Abstract
Dissemination and implementation (D&I) science is the practice of taking evidence-based interventions (EBI) and sustainably incorporating them in routine clinical practice. As a relatively young field, D&I techniques are underutilized in cardiothoracic surgery. This review offers an overview of D&I science from the context of the cardiothoracic surgeon. First, we provide a general introduction to D&I science and basic terminology that is used in the field. Second, to illustrate D&I techniques in a real-world example, we discuss a case study for implementing lung protective management (LPM) strategies for lung donor optimization nationally. Finally, we discuss challenges to successful implementation that are unique to cardiothoracic surgery and give several examples of EBIs that have been poorly implemented into surgical practice. We also provide examples of successful D&I interventions – including de-implementation strategies – from other surgical subspecialties. We hope that this review offers additional tools for cardiothoracic surgeons to explore when introducing EBIs into routine practice.
Keywords: Dissemination and implementation science
Graphical Abstract
Cardiothoracic surgeons have long prided themselves on providing strong, evidence-based care. Central to providing such care is (1) identifying novel evidence-based interventions (EBIs) through various avenues of research and (2) translating these EBIs into routine, widespread practice. It is widely accepted that only half of EBIs become incorporated into real-world practice, taking an average of 17 years to do so1–3. Cardiothoracic surgery –surgery in general – is not immune from the challenges with implementing new practices. For example, randomized controlled trials are infrequent in surgery and lead to concerns about generalizability; new techniques can have variable learning curves that challenge implementation in real-world practice4; new technology can be prohibitively expensive in certain practice environments. The old paradigm of “translational research” through which researchers simply deposit manuscripts into journals and expect widespread adoption is woefully inadequate and ineffective by modern standards5–7.
Dissemination and implementation (D&I) science is an emerging field that seeks to address several of the challenges associated with enacting new EBIs. In this review, we give a brief introduction to D&I research and terminology. We then present a brief case study of a national implementation intervention to promote lung protective management (LPM) algorithms for lung transplant donor optimization as a demonstration of how D&I techniques can be integrated into cardiothoracic surgical research. We conclude with a discussion about further opportunities to integrate D&I into the field.
What is D&I Science?
Formally, D&I research is the “scientific study of methods to promote the systematic uptake of research findings and other evidence-based practices into routine practice, and, hence, to improve the quality and effectiveness of health services and care”8. At its core, D&I research aims to correct the slow uptake of evidence-based research into routine practice and help ensure that EBIs are implemented as intended. D&I science is often conceptualized as the end phase of translational research, where scientific discoveries are applied and tested in real-world settings5,9. It takes treatments from the context of rigorous clinical trials to the more relevant context of everyday practice10. Even more simply, D&I takes a new “thing” (i.e., procedure, treatment guideline, etc.) and attempts to maximize the number of providers who know about the “thing” (dissemination) and the number of providers using the “thing” (implementation)11.
How is information disseminated?
Dissemination research is the focused examination of approaches for spreading evidence-based interventions to the target audience via determined channels using planned strategies 12–14. In other words, it is the study of how new information or practices are spread through a field. Effective dissemination of new EBIs across an entire field is challenging. While it may be unclear who holds the obligation to disseminate, 56% of academic researchers “strongly agree” that it is the researcher’s obligation to disseminate their own research15. Despite this, a minority of researchers believe that they do a “good” job disseminating their research findings16. One reason for this is that passive dissemination (like depositing a manuscript in an academic journal) does not work well in isolation7. For example, while academic journals and conferences are the most common avenues through which academic researchers disseminate their knowledge, local and state health practitioners rely primarily on seminars, workshops, and professional associations for dissemination of new evidence17. Similarly, while researchers tend to rank academic journals and reports to funders as their most common dissemination strategies, face-to-face meetings with stakeholders are believed to be the most likely dissemination strategies to influence practice and policy15. Therefore, effectively disseminating new research findings from academia to general practice require more innovative strategies.
Recent technological advances have addressed several barriers to dissemination. For example, practitioners can find information on modern techniques far easier with online as opposed to print academic journals. However, contextual issues – like private practice providers who do not have subscriptions to these journals – remain pertinent. Several production groups have also tried to improve high-quality dissemination efforts (i.e., national treatment guidelines, Cochrane reviews, UpToDate, etc.). These resources, while excellent, still face significant dissemination challenges18. Finally, platforms like academic Twitter have allowed for rapid dissemination of new research. However, limitations of these platforms – such as an overwhelming volume of material and unsolicited or editorialized content – are important to recognize and harbor inherent risk. “Design for Dissemination” (D4D) strategies may help to lessen the current dissemination gaps that exist in surgery7.
How are interventions implemented?
Implementation research focuses on the process of putting to use or integrating evidence-based interventions within a routine practice setting14. In other words, it is the examination of how new practices are actually enacted. Cardiothoracic surgery has several examples of EBIs that have been slowly adopted into routine care. One of the most notable examples is lung cancer screening. The National Lung Screening Trial (NLST), originally published in 2011, demonstrated that annual low-dose computed tomography (CT) screening reduces lung cancer deaths in individuals with high-risk smoking histories19. Nearly a decade later, less than 15% of screening-eligible adults in the United States receive appropriate lung cancer screening20.
Implementation science emphasizes effectiveness over efficacy21. Traditional biomedical research has focused on efficacy; the benefit of a drug or intervention in a well-controlled clinical environment. Effectiveness, on the other hand, focuses on the benefit of that drug or intervention in the real-world, including ways to promote sustainable adoption across several practice environments. It is important to distinguish these terms when implementing new practices as failure of effectiveness is different from failure of efficacy. For example, a new cancer drug may prolong survival in a randomized-controlled trial (i.e., efficacy); however, if that drug is not implemented correctly to the population of patients that it is designed to treat (for instance, because of low prescription rates or incorrect prescribing practices), then the apparent efficacy of that drug will be much lower on a population level. The failure of the drug, in that case, has nothing to do with the drug’s efficacy but rather an inability to implement that drug into routine, real-world practice (i.e., effectiveness).
