Abstract
INTRODUCTION:
Although dementia prevalence differs by race, it remains unclear whether cognition and neuropsychiatric symptom severity differ between Black and White individuals with dementia.
METHODS:
Using National Alzheimer’s Coordinating Center (NACC) data, we evaluated dementia prevalence in non-Hispanic Black and White participants and compared their clinico-demographic characteristics. We examined race differences in cognition, neuropsychiatric symptoms, and functional abilities in participants with dementia using multivariable linear and logistic regression models.
RESULTS:
We included 5,700 Black and 31,225 White participants across 39 Alzheimer’s Disease Centers. Of these, 1,528 (27%) Black and 11,267 (36%) White participants had dementia diagnoses. Despite having lower dementia prevalence, risk factors were more prevalent among Black participants. Black participants with dementia showed greater cognitive deficits, neuropsychiatric symptoms/severity, and functional dependence.
DISCUSSION:
Despite lower dementia prevalence, Black participants with dementia had more dementia risk factors, as well as greater cognitive impairment and neuropsychiatric symptom severity than White participants.
Keywords: African American, dementia, racial/ethnic disparities, neuropsychiatric symptoms, cognition
1. Background
Alzheimer’s disease and related dementias (ADRD) are a group of disorders characterized by a decline in cognitive functioning leading to loss of independence [1]. Currently, Alzheimer’s disease (AD) is the sixth leading cause of death in the U.S. and the fifth leading cause of death among adults age 65 and older [2]. Recent estimates suggest that 6.1 million people had clinical AD or mild cognitive impairment (MCI) due to AD in 2017, a number expected to grow to 15 million by 2060 [3]. Alongside the increased prevalence of ADRD in the U.S., changes in the demographic composition of the country are also anticipated in coming decades. Current projections suggest that, by 2050, 42% of the nation’s older adults will be racial/ethnic minorities [4,5]. Given the expected growth rate of ADRD is highest among racial/ethnic minority groups, this confluence of factors warrants vigilant attention to address existing healthcare disparities and ensure equitable access to care is available for at-risk groups [1].
According to the U.S. Census Bureau [6], the U.S. population is approximately 76.5% White and 13.4% Black, excluding those who identify as bi- or multiracial. Among those 65 years and older, Black individuals have the highest reported risk of ADRD relative to other racial groups [7]. For example, although empirical estimates suggest the prevalence of AD in Black individuals is highly variable, ranging from 14% to 500% greater than their White counterparts [8], the most common and conservative finding is that Black individuals are roughly 1.5-2 times as likely to develop AD compared to White individuals [9–11].
Among the factors that may contribute to these observed discrepancies, dementia risk factors deserve close consideration. Although race is not a biological distinction, certain physiological characteristics, diseases, and lifestyle factors associated with dementia risk differentially impact members of racial/ethnic minority groups [2,12–17]. Here, we define dementia risk factors as a set of physiological variables (e.g., body mass index [BMI], blood pressure), cardiovascular conditions (e.g., hypertension, diabetes mellitus, stroke, myocardial infarction, congestive heart failure), and lifestyle factors (e.g., smoking) that have been consistently associated with dementia and late-life cognitive decline [18–20]. The emergence of these dementia risk factors may be driven by more distal factors such as socioeconomic status, healthcare access/insurance coverage, clinical presentation, and timing of diagnosis [12,15,21–23]. Beyond consideration of the race-specific distribution of dementia risk factors, the co-occurrence of neuropsychiatric symptoms deserves additional attention to further address barriers to accurate and early diagnosis of ADRD among racial/ethnic minority groups.
Neuropsychiatric symptoms are common in ADRD [24]. These symptoms are interdependent and present in a heterogeneous manner across individuals [25]. For example, neuropsychiatric symptoms accompanying ADRD may include hallucinations, delusions, agitation, aggression, apathy, depression, and insomnia [26]. Clinical presentation of ADRD may also vary by racial/ethnic group or may be impacted by cultural factors. For instance, some research has suggested that Black individuals more frequently report hallucinations in conjunction with probable AD diagnosis [27]. Additionally, Black participants with AD reported more frequent insomnia, greater functional impairment, and a shorter duration of illness at the time of initial diagnosis in another study [28]. These variations in clinical presentation may undermine diagnostic accuracy, as there is evidence that missed diagnosis of ADRD is more common among older Black and Hispanic/Latinx individuals than among older White individuals [29–30].
Given the well-documented health disparities that exist between racial groups in the U.S., further investigation into how such disparities impact the rate of cognitive diagnoses secondary to AD is needed. Beyond differences in cognitive performance that may underlie such diagnostic discrepancies among racial groups, possible racial differences in neuropsychiatric symptoms and functional ability deserve attention to better assess confounds to dementia diagnosis. Substantial gaps in the scientific literature remain regarding the impact of racial/ethnic factors on ADRD, due in part to underrepresentation of non-White participants in population-based cohort studies investigating the national prevalence of ADRD [2,4,23], which only serves to magnify bias and inequity within the healthcare system. The present study is the first, to our knowledge, to retrospectively investigate racial representation and neuropsychiatric symptoms of non-Hispanic Black and White individuals within the Uniform Data Set (UDS) of the National Alzheimer’s Coordinating Center (NACC). Specifically, we aimed to: (1) determine the rates of dementia diagnosis among non-Hispanic Black and White participants in the full sample, and (2) examine racial differences in cognitive performance, neuropsychiatric symptoms, and functional abilities among participants with dementia. Importantly, the NACC, which aggregates data from Alzheimer’s Disease Research Centers (ADRCs) across the United States, is not necessarily representative of the population of Black and White individuals living within the community. The recent finding that ADRC recruitment and enrollment factors differ between Black and White participants highlights a potential source of bias that may influence clinic-based studies of race differences [31]. However, given that data from ADRCs are widely used to make inferences about ADRD, it is important to understand how Black and White individuals in this clinic-based sample differ with respect to important clinical characteristics.
2. Methods
2.1. National Alzheimer’s Coordinating Center Uniform Data Set
Data for this retrospective study were obtained from the UDS of the NACC, a public dataset established in 1999 by the National Institute on Aging that centralizes data across all ADRCs, including but not limited to demographic information, personal and family histories of AD, etiologic diagnoses, cognitive statuses and functioning [32], and neuropathology measures. The UDS data should be viewed as a case series with ongoing data collection, including data up to the quarterly data freeze date of March 1, 2020. Replication efforts can utilize data before or after any of these scheduled freeze dates.
2.2. Study Design and Participant Selection
Extracted UDS data for this analysis represent 39 ADRCs. This study considered all participants meeting inclusion criteria from September 1, 2005 up to March 1, 2020 (total N=36,925; dementia only N=12,795), with the exception of analyses related specifically to cognitive test scores (Supplemental files 1–2). Only initial visit cognitive data from tests included in the UDS Neuropsychological Battery (UDSNB) 3.0 revision were included in analyses. Unlike the full sample, individuals who completed the UDSNB 3.0 revision were initially seen between March 1, 2015 and March 1, 2020. To maximize our sample, we did not exclude those who completed cognitive tests prior to 2015, but removed participants on a test-by-test basis that included all tests that were present in both the earlier battery and the 3.0 revision (dementia only cognitive analysis N=11,026; Supplemental files 2–3). As we only used and analyzed data from the initial (index) visit for all eligible participants, this study was cross-sectional. Inclusion criteria included baseline data availability and non-Hispanic Black or non-Hispanic White race. We excluded all other reported races, including bi-and multiracial participants (e.g., White Hispanic, Black Hispanic), as well as participants for whom English was a second language. The exclusion of the Hispanic ethnicity was decided upon because it was not mutually exclusive, and beyond the scope of our study.
