Abstract
In the last few decades, antimicrobial resistance (AMR) has been a worldwide concern. The excessive use of antibiotics affects animal and human health. In the last few years, livestock production has used antibiotics as food supplementation. This massive use can be considered a principal factor in the accelerated development of genetic modifications in bacteria. These modifications are responsible for AMR and can be widespread to pathogenic and commensal bacteria. In addition, these antibiotic residues can be dispersed by water and sewer water systems, the contamination of soil and, water and plants, in addition, can be stocked in tissues such as muscle, milk, eggs, fat, and others. These residues can be spread to humans by the consumption of water or contaminated food. In addition, studies have demonstrated that antimicrobial resistance may be developed by vertical and horizontal gene transfer, producing a risk to public health. Hence, the World Health Organization in 2000 forbid the use of antibiotics for feed supplementation in livestock. In this context, to obtain safe food production, one of the potential substitutes for traditional antibiotics is the use of antimicrobial peptides (AMPs). In general, AMPs present anti-infective activity, and in some cases immune response. A limited number of AMP-based drugs are now available for use in animals and humans. This use is still not widespread due to a few problems like in-vivo effectiveness, stability, and high cost of production. This review will elucidate the different AMPs applications in animal diets, in an effort to generate safe food and control AMR.
Keywords: antimicrobial resistance, growth promoters, antimicrobial peptides, livestock, feed supplementation
Introduction
In the last decades, antimicrobial resistance (AMR) has been a worldwide concern. The indiscriminate use of such drugs for a long time led to the formation of significant reservoirs of microorganisms with AMR genes in human and animal production (World Health Organization., 2014; Sharma et al., 2018).
The use of antimicrobials in animal feedstuff as therapeutic, metaphylactic, prophylactic, and growth promoter agents started in the year 1950, to boost food production (Krishnasamy et al., 2015; Woolhouse et al., 2015; Lagha et al., 2017; Magouras et al., 2017). The indiscriminate use of such drugs for a long time led to the formation of significant reservoirs of microorganisms with AMR genes in livestock production (World Health Organization., 2014; Sharma et al., 2018). Moreover, drug-resistant bacteria can disseminate in two ways: through direct contact with animals and humans or indirectly through the food chain, and contaminated environment (Soucy et al., 2015; Lagha et al., 2017; Magouras et al., 2017; Vidovic and Vidovic, 2020). In 2014, the World Health Organization (WHO), emphasized the abusive use of antibiotics in the treatment of infectious diseases can result in bacteria with genes resistant to these drugs (Brown et al., 2017) (Table 1). Hence, in 2000, the WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance (AGISAR) (2011) classified AMR as a global public health concern, recommending the eradication of the use of antibiotics for feed supplementation in livestock.
Table 1.
Antimicrobial agents used in animals and humans.
| Antimicrobial class | Antibiotics | Animal use |
Activity human |
References |
|---|---|---|---|---|
| Aminoglycosides | Gentamicin B | Therapeutic use for poultry and swine | Yes | Heuer et al. (2009) |
| Lasalocid | AGP* | No | Heuer et al. (2009) | |
| Neomycin | Therapeutic use and AGP in cattle, swine, poultry and aquiculture | Yes | Jones and Ricke (2003) | |
| Streptomycin B | Feed supplementation for aquiculture | Yes | National Research Council. (1999) | |
| Amphenicols | Florfenicol | Therapeutic use in cattle and swine | No | Dibner and Richards (2005) |
| Carbomycin B | Feed supplementation for aquiculture | Yes | Bywater (2005) | |
| Aminocoumarins | Novobiocin | Therapeutic use in bovine mastitis | Yes | Katsunuma et al. (2007) |
| Aminopenicillins | Amoxicillin, B ampicillin B | Therapeutic use in cattle, mastitis, swine, poultry and aquiculture | Yes | Aarestrup et al, 2007 |
| Arsenicals | Roxarsone | AGP for poultry, swine and therapeutic use in swine | No | Witte (2000) |
| Beta-lactams | Procaine penicillin | AGP in poultry and swine | Yes | Witte (2000) |
| Cyclopolypeptides | Colistin | Feed supplementation for cattle, swine and broiler | Yes | Witte (2000) |
| Diaminopyrimidines | Ormetoprim | AGP and therapeutic use for poultry | No | Andleeb et al. (2020) |
| Elfamycins | Efrotomycin | AGP for swine | No | Bywater (2005) |
| Fluoroquinolones | Enrofloxacin B | Therapeutic use for cattle, swine | No | Bywater (2005) |
| Flumequin B | Therapeutic use in aquaculture | No | Dibner and Richards (2005) | |
| Glycopeptides | Ardacin | AGP for broilers | No | Arestrup et al. (2001) |
| Avoparcin B | AGP | No | Arestrup et al. (2001) | |
| Ionophores | Narasin | Feed supplementation and therapeutic use for poultry and AGP for cattle | No | Katsunuma et al. (2007) |
| Maduramycin | Feed supplementation for poultry | No | Jones and Ricke (2003) | |
| Monensin | AGP in cattle and poultry | No | Jones and Ricke (2003) | |
| Salinomycin | AGP and therapeutic use for swine | No | Witte (2000) | |
| Lincosamides | Lincomycin | Therapeutic use for poultry and swine | Rare | Heuer et al. (2009) |
| Macrolides | Macrolides | Therapeutic use for poultry | No | Bywater (2005) |
| Tylosin B | AGP for swine and therapeutic use for mastitis | No | McEwen and Fedorka-Cray (2002) | |
| Oleandomycin B | AGP for swine and poultry | Yes | Andleeb et al. (2020) | |
| Erythromycinb | AGP in cattle, poultry, swine and therapeutic use in aquaculture | Yes | Dibner and Richards (2005) | |
| Spiramycin B | AGP for swine and therapeutic use in bovine mastitis | Yes | Witte (2000) | |
| Nitrofurans | Furazolidone | Therapeutic use in aquaculture | Yes | Dibner and Richards (2005) |
| Orthosomysins | Avilamycin | AGP for broilers | No | Arestrup et al. (2001) |
| Phosphoglycolipids | Bambermycin | AGP | No | Butaye et al. (2003) |
| Pleuromutilins | Tiamulin | Therapeutic use and AGP for swine | No | McEwen and Fedorka-Cray (2002) |
| Polypeptides | Bacitracin/zinc bacitracin | AGP and therapeutic use in several livestock infections | Yes | Butaye et al. (2003) |
| Quinolones | Oxolinic acid B | Feed supplementation for aquiculture | No | Andleeb et al. (2020) |
| Quinoxalines | Carbadox | Therapeutic use in swine | No | Butaye et al. (2003) |
| Olaquindox | AGP an therapeutic use in swine | No | Katsunuma et al. (2007) | |
| Streptogramins | Pristinamycin | AGP | Yes | Andleeb et al. (2020) |
| Virginiamycin | AGP for broilers | Yes | McEwen and Fedorka-Cray (2002) | |
| Streptothricins | Nourseothricin | AGP for swine | No | Katsunuma et al. (2007) |
| Sulfonamides | Sulfonamides | Therapeutic use in aquiculture, and AGP in poultry and swine | Yes | National Research Council. (1999) |
| Tetracylines | Tetracyclines (oxy- and chlor-) B | AGP in cattle, poultry, swine and therapeutic use for livestock infection | Yes | National Research Council. (1999) |
*Antimicrobial growth promoters.
In this sense, the use of alternative treatments such as phages therapy (Ferriol-González and Domingo-Calap, 2021; Loponte et al., 2021) and antimicrobial peptides treatment (Vieco-Saiz et al., 2019; Silveira et al., 2021) are considered to combat the advance of resistant microorganisms. In this review, we described information about antimicrobial peptides treatment.
Thus, the use of antimicrobial peptides (AMPs) suggests a possible alternative to traditional antibiotics, given their several modes of action, facility for degradation in nature, avoiding the accumulation, low resistance frequency, host immunity enhancement, and ability to neutralize the activity of many microbes (Jenssen et al., 2006; Zhao et al., 2016; Li et al., 2018). AMPs can be found in all organisms and demonstrated activity against several microorganisms even cancer cells (Saido-Sakanaka et al., 2004; Brogden, 2005; Hwang et al., 2011; Rodrigues G. et al., 2019; Rodrigues G. R. et al., 2019; Spohn et al., 2019; Vilas et al., 2019; Cardoso et al., 2020). Likewise, AMPs have sequences with variable structures, and mechanisms of action (Gomes et al., 2018; Spohn et al., 2019; Cardoso et al., 2020). Due to their cationic characteristics, AMPs may be capable of set electrostatic interactions with the external bacterial membrane, which is generally present negatively charged phospholipids (Hancock and Chapple, 1999; Shai, 2002). AMPs have the capacity to connect the outer membrane and act in the disturbed. In addition, they can also be translocated across the membrane and also react to internal targets (Hancock and Sahl, 2006). Furthermore, these peptides present the ability to stimulate the host's immune system indirectly (Hancock, 2001; Ward et al., 2013; Wang et al., 2016; Ageitos et al., 2017).
Therefore, this review will examine the different applications of AMPs supplemented in ruminants and non-ruminant feed, in an attempt to increase food production safety and control AMR.
Antimicrobial Resistance and Environmental Problems
The discovery of penicillin represented an unprecedented milestone for modern medicine, transforming human history (Swann, 1983). Penicillin over the years has been collaborated to a massive reduction in mortality and caused an increase in life expectancy, besides offering essential support for invasive surgeries, and chemotherapy treatments (Blair and Piddock, 2009). Likewise, antibiotics also brought benefits to animal health when used as feed supplementation improving the growth and rentability of animal production (Cheng et al., 2014; Lhermie et al., 2017).
However, the antimicrobials used for animal food supplementation are the same as those administered as medicine for humans (World Health Organization., 2014; Sharma et al., 2018; Wu et al., 2018; Medina et al., 2020). The abusive use of antibiotics is the major factor in developing genetic modifications in bacteria. That is the main cause of antimicrobial resistance (AMR), which can be widespread in pathogenic and commensal bacteria (Thomas and Nielsen, 2005; Founou et al., 2016; Aslam et al., 2018; Li et al., 2018; Innes et al., 2020). AMR can be diffused into the food chain, by animal contact, or by environmental routes (Li et al., 2018; Scott et al., 2019) (Figure 1). Additionally, most of these drugs are not totally degraded in the body of animals and humans, and those residues are eliminated by excreted urine and feces, which then accumulate in soils, wastewater, manure causing profound, and complex impacts (Lim et al., 2013; Wu et al., 2014; Thanner et al., 2016; Li et al., 2018). Contact with or ingestion of antibiotic residues can give rise to several health problems, such as allergic hypersensitivity reactions, hepatotoxicity, nephropathy, mutagenicity, carcinogenicity, and antibiotic resistance (Mensah et al., 2014).
Figure 1.

Representation of antibiotic-resistant bacteria in different ecosystems: antimicrobial usage may select the genes encoding resistance. Drug-resistant bacteria can spread when domestic animals receive antibiotics and develop antibiotic-resistant bacteria in their gastrointestinal tracts (GIT). This contamination can occur through humans, among vulnerable patients in hospitals or with contact with contaminated surfaces. These bacteria can be propagated in humans either through the food supply chain (meat and dairy products) or by direct animal contact. In addition, they can spread via water containing animal feces used for animal crops, or drug-resistant bacteria can remain on crops and be eaten. In this way, bacteria can remain in human and animal guts and spread in the community.
