Abstract
Trace metals are essential micronutrients required for survival across all kingdoms of life. From bacteria to animals, metals have critical roles as both structural and catalytic cofactors for an estimated third of the proteome, representing a major contributor to the maintenance of cellular homeostasis. The reactivity of metal ions engenders them with the ability to promote enzyme catalysis and stabilize reaction intermediates. However, these properties render metals toxic at high concentrations and, therefore, metal levels must be tightly regulated. Having evolved in close association with bacteria, vertebrate hosts have developed numerous strategies of metal limitation and intoxication that prevent bacterial proliferation, a process termed nutritional immunity. In turn, bacterial pathogens have evolved adaptive mechanisms to survive in conditions of metal depletion or excess. In this Review, we discuss mechanisms by which nutrient metals shape the interactions between bacterial pathogens and animal hosts. We explore the cell-specific and tissue-specific roles of distinct trace metals in shaping bacterial infections, as well as implications for future research and new therapeutic development.
Subject terms: Pathogens, Bacterial infection
Trace metals are essential micronutrients required for survival across all kingdoms of life. In this Review, Murdoch and Skaar discuss the strategies whereby vertebrate hosts limit metal or induce excess metal to prevent bacterial proliferation, a process termed nutritional immunity, and they discuss adaptive mechanisms that bacterial pathogens have evolved to survive in conditions of metal depletion or excess.
Introduction
Trace metals including zinc (Zn), iron (Fe), manganese (Mn) and copper (Cu) are essential for all forms of life. The importance of nutrient metals in vertebrate health is underscored by the fact that both metal deficiency and metal excess lead to the development of numerous pathologies and represent a major public health burden1. The interaction with metals alters the physico-chemical properties of proteins, thereby promoting catalysis of enzymatic reactions, stabilizing protein structure and/or facilitating electron transport2,3. These metal-associated proteins, referred to as metalloproteins, function in a diverse set of cellular processes, including respiration, transcription, signal transduction and proliferation4. Despite the necessity of trace metals in cellular activities, excess metals are toxic, likely due to the generation of redox-active molecules or from the mis-metalation of metalloproteins, which abrogates their enzymatic function. Organisms maintain appropriate physiological levels of metals by modulating uptake, use, storage and export at the cellular and systemic levels. Tissue metal levels in vertebrate hosts are mainly controlled by absorption from dietary sources in the intestinal tract, whereas bacterial pathogens acquire metal from the extracellular and intracellular environments of host tissues during infection5.
Metal bioavailability exerts a strong selective pressure at the infectious interface giving rise to an evolutionary arms race between host and pathogen that shapes metal sequestration strategies6. Across all forms of life, metals such as Fe, Zn, Mn, magnesium, cobalt and molybdenum are required for enzymatic function of upwards of 36% of proteins in every enzyme class7,8. Given this vital requirement of metals for cellular function in both vertebrate hosts and bacterial pathogens, it is not surprising that vertebrates undergo drastic alterations in metal metabolism in response to bacterial infections. Vertebrates have developed numerous strategies to starve bacteria of nutrient metals via a process termed nutritional immunity6,9,10. Current research efforts are broadening the scope of nutritional immunology, with newly identified metal-binding molecules and metal transport systems in bacterial pathogens, as well as an appreciation of non-metal nutrients that dictate host–pathogen interactions. Moreover, knowledge of the effects of metal starvation on bacterial physiology is deepening with exciting discoveries in the areas of cell wall modifications and expression of virulence factors. Due to the essentiality of nutrient metal maintenance systems, understanding the role of trace metals in bacterial infections remains a critical area of research to identify novel therapeutic strategies. Recent developments in drug design include the emergence of siderophore-conjugated antibiotics, which harness bacterial metal uptake systems to aid in drug delivery. In this Review, we discuss the elaborate mechanisms by which vertebrate hosts and bacterial pathogens maintain cellular metal homeostasis, providing a timely update to the Review previously detailing host–pathogen–metal interactions9. We examine the role of trace metals (Fe, Zn, Mn and Cu) in mediating host–pathogen interactions in cell-specific and tissue-specific host niches. Finally, we explore emerging concepts in nutritional immunity, including the paradox of metal starvation and intoxication at the host–pathogen nexus and novel mechanisms used for bacterial metal acquisition.
Fe in infection and immunity
Fe is a redox-active trace metal that has important roles in most biological systems, supporting vital processes such as DNA replication, transcription and central metabolism9. Although essential for life, levels of Fe must be tightly regulated, especially considering that ferric Fe participates in Fenton-type redox chemistry and can be detrimental to macromolecular function11. Under aerobic conditions, Fe typically exists in the insoluble ferric form (Fe3+), whereas the soluble ferrous Fe (Fe2+) exists under anaerobic or acidic conditions11. Fe can be kinetically trapped in molecules such as haem or associated with host metalloproteins and storage molecules. However, Fe sources can also be exchangeable whereby Fe is tightly bound but still labile. The requirement of Fe by both vertebrate hosts and bacterial pathogens has led to the development of sophisticated host and bacterial systems to liberate, sequester and scavenge Fe within host niches. Host strategies for maintaining Fe pools during infection have been recently reviewed9,12 and, hence, these mechanisms are discussed only briefly below.
Host-imposed Fe restriction during infection
Vertebrate tissues present an Fe-restricted environment for bacterial pathogens (Fig. 1). Most intracellular Fe is incorporated into metalloproteins or stored in association with ferritin, thereby protecting the host from Fe toxicity while restricting access to invading bacteria12. The majority of extracellular Fe is stored in the tetrapyrrole cofactor haem, which is complexed to haemoglobin in circulating erythrocytes and is responsible for oxygen binding. If haemoglobin or haem is released from erythrocytes, it is rapidly bound by host haemoproteins, haptoglobin or haemopexin (HPX), further preventing Fe use by pathogens13. Circulating ferric Fe that is not associated with haem is bound by the serum protein transferrin. The concerted action of these proteins renders host Fe pools largely inaccessible to bacteria that lack high-affinity Fe uptake pathways or systems9,12,14. In fact, humans with genetic defects in Fe-handling systems have a substantially increased risk for infectious disease, highlighting the critical requirement for Fe-dependent nutritional immunity in host protection against bacterial pathogens15 (see Box 1).
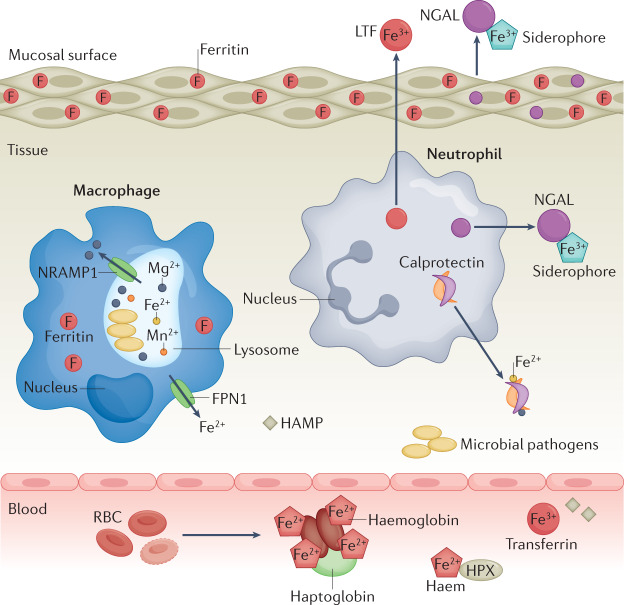
Fig. 1. Host Fe-limitation strategies.
Following invasion by pathogenic bacteria, the host limits bacterial access to iron (Fe) through a systemic reprogramming of Fe homeostasis by sequestering Fe in macrophages, hepatocytes and enterocytes, while simultaneously reducing uptake of Fe from the diet. Within the tissue environment, Fe sources are limited through concerted action of host secreted factors including Fe-chelating molecules calprotectin, neutrophil gelatinase-associated lipocalin (NGAL) and lactoferrin (LTF) as well as intracellular Fe storage protein ferritin. In circulation, Fe is associated with haemoglobin in red blood cells (RBCs) or bound by transferrin. Lysis of RBCs results in release of haemoglobin and free haem–Fe, which is rapidly bound by haptoglobin and haemopexin (HPX) to prevent usage by bacterial pathogens. Secreted hormone hepcidin (HAMP) prevents host Fe export through binding and subsequent internalization of Fe transporter ferroportin 1 (FPN1). Intracellular pathogens within macrophages are starved of Fe through the action of natural resistance-associated macrophage protein 1 (NRAMP1) in the phagolysosome. Fe2+, ferrous iron; Fe3+, ferric iron.
Immune cells, including macrophages and neutrophils, have important roles in restricting metal access to invading pathogens. In macrophages, the natural resistance-associated macrophage protein 1 (NRAMP1) is an antiporter localized to late endosomal or lysosomal membranes16. For intracellular pathogens including Mycobacterium tuberculosis and Salmonella enterica subsp. enterica serovar Typhimurium, NRAMP reduces metal availability by redirecting storage of cellular Fe, Mn and magnesium from the phagolysosome to the cytoplasm17–19. Moreover, neutrophils secrete numerous Fe-scavenging proteins including lactoferrin (LTF), calprotectin and neutrophil gelatinase-associated lipocalin (NGAL; also known as lipocalin 2) to limit bacterial growth20. Notably, secretion of metal-scavenging proteins is not limited to immune cells. Host mucosal epithelial cells such as those found in the intestinal tract also secrete metal-scavenging proteins including NGAL, which can inhibit bacterial growth of enteric pathogens21, thereby having an important role in host innate immunity.
Box 1 Host genetics and diet.
Trace metals are obtained through dietary sources in vertebrate hosts. Following absorption in the gastrointestinal tract, cellular and systemic metal levels are regulated by numerous well-defined metabolic circuits. Both conditions of metal deficiency and metal excess lead to increased risk of infection in humans, and these perturbations in metal homeostasis can be the result of either dietary or inherited factors. However, the mechanisms by which dietary metals and host genetic impairments in metal metabolism affect critical factors including colonization resistance, bacterial virulence as well as immune responses to bacterial pathogens are not fully understood, representing a promising field of future research using animal models with genetic and environmental manipulation of metal levels.
Human genetic polymorphisms in genes required for maintaining iron (Fe) homeostasis often cause the Fe overload disorders β-thalassaemia and haemochromatosis and lead to increased susceptibility to infectious disease199. More specifically, β-thalassaemia leads to increased sensitivity to bacterial infection by Enterobacteriaecia, Salmonella enterica and Listeria monocytogenes200,201. Haemochromatosis leads to increased susceptibility of hosts to numerous opportunistic pathogens such as Vibrio vulnificus and non-pigmented Yersinia pestis202,203. Dietary changes that result in altered metal levels in the gut lead to substantial alterations in microbial ecology that may facilitate gut colonization by enteric pathogens such as S. enterica subsp. enterica serovar Typhimurium, Citrobacter rodentium, Enterobacter faecalis and Clostridioides difficile5,204,205. In fact, excess Fe can lead to pathogenic Yersinia enterocolitica colonization206. Similar to Fe, high levels of dietary zinc (Zn) can lead to expansion of distinct gut commensals and increased colonization by C. difficile205. Due to host restriction of other metals including manganese (Mn), increased dietary Mn is likely to also contribute to bacterial pathogenesis.
Conversely, there are well-described links between deficiencies of Fe, Mn, copper (Cu) and Zn and bacterial infection. Zn is generally important in hosts for growth and immune development as well as in pathogen killing. Zn deficiency is associated with increased susceptibility to L. monocytogenes, pneumococcal pneumonia and infection by enteroaggregative Escherichia coli. Further, Zn deficiency results in increased expression of virulence factors of enteric pathogens including enteropathogenic E. coli207,208. Animals deficient in Zn uptake exhibit increased susceptibility to bacterial infection209, as is seen in Zip8-knockout mice infected with Streptococcus pneumonia210. Reduced levels of Cu are associated with neutropenia and, therefore, increase infectious disease risks by pathogens such as S. enterica and Mannheimia haemolytica in animal models211,212. Although less studied, deficiencies in dietary Mn have also been linked to increased risk of infection by Staphylococcus aureus213. Collectively, these findings demonstrate that levels of dietary metals must be tightly regulated to prevent expansion of gut pathogens while maintaining a healthy immune response to invading bacteria.
Bacterial acquisition of Fe
Bacterial pathogens have evolved numerous strategies to acquire Fe in host environments (Fig. 2). The prominent strategies that bacteria use to acquire Fe include the secretion of siderophores and the uptake of siderophore–Fe, haem–Fe or kinetically labile ferrous Fe (ref.9). The extremely Fe-limited host environment acts as a signal to bacterial pathogens, resulting in the transcriptional upregulation of Fe-acquisition machinery as well as many virulence factors that facilitate host colonization. These changes in gene expression are predominantly mediated by Fe-responsive transcription factors, such as the ferric uptake regulator (Fur) or diphtheria toxin repressor (DtxR)22,23. Yet little is known regarding the direct effect of metals on the activity of proteins involved in these uptake systems. Interestingly, some bacteria, such as Lactobacillus plantarum and Borrelia burgdorferi, have evolved elegant mechanisms to circumvent the need for nutrient Fe altogether via the incorporation of non-Fe metals such as Mn into metalloproteins24,25. The host also uses mechanisms to sequester nutrient Mn (see below). In some cases, obligate human bacterial pathogens, including Neisseria and Moraxella spp., exhibit species-specific binding to Fe-binding molecules such as transferrin and LTF through transferrin or LTF receptors, thus facilitating bacterial scavenging of host-restricted Fe pools26,27.
