Significance
Stimulants such as methylphenidate (Ritalin) are often used clinically to treat attention deficit hyperactivity disorder (ADHD) and generally to improve selective attention. Their systemic administration provides a causal manipulation of the attentional system, allowing measurements of the neuronal population changes that accompany artificially manipulated selective attention. We found that orally administering methylphenidate to rhesus monkeys selectively improved visual performance at only their attended spatial location, and that correspondingly specific changes in the shared variability of groups of neurons in visual area V4 occurred only when attention was directed toward the receptive fields of those neurons. Critically, methylphenidate changed behavior exactly when it changed the shared variability of the neuronal responses, suggesting that it may work through naturally selective cognitive mechanisms.
Keywords: attention, attention deficit disorder with hyperactivity, methylphenidate, population coding, visual cortex
Abstract
Most systems neuroscience studies fall into one of two categories: basic science work aimed at understanding the relationship between neurons and behavior, or translational work aimed at developing treatments for neuropsychiatric disorders. Here we use these two approaches to inform and enhance each other. Our study both tests hypotheses about basic science neural coding principles and elucidates the neuronal mechanisms underlying clinically relevant behavioral effects of systemically administered methylphenidate (Ritalin). We discovered that orally administered methylphenidate, used clinically to treat attention deficit hyperactivity disorder (ADHD) and generally to enhance cognition, increases spatially selective visual attention, enhancing visual performance at only the attended location. Further, we found that this causal manipulation enhances vision in rhesus macaques specifically when it decreases the mean correlated variability of neurons in visual area V4. Our findings demonstrate that the visual system is a platform for understanding the neural underpinnings of both complex cognitive processes (basic science) and neuropsychiatric disorders (translation). Addressing basic science hypotheses, our results are consistent with a scenario in which methylphenidate has cognitively specific effects by working through naturally selective cognitive mechanisms. Clinically, our findings suggest that the often staggeringly specific symptoms of neuropsychiatric disorders may be caused and treated by leveraging general mechanisms.
Studying the behavioral and neuronal effects of stimulants such as methylphenidate is important for both translational and basic science reasons. It is of translational importance because stimulants are widely used by adults and children, but their neuronal mechanisms remain unclear (1). More than 6% of children in the United States are prescribed stimulants to treat attention deficit hyperactivity disorder (ADHD) (2). Additionally, one-fifth of polled Nature readers report using these stimulants without prescription to enhance performance (3), with this number thought to be much larger among college students (4). These stimulants are frequently used both with and without prescription with the intention of improving selective attention, which allows one to focus on a desired target and tune out distractors (5). However, despite the frequent goal of achieving selective changes in performance, most behavioral and neuroscientific studies of stimulants have focused on examining overall performance changes related to global processes such as motivation and vigilance (1, 4, 6–13).
Studying stimulants is also important because it provides a strong, causal test of basic science hypotheses about how groups of neurons affect visually guided behaviors. In a previous study (14), we demonstrated that there is a robust relationship between the magnitude of correlated variability in visual cortex [the shared trial-to-trial variability of pairs of neurons in response to repeated presentations of the same stimulus (15)] and the ability of rhesus monkeys to detect changes in the orientation of a visual stimulus. This relationship between neuronal populations in visual area V4 and performance persisted whether correlated variability and behavior were changed by spatial attention on fast timescales, perceptual learning over several weeks, or factors outside experimenter control. These observations led to the hypothesis that a cognitive process, neuropsychiatric disorder, or causal manipulation should affect performance on this task precisely when it affects correlated variability in V4. Methylphenidate as a causal manipulation comprises a strong test of this hypothesis because it has widespread effects on the dopamine system throughout the brain (16, 17), and it is unknown whether a systemically administered stimulant can have such specific effects on neuronal activity.
Results
To test our basic science hypotheses and investigate the clinically relevant behavioral and neuronal effects of methylphenidate, we administered methylphenidate and recorded populations of V4 neurons in rhesus monkeys trained to perform a perceptually challenging visual task with a spatial attention component. We chose oral administration because this is the most common means of methylphenidate administration (10) and to test the effects of a systemic manipulation of the attentional system on the activity of a neuronal population in sensory cerebral cortex.