Presently, 700,000 annual worldwide death are associated with AMR, and the number of deaths in 2050 is estimated to reach 10 million (Aria and Murray, 2009; Munita and Arias, 2016; World-Health-Organisation [WHO], 2018; Ghosh et al., 2019). Considering all this information, the WHO recommended the suspension or elimination of the use of antimicrobial agents in animal feed supplementation. Following the recommendations of the WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance (AGISAR) (2011), countries of the European Union forbade feed supplementation with antibiotics in livestock production in 2006 (Magouras et al., 2017). In an attempt to standardize the measures to be taken and the information generated, surveillance and monitoring programs were created, advised by the WHO the OIE (OIE World Organisation for Animal Health., 2012), and the Food and Agriculture Organization (FAO) (FAO et al., 2018).
Antimicrobial Peptides As An Alternative For Livestock Treatment
Livestock production is a sector that has expanded immensely, in an attempt to keep up with meat consumption. According to the FAO, cattle (including meat and dairy), pigs, and poultry together represent approximately 80% of the meat production (FAO, 2016, 2018). Current meat production is 200 million tons, and in 2050, this production will need to expand to 470 million tons, under current rates and predictions (Clifford et al., 2018). This rise causes concern regarding the quality of the meat produced (Vieco-Saiz et al., 2019), as accelerated production on large farms can cause health problems like weight loss, mastitis, and other infectious diseases (Krehbiel, 2013; Li et al., 2018; Sharma et al., 2018). Furthermore, farmers have been using antibiotics in their livestock production in an effort to prevent animal health problems, but the broad use of antimicrobials is one of the causes of the development of resistant microorganisms (World Health Organization., 2014; Sharma et al., 2018).
As described above AMPs, in general, demonstrated efficient activity against antimicrobial infection, due to the rapid action against pathogens, non-specific action, these result in a low resistance rate (Wimley and Hristova, 2011; Maria-Neto et al., 2015; Ageitos et al., 2017; Li et al., 2018). According to this, the overexposure of AMPs to the pathogens can generate the development of AMP-resistant strains.
Antimicrobial Peptides Issues
AMPs demonstrated an efficient result acting as antimicrobial and immunomodulation activity. Despite this, AMPs may present some issues like bacterial resistance (Fry, 2018). This mechanism is unclear, but studies described that bacterial AMPs resistance cause alterations in membranes, cell walls, and cellular metabolism. In the case of membrane modification, bacteria can switch the AMP target, decreasing AMPs interactions with membrane components (Huhand and Kwon, 2011; Zucca et al., 2011). Also, these modifications can affect the permeability and fluidity of the membrane (Li et al., 2007; Otto, 2009).
Other resistance mechanisms result in a modification of bacterial ionic cell wall potential in specific interaction spots that can reduce the binding of antibiotic peptides (Henderson et al., 2014). In addition, AMPs activities against the bacteria could generate high metabolic stress levels like the production of proteases, modification of surface structures, and biofilm (Yeaman and Yount, 2003). Furthermore, AMPs also present problems related to high production costs compared with antibiotics, susceptible to enzymatic and pH degradation. AMPs that act in the gastrointestinal tract (GIT) occur in intestinal absorption, bioavailability, distribution, renal clearance, and peptide elimination (Fry, 2018; Meade et al., 2020).
In general, these issues can be avoided using computational strategies to overcome challenges associated with the high cost of production, the potency of AMPs, and reduce the rate of resistance, degradation, toxicity, and instability (Cardoso et al., 2020; Dijksteel et al., 2021). Another option is the use of multi-omics (including genomics, transcriptomics, and proteomics) which allows identifying a novel sequence of AMPs (Chen et al., 2019; Burgos-Toro et al., 2021).
Problems related above are responsible for the low number of peptides approved in a clinical trial because the efficiency of the results in vitro does not always the same as in vivo. Nevertheless, AMPs remain a great option to control microbial infections. Table 2 summarized some AMPs recently approved or in advanced clinical trials (Dijksteel et al., 2021).
Table 2.
AMPs recently tested and approved by FDA.
| Peptide | Description | Target | Phase | Clinical Trial ID | Mechanism | References |
|---|---|---|---|---|---|---|
| Topical | ||||||
| PXL01 | Analog of Lactoferrin | Postsurgical adhesions | II | NCT01022242 | Immunomodulation | Edsfeldt et al., 2017 |
| Wap-8294A2 (Lotilibcin) |
Produced by Lysobacter species | Gram-positive bacteria | II/III | Membrane disruption | Itoh et al., 2018 | |
| Novexatin (NP213) | Cyclic Cationic peptide | Fungal nail infection | II | NCT02933879 | Membrane disruption | Mercer et al., 2020 |
| Melamine | Chimeric peptide | Contact lenses microbials | II/III | Membrane disruption | Yasir et al., 2019 | |
| Mel4 | Derivative of melamine | Contact lenses microbials | II/III | ACTRN1261500072556 | Membrane disruption | Yasir et al., 2020 |
| D2A21 | Synthetic peptide | Burn wound infections | III | Membrane disruption | Muchintala et al., 2020 | |
| Delmitide (RDP58) | Derivative of HLA | Inflammatory bowel disease | II | Immunomodulation | Travis et al., 2005 | |
| XOMA-629 (XMP-629) | Derivative of BPI | Impetigo/acne rosacea | III | Immunomodulation | Easton et al., 2009 | |
| PL-5 | Synthetic peptide | Skin infections | Membrane disruption | Miyake et al., 2004 | ||
| LTX-109 | Synthetic tripeptide | MRSA/impetigo | I/II | NCT01803035; NCT01158235 | Membrane disruption | Isaksson et al., 2011; Sivertsen et al., 2014 |
| Intravenous | ||||||
| hLF1-11 | Fragment of human lactoferrin | Bacterial/fungal infections | I/II | NCT00430469 | Membrane disruption/immunomodulation | Brouwer et al., 2018 |
| EA-230 | Oligopeptide | Sepsis | II | NCT03145220 | Immunomodulation | van Groenendael, 2018 |
| DPK-060 | Derivative of Kininogen | Acute external otitis | II | NCT01447017 | Membrane disruption/immunomodulation | Håkansson et al., 2019 |
| Friulimicin | Cyclic lipopeptide | MRSA/pneumonia | I | NCT00492271 | Membrane disruption | Schneider et al., 2009 |
| Murepavadin (POL7080) | Analog of Protegrin | P. aeruginosa, K. pneumoniae | II | EUCTR2017- | Binding to LptD | Srinivas et al., 2010 |
| IDR-1 | Bactenecin | Infection prevention | I | Immunomodulation | Yu et al., 2009 | |
| Ghrelin | Endogenous peptide | Chronic respiratory infection | II | NCT00763477 | Immunomodulation | Gualillo et al., 2003 |
| PMX-30063 (Brilacidin) | Defensin mimetic | Acute bacterial skin infection | II | NCT01211470; NCT02052388 | Membrane disruption/immunomodulation | Mensa et al., 2014 |
| Oral | ||||||
| Ramoplanin (NTI-851) | Glycolipodepsipeptide | C. difficile | III | Inhibition of cell wall synthesis | Fulco and Wenzel, 2006 | |
| SGX942 (Dusquetide) | Analog of IDR-1 | Oral mucositis | III | NCT03237325 | Immunomodulation | Kudrimoti et al., 2016 |
| GSK1322322 (Lanopepden) | Synthetic hydrazide | Bacterial skin infection | II | NCT01209078 | Peptide deformylase inhibitor | Peyrusson et al., 2015 |
| NVB-302 | Lantibiotic | C. difficile | I | ISRCTN40071144 | Inhibition of cell wall synthesis | Crowther et al., 2013 |
| Nisin bacteria | Polycyclic lantibiotic | Gram-positive | NCT02928042; NCT02467972 | Depolarization of cell membrane | Prince et al., 2016 | |
AMP to Control Microbiota in Livestock Production
The microbiota profile relates to the growth performance of animals since the presence of specific groups of microorganisms promotes the absorption of nutrients inside the gastrointestinal tract (Yadav and Jha, 2019). The modulation of microbiota may also lead to the reduction of pathogenic species, decreasing the frequency and lethality of some diseases (Cheema et al., 2011; Wang et al., 2015b; Yadav and Jha, 2019).
Despite that, several diseases affected the livestock production causing intestinal mucosa inflammation, and diarrhea associated with morphological changes in the intestinal epithelium. These pathologies are caused by toxins produced by bacteria (Xiao et al., 2015). For decades, all diseases were treated using antibiotics which boosted the increase of antibiotic-resistant microorganisms. This increase in resistant bacteria in the animal microbiota has been demonstrated in resistome studies (Wang et al., 2021). Resitsome studies described the existence of a broad spectrum of antimicrobial resistance genes (ARGs) in the digestive tract of food-producing animals. The presence of ARGs is not necessarily associated with the direct use of antibiotics but can occur with the administration through feed or water or by injectable antimicrobials (Ma et al., 2021).
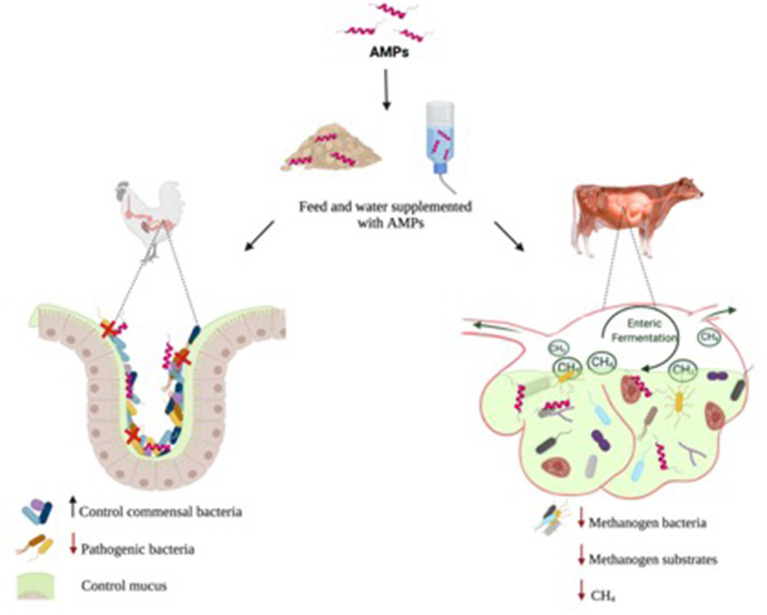
In this context, the uses of AMPs utilization have demonstrated their ability to recover and maintain the GIT of animals by epithelial barrier integrity stabilization and by boosting intestinal epithelium colonization susceptibility (Murphy et al., 1993; Gallo et al., 1994; Podolsky, 2000; Tollin et al., 2003; Xiao et al., 2015) (Figure 2). Furthermore, some AMPs can act by inhibiting LPS-induced pro-inflammatory cytokine production, behaving as chemokines, or modulating the dendritic cell and T cell response (Mookherjee et al., 2006; Xiao et al., 2015).
Figure 2.
