Abstract
Herein we report the copper-catalyzed silylation of propargylic difluorides to generate axially chiral, tetrasubstituted monofluoroallenes in both good yields (27 examples >80%) and enantioselectivities (82–98% ee). Compared to previously reported synthetic routes to axially chiral allenes (ACAs) from prochiral substrates, a mechanistically distinct reaction has been developed: the enantiodiscrimination between enantiotopic fluorides to set an axial stereocenter. DFT calculations and vibrational circular dichroism (VCD) suggest that β-fluoride elimination from an alkenyl copper intermediate likely proceeds through a syn-β-fluoride elimination pathway rather than an anti-elimination pathway. The effects of the C1-symmetric Josiphos-derived ligand on reactivity and enantioselectivity were investigated. Not only does this report showcase that alkenyl copper species (like their alkyl counterparts) can undergo β-fluoride elimination, but this elimination can be achieved in an enantioselective fashion.
Graphical Abstract
INTRODUCTION
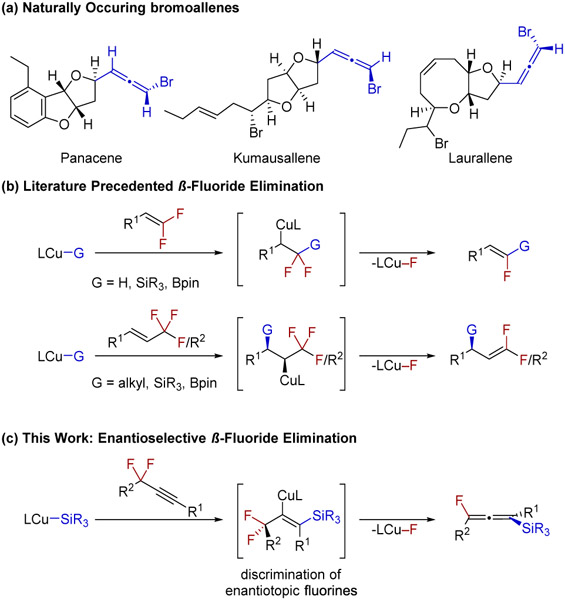
The pursuit of synthetic methods to access structurally diverse allenes stems from their applications in both medicinal and materials chemistry as well as their ability to serve as a reactive functional group for further synthetic manipulations.1,2 These cumulenes can exhibit axial chirality, and most of the naturally occurring allenic compounds that have been isolated are nonracemic.1a-c Although enantiopure bromoallenes have been discovered in nature (Scheme 1a) and axially chiral bromo-, chloro-, and iodoallenes have been synthesized, the corresponding axially chiral fluoroallenes are largely unknown.3,4 Specifically, to the best of our knowledge, there is a single report where an enantioenriched tetrasubstituted, axially chiral monofluoroallene has been prepared, albeit in modest enantioselectivity.4c Due to a lack of general synthetic routes toward this chiral, fluorinated motif, their potential applications have remained unexplored.
Scheme 1.
Allenyl Halide Natural Products and Synthesis of Monofluoro ACAs via Enantioselective β-Fluoride Elimination
An emerging route toward the catalytic synthesis of ACAs has been from prochiral substrates.5 This strategy overcomes the requirement for a stoichiometric amount of a chiral auxiliary and/or enantioenriched substrates. Prochiral substrates that have been transformed into ACAs include propargylic electrophiles, 1,3-enynes, terminal alkynes, 1,3-dienes, racemic allenes, and vinyl triflates.6 In the case of vinyl triflates, it was demonstrated that β-hydride elimination occurred in an enantioselective fashion, revealing a new mechanistic route for the synthesis of ACAs.6f Of the classes of ACAs (1,3-di-, tri-, and tetrasubstituted), tetrasubstituted ACAs remain difficult to synthesize in high enantiopurity.7
Moreover, the incorporation of a functional group directly attached to ACAs that permits further transformations has gained popularity as evidenced by recent reports of boryl and silyl substituted ACAs.8,9 Access to tetrasubstituted, boryl, or silyl monofluoro ACAs would permit an array of further transformations that could generate quaternary, fluorine-containing stereocenters by way of axial-to-point chirality transfer.3a,10
The development of defluorination methods of (poly)-fluorinated compounds has emerged as a complementary strategy to access complex, fluorine-functionalized motifs that have typically been accessed from nonfluorinated substrates.11 Of the metals that catalyze such defluorination reactions, copper has been shown to be exceptionally competent, as evidenced by the numerous reports of catalytic hydrodefluorinations, defluoroborylations, and defluorosilylations of fluoroarenes and fluoroolefins.12 An emerging trend in this field is the enantioselective defluorination of allylic CF3 or CF2R groups, forging stereocenters adjacent to mono- and difluoroolefins (Scheme 1b).13 Recently, a rare example of enantioselective defluorination via oxidative addition to a gem-difluoride has been reported as a method to generate products with a fluorine-containing stereogenic unit.14 We envisioned that a mechanistically different approach to the desymmetrization of difluoromethylene groups, proceeding through an enantioselective β-fluoride elimination reaction, might be employed in the enantioselective synthesis of monofluoro ACAs (Scheme 1c).
To this end, we hypothesized that reaction of propargylic difluorides with a suitable chiral copper nucleophile would form an alkenyl copper species that could undergo an enantioselective β-fluoride elimination to generate tetrasubstituted monofluoro ACAs (Scheme 1c). Potential obstacles toward achieving this transformation included controlling both the regioselectivity15 and enantioselectivity of the process, avoiding undesired reactivity of the alkenyl copper intermediate,16 and preventing further silylation of the product.17 Herein we demonstrate that β-fluoride elimination from an alkenyl copper species is possible and the discrimination of enantiotopic fluorides is a viable elementary process to achieve asymmetric synthesis of ACAs. DFT studies predict that this elimination proceeds through a syn-elimination pathway, which is in contrast to some studies of alkyl copper species that undergo β-fluoride elimination.12b,g-i,13b,c,f,g
RESULTS AND DISCUSSION
We began our investigation by determining if β-fluoride elimination was feasible from a vinyl copper intermediate. In the presence of a suitable base, the borylation of 1a with B2pin2 generated the desired boryl monofluoroallene in 46% NMR yield (eq 1); however, attempts at isolating this product were
 |
(1) |
unsuccessful.8b We hypothesized that the corresponding silyl monofluoroallene of 1a would be isolable and gratifyingly discovered that the silylation of 1a with PhMe2SiBpin (2) led to 1b. After a brief optimization of conditions, over 40 chiral ligands were examined for this transformation. Of the chiral ligands employed, only four gave the desired allene in greater than 20% ee.
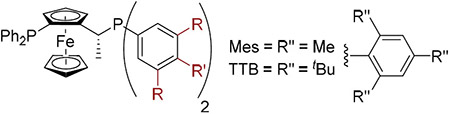
Fortunately (R,S)-Josiphos afforded 1b in a modest yield (71%) and promising enantioselectivity (20% ee). A series of Josiphos ligands were generated to evaluate their steric and electronic effects (see Supporting Information Tables S1 and S2) on the reaction. Josiphos ligands containing aryl groups on both phosphorus atoms demonstrated good reactivity and enantioselectivity, specifically when the alkyldiarylphosphorus moiety possessed bulky 3,5-substituted arenes (Table 1, compare entries 2 and 3; Supporting Information Tables S2 and S3). However, once the 3,5-substituents became sterically too demanding, for example, with the TTB derivative, the reactivity dropped significantly (entry 5). Moreover, it appeared that steric rather than electronic factors played a decisive role in determining the enantioselectivity of the transformation (compare entries 3 and 8).
Table 1.
Structural Effect of Josiphos on Transformationa

| |||
|---|---|---|---|
| entry | R; R' | yield (SM %)b | ee (%)c |
| 1 | Me; H | 59% (23) | 45 |
| 2 | Me; OMe | 31% (14) | 44 |
| 3 | tBu; OMe | 41% (<1) | 70 |
| 4 | Mes; H | 58% (38) | 75 |
| 5 | TTB; H | 3% (79) | nd |
| 6 | TMS; H | 73% (<1) | 75 |
| 7 | TES; H | 66% (<1) | 83 |
| 8 | CF3; H | 43% (<2) | 71 |
| 9 | iPr(F7); H | 44% (43) | 86 |
| |||
Standard conditions: 1a (0.10 mmol, 1.0 equiv), 2 (0.135 mmol, 1.35 equiv), CuCl (5 mol %), L (6 mol %), THF (1.0 mL), 65 °C, 24 h.
Yield was determined by 19F NMR of crude reaction, using PhF as an internal standard.
Determined by HPLC with a chiral stationary phase.
Further optimization led to simplified reaction conditions (Table 2). By lowering the temperature and changing the solvent, the enantiomeric excess of 1b improved to 90%, but the overall conversion of 1a dropped (entry 1). Increasing the amount of phenoxide base resulted in decoordination of the ligand, determined by 31P NMR spectroscopy, and erosion of the ee of 1b.18 By use of an insoluble source of fluoride (CsF), an increase in the chemical yield of 1b was achieved (83%, entry 2). Possible roles of CsF could be either trapping FBpin19 and/or releasing CsOAr from ArOBpin.20 Fortunately, the transformation proceeded with CsF as the sole base, removing the chance of phosphine decoordination due to excess phenoxide.21 The reaction proceeded well in nonpolar, low coordinating solvents (entries 4–7), and the removal of MeCN led to in an increase in catalytic activity (entries 8, 9). Switching to CuOTf·1/2C6H6 as the copper source afforded the desired allene in almost quantitative yield (entry 10).
Table 2.
