Abstract
Introduction
Bosutinib, a dual Abelson/Src inhibitor, was investigated in individuals with dementia with Lewy bodies (DLB).
Methods
A single site, randomized, double‐blind, placebo‐controlled study of the effects of oral bosutinib, 100 mg once daily for 12 weeks on primary safety and pharmacokinetics and secondary biomarker outcomes.
Results
Twenty‐six participants were randomized and included male and female (12:1) in the bosutinib arm and all male (13) in the placebo arm. The average age was 72.9 ± 8.1 (year ± standard deviation). There were no serious adverse events and no dropouts. Bosutinib was measured in the cerebrospinal fluid (CSF) and inhibited Abelson. Bosutinib reduced CSF alpha‐synuclein and dopamine catabolism.
Discussion
Bosutinib is safe and well tolerated and penetrates the blood–brain barrier to inhibit Abelson and reduce CSF alpha‐synuclein and dopamine catabolism, suggesting that bosutinib (100 mg) may be at or near the lowest effective dose in DLB. These results will guide adequately powered studies to determine the efficacy of a dose range of bosutinib and longer treatment in DLB.
Highlights
Bosutinib is a dual Abl/Src inhibitor that penetrates the blood brain barrier
Bosutinib is safe and tolerated in individuals with dementia with Lewy bodies
Bosutinib engages its target via inhibition of Abl and Src
Bosutinib reduces CSF alpha‐synuclein and attenuates breakdown of dopamine
Bosutinib improves activities of daily living in dementia with Lewy bodies
Keywords: Abelson, activities of daily living, alpha‐synuclein, dementia with Lewy bodies, dopamine
1. INTRODUCTION
Bosutinib monohydrate (Bosulif@, Pfizer, SKI‐606) is a dual inhibitor of the tyrosine kinases (TKs) Abelson (Abl)/Src and is approved to treat Philadelphia chromosome‐positive chronic myelogenous leukemia (CML) in a chronic phase at 500 mg/kg orally once daily. 1 , 2 , 3 , 4 Abl is elevated in Alzheimer's disease (AD) 5 , 6 and nigrostriatum of Parkinson's disease (PD). 7 TKs are activated via phosphorylation 5 , 8 , 9 , 10 and hippocampal injection of amyloid beta (Aβ) fibrils increases Abl levels 11 but Abl inhibition prevents Aβ fibril‐ and hydrogen peroxide (H2O2)‐induced cell death. 12 Abl knockout protects dopaminergic neurons against 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP) toxicity in PD models. 9 In animal models Abl/Src inhibition is an optimal strategy to reduce misfolded proteins and protect dopaminergic neurons. 7 , 13
Dementia with Lewy bodies (DLB) is characterized by loss of dopaminergic neurons and accumulation of misfolded alpha‐synuclein in Lewy bodies (LBs). Alpha‐synuclein, hyper‐phosphorylated tau (p‐tau), and Aβ aggregates are present in neurodegenerative diseases. 14 , 15 , 16 We previously demonstrated that bosutinib enters the brain and inhibits Abl at lower daily doses (5 mg/kg) than the CML daily dose (80 mg/kg) in animals. 17 We also demonstrated that bosutinib clears misfolded proteins, including alpha‐synuclein, p‐tau, and Abeta and via autophagy 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 reduces inflammation 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 and improves motor and cognitive behavior. 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 Therefore, bosutinib provides a potential therapeutic strategy that promotes autophagy to clear neurotoxic protein aggregates. 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 26 Based on supportive preclinical evidence, we investigated the effects of a lower oral daily dose of bosutinib, 100 mg, on safety, tolerability, and pharmacokinetics (PK) as a primary objective and biomarkers as secondary outcomes in patients with mild to moderate DLB.
RESEARCH IN CONTEXT
Systematic Review: Dementia with Lewy bodies (DLB) has no cure. Preclinical evidence demonstrated that a US Food and Drug Administration‐approved leukemia drug Abl/Src inhibitor called bosutinib (500 mg), alleviates DLB pathology. It is not known whether bosutinib enters the brain or benefits DLB patients. Individuals with DLB were randomized (1:1) into placebo (n = 13) and low oral daily dose (100 mg) of bosutinib (n = 13) for 12 weeks to determine bosutinib effects on safety, cerebrospinal fluid (CSF) concentration, and Abl inhibition.
Interpretation: This study met its primary objectives that bosutinib was safe and well tolerated and it entered the central nervous system and inhibited Abl. Bosutinib alleviated DLB pathology via reduction of CSF alpha‐synuclein and dopamine breakdown, and improved activities of daily living.
Future Direction: This study determined the lowest effective dose of bosutinib in DLB and it will guide larger multicenter studies using a dose range of bosutinib (100 to 400 mg) and longer treatment periods (> 6 months) to determine its safety and efficacy in DLB.
2. RESULTS
2.1. Enrollment and demographics
Of 120 subjects approached, 39 were screened, 13 did not meet inclusion/exclusion criteria, and 26 were randomized (Figure 1 and Table 1) and included male and female participants (12:1) in the bosutinib arm and all male participants (13) in the placebo arm with an average age 72.9 ± 8.1 (year ± standard deviation [SD]). All 26 participants completed all study procedures per protocol. Montreal Cognitive Assessment (MoCA) scores were 24.85 ± 3.5 (mean ± SD) in bosutinib and 23.92 ± 3.7 (mean ± SD) in placebo. The levodopa equivalent daily dose (LEDD) at enrollment to 12 weeks was 248.15 ± 296.33 mg (mean ± SD) in bosutinib and 417.35 ± 344.66 mg (mean ± SD) in placebo. Acetylcholinesterase inhibitors (AChEI), including therapeutic doses of transdermal rivastigamine (Exelon® patch) and donepezil and monoamine oxidase‐B (MAO‐B) inhibitor, rasagiline (Azilect) were used.
FIGURE 1.
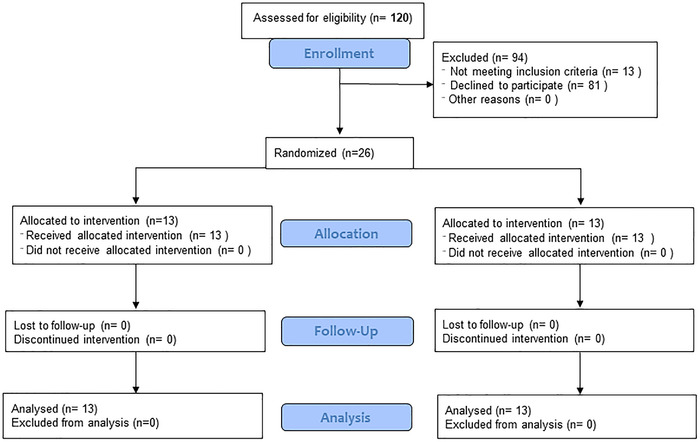
Consolidated Standards of Reporting Trials (CONSORT) diagram of phase 2, randomized, double‐blind, placebo‐controlled study to evaluate bosutinib effects on safety, tolerability, biomarkers, and clinical outcomes in dementia with Lewy bodies
TABLE 1.
