Abstract
Background
Physicians often prescribe opioids for pain in the acute care setting. Nausea and vomiting are well‐described adverse events, occurring in over one‐third of patients. Prophylactic antiemetics may be one option to reduce opioid‐associated nausea and vomiting. However, these medications also have their own adverse effects, so it is important to understand their efficacy and safety prior to routine use. This is a review of randomized controlled trials comparing prophylactic antiemetics versus placebo or standard care for preventing opioid‐associated nausea and vomiting.
Objectives
To assess the effects of prophylactic antiemetics for nausea and vomiting in adults (aged 16 years or older) receiving intravenous opioids in the acute care setting.
Search methods
We searched CENTRAL (the Cochrane Library), MEDLINE (OVID), Embase (OVID) from inception to January 2022, and Google Scholar (17 January 2022). We also searched the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) and screened reference lists.
Selection criteria
We included randomized controlled trials of prophylactic antiemetics versus placebo or standard care in adults prior to receiving an intravenous opioid.
Data collection and analysis
Two review authors (MG, JNC) independently determined the eligibility of each study according to the inclusion criteria. Two review authors (MG, GDP) then independently extracted data, assessed risk of bias, and determined the certainty of evidence using GRADE. Our primary outcomes were the occurrence of nausea, vomiting, and adverse events. Secondary outcomes included nausea severity, number of vomiting episodes, and number of participants requiring antiemetic rescue therapy. We presented outcomes as risk ratios (RR) for dichotomous data (e.g. presence of vomiting, presence of nausea, number of participants requiring rescue medication, adverse events) and mean difference (MD) or standardized mean difference for continuous data (e.g. number of vomiting episodes, nausea severity) with 95% confidence intervals (CI).
Main results
We included three studies involving 527 participants (187 women and 340 men) with a mean age of 42 years. All studies used intravenous metoclopramide (10 mg) as the intervention and a placebo for the comparator. No studies assessed any other antiemetic or compared the intervention to standard care.
Compared to placebo, metoclopramide did not reduce vomiting (RR 1.18, 95% CI 0.26 to 5.32; low‐certainty evidence) or nausea (RR 0.55; 95% CI 0.15 to 2.03; low‐certainty evidence) and there was no difference in adverse events (RR 2.34, 95% CI 0.47 to 11.61; low‐certainty evidence). No data were available regarding the number of vomiting episodes. Metoclopramide did reduce the severity of nausea compared with placebo (MD −0.49, 95% CI −0.75 to −0.23; low‐certainty evidence) but did not reduce the need for rescue medication (RR 1.86, 95% CI 0.17 to 20.16; low‐certainty evidence).
Two studies were at unclear risk of bias for random sequence generation, one for blinding of outcome assessors, one for incomplete outcome data, and two for selective reporting. The studies were at low risk of bias for all remaining components.
Authors' conclusions
There was no evidence that prophylactic metoclopramide affected the risk of vomiting, nausea, or the need for rescue medication when provided prior to intravenous opioids in the acute care setting. There was a clinically insignificant difference in nausea severity when comparing prophylactic metoclopramide with placebo. Overall, the evidence was of low certainty. Future research could better delineate the effects of prophylactic antiemetics on specific populations, and new studies are needed to evaluate the use of other prophylactic antiemetic agents, for which there were no data.
Plain language summary
Prophylactic antiemetics for adults receiving intravenous opioids in the acute care setting
Key messages
Metoclopramide did not reduce the risk of vomiting, nausea, or the need for rescue medication when provided prior to intravenous opioids in the emergency department.
In terms of the severity of nausea, metoclopramide did not help patients any more than placebo (sham treatment).
What is opioid‐induced emesis?
Physicians often give patients opioids for pain in emergency departments, but over a third experience the side effects of nausea and vomiting (emesis). Some experts have suggested that taking antiemetics before receiving the opioid (that is, as a prophylactic) could prevent these symptoms from occurring. However, these medications have their own side effects, so it is important to understand whether they are effective and safe before routinely using them.
What did we want to find out?
This review looks at whether taking antiemetics (medications to treat or prevent nausea and vomiting) before receiving an intravenous opioid reduces the risk of experiencing nausea and vomiting as side effects.
What did we do?
We looked for studies involving adults (aged 16 years or older) who received prophylactic antiemetics compared with either placebo or standard care before receiving an intravenous opioid.
What did we find?
We found three studies with a total of 527 patients. All the studies used metoclopramide as the antiemetic. Compared with placebo, metoclopramide did not reduce the risk of vomiting, nausea, or the need for an antiemetic later on. There was also no difference in side effects between those who received antiemetics and those who did not.
What are the limitations of the evidence?
The studies investigated only one medication (metoclopramide) and did not report all the information we were interested in. The intervention probably makes little or no difference in terms of experiencing nausea or vomiting.
How up to date is this evidence?
This evidence is up to date to 17 January 2022.
Summary of findings
Summary of findings 1. Prophylactic dopamine antagonist antiemetic medications versus placebo in adults receiving intravenous opioids.
| Prophylactic dopamine antagonist antiemetic medications versus placebo in adults receiving intravenous opioids | |||||
|
Patient or population: adults receiving intravenous opioids Settings: emergency department Time point: enrolment to emergency department discharge or hospital admission Intervention: dopamine receptor antagonists Comparison: placebo | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with placebo | Risk with dopamine receptor antagonists | ||||
| Vomiting (≥1 episode) | 16 per 1000 | 18 per 1000 (4 to 83) | RR 1.18 (0.26 to 5.32) | 527 (3 RCTs) | ⨁⨁◯◯ Low a,b |
| Nausea (≥1 episode) | 37 per 1000 | 20 per 1000 (6 to 75) | RR 0.55 (0.15 to 2.03) | 336 (2 RCTs) | ⨁⨁◯◯ Low a,b |
| Adverse events | 8 per 1000 | 18 per 1000 (4 to 90) | RR 2.34 (0.47 to 11.61) | 527 (3 RCTs) | ⨁⨁◯◯ Low a,b |
| Number of vomiting episodes | NA | NA | NA | NA | — |
| Nausea severity (postintervention) Scale from: 0 to 10 | The mean nausea severity (postintervention) was 0 | MD 0.49 lower (0.75 lower to 0.23 lower) | — | 191 (1 RCT) | ⨁⨁◯◯ Low a,b |
| Rescue medication | 4 per 1000 | 7 per 1000 (1 to 78) | RR 1.86 (0.17 to 20.16) | 527 (3 RCTs) | ⨁⨁◯◯ Low a,b |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; NA: not available; MD: mean difference | |||||
|
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded once for study limitations, due to unclear risk of bias in multiple areas: allocation (2 studies), blinding (1 study), incomplete outcome data (1 study), and selective reporting (1 study).
bDowngraded once for imprecision because the optimal information size criterion was not met.
Background
Description of the condition
Pain is a common reason for presenting to the emergency department (ED). Studies have found that pain‐related presentations can comprise 45% to 78% of ED visits in the USA (Chang 2014; Johnston 1998; Mura 2017; Tanabe 1999). Managing these conditions often involves an opioid. In the USA, one study found that 53.4 out of every 1000 people in the ED were given an intravenous (IV) opioid for pain (Rui 2019). In Australia, another study reported that 32.7% of people treated for pain received an opioid in the ED (Fry 2011).
Opioids are potent analgesics that function by binding to mu, kappa, or delta opioid receptors in the brain, spinal cord, or digestive tract (Lesniak 2011; Mansour 1994). Because of the wide distribution of receptors, there is a potentially broad array of side effects, including respiratory depression, drowsiness, pruritis, constipation, and nausea and vomiting (Mallick‐Searle 2017). Opioid‐induced nausea and vomiting is a complex process involving the vestibular apparatus, chemoreceptor trigger zone, and the gastrointestinal tract (Coluzzi 2012). Studies have suggested that approximately 40% of people who receive an opioid will experience nausea, and 15% to 25% may experience vomiting (Mallick‐Searle 2017). These symptoms can be highly unpleasant for the people experiencing them. In fact, one postoperative study found that patients believed that avoiding nausea and vomiting was more important than controlling the pain itself (Macario 1999).
