Abstract
Several Indigenous edible vegetables in sub-Saharan countries have potential bioactive compounds, including underutilized Biden pilosa (BP). Bioactives from Indigenous edible vegetables are re-evolving as an alternative medicine potential for drug formulations. BP has also been used to mitigate over 40 different diseases in people and animals as herbal medication. Due to globalization and urbanization, people move from more active to more sedentary lifestyles, home-cooked meals to fast foods and snacks, organic foods to processed food with high sugar, salt, and fat. The consumption of native fruits and vegetables is now replaced by a highly processed calory diet, leading to metabolic syndromes such as diabetes, obesity, and other diet-related non-communicable diseases. Hence this article was designed to investigate the existing reports on the use, knowledge and the need to utilize the potentiality of BP further to overcome nutritional deficiencies, food scarcity and mitigation of medical conditions in sub-Saharan Africa. The use of plant-based drugs will aid to decrease the health capitation load as most countries do not have enough funds for purchasing synthetic chemicals used to mitigate diseases.
Keywords: Blackjack, Nutrients, Bioactive: sub-Saharan Africa: Bidens pilosa, Indigenous vegetable
Graphical abstract
Blackjack; Nutrients; Bioactive: sub-Saharan Africa: Bidens pilosa; Indigenous vegetable.
1. Introduction
The blackjack plant scientifically known as Biden Pilosa initiated from South America and then flourished in all tropical and subtropical areas of the global climates [1, 2]. It is unintentionally introduced in the field through farming activities or intentionally for decorative functions. The plant is normally found in the field as a weed, and it is cultivated on a small scale in some countries such as Nigeria, Benin and Zimbabwe for food and medicine [3]. Generally, blackjack is considered a major crop weed and a threat to other plants, as reported by other researchers, and it is widely distributed in tropical Africa [4, 5, 6]. In this case, it is described as a dangerous plant to many other vegetations due to its nature as an invasive weed and hence destroyed as a weed to protect other important flora [6, 7]. Biden Pilosa has been reported to occur in other parts of the world due to its characteristic nature of hardiness, ability to thrive in almost any environment and explosive reproductive potential [6]. Therefore, they are found in other parts of the world apart from Africa, such as Martinique, Dominica, the Caribbean, Mexico, Bolivia, Quechua, Quechua, India, Taiwan, Brazil, Australia, US and Peru [8].
Regardless of its troublesome characteristics, blackjack is well cherished for its medicinal and nutritional potentials [6]. In the medicinal aspect, it is well known and used in treating various diseases such as indigestion, diarrhoea, dysentery, wounds and respiratory infections [6]. The scientific evidence in the literature that BP is an effective treatment for these medical conditions are available, but more studies are required. Also, some African continent countries, including Mozambique, South Africa, Zimbabwe, Zambia, Botswana, Kenya and Congo, reported using indigenous vegetables [9, 10, 11]. Despite all these potentials, blackjack (Biden Pilosa) is underutilized in sub-Saharan Africa due to its classification as a weed or a wild plant [12], which creates a negative perception to the community concerning the consumption of wild or weed plants [13, 14]. To date, no literature addresses consumer preferences for Biden pilosa for reported applications which is a serious omission in the literature. Preference of BP is hindered by its classification as a weed or a wild plant [12] and the negative perception concerning the consumption of wild or weed plants. Therefore future studies on blackjack need to fill this knowledge gap. Due to the negative perception on the consumption of blackjack it is used at the time of scarcity only, particularly when other vegetables are not available [15]. Based on this situation, blackjack is not preferable to many communities despite its benefit in many parts of the world [7]. Therefore, this study aims at providing information to scholars, researchers, policy makers and the general public on the potential benefits of blackjack. In connection to this, the following questions were addressed: -
(i.) What is the nutritional benefit of a blackjack plant?
(ii.) What is the medicinal value of the blackjack plant?
(iii.) Does the blackjack plants have any other applications?
(iv.) What is the safety of using a blackjack plant?
2. Methodology
Multiple search terms and keywords related to blackjack (Biden Pilosa) were used, including health advantages, medicinal value, indigenous vegetable use, safety, nutritional value, and applications. Also, those with references to sub-Saharan Africa and other world places. The studies evaluated by this study are provided in Table 1.
Table 1.
Studies evaluated to extract data on the potential of underutilized Blackjack.
| Author | Study site | Year of study | Study design | Findings | Remarks |
|---|---|---|---|---|---|
|
Medicinal Application | |||||
| Peter Geissberger and Urs Séquin [2] | Basel, Switzerl and Sample of B. Pilosa was collected from Ukerewe Tanzania | 1990 | Experimental | Extracts have antimicrobial and anti-inflammatory activities | Though mild antimicrobial activities, the extract can be used as anti-inflammatory agents |
| Longo et al. [24] | Cameroon | 2008 | Experiment | Enhancement of labour due to the presence of biologically active compounds which act directly in the uterine muscle | Use of B. Pilosa for labour enhancement |
| De Avila et al. [25] | Brazil | 2015 | Experiment | Formulation of B. Pilosa extract for the treatment of intestinal injury in patients undergoing chemotherapy or radiotherapy | B. Pilosa can reduce intestinal injury caused by side effects of chemotherapy and radiotherapy |
| Abd El-Ghani [26] | Nigeria | 2016 | Theoretical | Indigenous vegetable use for medical application in Nigeria, including B. Pilosa, documented | Medical application |
| Hsu et al. [27] | Taiwan | 2009 | Experiment | B. Pilosa aqueous extract can control type 2 diabetes | Utilization of B. Pilosa aqueous extract to control type 2 diabetes |
| Ubillas et al. [28] | USA | 2000 | Experiment | Aerial part extract of B. Pilosa contain acetylenic glucosides | Utility of aerial part extract to control type 2 diabetes |
| Liang et al. [29] | - | 2016 | Experiment | Bioactives in B. Pilosa has antiobesity properties | Use of bioactive from B. Pilosa to control obesity |
| Chung et al. [30] | - | 2016 | Experiment | B. Pilosa extract has antibacterial properties | B. Pilosa extract can be used to treat gut bacteria and coccidiosis in chickens |
| Dimo et al [31] | Cameron | 2001 | Experiment | Aqueous and methylene chloride extracts of B. Pilosa leaf has antihyperglycemic and antihypertensive activity | Aqueous and methylene chloride extracts of B. Pilosa Leaf can be used to reverse high blood and hyperglycaemia |
| Dimo et al [32] | Cameron | 2002 | Experiment | Methanol extract improves masculine sensitivity | Use of methanol extract of B. Pilosa to improve masculine sensitivity |
| Gavhi et al. [33] | South Africa | 2019 | Experiment | B. Pilosa extract possesses bioactive with antihypertensive properties | B. Pilosa extract can be used to control blood pressure |
| Mokganya [34] | South Africa | 2019 | Survey | B. Pilosa has medicinal value | B. Pilosa has medicinal value, therefore can be used by the local paper |
| Yang et al. [35] | Taiwan | 2006 | Experiment | B. Pilosa extract can protect normal human erythrocytes against oxidative damage in vitro | Use of B. Pilosa extract to protect normal human erythrocytes against oxidative damage |
| Lai et al. [36] | Taiwan | 2014 | Clinical evaluation (Human) | B. Pilosa formulation exhibited better glycemic control than diabetic drugs | B. Pilosa formulation had better glycemic control than diabetic drugs; therefore, its use in the control of glycemia |
| Sigh et al |
India |
2017 |
Experiment |
B. Pilosa extracts have antimicrobial activity against E. Coli and cytotoxic against human epidermoid carcinoma |
Use of B. Pilosa as antimicrobial and antitumor activity |
|
Application in Agriculture | |||||
| Batish et al. [37] | India | 2001 | Experiment | B. Pilosa can exert inhibitory effect and hence control the development of weeds | The use of B. Pilosa to control other weeds |
| Tembo et al. [38] | Tanzania and Malawi | 2018 | Experiment | Safety of botanical pesticides such as bioactive from B. Pilosa | The use of bioactive from B. Pilosa as botanical pesticides |
| Taffner and Coallegues [39] | Uganda, East Africa | 2020 | Complementary scrutinises amplicon and isolate libraries | An unusually large core microbiome shared by plants, including procaryotic families such as comamonadaceae, bacillus, sphingobium, pseudomonas, and one archaeon from the soil crenarchaeotic group. Microbiome composition did not differ significantly for plant species but differed for microhabitats. The diversity was, in general, higher for bacteria (27,697 ASVs/H = 6.91) than for archaea (2,995 ASVs/H = 4.91); both groups form a robust network of copiotrophic bacteria and oligotrophic archaea |
Indigenous leafy green vegetable crops can better cope with biotic and abiotic stresses. Therefore, it can properly be used for Plant Growth, Health, and Resilience |
| Ahmed et al. [40] |
Egypt |
2021 |
Field experiment |
B. Pilosa extract have insecticidal properties |
Use of B. Pilosa extract as insecticides |
|
Nutritional Value of B. P | |||||
| Yang and Keding [41] | - | 2009 | Theoretical | B. Pilosa has micronutrient potential as other vegetables such as folate and vitamin B9 | Use of B. Pilosa as a supplement for folate and vitamin B9 |
| Faber et al. [42] | South Africa | 2010 | Qualitative explorative stage (field walks, semi-structured interviews with key informants, focus group discussions) | B. Pilosa, as a wild vegetable, was consumed individually or mixed with other leaves. | Possibility for its application for food in other areas. |
| Odhavi et al. [43] | South Africa | 2007 | Experiment | Leafy vegetables including B. Pilosa contain mineral elements (Ca, P, Na, Zn, Mg, Mn and Fe) and antioxidant levels | Offer indication that these traditional vegetables, requiring no formal cultivation, could contribute to improve the nutritional content of rural and urban communities |
| Singh et al. [44] | India | 2013 | Theoretical | Availability of indigenous vegetables with nutritional benefits | Indigenous vegetables for food and nutritional security |
| Manduna and Vibrans [45] |
Zimbabwe |
2018 |
A survey using focus group discussion and interview |
Availability of indigenous vegetables with nutritional benefits |
B. Pilosa is among the preferred wild plant used as vegetables and eaten frequently |
|
Safety of usage of B. P | |||||
| Liang et al. [46] |
Taiwan |
2020 |
Experiment |
B. Pilosa has no effects in Mice and Chicken |
Indicating its safety for medical application |
|
Other Application of B. Pilosa | |||||
| Ajayi et al. [47] | Nigeria | 2019 | Experiment | B. Pilosa has a good property for corrosion inhibition of surfaces made of mild steel in an acidic medium | Use of bioactive from B. Pilosa as a corrosion inhibitor |
| Alaneme et al. [48] |
Nigeria |
2016 |
Experiment |
B. Pilosa has a good property for corrosion inhibition of surfaces made of aluminium composites in an acidic medium |
Use of bioactive such as tannins and flavonoids from B. Pilosa as a corrosion inhibitor |
|
BP Suppression | |||||
| George [49] | Zimbabwe | 2020 | Field experiment | Herbicides used to control B. Pilosa | Synthetic herbicide such as atrazine to control B.P and other weeds |
| Daba et al. [50] | Ethiopia | 2018 | Field experiment | inhibitory potential of essential oils extracted from eight locally plants and three inert minerals against common weed species of coffee with an stress on Bidens pilosa | Propose the use of this plant extract to control B. Pilosa and other weeds |
| Wang et al. [51] | - | 2013 | Field experiment | Litchi extract can control B. Pilosa as weeds | Propose the use of this plant extract to control B. Pilosa and other weeds |
2.1. Exclusion and inclusion criteria
Any paper which reports on the blackjack (Biden Pilosa), health benefits, medicinal value, indigenous vegetables, usage, safety, nutritional values or its applications were included or else excluded in the study. Most of the literature reviewed was from 2000 – 2021 but included a few sources with valuable information published in 1991, 1999, 1988, 1961 and 1962. Blackjack (B. Pilosa) has been reported to contain bioactive molecules responsible for all properties exhibited [6, 16, 17, 18, 19, 20]. The reports of bioactive such as polyenes, tannins and flavonoids are available [21, 22, 23]. B. Pilosa has been utilized in different applications, including food, supplements, botanical pesticides, and other applications such as corrosion inhibitors in an acidic medium on various surfaces. Table 1 presents studies evaluated to provide data on the potential of blackjack in various applications.
2.2. Botanical properties of the genus of Blackjack
Bidens is a flowering plant genus with over 230 species belonging to the Asteraceae family. The plant's fruits are beggarticks, blackjack, burr marigolds, cobblers pegs, Spanish needles, stickseeds, and tickseed sunflowers [52]. This genus is distinguished by the number of thorny bristles that stick onto animal coats or human garments. The number of thorns ranges from two to four; some species possess lengthy ray flowers and serrated segments, while others have undivided lance-shaped leaves with short ray flowers or none at all, while the majority have yellow disk flowers. These features differentiate one member from others in this family. Taxonomical classifications of genus Biden and Biden Pilosa L., a widely distributed species, are presented in Table 2.