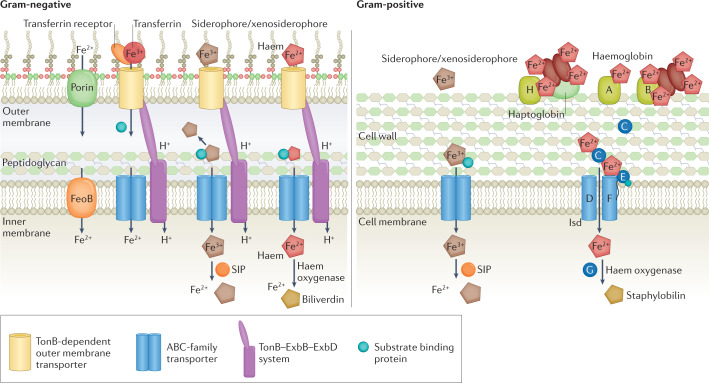
Fig. 2. Bacterial Fe homeostasis at the host–pathogen interface.
Bacterial pathogens can scavenge iron (Fe) through concerted action of secreted siderophores, uptake of host haem or Fe-containing molecules (transferrin and calprotectin) and uptake of ferrous Fe (Fe2+). Gram-negative pathogens uptake Fe into periplasm via activity of porins or TonB-dependent outer membrane transporters. Periplasmic Fe is subsequently transported via FeoB (Fe2+) or ATP-binding cassette (ABC)-family transporters (ferric Fe (Fe3+)) through inner membrane into cytosol. Gram-positive bacteria acquire Fe-bound siderophores or xenosiderophores using ABC-family transporters in the cell membrane and host haem–Fe via activity of Fe-regulated surface determinant system (Isd) transport systems. In the cytosol, siderophore-interacting proteins (SIPs) reduce Fe-loaded siderophores for liberation of Fe2+. Iron is liberated from haem through activity of haem oxygenases, releasing biliverdin and staphylobilin as by-products in the cytosol.
Circulating haem makes up the largest potential reservoir of Fe for bacterial pathogens in the vertebrate host13. Haem consists of an Fe atom coordinated by a tetrapyrrole ring and interacts with host proteins, which makes haem unavailable to potentiate toxicity or be acquired by pathogens. Because haem is a valuable Fe source and some bacteria are unable to synthesize haem de novo, pathogens have evolved elaborate mechanisms to import, catabolize and release Fe from haem. To access haem, several pathogenic bacteria including strains of Pseudomonas, Staphylococci, Streptococci and Escherichia secrete haemolysins that integrate into erythrocyte membranes and result in osmotic lysis28,29. Bacterial pathogens capture liberated haem or haemoproteins using either cell wall-anchored receptors (Gram-positive bacteria), TonB-dependent receptors (Gram-negative bacteria) or haemophores, which are secreted proteins that complex haem30. Subsequently, Fe must be liberated from the tetrapyrrole ring of haem via the activity of haem oxygenases in the bacterial cytosol30. Haem oxygenases are classified into five different enzyme families including the HO-1 family, the IsdG family, ChuZ, ChuW and HutW31,32.
Many Gram-positive bacteria use the well-characterized Fe-regulated surface determinant system (Isd) to scavenge host haem. The prototypical Isd system is described in Staphylococcus aureus and consists of ten genes encoding cell wall-anchored proteins (IsdABCH), a membrane transport system (IsdDEF), haem oxygenases (IsdG and IsdI) and a transpeptidase (SrtB). The Bacillus anthracis Isd proteins include IsdX1 and IsdX2, which are secreted haemophores that bind haemoglobin, haptoglobin–haemoglobin or haem33–35. More recently, additional Isd proteins have been identified including an autolysin, IsdP, that reorganizes the cell wall to improve haem acquisition in Staphylococcus lugdunensis36. Acquisition of host haem is necessary for full infection, as mutants lacking Isd components have reduced virulence37. Isd-independent haem acquisition systems in other species include the ECF transporter LhaSTA in S. lugdunenesis38 and HtsABC and HmuUV in Corynebacterium diphtheriae and Streptococcus spp.39,40. Bacterial haem acquisition permits bacterial survival amidst the presence of host Fe-chelating molecules. For instance, in the presence of the Fe-sequestering protein calprotectin, haem availability is essential for the survival of S. aureus and Pseudomonas aeruginosa, highlighting the importance of haem as an Fe source within the host41. The host counteracts bacterial haem scavenging using haptoglobin, which binds haemoglobin and reduces accessibility of haem while promoting clearance by host cells. Recent studies have provided insights into how pathogens can compete with host clearance of haptoglobin–haemoglobin complexes. Although host haptoglobin reduces IsdH haemoglobin binding42, evidence suggests that IsdH can bind the haptoglobin–haemoglobin complex, preventing recognition by macrophage CD136 and, thus, blocking subsequent internalization and clearance43. The recognition of host haemoprotein complexes and bacterial mechanisms to subvert host haem recycling is a growing area of research.
In Gram-negative bacteria, haem uptake systems are more diverse as the outer membrane presents an additional barrier for haem import. Host haem or haemoproteins are recognized by outer membrane receptors that bind haem directly or bind haem-bound secreted haemophores for trafficking into the periplasm. From the periplasm, haem is transported to the cytoplasm via ABC transporters within the inner membrane. Some Gram-negative pathogens encode multiple haem-acquisition systems such as the Phu and Has systems in P. aeruginosa44, suggesting that multiple non-redundant haem uptake systems benefit bacterial virulence. In the nosocomial pathogen Acinetobacter baumannii, the haemophore HphA is recognized by the two-component system receptor HphR45. Moreover, recent findings demonstrate that haem uptake efficiency is dependent upon bacterial toxin production in Vibrio cholerae46, raising the possibility that virulence factors may have a role in enhancing bacterial haem uptake in vivo.
Bacteria acquire non-haem Fe through the secretion of diverse low molecular-weight Fe-binding molecules called siderophores. To date, more than 500 distinct siderophores have been identified47, and many pathogenic bacteria produce several types of siderophores with distinct molecular features, highlighting the importance of these molecules to access Fe within the host. Siderophores are released from the bacterial cell and bind the ferric form (Fe3+) with remarkably high affinity, often surpassing the affinity of host Fe-binding proteins including transferrin and LTF48. The production and utilization of siderophores give bacteria a competitive advantage in gaining Fe necessary for growth, while simultaneously impacting the host’s ability to scavenge Fe for immune cells to generate reactive oxygen species (ROS)49. Siderophores are captured by dedicated bacterial uptake systems and release Fe within the cytoplasm or periplasm50. Conventional thinking has been that bacteria are uniformly Fe starved within the host. Recent work has demonstrated heterogeneous production of specific siderophores during infection, suggesting that either bacteria experience distinct Fe levels within host tissue or additional, as yet unidentified, regulatory strategies for siderophore production or secretion exist51–56. Additionally, the uptake of siderophores produced by other commensal microorganisms, termed xenosiderophores, are likely to contribute to Fe acquisition in bacterial pathogens in tissues with dense microbial communities such as the gastrointestinal tract or oral cavity. This is important because some bacterial pathogens, such as Campylobacter jejuni, are unable to produce siderophores, and rely instead on the uptake of xenosiderophores to colonize host niches57–59. Opportunistic pathogens may also rely on the use of host molecules such as neurotransmitters as siderophores to promote proliferation in Fe-depleted tissues60. To counteract bacterial siderophore-mediated Fe acquisition, the host secretes the siderophore-binding protein NGAL (also known as lipocalin 2 or siderocalin) from neutrophils and epithelial cells. The full repertoire of siderophore-binding molecules produced by the host is unknown and serves as a promising area for future research, as new host proteins are being described to target bacterial siderophores61,62. To evade NGAL binding of siderophores, certain bacterial species have evolved structurally unrecognizable ‘stealth siderophores’. For example, B. anthracis, Salmonella spp. and Klebsiella spp. express stealth siderophores such as petrobactin, aerobactin, salmochelin and yersinibactin.
Although most Fe is taken up by pathogens in a chelated ferric form (Fe3+), bacteria encode systems that enable the uptake of ferrous Fe (Fe2+). Ferrous Fe predominates in environments that are highly acidic, reducing and/or anaerobic, making Fe2+ uptake systems critical for bacterial colonization of host tissue. The canonical feo operon encodes three proteins (FeoA, FeoB and FeoC) and is the archetypal Fe2+ uptake system in bacteria63,64. FeoB is a membrane transporter containing a soluble G protein domain and is predicted to transport Fe across the membrane dependent upon GTP hydrolysis. The roles of small soluble cytoplasmic FeoA and FeoC remain uncharacterized63. Mutations in FeoB result in decreased colonization and virulence of several pathogens, including Legionella pneumophila, S. Typhimurium, Helicobacter pylori, P. aeruginosa and C. jejuni63,65,66. However, not all pathogens rely solely on FeoB for Fe2+ uptake, suggesting alternative mechanisms for ferrous Fe acquisition. In A. baumannii, FeoB is required for survival in human serum and mouse RAW macrophages67, but seems to be dispensable for bacteraemia in mice68. Rather, the Fe3+ transporter, TonB3, has been implicated as critical for virulence67–69. Moreover in P. aeruginosa, a double mutant of feoB and TonB-dependent Fe import gene, tonB1, demonstrates a more attenuated virulence phenotype66. These findings highlight that Fe import may have host-specific and tissue-specific consequences on pathogenicity, and that other FeoB-independent mechanisms for uptake of kinetically labile ferric or ferrous Fe may have important cooperative roles in pathogenesis70. To date, alternative ferrous Fe import systems have been identified, including ZupT, YfeABCD, FutABC and EfeUOB71–76. Moreover, in the Gram-negative pathogen L. pneumophila, the type IV effector MavN transports intracellular Fe, Mn, cobalt and Zn in macrophage vacuoles, suggesting that intracellular pathogens also have adapted the use of secretion systems for Fe uptake77,78.
Zn and Mn at the infection interface
The essentiality of nutrient metals extends beyond Fe to encompass other critical nutrient metals such as Zn and Mn. Zn is the second most abundant trace metal in humans and is predicted to metalate 9–10% of eukaryotic proteomes and 4–8% of bacterial proteomes79,80. Similarly, Mn has important roles in protein function, including in bacterial superoxide dismutase which is required to detoxify ROS during infection81. In certain bacterial species, Mn can replace Fe in metalloproteins, circumventing host-imposed Fe restriction and reducing oxidative damage to proteins24. Below, we discuss host and bacterial mechanisms of Zn and Mn sequestration.
Host sequestration of Zn and Mn by S100 proteins
The vertebrate host uses several strategies to regulate systemic Zn levels including modulating cellular uptake and secretion of Zn by families of transporters (ZIP importers and ZnT exporters) as well as extracellular scavenging of free Zn by secreted proteins82 (Fig. 3). The vertebrate S100 protein family contributes to the extracellular chelation of metals including Zn and Mn. S100 proteins contain an EF-hand motif and bind Ca2+, and they have been implicated in diverse cellular processes including proliferation, apoptosis and energy metabolism. A unifying feature of S100 proteins is their ability to dimerize, generating high-affinity metal-binding sites at dimer interfaces. A subset of S100 proteins are released extracellularly and have roles in infection and inflammation83,84. The S100A8 and S100A9 heterodimer, also known as calprotectin, is involved in the immune response to bacterial pathogens. Secreted calprotectin binds to and sequesters Zn, Mn, Ni and Fe in the extracellular milieu through the action of two metal-binding sites termed site I and site II that are formed at the S100A8–S100A9 interface20,85,86. Site I binds Zn and Mn with high affinity as well as Fe and Ni, whereas site II binds Zn and Ni86,87. Calprotectin exerts antimicrobial activity in vitro that can be alleviated by the addition of exogenous metals, illustrating that calprotectin prevents bacterial replication via metal sequestration. The protein is highly expressed in immune cells including neutrophils, macrophages and dendritic cells as well as epithelial cells20. Calprotectin comprises approximately 50% of the protein content in neutrophils and protects against an array of bacterial pathogens including S. aureus, A. baumannii, Clostridioides difficile, M. tuberculosis, Aspergillus fumigatus and Candida albicans20. Apart from infection, calprotectin has demonstrated roles in modulating the composition of the commensal bacterial community in the intestine and the development of the immune system88.
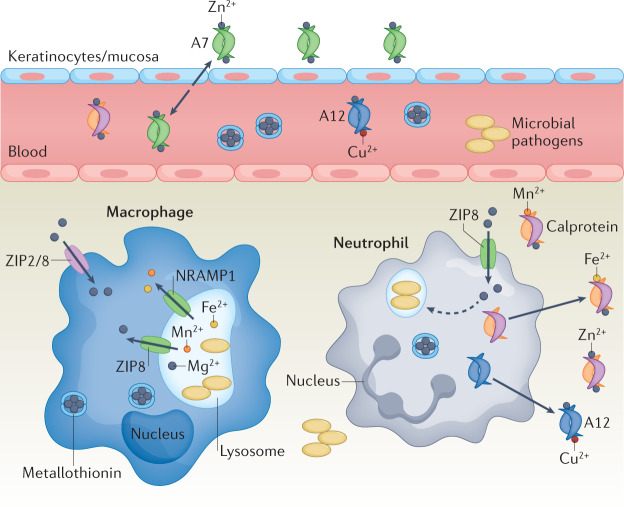
Fig. 3. Host sequestration of Zn and Mn.
