Summary
Background
Data are limited regarding long-term consequences of invasive GBS (iGBS) disease in early infancy, especially from low- and middle-income countries (LMIC) where most cases occur. We aimed to estimate risk of neurodevelopmental impairment (NDI) in children with a history of iGBS disease.
Methods
A multi-country matched cohort study was undertaken in South Africa, India, Mozambique, Kenya, and Argentina from October 2019 to April 2021. The exposure of interest was defined as a history of iGBS disease (sepsis or meningitis) before 90 days of age, amongst children now aged 1·5–18 years. Age and sex-matched, children without history of GBS were also recruited. Age-appropriate, culturally-adapted assessments were used to define NDI across multiple domains (cognitive, motor, hearing, vision, emotional-behaviour, growth). Pooled NDI risk was meta-analysed across sites. Association of iGBS exposure and NDI outcome was estimated using modified Poisson regression with robust variance estimator.
Findings
Amongst 138 iGBS survivors and 390 non-iGBS children, 38·1% (95% confidence interval [CI]: 30·0% – 46·6%) of iGBS children had any NDI, compared to 21·7% (95% CI: 17·7% - 26·0%) of non- iGBS children, with notable between-site heterogeneity. Risk of moderate/severe NDI was 15·0% (95% CI: 3·4% - 30·8%) among GBS-meningitis, 5·6% (95% CI: 1·5% - 13·7%) for GBS-sepsis survivors. The adjusted risk ratio (aRR) for moderate/severe NDI among iGBS survivors was 1.27 (95% CI: 0.65, 2.45), when compared to non-GBS children. Mild impairment was more frequent in iGBS (27.6% (95% CI: 20.3 – 35.5%)) compared to non-GBS children (12.9% (95% CI: 9.7% - 16.4%)). The risk of emotional-behavioural problems was similar irrespective of iGBS exposure (aRR=0.98 (95% CI: 0.55, 1.77)).
Interpretation
Our findings suggest that iGBS disease is on average associated with a higher risk of moderate/severe NDI, however substantial variation in risk was observed between sites and data are consistent with a wide range of values. Our study underlines the importance of long-term follow-up for at-risk neonates and more feasible, standardised assessments to facilitate diagnosis in research and clinical practice.
Funding
This work was supported by a grant (INV-009018) from the Bill & Melinda Gates Foundation to the London School of Hygiene &Tropical Medicine.
Keywords: Group B streptococcus, Meningitis, Sepsis, Infants, children, Impairment, Neurodevelopment, Disability
Research in context.
Evidence before this study
Group B Streptococcus (GBS) sepsis and meningitis are important causes of mortality in neonates and young infants, with data gaps regarding long-term outcomes. A systematic review of neurodevelopmental impairment (NDI) risk among invasive GBS (iGBS) survivors found 18 studies assessing moderate and/or severe NDI outcomes after iGBS-meningitis in children, with assessments performed primarily before the age of 2 years. Three important data gaps were highlighted: (1) outcomes after iGBS-sepsis; (2) risk of milder outcomes, which often become more apparent later in childhood; (3) geographic representativeness of data, with data gaps especially in low or middle-income countries (LMIC), where the majority of iGBS cases occur. Furthermore, few studies involved a comparison group, limiting ability to quantify an association (i.e., relative risk or odds ratio). A large registry-based cohort study from Denmark and the Netherlands, published in 2021, quantified NDI risk after iGBS, including milder outcomes, and reported a two-fold increase in risk of moderate or severe NDI by 10 years of age. A Pubmed search, with search terms similar to the previous systematic review related to “Streptococcus agalactiae [MesH]”, “group B streptococcus”, and “disability”, “impairment”, with no restrictions for date (until 2021) and language, did not identify any additional published studies on NDI in children with history of iGBS and still no studies from LMICs were found.
Added value of this study
Our study addresses these priority data gaps identified, being a multi-country study in LMICs (South Africa, India, Mozambique, Kenya, and Argentina) to quantify severity of NDI risk in iGBS survivors, including after sepsis, beyond early childhood (median age 6 years old). Children without history of iGBS, matched on age and sex to iGBS children, were recruited in each site. Amongst 138 children with a history of iGBS, there was an increased risk of moderate/severe NDI, compared with 390 children with no history of iGBS, although estimates showed between-country heterogeneity.
Implications of all the available evidence
We found that NDI was frequent after iGBS, consistent with previous studies on GBS-meningitis and providing novel findings that GBS-sepsis survivors also have substantial NDI. Since sepsis is the most common iGBS disease clinical presentation, this has important implications for disease burden estimates and cost-effectiveness analyses. To inform both individual clinical care and public health planning, standardised developmental assessment tests are needed which are more feasible, adaptable to context, and freely accessible. Better detection also needs to link to better healthcare and education for these children, plus support for their families, requiring context-specific implementation research.
Alt-text: Unlabelled box
Introduction
Achieving the developmental and educational potential for every child is the ambition of every family, foundational for promoting human capital growth in every country, and is reflected in the Sustainable Development Goals (SDGs) and other United Nations frameworks with the mantra survive and thrive.1, 2, 3, 4 However, the lack of standardised multi-country data impedes tracking of related targets: for example, global estimates of sub-optimal early child development (ECD) often use childhood stunting as a surrogate.5 Accurate measurement of neurodevelopmental impairments (NDI) is crucial to strengthening quantification of burden of disease (e.g., to inform disability adjusted life years (DALYs6)) and cost-effectiveness, such as included in the Full Value of Vaccine Analyses.7
Long-term sequelae after severe infections in the first weeks of life are a preventable cause of NDI. Invasive Group B Streptococcus (iGBS) infections in early infancy leading to neonatal and infant mortality are well documented, 8 but the risk of NDI in children after iGBS disease has been under studied. A recent meta-analysis reported 18% of iGBS-meningitis survivors had moderate/severe NDI at a median age of 18 months,9 and too few studies on NDI after iGBS-sepsis were identified to be meta-analysed.9 Only a handful of studies reported on outcomes in children older than 2 years old. Relevant domains such as hearing and vision were not reported for most studies nor were milder impairment outcomes (e.g., emotional-behavioral, mild cognition), which are more accurately detected later in childhood.9 Whilst recent data from a large registry-based cohort study in Denmark and the Netherlands provided more comprehensive quantification of the risk compared to the previous studies,10 a major data gap remains for low- and middle-income countries (LMIC), where the majority of the iGBS disease cases occur. In the previous meta-analysis, only three of the 18 studies were from middle-income countries and none were from low-income countries.9 Additionally, comparator groups were often missing, but are needed to understand causality of iGBS on NDI outcomes.
We designed a multi-country matched cohort study to address this priority gap of LMIC data on NDI after iGBS. In this paper, we focus on the objective to estimate the risk of NDI; the other objectives of the study have been published (e.g. acute costs of care11) or will be reported separately (e.g, long term economic outcomes).12 Here, our focus is to (1) describe the risk of moderate/severe and mild NDI among iGBS survivors by site (South Africa,13 Mozambique,14 India,15 combined with Kenya, Argentina), syndrome (meningitis, sepsis) and age; (2) estimate risk of multi-domain NDI and emotional-behavioural problems after iGBS exposure; (3) quantify domain-specific NDI and growth outcomes.