Reaction Optimizationa

|
|||||
|---|---|---|---|---|---|
| entry | Cu | base (mol %) | solvent | yield (SM %)b | ee (%)c |
| 1 | Cu(MeCN)4BF4 | A (20) | PhMe | 50% (49) | 90 |
| 2 | “ | A (20), CsF (100) | “ | 83% (13) | 90 |
| 3 | “ | CsF (150) | “ | 75% (11) | 91 |
| 4 | Cu(MeCN)4BF4 | “ | THF | 39% (48) | 74 |
| 5 | “ | “ | dioxane | 65% (20) | 91 |
| 6 | “ | “ | cyclohexane | 50% (31) | 87 |
| 7 | “ | “ | MTBE | 75% (10) | 92 |
| 8 | “d | “ | “ | 74% (<1) | 91 |
| 9 | Cu(MeCN)4OTfd | “ | “ | 82% (4) | 90 |
| 10 | CuOTf·1/2C6H6 | CsF (160) | 98% (–) | 90 | |
Standard conditions: 1a (0.10 mmol, 1.0 equiv), 2 (0.135 mmol, 1.35 equiv), [Cu] (5 mol %), L (6 mol %), solvent (1.5 mL), 32 °C, 24 h. A = NaO(2-OMeC6H4).
Yield was determined by 19F NMR of crude reaction, using PhF as an internal standard.
Determined by HPLC with a chiral stationary phase.
MeCN removed before reaction.
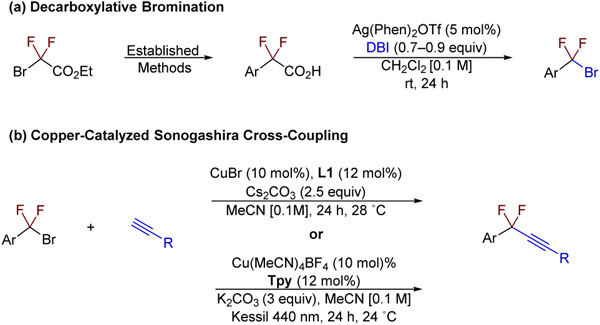
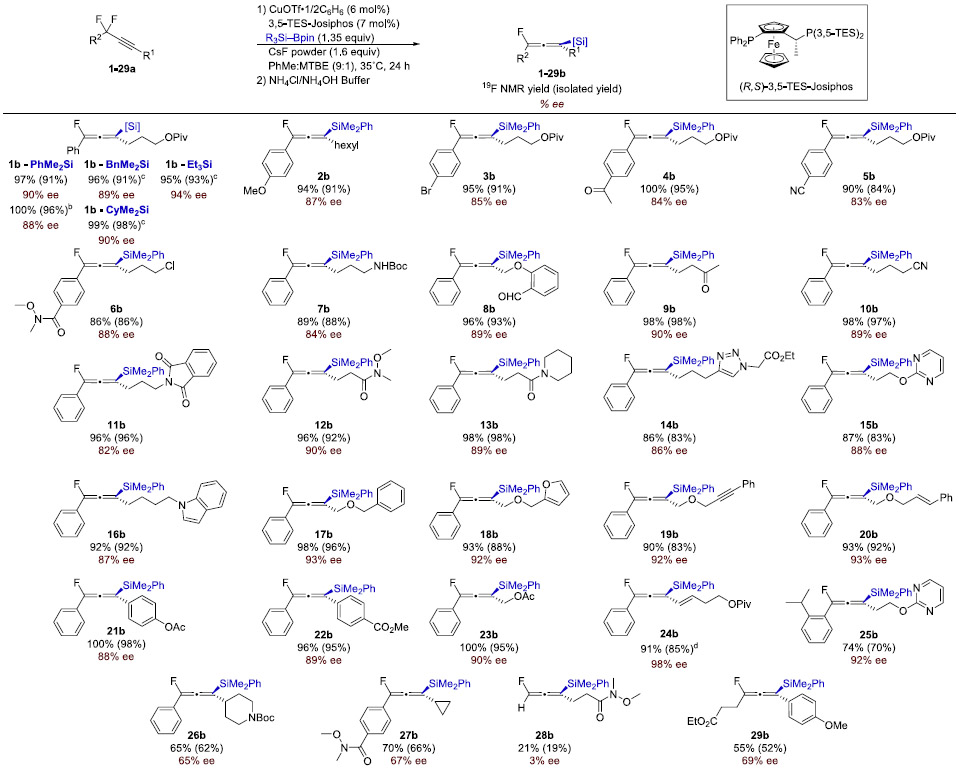
With the optimal conditions established, an alternative synthetic route employing readily available material to prepare propargylic difluorides was developed (Scheme 2). The decarboxylative bromination of difluorocarboxylic acids and the copper-catalyzed Sonogashira cross-coupling of terminal alkynes with difluorobenzyl bromides afforded difluoroalkynes, which were subjected to defluorosilylation under the optimized conditions. A range of functional groups were tolerated in the copper catalyzed transformation, affording the desired allenes 1–24b in high yields (83–98%) and in good enantioselectivities (82–98%) after isolation (Table 3). Notably, alkynes (19b), alkenes (20b), enynes (24b), aldehydes (8b), ketones (9b), propargylic acetates (23b) as well as alkyl and aryl halides (3b and 6b) were tolerated. Coordinating heterocycles (14–16b, 18b, and 25b), amides (7b, 11–13b), and nitriles (5b and 10b) also did not hamper catalysis. Although changing the electronics of the aryl ring slightly decreased the enantiomeric excess of the reaction (1–6b), increasing the steric bulk of the aryl group was well tolerated (25b). Although many functional groups were tolerated, the reaction proved more sensitive to alterations of the substituents directly attached to the allene, which could affect the barrier of silylation of the alkyne (26–27b) or impact the C─F bond strength (28–29b).
Scheme 2.
Improved Synthesis of Propargylic Difluorides
Table 3.
Scope of Asymmetric β-Fluoride Eliminationa
|
Standard conditions: 1–25a (0.20 mmol, 1.0 equiv), 2 (0.27 mmol, 1.35 equiv), CuOTf·½C6H6 (6 mol %), L (7 mol %), CsF (1.6–1.8 equiv), 9:1 PhMe:MTBE or MTBE (3.0 mL), 35–45 °C, 24 h. Reported yields are of isolated allene. Enantiomeric excess was determined by HPLC with a chiral stationary phase.
1a (6.0 mmol), CsF(25%)–CaF2 (1.6 equiv), 30 h.
1a (2.5–3.0 mmol), R3SiBpin (1.35–1.45 equiv), Cu (8–9 mol %), 3,5-TripJosiphos (9–10 mol %), CsF(25%)–CaF2 (1.8–2.5 equiv), MTBE, 28–45°C, 30–48 h.
NaO(2-OMeC6H4) (30 mol %), CsF (1.0 equiv), PhMe, 27 °C.
To explore the scalability of this method, 1b was synthesized on a 6 mmol scale without a significant loss in enantioselectivity or chemical yield. It was discovered that other silylboranes could be utilized for this transformation when (R,S)-3,5-Trip-Josiphos was employed as the ligand (1b-BnMe2Si, 1b-CyMe2Si, and 1b-Et3Si). These allenyl silanes were synthesized on gram scales with comparable yields and enantioselectivities to 1b-PhMe2Si.
Determination of the absolute configuration of allene 10b was achieved using vibrational circular dichroism (VCD).22 After a careful conformational search (see Supporting Information for details) at four levels of theory, B3LYP/6-31G(d), B3PW91/6-31G(d), B3LYP/cc-pVTZ, and B3PW91/cc-pVTZ,23 the resulting conformers were Boltzmann averaged and plotted with a line width of 5 cm−1 to produce the final theoretical spectra. The IR and VCD spectra were then frequency scaled23 for comparison to the experimental data. Calculations at all four levels of theory matched well, proving the absolute configuration of 10b to be S. Of the four methods employed, the best agreement with experimental data was from the B3PW91/cc-pVTZ level. The comparison of experimental and theoretical spectra was quantified24 using BioTools (Jupiter, FL) CompareVOA software, with high neighborhood similarity for IR (90.4) and VCD (69.6), ESI (enantiomeric similarity index) for VCD (62.9), and a confidence level of 99%. Of particular note was the asymmetric allene C─C─C stretch observed at 1933 cm−1, which was one of many closely correlated bands between experiment and theory. In addition to allene 10b, the absolute configuration of 1b-Et3Si, 12b, 17b, and 25b were also determined to be S by VCD analysis (see Supporting Information).
