Abstract
Motor impairments are pervasive and persistent in children with Autism Spectrum Disorder (ASD) throughout childhood and adolescence. Based on recent studies examining motor impairments in children with ASD between 5 and 15 years (i.e. SPARK study sample), 87–88% of this population is at-risk for a motor impairment, these problems persisted until 15 years, and related to their core (social communication skills and repetitive behaviors) and comorbid (language, cognitive, and functional) impairments. Persistent motor impairments extending into adolescence/adulthood could negatively impact their independent daily living skills, physical fitness/activity levels, and physical/mental health. While multiple studies have examined relations between motor dimensions and core/comorbid impairments in young children with ASD, few studies have examined such relations in school-age children/adolescents with ASD. This paper conducts a further multidimensional study of which motor domains (i.e., gross-motor including visuo-motor or multi-limb coordination/planning, fine-motor or general coordination skills) best distinguish subgroups of school-age children/adolescents with ASD and help predict core and comorbid impairments after accounting for age and sex. Visuomotor, fine-motor and certain general coordination skills were better at explaining variations in / predicting social communication impairments whereas fine-motor skills were slightly better at explaining variations in / predicting repetitive behavior severity. Multi-limb coordination/planning and fine-motor skills explained variations in /predicted cognitive delays whereas visuomotor and fine-motor skills explained variations in and better predicted language delays. All 3 motor dimensions explained variations in / predicted functional delays. This study provides further evidence for inclusion of motor impairments within the ASD definition (criteria or specifiers).
Lay Summary
Gross-motor skills were related to social communication and functional delays of children with ASD (visuomotor skills related to language delays and multilimb coordination/planning skills related to cognitive delays). Fine-motor skills were related to repetitive behavior severity, language, cognitive, and functional delays in ASD. Diagnosticians should recommend systematic motor screening, further evaluations, and treatments for children at-risk for and diagnosed with ASD. Motor advocacy and enhanced public/clinical community awareness is needed to fulfill the unmet motor needs of children with ASD.
Introduction
With 1 in 44 children diagnosed with Autism Spectrum Disorder (ASD), it is one of the most common pediatric developmental disorders (Maenner et al., 2021). There is an enormous financial and psychological burden on families caring for children with ASD with average annual medical, non-medical, and productivity costs in the US ranging from ~268 to 461 billion (Leigh & Du, 2015). Parents of children with ASD struggle to receive an early diagnosis and comprehensive assessments for their child in order to gain access to various evidence-based early interventions for the multiple core and comorbid problems that occur in the early years (Shaw et al., 2020; Landa, 2008). Together, these issues make it urgent for clinical researchers to recognize and bring to forefront issues of under-diagnosis and under-treatment so that healthcare can be improved to provide appropriate assessments and interventions early on in life with the hope of improved future outcomes.
ASD is traditionally considered a social communication disorder; but recently has been recognized by many as a complex, multisystem disorder with multiple core and comorbid impairments (Elsabbagh & Johnson, 2016; Srinivasan & Bhat, 2013). Children with ASD present with social communication impairments and repetitive behaviors that together form the defining criteria for ASD (American Psychiatric Association, 2013). Apart from core impairments, children with ASD also present with cognitive and language impairments that are captured within the ASD definition through the use of “specifiers”, for example, a child may receive a diagnosis of “ASD with a language/intellectual impairment” (American Psychiatric Association, 2013). Sensory processing issues were more recently included within ASD criteria under the repetitive behaviors category. However, motor issues in ASD are still not recognized within the ASD defining criteria or specifiers. In this study, we examine variations in, predictive validity of, and associations between various motor dimensions (e.g., visuomotor, balance/postural control, complex motor coordination, etc.) and the core/comorbid impairments across the spectrum of children and adolescents with ASD. These findings will shed light on whether motor issues should be generally recognized as part of ASD specifiers or if certain motor issues should be part of the core impairments of ASD. It would also help to hone in on the types of motor skills that should be evaluated and treated when providing services to children and adolescents with ASD.
Recent findings from population-based studies such as the SPARK study in the United States and the West Australian Autism Register study in Australia confirm motor impairments in 79–88% of children with ASD (Licari et al., 2019; Bhat, 2020). These numbers align with smaller studies that also report similar high proportions of motor impairment in their ASD samples (Green et al., 2009; Miller et al., 2021). Moreover, the first author’s recent publications based on the SPARK cohort confirm that motor impairments in children with ASD persist between 5 and 15 years and increase with increasing severity of core impairments in social communication skills and repetitive behavior severity as well as comorbid, cognitive, language, and functional impairments (N=13,887) (Bhat, 2020, 2021). Persistent motor impairments extending into adolescence and adulthood not only negatively impact the ASD population’s independent daily living skills but also their physical fitness/activity levels and overall physical/mental health and well-being (Srinivasan, Pescatello, & Bhat, 2014, Bremer & Cairney, 2020, Ketcheson, Hauk, & Ulrich, 2017). The present study is a further extension of our earlier work to highlight how different motor domains/dimensions: gross-motor (i.e., visuo-motor or multi-limb coordination/planning), fine-motor, and general coordination skills (i.e., sport practice, motor speed, competence or clumsiness, and fatigability) help distinguish subgroups of children with ASD based on aforementioned core and comorbid impairments. Additionally, how are the different types of motor impairment associated with and predictive of other system impairments in school-age children with ASD?
There is copious literature on the range of motor impairments reported in children and adolescents with ASD that distinguish them from children with other / no diagnoses as reviewed earlier in Bhat, 2020 and 2021. Motor impairments in ASD include gross-motor problems in static/dynamic balance, motor coordination (i.e., visuomotor and multilimb/bilateral coordination), dyspraxia/motor planning problems, as well as problems with fine-motor problems in precision/integration and hand dexterity, etc. (Dewey, Cantell, & Crawford, 2007; Bhat, Galloway, & Landa, 2011, Shield et al., 2017; Bhat et al., 2018; Kaur et al., 2013, 2018; Jansiewicz et al., 2006; Fleury, Kushki, Tanel, Anagnostou & Chau, 2013; Ament et al., 2014; Kushki, Chau, Anagnostou, 2011; Fournier, Hass, Naik, Lodha, & Cauraugh, 2010). Definitions for various motor terms are provided in the supplementary materials. Moreover, there is mounting evidence for how motor impairments in infants at-risk for and children with ASD are associated with / predictive of core and comorbid impairments in ASD including social communication, receptive/expressive language, cognitive, and functional impairments as well as repetitive behavior severity (Macdonald, Lord, & Ulrich, 2013a, 2013b, 2014; Bhat et al., 2018; Srinivasan & Bhat, 2016; Shield et al., 2017; Kaur, Srinivasan & Bhat, 2015, 2018; LeBarton & Landa, 2019; Licari et al., 2019; Bhat, 2021; Radonovich, Fournier, & Hass, 2013). Both, fine- and gross-motor skills of children with ASD between 14–33 months (based on the Mullen Scales of Early Learning) predicted their concurrent autism severity using the diagnostic tool, Autism Diagnostic Observation Schedule (ADOS) (Macdonald et al., 2013a). While motor impairments in ASD are present across the ASD spectrum the prevalence and severity of motor impairment is greater in children with greater cognitive impairments (Green et al., 2009; Kaur et al., 2018; Licari et al., 2019; Bhat, 2021; Ketcheson, Hauk, & Ulrich, 2021). Additionally, greater motor impairment in children with ASD is linked to lower functional independence (Macdonald et al., 2013b; Licari et al., 2019; Bhat, 2021) as well as higher rates of repetitive behaviors (Radonovich et al., 2013; Bhat, 2021). When comparing fine and gross-motor performance in young children with ASD between 13 and 33 months, the fine-motor subtest of the Mullen scales predicted concurrent overall adaptive functioning, social, and communication functioning, and daily living skills performance whereas gross-motor skills predicted concurrent daily living skills performance only on the Vineland Adaptive Behavioral Scales (VABS) measure (Macdonald et al., 2013b). In contrast, in even younger infants at-risk for ASD between 7 to 36 months, early gross-motor but not fine-motor trajectories predicted future expressive language development (Leonard, Bedford, Pickles, & Hill, 2015). Overall, motor impairments generally differentiate children with ASD from children with no/other diagnoses and relate to core and comorbid impairments of ASD. However, majority of the aforementioned studies have been conducted in young children at-risk for ASD or those who were newly diagnosed with ASD. Few studies have reported relationships between fine- and gross-motor impairments and core / comorbid impairments in school-age children and adolescents with ASD; which will be the focus of the present study.
While many studies have reported associations between motor and other system impairments in children with ASD, even fewer studies have conducted comprehensive, multidimensional motor assessments to relate a variety of motor domains to the core and comorbid impairments of young and older children with ASD. Children with ASD have consistently shown associations between imitation/praxis performance and autism severity (based on ADOS scores) even after controlling for basic motor skill (Dewey et al., 2007; Dziuk et al., 2007; Dowell, Mahone, & Mostofsky, 2009; Stieglitz Ham et al., 2011; Macneil & Mostofsky, 2012; Kaur et al., 2018). Children with ASD also showed specific impairments in catching and balance skills that increased the risk of an ASD outcome indicating that visuomotor skills were particularly affected in children with ASD compared to those with Attention Deficit Hyperactivity Disorder (ADHD, Ament et al., 2014). Similarly, children with ASD had greater manual dexterity problems (requires hand-eye coordination) compared to children with Specific Language Impairment (SLI, McPhillips et al., 2014). The most consistent finding so far has been the relationship between early fine motor performance and concurrent and future language development and the ability to predict future language delays (Bhat et al., 2012; Bedford et al., 2015; Choi, Leech, Tager-Flusberg, & Nelson, 2019; Bal et al., 2020). Bedford et al. (2015) found that gross-motor development at 2 years of age was predictive of receptive and expressive language development in children with ASD between 2 and 9 years of age. Choi et al. (2019) and Bal et al. (2020) found that fine motor skills of children with ASD during early infancy and childhood (6 months to 2 years) were predictive of their future expressive language skills at 3 or 19 years of age even after controlling for visual reception skills. Using the Peabody Developmental Motor Scale (PDMS), a multidimensional motor tool, LeBarton & Landa (2019) found that postural control and reach-grasp performance (visuomotor skills) at 6 months predicted expressive language in toddlers at 30–36 months and ASD outcomes at 24–36 months. Using the PDMS measure, Wu et al. found that early motor performance between 24–42 months (including stationary, locomotion, object manipulation, grasping, and visuomotor integration) was predictive of concurrent receptive and expressive language delay in newly diagnosed children with ASD (Wu, Tsao, Huang, Yang, & Li, 2021). Overall, evidence suggests that fine-motor skills are related to and predictive of language delays and ASD outcomes and gross-motor skills, specifically visuomotor, fine-motor, and balance/postural control skills maybe related to the core impairments of children with ASD. However, the aforementioned studies involved small, biased samples with limited ranges of functioning, cognitive, or verbal capacities. Additionally, many studies examining relations between multiple motor dimensions and core/comorbid impairments involve young children at-risk for and newly diagnosed with ASD. Hence, the present study will extend the past work to study such associations/predictive relations between various motor dimensions and core/comorbid impairments in a broad and variable spectrum of school-age children / adolescents with ASD.
The SPARK study is a large population-based study engaging a large group of parents of school-age children and adolescents with ASD within the US using the Developmental Coordination Disorder Questionnaire (DCD-Q, Schoemaker et al., 2006), a motor screener, the Social Communication Questionnaire - Lifetime (SCQ, Berument et al., 1999), a social communication delay screener, and the Repetitive Behaviors Scale (RBS-R, Lam & Aman, 2006), a measure of various repetitive behaviors and restricted interests. Additionally, parents provided information on the child’s current language, functional, and cognitive abilities compared to same-age peers. This rich dataset includes basic demographic information, birth history, and diagnostic information as well. The present study examined the SPARK study dataset, version 3 to analyze the DCD-Q motor measure and its subscales and items. The standard domain / subscale scores of DCD-Q (control during movement/gross-motor, fine-motor, and general coordination) were compared across multiple subgroups of children with ASD and effect sizes were calculated for differences between subgroups based on SCQ, RBS-R, and parent-reported current abilities (cognitive, language, and functional delay) data. In terms of hypothesis, DCD-Q total scores (i.e., risk for motor impairment) and subscale scores (fine-motor, gross-motor, and general coordination scores) will be lower in children with ASD with higher SCQ and RBS-R scores as well as greater language, cognitive, and functional impairments. Internal consistency of the DCD-Q items will be high and will confirm its validity in screening motor problems of school-age children with ASD using the DCD-Q. New DCDQ subdomains will be obtained through factor analysis (FA). These new subdomains will also be examined for differences across various subgroups. Gross-motor performance (i.e., visuo-motor coordination) will relate more/be more predictive of core social impairments of ASD and fine-motor performance will relate more/be predictive of repetitive behaviors as well as general cognitive and language delays.
Methods
SPARK Study Procedures and Data Access
The SPARK research team recruited families with one or more children with ASD through 21 clinical sites across the US using a diverse social media strategy (Feliciano et al., 2018). Families who volunteered for the study completed several online questionnaires on the SPARK website (https://sparkforautism.org/registration/account_information/). They also received information on local research studies they could participate in. The first author’s lab signed up with the SPARK study to utilize their participant match resource after receiving approval for ongoing studies from the University of Delaware (UD)’s Human Subjects Review Board. UD also signed an authorization agreement with the Simons Foundation; after which the first author was given access to version 3 of the SPARK study database.
SPARK Forms and Measures
The SPARK database comprises of multiple parent questionnaires such as the basic medical screening form, individual data form, and background history form. The basic medical screening form includes demographic information, birth history, professional diagnosis of ASD and other disorders, as well as other general medical conditions. The individual data form provides details about the child’s ASD diagnosis, whether a professional provided the diagnoses, whether there is a cognitive impairment, whether there is an Individualized Education Plan (IEP) for the child, and whether the child receives ASD services. The background history form lists the various intervention services received by the child as well as data on cognitive, language, and functional age level of each participant (i.e., above, at, slightly below, or significantly below same-age peers). Table 1 summarizes the type of SPARK study data used for our analysis. Apart from participant information, data from the following 3 parent questionnaires were analyzed: a) the Developmental Coordination Disorder Questionnaire (DCD-Q, Schoemaker et al., 2006), b) the Social Communication Questionnaire – Lifetime (SCQ, Berument et al., 1999), and c) the Repetitive Behaviors Scale – Revised (RBS-R, Lam & Aman, 2006).
