Abstract
Background:
Alzheimer’s disease (AD) is the most common form of dementia worldwide, with approximately 6 million cases reported in America in 2020. The clinical signs of AD include cognitive dysfunction, apathy, anxiety and neuropsychiatric signs, and pathogenetic mechanisms that involve amyloid peptide-β extracellular accumulation and tau hyperphosphorylation. Unfortunately, current drugs to treat AD can provide only symptomatic relief but are not disease-modifying molecules able to revert AD progression. The endogenous modulator adenosine, through A2A receptor activation, plays a role in synaptic loss and neuroinflammation, which are crucial for cognitive impairment and memory damage.
Objective:
In this review, recent advances covering A2A adenosine receptor antagonists will be extensively reviewed, providing a basis for the rational design of future A2A inhibitors.
Methods:
Herein, the literature on A2A adenosine receptors and their role in synaptic plasticity and neuroinflammation, as well as the effects of A2A antagonism in animal models of AD and in humans, are reviewed. Furthermore, current chemical and structure-based strategies are presented.
Results:
Caffeine, the most widely consumed natural product stimulant and an A2A antagonist, improves human memory. Similarly, synthetic A2A receptor antagonists, as described in this review, may provide a means to fight AD.
Conclusion:
This review highlights the clinical potential of A2A adenosine receptor antagonists as a novel approach to treat patients with AD.
Keywords: Alzheimer’s disease, A2A receptors, A2A antagonists, cognitive impairment, drug design, neuroinflammation
1. INTRODUCTION
Alzheimer’s disease (AD), an age-associated pathology, is one of the principal causes of dementia in the elderly and the fifth main cause of death in patients in the ≥65 year age group. The number of people in America suffering from AD reached an impressive 5.8 million in 2020 and is expected to triple by 2050 [1]. Two types of AD have been described that include sporadic and familial forms, presenting late and early onset, respectively, with the latter being responsible for less than 1% of cases [1]. In the case of sporadic AD, the principal risk factor is age, followed by an ApoE-ε4 genetic polymorphism.
In familial AD, mutations affecting amyloid precursor protein (APP), and presenilin 1 and 2 (PS1 and PS2) genes, provoke beta-amyloid peptide (Aβ) accumulation and plaque formation with a more rapid pathological development [2]. The clinical signs of AD include cognitive dysfunction, apathy, anxiety and neuropsychiatric signs [3, 4]. The pathogenetic mechanisms of AD involve Aβ extracellular accumulation and hyperphosphorylation of tau protein [5]. Aβ peptides originate from amyloid precursor protein (APP) following sequential cleavage by β- and γ-secretases [2, 6]. According to the amyloid hypothesis, the Aβ plaque generation is the main cause of AD, inducing neurotoxicity and synaptic dysfunction, and it is responsible for memory decline [7]. During the pathological progression, Aβ aggregation increases phosphorylation of tau, the most important microtubule-related protein, playing a crucial role in microtubule formation and being relevant for neuronal plasticity and axonal outgrowth [8, 9]. Hyperphosphorylated tau protein is released from microtubules and produces intracellular, neurotoxic, and insoluble neurofibrillary tangles (NFT-s) [10]. All of these processes induce neuronal death and neuroinflammation, the major hallmarks of AD [11]. Approved drugs for AD primarily increase acetylcholine (ACh) transmission as well as reduce glutamate excitotoxicity, and comprise donepezil, rivastigmine, galantamine and memantine [12, 6]. However, these drugs can induce beneficial effects in the short term but are not able to stop or reverse AD progression. Furthermore, these drugs have various liabilities upon long-term use. The production of effective disease-modifymg drugs able to impede or cure AD appears to be a difficult goal to reach, as demonstrated by the numerous molecules that entered but eventually failed in clinical trials [4, 13]. Recently, a novel antibody decreasing extra-neuronal Aβ protein accumulation in the brain, called aducanumab, has been approved by the US Food and Drug Administration (FDA) [14]. However, the utility of this therapy is under debate due to the conflicting results of the two clinical trials, EMERGE and ENGAGE, carried out to test its efficacy in patients with mild cognitive impairment (MCI) and early dementia. There is considerable skepticism from the scientific community regarding this new and expensive Biogen drug, aducanumab [15–17]. Overall, the management of AD has become a global concern due to a worldwide increase in life expectancy [1]. Unfortunately, AD incidence, prevalence and mortality, as well as the costs of patient care, and its influence on society, are dramatically increasing [1]. Therefore, in the context of exploring new therapies to treat AD, in this review, we will discuss the potential involvement of the A2A adenosine receptor in this pathology, leading to the suggestion that A2A antagonists might be a strategic approach to discover new drugs.
2. A2A ADENOSINE RECEPTORS IN AD
A2A receptors belong to the P1 purinergic receptor family of four G protein-coupled receptors (GPCRs) that respond to the endogenous modulator adenosine, comprising A1, A2B and A3 subtypes [18]. One structural characteristic of the A2A subtype not shared by other receptor family members is a long intracellular carboxy terminal tail, where phosphorylation and palmitoylation processes may induce receptor desensitization and internalization [19]. This subtype is able to associate with different receptors, such as A1 adenosine and D2 dopamine receptors, to form heteromers having distinct pharmacological characteristics with respect to monomers [20]. The A2A receptor is distributed in both the central nervous system and the periphery. The highest expression is in the striatum, the olfactory tubercle, the immune system, and at lower levels in the cerebral cortex, hippocampus, heart, lung, and vasculature [21].
At the neuronal presynaptic level, the main effect of A2A receptor activation is to increase glutamate release, thus contributing to excitotoxicity. Postsynaptically, the A2A receptor promotes alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) activation and N-methyl-D-aspartate (NMDA) phosphorylation mGluR5-dependent (Fig. 1). In astrocytes, the A2A receptor provokes activation, proliferation and reduction of glutamate uptake, by regulating the expression of glutamate transporters GLT-1 and GLAST. In microglia, its activation is responsible for proliferation and the release of inflammatory mediators [22]. In the peripheral immune system, the A2A receptor is present at high levels in almost all immune cells, e.g., neutrophils, monocytes, macrophages, dendritic and T cells, as well as platelets, and blood vessels, where it produces important non-redundant anti-inflammatory, antiaggregatory, and vasodilatory actions, respectively [23]. A2A receptor stimulation triggers activation of Golf proteins in the striatum and Gs, proteins in both the brain and the periphery. Thus, it is responsible for increasing cyclic AMP and PKA phosphorylation, as well as regulation of downstream Akt and MAPK pathways [24–29]. Due to the widespread distribution of the A2A receptor and the increase of its endogenous ligand in both inflammation and cancer, it affects diverse pathologies spanning neurodegenerative, autoimmune, inflammatory and malignant diseases. Specifically, adenosine controls multiple processes in the brain, from sleep to seizures, and cognitive and memory functions, through A1 and A2A receptor activation to modulate the activity of excitatory glutamatergic synapses [30].
Fig. (1).
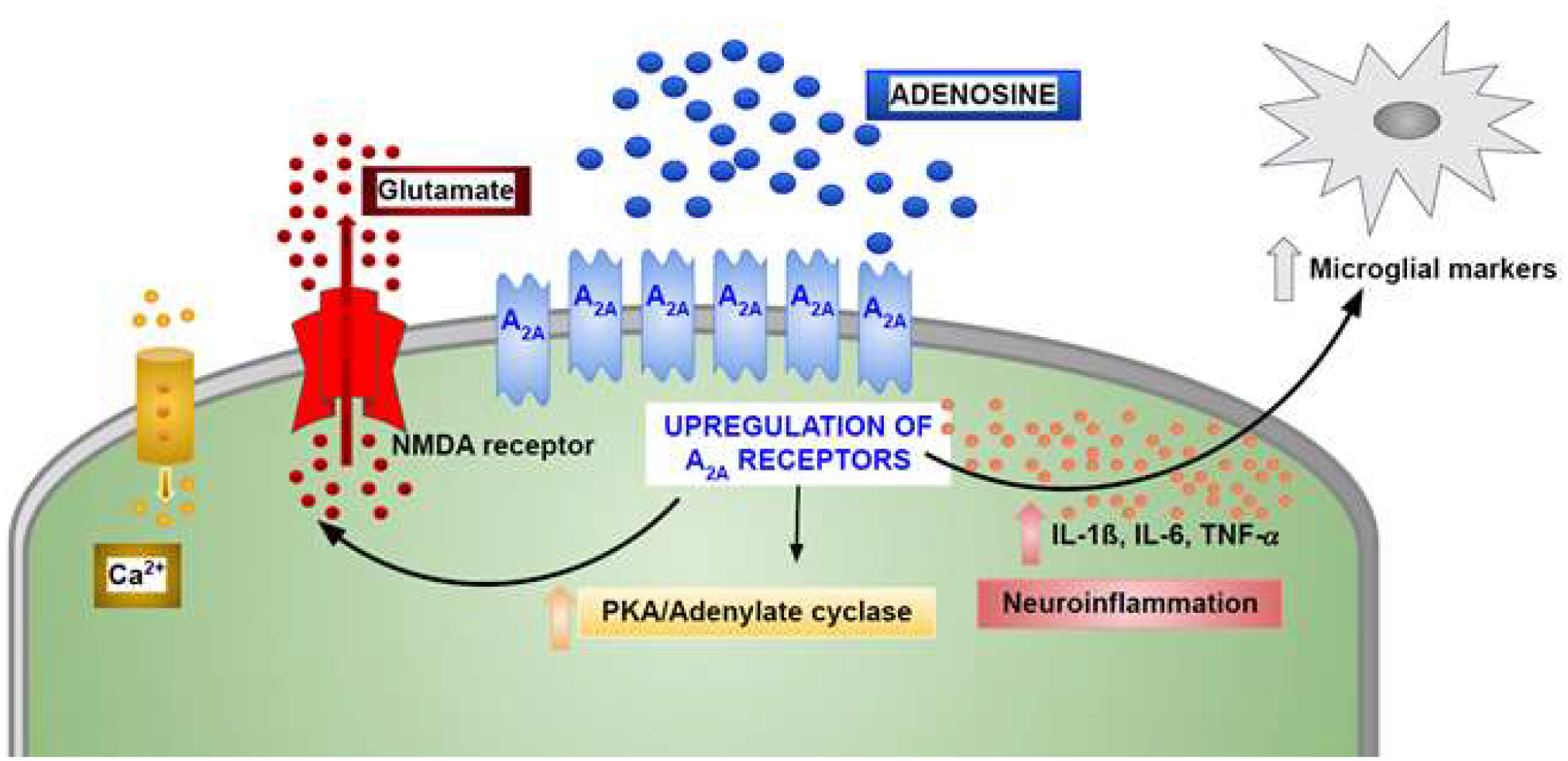
Schematic overview of A2a receptor signaling involved in brain-related mechanisms of AD pathology.
