Abstract
Background
Most people with Parkinson’s disease (PD) experience at least one fall during the course of their disease. Several interventions designed to reduce falls have been studied. An up‐to‐date synthesis of evidence for interventions to reduce falls in people with PD will assist with informed decisions regarding fall‐prevention interventions for people with PD.
Objectives
To assess the effects of interventions designed to reduce falls in people with PD.
Search methods
CENTRAL, MEDLINE, Embase, four other databases and two trials registers were searched on 16 July 2020, together with reference checking, citation searching and contact with study authors to identify additional studies. We also conducted a top‐up search on 13 October 2021.
Selection criteria
We included randomised controlled trials (RCTs) of interventions that aimed to reduce falls in people with PD and reported the effect on falls. We excluded interventions that aimed to reduce falls due to syncope.
Data collection and analysis
We used standard Cochrane Review procedures. Primary outcomes were rate of falls and number of people who fell at least once. Secondary outcomes were the number of people sustaining one or more fall‐related fractures, quality of life, adverse events and economic outcomes. The certainty of the evidence was assessed using GRADE.
Main results
This review includes 32 studies with 3370 participants randomised. We included 25 studies of exercise interventions (2700 participants), three studies of medication interventions (242 participants), one study of fall‐prevention education (53 participants) and three studies of exercise plus education (375 participants). Overall, participants in the exercise trials and the exercise plus education trials had mild to moderate PD, while participants in the medication trials included those with more advanced disease. All studies had a high or unclear risk of bias in one or more items. Illustrative risks demonstrating the absolute impact of each intervention are presented in the summary of findings tables.
Twelve studies compared exercise (all types) with a control intervention (an intervention not thought to reduce falls, such as usual care or sham exercise) in people with mild to moderate PD. Exercise probably reduces the rate of falls by 26% (rate ratio (RaR) 0.74, 95% confidence interval (CI) 0.63 to 0.87; 1456 participants, 12 studies; moderate‐certainty evidence). Exercise probably slightly reduces the number of people experiencing one or more falls by 10% (risk ratio (RR) 0.90, 95% CI 0.80 to 1.00; 932 participants, 9 studies; moderate‐certainty evidence).
We are uncertain whether exercise makes little or no difference to the number of people experiencing one or more fall‐related fractures (RR 0.57, 95% CI 0.28 to 1.17; 989 participants, 5 studies; very low‐certainty evidence). Exercise may slightly improve health‐related quality of life immediately following the intervention (standardised mean difference (SMD) ‐0.17, 95% CI ‐0.36 to 0.01; 951 participants, 5 studies; low‐certainty evidence). We are uncertain whether exercise has an effect on adverse events or whether exercise is a cost‐effective intervention for fall prevention.
Three studies trialled a cholinesterase inhibitor (rivastigmine or donepezil). Cholinesterase inhibitors may reduce the rate of falls by 50% (RaR 0.50, 95% CI 0.44 to 0.58; 229 participants, 3 studies; low‐certainty evidence). However, we are uncertain if this medication makes little or no difference to the number of people experiencing one or more falls (RR 1.01, 95% CI 0.90 to 1.14; 230 participants, 3 studies) and to health‐related quality of life (EQ5D Thermometer mean difference (MD) 3.00, 95% CI ‐3.06 to 9.06; very low‐certainty evidence). Cholinesterase inhibitors may increase the rate of non fall‐related adverse events by 60% (RaR 1.60, 95% CI 1.28 to 2.01; 175 participants, 2 studies; low‐certainty evidence). Most adverse events were mild and transient in nature. No data was available regarding the cost‐effectiveness of medication for fall prevention.
We are uncertain of the effect of education compared to a control intervention on the number of people who fell at least once (RR 10.89, 95% CI 1.26 to 94.03; 53 participants, 1 study; very low‐certainty evidence), and no data were available for the other outcomes of interest for this comparisonWe are also uncertain (very low‐certainty evidence) whether exercise combined with education makes little or no difference to the number of falls (RaR 0.46, 95% CI 0.12 to 1.85; 320 participants, 2 studies), the number of people sustaining fall‐related fractures (RR 1.45, 95% CI 0.40 to 5.32,320 participants, 2 studies), or health‐related quality of life (PDQ39 MD 0.05, 95% CI ‐3.12 to 3.23, 305 participants, 2 studies). Exercise plus education may make little or no difference to the number of people experiencing one or more falls (RR 0.89, 95% CI 0.75 to 1.07; 352 participants, 3 studies; low‐certainty evidence). We are uncertain whether exercise combined with education has an effect on adverse events or is a cost‐effective intervention for fall prevention.
Authors' conclusions
Exercise interventions probably reduce the rate of falls, and probably slightly reduce the number of people falling in people with mild to moderate PD.
Cholinesterase inhibitors may reduce the rate of falls, but we are uncertain if they have an effect on the number of people falling. The decision to use these medications needs to be balanced against the risk of non fall‐related adverse events, though these adverse events were predominantly mild or transient in nature.
Further research in the form of large, high‐quality RCTs are required to determine the relative impact of different types of exercise and different levels of supervision on falls, and how this could be influenced by disease severity. Further work is also needed to increase the certainty of the effects of medication and further explore falls prevention education interventions both delivered alone and in combination with exercise.
Plain language summary
Interventions for preventing falls in Parkinson's disease
Review Question
In this review we assessed the evidence on the effect of interventions designed to reduce falls in people with Parkinson’s disease (PD). The interventions included exercise, medication, fall‐prevention education and exercise plus education combined. We excluded interventions that aimed to reduce falls due to syncope (e.g. dizziness and fainting). The evidence in this review is current to 16 July 2020.
Background
In people with PD, the emergence of frequent falls is one of the most serious disease milestones. Information about effective fall‐prevention strategies will aid the implementation of fall‐prevention interventions.
Study characteristics
We included 32 randomised controlled trials with 3370 participants. Of these, 25 studies with 2700 participants were exercise trials. Three studies with 242 participants were medication trials. One study with 53 participants was an education trial. Three studies with 375 participants were exercise plus education trials. Overall, the exercise and exercise plus education studies included people with mild to moderate PD.
Key results
Twelve studies compared exercise with a control intervention not thought to reduce falls. Exercise probably reduces the number of falls by around 26%. Exercise probably slightly reduces the number of people experiencing one or more falls by around 10%. Exercise may slightly improve health‐related quality of life immediately after the exercise program. However, we are uncertain if it reduces the number of fall‐related fractures, if it has an effect on the number of adverse events or if it is a cost‐effective intervention for fall prevention.
Three studies compared a cholinesterase inhibitor (either rivastigmine or donepezil) with placebo medication (an inactive treatment) and found that this medication may reduce the rate of falls by around 50%. However, the effect of this medication on the number of people experiencing one or more falls, and on health‐related quality of life was uncertain. Cholinesterase inhibitor medication may increase the number of non fall related adverse events by around 60%. There was no information about the cost‐effectiveness of medication for fall prevention.
One study compared education alone and three studies compared exercise plus education with a control group. Exercise plus education may make little or no difference to the number of people experiencing one or more falls. However, we are uncertain of the effects of these interventions on the other fall and non‐fall outcomes.
Certainty of the evidence
All studies had high or unclear risk of bias in at least one area. This could have influenced how the studies were conducted and how the outcomes were assessed.
For the exercise interventions, the certainty of the evidence for the rate of falls and the number of people experiencing one or more falls was moderate. The certainty of the evidence was low or very low for all other outcomes.
For medication, the education and the exercise plus education interventions, the certainty of the evidence was low to very low for all outcomes.
Summary of findings
Background
Description of the condition
People with Parkinson’s disease (PD) fall frequently and recurrently with approximately 60% of individuals falling each year and two thirds of these people falling recurrently (Allen 2013; Bloem 2001; Latt 2009; Paul 2013; Pickering 2007). These rates are double those reported for the general older population (Sherrington 2019). In addition, falls in people with PD are associated with injury (Paul 2017; Walker 2013; Wielinski 2005), with the incidence of hip fracture reported to be two (Kalilani 2016) to four times (Walker 2013) that of older people of the same age without PD. It is not surprising that falls are associated with escalating healthcare costs (Paul 2017; Pressley 2003), and are major contributors to reduced health‐related quality of life (Rascol 2015; Soh 2011).
A large number of fall risk factors have been identified in people with PD (Canning 2014; Fasano 2017) . Consistently identified risk factors include a history of past falls (Allcock 2009; Latt 2009; Paul 2013; Pickering 2007); disease severity (Allcock 2009; Kerr 2010; Latt 2009; Paul 2013; Pickering 2007), which are fixed and not remediable. However, a number of risk factors which contribute to loss of balance and falls have the potential to be modified with exercise or pharmaceutical interventions (Allen 2011; Fasano 2017; Shen 2016; Tomlinson 2013), which may in turn reduce falls. These include: freezing of gait (i.e. an episodic inability to initiate or continue walking) (Kerr 2010; Latt 2009; Paul 2013); balance deficits, mobility impairments and lower limb muscle strength deficits (Kerr 2010; Latt 2009; Paul 2013); fear of falling (Mak 2009), and cognitive deficits (Allcock 2009; Latt 2009; Paul 2013). While falls are commonly monitored as adverse events in intervention trials (Nieuwboer 2007; van Nimwegen 2013), only recently have interventions designed primarily to reduce falls in people with PD been developed and investigated (e.g. Canning 2015a; Chivers Seymour 2019; Li 2012; Mirelman 2016; Morris 2015).
Description of the intervention
Interventions designed to reduce falls in people with PD include exercise and/or movement strategy training, pharmacological and/or surgical management, increasing knowledge about fall prevention (education), environmental modifications, assistive technology, management of urinary incontinence, fluid or nutrition therapy, psychological interventions, social environment, and any other intervention designed to reduce falls in this population. Interventions are classified as single interventions (e.g. exercise), multiple interventions (e.g. exercise plus environmental modifications) or multifactorial interventions (i.e. multiple interventions tailored to the individual's identified risk factors).
How the intervention might work
Each intervention type is designed to target specific, potentially remediable fall risk factors. Exercise interventions aim to reduce falls by targeting physical and/or cognitive risk factors, including poor balance, reduced muscle strength and freezing of gait (Canning 2014; Mirelman 2016). Cholinesterase inhibitors address the central nervous system (CNS) cholinergic neuron loss associated with PD and may reduce falls by enhancing cognitive and attentional resources (Chung 2010), and/or reducing gait variability contributing to falls (Henderson 2016). Education interventions aim to increase awareness of the risk of falls and may include behaviour modification to avoid high‐risk activities (Stack 2013), while environmental modifications focus on reducing environmental hazards, such as poor lighting, or slippery surfaces (Bhidayasiri 2015).
Why it is important to do this review
Recently, a number of large‐scale randomised controlled trials and several smaller trials specifically testing interventions designed to reduce falls in people with PD have been published. In addition, participants with PD are excluded from the Cochrane Reviews of interventions for preventing falls in older people living in the community (Hopewell 2018; Sherrington 2019). Further, while falls as an outcome is addressed in Cochrane Reviews of physiotherapy interventions for PD (Tomlinson 2013; Tomlinson 2014), these reviews do not differentiate between physiotherapy interventions primarily designed to reduce falls versus other interventions. In addition, the scope of the physiotherapy reviews is limited to physical interventions. Therefore, there is a need to systematically review the literature to identify trials of all interventions aimed at reducing falls in people with PD and summarise this evidence for people with PD, clinicians, researchers and policymakers.
Objectives
To assess the effects of interventions designed to reduce the incidence of falls in people with Parkinson's disease (PD).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐randomised trials, including cluster‐ and cross‐over trials, evaluating the effects of interventions on falls in people with PD. Eligible randomised cross‐over trials of exercise interventions had the first phase data only included in order to minimise the risk of carry‐over effects of the interventions. For eligible randomised cross‐over trials of medication interventions we included data from both phases as washout phases ensured no carry‐over effects. We did not include studies published only in abstract form.
Types of participants
We included trials of participants with idiopathic PD who had been diagnosed by the UK Parkinson’s Disease Brain Bank criteria (Hughes 1992), or by a clinical definition. No restrictions were made with regard to gender, age or disease duration. We included studies reporting an intervention carried out in a mixed sample of participants, including people with idiopathic PD, if separate data were available for participants with idiopathic PD.
Types of interventions
We included interventions where a stated primary or secondary aim was to reduce falls in people with PD. Therefore, any intervention which did not have a stated aim of preventing falls, and which reported falls as an adverse event, was not included. We did not include interventions designed to primarily address syncopal falls (e.g. falls associated with neurogenic postural hypotension) as the aetiology and intervention for syncopal falls are different from falls arising from loss of balance due to physical, cognitive and emotional risk factors associated with PD (Fasano 2012; van der Marck 2014). We included studies where a fall‐prevention intervention was compared with ‘usual care’ (i.e. no change in usual activities or treatments), a ‘placebo’ or other control intervention (i.e. an intervention not thought to have an effect on falls, such as very gentle or 'sham' exercise), or another fall‐prevention intervention.
We grouped interventions using the fall‐prevention classification taxonomy developed by the Prevention of Falls Network Europe (ProFaNE) (Lamb 2011). Interventions were classified according to intervention type: exercises, medication (drug target, i.e. withdrawal, dose reduction or increase, substitution, provision), surgery, management of urinary incontinence, fluid or nutrition therapy, psychological interventions, environment/assistive technology, social environment, interventions to increase knowledge (education), or other interventions. Interventions were also classified according to combination of intervention types: single, multiple (more than one intervention type) or multifactorial (more than one intervention type specifically targeting person‐specific fall risk factors). Full details are available in the ProFaNE Taxonomy Manual (Lamb 2011).
We used the ProFaNE taxonomy (Lamb 2011) to categorise exercise types. Exercise categories were: i) gait, balance and functional training; ii) resistance training (including muscle power training); iii) flexibility exercise; iv) 3D exercise (e.g. Tai Chi); v) general physical activity; vi) endurance exercise, and vii) other forms of exercise (including where the exercise was not described in sufficient detail to allocate a category) (Table 7).
3. Exercise categories (based on ProFaNE): definition and application.
| Exercise Category | ProFaNE exercise description | How the criteria were applied in this review* |
| Gait, balance and functional training | Gait training involves specific correction of walking technique (e.g., posture, stride length and cadence) and changes of pace, level and direction. Balance training involves the efficient transfer of bodyweight from one part of the body to another or challenges specific aspects of the balance systems (e.g. vestibular systems). Balance retraining activities range from the re‐education of basic functional movement patterns to a wide variety of dynamic activities that target more sophisticated aspects of balance. Functional training utilises functional activities as the training stimulus, and is based on the theoretical concept of task specificity. All gait, balance and functional training should be based on an assessment of the participant's abilities prior to starting the program; tailoring of the intervention to the individuals abilities; and progression of the exercise program as ability improves. | Selected as the primary exercise category when the majority of the exercise was conducted in standing and when the intervention focus and the majority of time spent was on exercise in this category. Movement strategy training and cueing are included in this category. |
| Resistance training | The term Resistance Training covers all types of weight training.i.e contracting the muscles against a resistance to overload and bring about a training effect in the muscular system. The resistance is an external force, which can be ones own body placed in an unusual relationship to gravity (e.g. prone back extension) or an external resistance (e.g. free weight). All strength/resistance training should be based on an assessment of the participant's abilities prior to starting the program; tailoring of the intervention to the individuals abilities; and progression of the exercise program as ability improves. | Selected as the primary category for interventions where additional resistance was used or where it was clear that overload was sufficient without external resistance and where the intervention focus and the majority of time spent was on exercise in this category. |
| Flexibility | Flexibility training is the planned process by which stretching exercises are practised and progressed to restore or maintain the optimal Range Of Movement (ROM) available to a joint or joints. The ranges of motion used by flexibility programs may vary from restoration/maintenance of the entire physiological range of motion, or alternatively, maintenance of range that is essential to mobility or other functions. | Selected as the primary category for interventions where flexibility training was a stated aim of the intervention and where the intervention focus and the majority of time spent was on exercise in this category. |
| 3D | 3D training involves constant movement in a controlled, fluid, repetitive way through all 3 spatial planes or dimensions (forward and back, side to side, and up and down). Tai Chi and Qi Gong incorporate specific weight transferences and require upright posture and subtle changes of head position and gaze direction. Dance involves a wide range of dynamic movement qualities, speeds and patterns. | Selected as the primary exercise category where the intervention focus and the majority of time was spent on exercise in this category (e.g., Tai Chi or dance). |
| General Physical activity | Physical activity is any bodily movement produced by skeletal muscle contraction resulting in a substantial increase in energy expenditure. Physical activity has occupational, transportation and recreational components and includes pursuits like golf, tennis and swimming. It also includes other activities and pastimes like gardening, cutting wood and carpentry. Physical activity can provide progressive health benefits and is a catalyst for improving health attitudes, health habits and lifestyle. Increasing habitual physical activity should be with specific recommendations as to duration, frequency and intensity if a physical or mental health improvement is indicated. | Selected as the primary category where the intervention focus and the majority of time was spent on exercise in this category (e.g. unstructured physical activity, including unstructured waking). |
| Endurance | Endurance training is aimed at cardiovascular conditioning and is aerobic in nature and simultaneously increases the heart rate and the return of blood to the heart. | Selected as the primary category for interventions where the intervention focus and the majority of time spent was on structured aerobic training (e.g. exercise with a target heart rate range). |
| Other | Other kind of exercises not described. | Selected as the primary category if the intervention did not meet the other categories listed and where the intervention focus and the majority of time was spent in this category. This category included interventions where the exercise was not described in sufficient detail to allocate a category. |
*Interventions were allocated primary categories using categorisation based on Sherrington 2019.
Types of outcome measures
We included studies that reported the rate or number of falls, or the number of participants experiencing at least one fall during the follow‐up. We included studies that recorded falls either prospectively or retrospectively.
Primary outcomes
Rate (number) of falls
Number of people who fell at least once (i.e. the number of fallers)
Secondary outcomes
Number of participants sustaining one or more fall‐related fractures
Quality of life
Rate (number) and type of adverse events (excluding falls)
Economic outcomes
Adverse events were only included in meta‐analyses when they were monitored using the same methods in all groups over the entire study period. We used the rate of adverse events excluding falls, as the rate of falls is presented separately in the analyses.
Timing of outcome measurement
One time point from each study was used for the primary outcomes. Where studies reported outcomes measured at multiple time periods, we used the longest time period available unless outcomes were monitored for over 12 months, in which case we used results reported at 12 months if these were available. We chose a 12‐month limit as nearly all fall studies in PD measure falls for 12 months or less. Where studies reported falls data for different time periods, we combined the data for the different time periods when possible. If this was not possible, we used the data from the time period closest to the end of the intervention period. For the quality of life outcomes, we used data from immediately after the end of the intervention, and data from follow‐up at a later time in separate analyses.
Search methods for identification of studies
Electronic searches
We performed searches up until the 16 July 2020 and conducted a top‐up search on the 13 October 2021. Studies identified in the top‐up search were added to 'Studies awaiting classification.' We searched the Cochrane Movement Disorders Group Trial Register and the Cochrane Central Register of Controlled Trials (CENTRAL, in The Cochrane Library; 2021, issue 11), MEDLINE (OvidSP from 1946), Embase (OvidSP from 1947), CINAHL (Cumulative Index to Nursing and Allied Health Literature) (EBSCO from 1982), PsycINFO (OvidSP from 1806), AMED (OvidSP from 1985), and the Physiotherapy Evidence Database (PEDro)(The University of Sydney, https://pedro.org.au/).
The full search strategy for each database can be found in Appendix 1.
Searching other resources
To identify any further published or ongoing trials, we:
searched trial registers: ClinicalTrials.gov (http://clinicaltrials.gov/), and the World Health Organization's International Clinical Trials Registry Platform Search Portal (http://apps.who.int/trialsearch/) (January 20, 2022) (see Appendix 1);
checked reference lists of relevant articles;
contacted trialists and researchers in the field;
used Science Citation Index Cited Reference Search;
checked studies included in the Cochrane Review of interventions for preventing falls in older people living in the community (Gillespie 2012; Hopewell 2018; Sherrington 2019) and the Cochrane Review of interventions for preventing falls in older people in care facilities and hospitals (Cameron 2018) for any trial which includes a subgroup of people with PD.
We did not apply any language restrictions.
Data collection and analysis
The intended methods for data collection and analysis for this review are published in our protocol (Canning 2015b). These are based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Review authors CC and NL separately screened the search results (title, abstract and descriptors) to identify studies for possible inclusion. Trial register results were excluded at this stage and searched separately through the trials registries as previously described. Any study that either researcher identified for possible inclusion was progressed to full‐text screening. CC and NL then separately assessed the eligibility of studies based on full text. Where a researcher involved in selecting studies was an author of a potentially eligible study, review author AN replaced them to assess the eligibility of that study. Again, disagreements were resolved through discussion or third‐party adjudication. Study authors were contacted for additional information if necessary.
Data extraction and management
Information for the included studies' table was extracted by pairs of review authors (LA, NA and TY).
Review authors NA and GV independently extracted data using a pre‐tested data extraction form (based on the one used in Sherrington 2019). Disagreement was resolved by consensus or third‐party adjudication. Review authors were not blinded to authors or sources.
The following information was collected.
General information: review author's name, study ID and first author of study.
Study details: study design and interventions, sample size, baseline fall rates, number of dropouts, cluster randomisation.
Rate of falls, number of people experiencing one or more falls, number of people experiencing one or more fall‐related fractures, rate and type of adverse events, quality of life, and cost and cost‐effectiveness information related to fall outcomes. Where data were provided in graphical form, we used the software program Web‐PlotDigitizer to extract the data (WebPlotDigitizer 2020).
We collected data from full‐text journal articles. Where a study had more than one journal article published, we consulted all articles for details. Where there was insufficient information reported, we contacted the study authors, requesting additional details.
Assessment of risk of bias in included studies
Pairs of review authors (NA, SK and NL) independently assessed risk of bias using the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017) and using a pre‐tested risk of bias assessment form. Review authors were not blinded to author and source institution. Review authors did not assess their own studies. Disagreement was resolved by consensus or third‐party adjudication.
We assessed the following domains, using the criteria developed by Gillespie 2012 for judging risk of bias in fall‐prevention trials (as outlined in Table 8): random sequence generation (selection bias); allocation concealment (selection bias); blinding of participants and personnel (performance bias); blinding of outcome assessment (detection bias) for falls and the number of people who fell at least once, and for fractures separately; incomplete outcome data (attrition bias) for falls and the number of people who fell at least once separately, and selective outcome reporting bias. We assessed bias in the recall of falls due to unreliable methods of ascertainment (Hannan 2010). We also used the specific criteria for assessing attrition bias in falls trials developed by Gillespie 2012 (Appendix 2). Additionally, we assessed the trials for any other potential sources of bias.
4. Risk of bias assessment tool.
| Domain | Criteria for judging risk of bias |
| Random sequence generation: selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence | • Judgement of ’low risk’ if the trial authors described a random component in the sequence generation, e.g. referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation. • Judgement of ’high risk’ if the trial used a systematic nonrandom method, e.g. date of admission; odd or even date of birth; case record number; clinician judgement; participant preference; patient risk factor score or test results; availability of intervention. • Judgement of ’unclear risk’ if there is insufficient information about the sequence generation process to permit judgement of ’low risk’ or ’high risk’. |
| Allocation concealment: selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment | • Judgement of ’low risk’ in studies using:
◦ individual randomisation if the trial described allocation concealment as by central allocation (telephone, internet‐based or pharmacy‐controlled randomisation); sequentially‐numbered identical drug containers; sequentially numbered, opaque, sealed envelopes; ◦ cluster randomisation if allocation of all cluster units performed at the start of the study and individual participant recruitment was completed prior to assignment of the cluster, and the same participants were followed up over time or individual participants were recruited after cluster assignment, but recruitment carried out by a person unaware of group allocation and participant characteristics (e.g. fall history) or individual participants in intervention and control arms were invited by mail questionnaire with identical information. • Judgement of ’high risk’ in studies using: ◦ individual randomisation if investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, e.g. using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes unsealed, non‐opaque, or not sequentially numbered; alternation or rotation; date of birth; case record number; or any other explicitly unconcealed procedure; ◦ cluster‐randomisation if individual participant recruitment was undertaken after group allocation by a person who was unblinded and may have had knowledge of participant characteristics. • Judgement of ’unclear risk’ if insufficient information to permit judgement of ’low risk’ or ’high risk’. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, e.g. if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed. |
| Blinding of participants and personnel: performance bias due to knowledge of the allocated interventions by participants and personnel carrying out the interventions | • Judgement of ’low risk’ if blinding of participants and personnel implementing the interventions was ensured, and unlikely that the blinding could have been broken. • Judgement of ’high risk’ if participants or intervention delivery personnel, or both, were not blinded to group allocation (e.g. exercise intervention), and the outcomes (falls and fractures) are likely to be influenced by lack of blinding. • Judgement of ’unclear risk’ if there is insufficient information to make a judgement of ’low risk’ or ’high risk’. |
| Blinding of outcome assessment: detection bias due to knowledge of the allocated interventions by outcome assessors | • Falls, fallers:
◦ judgement of ’low risk’ if outcomes were recorded/confirmed in all allocated groups using the same method and the personnel recording/confirming outcomes were blind to group allocation;
◦ judgement of ’high risk’ if outcomes were not recorded/confirmed in all allocated groups using the same method or the personnel recording/confirming outcomes were NOT blind to group allocation;
◦ judgement of ’unclear’ if there is insufficient information to make a judgement of ’low risk’ or ’high risk’. • Fractures: ◦ judgement of ’low risk’ if fractures were recorded/confirmed in all allocated groups using the same method and fractures were confirmed by the results of radiological examination or from primary care case records and the personnel recording/confirming fractures were blind to group allocation; ◦ judgement of ’high risk’ if fractures were not recorded/ confirmed in all allocated groups using the same method or the only evidence for fractures was from self reports from participants or carers; ◦ judgement of ’unclear risk’ if there is insufficient information to make a judgement of ’low risk’ or ’high risk’. |
| Incomplete outcome data: attrition bias due to amount, nature or handling of incomplete outcome data | • Judgement of ’low risk’ if there are no missing outcome data, or less than 20% of outcome data are missing and losses are balanced in numbers across intervention groups with similar reasons for missing data across groups or missing data have been imputed using appropriate methods. • Judgement of ’high risk’ if greater than 20% of outcome data missing, or reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups, or ‘as treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation or potentially inappropriate application of simple imputation. • Judgement of ’unclear risk’ if there is insufficient information to make a judgement of ’low risk’ or ’high risk’. See Appendix 2 for details |
| Selective reporting: reporting bias due to selective outcome reporting | • Judgement of ’low risk’ if the study protocol is available (i.e., published protocol or trial registry) and all prespecified study outcomes are reported in the prespecified way or the study protocol is unavailable, but it is clear the published report includes all expected outcomes. • Judgement of ’high risk’ if not all prespecified study outcomes are reported, or one or more primary outcomes are reported in ways which were not prespecified, or one or more outcomes are reported incompletely, or the study fails to include results for a key outcome that would be expected to be reported. • Judgement of ’unclear risk’ if there is insufficient information to make a judgement of ’low risk’ or ’high risk’. |
| Method of ascertaining falls: bias in the recall of falls due to unreliable methods of ascertainment | • Judgement of ’low risk’ if the study used some form of concurrent collection of data about falling, e.g. participants given postcards to fill in daily and mail back monthly, calendar to mark monthly, or more frequent, follow‐up by the researchers. • Judgement of ’high risk’ if ascertainment relied on participant recall at longer intervals than 1 month during the study or at its conclusion. • Judgement of ’unclear risk’ if there was retrospective recall over a short period only, or if the trial authors did not describe details of ascertainment, i.e. insufficient information was provided to allow a judgement of ’low risk’ or ’high risk’. |
We adapted this from Table 8.5.a 'The Cochrane Collaboration's tool for assessing risk of bias’ and Table 8.5.d 'Criteria for judging risk of bias in the 'Risk of bias’ assessment tool’ (Higgins 2017) and from Sherrington 2019.
We rated the risk of bias in each domain as high, low or unclear.
Measures of treatment effect
We reported treatment effect for rate of falls and rate of adverse events as a rate ratio (RaR) and 95% confidence interval (CI). The RaR compares the rate of events (falls or adverse events) between two groups in any given trial, where rate of events is the total number of events per unit of person time that events were monitored (e.g. falls per person year). If the RaR was reported in the included trial (e.g. incidence rate ratio or hazard ratio (HR)), we used the reported values. If both adjusted and unadjusted RaRs were reported, we used the unadjusted RaR, unless the adjustment was for clustering. If a RaR was not reported, but appropriate raw data were available, we used Excel to calculate a RaR and 95% CI. To do this, we used the reported rate of events (per person year) in each group or the reported total number of events in each group. If the rate of events in each group was not reported, where possible we calculated this as events per person year from the total number of events in that group, the length of time events were monitored and the number of participants contributing to the data. If there were no participants lost to follow‐up, or data were only available for participants completing the study, we assumed that participants' data had been collected for the maximum possible period of time.
It is possible that individual multiple fallers may have excessive influence on the rate of falls results. To investigate this possibility, we recorded procedures used by investigators to decrease this influence, such as randomisation stratified by fall history or analyses adjusted for previous falls. We also extracted baseline falling rates for each group (where available).
For the number of people who fell at least once and number of participants experiencing fall‐related fractures, we reported a risk ratio (RR) and 95% CI. The RR compares the number of people experiencing events (i.e. participants who fell once or more, or participants who experienced one or more fall‐related fractures) between groups. If the RR and 95% CI was reported (including relative risk, HR for first fall or odds ratio (OR)), we used the reported values. If both adjusted and unadjusted RRs were reported, we used the unadjusted RR, unless the adjustment was for clustering. If a RR was not reported, but data were available to calculate the relative risk and 95% CI, then this was calculated using the calculator function in RevMan 5.4. For these calculations, we used the number of participants reported contributing data to each group. If the number of participants contributing data was not known, we used the number randomised to each group.
Quality of life was reported as a continuous outcome. For these data we calculated mean differences (MD) with 95% CIs where data using one measurement were pooled, or standardised mean differences (SMD) and 95% CIs where data using different outcome measures were pooled. Where study authors reported median and interquartile range (IQR), the mean and standard deviation (SD) was estimated by review authors. For studies with smaller sample size (e.g. 40 participants), this was conducted using the technique described by Wan 2014. For larger trials (e.g. over 100 participants), this was conducted using the technique described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017).
Where comprehensive economic evaluations were incorporated in the included studies, we reported the incremental cost per fall prevented and/or per quality‐adjusted life‐year (QALY) gained by the intervention compared with the comparator group, as stated by the authors. We also extracted from studies reporting a cost analysis or cost description, the type of resource use (e.g. delivering the intervention, hospital admissions, outpatient visits) and the intervention and healthcare service costs per participant in each group.
Unit of analysis issues
We incorporated studies with more than one intervention arm compared with a control group, and therefore needed to avoid 'double‐counting' of control participants from these studies in any one meta‐analysis. To achieve this, each intervention was included in a separate comparison. For the RaRs and RRs, the standard errors (SEs) of the natural log of the between‐group difference were increased by 25% and participant numbers in the control group were allocated in proportion to the participant numbers in each intervention arm. For example, if a study had 70 participants in exercise group A, 70 in exercise group B and 70 in a control group, the SEs of the natural log of the between‐group difference in the exercise A versus control and exercise B versus control were increased by 25% and the number of control participants was shown as 35 in each comparison. For the continuous data (i.e. quality of life), the number of participants in the control group was divided equally among the comparisons and the control mean and SD were unchanged (Higgins 2017).
Data from cluster‐randomised trials were adjusted for clustering (Higgins 2017), if this had not already been done by the trial authors. If no estimate of the intra‐class correlation coefficient (ICC) was available, we used an ICC of 0.01 as reported by Smeeth 2002.
Dealing with missing data
We provided an overview of missing data from our selected studies in raw data tables. We did not use a cut‐off for missing data as an inclusion criterion. When outcome data were not reported, we contacted the study authors to request the data. We addressed the potential impact of missing data in the assessment of risk of bias.
Assessment of heterogeneity
We performed meta‐analyses when we considered study interventions to be similar enough to pool results. We assessed heterogeneity of these meta‐analyses by visual inspection of forest plots, as well as considering both the Chi2 test (with statistical significance set at P < 0.10) and the I2 statistic. We interpreted the I2 statistic according to Higgins 2017 who suggested: 0% to 40% may not be important; 30% to 60% may indicate moderate heterogeneity; 50% to 90% may indicate substantial heterogeneity; and 75% to 100% may indicate considerable heterogeneity. We performed subgroup analyses to determine whether heterogeneity was explained by study and/or participant characteristics.
Assessment of reporting biases
We minimised reporting bias by comprehensively searching multiple databases, searching for studies in languages other than English, and searching the grey literature and trial registries. We observed funnel plots for outcomes with more than 10 data points and considered reporting bias when using the GRADE approach to inform the certainty of the evidence in the summary of findings tables.
Data synthesis
We performed separate analyses to pool results of studies comparing an active fall‐prevention intervention with either ‘usual care’ or a ‘placebo’ control intervention, and studies comparing two active fall‐prevention interventions. We grouped similar intervention types together using the fall‐prevention classification taxonomy for intervention descriptors developed by ProFaNE (Lamb 2011). Furthermore, similar exercise interventions were grouped together according to ProFaNE exercise categories (Lamb 2011) (Table 7). Where meta‐analyses were appropriate (i.e. studies with comparable interventions and participant characteristics), we pooled results using fixed‐effect models, except where the review authors felt that it was unlikely that there would be a single true effect of the intervention on falls (i.e. exercise interventions and exercise plus education interventions), in which case random‐effects models were used. We considered it to be inappropriate to perform meta‐analyses where two active fall‐prevention interventions were compared. When meta‐analyses were not performed, trial‐level data are presented in forest plots and tables and narrative reviews are provided.
Where appropriate, pooled RaRs (for falls and adverse events) and pooled RRs (number of people who fell at least once and number of people sustaining one or more fall‐related fractures) were calculated using the generic inverse variance method in Review Manager software (RevMan 5.4). This involves entering the natural logarithm of the RaR or RR and its SE for each study. These values were calculated using Excel with the method developed for the Gillespie and colleagues Cochrane Review of interventions to prevent falls (Gillespie 2012).
The continuous quality of life outcomes were presented as MDs where one outcome measure was pooled, or SMDs where different outcome measures were pooled. Where SMDs were presented, the SMD was converted back to an MD in the summary of findings tables. This was done for the most commonly used outcome measure, using the SD from the baseline scores of the largest included study.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses to determine whether intervention impacts on primary outcomes varied according to baseline level of fall risk (increased fall risk due to previous fall or specified high fall risk versus fall risk not specified), or disease severity. For exercise trials, subgroup analysis was undertaken for the type of exercise (ProFaNE exercise category) and the proportion of exercise that was supervised.
For the subgroup analyses on disease severity, we extracted and pooled subgroup data from included studies that reported results by disease severity subgroups, and pooled these data using random‐effects meta‐analyses. This was because we were unable to categorise studies based on disease severity as most studies used populations with a range of disease severity and used different definitions of disease severity.
We used the random‐effects model to pool data in all analyses testing for subgroup differences due to the high risk of false‐positive results when comparing subgroups in a fixed‐effect model (Higgins 2017). We used the test for subgroup differences available in RevMan 5.4 to determine whether there was evidence of a difference in treatment effects between subgroups.
Sensitivity analysis
We performed sensitivity analyses to explore the impact of risk of bias on pooled estimates of treatment effect for the primary outcomes. We removed studies from pooled analyses if they were assessed as having high risk of bias in any item, or as having high or unclear risk of bias in a key domain: random‐sequence generation (selection bias), allocation concealment (selection bias), blinding of outcome assessors (detection bias), and incomplete outcome data (attrition bias) (see Higgins 2017). We performed a sensitivity analyses to explore the impact of fall monitoring time by removing studies from pooled analyses that monitored falls for less than three months. Additionally, we performed sensitivity analyses on comparisons with a high heterogeneity (I2 > 50%) by removing the studies that were responsible for the high levels of heterogeneity. We explored the impact of the model of meta‐analysis chosen by performing sensitivity analyses using fixed‐effect rather than random‐effects analyses on the exercise versus control and exercise plus education versus control studies and using random effects rather than fixed effects analyses on the cholinesterase inhibitor versus placebo studies. Additionally, we considered there was some subjectivity in the classification of exercise categories, so we performed a sensitivity analysis where studies that utilised functional strength training (e.g. using body weight, weighted vests and/or ankle weights) were re‐classified from resistance exercise to gait, balance and functional training.
Summary of findings and assessment of the certainty of the evidence
Summary of findings tables were prepared for each comparison where interventions were compared with control or placebo interventions. The certainty of the evidence in these tables for all outcomes where meta‐analyses had been conducted was assessed using the GRADE approach (Schűnemann 2013), utilising GRADEpro GDT (GRADEPro GDT 2015). This approach categorises the certainty of the evidence as high, moderate, low or very low depending on the evaluation of five factors: risk of bias; inconsistency of the effect; indirectness; imprecision; and publication bias. The certainty of the evidence and effect size were then used to determine the appropriate standardised statements to describe the certainty of the evidence (Cochrane Norway 2017). Decisions regarding whether to downgrade the evidence are described in the footnotes of the summary of findings tables.
Results
Description of studies
Results of the search
A flow diagram of the study selection process is shown in Figure 1. A total of 3459 records were downloaded, with the number from each database as follows: Cochrane Movement Disorders Group Trial Register and CENTRAL (663); MEDLINE (687), Embase (1665), CINHAL (174), PsycINFO (159), AMED (40) and PEDro (71).
1.
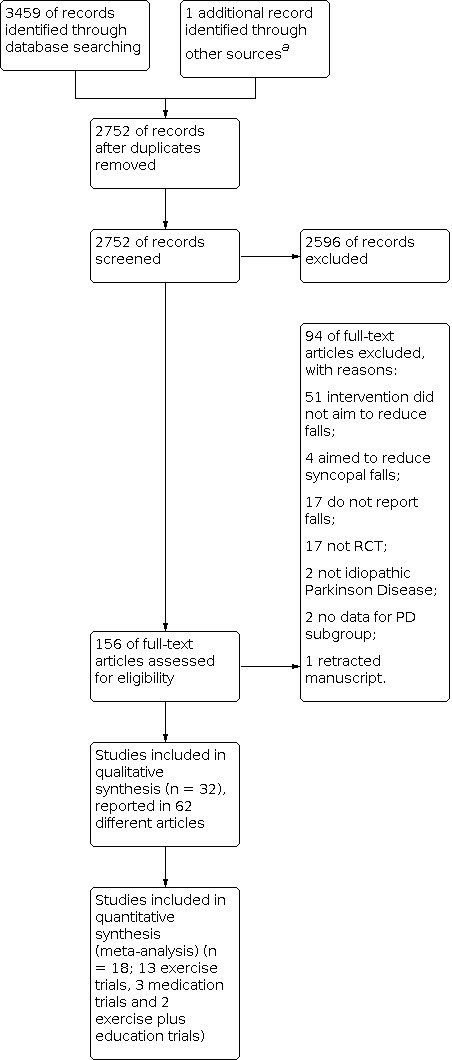
Study flow diagram.
a Ashburn 2019 was identified through contacting researchers in the field.
Following removal of duplicates, we screened the abstracts and titles of 2752 papers, resulting in 156 full‐text papers being considered. From these we removed 89 papers, leaving 67 reports of 36 studies. We contacted the authors of four studies (one with two reports (Hill 2015)) to request additional information regarding eligibility of the study (Hill 2015; Kurlan 2015; Sparrow 2016; Thaut 2019). We received responses from three (Hill 2015; Sparrow 2016;Thaut 2019). Three studies were excluded from the review (Hill 2015; Kurlan 2015; Sparrow 2016). Subsequently, a fourth study was excluded (Sato 2011) as the integrity of the data has been questioned (Bolland 2016) and the publication has been retracted by the journal. Information about the excluded studies is in the Characteristics of excluded studies. Consequently, there were 32 studies reported in 62 articles in the review. A flow‐diagram of the study selection process is in Figure 1.
Following the 'top‐up' search on 13 October 2021, an additional two eligible trials were identified. One trialled peroneal nerve functional electrical stimulation and the other trialled perturbation training. These have been added to the "Studies awaiting classification."
Included studies
This review includes 32 studies with 3370 participants randomised. There were 29 studies of a single intervention and three studies of multiple interventions. In the single intervention studies there were 25 studies of exercise (2700 participants randomised), three studies of cholinesterase inhibitors (242 participants randomised) and one study of education (53 participants with PD). The three studies of multiple interventions all trialled exercise plus education (375 participants randomised). Details of the studies are presented in the Characteristics of included studies.
We contacted the authors of 24 included studies for further information: 18 exercise studies, three medication studies, one education study and two exercise plus education studies. For the exercise studies, nine authors responded, and six authors provided further information that was used in the review (Ashburn 2007; Chivers Seymour 2019; Goodwin 2011; Harro 2014; Paul 2014; Thaut 2019). The remaining three authors were unable to provide the requested information (Martin 2015; Munneke 2010; Protas 2005). The authors for all three medication studies were contacted for further information, and two responded, providing information that was used in the review (Chung 2010; Henderson 2016). There was no response to our request for further information about the education study (Ward 2004). One of the two authors contacted regarding the exercise plus education studies responded with information that was used in the review (Morris 2015), but there was no response from the other author (Cattaneo 2019).
Trial design
All included studies were randomised controlled trials (RCTs), with one exercise study being cluster randomised by community hospitals and their catchment areas (Munneke 2010). The exercise studies had a total of 54 groups, with 10 exercise studies having two groups, one of which was a control group (i.e. usual care, or sham exercise) (Ashburn 2007; Canning 2015a; Chivers Seymour 2019; Gao 2014; Goodwin 2011; Martin 2015; Paul 2014; Protas 2005; Song 2018; Wong‐Yu 2015). A further 11 studies had two groups which compared two different exercise interventions (Gandolfi 2017; Gandolfi 2019; Harro 2014; Mirelman 2016; Munneke 2010; Penko 2019; Shen 2015; Smania 2010; Thaut 2019; Volpe 2014a; Volpe 2014b). There were four studies that compared three groups; two of these had two exercise groups and one control group (Li 2012; Sedaghati 2016) and two had three exercise groups (Pelosin 2017; Ricciardi 2015). All three medication studies had two groups and compared a cholinesterase inhibitor with a placebo (Chung 2010; Henderson 2016; Li 2015a). One of these studies was a randomised cross‐over trial (Chung 2010), the two others had parallel arms. The education study compared personalised health education, including education about falls prevention, with a control group (Ward 2004). Two of the exercise plus education studies compared the intervention with a control group (Morris 2017; Cattaneo 2019) while the third study had two intervention groups and one control group (Morris 2015).
Trial size
The median number of participants randomised per study in the exercise studies was 60 (interquartile range (IQR) 34 to 130), with sample size ranging from 18 (Protas 2005) to 474 (Chivers Seymour 2019). For the medication studies, the median number of participants randomised per study was 89 (IQR 56 to 109.5), with sample size ranging from 23 (Chung 2010) to 130 (Henderson 2016). There were 53 participants with PD in the education study (Ward 2004). The exercise plus education studies had a median of 133 participants randomised (IQR 83 to 172), with sample size ranging from 32 (Cattaneo 2019) to 210 (Morris 2015).
Trial setting
Of the exercise studies, 13 were conducted at a facility with full supervision (Gao 2014; Harro 2014; Li 2012; Mirelman 2016; Paul 2014; Pelosin 2017; Penko 2019; Protas 2005; Ricciardi 2015; Sedaghati 2016; Smania 2010; Volpe 2014a; Volpe 2014b); five were conducted partially at a facility and partially at home with four of these having an average of 35% (range 13% to 55%) of sessions supervised (Canning 2015a; Goodwin 2011; Shen 2015; Wong‐Yu 2015), and the proportion of supervision in the remaining study was unclear (Gandolfi 2019). Five studies were conducted entirely in the participants’ homes, and in four of these there was an average of 10% (range 5% to 18%) of sessions supervised (Ashburn 2007; Chivers Seymour 2019; Martin 2015; Song 2018). The proportion of supervision in the remaining home‐based study was unclear (Thaut 2019). One exercise study included both a group that attended a facility with full supervision, and a group that was home‐based and fully supervised in pairs via telehealth (Gandolfi 2017). There was one study where the setting of the study was unclear (Munneke 2010).
Of the three exercise plus education studies, two were conducted partially at a facility and partially at home (Cattaneo 2019; Morris 2015). One of these had 14% of the exercise supervised, and the education session delivered in a group setting (Cattaneo 2019). The remaining two studies both had 50% of the exercise sessions supervised, and the education sessions delivered individually (Morris 2015; Morris 2017), with one of these studies delivered wholly in participants’ homes (Morris 2017).
Participants
In the exercise studies, 2601 participants contributed data for the rate of falls (1456 in the exercise versus control meta‐analysis) and 1044 participants for the number of people who fell at least once (932 in the exercise versus control meta‐analysis). In the cholinesterase inhibitor versus placebo studies, 229 participants contributed data for the rate of falls outcome and 230 contributed data for the number of people who fell at least once. The study of an education intervention versus control did not report the rate of falls and included 53 participants in the number of people who fell at least once outcome. The three studies of exercise plus education versus control included 352 participants (320 participants from two RCTs for the rate of falls meta‐analysis and 352 participants from three RCTs in the number of people who fell at least once meta‐analysis). The inclusion and exclusion criteria and other participant details are presented in the Characteristics of included studies table.
The included studies described disease severity in a variety of ways, and overall, participants in the included studies had mild to moderate PD (see Characteristics of included studies), though the increased fall rates and inclusion of people with impaired cognition in the medication trials indicates these participants had more advanced disease overall than participants in the trials of other interventions.
For the exercise studies the average disease duration was 7.9 years and the average age was 68.3 years. Thirteen exercise studies specified that participants had to either have a recent history of one or more falls, or a fall risk factor to be included (Ashburn 2007; Canning 2015a; Chivers Seymour 2019; Gao 2014; Goodwin 2011; Mirelman 2016; Penko 2019; Protas 2005; Sedaghati 2016; Smania 2010; Thaut 2019; Volpe 2014a; Volpe 2014b). One study included only participants with no history of falls (Wong‐Yu 2015). For the medication studies, the average disease duration was 7.9 years and average age was 68.3 years. Two of the three cholinesterase inhibitor versus placebo studies specified that participants required a history of falls to be included (Henderson 2016; Li 2015a), with one study requiring at least one fall in the prior year (Henderson 2016), and the other requiring two or more falls or near falls each week, without freezing of gait (Chung 2010).
The single study of an education intervention did not report age, disease severity or disease duration for the PD subgroup, and did not require a history of falls for participation (Ward 2004).
Of the three studies of exercise plus education versus control, one included people with and without PD and reported data for, but not the characteristics of the PD subgroup (Cattaneo 2019). The remaining two studies included people with mild to moderately severe PD with an average age of 69 years (Morris 2015; Morris 2017). An average disease duration of 6.7 years was reported in one of these studies (Morris 2015). There was no requirement for participants in any of these studies to have a history of falls.
Most studies excluded participants with significant cognitive impairment (usually defined as a Mini‐mental State Examination score of below 24). There was one exercise study (Mirelman 2016) and one exercise plus education study (Cattaneo 2019) that included participants with mild cognitive impairment (Mini‐mental State Examination ≥ 21). Two studies only excluded people with dementia; one medication study (Henderson 2016) and the education study (Ward 2004). Another medication study (Li 2015a) recruited only people with cognitive impairment.
Interventions
In the exercise studies, exercise was compared with a control intervention (i.e. usual care or an intervention not expected to have an effect on falls, such as ‘sham’ exercise or upper limb exercise) in 12 studies (Ashburn 2007; Canning 2015a; Chivers Seymour 2019; Gao 2014; Goodwin 2011; Li 2012; Martin 2015; Paul 2014; Protas 2005; Sedaghati 2016; Song 2018; Wong‐Yu 2015), and with an alternative form of exercise in 15 studies (Gandolfi 2017; Gandolfi 2019; Harro 2014; Li 2012; Mirelman 2016; Munneke 2010; Pelosin 2017; Penko 2019; Ricciardi 2015; Sedaghati 2016; Shen 2015; Smania 2010; Thaut 2019; Volpe 2014a; Volpe 2014b). Three of these studies compared more than one exercise intervention with a control intervention (Li 2012; Pelosin 2017; Sedaghati 2016). Overall, there were 42 exercise interventions and 12 control interventions.
The exercise interventions were grouped into categories based on the ProFaNE taxonomy (Table 7). The features of the exercise interventions are presented in Table 9. Most exercise interventions (34/42, 81%) were categorised as primarily gait, balance and functional training. PD‐specific exercises such as movement strategy training and cueing were included in this category. There were three resistance training interventions (7%) (Li 2012; Paul 2014; Shen 2015). Two interventions (5%) were of 3D exercise (Tai Chi; Li 2012; Gao 2014) and one intervention (2%) utilised flexibility exercises (Smania 2010). A further two interventions (5%) were from a study that compared physiotherapy provided by therapists with specific PD training according to evidence‐based guidelines with physiotherapy provided by usual therapists, but the specific details of the interventions were not provided (Munneke 2010). The duration of the exercise interventions ranged from 6 to 26 weeks (mean (SD) 11.3 (SD 6.9) weeks).
5. Features of exercise interventions.
| Study ID | Exercise description | Primary exercise category | Duration of exercise intervention (weeks) | Group/Individual | Location | % supervision* |
| Exercise trials | ||||||
|
Ashburn 2007 |
Functional strength, range of movement, balance and walking exercise. | Gait, balance and functional training | 6 | Individual | Home‐based | 18% |
|
Canning 2015a |
Functional strength, balance and cueing exercise (some participants attended monthly group classes). | Gait, balance and functional training | 24 | Both (most individual but some participants attended monthly exercise classes) | Both (most home‐based (but classes were held at a facility) | 13% |
|
Chivers Seymour 2019 |
Functional strength and balance exercise and strategies for fall and freezing avoidance. | Gait, balance and functional training | 26 | Individual | Home‐based | 7% |
| Gandolfi 2017 | Virtual reality balance training delivered via telehealth |
Gait, balance and functional training | 7 | Group (pairs) | Home‐based | 100% |
| Gandolfi 2017 | Sensory‐integration balance training | Gait, balance and functional training | 7 | Individual | Facility‐based | 100% |
| Gandolfi 2019 | Trunk‐specific exercise | Gait, balance and functional training | 4 | Individual | Both | Unclear ‐ 100% at facility, number of unsupervised home‐sessions prescribed unclear |
| Gandolfi 2019 | General exercise | Gait, balance and functional training | 4 | Individual | Both | Unclear ‐ 100% at facility, number of unsupervised home‐sessions prescribed unclear |
|
Gao 2014 |
Tai Chi classes. | 3D (Tai Chi) | 12 | Group | Facility‐based | 100% |
|
Goodwin 2011 |
Functional strength and balance exercise. | Gait, balance and functional training | 10 | Both | Both | 33% |
|
Harro 2014 |
Rhythmic auditory cued overground walking. | Gait, balance and functional training | 6 | Group | Facility‐based | 100% |
|
Harro 2014 |
Treadmill‐based gait training. | Gait, balance and functional training | 6 | Individual | Facility‐based | 100% |
|
Li 2012 |
Tai Chi classes. | 3D (Tai Chi) | 24 | Group | Facility‐based | 100% |
|
Li 2012 |
Functional strength exercise with weighted vests and ankle weights. | Resistance training | 24 | Group | Facility‐based | 100% |
|
Martin 2015 |
Exercises to address freezing of gait and associated falls, and walking using cues. | Gait, balance and functional training | 24 | Individual | Home‐based | 5% |
|
Mirelman 2016 |
Treadmill training in a virtual reality environment. | Gait, balance and functional training | 6 | Individual | Facility‐based | 100% |
|
Mirelman 2016 |
Treadmill‐based gait training. | Gait, balance and functional training | 6 | Individual | Facility‐based | 100% |
|
Munneke 2010 |
Physiotherapy provided by ParkinsonNet therapists. | Other ‐ ParkinsonNet trained therapists | 24 | Individual | Unclear | ND |
|
Munneke 2010 |
Physiotherapy usual care. | Other ‐ usual therapists | 24 | Individual | Unclear | ND |
|
Paul 2014 |
Progressive lower limb muscle power training using strength training machines. | Resistance training | 12 | Group (pairs) | Facility‐based | 100% |
| Pelosin 2017 | High frequency treadmill training (5 times per week for 10 sessions) | Gait, balance and functional training | 2 | Individual | Facility‐based | 100% |
| Pelosin 2017 | Intermediate frequency treadmill training (3 times per week for 10 sessions) | Gait, balance and functional training | 3.3 | Individual | Facility‐based | 100% |
| Pelosin 2017 | Low frequency treadmill training (2 times per week for 10 sessions) | Gait, balance and functional training | 5 | Individual | Facility‐based | 100% |
| Penko 2019 | Gait and cognitive training practised together | Gait, balance and functional training | 8 | Individual | Facility‐based | 100% |
| Penko 2019 | Gait and cognitive training practised separately | Gait, balance and functional training | 8 | Individual | Facility‐based | 100% |
|
Protas 2005 |
Gait and stepping training. | Gait, balance and functional training | 8 | Individual | Facility‐based | 100% |
|
Ricciardi 2015 |
Strength, balance and gait training targeting the more affected side. | Gait, balance and functional training | 12 | Unclear | Facility‐based | 100% |
|
Ricciardi 2015 |
Strength, balance and gait training targeting the less affected side. | Gait, balance and functional training | 12 | Unclear | Facility‐based | 100% |
|
Ricciardi 2015 |
Functional strength, balance and gait training. | Gait, balance and functional training | 12 | Unclear | Facility‐based | 100% |
|
Sedaghati 2016 |
Progressive balance and gait training with a balance pad (i.e. foam to stand on). | Gait, balance and functional training | 10 | Unclear | Facility‐based | 100% |
|
Sedaghati 2016 |
Progressive balance and gait training without a balance pad. | Gait, balance and functional training | 10 | Unclear | Facility‐based | 100% |
|
Shen 2015 |
Balance and gait training. | Gait, balance and functional training | 12 | Unclear | Both | 55% |
|
Shen 2015 |
Lower limb resistance training using strength training machines (facility) and functional strength training (home) | Resistance training | 12 | Unclear | Both | 55% |
|
Smania 2010 |
Balance exercises. | Gait, balance and functional training | 7 | Individual | Facility‐based | 100% |
|
Smania 2010 |
Flexibility and coordination exercises not aimed at improving balance. | Flexibility | 7 | Individual | Facility‐based | 100% |
| Song 2018 | Stepping videogame exercise | Gait, balance and functional training | 12 | Individual | Home‐based | 8% |
| Thaut 2019 | Gait training with rhythmic auditory stimulation throughout intervention period | Gait, balance and functional training | 24 | Individual | Home‐based | Unclear |
| Thaut 2019 | Gait training with rhythmic auditory stimulation, with no training in middle 8 weeks of intervention period | Gait, balance and functional training | 16 | Individual | Home‐based | Unclear |
|
Volpe 2014a |
Balance training using external perturbations wearing a proprioceptive stabiliser. | Gait, balance and functional training | 8 | Individual | Facility‐based | 100% |
|
Volpe 2014a |
Balance training using external perturbations with a sham proprioceptive stabiliser. | Gait, balance and functional training | 8 | Individual | Facility‐based | 100% |
|
Volpe 2014b |
Hydrotherapy with perturbation‐based balance training. | Gait, balance and functional training | 8 | Unclear | Facility‐based | 100% |
|
Volpe 2014b |
Land‐based therapy with perturbation‐based balance training. | Gait, balance and functional training | 8 | Unclear | Facility‐based | 100% |
|
Wong‐Yu 2015 |
Strength and balance exercise, including dance and modified Wing Chun martial art. | Gait, balance and functional training | 8 | Both | Both | 40% |
| Exercise plus education trials | ||||||
| Cattaneo 2019 | Tailored mobility and balance exercises (plus fall prevention education). | Gait, balance and functional training | 8 | Individual | Home‐based | 14% |
|
Morris 2015 |
Functional progressive resistance training with weighted vests and resistance bands. | Resistance training | 8 | Individual | Both | 50% |
|
Morris 2015 |
Movement strategy training. | Gait, balance and functional training | 8 | Individual | Both | 50% |
|
Morris 2017 |
Functional strength, movement strategy training (plus falls prevention education). | Gait, balance and functional training | 6 | Individual | Home‐based | 50% |
* % supervision calculated according to the % of exercise sessions supervised.
ND: no useable data
In the medication studies, three trials compared a cholinesterase inhibitor with a placebo. Two of these studies trialled rivastigmine, for either eight months (Henderson 2016) or 12 months (Li 2015a). The other trialled donepezil for six weeks (Chung 2010).
The education study (Ward 2004) provided individualised education and information in the form of a 12‐month health action plan, designed to improve each participant’s physical, social and psychological well‐being, including addressing fall risk. The education was delivered in participants’ homes by an occupational therapist through one home visit and a subsequent phone call.
In all three of the exercise plus education studies, the intervention was compared with a control intervention (Cattaneo 2019; Morris 2015; Morris 2017), with one of these also comparing with an alternative form of exercise plus education (Morris 2015). Two studies used home‐based exercise that was categorised as gait, balance and functional training (Cattaneo 2019; Morris 2017). The remaining study conducted the exercise interventions at a facility and at home, with one intervention categorised as gait, balance and functional training and the other as resistance training (Morris 2015). The features of the exercise interventions are presented in Table 9. The fall‐prevention education was provided individually at the time of the weekly supervised exercise session in two studies (Morris 2015; Morris 2017). In the remaining study there was a single one‐hour group education session about fall prevention which occurred before the exercise program was prescribed (Cattaneo 2019).
Outcomes
The source and time period of the data used for the generic inverse variance analysis (falls, fractures and adverse events (adverse events for the medication studies only)) outcomes for each study is shown in Table 10 and Table 11. Raw data for these outcomes and baseline falls data, when available, are shown in Table 11 and Table 12, respectively. Raw quality of life data is in Table 13. Data from studies reporting an economic analysis related to the cost of the intervention and/or fall outcomes is in Table 14, and information related to adverse events is in Table 15.
6. Source of data for generic inverse variance analysis (see footnotes for explanations of codes).
| Study ID and comparison | Source for rate ratio: rate of falls | Source for risk ratio: number of fallers | Source for risk ratio: number with fractures | Source for risk ratio: number with adverse events |
| Exercise trials | ||||
|
Ashburn 2007 Gait, balance and functional training vs Control |
3* | 7 | 7 | NA |
|
Canning 2015a+ Gait, balance and functional training vs Control |
1 | 5 | 7 | NA |
|
Chivers Seymour 2019‡ Gait, balance and functional training vs Control |
1a++ | NA | 7 | NA |
|
Gandolfi 2017 Gait, balance and functional training (virtual reality telerehabilitation) vs Gait, balance and functional training (balance training in a facility) |
3‡‡‡ | NA | NA | NA |
|
Gandolfi 2019 Gait, balance and functional training (trunk‐specific exercises) vs Gait, balance and functional training (general exercises) |
3‡‡‡ | NA | NA | NA |
|
Gao 2014 3D exercise (Tai Chi) vs Control |
3 | 7 | NA | NA |
|
Goodwin 2011‡‡ Gait, balance and functional training vs Control |
1a++ | 6a | 7 | NA |
|
Harro 2014 Gait, balance and functional training (cueing training) vs Gait, balance and functional training (treadmill‐based gait training) |
3 | 7 | NA | NA |
|
Li 2012 3D exercise (Tai Chi) vs Resistance training (functional strength) and 3D exercise (Tai Chi) vs Control |
1 | 7 | NE | NA |
|
Li 2012 Resistance training (functional strength) vs Control |
3 | 7 | NE | NA |
|
Martin 2015 Gait, balance and functional training vs Control |
1* | 7 | NA | NA |
|
Mirelman 2016 Gait, balance and functional training (virtual reality treadmill training) vs Gait, balance and functional training (treadmill‐based gait training) |
1a | NA | NA | NA |
|
Munneke 2010 Other exercise (ParkinsonNet therapists) vs Other exercise (standard therapists) |
3c | NA | NA | NA |
|
Paul 2014 Resistance training vs Control |
1** | 5 | 7 | NA |
|
Pelosin 2017 Gait, balance and functional training (treadmill training at high frequency) vs Gait balance and functional training (treadmill training at intermediate frequency) vs Gait, balance and functional training (treadmill training at low frequency) |
3‡‡‡ | NA | NA | NA |
|
Penko 2019 Gait, balance and functional training (Gait and cognitive training practised together) vs Gait, balance and functional training (Gait and cognitive training practised separately) |
3‡‡‡ | NA | NA | NA |
|
Protas 2005 Gait, balance and functional training vs Control |
3 | 7 | NA | NA |
|
Ricciardi 2015 Gait, balance and functional training (best side therapy) vs Gait, balance and functional training (worst side therapy) vs Gait, balance and functional training (standard therapy) |
3 | NA | NA | NA |
|
Sedaghati 2016 Gait, balance and functional training (with a balance pad) vs Gait, balance and functional training (without a balance pad) vs Control |
3 | NA | NA | NA |
|
Shen 2015*** Gait, balance and functional training vs Resistance training |
1a | 7 | 7 | NA |
|
Smania 2010 Gait, balance and functional training vs Flexibility exercise |
3 | NA | NA | NA |
|
Song 2018 Gait, balance and functional training vs Control |
1 | 7 | NA | NA |
|
Thaut 2019 Gait, balance and functional training (rhythmic auditory stimulation training throughout intervention period) vs Gait, balance and functional training (rhythmic auditory stimulation training with no training in middle 8 weeks of intervention period) |
NA | 7 | NA | NA |
|
Volpe 2014a Gait, balance and functional training (with proprioceptive stabiliser) vs Gait, balance and functional training (without proprioceptive stabiliser) |
3 | NA | NA | NA |
|