Improving dissemination and implementation
Understanding and addressing the patient-, provider-, and context-related factors that lead to gaps between idealized versus real-world clinical practice are important tenets of D&I research. To address this gap, D&I investigators test implementation strategies which can target these factors22. To further determine whether these strategies are successful, researchers focus on a set of outcomes (so-called implementation outcomes) which differ from the typical patient health outcomes observed in clinical research. For example, feasibility, or the perception of how easy it will be to implement an EBI, may impact whether an intervention is adopted by a clinician. If the intervention, although beneficial for patients, is too complicated or time consuming – and thus not feasible – it is unlikely that a clinician will use it. Another common implementation outcome is fidelity, or the extent to which a clinician implements an intervention as intended23. If an intervention is not implemented with fidelity - for instance, if a surgeon leaves out a critical step in a procedure - it may not be as effective. Conversely, adaptation, or the extent to which interventions are modified to fit the context in which they are implemented, is also considered an important implementation outcome. A surgeon may adapt a procedure to address the specific needs of a patient, thus enhancing the effectiveness of the procedure for that patient. The overall implementation success depends on how well each of these outcomes is addressed23.
D&I investigators use a mixture of theories, models, and frameworks to guide research14. While a full description of these theories and frameworks is outside the scope of this review, Tabak and colleagues provide a comprehensive evaluation elsewhere24. The purpose of these frameworks is to provide analytic structure to D&I questions, allowing for better identification of contextual factors that may influence successful implementation endeavors, specify relevant outcomes, and identify processes for adequately implementing new EBIs in practice. One such framework is the Conceptual Model of Implementation Research developed by Proctor and colleagues25. This framework highlights various implementation outcomes that researchers should measure during D&I studies (Figure 1)23. The framework details a full set of implementation outcomes (like feasibility, acceptability, and sustainability) in addition to traditional service outcomes (like readmissions, survival, and recurrence). Adding implementation outcomes to early clinical testing may aid in rapid dissemination of the new technology after appropriate validation26.
Figure 1:
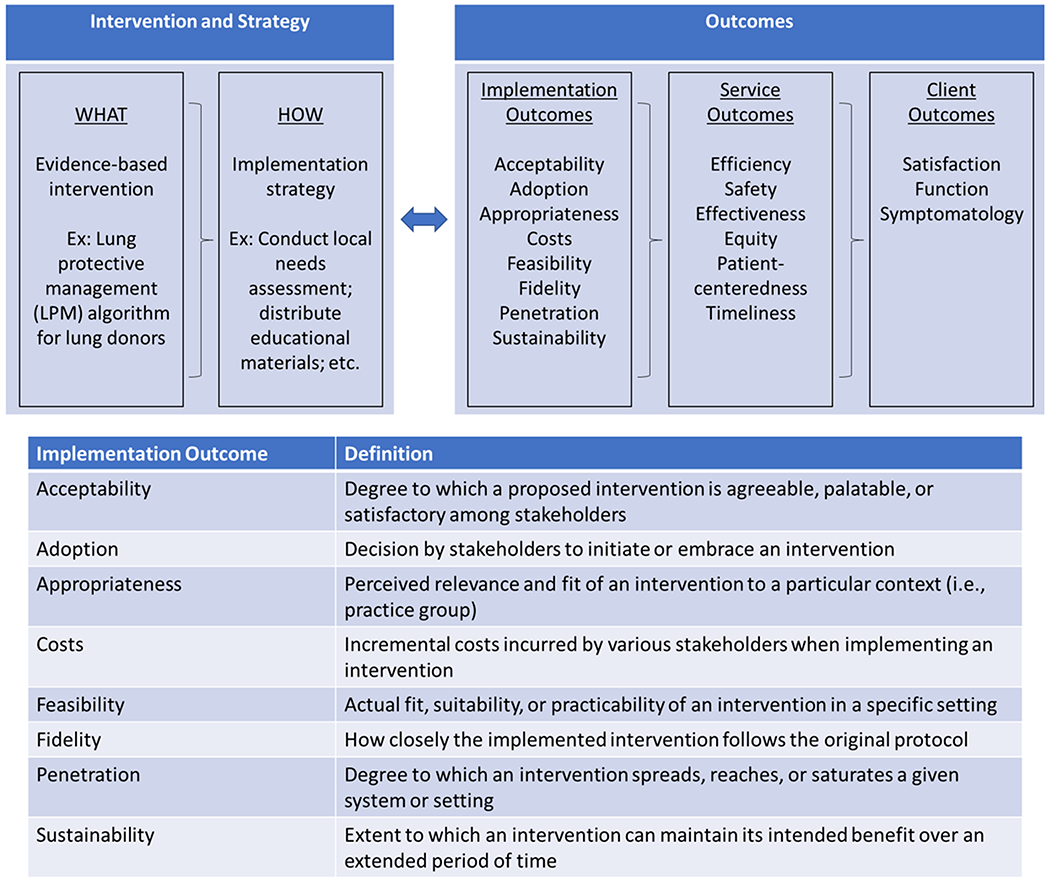
Conceptual Model of Implementation Research Framework. Framework adapted from Proctor and colleagues23,25. Definitions also adapted from Brownson and colleagues14.
D&I science leverages a mixture of common research approaches, including quantitative, qualitative, and mixed method designs27. Several novel, randomized designs have also emerged to suit the demands of D&I research such as hybrid designs, which measure both implementation outcomes and clinical outcomes. Such hybrid designs may be particularly useful for cardiothoracic surgeons to consider early during EBI development because they allow the researchers to test efficacy while also planning for the wide dissemination and implementation of an intervention.
Cardiothoracic surgeons with an interest in D&I may find it a practical time to become involved in such research since several organizations are eagerly supporting D&I endeavors. The National Institutes of Health (NIH), including the National Cancer Institute (NCI) and the National Heart, Lung, and Blood Institute (NHLBI), offer many funding opportunities for “rigorous, cutting-edge dissemination and implementation research,” including R01 opportunities28. Other opportunities are available through organizations like the Patient-Centered Outcomes Research Institute (PCORI) and the Veterans Health Administration (VHA). Cardiothoracic surgeons should be aware of this trend among funding agencies as future funding may require D&I techniques.