Table 2.
Taxonomical classification of genus Biden and Biden Pilosa L. a widely distributed species.
| Scientific classifications | |
|---|---|
| Kingdom | Plantae |
| Clade | Tracheophytes |
| Clade | Angiosperms |
| Clade | Eudicots |
| Clade | Asterids |
| Order | Asterales |
| Family | Asteraceae |
| Subfamily | Helianthodae |
| Tribe | Coreopsideae |
| Genus | Bidens |
|
| |
|
Kingdom |
Plantae |
| Division | Magnoliophyta |
| Class | Magnoliosida |
| Order | Asterales |
| Family | Asteraceae |
| Genus | Biden |
| Species | Biden Pilosa L. |
2.3. Morphological presentation of Biden Pilosa
Morphologically Biden Pilosa possesses lobed, serrate or separated opposite green leaves, yellow or white flowers and barbed achenes [53]. Figure 1 represents the morphology of Biden Pilosa.
Figure 1.
Morphological representation of Biden Pilosa.
2.3.1. Climatic conditions favourable for the growth of blackjack plants
Blackjack plants flourish well in hot weather conditions with temperatures ranging from 25 to 38 °C [54]. These plants fail to tolerate frost but flourish in sunny or slightly shaded areas [55]. The optimum growth rainfall for a blackjack plant is abundant, ranging from 500 mm to 800 mm, that means cannot withstand drought conditions [56]. Blackjack plants can flourish soils with light, moderate and heavy rains and flourish in loose soil containing high organic matter. In addition, blackjack can tolerate saline soil as it can survive in the soil with pH ranging from 4 to 9 [39]. Generally, blackjack plants grow in rich soil in the wild, planting fields, and home gardens. Additionally, they can be found on river banks throughout the year [42].
2.3.2. Bioactive compounds in blackjack plants
There are several main chemical compounds (about 300 constituents) belonging to phytosterols, fatty acids, pheophytins, terpenes, phenolic acids, okanin glycosides, chalcones, aurones, flavone glycosides, flavonoids, polyacetylene glycosides, and polyacetylenes identified and isolated from different parts of blackjack plant [57, 58]. Table 3 presents some bioactive molecules from blackjack and their properties.
Table 3.
Representatives of bioactive molecules from blackjack and their properties.
| Compound | Properties | References |
|---|---|---|
| 2-b-D-Glucopyranosyloxy-1-hydroxyltrideca-3,5,7,9,11- pentryne | Anti-inflammatory and antibacterial | [18, 59] |
| Astragalin | Anticandidal | [60, 61] |
| Axillaroside | Antibacterial and antifungal | [16] |
| Iso-Vanillin | Antimicrobial | [62] |
| Pyrocatechin | Plasticizer | [62] |
| Linalool, b-Linalool | Antitumour | [21, 63] |
| Daucene | Antibacterial, antioxidant and antifungal | [64] |
| Sandaracopimara-8(14),15-diene | Antibacterial, antioxidant and antifungal | [64, 65] |
| Squalene | Anti-inflammatory | [66] |
| B-caryophyllene and s-cadinene | Antioxidant | [21] |
| Pyrocatechin, salicylic acid, p-vinylguaiacol, dimethoxyphenol, eugenol, 4-ethyl-1,2-benzenediol, iso-vanillin, 2-hydroxy-6-methylbenzaldehyde, vanillin, vanillic acid, p-hydroxybenzoic acid, protocatechuic acid, p-coumaric acid, ferulic acid, and caffeic acid | Antifungal and plant growth inhibitory | [62] |
| Chalcone (ONC) and okanin flavanone (ONF) | Antioxidant | [67] |
| 4-O-acetyl-3-O-caffeoyl-2-C-methyl-D-erythronate; (12R)-tridecane-2E,8E-diene-4,6-diyne-1,14-diol-12-O-β-D-glucopyranoside; (10S)-tridecane-2E,12-diene-4,6,8-triyne-1-ol-10-O-β-D-glucopyranoside | Angiogenic activity | [68] |
| 7-phenyl-hepta-4,6-diyne-2-ol | Anti-inflammatory and antioxidant | [16, 66] |
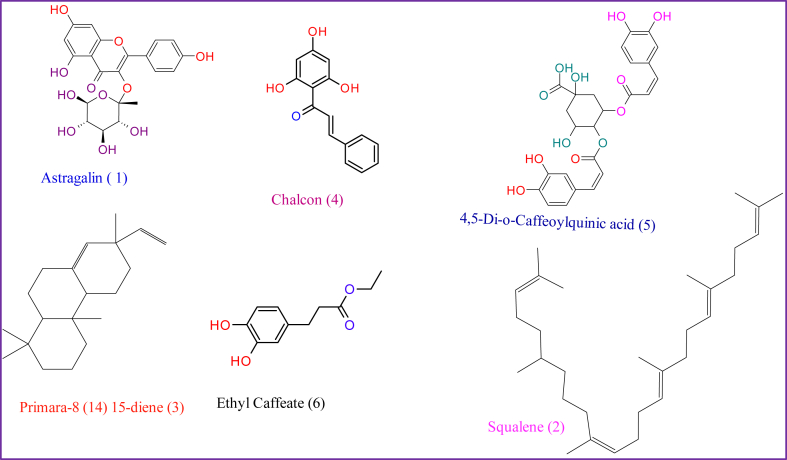
The use of extract from natural products such as blackjack in treating various diseases has been proven the best alternative due to the spread of drug-resistant pathogens, which is the major public health threat globally [69]. Microorganisms such as bacteria, for instance, have demonstrated an outstanding capability to tolerate and adapt to their surroundings, such as the development of various mechanisms of resistance to a wide range of antimicrobial drugs. In this case, the investigation of potential novel and effective antimicrobial drugs is required, such as natural products. Figure 2: Represents structures of selected bioactive compounds from Biden Pilosa; (1)-Astragalin, (2)- Squalene; (3)- Pimara-8(14)15 -diene; (4)- Chalcon; (5)-4,5-Di-o- Caffeoylquinic acid; (6)- Ethyl caffeate.
Figure 2.
Represents structures of selected bioactive compounds from Biden Pilosa; (1)-Astragalin, (2)- Squalene; (3)- Pimara-8(14)15 -diene; (4)- Chalcon; (5)-4,5-Di-o- Caffeoylquinic acid; (6)- Ethyl caffeate.
2.4. Structural properties of selected bioactive from Biden pilosa
Bioactives from natural products such as Biden pilosa demonstrated a vital role in eradicating medical conditions. The presence of particular groups in the structure of bioactive contributes to their ability to treat diseases. The inclusion of a b-unsaturated ester and catechol moiety in the structure of ethyl caffeate, for example, contributes to its anti-inflammatory effect. These structural properties are required for preventing the development of the NFkB DNA complex [70].
2.4.1. Astragalin mechanism of anticandidal
Astragalin, a flavonoid found in various plants, including Biden pilosa, have shown anticandidal activity, indicating its use as an anticandidal agent. Astragalin has promising antibacterial properties and can inhibit fungous biofilm development, which is a key factor for its pathogenicity without affecting the yeast-hyphae transition [71]. It is important to investigate the anticandidal mechanisms of astragalin in vitro and in vivo before its use in developing alternative therapeutic candidates [71]. Astragalin is not toxic to human fibroblasts cells, indicating the safety of use, likely application singly or as a mixture with current commercial antifungals in treating C. albicans infections. Therefore, possibility to provide a substantial antimicrobial effect at a lower dosage.
2.4.2. Effect of astragalin on cell membrane integrity
Results of the investigation of 30 min astragalin treatment revealed strong absorbance indicators for nucleic acids (A260) and proteins (A280), indicating the potential influence of astragalin [71]. A strong effect of astragalin in the destruction of the fungus membrane veracity is highlighted, thus contributing to its antifungal activity [71]. In the parasite Trypanosoma cruzi, astragalin has demonstrated the capability to alter the morphology of the cell membrane [72, 73]. Results of a study by Ivanov et al. (2020) [71] disagree with a previous theoretical study that predicted astragalin to work as a membrane integrity agonist [74], suggesting that theoretical docking studies should be supplemented with in vitro testing before predicting bioactivities of diverse substances.
2.4.3. Astragalin impacts yeast to hyphal transition
Ivanov et al. (2020) revealed that astragalin had no antioxidant activity in C. albicans. In an analogous study, just 6% of the flavonoids apigenin and apigetrin inhibited ROS activity, compared to 40% suppression of ROS activity by apigenin and apigetrin flavonoids [75]. The growth of fungous cells with or without astragalin performed was inspected, results revealed a marginal reduction in the proportion of hyphal cells following 4-hour treatment with astragalin at MIC [71]. A similar study by Candiracci and Colleagues demonstrated the capability of honey flavonoid extract comprising kaempferol to cause a reduction in the proportion of cells switching from yeast to hyphal [76].
2.4.4. Astragalin's ability to interfere with sterol biosynthesis
The MIC of astragalin against C. albicans was investigated before and after introducing exogenous ergosterol to the growth media to see if it interacts with fungal ergosterol [72]. The MIC increased thrice after ergosterol introduction, whereas the astragalin MIC was unaffected by ergosterol addition [72].
2.5. NF-kB/DNA complex inhibition by ethyl caffeate
Chiang and Colleagues studied the relationship between the structure of ethyl caffeate extracted from Bidens pilosa and its activity [70]. The study suggests that inhibition of NFkB DNA complex formation is contributed by a b-unsaturation in ester and catechol in ethyl caffeate. Chiang and Colleagues utilized ethyl caffeate and its analogues such as catechol in an in vitro NF-kB to DNA binding to describe the role of ethyl caffeate in the inhibition of NFkB to DNA complex formation [70]. It was revealed that the binding of NF-kB to DNA was inhibited by 50 mM of ethyl caffeate. On the other hand, Catechol was observed to block the binding at 400 mM, whereas the concentration of 400 mM of ethyl cinnamate failed to inhibit NF-kB to DNA binding; this may be due to lack of catechol moiety in the ethylcinnamate molecule [70]. Thus, the catechol part and the a b-unsaturation at ester together may play important roles in NF-kB to DNA binding and inhibition [70]. This study indicates that the structure of a bioactive is essential for the activity it plays, for the bioactive used for mitigation of various health conditions.
3. Squalene anti-inflammatory mechanism
Squalene has anti-inflammatory and antioxidant properties [77]. It was also shown that squalene works by inhibiting the over provocation of neutrophils, monocytes, and macrophages by targeting pro-inflammatory mediators and PPAR signalling pathways [77]. To exert its antioxidant capacity, squalene targets LPS-induced intracellular ROS generation in murine peritoneal macrophages, resulting in the highly fluorescent chemical DCF [78]. After an 18-hour incubation period in the presence of 50 M squalene, intracellular ROS levels were significantly reduced (P 0.05) [78]. According to some research, squalene's antioxidant activity is mediated through the protein production of Nrf2, a crucial transcription factor that controls the cellular antioxidant response [79]. The results of an 18-hour incubation with 50 mM squalene show a significant increase in Nrf2 protein expression in LPS-treated cells (P 0.01), approaching that of untreated cells [78].
3.1. Nitrite production and iNOS and COX-2 protein expression suppression
LPS can cause NO production and release in murine macrophages, according to Gordon and Taylor (2005) [80,81]. Cárdeno et al. (2015) discovered a significant increase in nitrites in the medium of LPS-treated murine peritoneal macrophages as an indicator of NO generation; however, squalene was shown to reverse this effect (P 0.001) [78]. As a result of the 18-hour incubation with 25 and 50 M squalene, the levels of iNOS protein expression in LPS-treated cells were reduced (P 0.05 and P 0.01) [78]. In murine peritoneal macrophages, LPS increased COX-2 protein expression; however, 18-hour incubation with 25 and 50 M squalene resulted in significant reductions in COX-2 protein expression in LPS-treated cells, with a pattern strikingly similar to that reported for iNOS protein expression.
3.2. Modulation of MAPK and NF-κB signalling
MAPKs are responsible for many inflammatory mediators, such as iNOS and COX-2 [82]. As a result, the potential of squalene to target the activity of MAPK pathways was studied to determine the molecular mechanism underlying its ability to reduce pro-inflammatory signals [78]. LPS stimulation resulted in increased levels of phosphorylated JNK and p38 MAPK expression in murine peritoneal macrophages, according to the findings [78]. After 18 h of treatment with 25 and 50 M of squalene, there was a significant drop in phosphorylated JNK (P 0.05) but not p38 MAPK expression in LPS-treated cells, reaching a level comparable to that of untreated cells [78]. Other transcriptional systems, such as NF–B, a critical effector pathway involved in inflammation response, may be controlled by LPS, according to Andreakos et al. (2004) [83], and NF–B has been identified as a crucial regulator of cellular responses to oxidative stress in mammalian cells [84]. LPS stimulation decreased the inhibitory protein IB in the cytoplasm while increasing the p65 NF–B protein in the nucleus of murine peritoneal macrophages. However, after 18 h of incubation with 25 and 50 M squalene, IB increased significantly (P 0.05), while p65 decreased significantly in LPS-treated cells (P 0.05), reaching comparable levels to untreated cells [84].