Demographics and summary of all reported adverse events in bosutinib and placebo groups
| Demographics | |||
|---|---|---|---|
| Bosutinib (100 mg) | Placebo | P‐value | |
| Total enrolled | N = 13 | N = 13 | NA |
| Total finished end of treatment | 13 (100%) | 13 (100%) | 1 |
| Total dropped out | 0 (0%) | 0 (0%) | 1 |
| Average age (years) ±SD | 71.43 ± 7.94 | 74.45 ± 8.22 | 0.35 |
| Weight (kg) ±SD | 78.22 ± 7.63 | 82.61 ± 19.12 | 0.45 |
| Height (cm) ±SD | 174.28 ± 8.29 | 174.15 ± 4.34 | 0.96 |
| Body mass index (BMI) ± SD | 25.82 ± 2.75 | 27.06 ± 5.39 | 0.47 |
| Male | 12 (92.3%) | 13 (100%) | 1 |
| Female | 1 (7.7%) | 0 (0%) | |
| Race | 12 White (92.3%) | 12 White (92.3%) | 1 |
| 1 Black (7.7%) | 1 Asian (7.7%) | ||
| Montreal Cognitive Assessment (MoCA) at screening mean ± SD | 24.85 ± 3.5 | 23.92 ± 3.7 | 0.52 |
| Levodopa equivalent daily dose (LEDD) at baseline | 248.15 mg ± 296.33 | 417.35 mg ± 344.66 | 0.19 |
| LEDD at 12 weeks | 248.15 mg ± 296.33 | 417.35 mg ± 344.66 | 0.19 |
| Acetylcholinesterase inhibitors | No change | No change | |
| Adverse events (AEs) | |||
|---|---|---|---|
| System organ class | |||
| Preferred term | Number of events (%) | Number of events (%) | |
| Gastrointestinal disorders | |||
| Impaction | 0 (0%) | 1 (7.7%) | |
| General disorders | |||
| Falls | 3 (23.1%) | 3 (23.1%) | |
| Pain | 3 (23.1%) | 1 (7.7%) | |
| Flu | 0 (0%) | 1 (7.7%) | |
| Hepatic disorders | |||
| Liver transaminases | 1 (7.7%) | 0 (0%) | |
| Nervous system disorders | |||
| Post‐lumbar puncture headache | 1 (7.7%) | 0 (0%) | |
| Dizziness | 1 (7.7%) | 1 (7.7%) | |
| Renal and urinary disorders | |||
| Urinary incontinence | 1 (7.7%) | 0 (0%) | |
| Urinary tract infection | 1 (7.7%) | 0 (0%) | |
| Respiratory, thoracic, and mediastinal disorders | |||
| Upper respiratory infection | 0 (0%) | 1 (7.7%) | |
| Skin and subcutaneous disorder | |||
| Lesion | 0 0%) | 1 (7.7%) | |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; SD, standard deviation
2.2. Adverse events
There were no serious adverse events (SAEs) and no dropouts (Table 1). There was no prolongation of QTc intervals in both groups (Table S1 in supporting information). AEs were 11 in bosutinib and 9 in placebo (P = 0.68). The most common adverse event (AE) was fall, which was equal between groups. Dizziness was reported in both bosutinib and placebo. Pain was reported in bosutinib. There was one event of transient borderline liver enzyme elevation, including plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST), and one event of post lumbar puncture (LP) headache in bosutinib. Urinary tract infection and incontinence were reported in bosutinib, and skin lesion, impaction, flu, and upper respiratory tract infection in placebo.
2.3. Primary outcomes: pharmacokinetics and pharmacodynamics
An open label physiologically based population pharmacokinetic (popPK) study was performed and participants (n = 26) were randomized (1:1:1) into seamless random single dose (RSD) of bosutinib, 100 mg (n = 9), 200 mg (n = 9), or placebo (n = 8). LP was performed at 1, 2, 3, and 4 hours after bosutinib/placebo dosing. All 26 participants were then re‐randomized 1:1 into two groups (n = 13/per group) and double‐blind treatment of an oral daily dose of bosutinib, 100 mg, or matching placebo for 12 weeks. Another LP was performed at 1, 2, 3, and 4 hours at week 12 and bosutinib concentration was measured in plasma and cerebrospinal fluid (CSF). A dose‐dependent increase of bosutinib, 100 mg (Cmax = 13.5 ± 15.1 ng/mL) and 200 mg (Cmax = 19.6 ± 12.1 ng/mL) was detected in the plasma after a single dose (Table 2); and a similar dose‐dependent increase of CSF bosutinib, 100 mg (Cmax = 0.06 ±0.04 ng/mL) and 200 mg (Cmax = 0.13 ± 0.06 ng/mL) was observed. Exposure in the plasma was also dose dependent and the area under the curve between 0 and 4 hours (AUC0‐4) was 25 ± 11.8 and 36.9 ± 2.81 ng/mL x hour with bosutinib 100 and 200 mg, respectively. Exposure in the CSF showed AUC0‐4 was 0.14 ± 0.04 and 0.24 ± 0.03 ng/mL x hour with bosutinib 100 and 200 mg, respectively. Calculation of the predicted single dose of bosutinib based on detection of bosutinib, 500 mg (Cmax = 87.9 ± 30.8 ng/mL) in normal individuals showed almost identical plasma levels of 100 mg (Cmax = 17.6 ± 6.16 ng/mL) and the predicted concentration of plasma bosutinib dose‐dependently increased after a single dose of 200, 300, and 400 mg (Table 2). Exposure showed that AUC0‐24 also proportionally increased and Tmax was around 6 hours between 0 and 24 hours in previous studies 27 , 28 and was reduced to 3 to 4 hours between 0 and 4 hours in the current study.
TABLE 2.