During infection, metals such as zinc (Zn) and manganese (Mn) are sequestered by host immune cells via concerted action of transporters and secreted molecules177. Zn is imported into innate immune cells by ZIP-family transporters, including ZIP2 and ZIP8. Within the cell and in circulation, metallothioneins bind and sequester Zn. Extracellular Zn is limited by secretion of Zn-chelating S100 proteins. S100A7 is secreted at epithelial surfaces and keratinocytes, whereas S100A12 and calprotectin (S100A8–S100A9) are secreted by innate immune cells such as neutrophils. S100A7 binds Zn, whereas S100A12 binds Zn and copper (Cu). Calprotectin functions to limit the extracellular availability of several transition metals and binds Mn, iron (Fe) and Cu intracellularly in macrophages, membrane protein resistance-associated macrophage protein 1 (NRAMP1) effluxes Mn from phagosome and ZIP8 effluxes Zn, limiting availability for pathogens. Fe2+, ferrous iron.
Other S100 proteins, such as S100A7 and S100A12, are expressed predominantly in non-immune cells and have antimicrobial functions. S100A7 (also known as psoriasin) is secreted as a homodimer from keratinocytes and mucosal surfaces and has a high affinity for Zn. S100A7 displays antimicrobial activities against pathogenic bacteria using both metal-dependent and metal-independent strategies89–91. S100A12, also known as calgranulin C, is expressed in keratinocytes, monocytes and neutrophils, and forms homodimers that bind both Zn and Cu at the dimer interface. Recombinant S100A12 exhibits Zn-dependent antimicrobial activity against P. aeruginosa, Escherichia coli and C. albicans20. Although host molecules sequester metals from pathogens to prevent bacterial proliferation, metal limitation can lead to beneficial adaptations in pathogens. For instance, Zn-starved M. tuberculosis has increased resistance to ROS and increased proliferation in vivo, suggesting that host Zn restriction primes bacterial cells for immune attack92.
Bacterial acquisition of Zn and Mn
Zn and Mn uptake systems are critical for host colonization by bacterial pathogens93–97 (Fig. 4). The mechanisms of Zn and Mn transport into the cytoplasm are well described in many pathogenic bacteria, yet the mechanisms of Zn and Mn trafficking across the Gram-negative outer membrane remain largely unknown. It was previously thought that Zn and Mn passively diffuse through non-selective porins in the outer membrane, yet evidence now suggests that specific transporters exist to traffic these metals such as the Mn-specific transporter MnoP identified in Bradyrhizobium japonicum98, highlighting selectivity of membranes to trace metals.
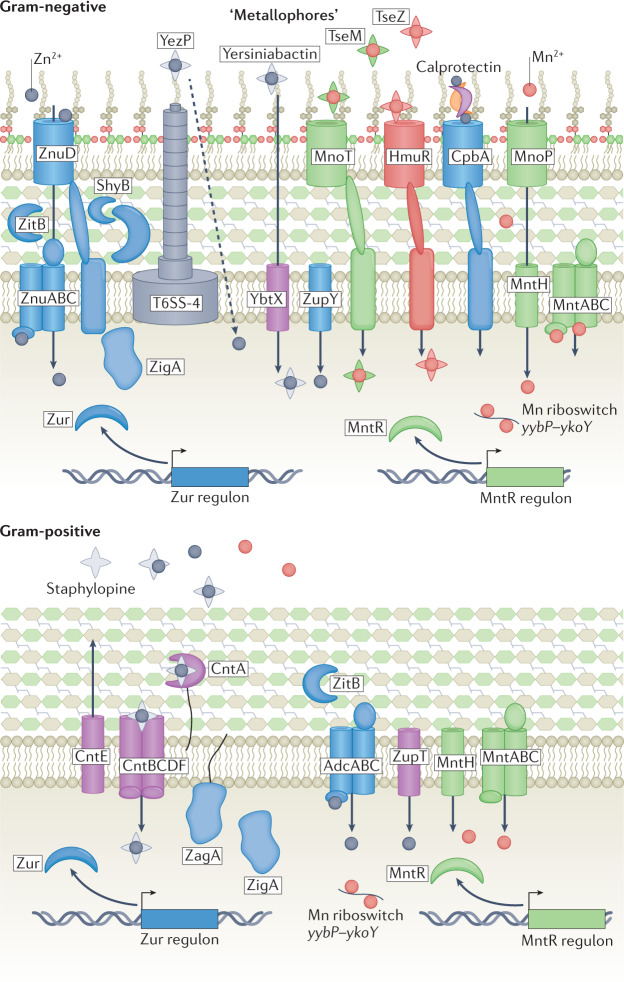
Fig. 4. Bacterial acquisition of Zn and Mn.
Pathogenic bacteria acquire zinc (Zn) using ATP-binding cassette (ABC) transporters ZnuABC (Gram-negative bacteria) and AdcABC (Gram-positive bacteria). Zn is transported through outer membrane using TonB-dependent transporter ZnuD in Gram-negative bacteria and ZupT in Gram-positive bacteria. Additional Zn acquisition systems include type 6 secretion system (T6SS)-secreted zincophore protein YezP in Yersinia pseudotuberculosis, transport of yersiniabactin–Zn in Yersinia pestis by TonB-dependent transporter MnoT, TseZ and TseM in Burkholderia thailandensis and outer membrane receptor CbpA that binds calprotectin in Neisseria meningitidis. Manganese (Mn) is taken up by outer membrane pore MnoP in Gram-negative bacteria and imported into cytosol by MntABC or MntH transporters. In Gram-positive bacteria, Mn is transported across cell membrane by MntH or MntABC transporters. The yybP–ykoY riboswitch family binds Mn with high affinity and modulates Mn homeostasis in bacterial pathogens including Neisseria and Streptococcus spp.
Unlike the passive transport of Mn across the membrane through MnoP, transport of Zn is an energy-dependent process mediated by the TonB–ExbB–ExbD systems in Gram-negative bacteria. Gram-negative pathogenic bacteria, including Neisseriaceae, Acinetobacteriaceae, Bordetellaceae and Moraxellaceae, use the TonB-dependent Zn transporter ZnuD for Zn uptake, with some strains encoding multiple copies99,100. Structural studies have illustrated that ZnuD takes up free Zn but do not exclude the possibility that it can transport chelated Zn. Zn acquisition facilitated by ZnuD has been implicated in bacterial fitness by protecting against neutrophil killing and oxidative stress as well as Zn restriction from calprotectin101. ZnuD was initially mis-annotated as a haem uptake transporter as it shares structural homology with the haem uptake transporters HasR, ShuA and HmbR102, but does not bind haem101. Expression of znuD is regulated by both Fur and Zur, suggesting that increased levels of Zn may be necessary under conditions of Fe starvation as endogenous haem biosynthetic enzymes require Zn102.
Zn and Mn ions are transported through the inner membrane of Gram-negative bacteria and the cytoplasmic membrane of Gram-positive bacteria primarily by NRAMP-family transporters and ATP-binding cassette (ABC) importers (Fig. 4). NRAMP transporters are found across all kingdoms of life and are designated MntH in bacteria. ABC transporters have a cytosolic dimeric ATPase, a membrane-spanning dimeric permease and a monomeric substrate-binding protein (SBP). Notable Mn-specific ABC-type transporter systems include PsaABC of S. pneumoniae and the MntABC (SitABC) of S. aureus, whereas some FeoB orthologues can also transport Mn103. The widely conserved ZnuABC system encodes the high-affinity Zn uptake ABC transport system. Uptake of Zn and Mn by ABC importers is analogous to the uptake of haem and siderophores described above. Specialized proteins, such as ZinT or polyhistidine triad (Pht) proteins, aid in the capture of Zn by the ZnuABC system in numerous pathogenic bacteria such as E. coli and S. enterica104–108. Alternatively, some bacterial pathogens express the low-affinity Zn transporter ZupT109–111 or acquire Zn through the action of inner membrane transporters such as ZevAB and ZurAM112,113.
Bacterial pathogens can also use secreted small molecules to sequester Zn. Recent reports provide evidence of production and secretion of Zn-binding small molecules analogous to Fe siderophores as well as the assignment of Zn-binding properties to established siderophores, suggesting that some siderophores may be more accurately referred to as ‘metallophores’. Bacterial Fe-chelating siderophores are well described, yet the specificity of siderophores to differing metals has not been studied in detail, leaving open the possibility that siderophores may function more broadly in scavenging trace metals. Specifically, siderophores including pyridine-2,6-bis(thiocarboxylic acid) (PDTC), pyochelin, micacocidin and yersiniabactin bind Zn with high affinity114–120. In fact, scavenging of Zn by yersiniabactin promotes Enterobactericae colonization in the inflamed gut, illustrating the importance of siderophore-mediated Zn acquisition in host colonization121. In addition to Zn-binding siderophores, secreted small molecules, termed ‘zincophores’, have been identified from numerous pathogenic bacteria. Streptomyces coelicolor produces a siderophore-like molecule, coelibactin, that is likely to function as a zincophore122. S. aureus produces and secretes staphylopine with broad metal chelating abilities123,124, and P. aeruginosa encodes Zn-binding pseudopaline. The eukaryotic fungal pathogen C. albicans secretes a Zn-scavenging molecule, Pra1 (ref.125), and, finally, Pseudomonas putida produces the small Zn-binding siderophore PDTC126,127. Additionally, some bacterial pathogens can use host Zn-sequestering molecules such as calprotectin and S100A7 to scavenge Zn128–130. The outer membrane receptor CpbA in N. meningitidis is expressed during Zn starvation131 and capable of binding human calprotectin128. Homologues of CpbA have been identified in Neisseria gonorrhoeae, suggesting that these Zn piracy mechanisms may be broadly conserved128,130.
Some bacteria encode more nuanced strategies to acquire Zn. Type VI secretion systems (T6SSs) are multiprotein complexes that mediate the transfer of effector proteins into neighbouring cells in Gram-negative bacteria to facilitate host–bacteria and inter-bacterial interactions. Recent studies have illustrated that T6SSs can be co-opted for Zn and Mn acquisition in Yersinia pseudotuberculosis and Burkholderia thailandensis. The oxidative stress regulator, OxyR, and ZntR induce expression of T6SS4 in Y. pseudotuberculosis, thereby leading to the excretion of a Zn-binding molecule YezP that aids in Zn uptake132,133. Similarly, B. thailandensis secretes a Zn-binding protein, TseZ, via T6SS4 which is imported via the haem transporter HmuR to metallate CuZn superoxide dismutase enzymes. B. thailandensis also expresses a Mn-specific TonB receptor, MnoT, and a Mn-binding secreted protein, TseM134. Collectively these findings highlight the evolution of T6SSs in metal acquisition.
The assembly of T6SSs requires reorganization of the bacterial cell wall, highlighting the importance of cell envelope modifications during conditions of metal limitation135. To this end, in A. baumannii cell wall modifications through Zur-dependent expression of the endopeptidase zrlA, as well as increased outer membrane vesicle formation, have been noted in conditions of Zn deficiency135–137. Notably, cell wall reorganization is not specific to Zn but is also observed in conditions of Fe starvation, as is seen for IsdA and IsdB membrane localization to acquire haem–Fe in S. aureus138. These findings suggest that cell wall reorganization is necessary for bacterial fitness in metal-restricted host environments.
Once nutrient metals have entered the cell, specialized proteins, deemed metallochaperones, mediate the transfer of cognate metals to client metalloenzymes. G3E family P-loop GTPases are candidate metallochaperones for metals including Ni and Zn, and have demonstrated roles in bacterial metal homeostasis and virulence139–141. In bacteria, COG0523 proteins such as YeiR, ZigA and ZagA bind to Zn and hydrolyse GTP141–143. Bacterial COG0523 proteins are necessary to respond to conditions of Zn limitation, similar to that experienced in the host environment. In A. baumannii, ZigA facilitates the mobilization of Zn from histidine pools and is required for full bacterial virulence in a mouse model of pneumonia141. Despite these findings, metallochaperone client proteins and their impact on trafficking trace metals to bacterial enzymes during infection remains largely unknown.
Host-imposed metal intoxication
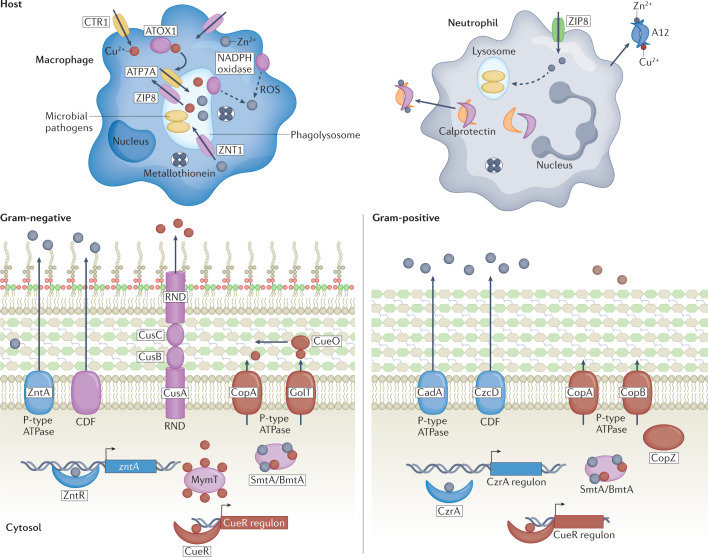
Nutritional immunity classically refers to host restriction of nutrient metals; however, vertebrates also use the toxic properties of metals to limit bacterial infection (Fig. 5). Although not discussed at length below, several metals including Mn and host metal-containing molecules such as haem can be detrimental to bacterial survival at high concentrations31,144. In S. pneumoniae, a riboswitch senses Mn levels and prevents toxicity of excess Mn through expression of a Mn exporter145. Bacterial pathogens including the enteric pathogens C. difficile and Enterococcus faecalis are sensitive to increased haem levels in the gut and encode systems to export haem under these conditions146–148. Perhaps the most well-known examples of metal intoxication include host-induced excess of Cu and Zn by specific cell types, most notably immune cells, and will be further discussed below.