Under basal activity, adenosine activates the A1 receptor, inducing a reduction in hippocampal glutamate signaling. However, it may also stimulate presynaptic A2A receptors, which remove the A1-mediated inhibition, thus increasing glutamate release [31]. At the postsynaptic level, the A2A receptor facilitates long-term potentiation (LTP) by regulating NMDA receptor activation and intracellular Ca2+ [22, 32–35]. It is well known that synaptic dysfunction and degeneration in the temporal lobe represents the first hallmark of cognitive disability, prior to Aβ plaque and tangle formation [36]. Indeed, patients with MCI and early AD present a loss of synapses in the hippocampus and in the posterior cingulate gyrus, suggesting that this event represents an initial point leading to memory damage [37–39].
Due to the involvement of the A2A adenosine receptor in glutamatergic synaptic physiology, a link between this subtype and AD has been revealed It has been reported that in aging, AD animal models and AD patients, A2A adenosine receptor expression is increased in both hippocampal neurons and astrocytes [30, 40–46], thus raising glutamate release [47, 48], calcium influx, long-term potentiation (LTP)-to- long-term depression (LTD) shift [49], and cognitive impairment [50]. Of historical interest, the first indication that the A2A receptor might be increased in the Alzheimer’s brain was evident m a comparison of specific A2A receptor photoaffinity labeling in post--mortem striatal membranes [51]. In contrast, A2A receptor antagonism attenuates hippocampus-dependent memory disabilities and LTP alterations in aged animals [52, 53] and AD models [54–57]. In addition, genetic silencing of A2A adenosine receptors can ameliorate synaptic damage present in AD models [58–60]. Interestingly, adenosine levels in postmortem AD brains are greater in the parietal and temporal lobes compared to the frontal cortex, suggesting increased A2A receptor stimulation in overexpressing regions [61]. The level of A2A receptor expression in human correlates with disease states, and thus, it is considered a biomarker reflective of susceptibility and progression of brain diseases [62]. The correlation of single nucleotide polymorphisms (SNPs) of the A2A receptor with neuropsychiatric and neurodegenerative disorders has been documented [62].
Another important A2A adenosine receptor function involved in AD concerns its modulation of neuroinflammation through its effects on glial cells [63, 64]. In particular, astrocytes of AD patients displayed elevated levels of the A2A adenosine receptor, and its genetic knockdown in young and aging mice increased long-term memory [44]. Functionally, A2A receptors in astrocytes were essential for the fine-tuning of inhibitory and excitatory modulation of synaptic transmission by affecting GABA and glutamate uptake. Specifically, A1 and A2A receptors, present as heteromers, regulated GABA transport in an opposite fashion, with the A1 inhibiting and the A2A promoting it [65]. In addition, the A2A receptor, which is essential for the reduction of glutamate transporters GLAST and GLT-I induced by Aβ peptide, impaired glutamate uptake [66]. A2A receptor upregulation in primary cortical astrocytes was recently reported to alter the astrocytic transcriptome with an important effect on genes relevant for inflammation and angiogenesis [67]. In addition to astrocytes, cells relevant to neuroinflammation that overexpress the A2A receptor are activated microglia, which also express another AD target receptor, i.e., the NMDA receptor. It has been reported that A2A and NMDA receptor subtypes interact, producing a novel entity, overexpressed in hippocampal cells from the APPSw, Ind mice, characterized by cross-antagonism, where A2A receptor antagonism may hamper the overstimulation of NMDA receptor activity [68, 69]. In addition, this approach to counter A2A receptor-mediated cytokine release may attenuate neuroinflammation to ameliorate memory dysfunction [70, 71]. Several Works have reported the relevance of A2A adenosine receptor antagonists for the restoration of function following the synaptic loss and cognitive disability in animal models of AD, suggesting a strategy to counteract synaptic toxicity [44]. Interestingly, caffeine, the most consumed A2A receptor antagonist following chronic ingestion through coffee and other foods, as well as genetic removal of A2A receptors in KO (knockout) mice, reduced hippocampal tau hyperphosphorylation, counteracted neuroinflammation and reverted the related memory deficit [56, 59, 72, 73]. Accordingly, in animal models of AD, exposure to low doses of the selective A2A antagonist, istradefylline (a commercially available new co-therapy for Parkinson’s disease (PD)), increased spatial memory and habituation, giving an important proof-of-concept that A2A receptor blockade might be a novel target to fight cognitive impairments in AD patients [74, 75]. This result suggests a great potential for drug development against dementia targeting the A2A receptor, for example, by repurposing istradefylline. Istradefylline has passed multiple clinical safety studies and is already approved in Japan, Korea and the US (as Nouriast) [76–79].
3. MEDICINAL CHEMISTRY OF A2A RECEPTOR ANTAGONISTS
The focus on A2A receptor antagonist therapeutic development was for PD during the 1990s and 2000s, which has now shifted toward immuno-oncology, as well as other neurodegenerative diseases, such as AD [80]. A wide range of chemotypes is now known to antagonize the A2A receptor [81, 82]. The appeal of A2A receptor antagonists to drug discovery programs is also strengthened by the precedent of widespread use of caffeine without serious adverse effects, and the non-lethal and well-tolerated phenotype of A2A receptor-KO mice. The ability of A2A receptor-selective PET tracers to determine the pharmacodynamics and receptor occupancy in the human brain has also aided drug discovery in this field [83].
The chemical modification of caffeine (1,3,7-trimethylxanthine, 1, Fig. 2) and naturally-occurring alkylxanthines has a long history [73], which predates knowledge of adenosine receptors. Early therapeutic targets included treatment of asthma (by analogy to the very efficacious antiasthmatic drug theophylline (Table 1), which has since fallen out of favor as a preferred treatment) and intermittent claudication (e.g., pentoxifylline). From 1968 to 1980, it became apparent that caffeine at μM concentrations blocks the action of adenosine at its newly discovered receptor(s) [84, 73]. Although a nonselective adenosine receptor antagonist, caffeine, is being examined clinically for the treatment of AD [clinicaltrials.gov, NCT04570085, accessed July 26, 2021], earlier studies indicated that caffeine intake correlates inversely with the occurrence of AD [85, 86] but a recent analysis found no correlation [87]. Currently, the major focus of selective A2A antagonist development is on immuno-oncology, and some previous candidate molecules for PD treatment have been repurposed for cancer [88]. However, it is conceivable that these anticancer agents, if successful, could later generate renewed interest for neuroprotection.
Fig. (2).