Methods
Study design and participants
Details regarding the research protocol and methods have been published separately.12 In summary, five sites were identified in three regions with high burden of GBS through a call for data request in 2018 (for more information see 12): sub-Saharan Africa (Kenya, Mozambique, South Africa), Asia (India), and Latin America (Argentina) (Figure 1). We used a matched cohort design; children with a history of invasive GBS (iGBS) disease, either GBS-sepsis or GBS-meningitis, in the first 90 days after birth, and who were at least 18 months old at time of recruitment were eligible for the study. Children were recruited from October 2019 to April 2021. Case definition for iGBS, inclusion and exclusion criteria, and expected number of iGBS survivors for each of the sites are given in Supplemental Table S1. iGBS children were identified via hospital records (Argentina, India, South Africa, Kenya), or via a microbiological surveillance system (Mozambique). iGBS survivors were grouped by clinical syndrome: sepsis or meningitis. A comparison cohort of children with no history of iGBS disease was identified in each site via hospital-birth registries (Argentina, India, South Africa) or a Health and Demographic Surveillance System (Kenya, Mozambique) and matched on birth month and year (18 months – 18 years old) and sex. We aimed to assess NDI outcomes in 200 iGBS survivors, based on the maximum number of cases expected to be identified across all 5 sites, and 600 non-GBS children (using a matching ratio of 3:1); assuming 26% moderate/severe NDI among iGBS survivors and 10% in the non-iGBS comparison group, this gives more than 90% power at a 5% significance level to detect a difference using a two-sided test of binomial proportions.12
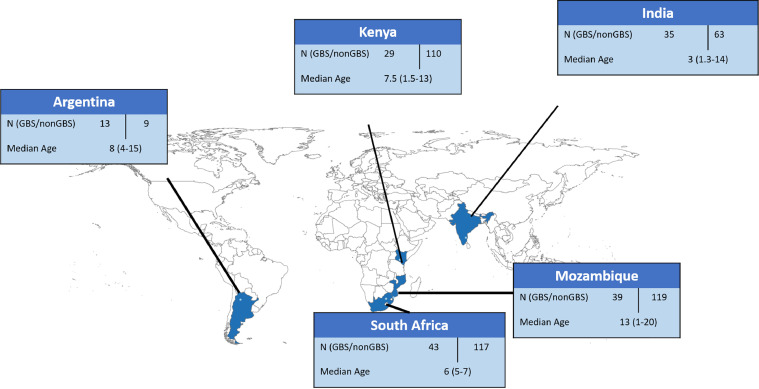
Figure 1.
Map of multi-country iGBS long-term follow-up studies: South Africa, Mozambique, Kenya, Argentina.
Written informed consent was obtained from parents/guardians before or at the in-person assessment visit. Assent, in addition to consent, was obtained from children based on local guidelines.
At a face-to-face appointment, the parent/guardian was asked to complete a demographic, health, and economic questionnaire (Supplemental methods SM1). Anthropometric measurements were performed. Age-specific neurodevelopmental assessment tools were administered to each child to assess NDIs (Table 1). Data were collected on paper forms or using a customized app. The customized Android tablet-based app was developed in collaboration with International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b,), and included questionnaires and neurodevelopment assessment tools, translated into local language where relevant (Supplemental methods SM2).12
Table 1.
NDI assessment matrix.
| Age band | Motor | Cognition | Vision | Hearing | Emotional-behavioural | |
|---|---|---|---|---|---|---|
| South Africa |
5-9 |
GMDS-ER – composite locomotor & hand-eye coordination scales |
GMDS-ER – composite hearing & language, eye-hand coordination, performance, reasoning scales |
Tumbling E |
OAE screening + clinical determination |
Pre-school CBCL (<6) – total problem score School age CBCL (6 – 18) – total problem score |
| Mozambique | <5 |
MDAT – gross motor scale* |
MDAT – fine motor-cognitive scale* |
Clinician screening + Peek acuity app |
Clinician screening + clinical determination (severe only) |
|
| 5-9 |
No motor for ≥6 from CANTAB as reference populations not available |
CANTAB composite of spatial span and working memory |
||||
| 10+ |
||||||
| India | <5 |
BSID – motor scale⁎⁎ BOT short form⁎⁎ |
BSID – composite language & cognitive scales⁎⁎ WPPSI⁎⁎ |
Visual screening + clinical determination |
Hearing screening + clinical determination |
|
| 5-9 |
BOT short form |
WPPSI – IQ score WISC5 –IQ score |
Tumbling E |
|||
| 10+ |
WISC 5 – IQ score |
|||||
| Kenya | <5 |
KDI – motor scale | KDI – eye-hand coordination scale | LEA Symbols | ABR |
|
| 5-9 |
composite score of Bead threading & ball balance |
RCM full scale |
Tumbling E |
|||
| 10+ |
||||||
| Argentina | <5 |
Clinical assessment of motor | WPPSI – IQ score | Tumbling E | Hearing screening + clinical determination | |
| 5-9 |
WPPSI – IQ score WISC 4–IQ score |
|||||
| 10+ | WISC 4 – IQ score |
ABR = Auditory Brainstem Response; BOT = Bruininks-Oseretsky Test; BSID = Bayley Scales of Infant and Toddler Development; CANTAB = Cambridge Neuropsychological Test Automated Battery CBCL Child Behavior Checklist; GMDS-ER = Griffiths Mental Development Scales – Extended Revised; KDI = Kilifi Developmental Inventory; MDAT = Malawi Developmental Assessment Tool; RCM = Raven's colored matrices; WISC = Wechsler Abbreviated Scale of Intelligence; WPPSI = Wechsler Preschool and Primary Scales of Intelligence.
BSID assessment up to 42 months.
†WPPSI assessment in Argentina 3-7 years. WPPSI assessment in India 4-7 years.
‡BOT assessment ≥4 years.
¥WISC 4 and WISC 5 assessment ≥7 years.
Each site used various neurodevelopmental assessments, with 26 tools in total across the 5 study sites. Each site selected assessment tools for their setting based on child's age, cultural appropriateness, validation of instrument for their population, and technical capacity. We developed a matrix by age bands (<5 years, 5-9 years, ≥10 years) and domain or item to allow combining of the various NDI outcomes (Table 1). Cognitive and motor scores were normalised using standard reference population, by assessment and site, to set thresholds for mild, moderate, and severe impairments, except for motor severity in Argentina (Supplemental Table S2). In Argentina, functional impact was used to categorise severity of motor impairment.
Vision and hearing impairments were defined using WHO severity categorisation (Table S2). For vision, children were assessed with age-appropriate methods, such as Tumbling E Chart (South Africa, Kenya, India, Argentina), LEA symbols (Kenya). For hearing, methods of assessment varied: in Kenya, Auditory Brainstem Response (ABR) was completed on all children; in Argentina, India, and South Africa, hearing screening identified individuals with any potential hearing impairment and those who failed the screening were assessed with a full audiometry. In Mozambique, no formal vision or hearing screening was done, however, in the three children suspected of vision problems, the Peek acuity app was used to classify impairment severity as per the defined categories.16 If there was clinical suspicion of a hearing problem, children were referred for formal hearing evaluation.
The Child Behaviour Checklist (CBCL) assessment was used by all sites to measure emotional-behavioural outcomes.17,18 Having any emotional-behavioural problem was defined by a total score on CBCL that was above 97th percentile. Percentiles were based on normative age- and sex-samples.12,19
NDI severity was defined to be consistent with Global Burden of Disease (GBD)20 and other relevant studies.9 Moderate/severe NDI defined based on at least one of the following criteria (Table S2):
-
•
Scored 2 standard deviations (SD) below the standardized reference mean in cognition AND/OR motor composite measures
-
•
moderate to severe hearing impairment per WHO criteria21
-
•
moderate to severe vision impairment per WHO criteria22
-
•
mild impairment (≥-2 SD and <-1 SD below the standardized reference mean) in at least 3 domains (motor, cognition, hearing, vision)20
Nutritional outcomes of stunting and moderate-to-severe malnutrition were derived from measurements of each child's height and weight (Table S2). Stunting was defined as height-for-age Z-score less than 2 SD below the median of the WHO child growth standard.23 Moderate-to-severe malnutrition was defined as stunting and/or wasting (more than 2 SD below the median weight-for-height of the WHO child growth standard) and/or underweight (more than 2 SD below median BMI-for-age of the WHO growth reference for school-aged children and adolescents).23
Statistical analysis
All analyses were performed in StataMP V15·1. Median age was reported; the remaining demographic and health data variables were categorical and presented as percentages.
Risks for the following outcomes were estimated for each study site and by exposure group: moderate/severe NDI, mild NDI, domain (eg., motor, cognition, hearing, vision)-specific outcomes, any emotional behavior problems (clinical range of CBCL total problem scale), stunting and moderate-to-severe malnutrition.