Fig. 5. Metal intoxication.
Metal intoxication is used by vertebrate hosts to combat bacterial proliferation. Following infection, innate immune cells accumulate zinc (Zn) in cytoplasm through ZIP8-mediated import and into phagolysosome via ZNT1. Zn accumulation induces generation of reactive oxygen species (ROS) by NADPH oxidase and NADPH oxidase may liberate Zn from host metallothionein. Copper (Cu) is imported into cytosol of phagocytic cells, including macrophages, by transporter CTR1. Cu is subsequently shuttled by ATOX1 to phagolysosomal membrane, where it is then transported into phagolysosome by ATP7A. Bacteria have evolved diverse mechanisms to withstand Zn and Cu toxicity, including efflux by cation diffusion facilitators (CDF), RND and P-type family ATPase transporters. Zn exporters ZntA, CadA and CzcD alleviate Zn toxicity in pathogenic bacteria. CopA and GolT export excess cytosolic Cu to prevent accumulation and reduce cellular redox stress. Bacterial metallothioneins including MymT in Mycobacteria and SmtA or BmtA bind and sequester cytosolic Cu (MymT, SmtA or BmtA) and Zn (SmtA or BmtA). Zn levels in cytosol are sensed by transcriptional regulator ZntR (Gram-negative bacteria) or CzrA (Gram-positive bacteria). Cytosolic levels of Cu are maintained at very low concentration and are typically regulated through transcriptional regulators including CueR. In Escherichia coli, periplasmic copper oxidase CueO is used to detoxify Cu.
Cu toxicity in bacteria
Cu is an essential metal that is the most reactive metal in the Irving William series. Cu functions as a cofactor for enzymes that function in oxidative phosphorylation, pigmentation, superoxide dismutation and Fe homeostasis. Notably, Cu can transition from two oxidation states Cu+ and Cu2+, which readily interact with biological ligands and therefore have important roles in redox reactions. Due to these properties, increased levels of Cu have potent antimicrobial activity against bacterial pathogens. Within macrophages, Cu accumulates in the phagolysosome of immune cells to combat infection by a defined pathway149 (Fig. 5). Although the mechanisms of Cu toxicity in bacteria are incompletely understood, it is appreciated that excess Cu disrupts protein maturation and functions by disrupting Fe–S clusters, while also perturbing proper protein folding by catalysing the formation of non-native disulfide bonds150. Additionally, during protein maturation, Cu can mis-metalate metalloproteins due to its strong affinity for thiol ligands. To counteract mis-metalation, bacteria can induce the expression of metallochaperones that have higher specificity for cognate partners such as SufA in E. coli151–153. Cu excess can also result in several membrane and cell surface disruptions including defects in lipoprotein and peptidoglycan maturation. Notably, Cu accumulation can generate ROS in vitro under aerobic conditions through the Haber–Weiss reaction and Fenton-like chemistry154, yet the contribution of Cu to generating ROS in vivo still remains unclear.
To avoid the toxicity of Cu, pathogenic bacteria have evolved mechanisms of Cu handling and detoxification to maintain a cytosolic environment free of Cu. The periplasm of Gram-negative bacteria contains the most numerous and diverse Cu-dependent enzymes, and thus is most at risk for Cu toxicity. To combat this pressure, bacteria have evolved multi-copper oxidases whose function is not completely understood. In E. coli, the multi-copper oxidase CueO is thought to convert Cu+ to the less toxic Cu2+ and oxidize the precursor of enterobactin, thereby preventing the generation of toxic cuprous ions155,156. One means of relieving Cu excess is through Cu export. Cu is primarily exported from bacteria via P-type family ATPases, examples of which include CopA of E. coli, CopA1 and CopA2 of P. aeruginosa, CopA, CopB, CopL and CopZ of S. aureus, and CopA and GolT of S. Typhimurium157–159. Cu is delivered to these ATP-dependent pumps via soluble or more recently discovered membrane-bound Cu metallochaperones160. Another example of Cu exporters found in bacteria is the E. coli CusABC complex. Other mechanisms for preventing Cu toxicity includes Cu binding to cysteine-rich metallothioneins, such as MymT in Mycobacteriaceae161 and CusF in E. coli162. Moreover, recent evidence demonstrates Cu2+ binding by Fe-scavenging siderophores, such as yersiniabactin163. This prevents Cu toxicity by restricting the formation of Cu+ and protecting E. coli from ROS in macrophages117. In M. tuberculosis, production of the VapBC4 toxin activates stress survival pathways, which increases bacterial Cu resistance in macrophages164, shedding light on the importance of toxin production on bacterial resistance to metal intoxication. The critical role of Cu detoxification strategies in bacterial pathogens is underscored by the fact that bacteria harbouring mutations in key Cu tolerance genes often display decreased virulence in an animal host and increased killing by host immune cells165.
Zn toxicity in immune cells
Although Zn sequestration has demonstrated roles in host defence against bacterial infection, growing evidence supports a model whereby host-imposed Zn excess enhances intracellular bacterial killing within immune cells. Loss of Zn detoxification machinery in several bacterial pathogens, including Group A and B streptococci166, M. tuberculosis and S. pneumoniae, results in decreased intracellular survival167–170 or in vivo survival in mouse models of infection169. Zn levels increase in macrophages infected with mycobacteria171, and Zn specifically localizes to phagosomes containing internalized M. tuberculosis in human macrophages167. However, the molecular mechanisms by which Zn is trafficked to phagosomes within macrophages remain largely unexplored. It is speculated that Zn may be released from cytosolic Zn–metallothionein complexes by NADPH phagocyte oxidase prior to transport into endocytic compartments by an SLC30-family transporter or fusion with Zn-containing vesicles (zincosomes)167. Recent evidence suggests that Zn transporters such as SLC30a1 partially mediate intracellular Zn transfer in a lipopolysaccharide (LPS)-dependent manner in macrophages172. Zn accumulation has also been observed in neutrophil lysosomes and azurophilic granules in response to Group A Streptococcus (GAS) infection169, and exposure of GAS to human neutrophils leads to an upregulation of Zn-efflux genes170. Interestingly, neutrophil expression of ZnT exporters remained unchanged in these conditions, suggesting that Zn-loaded granules fuse with the phagosome containing bacteria.
The mechanisms of Zn toxicity are not completely understood. Zn is a highly competitive metal for protein binding but is redox-inert due to a stably filled 3d orbital173,174. The focus of Zn toxicity has been placed on the ability of Zn to mis-metalate proteins, thereby disrupting enzymatic activity. Proteins such as topoisomerase I bind Fe and Zn, and evidence indicates that excess Zn can compete for binding for Fe in vivo169, which raises the possibility that Zn and Fe may have similar binding sites in critical enzymes. Excess Zn inhibits glycolytic enzymes and phosphoglucomutase in GAS, resulting in attenuated growth and decreased capsule formation175. Furthermore, Zn excess in E. coli disrupts Fe–S clusters in dehydratases through inhibition of Fe–S cluster assembly proteins such as IscU, IscA and ferredoxin176. Due to the role of Fe–S cluster proteins in diverse cellular pathways ranging from DNA replication to energy metabolism, Zn toxicity through impairment of Fe–S protein biogenesis would have broad impacts on the cell, but these remain to be explored.
Intracellular levels of Zn are sensed by transcriptional regulators, and excess Zn is exported via the activity of P-type family ATPases, RND-family transporters and/or cation diffusion facilitators (CDF)177. In E. coli, the metalloregulator ZntR facilitates the response to Zn excess. In Gram-positive species such as Bacillus subtilis, the transporter CzcD is upregulated by CzrA to export Zn in conditions of Zn intoxication177. Additionally, Zn buffering in the cytosol is mediated by metallothioneins, histidine and low molecular-weight thiols174. S. Typhimurium subverts host Zn intoxication in phagocytes by evading Zn-containing vesicles through an unknown mechanism that is likely to be associated with effector molecules encoded from Salmonella pathogenicity island 1 (ref.178). Collectively, these strategies ensure that intracellular Zn abundance is maintained at appropriate levels to facilitate bacterial colonization and proliferation.
Nutritional immunity-based therapeutics
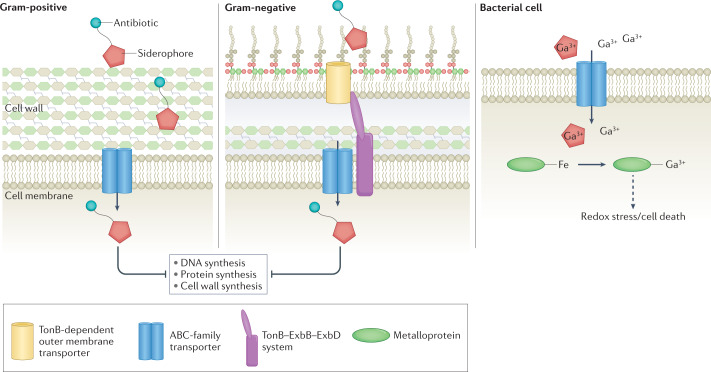
Due to the emergence of multidrug-resistant bacterial pathogens, the discovery of novel therapeutic strategies targeting bacterial pathogens is an urgent area of research (Fig. 6). Numerous drugs have been developed to target siderophore uptake systems to treat drug-resistant strains179. Another therapeutic strategy involves the use of non-ferrous Fe to target bacterial pathogens. Gallium (Ga3+) can compete with ferric Fe for siderophore binding and can further arrest Fe-dependent cellular metabolic processes. Treatment of S. aureus and A. baumannii with Ga3+ results in antimicrobial activity in vivo and in vitro, potentially via binding to secreted siderophores or competing with Fe in important proteins, which leads to redox stress and cell death180. Ga3+-conjugated siderophores, including Desferal (DFO) and pyochelin, are taken in by bacterial cells, thereby preventing uptake of Fe-conjugated siderophores and starving bacterial cells of Fe181. Siderophore-mimicking antibiotics have gained traction with the US Food and Drug Administration (FDA) approval of cefiderocol (Fetroja)179, which exploits the natural bacterial siderophore uptake systems to increase efficacy of the antibiotic, signifying the feasibility of targeting metal uptake systems in bacteria to use as human therapies. The FDA approval and clinical use of cefiderocol represent a landmark event in microbiology as an example of how basic research into microbial metal metabolism can lead to the development of new therapeutic strategies to combat bacterial pathogens. Recent work has attempted to harness the physical and magnetic properties of metal to treat antibiotic-resistant bacterial biofilms. Biofilms treated with magneto-responsive gallium-based liquid metal droplets and exposed to a low-intensity rotating magnetic field resulted in rupturing of bacterial cells and biofilm matrix182.
Fig. 6. Therapeutic interventions harnessing nutritional immunity.
Targeting of bacterial metal uptake systems through use of ‘Trojan horse’ antimicrobials presents a rapidly growing area of therapeutic development against infection. Conjugation of siderophores to antibiotics or alternative metals, such as gallium (Ga3+), ensures uptake by bacterial metal transport systems. Once internalized, antibiotics prevent DNA, protein or cell wall synthesis. Gallium-conjugated siderophores result in iron (Fe) starvation of bacteria and uptake of free gallium results in mis-metalation of Fe–metalloproteins within the cell leading to redox stress. ABC, ATP-binding cassette.
Due to the importance of the metal uptake machinery for bacterial growth in the host environment, metal transporters and trafficking proteins are promising drug targets for novel therapeutic development. Metal receptors are some of the most abundant proteins on the bacterial cell surface during infection, further adding strength to their utility as vaccine candidates and drug targets. Examples include screening strategies to identify small-molecule inhibitors of FeoB183–185, or antibodies that target IsdA and IsdB and prevent S. aureus from binding to haem, which has led to protection in mouse models of infection186,187. Similarly, ZnuD is a promising target for vaccine development in several bacterial pathogens including A. baumannii and N. meningitidis188,189. However, these transporter proteins are often not homogeneously expressed in bacterial populations, which may present technical challenges in targeting these proteins. An alternative strategy to circumvent this issue is to manipulate levels of host proteins that bind nutrient metals with high affinity, such as transferrin, to starve invading bacteria of key resources190,191. Collectively, these avenues of new therapeutic development targeting metal homeostasis at the host–pathogen interface pose promising advances to improve human health by harnessing nutritional immunity.