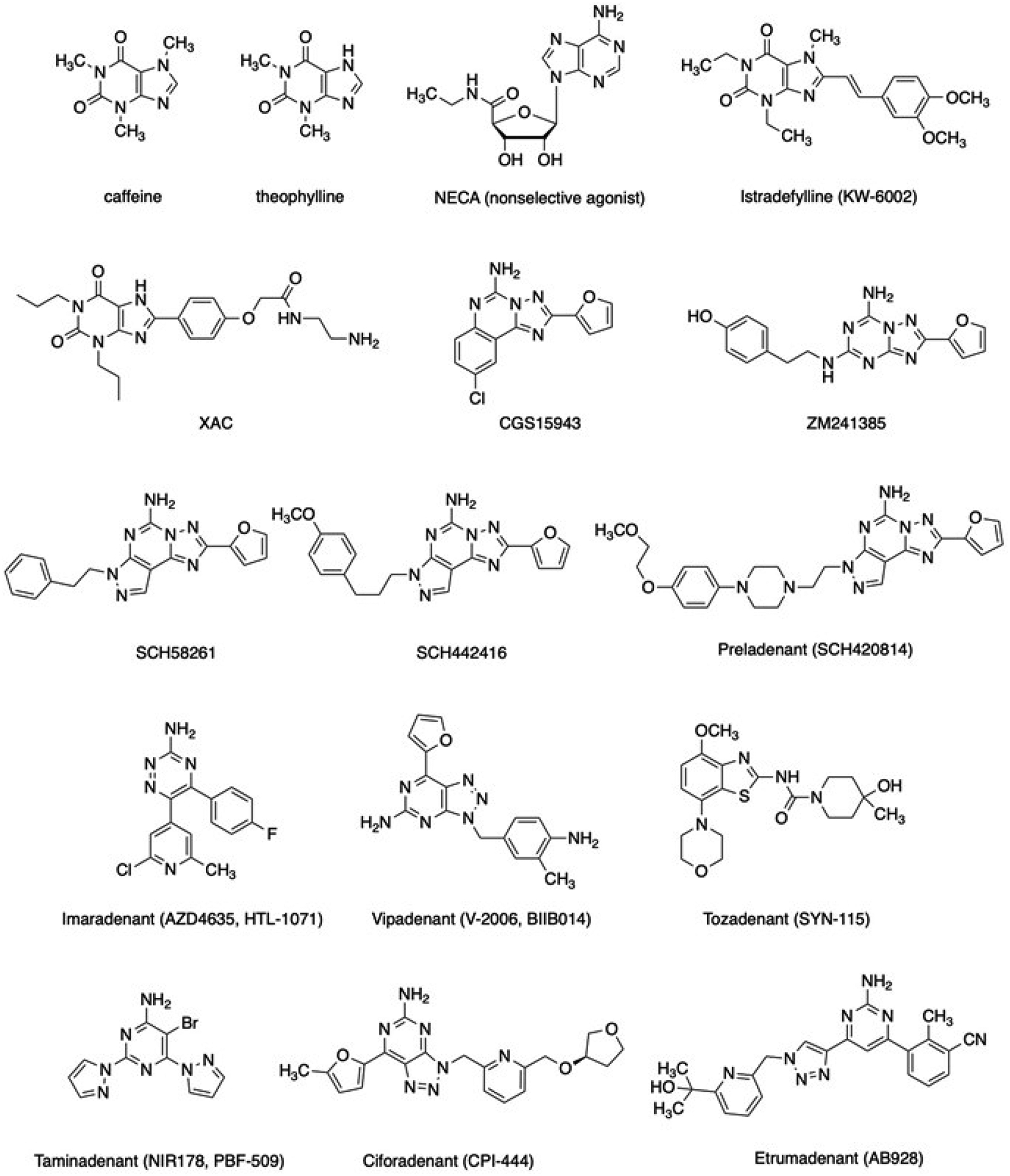
Structures of key A2A adenosine receptor ligands, including antagonists that are now or were recently in clinical trials for PD and/or immuno-oncology.
Table 1.
Affinity selected A2A AR antagonists mentioned in the text (Ki values in nM from binding assays, human, unless noted, (r), rat).
| - | Ki | - | |||
|---|---|---|---|---|---|
| - | A1AR | A2AAR | A2BAR | A3AR | Ref. |
| CGS15943 | 3.5 | 0.15 | 71 | 51 | [117, 82] |
| - | 6 (r) | 1.2 (r) | - | - | |
| ZM241385 | 255 | 0.8 | 50 | >10,000 | [82] |
| SCH58261 | 594 | 1.1 | >10,000 | >10,000 | [117] |
| SCH442416 | 1111 | 0.048 | >10,000 | >10,000 | [118] |
| 1815 (r) | 0.50 (r) | - | >10,000 (r) | ||
| Preladenant | 1474 | 1.1 | >1700 | >1000 | [108, 119] |
| XAC | 6.82 1.2 (r) |
18.4 63 (r) |
7.75 | 25.6 >10,000 (r) |
[120] |
| Istradefylline | 2830 | 36 | 1800 | >3000 | [121, 117] |
| CSC | >10,000 | 38 | 8200 | >10,000 | [117, 122] |
| Imaradenant | 160 | 1.7 | 64 | >10,000 | [123] |
| Taminadenant | 2500 | 12 | 1000 | 5000 | [124] |
| Ciforadenant | 192 | 3.54 | 1530 | 2460 | [125] |
| Etrumadenant | 64 | 1.5 | 2.0 | 489 | [126] |
| Vipadenant | 68 | 1.3 | 63 | 1005 | [127] |
| Tozadenant | 1350 | 5.0 | 700 | 1570 | [82] |
The screening of many xanthine analogues at the rat A1 and A2A receptors became feasible with the advent of adenosine receptor-specific, high-affinity radioligands. Although moderately selective for the rat A1 receptor [89], [3H]xanthine amine congener (XAC, Table 1) was of sufficient affinity to be demonstrated in human platelets as the first A2A receptor antagonist radioligand [90]. [3H]NECA was useful as an agonist A2A radioligand in rat brain membranes when A1 receptors were blocked selectively [91]. Other radioligands and PET ligands (for in vivo imaging of the brain A2A receptor) followed [81, 83, 92–95]. Antagonist selectivity for the A1 receptor was achieved first among the four receptor subtypes [96], followed in 1993 with the first A2A receptor-selective xanthine antagonists, the 8-styrylxanthines. Substitution of the xanthine scaffold can be directed toward increased affinity at each of the four adenosine receptors [96]. For the A2A receptor, the 8-styrylxanthines have achieved moderate A2A selectivity [97], e.g., 8-(3-chlorostyryl)-caffeine (CSC), which also inhibits MAO-B [98]. The most notable 8-styrylxanthine was introduced as KW-6002, now known as istradefylline [97, 76]. Based on its A2A antagonism and bioavailability in the brain when taken orally, istradefylline is currently approved as a co-therapy for PD with levodopa/carbidopa that reduces off-time episodes in patients [99].
One of the earliest efforts to identify A2A receptor-selective non-xanthine antagonists led to reports of CGS15943, a slightly A2A receptor-selective antagonist, of which the [1, 2, 4]triazolo[1,5-c]quinazolin-5-amine scaffold was inspired by multiple nitrogen substitutions of the xanthine scaffold and a phenyl ring fusion [100]. Screening of chemical libraries, first by experimentally-based or high throughput screeningand eventually by structure-based in silico methods, greatly increased the diversity of chemotypes known to bind to the A2A receptor selectively [101–103].
As mentioned above, CGS15943 showed high A2A receptor affinity but only marginal receptor subtype selectivity, and its initial chemical modification was not successful in achieving high selectivity. In fact, N5-acyl derivatives of CGS15943 have been shown to have high affinity at the human A3 receptor, and to some degree, the human A2B receptor. An early parallel effort by the pharmaceutical industry identified ZM241385 as a moderately selective A2A antagonist bearing a [1, 2, 4]triazolo[2,3-a] [1, 3, 5]triazin-5-ylamino] scaffold [104]. ZM241385 was later shown to be potent at the A2B receptor [105]; thus, ZM241385 is a less than optimal pharmacological tool compound for the A2A receptor. However, it has been utilized in the first and numerous subsequent A2A receptor X-ray structures, as described below [106, 107]. Its p-hydroxy-(2-phenylethyl) amino moiety points toward the extracellular regions in the human A2A receptor structures, although its precise position in this flexible region of the A2A receptor protein has proven variable.
In addition to CGS15943 and ZM241385, a heterocyclic scaffold that proved highly productive in the search for novel A2A antagonists was the 7H-pyrazolo[4,3-e] [1, 2, 4]triazolo[1,5-c]pyrimidin-5-amines, first introduced by Baraldi and colleagues, an academic lab research group working closely with the pharmaceutical industry [81]. Among the early antagonists bearing this scaffold were SCH58261 and SCH442416 [26, 92, 108]. Further derivatization of this scaffold identified the congener Preladenant (SCH420814), which progressed through Phase 3 clinical trials for PD [109]. Unfortunately, only a subset of the clinical results indicated efficacy, so its intended use in PD was abandoned. Later, the same compound was repurposed for cancer immunotherapy as MK-3814, as a monotherapy or in combination with an anti-PD-1 monoclonal antibody (Pembrolizumab) [88].
The elucidation of the three-dimensional structure of the human A2A receptor, initially in 2008 and followed by dozens of X-ray and cryo-electron microscopic structures, has greatly facilitated the discovery of A2A receptor antagonists [106, 110, 111]. The X-ray structure of the receptor complex with caffeine was reported in 2017 [112]. In fact, the human A2A receptor has become one of the most highly probed GPCR structures, after the β2 adrenergic receptor, and the result of this detailed knowledge of its structure and function at the atomistic level (including at a resolution of 1.8 Å that reveals specific water molecules in the binding site [107]) has enabled the identification of many new potential therapeutic agents [73, 111]. The X-ray structures and free energy calculations can guide the modification of known A2A antagonists to enhance affinity in a rational, structure-based fashion [113, 114].
A2A antagonist AZD4635 (HTL1071, Imaradenant), a 5,6-diaryl-1,2,4-triazin-3-amine derivative, is in clinical trials for cancer immunotherapy [115]. Prior to its application to cancer treatment, it was considered for the treatment of ADHD. It was the first A2A antagonist designed by structure-based methods that has progressed to human testing, displaying a Ki value of 1.7 nM human A2A receptor and showing >30-fold selectivity over other adenosine receptors [111].
Other scaffolds that provide A2A antagonists, including some that have entered clinical trials, include (Fig. 2) Vipadenant (BIIB014, a [1–3]triazolo[4,5-d]pyrimidin-5-amine); Tozadenant (SYN115, a 4,7-disubstituted benzo[d]thiazole); Taminadenant (NIR178, PBF-509, a 2,6-disubstituted pyrimidin-4-amine); Ciforadenant (CPI-444, V81444, a [1–3]triazolo[4,5-d]pyrimidin-5-amine); EOS100850 (structure not disclosed); and mixed A2A/A2B antagonist Etrumadenant (AB928, a 1,2,3-triazol-4-yl-pyrimidine). Tozadenant caused unanticipated toxicity in a clinical trial for PD, leading to five deaths from drug-induced agranulocytosis out of 409 patients [79]. Similar toxicity had not been observed with other A2A antagonists in clinical trials. An A2A antagonist of undisclosed structure (Inupadenant, EOS-850) is in Phase 1 clinical trial for use against solid tumors [116].