For cognitive data at the initial visits, each participant was characterized as “normal cognition”, “impaired not MCI”, “MCI”, or “dementia”. For cognitive, neuropsychiatric, and daily functioning analyses, participants with all-cause dementia (not specified by underlying etiology) were included. Participants were excluded from analyses of cognitive data only, if they presented with primary sensory deficits (e.g., visual and/or hearing impairments even after correction), or if they were not tested in English, due to the impact of these factors on test performance. Only initial visit cognitive data from tests comprising the UDS Neuropsychological Battery (UDSNB) 3.0 revision were included in analyses. Unlike the full sample, individuals who completed the UDSNB 3.0 revision were initially seen between March 1, 2015 and March 1, 2020. However, earlier participants completed certain tests that were later included in the UDSNB 3.0 revision, such as the Trail Making Test (TMT A & B).
2.3. Cognitive, Demographic, and Clinical Variables
Cognitive data were used from UDSNB 3.0 tests, as well as two informant-report measures, which included the following tests: the Montreal Cognitive Assessment (MoCA), TMT A & B, Digit Span Forward (DSF) and Backward (DSB), Verbal Fluency Phonemic Test, Category Fluency, Multilingual Naming Test (MINT), Craft Story 21 (Immediate & Delayed Recall), and Benson Figure (Copy & Recall). Informant-report measures included the Neuropsychiatric Inventory Questionnaire (NPIQ) and the Functional Activities Questionnaire (FAQ). UDSNB 3.0-specific normative data was used to calibrate measures (excluding MoCA, NPIQ, and FAQ) for age, sex, and education [33–34]. Additionally, we extracted relevant demographic and clinical variables from the visit concurrent with the cognitive assessment (see Tables 1 and 2). For a detailed description of cognitive variables, please see https://naccdata.org/data-collection/forms-documentation/uds-3.
Table 1.
Demographic and clinical characteristics and dementia status at baseline among the full National Alzheimer’s Coordinating Center sample, stratified by race, 2005-2020.
| Characteristic | Black (N = 5,700) |
White (N = 31,225) |
t (d) | χ2 (OR) |
|---|---|---|---|---|
| Demented at Baseline Visit | 1,528 (26.8) | 11,267 (36.1) | -- | 183.17 (0.65)*** |
| Demographic Variables | ||||
| Age (years), M (SD) | 72.12 (9.08) | 71.77 (10.69) | 2.33 (0.035)*** | -- |
| Education (years), M (SD) | 13.98 (3.29) | 15.72 (2.87) | −41.11 (.564)*** | -- |
| Sex, No. (%)† | ||||
| Female | 4,138 (72.6) | 14,560 (46.6) | -- | 724.31 (.14)*** |
| Male | 1,562 (27.4) | 16,665 (53.4) | ||
| APOE ε4 alleles, No. (%)‡ | ||||
| 0 allele | 1,947 (54.4) | 13,484 (58.9) | -- | 27.48 (1.20)*** |
| 1 allele | 1,373 (38.4) | 7,798 (34.1) | ||
| 2 alleles | 258 (7.2) | 1,599 (7.0) | ||
| Physiological & Lab Variables, M (SD) | ||||
| Body mass index, kg/m2 | 29.44 (6.42) | 26.63 (4.94) | 35.44 (0.49)*** | -- |
| Systolic BP, mm Hg | 138.36 (20.02) | 133.36 (18.55) | 17.60 (0.26)*** | -- |
| Diastolic, mm Hg | 77.57 (11.67) | 74.96 (10.43) | 16.23 (0.24)*** | -- |
| Hypercholesterolemia, No. (%)§ | 2,898 (51.7) | 15,344 (49.7) | -- | 7.39 (1.08)** |
| Cardiovascular Disease, No. (%) | ||||
| Hypertension§ | 4,183 (73.7) | 14,089 (45.3) | -- | 1,546.32 (3.38)*** |
| Diabetes mellitus§ | 1,500 (26.5) | 2,879 (9.2) | -- | 1,359.86 (3.54)*** |
| Stroke§ | 428 (7.5) | 1,206 (3.9) | -- | 151.72 (2.02)*** |
| Myocardial infarction§ | 301 (5.3) | 1,689 (5.4) | -- | 0.14 (0.98) |
| Congestive heart failure§ | 30 (2.3) | 58 (0.8) | -- | 23.18 (2.85)*** |
| Medication Use, No. (%) | ||||
| Antihypertensive | 3,765 (66.7) | 15,239 (49.3) | -- | 576.71 (2.06)*** |
| Cholesterol-lowering | 2,218 (39.3) | 12,736 (41.2) | -- | 7.40 (0.92)** |
| Diabetes medication | 1,109 (19.6) | 2,207 (7.1) | -- | 903.94 (3.18)*** |
| Smoking Status | ||||
| Cigarette use (years), M (SD) | 11.93 (17.24) | 9.53 (14.63) | 10.81 (0.15)*** | -- |
| Current cigarette use (years), No. (%) | 481 (8.5) | 1,209 (3.9) | -- | 229.06 (2.28)*** |
Values are displayed as M (SD) for continuous variables, and No. (%) for all categorical variables; percentages are within-column (e.g., race); OR analyses conducted with Black coded 1 and White coded 0.
CONSORT flow diagram represented in Supplemental file 1.
ϕ displayed for sex comparison effect size;
OR shown reflects binary comparison (e.g., 0 vs. ≥ 1 APOE ε4 allele);
Frequencies reflect any history (e.g., remote, recent, or active).
Abbreviations: APOE ε4, Apolipoprotein E ε4; M, Mean; No., Number; OR, Odds ratio; SD, Standard deviation.
p<.01;
p<.001
Table 2.
Demographic and clinical characteristics among National Alzheimer’s Coordinating Center participants with dementia at baseline, stratified by race, 2005-2020.
| Characteristic | Black (N = 1,528) |
White (N = 11,267) |
t (d) | χ2 (OR) |
|---|---|---|---|---|
| Demographic Variables | ||||
| Age (years), M (SD) | 76.04 (9.11) | 72.24 (10.50) | 13.47 (0.39)*** | -- |
| Education (years), M (SD) | 12.75 (3.56) | 15.12 (3.01) | 28.10 (0.72)*** | -- |
| Sex † | ||||
| Female, No. (%) | 1,021 (66.8) | 5,509 (48.9) | -- | 172.99 (.12)*** |
| Male, No. (%) | 507 (33.2) | 5,758 (51.2) | ||
| APOE ε4 alleles, No. (%)‡ | ||||
| 0 allele | 318 (36.1) | 3,839 (47.3) | ||
| 1 allele | 433 (49.1) | 3,358 (41.4) | -- | 41.22 (1.59)*** |
| 2 alleles | 130 (14.8) | 916 (11.3) | ||
| Physiological & Lab Variables, M (SD) | ||||
| Body mass index, kg/m2 | 27.01 (5.81) | 26.27 (4.83) | 5.01 (0.14)*** | -- |
| Systolic BP, mm Hg | 139.59 (21.20) | 133.83 (19.11) | 10.38 (0.29)*** | -- |
| Diastolic BP, mm Hg | 76.90 (11.96) | 75.25 (10.72) | 5.32 (0.15)*** | -- |
| Hypercholesterolemia, No. (%)§ | 751 (50.3) | 5,506 (49.4) | -- | 0.36 (1.03) |
| Cardiovascular Disease, No. (%) | ||||
| Hypertension§ | 1,148 (75.4) | 5,135 (45.8) | -- | 469.73 (3.63)*** |
| Diabetes mellitus§ | 379 (25.0) | 1,043 (9.3) | -- | 330.94 (3.25)*** |
| Stroke§ | 207 (13.6) | 598 (5.3) | -- | 155.24 (2.80)*** |
| Myocardial infarction§ | 100 (6.6) | 679 (6.1) | -- | 0.64 (1.09) |
| Congestive heart failure§ | 86 (5.7) | 266 (2.4) | -- | 54.05 (2.47)*** |
| Medication Use, No. (%) | ||||
| Antihypertensive | 1,031 (68.4) | 5,392 (48.2) | -- | 216.28 (2.32)*** |
| Cholesterol-lowering | 620 (41.1) | 4,522 (40.5) | -- | 0.26 (1.03) |
| Diabetes medication | 272 (18.0) | 775 (6.9) | -- | 216.68 (2.96)*** |
| Smoking Status | ||||
| Cigarette use (years), M (SD) | 11.55 (17.65) | 9.71 (15.35) | 4.13 (0.11)*** | -- |
| Current cigarette use, No. (%) | 103 (6.8) | 483 (4.3) | -- | 18.62 (1.62)*** |
Values are displayed as M (SD) for continuous variables, and No. (%) for all categorical variables; percentages are within-column (e.g., race); OR analyses conducted with Black coded 1 and White coded 0.