Description of the intervention
Antiemetic medications function by binding to receptors in the central nervous system and gastrointestinal system to reduce symptoms of nausea and vomiting (Roila 1995). Medication classes used for preventing nausea and vomiting include serotonin receptor antagonists (e.g. ondansetron), dopamine receptor antagonists (e.g. metoclopramide), neurokinin receptor antagonists (e.g. aprepitant), corticosteroids (e.g. dexamethasone), histamine receptor antagonists (e.g. promethazine), and anticholinergics (e.g. scopolamine) (Gan 2020).
A prior Cochrane Review found that prophylactic antiemetics were effective for preventing postoperative nausea and vomiting (Carlisle 2006). Recent guidelines have discussed this treatment in the postoperative setting (Gan 2020), and it has also been used in the ED. Following administration of IV opioids in the ED, one study found that 23% of people received a prophylactic dopamine receptor antagonist (Yeoh 2009), while another found that 41% received prophylactic serotonin antagonists (Bakhsh 2019).
However, these medication classes also carry a risk of adverse events. Serotonin and neurokinin receptor antagonists may cause headache and constipation (Coluzzi 2012; Diemunsch 2009), whereas dopamine receptor antagonists are associated with extrapyramidal symptoms and sedation (Friedman 2016; Leow 2006; Parlak 2005). Corticosteroids may increase blood glucose (Coluzzi 2012), and histamine receptor antagonists and anticholinergics may result in dry mouth, visual disturbances, and sedation.
How the intervention might work
Central and peripheral mechanisms explain the nausea and vomiting that may occur following administration of opioid medications (Coluzzi 2012). Opioids act centrally and may trigger the release of neurotransmitters (e.g. serotonin, dopamine), which stimulate the chemoreceptor trigger zone. Additionally, a peripheral mechanism involving opioid inhibition of gut motility may further promote chemoreceptor activity. Antiemetics can counteract these mechanisms by targeting neurotransmitter receptors or promoting gut motility, thereby preventing the nausea and vomiting associated with opioids. The mechanisms of antiemetics by medication class are as follows (Coluzzi 2012; Gan 2020; Weibel 2020).
Serotonin receptor antagonists: blockade of 5‐hydroxytryptamine subtype 3 (5HT3) receptors prevents binding of the neurotransmitter serotonin at the chemoreceptor trigger zone and peripherally in the gut.
Dopamine receptor antagonists: blockade of dopamine subtype 2 (D2) receptors prevents binding of the neurotransmitter dopamine at the chemoreceptor trigger zone.
Neurokinin receptor antagonists: blockade of neurokinin subtype 1 (NK1) receptors prevents binding of the neurotransmitter substance P at the chemoreceptor trigger zone.
Corticosteroids: though not fully understood, corticosteroids are thought to suppress the production of arachidonic acid, resulting in decreased activity at the vomiting center.
Histamine receptor antagonists: blockade of histamine subtype 1 (H1) receptors prevents binding of the tissue hormone histamine at the vomiting center and vestibular apparatus.
Anticholinergics: blockade of muscarinic receptors prevents binding of the neurotransmitter acetylcholine at the vomiting center and gut.
Why it is important to do this review
Up to 40% of people receiving opioids may develop nausea and vomiting, which can be very distressing for them (Mallick‐Searle 2017). Additionally, opioid‐associated emesis may outweigh the potential analgesic benefit in terms of cost‐effectiveness (Rainer 2000). Prophylactic antiemetics have been proposed as an option for prevention, with studies demonstrating their use in up to 41% of cases (Bakhsh 2019; Yeoh 2009). However, it is important to balance this with the risk of adverse events. Several published studies have assessed the efficacy of this intervention. This review evaluated both the efficacy and safety.
Objectives
To assess the effects of prophylactic antiemetics for nausea and vomiting in adults (aged 16 years or older) receiving intravenous opioids in the acute care setting.
Methods
Criteria for considering studies for this review
Types of studies
This review included randomized controlled trials (RCTs) only. RCTs are the best design to minimize bias when evaluating the effects of an intervention. We did not include quasi‐randomized studies or non‐randomized studies due to the risk of bias inherent to such designs.
Types of participants
We included adults (aged 16 years or over) who received an intravenous opioid in the acute care setting. Any intravenous opioids was eligible for inclusion (e.g. morphine, hydromorphone, fentanyl, sufentanil, tramadol). We defined the acute care setting as the ED or an urgent care clinic.
Types of interventions
The intervention consisted of antiemetic medication given prophylactically via any route (e.g. oral, sublingual, intramuscular, intravenous) to prevent opioid‐induced nausea and vomiting. We planned to complete analyses for outcomes by antiemetic medication class.
Serotonin receptor antagonists.
Dopamine receptor antagonists.
Neurokinin receptor antagonists.
Corticosteroids.
Histamine receptor antagonists.
Anticholinergics.
The comparators could consist of placebo or standard care without an antiemetic agent.
Types of outcome measures
Research in opioids for ED pain management recommends assessing outcomes after a sufficiently long time period to ensure adequate assessment of adverse events (Rainer 2000). We assessed outcomes from enrollment to ED discharge or hospital admission, as avoiding nausea and vomiting throughout acute treatment is important to facilitate disposition from the ED. We considered the following outcomes.
Primary outcomes
Vomiting: defined as the number of participants experiencing one or more episodes of vomiting from enrollment to ED discharge or hospital admission after receiving the medication
Nausea: defined as the number of participants experiencing one or more episodes of nausea occurring from enrollment to ED discharge or hospital admission after receiving the medication
Adverse events: defined as the number of participants experiencing at least one adverse event, as defined by included studies, from enrollment to ED discharge or hospital admission after receiving the medication. Adverse events were categorized as extrapyramidal symptoms, headache, sedation/drowsiness, vertigo/dizziness, and other adverse events. We planned to present these both as total events and by category.
Secondary outcomes
Number of vomiting episodes from enrollment to ED discharge or hospital admission after receiving the medication
Nausea severity: defined using a numeric rating scale or a previously validated visual analog scale (Meek 2009), occurring from enrollment to ED discharge or hospital admission after receiving the medication. If studies reported a visual analog scale or numeric rating scale that used either more than or fewer than 10 points, we planned to convert scores proportionally to a 10‐point numeric rating scale.
Number of participants requiring antiemetic rescue medication from enrollment to ED discharge or hospital admission after receiving the medication
Search methods for identification of studies
Electronic searches
The Cochrane Pain, Palliative, and Supportive Care Review Group's Information Specialist searched the following electronic databases for RCTs. There were no restrictions on language or year of publication.
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (Issue 12 of 12, 2021).
MEDLINE Ovid (1946 to 14 January 2022).
Embase Ovid (1974 to 14 January 2022).
Google Scholar ‐ scholar.google.com (17 January 2022, initial 220 articles) (Bramer 2017).
The search strategies are included in Appendix 1.
Searching other resources
We also searched www.clinicaltrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch) for ongoing trials. In addition, we handsearched reference lists of reviews and retrieved articles for additional studies, and we performed citation searches on key articles. We contacted experts in the field for unpublished and ongoing trials, defining experts as the primary or corresponding authors of included studies. We contacted study authors for additional information where necessary.
Data collection and analysis
Selection of studies
Two review authors (MG, JNC) independently determined the eligibility of each study identified by the search. The review authors excluded studies that clearly did not satisfy inclusion criteria and obtained full copies of the remaining studies. Two review authors (MG, JNC) independently read these records to select relevant studies, and in the event of disagreement, a third author was available to adjudicate (GDP). We did not anonymize the studies in any way before assessment. We created a PRISMA flowchart to show the status of identified studies (Moher 2009). We planned to include studies in the review irrespective of whether they reported measured outcome data in a way amenable to meta‐analysis.
Data extraction and management
Two review authors (MG, GDP) independently extracted data using a standard, piloted form and checked for agreement before entry into Review Manager (Review Manager 2020). In the event of disagreement, a third author was available to adjudicate (JNC). We collated multiple reports of the same study when present, so that each study rather than each report was the unit of interest in the review. We collected characteristics of the included studies in sufficient detail to populate a 'Characteristics of included studies' table. We extracted the following information.