CONCLUSION
The A2A adenosine receptor is one of the major players coming from the purine field in the treatment of neurodegenerative diseases, such as PD and AD. It shows various pharmacological properties, including antioxidant, anti-inflammatory, cytoprotective, antitumoral, antiplatelet, hepatoprotective, and antifibrotic activities. Due to its relevant biological effects, numerous chemical design approaches have been reported. Diverse heterocycles have been reported as potent and selective A2A antagonists, and some have entered clinical trials, either for PD or immuno-oncology. Nonselective antagonist caffeine is being examined as a clinically useful agent for AD. Despite the clinical trials of various selective A2A antagonists for PD, only one with FDA approval is a caffeine analogue, istradefylline. However, the clinical use of this antagonist offers the opportunity for further exploration of its neuroprotective properties. From another point of view, due to the complex nature of neurodegenerative diseases, it is obvious that “simple” targeting of A2A adenosine receptor antagonists for their therapy would not be enough. Thus, a multitarget-directed ligand approach involving A2A adenosine receptor antagonists has been suggested. However, the rational design of compounds that interact with several targets is very challenging. Up to now, dual-target A2A antagonists/D2 agonists, A2A /MAO-B inhibitors as well as triple-target A1/ A2A/MAO-B blockers and A1/A2A/H3 antagonists have been reported [122, 128–131]. This review offers a comprehensive overview of A2A antagonist receptor synthesis in the context of drug discovery for AD therapy.
FUNDING
The authors acknowledge financial support from the NIDDK Intramural Research Program (ZIAD-K031117) and the University of Ferrara (FAR 2020).
Footnotes
Publisher's Disclaimer: DISCLAIMER: The above article has been published, as is, ahead-of-print, to provide early visibility but is not the final version. Major publication processes like copy editing, proofing, typesetting and further review are still to be done and may lead to changes in the final published version, if it is eventually published. All legal disclaimers that apply to the final published article also apply to this ahead-of-print version.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- [1].Salahuddin P; Fatima MT; Uversky VN; Khan RH; Islam Z; Furkan M The role of amyloids in Alzheimer’s and Parkinson’s diseases. Int. J. Biol. Macromol, 2021, 190, 44–55. 10.1016/j.ijbiomac.2021.08.197 [DOI] [PubMed] [Google Scholar]
- [2].Bertram L; Tanzi RE The genetics of Alzheimer’s disease. Prog. Mol. Biol. Transl. Sci, 2012, 107, 79–100. 10.1016/B978-0-12-385883-2.00008-4 [DOI] [PubMed] [Google Scholar]
- [3].Johansson M; Stomrud E; Lindberg O; Westman E; Johansson PM; van Westen D; Mattsson N; Hansson O Apathy and anxiety are early markers of Alzheimer’s disease. Neurobiol. Aging, 2020, 85, 74–82. 10.1016/j.neurobiolaging.2019.10.008 [DOI] [PubMed] [Google Scholar]
- [4].Cummings J New approaches to symptomatic treatments for Alzheimer’s disease. Mol. Neurodegener, 2021, 16(1), 2. 10.1186/s13024-021-00424-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vaz M; Silvestre S Alzheimer’s disease: Recent treatment strategies. Eur. J. Pharmacol, 2020, 887, 173554. 10.1016/j.ejphar.2020.173554 [DOI] [PubMed] [Google Scholar]
- [6].Stoiljkovic M; Horvath TL; Hajós M Therapy for Alzheimer’s disease: Missing targets and functional markers? Ageing Res. Rev, 2021, 68, 101318. 10.1016/j.arr.2021.101318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sonawane SK; Uversky VN; Chinnathambi S Baicalein inhibits heparin-induced Tau aggregation by initializing non-toxic Tau oligomer formation. Cell Commun. Signal, 2021, 19(1), 16. 10.1186/s12964-021-00704-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Arendt T; Bullmann T Neuronal plasticity in hibernation and the proposed role of the microtubule-associated protein tau as a “master switch” regulating synaptic gain in neuronal networks. Am. J. Physiol. Regul. Integr. Comp. Physiol, 2013, 305(5), R478–R489. 10.1152/ajpregu.00117.2013 [DOI] [PubMed] [Google Scholar]
- [9].Gomes LA; Hipp SA; Rijal Upadhaya A; Balakrishnan K; Ospitalieri S; Koper MJ; Largo-Barrientos P; Uytterhoeven V; Reichwald J; Rabe S; Vandenberghe R; von Arnim CAF; Tousseyn T; Feederle R; Giudici C; Willem M; Staufenbiel M; Thai DR Aβ-induced acceleration of Alzheimer-related τ-pathology spreading and its association with prion protein. Acta Neuropathol, 2019, 138(6), 913–941. 10.1007/s00401-019-02053-5 [DOI] [PubMed] [Google Scholar]
- [10].Honson NS; Kuret J Tau aggregation and toxicity in tauopathic neurodegenerative diseases. J. Alzheimers Dis, 2008, 14(4), 417–422. 10.3233/JAD-2008-14409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ittner LM; Götz J Amyloid-β and tau--a toxic pas de deux in Alzheimer’s disease. Nat. Rev. Neurosci, 2011, 12(2), 65–72. 10.1038/nrn2967 [DOI] [PubMed] [Google Scholar]
- [12].Park KW; Kim EJ; Han HJ; Shim YS; Kwon JC; Ku BD; Park KH; Yi HA; Kim KK; Yang DW; Lee HW; Kang H; Kwon OD; Kim S; Lee JH; Chung EJ; Park SW; Park MY; Yoon B; Kim BC; Seo SW; Choi SH Efficacy and tolerability of rivastigmine patch therapy in patients with mild-to-moderate Alzheimer’s dementia associated with minimal and moderate ischemic white matter hyperintensities: A multicenter prospective open-label clinical trial. PLoS One, 2017, 12(8), e0182123. 10.1371/journal.pone.0182123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Long JM; Holtzman DM Alzheimer disease: An update on pathobiology and treatment strategies Cell, 2019, 179(2), 312–339. 10.1016/j.cell.2019.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mullard A FDA approval for Biogen’s aducanumab sparks Alzheimer disease firestorm. Nat. Rev. Drug Discov, 2021, 20(7), 496. [DOI] [PubMed] [Google Scholar]
- [15].Kuller LH; Lopez OL ENGAGE and EMERGE: Truth and consequences? Alzheimers Dement., 2021, 17(4), 692–695. 10.1002/alz.12286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Decourt B; Boumelhem F; Pope ED III; Shi J; Mari Z; Sabbagh MN Critical appraisal of amyloid lowering agents in AD. Curr. Neurol. Neurosci. Rep, 2021, 21(8), 39. 10.1007/s11910-021-01125-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fillit H; Green A Aducanumab and the FDA - where are we now? Nat. Rev. Neurol, 2021, 17(3), 129–130. 10.1038/s41582-020-00454-9 [DOI] [PubMed] [Google Scholar]
- [18].Borea PA; Gessi S; Merighi S; Vincenzi F; Varani K Pharmacology of adenosine receptors: The state of the art. Physiol. Rev, 2018, 98(3), 1591–1625. 10.1152/physrev.00049.2017 [DOI] [PubMed] [Google Scholar]
- [19].Fredholm BB; Arslan G; Halldner L; Kull B; Schulte G; Wasserman W Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch. Pharmacol, 2000, 362(4–5), 364–374. 10.1007/s002100000313 [DOI] [PubMed] [Google Scholar]
- [20].Ferré S; Navarro G; Casadó V; Cortés A; Mallol J; Canela EI; Lluís C; Franco R G protein-coupled receptor heteromers as new targets for drug development. Prog. Mol. Biol. Transl. Sci, 2010, 91, 41–52. 10.1016/S1877-1173(10)91002-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Borea PA; Gessi S; Merighi S; Vincenzi F; Varani K Pathological overproduction: the bad side of adenosine. Br. J. Pharmacol, 2017, 174(13), 1945–1960. 10.1111/bph.13763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Temido-Ferreira M; Coelho JE; Pousinha PA; Lopes LV Novel players in the aging synapse: Impact on cognition. J. Caffeine Adenosine Res, 2019, 9(3), 104–127. 10.1089/caff.2019.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Antonioli L; Fornai M; Blandizzi C; Pacher P; Haskó G Adenosine signaling and the immune system: When a lot could be too much. Immunol. Lett, 2019, 205, 9–15. 10.1016/j.imlet.2018.04.006 [DOI] [PubMed] [Google Scholar]
- [24].Ke RH; Xiong J; Liu Y; Ye ZR Adenosine A2a receptor induced gliosis via Akt/NF-kappaB pathway in vitro. Neurosci. Res, 2009, 65(3), 280–285. 10.1016/j.neures.2009.08.002 [DOI] [PubMed] [Google Scholar]