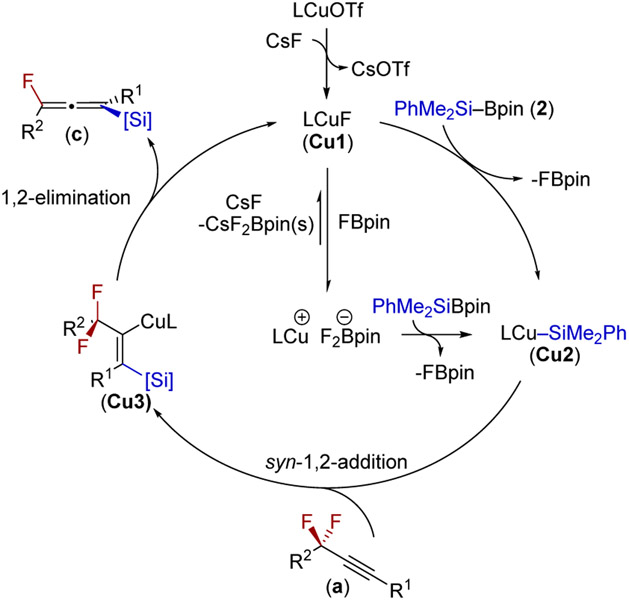
On the basis of previous reports regarding CuF12c,e,16a,21,25 and copper silyl species,15b,f,17d,26 we propose the following mechanism for the copper-catalyzed reaction (Figure 1). First, a complex between Josiphos and [CuOTf] undergoes salt metathesis with CsF to generate JosiphosCuF (Cu1).16a,21,25d σ-Bond metathesis with PhMe2SiBpin (2) generates JosiphosCuSiMe2Ph (Cu2) and releases FBpin.12c-e Subsequent coordination and silylation of the triple bond generates an alkenyl Cu species (Cu3).15a,e,27 A β-fluoride elimination12g regenerates Cu1, which is trapped by FBpin,19,28 215c or decomposes the formed allenylsilane (c). As FBpin is more Lewis acidic than B2pin2,28 the same is likely true with 2. By using a judicious amount of CsF and a nonpolar solvent, we propose that the precipitation of Cs[F2Bpin]19,20 drives this reaction forward. 1H, 19F, and 11B NMR studies have identified Cu1, Cu2, LCuF2Bpin, as well as LCuOH and confirmed the generation of Cu2 from both Cu1 and LCuF2Bpin (see Supporting Information section 7). Cu2 was also shown to react with alkyne 1a, generating both allene 1b and FBpin. It appears that LCuF2Bpin acts as a reservoir of CuIF, and under catalytic conditions, a monomeric or dimeric CuF was not observed. Over the course of the reaction only Cu2, LCuF2Bpin, and LCuOH were observed, which converged to Cu2 after the alkyne has been consumed (see Supporting Information section 7). Although the exact structure of Cu1 is unknown, the speciation of Cu1 appears to be both solvent and temperature dependent, in which the former has been observed for other Josiphos copper halide complexes (see Supporting Information section 7).29 On the basis of our experiments, we propose that the silylation of the alkyne and the β-fluoride elimination reactions are the rate- and selectivity-determining steps, respectively. Using (R,S)-3,5-TES-JosiphosCuF2Bpin as a catalyst, the desired allene 1b was obtained in a similar yield and enantiomeric excess, demonstrating its catalytic competence (see Supporting Information section 7).
Figure 1.
Proposed catalytic cycle.
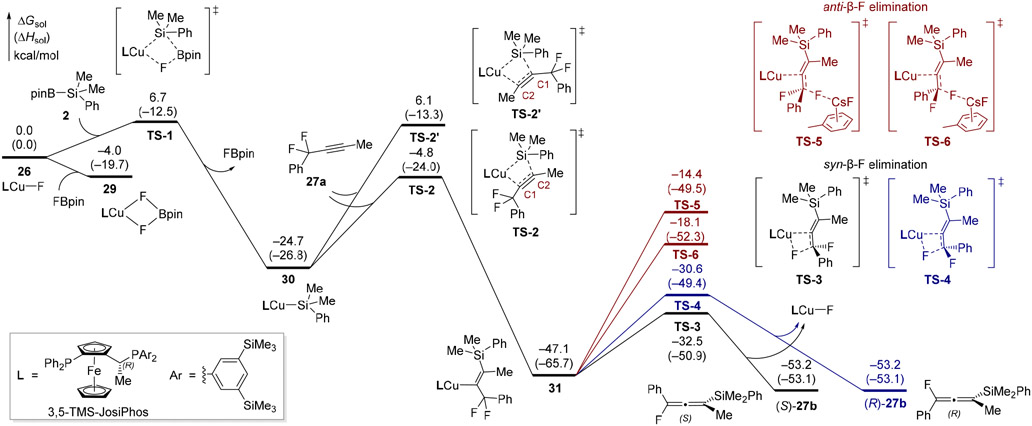
Density functional theory (DFT) calculations were performed to investigate the reaction mechanism and origin of enantioselectivity of this Cu-catalyzed asymmetric silylation of propargylic difluorides. The DFT calculations were performed at the M06/SDD(Cu,Fe,Cs)-6-311+G(d,p)/SMD-(toluene)//B3LYP-D3(zero)/SDD(Cu,Fe,Cs)-6-31G(d) level of theory using difluoroalkyne 27a and PhMe2SiBpin (2) as model substrates. The (R,S)-3,5-TMS-Josiphos ligand was used in the DFT calculations for simplicity because the use of this ligand in the ligand screening provided only slightly lower ee than using (R,S)-3,5-TES-Josiphos (Table 1, entries 6 and 7). On the basis of the proposed catalytic cycle, the computed reaction energy profile is shown in Figure 2. The association of FBpin to monomeric LCuF (26) to form a heterodimer (29) is exergonic by 4.0 kcal/mol, suggesting that the more stable complex 29 can be an off-cycle reservoir of CuIF. Although the dimerization of LCuF is exergonic by 7.3 kcal/mol, its formation is expected to be less favorable than forming 29 due to the low concentrations of LCuF under catalytic conditions (see Figure S1 for detailed discussions about the equilibrium of 26, 29, and the dimer of LCuF).
Figure 2.
Computed reaction energy profiles of the Cu-catalyzed silylation and asymmetric β-fluoride elimination. Gibbs free energies and enthalpies (in kcal/mol) are with respect to the monomeric copper fluoride 26.
The σ-bond metathesis between monomeric LCuF (26) and PhMe2SiBpin30 (2) takes place via a four-membered cyclic transition state (TS-1) to form silyl copper intermediate 30 and FBpin. This step requires a low activation barrier of 6.7 kcal/mol with respect to 26 and is exergonic by 24.7 kcal/mol. Migratory insertion of alkyne 27a into the silyl copper (TS-2) gives alkenyl copper species 31. This migratory insertion is highly regioselective for the formation of Cu─C bond at the alkyne terminus adjacent to the difluoromethylene. The transition state leading to the other regioisomer, TS-2′, is 10.9 kcal/mol higher in energy than TS-2. The high level of regioselectivity is due to steric repulsions between the silyl and the more hindered alkyne terminus (C1) in TS-2′ as well as inductive effects of the difluoro substituents that stabilize the building of negative charge at C1 in TS-2. From 31, both syn12g-i and anti13b β-fluoride elimination pathways were calculated. The syn-elimination of either of the two diastereotopic β-F in 31 (via TS-3 and TS-4) involves a four-membered cyclic transition state, while the anti-β-fluoride elimination is facilitated by CsF as a Lewis acid (via TS-5 and TS-6). The FBPin-facilitated anti-elimination was also computed and is also less favorable than the syn-elimination (see Figure S3). The syn-elimination pathways require much lower barriers than the anti-elimination, which is in contrast to a computational study by Hoveyda and Torker that suggested the β-fluoride elimination from alkyl copper species favors the anti-pathway due to Lewis acid (i.e., Na+) coordination to the F− leaving group and the Bpin group on the substrate.13b In the present study, the lack of such chelating Lewis-acid coordination in the anti-pathway, the weaker Lewis acidity of CsF, and the strain release effect that alleviates steric repulsions between the SiMe2Ph group and the Cu in the syn-elimination transition state changed the reaction mechanism to favor the syn-elimination.31
Among the three key elementary steps in the catalytic cycle, the alkyne migratory insertion (TS-2) has the highest activation free energy (ΔG‡ = 19.9 kcal/mol with respect to 30). This finding is consistent with our experimental results that suggest this step being the rate-determining step (vide supra). The enantioselectivity-determining step is the syn-β-fluoride elimination. TS-3, which leads to the (S)-enantiomer of the monofluoroallene product, is 1.9 kcal/mol more stable than TS-4 that leads to the (R)-enantiomer. The predicted enantioselectivity is consistent with the absolute configuration of the product identified by the VCD analysis.
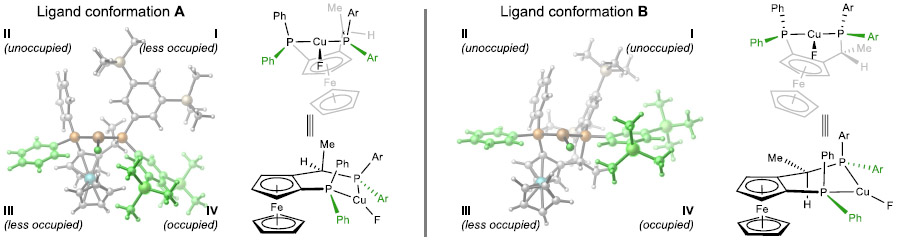
Next, we performed a detailed analysis to investigate the effects of the Josiphos ligand on the reactivity and enantioselectivity of the β-fluoride elimination. Because of the conformational flexibility of the (R,S)-3,5-TMS-Josiphos ligand,32 a careful conformational search was performed for all intermediates and transition states in the catalytic cycle. These calculations revealed at least four different conformers of the 3,5-TMS-Josiphos-supported copper complexes. The two most stable and catalytically active ligand conformations A and B are shown in Table 4 (see Figures S4-S6 for all possible ligand conformations). Ligand conformation A involves a twist-boat-type six-membered ring and is more favorable in the copper fluoride (26), the σ-bond metathesis transition state (TS-1), and the silyl copper intermediate (30). In more sterically encumbered structures, including the migratory insertion transition state (TS-2), alkenyl copper (31), and the β-fluoride elimination transition states (TS-3 and TS-4), ligand conformation B becomes more favorable. This ligand conformation involves a half-chair type six-membered ring, which points the Ar and Ph groups in quadrants I and II away from the Cu center. As such, the bulky SiMe2Ph group is placed between these unoccupied quadrants to minimize steric repulsions between the ligand and the SiMe2Ph group on the substrate.
Table 4.