3.3. Alteration of MPO and HO-1 gene expression
In previous investigations, squalene has been shown to reduce MPO and increase HO-1 gene expression in human neutrophils and monocytes treated with LPS. MPO is a human protein found mostly in neutrophil azurophilic granules and monocyte lysosomes [85, 86, 87]. MPO is generated when LPS is activated, and it catalyzes the development of a potent oxidant like hypochlorous acid, a 14-reactive chlorine species that accumulate in chronic inflammation sites [87]. The results show that after 18 h of treatment with 50 M squalene, the mRNA level of MPO in LPS-treated human monocytes and neutrophils decreased (P 0.001). The anti-inflammatory gene HO-1, on the other hand, was found to increase in both human monocytes (P 0.05) and neutrophils (P 0.001) treated with LPS.
3.4. Alteration of TLR4 and pro-inflammatory cytokines
TLR4 is activated by LPS, leading to the generation of important pro-inflammatory and immunoregulatory cytokines that can activate the innate immune response [88]. According to the findings of Cárdeno and Coallegues (2015), 18 h of treatment with 50 M squalene reduces TLR4 gene expression in LPS-treated human leukocytes, but more so in monocytes (P 0.001) [87]. It was also shown that incubation with 50 M squalene for 18 h inhibited the expression of pro-inflammatory cytokine genes TNF- and IL-1, but not IL-6 or IL-10.
4. iNOS and COX-2 enzymes overexpression
The effects of squalene on gene expression of enzymes involved in the LPS-induced inflammatory response in human monocytes and neutrophils revealed that 18 h of incubation with 50 M of squalene reduced iNOS and COX-2 gene expression in LPS-treated human monocytes (P 0.001), but not COX-2 gene expression in LPS-treated human neutrophils [71].
4.1. Alteration of MMPs and PPARγ gene expression
In LPS-treated human monocytes and neutrophils, Khokha and colleagues (2013) discovered that squalene could regulate MMPs and PPAR gene expression [89]. MMPs control ligand-receptor connections, effector function, immune cell growth, and migration, all of which are important in maintaining an inflammatory response [89]. The effects of squalene on three major MMPs were examined. There have been reports of PPAR inhibiting LPS-mediated MMPs and pro-inflammatory cytokines [90, 91]. MMP-1 (P 0.001) and MMP-9 (P 0.01) gene appearance in LPS-treated human monocytes, as well as MMP-1 and MMP-3 gene expression in LPS-treated human neutrophils (P 0.001), were shown to be significantly reduced after 18 h of incubation with 50 M of squalene. In contrast, these findings are supported by a substantial increase in PPAR gene expression in LPS-treated human monocytes and neutrophils (P 0.001) [71]. As a result, squalene's potential in treating inflammatory disorders is defined by the overactivation of neutrophils, monocytes, and macrophages, allowing for the effective termination of the inflammatory response.
4.2. Anti-HIV mechanism of natural products
As a result of the failure of existing anti-AIDS techniques due to multidrug resistance, most treatment plans look to natural items with anti-HIV potential [92]. This necessitates the development of novel and effective anti-AIDS medications derived from natural sources [92]. WHO proposes that ethnomedicines and several other natural elements be thoroughly tested to achieve the goal of preventing HIV [93, 94]. Natural materials used as medication are classified according to their mode of action or the activity of secondary metabolites [92]. In the fight against HIV, scientists look for chemicals that can stop HIV from replicating. Almost all anti-HIV medications, for instance, target viral proteins such as viral protease, integrase, and reverse transcriptase [95]. Natural goods can be immunoregulators, antioxidants, or anti-inflammatory, and their use could help in HIV prevention.
5. Utility of anti oxidant potential of natural products
Many reactive oxygen species (ROS) are produced in patients with AIDS due to changes in antioxidant enzyme levels [96], which leads to DNA damage and lipid peroxidation [97, 98]. ROS may also boost the nuclear factor kappa B (NF–B factor), which aids in HIV transcription and promotes its proliferation [99]. Antioxidants are substances that inhibit the production of reactive oxygen species (ROS) and protect cellular DNA. N-Acetylcysteine is an amino acid that functions as an antioxidant and treats HIV infection [69]. Antioxidants such as selenium, lipoic acid, vitamin C, -carotene, and vitamin E have been used to help people with HIV and AIDS [100, 101]. Cyanidin-3-glucoside and peonidin, two antioxidants, are also used to prevent AIDS [102, 103].
5.1. Immunomodulators from plants
Immunomodulators are substances that stimulate the cellular and humoral immune systems in response to any pathogenic infection [103, 104, 105]. They signal the antigen to T cells, initiating an immunological response. T cells also stimulate B cells to produce antibodies that bind to the antigen, and T cells activate killer T cells that attack infections [106]. Glycosides, alkaloids, tannins, terpenoids, coumarins, flavonoids, polysaccharides, and lignans are only a few naturally occurring chemicals showing immunomodulatory activities in HIV [107, 108]. In persons living with HIV [103], ellagic acid, a phenol from Punica granatum [109], curcumin from Curcuma longa [81], Plantago major's aucubin glycoside [110], vanillic acid, and ferulic acid [103] all showed immunostimulant properties. Plantago major chlorogenic acid [109]. Centaurein flavonoid from Bidens pilosa showed excellent immunomodulatory potential in AIDS [103].
5.2. Application of blackjack plants
There are about 240 Biden species known globally [111]. Reports of the traditional use of Biden pilosa for foods and medicines without obvious adverse effects are available [112]. The possibility of further exploring this plant's usefulness to humans and animals as food, medicine, and other applications can be archived. Every part of a plant is useful to humans, for example, the sap, flower; the leaves and roots of a perennial yellow-flowered plant are a warehouse of vitamins, with leaves being used as a source of vitamins A, E, and C before the plant's flower [5], roots being dry, baked, percolated, and used as a coffee substitute [30], sap being a source of latex [31, 32], and flowers having a high carotene content [113]. Nevertheless, blackjack plants and other indigenous vegetables are underutilized in many parts of sub-Saharan Africa, but the Network Vegetable Production in Africa (NEVEPA) aids in the protection and usage of indigenous vegetables through its activities [114]. The German Government funded the project and implemented it by the German Agency for Technical Cooperation (GATZ) [20]. This project aims to assist African countries in establishing national vegetable production networks and to enable communication between networks in different countries. Initial efforts were made in Tanzania, with later networks forming in Ethiopia and Uganda. As the knowledge and know-how on the indigenous vegetables are underutilized, these networks will improve information exchange in the vegetable production sector [12]; this could inform the general population about the possible benefits and thus their use.
5.3. Use of blackjack plants as vegetables
The consumption of vegetables is healthy and protective to the body due to the different nutrients contained. Vegetables are "protective foods" since they provide vital micronutrients and vitamins to the daily diet [44]. They are rich in phytochemicals, linked to a reduced risk of cardiovascular, digestive, colon cancer, anaemia, weariness, blindness and other immune-related disorders [44]. The World Health Report of 2002 revealed that poor fruit and vegetable diet are responsible for about 31% of ischemic heart disease and 11% of stroke globally [115]. Generally, it is projected that if fruits and vegetable consumption increases substantially, up to 2.7 million lives may be saved each year [115, 116]. Indigenous vegetables are region-specific, have a low acceptance rate, and are part of the subculture [44]. Therefore, blackjack plants are among the indigenous vegetables consumed mostly by rural communities in different parts of the world. It occurs naturally as a weed or cultivated at the home garden [117], and due to its seasonality, nature can also be commercialized. As one of the African indigenous vegetables, it contains a high amount of nutrients, it is easily accessible to the environment and cheap for underprivileged communities. It contains minerals and vitamins [43, 44, 118, 119]. Therefore, consumption of blackjack as a vegetable contributes to global initiatives of WHO, which calls for the increase of fruits and vegetable consumption in African nations [42]. In this case, blackjack has been used as an edible vegetable in different parts of the world where it is consumed regularly, like in South Africa or as famine food in some countries [120]. It acts as a protective substance as it contains many essential nutrients, including beta-carotene, vitamin A, vitamin C and medium in vitamin E and protein [112, 119]. In addition, the presence of various minerals in Biden pilosa has been reported [43]. Table 4 represents minerals and contents presented in BP contained in mg per 100g of dried weight.
Table 4.
Reported Minerals and its Amount in Blackjack.
| Mineral | Calcium | Phosphorus | Sodium | Manganese | Copper | Zinc | Magnesium | Iron |
|---|---|---|---|---|---|---|---|---|
| Amount (mg/100g) | 1354 | 504 | 290 | 21 | 10 | 22 | 658 | 17 |
Moreover, blackjack can also be used as a spice, feed, herbal tea, and medication. The leaves can be used to prepare blackjack tea and juice, as reported by other researchers [121]. The use of natural products with medicinal properties has traditionally been used in several countries and particularly in Africa for nutritional anaemia, blood flow improvement, malaria prevention, toothache relief, improved eye health, and wound healing, including wounds encountered by HIV patients [122]. The current need globally to move from persistent synthetic drugs to biodegradable natural herbs for sustainability is preferable, as supported by Arthur et al. (2012) and Benli et al. (2008) [123,124].
5.4. Medicinal application
Researchers reported antidiabetic, antiobesity and antihypertensive properties of Biden Pilosa worldwide, indicating the possibility of its application in the mitigation of diabetes, hypertension, and obesity. Therefore, adopting blackjack in diet may help alleviate metabolic syndrome, leading to a healthier society. However, little information is available on the medicinal application of bioactive from blackjack in most countries of sub-Saharan Africa. Such information is vital as it will enhance the reduction of synthetic drugs, which may sometimes become resistant to diseases and pollute the environment. Blackjack has been reported to be used to mitigate different medical conditions in humans and other organisms. For example, reports of detection and quantification of a compound with anticancerous potential, paclitaxel, from blackjack leaf extract are available [125]. Blackjack extracts also have shown pharmacological activities such as antibacterial, antifungal, and antiulcer [126]; this calls for further research. A study by Sigh and Coallegues (2017) examined the antimicrobial activity of Biden Pilosa against E. coli; results indicated the highest antimicrobial activity MIC of 80 μg/mL and IC50 110.04 μg/mL, indicating the possibility of its application as or in combination with the antimicrobial agent [125].
Similarly, Chang et al. (2015) investigated the efficiency of blackjack against eimeriosis on a green chicken farm. Results indicated that feed supplemented with blackjack, at the dose of 0.025% of feed or more, resulted in a significant reduction in eimeria infection [127]. Apart from reduced infection, the treatment increased body weight at a reduced feed conversion ratio, leading to higher growth performance. Also, it lowered morbidity/mortality rate, decreased oocysts per gram of faeces and gut pathology and augmented by the anticoccidial index [127]. Collectively, these data demonstrated the potential of Biden Pilosa to control chicken eimeriosis on chicken farms. Biden Pilosa can, therefore, be used as an effective means to control eimeriosis in chicken and used as supplements to ensure health.
5.5. The antimetabolic function of blackjack
Humans have created various health problems due to unhealthy eating habits, physical inactivity and inadequate intake of nutrients such as minerals and vitamins [128]. The problems may lead to long term illness, mortality and several metabolic syndromes worldwide [129, 130]. The metabolic syndrome results in several health risks for hypertension, diabetes and cardiovascular diseases. These disorders can be controlled using medications, but also indigenous substances can be used. Therefore, bioactive such as Polyynes in blackjack have been prophylactically and therapeutically active against metabolic-related syndrome like diabetes and adipogenesis [27, 131].
Regarding diabetes, Ubillas and Coallegues (2000) reported the anti-diabetic efficacy of blackjack on type two diabetes [23, 28, 132]. In their study, it was observed that the hydroethanolic extract of blackjack at a dose of 1 g/kg Body Weight (BW) reduced Fasting Blood Glucose (FBG) in db/db mice [28]. In this study, two polyynes were isolated and then mixed in 3:2 ratio and effectively decreased porphobilinogen (PBG) level and food consumption in mice, indicating that there is no acute porphyria attack and the polyynes were responsible for the anti-diabetic activity of blackjack as similarly reported by other researchers [133].
On the other hand, blackjack has been reported by several scholars and researchers to function properly in dealing with obesogenic properties. Generally, obesity is recognized as an imminent pandemic with negative health consequences worldwide [134, 135]. In 2014, it was predicted that 1.9 billion people globally were overweight, with over 600 million being obese [136]. Obesity is a health problem affecting people in every country on the planet [137]. The main cause of obesity is an unhealth lifestyle coupled with food transition where traditional and indigenous diet has mostly been replaced by unhealth diets such as fast foods and snacks, processed food with high sugar, salt and fat [138]. It is currently classified as a risk factor for several non-communicable diseases such as hypertension, diabetes, numerous types of cancer, and coronary heart diseases [139, 140]. Therefore, obesity and its related comorbidities need intervention, such as including vegetables in the diet with antimetabolic properties such as blackjack.