- [25].Kull B; Svenningsson P; Fredholm BB Adenosine A(2A) receptors are colocalized with and activate g(olf) in rat striatum. Mol. Pharmacol, 2000, 58(4), 771–777. 10.1124/mol.58.4.771 [DOI] [PubMed] [Google Scholar]
- [26].Preti D; Baraldi PG; Moorman AR; Borea PA; Varani K History and perspectives of A2A adenosine receptor antagonists as potential therapeutic agents. Med. Res. Rev, 2015, 35(4), 790–848. 10.1002/med.21344 [DOI] [PubMed] [Google Scholar]
- [27].Baraldi PG; Tabrizi MA; Gessi S; Borea PA Adenosine receptor antagonists: Translating medicinal chemistry and pharmacology into clinical utility. Chem. Rev, 2008, 108(1), 238–263. 10.1021/cr0682195 [DOI] [PubMed] [Google Scholar]
- [28].Schulte G; Fredholm BB Signalling from adenosine receptors to mitogen-activated protein kinases. Cell. Signal, 2003, 15(9), 813–827. 10.1016/S0898-6568(03)00058-5 [DOI] [PubMed] [Google Scholar]
- [29].Schwindinger WF; Mihalcik LJ; Giger KE; Betz KS; Stauffer AM; Linden J; Herve D; Robishaw JD Adenosine A2A receptor signaling and golf assembly show a specific requirement for the gamma7 subtype in the striatum. J. Biol. Chem, 2010, 285(39), 29787–29796. 10.1074/jbc.M110.142620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gonçalves FQ; Lopes JP; Silva HB; Lemos C; Silva AC; Gonçalves N; Tomé AR; Ferreira SG; Canas PM; Rial D; Agostinho P; Cunha RA Synaptic and memory dysfunction in a β-amyloid model of early Alzheimer’s disease depends on increased formation of ATP-derived extracellular adenosine. Neurobiol. Dis, 2019, 132, 104570–104634. 10.1016/j.nbd.2019.104570 [DOI] [PubMed] [Google Scholar]
- [31].Lopes LV; Cunha RA; Kull B; Fredholm BB; Ribeiro JA Adenosine A(2A) receptor facilitation of hippocampal synaptic transmission is dependent on tonic A(1) receptor inhibition. Neuroscience, 2002, 112(2), 319–329. 10.1016/S0306-4522(02)00080-5 [DOI] [PubMed] [Google Scholar]
- [32].Rebola N; Canas PM; Oliveira CR; Cunha RA Different synaptic and subsynaptic localization of adenosine A2A receptors in the hippocampus and striatum of the rat. Neuroscience, 2005, 132(4), 893–903. 10.1016/j.neuroscience.2005.01.014 [DOI] [PubMed] [Google Scholar]
- [33].Rebola N; Lujan R; Cunha RA; Mulle C Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron, 2008, 57(1), 121–134. 10.1016/j.neuron.2007.11.023 [DOI] [PubMed] [Google Scholar]
- [34].Costenla AR; Diógenes MJ; Canas PM; Rodrigues RJ; Nogueira C; Maroco J; Agostinho PM; Ribeiro JA; Cunha RA; de Mendonça A Enhanced role of adenosine A(2A) receptors in the modulation of LTP in the rat hippocampus upon ageing. Eur. J. Neurosci, 2011, 34(1), 12–21. 10.1111/j.1460-9568.2011.07719.x [DOI] [PubMed] [Google Scholar]
- [35].Tebano MT; Martire A; Rebola N; Pepponi R; Domenici MR; Grò MC; Schwarzschild MA; Chen JF; Cunha RA; Popoli P Adenosine A2A receptors and metabotropic glutamate 5 receptors are co-localized and functionally interact in the hippocampus: A possible key mechanism in the modulation of N-methyl-D-aspartate effects. J. Neurochem, 2005, 95(4), 1188–1200. 10.1111/j.1471-4159.2005.03455.x [DOI] [PubMed] [Google Scholar]
- [36].Terry RD; Masliah E; Salmon DP; Butters N; De-Teresa R; Hill R; Hansen LA; Katzman R Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol, 1991, 30(4), 572–580. 10.1002/ana.410300410 [DOI] [PubMed] [Google Scholar]
- [37].Selkoe DJ Alzheimer’s disease is a synaptic failure. Science, 2002, 298(5594), 789–791. 10.1126/science.1074069 [DOI] [PubMed] [Google Scholar]
- [38].Scheff SW; Price DA; Schmitt FA; DeKosky ST; Mufson EJ Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology, 2007, 68(18), 1501–1508. 10.1212/01.wnl.0000260698.46517.8f [DOI] [PubMed] [Google Scholar]
- [39].Scheff SW; Price DA; Ansari MA; Roberts KN; Schmitt FA; Ikonomovic MD; Mufson EJ Synaptic change in the posterior cingulate gyrus in the progression of Alzheimer’s disease. J. Alzheimers Dis, 2015, 43(3), 1073–1090. 10.3233/JAD-141518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Arendash GW; Schleif W; Rezai-Zadeh K; Jackson EK; Zacharia LC; Cracchiolo JR; Shippy D; Tan J Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain beta-amyloid production. Neuroscience, 2006, 142(4), 941–952. 10.1016/j.neuroscience.2006.07.021 [DOI] [PubMed] [Google Scholar]
- [41].Albasanz JL; Perez S; Barrachina M; Ferrer I; Martín M Up-regulation of adenosine receptors in the frontal cortex in Alzheimer’s disease. Brain Pathol, 2008, 18(2), 211–219. 10.1111/j.1750-3639.2007.00112.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Canas PM; Duarte JM; Rodrigues RJ; Köfalvi A; Cunha RA Modification upon aging of the density of presynaptic modulation systems in the hippocampus. Neurobiol. Aging, 2009, 30(11), 1877–1884. 10.1016/j.neurobiolaging.2008.01.003 [DOI] [PubMed] [Google Scholar]
- [43].Espinosa J; Rocha A; Nunes F; Costa MS; Schein V; Kazlauckas V; Kalinine E; Souza DO; Cunha RA; Porciúncula LO Caffeine consumption prevents memory impairment, neuronal damage, and adenosine A2A receptors upregulation in the hippocampus of a rat model of sporadic dementia. J. Alzheimers Dis, 2013, 34(2), 509–518. 10.3233/JAD-111982 [DOI] [PubMed] [Google Scholar]
- [44].Orr AG; Hsiao EC; Wang MM; Ho K; Kim DH; Wang X; Guo W; Kang J; Yu GQ; Adame A; Devidze N; Dubai DB; Masliah E; Conklin BR; Mucke L Astrocytic adenosine receptor A2A and Gs-coupled signaling regulate memory. Nat. Neurosci, 2015, 18(3), 423–434. 10.1038/nn.3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Merighi S; Battistello E; Casetta I; Gragnaniello D; Poloni TE; Medici V; Cirrincione A; Varani K; Vincenzi F; Borea PA; Gessi S Upregulation of cortical A2A adenosine receptors is reflected in platelets of patients with Alzheimer’s disease. J. Alzheimers Dis, 2021, 80(3), 1105–1117. 10.3233/JAD-201437 [DOI] [PubMed] [Google Scholar]
- [46].Gessi S; Poloni TE; Negro G; Varani K; Pasquini S; Vincenzi F; Borea PA; Merighi S 2A adenosine receptor as a potential biomarker and a possible therapeutic target in Alzheimer’s Disease. Cells, 2021, 10(9), 2344. 10.3390/cells10092344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lopes LV; Cunha RA; Ribeiro JA Increase in the number, G protein coupling, and efficiency of facilitatory adenosine A2A receptors in the limbic cortex, but not striatum, of aged rats. J. Neurochem, 1999, 73(4), 1733–1738. 10.1046/j.1471-4159.1999.731733.x [DOI] [PubMed] [Google Scholar]
- [48].Rebola N; Sebastião AM; de Mendonca A; Oliveira CR; Ribeiro JA; Cunha RA Enhanced adenosine A2A receptor facilitation of synaptic transmission in the hippocampus of aged rats. J. Neurophysiol, 2003, 90(2), 1295–1303. 10.1152/jn.00896.2002 [DOI] [PubMed] [Google Scholar]
- [49].Viana da Silva S; Haberl MG; Zhang P; Bethge P; Lemos C; Gonçalves N; Gorlewicz A; Malezieux M; Gonçalves FQ; Grosjean N; Blanchet C; Frick A; Nägerl UV; Cunha RA; Mulle C Early synaptic deficits in the APP/PS1 mouse model of Alzheimer’s disease involve neuronal adenosine A2A receptors. Nat. Commun, 2016, 7, 11915. 10.1038/ncomms11915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Pagnussat N; Almeida AS; Marques DM; Nunes F; Chenet GC; Botton PHS; Mioranzza S; Loss CM; Cunha RA; Porciúncula LO Adenosine A(2A) receptors are necessary and sufficient to trigger memory impairment in adult mice. Br. J. Pharmacol, 2015, 172(15), 3831–3845. 10.1111/bph.13180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ji X-D; Stiles GL; van Galen PJM; Jacobson KA Characterization of human striatal A2-adenosine receptors using radioligand binding and photoaffinity labeling. J. Recept. Res, 1992, 12(2), 149–169. 10.3109/10799899209074789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Costa MS; Botton PH; Mioranzza S; Souza DO; Porciúncula LO Caffeine prevents age-associated recognition memory decline and changes brain-derived neurotrophic factor and tirosine kinase receptor (TrkB) content in mice. Neuroscience, 2008, 153(4), 1071–1078. 10.1016/j.neuroscience.2008.03.038 [DOI] [PubMed] [Google Scholar]