Antimicrobial peptides (AMPs) affects intestinal mucosa and rumen: AMP as growth promoters in poultry and swine intestinal can act altering the composition of the microbiota to reduce competition for nutrients, reduce pathogen, and control mucus. In rumen the AMPs as growth promoters acting reducing the methanogen bacteria and substrates. In addition, decrease the rate of methane (CH4) production and release.
Likewise, antibiotics have been used in ruminants with the goal to control the ruminal microbiota reducing losses during the enteric fermentation process. Moreover, ruminants are relevant sources of greenhouse gas (GHG) emissions (Eisler et al., 2014; Reisinger and Clark, 2018). The CH4 liberated for enteric fermentation suggests that 90% GHG is present in the atmosphere (Lan and Yang, 2019; Leahy et al., 2019). Other problems related to CH4 are the conversion to ammonia by rumen fermentation and its further excretion as urea in the urine can accumulate in the soil, and also cause groundwater pollution (Firkins et al., 2007) (Figure 2).
In this context, AMPs are used as a sustainable alternative to the rising production and mitigated contaminants. Peptides like LL32, Lpep 19-2.5, and NK2 derivatives of porcine NK-lysin have demonstrated activity against methanogenic archaeal strains and also observed in the control of rumen fermentation (Bang et al., 2012). This modulation can occur as an influence on electron flow, acting as the hydrogen acceptor to effectively compete with rumen methane production, or killing some nitrate-reducing Gram-positive bacteria (Bang et al., 2012; Shen et al., 2016, 2017; Varnava et al., 2017). Besides, some peptides use rumen microbiota to reduce amino acid deamination and methanogenesis, without having a negative impact on dry matter digestibility or volatile fatty acid production (Varnava et al., 2017). Additionally, the sheep feed supplemented with peptides showed a decrease in methane emission of 10% (Callaway et al., 1997; Shen et al., 2016). Thus, the use of AMPs in livestock can be an alternative method to solve problems with digestibility and microbiota, improving the sustainability of livestock production (Santoso et al., 2004; Sar et al., 2005; Wang et al., 2015a,b; Vieco-Saiz et al., 2019).
AMPs Used as Growth Promoters
AMPs in feed supplementation have been extensively evaluated in several studies, and some characteristics are listed in Table 3. The peptide microcin J25 (MccJ25), a bacterial RNA polymerase inhibitor, increases the broilers' growth and attenuates the injuries to the intestine morphology caused by microbial infection. The application of MccJ25 in a range from 0.5 to 1.0 mg.kg−1 was able to reduce body weight loss by up to 70%, in comparison to 54.6% with antibiotic treatment (Wang et al., 2020). The recombinant cecropin A-D-Asn is formed by a chimeric peptide, from cecropin A, and cecropin D C-termini. Moreover, asparagine residue was added and amidated in C-terminus. The inclusion of 6 mg/kg−1 of the peptide to the basal feed of broilers boosts by 20% the weight when compared with feed without peptide addition (Wen and He, 2012).
Table 3.
AMPs using livestock production.
| AMP | Source | Activity | Target bacteria | Animal | References |
|---|---|---|---|---|---|
| Microcin J25 | E. coli | Immune Regulation, and Intestinal Microbiota | Escherichia coli, Salmonella CVCC519 | Broiler | Wang et al. (2020) and Iseppi et al. (2021) |
| Pediocin A | Pediococcus pentosaceus | Dietary supplementation | Clostridium perfringens | Broilers | Grilli et al. (2009) and Hernández-González et al. (2021) |
| Gallinacin-6 | Gallus gallus domesticus | Antimicrobial | Campylobacter jejuni, Salmonella enterica, Clostridium perfringens, E. coli | Broilers | van Dijk et al. (2007) |
| Plectasin | Pseudoplectania nigrella | Dietary supplementation | Broilers | Ma et al. (2019) | |
| RSRP | Oryctolagus cuniculus—sacculus rotundus | Dietary supplementation Intestinal mucosal immune responses |
Reducing the viable counts of E. coli | Broilers | Liu et al. (2008) |
| Lactoferrin (bLf) | Bos taurus | Dietary supplementation Intestinal mucosal |
Reducing the total viable counts of E. coli and Salmonella | Broilers | Tang et al. (2008); Messaoudi et al. (2012); Aguirre et al. (2015) |
| SMXD51 | Lactobacillus salivarius | Intestinal Microbiota | Campylobacter jejuni | Poultry | Cao et al. (2007); Ceotto-Vigoder et al. (2016) |
| BT | Brevibacillus texasporus | Dietary supplementation Intestinal mucosal |
Salmonella enterica serovar Enteritidis. | Neonatal poultry | Kogut et al. (2013) |
| Nissin* | Lactococcus sp. Streptococcus sp. | Food preservation; Antimicrobial | E. coli, Staphylococcus aureus, Streptococcus agalactiae, S. dysagalactiae, S. uberis S. aureus biofilm | Cattle | Santoso et al. (2004); Sar et al. (2005); Cao et al. (2007); Ceotto-Vigoder et al. (2016); Shen et al. (2016, 2017); Shin et al. (2016); Hernández-González et al. (2021) |
| Lysostaphin | Staphylococcus sp. | Antimicrobial | S. aureus biofilm | Cattle | Ceotto-Vigoder et al. (2016) |
| AP-CECT712 | Enterococcus faecalis | Antimicrobia | S. aureus, S. dysgalactiae, S. uberis, S. agalactia | Cattle | Sparo et al. (2009) |
| Colicin | E. coli | Antimicrobial | E. coli | Swine | Stahl et al. (2004); Cutler et al. (2007) |
| Porcine (pBD-1) |
Porcine blood | Antimicrobial, immune responses | Bordetella pertussis | Newborn Piglets | Elahi et al. (2006) |
| Cathelicidin-BF (C-BF) |
Bungarus fascia | Intestinal immune responses | Weanling piglets | Wang et al. (2008); Yi et al. (2015) |
*Commercial use—FDA liberation.
Pediocin A was administrated in poultry food and demonstrated efficient results as a growth promoter (Daeschel and Klaenhammer, 1985). A similar result with a gain of body weight was described using the combination of bacteriocins (divercin AS7 and nisin) as a food additive for broilers (Józefiak et al., 2013; Hernández-González et al., 2021). In vivo studies have shown AMPs also improve growth performance and digestive capacity in poultry and pigs (Wang et al., 2016). The use of AMP-A3 and AMP-P5 (both derived from the amino acid substitution of the Helicobacter pylori HP and the cecropin-magainin2 fusion, respectively), can raise the F:G ratio of weanling pigs and broilers, with additional benefits concerning nutrient uptake and intestinal morphology. The AMP-A3 (90 mg.kg−1) and AMP-P5 (60 mg.kg−1), display effective results showing elevated weight gain and reduced intestinal damage (Yoon et al., 2012, 2013, 2014; Choi et al., 2013a,b).
Ren et al. (2019) demonstrated the use of the recombinant swine defensin PBD-mI with a molecular mass of 5.4 kDa, and LUC-n with a molecular mass of 21.18 kDa, in 18 4-month-old Chuanzhong black goats. The animals were split into three groups (basal diet; basal diet + 2g AMP/goat/day; basal diet + 3g AMP/goat/day), and rumen fluid was collected and analyzed. Dietary supplementation with both AMPs demonstrated that the goats enhanced rumen microbiota diversity, updated ruminal fermentation, improved efficiency of food usage, and boosted growth performance. Although studies demonstrated positive results of AMPs in feed supplementation for poultry and pigs, the same is not observed for ruminants.
Use of AMPs to Control Infectious Disease
AMPs present an important role in controlling infection disease and the immunity system of non-ruminants maintaining (Hernández-González et al., 2021). Daneshmand et al. (2019), demonstrated that the use of a lactoferrin-derived peptide, cLF36 utilization can diminish infection by modulating the expression of cytokines IL-2 and IL-6 and mucine in broilers challenged with enterotoxigenic Escherichia coli. Adding 20 mg.kg−1 of cLF36 in feed reduced the population of E. coli and Clostridium spp. by 25% and 20%, respectively. Besides, the number of beneficial Lactobacillus spp. and Bifidobacterium spp. increased by up to 36%. Moreover, sublacin, a peptide obtained from Bacillus, may decrease harmful bacteria without causing any change in the Lactobacillus community. The peptide was supplemented with water (5.76 mg. L−1) (Wang et al., 2015a,b).
Another host defense peptide, ß-defensin-1 (pDB-1), has potential veterinary application. This peptide has shown its expression in the respiratory tract of old pigs, and demonstrated to be resistant against the infection of the respiratory pathogen Bordetella pertussis. Otherwise, newborn piglets do not seem to have pDB-1, and are susceptible to the disease. Thus, the application of 500 μg of tpDB-1 to the respiratory tract of these piglets was able to totally inhibit clinical symptoms (Elahi et al., 2006).
Furthermore, the peptide C-BF, which originates from Bungarus fasciatus venom, also demonstrated beneficial results in controlling bacterial disease in animal production (Elahi et al., 2006). C-BF used 0.5 mg.kg−1 in piglets via intraperitoneal application, and the peptide minimized the inflammatory molecule's TNF-α and IL-6. The level of cell apoptosis and intestinal barrier damage caused by bacterial lipopolysaccharide also decreased (Zhang et al., 2017). S100A8 and S100A9 showed beneficial results against ruminant infections. These peptides reduced uterine inflammation (which appears after calving in association with bacterial contamination) and modulated the early endometrial response against infection in Holstein–Friesian cows (Swangchan-Uthai et al., 2013).
Another application for AMPs is in aquaculture, a sector which dedicated to producing aquatic plants and animals, with a recent growth rate higher than any other land-based livestock (Gyan et al., 2020; León et al., 2020). In vitro study demonstrated high efficacy of synthetic peptides (frog caerin1.1, European sea bass dicentracin (Dic) and NK-lysin peptides (NKLPs) and tongue sole NKLP27) against viral fish pathogens, such as nodavirus (NNV), viral septicemia hemorrhagic virus (VHSV), infectious pancreatic necrosis virus (IPNV) and spring viremia carp virus (SVCV) (León et al., 2020).
In addition, Table 2 summarized many AMPs used in veterinary treatment with an efficient result.
Application of Amps In Different Sectors
AMPs presented beneficial results in the control of microbial infections and in food supplementation. However, peptides have different functions in the food industry (Bemena et al., 2014; Rai et al., 2016), and artificial breeding in livestock (Schulze et al., 2014, 2020; Speck et al., 2014; Shaoyong et al., 2019).
The food industry normally uses nitrites and sulfur dioxide (chemical preservatives), which can cause negative effects on human health and the nutritional level of food (Bemena et al., 2014). Recently, AMPs have been used instead, to maintain the properties of the food without modifying quality, besides not being harmful (Wang et al., 2016). The lactic acid bacteria are a good example because they are recognized as safe by the Food and Drug Administration, and are extensively used in human and animal food as a preservative, and to control pathogenic and spoilage bacteria (Rai et al., 2016; Venegas-Ortega et al., 2019; Iseppi et al., 2021).
AMPs are also being studied and applied to semen preservation in the artificial breeding process. A recent study used two synthetics cyclic hexapeptides, c-WFW and c-WWW, and magainin II (MK5E). These peptides were tested for boar semen preservation, indicating that cyclic hexapeptides can be promising candidates, due to proteolytic stability, capacity to control bacterial proliferation, and synergistic interaction with conventional antibiotics. The peptide ε-PL also showed effective results at a low concentration (0.16 g. L−1), suggesting that it could be a possible substitute for gentamicin to enhance sperm quality parameters, sperm capacitation, and in vitro fertilization by reducing bacterial concentrations (Shaoyong et al., 2019).
