colors. Dashed horizontal lines represent scheduled study duration (28 days) of each subject and solid vertical lines represent the scheduled date of each protocol amendment. Black squares (■) denote scheduled dropout of each subject
Study adherence
Overall, the subjects took 90.8% ± 19.2% of the total drug amount (Table 1, Figure 3), whereas adherence to the schedule was 69.1 ± 27.0% (Table 1, Figure 4). Similarly, subjects reported 97.6 ± 4.9% of the total body temperature measurements (Table 1, Figure 5), whereas adherence to the schedule was 59.0% ± 25.0% (Table 1, Figure 6). In both centers, adherence to the procedural schedule remarkably decreased after the major protocol amendment (i.e., study protocol versions 1.1 to 2.0) where procedural schedules were changed (Figure 6).
FIGURE 3.
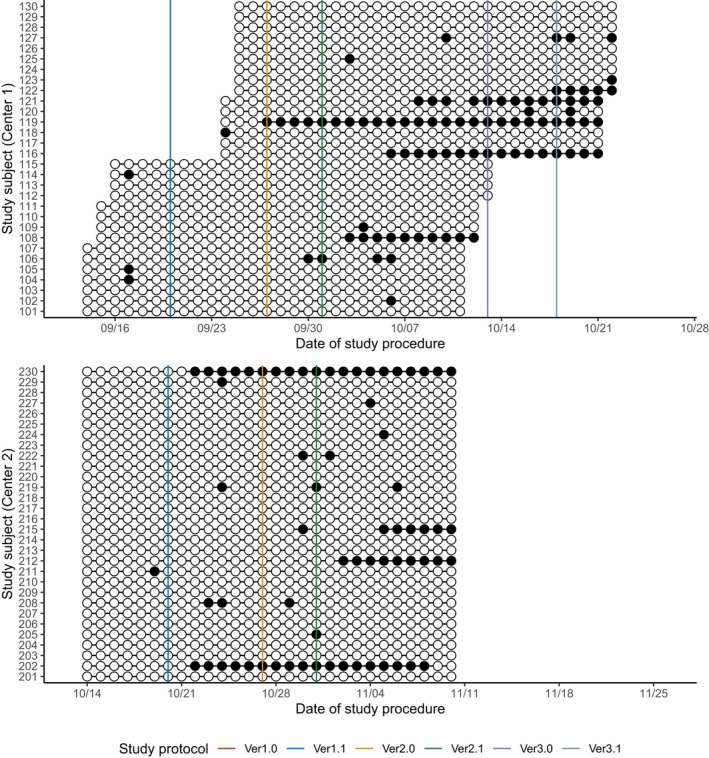
Summary of drug adherence: adherence to the right drug. White circles (○) represent the administration of the correct study drug while black circles (●) represent the incorrect conducts. Solid vertical lines represent the scheduled date of each protocol amendment. There were no changes to the administration of drug during the study
FIGURE 4.
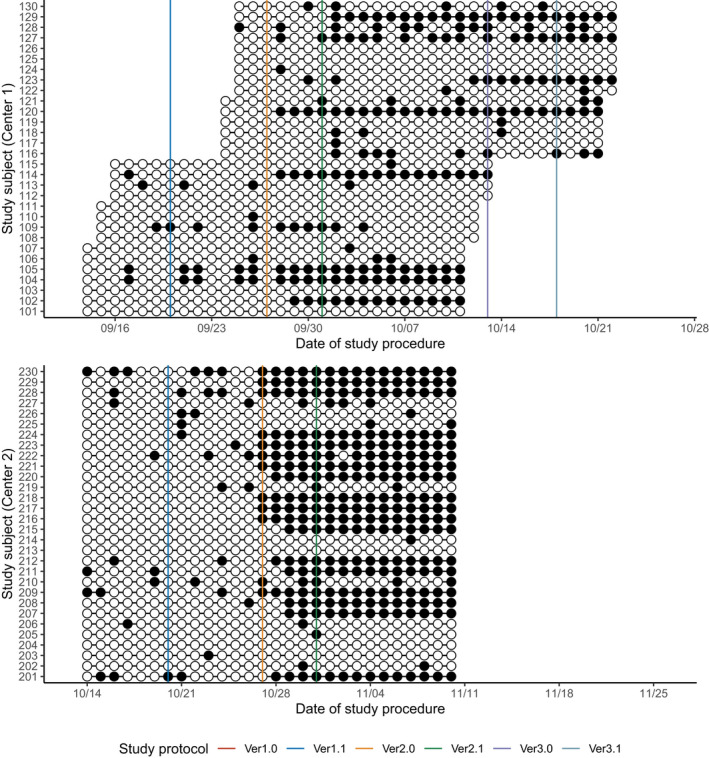
Summary of the drug adherence: adherence to the drug administration schedule. White circles (○) represent the administration of the study drug at the right schedule (within the scheduled time window) while black circles (●) represent the incorrect conducts. Solid vertical lines represent the scheduled date of each protocol amendment. There were no changes to the administration of drug during the study
FIGURE 5.
