Abstract
Design
Realist synthesis.
Study background
Large-scale hospital improvement initiatives can standardise healthcare across multiple sites but results are contingent on the implementation strategies that complement them. The benefits of these implemented interventions are rarely able to be replicated in different contexts. Realist studies explore this phenomenon in depth by identifying underlying context–mechanism–outcome interactions.
Objectives
To review implementation strategies used in large-scale hospital initiatives and hypothesise initial programme theories for how they worked across different contexts.
Methods
An iterative, four-step process was applied. Step 1 explored the concepts inherent in large-scale interventions using database searches and snowballing. Step 2 identified strategies used in their implementation. Step 3 identified potential initial programme theories that may explain strategies’ mechanisms. Step 4 focused on one strategy-theory pairing to develop and test context–mechanism–outcome hypotheses. Data was drawn from searches (March–May 2020) of MEDLINE, Embase, PubMed and CINAHL, snowballed from key papers, implementation support websites and the expertise of the research team and experts. Inclusion criteria: reported implementation of a large-scale, multisite hospital intervention. RAMESES reporting standards were followed.
Results
Concepts were identified from 51 of 381 articles. Large-scale hospital interventions were characterised by a top–down approach, external and internal support and use of evidence-based interventions. We found 302 reports of 28 different implementation strategies from 31 reviews (from a total of 585). Formal theories proposed for the implementation strategies included Diffusion of Innovation, and Organisational Readiness Theory. Twenty-three context–mechanism–outcome statements for implementation strategies associated with planning and assessment activities were proposed. Evidence from the published literature supported the hypothesised programme theories and were consistent with Organisational Readiness Theory’s tenets.
Conclusion
This paper adds to the literature exploring why large-scale hospital interventions are not always successfully implemented and suggests 24 causative mechanisms and contextual factors that may drive outcomes in the planning and assessment stage.
Keywords: quality in health care, change management, health policy
Strengths and limitations of this study.
An iterative process was used to search, extract data, validate and analyse results using evidence and expertise from researchers and partners.
RAMESES Reporting Standards were used to ensure rigour of each staged step.
In spite of a systematic and thorough search for literature, only 51 papers were found; while large-scale hospital interventions abound, implementation activities and outcomes are not commonly reported.
The wealth of data constrained our study to consider only a single formal theory, and a subset of implementation strategies.
Introduction
The implementation of large-scale, multisite, hospital-based improvement initiatives, developed from high quality evidence have the potential to standardise practice, improve safety, continuity and quality of care for patients, reduce unnecessary, unwarranted treatments and provide better value for money.1 Large-scale hospital interventions, as discussed here, are projects that are typically intended to be implemented across multiple hospitals (eg, all public hospitals in a region). They are usually ‘top down’ in nature, in contrast to local, clinician-initiated ‘grass-roots’ projects. The mandate to implement these initiatives is typically from the hospitals’ funding or governing bodies (eg, State Health Departments, or local health networks), or high-level clinical agencies (eg, a national Quality and Safety Commission). Such interventions may be supported by additional staff and resources and align with other high-level health system priorities. The focus of these initiatives is improvement of care and did not include mandated, enforceable health orders.
The QUARISMA intervention in Quebec, Canada, for example, was implemented in 32 hospitals.2 The intervention was based on best practice guidelines derived from recommendations of the Society of Obstetricians and Gynaecologists. The hospitals that implemented it, successfully and safely reduced the rates of clinically unwarranted caesarean sections in low risk mothers.2 Another example of a large-scale hospital intervention is the WHO’s surgical safety check-list3 which was successfully adopted in six high performing hospitals in The Netherlands. This significantly reduced surgical complications and mortality.4
Large-scale interventions are expensive and time consuming to implement.5 Their success is contingent on the implementation programmes that accompany them6; that is, the suite of individual implementation strategies designed to prepare the hospitals for change, and equip the focal stakeholders to adopt new practices and adapt or discard old ones. Recent systematic reviews have identified a range of strategies linked to successful implementation programmes, such as conducting a needs assessment, recruitment of champions or opinion leaders, use of audit and feedback, engaging organisational leaders and developing implementation teams (eg,7 8). For large-scale interventions, these implementation programmes are often required to fit a range of hospitals of different size, geographical and socio-demographic contexts, and health consumer needs.
In recent years, implementation strategies have been compiled, described and categorised9 but research has failed to explain why strategies that work as intended in one context (eg,10) may be a failure in another (eg,11). Results suggest that those designing implementation strategies have failed to take into account local contextual features,12 and that contextual features are poorly conceptualised and defined in reporting.13 Moreover, the underlying mechanism of action, working within that context, is only rarely defined, implying that the way strategies work is poorly understood. A programme theory that lies beneath the implementation programme and that articulates how the strategies are thought to work is often not explicitly stated. Davies and colleagues showed in their review of 235 guideline dissemination and improvement projects in healthcare, only 23% used theory of any kind to inform the development of the implementation strategies.14 This, they argue, can result in a poor choice of implementation strategy for the context (eg, settling for a ‘default strategy’ such as an education session15) and corresponding poor results.
Realist approaches take a deep dive into why programmes work as intended some of the time but not all of the time.16 A realist approach asserts that all programmes have an underlying programme theory that explains how the strategies bring about intended or unintended results. This holds the promise of unpicking the link between the context and outcomes. A realist synthesis is the ideal approach to understand implementation programmes for large-scale hospital interventions, as it explores the links between strategies, mechanisms of action, contexts, the responses of clinicians and outcomes. Terms used in this synthesis referring to types of theories are defined in box 1.
Box 1. Types of theories referred to in this paper.
Formal theories: here, this refers to general implementation science theories that have been used to explain how implementation strategies work broadly and for which there is some empirical support. Also called mid-range theories.42
Programme theory: a theory that explains how and why particular types of interventions work to generate the outcome/s of interest.16
Initial programme theory: a programme theory that is hypothesised, tested and refined as a result of the realist synthesis to explain how the focal type of intervention generates the outcome/s of interest.
Potential initial programme theories: a suite of programme theories being considered as an initial programme theory.
A realist synthesis is a generative process, first understanding the nature of the implementation programme and then proposing potential initial programme theories around the way a programme works. These initial programme theories, configured as contexts (circumstances under which the programme works), mechanisms (generative causes of how programmes elicit results) and outcomes (the results of the programme), are then tested using published literature.16 The context–mechanism–outcome configurations (CMOs) that are found through analysis of the literature can be explored and used to formulate and refine initial programme theories which explain how and under what circumstances programmes achieve different outcomes. Consequently, realist research does not apply value judgements on programme outcomes such as ‘successful’ or ‘unsuccessful’. Instead, it acknowledges that programmes produce intended and unintended outcomes.17
The aim of this realist synthesis was to synthesise evidence and generate initial programme theories that explain how implementation strategies work in large-scale hospital interventions; in other words, to gather evidence on what works as intended for whom, in what circumstances and why. This realist synthesis is divided into two parts. First, we scope the literature seeking to understand the concepts and features of implementation programmes for large-scale hospital interventions to understand the sorts of formal theories that may be relevant here. Second, we focus on a single group of implementation strategies and generate initial programme theories18 and CMO configurations to test against the literature.
Both parts of the synthesis are part of a larger project19 examining seven Leading Better Value Care projects implemented in metropolitan, remote and regional-based hospitals (n=100) across New South Wales (NSW), Australia, between 2016 and 2018.19 These projects are based on a value-based care paradigm and address unwarranted clinical variation, and preventable hospitalisations across seven high impact conditions.20 Early results from this project showed that implementation strategies accompanying the projects were variably successful across sites at eliciting buy-in and adoption of the interventions. This current study is informing a realist evaluation of the implementation strategies used to build a nuanced model to support future large-scale hospital implementations; specifically, by defining relevant concepts and proposing initial programme theories.