- [53].Prediger RDS; Batista LC; Takahashi RN Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats. Involvement of adenosine A1 and A2A receptors. Neurobiol. Aging, 2005, 26(6), 957–964. 10.1016/j.neurobiolaging.2004.08.012 [DOI] [PubMed] [Google Scholar]
- [54].Arendash GW; Cao C Caffeine and coffee as therapeutics against Alzheimer’s disease. J. Alzheimers Dis, 2010, 20(Suppl. 1), S117–S126. 10.3233/JAD-2010-091249 [DOI] [PubMed] [Google Scholar]
- [55].Dall’Igna OP; Fett P; Gomes MW; Souza DO; Cunha RA; Lara DR Caffeine and adenosine A(2a) receptor antagonists prevent beta-amyloid (25–35)-induced cognitive deficits in mice. Exp. Neurol, 2007, 203(1), 241–245. 10.1016/j.expneurol.2006.08.008 [DOI] [PubMed] [Google Scholar]
- [56].Laurent C; Eddarkaoui S; Derisbourg M; Leboucher A; Demeyer D; Carrier S; Schneider M; Hamdane M; Müller CE; Buée L; Blum D Beneficial effects of caffeine in a transgenic model of Alzheimer’s disease-like tau pathology. Neurobiol. Aging, 2014, 35(9), 2079–2090. 10.1016/j.neurobiolaging.2014.03.027 [DOI] [PubMed] [Google Scholar]
- [57].Cunha GMA; Canas PM; Melo CS; Hockemeyer J; Müller CE; Oliveira CR; Cunha RA Adenosine A2A receptor blockade prevents memory dysfunction caused by beta-amyloid peptides but not by scopolamine or MK-801. Exp. Neurol, 2008, 210(2), 776–781. 10.1016/j.expneurol.2007.11.013 [DOI] [PubMed] [Google Scholar]
- [58].Kaster MP; Machado NJ; Silva HB; Nunes A; Ardais AP; Santana M; Baqi Y; Müller CE; Rodrigues AL; Porciúncula LO; Chen JF; Tomé ÂR; Agostinho P; Canas PM; Cunha RA Caffeine acts through neuronal adenosine A2A receptors to prevent mood and memory dysfunction triggered by chronic stress. Proc. Natl. Acad. Sci. USA, 2015, 112, 7833–7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Laurent C; Burnouf S; Ferry B; Batalha VL; Coelho JE; Baqi Y; Malik E; Mariciniak E; Parrot S; Van der Jeugd A; Faivre E; Flaten V; Ledent C; D’Hooge R; Sergeant N; Hamdane M; Humez S; Müller CE; Lopes LV; Buée L; Blum D A2A adenosine receptor deletion is protective in a mouse model of Tauopathy. Mol. Psychiatry, 2016, 21(1), 97–107. 10.1038/mp.2014.151 [DOI] [PubMed] [Google Scholar]
- [60].Temido-Ferreira M; Ferreira DG; Batalha VL; Marques-Morgado I; Coelho JE; Pereira P; Gomes R; Pinto A; Carvalho S; Canas PM; Cuvelier L; Buée-Scherrer V; Faivre E; Baqi Y; Müller CE; Pimentel J; Schiffmann SN; Buée L; Bader M; Outeiro TF; Blum D; Cunha RA; Marie H; Pousinha PA; Lopes LV Age-related shift in LTD is dependent on neuronal adenosine A2A receptors interplay with mGluR5 and NMDA receptors. Mol. Psychiatry, 2020, 25(8), 1876–1900. 10.1038/s41380-018-0110-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Alonso-Andrés P; Albasanz JL; Ferrer I; Martín M Purine-related metabolites and their converting enzymes are altered in frontal, parietal and temporal cortex at early stages of Alzheimer’s disease pathology. Brain Pathol, 2018, 28(6), 933–946. 10.1111/bpa.12592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Moreira-de-Sá A; Lourenço VS; Canas PM; Cunha RA Adenosine A2A receptors as biomarkers of brain diseases. Front. Neurosci, 2021, 15, 702581. 10.3389/fnins.2021.702581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Illes P; Rubini P; Ulrich H; Zhao Y; Tang Y Regulation of microglial functions by purinergic mechanisms in the healthy and diseased CNS. Cells, 2020, 9(5), 1108. 10.3390/cells9051108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Merighi S; Poloni TE; Terrazzan A; Moretti E; Gessi S; Ferrari D Alzheimer and purinergic signaling: Just a matter of inflammation? Cells, 2021, 10(5), 1267. 10.3390/cells10051267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Cristóvão-Ferreira S; Navarro G; Brugarolas M; Pérez--Capote K; Vaz SH; Fattorini G; Conti F; Lluis C; Ribeiro JA; McCormick PJ; Casadó V; Franco R; Sebastião AM A1R-A2AR heteromers coupled to Gs and Gi/0 proteins modulate GABA transport into astrocytes. Purinergic Signal, 2013, 9(3), 433–449. 10.1007/s11302-013-9364-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Matos M; Augusto E; Machado NJ; dos Santos-Rodrigues A; Cunha RA; Agostinho P Astrocytic adenosine A2A receptors control the amyloid-β peptide-induced decrease of glutamate uptake. J. Alzheimers Dis, 2012, 31(3), 555–567. 10.3233/JAD-2012-120469 [DOI] [PubMed] [Google Scholar]
- [67].Paiva I; Carvalho K; Santos P; Cellai L; Pavlou MAS; Jain G; Gnad T; Pfeifer A; Vieau D; Fischer A; Buée L; Outeiro TF; Blum D A2A R-induced transcriptional deregulation in astrocytes: An in vitro study. Glia, 2019, 67(12), 2329–2342. 10.1002/glia.23688 [DOI] [PubMed] [Google Scholar]
- [68].Franco R; Rivas-Santisteban R; Casanovas M; Lillo A; Saura CA; Navarro G Adenosine A2A receptor antagonists affects NMDA glutamate receptor function. Potential to address neurodegeneration in Alzheimer’s disease. Cells, 2020, 9(5), 1075. 10.3390/cells9051075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Franco R; Lillo A; Rivas-Santisteban R; Reyes-Resina I; Navarro G Microglial Adenosine Receptors: From Pre-conditioning to Modulating the M1/M2 Balance in Activated Cells. Cells, 2021, 10(5), 1124. 10.3390/cells10051124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Saura J; Angulo E; Ejarque A; Casadó V; Tusell JM; Moratalla R; Chen J-FF; Schwarzschild MA; Lluis C; Franco R; Serratosa J Adenosine A2A receptor stimulation potentiates nitric oxide release by activated microglia. J. Neurochem, 2005, 95(4), 919–929. 10.1111/j.1471-4159.2005.03395.x [DOI] [PubMed] [Google Scholar]
- [71].Colella M; Zinni M; Pansiot J; Cassanello M; Mairesse J; Ramenghi L; Baud O Modulation of microglial activation by adenosine A2a receptor in animal models of perinatal brain injury. Front. Neurol, 2018, 9, 605. 10.3389/fneur.2018.00605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Carvalho K; Faivre E; Pietrowski MJ; Marques X; Gomez-Murcia V; Deleau A; Huin V; Hansen JN; Kozlov S; Danis C; Temido-Ferreira M; Coelho JE; Mériaux C; Eddarkaoui S; Gras SL; Dumoulin M; Cellai L; Landrieu I; Chern Y; Hamdane M; Buée L; Boutillier AL; Levi S; Halle A; Lopes LV; Blum D Exacerbation of C1q dysregulation, synaptic loss and memory deficits in tau pathology linked to neuronal adenosine A2A receptor. Brain, 2019, 142(11), 3636–3654. 10.1093/brain/awz288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Jacobson KA; Gao ZG; Matricon P; Eddy MT; Carlsson J Adenosine A2A receptor antagonists: From caffeine to selective non-xanthines. Br. J. Pharmacol, 2020, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Orr AG; Lo I; Schumacher H; Ho K; Gill M; Guo W; Kim DH; Knox A; Saito T; Saido TC; Simms J; Toddes C; Wang X; Yu GQ; Mucke L Istradefylline reduces memory deficits in aging mice with amyloid pathology. Neurobiol. Dis, 2018, 110, 29–36. 10.1016/j.nbd.2017.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Merighi S; Poloni TE; Pelloni L; Pasquini S; Varani K; Vincenzi F; Borea PA; Gessi S An open question: Is the A2A adenosine receptor a novel target for Alzheimer’s disease treatment? Front. Pharmacol, 2021, 12, 652455. 10.3389/fphar.2021.652455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kondo T; Mizuno Y A long-term study of istradefylline safety and efficacy in patients with Parkinson disease. Clin. Neuropharmacol, 2015, 38(2), 41–46. 10.1097/WNF.0000000000000073 [DOI] [PubMed] [Google Scholar]
- [77].Saki M; Yamada K; Koshimura E; Sasaki K; Kanda T In vitro pharmacological profile of the A2A receptor antagonist istradefylline. Naunyn Schmiedebergs Arch. Pharmacol, 2013, 386(11), 963–972. 10.1007/s00210-013-0897-5 [DOI] [PubMed] [Google Scholar]
- [78].Mizuno Y; Kondo T Adenosine A2A receptor antagonist istradefylline reduces daily OFF time in Parkinson’s disease. Mov. Disord, 2013, 28(8), 1138–1141. 10.1002/mds.25418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Chen JF; Cunha RA The belated US FDA approval of the adenosine A2A receptor antagonist istradefylline for treatment of Parkinson’s disease. Purinergic Signal, 2020, 16(2), 167–174. 10.1007/s11302-020-09694-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Chen JF; Schwarzschild MA Do caffeine and more selective adenosine A2A receptor antagonists protect against dopaminergic neurodegeneration in Parkinson’s disease? Parkinsonism Relat Disord, 2020, 80 Suppl 1(Suppl 1), S45–S53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Baraldi PG; Preti D; Borea PA; Varani K Medicinal chemistry of A3 adenosine receptor modulators: Pharmacological activities and therapeutic implications. J. Med. Chem, 2012, 55(12), 5676–5703. 10.1021/jm300087j [DOI] [PubMed] [Google Scholar]