On alternating days, a monkey drank either sugar water with methylphenidate mixed in or a placebo of only sugar water (18). The sugar water with or without methylphenidate was administered 30 min prior to behavioral testing (19).
The heart of our analytical approach is to compare pairs of experimental sessions with matched stimulus and task parameters (Methods) that were conducted on adjacent days. Each pair of sessions included one in which we administered methylphenidate and one in which we administered a placebo control. We used a paired-days paradigm to control for potential fluctuations in electrical signal and environmental factors (14).
We used between 2 and 6 mg/kg (Methods) (20–24), and the data from all dosages were included together in the analyses to avoid best-dose analyses (25) (while our goal was to use the systemic administration of methylphenidate as a causal test of our hypotheses, not to test for dose-dependent effects, we have included analyses per dosage in SI Appendix, Figs. S1B and S2 A and B).
To measure the effects of methylphenidate on selective attention, we trained three rhesus monkeys to perform the visual change-detection task that we used to manipulate spatial attention in our previous work (Fig. 1A) (14, 26). The monkey fixated a central point while two peripheral Gabor stimuli flashed on and off. At a random and unsignaled time, the orientation of one stimulus changed slightly. The monkey was rewarded for making an eye movement toward the changed stimulus. We manipulated spatial attention using a classic Posner spatial attention paradigm (27): Before each block of trials, the monkey was cued to attend to the location where the orientation change was most likely to happen. The orientation change occurred at the attended location 80% of the time, and the animal was rewarded for detecting changes at both the attended and unattended location. The attended location alternated between the left and right locations on each new block of trials.
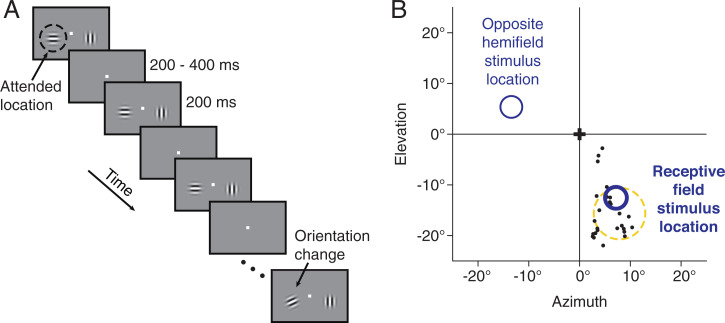
Fig. 1.
Behavioral and recording methods. (A) Orientation change-detection task with a spatial attention manipulation. This task is similar to one we have used in previous studies linking correlated variability in V4 to attention and performance (14, 26). The monkey was required to fixate a central spot while two Gabor stimuli flashed on and off, one in the left visual hemifield and one in the right. The monkeys were rewarded for detecting a subtle orientation change that occurred at either the attended location (80% of trials) or the unattended location. The orientation change occurred at a randomized location and time. The attended location was cued using unanalyzed instruction trials at the beginning of each block of trials. The starting orientation of each of the two stimuli was selected randomly per stimulus and per trial from a set of 4 to 12 orientations. (B) Physiological methods. For monkeys 2 and 3, we recorded from chronically implanted microelectrode arrays in visual area V4. We recorded the responses of a few dozen V4 neurons simultaneously. The receptive fields of the recorded neurons typically overlapped both each other and the location of one of the Gabor stimuli (the receptive field stimulus location). The figure depicts, for an example recording session, the centers of the receptive fields of the recorded neurons (black dots), a typical receptive field size and location (dashed yellow circle), and the locations of the two Gabor stimuli (dark blue circles).
For two of the monkeys, we simultaneously recorded the activity of a few dozen neurons in visual area V4 using chronically implanted microelectrode arrays. The two visual stimuli were positioned such that one stimulus overlapped the receptive fields of the recorded V4 neurons (Fig. 1B) and the other was in the opposite hemifield.
Methylphenidate Improves Motivation.