Methods
We followed the Reporting Standards for realist syntheses recommended by the RAMESES group.17 We used a combination of academic database and grey literature searches, data extraction and fortnightly research team discussions to collate evidence for the synthesis. Throughout the work, research team discussions around data extraction and interpretation were informed by ongoing discussions with partners at the NSW Ministry of Health, Agency for Clinical Innovation and Bureau of Health Information who were experienced in design and implementation of large-scale hospital initiatives, and colleagues from Macquarie University’s Centre for the Health Economy. All searches were conducted between March and August 2020. Table 1 shows the four iterative steps of our method.
Table 1.
The four iterative steps used to search, find, extract and synthesise evidence to generate initial programme theories that explain how implementation strategies work in large-scale hospital interventions
| Step | Purpose | Research questions | Activities |
1
2 |
To conceptualise implementation of large-scale hospital interventions. | What are the key concepts and features of large-scale hospital initiatives and their implementation? What mechanisms might these suggest are key to driving the programme? |
Build an initial list of concepts and associated features based on research team’s research and clinical experience. Add to the list through a search of published literature on implementation of large-scale hospital interventions. Consider antecedents and outcomes of each feature to identify putative relevant mechanisms. |
| To scope suites of implementation strategies used with large-scale hospital interventions. | What implementation strategies are used for large-scale hospital initiatives? How do they fit with Step 1? What do they tell us about possible mechanisms, contexts and the underlying programme theories? |
Collation of implementation strategies extracted from Step 1 literature. Search of additional published literature including extracted studies from systematic reviews. Grey literature search: targeted websites and search terms. Strategies aggregated and sorted then mapped to ERIC implementation strategies. |
|
3
4 |
Identify potential initial programme theories. | What formal theories might explain the mechanisms of action for the strategies listed? | Identification of formal theories from the published literature. Consideration of theories in the context of implementation strategies we have listed. Refinement of the initial programme theory-implementation strategy pairing through research team workshops using all data generated from the project. |
| Focus on a promising implementation strategy-theory pairing and development of CMOs. | What context–mechanism–outcome configurations can we develop and test with the literature around implementation strategies linked to Organisational Readiness Theory? | Research team workshop to develop initial CMO statements. Testing and refinement of CMO statements through review of literature from Steps 1–3. Final iterations of CMOs. |
CMO, context–mechanism–outcome configurations; ERIC, Expert Recommendations for Implementing Change.
Step 1: conceptualising large-scale hospital interventions
The first step towards generating initial programme theories in a realist synthesis is to identify the key concepts of the topic of interest. Concepts are tightly linked to programme theories as they help to understand where key mechanisms leading to expected outcomes are likely to occur.18 Here, we identified and defined key concepts associated with the implementation of large-scale hospital initiatives by exploring the focal stakeholder cohort, arena of action, social processes, intended outcomes and the nature of support for the programme.
This step drew data from three sources: the research team’s knowledge, expert consultation and a published literature search across three iterative stages. The research team (JCL, MNS, EFA, CP, HMN) was made up of four experienced health services researchers, two with clinical backgrounds, one sociology and the other psychology, and a research assistant (HMN). The team was actively mentored, and work validated by an experienced realist researcher (RH).
First we built a list of concepts and associated features characterising implementation programmes for large-scale hospital interventions from key articles (eg,1) our own research and clinical experience. This was done by the research team in two 1-hour meetings. This list was verified and expanded through ongoing discussions with partners involved in large-scale, multisite initiatives at the NSW Ministry of Health (senior policymakers), Agency for Clinical Innovation (senior implementation support strategists) and the Bureau of Health Information (senior data management and analysis professionals). Discussions occurred as one-on-one interactions (via email) or part of project meetings/updates.
Next, we examined the published literature for evidence to support or refute our list and to look for other concepts and features we had not considered. We searched MEDLINE, PubMed, Embase and CINAHL, using the search string: health AND ((((implementation OR driver) OR change) AND large-scale) AND ((innovation OR intervention) OR program)) AND hospital. We set limits on English language but no date limits were set. We snowballed papers from the reference lists and added known key papers not captured by the search, and included individual studies reported in reviews. We assessed whether each of the concepts and features on our list were supported by the literature, noting each as being reported ‘always’, ‘nearly always’, ‘often’, ‘sometimes’, ‘rarely’ or ‘not at all’.
Using an iterative approach, the research team refined our definition of large-scale hospital interventions as we built up the list of associated concepts and features. Finally, antecedents and intended outcomes of the features as a whole and individually were developed and considered to further explore possible mechanisms that may be relevant. Articles that we included involved implementation across multiple hospital sites for interventions aimed at improving patient safety or quality of care. We did not include programmes situated outside the hospital setting (eg, implemented solely in community-based health services), interventions at only one site, locally driven interventions (eg, internally developed, ward-based improvements) or tightly controlled research trials that were not considered ‘real world interventions’ (eg, randomised controlled trials). We did consider pragmatic trials if they met other parts of our definition. A data extraction sheet was used to organise concepts described in the papers found. Papers not reporting implementation strategies or activities were not included.
Step 2: 'scoping suites of implementation strategies
Our next task was to identify and collate all implementation strategies that were reported as part of these types of large-scale interventions. Together with the concepts and features of the initiatives found in Step 1, this list of strategies and any information reported on how they were intended to work, were needed to understand possible contexts and mechanisms leading to outcomes.
We started our search for implementation strategies with the papers found in Step 1. Next we scanned papers found in an existing systematic review of implementation strategies used in hospital avoidance interventions for people with chronic conditions, choosing projects that met our large-scale, multisite criteria.21 We also searched more broadly for systematic reviews looking at implementation strategies targeting other cohorts of patients (Web of Science (all databases selected): ‘implementation’ AND ‘systematic review’). We included protocol papers hoping these might provide a fuller rationale for their choice of strategies. We also included selected grey literature from a targeted search of implementation materials from agencies known to actively support large-scale implementation programmes: UK’s National Health Service, Canada’s Advance Care Planning, NSW Agency for Clinical Innovation, Australian Medical Research Council, Enhanced Recovery After Surgery Society, and WHO. We set up a data extraction matrix, recording reported implementation strategies for each project. We also ran a Google search on ‘implementation guide’ and ‘implementation healthcare guides.’ Implementation strategies were collated and reviewed in each source.
Initially, we used our own descriptors for the strategies, but then aggregated similar strategies and mapped them to the Expert Recommendations for Implementing Change (ERIC)9 taxonomy of 73 implementation strategies. Any strategies that did not map to an ERIC strategy were still included but noted.
Step 3: identifying potential initial program theories
In this next step, the research team held two 2-hour meetings to workshop ideas towards identifying potential initial programme theories.18 Many theories were proposed in the workshop, mainly from our prior research experience and discussed one by one. We also read up on theories proposed by other realist researchers and added them for consideration. This work was being done in parallel with the realist evaluation of the actual state-wide initiative so this also guided our thinking. This resulted in a short list of promising theories.
The process included consideration of all the data generated so far in the project as well as searching published literature around known formal theories; in particular, we examined together the concepts identified in Step 1 and the implementation strategies identified in Step 2. That is, we considered what existing formal theories or types of theories might be relevant to explain particular implementation strategies given the concepts and putative mechanisms we had identified. For example, an implementation strategy of conducting a local needs assessment, fitted with the concept of facilitation through provision of resources and the feature ensuring a formal period of planning. Organisational Readiness Theory was identified as a formal theory that promised to explain how this implementation strategy of conducting a local needs assessment would work across different contexts. These formal theories became the basis for our initial programme theories and were matched with implementation strategies using this process. Theories were retained or excluded on their ability to broadly describe what was happening in one or more implementation strategies, how and why across a range of contexts, and a range of levels (micro, meso and macro).
Step 4: further scoping and focus on a key strategy
As realist syntheses aim to explain how and why a programme works and have the potential to generate vast amounts of data to do this well, it was necessary to carefully scope the results generated and narrow our focus. Following the example of other realist syntheses,18 22–24 we looked for a single set of implementation strategies and their accompanying initial programme theory that (a) was deemed highly important in informing our parallel tranche of work—the realist evaluation of the Leading Better Value Care projects in NSW, Australia—and (b) had not already been researched using realist methodology.