Pharmacokinetics of a single dose of bosutinib 100 and 200 mg and multiple doses (100 mg once daily) for 3 months in plasma and cerebrospinal fluid (CSF) and the corresponding predicted values in plasma and CSF
| Expected/observed | Expected/observed | Predicted | Predicted | Predicted | Predicted | ||||
|---|---|---|---|---|---|---|---|---|---|
| Patients (n) | 9 | 9 | Subjects [n] | 6 | 6 | 6 | 6 | 6 | |
| Single oral dose | 100 mg | 200 mg | 500 mg | 400 mg | 300 mg | 200mg | 100mg | ||
| Plasma | Cmax (ng/mL) | 13.5 ± 15.1 | 19.6 ± 12.1 | Cmax (ng/mL) | 87.9 ± 30.8 | 70.3 ± 24.6 | 52.7 ± 18.5 | 35.2 ± 12.3 | 17.6 ± 6.16 |
| Cmax (nM) | 25.5 ± 28.5 | 37 ± 14.5 | |||||||
| Tmax (hrs) | 3 | 4 | Tmax (hrs) | 3.50 (2.00–6.00) | 3.50 (2.00–6.00) | 3.50 (2.00–6.00) | 3.50 (2.00‐6.00) | 3.50 (2.00‐6.00) | |
| AUC0‐4 (ng/mL*hr) | 25.4 ± 11.8 | 36.9 ± 2.81 | |||||||
| AUC0‐4 (nM*hr) | 47.9 ± 22.3 | 69.6 ± 5.3 | AUC0‐24 [ng/h/mL] | 2190 ± 661 | 1752 ± 529 | 1314 ± 397 | 876 ± 264 | 438 ± 132 | |
| CSF | Cmax (ng/mL) | 0.06 ± 0.04 | 0.13 ± 0.06 | t ½ [h] | 39.70 ± 8.49 | 31.8 ± 6.8 | 23.8 ± 5.09 | 15.9 ± 3.4 | 7.94 ± 1.7 |
| Cmax (nM) | 0.11 ± 0.08 | 0.25 ± 0.09 | |||||||
| Tmax (hrs) | 2 | 4 | |||||||
| AUC0‐4 (ng/mL*hr) | 0.14 ± 0.04 | 0.24 ± 0.03 | |||||||
| AUC0‐4 (nM*hr) | 0.26 ± 0.07 | 0.44 ± 0.06 | |||||||
| Expected/observed | Predicted | Predicted | Predicted | Predicted | |||||
|---|---|---|---|---|---|---|---|---|---|
| Patients (n) | 13 | 13 | 13 | 13 | 13 | ||||
| Multiple oral doses | 100 mg | 200 mg | 300 mg | 400 mg | 500 mg | ||||
| Plasma | Cmax (ng/mL) | 29.93 ± 10.27 | 59.9 ± 20.5 | 89.8 ± 30.8 | 120 ± 41.1 | 150 ± 51.4 | |||
| Cmax (nM) | 56.43 ± 19.34 | 113 ± 38.7 | 169 ± 58 | 226 ± 77.4 | 282 ± 96.7 | ||||
| Tmax (hrs) | 4 | 4 | 4 | 4 | 4 | ||||
| AUC0‐4 (ng/mL*hr) | 73.1 ± 6.63 | 146 ± 13.3 | 219 ± 19.9 | 292 ± 26.5 | 366 ± 33.2 | ||||
| AUC0‐4 (nM*hr) | 137.8 ± 12.5 | 276 ± 25 | 413 ± 37.5 | 551 ± 50 | 689 ± 62.5 | ||||
| CSF | Cmax (ng/mL) | 0.5 ± 0.17 | 1.0 ± 0.45 | 1.5 ± 0.52 | 2.0 ± 0.69 | 2.5 ± 0.86 | |||
| Cmax (nM) | 0.94 ± 0.32 | 1.9 ± 0.64 | 2.82 ± 0.96 | 3.76 ± 0.1.28 | 4.7 ± 1.6 | ||||
| Tmax (hrs) | 3 | 3 | 3 | 3 | 3 | ||||
| AUC0‐4 (ng/mL*hr) | 1.15 ± 0.15 | 2.3 ± 0.59 | 3.45 ± 0.47 | 4.6 ± 0.63 | 5.75 ± 0.79 | ||||
| AUC0‐4 (nM*hr) | 2.16 ± 0.21 | 4.32 ± 0.59 | 6.48 ± 0.89 | 8.64 ± 1.18 | 10.8 ± 1.49 |
Notes: AUC0‐4: area under the curve at 0 to 4 hours; Tmax: maximum time; Cmax: maximum concentration, T1/2: elimination.
The plasma level of multiple doses of bosutinib, 100 mg, increased more than 2‐fold in the plasma (Cmax = 29.93 ± 10.97 ng/mL) and 9‐fold in the CSF (Cmax = 0.5 ± 0.17 /mL) compared to a single dose (Table 2). Exposure after multiple doses increased almost 3‐fold in the plasma (AUC0‐4 = 73.1 ± 6.63 ng/mL x hour) and 10‐fold in the CSF (AUC0‐4 = 1.15 ± 0.16 ng/mL x hour) compared to a single dose. Multiple doses resulted in Tmax increases from 3 to 4 hours in the plasma and 2 to 3 hours in the CSF compared to a single dose. Estimation of CSF concentration of bosutinib based on observed exposure levels of multiple doses of bosutinib, 100 mg (AUC0‐4 = 2.16 ± 0.21 nM x hour) in individuals with DLB is predicted to increase (Table 2) in a dose proportional manner in 200 mg (AUC0‐4 = 4.32 ± 0.59 nM x hour) 300 mg (AUC0‐4 = 6.48 ± 0.89 nM x hour) and 400 mg (AUC0‐4 = 8.64 ± 1.18 nM x hour), achieving adequate concentrations > the maximal inhibitory coefficient of 50% (IC50 1.2 nM) of Abl/SRC 1 , 2 , 3 , 4 .
Pan‐tyrosine (Tyr) phosphorylated Abl (activated) as measured by mean differences between baseline and 12 weeks was significantly reduced in plasma (Figure 2A) and CSF (Figure 2B) in bosutinib, 100 mg, compared to placebo. Active Src, which is phosphorylated at Tyr416, was also significantly reduced in plasma (Figure 2C). We could not demonstrate differences in p‐Src in CSF (Figure 2D), likely because CNS exposure was below that required to see an effect.
FIGURE 2.
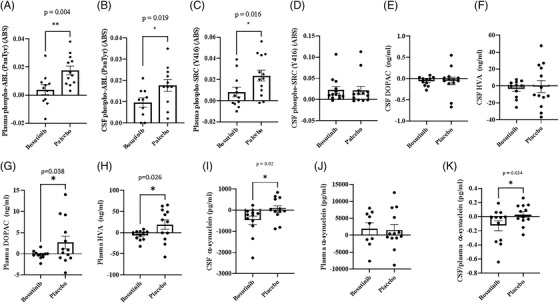
The effects of bosutinib on biomarkers. Histograms represent (A) plasma and (B) cerebrospinal fluid (CSF) levels of pan‐tyrosine phosphorylated Abl, (C) plasma and (D) CSF levels of phosphorylated Src at tyrosine (Y) 416, (E) CSF levels of 3,4‐dihydroxyphenylacetic acid (DOPAC) and (F) homovanillic acid (HVA), (G) plasma levels of DOPAC and (H) HVA, (I) CSF levels of alpha‐synuclein, (J) plasma levels of alpha‐synuclein, (K) the ratio of CSF/plasma alpha‐synuclein levels. n = 11 to 13 per group
2.4. Secondary biomarker objectives
There was no statistically significant difference in dopamine metabolite levels, including 3,4‐dihydroxyphenylacetic acid (DOPAC; Figure 2E, Table S2 in supporting information) and homovanillic acid (HVA) in the CSF (Figure 2F). However, plasma levels of DOPAC (Figure 2G) and HVA (Figure 2H) were significantly reduced in bosutinib, 100 mg, compared to placebo. Furthermore, the levels of alpha‐synuclein was significantly reduced in the CSF (Figure 2I) but not in plasma (Figure 2J), and the CSF:plasma of alpha‐synuclein (Figure 2K) was reduced in bosutinib, 100 mg, compared to placebo. There were no differences in CSF or plasma oligomeric alpha‐synuclein (Figure S1A‐D in supporting information), Aβ40 and Aβ42 (Figure S2E‐J in supporting information) as well as total tau and p‐tau181 (Figure S2A‐H) between bosutinib and placebo.