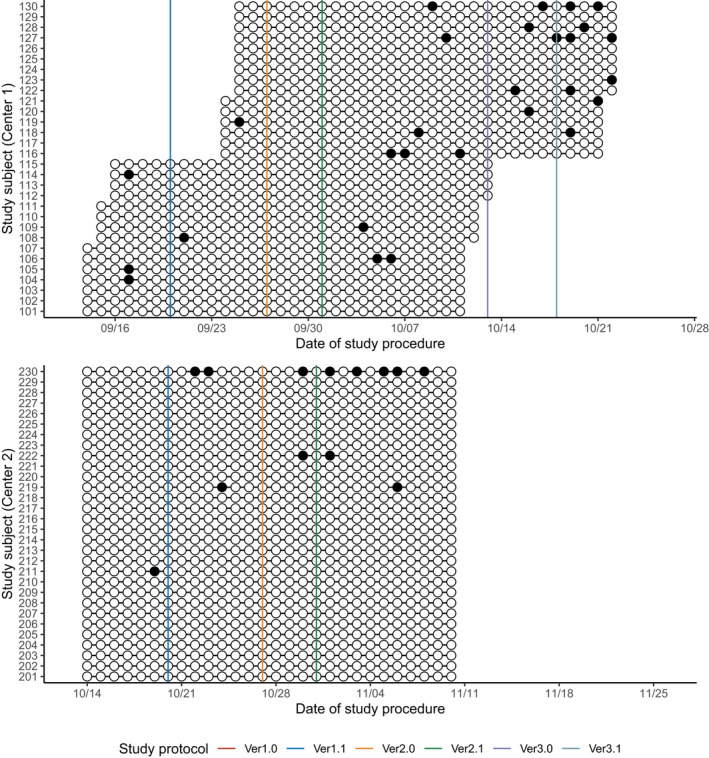
Summary of procedural adherence: whether body temperature was measured. White circles (○) represent the measurement of the body temperature while black circles (●) represent the missing measurements. Solid vertical lines represent the scheduled date of each protocol amendment. The scheduled time for body temperature measurements was changed at the major amendments
FIGURE 6.
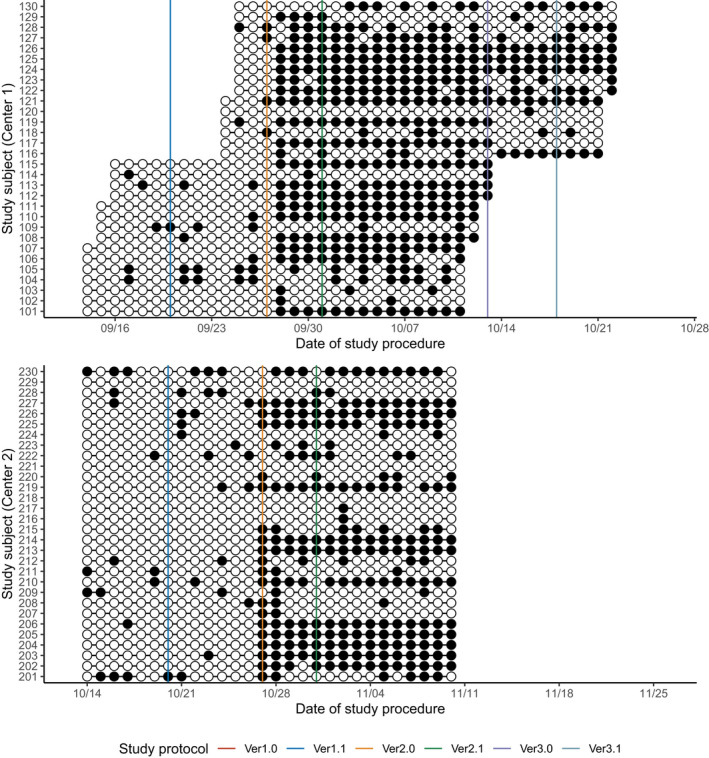
Summary of procedural adherence: adherence to the procedural schedule. White circles (○) represent the measurement of the body temperature at the right schedule (within the scheduled time window) while black circles (●) represent the incorrect conducts. Solid vertical lines represent the scheduled date of each protocol amendment. The scheduled time for body temperature measurements was changed at the major amendments
DISCUSSION
The blockchain‐based dynamic consent platform was able to retain consent from subjects for 4 weeks with a prompt response within an hour. Adherence results demonstrated that subjects could perform home‐based drug administration with an ~90% overall adherence rate. However, compliance with the drug and procedural schedule was ~70%, which was much lower than the overall adherence rate.