Study characteristics
Study date
Study design
Study setting
Study country
Study duration
Details of blinding and allocation concealment
Length of follow‐up
Publication type
Study funding source
Study author conflicts of interest
Participants
Total number of participants in each group
Inclusion criteria
Exclusion criteria
Mean or median age
Gender distribution
Existing comorbidities
Reason for presenting to the acute care setting
Initial opioid and dose
Intervention
Number of intervention groups
Type, dose, and route of intervention
Control group (i.e. placebo or standard care)
Concomitant medications
Rescue medications
Outcomes
Occurrence of and number of vomiting episodes in each group
Occurrence of nausea in each group
Nausea severity (assessed via a visual analog scale)
Number of participants receiving antiemetic rescue medication
Adverse events
Analysis
Statistical techniques used
Subgroup analyses
Number and percentage lost to follow‐up
Assessment of risk of bias in included studies
Two authors (MG, GDP) independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), resolving any disagreements through discussion. A third reviewer (JNC) was available to adjudicate if needed. We completed a risk of bias table for each included study using the RoB 1 in RevMan (Review Manager 2020).
We assessed the following biases for each included study.
-
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as being at:
low risk of bias – any truly random process, e.g. random number table; computer random number generator;
unclear risk of bias – insufficient detail about the method of randomization to be able to judge the generation as conferring a low or high risk of bias;
high risk of bias – studies using a non‐random process, e.g. odd or even date of birth; hospital or clinic record number. We excluded studies at high risk of bias for random sequence generation.
-
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of (or during) recruitment, or changed after assignment. We assessed the methods as being at:
low risk of bias – e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes;
unclear risk of bias – insufficient detail about the method of randomization to be able to judge the generation as being at low or high risk of bias;
high risk of bias – studies that did not conceal allocation (e.g. open list). We excluded studies at high risk of bias for allocation concealment.
-
Blinding of participants and personnel (checking for possible performance bias). We assessed the methods used to blind study participants and personnel as to which intervention a participant received. We assessed methods as being at:
low risk of bias – study stated that it was blinded and describes the method used to achieve blinding, such as identical tablets matched in appearance or smell, or a double‐dummy technique;
unclear risk of bias – study stated that it was blinded but did not provide an adequate description of how it was achieved;
high risk of bias – study stated that it was not double‐blinded.
-
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors as to which intervention a participant received. We assessed the methods as being at:
low risk of bias – study had a clear statement that outcome assessors were unaware of treatment allocation, and ideally described how this was achieved;
unclear risk of bias – study stated that outcome assessors were blind to treatment allocation but lacked a clear statement on how it was achieved;
high risk of bias – study stated that outcome assessors were not blinded.
-
Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as being at:
low risk of bias – no missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring is unlikely to introduce bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; missing data have been imputed using 'baseline observation carried forward’ analysis;
unclear risk of bias – insufficient reporting of attrition/exclusions to permit a judgement of low or high risk (e.g. number randomized not stated, no reasons for missing data provided, or the study did not address this outcome);
high risk of bias – reason for missing outcome data is likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; ‘as‐treated’ analysis done with substantial departure from the intervention assigned at randomization compared to the one received; potentially inappropriate application of simple imputation.
-
Selective reporting (checking for reporting bias). We assessed reporting biases due to selective outcome reporting. We judged studies as being at:
low risk of bias – the study protocol was available, and all the study’s prespecified (primary and secondary) outcomes that were of interest in the review had been reported in the prespecified way; the study protocol was not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified;
unclear risk of bias – insufficient information available to permit a judgement of low or high risk;
high risk of bias – not all the study’s prespecified primary outcomes had been reported; one or more primary outcomes had been reported using measurements, analysis methods or subsets of the data (e.g. subscale) that were not prespecified; one or more reported primary outcomes were not prespecified (unless clear justification for their reporting was provided, such as an unexpected adverse effect); one or more outcomes of interest in the review had been reported incompletely so that they could not be entered in a meta‐analysis; the study report failed to include results for a key outcome that would be expected to have been reported for such a study.
Measures of treatment effect
We used risk ratios (RRs) to measure treatment effects for dichotomous data (e.g. presence of vomiting, presence of nausea, number of participants requiring rescue medication, adverse events). We planned to calculate treatment effects for continuous data (e.g. number of vomiting episodes, nausea severity) using the mean difference (MD) or the standardized mean difference (SMD) if studies used different scales to measure the same outcome. We reported all treatment effects with 95% confidence intervals (CIs) and used forest plots to present the data. We reported the number needed to treat for an additional beneficial outcome (NNTB) or for an additional harmful outcome (NNTH).
Unit of analysis issues
We suspected that finding cluster‐randomized or cross‐over trials was unlikely based on historical findings from a similar review on this topic (Simpson 2011), but we planned to include them if the other inclusion criteria were met. We planned to analyze cluster‐randomized and cross‐over randomized trials using generic inverse‐variance methods (Higgins 2020).
We planned to seek direct estimates of the effect from an analysis accounting for the cluster design for cluster‐randomized trials. We planned to use the approximately correct analysis approach presented in the Cochrane Handbook where the analysis in a cluster‐randomized trial did not account for the cluster design (Higgins 2020).
We considered that the intervention was likely to have carry‐over effects for RCTs with a cross‐over design. We therefore planned to use data only from the first period and analyze the data as a parallel‐group trial, as outlined in Higgins 2020. We planned to pool data for antiemetic arms (serotonin antagonists or dopamine antagonists) into a single treatment group where cross‐over RCTs included more than two groups. We planned to consult with a statistician where cross‐over RCTs with dichotomous outcomes would require more complicated methods (Elbourne 2002).
Dealing with missing data
We used intention‐to‐treat (ITT) data to minimize the impact of missing information due to study participant attrition. We contacted investigators to verify missing study characteristics and numerical outcome data. We planned to impute standard deviations from available reported variances using standard errors, confidence intervals, t statistics, or P values for continuous data reported without standard deviations. We planned to impute missing standard deviations using the average from included studies if a study did not report variances. We planned to further assess outcome data including imputed values via sensitivity analysis.
Assessment of heterogeneity
We assessed the Chi2 test and I2 statistic to assess heterogeneity of pooled studies. We used N − 1 degrees of freedom and considered P values of less than 0.10 to indicate statistically significant heterogeneity (i.e. variation in effect estimates beyond chance) for the Chi2 test. We used the I2 statistic to classify heterogeneity as follows (Deeks 2020).
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
We planned to assess funnel plots if there were sufficient studies to support this, considering small study effects and non‐reporting biases as explanations for the asymmetry where present (Higgins 2011). Additional explanations of funnel plot asymmetry might have been inflated effects in smaller studies, true heterogeneity, artefactual causes, and chance (Egger 1997). Lastly, we planned to consider missing outcome data and contact study authors to obtain them. However, because the review included only three studies, a funnel plot was inappropriate.
Data synthesis
We pooled outcomes using a random‐effects model, chosen irrespective of the assessment of statistical heterogeneity, to address any remaining unexplained clinical differences after our investigation of heterogeneity. We planned to account for clinical heterogeneity, as necessary, via subgroup analyses. We planned to pool adverse events for analysis and measure events per participant (i.e. a participant experiencing more than one adverse event would be treated as a single event).
Subgroup analysis and investigation of heterogeneity
We planned to use a subgroup analysis to explore sources of heterogeneity (defined as I2 statistic greater than 59%) only for the primary outcome measure. A priori, we decided a subgroup analysis would take into account treatment setting (ED compared with urgent care clinic), based on the rationale that participants treated in an ED may present with more severe pain conditions and associated nausea. Additionally, we planned to perform subgroup analyses by drug within each medication class to inform clinicians of efficacy and safety. Subgroups would be compared with each other using the difference in effects between subgroups approach presented in the Cochrane Handbook (Deeks 2020). We planned to treat our subgroup analysis as observational comparisons, measuring treatment effects using risk difference, because participants were unlikely to be randomized into the subgroups. However, this was not completed because all studies used the same medication and took place in the ED setting.
Sensitivity analysis
We planned to perform sensitivity analyses to examine the impact of excluding studies that were industry‐funded, unpublished, or at high risk of bias in any category according to the risk of bias assessment. However, no studies met these criteria.
Assessment of the certainty of the evidence
Two review authors (MG, GDP) independently rated the certainty of the body of evidence for the outcomes. We used GRADE software and the guidelines provided in the Cochrane Handbook to rank the certainty of the evidence as follows (GRADEpro GDT 2015; Schünemann 2020).