Volpe 2014b Gait, balance and functional training (hydrotherapy) vs Gait, balance and functional training (land‐based therapy) |
3 | NA | NE | NA |
|
Wong‐Yu 2015 Gait, balance and functional training vs Control |
1 | 6 | NA | NA |
| Medication trials | ||||
|
Chung 2010 Donepezil vs placebo |
3 | 7 | NE | 3 |
|
Henderson 2016 Rivastigmine vs placebo |
1* | 7 | NA | 3 |
|
Li 2015a Rivastigmine vs placebo |
3 | 6 | NA | ND |
| Education trial | ||||
|
Ward 2004 Personalised education vs control (standardised printed information) |
NA | 6a | NA | NA |
| Exercise plus education trials | ||||
|
Cattaneo 2019 Gait, balance and functional training + education vs Control |
NA | 4 | NA | NA |
|
Morris 2015 Resistance training (functional strength) + education vs Control and Gait, balance and functional training (movement strategy training) + education vs Control |
1 | 5 | 7 | NA |
|
Morris 2015 Resistance training (functional strength) + education vs Gait, balance and functional training (movement strategy training) + education |
3 | 7 | 7 | NA |
|
Morris 2017 Gait, balance and functional training + education vs Control |
1 | 5 | 7 | NA |
ND: no useable data; NA: not applicable (not reported as an outcome in the trial OR not applicable for adverse events for exercise and exercise plus education trials as these were not pooled); NE (no events in either group.)
*One participant with excessive number of falls removed from analysis.
**Two participants with excessive number of falls assigned a value of 10 falls.
***One participant from the balance group and 2 from the resistance group with excessive number of falls at baseline removed from the analysis.
+randomisation stratified by falls history
++adjusted for previous falls
+++Incidence rate ratio using Poisson‐Inverse Gaussian regression, with unpublished 95% confidence interval provided by trial authors.
‡0 to 6 months data used as 0 to 12 months not available
‡‡0 to 10 weeks data used for rate ratio as 0 to 20 weeks not available
‡‡‡the separate time periods of falls data were combined
Codes for source of rate ratio:
1. Incidence rate ratio reported by trial authors
2. Hazard ratio/relative hazard (multiple events) reported by trial authors
3. Incidence rate ratio calculated by review authors
a. Adjusted for confounders by trial authors
b. Adjusted for clustering by trial authors
c. Adjusted for clustering by review authors
Codes for source of risk ratio:
4. Hazard ratio/relative hazard (first fall only) reported by trial authors
5. Relative risk reported by trial authors
6. Odds ratio reported by trial authors
7. Relative risk calculated by review authors
a. Adjusted for confounders by trial authors
b. Adjusted for clustering by trial authors
c. Adjusted for clustering by review authors
7. Raw data for rate ratios and risk ratios.
| Study ID and comparison | Intervention group: falls per person year | Intervention group: number (%) of fallers | Intervention group: number (%) of people sustaining one or more fall‐related fractures | Intervention group: non‐fall‐related adverse events per person year | Intervention group: number in analysis | Control group: falls per person year | Control group: number (%) of fallers | Control group: number (%) of people sustaining one or more fall‐related fractures | Control group: non‐fall‐related adverse events per person year | Control group: number in analysis | Length of falls/adverse events monitoring |
| Exercise trials | |||||||||||
|
Ashburn 2007 Gait, balance and functional training vs Control |
6.3 | 46 (73%) | 2 (3%) | NA | Rate of falls and number of fallers: 63 Number sustaining fracture: 67 |
7.9* | 49 (78%) | 6 (9%) | NA | Rate of falls: 62 Number of fallers: 63 Number sustaining fracture: 67 |
6 months |
|
Canning 2015a Gait, balance and functional training vs Control |
8.2 | 75 (65%) | 3 (3%) | NA | 115 | 14.0 | 81 (70%) | 4 (3%) | NA | 116 | 6 months |
|
Chivers Seymour 2019 Gait, balance and functional training vs Control |
0‐6 months: 6.8 6‐12 months: 5.4 |
NA | 0‐6 months: 5 (2%) 6‐12 months: 7 (5%) |
NA | 0‐6 months: 231 6‐12 months: 127 |
0‐6 months: 5.4 6‐12 months: 5.6 |
NA | 0‐6 months: 9 (4%) 6‐12 months: 3 (2%) |
NA | 0‐6 months: 230 6‐12 months: 147 |
12 months, divided into 0‐6 and 6‐12 month time periods |
|
Gandolfi 2017 Gait, balance and functional training (virtual reality telerehabilitation) vs Gait, balance and functional training (balance training in a facility) |
4.0/8.5 | NA | NA | NA | 36/34 | NA | NA | NA | NA | NA | 2 months‡‡ |
|
Gandolfi 2019 Gait, balance and functional training (trunk‐specific exercises) vs Gait, balance and functional training (general exercises) |
6.3/2.9 | NA | NA | NA | 19/18 | NA | NA | NA | NA | NA | 2 months‡‡ |
|
Gao 2014 3D exercise (Tai Chi) vs Control |
0.6 | 8 (22%) | NA | NA | 37 | 1.3 | 19 (49%) | NA | NA | 39 | 6 months |
|
Goodwin 2011 Gait, balance and functional training vs Control |
0‐10 weeks: 93.9 10‐20 weeks: 34.5 |
0‐20 weeks: 52 (85%) |
0‐20 weeks: 0 (0%) | NA | 61 | 0‐10 weeks: 168.1 10‐20 weeks: 155.4 |
0‐20 weeks: 55 (86%) |
0‐20 weeks: 1 (2%) |
NA | 64 | 20 weeks |
|
Harro 2014 Gait, balance and functional training (cueing training) / Gait, balance and functional training (treadmill‐based gait training) |
0.4/1.0 | 2 (20%)/4 (40%) | NA | NA | 10/10 | NA | NA | NA | NA | NA | 6 months |
|
Li 2012 3D exercise (Tai Chi) / Resistance training vs Control |
1.9/4.1 | 19 (29%)/31 (48%) | 0 (0%)/0 (0%) | NA | 65/65 | 5.7 | 26 (40%) | 0 (0%) | NA | 65 | 6 months |
|
Martin 2015 Gait, balance and functional training vs Control |
166.4 (using fall rate data from week 24‐28)* | 10 (100%) | NA | NA | Fall rate: 9 Number of fallers: 10 |
140.4 (using fall rate data from week 24‐28) | 9 (100%) | NA | NA | Fall rate: 8 Number of fallers: 9 |
6 months |
|
Mirelman 2016 Gait, balance and functional training (virtual reality treadmill training) / Gait, balance and functional training (treadmill‐based gait training) |
ND | NA | NA | NA | 66/64 | NA | NA | NA | NA | NA | 6 months |
|
Munneke 2010 Other exercise (ParkinsonNet therapists) / Other exercise (standard therapists) |
1.5/1.4 | NA | NA | NA | 329/312 | NA | NA | NA | NA | NA | 24 weeks |
|
Paul 2014 Resistance training vs Control |
6.5 | 7 (37%) | 1 (5%) | NA | 19 | 11.6 | 12 (63%) | 0 (0%) | NA | 19 | 6 months |
|
Pelosin 2017 Gait, balance and functional training (treadmill training at high frequency) vs Gait balance and functional training (treadmill training at intermediate frequency) vs Gait, balance and functional training (treadmill training at low frequency) |
Unclear, as timeframe for falls monitoring not reported | NA | NA | NA | 10/10/10 | NA | NA | NA | NA | NA | Unclear |
|
Penko 2019 Gait, balance and functional training (Gait and cognitive training practised together) vs Gait, balance and functional training (Gait and cognitive training practised separately) |
9.1/8.2 | NA | NA | NA | 10/9 | NA | NA | NA | NA | NA | 2 months‡‡ |
|
Protas 2005 Gait, balance and functional training vs Control |
23.1 | 5 (56%) | ND | NA | 9 | 37.6 | 6 (67%) | ND | NA | 9 | 2 weeks |
|
Ricciardi 2015 Gait, balance and functional training (best side therapy) / Gait, balance and functional training (worst side therapy) / Gait, balance and functional training (standard therapy) |
11.1/7.2/4.9 | NA | NA | NA | 9/9/9 | NA | NA | NA | NA | NA | 16 weeks |
|
Sedaghati 2016 Gait, balance and functional training (with a balance pad) / Gait, balance and functional training (without a balance pad) vs Control |
1.04/4.16 | NA | NA | NA | 15/14 | 7.8 | NA | NA | NA | 15 | 10 weeks |
|
Shen 2015 Gait, balance and functional training / Resistance training |
0.41/1.02 | 6 (27%)/13 (57%) | 1 (5%)/1 (4%) | NA | Fall rate: 21/21 Number of fallers and fractures: 22/23 |
NA | NA | NA | NA | NA | 15 months |
|
Smania 2010 Gait, balance and functional training / Flexibility exercise |
15.6/49.2 | NA | NA | NA | 28/27 | NA | NA | NA | NA | NA | 1 month |
|
Song 2018 Gait, balance and functional training vs Control |
9.4 | 16 (55%) | NA | NA | 29 | 8.6 | 17 (68%) | NA | NA | 25 | 6 months |
|
Thaut 2019 Gait, balance and functional training (rhythmic auditory stimulation training throughout intervention period) vs Gait, balance and functional training (rhythmic auditory stimulation training with no training in middle 8 weeks of intervention period) |
NA | 24 (96%)/22 (100%) | NA | NA | 25/22 | NA | NA | NA | NA | ||
|
Volpe 2014a Gait, balance and functional training (with proprioceptive stabiliser) / Gait, balance and functional training (without proprioceptive stabiliser) |
11.4/18.6 | NA | NA | NA | 20/20 | NA | NA | NA | NA | NA | 4 months |
|
Volpe 2014b Gait, balance and functional training (hydrotherapy) / Gait, balance and functional training (land‐based therapy) |
3.6/9.6 | NA | 0 (0%)/0 (0%) | NA | 17/17 | NA | NA | NA | NA | NA | 2 months |
|
Wong‐Yu 2015 Gait, balance and functional training vs Control |
0.38 | 6 (19%) | NA | NA | 32 | 0.38 | 8 (22%) | NA | NA | 36 | 6 months |
| Medication trials | |||||||||||
|
Chung 2010 Donepezil vs placebo+ |
47.45 | 18 (95%) | 0 (0%) | 3.0 | Fall rates and number of fallers and fractures:19 Adverse events: 23 |
91.25 | 16 (84%) | 0 (0%) | 1.1 | Fall rates and number of fallers and fractures: 19 Adverse events: 23 |
12 weeks |
|
Henderson 2016 Rivastigmine vs placebo |
16.8* | 56 (86%) | NA | 4.4 | Fall rate and adverse events: 64 Number of fallers and fractures:65 |
28.8 | 56 (86%) | NA | 2.8 | 65 | 8 months |
|
Li 2015a Rivastigmine vs placebo |
1.82 | 13 (32%) | NA | ND | 41 | 4.26 | 24 (60%) | NA | ND | 40 | 12 months |
| Education trial | |||||||||||
|
Ward 2004 Personalised education vs control (standardised printed information) |
NA | ND | NA | NA | 27 | NA | ND | NA | NA | 26 | 12 months |
| Education plus exercise trials | |||||||||||
|
Cattaneo 2019 Gait, balance and functional training plus education vs Control |
NA | ND | NA | NA | 15 | NA | ND | NA | NA | 17 | 6 months |
|
Morris 2015 Resistance training / Gait, balance and functional training (movement strategy training) vs Control |
2.8/6.6 | 36 (52%)/44 (66%) | 3 (4%)/3 (4%) | NA | 69/67 | 18.6 | 37 (63%) | 2 (3%) | NA | 59 | 12 months |
|
Morris 2017 Gait, balance and functional training plus education vs Control |
21.9 | 39 (61%) | 2 (3%) | NA | 64 | 14.2 | 43 (72%) | 1 (2%) | NA | 60 | 12 months |
ND: no useable data; NA: not applicable (not reported as an outcome in the trial OR not applicable for adverse events for exercise and education trials as these were not pooled).
*outlier with excessive number of falls excluded
+randomised cross‐over trial
‡‡the two separate months of falls data were combined
8. Baseline fall data.
| Study ID and groups | Intervention group: Number of participants | Intervention group: falls per person year | Intervention group: number (%) of fallers | Control group: number of participants | Control group: falls per person year | Control group: number (%) of fallers | Randomisation stratified by fall history | Timeframe for baseline falls monitoring |
| Exercise trials | ||||||||
|
Ashburn 2007 Gait, balance and functional training vs Control |
70 | 60 | 70 (100%) | 72 | 61 | 72 (100%) | No | 12 months, measured retrospectively |
|
Canning 2015a Gait, balance and functional training vs Control |
115 | 2 | 90 (78%) | 116 | 2 | 90 (78%) | Yes | 12 months, measured retrospectively |
|
Chivers Seymour 2019 Gait, balance and functional training vs Control |
3 months prospective: 237 12 months retrospective: 238 |
3 months prospective: 23.6 12 months retrospective: 26 |
12 months retrospective: 238 (100%) | 3 months prospective and 12 months retrospective: 236 |
3 months prospective: 12 12 months retrospective: 19 |
236 (100%) | No | 3 months, measured prospectively prior to commencing intervention and 12 months, measured retrospectively |
|
Gandolfi 2017 Gait, balance and functional training (virtual reality telerehabilitation) vs Gait, balance and functional training (balance training in a facility) |
38/38 | 6.9/22.1 | ND | NA | NA | NA | No | 1 month, unclear if measured prospectively or retrospectively |
|
Gandolfi 2019 Gait, balance and functional training (trunk‐specific exercises) vs Gait, balance and functional training (general exercises) |
19/18 | 19.6/7.9 | ND | NA | NA | NA | No | 1 month, unclear if measured prospectively or retrospectively |
|
Gao 2014 3D exercise (Tai Chi) vs Control |
40 | ND | ND | 40 | ND | ND | No | ND |
|
Goodwin 2011 Gait, balance and functional training vs Control |
Rate of falls analysis: 60 Number of fallers analysis: 64 |
137.8 | 55 (86%) | Rate of falls analysis: 62 Number of fallers analysis: 66 |
156.2 | 54 (82%) | No | 10 weeks, measured prospectively prior to commencing intervention |
|
Harro 2014 Gait, balance and functional training (cueing training) / Gait, balance and functional training (treadmill‐based gait training) |
10/10 | 1/1.4 | 3 (30%)/5 (50%) | NA | NA | NA | No | 6 months, measured retrospectively |
|
Li 2012 3D exercise (Tai Chi) / resistance training (functional strength) vs Control |
65/65 | ND | ND | 65 | ND | ND | No | ND |
|
Martin 2015 Gait, balance and functional training vs Control |
Rate of falls analysis: 11* Number of fallers analysis: 12 |
202.8 | 9 (75%) | 9 | 150.8 | 6 (67%) | No | 5 weeks, measured prospectively from the point of study entry ‐ unclear if this overlaps with the first 3 weeks of the intervention period |
|
Mirelman 2016 Gait, balance and functional training (virtual reality treadmill training) / Gait, balance and functional training (treadmill‐based gait training) |
66/64 | 36.5/38.5 | 66 (100%)/64 (100%) | NA | NA | NA | No | 6 months, measured retrospectively |
|
Munneke 2010 Other exercise (ParkinsonNet therapists) / Other exercise (standard therapists) |
358/341 | ND | ND | NA | NA | NA | No | ND |
|
Paul 2014 Resistance training vs Control |
20 | ND | 5 (25%) | 20 | ND | 7 (35% | No | 12 months, measured retrospectively |
|
Pelosin 2017 Gait, balance and functional training (treadmill training at high frequency) vs Gait balance and functional training (treadmill training at intermediate frequency) vs Gait, balance and functional training (treadmill training at low frequency) |
10/10/10 | Unclear, as timeframe for falls monitoring not reported | NA | NA | NA | NA | No | Unclear |
|
Penko 2019 Gait, balance and functional training (Gait and cognitive training practised together) vs Gait, balance and functional training (Gait and cognitive training practised separately) |
10/9 | 28/7.4 | ND | NA | NA | NA | No | 30 days, measured retrospectively |
|
Protas 2005 Gait, balance and functional training vs Control |
9 | 66.4 | 5 (56%) | 9 | 66.4 | 6 (67%) | No | 2 weeks, measured prospectively |
|
Ricciardi 2015 Gait, balance and functional training (best side therapy) / Gait, balance and functional training (worst side therapy) / Gait, balance and functional training (standard therapy) |
9/9/10 | ND | ND | NA | NA | NA | No | ND |
|
Sedaghati 2016 Gait, balance and functional training (with a balance pad) / Gait, balance and functional training (without a balance pad) vs Control |
15/14 | 6.8/6.7 | ND | 15 | 6.2 | ND | No | 10 weeks, unclear if measured retrospectively or prospectively |
|
Shen 2015 Gait, balance and functional training / Resistance training |
22/23 | 0.57/0.76** | 9 (41%)/10 (43%) | NA | NA | NA | No | 12 months, measured retrospectively |
|
Smania 2010 Gait, balance and functional training / Flexibility exercise |
28/27 | 51.6/55.2 | ND | NA | NA | NA | No | 1 month, measured prospectively |
|
Song 2018 Gait, balance and functional training vs Control |
31 | ND | 17 (55%) | 29 | ND | 16 (55%) | No | 6 months, measured retrospectively |
|
Thaut 2019 Gait, balance and functional training (rhythmic auditory stimulation training throughout intervention period) vs Gait, balance and functional training (rhythmic auditory stimulation training with no training in middle 8 weeks of intervention period) |
25/22 | 4.5/4.2 | ND | NA | NA | NA | No | 12 months, measured retrospectively |
|
Volpe 2014a Gait, balance and functional training (with proprioceptive stabiliser) / Gait, balance and functional training (without proprioceptive stabiliser) |
20/20 | ND | 16 (80%)/12 (60%) | NA | NA | NA | No | 2 months, measured prospectively |
|
Volpe 2014b Gait, balance and functional training (hydrotherapy) / Gait, balance and functional training (land‐based therapy) |
17/17 | 18/12.6 | 17 (100%)/17 (100%) | NA | NA | NA | No | Rate of falls: 2 months, measured prospectively Number of fallers: 12 months, measured retrospectively |
|
Wong‐Yu 2015 Gait, balance and functional training vs Control |
32 | 0 | 0 (0%) | 38 | 0 | 0 (0%) | No | 6 months, measured retrospectively |
| Medication trials | ||||||||
|
Chung 2010 Donepezil vs placebo |
19 | ND | 19 (100%) | 19 | ND | 19 (100%) | No | Unclear: participants had all fallen or nearly fallen 2 or more times per week, measured retrospectively |
|
Henderson 2016 Rivastigmine vs placebo |
65 | 5.0 | 65 (100%) | 65 | 5.5 | 65 (100%) | No | 12 months, measured retrospectively |
|
Li 2015a Rivastigmine vs placebo |
41 | 3.6 | 22 (54%) | 40 | 3.8 | 23 (58%) | No | Unclear |
| Education trial | ||||||||
|
Ward 2004 Personalised education vs control (standardised printed information) |
27 | ND | ND | 26 | ND | ND | No | ND |
| Exercise plus education trials | ||||||||
|
Cattaneo 2019 Gait, balance and functional training plus education vs Control |
15 | ND | ND | 17 | ND | ND | No | ND |
|
Morris 2015 Resistance training (functional strength) / Gait, balance and functional training (movement strategy training) vs Control |
70/69 | ND | 38 (54%)/40 (58%) | 71 | ND | 38 (54%) | No | 12 months, measured retrospectively |
|
Morris 2017 Gait, balance and functional training plus education vs Control |
67 | ND | 38 (57%) | 66 | ND | 35 (53%) | No | 12 months, measured retrospectively |
ND: no useable data; NA: not applicable
*One participant with excessive number of falls removed from analysis
**One participant from the balance group and 2 from the resistance group with excessive number of falls removed from the analysis
9. Raw data for quality of life.
| Study ID and comparison | Intervention group baseline n, mean (SD) | Control group baseline n, mean (SD) | Intervention group post timeframe, n, mean (SD) | Control group post timeframe, n, mean (SD) | Intervention group follow‐up timeframe, n, mean (SD) | Control group follow‐up timeframe, n, mean (SD) |
| Exercise trials | ||||||
| Parkinson’s Disease Questionnaire 39 (PDQ39) and 8 (PDQ8) (range 0‐100)* | ||||||
|
Canning 2015a Gait, balance and functional training vs Control |
115, 28 (13.9) |
116, 30.7 (15.4) |
26 weeks, 104, 29.7 (14.8) |
26 weeks, 115, 32.5 (15.9) |
NA | NA |
|
Chivers Seymour 2019 Gait, balance and functional training vs Control |
126, 27.4 (14.3) |
153, 28.7 (15.9) |
6 months, 126, 28.3 (15.0) |
6 months, 153, 29.5 (16.5) |
12 months, 77, 29.1 (15.4) |
12 months, 100, 31.7 (15.5) |
|
Gandolfi 2017 (PDQ8) Gait, balance and functional training (virtual reality telerehabilitation) vs Gait, balance and functional training (balance training in a facility) |
36, 30.7 (15.5)/ 34, 30.5 (16.0) |
NA | 7 weeks, 36, 24.1 (14.8)/ 34, 24.2 (15.9) |
NA | 11 weeks, 36, 25.8 (14.9)/ 34, 23.9 (13.2) |
NA |
|
Gandolfi 2019 (PDQ8) Gait, balance and functional training (trunk‐specific exercises) vs Gait, balance and functional training (general exercises) |
19, 25.5 (11.8)/ 18, 18.7 (10.8) |
NA | 4 weeks, 19, 21.5 (10.0)/ 18, 15.3 (8.6) |
NA | 8 weeks, 19, 23.0 (12.6)/ 18, 21.0 (8.8) |
NA |
|
Harro 2014 Gait, balance and functional training (cueing training) / Gait, balance and functional training (treadmill‐based gait training) |
11, 31.1 (14.8)/ 11, 40.1 (17.5 |
NA | 6 weeks, 10, 27.5 (17.9)/ 10, 27.4 (10.0) |
NA | 3 months, 10, 25.4 (15.0)/ 9, 30.0 (12.9) |
NA |
|
Li 2012 (PDQ8) 3D exercise (Tai Chi) / Resistance training vs Control |
65, 25.1 (16.8)/ 65, 25.3 (14.7) |
65, 25.2 (16.3) |
6 months, 65, 15.5 (11.4)/ 65, 21.4 (12.7) |
6 months, 65, 25.1 (15.6) |
NA | NA |
|
Volpe 2014a** Gait, balance and functional training (with proprioceptive stabiliser) / Gait, balance and functional training (without proprioceptive stabiliser) |
20, 62.7 (19.5)/ 20, 61.4 (38.9) |
NA | 2 months, 20, 44.0 (22.3)/ 20, 58.5 (37.9) |
NA | 4 months, 20, 53.7 (22.3)/ 20, 61.0 (35.1) |
NA |
|
Volpe 2014b Gait, balance and functional training (hydrotherapy) / Gait, balance and functional training (land‐based therapy) |
17, 60.3 (19.9)/ 17, 64.4 (28.6) |
NA | 2 months 17, 41.9 (20.9)/ 17, 56.4 (26.8) |
NA | NA | NA |
| EQ5D Thermometer (0‐100) | ||||||
|
Ashburn 2007 Gait, balance and functional training vs Control |
70, 63.1 (17.1) |
71, 64.6 (14.5) |
8 weeks, 67, 61.3 (19.8) |
8 weeks, 66, 61.7 (14.5) |
6 months, 65, 63.0 (18.7) |
6 months, 64, 56.6 (16.9) |
| EQ5D Index score (range 0‐1) | ||||||
|
Goodwin 2011** Gait, balance and functional training vs Control |
61, 0.7 (0.1) |
63, 0.7 (0.1) |
10 weeks, 61, 0.7 (0.1) |
10 weeks, 63, 0.7 (0.1) |
20 weeks, 61, 0.8 (0.3) |
20 weeks, 62, 0.7 (0.3) |
|
Munneke 2010 Other exercise (ParkinsonNet therapists) / Other exercise (standard therapists) |
358, 0.65 (0.20)/ 341, 0.65 (0.22) |
NA | 16 weeks, 295, 0.66 (0.20)/ 294, 0.65 (0.23) |
NA | 24 weeks, 262, 0.68 (0.21)/ 259, 0.66 (0.23) |
NA |
| SF12 and SF36 Physical Composite Score (range 0‐100) | ||||||
|
Canning 2015a (SF12) Gait, balance and functional training vs control |
115, 42.3 (7.6) |
116, 42.9 (7.9) |
26 weeks, 104, 41.3 (8.8) |
26 weeks, 115, 40.2 (7.8) |
NA | NA |
|
Mirelman 2016 (SF36) Gait, balance and functional training (virtual reality treadmill training) / Gait, balance and functional training (treadmill‐based gait training) |
66, 49 (2.5)/ 64, 44.8 (2.5) |
NA | 6 weeks, 66, 52 (2.5)/ 64, 46.5 (2.5) |
NA | 6 months, 66, 50.5 (2.5)/ 64, 48 (2.5) |
NA |
| SF12 Mental Composite Score (range 0‐100) | ||||||
|
Canning 2015a Gait, balance and functional training vs Control |
115, 51.6 (6.5) |
116, 50.5 (6.8) |
26 weeks, 104, 51.2 (6.4) |
26 weeks, 115, 50.3 (6.7) |
NA | NA |
| Medication Trials | ||||||
| EQ5D Thermometer (0‐100) | ||||||
|
Henderson 2016 Rivastigmine vs placebo |
65, 64 (17) |
65, 65 (17) |
32 weeks, 58, 66 (16) |
32 weeks, 63, 63 (18) |
NA | NA |
| EQ 5D Index score (range 0‐1) | ||||||
|
Henderson 2016 Rivastigmine vs placebo |
65, 0.72 (0.19) |
65, 0.71 (0.18) |
32 weeks, 58, 0.66 (0.21) |
32 weeks, 63, 0.66 (0.19) |
NA | NA |
| Education plus exercise trials | ||||||
| Parkinson’s Disease Questionnaire 39 (PDQ39) (range 0‐100)* | ||||||
|
Morris 2015 Resistance training / Gait, balance and functional training (movement strategy training) vs Control |
70, 20.8 (13.6)/ 69, 19.4 (12.8) |
71, 22.1 (12.5) |
3 months, 67, 18.9 (13.5)/ 64, 16.9 (14.0) |
3 months, 54, 18.5 (12.6) |
14 months, 67, 20.0 (13.6)/ 66, 20.8 (14.1) |
14 months, 57, 24.1 (13.1) |
|
Morris 2017 Gait, balance and functional training plus education vs Control |
67, 23 (14) |
66, 24 (15) |
6 weeks, 62, 21 (14) |
6 weeks, 58, 20 (14) |
58 weeks, 55, 22 (13) |
58 weeks, 53, 22 (14) |
| EQ5D Thermometer (0‐100) | ||||||
|
Morris 2015 Resistance training / Gait, balance and functional training (movement strategy training) vs Control |
70, 74.1 (16.7)/ 69, 73.9 (15.9) |
71, 72.7 (14.6) |
3 months, 67, 71.8 (16.4)/ 64, 76.5 (16.4) |
3 months, 54, 74.7 (16.0) |
14 months, 67, 75.4 (14.1)/ 66, 75.0 (13.5) |
14 months, 57, 72.8 (16.0) |
|
Morris 2017 Gait, balance and functional training vs Control |
67, 73 (15) |
66, 72 (16) |
6 weeks, 62, 68 (15) |
6 weeks, 58, 76 (12) |
58 weeks, 55, 72 (17) |
58 weeks, 53, 71 (14) |
| EQ5D Index score (range 0‐1) | ||||||
|
Morris 2017 Gait, balance and functional training vs Control |
67, 0.67 (0.27) |
66, 0.63 (0.28) |
6 weeks, 62, 0.66 (0.29) |
6 weeks, 58, 0.65 (0.27) |
58 weeks, 55, 0.67 (0.25) |
58 weeks, 53, 0.64 (0.3) |
NA: not applicable
*High score = worse quality of life
** Median and interquartile range reported by trial authors and converted to mean and standard deviation by review authors: Volpe 2014a using technique described by Wan 2014 and Goodwin 2011 using the technique described in the Cochrane Handbook (Higgins 2017). Conversion techniques differed due to the different sample sizes in the trials.
PDQ8 = Parkinson’s Disease Questionnaire 8
10. Studies reporting an economic analysis related to the cost of the intervention and/or fall outcomes.
|
Study ID, (source if not primary reference), sample, comparison, type of evaluation |
Intervention(s) and comparator (n in analyses) | Perspectives, type of currency, price year, time horizon | Cost items measured | Intervention costs per participant | Healthcare service costs per participant |
Incremental cost per fall prevented/ per QALY gained |
| Exercise trials | ||||||
|
Canning 2015a (Farag 2016) People with PD who had fallen at least once in the past year or were at risk of falls. Gait, balance and functional training vs control Evaluated with cost‐effectiveness analyses |
Exercise (balance, lower limb strength, and when required cueing), 3 X week, 24 weeks, with 6‐10 sessions supervised either individually or in a group setting (n = 113) vs usual care control (n = 113) | Health system perspective, Australian dollar, 2012, During 6‐month trial period |
Intervention costs (staff time, travel, equipment) Health service use costs (hospital, medical, allied health) Medication costs |
$A1,010 (€642) |
Exercise group $A4,604 (€2,925) Control group $A3,920 (€2,491) |
Cost per fall prevented $A574 (€365) Cost per QALY gained $A338,800 (€215,277) |
|
Chivers Seymour 2019 (Ashburn 2019, Xin 2020) People with PD who had fallen at least once in the past year. Gait, balance and functional training vs control Evaluated with cost‐effectiveness analyses |
Exercise (balance and lower limb strengthening exercises, plus strategies for preventing falls and reducing freezing of gait), 30 min per day for 6 months, including 12 x 1‐1.5 hour supervised sessions with a physiotherapist (n = 238) vs usual care control (n = 236) | United Kingdom National Health Service and Personal Social Services perspectives, Pound Stirling, 2016, During 6 month intervention period |
Intervention costs (physiotherapist salaries, training, travel, equipment and consumables) Health service use (hospital, primary care, social service) Medication costs collected but not included in analyses |
£650 (€765) | Exercise group £3,137 (€3,905) Control group £3,069 (€3,613) |
Cost per QALY gained £120,659 (€142,063) |
|
Gandolfi 2017 People with PD, both fallers and non‐fallers. Gait, balance and functional training (virtual reality telerehabilitation) vs Gait, balance and functional training (balance training in a facility) Evaluated with cost analysis |
Virtual reality balance training (using Nintendo Wii Fit system, Nintendo Co., Ltd., Kyoto, Japan) delivered via telehealth (using Skype, Microsoft, USA), delivered in pairs (n = 36) vs sensory‐integration balance training delivered in‐person, individually (n = 34), both interventions 50 mins, 3 X week, 7 weeks | Cost of rehabilitation perspective, Euros, Price year not reported, During assessments and 7 week intervention period |
Direct costs (personnel for screening, assessments and intervention, plus resource utilisation). Indirect costs (utilities, facilities) |
Virtual reality via telehealth balance training (delivered in pairs) €383.55 sensori‐integration balance training (delivered individually) €602.10 |
Not reported | Not reported |
|
Goodwin 2011 (Fletcher 2012) People with PD and 2 or more falls in the preceding year. Gait, balance and functional training vs control Evaluated with cost‐effectiveness analyses |
Exercise (balance, lower limb and trunk strength, 1 X week supervised group and 2 X week independent at home for 10 weeks (n=48) vs usual care control (n = 45). Economic analyses conducted with intervention n = 48 and control n = 45 |
United Kingdom National Health Service and Personal Social Services perspectives, Pound sterling, 2008/9, During 20 weeks (10 weeks intervention and 10 weeks follow‐up) |
Intervention costs (staff time, travel, equipment, venue hire) Health service use (hospital, primary care, social service) Medication costs |
£76 (€89) | Exercise group Health care cost £1,198 (€1,410) Health and social care cost £1,444 (€1,700) Control group Health care cost £1,320 (€1,554) Health and social care cost £1,479 (€1,741) |
Cost per QALY gained for total health care costs ‐£4,885 (‐€5,752) Cost per QALY gained for combined total health care and social care costs ‐£1,358 (‐€1,599) |
|
Li 2012 (Li 2015b) People with PD, both fallers and non‐fallers. 3D exercise (Tai Chi) / resistance training (functional strength) vs control Evaluated with cost‐effectiveness analyses |
Tai Chi (n=65) vs resistance training (n=65) vs stretching (control) (n=65), all group classes for 60 minutes, 2 X week, 24 weeks | Societal perspective, United States dollar, 2011, During 9 months (6 months intervention and 3 months follow‐up) |
Intervention costs (program promotion, recruitment, staff time, insurance, equipment, room hire, printed materials) Non‐intervention costs (PD medication, physical therapy, medical treatment for falls, participant travel) |
Tai chi $US1,080 (€952) Resistance $US1,186 (€1,046) Stretching $US1,155 (€1,019) |
PD medication, physical therapy, medical treatment for falls and participant travel costs: Tai chi $US272 (€240) Resistance $US310 (€273) Stretching $US726 (€640) |
Tai chi vs stretching (control): Cost per fall prevented ‐$US175 (‐€154) Cost per QALY gained ‐$US3,394 (‐€2,993) Resistance vs Tai Chi: Cost per fall prevented $US100 (€88) Cost per QALY gained $US1,236 (€1,090) |
|
Munneke 2010 People with PD, both fallers and non‐fallers. Other exercise (ParkinsonNet therapists) vs Other exercise (standard therapists) Evaluated with cost analysis |
Treatment from ParkinsonNet trained physiotherapists (n=343 to 350)* vs usual care (treatment from physiotherapists without specific PD training) (n=332‐340)*, both groups 24 weeks intervention period | Societal perspective, Euro, Price year not reported, but data collected 2005‐2007, During 24 weeks intervention |
Health care costs (physiotherapy, medication, consultation, day‐hospital rehabilitation, admission to hospital, home‐care (paid services), informal care, costs due to lost productivity of the care‐partner). | Physiotherapy cost: ParkinsonNet group €297 Usual care group €310 |
Excluding physiotherapy: ParkinsonNet group €2,674 Usual care group €3,424 |
Not calculated |
| Exercise plus education trial | ||||||
|
Morris 2017 People with PD, both fallers and non‐fallers. Gait, balance and functional training plus education vs control Evaluated with cost analysis |
Exercise (strength training (lower limb and trunk), movement strategy training) and falls prevention education, 1 X week 60 mins supervised and 1 X week 60 mins independent practice for 6 weeks (n=67) vs Life Skills program (control) (n=66) | Health system perspective, Australian dollar, 2016, During 12 months follow‐up |
Intervention costs (travel, home visits, therapist training, equipment). Life skills control intervention was considered as a placebo and therefore had no costs attributed to it. Medical costs associated with falling events (medical, medical ancillary, diagnostic and hospitalisation costs) |
$A1,596 (€1,013) | Not reported | Not calculated as there was no difference between the groups |
*Different participant numbers for different cost components
Where costs were reported in a currency other than EUR, the cost was converted to EUR (€) on December 23, 2021.
QALY = quality‐adjusted life‐year
11. Adverse events.
| Study ID and comparison | Information related to adverse events |
| Exercise trials | |
|
Ashburn 2007 Gait, balance and functional training vs Control |
No participants fell while performing the exercise program. |
|
Canning 2015a Gait, balance and functional training vs Control |
Two participants had non‐injurious falls during unsupervised exercise at home. |
|
Chivers Seymour 2019 Gait, balance and functional training vs Control |
No participants fell while performing the exercise program, and no adverse events were associated with the intervention. 0‐6 months hospitalisations: 9 PDSAFE exercise group participants (1 participant with 2 hospitalisations); 20 control group participants. 6‐12 months hospitalisations: 18 PDSAFE exercise group participants (2 participants with 2 hospitalisations); 21 control group participants (2 participants with 2 hospitalisations). |
|
Gandolfi 2017 Gait, balance and functional training (virtual reality telerehabilitation) vs Gait, balance and functional training (balance training in a facility) |
No adverse events were reported during the study. |
|
Gandolfi 2019 Gait, balance and functional training (trunk‐specific exercises) vs Gait, balance and functional training (general exercises) |
No adverse events or safety concerns were reported during the study. |
|
Gao 2014 3D exercise (Tai Chi) vs Control |
Not reported |
|
Goodwin 2011 Gait, balance and functional training vs Control |
No adverse events occurred during the exercise sessions. |
|
Harro 2014 Gait, balance and functional training (cueing training) / Gait, balance and functional training (treadmill‐based gait training) |
No adverse events during the intervention. |
|
Li 2012 3D exercise (Tai Chi) / Resistance training vs Control |
Tai‐chi (n=65): 3 in class events ‐ 2 falls, 1 muscle soreness or pain; 24 out of class events ‐ 19 falls, 4 low back pain, 1 ankle sprain. Functional strength training (n=65): 14 in class events ‐ 4 falls, 4 muscle soreness or pain, 3 dizziness or faintness, 3 symptoms of hypotension; 41 out of class events ‐ 31 falls, 3 chest pain, 1 hypotension, 4 low back pain, 2 ankle sprains. Stretching (n=65): 9 in‐class events ‐ 5 falls, 1 muscle soreness or pain, 2 dizziness or faintness, 1 symptoms of hypotension; 36 out of class events ‐ 26 falls, 2 chest pain, 2 hypotension, 5 low back pain, 1 ankle sprain. Nb ‐ out of class events are those that occurred during habitual activity or during an assessment. Participants did not perform any intervention outside the class. |
|
Martin 2015 Gait, balance and functional training vs Control |
Not reported |
|
Mirelman 2016 Gait, balance and functional training (virtual reality treadmill training) vs Gait, balance and functional training (treadmill‐based gait training) |
No serious adverse events during training. Adverse events other than those that occurred during intervention were recorded for both groups, but were not reported separately for the participants with Parkinson's disease. |
|
Munneke 2010 Other exercise (ParkinsonNet therapists) / Other exercise (standard therapists) |
None reported, though not collected systematically. |
|
Paul 2014 Resistance training (muscle power training) vs Control |
Power training (n=20): 1 exacerbation of pre‐existing low back pain, 1 pelvic fracture unrelated to the intervention, and 6 participants required modification to training loads due to transient pain, joint inflammation or illness. Control low intensity exercise (n=20): 2 participants had exacerbations of pre‐existing hernias, though this was not attributable to the low intensity exercise. |
|
Pelosin 2017 Gait, balance and functional training (treadmill training at high frequency) vs Gait balance and functional training (treadmill training at intermediate frequency) vs Gait, balance and functional training (treadmill training at low frequency) |
Not reported |
|
Penko 2019 Gait, balance and functional training (Gait and cognitive training practised together) vs Gait, balance and functional training (Gait and cognitive training practised separately) |
Not reported |
|
Protas 2005 Gait, balance and functional training vs Control |
Not reported |
|
Ricciardi 2015 Gait, balance and functional training (best side therapy) / Gait, balance and functional training (worst side therapy) / Gait, balance and functional training (standard therapy) |
Not reported |
|
Sedaghati 2016 Gait, balance and functional training (with a balance pad) / Gait, balance and functional training (without a balance pad) vs Control |
Not reported |
|
Shen 2015 Gait, balance and functional training / Resistance training |
No adverse events related to the intervention in either group. |
|
Smania 2010 Gait, balance and functional training / Flexibility exercise |
Not reported |
|
Song 2018 Gait, balance and functional training vs Control |
Adverse events were reported for the intervention group. Six participants ceased the stepping training: two ceased exercise due to it exacerbating pre‐existing lower back pain; two died; one sustained a knee injury from a fall unrelated to the intervention; one ceased for personal reasons. Additionally, one participant experienced a non‐injurious fall while undertaking the intervention and eight participants reported an increase in pre‐existing pain (e.g. lower back pain, knee pain, foot pain) but felt that the exacerbation was unrelated to the intervention. |
|
Thaut 2019 Gait, balance and functional training (rhythmic auditory stimulation training throughout intervention period) vs Gait, balance and functional training (rhythmic auditory stimulation training with no training in middle 8 weeks of intervention period) |
Participants who dropped out did so for reasons unrelated to adverse events. |
|
Volpe 2014a Gait, balance and functional training (with proprioceptive stabiliser) / Gait, balance and functional training (without proprioceptive stabiliser) |
No major adverse event related to the intervention. |
|
Volpe 2014b Gait, balance and functional training (hydrotherapy) / Gait, balance and functional training (land‐based therapy) |
Not reported |
|
Wong‐Yu 2015 Gait, balance and functional training vs Control |
No adverse events related to the intervention. |
| Medication trials | |
|
Chung 2010 Donepezil vs placebo |
Donepezil (n=23): Eight participants (35%) reported 16 side effects (e.g. dehydration, gastrointestinal upset, headache, sleep disturbance, muscle cramps, orthostatic hypotension, weight loss). Placebo (n=23): Five participants (22%) reported 6 side effects (e.g. gastrointestinal upset, headache, sleep disturbance). These side effects were reported to be transient in most cases. |
|
Henderson 2016 Rivastigmine vs placebo |
Rivastigmine (n=64): 187 adverse events (excluding falls) Placebo (n=65): 122 adverse events (excluding falls) Adverse events included cardiac disorders, endocrine disorders, gastrointestinal disorders, general disorders and administration site disorders, immune system disorders, infections and infestations, injury, poisoning and procedural complications, investigations, metabolism and nutrition disorders, musculoskeletal and connective tissue disorders, neoplasms benign, malignant and unspecified, nervous system disorders, psychiatric disorders, renal and urinary disorders, respiratory, thoracic and mediastinal disorders, skin and subcutaneous tissue disorders, surgical medical procedures, vascular disorders. About one third of participants in the rivastigmine group complained of nausea. Most adverse events were categorised as mild and were considered to be unrelated to the intervention. There were 27 adverse events that were classified as serious; 14 in the rivastigmine group and 13 in the placebo group. Two of these events in the rivastigmine group were considered to be probably related to the rivastigmine. Twenty‐three participants in the rivastigmine group and 19 participants in the placebo group stopped taking the trial medication due to adverse events. |
|
Li 2015a Rivastigmine vs placebo |
Two participants withdrew due to adverse reactions, however details not provided. |
| Education trial | |
|
Ward 2004 Personalised education vs control (standardised printed information) |
Not reported |
| Exercise plus education trials | |
|
Cattaneo 2019 Gait, balance and functional training plus education vs Control |
Not reported |
|
Morris 2015 Resistance training / Gait, balance and functional training (movement strategy training) vs Control |
Functional strength training group (n=70): 25 occasions of new muscle soreness lasting > 24 hours Movement strategy training group (n=69): 11 occasions of new muscle soreness lasting > 24 hours, 1 fall and 2 occasions of dizziness during the intervention. |
|
Morris 2017 Gait, balance and functional training vs Control |
No adverse events related to the intervention. |
In the exercise studies, a RaR for the rate of falls was reported in 10 studies, and could be calculated in an additional 14. There was one study where the rate of falls was reported for some comparisons but required calculation in other comparisons (Li 2012). The RR for the number of people experiencing a fall was reported in four studies and could be calculated in an additional nine. Data to calculate the risk of fractures (number sustaining one or more fall‐related fractures) were reported in six studies and a further two studies reported there were no fractures in either group (Li 2012; Volpe 2014b). Six exercise studies reported an economic analysis related to the cost of the intervention and/or falls outcomes.
Information regarding adverse events related to the exercise intervention was provided by 15 studies. Only three of these studies (Li 2012; Mirelman 2016; Paul 2014) reported adverse events more broadly and monitored for adverse events using the same methods in all groups over the entire study period. Of these three, one included participants with and without PD and did not report these data separately for the PD group participants (Mirelman 2016). Ten studies did not report whether there were adverse events.
Health‐related quality of life was reported in 12 exercise studies, with one of these studies reporting more than one quality of life outcome. The most commonly reported outcome was the Parkinson’s Disease Questionnaire, with the 39 item (PDQ39) questionnaire reported by five studies and the eight‐item (PDQ8) questionnaire reported by three studies. The EQ5D was reported in three studies, with one study reporting the EQ5D thermometer score and two studies reporting the EQ5D index score. The Physical Composite Score from the SF12 was reported in one study and from the SF36 in an additional study. The Mental Composite Score from the SF12 was reported in one study.
Of the three cholinesterase inhibitor versus placebo studies, one reported the rate of falls (Henderson 2016), and this variable could be calculated in the remaining two (Chung 2010; Li 2015a). One study reported the risk of falling (Li 2015a), with this calculated in the remaining two. One of these studies reported data related to fractures, however a risk ratio (RR) could not be calculated as there were no events (Chung 2010). Two studies monitored adverse events using the same methods in all groups over the entire study period and reported enough data to enable calculation of the rate of adverse events excluding falls (Chung 2010; Henderson 2016). Health‐related quality of life was reported in the form of the EQ5D thermometer and index score in one study (Henderson 2016). None of these studies reported an economic analysis related to fall outcomes.
The education study reported an odds ratio for the risk of falling but did not report rate of falls, risk of fractures, adverse events, quality of life or economic data (Ward 2004).
In the exercise plus education studies, a RaR ratio for the rate of falls was reported in two studies (Morris 2015; Morris 2017) and a RR for the number of people who fell at least once was reported in all three. One of these studies compared two intervention groups and a control group and both the risk of falling and rate of falls was reported for two comparisons but required calculation for a third comparison (Morris 2015). Two studies reported data to calculate the risk of sustaining one or more fall‐related fractures as well as health‐related quality of life at post‐test and at follow‐up (Morris 2015; Morris 2017). These studies also reported information about adverse events related to the intervention, and one study reported information about the cost of the intervention (Morris 2017).
Excluded studies
There were four studies that initially appeared to meet the inclusion criteria but were subsequently excluded (see Characteristics of excluded studies). Two exercise studies were excluded, one because it did not meet the inclusion criteria for the types of outcome measures (Kurlan 2015), and the other because it was a randomised cross‐over trial where falls data were not collected during the control period (Sparrow 2016). Another study (Hill 2015), investigated the effect of inpatient and staff education and included participants with a wide range of diagnoses. Data for just the participants with PD were not available. The fourth excluded study explored the effect of sunlight exposure in increasing 25‐hydroxyvitamin D and reducing hip fractures in people with PD (Sato 2011). This study was excluded as the integrity of the data has been questioned (Bolland 2016), and the publication of the study has been retracted by the journal.
Ongoing studies
We identified 30 ongoing studies; 20 trialling exercise interventions, one trialling medication, three trialling deep brain stimulation, one trialling deep brain stimulation plus physiotherapy, three trialling a model of care, one trialling a multifactorial intervention (environmental modification, exercise and behavioural strategies), and one trialling osteopathic manipulative medicine (see Characteristics of ongoing studies).
Studies that are currently open to recruitment include: 14 exercise studies (NCT04300023; NCT04108741; NCT03972969; NCT04946812; NCT04897256; NCT04874051; NCT04848077; NCT04665869; NCT04634331; DRKS00024982; ChiCTR2000038852; NCT05172661 – by invitation only; ACTRN12620001135909; NCT04613141 – by invitation only), one medication study (NCT04226248), one deep brain stimulation study (NCT04408573), one deep brain stimulation plus physiotherapy study (NCT04953637), two model of care studies (NCT04694443; NCT04555720 – by invitation only), and the multifactorial intervention study (ACTRN12619000415101). Two exercise studies (NCT04389138; NCT04300348) and 1onemodel of care study (NCT05127057) were not yet recruiting.
The exercise studies have a median target sample size of 48 (range 16 to 452) and two of the studies (10%) specified a history of falls or increased fall risk as an inclusion criterion. Seventeen studies are investigating forms of gait, balance and functional training, with two of these studies investigating treadmill training with virtual reality versus treadmill training alone (NCT04108741; NCT03727529); three investigating structured exercise programs versus control (NCT04389138; NCT03972969; ACTRN12620001135909), two investigating exercise in a virtual reality environment versus exercise alone (NCT04874051; NCT04634331); two investigating balance plus cognitive dual task training versus balance training alone (NCT05172661; ChiCTR2000038852); one investigating split belt treadmill training compared to usual treadmill training (NCT04946812); one investigating walking with haptic feedback plus an exercise program versus control (NCT04613141); one investigating walking with auditory feedback plus an exercise program versus the same intervention without the feedback (NCT04300348); one investigating a combined brisk walking and balance program versus flexibility and strength exercise (NCT04665869); one investigating walking with a robotic device versus control (NCT03751371); one investigating volitional and reactive step training using an exergame as well as slip and trip training versus control (ACTRN12618001515280); one investigating exercises focused on turning versus control (NCT04897256) and one investigating exercise including eye movement training versus exercise alone (DRKS00024982). Of the remaining studies, one is investigating home‐based cycling versus control (NCT04300023), one is investigating different proportional increases in daily step count supported via a smartphone app (NCT04848077), and one is investigating muscle power training versus control (RBR‐5w2sqt).
The medication study is investigating a cholinesterase inhibitor (rivastigmine) versus placebo (NCT04226248), has a target sample size of 600 and specifies a history of falls or increased fall risk as an inclusion criterion.
The deep brain stimulation studies have a median target sample size of 15 (range 10 to 30) and none of them specify fall risk as part of the inclusion criteria. One study is investigating cyclic stimulation versus continuous stimulation (NCT04408573), one is investigating flexible subthalamic nucleus stimulation versus standard subthalamic nucleus stimulation (NCT04116177), and the third is investigating segmented (steered) contacts versus a contact combination in ring mode (NCT04093544). The deep brain stimulation plus physiotherapy study has a target sample size of 60 and does not include fall risk in the inclusion criteria (NCT04953637). It is comparing deep brain stimulation plus physiotherapy targeting gait and balance with deep brain stimulation plus encouragement to be active.
The three studies trialling models of care are all comparing a care model with control usual care. They have a median target sample size of 200 (range 76 to 214) and none of them specify fall risk as part of the inclusion criteria. One study is trialling a multicomponent model of care including case management, information technology infrastructure and empowerment of patients, care‐partners and therapists (NCT05127057). The second is trialling multidisciplinary telehealth in conjunction with standard in‐person consultations (NCT04694443). The third study is trialling interdisciplinary care including the development of a treatment plan (NCT04555720).
The multifactorial intervention versus control study has a target sample size of 40, and includes a history of falls in the inclusion criteria (ACTRN12619000415101). The study of osteopathic manipulative medicine versus control has a target sample size of 50, and does not include a history of falls as part of the inclusion criterion (NCT02107638).
Risk of bias in included studies
The results of the risk of bias assessment for each included study is shown in the Characteristics of included studies, in Figure 2 and Figure 3.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
In the exercise studies, we judged the risk of bias in the generation of the allocation sequence to be low in 72% (n = 18/25) and unclear in 28% (n= 7/25) of studies. The method of concealment of the allocation sequence prior to group assignment was assessed to be at low risk of bias in 52% (n = 13/25) and unclear risk in 48% (12/25). In the three medication studies, the risk of selection bias was low in one study (33%) and unclear in the remaining two (67%) for these items. The education study had low risk of bias for random sequence generation and unclear risk for allocation concealment. In the three exercise plus education studies we judged the risk of bias in the generation of the allocation sequence to be low in all (100%), but the method of concealment of the allocation sequence prior to group assignment was assessed to be at low risk of bias in two studies (67%) and unclear in the other (33%).
Blinding
Blinding of participants and personnel
In most exercise intervention studies (92%, n = 23/25), the education study and the three exercise plus education studies, it was not possible to blind the participants or personnel to group allocation. The risk of performance bias was assessed as unclear in these studies, as the effect of awareness of group allocation in an exercise and/or education study is unclear. The remaining two exercise studies (8%) were able to blind participants and personnel to group allocation and were therefore assessed as at low risk of performance bias. Two (67%) of the medication studies described how blinding of participants and personnel was ensured, and so were assessed as being at low risk of bias. The remaining study did not describe how blinding was achieved and therefore the risk of bias was assessed as unclear.
Blinding of outcome assessment
We assessed the risk of bias for blinding of outcome assessment (detection bias) separately for the falls outcomes and for the fractures outcome.
1. Rate of falls and risk of falling
In the exercise studies, the risk of detection bias related to the measurement of falls outcomes was judged as low in 24% (n = 6/25), unclear in 68% (n = 17/25) and high in 8% (n = 2/25). The risk of bias was low in all three medication studies, since interventions were placebo matched and personnel collecting outcomes were blinded to group allocation. The risk of detection bias for the falls outcomes was unclear in the education study and in one of the exercise plus education studies (33%). It was judged as being at low risk of bias for the remaining two exercise plus education studies (67%).
2. Risk of one or more fall‐related fractures
Eight exercise studies, one medication study and two exercise plus education studies reported data relating to fractures. The risk of detection bias relating to the methods of ascertainment of fractures was unclear in all these studies.
Incomplete outcome data
We assessed the risk of bias for incomplete outcome data (attrition bias) separately for the rate of falls the risk of falling.
1. Rate of falls
The risk of attrition bias in the exercise studies for data relating to the rate of falls were assessed as low in 76% (n = 19/25 studies), unclear in 12% (n = 3/25) and high in the remaining 12% (n = 3/25) of studies. In the medication studies, it was assessed as low in one study (33%), unclear in one study (33%) and high in one study (33%). In the two exercise and education studies that reported rate of falls, the risk of attrition bias was assessed as low in one and high in the other.
2. Risk of falling
The risk of attrition bias in the exercise studies where data were reported relating to the risk of falling (number of people who fell at least once) was assessed as low in 73% (n = 11/15), unclear in 20% (n = 3/15) and high risk of bias in 7% (n = 1/15). In the medication studies, it was assessed as low in two studies (67%), and unclear in one study (33%). The risk of attrition bias in the risk of falling data in the education study was unclear, and in the three exercise plus education studies was low.
Selective reporting
We assessed the risk of bias due to selective reporting of the outcomes included in this review. In the exercise studies, the risk of bias was assessed as low in 44% (n = 11/25), unclear in 48% (n = 12/25) and high in 8% (n = 2/25). In the three medication studies, the risk of bias from selective reporting was unclear in two (67%) studies and high in one (25%) study. In the education study, the risk of bias due to selective reporting was unclear. In all three exercise plus education studies, the risk was assessed as low.
Bias in the recall of falls due to less reliable methods of ascertainment
We assessed the risk of bias in the recall of falls in the exercise studies as being low in 52% (n = 13/25), and unclear in the remaining 48% (n = 12/25). In the medication studies, the risk of bias in the recall of falls was assessed as low in two (67%) studies and unclear in the remaining study (33%). The education study was assessed as having a high risk of bias in the recall of falls as ascertainment of falls relied on participant recall from the prior two months. Two of the exercise plus education studies was assessed as having a low risk of bias (67%), and the risk of bias in the other was unclear (33%).
Other potential sources of bias
In undertaking the GRADE assessment, we downgraded the certainty of evidence based on the risk of bias for the following comparisons.
1. Health‐related quality of life for exercise versus control immediately after the intervention.
2. Health‐related quality of life for exercise versus control at follow‐up.
3. Number of people who fell at least once outcome for cholinesterase inhibitors versus placebo.
4. Number of people who fell at least once outcome for education versus usual care.
5. Rate of falls outcome for exercise plus education versus control.
6. Health‐related quality of life for exercise plus education versus control immediately after the intervention.
7. Health‐related quality of life for exercise plus education versus control at follow‐up.
Further details are provided in the summary of findings tables: Table 1 (exercise compared to control); Table 2 (cholinesterase inhibitor compared to placebo); Table 5 (education compared to control) and Table 6 (exercise plus education compared to control).
Summary of findings 1. Summary of findings for exercise compared to control.
| Exercise (all types) compared with control (e.g. usual activities) for preventing falls in people with Parkinson's disease | ||||||
|
Patient or population: People with Parkinson's disease Settings: Any Intervention: Exercise of all types Comparison: Control ‐ usual care or a non‐active intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Exercise (all types) | |||||
|
Rate of Falls (falls per person‐year) Follow‐up: range 2 weeks to 12 months |
All exercise trials population | Rate ratio 0.74 (0.63 to 0.87) | 1456 (12 RCTs) | ⊕⊕⊕⊝ moderatea | Overall, exercise probably reduces the number of falls by 26% (95% CI 37% reduction to 13% reduction). |
|
|
8250 falls per 1000 people |
6105 falls per 1000 people (5198 to 7178) | |||||
|
Number of people who experienced one or more falls Follow‐up: range 2 weeks to 12 months |
All exercise trials population | Risk ratio 0.90 (0.80 to 1.00) | 932 (9 RCTs) | ⊕⊕⊕⊝ moderatea | Overall, exercise probably slightly reduces the number of people experiencing one or more falls by 10% (95% CI 20% reduction to no change). |
|
|
634 fallers per 1000 people |
571 fallers per 1000 people (507 to 634) | |||||
|
Number of people sustaining one or more fall‐related fractures Follow‐up: range 20 weeks to 12 months |
All exercise trials population | Risk ratio 0.57 (0.28 to 1.17) | 989 (5 RCTs) | ⊕⊝⊝⊝ very lowb | The evidence is of very low certainty, hence we are uncertain of the findings that exercise may make little or no difference in the number of people experiencing one or more fall‐related fractures. | |
|
40 people with fracture per 1000 |
23 people with fracture per 1000 (11 to 47) | |||||
|
Quality of life immediately after the intervention assessed with various measures Follow‐up: range 8 weeks to 6 months A lower score indicates better quality of life |
‐ | The mean quality of life score in the intervention groups was 0.17 standard deviations lower (0.36 lower to 0.01 higher). | 951 (5 RCTs) | ⊕⊕⊝⊝ lowc | Overall, exercise may slightly improve quality of life by 2.6 points in the PDQ39 score (MD = 2.6 lower, 95% CI 5.5 lower to 0.2 higher). Of note is that the 95% CI includes the possibility of both increased and no change in quality of life. The SMD was converted back to MD using the PDQ39 scale (0‐100), using the pooled SD from the baseline scores of the largest included trial (Chivers Seymour 2019). The MID for the PDQ39 is about 1.6 (Peto 2001). SMD was calculated from 2 trials using the PDQ39, 1 trial using the PDQ8, 1 trial using the EQ‐5D visual analogue scale and 1 trial using the EQ‐5D index score. |
|
| Adverse events | Adverse events were reported inconsistently and often only for the exercise group. Three studies reported there were no adverse events related to the exercise intervention and one reported there were no falls during exercise. The remaining four studies reported minor adverse events such as muscle or joint soreness and non‐injurious falls. | Not estimable | 1242 (8 RCTs) |
⊕⊝⊝⊝ very lowd | The evidence is of very low certainty, hence we are uncertain whether exercise has an effect on adverse events. |
|
| Economic outcomes | We were unable to compare ICERs due to variations in the methods used, however reported ICERs suggest that exercise may be cost‐effective in preventing falls. | Not estimable | 923 (4 RCTs) |
⊕⊝⊝⊝ very lowd | The evidence is of very low certainty, hence we are uncertain whether exercise is a cost‐effective intervention for falls prevention. |
|
| *The assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; ICERs: incremental cost‐effectiveness ratios; MD: mean difference; MID: minimally important difference; PDQ8: The Parkinson's Disease Questionnaire ‐ 8 items; PDQ39: The Parkinson's Disease Questionnaire ‐ 39 items; RCTs: randomised controlled trials; SD: standard deviation; SMD: standardised mean difference | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded due to indirectness as most of the included participants had mild to moderate disease and good cognition. There was no downgrading for risk of bias as most trials had low or unclear risk of bias and the unclear risk of bias (predominantly performance bias and detection bias) unlikely to lower the confidence in the estimation of the effect.
bDowngraded due to indirectness as most of the included participants had mild to moderate disease and good cognition. Downgraded by two levels due to imprecision as there was a small number of events and a wide confidence interval. There was no downgrading for risk of bias as most trials had low or unclear risk of bias and the unclear risk of bias (predominantly performance bias and detection bias) unlikely to lower the confidence in the estimation of the effect.
cDowngraded by one level due to risk of bias as most trials were at high or unclear risk of bias for performance bias and detection bias as quality of life is a self‐reported measure. Downgraded by a further level due to indirectness as most of the included participants had mild to moderate disease and good cognition.
dDowngraded by three levels due to incomplete data.
Summary of findings 2. Summary of findings for cholinesterase inhibitors compared to placebo.
| Cholinesterase inhibitors compared with placebo medication for preventing falls in people with Parkinson's disease | ||||||
|
Patient or population: People with Parkinson's disease Settings: Any Intervention: cholinesterase inhibitor medication (rivastigmine, donepezil) Comparison: placebo medication | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Cholinesterase inhibitor | |||||
|
Rate of falls (falls per person‐year) Follow‐up: range 12 weeks to 12 months |
Cholinesterase inhibitor trial population | Rate ratio 0.50 (0.44 to 0.58) | 229 (3 RCTs) | ⊕⊕⊝⊝ lowa | Overall, cholinesterase inhibitors may reduce the number of falls by 50% (95% CI 42% reduction to 56% reduction). |
|
|
28,800 falls per 1000 |
14,400 falls per 1000 (12,672 to 16,704) | |||||
|
Number of people who experienced one or more falls Follow‐up: range 12 weeks to 12 months |
Cholinesterase inhibitor trial population | Risk rato 1.01 (0.90 to 1.14) | 230 (3 RCTs) | ⊕⊝⊝⊝ verylowb | The evidence is of very low certainty, hence we are uncertain of the finding that cholinesterase inhibitors make little or no difference to the number of people experiencing one or more falls. | |
|
774 fallers per 1000 |
782 fallers per 1000 (697 to 882) | |||||
|
Number of people sustaining one or more fall‐related fractures Follow‐up: 12 weeks |
Reported in one study, with no fractures in either group. | Not estimable | 23 (1 RCT) |
⊕⊝⊝⊝ verylowc | The evidence is of very low certainty, hence we are uncertain whether cholinesterase inhibitors make little or no difference to the number of people sustaining one or more fall‐related fractures. | |
|
Quality of Life immediately after the intervention (EQ5D Thermometer, scale 0 to 100; and EQ5D Index Score, scale 0‐1, high score is better quality of life) Follow‐up: 8 months |
The mean EQ5D thermometer score was 63 and the mean EQ5D Index Score was 0.66 in the placebo group. | In the cholinesterase inhibitor group the mean EQ5D Thermometer Score was 3 points higher (3.06 lower to 9.06 higher) and the mean EQ5D Index Score was 0.01 points lower (0.08 lower to 0.07 higher). | 121 (1 RCT) | ⊕⊝⊝⊝ very lowd | The evidence is of very low certainty, hence we are uncertain of the finding that cholinesterase inhibitors may make little or no difference to health‐related quality of life immediately after the intervention. | |
|
Rate of adverse events excluding falls (per person year) Follow‐up: range 12 weeks to 8 months |
Cholinesterase inhibitor trial population | Rate ratio 1.60 (1.28 to 2.01) | 175 (2 RCTs) | ⊕⊕⊝⊝ lowe | Overall, cholinesterase inhibitors may increase the number of non fall‐related adverse events by 60% (95% CI 28% increase to 101% increase). |
|
|
1970 adverse events per 1000 |
3152 adverse events per 1000 (2,521 to 3,960) | |||||
| Economic outcomes | No data reported for this outcome | |||||
| *The assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MID: minimally important difference; RCTs: randomised controlled trials. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by two levels for imprecision due to the relatively small sample size. There was no downgrading for risk of bias as the sensitivity analyses to remove trials at high risk of bias in any item, or high/unclear risk of bias in any domain, made little difference to the result (Table 3).
bDowngraded by one level due to risk of bias as results changed when removing the two trials with a high risk of bias in any item (Henderson 2016, Chung 2010) (Table 4). Downgraded an additional two levels due to imprecision because of the relatively small sample size. There was no downgrading for inconsistency as results were essentially unchanged with removal of the comparison responsible for the high heterogeneity (Li 2015a) (Table 4).
cDowngraded by two levels for imprecision due to the very small sample size. Downgraded a further one level as only one of the three studies included in the review for this comparison contributed to the outcome.
dDowngraded by two levels for imprecision due to the relatively small sample size. Downgraded a further one level as only one of the three studies included in the review for this comparison contributed to the outcome.
eDowngraded by two levels for imprecision due to the relatively small sample size.
Summary of findings 3. Summary of findings for education compared to control.
| Health education compared with usual care for preventing falls in people with Parkinson's disease | ||||||
|
Patient or population: People with Parkinson's disease Settings: Any Intervention: Education about falls prevention Comparison: Usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Health education | |||||
| Rate of falls (falls per person‐year) | No data reported for this outcome | |||||
|
Number of people who experienced one or more falls Follow‐up: 12 months |
All exercise trials population* | Risk ratio 10.89 (1.26 to 94.03) | 53 (1 RCT) | ⊕⊝⊝⊝ very lowa | The evidence is of very low certainty, hence we are uncertain of the finding that health education increases the number of people who experience one or more falls. | |
| 634 fallers per 1000 people | 6,911 per 1000 (824 to 59,596) | |||||
| Number of people sustaining one or more fall‐related fractures | No data reported for this outcome | |||||
| Quality of life | No data reported for this outcome | |||||
| Adverse events | No data reported for this outcome | |||||
| Economic outcomes | No data reported for this outcome | |||||
| *The assumed risk is the median control group risk across exercise versus control studies, as there were no data to calculate the illustrative risk in the health education trial. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RCT: randomised controlled trial | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded three levels due to risk of bias (single study with high risk of bias for method of ascertaining falls (recall bias) and unclear risk for allocation concealment, performance bias, detection bias, attrition bias and reporting bias). Also downgraded for imprecision due to the relatively small sample size and very wide confidence interval.
Summary of findings 4. Summary of findings for exercise plus education compared to control.
| Exercise (all types) plus education for falls prevention compared with control (e.g. usual activities) for preventing falls in people with Parkinson's disease | ||||||
|
Patient or population: People with Parkinson's disease Settings: Any Intervention: Exercise of all types plus fall‐prevention education Comparison: Control ‐ usual care or a non‐active intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Exercise plus education | |||||
|
Rate of Falls (falls per person‐year) Follow‐up: 12 months |
Exercise plus education trials population | Rate ratio 0.46 (0.12 to 1.85) | 320 (2 RCTs) | ⊕⊝⊝⊝ very lowa | The evidence is of very low certainty, hence we are uncertain of the finding that exercise plus education makes little or no difference to the number of falls. | |
| 16,400 falls per 1000 people | 7,544 per 1000 (1968 to 30,340) | |||||
|
Number of people who experienced one or more falls Follow‐up: range 6 months to 12 months |
Exercise plus education trials population | Risk Ratio 0.89 (0.75 to 1.07) | 352 (3 RCTs) | ⊕⊕⊝⊝ lowb | Overall, exercise plus education may make little or no difference to the number of people experiencing one or more falls (11% reduction (95% CI 25% reduction to 7% increase)). |
|
| 672 per 1000 | 598 per 1000 (504 to 719) | |||||
|
Number of people sustaining one or more fall‐related fractures Follow‐up: 12 months |
Exercise plus education trials population | Risk ratio 1.45 (0.40 to 5.32) | 320 (2 RCTs) | ⊕⊝⊝⊝ very lowc | The evidence is of very low certainty, hence we are uncertain of the finding that exercise plus education makes little or no difference to the number of people experiencing one or more fall‐related fractures. | |
| 25 per 1000 | 36 per 1000 (10 to 133) | |||||
|
Quality of life immediately after the intervention assessed with the PDQ39 (range 0 to 100) Follow‐up: 6 weeks A lower score indicates better quality of life |
‐ | The mean PDQ39 in the intervention groups was 0.05 points higher (3.12 lower to 3.23 higher) | 305 (2 RCTs) | ⊕⊝⊝⊝ very lowd | The evidence is of very low certainty, hence we are uncertain of the finding that exercise plus education makes little or no difference to health‐related quality of life immediately after the intervention. | |
| Adverse events | Adverse events related to the exercise intervention only were reported. One study reported there were no adverse events, while the other reported minor adverse events such as muscle soreness and a fall while exercising. | Not estimable | 343 (2 RCTs) |
⊕⊝⊝⊝ very lowe | The evidence is of very low certainty, hence we are uncertain whether exercise plus education has an effect on adverse events. | |
| Economic Outcomes | Costs per fall prevented were not calculated as there was no reduction in falls in this study | Not estimable | 133 (1 RCT) |
⊕⊝⊝⊝ very lowf | The evidence is of very low certainty, hence we are uncertain whether exercise plus education is a cost‐effective intervention for falls prevention. | |
| *The assumed risk the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MID: minimally important difference; PDQ39: The Parkinson's Disease Questionnaire ‐ 39 items; RCTs: randomised controlled trials | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by three levels due to risk of bias as results changed when removing the study with a high risk of bias on assessor blinding (Morris 2017) and for inconsistency due to a high level of heterogeneity, with the result changed when the comparison responsible for the high heterogeneity (Morris 2017) was removed (Table 3). Downgraded for imprecision due to the wide confidence interval and small sample size and indirectness as most of the included participants had mild to moderate disease and good cognition. Additionally downgraded as the result changed when fixed effects analysis was used (Table 3).
bDowngraded one level for imprecision due to the relatively small sample size and an additional level for indirectness as most of the included participants had mild to moderate disease and good cognition. There was no downgrading for risk of bias as the sensitivity analyses to remove trials at high risk of bias in any item, or high/unclear risk of bias in any domain, made little difference to the result (Table 4).
cDowngraded by two levels for imprecision due to the relatively small sample size, the small number of events and the very wide confidence interval. Downgraded a further level for indirectness as most of the included participants had mild to moderate disease and good cognition.
dDowngraded by one level due to risk of bias as the studies included for this outcome were at unclear risk of bias for performance bias and high risk of bias for detection bias as quality of life is a self‐reported measure. Downgraded by one level for imprecision due to the relatively small sample size and wide confidence interval. Downgraded a further level for indirectness as most of the included participants had mild to moderate disease and good cognition.
eDowngraded by three levels due to incomplete data and serious risk of bias from reporting bias.
fDowngraded by two levels for imprecision due to the small sample size. Downgraded a further level for indirectness as most of the included participants had mild to moderate disease and good cognition. Downgraded a further one level as only one of the three studies included in the review for this comparison contributed to the outcome.
Effects of interventions
See: Table 1; Table 2; Table 5; Table 6
Effects of exercise interventions
Exercise interventions versus control
See: Table 1.
Rate of falls (falls per person‐year)
Compared to a control intervention (i.e. usual care or an intervention not expected to have an effect on falls, such as ‘sham’ exercise or upper limb exercise), exercise (all types combined) probably reduces the rate of falls by 26% (RaR 0.74, 95% confidence interval (CI) 0.63 to 0.87; 1456 participants, 12 studies, I2 = 30%; moderate‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Exercise vs control (rate of falls), Outcome 1: Rate of falls
Subgroup analysis by exercise type (based on ProFaNE categories, see Table 7 and Table 9) did not find a difference in the effects of different types of exercise on fall rates (test for subgroup differences: Chi2 = 4.92, df = 2, P = 0.09, I2 = 59.3%; Analysis 1.2). Studies of gait, balance and functional training versus control had a RaR of 0.80 (95% CI 0.67 to 0.95; 1146 participants, 9 studies, I2 = 24%); studies of resistance training versus control had a RaR of 0.72 (95% CI 0.55 to 0.94; 136 participants, 2 studies, I2 = 0%); and studies of 3D exercise (Tai Chi) versus control had a RaR of 0.41 (95% CI 0.23 to 0.72; 174 participants, 2 studies, I2 = 0%).
1.2. Analysis.