Consequently, blackjack, regardless of its status as a weed or wild plant, has been studied and found to have ant-obesity properties [141]. A study was conducted to investigate blackjack's effectiveness as an antiobesity agent using Institute of Cancer Research (ICR) mice, where ICR mice were fed with blackjack extract at different doses for 24 weeks. The result indicated that blackjack extract significantly reduced crude fat content in ICR mice at different rates [141]. It was further revealed that blackjack extract has anti-obesity properties due to reducing fat content [141]. Furthermore, the study examined the outcome of blackjack on body weight and body composition using ob/ob mice [25]. Using the mouse model of obesity, the blackjack extract was fed to mice for five consecutive weeks at different doses [30]. The result showed the positive effect of blackjack extract on body weight and body composition. Blackjack dependently decreases the body weight of visceral and subcutaneous fat depending on the dose administered [25, 142]. Therefore, the two experiments conducted on blackjack indicate that the plant has an antiobesity function. It is, therefore, imperative for researchers to conduct more research for more information.
In addition, blackjack has also been reported to have the ability to treat hypertension [143, 144]. Generally, hypertension is the world-leading cause of illness which has drawn high attention to the public health sector. It is the major cause of mortality and morbidity globally [145]. In sub-Saharan Africa, hypertension is estimated to be as high as 38%, and it is believed that between 10 to 20 million persons among 650 million in sub-Saharan Africa suffer from hypertension [146, 147]. Diet is an important adjustable risk factor for hypertension development. Therefore, in comparative situation an individual whose diet contain enough fruit and vegetable has lower blood pressure level and minimum risk of hypertension than those who consume fast foods, snacks, processed food with high sugar, salt and fat regularly [148].
In this case, sub-Saharan Africa has a good potential to include fruits and vegetables in the diet at a low cost by using indigenous plants available in their environment. Blackjack is, therefore, among indigenous leaf vegetables freely available in the environment, and it has been reported to have bioactive molecules to mitigate hypertension [149]. The potential of blackjack for the mitigation of hypertension was reported by several scholars [32, 150, 151]. There are existing reports of the test of blackjack extracts for its antihypertensive and many hypertension models, including spontaneously hypertensive rats (SHR), salt-loading and fructose-induced hypertensive rats [32, 150, 151], and even human cells, have been shown to have hypotensive effects [152]. These studies show the positive response towards Biden pilosa in the management of hypertensive. The clinical trial of Biden pilosa in treating different diseases has been done on both humans and other animals to test the efficacy in the treatment and control of infectious and non-communicable diseases. Table 5 presents clinical trials conducted on Biden pilosa extracts.
Table 5.
Presents clinical trials conducted using Biden pilosa extracts.
| Activity | Method | Result | References |
|---|---|---|---|
| Anticancer | Extract from the whole plant using n-hexane, chloroform and methanol (E1-E3). The extracts were fractioned by column chromatography with ethyl acetate, acetone and water (F1 – F3) | Results show that E1 have notable anticancer activity, and E3 bears maximum antipyretic activity. | [153, 154] |
| Anti-coccidial | Some of the chickens were affected by E. tenella. Chickens were fed standard daily feed for 21days. The feed contains commercial anti-coccidial agent salinomycin or 0.05%, 0.01% and 0.002% B. pilosa powder. | Compared to the control group, the results show that feeding resulted in a 60% survival rate (100 per cent). Chickens infected with E. tenella and fed salinomycin-containing diet had a 90% survival rate. Infected chickens fed feed with B. pilosa at doses of 0.05 per cent, 0.01 per cent, and 0.002 per cent had survival rates of 100 per cent, 100 per cent, and 60 per cent, respectively. At 100 ppm or higher, B. pilosa causes a decrease in oocyst excretion; gut pathology; prophylactic duration was 3 days. | [155] |
| Anti-diabetic | Treatment was divided into groups: ranging from 200-800 mg/kg. At the end of week IV, biochemical tests for blood glucose monitoring was conducted; Alanine aminotransferase (ALT) and Aspartate aminotransferase (AST). | Results show Bidens pilosa maintained hypoglycemia for two weeks, and later status was lost. T1DM rats treated with 200 mg/kg indicates better recovery, followed by 400 mg/kg, indicating lower doses are more efficient. | [156] |
| Antimicrobial | Agar dilution method, with the root of the South African ecotype, methanol: acetone: water extracts | The extracts suppressed all the bacteria and some fungi species, except for water extract | [157] |
| Antihyportensive | Extracts evaluated aqueous with 150–350 mg/kg; methylene chloride with 150–300 mg/kg; pilosa by use fructose-induced hypertension in rats. | Results show that Bidens pilosa has hypotensive potential whose mechanism of action is not related to insulin sensitivity | [158] |
5.6. Safety of blackjack consumption
The consumption of blackjack as food in some parts of Africa was suggested in 1975 by the Food and Agriculture Organization of the United Nations [142, 159]. In this case, blackjack is recognized as safe for consumption by humans and other animals. In addition, blackjack is also recognized as a safe medicinal plant for treating different types of sickness in human beings and other animals. Although there is no evidence of toxicological study on humans and animals for blackjack conducted in sub-Saharan African countries, some evidence from several seminal studies proves that Biden Pilosa is safe for consumption for food and medicinal purposes. Table 6 represents some studies conducted to indicate the safety of blackjack.
Table 6.
Reported studies showing the safety of blackjack consumption.
| Study | Findings | Remarks | Reference |
|---|---|---|---|
| A 90-day trial, administration B. pilosa-based health food at a daily dose of 400 mg/individual mice, three times a day | Results indicated no adverse effects with the increased insulin level and decreased level of total cholesterol (TC). | No potential harm; All parameters were in the physiological range, hence | [160, 161] |
| In mice, administration of B. pilosa whole plant at 1 g/kg bodyweight for 24 weeks, a dosage of close to 10% of food was done. | No toxicity was observed, as evidenced by retaining their normal ways of life. | Normal patterns were observed, indicating no toxic effects | [162] |
| A trial where an aqueous extract of B. pilosa leaves (10 g/kg BW) given to rats | No fatality or changes in rats observed | Indicating no toxicity | [163] |
| A regular dose of the aqueous extract of B. pilosa leaves at 0.8 g/kg BW was given to rats | No noticeable 28-day toxicity in rats was exhibited, as evidenced by survival, BW, and gross examination of vital organs | Indicating no toxicity | [24] |
| A 24-week oral treatment with BP at doses of 0–2.5%; 5% and 10% was investigated in mice. | No significant difference was observed. | Indicating no toxicity | [46] |
5.7. Application of blackjack in agriculture
Among the agricultural benefits of blackjack comes from applying its bioactive such as alkaloids, as a herbicide in organic agriculture [164]. The motivation to research alkaloids in botanical pesticides has intensified due to environmental friendliness, low toxicity, and biodegradability compared to typical chemical pesticides while concurrently exhibiting higher effectiveness after certain structural modifications [164, 165]. This is significant to the sustainability of ecosystems as the products will help to decrease the chemical load in our environments. A study conducted by Deba and colleagues examined the option of utilizing Bidens Pilosa extracts for plant fungus and weed control. The results indicated that the extract of Biden Pilosa shows strong phytotoxic action against the growth of Crus-galli, Raphanus Sativus and Echinochloa and antifungal activity against phytopathogens in bioassays. Therefore, this result shows that extract of this plant can be used for treatments of plant diseases and replace chemical agents that pollute the environment. Also, the plant growth inhibitory activity exhibited by extracts can be utilized positively to control other weeds.
5.8. Corrosion inhibitor
Apart from nutritional and medicinal properties, blackjack has shown promising corrosion inhibitors in an acidic medium. Olusegun and Coallegues (2018) assessed the corrosion inhibition activity of Biden Pilosa in an acidic medium [166]. The results indicated that the extract showed inhibition efficiency of 97% at 303 K through decreased with increasing temperature indicating the inhibition mechanism was physisorption. There is a need for further research on the possibilities of utilising and improving Biden Pilosa extract in various mediums as corrosion inhibitors. In an acidic medium, Biden Pilosa extract could inhibit corrosion on a composite matrix made from silicon carbide reinforced with the aluminium matrix, as presented by Alanemea et al. (2016) [48]. Concerning this study, bioactive compounds present in extracts such as flavonoids and tannins are indicators of its corrosion inhibition. The results indicated that Biden Pilosa extract was able to reduce corrosion rate. However, the influence of increased concentration of the Biden Pilosa extract on progressive reduction in corrosion was not established. This necessitates the need for further studies. Similarly, the Biden Pilosa extract corrosion inhibition on the aluminium environment occurred through physical adsorption [48]. Other researchers also reported corrosion inhibition of Biden Pilosa extract on mild steel in an acidic medium [47]. This information justifies that Biden Pilosa is important in medicine, nutrition and other applications such as corrosion inhibition.
5.9. Traditional uses and ethnopharmacology of Biden pilosa
Since prehistoric times, humans have been employing extracts from natural products to treat medical conditions. In this case, traditional Chinese medicine, Ayurveda; Kampo; traditional Korean medicine, and Unani have been practised in different parts of the world and have evolved into well-organized medical systems [167, 168, 169, 170]. With the advancement of science and technology and the necessity to obtain lead compounds for drug development, research focusing on safety, pharmacological activity, and extract composition becomes more important. These studies discovered that the extract from natural products possesses pharmacological activities that traditional healers utilize through their indigenous knowledge [171] and trials to solve societal problems [172].
The use of traditional medicine may be motivated by the absence of clinical settings [173] and other factors such as community perception. Currently, drug resistance is among the motivation for using alternative or traditional medicine. On the other hand, the ability of traditional healers to treat diseases using natural products, initial studies revealed the presence of drug lead in natural products and natural products possession of bioactivities attracted medical practitioners and led to the development of modern technology medicine from natural products (alternative medicine) [167]. Therefore, these studies confirmed that the traditional usage of natural product extract utilized the variety of biological potential provided by these extracts. Natural product extracts are used in various regions, such as cold or hot extracts, and some use the entire plant, while others use sections of the plant such as roots, leaves, flowers, barks, and shoots. Table 7 summarizes various ways of traditional usages of Blackjack in named places. The report of medicinal plants and finished marketed herbal products used in the treatment of deseases such as malaria, tuberculosis and other deseases are available globally [174, 175, 176, 177, 178, 179].
Table 7.
Summarizes differences between the traditional usages of blackjack in named places.
| Traditional usages | Place or region | References |
|---|---|---|
| Dizziness mitigation | West Africa | [180] |
| Mitigation of migraines | South Africa | |
| Mitigation of sexually transmitted diseases | [181] | |
| Treatment of TB | [182] | |
| Mitigation of headache and rheumatism | Tropical Areas | [183] |
| Use of flowers or whole plant for treatment of TB | Uganda; Ghana; South Africa | [172, 174, 184] |
| Use to induce labor | Tanzania | [185, 186] |
| Use to induce abortion | Tanzania | |
| Vegetables | Kenya, Congo, Botswana, Zambia, Zimbabwe, South Africa and Mozambique | [9, 10, 11, 187] |
| Management of AIDS in clients | Uganda, Tanzania, Malawi | [122, 175, 188] |
| Use of roots for the treatment of malaria, TB and related symptoms | Uganda | [174] |
| Use of grounded leaves as insecticides and for flu urinary tract infections mitigation. Infected wounds of the skin and upper respiratory tract infections. |
Kenya (Giriama) | [189] |
| Crushed leaves, Leaf sap, Powder from seeds, Leaf extract Jaundice/dysentery, burns, anaesthetic and Swollen spleens |
Cote d’ Ivoire Tanzania Nigeria |
|
| Use of concoction of the whole plan, as poison antidote; ease child delivery; relieve pain from a hernia; | Congo | |
| Use of suspension of powdered leaves, the concoction of leaf for abdominal pain, Arthritis and malaria | South Africa (Zulu) | |
| The use of leaf tea to mitigate stomach, mouth ulcers, diarrhoea, headaches, and hangovers. | Zimbabwe (Manyika) | |
| Use of crushed leaves; decoction of leaf powder; herbal tea; blood clotting agent; ear infection; kidney problems; headache and flatulence; | Uganda | |
| Management of childhood diseases | South Africa | [190] |
5.10. Challenges of application Biden Pilosa
The potential of Biden Pilosa for food and as a source of supplement is supported by its nutritional values [7, 41, 44, 191]. Also, it has been utilized for the mitigation of different metabolic syndromes such as diabetes and obesity and other medical conditions such as hypertension [160]. Also, Biden Pilosa is applicable in the agricultural sector, where it is used to control other weeds and improve soil properties [6, 17]. Apart from its usefulness, its application is hindered by its categorization as inversive species (weeds) [192]. The information on the potential of Biden Pilosa is inadequate in such a way that researchers find a way to suppress its growth. A study by Wang and Coallegues (2013) reported the ability of leaf powder of Litchi chinensis (Litchi), which is used as a naturally occurring herbicide, to inhibit the growth of weeds, including Bidens pilosa [51]. Kaur (2002) [37] studied Parthenium hysterophorus extract's ability to inhibit the growth of Biden Pilosa. The results indicated the potential of usage of the extract for the management of other weeds in future.