Despite the main purpose of providing sufficient information to the subjects, the practicability of dynamic consent is often questioned. One concern is that subjects are exposed to “unwanted information,” which could be a large burden when participating in a study. 20 , 21 Another concern is that accessing information via the web or through an application can be challenging for several vulnerable patient groups (e.g., elderly). 22
The results of our study revealed that the information given on the online platform could often be ignored or misunderstood. We noted that many subjects gave consent to the amended study protocols without an accurate understanding of the content. Most subjects in center 1 stuck to the initial procedure (i.e., body temperature measurement in the morning). A similar phenomenon was noted in center 2. In addition, several subjects in center 2 confused changes in body temperature measurement with those in drug administration. This resulted in unintended protocol deviations in the drug schedule, which could be a highly risky situation in real clinical trial settings.
The protocol deviations strongly suggested that the system to verify the accurate understanding of the subjects is required. We found that compliance with the study procedures was high at the beginning of the study, when the investigators directly gave instructions on the study procedures. The compliance dramatically decreased after the notification of the protocol amendment via the application. This implied that several systemic components that can aid efficient online communication are necessary. Such components include audio or video component comprehension quizzes. 23 , 24 It was supported by several opinions from the subjects in our study that the content provided in the electronic file was difficult to understand.
To our knowledge, this was the first trial to utilize virtual drugs to evaluate study adherence in a decentralized clinical trial. As most decentralized clinical trials involving home‐based drug administration and remote monitoring, 25 , 26 the accurate tracking of drug behavior is important. The virtual drugs enabled tracking drug adherence with little cost and risk. This approach can be applied to further research, such as remote delivery of the medication. 27
We also showed the practicality of blockchain‐based platforms to ensure data integrity in decentralized settings. 5 , 28 METORY provided stable interactions between subjects and investigators. All consent information and procedures were tracked through the blockchain platform and free from tampering. Utilization of the private blockchain made it possible to provide separate authorization by centers and personnel.
Our study had some limitations. The virtual drugs could not accurately reflect the events after administering the actual drugs, especially for adverse events. The absence of comparators (i.e., paper‐based consent) also restricted the interpretation of the results. Notwithstanding, our study was able to demonstrate the real‐world feasibility of a blockchain‐based dynamic consent platform with quantitative outcomes.
In conclusion, we evaluated a blockchain‐based dynamic consent platform in real clinical trial settings. The results suggested that major changes should be avoided unless subjects’ proper understanding is warranted.
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
K.Y.H., M.‐G.K., and S.L. wrote the manuscript. K.Y.H., S.J., M.‐G.K., and S.L. designed the research. K.Y.H., S.J.M., S.J., M.‐J.K., W.Y., and M.J. performed the research. K.Y.H., S.J., M.‐J.K., and M.J. analyzed data.
Supporting information
Supplementary Material
Huh KY, Moon SJ, Jeong S‐U, et al. Evaluation of a blockchain‐based dynamic consent platform (METORY) in a decentralized and multicenter clinical trial using virtual drugs. Clin Transl Sci. 2022;15:1257‐1268. doi: 10.1111/cts.13246
Min‐Gul Kim and SeungHwan Lee should be considered joint corresponding author.
Funding information
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number: HI19C0332)
Contributor Information
Min‐Gul Kim, Email: mgkim@jbnu.ac.kr.
SeungHwan Lee, Email: leejh413@snu.ac.kr.
REFERENCES
- 1. Zheng Z, Xie S, Dai H, Chen X, Wang H. An overview of blockchain technology: Architecture, consensus, and future trends. 2017 IEEE international congress on big data (BigData congress). 2017;557‐564.