Comparison 1: Exercise vs control (rate of falls), Outcome 2: Rate of falls subgrouped by ProFaNE exercise categories
Subgroup analysis by the proportion of exercise sessions that were supervised by a therapist (see features of exercise interventions in Table 9) found a difference in the effect of exercise (test for subgroup differences: Chi2 = 5.95, df = 1, P = 0.01, I2 = 83.2%; Analysis 1.3) with a greater reduction in the rate of falls in studies where participants were fully supervised during exercise (RaR 0.56, 95% CI 0.41 to 0.77; 373 participants, 5 studies, I2 = 21%) compared with studies where participants were not fully supervised (RaR 0.85, 95% CI 0.75 to 0.97; 1083 participants, 7 studies, I2 = 0%).
1.3. Analysis.

Comparison 1: Exercise vs control (rate of falls), Outcome 3: Rate of falls ‐ subgrouped by % supervision (100% supervision vs <100% supervision)
Subgroup analysis by fall risk at baseline (higher fall risk participants compared with unspecified fall risk participants) did not find a difference in the effect of exercise on fall rates in studies with different inclusion criteria (test for subgroup differences: Chi2 = 0.03, df = 1, P = 0.86, I2 = 0%; Analysis 1.4). Studies where all participants were at a high risk of falls (past falls history or identified fall risk factors) had a RaR of 0.73 (95% CI 0.59 to 0.91; 1082 participants, 7 studies, I2 = 48%) whereas studies that did not use fall risk as an inclusion criterion had a RaR of 0.71 (95% CI 0.56 to 0.90; 374 participants, 5 studies, I2 = 0%).
1.4. Analysis.

Comparison 1: Exercise vs control (rate of falls), Outcome 4: Rate of falls ‐ subgrouped by baseline fall risk (increased fall risk vs fall risk not specified)
Most studies included participants with varying disease severity and methods for classifying disease severity varied between studies. Therefore, we performed a subgroup analysis using data from two studies (Canning 2015a; Chivers Seymour 2019) that reported subgroup analyses based on disease severity for the rate of falls (raw data reported in Table 16). Canning 2015 reported a lower disease severity (Unified Parkinson's Disease Rating Scale (UPDRS) motor score 26 or under, equivalent to an MDS‐UPDRS motor score of 33 or under (Hentz 2015)), and a higher disease severity (UPDRS motor score 27 or over, equivalent to an MDS‐UPDRS motor score of 34 or over (Hentz 2015)). Chivers Seymour 2019 reported three subgroups: low disease severity (MDS‐UPDRS motor score 22 or lower); moderate disease severity (MDS‐UPDRS motor score 23 to 38), and higher disease severity (MDS‐UPDRS motor score 39 and over) (data in Ashburn 2019). Due to the differing disease severity cut points, we pooled the low and moderate disease severity subgroups (lower disease severity) and compared them with the higher disease severity subgroups. Results showed there may be a differential intervention effect by disease severity (test for subgroup differences: Chi2 = 7.67, df = 1, P = 0.006, I2 = 87%) with an increase in fall rates with exercise in the higher disease severity subgroup (RaR 1.47, 95% CI 1.11 to 1.94; participant numbers not reported, I2 = 0%), and a slight decrease in fall rates with exercise in the lower disease severity subgroups (RaR 0.65, 95% CI 0.39 to 1.08; participant numbers not reported, I2 = 76%; Analysis 1.5). Notably, both these studies provided minimal physiotherapy supervision (Canning 2015a 13%; Chivers Seymour 2019 7%) and the exercise was performed either wholly (Chivers Seymour 2019), or mostly (Canning 2015a) at home.
12. Raw data for rate ratios and risk ratios for pooled subgroups based on disease severity.
| Study ID and comparison | Subgroup definition, number of participants (n) | Intervention lower severity group: falls per person year | Intervention higher severity group: falls per person year | Intervention lower severity group: number (%) of fallers | Intervention higher severity group: number (%) of fallers | Control lower severity group: falls per person year | Control higher severity group: falls per person year | Control lower severity group: number (%) of fallers | Control higher severity group: number (%) of fallers | Length of falls monitoring |
|
Ashburn 2007 Gait, balance and functional training vs Control |
Lower disease severity: Hoehn and Yahr stages 2 and 3, n = 96 Higher disease severity: Hoehn and Yahr stage 4, n = 30 |
NA | NA | 31 (66%) | 15 (94%) | NA | NA | 37 (76%) | 12 (86%) | 6 months |
|
Canning 2015a Gait, balance and functional training vs Control |
Lower disease severity: UPDRS motor score ≤ 26, n = 122 Higher disease severity: UPDRS motor score ≥ 27, n = 109 |
ND | ND | ND | ND | ND | ND | ND | ND | 6 months |
|
Chivers Seymour 2019 (data reported in Ashburn 2019) Gait, balance and functional training vs Control |
Lower disease severity: includes both the low disease severity subgroup ‐ MDS‐UPDRS motor score ≤ 22, n = 152 and moderate disease severity subgroup ‐ MDS‐UPDRS motor score 23 ‐ 28, n = 155 Higher disease severity: MDS‐UPDRS motor score ≥ 39, n = 152 |
ND | ND | NA | NA | ND | ND | NA | NA | 6 months |
ND: no useable data; NA: not applicable (not reported as an outcome in the trial).
1.5. Analysis.

Comparison 1: Exercise vs control (rate of falls), Outcome 5: Rate of falls ‐ pooled disease severity subgroup analyses_UPDRS
Number of people who experienced one or more falls (risk of falling)
Compared to a control intervention, exercise (all types combined) probably slightly reduces the number of people experiencing one or more falls by 10% (risk ratio (RR) 0.90, 95% CI 0.80 to 1.00, P = 0.05; 932 participants, 9 studies, I2 = 0%; moderate‐certainty evidence; Analysis 2.1). There was one study (Martin 2015) where all participants in both groups fell, and so these data could not be included in the meta‐analyses (Higgins 2017).
2.1. Analysis.

Comparison 2: Exercise vs control (number of fallers), Outcome 1: Number of fallers
Subgroup analysis by exercise type (based on ProFaNE categories, see Table 7 and Table 9) did not show a difference in the effects of different types of exercise on the number of people who fell at least once (test for subgroup differences: Chi2 = 3.14, df = 2, P = 0.21, I2 = 36.2%; Analysis 2.2). Studies of gait, balance and functional training versus control had a RR of 0.92 (95% CI 0.81 to 1.04; 622 participants, 6 studies, I2 = 0%); studies of resistance training versus control had a RR of 0.87 (95% CI 0.43 to 1.74; 136 participants, 2 studies, I2 = 65%); and studies of 3D exercise (Tai Chi) versus control had a RR of 0.59 (95% CI 0.36 to 0.95; 174 participants, 2 studies, I2 = 12%).
2.2. Analysis.

Comparison 2: Exercise vs control (number of fallers), Outcome 2: Number of fallers subgrouped by ProFaNE exercise categories
Subgroup analysis by the proportion of exercise sessions that were supervised by a therapist (see features of exercise interventions in Table 9) did not show a difference in the effect of exercise on the number of people experiencing one or more falls in studies where participants were fully supervised during exercise (RR 0.75, 95% CI 0.53 to 1.06; 328 participants, 4 studies, I2 = 36%) compared with studies where participants were not fully supervised (RR 0.92, 95% CI 0.82 to 1.04; 604 participants, 5 studies, I2 = 0%); test for subgroup differences Chi2 = 1.24, df = 1, P = 0.27, I2 = 19.3%; Analysis 2.3).
2.3. Analysis.

Comparison 2: Exercise vs control (number of fallers), Outcome 3: Number of fallers ‐ subgrouped by % supervision (100% supervision vs <100% supervision)
Subgroup analysis by fall risk at baseline did not show a difference in the effect of exercise on the number of people experiencing one or more falls where all participants were at a high risk of falls (past falls history or identified fall risk factors; RR 0.89, 95% CI 0.76 to 1.04; 576 participants, 5 studies, I2 = 15%) compared with studies that did not use fall risk as an inclusion criterion (RR 0.86, 95% CI 0.67 to 1.11; 356 participants, 4 studies, I2 = 0%; test for subgroup differences: Chi2 = 0.06, df = 1, P = 0.81, I2 = 0%; Analysis 2.4).
2.4. Analysis.

Comparison 2: Exercise vs control (number of fallers), Outcome 4: Number of fallers ‐ subgrouped by baseline fall risk (increased fall risk vs fall risk not specified)
As for the rate of falls, most studies were not able to be included in subgroup analysis on the effect of exercise on the risk of falls by disease severity. However, we pooled data from two studies (Ashburn 2007; Canning 2015a) that reported subgroup analyses based on disease severity (raw data presented in Table 16). Canning 2015a reported a lower disease severity (UPDRS motor score 26 or under) and a higher disease severity (UPDRS motor score 27 or over. Ashburn 2007 reported a lower disease severity (Hoehn and Yahr stage 2 or 3), and a higher disease severity (Hoehn and Yahr stage 4) subgroup. Results showed there may be a differential intervention effect by disease severity (test for subgroup differences: Chi2 = 8.14, df = 1, P = 0.004, I2 = 87.7%; Analysis 2.5). The results show there may be a slight reduction in the number of people who experienced one or more falls with exercise in the lower disease severity subgroup (RR 0.78, 95% CI 0.62 to 0.98; 218 participants; I2 = 31%), but there may be a slight increase in the proportion of people who fell at least once with exercise in the higher disease severity subgroup (RR 1.19, 95% CI 1.00 to 1.41; 139 participants; I2 = 0%). Notably, both these studies provided minimal physiotherapy supervision (Ashburn 2007 18%; Canning 2015a 13%) and the exercise was performed either wholly (Ashburn 2007) or mostly (Canning 2015a) at home.
2.5. Analysis.

Comparison 2: Exercise vs control (number of fallers), Outcome 5: Number of fallers ‐ pooled disease severity subgroup analyses
Number of people who experienced one or more fall‐related fractures
We are uncertain of the finding that exercise may make little or no difference in the number of people experiencing one or more fall‐related fractures compared to control (RR 0.57, 95% CI 0.28 to 1.17; 989 participants, 5 studies, I2 = 0%; very low‐certainty evidence; Analysis 3.1).
3.1. Analysis.

Comparison 3: Exercise vs control (number of people sustaining one or more fall‐related fractures), Outcome 1: Number of people sustaining one or more fall‐related fractures
Health‐related quality of life
Immediately post intervention, exercise interventions compared to control may slightly improve health‐related quality of life (standardised mean difference (SMD) ‐0.17, 95% CI ‐0.36 to 0.01; 951 participants, 5 studies; I2 = 48%; low certainty evidence; Analysis 4.1). When the SMD is converted back to a mean difference (MD) in the PDQ39, the difference is ‐2.6 (95% CI ‐5.5 to 0.2), showing the MD exceeds the minimally important difference (MID) of ‐1.6 (Peto 2001), however the 95% CI includes scores both larger and smaller than the MID.
4.1. Analysis.

Comparison 4: Exercise vs control (health‐related quality of life), Outcome 1: Health‐related quality of life ‐ combined measures post intervention
We are uncertain of the finding that exercise improves health‐related quality of life at follow‐up (range 20 weeks to 12 months; SMD ‐0.27, 95% CI ‐0.46 to – 0.08; 429 participants, 3 studies; I2 = 0%; very low‐certainty evidence; Analysis 4.2). When the SMD is converted back to a mean difference (MD) in the PDQ39, the difference is ‐4.1 (95% CI ‐7.0 to – 1.2), which exceeds the minimally important difference of ‐1.6 (Peto 2001).
4.2. Analysis.

Comparison 4: Exercise vs control (health‐related quality of life), Outcome 2: Health‐related quality of life ‐ combined measures follow‐up
Exercise versus exercise
The results of studies comparing different types of exercise are presented for rate of falls in Analysis 5.1, for the number of people experiencing one or more falls in Analysis 6.1 and for health‐related quality of life in Analysis 7.1 (post intervention) and Analysis 7.2 (follow‐up). We did not undertake any meta‐analyses for these outcomes due to the substantial variability between exercise programs.
5.1. Analysis.

Comparison 5: Exercise vs exercise (rate of falls), Outcome 1: Rate of falls, different types of exercise compared
6.1. Analysis.

Comparison 6: Exercise vs exercise (number of fallers), Outcome 1: Number of fallers, different types of exercise compared
7.1. Analysis.

Comparison 7: Exercise vs exercise (health‐related quality of life), Outcome 1: Quality of life ‐ combined measures post intervention, different types of exercise compared
7.2. Analysis.

Comparison 7: Exercise vs exercise (health‐related quality of life), Outcome 2: Quality of life ‐ combined measures follow‐up, different types of exercise compared
Some studies did find greater effects of one exercise compared to another. Treadmill walking in a virtual reality environment was found to reduce the rate of falls and improve health‐related quality of life compared to treadmill walking alone (Mirelman 2016, n = 130). Additionally, Li 2012 (n = 130) found a reduction in the number of people who fell at least once and improved health‐related quality of life following Tai Chi classes compared to functional resistance training.
The remaining studies showing effects were relatively small (range 27 to 70 participants) and their results require confirmation in different, larger studies. Gandolfi 2017 found that home‐based balance exercises using video games and delivered via telerehabilitation reduced the rate of falls compared to facility‐based balance training without the video games. Ricciardi 2015 compared standard strength, balance and gait training exercise with the same exercise targeting the more affected side, and with the same exercise targeting the less affected side. Results suggested that standard training led to a greater reduction in falls compared to training focused on the less affected side, but there were no other between group differences. Sedaghati 2016 found that balance and gait training with a ‘balance pad’ (i.e. foam mat) led to a greater reduction in the rate of falls than the same exercises without the ‘balance pad’. Similarly, Volpe 2014a reported that balance training with external perturbations was more effective in reducing the rate of falls if it was conducted while participants wore an active proprioceptive stabiliser (a device providing focal vibrations on the 7th cervical vertebra and both soleus tendons) compared to wearing inactive (placebo) devices. Smania 2010 found greater effects on the rate of falls from balance exercises compared to flexibility and co‐ordination exercises not targeting balance.
One study reported one fall‐related fracture in each group when comparing gait, balance and functional training with resistance training (Shen 2015).
Adverse events
Details regarding adverse events are presented in Table 15. Adverse events related to the exercise intervention were reported in 15 studies (2311 participants), with four of these reporting minor adverse events (Canning 2015a; Li 2012; Paul 2014; Song 2018). Canning 2015a reported that two participants experienced non‐injurious falls while undertaking unsupervised exercise at home. Li 2012 reported 26 in‐class adverse events including: two falls and one muscle soreness or pain in the Tai Chi group; four falls and four muscle soreness or pain in the functional strength training group; five falls and one muscle soreness or pain the control (stretching) group. The remaining in‐class adverse events were dizziness/faintness or symptoms of hypotension (six in the functional strength training group and three in the control group). Paul 2014 reported that in the muscle power training group there was one participant who experienced an exacerbation of pre‐existing low back pain and six participants who required modification to training loads due to transient pain, joint inflammation or illness. Song 2018 reported the stepping intervention exacerbated pre‐existing low back pain in two participants, and one participant sustained a non‐injurious fall while performing the stepping exercise. The remaining studies either reported there were no falls during the intervention (Ashburn 2007), no adverse events (Chivers Seymour 2019; Gandolfi 2017; Gandolfi 2019; Goodwin 2011; Harro 2014; Munneke 2010; Shen 2015; Wong‐Yu 2015), or no serious adverse events (Mirelman 2016; Volpe 2014a) related to the intervention.
Adverse events not attributable to the exercise intervention were monitored equally in all groups and reported in three studies (Li 2012; Mirelman 2016; Paul 2014), though one of these studies included participants with and without PD and did not report these data separately for the PD group participants (Mirelman 2016). Li 2012 reported all adverse events were minor to moderate and included falls, muscle soreness and pain, hypotension, chest pain, low back pain and ankle sprain. There were 27 adverse events occurring in the Tai Chi group, 55 in the resistance training group, and 45 in the control (stretching) group. Paul 2014 reported a pelvic fracture in one muscle power training participant and exacerbations of hernias in two control (sham exercise) participants.
Economic analysis
Six exercise studies reported costs or cost‐effectiveness data related to fall outcomes (Table 14) (Canning 2015a; Chivers Seymour 2019; Gandolfi 2017; Goodwin 2011; Li 2012; Munneke 2010). These included intervention costs, healthcare service costs and/or results of study‐based incremental costs per fall prevented/quality‐adjusted life‐year (QALY) gained. We were unable to compare incremental cost‐effectiveness ratios (ICERs) as the perspectives taken, the cost items measured, and the type of healthcare resources included in the calculations varied. Nonetheless, results from the three studies that delivered exercise at a relatively low cost and took an extensive health system perspective (Canning 2015a; Chivers Seymour 2019; Goodwin 2011) reported ICERs suggesting that exercise may be cost‐effective in preventing falls and improving health. For example, the Canning 2015a study reported a cost of $A574 per fall prevented (Farag 2016) and the Goodwin 2011 study reported total healthcare costs of ‐£4,885 per quality‐adjusted life‐year (QALY) gained (Fletcher 2012).
Effects of medication interventions versus placebo
See: Table 2.
Two different cholinesterase inhibitors were trialled in comparison to placebo: rivastigmine and donepezil.
Rate of falls (falls per person year)
Cholinesterase inhibitors may reduce the rate of falls by 50% when compared to a placebo medication (RaR 0.50, 95% CI 0.44 to 0.58; 229 participants, 3 studies, I2 = 3%; low‐certainty evidence; Analysis 8.1). Subgroup analyses indicated that there was no difference in the effect on fall rates between rivastigmine and donepezil (test for subgroup differences: Chi2 = 0.22, df = 1, P = 0.64, I2 = 0%; Analysis 8.2; random effects meta‐analysis). We were unable to conduct any subgroup analyses based on fall risk at baseline or disease severity. All three studies included participants at high risk of falls, with two studies (Henderson 2016; Chung 2010) specifying a history of falls in the inclusion criteria and the third study (Li 2015a) including only participants with cognitive impairment, which is known to be a risk factor for falls in people with PD (Latt 2009; van der Marck 2014). None of the studies reported a subgroup analysis falls RaR for disease severity, however one study (Chung 2010) reported an observation that the five participants with the most frequent falls showed the most improvement after six weeks on donepezil (19 participants, no statistics provided).
8.1. Analysis.

Comparison 8: Cholinesterase inhibitor vs placebo (rate of falls), Outcome 1: Rate of falls
8.2. Analysis.

Comparison 8: Cholinesterase inhibitor vs placebo (rate of falls), Outcome 2: Rate of falls ‐ subgrouped by medication
Number of people who experienced one or more falls (risk of falling)
We are uncertain of the finding of little or no difference in the number of people experiencing one or more falls following a cholinesterase inhibitor compared to placebo (RR 1.01, 95% CI 0.90 to 1.14; 230 participants, 3 studies, I2 = 72%; very low‐certainty evidence; Analysis 9.1). Subgroup analyses indicated that there was no difference in the effect on the risk of falls between rivastigmine and donepezil (test for subgroup differences: Chi2 = 1.08, df = 1, P = 0.30, I2 = 7%; Analysis 9.2; random‐effects meta‐analysis). As for the rate of falls, we were unable to conduct any subgroup analyses based on fall risk at baseline or disease severity.
9.1. Analysis.

Comparison 9: Cholinesterase inhibitor vs placebo (number of fallers), Outcome 1: Number of fallers
9.2. Analysis.

Comparison 9: Cholinesterase inhibitor vs placebo (number of fallers), Outcome 2: Number of fallers ‐ subgrouped by medication
Number of people who experienced one or more fall‐related fractures
There were insufficient data from the cholinesterase inhibitor versus placebo studies to pool for the number of people sustaining one or more fall‐related fractures. One study reported no fractures in either group (Chung 2010), and the remaining two studies did not report fractures as an outcome (Henderson 2016; Li 2015a).
Health‐related quality of life
We are uncertain whether cholinesterase inhibitors make little or no difference to health‐related quality of life compared to a placebo immediately post intervention. One study reported two health‐related quality of life outcomes (EQ5D thermometer score MD 3.00, 95% CI ‐3.06 to 9.06; EQ5D index score MD ‐0.01, 95% CI ‐0.08 to 0.07; 121 participants, 1 study; very low‐certainty evidence; Analysis 10.1 and Analysis 10.2). The minimally important difference for the EQ5D index score is about 0.07 (95% CI 0.01 to 0.14) (Walters 2005).
10.1. Analysis.

Comparison 10: Cholinesterase inhibitor vs placebo (health‐related quality of life), Outcome 1: Quality of life EQ5D thermometer post intervention
10.2. Analysis.

Comparison 10: Cholinesterase inhibitor vs placebo (health‐related quality of life), Outcome 2: Quality of life EQ5D Index Score post intervention
None of the studies of medication interventions measured health‐related quality of life at follow‐up.
Rate of adverse events excluding falls (adverse events per person‐year)
Details regarding adverse events are in Table 15. Two of the medication studies (Chung 2010; Henderson 2016) reported adverse events. Most adverse events were considered to be mild and transient in nature. However, Henderson 2016 reported 27 serious adverse events (14 in the rivastigmine group and 13 in the placebo group), with two of these events (both a worsening of PD impairments) in the rivastigmine group considered likely to be related to the rivastigmine intervention.
Meta‐analysis shows that cholinesterase inhibitors may increase the rate of adverse events excluding falls by 60% when compared to a placebo medication (RaR 1.60, 95% CI 1.28 to 2.01; 175 participants, 2 studies, I2 = 16%; low‐certainty evidence; Analysis 11.1).
11.1. Analysis.

Comparison 11: Cholinesterase inhibitor vs placebo (rate of adverse events excluding falls), Outcome 1: Rate of adverse events excluding falls
Economic analysis
None of the medication studies reported an economic analysis.
Effects of education versus usual care
See: Table 5.
The single included study of an education intervention compared to usual care only provided data related to this review for the number of people who experienced one or more falls (risk of falling).
Number of people who experienced one or more falls (risk of falling)
We are uncertain whether education increases the number of people experiencing one or more falls (RR 10.89, 95% CI 1.26 to 94.03; 53 participants, 1 study; very low certainty evidence; Analysis 12.1).
12.1. Analysis.

Comparison 12: Education vs usual care (number of fallers), Outcome 1: Number of fallers
Effects of exercise plus education interventions
Exercise plus education versus control
See: Table 6.
Rate of falls (falls per person‐year)
We are uncertain whether exercise plus education compared to a control intervention makes little or no difference to the rate of falls (RaR 0.46, 95% CI 0.12 to 1.85; 320 participants, 2 studies, I2 = 87%; very low certainty evidence; Analysis 13.1).
13.1. Analysis.

Comparison 13: Exercise and education vs control (rate of falls), Outcome 1: Rate of falls
Number of people who experienced one or more falls (risk of falling)
Exercise plus education compared to a control intervention may make little or no difference to the number of people experiencing one or more falls (RR 0.89, 95% CI 0.75 to 1.07; 352 participants, 3 studies, I2 = 0%; low‐certainty evidence; Analysis 14.1).
14.1. Analysis.

Comparison 14: Exercise and education vs control (number of fallers), Outcome 1: Number of fallers
It was not possible to perform subgroup analyses based on the per cent of exercise supervision, as all studies utilised exercise programs with 50% or less of the exercise supervised. None of the included studies used fall risk as an inclusion criterion. One study (Cattaneo 2019) did not provide any information about disease severity, with the remaining two including predominantly people with mild to moderate disease, so subgroup analyses based on these factors was also not possible.
Number of people who experienced one or more fall‐related fractures
We are uncertain whether an exercise plus education intervention changes the number of people experiencing one or more fall‐related fractures (RR 1.45, 95% CI 0.40 to 5.32; 320 participants; very low‐certainty evidence; Analysis 15.1).
15.1. Analysis.

Comparison 15: Exercise and education vs control (number of people sustaining one or more fall‐related fractures), Outcome 1: Number of people sustaining one or more fall‐related fractures
Two studies reported more than one health‐related quality of life outcomes (PDQ39, EQ5D visual analogue scale and the EQ5D Index Score) (Morris 2015; Morris 2017). We are uncertain whether an exercise plus education intervention makes little or no difference to health‐related quality of life after the intervention and at follow‐up. Results for the PD‐specific tool (the PDQ39) shows little or no change (post intervention MD 0.05, 95% CI ‐3.12 to 3.23, 305 participants, 2 studies, very low‐certainty evidence, Analysis 16.1; follow‐up MD ‐2.25, 95% CI ‐5.45 to 0.96, 299 participants, 2 studies, very low‐certainty evidence, Analysis 16.2). The minimally important difference for the PDQ39 is about ‐1.6 (Peto 2001).
16.1. Analysis.

Comparison 16: Exercise and education vs control (health‐related quality of life), Outcome 1: Health‐related quality of life ‐ Parkinson's Disease Questionnaire (PDQ39) post intervention
16.2. Analysis.

Comparison 16: Exercise and education vs control (health‐related quality of life), Outcome 2: Health‐related quality of life ‐ Parkinson's Disease Questionnaire (PDQ39) at follow‐up
Health‐related quality of life
We are uncertain whether exercise plus education makes little or no difference to health‐related quality of life immediately post intervention (PDQ39 MD 0.05, 95% CI ‐3.12 to 3.23; 305 participants, 2 studies; I2 = 0%; very low‐certainty evidence; Analysis 16.1) or at 12 months follow‐up (PDQ39 MD ‐2.25, 95% CI ‐5.45 to 0.96; 299 participants, 2 studies; I2 = 0; very low‐certainty evidence; Analysis 16.2).
Exercise plus education versus exercise plus education
One study (Morris 2015) compared two different types of exercise; gait, balance and functional training in the form of movement strategy training versus resistance training in the form of functional resistance training with weighted vests and resistance bands. Both exercise interventions were delivered with the same fall‐prevention education. The results for the rate of falls are presented in Analysis 17.1, for the number of people experiencing one or more falls in Analysis 18.1, for the number of people sustaining fall‐related fracture in Analysis 19.1 and for health‐related quality of life in Analysis 20.1 (post intervention) and Analysis 20.2 (follow‐up).
17.1. Analysis.

Comparison 17: Exercise and education vs exercise and education (rate of falls), Outcome 1: Rate of falls
18.1. Analysis.

Comparison 18: Exercise and education vs exercise and education (number of fallers), Outcome 1: Number of fallers
19.1. Analysis.

Comparison 19: Exercise and education vs exercise and education (number of people sustaining one or more fall‐related fractures), Outcome 1: Number of people sustaining one or more fall‐related fractures
20.1. Analysis.

Comparison 20: Exercise and education vs exercise and education (health‐related quality of life), Outcome 1: Health‐related quality of life ‐ Parkinson's Disease Questionnaire (PDQ39) post intervention
20.2. Analysis.

Comparison 20: Exercise and education vs exercise and education (health‐related quality of life), Outcome 2: Health‐related quality of life ‐ Parkinson's Disease Questionnaire (PDQ39) at follow‐up
This study found that resistance training plus education reduced the rate of falls compared to movement strategy training, but there was no effect on the number of people who fell at least once, the number of people experiencing a fall‐related fracture or on health‐related quality of life (Morris 2015, n = 136).
Adverse events
Two studies of an exercise plus education intervention reported information related to adverse events (Table 15). Morris 2015 reported one fall and two occasions of dizziness during movement strategy training intervention along with 36 occasions of new muscle soreness lasting more than 24 hours (11 in the movement strategy training group and 25 in the functional strength training group). The remaining study stated that there were no adverse events related to the intervention (Morris 2017).
Economic analysis
One study of an exercise plus education intervention provided information regarding the cost of the intervention (Table 14). However, costs per fall prevented were not calculated as there was no reduction in falls in this study (Morris 2017).
Sensitivity analyses
We conducted sensitivity analyses for the pooled falls outcomes for exercise versus control, cholinesterase inhibitor versus placebo and exercise plus education versus control. A summary of these results is in Table 3 and Table 4. The results of the sensitivity analyses can be seen in Analyses 21 to 30, as per the list below.
1. Sensitivity analysis: exploring impact on results (rate of falls outcome).
| Sensitivity analysis | Pooled impact of intervention on fall rate, Rate ratio, 95% CI |
| Exercise trials vs control | |
| Primary analysis, all trials, random effects meta‐analysis | 0.74, 0.63 to 0.87; participants = 1456; trials = 12 |
| Sensitivity analysis 1, removing trials with high risk of bias in any item | 0.74, 0.61 to 0.90; participants = 1,245; trials = 9 |
| Sensitivity analysis 2, removing trials with unclear or high risk of bias on random sequence generation | 0.90, 0.76 to 1.05; participants = 995; trials = 7 |
| Sensitivity analysis 3, removing trials with unclear or high risk of bias on allocation concealment | 0.80, 0.70 to 0.91; participants = 1299; trials = 8 |
| Sensitivity analysis 4, removing trials with unclear or high risk of bias on assessor blinding | 0.92, 0.73 to 1.16; participants = 692; trials = 2 |
| Sensitivity analysis 5, removing trials with unclear or high risk of bias on incomplete outcome data | 0.77, 0.65 to 0.92; participants = 1260; trials = 11 |
| Sensitivity analysis 6, removing trials with less than three months falls monitoring | 0.79, 0.68 to 0.92; participants = 1268; trials = 9 |
| Sensitivity analysis 8, all exercise trials, fixed effects meta‐analysis | 0.79, 0.71 to 0.88; participants = 1456; trials = 12 |
| Primary analysis, subgrouped by exercise type Gait, balance and functional training Resistance training 3D exercise |
0.80, 0.67 to 0.95; participants = 1146; trials = 9 0.72, 0.55 to 0.94; participants = 137; trials = 2 0.41, 0.23 to 0.72; participants = 174; trials = 2 Test for subgroup differences Chi2 = 4.92, df = 2 (P = 0.09), I2 = 59.3% |
| Sensitivity analysis 10, classification of interventions that included functional strength training from resistance training to gait, balance and functional training Gait, balance and functional training Resistance training 3D exercise |
0.78, 0.68 to 0.91; participants = 1244; trials = 10 0.84, 0.28 to 2.53; participants = 38; trials = 1 0.41, 0.23 to 0.72; participants = 174; trials = 2 Test for subgroup differences Chi2 = 4.8, df = 2 (P = 0.09), I2 = 58.3% |
| Medication trials ‐ cholinesterase inhibitor vs placebo | |
| Primary analysis, all trials, fixed effects meta‐analysis | 0.50, 0.44 to 0.58; participants = 229; trials = 3 |
| Sensitivity analysis 1, removing trials with high risk of bias in any item | 0.43, 0.32 to 0.56; participants = 81; trials = 1 |
| Sensitivity analysis 2, removing trials with unclear or high risk of bias on random sequence generation | 0.60, 0.38 to 0.96; participants = 129; trials = 1 |
| Sensitivity analysis 3, removing trials with unclear or high risk of bias on allocation concealment | 0.60, 0.38 to 0.96; participants = 129; trials = 1 |
| Sensitivity analysis 5, removing trials with unclear or high risk of bias on incomplete outcome data | 0.60, 0.38 to 0.96; participants = 129; trials = 1 |
| Sensitivity analysis 9, all cholinesterase inhibitor trials, random effects meta‐analysis | 0.50, 0.43 to 0.58; participants = 229; trials = 3 |
| Exercise plus education trials vs control | |
| Primary analysis, all trials, random effects meta‐analysis | 0.46, 0.12 to 1.85; participants = 320; trials = 2 |
| Sensitivity analysis 1, removing trials with high risk of bias in any item | 1.58, 0.74 to 3.40; participants = 124; trials = 1 |
| Sensitivity analysis 4, removing trials with unclear or high risk of bias on assessor blinding | 0.24, 0.10 to 0.61; participants = 196; trials = 1 |
| Sensitivity analysis 5, removing trials with unclear or high risk of bias on incomplete outcome data | 1.58, 0.74 to 3.40; participants = 124; trials = 1 |
| Sensitivity analysis 7, removing the comparison responsible for the high level of heterogeneity (Morris 2017) | 0.24, 0.10 to 0.61; participants = 196; trials = 1 |
| Sensitivity analysis 8, all exercise plus education trials, fixed effects meta‐analysis | 0.54, 0.33 to 0.89; participants = 320; trials = 2 |
2. Sensitivity analysis: exploring impact on results (number of people who experienced one or more falls outcome).
| Sensitivity analysis | Pooled impact of intervention on risk of falling, Risk ratio, 95% CI |
| Exercise trials vs control | |
| Primary analysis, all exercise trials, random effects meta‐analysis | 0.90, 0.80 to 1.00; participants = 932; trials = 9 |
| Sensitivity analysis 1, removing trials with high risk of bias in any item | 0.87, 0.75 to 1.02; participants = 721; trials = 6 |
| Sensitivity analysis 2, removing trials with unclear or high risk of bias on random sequence generation | 0.89, 0.76 to 1.04; participants = 516; trials = 5 |
| Sensitivity analysis 3, removing trials with unclear or high risk of bias on allocation concealment | 0.91, 0.81 to 1.03; participants = 838; trials = 7 |
| Sensitivity analysis 4, removing trials with unclear or high risk of bias on assessor blinding | 0.93, 0.78 to 1.11; participants = 231; trials = 1 |
| Sensitivity analysis 5, removing trials with unclear or high risk of bias on incomplete outcome data | 0.89, 0.79 to 1.00; participants = 736; trials = 8 |
| Sensitivity analysis 6, removing trials with less than three months falls monitoring | 0.89, 0.77 to 1.02; participants = 789; trials = 7 |
| Sensitivity analysis 8, all exercise trials, fixed effects meta‐analysis | 0.90, 0.80 to 1.00; participants = 932; trials = 9 |
| Primary analysis, subgrouped by exercise type Gait, balance and functional training Resistance training 3D exercise |
0.92, 0.81 to 1.04; participants = 622; trials = 6 0.87, 0.43 to 1.74; participants = 136; trials = 2 0.59, 0.36 to 0.95; participants = 174; trials = 2 Test for subgroup differences Chi2 = 3.14, df = 2 (P = 0.21), I2 = 36.2% |
| Sensitivity analysis 10, classification of interventions that included functional strength training from resistance training to gait, balance and functional training Gait, balance and functional training Resistance training 3D exercise |
0.93, 0.83 to 1.05; participants = 720; trials = 7 0.58, 0.30 to 1.13; participants = 38; trials = 1 0.59, 0.36 to 0.95; participants = 174; trials = 2 Test for subgroup differences Chi2 = 5.02, df = 2 (P = 0.08), I2 = 60.1% |
| Medication trials ‐ cholinesterase inhibitor vs placebo | |
| Primary analysis, all trials, fixed effects meta‐analysis | 1.01, 0.90 to 1.14; participants = 230; trials = 3 |
| Sensitivity analysis 1, removing trials with high risk of bias in any item | 0.31, 0.12 to 0.78; participants = 81; trials = 1 |
| Sensitivity analysis 2, removing trials with unclear or high risk of bias on random sequence generation | 1.00, 0.87 to 1.15; participants = 130; trials = 1 |
| Sensitivity analysis 3, removing trials with unclear or high risk of bias on allocation concealment | 1.00, 0.87 to 1.15; participants = 130; trials = 1 |
| Sensitivity analysis 5, removing trials with unclear or high risk of bias on incomplete outcome data | 1.03, 0.92 to 1.16; participants = 149; trials = 2 |
| Sensitivity analysis 7, removing the comparison responsible for the high level of heterogeneity (Li 2015a) | 1.03, 0.92 to 1.16; participants = 149; trials = 2 |
| Sensitivity analysis 9, all cholinesterase inhibitor trials, random effects meta‐analysis | 0.95, 0.70 to 1.28; participants = 230; trials = 3 |
| Exercise plus education trials vs control | |
| Primary analysis, all trials, random effects meta‐analysis | 0.89, 0.75 to 1.07; participants = 352; trials = 3 |
| Sensitivity analysis 1, removing trials with high risk of bias in any item | 0.84, 0.65 to 1.08; participants = 156; trials = 2 |
| Sensitivity analysis 3, removing trials with unclear or high risk of bias on allocation concealment | 0.90, 0.75 to 1.08; participants = 320, trials = 2 |
| Sensitivity analysis 4, removing trials with unclear or high risk of bias on assessor blinding | 0.93, 0.73 to 1.19; participants = 228, trials = 2 |
| Sensitivity analysis 8, all exercise plus education trials, fixed effects meta‐analysis | 0.89, 0.75 to 1.07; participants = 352; trials = 3 |
1. Sensitivity analysis 1, removing studies with high risk of bias in any item, presented in Analysis 21.1 to Analysis 21.6.
21.1. Analysis.