Case study: lung protective management algorithms for lung transplant donation
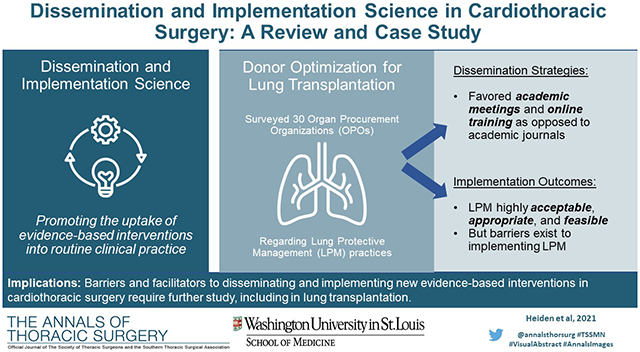
To further exemplify D&I techniques in cardiothoracic surgery, we performed a study examining the potential for implementing an innovative LPM algorithm in lung transplant donors. Lung transplantation is a viable treatment option for patients with end-stage lung disease. Despite significant advances in transplant outcomes over the last several decades, there remains a chronic shortage of available donor organs resulting in long wait times and significant waitlist mortality29. Only 20% of all potential donor lungs are utilized for lung transplantation (the lung utilization rate); this is well below both liver and kidney utilization rates which exceed 50% and 90%, respectively29. Therefore, optimizing donor lung utilization rates is critical.
Previous work by our group has demonstrated that LPM in lung donors results in significantly higher lung utilization rates30. Since 2008, our local organ procurement organization (OPO, Mid-America Transplant) has utilized a simple yet effective LPM algorithm for potential lung donors (see Chang and colleagues for full protocol details)30. Since adoption of this algorithm, lung utilization rates by our OPO have nearly doubled from 19.8% to 33.9%30. Implementation of LPM protocols on a national scale would therefore drastically increase the number of available organs for lung transplant.
We conducted this study to better understand both dissemination and implementation practices by OPOs and other procurement stakeholders across the US. We also attempted to identify barriers and facilitators to effective implementation within this context. Fifty-seven OPOs manage all donor and transplant activities in their respective regions in the US. Using a sequential exploratory mixed methods approach, we initially conducted key informant interviews with 15 clinicians working in OPOs to identify potential barriers and facilitators of adopting the LPM algorithm, and further conducted two on-site visits to better assess barriers to LPM implementation. Then, we distributed surveys to each OPO site, focusing on two stakeholder groups: medical directors and transplant coordinators. The survey employed Proctor’s Conceptual Model of Implementation Research to assess several implementation outcomes following a video-training session on LPM strategies, including awareness, acceptability, appropriateness, and feasibility25. We used items from the Acceptability of Intervention Measure (AIM), Intervention Appropriateness Measure (IAM), and Feasibility of Intervention Measure (FIM) to measure each outcome31.
Surveys were completed by 40 respondents representing 30 of the 57 (52.6%) OPOs nationally. Respondent characteristics and current donor management practices are shown in Table 1. A majority of responding OPOs managed between 101-200 brain dead donors annually for an average donor management duration of 24-48 hours. The average estimated lung utilization rate among these OPOs was 30.0%. Current donor management practices varied across OPOs with 74.3% performing routine chest x-ray (within 3 hours of offer, and repeating every 12 hours), 94.6% performing bronchoscopy on all donors, and 27.8% performing percussive ventilation techniques. Antibiotic management was routinely performed (89.2%) whereas bronchodilator administration was performed less frequently (48.7%).
Table 1:
Survey respondent characteristics and current donor management practices
| Survey Question | Responses |
|---|---|
| Responses | |
| Respondents (number of individuals) | 40 |
| OPOs (number of OPOs represented) | 30 |
|
| |
| Years at organization (%) | |
| <5 | 4 (10.0) |
| 5-9 | 14 (35.0) |
| ≥10 | 22 (55.0) |
|
| |
| Estimated number of brain dead donors (%) | |
| 0-100 | 9 (23.7) |
| 101-200 | 19 (50.0) |
| 201-300 | 4 (10.5) |
| >300 | 6 (15.8) |
|
| |
| Estimated lung utilization rate (median, IQR) | 30.0 (15.0) |
|
| |
| Where are brain dead donors managed? (%) | |
| Hospitals | 27 (71.1) |
| Hospitals and/or SDCFs | 11 (29.0) |
|
| |
| Who manages brain dead donors? (%) | |
| Coordinators | 35 (92.1) |
| Other | 3 (7.9) |
|
| |
| Average duration of lung donor management (%) | |
| 24-48 hours | 26 (68.4) |
| 49-72 hours | 12 (31.6) |
|
| |
| Mode of ventilation (%) | |
| Pressure Regulated Volume Control (PRVC) | 15 (40.5) |
| Airway Pressure Release Ventilation (APRV) | 4 (10.8) |
| Assist Control Ventilation (ACV) | 6 (16.2) |
| Combination of modes or other | 12 (32.4) |
|
| |
| Donor management (%) | |
| Perform CXR w/in 3 hrs of offer, repeating every 12 hrs | 26 (74.3) |
| Perform early bronchoscopy on all donors | 35 (94.6) |
| Perform intrapulmonary percussive ventilation | 10 (27.8) |
| Perform ABG every 4 hours or with ventilatory changes | 36 (97.3) |
| Perform lung recruitment for PaO2/FiO2 < 300 | 36 (97.3) |
|
| |
| Antibiotic management (%) | |
| Administered routinely | 33 (89.2) |
| Administered as needed | 4 (10.8) |
| Not administered | 0 (0.0) |
|
| |
| Bronchodilator management (%) | |
| Administered routinely | 18 (48.7) |
| Administered as needed | 17 (46.0) |
| Not administered | 2 (5.4) |
ABG=arterial blood gas, CXR=chest x-ray, FiO2= fraction of inspired oxygen, PaO2=partial pressure of oxygen, SDCF=specialized donor care facility
Preferred information dissemination strategies among OPOs are shown in Table 2. The most common method to learn about advances in lung donor management was through academic meetings (100% of respondents). Only 62.5% of respondents endorsed learning about advances in donor management from academic journals. In terms of preferences for the most effective dissemination techniques, 40.6% preferred online training platforms and 28.1% preferred conferences. Only 1 respondent (3.1%) listed academic journals as the preferred dissemination method.