Results
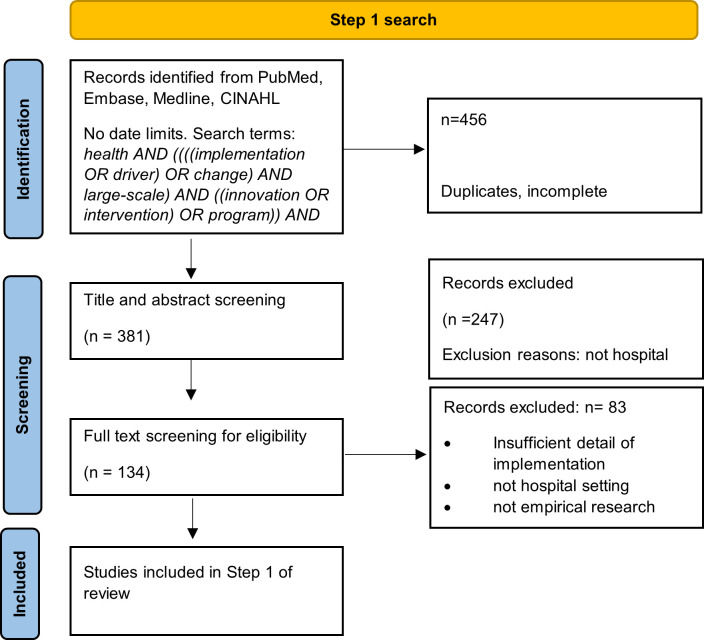
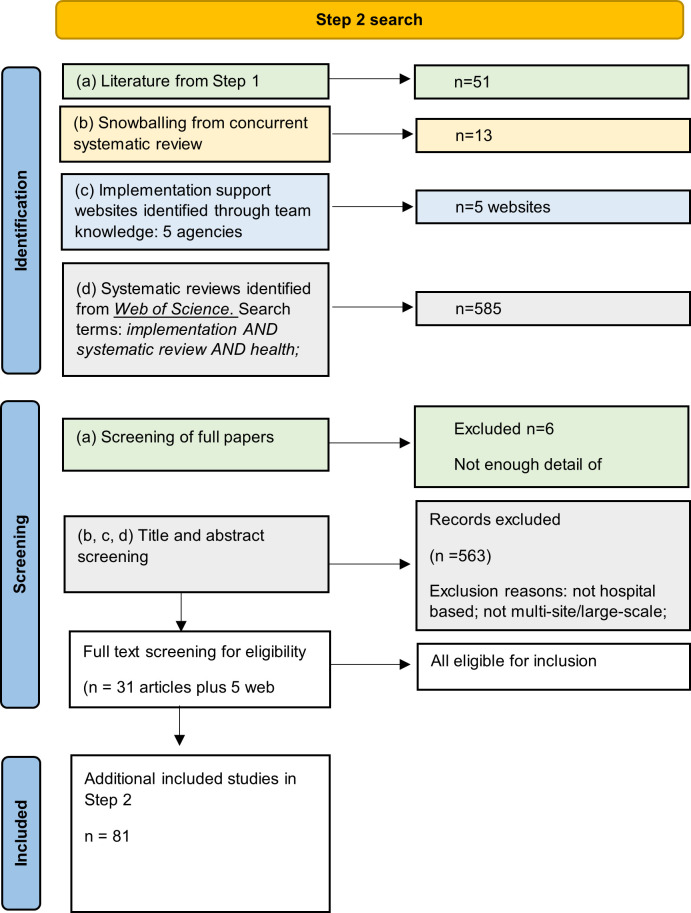
Results of the activities used to synthesise evidence and generate initial programme theories that explain how implementation strategies work in large-scale hospital interventions are outlined below. The process was driven by the fortnightly research team meetings, separate 2-hour workshops, validation by other authors and stakeholders, and iterative refinements. Table 2 summarises the results from the four steps. Figures 1 and 2 show the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)-style flow diagrams for Steps 1 and 2, respectively.
Table 2.
Summary of search strategy, activities and results at each of the four steps
| Step | Purpose | Activity | Interim results | Final result |
| 1 | To conceptualise implementation of large-scale hospital interventions. | Build an initial list of concepts and associated features based on research team’s research and clinical experience, validated by key informants on the wider project. Search databases for implementation of large-scale hospital interventions, screen title and abstract for relevance, data extraction. |
5 concepts and 12 features listed. 381 articles found. 51 relevant articles. Exclusions: not hospital-based, not large-scale, implementation not described. 4 additional features identified from the literature. |
5 concepts and 16 features identified and described. |
| 2 | To scope suites of implementation strategies used with large-scale hospital interventions. | Extracted data from Step 1 literature that report implementation strategies. Search for published literature on implementation and screen for large-scale hospital criteria. Include known literature. Individual studies extracted from reviews. Search of targeted websites and other grey literature. Strategies aggregated and sorted then mapped to ERIC implementation strategies. |
45 articles. 585 reviews found: 31 found to include relevant studies some reporting on multiple implementation strategies. Data extracted from 5 sets of grey literature documents. |
302 reports of 28 different implementation strategies identified and collated. 28 implementation strategies mapped to ERIC taxonomy, 1 did not map. |
| 3 | Identify potential initial programme theory areas. | Identification of potential initial programme theories using all data generated from the project so far plus other realist studies, compilations of programme theories and literature describing individual formal theories. | 3 broad domains of action identified. | 5 initial programme theories mapped to implementation strategies. |
| 4 | Focus on a promising implementation strategy-theory pairing and development of CMOs. | Research team workshop to develop initial CMO statements informed by Organisational Readiness Theory. Testing and refinement of CMO statements through review of literature from Steps 1–3. Final iterations of CMOs. |
All data collected so far. 51 articles+4 articles that focused on organisational readiness assessment. |
24 CMOs were hypothesised and literature used to support or refute them. |
CMO, context–mechanism–outcome configurations; ERIC, Expert Recommendations for Implementing Change.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses-style flowchart for data sources in Step 1.
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses-style flowchart for data sources in Step 2.
Concepts associated with large-scale hospital intervention implementation programmes (step 1)
The research team initially listed 5 concepts associated with 12 features of large-scale hospital interventions, which grew to a final set of 16 features after further scoping of the literature. Over 400 titles and abstracts were accessed via database searching and data were extracted from a subset of 51 full-text articles that met our inclusion criteria. Table 2 summarises results of Step 1 and online supplemental file 1 shows the full data extraction sheets.
bmjopen-2021-058158supp001.pdf (159.1KB, pdf)
The five concepts of large-scale hospital improvement initiative implementation were: (i) External, top–down source, (ii) Evidence-based interventions, (iii) Safety and quality focus, (iv) Facilitation through provision of resources and (v) Harnessing of local resources and encouraging adaptation. Between two and four features of each were identified.
External, top–down source
Features found associated with this concept were that the interventions being implemented were externally developed: either by peak agencies or research institutes (eg, WHO,25 American College of Surgeons26), quality collaboratives (eg, Michigan Surgical Quality Collaborative,27 German Quality Network28) or in one case, mandated, evidence-informed policy (eg, US Veterans’ Affairs (VA) National Disclosure Policy29). Support for implementation for the intervention itself was frequently built into this package by the external source: interventions were often presented as a ‘bundle’ of interventions all aimed at addressing a single issue (eg, surgical site infections,30 treatment of blunt chest injury31). Checklists and implementation guides may also be provided by the external agency that developed the intervention. Contrary to our expectations, the offer of incentives or disincentives for implementation was rarely reported.
Evidence-based interventions
All interventions were identified by the authors as being evidence-based, although the evidence (eg, the randomised control trial on which the intervention was built) itself was rarely cited. Contrary to the expectations of our research team, de-implementation of processes and practices that presumably were no longer ‘best practice’ was rarely reported. This applied even to upgraded information technology systems where legacy systems were allowed to remain alongside the new programmes.32
Safety and quality focus
A clear aim of improving patient outcomes was consistently found, often by making a case for change from baseline data. Implicit in most programmes was the assumption that a positive safety culture, that saw improvement of patient outcomes as core business was present at the site. Also implicit was that there was consensus at each site that the intervention was needed, and that the implementation support provided would be acceptable.