Comparison 21: Sensitivity analysis 1: excluding studies at a high risk of bias in any item, Outcome 1: Rate of falls ‐ exercise vs control
21.6. Analysis.

Comparison 21: Sensitivity analysis 1: excluding studies at a high risk of bias in any item, Outcome 6: Number of fallers ‐ exercise and education vs control
2. Sensitivity analysis 2, removing studies with unclear or high risk of bias on random sequence generation, presented in Analysis 22.1 to Analysis 22.4.
22.1. Analysis.

Comparison 22: Sensitivity analysis 2: excluding studies with unclear or high risk of bias on random sequence generation, Outcome 1: Rate of falls ‐ exercise vs control
22.4. Analysis.

Comparison 22: Sensitivity analysis 2: excluding studies with unclear or high risk of bias on random sequence generation, Outcome 4: Number of fallers ‐ cholinesterase inhibitor vs placebo
3. Sensitivity analysis 3, removing studies with unclear or high risk of bias on allocation concealment, presented in Analysis 23.1 to Analysis 23.5.
23.1. Analysis.

Comparison 23: Sensitivity analysis 3: excluding studies with unclear or high risk of bias on allocation concealment, Outcome 1: Rate of falls ‐ exercise vs control
23.5. Analysis.

Comparison 23: Sensitivity analysis 3: excluding studies with unclear or high risk of bias on allocation concealment, Outcome 5: Number of fallers ‐ exercise and education vs control
4. Sensitivity analysis 4, removing studies with unclear or high risk of bias on assessor blinding, presented in Analysis 24.1 to Analysis 24.4.
24.1. Analysis.

Comparison 24: Sensitivity analysis 4, excluding studies with unclear or high risk of bias on assessor blinding, Outcome 1: Rate of falls ‐ exercise vs control
24.4. Analysis.

Comparison 24: Sensitivity analysis 4, excluding studies with unclear or high risk of bias on assessor blinding, Outcome 4: Number of fallers ‐ exercise and education vs control
5. Sensitivity analysis 5, removing studies with unclear or high risk of bias on incomplete outcome data, presented in Analysis 25.1 to Analysis 25.5.
25.1. Analysis.

Comparison 25: Sensitivity analysis 5, excluding studies with unclear or high risk of bias on incomplete outcome data, Outcome 1: Rate of falls ‐ exercise vs control
25.5. Analysis.

Comparison 25: Sensitivity analysis 5, excluding studies with unclear or high risk of bias on incomplete outcome data, Outcome 5: Rate of falls ‐ exercise and education vs control
6. Sensitivity analysis 6, removing studies with less than three months falls monitoring, presented in Analysis 26.1 and Analysis 26.2.
26.1. Analysis.

Comparison 26: Sensitivity analysis 6, excluding studies with less than three months falls monitoring, Outcome 1: Rate of falls ‐ exercise vs control
26.2. Analysis.

Comparison 26: Sensitivity analysis 6, excluding studies with less than three months falls monitoring, Outcome 2: Number of fallers ‐ exercise vs control
7. Sensitivity analysis 7, removing the comparisons responsible for high levels of heterogeneity, presented in Analysis 27.1 and Analysis 27.2.
27.1. Analysis.

Comparison 27: Sensitivity analysis 7, excluding comparisons responsible for the high level of heterogeneity, Outcome 1: Number of fallers ‐ cholinesterase inhibitor vs placebo
27.2. Analysis.

Comparison 27: Sensitivity analysis 7, excluding comparisons responsible for the high level of heterogeneity, Outcome 2: Rate of falls ‐ exercise and education vs control
8. Sensitivity analysis 8, fixed‐effect meta‐analysis, presented in Analysis 28.1 to Analysis 28.4.
28.1. Analysis.

Comparison 28: Sensitivity analysis 8, fixed‐effect meta‐analysis, Outcome 1: Rate of falls ‐ exercise vs control
28.4. Analysis.

Comparison 28: Sensitivity analysis 8, fixed‐effect meta‐analysis, Outcome 4: Number of fallers ‐ exercise and education vs control
9. Sensitivity analysis 9, random effects meta‐analysis, presented in Analysis 29.1 and Analysis 29.2.
29.1. Analysis.

Comparison 29: Sensitivity analysis 9, random effects meta‐analysis, Outcome 1: Rate of falls ‐ cholinesterase inhibitor vs placebo
29.2. Analysis.

Comparison 29: Sensitivity analysis 9, random effects meta‐analysis, Outcome 2: Number of fallers ‐ cholinesterase inhibitor vs placebo
10. Sensitivity analysis 10, reclassifying studies that utilised functional strength training from resistance exercise to gait, balance and functional training, presented in Analysis 30.1 and Analysis 30.2.
30.1. Analysis.

Comparison 30: Sensitivity analysis 10, reclassifying functional resistance training from resistance training to gait, balance and functional training, Outcome 1: Rate of falls ‐ exercise vs control
30.2. Analysis.

Comparison 30: Sensitivity analysis 10, reclassifying functional resistance training from resistance training to gait, balance and functional training, Outcome 2: Number of fallers ‐ exercise vs control
As shown in Table 3 and Table 4, generally these sensitivity analyses made little difference to the results of the primary pooled analyses, indicating that overall the review’s methods and findings are robust. The exception to this was the cholinesterase inhibitor versus placebo, number of people who fell at least once and exercise plus education versus control, number of falls outcomes.
In the cholinesterase inhibitor versus placebo number of people who fell at least once outcome, removing studies with a high risk of bias in any item (Sensitivity analysis 1) removed the two largest of the three included studies, and resulted in a much lower risk ratio (all studies RR 1.01, 95% CI 0.90 to 1.14; 249 participants, 3 studies; Analysis 9.1 versus Sensitivity analysis 1 RR 0.31, 95% CI 0.12 to 0.78; 81 participants, 1 study; Analysis 21.4). The remaining smaller study (Chung 2010) had a much greater effect size in favour of medication and much wider confidence intervals than the other two studies. Consequently, the certainty of the evidence for this comparison was downgraded (see Table 2).
21.4. Analysis.

Comparison 21: Sensitivity analysis 1: excluding studies at a high risk of bias in any item, Outcome 4: Number of fallers ‐ cholinesterase inhibitor vs placebo
In the exercise plus education versus control rate of falls outcome (all studies RaR 0.46, 95% CI 0.12 to 1.85; 320 participants, 2 studies; Analysis 13.1), results were substantially changed by: i) removing studies with a high or unclear risk of bias on assessor blinding (Sensitivity analysis 4 RaR 0.24, 95% CI 0.10 to 0.61; 196 participants, 1 study; Analysis 24.3); ii) removing comparisons responsible for the high level of heterogeneity (Sensitivity analysis 7 RaR 0.24, 95% CI 0.10 to 0.61; 196 participants, 1 study; Analysis 27.2), and conducting a fixed effects meta‐analysis (Sensitivity analysis 8 RaR 0.54, 95% CI 0.33 to 0.89; 320 participants, 2 studies; Analysis 28.3). These sensitivity analyses changed the result from indicating little or no difference to the number of falls to indicating a reduction in the rate of falls (Table 3). The certainty of the evidence for this comparison was therefore downgraded (see Table 6).
24.3. Analysis.

Comparison 24: Sensitivity analysis 4, excluding studies with unclear or high risk of bias on assessor blinding, Outcome 3: Rate of falls ‐ exercise and education vs control
28.3. Analysis.

Comparison 28: Sensitivity analysis 8, fixed‐effect meta‐analysis, Outcome 3: Rate of falls ‐ exercise and education vs control
Heterogeneity
There was substantial heterogeneity in this review’s primary analysis for the effect of cholinesterase inhibitors versus placebo on the risk of falling (Chi2 = 7.23, df = 2, P = 0.03, I2 = 72%; Analysis 9.1). We were unable to use our pre‐specified subgroup analyses to explore this heterogeneity. However, removal of one of the three studies (Li 2015a) reduces the heterogeneity to a level where it is unlikely to be important (Chi2 = 0.78, df = 1, P = 0.38, I2 = 0%; Analysis 27.1) with minimal change to the RR (all studies RR 1.01, 95% CI 0.90 to 1.14; Li 2015a excluded RR 1.03, 95% CI 0.92 to 1.16). It is possible that this heterogeneity may be, in part, due to the differing inclusion criteria of these three studies, with Li 2015a including only participants with cognitive impairment (including dementia), while Henderson 2016 excluded participants with dementia and Chung 2010 excluded participants with substantial cognitive impairment (Mini‐mental State Examination score < 25). Further research is required to explore potential sources of heterogeneity in this outcome. However, given the overall stability of the results, we consider the meta‐analyses we have undertaken to be appropriate.
There was also considerable heterogeneity in this review’s primary analysis for the effect of exercise plus education versus control on the rate of falls (Chi2 = 15.16, df = 2, P = 0.0005, I2 = 87%; Analysis 13.1). Removal of one study (Morris 2017) reduced the heterogeneity to a moderate level (Chi2 = 1.96, df = 1, P = 0.16, I2 = 49%; Analysis 27.2), but altered the result from indicating little or no difference to a reduction in the number of falls (all studies RaR 0.46, 95% CI 0.12 to 1.85; Morris 2017 excluded RaR 0.24, 95% CI 0.10 to 0.61). The lack of stability in this result led to the downgrading of the certainty of this evidence. We were unable to conduct the pre‐planned subgroup analyses to explore this heterogeneity. However, the authors of Morris 2017 suggested the lack of effect on falls seen in this trial could be due to an insufficient dose of exercise plus education. As for the cholinesterase inhibitor outcome, further research is required to explore potential sources of heterogeneity in this outcome.
There was no evidence of important heterogeneity in the remaining exercise versus control, cholinesterase inhibitor versus placebo or exercise plus education versus control primary outcomes.
Small sample bias
Funnel plots were generated for the exercise versus control comparisons for both the rate of falls and the number of people who fell at least once (Figure 4 and Figure 5). These plots do show some asymmetry. However, we did not consider it sufficient to downgrade the evidence for these outcomes. There were too few comparisons to warrant generating funnel plots for the other outcomes.
4.

Funnel plot of comparison: 1 Exercise vs control (rate of falls), outcome: 1.1 Rate of falls.
5.

Funnel plot of comparison: 2 Exercise vs control (number of fallers), outcome: 2.1 Number of fallers.
Discussion
Summary of main results
This review explores interventions to prevent falls in people with Parkinson's disease (PD) and includes 25 studies of exercise (2700 participants), three studies of medication (242 participants), one study of education (53 participants with PD) and three studies of exercise combined with education (375 participants). All studies were of a single intervention, except for the three studies that investigated exercise combined with education.
Exercise versus control
There is moderate‐certainty evidence that exercise programs probably reduce the rate of falls (reported in 12 studies) in people with PD, and that the number of people experiencing one or more falls (reported in 9 studies) is probably slightly reduced (see Table 1). For the rate of falls, there was an illustrative rate of 8250 falls per 1000 person‐years in people with PD in the control group, with 2145 (26%) fewer falls per 1000 person‐years in the exercise group (95% confidence interval (CI) 1072 (13%) to 3052 (37%) fewer). For the number of people who fell at least once, there was an illustrative risk of 634 fallers per 1000 people with PD in the control group, with 63 (10%) fewer fallers per 1000 people with PD in the exercise group (95% CI 127 (20%) to 0 (0%) fewer). The larger benefit on the rate of falls compared to the number of people who fell at least once suggests that while exercise probably reduces the number of falls people with PD experience, it often does not eliminate falls altogether.
The test for subgroup differences when grouped by exercise type did not show any subgroup differences for the rate of falls or the number of people who fell at least once compared to a control intervention. However, the exercise intervention delivered in most comparisons was gait, balance and functional training, (10 (71%) for the rate of falls and 6 (60%) for the number of people who fell at least once) meaning there were unlikely to be sufficient numbers of studies of alternative intervention types to find a difference if one exists. Subgroup analyses based on the baseline fall risk also did not find an effect on either fall outcome.
Subgroup analysis suggested that exercise programs that are fully supervised by a therapist may reduce the number of falls more so than exercise that was partially supervised; though this was not found for the number of people who fell at least once. Improved results with supervision could be due to several factors, such as feedback on exercise performance, encouragement and increased exercise intensity and challenge. However, fully supervised exercise is not sustainable in the context of a long‐term, neurodegenerative condition. Further work is needed to design and explore methods of identifying the appropriate level of supervision required by individuals with PD to achieve optimal outcomes throughout their disease course. In addition, identifying methods of optimising semi‐supervised exercise and service delivery aimed at fall prevention, such as using intermittent, in‐person, supervised therapy interspersed with therapy supported by telehealth (Pelicioni 2020) and/or feedback‐based technology (Canning 2020) is required.
Pooling of reported subgroups based on disease severity (two randomised controlled trials (RCTs) for each fall outcome) showed differences suggesting that exercise interventions may reduce the rate of falls and the number of people who fell at least once in participants with lower disease severity, but increase them in people with higher disease severity. There is no clear explanation for this, however there is evidence that people with more advanced disease may adhere to prescribed exercise, but compensate by reducing other exercise, which could result in an inadequate dose of exercise overall (Canning 2015a). In contrast, it is possible that improvements in mobility in the more severely affected group leads to people having more exposure to situations where they are at risk of falls (Canning 2015a; Del Din 2020). This issue requires investigation taking a precision medicine approach (Canning 2020; Nonnekes 2018), where interventions are more specifically targeted to the individual’s clinical presentation, risk factors, lifestyle and environment. In addition, analysis of fall rates relative to activity exposure will contribute to further understanding of the effectiveness of interventions designed to reduce falls (Del Din 2020).
Exercise may slightly improve health‐related quality of life compared to control immediately after the intervention (low‐certainty evidence), with conversion of the pooled result to the PDQ39 score showing that the mean difference (‐2.6) may be greater than the minimally important difference (‐1.6) (Peto 2001), though the 95% CI included scores that were smaller than the minimally important difference (‐5.5 to 0.2). However, we are uncertain whether exercise improves health‐related quality of life at follow‐up (range 20 weeks to 12 months; very low‐certainty evidence). We are also uncertain whether exercise makes little or no difference to the number of people sustaining a fall‐related fracture (very low‐certainty evidence).
Exercise versus control and exercise versus exercise
Most exercise studies (15) monitored adverse events related to the exercise intervention. Minor adverse events related to the exercise intervention were reported in four studies, primarily non‐injurious falls, excessive muscle soreness, or pain, dizziness or hypotension. Nine studies reported that there were no adverse events related to the intervention, and two reported that there were no serious adverse events. Only three studies additionally monitored for adverse events unrelated to the intervention using the same methods in all groups across the entire study period (Li 2012; Mirelman 2016; Paul 2014), though two additional studies also mentioned non‐intervention‐related adverse events (Chivers Seymour 2019; Song 2018). Overall, these results suggest that exercise is likely to be a low‐risk intervention.
Six exercise studies included in this review reported an economic evaluation. Four of these gave an indication of value for money for the interventions tested, however there were variations in the methods used which made it difficult to compare studies. There was some evidence that exercise for fall prevention in people with PD can be cost‐effective during the study period and a short time beyond. The relative cost‐effectiveness of different fall‐prevention intervention approaches in people with PD requires further exploration.
Medication versus placebo
There is low‐certainty evidence that cholinesterase inhibitors may reduce the rate of falls (reported in three RCTs) compared to placebo medication (see Table 2). Based on an illustrative rate of 28,800 falls per 1000 person‐years in the placebo group, there were 14,400 (50%) fewer falls per 1000 person‐years in the cholinesterase inhibitor group (95% CI 12,096 (42%) to 16,128 (56%) fewer). However, we are uncertain whether this medication makes little or no difference to the number of people who fell at least once and to health‐related quality of life immediately after the intervention (very low‐certainty evidence).
We were unable to conduct the pre‐planned subgroup analyses based on fall risk at baseline or disease severity as all three studies included participants at high risk of falls, and all participants were similar in terms of disease severity.
There is low‐certainty evidence that cholinesterase inhibitors may increase the rate of non fall‐related adverse events (reported in two RCTs) compared to placebo medication. Based on an illustrative rate of 1970 adverse events per 1000 person‐years in the placebo group, there were 1182 (60%) more adverse events per 1000 person‐years in the cholinesterase inhibitor group (95% CI 552 (28%) to 1990 (101%) more). Most adverse events were mild and transient in nature, such as nausea and headache.
Education versus control
There was only one study of an education intervention compared to usual care, which provided data only for the number of people who fell at least once (Table 5). This study provided very low‐certainty evidence; hence we are uncertain of the finding that education increases the number of people with PD who fall. The very wide confidence interval means that these data are not informative.
Exercise plus education versus control and exercise plus education versus exercise plus education
We are uncertain whether exercise plus education compared to control makes little or no difference to the rate of falls, the number of people sustaining fall‐related fractures and health‐related quality of life (all very low ‐certainty evidence, see Table 6). Exercise plus education may make little or no difference to the number of people experiencing one or more falls (low‐certainty evidence). Based on an illustrative risk of 672 fallers per 1000 people with PD in the control group, there may be 74 (11%) fewer fallers per 1000 people with PD in the exercise plus education group (95% CI 168 (25%) fewer to 47 (7%) more).
Two of the three exercise plus education studies reported adverse events related to the exercise intervention, with one study reporting minor adverse events (Morris 2015) and the other reporting there were no adverse events (Morris 2017). This concurs with the result from the exercise studies, further supporting that exercise may be a low risk intervention.
Overall completeness and applicability of evidence
Trial design and participants
Of the 32 studies included in this review, 25 were of exercise interventions, three were medication interventions, one was an education intervention and three were exercise plus education interventions. Overall, most participants had mild to moderate PD, though the participants in the medication trials had greater disease severity than the trials of the other interventions.
In the exercise studies, 13 studies (52%) recruited participants with a recent history of falls or one or more risk factors for falling. One study (4%) recruited only participants with no recent fall history. Most participants in the exercise studies had mild to moderate disease severity, and minimal or no cognitive impairment, with only one study including people with mild cognitive impairment. These factors combined suggest that overall, many of the participants included in this review were at relatively low risk of falls and the results of this review are unlikely to be applicable to people with a high risk of falls, moderately severe to severe disease or with substantially impaired cognition.
In medication studies, three studies compared a cholinesterase inhibitor with a placebo. Two of these studies recruited only participants with a history of falls, but had vastly different inclusion criteria. One study included both occasional and frequent fallers, requiring one or more falls in the prior year, and excluded people with dementia. Another included only frequent fallers, requiring two or more falls or near falls each week without freezing of gait, and excluded those with cognitive impairment. The remaining study included only people with cognitive impairment, including dementia. Given cognitive impairment is a known risk factor for falls (Allcock 2009; Latt 2009; Paul 2013), the participants in this study were at increased risk of falls. This suggests that these results can be applied to people with PD who are at risk of falls, including people with impaired cognition.
The single education intervention trial did not report any information regarding disease severity and included both people with and without a history of falls. They also included people with some level of cognitive impairment, excluding only those with dementia.
The three studies of exercise plus education included both fallers and non‐fallers and excluded people with cognitive impairment. Two studies reported information related to disease severity, with most participants having mild to moderate disease. Therefore, the results of this review for exercise plus education interventions are unlikely to be applicable to people with moderately severe to severe disease or with substantially impaired cognition.
The illustrative fall rates and fall risk based on control/placebo group fall rates and risk varied between exercise, medication and exercise plus education studies. The illustrative fall risk (i.e. number of people who fell at least once) varied from 634 fallers per 1000 people in the exercise studies, to 672 per 1000 people in the exercise plus education study, and 774 fallers per 1000 people in the cholinesterase inhibitor studies. While somewhat variable, these values are broadly similar to the previously reported average of 60.5% (i.e. 605 fallers per 1000 people) of people with PD falling in any one year (Allen 2013). The illustrative fall rates (i.e. number of falls) had a higher variability, ranging from 8250 falls per 1000 people per year in the exercise studies, to 16,400 falls per 1000 people per year in the exercise plus education studies and up to 28,800 falls per 1000 people per year in the cholinesterase inhibitor studies. Even greater variability than this was reported in a previously published review (4700 to 67,600 falls per 1000 people per year reported in Allen 2013). The variability in the illustrative fall risk for both fall measures reflects, at least in part, the varying inclusion criteria of the different studies.
Setting
Around half the exercise studies included in this review were conducted at a facility and fully supervised, including supervised group exercise. Of the remaining studies, five included both facility and home‐based exercise, and five were solely home‐based, with solely home‐based trials having less than 50% of sessions supervised and some trials reporting as little as 5% of sessions supervised. The subgroup analysis comparing studies with 100% supervision with those with < 100% supervision found subgroup differences for the rate of falls, suggesting that exercise interventions for people with PD may be more effective in reducing falls if they are fully supervised. However, it is unlikely that fully‐supervised exercise will be cost‐effective in the long term. Therefore, identifying individuals who can exercise effectively using a semi‐supervised model has the potential to improve sustainability.
The single study of an education intervention included an individual home‐visit and a follow‐up phone call. One of the three studies of exercise plus education interventions involved home‐based exercise, while the others used a combination of facility and home‐based. Two studies provided supervision of 50% of the exercise, and also provided the education individually to participants alongside the exercise. The other study provided three supervised exercise sessions at a facility, followed by fully home‐based and unsupervised exercise. This study provided a single, one hour group education session at a facility before participants began the exercise program.
Interventions
We classified the exercise interventions according to the ProFaNE guidelines (Lamb 2011; Table 7 and Table 9). Most studies were categorised as gait, balance and functional training, with few studies of resistance training, 3D exercise, flexibility exercise or other exercise. Subgroup analyses for rate of falls and number of people who fell at least once versus control found there was no evidence for one category of exercise being superior to another. However, the small number of studies that were not categorised as gait, balance and functional training meant that our ability to find a difference between exercise interventions, should a difference exist, was limited. In addition, people with PD experience risk factors for falls over and above those attributable to ageing; such as freezing of gait, difficulty performing dual tasks and specific problems with reactive balance (van der Marck 2014). Therefore, exercise programs may include specific exercises designed to address these PD‐specific risk factors, but these details are missed when the exercise is placed in the broader category of gait, balance and functional training. Furthermore, many of the studies included in this review used various combinations of exercise types (e.g. balance, functional strength training and cueing for freezing of gait). These studies arguably reflect programs that are offered in clinical practice, and fit well into the category of gait, balance and functional training. However, other studies trialled specific single interventions, such as cueing (Martin 2015) or step training (Song 2018). Therefore, the use of a broad exercise category to include combinations of exercises and individual exercise as well as PD‐specific and non‐PD specific exercise limited our ability to explore differences between types of exercise.
Some subjectivity in the classification of exercise interventions is also apparent. In particular, we considered that functional strength exercises performed largely in standing using body weight or equipment, such as weighted vests and ankle weights, potentially could have been categorised as gait, balance and functional training rather than resistance exercise. Sensitivity analyses to re‐categorise these studies as gait, balance and functional training for the primary outcomes makes little difference to the test for subgroup differences. However, it should be noted that this re‐classification leaves only one study with a small sample size and wide confidence intervals in the resistance training category (Paul 2014).
The length of the interventions in the exercise studies was short compared to those reported for community dwelling people in the general older population (Sherrington 2019). In the present review, exercise interventions varied from six weeks to six months, with the intervention conducted over 12 weeks or less in 72% of studies. In the aforementioned review of exercise for older people, most exercise programs were 12 weeks or longer, with nearly one third of studies trialling programs of 12 months or more (Sherrington 2019).
Three medication studies compared a cholinesterase inhibitor to a placebo, with two trialling rivastigmine and one trialling donepezil. The length of time that medications were administered in these studies was highly variable, at six weeks, eight months and 12 months. There was no evidence of subgroup differences based on which cholinesterase inhibitor was trialled for either fall outcome.
The education study provided participants with a 12‐month action plan, which included fall‐prevention strategies and was delivered to them in their home by an occupational therapist in a single home visit with a follow‐up phone call.
The three studies of exercise plus education used differing approaches. One study utilised a single one‐hour falls‐prevention education session delivered to small groups of participants (two to four) by a physiotherapist. This was followed by three individual exercise sessions where the participant was taught mobility and balance exercises and asked to perform them on their own two to three times per week at home for two months. The remaining two studies both utilised individual functional progressive resistance training and movement strategy training, though one trialled these individually in two separate intervention groups for eight weeks, while the other combined these exercise interventions for six weeks. Both these studies incorporated falls prevention education into one weekly supervised session, with the other exercise session performed by participants independently.
Outcomes
We extracted data for the rate of falls, number of people who fell at least once, number of people sustaining a fall‐related fracture, health‐related quality of life, rate of adverse events and economic evaluations related to fall outcomes. Most studies of exercise versus control intervention and all the medication versus placebo studies reported both the rate of falls and the number of people who fell at least once. However, less than half of the exercise versus control studies reporting rate of falls also reported the number of people sustaining a fall‐related fracture and/or health‐related quality of life at post intervention. Similarly, only one medication study reported fracture data and health‐related quality of life at post‐test. The education study reported the risk of falling but no other data relevant to this review. All the exercise and education studies reported the number of people who fell at least once, however only two studies reported the rate of falls, the number of people sustaining a fall‐related fracture and health‐related quality of life.
There were some inconsistencies in the way studies defined and collected falls data. Most, but not all studies, defined a fall according to the definition developed by ProFaNE; that is “an unexpected event in which the participant comes to rest on the ground, floor, or lower level” (Lamb 2005) while some studies applied the more stringent criterion of “without overwhelming external force or a major internal event” (Gibson 1987). Some studies omitted to provide any clear definition, and some did not use the ProFaNE recommended protocol for ascertaining falls data (i.e. daily recording of falls with follow‐up at least monthly by researchers blinded to group allocation) (Lamb 2005). While collecting falls data in this way can be burdensome and resource intensive (Iliffe 2015), relying on recall is likely to result in an under‐reporting of falls compared to data that are recorded daily and returned monthly (Hannan 2010). Notably, most studies in this review relied on recall over the prior 6 to 12 months for baseline fall measures. One study (Chivers Seymour 2019) collected baseline fall data prospectively for three months using falls diaries, providing shorter‐term but more accurate baseline fall data. Comparability of studies would be enhanced by the adoption of a standard falls definition and method for ascertaining falls data which may be automated (van der Marck 2011). In the future, further automation of fall detection is likely to be achieved by the use of body worn sensors to monitor falls in daily life, potentially increasing the robustness of falls data (Silva de Lima 2020).
While nearly all exercise studies reported on adverse events related to the intervention, very few measured adverse events in the same way in all groups throughout the study period. This contrasts with the medication studies, where the rate of adverse events in the medication and placebo group were reported in all except one study. The lack of rigorous adverse event monitoring in the exercise studies could be due to lack of resources coupled with the high burden of reporting on participants, who are often also required to keep records of completed exercise along with falls diaries. In contrast, adverse event reporting in medication studies is viewed as a routine component of study protocols and resources allocated accordingly. Additionally, researchers of exercise interventions may consider that the relationship between any particular adverse event and the exercise intervention is more clear‐cut than in medication studies, and therefore safety of the intervention can be surmised from collecting only adverse events that are directly related to the intervention (e.g. injuries or falls when exercising). While exercise interventions generally appear to involve low risk to participants, more consistent monitoring of adverse events is required to provide stronger evidence of this safety.
Economic evaluations related to the cost of the intervention and/or fall outcomes were reported in six exercise studies and one exercise plus education study. Three of the exercise studies reported the cost per fall prevented and/or quality‐adjusted life‐year (QALY) gained. However, these evaluations used a variety of methods, perspectives, time horizons and cost items, making it difficult to compare economic results across studies and intervention types.
Ongoing studies
The design of the 30 identified ongoing studies may help to guide future research priorities for people with PD. Twenty ongoing studies will evaluate the effectiveness of exercise interventions, however only three of these studies have a target sample size of over 100 participants, indicating that most of these studies will be underpowered to find an effect on falls. Most of the exercise studies are exploring an exercise intervention that can be classified as gait, balance and functional training. Around half of the studies are evaluating the relative impact of different exercise programs. Only one exercise study has registered adverse events as an outcome, and these adverse events will be measured only during the intervention (NCT03751371). Additionally, only one ongoing study is exploring the effect of a multifactorial intervention including exercise, where the exercise is combined with environmental modification and behavioural strategies (ACTRN12619000415101).
The ongoing medication study is a phase III trial powered to find an effect on falls. This large‐scale study is comparing rivastigmine with a placebo medication and has a target sample size of 600 participants (NCT04226248).
Several ongoing studies are exploring interventions that were not included in this review, as we did not find any published studies that met the inclusion criteria. In addition to the multifactorial intervention, three small randomised cross‐over trials are investigating the effect of differing regimens of deep brain stimulation on falls in people with PD. An additional study is exploring the effect of deep brain stimulation combined with physiotherapy and another the effect of osteopathic manipulative medicine compared to education. None of these studies are powered to find an effect on falls. However, the three studies exploring different models of care have larger sample sizes, with two of these large enough to find an effect on falls (NCT05127057, n = 214; NCT04555720, n = 200).
Of note, none of the ongoing studies specifically identify fall‐related fractures as an outcome measure, few mention adverse events and only one (rivastigmine versus placebo NCT04226248) is planning a cost‐effectiveness analysis. This, along with the under‐powered sample size of most ongoing studies, highlights areas for future research. However, such research will be costly, requiring large numbers of participants.
Quality of the evidence
This review containing 32 studies (3370 participants) provides moderate‐certainty evidence regarding the effect of exercise on falls in people with PD, however the evidence regarding the effect of medication, education alone or education plus exercise is less certain, ranging from low to very low.
We assessed the certainty of the evidence using the GRADE approach, and have summarised the results in four summary of findings tables: Table 1 (exercise compared to control); Table 2 (cholinesterase inhibitors compared to placebo); Table 5 (education compared to control); Table 6 (exercise plus education compared to control).
All studies had high or unclear risk of bias in at least one area. Of note is the unclear risk of bias due to knowledge of the allocated interventions (i.e. performance bias) in most studies with an exercise intervention and in all studies incorporating education. In studies where exercise and/or education is compared to usual care or no intervention, it is not possible to blind to participants or personnel regarding whether they are involved in the intervention (exercise/education) group or not. However, the extent to which this knowledge impacts study results is unclear.
The certainty of the evidence was downgraded for indirectness in the exercise versus control and the exercise plus education versus control outcomes. This was because the included participants had overall mild to moderate disease and good cognition. Therefore, they were not representative of the population with PD seeking falls prevention interventions (Domingos 2015), as many of these people have more advanced disease and impaired cognition.
Sensitivity analysis revealed overall stability in the results for the falls outcomes in the exercise versus control, the cholinesterase inhibitor versus placebo and the exercise plus education versus control comparisons (Table 3 and Table 4). This indicates these results are robust despite the variable risk of bias across studies and are largely unchanged by the methodological choices made in undertaking the review.
There were two comparisons of outcomes where sensitivity analysis made a substantial change to the primary analysis: the number of people who fell at least once in the cholinesterase inhibitor versus placebo comparison and the rate of falls in the exercise plus education versus control comparison. The results of these sensitivity analyses led to the downgrading of the certainty of the evidence for these outcomes.
Potential biases in the review process
It is possible that some relevant studies may have been missed from this review. We attempted to minimise the risk of this occurring by comprehensively searching multiple databases, searching for studies in languages other than English, and searching the reference lists of other reviews, grey literature and trial registries. While the literature searches were run in July 2020, we ran a top‐up search in October 2021, which identified two additional small studies (n < 70). We believe the incorporation of these studies will not change the results of this review (Studies awaiting classification). These studies will be considered for inclusion when we update this review. Additionally, pairs of review authors who were blind to each other’s results both performed screening and data extraction for each study. In selecting studies for inclusion, we followed our protocol methods and excluded studies without usable data. There is a chance this could introduce bias due to selective outcome reporting.
Another potential source of bias is in the categorisation of the exercise types. We classified the exercise interventions according to the ProFaNE guidelines (Lamb 2011). We recognise there is some subjectivity in this system, and the category of gait, balance and functional training is very broad. For example, functional strength exercises performed largely in standing using body weight as well as equipment such as weighted vests and ankle weights potentially could have been categorised as resistance exercise or gait, balance and functional training. Nonetheless, sensitivity analyses that explored the effect of reclassifying these resistance exercise interventions as gait, balance and functional training did not make any important differences to the results evaluating subgroup differences based on exercise category. However, this reclassification left only one study in the resistance training category, along with the two studies in the 3D exercise category. Any possible bias in the categorisation of exercise type therefore remains unclear.
It is also possible that our use of falls data from the longest available time‐period (up to 12 months) for each study introduced bias. The length of intervention and follow‐up varied between studies. While we used a commonly‐used approach to combine all the available data (e.g., Sherrington 2019; Cameron 2018), this means that we have combined data that for some studies was collected only or predominantly during the intervention period, with data from other studies which were collected in a non‐intervention follow‐up period. We acknowledge that this is a limitation, as it could be expected that the effect of the intervention may vary over time, and that the amount of falls data returned by participants would reduce over time (Hunter 2018). Future work could explore if the results from this approach vary with results from combining data only from the intervention period and only from a non‐intervention follow‐up period.
Agreements and disagreements with other studies or reviews
This review is the first Cochrane Review to report the effect of interventions to prevent falls in people with PD. For the exercise interventions, this review extends the findings of a review of RCTs reported by Shen 2016. Shen 2016 restricted the type of exercise intervention to those that were aimed at enhancing balance and gait (including gait, balance or strength exercise) compared to a control group. Additionally, in contrast to the present review, Shen 2016 included studies that reported falls as part of monitoring for adverse events (e.g. Nieuwboer 2007). While Shen 2016 had fewer included studies, the results supported the current finding that exercise probably reduces the rate of falls. However, the Shen 2016 review found a greater reduction in falls than the present review (RaR 0.49, 95% CI 0.33 to 0.72, 605 participants, 4 studies). Furthermore, while the present review found evidence that exercise probably slightly reduces the number of people who fell at least once, Shen 2016 did not find this effect (RR 0.94, 95% CI 0.82 to 1.07, 707 participants, 4 studies). These differences in results are likely to be due to the differing inclusion criteria along with the inclusion of more recently published studies in the current review.
Subgroup analyses in this Cochrane Review suggest that exercise interventions may reduce falls in people with milder disease, but may increase them in people with more advanced disease. This result agrees with a previously published narrative review (Hulbert 2019) which reported a reduction in fall rate following exercise in participants with less severe disease, with this reduction no longer apparent when results are combined across the spectrum of disease.
Subgroup analyses in this review suggest that fully supervised exercise may be more effective in reducing the number of falls than exercise that is partially supervised. This is in contrast to a recent review (Flynn 2019) that found home‐based exercise programs with minimal supervision were effective in improving balance‐related activities, while home‐based programs that were fully supervised were not effective. The difference in result may be because most of the studies in the present review were fully supervised at a facility, whereas only home‐based programs were included in the Flynn 2019 review. Notably, the fully supervised home‐based programs included in Flynn 2019 were of a lower dose than the partially supervised home‐based programs, suggesting that the resource requirement involved in providing fully supervised exercise at home could lead to a lower dose of intervention. Given the need for fall‐prevention interventions for people with PD to be sustainable over the long term, this possible interaction between dose, supervision and exercise location warrants consideration.
We are unaware of any published reviews exploring the effects of non‐exercise interventions on falls.
Authors' conclusions
Implications for practice.
Overall, the results of this review indicate that exercise interventions probably reduce the rate of falls and probably slightly reduce the number of people falling in people with Parkinson's disease (PD) (moderate‐certainty evidence). Furthermore, results suggest that fully supervised exercise may be more effective for reducing the number of falls than partially supervised exercise. Notably, this evidence applies to people with mild to moderate PD, minimal cognitive impairment and relatively low risk of falls.
The effect of exercise on falls in people with more advanced disease is unclear. Pooling of subgroups from three studies of minimally supervised exercise interventions (Ashburn 2007, Canning 2015a and Chivers Seymour 2019), suggests that this form of exercise may be used effectively to reduce falls in people with milder disease, but not in those with more advanced disease. This raises a challenge as most people with PD who present for exercise interventions (e.g. physiotherapy) in clinical practice have more advanced disease, cognitive impairment and recurrent falls. While there is currently no evidence that exercise can reduce falls in people with more advanced disease, exercise is known to have numerous other benefits (WHO 2020). Therefore, safety, supervision (either from a clinician or trained care partner) and monitoring are important considerations when prescribing any exercise intervention for people with PD, particularly for those with more advanced disease.
The type of exercise that is best to reduce falls is uncertain, with most studies in this review categorised as gait, balance and functional training, with some studies including specific exercises aimed at managing freezing of gait. Notably, there were only two studies in the exercise versus control analyses of 3D exercise (e.g. Tai Chi), and three studies in the resistance exercise category; two of which involved functional resistance training so could have been categorised as gait, balance and functional training. Current evidence therefore suggests that exercise interventions should include gait, balance and functional training. Notably, 3D exercise such as Tai Chi also challenges balance and could also be considered.
Cholinesterase inhibitors may reduce the rate of falls in people with PD who are at risk of falls, including those with impaired cognition. However, we found very low‐certainty evidence that this medication makes little or no difference to the number of people falling. Any benefits of a cholinesterase inhibitor needs to be balanced against the potential side effects, with low‐certainty evidence that it may increase non fall‐related adverse events. Notably, these adverse events were described as mostly mild and transient in nature, though they can be serious. People with PD and their families can therefore make an informed decision about whether to trial cholinesterase inhibitors and can be monitored for any benefit on falls as well as the development of any side effects.
There is currently insufficient evidence to determine the effects of education alone or exercise plus education on falls in people with PD.
Implications for research.
Further research is required to elucidate the relative impact of different types of exercise (or combinations of exercise) on falls in people with PD. In particular, studies specifically designed to target fall risk factors unique to PD (e.g. freezing of gait), along with progressive resistance/muscle power training and of 3D exercise (such as Tai Chi) will progress our understanding in this area. PD‐specific adaptation of the ProFaNE exercise categories may facilitate this process. Additionally, this review confirmed the findings of Domingos 2015 that most fall‐prevention exercise trials published to date have systematically excluded people with cognitive impairment, despite the fact that cognitive impairment is common amongst people with PD as the disease progresses (Hely 2008), and is known to be a risk factor for falls (Fasano 2017). Consequently, there is little evidence about fall‐prevention interventions for the large proportion of people with impaired cognition and more advanced disease. Therefore, further work is needed to confirm the relative impact of exercise interventions on falls in people with differing levels of disease severity, and to design and evaluate exercise interventions for people with cognitive impairment. This work should examine other factors such as intervention supervision, location and dose. Such studies will need to be very large in order to be adequately powered to detect if there are differing effects between interventions and/or differing effects of interventions according to disease severity. Additionally, studies should include cost‐effectiveness analyses related to fall outcomes in order to inform decisions made by healthcare funders and providers. There is also a need to investigate strategies to implement effective fall‐prevention exercise programs into the routine care of people with mild to moderate PD. A precision medicine approach to these investigations may facilitate translation of research to practice (Canning 2020; Nonnekes 2018).
While the certainty of the evidence for exercise interventions on fall outcomes was moderate, there was less certainty about the effect of other types of intervention, including medications. The effect size reported in the three studies that examined the effect of cholinesterase inhibitors was large, with an estimated reduction in fall rate of 50%. This was not paralleled by a reduction in the number of fallers. It is presumably much easier to reduce the number of falls, but not prevent all falls. The rather marked reduction in rates of falls in the cholinesterase inhibitor studies is not paralleled by common clinical experience in daily practice, suggesting that the effect sizes were perhaps inflated in the clinical trials, possibly because the high rate of adverse effects led to some unblinding. Further research is required to determine the effects of medication on falls and other related outcomes (e.g. fractures, adverse events and cost‐effectiveness related to falls) in people with PD. One large‐scale medication study is currently ongoing, trialling a cholinesterase inhibitor (rivastigmine) and including a cost‐effectiveness analysis (NCT04226248).
There were only three studies in this review of multiple component interventions (i.e. interventions where there are two or more components, where the same components are provided to all individuals), with all combining exercise plus fall‐prevention education. There were no studies of multifactorial interventions (i.e. interventions where there are two or more components, but the component interventions are applied according to each individual’s fall risk factors). Evidence suggests that in the general older population, multifactorial interventions may reduce the rate of falls compared with a control group, and that multiple component interventions (mostly including exercise) may reduce the rate of falls and the number of people falling compared to a control group (Hopewell 2018). Given that exercise probably reduces the number of falls by around 26%, and probably slightly reduces the number of people falling by around 10%, falls remain a significant problem for people with PD even following effective exercise intervention. This along with the complexity of PD impairments and the wide variety of risk factors for falls (van der Marck 2014), suggests multicomponent and multifactorial fall‐prevention interventions warrant exploration. There is one small ongoing study exploring the effects of a multifactorial intervention including exercise, environmental modification and behavioural strategies in people with PD (ACTRN12619000415101). While this study is measuring falls, it is not powered to detect an effect on falls. Large scale studies are required to determine the effect of multiple component and multifactorial fall‐prevention interventions in people with PD.
Further work is also required to determine the effects of interventions on adverse events, including fall‐related fractures. Adverse events and fall‐related fractures are costly to both healthcare systems and to individuals and their families. These outcomes should be carefully considered when designing fall‐prevention studies, including exercise studies.
Most exercise studies included in this review were of relatively short duration. This contrasts with the studies included in a recent Cochrane Review examining exercise studies to prevent falls in older people living in the community (Sherrington 2019), where the intervention was one year or more in 30% of studies. Furthermore, in the general older population, exercise programs that are of a higher dose (i.e. > 3 hours per week) have been found to have greater benefit in reducing the rate of falls (Sherrington 2017). In people with PD, the lack of an effect on falls seen in the Morris 2017 exercise plus education study, following an effective fall‐prevention exercise intervention by the same research team two years prior (Morris 2015), has been suggested to be due to an insufficient dose of exercise in the latter study (Hulbert 2019; Morris 2017). The present review did not conduct a subgroup analysis to explore any effect of the dose of exercise on falls outcomes. Given falls are a long‐term problem, the effectiveness and sustainability of interventions over the long term, as well as any dose response relationship between exercise and falls warrants investigation.
Alternative research methods could assist in furthering the understanding of the effectiveness of interventions to prevent falls, including the differential effects of interventions in people of different disease severities and characteristics. For example, individual participant data meta‐analysis would allow exploration of subgroups using individual‐level rather than trial‐level characteristics. Furthermore, in the present review, the risk of bias due to knowledge of the allocated interventions (i.e. performance bias) was assessed as unclear in most exercise and in all education studies, as participants and exercise delivery personnel were not blinded to group allocation, but the impact of this non‐blinding was unclear. Randomised controlled trials (RCTs) of exercise and education interventions, particularly where exercise/education is compared with another intervention or a sham intervention, could aim to blind both participants and personnel to knowledge of the hypothesised outcome. Evaluation of the success of this blinding may help to determine the risk of performance bias in any given study.
The high rate of falls in some people with PD, and the complex relationship between falls, disease severity and physical activity levels present statistical challenges. The distribution of falls in PD is typically skewed due to participants who fall frequently, including a small number of participants who fall multiple times per day. These very frequent fallers can have undue influence on the outcomes of statistical tests, such as negative binomial regression. Alternative statistical methods, such as Poisson inverse gaussian regression (as used in Canning 2015a), may provide a better model to fit datasets of falls in people with PD. An additional challenge is the non‐linear association between fall rates and disease severity, and the influence of physical activity on this association (Del Din 2020). Early in the disease, people tend to maintain their pre‐disease activity levels and fall infrequently. As the disease progresses, falls also increase up until the stage where the individual becomes less mobile, and therefore falls less often as they are mostly bed or chair bound (Mactier 2015). Furthermore, increasing the amount of physical activity as part of an exercise intervention – which is by itself a desired effect – may paradoxically be paralleled by an increase in falls, which by definition occur mainly in active people. Alternative measures of fall rate, such as the fall rate relative to activity exposure index (Del Din 2020) would provide a way of assessing fall rates that takes the individual’s level of activity into account.
Studies in this review used a variety of fall and adverse event definitions, as well as methods of fall and adverse event ascertainment. Standardisation of definitions and methods of ascertainment remains a challenge for researchers, requiring consensus. Technological advances may provide more robust methods of falls data collection, however, development of protocols for data collection and validation of algorithms is required (Silva de Lima 2020).
What's new
| Date | Event | Description |
|---|---|---|
| 8 June 2022 | Amended | Minor typographical error amended. |
History
Protocol first published: Issue 3, 2015 Review first published: Issue 6, 2022
Acknowledgements
We are grateful to the authors of Sherrington 2019, for the development of methods and procedures used in this review. We also thank the authors who provided us with additional data and information for this review. The following people conducted the editorial process for this article.
Sign‐off Editor (final editorial decision): Robert Boyle, Network Editor, Cochrane
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Colleen Ovelman, Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks and supported editorial team): Leticia Rodrigues, Cochrane Central Editorial Service
Copy Editor (copy editing and production): [NAME, AFFILIATION]
Peer‐reviewers (provided comments and recommended an editorial decision): Avril Mansfield, KITE‐Toronto Rehabilitation Institute, University Health Network (clinical review), Margaret Mak, Department of Rehabilitation Sciences, The Hong Kong Polytechnic University (clinical review), Nicola O'Malley (clinical review), Robin Featherstone, Cochrane Central Editorial Service (search review), Nuala Livingstone, Cochrane Evidence Production and Methods Directorate (methods). 1 of additional peer reviewers provided consumer peer review but chose not to be publicly acknowledged.
Appendices
Appendix 1. Search strategies
MEDLINE (1946 to present, OvidSP)
1. Accidental Falls/
2. (falls or faller$1).tw.
3. 1 or 2
4. Parkinson Disease/
5. Parkinson*.ti.
6. Parkinson*.ab.
7. PD.ti.
8. PD.ab.
9. 4 or 5 or 6 or 7 or 8
10. 3 and 9
11. randomized controlled trial.pt.
12. randomized.ab.
13. placebo.ab.
14. drug therapy.fs.
15. randomly.ab.
16. trial.ab.
17. groups.ab.
18. 11 or 12 or 13 or 14 or 15 or 16 or 17
19. 10 and 18
The Cochrane Movement Disorders Group Trials Register and The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 11, 2021)
1. MeSH descriptor: [Accidental Falls] explode all trees
2. Fall*
3. 1 or 2
4. MeSH descriptor [Parkinson Disease] explode all trees
5. 3 and 4
(Only trials)
Embase (1947 to present, OvidSP)
1. Accidental Falls/
2. (falls or faller$1).tw.
3. 1 or 2
4. Parkinson Disease/
5. Parkinson*.ti.
6. Parkinson*.ab.
7. PD.ti.
8. PD.ab.
9. 4 or 5 or 6 or 7 or 8
10. 3 and 9
11. randomized controlled trial.pt.
12. randomized.ab.
13. placebo.ab.
14. drug therapy.fs.
15. randomly.ab.
16. trial.ab.
17. groups.ab.
18. 11 or 12 or 13 or 14 or 15 or 16 or 17
19. 10 and 18
CINAHL (Cumulative Index to Nursing and Allied Health Literature) (1982 to present, EBSCO)
1. (MM "Accidental Falls")
2. (MM "Parkinson Disease")
3. PD
4. 2 or 3
5. 1 and 4
6. (MH "Randomized Controlled Trials+")
7. (MM "Placebos")
8. (MH "Drug Therapy+")
9. 6 or 7 or 8
10. 5 and 9
PsycINFO (1806 to present, OvidSP)
1. Accidental Falls/
2. (falls or faller$1).tw.
3. 1 or 2
4. Parkinson Disease/
5. Parkinson*.ti.
6. Parkinson*.ab.
7. PD.ti.
8. PD.ab.
9. 4 or 5 or 6 or 7 or 8
10. 3 and 9
11. randomized controlled trial.pt.
12. randomized.ab.
13. placebo.ab.
14. drug therapy.fs.
15. randomly.ab.
16. trial.ab.
17. groups.ab.
18. 11 or 12 or 13 or 14 or 15 or 16 or 17
19. 10 and 18
AMED (1985 to present, Ovid SP)
1. Accidental Falls/
2. (falls or faller$1).tw.
3. 1 or 2
4. Parkinson Disease/
5. Parkinson*.ti.
6. Parkinson*.ab.
7. PD.ti.
8. PD.ab.
9. 4 or 5 or 6 or 7 or 8
10. 3 and 9
11. randomized controlled trial.pt.
12. randomized.ab.
13. placebo.ab.
14. (Herbal drugs/ or Plants medicinal/ or Drug therapy/ or Phytotherapy/ or "Therapeutic Use"/ or Plant extracts/)
15. randomly.ab.
16. trial.ab.
17. groups.ab.
18. 11 or 12 or 13 or 14 or 15 or 16 or 17
19. 10 and 18
The Physiotherapy Evidence Database (PEDro, The University of Sydney)
1. Abstract & Title: fall* Parkinson*
2. Subdiscipline: neurology
3. Method: clinical trial
ClinicalTrials.gov
(Parkinson’s disease OR Parkinson disease) AND (fall OR fallers)
World Health Organization ICTRP
Parkinson’s disease OR Parkinson disease AND fall*
Appendix 2. Risk of bias assessment methods for incomplete outcome data (attrition bias)
We used the same criteria as that reported in Sherrington 2019 to judge risk of bias due to incomplete outcome data.
Rate of falls
For studies reporting falls as an outcome, we first calculated a rate ratio (RaR1) by dividing falls per person year in the intervention group by falls per person year in the control group. If these data or the numbers lost to follow‐up in each group were not available we assessed the risk of bias as ’Unclear’. We estimated a second rate of falling for all participants randomised (RaR2) by using the conservative assumption that participants lost to follow‐up in the intervention group had the same rate of falls as observed in the control group, and vice versa.
A ratio of these rate ratios (RaR2/RaR1) of greater than 1.15 or less than 0.85 was assessed as ’High risk’ indicating the possibility of clinically important bias; studies with values between 0.85 and 1.15 were assessed as ’Low risk’.
Number of people who fell at least once
For risk of falling, we first calculated for intervention and control groups in each study a risk of falling and a risk of falling ratio (RR1) using for each group the number of participants falling divided by the number analysed. Where the number analysed in each group was not provided, we used as denominator the number in each group providing complete data on falling throughout the study period. Where these data were not specifically mentioned, we used the number of participants randomised less the number lost to follow‐up as the denominator. Using the conservative assumption that participants lost to follow‐up in the intervention group had experienced the risk of falling observed in the control group, and vice‐versa, we calculated an estimated risk of falling ratio for all participants randomised (RR2). We added an imputed number of fallers in each group (the number of lost participants who might have experienced a fall) to the observed number of fallers in each group. The number randomised to that group was used as the denominator. A ratio of the risk ratios RR2/RR1 of greater than 1.15 or less than 0.85 was assessed as ’High risk’ indicating the possibility of clinically important bias; values between 0.85 and 1.15 were assessed as ’Low risk’. When data were not available to calculate RR1 and RR2, risk was assessed as ’Unclear’.
Data and analyses
Comparison 1. Exercise vs control (rate of falls).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Rate of falls | 12 | 1456 | Rate Ratio (IV, Random, 95% CI) | 0.74 [0.63, 0.87] |
| 1.2 Rate of falls subgrouped by ProFaNE exercise categories | 12 | 1456 | Rate Ratio (IV, Random, 95% CI) | 0.74 [0.63, 0.87] |
| 1.2.1 Gait, balance and functional training vs Control | 9 | 1146 | Rate Ratio (IV, Random, 95% CI) | 0.80 [0.67, 0.95] |
| 1.2.2 Resistance training vs control | 2 | 136 | Rate Ratio (IV, Random, 95% CI) | 0.72 [0.55, 0.94] |
| 1.2.3 3D exercise (Tai Chi) vs Control | 2 | 174 | Rate Ratio (IV, Random, 95% CI) | 0.41 [0.23, 0.72] |
| 1.3 Rate of falls ‐ subgrouped by % supervision (100% supervision vs <100% supervision) | 12 | Rate Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.3.1 100% supervision | 5 | 373 | Rate Ratio (IV, Random, 95% CI) | 0.56 [0.41, 0.77] |
| 1.3.2 < 100% supervision | 7 | 1083 | Rate Ratio (IV, Random, 95% CI) | 0.85 [0.75, 0.97] |
| 1.4 Rate of falls ‐ subgrouped by baseline fall risk (increased fall risk vs fall risk not specified) | 12 | Rate Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.4.1 Higher fall risk participants | 7 | 1082 | Rate Ratio (IV, Random, 95% CI) | 0.73 [0.59, 0.91] |
| 1.4.2 Unspecified fall risk participants | 5 | 374 | Rate Ratio (IV, Random, 95% CI) | 0.71 [0.56, 0.90] |
| 1.5 Rate of falls ‐ pooled disease severity subgroup analyses_UPDRS | 2 | Rate Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.5.1 Higher disease severity participants | 2 | Rate Ratio (IV, Random, 95% CI) | 1.47 [1.11, 1.94] | |
| 1.5.2 Lower disease severity participants | 2 | Rate Ratio (IV, Random, 95% CI) | 0.65 [0.39, 1.08] |
Comparison 2. Exercise vs control (number of fallers).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Number of fallers | 9 | 932 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.80, 1.00] |
| 2.2 Number of fallers subgrouped by ProFaNE exercise categories | 9 | 932 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.80, 1.00] |
| 2.2.1 Gait, balance and functional training vs Control | 6 | 622 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.81, 1.04] |
| 2.2.2 Resistance training vs control | 2 | 136 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.43, 1.74] |
| 2.2.3 3D exercise (Tai Chi) vs control | 2 | 174 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.36, 0.95] |
| 2.3 Number of fallers ‐ subgrouped by % supervision (100% supervision vs <100% supervision) | 9 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.3.1 100% supervision | 4 | 328 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.53, 1.06] |
| 2.3.2 < 100% supervision | 5 | 604 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.82, 1.04] |
| 2.4 Number of fallers ‐ subgrouped by baseline fall risk (increased fall risk vs fall risk not specified) | 9 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.4.1 Higher fall risk participants | 5 | 576 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.76, 1.04] |
| 2.4.2 Unspecified fall risk participants | 4 | 356 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.67, 1.11] |
| 2.5 Number of fallers ‐ pooled disease severity subgroup analyses | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.5.1 Higher disease severity participants | 2 | 139 | Risk Ratio (IV, Random, 95% CI) | 1.19 [1.00, 1.41] |
| 2.5.2 lower disease severity participants | 2 | 218 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.62, 0.98] |
Comparison 3. Exercise vs control (number of people sustaining one or more fall‐related fractures).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Number of people sustaining one or more fall‐related fractures | 5 | 989 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.28, 1.17] |
Comparison 4. Exercise vs control (health‐related quality of life).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Health‐related quality of life ‐ combined measures post intervention | 5 | 951 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.36, 0.01] |
| 4.2 Health‐related quality of life ‐ combined measures follow‐up | 3 | 429 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.46, ‐0.08] |
Comparison 5. Exercise vs exercise (rate of falls).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Rate of falls, different types of exercise compared | 14 | Rate Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1.1 Gait, balance and functional training vs gait, balance and functional training | 10 | Rate Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1.2 Gait, balance and functional training vs resistance training | 1 | Rate Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1.3 Gait, balance and functional training vs flexibility | 1 | Rate Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1.4 3D exercise (Tai Chi) vs resistance training | 1 | Rate Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1.5 Other exercise vs Other exercise | 1 | Rate Ratio (IV, Fixed, 95% CI) | Totals not selected |
Comparison 6. Exercise vs exercise (number of fallers).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Number of fallers, different types of exercise compared | 4 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1.1 Gait, balance and functional training vs gait, balance and functional training | 2 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1.2 Gait, balance and functional training vs resistance training | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1.3 3D exercise (Tai Chi) vs resistance training | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected |
Comparison 7. Exercise vs exercise (health‐related quality of life).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Quality of life ‐ combined measures post intervention, different types of exercise compared | 8 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1.1 Gait, balance and functional training vs Gait, balance and functional training | 6 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1.2 3D exercise (Tai Chi) vs Resistance exercise | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1.3 Other exercise vs Other exercise | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.2 Quality of life ‐ combined measures follow‐up, different types of exercise compared | 5 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.2.1 Functional gait, balance and strength training vs Functional gait, balance and strength training | 5 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 8. Cholinesterase inhibitor vs placebo (rate of falls).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Rate of falls | 3 | 248 | Rate Ratio (IV, Fixed, 95% CI) | 0.50 [0.44, 0.58] |
| 8.2 Rate of falls ‐ subgrouped by medication | 3 | 248 | Rate Ratio (IV, Random, 95% CI) | 0.50 [0.43, 0.58] |
| 8.2.1 Rivastigmine vs placebo | 2 | 210 | Rate Ratio (IV, Random, 95% CI) | 0.48 [0.35, 0.66] |
| 8.2.2 Donepezil vs placebo | 1 | 38 | Rate Ratio (IV, Random, 95% CI) | 0.52 [0.44, 0.62] |
Comparison 9. Cholinesterase inhibitor vs placebo (number of fallers).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Number of fallers | 3 | 249 | Risk Ratio (IV, Fixed, 95% CI) | 1.01 [0.90, 1.14] |
| 9.2 Number of fallers ‐ subgrouped by medication | 3 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.70, 1.28] | |
| 9.2.1 Rivastigmine vs placebo | 2 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.20, 1.90] | |
| 9.2.2 Donepezil vs placebo | 1 | Risk Ratio (IV, Random, 95% CI) | 1.13 [0.90, 1.40] |
Comparison 10. Cholinesterase inhibitor vs placebo (health‐related quality of life).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Quality of life EQ5D thermometer post intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1.1 Rivastigmine vs placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.2 Quality of life EQ5D Index Score post intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.2.1 Rivastigmine vs placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 11. Cholinesterase inhibitor vs placebo (rate of adverse events excluding falls).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Rate of adverse events excluding falls | 2 | 175 | Rate Ratio (IV, Fixed, 95% CI) | 1.60 [1.28, 2.01] |
Comparison 12. Education vs usual care (number of fallers).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Number of fallers | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected |
Comparison 13. Exercise and education vs control (rate of falls).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Rate of falls | 2 | 320 | Rate Ratio (IV, Random, 95% CI) | 0.46 [0.12, 1.85] |
Comparison 14. Exercise and education vs control (number of fallers).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Number of fallers | 3 | 352 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.75, 1.07] |
Comparison 15. Exercise and education vs control (number of people sustaining one or more fall‐related fractures).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Number of people sustaining one or more fall‐related fractures | 2 | 320 | Risk Ratio (IV, Random, 95% CI) | 1.45 [0.40, 5.32] |
Comparison 16. Exercise and education vs control (health‐related quality of life).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 16.1 Health‐related quality of life ‐ Parkinson's Disease Questionnaire (PDQ39) post intervention | 2 | 305 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐3.12, 3.23] |
| 16.2 Health‐related quality of life ‐ Parkinson's Disease Questionnaire (PDQ39) at follow‐up | 2 | 299 | Mean Difference (IV, Random, 95% CI) | ‐2.25 [‐5.45, 0.96] |
Comparison 17. Exercise and education vs exercise and education (rate of falls).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 17.1 Rate of falls | 1 | Rate Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 17.1.1 Gait, balance and functional training plus education vs resistance training plus education | 1 | Rate Ratio (IV, Fixed, 95% CI) | Totals not selected |
Comparison 18. Exercise and education vs exercise and education (number of fallers).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 18.1 Number of fallers | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 18.1.1 Gait, balance and functional training plus education vs resistance training plus education | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected |
Comparison 19. Exercise and education vs exercise and education (number of people sustaining one or more fall‐related fractures).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 19.1 Number of people sustaining one or more fall‐related fractures | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 19.1.1 Gait, balance and functional training plus education vs resistance training plus education | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected |
Comparison 20. Exercise and education vs exercise and education (health‐related quality of life).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 20.1 Health‐related quality of life ‐ Parkinson's Disease Questionnaire (PDQ39) post intervention | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 20.1.1 Gait, balance and functional training plus education vs resistance training plus education | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 20.2 Health‐related quality of life ‐ Parkinson's Disease Questionnaire (PDQ39) at follow‐up | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 20.2.1 Gait, balance and functional training plus education vs resistance training plus education | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 21. Sensitivity analysis 1: excluding studies at a high risk of bias in any item.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 21.1 Rate of falls ‐ exercise vs control | 9 | 1245 | Rate Ratio (IV, Random, 95% CI) | 0.74 [0.61, 0.90] |
| 21.2 Number of fallers ‐ exercise vs control | 6 | 721 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.75, 1.02] |
| 21.3 Rate of falls ‐ cholinesterase inhibitor vs placebo | 1 | 81 | Rate Ratio (IV, Fixed, 95% CI) | 0.43 [0.32, 0.56] |
| 21.3.1 Rivastigmine vs placebo | 1 | 81 | Rate Ratio (IV, Fixed, 95% CI) | 0.43 [0.32, 0.56] |
| 21.4 Number of fallers ‐ cholinesterase inhibitor vs placebo | 1 | 81 | Risk Ratio (IV, Fixed, 95% CI) | 0.31 [0.12, 0.78] |
| 21.4.1 Rivastigmine vs placebo | 1 | 81 | Risk Ratio (IV, Fixed, 95% CI) | 0.31 [0.12, 0.78] |
| 21.5 Rate of falls ‐ exercise and education vs control | 1 | 124 | Rate Ratio (IV, Random, 95% CI) | 1.58 [0.74, 3.40] |
| 21.6 Number of fallers ‐ exercise and education vs control | 2 | 156 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.65, 1.08] |
21.2. Analysis.