CONSORT flow diagram represented in Supplemental file 2.
ϕ displayed for sex comparison effect size;
OR shown reflects binary comparison (e.g., 0 vs. ≥ 1 APOE ε4 allele);
Frequencies reflect any history (e.g., remote, recent, or active).
Abbreviations: APOE ε4, Apolipoprotein E ε4; BP, Blood pressure; M, Mean; No., Number; OR, Odds ratio; SD, Standard deviation.
p<.001
2.4. Statistical Analysis
Analyses were performed with SPSS Statistics software, version 26 (IBM). Data visualization was completed using GraphPad Prism version 8 (GraphPad Software, La Jolla, California USA). Chi-square (χ2) tests of independence were used to examine the relation between race and dementia status for the full sample, as well as the associations among race and medical, clinical, and demographical categorical variables. Independent samples t-tests were used to compare continuous patient characteristics, stratified by race.
As noted above, cognitive, neuropsychiatric, and daily functioning variables were only examined in the dementia sample. To analyze differences in performance across cognitive variables between Black and White participants, we used univariate general linear models with and without adjusting for age, sex, and education. To examine race differences in neuropsychiatric symptoms (NPIQ) and daily functioning (FAQ), we used χ2 tests of independence as well as unadjusted and adjusted (for age, sex, and education) binary logistic models. For each FAQ item, ‘Normal’ and ‘Has difficulty, but does by self’ responses were coded as ‘0’ and ‘Requires assistance’ and ‘Dependent’ responses were coded as ‘1’. In addition, NPIQ (symptom count and severity, separately) and FAQ items were summed across domains (where higher scores reflect greater functional dependence/impairment) and compared racial groups using corrected (for age, sex, and education) and uncorrected univariate general linear models. Partial eta squared () and exponentiated betas (eβ; analogous with odds ratios) were used as estimates of effect size for between-group general linear, and binary logistic regression models, respectively. Based on preliminary data screening, NPIQ total severity, FAQ total, and all cognitive variables were rank-transformed to correct for distribution non-normality. Analysis also revealed no pattern of systematic missingness (between races) among variables (NPIQ: both White and Black = 3.6% missingness; FAQ: White = 35.5%, Black = 39.5% missingness, ps>.05).
3. Results
A total of 5,700 non-Hispanic Black (15%) and 31,225 non-Hispanic White (85%) individuals who participated in the UDS from 2005-2020 were included in the final analytic sample. Tables 1 and 2 report demographic and clinical data for the full sample and the subset of individuals with dementia. At the index (baseline) visit, 26.8% (N=1,528) of Black participants were diagnosed with dementia, whereas 36.1% (N=11,267) of White participants were diagnosed with dementia (χ2=183.17; P<.001) (Table 1). Analysis also showed that Black participants had 35% lower odds of having a dementia diagnosis at the initial visit relative to White participants. The three most common dementia primary etiologies for the Black participants were AD (N=1,292, 84.6%), vascular dementia (N=76, 5.0%), and Lewy body disease (LBD; N=61, 4.0%), while the primary etiologies for White participants were AD (N=7,936, 70.4%), frontotemporal lobar degeneration (N=1,611, 14.3%), and LBD (N=758, 6.7%). The frontotemporal lobar degeneration classification included all frontotemporal dementias and primary progressive aphasia, with the exclusion of amyotrophic lateral sclerosis.
Despite the lower dementia prevalence, dementia risk factors were more widespread among Black participants compared to White participants in the full sample (Table 1), including significantly fewer years of education, and greater rates of hypertension and diabetes. Further analysis revealed a disproportionately higher number of Black participants with ≤12 years of education (N=2,223, 39%) compared to White participants (N=6,247, 20%). Additionally, we note that Black men were considerably underrepresented, comprising only 27% of the full sample of Black participants. By comparison, men represented just over half (53%) of White participants.
Important race differences were also present in the subset of NACC participants diagnosed with dementia. Specifically, Black participants with dementia were significantly older, more likely to have ≤12 years and less likely to have ≥16 years of education, and more likely to possess one or more copies of the APOE ε4 allele. Additionally, vascular risk factors, including BMI, hypertension, and diabetes, were more prevalent among Black participants with dementia (Table 2).
Regarding cognition, Black participants with dementia generally had lower scores on the MoCA screening instrument, compared to White participants (mean difference: 2.5 points). Further analysis of race difference among cognitive measures (Table 3) revealed a pattern of comparatively lower performances on measures of processing speed (TMT A), executive function/working memory (TMT B, DSB), and language (Category Fluency, Verbal Fluency Phonemic Test, MINT) among Black participants, after adjusting for age, sex, and education, though these effects were generally small. Post-hoc analyses indicated that these racial differences were generally present across varying levels of dementia severity, as measured by the CDR® Dementia Staging Instrument (Figure 1). Notably, Black and White participants with dementia did not differ on measures of memory or visuospatial abilities.
Table 3.
Cognition among National Alzheimer’s Coordinating Center participants with dementia at baseline, stratified by race.
| Black (N = 1,311)† | White (N = 9,715)† | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|---|
| Measure | M (SD) | Med (IQR) | M (SD) | Med (IQR) | F() | F() |
| MoCA‡ | 13.03 (5.99) | 13.00 (6.00) | 15.52 (6.22) | 16.00 (7.00) | 23.15 (.013)*** | 11.403 (.006)*** |
| TMT A | 12.74 (32.33) | 18.91 (30.68) | 19.86 (37.03) | 33.55 (26.10) | 1007.29 (.012)*** | 218.98 (.024)*** |
| TMT B | 18.48 (22.09) | 23.06 (36.06) | 20.22 (28.32) | 27.13 (42.76) | 19.57 (.003)*** | 89.00 (.013)*** |
| DSF | 42.99 (10.22) | 41.43 (16.93) | 42.12 (10.49) | 41.67 (13.37) | 0.35 (<.001) | 1.95 (.001) |
| DSB | 35.59 (9.85) | 34.77 (13.33) | 36.84 (10.74) | 36.36 (13.05) | 1.51 (<.001) | 11.95 (.007)*** |
| AN | 30.07 (9.52) | 30.00 (15.95) | 30.55 (11.08) | 30.26 (14.31) | 2.09 (<.001) | 27.26 (.003)*** |
| LF (F & L) | 36.22 (10.50) | 34.28 (16.51) | 36.81 (11.88) | 36.31 (15.05) | 0.56 (<.001) | 5.00 (.003)* |
| MINT | 16.05 (35.94) | 27.27 (20.42) | 23.98 (33.01) | 34.44 (28.57) | 12.48 (.007)*** | 13.36 (.008)*** |
| BF Copy | 29.89 (30.36) | 39.29 (18.94) | 27.67 (36.48) | 41.11 (18.57) | 0.24 (<.001) | 2.43 (.001) |
| BF DR | 22.57 (13.91) | 19.69 (25.89) | 22.92 (15.28) | 19.70 (23.64) | 0.04 (<.001) | 3.14 (.002) |
| CS21 IR | 30.10 (10.70) | 28.20 (17.17) | 30.07 (10.40) | 29.11 (12.91) | <0.01 (<.001) | 1.61 (.001) |
| CS21 DR | 28.12 (9.48) | 26.14 (19.61) | 28.50 (9.89) | 26.14 (15.03) | 0.10 (<.001) | 3.33 (.002) |
With the exception of MoCA (which used raw scores), all cognitive data were transformed into standardized T-scores, calibrated for age, sex, and years of education. Adjusted statistical models include age, sex, and education as covariates. Cognitive variables were rank transformed due to distribution non-normality.