Facilitation through provision of resources
As well as implementation guides and intervention resources, external support was seen in many projects in the form of new equipment, customised forms for documentation, and care pathways. Project officers skilled in the intervention and tasked with data collection or training were funded in some projects, often budgeted as part of an associated research component (eg,33). Partnership agreements with external agencies facilitated implementation by providing access to specialist advice. Funding for the projects was often a mix of external (eg, VA (USA)29), internal (eg, Hornsby Ku-ring-gai District Hospital (Australia)33) and research-based (eg, National Institutes of Health grants (USA)).34 Facilitation was not always a feature. All studies relied on the goodwill of clinicians, and some did not factor in any quarantined time for implementation activities such as performing audits. Interventions developed by clinical collaboratives were often framed as partnerships, including access to practical support and expert advice (in-kind) for the implementation and monitoring of outcomes, some allowing dissemination of learnings from other sites, and benchmarking. Data was often provided to make a case for change, and support for ongoing audit and feedback were common features.
Harnessing local resources and encouraging adaptation
The provision of a lead-in time for each site to assess for readiness and local needs was sometimes reported, and internal support for implementation from senior management was reported in most papers. Design amenable to adaptation to fit different local practices, patient cohorts or workflows, developed by clinically based implementation teams, was also frequently reported. Clinical leadership, mentoring, supervision and in-house education were also key features.
Following this, features were refined by determining their antecedents and intended outcomes, to help with the next step of defining associated implementation strategies, mechanisms and potential initial programme theories. Online supplemental file 2 shows the results of this step.
bmjopen-2021-058158supp002.pdf (101.9KB, pdf)
Collated suites of implementation strategies (step 2)
We found 302 reports of 28 different implementation strategies associated with large-scale hospital interventions from 45 peer-reviewed papers and five sets of grey literature documents (each linked to a single website). Figure 2 shows the PRISMA flow diagram for this step. All of the strategies except one mapped to one or a combination of strategies in the ERIC taxonomy.9 The strategy that did not map was Aligned with organisational/District and Departmental priorities. Some strategies that were similar were combined as descriptions in the articles were not sufficient to determine exact details (eg, Involve executive boards was combined with obtain formal commitments as it was often the executive group which was negotiating on behalf of the site.) The 28 strategies are summarised in table 3 and shown in full in online supplemental file 3. Most frequently reported or recommended strategies were: Promote adaptability/purposely re-examine the implementation (n=34); Involve executive boards/obtain formal commitments (n=24); and Assess for readiness and identify barriers and facilitators (n=24).
Table 3.
List of implementation strategies and their frequency, found in the set of 51 grey and black literature documents
| ERIC taxonomy ERIC,9 |
Implementation strategy | Frequency (n=51 sources) |
| Access new funding | Extra staffing as needed; salary support | 6 |
| Assess for readiness and identify barriers and facilitators | Readiness | 24 |
| Audit and provide feedback | Audit and feedback | 11 |
| Build a coalition; create new clinical teams; create a learning collaborative | Multidisciplinary involvement; clinical leadership | 16 |
| Capture and share local knowledge | Community of practice/knowledge network of clinicians | 11 |
| Change physical structure and equipment (a) | Funding for equipment | 6 |
| Change physical structure and equipment (b) | Tools to improve communication | 4 |
| Conduct cyclical small tests of change | PDSA cycles | 5 |
| Conduct local consensus discussions; facilitator | Local facilitator/project officer | 10 |
| Conduct local needs assessment | Identify resources required | 12 |
| Create a learning collaborative | Engaging stakeholders | 7 |
| Develop a formal implementation blueprint (a) | Implementation guides | 14 |
| Develop a formal implementation blueprint (b) | Intervention toolkit | 10 |
| Develop academic partnerships; use an implementation advisor; use advisory boards and workgroups | Support from external experts/external support | 14 |
| Develop and implement tools for quality monitoring | Monitoring | 6 |
| Develop educational materials; distribute educational materials | Education | 18 |
| Develop resource sharing agreements | Resources shared | 1 |
| Distribute educational materials | Clinical practice guidelines | 8 |
| Facilitation | Problem solving | 2 |
| Identify and prepare champions | Champion | 4 |
| Inform local opinion leaders | Opinion leaders | 7 |
| Involve executive boards; obtain formal commitments | Executive sponsorship/engagement with the state-wide collective | 24 |
| Organise clinician implementation team meetings | Quarantined time for skill acquisition | 4 |
| Promote adaptability; purposely re-examine the implementation | Local adaptation | 34 |
| Provide clinical supervision | Mentoring/supervision/coaching | 16 |
| Recruit, designate, and train for leadership | Clinical leadership | 10 |
| Use data experts | Information technology and communication support for new processes | 6 |
| (No ERIC equivalent) | Align with organisational/district or departmental priorities | 12 |
| Total | 302 |
ERIC, Expert Recommendations for Implementing Change; PDSA, Plan, Do, Study, Act.
bmjopen-2021-058158supp003.pdf (101.7KB, pdf)
Identify potential initial programme theories (step 3)
The research team workshop started by considering both the concepts and features from Step 1 and the strategies from Step 2 to identify high level domains in which our potential initial programme theories and their underlying mechanisms would be expected to work. Four of these domains were identified: social processes and influences; assessment and planning; accessing resources; and partnering outside the organisation. Domains were not seen as mutually exclusive but connected and interdependent. A list of formal theories that addressed these domains was compiled through researcher knowledge and discussion, searching other published realist studies, literature on programme theories and online searches. Five formal theories that explained in a very broad sense, how various strategies might be expected to work were selected through discussion. The theories that were selected were: Organisational Readiness Theory, Social Cognitive Theory, Partnership Synergy Theory, Diffusion of Innovation and the Theory of Planned Behaviour. Table 4 summarises the selected formal theories. Table 5 shows the strategies, concepts, domains and their matched theories.
Table 4.
Summaries of formal theories selected as potential initial programme theories to explain mechanisms across different contexts of the implementation strategies identified
| Theory | Overview (sources) |
| Organisational Readiness Theory | Readiness for change refers to organisational members’ shared resolve to implement a change (change commitment) and shared belief in their collective capability to do so (change efficacy).35 |
| Social Cognitive Theory | Behaviour is influenced by three mechanisms operating in concert: direct personal agency; proxy agency that relies on others to act on one’s behalf to attain the desired goals; and collective agency where the larger group acts.43 |
| Partnership Synergy Theory | Partners who effectively collaborate and share knowledge, skills and perspectives are able to achieve more value than the sum of the individual parts contributed.44 |
| Diffusion of Innovation | Explains how an innovation, new idea or product spreads, mediated by social processes within a population over time. A slow start by innovators and early adopters demonstrates the innovation in practice, increasing confidence. A tipping point is reached after a time when the majority take up the new practice. A small group of conservative and risk aversive ‘laggards’ will be the last to adopt.45 |
| Theory of Planned Behaviour | Three independent constructs determine a person’s intention to perform a specific behaviour: ‘attitude’ refers to how positively or negatively a person perceives the behaviour; ‘social norm’ refers to the perceived pressure from others to perform the behaviour; ‘perceived behaviour control’ relates to how easy or difficult the person thinks it will be to perform the behaviour.46 |
Table 5.