To investigate the many clinically relevant behavioral effects of methylphenidate (4, 6, 7, 10, 12) in our controlled laboratory setting, we measured many aspects of the monkeys’ behavior and quantitatively compared days on which we administered methylphenidate to their corresponding placebo control days. The most dramatic change was in the amount of time the monkeys engaged in the behavioral task. For our behavioral datasets (Methods), the monkeys controlled the length of the session: The experiment ended when the monkey had not fixated the central spot to initiate a trial for 10 min. Even when we matched the total amount of liquid the monkeys received prior to drug and placebo control days to control for any effect of the prior day’s juice intake (SI Appendix, Fig. S1A), the monkeys performed the task nearly twice as long on drug than control days (Fig. 2). The methylphenidate dosage did not significantly affect working time (SI Appendix, Fig. S1B; though see refs. 22 and 24).
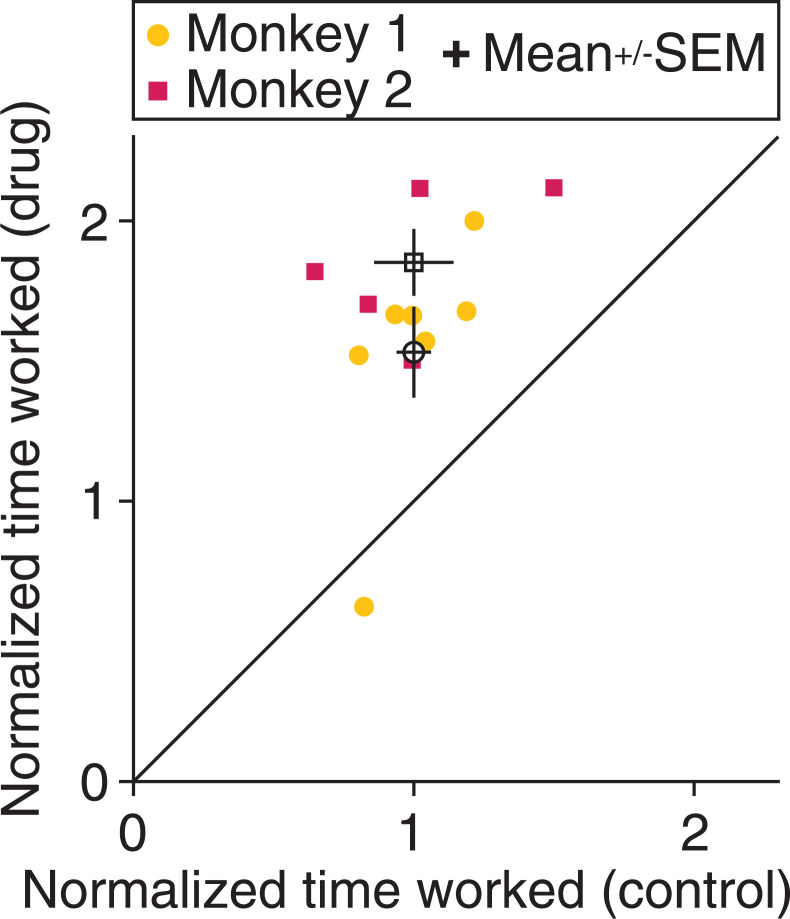
Fig. 2.
Methylphenidate improves measures of general processes like motivation or work ethic. For a subset of days on which we followed a strict protocol for measuring time engaged on the change-detection task (Methods), the plot depicts the amount of time that the monkey engaged in the task each day. The time worked is normalized per monkey to the mean time that the monkey worked across all placebo control days. Each point is the normalized working time for a drug day (y axis) and its matched control day (x axis; adjacent control day with identical stimulus parameters) for each monkey (marker symbols). The open symbols are the mean for each monkey, and error bars represent SEM. Both animals worked significantly longer on drug than control days [paired t tests; monkey 1: n = 7 pairs of days, t(6) = −4.1, P = 6.1 × 10−3; monkey 2: n = 5 pairs of days, t(4) = −6.6, P = 2.7 × 10−3].
Methylphenidate Increases Selective Attention.