Table 2:
Dissemination practices and strategies
| Survey question | Responses |
|---|---|
| How do you learn about advances in lung donor management? (%)a | |
| Scientific journals | 20 (62.5) |
| Conferences | 32 (100.0) |
| Email broadcasting | 11 (34.3) |
| From my organization | 9 (28.1) |
|
| |
| In your opinion, what is the best way to disseminate new evidence-based practices? (%) | |
| An educative/illustrative video | 3 (9.4) |
| Conferences | 9 (28.1) |
| Research briefs | 3 (9.4) |
| Newsletter | 1 (3.1) |
| Online training platform | 13 (40.6) |
| Other | 3 (9.4) |
|
| |
| How was current lung donor protocol developed? (%)a | |
| Review of published evidence on lung donor management | 23 (62.1) |
| Personal clinical experiences | 11 (29.7) |
| External collaboration | 14 (37.8) |
| From already existing protocols | 12 (32.4) |
| Experimental trial (Randomized control trial, etc.) | 5 (13.5) |
| Medical director expertise | 4 (10.8) |
|
| |
| What factors influence current lung donor protocol? (%)a | |
| Experience of Staff | 21 (58.3) |
| Availability and location of transplant centers | 11 (30.6) |
| Expectations of transplant hospitals and surgeon | 24 (66.7) |
| Lung transplant rate | 15 (41.7) |
Respondents could choose multiple responses
The methods for developing current lung donor protocols were also assessed. In general, staff experience (58.3%) and expectations from surgeons (66.7%) were the most common factors influencing the current lung donor protocol. Factors most commonly influencing the creation of current lung donor protocols were published literature (62.1%), external collaboration (37.8%), prior protocols (32.4%), and personal experience (29.7%).
Following a brief video-based training session, we assessed several implementation outcomes and barriers to implementation for the LPM algorithm. In general, the LPM algorithm was viewed as highly acceptable, appropriate, and feasible by the OPOs (Table 3). Awareness of the LPM algorithm was also high among OPOs with only 18.8% being “unfamiliar” (Table 4). “Definitely” and “likely” needing additional training were reported by 9.4% and 43.8% of respondents, respectively, and 34.4% of respondents anticipated a need for additional resources. Commonly perceived barriers to implementation of the LPM algorithm were staff specific training (57.1%), requirement of ongoing support or supervision (25.0%), additional personnel (14.3%), and additional material resources (14.3%). Staff specific training as a perceived barrier was commonly shared by stakeholders at OPOs during interviews. In explaining how challenging it may be to provide training, one person shared: “We have a large team that works, you know, different shifts. So getting everyone together in one room especially in our large service area isn’t really feasible.” Some stakeholders thought that these barriers could be overcome if ongoing support and supervision was made available to OPO staff. A stakeholder explained:
Table 3:
Acceptability, appropriateness, and feasibility of LPM algorithm among OPOs
| Survey Question | Yes | Maybe | Not Sure | No |
|---|---|---|---|---|
| Does the LPM algorithm… (%) | ||||
| Meet your approvala | 21 (65.6) | 7 (21.9) | 3 (9.4) | 1 (3.1) |
| Seem appealinga | 23 (71.9) | 7 (21.9) | 2 (6.3) | 0 (0.0) |
| Seem fittingb | 26 (81.3) | 3 (9.4) | 2 (6.3) | 1 (3.1) |
| Seem easy to usec | 26 (81.3) | 4 (12.5) | 1 (3.1) | 1 (3.1) |
| Seem implementablec | 28 (87.5) | 2 (6.3) | 2 (6.3) | 0 (0.0) |
| Seem realisticc | 28 (87.5) | 2 (6.3) | 2 (6.3) | 0 (0.0) |
Adapted from Weiner and colleagues31.
Acceptability of Intervention Measure (AIM)
Intervention Appropriateness Measure (IAM)
Feasibility of Intervention Measure (FIM)
Table 4:
Awareness and barriers to implementation of LPM algorithm among OPOs
| Survey Question | Responses |
|---|---|
| How familiar were you with LPM? (“Awareness”) (%) | |
| Used it | 7 (21.9) |
| Read about it | 10 (31.3) |
| Heard about it | 9 (28.1) |
| Not familiar at all | 6 (18.8) |
|
| |
| Would your staff need additional training to implement LPM? (%) | |
| Definitely | 3 (9.4) |
| Likely | 14 (43.8) |
| Not at all | 15 (46.9) |
|
| |
| Does your organization have the necessary resources to implement LPM? (%) | |
| Have the necessary resources | 20 (62.5) |
| May need additional resources | 11 (34.4) |
| Unknown | 1 (3.1) |
|
| |
| What are the most significant barriers to LPM implementation? (%) | |
| Staff specific training | 16 (57.1) |
| Too time-consuming | 2 (7.1) |
| Requires ongoing support and supervision | 7 (25.0) |
| Requires additional personnel | 4 (14.3) |
| Requires additional material resources | 4 (14.3) |
| Not flexible enough for specific patient | 1 (3.6) |
| Do not have enough information to determine | 6 (21.4) |
“And so we’re looking at ways that we might be able to create that same type of environment where you’re working alongside with your colleagues and seeing what each other are doing to help improve you know, you want more stability and oxygenation, so that’s kind of evolving. But I think something like that would be ideal.”
The findings of our study demonstrate how D&I might be integrated into cardiothoracic surgery. As hypothesized, OPOs prefer mechanisms of information dissemination that are different from the traditional research pipeline (like seminars, online modules, conferences, etc.). For current lung transplant researchers, this suggests that more active forms of dissemination are needed for implementation of new practices. More importantly, implementation should not be merely perceived as introduction of a new finding into practice, but rather to implement in a way that remains sustainable over time, as highlighted by the need for continued support and supervision by OPOs in implementing the LPM. Understanding preferred and effective methods for information dissemination among all involved stakeholders is important for high-level, cutting-edge care.
Other Examples of D&I in surgery
Various surgical subspecialties have begun to explore the field of D&I science with some success, although mostly outside of cardiothoracic surgery. One example is the rapid implementation of World Health Organization (WHO) surgical safety checklist32. In 2009, Haynes and colleagues demonstrated a significant reduction in mortality and peri-operative complications after introduction of the checklist to a diverse set of practice environments33. Within several years of this study, safety checklist use expanded rapidly to more than 3,900 hospitals in 122 countries34. Such successful and rapid implementation of new health practices like the WHO checklist are rare. Therefore, better understanding of examples like this is imperative to D&I investigators so they can harness such techniques in future implementation endeavors. However, implementation of the WHO checklist also displays the complexities of D&I research. For example, some have questioned if surgical safety checklists are beneficial across all contexts35. Others have noted the ongoing challenges of successfully implementing the checklist in low resource settings, even despite its relative simplicity36. These issues highlight the range of factors that can challenge successful implementation.