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
The GRADE system considers study design as a baseline marker of certainty. Randomized controlled trials are considered to produce high‐certainty evidence and can be downgraded for important limitations. Observational trials are considered low‐certainty evidence and can be upgraded for strengths (Schünemann 2013). These limitations and strengths are assessed along five domains (study limitations [risk of bias], unexplained heterogeneity and inconsistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence for each outcome. We decreased the grade rating if we identified the following:
Serious or very serious study limitations.
Serious or very serious inconsistency of results.
Serious or very serious uncertainty about directness.
Serious or very serious imprecision.
Probability of reporting bias.
Summary of findings tables
We included one summary of findings table for dopamine antagonist antiemetics versus placebo. Additional tables were planned for serotonin antagonist antiemetics versus placebo; dopamine antagonist antiemetics versus standard care; and serotonin antagonist antiemetics versus standard care. However, these summary of findings tables were unnecessary because all studies used only dopamine antagonist antiemetics and placebo.
We planned to include key information concerning the certainty of evidence, the magnitude of effect of the interventions examined, and the sum of available data for the following outcomes.
Vomiting: defined as one or more episodes of vomiting from enrollment to ED discharge or hospital admission after receiving the medication.
Nausea: defined as one or more episodes of nausea occurring from enrollment to ED discharge or hospital admission after receiving the medication.
Adverse events: defined by the individual studies (e.g. extrapyramidal symptoms, headache, sedation/drowsiness, vertigo/dizziness, other adverse events), occurring from enrollment to ED discharge or hospital admission after receiving the medication.
Number of vomiting episodes from enrollment to ED discharge or hospital admission after receiving the medication.
Nausea severity: defined using the previously validated visual analog scale (Meek 2009), or a numeric rating scale, occurring from enrollment to ED discharge or hospital admission after receiving the medication.
Number of participants requiring antiemetic rescue medication from enrollment to ED discharge or hospital admission after receiving the medication.
Summary of findings and assessment of the certainty of the evidence
Two review authors (MG, GDP) independently rated the certainty of the body of evidence for the outcomes. We used GRADE software and the guidelines provided in the Cochrane Handbook to rank the certainty of the evidence (GRADEpro GDT 2015; Schünemann 2020) as follows.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
The GRADE system considers study design as a baseline marker of certainty. Randomized controlled trials are considered to be high‐certainty evidence and can be downgraded for important limitations. Observational trials are considered low‐certainty evidence and can be upgraded for strengths (Schünemann 2013). These limitations and strengths are assessed along five domains (study limitations [risk of bias], unexplained heterogeneity and inconsistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence for each outcome. We decreased the grade rating if we identified the following.
Serious or very serious study limitations.
Serious or very serious inconsistency of results.
Serious or very serious uncertainty about directness.
Serious or very serious imprecision.
Probability of reporting bias.
Results
Description of studies
Results of the search
Our search identified 3785 total records. After removing duplicates, we reviewed 3165 unique records. Of those, 3157 were not relevant to the review, so we excluded them during the initial screening stage. We reviewed eight full‐text articles and excluded five records for specific reasons (Characteristics of excluded studies). We selected three studies for inclusion in this review (Characteristics of included studies and Figure 1). No ongoing trials were identified.
1.
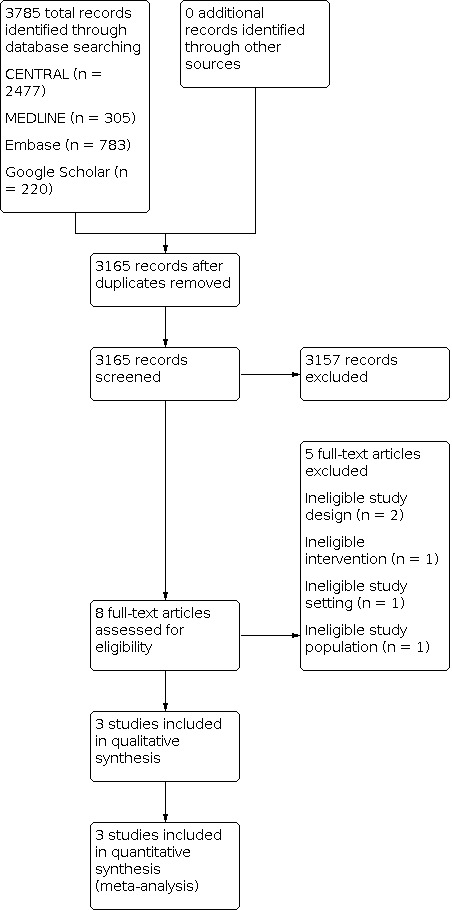
PRISMA Study flow diagram.
Included studies
We included three studies (Characteristics of included studies), involving 527 participants with a mean age of 42 years. There were 187 women and 340 men. One study took place in Malaysia (Choo 2019), one in New Zealand (Lambie 1999), and one in Australia (Talbot‐Stern 2000). All three studies were conducted in the ED. Reported exclusion criteria were: history of vomiting since the time of injury (Choo 2019; Lambie 1999; Talbot‐Stern 2000); receiving an opioid or antiemetic prior to arrival (Choo 2019; Lambie 1999; Talbot‐Stern 2000); known allergy to metoclopramide (Choo 2019; Lambie 1999); concurrently taking a medication with antiemetic action (Choo 2019; Lambie 1999); pregnancy (Choo 2019; Lambie 1999); lactation (Choo 2019); injuries to the brain, chest, abdomen, or pelvis (Choo 2019); alteration in level of consciousness (Choo 2019); hemodynamic instability (Choo 2019); known case of vertiginous disorder (Choo 2019); currently undergoing chemotherapy or radiotherapy (Choo 2019); gastrointestinal conditions that may predispose to vomiting such as a small bowel obstruction (Talbot‐Stern 2000); family or personal history of Parkinsonism or dystonia (Talbot‐Stern 2000); current use of psychotropic agents (Talbot‐Stern 2000); prisoners (Talbot‐Stern 2000); wards of the state (Talbot‐Stern 2000); or members of the armed service (Talbot‐Stern 2000). One study used intravenous morphine as the opioid (Lambie 1999), one used a combination of intravenous morphine and pethidine (Talbot‐Stern 2000), and one used tramadol as the opioid (Choo 2019). All three studies used metoclopramide 10 mg given intravenously as the intervention and 0.9% normal saline as the placebo. Two studies used a 60‐min follow‐up period (Choo 2019; Talbot‐Stern 2000), while one study followed up participants for 120 min (Lambie 1999).
Excluded studies
We excluded five studies (Characteristics of excluded studies): two because they were not RCTs (Culver 2017; Okamoto 2007), one because it did not include adult patients (Bradshaw 2006), one because it did not take place in an acute care setting (Conner 1977), and one because it did not assess the role of antiemetics (Hersh 2016).
Risk of bias in included studies
The results of our risk of bias assessment are summarized in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We classified one study as being at low risk of bias for random sequence generation, as authors described the use of a computerized random number generator (Choo 2019). Two studies were at unclear risk of bias for random sequence generation because they did not adequately describe how randomization was performed (Lambie 1999; Talbot‐Stern 2000).
Allocation concealment
All three studies were at low risk for allocation bias because they were coded independently prior to assignment (Choo 2019; Lambie 1999; Talbot‐Stern 2000).
Blinding
Blinding of participants and personnel
We classified all three studies as being at low risk of bias for blinding of participants and personnel, as the syringes for the intervention and control medications were indistinguishable (Choo 2019; Lambie 1999; Talbot‐Stern 2000).
Blinding of outcome assessment
Two studies were at low risk of blinding for outcome assessment because they stated that the treatment allocations were blinded until after data were collected (Choo 2019; Talbot‐Stern 2000). Lambie 1999 did not explicitly state if outcome assessors were blinded, so we judged it to be at unclear risk of bias.
Incomplete outcome data
Two studies were at low risk of bias for incomplete outcome data because no participants were lost to follow‐up (Choo 2019; Lambie 1999), while one study was at unclear risk of bias because four data sheets were missing (Talbot‐Stern 2000).
Selective reporting
One study was at low risk of bias for selective reporting (Choo 2019), while two studies were at unclear risk of bias because they were not preregistered, and no protocol was available (Lambie 1999; Talbot‐Stern 2000).
Other potential sources of bias
No other potential sources of bias were identified.