Table 1:
Sample size and demographic information for the SPARK sample used in this analysis.
| Sample size | |
|---|---|
| Original dataset | 16,705 (corresponds to the number of completed DCDQ questionnaires) |
| Exclusion | Based on Bhat, 2021 |
| Final dataset | 13,887 [total excluded = 2,818 (16.9%)] |
| Demographics | |
| Gender | Female: 2,717 (19.6%), Male: 11,170 (80.4%) |
| Race | More than one: 1,457 (10.5%), Asian: 237 (1.7%), African American: 662 (4.8%), Native American: 54 (0.39%), Native Hawaiian: 16 (0.12%), White: 10,591 (76.3%), Other: 465 (3.3%), Missing: 405 (2.9%) |
| Ethnicity | Hispanic: 2,297 (16.5%), Not Hispanic: 11,206 (80.7%), Missing: 384 (2.8%) |
| Annual household income [$] | ≤20K: 1,611 (11.6%), 21K–35K: 2,002 (14.4%), 36K–50K: 1,869 (13.5%), 51K–65K: 1,400 (10.1%), 66K–80K: 1,469 (10.6%), 81K–100K: 1,496 (10.8%), 101K–130K: 1,529 (11.0%), 131K–160K: 702 (5.1%), ≥161K: 1,151 (8.3%), Missing: 658 (4.7%) |
| Age A at evaluation [Years] | 5<A≤6: 2,418 (17.4%), 6<A≤7: 1,492 (10.7%), 7<A≤8: 1,457 (10.5%), 8<A≤9: 1,402 (10.1%), 9<A≤10: 1,285 (9.3%), 10<A≤11: 1,220 (8.8%), 11<A≤12: 1,071 (7.7%), 12<A≤13: 1,037 (7.5%), 13<A≤14: 905 (6.5%), 14<A≤15: 857 (6.2%), 15<A<16: 743 (5.4%), Missing: 0 (0.0%) Mean: 9.45, Standard deviation: 3.2 |
| Comorbid diagnosis ψ | ADHD: 5,745 (41.4%), Motor delay or DCD: 2,428 (17.5%), Learning disability: 3,123 (22.5%), Cognitive impairment: 2,073 (14.9%), Language disorders: 6,170 (44.4%) |
| Services received ψ | ASD services: 12,061 (86.9%), IEP for ASD: 11,696 (84.2%), Behavioral / Developmental: 8,886 (64.0%), Speech and language therapy: 11,602 (83.5%), Social skills: 6,452 (46.5%), Occupational therapy (OT): 11,253 (81.0%), Physical therapy (PT): 4,713 (33.9%), Recreational therapies (RT): 1,989 (14.3%) |
Each row is independent, i.e., the percentages do not need to sum to 100.
Inclusion/Exclusion Criteria
Out of the total 150,064 individuals in the SPARK database, which include children with ASD and their family members, parents of 16,705 children with ASD completed the DCDQ form; hence that was our base sample. The final sample included 13,887 children with ASD was obtained after applying various filters already reported in the earlier companion paper, Bhat, 2021. Compared to the sample analyzed in Bhat, 2020, the current study sample includes participants with cognitive /intellectual impairment because one of our questions focuses on how multiple motor dimensions differ in children with ASD as a function of cognitive abilities.
DCD-Q
The DCD-Q is a 15-item parent questionnaire used to screen for gross- and fine-motor performance during everyday functions/play within the child’s natural environment (Schoemaker et al., 2006). The questionnaire includes various motor skills such as ball skills (e.g., hitting or catching a ball), complex body coordination skills (e.g., jumping, running, etc.), fine motor skills (e.g., writing, cutting, etc.), and general motor abilities (e.g., quickness, clumsiness, fatigability, etc.). These skills are categorized into three subscales: control during movement, fine motor coordination, and general coordination. The total final score is the sum of the individual subscale scores. Definite motor impairment or suspect DCD (<10th percentile) is determined based on the final score cutoffs which differ for different age groups. For example, these cutoffs include a score < 47 for children between 5 years to < 8 years, a score below 56 for children between 8 years to < 10 years, and a score < 58 for children between 10–15 years. Based on these criteria, an assignment of risk for DCD (1 = Yes, 0 = No) is provided for each participant. A DCD diagnosis is typically confirmed with a follow-up, standardized motor assessment, and clinical judgment of a trained movement clinician. Note that the positive predictive value of the DCD-Q with a clinical motor assessment such as the Movement ABC is 80–92% (i.e., 80–92% of the children who are at-risk for a motor impairment using the DCD-Q are likely to perform poorly on the M-ABC motor measure) (Green et al., 2009; van Damme, Vancampfort, Thoen, Sanchez, Biesen, 2021). The risk for DCD/motor impairment in this sample has already been reported in previous papers - Bhat 2020, 2021. This paper focuses on the various DCD-Q subdomain scores and the effect sizes for differences between subgroups based on various core and comorbid impairments as well as associations between DCD-Q subdomain scores and other impairments.
SCQ
The SCQ is a common 40-item parent questionnaire (Yes/No format) to screen for autistic traits in children above 4 years of age with a mental age of at least 2 years (Berument et al., 1999). It is based on the popular diagnostic interview, the Autism Diagnostic Interview-Revised (ADI-R). The SCQ has two versions – Lifetime (used to support a diagnosis) and - Current (used to support an evaluation of current difficulties). The Lifetime version provides a total SCQ score. If the total score is >12, it indicates a social communication delay and higher likelihood to be on the autism spectrum. The cut-off of 12 is a research recommended, sensitive cut-off and was implemented in this study (Lee et al., 2010; Daniels et al., 2012; Zwaigenbaum et al., 2015; Marvin et al., 2017). The total SCQ score was also used as a measure of social communication impairment.
RBS-R
The RBS-R is a 43-item parent report measure to characterize the repetitive behaviors of children with ASDs. It has high internal consistency and medium reliability (Lam & Aman, 2006). Each item/question is scored on a 4-point scale: 0 (no such behavior), 1 (mild problem), 2 (moderate problem), and 3 (severe problem), therefore the total score ranges between 0 and 129 with higher score indicating more repetitive behavior. It has six subscales on the child’s stereotyped (I), self-injurious (II), compulsive behaviors (III), ritualistic (IV), sameness behaviors (V), and restricted interests (VI). The total RBS score was use as a measure of repetitive behavior severity.
Subgrouping Analysis
As reported in Bhat, 2021, the sample was divided into 5 subgroups/categories (C) based on scoring ranges defined for SCQ scores, RBS-R scores, and DCD-Q scores using each measure’s sample mean and standard deviations (see grouping details in Bhat, 2021 and Table S1 in supplementary materials). Subgrouping categories included very low (C1), low (C2), high (C3), very high (C4), and extremely high (C5) for both social communication impairments (SCI) or repetitive behavior (RB) severity. Missing data for scores was only 0.6% for the RBS-R scores, but no data was missing for the DCD-Q and SCQ scores because presence of valid DCD-Q and SCQ scores was a key inclusion criterion.
Data extracted from parent reported levels of cognitive, functional, and language delays are presented in Table S1. As reported in Bhat, 2021, the parent-reported outcome data were divided into 3 subgroups based on the reported level of cognitive, functional, and language delay compared to peers (i.e., at or above (C1), slightly below (C2), or significantly below (C3) peers). ~5% cognitive, ~3% functional, and ~3% language delay information was missing for the present sample.
Demographic Information
Key demographic information for this sample is a presented in Table 1. The sample had ~80% males and ~20% females. In terms of race, ~76% were White, ~10% were multi-racial, and ~5% were African American. In terms of ethnicity, ~16% were Hispanic and ~81% were non-Hispanic. The sample was generally evenly distributed in terms of annual household income. Note that certain items were not reported, hence those participants were labeled as having missing data (up to ~5% of the sample). As reported in Bhat, 2021 and also shown in Table S1, 88.2% of children had a risk for motor impairment based on their DCD-Q performance. Many children held formal comorbid diagnoses – specifically, ~41% had ADHD, ~17% had motor delay or DCD, ~22% had learning disability, ~15% had cognitive impairment, and ~44% had language disorders. In terms of services received, ~87% received ASD services, ~84% had an IEP for ASD, ~64% received behavioral or developmental services, ~83% received speech and language therapy services, ~81% received OT services, ~46% received social skills training, only ~34% received PT services, and only ~14% received recreational therapies.
Statistical Analysis
Statistical analyses were conducted using JMP Pro 15.0 (JMP, Inc). The original factor analysis (FA) of the DCD-Q measure by Rivard et al. (2012) provided 3 standard subdomains (CDM – Control During Movement, FM – Fine Motor, and GC – General Coordination). However, to identify unique patterns of motor impairment in children with ASD, an FA of the SPARK ASD sample was conducted using the maximum likelihood estimation and Varimax orthogonal rotation method. This method determines where each DCD-Q question/item most likely belongs in terms of a factor/subdomain. Additional DCD-Q subdomains were identified compared to the subdomains determined from the original FA (Rivard et al., 2012).
Internal consistency (reliability) or degree of homogeneity among the 15 DCD-Q questions/items was also evaluated by calculating Cronbach’s alpha for the whole sample. The criterion for sufficient homogeneity among the items was set to 0.7 (Bland and Altman, 1997). If deletion of an item results in significantly higher alpha, that item is problematic for the measure and perhaps could be removed to consolidate the tool. On the other hand, if deletion of an item results in significantly lower alpha, that item is very important for the measure, and hence must be preserved.
Spearman rank correlations were calculated between the DCD-Q subdomains and subgroup assignments based on SCQ, RBS-R scores (1 to 5) or language, cognitive, and functional delay levels (1 to 3). Ordinal logistic regression analyses were used to predict subgroup assignment (i.e., subgroup 1 to 5 based on SCI and RB severity or subgroup 1 to 3 based on language, cognitive, and functional delays). For each regression model, age and sex effects were also included and accounted for. Four different models were analyzed for each type of subgroup assignment based on SCI, RB severity and the 3 delay types (20 models in total) using total scores, original subdomain scores, FA-based subdomain scores, and item-level DCD-Q scores as predictors. The Wald’s chi-squared test values are reported for predictors that significantly contributed to a given model.
One-way Analysis of Variances (ANOVAs) comparing DCD-Q scores across subdomains (standard and FA-based) between categories of SCI and RB severity as well as language, cognitive, and functional delays were conducted. Statistically significant differences are being reported based on significant Tukey post-hoc testing along with confidence intervals that do not include a zero value.
For all analyses, statistical significance was modified based on p-value thresholds set after Bonferroni corrections. The magnitude of group differences between the extreme categories (C1 vs. C5 or C1 vs. C3) based on SCI, RB, Cognitive delay, Functional delay, or Language delay was calculated using the Cohen’s d effect size estimate (small effect: <0.5, medium: >0.5 but <0.8, large: ≥0.8). This helped in understanding which DCD-Q items / standard subdomains / FA-based subdomains / most explained the variation in the SPARK ASD sample based on core (SCI / RB variations) or general/comorbid (cognitive, language, functional) impairments.
Results
Factor Analysis
Based on factor analyses of the DCD-Q conducted in the general population (Rivard et al., 2012), DCD-Q items were grouped into three standard subdomains – Control During Movement (CDM – Items/Questions Q01 to Q06), Fine Motor (FM – Q07 to Q10), and General Coordination (GC – Q11 to Q15). In the present study, FA of the SPARK ASD sample was carried out with 3 to 7 factors. A 5-factor solution was chosen because it explained an optimal amount of variance (58%, Table 2). With 3 or 4 factors, more items belonged to each of the factors, but the explained variance was lower (~52% and ~55%, respectively). With 6 or 7 factors, only slightly more variance was explained as compared to 5 factors (59.5% and 60%, respectively), but none of the DCD-Q items belonged to these extra factors. Therefore, the 5-factor solution was considered ideal.
Table 2:
Factor analysis to assess the factor structure based on maximum likelihood method. For each question, shown value indicates the factor to which the question most likely belongs. Order of these factors is based on the percent variance explained by each factor and is therefore different from the factors shown in the other tables. For example, Factor 1 is similar to the Fine Motor (FM) subdomain. Factor 2 is similar to the Control During Movement 1 (CDM1) subdomain. Factor 3 is similar to the Control During Movement 2 (CDM2) subdomain. Factor 4 is similar to the General Coordination 1 (GC1) subdomain. Factor 5 is similar to the General Coordination 2 (GC2) subdomain. Note that Q13 could belong in any of the Factors 1, 4, or 5.
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | |
|---|---|---|---|---|---|
| Variance explained [%] | 18.9 | 15.1 | 11.3 | 6.7 | 6.2 |
| DCD-Q Items | |||||
| Q1. Throw | 0.69 | ||||
| Q2. Catches | 0.84 | ||||
| Q3. Hits | 0.70 | ||||
| Q4. Jumps | 0.69 | ||||
| Q5. Runs | 0.74 | ||||
| Q6. Plans | 0.45 | ||||
| Q7. Writes fast | 0.75 | ||||
| Q8. Writes legibly | 0.85 | ||||
| Q9. Effort pressure | 0.76 | ||||
| Q10. Cuts | 0.68 | ||||
| Q11. Likes sports | 0.42 | ||||
| Q12. Learning new | 0.65 | ||||
| Q13. Quick competent | 0.33 | 0.33 | 0.31 | ||
| Q14. Bull in a china shop | 0.59 | ||||
| Q15. Fatigues easily | 0.54 | ||||
Items Q01 to Q03 (Throw, Catches, Hits) loaded cleanly on Factor 2, which was termed Control During Movement 1 (CDM1) or visuomotor items. Items Q04 to Q06 (Jumps, Runs, Plans) loaded cleanly on Factor 3, which was termed Control During Movement 2 (CDM2) or multilimb coordination/planning items. Items Q07 to Q10 (Writes fast, Writes legibly, Effort pressure, Cuts) loaded cleanly on Factor 1, which were the Fine Motor (FM) items in the original DCD-Q as well. Items Q11 to Q12 (Likes sports, Learning new) loaded cleanly on Factor 5, which was termed General Coordination 1 (GC1). Items Q14 to Q15 (Bull in a china shop, Fatigues easily) loaded cleanly on Factor 4, which was termed General Coordination 2 (GC2). Item Q13 (Quick competent) showed factorial complexity, with about equal factor loadings on Factors 1 (FM), 4 (GC1), and 5 (GC2); hence for simplicity, it was assigned to GC1. Overall, Q01-Q03 / visuomotor items and Q04-Q06 / multilimb coordination/planning items belonged to two distinct subdomains, indicating that they might be able to capture distinct information in the SPARK ASD sample. Correlations among items are shown in Table S2 and support this observation, where Q01-Q03 correlate more with each other compared to Q04-Q06, and vice versa.
Visuomotor, multilimb coordination/planning, fine-motor, and general coordination scores worsen with and explain variations in core impairments (SCI and RB severity)
Bhat (2021) reported the variation in DCD-Q total score and standard subdomain scores (CDM, FM, and GC) as a function of core impairments (SCI and RB severity, shown in Table S3–S6 of supplementary materials). Similar analysis is now extended to the FA-based subdomains (CDM1, CDM2, FM, GC1, and GC2). Note that the standard FM subdomain is identical to the FA-based FM subdomain. As shown in Figure 1, the new FA-based subdomain DCD-Q scores (CDM1, CDM2, GC1, and GC2) worsened across subgroups with increasing SCI and RB severity (p-values < 0.001, Tables S7–S10). Certain subgroup comparisons were an exception to these findings (1 CDM1-SCI, 1 CDM1-RB severity, 1 CDM2-SCI, 1 CDM2-RB severity, 1 GC1-SCI, 2 for GC1-RB severity, 2 for GC2-SCI, and 1 for GC2-RB severity, see details in the footnotes of Tables S7–S10).