2.5. Exploratory clinical outcomes
There was no statistically significant difference in Alzheimer's Disease Assessment Scale–Cognitive subscale (ADAS‐Cog) and no change in MoCA scores between patients on bosutinib and placebo at baseline to 12 weeks (Table 3). The mean difference in ADAS‐Cog scores decreased at 12 to 16 weeks (–4.67 points) between groups, suggesting an increase (albeit non‐significant) in cognitive dysfunction in the wash‐out period. There was a trend showing that participants in the bosutinib group completed the Trail Making Test (TMT)‐Part B faster (–43 seconds) than the placebo group at 12 weeks, and they declined (–25 seconds) at 16 weeks. There was no difference in motor performance in the Timed Up and Go (TUG) test and the Unified Parkinson's Disease Rating Scale (UPDRS) Part I, II, and III (Table 3). There was no difference in behavioral outcomes, using Neuropsychiatric Inventory (NPI), Problem Behaviours Assessment (PBA), and Irritability/Apathy Scale (IAS) for both patients and caregivers (Table 3). Remarkably, there was a significant improvement (–5 points) in the mean difference scores in Alzheimer's Disease Cooperative Study‐Activities of Daily Living (ADCS‐ADL) between baseline and 12 weeks (Table 3), and this difference was not observed at week 16. There was no difference in Clinician Assessment of Fluctuation (CAF) between groups.
TABLE 3.
Clinical outcomes at baseline, end of treatment (12 weeks), and wash‐out period (16 weeks) comparing bosutinib and placebo
| Changes | Clinical endpoints | Bosutinib vs. placebo | P‐value Wilcoxon |
|---|---|---|---|
| 12 week‐ baseline | Montreal Cognitive Assessment (MoCA) | 0 (−1, 2) | 0.773 |
| Trail Making Test (TMT)‐Part B | −43 (−113, 22) | 0.151 | |
| Timed Up and Go | 0 (−3, 2) | 0.725 | |
| Unified Parkinson's Disease Rating Scale (UPDRS‐Cognition)‐Part I | 0 (−2, 1) | 0.795 | |
| Unified Parkinson's Disease Rating Scale (Activities of Daily Living)‐Part II | −1 (−3, 2) | 0.622 | |
| Unified Parkinson's Disease Rating Scale (Motor)‐Part III | 0 (−4, 4) | 0.938 | |
| Unified Parkinson's Disease Rating Scale (SUM Part II+III) | −1 (−6, 5) | 0.738 | |
| Unified Parkinson's Disease Rating Scale (total Part I+III) | −2 (−7, 6) | 0.777 | |
| Alzheimer's Disease Assessment Scale–Cognitive subscale (ADAS‐Cog) | −0.66 (−4.67, 3.33) | 0.817 | |
| Irritability and Apathy Scale (IAS)‐_Partner_APATHY | −1 (−4, 2) | 0.571 | |
| Irritability and Apathy Scale (IAS)‐_Participant_APATHY | 0 (−3, 4) | 0.836 | |
| Irritability and Apathy Scale (IAS)‐_Partner_IRRITABILITY | 1 (−1, 4) | 0.255 | |
| Irritability and Apathy Scale (IAS)‐_Participant_IRRITABILITY | 0 (−2, 3) | 0.777 | |
| Alzheimer's Disease Cooperative Study‐Activities of Daily Living (ADCS‐ADL) | −5 (−8, 0) | 0.037 | |
| Neuropsychiatric Inventory (NPI) frequency x severity | 0 (−8, 9) | 0.939 | |
| Neuropsychiatric Inventory (NPI)_Caregiver Distress | 2 (−1, 6) | 0.127 | |
| Problem Behaviours Assessment (PBA) | 1 (−14, 10) | 0.898 | |
| Clinician Assessment of Fluctuation (CAF) | 1 (−2,3) | 0.378 | |
| 16 week‐ baseline | Montreal Cognitive Assessment (MoCA) | 1 (−1, 3) | 0.234 |
| Trail Making Test (TMT)‐Part B | −25 (−74, 20) | 0.27 | |
| Timed Up and Go | 0 (−2, 1) | 1 | |
| Unified Parkinson's Disease Rating Scale (UPDRS‐Cognition)‐Part I | −1 (−2, 1) | 0.328 | |
| Unified Parkinson's Disease Rating Scale (Activities of Daily Living)‐Part II | 0 (−2, 2) | 0.815 | |
| Unified Parkinson's Disease Rating Scale (Motor)‐Part III | −3 (−9, 2) | 0.193 | |
| Unified Parkinson's Disease Rating Scale (SUM Part II+III) | −3 (−9, 3) | 0.271 | |
| Unified Parkinson's Disease Rating Scale (total Part I+III) | −4 (−10, 2) | 0.165 | |
| Alzheimer's Disease Assessment Scale–Cognitive subscale (ADAS‐Cog) | −4.67 (−10.34, 1) | 0.111 | |
| Irritability/Apathy Scale (IAS)‐_Partner_APATHY | 0 (−4, 4) | 0.836 | |
| Irritability/Apathy Scale (IAS)‐_Participant_APATHY | 0 (−4, 3) | 0.918 | |
| Irritability/Apathy Scale (IAS)‐_Partner_IRRITABILITY | 1 (−3, 5) | 0.553 | |
| Irritability/Apathy Scale (IAS)‐_Participant_IRRITABILITY | −2 (−4, 0) | 0.07 | |
| Alzheimer's Disease Cooperative Study‐Activities of Daily Living (ADCS‐ADL) | −2 (−8, 5) | 0.837 | |
| Neuropsychiatric Inventory (NPI) frequency x severity | 2 (−6, 10) | 0.608 | |
| Neuropsychiatric Inventory (NPI)_Caregiver Distress | 3 (−2, 8) | 0.142 | |
| Problem Behaviours Assessment (PBA) | 2 (−7, 11) | 0.589 | |
| Clinician Assessment of Fluctuation (CAF) | 1 (−1, 3) | 0.288 |
3. DISCUSSION
The study met 80% (26/30) of the enrollment target despite the COVID‐19 pandemic, which resulted in major disruptions in recruitment. Participants were male (25) and female (1) with an average age 72.9 ± 8.1 (year ± SD). This study enrolled 5 participants (19.2%) who ethnically self‐identified as Spanish (n = 3), Black (n = 1), and Asian (n = 1), therefore enrolling (19.2%) underrepresented minorities; however, there was an obvious lack of female representation. No dropouts or SAEs were reported, indicating that patients tolerated bosutinib. All patients complied with study procedures, including LPs. The placebo group received a higher level of LEDD (60%) than the bosutinib group, but all patients were stable on standard of care, including AChEI, rasagiline, and selective serotonin re‐uptake inhibitors (SSRIs). There were no SAEs and no dropouts. This study showed no cardiovascular and no significant AEs between groups, including 11 AEs in bosutinib and 9 AEs in placebo. The most common reported AE among the groups was fall, which was equal between groups, but one fall occurred between screening and baseline visits (prior to study drug) in bosutinib and two falls in placebo. All other AEs were < 10%, including one event of transient borderline liver transaminases elevation, ALT and AST, which resolved without medical intervention. Bosutinib was FDA approved in 2012 for CML and several clinical trials showed that oral bosutinib (>400 mg daily) is well tolerated with generally transient and self‐limited toxicity profile. 29 The prescribing information warnings and precautions include diarrhea and elevation of liver transaminases, cardiovascular and renal toxicity, fluid retention, and nasopharyngitis. 30 , 31 , 32 , 33 , 34 This study used a lower dose of bosutinib, 100 mg, compared to the CML approved dose and therefore we found only limited toxicity.