Sample sizes varied according to cognitive test (Supplemental files 2–3);
MoCA not adjusted for education.
Abbreviations: AN, Animal naming; BF, Benson figure; CS21, Craft Story 21-Item; DR, Delayed recall; DSF, Digit Span Forward; DSB, Digit Span Backward; IQR, Interquartile range; IR, Immediate recall; LF, Letter fluency; M, Mean; Med, Median; MINT, Multilingual naming test; MoCA, Montreal Cognitive Assessment; , Partial eta squared; SD, Standard deviation; TMT, Trail Making Test.
p<.05;
p≤.001
Figure 1. Cognitive performance and neuropsychiatric symptom severity among Black and White participants with dementia, stratified by CDR category.
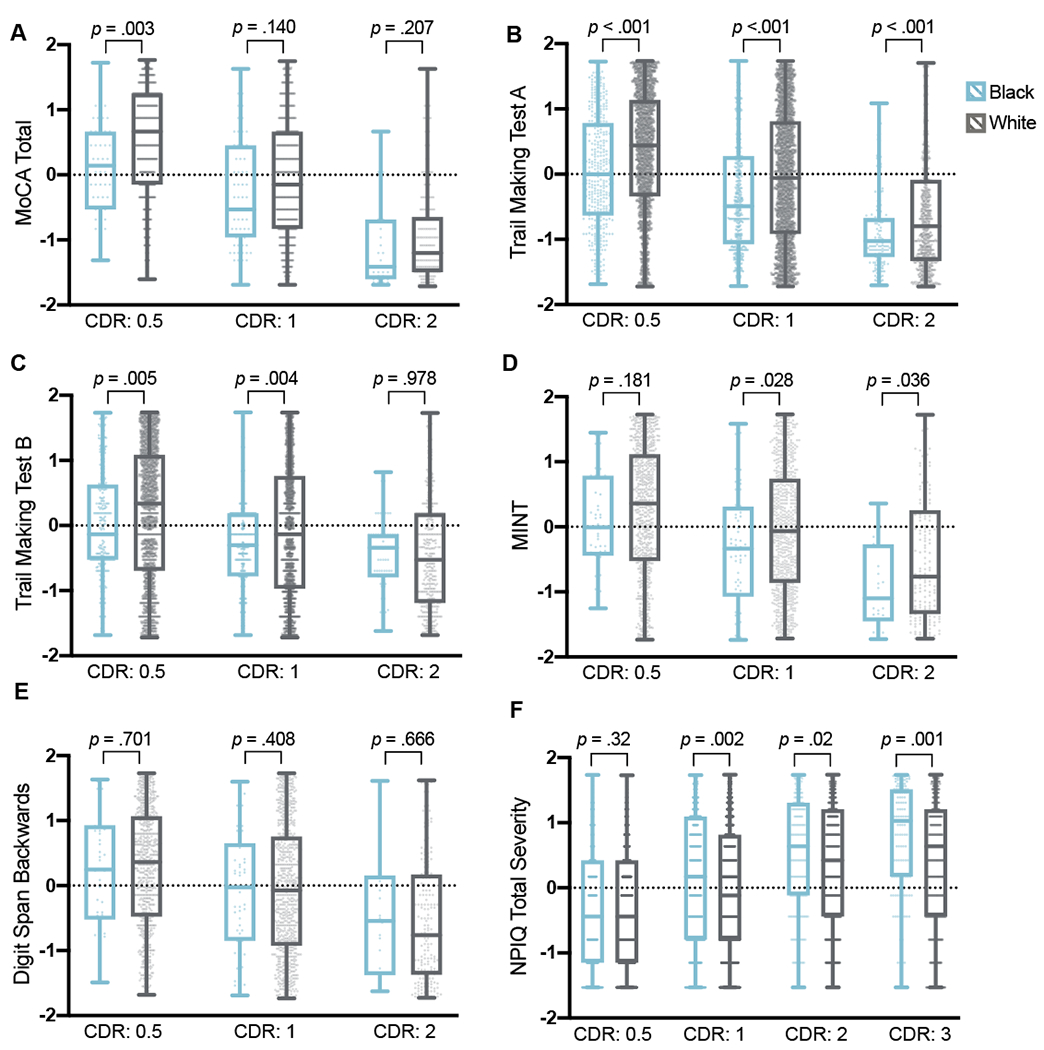
Differences in rank-transformed variables were examined with independent samples t-test at each CDR level, stratified by race.
Abbreviations: CDR, CDR® Dementia Staging Instrument (0 = No impairment; 0.5 = Questionable or very mild dementia; 1 = Mild dementia; 2 = Moderate dementia); MINT, Multilingual Naming Test; MoCA, Montreal Cognitive Assessment; NPIQ, Neuropsychiatric Inventory Questionnaire.
Neuropsychiatric symptoms were also more likely to occur in Black participants with dementia than in White participants with dementia, measured by the NPIQ informant reports. In models adjusted for age, sex, and education, the odds of experiencing delusions and hallucinations were approximately double among Black individuals with dementia (Table 4). Other symptoms, including agitation/aggression, disinhibition, irritability/lability, motor disturbances, abnormal nocturnal behavioral, and appetite/eating changes, were also more likely to occur in Black participants with dementia after accounting for demographic covariates. Black and White participants with dementia did not differ with regard to affective or anxiety symptoms, or apathy and indifference. Black participants with dementia reported approximately one point greater functional impairment on the FAQ (Table 5). However, this significant difference was not retained in the adjusted model. As with cognitive data, effects were small across FAQ analyses.
Table 4.
Neuropsychiatric presentations among National Alzheimer’s Coordinating Center participants with dementia at baseline, stratified by race.