6. Conclusions and future perspectives
Blackjack is among indigenous leaves used as vegetables in Africa with potential applications regardless of its classification as weed or wild plants. It offers significant health benefits to both human beings and other animals. Blackjack is the main nutrient and medicinal source, important for health and immune enhancement. Several researchers have reported the plant to solve non-communicable diseases such as obesity, hypertension and diabetes. It can also improve soil conditions, leading to more production of crops and a healthier society.
Moreover, it can be utilized as a potential corrosion inhibitor in an acidic medium. However, due to inadequate information, this plant has been underutilized and destroyed as a weed to protect other plants that society thinks are more important than Blackjack. This calls for consensus among the researchers to have one voice regarding its utility. There will be an improvement in nutrition security and reduction of synthetic drugs that are persistent in the environment and prone to resistance. In addition, the increased difficulties in treating diseases caused by microorganisms and the emergence of drug-resistant strains challenge globally. In the future, bioactive from natural products such as Bidens species can be used to treat both communicable and non-communicable diseases. Specifically, researchers should focus on applying Biden pilosa extracts in the clinical setting to mitigate leading killer and chronic diseases such as tuberculosis, cancer, diabetes, and hypertension. There is a notable lack of knowledge on consumer preferences for Biden pilosa with no published studies on the topic. Research on consumer preferences, and attitudes towards Biden pilosa is needed in order to tailor policies to raise awareness on the potential benefits of the Blackjack.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors of this paper acknowledge and appreciate the contribution of anonymous reviewers of this paper. The critical reviews and feedback made by the anonymous reviewers improved the clarity and quality of this paper.
References
- 1.Anderson R.C. Evolution and origin of the Central Grassland of North America: climate, fire, and mammalian grazers1. J. Torrey Botan. Soc. 2006;133(4):626–647. [Google Scholar]
- 2.Sequin PGaU Constituents of Bidens pilosa L." Do the components found so far explain the use of this plant in traditional medicine? Acta Trop. 1991;48(1991):251–261. doi: 10.1016/0001-706x(91)90013-a. [DOI] [PubMed] [Google Scholar]
- 3.Grubben G., Denton O. Vol. 61. PROTA Foundation; Wageningen, Netherlands: 2004. p. 108. (Plant Resources of Tropical Africa 2. Vegetables). J Backhuys Publishers, Leiden, Netherlands/CTA, Wageningen, Netherlands. [Google Scholar]
- 4.Mahmoud T., Gairola S., El-Keblawy A. Parthenium hysterophorus and Bidens pilosa, two new records to the invasive weed flora of the United Arab Emirates. J. New Biol. Rep. 2015;4(1):26–32. [Google Scholar]
- 5.McCarthy B.C. Identification and control of invasive plant species 1999. Ann. Report. 1999;18:1999. [Google Scholar]
- 6.Arthur G.D., Naidoo K., Coopoosamy R.M. Bidens pilosa L.: agricultural and pharmaceutical importance. J. Med. Plants Res. 2012;6(17):3282–3287. [Google Scholar]
- 7.Mboya R.M. The nutritional and health potential of blackjack (bidens pilosa l.): a review – promoting the use of blackjack for food. Int. J. Appl. Res. Publ. Health Manag. 2019;4(1) [Google Scholar]
- 8.Duke J.A. CRC Press; 2008. Duke's Handbook of Medicinal Plants of Latin America. [Google Scholar]
- 9.Maundu P., Achigan-Dako E., Morimoto Y. Routledge; 2009. Biodiversity of African Vegetables. African Indigenous Vegetables in Urban Agriculture; pp. 97–136. [Google Scholar]
- 10.Otang-Mbeng W., Mashabela M.N. A review of beneficial phytochemicals and postharvest studies on some indigenous leafy vegetables from the Mpumalanga Province of South Africa. J. Med. Plants-Int. J. Phytomed. Relat. Industr. 2020;12(4):533–544. [Google Scholar]
- 11.Shayanowako A.I.T., Morrissey O., Tanzi A., Muchuweti M., Mendiondo G.M., Mayes S., et al. African leafy vegetables for improved human nutrition and food system resilience in southern Africa: a scoping review. J. Sustainab. 2021;13(5):2896. [Google Scholar]
- 12.Williams J.T. Bioversity International; 2002. Global Research on Underutilized Crops: an Assessment of Current Activities and Proposals for Enhanced Cooperation. [Google Scholar]
- 13.Bharucha Z., Pretty J. The roles and values of wild foods in agricultural systems. J. Philosophic. Transact. Royal Soc. B: Biol. Sci. 2010;365(1554):2913–2926. doi: 10.1098/rstb.2010.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amer W.M. The worst invasive species to Egypt. J. Invas. Alien Spec.: Observat. Issues Around World. 2021;1:112–138. [Google Scholar]
- 15.Musaweti. Bidens Pilosa (Blackjack). Factsheet - Bidens pilosa (Blackjack).
- 16.Wang R., Wu Q.-X., Shi Y.-P. Polyacetylenes and flavonoids from the aerial parts of Bidens pilosa. J. Planta Med. 2010;76(9):893–896. doi: 10.1055/s-0029-1240814. [DOI] [PubMed] [Google Scholar]
- 17.Mwine T.J., Van Damme P., Gerard K., Charles K. Ethnobotanical survey of pesticidal plants used in South Uganda: case study of Masaka district. J. Med. Plants Res. 2011;5(7):1155–1163. [Google Scholar]
- 18.Zhao X., Mei W., Gong M., Zuo W., Bai H., Dai H.J.M. Antibacterial activity of the flavonoids from Dalbergia odorifera on Ralstonia solanacearum. J. Mol. 2011;16(12):9775–9782. doi: 10.3390/molecules16129775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang Y., Shen X-f, Chen Y.J.A. Weed control and influence of the following crops with tebuthiuron in sugarcane fields. J. Agrochem. 2012;12:919–922. [Google Scholar]
- 20.Padulosi S., Bergamini N., Lawrence T. Proceedings of an International Conference, Frankfurt, 14-16 June, 2011. 2012. On-farm conservation of neglected and underutilized species: status, trends and novel approaches to cope with climate change. [Google Scholar]
- 21.Deba F., Xuan T.D., Yasuda M., Tawata S. Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidens pilosa Linn. var. Radiata. Food Control. 2008;19(4):346–352. [Google Scholar]
- 22.Bartolome A.P., Villaseñor I.M., Yang W.-C. Bidens Pilosa L.(Asteraceae): botanical properties, traditional uses, phytochemistry, and pharmacology. Evid.-based Compl. Altern. Med. 2013;2013 doi: 10.1155/2013/340215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang C.L., Lin Y., Bartolome A.P., Chen Y.-C., Chiu S.-C., Yang W.-C., et al. Herbal therapies for type 2 diabetes mellitus: chemistry, biology, and potential application of selected plants and compounds. Evid.-Based Compl. Altern. Med. 2013;2013 doi: 10.1155/2013/378657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frida L., Rakotonirina S., Rakotonirina A., Savineau J.-P. In vivo and in vitro effects of Bidens pilosa L.(Asteraceae) leaf aqueous and ethanol extracts on primed-oestrogenized rat uterine muscle. Afr. J. Tradit., Complementary Altern. Med. 2008;5(1):79–91. [PMC free article] [PubMed] [Google Scholar]
- 25.Paulo Henrique Marcelino de Ávila P.H.M., de Ávila R.I., dos Santos Filho E.X., Bastos C.C.C., Batista A.C., Mendonca E.F., et al. Mucoadhesive formulation of Bidens pilosa L.(Asteraceae) reduces intestinal injury from 5-fluorouracil-induced mucositis in mice. J. Toxicol. Rep. 2015;2:563–573. doi: 10.1016/j.toxrep.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abd El-Ghani M.M.J.A. Traditional medicinal plants of Nigeria: an overview. J Agric. Biol. J. North Am. 2016;7(5):220–247. [Google Scholar]
- 27.Hsu Y.-J., Lee T.-H., Chang C.L.-T., Huang Y.-T., W-CJJoe Yang. Anti-hyperglycemic effects and mechanism of Bidens pilosa water extract. J. Ethnopharmacol. 2009;122(2):379–383. doi: 10.1016/j.jep.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 28.Ubillas R.P., Mendez C.D., Jolad S.D., Luo J., King S.R., Carlson T.J., et al. Antihyperglycemic acetylenic glucosides from Bidens pilosa. J. Planta Med. 2000;66(1):82–83. doi: 10.1055/s-0029-1243117. [DOI] [PubMed] [Google Scholar]
- 29.Liang Y.-C., Yang M.-T., Lin C.-J., Chang C.L.-T., Yang W.-C. Bidens pilosa and its active compound inhibit adipogenesis and lipid accumulation via down-modulation of the C/EBP and PPARγ pathways. Sci. Rep. 2016;6(1):24285. doi: 10.1038/srep24285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang C.L., Chung C.-Y., Kuo C.-H., Kuo T.-F., Yang C.-W., W-CJPo Yang. Beneficial effect of Bidens pilosa on body weight gain, food conversion ratio, gut bacteria and coccidiosis in chickens. PloS One. 2016;11(1) doi: 10.1371/journal.pone.0146141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimo T., Azay J., Tan P.V., Pellecuer J., Cros G., Bopelet M., et al. Effects of the aqueous and methylene chloride extracts of Bidens pilosa leaf on fructose-hypertensive rats. J. Ethnopharmacol. 2001;76(3):215–221. doi: 10.1016/s0378-8741(01)00229-x. [DOI] [PubMed] [Google Scholar]
- 32.Dimo T., Rakotonirina S.V., Tan P.V., Azay J., Dongo E., Cros G. Leaf methanol extract of Bidens pilosa prevents and attenuates the hypertension induced by high-fructose diet in Wistar rats. J. Ethnopharmacol. 2002;83(3):183–191. doi: 10.1016/s0378-8741(02)00162-9. [DOI] [PubMed] [Google Scholar]
- 33.F G. University of Nevada; 2019. The Nutritional Knowledge and Consumption of Blackjack by Hypertensive Patients in Vhembe District, Limpopo Province, South Africa. [Google Scholar]
- 34.Mokganya M., Tshisikhawe M. Medicinal uses of selected wild edible vegetables consumed by Vhavenda of the Vhembe District Municipality, South Africa. South Afr. J. Bot. 2019;122:184–188. [Google Scholar]
- 35.Yang H.-L., Chen S.-C., Chang N.-W., Chang J.-M., Lee M.-L., Tsai P.-C., et al. Protection from oxidative damage using Bidens pilosa extracts in normal human erythrocytes. Food Chem. Toxicol. 2006;44(9):1513–1521. doi: 10.1016/j.fct.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Lai B.-Y., Chen T.-Y., Huang S.-H., Kuo T.-F., Chang T.-H., Chiang C.-K., et al. Bidens pilosa formulation improves blood homeostasis and β-cell function in men: a pilot study. J. Evid.-based Compl. Altern. Med. 2015;2015 doi: 10.1155/2015/832314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Batish D.R., Singh H., Kohli R., Saxena D., Kaur S.J.E., botany e. Allelopathic effects of parthenin against two weedy species, Avena fatua and Bidens pilosa. Environ. Exp. Bot. 2002;47(2):149–155. [Google Scholar]
- 38.Tembo Y., Mkindi A.G., Mkenda P.A., Mpumi N., Mwanauta R., Stevenson P.C., et al. Pesticidal plant extracts improve yield and reduce insect pests on legume crops without harming beneficial arthropods. Front. Plant Sci. 2018;9:1425. doi: 10.3389/fpls.2018.01425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taffner J., Laggner O., Wolfgang A., Coyne D., Berg G. Exploring the microbiota of east African indigenous leafy greens for plant growth, health, and resilience. Front. Microbiol. 2020;11:2968. doi: 10.3389/fmicb.2020.585690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed S.S., Abdel-Aziz S.Y., Taha A., Elhefny A. Insecticidal effects of two plant extracts of (bidens pilosa and rumex dentatus) and neem oil against certain stored grains insects. Egypt. Acad. J. Biol. Sci. F Toxicol. 2021;13(1):149–158. [Google Scholar]