Theory areas associated with implementation strategies
| ERIC strategy | Domain | Associated concepts (bold) and intended outcomes | Associated initial programme theories |
| Develop a formal implementation blueprint | Baseline assessment and planning |
Clear implementation plan or blueprint for change
Clear aim of improving patient outcomes: Clear communication of expectations across sites; tool for planning changes. Provide support for comparison across sites implementing the intervention |
Social Cognitive Theory |
| Conduct cyclical small tests of change | Ongoing assessment | Designed with adaptation to local settings in mind: Incremental changes easier than multifaceted ones. | Social Cognitive Theory |
| Promote adaptability; purposely re-examine the implementation | Ongoing assessment | Designed with adaptation to local settings in mind: Negotiation, needs assessment, ownership of change. | Social Cognitive Theory |
| Build a coalition; create new clinical teams | Partnering | Facilitate access to reputable advice and problem-solving assistance: Inclusion, trust, common goal, breadth of expertise. | Partnership Synergy Theory |
| Develop academic partnerships; use an implementation advisor; use advisory boards and workgroups | Partnering | Facilitate access to reputable advice and problem-solving assistance: Breadth of expertise, social support. | Partnership Synergy Theory |
| Align with other priorities | Social processes | Formal period of planning and needs assessment: Assess the fit with current workflow, personal and organisational goals aligned. |
Organisational Readiness Theory
Social Cognitive Theory |
| Conduct local needs assessment | Baseline assessment and planning |
Formal period of planning and needs assessment: Assessing readiness; understanding implications of change on workflow and practice. Designed with adaptation to local settings in mind |
Organisational Readiness Theory |
| Assess for readiness and identify barriers and facilitators | Baseline assessment and planning | Formal period of planning and needs assessment: Setting up conditions that support change. | Organisational Readiness Theory |
| Change physical structure and equipment | Accessing resources | Provide or facilitate practical support in the form of resources and equipment: Aligning structure with process. | Partnership Synergy Theory |
| Use data experts | Partnering | Provide data support for new or changed IT systems, baseline audits and ongoing monitoring: Partnership with experts to support change. | Partnership Synergy Theory |
| Develop resource sharing agreements | Partnering | Provide or facilitate practical support in the form of resources and equipment: Working with others to effect change. | Partnership Synergy Theory |
| Develop educational materials | Baseline assessment and planning | Formal period of planning and needs assessment: Setting up educational support/conditions that support change. | Organisational Readiness Theory |
| Distribute educational materials | Accessing resources | Provide practical support in the form of education and skill acquisition: Knowledge and skill acquisition, increase in personal and collective competence and confidence. | Social Cognitive Theory |
| Provide clinical supervision | Social processes and influences |
Provide practical support in the form of education and skill acquisition: Social support, role modelling, and practice of new behaviours. Provide social support from executive sponsorship, supervised practice, project officers, opinion leaders or champions |
Social Cognitive Theory |
| Access new funding | Accessing resources | Provide practical support in the form of resources and equipment: Setting up conditions that support change. | Partnership Synergy Theory |
| Create a learning collaborative | Social processes | Provide social support from executive sponsorship, supervised practice, project officers, opinion leaders or champions: Social influences supporting change and learning. |
Diffusion of innovation
Organisational Readiness Theory |
| Facilitation | Social processes | Provide social support from executive sponsorship, supervised practice, project officers, opinion leaders or champions: Breadth of expertise, social support. | Diffusion of innovation |
| Identify and prepare champions; inform local opinion leaders | Social processes | Provide social support from executive sponsorship, supervised practice, project officers, opinion leaders or champions: Social influence supporting change. |
Diffusion of innovation
Organisational Readiness Theory |
| Involve executive boards; obtain formal commitments | Social processes | Provide social support from executive sponsorship, supervised practice, project officers, opinion leaders or champions: Trust, social support, legitimacy, accountability. |
Social Cognitive Theory
Diffusion of Innovation Organisational Readiness Theory |
| Recruit, designate, and train for leadership | Social processes |
Provide social support from executive sponsorship, supervised practice, project officers, opinion leaders or champions: Social influence supporting change. Provide or facilitate practical support in the form of education and skill acquisition: |
Social Cognitive Theory
Diffusion of innovation |
| Organise clinician implementation team meetings | Social processes | Provide social support from executive sponsorship, supervised practice, project officers, opinion leaders or champions: Social influence supporting change, setting common goals and expectations. |
Organisational Readiness Theory
Theory of Planned Behaviour |
| Conduct local consensus discussions | Social processes |
Provide social support from executive sponsorship, supervised practice, project officers, opinion leaders or champions: Social influence supporting the setting of clear objectives, building local trust, planning. Designed with adaptation to local settings in mind |
Organisational Readiness Theory
Partnership Synergy Theory Theory of Planned Behaviour |
| Audit and provide feedback | Baseline assessment and planning Ongoing assessment |
Formal period of planning and needs assessment: Setting up tension for change. Provide support for comparison across sites implementing the intervention: Standardised collection of data sets up a tension for change, diagnoses areas for individual sites to work on, and tracks progress locally and across sites. |
Organisational Readiness Theory |
| Capture and share local knowledge | Social processes | Support for comparison across sites implementing the intervention: Increase the breadth of expertise, social support. | Social Cognitive Theory |
| Develop and implement tools for quality monitoring | Baseline assessment and planning Ongoing assessment |
Support for implementation built into intervention: Setting up conditions that foster change and decrease participant effort. Provide support for comparison across sites implementing the intervention |
Organisational Readiness Theory |
ERIC, Expert Recommendations for Implementing Change; IT, information technology.
CMO statements from the organisational readiness theory (step 4)
Weiner defines organisational readiness as a multilevel and multifaceted construct referring to organisational members’ shared commitment to change—encompassing both willingness and capacity.35 This readiness for change is crucial in producing collective engagement; that is achieving buy in and commitment from those at the front lines enacting the change. This engagement results in valuable implementation outcomes: a collective commitment to initiate change, greater effort to make the change successful, greater perseverance when barriers are encountered and an increase in pro-social collaborative behaviours that promote the change.35 Holt, Amenakis and colleagues,36 state the most potent mechanisms were shared perceptions and beliefs among stakeholders in the organisation that (a) they are capable of implementing the proposed change (ie, change-specific efficacy), (b) the proposed change is appropriate for the organisation (ie, appropriateness), (c) leaders are committed to the proposed change (ie, management support) and (d) the proposed change is beneficial to organisational members (ie, personal valence). Perceptions about resources are considered the active means to achieve readiness rather than the resources themselves.35
In an iterative process undertaken by the research team, CMOs were configured, to understand what circumstances (context) needed to be present in an implementation strategy to trigger an identified mechanism leading to an outcome. Since many of the strategies overlapped in their mechanisms and outcomes, we considered them both together and separately. We limited our enquiry to how the mechanisms worked on the implementers within an organisation; that is, the people delivering the intervention directly to patients, rather than the designers or facilitators of the intervention. The outcomes associated with the Theory of Organisational Readiness were all around engagement, buy-in and commitment to the change.
At the same time as the CMO statements were being configured, articles that reported enough detail on these strategies were reviewed for evidence looking for specific contextual factors (external, organisational or individual13) and mechanisms. A further search specifically for implementation projects across multiple sites that reported using organisational readiness theory was also performed, yielding another four papers. The final column of table 6 indicates the articles that give evidence to support or not support the CMO configurations.
Table 6.