In analyses combining data from the sites, pooled risks of moderate/severe NDI, any NDI, emotional-behavioural, and nutritional outcomes among exposed and unexposed cohorts were based on random-effects meta-analysis applying the DerSimonian and Laird method for pooled proportion estimates with 95% confidence intervals (CI).24 Estimates were stratified by age (<5 years old; ≥5 years old); 5-9 and 10+ age bands were combined due to small sample size and within site age distribution. Risks of NDI outcomes were also calculated among GBS-meningitis and GBS-sepsis survivors separately. To quantify the association between iGBS disease and NDI outcomes, a modified random-effects Poisson regression model with robust variance estimator was used to estimate risk ratios (RRs) adjusted for matching variables (age and sex), as well as study site, primary carer's education (< secondary; secondary or higher), and gestational age (term birth ≥37 weeks; preterm birth <37 weeks; missing).25 Preterm birth was defined as gestational age <37 weeks. Only iGBS survivors with matched non-GBS comparator were included. The modified Poisson regression model, rather than the logistic model described in 12, was used because for non-rare outcomes odds ratio does not properly approximate the risk ratio (RR).26 RR were calculated separately for children younger than 5 years old and for children 5 years and older. For comparison, results of the logistic model are presented in Supplemental Table S4.
Estimates of NDI risk and regression analyses with the combined dataset did not include data from all study sites due to potential selection bias. In particular, a high proportion of children identified as eligible for recruitment were not contacted after completion of data collection in South Africa (57%) and Argentina (50%). Whilst in South Africa, there were no significant differences in human immunodeficiency virus (HIV) exposure, socio-economic status or NDI outcome at one year of age between those contacted and those not reachable, in Argentina such an analysis was not possible. For this reason, data from Argentina were not included in these analyses. A sensitivity analysis on the association between iGBS disease and moderate/severe NDI that included the Argentina data was performed.
Ethics
The overarching protocol for this multi-country observational study was granted ethical approval at the London School of Hygiene & Tropical Medicine (approval number 16246). Institutional review boards in each of the study sites granted ethics approval (Argentina approval number Protocol EGB-1; India approval numbers 11723 (Christian Medical College (CMC) Vellore), 2019-7034 (Indian Council of Medical Research (ICMR)); Kenya approval number SERU/CGMR-C/164/3882; Mozambique approval numbers 98/CNBS/2019; South Africa approval number M190241), as well as the institutional review board of the World Health Organization (WHO) (approval number ERC.0003169).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, writing of the manuscript, or decision to submit the manuscript for publication. The funders had no access to the dataset of this study. PP, JC, EHP, JEL had access to the dataset. PP and JEL decided to submit for publication.
Results
Participants
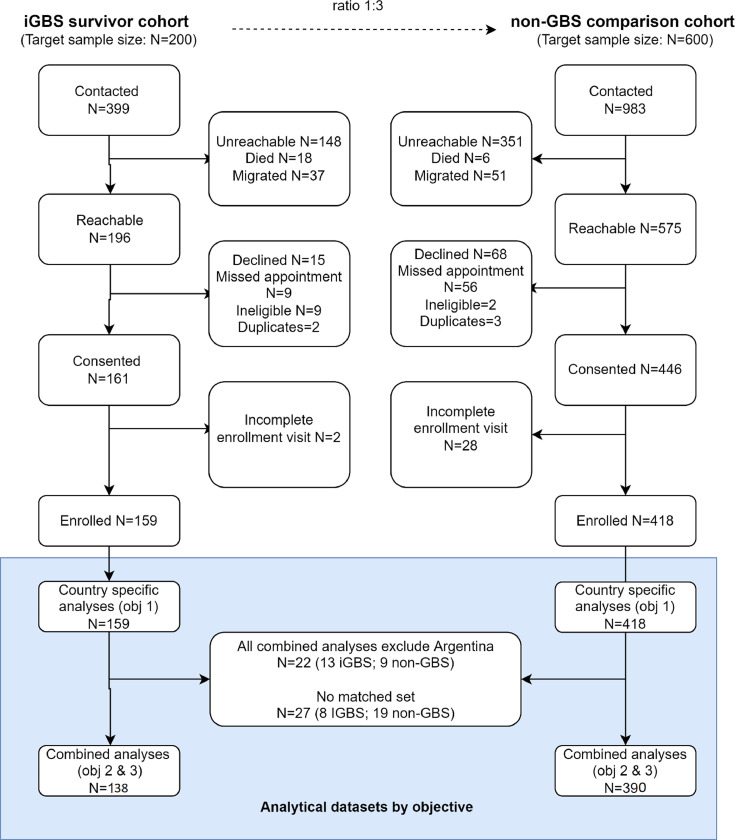
iGBS survivors (N=399) and eligible unexposed children (N=983) were identified across five sites (Figure 1), with 196 and 575 being reached, respectively. Overall, 37% were unreachable and another 14% were excluded due to migration or death in the iGBS cohort. Similarly, 36% were unreachable in the non-GBS comparison cohort; exclusion due to death or migration in the unexposed group was 6%. After contact, another 35 (9%) and 129 (13%) in iGBS and non-GBS cohorts, respectively, were excluded due to other reasons, such as declined participation, duplicate records or missed appointments. Argentina enrolled only 50% of their target number iGBS survivors (Table S1) due to strict COVID-19 social distancing measures during their expected enrolment period (N = 13 enrolled participants / 26 expected iGBS survivors). Whilst South Africa had a high proportion of children who could not be contacted (103/180; 57%), the other sites reported that less than 15% of the children were not contactable. Thirty children were excluded due to incomplete NDI assessments during the study visit.
A total of 159 iGBS survivors and 418 children with no history of iGBS disease completed questionnaires and neurodevelopmental assessments. It was not possible to identify a matched unexposed child for 16 exposed children. Of the remaining 143 exposed children, 22 were matched to 1 unexposed child, 26 were matched to 2 unexposed children, 86 were matched to 3 unexposed children, and 9 were matched to 4 or more unexposed children. The age of exposed children ranged from 1-18 years (1-20 years in total cohort); the median ages were 3, 6, 7, 8, and 13 years in India, South Africa, Kenya, Argentina, and Mozambique, respectively. There were slightly more males (51·3%) than females. Prematurity and low birth weight were more frequent among the iGBS cohort compared to the comparison unexposed group in India (3/35 in iGBS vs 3/63 in non-GBS), Kenya (5/29 vs 6/110), and South Africa (9/43 vs 17/117) (Table 2).
Table 2.
Descriptive characteristics amongst survivors of invasive Group B Streptococcal (iGBS) in infancy and comparison cohort, stratified by country.