| NPIQ Domain No. (%) |
Black (N = 1,528)† |
White (N = 11,267)† |
χ2 (ϕ) | Unadjusted eβ (95% CI) |
Adjusted eβ (95% CI) |
|---|---|---|---|---|---|
| Symptom present: | |||||
|
| |||||
| Agitation/aggression | 707 (48.0) | 3,820 (35.2) | 92.00 (.09) | 1.70 (1.53, 1.90)*** | 1.69 (1.51, 1.90)*** |
| Anxiety | 565 (38.4) | 4,568 (42.1) | 7.40 (−.03) | 0.86 (0.77, 0.96)** | 0.90 (0.80, 1.01) |
| Apathy/indifference | 655 (44.5) | 5,234 (48.2) | 7.24 (−.02) | 0.86 (0.77, 0.96)** | 0.95 (0.85, 1.07) |
| Appetite/eating problems | 539 (36.6) | 3,365 (31.0) | 18.74 (.04) | 1.29 (1.15, 1.44)*** | 1.39 (1.24, 1.57)*** |
| Delusions | 461 (31.3) | 1,576 (14.5) | 264.78 (.15) | 2.68 (2.37, 3.03)*** | 2.27 (1.99, 2.58)*** |
| Depression/dysphoria | 608 (41.4) | 4,462 (41.1) | 0.03 (<.01) | 1.01 (0.90, 1.13) | 1.04 (0.92, 1.17) |
| Disinhibition | 394 (26.8) | 2,800 (25.8) | 0.66 (.01) | 1.05 (0.93, 1.19) | 1.21 (1.06, 1.38)** |
| Elation/euphoria | 99 (6.7) | 772 (7.1) | 0.30 (−0.01) | 0.94 (0.76, 1.17) | 1.19 (0.94, 1.49) |
| Hallucinations | 264 (17.9) | 972 (9.0) | 115.84 (.10) | 2.22 (1.92, 2.58)*** | 2.03 (1.73, 2.38)*** |
| Irritability/lability | 671 (45.6) | 4,435 (40.9) | 12.09 (.03) | 1.21 (1.09, 1.36)*** | 1.25 (1.12, 1.40)*** |
| Motor disturbances | 476 (32.3) | 2,709 (25.0) | 36.83 (.06) | 1.44 (1.28, 1.62)*** | 1.58 (1.39, 1.78)*** |
| Nighttime behaviors | 568 (38.7) | 3,360 (31.0) | 34.74 (.05) | 1.40 (1.25, 1.57)*** | 1.49 (1.33, 1.68)*** |
|
| |||||
| Measure, M (SD) | Black | White | Unadjusted F() | Adjusted F() | |
|
| |||||
| NPIQ total severity | 6.74 (5.92) | 5.57 (5.02) | 41.63 (.003)*** | 59.46 (.005)*** | |
| NPIQ total symptoms | 4.09 (2.84) | 3.53 (2.55) | 60.49 (.005)*** | 80.55 (.007)*** | |
Values are displayed as No. (%) for all NPIQ variables, within each column (e.g., within race).
Adjusted statistical models include age, sex, and education as covariates. NPIQ total severity scores were rank transformed due to distribution non-normality.
Sample sizes varied nominally according to NPIQ symptom domain (Supplemental file 3).
Abbreviations: eβ, Exponentiated beta; NPIQ, Neuropsychiatric Inventory Questionnaire; M, Mean; No., Number; , Partial eta squared; SD, Standard deviation.
p<.01;
p<.001
Table 5.
Functional abilities among National Alzheimer’s Coordinating Center participants with dementia at baseline, stratified by race.
| FAQ Domain, No. (%) | Black (N =1,528)† |
White (N = 11,267)† |
Unadjusted eβ (95% CI) |
Adjusted eβ (95% CI) |
|---|---|---|---|---|
| Functionally dependent for: | ||||
|
| ||||
| Attending to TV/reading | 263 (17.8) | 1,620 (14.7) | 1.26 (1.09, 1.45)** | 1.05 (0.91, 1.23) |
| Kitchen appliances (stove) | 381 (26.2) | 2,317 (21.5) | 1.30 (1.14, 1.47)*** | 1.12 (0.98, 1.28) |
| Managing finances | 792 (57.8) | 5,479 (55.7) | 1.09 (0.97, 1.22) | 0.97 (0.86, 1.09) |
| Meal preparation | 559 (40.9) | 3,510 (37.0) | 1.18 (1.05, 1.33)** | 1.04 (0.92, 1.18) |
| Playing games/hobbies | 384 (32.2) | 2,543 (25.6) | 1.38 (1.21, 1.57)*** | 1.24 (1.09, 1.42)** |
| Remembering events/tasks | 625 (42.1) | 3,766 (34.1) | 1.40 (1.26, 1.57)*** | 1.19 (1.06, 1.33)** |
| Shopping | 557 (38.4) | 3,484 (32.4) | 1.30 (1.16, 1.46)*** | 1.12 (0.99, 1.26) |
| Taxes/paperwork | 802 (64.6) | 5,906 (62.5) | 1.10 (0.09, 1.24) | 0.92 (0.81, 1.05) |
| Tracking current events | 468 (32.1) | 2,453 (22.4) | 1.64 (1.45, 1.84)*** | 1.30 (1.15, 1.47)*** |
| Transportation/travel | 778 (53.5) | 5,333 (48.7) | 1.21 (1.09, 1.35)*** | 0.99 (0.88, 1.11) |
|
| ||||
| Measure | Black | White | Unadjusted F() | Adjusted F() |
|
| ||||
| FAQ total, M (SD) | 19.51 (9.27) | 18.34 (8.88) | 18.18 (.002)*** | 2.80 (<.001) |
Values are displayed as No. (%) for all FAQ variables, within each column (e.g., race).
Adjusted statistical models include age, sex, and education as covariates.
FAQ total scores rank transformed due to distribution non-normality.
Sample sizes varied nominally according to FAQ domain (Supplemental file 3).
Abbreviations: eβ, Exponentiated beta; FAQ, Functional Activities Questionnaire; M, Mean; , Partial eta squared; SD, Standard deviation.
p<.01;
p≤.001
4. Discussion
This study retrospectively investigated the racial representation of non-Hispanic Black and White individuals within a national ADRD medical research database, and examined race differences in cognitive performance, neuropsychiatric symptoms, and functional impairment. The prevalence of dementia diagnoses among non-Hispanic Black participants was significantly lower than that of non-Hispanic White participants among the NACC dataset, an unexpected result given that the prevalence of dementia among Black individuals tends to be higher than that of White individuals in community-based samples [35]. This finding was especially surprising given that known risk factors for dementia (e.g., hypertension, diabetes) were consistently higher among Black participants in this sample, mirroring prior literature [36].
Considering ADRDs are currently diagnosed on the basis of clinical symptoms, the unexpectedly low prevalence of dementia among Black participants may be explained, at least in part, by the methodology used to make such judgments. For instance, the use of race-based norms has recently been criticized for its potential for systemic bias [37]. Although race-based norms were developed to reduce harm from potential overidentification of cognitive impairment among racial/ethnic minorities, it is becoming increasingly clear that race represents a crude proxy for social determinants of health, such as early-life experiences, disparate educational quality, socioeconomic status, actual and perceived discrimination, experiences of segregation, and neighborhood disadvantage. Similar criticisms have been offered about education-based norms, such that years of education is an imprecise measure of educational experience among racial/ethnic minorities [38]. Studies have demonstrated that quality of education, as measured by literacy, is a more sensitive predictor of memory decline in racial/ethnic minority samples than years of education [39–41]. This may have been a factor in the varying prevalence rates of dementia diagnoses in the current study given that, while Black participants had fewer years of education, educational quality was not a consideration.
When faced with the substantial limitations of the available normative data, clinicians are left to their own judgments to determine whether racial/ethnic minority individuals meet clinical criteria for a dementia diagnosis. This may result in unconscious bias, given that biases are often overlooked or disregarded when interpreting race differences and are exacerbated in the context of inadequate normative data and diagnostic criteria [42]. These clinical scenarios likely increase the risk of clinicians acting based on heuristics which are differentially susceptible to bias [42]. Another possible ramification is that racial/ethnic minority groups may require more severe clinical presentations to warrant a diagnosis of dementia when clinicians default to their own judgments. Our present findings potentially corroborate this hypothesis, as the Black participants with dementia were significantly older, less educated, more likely to possess copies of the APOE ε4 allele, and had greater risk factors typically associated with cognitive decline, compared to their White counterparts. Moreover, they typically have comparable or worse cognitive performance, comparable functional impairment, and more behavioral and psychological symptoms of dementia. This is consistent with numerous studies indicating that Black individuals are not being diagnosed with AD or seeking treatment until the disease process is more advanced [17,43–46].