Concluding Remarks and Prospects
The excessive use of antibiotics as a growth promoter in livestock causes microbial resistance, which is associated with increased consumption of animal protein, while production has difficulties in keeping up with this demand (Eisler et al., 2014).
Hence, various countries prohibited antibiotics in animal supplementation, thus stimulating the expansion of research to sustainable approaches (Wang et al., 2016; Li et al., 2018; Leahy et al., 2019). Besides that, livestock products have faced challenges such as reduced productivity, loss of biodiversity, rising GHG emissions, sick animals, and diseases that can cause human illness (Grace et al., 2012; Michalk et al., 2019). Thus, sustainable animal production is the next step to increasing healthy livestock production and at the same time reducing environmental impacts (Kemp and Michalk, 2011; Godfray and Garnett, 2015; Vidovic and Vidovic, 2020).
Herein, we demonstrated positive results in the use of AMPs, which have shown to be promising in controlling microbial infection (Stahl et al., 2004; Ceotto-Vigoder et al., 2016), and methane gas emissions (Santoso et al., 2004; Sar et al., 2005), while also providing in-feed supplementation (Wang et al., 2008, 2016; Ren et al., 2019).
In this context, synthetic biology (SB) is an approach responsible for improving or completely creating systems and organisms, providing novel diagnostic tools, and enabling the economic production of new therapeutics drugs (Weber and Fussenegger, 2012; Takano and Breitling, 2014). SB has the skills to produce antibiotic drug advances, using different approaches like synthetic gene circuits (Weber et al., 2008) and protein engineering (King et al., 2016). It can foster the development of new drugs using faster and more efficient protocols, allowing the development of more accessible medicines that demonstrate greater precision (Noel, 2010; Jakobus et al., 2012). The rational design seeks to improve AMP sequence optimization and enhance biological activities, aiming to develop new drugs with high specificity against microorganisms and a reduction in adverse effects (Porto et al., 2012; Cardoso et al., 2020). In this context, computational tools like quantitative structure-activity relationship (QSAR), de novo, linguistic, pattern insertion, and evolutionary/genetic algorithms are very useful in designing AMP variants (Chen and Bahar, 2004; Loose et al., 2006; Hiss et al., 2010; Mitchell, 2014; Torres and De La Fuente-Nunez, 2019). In addition, these computational tools can be used separately or in association to construct novel peptide-based drug candidates (Cardoso et al., 2020).
In addition, AMPs can be used associated with nanoparticles (NPs) (Sharma et al., 2018). They could have several shapes and formulations (e.g., nitric oxide-releasing nanoparticles, chitosan-containing, and metal-containing nanoparticles) (Huhand and Kwon, 2011; Pelgrift and Friedman, 2013), and delivery systems, such as microencapsulation (Ganesh and Hettiarachchy, 2016; Kaikabo et al., 2016; Suresh et al., 2018), improving the bacterial control system. NPs can boost the effectiveness in the treatment of infectious diseases, besides protecting the peptide from degradation in the physiological environment (Rodrigues G. et al., 2019; Rodrigues G. R. et al., 2019). These tools are able to produce new drugs with fewer side effects, low costs, and with ability to abolish or control infectious diseases.
Different studies have been executed in the search for AMPs with anti-infective activities, but it is essential that these studies proceed to in vivo models and also to clinical trials.
All alternate strategies suggested can be successfully implemented with the prudent use of antibiotics, and strengthen the supervision associated with policies and regulation of use. These steps will allow farmers and veterinarians to prescribe treatment options for livestock production without causing chain effects. Thus, the use of AMPs in livestock allows the safe production of quality food, contributing to the maximization of agricultural output in a sustainable and economically satisfactory way.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by grants from Fundação de Apoio à Pesquisa do Distrito Federal (FAPDF), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (MC 88887.351521/2019-00), Conselho Nacional de Desenvolvimento e Tecnológico (CNPq), and Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT), Brazil.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Ageitos J. M., Sánchez-Pérez A., Calo-Mata P., Villa T. G. (2017). Antimicrobial peptides (AMPs): ancient compounds that represent novel weapons in the fight against bacteria. Biochem. Pharmacol. 133, 117–138. 10.1016/j.bcp.2016.09.018 [DOI] [PubMed] [Google Scholar]
- Aguirre A. T. A., Acda S. P., Angeles A. A., Oliveros M. C. R., Merca F. E., Cruz F. A. (2015). Effect of bovine lactoferrin on growth performance and intestinal histologic features of broiler. Philipp. J. Vet. Anim. Sci. 41, 12–20. [Google Scholar]
- Andleeb S., Jamal M., Bukhari S. M., Sardar S., Majid M. (2020). “Trends in antimicrobial use in food animals, aquaculture, and hospital waste,” in Antibiotics and Antimicrobial Resistance Genes, ed M. Z. Hashmi (Cham: Springer; ), 95–138. 10.1007/978-3-030-40422-2_5 [DOI] [Google Scholar]
- Arestrup F. M., Seyfarth A. M., Emborg H. N., Pedersen K., Hendriksen R. S., Bager F. (2001). Effect of abolishment of the use of antimicrobial resistance in fecal Enterococci from food animals in Denmark. Antimicrob. Agents Chemother. 45, 2054–2059. 10.1128/AAC.45.7.2054-2059.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarestrup F. M., Knöchel S., Hasman H. (2007). Antimicrobial susceptibility of Listeria monocytogenes from food products. Foodborne Pathog. Dis. 4, 216–221. 10.1089/fpd.2006.0078 [DOI] [PubMed] [Google Scholar]
- Aria C. A., Murray B. E. (2009). Antibiotic-resistant bugs in the 21st century—a clinical super-challenge. N. Engl. J. Med. 360:439–443. 10.1056/NEJMp0804651 [DOI] [PubMed] [Google Scholar]
- Aslam B., Wang W., Arshad M. I., Khurshid M., Muzammil S., Rasool M. H., et al. (2018). Antibiotic resistance: a rundown of a global crisis. Infect. Drug Resist. 11, 1645. 10.2147/IDR.S173867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang C., Schilhabel A., Weidenbach K., Kopp A., Goldmann T., Gutsmann T., et al. (2012). Effects of antimicrobial peptides on methanogenic archaea. Antimicrob. Agents Chemother. 56, 4123–4130. 10.1128/AAC.00661-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemena L. D., Mohamed L. A., Fernandes A. M., Lee B. H. (2014). Applications of bacteriocins in food, livestock health and medicine. Int. J. Curr. Microbiol. Appl. Sci. 3, 924–949. [Google Scholar]
- Blair J. M., Piddock L. J. (2009). Structure, function and inhibition of RND efflux pumps in Gram-negative bacteria: an update. Curr. Opin. Microbiol. 12, 512–519. 10.1016/j.mib.2009.07.003 [DOI] [PubMed] [Google Scholar]
- Brogden K. A. (2005). Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3, 238–250. 1098. 10.1038/nrmicro1098 [DOI] [PubMed] [Google Scholar]
- Brouwer C. P. J. M., Roscini L., Cardinali G., Corte L., Pierantoni D. C., Robert V., et al. (2018). Structure-activity relationship study of synthetic variants derived from the highly potent human antimicrobial peptide hLF (1-11). Cohesive J. Microbiol. Infect. Dis. 1, 1–19. 10.31031/CJMI.2018.01.000512 [DOI] [Google Scholar]
- Brown K., Uwiera R. R., Kalmokoff M. L., Brooks S. P., Inglis G. D. (2017). Antimicrobial growth promoter use in livestock: a requirement to understand their modes of action to develop effective alternatives. Int. J. Antimicrob, Agents 49, 12–24. 10.1016/j.ijantimicag.2016.08.006 [DOI] [PubMed] [Google Scholar]
- Burgos-Toro A., Dippe M., Vásquez A. F., Pierschel E., Wessjohann L. A., Fernández-Niño M. (2021). “Multi-omics data mining: A novel tool for biobrick design,” in Synthetic Genomics-From Natural to Synthetic Genomes (IntechOpen: ). [Google Scholar]
- Butaye P., Devriese L. A., Haesebrouck F. (2003). Antimicrobial growth promoters used in animal feed: effects of less well-known antibiotics on gram-positive bacteria. Clin. Microbiol. Rev. 16, 175–188. 10.1128/CMR.16.2.175-188.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bywater R. J. (2005). Identification and surveillance of antimicrobial resistance dissemination in animal production. Poult. Sci. J. 84, 644–648. 10.1093/ps/84.4.644 [DOI] [PubMed] [Google Scholar]
- Callaway T. R., Carneiro De Melo A. M. S., Russell J. B. (1997). The effect of nisin and monensin on ruminal fermentations in vitro. Curr. Microbiol. 35, 90–96. 10.1007/s002849900218 [DOI] [PubMed] [Google Scholar]
- Cao L. T., Wu J. Q., Xie F., Hu S. H., Mo Y. (2007). Efficacy of nisin in treatment of clinical mastitis in lactating dairy cows. Int. J. Dairy Sci. 90, 3980–3985. 10.3168/jds.2007-0153 [DOI] [PubMed] [Google Scholar]
- Cardoso M. H., Orozco R. Q., Rezende S. B., Rodrigues G., Oshiro K. G., Cândido E. S., et al. (2020). Computer-aided design of antimicrobial peptides: are we generating effective drug candidates? Front. Microbiol. 10, 3097. 10.3389/fmicb.2019.03097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceotto-Vigoder H., Marques S. L. S., Santos I. N. S., Alves M. D. B., Barrias E. S., Potter A., et al. (2016). Nisin and lysostaphin activity against preformed biofilm of Staphylococcus aureus involved in bovine mastitis. J. Appl. Microbiol. 121, 101–114. 10.1111/jam.13136 [DOI] [PubMed] [Google Scholar]
- Cheema U. B., Younas M., Sultan J. I., Iqbal A., Tariq M., Waheed A. (2011). Antimicrobial peptides: an alternative of antibiotics in ruminants. Adv. Agric. Biotechnol. 2, 15–21. [Google Scholar]
- Chen S. C., Bahar I. (2004). Mining frequent patterns in protein structures: a study of protease families. J. Bioinform. 20, i77–i85. 10.1093/bioinformatics/bth912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Yi Y., You X., Liu J., Shi Q. (2019). High-throughput identification of putative antimicrobial peptides from multi-omics data of the lined seahorse (Hippocampus erectus). Marine Drugs 18, 30. 10.3390/md18010030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G., Hao H., Xie S., Wang X., Dai M., Huang L., et al. (2014). Antibiotic alternatives: the substitution of antibiotics in animal husbandry? Front. Microbiol. 5, 217. 10.3389/fmicb.2014.00217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. C., Ingale S. L., Kim J. S., Park Y. K., Kwon I. K., Chae B. J. (2013a). Effects of dietary supplementation with an antimicrobial peptide-P5 on growth performance, nutrient retention, excreta and intestinal microflora and intestinal morphology of broilers. Anim. Feed. Sci. Technol. 185, 78–84. 10.1016/j.anifeedsci.2013.07.005 [DOI] [Google Scholar]