| South Africa |
Mozambique |
India |
Kenya |
Argentina |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| iGBS Survivors (N=43) | non-iGBS comparison group (N=117) | iGBS Survivors (N=39) | non-iGBS comparison group (N=119) | iGBS Survivors (N=35) | non-iGBS comparison group (N=63) | iGBS Survivors (N=29) | non-iGBS comparison group (N=110) | iGBS Survivors (N=13) | non-iGBS comparison group (N=9) | |
| Clinical syndrome, n (%) | ||||||||||
| Sepsis | 30 (69·8) | 22 (56·4) | 31 (88·6) | 25 (86·2) | 10 (76·9) | |||||
| Meningitis | 13 (30·2) | 17 (43·6) | 4 (11·4) | 4 (13·8) | 3 (23·1) | |||||
| GBS onset, n (%) | ||||||||||
| Early | 22 (51·2) | 7 (18·0) | 33 (94·3) | 9 (31·0) | 9 (69·2) | |||||
| Late | 21 (48·8) | 32 (82·0) | 2 (5·7) | 20 (69·0) | 4 (30·8) | |||||
| Age (in years) at assessment, median (range) | 6 (5 – 7) | 6 (5 – 7) | 13 (1 – 17) | 13 (1 – 18) | 3 (1 – 14) | 3 (1 – 14) | 7 (1 – 13) | 7 (1 – 13) | 8 (4 – 15) | 8 (4 – 15) |
| Sex, n (%) | ||||||||||
| Male | 22 (51.2) | 60 (51.3) | 19 (48.7) | 53 (48.2) | 16 (45.7) | 26 (41.3) | 18 (62.1) | 52 (52.0) | 7 (53.9) | 4 (44.4) |
| Female | 21 (48.8) | 57 (48.7) | 20 (54.5) | 57 (51.8) | 19 (54.3) | 37 (58.7) | 11 (37.9) | 48 (48.0) | 6 (46.2) | 5 (55.6) |
| Birthweight, n(%)* | ||||||||||
| ≥2500g | 33 (76·7) | 102 (87·2) | 24 (61·5) | 36 (30·2) | 29 (82·9) | 50 (79·4) | 16 (55·2) | 87 (79·1) | 12 (92·3) | 6 (66·7) |
| <2500g | 10 (23·3) | 15 (12·8) | 5 (12·8) | 5 (4·2) | 6 (17·1) | 13 (20·6) | 10 (34·5) | 10 (9·1) | 1 (7·7) | 2 (22·2) |
| Don't know | 10 (25·6) | 78 (65·6) | 0 (0·0) | 0 (0·0) | 3 (10·3) | 13 (11·8) | 0 (0·0) | 1 (11·1) | ||
| Prematurity (<37 weeks), n (%) | 9 (20·9) | 17 (14·5) | 1 (2·6)* | 1 (0·8)* | 3 (8·6) | 3 (4·8) | 5 (17·2) | 6 (5·5) | 1 (7·7) | 2 (22·2) |
| Self reported HIV, n(%) | ||||||||||
| No | 43 (0.0) | 116 (99.2) | 5 (12.8) | 16 (13.5) | 28 (84.9) | 56 (88.9) | 21 (72.4) | 41 (41.0) | 5 (38.5) | 2 (22.2) |
| Yes | 1 (0.8) | 0 (0.0) | 3 (2.5) | 1 (3.0) | 0 (0.0) | 2 (6.9) | 3 (3.0) | 0 (0.0) | 0 (0.0) | |
| Missing | 34 (87.2) | 100 (84.0) | 4 (12.1) | (11.1) | 6 (20.8) | 56 (56.0) | 8 (61.5) | 7 (77.8) | ||
| Birth order, n (%) | ||||||||||
| First born | 14 (32·6) | 50 (42·7) | 13 (33·3) | 38 (31·9) | 30 (85·7) | 33 (52·4) | 11 (37·9) | 18 (16·4) | 10 (76·9) | 6 (66·7) |
| Second born | 14 (32·6) | 33 (28·2) | 9 (23·1) | 22 (18·5) | 5 (14·3) | 25 (39·7) | 7 (24·1) | 21 (19·1) | 2 (15·4) | 1 (11·1) |
| Third born and higher | 15 (34·9) | 34 (29·1) | 17 (43·6) | 59 (49·6) | 0 (0·0) | 5 (7·9) | 11 (37·9) | 71 (64·5) | 1 (7·7) | 2 (22·2) |
| Highest education for main caregiver, n (%) | ||||||||||
| <Primary | 7 (17·9) | 90 (75·6) | 2 (5·7) | 0 (0·0) | 25 (86·2) | 100 (90·9) | 1 (7·7) | 0 (0·0) | ||
| Primary | 1 (2·33) | 1 (0·9) | 18 (46·3) | 28 (23·5) | 4 (11·4) | 11 (17·5) | 4 (13·8) | 8 (7·3) | 4 (30·8) | 4 (44·4) |
| Secondary | 32 (74·4) | 84 (71·8) | 10 (25·6) | 0 (0·0) | 7 (20·0) | 14 (22·2) | 0 (0·0) | 2 (1·8) | 6 (46·1) | 4 (44·4) |
| Higher education (University/technical/) | 10 (23·3) | 32 (27·3) | 4 (10·3) | 1 (0·8) | 22 (62·9) | 38 (60·3) | 2 (15·4) | 1 (11·1) | ||
Objective 1: NDI outcomes by site, syndrome, age
The majority (74·3%) of the exposed children had a history of GBS-sepsis, and about a quarter (25·7%) had GBS-meningitis. GBS-meningitis was diagnosed by GBS-positive cerebrospinal fluid (CSF) culture in most cases (67·4%), but five cases defined as meningitis had suggestive CSF leucocyte count of >20 × 106/l plus GBS-positive blood culture and nine had GBS-positive blood culture plus suggestive clinical symptoms of meningitis. Children with early-onset iGBS disease were more likely to present with sepsis (71/80; 88·7%), whereas, 40·5% (32/79) and 59·5% (47/79) of the late-onset cases were GBS-meningitis and sepsis, respectively.
Figure 2.
Participant flow of iGBS cases and non-iGBS children recruited in multi-country study. Of the 159 iGBS survivors (43 South Africa, 39 Mozambique, 35 India, 29 Kenya, 13 Argentina) included in the country specific descriptive analyses (objective 1), 138 iGBS survivors were included to estimate pooled absolute and relative risk (43 South Africa, 33 Mozambique, 33 India, 29 Kenya).
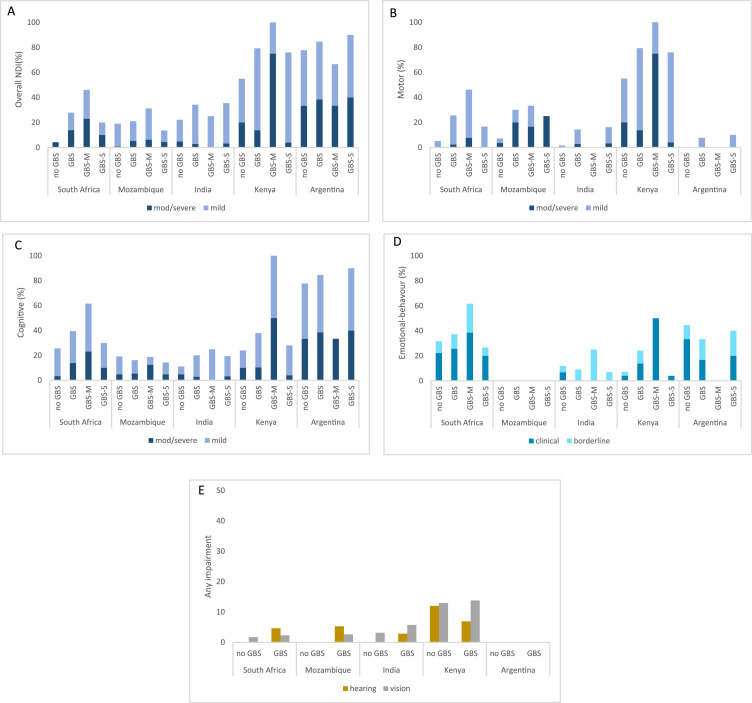
Risk of moderate/severe NDI among iGBS survivors overall varied by site: 2.9% in India (1/35), 3·4% in Kenya (3/29), 7·7% Mozambique (3/39), 13·9% in South Africa (6/43), and 23·1% Argentina (3/13;). Frequency of mild impairment also varied greatly between the sites, ranging from 18% in Mozambique to 61% in Argentina (Figure 3, Supplemental Table S3).
Figure 3.
Multi-domain and domain specific impairment among non-iGBS children, any iGBS, GBS-sepsis, and GBS-meningitis, stratified by country. Proportion of children with different NDI outcomes in South Africa, Mozambique, India, Kenya, and Argentina for GBS exposed and unexposed children. (A) Overall impairment (moderate/severe and mild). (B) Motor impairment (moderate/severe and mild).(C) Cognitive impairment (moderate/severe and mild). (D) Emotional-behavioural problem (any problem in clinical range). (E) Hearing and vision impairment (any impairment). GBS=group B Streptococcus. GBS-M=GBS meningitis. GBS-S=GBS sepsis. NDI=neurodevelopmental impairment.
Among GBS-sepsis survivors, moderate/severe NDI risk ranged from <5% in India (1/31; 3·2%) and Kenya (1/25; 4·2%) to 25% in Mozambique (1/4) (Figure 3, Table S3). A more limited number of children were recruited after GBS-meningitis (N=41). Similar to GBS-sepsis survivors, risk of moderate/severe NDI after iGBS-meningitis ranged from no moderate/severe impairment in India (N = 4) to 50% in Kenya (Figure 3, Table S3).