Bosutinib was measured in the CSF at low (< 2% of plasma levels) but adequate concentrations (dose‐dependent) to engage its target Abl. A single dose of bosutinib, 100 and 200 mg, resulted in a dose‐dependent increase in plasma and CSF bosutinib levels, but there was a significant difference with multiple doses (12 weeks) of bosutinib, 100 mg, which resulted in more than 2‐fold elevation in the plasma and 9‐fold elevation in the CSF. Exposure was also increased almost 3‐fold in the plasma and 10‐fold in the CSF and Tmax increased in the plasma and CSF after multiple doses. Prior studies of a single dose of bosutinib, 500 mg, with food in healthy subjects showed that bosutinib absorption was slow, with a median time‐to‐peak (Tmax) concentration of 6 hours, and although bosutinib exhibited dose‐proportional increases in AUC0‐24, 27 the volume of distribution was large, suggesting that bosutinib is distributed extensively into the tissues with low bioavailability. 27 , 35 Bosutinib also exhibits high plasma protein binding (94%). 35 These studies are consistent with our results suggesting that multiple doses may have affected the bioavailability, which subsequently resulted in enhanced detection of plasma and CSF bosutinib after 12 weeks compared to a single dose. Furthermore, this study evaluated the exposure of bosutinib over 4 hours (AUC0‐4), therefore plasma and CSF bosutinib PK parameters (Cmax, Tmax, and AUC) may have been underestimated whereas AUC0‐∞ values were not calculable. Administration of oral bosutinib, 100 mg, once daily resulted in direct target engagement via inhibition of plasma Abl and Src and CSF Abl in DLB patients, suggesting that this may be at or near the lowest effective therapeutic dose. The CSF concentration after multiple ascending doses of bosutinib (200, 300, 400 mg) is predicted to increase in a dose‐proportional manner and achieve drug exposure and greater concentrations than the IC50 (1.2 nM) required to inhibit Abl/Src. 1 , 2 , 3 , 4 Therefore, a dose range of bosutinib 100 to 400 mg daily, which has an established and acceptable safety profile, will guide the design of future studies to determine a safe and effective dose in DLB.
Plasma but not CSF levels of DOPAC and HVA were significantly reduced in bosutinib, 100 mg, compared to placebo. Both HVA and DOPAC are primary metabolites of dopamine and plasma HVA and DOPAC provide excellent controls for changes of CNS dopamine metabolism in this study. The brain contributes a small percentage (15%) of circulating plasma HVA, 36 which is mostly due to intestinal dopamine metabolism. CSF HVA peaks in PD patients around 1.5 to 2 hours after levodopa (200 mg) administration and it remains constant up to 4 hours, 37 suggesting that the absence of change of CSF HVA may be due to levodopa treatment in DLB patients. Conversely, DOPAC is almost exclusively derived from CNS metabolism of several monoamines, including dopamine, and it is secreted into the circulation and discarded via urination. Therefore, the decrease in plasma DOPAC may indicate that plasma DOPAC was secreted from the CNS. Taken together, these data may reflect changes in both CNS and peripheral dopamine levels.
This effect on dopamine metabolism is concurrent with a decrease of CSF alpha‐synuclein and CSF:plasma levels of alpha‐synuclein suggesting that less alpha‐synuclein may be produced peripherally or transported from the brain to plasma. Alpha‐synuclein may be transported into the plasma via brain‐derived exosomes, 38 which constitute a possible waste disposal mechanism. The level of CSF alpha‐synuclein is decreased in PD, but no data suggest the same in DLB. Additionally, it is still not understood whether a clinically effective drug should lead to an increase or further decrease of CSF alpha‐synuclein either in PD or DLB. Therefore, within the specific context of this longitudinal (3 months) measurement, our data indicate significant reduction of CSF alpha‐synuclein in response to bosutinib treatment in DLB. In animal models, accumulation of alpha‐synuclein aggregates impairs dopamine transmission but elimination of these aggregates enhances dopamine release and use. 39 Loss of dopaminergic neurons and aggregation of alpha‐synuclein are the main pathological characteristics of DLB 40 and these features may be present with p‐tau and Aβ. No effects were observed on p‐tau, Aβ, or oligomeric alpha‐synuclein between study groups. We documented extensive preclinical evidence that Abl/Src inhibition via bosutinib clears misfolded proteins, including alpha‐synuclein via autophagy and improves motor and cognitive behavior in models of neurodegeneration. 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 Bosutinib, 100 mg, may have resulted in minimal significant effects compared to placebo due to either the low dose or the short duration of the trial.
This study was underpowered (by design) for clinical measures, which were only exploratory to guide future trial designs of bosutinib in DLB. Remarkably, there was a significant improvement (–5 points) in activities of daily living (ADL) in the bosutinib group and this effect was not detected in the wash‐out period, suggesting possible effect of bosutinib on ADL. During the treatment period, a trending improvement in TMT‐B, which assesses executive functioning among multiple domains, 41 was observed and it attenuated in the washout period. Furthermore, the severity in cognitive dysfunction as measured by ADAS‐Cog was not different during the 12‐week treatment period, but strongly trended toward a decline (–4.75) between groups during wash‐out. An open label study (12 months) suggested that bosutinib was associated with less decline in Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) but withdrawal of bosutinib worsened cognitive performance. 42 There was no difference in motor symptoms as measured by UPDRS, and no behavioral differences were observed between groups.
In conclusion, this was a single site study with 13 DLB patients per group who were treated with a low dose of bosutinib, 100 mg, for 12 weeks. One limitation of this study is lack of female participants. This phase 2 study met its primary objectives and determined bosutinib PK, evidence of direct target engagement of Abl/Src, and effects on key DLB‐associated biomarkers, including dopamine metabolism and alpha‐synuclein. This study determined the effects of the lowest available dose of bosutinib to engage Abl in DLB and provided data for PK prediction of higher doses of bosutinib levels in the CSF. Larger multicenter studies using a dose range of bosutinib (100 to 400 mg) and longer treatment periods (> 6 months) are needed to determine the safe and effective dose in DLB.