- 41.Yang R.-Y., Keding G.B. Routledge; 2009. Nutritional Contributions of Important African Indigenous Vegetables. African Indigenous Vegetables in Urban Agriculture; pp. 137–176. [Google Scholar]
- 42.Faber M.O.A., Van Jaarsveld P.J., Jansen van Rensburg W.S. African leafy vegetables consumed by households in the Limpopo and KwaZulu-Natal provinces in South Africa. S. Afr. J. Clin. Nutr. 2010;2010(23):1. [Google Scholar]
- 43.Odhava SB B., Akulaa Us, Baijnathc H. Preliminary assessment of nutritional value of traditional leafy vegetables in KwaZulu-Natal, South Africa. J. Food Compos. Anal. 2007;20:430–435. 2007. [Google Scholar]
- 44.Shrawan Singh D.R.S., Singh L.B., Chand Subhash, Dam Roy S. Indigenous vegetables for food and nutritional security in Andaman and Nicobar Islands, India. Int. J. Agric. Food Sci. Technol. 2013;4:503–512. 2013. [Google Scholar]
- 45.Manduna I., Vibrans H. Consumption of wild-growing vegetables in the Honde valley, Zimbabwe. Econ. Bot. 2018;72(4):436–449. [Google Scholar]
- 46.Liang Y.-C., Lin C.-J., Yang C.-Y., Chen Y.-H., Yang M.-T., Chou F.-S., et al. Toxicity study of Bidens pilosa in animals. J. Tradit. Compl. Med. 2020;10(2):150–157. doi: 10.1016/j.jtcme.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ajayi S., Ademosun T., Okoro E., Ajanaku C., Mordi R., Ajanaku K., editors. Journal of Physics: Conference Series. IOP Publishing; 2019. Corrosion inhibitory properties of Biden pilosa plant extract on mild steel in acidic media. [Google Scholar]
- 48.Alaneme K., Osasona B., Okotete E., Olusegun S., Donatus U. Corrosion inhibition behaviour of Biden pilosa extract on aluminium matrix composites in 1M HCl solution. J. Assoc. Prof. Eng. Trinidad Tobago. 2016;44(2):35–42. [Google Scholar]
- 49.George M.H. Evaluation of the efficacy of herbicides during transition to conservation agriculture in Zimbabwe. World. 2020;7(2):237–242. [Google Scholar]
- 50.Daba A., Tadesse M., Mohammed A. Evaluation of botanical herbicides against common weed Species of Coffee (Coffea arabica l.) with emphasis on bidens pilosa at southwestern Ethiopia. Ethiop. J. Agric. Sci. 2018 [Google Scholar]
- 51.Wang C., Jhan Y., Yen L., Su Y., Chang C., Wu Y., et al. The allelochemicals of litchi leaf and its potential as natural herbicide in weed control. Allelopathy J. 2013;32(2):157. [Google Scholar]
- 52.Glimn-Lacy J., Kaufman P.B. Flowering Plant Families; 2006. Aster Family (Asteraceae). J Botany Illustrated: Introduction to Plants, Major Groups; p. 119. [Google Scholar]
- 53.Bartolome A.P., Villaseñor I.M., Yang W.-C. Bidens pilosa L.(Asteraceae): botanical properties, traditional uses, phytochemistry, and pharmacology. J. Evid.-based Compl. Altern. Med. 2013;2013 doi: 10.1155/2013/340215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rastogi J., Rawat D., Chandra S. Diversity of invasive alien species in Pantnagar flora. Trop. Plant Res. 2015;2(3):282–287. [Google Scholar]
- 55.Chivinge Studies on the germination and seedling emergence of Bidens pilosa and its response to fertilizer application. Invasive Species Compendium. 1996 [Google Scholar]
- 56.Graber J.W. Distribution, habitat requirements, and life history of the black-capped vireo (Vireo atricapilla) J. Ecol. Monogr. 1961;31(4):313–336. [Google Scholar]
- 57.Xuan T.D., TDJJopi Khanh. Chemistry and pharmacology of Bidens pilosa: an overview. J. Pharmaceut. Investig. 2016;46(2):91–132. doi: 10.1007/s40005-016-0231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ndiege M.L., Kengara F., Maiyoh G.K. Characterization of phenolic compounds from leaf extract of bidens pilosa Linn. Var. Radiata. South Asian Res. J. Nat. Prod. 2021:44–58. [Google Scholar]
- 59.Le J., Lu W., Xiong X., Wu Z., Chen W.J.M. Anti-inflammatory constituents from Bidens frondosa. J. Mol. 2015;20(10):18496–18510. doi: 10.3390/molecules201018496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Riaz A., Rasul A., Hussain G., Zahoor M.K., Jabeen F., Subhani Z., et al. Astragalin: a bioactive phytochemical with potential therapeutic activities. J. Adv. Pharmacol. Sci. 2018;2018 doi: 10.1155/2018/9794625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ivanov M., Kannan A., Stojkovic D., Glamoclija J., Grdadolnik S.G., Sanglard D., et al. Revealing the astragalin mode of anticandidal action. EXCLI J. 2020;19:1436. doi: 10.17179/excli2020-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deba F., Xuan T.D., Yasuda M., Tawata S. Management. Herbicidal and fungicidal activities and identification of potential phytotoxins from Bidens pilosa L. var. radiata Scherff. Weed Biol. Manag. 2007;7(2):77–83. [Google Scholar]
- 63.Chang M.-Y., Shen Y.-L. Linalool exhibits cytotoxic effects by activating antitumor immunity. J. Mol. 2014;19(5):6694–6706. doi: 10.3390/molecules19056694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ogunbinu A.O., Flamini G., Cioni P.L., Adebayo M.A., Ogunwande I.A. Constituents of Cajanus cajan (L.) Millsp., Moringa oleifera Lam., Heliotropium indicum L. And bidens pilosa L. from Nigeria. Nat. Prod. Commun. 2009;4(4) [PubMed] [Google Scholar]
- 65.Fukuta M., Dang Xuan T., Deba F., Tawata S., Dang Khanh T., Min Chung I.J. Comparative efficacies in vitro of antibacterial, fungicidal, antioxidant, and herbicidal activities of momilatones A and B. J. Plant Interact. 2007;2(4):245–251. [Google Scholar]
- 66.Chang M.H., Wang G.J., Kuo Y.H., Lee C.K. The low polar constituents from Bidens pilosa L. var. minor (Blume) Sherff. J. Chin. Chem. Soc. 2000;47(5):1131–1136. [Google Scholar]
- 67.Kabanda M.M., Gbashi S., Madala N.E. Proportional coexistence of okanin chalcone glycoside and okanin flavanone glycoside in Bidens pilosa leaves and theoretical investigation on the antioxidant properties of their aglycones. Free Radic. Res. 2021;55(1):53–70. doi: 10.1080/10715762.2020.1859107. [DOI] [PubMed] [Google Scholar]
- 68.Zhu Y.J., Li C.H., Yu S.H., Jiang H.Q., Tian Z.H., Wang W.Q., et al. Two new polyacetylene glucosides and a new caffeoyl derivative with angiogenic activity from Bidens parviflora Willd. Phytochem. Lett. 2021;42:82–86. [Google Scholar]
- 69.Ashafa A., Afolayan A. Screening the root extracts from Biden pilosa L. var. radiata (Asteraceae) for antimicrobial potentials. J. Med. Plants Res. 2009;3(8):568–572. [Google Scholar]
- 70.Chiang Yi-Ming, Kuo Yueh-Hsiung, Lo Chiu-Ping, Chen Yi-Ping, Yang N.-S., Wang Sheng-Yang, et al. Ethyl caffeate suppresses NF-jB activation and its downstream inflammatory mediators, iNOS, COX-2, and PGE2 in vitro or in mouse skin. Br. J. Pharmacol. 2005;2005(146):352–363. doi: 10.1038/sj.bjp.0706343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ivanov M., Kannan A., Stojkovic D., Glamoclija J., Golic Grdadolnik S., Sanglard D., et al. Revealing the astragalin mode of anticandidal action. EXCLI J. 2020;19:1436–1445. doi: 10.17179/excli2020-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ivanov M., Kannan A., Stojkovic D., Glamoclija J., Grdadolnik S.G., Sanglard D., et al. Revealing the astragalin mode of anticandidal action. EXCLI J. 2020;19:1436. doi: 10.17179/excli2020-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nardella F., Gallé J.-B., Bourjot M., Weniger B., Vonthron-Sénécheau C. Springer; 2018. Antileishmanial and Antitrypanosomal Activities of Flavonoids. Natural Antimicrobial Agents; pp. 163–194. [Google Scholar]
- 74.Ammar O. In silico pharmacodynamics, toxicity profile and biological activities of the Saharan medicinal plant Limoniastrum feei. Braz. J. Pharmac. Sci. 2017;53 [Google Scholar]
- 75.Smiljkovic M., Stanisavljevic D., Stojkovic D., Petrovic I., Vicentic J.M., Popovic J., et al. Apigenin-7-O-glucoside versus apigenin: insight into the modes of anticandidal and cytotoxic actions. EXCLI J. 2017;16:795. doi: 10.17179/excli2017-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Candiracci M., Piatti E., Dominguez-Barragán M., García-Antrás D., Morgado B., Ruano D., et al. Anti-inflammatory activity of a honey flavonoid extract on lipopolysaccharide-activated N13 microglial cells. J. Agric. Food Chem. 2012;60(50):12304–12311. doi: 10.1021/jf302468h. [DOI] [PubMed] [Google Scholar]
- 77.Cárdeno Ana, Aparicio-Soto Marina, Montserrat-de la Paz Sergio, Bermúdez Beatriz, Francisco J., Muriana G., Alarcón-de-la-Lastra Catalina. Squalene targets pro- and anti-inflammatory mediators and pathways to modulate over-activation of neutrophils, monocytes and macrophages. J. Funct.Foods. 2015;14:779–790. 2015. [Google Scholar]
- 78.Cárdeno Ana, Aparicio-Soto Marina, Montserrat-de la Paz Sergio, Bermúdez Beatriz, Francisco J., Muriana G., Alarcón-de-la-Lastra Catalina. Squalene targets pro- and anti-inflammatory mediators and pathways to modulate over-activation of neutrophils, monocytes and macrophages. J. Funct.Foods. 2015 [Google Scholar]
- 79.Jaiswal A.K. Nrf2 signaling in coordinated activation of antioxidant gene expression. J. Free Rad. Biol. Med. Conf. Surv. 2004;36(10):1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 80.Gordon S., Taylor P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005;5(12):953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 81.Taylor P.R., Martinez-Pomares L., Stacey M., Lin H.-H., Brown G.D., Gordon S. Macrophage receptors and immune recognition. J. Annu. Rev. Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 82.Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—from molecular mechanisms to therapeutic benefits. J. Biochimica et Biophysica Acta -Proteins Proteom. 2005;1754(1-2):253–262. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 83.Andreakos E., Foxwell B., Feldmann M. Is targeting Toll-like receptors and their signaling pathway a useful therapeutic approach to modulating cytokine-driven inflammation? J. Immunol. Rev. 2004;202(1):250–265. doi: 10.1111/j.0105-2896.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- 84.Helenius M., Kyrylenko S., Vehviläinen P., Salminen A.J.A., Signaling R. Characterization of aging-associated up-regulation of constitutive nuclear factor-κB binding activity. Antioxidants Redox Signal. 2001;3(1):147–156. doi: 10.1089/152308601750100669. [DOI] [PubMed] [Google Scholar]
- 85.Cárdeno A., Aparicio-Soto M., Montserrat-de la Paz S., Bermudez B., Muriana F.J., Alarcón-de-la-Lastra C. Squalene targets pro-and anti-inflammatory mediators and pathways to modulate over-activation of neutrophils, monocytes and macrophages. J. Funct. Foods. 2015;14:779–790. [Google Scholar]
- 86.Jara C.P., Mendes N.F., Prado TPd, de Araújo E.P. Bioactive fatty acids in the resolution of chronic inflammation in skin wounds. J. Adv. Wound Care. 2020;9(8):472–490. doi: 10.1089/wound.2019.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cárdeno A., Aparicio-Soto M., Montserrat-de la Paz S., Bermudez B., Muriana F.J., Alarcón-de-la-Lastra C. Squalene targets pro-and anti-inflammatory mediators and pathways to modulate over-activation of neutrophils, monocytes and macrophages. J. Funct. Foods. 2015;14:779–790. [Google Scholar]
- 88.Chen H., Sohn J., Zhang L., Tian J., Chen S., Bjeldanes L.F. Anti-inflammatory effects of chicanine on murine macrophage by down-regulating LPS-induced inflammatory cytokines in IκBα/MAPK/ERK signaling pathways. J. Eur. J. Pharmacol. 2014;724:168–174. doi: 10.1016/j.ejphar.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khokha R., Murthy A., Weiss A. Metalloproteinases and their natural inhibitors in inflammation and immunity. J. Nat. Rev. Immunol. 2013;13(9):649–665. doi: 10.1038/nri3499. [DOI] [PubMed] [Google Scholar]