Other areas of surgical research would benefit from more extensive implementation. For example, handwashing is a simple and effective tool to prevent nosocomial infections. Despite this, hand hygiene is a routinely disregarded practice across several areas of medicine37,38. Similarly, new persistent opioid abuse is significant after major and minor surgical procedures39. Despite renewed efforts to decrease the amount of opiates prescribed after surgery, over-prescribing practices remain high40,41. Addressing these gaps in evidence-based practice are important.
Areas for D&I research in cardiothoracic surgery
A number of areas in cardiothoracic surgery may benefit from D&I techniques. In thoracic surgery, there are several areas where implementation of EBIs has been suboptimal. For example, video-assisted thoracic surgery (VATS) has existed for several decades and the approach has been shown to reduce both operative morbidity and mortality compared to open thoracotomy42. Despite this, recent studies have highlighted that open resections are still extraordinarily common nationwide43. Capitalizing on established implementation strategies from other fields may allow for better adoption of VATS, particularly at non-academic, low-volume centers44. Another example in thoracic surgery is the poor implementation of lung volume reduction surgery for patients with emphysema. Subgroup analyses of the National Emphysema Treatment Trial demonstrated that patients with upper lobe predominant emphysema and low exercise capacity benefit from apical lung volume reduction45. Despite subsequent analyses confirming this observation46,47, lung volume reduction surgery remains vastly underutilized48. A final example of poor implementation in thoracic surgery is the underwhelming adoption of lung cancer screening programs. As discussed previously, nearly a decade after the NLST, less than 15% of screening-eligible adults in the United States receive appropriate lung cancer screening20. Better implementation of lung cancer screening programs will be needed in light of recent recommendations from the United States Preventative Services Taskforce to expand screening eligibility49.
There are additional areas for better implementation in lung transplantation, apart from our case study. For example, prone ventilation of the donor has been found to significantly improve donor oxygenation and augment lung utilization rates50. Disseminating and implementing this technique across all donors could therefore address the chronic shortage of donor organs. D&I techniques could help to address the feasibility and practicality of this relatively benign intervention, focusing on certain practice contexts (i.e., rural hospitals with smaller ICUs and fewer staff) where prone ventilation may be less common and more challenging.
Cardiac surgery also has several examples of meager implementation. For example, the gold-standard conduit for CABG is the internal mammary artery (IMA) due to superior long-term patency rates. While the advantages of IMA selection were well-established by the mid 1980s51, widespread use of the IMA lagged by at least a decade52,53. The adoption of IMA bypass grafting is an example of slow implementation that modern D&I investigators seek to prevent. Another example is the use of off-pump CABG. While this technique may be of benefit in select patients, building evidence suggests that de-implementation of this procedure may be warranted due to lower graft patency rates54.
Opportunities to advance D&I science are possible in other settings. For example, sustainability is the “extent to which an evidence-based intervention can deliver its intended benefits over an extended period of time after external support from the donor agency is terminated”14,55. In thoracic surgery, the sustainability of collecting patient-reported outcomes after pulmonary resection has been a challenge for several groups – particularly after external research funding expires56. At academic hospitals, sustaining various service-specific practices (like Enhanced Recover After Surgery protocols) can be challenging as providers teams – particularly residents and fellows – change frequently57.
Another possibility is de-implementation, or stopping or abandoning practices that have not proved to be effective and are possibly harmful, which has emerged as a new line of research within D&I14,58. For example, de-implementation has been studied outside of cardiothoracic surgery in the context of the Choosing Wisely® campaign, particularly in low-value breast cancer surgeries59–61. The Choosing Wisely® campaign is an initiative of the American Board of Internal Medicine that invites (or challenges) various professional organizations to critically evaluate low-value practices in their field62. The campaign is meant to de-implement mis- or over-used tests and procedures that result in little patient benefit or potentially even harm63. Several procedures for breast cancer like re-excision of close (but negative) margins and completion axillary lymph node dissection (ALND) are unnecessary but still commonly practiced. Smith and colleagues identified several barriers and facilitators to de-implementation of these procedures among breast cancer surgeons59. Factors like patient autonomy and “social influence” from other medical providers (like medical oncologists) dictated continued use of these low-value procedures. Understanding the factors that influence surgeon behavior are critical components of successful implementation research38,59.
The Society of Thoracic Surgery (STS) also contributed to the Choosing Wisely® campaign. After considering seventeen potential candidates, five interventions were deemed to be unnecessary by the STS (Table 5). Interestingly, all of these “low-value” interventions were lab or imaging tests, not procedures62. Despite initial enthusiasm and widespread buy-in, it remains unclear how successfully these interventions are being de-implemented on a national scale64. Cardiothoracic surgery should consider other de-implementable practices in the field, including low-value procedures. For example, de-implementation of off-pump coronary artery bypass grafting (CABG) has been recommended by various groups but remains common in practice54. Performing surveillance imaging too frequently after lung cancer resection may be another common practice to de-implement65. In considering de-implementation, surgeons should take into account three tenets: (1) does the intervention lack evidence or cause harm; (2) are there more effective or efficient interventions available; and (3) has the issue of concern (i.e., condition being treated) dissipated66. Interventions that violate any of these tenets may warrant further scrutiny for potential de-implementation.
Table 5:
Choosing Wisely® campaign in cardiothoracic surgery
| Cardiac | |
|---|---|
| Carotid disease | Avoid routine evaluation for carotid artery disease prior to cardiac surgery in the absence of symptoms or other high-risk factors |
| Echocardiogram | Avoid routine echocardiogram prior to discharge following valve replacement surgery |
| Pulmonary function tests (PFTs) | Avoid routine PFTs in patients undergoing cardiac surgery in the absence of respiratory symptoms |
| Thoracic | |
| Stress tests | Avoid routine pre-operative stress testing in patients with good functional status and no cardiac history |
| Brain imaging | Avoid routine brain imaging in patients with suspected or biopsy-proven clinical stage I NSCLC without neurological symptoms |
Adapted from Wood and colleagues69.
Limitations of D&I Science
It is worth noting that while D&I is considered a relatively “young” and new field, it is not entirely novel. In particular, D&I has roots in and draws upon several less scientific and less elaborate mechanisms of dissemination and implementation that have been in place for decades in several fields, including cardiothoracic surgery67. For example, various collaboratives (like the Virginia Cardiac Services Quality Initiative, the Michigan Society of Thoracic and Cardiovascular Surgeons, etc.) have long contributed to disseminating EBIs in cardiothoracic surgery, but often with a less systematic approach and a more focused clinical area. D&I science builds on this legacy by providing objective methods to promote and sustain beneficial interventions, focusing particularly on barriers and facilitators to early implementation68.