4. METHODS
4.1. Standard protocol approvals and registrations
This is a single site study that was conducted by the Translational Neurotherapeutics Program (TNP) at Georgetown University Medical Center (GUMC). This study was approved by the Institutional Review Board (IRB# 000017) at GUMC. The study was conducted under FDA Investigational New Drug (IND) # 142061 and clinical trial number NCT03888222.
4.2. Randomization and blinding
This study used a block randomization using the blockrand function in R software (version 3.4) to randomize 26 participants into the two treatment groups. The block size varies between baseline and 12 weeks and the randomization was done within blocks to ensure a balance in sample sizes across groups blocks. All site staff, investigators, raters, participants, and caregivers were blinded to dose and treatment.
4.3. Participants
Participants provided informed consent and complied with all study procedures. Participants included males and females, multi‐racial groups, age of 25 to 90 years, medically stable, who had clinical diagnosis of DLB according to McKeith et al. 43 with both dementia MoCA≥18 and Parkinsonian defined as bradykinesia in combination with rest tremor, rigidity or both UPDRS I‐III (ON) is < 50 and UPDRS‐III between 20 and 40 and at least one other symptom such as fluctuation, visual hallucinations, or REM sleep behavioral disorder (RBD) and abnormal DaTScan (historical). Participants were stable on levodopa no more than 800 mg daily and dopamine agonists were allowed and participants were stabilized with 1 mg rasagiline (Azilect) at least 4 weeks before enrollment. Participants had stable concomitant medical and/or psychiatric illnesses and QTc interval 350 to 470 ms, inclusive, and they consented to LP at baseline and 12 weeks.
4.4. Study design and objectives
To primarily evaluate the effects of 100 mg bosutinib on safety, tolerability, and biomarkers in individuals with mild to moderate DLB. The primary objective included measurement of bosutinib in the CSF. Participants (n = 26) were randomized (1:1:1) into seamless RSD of 100 mg bosutinib (n = 9), 200 mg bosutinib (n = 9), or placebo (n = 8). LP was performed at 1, 2, 3, and 4 hours after dosing. Participants were then re‐randomized (1:1) into placebo and bosutinib, 100 mg, for 12 weeks followed by 4 weeks wash‐out. Another mandatory LP was performed at 1, 2, 3, and hours at 12 weeks. Safety was measured using the occurrence of AEs and SAEs deemed to be possibly, probably, or definitely related to the study drug.
4.5. Data analysis
Baseline characteristics were descriptively summarized using mean ± SD for continuous variables such as age and drug dose and frequencies and percentages for categorical variables by the two treatment groups. All measurements were longitudinal at baseline and 3 months at end of treatment. For comparisons between the two groups, either two‐sample t‐tests for continuous variables or Pearson's Chi‐squared test for the binary variables were used. AEs were summarized using frequencies and percentages by the two treatment groups. Exploratory biomarkers are presented as mean ± SD in charts and graphed as mean ± standard error of the mean. The changes in biomarkers within each group were compared using a one‐tailed paired Wilcoxon matched‐pairs signed rank test while the changes in biomarkers across each group were compared using a one‐tailed unpaired t‐test with Welch's corrections. Asterisks denote actual P‐value significances (*P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001) between groups or within groups and are noted in the individual figures. Biomarker statistical analysis was performed using GraphPad Prism, version 9.1.2 (GraphPad Software Inc.).
Exploratory clinical endpoints by the groups at baseline, 12 weeks, and 16 weeks were summarized using sample mean ± SDs along with trajectory plots of changes over visits. For each treatment group, a paired Wilcoxon signed‐rank test was used to test whether there were changes in each clinical endpoint between baseline and 12 weeks, baseline and 16 weeks. Changes from the baseline between the two treatment groups were evaluated using analysis of covariance (ANCOVA) where the baseline value was the fixed covariate. For biomarker endpoint comparisons, one‐sided type I error of 5% and 90% confidence interval (CI) was used, and two‐sided type I error of 5% and 95% CI for clinical endpoint comparisons was used. This exploratory endpoint was designed for proof‐of‐concept and no multiplicity correction was applied. This is a small proof of concept study to primarily determine PK/PD of bosutinib, so no formal power analysis was done. Biomarkers were secondary and behavioral; cognitive and motor outcomes were exploratory to guide future larger studies. Due to the small number of patients and the large exploratory clinical measures no correction for multiple statistical analyses could be meaningfully done.
4.6. Data sharing
The final data, study protocol, and all interpretations will be available to the scientific and non‐scientific community and clinicians. Investigators adhered to the Privacy Rule under the Health Insurance Portability and Accountability Act (HIPAA).
4.7. Plasma and CSF collection
Blood draw (10 mL) and LP ≈15 mL CSF were performed ≈2 hours after the last levodopa dose and at 1, 2, 3, or 4 hours after administration of bosutinib as we previously described. 44
4.8. Total alpha‐synuclein ELISA
Solid phase sandwich enzyme‐linked immunosorbent assay (ELISA) was used to measure CSF and plasma alpha‐synuclein according to manufacturer's protocol (Cat#844101, BioLegend) and as we previously described. 44
4.9. Oligomeric alpha‐synuclein ELISA
Quantitative ELISA was performed on CSF and plasma samples to measure alpha‐synuclein oligomer according to manufacturer's protocol (Cat# DEIA‐BJ882, Creative Diagnostics).
4.10. Phospho‐Abl (panTyr) ELISA
PathScan® phospho‐Abl solid phase sandwich ELISA was performed on CSF and plasma samples (100 μL), according to manufacturer's protocol (Cat# 7903, Cell Signaling Technologies) as we previously described. 45
4.11. Phospho‐Src (Y416) ELISA
PathScan phospho‐Src (CAT#7953, Cell Signaling Technologies). CSF or plasma samples were added to phospho‐Src polyclonal rabbit coated micro‐wells that capture Src proteins when phosphorylated at Tyr 416 according to PathScan phospho‐Src manufacturer's protocol (CAT#7953, Cell Signaling Technologies).
4.12. Aβ40, Aβ42, total tau, and p‐tau 181 ELISA
CSF and plasma samples were analyzed in parallel using the same reagents using Milliplex ELISA (Cat. #HNABTMAG‐68 K, Millipore) according to protocol as we previously described. 45
4.13. Mass spectrometry to measure bosutinib
Plasma and CSF (100 μI) samples were mixed with 10 μI of optimized internal standard followed by pipetting 400 μI of acetonitrile (ACN) containing 0.625% (v/v) formic acid. The samples were dried by use of a centrifugal evaporator miVac Duo (Genevac) and then reconstituted in 20 μI of the mobile phase A and analyzed by ultra‐high‐performance liquid chromatography tandem mass spectrometry with electrospray ionization (UHPLC‐ESI‐MS/MS) operating in optimal conditions.