Relative Free Energies of Conformers of (R,S)-3,5-TMS-Josiphos-Supported Copper Complexes and Transition Statesa
| |||||||
|---|---|---|---|---|---|---|---|
|
syn-β-fluoride elimination |
|||||||
| ligand conformation | LCuF (26) | σ-bond metathesis (TS-1) | silyl copper (30) | migratory insertion (TS-2) | alkenyl copper (31) | TS-3 | TS-4 |
| A | 0.0 | 6.7 | −24.7 | 2.9 | −42.8 | −26.7 | −28.4 |
| B | 4.8 | 10.8 | −23.5 | −4.8 | −47.1 | −32.5 | −30.6 |
All Gibbs free energies are in kcal/mol with respect to the monomeric copper fluoride 26. Bold numbers indicate the favorable ligand conformation.
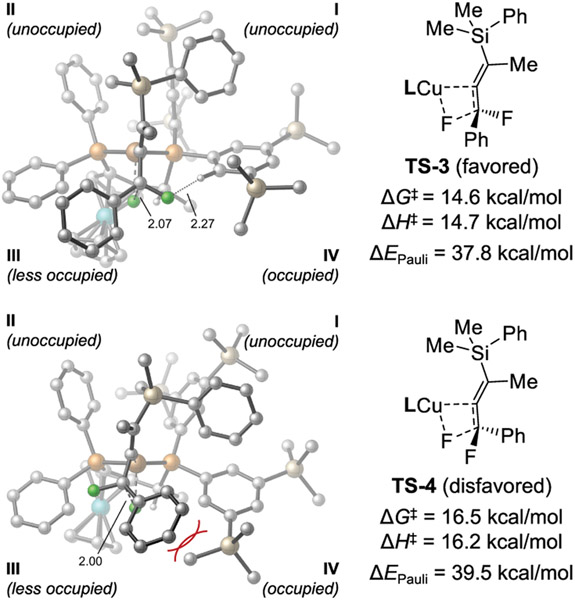
Ligand conformation B not only stabilizes the silylalkenyl copper species but also plays a significant role in controlling the enantioselectivity of the β-fluoride elimination. The P-phenyl group in quadrant III and the P-3,5-TMS-phenyl group in quadrant IV point toward the Cu center and thus occupy these quadrants. The larger size of the 3,5-TMS-phenyl compared to phenyl indicates that the ligand–substrate repulsions in quadrant IV would be more pronounced than those in quadrant III. Indeed, quadrant diagrams of the β-fluoride elimination transition states (Figure 3) support this hypothesis. In the less favorable transition state TS-4, the phenyl group on the substrate is located in the more occupied quadrant IV, leading to steric repulsion with a TMS group on the ligand. By contrast, in the more favorable β-fluoride elimination transition state TS-3, the much smaller fluoro group is located in quadrant IV, and thus the ligand–substrate steric repulsions are diminished. Next, we performed energy decomposition analysis (EDA)33 calculations to quantitatively analyze the ligand–substrate noncovalent interactions in TS-3 and TS-4 (see Supporting Information for computational details). The EDA calculations revealed that the dominant factor controlling the enantioselectivity is the Pauli repulsion (i.e., steric repulsion) between the (R,S)-3,5-TMS-Josiphos ligand and the substrate. The Pauli repulsion energy (ΔEPauli) in TS-4 is 1.7 kcal/mol higher than that in TS-3 and thus destabilizes the former transition state.
Figure 3.
Origin of enantioselectivity in syn-β-fluoride elimination. Most hydrogen atoms are omitted for clarity. Gibbs free energies and enthalpies are with respect to 31. ΔEPauli is the Pauli repulsion energy between the substrate and the Josiphos ligand from EDA calculations.
Finally, we calculated the enantioselectivity-determining syn-β-F elimination transition states of the reaction of the same substrate (27a) catalyzed by a SegPhos-supported Cu complex. The computed enantioselectivity is diminished (ΔΔG‡ = 0.2 kcal/mol, Figure S7), indicating the C2-symmetric SegPhos ligand is not effective for asymmetric induction. This prediction is consistent with the low ee of 12% obtained experimentally at 65°C with 5 mol % CuCl, 40 mol % sodium phenoxide, and 6 mol % SegPhos ligand. Taken together, these ligand effect analyses revealed the unique roles of the conformationally flexible C1-symmetric Josiphos ligand, where it lowers the activation barrier for the rate-determining alkyne migratory insertion step and improves the enantioselectivity of the β-F elimination.
CONCLUSION
The first copper-catalyzed, enantioselective β-fluoride elimination has been achieved. The resulting monofluoro ACAs represent the first examples of fluorine-containing, chiral tetrasubtituted allenes.34 It is expected that such a motif will find value in pharmaceutical and agrochemical chemistry in addition to being a valuable building block for the generation of more elaborate fluorine-containing stereocenters. DFT calculations of the reaction mechanisms predicted that this elimination occurs in a syn-fashion, which is promoted by strain release of the Z-β-silylalkenyl copper intermediate. The unique roles of the C1-symmetric Josiphos-derived ligand in promoting the reactivity and enantioselectivity were investigated. It is believed that lessons learned from this desymmetrization could be leveraged for the creation of other fluorine-containing stereocenters via defluorination pathways.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the National Institutes of Health (Grants R35 GM118190 and R35 GM128779) for financial support. T.J.O. thanks the NSF (Grant DGE 1752814) for a predoctoral fellowship. The College of Chemistry CheXray (NIH Shared Instrumentation Grant S10-RR027172) is acknowledged for X-ray crystallographic data, and we thank Dr. Hasan Celik and UC Berkeley’s NMR facility in the College of Chemistry (CoC-NMR) for spectroscopic assistance. Instruments in the CoC-NMR are supported in part by Grant NIH S10OD024998. DFT calculations were carried out at the Center for Research Computing at the University of Pittsburgh, the Extreme Science and Engineering Discovery Environment (XSEDE), and the TACC Frontera Supercomputer.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.1c05769.
Synthesis procedures, characterization data for all new compounds, additional optimization data, computational details, and Cartesian coordinates of all computed structures (PDF)
The authors declare no competing financial interest.
Contributor Information
Thomas J. O’Connor, Department of Chemistry, University of California, Berkeley, California 94720, United States
Binh Khanh Mai, Department of Chemistry, University of Pittsburgh, Pittsburgh, Pennsylvania 15260, United States.
Jordan Nafie, BioTools, Inc., Jupiter, Florida 33458, United States.
Peng Liu, Department of Chemistry, University of Pittsburgh, Pittsburgh, Pennsylvania 15260, United States.
F. Dean Toste, Department of Chemistry, University of California, Berkeley, California 94720, United States.
REFERENCES
- (1).(a) Modern Allene Chemistry; Krause N, Hashmi ASK, Eds.; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]; (b) Rivera-Fuentes P; Diederich F Allenes in molecular materials. Angew. Chem., Int. Ed 2012, 51, 2818–28. [DOI] [PubMed] [Google Scholar]; (c) Hoffmann-Roder A; Krause N Synthesis and properties of allenic natural products and pharmaceuticals. Angew. Chem., Int. Ed 2004, 43, 1196–216. [DOI] [PubMed] [Google Scholar]; (d) Cai F; Pu X; Qi X; Lynch V; Radha A; Ready JM Chiral allene-containing phosphines in asymmetric catalysis. J. Am. Chem. Soc 2011, 133, 18066–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).(a) Allen AD; Tidwell TT Ketenes and other cumulenes as reactive intermediates. Chem. Rev 2013, 113, 7287–342. [DOI] [PubMed] [Google Scholar]; (b) Sutherland DR; Kinsman L; Angiolini SM; Rosair GM; Lee AL Gold(I)-Catalysed Hydroarylation of 1,3-Disubstituted Allenes with Efficient Axial-to-Point Chirality Transfer. Chem. - Eur. J 2018, 24, 7002–7009. [DOI] [PubMed] [Google Scholar]; (c) Ma S Electrophilic addition and cyclization reactions of allenes. Acc. Chem. Res 2009, 42, 1679–88. [DOI] [PubMed] [Google Scholar]; (d) Liu L; Ward RM; Schomaker JM Mechanistic Aspects and Synthetic Applications of Radical Additions to Allenes. Chem. Rev 2019, 119, 12422–12490. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Krause N; Winter C Gold-catalyzed nucleophilic cyclization of functionalized allenes: a powerful access to carbo- and heterocycles. Chem. Rev 2011, 111, 1994–2009. [DOI] [PubMed] [Google Scholar]; (f) Adams CS; Weatherly CD; Burke EG; Schomaker JM The conversion of allenes to strained three-membered heterocycles. Chem. Soc. Rev 2014, 43, 3136–63. [DOI] [PubMed] [Google Scholar]; (g) Yu S; Ma S Allenes in catalytic asymmetric synthesis and natural product syntheses. Angew. Chem., Int. Ed 2012, 51, 3074–112. [DOI] [PubMed] [Google Scholar]