Even though we administered methylphenidate systemically, methylphenidate improved behavioral performance on our challenging visual change-detection task at only the attended location (Fig. 3A). Methylphenidate did not increase performance at the unattended location (Fig. 3B), though it is possible that a task that produces a more dynamic range of unattended performance might reveal methylphenidate effects at the unattended location as well. Overall, methylphenidate increased the selective effects of attention (the difference in performance between the attended and unattended locations; Fig. 3C). Comparing the attention conditions directly demonstrates that the methylphenidate effects were different at the attended versus unattended locations (Fig. 3C). The methylphenidate dosage did not significantly affect the animal’s performance on the change-detection task (SI Appendix, Fig. S2 A and B). There was no indication of a relationship between performance and motivation effects, suggesting distinct mechanisms (SI Appendix, Fig. S2 C and D). The number of days after a drug day that the placebo day took place did not significantly affect either performance or motivation (SI Appendix, Fig. S3). The positive effect of methylphenidate on performance at the attended location was due to both improved visual sensitivity (improving the monkey’s ability to see the difference between the original and changed stimuli in our task; SI Appendix, Fig. S4A) and decreased criterion (increasing the readiness of the animal to move its eyes; SI Appendix, Fig. S4B).
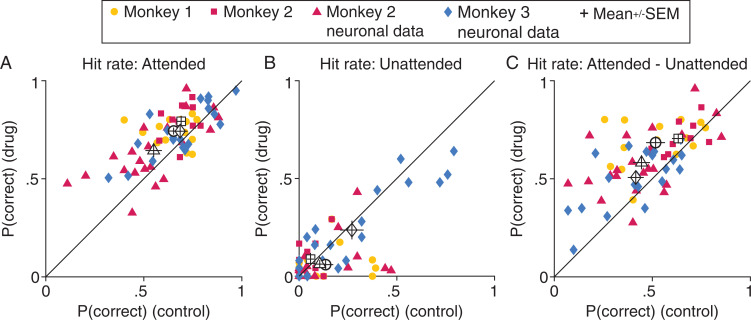
Fig. 3.
Methylphenidate selectively improves performance at the attended location. (A) All three monkeys (marker symbols) were better able to detect subtle orientation changes at the attended location on drug days (y axis; numbers represent the hit rate: number of hits divided by hits plus misses) compared with paired control days (x axis). Attended performance per stimulus location (left or right location; Fig. 1A) is plotted separately per day. The open symbols and error bars depict the mean and SEM for each dataset. The drug-related improvement was significant for each dataset [paired t tests; monkey 1: n = 14 (7 pairs of days × 2 stimulus locations per pair), t(13) = −2.5, P = 0.025; monkey 2: n = 10, t(9) = −3.3, P = 9.2 × 10−3; monkey 2 neuronal dataset: n = 22, t(21) = −3.1, P = 5.6 × 10−3; monkey 3 neuronal dataset: n = 20, t(19) = −2.6, P = 0.019]. (B) Methylphenidate does not significantly change performance at the unattended location [paired t tests; monkey 1: t(13) = 1.8, P = 0.093; monkey 2: t(9) = −1.0, P = 0.34; monkey 2 neuronal dataset: t(21) = 1.4, P = 0.17; monkey 3 neuronal dataset: t(19) = 1.3, P = 0.22]. Conventions are as in A. (C) Comparing the results in A and B illustrates that methylphenidate increases the selective effect of attention, defined here as the attention-related difference in hit rate [paired t tests; monkey 1: t(13) = −3.5, P = 4.0 × 10−3; monkey 2: t(9) = −2.8, P = 0.019; monkey 2 neuronal dataset: t(21) = −3.6, P = 1.8 × 10−3; monkey 3 neuronal dataset: t(19) = −2.9, P = 8.5 × 10−3]. Conventions are as in A.
Methylphenidate Improves Performance When It Changes Neuronal Correlated Variability.
This spatial specificity in the behavioral effect of methylphenidate was reflected in the V4 neuronal population responses. Consistent with our basic science hypothesis about a general neural coding principle (14), methylphenidate improves performance exactly when it changes correlated variability in visual cortex [the average spike count correlation across all simultaneously recorded pairs of V4 neurons; spike count correlation, also called noise correlation, quantifies the trial-to-trial response variability that is shared between a pair of neurons in response to repeated presentations of the same stimulus (15)].