4.14. Quantification of dopamine metabolites DOPAC and HVA
Concentrations of DOPAC and HVA in the CSF samples were measured by UHPLC‐MS/MS after derivatization with benzoyl chloride were measured by Pronexus Analytical AB, as we previously described. 46
4.15. Clinical assessments
All participants were tested in the “ON” state < 2 hours since the last dose of levodopa. A single rater conducted all clinical exams in all participants across all study visits and ON state was also verified with the participant and the objective report of study partner and study investigator/rater. All clinical assessments were performed at baseline and 12 weeks via, TUG, MoCA and MDS‐UPDRS, ADAS‐Cog, ADCS‐ADL, CAF, IAS, PBA, and NPI.
CONFLICTS OF INTERESTS
Charbel Moussa is an inventor on a Georgetown University (GU) US and International Patent to use bosutinib in neurodegenerative diseases, including alpha‐synucleinopathies. GU exclusively licensed bosutinib use patent to KeifeRx LLC. Charbel Moussa and Fernando Pagan are founders and shareholders of KeiferX LLC, and Charbel Moussa and Jaeil Ahn are paid consultants to KeifeRX LLC. All other authors declare no conflict with this manuscript.
AUTHOR CONTRIBUTIONS
Fernando L. Pagan is principal investigator; Yasar Torres‐Yaghi, Barbara Wilmarth, and R. Scott Turner recruited patients; Dalila Ferrante and Sara Matar managed data; Michaeline L. Hebron and Jaeil Ahn analyzed data; Charbel Moussa conceived of the study and wrote the manuscript.
Supporting information
FigureS1
FigureS2
TableS1
TableS2
ACKNOWLEDGMENTS
The authors acknowledge Jan Kehr and Staffan Schmidt at Pronexus Analytical AB, Bromma, Sweden for LC‐MS/MS for analysis of CSF HVA and DOPAC and the determination of bosutinib PK. The authors are grateful to all patients and caregivers who participated in this study, the research pharmacy at MGH, and to the nurses and staff of the CRU, which has been funded in whole or in part with federal funds (UL1TR000101 previously UL1RR031975) from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through the Clinical and Translational Science Awards Program (CTSA). The authors acknowledge and thank the members of the independent DSMB, including Drs Jill Farmer, Vasillios Papademetriou, Katherine Freeman‐Costin, and Robert Bies, as well as the study monitor, NEMA Research. This work was supported by the Alzheimer's Association Part the Cloud grant PTC‐19‐604235 to Charbel Moussa.
Pagan FL, Torres‐Yaghi Y, Hebron ML, et al. Safety, target engagement, and biomarker effects of bosutinib in dementia with Lewy bodies. Alzheimer's Dement. 2022;8:e12296. 10.1002/trc2.12296
REFERENCES
- 1. Keller VAG, Brummendorf TH. Novel aspects of therapy with the dual Src and Abl kinase inhibitor bosutinib in chronic myeloid leukemia. Expert Rev Anticancer Ther. 2012;12(9):1121‐1127. [DOI] [PubMed] [Google Scholar]
- 2. Cortes JE, Kantarjian HM, Brummendorf TH, et al. Safety and efficacy of bosutinib (SKI‐606) in chronic phase Philadelphia chromosome‐positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood. 2011;118(17):4567‐4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Puttini M, Coluccia AM, Boschelli F, et al. In vitro and in vivo activity of SKI‐606, a novel Src‐Abl inhibitor, against imatinib‐resistant Bcr‐Abl+ neoplastic cells. Cancer Res. 2006;66(23):11314‐11322. [DOI] [PubMed] [Google Scholar]
- 4. Musumeci F, Schenone S, Brullo C, Botta M. An update on dual Src/Abl inhibitors. Future Med Chem. 2012;4(6):799‐822. [DOI] [PubMed] [Google Scholar]
- 5. Tremblay MA, Acker CM, Davies P. Tau phosphorylated at tyrosine 394 is found in Alzheimer's disease tangles and can be a product of the Abl‐related kinase, Arg. J Alzheimers Dis. 2010;19(2):721‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schlatterer SD, Acker CM, Davies P. c‐Abl in neurodegenerative disease. J Mol Neurosci. 2011;45:445‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fowler AJ, Hebron M, Missner AA, et al. Multikinase Abl/DDR/Src inhibition produces optimal effects for tyrosine kinase inhibition in neurodegeneration. Drugs R D. 2019;19(2):149‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Imam SZ, Zhou Q, Yamamoto A, et al. Novel regulation of parkin function through c‐Abl‐mediated tyrosine phosphorylation: implications for Parkinson's disease. J Neurosci. 2011;31(1):157‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ko HS, Lee Y, Shin JH, et al. Phosphorylation by the c‐Abl protein tyrosine kinase inhibits parkin's ubiquitination and protective function. Proc Natl Acad Sci U S A. 2010;107(38):16691‐16696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jing Z, Caltagarone J, Bowser R. Altered subcellular distribution of c‐Abl in Alzheimer's disease. J Alzheimers Dis. 2009;17(2):409‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cancino GI, Toledo EM, Leal NR, et al. STI571 prevents apoptosis, tau phosphorylation and behavioural impairments induced by Alzheimer's beta‐amyloid deposits. Brain. 2008;131(Pt 9):2425‐2442. [DOI] [PubMed] [Google Scholar]
- 12. Alvarez AR, Sandoval PC, Leal NR, Castro PU, Kosik KS. Activation of the neuronal c‐Abl tyrosine kinase by amyloid‐beta‐peptide and reactive oxygen species. Neurobiol Dis. 2004;17(2):326‐336. [DOI] [PubMed] [Google Scholar]
- 13. Tsuji S, Hase T, Yachie‐Kinoshita A, et al. Artificial intelligence‐based computational framework for drug‐target prioritization and inference of novel repositionable drugs for Alzheimer's disease. Alzheimers Res Ther. 2021;13(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walker L, McAleese KE, Thomas AJ, et al. Neuropathologically mixed Alzheimer's and Lewy body disease: burden of pathological protein aggregates differs between clinical phenotypes. Acta Neuropathol. 2015;129(5):729‐748. [DOI] [PubMed] [Google Scholar]
- 15. Bassil F, Brown HJ, Pattabhiraman S, et al. Amyloid‐beta (Abeta) plaques promote seeding and spreading of alpha‐synuclein and tau in a mouse model of lewy body disorders with abeta pathology. Neuron. 2020;105(2):260‐275 e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jellinger KA. Dementia with Lewy bodies and Parkinson's disease‐dementia: current concepts and controversies. J Neural Transm (Vienna). 2018;125(4):615‐650. [DOI] [PubMed] [Google Scholar]