- (3).(a) Yokobori U; Ohmiya H; Sawamura M Synthesis of Allenylsilanes through Copper-Catalyzed γ-Selective Coupling between γ-Silylated Propargylic Phosphates and Alkylboranes. Organometallics 2012, 31, 7909–7913. [Google Scholar]; (b) Whitehead DC; Yousefi R; Jaganathan A; Borhan B An organocatalytic asymmetric chlorolactonization. J. Am. Chem. Soc 2010, 132, 3298–300. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Vaz B; Pereira R; Perez M; Alvarez R; de Lera AR Stereoselective Stille coupling of enantiopure haloallenes and alkenylstannanes for the synthesis of allenyl carotenoids. Experimental and computational studies. J. Org. Chem 2008, 73, 6534–41. [DOI] [PubMed] [Google Scholar]; (d) Schade MA; Yamada S; Knochel P Synthesis of polyfunctional allenes by successive copper-mediated substitutions. Chem. - Eur. J 2011, 17, 4232–7. [DOI] [PubMed] [Google Scholar]; (e) Ohno H; Ando K; Hamaguchi H; Takeoka Y; Tanaka T A highly cis-selective synthesis of 2-ethynylaziridines by intramolecular amination of chiral bromoallenes: improvement of stereoselectivity based on the computational investigation. J. Am. Chem. Soc 2002, 124, 15255–66. [DOI] [PubMed] [Google Scholar]; (f) Marshall JA; Grant CM Formation of Transient Chiral Allenylindium Reagents from Enantioenriched Propargylic Mesylates through Oxidative Transmetalation. Applications to the Synthesis of Enantioenriched Homopropargylic Alcohols. J. Org. Chem 1999, 64, 696–697. [DOI] [PubMed] [Google Scholar]; (g) Li H; Grassi D; Guenee L; Burgi T; Alexakis A Copper-catalyzed propargylic substitution of dichloro substrates: enantioselective synthesis of trisubstituted allenes and formation of propargylic quaternary stereogenic centers. Chem. - Eur. J 2014, 20, 16694–706. [DOI] [PubMed] [Google Scholar]; (h) Kim MJ; Sohn TI; Kim D; Paton RS Concise substrate-controlled asymmetric total syntheses of dioxabicyclic marine natural products with 2,10-dioxabicyclo-[7.3.0]dodecene and 2,9-dioxabicyclo[6.3.0]undecene skeletons. J. Am. Chem. Soc 2012, 134, 20178–88. [DOI] [PubMed] [Google Scholar]; (i) Evans PA; Murthy VS; Roseman JD; Rheingold AL Enantioselective Total Synthesis of the Nonisoprenoid Sesquiterpene (−)-Kumausallene. Angew. Chem., Int. Ed 1999, 38, 3175–3177. [PubMed] [Google Scholar]; (j) Caporusso AM; Zampieri A; Aronica LA; Banti D Stereoselective synthesis of chiral 3-aryl-1-alkynes from bromoallenes and heterocuprates. J. Org. Chem 2006, 71, 1902–10. [DOI] [PubMed] [Google Scholar]; (k) Caporusso AM; Filippi S; Barontini F; Salvadori P Coupling of chiral 1-bromo-1,2-dienes with zinc-based cuprates: a new procedure for the regio and stereoselective synthesis of functionalized acetylenic compounds. Tetrahedron Lett. 2000, 41, 1227–1230. [Google Scholar]
- (4).(a) Wang X; Wu Z; Wang J alpha-Fluoroallenoate Synthesis via N-Heterocyclic Carbene-Catalyzed Fluorination Reaction of Alkynals. Org. Lett 2016, 18, 576–9. [DOI] [PubMed] [Google Scholar]; (b) Mae M; Hong JA; Xu B; Hammond GB Highly regioselective synthesis of gem-difluoroallenes through magnesium organocuprate SN2′ substitution. Org. Lett 2006, 8, 479–82. [DOI] [PubMed] [Google Scholar]; (c) Li T; Zhou C; Yan X; Wang J Solvent-Dependent Asymmetric Synthesis of Alkynyl and Monofluoroalkenyl Isoindolinones by CpRh(III) -Catalyzed C-H Activation. Angew. Chem., Int. Ed 2018, 57, 4048–4052. [DOI] [PubMed] [Google Scholar]; (d) Hammond GB Functionalized Fluorinated Allenes. In Fluorine-Containing Synthons; American Chemical Society, 2005; Vol. 911, pp 204–215;. [Google Scholar]; (e) Castelhano AL; Krantz A A novel route to allenyl fluorides. Synthesis of 4-amino-7-fluorohepta-5,6-dienoic acid, the first fluoroallenyl amino acid. J. Am. Chem. Soc 1987, 109, 3491–3493. [Google Scholar]; (f) Lan Y; Hammond GB Functionalization of monofluoroallene and the synthesis of aryl-substituted conjugated fluorodienes. Org. Lett 2002, 4, 2437–9. [DOI] [PubMed] [Google Scholar]; (g) Pacheco MC; Purser S; Gouverneur V The chemistry of propargylic and allylic fluorides. Chem. Rev 2008, 108, 1943–81. [DOI] [PubMed] [Google Scholar]; (h) Zapata AJ; Gu Y; Hammond GB The first alpha-fluoroallenylphosphonate, the synthesis of conjugated fluoroenynes, and the stereoselective synthesis of vinylfluorophosphonates using a new multifunctional fluorine-containing building block. J. Org. Chem 2000, 65, 227–34. [DOI] [PubMed] [Google Scholar]
- (5).(a) Neff RK; Frantz DE Recent Advances in the Catalytic Syntheses of Allenes: A Critical Assessment. ACS Catal. 2014, 4, 519–528. [Google Scholar]; (b) Chu W-D; Zhang Y; Wang J Recent advances in catalytic asymmetric synthesis of allenes. Catal. Sci. Technol 2017, 7, 4570–4579. [Google Scholar]; (c) Brummond K; DeForrest J Synthesizing Allenes Today (1982–2006). Synthesis 2007, 2007, 795–818. [Google Scholar]; (d) Ogasawara M Catalytic enantioselective synthesis of axially chiral allenes. Tetrahedron: Asymmetry 2009, 20, 259–271. [Google Scholar]
- (6).(a) Wang Y; Zhang W; Ma S A room-temperature catalytic asymmetric synthesis of allenes with ECNU-Phos. J. Am. Chem. Soc 2013, 135, 11517–20. [DOI] [PubMed] [Google Scholar]; (b) Trost BM; Zell D; Hohn C; Mata G; Maruniak A Enantio- and Diastereoselective Synthesis of Chiral Allenes by Palladium-Catalyzed Asymmetric [3+2] Cycloaddition Reactions. Angew. Chem., Int. Ed 2018, 57, 12916–12920. [DOI] [PubMed] [Google Scholar]; (c) Trost BM; Schultz JE; Chang T; Maduabum MR Chemo-, Regio-, Diastereo-, and Enantioselective Palladium Allylic Alkylation of 1,3-Dioxaboroles as Synthetic Equivalents of alpha-Hydroxyketones. J. Am. Chem. Soc 2019, 141, 9521–9526. [DOI] [PubMed] [Google Scholar]; (d) Liu H; Leow D; Huang KW; Tan CH Enantioselective synthesis of chiral allenoates by guanidine-catalyzed isomerization of 3-alkynoates. J. Am. Chem. Soc 2009, 131, 7212–3. [DOI] [PubMed] [Google Scholar]; (e) Jiang Y; Diagne AB; Thomson RJ; Schaus SE Enantioselective Synthesis of Allenes by Catalytic Traceless Petasis Reactions. J. Am. Chem. Soc 2017, 139, 1998–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Crouch IT; Neff RK; Frantz DE Pd-catalyzed asymmetric beta-hydride elimination en route to chiral allenes. J. Am. Chem. Soc 2013, 135, 4970–3. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Chu WD; Zhang L; Zhang Z; Zhou Q; Mo F; Zhang Y; Wang J Enantioselective Synthesis of Trisubstituted Allenes via Cu(I)-Catalyzed Coupling of Diazoalkanes with Terminal Alkynes. J. Am. Chem. Soc 2016, 138, 14558–14561. [DOI] [PubMed] [Google Scholar]; (h) Bayeh-Romero L; Buchwald SL Copper Hydride Catalyzed Enantioselective Synthesis of Axially Chiral 1,3-Disubstituted Allenes. J. Am. Chem. Soc 2019, 141, 13788–13794. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Adamson NJ; Jeddi H; Malcolmson SJ Preparation of Chiral Allenes through Pd-Catalyzed Intermolecular Hydroamination of Conjugated Enynes: Enantioselective Synthesis Enabled by Catalyst Design. J. Am. Chem. Soc 2019, 141, 8574–8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).(a) Wu S; Huang X; Wu W; Li P; Fu C; Ma S A C-H bond activation-based catalytic approach to tetrasubstituted chiral allenes. Nat. Commun 2015, 6, 7946. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Scheipers I; Muck-Lichtenfeld C; Studer A Palladium-Catalyzed Decarboxylative gamma-Arylation for the Synthesis of Tetrasubstituted Chiral Allenes. Angew. Chem., Int. Ed 2019, 58, 6545–6548. [DOI] [PubMed] [Google Scholar]; (c) Qian D; Wu L; Lin Z; Sun J Organocatalytic synthesis of chiral tetrasubstituted allenes from racemic propargylic alcohols. Nat. Commun 2017, 8, 567. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Hayashi T; Tokunaga N; Inoue K Rhodium-catalyzed asymmetric 1,6-addition of aryltitanates to enynones giving axially chiral allenes. Org. Lett 2004, 6, 305–7. [DOI] [PubMed] [Google Scholar]; (e) Hashimoto T; Sakata K; Tamakuni F; Dutton MJ; Maruoka K Phase-transfer-catalysed asymmetric synthesis of tetrasubstituted allenes. Nat. Chem 2013, 5, 240–4. [DOI] [PubMed] [Google Scholar]; (f) Liao Y; Yin X; Wang X; Yu W; Fang D; Hu L; Wang M; Liao J Enantioselective Synthesis of Multisubstituted Allenes by Cooperative Cu/Pd-Catalyzed 1,4-Arylboration of 1,3-Enynes. Angew. Chem., Int. Ed 2020, 59, 1176–1180. [DOI] [PubMed] [Google Scholar]; (g) Armstrong RJ; Nandakumar M; Dias RMP; Noble A; Myers EL; Aggarwal VK Enantiodivergent Synthesis of Allenes by Point-to-Axial Chirality Transfer. Angew. Chem., Int. Ed 2018, 57, 8203–8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).(a) Sang HL; Yu S; Ge S Copper-catalyzed asymmetric hydroboration of 1,3-enynes with pinacolborane to access chiral allenylboronates. Org. Chem. Front 2018, 5, 1284–1287. [Google Scholar]; (b) Huang Y; Del Pozo J; Torker S; Hoveyda AH Enantioselective Synthesis of Trisubstituted Allenyl-B(pin) Compounds by Phosphine-Cu-Catalyzed 1,3-Enyne Hydroboration. Insights Regarding Stereochemical Integrity of Cu-Allenyl Intermediates. J. Am. Chem. Soc 2018, 140, 2643–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gao DW; Xiao Y; Liu M; Liu Z; Karunananda MK; Chen JS; Engle KM Catalytic, Enantioselective Synthesis of Allenyl Boronates. ACS Catal. 2018, 8, 3650–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).(a) Wang M; Liu ZL; Zhang X; Tian PP; Xu YH; Loh TP Synthesis of Highly Substituted Racemic and Enantioenriched Allenylsilanes via Copper-Catalyzed Hydrosilylation of (Z)-2-Alken-4-ynoates with Silylboronate. J. Am. Chem. Soc 2015, 137, 14830–3. [DOI] [PubMed] [Google Scholar]; (b) Nishimura T; Makino H; Nagaosa M; Hayashi T Rhodium-catalyzed enantioselective 1,6-addition of arylboronic acids to enynamides: asymmetric synthesis of axially chiral allenylsilanes. J. Am. Chem. Soc 2010, 132, 12865–7. [DOI] [PubMed] [Google Scholar]; (c) Liu ZL; Yang C; Xue QY; Zhao M; Shan CC; Xu YH; Loh TP Copper-Catalyzed Asymmetric Silylation of Propargyl Dichlorides: Access to Enantioenriched Functionalized Allenylsilanes. Angew. Chem., Int. Ed 2019, 58, 16538–16542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).(a) Danheiser RL; Carini DJ; Fink DM; Basak A Scope and stereochemical course of the (trimethylsilyl)cyclopentene annulation. Tetrahedron 1983, 39, 935–947. [Google Scholar]; (b) Brawn RA; Panek JS Preparation and use of enantioenriched allenylsilanes for the stereoselective synthesis of homopropargylic ethers. Org. Lett 2007, 9, 2689–92. [DOI] [PubMed] [Google Scholar]; (c) Marshall JA; Maxson K Stereoselective synthesis of stereotriad subunits of polyketides through additions of nonracemic allenylsilanes to (R)- and (S)-2-methyl-3-oxygenated propanals. J. Org. Chem 2000, 65, 630–3. [DOI] [PubMed] [Google Scholar]; (d) Brawn RA; Panek JS Synthesis of enantioenriched homopropargylic sulfonamides by a three component reaction of aldehydes, sulfonamides, and chiral allenylsilanes. Org. Lett 2009, 11, 4362–5. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Danheiser RL; Kwasigroch CA; Tsai YM Application of allenylsilanes in [3+2] annulation approaches to oxygen and nitrogen heterocycles. J. Am. Chem. Soc 1985, 107, 7233–7235. [Google Scholar]; (f) Masse CE; Panek JS Diastereoselective Reactions of Chiral Allyl and Allenyl Silanes with Activated C:X .pi.-Bonds. Chem. Rev 1995, 95, 1293–1316. [Google Scholar]; (g) Fleming I; Terrett NK Stereospecific syntheses and reactions of allyl- and allenyl-silanes. J. Organomet. Chem 1984, 264, 99–118. [Google Scholar]; (h) Danheiser RL; Carini DJ (Trimethylsilyl)allenes as propargylic anion equivalents: synthesis of homopropargylic alcohols and ethers. J. Org. Chem 1980, 45, 3925–3927. [Google Scholar]; (i) Brawn RA; Panek JS Stereoselective C-glycosidations with achiral and enantioenriched allenylsilanes. Org. Lett 2010, 12, 4624–7. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Danheiser RL; Carini DJ; Basak A (Trimethylsilyl)-cyclopentene annulation: a regiocontrolled approach to the synthesis of five-membered rings. J. Am. Chem. Soc 1981, 103, 1604–1606. [Google Scholar]; (k) Spiteri C; Moses JE Copper-catalyzed azide-alkyne cyclo-addition: regioselective synthesis of 1,4,5-trisubstituted 1,2,3-triazoles. Angew. Chem., Int. Ed 2010, 49, 31–3. [DOI] [PubMed] [Google Scholar]; (l) Cai B; Evans RW; Wu J; Panek JS Total Synthesis of Nuclear Factor of Activated T-Cells-68 (NFAT-68): Sequential Use of Chiral Allenylsilane and Titanium Alkoxide-Mediated Reductive Coupling Bond Construction. Org. Lett 2016, 18, 4304–7. [DOI] [PubMed] [Google Scholar]; (m) Danheiser RL; Carini DJ; Kwasigroch CA Scope and stereochemical course of the addition of (trimethylsilyl)-allenes to ketones and aldehydes. A regiocontrolled synthesis of homopropargylic alcohols. J. Org. Chem 1986, 51, 3870–3878. [Google Scholar]; (n) Weinreb SM Thermal and Lewis Acid Catalyzed Intramolecular Ene Reactions of Allenylsilanes. Synthesis 1998, 1998, 509–521. [Google Scholar]; (o) Ohmiya H; Ito H; Sawamura M General and functional group-tolerable approach to allenylsilanes by rhodium-catalyzed coupling between propargylic carbonates and a silylboronate. Org. Lett 2009, 11, 5618–20. [DOI] [PubMed] [Google Scholar]; (p) Curtis-Long MJ; Aye Y Vinyl-, propargyl-, and allenylsilicon reagents in asymmetric synthesis: a relatively untapped resource of environmentally benign reagents. Chem. - Eur. J 2009, 15, 5402–16. [DOI] [PubMed] [Google Scholar]
- (11).(a) Wang H; Jui NT Catalytic Defluoroalkylation of Trifluoromethylaromatics with Unactivated Alkenes. J. Am. Chem. Soc 2018, 140, 163–166. [DOI] [PubMed] [Google Scholar]; (b) Vogt DB; Seath CP; Wang H; Jui NT Selective C-F Functionalization of Unactivated Trifluoromethylarenes. J. Am. Chem. Soc 2019, 141, 13203–13211. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Thornbury RT; Toste FD Palladium-Catalyzed Defluorinative Coupling of 1-Aryl-2,2-Difluoroalkenes and Boronic Acids: Stereoselective Synthesis of Monofluorostilbenes. Angew. Chem., Int. Ed 2016, 55, 11629–11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).(a) Hu J; Zhao Y; Shi Z Highly tunable multi-borylation of gem-difluoroalkenes via copper catalysis. Nature Catal. 2018, 1, 860–869. [Google Scholar]; (b) Zhang J; Dai W; Liu Q; Cao S Cu-Catalyzed Stereoselective Borylation of gem-Difluoroalkenes with B2pin2. Org. Lett 2017, 19, 3283–3286. [DOI] [PubMed] [Google Scholar]; (c) Tan D-H; Lin E; Ji W-W; Zeng Y-F; Fan W-X; Li Q; Gao H; Wang H Copper-Catalyzed Stereoselective Defluorinative Borylation and Silylation of gem-Difluoroalkenes. Adv. Synth. Catal 2018, 360, 1032–1037. [Google Scholar]; (d) Sakaguchi H; Uetake Y; Ohashi M; Niwa T; Ogoshi S; Hosoya T Copper-Catalyzed Regioselective Monodefluoroborylation of Polyfluoroalkenes en Route to Diverse Fluoroalkenes. J. Am. Chem. Soc 2017, 139, 12855–12862. [DOI] [PubMed] [Google Scholar]; (e) Sakaguchi H; Ohashi M; Ogoshi S Fluorinated Vinylsilanes from the Copper-Catalyzed Defluorosilylation of Fluoroalkene Feedstocks. Angew. Chem., Int. Ed 2018, 57, 328–332. [DOI] [PubMed] [Google Scholar]; (f) Niwa T; Ochiai H; Hosoya T Copper-Catalyzed ipso-Borylation of Fluoroarenes. ACS Catal. 2017, 7, 4535–4541. [Google Scholar]; (g) Hu J; Han X; Yuan Y; Shi Z Stereoselective Synthesis of Z Fluoroalkenes through Copper-Catalyzed Hydrodefluorination of gem-Difluoroalkenes with Water. Angew. Chem., Int. Ed 2017, 56, 13342–13346. [DOI] [PubMed] [Google Scholar]; (h) Andrella NO; Xu N; Gabidullin BM; Ehm C; Baker RT Selective Copper Complex-Catalyzed Hydrodefluorination of Fluoroalkenes and Allyl Fluorides: A Tale of Two Mechanisms. J. Am. Chem. Soc 2019, 141, 11506–11521. [DOI] [PubMed] [Google Scholar]; (i) Kojima R; Kubota K; Ito H Stereodivergent hydrodefluorination of gem-difluoroalkenes: selective synthesis of (Z)- and (E)-monofluoroalkenes. Chem. Commun 2017, 53, 10688–10691. [DOI] [PubMed] [Google Scholar]