Methylphenidate decreased the correlated variability of the recorded V4 neurons only when the animal attended to the stimulus within the receptive fields of the recorded neurons (Fig. 4A). It did not decrease the correlated variability when the animal did not attend to the stimulus within the neuronal receptive fields (Fig. 4B), such that it overall increased the selective effects of attention (the difference in correlated variability between the attended and unattended locations; Fig. 4C). These data illustrate a consistent, quantitative relationship between behavioral performance and correlated variability per monkey (Fig. 4D), with methylphenidate simply moving the attended behavior and neurons along that quantitative relationship. In other words, the extent to which methylphenidate improved performance at the attended location was matched by the extent to which methylphenidate decreased correlated variability. There was a strong relationship between correlated variability and both visual sensitivity and criterion (SI Appendix, Fig. S5 A and B; also see ref. 28). In contrast, there was no detectable relationship between performance and firing rate for either the drug or placebo control days (SI Appendix, Fig. S5C).
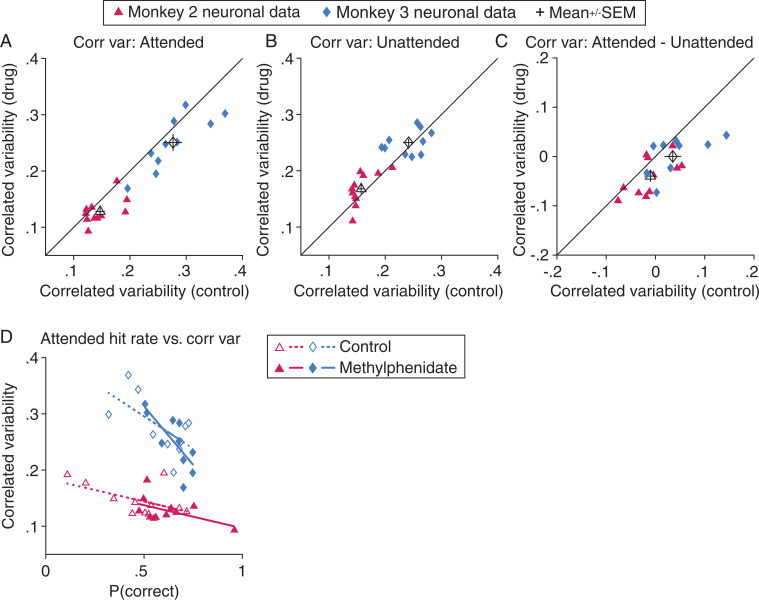
Fig. 4.
Consistent with our basic science hypothesis, methylphenidate improves performance exactly when it changes correlated variability in visual cortex. (A) Methylphenidate reduces V4 correlated variability when the animal pays attention to the joint receptive fields of the recorded neurons. The plot depicts the average noise correlation between all simultaneously recorded neurons on matched drug days (y axis) and placebo control days (x axis) for the monkey 2 and monkey 3 neuronal datasets (marker symbols; Methods). The mean correlated variability is consistently lower when the receptive field location is attended [paired t tests; monkey 2: n = 11 (11 pairs of days × 1 receptive field stimulus location), t(10) = 2.6, P = 0.025; monkey 3: n = 10, t(9) = 2.9, P = 0.018]. The open symbols and error bars depict the mean and SEM for each dataset. (B) Methylphenidate does not significantly change V4 correlated variability when the receptive field location is unattended [paired t tests; monkey 2: t(10) = −1.7, P = 0.13; monkey 3: t(9) = −0.89, P = 0.40]. Conventions are as in A. (C) Comparing the results in A and B illustrates that methylphenidate increases the selective effect of attention, defined here as the attention-related difference in correlated variability [paired t tests; monkey 2: t(10) = 2.9, P = 0.015; monkey 3: t(9) = 2.7, P = 0.025]. (D) There is a single, robust relationship between attended behavioral performance (hit rate; x axis) and attended mean correlated variability (y axis) for monkey 2 (correlation coefficient; R = −0.60, P = 3.0 × 10−3; correlation was indistinguishable between control and drug conditions, depicted with open and filled symbols, respectively; control: R = −0.55, P = 0.081; drug: R = −0.50, P = 0.11; Fisher z Pearson–Filon test of the difference between dependent but nonoverlapping correlation coefficients: zpf = −0.14, P = 0.89) and monkey 3 (correlation coefficient; R = −0.69, P = 7.9 × 10−4; correlation was indistinguishable between control and drug conditions; control: R = −0.63, P = 0.053; drug: R = −0.76, P = 0.011; Fisher z Pearson–Filon test: zpf = 0.70, P = 0.49). As with natural cognitive processes (control data; also see ref. 14), systemically administered methylphenidate improves behavioral performance according to the correlated variability change it induces. Best fit lines are depicted for control (dashed lines) and methylphenidate data (solid lines).