Effects of interventions
See: Table 1
Prophylactic dopamine antagonist antiemetics versus placebo
Primary outcomes
Vomiting
Three studies reported the incidence of vomiting from enrollment to ED discharge or hospital admission (Choo 2019; Lambie 1999; Talbot‐Stern 2000). There was no evidence that prophylactic antiemetics affected the risk of vomiting (RR 1.18, 95% CI 0.26 to 5.32; P = 0.83; Table 1; Analysis 1.1; Figure 4). We downgraded the certainty of the evidence to low: once for serious study limitations due to unclear risk of bias in multiple areas (allocation, blinding, incomplete outcome data, and selective reporting) and once for imprecision because the optimal information size criterion was not met.
1.1. Analysis.

Comparison 1: Dopamine receptor antagonists versus placebo, Outcome 1: Vomiting
4.

Nausea
Two studies reported the incidence of nausea from enrollment to ED discharge or hospital admission (Lambie 1999; Talbot‐Stern 2000). There was no evidence that prophylactic antiemetics affected this outcome (RR 0.55, 95% CI 0.15 to 2.03; P = 0.37; Analysis 1.2; Figure 5). We downgraded the certainty of the evidence to low: once for serious study limitations due to unclear risk of bias in multiple areas (allocation, blinding, incomplete outcome data, and selective reporting) and once for imprecision because the optimal information size criterion was not met.
1.2. Analysis.

Comparison 1: Dopamine receptor antagonists versus placebo, Outcome 2: Nausea
5.

Adverse events
Three studies reported the incidence of adverse events from enrollment to ED discharge or hospital admission (Choo 2019; Lambie 1999; Talbot‐Stern 2000). There was no evidence that prophylactic antiemetics affected the risk of adverse events (RR 2.34, 95% CI 0.47 to 11.61; P = 0.30; Analysis 1.3; Figure 6). Further analyses showed no effect on extrapyramidal symptoms (RR 2.81, 95% CI 0.12 to 67.71; P = 0.52; Analysis 1.4), sedation/drowsiness (RR 2.81, 95% CI 0.12 to 67.71; P = 0.52; Analysis 1.5), vertigo/dizziness (RR 4.69, 95% CI 0.23 to 95.66; P = 0.32; Analysis 1.6), or other adverse events (RR 0.47, 95% CI 0.04 to 5.03; P = 0.53; Analysis 1.7). None of the studies reported headaches in either group. We downgraded the certainty of the evidence to low: once for serious study limitations due to unclear risk of bias in multiple areas (allocation, blinding, incomplete outcome data, and selective reporting) and once for imprecision because the optimal information size criterion was not met.
1.3. Analysis.

Comparison 1: Dopamine receptor antagonists versus placebo, Outcome 3: Adverse events
6.

1.4. Analysis.

Comparison 1: Dopamine receptor antagonists versus placebo, Outcome 4: Adverse events (extrapyramidal symptoms)
1.5. Analysis.

Comparison 1: Dopamine receptor antagonists versus placebo, Outcome 5: Adverse events (sedation/drowsiness)
1.6. Analysis.

Comparison 1: Dopamine receptor antagonists versus placebo, Outcome 6: Adverse events (vertigo/dizziness)
1.7. Analysis.

Comparison 1: Dopamine receptor antagonists versus placebo, Outcome 7: Adverse events (other)
Secondary outcomes
Number of vomiting episodes
No studies reported the number of vomiting episodes between groups.
Nausea severity
Two studies reported postintervention nausea severity. Choo 2019 reported this outcome on a scale from 0 to 10 (MD −0.49, 95% CI −0.75 to −0.23; Analysis 1.8). Talbot‐Stern 2000 reported the presence of nausea of mild or moderate severity at 30 min and 60 min, but without clarifying the tool used. In this study, there were four mild cases of nausea in the placebo group, while there was one mild and one moderate case of nausea in the intervention group at 30 min. At 60 min, there were two cases of mild nausea in the placebo group, compared to two cases of mild nausea and one case of moderate nausea in the intervention group. We downgraded the certainty of the evidence to low: once for serious study limitations due to unclear risk of bias in multiple areas (allocation, blinding, incomplete outcome data, and selective reporting) and once for imprecision because the optimal information size criterion was not met.
1.8. Analysis.

Comparison 1: Dopamine receptor antagonists versus placebo, Outcome 8: Nausea severity
Antiemetic rescue medication
Three studies reported the use of antiemetic rescue medication use from enrollment to ED discharge or hospital admission (Choo 2019; Lambie 1999; Talbot‐Stern 2000). There was no evidence that prophylactic antiemetics affected the risk of requiring rescue medication (RR 1.86, 95% CI 0.17 to 20.16; P = 0.61; Analysis 1.9). We downgraded the certainty of the evidence to low: once for serious study limitations) due to unclear risk of bias in multiple areas (allocation, blinding, incomplete outcome data, and selective reporting) and once for imprecision because the optimal information size criterion was not met.
1.9. Analysis.

Comparison 1: Dopamine receptor antagonists versus placebo, Outcome 9: Rescue medication
Other comparisons
We did not perform analyses for other comparisons because all studies used the same medication.
Subgroup analyses
We did not perform subgroup analyses because all studies used the same medication and took place in the ED setting.
Sensitivity analyses
We did not perform sensitivity analyses because none of the studies were industry funded or deemed to be at high risk of bias.
Discussion
Summary of main results
All three trials meeting our inclusion criteria for this review assessed metoclopramide. However, the data demonstrated no meaningful benefit to administering prophylactic metoclopramide prior to intravenous opioids. Compared to placebo, prophylactic metoclopramide did not reduce the risk of vomiting, nausea, or need for rescue antiemetic medication, nor did it increase the risk of adverse events.
Overall completeness and applicability of evidence
Several factors may potentially influence the external validity of this review. First, the overall sample size of included studies was relatively small, resulting in moderately wide confidence intervals, so it is still possible that a treatment effect exists that was not detected due to the limited data available. Additionally, all three studies used metoclopramide, so it is possible that a different antiemetic agent could produce a benefit. Moreover, the type of opioids differed: Lambie 1999 and Talbot‐Stern 2000 used morphine, while Choo 2019 used tramadol. The length of follow‐up also differed, with Choo 2019 and Talbot‐Stern 2000 assessing outcomes at 60 min and Lambie 1999 at 120 min. Results may have differed according to the specific opioid or time period used.
Quality of the evidence
Overall, the certainty of the evidence was low for the outcomes of vomiting, nausea, adverse events, nausea severity, and the need for rescue medication. This rating was primarily influenced by unclear risk of bias in multiple areas (allocation, blinding, incomplete outcome data, and selective reporting) and imprecision because the optimal information size criterion was not met. While unlikely, we could not rule out the risk of selection bias because neither Lambie 1999 nor Talbot‐Stern 2000 adequately described the randomization sequence, nor did they preregister their study or publish a protocol. Similarly, there is a potential risk regarding detection bias, as Lambie 1999 did not adequately describe blinding of the outcome assessors.
Potential biases in the review process
We minimized potential biases in the review process by searching for published studies and ongoing clinical trials across several sources without restrictions on date of publication or language. Two review authors independently screened, identified, extracted data, and completed the risk of bias assessments.
Agreements and disagreements with other studies or reviews
Our review is consistent with one prior systematic review and meta‐analysis comparing prophylactic metoclopramide versus placebo in adult and pediatric ED patients receiving intravenous morphine (Simpson 2011). Those review authors found no evidence of a difference in rates of vomiting. Our study adds to these data by focusing on the adult population, adding one new study (Choo 2019), and assessing additional clinically relevant outcomes.
Authors' conclusions
Implications for practice.
For people receiving opioids
There is low‐certainty evidence that prophylactic metoclopramide does not reduce vomiting, nausea, or the need for rescue or antiemetic medications compared with placebo. Therefore, current data do not support the routine use of prophylactic metoclopramide in patients receiving intravenous opioids. Importantly, the overall rates of vomiting and nausea after intravenous opioids were very low across all three studies, suggesting that a very large sample would be necessary to detect a statistically significant difference. While no differences were identified for adverse events, it is possible a larger sample size could change this result, so it would be important to weigh the risks and benefits of such treatment if future research were to demonstrate a benefit. There is a lack of evidence regarding the harms or benefits of other antiemetic agents.