- 90.Mencarelli A., Distrutti E., Renga B., D'Amore C., Cipriani S., Palladino G., et al. Probiotics modulate intestinal expression of nuclear receptor and provide counter-regulatory signals to inflammation-driven adipose tissue activation. PloS One. 2011;6(7) doi: 10.1371/journal.pone.0022978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang W., Wang S., Ma X., Gong J. Recent advances in catalytic hydrogenation of carbon dioxide. J. Chem. Soc. Rev. 2011;40(7):3703–3727. doi: 10.1039/c1cs15008a. [DOI] [PubMed] [Google Scholar]
- 92.Kaur Ramandeep, Sharma Pooja, Gupta Girish K., Kumar Dinesh, Ntie-Kang Fidele. Structure-activity-relationship and mechanistic insights for anti-HIV natural products. Molecules. 2020;2020(25):2070. doi: 10.3390/molecules25092070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sharifi-Rad J. Herbal antibiotics: moving back into the mainstream as an alternative for" superbugs. J. Cell Mol. Biol. 2016;62(9):1–2. [PubMed] [Google Scholar]
- 94.Organization W.H. In vitro screening of traditional medicines for anti-HIV activity: memorandum from a WHO meeting. J. Bull World Health Organ. 1989;87:613–618. [PMC free article] [PubMed] [Google Scholar]
- 95.Farnsworth N.R. The role of ethnopharmacology in drug development. J. Bioact. Comp. Plants. 1990;154:2–21. doi: 10.1002/9780470514009.ch2. [DOI] [PubMed] [Google Scholar]
- 96.Pace G.W., Leaf C.D. The role of oxidative stress in HIV disease. J. Free Rad. Biol. Med. 1995;19(4):523–528. doi: 10.1016/0891-5849(95)00047-2. [DOI] [PubMed] [Google Scholar]
- 97.Olinski R., Gackowski D., Foksinski M., Rozalski R., Roszkowski K., Jaruga P. Oxidative DNA damage: assessment of the role in carcinogenesis, atherosclerosis, and acquired immunodeficiency syndrome. J. Free Rad. Biol. Med. Conf. Surv. 2002;33(2):192–200. doi: 10.1016/s0891-5849(02)00878-x. [DOI] [PubMed] [Google Scholar]
- 98.Rozalski R. Oxidative DNA damage: assessment of the role in carcinogenesis, atherosclerosis, and acquired immunodeficiency s. J. Free Rad. Biol. Med. Conf. Surv. 2002;33(2):192–200. doi: 10.1016/s0891-5849(02)00878-x. [DOI] [PubMed] [Google Scholar]
- 99.Torre D., Pugliese A., Speranza F. Role of nitric oxide in HIV-1 infection: friend or foe? The Lancet Infect. Dis. 2002;2(5):273–280. doi: 10.1016/s1473-3099(02)00262-1. [DOI] [PubMed] [Google Scholar]
- 100.Sappey C., Legrand-Poels S., Best-Belpomme M., Favier A., Rentier B., Piette J. Stimulation of glutathione peroxidase activity decreases HIV type 1 activation after oxidative stress. J. AIDS Res. Hum. Retrovir. 1994;10(11):1451–1461. doi: 10.1089/aid.1994.10.1451. [DOI] [PubMed] [Google Scholar]
- 101.Bailly F., Cotelle P. Anti-HIV activities of natural antioxidant caffeic acid derivatives: toward an antiviral supplementation diet. J. Curr. Med. Chem. 2005;12(15):1811–1818. doi: 10.2174/0929867054367239. [DOI] [PubMed] [Google Scholar]
- 102.Rechner A.R., Kroner C. Anthocyanins and colonic metabolites of dietary polyphenols inhibit platelet function. J. Thrombosis Res. 2005;116(4):327–334. doi: 10.1016/j.thromres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 103.Brindha P. Role of phytochemicals as immunomodulatory agents: a review. J. Int. J. Green Pharm. 2016;10(1) [Google Scholar]
- 104.Agarwal S., Singh V. Immunomodulators: a review of studies on Indian medicinal plants and synthetic peptides. Part-I: medicinal plants. J. Proc. Indian Nat. Sci. Acad.-Part B: Biol. Sci. 1999;65(3-4):179–204. [Google Scholar]
- 105.Agrawal S., Singh V. Immunomodulators: a review of studies on indian medicinal plants and synthetic peptides: Part II-Synthetic peptides. J. Proc. Indian Nat. Sci. Acad.-Part B: Biol. Sci. 1999;65(6):377–392. [Google Scholar]
- 106.Holtmeier W., Kabelitz D. γδ T cells link innate and adaptive immune responses. J. Mechanis. Epithelial Defense. 2005;86:151–183. doi: 10.1159/000086659. [DOI] [PubMed] [Google Scholar]
- 107.Harborne A. Springer science & business media; 1998. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis. [Google Scholar]
- 108.Okwu D. Phytochemical and vitamin content of indigenous spices of South Eastern Nigeria. J. Sustain Agric. Environ. 2004;6:30–34. [Google Scholar]
- 109.Seeram N.P., Adams L.S., Henning S.M., Niu Y., Zhang Y., Nair M.G., et al. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005;16(6):360–367. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 110.Chiang L.-C., Ng L.T., Chiang W., Chang M.-Y., Lin C.-C. Immunomodulatory activities of flavonoids, monoterpenoids, triterpenoids, iridoid glycosides and phenolic compounds of Plantago species. J. Planta Med. 2003;69(7):600–604. doi: 10.1055/s-2003-41113. [DOI] [PubMed] [Google Scholar]
- 111.Bartolome A.P., Villaseñor I.M., Yang W.C. Bidens pilosa L. (Asteraceae): botanical properties, traditional uses, phytochemistry, and pharmacology. Evid. base Compl. Alternative Med. 2013;2013:340215. doi: 10.1155/2013/340215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mboya R.M. The nutritional and health potential of blackjack (bidens pilosa l.): a review–promoting the use of blackjack for food. J. Int. J. Appl. Res. Publ. Health Manag. 2019;4(1):47–66. [Google Scholar]
- 113.Zamora-Martínez M.C., de Pascual Pola C.N. Medicinal plants used in some rural populations of Oaxaca, Puebla and Veracruz, Mexico. J. Ethnopharmacol. 1992;35(3):229–257. doi: 10.1016/0378-8741(92)90021-i. [DOI] [PubMed] [Google Scholar]
- 114.Habwe F.O.W., Abukutsa-Onyango Mary K., Oluoch Mary O., Mel O. Iron content of the formulated East African indigenous vegetable recipes. J. Afr. J. Food Sci. 2009;3(12):393–397. [Google Scholar]
- 115.WHO . WHO; 2002. The World Health Report 2002 Reducing Risks, Promoting Healthy Life. [DOI] [PubMed] [Google Scholar]
- 116.Ramya V., Patel P. Health benefits of vegetables. IJCS. 2019;7(2):82–87. [Google Scholar]
- 117.Peevy F.A. Evaluation of placements of 2, 4, 5-T basal spray for control of blackjack oak. J. Weeds. 1962;10(1):74–75. [Google Scholar]
- 118.Mary A.-O. The diversity of cultivated African leafy vegetables in three communities in western Kenya. AJFAND. 2007 [Google Scholar]
- 119.Uusiku N.P., Oelofse A., Duodu K.G., Bester M.J., Faber M.J. Nutritional value of leafy vegetables of sub-Saharan Africa and their potential contribution to human health: a review. J. Food Composit. Anal. 2010;23(6):499–509. [Google Scholar]
- 120.Faber M., Van Jaarsveld P., Wenhold F., Rensburg V. African leafy vegetables consumed by households in the Limpopo and KwaZulu-Natal provinces in South Africa. J. South Afr. J. Clin. Nutr. 2010;23(1) [Google Scholar]
- 121.Shrawan Singh D.R.S., Singh L.B., Chand Subhash, Dam Roy S. Indigenous vegetables for food and nutritional security in Andaman and Nicobar Islands, India. Int. J. Agric. Food Sci. Technol. 2013;4(5):503–512. [Google Scholar]
- 122.Rodas-Moya S., Kodish S., Manary M., Grede N., De Pee S. Preferences for food and nutritional supplements among adult people living with HIV in Malawi. J. Publ. Health Nutr. 2016;19(4):693–702. doi: 10.1017/S1368980015001822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yip L., Pei S., Hudson J., Towers G.J. Screening of medicinal plants from Yunnan Province in southwest China for antiviral activity. J. Ethnopharmacol. 1991;34(1):1–6. doi: 10.1016/0378-8741(91)90182-d. [DOI] [PubMed] [Google Scholar]
- 124.Arthur G., Naidoo K., Coopoosamy R.J. Bidens pilosa L.: agricultural and pharmaceutical importance. J. Med. Plants Res. 2012;6(17):3282–3291. [Google Scholar]
- 125.Singh G., Passsari A.K., Singh P., Leo V.V., Subbarayan S., Kumar B., et al. Pharmacological potential of Bidens pilosa L. and determination of bioactive compounds using UHPLC-QqQ LIT-MS/MS and GC/MS. BMC Compl. Altern. Med. 2017;17(1):1–16. doi: 10.1186/s12906-017-2000-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ndiege M.L., Kengara F., Maiyoh G.K. Characterization of phenolic compounds from leaf extract of bidens pilosa Linn. Var. Radiata. J South Asian Res. J. Nat. Prod. 2021:44–58. [Google Scholar]
- 127.Chang C.L.-T., Yang C.-Y., Muthamilselvan T., Yang W.-C. Field trial of medicinal plant, Bidens pilosa, against eimeriosis in broilers. J. Sci. Rep. 2016;6(1):1–7. doi: 10.1038/srep24692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ignarro L.J., Balestrieri M.L., Napoli C. Nutrition, physical activity, and cardiovascular disease: an update. J. Cardiovasc. Res. 2007;73(2):326–340. doi: 10.1016/j.cardiores.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 129.Farhud D.D. Impact of lifestyle on health. J. Iran. J. Publ. Health. 2015;44(11):1442. [PMC free article] [PubMed] [Google Scholar]
- 130.Nilsson P.M., Tuomilehto J., Rydén L. The metabolic syndrome–What is it and how should it be managed? J. Eur. J. Prevent. Cardiol. 2019;26(2_suppl):33–46. doi: 10.1177/2047487319886404. [DOI] [PubMed] [Google Scholar]
- 131.Lin Y., Yang M.-T., Minh H.T.N., Yang W.-C. 2020. Polyynes in Food. [Google Scholar]
- 132.Ezuruike U.F. University College London; 2015. Evaluation of Herb-Drug Interactions in Nigeria with a Focus on Medicinal Plants Used in Diabetes Management: UCL. [Google Scholar]
- 133.Jiralerspong S., Palla S.L., Giordano S.H., Meric-Bernstam F., Liedtke C., Barnett C.M., et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J. Clin. Oncol. 2009;27(20):3297. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kopelman P.G. Obesity as a medical problem. J. Nat. 2000;404(6778):635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 135.Mendis S.C. Oleg the global burden of cardiovascular diseases: a challenge to improve. J. Curr. Cardiol. Rep. 2014;16(5):486. doi: 10.1007/s11886-014-0486-3. [DOI] [PubMed] [Google Scholar]
- 136.Abu-Abid S., Szold A., Klausner J. Obesity and cancer. J. Med. 2002;33(1-4):73–86. [PubMed] [Google Scholar]
- 137.Organization W.H. World Health Organization; 2002. The World Health Report 2002: Reducing Risks, Promoting Healthy Life. [DOI] [PubMed] [Google Scholar]
- 138.Steyn N., Bradshaw D., Norman R., Joubert J., Schneider M., Steyn K., et al. Dietary changes and the health transition in South Africa: implications for health policy. JTdbomCsfsdcRF. 2006:259–304. [Google Scholar]
- 139.Boutayeb A., Boutayeb S. The burden of non communicable diseases in developing countries. Int. J. Equity Health. 2005;4(1):1–8. doi: 10.1186/1475-9276-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Angkurawaranon C., Jiraporncharoen W., Chenthanakij B., Doyle P., Nitsch D. Urbanization and non-communicable disease in Southeast Asia: a review of current evidence. J. Public Health. 2014;128(10):886–895. doi: 10.1016/j.puhe.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 141.Liang Y.-C., Yang M.-T., Lin C.-J., Chang C.L.-T., Yang W.-C. Bidens pilosa and its active compound inhibit adipogenesis and lipid accumulation via down-modulation of the C/EBP and PPARγ pathways. J. Sci. Rep. 2016;6(1):1–10. doi: 10.1038/srep24285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kuo T.F., Yang G., Chen T.Y., Wu Y.C., Tran Nguyen Minh H., Chen L.S., et al. Bidens pilosa: nutritional value and benefits for metabolic syndrome. J. Food Front. 2021;2(1):32–45. [Google Scholar]
- 143.Gavhi F. The Nutritional Knowledge and Consumption of Blackjack by Hypertensive Patients in Vhembe District, Limpopo Province, South Africa 2019.