Comparison 21: Sensitivity analysis 1: excluding studies at a high risk of bias in any item, Outcome 2: Number of fallers ‐ exercise vs control
21.3. Analysis.

Comparison 21: Sensitivity analysis 1: excluding studies at a high risk of bias in any item, Outcome 3: Rate of falls ‐ cholinesterase inhibitor vs placebo
21.5. Analysis.

Comparison 21: Sensitivity analysis 1: excluding studies at a high risk of bias in any item, Outcome 5: Rate of falls ‐ exercise and education vs control
Comparison 22. Sensitivity analysis 2: excluding studies with unclear or high risk of bias on random sequence generation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 22.1 Rate of falls ‐ exercise vs control | 7 | 995 | Rate Ratio (IV, Random, 95% CI) | 0.90 [0.76, 1.05] |
| 22.2 Number of fallers ‐ exercise vs control | 5 | 516 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.76, 1.04] |
| 22.3 Rate of falls ‐ cholinesterase inhibitor vs placebo | 1 | 129 | Rate Ratio (IV, Fixed, 95% CI) | 0.60 [0.38, 0.96] |
| 22.3.1 Rivastigmine vs placebo | 1 | 129 | Rate Ratio (IV, Fixed, 95% CI) | 0.60 [0.38, 0.96] |
| 22.4 Number of fallers ‐ cholinesterase inhibitor vs placebo | 1 | 130 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.87, 1.15] |
| 22.4.1 Rivastigmine vs placebo | 1 | 130 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.87, 1.15] |
22.2. Analysis.

Comparison 22: Sensitivity analysis 2: excluding studies with unclear or high risk of bias on random sequence generation, Outcome 2: Number of fallers ‐ exercise vs control
22.3. Analysis.

Comparison 22: Sensitivity analysis 2: excluding studies with unclear or high risk of bias on random sequence generation, Outcome 3: Rate of falls ‐ cholinesterase inhibitor vs placebo
Comparison 23. Sensitivity analysis 3: excluding studies with unclear or high risk of bias on allocation concealment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 23.1 Rate of falls ‐ exercise vs control | 8 | 1299 | Rate Ratio (IV, Random, 95% CI) | 0.80 [0.70, 0.91] |
| 23.2 Number of fallers ‐ exercise vs control | 7 | 838 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.81, 1.03] |
| 23.3 Rate of falls ‐ cholinesterase inhibitor vs placebo | 1 | 129 | Rate Ratio (IV, Fixed, 95% CI) | 0.60 [0.38, 0.96] |
| 23.3.1 Rivastigmine vs placebo | 1 | 129 | Rate Ratio (IV, Fixed, 95% CI) | 0.60 [0.38, 0.96] |
| 23.4 Number of fallers ‐ cholinesterase inhibitor vs placebo | 1 | 130 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.87, 1.15] |
| 23.4.1 Rivastigmine vs placebo | 1 | 130 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.87, 1.15] |
| 23.5 Number of fallers ‐ exercise and education vs control | 2 | 320 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.75, 1.08] |
23.2. Analysis.

Comparison 23: Sensitivity analysis 3: excluding studies with unclear or high risk of bias on allocation concealment, Outcome 2: Number of fallers ‐ exercise vs control
23.3. Analysis.

Comparison 23: Sensitivity analysis 3: excluding studies with unclear or high risk of bias on allocation concealment, Outcome 3: Rate of falls ‐ cholinesterase inhibitor vs placebo
23.4. Analysis.

Comparison 23: Sensitivity analysis 3: excluding studies with unclear or high risk of bias on allocation concealment, Outcome 4: Number of fallers ‐ cholinesterase inhibitor vs placebo
Comparison 24. Sensitivity analysis 4, excluding studies with unclear or high risk of bias on assessor blinding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 24.1 Rate of falls ‐ exercise vs control | 2 | 692 | Rate Ratio (IV, Random, 95% CI) | 0.92 [0.73, 1.16] |
| 24.2 Number of fallers ‐ exercise vs control | 1 | 231 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.78, 1.11] |
| 24.3 Rate of falls ‐ exercise and education vs control | 1 | 196 | Rate Ratio (IV, Random, 95% CI) | 0.24 [0.10, 0.61] |
| 24.4 Number of fallers ‐ exercise and education vs control | 2 | 228 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.73, 1.19] |
24.2. Analysis.

Comparison 24: Sensitivity analysis 4, excluding studies with unclear or high risk of bias on assessor blinding, Outcome 2: Number of fallers ‐ exercise vs control
Comparison 25. Sensitivity analysis 5, excluding studies with unclear or high risk of bias on incomplete outcome data.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 25.1 Rate of falls ‐ exercise vs control | 11 | 1260 | Rate Ratio (IV, Random, 95% CI) | 0.77 [0.65, 0.92] |
| 25.2 Number of fallers ‐ exercise vs control | 8 | 736 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.79, 1.00] |
| 25.3 Rate of falls ‐ cholinesterase inhibitor vs placebo | 1 | 129 | Rate Ratio (IV, Fixed, 95% CI) | 0.60 [0.38, 0.96] |
| 25.3.1 Rivastigmine vs placebo | 1 | 129 | Rate Ratio (IV, Fixed, 95% CI) | 0.60 [0.38, 0.96] |
| 25.4 Number of fallers ‐ cholinesterase inhibitor vs placebo | 2 | 168 | Risk Ratio (IV, Fixed, 95% CI) | 1.03 [0.92, 1.16] |
| 25.4.1 Rivastigmine vs placebo | 1 | 130 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.87, 1.15] |
| 25.4.2 Donepezil vs placebo | 1 | 38 | Risk Ratio (IV, Fixed, 95% CI) | 1.13 [0.90, 1.40] |
| 25.5 Rate of falls ‐ exercise and education vs control | 1 | 124 | Rate Ratio (IV, Random, 95% CI) | 1.58 [0.74, 3.40] |
25.2. Analysis.

Comparison 25: Sensitivity analysis 5, excluding studies with unclear or high risk of bias on incomplete outcome data, Outcome 2: Number of fallers ‐ exercise vs control
25.3. Analysis.

Comparison 25: Sensitivity analysis 5, excluding studies with unclear or high risk of bias on incomplete outcome data, Outcome 3: Rate of falls ‐ cholinesterase inhibitor vs placebo
25.4. Analysis.

Comparison 25: Sensitivity analysis 5, excluding studies with unclear or high risk of bias on incomplete outcome data, Outcome 4: Number of fallers ‐ cholinesterase inhibitor vs placebo
Comparison 26. Sensitivity analysis 6, excluding studies with less than three months falls monitoring.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 26.1 Rate of falls ‐ exercise vs control | 9 | 1268 | Rate Ratio (IV, Random, 95% CI) | 0.79 [0.68, 0.92] |
| 26.2 Number of fallers ‐ exercise vs control | 7 | 789 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.77, 1.02] |
Comparison 27. Sensitivity analysis 7, excluding comparisons responsible for the high level of heterogeneity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 27.1 Number of fallers ‐ cholinesterase inhibitor vs placebo | 2 | 168 | Risk Ratio (IV, Fixed, 95% CI) | 1.03 [0.92, 1.16] |
| 27.1.1 Rivastigmine vs placebo | 1 | 130 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.87, 1.15] |
| 27.1.2 Donepezil vs placebo | 1 | 38 | Risk Ratio (IV, Fixed, 95% CI) | 1.13 [0.90, 1.40] |
| 27.2 Rate of falls ‐ exercise and education vs control | 1 | 196 | Rate Ratio (IV, Random, 95% CI) | 0.24 [0.10, 0.61] |
Comparison 28. Sensitivity analysis 8, fixed‐effect meta‐analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 28.1 Rate of falls ‐ exercise vs control | 12 | 1456 | Rate Ratio (IV, Fixed, 95% CI) | 0.79 [0.71, 0.88] |
| 28.2 Number of fallers ‐ exercise vs control | 9 | 932 | Risk Ratio (IV, Fixed, 95% CI) | 0.90 [0.80, 1.00] |
| 28.3 Rate of falls ‐ exercise and education vs control | 2 | 320 | Rate Ratio (IV, Fixed, 95% CI) | 0.54 [0.33, 0.89] |
| 28.4 Number of fallers ‐ exercise and education vs control | 3 | 352 | Risk Ratio (IV, Fixed, 95% CI) | 0.89 [0.75, 1.07] |
28.2. Analysis.