- 2. Shi S, He D, Li L, et al. Applications of blockchain in ensuring the security and privacy of electronic health record systems: A survey. Comput Secur. 2020;97:101966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khin NA, Francis G, Mulinde J, et al. Data integrity in global clinical trials: discussions from Joint US food and drug administration and UK medicines and healthcare products regulatory agency good clinical practice workshop. Clin Pharmacol Ther. 2020;108:949‐963. [DOI] [PubMed] [Google Scholar]
- 4. Benchoufi M, Ravaud P. Blockchain technology for improving clinical research quality. Trials. 2017;18:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wong DR, Bhattacharya S, Butte AJ. Prototype of running clinical trials in an untrustworthy environment using blockchain. Nat Commun. 2019;10:917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaye J, Whitley EA, Lund D, et al. Dynamic consent: a patient interface for twenty‐first century research networks. Eur J Hum Genet. 2015;23:141‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Albanese G, Calbimonte J‐P, Schumacher M, Calvaresi D. Dynamic consent management for clinical trials via private blockchain technology. J Ambient Intelligence Humanized Comput. 2020;11:4909‐4926. [Google Scholar]
- 8. Velmovitsky PE, Miranda PASES, Vaillancourt H, et al. A blockchain‐based consent platform for active assisted living: modeling study and conceptual framework. J Med Internet Res. 2020;22:e20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khozin S, Coravos A. Decentralized trials in the age of real‐world evidence and inclusivity in clinical investigations. Clin Pharmacol Ther. 2019;106:25‐27. [DOI] [PubMed] [Google Scholar]
- 10. De Brouwer W, Patel CJ, Manrai AK, Rodriguez‐Chavez IR, Shah NR. Empowering clinical research in a decentralized world. NPJ Digit Med. 2021;4:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benchoufi M, Porcher R, Ravaud P. Blockchain protocols in clinical trials: Transparency and traceability of consent. F1000Res. 2017;6:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakamoto S. A peer‐to‐peer electronic cash system. Bitcoin Project website. https://bitcoin.org/bitcoin.pdf. Accessed February 14, 2022.
- 13. Zhao W. Blockchain technology: development and prospects. Natl Sci Rev. 2019;6:369‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maslove DM, Klein J, Brohman K, Martin P. Using blockchain technology to manage clinical trials data: a proof‐of‐concept study. JMIR Med Inform. 2018;6:e11949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hang L, Kim B, Kim K, Kim D. A permissioned blockchain‐based clinical trial service platform to improve trial data transparency. Biomed Res Int. 2021;2021:5554487. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16. Zhuang Y, Sheets L, Shae Z, Tsai JJP, Shyu CR. Applying blockchain technology for health information exchange and persistent monitoring for clinical trials. AMIA Annu Symp Proc. 2018;2018:1167‐1175. [PMC free article] [PubMed] [Google Scholar]
- 17. Lunt H, Connor S, Skinner H, Brogden G. Electronic informed consent: the need to redesign the consent process for the digital age. Intern Med J. 2019;49:923‐929. [DOI] [PubMed] [Google Scholar]
- 18. Nouri SS, Avila‐Garcia P, Cemballi AG, et al. Assessing mobile phone digital literacy and engagement in user‐centered design in a diverse, safety‐net population: mixed methods study. JMIR Mhealth Uhealth. 2019;7:e14250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Androulaki E, Barger A, Bortnikov V, et al. Hyperledger fabric: a distributed operating system for permissioned blockchains. Proceedings of the Thirteenth EuroSys Conference. Article 30 (2018). arXiv.org Preprint. https://doi.org/ 10.1145/3190508.3190538.. [DOI]
- 20. Steinsbekk KS, Kåre Myskja B, Solberg B. Broad consent versus dynamic consent in biobank research: is passive participation an ethical problem? Eur J Hum Genet. 2013;21:897‐902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Teare HJA, Prictor M, Kaye J. Reflections on dynamic consent in biomedical research: the story so far. Eur J Hum Genet. 2021;29:649‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wee R. Dynamic consent in the digital age of biology. J Prim Health Care. 2013;5:259‐261. [PubMed] [Google Scholar]
- 23. Vanaken HI, Masand SN. Awareness and collaboration across stakeholder groups important for econsent achieving value‐driven adoption. Ther Innov Regul Sci. 2019;53:724‐735. [DOI] [PubMed] [Google Scholar]
- 24. Madathil KC, Koikkara R, Dorlette‐Paul M, et al. An investigation of format modifications on the comprehension of information in consent form when presented on mobile devices. Proc Hum Fact Ergonom Soc Annual Meeting. 2012;56:921‐925. [Google Scholar]
- 25. Banks MA. Core Concept: In the wake of COVID‐19, decentralized clinical trials move to center stage. Proc Natl Acad Sci USA. 2021;118:e2119097118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Izmailova ES, Ellis R, Benko C. Remote monitoring in clinical trials during the COVID‐19 pandemic. Clin Transl Sci. 2020;13:838‐841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stoa MK, Frail CK, Farley JF, Pestka DL, Blanchard CM. Adaptations made to delivery of comprehensive medication management in the community pharmacy setting during COVID‐19. Explor Res Clin Soc Pharm. 2021;4:100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hashem H, Abufaraj M, Tbakhi A, Sultan I. Obstacles and considerations related to clinical trial research during the COVID‐19 Pandemic. Front Med (Lausanne). 2020;7:598038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