Given that cognitive, neuropsychiatric, and functional impairment become more severe with advancing AD staging, the characteristics of the Black participants within this study are consistent with the current literature. In addition, research purports that Black individuals seek medical treatment for AD at a more advanced stage because of social attitudes and beliefs held within the Black American community that memory loss is a typical part of normal aging [4,47–49]. Consequently, Black individuals are more likely to seek medical attention for neuropsychiatric symptoms such as hallucinations, delusions, and personality changes rather than memory concerns [47–48,50]. Accordingly, studies have demonstrated the neuropathology of Black individuals with AD is more likely to be of mixed AD/LBD pathology rather than AD/vascular compared to matched (age, sex, education, and cognition) White counterparts [48]. Though clinically defined LBD was not seen at elevated rates in the current study, it remains possible that the increased tendency for Black individuals to seek medical attention in the context of neuropsychiatric symptoms may explain the elevated rates of hallucinations, delusions, and agitation, observed among Black participants in this dataset. The extent to which differences in disease course and severity may be attributed to racial bias arising from currently accepted approaches for dementia diagnosis remains unclear.
The lower prevalence of dementia diagnoses among Black participants may be partially attributable to underrepresentation of Black men in this sample, who comprised only 27% of the Black participants. However, the observation that dementia risk factors, cognitive performance, neuropsychiatric symptoms, and functional abilities are equivalent or worse among the Black participants continues to suggest a potential diagnostic bias. This underrepresentation is not unique to the NACC, and is consistent with a general under-engagement and/or exclusion of Black individuals in medical research, including clinical trials [51–56]. In support of this explanation, recent studies have found that Black participants are less likely to enroll in AD trials, and have higher dropout rates, compared to their White counterparts [54,57].
The current study has several important strengths, including the large sample of Black participants from which we can expect stable prevalence estimates, and the multi-racial assessment of cognition, neuropsychiatric symptoms, and functional abilities all within the same cohort. However, several study limitations should also be noted. First, it is important to note that this study was conducted with a clinic-based sample derived from ADRCs across the United States. Although it is critical that we understand ADRD-related race differences in this widely-studied group of older adults, the results of our analyses may not generalize to the broader population of Black and White older adults. Data analyses were cross-sectional and cannot attest to possible longitudinal changes in dementia prevalence within the NACC UDS. This study also presented a targeted comparison between non-Hispanic Black and non-Hispanic White participants and, therefore, is limited in its generalizability about prevalence rates for other minority populations. Given that the present study examined a circumscribed racial minority group, future studies may expand these findings by comparing rates and risk factors in other racial/ethnic minority groups. Due to the blinding of the ADRCs for participant privacy, we were unable to conduct more fine-grained analyses to determine if dementia underdiagnosis among this sample is related to the particular geographic locations of the contributing ADRCs, which is particularly relevant considering that the ADRCs do not provide equal contributions to the NACC UDS.
In sum, it is becoming increasingly clear that sociocultural factors contribute to inadequate or delayed treatment of ADRD in racial/ethnic minority populations. The current findings corroborate this assertion in that non-Hispanic Black participants in a national ADRD medical research database had a lower prevalence of dementia diagnoses, despite having comparable or worse dementia risk factors, and comparatively greater cognitive impairment, neuropsychiatric symptoms, and functional limitations. There are multiple avenues through which clinicians and researchers can begin remedying these inequities in ADRD diagnosis, treatment, and research. These efforts include increasing representation of Black individuals in ADRD research by facilitating closer partnerships with minority communities and working with local civic leaders and organizations, as well as targeted oversampling [58]. It will also be critical to develop regression-based normative approaches to aide neuropsychologists in accounting for known social determinants of brain health. In addition, biological diagnostic approaches may help facilitate more accurate prevalence estimates of ADRD in diverse populations. Finally, it is incumbent on the medical community to cultivate trust and provide education on ADRD to promote more equitable access to healthcare and increased enrollment in clinical research for racial/ethnic minority populations.
Supplementary Material
Acknowledgments:
The authors gratefully acknowledge Dr. Christopher Howard for his contributions to study conceptualization. We also acknowledge the NACC grant, Center, and research consultants.
The NACC database is funded by NIA/NIH Grant U24 AG072122. NACC data are contributed by the NIA-funded ADRCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI Robert Vassar, PhD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Funding/Disclosures:
JL received supported for the Alfred Adler Scholarship for the present manuscript. JL is a Board Member for the Journal of Alzheimer’s Disease (unpaid). KW received research funding and salary support from the NIH/National Institute on Aging Intramural Research Program for the present manuscript. KW received grant funding and travel support from the NIH/National Institute of Aging (K23 AG064122). KW received lecture honoraria from Boston University Medical Center. KW serves as the Programing Chair-Elect for the National Academy of Neuropsychology (NAN). SLA, VAD, TR, ZJR, and JME have nothing to disclose.
Abbreviations:
- AD
Alzheimer’s disease
- ADRC
Alzheimer’s disease research center
- ADRD
Alzheimer’s disease and related dementias
- DSB
Digit Span Backward
- DSF
Digit Span Forward
- FAQ
Functional Activities Questionnaire
- LBD
Lewy body disease
- MCI
Mild cognitive impairment
- MoCA
Montreal Cognitive Assessment
- MINT
Multilingual naming test
- NACC
National Alzheimer’s Coordinating Center
- NPIQ
Neuropsychiatric Inventory Questionnaire
- TMT
Trail Making Test
- UDS
Uniform Data Set
- UDSNB
UDS neuropsychological battery
Footnotes
Declarations of interest: none
Due to the use of NACC data, compliance with the National Institutes of Health (NIH) Public Access Policy requires proper submission of this work to PubMed Central (PMC). All cited articles in this manuscript contain human and/or animal work approved by institutional review boards (IRBs) prior to publication. This study was considered exempt by the relevant IRB. Per the University of Washington Human Subjects Division, the NACC database itself is exempt from IRB review and approval because it does not involve human subjects, as defined by federal and state regulations. However, all contributing ADRCs are required to obtain informed consent from their participants and maintain their own separate IRB reviews and approvals from their institutions prior to submitting data to NACC.
Data Availability Statement:
NACC data is freely available upon request to researchers as a full document or specific set of variables. Further information may be retrieved from https://naccdata.org.
References
- [1].Matthews KA, Xu W, Gaglioti AH, Holt JB, Croft JB, Mack D, McGuire LC, Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015-2060) in adults aged ≥65 years. Alzheimers Dement 2019;15(1):17–24. 10.1016/j.jalz.2018.06.3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement 2020;16(3):391+. Retrieved December 3, 2020, from https://www.alz.org/media/Documents/alzheimers-facts-and-figures.pdf [Google Scholar]
- [3].Brookmeyer R, Abdalla N, Kawas CH, Corrada MM, Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimers Dement 2018;14(2):121–129. 10.1016/j.jalz.2017.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Barnes LL, Bennett DA, Alzheimer’s disease in African Americans: Risk factors and challenges for the future. Health Aff (Millwood) 2014;33(4):580–586. 10.1377/hlthaff.2013.1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Alzheimer’s Association, 2018 Alzheimer’s disease facts and figures. Alzheimers Dement 2018:14(3):367–429. Retrieved from alz.org/facts (accessed May 11, 2021). [Google Scholar]
- [6].U.S. Census Bureau 2019, QuickFacts: United States. Retrieved June 5, 2020, from https://www.census.gov/quickfacts/fact/table/US/PST045219.
- [7].Centers for Disease Control and Prevention 2018, U.S. burden of Alzheimer’s disease, related dementias to double by 2060. Retrieved June 5, 2020, from https://www.cdc.gov/media/releases/2018/p0920-alzheimers-burden-double-2060.html.