- [82].de Lera Ruiz M; Lim YH; Zheng J Adenosine A2A receptor as a drug discovery target. J. Med. Chem, 2014, 57(9), 3623–3650. 10.1021/jm4011669 [DOI] [PubMed] [Google Scholar]
- [83].Sun MJ; Liu F; Zhao YF; Wu XA In vivo In vivo positron emission tomography imaging of adenosine A2A receptors. Front. Pharmacol, 2020, 11, 599857. 10.3389/fphar.2020.599857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Shimizu H; Daly JW; Creveling CR A radioisotopic method for measuring the formation of adenosine 3′,5′-- cyclic monophosphate in incubated slices of brain. J. Neurochem, 1969, 16(12), 1609–1619. 10.1111/j.1471-4159.1969.tb10360.x [DOI] [PubMed] [Google Scholar]
- [85].Wierzejska R Can coffee consumption lower the risk of Alzheimer’s disease and Parkinson’s disease? A literature review. Arch. Med. Sci, 2017, 13(3), 507–514. 10.5114/aoms.2016.63599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Cellai L; Carvalho K; Faivre E; Deleau A; Vieau D; Buée L; Blum D; Meriaux C; Gomez-Murcia V The adenosinergic signaling: A complex but promising therapeutic target for Alzheimer’s disease. Front. Neurosci, 2018, 12(12), 520. 10.3389/fnins.2018.00520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Bae JM History of coffee consumption and risk of Alzheimer’s disease: A meta-epidemiological study of population-based cohort studies. Dement. Neurocogn. Disord, 2020, 19(3), 108–113. 10.12779/dnd.2020.19-3.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Yu F; Zhu C; Xie Q; Wang Y Adenosine A2A receptor antagonists for cancer immunotherapy. J. Med. Chem, 2020, 63(21), 12196–12212. 10.1021/acs.jmedchem.0c00237 [DOI] [PubMed] [Google Scholar]
- [89].Jacobson KA; Ukena D; Kirk KL; Daly JW [3H]xanthine amine congener of 1,3-dipropyl-8-phenylxanthine: An antagonist radioligand for adenosine receptors. Proc. Natl. Acad. Sci. USA, 1986, 83(11), 4089–4093. 10.1073/pnas.83.11.4089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Ukena D; Jacobson KA; Kirk KL; Daly JWAA [3H]amine congener of l,3-dipropyl-8-phenylxanthine. A new radioligand for A2 adenosine receptors of human platelets. FEBS Lett, 1986, 199(2), 269–274. 10.1016/0014-5793(86)80493-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Bruns RF; Lu GH; Pugsley TA Characterization of the A2 adenosine receptor labeled by [3H]NECA in rat striatal membranes. Mol. Pharmacol, 1986, 29(4), 331–346. [PubMed] [Google Scholar]
- [92].Dionisotti S; Ongini E; Zocchi C; Kull B; Arslan G; Fredholm BB Characterization of human A2A adenosine receptors with the antagonist radioligand [3H]-SCH 58261. Br. J. Pharmacol, 1997, 121(3), 353–360. 10.1038/sj.bjp.0701119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Khanapur S; Paul S; Shah A; Vatakuti S; Koole MJ; Zijlma R; Dierckx RA; Luurtsema G; Garg P; van Waarde A; Elsinga PH Development of [18F]-labeled pyrazolo [4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine (SCH442416) analogs for the imaging of cerebral adenosine A2A receptors with positron emission tomography. J. Med Chem, 2014, 57(15), 6765–6780. 10.1021/jm500700y [DOI] [PubMed] [Google Scholar]
- [94].Ishibashi K; Miura Y; Wagatsuma K; Toyohara J; Ishiwata K; Ishii K Occupancy of adenosine A2A receptors by istradefylline in patients with Parkinson’s disease using 11C-preladenant PET. Neuropharmacology, 2018, 143, 106–112. 10.1016/j.neuropharm.2018.09.036 [DOI] [PubMed] [Google Scholar]
- [95].Lai TH; Toussaint M; Teodoro R; Dukić-Stefanović S; Gündel D; Ludwig FA; Wenzel B; Schröder S; Sattler B; Moldovan RP; Falkenburger BH; Sabri O; Deuther-Conrad W; Brust P Improved in vivo PET imaging of the adenosine A2A receptor in the brain using [18F]FLUDA, a deuterated radiotracer with high metabolic stability. Eur. J. Nucl. Med. Mol. Imaging, 2021, 48(9), 2727–2736. 10.1007/s00259-020-05164-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Jacobson KA; IJzerman AP; Müller CE Medicinal chemistry of P2 and adenosine receptors: Common scaffolds adapted for multiple targets. Biochem. Pharmacol, 2021, 187, 114311. 10.1016/j.bcp.2020.114311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Nonaka Y; Shimada J; Nonaka H; Koike N; Aoki N; Kobayashi H; Kase H; Yamaguchi K; Suzuki F Photoisomerization of a potent and selective adenosine A2 antagonist, (E)-1,3-Dipropyl-8-(3,4-dimethoxystyryl)-7-methylxanthine. J. Med. Chem, 1993, 36(23), 3731–3733. 10.1021/jm00075a031 [DOI] [PubMed] [Google Scholar]
- [98].Van der Walt MM; Terre’Blanche G; Petzer A; Lourens AC; Petzer JP The adenosine A(2A) antagonistic properties of selected C8-substituted xanthines. Bioorg. Chem, 2013, 49, 49–58. 10.1016/j-bioorg.2013.06.006 [DOI] [PubMed] [Google Scholar]
- [99].Berger AA; Winnick A; Welschmeyer A; Kaneb A; Berardino K; Cornett EM; Kaye AD; Viswanath O; Urits I IIstradefylline to treat patients with Parkinson’s disease experiencing “Off’ Episodes: A comprehensive review. Neurol. Int, 2020, 12(3), 109–129. 10.3390/neurolint12030017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Francis JE; Cash WD; Psychoyos S; Ghai G; Wenk P; Friedmann RC; Atkins C; Warren V; Furness P; Hyun JL Structure-activity profile of a series of novel triazoloquinazoline adenosine antagonists. J. Med. Chem, 1988, 31(5), 1014–1020. 10.1021/jm00400a022 [DOI] [PubMed] [Google Scholar]
- [101].Daly JW; Hong O; Padgett WL; Shamim MT; Jacobson KA; Ukena D Non-xanthine heterocycles: Activity as antagonists of A1- and A2-adenosine receptors. Biochem. Pharmacol, 1988, 37(4), 655–664. 10.1016/0006-2952(88)90139-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Katritch V; Jaakola VP; Lane JR; Lin J; Ijzerman AP; Yeager M; Kufareva I; Stevens RC; Abagyan R Structure-based discovery of novel chemotypes for adenosine A(2A) receptor antagonists. J. Med. Chem, 2010, 53(4), 1799–1809. 10.1021/jm901647p [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Carlsson J; Yoo L; Gao ZG; Irwin JJ; Shoichet BK; Jacobson KA Structure-based discovery of A2A adenosine receptor ligands. J. Med. Chem, 2010, 53(9), 3748–3755. 10.1021/jm100240h [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Poucher SM; Keddie JR; Singh P; Stoggall SM; Caulkett PW; Jones G; Coll MG The in vitro pharmacology of ZM 241385, a potent, non-xanthine A2a selective adenosine receptor antagonist. Br. J. Pharmacol, 1995, 115(6), 1096–1102. 10.1111/j.1476-5381.1995.tb15923.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Kim YC; de Zwart M; Chang L; Moro S; von Frijtag Drabbe Künzel JK; Melman N; IJzerman AP; Jacobson KA Derivatives of the triazoloquinazoline adenosine antagonist (CGS 15943) having high potency at the human A2B and A3 receptor subtypes. J. Med. Chem, 1998, 41(15), 2835–2845. 10.1021/jm980094b [DOI] [PubMed] [Google Scholar]
- [106].Jaakola VP; Griffith MT; Hanson MA; Cherezov V; Chien EY; Lane JR; Ijzerman AP; Stevens RC The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science, 2008, 322(5905), 1211–1217. 10.1126/science.1164772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Liu W; Chun E; Thompson AA; Chubukov P; Xu F; Katritch V; Han GW; Roth CB; Heitman LH; IJzerman AP; Cherezov V; Stevens RC Structural basis for allosteric regulation of GPCRs by sodium ions. Science, 2012, 337(6091), 232–236. 10.1126/science.1219218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Neustadt BR; Liu H; Hao J; Greenlee WJ; Stamford AW; Foster C; Arik L; Lachowicz J; Zhang H; Bertorelli R; Fredduzzi S; Varty G; Cohen-Williams M; Ng K Potent and selective adenosine A2A receptor antagonists: 1,2,4-Triazolo[1,5-c]pyrimidines. Bioorg. Med. Chem. Lett, 2009, 19(3), 967–971. 10.1016/j.bmcl.2008.11.075 [DOI] [PubMed] [Google Scholar]