Figure 1:
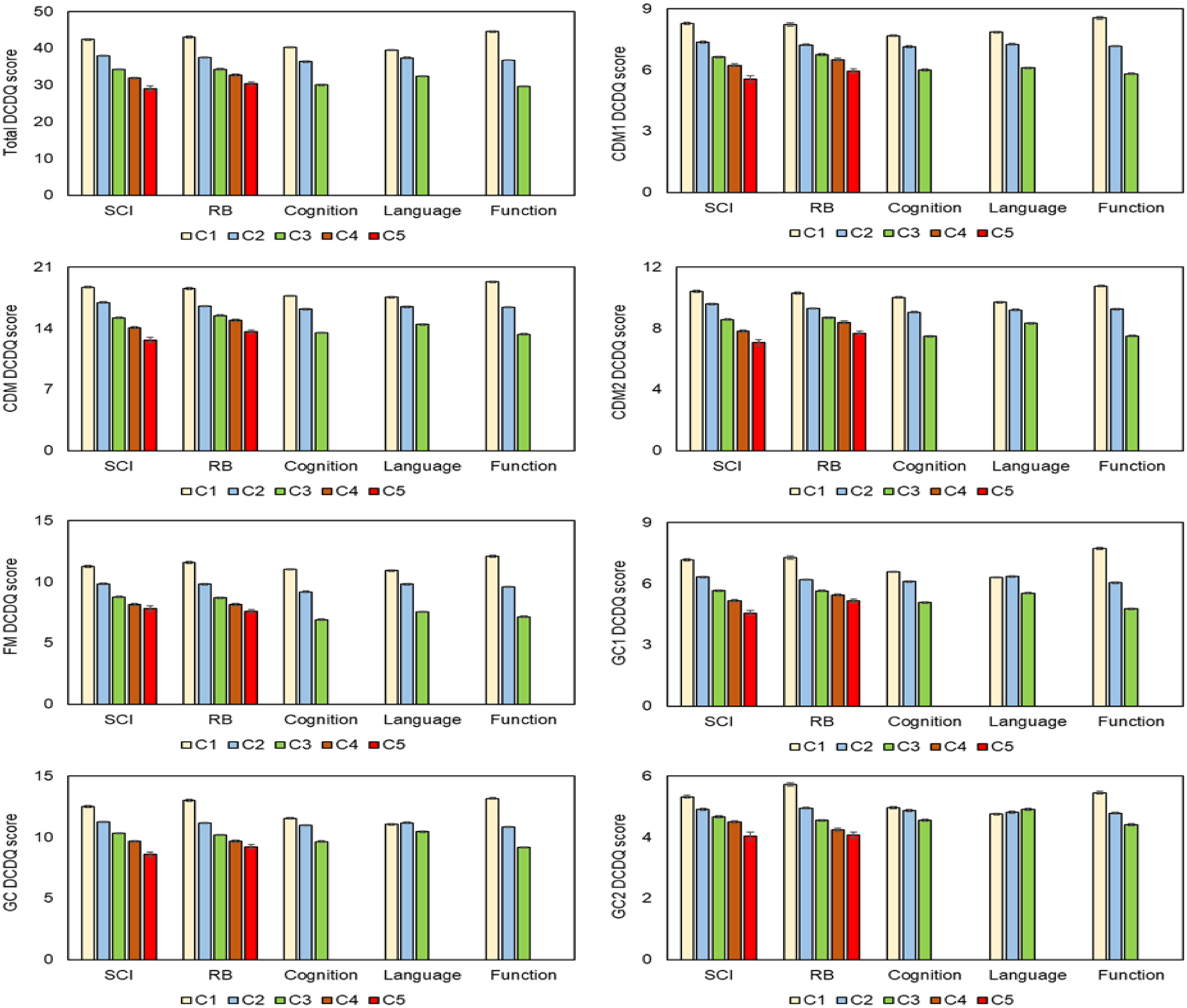
DCD-Q total, standard subdomain (CDM, FM, GC), and factor analysis-based subdomain (CDM1, CDM2, FM, GC1, GC2) scores as a function of SCI, RB severity, cognitive, language, and functional impairment categories (C1 to C5) in children with ASD. Note that FM subplot is the same for the standard subdomains and the factor analysis-based subdomains. The total and standard subdomain subplots are taken from Bhat, 2021. CDM: Control During Movement, FM: Fine Motor, GC: General Coordination.
Bhat (2021) had also reported DCD-Q vs. impairment category correlations for DCD-Q total scores only. Additional correlations between SCQ / RBS-R scores and DCD-Q subdomain scores (standard and FA-based) as well as DCD-Q item-level correlations highlight which subdomains / items best correlate with core impairments in ASD (Table 3). The standard subdomain CDM and the FA-based subdomains CDM1/visuomotor and CDM2/multilimb coordination/planning moderately correlated with SCI (r = −0.24 to −0.28, ps < 0.0001); however, they correlated less with RB severity (r = −0.17 to −0.19, ps < 0.0001). Instead, the standard FM and GC subdomains moderately correlated with RB severity (r = −0.22 to −0.23, ps < 0.0001).
Table 3:
Correlations across DCD-Q subdomains/items, measure-based impairment categories (SCI and RB severity), and parent-reported delay categories (Cognitive, Language, and Functional delays).
| DCD score type | SCI category | RB category | Cognitive delay category | Language delay category | Functional delay category |
|---|---|---|---|---|---|
| Correlations using the total DCD-Q score | |||||
| Total (Q01 to Q15) | −0.30 | −0.25 | −0.34 | −0.25 | −0.44 |
| Correlations using the standard subdomains (CDM, FM, GC) | |||||
| CDM (Q01 to Q06) | −0.28 | −0.19 | −0.29 | −0.22 | −0.36 |
| FM (Q07 to Q10) | −0.24 | −0.23 | −0.39 | −0.34 | −0.40 |
| GC (Q11 to Q15) | −0.23 | −0.22 | −0.17 | −0.05 | −0.33 |
| Correlations using the factor analysis-based subdomains (CDM1, CDM2, FM, GC1, GC2) | |||||
| CDM1 (Q01 to Q03) | −0.24 | −0.17 | −0.22 | −0.24 | −0.32 |
| CDM2 (Q04 to Q06) | −0.26 | −0.17 | −0.29 | −0.16 | −0.33 |
| FM (Q07 to Q10) | −0.24 | −0.23 | −0.39 | −0.34 | −0.40 |
| GC1 (Q11 to Q13) | −0.26 | −0.19 | −0.22 | −0.12 | −0.38 |
| GC2 (Q14 to Q15) | −0.13 | −0.19 | −0.07 | 0.03 | −0.16 |
| Correlations using the DCD-Q items | |||||
| Q01. Throw | −0.21 | −0.12 | −0.18 | −0.17 | −0.28 |
| Q02. Catches | −0.20 | −0.17 | −0.18 | −0.20 | −0.27 |
| Q03. Hits | −0.22 | −0.16 | −0.21 | −0.26 | −0.29 |
| Q04. Jumps | −0.21 | −0.16 | −0.21 | −0.10 | −0.27 |
| Q05. Runs | −0.20 | −0.12 | −0.16 | −0.03 | −0.23 |
| Q06. Plans | −0.25 | −0.16 | −0.36 | −0.28 | −0.35 |
| Q07. Writes fast | −0.23 | −0.19 | −0.36 | −0.28 | −0.35 |
| Q08. Writes legibly | −0.18 | −0.17 | −0.33 | −0.28 | −0.34 |
| Q09. Effort pressure | −0.22 | −0.25 | −0.29 | −0.24 | −0.32 |
| Q10. Cuts | −0.21 | −0.20 | −0.34 | −0.33 | −0.37 |
| Q11. Likes sports | −0.22 | −0.11 | −0.15 | −0.07 | −0.24 |
| Q12. Learning new | −0.20 | −0.16 | −0.19 | −0.11 | −0.28 |
| Q13. Quick competent | −0.19 | −0.22 | −0.17 | −0.08 | −0.37 |
| Q14. Bull in a china shop | −0.13 | −0.19 | −0.09 | −0.05 | −0.17 |
| Q15. Fatigues easily | −0.09 | −0.14 | −0.04 | 0.07 | −0.11 |
SCI: Social Communication Impairment, RB = Repetitive Behaviors, CDM: Control During Movement, FM: Fine Motor, GC: General Coordination. All correlations are statistically significant with a p-value of 0.0023 (after Bonferroni correction) or lower. Correlation values ≥0.2 or ≤−0.2 are highlighted to show important relations.
Ordinal logistic regression analyses were used to predict subgroup assignment based on SCI and RB severity using age, sex, and DCD-Q total, original subdomain, FA-based subdomain, and item-level scores as predictors. Wald chi-squared test shows the contributions of significant predictors in Table 4. For the SCI categories model using standard subdomain score predictors, in the order of most to least importance, child’s age, CDM, FM, and GC DCD-Q scores were significant contributors to the model. For the SCI categories model using FA-based subdomain score predictors, in the order of most to least importance, child’s age, FM, CDM1, CDM2, and GC1 DCD-Q scores were significant contributors to the model. For the SCI categories model using item score predictors, child’s age as well as 9 out of 15 items including Q06/plans, Q03/hits ball, and Q09/writing effort were significant contributors to the model. For the RB categories model, using standard subdomain score predictors, in the order of most to least importance, FM, GC, child’s age, and CDM DCD-Q scores were significant contributors to the model. For the RB categories model using FA-based subdomain score predictors, in the order of most to least importance, FM, GC2, child’s age, GC1, and CDM2 DCD-Q scores were significant contributors to the model. For the RB categories model using item score predictors, child’s age, and 10 out of 15 items including Q09/writing effort, Q13/quick competent, and Q14/bull in china shop were significant contributors to the model.Effect sizes for differences across categories based on SCI and RB severity are reported in Table 5 (C1 vs. C5), Table S11 (for all category pairs), and are also plotted in Figures 2 and 3 for visual comparisons. When examining effect sizes for DCD-Q total scores for extreme categories (C1 vs. C5) based on SCI and RB severity, the differences had large effects. Additionally, effect sizes for the standard subdomain scores across extreme categories were large for the following pairs: (CDM – SCI, GC – SCI, CDM – RB, FM – RB, GC – RB) and moderate for FM – SCI along with no small effects. When examining effect sizes for the FA-based subdomain scores across extreme categories, large effects were seen for the following pairs: (CDM1 – SCI, CDM2 – SCI, GC1 – SCI, FM – RB); moderate effects seen for the following pairs: (FM – SCI, GC2 – SCI, CDM1 – RB, CDM2 – RB, GC1 – RB, GC2 - RB), and no small effects were seen.
Table 4:
Wald chi-square statistic to predict the impairment categories based on SCI and RB severity as well as Cognitive, Language, and Functional delay using age, sex, and DCD-Q total/subdomains/item scores within ordinal logistic regression analyses. Predictor significance is based on FDR-corrected p-values.
| DCD score type | SCI category | RB category | Cognitive delay category | Language delay category | Functional delay category |
|---|---|---|---|---|---|
| N | 13,887 | 13,803 | 13,197 | 13,423 | 13,449 |
| Predictions using age, sex, and total DCD-Q score | |||||
| Age | 343.3** | 64.7** | 78.5** | 744.1** | |
| Sex | 53.7** | 9.1** | |||
| Total (Q01 to Q15) | 1368.5** | 941.8** | 1467.3** | 745.3** | 2501.9** |
| Predictions using age, sex, and the standard subdomains (CDM, FM, GC) | |||||
| Age | 358.4** | 60.7** | 171.3** | 584.8** | |
| Sex | 85.7** | 5.1* | 15.8** | ||
| CDM (Q01 to Q06) | 268.6** | 4.3* | 225.4** | 306.7** | 238.9** |
| FM (Q07 to Q10) | 199.3** | 214.4** | 1170.6** | 859.5** | 744.2** |
| GC (Q11 to Q15) | 28.2** | 205.2** | 73.5** | 335.0** | 116.1** |
| Predictions using age, sex, and factor analysis-based subdomains (CDM1, CDM2, FM, GC1, GC2) | |||||
| Age | 349.6** | 59.9 | 112.9** | 515.5** | |
| Sex | 87.0** | 5.7* | 18.7** | ||
| CDM1 (Q01 to Q03) | 95.6** | 107.5** | 17.4** | ||
| CDM2 (Q04 to Q06) | 51.5** | 8.2** | 182.4** | 62.3** | 76.9** |
| FM (Q07 to Q10) | 191.3** | 221.3** | 1130.6** | 841.9** | 689.8** |
| GC1 (Q11 to Q13) | 37.3** | 28.8** | 6.2* | 73.4** | 271.3** |
| GC2 (Q14 to Q15) | 163.1** | 74.4** | 221.0** | 5.7* | |
| Predictions using age, sex, and DCD-Q questions (items) | |||||
| Age | 375.6** | 20.7** | 187.7** | 365.8** | 23.0** |
| Sex | 80.6** | 14.5** | |||
| Q01. Throw | 31.3** | 8.4** | 8.4** | ||
| Q02. Catches | 23.1** | 7.3** | |||
| Q03. Hits | 50.1** | 5.0* | 14.0** | 115.0** | 15.2** |
| Q04. Jumps | 9.3** | 6.6* | 10.4** | ||
| Q05. Runs | 45.6** | 35.4** | |||
| Q06. Plans | 125.2** | 6.6* | 544.7** | 462.7** | 189.4** |
| Q07. Writes fast | 28.5** | 204.0** | 115.4** | 42.6** | |
| Q08. Writes legibly | 9.8** | 20.1** | 26.4** | 10.5** | 14.1** |
| Q09. Effort pressure | 35.5** | 178.4** | |||
| Q10. Cuts | 10.4** | 103.4** | 185.3** | 107.6** | |
| Q11. Likes sports | 22.0** | 6.4* | |||
| Q12. Learning new | 13.4** | ||||
| Q13. Quick competent | 26.4** | 101.1** | 6.7* | 72.5** | 512.1** |
| Q14. Bull in a china shop | 8.3** | 62.9** | 7.3** | ||
| Q15. Fatigues easily | 33.4** | 27.3** | 155.5** | 10.8** | |
Note that **indicates p < 0.007, *indicates p<0.05). SCI: Social Communication Impairment, RB = Repetitive Behaviors, CDM: Control During Movement, FM: Fine Motor, GC: General Coordination, FDR: False Discovery Rate.