- (13).(a) Wang M; Pu X; Zhao Y; Wang P; Li Z; Zhu C; Shi Z Enantioselective Copper-Catalyzed Defluoroalkylation Using Arylboronate-Activated Alkyl Grignard Reagents. J. Am. Chem. Soc 2018, 140, 9061–9065. [DOI] [PubMed] [Google Scholar]; (b) Paioti PHS; Del Pozo J; Mikus MS; Lee J; Koh MJ; Romiti F; Torker S; Hoveyda AH Catalytic Enantioselective Boryl and Silyl Substitution with Trifluoromethyl Alkenes: Scope, Utility, and Mechanistic Nuances of Cu-F beta-Elimination. J. Am. Chem. Soc 2019, 141, 19917–19934. [DOI] [PubMed] [Google Scholar]; (c) Kojima R; Akiyama S; Ito H A Copper(I)-Catalyzed Enantioselective gamma-Boryl Substitution of Trifluoromethyl-Substituted Alkenes: Synthesis of Enantioenriched gamma,gamma-gem-Difluoroallylboronates. Angew. Chem., Int. Ed 2018, 57, 7196–7199. [DOI] [PubMed] [Google Scholar]; (d) Jang YJ; Rose D; Mirabi B; Lautens M Rhodium-Catalyzed Enantioselective Defluorinative alpha-Arylation of Secondary Amides. Angew. Chem., Int. Ed 2018, 57, 16147–16151. [DOI] [PubMed] [Google Scholar]; (e) Huang Y; Hayashi T Rhodium-Catalyzed Asymmetric Arylation/Defluorination of 1-(Trifluoromethyl)alkenes Forming Enantioenriched 1,1-Difluoroalkenes. J. Am. Chem. Soc 2016, 138, 12340–3. [DOI] [PubMed] [Google Scholar]; (f) Gao P; Yuan C; Zhao Y; Shi Z Copper-Catalyzed Asymmetric Defluoroborylation of 1-(Trifluoromethyl)Alkenes. Chem. 2018, 4, 2201–2211. [Google Scholar]; (g) Akiyama S; Kubota K; Mikus MS; Paioti PHS; Romiti F; Liu Q; Zhou Y; Hoveyda AH; Ito H Catalytic Enantioselective Synthesis of Allylic Boronates Bearing a Trisubstituted Alkenyl Fluoride and Related Derivatives. Angew. Chem., Int. Ed 2019, 58, 11998–12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).(a) Butcher TW; Yang JL; Amberg WM; Watkins NB; Wilkinson ND; Hartwig JF Desymmetrization of difluoromethylene groups by C-F bond activation. Nature 2020, 583, 548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).(a) Garcia-Rubia A; Romero-Revilla JA; Mauleon P; Gomez Arrayas R; Carretero JC Cu-catalyzed silylation of alkynes: a traceless 2-pyridylsulfonyl controller allows access to either regioisomer on demand. J. Am. Chem. Soc 2015, 137, 6857–65. [DOI] [PubMed] [Google Scholar]; (b) Hazra CK; Fopp C; Oestreich M Copper(I)-catalyzed regioselective addition of nucleophilic silicon across terminal and internal carbon-carbon triple bonds. Chem. - Asian J 2014, 9, 3005–10. [DOI] [PubMed] [Google Scholar]; (c) Zhao M; Shan CC; Wang ZL; Yang C; Fu Y; Xu YH Ligand-Dependent-Controlled Copper-Catalyzed Regio- and Stereoselective Silaboration of Alkynes. Org. Lett 2019, 21, 6016–6020. [DOI] [PubMed] [Google Scholar]; (d) Meng F; Jang H; Hoveyda AH Exceptionally E- and beta-selective NHC-Cu-catalyzed proto-silyl additions to terminal alkynes and site- and enantioselective proto-boryl additions to the resulting vinylsilanes: synthesis of enantiomerically enriched vicinal and geminal borosilanes. Chem. - Eur. J 2013, 19, 3204–14. [DOI] [PubMed] [Google Scholar]; (e) Fujihara T; Tani Y; Semba K; Terao J; Tsuji Y Copper-catalyzed silacarboxylation of internal alkynes by employing carbon dioxide and silylboranes. Angew. Chem., Int. Ed 2012, 51, 11487–90. [DOI] [PubMed] [Google Scholar]; (f) Wang P; Yeo XL; Loh TP Copper-catalyzed highly regioselective silylcupration of terminal alkynes to form alpha-vinylsilanes. J. Am. Chem. Soc 2011, 133, 1254–6. [DOI] [PubMed] [Google Scholar]
- (16).(a) Uehling MR; Suess AM; Lalic G Copper-catalyzed hydroalkylation of terminal alkynes. J. Am. Chem. Soc 2015, 137, 1424–7. [DOI] [PubMed] [Google Scholar]; (b) Uehling MR; Rucker RP; Lalic G Catalytic anti-Markovnikov hydrobromination of alkynes. J. Am. Chem. Soc 2014, 136, 8799–803. [DOI] [PubMed] [Google Scholar]; (c) Shi SL; Buchwald SL Copper-catalysed selective hydroamination reactions of alkynes. Nat. Chem 2015, 7, 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Mailig M; Hazra A; Armstrong MK; Lalic G Catalytic Anti-Markovnikov Hydroallylation of Terminal and Functionalized Internal Alkynes: Synthesis of Skipped Dienes and Trisubstituted Alkenes. J. Am. Chem. Soc 2017, 139, 6969–6977. [DOI] [PubMed] [Google Scholar]; (e) Chemla F; Ferreira F The Chemistry of Organocopper Compounds; Rappoport Z, Marek I, Eds.; Patai Series; Wiley-Chichester, 2009; 527 pp, DOI: 10.1002/9780470682531.pat0444. [DOI] [Google Scholar]; (f) Whittaker AM; Lalic G Monophasic catalytic system for the selective semireduction of alkynes. Org. Lett 2013, 15, 1112–5. [DOI] [PubMed] [Google Scholar]; (g) Barbero A; Pulido FJ Allylsilanes and vinylsilanes from silylcupration of carbon-carbon multiple bonds: scope and synthetic applications. Acc. Chem. Res 2004, 37, 817–25. [DOI] [PubMed] [Google Scholar]
- (17).(a) Yoshida H; Hayashi Y; Ito Y; Takaki K Inverse regioselectivity in the silylstannylation of alkynes and allenes: copper-catalyzed three-component coupling with a silylborane and a tin alkoxide. Chem. Commun 2015, 51, 9440–2. [DOI] [PubMed] [Google Scholar]; (b) Xu YH; Wu LH; Wang J; Loh TP Synthesis of multi-substituted vinylsilanes via copper(I)-catalyzed hydrosilylation reactions of allenes and propiolate derivatives with silylboronates. Chem. Commun 2014, 50, 7195–7. [DOI] [PubMed] [Google Scholar]; (c) Tani Y; Yamaguchi T; Fujihara T; Terao J; Tsuji Y Copper-catalyzed Silylative Allylation of Ketones and Aldehydes Employing Allenes and Silylboranes. Chem. Lett 2015, 44, 271–273. [Google Scholar]; (d) Tani Y; Fujihara T; Terao J; Tsuji Y Copper-catalyzed regiodivergent silacarboxylation of allenes with carbon dioxide and a silylborane. J. Am. Chem. Soc 2014, 136, 17706–9. [DOI] [PubMed] [Google Scholar]; (e) He ZT; Tang XQ; Xie LB; Cheng M; Tian P; Lin GQ Efficient Access to Bicyclo[4.3.0]nonanes: Copper-Catalyzed Asymmetric Silylative Cyclization of Cyclohexadienone-Tethered Allenes. Angew. Chem., Int. Ed 2015, 54, 14815–8. [DOI] [PubMed] [Google Scholar]; (f) Fujihara T; Sawada A; Yamaguchi T; Tani Y; Terao J; Tsuji Y Boraformylation and Silaformylation of Allenes. Angew. Chem., Int. Ed 2017, 56, 1539–1543. [DOI] [PubMed] [Google Scholar]; (g) Chang XH; Liu ZL; Luo YC; Yang C; Liu XW; Da BC; Li JJ; Ahmad T; Loh TP; Xu YH Copper-catalyzed silylation reactions of propargyl epoxides: easy access to 2,3-allenols and stereodefined alkenes. Chem. Commun 2017, 53, 9344–9347. [DOI] [PubMed] [Google Scholar]
- (18).Lee J; Radomkit S; Torker S; Del Pozo J; Hoveyda AH Mechanism-based enhancement of scope and enantioselectivity for reactions involving a copper-substituted stereogenic carbon centre. Nat. Chem 2018, 10, 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Tian YM; Guo XN; Kuntze-Fechner MW; Krummenacher I; Braunschweig H; Radius U; Steffen A; Marder TB Selective Photocatalytic C-F Borylation of Polyfluoroarenes by Rh/Ni Dual Catalysis Providing Valuable Fluorinated Arylboronate Esters. J. Am. Chem. Soc 2018, 140, 17612–17623. [DOI] [PubMed] [Google Scholar]
- (20).Somerville RJ; Hale LVA; Gomez-Bengoa E; Bures J; Martin R Intermediacy of Ni-Ni Species in sp(2) C-O Bond Cleavage of Aryl Esters: Relevance in Catalytic C-Si Bond Formation. J. Am. Chem. Soc 2018, 140, 8771–8780. [DOI] [PubMed] [Google Scholar]
- (21).Dang H; Mailig M; Lalic G Mild copper-catalyzed fluorination of alkyl triflates with potassium fluoride. Angew. Chem., Int. Ed 2014, 53, 6473–6. [DOI] [PubMed] [Google Scholar]
- (22).(a) He Y; Wang B; Dukor RK; Nafie LA Determination of absolute configuration of chiral molecules using vibrational optical activity: a review. Appl. Spectrosc 2011, 65, 699–723. [DOI] [PubMed] [Google Scholar]; (b) Merten C; Golub TP; Kreienborg NM Absolute Configurations of Synthetic Molecular Scaffolds from Vibrational CD Spectroscopy. J. Org. Chem 2019, 84, 8797–8814. [DOI] [PubMed] [Google Scholar]; (c) Polavarapu PL; Santoro E Vibrational optical activity for structural characterization of natural products. Nat. Prod. Rep 2020, 37, 1661–1699. [DOI] [PubMed] [Google Scholar]; (d) Chamberlain BT; Vincent M; Nafie J; Muller P; Greka A; Wagner FF Multigram Preparation of BRD4780 Enantiomers and Assignment of Absolute Stereochemistry. J. Org. Chem 2021, 86, 4281–4289. [DOI] [PubMed] [Google Scholar]