Context–mechanism–outcome configurations for implementation strategies aligning with Organisational Readiness Theory. The broad context is for individual and collective implementers of large-scale hospital interventions
| Implementation strategy (ERIC wording) | Context | Mechanism | Outcome | Component of Organisational Readiness Theory | Evidence from the literature on large-scale hospital projects |
| Baseline audit results shared with implementers (Audit and provide feedback) |
When implementers see their baseline audit results and perceive that current practice is not optimal | …a tension for change is developed leading to | … members being more likely to engage in the project | Appropriateness Personal valence |
Support10 47 |
| Clear evidence provided on effectiveness of intervention (Audit and provide feedback) |
When implementers see clear evidence that the intervention is effective and will improve patient care | … implementers value the change | … members are more likely to engage in the project | Appropriateness Personal valence |
Support48 |
| When implementers do not see clear evidence of the effectiveness of the intervention / do not see the link with improved outcomes for patients | … implementers do not value the change | … members are less likely to engage in the project | Appropriateness Personal valence |
Limited support39 40 | |
| Sharing the positive experience of early adopters of the intervention (Create a learning collaborative) |
When implementers are told of the success of early adopters at other sites | …a tension for change is developed and perceptions of feasibility at their own site will improve leading to | … members being more likely to engage in the project | Appropriateness Personal valence |
Support49 |
| A lead-in period is provided when local needs are assessed (Conduct local needs assessment) |
When local needs of implementers are assessed before any proposed change | … confidence in capability rises, resulting in greater levels of commitment and collaboration | … resulting in more effective implementation | Appropriateness Change-specific efficacy |
Support 10 31 38 50–52 |
| When local needs are not accurately assessed (eg, time needed for new practice underestimated) | … confidence in capability falls, resulting in poorer levels of commitment and collaboration | … resulting in poor adoption and outcomes | Not supported37
Support38 |
||
| Executive and management are engaged and support the intervention (Involve executive boards; obtain formal commitments) |
Executive /management support that is visible to the implementers | … increases perceptions of feasibility and organisational capacity | … resulting in increased engagement | Management support | Support10 52–55 |
| Commitment to support the change from executive level is communicated to implementers | … increases perceptions of feasibility and organisational capacity | … resulting in increased engagement | Support28 48 50–52 | ||
| Executive /management support is inadequate or not visible to the implementers | … decreases perceptions of feasibility and value of the change | … resulting in lack of engagement | Support32 38 | ||
| Executive /management support is inadequate or distant, but local or within team leadership is seen as strong and autonomous | … does not decrease perceptions of feasibility and value of the change | … and does not impact intention to commit | Supported56 | ||
| Executive /management support is inadequate, but local or within team leadership is seen as strong | … increases perceptions of siloed change, decreasing perceptions of feasibility | … resulting in lower staff buy-in and commitment | Supported55 | ||
| Executive /management support is inadequate, and local or within team leadership is also inadequate/ non participatory | … decreases perceptions of feasibility and value of the change | … resulting in lack of engagement | Supported55 | ||
| Support from external agencies/peak bodies for the intervention (Develop academic partnerships; use an implementation advisor; use advisory boards and workgroups) |
When external support and/or endorsement of the proposed change is present | implementers may value the change more favourably or feel a greater tension for change | …resulting in increased engagement and commitment | Appropriateness | Support10 27 48 50 53 57 |
| Clear and consistent communication with identified/designated leaders of the intervention (Identify and prepare champions; Recruit, designate, and train for leadership) |
Consistent messages and actions from leaders, opinion leaders and champions | … increase perceptions of organisational capacity | … resulting in more effective engagement | Management support Appropriateness |
Support50 57 |
| Mixed or missed information from leaders, opinion leaders and champions | … decrease perceptions of organisational capacity and disempowerment | … resulting in poorer engagement | Support38 58 | ||
| Align intervention with other organisational priorities | When the proposed change aligns with other organisational or national priorities | … implementers may value the change more favourably and see their efforts as contributing to a larger, more significant programme | … resulting in more effective engagement | Personal valence Appropriateness | Support48 57 |
| When the proposed change is part of a collaborative effort across multiple sites | … stakeholders’ perceptions of the value of the change may increase | … resulting in greater commitment | Support27 28 48 59 | ||
| Align with known concerns/priorities of implementers | When the proposed change aligns with the personal priorities of implementers | …the change is valued more highly by implementers | … resulting in more effective engagement | Personal valence | Support32 51 |
| When the proposed change does not align with personal or group priorities/ do not make sense | … the value of the change is discounted | …resulting in poor engagement | Individual and group valence | Support58 | |
| Provide opportunities for formal and informal planning and knowledge exchange around the intervention (Create a learning collaborative; Capture and share local knowledge) |
When there is appropriate and timely information sharing through social interaction, and shared experience | … may increase collective vision and purpose | … resulting in greater engagement and persistence | Support10 38 47 51 52 57 | |
| Providing appropriate education (Develop educational materials) |
Development of educational packages appropriately pitched at key implementers | …… increase perceptions of feasibility and organisational capacity | … members are more likely to engage in the project | Change-specific efficacy | Support31 47 51 57 59 |
| Development of educational packages not tailored to specific group’s knowledge base perceived as inappropriate | … decreases perceptions of capability | …members are less likely to engage or commit to the project | Support38 | ||
| Providing appropriate implementation support (Facilitation: Develop a formal implementation blueprint) |
Provision or preparation of implementation blueprints or plans | …… increase perceptions of feasibility and organisational capacity | … members are more likely to engage in the project | Change-specific efficacy | Support31 |
| Appealing to past successes | In spite of previous successes and capabilities, if local needs and capabilities are not considered adequate by those enacting this specific change proposed …. | … collective capability will be seen as deficient | … levels of commitment will be poor | Change-specific efficacy | No evidence found |
ERIC, Expert Recommendations for Implementing Change.
Online supplemental file 4 shows the RAMESES checklist for this synthesis. Online supplemental file 5 shows the full search string used in the early steps.
bmjopen-2021-058158supp004.pdf (47.3KB, pdf)
bmjopen-2021-058158supp005.pdf (66.6KB, pdf)
Discussion
In this realist review of implementation strategies for large-scale hospital interventions we have used a four-step process to build a clearer picture of the nature and purpose of implementation and identify likely mechanisms driving intended and unintended outcomes. In the final step we focused on early implementation strategies around baseline assessment and planning to define and test CMO statements explaining outcomes.
In Step 1, we articulated the key concepts associated with implementation programmes of large-scale hospital interventions. Providing practical and social support figured prominently, as did establishing credibility, level of evidence and intended outcomes of the intervention through clear blueprints and collaborative learning and planning activities. While many of the interventions themselves were prescriptive (eg, surgical checklists10–12), the need for implementation to include local needs assessments and tailored activities was also clear. In Step 2, we identified suites of implementation strategies for large-scale hospital interventions and found them to be multifaceted, directed at both individual and organisational levels, and often interdependent. For example, while nearly all the large-scale projects reported education and local leadership, these would only be successful as strategies if they were combined with executive support for the project, and a collective sense of the need for change. It can be argued that the precursor to all implementation strategies is the engagement of the implementers, as without their commitment to change, no substantive change can be achieved. The choice to use Organisational Readiness Theory to further develop the initial programme theories was prompted by this observation.
Organisational Readiness Theory postulates that engagement and commitment to any proposed change will be strongly influenced by individual and collective perceptions around the need for the intervention, its quality and effectiveness, the level of support from management and executive that is apparent and the feasibility of using it. Support for the hypothesised CMOs was found across multiple projects providing strong evidence of the theory’s applicability in large-scale hospital interventions.
Evidence found in our set of literature almost all supported CMOs that led to positive, desirable implementation outcomes of engagement and commitment. There was some refuting evidence that pointed to the interdependence of some factors, and that at times one contextual factor could interact and outweigh another. For example, Wyld and colleagues found that although all stakeholders involved with a new biobank highly valued the initiative, doctors tasked with collecting the samples felt early consultation, management support and consideration of the feasibility for them had been lacking.37 In spite of this, the implementation of the programme had been successful with almost universal adherence to the new processes by the doctors. Possibly, the patients’ altruistic enthusiasm for the initiative, that was often voiced to the doctors during the informed consent process may have put greater value on the initiative, outweighing the doctors’ difficulty.
Evidence for contextual factors that triggered mechanisms leading to poorer outcomes were also found. Bayley and colleagues note the mismatch in perceptions of feasibility found between managers and implementers, and between different healthcare professionals contributing to the multidisciplinary team effort of implementing stroke rehabilitation guidelines.38 This same project found that perceptions of feasibility were also negatively affected by overly complicated statements of the intervention and called for a ‘plain English’ version that would be more accessible for busy clinicians. Both contextual factors were considered barriers as this collective perception of lack of support and lack of feasibility triggered disengagement and lack of commitment to the change.
Some authors, lacking high quality evidence, suggested the cause of poor implementation outcomes might be linked to contextual factors. For example, Reames and colleagues suggest that the failure of their large-scale hospital intervention to effect change might be because it required staff to follow processes that were not strongly associated with clear patient improvements.39 This perception of lack of effectiveness would not trigger the mechanism of building a tension for change, but complacency leading to poor adoption of the intervention. Wand and colleagues found that disagreement voiced by a senior clinician at one site in the early planning stages of an intervention was likely to adversely affect the project’s success there unless it could be resolved.40 Here the perception of implementers would be that the intervention was not feasible or appropriate, and trigger disengagement. While these examples do not claim to be high level evidence, but rather the informed opinion of the authors, they are intuitively correct and consistent with the other evidence found in this study.