Discussion
Cognitive processes like attention can affect performance in a highly selective manner, improving detection of specific stimuli (5). This selectivity is often the goal of stimulant use. People use stimulants both with and without prescription with the goal of enhancing selective cognitive processes such as the ability to focus on one task or one aspect of the environment while ignoring distractions (3, 6, 12, 13). Yet, while we have progressed our understanding of the neuronal mechanisms underlying the effects of these drugs on memory, learning, cognitive flexibility, motivation, and impulsivity (19, 20, 22–24, 29–35), we have only begun to understand the neuronal effects of these stimulants on selective attention in the context of a controlled laboratory setting (36–39). The neural mechanisms underlying stimulant-related changes in selective cognition have remained a mystery; here, we report how changes in neuronal population responses correspond to increased selective attention with ADHD drugs.
In addition to finding that methylphenidate selectively increased performance at the attended location (Fig. 3), we found that methylphenidate increased overall motivation to perform the task (Fig. 2). A reasonable hypothesis regarding these two behavioral effects is that one was causally related to the other; for example, increased motivation to perform the task may have led to increased selective attention. Alternatively, methylphenidate may have concurrently increased both motivation and selective attention through related or independent neural pathways. To test the extent to which changes in motivation and selective attention were related, we analyzed whether there was any correlation between the magnitudes of these two effects. We did not find any indication of a relationship between methylphenidate-related changes in motivation and performance (SI Appendix, Fig. S2 C and D). Our findings suggest that distinct neural mechanisms underlie general effects on motivation and specific effects on performance at the attended location associated with methylphenidate.
Prior studies also found evidence that the many effects of methylphenidate may be mediated by distinct mechanisms. For example, there are different dose–response curves for distinct cognitive effects (22, 40). Rajala and colleagues (22) found that the dose–response curves for performance on a saccade task and session length had differing peaks, suggesting distinct mechanisms.
Our results demonstrated that methylphenidate improved behavioral performance exactly when it changed correlated variability in visual cortex (Fig. 4D), but that those behavioral improvements were unrelated to mean firing rate (SI Appendix, Fig. S5C). While we found that correlated variability was related to both sensitivity and criterion, we were not able to distinguish between multiple potential relationships between these three variables (SI Appendix, Fig. S5 A and B). This is not surprising, as prior studies have found that sensitivity and criterion are often strongly yoked (14, 41, 42). Experimental paradigms aimed at isolating the effects of sensitivity versus criterion (28) may be able to shed light on the potential effects of methylphenidate on these distinct behavioral signatures. Prior studies that have measured how stimulants affect hit rate versus false alarm rate suggest that different stimulants and dosages can have distinct effects on sensitivity versus criterion (43, 44).
Our results demonstrate that a systemic manipulation can selectively change behavior and the underlying neural mechanisms. They support the hypothesis that the spatially selective behavioral and neuronal changes we observed involved an interaction between the diffuse activity of neurotransmitters at the level of top-down control areas (as suggested by in vitro and in vivo measurements of stimulant effects; for reviews, see refs. 1, 16, and 45) and the localized activity of neurotransmitters at the level of early sensory areas like V4 [as suggested by in vitro and in vivo studies of attention effects (46); for reviews, see refs. 17, 47, and 48]. While electrophysiological studies have differed in their findings regarding the role of prefrontal cortex in mediating the behavioral effects of methylphenidate (19, 24, 31, 39, 46), the selective changes we observed here support that methylphenidate can interact with frontoparietal networks (49, 50) through dopaminergic projections (17, 51) to enhance selective attention processing (1, 52–54). Determining how ADHD drugs act through different sites within the brain’s attentional network to enhance selective attention remains an exciting future avenue for both basic and translational neuroscience.