- Choi S. C., Ingale S. L., Kim J. S., Park Y. K., Kwon I. K., Chae B. J. (2013b). An antimicrobial peptide-A3: effects on growth performance, nutrient retention, intestinal and fecal microflora and intestinal morphology of broilers. Br. Poult. Sci. 54, 738–746. 10.1080/00071668.2013.838746 [DOI] [PubMed] [Google Scholar]
- Clifford K., Desai D., Prazeres da Costa C., Meyer H., Klohe W.inkler A. S., Rahman T., et al. (2018). Antimicrobial resistance in livestock and poor-quality veterinary medicines. Bull. World Health Orga. 96, 662–664. 10.2471/BLT.18.209585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther G. S., Baines S. D., Todhunter S. L., Freeman J., Chilton C. H., Wilcox M. H. (2013). Evaluation of NVB302 versus vancomycin activity inan in vitro human gut model of Clostridium difficile infection. J. Antimicrob. Chemother. 68, 168–176. 10.1093/jac/dks359 [DOI] [PubMed] [Google Scholar]
- Cutler S. A., Lonergan S. M., Cornick N., Johnson A. K., Stahl C. H. (2007). Dietary inclusion of colicin e1 is effective in preventing postweaning diarrhea caused by F18-positive Escherichia coli in pigs. Antimicrob. Agents Chemother. 51, 3830–3835. 10.1128/AAC.00360-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daeschel M. A., Klaenhammer T. R. (1985). Association of a 13.6-megadalton plasmid in Pediococcus pentosaceus with bacteriocin activity. Appl. Environ. Microbiol. 50, 1538–1541. 10.1128/aem.50.6.1538-1541.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshmand A., Kermanshahi H., Sekhavati M. H., Javadmanesh A., Ahmadian M. (2019). Antimicrobial peptide, cLF36, affects performance and intestinal morphology, microflora, junctional proteins, and immune cells in broilers challenged with E. coli. Sci. Rep. 9, 14176. 10.1038/s41598-019-50511-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner J. J., Richards J. D. (2005). Antibiotic growth promoters in agriculture: history and mode of action. Poult. Sci. 84, 634–643. 10.1093/ps/84.4.634 [DOI] [PubMed] [Google Scholar]
- Dijksteel G. S., Ulrich M. M., Middelkoop E., Boekema B. K. (2021). lessons learned from clinical trials using antimicrobial peptides (AMPs). Front. Microbiol. 12, 287. 10.3389/fmicb.2021.616979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton D. M., Nijnik A., Mayer M. L., Hancock R. E. W. (2009). Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends Biotechnol. 27, 582–590. 10.1016/j.tibtech.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edsfeldt S., Holm B., Mahlapuu M., Reno C., Hart D. A., Wiig M. (2017). PXL01 in sodium hyaluronate results in increased PRG4 expression: a potential mechanism for anti-adhesion. Ups. J. Med. Sci. 122, 28–34. 10.1080/03009734.2016.1230157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisler M. C., Lee M. R., Tarlton J. F., Martin G. B., Beddington J., Dungait J. A., et al. (2014). Agriculture: steps to sustainable livestock. Nat. News 507, 32–34. 10.1038/507032a [DOI] [PubMed] [Google Scholar]
- Elahi S., Buchanan R. M., Attah-Poku S., Townsend H. G., Babiuk L. A., Gerdts V. (2006). The host defense peptide beta-defensin 1 confers protection against Bordetella pertussis in newborn piglets. Infect. Immun. 74, 2338–2352. 10.1128/IAI.74.4.2338-2352.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (2016). The FAO Action Plan on Antimicrobial Resistance. Rome: Food and Agriculture Organization of the United Nations. 3–25. Available online at: http://www.fao.org/3/a-i5996e.pdf (accessed May 25, 2021).
- FAO (2018). World Livestock: Transforming the Livestock Sector Through the Sustainable Development Goals. Rome: FAO. 222. [Google Scholar]
- FAO IFAD, UNICEF, WFP, and WHO. (2018). The State of Food Security and Nutrition in the World 2018. Building Climate Resilience for Food Security and Nutrition. Rome: FAO. [Google Scholar]
- Ferriol-González C., Domingo-Calap P. (2021). Phage therapy in livestock and companion animals. Antibiotics 10, 559. 10.3390/antibiotics10050559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firkins J. L., Yu Z., Morrison M. (2007). Ruminal nitrogen metabolism: perspectives for integration of microbiology and nutrition for dairy. J. Dairy Sci. 90, E1–E16. 10.3168/jds.2006-518 [DOI] [PubMed] [Google Scholar]
- Founou L. L., Founou R. C., Essack S. Y. (2016). Antibiotic resistance in the food chain: a developing country-perspective. Front. Microbiol. 7, 1881. 10.3389/fmicb.2016.01881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry D. E. (2018). Antimicrobial peptides. Surg. Infect. 19, 804–811. 10.1089/sur.2018.194 [DOI] [PubMed] [Google Scholar]
- Fulco P., Wenzel R. P. (2006). Ramoplanin: a topical lipoglycodepsipeptide antibacterial agent. Expert Rev. Anti Infect. Ther. 4, 939–945. 10.1586/14787210.4.6.939 [DOI] [PubMed] [Google Scholar]
- Gallo R. L., Ono M., Povsic T., Page C., Eriksson E., Klagsbrun M., et al. (1994). Syndecans, cell surface heparan sulfate proteo- glycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc. Natl. Acad. Sci. U. S. A. 91, 11035-11039. 10.1073/pnas.91.23.11035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh V., Hettiarachchy N. S. (2016). A review: supplementation of foods with essential fatty acids—can it turn a breeze without further ado? Crit. Rev. Food Sci. Nutr. 56, 1417–1427. 10.1080/10408398.2013.765383 [DOI] [PubMed] [Google Scholar]
- Ghosh C., Sarkar P., Issa R., Haldar J. (2019). Alternatives to conventional antibiotics in the Era of antimicrobial resistance. Trends Microbiol. 27, 323–338. 10.1016/j.tim.2018.12.010 [DOI] [PubMed] [Google Scholar]
- Godfray H. C. J., Garnett T. (2015). Food security and sustainable intensification. Philos. Trans. R. Soc. B. 369, 20120273. 10.1098/rstb.2012.0273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes B., Augusto M. T., Felício M. R., Hollmann A., Franco O. L., Gonçalves S., et al. (2018). Designing improved active peptides for therapeutic approaches against infectious diseases. Biotechnol. Adv. 36, 415–429. 10.1016/j.biotechadv.2018.01.004 [DOI] [PubMed] [Google Scholar]
- Grace D., Mutua F., Ochungo P., Kruska R. L., Jones K., Brierley L., et al. (2012). Mapping of Poverty and Likely Zoonoses Hotspots. Available online at: https://hdl.handle.net/10568/2116 (accessed May 10, 2021).
- Grilli E., Messina M. R., Catelli E., Morlacchini M., Piva A. (2009). Pediocin A improves growth performance of broilers challenged with Clostridium perfringens. Poult. Sci. J. 88, 2152–2158. 10.3382/ps.2009-00160 [DOI] [PubMed] [Google Scholar]
- Gualillo O., Lago Gómez-Reino J., Casanueva F. F., Dieguez C. (2003). Ghrelin, a widespread hormone: insights into molecular and cellular regulation of its expression and mechanism of action. FEBS Lett. 552, 105–109. 10.1016/S0014-5793(03)00965-7 [DOI] [PubMed] [Google Scholar]
- Gyan W. R., Yang Q., Tan B., Jan S. S., Jiang L., Chi S., et al. (2020). Effects of antimicrobial peptides on growth, feed utilization, serum biochemical indices and disease resistance of juvenile shrimp, Litopenaeus vannamei. Aquac Res 51, 1222–1231. 10.1111/are.14473 [DOI] [Google Scholar]
- Hancock R., Chapple D. (1999). MINIREVIEW peptide antibiotics. Antimicrob. Agents Chemother. 43, 1317–1323. 10.1128/AAC.43.6.1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E. (2001). Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 1, 156–164. 10.1016/S1473-3099(01)00092-5 [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Sahl H. G. (2006). Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24, 1551–1557. 10.1038/nbt1267 [DOI] [PubMed] [Google Scholar]
- Håkansson J., Ringstad L., Umerska A., Johansson J., Andersson T., Boge L., et al. (2019). Characterization of the in vitro, ex vivo, and in vivo efficacy of the antimicrobial peptide dpk-060 used for topical treatment. Front. Cell. Infect. Microbiol. 9,174. 10.3389/fcimb.2019.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson J. C., Fage C. D., Cannon J. R., Brodbelt J. S., Keatinge-Clay A. T., Trent M. S. (2014). Antimicrobial peptide resistance of Vibrio cholerae results from an LPS modification pathway related to nonribosomal peptide synthetases. ACS Chem. Biol. 9, 2382–2392. 10.1021/cb500438x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-González J. C., Martínez-Tapia A., Lazcano-Hernández G., García-Pérez B. E., Castrejón-Jiménez N. S. (2021). Bacteriocins from lactic acid bacteria. A powerful alternative as antimicrobials, probiotics, and immunomodulators in veterinary medicine. Animals 11, 979. 10.3390/ani11040979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer O. E., Kruse H., Grave K., Collignon P., Karunasagar I., Angulo F. J. (2009). Human health consequences of use of antimicrobial agents in aquaculture. Clin. Infect. Dis. 49, 1248–1253. 10.1086/605667 [DOI] [PubMed] [Google Scholar]
- Hiss J. A., Hartenfeller M., Schneider G. (2010). Concepts and applications of “natural computing” techniques in de novo drug and peptide design. Curr. Pharm. Des. 16, 1656–1665. 10.2174/138161210791164009 [DOI] [PubMed] [Google Scholar]
- Huhand Y. A. J., Kwon J. (2011). Nanoantibiotics': a new paradigm for treating infectious dis-eases using nanomaterials in the antibiotics-resistant era. J. Control Release 156, 128–145. 10.1016/j.jconrel.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Hwang B., Hwang J. S., Lee J., Lee D. G. (2011). The antimicrobial peptide, psacotheasin induces reactive oxygen species and triggers apoptosis in Candida albicans. Biochem. Biophys. Res. Commun. 405, 267–271. 10.1016/j.bbrc.2011.01.026 [DOI] [PubMed] [Google Scholar]
- Innes G. K., Randad P. R., Korinek A., Davis M. F., Price L. B., So A. D., et al. (2020). External societal costs of antimicrobial resistance in humans attributable to antimicrobial use in livestock. Annu. Rev. Public Health 41, 111–157. 10.1146/annurev-publhealth-040218-043954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksson J., Brandsdal B. O., Engqvist M., Flaten G. E., Svendsen J. S. M., Stensen W. (2011). A synthetic antimicrobial peptidomimetic (LTX 109): stereochemical impact on membrane disruption. J. Med. Chem. 54, 5786–5795. 10.1021/jm200450h [DOI] [PubMed] [Google Scholar]
- Iseppi R., Andrea L., Sabia C. (2021). Bacteriocin-producing probiotic bacteria: a natural solution for increasing efficiency and safety of livestock food production. Front. Microbiol. 12, 675483. 10.3389/fmicb.2021.675483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H., Tokumoto K., Kaji T., Paudel A., Panthee S., Hamamoto H., et al. (2018). Total synthesis and biological mode of action of WAP-8294A2: A menaquinone-targeting antibiotic. J. Org. Chem. 83, 6924–6935. 10.1021/acs.joc.7b02318 [DOI] [PubMed] [Google Scholar]