- [8].Froehlich TE, Bogardus ST, Inouye SK, Dementia and race: Are there differences between African Americans and Caucasians? J Am Geriatr Soc 2001;49(4):477–484. 10.1046/j.1532-5415.2001.49096.x [DOI] [PubMed] [Google Scholar]
- [9].Alzheimer’s Association 2011, 2010 Alzheimer’s Disease Facts and Figures. Retrieved June 5, 2020, from http://www.alz.org/documents_custom/report_alzfactsfigures2010.pdf.
- [10].Alzheimer’s Association 2014, 2013 Alzheimer’s Disease Facts and Figures. Retrieved June 5, 2020, from http://www.alz.org/alzheimers_disease_facts_and_figures.asp.
- [11].Power MC, Bennett EE, Turner RW, Dowling NM, Ciarleglio A, Glymour MM, Gianattasio KZ, Trends in relative incidence and prevalence of dementia across non-Hispanic Black and White individuals in the United States, 2000-2016 [Manuscript published online November 30, 2020]. JAMA Neurol. 10.1001/jamaneurol.2020.4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chin AL, Negash S, Hamilton R, Diversity and disparity in dementia: The impact of ethnoracial differences in Alzheimer’s disease. Alzheimers Dis Assoc Disord 2011;25(3):187–195. 10.1097/WAD.0b013e318211c6c9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].O’Bryant SE, Xiao G, Edwards M, Devous M, Gupta VB, Martins R, Zhang F, Barber R, Biomarkers of Alzheimer’s disease among Mexican Americans. J Alzheimers Dis 2013;34(4):841–849. https://doi.org/3233/JAD-122074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Howell JC, Watts KD, Parker MW, Wu J, Kollhoff A, Wingo TS, Dorbin CD, Qiu D, Hu WT, Race modifies the relationship between cognition and Alzheimer’s disease cerebrospinal fluid biomarkers. Alzheimers Res Ther 2017;9:88. 10.1186/s13195-017-0315-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].O’Bryant SE, Johnson L, Balldin V, Edwards M, Barber R, Williams B, Devous M, Cushings B, Knebl J, Hall J, Characterization of Mexican Americans with mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis 2013;33:373–379. 10.3233/JAD-2012-121420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Roses AD, Lutz MW, Saunders AM, Goldgaber D, Saul R, Sundseth SS, Akkari PA, Roses SM, Gottschalk WK, Whitfield KE, Vostrov AA, Hauser MA, Allingham RR, Burns DK, Chiba-Falek O, Welsh-Bohmer KA, African American TOMM40’523-APOE haplotypes are admixture of West African and Caucasian alleles. Alzheimers Dement 2014;10(6):592–601. 10.1016/j.jalz.2014.06.009 [DOI] [PubMed] [Google Scholar]
- [17].Yu L, Lutz MW, Wilson RS, Burns DK, Roses AD, Saunders AM, Yang J, Gaiteri C, De Jager PL, Barnes LL, Bennett DA, APOE ε4-TOMM40 ‘523 haplotypes and the risk of Alzheimer’s diseases in older Caucasian and African Americans. PLos One 2017;12(7):e0180356. 10.1371/journal.pone.0180356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gottesman RF, Albert MS, Alonso A, Coker LH, Coresh J, Davis SM, Deal JA, McKhann GM, Mosley TH, Sharrett AR, Schneider ALC, Windham BG, Wruck LM, Knopman DS, Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol 2017;388(10046):797–805. 10.1001/jamaneurol.2017.1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H, Summary of evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement 2015;11(6):718–726. 10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- [20].Shiekh SI, Forbes H, Mathur R, Smeeth L, Pearce N, Warren-Gash C, Ethnicity and risk of diagnosed dementia after stroke: A cohort study using the Clinical Practice Research Datalink. J Epidemiol Community Health 2020;74(2):114–119. 10.1136/jech-2019-212825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cooper C, Tandy AR, Balamurali TB, Livingston G, A systematic review and meta-analysis of ethnic differences in use of dementia treatment, care, and research. Am J Geriatr Psychiatry 2010;18:193–203. 10.1097/JGP.0b013e3181bf9caf [DOI] [PubMed] [Google Scholar]
- [22].O’Bryant SE, Humphreys JD, Schiffer RB, Sutker PB, Presentation of Mexican Americans to a memory disorder clinic. J Psychopathology Behav Assess 2007;29:137–140. 10.1007/s10862-006-9042-9 [DOI] [Google Scholar]
- [23].Babulal GM, Quiroz YT, Albensi BC, Arenaza-Urquijo E, Astell AJ, Babiloni C, Bahar-Fuchs A, et al. , Perspectives on ethnic and racial disparities in Alzheimer’s disease and related dementias: Update and areas of immediate need. Alzheimers Dement 2019;15(2):292–312. 10.1016/j.jalz.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chakraborty S, Lennon JC, Malkaram SA, Zeng Y, Fisher DW, Dong H, Serotonergic system, cognition, and BPSD in Alzheimer’s disease. Neurosci Lett 2019;704:36–44. 10.1016/j.neulet.2019.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ehrenberg AJ, Suemoto CK, França Resende EDP, Petersen C, Leite REP, Rodriguez RD, Ferretti-Rebustini R.E.d.L., You M, Oh J, Nitrini R, Pasqualucci CA, Jacob-Filho W, Kramer JH, Gatchel JR, Grinberg LT, Neuropathologic correlates of psychiatric symptoms in Alzheimer’s disease. J Alzheimer Dis 2018;66(1):115–126. 10.3233/JAD-180688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gottesman RT, Stern Y, Behavioral and psychiatric symptoms of dementia and rate of decline in Alzheimer’s disease. Front Pharmacol 2019;10. 10.3389/fphar.2019.01062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bassiony MM, Steinberg MS, Warren A, Rosenblatt AS, Baker AS, Lyketsos CG, Delusions and hallucinations in Alzheimer’s disease: Prevalence and clinical correlates. Int J Geriatr Psychiatry 2000;15:99–107. [DOI] [PubMed] [Google Scholar]
- [28].Hargrave R, Stoeklin M, Haan M, Reed B, Clinical aspects of Alzheimer’s disease in Black and White patients. J Natl Med Assoc 1998;90(2):78–84. [PMC free article] [PubMed] [Google Scholar]
- [29].Clark PC, Kutner NG, Goldstein FC, Peterson-Hazen S, Garner V, Zhang R, Bowles T, Impediments to timely diagnosis of Alzheimer’s disease in African Americans. J Am Geriatr Soc 2005;53(11):2012–2017. 10.1111/j.1532-5415.2005.53569.x [DOI] [PubMed] [Google Scholar]
- [30].Fitten LJ, Ortiz F, Ponton M, Frequency of Alzheimer’s disease and other dementias in a community outreach sample of Hispanics. J Am Geriatr Soc 2001;49(10):1301–1308. 10.1046/j.1532-5415.2001.49257.x [DOI] [PubMed] [Google Scholar]
- [31].Gleason CE, Norton D, Zuelsdorff M, Benton SF, Wyman MF, Nystrom N, Lambrou N, Salazar H, Koscik RL, Jonaitis E, Carter F, Harris B, Gee A, Chin N, Ketchum F, Johnson SC, Edwards DF, Carlsson CM, Kukull W, Asthana S, Association between enrollment factors and incident cognitive impairment in Blacks and Whites: Data from the Alzheimer’s Disease Center. Alzheimers Dement 2019;15(12):1533–1545. 10.1016/j.jalz.2019.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris JC, The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): The neuropsychologic test battery. Alzheimer Dis Assoc Disord 2009;23(2):91–101. 10.1097/WAD.0b013e318191c7dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Weintraub S, Besser L, Dodge HH, Teylan M, Ferris S, Goldstein FC, Giordani B, Kramer J, Loewenstein D, Marson D, Mungas D, Salmon D, Welsh-Bohmer K, Zhou XH, Shirk SD, Kukull WA, Phelps C, Morris JC, Version 3 of the Alzheimer Disease Centers’ neuropsychological test battery in the Uniform Data Set (UDS). Alzheimer Dis Assoc Disord 2018;32:10–17. 10.1097/WAD.0000000000000223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].National Alzheimer’s Coordinating Center. Normative data for the UDS3 neuropsychological batteries. Retrieved November 11, 2020, from https://www.alz.washington.edu/NONMEMBER/UDS/DOCS/VER3/C1andC2normative.html