For clinicians
While antiemetics continue to be used for treating nausea or vomiting after opioid delivery, the current data do not support clinicians routinely prescribing prophylactic metoclopramide to prevent these adverse events in patients being given intravenous opioids. As the data were limited to metoclopramide, it remains unclear whether other antiemetics may be beneficial. Clinicians should be aware of the current data regarding metoclopramide and consider devoting their efforts elsewhere, including toward addressing the underlying etiology of the pain as well as earlier recognition and treatment of nausea or vomiting resulting from intravenous opioids. There is a lack of evidence regarding the harms or benefits of other antiemetic agents.
For policymakers
Based on the findings from this study, prophylactic metoclopramide does not appear to meaningfully prevent nausea or vomiting in people receiving intravenous opioids. Policymakers should be aware of these findings, and future policies for intravenous opioid and nausea or vomiting management should not include recommendations for prophylactic antiemetics. There is a lack of evidence regarding the harms or benefits of other antiemetic agents.
For funders
While current data do not demonstrate evidence of a benefit for prophylactic antiemetics, published studies are currently restricted to metoclopramide. Funders should be aware of these limitations and consider additional research funding to assess the safety and efficacy of other antiemetics and alternate interventions to reduce the risk of nausea or vomiting in people receiving intravenous opioids.
Implications for research.
General implications
The current data show no evidence of a benefit with regard to dopamine antagonist antiemetics compared with placebo. While these results do not currently support the use of prophylactic antiemetics, future research is needed to better delineate whether other agents may be more effective or if specific patient populations may benefit from this treatment.
Design
Future research may be beneficial to better delineate the effect of prophylactic antiemetics for specific populations. For example, while the overall rate of vomiting and nausea was low in the included studies, it is unclear whether patients with a higher likelihood or prior history of opioid‐induced nausea and vomiting could benefit from prophylactic antiemetics.
Additionally, as all three studies assessed a single agent, other agents (e.g. serotonin receptor antagonists, neurokinin receptor antagonists, corticosteroids, histamine receptor antagonists, or anticholinergics) may yet demonstrate a benefit. Future studies should therefore assess the efficacy and safety of these other antiemetic agents in comparison with placebo and other agents.
Moreover, investigators should ensure that they preregister their studies to ensure transparency and adequately describe randomization and blinding.
Measurement (endpoints)
Only two studies reported nausea severity postintervention, and they used different scales (Choo 2019; Talbot‐Stern 2000). Future studies should use consistent and previously validated scales for assessing nausea. Authors should also ensure that studies are powered to identify a clinically meaningful difference in nausea severity.
Finally, most studies were underpowered to detect a difference in adverse events. Future studies should be adequately powered to identify a difference in adverse events in order to inform a determination of the risk and benefit of this intervention. It will also be important for authors to use a standardized tool and adequate follow‐up when assessing adverse events.
What's new
| Date | Event | Description |
|---|---|---|
| 26 January 2021 | Amended | Examples of 'anti‐emetics' amended to 'antiemetics'. |
History
Protocol first published: Issue 1, 2021
Acknowledgements
Cochrane Review Group funding acknowledgement: this project was funded by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
The authors would like to acknowledge the following peer reviewers: Peter Cole, Pierre Diemunsch, Sina Grape, Kyle Kirkham, and Danial Sayyad.
We thank Anne Littlewood, Information Specialist for Cochrane Oral Health Review Group, for peer reviewing the search strategy.
Sign‐off Editor (final editorial decision): Dr Neil O'Connell, PaPaS Co‐ordinating Editor, and Reader at Brunel University London
Managing Editor (provided editorial guidance to authors, edited the article): Anna Erskine (Oxford University Hospitals (OUH) NHS Foundation Trust, Oxford, UK).
Assistant Managing Editor (selected peer reviewers, collated peer‐reviewer comments, conducted editorial checks and supported editorial team): Kerry Harding (Oxford University Hospitals (OUH) NHS Foundation Trust, Oxford, UK)
Contact Editor (editorial guidance): McKenzie Ferguson, Department of Pharmacy Practice, School of Pharmacy, Southern Illinois University Edwardsville, USA Information Specialist (searching support): Joanne Abbott (Oxford University Hospitals (OUH) NHS Foundation Trust, Oxford, UK) Copy‐editing (initial copy‐edit and final proofread): Meggan Harris, Copy Edit Support Group Peer‐reviewers (provided comments and recommended an editorial decision): Pierre Diemunsch, Anesthesia & Perioperative Medicine, University Hospital, Strasbourg, France (clinical review), Sina Grape, Department of Anesthesia, Valais Hospital, Sion, Switzerland (clinical review), Julia Robertson, Griffith University (consumer review).
Appendices
Appendix 1. Search strategies
CENTRAL (Cochrane Library) ‐ 2,477 Results
#1 MeSH descriptor: [Analgesics, Opioid] explode all trees
#2 ((opioid* or opiat* or morphine or buprenorphine or dipipanone or diamorphine or alfentanil or fentanyl or remifentanil or sufentanil or methadone or oxycodone or papaveretum or pentazocine or pethidine or tapentadol or tramadol)):ti,ab,kw (Word variations have been searched)
#3 MeSH descriptor: [Administration, Intravenous] explode all trees
#4 (intravenous*):ti,ab,kw (Word variations have been searched)
#5 #1 or #2
#6 #3 or #4
#7 MeSH descriptor: [Antiemetics] explode all trees
#8 ((antiemetic* or anti‐emetic*) or (prevent* next nausea) or (prevent* next sickness) or (prevent* next vomit*)):ti,ab,kw (Word variations have been searched)
#9 MeSH descriptor: [Serotonin 5‐HT3 Receptor Antagonists] explode all trees
#10 MeSH descriptor: [Dopamine D2 Receptor Antagonists] explode all trees
#11 MeSH descriptor: [Neurokinin‐1 Receptor Antagonists] explode all trees
#12 MeSH descriptor: [Adrenal Cortex Hormones] explode all trees
#13 MeSH descriptor: [Histamine H1 Antagonists] explode all trees
#14 MeSH descriptor: [Cholinergic Antagonists] explode all trees
#15 ((ondansetron or granisetron or dolasetron or palonosetron or tropisetron)):ti,ab,kw (Word variations have been searched)
#16 ((metoclopramide or promethazine or prochlorperazine or droperidol or haloperidol)):ti,ab,kw (Word variations have been searched)
#17 ((aprepitant or rolapitant)):ti,ab,kw (Word variations have been searched)
#18 (dexamethasone):ti,ab,kw (Word variations have been searched)
#19 ((diphenhydramine or dimenhydrinate or promethazine)):ti,ab,kw (Word variations have been searched)
#20 ((scopolamine or hyoscine)):ti,ab,kw (Word variations have been searched)
#21 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20
#22 #5 and #6
#23 #21 and #22
MEDLINE (OVID) ‐ 305 Results
1 exp Analgesics, Opioid/
2 (opioid* or opiat* or morphine or buprenorphine or dipipanone or diamorphine or alfentanil or fentanyl or remifentanil or sufentanil or methadone or oxycodone or papaveretum or pentazocine or pethidine or tapentadol or tramadol).tw.
3 exp Administration, Intravenous/
4 intravenous*.tw.
5 1 or 2
6 3 or 4
7 5 and 6
8 exp Antiemetics/
9 (antiemetic* or anti‐emetic* or (prevent* adj (nausea or vomit* or sickness))).tw.
10 exp serotonin 5‐ht3 receptor antagonists/
11 exp Dopamine D2 Receptor Antagonists/
12 exp Neurokinin‐1 Receptor Antagonists/
13 exp Adrenal Cortex Hormones/
14 exp Histamine H1 Antagonists/
15 exp Cholinergic Antagonists/
16 (ondansetron or granisetron or dolasetron or palonosetron or tropisetron).tw.
17 (metoclopramide or promethazine or prochlorperazine or droperidol or haloperidol).tw.
18 (aprepitant or rolapitant).tw.
19 dexamethasone.tw.
20 (diphenhydramine or dimenhydrinate or promethazine).tw.
21 (scopolamine or hyoscine).tw.
22 or/8‐21
23 (precaution* or prevent* or prophyla*).tw.
24 22 and 23
25 7 and 24
26 randomized controlled trial.pt.
27 controlled clinical trial.pt.
28 randomized.ab.
29 placebo.ab.
30 drug therapy.fs.
31 randomly.ab.
32 trial.ab.
33 26 or 27 or 28 or 29 or 30 or 31 or 32
34 exp animals/ not humans.sh.