It is similarly important to recognize that D&I is not a panacea for solving all social and clinical challenges in medicine. For example, while D&I researchers strive to identify barriers to successful implementation, only a subset of these barriers may be modifiable. Similarly, facilitators to successful implementation, while critical to understand, are heavily context dependent. Akin to any scientific endeavor, the success of D&I researchers in producing meaningful change is subject to uncertainty. Nonetheless, even partial success may be an achievable and appropriate outcome. For example, instead of implementing certain complex techniques across all practice domains (i.e., in small or rural hospitals), it may be more feasible and appropriate to focus initial implementation efforts where more resources exist or where there is the greatest need. D&I techniques can help to explore such subtleties.
Conclusion
Cardiothoracic surgeons have traditionally been at the forefront of evidence-based surgical practice. Over the last several decades, the volume of evidence that supports various practice changes has been growing immensely. Consequently, it is becoming increasingly difficult to take EBIs from the “bench” to “bedside,” especially on a national or international scale. This is evident in the fact that several interventions with poor evidence are still practiced69 and other interventions with strong evidence are not practiced43,46. Barriers and facilitators to disseminating and implementing new evidence-based interventions in cardiothoracic surgery are understudied. Both dissemination and implementation techniques can help to lessen this gap between idealized and real-world practice and allow cardiothoracic researchers to “reach” a larger pool of surgeons.
Source of Funding:
Funded in part by NIH 5T32HL007776-25 (BTH), 1 I01 HX002475-01A2 (VP), UL1TR002345 (VRM), and the Center for Dissemination and Implementation in the Institute for Public Health at Washington University in St. Louis (VRM)
Footnotes
Conflict of Interest: Pending patent (DK) entitled “Compositions and methods for detecting CCR2 receptors” (application number 15/611,577)
References
- 1.Morris ZS, wooding S, Grant J. The answer is 17 years, what is the question: Understanding time lags in translational research. J R Soc Med. 2011;104(12):510–520. doi: 10.1258/jrsm.2011.110180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer MS, Damschroder L, Hagedorn H, Smith J, Kilbourne AM. An introduction to implementation science for the non-specialist. BMC Psychol. 2015;3(1). doi: 10.1186/S40359-015-0089-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balas EA, Boren SA. Managing Clinical Knowledge for Health Care Improvement. Yearb Med Inform. 2000;(1):65–70. [PubMed] [Google Scholar]
- 4.Arroyo NA, Gessert T, Hitchcock M, et al. What Promotes Surgeon Practice Change? A Scoping Review of Innovation Adoption in Surgical Practice. Ann Surg. 2021;273(3):474–482. doi: 10.1097/SLA.0000000000004355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sampson UKA, Mensah GA, Narula J. Implementation research: An imperative for improving global health and health inequities. Glob Heart. 2015;10(1):1–2. doi: 10.1016/j.gheart.2014.12.002 [DOI] [PubMed] [Google Scholar]
- 6.Bero LA, Grilli R, Grimshaw JM, Harvey E, Oxman AD, Thomson MA. Getting research findings into practice. Closing the gap between research and practice: An overview of systematic reviews of interventions to promote the implementation of research findings. Br Med J. 1998;317(7156):465–468. doi: 10.1136/bmj.317.7156.465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brownson RC, Jacobs JA, Tabak RG, Hoehner CM, Stamatakis KA. Designing for dissemination among public health researchers: Findings from a national survey in the United States. Am J Public Health. 2013;103(9):1693–1699. doi: 10.2105/AJPH.2012.301165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eccles MP, Mittman BS. Welcome to implementation science. Implement Sci. 2006;1(1):1. doi: 10.1186/1748-5908-1-1 [DOI] [Google Scholar]
- 9.Burnham JP, Geng E, Venkatram C, Colditz GA, McKay VR. Putting the Dissemination and Implementation in Infectious Diseases. Clin Infect Dis. 2020;71(1):218–225. doi: 10.1093/cid/ciz1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geng EH, Peiris D, Kruk ME. Implementation science: Relevance in the real world without sacrificing rigor. PLOS Med. 2017;14(4):e1002288. doi: 10.1371/journal.pmed.1002288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curran GM. Implementation science made too simple: a teaching tool. Implement Sci Commun. 2020;1(1). doi: 10.1186/s43058-020-00001-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diffusion LOMASJ, Dissemination, and Implementation: Who Should Do What? Ann N Y Acad Sci. 1993;703(1):226–237. doi: 10.1111/j.1749-6632.1993.tb26351.x [DOI] [PubMed] [Google Scholar]
- 13.MacLean DR. Positioning dissemination in public health policy. Can J Public Health. 1996;87 Suppl 2:S40–3. [PubMed] [Google Scholar]
- 14.Rabin BA, Brownson RC. Terminology for Dissemination and Implementation Research. In: Dissemination and Implementation Research in Health. 2nd ed. Oxford University Press; 2017. doi: 10.1093/oso/9780190683214.003.0002 [DOI] [Google Scholar]
- 15.Knoepke CE, Ingle MP, Matlock DD, Brownson RC, Glasgow RE. Dissemination and stakeholder engagement practices among dissemination & implementation scientists: Results from an online survey. Conejero JA, ed. PLoS One. 2019;14(11):e0216971. doi: 10.1371/journal.pone.0216971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tabak RG, Stamatakis KA, Jacobs JA, Brownson RC. What predicts dissemination efforts among public health researchers in the United States? Public Health Rep. 2014;129(4):361–368. doi: 10.1177/003335491412900411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brownson RC, Fielding JE, Green LW. Building Capacity for Evidence-Based Public Health: Reconciling the Pulls of Practice and the Push of Research. In: Annual Review of Public Health. Vol 39. Annual Reviews Inc.; 2018:27–53. doi: 10.1146/annurev-publhealth-040617-014746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimshaw JM, Thomas RE, MacLennan G, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess (Rockv). 2004;8(6). doi: 10.3310/hta8060 [DOI] [PubMed] [Google Scholar]