Objective 2: Risk of NDI and association with GBS
When combing data from four study sites (excluding Argentina), the pooled risk of moderate/severe NDI for all iGBS was 8·8% (95% CI: 4·4% – 14·5%) and risk of any NDI was nearly four times higher (38·1% (30·0% – 46·6%)), with varied age composition (Table 3). Inclusion of Argentina did not change the moderate/severe NDI risk 9·0% (3·5% -16·1%). Risk of moderate/severe NDI was 5·5% (0·0% - 16·0%) in younger than 5 year olds and 11·2% (5·3% - 18·5%) in those aged 5 and older. The pooled estimate of risk of moderate/severe NDI among GBS-sepsis was 6·9% (95% CI: 2·0% - 13·7%). GBS-meningitis had a 15·0% (3·4% - 30·8%) risk of moderate/severe NDI (Table 3).
Table 3.
Pooled estimates of neurodevelopmental outcomes and relative risk amongst survivors of iGBS in early infancy and matched non-GBS cohort.
| iGBS survivors |
Non-GBS cohort | Relative risk |
|||||
|---|---|---|---|---|---|---|---|
| GBS-meningitis | GBS-sepsis | Total iGBS | n | RR | aRR* | ||
| Total cohort | |||||||
| Moderate/severe neurodevelopmental impairment | 15·0% (3·4% - 30·8%) | 5·6% (1·5% - 13·7%) | 8·8% (4·4% – 14·5%) | 5·9% (3·7% - 8·6%) | 42 | 1·35 (0·73, 2·51) | 1.27 (0.65, 2.45) |
| Mild neurodevelopmental impairment | 23.9% (9.6% - 41.2%) | 27.9% (19.3% - 37.3%) | 27.6% (20.3 – 35.5%) | 12.9% (9.7% - 16.4%) | 106 | 1.84 (1.34, 2.53)⁎⁎ | 1.96 (1.41, 2.74)⁎⁎ |
| Any neurodevelopmental impairment | 45·7% (28·1% – 63·9%) | 35·2% (26·0% - 45·0%) | 38·1% (30·0% – 46·6%) | 21·7% (17·7% - 26·0%) | 148 | 1·69 (1·32, 2·17)⁎⁎ | 1.74 (1.34, 2.26)⁎⁎ |
| Any behavioural problems | 12·1 (1·7% - 27·5%) | 4·5% (0·8% - 10·2%) | 10·4% (5·5% - 16·5%) | 5·5% (3·3% - 8·1%) | 47 | 1·03 (0·58, 1·85) | 0.98 (0.55, 1.77) |
| Stunting | 1·2% (0·0% - 11·5%) | 6·2% (1·8% - 12·3%) | 6·0% (2·3% - 11·0%) | 8·5% (5·7% - 11·8%) | .. | .. | |
| < 5years | |||||||
| Moderate/severe neurodevelopmental impairment | 16·2% (0·0% - 56·3%) | 0·0% (0·0% - 7·5%) | 3·9% (0·0% – 13·9%) | 11·6% (5·5% - 19·4%) | 15 | 0·73 (0·23, 2·35) | 0.90 (0.17, 4.59) |
| Mild neurodevelopmental impairment | 21.5% (0.0% - 62.7%) | 43.5% (25.6% - 62.2%) | 40.9% (25.5% - 57.2%) | 9.2% (3.6% - 16.4%) | 27 | 4.50 (2.35, 8.60) | 3.95 (1.93, 8.06)⁎⁎ |
| Any neurodevelopmental impairment | 52·5% (13·2% – 90·5%) | 48·5% (30·2% - 67·0%) | 49·0% (33·0% – 65·2%) | 21·6% (13·4% – 30·9%) | 42 | 2·50 (1·62, 3·85)⁎⁎ | 2.41 (1.48, 3.91)⁎⁎ |
| Any behavioural problems | 0.0% (0.0% - 44.4%) | 3.5% (0.0% - 14.4%) | 3.5% (0.0% - 13.1%) | 11.2% (4.9% - 19.1%) | 7 | 0·38 (0·04, 3·42) | 0.26 (0.02, 2.96) |
| ≥ 5 years old | |||||||
| Moderate/severe neurodevelopmental impairment | 10·9% (0·2% - 29·2%) | 6·0% (0·8% - 13·9%) | 9·5% (3·9% – 16·8%) | 3·6% (1·6% - 6·3%) | 27 | 1·93 (0·92, 4·08)* | 1.94 (0.87, 4.35) |
| Mild neurodevelopmental impairment | 23.5% (6.7% - 44.4%) | 20.2% (10.9% - 31.1%) | 22.0% (13.8% - 31.2%) | 12.7% (9.0% -16.9%) | 79 | 1.37 (0.93, 2.03) | 1.78 (1.18, 2.71)⁎⁎ |
| Any neurodevelopmental impairment | 39·8% (19·4% – 61·7%) | 28·6% (18·0% - 40·5%) | 33·4% (24·0% – 43·5%) | 21·0% (16·4% - 25·9%) | 106 | 1·50 (1·10, 2·05)⁎⁎ | 1.81 (1.30, 2.52)⁎⁎ |
| Any behavioural problems | 23.3% (6.6% - 44.3%) | 18.5% (9.3% - 29.6%) | 15.9% (8.6% - 24.6%) | 8.9% (5.7% - 12.6%) | 40 | 1·18 (0·65, 2·14) | 1·15 (0·64, 2·08) |
RR=Relative risk adjusted for matching variables (age, sex) and study site. aRR= adjusted relative risk, adjusted for matching variables study site, caregiver education, and prematurity
significant at p<0·1
significant at p<0·05.
Among the non-GBS cohort, any NDI risk and moderate/severe NDI risk was high: 21·7% (17·7% - 26·0%) and 5·9% (3·7% - 8·6%), respectively (Table 3). Compared with the non-GBS cohort, children with a history of iGBS had a 70% increased risk of any NDI (adjusted risk ratio (aRR)=1·74 (95% CI: 1·34, 2·26)) after adjustment for prematurity and caregiver education. The aRR for moderate/severe NDI was 1·27 (0·65, 2·45). In children aged less than 5 years, there is a 2·4 increased risk of any NDI associated with iGBS disease (aRR=2·41 (1·48, 3.91)), but no association in moderate/severe NDI risk (aRR=0.90 (0·17, 4.59)). For children 5 years or older, there was an increase risk of any NDI (aRR=1.81 (1.30, 2.52)) among iGBS survivors compared to non-GBS comparators. For moderate/severe NDI, the aRR was 1.94 (0.87, 4.35).
Objective 3: Domain specific NDI and growth outcomes
Frequency of impairment varied by neurodevelopmental domain (Figure 3, Table 2). Cognitive impairment was more common among iGBS survivors (range 2.9 – 38.5%) than motor, hearing, and vision impairment in all the sites, except in India, where only mild cognitive impairment was detected. Hearing impairment was identified in all the study sites, except for in Argentina where hearing assessments could not be performed on all children due to COVID-19 related social distancing measures, and ranged from 2·8% in India to 6·9% in Kenya. Although in Mozambique hearing assessments were only performed in children for whom there was a clinical suspicion of hearing problems (n=3), two children with history of iGBS had moderate/severe impairment (2/39; 5.1%) and one child had mild hearing impairment (1/39; 2.6%). Vision impairment was infrequently diagnosed among iGBS survivors in this study, being detected only in India (2/35; 5·7%) and Kenya (2/25; 13·8%).
Emotional-behavioural problems were identified in iGBS survivors in Kenya (2/25; 6·95%), Argentina (2/13; 16·7%), and South Africa (11/43; 25·6%). A high proportion of children in the non-GBS group had any behavioural problems (Figure 3, Table S3). In India, although no emotional-behavioural problems were identified among the iGBS survivors, 6·5% (4/63) of the non-GBS cohort were identified with behavioural problems. In Mozambique, no emotional-behavioural problems were identified in either the exposed or unexposed cohorts (Figure 3, Table S3). In the combined analysis, emotional-behaviour problems were similar between iGBS survivors and non-GBS children (aRR=1·02 (0·57, 1·83)) (Table 3).