Conclusions and future perspectives
Nutritional immunity shapes host–pathogen interactions, and the specific impacts of metal limitation on bacterial processes are becoming increasingly appreciated. Mounting evidence suggests a much larger arsenal of host and bacterial factors involved in metal sequestration. Identification of additional host proteins beyond the S100 proteins and NGAL as well as identification of bacterial non-Fe-binding metallophores would greatly expand the field of bacterial pathogenesis and nutritional immunity. Our understanding of nutritional immunity and the ever-evolving battle for nutrient metals at the host–pathogen interface will vastly expand our knowledge of bacterial metal metabolism and provide novel therapeutic targets for future drug development. Host-imposed metal starvation prevents population of critical bacterial enzymes with necessary co-factors, thereby hindering bacterial growth. We speculate that metallochaperones such as ZigA in organisms such as A. baumannii and S. aureus have important roles during bacterial pathogenesis, potentially shuttling trace metals to essential client metalloenzymes required for host colonization and persistance139–141. The kinetics of metal allocation and the transcriptional and translational response to altered metal levels in bacterial pathogens are fruitful areas of study that will inform how pathogens prioritize and distribute metals to facilitate infection. To this end, sequential Fur-regulated and Zur-regulated responses to metal deprivation have been studied extensively in B. subtilis192, and have revealed Fe-sparing responses and the molecular mechanisms of Zn mobilization during nutrient starvation193.
Regulation of cellular metal concentrations is a delicate balance in both host and bacterial systems. Opposing strategies of both limitation and intoxication are used by vertebrates to control bacterial proliferation in host tissue environments. Fluctuations in relative metal availabilities encountered by bacteria in host tissues present a challenge in properly metalating metalloproteins. Moreover, the redox properties of metals, including Cu and Fe, shape host immune responses to infection by promoting the production of oxidants. Although methods of coordinating seemingly contradicting processes to mediate bacterial infection remain incompletely understood, cellular compartmentalization is emerging as a prominent factor in dictating metal-mediating killing. Toxicity of metals, such as Zn and Cu, is often restricted to phagosomes as intracellular killing strategies. This is likely to have evolved to protect host tissues while harnessing the toxic properties of metals to specifically target invading microorganisms. Metal starvation commonly occurs in routes of entry and systemic transfer such at mucosal barriers and in the blood, respectively.
Importantly, limitation of non-metal nutrients also has a critical role in restricting bacterial pathogenicity by stressing the metabolic needs of bacterial pathogens in host tissue environments. Numerous pathogens including S. aureus, enteropathogenic E. coli and M. tuberculosis rely heavily on host amino acids and fatty acids as essential nutrients to establish infection194–197. Recent studies identify the presence of metal storage organelles within bacteria, which could serve both as a means to prevent metal toxicity as well as to provide metals in conditions of nutrient starvation198. Such systems may be strategies to balance metal excess and limitation in bacterial pathogens. Further research is necessary to understand the intersection between metal and non-metal nutrient limitation in preventing bacterial infections and how these processes can collectively be harnessed to develop novel antimicrobial therapies.
Acknowledgements
The authors thank the members of the Skaar laboratory for critical reading of this manuscript. The writing of this manuscript was supported through fellowships to C.C.M. through the National Institutes of Health (NIH) (F32GM142246) and research grants to E.P.S. (R01, AI150702, R01 AI073843, R01AI101171, R01AI138581, R01AI145992).
Glossary
- Mis-metalation
The association of metalloproteins with non-cognate metals, often abrogating enzymatic function.
- Siderophore
A secreted low molecular-weight molecule that binds and sequesters iron (Fe), and potentially other metals.
- Ferritin
An intracellular protein used in the storage of labile cellular iron (Fe).
- Haptoglobin
A host protein that binds haemoglobin in circulation, preventing oxidative activity and use by pathogens.
- Haemopexin
(HPX). A host serum protein that binds circulating haem with high affinity.
- Biliverdin
A green pigmented by-product of haem catabolism by haem oxygenases.
- Staphylobilin
A by-product of haem catabolism by IsdG family haem oxygenases.
- Superoxide dismutase
An enzyme that catalyses the formation of hydrogen peroxide from superoxide.
- EF-hand motif
A helix–loop–helix calcium binding structural domain present in S100 proteins.
- G3E family P-loop GTPases
A conserved family of proteins that bind and hydrolyse GTP with known roles in bacterial metallocentre biosynthesis.
- COG0523 proteins
A subgroup of G3E P-loop GTPase proteins predicted to function as metallochaperones.
- Riboswitch
A regulatory RNA segment that binds metals and modulates expression of the full transcript.
- Fe–S clusters
Inorganic redox-active protein cofactors that play structural and functional roles in metalloproteins formed by complexes of iron (Fe) and sulfides.
- P-type family ATPases
A class of autocatalytic ATP-hydrolysing transporters found across all kingdoms of life.
- Metallothioneins
Low molecular-weight cysteine-rich proteins that bind metals such as zinc (Zn), copper (Cu) and cadmium.
- Phosphoglucomutase
An enzyme that catalyses the transfer of phosphate between the C1 and C6 positions on glucose monomers.
- RND-family transporters
Gram-negative bacterial efflux transporters that span the inner and outer membrane and use the proton gradient to move substrates from the cytosol to the extracellular space.
Author contributions
C.C.M. researched data for the article. E.P.S. and C.C.M contributed substantially to discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Peer review
Peer review information
Nature Reviews Microbiology thanks Mark O’Brian, Isabelle Schalk and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Van Gossum A, Neve J. Trace element deficiency and toxicity. Curr. Opin. Clin. Nutr. Metab. Care. 1998;1:499–507. doi: 10.1097/00075197-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Andreini C, Bertini I, Cavallaro G, Holliday GL, Thornton JM. Metal ions in biological catalysis: from enzyme databases to general principles. J. Biol. Inorg. Chem. 2008;13:1205–1218. doi: 10.1007/s00775-008-0404-5. [DOI] [PubMed] [Google Scholar]
- 3.Maret W. Metalloproteomics, metalloproteomes, and the annotation of metalloproteins. Metallomics. 2010;2:117–125. doi: 10.1039/B915804A. [DOI] [PubMed] [Google Scholar]
- 4.Andreini C, Banci L, Bertini I, Rosato A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006;5:196–201. doi: 10.1021/pr050361j. [DOI] [PubMed] [Google Scholar]
- 5.Lopez CA, Skaar EP. The impact of dietary transition metals on host–bacterial interactions. Cell Host Microbe. 2018;23:737–748. doi: 10.1016/j.chom.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antelo GT, Vila AJ, Giedroc DP, Capdevila DA. Molecular evolution of transition metal bioavailability at the host–pathogen interface. Trends Microbiol. 2021;29:441–457. doi: 10.1016/j.tim.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waldron KJ, Rutherford JC, Ford D, Robinson NJ. Metalloproteins and metal sensing. Nature. 2009;460:823–830. doi: 10.1038/nature08300. [DOI] [PubMed] [Google Scholar]
- 8.Cunrath O, Geoffroy VA, Schalk IJ. Metallome of Pseudomonas aeruginosa: a role for siderophores. Env. Microbiol. 2016;18:3258–3267. doi: 10.1111/1462-2920.12971. [DOI] [PubMed] [Google Scholar]
- 9.Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen–host interface. Nat. Rev. Microbiol. 2012;10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinberg ED. Nutritional immunity. Host’s attempt to withold iron from microbial invaders. JAMA. 1975;231:39–41. doi: 10.1001/jama.1975.03240130021018. [DOI] [PubMed] [Google Scholar]
- 11.Wardman P, Candeias LP. Fenton chemistry: an introduction. Radiat. Res. 1996;145:523–531. doi: 10.2307/3579270. [DOI] [PubMed] [Google Scholar]
- 12.Haschka D, Hoffmann A, Weiss G. Iron in immune cell function and host defense. Semin. Cell Dev. Biol. 2021;115:27–36. doi: 10.1016/j.semcdb.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Choby JE, Skaar EP. Heme synthesis and acquisition in bacterial pathogens. J. Mol. Biol. 2016;428:3408–3428. doi: 10.1016/j.jmb.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iatsenko I, Marra A, Boquete JP, Pena J, Lemaitre B. Iron sequestration by transferrin 1 mediates nutritional immunity in Drosophila melanogaster. Proc. Natl Acad. Sci. USA. 2020;117:7317–7325. doi: 10.1073/pnas.1914830117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganz T. Iron and infection. Int. J. Hematol. 2018;107:7–15. doi: 10.1007/s12185-017-2366-2. [DOI] [PubMed] [Google Scholar]
- 16.Cellier MF, Courville P, Campion C. Nramp1 phagocyte intracellular metal withdrawal defense. Microbes Infect. 2007;9:1662–1670. doi: 10.1016/j.micinf.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Brenz Y, Ohnezeit D, Winther-Larsen HC, Hagedorn M. Nramp1 and NrampB contribute to resistance against Francisella in Dictyostelium. Front. Cell Infect. Microbiol. 2017;7:282. doi: 10.3389/fcimb.2017.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunrath O, Bumann D. Host resistance factor SLC11A1 restricts Salmonella growth through magnesium deprivation. Science. 2019;366:995–999. doi: 10.1126/science.aax7898. [DOI] [PubMed] [Google Scholar]
- 19.Wessling-Resnick M. Nramp1 and other transporters involved in metal withholding during infection. J. Biol. Chem. 2015;290:18984–18990. doi: 10.1074/jbc.R115.643973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monteith AJ, Skaar EP. The impact of metal availability on immune function during infection. Trends Endocrinol. Metab. 2021;32:916–928. doi: 10.1016/j.tem.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh V, et al. Microbiota-inducible innate immune, siderophore binding protein lipocalin 2 is critical for intestinal homeostasis. Cell Mol. Gastroenterol. Hepatol. 2016;2:482–498.e6. doi: 10.1016/j.jcmgh.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hantke K. Iron and metal regulation in bacteria. Curr. Opin. Microbiol. 2001;4:172–177. doi: 10.1016/S1369-5274(00)00184-3. [DOI] [PubMed] [Google Scholar]
- 23.Wakeman CA, Skaar EP. Metalloregulation of Gram-positive pathogen physiology. Curr. Opin. Microbiol. 2012;15:169–174. doi: 10.1016/j.mib.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Posey JE, Gherardini FC. Lack of a role for iron in the Lyme disease pathogen. Science. 2000;288:1651–1653. doi: 10.1126/science.288.5471.1651. [DOI] [PubMed] [Google Scholar]
- 25.Sabine DB, Vaselekos J. Trace element requirements of Lactobacillus acidophilus. Nature. 1967;214:520. doi: 10.1038/214520a0. [DOI] [PubMed] [Google Scholar]
- 26.Gray-Owen SD, Schryvers AB. Bacterial transferrin and lactoferrin receptors. Trends Microbiol. 1996;4:185–191. doi: 10.1016/0966-842X(96)10025-1. [DOI] [PubMed] [Google Scholar]
- 27.Ostan NK, et al. Lactoferrin binding protein B — a bi-functional bacterial receptor protein. PLoS Pathog. 2017;13:e1006244. doi: 10.1371/journal.ppat.1006244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linhartova I, et al. RTX proteins: a highly diverse family secreted by a common mechanism. FEMS Microbiol. Rev. 2010;34:1076–1112. doi: 10.1111/j.1574-6976.2010.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martins R, et al. Heme drives hemolysis-induced susceptibility to infection via disruption of phagocyte functions. Nat. Immunol. 2016;17:1361–1372. doi: 10.1038/ni.3590. [DOI] [PubMed] [Google Scholar]