35 33 not 34
36 25 and 35
Embase (OVID) ‐ 783 Results
1 exp narcotic analgesic agent/
2 (opioid* or opiat* or morphine or buprenorphine or dipipanone or diamorphine or alfentanil or fentanyl or remifentanil or sufentanil or methadone or oxycodone or papaveretum or pentazocine or pethidine or tapentadol or tramadol).tw.
3 exp Administration, Intravenous/
4 intravenous*.tw.
5 1 or 2
6 3 or 4
7 5 and 6
8 exp antiemetic agent/
9 (antiemetic* or anti‐emetic* or (prevent* adj (nausea or vomit* or sickness))).tw.
10 exp serotonin 3 antagonist/
11 exp dopamine 2 receptor blocking agent/
12 exp neurokinin 1 receptor antagonist/
13 exp corticosteroid/
14 exp histamine H1 receptor antagonist/
15 exp cholinergic receptor blocking agent/
16 (ondansetron or granisetron or dolasetron or palonosetron or tropisetron).tw.
17 (metoclopramide or promethazine or prochlorperazine or droperidol or haloperidol).tw.
18 (aprepitant or rolapitant).tw.
19 dexamethasone.tw.
20 (diphenhydramine or dimenhydrinate or promethazine).tw.
21 (scopolamine or hyoscine).tw.
22 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 17 or 18 or 19 or 20 or 21
23 (precaution* or prevent* or prophyla*).tw.
24 22 and 23
25 7 and 24
26 random$.tw.
27 factorial$.tw.
28 crossover$.tw.
29 cross over$.tw.
30 cross‐over$.tw.
31 placebo$.tw.
32 (doubl$ adj blind$).tw.
33 (singl$ adj blind$).tw.
34 assign$.tw.
35 allocat$.tw.
36 volunteer$.tw.
37 Crossover Procedure/
38 double‐blind procedure.tw.
39 Randomized Controlled Trial/
40 Single Blind Procedure/
41 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40
42 (animal/ or nonhuman/) not human/
43 41 not 42
44 25 and 43
Google Scholar ‐ 220 Results
Advanced Search Mode
With the words: antiemetic intravenous opioid
Without the words: child children
Data and analyses
Comparison 1. Dopamine receptor antagonists versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Vomiting | 3 | 527 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.26, 5.32] |
| 1.2 Nausea | 2 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.15, 2.03] |
| 1.3 Adverse events | 3 | 527 | Risk Ratio (M‐H, Random, 95% CI) | 2.34 [0.47, 11.61] |
| 1.4 Adverse events (extrapyramidal symptoms) | 3 | 527 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.12, 67.71] |
| 1.5 Adverse events (sedation/drowsiness) | 3 | 527 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.12, 67.71] |
| 1.6 Adverse events (vertigo/dizziness) | 3 | 527 | Risk Ratio (M‐H, Random, 95% CI) | 4.69 [0.23, 95.66] |
| 1.7 Adverse events (other) | 3 | 527 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.04, 5.03] |
| 1.8 Nausea severity | 1 | 191 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.75, ‐0.23] |
| 1.9 Rescue medication | 3 | 527 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [0.17, 20.16] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Choo 2019.
| Study characteristics | ||
| Methods | RCT Country: Malaysia |
|
| Participants | Adult patients (age ≥ 18 years) presenting to the ED with traumatic injuries of the extremities requiring tramadol (50 mg, up to 2 doses) for pain relief Baseline demographic data: mean age of 39 years, 24.1% female Study period: 6 months (Exact dates not reported) |
|
| Interventions | Intervention: 96 patients received metoclopramide 10 mg IV Control: 95 patients received 0.9% normal saline |
|
| Outcomes | Primary outcome: mean change in nausea severity using a 100 mm visual analogue scale at 60 min Secondary outcomes
|
|
| Notes | No funding support or author COIs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computerized random number generator was used. |
| Allocation concealment (selection bias) | Low risk | The allocation list was kept by a separate pharmacist. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The intervention and control medications were identical. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Treatment allocations were blinded until after study completion. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All data were reported. |
| Other bias | Low risk | None |
Lambie 1999.
| Study characteristics | ||
| Methods | RCT Country: New Zealand |
|
| Participants | Adult patients (age ≥ 18 years) presenting to the ED with musculoskeletal trauma requiring morphine for pain relief | |
| Interventions | Intervention: 111 patients received metoclopramide 10 mg IV Control: 103 patients received 0.9% normal saline Baseline demographic data: mean age of 47 years, 49.5% female Study period: March 1998 to October 1998 |
|
| Outcomes | Primary outcome: occurrence of vomiting at 120 min Secondary outcomes
|
|
| Notes | Funding support and COIs not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors did not explicitly define how randomization was performed. |
| Allocation concealment (selection bias) | Low risk | Syringes were created by a pharmacist and coded prior to assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators were blinded to the corresponding codes. The intervention and control medications were identical in appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not stated in the study protocol. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | The study was not preregistered, and no protocol was available. |
| Other bias | Low risk | None |
Talbot‐Stern 2000.
| Study characteristics | ||
| Methods | RCT Country: Australia |
|
| Participants | Adult patients (age ≥ 16 years) presenting to the ED with acute pain requiring intravenous morphine and pethidine for pain control | |
| Interventions | Intervention: 63 patients received metoclopramide 10 mg IV Control: 59 patients received 0.9% normal saline Baseline demographic data: mean age of 40 years, 28.7% female Study period: 12 months (Exact dates not reported) |
|
| Outcomes | Incidence of nausea at 30 min and 60 min, incidence of vomiting at 30 min and 60 min, number of episodes of vomiting, and adverse events | |
| Notes | Funding support and COIs not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors did not explicitly define how randomization was performed. |
| Allocation concealment (selection bias) | Low risk | Syringes were prepared by a pharmacist and coded prior to assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The intervention and control medications were identical in appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Treatment allocations were blinded until after study completion. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Four data sheets were missing. |
| Selective reporting (reporting bias) | Unclear risk | The study was not preregistered and no protocol was available |
| Other bias | Low risk | None |
COI, conflict of interest; ED, emergency department; IV, intravenous; RCT: randomized controlled trial.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bradshaw 2006 | Wrong population (not adult patients) |
| Conner 1977 | Wrong setting (not in the acute care setting) |
| Culver 2017 | Wrong study design (not an RCT) |
| Hersh 2016 | Wrong intervention (not an antiemetic) |
| Okamoto 2007 | Wrong study design (not an RCT) |
RCT, randomized control trial.
Differences between protocol and review
We changed the participant definition of adult from aged 18 years and over to aged 16 years and over. This was because the definition of adult varies across countries and age ≥ 16 years would be inclusive of the age ≥ 18 years threshold. We do not believe there is a meaningful difference in physiology between people aged 18 years versus 16‐17 years.
Contributions of authors
MG: idea synthesis, literature review, protocol design, protocol drafting, and review.
GDP: idea synthesis, literature review, protocol design, protocol drafting, and review.
JNC: idea synthesis, literature review, protocol design, protocol drafting, and review.
MG will be responsible for future updates.
Sources of support
Internal sources
No sources of support provided
External sources
-
National Institute for Health Research (NIHR), UK
Cochrane Infrastructure funding to the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS)
Declarations of interest
MG: none known. MG is an emergency medicine physician who manages pain in the acute care setting.
JNC: none known. JNC is an emergency medicine physician who manages pain in the acute care setting.
GDP: none known. GDP is a clinical pharmacist who works in the Emergency Department.