Frequencies of stunting and moderate/severe malnutrition were similar between the iGBS and the non-GBS cohort, but varied between the five sites (Table 3, Table S3). Less than 5% of the children in South Africa were moderate/severe malnourished (4/160)), while 17% of the children in Kenya (19/107) were malnourished.
Discussion
Our study addresses three important data gaps regarding the burden of iGBS disease:9 (1) long-term outcomes after iGBS-sepsis; (2) risk of milder outcomes, which become more apparent later in childhood; (3) paucity of data in LMIC, despite these countries having the majority of the iGBS burden. This first LMIC-based multi-country study of longer term outcomes after iGBS found NDI was more frequent among iGBS survivors.
We identified few children with hearing impairments, however not all the sites were able to assess all children with sensitive hearing tests, so these results may underestimate the frequency of hearing loss. Frequencies of emotional-behavioural problems based on standardised CBCL were similar in children with or without history of iGBS and highly variable between sites. Notably, no study participant in Mozambique was diagnosed with emotional-behavioural problems, possibly reflecting the cultural interpretation of behavioural constructs within the CBCL and the need for further context-specific adaptation.
After GBS-meningitis, we estimated a 15% risk of moderate/severe NDI, which is comparable to previously reported studies, including a recent registry-based study undertaken in Demark and the Netherlands that reported 10-15% risk of moderate/severe NDI in GBS-meningitis children aged 10 years. Although the number of iGBS-meningitis survivors in the combined dataset was small (N=41), it is similar to the total sample size for the middle-income countries reported in the review and meta-analysis performed by Kohli-Lynch et al. (N=38).9
Among iGBS-sepsis survivors, we estimated 5% risk of moderate/severe NDI, with considerable between-site heterogeneity. This risk is similar to the 5% reported in Denmark and 8% in Netherlands after iGBS sepsis by the age of 10 years old. Although risk of NDI after GBS-sepsis is lower than risk after meningitis, GBS-sepsis is more common than meningitis, and therefore is likely to contribute more to the total GBS-associated NDI burden.
Milder developmental impairment and emotional-behavioural findings were high for the iGBS exposed cohort (27%) and the comparison cohort (13%), but varied markedly by study site. This aligns with the estimate that 40% of children, with up to 60% in sub-Saharan Africa, will not reach developmental potential by 5 years of age.5 Variability in the background risks highlight the importance of a matched counterfactual to quantify excess risk of NDI due to specific exposures, such as iGBS disease.
A strength of our study is that it was specifically designed to address the paucity of NDI data after iGBS disease in LMIC settings. In particular, this multi-country study represents three geographic regions (Latin America, Africa, Asia), implemented standardised training, applied the same tools/assessments in multiple study sites when possible (e.g., CBCL, WPPSI), and used a customizable data capture app. We included an unexposed comparison group; the importance of which is underscored by the variable background risk between-sites, which might reflect true differences in baseline NDI risk and underlines the need for robust comparison cohorts in future studies. This study also has limitations. Exposure to iGBS disease in this study was assessed retrospectively and, therefore, we cannot rule out misclassification of exposure for some children. Meningitis is underdiagnosed, especially in neonates who may not have localising signs, and hence lumbar punctures are meant to be done for all suspected cases. If bacteraemia is detected but lumbar puncture is not performed, misclassification might occur from true meningitis to being considered to be sepsis. This misclassification is more common in low income/resource-limited settings and for very sick neonates where lumbar puncture may not be done, or done after antibiotic administration. Longer term cohorts with standard diagnostic criteria of GBS exposure and prospective definition of exposure status are needed. Of note, when assessing the relative risk of the different NDI outcomes, data from GBS sepsis and meningitis cases were combined. Furthermore, the comparison group sample size was smaller than expected due to recruitment challenges during the COVID-19 pandemic. While this study addresses key data gaps, children enrolled might not be representative of all iGBS survivors and selection bias might have occurred especially in South Africa, where a higher proportion could not be contacted. However, analysing key demographic and health history variables, there were no statistical difference between the South African children who enrolled in the study and those who were not reachable.13 In Argentina, only a small number could to be contacted during the pandemic, and for this reason children from this site were not included in pooled analyses. We were unable to adjust for HIV-exposure or HIV status, which are risks for NDI27,28, due to limited data. In South Africa where HIV-exposure data was available,29 iGBS survivorship was associated with increased NDI even after controlling for HIV-exposure.13 Finally, we adjusted for prematurity as a potential confounder,30, 31, 32 however we note that gestational age determination in our study was largely measured using last menstrual period, which is less accurate33 and tends to overestimate prematurity.34 Of note, complete case analysis, where only participants with information on gestational age variable were included, was performed and similar results were obtained (data not shown). Comparable measurement of NDI in different populations is challenging. There is a plethora of available assessment tools (>100 in LMIC) and many of the widely used tools are expensive, complex to use, and reporting of impairment often omits important domains, especially hearing and vision.35 Additionally, many have not been adapted formally for LMIC context and diverse cultural setting thus limiting their use in different populations. Whilst attempts were made to use comparable NDI assessment in this study, there were 26 different tools used across sites due to local practices and expertise, and hence comparability remains limited and may be further affected by instrument validity, technical capacity and cultural interpretation. We undertook domain mapping of assessment tools to better align measurement equivalence and comparability of the NDI outcomes between different ages, assessment tools and sites, before individual-level data from each site were further combined for analysis. However, in Mozambique, detailed motor assessment in older children was not possible due to unavailability of population standard for children under 18 years of age and no formal screening of hearing and vision were performed, which may have underestimated mild to moderate impairment in this site. In Argentina, the use of clinical motor assessment is rather different and less sensitive compared to the tools used in the other sites that provided detailed evaluation of various motors skills. Cognitive tests in Kenya only measure non-verbal skills and therefore may have underestimated cognitive impairments in the population as language was not included.
Our findings demonstrate a higher than previously recognised burden of both mild and moderate/severe NDI in iGBS survivors with implications for both public health programmes and research. Implementation research is needed to develop context-specific approaches to follow-up developmentally at-risk newborns in existing child health programmes. Our findings of higher risk of mild NDI identified in children 5 years and older underlines the need for follow-up to school age. To ensure more accurate identification of children with NDI there is a need for a toolkit of standardised assessments that assess all domains, can be freely used and are easily adapted for different context. WHO's new Global Scale for Early Development (GSED) aims to enable comparable monitoring of childhood development for children less than 3 year olds and the Early Childhood Development Index targets 3-5 year olds. However, these tools miss older, school-ages and omit vision and hearing.
Our findings show that long-term impairment after iGBS disease is frequent, confirming previous findings after GBS-meningitis. Importantly among GBS-sepsis survivors, our new data show that moderate and/or severe NDI is also high in LMIC. Given that sepsis is the most common iGBS clinical presentation, these results should be considered in disease burden estimates and cost-effectiveness analyses of preventative approaches. Whilst our estimate of risk ratio for moderate/severe NDI outcome is uncertain, our multivariate analyses suggest an increased risk of any and moderate/severe NDI in iGBS survivors compared to unexposed children. Additionally, there was marked heterogeneity in NDI risk between sites. To increase the robustness of these findings, more multi-site studies are needed, especially in LMIC given the highest burden is in these settings.
To inform both clinical practice and public health policy, there is a need for standardised developmental assessment tests that are more feasible for routine use, freely accessible and adaptable to context. Importantly hearing and vision domains should be included, and the age range needs to extend beyond early childhood (0-3 years). Better detection also needs to link to better healthcare and education for these children and support of their families, including context-specific implementation research.
Funding
This work was supported by a grant (INV-009018) from the Bill & Melinda Gates Foundation to the London School of Hygiene &Tropical Medicine.
Contributors
JEL conceived the idea, obtained the funding and oversaw the process. PP was responsible for the oversight of the LMIC studies, design of the app and analyses of the NDI data. JC oversaw the NDI tool assessment mapping, design and use of the app and training for new tools as well as inputting to the NDI analyses. JEL, PP, SP, MJ, ZD, CRN, AA, SS, QB, AB, and RL developed the multi-site protocol. ZD and SL provided NDI risk data from South Africa; SS and HBJ provided NDI risk data from India; AA and CN provided risk data from Kenya; RL and CSY provided data from Argentina; QB, JB and AB provided data from Mozambique. PP conducted analysis with JC and FS; FS provided data management. AKMTH and QSR developed the data collection app and provided data management support. EHP and BG input conceptually to the analyses and EHP provided statistical oversight. PP prepared the first draft of the manuscript with JC, BG, and JEL; KM provided significant input on initial draft. PP and JEL had final responsibility for the decision to submit for publication. All authors provided input to the paper, reviewed the manuscript, and approved the final version.