- Jakobus K., Wend S., Weber W. (2012). Synthetic mammalian gene networks as a blueprint for the design of interactive biohybrid materials. Chem. Soc. Rev. 41, 1000–1018. 10.1039/C1CS15176B [DOI] [PubMed] [Google Scholar]
- Jenssen H., Hamill P., Hancock R. E. (2006). Peptide antimicrobial agents. Clin. Microbiol. Rev. 19, 491–511. 10.1128/CMR.00056-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F. T., Ricke S. C. (2003). Observations on the history of the development of antimicrobials and their use in poultry feeds. Poult. Sci. J. 82, 613–617. 10.1093/ps/82.4.613 [DOI] [PubMed] [Google Scholar]
- Józefiak D., Kierończyk B., Juśkiewicz J., Zduńczyk Z., Rawski M., Długosz J., et al. (2013). Dietary nisin modulates the gastrointestinal microbial ecology and enhances growth performance of the broiler chickens. PLoS ONE 8, e85347. 10.1371/journal.pone.0085347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaikabo A. A., Sabo Mohammed A., Abas F. (2016). Chitosan nanoparticles as carriers for the delivery of ΦKAZ14 bacteriophage for oral biological control of colibacillosis in chickens. Molecules 21, 256. 10.3390/molecules21030256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsunuma Y., Hanazumi M., Fujisaki H., Minato H., Hashimoto Y., Yonemochi C. (2007). Associations between the use of antimicrobial agents for growth promotion and the occurrence of antimicrobial-resistant Escherichia coli and enterococci in the feces of livestock and livestock farmers in Japan. J. Gen. Appl. Microbiol. 53, 273–279. 10.2323/jgam.53.273 [DOI] [PubMed] [Google Scholar]
- Kemp D. R., Michalk D. L. (eds.). (2011). Development of Sustainable Livestock Systems on Grasslands in North-Western China ACIAR Proceedings No. 134. Canberra, ACT: Australian Centre for International Agricultural Research. [Google Scholar]
- King J. R., Edgar S., Qiao K., Stephanopoulos G. (2016). Accessing nature's diversity through metabolic engineering and synthetic biology. F1000 Res. 5, 1–11. 10.12688/f1000research.7311.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogut M. H., Genovese K. J., He H., Swaggerty C. L., Jiang Y. (2013). Modulation of chicken intestinal immune gene expression by small cationic peptides as feed additives during the first week posthatch. Clin. Vaccine Immunol. 20, 1440–1448. 10.1128/CVI.00322-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krehbiel C. (2013). The role of new technologies in global food security: improvinganimal production efficiency and minimizing impacts. Anim. Front. 3, 4–7. 10.2527/af.2013-0017 [DOI] [Google Scholar]
- Krishnasamy V., Otte J., Silbergeld E. (2015). Antimicrobial use in Chinese swine and broiler poultry production. Antimicrob. Resist. Infect. Control 4, 17. 10.1186/s13756-015-0050-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudrimoti M., Curtis A., Azawi S., Worden F., Katz S., Adkins D., et al. (2016). Dusquetide: a novel innate defense regulator demonstrating a significant and consistent reduction in the duration of oral mucositis in preclinical data and a randomized, placebo-controlled Phase 2a clinical study. J. Biotechnol. 239, 115–125. 10.1016/j.jbiotec.2016.10.010 [DOI] [PubMed] [Google Scholar]
- Lagha A. B., Haas B., Gottschalk M., Grenier D. (2017). Antimicrobial potential of bacteriocins in poultry and swine production. Vet. Res. 48, 22. 10.1186/s13567-017-0425-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W., Yang C. (2019). Ruminal methane production: associated microorganisms and the potential of applying hydrogen-utilizing bacteria for mitigation. Sci. Total Environ. 654, 1270–1283. 10.1016/j.scitotenv.2018.11.180 [DOI] [PubMed] [Google Scholar]
- Leahy S. C., Doyle N., Mbandlwa P., Attwood G. T., Li Y., Ross P., et al. (2019). Use of lactic acid bacteria to reduce methane production in ruminants, a critical review. Front. Microbiol. 10, 2207. 10.3389/fmicb.2019.02207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- León R., Ruiz M., Valero Y., Cárdenas C., Guzman F., Vila M., et al. (2020). Exploring small cationic peptides of different origin as potential antimicrobial agents in aquaculture. Fish Shellfish Immunol. 98, 720–727. 10.1016/j.fsi.2019.11.019 [DOI] [PubMed] [Google Scholar]
- Lhermie G., Gröhn Y. T., Raboisson D. (2017). Addressing antimicrobial resistance: an overview of priority actions to prevent suboptimal antimicrobial use in food-animal production. Front. Microbiol. 7, 2114. 10.3389/fmicb.2016.02114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Cha D. J., Lai Y., Villaruz A. E., Sturdevant D. E., Otto M. (2007). The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol. Microbiol. 66, 1136–1147. 10.1111/j.1365-2958.2007.05986.x [DOI] [PubMed] [Google Scholar]
- Li Z., Hu Y., Yang Y., Lu Z., Wang Y. (2018). Antimicrobial resistance in livestock: antimicrobial peptides provide a new solution for a growing challenge. Anim. Front. 8, 21–29. 10.1093/af/vfy005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. J., Seo C. K., Kim T. H., Myung S. W. (2013). Occurrence and ecological hazard assessment of selected veterinary medicines in livestock wastewater treatment plants. J. Environ. Sci. Health B. 48, 658–670. 10.1080/03601234.2013.778604 [DOI] [PubMed] [Google Scholar]
- Liu T., She R., Wang K., Bao H., Zhang Y., Luo D., et al. (2008). Effects of rabbit Sacculus rotundus antimicrobial peptides on the intestinal mucosal immunity in chickens. Poult. Sci. 87, 250–254. 10.3382/ps.2007-00353 [DOI] [PubMed] [Google Scholar]
- Loose C., Jensen K., Rigoutsos I., Stephanopoulos G. (2006). A linguistic model for the rational design of antimicrobial peptides. Nature 443, 867–869. 10.1038/nature05233 [DOI] [PubMed] [Google Scholar]
- Loponte R., Pagnini U., Iovane G., Pisanelli G. (2021). Phage therapy in veterinary medicine. Antibiotics 10, 421. 10.3390/antibiotics10040421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J. L., Zhao L. H., Sun D. D., Zhang J., Guo Y. P., Zhang Z. Q., et al. (2019). Effects of dietary supplementation of recombinant plectasin on growth performance, intestinal health and innate immunity response in broilers. Probiotics Antimicrob. Proteins 12, 214–223. 10.1007/s12602-019-9515-2 [DOI] [PubMed] [Google Scholar]
- Ma T., McAllister T. A, Guan L. L. (2021). A review of the resistome within the digestive tract of livestock. Anim. Sci. Biotechnol. 12, 1–20. 10.1186/s40104-021-00643-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magouras I., Carmo L. P., Stärk K. D., Schüpbach-Regula G. (2017). Antimicrobial usage and-resistance in livestock: where should we focus? Front. Vet. Sci. 4, 148. 10.3389/fvets.2017.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria-Neto S., de Almeida K. C., Macedo M. L. R., Franco O. L. (2015). Understanding bacterial resistance to antimicrobial peptides: from the surface to deep inside. Biochimica et Biophys. Acta Biomemb. 1848, 3078–3088. 10.1016/j.bbamem.2015.02.017 [DOI] [PubMed] [Google Scholar]
- McEwen S. A., Fedorka-Cray P. J. (2002). Antimicrobial use and resistance in animals. Clin. Infect. Dis. 34(Suppl. 3), S93–S106. 10.1086/340246 [DOI] [PubMed] [Google Scholar]
- Meade E., Slattery M. A., Garvey M. (2020). Bacteriocins, potent antimicrobial peptides and the fight against multi drug resistant species: resistance is futile? Antibiotics 9, 32. 10.3390/antibiotics9010032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina M. J., Legido-Quigley H., Hsu L. Y. (2020). Antimicrobial Resistance in One Health. Global Health Security. Cham: Springer. 209–229. 10.1007/978-3-030-23491-1_10 [DOI] [Google Scholar]
- Mensa B., Howell G., Scott R., DeGrado W. (2014). Comparative mechanistic studies of brilacidin, daptomycin, and the antimicrobial peptide LL16. Antimicrob. Agents Chemother. 58, 5136–5145. 10.1128/AAC.02955-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensah S. E., Koudande O. D., Sanders P., Laurentie M., Mensah G. A., Abiola F. A. (2014). Antimicrobial residues in foods of animal origin in Africa: public health risks. Rev. Sci. Tech. 33, 987–996. 10.20506/rst.33.3.2335 [DOI] [PubMed] [Google Scholar]
- Mercer D. K., Robertson J. C., Miller L., Stewart C. S., O'Neil D. A. (2020). NP213 (NovexatinR): a unique therapy candidate for onychomycosis with a differentiated safety and efficacy profile. Med. Mycol. 58, 1064–1072. 10.1093/mmy/myaa015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi S., Madi A., Prévost H., Feuilloley M., Manai M., Dousset X., et al. (2012). In vitro evaluation of the probiotic potential of Lactobacillus salivarius SMXD51. Anaerobe 18, 584–589. 10.1016/j.anaerobe.2012.10.004 [DOI] [PubMed] [Google Scholar]
- Michalk D. L., Kemp D. R., Badgery W. B., Wu J., Zhang Y., Thomassin P. J. (2019). Sustainability and future food security—A global perspective for livestock production. Land Degrad. Dev. 30, 561–573. 10.1002/ldr.3217 [DOI] [Google Scholar]
- Mitchell J. B. (2014). Machine learning methods in chemoinformatics. Wiley Interdiscipl. Rev. Comput. Mol. Sci. 4, 468–481. 10.1002/wcms.1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake O., Ochia A., Hashimoto W., Murata K. (2004). Origin and diversity of alginate lyases of families PL-5 and−7 in Sphingomonas sp.strain A1. J. Bacteriol. 186, 2891–2896. 10.1128/JB.186.9.2891-2896.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookherjee N., Brown K. L., Bowdish D. M., Doria S., Falsafi R., Hokamp K., et al. (2006). Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J. Immunol. 176, 2455–2464. 10.4049/jimmunol.176.4.2455 [DOI] [PubMed] [Google Scholar]
- Muchintala D., Suresh V., Raju D., Sashidhar R. B. (2020). Synthesis and characterization of cecropin peptide-based silver nanocomposites: its antibacterial activity and mode of action. Mater. Sci. Eng. C. 110, 110712. 10.1016/j.msec.2020.110712 [DOI] [PubMed] [Google Scholar]
- Munita J. M., Arias C. A. (2016). Mechanisms of antibiotic resistance. Microbiol Spectr. 4, 10.1128/microbiolspec. VMBF-0016- 2015. 10.1128/microbiolspec [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C. J., Foster B. A., Mannis M. J., Selsted M. E., Reid T. W. (1993). Defensins are mitogenic for epithelial cells and fibroblasts. J. Cell Physiol. 155, 408–413. 10.1002/jcp.1041550223 [DOI] [PubMed] [Google Scholar]
- National Research Council . (1999). The Use of Drugs in Food Animals: Benefits and Risks. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Noel J. P. (2010). Synthetic metabolism goes green. Nature 468, 380–381. 10.1038/468380a [DOI] [PubMed] [Google Scholar]
- OIE World Organisation for Animal Health. (2012). Terrestrial Animal Health Code. 21st Edn. Paris: OIE. Available online at: http://www.oie.int/international-standard-setting/terrestrial-code/ (accessed March 30, 2021).