- 30.Anzaldi LL, Skaar EP. Overcoming the heme paradox: heme toxicity and tolerance in bacterial pathogens. Infect. Immun. 2010;78:4977–4989. doi: 10.1128/IAI.00613-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richard KL, Kelley BR, Johnson JG. Heme uptake and utilization by Gram-negative bacterial pathogens. Front. Cell Infect. Microbiol. 2019;9:81. doi: 10.3389/fcimb.2019.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brimberry M, Toma MA, Hines KM, Lanzilotta WN. HutW from Vibrio cholerae is an anaerobic heme-degrading enzyme with unique functional properties. Biochemistry. 2021;60:699–710. doi: 10.1021/acs.biochem.0c00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maresso AW, Chapa TJ, Schneewind O. Surface protein IsdC and Sortase B are required for heme-iron scavenging of Bacillus anthracis. J. Bacteriol. 2006;188:8145–8152. doi: 10.1128/JB.01011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maresso AW, Garufi G, Schneewind O. Bacillus anthracis secretes proteins that mediate heme acquisition from hemoglobin. PLoS Pathog. 2008;4:e1000132. doi: 10.1371/journal.ppat.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skaar EP, Gaspar AH, Schneewind O. Bacillus anthracis IsdG, a heme-degrading monooxygenase. J. Bacteriol. 2006;188:1071–1080. doi: 10.1128/JB.188.3.1071-1080.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farrand AJ, et al. An iron-regulated autolysin remodels the cell wall to facilitate heme acquisition in Staphylococcus lugdunensis. Infect. Immun. 2015;83:3578–3589. doi: 10.1128/IAI.00397-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pishchany G, et al. IsdB-dependent hemoglobin binding is required for acquisition of heme by Staphylococcus aureus. J. Infect. Dis. 2014;209:1764–1772. doi: 10.1093/infdis/jit817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jochim A, et al. An ECF-type transporter scavenges heme to overcome iron-limitation in Staphylococcus lugdunensis. eLife. 2020 doi: 10.7554/eLife.57322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allen CE, Schmitt MP. HtaA is an iron-regulated hemin binding protein involved in the utilization of heme iron in Corynebacterium diphtheriae. J. Bacteriol. 2009;191:2638–2648. doi: 10.1128/JB.01784-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu H, Liu M, Lei B. The surface protein Shr of Streptococcus pyogenes binds heme and transfers it to the streptococcal heme-binding protein Shp. BMC Microbiol. 2008;8:15. doi: 10.1186/1471-2180-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zygiel EM, Obisesan AO, Nelson CE, Oglesby AG, Nolan EM. Heme protects Pseudomonas aeruginosa and Staphylococcus aureus from calprotectin-induced iron starvation. J. Biol. Chem. 2021;296:100160. doi: 10.1074/jbc.RA120.015975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mikkelsen JH, Runager K, Andersen CBF. The human protein haptoglobin inhibits IsdH-mediated heme-sequestering by Staphylococcus aureus. J. Biol. Chem. 2020;295:1781–1791. doi: 10.1074/jbc.RA119.011612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saederup KL, et al. The Staphylococcus aureus protein IsdH inhibits host hemoglobin scavenging to promote heme acquisition by the pathogen. J. Biol. Chem. 2016;291:23989–23998. doi: 10.1074/jbc.M116.755934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ochsner UA, Johnson Z, Vasil ML. Genetics and regulation of two distinct haem-uptake systems, phu and has, in Pseudomonas aeruginosa. Microbiology. 2000;146:185–198. doi: 10.1099/00221287-146-1-185. [DOI] [PubMed] [Google Scholar]
- 45.Bateman TJ, et al. A Slam-dependent hemophore contributes to heme acquisition in the bacterial pathogen Acinetobacter baumannii. Nat. Commun. 2021;12:6270. doi: 10.1038/s41467-021-26545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rivera-Chavez F, Mekalanos JJ. Cholera toxin promotes pathogen acquisition of host-derived nutrients. Nature. 2019;572:244–248. doi: 10.1038/s41586-019-1453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hider RC, Kong X. Chemistry and biology of siderophores. Nat. Prod. Rep. 2010;27:637–657. doi: 10.1039/b906679a. [DOI] [PubMed] [Google Scholar]
- 48.Sheldon JR, Laakso HA, Heinrichs DE. Iron acquisition strategies of bacterial pathogens. Microbiol. Spectr. 2016 doi: 10.1128/microbiolspec.VMBF-0010-2015. [DOI] [PubMed] [Google Scholar]
- 49.Miethke M, Marahiel MA. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 2007;71:413–451. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schalk IJ, Guillon L. Fate of ferrisiderophores after import across bacterial outer membranes: different iron release strategies are observed in the cytoplasm or periplasm depending on the siderophore pathways. Amino Acids. 2013;44:1267–1277. doi: 10.1007/s00726-013-1468-2. [DOI] [PubMed] [Google Scholar]
- 51.Perry WJ, et al. Staphylococcus aureus exhibits heterogeneous siderophore production within the vertebrate host. Proc. Natl Acad. Sci. USA. 2019;116:21980–21982. doi: 10.1073/pnas.1913991116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beasley FC, Marolda CL, Cheung J, Buac S, Heinrichs DE. Staphylococcus aureus transporters Hts, Sir, and Sst capture iron liberated from human transferrin by Staphyloferrin A, Staphyloferrin B, and catecholamine stress hormones, respectively, and contribute to virulence. Infect. Immun. 2011;79:2345–2355. doi: 10.1128/IAI.00117-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beasley FC, et al. Characterization of staphyloferrin A biosynthetic and transport mutants in Staphylococcus aureus. Mol. Microbiol. 2009;72:947–963. doi: 10.1111/j.1365-2958.2009.06698.x. [DOI] [PubMed] [Google Scholar]
- 54.Sheldon JR, Heinrichs DE. Recent developments in understanding the iron acquisition strategies of Gram positive pathogens. FEMS Microbiol. Rev. 2015;39:592–630. doi: 10.1093/femsre/fuv009. [DOI] [PubMed] [Google Scholar]
- 55.Sheldon JR, Marolda CL, Heinrichs DE. TCA cycle activity in Staphylococcus aureus is essential for iron-regulated synthesis of staphyloferrin A, but not staphyloferrin B: the benefit of a second citrate synthase. Mol. Microbiol. 2014;92:824–839. doi: 10.1111/mmi.12593. [DOI] [PubMed] [Google Scholar]
- 56.Sheldon JR, Skaar EP. Acinetobacter baumannii can use multiple siderophores for iron acquisition, but only acinetobactin is required for virulence. PLoS Pathog. 2020;16:e1008995. doi: 10.1371/journal.ppat.1008995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pletzer D, Braun Y, Weingart H. Swarming motility is modulated by expression of the putative xenosiderophore transporter SppR-SppABCD in Pseudomonas aeruginosa PA14. Antonie Van. Leeuwenhoek. 2016;109:737–753. doi: 10.1007/s10482-016-0675-8. [DOI] [PubMed] [Google Scholar]
- 58.Xu F, Zeng X, Haigh RD, Ketley JM, Lin J. Identification and characterization of a new ferric enterobactin receptor, CfrB, in Campylobacter. J. Bacteriol. 2010;192:4425–4435. doi: 10.1128/JB.00478-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ellermann M, Arthur JC. Siderophore-mediated iron acquisition and modulation of host–bacterial interactions. Free Radic. Biol. Med. 2017;105:68–78. doi: 10.1016/j.freeradbiomed.2016.10.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perraud Q, et al. Opportunistic use of catecholamine neurotransmitters as siderophores to access iron by Pseudomonas aeruginosa. Environ. Microbiol. 2022;24:878–893. doi: 10.1111/1462-2920.15372. [DOI] [PubMed] [Google Scholar]
- 61.Correnti C, Strong RK. Mammalian siderophores, siderophore-binding lipocalins, and the labile iron pool. J. Biol. Chem. 2012;287:13524–13531. doi: 10.1074/jbc.R111.311829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zsila F, Beke-Somfai T. Human host-defense peptide LL-37 targets stealth siderophores. Biochem. Biophys. Res. Commun. 2020;526:780–785. doi: 10.1016/j.bbrc.2020.03.162. [DOI] [PubMed] [Google Scholar]
- 63.Lau CK, Krewulak KD, Vogel HJ. Bacterial ferrous iron transport: the Feo system. FEMS Microbiol. Rev. 2016;40:273–298. doi: 10.1093/femsre/fuv049. [DOI] [PubMed] [Google Scholar]
- 64.Sestok AE, Linkous RO, Smith AT. Toward a mechanistic understanding of Feo-mediated ferrous iron uptake. Metallomics. 2018;10:887–898. doi: 10.1039/C8MT00097B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Costa LF, et al. Iron acquisition pathways and colonization of the inflamed intestine by Salmonella enterica serovar Typhimurium. Int. J. Med. Microbiol. 2016;306:604–610. doi: 10.1016/j.ijmm.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Minandri F, et al. Role of iron uptake systems in Pseudomonas aeruginosa virulence and airway infection. Infect. Immun. 2016;84:2324–2335. doi: 10.1128/IAI.00098-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Runci F, et al. Contribution of active iron uptake to Acinetobacter baumannii pathogenicity. Infect. Immun. 2019 doi: 10.1128/IAI.00755-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Subashchandrabose S, et al. Acinetobacter baumannii genes required for bacterial survival during bloodstream infection. mSphere. 2016 doi: 10.1128/mSphere.00013-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nagy TA, Moreland SM, Detweiler CS. Salmonella acquires ferrous iron from haemophagocytic macrophages. Mol. Microbiol. 2014;93:1314–1326. doi: 10.1111/mmi.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perez N, Johnson R, Sen B, Ramakrishnan G. Two parallel pathways for ferric and ferrous iron acquisition support growth and virulence of the intracellular pathogen Francisella tularensis Schu S4. Microbiologyopen. 2016;5:453–468. doi: 10.1002/mbo3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bearden SW, Staggs TM, Perry RD. An ABC transporter system of Yersinia pestis allows utilization of chelated iron by Escherichia coli SAB11. J. Bacteriol. 1998;180:1135–1147. doi: 10.1128/JB.180.5.1135-1147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grass G, et al. FieF (YiiP) from Escherichia coli mediates decreased cellular accumulation of iron and relieves iron stress. Arch. Microbiol. 2005;183:9–18. doi: 10.1007/s00203-004-0739-4. [DOI] [PubMed] [Google Scholar]
- 73.Katoh H, Hagino N, Grossman AR, Ogawa T. Genes essential to iron transport in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 2001;183:2779–2784. doi: 10.1128/JB.183.9.2779-2784.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cao J, Woodhall MR, Alvarez J, Cartron ML, Andrews SC. EfeUOB (YcdNOB) is a tripartite, acid-induced and CpxAR-regulated, low-pH Fe2+ transporter that is cryptic in Escherichia coli K-12 but functional in E. coli O157:H7. Mol. Microbiol. 2007;65:857–875. doi: 10.1111/j.1365-2958.2007.05802.x. [DOI] [PubMed] [Google Scholar]
- 75.Grosse C, et al. A new ferrous iron-uptake transporter, EfeU (YcdN), from Escherichia coli. Mol. Microbiol. 2006;62:120–131. doi: 10.1111/j.1365-2958.2006.05326.x. [DOI] [PubMed] [Google Scholar]
- 76.Kammler M, Schon C, Hantke K. Characterization of the ferrous iron uptake system of Escherichia coli. J. Bacteriol. 1993;175:6212–6219. doi: 10.1128/jb.175.19.6212-6219.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Christenson ET, et al. The iron-regulated vacuolar Legionella pneumophila MavN protein is a transition-metal transporter. Proc. Natl Acad. Sci. USA. 2019;116:17775–17785. doi: 10.1073/pnas.1902806116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Isaac DT, Laguna RK, Valtz N, Isberg RR. MavN is a Legionella pneumophila vacuole-associated protein required for efficient iron acquisition during intracellular growth. Proc. Natl Acad. Sci. USA. 2015;112:E5208–E5217. doi: 10.1073/pnas.1511389112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andreini C, Banci L, Bertini I, Rosato A. Zinc through the three domains of life. J. Proteome Res. 2006;5:3173–3178. doi: 10.1021/pr0603699. [DOI] [PubMed] [Google Scholar]
- 80.Rahman MT, Karim MM. Metallothionein: a potential link in the regulation of zinc in nutritional immunity. Biol. Trace Elem. Res. 2018;182:1–13. doi: 10.1007/s12011-017-1061-8. [DOI] [PubMed] [Google Scholar]
- 81.Juttukonda LJ, Skaar EP. Manganese homeostasis and utilization in pathogenic bacteria. Mol. Microbiol. 2015;97:216–228. doi: 10.1111/mmi.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kambe T, Tsuji T, Hashimoto A, Itsumura N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015;95:749–784. doi: 10.1152/physrev.00035.2014. [DOI] [PubMed] [Google Scholar]
- 83.Kozlyuk N, et al. S100 proteins in the innate immune response to pathogens. Methods Mol. Biol. 2019;1929:275–290. doi: 10.1007/978-1-4939-9030-6_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zackular JP, Chazin WJ, Skaar EP. Nutritional immunity: S100 proteins at the host–pathogen interface. J. Biol. Chem. 2015;290:18991–18998. doi: 10.1074/jbc.R115.645085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Damo SM, et al. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc. Natl Acad. Sci. USA. 2013;110:3841–3846. doi: 10.1073/pnas.1220341110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nakashige TG, Zygiel EM, Drennan CL, Nolan EM. Nickel sequestration by the host-defense protein human calprotectin. J. Am. Chem. Soc. 2017;139:8828–8836. doi: 10.1021/jacs.7b01212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakashige TG, Zhang B, Krebs C, Nolan EM. Human calprotectin is an iron-sequestering host-defense protein. Nat. Chem. Biol. 2015;11:765–771. doi: 10.1038/nchembio.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Willers M, et al. S100A8 and S100A9 are important for postnatal development of gut microbiota and immune system in mice and infants. Gastroenterology. 2020;159:2130–2145.e5. doi: 10.1053/j.gastro.2020.08.019. [DOI] [PubMed] [Google Scholar]
- 89.Buchau AS, et al. S100A15, an antimicrobial protein of the skin: regulation by E. coli through Toll-like receptor 4. J. Invest. Dermatol. 2007;127:2596–2604. doi: 10.1038/sj.jid.5700946. [DOI] [PubMed] [Google Scholar]
- 90.Glaser R, et al. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat. Immunol. 2005;6:57–64. doi: 10.1038/ni1142. [DOI] [PubMed] [Google Scholar]
- 91.Lee KC, Eckert RL. S100A7 (psoriasin) — mechanism of antibacterial action in wounds. J. Invest. Dermatol. 2007;127:945–957. doi: 10.1038/sj.jid.5700663. [DOI] [PubMed] [Google Scholar]