- 144.Mokganya M., Tshisikhawe M. Medicinal uses of selected wild edible vegetables consumed by Vhavenda of the Vhembe District Municipality, South Africa. South Afr. J. Bot. 2019;122:184–188. [Google Scholar]
- 145.Lopez A.D., Mathers C.D., Ezzati M., Jamison D.T., Murray C.J. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. J. Lancet. 2006;367(9524):1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 146.Ataklte F., Erqou S., Kaptoge S., Taye B., Echouffo-Tcheugui J.B., Kengne A.P. Burden of undiagnosed hypertension in sub-saharan Africa: a systematic review and meta-analysis. J. Hypert. 2015;65(2):291–298. doi: 10.1161/HYPERTENSIONAHA.114.04394. [DOI] [PubMed] [Google Scholar]
- 147.Steyn K., Bradshaw D., Norman R., Laubscher R. Determinants and treatment of hypertension in South Africans: the first demographic and health survey. South Afr. Med. J. = Suid-Afrikaanse tydskrif vir geneeskunde. 2008;98(5):376–380. [PubMed] [Google Scholar]
- 148.Sacks F.M., Kass E.H. Low blood pressure in vegetarians: effects of specific foods and nutrients. Am. J. Clin. Nutr. 1988;48(3 Suppl):795–800. doi: 10.1093/ajcn/48.3.795. [DOI] [PubMed] [Google Scholar]
- 149.Oyunchimeg B., Hwang J.H., Ahmed M., Choi S., Han D. Complementary and alternative medicine use among patients with cancer in Mongolia: a National hospital survey. BMC Compl. Alternative Med. 2017;17(1):58. doi: 10.1186/s12906-017-1576-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dimo T., Nguelefack T.B., Kamtchouing P., Dongo É., Rakotonirina A., Rakotonirina S.V. Effets hypotensifs de l'extrait au méthanol de Bidens pilosa Linn chez les rats hypertendus. Compt. Rendus Acad. Sci. III Sci. Vie. 1999;322(4):323–329. doi: 10.1016/s0764-4469(99)80068-7. [DOI] [PubMed] [Google Scholar]
- 151.Dimo T., Azay J., Tan P.V., Pellecuer J., Cros G., Bopelet M., et al. Effects of the aqueous and methylene chloride extracts of Bidens pilosa leaf on fructose-hypertensive rats. J. Ethnopharmacol. 2001;76(3):215–221. doi: 10.1016/s0378-8741(01)00229-x. [DOI] [PubMed] [Google Scholar]
- 152.Yang H.-L., Chen S., Chang N., Chang J., Lee M.-L., Tsai P., et al. Protection from oxidative damage using Bidens pilosa extracts in normal human erythrocytes. J. Food Chem. Toxicol. : Int. J. Publ. Br. Industr. Biol. Res. Assoc. 2006;44 9:1513–1521. doi: 10.1016/j.fct.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 153.Dey A., Doss A.G., Natarajan S., Rajappan M., Smith A., Sundararajan P. Studies of anticancer and antipyretic activity of Bidens pilosa check for this species in other resources whole plant. J. Afr. Health Sci. 2006:27–30. doi: 10.5555/afhs.2006.6.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dey A, Doss AG, Natarajan S, Rajappan M, Smith A, Sundararajan P. Studies of anticancer and antipyretic activity of Bidens pilosa check for this species in other resources whole plant. J. Afr. Health Sci..27–30. [DOI] [PMC free article] [PubMed]
- 155.Chang C.L.-T., Yang C.-Y., Muthamilselvan T., Yang W.-C. Field trial of medicinal plant, Bidens pilosa, against eimeriosis in broilers. J. Sci. Rep. 2016;6(1):1–7. doi: 10.1038/srep24692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ajagun-Ogunleye M.O., Tirwomwe M., Mitaki R.N., Ejekwumadu J.N., Kasozi K.I., Pantoglou J., et al. Hypoglycemic and high dosage effects of bidens pilosa in type-1 diabetes mellitus. J. Diabet. Mellitus. 2015;5(3):146. [Google Scholar]
- 157.Ashafa A., Afolayan A. Screening the root extracts from Biden pilosa L. var. radiata (Asteraceae) for antimicrobial potentials. J. Med. Plants Res. 2009;3(8):568–572. [Google Scholar]
- 158.Bairwa K., Kumar R., Sharma R.J., Roy R.K. An updated review on Bidens pilosa L. J. Der. Pharma Chemica. 2010;2(3):325–337. [Google Scholar]
- 159.Manduna I.V. Heike consumption of wild-growing vegetables in the honde valley, Zimbabwe. J. Econom. Bot. 2018;72(4):436–449. [Google Scholar]
- 160.Kuo T.F., Yang G., Chen T.Y., Wu Y.C., Tran Nguyen Minh H., Chen L.S., et al. Bidens pilosa: nutritional value and benefits for metabolic syndrome. Food Front. 2021;2(1):32–45. [Google Scholar]
- 161.Lai B.-Y., Chen T.-Y., Huang S.-H., Kuo T.-F., Chang T.-H., Chiang C.-K., et al. Bidens pilosa formulation improves blood homeostasis and β-cell function in men: a pilot study. Evid. base Compl. Alternative Med. 2015;2015 doi: 10.1155/2015/832314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liang Y.-C., Lin C.-J., Yang C.-Y., Chen Y.-H., Yang M.-T., Chou F.-S., et al. Toxicity study of Bidens pilosa in animals. J. Tradit. Compl. Med. 2020;10(2):150–157. doi: 10.1016/j.jtcme.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.de Ávila P.H.M., de Ávila R.I., dos Santos Filho E.X., Bastos C.C.C., Batista A.C., Mendonca E.F., et al. Mucoadhesive formulation of Bidens pilosa L.(Asteraceae) reduces intestinal injury from 5-fluorouracil-induced mucositis in mice. Toxicol Rep. 2015;2:563–573. doi: 10.1016/j.toxrep.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wu Y., Ren D., Gao C., Li J., Du B., Wang Z., et al. Recent advances for alkaloids as botanical pesticides for use in organic agriculture. J. Int. J. Pest Manag. 2021:1–11. [Google Scholar]
- 165.George M.H. Evaluation of the efficacy of herbicides during transition to conservation agriculture in Zimbabwe. J. World Aquac. 2020;7(2):237–242. [Google Scholar]
- 166.Olusegun S., Joshua T., Bodunrin M., Aribo S.J.N. Science. Inhibition of mild steel corrosion in HCl solution by plant extract of Biden pilosa. J. Nat. Sci. 2018;16(1):1–8. [Google Scholar]
- 167.Yuan H., Ma Q., Ye L., Piao G. The traditional medicine and modern medicine from natural products. Molecules (Basel, Switzerland) 2016;21(5):559. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bartolome Arlene P., Villaseñor Irene M., Yang Wen-Chin. Bidens pilosa L. (Asteraceae): botanical properties, traditional uses, phytochemistry, and pharmacology. Hindawi Publish. Corpor. Evid.-Based Compl. Altern. Med. 2015;2013:51. doi: 10.1155/2013/340215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Moshi Mainen J., Otieno Donald F., Mbabazi Pamela K., Weisheit Anke. Ethnomedicine of the Kagera region, north western Tanzania. J. Ethnobiol. Ethnomed. 2010;6(19) doi: 10.1186/1746-4269-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bunalema Lydia, Obakiro Samuel, John R., Tabuti S., Paul Waako. Knowledge on plants used traditionally in the treatment of tuberculosis in Uganda. J. Ethnopharmacol. 2014;151:999–1004. doi: 10.1016/j.jep.2013.12.020. 2014. [DOI] [PubMed] [Google Scholar]
- 171.Yadav S.S., Singh M.K., Singh P.K., Kumar V. Traditional knowledge to clinical trials: a review on therapeutic actions of Emblica officinalis. Biomed. Pharmacother. 2017;93:1292–1302. doi: 10.1016/j.biopha.2017.07.065. [DOI] [PubMed] [Google Scholar]
- 172.Borokini Temitope I., Lawal Ibrahim O. Traditional medicine practices among the Yoruba people of Nigeria: a historical perspective. JMPS. 2014;2(6):20–33. [Google Scholar]
- 173.Bodeker G., Ong C.-K. World Health Organization; 2005. WHO Global Atlas of Traditional, Complementary and Alternative Medicine. [Google Scholar]
- 174.Tabuti John R.S., Kukunda Collins B., Waakoc Paul J. Medicinal plants used by traditional medicine practitioners in the treatment of tuberculosis and related ailments in Uganda. J. Ethnopharmacol. 2010;127:130–136. doi: 10.1016/j.jep.2009.09.035. 2010. [DOI] [PubMed] [Google Scholar]
- 175.Nyamukuru Antonia, Tabuti John R.S., Lamorde Mohammed, Aduma Philip R., Kato Benard, Sekagya Yahaya. Medicinal plants and traditional treatment practices used in the management of HIV/AIDS clients in Mpigi District, Uganda. J. Herb. Med. 2016 [Google Scholar]
- 176.Ribeiro R.V., Bieski I.G.C., Balogun S.O., de Oliveira Martins D.T. Ethnobotanical study of medicinal plants used by Ribeirinhos in the North Araguaia microregion, Mato Grosso, Brazil. JJoe. 2017;205:69–102. doi: 10.1016/j.jep.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 177.de Carvalho Silva A., de Araújo C.R., Vázquez L.L. Springer; 2019. Oleraceous. Natural Enemies of Insect Pests in Neotropical Agroecosystems; pp. 341–354. [Google Scholar]
- 178.Panda S.K., Luyten W.J.P. Antiparasitic activity in Asteraceae with special attention to ethnobotanical use by the tribes of Odisha, India. Parasite. 2018;25 doi: 10.1051/parasite/2018008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Moro C.A.Z. Pharmaceutical preparations in the 19th and 20th centuries in the Eastern region of Cuba. J. Revista Cubana de Farmacia. 2016;50(1):171–180. [Google Scholar]
- 180.Mahabir D., Gulliford M.C. Use of medicinal plants for diabetes in Trinidad and Tobago. J. Revista Panamericana de Salud Pública. 1997;1:174–179. doi: 10.1590/s1020-49891997000300002. [DOI] [PubMed] [Google Scholar]
- 181.Maema L., Potgieter M., Samie A. Ethnobotanical survey of invasive alien plant species used in the treatment of sexually transmitted infections in Waterberg district, South Africa. South Afr. J. Bot. 2019;122:391–400. doi: 10.1016/j.dib.2019.104281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lawal I., Grierson D., Afolayan A. Phytotherapeutic information on plants used for the treatment of tuberculosis in Eastern Cape Province, South Africa. J. Evid.-Based Compl. Altern. Med. 2014;2014 doi: 10.1155/2014/735423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ndukwu B., Ben-Nwadibia N. Ethnomedicinal aspects of plants used as spices and condiments in the Niger delta area of Nigeria. J. Ethnobotanical Leaflets. 2005;2005(1):10. [Google Scholar]
- 184.Nguta Joseph Mwanzia, Regina Appiah-Opong, Nyarko Alexander K., Dorothy Yeboah-Manu, Addo Phyllis G.A. Medicinal plants used to treat TB in Ghana. Int. J. Mycobacteriol. 2015;4:1 6–12 3. doi: 10.1016/j.ijmyco.2015.02.003. 2015. [DOI] [PubMed] [Google Scholar]
- 185.Rasch Vibeke, Sørensen Pernille H., Wang Anna R., Tibazarwa Flora, Jäger Anna K. Unsafe abortion in rural Tanzania – the use of traditional medicine from a patient and a provider perspective. Rasch et al BMC Pregnancy and Childbirth. 2014;2014(14):429. doi: 10.1186/s12884-014-0419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nikolajsen Tine, Nielsen Frank, Rasch Vibeke, Sørensen Pernille H., Ismail Flora, Kristiansen Uffe, et al. Uterine contraction induced by Tanzanian plants used to induce abortion. J. Ethnopharmacol. 2011;137:921–925. doi: 10.1016/j.jep.2011.05.026. 2011. [DOI] [PubMed] [Google Scholar]
- 187.Mushagalusa Kasali F., Ahadi Irenge C., Murhula Hamuli P., Birindwa Mulashe P., Murhula Katabana D., Mangambu Mokoso J.D.D., et al. Ethnopharmacological survey on treatment of hypertension by traditional healers in Bukavu city, DR Congo. J. Evid.-Based Compl. Altern. Med. 2021:2021. doi: 10.1155/2021/6684855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lamorde Mohammed, Tabuti John R.S., Obua Celestino, Kukunda-Byobona Collins, Lanyero Hindam, Byakika-Kibwika Pauline, et al. Medicinal plants used by traditional medicine practitioners for the treatment of HIV/AIDS and related conditions in Uganda. J. Ethnopharmacol. 2010 doi: 10.1016/j.jep.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 189.Arthur G., Naidoo K., Coopoosamy R. Bidens pilosa L.: agricultural and pharmaceutical importance. J. Med. Plants Res. 2012;6(17):3282–3291. [Google Scholar]
- 190.Ndhlovu P.T., Omotayo A.O., Otang-Mbeng W., Aremu A.O. Ethnobotanical review of plants used for the management and treatment of childhood diseases and well-being in South Africa. South Afr. J. Bot. 2021;137:197–215. [Google Scholar]
- 191.Swai K.W.I. Consumption of traditional vegetables in central and Northeastern Tanzania. Ecol. Food Nutr. 2006;45(2):87–103. [Google Scholar]
- 192.Chou C. Role of allelopathy in sustainable agriculture: use of allelochemicals as naturally occurring bio-agrochemicals. Allelopathy J. 2010;25(1) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.