Comparison 28: Sensitivity analysis 8, fixed‐effect meta‐analysis, Outcome 2: Number of fallers ‐ exercise vs control
Comparison 29. Sensitivity analysis 9, random effects meta‐analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 29.1 Rate of falls ‐ cholinesterase inhibitor vs placebo | 3 | 248 | Rate Ratio (IV, Random, 95% CI) | 0.50 [0.43, 0.58] |
| 29.1.1 Rivastigmine vs placebo | 2 | 210 | Rate Ratio (IV, Random, 95% CI) | 0.48 [0.35, 0.66] |
| 29.1.2 Donepezil vs placebo | 1 | 38 | Rate Ratio (IV, Random, 95% CI) | 0.52 [0.44, 0.62] |
| 29.2 Number of fallers ‐ cholinesterase inhibitor vs placebo | 3 | 249 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.70, 1.28] |
| 29.2.1 Rivastigmine vs placebo | 2 | 211 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.20, 1.90] |
| 29.2.2 Donepezil vs placebo | 1 | 38 | Risk Ratio (IV, Random, 95% CI) | 1.13 [0.90, 1.40] |
Comparison 30. Sensitivity analysis 10, reclassifying functional resistance training from resistance training to gait, balance and functional training.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 30.1 Rate of falls ‐ exercise vs control | 12 | 1456 | Rate Ratio (IV, Random, 95% CI) | 0.74 [0.63, 0.87] |
| 30.1.1 Gait, balance and functional training vs Control | 10 | 1244 | Rate Ratio (IV, Random, 95% CI) | 0.78 [0.68, 0.91] |
| 30.1.2 Resistance training vs control | 1 | 38 | Rate Ratio (IV, Random, 95% CI) | 0.84 [0.28, 2.53] |
| 30.1.3 3D exercise (Tai Chi) vs Control | 2 | 174 | Rate Ratio (IV, Random, 95% CI) | 0.41 [0.23, 0.72] |
| 30.2 Number of fallers ‐ exercise vs control | 9 | 932 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.80, 1.00] |
| 30.2.1 Gait, balance and functional training vs Control | 7 | 720 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.83, 1.05] |
| 30.2.2 Resistance training vs control | 1 | 38 | Risk Ratio (IV, Random, 95% CI) | 0.58 [0.30, 1.13] |
| 30.2.3 3D exercise (Tai Chi) vs control | 2 | 174 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.36, 0.95] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ashburn 2007.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: home, UK N = 142 Sample: recruited from clinical registers of three PD specialists in two National Health Service (NHS) trusts (39% women) Age (years): mean (SD) intervention group 72.7 (9.6), control group 71.6 (8.8) Inclusion criteria: diagnosis of idiopathic PD; independently mobile; living at home in the community; > 1 fall in the previous 12 months; passed a screening test for gross cognitive impairment Exclusion criteria: pain preventing participation in assessments; an acute medical condition Disease severity at baseline: HY stage 2 to 4, UPDRS motor score mean (SD) 21.0 (10.2) |
|
| Interventions | Exercise 1. Exercise: 6‐week home‐supervised exercises designed with six levels of progression comprising of strength (lower limb), range of movement, balance training and walking exercises. Plus strategy training for falls prevention and movement initiation and compensation. The supervised exercises were performed for 60 minutes, 1x/week for 6 weeks. Plus, home unsupervised exercises (minutes not reported), 7x/week for 6 months 2. Control: usual care (usual care for the vast majority comprised contact with a local PD nurse) |
|
| Outcomes | 1. Rate of falls (data provided by trial authors on request) 2. Number of fallers 3. Number reporting a fall‐related fracture 4. Quality of life (EQ‐5D) Other outcomes reported but not included in this review |
|
| Duration of the study | 6 months | |
| Funding source | Action Medical Research, and the John and Lucille Van Geest Foundation | |
| Notes | Fall data collected: at 8 weeks and 6 months follow‐up by monthly falls diaries | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of generating the randomisation list not described. Quote: "Randomisation was stratified by NHS Trust using blocks of size four." |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was described as by central allocation. Quote: "After the baseline assessment by the assessor, the treating physiotherapist obtained the random allocation by telephoning the Medical Statistics Group at the University of Southampton, Southampton, UK.” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and intervention (exercise) delivery personnel not blinded to group allocation but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | Unclear risk | Unclear if personnel collecting fall information were blinded to group allocation. |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | The evidence for fractures was from self‐reports from participants or carers. Quote: "Participants were also asked to record injuries as a result of falls (cuts and bruises, fractures or other trauma) and whether they attended the hospital, sought other forms of medical help or self‐managed their injuries.” |
| Incomplete outcome data (attrition bias) Falls | Low risk | See appendix for method of assessment |
| Incomplete outcome data (attrition bias) Fallers | Low risk | See appendix for method of assessment |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (ISRCTN63503875) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Method of ascertaining falls (recall bias) Falls and fallers | Low risk | The study used concurrent collection of data about falling with monthly, or more frequent, follow‐up by the researchers. Quote: “Fall events that were experienced during the trial period were recorded prospectively using self‐completed diaries. Each month, participants were sent a falls diary sheet, consisting of daily numbered date boxes. Individuals recorded “F” for a “fall” and “NF” for a “near fall”whenever these occurred, and returned the sheets to the secretary in a stamped addressed envelope.” |
Canning 2015a.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: home and facility, Australia N = 231 Sample: recruited from metropolitan Sydney and regional and rural New South Wales (NSW), via Parkinson’s NSW consumer support groups, newspaper advertisements, and referrals from neurologists and physical therapists (42% women) Age (years): mean (SD) intervention group 71.4 (8.1), control group 69.9 (9.3) Inclusion criteria: diagnosis of idiopathic PD; age 40 years or older; ability to walk independently with or without a walking aid; stable antiparkinsonian medication for at least 2 weeks; ≥ 1 fall in the past year or at risk of falls based on physical assessment Exclusion criteria: cognitive impairment (Mini‐mental State Examination score of < 24); unstable cardiovascular disease, or other uncontrolled chronic conditions that would interfere with the safety and conduct of the training and testing protocol Disease severity at baseline: HY stage 2 to 4, UPDRS motor score mean (SD) 26.3 (9.5) |
|
| Interventions | Exercise 1. Exercise: PD‐WEBB program including balance and lower limb strengthening exercises. Plus, cueing strategies for participants reporting freezing of gait. Home‐based exercises (40‐60 minutes, 3x/week for 24 weeks ‐ including 6 to 10 sessions supervised by a physiotherapist, either in a 1x month exercise class and/or at home), plus usual care and a booklet containing standardised fall‐prevention advice 2. Control: usual care and a booklet containing standardised fall‐prevention advice Usual care could include medical practitioner and community services) |
|
| Outcomes | 1. Rate of falls 2. Number of fallers 3. Number reporting a fall‐related fracture 4. Quality of life (SF‐12v2, SF‐6D, PDQ‐39) Economic analysis reported in Farag 2016: 1. Cost of delivering the intervention 2. Cost of health service use 3. Incremental cost per QALY gained 4. Incremental cost per fall prevented Other outcomes reported but not included in this review |
|
| Duration of the study | 6 months | |
| Funding source | Australian National Health and Medical Research Council (NHMRC ID: 512326), and the Harry Secomb Foundation | |
| Notes | Fall data collected: during the 6‐month intervention period by monthly falls diaries | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component in the sequence generation was described. Quote: "Participants were randomized to intervention group or control group after the baseline assessments. Randomization was stratified by fall history (0‐9/≥10 falls in the previous 12 months) using a computer‐generated random‐number schedule with variable block sizes of 2 and 4." |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was described as by central allocation. Quote: “Randomization was performed centrally by an investigator not involved in the recruitments or assessments (C.S.).” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and intervention (exercise) delivery personnel not blinded to group allocation but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | Low risk | Outcomes were recorded/confirmed in all allocated groups using the same method and the personnel recording/confirming outcomes were blind to group allocation. |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Insufficient information to permit judgement. Fractures diagnosed following visit to medical practitioner, emergency department or hospital admission, however fractures were self‐reported and not confirmed by the results of radiological examination or from primary care case records. |
| Incomplete outcome data (attrition bias) Falls | Low risk | See appendix for method of assessment |
| Incomplete outcome data (attrition bias) Fallers | Low risk | See appendix for method of assessment |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (ACTRN12608000303347) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Method of ascertaining falls (recall bias) Falls and fallers | Low risk | The study used concurrent collection of data about falling with monthly, or more frequent, follow‐up by the researchers. Quote: “All participants will receive monthly calendars on entry to the study, with instructions to record the following events: number of falls...” and “All participants will also be telephoned monthly to record any changes in medications, use of health resources and verify any falls details.” |
Cattaneo 2019.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: facility and home, Italy N = 32 (PD subgroup) Sample: recruited from three Italian field centres by a group of researchers and clinicians (physiotherapists and medical doctors ‐ the NEUROFALL group) (37% women in whole sample ‐ not reported for PD subgroup) Age (years): mean (SD) intervention group 61 (15), control group 63 (11) (whole sample) Inclusion criteria: diagnosis of PD; able to walk 10 metres independently with or without a mobility aid; willing to commit to the educational program; able to give written informed consent Exclusion criteria: major depression; severe bone/joint disorder interfering with mobility; cognitive impairment (Mini‐mental State Examination score < 21) Disease severity at baseline: not reported |
|
| Interventions | Exercise plus education 1. Education and exercise: one, one‐hour education session about fall‐prevention delivered by a physical therapist to a small group ranging in size from two to four people. Exercise focused on mobility and balance and was tailored to the individual. Three, one‐hour supervised sessions, followed by home‐based unsupervised exercise two to three times per week for two months 2. Control: usual treatments, plus two, one‐hour sessions to learn stretching exercises, followed by independent performance of stretching exercises at home for two months |
|
| Outcomes | 1. Number of fallers Other outcomes reported but not included in this review |
|
| Duration of the study | 6 months | |
| Funding source | Italian Ministry of Health (RF‐2010‐2318552) | |
| Notes | Fall data collected: with a fall diary and a second monthly phone call for 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component in the sequence generation was described. Quote: “…using a computer generated randomization list generated before commencement of the study… using random block sizes of 4.” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement as a method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and intervention (exercise and education) delivery personnel not blinded to group allocation but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | Low risk | Outcomes were recorded/confirmed in all allocated groups using the same method and the personnel recording/confirming outcomes were blind to group allocation. Quote: "Data were collected by trained interviewers blinded to the intervention not located in the clinical centers where the assessments were made." |
| Incomplete outcome data (attrition bias) Fallers | Low risk | See appendix for method of assessment. Data not available for calculation ‐ however, no dropouts in either group. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT03570268) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Method of ascertaining falls (recall bias) Falls and fallers | Unclear risk | The study used concurrent collection of data about falling however follow‐up by the researchers was every 2 months. The effect of the longer time frame for researcher follow‐up is unclear Quote: "Each patient was given a fall diary and was followed for 6 months with telephone contacts approximately at 2, 4, and 6 months." |
Chivers Seymour 2019.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: home, UK N = 474 Sample: recruited from Parkinson’s services in NHS hospitals and clinics, as well as community and social services and the study website (44% women) Age (years): mean (SD) intervention group 71 (7.7), control group 73 (7.7) Inclusion criteria: diagnosis of idiopathic PD using the UK Brain Bank criteria; living in the community; ability to walk independently with or without a walking aid; ≥ 1 fall in the past year, Mini‐mental State Examination score of ≥ 24, able to give informed consent, understand and follow commands, considered able to participate in an exercise and strategy (PDSAFE) program Exclusion criteria: living in a care home; needs assistance from another person to walk indoors; wheelchair bound or bedridden unless aided Disease severity at baseline: HY stage 1 to 4; UPDRS motor score mean (SD) 32.5 (16.3) |
|
| Interventions | Exercise 1. Exercise: PDSAFE program consisting of balance and lower limb strengthening exercises, plus strategies for preventing falls and reducing freezing of gait. Individually‐tailored home‐based exercises (30 minutes, daily for 6 months ‐ including 12 x 1‐1.5‐hour supervised sessions with a physiotherapist, with more supervised sessions early in the program) 2. Control: received a Parkinson’s UK DVD with information about PD. At the end of the trial the control participants received a single session about fall prevention and a booklet about falls management Both groups received usual care (including medical management) and took part in their usual activities, such as exercise or social groups |
|
| Outcomes | 1. Rate of falls 2. Number reporting a fall‐related fracture 3. Quality of life (PDQ39) Other outcomes reported but not included in this review |
|
| Duration of the study | 12 months | |
| Funding source | National Institute for Health Research HTA program (project number 10/57/21) and National Institute for Health Research Newcastle CRF Infrastructure funding | |
| Notes | Fall data collected: for 3 months prior to randomisation and for the 12‐month trial period using monthly falls diaries | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component in the sequence generation was described. Quote: “Random allocations were computer‐generated, stratified by centre and allocated in blocks with random size of 2, 4, 6 or 8.” |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was described as by central allocation. Quote: “...randomly assigned (50:50) to either the intervention or control group, using an online procedure set up by OCTRU (a UKCRC registered trials unit). The allocations were sent to the trial manager who informed a treating therapist, to ensure allocation concealment from trial recruiters and assessors.” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and intervention (exercise) delivery personnel not blinded to group allocation but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | Low risk | Outcomes were recorded/confirmed in all allocated groups using the same method and the personnel recording/confirming outcomes were blind to group allocation. |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Insufficient information to permit judgement. Unclear how data regarding fractures was collected. |
| Incomplete outcome data (attrition bias) Falls | Low risk | See appendix for method of assessment |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (ISRCTN48152791) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Method of ascertaining falls (recall bias) Falls and fallers | Low risk | The study used concurrent collection of data about falling with monthly, or more frequent, follow‐up by the researchers. Quote from Goodwin (2015) protocol: "Fall data will be collected using monthly, prospective, self‐report diaries for twelve months following randomisation and will include falls, near falls and injuries. In addition to the diary, participants will be provided forms to completed details of any falls such as location and subsequent treatment. The diaries will be delivered by the assessor at assessment visits and returned each month in a prepaid envelope and with telephone reminders when not received within three weeks.” |
Chung 2010.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: USA N = 23 Sample: recruited from Oregon Health & Sciences University Movement Disorders Clinic (21% women) Age (years): mean (SD) 68.3 (10.8) Inclusion criteria: diagnosed with probable idiopathic PD; responsive to levodopa replacement therapy; baseline frequency of falling or nearly falling ≥2 times per week; ambulatory about the home either independently or with a walker or cane Exclusion criteria: freezing or non‐CNS contributors to falls such as orthostasis, arthritic impairments, or neuropathy; currently using cholinesterase inhibitors or drugs with anticholinergic or sedative‐hypnotic properties; cognitive impairment (Mini‐mental State Examination score of <25); unstable medical or psychiatric problems; Hoehn and Yahr stage 5 Disease severity at baseline: HY mean (SD) 3.2 (0.4), UPDRS motor score mean (SD) 24.7 (8.6) |
|
| Interventions | Medication: cholinesterase inhibitor 1. Donepezil (5 mg) for 3 weeks, increasing to 10 mg for 3 weeks. Plus washout period for 3 wks. Plus placebo (5 mg) for 3 weeks, increasing to 10 mg for 3 weeks 2. Placebo (5 mg) for 3 weeks, increasing to 10 mg for 3 weeks. Plus washout period for 3 weeks. Plus Donepezil (5 mg) for 3 weeks, increasing to 10 mg for 3 weeks |
|
| Outcomes | 1. Rate of falls 2. Number of fallers (data provided by trial authors on request) 2. Number reporting a fall‐related fracture 3. Number and type of adverse events Other outcomes reported but not included in this review |
|
| Duration of the study | 15 weeks | |
| Funding source | Pfizer Inc ‐ this was an investigator‐initiated project and Pfizer Inc did not design or monitor the study or receive the data or influence the writing of the manuscript. Also supported by a Veterans Administration Career development Award, US Public Health Service Grant (ULIRR024140‐02), and the NIH (R01‐NS21062 and NIA AG006457) | |
| Notes | Fall data collected: at baseline and daily onto postcards which accumulated data for 1 week of monitoring, and collected for 6 weeks per phase. Postcards were mailed back to the investigator weekly | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of generating the randomisation list not described. Quote: "The trial was a randomized, crossover, double‐blind study." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and personnel implementing the interventions ensured, and unlikely that the blinding could have been broken. Quote: "Drug and placebo tablets were identical in appearance and were provided by Pfizer." |
| Blinding of outcome assessment (detection bias) Falls and fallers | Low risk | Outcomes were recorded/confirmed in all allocated groups using the same method and the personnel recording/confirming outcomes were blind to group allocation. |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Unclear how data regarding fractures was collected. |
| Incomplete outcome data (attrition bias) Falls | High risk | See appendix for method of assessment |
| Incomplete outcome data (attrition bias) Fallers | Low risk | See appendix for method of assessment |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is available (NCT00611481) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way, however the protocol was registered after the trial was completed. |
| Method of ascertaining falls (recall bias) Falls and fallers | Low risk | The study used concurrent collection of data about falling with monthly, or more frequent, follow‐up by the researchers. Quote: “The primary outcomes were fall and near‐fall frequency determined using daily event recording by the subjects onto postcards which accumulated data for 1week of monitoring, and collected for 6 weeks per phase. Postcards were mailed back to the investigator weekly.” |
Gandolfi 2017.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: home (virtual reality telerehabilitation group) and facility (sensory‐integration balance training group), Italy N = 76 Sample: recruited from four neurorehabilitation units in Veneto, Italy (predominantly rural areas) (33% women) Age (years): mean (SD) virtual reality telerehabilitation group 67.5 (7.2), sensory‐integration balance training group 69.8 (9.4) Inclusion criteria: diagnosis of PD according to the UK Brain bank criteria; aged over 18 years; modified Hoehn and Yahr stage 2.5 to 3; stable medication for the past month; able to transfer and maintain upright standing for at least 10 minutes; presence of a caregiver Exclusion criteria: cardiovascular, orthopaedic and otovestibular disorders; visual or other neurological conditions that could interfere with balance; severe dyskinesias or on‐off fluctuations; Mini‐mental State Examination score < 24/30; severe depression measured on the Geriatric Depression scale. Disease severity at baseline: HY stage 2.5 to 3, UPDRS total score mean (SD) 47.4 (24.1) |
|
| Interventions | Exercise 1. Virtual reality telerehabilitation balance training: Nintendo Wii Fit exergames (Nintendo Co., Ltd., Kyoto, Japan) delivered via telehealth (Skype, Microsoft, USA) to participants in their homes, two participants at a time (50 min, 3x/week for 7 weeks) 2. Sensory integration balance training: balance exercises under different sensory conditions, delivered individually at a facility (50 minutes, 3x/week for 7 weeks) |
|
| Outcomes | 1. Rate of falls 2. Quality of life (PDQ8) 3. Cost of delivering the intervention Other outcomes reported but not included in this review |
|
| Duration of the study | 11 weeks | |
| Funding source | Ricerca Sanitaria Finalizzata Regionale, 2010 (grant no. 319/10) | |
| Notes | Fall data collected: for the prior 1 month in a self‐report logbook, measured at 7 weeks (post intervention) and 11 weeks (follow‐up) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component in the sequence generation was described. Quote: "After screening, a list was generated using computer‐generated random number tables (allocation ratio 1:1). Eligible patients were consecutively entered into the list." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement as a method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and intervention (two different exercise interventions) delivery personnel not blinded to group allocation but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | Low risk | Outcomes were recorded/confirmed in all allocated groups using the same method and the personnel recording/confirming outcomes were blind to group allocation. Quote: "At each study center, outcomes were assessed by a single examiner blinded to treatment assignment." |
| Incomplete outcome data (attrition bias) Falls | Low risk | See appendix for method of assessment. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ as no published trial protocol or trial registration available. |
| Method of ascertaining falls (recall bias) Falls and fallers | Unclear risk | Deatails of ascertainment were not described. Quote: "The number of falls in the previous month was recorded in a self‐report log." |
Gandolfi 2019.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: facility, Italy N = 37 Sample: recruited from outpatients attending neurology and neurorehabilitation clinics at Azienda Ospedaliera Universitaria Integrata, Verona (35% women) Age (years): mean (SD) trunk exercise group 72.4 (6.4), general exercise group 70.7 (6.6) Inclusion criteria: diagnosis of PD; aged 18 years or over; Mini‐mental State Examination ≥ 24; ≥ 5 degrees of forward trunk flexion during standing and walking that completely subsided when recumbent; Hoehn and Yahr Stage ≤ 4 when "ON" medication; taking their usual antiparkinsonian medication. Exclusion criteria: severe dyskinesia or "on‐off" fluctuations; PD medication modification in the prior 3 months; history of major spinal surgery or muscle and/or skeletal spine diseases; need for assistive devices to rise from a chair or bed; other neurological, orthopaedic or cardiovascular co‐morbidities that could interfere with postural control. Disease severity at baseline: HY stage median (Q25; Q75) 2.5 (1.5; 3), UPDRS total score mean (SD) 62.43 (24.6) |
|
| Interventions | Exercise 1. Trunk exercise group: active self correction exercises with and without visual or proprioceptive feedback, trunk stabilisation exercises, dual‐task training while maintaining improved posture (60 minutes, 2x/week for 4 weeks, individual therapy from a physiotherapist). 2. General exercise: joint mobilisation; muscle strengthening and stretching; overground gait training and balance exercises (60 minutes, 2x/week for 4 weeks, individual therapy from a physiotherapist). For both groups, three sessions were performed as 'self practice' at the participants' home and monitored by daily phone calls by the treating physiotherapist. It is unclear how often the participants were expected to perform the exercises at home. |
|
| Outcomes | 1. Rate of falls 2. Quality of life (PDQ‐8) Other outcomes reported but not included in this review |
|
| Duration of the study | 8 weeks | |
| Funding source | Brain Research Foundation Verona ONLUS (grant no. 1/2017) | |
| Notes | Fall data collected: for the prior 1 month, measured at 4 weeks (post intervention) and 8 weeks (follow‐up) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component in the sequence generation was described. Quote: "Eligible patients were assigned to either the EG or the CG by a simple randomization scheme using an automated randomization system (www.randomization.com).” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ as it is unclear of the investigator involved in group allocation was also involved in recruitment. Quote: "Group allocation was kept concealed. The randomization list was locked in a desk drawer accessible only to the principal investigator." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Intervention delivery personnel (two different exercise interventions) were not blinded to group allocation but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | Low risk | Outcomes were recorded/confirmed in all allocated groups using the same method and the personnel recording/confirming outcomes were blind to group allocation. Quote: "The same blinded examiner measured primary and secondary outcomes at each session." |
| Incomplete outcome data (attrition bias) Falls | Low risk | See appendix for method of assessment. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT03741959) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
| Method of ascertaining falls (recall bias) Falls and fallers | Unclear risk | Deatails of ascertainment were not described. Quote: "Secondary outcomes were… the number of falls in the previous month” |
Gao 2014.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: facility, China N = 80 Sample: recruited by screening admissions at the West China Hospital (34.2% women) Age (years): mean (SD) intervention group 69.5 (7.3), control group 68.3 (8.5) Inclusion criteria: diagnosis of idiopathic PD; over 40 years old; able to walk independently; ≥ 1 fall during the past 12 months Exclusion criteria: cognitive impairment (Mini‐mental state examination score < 24); serious medical problem such as heart failure or severe hypertension; unable to endure moderate exercise for 60 minutes Disease severity at baseline: UPDRS motor score mean (SD) 31.2 (10.7) |
|
| Interventions | Exercise 1. Exercise: 24‐form Yang Style Tai Chi. Group supervised by a Tai Chi instructor (60 minutes, 3x/week for 12 weeks) 2. Control: no intervention |
|
| Outcomes | 1. Rate of falls 2. Number of fallers Other outcomes reported but not included in this review |
|
| Duration of the study | 6 months | |
| Funding source | No funding | |
| Notes | Fall data collected: during the 6 months follow‐up period starting after the end of intervention by monthly phone calls | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement. Method of generating the randomisation list not described. Quote: "Each patient was given a random number following a random number table and ordered by their assigned numbers. The patients were then assigned to groups by taking the first patient in the order list for the Tai Chi group, the next patient for the control group, and so on until all were assigned." |
| Allocation concealment (selection bias) | Unclear risk | Unclear if investigators enrolling participants could possibly foresee assignments. Quote: "Each patient was given a random number following a random number table and ordered by their assigned numbers." Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and intervention (exercise) delivery personnel not blinded to group allocation but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | Unclear risk | Unclear if personnel collecting fall information blinded to group allocation. |
| Incomplete outcome data (attrition bias) Falls | Low risk | See appendix for method of assessment |
| Incomplete outcome data (attrition bias) Fallers | Low risk | See appendix for method of assessment |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ as unable to find a published protocol or trial registration. |
| Method of ascertaining falls (recall bias) Falls and fallers | Low risk | The study used concurrent collection of data about falling with monthly, or more frequent, follow‐up by the researchers. Quote: “A notebook was given to every patient to record the amount and the description of the falls. Every patient was telephoned once a month to get the details about experience of falls such as the number of falls, how and where they fell and the injuries they suffered.” |
Goodwin 2011.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: facility and home, UK N = 130 Sample: recruited from specialist PD clinicians and DeNDRoN (Dementia and Neurodegenerative Disease Research Network) research nurses from four acute hospital trusts and one community trust, general practices in three primary care organisations and local PD support groups (43% women) Age (years): mean (SD) intervention group 72.0 (8.6), control group 70.1 (8.3) Inclusion criteria: diagnosis of idiopathic PD using the UK Brain Bank criteria; self‐reported history of ≥ 2 falls in the preceding year; ability to mobilise independently indoors, with or without a walking aid; being resident in Devon or registered with a Devon general practitioner Exclusion criteria: required supervision or assistance to mobilise indoors; significant comorbidity or symptoms that affected ability or safety to exercise (e.g., unstable angina, significant postural hypotension, severe pain); unable to follow written or verbal instructions in English Disease severity at baseline: HY stage 1 to 4, mean (SD) 2.5 (0.9) |
|
| Interventions | Exercise 1. Exercise: strength (lower limb and trunk) and balance training exercises. Group supervised by a physiotherapist (60 minutes, 1x/week for 10 weeks); plus, home unsupervised exercises (2x/week for 10 weeks); plus, usual care 2. Control: usual care (usual care could include medical and medication management, physiotherapy, occupational therapy or speech therapy) |
|
| Outcomes | 1. Rate of falls 2. Number of fallers 3. Number reporting a fall‐related fracture 5. Quality of life (EQ‐5D) Economic analysis reported in Fletcher 2012: 1. Cost of delivering the intervention 2. Cost of health and social service use 3. Incremental cost per QALY gained Other outcomes reported but not included in this review |
|
| Duration of the study | 30 weeks | |
| Funding source | National Institute for Health Research Researcher development Award (grant No RDA/02/06/41) awarded to VG | |
| Notes | Fall data collected: during the 10‐week baseline period, the 10‐week intervention period and the 10‐week follow‐up period via weekly diaries Economic analysis reported in pounds sterling (price year 2008/09) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component in the sequence generation was described. Quote: "The randomisation sequence was created using computer generated random number tables, with 1:1 allocation of individuals to either the intervention group or the control group." |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was described as by central allocation. Quote: "Once a cohort had been recruited and assessed, telephone randomisation procedures were used, using a service independent from the study data collection, for allocation assignment." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and intervention (exercise) delivery personnel not blinded to group allocation but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | High risk | Personnel recording outcomes not blinded to group allocation. Quote: "It was not possible to blind the outcome assessor to participant allocation.” |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Unclear how data regarding fractures was collected. |
| Incomplete outcome data (attrition bias) Falls | Low risk | See appendix for method of assessment |
| Incomplete outcome data (attrition bias) Fallers | Low risk | See appendix for method of assessment |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (ISRCTN50793425) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Method of ascertaining falls (recall bias) Falls and fallers | Low risk | The study used concurrent collection of data about falling with monthly, or more frequent, follow‐up by the researchers. Quote: “Falls and fall related injuries were self‐reported and collected via weekly diaries and returned in prepaid envelopes by the study participants each week for 30 weeks.” |
Harro 2014.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: facility, USA N = 22 Sample: recruited from local chapter of the National Parkinson Foundation, the Mercy Health Hauenstein NeuroScience Center and local retirement communities (35% women) Age (years): mean (SD) speed‐dependent treadmill training group 64.9 (9.5), rhythmic auditory‐cued overground training group 67.3 (11.5) Inclusion criteria: age of 18–89 years; diagnosis of idiopathic PD; stage 1–3 on the Hoehn and Yahr scale; ability to walk continuously without physical assistance for five minutes with or without an assistive device; stable PD medication schedule and dosing over the past month as reported by the participant’s neurologist; functional vision and hearing sufficient to perceive cues with or without aides/glasses Exclusion criteria: impaired cognitive functioning (a score of 20 or less on the Saint Louis Mental Status Examination (SLUMS)); history of other neurologic or vestibular disorders; current orthopedic conditions that would affect the ability to walk; history of PD‐related deep brain stimulation; inability to speak and read English; unstable medical status; inability to engage in moderate exercise Disease severity at baseline: HY stage 1 to 3, mean (SD) 1.9 (0.6) |
|
| Interventions | Exercise 1. Exercise: progressive speed‐dependent treadmill training. Individual, fully supervised treatment (30 minutes, 3x/week for 6 weeks) 2. Exercise: progressive rhythmic auditory‐cued overground training. Group treatment (5 participants per group) at an indoor track (30 minutes, 3x/week for 6 weeks) |
|
| Outcomes | 1. Rate of falls 2. Number of fallers 3. Quality of life (PDQ39) |
|
| Duration of the study | 6 months | |
| Funding source | Saint Mary's Healthcare Doran Foundation | |
| Notes | Fall data collected: at baseline (considering 6 months prior to training) and 6 months after training by monthly fall diaries | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component in the sequence generation was described. Quote: "...and then were randomly assigned using computer generated numbers into one of two groups.” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and intervention (exercise) delivery personnel not blinded to group allocation but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | Unclear risk | Unclear if personnel collecting fall information blinded to group allocation. |
| Incomplete outcome data (attrition bias) Falls | High risk | See appendix for method of assessment |
| Incomplete outcome data (attrition bias) Fallers | Low risk | See appendix for method of assessment |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement as unable to find a published protocol or trial registration. |
| Method of ascertaining falls (recall bias) Falls and fallers | Low risk | The study used concurrent collection of data about falling with monthly, or more frequent, follow‐up by the researchers. Quote: “Each participant completed a monthly fall calendar, denoting any falls that occurred during the month. A fall was defined as occurring when a participant loses their balance causing them to hit the ground or another object at a lower level. If a fall occurred, the participant was required complete a fall report form to describe the nature and activity engaged during the fall and if any injuries incurred as a result of the fall.” |
Henderson 2016.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: UK N = 130 Sample: recruited from local centres, hospital clinics, from the Parkinson’s Register of the Dementias and Neurodegenerative Diseases Research Network (ProDeNDRoN) database and via advertising through the Parkinson’s UK charity research network and local media (based at North Bristol NHS Trust Hospital) (38% women) Age (years): median (range) intervention group 71 (54‐90) control group 69 (46‐88) Inclusion criteria: idiopathic PD (diagnosed by a movement disorder specialist); Hoehn and Yahr stage 2–3; stable on antiparkinsonian drugs for 2 weeks before enrolment; able to walk 18 metres without an aid; ≥ 1 fall in the past year; no previous exposure to an acetylcholinesterase inhibitor; no dementia Exclusion criteria: did not speak English; had an absolute contraindication to, or had previously taken, acetylcholinesterase inhibitors; any other neurological, visual, or orthopaedic problem that meaningfully interfered with gait; dementia Disease severity at baseline: HY stage 2 to 3; MDS‐UPDRS motor score mean (SD) 40.0 (14.5) |
|
| Interventions | Medication: cholinesterase inhibitor 1. Oral rivastigmine dosage optimisation (3–12 mg/day) for up to 16 weeks. Plus maintenance treatment (the highest tolerated dose) for 16 weeks 2. Placebo dosage optimisation (3–12 mg/day) for up to 16 weeks. Plus maintenance treatment (the highest tolerated dose) for 16 weeks |
|
| Outcomes | 1. Rate of falls 2. Number of fallers 3. Number and type of adverse events 4. Quality of life (EQ‐5D‐5L both visual analogue score and index score) Other outcomes reported but not included in this review |
|
| Duration of the study | 12 months | |
| Funding source | Parkinson's UK | |
| Notes | Fall data collected: at baseline and by monthly falls diaries and phone calls for 12 months. Falls outcome reported for the first 8 months of this data collection period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component in the sequence generation was described. Quote: "the randomization sequence, which was computer generated by the Bristol Randomised Trials Collaboration (BRTC) clinical trials unit using a web‐based program...” |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was described as by central allocation. Quote: "Participants were enrolled and tested by an investigator who had no access to the randomization sequence... A treatment pack number was issued via a secure website that matched the number to a drug pack held in the pharmacy to ensure concealment of allocation.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and personnel implementing the interventions ensured, and unlikely that the blinding could have been broken. Quote: “Patients were randomly assigned (1:1) to oral rivastigmine or placebo capsules matched to those for rivastigmine in colour and weight.”; “Identical titration was performed for those taking placebo to maintain masking.” |
| Blinding of outcome assessment (detection bias) Falls and fallers | Low risk | Outcomes were recorded/confirmed in all allocated groups using the same method and the personnel recording/confirming outcomes were blind to group allocation. |
| Incomplete outcome data (attrition bias) Falls | Low risk | See appendix for method of assessment |
| Incomplete outcome data (attrition bias) Fallers | Low risk | See appendix for method of assessment |
| Selective reporting (reporting bias) | High risk | The study protocol is available (ISRCTN 19880883) but not all the secondary outcomes of interest have been reported in the pre‐specified way |
| Method of ascertaining falls (recall bias) Falls and fallers | Low risk | The study used concurrent collection of data about falling with monthly, or more frequent, follow‐up by the researchers. Quote: “We measured occurrence of falls with use of monthly falls diaries, which patients posted monthly to the investigators. We telephoned participants every month to corroborate fall information.” |
Li 2012.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: facility, USA N = 195 Sample: recruited from four Oregon cities (Eugene, Corvallis, Salem, and Portland) by means of newspaper advertisements, referrals from neurologists or physical therapists, and information distributed to local Parkinson’s disease support groups (37% women) Age (years): Mean (SD) Tai Chi intervention group 68 (9), strength training intervention group 69 (8), control group 69 (9) Inclusion criteria: diagnosis of PD; Hoehn and Yahr stage 1 to 4; age of 40 to 85 years; at least one score of 2 or more for at least one limb for the tremor, rigidity, postural stability, or bradykinesia items in the motor section of the Unified Parkinson’s Disease Rating Scale; stable medication use; ability to stand unaided and walk with or without an assistive device; medical clearance for participation; willingness to be assigned to any of the three interventions Exclusion criteria: current participation in any other behavioral or pharmacologic study or instructor‐led exercise program; cognitive impairment (Mini–Mental State examination score <24); debilitating conditions or vision impairment that would impede full participation in the study; unavailability during the study period. Disease severity at baseline: HY stage 1 to 4; UPDRS motor score mean (SD) 15.2 (5.9) |
|
| Interventions | Exercise 1. Exercise: Tai Chi Group supervised by a Tai Chi instructor (60 min, 2x/week for 24 weeks) 2. Exercise: strength training (lower limb). Group supervised by an instructor (60 min, 2x/week for 24 weeks) 3. Control: Stretching. Group supervised by an instructor (60 min, 2x/week for 24 weeks) |
|
| Outcomes | 1. Rate of falls 2. Number of fallers 3. Number and type of adverse events 4. Quality of life (PDQ‐8) (Li 2014) Economic analysis reported in Li 2015: 1. Cost of delivering the intervention 2. Cost of health service use 3. Incremental cost per QALY gained 4. Incremental cost per fall prevented Other outcomes reported but not included in this review |
|
| Duration of the study | 9 months | |
| Funding source | National Institiute of Neurological Disorders and Stroke | |
| Notes | Fall data collected: during the 6‐month intervention period and at the 3‐month follow‐up period by monthly falls diaries Economic analysis reported in US dollar (price year 2011) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of generating the randomisation list not described. Quote: "...randomly assigned to one of the interventions, in a ratio of 1:1:1, without stratification, with the use of permuted‐block randomization." |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was described by central allocation. Quote from protocol: "Concealment of allocation will be implemented. The randomization schedule, generated by the project data analyst, will be kept by a project staff who will deliver it, in a sealed envelope, to a research assistant who will then assign qualified individuals to intervention groups.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and intervention (exercise) delivery personnel not blinded to group allocation, but were unaware of which was the control group. Quote: “To reduce potential expectation bias, participants will be informed that the study will be comparing three different exercises and that they will be assigned to an exercise group at random.” And “Because of the behavioral trial, blinding instructors will not be possible. However, the instructors will not be provided with any information related to the objectives of the study..." |
| Blinding of outcome assessment (detection bias) Falls and fallers | Unclear risk | Personnel collecting fall information not blinded to group allocation, but were unaware of which was the control group. Quotes: “Because of the behavioral trial, blinding instructors will not be possible. However, the instructors will not be provided with any information related to the objectives of the study, nor will they participate in any outcome assessments.” |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Insufficient information to permit judgement. No fractures reported and unclear how data regarding fractures was collected. |
| Incomplete outcome data (attrition bias) Falls | Unclear risk | Data not available to assess. |
| Incomplete outcome data (attrition bias) Fallers | Unclear risk | Data not available to assess. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT00611481) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Method of ascertaining falls (recall bias) Falls and fallers | Low risk | The study used concurrent collection of data about falling with monthly, or more frequent, follow‐up by the researchers. Quote: ”Falls were monitored by means of daily “fall calendars” that were maintained by the study participants and collected monthly throughout the intervention or until a participant withdrew from the study.” |
Li 2015a.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: China N = 89 (subgroup with cognitive impairment only as they were given the intervention) Sample: recruited from the PD collaborative study carried out in the neurology department of Weihai Municipal Hospital (37% women) Age (years): Mean (95% CI) intervention group 67.5 (52.7‐71.1) control group 66.9 (53.8‐70.3) Inclusion criteria: diagnosis of PD in accordance with the UK Brain Bank criteria; cognitive impairment, including PD dementia Exclusion criteria: the presence of other conditions that can lead to cognitive dysfunction, such as delirium, stroke, severe depression, metabolic abnormalities, drug side effects, and head trauma Disease severity at baseline: HY stage 1 to 5; MDS‐UPDRS motor score mean 20.6 (SD not reported) |
|
| Interventions | Medication: cholinesterase inhibitor 1. Oral rivastigmine (3mg twice daily) for 12 months 2. Placebo (3mg twice daily) for 12 months |
|
| Outcomes | 1. Rate of falls 2. Number of fallers Other outcomes reported but not included in this review |
|
| Duration of the study | 12 months | |
| Funding source | Development Plan of Medical and Health Sciences of Shandong Province (No. 2007HW020) | |
| Notes | Fall data collected: at baseline and every week by phone calls or follow‐up evaluations for 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of generating randomisation list not described. Quote: "The trial was a randomized, double‐blind, placebo‐controlled study of 12 months duration.” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement. No details provided relating to how double blinding to group allocation (medication/placebo) was performed. |
| Blinding of outcome assessment (detection bias) Falls and fallers | Low risk | Outcomes were recorded/confirmed in all allocated groups using the same method and the personnel recording/confirming outcomes were blind to group allocation. |
| Incomplete outcome data (attrition bias) Falls | Unclear risk | Data not available to assess. |
| Incomplete outcome data (attrition bias) Fallers | Unclear risk | Data not available to assess. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement as unable to find a published protocol or trial registration. |
| Method of ascertaining falls (recall bias) Falls and fallers | Unclear risk | It appears that retrospective recall was required over a short period (one week). Quote: "Phone calls or follow‐up evaluations were conducted every week to record the data related to falls.” |
Martin 2015.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: home, New Zealand N = 21 Sample: recruited from the New Zealand Brain Research Institute database (38% women) Age (years): mean (SD) Immediate start intervention group 72 (5.1) Delayed start intervention group 72 (5.8) Inclusion criteria: diagnosis of PD by a movement disorder specialist; aged over 65 years; presence of FOG as indicated by answering “yes” to question 1 on New Freezing of Gait Questionnaire (NFOGQ); independently mobile with or without walking aid; stable PD medication regimen at the time of recruitment Exclusion criteria: cognitive impairment (Mini Mental State Examination Score of <24); comorbidities that would prohibit safe participation in exercise; unable to press metronome buttons, or hear a metronome adequately Disease severity at baseline: HY stage mean (SD) 2.8 (0.6) |
|
| Interventions | Exercise 1. Exercise: immediate start (2 week wait period) ‐ Cued Up! program including home‐based cued exercises and practice of functional movements associated with freezing of gait (FOG) using cues along with strategies for preventing FOG (30‐60 min for 24 weeks ‐ including 6 home visits by a physiotherapist within the first 4 weeks of the 24‐week intervention period followed by weekly phone calls for the remaining 20 weeks) 2. Exercise: delayed start (24 week wait period) ‐ Cued Up! program including home‐based cued exercises and practice of functional movements associated with FOG using cues along with strategies for preventing FOG (30‐60 min. for 24 weeks ‐ including 6 home visits by a physiotherapist within the first 4 weeks of the 24‐ week intervention period followed by weekly phone calls for the remaining 20 weeks) |
|
| Outcomes | 1. Rate of falls 2. Number of fallers Other outcomes reported but not included in this review |
|
| Duration of the study | 12 months | |
| Funding source | Canterbury Multiple Sclerosis and Parkinson's Disease Society, Physiotherapy New Zealand's Older Adult and Neurology Special Interest Groups, and the Hope Foundation for Research on Ageing | |
| Notes | Fall data collected: at baseline (weeks 1‐5), mid active (weeks 9‐13) and end of active (weeks 24‐28) by monthly falls diaries | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component in the sequence generation was described. Quote: "Participants were randomized to immediate‐start (IS), n =1 2, or 6‐month delayed‐start (DS), n = 9, groups by a computerized random number generator.” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and intervention (exercise) delivery personnel not blinded to group allocation but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | Unclear risk | Unclear if personnel collecting fall information blinded to group allocation. |
| Incomplete outcome data (attrition bias) Falls | Low risk | Based on fall rates reported for weeks 24‐28. See appendix for method of assessment |
| Incomplete outcome data (attrition bias) Fallers | Low risk | See appendix for method of assessment |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ as unable to find a published protocol or trial registration. |
| Method of ascertaining falls (recall bias) Falls and fallers | Low risk | The study used concurrent collection of data about falling with monthly, or more frequent, follow‐up by the researchers. Quote: “Participants used a daily diary to record whether a fall had occurred and the number of falls that occurred each day. Family or care givers were also instructed on use of the falls diary to help with its completion. Participants posted diaries to the researcher each month. Telephone calls were made to prompt participants if diaries were not received.” |
Mirelman 2016.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: facility; Israel, Belgium, UK, Italy, the Netherlands N = 130 (PD subgroup) Sample: recruited via flyers, advertising, presentations at local residential and community senior centres, review of medical records at local outpatient clinics, and word of mouth (52% women) Age (years): mean (SD) intervention group 71.0 (6.3) control group 71.0 (6.1) (PD subgroup) Inclusion criteria: aged 60−90 years; able to walk for at least 5 minutes unassisted; stable medication for the past month; ≥ 2 falls within 6 months before screening; diagnosis of PD in accordance with the UK Brain Bank criteria; HY stage 2‐3; taking antiparkinsonian medication Exclusion criteria: psychiatric comorbidity (e.g., major depressive disorder as in accordance with DSM IV criteria); history of stroke, traumatic brain injury, or other neurological disorders; acute lower back or lower extremity pain; peripheral neuropathy; rheumatic and orthopaedic diseases; or a clinical diagnosis of dementia or severe cognitive impairment (Mini Mental State Exam score <21). Disease severity at baseline: HY stage 2 to 3, UPDRS motor score mean (SD) 30.7 (13.7) |
|
| Interventions | Exercise 1. Exercise: treadmill training plus non‐immersive virtual reality. Individual treatment supervised by a trainer (45 minutes, 3x/week for 6 weeks) 2. Control: treadmill training. Individual treatment supervised by a trainer (45 minutes, 3x/week for 6 weeks) |
|
| Outcomes | 1. Rate of falls 2. Number and type of adverse events 3. Quality of life (SF‐36) Other outcomes reported but not included in this review |
|
| Duration of the study | 6 months | |
| Funding source | European Commission | |
| Notes | Fall data collected: at baseline (considering 6 months before intervention) and during the 6 months after the end of training by falls calendar (monthly paper version, web‐based calendar, or a smartphone application) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component in the sequence generation was described. Quote: "By use of computer‐based allocation, participants were randomly assigned to receive either treadmill training plus VR or treadmill training alone.” |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was described as by central allocation. Quote: "Allocation was done by the study contract research organisation (Advanced Drug and Device Services [ADDS], Brno, Czech Republic), a third partly not involved in study procedures on site.” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and intervention (exercise) delivery personnel not blinded to group allocation but impact of non‐blinding unclear |
| Blinding of outcome assessment (detection bias) Falls and fallers | Low risk | Outcomes were recorded/confirmed in all allocated groups using the same method and the personnel recording/confirming falls were blind to group allocation. Quote: "All outcome measures (ie falls and secondary outcomes) were assessed by blinded assessors.” |
| Incomplete outcome data (attrition bias) Falls | Unclear risk | Parkinson's disease‐specific data not available to assess. |
| Incomplete outcome data (attrition bias) Fallers | Unclear risk | Parkinson's disease‐specific data not available to assess. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT01732653) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Method of ascertaining falls (recall bias) Falls and fallers | Low risk | The study used concurrent collection of data about falling with monthly, or more frequent, follow‐up by the researchers. Quote: “Participants received a falls calendar...Research staff contacted all participants every month to maximise compliance.” |
Morris 2015.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: facility and home, Australia N = 210 Sample: recruited from PD support groups, neurologists, medical practitioners, movement disorders clinics, and by advertisements in PD Association newsletters (33% women) Age (years): mean (SD) progressive resistance strength training intervention group 67.4 (10.4), movement strategy training intervention group 68.4 (9.9), control group 67.9 (8.4) Inclusion criteria: Mini Mental State Examination ≥ 24; HY stage < 5; diagnosis of PD; being medically able and safe to perform the interventions Exclusion criteria: deep brain stimulation Disease severity at baseline: HY stage 1 to 4 (median 2.5), UPDRS motor score mean (SD) 15.2 (6.2) |
|
| Interventions | Exercise plus education 1. Exercise: progressive resistance strength training (lower limb and trunk). Supervised by a physiotherapist (120 minutes, 1x/week for 8 weeks). Plus, home‐strengthening exercises at very similar duration of the outpatient therapy sessions. Plus falls prevention education 2. Exercise: movement strategy training. Supervised by a physiotherapist (120 minutes, 1x/week for 8 weeks). Plus, home strategies exercise at very similar duration of the outpatient therapy sessions. Plus falls prevention education 3. Control: life‐skill sessions. Groups conducted by physiotherapists, occupational therapists, speech pathologists or social workers with no contents related to fall or mobility (120 minutes, 1x/week for 8 weeks). Plus, home programs with similar life skill activities at very similar duration of the outpatient sessions. |
|
| Outcomes | 1. Rate of falls 2. Number of fallers 3. Number reporting a fall‐related fracture 5. Quality of life (PDQ39 and VAS of the Euroqol‐5D) Other outcomes reported but not included in this review |
|
| Duration of the study | 12 months | |
| Funding source | Michael J Fox Foundation (US) Clinical Discovery Grant | |
| Notes | Fall data collected: during 12 months after the end of the intervention by falls diaries | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component in the sequence generation was described. Quote: "...computer generated random allocation sequence with sequentially numbered envelopes..." |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was described. Quote: "Participants were notified of their group allocation and enrolled by a research assistant who was not informed of the trial aims and did not provide therapy or testing.” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and intervention (exercise) delivery personnel not blinded to group allocation but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | Low risk | Outcomes were recorded/confirmed in all allocated groups using the same method and the personnel recording/confirming outcomes were blind to group allocation. Quote: "All therapists who performed assessments were kept blind to group allocation. Therapists delivering interventions did not assess participants or record outcomes measures." |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Insufficient information to permit judgement. Unclear how data regarding fractures was collected. |
| Incomplete outcome data (attrition bias) Falls | High risk | See appendix for method of assessment |
| Incomplete outcome data (attrition bias) Fallers | Low risk | See appendix for method of assessment |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (ACTRN12606000344594) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Method of ascertaining falls (recall bias) Falls and fallers | Low risk | The study used concurrent collection of data about falling with monthly, or more frequent, follow‐up by the researchers. Quote (from McGinley 2012): “Falls were monitored using a Falls Calendar protocol. This required people to enter falls on a calendar as they occurred and to telephone a falls hotline to answer questions relating to fall circumstances and consequences.” |
Morris 2017.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: home, Australia N = 133 Sample: recruited via hospital‐based neurologists and the state Parkinson’s support group (40% women) Age (years): mean (SD) intervention group 71.0 (8), control group 71.0 (10) Inclusion criteria: diagnosis of idiopathic PD; modified HY stage ≤ 4; community dwelling Exclusion criteria: cognitive impairment (Mini Mental State Examination < 24); other health conditions that preclude safe participation in the exercise program; insufficient English to follow instructions; an unwillingness to be assessed and treated at home Disease severity at baseline: HY stage 1 to 4, MDS‐UPDRS motor score mean (SD) 35.5 (15) |
|
| Interventions | Exercise plus education 1. Exercise: home program comprised of progressive resistance strength training (lower limb and trunk), movement strategy training and falls prevention education.Supervised by a therapist who was guided by a physiotherapist (60 minutes, 1x/week for 6 weeks). Plus, unsupervised session prescribed by a physiotherapist (60 minutes, 1x/week for 6 weeks) 2. Control: non‐specific life skills program. Delivered by trained allied health professionals, including occupational therapists, physiotherapists and speech pathologists with no contents related to physical activity, exercise, walking, or fall risk education at comparable length of the intervention group. Plus, self‐directed homework sessions at comparable length of the intervention group |
|
| Outcomes | 1. Rate of falls 2. Number of fallers 3. Number reporting a fall‐related fracture 4. Quality of life (PDQ39 and EQ‐5D‐3L) Economic analysis 1. Cost of delivering the intervention 2. Cost of fall‐related injury Other outcomes reported but not included in this review |
|
| Duration of the study | 12 months | |
| Funding source | National Health and Medical Research Council Project Grant (no. 509129) | |
| Notes | Fall data collected: from the initial pre‐intervention assessment until the follow‐up assessment 12 months after the intervention by monthly falls diaries Economic analysis reported in AUD dollar (price year 2016, hospital costs 20112/13) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component in the sequence generation was described. Quote: "Randomisation was stratified according to referral source, and performed by an independent entity using a computerised random number generator.” |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was described as by central allocation. Quote: "Randomisation was stratified according to referral source, and performed by an independent entity using a computerised random number generator.” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and intervention (exercise) delivery personnel not blinded to group allocation but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | Unclear risk | Unclear if personnel collecting fall information blinded to group allocation. |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Insufficient information to permit judgement. Fractures were collected as injurious falls as part of the falls diaries, with injurious falls “defined as any fall that required medical attention or healthcare utilization,” however fractures were self‐reported and not confirmed by the results of radiological examination or from primary care case records. |
| Incomplete outcome data (attrition bias) Falls | Low risk | See appendix for method of assessment |
| Incomplete outcome data (attrition bias) Fallers | Low risk | See appendix for method of assessment |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (ACTRN12608000390381) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Method of ascertaining falls (recall bias) Falls and fallers | Low risk | The study used concurrent collection of data about falling with monthly, or more frequent, follow‐up by the researchers. Quote: “...via monthly falls calendars returned via pre‐paid mail. Each participant was required to record any falls incidents by marking the date on the calendar and indicating whether the fall was injurious (defined as any fall that required medical attention or healthcare utilisation). Telephone calls were made to remind participants to return their calendars and to investigate any injurious falls.” |
Munneke 2010.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: location unclear, the Netherlands N = 699 Sample: recruited from the clusters (community hospitals) in the vicinity of the three participating university medical centres (Radboud University Nijmegen Medical Centre, VU University of Amsterdam and Leiden University Medical Centre) (42% women) Age (years): mean (SD) ParkinsonNet clusters 68.8 (7.9), usual care clusters 68.4 (7.5) Inclusion criteria: diagnosis of idiopathic Parkinson’s disease by a neurologist on the basis of the UK Brain Bank criteria; living independently in the community; ability to complete the questionnaires; absence of comorbidity that interfered with daily functioning Exclusion criteria: cognitive impairment (Mini‐mental State Examination score <24); presence of major psychiatric disorders Disease severity at baseline: HY stage 1 to 4, UPDRS motor score mean (SD) 28.6 (12.1) |
|
| Interventions | Exercise 1. ParkinsonNet clusters: physiotherapists provided patients with evidence‐based recommendations. Plus, specific training of physiotherapists, structuring of the referral process and optimisation of communication between the participating health professionals 2. Usual care clusters: physiotherapists provided patients with usual care, and did not receive any of the components of the ParkinsonNet intervention |
|
| Outcomes | 1. Rate of falls 2. Quality of life (EQ‐5D, PDQ‐39 mobility subscore only) Other outcomes reported but not included in this review |
|
| Duration of the study | 24 weeks | |
| Funding source | ZonMw, Netherlands Organisation for Scientific Research, Dutch Parkinson's Disease Society, National Parkinson Foundation, and Stichting Robuust | |
| Notes | Fall data collected during 24 weeks by a falls calculator | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component in the sequence generation was described. Quote: "An independent biostatistician (GFB) who was not involved in recruitment randomly allocated clusters by use of a variance minimisation algorithm.” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of low risk or high risk as it is unclear if the cluster randomisation was performed prior to the start of the study. Quote: "An independent biostatistician (GFB) who was not involved in recruitment randomly allocated clusters by use of a variance minimisation algorithm.” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants blinded to group allocation, but personnel implementing the intervention not blinded, and impact of non‐blinding unclear. Quote: “Participants did not know which cluster they were in, and there was minimum risk of contamination.” |
| Blinding of outcome assessment (detection bias) Falls and fallers | Unclear risk | Unclear if personnel collecting fall information blinded to group allocation. |
| Incomplete outcome data (attrition bias) Falls | Low risk | See appendix for method of assessment |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT00330694) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Method of ascertaining falls (recall bias) Falls and fallers | Unclear risk | Falls were monitored with a falls calculator, but details of this were not reported. |
Paul 2014.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: facility, Australia N = 40 Sample: recruited from Parkinson’s support groups and neurology clinics (38% women) Age (years): mean (SD) intervention group 68.1 (5.6), control group 64.5 (7.4) Inclusion criteria: idiopathic PD; aged over 40 years; able to walk independently with or without an aid Exclusion criteria: significant cognitive impairment (Mini‐mental State Examination score <24); any unstable cardiovascular, orthopaedic or neurological conditions that would interfere with the safety of assessment and/or interpretation of results Disease severity at baseline: HY stage mean (SD) 1.95 (0.8), MDS‐UPDRS motor score mean (SD) 36.4 (12.5) |
|
| Interventions | Exercise 1. Exercise: muscle power training (lower limb). Group (pairs) supervised by a physiotherapist (45 minutes, 2x/week for 12 weeks) 2. Control: low‐intensity exercises (lower limb and trunk). Home unsupervised exercises (2x/week for 12 weeks) |
|
| Outcomes | 1. Rate of falls 2. Number of fallers 3. Number reporting a fall‐related fracture Other outcomes reported but not included in this review |
|
| Duration of the study | 6 months | |
| Funding source | Parkinson's NSW Unity Walk Research Grant (ID: 2010‐02589) and a University of Sydney Bridging Support Grant | |
| Notes | Fall data collected: for six months by monthly falls diaries | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component in the sequence generation was described. Quote: "Randomization was done in blocks of four using a computer‐generated random number schedule." |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was described as by central allocation. Quote: "Randomization was performed off‐site by an investigator not involved in recruitment or assessment." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and intervention (exercise) delivery personnel not blinded to group allocation but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | Unclear risk | Unclear if personnel collecting fall information blinded to group allocation. |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Insufficient information to permit judgement. Unclear how data regarding fractures was collected. |
| Incomplete outcome data (attrition bias) Falls | Low risk | See appendix for method of assessment |
| Incomplete outcome data (attrition bias) Fallers | Low risk | See appendix for method of assessment |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (ACTRN12611000986976) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Method of ascertaining falls (recall bias) Falls and fallers | Low risk | The study used concurrent collection of data about falling with monthly, or more frequent, follow‐up by the researchers. Quote: "The number of falls sustained by each person was monitored prospectively over six months using monthly falls diaries.” |
Pelosin 2017.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: Facility, Italy N = 30 Sample: recruited from the outpatient Movement Disorders Clinic of the University of Genoa, Italy (% women not reported) Age (years): mean (SD) high frequency treadmill training group 69.9 (4.5), intermediate frequency treadmill training group 73.7 (8.3), low‐frequency treadmill training group 73.1 (6.8) Inclusion criteria: diagnosis of idiopathic PD according to the United Kingdom PD Society Brain Bank criteria; Hoehn and Yahr stage 1 to 2.5; stable medication regime for at least three months; ability to walk for six minutes without assistance. Exclusion criteria: past history of neurological conditions other than PD; deep brain stimulation; presence of freezing of gait; Mini‐mental State examination Score <24; presence of cardiovascular dysfunction; orthopaedic conditions restricting exercise training. Disease severity at baseline: HY stage mean (SD) 2.2 (0.5); MDS‐UPDRS motor score mean (SD) 31.4 (5.9) |
|
| Interventions | Exercise 1. Exercise: high‐frequency treadmill training (45 minutes, 5x/week for 10 sessions) 2. Exercise: intermediate‐frequency treadmill training (45 minutes, 3x/week for 10 sessions) 3. Exercise: low‐frequency treadmill training (45 minutes, 2x/week for 10 sessions) Treadmill training for all groups started at 90% of comfortable overground waking speed, and was increased by 5% every two sessions, aiming to reach 115% for the last 2 sessions |
|
| Outcomes | 1. Rate of falls Other outcomes reported but not included in this review |
|
| Duration of the study | 5 months | |
| Funding source | none reported | |
| Notes | Fall data collected: via a monthly calendar with a weekly phone call | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component in the sequence generation was described. Quote: "…participants were randomized using a computerized random number generator (block size=3) in a 1:1:1 ratio into one of the three intervention groups.” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement as a method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and intervention (exercise) delivery personnel not blinded to group allocation but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | Unclear risk | Unclear if personnel collecting fall information blinded to group allocation. |
| Incomplete outcome data (attrition bias) Falls | Low risk | See appendix for method of assessment |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ as no published trial protocol or trial registration available. |
| Method of ascertaining falls (recall bias) Falls and fallers | Unclear risk | The study used concurrent collection of data about falling with weekly follow‐up by the researchers. Quote: "…number of falls was determined by means of a monthly calendar, in which all participants were instructed to record, the number of falls for every single day. In addition, patients were constantly monitored by a weekly phone call.” |
Penko 2019.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: facility, USA N = 21 Sample: recruited from the Cleveland Clinic (Cleveland, Ohio) and the surrounding area (32% women) Age (years): mean (SD) single‐modal group 64.6 (8.5), multimodal group 57.8 (8.2) Inclusion criteria: clinical diagnosis of PD; Hoehn and Yahr stage 2 to 4; at least 2 falls in the prior 12 months; ability to walk a minimum of 300 feet with or without a walking aid. Exclusion criteria: any musculoskeletal contraindication to exercise; a history of neurological disease other than PD; ≥3 errors on the short Portable Mental Status Questionnaire; inability to follow 2‐step commands; uncontrolled cardiovascular risk factors classifying the individual as a high‐risk exerciser as per the American College of Sports Medicine; having undergone any surgical procedure for the treatment of PD (e.g. deep brain stimulation). Disease severity at baseline: HY stage mean (SD) 2.3 (0.5), MDS‐UPDRS motor score mean (SD) 36.6 (11.2) |
|
| Interventions | Exercise 1. Exercise: single‐modal training ‐ gait training and cognitive training performed separately (45 minutes, 3x/week for 8 weeks) 2. Exercise: multimodal training ‐ gait training and cognitive training performed simultaneously (45 minutes, 3x/week for 8 weeks) Cognitive training was the same for both groups and involved tasks targeting executive function, attention, memory and language. Gait training was the same for both groups and focused on improving gait quality (e.g. velocity and step length) |
|
| Outcomes | 1. Rate of falls Other outcomes reported but not included in this review |
|
| Duration of the study | 12 weeks | |
| Funding source | Davis Phinney Foundation | |
| Notes | Fall data collected: for the past 30 days, measured at 8 weeks (post intervention) and 12 weeks (follow‐up), via recall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component in the sequence generation was described. Quote from Rosenfeldt 2019: "…participants were randomized via a nonreplenished envelope pull into the SMT or MMT group” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement as a method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and intervention (two different exercise and cognitive training interventions) delivery personnel not blinded to group allocation but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | Unclear risk | Unclear if personnel collecting fall information blinded to group allocation. |
| Incomplete outcome data (attrition bias) Falls | Low risk | See appendix for method of assessment |
| Selective reporting (reporting bias) | High risk | The study protocol is available (NCT02538029) but not all the secondary outcomes of interest (quality of life) have been reported in the pre‐specified way. Additionally, falls are reported but are not listed as an outcome in the protocol. |
| Method of ascertaining falls (recall bias) Falls and fallers | Unclear risk | At baseline and follow‐up there was retrospective recall over 30 days. There was shorter recall during the intervention period, however both post test and follow‐up fall data has been used in the analysis. Quote: "Fall frequency over the past 30 days were assessed via participant recall, and individuals were prompted by study personnel asking, “How many times have you come to rest inadvertently on the ground or other lower level surface in the past 30 days?” and "...participants were asked if a fall occurred at each intervention visit." |
Protas 2005.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: facility, USA N = 18 Sample: recruited from VA Parkinson’s Disease Research, Education and Clinical Center (PADRECC) (0% women) Age (years): mean (SD) intervention group 71.3 (7.4), control group 73.7 (8.5) Inclusion criteria: idiopathic PD; postural instability‐gait difficulty predominant PD; experiences with freezing episodes, and/or a history of falls; stable regimen of antiparkinsonian medications; ability to stand and walk with or without assistance; HY stage 2 or 3; scores of moderate or higher on all scales of the Neurobehavioral Cognitive Status Examination (Cognistat) Exclusion criteria: Not reported Disease severity at baseline: HY stage 2 to 3, UPDRS motor score mean (SD) 29.4 (10.8) |
|
| Interventions | Exercise 1. Exercise: gait and step training. Individual treatment supervised by a physiotherapist (60 minutes, 3x/week for 8 weeks) 2. Control: usual care |
|
| Outcomes | 1. Rate of falls 2. Number of fallers Other outcomes reported but not included in this review |
|
| Duration of the study | 8 weeks | |
| Funding source | Parkinson's Disease Research, Education, and Clinical Center, Michael E. Debakey Veterans Affairs Medical center, Houston, TX (Department of veterans Affairs #B2728‐R) | |