- 19.The National Lung Screening Trial Research Team. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richards TB, Soman A, Thomas CC, et al. Screening for Lung Cancer — 10 States, 2017. MMWR Morb Mortal Wkly Rep. 2020;69(8):201–206. doi: 10.15585/mmwr.mm6908a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glasgow RE, Vinson C, Chambers D, Khoury MJ, Kaplan RM, Hunter C. National institutes of health approaches to dissemination and implementation science: Current and future directions. Am J Public Health. 2012;102(7):1274–1281. doi: 10.2105/AJPH.2012.300755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: Results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10(1):21. doi: 10.1186/s13012-015-0209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: Conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Heal Ment Heal Serv Res. 2011;38(2):65–76. doi: 10.1007/s10488-010-0319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabak RG, Khoong EC, Chambers DA, Brownson RC. Bridging research and practice: Models for dissemination and implementation research. Am J Prev Med. 2012;43(3):337–350. doi: 10.1016/j.amepre.2012.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Proctor EK, Landsverk J, Aarons G, Chambers D, Glisson C, Mittman B. Implementation research in mental health services: An emerging science with conceptual, methodological, and training challenges. Adm Policy Ment Heal Ment Heal Serv Res. 2009;36(1):24–34. doi: 10.1007/s10488-008-0197-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: Combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50(3):217–226. doi: 10.1097/MLR.0b013e3182408812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazzucca S, Tabak RG, Pilar M, et al. Variation in research designs used to test the effectiveness of dissemination and implementation strategies: A review. Front Public Heal. 2018;6(FEB). doi: 10.3389/fpubh.2018.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Funding Opportunities | Division of Cancer Control and Population Sciences (DCCPS). Accessed March 31, 2021. https://cancercontrol.cancer.gov/is/funding
- 29.Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2017 Annual Data Report: Lung. Am J Transplant. 2019;19:404–484. doi: 10.1111/ajt.15279 [DOI] [PubMed] [Google Scholar]
- 30.Chang SH, Kreisel D, Marklin GF, et al. Lung Focused Resuscitation at a Specialized Donor Care Facility Improves Lung Procurement Rates. Ann Thorac Surg. 2018;105(5):1531–1536. doi: 10.1016/j.athoracsur.2017.12.009 [DOI] [PubMed] [Google Scholar]
- 31.Weiner BJ, Lewis CC, Stanick C, et al. Psychometric assessment of three newly developed implementation outcome measures. Implement Sci. 2017;12(1):108. doi: 10.1186/s13012-017-0635-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hull L, Athanasiou T, Russ S. Implementation Science. Ann Surg. 2017;265(6):1104–1112. doi: 10.1097/SLA.0000000000002013 [DOI] [PubMed] [Google Scholar]
- 33.Haynes AB, Weiser TG, Berry WR, et al. A Surgical Safety Checklist to Reduce Morbidity and Mortality in a Global Population. N Engl J Med. 2009;360(5):491–499. doi: 10.1056/NEJMsa0810119 [DOI] [PubMed] [Google Scholar]
- 34.Conley DM, Singer SJ, Edmondson L, Berry WR, Gawande AA. Effective surgical safety checklist implementation. J Am Coll Surg. 2011;212(5):873–879. doi: 10.1016/j.jamcollsurg.2011.01.052 [DOI] [PubMed] [Google Scholar]
- 35.Urbach DR, Govindarajan A, Saskin R, Wilton AS, Baxter NN. Introduction of Surgical Safety Checklists in Ontario, Canada. N Engl J Med. 2014;370(11):1029–1038. doi: 10.1056/NEJMsa1308261 [DOI] [PubMed] [Google Scholar]
- 36.Delisle M, Pradarelli JC, Panda N, et al. Variation in global uptake of the Surgical Safety Checklist. Br J Surg. 2020;107(2):e151–e160. doi: 10.1002/bjs.11321 [DOI] [PubMed] [Google Scholar]
- 37.Srigley JA, Corace K, Hargadon DP, et al. Applying psychological frameworks of behaviour change to improve healthcare worker hand hygiene: A systematic review. J Hosp Infect. 2015;91(3):202–210. doi: 10.1016/j.jhin.2015.06.019 [DOI] [PubMed] [Google Scholar]
- 38.Telem DA, Dimick J, Skolarus TA. Dissecting Surgeon Behavior: Leveraging the Theoretical Domains Framework to Facilitate Evidence-based Surgical Practice. Ann Surg. 2018;267(3):432–434. doi: 10.1097/SLA.0000000000002506 [DOI] [PubMed] [Google Scholar]
- 39.Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in us adults. JAMA Surg. 2017;152(6):e170504. doi: 10.1001/jamasurg.2017.0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agarwal AK, Lee D, Ali Z, et al. Patient-Reported Opioid Consumption and Pain Intensity After Common Orthopedic and Urologic Surgical Procedures With Use of an Automated Text Messaging System. JAMA Netw Open. 2021;4(3):e213243. doi: 10.1001/jamanetworkopen.2021.3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subramanian MP, Sahrmann JM, Nickel KB, et al. Assessment of Preoperative Opioid Use Prevalence and Clinical Outcomes in Pulmonary Resection. Ann Thorac Surg. Published online 2020. doi: 10.1016/j.athoracsur.2020.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernandez-Vaquero D, Vigil-Escalera C, Pérez-Méndez I, et al. Survival After Thoracoscopic Surgery Or Open Lobectomy. Systematic Review And Meta-Analysis. Ann Thorac Surg. 2020;0(0). doi: 10.1016/j.athoracsur.2020.05.144 [DOI] [PubMed] [Google Scholar]
- 43.Subramanian MP, Liu J, Chapman WC, et al. Utilization Trends, Outcomes, and Cost in Minimally Invasive Lobectomy. In: Annals of Thoracic Surgery. Vol 108. Elsevier USA; 2019:1648–1655. doi: 10.1016/j.athoracsur.2019.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kozower BD, Stukenborg GJ. The relationship between hospital lung cancer resection volume and patient mortality risk. Ann Surg. 2011;254(6):1032–1037. doi: 10.1097/SLA.0b013e31821d4bdd [DOI] [PubMed] [Google Scholar]