- 17. Lonskaya I, Hebron ML, Desforges NM, Franjie A, Moussa CE. Tyrosine kinase inhibition increases functional parkin‐Beclin‐1 interaction and enhances amyloid clearance and cognitive performance. EMBO Molecular Medicine. 2013;5(8):1247‐1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hebron ML, Lonskaya I, Olopade P, ST Selby, Pagan F, Moussa CE. Tyrosine kinase inhibition regulates early systemic immune changes and modulates the neuroimmune response in alpha‐synucleinopathy. J Clin Cell Immunol. 2014;5:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lonskaya I, Desforges NM, Hebron ML, Moussa CE. Ubiquitination increases parkin activity to promote autophagic alpha‐synuclein clearance. PLoS One. 2013;8(12):e83914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wenqiang C, Lonskaya I, Hebron ML, et al. Parkin‐mediated reduction of nuclear and soluble TDP‐43 reverses behavioral decline in symptomatic mice. Hum Mol Genet. 2014;23(18):4960‐4969. [DOI] [PubMed] [Google Scholar]
- 21. Hebron M, Lonskaya I, Moussa CE. Nilotinib reverses loss of dopamine neurons and improves motor behavior via autophagic degradation of α‐synuclein in Parkinson's disease models. Hum Mol Genet. 2013;22:3315‐3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lonskaya I, Hebron M, Chen W, Schachter J, Moussa C. Tau deletion impairs intracellular beta‐amyloid‐42 clearance and leads to more extracellular plaque deposition in gene transfer models. Molecular Neurodegeneration. 2014;9:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moussa CE. Parkin is dispensable for mitochondrial function, but its ubiquitin ligase activity is critical for macroautophagy and neurotransmitters: therapeutic potential beyond Parkinson's disease. Neuro‐Degenerative Diseases. 2015;15:259‐270. [DOI] [PubMed] [Google Scholar]
- 24. Lonskaya I, Hebron ML, Selby ST, Turner RS, Moussa CE. Nilotinib and bosutinib modulate pre‐plaque alterations of blood immune markers and neuro‐inflammation in Alzheimer's disease models. Neuroscience. 2015;304:316‐327. [DOI] [PubMed] [Google Scholar]
- 25. Ma L, Manaenko A, Ou YB, Shao AW, Yang SX, Zhang JH. Bosutinib attenuates inflammation via inhibiting salt‐inducible kinases in experimental model of intracerebral hemorrhage on mice. Stroke. 2017;48(11):3108‐3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Imamura K, Izumi Y, Watanabe A, et al. The Src/c‐Abl pathway is a potential therapeutic target in amyotrophic lateral sclerosis. Sci Transl Med. 2017;9(391):eaaf3962. [DOI] [PubMed] [Google Scholar]
- 27. Hanaizi Z, Unkrig C, Enzmann H, et al. The European medicines agency review of bosutinib for the treatment of adult patients with chronic myelogenous leukemia: summary of the scientific assessment of the committee for medicinal products for human use. Oncologist. 2014;19(4):421‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abbas R, Hug BA, Leister C, Gaaloul ME, Chalon S, Sonnichsen D. A phase I ascending single‐dose study of the safety, tolerability, and pharmacokinetics of bosutinib (SKI‐606) in healthy adult subjects. Cancer Chemother Pharmacol. 2012;69(1):221‐227. [DOI] [PubMed] [Google Scholar]
- 29. Hill BG, Kota VK, Khoury HJ. Bosutinib: a third generation tyrosine kinase inhibitor for the treatment of chronic myeloid leukemia. Expert Rev Anticancer Ther. 2014;14(7):765‐770. [DOI] [PubMed] [Google Scholar]
- 30. Doan V, Wang A, Prescott H. Bosutinib for the treatment of chronic myeloid leukemia. Am J Health Syst Pharm. 2015;72(6):439‐447. [DOI] [PubMed] [Google Scholar]
- 31. Bethelmie‐Bryan B, Lord K, Holloway S, Khoury HJ. Bosutinib treatment for Philadelphia chromosome‐positive leukemias. Future Oncol. 2014;10(2):179‐185. [DOI] [PubMed] [Google Scholar]
- 32. Chuah C, Koh LP, Numbenjapon T, et al. Efficacy and safety of bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia in the Asian subpopulation of the phase 3 BFORE trial. Int J Hematol. 2021;114(1):65‐78. [DOI] [PubMed] [Google Scholar]
- 33. Bosutinib . LiverTox: Clinical and Research Information on Drug‐Induced Liver Injury. Bethesda (MD) 2012. [PubMed]
- 34. Hochhaus A, Gambacorti‐Passerini C, Abboud C, et al. Bosutinib for pretreated patients with chronic phase chronic myeloid leukemia: primary results of the phase 4 BYOND study. Leukemia. 2020;34(8):2125‐2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abbas R, Hsyu PH. Clinical pharmacokinetics and pharmacodynamics of bosutinib. Clin Pharmacokinet. 2016;55(10):1191‐1204. [DOI] [PubMed] [Google Scholar]
- 36. Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004;56(3):331‐349. [DOI] [PubMed] [Google Scholar]
- 37. Stefani A, Pierantozzi M, Olivola E, et al. Homovanillic acid in CSF of mild stage Parkinson's disease patients correlates with motor impairment. Neurochem Int. 2017;105:58‐63. [DOI] [PubMed] [Google Scholar]
- 38. Ngolab J, Trinh I, Rockenstein E, et al. Brain‐derived exosomes from dementia with Lewy bodies propagate alpha‐synuclein pathology. Acta Neuropathol Commun. 2017;5(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wegrzynowicz M, Bar‐On D, Calo L, et al. Depopulation of dense alpha‐synuclein aggregates is associated with rescue of dopamine neuron dysfunction and death in a new Parkinson's disease model. Acta Neuropathol. 2019;138(4):575‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Braak H, Del Tredici K. Neuropathological staging of brain pathology in sporadic Parkinson's disease: separating the wheat from the chaff. J Parkinsons Dis. 2017;7(s1):S73‐S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Llinas‐Regla J, Vilalta‐Franch J, Lopez‐Pousa S, Calvo‐Perxas L, Torrents Rodas D, Garre‐Olmo J. The trail making test. Assessment. 2017;24(2):183‐196. [DOI] [PubMed] [Google Scholar]
- 42. Mahdavi KD, Jordan SE, Barrows HR, et al. Treatment of dementia with bosutinib: an open‐label study of a tyrosine kinase inhibitor. Neurol Clin Pract. 2021;11(3):e294‐e302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology. 2017;89(1):88‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pagan FL, Hebron ML, Wilmarth B, et al. Nilotinib effects on safety, tolerability, and potential biomarkers in parkinson disease: a phase 2 randomized clinical trial. JAMA Neurol. 2020;77(3):309‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Turner RS, Hebron ML, Lawler A, et al. Nilotinib effects on safety, tolerability, and biomarkers in Alzheimer's disease. Ann Neurol. 2020;88:183‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pagan FL, Hebron ML, Wilmarth B, et al. Pharmacokinetics and pharmacodynamics of a single dose Nilotinib in individuals with Parkinson's disease. Pharmacol Res Perspect. 2019;7(2):e00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FigureS1
FigureS2
TableS1
TableS2