Table 5:
Subgroup effect sizes across DCD-Q subdomains/items, measures-based impairment categories (SCI and RB severity), and parent-reported delay categories (Cognitive, Language, and Functional delays).
| DCD score type | SCI category | RB category | Cognitive delay category | Language delay category | Functional delay category |
|---|---|---|---|---|---|
| Effect sizes using the total DCD-Q score | |||||
| Total (Q01 to Q15) | 1.11 | 1.01 | 0.89 | 0.60 | 1.36 |
| Effect sizes using the standard subdomains (CDM, FM, GC) | |||||
| CDM (Q01 to Q06) | 1.07 | 0.83 | 0.75 | 0.54 | 1.08 |
| FM (Q07 to Q10) | 0.76 | 0.88 | 0.99 | 0.80 | 1.22 |
| GC (Q11 to Q15) | 0.88 | 0.84 | 0.46 | 0.15 | 1.01 |
| Effect sizes using the factor analysis-based subdomains (CDM1, CDM2, FM, GC1, GC2) | |||||
| CDM1 (Q01 to Q03) | 0.86 | 0.71 | 0.54 | 0.57 | 0.91 |
| CDM2 (Q04 to Q06) | 1.02 | 0.77 | 0.77 | 0.39 | 0.99 |
| FM (Q07 to Q10) | 0.76 | 0.88 | 0.99 | 0.80 | 1.22 |
| GC1 (Q11 to Q13) | 0.90 | 0.71 | 0.55 | 0.28 | 1.14 |
| GC2 (Q14 to Q15) | 0.55 | 0.70 | 0.18 | −0.07 | 0.46 |
| Effect sizes using the DCD-Q items | |||||
| Q01. Throw | 0.84 | 0.51 | 0.48 | 0.42 | 0.79 |
| Q02. Catches | 0.72 | 0.72 | 0.45 | 0.47 | 0.79 |
| Q03. Hits | 0.73 | 0.68 | 0.51 | 0.62 | 0.82 |
| Q04. Jumps | 0.82 | 0.70 | 0.56 | 0.25 | 0.77 |
| Q05. Runs | 0.79 | 0.51 | 0.40 | 0.06 | 0.65 |
| Q06. Plans | 0.97 | 0.75 | 0.98 | 0.71 | 1.05 |
| Q07. Writes fast | 0.70 | 0.66 | 0.89 | 0.67 | 1.05 |
| Q08. Writes legibly | 0.46 | 0.66 | 0.83 | 0.66 | 0.99 |
| Q09. Effort pressure | 0.69 | 0.90 | 0.74 | 0.56 | 0.94 |
| Q10. Cuts | 0.76 | 0.82 | 0.86 | 0.80 | 1.13 |
| Q11. Likes sports | 0.81 | 0.42 | 0.41 | 0.20 | 0.70 |
| Q12. Learning new | 0.68 | 0.55 | 0.46 | 0.25 | 0.82 |
| Q13. Quick competent | 0.63 | 0.77 | 0.42 | 0.21 | 1.14 |
| Q14. Bull in a china shop | 0.51 | 0.64 | 0.20 | 0.09 | 0.45 |
| Q15. Fatigues easily | 0.42 | 0.54 | 0.10 | −0.19 | 0.30 |
SCI: Social Communication Impairment, RB = Repetitive Behaviors, CDM: Control During Movement, FM: Fine Motor, GC: General Coordination. Effect sizes ≥0.8 or ≤−0.8 (large) are highlighted in green and 0.5 to 0.8 or −0.5 to −0.8 (moderate) are highlighted in gold to show how motor performance (DCD-Q) distinguishes the extreme categories based on other impairments/delays.
Figure 2:
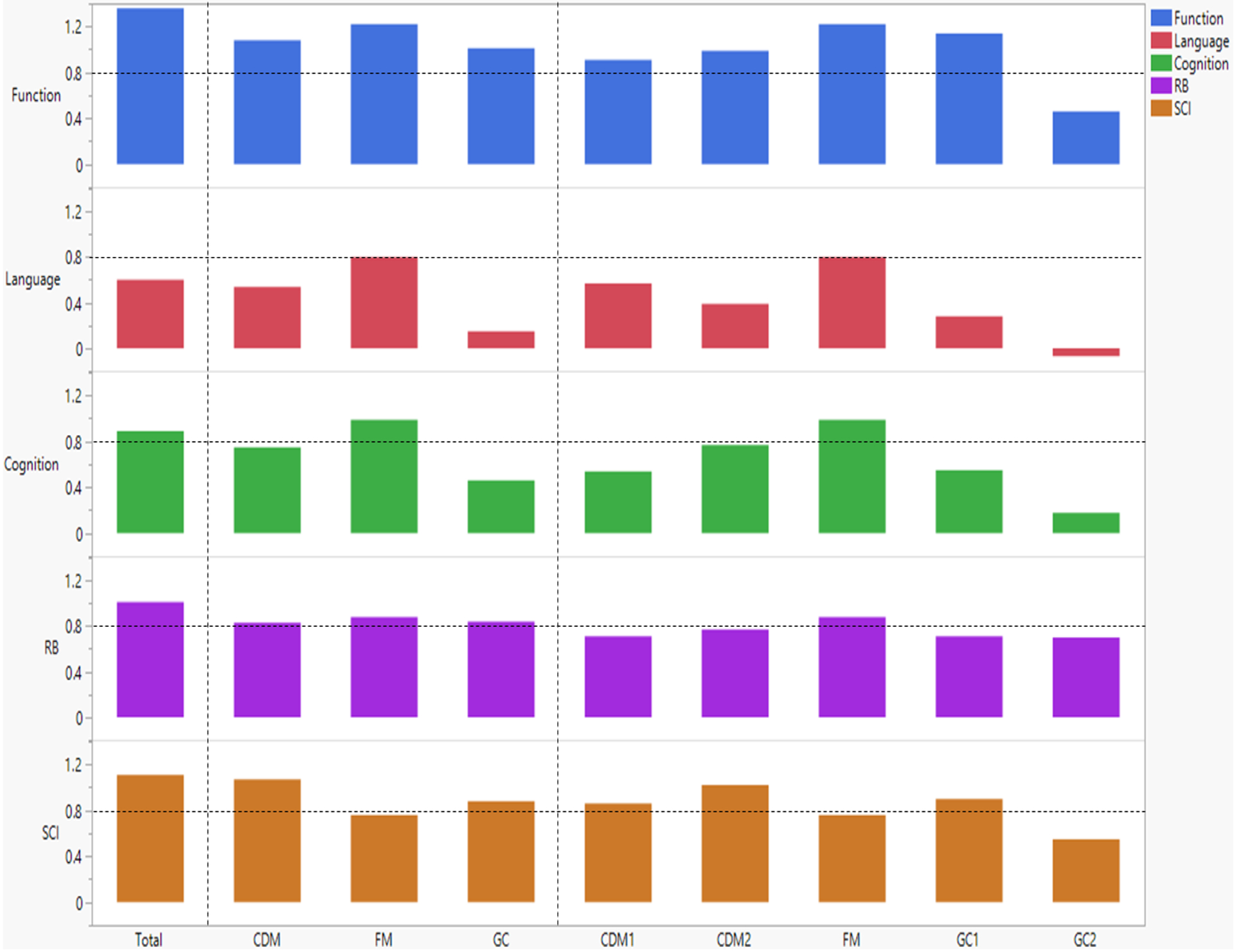
Effect sizes for DCD-Q total score, standard subdomains (CDM, FM, GC), and factor analysis-based subdomains (CDM1, CDM2, FM, GC1, GC2). Horizontal dashed lines are added to illustrate high effect sizes (≥0.8 or ≤−0.8). CDM: Control During Movement, FM: Fine Motor, GC: General Coordination.
Figure 3:
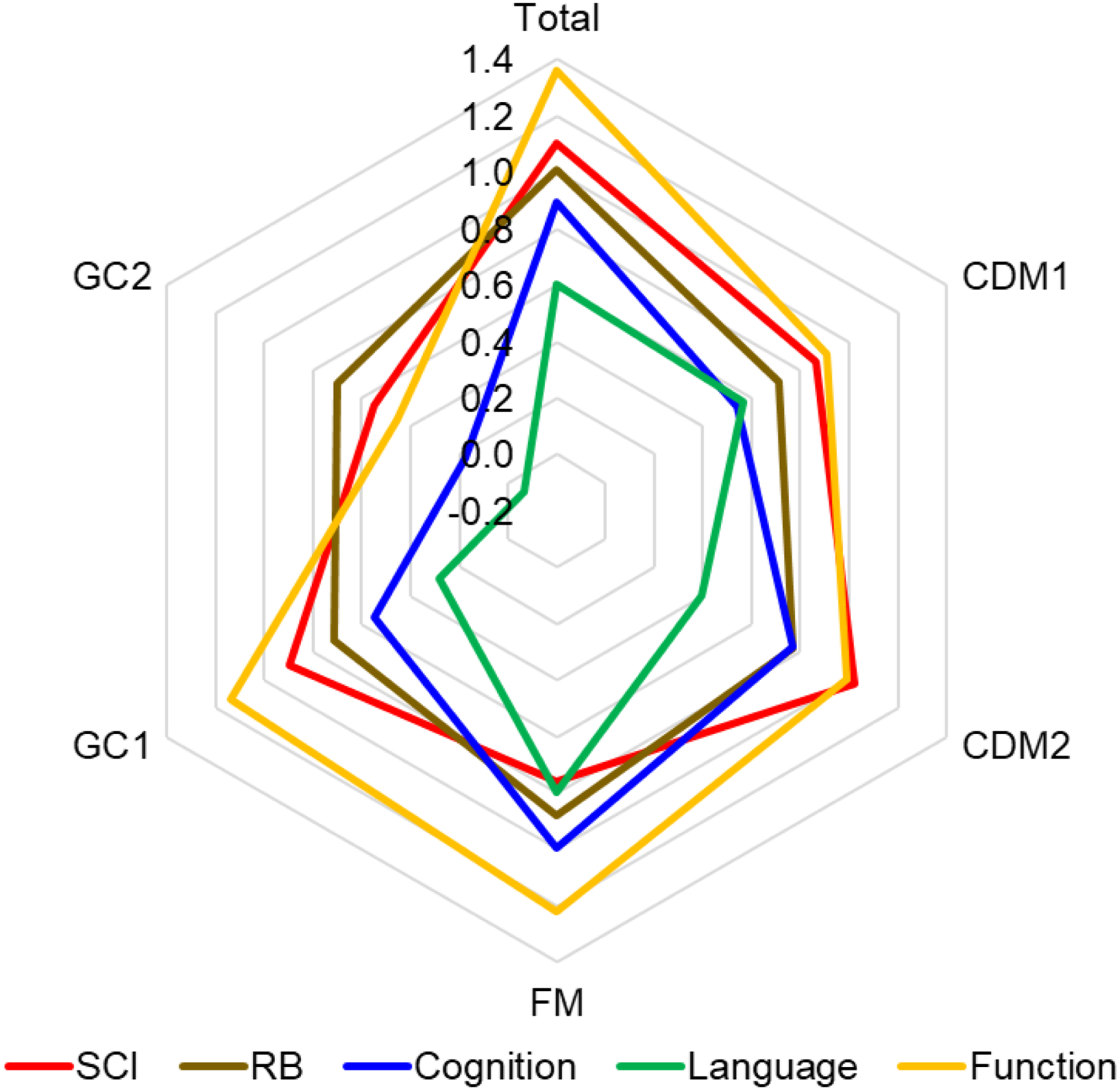
Radar chart showing the effect sizes for DCD-Q total score and factor analysis-based subdomains (CDM1, CDM2, FM, GC1, GC2) to compare effects across impairments/delays. CDM: Control During Movement, FM: Fine Motor, GC: General Coordination.
Similar effect size analyses were also conducted for the 15 individual items as shown in Table 5 (C1 vs. C5), Table S11 (for all category pairs) and are also plotted in Figures S1 and S2 for visual comparisons. When examining effect sizes for individual items across extreme categories, large effects were seen for the following pairs: Q01 (throws), Q04 (jumps), Q06 (plans), and Q11 (likes sports) – SCI, Q09 (writing effort) and Q10 (cuts) – RB. Small effects were seen for Q08 (write legibly) and Q15 (fatigues easily) – SCI and Q11 (likes sports) – RB. Moderate effects were seen for all the remaining item – SCI/RB impairment pairs. Overall, 3 gross-motor items (Q01/throw, Q04/jumps, Q06/plans) and 1 general coordination (GC/GC1) item (Q11/likes sports) explained SCI variations with large effects whereas two fine-motor items explained RB variations with large effects (Q09/writing effort, Q10/cuts). Together, these findings revealed that gross-motor, followed by fine-motor, and certain general coordination skills were associated with, predictive of and better explained SCI variations and fine-motor and certain general coordination skills were slightly more associated with, predictive of, and better explained variations in RB severity.
Visuomotor, multilimb coordination/planning, fine-motor, and general coordination scores worsen with and explain variations in general/comorbid impairments (cognitive, language, and functional delays)
Bhat (2021) reported the variation in DCD-Q total score and standard subdomain scores (CDM, FM, and GC) as a function of comorbid impairments (cognitive, language, and functional delays, Tables S3–S6). Similar analysis is now extended to the FA-based subdomains (CDM1, CDM2, FM, GC1, and GC2). Note that the standard FM subdomain is identical to the FA-based FM subdomain. As shown in Figure 1, the new FA-based subdomain DCD-Q scores (CDM1, CDM2, GC1, and GC2) worsened with increasing cognitive, language and functional delay (p-values < 0.0021, Tables S7–S10). There were 4 exceptions to this trend (GC1-language delay, 1 for GC2-cognitive delay, and 2 for GC2-language delay, see details in the footnotes of Tables S9–S10).
Bhat (2021) reported DCD-Q vs. impairment category correlations for DCD-Q total scores only. Additional correlations between cognitive, language and functional delay categories and DCD-Q subdomain scores (standard and FA-based) and DCD-Q items were conducted to highlight which subdomains / items best correlate with general/comorbid impairments (Table 3). The standard subdomain CDM and the FA-based subdomains CDM1/visuomotor and CDM2/multilimb coordination/planning moderately correlated with cognitive delay (r = −0.22 to −0.29, ps < 0.0001) and functional delay (r = −0.32 to −0.36, ps < 0.0001). CDM and CDM1 moderately correlated with language delay (r = −0.22 to −0.24, p < 0.0001); however, CDM2 correlated less with language delay (r = −0.16, p < 0.0001). The FM subdomain is moderately associated with all 3 general/comorbid impairments (r = −0.34 to −0.40, ps < 0.0001). The standard GC subdomain only moderately correlated with functional delay (r = −0.33, p < 0.0001) and less with cognitive or language delays (r = −0.05 to −0.17, ps < 0.0001). GC1 subdomain moderately correlated with functional delay (r = −0.33, p < 0.0001) and cognitive delay (r = −0.22, p < 0.0001) but less with language delay (r = −0.12, p < 0.0001). GC2 items correlated less with the general/comorbid impairments (r = 0.03 to −0.16, cognitive p < 0.0001, language p = 0.0023, functional p < 0.0001).