| Notes | Fall data collected: 2 weeks prior to and after the 8‐week intervention period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of generating the randomisation list not described. Quote: "...was randomly assigned to either the gait and step training intervention group or a control group..." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and intervention (exercise) delivery personnel not blinded to group allocation but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | High risk | Personnel recording/confirming falls were not blind to group allocation. Quote: "A physical therapist who was not blinded to group assignment obtained fall records.” |
| Incomplete outcome data (attrition bias) Falls | Low risk | See appendix for method of assessment |
| Incomplete outcome data (attrition bias) Fallers | Low risk | See appendix for method of assessment |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ as unable to find a published protocol or trial registration. |
| Method of ascertaining falls (recall bias) Falls and fallers | Low risk | The study used concurrent collection of data about falling with monthly, or more frequent, follow‐up by the researchers. Quote: “Each subject was contacted daily by telephone for a period of 2 weeks prior to starting the 8 week training or control sessions. The patient was asked if he fell that day, under what circumstances, and whether or not the fall resulted in any injuries.” |
Ricciardi 2015.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: facility, UK N = 28 Sample: did not report source of patients (32% women) Age (years): mean (SD) worst side group 66 (6.1), best side group 69 (5.8) control group 70 (4.9) Inclusion criteria: diagnosis of PD according to UK Brain Bank criteria; HY stage 2 or 3; medical treatment and clinical condition stable for at least 4 weeks Exclusion criteria: cognitive impairment (Mini Mental State Examination score <24); orthopedic or major disease interfering with gait and balance; history of psychiatric or neurological illnesses (other than PD); depression (Hamilton Depression Rating Scale >17) Disease severity at baseline: HY stage 2 to 3, UPDRS motor score mean (SD) 27.9 (10.3) |
|
| Interventions | Exercise 1. Exercise: strength, balance and gait training targeting the most affected body side, with doubled number of repetitions for the most affected side (60 min, 2x/week for 3 months) 2. Exercise: strength, balance and gait training targeting the least affected side, with doubled number of repetitions for the least affected side (60 min, 2x/week for 3 months) 3. Control (standard treatment): strength, balance and gait training targeting both sides, with the same number of repetitions for both body sides (60 min, 2x/week for 3 months) |
|
| Outcomes | 1. Rate of falls 2. Quality of life (EQ‐5D) Other outcomes reported but not included in this review |
|
| Duration of the study | 16 weeks | |
| Funding source | Not reported | |
| Notes | Fall data collected: throughout the duration of the study by falls diaries | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component in the sequence generation was described. Quote: "By means of random number generator, patients were randomly assigned to one of the three study groups:” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants blinded to group allocation, but not delivery personnel, but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | Unclear risk | Unclear if personnel collecting fall information blinded to group allocation. |
| Incomplete outcome data (attrition bias) Falls | Low risk | See appendix for method of assessment |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement as unable to find a published protocol or trial registration. |
| Method of ascertaining falls (recall bias) Falls and fallers | Unclear risk | Falls diary completed by all participants, however no description of any researcher follow‐up. Quote: “Patients and their next of kin were asked to keep a diary of falls during all the study period.” |
Sedaghati 2016.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: facility, Iran N = 47 Sample: recruited from university afÂÂfiliated neurology clinics and private neurology offices in Kashan (30% women) Age (years): mean (SD) progressive balance and gait training with balance pad intervention group 59.1 (8.4), progressive balance and gait training without balance pad intervention group 58.8 (8.1), control group 57.2 (6.9) Inclusion criteria: diagnosis of idiopathic PD for three years; able to walk independently; aged between 50 and 70 years; consumed the same anti‐PD medication for past 2 weeks; history of falling in the past year Exclusion criteria: significant cognitive impairment (Mini Mental State Examination < 24); other neurological/musculoskeletal/ cardiopulmonary/metabolic conditions that would interfere with safe conduction of training or exercise program. Disease severity at baseline: HY stage 2 to 3, mean (SD) 2.6 (0.5) |
|
| Interventions | Exercise 1. Exercise: progressive balance and gait training activities with balance pad. Wholly‐supervised exercises (60 minutes, 3x/week for 10 weeks) 2. Exercise: progressive balance and gait training activities with no balance pad. Wholly‐supervised exercises (60 minutes, 3x/week for 10 weeks) 3. Control: received their usual care by a neurologist |
|
| Outcomes | 1. Rate of falls Other outcomes reported but not included in this review |
|
| Duration of the study | 10 weeks | |
| Funding source | Not reported | |
| Notes | Fall data collected: at baseline and after a 10‐week follow‐up intervention by direct questioning | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of generating randomisation list not described. Quote: "After baseline assessment, participants were randomly allocated to control and two exercise groups.” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and intervention (exercise) delivery personnel not blinded to group allocation but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | Unclear risk | Unclear if personnel collecting fall information blinded to group allocation. |
| Incomplete outcome data (attrition bias) Falls | Low risk | See appendix for method of assessment |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement as unable to find a published protocol or trial registration. The published trial registration number appears to be incorrect. |
| Method of ascertaining falls (recall bias) Falls and fallers | Unclear risk | No information about how or when this direct questioning occurred. Quote: “The number of falls were recorded by direct questioning.” |
Shen 2015.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: facility and home, Hong Kong N = 51 Sample: recruited from Hong Kong Parkinson’s Disease Association, a patient self‐help group, and the Movement Disorders Clinic of a local hospital (44% women) Age (years): mean (SD) intervention group 63.3 (8), control group 65.3 (8.5) Inclusion criteria: diagnosis of idiopathic PD; stable after taking anti‐Parkinsonian medication; ability to walk independently for 10 metres; cognitive impairment (Mini‐mental State Examination score > 23) Exclusion criteria: motor fluctuations; any disorders that would affect balance and locomotion, such as neurological conditions other than PD; uncompensated cardiovascular disease; visual disturbance; a recent musculoskeletal disorder in the back or the lower limbs. Disease severity at baseline: HY stage 2 to 3, UPDRS motor score mean (SD) 23.6 (7.4) |
|
| Interventions | Exercise 1. Exercise: balance and gait training. Laboratory‐based supervised by a physiotherapist (60 minutes, 3x/week for 4 weeks). Followed by unsupervised home based training with the same emphases as the laboratory‐based phase (20 minutes, 5x/week for 4 weeks). Followed by laboratory‐based supervised by a physiotherapist (60 minutes, 3x/week for 4 weeks) 2. Control: strength training (lower limb). Laborator‐ based supervised by a physiotherapist (60 minutes, 3x/week for 4 weeks). Followed by unsupervised home‐based training with the same emphases as the laboratory‐based phase (20 minutes, 5x/week for 4 weeks). Followed by laboratory‐based supervised by a physiotherapist (60 minutes, 3x/week for 4 weeks) |
|
| Outcomes | 1. Rate of falls 2. Number of fallers 3. Number reporting a fall‐related fracture Other outcomes reported but not included in this review |
|
| Duration of the study | 15 months | |
| Funding source | SK Yee Medical Foundation (5‐ZH61) and Hong Kong Parkinson's Disease Foundation (5‐ZH76) | |
| Notes | Fall data collected: over 3, 6, and 15 months after treatment commencement | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component in the sequence generation was described. Quote: "They were randomly assigned (by drawing lots) to 1 of 2 groups:…” |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was described. Quote: "Randomization was done by a researcher who was not involved in any other aspect of the study.” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and intervention (exercise) delivery personnel not blinded to group allocation but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | Unclear risk | Unclear if personnel collecting fall information blinded to group allocation. |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Insufficient information to permit judgement. Unclear how data regarding fractures was collected. |
| Incomplete outcome data (attrition bias) Falls | High risk | See appendix for method of assessment |
| Incomplete outcome data (attrition bias) Fallers | High risk | See appendix for method of assessment |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ as unable to find a published protocol or trial registration. |
| Method of ascertaining falls (recall bias) Falls and fallers | Unclear risk | It appears that retrospective recall may have been required each month. Quote: ”Following the baseline assessment, all the subjects were contacted by phone monthly to record any fall occurrences until the end of the study period or dropout from the study during the 12‐week intervention period.” |
Smania 2010.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: facility, Italy N = 64 Sample: recruited from the PD outpatient department of the G.B. Rossi University Hospital Neurological Rehabilitation (47% women) Age (years): mean (SD) intervention group 67.6 (7.4), control group 67.3 (7.2) Inclusion criteria: idiopathic PD; HY stage 3‐4; able to rise from chairs or beds without assistance; no other neurological conditions; sufficient cognition (Mini Mental State Examination score >23) Exclusion criteria: unstable cardiovascular disease or other chronic conditions that could interfere with their safety during testing or training procedures; severe dyskinesia or “on‐off” phases. Disease severity at baseline: HY stage 3 to 4, UPDRS total score mean (SD) 44.6 (14.2) |
|
| Interventions | Exercise 1. Exercise: balance exercises. Individual treatment supervised by a physiotherapist (50 minutes, 3x/week for 7 weeks) 2. Control: exercises not specifically aimed at improving postural reactions. Individual treatment supervised by a physiotherapist (50 minutes, 3x/week for 7 weeks) |
|
| Outcomes | 1. Rate of falls Other outcomes reported but not included in this review |
|
| Duration of the study | 3 months | |
| Funding source | No funding | |
| Notes | Fall data collected: during the 4‐week baseline period, the last 4‐week intervention period and the 4‐week follow‐up period by falls diaries | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of generating the randomisation list not described. Quote: "...according to a simple randomization scheme using a randomization list locked in a desk drawer accessible only to the principal investigator..." |
| Allocation concealment (selection bias) | Unclear risk | Principal investigator’s role not described elsewhere. Quote: "…according to a simple randomization scheme using a randomization list locked in a desk drawer accessible only to the principal investigator.” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and intervention (exercise) delivery personnel not blinded to group allocation but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | Unclear risk | Unclear if personnel collecting fall information blinded to group allocation. |
| Incomplete outcome data (attrition bias) Falls | High risk | See appendix for method of assessment |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ as unable to find a published protocol or trial registration. |
| Method of ascertaining falls (recall bias) Falls and fallers | Low risk | The study used concurrent collection of data about falling with monthly, or more frequent, follow‐up by the researchers. Quote: “Each participant was requested to record any falls in a diary for 1 month prior to the start of each evaluation session.” |
Song 2018.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: home, Australia N = 60 Sample: recruited from metropolitan Sydney, via Parkinson’s disease support groups and neurology clinics (60% women) Age (years): mean (SD) intervention group 68 (7), control group 65 (7) Inclusion criteria: diagnosis of idiopathic PD; living in the community; age 40 years or older; ability to walk unaided for at least 30 metres; stable antiparkinsonian medication for at least 2 weeks Exclusion criteria: cognitive impairment (Mini‐mental State Examination score of < 24); medical conditions which would preclude or interfere with physical assessment or stepping training Disease severity at baseline: MDS‐UPDRS motor score mean (SD) 32 (12) |
|
| Interventions | Exercise 1. Exercise: home‐based stepping training exercise video game (at least 15 minutes, 3x/week for 12 weeks ‐ including 3 sessions supervised by a therapist, with two of these supervised sessions at the beginning and one in the middle of the intervention period), plus usual activities and health care 2. Control: maintain usual activities and healthcare |
|
| Outcomes | 1. Rate of falls 2. Number of fallers Other outcomes reported but not included in this review |
|
| Duration of the study | 6 months | |
| Funding source | Parkinson's New South Wales Bendigo Bank Parkinson's Research Grant and a University of Sydney Bridging Support Grant | |
| Notes | Fall data collected: during the 6‐month intervention period by monthly falls diaries | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component in the sequence generation was described. Quote: "The random allocation was conducted using a computer‐generated table with randomly permuted blocks..." |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was described as by central allocation. Quote: “The trial manager emailed the allocating researcher, who was located offsite and was not involved in recruitment, intervention or outcome assessment." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and intervention (exercise) delivery personnel not blinded to group allocation but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | Unclear risk | Unclear if personnel collecting fall information were blinded to group allocation. |
| Incomplete outcome data (attrition bias) Falls | Low risk | See appendix for method of assessment |
| Incomplete outcome data (attrition bias) Fallers | Low risk | See appendix for method of assessment |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (ACTRN12613000688785) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Method of ascertaining falls (recall bias) Falls and fallers | Low risk | The study used concurrent collection of data about falling with monthly, or more frequent, follow‐up by the researchers. |
Thaut 2019.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: home, USA N = 60 Sample: study participants were randomly selected from referral lists of local Parkinson's disease support groups and neurology practices (62% women) Age (years): Mean (SD) intervention group 71 (7), control group 73 (8) Inclusion criteria: diagnosis of idiopathic PD; HY stage 3 or 4; at least two falls in the past year; a stable antiparkinson medication regime; able to walk independently at least 50 metres. Exclusion criteria: other neurological or orthopaedic conditions; medically diagnosed hearing loss; dementia (Mini‐mental State Examination score of < 24) Disease severity at baseline: HY stage mean 3.5 (SD not reported) |
|
| Interventions | Exercise 1. Exercise: walking in a home‐based environment with rhythmic auditory stimulation via click‐embedded music. Individual, level of supervision unclear (30 minutes, 7x/week for 24 weeks) 2. Exercise: walking in a home‐based environment with rhythmic auditory stimulation via click‐embedded music. Individual, level of supervision unclear (30 minutes, 7x/week for 16 weeks; 8 weeks intervention, 8 weeks no intervention, 8 weeks intervention) All participants received standard care and optimal medical treatment during the study |
|
| Outcomes | 1. Number of fallers | |
| Duration of the study | 24 weeks | |
| Funding source | The Charlene B. Flood Memorial Fund, San Diego California | |
| Notes | Fall data collected: during the 24 week intervention period, details of ascertainment not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component in the sequence generation was described. Quote: "Subjects were randomly selected and assigned in an intent‐to‐treat design to the experimental and control conditions using a computerized random selector program." |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was described as by central allocation. Quote: "Subjects were randomly selected and assigned in an intent‐to‐treat design to the experimental and control conditions using a computerized random selector program implemented by a computer specialist external to the study to assure allocation concealment." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and intervention (exercise) delivery personnel not blinded to group allocation but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | Unclear risk | Unclear if personnel collecting fall information blinded to group allocation. Quote: "The Fall Index was computed based on self‐reports by subjects or caregivers” |
| Incomplete outcome data (attrition bias) Falls | Unclear risk | Data not available to assess. |
| Incomplete outcome data (attrition bias) Fallers | Unclear risk | Data not available to assess. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT03316365) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Method of ascertaining falls (recall bias) Falls and fallers | Unclear risk | Details of ascertainment are not described. |
Volpe 2014a.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: facility, Italy N = 40 Sample: recruited from the Neurorehabilitation Unit of ‘‘S. Raffaele Arcangelo’’ Hospital (60% women) Age (years): median (Q1; Q3) intervention group 66.5 (64.0; 78.0) control group 69.5 (65.0; 73.8) Inclusion criteria: diagnosis of PD; HY stages 2 and 3 on levodopa; ≥ 1 fall in the past year; presence of postural alterations; presence of postural instability; ability to attend a physiotherapy venue; absence of cognitive impairment (Mini‐mental State Examination > 24/30); stable medications Exclusion criteria: medication‐induced dyskinesias; presence of co‐morbidities preventing mobility or safe exercise (including clinically evident neuropathy and major medical conditions such as malignancies); history of deep brain stimulation surgery; other conditions affecting stability (e.g. poor visual acuity or vestibular dysfunction); HY stage ≥4 on levodopa; an inability to travel to the physiotherapy venues Disease severity at baseline: HY stage 2 to 3, UPDRS motor score median intervention group = 42, control group = 39.5 |
|
| Interventions | Exercise 1. Exercise: perturbation‐based balance training program wearing 3 proprioceptive devices. Individual treatment supervised by a physiotherapist (60 minutes, 5x/week for 8 weeks) 2. Control: perturbation‐based balance training program wearing 3 inactive devices. Individual treatment supervised by a physiotherapist (60 minutes, 5x/week for 8 weeks) |
|
| Outcomes | 1. Rate of falls 3. Quality of life (PDQ‐39) Other outcomes reported but not included in this review |
|
| Duration of the study | 4 months | |
| Funding source | No funding | |
| Notes | Fall data collected: at baseline, within 1 week after the intervention period and at two months after the end of treatment by falls diaries | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of generating the randomisation list not described. Quote: "A blocked stratified randomization procedure conducted by a third party and based on the Hoehn & Yahr score was used to allocate participants to one of the two treatment groups…” |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was described. Quote: "A blocked stratified randomization procedure conducted by a third party...” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and personnel implementing the intervention assured. Quote: "...patients were blinded to the group allocation during the whole duration of the study. The study coordinator responsible for WPS placing (M.G.G.) was not blinded to group allocation, but she was not involved in rehabilitation procedures or outcome assessment. The therapists providing the interventions were blinded and not involved in other aspects of the trial (i.e., aims, hypotheses or predictions of the study were not disclosed). Both active and placebo WPSs were identical and did not cause any recognizable sensory sensation, thus guarantying patients’ blindness.” |
| Blinding of outcome assessment (detection bias) Falls and fallers | Low risk | Outcomes were recorded/confirmed in all allocated groups using the same method and the personnel recording/confirming falls were blind to group allocation. Quote: "The two trained assessors and patients were blinded to the group allocation during the whole duration of the study” |
| Incomplete outcome data (attrition bias) Falls | Low risk | See appendix for method of assessment |
| Incomplete outcome data (attrition bias) Fallers | Low risk | See appendix for method of assessment |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement as unable to find a published protocol or trial registration. |
| Method of ascertaining falls (recall bias) Falls and fallers | Unclear risk | Details of ascertainment were not described. Quote: “Falls were recorded by means of fall diaries of the previous two months.” |
Volpe 2014b.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: facility, Italy N = 34 Sample: did not describe the source of patients Age (years): mean (SD) intervention group 68 (7) control group 66 (8) Inclusion criteria: diagnosis of ‘clinically probable’ idiopathic Parkinson’s disease; HY stage 2.5 and 3; ability to walk without any assistance; at least two falls in the last year; Mini‐mental State Examination score ≥ 25; no relevant comorbidity or vestibular/ visual dysfunctions, limiting locomotion or balance; stable dopaminergic therapy in the last four weeks Exclusion criteria: history of deep brain stimulation surgery and other conditions limiting hydrotherapy (for example cardio pulmonary disease). Disease severity at baseline: HY stage 2.5 to 3, UPDRS motor score mean (SD) 40.6 (10.8) |
|
| Interventions | Exercise 1. Exercise: hydrotherapy focused on perturbation‐based balance training (60 minutes, 5x/week for 8 weeks) 2. Control: land‐based treatment focused on perturbation‐based balance training (60 minutes, 5x/week for 8 weeks) |
|
| Outcomes | 1. Rate of falls 2. Number reporting a fall‐related fracture 3. Quality of life (PDQ‐39) Other outcomes reported but not included in this review |
|
| Duration of the study | 10 weeks | |
| Funding source | No funding | |
| Notes | Fall data collected: Falls which occurred two months prior to the trial and during the 2 month trial period were collected by falls diary or telephone interview | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component in the sequence generation was described. Quote: "For the allocation of the participants, a computer‐generated list of binary random numbers was used.” |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was described as by central allocation. Quote: "The sequence was concealed and the following number (0: Group 1; 1: Group 2) was disclosed by a person not involved in the enrolment process, every time a new patient was added.” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and intervention (exercise) delivery personnel not blinded to group allocation but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | Unclear risk | Unclear if personnel collecting fall information blinded to group allocation. |
| Blinding of outcome assessment (detection bias) Fractures | Unclear risk | Insufficient information to permit judgement. No fractures reported (as no injurious falls) and unclear how data regarding fractures was collected. |
| Incomplete outcome data (attrition bias) Falls | Low risk | See appendix for method of assessment |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement as unable to find a published protocol or trial registration. |
| Method of ascertaining falls (recall bias) Falls and fallers | Unclear risk | The study used some form of concurrent collection of data about falling‐ i.e. falls diaries, but frequency of follow‐up by the researchers was not reported. |
Ward 2004.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: home, UK N = 53 (PD subgroup) Sample: recruited from General Practices based within the city of Nottingham boundaries (45% women, all participants) Age (years): median (range) education group 63 (29‐89) control group 65 (22‐86), all participants Inclusion criteria: aged over 15 years with one of the following possible recorded diagnoses: PD and other causes of progressive parkinsonism, multiple sclerosis, motor neurone disease, Huntington's disease and other degenerative disorders affecting the central nervous system, muscles or peripheral nerves (note ‐ only the PD subgroup was included in this review) Exclusion criteria: dementing disorders such as Alzheimer's Disease; clinical features appeared incompatible with the recorded diagnosis; neurological complications of primarily non‐neurological conditions such as diabetes mellitus; additional causes of severe disability Disease severity at baseline: not reported |
|
| Interventions | Health education, including falls prevention 1. Education: education visit from the research occupational therapist (OT) to provide personalized advice and information based on a multidisciplinary expert panel discussion, a tailored version of the standard information package, and a leaflet offering information about the participant's condition and about self‐help organisations. Plus, an action plan most likely to promote each individual's physical, social and psychological well‐being, taking into account their risk of falls. Plus, a single follow‐through phone call from the OT to confirm and reinforce the educational content of the visit 2. Control: information visit from the OT to provide standardised printed information package on generic services and condition‐specific self‐help organisations. Participants raising any specific queries during the information visit were advised to consult routine sources of advice |
|
| Outcomes | 1. Number of fallers Other outcomes reported but not included in this review |
|
| Duration of the study | 12 months | |
| Funding source | Department of Health Policy Research Program | |
| Notes | Fall data collected: at baseline and 12‐month follow‐up by two‐monthly phone calls | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component in the sequence generation was described. Quote: "Each participant was allocated consecutively to a group by consulting a computer‐generated random number series.” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. Quote: "Following a baseline assessment visit from a trained interviewer with no health or social care qualifications, participants were randomized to either the education group (EG) or the comparison group (CoG)." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and intervention (education) delivery personnel not blinded to group allocation but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | Unclear risk | Unclear if personnel collecting fall information blinded to group allocation. |
| Incomplete outcome data (attrition bias) Fallers | Unclear risk | Data not available to assess. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement as unable to find a published protocol or trial registration. |
| Method of ascertaining falls (recall bias) Falls and fallers | High risk | Ascertainment relied on participant recall at longer intervals than one month during the study. Quote: “...falls reported at two monthly phone calls during 12 months of follow‐up.” |
Wong‐Yu 2015.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Setting: facility and home, Hong Kong N = 70 Sample: recruited from the Hong Kong PD Association (a patient self‐help group) and movement disorder clinics (43% women) Age (years): mean (SD) intervention group 60.2 (9), control group 61.9 (8.5) Inclusion criteria: diagnosis of PD according to the United Kingdom PD Brain Bank Criteria; at least 30 years of age; stable on anti‐PD medications; no fall history in the previous 6 months; could walk 30mutes with or without a cane Exclusion criteria: musculoskeletal or cardiopulmonary disorders; had undergone neurosurgery; neurologic conditions other than PD; cognitive deficits on the Mini‐mental State Examination (<24); had joined another exercise program in the previous 3 months. Disease severity at baseline: HY stage mean (SD) 2.4 (0.3), MDS‐UPDRS motor score mean (SD) 29.7 (10.6) |
|
| Interventions | Exercise 1. Exercise: task‐ and context‐specific multisystem balance program and lower limb strength training. Group supervised by a physiotherapist and an assistant (120 minutes, 1x/week for 8 weeks). Plus, home exercise guided by handouts and DVDs (3 hours/week) 2. Control: upper limb training. Group supervised by a physiotherapist and an assistant (120 minutes, 1x/week for 8 weeks). Plus, home exercise guided by handouts and DVDs (3 hours/week) |
|
| Outcomes | 1. Rate of falls 2. Number of fallers Other outcomes reported but not included in this review |
|
| Duration of the study | 8 months | |
| Funding source | Hong Kong Parkinson's Disease Foundation (no. 8‐ZH89). | |
| Notes | Fall data collected: at 1‐week pre‐training, immediately post‐training and at the 6‐month post‐training follow‐up by fall diaries |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component in the sequence generation was described. Quote: "Before the baseline assessment, a team member not involved in this study used the Research Randomizer to make a randomized assignment of eligible participants into either a balance (BAL) or an active control (CON) group.” |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was described as by central allocation. Quote: "Before the baseline assessment, a team member not involved in this study used the Research Randomizer to make a randomized assignment of eligible participants into either a balance (BAL) or an active control (CON) group.” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and intervention (exercise) delivery personnel not blinded to group allocation but impact of non‐blinding unclear. |
| Blinding of outcome assessment (detection bias) Falls and fallers | Unclear risk | Unclear if personnel collecting fall information blinded to group allocation. |
| Incomplete outcome data (attrition bias) Falls | Low risk | See appendix for method of assessment |
| Incomplete outcome data (attrition bias) Fallers | Low risk | See appendix for method of assessment |
| Selective reporting (reporting bias) | High risk | The study protocol is available (NCT01799681) and pre‐specified outcomes of interest (falls and fallers) were specified to be reported over 12 months, but have been reported over 6 months. A pre‐specified secondary outcome of interest (PDQ‐39) has not been reported. |
| Method of ascertaining falls (recall bias) Falls and fallers | Unclear risk | The study used concurrent collection of data about falling however it is unclear if there was any follow‐up by the researchers. Quote: “Fall diaries were provided, and subjects were instructed to complete a standard form on the date and location of the fall, fall activities, landing body parts, perceived causes, and related injuries, as soon as possible after each fall event.” |
CNS: central nervous system; DSM IV: Diagnostic and Statistical Manual of Mental Disorders, fourth edition;HY stage: Hoehn and Yahr Stage; MDS‐UPDRS: Movement Disorders Society Sponsored Revision of the Unified Parkinson's disease Rating Scale;PD: Parkinson's disease; RCT: randomised controlled trial; QALY: quality‐adjusted life years: SF36: Short Form 36; SD: standard deviation; UPDRS: Unified Parkinson's Disease Rating Scale;
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allen 2010 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Bevilacqua 2020 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Bueno 2017 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Cakit 2007 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Calabro 2019 | No falls reported. |
| Celiker 2018 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Chang 2019 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Cherup 2019 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Chomiak 2017 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Chou 2017 | Not RCT. |
| Citrome 2018 | No falls reported. |
| Cosentino 2013 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Cummings 2013 | Not idiopathic Parkinson's disease participants. |
| da Silva 2019 | No falls reported. |
| Deepa 2019 | Not RCT. |
| de Lucena 2017 | No falls reported. |
| de Natale 2017 | Not RCT. |
| Duncan 2018 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Elmer 2018 | No falls reported. |
| El‐Tamawy 2013 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Emre 2010 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Galli 2018 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Geroin 2018 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Giardini 2018 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Giladi 2013 | Not RCT. |
| Grobbelaar 2017 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Gu 2013 | No falls reported. |
| Gurevich 2007 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Hackney 2007 | No falls reported. |
| Hauser 2013 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Hauser 2016 | Intervention (droxidopa medication) targets syncopal falls. |
| Hawkins 2018 | No falls reported. |
| Hewitt 2018 | Not idiopathic Parkinson's disease. |
| Hill 2015 | Separate data for the participants with Parkinson's disease not available. |
| Hiller 2018 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Hubble 2018 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Hubble 2019 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Kalyani 2020 | Not RCT. |
| Kanegusuku 2017 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Klamroth 2019 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Kurlan 2015 | No falls reported. |
| Lang 2016 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Lees 2017 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| LeWitt 2019 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Li 2019 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Lieberman 2019 | No falls reported. |
| Litvinenko 2007 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Litvinenko 2008 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Mancini 2019 | No falls reported. |
| Marumoto 2019 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| McDonald 2018 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Mezzarobba 2018 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Mi 2019 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Miller 2019 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Moro 2010 | No falls reported. |
| Myers 2019 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Negrini 2017 | Not RCT. |
| Nieuwboer 2007 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Oertel 2013 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Okun 2012 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Olanow 2020 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Ozgonenel 2016 | Not RCT. |
| Perez 2017 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Pohl 2020 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Postuma 2008 | Not RCT. |
| Rascol 2016 | Not RCT. |
| Rawson 2019 | Not RCT. |
| Sato 2011 | Publication of the trial retracted by the journal due to concerns regarding the integrity of the data. |
| Sato 2013 | Not RCT. |
| Schenkman 2018 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Scianni 2015 | Not RCT. |
| Sedaghati 2018 | Not RCT. |
| Silva‐Batista 2018 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Simuni 2020 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Sparrow 2016 | Randomised cross‐over trial that did not collect falls data during the control period. |
| St George 2015 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Stozek 2003 | No falls reported. |
| Strouwen 2017 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Thevathasan 2010 | Not RCT. |
| Toole 2005 | No falls reported. |
| van Nimwegen 2013 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Van Puymbroeck 2018 | No falls reported. |
| Vercruysse 2014 | Not RCT. |
| Walter 2019 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Wass 2008 | Not RCT. |
| Welter 2015 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Whone 2019 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
| Wong 2016 | Not RCT. |
| Yuan 2020 | No falls reported. |
| Zhang 2018 | Intervention not aiming to reduce falls in people with Parkinson's disease. |
RCT: randomiused controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
Lurie 2020.
| Methods | RCT |
| Participants | Setting: multicentre across 8 outpatient physiotherapy clinics in the USA. N = 66 in the PD subgroup Sample: recruited from patients referred for physiotherapy for gait and/or balance problems at participating clinics. Age: not reported for the PD group. Inclusion criteria (PD group): aged 65 years or older; one of the following fall risk factors, timed up and go ≥ 8 seconds, Dynamic Gait Index ≤ 22/24, Berg BalancecScale < 54/56. Exclusion criteria: primary problem positional vertigo; not able to undertake intervention due to severe physical limitations Disease severity at baseline: not reported |
| Interventions | Exercise 1. Exercise: surface perturbation treadmill training plus multi‐modal balance training. For the perturbation training, participants wore a harness and practised responding to perturbations (forwards, backwards and occasionally sideways) using the ActiveStep system. Multimodal balance training included strength, flexibility and balance exercise, gait training and education. Participants attended a supervised session around 2 to 3 times per week for 4 to 6 weeks, approximately 45 minutes per session with 15 minutes of this perturbation training, plus home unsupervised sessions 4 to 5 times per week. 2. Exercise: multi‐modal balance training alone, including strength, flexibility and balance exercise, gait training and education. Participants attended a supervised session around 2 to 3 times per week for 4 to 6 weeks, approximately 45 minutes per session, plus home unsupervised sessions 4 to 5 times per week. |
| Outcomes | 1. Number of fallers Other outcomes reported but not included in this review |
| Notes | At randomisation, 34 participants were in the perturbation group and 32 in the standard care group. At 3 months there was data for 24 participants in the perturbation group and 25 in the standard care group. Participants having any fall at 3 months: 9 (37%) perturbation group; 8 (32%) standard care group. Fall data collected using a fall diary and 3‐monthly telephone calls for 12 months. Funding source: Agency for Healthcare Research and Quality |
Taylor 2021.
| Methods | RCT |
| Participants | Setting: two hospitals in the UK. N = 64 Sample: recruited through Parkinson's Society and other local publicity (28% women) Age (years): Mean (SD) intervention group 69.3 (8.7), control group 71.3 (7.8) Inclusion criteria: ≥ 18 years; idiopathic PD; Hoehn and Yahr stage 1 to 4; bradykinesia demonstrated by slow gait over 10 metres at < 1.25 ms‐1; gait abnormality (e.g. reduced stride length); able to walk 10 metres independently with or without an aid; able to stand up from sitting independently; medically stable; able to understand and comply with assessment and intervention Exclusion criteria: treatments other than usual PD medications; uncontrolled epilepsy; pregnancy; active medical implanted devices; other neurological causes of 'dropped foot'; severe osteoarticular pathology; malignancy; dermatological problems in the area of electrode placement; significant cognitive impairment Disease severity at baseline: Hoehn and Yahr stage 1 to 4, mean (SD) 2.4 (0.8) |
| Interventions | FES 1. FES: FES to the common peroneal nerve of one leg, set up to correct any problems with dorsiflexion and eversion during walking. The device was worn daily when walking for 18 weeks. The intervention was in addition to standard care. 2. Control: standard care including medical care, specialist nurses and exercise classes. |
| Outcomes | 1. Rate of falls 2. Number of fallers 3. Quality of life Other outcomes reported but not included in this review |
| Notes | Falls data collected at 18 weeks and 22 weeks by falls diaries Number of falls during the intervention (0 to 18 weeks), median (IQR): FES 3.0 (10.8); control 2.0 (3.0) Number of falls during follow‐up (18 to 22 weeks), median (IQR): FES 0.0 (2.7); control 0.0 (1.3) Number of people who fell during the intervention (0 to 18 weeks): FES 14 (61%); control 17 (63%) Number of people who fell during follow‐up (18 to 22 weeks): FES 11 (42%); control 10 (42%) Funding source: National Institute for Health Research, Research for Patient Benefit funding stream. |
FES: functional electrical stimulation; IQR: interquartile range; PD: Parkinson's diease; RCT: randomised controlled trial; SD: standard deviation.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12618001515280.
| Study name | SAFE‐PD ‐ Stepping to Avoid Fall Events in Parkinson’s disease |
| Methods | RCT |
| Participants | Target sample size: 44 Inclusion criteria: diagnosed with Parkinson’s disease (according to UK PD Society Brain Bank Criteria); stable on anti‐Parkinsonian medications for at least 1 month; Living independently in the community or retirement village; able to communicate in English language. Exclusion criteria: Hoehn & Yahr stage >3; diagnosis of other neurological and/or significant cognitive impairments (Montreal Cognitive Assessment (MOCA) < 19); atypical Parkinsonism; inability to stand or walk 30 m without assistance; less than 6 months post deep brain stimulation surgery; medical conditions which would preclude physical assessment or training using perturbation (e.g. duodopa); history of weekly (12+) falls in past 3 months. |
| Interventions | 1. Volitional and reactive step training using "home‐based exergames" for 80+ minutes per week (3 or 4 sessions per week) for 12 weeks. Participants will also visit the research laboratory to undertake three individual sessions (one per month, 120 minutes in total) with each session focusing on balance recovery progressively from 1) slips, 2) trips and 3) mix of trips and slips. 2. Control: Usual activities |
| Outcomes | 1. Rate of falls Other outcomes not relevant to this review |
| Starting date | September 11, 2018 |
| Contact information | Prof Stephen Lord, Neuroscience Research Australia, Email: s.lord@neura.edu.au |
| Notes |
ACTRN12619000415101.
| Study name | The Integrate program for safe mobility in Parkinson's disease |
| Methods | RCT |
| Participants | Target sample size: 40 Inclusion criteria: at least 2 falls in the prior 6 months; no change in Parkinson's Disease medications 2 weeks prior to commencing the study; ability to walk independently at least 10 metres with or without an aid; participants with significant cognitive impairment (Montreal Cognitive assessment <19 or a level of functional cognition that the researchers deem requires assistance to participate) will require a care partner who is willing to participate with them to assist them with the intervention. Exclusion criteria: medical conditions which would preclude or interfere with study safety and conduct; severe cognitive impairment (Montreal Cognitive Assessment < 5 ). |
| Interventions | 1.A multifactorial home‐based program designed to improve safe mobility and reduce falls in people with Parkinson's disease, consisting of environmental modification, behavioral modification and exercise. Participants will receive 8‐12 therapy home visits (physiotherapy/occupational therapy) over a 6 month period depending on their need. 2. Control usual care |
| Outcomes | 1. Rate of falls 2. Number of fallers Other outcomes not relevant to this review |
| Starting date | July 1, 2019 |
| Contact information | Dr Natalie Allen The University of Sydney, Australia Email: natalie.allen@sydney.edu.au |
| Notes |
ACTRN12620001135909.
| Study name | A Randomised trial of exercise therapy for Parkinson’s disease |
| Methods | RCT |
| Participants | Target sample size: 16 Inclusion criteria: Parkinson’s disease, Modified Hoehn & Yahr stage 3 or less when tested ON, age 30‐75 years, sedentary lifestyle (low levels of aerobic physical activity, defined by the American College of Sports Medicine recommendation for older adults as any level below recommended weekly amount of aerobic exercise), receiving a stable dopaminergic medication dose for at least one month before the study, or else De‐novo – not receiving PD medication. Exclusion criteria: judged unsafe to exercise by medical practitioner, taking beta‐blockers, taking anti‐psychotics, unable to cycle, use a treadmill or perform stretching exercises due to neurological conditions or co‐morbidities, unable to fill out questionnaires due to poor vision or other reasons, unable to independently transport self to the exercise venue, unable to read, psychiatric conditions or major depression, Mini Mental Status Examination score of less than 24, contra‐indications to aerobic exercise, such as diagnosed cardiac diseases (e.g. unstable angina, heart block, arrhythmia’s, uncontrolled hypertension), poorly controlled diabetes. |
| Interventions | 1. Multimodal exercise program supervised at a clinic including strength training, aerobic exercise at a moderate intensity, balance training and falls education, 60 minutes, 2 times per week for 3 months. 2. Control: stretching, flexibility and relaxation exercises and falls education independently at home, 60 minutes, 2 times per week for 3 months. |
| Outcomes | 1. Rate of falls 2. Number of fallers 3. Health‐related quality of life (PDQ39) Other outcomes not relevant to this review |
| Starting date | September 11, 2020 |
| Contact information | Prof Meg Morris La Trobe University, Australia Email:m.morris@latrobe.edu.au |
| Notes |
ChiCTR2000038852.
| Study name | Study on the effect and mechanism of cognitive‐cup‐tapping‐balance‐training on fall prevention in community Parkinson's patients: a randomized controlled trial |
| Methods | RCT |
| Participants | Target sample size including subset of people with Parkinson's disease: 87 Inclusion for participants with Parkinson's disease: Parkinson's disease diagnosed more than 6 months prior, aged between 40 and 80 years; Hoehn and Yahr stage 1 to 4, stable response to anti‐Parkinson's drugs, able to walk independently at least 30m with or without walking aids, normal vision. Exclusion criteria for participants with Parkinson's disease: Mini Mental State examination score < 24, undergone neurosurgical procedures such as deep brain stimulation, musculoskeletal problems, cardiopulmonary diseases or other neurological diseases that may affect balance or exercise, lower extremity peripheral neuropathy, severe hearing or language impairment leading to an inability to understand commands and express needs, impaired visual function (such as significantly reduced colour resolution, contrast sensitivity, spatial resolution, etc.) or visual hallucinations after medication. |
| Interventions | 1. Dual task training involving cognitive cup‐tapping balance training. 2. Single task training involving cup tapping balance training 3. Control: education |
| Outcomes | 1. Number of fallers Other outcomes not relevant to this review |
| Starting date | October 20, 2020 |
| Contact information | Prof Jia Han Shanghai University of Sport Email: Jia.Han@canberra.edu.au |
| Notes |
DRKS00024982.
| Study name | Effects of an activity‐oriented physiotherapy exercise programme with and without eye movement training on dynamic balance and fall risk in people with Parkinson’s disease: a randomised controlled pilot trial |
| Methods | RCT |
| Participants | Target sample size: 46 Inclusion criteria: idiopathic Parkinson's disease, Hoehn & Yahr stage 1‐3 when ON medication, aged 30‐80 years, able to walk independently, mini‐mental state exam score ≥ 24/30, stable dopaminergic medication for at least 3 weeks, German speaking and writing. Exclusion criteria: concomitant diseases, photosensitivity, gait disorder for reasons other than Parkinson's disease, recent surgery, intraocular implants, strabismus, nystagmus, severe drooping eyelids, untreated pain, uncorrected visual or hearing impairment, pregnancy, recent deep brain stimulation or a change in DBS parameters within the previous year, severe motor fluctuations, initiation of a new dopaminergic medication or planned adjustment thereof within the study period. |
| Interventions | 1. Activity oriented exercise program plus eye movement training, 30 minutes, 4 times per week for 4 weeks. 2. Activity oriented exercise program without eye movement training, 30 minutes, 4 times per week for 4 weeks. |
| Outcomes | 1. Rate of falls 2. Health‐related quality of life (PDQ39) Other outcomes not relevant to this review |
| Starting date | April 25, 2021 |
| Contact information | Dr Barbara Seebacher Reha Zentrum Münster, Austria Email: barbara.seebacher@reha‐muenster.at |
| Notes |
NCT02107638.
| Study name | Effect of osteopathic manipulative medicine on Parkinson disease |
| Methods | RCT |
| Participants | Target sample size: 50 Inclusion criteria: diagnosed with PD, over 40 years old. Exclusion criteria: no diagnosis of PD, presence of other diagnosed neurological diseases or disorders, wheelchair bound or presence of physical deformities that would prevent completion of the assessment tools |
| Interventions | 1. Osteopathic manipulative medicine, twice per week for 6 weeks 2. Counseling on PD‐related issues, once per week for 6 weeks (face to face time matched with intervention group) |
| Outcomes | 1. Fall rate Other outcomes not relevant to this review |
| Starting date | April 15, 2014 |
| Contact information | Sheldon Yao New York Institiute of Technology, USA Email: cmomm1@nyit.edu |
| Notes |
NCT03727529.
| Study name | Immersive virtual reality to improve gait in Parkinson's disease (NMSK‐LH02) |
| Methods | RCT |
| Participants | Target sample size: 46 Inclusion criteria: PD diagnosis according to UK Brain bank criteria, Hoehn and Yahr score of 1 to 3, optimal drug treatment for at least 4 weeks at the time of inclusion, in ON phase during assessments and treatment sessions Exclusion criteria: other pathologies that increase risk of falling, other pathologies that increase risk of nausea and vertigo, contraindication to physical exercise, freezing of gait |
| Interventions | 1. Treadmill walking wearing a virtual reality headset with a simple, virtual environment 2. Treadmill walking alone |
| Outcomes | 1. Rate of falls 2. Health‐related quality of life (PDQ39) Other outcomes not relevant to this review |
| Starting date | January 30, 2019 |
| Contact information | Dr Alexis Lheureux, Cliniques Universitaires Saint Luc, Belgium Email: alexis.lheureux@uclouvain.be |
| Notes |
NCT03751371.
| Study name | Robotic walking device to improve mobility in Parkinson's disease |
| Methods | RCT |
| Participants | Target sample size: 46 Inclusion criteria: diagnosis of idiopathic PD, aged 50 to 80 years, able to ambulate without assistance (Hoehn & Yahr stages 1‐3), on stable doses of Parkinson's medications for at least 4 weeks prior to the study Exclusion criteria: other significant cardiac, neurological or orthopedic problems that affect gait, weight more than 220 pounds and height greater than 6'8", electronic medical devices embedded in the body, participating in any physical therapy, unable to understand instructions required by the study |
| Interventions | 1. Home‐ and community‐based training with Honda Walking Assist device 2 times per week for 45 to 60 minutes for 8 weeks 2. Usual care control |
| Outcomes | 1. Number of falls as measured by accelerometers 2. Number of adverse events (including falls) during training Other outcomes not relevant to this review |
| Starting date | May 15, 2019 |
| Contact information | Raquel Minarsch The Ohio State University, Ohio, USA Email: raquel.minarsch@osumc.edu |
| Notes |
NCT03972969.
| Study name | Highly challenging balance program to reduce fall rate in PD |
| Methods | RCT |
| Participants | Target sample size: 162 Inclusion criteria: physician diagnosed idiopathic PD, at least 2 of the 3 cardinal signs of PD (resting tremor, rigidity, bradykinesia), response to dopaminergic medication Exclusion criteria: angina pectoris, history of myocardial infarction within 6 months, history of ventricular dysrhythmia requiring current therapy |
| Interventions | 1: Facility‐based structured exercise with instruction and encouragement for 3 months 2: Home‐based structured exercise with instruction and encouragement for 3 months 3. Control: general health education for 3 months |
| Outcomes | 1. Fall rates Other outcomes not relevant to this review |
| Starting date | October 1, 2019 |
| Contact information | David Sparrow VA Boston Healthcare System Jamaica Plain Campus, Jamaica Plain, Massachusetts, USA Email: david.sparrow@va.gov |
| Notes |
NCT04093544.
| Study name | Expanding the therapeutic window of deep brain stimulation in Parkinson's disease by means of directional leads |
| Methods | Randomised cross‐over trial |
| Participants | Target sample size: 20 Inclusion criteria: diagnosis of PD according to the British Parkinson's Disease Society Brain Bank criteria, who fulfilled the inclusion and exclusion criteria proposed by the core assessment programme for surgical interventional therapies in PD panel, symptoms responsive to L‐ dopa medications, but who have significant impairment related to PD that is no longer well controlled with pharmacotherapy, considered as subthalamic nucleus deep brain stimulation (STN‐DBS) candidates as per current standard of care, aged 18 to 80 years, quality of life and social functioning influenced by levodopa‐responsive symptoms, no major comorbidities Exclusion criteria: people with other significant neurologic or psychiatric illnesses or cognitive deficit |
| Interventions | 1. Stimulation using the best segmented (steered) contacts 2. Stimulation using the best contact combination in ring mode (control) |
| Outcomes | 1. Rate of falls 2. Number/incidence of adverse events 3. Health‐related quality of life (PDQ39) Other outcomes not relevant to this review |
| Starting date | May 15, 2018 |
| Contact information | Prof Alfonso Fasano, University of Toronto, Canada Email: alfonso.fasano@uhn.ca |
| Notes |
NCT04108741.
| Study name | Augmented reality treadmill training in patients with Parkinson's disease (Falls in PD) |
| Methods | RCT |
| Participants | Target sample size: 32 Inclusion criteria: ability to provide informed consent, PD without dementia or hallucinations, at least one fall within the past 3 months or postural instability, gait disorder, Hoehn and Yahr stage II to IV, able to perform the treadmill therapy. Exclusion criteria: contraindications to treadmill training, dementia (Montreal cognitive assessment < 20) |
| Interventions | 1. Augmented reality treadmill training, for 30 minutes, 3 days per week for 3 weeks 2. Treadmill training, for 30 minutes, 3 times per week for 3 weeks |
| Outcomes | 1. Fall rate Other outcomes not relevant to this review |
| Starting date | March 15, 2020 |
| Contact information | Prof Veit Mylius, Klinik Valens, Saint Gallen, Switzerland Email: veit.mylius@kliniken‐valens.ch |
| Notes |
NCT04116177.
| Study name | Flexible vs. standard deep brain stimulation programming in Parkinson disease patients |
| Methods | Randomised crossover trial |
| Participants | Target sample size: 10 Inclusion criteria: diagnosis of PD according to the British Parkinson's Disease Society, fulfil the inclusion and exclusion criteria proposed by the core assessment programme for surgical interventional therapies in PD panel, symptoms responsive to L‐dopa medications, but who have significant impairment related to PD that is no longer well controlled with pharmacotherapy, considered as subthalamic nucleus‐DBS candidates as per current standard of care, quality of life and social functioning influenced by levodopa‐responsive signs, no major comorbidities. Exclusion criteria: people with other significant neurologic or psychiatric illnesses or cognitive deficit |
| Interventions | 1. Flexible subthalamic nucleus stimulation using all available stimulation strategies provided by the VerciseTM system including stimulation of contacts 1‐8 and variable pulse width and frequency 2. Control: standard subthalamic nucleus stimulation using contact 3‐6 to achieve best therapeutic stimulation |
| Outcomes | 1. Rate of falls 2. Health‐related quality of life (PDQ39) Other outcomes not relevant to this review |
| Starting date | September 2016 |
| Contact information | Prof Alfonso Fasano, University of Toronto, Canada Email: alfonso.fasano@uhn.ca |
| Notes |
NCT04226248.
| Study name | CHIEF PD (CHolinesterase Inhibitor to prEvent Falls in Parkinson's Disease) |
| Methods | RCT, Phase III |
| Participants | Target sample size: 600 Inclusion criteria: diagnosis of idiopathic Parkinson's disease, Modified Hoehn and Yahr stage 1‐4 disease, have experienced a fall in the previous year, able to walk ≥10m without aids or assistance, over 18 years of age. Exclusion criteria: previous cholinesterase inhibitor use in 12 months prior to enrolment, hypersensitivity to rivastigmine, dementia diagnosed according to MDS criteria, inability to attend or comply with treatment or follow‐up scheduling, non‐English‐speaking, Falling 4 or more times per day, unwillingness to use an acceptable method of contraception for the duration of the trial if they are of childbearing potential, pregnancy and/or breastfeeding |
| Interventions | 1. Rivastigmine transdermal patch for 12 months. 2. Placebo matched transdermal patch for 12 months |
| Outcomes | 1. Fall rate 2. Health‐related quality of life (EuroQoL 5D‐5L health status questionnaire) 3. Cost‐effectiveness by NGS resource use Other outcomes not relevant to this review |
| Starting date | January 2, 2020 |
| Contact information | Dr Sandra Neumann, Royal United Hospitals Bath NHS Foundation Trust, UK, Email: chief‐pd@bristol.ac.uk |
| Notes |
NCT04300023.
| Study name | In‐home cycling for individuals with PD |
| Methods | RCT |
| Participants | Target sample size: 52 (40 in study 1 and 12 in study 2) Inclusion criteria: diagnosis of idiopathic PD, vision at not corrected to 20/40 or better, able to walk at least 10m continuously, no reported vestibular or neurological disease other than PD, score of at least 78 on the telephone adaptation of the modified mini‐mental state exam, English speaking. Exclusion criteria: contraindications to exercise, history of muscular or orthopaedic diagnosis, inability to participate in the full duration of the study, currently exercising for 20 or more minutes per week. |
| Interventions | Study 1: 1. Cycling group (30 minute sessions on exercise bike at home while engaged in social interaction with researcher) for 6 months 2. Wait list control Study 2: 1. Social cycling group (30 minute sessions on exercise bike at home while engaged in social interaction with researcher) for 6 months 2. Solo cycling (30 minute sessions on exercise bike at home) for 6 months |
| Outcomes | 1. Change in fall History using the Fall history Questionnaire Other outcomes not relevant to this review |
| Starting date | October 2020 |
| Contact information | Dr Kristen Pickett University of Wisconsin, Madison, USA Email: kristen.pickett@wisc.edu |
| Notes |
NCT04300348.
| Study name | Improving walking with Heel‐To‐Toe device |
| Methods | RCT |
| Participants | Target sample size: 40 Inclusion criteria: Parkinson's disease, able to walk independently without a walking aid. Exclusion criteria: exercising three or more time per week, any additional illness that restricted function, difficulty reading, understanding or speaking either French or English. |
| Interventions | 1. Walking with auditory feedback using a device triggered by a strong heel strike, 10 minutes per day for 3 months plus a workbook of simple exercises aimed to improve walking. 2. Walking without auditory feedback, 10 minutes per day for 3 months, plus a workbook of simple exercises aimed to improve walking. The control group will wear the same device, but it won't provide auditory cues. |
| Outcomes | 1. Rate of falls
2. Health‐related quality of life (EQ‐5D‐3L) Other outcomes not relevant to this review |
| Starting date | February 15, 2021 |
| Contact information | Ahmed Abou‐Sharkh McGill University, Canada Email: ahmed.abou‐sharkh@mail.mcgill.ca |
| Notes |
NCT04389138.
| Study name | Is Physiotherapy Effective for People with Early Parkinson's (PEEP) |
| Methods | RCT |
| Participants | Target sample size: 40 Inclusion criteria: PD diagnosed < 4 years, PD diagnosis as per UK Brain bank criteria, aged 18 years or over, willingness to attend physiotherapy, ability to transfer and walk independently, stable PD medication (not commenced or altered in last 2 months, or not yet on medication), changes to PD medication not planned in next 6 months. Exclusion criteria: Hoehn‐Yahr stage 4 to 5, lacking capacity to consent, meets criteria for commencement of the Gold Standards Framework, more than 1 fall in the prior 3 months, freezing of gait, already had outpatient or community physiotherapy for PD. |
| Interventions | 1. Physiotherapy intervention based on the European Physiotherapy Guideline for Parkinson's disease, 1 assessment and 4 therapy sessions delivered over 6 months. 2. Control group: usual care |
| Outcomes | 1. Fall rate 2. Health‐related quality of life (PDQ39) Other outcomes not relevant to this review |
| Starting date | May 2020 |
| Contact information | Robert Skelly University Hospitals of Derby and Burton NHS Foundation Trust, UK Email: rob.skelly@nhs.net |
| Notes |
NCT04408573.
| Study name | Cycling deep brain stimulation on Parkinson's disease gait (DBS) |
| Methods | randomised cross‐over trial |
| Participants | Target sample size: 30 Inclusion criteria: diagnosis of idiopathic PD, currently receiving deep brain stimulation as a PD treatment, Hoehn & Yahr stage between 2‐4 during off‐medication, underlying gait disorders despite optimal medical and stimulation treatment, willingness to comply with all study procedures Exclusion criteria: active moderate/severe psychiatric condition, active infection or other uncontrolled moderate/grave comorbidities, treatment with experimental drug, pregnancy or breast‐feeding |
| Interventions | 1. Two weeks of regular continuous high frequency (>130Hz) stimulation, 2 weeks of cycling high frequency (>130Hz) stimulation (40 seconds on, 2 seconds off), 2 weeks of low‐frequency (80Hz) continuous stimulation and 2 weeks of cycling low frequency (80Hz) stimulation (40 seconds on, 2seconds off) 2. Control: regular continuous high‐frequency stimulation |
| Outcomes | 1. Rate of falls 2. Health‐related quality of life (PDQ39) Other outcomes not relevant to this review |
| Starting date | June 19, 2020 |
| Contact information | Dr Rubens G Cury, University of Sao Paulo General Hospital, Email: rubens_cury@usp.br |
| Notes |
NCT04555720.
| Study name | The Benchmark Clinic: an interdisciplinary comprehensive care model for people with Parkinson disease |
| Methods | RCT |
| Participants | Target sample size: 200 Inclusion criteria: Parkinson's disease, over 30 years, caregiver willing to participate as well as able to provide consent. Exclusion criteria: atypical Parkinsonism. |
| Interventions | 1. Interdisciplinary care including social work, physical therapy, occupational therapy, speech therapy and pharmacy with a single clinic visit including development of a treatment plan in conjunction with the treating doctor. 2. Control: usual care, with standard neurologist appointment |
| Outcomes | 1. Rate of falls Other outcomes not relevant to this review |
| Starting date | February 3, 2021 |
| Contact information | Dr Kyle Mitchell Duke University, North Carolina, United States Email: kyle.mitchell@duke.edu |
| Notes |
NCT04613141.
| Study name | The WalkingTall Study: comparing WalkingTall with Parkinson's Disease (WalkingTall‐PD) with mobility‐plus to reduce falls and improve mobility. (WalkingTall‐PD) |
| Methods | RCT |
| Participants | Target sample size: 60 Inclusion criteria: idiopathic Parkinson's disease, Hoehn and Yahr stage 1‐4, ability to walk 18 meters with or without an aid, at least one fall in the past 6 months, or at least 2 falls in the past 12 months, or severe mobility impairment such as freezing of gait, or history of near falls, being stable on anti‐Parkinsonian medications for > 1 month, living independently in the community or retirement village, able to communicate in English language. Exclusion criteria: other neurological and/ or significant cognitive impairments (Montreal Cognitive Assessment < 19 points), atypical Parkinsonism, less than 6 months post deep brain stimulation surgery, > 12 falls in the past 6 months, insufficient foot/ ankle sensation, unable to speak English, another medical condition besides Parkinson's disease that significantly impairs mobility, balance or ability to exercise safely, participating in a different study to improve mobility or prevent falls. |
| Interventions | 1. Walking Tall‐PD program involving smart socks that deliver haptic stimuli timed with preferred cadence and auditory cues via a smartphone app.This is combined with stepping, walking and balance training via the app. 2. Control: Sham exercise using non‐slip socks and a paper‐based low intensity exercise program plus Parkinson's disease health information. |
| Outcomes | 1. Rate of falls 2. Health‐related quality of life (EQ‐5D) Other outcomes not relevant to this review |
| Starting date | July 15, 2021 |
| Contact information | Dr Matthew Brodie Neuroscience Research Australia Email: a.m.brodie@unsw.edu.au |
| Notes |
NCT04634331.
| Study name | Dual‐task Augmented Reality Treatment for Parkinson's disease (DART) |
| Methods | RCT |
| Participants | Target sample size: 50 Inclusion criteria: idiopathic Parkinson's disease, self‐reported gait or balance deficits, Hoehn and Yahr stage 1‐3, Ability to walk >10 minutes continuously. Exclusion criteria: dementia or any neurocognitive impairment that compromises the ability to provide informed consent, >2 errors on the Short Portable Mental Status Questionnaire, deep brain stimulation, musculoskeletal or cardiopulmonary issue that limits ability to engage in exercise, neurological disease other than Parkinson's disease that impacts motor or cognitive function. |
| Interventions | 1. Augmented reality multi‐modal training administered using an augmented reality headset, 2 times per week for 8 weeks. 2. Traditional multimodal training, 2 times per week for 8 weeks. |
| Outcomes | 1. Rate of falls Other outcomes not relevant to this review |
| Starting date | December 10, 2020 |
| Contact information | Ryan Kaya Cleveland Clinic, Cleveland, United States Email: KAYAR@ccf.org |
| Notes |
NCT04665869.
| Study name | Long‐term effects of combined balance and brisk walking in Parkinson's disease |
| Methods | RCT |
| Participants | Target sample size: 70 Inclusion criteria: Parkinson's disease, Hoehn and Yahr stage 2 or 3, able to walk 30 metres. Exclusion criteria: neurological condition (other than Parkinson's disease), musculoskeletal conditions affecting gait, balance or function, deep brain stimulation, cognitive impairment with Montreal Cognitive Assessment score <24, on‐off motor fluctuations. |
| Interventions | 1. Combined balance and brisk walking program for 90 minutes, 2 to 3 times per week. Group supervision provided weekly for weeks 1 to 6, then monthly for weeks 7 to 26. 2. Flexibility and strength exercises for 90 minutes, 2 to 3 times per week.Group supervision provided weekly for weeks 1 to 6, then monthly for weeks 7 to 26. |
| Outcomes | 1. Rate of falls
2. Number of fallers
3. Health‐related quality of life (PDQ39) Other outcomes not relevant to this review |
| Starting date | March 15, 2021 |
| Contact information | Prof Margaret Mak Hong Kong Polytechnic University, Hong Kong Email: margaret.mak@polyu.edu.hk |
| Notes |
NCT04694443.
| Study name | Multidisciplinary home‐based Tele‐rehabilitation Intervention (TeleFall) |
| Methods | RCT |
| Participants | Target sample size: 76 Inclusion criteria: no dementia, idiopathic Parkinson's disease, able to walk with a Hoehn Yahr stage < 3. Exclusion criteria: non‐ambulatory, diagnosed with significant comorbidity (psychiatric, systemic, hearing or visual disturbances). |
| Interventions | 1. Multidisciplinary telehealth including physical therapy, neurologist, nurse and psychologist plus standard in‐office visits. 2. Control usual care with standard in‐office visits |
| Outcomes | 1. Rate of falls 2. Number of fallers 3. Health‐related quality of life (PDQ39) Other outcomes not relevant to this review |
| Starting date | January 1, 2020 |
| Contact information | Dr Esther Cubo Hospital Universitario de Burgos, Spain Email: mcubo@saludcastillayleon.es |
| Notes |
NCT04848077.
| Study name | STEPWISE Parkinson: Smartphone based Exercise solution for Patients with Parkinson's disease (STEPWISE) |
| Methods | RCT |
| Participants | Target sample size: 452 Inclusion criteria: idiopathic PD, Hoehn and Yahr 1‐3, able to understand the Dutch language, able to walk independently, equal to or less than 120 minutes of sports/outdoor activities per day, less than 7000 steps/day during 1‐month baseline. Exclusion criteria: weekly falls in the previous 3 months, medical conditions that hamper mobility other than Parkinson's disease, living in a nursing home, cognitive impairments that hamper use of the motivational app, not in the possession of a suitable smartphone. |
| Interventions | 1. Very large proportional increase in daily steps, encouraged via a smartphone app over 1 year. 2. Large proportional increase in daily steps, encouraged via a smartphone app over 1 year. 3. Medium proportional increase in daily steps, encouraged via a smartphone app over 1 year. 4. Small proportional increase in daily steps, encouraged via a smartphone app over 1 year. |
| Outcomes | 1. Rate of falls 2. Health‐related quality of life (PDQ39) Other outcomes not relevant to this review |
| Starting date | May 18, 2021 |
| Contact information | Sabine Schootemeijer Radboud University Medical Center, Email: sabine.schootemeijer@radboudumc.nl |
| Notes |
NCT04874051.
| Study name | Sensor‐based assessment and rehabilitation of Balance in Neurological Diseases (BALANCE) |
| Methods | RCT |
| Participants | Target sample size: 120 overall, with a subset of these with Parkinson's disease Inclusion criteria for participants with Parkinson's disease: Berg Balance Scale < 50/56, able to stand without support for 1 minute, Functional Independence measure < 100/126, Barthel Index < 80/100, Hoehn and Yahr stage 1.5 to 3, Subitem "freezing" when walking"of the UPDRS ≤ 2. Exclusion criteria: untreated epilepsy, major depressive disorder, fractures, dementia, ideomotor apraxia, neglect, severe impairment of verbal comprehension, severe acoustic and visual disorders. |
| Interventions | 1. Balance exercise using exercise in a virtual reality environment, 60 minutes, 5 times per week for 3 weeks 2. Balance exercise without the virtual reality environment, 60 minutes, 5 times per week for 3 weeks |
| Outcomes | 1. Rate of falls Other outcomes not relevant to this review |
| Starting date | September 2, 2019 |
| Contact information | Dr Andrea Turolla San Camillo IRCCS Email: andrea.turolla@ospedalesancamillo.net |
| Notes |
NCT04897256.
| Study name | Mobility in daily life and Falls in Parkinson's disease: potential for rehabilitation |
| Methods | RCT |
| Participants | Target sample size: 60 Inclusion criteria: Idiopathic Parkinson's disease, excellent response to levodopa, Hoehn & Yahr stages 2 to 4, aged 55 to 85 years. Exclusion criteria: major musculoskeletal or neurological disorders, structural brain disease, epilepsy, acute illness or health history, other than Parkinson's disease, medical condition that precludes exercise, MoCA ≤ 21 or inability to follow directions, excessive use of alcohol or recreational drugs, recent change in medication, inability to stand and walk for 2 minutes without an assistive device. |
| Interventions | 1. Turning boot camp exercise program, with supervised classes for 1 hour, 3 times per week for 6 weeks. Classes include exercises that involve weight shifting and increasing axial rotation. 2. Control: Usual care |
| Outcomes | 1. Rate of falls 2. Health‐related quality of life (PDQ39) Other outcomes not relevant to this review |
| Starting date | September 13, 2021 |
| Contact information | Austin Prewitt Oregon Health and Science University Email: balance@ohsu.edu |
| Notes |
NCT04946812.
| Study name | Split‐belt treadmill training to rehabilitate freezing of gait and balance in Parkinson's disease |
| Methods | RCT |
| Participants | Target sample size: 28 Inclusion criteria: Idiopathic Parkinson's disease, Hoehn & Yahr Stage 2‐3 when on levodopa, freezing of gait that is resistant to dopaminergic therapy, disease duration 5 to 15 years, stable clinical response to medications or stimulation parameters (if DBS) for at least 3 months, mini‐mental state examination >24/30, able to walk on a motor‐driven treadmill. Exclusion criteria: Severe imbalance that limits walking ability (Hoehn &Yahr score above 3), orthopaedic conditions and other systemic disease affecting walking, cardiac conditions limiting the ability to walk uninterrupted for 1 hour, other neurological disorders, not fluent in English. |
| Interventions | 1. Split‐belt treadmill training, where the velocity of the belt will be reduced on the least affected side by 25%, starting at 20 minutes per session and increasing over 18 sessions conducted across 3 weeks. 2. Tied‐belt treadmill training, starting at 20 minutes per session and increasing over 18 sessions conducted across 3 weeks. |
| Outcomes | 1. Rate of falls 2. Number of fallers 3. Health‐related quality of life (PDQ39) Other outcomes not relevant to this review |
| Starting date | March 27, 2020 |
| Contact information | Prof Alfonso Fasano Toronto Western Hospital Email: alfonso.fasano@uhn.ca |
| Notes |
NCT04953637.
| Study name | Physiotherapy and deep brain Stimulation in Parkinson's disease |
| Methods | RCT |
| Participants | Target sample size: 60 Inclusion criteria: Parkinson's disease and eligible for deep Brain stimulation surgery, able to give informed consent, aged 18 years or older. Exclusion criteria: ongoing orthopaedic conditions potentially impacting on global mobility, live >50km from downtown Toronto, severe cognitive deficits (Montreal Cognitive Assessment score <17), already receiving physiotherapy treatment (or that has been receiving it during the three months prior to enrolment). |
| Interventions | 1. DBS plus physiotherapy focused on gait and balance. Physiotherapy starts 4 months after DBS surgery and occurs for one hour, 3 times per week for 8 weeks. 2. Control: DBS surgery plus encouragement to keep an active lifestyle through a home exercise video which they are encouraged to perform 3 times per week for 8 weeks. |
| Outcomes | 1. Rate of falls 2. Number of fallers 3. Health‐related quality of life (PDQ39) Other outcomes not relevant to this review |
| Starting date | April 15, 2021 |
| Contact information | Prof Alfonso Fasano Toronto Western Hospital Email: alfonso.fasano@uhn.ca |
| Notes |
NCT05127057.
| Study name | Proactive and Integrated Management and Empowerment in Parkinson's disease (PRIME‐UK): A New Model of Care (PRIME‐RCT) (PRIME‐RCT) |
| Methods | RCT |
| Participants | Target sample size: 214 Inclusion criteria: diagnosis of Parkinsonism, ability to provide informed consent or have another person who can act as a personal consultee, aged 18 years or older, lives within the geographical catchment area of Royal United Hospital Bath NHS Foundation Trust, UK. Exclusion criteria: drug, infection or toxin induced parkinsonism, lack capacity to participate and do not have anyone who can be a consultee to provide advice regarding the patient's wishes and views, current medical, cognitive or psychosocial issue or co‐enrolment in other study that, in the opinion of the site investigator, would interfere with adherence to study requirements. |
| Interventions | 1. PRIME Parkinson Care: a multi‐component model of care including case management, empowerment of patients and care‐partners, empowerment of healthcare professionals, IT infrastructure. 2. Control: Usual care |
| Outcomes | 1. Rate of falls 2. Number of fallers 3. Health‐related quality of life (PDQ39 and EuroQol 5D‐5L) Other outcomes not relevant to this review |
| Starting date | March 1, 2022 |
| Contact information | Dr Emily Henderson University of Bristol Email: prime‐parkinson@bristol.ac.uk |
| Notes |
NCT05172661.
| Study name | Effects of physical‐cognitive training with different task models in Parkinson's disease with mild cognitive impairment |
| Methods | RCT |
| Participants | Target sample size: 28 Inclusion criteria: idiopathic Parkinson's disease, decreased cognitive functions that do not interfere with functional independence, Montreal Cognitive Assessment 21‐25, able to walk independently without walking devices for at least 10 metres and with the ability to turn 180°. Exclusion criteria: dementia, other diseases that may influence cognitive functions or walking performance, history of brain surgery, modification of medications during the exercise intervention. |
| Interventions | 1. Integrated motor‐cognitive training, performing postural and cognitive tasks simultaneously for 70 minutes, 2 times per week for 6 weeks. 2. Consecutive training, performing postural and cognitive tasks separately for the same duration, 70 minutes, 2 times per week for 6 weeks. |
| Outcomes | 1. Fall rate Other outcomes not relevant to this review |
| Starting date | November 26, 2021 |
| Contact information | National Taiwan University Hospital, Taipei, Taiwan |
| Notes |
RBR‐5w2sqt.
| Study name | Effects of strength exercises with elastic bands and tubes on the difficulty of movements, quality of life, sleep, memory, depressive symptoms, balance and risk of falls of patients with Parkinson's disease |