- Otto M. (2009). Bacterial sensing of antimicrobial peptides. Bacterial Sens. Signal. 16, 136–149. 10.1159/000219377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelgrift R. Y., Friedman A. J. (2013). Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 65, 1803–1815. 10.1016/j.addr.2013.07.011 [DOI] [PubMed] [Google Scholar]
- Peyrusson F., Butler D., Tulkens P. M., Van Bambeke F. (2015). Cellular pharmacokinetics and intracellular activity of the novel peptide deformylase inhibitor GSK1322322 against Staphylococcus aureus laboratory and clinical strains with various resistance phenotypes: studies with human THP-1monocytes and J774 murine macrophages. Antimicrob. Agents Chemother. 59, 5747–5760. 10.1128/AAC.00827-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K. (2000). Healing after inflammatory injury–coordination of a regulatory peptide network. Aliment. Pharmacol. Ther. 14, 87–93. 10.1046/j.1365-2036.2000.014s1087.x [DOI] [PubMed] [Google Scholar]
- Porto W. F., Silva O. N., Franco O. L. (2012). “Prediction and rational design of antimicrobial peptides,” in Protein Structure, 1st Edn., ed E. Faraggi (InTech), 20. [Google Scholar]
- Prince A., Sandhu P., Ror P., Dash E., Sharma S., Arakha M., et al. (2016). Lipid-II independent antimicrobial mechanism of nisin depends on its crowding and degree of oligomerization. Sci. Rep. 6, 1–15. 10.1038/srep37908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai M., Pandit R., Gaikwad S., Kövics G. (2016). Antimicrobial peptides as natural bio-preservative to enhance the shelf-life of food. J. Food Sci. Technol. 53, 3381–3394. 10.1007/s13197-016-2318-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisinger A., Clark H. (2018). How much do direct livestock emissions actually contribute to global warming? Glob. Change Biol. 24, 1749–1761. 10.1111/gcb.13975 [DOI] [PubMed] [Google Scholar]
- Ren Z., Yao R., Liu Q., Deng Y., Shen L., Deng H., et al. (2019). Effects of antibacterial peptides on rumen fermentation function and rumen microorganisms in goats. PLoS ONE 14, e221815. 10.1371/journal.pone.0221815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues G., Silva G. G. O., Buccini D. F., Duque H. M., Dias S. C., Franco O. L. (2019). Bacterial proteinaceous compounds with multiple activities toward cancers and microbial infection. Front. Microbiol. 10, 1690. 10.3389/fmicb.2019.01690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues G. R., López-Abarrategui C., de la Serna Gómez I., Dias S. C., Otero-González A. J., Franco O. L. (2019). Antimicrobial magnetic nanoparticles based-therapies for controlling infectious diseases. Int. J. Pharm. 555, 356–367. 10.1016/j.ijpharm.2018.11.043 [DOI] [PubMed] [Google Scholar]
- Saido-Sakanaka H., Ishibashi J., Momotani E., Amano F., Yamakawa M. (2004). In vitro and in vivo activity of antimicrobial peptides synthesized based on the insect defensin. Peptides 25, 19–27. 10.1016/j.peptides.2003.12.009 [DOI] [PubMed] [Google Scholar]
- Santoso B., Mwenya B., Sar C., Gamo Y., Kobayashi T., Morikawa R., et al. (2004). Effects of supplementing galacto-oligosaccharides, Yucca schidigera or nisin on rumen methanogenesis, nitrogen and energy metabolism in sheep. Livest. Prod. Sci. 91, 209–217. 10.1016/j.livprodsci.2004.08.004 [DOI] [Google Scholar]
- Sar C., Mwenya B., Pen B., Morikawa R., Takaura K., Kobayashi T., et al. (2005). Effect of nisin on ruminal methane production and nitrate/nitrite reduction in vitro. Aust. J. Agric. Res. 56, 803–810. 10.1071/AR04294 [DOI] [Google Scholar]
- Schneider T., Gries K., Josten M., Wiedemann I., Pelzer S., Labischinski H., et al. (2009). The lipopeptide antibiotic friulimicin B inhibits cell wall biosynthesis through complex formation with bactoprenol phosphate. Antimicrob. Agents Chemother. 53, 1610–1618. 10.1128/AAC.01040-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze M., Junkes C., Mueller P., Speck S., Ruediger K., Dathe M., et al. (2014). Effects of cationic antimicrobial peptides on liquid-preserved boar spermatozoa. PLoS ONE 9, e100490. 10.1371/journal.pone.0100490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze M., Nitsche-Melkus E., Hensel B., Jung M., Jakop U. (2020). Antibiotics and their alternatives in artificial breeding in livestock. Anim. Reprod. Sci. 220, 106284. 10.1016/j.anireprosci.2020.106284 [DOI] [PubMed] [Google Scholar]
- Scott H. M., Acuff G., Bergeron G., Bourassa M. W., Gill J., Graham D. W., et al. (2019). Critically important antibiotics: criteria and approaches for measuring and reducing their use in food animal agriculture. Ann. N. Y. Acad. Sci. 1441, 8. 10.1111/nyas.14058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shai Y. (2002). Mode of action of membrane active antimicrobial peptides. Pep. Sci. 66, 236–248. 10.1002/bip.10260 [DOI] [PubMed] [Google Scholar]
- Shaoyong W., Li Q., Ren Z. Q., Wei C. S., Chu G. Y., Dong W. Z., et al. (2019). Evaluation of ε-polylysine as antimicrobial alternative for liquid-stored boar semen. Theriogenology 130, 146. 10.1016/j.theriogenology.2019.03.005 [DOI] [PubMed] [Google Scholar]
- Sharma C., Rokana N., Chandra M., Singh B. P., Gulhane R. D., Gill J. P. S., et al. (2018). Antimicrobial resistance: its surveillance, impact, and alternative management strategies in dairy animals. Front. Vet. Sci. 4, 237. 10.3389/fvets.2017.00237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Liu Z., Yu Z., Zhu W. (2017). Monensin and nisin affect rumen fermentation and microbiota differently in vitro. Front. Microbiol. 8, 1111. 10.3389/fmicb.2017.01111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J. S., Liu Z., Chen Y. Y., Lv P. A., Zhu W. Y. (2016). Effects of nisin on in vitro fermentation, methanogenesis and functional microbial populations of the rumen. Acta Microbiol. Sin. 56, 1348–1357. 10.13343/j.cnki.wsxb.20150559 [DOI] [PubMed] [Google Scholar]
- Shin J. M., Gwak J. W., Kamarajan P., Fenno J. C., Rickard A. H., Kapila Y. L. (2016). Biomedical applications of nisin. J. Appl. Microbiol. 120, 1449–1465. 10.1111/jam.13033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira R. F., Roque-Borda C. A., Vicente E. F. (2021). Antimicrobial peptides as a feed additive alternative to animal production, food safety and public health implications: an overview. Anim. Nutr. 7, 896–904. 10.1016/j.aninu.2021.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivertsen A., Isaksson J., Leiros H.-K. S., Svenson J., Svendsen J.-S., Brandsdal B. O. (2014). Synthetic cationic antimicrobial peptides bind with their hydrophobic parts to drug site II of human serum albumin. BMC Struct. Biol. 14:4. 10.1186/1472-6807-14-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy S. M., Huang J., Gogarten J. P. (2015). Horizontal gene transfer: building the web of life. Nat. Rev. Genet. 16, 472–482. 10.1038/nrg3962 [DOI] [PubMed] [Google Scholar]
- Sparo M. D., Jones D. G., Sánchez Bruni S. F. (2009). In vitro efficacy of the novel peptide CECT7121 against bacteria isolated from mastitic dairy cattle. Lett. Appl. Microbiol. 48, 187–192. 10.1111/j.1472-765X.2008.02507.x [DOI] [PubMed] [Google Scholar]
- Speck S., Courtiol A., Junkes C., Dathe M., Müller K., Schulze M. (2014). Cationic synthetic peptides: assessment of their antimicrobial potency in liquid preserved boar semen. PLoS ONE 9, e105949. 10.1371/journal.pone.0105949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spohn R., Daruka L., Lázár V., Martins A., Vidovics F., Grézal G., et al. (2019). Integrated evolutionary analysis reveals antimicrobial peptides with limited resistance. Nat. Commun. 10, 1–13. 10.1038/s41467-019-12364-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas N., Jetter P., Ueberbacher B. J., Werneburg M., Zerbe K., Steinmann J., et al. (2010). Peptidomimetic antibiotics target outer-membrane biogenesis in Pseudomonas aeruginosa. Science 327, 1010–1013. 10.1126/science.1182749 [DOI] [PubMed] [Google Scholar]
- Stahl C. H., Callaway T. R., Lincoln L. M., Lonergan S. M., Genovese K. J. (2004). Inhibitory activities of colicins against Escherichia coli strains responsible for postweaning diarrhea and edema disease in swine. Antimicrob. Agents Chemother. 48, 3119–3121. 10.1128/AAC.48.8.3119-3121.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh G., Das R. K., Kaur Brar S., Rouissi T., Avalos Ramirez A., Chorfi Y., et al. (2018). Alternatives to antibiotics in poultry feed: molecular perspectives. Crit. Rev. Microbiol. 44, 318–335. 10.1080/1040841X.2017.1373062 [DOI] [PubMed] [Google Scholar]
- Swangchan-Uthai T., Chen Q., Kirton S. E., Fenwick M. A., Cheng Z., Patton J., et al. (2013). Influence of energy balance on the antimicrobial peptides S100A8 and S100A9 in the endometrium of the post-partum dairy cow. Reproduction 145, 527. 10.1530/REP-12-0513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann J. P. (1983). The search for synthetic penicillin during World War II. Br. J. Hist. Sci. 16, 154–190. 10.1017/S0007087400026789 [DOI] [PubMed] [Google Scholar]
- Takano E., Breitling R. (2014). Antimicrobial Resistance – A New Drug Perspective Using Synthetic Biology. Houston, TX: Emerging and Persistent Infectious Diseases (EPID): Focus on Antimicrobial Resistance. [Google Scholar]
- Tang Z., Yin Y., Zhang Y., Huang R., Sun Z., Li T., et al. (2008). Effects of dietary supplementation with an expressed fusion peptide bovine lactoferricin–lactoferrampin on performance, immune function and intestinal mucosal morphology in piglets weaned at age 21 d. Br. J. Nutr. 101, 998–1005. 10.1017/S0007114508055633 [DOI] [PubMed] [Google Scholar]
- Thanner S., Drissner D., Walsh F. (2016). Antimicrobial resistance in agriculture. MBio 7, e02227–e02215. 10.1128/mBio.02227-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. M., Nielsen K. M. (2005). Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat. Rev. Microbiol. 3, 711–721. 10.1038/nrmicro1234 [DOI] [PubMed] [Google Scholar]
- Tollin M., Bergman P., Svenberg T., Jornvall H., Gudmundsson G. H., Agerberth B. (2003). Antimicrobial peptides in the first line defence of human colon mucosa. Peptides 24, 523–530. 10.1016/S0196-9781(03)00114-1 [DOI] [PubMed] [Google Scholar]