Data sharing statements
Data jointly owned by all the teams based on a jointly signed data sharing agreement. The tools and materials used in this study will be available at time of publication. Datasets are available from the corresponding author on request.
Members of GBS long term outcomes LMIC collaborative group
Shabir A. Madhi (Medical Research Council: Vaccines and Infectious Diseases Analytical Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa; Department of Science and Technology/National Research Foundation: Vaccine Preventable Diseases, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa), Ziyaad Dangor (Medical Research Council: Vaccines and Infectious Diseases Analytical Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa), Shannon Leahy (Department of Paediatrics and Child Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa), Lois Harden (Brain Function Research Group, School of Physiology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa), Azra Ghoor (Department of Paediatrics and Child Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa), Sibongile Mbatha (Department of Paediatrics and Child Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa), Sarah Lowick (Department of Paediatrics and Child Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa), Barbara Laughton (Department of Paediatrics and Child Health, Stellenbosch University, Tygerberg, Western Cape, South Africa), Tamara Jaye (Department of Paediatrics and Child Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa), Sanjay G Lala (Department of Paediatrics and Child Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa), Pamela Sithole (Medical Research Council: Vaccines and Infectious Diseases Analytical Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa), Jacqueline Msayi (Medical Research Council: Vaccines and Infectious Diseases Analytical Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa), Ntombifuthi Kumalo (Medical Research Council: Vaccines and Infectious Diseases Analytical Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa), Tshepiso Nompumelelo Msibi (Medical Research Council: Vaccines and Infectious Diseases Analytical Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa), Sridhar Santhanam (Department of Neonatology, Christian Medical College, Vellore, India), Hima B. John (Department of Neonatology, Christian Medical College, Vellore, India), Asha Arumugam (Department of Neonatology, Christian Medical College, Vellore, India), Nandhini Murugesan (Department of Neonatology, Christian Medical College, Vellore, India), Nandhini Rajendraprasad (Department of Neonatology, Christian Medical College, Vellore, India), Mohana Priya (Department of Neonatology, Christian Medical College, Vellore, India), Amina Abubakar (Neuroscience Research Group, Department of Clinical Sciences, KEMRI-Wellcome Trust, Kilifi, Kenya; Institute for Human Development, Aga Khan University, Nairobi, Kenya), Carophine Nasambu (Neuroscience Research Group, Department of Clinical Sciences, KEMRI-Wellcome Trust, Kilifi, Kenya), Adam Mabrouk Adan (KEMRI/Wellcome Trust Research Programme, Kilifi, Kenya), Patrick Vidzo Katana (KEMRI/Wellcome Trust Research Programme, Kilifi, Kenya), Eva Mwangome (KEMRI/Wellcome Trust Research Programme, Kilifi, Kenya), Charles R. Newton (Department of Psychiatry, Medical Sciences Division, University of Oxford, Oxford, UK; KEMRI/Wellcome Trust Research Programme), Quique Bassat (Centro de Investigação em Saúde de Manhiça, Maputo, Mozambique; ISGlobal, Hospital Clínic, Universitat de Barcelona, Barcelona, Spain; ICREA, Pg. Lluís Companys 23, 08010 Barcelona, Spain; Pediatrics Department, Hospital Sant Joan de Déu [University of Barcelona], Barcelona, Spain; Consorcio de Investigación Biomédica en Red de Epidemiología y Salud Pública [CIBERESP], Madrid, Spain), Azucena Bardají (Centro de Investigação em Saúde de Manhiça, Maputo, Mozambique; ISGlobal, Hospital Clínic, Universitat de Barcelona, Barcelona, Spain), Justina Bramugy (Centro de Investigaçção em Saúde de Manhiça, Maputo, Mozambique), Humberto Mucasse (Centro de Investigação em Saúde de Manhiça, Maputo, Mozambique), Celine Aerts (ISGlobal, Hospital Clíínic, Universitat de Barcelona, Barcelona, Spain), Sergio Massora (Centro de Investigação em Saúde de Manhiça, Maputo, Mozambique), Romina Libster (Fundación INFANT, Buenos Aires, Argentina), Clara Sánchez Yanotti (Fundación INFANT, Buenos Aires, Argentina), Valeria Medina (Instituto de Maternidad y Ginecología Nuestra Señora de las Mercedes, Argentina), Andrea Rojas (Instituto de Maternidad y Ginecología Nuestra Señora de las Mercedes, Argentina), Daniel Amado (Instituto de Maternidad y Ginecología Nuestra Señora de las Mercedes, Argentina), Conrado J. Llapur (Instituto de Maternidad y Ginecología Nuestra Señora de las Mercedes, Argentina), A. K. M. Tanvir Hossain (Maternal and Child Health Division, International Centre for Diarrhoeal Disease Research, Bangladesh [icddr,b], Dhaka, Bangladesh), Qazi Sadeq-ur Rahman (Maternal and Child Health Division, International Centre for Diarrhoeal Disease Research, Bangladesh [icddr,b], Dhaka, Bangladesh).
Declaration of interests
SRP reports additional finding from Bill & Melinda Gates Foundation on Covid-19 related projects. ZD and SL report subawards from the London School of Hygiene & Tropical Medicine (LSHTM) to the Wits Health Consortium. SS and HBJ report subawards from LSHTM to Christian Medical College. QB and AB reports subawards from LSHTM to ISGlobal and Centro de Investigação em Saúde de Manhiça. AA and CN reports subawards from the LSHTM to the University of Oxford. CRN. reports subawards from LSHTM to Kenya Medical Research Institute, Kilifi, Kenya. CSY reports subawards from LSHTM to Fundación INFANT. RL reports subawards from LSHTM to Fundación INFANT. a grant from Program for Appropriate Technology in Health for a respiratory syncytial virus (RSV) costing study, grants to Fernando Polack from the Bill & Melinda Gates Foundation for estimating the RSV burden of disease, consulting fees and participating for serving on Pfizer’s GBS advisory board and Janssen’s RSV advisory board, payment or honoraria for Janssen’s RSV lecture and Merck’s human papillomavirus lecture, and stock or stock options from iTRIALS. FS reports non-financial support from The Federal University of São Paulo for submitted work and employment by the UK NSC hosted by the Department of Health, who developed the maternal GBS screening policy recommendation in the UK. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Acknowledgements
Firstly, and most importantly we thank the women and children participating in the GBS study and their families, without whom this work would not have been possible. We thank all the site study staff for their hard work. Many thanks to Claudia da Silva for administrative support. We express appreciation to the GBS scientific advisory group, and we are particularly grateful to the project officer at the Bill & Melinda Gates Foundation, Ajoke Sobanjo-ter Meulen for her insightful support and advice throughout this work. The findings and conclusions in this report are those of the authors, and do not necessarily represent the official position of any of the agencies or organisations listed.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2022.101358.
Contributor Information
Proma Paul, Email: proma.paul@lshtm.ac.uk.