| Methods | RCT |
| Participants | Target sample size: 50 Inclusion criteria: diagnosis of Parkinson’s disease according to the UK PD Brain Bank Diagnostic Criteria; stages of 1 to 3 according to the modified Hoehn & Yahr scale; stable antiparkinsonian medication regimen for at least 4 weeks before the intervention; literate; independent in basic daily living activities according to SE higher or equal to 80%; age of 40 years or more; be a resident of Fortaleza, Brazil. Exclusion criteria: BMI greater than 40 and less than 20; diagnosis of Chron's Disease and Ulcerative Colitis; diagnosis of multiple sclerosis, ADEM, Parkinsonism plus, cerebrovascular disease with motor sequelae, Guillain‐Barre; dementia syndrome of any etiology according to MSD‐V; schizophrenia with hospitalisation or psychotic episode or suicidal ideation in the last 6 months; bipolar affective disorder with hospitalisation or episode of mania or episode of hypomania or episode of depression in the last 6 months; depression with hospitalisation or suicidal ideation or psychotic episode in the last 6 months; myocardial infarction with or without ST elevation in the last 12 months; myocardial revascularisation surgery or percutaneous angioplasty in the last 12 months; uncontrolled arrhythmia; severe or oxygen dependent COPD; cardiac insufficiency with reduced functional class III or IV; resting Blood Pressure greater than or equal to 160 x 100 mmHg; implantable cardioversion defibrillator (ICD); severe chronic kidney disease (creatinine clearance less than 30ml/min); proliferative retinopathy secondary to diabetes; peripheral neuropathy with motor impairment; moderate to severe hearing impairment (inability to maintain a dialogue or need for lip reading); moderate to severe visual impairment (minimum visual acuity 20/70 ‐ Snellen); cancer in activity or in treatment; history of conventional surgery or DBS for Parkinson's disease; alcohol consumption greater than 14 drinks per week; live with people who participate in the same study; thromboembolism without anticoagulation regimen; significant weight loss (10% of usual weight) in the last 6 months; lack of family support to participate in the study; bariatric surgery history; exercise at moderate intensity for at least 3 x week; glycated haemoglobin higher than 12. |
| Interventions | 1. Muscle power training with elastic bands and resistance tubes for 60 minutes, 2 x per week for 12 weeks. 2. Group health education about living well with Parkinson's disease, once a week, for 12 weeks. |
| Outcomes | 1. Rate of falls in the past month 2. PDQ39 Other outcomes not relevant to this review |
| Starting date | Not yet recruiting |
| Contact information | Danielle Pessoa Lima Faculdade de Medicina da Universidade Federal do Ceará, Brazil |
| Notes |
BMI: body, mass index;COPD: chronic obstructive pulmonary disease; EQ‐5D : European Quality of Life 5 Dimension; PDQ39: Parkinson's Disease Questionnaire‐39; PDQ8: Parkinson's Disease Questionnaire‐8;RCT: randomised controlled trial;
Differences between protocol and review
Types of interventions
We excluded interventions designed to primarily address syncopal falls (e.g. falls associated with neurogenic postural hypotension) as the aetiology and intervention for syncopal falls are different from falls arising from loss of balance due to physical, cognitive and emotional risk factors associated with PD (van der Marck 2014, Fasano 2012).
Types of outcomes
When reporting adverse events, we reported a rate ratio instead of a risk ratio, as the data reported in the included studies were for the rate of adverse events. We decided to exclude falls from the rate of adverse events, as falls were analysed separately and including them obscured data relating to other types of adverse events.
Data synthesis ‐ decisions for pooling data
For randomised cross‐over trials of exercise interventions, we used first phase data as per our protocol to avoid the possibility of carry‐over effects from the first phase exercise intervention to the second phase. However, for the medication trials, we used data from the end of the second phase, as the washout period between phases was likely to mean the effects of the medication in the first phase had ceased.
We incorporated trials with more than one intervention arm compared with a control group. To avoid 'double counting' of control participants from these trials in any one meta‐analysis, the participant numbers in the control group were allocated in proportion to the participant numbers in each intervention arm. Additionally, to adjust the rate ratios and risk ratios for this, we increased the standard errors of the natural log by 25%.
We had originally planned to perform only fixed‐effect meta‐analyses. However, the review authors felt that it was unlikely that there would be a single true effect of exercise interventions on falls in people with PD. Therefore, when meta‐analyses of exercise interventions or of exercise plus education interventions were performed, a random‐effects model was used. We then undertook sensitivity analyses to assess if fixed‐effect analyses would influence the results.
GRADE assessment
We undertook GRADE assessments to evaluate the certainty of the evidence for comparisons of an intervention versus control or placebo where meta‐analyses had been conducted.
Summary of findings tables
We produced summary of findings tables for each comparison of an intervention versus control or placebo.
Contributions of authors
All authors have contributed to the production of this review.
NA was involved in screening, data extraction, data analysis, and co‐led the writing of the review and acted as guarantor of the review.
CC was involved in screening, data extraction, data analysis and co‐led the writing of the review.
LA, SK and GV were involved in data extraction, contributed to writing the review and commented on drafts of the review.
BB contributed to writing the review and commented on drafts of the review.
NL and TY were involved in screening, data extraction, contributed to writing the review and commented on drafts of the review.
AN was involved in screening, contributed to writing the review and commented on drafts of the review.
CS was involved in data analysis, contributed to writing the review and commented on drafts of the review.
Sources of support
Internal sources
No sources of support provided
External sources
No sources of support provided
Declarations of interest
No review author was involved in study selection or processing of risk of bias of any trials in which they are involved.
Several authors (CS, NA, CC) are currently running trials of fall‐prevention interventions; including the following ongoing trial in this review (ACTRN12619000415101).
NA is an author of several trials considered in this review, including two included trials (Canning 2015a, Song 2018).
CC is an author of several trials considered in this review, including three included trials (Canning 2015a, Paul 2014, Song 2018).
LA has no known conflict of interest.
BB is an author of several trials considered in this review, including two included trials (Munneke 2010, Mirelman 2016).
SK is an author of several trials considered in this review, including one included trial (Munneke 2010).
NL has no known conflicts of interest.
AN is an author of several trials considered in this review, including one included trial (Mirelman 2016).
GV has no known conflicts of interest.
TP has no known conflicts of interest.
CS is an author of several trials considered in this review, including three included trials (Canning 2015a, Paul 2014, Song 2018).
Edited (no change to conclusions)
References
References to studies included in this review
Ashburn 2007 {published and unpublished data}
- Ashburn A, Fazakarley L, Ballinger C, Pickering R, McLellan LD, Fitton C. A randomised controlled trial of a home based exercise programme to reduce the risk of falling among people with Parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry 2007;78(7):678-84. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburn A, Stack E, Ballinger C, Fazakarley L, Fitton C. The circumstances of falls among people with Parkinson’s disease and the use of Falls Diaries to facilitate reporting. Disability and Rehabilitation 2008;30(16):1205-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Canning 2015a {published data only}
- Canning CG, Sherrington C, Lord SR, Close JC, Heritier S, Heller GZ, et al. Exercise for falls prevention in Parkinson disease: a randomized controlled trial. Neurology 2015;84(3):304-12. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning CG, Sherrington C, Lord SR, Fung VS, Close JC, Latt MD, et al. Exercise therapy for prevention of falls in people with Parkinson's disease: a protocol for a randomised controlled trial and economic evaluation. BMC Neurology 2009;9:4. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag I, Sherrington C, Hayes A, Canning CG, Lord SR, Close JC, et al. Economic evaluation of a falls prevention exercise program among people With Parkinson's disease. Movement Disorders 2016;31(1):53-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cattaneo 2019 {published data only (unpublished sought but not used)}
- Cattaneo D, Gervasoni E, Pupillo E, Blanchi E, Aprile I, Imbimbo I, et al. Educational and exercise intervention to prevent falls and improve participation in subjects with neurological conditions: the NEUROFALL randomized controlled trial. Frontiers in Neurology 2019;10:865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chivers Seymour 2019 {published and unpublished data}
- Ashburn A, Pickering R, McIntosh E, Hulbert S, Rochester L, Roberts HC, et al. Exercise- and strategy-based physiotherapy-delivered intervention for preventing repeat falls in people with Parkinson's: the PDSAFE RCT. Health Technology Assessment NIHR Library 2019;23(36):1-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivers Seymour K, Pickering R, Rochester L, Roberts HC, Ballinger C, Hulbert S, et al. Multicenter, randomised controlled trial of PDSAFE, a physiotherapist-delivered fall prevention program for people with Parkinson's. Journal of Neurology, Neurosurgery, and Psychiatry 2019;90(7):774-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin VA, Pickering R, Ballinger C, Roberts H, McIntosh E, Lamb S, et al. A multi-centre, randomised controlled trial of the effectiveness of PDSAFE to prevent falls among people with Parkinson’s: study protocol. BMC Neurology 2015;15:81. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert S, Chivers-Seymour K, Summers R, Lamb S, Goodwin V, Rochester L, et al. 'PDSAFE' - a multi-dimensional model of falls rehabilitation for people with Parkinson's. A mixed methods analysis of therapists' delivery and experience. Physiotherapy 2020;August 30:online ahead of print. [DOI: 10.1016/physio.2020.08.006] [DOI] [PubMed] [Google Scholar]
- Hulbert S, Rochester L, Nieuwboer A, Goodwin V, Fitton C, Chivers-Seymour K, et al. "Staying safe" - a narrative review of falls prevention in people with Parkinson's - "PDSAFE". Disability and Rehabilitation 2019;41(21):2596-605. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Rowsell A, Ashburn A, Fitton C, Goodwin VA, Hulbert S, Lamb SE, et al. Participant expectations and experiences of a tailored physiotherapy intervention for people with Parkinson's and a history of falls. Disability and Rehabilitation 2020;online ahead of print June 23:1-9. [DOI: 10.1080/09638288.2020.1779824] [DOI] [PubMed] [Google Scholar]
- Xin Y, Ashburn A, Pickering RM, Chivers Seymour K, Hulbert S, Fitton C, et al. Cost-effectiveness of the PDSAFE personalised physiotherapy intervention for fall prevention in Parkinson's: an economic evaluation alongside a randomised controlled trial. BMC Neurology 2020;20(295):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chung 2010 {published and unpublished data}
- Chung KA, Lobb BM, Nutt JG, Horak FB. Effects of a central cholinesterase inhibitor on reducing falls in Parkinson disease. Neurology 2010;75(14):1263-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gandolfi 2017 {published data only}
- Gandolfi M, Geroin C, Dimitrova E, Boldrini P, Waldner A, Bonadiman S, et al. Virtual reality telerehabilitation for postural instability in Parkinson's disease: a multicenter, single-blind, randomized, controlled trial. BioMed Research International 2017;2017:Article ID 7962826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gandolfi 2019 {published data only}
- Gandolfi M, Tinazzi M, Magrinelli F, Busselli G, Dimitrova E, Polo N, et al. Four-week trunk-specific exercise program decreases forward trunk flexion in Parkinson's disease: a single-blinded, randomized controlled trial. Parkinsonism and Related Disorders 2019;64:268-74. [DOI] [PubMed] [Google Scholar]
Gao 2014 {published data only}
- Gao Q, Leung A, Yang Y, Wei Q, Guan M, Jia C, et al. Effects of Tai Chi on balance and fall prevention in Parkinson's disease: a randomized controlled trial. Clinical Rehabilitation 2014;28(8):748-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Goodwin 2011 {published data only (unpublished sought but not used)}
- Fletcher E, Goodwin VA, Richards SH, Campbell JL, Taylor RS. An exercise intervention to prevent falls in Parkinson's: an economic evaluation. BMC Health Services Research 2012;12:426. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin VA, Richards SH, Henley W, Ewings P, Taylor AH, Campbell JL. An exercise intervention to prevent falls in people with Parkinson's disease: a pragmatic randomised controlled trial. Journal of Neurology, Neurosurgery, and Psychiatry 2011;82(11):1232-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Harro 2014 {published and unpublished data}
- Harro CC, Shoemaker MJ, Frey O, Gamble AC, Harring KB, Karl KL, et al. The effects of speed-dependent treadmill training and rhythmic auditory-cued overground walking on balance function, fall incidence, and quality of life in individuals with idiopathic Parkinson's disease: a randomized controlled trial. NeuroRehabilitation 2014;34(3):541-56. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Harro CC, Shoemaker MJ, Frey OJ, Gamble AC, Harring KB, Karl KL, et al. The effects of speed-dependent treadmill training and rhythmic auditory-cued overground walking on gait function and fall risk in individuals with idiopathic Parkinson's disease: a randomized controlled trial. NeuroRehabilitation 2014;34(3):557-572. [PMID: ] [DOI] [PubMed] [Google Scholar]
Henderson 2016 {published and unpublished data}
- Henderson EJ, Lord SR, Brodie MA, Gaunt DM, Lawrence AD, Close JC, et al. Rivastigmine for gait stability in patients with Parkinson's disease (ReSPonD): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurology 2016;15(3):249-58. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Henderson EJ, Lord SR, Close JC, Lawrence AD, Whone A, Ben-Shlomo Y. The ReSPonD trial - rivastigmine to stabilise gait in Parkinson's disease a phase II, randomised, double blind, placebo controlled trial to evaluate the effect of rivastigmine on gait in patients with Parkinson's disease who have fallen. BMC Neurology 2013;13:188. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2012 {published data only (unpublished sought but not used)}
- Li F, Harmer P, Fitzgerald K, Eckstrom E, Stock R, Galver J, et al. Tai chi and postural stability in patients with Parkinson's disease. New England Journal of Medicine 2012;366(6):511-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Harmer P, Liu Y, Eckstrom E, Fitzgerald K, Stock R, et al. A randomized controlled trial of patient-reported outcomes with tai chi exercise in Parkinson's disease. Movement Disorders 2014;29(4):539-45. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Harmer P. Economic evaluation of a Tai Ji Quan intervention tor educe falls in people with Parkinson disease, Oregon, 2008-2011. Preventing Chronic Disease 2015;12:E120. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2015a {published data only (unpublished sought but not used)}
- Li Z, Yu Z, Zhang J, Wang J, Sun C, Wang P, et al. Impact of rivastigmine on cognitive dysfunction and falling in Parkinson's disease patients. European Neurology 2015;74:86-91. [PMID: ] [DOI] [PubMed] [Google Scholar]
Martin 2015 {published data only (unpublished sought but not used)}
- Martin T, Weatherall M, Anderson TJ, MacAskill MR. A randomized controlled feasibility trial of a specific cueing program for falls management in persons with Parkinson disease and freezing of gait. Journal of Neurologic Physical Therapy 2015;39(3):179-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mirelman 2016 {published data only (unpublished sought but not used)}
- Bekkers EM, Mirelman A, Alcock L, Rochester L, Nieuwhof F, Bloem BR, et al. Do patients with freezing of gait respond differently than those without to treadmill training augmented by virtual reality? Neurorehabilitation and Neural Repair 2020;34(5):440-9. [DOI] [PubMed] [Google Scholar]
- Del Din S, Galna B, Lord S, Nieuwboer A, Bekkers EM, Pelosin E, et al. Fall risk in relation to activity exposure in high risk older adults. Journals of Gerontology. SEries A Biological Sciences and Medical Sciences 2020;75(6):1198-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidan I, Rosenberg-Katz K, Jacob Y, Giladi N, Hausdorff JM, Mirelman A. Disparate effects of training on brain activation in Parkinson disease. Neurology 2017;89:1804-10. [DOI] [PubMed] [Google Scholar]
- Mirelman A, Rochester L, Maidan I, Del Din S, Alcock L, Nieuwhof F, et al. Addition of a non-immersive virtual reality component to treadmill training to reduce fall risk in older adults (V-TIME): a randomised controlled trial. Lancet 2016;388(10050):1170-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Mirelman A, Rochester L, Reelick M, Nieuwhof F, Pelosin E, Abbruzzese G, et al. V-TIME: a treadmill training program augmented by virtual reality to decrease fall risk in older adults: study design of a randomized controlled trial. BMC Neurology 2013;13:15. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosin E, Cerulli C, Ogliastro C, Lagravinese G, Mori L, Bonassi G, et al. A multimodal training modulates short afferent inhibition and improves complex walking in a cohort of faller older adults with an increased prevalence of Parkinson's disease. Journals of Gerontology. SEries A Biological Sciences and Medical Sciences J Gerontol A Biol Sci Med Sci 2020;75(4):722-8. [DOI] [PubMed] [Google Scholar]
Morris 2015 {published and unpublished data}
- McGinley JL, Martin C, Huxham FE, Menz HB, Danoudis M, Murphy AT, et al. Feasibility, safety, and compliance in a randomized controlled trial of physical therapy for Parkinson's disease. Parkinson's Disease 2012;2012:1-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris ME, Menz HB, McGinley JL, Huxham FE, Murphy AT, Iansek R, et al. Falls and mobility in Parkinson's disease: protocol for a randomised controlled clinical trial. BMC Neurology 2011;11:93. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris ME, Menz HB, McGinley JL, Watts JJ, Huxham FE, Murphy AT, et al. f randomized controlled trial to reduce Falls in people with Parkinson's disease. Neurorehabilitation and Neural Repair 2015;29(8):777-85. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Watts JJ, McGinley JL, Huxham F, Menz HB, Iansek R, Murphy AT, et al. Cost effectiveness of preventing falls and improving mobility in people with Parkinson disease: protocol for an economic evaluation alongside a clinical trial. BMC Geriatrics 2008;8(23):1-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morris 2017 {published data only}
- Morris ME, Martin C, McGinley JL, Huxham FE, Menz HB, Taylor NF, et al. Protocol for a home-based integrated physical therapy program to reduce falls and improve mobility in people with Parkinson's disease. BMC Neurology 2012;12:54. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris ME, Taylor NF, Watts JJ, Evans A, Horne M, Kempster P, et al. A home program of strength training, movement strategy training and education did not prevent falls in people with Parkinson's disease: a randomised trial. Journal of Physiotherapy 2017;63(2):94-100. [PMID: ] [DOI] [PubMed] [Google Scholar]
Munneke 2010 {published data only (unpublished sought but not used)}
- Keus SH, Nijkrake MJ, Borm GF, Kwakkel G, Roos RA, Berendse HW, et al. The ParkinsonNet trial: design and baseline characteristics. Movement Disorders 2010;25(7):830-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Munneke M, Nijkrake MJ, Keus SH, Kwakkel G, Berendse HW, Roos RA, et al. Efficacy of community-based physiotherapy networks for patients with Parkinson's disease: a cluster randomised trial. Lancet Neurology 2010;9(1):46-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Nijkrake MJ, Keus SH, Overeem S, Oostendorp RA, Vliet Vlieland TP, Mulleners W, et al. The ParkinsonNet concept: development, implementation and initial experience. Movement Disorders 2010;25(7):823-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Paul 2014 {published and unpublished data}
- Paul SS, Canning CG, Song J, Fung VS, Sherrington C. Leg muscle power is enhanced by training in people with Parkinson'sdisease: a randomized controlled trial. Clinical Rehabilitation 2014;28(3):275-88. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pelosin 2017 {published data only (unpublished sought but not used)}
- Pelosin E, Avanzino L, Barella R, Bet C, Magioncalda E, Trompetto C, et al. Treadmill training frequency influences walking improvement in subjects with Parkinson's disease: a randomized pilot study. European Journal of Physical and Rehabilitation Medicine 2017;53(2):201-8. [DOI] [PubMed] [Google Scholar]
Penko 2019 {published data only (unpublished sought but not used)}
- Penko AL, Barkley JE, Rosenfeldt AB, Alberts JL. Multimodal training reduces fall frequency as physical activity increases in individuals with Parkinson's disease. Journal of Physical Activity and Health 2019;16:1085-91. [DOI] [PubMed] [Google Scholar]
- Rosenfeldt A, Penko AL, Bazyk AS, Streicher MC, Dey T, Alberts JL. The 2-min walk test detects dual-task deficits in individuals with Parkinson's disease. Journal of Aging and Physical Activity 2019;27(4):843-7. [DOI] [PubMed] [Google Scholar]
- Rosenfeldt AB, Penko AL, Streicher MC, Zimmerman NM, Miller Koop M, Alberts JL. Improvements in temporal and postural aspects of gait vary following single- and multi-modal training in individuals with Parkinson's disease. Parkinsonism and Related Disorders 2019;64:280-5. [DOI] [PubMed] [Google Scholar]
Protas 2005 {published data only (unpublished sought but not used)}
- Protas EJ, Mitchell K, Williams A, Qureshy H, Caroline K, Lai EC. Gait and step training to reduce falls in Parkinson's disease. NeuroRehabilitation 2005;20(3):183-90. [PMID: ] [PubMed] [Google Scholar]
Ricciardi 2015 {published data only (unpublished sought but not used)}
- Ricciardi L, Ricciardi D, Lena F, Plotnik M, Petracca M, Barricella S, et al. Working on asymmetry in Parkinson's disease: randomized, controlled pilot study. Neurological Sciences 2015;36(8):1337-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sedaghati 2016 {published data only (unpublished sought but not used)}
- Sedaghati P, Daneshmandi H, Karimi N, Barati A. A selective corrective exercise to decrease falling and improve functional balance in idiopathic Parkinson's disease. Trauma Monthly 2016;21(1):e23573. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shen 2015 {published data only}
- Shen X, Mak MK. Balance and gait training with augmented feedback improves balance confidence in people with Parkinson's disease: a randomized controlled trial. Neurorehabilitation and Neural Repair 2014;28(6):524-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Shen X, Mak MK. Technology-assisted balance and gait training reduces falls in patients with Parkinson's disease: a randomized controlled trial with 12-month follow-up. Neurorehabilitation and Neural Repair 2015;29(2):103-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
Smania 2010 {published data only (unpublished sought but not used)}
- Smania N, Corato E, Tinazzi M, Stanzani C, Fiaschi A, Girardi P, et al. Effect of balance training on postural instability in patients with idiopathic Parkinson's disease. Neuroherabilitation and Neural Repair 2010;24(9):826-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Song 2018 {published data only}
- Song J, Paul SS, Caetano MJ, Smith S, Dibble LE, Love R, et al. Home-based step training using videogame technology in people with Parkinson's disease: a single-blinded randomised controlled trial. Clinical Rehabilitation 2018;32(3):299-311. [DOI] [PubMed] [Google Scholar]
Thaut 2019 {published and unpublished data}
- Thaut MH, Rice RR, Braun Janzen T, Hurt-Thaut CP, McIntosh GC. Rhythmic auditory stimulation for reduction of falls in Parkinson's disease: a randomized controlled study. Clinical Rehabilitation 2019;33(1):34-43. [DOI] [PubMed] [Google Scholar]
Volpe 2014a {published data only (unpublished sought but not used)}
- Volpe D, Giantin MG, Fasano A. A wearable proprioceptive stabilizer (Equistasi®) for rehabilitation of postural instability in Parkinson's disease: a phase II randomized double-blind, double-dummy, controlled study. PLOS One 2014;9(11):e112065. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Volpe 2014b {published data only (unpublished sought but not used)}
- Volpe D, Giantin MG, Maestri R, Frazzitta G. Comparing the effects of hydrotherapy and land-based therapy on balance in patients with Parkinson's disease: a randomized controlled pilot study. Clinical Rehabilitation 2014;28(12):1210-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ward 2004 {published data only (unpublished sought but not used)}
- Ward CD, Turpin G, Dewey ME, Fleming S, Hurwitz B, Ratib S, et al. Education for people with progressive neurological conditions can have negative effects: evidence from a randomized controlled trial. Clinical Rehabilitation 2004;18(7):717-25. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wong‐Yu 2015 {published data only}
- Wong-Yu IS, Mak MK. Task- and context-specific balance training program enhances dynamic balance and functional performance in parkinsonian nonfallers: a randomized controlled trial with six-month follow-up. Archives of Physical Medicine and Rehabilitation 2015;96(12):2103-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Wong-Yu ISK, Mak MK. Multi-dimensional balance training programme improves balance and gait performance in people with Parkinson's disease: a pragmatic randomized controlled trial with 12-month follow-up. Parkinsonism and Related Disorders 2015;21(6):615-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Wong-Yu ISK, Mak MK. Multisystem balance training reduces injurious fall risk in Parkinson disease. American Journal of Physical Medicine and Rehabilitation 2019;98(3):239-44. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allen 2010 {published data only}
- Allen NE, Canning CG, Sherrington C, Lord SR, Latt M D, Close JC, et al. The effects of an exercise program on fall risk factors in people with Parkinson's disease: a randomized controlled trial. Movement Disorders 2010;25(9):1217-25. [DOI] [PubMed] [Google Scholar]
Bevilacqua 2020 {published data only}
- Bevilacqua R, Maranesi E, Di Rosa M, Luzi R, Casoni E, Rinaldi N, et al. Rehabilitation of older people with Parkinson's disease: an innovative protocol for RCT study to evaluate the potential of robotic-based technologies. BMC Neurology 2020;20(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bueno 2017 {published data only}
- Bueno ME, dos Reis Andrello AC, Terra MB, dos Santos HB, Marquioli JM, Santos SM. Comparison of three physical therapy interventions with an emphasis on the gait of individuals with Parkinson's disease [Fisioterapia em Movimento]. [Physical Therapy in Movement] 2017;30(4):691-701. [Google Scholar]
Cakit 2007 {published data only}
- Cakit BD, Saracoglu M, Genc H, Erdem HR, Inan L. The effects of incremental speed-dependent treadmill training on postural instability and fear of falling in Parkinson's disease. Clinical Rehabilitation 2007;21(8):698-705. [DOI] [PubMed] [Google Scholar]
Calabro 2019 {published data only}
- Calabro RS, Naro A, Filoni S, Pullia M, Billeri L, Tomasello P, et al. Walking to your right music: a randomized controlled trial on the novel use of treadmill plus music in Parkinson's disease. Journal of Neuroengineering & Rehabilitation 2019;16(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Celiker 2018 {published data only}
- Celiker O, Demir G, Kocaoglu M, Altug F, Acar F. Comparison of subthalamic nucleus vs. globus pallidus interna deep brain stimulation in terms of gait and balance; a two year follow-up study. Turkish Neurosurgery 2018;29(3):355-61. [DOI] [PubMed] [Google Scholar]
Chang 2019 {published data only}
- Chang MC, Chun MH. The effect of balance training with Tetra-ataxiometric posturography on balance function in patients with Parkinsonism. Neurorehabilitation 2019;45(3):379-84. [DOI] [PubMed] [Google Scholar]
Cherup 2019 {published data only}
- Cherup NP, Buskard A, Strand L, Roberson KB, Michiels E R, Kuhn JE, et al. Power vs strength training to improve muscular strength, power, balance and functional movement in individuals diagnosed with Parkinson's disease. Experimental Gerontology 2019;128:110740. [DOI] [PubMed] [Google Scholar]
Chomiak 2017 {published data only}
- Chomiak T, Watts A, Meyer N, Pereira FV, Hu B. A training approach to improve stepping automaticity while dual-tasking in Parkinson's disease. Medicine 2017;96(5):e5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chou 2017 {published data only}
- Chou KL, Elm JJ, Wielinski CL, Simon DK, Aminoff MJ, Christine CW et, al. Factors associated with falling in early, treated Parkinson's disease: the NET-PD LS1 cohort. Journal of the Neurological Sciences 2017;377:137-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Citrome 2018 {published data only}
- Citrome L, Norton JC, Chi-Burris K, Demos G. Pimavanserin for the treatment of Parkinson's disease psychosis: number needed to treat, number needed to harm, and likelihood to be helped or harmed. CNS Spectrums 2018;23(3):228-38. [DOI] [PubMed] [Google Scholar]
Cosentino 2013 {published data only}
- Cosentino G, Valentino F, Pozzi NG, Brighina F, Fierro B, Savettieri G, et al. Transcranial direct current stimulation for treatment of freezing of gait in Parkinson's disease. A cross-over study. Journal of the Neurological Sciences 2013;333:e83. [DOI] [PubMed] [Google Scholar]
Cummings 2013 {published data only}
- Cummings J, Isaacson S, Mills R, Williams H, Chi-Burris K, Dhall R, et al. Antipsychotic efficacy and motor tolerability in a phase III placebo-controlled study of pimavanserin in patients with Parkinson's Disease psychosis (Acp-103-020). Journal of the Neurological Sciences 2013;333:e119-20. [Google Scholar]
da Silva 2019 {published data only}
- da Silva LP, Souza Duarte MP, Cassia Batista de Souza C, dos Santos Accioly Lins CC, das Gracas Wanderley de Sales Coriolano M, Lins OG. Effects of mental practice associated with motor physical therapy on gait and risk of falls in Parkinson's disease: a pilot study. Fisioterapia e Pesquisa 2019;26(2):120-7. [Google Scholar]
Deepa 2019 {published data only}
- Deepa S, Ramana K. External cueing on gait parameters in Parkinson's disease. International Journal of Research in Pharmaceutical Sciences 2019;10(3):2452-6. [Google Scholar]
de Lucena 2017 {published data only}
- Lucena Trigueiro LC, Gama GL, Ribeiro TS, Macedo Ferreira LG, Galvao ER, Souza e Silva EM, et al. Influence of treadmill gait training with additional load on motor function, postural instability and history of falls for individuals with Parkinson's disease: A randomized clinical trial. Journal of Bodywork and Movement Therapies 2017;21(1):93-100.. [DOI] [PubMed] [Google Scholar]
de Natale 2017 {published data only}
- Natale ER, Paulus KS, Aiello E, Sanna B, Manca A, Sotgiu G, et al. Dance therapy improves motor and cognitive functions in patients with Parkinson's disease. NeuroRehabilitation 2017;40(1):141-4. [DOI] [PubMed] [Google Scholar]
Duncan 2018 {published data only}
- Duncan RP, Van Dillen LR, Garbutt JM, Earhart GM, Perlmutter JS. Physical therapy and deep brain stimulation in Parkinson's Disease: protocol for a pilot randomized controlled trial. Pilot & Feasibility Studies 2018;4:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elmer 2018 {published data only}
- Elmer LW, Juncos JL, Singer C, Truong DD, Criswell SR, Parashos S, et al. Pooled analyses of phase III studies of ADS-5102 (Amantadine) extended-release capsules for dyskinesia in Parkinson's disease. CNS Drugs 2018;32(4):387-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Tamawy 2013 {published data only}
- El-Tamawy MS, Shehata HS, Shalaby NM, Nawito A, Esmail EH. Can repetitive transcranial magnetic stimulation help on-freezers with Parkinson's disease? Egyptian Journal of Neurology, Psychiatry and Neurosurgery 2013;50(4):355-60. [Google Scholar]
Emre 2010 {published data only}
- Emre M, Tsolaki M, Bonuccelli U, Destee A, Tolosa E, Kutzelnigg A, et al. Memantine for patients with Parkinson's disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurology 2010;9(10):969-77. [DOI] [PubMed] [Google Scholar]
Galli 2018 {published data only}
- Galli M, Vicidomini C, Rozin Kleiner AF, Vacca L, Cimolin V, Condoluci C, et al. Peripheral neurostimulation breaks the shuffling steps patterns in Parkinsonian gait: a double blind randomized longitudinal study with automated mechanical peripheral stimulation. European Journal of Physical & Rehabilitation Medicine. 2018;54(6):860-5. [DOI] [PubMed] [Google Scholar]
Geroin 2018 {published data only}
- Geroin C, Nonnekes J, Vries NM, Strouwen C, Smania N, Tinazzi M, et al. Does dual-task training improve spatiotemporal gait parameters in Parkinson's disease? Parkinsonism & Related Disorders 2018;55:86-91. [DOI] [PubMed] [Google Scholar]
Giardini 2018 {published data only}
- Giardini M, Nardone A, Godi M, Guglielmetti S, Arcolin I, Pisano F, et al. Instrumental or physical-exercise rehabilitation of balance improves both balance and gait in Parkinson's disease. Neural Plasticity 2018;2018:5614242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giladi 2013 {published data only}
Grobbelaar 2017 {published data only}
- Grobbelaar R, Venter R, Welman KE. Backward compared to forward over ground gait retraining have additional benefits for gait in individuals with mild to moderate Parkinson's disease: a randomized controlled trial. Gait & Posture 2017;58:294-9. [DOI] [PubMed] [Google Scholar]
Gu 2013 {published data only}
- Gu S, Song Z, Fan X, Chen R, Zheng W, Yan W. Effect of PD-WEBB training on balance impairment and falls in people with Parkinson's disease. [Chinese]. Zhong nan da xue xue bao (Journal of Central South University, Medical sciences) 2013;38(11):1172-6. [DOI] [PubMed] [Google Scholar]
Gurevich 2007 {published data only}
- Gurevich T, Peretz C, Moore O, Weizmann N, Giladi N. The effect of injecting botulinum toxin type a into the calf muscles on freezing of gait in Parkinson's disease: a double blind placebo-controlled pilot study. Movement Disorders 2007;22(6):880-3. [DOI] [PubMed] [Google Scholar]
Hackney 2007 {published data only}
- Hackney ME, Kantorovich S, Earhart GM. A study on the effects of Argentine tango as a form of partnered dance for those with Parkinson Disease and the healthy elderly. American Journal of Dance Therapy 2007;29(2):109-27. [Google Scholar]
Hauser 2013 {published data only}
- Hauser RA, Hsu A, Kell S, Espay AJ, Sethi K, Stacy M, et al. Extended-release carbidopa-levodopa (IPX066) compared with immediate-release carbidopa-levodopa in patients with Parkinson's disease and motor fluctuations: a phase 3 randomised, double-blind trial. Lancet Neurology 2013;12(4):346-56. [DOI] [PubMed] [Google Scholar]
Hauser 2016 {published and unpublished data}
- Francois C, Hauser RA, Aballea S, Dorey J, Kharitonova E, Hewitt LA. Cost-effectiveness of droxidopa in patients with neurogenic orthostatic hypotension: post-hoc economic analysis of Phase 3 clinical trial data. Journal of Medical Economics 2016;19(5):515-25. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hauser RA, Heritier S, Rowse GJ, Hewitt LA, Isaacson SH. Droxidopa and reduced falls in a trial of Parkinson disease patients with neurogenic orthostatic hypotension. Clinical Neuropharmacology 2016;39(5):220-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser RA, Hewitt LA, Isaacson S. Droxidopa in patients with neurogenic orthostatic hypotension associated with Parkinson's disease (NOH306A). Journal of Parkinson's Disease 2014;4(1):57-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hauser RA, Isaacson S, Lisk JP, Hewitt LA, Rowse G. Droxidopa for the short-term treatment of symptomatic neurogenic orthostatic hypotension in Parkinson's disease (nOH206B). Movement Disorders 2015;30(5):646-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hawkins 2018 {published data only}
- Hawkins B L, Van Puymbroeck M, Walter A, Sharp J, Woshkolup K, Urrea-Mendoza E, et al. Perceived activities and participation outcomes of a yoga intervention for individuals with Parkinson's disease: a mixed methods study. International Journal of Yoga Therapy 2018;28(1):51-61. [DOI] [PubMed] [Google Scholar]
Hewitt 2018 {published data only}
- Hewitt J, Goodall S, Clemson L, Henwood T, Refshauge K. Progressive resistance and balance training for falls prevention in long-term residential aged care: a cluster randomized trial of the Sunbeam Program. Journal of the American Medical Directors Association 2018;19(4):361-9. [DOI] [PubMed] [Google Scholar]
Hill 2015 {published data only (unpublished sought but not used)}
- Hill A, McPhail SM, Waldron N, Etherton-Beer C, Ingram K, Flicker L, et al. Fall rates in hospital rehabilitation units after individualised patient and staff education programmes: a pragmatic, stepped-wedge, cluster-randomised controlled trial. Lancet 2015;385:2592-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hill A, Waldron N, Etherton-Beer C, McPhail SM, Ingram K, Flicker L, et al. A stepped-wedge cluster randomised controlled trial for evaluating rates of falls among inpatients in aged care rehabilitation units receiving tailored multimedia education in addition to usual care: a trial protocol. BMJ Open 2014;4(1):e004195. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hiller 2018 {published data only}
- Hiller AL, Murchison CF, Lobb BM, O'Connor S, O'Connor M, Quinn J F. A randomized, controlled pilot study of the effects of vitamin D supplementation on balance in Parkinson's disease: does age matter? PLOS One [Electronic Resource] 2018;13(9):e0203637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hubble 2018 {published data only}
- Hubble RP, Naughton G, Silburn PA, Cole MH. Trunk exercises improve gait symmetry in Parkinson disease: a blind phase ii randomized controlled trial. American Journal of Physical Medicine & Rehabilitation 2018;97(3):151-9. [DOI] [PubMed] [Google Scholar]
Hubble 2019 {published data only}
- Hubble RP, Silburn PA, Naughton GA, Cole MH. Trunk exercises improve balance in Parkinson disease: a phase II randomized controlled yrial. Journal of Neurologic Physical Therapy 2019;43(2):96-105. [DOI] [PubMed] [Google Scholar]
Kalyani 2020 {published data only}
- Kalyani HH, Sullivan KA, Moyle GM, Brauer S, Jeffrey ER, Kerr GK. Dance improves symptoms, functional mobility and fine manual dexterity in people with Parkinson disease: a quasi-experimental controlled efficacy study. European Journal of Physical & Rehabilitation Medicine 2020;08:08. [DOI] [PubMed] [Google Scholar]
Kanegusuku 2017 {published data only}
- Kanegusuku H, Silva-Batista C, Pecanha T, Nieuwboer A, Silva ND Jr, Costa LA, et al. Effects of progressive resistance training on cardiovascular autonomic regulation in patients with Parkinson's disease: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2017;98(11):2134-41. [DOI] [PubMed] [Google Scholar]
Klamroth 2019 {published data only}
- Klamroth S, Gasner H, Winkler J, Eskofier B, Klucken J, Pfeifer K, et al. Interindividual balance adaptations in response to perturbation treadmill training in persons with Parkinson disease. Journal of Neurologic Physical Therapy 2019;43(4):224-32. [DOI] [PubMed] [Google Scholar]
Kurlan 2015 {published data only (unpublished sought but not used)}
- Kurlan R, Evans R, Wrigley S, McPartland S, Bustami R, Cotter A. Tai Chi in Parkinson's disease: a preliminary randomized, controlled, and rater-blinded study. Advances in Parkinson's Disease 2015;4:9-12. [Google Scholar]
Lang 2016 {published data only}
- Lang AE, Rodriguez RL, Boyd JT, Chouinard S, Zadikoff C, Espay AJ, et al. Integrated safety of levodopa-carbidopa intestinal gel from prospective clinical trials. Movement Disorders 2016;31(4):538-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lees 2017 {published data only}
- Lees AJ, Ferreira J, Rascol O, Reichmann H, Stocchi F, Tolosa E, et al. Opicapone for the management of end-of-dose motor fluctuations in patients with Parkinson's disease treated with L-DOPA. Expert Review of Neurotherapeutics 2017;17(7):649-59. [DOI] [PubMed] [Google Scholar]
LeWitt 2019 {published data only}
- LeWitt PA, Hauser RA, Pahwa R, Isaacson SH, Fernandez HH, Lew M, et al. Safety and efficacy of CVT-301 (levodopa inhalation powder) on motor function during off periods in patients with Parkinson's disease: a randomised, double-blind, placebo-controlled phase 3 trial. The Lancet Neurology 2019;18(2):145-54. [DOI] [PubMed] [Google Scholar]
Li 2019 {published data only}
- Li Z, Zhuang J, Jiang Y, Xiao G, Jie K, Wang T, et al. Study protocol for a single-blind randomised controlled trial to evaluate the clinical effects of an Integrated Qigong exercise intervention on freezing of gait in Parkinson's disease. BMJ Open 2019;9(9):e028869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lieberman 2019 {published data only}
- Lieberman A, Lockhart TE, Olson MC, Smith Hussain VA, Frames CW, Sadreddin A, et al. Nicotine Bitartrate reduces falls and freezing of gait in Parkinson disease: a reanalysis. Frontiers in Neurology 2019;10:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Litvinenko 2007 {published data only}
- Litvinenko IV, Odinak MM, Mogilnaya VI, Yu Emelin A. Efficacy and safety of galantamine (reminyl) in the treatment of dementia in patients with Parkinson's disease (open-label controlled trial). [Russian]. Zhurnal Nevrologii i Psihiatrii imeni S.S 2007;107(12):25-33. [PubMed] [Google Scholar]
Litvinenko 2008 {published data only}
- Litvinenko IV, Odinak MM, Mogil'naya V I, Emelin AY. Efficacy and safety of galantamine (reminyl) for dementia in patients with Parkinson's disease (an open controlled trial). Neuroscience and Behavioral Physiology 2008;38(9):937-45. [DOI] [PubMed] [Google Scholar]
Mancini 2019 {published data only}
- Mancini M, Chung K, Zajack A, Martini DN, Ramsey K, Lapidus J, et al. Effects of augmenting cholinergic neurotransmission on balance in Parkinson's disease. Parkinsonism & Related Disorders 2019;69:40-7. [DOI] [PubMed] [Google Scholar]
Marumoto 2019 {published data only}
- Marumoto K, Yokoyama K, Inoue T, Yamamoto H, Kawami Y, Nakatani A, et al. Inpatient enhanced multidisciplinary care effects on the quality of life for Parkinson disease: a quasi-randomized controlled trial. Journal of Geriatric Psychiatry and Neurology 2019;32(4):186-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2018 {published data only}
- McDonald J, Pourcher E, Nadeau A, Corbeil P. A randomized trial of oral and transdermal rivastigmine for postural instability in Parkinson disease dementia. Clinical Neuropharmacology 2018;41(3):87-93. [DOI] [PubMed] [Google Scholar]
Mezzarobba 2018 {published data only}
- Mezzarobba S, Grassi M, Pellegrini L, Catalan M, Kruger B, Furlanis G, et al. Action observation plus sonification. A novel therapeutic protocol for Parkinson's patient with freezing of gait. Frontiers in Neurology 2018;8(Jan):723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mi 2019 {published data only}
- Mi TM, Garg S, Ba F, Liu AP, Wu T, Gao LL, et al. High-frequency rTMS over the supplementary motor area improves freezing of gait in Parkinson's disease: a randomized controlled trial. Parkinsonism & Related Disorders 2019;68:85-90. [DOI] [PubMed] [Google Scholar]
Miller 2019 {published data only}
- Miller Koop M, Rosenfeldt AB, Alberts JL. Mobility improves after high intensity aerobic exercise in individuals with Parkinson's disease. Journal of the Neurological Sciences 2019;399:187-93. [DOI] [PubMed] [Google Scholar]
Moro 2010 {published data only}
Myers 2019 {published data only}
- Myers PS, Harrison EC, Rawson KS, Horin AP, Sutter EN, McNeely ME, et al. Yoga Improves balance and low-back pain, but not anxiety, in people with Parkinson's disease. International Journal of Yoga Therapy 2019;04:04. [DOI] [PMC free article] [PubMed] [Google Scholar]
Negrini 2017 {published data only}
- Negrini S, Bissolotti L, Ferraris A, Noro F, Bishop M, Villafane J H. Nintendo Wii Fit for balance rehabilitation in patients with Parkinson's disease: a comparative study. Journal of Bodywork and Movement Therapies 2017;21(1):117-23. [DOI] [PubMed] [Google Scholar]
Nieuwboer 2007 {published data only}
- Nieuwboer A, Kwakkel G, Rochester L, Jones D, Wegen E, Willems AM, et al. Cueing training in the home improves gait-related mobility in Parkinsons disease: the RESCUE trial. Journal of Neurology, Neurosurgery and Psychiatry 2007;78(2):134-40.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oertel 2013 {published data only}
- Oertel W, LeWitt P, Giladi N, Ghys L, Grieger F, Boroojerdi B. Treatment of patients with early and advanced Parkinson's disease with rotigotine transdermal system: age-relationship to safety and tolerability. Parkinsonism & Related Disorders 2013;19(1):37-42. [DOI] [PubMed] [Google Scholar]
Okun 2012 {published data only}
- Okun MS, Gallo BV, Mandybur G, Jagid J, Foote KD, Revilla FJ, et al. Subthalamic deep brain stimulation with a constant-current device in Parkinson's disease: an open-label randomised controlled trial. Lancet Neurology. 2012;11:140-9. [DOI] [PubMed] [Google Scholar]
Olanow 2020 {published data only}
- Olanow CW, Factor SA, Espay AJ, Hauser RA, Shill HA, Isaacson S, et al. Apomorphine sublingual film for off episodes in Parkinson's disease: a randomised, double-blind, placebo-controlled phase 3 study. Lancet Neurology 2020;19(2):135-44. [DOI] [PubMed] [Google Scholar]
Ozgonenel 2016 {published data only}
- Ozgonenel L, Cagirici S, Cabalar M, Durmusoglu G. Use of game console for rehabilitation of Parkinson's disease. Balkan Medical Journal 2016;33(4):396-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perez 2017 {published data only}
- Perez de la Cruz S. Effectiveness of aquatic therapy for the control of pain and increased functionality in people with Parkinson's disease: a randomized clinical trial. European Journal of Physical & Rehabilitation Medicine. 2017;53(6):825-32. [DOI] [PubMed] [Google Scholar]
Pohl 2020 {published data only}
- Pohl P, Wressle E, Lundin F, Enthoven P, Dizdar N. Group-based music intervention in Parkinson's disease - findings from a mixed-methods study. Clinical Rehabilitation 2020;34(4):533-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Postuma 2008 {published data only}
- Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. REM sleep behaviour disorder in Parkinson's disease is associated with specific motor features. Journal of Neurology, Neurosurgery & Psychiatry 2008;79(10):1117-21. [DOI] [PubMed] [Google Scholar]
Rascol 2016 {published data only}
- Rascol O, Hauser RA, Stocchi F, Fitzer-Attas CJ, Sidi Y, Abler V, et al. Long-term effects of rasagiline and the natural history of treated Parkinson's disease. Movement Disorders 2016;31(10):1489-96. [DOI] [PubMed] [Google Scholar]
Rawson 2019 {published data only}
- Rawson KS, McNeely ME, Duncan RP, Pickett KA, Perlmutter JS, Earhart GM. Exercise and Parkinson disease: comparing tango, treadmill, and stretching. Journal of Neurologic Physical Therapy 2019;43(1):26-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sato 2011 {published data only}
- Sato Y, Iwamoto J, Honda Y. Amelioration of osteoperosis and hypovitaminosis D by sunlight exposure in Parkinson's disease. Parkinsonism and Related Disorders 2011;17(1):22-6. [DOI] [PubMed] [Google Scholar]
Sato 2013 {published data only}
- Sato Y, Iwamoto J, Honda Y, Amano N. Vitamin D reduces falls and hip fractures in vascular Parkinsonism but not in Parkinson's disease. Therapeutics and Clinical Risk Management 2013;9(1):171-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Schenkman 2018 {published data only}
- Schenkman M, Moore CG, Kohrt WM, Hall DA, Delitto A, Comella CL, et al. Effect of high-intensity treadmill exercise on motor symptoms in patients with De Novo Parkinson disease a phase 2 randomized clinical trial. JAMA Neurology 2018;75(2):219-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scianni 2015 {published data only}
- Scianni A. Tai Chi improves balance and prevents falls in people with Parkinson's disease. Journal of Physiotherapy 2015;61(1):44. [DOI] [PubMed] [Google Scholar]
Sedaghati 2018 {published data only}
- Sedaghati P, Goudarzian M, Daneshmandi H, Ardjmand A. Effects of alexander-based corrective techniques on forward flexed posture, risk of fall, and fear of falling in idiopathic Parkinson's disease. Archives of Neuroscience 2018;5(2):e61274. [Google Scholar]
Silva‐Batista 2018 {published data only}
- Silva-Batista C, Corcos DM, Kanegusuku H, Piemonte ME, Gobbi LT, Lima-Pardini AC, et al. Balance and fear of falling in subjects with Parkinson's disease is improved after exercises with motor complexity. Gait & Posture 2018;61:90-7. [DOI] [PubMed] [Google Scholar]
Simuni 2020 {published data only}
- Simuni T. Isradipine versus placebo in early Parkinson disease a randomized trial. Annals of Internal Medicine 2020;172(9):591-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sparrow 2016 {published data only (unpublished sought but not used)}
- Saprrow D, DeAngelis TR, Hendron K, Thomas CA, Saint-Hilaire S, Ellis T. Highly challenging balance program reduces fall rate in Parkinson disease. Journal of Neurologic Physical Therapy 2016;40(1):24-30. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
St George 2015 {published data only}
- St George RJ, Carlson-Kuhta P, King LA, Burchiel KJ, Horak FB. Compensatory stepping in Parkinson's disease is still a problem after deep brain stimulation randomized to STN or GPi. Journal of Neurophysiology 2015;114(3):1417-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stozek 2003 {published data only}
- Stozek J, Rudzinska M, Longawa K, Szczudlik A. The effect of the complex rehabilitation on posture and gait in Parkinson disease. [Polish]. Neurologia i Neurochirurgia Polska 2003;37 Suppl 5:67-81. [PubMed] [Google Scholar]
Strouwen 2017 {published data only}
- Strouwen C, Molenaar Ealm Munks L, Keus SH, Zijlmans JC, Vandenberghe W, Bloem BR, et al. Training dual tasks together or apart in Parkinson's disease: results from the DUALITY trial. Movement Disorders 2017;32(8):1201-10. [DOI] [PubMed] [Google Scholar]
Thevathasan 2010 {published data only}
Toole 2005 {published data only}
- Toole T, Maitland CG, Warren E, Hubmann MF, Panton L. The effects of loading and unloading treadmill walking on balance, gait, fall risk, and daily function in Parkinsonism. NeuroRehabilitation 2005;20(4):307-22. [PubMed] [Google Scholar]
van Nimwegen 2013 {published data only}
- Nimwegen M, Speelman AD, Overeem S, de Warrenburg BP, Smulders K, Dontje ML, et al. Promotion of physical activity and fitness in sedentary patients with Parkinson's disease: randomised controlled trial. BMJ 2013;346:f576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Puymbroeck 2018 {published data only}
- Van Puymbroeck M, Walter AA, Hawkins BL, Sharp JL, Woschkolup K, Urrea-Mendoza E, et al. Functional improvements in Parkinson's disease following a randomized trial of yoga. Evidence-Based Complementary & Alternative Medicine: eCAM 2018;2018:8516351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vercruysse 2014 {published data only}
- Vercruysse S, Vandenberghe W, Munks L, Nuttin B, Devos H, Nieuwboer A. Effects of deep brain stimulation of the subthalamic nucleus on freezing of gait in Parkinson's disease: a prospective controlled study. Journal of Neurology, Neurosurgery and Psychiatry 2014;85(8):872-8. [DOI] [PubMed] [Google Scholar]
Walter 2019 {published data only}
- Walter AA, Adams EV, Van Puymbroeck M, Crowe BM, Urrea-Mendoza E, Hawkins BL, et al. Changes in nonmotor symptoms following an 8-week yoga intervention for people with Parkinson’s disease. International Journal of Yoga Therapy 2019;29(1):91-9. [DOI] [PubMed] [Google Scholar]
Wass 2008 {published data only}
- Wass S, Webster PJ, Nair BR. Delirium in the elderly: a review. Oman Medical Journal 2008;23(3):150-7. [PMC free article] [PubMed] [Google Scholar]
Welter 2015 {published data only}
- Welter ML, Demain A, Ewenczyk C, Czernecki V, Lau B, El Helou A, et al. PPNa-DBS for gait and balance disorders in Parkinson's disease: a double-blind, randomised study. Journal of Neurology 2015;262(6):1515-25. [DOI] [PubMed] [Google Scholar]
Whone 2019 {published data only}
- Whone A, Luz M, Boca M, Woolley M, Mooney L, Dharia S, et al. Randomized trial of intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson's disease. Brain 2019;142(3):512-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2016 {published data only}
- Wong RK, Tsang DS, Lau CK, Chan AY, Chan DT, Zhu X, et al. Effectiveness of occupational therapy multi-domain group therapy program for Parkinson's disease. Movement Disorders 2016;31:S159. [Google Scholar]
Yuan 2020 {published data only}
- Yuan RY, Chen SC, Peng CW, Lin YN, Chang YT, Lai CH. Effects of interactive video-game-based exercise on balance in older adults with mild-to-moderate Parkinson's disease. Journal of Neuroengineering & Rehabilitation 2020;17(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2018 {published data only}
- Zhang Z, Shao M, Chen S, Liu C, Peng R, Li Y, et al. Adjunct rasagiline to treat Parkinson's disease with motor fluctuations: a randomized, double-blind study in China. Translational Neurodegeneration 2018;7(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Lurie 2020 {published and unpublished data}
- Lurie JD, Zagaria AB, Ellis L, Pidgeon D, Gill-Body KM, Burke C, et al. Surface perturbation training to prevent falls in older adults: a highly pragmatic, randomized controlled trial. Physical Therapy 2020;100(7):1153-62. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2021 {published data only}
- Taylor PN, Sampson T, Beare B, Donavon-Hall M, Thomas PW, Marques E, et al. The effectiveness of peroneal nerve functional electrical stimulation for the reduction in bradykinesia in Parkinson's disease: a feasibility study for a randomised control trial. Clinical Rehabilitation 2021;35(4):546-57. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12618001515280 {published data only}
- ACTRN12618001515280. SAFE-PD - Stepping to Avoid Fall Events in Parkinson’s disease. https://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12618001515280 (first receieved september 10, 2018).
ACTRN12619000415101 {published data only}
- ACTRN12619000415101. The Integrate program for safe mobility in Parkinson's disease.. https://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12619000415101 (first received March 13, 2019).
ACTRN12620001135909 {published data only}
- ACTRN12620001135909. A randomised trial of exercise therapy for Parkinson’s disease. https://trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12620001135909 (first received October 30, 2020).
ChiCTR2000038852 {published data only}
- ChiCTR2000038852. Study on the effect and mechanism of cognitive-cup-tapping-balance-training on fall prevention in community Parkinson's patients: a randomized controlled trial. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2000038852 (first received October 7, 2020).
DRKS00024982 {published data only}
- DRKS00024982. Effects of an activity-oriented physiotherapy exercise programme with and without eye movement training on dynamic balance and fall risk in people with Parkinson’s disease: a randomised controlled pilot trial. https://trialsearch.who.int/Trial2.aspx?TrialID=DRKS00024982 (first received April 8, 2021).
NCT02107638 {published data only}
- NCT02107638. Effect of osteopathic manipulative medicine on Parkinson disease. https://clinicaltrials.gov/ct2/show/NCT02107638 (first receieved April 8, 2014).
NCT03727529 {published data only}
- NCT03727529. Immersive virtual reality to improve Gait in Parkinson's disease (NMSK-LH02). https://clinicaltrials.gov/ct2/show/NCT03727529 (first receieved November 1, 2018).
NCT03751371 {published data only}
- NCT03751371. Robotic walking device to improve mobility in Parkinson's disease. https://clinicaltrials.gov/ct2/show/NCT03751371 (first receieved November 23, 2018).
NCT03972969 {published data only}
- NCT03972969. Highly challenging balance program to reduce fall rate in PD. https://clinicaltrials.gov/ct2/show/NCT03972969 (first receieved June 4, 2019).
NCT04093544 {published data only}
- NCT04093544. Expanding the therapeutic window of deep brain stimulation in Parkinson's disease by means of directional leads. https://clinicaltrials.gov/ct2/show/NCT04093544 (first receieved September 8, 2019).
NCT04108741 {published data only}
- NCT04108741. Augmented reality treadmill training in patients with Parkinson's disease (Falls in PD). https://clinicaltrials.gov/ct2/show/NCT04108741 (first receieved september 30, 2019).
NCT04116177 {published data only}
- NCT04116177. Flexible vs. standard deep brain stimulation programming in Parkinson disease patients. https://clinicaltrials.gov/ct2/show/NCT04116177 (first receieved October 4, 2019).
NCT04226248 {published data only}
- NCT04226248. CHIEF PD (CHolinesterase Inhibitor to prEvent Falls in Parkinson's Disease). https://clinicaltrials.gov/ct2/show/NCT04226248 (first receieved January 13, 2020).
NCT04300023 {published data only}
- NCT04300023. In-home cycling for individuals with PD. https://clinicaltrials.gov/ct2/show/NCT04300023 (first receieved March 9, 2020).
NCT04300348 {published data only}
- NCT04300348. Improving walking with Heel-To-Toe device. https://clinicaltrials.gov/ct2/show/NCT04300348 (first received March 9, 2020).
NCT04389138 {published data only}
- NCT04389138. Is physiotherapy effective for people with early Parkinson's (PEEP). https://clinicaltrials.gov/ct2/show/NCT04389138 (first receieved May 15, 2020).
NCT04408573 {published data only}
- NCT04408573. Cycling deep brain stimulation on Parkinson's disease gait (DBS). https://clinicaltrials.gov/ct2/show/NCT04408573 (first receieved May 29, 2020).
NCT04555720 {published data only}
- NCT04555720. The Benchmark Clinic: an interdisciplinary comprehensive care model for people with Parkinson disease. https://clinicaltrials.gov/ct2/show/NCT04555720 (first received September 21, 2020).
NCT04613141 {published data only}
- NCT04613141. The WalkingTall Study: comparing WalkingTall with Parkinson's Disease (WalkingTall-PD) with mobility-plus to reduce falls and improve mobility. (WalkingTall-PD). https://clinicaltrials.gov/ct2/show/NCT04613141 (first received November 3, 2020).
NCT04634331 {published data only}
- NCT04634331. Dual-task Augmented Reality Treatment for Parkinson's disease (DART). https://clinicaltrials.gov/ct2/show/NCT04634331 (first received November 18, 2020).
NCT04665869 {published data only}
- NCT04665869. Long-term effects of combined balance and brisk walking in Parkinson's disease. https://clinicaltrials.gov/ct2/show/NCT04665869 (first received December 14, 2020).
NCT04694443 {published data only}
- NCT04694443. Multidisciplinary home-based Tele-rehabilitation Intervention (TeleFall). https://clinicaltrials.gov/ct2/show/NCT04694443 (first received January 5, 2021).
NCT04848077 {published data only}
- NCT04848077. STEPWISE Parkinson: a Smartphone based exercise solution for patients with Parkinson's disease (STEPWISE). https://clinicaltrials.gov/ct2/show/NCT04848077 (first received April 19, 2021).
NCT04874051 {published data only}
- NCT04874051. Sensor-based assessment and rehabilitation of balance in neurological diseases (BALANCE). https://clinicaltrials.gov/ct2/show/NCT04874051 (first received May 5, 2021).
NCT04897256 {published data only}
- NCT04897256. Mobility in daily life and falls in Parkinson's disease: potential for rehabilitation. https://clinicaltrials.gov/ct2/show/NCT04897256 (first received May 21, 2021).
NCT04946812 {published data only}
- NCT04946812. Split-belt treadmill training to rehabilitate freezing of gait and balance in Parkinson's disease. https://clinicaltrials.gov/ct2/show/NCT04946812 (first received July 1, 2021).
NCT04953637 {published data only}
- NCT04953637. Physiotherapy and deep brain stimulation in Parkinson's disease. https://clinicaltrials.gov/ct2/show/NCT04953637 (first received July 8, 2021).
NCT05127057 {published data only}
- NCT05127057. Proactive and Integrated Management and Empowerment in Parkinson's disease (PRIME-UK): a New model of care (PRIME-RCT) (PRIME-RCT). https://clinicaltrials.gov/ct2/show/NCT05127057 (first received November 19, 2021).
NCT05172661 {published data only}
- NCT05172661. Effects of physical-cognitive training with different task models in Parkinson's disease with mild cognitive impairment. https://clinicaltrials.gov/ct2/show/NCT05172661 (first received December 29, 2021).
RBR‐5w2sqt {published data only}
- RBR-5w2sqt. Effects of strength exercises with elastic bands and tubes on the difficulty of movements, quality of life, sleep, memory, depressive symptoms, balance and risk of falls of patients with Parkinson's disease. http://www.ensaiosclinicos.gov.br/rg/RBR-5w2sqt/ (first receieved february 24, 2020).
Additional references
Allcock 2009
- Allcock LM, Rowan EN, Steen IN, Wesnes K, Kenny RA, Burn DJ. Impaired attention predicts falling in Parkinson's disease. Parkinsonism & Related Disorders 2009;15(2):110-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Allen 2011
- Allen NE, Sherrington C, Paul SS, Canning CG. Balance and falls in Parkinson's disease: a meta-analysis of the effect of exercise and motor training. Movement Disorders 2011;26(9):1605-15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Allen 2013
- Allen NE, Schwarzel AK, Canning CG. Recurrent falls in Parkinson's disease: a systematic review. Parkinson's Disease 2013;2013:906274. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ashburn 2019
- Ashburn A, Pickering R, McIntosh E, Hulbert S, Rochester L, Roberts HC, et al. Exercise- and strategy-based physiotherapy-delivered intervention for preventing repeat falls in people with Parkinson's: the PDSAFE RCT. Health Technology Assessment 2019;23(36):1-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhidayasiri 2015
- Bhidayasiri R, Jitkritsadakul O, Boonrod N, Sringean J, Calne SM, Hattori N, et al. What is the evidence to msupport home environmental adaptation in Parkinson's disease? A call for multidisciplinary interventions. Parkinsonism and Related Disorders 2015;21(10):1127-32. [DOI] [PubMed] [Google Scholar]
Bloem 2001
- Bloem BR, Grimbergen YA, Cramer M, Willemsen M, Zwinderman AH. Prospective assessment of falls in Parkinson's disease. Journal of Neurology 2001;248(11):950-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bolland 2016
- Bolland MJ, Avenell A, Gamble GD, Grey A. Systematic review and statistical analysis of the integrity of 33 randomized controlled trials. Neurology 2016;87:2391-402. [DOI] [PubMed] [Google Scholar]
Cameron 2018
- Cameron ID, Dyer SM, Panagoda CE, Murray GR, Hill KD, Cumming RG, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database of Systematic Reviews 2018, Issue 9. Art. No: CD005465. [DOI: 10.1002/14651858.CD005465.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Canning 2014
- Canning CG, Paul SS, Nieuwboer A. Prevention of falls in Parkinson's disease: a review of fall risk factors and the role of physical interventions. Neurodegenerative Disease Management 2014;4(3):203-21. [DOI] [PubMed] [Google Scholar]
Canning 2020
- Canning CG, Allen NE, Nackaerts E, Paul SS, Nieuwboer A, Gilat M. Virtual reality in research and rehabilitation of gait and balance in Parkinson disease. Nature Reviews Neurology 2020;16(8):409-25. [DOI: 10.1038/s41582-020-0370-2] [DOI] [PubMed] [Google Scholar]
Cochrane Norway 2017
- Cochrane Norway. How to write a plain language summary of a Cochrane intervention review. https://www.cochrane.no/sites/cochrane.no/files/public/uploads/checklist_for_cochrane_pls_28th_feb_2017_0.pdf (accessed 21 October 2020).
Del Din 2020
- Del Din S, Galna B, Lord S, Nieuwboer A, Bekkers EM, Pelosin E, et al. Fall risk in relation to activity exposure in high-risk older adults. journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2020;75(6):1198-205. [DOI: 10.1093/gerona/glaa007] [DOI] [PMC free article] [PubMed] [Google Scholar]
Domingos 2015
- Domingos JM, Godinho C, Dean J, Coelho M, Pinto A, Bloem BR, et al. Cognitive impairment in fall-related studies in Parkinson's disease. Journal of Parkinson's Disease 2015;5(3):453-69. [DOI: 10.3233/JPD-150590] [DOI] [PMC free article] [PubMed] [Google Scholar]
Farag 2016
- Farag I, Sherrington C, Hayes A, Canning CG, Lord SR, Close JC, et al. Economic evaluation of a falls prevention exercise program among people With Parkinson's disease. Movement Disorders 2016;31(1):53-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fasano 2012
- Fasano A, Plotnik M, Bove F, Berardelli A. The neurobiology of falls. Neurological Sciences 2012;33:1215-1223. [DOI: 10.1007/s10072-012-1126-6] [DOI] [PubMed] [Google Scholar]
Fasano 2017
- Fasano A, Canning CG, Hausdorff JM, Lord S, Rochester L. Falls in Parkinson's disease: A complex and evolving picture. Movement Disorders 2017;32(11):1524-36. [DOI: 10.1002/mds.27195] [DOI] [PubMed] [Google Scholar]
Fletcher 2012
- Fletcher E, Goodwin VA, Richards SH, Campbell JL, Taylor RS. An exercise intervention to prevent falls in Parkinson's: an economic evaluation. BMC Health Services Research 2012;12:426. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flynn 2019
- Flynn A, Allen NE, Dennis S, Canning CG, Preston E. Home-based prescribed exercise improves balance-related activities in people with Parkinson's disease and has benefits similar to centre-based exercise: a systematic review. Journal of Physiotherapy 2019;65:189-99. [DOI: 10.1016/j.jphys.2019.08.003] [DOI] [PubMed] [Google Scholar]
Gibson 1987
- Gibson M, Andres B, Isaacs B, Radebaugh T, Worm-Peterson J. The prevention of falls in later life: a report of the Kellogg International Work Group on the prevention of falls by the elderly. Danish Medical Bulletin 1987;34(suppl 4):1-24. [PubMed] [Google Scholar]
Gillespie 2012
- Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, et al. Interventions for preventing falls in older people living in the community. Cochrane Database of Systematic Reviews 2012, Issue 9. Art. No: CD007146. [DOI: 10.1002/14651858.CD007146.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEPro GDT 2015 [Computer program]
- Hamilton (ON): McMaster University GRADEpro GDT. McMaster University (developed by Evidence Prime). Hamilton (ON): McMaster University, 2015.
Hannan 2010
- Hannan MT, Gagnon MM, Aneja J, Jones RN, Cupples LA, Lipsitz LA, et al. Optimizing the tracking of falls in studies of older participants: comparison of quarterly telephone recall with monthly falls calendars in the MOBILIZE Boston Study. American Journal of Epidemiology 2010;171(9):1031-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hely 2008
- Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Movement Disorders 2008;23(6):837-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hentz 2015
- Hentz JG, Mehta SH, Shil HA, Driver-Dunckley E, Beach TG, Adler CH. Simplified conversion method for Unified Parkinson's Disease Rating Scale motor examinations. Movement Disorders 2015;30(14):1967-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Deeks JJ, Altman DG. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook-5-1.cochrane.org. [Google Scholar]
Higgins 2017
- Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook. [Google Scholar]
Hopewell 2018
- Hopewell S, Adedire O, Copsey BJ, Boniface GJ, Sherrington C, Clemson L, et al. Multifactorial and multiple component interventions for preventing falls in older people living in the community. Cochrane Database of Systematic Reviews 2018, Issue 7. Art. No: CD012221. [DOI: 10.1002/14651858.CD012221.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 1992
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. Journal of Neurology, Neurosurgery, and Psychiatry 1992;55(3):181-4. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hulbert 2019
- Hulbert S, Rochester L, Nieuwboer A, Goodwin V, Fitton C, Chivers-Seymour K, et al. "Staying safe" - a narrative review of falls prevention in people with Parkinson's - "PDSAFE". Disability and Rehabilitation 2019;41(21):2596-605. [DOI: 10.1080/09638288.2018.1471167] [DOI] [PubMed] [Google Scholar]
Hunter 2018
- Hunter H, Rochester L, Morris R, Lord S. Longitudinal falls data in Parkinson’s disease: feasibility of fall diaries and effect of attrition. Disability and Rehabilitation 2018;40(19):2236-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Iliffe 2015
- Iliffe S, Kendrick D, Morris R, Griffin M, Haworth D, Carpenter H, et al. Promoting physical activity in older people in general practice: ProAct65+ cluster randomised controlled trial. British Journal of General Practice 2015;65(640):e731-738. [DOI: 10.3399/bjgp15X687361] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalilani 2016
- Kalilani L, Asgharnejad M, Palokangas T, Durgin T. Comparing the incidence of falls/fractures in Parkinson's disease patients in the US population. PLOS One 2016;11(9):1-11. [DOI: 10.1371/journal.pone.0161689] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerr 2010
- Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn PA. Predictors of future falls in Parkinson disease. Neurology 2010;75(2):116-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lamb 2005
- Lamb SE, Jorstad-Stein EC, Hauer K, Becker C. Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. Journal of the American Geriatrics Society 2005;53(9):1618-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lamb 2011
- Lamb SE, Becker C, Gillespie LD, Smith JL, Finnegan S, Potter R, et al. Reporting of complex interventions in clinical trials: development of a taxonomy to classify and describe fall-prevention interventions. Trials 2011;12:125. [DOI: 10.1186/1745-6215-12-125] [DOI] [PMC free article] [PubMed] [Google Scholar]
Latt 2009
- Latt MD, Lord SR, Morris JG, Fung VS. Clinical and physiological assessments for elucidating falls risk in Parkinson's disease. Movement Disorders 2009;24(9):1280-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Li 2015b
- Li F, Harmer P. Economic evaluation of a Tai Ji Quan intervention to reduce falls in people with Parkinson disease, Oregon, 2008-2011. Preventing Chronic Disease 2015;12:E120. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mactier 2015
- Mactier K, Lord S, Godfrey A, Burn D, Rochester L. The relationship between real world ambulatory activity and falls in incident Parkinson's disease: influence of classification scheme. Parkinsonism & Related Disorders 2015;21(3):236-42. [DOI: 10.1016/j.parkreldis.2014.12.014] [DOI] [PubMed] [Google Scholar]
Mak 2009
- Mak MK, Pang MY. Fear of falling is independently associated with recurrent falls in patients with Parkinson’s disease: a 1-year prospective study. Journal of Neurology 2009;256(10):1689-95. [DOI: 10.1007/s00415-009-5184-5] [DOI] [PubMed] [Google Scholar]
Nonnekes 2018
- Nonnekes J, Nieuwboer A. Towards personlized rehabilitation for gait impairments in Parkinson's disease. Journal of Parkinson's Disease 2018;8(s1):S101-S106. [DOI: 10.3233/JPD-181464] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paul 2013
- Paul SS, Canning CG, Sherrington C, Lord SR, Close JC, Fung VS. Three simple clinical tests to accurately predict falls in people with Parkinson's disease. Movement Disorders 2013;28(5):655-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
Paul 2017
- Paul SS, Harvey L, Canning CG, Boufous S, Lord SR, Close JC, et al. Fall-related hospitalization in people with Parkinson's disease. European Journal of Neurology 2017;24(3):523-9. [DOI: 10.1111/ene.13238] [DOI] [PubMed] [Google Scholar]
Pelicioni 2020
- Pelicioni PH, Schulz-Moore JS, Hale L, Canning CG, Lord SR. Lockdown during COVID-19 and the increase of frailty in people with neurological conditions. Frontiers in Neurology 2020;11:604299. [DOI: 10.3389/fneur.2020.604299] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peto 2001
- Peto V, Jenkinson C, Fitzpatrick R. Determining minimally important differences for the PDQ-39 Parkinson's disease questionnaire. Age and Ageing 2001;30(4):299-302. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pickering 2007
- Pickering RM, Grimbergen YA, Rigney U, Ashburn A, Mazibrada G, Wood B, et al. A meta-analysis of six prospective studies of falling in Parkinson's disease. Movement Disorders 2007;22(13):1892-900. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pressley 2003
- Pressley JC, Louis ED, Tang MX, Cote L, Cohen PD, Glied S, et al. The impact of comorbid disease and injuries on resource use and expenditures in Parkinsonism. Neurology 2003;60(1):87-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rascol 2015
- Rascol O, Perez-Lloret S, Damier P, Delval A, Derkinderen P, Destee A, et al. Falls in ambulatory non-demented patients with Parkinson's disease. Journal of Neural Transmission 2015;122(10):1447-55. [DOI: 10.1007/s00702-015-1396-2] [DOI] [PubMed] [Google Scholar]
RevMan 5.4 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.4.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Schűnemann 2013
- Schűnemann H, Brozek J, Guyatt G, Oxman A, editors. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from https://gdt.gradepro.org/app/handbook/handbook.html. [Google Scholar]
Shen 2016
- Shen X, Wong IS, Mak MK. Effects of exercise on falls, balance and gait ability in Parkinson’s diseases: a meta-analysis. Neurorehabilitation and Neural Repair 2016;30(6):512-27. [DOI: 10.1177/1545968315613447] [DOI] [PubMed] [Google Scholar]
Sherrington 2017
- Sherrington C, Michaleff ZA, Fairhall N, Paul SS, Tiedemann A, Whitney J, et al. Exercise to prevent falls in older adults: an updated systematic review and meta-analysis. British Journal of Sports Medicine 2017;51:1749-57. [DOI: 10.1136/bjsports-2016-096547] [DOI] [PubMed] [Google Scholar]
Sherrington 2019
- Sherrington C, Fairhall NJ, Wallbank GK, Tiedemann A, Michaleff ZA, Howard K, et al. Exercise for preventing falls in older people living in the community. Cochrane Database of Systematic Reviews 2019, Issue 1. Art. No: CD012424. [DOI: 10.1002/14651858.CD012424.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Silva de Lima 2020
- Silva de Lima AL, Smits T, Darweesh SK, Valenti G, Milosevic M, Pijl M, et al. Home-based monitoring of falls using wearable sensors in Parkinson's disease. Movement Disorders 2020;35(1):109-15. [DOI: 10.1002/mds.27830] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smeeth 2002
- Smeeth L, Ng ES. Intraclass correlation coefficients for cluster randomized trials in primary care: data from the MRC Trial of the Assessment and Management of Older People in the Community. Controlled Clinical Trials 2002;23(4):409-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
Soh 2011
- Soh SE, Morris ME, McGinley JL. Determinants of health-related quality of life in Parkinson's disease: a systematic review. Parkinsonism & Related Disorders 2011;17(1):1-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stack 2013
- Stack EL, Roberts HC. Slow down and concentrate: time for a paradigm shift in fall prevention among people with Parkinson’s disease? Parkinson's Disease 2013;2013:Article ID 704237. [DOI: 10.1155/2013/704237] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomlinson 2013
- Tomlinson CL, Patel S, Meek C, Herd CP, Clarke CE, Stowe R, et al. Physiotherapy versus placebo or no intervention in Parkinson's disease. Cochrane Database of Systematic Reviews 2013, Issue 9. Art. No: CD002817. [DOI: 10.1002/14651858.CD002817.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomlinson 2014
- Tomlinson CL, Herd CP, Clarke CE, Meek C, Patel S, Stowe R, et al. Physiotherapy for Parkinson's disease: a comparison of techniques. Cochrane Database of Systematic Reviews 2014, Issue 6. Art. No: CD002815. [DOI: 10.1002/14651858.CD002815.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Marck 2011
- Marck MA, Overeem S, Klok PC, Bloem BR, Munneke M. Evaluation of the falls telephone: an automated system for enduring assessment of falls. Journal of the American Geriatrics Society 2011;59(2):340-4. [DOI: 10.1111/j.1532-5415.2010.03263.x] [DOI] [PubMed] [Google Scholar]
van der Marck 2014
- Marck MA, Klok MP, Okun MS, Giladi N, Munneke M, Bloem B, on behalf of the NPF Task Force. Consensus-based clinical practice recommendations for the examination and management of falls in patients with Parkinson's disease. Parkinsonism and Related Disorders 2014;20:360-9. [DOI] [PubMed] [Google Scholar]
Walker 2013
- Walker RW, Chaplin A, Hancock RL, Rutherford R, Gray WK. Hip fractures in people with idiopathic Parkinson's disease: incidence and outcomes. Movement Disorders 2013;28(3):334-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Walters 2005
- Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Quality of Life Research 2005;14(6):1523-32. [DOI] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14:135. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
WebPlotDigitizer 2020 [Computer program]
- WebPlotDigitizer, version 4.3. Rohatgi A. https://automeris.io/WebPlotDigitizer, 2020.
WHO 2020
- World Health Organization. WHO guidelines on physical activity and sedentary behaviour. Geneva: World Health Organization, 2020. [LICENCE: CC BY-NC-SA 3.0 IGO] [Google Scholar]
Wielinski 2005
- Wielinski CL, Erickson-Davis C, Wichmann R, Walde-Douglas M, Parashos SA. Falls and injuries resulting from falls among patients with Parkinson's disease and other parkinsonian syndromes. Movement Disorders 2005;20(4):410-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Xin 2020
- Xin Y, Ashburn A, Pickering RM, Chivers Seymour K, Hulbert S, Fitton C, et al. Cost-effectiveness of the PDSAFE personalised physiotherapy intervention for fall prevention in Parkinson's: an economic evaluation alongside a randomised controlled trial. BMC Neurology 2020;20(295):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Canning 2015b
- Canning CG, Allen NE, Bloem BR, Keus SHJ, Munneke M, Nieuwboer A, et al. Interventions for preventing falls in Parkinson's disease. Cochrane Database of Systematic Reviews 2015, Issue 3. Art. No: CD011574. [DOI: 10.1002/14651858.CD011574] [DOI] [PMC free article] [PubMed] [Google Scholar]