More broadly, our study illustrates that when it comes to combining basic science and translational approaches, the whole is greater than the sum of its parts. We discovered behavioral effects of a drug that is widely used, and we leveraged that drug to conduct a strong causal test of a basic science hypothesis that has wide implications for neural coding in many species, systems, and brain areas (14, 55). Extending this framework to study potential treatments of disorders that affect cognition has the potential to simultaneously transform our understanding of both basic neural mechanisms and clinical outcomes.
Methods
The subjects were three adult male rhesus monkeys (Macaca mulatta): monkeys 1, 2, and 3 (7.5, 9.0, and 9.5 kg, respectively). All animal procedures were approved by the Institutional Animal Care and Use Committees of the University of Pittsburgh and Carnegie Mellon University. Each animal was implanted with a titanium head post prior to beginning behavioral training.
Methylphenidate Administration.
We tested the behavioral and electrophysiological effects of methylphenidate hydrochloride (Mallinckrodt Pharmaceuticals). Methylphenidate was administered on alternating data collection days (these did not include days on which data were not collected or days on which an insufficient number of trials were collected—see Data Analysis) for several weeks, providing a minimum of a 24-h washout period following drug administration prior to collecting control-day data (20). A 24-h washout period between drug and control days was selected based on measurements of orally administered methylphenidate plasma concentrations in rhesus monkeys that determined the drug’s half-life to be less than 2 h (56), such that it is undetectable after 12 h (21).
On drug-administration days, the methylphenidate was dissolved in 10 mL of sugar water (200 mg/mL) and administered orally (the method of dissolving the drug in a flavored liquid for oral administration was adapted from ref. 18). On control days, 10 mL of sugar water alone (200 mg/mL) was administered orally. For the data in this study, the methylphenidate in sugar water or the sugar water alone was always administered 30 min prior to the monkey beginning the change-detection task [based on prior studies that used similar rhesus monkey behavioral session timing after oral stimulant administration (19, 22, 23)].
A maximum dosage of 8.0 mg/kg was predetermined based on prior studies performed in rhesus monkeys (18, 19, 22, 23, 57). The dosages included in the analyses were 2.0, 3.0, 4.0, 5.0, and 6.0 mg/kg (SI Appendix, Fig. S2 A and B). Dosages of 6.0 and 7.0 mg/kg sometimes led to agitation that prevented the monkeys from being able to perform the task. This occurred with one out of one test of 6.0 mg/kg for monkey 1, one out of two tests of 6.0 mg/kg for monkey 2, and one out of one test of 7.0 mg/kg for monkey 2. Due to these effects, we did not test higher than 5.0 mg/kg with monkey 3, and we never tested a dosage higher than 7.0 mg/kg. The mean analyzed dosage was 3.8 mg/kg [doses of 3.0 mg/kg in rhesus macaques result in similar plasma levels as therapeutic doses of 0.3 mg/kg in humans (56)].
Agitation or drowsiness leading to the inability to collect behavioral data has been previously reported at higher stimulant dosages (20, 22). Here, the agitating effect of higher dosages described above manifested as an increase in erratic eye movements, resulting in an inability to fixate and initiate behavioral trials. This decrease in stimulant efficacy at higher dosages follows the characteristic inverted U-shaped pharmacological dose–response curve (58) that has been well-documented for stimulants (19, 22, 33, 59, 60) (for reviews, see refs. 12, 17, and 61).
Data from all dosages were combined for each analysis to avoid best-dose analysis (25), as our goal was to use methylphenidate as a causal mechanism to test our hypotheses, not to test for dose-dependent effects (see ref. 22 for analyses of methylphenidate dose-dependent effects in rhesus monkeys).