Ordinal logistic regression analyses were used to predict subgroup assignment based on cognitive, language, and functional delays using age, sex, and DCD-Q total, original subdomain, FA-based subdomain, and item-level scores as predictors. Wald chi-squared test shows the contributions of significant predictors in Table 4. For the cognitive delay model using standard subdomain score predictors, in the order of most to least importance, FM, CDM, age, sex and GC DCD-Q scores were significant contributors to the model. For the cognitive delay model using FA-based subdomain score predictors, in the order of most to least importance, FM, CDM2, age, sex, and GC2, and GC1 DCD-Q scores were significant contributors to the model. For the cognitive delay model using item score predictors, child’s age and sex as well as 10 out of 15 items including Q06/plans, Q07/writes fast, and Q10/cuts were significant contributors to the model. For the language delay model using standard subdomain score predictors, in the order of most to least importance, FM, age, GC, and CDM DCD-Q scores were significant contributors to the model. For the language delay model using FA-based subdomain score predictors, in the order of most to least importance, FM, age, GC2, CDM1, GC1, and lastly CDM2 DCD-Q scores were significant contributors to the model. For the language delay model using item score predictors, child’s age as well as 12 out of 15 items including Q06/plans, Q07/writes fast, and Q10/cuts were significant contributors to the model. For the functional delay model using standard subdomain score predictors, in the order of most to least importance FM, CDM, and GC DCD-Q scores were significant contributors to the model. Notably, age was not a contributor to this model. For the functional delay model using FA-based subdomain score predictors, in the order of most to least importance, child’s age, FM, GC1, CDM2, CDM1, and GC2 DCD-Q scores were significant contributors to the model. For the functional delay model using item score predictors, child’s age, sex as well as 9 out of 15 items including Q13/quick and competent, Q07/writes fast, and Q10/cuts were significant contributors to the model.Effect sizes for differences across extreme categories based on cognitive, language, and functional delays are reported in Table 5 (C1 vs. C3), Table S11 (all category pairs) and are also plotted in Figures 2 and 3 for visual comparisons. Effects sizes for DCD-Q total scores across extreme categories were large for cognitive and functional delays, but moderate for language delay. Effect sizes for the standard subdomain scores across extreme categories were large for the following pairs: (FM – Cognitive, FM – Language, CDM – Functional, FM – Functional, and GC - Functional); moderate for the following pairs: (CDM – Cognitive and CDM – Language), and small for the following pairs: (GC – Cognitive and GC – Language). When examining effect sizes for FA-based subdomains across extreme categories, large effects were observed for the following pairs: (FM – Cognitive, FM – Language, CDM1 – Functional, CDM2 – Functional, FM – Functional, and GC1 - Functional); moderate effects for the following pairs: (CDM1 – Cognitive, CDM2 – Cognitive, GC1 – Cognitive, and CDM1 – Language), and small effects were seen for the following pairs: (GC2 – Cognitive, CDM2 – Language, GC1 – Language, GC2 – Language, and GC2 – Functional).
For individual DCD-Q items, large effects were observed for the following pairs: (Q06, Q07, Q08, Q10 – Cognitive, Q10 – Language, Q03, Q06, Q07, Q08, Q09, Q10, Q12, Q13 – Functional), moderate effects for the following pairs: (Q03, Q04, Q09 – Cognitive, Q03, Q06, Q07, Q08, Q09 – Language, Q01, Q02, Q04, Q5, Q11 – Functional), and small effects were seen for the following pairs: (Q01, Q02, Q05, Q11, Q12, Q13, Q14, Q15 – Cognitive, Q01, Q02, Q04, Q05, Q11, Q12, Q13, Q14, Q15 – Language, Q14, Q15 – Functional). Overall, fine-motor skills (Q07-Q10/related to writing/cutting) were best associated with, predictive of, and better explained variations in cognitive and language delays. Additionally, multilimb coordination/planning skills (Q03/hits ball, Q04/jumps, and Q06/plans) were also better associated with, predictive of, and better explained variations in cognitive delays. All three types of motor skills (CDM, FM, and GC) were associated with, predictive of, and better explained variations in functional delays (except Q14 and Q15). Finally, GC/GC2 items such as Q14 (Bull in china shop) and Q15 (Fatigues easily) did not correlate well with the 3 comorbid impairments.
Internal Consistency of the DCD-Q
For the entire DCD-Q items set, Cronbach’s alpha coefficient was 0.894 (significantly higher than the criterion of 0.7). Deletion of items Q14 or Q15 resulted in slightly higher alpha (0.895 and 0.897, respectively). Deletion of any of the other items (Q01 to Q13) resulted in slightly lower alpha (0.884 to 0.888). This indicates that Q14 and Q15 are slightly less important compared to the other items, but none of the items are problematic for the measure, and removal of no one item consolidated the total DCD-Q score.
For the standard sub-domains, Cronbach’s alpha coefficients were as follows: 0.858 for CDM, 0.879 for FM and 0.692 for GC. Deletion of items from CDM resulted in minor changes in alpha (range = 0.823 to 0.850, most important item = Q01 throws ball), indicating that the CDM items are fairly homogeneous. Deletion of items from FM resulted in minor changes in alpha values (range = 0.827 to 0.861, most important item = Q08 prints letters), indicating that the FM items are fairly homogeneous. Deletion of items from GC resulted in moderate changes in alpha (range = 0.599 to 0.673, most important item = Q12 learns new skills).
For the FA-based sub-domains, Cronbach’s alpha coefficients were as follows: 0.863 for CDM1, 0.789 for CDM2, 0.879 for FM, 0.691 for GC1, and 0.522 for GC2. The very low value for GC2 indicates that its items (Q14 to Q15) are not homogeneous. Deletion of items from CDM1 resulted in moderate changes in alpha (range = 0.763 to 0.830, most important item = Q02 catches ball). Deletion of items from CDM2 resulted in large changes in alpha (range = 0.640 to 0.800, most important item = Q04 jumps over obstacles). Deletion of items from FM resulted in minor changes in alpha values (range = 0.827 to 0.861, most important item = Q08 prints letters), indicating that FM items are fairly homogeneous. Deletion of items from GC1 resulted in large changes in alpha (range = 0.485 to 0.681, most important item = Q12 learns new skills). Finally, deletion of items from GC2 could not be tested as there are only two questions.
Discussion
The present study is a further analysis of the Developmental Coordination Disorder Questionnaire (DCD-Q) measure obtained from one of the largest ASD cohorts in the US, the SPARK study sample of children with ASD between 5 and 15 years. The earlier companion paper, Bhat (2021), examined the risk of motor impairment and changes in DCD-Q total scores across subcategories of children with ASD with increasing levels of core and comorbid impairments (social communication, repetitive behavior severity, as well as cognitive, language, functional impairments). This paper conducts a multidimensional assessment of motor abilities: visuomotor (CDM1), multilimb coordination/planning (CDM2), fine-motor, and general coordination (GC1 or GC2) to identify which motor impairments are associated with, predictive of, and better explain variations in core and/or comorbid impairments in school-age children with ASD. We conducted factor analysis to identify unique motor impairments observed in the SPARK study sample. Gross-motor including visuomotor (CDM1), multilimb coordination/planning (CDM2), fine-motor (FM), and certain general coordination skills (GC1) were associated with, predictive of, and better explained the variations in social communication impairment in ASD. In contrast, fine-motor (FM) and certain general coordination (GC) skills were slightly better associated with, predictive of, and better explained the variations in repetitive behavior severity in ASD compared to gross-motor skills. In terms of comorbid impairments, gross-motor skills, especially, multilimb coordination/planning (particularly, Q06/plans) and fine-motor skills were associated with, predictive of, and better explained variations in cognitive impairments. Fine-motor and gross, visuomotor skills were most associated with and best explained variations in language delay. Both, fine- and gross-motor (visuomotor and multi-limb coordination/planning) skills and certain general coordination skills (Q11/likes sports, Q12/learns new skills, and Q13/quick/competent) best explained variations in functional delay.
Gross- and fine-motor skills are related to both core impairments in ASD
In the previous paper (Bhat, 2021), general motor performance (using total DCD-Q scores) correlated equally with social communication impairments (using SCQ total scores) as well as repetitive behavior severity (using total RBS-R scores) (Bhat, 2021). However, the present study attempted to distinguish gross- and fine-motor relations with core ASD impairments. Gross-motor (visuomotor (CDM1) and multilimb coordination/planning (CDM2)), fine-motor, and general coordination skills (liking sports, learning new motor skills, and being quick/competent (GC1)) were similarly associated with social communication impairments in the SPARK ASD sample (r=−0.24 to −0.26). Gross-motor and certain general coordination skills were better at explaining variations in social communication impairment (effect size = 0.86–1.02) compared to fine-motor skills (effect size = 0.76). While all three motor skills (CDM, FM, and GC) contributed to the social communication impairment models, gross-motor skills made stronger contributions when predicting social communication impairments after accounting for age and sex effects. Motor delays are one of the earliest developmental markers noted in children who eventually develop ASD or related language delays (Landa & Garrett-Mayer, 2006; Bhat et al., 2012; Flanagan et al., 2012; Sacrey et al., 2015). These delays in turn affect a child’s ability to explore their environment (objects during play) as well as their ability to communicate and interact with caregivers during early learning contexts (Kaur et al., 2015; Bhat et al., 2006, 2007; Srinivasan & Bhat, 2016, 2019, 2020; Iverson et al., 2018a, 2018b). Early social communication acts of head turning, physical approach through walking, reaching, showing, giving, and pointing require motor coordination and postural control (head and trunk control) and are known to be affected in young children with ASD (Nickel et al., 2013; Kaur et al., 2015; Srinivasan & Bhat, 2016). The gap in fine- and gross-motor development increases with development with motor performance in school-age children with ASD being at the level of peers half their age (Lloyd, Macdonald, & Lord, 2013, Staples & Reid, 2010).
In preschool and elementary school years, children spend a lot of time on the playground engaging in small and large group activities to connect with other children, play together, make friends, and build relationships (Bhat et al., 2011). Poor early motor skills and a growing motor gap leads to a lower sense of self-worth and poor perceived competence in children with motor problems (Piek, Baynam, & Barrett, 2006). This could further escalate the lack of social connectedness in children with ASD due to reduced movement play and lack of opportunity to build friendships. These findings join other smaller studies on relations between motor skills and social communication performance in children with ASD using standardized assessments of motor and social communication/social cognitive development as well as ASD severity (Macdonald et al., 2013a; Licari et al., 2019; Hellendoorn et al., 2015).
In contrast to gross-motor skills, fine-motor skills were only slightly better associated with (r = −0.23) and better explained variations (effect size = 0.88) in repetitive behavior severity than gross-motor skills (r = −0.17, effect size = 0.71–0.77). Once again, while all three motor skills (CDM, FM, and GC) contributed to the repetitive behavior severity models, fine-motor skills made strongest contributions when predicting repetitive behavior severity after accounting for age and sex effects. Apart from Bhat, 2021, there are only a handful of studies reporting associations between motor performance and repetitive behaviors in individuals with ASD (Bhat, 2021). Uljarevic et al. found that early motor milestone attainment (mainly standing and toe walking) predicted repetitive sensory mannerisms and insistence on sameness scores using the Social Responsiveness Scale in a large sample of school-aged children between 2 to 18 years (Uljarevic, Hedley, Alvares, Varcin, Whitehouse, 2019). In contrast, Ravizza et al. found that stereotyped movements on the RBS-R measure was more associated with fine motor performance on a rhythmic finger tapping task and not a spatial attention task in adolescents with ASD (Ravizza, Solomon, Ivry, & Carter, 2013). Shared underlying processes were said to be impaired in adolescents with ASD leading to impairments in motor control as well as presence of repetitive behaviors. Overall, both fine and gross-motor skills seemed to correlate /predict / explain variations in core ASD impairments in social communication and repetitive behavior severity. These findings provide supportive evidence for why motor challenges should be among the core symptoms of ASD.
Fine-motor and gross-motor, multilimb coordination/planning skills are related to cognitive impairments in children with ASD
Fine-motor (r=−0.39, effect size = 0.99) and gross-motor skills (multi-limb coordination/planning (CDM2), r=−0.29, effect size = 0.77) were more associated with, predictive of, and explained cognitive variations in children with ASD. Motor impairments are greater in magnitude and/or prevalence in children with ASD with cognitive impairment compared to those without (Green et al., 2009; Licari et al., 2019; Bhat, 2021; Ketcheson et al., 2021). It is important to note that motor impairments are observed across the autism spectrum regardless of IQ (Jansiewicz et al., 2006); however, children with accompanying cognitive impairment have more motor difficulties. Kaur et al. (2018) reported impaired fine-motor performance (fine manual and manual dexterity) on the Bruininks-Oseretsky test of Motor Proficiency as well as more imitation-based praxis errors (mirroring, overflow, and total) when copying complex gross-motor actions during praxis subtests of the Sensory Integration and Praxis test, in children with ASD with low IQ compared to children with ASD with high IQ. Additionally, while performing multilimb actions of clapping, marching, and drumming, children with ASD with low IQ had greater movement variability compared to those with high IQ. Both, fine-motor and multilimb coordination/planning skills involve complex action sequences requiring significant multitasking / executive functioning / cognitive abilities. For this reason, it is not surprising that these skills were more related to the cognitive variations in children with ASD.
Fine-motor and gross-motor (visuomotor) skills are related to language delays in children with ASD
Fine-motor (r=−0.34, effect size = 0.8) and gross-motor skills (visuomotor (CDM1), r=−0.24, effect size = 0.57) were more associated with, predictive of, and explained language variations in children with ASD. The relationship between fine-motor and language development in young and older children with and without ASD is probably the most well-reported in the literature (Bedford et al., 2015; Iverson, 2018; Choi et al., 2018; LeBarton & Landa, 2019; Gonzalez et al., 2019; Bal et al., 2020; Shield et al., 2018; Bhat et al., 2018; Gernsbacher et al., 2008). Early motor skills such as reaching, sitting, crawling, walking, etc. provide opportunities to explore new spaces and to interact with objects and caregivers, which in turn affords new perceptual information (e.g., early limb movements improve awareness of limb/bodily constraints, vertical height during walking offers a new perspective of surroundings), access to distal spaces and awareness of objects and people in it, more opportunities / instances to communicate/learn as well as more complex linguistic input from caregivers (Bhat et al., 2006, 2007; Gonzalez et al., 2019; Iverson, 2018b). While typically developing infants show a surge in rhythmic arm movements before the onset of babbling, such co-occurrence was not seen in infants at-risk for ASD (Iverson, 2018b). Early object interactions such as manual shaking/transfers, looking, and mouthing in an upright sitting position provides opportunities for caregivers to initiate communication bids and label the name and actions of objects to promote verbal and nonverbal communication in their infants. Infants at-risk for ASD or those who go onto develop ASD later are known to have different, reduced or less variable object exploration behaviors in the first year of life compared to infants without ASD (Kaur et al., 2015; Iverson et al., 2019). Additionally, infants at-risk for ASD and those who go onto develop ASD produce fewer early (showing, giving, requesting, yes/no using head movements) and late (symbolic play-based gestures) gestures compared to infants without ASD even as early as 9 or 14 months and these differences persisted until 3 years (Iverson et al., 2018a; Leezenbaum, Campbell, Butler, & Iverson, 2014). Together, these altered language learning contexts early on in life may reduce the number of opportunities for caregiver labeling / scaffolding to facilitate early vocabulary and subsequent language development.
In contrast to the burgeoning literature on motor-language relations, there are fewer studies on how visuomotor skills (e.g., ball and balance skills) may be specifically impaired in children with ASD compared to other diagnoses such as ADHD and specific language impairment (SLI) (Dowell et al., 2009; Bhat et al., 2010; 2018; Whyatt & Craig, 2012; Hellendoorn et al., 2015; Ament et al., 2014; McPhillips et al., 2014; Macdonald et al., 2014). McPhillips et al. (2014) reported greater associations between visuomotor and language impairments in children with ASD than those with specific language impairments. More recently, Wu et al. (2021) reported associations between object manipulation and visuo-motor integration performance on the Peabody Developmental Motor Scale with receptive/expressive language development in children newly diagnosed with ASD. Shared perceptuo-motor requirements, developmental processes, and common underlying neural processes between visuomotor and language development, such as early caregiver observation and imitation skills as well as shared neural networks (explained later in more detail), provide a basis for the associations between these two developing systems.