Strengths and limitations
Our search for literature was systematic and thorough yet only resulted in 51 papers. This was because while large-scale hospital interventions abound, implementation activities and outcomes are not commonly reported.41 This meant many articles reporting interventions were not relevant to the present study. Even for papers reporting implementation, reporting of these strategies and the contexts in which they were used was often not detailed enough to develop theories. Notable was the lack of accounts of patient involvement in implementation plans. Articles that were found were mostly reporting successful implementations and this is an acknowledged bias of published literature. While CMOs are useful in explaining single factors, multiple contextual factors may arise that modify how mechanisms work. Lack of detail in reporting meant the O (outcomes) in our CMO configurations were high level and dichotomous: implementers were engaged or implementers were not engaged. Another limitation was the need to constrain our search and inquiry to a subset of strategies and a single formal theory. Strengths included the expertise of the research team (including clinical and implementation science expertise) and the systematic four step iterative investigation.
Conclusions
Large-scale hospital interventions hold the promise of standardising high quality, evidence-based care for large numbers of patients but must be supported with appropriate implementation strategies to support and effect change. The study has used realist methodology to tease out how initial planning activities can drive engagement and commitment and delineate the contextual factors required to trigger mechanisms. These findings, using Organisational Readiness Theory, will add to understandings around why large-scale projects work some of the time but not all of the time. Evidence has been presented around a set of CMO hypotheses, showing the importance of implementers’ perceptions around feasibility, support and value in triggering engagement and commitment to the proposed change.
Supplementary Material
Footnotes
Twitter: @JanetCLong
Contributors: JCL conceptualised the synthesis, and JCL, CP, HMN, MNS, EFA and RH contributed to the overall design. JCL, CP and HMN conducted the database search, article screening and data extraction. JCL conducted the synthesis and drafted the first manuscript. MNS, EFA, RH and JB contributed to the final versions of the manuscript. All authors read and approved the manuscript.JCL acts as guarantor of this study.
Funding: This systematic review was funded by the Medical Research Future Fund (MRFF) (APP1178554, CI Braithwaite).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. For this realist review all data relevant to the study are included in the article or uploaded as supplementary files.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Best A, Greenhalgh T, Lewis S, et al. Large-system transformation in health care: a realist review. Milbank Q 2012;90:421–56. 10.1111/j.1468-0009.2012.00670.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chaillet N, Dumont A, Abrahamowicz M, et al. A cluster-randomized trial to reduce cesarean delivery rates in Quebec. N Engl J Med 2015;372:1710–21. 10.1056/NEJMoa1407120 [DOI] [PubMed] [Google Scholar]
- 3. Haynes AB, Weiser TG, Berry WR, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med Overseas Ed 2009;360:491–9. 10.1056/NEJMsa0810119 [DOI] [PubMed] [Google Scholar]
- 4. de Vries EN, Prins HA, Crolla RMPH, et al. Effect of a comprehensive surgical safety system on patient outcomes. N Engl J Med 2010;363:1928–37. 10.1056/NEJMsa0911535 [DOI] [PubMed] [Google Scholar]
- 5. Greenhalgh T, Humphrey C, Hughes J, et al. How do you modernize a health service? A realist evaluation of whole-scale transformation in London. Milbank Q 2009;87:391–416. 10.1111/j.1468-0009.2009.00562.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamey G. What are the barriers to scaling up health interventions in low and middle income countries? A qualitative study of academic leaders in implementation science. Global Health 2012;8:11. 10.1186/1744-8603-8-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Braithwaite J, Marks D, Taylor N. Harnessing implementation science to improve care quality and patient safety: a systematic review of targeted literature. Int J Qual Health Care 2014;26:321–9. 10.1093/intqhc/mzu047 [DOI] [PubMed] [Google Scholar]
- 8. Rapport F, Clay-Williams R, Churruca K, et al. The struggle of translating science into action: foundational concepts of implementation science. J Eval Clin Pract 2018;24:1–10. 10.1111/jep.12741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the expert recommendations for implementing change (ERIC) project. Implement Sci 2015;10:21. 10.1186/s13012-015-0209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haynes AB, Edmondson L, Lipsitz SR, et al. Mortality trends after a voluntary Checklist-based surgical safety collaborative. Ann Surg 2017;266:923–9. 10.1097/SLA.0000000000002249 [DOI] [PubMed] [Google Scholar]
- 11. Urbach DR, Govindarajan A, Saskin R, et al. Introduction of surgical safety checklists in Ontario, Canada. N Engl J Med 2014;370:1029–38. 10.1056/NEJMsa1308261 [DOI] [PubMed] [Google Scholar]
- 12. Molina G, Jiang W, Edmondson L, et al. Implementation of the surgical safety checklist in South Carolina hospitals is associated with improvement in perceived perioperative safety. J Am Coll Surg 2016;222:725–36. 10.1016/j.jamcollsurg.2015.12.052 [DOI] [PubMed] [Google Scholar]
- 13. Rogers L, De Brún A, McAuliffe E. Defining and assessing context in healthcare implementation studies: a systematic review. BMC Health Serv Res 2020;20:591. 10.1186/s12913-020-05212-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davies P, Walker AE, Grimshaw JM. A systematic review of the use of theory in the design of guideline dissemination and implementation strategies and interpretation of the results of rigorous evaluations. Implement Sci 2010;5:14. 10.1186/1748-5908-5-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fixsen D, Naoom S, Blase K. Implementation research: a synthesis of the literature. Tamps, FL: University of South Florida, Louis de la Parte Florida Mental Health Institute, National Implementation Research Network, 2005. [Google Scholar]
- 16. Pawson R, Greenhalgh T, Harvey G, et al. Realist review--a new method of systematic review designed for complex policy interventions. J Health Serv Res Policy 2005;10 Suppl 1:21–34. 10.1258/1355819054308530 [DOI] [PubMed] [Google Scholar]
- 17. Wong G, Greenhalgh T, Westhorp G, et al. RAMESES publication standards: realist syntheses. BMC Med 2013;11:21. 10.1186/1741-7015-11-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shearn K, Allmark P, Piercy H, et al. Building realist program theory for large complex and messy interventions. Int J Qual Methods 2017;16:160940691774179. 10.1177/1609406917741796 [DOI] [Google Scholar]
- 19. Sarkies MN, Francis-Auton E, Long JC, et al. Implementing large-system, value-based healthcare initiatives: a realist study protocol for seven natural experiments. BMJ Open 2020;10:e044049. 10.1136/bmjopen-2020-044049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koff E, Lyons N. Implementing value-based health care at scale: the NSW experience. Med J Aust 2020;212:104–6. 10.5694/mja2.50470 [DOI] [PubMed] [Google Scholar]
- 21. Sarkies M, Long JC, Pomare C, et al. Avoiding unnecessary hospitalisation for patients with chronic conditions: a systematic review of implementation determinants for hospital avoidance programmes. Implement Sci 2020;15:91. 10.1186/s13012-020-01049-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cunningham U, Ward ME, De Brún A, et al. Team interventions in acute Hospital contexts: a systematic search of the literature using realist synthesis. BMC Health Serv Res 2018;18:536. 10.1186/s12913-018-3331-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rycroft-Malone J, McCormack B, Hutchinson AM, et al. Realist synthesis: illustrating the method for implementation research. Implement Sci 2012;7:33. 10.1186/1748-5908-7-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wong G, Greenhalgh T, Pawson R. Internet-Based medical education: a realist review of what works, for whom and in what circumstances. BMC Med Educ 2010;10:12. 10.1186/1472-6920-10-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Makene CL, Plotkin M, Currie S, et al. Improvements in newborn care and newborn resuscitation following a quality improvement program at scale: results from a before and after study in Tanzania. BMC Pregnancy Childbirth 2014;14:381. 10.1186/s12884-014-0381-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cima R, Dankbar E, Lovely J, et al. Colorectal surgery surgical site infection reduction program: a national surgical quality improvement program--driven multidisciplinary single-institution experience. J Am Coll Surg 2013;216:23–33. 10.1016/j.jamcollsurg.2012.09.009 [DOI] [PubMed] [Google Scholar]