- Torres M. D. T., De La Fuente-Nunez C. (2019). Toward computer-made artificial antibiotics. Curr. Opin. Microbiol. 51, 30–38. 10.1016/j.mib.2019.03.004 [DOI] [PubMed] [Google Scholar]
- Travis S., Yap L. M., Hawkey C., Warren B., Lazarov M., Fong T., et al. (2005). RDP58 is a novel and potentially effective oral therapy for ulcerative colitis. Inflamm. Bowel Dis. 11, 713–719. 10.1097/01.MIB.0000172807.26748.16 [DOI] [PubMed] [Google Scholar]
- van Dijk A., Veldhuizen E. J., Kalkhove S. I., Tjeerdsma-van Bokhoven J. L., Romijn R. A., Haagsman H. P. (2007). The β-defensin gallinacin-6 is expressed in the chicken digestive tract and has antimicrobial activity against food-borne pathogens. Antimicrob. Agents Chemother. 51, 912–922. 10.1128/AAC.00568-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Groenendael R., Kox M., van Eijk L. T., Pickkers P. (2018). Immunomodulatory and kidney-protective effects of the human chorionic gonadotropin derivate EA-230. Nephron. 140, 148–151. 10.1159/000490772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnava K. G., Ronimus R. S., Sarojini V. (2017). A review on comparative mechanistic studies of antimicrobial peptides against archaea. Biotechnol. Bioeng. 114, 2457–2473. 10.1002/bit.26387 [DOI] [PubMed] [Google Scholar]
- Venegas-Ortega M. G., Flores-Gallegos A. C., Martínez-Hernández J. L., Aguilar C. N., Nevárez-Moorillón G. V. (2019). Production of bioactive peptides from lactic acid bacteria: a sustainable approach for healthier foods. Compr. Rev. Food Sci. Food Saf. 18, 1039–1051. 10.1111/1541-4337.12455 [DOI] [PubMed] [Google Scholar]
- Vidovic N., Vidovic S. (2020). Antimicrobial resistance and food animals: influence of livestock environment on the emergence and dissemination of antimicrobial resistance. Antibiotics 9, 52. 10.3390/antibiotics9020052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieco-Saiz N., Belguesmia Y., Raspoet R., Auclair E., Gancel F., Kempf I., et al. (2019). Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front. Microbiol. 10, 57. 10.3389/fmicb.2019.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilas L. B., Campos M. L., Berlanda R. L. A., Franco O. L. (2019). Antiviral peptides as promising therapeutic drugs. Cell Mol. Life Sci. 76, 3525–3542. 10.1007/s00018-019-03138-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Song Q., Huang S., Wang Y., Cai S., Yu H., et al. (2020). Effect of antimicrobial peptide microcin J25 on growth performance, immune regulation, and intestinal microbiota in broiler chickens challenged with Escherichia coli and Salmonella. Animals 10, 345. 10.3390/ani10020345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Thacker P.A, Watford M., Qiao S. (2015a). Functions of antimicrobial peptides in gut homeostasis. Curr. Prot. Pept. Sci. 16, 582–591. 10.2174/1389203716666150630135847 [DOI] [PubMed] [Google Scholar]
- Wang S., Zeng X., Yang Q., Qiao S. (2016). Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. Int. J. Mol. Sci. 17, 603. 10.7150/ijms.13264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Zeng X. F., Wang Q. W., Zhu J. L., Peng Q., Hou C. L., et al. (2015b). The antimicrobial peptide sublancin ameliorates necrotic enteritis induced by Clostridium perfringens in broilers. J. Anim. Sci. 93, 4750–4760. 10.2527/jas.2015-9284 [DOI] [PubMed] [Google Scholar]
- Wang Y., Hong J., Liu X., Yang H., Liu R., Wu J., et al. (2008). Snake cathelicidin from Bungarus fasciatus is a potent peptide antibiotic. PLoS ONE 3, e3217. 10.1371/journal.pone.0003217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Lyu N., Liu F., Liu W. J., Bi Y., Zhang Z., et al. (2021). More diversified antibiotic resistance genes in chickens and workers of the live poultry markets. Environ. Int. 153, 106534. 10.1016/j.envint.2021.106534 [DOI] [PubMed] [Google Scholar]
- Ward B. P., Ottaway N. L., Perez-Tilve D., Ma D., Gelfanov V. M., Tschöp M. H., et al. (2013). Peptide lipidation stabilizes structure to enhance biological function. Mol. Metab. 2, 468–479. 10.1016/j.molmet.2013.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber W., Fussenegger M. (2012). Emerging biomedical applications of synthetic biology. Nat. Rev. Genet. 13, 21–35. 10.1038/nrg3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber W., Schoenmakers R., Keller B., Gitzinger M., Grau T., Daoud-El Baba M., et al. (2008). A synthetic mammalian gene circuit reveals antituberculosis compounds. Proc. Natl. Acad. Sci. U. S. A. 105, 9994–9998. 10.1073/pnas.0800663105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L. F., He J. G. (2012). Dose-response effects of an antimicrobial peptide, a cecropin hybrid, on growth performance, nutrient utilisation, bacterial counts in the digesta and intestinal morphology in broilers. Br. J. Nutr. 108, 1756–1763. 10.1017/S0007114511007240 [DOI] [PubMed] [Google Scholar]
- WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance (AGISAR) (2011). Critically Important Antimicrobials for Human Medicine – 3rd Revision 2011. 1–38. Available online at: http://apps.who.int/iris/bitstream/10665/77376/1/9789241504485_eng.pdf (accessed 15 April 2021).
- Wimley W. C., Hristova K. (2011). Antimicrobial peptides: successes,challenges and unanswered questions. J. Membr. Biol. 239, 27–34. 10.1007/s00232-011-9343-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte W. (2000). Selective pressure by antibiotic use in livestock. Int. J. Antimicrob. Agents 16, 19–24. 10.1016/S0924-8579(00)00301-0 [DOI] [PubMed] [Google Scholar]
- Woolhouse M., Ward M., van Bunnik B., Farrar J. (2015). Antimicrobial resistance in humans, livestock and the wider environment. Philos. Trans. R. Soc. B. Biol. Sci. 370, 20140083. 10.1098/rstb.2014.0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2014). WHO's First Global Report on Antibiotic Resistance Reveals Serious, Worldwide Threat to Public Health. Available online at: http://www.who.int/mediacentre/news/releases/2014/amr-report/en/ (accessed April 10, 2021).
- World-Health-Organisation [WHO] (2018). Cancer: World Cancer Report. Geneva: World-Health-Organisation. [Google Scholar]
- Wu W., Yu Q., You L., Chen K., Tang H., Liu J. (2018). Global cropping intensity gaps: increasing food production without cropland expansion. Land Use Policy 76, 515–525. 10.1016/j.landusepol.2018.02.03227956604 [DOI] [Google Scholar]
- Wu X. L., Xiang L., Yan Q. Y., Jiang Y. N., Li Y. W., Huang X. P., et al. (2014). Distribution and risk assessment of quinolone antibiotics in the soils from organic vegetable farms of a subtropical city, Southern China. Sci. Total Environ. 487, 399–406. 10.1016/j.scitotenv.2014.04.015 [DOI] [PubMed] [Google Scholar]
- Xiao H., Shao F., Wu M., Ren W., Xiong X., Tan B., et al. (2015). The application of antimicrobial peptides as growth and health promoters for swine. Anim. Sci. Biotechnol. 6, 1–6. 10.1186/s40104-015-0018-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S., Jha R. (2019). Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 10, 2 10.1186/s40104-018-0310-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasir M., Dutta D., Willcox M. D. P. (2019). Comparative mode of action of the antimicrobial peptide melimine and its derivative Mel4 against Pseudomonas aeruginosa. Sci. Rep. 9, 7063. 10.1038/s41598-019-42440-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasir M., Dutta D., Hossain K. R., Chen R., Ho K. K. K., Kuppusamy R., et al. (2020). Mechanism of action of surface immobilized antimicrobial peptides against Pseudomonas aeruginosa. Front. Microbiol. 10:3053. 10.3389/fmicb.2019.03053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman M. R., Yount N. Y. (2003). Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 55, 27–55. 10.1124/pr.55.1.2 [DOI] [PubMed] [Google Scholar]
- Yi H., Yu C., Zhang H., Song D., Jiang D., Du H., et al. (2015). Cathelicidin-BF suppresses intestinal inflammation by inhibiting the nuclear factor-κB signaling pathway and enhancing the phagocytosis of immune cells via STAT-1 in weanling piglets. Inter. Immunopharmacol. 28, 61–69. 10.1016/j.intimp.2015.05.034 [DOI] [PubMed] [Google Scholar]
- Yoon J. H., Ingale S. L., Kim J. S., Kim K. H., Lee S. H., Park Y. K., et al. (2012). Effects of dietary supplementation of antimicrobial peptide-A3 on growth performance, nutrient digestibility, intestinal and fecal microflora and intestinal morphology in weanling pigs. Anim. Feed Sci. Technol. 177, 98–107. 10.1016/j.anifeedsci.2012.06.009 [DOI] [Google Scholar]
- Yoon J. H., Ingale S. L., Kim J. S., Kim K. H., Lee S. H., Park Y. K., et al. (2014). Effects of dietary supplementation of synthetic antimicrobial peptide-A3 and P5 on growth performance, apparent total tract digestibility of nutrients, fecal and intestinal microflora and intestinal morphology in weanling pigs. Livest. Sci. 159, 53–60. 10.1016/j.livsci.2013.10.025 [DOI] [PubMed] [Google Scholar]
- Yoon J. H., Ingale S. L., Kim J. S., Kim K. H., Lohakare J., Park Y. K., et al. (2013). Effects of dietary supplementation with antimicrobial peptide-P5 on growth performance, apparent total tract digestibility, faecal and intestinal microflora and intestinal morphology of weanling pigs. J. Sci. Food Agric. 93, 587–592. 10.1002/jsfa.5840 [DOI] [PubMed] [Google Scholar]
- Yu H. B., Kielczewska A., Rozek A., Takenaka S., Li Y., Thorson L., et al. (2009). Sequestosome-1/p62 is the key intracellular target of innate defense regulator peptide. J. Biol. Chem. 284, 36007–36011. 10.1074/jbc.C109.073627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhang B., Zhang X., Wang X., Wu K., Guan Q. (2017). Effects of cathelicidin-derived peptide from reptiles on lipopolysaccharide-induced intestinal inflammation in weaned piglets. Vet. Immunol. Immunopathol. 192, 41–53. 10.1016/j.vetimm.2017.09.005 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zhang M., Qiu S., Wang J., Peng J., Zhao P., et al. (2016). Antimicrobial activity and stability of the D-amino acid substituted derivatives of antimicrobial peptide polybia-MPI. AMB Express 6, 122. 10.1186/s13568-016-0295-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucca M., Scutera S., Savoia D. (2011). New antimicrobial frontiers. Mini Rev. Med. Chem. 11, 888–900. 10.2174/138955711796575498 [DOI] [PubMed] [Google Scholar]