GBS long term outcomes LMIC collaborative group:
Shabir A. Madhi, Ziyaad Dangor, Shannon Leahy, Lois Harden, Azra Ghoor, Sibongile Mbatha, Sarah Lowick, Barbara Laughton, Tamara Jaye, Sanjay G Lala, Pamela Sithole, Jacqueline Msayi, Ntombifuthi Kumalo, Tshepiso Nompumelelo Msibi, Sridhar Santhanam, Hima B. John, Asha Arumugam, Nandhini Murugesan, Nandhini Rajendraprasad, Mohana Priya, Amina Abubakar, Carophine Nasambu, Adam Mabrouk Adan, Patrick Vidzo Katana, Eva Mwangome, Charles R. Newton, Quique Bassat, Azucena Bardají, Justina Bramugy, Humberto Mucasse, Celine Aerts, Sergio Massora, Romina Libster, Clara Sánchez Yanotti, Valeria Medina, Andrea Rojas, Daniel Amado, Conrado J. Llapur, A.K.M. Tanvir Hossain, and Qazi Sadeq-ur Rahman
Supplementary materials
References
- 1.Barbosa NG, Dos Reis H, Mantese OC, Mussi-Pinhata MM, Abdallah VO, Gontijo Filho PP. Early-onset neonatal sepsis by Group B Streptococcus in a Brazilian public hospital. Braz J Infect Dis. 2016;20(6):647–648. doi: 10.1016/j.bjid.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wertlieb D. Nurturing care framework for inclusive early childhood development: opportunities and challenges. Dev Med Child Neurol. 2019;61(11):1275–1280. doi: 10.1111/dmcn.14234. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . WHO; Geneva: 2015. Global Strategy for Women's, Children's and Adolescents' Health 2016–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black MM, Behrman JR, Daelmans B, et al. The principles of Nurturing Care promote human capital and mitigate adversities from preconception through adolescence. BMJ Glob Health. 2021;6(4) doi: 10.1136/bmjgh-2020-004436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu C, Black MM, Richter LM. Risk of poor development in young children in low-income and middle-income countries: an estimation and analysis at the global, regional, and country level. The Lancet Global Health. 2016;4(12):e916–ee22. doi: 10.1016/S2214-109X(16)30266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Institution of Health Metrics and Evaluation. Global Burden of Disease (GBD). http://www.healthdata.org/gbd/2019. Accessed 27 September 2021.
- 7.Hutubessy RCW, Lauer JA, Giersing B, et al. The Full Value of Vaccine Assessments (FVVA): A Framework to Assess and Communicate the Value of Vaccines for Investment and Introduction Decision Making (May 7, 2021). SSRN; 2021. [DOI] [PMC free article] [PubMed]
- 8.Seale AC, Bianchi Jassir F, Russell N, et al. Estimates of the burden of Group B Streptococcal disease worldwide for pregnant women, stillbirths and children. Clinical Infectious Diseases. 2017 doi: 10.1093/cid/cix664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohli-Lynch M, Russell N, Seale AC, et al. Neurodevelopmental impairment in children after Group B Streptococcus disease worldwide: systematic review and meta-analyses. Clinical Infectious Diseases. 2017 doi: 10.1093/cid/cix663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horváth-Puhó E, Kassel MNv, Gonçalves BP, et al. Mortality, neurodevelopmental impairments, and economic outcomes after invasive group B streptococcal disease in early infancy in Denmark and the Netherlands: a national matched cohort study. The Lancet Child & Adolescent Health. 2021;5(6):398–407. doi: 10.1016/S2352-4642(21)00022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aerts C, Leahy S, Mucasse H, et al. Quantifying the acute costs of neonatal bacterial sepsis and meningitis in Mozambique and South Africa. Clinical Infectious Diseases. 2021 doi: 10.1093/cid/ciab815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul P, Procter SR, Dangor Z, et al. Quantifying long-term health and economic outcomes for survivors of group B Streptococcus invasive disease in infancy: protocol of a multi-country study in Argentina, India, Kenya, Mozambique and South Africa. Gates Open Res. 2020;4:138. doi: 10.12688/gatesopenres.13185.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harden LM, Leahy S, Lala SG, et al. South African children: A matched cohort study of neurodevelopmental impairment in survivors of invasive Group B Streptococcus disease aged 5- to 8-years. Clinical Infectious Diseases. 2021 doi: 10.1093/cid/ciab814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bramugy J, Mucasse H, Massora S, et al. Short and long-term outcomes of GBS invasive disease in Mozambican children: Results of a matched cohort and retrospective observational study and implications for future vaccine introduction. Clinical Infectious Diseases. 2021 doi: 10.1093/cid/ciab793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.John HB, Arumugam A, Priya M, et al. South Indian children's neurodevelopmental outcomes after Group B Streptococcus invasive disease: A matched cohort study. Clinical Infectious Diseases. 2021 doi: 10.1093/cid/ciab792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bastawrous A. Increasing access to eye care ... there's an app for that. Peek: smartphone technology for eye health. Int J Epidemiol. 2016;45(4):1040–1043. doi: 10.1093/ije/dyw086. [DOI] [PubMed] [Google Scholar]
- 17.Achenbach T, Rescorla L. Manual for the ASEBA school-age forms & profiles: an integrated system of multi-informant assessment, 2001.
- 18.Achenbach T, Rescorla L. University of Vermont, Research Center for Children, Youth & Families; Burlington, VT: 2000. Manual for the ASEBA preschool forms & profiles: an integrated system of multi-informant assessment; child behavior checklist for ages 1 1/2-5; language development survey; caregiver - teacher report form. [Google Scholar]
- 19.Chandna J, Liu W-H, Dangor Z, et al. Emotional and behavioural outcomes in childhood for survivors of Group B Streptococcus invasive disease in infancy: findings from five LMIC. Clinical Infectious Diseases. 2021 doi: 10.1093/cid/ciab821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blencowe H, Vos T, Lee AC, et al. Estimates of neonatal morbidities and disabilities at regional and global levels for 2010: introduction, methods overview, and relevant findings from the Global Burden of Disease study. Pediatr Res. 2013;74(Suppl 1):4–16. doi: 10.1038/pr.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO | Grades of hearing impairment. 2022
- 22.Vision impairment and blindness. 2022
- 23.World Health Organization. Malnutrition in children. 2022
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Yu KF. What's the Relative Risk? A Method of Correcting the Odds Ratio in Cohort Studies of Common Outcomes. JAMA. 1998;280(19) doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 27.Wedderburn CJ YS, Rehman AM, Stadler JAM, Nhapi RT, Barnett W, Myer L, Gibb DM, Zar HJ, Stein DJ, Donald KA. Neurodevelopment of HIV-exposed uninfected children in South Africa: outcomes from an observational birth cohort study. Lancet Child Adolesc Health. 2019;3(11):803–813. doi: 10.1016/S2352-4642(19)30250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ntozini R CJ, Evans C, Chasekwa B, Majo FD, Kandawasvika G, Tavengwa NV, Mutasa B, Mutasa K, Moulton LH, Humphrey JH, Gladstone MJ, Prendergast AJ. SHINE Trial Team. Early child development in children who are HIV-exposed uninfected compared to children who are HIV-unexposed: observational sub-study of a cluster-randomized trial in rural Zimbabwe. J Int AIDS Soc. 2022;23(5):e25456. doi: 10.1002/jia2.25456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dangor Z, Lala SG, Cutland CL, et al. Burden of Invasive Group B Streptococcus Disease and Early Neurological Sequelae in South African Infants. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0123014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mwaniki MK, Atieno M, Lawn JE, Newton CR. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet. 2012;379(9814):445–452. doi: 10.1016/S0140-6736(11)61577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez de Gamarra-Oca L, Ojeda N, Gomez-Gastiasoro A, et al. Long-Term Neurodevelopmental Outcomes after Moderate and Late Preterm Birth: A Systematic Review. J Pediatr. 2021 doi: 10.1016/j.jpeds.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Hee Chung E, Chou J, Brown KA. Neurodevelopmental outcomes of preterm infants: a recent literature review. Transl Pediatr. 2020;9(Suppl 1):S3–S8. doi: 10.21037/tp.2019.09.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haider MM, Mahmud K., Blencowe H., et al. Gestational age data completeness, quality and validity in population-based surveys: EN-INDEPTH study. Popul Health Metrics. 2021;16 doi: 10.1186/s12963-020-00230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ambrose CS, Caspard H, Rizzo C, Stepka EC, Keenan G. Standard methods based on last menstrual period dates misclassify and overestimate US preterm births. J Perinatol. 2015;35(6):411–414. doi: 10.1038/jp.2015.25. [DOI] [PubMed] [Google Scholar]
- 35.Boggs D, Milner KM, Chandna J, et al. Rating early child development outcome measurement tools for routine health programme use. Arch Dis Child. 2019;104(Suppl 1):S22–S33. doi: 10.1136/archdischild-2018-315431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.