- 27. Vu JV, Collins SD, Seese E, et al. Evidence that a regional surgical collaborative can transform care: surgical site infection prevention practices for colectomy in Michigan. J Am Coll Surg 2018;226:91–9. 10.1016/j.jamcollsurg.2017.10.013 [DOI] [PubMed] [Google Scholar]
- 28. Schwarzkopf D, Rüddel H, Gründling M, et al. The German quality network sepsis: study protocol for the evaluation of a quality collaborative on decreasing sepsis-related mortality in a quasi-experimental difference-in-differences design. Implement Sci 2018;13:15. 10.1186/s13012-017-0706-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maguire EM, Bokhour BG, Wagner TH, et al. Evaluating the implementation of a national disclosure policy for large-scale adverse events in an integrated health care system: identification of gaps and successes. BMC Health Serv Res 2016;16:648. 10.1186/s12913-016-1903-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schweizer ML, Chiang H-Y, Septimus E, et al. Association of a bundled intervention with surgical site infections among patients undergoing cardiac, hip, or knee surgery. JAMA 2015;313:2162–71. 10.1001/jama.2015.5387 [DOI] [PubMed] [Google Scholar]
- 31. Kourouche S, Buckley T, Van C, et al. Designing strategies to implement a blunt chest injury care bundle using the behaviour change wheel: a multi-site mixed methods study. BMC Health Serv Res 2019;19:461. 10.1186/s12913-019-4177-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hendy J, Fulop N, Reeves BC, et al. Implementing the NHS information technology programme: qualitative study of progress in acute trusts. BMJ 2007;334:1360. 10.1136/bmj.39195.598461.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mudge AM, Banks MD, Barnett AG, et al. CHERISH (collaboration for hospitalised elders reducing the impact of stays in hospital): protocol for a multi-site improvement program to reduce geriatric syndromes in older inpatients. BMC Geriatr 2017;17:11. 10.1186/s12877-016-0399-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marcus RK, Lillemoe HA, Rice DC, et al. Determining the safety and efficacy of enhanced recovery protocols in major oncologic surgery: an institutional NSQIP analysis. Ann Surg Oncol 2019;26:782–90. 10.1245/s10434-018-07150-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weiner BJ. A theory of organizational readiness for change. Implement Sci 2009;4:67. 10.1186/1748-5908-4-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holt DT, Armenakis AA, Feild HS. Readiness for organizational change: the systematic development of a scale. The Journal of Applied Behavioral Science 2007;43:232–55. [Google Scholar]
- 37. Wyld L, Smith S, Hawkins NJ, et al. Introducing research initiatives into healthcare: what do doctors think? Biopreserv Biobank 2014;12:91–8. 10.1089/bio.2013.0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bayley MT, Hurdowar A, Richards CL, et al. Barriers to implementation of stroke rehabilitation evidence: findings from a multi-site pilot project. Disabil Rehabil 2012;34:1633–8. 10.3109/09638288.2012.656790 [DOI] [PubMed] [Google Scholar]
- 39. Reames BN, Krell RW, Campbell DA, et al. A checklist-based intervention to improve surgical outcomes in Michigan: evaluation of the keystone surgery program. JAMA Surg 2015;150:208–15. 10.1001/jamasurg.2014.2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wand T, Crawford C, Bell N, et al. Documenting the pre-implementation phase for a multi-site translational research project to test a new model emergency Department-based mental health nursing care. Int Emerg Nurs 2019;45:10–16. 10.1016/j.ienj.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 41. Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci 2013;8:139. 10.1186/1748-5908-8-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kislov R, Pope C, Martin GP, et al. Harnessing the power of theorising in implementation science. Implement Sci 2019;14:103. 10.1186/s13012-019-0957-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bandura A. Social cognitive theory in cultural context. Appl Psychol 2002;51:269–90. 10.1111/1464-0597.00092 [DOI] [Google Scholar]
- 44. Lasker RD, Weiss ES. Creating partnership synergy: the critical role of community stakeholders. J Health Hum Serv Adm 2003;26:119–39. [PubMed] [Google Scholar]
- 45. Rogers EM. Diffusion of innovations. New York: Free Press, 1983. [Google Scholar]
- 46. Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process 1991;50:179–211. 10.1016/0749-5978(91)90020-T [DOI] [Google Scholar]
- 47. Palomar M, Álvarez-Lerma F, Riera A, et al. Impact of a national multimodal intervention to prevent catheter-related bloodstream infection in the ICU: the Spanish experience. Crit Care Med 2013;41:2364–72. 10.1097/CCM.0b013e3182923622 [DOI] [PubMed] [Google Scholar]
- 48. Pronovost P. Interventions to decrease catheter-related bloodstream infections in the ICU: the keystone intensive care unit project. Am J Infect Control 2008;36:S171.e171–5. 10.1016/j.ajic.2008.10.008 [DOI] [PubMed] [Google Scholar]
- 49. Morrow E, Robert G, Maben J, et al. Implementing large-scale quality improvement: lessons from the productive ward: releasing time to care. Int J Health Care Qual Assur 2012;25:237–53. 10.1108/09526861211221464 [DOI] [PubMed] [Google Scholar]
- 50. Mansoori B, Erhard KK, Sunshine JL. Picture Archiving and Communication System (PACS) implementation, integration & benefits in an integrated health system. Acad Radiol 2012;19:229–35. 10.1016/j.acra.2011.11.009 [DOI] [PubMed] [Google Scholar]
- 51. Pun BT, Gordon SM, Peterson JF, et al. Large-Scale implementation of sedation and delirium monitoring in the intensive care unit: a report from two medical centers. Crit Care Med 2005;33:1199–205. 10.1097/01.ccm.0000166867.78320.ac [DOI] [PubMed] [Google Scholar]
- 52. van Harten WH, Goedbloed N, Boekhout AH, et al. Implementing large scale fast track diagnostics in a comprehensive cancer center, pre- and post-measurement data. BMC Health Serv Res 2018;18:85. 10.1186/s12913-018-2868-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cuypers M, Al-Itejawi HHM, van Uden-Kraan CF, et al. Introducing decision AIDS into routine prostate cancer care in the Netherlands: implementation and patient evaluations from the multi-regional JIPPA initiative. J Canc Educ 2020;35:1141–8. 10.1007/s13187-019-01572-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sharma N, Herrnschmidt J, Claes V, et al. Organizational readiness for implementing change in acute care hospitals: an analysis of a cross-sectional, multicentre study. J Adv Nurs 2018;74:2798–808. 10.1111/jan.13801 [DOI] [PubMed] [Google Scholar]
- 55. Rees GH. Organisational readiness and lean thinking implementation: findings from three emergency department case studies in New Zealand. Health Serv Manage Res 2014;27:1–9. 10.1177/0951484814532624 [DOI] [PubMed] [Google Scholar]
- 56. Zapka J, Simpson K, Hiott L, et al. A mixed methods descriptive investigation of readiness to change in rural hospitals participating in a tele-critical care intervention. BMC Health Serv Res 2013;13:33. 10.1186/1472-6963-13-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 2006;355:2725–32. 10.1056/NEJMoa061115 [DOI] [PubMed] [Google Scholar]
- 58. Flynn R, Rotter T, Hartfield D, et al. A realist evaluation to identify contexts and mechanisms that enabled and hindered implementation and had an effect on sustainability of a lean intervention in pediatric healthcare. BMC Health Serv Res 2019;19:912. 10.1186/s12913-019-4744-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nocera M, Shanahan M, Murphy RA, et al. A statewide nurse training program for a hospital based infant abusive head trauma prevention program. Nurse Educ Pract 2016;16:e1–6. 10.1016/j.nepr.2015.07.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-058158supp001.pdf (159.1KB, pdf)
bmjopen-2021-058158supp002.pdf (101.9KB, pdf)
bmjopen-2021-058158supp003.pdf (101.7KB, pdf)
bmjopen-2021-058158supp004.pdf (47.3KB, pdf)
bmjopen-2021-058158supp005.pdf (66.6KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. For this realist review all data relevant to the study are included in the article or uploaded as supplementary files.