- 45.Fishman A, Martinez F, Piantadosi S, Wise R, Hopkins Uni-versity J, Ries A. A Randomized Trial Comparing Lung-Volume–Reduction Surgery with Medical Therapy for Severe Emphysema. N Engl J Med. 2003;348(21):2059–2073. doi: 10.1056/NEJMoa030287 [DOI] [PubMed] [Google Scholar]
- 46.van Agteren JEM, Carson KV., Tiong LU, Smith BJ. Lung volume reduction surgery for diffuse emphysema. Cochrane Database Syst Rev. 2016;2016(10). doi: 10.1002/14651858.CD001001.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horwood CR, Mansour D, Abdel-Rasoul M, et al. Long-Term Results After Lung Volume Reduction Surgery: A Single Institution’s Experience. Ann Thorac Surg. 2019;107(4):1068–1073. doi: 10.1016/j.athoracsur.2018.10.014 [DOI] [PubMed] [Google Scholar]
- 48.Decker MR, Leverson GE, Jaoude WA, Maloney JD. Lung volume reduction surgery since the National Emphysema Treatment Trial: Study of Society of Thoracic Surgeons Database. J Thorac Cardiovasc Surg. 2014;148(6):2651–2658.e1. doi: 10.1016/j.jtcvs.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krist AH, Davidson KW, Mangione CM, et al. Screening for Lung Cancer. JAMA. 2021;325(10):962. doi: 10.1001/jama.2021.1117 [DOI] [PubMed] [Google Scholar]
- 50.Marklin GF, O’Sullivan C, Dhar R. Ventilation in the prone position improves oxygenation and results in more lungs being transplanted from organ donors with hypoxemia and atelectasis. J Hear Lung Transplant. 2021;40(2):120–127. doi: 10.1016/j.healun.2020.11.014 [DOI] [PubMed] [Google Scholar]
- 51.Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the Internal-Mammary-Artery Graft on 10-Year Survival and Other Cardiac Events. N Engl J Med. 1986;314(1):1–6. doi: 10.1056/nejm198601023140101 [DOI] [PubMed] [Google Scholar]
- 52.Canver CC. Conduit options in coronary artery bypass surgery. Chest. 1995;108(4):1150–1155. doi: 10.1378/chest.108.4.1150 [DOI] [PubMed] [Google Scholar]
- 53.Melly L, Torregrossa G, Lee T, Jansens JL, Puskas JD. Fifty years of coronary artery bypass grafting. J Thorac Dis. 2018;10(3):1960–1967. doi: 10.21037/jtd.2018.02.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gaudino M, Benedetto U, Bakaeen F, et al. Off-versus on-pump coronary surgery and the effect of follow-up length and surgeons’ experience: A meta-analysis. J Am Heart Assoc. 2018;7(21). doi: 10.1161/JAHA.118.010034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shediac-Rizkallah MC, Bone LR. Planning far the sustainability of community-based health programs: Conceptual frameworks and future directions for research, practice and policy. Health Educ Res. 1998;13(1):87–108. doi: 10.1093/her/13.1.87 [DOI] [PubMed] [Google Scholar]
- 56.Heiden BT, Subramanian MP, Nava R, et al. Routine Collection of Patient Reported Outcomes in Thoracic Surgery: A Quality Improvement Study. Ann Thorac Surg. Published online July 2, 2021. doi: 10.1016/J.ATHORACSUR.2021.05.091 [DOI] [PubMed] [Google Scholar]
- 57.Heiden BT, Semenkovich TR, Kozower BD. Guide to Enhanced Recovery for Cancer Patients Undergoing Surgery. Ann Surg Oncol. Published online 2021. doi: 10.1245/s10434-021-09882-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prasad V, Ioannidis JPA. Evidence-based de-implementation for contradicted, unproven, and aspiring healthcare practices. Implement Sci. 2014;9(1). doi: 10.1186/1748-5908-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith ME, Vitous CA, Hughes TM, Shubeck SP, Jagsi R, Dossett LA. Barriers and Facilitators to De-Implementation of the Choosing Wisely® Guidelines for Low-Value Breast Cancer Surgery. Ann Surg Oncol. 2020;27(8):2653–2663. doi: 10.1245/s10434-020-08285-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gleisner AL, Moss A, Friedman C, et al. De-Implementation of Axillary Dissection in Women with Breast Cancer is Largely Driven By Site-Level Contextual Effects. Ann Surg. 2020;Publish Ah. doi: 10.1097/SLA.0000000000004705 [DOI] [PubMed] [Google Scholar]
- 61.Wang T, Sabel MS, Dossett LA. A Framework for De-implementation in Surgery. Ann Surg. 2021;273(3):e105–e107. doi: 10.1097/SLA.0000000000004325 [DOI] [PubMed] [Google Scholar]
- 62.Morden NE, Colla CH, Sequist TD, Rosenthal MB. Choosing Wisely — The Politics and Economics of Labeling Low-Value Services. N Engl J Med. 2014;370(7):589–592. doi: 10.1056/nejmp1314965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choosing Wisely: Society of Thoracic Surgeons – The Journal of Healthcare Contracting. Accessed March 29, 2021. https://www.jhconline.com/choosing-wisely-society-of-thoracic-surgeons.html
- 64.Kerr EA, Kullgren JT, Saini SD. Choosing Wisely: How To Fulfill The Promise In The Next 5 Years. Health Aff. 2017;36(11):2012–2018. doi: 10.1377/hlthaff.2017.0953 [DOI] [PubMed] [Google Scholar]
- 65.Heiden BT, Subramanian MP, Puri V, Kozower BD. Striking a Balance: Surveillance of Non-Small Cell Lung Cancer after Resection. J Thorac Cardiovasc Surg. Published online December 18, 2020. doi: 10.1016/j.jtcvs.2020.10.166 [DOI] [PubMed] [Google Scholar]
- 66.McKay VR, Morshed AB, Brownson RC, Proctor EK, Prusaczyk B. Letting go: Conceptualizing intervention de-implementation in public health and social service settings. Am J Community Psychol. 2018;62(1-2):189–202. doi: 10.1002/ajcp.12258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lobb R, Colditz GA. Implementation Science and Its Application to Population Health. Annu Rev Public Health. 2013;34(1):235–251. doi: 10.1146/annurev-publhealth-031912-114444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kao LS. Implementation Science and Quality Improvement BT - Success in Academic Surgery: Health Services Research. In: Dimick JB, Greenberg CC, eds. Springer; London; 2014:85–100. doi: 10.1007/978-1-4471-4718-3_8 [DOI] [Google Scholar]
- 69.Wood DE, Mitchell JD, Schmitz DS, et al. Choosing wisely: Cardiothoracic surgeons partnering with patients to make good health care decisions. Ann Thorac Surg. 2013;95(3):1130–1135. doi: 10.1016/j.athoracsur.2013.01.008 [DOI] [PubMed] [Google Scholar]