- [35].Steenland K, Goldstein FC, Levey A, Wharton W, A meta-analysis of Alzheimer’s disease incidence and prevalence comparing African-American and Caucasians. J Alzheimers Dis 2016;50(1):71–76. 10.3233/JAD-150778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sundquist J, Winkleby MA, Pudaric S, Cardiovascular disease risk factors among older black, Mexican-American, and white women and men: an analysis of NHANES III, 1988–1994. JAGS 2001;49:109–116. 10.1046/j.1532-5415.2001.49030.x [DOI] [PubMed] [Google Scholar]
- [37].Possin KL, Tsoy E, Windon CC, Perils of Race-Based Norms in Cognitive Testing: The Case of Former NFL Players. [Manuscript published online December 21, 2020]. JAMA Neurol. 10.1001/jamaneurol.2020.4763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Glymour MM, Manly JJ, Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev 2008;18(3):223–254. 10.1007/s11065-008-9064-z [DOI] [PubMed] [Google Scholar]
- [39].Fyffe DC, Mukherjee S, Barnes LL, Manly JJ, Bennett DA, Crane PK, Explaining differences in episodic memory performance among older African Americans and Whites: the roles of factors related to cognitive reserve and test bias. J Int Neuropsychol Soc 2011;17(4):625–638. 10.1017/S1355617711000476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Manly JJ, Touradji P, Tang MX, Stern Y, Literacy and memory decline among ethnically diverse elders. J Clin Exp Neuropsychol 2003;25:680–690. 10.1076/jcen.25.5.680.14579 [DOI] [PubMed] [Google Scholar]
- [41].Manly JJ, Schupf N, Tang MX, Stern Y, Cognitive decline and literacy among ethnically diverse elders. J Geriatr Psychiatry Neurol 2005;18:213–217. 10.1177/0891988705281868 [DOI] [PubMed] [Google Scholar]
- [42].Marcelin JR, Siraj DS, Victor R, Kotadia S, Maldonado YA, The impact of unconscious bias in healthcare: How to recognize and mitigate it. J Infect Dis 2019;220:S62–S73. 10.1093/infdis/jiz214 [DOI] [PubMed] [Google Scholar]
- [43].Clark PC, Kutner NG, Goldstein FC, Peterson-Hazen S, Garner V, Zhang R, Bowles T, Impediments to timely diagnosis of Alzheimer’s disease in African Americans. J Am Geriatr Soc 2005;53:2012–2017. 10.1111/j.1532-5415.2005.53569.x [DOI] [PubMed] [Google Scholar]
- [44].Lerner AJ, McLendon MJ, Sami SA, Ogrocki PK, Adams KB, Smyth KA, Factors affecting usage patterns of memantine in Alzheimer disease. Alzheimer Dis Assoc Disord 2008;22:137–143. 10.1097/WAD.0b013e31815ccd68 [DOI] [PubMed] [Google Scholar]
- [45].Mehta KM, Yin M, Resendez C, Yaffe K, Ethnic differences in acetylcholinesterase inhibitor use for Alzheimer disease. Neurology 2005;65:159–162. 10.1212/01.wnl.0000167545.38161.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zuckerman IH, Ryder PT, Simoni-Wastila L, Shaffer T, Sato M, Zhao L, Stuart B, Racial and ethnic disparities in the treatment of dementia among Medicare beneficiaries. J Gerontol B Psychol Sci Soc Sci 2008; 63:S328–333. 10.1093/geronb/63.5.s328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mohamed S, Rosenheck R, Lyketsos CG, Schneider LS, Caregiver burden in Alzheimer disease: Cross-sectional and longitudinal patient correlates. Am J Geriatr Psychiatry 2010;18(10):917–927. 10.1097/JGP.0b013e3181d5745d [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Barnes LL, Leurgans S, Aggarwal NT, Shah RC, Arvanitakis Z, James BD, Buchman AS, Bennett DA, Schneider JA, Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology 2015;85(6):528–534. 10.1212/WNL.0000000000001834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chui HC, Gatz M, Cultural diversity in Alzheimer disease: The interface between biology, belief, and behavior. Alzheimer Dis Assoc Disord 2005;19(4):250–255. 10.1097/01.wad.0000190802.03717.20 [DOI] [PubMed] [Google Scholar]
- [50].Ornstein KA, Gaugler JE, Devanand DP, Scarmeas N, Zhu CW, Stern Y, Are there sensitive time periods for dementia caregivers? The occurrence of behavioral and psychological symptoms in the early stages of dementia. Int Psychogeriatr 2013;25(9):1453–1462. 10.1017/S1041610213000768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ballard EL, Gwyther LP, Edmonds HL, Challenges and opportunities: recruitment and retention of African Americans for Alzheimer disease research: Lessons learned. Alzheimer Dis Assoc Disord 2010;24 Suppl:S19–23. 10.1097/WAD.0b013e3181f12432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Williams MM, Scharff DP, Mathews KJ, Hoffsuemmer JS, Jackson P, Morris JC, Edwards DF, Barriers and Facilitators of African American Participation in Alzheimer Disease Biomarker Research. Alzheimer Dis Assoc Disord 2010;24 Suppl:S24–29. 10.1097/WAD.0b013e3181f14a14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lincoln KD, Chow T, Gaines BF, Fitzgerald T, Fundamental causes of barriers to participation in Alzheimer’s clinical research among African Americans. Ethn Health 2018;26(4):585–599. https://doi.org/1080/13557858.2018.1539222 [DOI] [PubMed] [Google Scholar]
- [54].Zhou Y, Elashoff D, Kremen S, Teng E, Karlawish J, Grill JD, African Americans are less likely to enroll in preclinical Alzheimer’s disease clinical trials. Alzheimers Dement 2016;3(1):57–64. 10.1016/j.trci.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Manly JJ, Gilmore-Bykovskyi A, Deters KD, Inclusion of underrepresented groups in preclinical Alzheimer disease trials – opportunities abound. JAMA Netw Open 2021;4(7):e2114606. 10.1001/jamanetworkopen.2021.14606 [DOI] [PubMed] [Google Scholar]
- [56].Gilmore-Bykovskyi AL, Jin Y, Gleason C, et al. , Recruitment and retention of underrepresented populations in Alzheimer’s disease research: A systematic review. Alzheimer Dement 2019;5:751–770. 10.1016/j.trci.2019.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kennedy RE, Cutter GR, Wang G, Schneider LS, Challenging assumptions about African American participation in Alzheimer disease trials. Am J Geriatr Psychiatry 2017;25:1150–1159. 10.1016/j.jagp.2017.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ighodaro E, Nelson PT, Kukull WA, Schmitt FA, Abner EL, Caban-Holt A, Bardach SH, Hord DC, Glover CM, Jicha GA, Van Eldik LJ, Byrd AX, Fernander A, Challenges and considerations related to studying dementia in Blacks/African Americans. J Alzheimers Dis 2017;60(1):1–10. 10.3233/JAD-170242 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
NACC data is freely available upon request to researchers as a full document or specific set of variables. Further information may be retrieved from https://naccdata.org.