New
References
References to studies included in this review
Choo 2019 {published data only}
- Choo KH, Manikam RA, Yoong KPY, Kandasamy VA.Prophylactic metoclopramide use in trauma patients given tramadol: a randomised, double-blinded, placebo-controlled trial [ ]. Hong Kong Journal of Emergency Medicine 2019;26(2):98-105. [Google Scholar]
Lambie 1999 {published data only}
- Lambie B, Chambers J, Herbison P.The role of prophylactic anti‐emetic therapy in emergency department patients receiving intravenous morphine for musculoskeletal trauma. Emergency Medicine Australasia 1999;11(4):240-3. [Google Scholar]
Talbot‐Stern 2000 {published data only}
- Talbot-Stern J, Paoloni R.Prophylactic metoclopramide is unnecessary with intravenous analgesia in the ED. American Journal of Emergency Medicine 2000;18(6):653-7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bradshaw 2006 {published data only}
- Bradshaw M, Sen A.Use of a prophylactic antiemetic with morphine in acute pain: randomised controlled trial. Emergency Medicine Journal 2006;23(3):210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Conner 1977 {published data only}
- Conner JT, Bellville JW, Wender R, Wapner S, Dorey FJ, Katz RL.Morphine and promethazine as intravenous premedicants. Anesthesia & Analgesia 1977;56(6):801-7. [DOI] [PubMed] [Google Scholar]
Culver 2017 {published data only}
- Culver MA, Richards EC, Jarrell DH, Edwards CJ.Use of prophylactic ondansetron with intravenous opioids in emergency department patients: a prospective observational pilot study. Journal of Emergency Medicine 2017;53(5):629-34. [DOI] [PubMed] [Google Scholar]
Hersh 2016 {published data only}
- Hersh E, Marino M, Schachtel B.CL-108 reduces the use of rescue antiemetic medications for opioid-induced nausea and vomiting. Pain Medicine 2018;19(4):423-4. [Google Scholar]
Okamoto 2007 {published data only}
- Okamoto Y, Tsuneto S, Matsuda Y, Inoue T, Tanimukai H, Tazumi K, et al.A retrospective chart review of the antiemetic effectiveness of risperidone in refractory opioid-induced nausea and vomiting in advanced cancer patients. Journal of Pain and Symptom Management 2007;34(2):217-22. [DOI] [PubMed] [Google Scholar]
Additional references
Bakhsh 2019
- Bakhsh HT, Perona SJ.Medical and nursing staff education reduces use of prophylactic ondansetron with opioids in the emergency department. Journal of Emergency Nursing 2019;45(3):273-7. [DOI] [PubMed] [Google Scholar]
Bramer 2017
- Bramer WM, Rethlefsen ML, Kleijnen J, Franco OH.Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Systematic Reviews 2017;6(1):245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carlisle 2006
- Carlisle JB, Stevenson CA.Drugs for preventing postoperative nausea and vomiting. Cochrane Database of Systematic Reviews 2006, Issue 3. Art. No: CD004125. [DOI: 10.1002/14651858.CD004125.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2014
- Chang HY, Daubresse M, Kruszewski SP, Alexander GC.Prevalence and treatment of pain in EDs in the United States, 2000 to 2010. American Journal of Emergency Medicine 2014;32(5):421-31. [DOI] [PubMed] [Google Scholar]
Coluzzi 2012
- Coluzzi F, Rocco A, Mandatori I, Mattia C.Non-analgesic effects of opioids: opioid-induced nausea and vomiting: mechanisms and strategies for their limitation. Current Pharmaceutical Design 2012;18(37):6043-52. [DOI] [PubMed] [Google Scholar]
Deeks 2020
- Deeks JJ, Higgins JP, Altman DG, editor(s).Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020 [ ]. Available from training.cochrane.org/handbook ; ( ): .
Diemunsch 2009
- Diemunsch P, Joshi GP, Brichant JF.Neurokinin-1 receptor antagonists in the prevention of postoperative nausea and vomiting. British Journal of Anaesthesia 2009;103(1):7-13. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C.Bias in meta-analysis detected by a simple, graphical test [ ]. BMJ 1997;13(315):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A .Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [DOI] [PubMed] [Google Scholar]
Friedman 2016
- Friedman BW, Cabral L, Adewunmi V, Solorzano C, Esses D, Bijur PE, et al.Diphenhydramine as adjuvant therapy for acute migraine: an emergency department-based randomized clinical trial. Annals of Emergency Medicine 2016;67(1):32-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fry 2011
- Fry M, Bennetts S, Huckson S.An Australian audit of ED pain management patterns. Journal of Emergency Nursing 2011;37(3):269-74. [DOI] [PubMed] [Google Scholar]
Gan 2020
- Gan TJ, Belani KG, Bergese S, Chung F, Diemunsch P, Habib AS, et al.Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesthesia & Analgesia 2020;131(2):411-48. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.) GRADEpro Guideline Development Tool[ ]. , Version accessed prior to 13 April 2022. Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.), . Available from www.gradepro.org.
Higgins 2011
- Higgins JP, Altman DG, Sterne JA.Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1.
Higgins 2020
- Higgins JP, Eldridge S, Li T.Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Johnston 1998
- Johnston CC, Gagnon AJ, Fullerton L, CommonC, Ladores M, Forlini S.One-week survey of pain intensity on admission to and discharge from the emergency department: a pilot study. Journal of Emergency Medicine 1998;16(3):377-82. [DOI] [PubMed] [Google Scholar]
Leow 2006
- Leow F, Knott J, Abu Hassan F, Taylor D, Udayasiri R.Risk and severity of akathisia following administration of metoclopramide in the emergency department. Journal of Pharmacy Practice and Research 2006;36:194-8. [Google Scholar]
Lesniak 2011
- Lesniak A, Lipkowski AW.Opioid peptides in peripheral pain control. Acta Neurobiologiae Experimentalis Journal 2011;71(1):129-38. [DOI] [PubMed] [Google Scholar]
Macario 1999
- Macario A, Weinger M, Carney S, Kim A.Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesthesia and Analgesia 1999;89(3):652-8. [DOI] [PubMed] [Google Scholar]
Mallick‐Searle 2017
- Mallick-Searle T, Fillman M.The pathophysiology, incidence, impact, and treatment of opioid-induced nausea and vomiting. Journal of the American Association of Nurse Practitioners 2017;29(11):704-10. [DOI] [PubMed] [Google Scholar]
Mansour 1994
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, et al.Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. Journal of Comparative Neurology 1994;350(3):412-38. [DOI] [PubMed] [Google Scholar]
Meek 2009
- Meek R, Kelly AM, Hu XF.Use of the visual analog scale to rate and monitor severity of nausea in the emergency department. Academic Emergency Medicine 2009;16(12):1304-10. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement [ ]. PLOS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mura 2017
- Mura P, Serra E, Marinangeli F, Patti S, Musu M, Piras I, et al.Prospective study on prevalence, intensity, type, and therapy of acute pain in a second-level urban emergency department. Journal of Pain Research 2017;10:2781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parlak 2005
- Parlak I, Atilla R, Cicek M.Rate of metoclopramide infusion affects the severity and incidence of akathisia. Emergency Medicine Journal 2005;22:621-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rainer 2000
- Rainer TH, Jacobs P, Ng YC, Cheung NK, Tam M, Lam PK, et al.Cost effectiveness analysis of intravenous ketorolac and morphine for treating pain after limb injury: double blind randomised controlled trial. BMJ 2000;321(7271):1247-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5)[ ]. , Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020. .
Roila 1995
- Roila F, Del Favero A.Ondansetron clinical pharmacokinetics. Clinical Pharmacokinetics 1995;29(2):95-109. [DOI] [PubMed] [Google Scholar]
Rui 2019
- Rui P, Schappert SM.Opioids prescribed at discharge or given during emergency department visits among adults in the United States, 2016 [ ]. US Department of Health and Human Services, Centers for Disease Control and Prevention; 2019 May. NCHS Data Brief. No 338. Available at: www.cdc.gov/nchs/data/databriefs/db338-h.pdf ; ( ): . [PubMed]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A.Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013 [ ]. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html ; ( ): .
Schünemann 2020
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al.Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Simpson 2011
- Simpson PM, Bendall JC, Middleton PM.Review article: prophylactic metoclopramide for patients receiving intravenous morphine in the emergency setting: a systematic review and meta-analysis of randomized controlled trials [ ]. Emergency Medicine Australasia 2011;23(4):452-7. [DOI] [PubMed] [Google Scholar]
Tanabe 1999
- Tanabe P, Buschmann M.A prospective study of ED pain management practices and the patient's perspective. Journal of Emergency Nursing 1999;25(3):171-7. [DOI] [PubMed] [Google Scholar]
Weibel 2020
- Weibel S, Rücker G, Eberhart LH, Pace NL, Hartl HM, Jordan OL, et al.Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis. Cochrane Database of Systematic Reviews 2020, Issue 10. Art. No: CD012859. [DOI: 10.1002/14651858.CD012859.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yeoh 2009
- Yeoh B, Taylor D, Taylor S.Education initiative improves the evidence-based use of metoclopramide following morphine administration in the emergency department. Emergency Medicine Australasia 2009;21:178-83. [DOI] [PubMed] [Google Scholar]