- [109].LeWitt PA; Aradi SD; Hauser RA; Rascol O The challenge of developing adenosine A2A antagonists for Parkinson disease: Istradefylline, preladenant, and tozadenant. Parkinsonism Relat. Disord, 2020, 80(Suppl. 1), S54–S63. 10.1016/j.parkreldis.2020.10.027 [DOI] [PubMed] [Google Scholar]
- [110].Congreve M; Andrews SP; Doré AS; Hollenstein K; Hurrell E; Langmead CJ; Mason JS; Ng IW; Tehan B; Zhukov A; Weir M; Marshall FH Discovery of 1,2,4-triazine derivatives as adenosine A(2A) antagonists using structure based drug design. J. Med. Chem, 2012, 55(5), 1898–1903. 10.1021/jm201376w [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Congreve M; de Graaf C; Swain NA; Tate CG Impact of GPCR Structures on Drug Discovery. Cell, 2020, 181(1), 81–91. 10.1016/j.cell.2020.03.003 [DOI] [PubMed] [Google Scholar]
- [112].Cheng RKY; Segala E; Robertson N; Deflorian F; Doré AS; Errey JC; Fiez-Vandal C; Marshall FH; Cooke RM Structures of human A1 and A2A adenosine receptors with xanthines reveal determinants of selectivity. Structure, 2017, 25(8), 1275–1285.e4. 10.1016/j.str.2017.06.012 [DOI] [PubMed] [Google Scholar]
- [113].Matricon P; Ranganathan A; Warnick E; Gao ZG; Rudling A; Lambertucci C; Marucci G; Ezzati A; Jaiteh M; Dal Ben D; Jacobson KA; Carlsson J Fragment optimization for GPCRs by molecular dynamics free energy calculations: Probing druggable subpockets of the A 2A adenosine receptor binding site. Sci. Rep, 2017, 7(1), 6398. 10.1038/s41598-017-04905-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Jespers W; Verdon G; Azuaje J; Majellaro M; Keränen H; García-Mera X; Congreve M; Deflorian F; de Graaf C; Zhukov A; Doré AS; Mason JS; Åqvist J; Cooke RM; Sotelo E; Gutiérrez-de-Terán H X-ray crystallography and free energy calculations reveal the binding mechanism of A2A adenosine receptor antagonists. Angew. Chem. Int. Ed Engl, 2020, 59(38), 16536–16543. 10.1002/anie.202003788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Voronova V; Peskov K; Kosinsky Y; Helmlinger G; Chu L; Borodovsky A; Woessner R; Sachsenmeier K; Shao W; Kumar R; Pouliot G; Merchant M; Kimko H; Mugundu G Evaluation of combination strategies for the A2AR inhibitor AZD4635 across tumor microenvironment conditions via a systems pharmacology model. Front. Immunol, 2021, 12, 617316. 10.3389/fimmu.2021.617316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Buisseret L; Rottey S; De Bono JS; Migeotte A; Delafontaine B; Manickavasagar T; Martinoli C; Wald N; Rossetti M; Gangolli EA; Wiegert E; McIntyre N; Lager JJ; Machiels JPH Phase 1 trial of the adenosine A2A receptor antagonist inupadenant (EOS-850): Update on tolerability, and antitumor activity potentially associated with the expression of the A2A receptor within the tumor. J. Clin. Oncol, 2021, 39(15)(Suppl.), 2562–2562. 10.1200/JCO.2021.39.15_suppl.2562 [DOI] [Google Scholar]
- [117].Armentero MT; Pinna A; Ferré S; Lanciego JL; Müller CE; Franco R Past, present and future of A(2A) adenosine receptor antagonists in the therapy of Parkinson’s disease. Pharmacol. Ther, 2011, 132(3), 280–299. 10.1016/j.pharmthera.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Todde S; Moresco RM; Simonelli P; Baraldi PG; Cacciari B; Spalluto G; Varani K; Monopoli A; Matarrese M; Carpinelli A; Magni F; Kienle MG; Fazio F Design, radiosynthesis, and biodistribution of a new potent and selective ligand for in vivo imaging of the adenosine A(2A) receptor system using positron emission tomography. J. Med. Chem, 2000, 43(23), 4359–4362. [2]. 10.1021/jm0009843 [DOI] [PubMed] [Google Scholar]
- [119].Pinna A Adenosine A2A receptor antagonists in Parkinson’s disease: progress in clinical trials from the newly approved istradefylline to drugs in early development and those already discontinued. CNS Drugs, 2014, 28(5), 455–474. 10.1007/s40263-014-0161-7 [DOI] [PubMed] [Google Scholar]
- [120].Kim YC; Ji X; Melman N; Linden J; Jacobson KA Anilide derivatives of an 8-phenylxanthine carboxylic congener are highly potent and selective antagonists at human A(2B) adenosine receptors. J. Med. Chem, 2000, 43(6), 1165–1172. 10.1021/jm990421v [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Weiss SM; Benwell K; Cliffe IA; Gillespie RJ; Knight AR; Lerpiniere J; Misra A; Pratt RM; Revell D; Upton R; Dourish CT Discovery of nonxanthine adenosine A2A receptor antagonists for the treatment of Parkinson’s disease. Neurology, 2003, 61(11)(Suppl. 6), S101–S106. 10.1212/01.WNL.0000095581.20961.7D [DOI] [PubMed] [Google Scholar]
- [122].Brunschweiger A; Koch P; Schlenk M; Pineda F; Küppers P; Hinz S; Köse M; Ullrich S; Hockemeyer J; Wiese M; Heer J; Müller CE 8-Benzyltetrahydropyrazino[2, 1-f]purinediones: water-soluble tricyclic xanthine derivatives as multitarget drugs for neurodegenerative diseases. ChemMedChem, 2014, 9(8), 1704–1724. 10.1002/cmdc.201402082 [DOI] [PubMed] [Google Scholar]
- [123].Borodovsky A; Wang Y; Ye M; Shaw JC; Sachsenmeier KF; Deng N; DelSignore KJ; Fretland AJ; Clarke JD; Goodwin RJ; Strittmatter N; Hay C; Sah VR Abstract 5580: Preclinical pharmacodynamics and antitumor activity of AZD4635, a novel adenosine 2A receptor inhibitor that reverses adenosine mediated T cell suppression. Cancer Res, 2017, 77(13 Supplement), 5580. 10.1158/1538-7445.AM2017-5580 [DOI] [Google Scholar]
- [124].Mediavilla-Varela M; Castro J; Chiappori A; Noyes D; Hernandez DC; Allard B; Stagg J; Antonia SJ A novel antagonist of the immune checkpoint protein adenosine A2A receptor restores tumor-infiltrating lymphocyte activity in the context of the tumor microenvironment. Neoplasia, 2017, 19(7), 530–536. 10.1016/j.neo.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Willingham SB; Ho PY; Hotson A; Hill C; Piccione EC; Hsieh J; Liu L; Buggy JJ; McCaffeiy I; Miller RAA 2AR antagonism with CPI-444 induces antitumor responses and augments efficacy to anti-PD-L1 and anti-CT-LA-4 in preclinical models. Cancer Immunol. Res, 2018, 6(10), 1136–1149. 10.1158/2326-6066.CIR-18-0056 [DOI] [PubMed] [Google Scholar]
- [126].Walters MJ; Tan JB; Becker A; Yi FF; Park T; Leleti MR; Rosen B; Sharif E; Debien L; Young S; Lim WH; Garrido-Shaqfeh S; Jaen JC; Powers JP In: Proceedings of the American Association for Cancer Research Annual Meeting 2017; 2017 Apr 1–5; Washington, DC. Philadelphia (PA): AACR; Abstract 4572; Characterization of the potent and selective A2AR antagonist AB928 for the treatment of cancer. Cancer Res, 2017, 77(13), 4572.(Suppl.) [Google Scholar]
- [127].Gillespie RJ; Bamford SJ; Botting R; Comer M; Denny S; Gaur S; Griffin M; Jordan AM; Knight AR; Lerpiniere J; Leonardi S; Lightowler S; McAteer S; Merrett A; Misra A; Padfield A; Reece M; Saadi M; Selwood DL; Stratton GC; Surry D; Todd R; Tong X; Ruston V; Upton R; Weiss SM Antagonists of the human A(2A) adenosine receptor. 4. Design, synthesis, and preclinical evaluation of 7-aryltriazolo[4,5-d]pyrimidines. J. Med. Chem, 2009, 52(1), 33–47. 10.1021/jm800961g [DOI] [PubMed] [Google Scholar]
- [128].Hagenow S; Affini A; Pioli EY; Hinz S; Zhao Y; Porras G; Namasivayam V; Müller CE; Lin JS; Bezard E; Stark H Adenosine A2AR/A1R Adenosine A2AR/A1R antagonists enabling additional H3R antagonism for the treatment of Parkinson’s disease. J. Med. Chem, 2021, 64(12), 8246–8262. 10.1021/acs.jmedchem.0c00914 [DOI] [PubMed] [Google Scholar]
- [129].Jaiteh M; Zeifman A; Saarinen M; Svenningsson P; Bréa J; Loza MI; Carlsson J Docking screens for dual inhibitors of disparate drug targets for Parkinson’s disease. J. Med. Chem, 2018, 61(12), 5269–5278. 10.1021/acs.jmedchem.8b00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Geldenhuys WJ; Van der Schyf CJ Rationally designed multi-targeted agents against neurodegenerative diseases. Curr. Med. Chem, 2013, 20(13), 1662–1672. 10.2174/09298673113209990112 [DOI] [PubMed] [Google Scholar]
- [131].Kampen S; Duy Vo D; Zhang X; Panel N; Yang Y; Jaiteh M; Matricon P; Svenningsson P; Brea J; Loza MI; Kihlberg J; Carlsson J Structure-guided design of G-Protein-coupled receptor polypharmacology. Angew. Chem. Int. Ed Engl, 2021, 60(33), 18022–18030. 10.1002/anie.202101478 [DOI] [PMC free article] [PubMed] [Google Scholar]