- 92.Dow A, et al. Zinc limitation triggers anticipatory adaptations in Mycobacterium tuberculosis. PLoS Pathog. 2021;17:e1009570. doi: 10.1371/journal.ppat.1009570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hesse LE, Lonergan ZR, Beavers WN, Skaar EP. The Acinetobacter baumannii Znu system overcomes host-imposed nutrient zinc limitation. Infect. Immun. 2019 doi: 10.1128/IAI.00746-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu Y, et al. Magnesium sensing regulates intestinal colonization of enterohemorrhagic Escherichia coli O157:H7. mBio. 2020 doi: 10.1128/mBio.02470-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Quan G, Xia P, Lian S, Wu Y, Zhu G. Zinc uptake system ZnuACB is essential for maintaining pathogenic phenotype of F4ac+ enterotoxigenic E. coli (ETEC) under a zinc restricted environment. Vet. Res. 2020;51:127. doi: 10.1186/s13567-020-00854-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zackular JP, et al. ZupT facilitates Clostridioides difficile resistance to host-mediated nutritional immunity. mSphere. 2020 doi: 10.1128/mSphere.00061-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ganguly T, Peterson AM, Kajfasz JK, Abranches J, Lemos JA. Zinc import mediated by AdcABC is critical for colonization of the dental biofilm by Streptococcus mutans in an animal model. Mol. Oral. Microbiol. 2021;36:214–224. doi: 10.1111/omi.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hohle TH, Franck WL, Stacey G, O’Brian MR. Bacterial outer membrane channel for divalent metal ion acquisition. Proc. Natl Acad. Sci. USA. 2011;108:15390–15395. doi: 10.1073/pnas.1110137108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Braymer JJ, Giedroc DP. Recent developments in copper and zinc homeostasis in bacterial pathogens. Curr. Opin. Chem. Biol. 2014;19:59–66. doi: 10.1016/j.cbpa.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mortensen BL, Rathi S, Chazin WJ, Skaar EP. Acinetobacter baumannii response to host-mediated zinc limitation requires the transcriptional regulator Zur. J. Bacteriol. 2014;196:2616–2626. doi: 10.1128/JB.01650-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Calmettes C, et al. The molecular mechanism of Zinc acquisition by the neisserial outer-membrane transporter ZnuD. Nat. Commun. 2015;6:7996. doi: 10.1038/ncomms8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kumar P, Sannigrahi S, Tzeng YL. The Neisseria meningitidis ZnuD zinc receptor contributes to interactions with epithelial cells and supports heme utilization when expressed in Escherichia coli. Infect. Immun. 2012;80:657–667. doi: 10.1128/IAI.05208-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dashper SG, et al. A novel Porphyromonas gingivalis FeoB plays a role in manganese accumulation. J. Biol. Chem. 2005;280:28095–28102. doi: 10.1074/jbc.M503896200. [DOI] [PubMed] [Google Scholar]
- 104.Bersch B, et al. New insights into histidine triad proteins: solution structure of a Streptococcus pneumoniae PhtD domain and zinc transfer to AdcAII. PLoS ONE. 2013;8:e81168. doi: 10.1371/journal.pone.0081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Graham AI, et al. Severe zinc depletion of Escherichia coli: roles for high affinity zinc binding by ZinT, zinc transport and zinc-independent proteins. J. Biol. Chem. 2009;284:18377–18389. doi: 10.1074/jbc.M109.001503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ilari A, et al. The Salmonella enterica ZinT structure, zinc affinity and interaction with the high-affinity uptake protein ZnuA provide insight into the management of periplasmic zinc. Biochim. Biophys. Acta. 2014;1840:535–544. doi: 10.1016/j.bbagen.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 107.Kehl-Fie TE, Skaar EP. Nutritional immunity beyond iron: a role for manganese and zinc. Curr. Opin. Chem. Biol. 2010;14:218–224. doi: 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Petrarca P, Ammendola S, Pasquali P, Battistoni A. The Zur-regulated ZinT protein is an auxiliary component of the high-affinity ZnuABC zinc transporter that facilitates metal recruitment during severe zinc shortage. J. Bacteriol. 2010;192:1553–1564. doi: 10.1128/JB.01310-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cerasi M, et al. The ZupT transporter plays an important role in zinc homeostasis and contributes to Salmonella enterica virulence. Metallomics. 2014;6:845–853. doi: 10.1039/C3MT00352C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grass G, Wong MD, Rosen BP, Smith RL, Rensing C. ZupT is a Zn(II) uptake system in Escherichia coli. J. Bacteriol. 2002;184:864–866. doi: 10.1128/JB.184.3.864-866.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sabri M, Houle S, Dozois CM. Roles of the extraintestinal pathogenic Escherichia coli ZnuACB and ZupT zinc transporters during urinary tract infection. Infect. Immun. 2009;77:1155–1164. doi: 10.1128/IAI.01082-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Corbett D, et al. Two zinc uptake systems contribute to the full virulence of Listeria monocytogenes during growth in vitro and in vivo. Infect. Immun. 2012;80:14–21. doi: 10.1128/IAI.05904-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rosadini CV, Gawronski JD, Raimunda D, Arguello JM, Akerley BJ. A novel zinc binding system, ZevAB, is critical for survival of nontypeable Haemophilus influenzae in a murine lung infection model. Infect. Immun. 2011;79:3366–3376. doi: 10.1128/IAI.05135-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bobrov AG, et al. The Yersinia pestis siderophore, yersiniabactin, and the ZnuABC system both contribute to zinc acquisition and the development of lethal septicaemic plague in mice. Mol. Microbiol. 2014;93:759–775. doi: 10.1111/mmi.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Braud A, Geoffroy V, Hoegy F, Mislin GL, Schalk IJ. Presence of the siderophores pyoverdine and pyochelin in the extracellular medium reduces toxic metal accumulation in Pseudomonas aeruginosa and increases bacterial metal tolerance. Environ. Microbiol. Rep. 2010;2:419–425. doi: 10.1111/j.1758-2229.2009.00126.x. [DOI] [PubMed] [Google Scholar]
- 116.Braud A, Hoegy F, Jezequel K, Lebeau T, Schalk IJ. New insights into the metal specificity of the Pseudomonas aeruginosa pyoverdine–iron uptake pathway. Environ. Microbiol. 2009;11:1079–1091. doi: 10.1111/j.1462-2920.2008.01838.x. [DOI] [PubMed] [Google Scholar]
- 117.Chaturvedi KS, Hung CS, Crowley JR, Stapleton AE, Henderson JP. The siderophore yersiniabactin binds copper to protect pathogens during infection. Nat. Chem. Biol. 2012;8:731–736. doi: 10.1038/nchembio.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kobayashi S, et al. Micacocidin A, B and C, novel antimycoplasma agents from Pseudomonas sp. II. Structure elucidation. J. Antibiot. 1998;51:328–332. doi: 10.7164/antibiotics.51.328. [DOI] [PubMed] [Google Scholar]
- 119.Kreutzer MF, et al. Biosynthesis of a complex yersiniabactin-like natural product via the mic locus in phytopathogen Ralstonia solanacearum. Appl. Environ. Microbiol. 2011;77:6117–6124. doi: 10.1128/AEM.05198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Price SL, et al. Yersiniabactin contributes to overcoming zinc restriction during Yersinia pestis infection of mammalian and insect hosts. Proc. Natl Acad. Sci. USA. 2021 doi: 10.1073/pnas.2104073118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Behnsen J, et al. Siderophore-mediated zinc acquisition enhances enterobacterial colonization of the inflamed gut. Nat. Commun. 2021;12:7016. doi: 10.1038/s41467-021-27297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao B, et al. Structural analysis of cytochrome P450 105N1 involved in the biosynthesis of the zincophore, coelibactin. Int. J. Mol. Sci. 2012;13:8500–8513. doi: 10.3390/ijms13078500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ghssein G, et al. Biosynthesis of a broad-spectrum nicotianamine-like metallophore in Staphylococcus aureus. Science. 2016;352:1105–1109. doi: 10.1126/science.aaf1018. [DOI] [PubMed] [Google Scholar]
- 124.Grim KP, et al. The metallophore staphylopine enables Staphylococcus aureus to compete with the host for zinc and overcome nutritional immunity. mBio. 2017 doi: 10.1128/mBio.01281-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Citiulo F, et al. Candida albicans scavenges host zinc via Pra1 during endothelial invasion. PLoS Pathog. 2012;8:e1002777. doi: 10.1371/journal.ppat.1002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cortese MS, et al. Metal chelating properties of pyridine-2,6-bis(thiocarboxylic acid) produced by Pseudomonas spp. and the biological activities of the formed complexes. Biometals. 2002;15:103–120. doi: 10.1023/A:1015241925322. [DOI] [PubMed] [Google Scholar]
- 127.Leach LH, Morris JC, Lewis TA. The role of the siderophore pyridine-2,6-bis(thiocarboxylic acid) (PDTC) in zinc utilization by Pseudomonas putida DSM 3601. Biometals. 2007;20:717–726. doi: 10.1007/s10534-006-9035-x. [DOI] [PubMed] [Google Scholar]
- 128.Jean S, Juneau RA, Criss AK, Cornelissen CN. Neisseria gonorrhoeae evades calprotectin-mediated nutritional immunity and survives neutrophil extracellular traps by production of TdfH. Infect. Immun. 2016;84:2982–2994. doi: 10.1128/IAI.00319-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Maurakis S, et al. The novel interaction between Neisseria gonorrhoeae TdfJ and human S100A7 allows gonococci to subvert host zinc restriction. PLoS Pathog. 2019;15:e1007937. doi: 10.1371/journal.ppat.1007937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stork M, et al. Zinc piracy as a mechanism of Neisseria meningitidis for evasion of nutritional immunity. PLoS Pathog. 2013;9:e1003733. doi: 10.1371/journal.ppat.1003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stork M, et al. An outer membrane receptor of Neisseria meningitidis involved in zinc acquisition with vaccine potential. PLoS Pathog. 2010;6:e1000969. doi: 10.1371/journal.ppat.1000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang T, et al. Type VI secretion system transports Zn2+ to combat multiple stresses and host immunity. PLoS Pathog. 2015;11:e1005020. doi: 10.1371/journal.ppat.1005020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang T, et al. ZntR positively regulates T6SS4 expression in Yersinia pseudotuberculosis. J. Microbiol. 2017;55:448–456. doi: 10.1007/s12275-017-6540-2. [DOI] [PubMed] [Google Scholar]
- 134.Si M, et al. Manganese scavenging and oxidative stress response mediated by type VI secretion system in Burkholderia thailandensis. Proc. Natl Acad. Sci. USA. 2017;114:E2233–E2242. doi: 10.1073/pnas.1614902114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lonergan ZR, et al. An Acinetobacter baumannii, zinc-regulated peptidase maintains cell wall integrity during immune-mediated nutrient sequestration. Cell Rep. 2019;26:2009–2018.e6. doi: 10.1016/j.celrep.2019.01.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim N, et al. The role of Zur-regulated lipoprotein A in bacterial morphology, antimicrobial susceptibility, and production of outer membrane vesicles in Acinetobacter baumannii. BMC Microbiol. 2021;21:27. doi: 10.1186/s12866-020-02083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Murphy SG, et al. Endopeptidase regulation as a novel function of the zur-dependent zinc starvation response. mBio. 2019 doi: 10.1128/mBio.02620-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pishchany G, Dickey SE, Skaar EP. Subcellular localization of the Staphylococcus aureus heme iron transport components IsdA and IsdB. Infect. Immun. 2009;77:2624–2634. doi: 10.1128/IAI.01531-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Haas CE, et al. A subset of the diverse COG0523 family of putative metal chaperones is linked to zinc homeostasis in all kingdoms of life. BMC Genomics. 2009;10:470. doi: 10.1186/1471-2164-10-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jordan MR, et al. Mechanistic insights into the metal-dependent activation of Zn(II)-dependent metallochaperones. Inorg. Chem. 2019;58:13661–13672. doi: 10.1021/acs.inorgchem.9b01173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nairn BL, et al. The response of Acinetobacter baumannii to zinc starvation. Cell Host Microbe. 2016;19:826–836. doi: 10.1016/j.chom.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Blaby-Haas CE, Flood JA, Crecy-Lagard V, Zamble DB. YeiR: a metal-binding GTPase from Escherichia coli involved in metal homeostasis. Metallomics. 2012;4:488–497. doi: 10.1039/c2mt20012k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chandrangsu P, Huang X, Gaballa A, Helmann JD. Bacillus subtilis FolE is sustained by the ZagA zinc metallochaperone and the alarmone ZTP under conditions of zinc deficiency. Mol. Microbiol. 2019;112:751–765. doi: 10.1111/mmi.14314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sousa Geros A, Simmons A, Drakesmith H, Aulicino A, Frost JN. The battle for iron in enteric infections. Immunology. 2020;161:186–199. doi: 10.1111/imm.13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Martin JE, et al. A Mn-sensing riboswitch activates expression of a Mn2+/Ca2+ ATPase transporter in Streptococcus. Nucleic Acids Res. 2019;47:6885–6899. doi: 10.1093/nar/gkz494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Knippel RJ, et al. Clostridioides difficile senses and hijacks host heme for incorporation into an oxidative stress defense system. Cell Host Microbe. 2020;28:411–421.e6. doi: 10.1016/j.chom.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Knippel RJ, et al. Heme sensing and detoxification by HatRT contributes to pathogenesis during Clostridium difficile infection. PLoS Pathog. 2018;14:e1007486. doi: 10.1371/journal.ppat.1007486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Saillant V, et al. A novel Enterococcus faecalis heme transport regulator (FhtR) senses host heme to control its intracellular homeostasis. mBio. 2021 doi: 10.1128/mBio.03392-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hodgkinson V, Petris MJ. Copper homeostasis at the host–pathogen interface. J. Biol. Chem. 2012;287:13549–13555. doi: 10.1074/jbc.R111.316406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Giachino A, Waldron KJ. Copper tolerance in bacteria requires the activation of multiple accessory pathways. Mol. Microbiol. 2020;114:377–390. doi: 10.1111/mmi.14522. [DOI] [PubMed] [Google Scholar]