Gross- and fine-motor skills have profound impact on functional independence of children with ASD
In the present study, visuomotor (CDM1), multilimb coordination/planning (CDM2), and fine motor skills moderately correlated with, predicted, and well-explained variations in parent-reported functional delays (r=−0.32 to −0.40, effect sizes = 0.91–1.22). There are few reports on relations between motor skills and daily functioning in young and older children with ASD (Macdonald et al., 2013b; Bhat, 2021; Licari et al., 2019; Jasmin et al., 2009). The Bhat, 2021 paper reported moderate associations between general motor performance and parent-reported functional delays in the SPARK ASD sample. Licari et al. (2019) reported that motor skill performance correlated with the daily living skills domain on the Vineland Adaptive Behavioral Scales (VABS) in a large Australian sample of children with ASD between 2 to 6 years and yet few children were being screened and treated for their motor problems. Macdonald et al. (2013b) found that fine motor skills of young children with ASD (14 to 49 months) were more predictive of all adaptive domains on the VABS whereas gross motor skills were more predictive of the daily living skills only. Jasmin et al. (2009) also reported similar associations between motor and daily living skills in preschoolers with ASD using 2 different functional measures - Functional Independence Measure (Wee FIM) and the VABS and the Peabody Developmental Motor Scales (PDMS) in preschoolers with ASD. Multiple motor dimensions including locomotion, object manipulation, grasping, and visuomotor integration correlated with the self-care domain of the WeeFIM and/or the personal/daily living skills domain of the VABS. Poor motor performance / perceived motor competence as well as dyspraxia or difficulty performing complex motor sequences in children with ASD may affect their ability to perform a variety of daily physical functions (fine and gross-motor) leading to negative cascading effects on their social, communication, and cognitive functioning.
Certain General Coordination Skills Linked to Core and Functional Impairments in ASD
General coordination skills (GC1) of liking sports participation, being able to learn new motor skills easily, and being quick/competent at various daily motor functions were moderately associated with, contributed to, and/or explained variations in core social communication skills as well as comorbid cognitive and functional impairments. The last 2 general coordination skills (GC2 = bull in china shop and fatigues easily) weakly correlated with core / comorbid impairments. This could be because they were the only items stated in reverse order to reduce respondent bias whereas the earlier 13 items were not. To be clear, Q01 to Q13 DCD-Q items are stated as follows “Your child catches a ball or hits a ball accurately, etc.” with better scores being a 5. On the other hand, the last 2 items (Q14 and Q15) are stated in reverse, “Your child would never be described as clumsy or does not fatigue easily” with better scores also being a 5. The wording describing the last 2 items could be confusing and may be contributing to their unreliability/ineffectiveness. It is possible that the skills stated in the last 2 questions are still valuable and important for describing motor problems in children. From an intervention perspective, children with ASD may be limited in their sports/physical activity participation, have challenges acquiring new motor skills, and are slower and need more time to complete complex (multistep) motor functions.
Neural Framework for Motor-Other System Relations in ASD
School-age children with ASD presented with visuomotor (CDM1), multilimb coordination/planning (CDM2), fine-motor and general coordination (GC1) impairments that were associated with their core as well as comorbid impairments. These findings fit with the framework of disrupted connectivity in individuals with ASD including structural and functional abnormalities within primary motor cortices, corticospinal tracts, interhemispheric connectivity, as well as connectivity between various cortical and subcortical regions including cortico-cerebellar, cortico-striatal, and thalamo-cortical connectivity (Turner, Frost, McKilroy, Liesenbardt, & Mueller, 2006; Courchesne et al., 2007; Just, Keller, Malavi, Kana, & Varma, 2012; Frazier, Keshavan, Minshew, & Hardan, 2012; Nair et al., 2013; Maximo, Cadena, & Kana, 2014; Carper, Solders, Treiber, Fishman, & Mueller, 2015; Vasa, Mostofsky, & Ewen, 2016; Chen et al., 2018; Floris & Howells, 2018). Specific brain abnormalities attributed to visuospatial/visuomotor (includes fine/gross-motor) disturbances include reduced/aberrant long-range fronto-occipital, fronto-parietal, cortico-subcortical functional connectivity (Villalobos et al., 2005; Just et al., 2012; Maximo et al., 2014; Nebel et al., 2016; Wang et al., 2019; Lidstone et al., 2021) along with increased local connectivity in frontal, temporal, parietal, and occipital cortices as well as overconnectivity in certain cortico-cortical and cortico-subcortical pathways (Maximo et al., 2014; Wang et al., 2019; Lidstone et al., 2021). Nebel et al. (2016) reported greater temporal asynchrony between motor (primary motor and ventral premotor, and parietal operculum) and visual cortices (Brodmann’s areas 17–19) in individuals with ASD implying aberrant functional connectivity between these regions. Additionally, visuo-motor asynchrony in individuals with ASD was associated with gestural imitation performance on the Florida Apraxia Battery as well as social communication performance on the Social Responsiveness Scale (SRS). Both, Wang et al. (2019) and Lidstone et al. (2021) have implicated increased cortico-cerebellar (including cerebellar-occipital and cerebellar-inferior parietal) connectivity as the basis for visuomotor disturbances in individuals with ASD. The aforementioned brain regions are not only important for visuomotor functions but also for imitation-based learning and social communication development and offer plausible explanations for motor - social communication relations in ASD.
Additionally, multilimb coordination/planning impairments (including fine/gross-motor) could also be explained by executive functioning (EF) problems that not only affect cognitive processing but also complex, social communication and perceptuo-motor skills. EF skills include inhibition, working memory, motor planning, and task switching/organization that are often impaired in children with ASD and could directly contribute to their difficulty performing complex, multistep motor skills/gestures also known as dyspraxia (Lai et al., 2017). Individuals with ASD have aberrant connectivity in the EF / fronto-parietal networks including prefrontal/frontal cortices (include superior, middle, and inferior frontal and medial prefrontal gyri), posterior parietal cortex, and anterior cingulate cortex, and insula which is also associated with poor performance on EF tasks as well as repetitive behavior severity (Dajani & Uddin, 2015; May & Kana, 2020). Wang et al. (2019) reported reduced functional connectivity between cerebellar-frontal/prefrontal and cerebellar-temporal cortices in individuals with ASD; which may also contribute to EF / perceptuo-motor difficulties. Taken together, connectivity disruptions found in the various aforementioned brain networks may contribute to the multisystem impairments (including motor issues) in ASD.
Implications for future DSM criteria
The overall implications of Bhat, 2020, 2021 and the present study are that motor impairments in children with ASD are pervasive (seen in 87–88% of the sample), persistent (did not change between 5 and 15 years) and associated with, predictive of, and/or explain variations in core and comorbid impairments in ASD. Gross-motor (visuomotor, multilimb coordination/planning) and fine-motor skills are uniquely affected in ASD and relate to various other system impairments. Motor impairments are also very well-explained by the popular disrupted connectivity framework of ASD. These multidimensional motor findings in the SPARK ASD sample join the multiple recent calls (Licari et al., 2019; van Damme et al., 2021; Miller et al., 2021) for inclusion of motor impairments within the definition of ASD either within the criteria (relations to social communication skills or repetitive behaviors) or specifiers (relations to cognitive and language impairments). If the motor system is represented within the ASD definition, it would strongly encourage diagnosticians (physicians, pediatricians, psychologists, and psychiatrists) to conduct early motor screening and diagnostic referrals to movement clinicians (OTs and PTs) to address the early motor delays and the increasing motor gaps in childhood, as well as the negative impacts of developmental dyspraxia on overall child development and functioning in adolescence and adulthood. Identifying motor problems early on through motor screening could make available interventions that prevent the cascading negative impacts on children’s concurrent and future cognitive, social communication, language, and functional development, physical fitness/activity levels, social participation, as well as physical/mental health and well-being.
Implications for Motor Screening, Assessment, and Intervention
Often motor evaluations and services (specifically, PT) are not recommended to children with ASD because most children acquire major motor milestones despite some delays. More strategic efforts will be needed to change the mindset of clinicians, educators, and researchers for them to often recommend and practice motor screening, assessments, and interventions to address the more complex and persistent fine and gross motor problems associated with developmental dyspraxia/DCD. Screening tools such as the Ages-Stages Questionnaire (ASQ), DCD-Q and Movement-ABC checklist provide quick tools to screen for motor delays in young infants and children (van Damme et al., 2021; Miller et al., 2021). In particular, the psychometrics of the DCD-Q using the internally consistent and validated 5-subdomain structure might work better for motor screening in children with ASD. If a risk for motor impairment is identified, then the next step would be to complete an age-appropriate, full motor assessment such as the Alberta Infant Motor Scale (AIMS) for infants, Peabody Developmental Motor Scale (PDMS) for infants and toddlers, or the Movement-ABC (MABC) or Test of Gross Motor Development (TGMD) for school-age children. This would determine the extent and nature of motor impairments which in concert with family/child’s goals for motor functioning could help determine the next steps for providing effective motor interventions.
Research conducted in the first author’s lab has evaluated effects of various creative movement (music and movement, yoga, dance) and physical activity interventions that were truly multisystem in nature due to key ingredients such as gross-motor coordination/balance training, interpersonal synchrony/turn-taking, as well as social interactions including, listening, singing/conversations, and leading/following (Srinivasan et al., 2015a, 2015b, 2016a, 2016b; Kaur & Bhat, 2019, 2021). Our recent systematic review found strong evidence for the positive effects of music and movement on the social communication, cognitive-behavioral, and perceptuo-motor skills of children with ASD (Amonkar, Su, Srinivasan, & Bhat, 2021). Such interventions may also facilitate changes in functional activation and connectivity to normalize brain functioning in ASD using recently identified neurobiomarkers (Su et al., 2020a, 2020b, 2021, 2022; Sharda et al., 2018). Our current work has found reduced superior temporal and inferior frontal activation in children with ASD compared to age-matched controls, during naturalistic motor tasks (Bhat et al., 2017, 2019; Su et al., 2020a, 2020b, 2021). These biomarkers are currently being utilized to assess changes in neural response following 8-week long, multisystem, movement-based interventions (Su, Srinivasan, Cleffi, & Bhat, 2021; Srinivasan, Su, Cleffi, & Bhat, 2021; Bhat, Su, Cleffi, & Srinivasan, 2021; Cleffi, Su, Srinivasan, & Bhat, 2022). Taken together, there is a need to diversify autism interventions through inclusion of evidence-based, multisystem approaches that target perceptuo-motor, social communication, and cognitive skills of children.
Finally, on the clinical front, a lot more remains to be done to increase community awareness about the under-diagnosis (only 15% have DCD co-diagnosis in the SPARK ASD sample) and under-treatment (PT in 32% and recreational therapies in 13% in the SPARK ASD sample) of motor problems in ASD. ABA therapists, special educators, as well as movement clinicians (OTs, PTs, adaptive physical educators) must utilize evidence-based movement interventions (age-appropriate motor skills training, physical activity/exercise, sports participation, or creative movement such as yoga and music and movement individually or in small groups) to make interventions more embodied (i.e., inclusive of movement) and socially embedded/relevant to promote motor, cognitive, and social communication development. Even early on in life, when receiving early intervention (EI), it will be important to identify the mild to moderate motor delays in infants/preschoolers at-risk for ASD and address them through parent-mediated interventions and/or IDEA-based EI services as many parents/clinicians might make the error of “waiting” when in fact, early delays can be easily addressed to avoid more significant motor deficits in the future. On the research front, there is a clear dearth of motor intervention studies in individuals with ASD across the lifespan to facilitate motor functioning/physical activity and to study its cascading effects on other developmental domains/functioning, physical/mental health, as well as its underlying neural mechanisms.
Limitations
This study relied on parent report questionnaires and parent reports of children’s abilities which could be influenced by reporting biases such as the Horn effect i.e., parents of children with greater impairments may have rated their child worse. However, parent reports are a feasible method to obtain information from a large sample to help elucidate the population scale patterns of a disorder. Moreover, clinical assessments using large-sized samples would be important to further confirm the pattern of motor impairment reported here. Even though this study used a large sized-sample the SPARK study seems biased towards recruiting white (~76%) families at this time. However, the income and age distributions were fairly even across the sample. Last but not the least, it would be valuable to study motor performance across the lifespan in individuals with ASD by adding more objective, clinical motor / functional assessments to the SPARK study battery (e.g., PDMS, MABC, TGMD, etc.).
Conclusion
The present study examined the SPARK ASD sample of children between 5 and 15 years to conduct a multidimensional motor assessment using factor analysis, subgrouping, and correlational/effect size analyses. Visuomotor, multilimb coordination/planning, fine motor, and general coordination impairments were uniquely distinguishing motor problems of children with ASD. Both, gross-and fine-motor skills as well as certain general coordination skills were associated with, predictive of, and explained variations in the core impairments of children with ASD as well as their comorbid impairments in cognition, language and functioning. The comprehensive analysis of motor impairments in a large ASD cohort across 3 studies (Bhat, 2020, 2021, and this paper) provide robust evidence for the inclusion of motor impairments within the definition of ASD, either under diagnostic criteria or specifiers. Given the growing calls to address motor problems in the ASD population, diagnosticians should begin recommending systematic motor screening, further evaluations, and treatments for children at-risk for and newly diagnosed with ASD. Parents, clinicians, and educators in early intervention and school systems should be made aware of the need to address motor delays/gaps in their children and should not be complacent with a “wait and see” strategy or inaction. Motor advocacy and enhanced public/clinical community awareness is urgently needed to begin moving the needle in the right direction for children and families who clearly have unmet motor needs that they too are potentially unaware of.
Refer to supplementary information for list of acronyms and abbreviations, list of motor definitions, Figures S1–S2, and Tables S1–S11.
Supplementary Material
Acknowledgments
The authors are grateful to all SPARK families, SPARK clinical sites, and SPARK staff and truly appreciate obtaining access to the phenotypic data on SFARI Base. Approved researchers can obtain the SPARK population dataset described in this study at https://base.sfari.org/ordering/phenotype/sfari-phenotype by applying for the same at the following website: https://base.sfari.org. This research is supported by the National Institutes of Mental Health through an R01 award (Grant #: 1R01MH125823-01, PI: Bhat, A.).
Financial Disclosure Statement:
The authors have received no other financial support for conducting this research. Authors have no other conflicts of interest to report.
Footnotes
Conflict of Interest Disclosure
The authors have no conflicts of interest to report.
Data Sharing Statement:
Approved researchers can obtain the SPARK population dataset (version 3) described in this study by applying to https://base.sfari.org.