- (23).Devlin FJ; Stephens PJ; Cheeseman JR; Frisch MJ Ab InitioPrediction of Vibrational Absorption and Circular Dichroism Spectra of Chiral Natural Products Using Density Functional Theory: Camphor and Fenchone. J. Phys. Chem. A 1997, 101, 6322–6333. [Google Scholar]
- (24).(a) Polavarapu PL; Covington CL Comparison of experimental and calculated chiroptical spectra for chiral molecular structure determination. Chirality 2014, 26, 539–52. [DOI] [PubMed] [Google Scholar]; (b) Debie E; De Gussem E; Dukor RK; Herrebout W; Nafie LA; Bultinck P A confidence level algorithm for the determination of absolute configuration using vibrational circular dichroism or Raman optical activity. ChemPhysChem 2011, 12, 1542–1549. [DOI] [PubMed] [Google Scholar]
- (25).(a) Cirriez V; Rasson C; Hermant T; Petrignet J; Diaz Alvarez J; Robeyns K; Riant O Copper-catalyzed addition of nucleophilic silicon to aldehydes. Angew. Chem., Int. Ed 2013, 52, 1785–8. [DOI] [PubMed] [Google Scholar]; (b) Gibbons SK; Hughes RP; Glueck DS; Royappa AT; Rheingold AL; Arthur RB; Nicholas AD; Patterson HH Synthesis, Structure, and Luminescence of Copper(I) Halide Complexes of Chiral Bis(phosphines). Inorg. Chem 2017, 56, 12809–12820. [DOI] [PubMed] [Google Scholar]; (c) Gurung SK; Thapa S; Kafle A; Dickie DA; Giri R Copper-catalyzed Suzuki-Miyaura coupling of arylboronate esters: transmetalation with (PN)CuF and identification of intermediates. Org. Lett 2014, 16, 1264–7. [DOI] [PubMed] [Google Scholar]; (d) Lee M; Nguyen M; Brandt C; Kaminsky W; Lalic G Catalytic Hydroalkylation of Allenes. Angew. Chem., Int. Ed 2017, 56, 15703–15707. [DOI] [PubMed] [Google Scholar]; (e) Mita T; Chen J; Sugawara M; Sato Y One-Pot Synthesis of α-Amino Acids from CO2 Using a Bismetal Reagent with Si–B Bond. Org. Lett 2012, 14, 6202–6205. [DOI] [PubMed] [Google Scholar]; (f) Shibasaki M; Kanai M Copper(I) Fluoride and Copper(II) Fluoride. In Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons, Ltd.: Hoboken, NJ, 2007; DOI: 10.1002/047084289X.rn00744. [DOI] [Google Scholar]; (g) Suess AM; Uehling MR; Kaminsky W; Lalic G Mechanism of Copper-Catalyzed Hydroalkylation of Alkynes: An Unexpected Role of Dinuclear Copper Complexes. J. Am. Chem. Soc 2015, 137, 7747–53. [DOI] [PubMed] [Google Scholar]; (h) Wyss CM; Tate BK; Bacsa J; Wieliczko M; Sadighi JP Dinuclear μ-fluoro cations of copper, silver and gold. Polyhedron 2014, 84, 87–95. [Google Scholar]
- (26).(a) Meng F-F; Xie J-H; Xu Y-H; Loh T-P Catalytically Asymmetric Synthesis of 1,3-Bis(silyl)propenes via Copper-Catalyzed Double Proto-Silylations of Polar Enynes. ACS Catal. 2018, 8, 5306–5312. [Google Scholar]; (b) Plotzitzka J; Kleeberg C [(NHC)Cu(I)-ER3] Complexes (ER3 = SiMe2Ph, SiPh3, SnMe3): From Linear, Mononuclear Complexes to Polynuclear Complexes with Ultrashort Cu(I)···Cu(I) Distances. Inorg. Chem 2016, 55, 4813–23. [DOI] [PubMed] [Google Scholar]; (c) Plotzitzka J; Kleeberg C [(18-C-6)K][(N identical withC)Cu(I)-SiMe2Ph], a Potassium Silylcyanocuprate as a Catalyst Model for Silylation Reactions with Silylboranes: Syntheses, Structures, and Catalytic Properties. Inorg. Chem 2017, 56, 6671–6680. [DOI] [PubMed] [Google Scholar]
- (27).(a) Ohmiya H; Yokobori U; Makida Y; Sawamura M General approach to allenes through copper-catalyzed gamma-selective and stereospecific coupling between propargylic phosphates and alkylboranes. Org. Lett 2011, 13, 6312–5. [DOI] [PubMed] [Google Scholar]; (b) Zhong C; Sasaki Y; Ito H; Sawamura M The synthesis of allenes by Cu(I)-catalyzed regio- and stereoselective reduction of propargylic carbonates with hydrosilanes. Chem. Commun 2009, 5850–2. [DOI] [PubMed] [Google Scholar]
- (28).(a) Kuehn L; Stang M; Wurtemberger-Pietsch S; Friedrich A; Schneider H; Radius U; Marder TB FBpin and its adducts and their role in catalytic borylations. Faraday Discuss. 2019, 220, 350–363. [DOI] [PubMed] [Google Scholar]; (b) Pietsch S; Neeve EC; Apperley DC; Bertermann R; Mo F; Qiu D; Cheung MS; Dang L; Wang J; Radius U; Lin Z; Kleeberg C; Marder TB Synthesis, Structure, and Reactivity of Anionic sp(2) -sp(3) Diboron Compounds: Readily Accessible Boryl Nucleophiles. Chem. - Eur. J 2015, 21, 7082–98. [DOI] [PubMed] [Google Scholar]
- (29).Harutyunyan SR; Lopez F; Browne WR; Correa A; Pena D; Badorrey R; Meetsma A; Minnaard AJ; Feringa BL On the mechanism of the copper-catalyzed enantioselective 1,4-addition of grignard reagents to alpha,beta-unsaturated carbonyl compounds. J. Am. Chem. Soc 2006, 128, 9103–18. [DOI] [PubMed] [Google Scholar]
- (30).The formations of several possible off-cycle adducts of CsF with LCuF and PhMe2SiBpin were calculated (see Figure S1). The DFT calculations suggest that although these adducts are slightly more stable than the monomeric species, the dissociation of these adducts are facile. For simpilicity, PhMe2SiBpin and FBpin were used as the reference in the computed reaction energy profile.
- (31).A Lewis base-promoted antielimination pathway, proposed in ref 13b, is unlikely in the present system because a Lewis base will not be able to coordinate to the highly sterically encumbered Cu in 31.
- (32).(a) Kobayashi K; Yamamoto Y; Miyaura N Pd/Josiphos-Catalyzed Enantioselective α-Arylation of Silyl Ketene Acetals and Mechanistic Studies on Transmetalation and Enantioselection. Organometallics 2011, 30, 6323–6327. [Google Scholar]; (b) Li C; Liu RY; Jesikiewicz LT; Yang Y; Liu P; Buchwald SL CuH-Catalyzed Enantioselective Ketone Allylation with 1,3-Dienes: Scope, Mechanism, and Applications. J. Am. Chem. Soc 2019, 141, 5062–5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).(a) Lu G; Liu RY; Yang Y; Fang C; Lambrecht DS; Buchwald SL; Liu P Ligand-Substrate Dispersion Facilitates the Copper-Catalyzed Hydroamination of Unactivated Olefins. J. Am. Chem. Soc 2017, 139, 16548–16555. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kang T; Erbay TG; Xu KL; Gallego GM; Burtea A; Nair SK; Patman RL; Zhou R; Sutton SC; McAlpine IJ; Liu P; Engle KM Multifaceted Substrate-Ligand Interactions Promote the Copper-Catalyzed Hydroboration of Benzylidenecyclobutanes and Related Compounds. ACS Catal. 2020, 10, 13075–13083. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Xi Y; Su B; Qi X; Pedram S; Liu P; Hartwig JF Application of Trimethylgermanyl-Substituted Bisphosphine Ligands with Enhanced Dispersion Interactions to Copper-Catalyzed Hydroboration of Disubstituted Alkenes. J. Am. Chem. Soc 2020, 142, 18213–18222. [DOI] [PubMed] [Google Scholar]
- (34).(a) Bizet V; Cahard D Asymmetric Fluorination Methods: Application in the Stereoselective Synthesis of Fluorinated Drugs. In Stereoselective Synthesis of Drugs and Natural Products; John Wiley & Sons, Inc., 2013. DOI: 10.1002/9781118596784.ssd044. [DOI] [Google Scholar]; (b) Wang J; Sánchez-Roselló M; Aceña JL; del Pozo C; Sorochinsky AE; Fustero S; Soloshonok VA; Liu H Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decade (2001–2011). Chem. Rev 2014, 114, 2432–2506. [DOI] [PubMed] [Google Scholar]; (c) Gillis EP; Eastman KJ; Hill MD; Donnelly DJ; Meanwell NA Applications of fluorine in medicinal chemistry. J. Med. Chem 2015, 58, 8315–8359. [DOI] [PubMed] [Google Scholar]; (d) Zhu Y; Han J; Wang J; Shibata N; Sodeoka M; Soloshonok VA; Coelho JAS; Toste FD Modern Approaches for Asymmetric Construction of Carbon–Fluorine Quaternary Stereogenic Centers: Synthetic Challenges and Pharmaceutical Needs. Chem. Rev 2018, 118, 3887–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.