- 151.Fung DK, Lau WY, Chan WT, Yan A. Copper efflux is induced during anaerobic amino acid limitation in Escherichia coli to protect iron–sulfur cluster enzymes and biogenesis. J. Bacteriol. 2013;195:4556–4568. doi: 10.1128/JB.00543-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Macomber L, Imlay JA. The iron–sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl Acad. Sci. USA. 2009;106:8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tan G, et al. Copper binding in IscA inhibits iron–sulphur cluster assembly in Escherichia coli. Mol. Microbiol. 2014;93:629–644. doi: 10.1111/mmi.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Macomber L, Rensing C, Imlay JA. Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli. J. Bacteriol. 2007;189:1616–1626. doi: 10.1128/JB.01357-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tree JJ, Kidd SP, Jennings MP, McEwan AG. Copper sensitivity of CueO mutants of Escherichia coli K-12 and the biochemical suppression of this phenotype. Biochem. Biophys. Res. Commun. 2005;328:1205–1210. doi: 10.1016/j.bbrc.2005.01.084. [DOI] [PubMed] [Google Scholar]
- 156.Grass G, et al. Linkage between catecholate siderophores and the multicopper oxidase CueO in Escherichia coli. J. Bacteriol. 2004;186:5826–5833. doi: 10.1128/JB.186.17.5826-5833.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li C, Li Y, Ding C. The role of copper homeostasis at the host–pathogen axis: from bacteria to fungi. Int. J. Mol. Sci. 2019 doi: 10.3390/ijms20010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sitthisak S, Knutsson L, Webb JW, Jayaswal RK. Molecular characterization of the copper transport system in Staphylococcus aureus. Microbiology. 2007;153:4274–4283. doi: 10.1099/mic.0.2007/009860-0. [DOI] [PubMed] [Google Scholar]
- 159.Fu Y, Chang FM, Giedroc DP. Copper transport and trafficking at the host–bacterial pathogen interface. Acc. Chem. Res. 2014;47:3605–3613. doi: 10.1021/ar500300n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fu Y, et al. A new structural paradigm in copper resistance in Streptococcus pneumoniae. Nat. Chem. Biol. 2013;9:177–183. doi: 10.1038/nchembio.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gold B, et al. Identification of a copper-binding metallothionein in pathogenic mycobacteria. Nat. Chem. Biol. 2008;4:609–616. doi: 10.1038/nchembio.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Franke S, Grass G, Rensing C, Nies DH. Molecular analysis of the copper-transporting efflux system CusCFBA of Escherichia coli. J. Bacteriol. 2003;185:3804–3812. doi: 10.1128/JB.185.13.3804-3812.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Katumba GL, Tran H, Henderson JP. The Yersinia high-pathogenicity island encodes a siderophore-dependent copper response system in uropathogenic Escherichia coli. mBio. 2022 doi: 10.1128/mBio.02391-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Barth VC, et al. Mycobacterium tuberculosis VapC4 toxin engages small ORFs to initiate an integrated oxidative and copper stress response. Proc. Natl Acad. Sci. USA. 2021 doi: 10.1073/pnas.2022136118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ladomersky E, Petris MJ. Copper tolerance and virulence in bacteria. Metallomics. 2015;7:957–964. doi: 10.1039/C4MT00327F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sullivan MJ, Goh KGK, Ulett GC. Cellular management of zinc in group B Streptococcus supports bacterial resistance against metal intoxication and promotes disseminated infection. mSphere. 2021 doi: 10.1128/mSphere.00105-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Botella H, et al. Mycobacterial p(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe. 2011;10:248–259. doi: 10.1016/j.chom.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Martin JE, et al. The zinc efflux activator SczA protects Streptococcus pneumoniae serotype 2 D39 from intracellular zinc toxicity. Mol. Microbiol. 2017;104:636–651. doi: 10.1111/mmi.13654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ong CL, Gillen CM, Barnett TC, Walker MJ, McEwan AG. An antimicrobial role for zinc in innate immune defense against group A Streptococcus. J. Infect. Dis. 2014;209:1500–1508. doi: 10.1093/infdis/jiu053. [DOI] [PubMed] [Google Scholar]
- 170.Ong CY, Berking O, Walker MJ, McEwan AG. New insights into the role of zinc acquisition and zinc tolerance in group A streptococcal infection. Infect. Immun. 2018 doi: 10.1128/IAI.00048-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Johnson MD, Kehl-Fie TE, Rosch JW. Copper intoxication inhibits aerobic nucleotide synthesis in Streptococcus pneumoniae. Metallomics. 2015;7:786–794. doi: 10.1039/C5MT00011D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Stocks CJ, et al. Frontline science: LPS-inducible SLC30A1 drives human macrophage-mediated zinc toxicity against intracellular Escherichia coli. J. Leukoc. Biol. 2021;109:287–297. doi: 10.1002/JLB.2HI0420-160R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Stojiljkovic I, Perkins-Balding D. Processing of heme and heme-containing proteins by bacteria. DNA Cell Biol. 2002;21:281–295. doi: 10.1089/104454902753759708. [DOI] [PubMed] [Google Scholar]
- 174.Capdevila DA, Wang J, Giedroc DP. Bacterial strategies to maintain zinc metallostasis at the host–pathogen interface. J. Biol. Chem. 2016;291:20858–20868. doi: 10.1074/jbc.R116.742023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ong CL, Walker MJ, McEwan AG. Zinc disrupts central carbon metabolism and capsule biosynthesis in Streptococcus pyogenes. Sci. Rep. 2015;5:10799. doi: 10.1038/srep10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li J, et al. Zinc toxicity and iron–sulfur cluster biogenesis in Escherichia coli. Appl. Environ. Microbiol. 2019 doi: 10.1128/AEM.01967-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lonergan ZR, Skaar EP. Nutrient zinc at the host–pathogen interface. Trends Biochem. Sci. 2019;44:1041–1056. doi: 10.1016/j.tibs.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kapetanovic R, et al. Salmonella employs multiple mechanisms to subvert the TLR-inducible zinc-mediated antimicrobial response of human macrophages. FASEB J. 2016;30:1901–1912. doi: 10.1096/fj.201500061. [DOI] [PubMed] [Google Scholar]
- 179.Zhanel GG, et al. Cefiderocol: a siderophore cephalosporin with activity against carbapenem-resistant and multidrug-resistant Gram-negative bacilli. Drugs. 2019;79:271–289. doi: 10.1007/s40265-019-1055-2. [DOI] [PubMed] [Google Scholar]
- 180.Hijazi S, et al. Antimicrobial activity of gallium compounds on ESKAPE pathogens. Front. Cell Infect. Microbiol. 2018;8:316. doi: 10.3389/fcimb.2018.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Greenwald J, et al. Real time fluorescent resonance energy transfer visualization of ferric pyoverdine uptake in Pseudomonas aeruginosa. A role for ferrous iron. J. Biol. Chem. 2007;282:2987–2995. doi: 10.1074/jbc.M609238200. [DOI] [PubMed] [Google Scholar]
- 182.Elbourne A, et al. Antibacterial liquid metals: biofilm treatment via magnetic activation. ACS Nano. 2020;14:802–817. doi: 10.1021/acsnano.9b07861. [DOI] [PubMed] [Google Scholar]
- 183.Shin M, et al. Characterization of an antibacterial agent targeting ferrous iron transport protein FeoB against Staphylococcus aureus and Gram-positive bacteria. ACS Chem. Biol. 2021;16:136–149. doi: 10.1021/acschembio.0c00842. [DOI] [PubMed] [Google Scholar]
- 184.Shin M, et al. Identification of a new antimicrobial agent against bovine mastitis-causing Staphylococcus aureus. J. Agric. Food Chem. 2021;69:9968–9978. doi: 10.1021/acs.jafc.1c02738. [DOI] [PubMed] [Google Scholar]
- 185.Veloria J, et al. Developing colorimetric and luminescence-based high-throughput screening platforms for monitoring the GTPase activity of ferrous iron transport protein B (FeoB) SLAS Discov. 2019;24:597–605. doi: 10.1177/2472555219844572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bennett MR, et al. Human mAbs to Staphylococcus aureus IsdA provide protection through both heme-blocking and Fc-mediated mechanisms. J. Infect. Dis. 2019;219:1264–1273. doi: 10.1093/infdis/jiy635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kim HK, et al. IsdA and IsdB antibodies protect mice against Staphylococcus aureus abscess formation and lethal challenge. Vaccine. 2010;28:6382–6392. doi: 10.1016/j.vaccine.2010.02.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hubert K, et al. ZnuD, a potential candidate for a simple and universal Neisseria meningitidis vaccine. Infect. Immun. 2013;81:1915–1927. doi: 10.1128/IAI.01312-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Qamsari MM, Rasooli I, Chaudhuri S, Astaneh SDA, Schryvers AB. Hybrid antigens expressing surface loops of ZnuD from Acinetobacter baumannii is capable of inducing protection against infection. Front. Immunol. 2020;11:158. doi: 10.3389/fimmu.2020.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bruhn KW, Spellberg B. Transferrin-mediated iron sequestration as a novel therapy for bacterial and fungal infections. Curr. Opin. Microbiol. 2015;27:57–61. doi: 10.1016/j.mib.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lin L, et al. Transferrin iron starvation therapy for lethal bacterial and fungal infections. J. Infect. Dis. 2014;210:254–264. doi: 10.1093/infdis/jiu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pi H, Helmann JD. Sequential induction of Fur-regulated genes in response to iron limitation in Bacillus subtilis. Proc. Natl Acad. Sci. USA. 2017;114:12785–12790. doi: 10.1073/pnas.1713008114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Smaldone GT, et al. A global investigation of the Bacillus subtilis iron-sparing response identifies major changes in metabolism. J. Bacteriol. 2012;194:2594–2605. doi: 10.1128/JB.05990-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Potter AD, et al. Host nutrient milieu drives an essential role for aspartate biosynthesis during invasive Staphylococcus aureus infection. Proc. Natl Acad. Sci. USA. 2020;117:12394–12401. doi: 10.1073/pnas.1922211117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Frank MW, et al. Host fatty acid utilization by Staphylococcus aureus at the infection site. mBio. 2020 doi: 10.1128/mBio.00920-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pandey AK, Sassetti CM. Mycobacterial persistence requires the utilization of host cholesterol. Proc. Natl Acad. Sci. USA. 2008;105:4376–4380. doi: 10.1073/pnas.0711159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pal RR, et al. Pathogenic E. coli extracts nutrients from infected host cells utilizing injectisome components. Cell. 2019;177:683–696.e18. doi: 10.1016/j.cell.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 198.Grant CR, et al. Distinct gene clusters drive formation of ferrosome organelles in bacteria. Nature. 2020 doi: 10.1038/s41586-022-04741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Weinberg ED. Iron availability and infection. Biochim. Biophys. Acta. 2009;1790:600–605. doi: 10.1016/j.bbagen.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 200.Ampel NM, Van Wyck DB, Aguirre ML, Willis DG, Popp RA. Resistance to infection in murine β-thalassemia. Infect. Immun. 1989;57:1011–1017. doi: 10.1128/iai.57.4.1011-1017.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wanachiwanawin W. Infections in E-β thalassemia. J. Pediatr. Hematol. Oncol. 2000;22:581–587. doi: 10.1097/00043426-200011000-00027. [DOI] [PubMed] [Google Scholar]
- 202.Quenee LE, et al. Hereditary hemochromatosis restores the virulence of plague vaccine strains. J. Infect. Dis. 2012;206:1050–1058. doi: 10.1093/infdis/jis433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wright AC, Simpson LM, Oliver JD. Role of iron in the pathogenesis of Vibrio vulnificus infections. Infect. Immun. 1981;34:503–507. doi: 10.1128/iai.34.2.503-507.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sanchez KK, et al. Cooperative metabolic adaptations in the host can favor asymptomatic infection and select for attenuated virulence in an enteric pathogen. Cell. 2018;175:146–158.e15. doi: 10.1016/j.cell.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zackular JP, et al. Dietary zinc alters the microbiota and decreases resistance to Clostridium difficile infection. Nat. Med. 2016;22:1330–1334. doi: 10.1038/nm.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Melby K, Slordahl S, Gutteberg TJ, Nordbo SA. Septicaemia due to Yersinia enterocolitica after oral overdoses of iron. Br. Med. J. 1982;285:467–468. doi: 10.1136/bmj.285.6340.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Okhuysen PC, Dupont HL. Enteroaggregative Escherichia coli (EAEC): a cause of acute and persistent diarrhea of worldwide importance. J. Infect. Dis. 2010;202:503–505. doi: 10.1086/654895. [DOI] [PubMed] [Google Scholar]
- 208.Xue Y, Osborn J, Panchal A, Mellies JL. The RpoE stress response pathway mediates reduction of the virulence of enteropathogenic Escherichia coli by zinc. Appl. Environ. Microbiol. 2015;81:3766–3774. doi: 10.1128/AEM.00507-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Liu MJ, et al. ZIP8 regulates host defense through zinc-mediated inhibition of NF-κB. Cell Rep. 2013;3:386–400. doi: 10.1016/j.celrep.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hall SC, et al. Critical role of zinc transporter (ZIP8) in myeloid innate immune cell function and the host response against bacterial pneumonia. J. Immunol. 2021;207:1357–1370. doi: 10.4049/jimmunol.2001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Jones DG, Suttle NF. The effect of copper deficiency on the resistance of mice to infection with Pasteurella haemolytica. J. Comp. Pathol. 1983;93:143–149. doi: 10.1016/0021-9975(83)90052-X. [DOI] [PubMed] [Google Scholar]
- 212.Newberne PM, Hunt CE, Young VR. The role of diet and the reticuloendothelial system in the response of rats to Salmonella typhilmurium infection. Br. J. Exp. Pathol. 1968;49:448–457. [PMC free article] [PubMed] [Google Scholar]
- 213.Juttukonda LJ, et al. Dietary manganese promotes staphylococcal infection of the heart. Cell Host Microbe. 2017;22:531–542.e8. doi: 10.1016/j.chom.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]