References
- Ament K, Mejia A, Buhlman R, Erklin S, Caffo B, Mostofsky S, & Wodka E (2014). Evidence for Specificity of Motor Impairments in Catching and Balance in Children with Autism. Journal of Autism and Developmental Disorders, 45(3), 742–751. 10.1007/s10803-014-2229-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th Edition: DSM-5 (5th ed.). American Psychiatric Publishing. [Google Scholar]
- Amonkar N, Su W, Bhat A, Srinivasan S (2021) Effects of creative movement therapies on social communication, behavioral-affective, sensorimotor, cognitive, and functional participation skills of individuals with autism spectrum disorder: A systematic review. Frontiers in Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal VH, Fok M, Lord C, Smith IM, Mirenda P, Szatmari P, Vaillancourt T, Volden J, et al. (2020) Predictors of longer-term development of expressive language in two independent longitudinal cohorts of language-delayed preschoolers with Autism Spectrum Disorder. Journal of Child Psychology & Psychiatry. 61(7):826–835. 10.1111/jcpp.13117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford R, Pickles A, & Lord C (2015). Early gross motor skills predict the subsequent development of language in children with autism spectrum disorder. Autism Research, 9(9), 993–1001. 10.1002/aur.1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, & Bailey A (1999). Autism screening questionnaire: Diagnostic validity. British Journal of Psychiatry, 175(5), 444–451. 10.1192/bjp.175.5.444 [DOI] [PubMed] [Google Scholar]
- Bhat A, Su W, Cleffi C, Srinivasan S (2021) A Hybrid Clinical Trial Delivery Model in the COVID-19 era. Physical Therapy, 101(8): pzab116. 10.1093/ptj/pzab116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat AN (2020). Is Motor Impairment in Autism Spectrum Disorder Distinct From Developmental Coordination Disorder? A Report From the SPARK Study. Physical Therapy, 100(4), 633–644. 10.1093/ptj/pzz190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat A (2021) Motor Impairment Increases in Children With Autism Spectrum Disorder as a Function of Social Communication, Cognitive and Functional Impairment, Repetitive Behavior Severity, and Comorbid Diagnoses: A SPARK Study Report. Autism Research,14(1):202–219. https://onlinelibrary.wiley.com/doi/epdf/10.1002/aur.2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat AN, Galloway JC, & Landa RJ (2012). Relation between early motor delay and later communication delay in infants at risk for autism. Infant Behavior and Development, 35(4), 838–846. 10.1016/j.infbeh.2012.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat AN, Landa RJ, & Galloway JCC (2011). Current Perspectives on Motor Functioning in Infants, Children, and Adults With Autism Spectrum Disorders. Physical Therapy, 91(7), 1116–1129. 10.2522/ptj.20100294 [DOI] [PubMed] [Google Scholar]
- Bhat A, Galloway JC, & Landa R (2010) Social and non-social visual attention patterns in infants at risk for autism and typically developing infants. Journal of Child Psychology and Psychiatry, 51(9), 989–997. 10.1111/j.1469-7610.2010.02262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat A & Galloway JC (2006) Toy-oriented changes in early arm movements of young infants: Hand Kinematics. Infant Behavior and Development, 29(3), 358–372. 10.1016/j.infbeh.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Bhat A, Galloway JC (2007) Toy-oriented changes in early arm movements III: Constraints on Joint Kinematics. Infant Behavior and Development, 30(3), 515–522. 10.1016/j.infbeh.2006.12.007 [DOI] [PubMed] [Google Scholar]
- Bhat AN, Srinivasan SM, Woxholdt C, & Shield A (2018). Differences in praxis performance and receptive language during fingerspelling between deaf children with and without autism spectrum disorder. Autism, 22(3), 271–282. 10.1177/1362361316672179 [DOI] [PubMed] [Google Scholar]
- Bhat A, Hoffman M, Trost S, Eilbott J, Pelphrey K (2017) Cortical Activation Patterns during Action Observation, Action Execution, & Interpersonal Synchrony in Adults: A functional Near-Infrared Spectroscopy (fNIRS) Study. Frontiers in Human Neuroscience. Volume 11. Article 431. 10.3389/fnhum.2017.00431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat A, McDonald N, Eilbott J, and Pelphrey K (2019) Exploring cortical activation and connectivity in infants with and without familial risk for autism during naturalistic social interactions: A preliminary study. Infant Behavior and Development. Special Issue in Brain Imaging 57:101337. 10.1016/j.infbeh.2019.101337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland JM & Altman DG (1997) Cronbach’s alpha. 314(7080):572. 10.1136/bmj.314.7080.572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer E & Cairney J (2020) Adaptive Behavior Moderates Health-Related Pathways in Children with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 50(2):491–499. 10.1007/s10803-019-04277-6 [DOI] [PubMed] [Google Scholar]
- Carper RA, Solders S, Treiber JM, Fishman I, & Müller R-A (2015). Corticospinal Tract Anatomy and Functional Connectivity of Primary Motor Cortex in Autism. Journal of the American Academy of Child & Adolescent Psychiatry, 54(10), 859–867. 10.1016/j.jaac.2015.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Wang J, Uddin LQ, Wang X, Guo X, Lu F, Duan X, Wu L, & Chen H (2018). Aberrant functional connectivity of neural circuits associated with social and sensorimotor deficits in young children with autism spectrum disorder. Autism Research, 11(12), 1643–1652. 10.1002/aur.2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi B, Leech KA, Tager-Flusberg H, & Nelson CA (2018). Development of fine motor skills is associated with expressive language outcomes in infants at high and low risk for autism spectrum disorder. Journal of Neurodevelopmental Disorders, 10(14), 1–11. 10.1186/s11689-018-9231-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleffi C, Su W, Srinivasan S, Bhat A (2022) Impact of the COVID-19 pandemic on Movement Intervention Research in Children with Autism Spectrum Disorder. Pediatric Physical Therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, & Morgan J (2007). Mapping Early Brain Development in Autism. Neuron, 56(2), 399–413. 10.1016/j.neuron.2007.10.016 [DOI] [PubMed] [Google Scholar]
- Daniels AM, Rosenberg RE, Anderson C, Law JK, Marvin AR, & Law PA (2011). Verification of Parent-Report of Child Autism Spectrum Disorder Diagnosis to a Web-Based Autism Registry. Journal of Autism and Developmental Disorders, 42(2), 257–265. 10.1007/s10803-011-1236-7 [DOI] [PubMed] [Google Scholar]
- Dajani DR & Uddin LQ (2015) Demystifying cognitive flexibility: Implications for clinical and developmental neuroscience. Trends in Neuroscience, 38(9):571–8. 10.1016/j.tins.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey D, Cantell M, & Crawford S (2007). Motor and gestural performance in children with autism spectrum disorders, developmental coordination disorder, and/or attention deficit hyperactivity disorder. Journal of the International Neuropsychological Society, 13(02), 246–256. 10.1017/s1355617707070270 [DOI] [PubMed] [Google Scholar]
- Dowell LR, Mahone EM, & Mostofsky SH (2009). Associations of postural knowledge and basic motor skill with dyspraxia in autism: implication for abnormalities in distributed connectivity and motor learning. Neuropsychology, 23(5), 563. 10.1037/a0015640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziuk MA, Larson JCG, Apostu A, Mahone EM, Denckla MB, & Mostofsky SH (2007). Dyspraxia in autism: association with motor, social, and communicative deficits. Developmental Medicine & Child Neurology, 49(10), 734–739. 10.1111/j.1469-8749.2007.00734.x [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, & Johnson MH (2016). Autism and the Social Brain: The First-Year Puzzle. Biological Psychiatry, 80(2), 94–99. 10.1016/j.biopsych.2016.02.019 [DOI] [PubMed] [Google Scholar]
- Flanagan J, Landa R, Bhat A, & Bauman M (2012) Head lag in infants at risk for autism: A preliminary study. American Journal of Occupational Therapy, 66(5), 577–585. 10.5014/ajot.2012.004192 [DOI] [PubMed] [Google Scholar]
- Feliciano P, Daniels AM, Green Snyder L, Beaumont A, Camba A, Esler A, Gulsrud AG, Mason A, Gutierrez A, Nicholson A, Paolicelli AM, McKenzie AP, Rachubinski AL, Stephens AN, Simon AR, Stedman A, Shocklee AD, Swanson A, Finucane B, … Chung WK (2018). SPARK: A US Cohort of 50,000 Families to Accelerate Autism Research. Neuron, 97(3), 488–493. 10.1016/j.neuron.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleury A, Kushki A, Tanel N, Anagnostou E, & Chau T (2013). Statistical persistence and timing characteristics of repetitive circle drawing in children with ASD. Developmental Neurorehabilitation, 16(4), 245–254. 10.3109/17518423.2012.758184 [DOI] [PubMed] [Google Scholar]
- Floris DL, & Howells H (2018). Atypical structural and functional motor networks in autism. Progress in Brain Research, 207–248. 10.1016/bs.pbr.2018.06.010 [DOI] [PubMed] [Google Scholar]
- Fournier KA, Hass CJ, Naik SK, Lodha N, & Cauraugh JH (2010). Motor Coordination in Autism Spectrum Disorders: A Synthesis and Meta-Analysis. Journal of Autism and Developmental Disorders, 40(10), 1227–1240. 10.1007/s10803-010-0981-3 [DOI] [PubMed] [Google Scholar]
- Frazier TW, Keshavan MS, Minshew NJ, & Hardan AY (2012). A Two-Year Longitudinal MRI Study of the Corpus Callosum in Autism. Journal of Autism and Developmental Disorders, 42(11), 2312–2322. 10.1007/s10803-012-1478-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernsbacher MA, Sauer EA, Geye HM, Schweigert EK, & Hill Goldsmith H (2008). Infant and toddler oral- and manual-motor skills predict later speech fluency in autism. Journal of Child Psychology and Psychiatry, 49(1), 43–50. 10.1111/j.1469-7610.2007.01820.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez SL, Alvarez V, & Nelson EL (2019). Do Gross and Fine Motor Skills Differentially Contribute to Language Outcomes? A Systematic Review. Frontiers in Psychology, 10, Article 2670. 10.3389/fpsyg.2019.02670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D, Charman T, Pickles A, Chandler S, Loucas T, Simonoff E, & Baird G (2009). Impairment in movement skills of children with autistic spectrum disorders. Developmental Medicine & Child Neurology, 51(4), 311–316. 10.1111/j.1469-8749.2008.03242.x [DOI] [PubMed] [Google Scholar]
- Hellendoorn A, Wijnroks L, van Daalen E, Dietz C, Buitelaar J, Leseman P (2015) Motor functioning, exploration, visuospatial cognition and language development in preschool children with autism. Research in Developmental Disabilities, 39:32–42. 10.1016/j.ridd.2014.12.033. [DOI] [PubMed] [Google Scholar]
- Iverson JM, Northrup JB, Leezenbaum NB, Parladé MV, Koterba EA, & West KL (2018a). Early communicative and language development in infant siblings of children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 48, 55–71. 10.1007/s10803-017-3297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson JM (2018b). Early Motor and Communicative Development in Infants With an Older Sibling With Autism Spectrum Disorder. Journal of Speech, Language, and Hearing Research, 61(11), 2673–2684. 10.1044/2018_jslhr-l-rsaut-18-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansiewicz EM, Goldberg MC, Newschaffer CJ, Denckla MB, Landa R, & Mostofsky SH (2006). Motor signs distinguish children with high functioning autism and Asperger’s syndrome from controls. Journal of Autism and Developmental Disorders, 36(5), 613–621. 10.1007/s10803-006-0109-y [DOI] [PubMed] [Google Scholar]
- Jasmin E, Couture M, McKinley P, Reid G, Fombonne E, Gisel E (2009) Sensori-motor and daily living skills of preschool children with autism spectrum disorders. Journal of Autism and Developmental Disorders;39(2):231–41. 10.1007/s10803-008-0617-z. [DOI] [PubMed] [Google Scholar]
- Just MA, Keller TA, Malave VL, Kana RK, & Varma S (2012). Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity. Neuroscience & Biobehavioral Reviews, 36(4), 1292–1313. 10.1016/j.neubiorev.2012.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur M, Eigsti Inge-Marie, Bhat A (2021) Effects of Creative Yoga Intervention on the Joint Attention, Social Communication, and Affective States of Children with Autism Spectrum Disorder. Research in Autism Spectrum Disorders, 88: 101860. 10.1016/j.rasd.2021.101860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur M, & Bhat A (2019). Creative Yoga Intervention Improves Motor and Imitation Skills of Children With Autism Spectrum Disorder. Physical Therapy, 99(11), 1520–1534. 10.1093/ptj/pzz115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur M, M. Srinivasan S, & N. Bhat A (2018). Comparing motor performance, praxis, coordination, and interpersonal synchrony between children with and without Autism Spectrum Disorder (ASD). Research in Developmental Disabilities, 72, 79–95. 10.1016/j.ridd.2017.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur M, Srinivasan S, & Bhat A (2015) Atypical object exploration skills in infants at-risk for autism during the first year of life. Frontiers in Psychology, 6, 1–15. (Special edition on goal-directed social and object interactions in infancy). 10.3389/fpsyg.2015.00798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketcheson L, Hauck J, & Ulrich D (2017). The effects of an early motor skill intervention on motor skills, levels of physical activity, and socialization in young children with autism spectrum disorder: A pilot study. Autism, 21(4), 481–492. 10.1177/1362361316650611 [DOI] [PubMed] [Google Scholar]
- Ketcheson LR, Pitchford A,E, Wentz CF (2021) The Relationship Between Developmental Coordination Disorder and Concurrent Deficits in Social Communication and Repetitive Behaviors Among Children with Autism Spectrum Disorder. Autism Research, 14(4):804–816. Epub 2021 Jan 9. 10.1002/aur.2469. [DOI] [PubMed] [Google Scholar]
- Kushki A, Chau T, & Anagnostou E (2011). Handwriting Difficulties in Children with Autism Spectrum Disorders: A Scoping Review. Journal of Autism and Developmental Disorders, 41(12), 1706–1716. 10.1007/s10803-011-1206-0 [DOI] [PubMed] [Google Scholar]
- Lai C, Lau Z Lui S, Lok E, Tam V, Chan Q, Cheng K, Lam S, Cheung E (2017) Meta-analysis of neuropsychological measures of executive functioning in children and adolescents with high-functioning autism spectrum disorder. Autism Research; 10(5):911–939. 10.1002/aur.1723. [DOI] [PubMed] [Google Scholar]
- Lam KSL, & Aman MG (2006). The Repetitive Behavior Scale-Revised: Independent Validation in Individuals with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders, 37(5), 855–866. 10.1007/s10803-006-0213-z [DOI] [PubMed] [Google Scholar]
- Landa R, & Garrett-Mayer E (2006). Development in infants with autism spectrum disorders: a prospective study. Journal of Child Psychology and Psychiatry, 47(6), 629–638. 10.1111/j.1469-7610.2006.01531.x [DOI] [PubMed] [Google Scholar]
- Landa R (2008) Diagnosis of autism spectrum disorders in the first 3 years of life. Nature Clinical Practice Neurology. 4(3):138–47. 10.1038/ncpneuro0731 [DOI] [PubMed] [Google Scholar]
- LeBarton ES, & Landa RJ (2019). Infant motor skill predicts later expressive language and autism spectrum disorder diagnosis. Infant Behavior and Development, 54, 37–47. 10.1016/j.infbeh.2018.11.003 [DOI] [PubMed] [Google Scholar]
- Lee H, Marvin AR, Watson T, Piggot J, Law JK, Law PA, Constantino JN, & Nelson SF (2010). Accuracy of phenotyping of autistic children based on internet implemented parent report. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 10.1002/ajmg.b.31103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leezenbaum N, Campbell S, Butler D, Iverson J (2014) Maternal verbal responses to communication of infants at low and heightened risk of autism. Autism. 18(6):694–703. 10.1177/1362361313491327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh P & Du J (2015) Brief Report: Forecasting the Economic Burden of Autism in 2015 and 2025 in the United States. Journal of Autism and Developmental Disorders, 45(12):4135–9. 10.1007/s10803-015-2521-7 [DOI] [PubMed] [Google Scholar]
- Leonard HC, Bedford R, Pickles A, & Hill EL (2015). Predicting the rate of language development from early motor skills in at-risk infants who develop autism spectrum disorder. Research in Autism Spectrum Disorders, 13–14, 15–24. 10.1016/j.rasd.2014.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licari MK, Alvares GA, Varcin K, Evans KL, Cleary D, Reid SL, Glasson EJ, Bebbington K, Reynolds JE, Wray J, & Whitehouse AJO (2019). Prevalence of Motor Difficulties in Autism Spectrum Disorder: Analysis of a Population‐Based Cohort. Autism Research, 13(2), 298–306. 10.1002/aur.2230 [DOI] [PubMed] [Google Scholar]
- Lidstone DE, Rochowiak R, Mostofsky SH, and Nebel MB (2021) A Data Driven Approach Reveals That Anomalous Motor System Connectivity is Associated With the Severity of Core Autism Symptoms. Autism Research, 00: 1–18. Online ahead of print. 10.1002/aur.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd M, MacDonald M, & Lord C (2013). Motor skills of toddlers with autism spectrum disorders. Autism, 17(2), 133–146. 10.1177/1362361311402230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald M, Lord C, & Ulrich DA (2013a). The Relationship of Motor Skills and Social Communicative Skills in School-Aged Children With Autism Spectrum Disorder. Adapted Physical Activity Quarterly, 30(3), 271–282. 10.1123/apaq.30.3.271 [DOI] [PubMed] [Google Scholar]
- MacDonald M, Lord C, & Ulrich D (2013b). The relationship of motor skills and adaptive behavior skills in young children with autism spectrum disorders. Research in Autism Spectrum Disorders, 7(11), 1383–1390. 10.1016/j.rasd.2013.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald M, Lord C, Ulrich D (2014) Motor skills and calibrated autism severity in young children with autism spectrum disorder. Adapted Physical Activity Quarterly, 31(2):95–105. 10.1123/apaq.2013-0068 [DOI] [PubMed] [Google Scholar]
- MacNeil LK, & Mostofsky SH (2012). Specificity of dyspraxia in children with autism. Neuropsychology, 26(2), 165–171. 10.1037/a0026955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May KE & Kana RK (2020) Frontoparietal Network in Executive Functioning in Autism Spectrum Disorder. Autism Research, 13(10):1762–1777. 10.1002/aur.2403 [DOI] [PubMed] [Google Scholar]
- Maenner et al. (2021). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018 Morbidity and Mortality Weekly Report - Surveillance Summaries, 70(11): 1–16. 10.15585/mmwr.ss7011a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin AR, Marvin DJ, Lipkin PH, & Law JK (2017). Analysis of Social Communication Questionnaire (SCQ) Screening for Children Less Than Age 4. Current Developmental Disorders Reports, 4(4), 137–144. 10.1007/s40474-017-0122-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximo J, Cadena E, Kana R (2014) The implications of brain connectivity in the neuropsychology of autism. Neuropsychology Reviews;24(1):16–31. 10.1007/s11065-014-9250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhillips M, Finlay J, Bejerot S, & Hanley M (2014). Motor Deficits in Children With Autism Spectrum Disorder: A Cross-Syndrome Study. Autism Research, 7(6), 664–676. 10.1002/aur.1408 [DOI] [PubMed] [Google Scholar]
- Miller HL, Sherrod GM, Mauk JE, Fears NE, Hynan LS, Tamplain P (2021) Shared Features or Co-occurrence? Evaluating Symptoms of Developmental Coordination Disorder in Children and Adolescents with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 51(10):3443–3455. 10.1007/s10803-020-04766-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Treiber J, Shukla D, Shih P, and Müller R (2013) Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain, 136(6), 1942–55. 10.1093/brain/awt079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebel MB, Eloyan A, Nettles CA, Sweeney KL, Ament K, Ward RE, Choe AS, Barber AD, Pekar JJ, & Mostofsky SH (2016). Intrinsic Visual-Motor Synchrony Correlates With Social Deficits in Autism. Biological Psychiatry, 79(8), 633–641. 10.1016/j.biopsych.2015.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel LR, Thatcher AR, Keller F, Wozniak RH, & Iverson JM (2013). Posture Development in Infants at Heightened versus Low Risk for Autism Spectrum Disorders. Infancy, 18(5), 639–661. 10.1111/infa.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piek JP, Baynam GB, Barrett NC (2006) The relationship between fine and gross motor ability, self-perceptions and self-worth in children and adolescents. Human Movement Science, 25 (1):65–75. [DOI] [PubMed] [Google Scholar]
- Radonovich K, Fournier K, Hass C (2013) Relationship between postural control and restricted, repetitive behaviors in autism spectrum disorders. Frontiers in Integrative Neuroscience. 7;7:28. 10.3389/fnint.2013.00028. eCollection 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza SM, Solomon M, Ivry RB, Carter CS (2013) Restricted and repetitive behaviors in autism spectrum disorders: the relationship of attention and motor deficits. Developmental Psychopathology; 25(3):773–84. 10.1017/S0954579413000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivard L, Missiuna C, McCauley D, & Cairney J (2012). Descriptive and factor analysis of the Developmental Coordination Disorder Questionnaire (DCDQ’07) in a population-based sample of children with and without Developmental Coordination Disorder. Child: Care, Health and Development, 40(1), 42–49. 10.1111/j.1365-2214.2012.01425.x [DOI] [PubMed] [Google Scholar]
- Sacrey LR, Zwaigenbaum L, Bryson S, Brian J, Smith IM, Roberts W, Szatmari P, Roncadin C, Garon N, Novak C, Vaillancourt T, McCormick T, MacKinnon B, Jilderda S, Armstrong V (2015) Can Parents’ Concerns Predict Autism Spectrum Disorder? A Prospective Study of High-Risk Siblings From 6 to 36 Months of Age. Journal of American Academy of Child & Adolescent Psychiatry, 54(6):470–8. 10.1016/j.jaac.2015.03.014 [DOI] [PubMed] [Google Scholar]
- Schoemaker MM, Flapper B, Verheij NP, Wilson BN, Reinders-Messelink HA, & de Kloet A (2006). Evaluation of the Developmental Coordination Disorder Questionnaire as a screening instrument. Developmental Medicine & Child Neurology, 48(08), 668. 10.1017/s001216220600140x [DOI] [PubMed] [Google Scholar]
- Sharda M, Tuerk C, Chowdhury R, Jamey K, Foster N, Custo-Blanch M, Tan M, Nadig A, Hyde K (2018) Music improves social communication and auditory-motor connectivity in children with autism. Translational Psychiatry;8(1):231. 10.1038/s41398-018-0287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw KA, Maenner MJ, Baio J, Washington A, Christensen DL, Wiggins L, Pettygrove S, Andrews JG, White T, Rosenberg C, Constantino J, Fitzgerald R, Zahorodny W, Shenouda J, Daniels JL, Salinas A, Durkin MS, Dietz P (2020) Early Identification of Autism Spectrum Disorder Among Children Aged 4 Years - Early Autism and Developmental Disabilities Monitoring Network, Six Sites, United States, 2016. Morbidity and Mortality Weekly Report - Surveillance Summaries, 69(3):1–11. 10.15585/mmwr.ss6903a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shield A, Knapke K, Henry M, Srinivasan S, & Bhat A (2017). Impaired praxis in gesture imitation by deaf children with autism spectrum disorder. Autism & Developmental Language Impairments, 2, 239694151774567. 10.1177/2396941517745674 [DOI] [Google Scholar]
- Srinivasan SM, & Bhat AN (2013). A review of “music and movement” therapies for children with autism: embodied interventions for multisystem development. Frontiers in Integrative Neuroscience, 7 (Article 22), 1–22. 10.3389/fnint.2013.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan SM, & Bhat AN (2016). Differences in object sharing between infants at risk for autism and typically developing infants from 9 to 15 months of age. Infant Behavior and Development, 42, 128–141. 10.1016/j.infbeh.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Bhat A (2019) Differences in means-end exploration between infants at risk for autism and typically developing infants in the first 15 months of life. Developmental Psychobiology, 61(2):203–215. 10.1002/dev.21810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Bhat A. (2020) Differences in caregiver behaviors of infants at-risk for autism and typically developing infants from 9 to 15 months of age. Infant Behavior and Development, 59:101445. 10.1016/j.infbeh.2020.101445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan SM, Eigsti I-M, Gifford T, & Bhat AN (2016a). The effects of embodied rhythm and robotic interventions on the spontaneous and responsive verbal communication skills of children with Autism Spectrum Disorder (ASD): A further outcome of a pilot randomized controlled trial. Research in Autism Spectrum Disorders, 27, 73–87. 10.1016/j.rasd.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan SM, Eigsti I-M, Neelly L, & Bhat AN (2016b). The effects of embodied rhythm and robotic interventions on the spontaneous and responsive social attention patterns of children with autism spectrum disorder (ASD): A pilot randomized controlled trial. Research in Autism Spectrum Disorders, 27, 54–72. 10.1016/j.rasd.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan SM, Kaur M, Park IK, Gifford TD, Marsh KL, & Bhat AN (2015a). The Effects of Rhythm and Robotic Interventions on the Imitation/Praxis, Interpersonal Synchrony, and Motor Performance of Children with Autism Spectrum Disorder (ASD): A Pilot Randomized Controlled Trial. Autism Research and Treatment, 2015, 1–18. 10.1155/2015/736516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan SM, Park IK, Neelly LB, & Bhat AN (2015b). A comparison of the effects of rhythm and robotic interventions on repetitive behaviors and affective states of children with Autism Spectrum Disorder (ASD). Research in Autism Spectrum Disorders, 18, 51–63. 10.1016/j.rasd.2015.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan SM, Pescatello LS, & Bhat AN (2014). Current Perspectives on Physical Activity and Exercise Recommendations for Children and Adolescents With Autism Spectrum Disorders. Physical Therapy, 94(6), 875–889. 10.2522/ptj.20130157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Su W, Cleffi C, Bhat A (2021) From Social distancing to Social connections: Insights from the Delivery of a Clinician-Caregiver co-mediated Telehealth-based Intervention in Children with Autism Spectrum Disorder. Frontiers in Psychiatry, 12:700247. 10.3389/fpsyt.2021.700247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieglitz Ham H, Bartolo A, Corley M, Rajendran G, Szabo A, & Swanson S (2010). Exploring the Relationship Between Gestural Recognition and Imitation: Evidence of Dyspraxia in Autism Spectrum Disorders. Journal of Autism and Developmental Disorders, 41(1), 1–12. 10.1007/s10803-010-1011-1 [DOI] [PubMed] [Google Scholar]
- Staples K and Reid G (2010) Fundamental movement skills and autism spectrum disorders. Journal of Autism and Developmental Disorders; 40(2):209–17. 10.1007/s10803-009-0854-9. [DOI] [PubMed] [Google Scholar]
- Su W, Srinivasan S, Cleffi C, Bhat A (2021) Short Report on Research Trends in the COVID-19 Pandemic and Use of Telehealth Interventions and Remote Brain Research in Children with ASD. Autism, Aug 25(6):1816–1822. 10.1177/13623613211004795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W-C, Culotta M, Tsuzuki D, Bhat A (2021) Differences in Movement Kinematics and Cortical Activation Between Children with or without Autism Spectrum Disorder During a Sway Synchrony Task: A Functional Near Infrared Spectroscopy Study. Scientific Reports, 11: 15035. 10.1038/s41598-021-94519-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W-C, Culotta M, Hoffman M, Trost S, Pelphrey K, Tsuzuki D, Bhat A (2020a) Developmental differences in cortical activation patterns during Action Observation, Action Execution, & Interpersonal Synchrony: A functional Near-Infrared Spectroscopy (fNIRS) Study. Frontiers in Human Neuroscience, Vol. 14. Article 57. Mar 3;14:57. 10.3389/fnhum.2020.00057.eCollection 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W-C, Culotta M, Hoffman M, Trost S, Pelphrey K, Tsuzuki D, Bhat A (2020b) Differences in cortical activation patterns during Action Observation, Action Execution, & Interpersonal Synchrony between children with and without ASD. An fNIRS pilot study. Plos One. Available Online: October 29, 2020. 10.1371/journal.pone.0240301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner KC, Frost L, Linsenbardt D, McIlroy JR, & Müller R-A (2006). Atypically diffuse functional connectivity between caudate nuclei and cerebral cortex in autism. Behavioral and Brain Functions, 2(1), 34. 10.1186/1744-9081-2-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uljarević M, Jo B, Frazier TW, Scahill L, Youngstrom EA, Hardan AY (2021) Using the big data approach to clarify the structure of restricted and repetitive behaviors across the most commonly used autism spectrum disorder measures. Molecular Autism, 12(1):39. 10.1186/s13229-021-00419-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Damme T, Vancampfort D, Thoen A, Sanchez C, Van Biesen D (2021) Evaluation of the Developmental Coordination Questionnaire (DCDQ) as a Screening Instrument for Co-occurring Motor Problems in Children with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders. Online ahead of print. 10.1007/s10803-021-05285-1 [DOI] [PubMed] [Google Scholar]
- van Etten H, Kaur M, Srinivasan S, Cohen S Bhat A, Dobkins K (2017) Increased Prevalence of Unusual Sensory Behaviors in Infants at Risk for, and Teens with, Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 47(1), 1–15. 10.1007/s10803-017-3227-9 [DOI] [PubMed] [Google Scholar]
- Vasa RA, Mostofsky SH, & Ewen JB (2016). The Disrupted Connectivity Hypothesis of Autism Spectrum Disorders: Time for the Next Phase in Research. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 1(3), 245–252. 10.1016/j.bpsc.2016.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos M, Mizuno A, Dahl B, Kemmotsu N, Müller R (2005) Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. Neuroimage;25(3):916–25. 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z Wang Y Sweeney JA, Gong Q, Lui S, Mosconi M (2019) Resting-State Brain Network Dysfunctions Associated With Visuomotor Impairments in Autism Spectrum Disorder. Frontiers in Integrative Neuroscience, 13:17. 10.3389/fnint.2019.00017. eCollection 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt CP, & Craig CM (2012). Motor Skills in Children Aged 7–10 Years, Diagnosed with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 42(9), 1799–1809. 10.1007/s10803-011-1421-8 [DOI] [PubMed] [Google Scholar]
- Wu Y, Tsao C, Huang H, Yang T, Li Y (2021) Relationship Between Motor Skills and Language Abilities in Children With Autism Spectrum Disorder. Physical Therapy, 101(5):pzab033. 10.1093/ptj/pzab033 [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bauman ML, Stone WL, Yirmiya N, Estes A, Hansen RL, McPartland JC, Natowicz MR, Choueiri R, Fein D, Kasari C, Pierce K, Buie T, Carter A, Davis PA, Granpeesheh D, Mailloux Z, Newschaffer C, Robins D, … Wetherby A (2015). Early Identification of Autism Spectrum Disorder: Recommendations for Practice and Research. Pediatrics, 136(Supplement), S10–S40. 10.1542/peds.2014-3667c [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Approved researchers can obtain the SPARK population dataset (version 3) described in this study by applying to https://base.sfari.org.