Behavioral Task.
The monkeys performed an orientation change-detection task (14, 26) with cued attention (27). All three monkeys were trained extensively on this task before the data presented here were recorded. Visual stimuli were presented on a cathode-ray tube monitor (calibrated to linearize intensity; 1,024 × 768 pixels; 120-Hz refresh rate) placed 57 cm from the monkey, using custom software written in MATLAB [Psychophysics Toolbox (62, 63)]. Eye position was monitored using an infrared eye tracker (EyeLink 1000; SR Research) as per previously published methods (14).
A monkey began a trial by fixing its gaze on a small spot presented in the center of the video display (Fig. 1A). Next, two peripheral drifting Gabor stimuli, one presented in the left visual hemifield and one presented in the right visual hemifield, synchronously flashed on (for 200 ms) and off (for an interval that was randomly selected from a uniform distribution with a range of 200 to 400 ms) until, at a random and unsignaled time, the orientation of one of the stimuli changed. The monkey received a liquid reward for making a saccade to the changed stimulus within 450 ms of its onset and was randomly administered extra rewards after correctly completed trials. If no orientation change occurred within a maximum of 12 to 15 stimulus presentations (∼10% of the trials), the trial was terminated and the monkey received a liquid reward simply for having maintained fixation throughout the trial (catch trials).
The size, two locations, temporal frequency, and spatial frequency of the Gabor stimuli were fixed for both days of a pair (the drug day and the paired placebo control day). The orientation change amount was also fixed for both days of a pair, and was the same for both stimulus locations and all trials. The starting orientation at which each stimulus was flashed multiple times before any orientation change occurred was selected randomly per trial and per stimulus location from a set of 4 to 12 different starting orientations.
The attended location alternated between the left and right stimulus locations (Fig. 1A) on each new block of 120 to 125 trials. Prior to a new block, the monkey was cued to attend to one stimulus location with 10 instruction trials in which a stimulus was only flashed at that one location. During each block, the orientation change occurred at the cued location on 80% of the trials and at the other location on 20% of the trials.
Datasets.
During the behavioral datasets (collected for monkey 1 and monkey 2 and illustrated with circle markers and square markers, respectively), no neuronal data were collected. The monkey controlled the length of each experimental session: The session ended when the monkey had not fixated the central fixation point to initiate a trial for 10 min. For each monkey, the two locations for the Gabor stimuli were selected based on the monkey demonstrating approximately equal performance at those two locations prior to beginning data collection.
During the neuronal datasets (collected for monkey 2 and monkey 3 and illustrated with triangle markers and diamond markers, respectively), psychophysical and neuronal data were collected simultaneously. For each monkey, the two locations for the Gabor stimuli were selected such that one location maximally overlapped the joint recorded receptive fields and the other location was in the opposite visual hemifield.
Neurophysiological Recordings.
For the neuronal datasets collected for monkey 2 and monkey 3, we recorded extracellularly per monkey using a single chronically implanted microarray (48 electrodes per array; Blackrock Microsystems) in visual area V4 (left hemisphere for monkey 2 and right hemisphere for monkey 3; each monkey also had a second chronically implanted microarray, the data from which are not included in this study), using previously published methods (14). We set the same spike-detection voltage threshold across all electrodes and all recording sessions and included all threshold crossings as the neuronal activity per electrode [the recorded “unit” (14, 64); Data Analysis]. The typical receptive field size plotted in Fig. 1B (dashed yellow circle) was calculated as the SD of a Gaussian fit.
Data Analysis.
Statistical details can be found in the figure legends (statistical tests used, n values, etc.). Experimental sessions were included in the analyses if a minimum of 200 change-detection trials were completed (correct or incorrect).
To determine the effect of methylphenidate on the amount of time a monkey engaged in the change-detection task (Fig. 2 and SI Appendix, Figs. S1 and S2 C and D), the behavioral datasets were analyzed. The time engaged in the task was calculated as the time between the start time of the first trial and the end time of the tenth from last correctly completed trial (excluding the last trials conservatively estimated the working time so as to not include potential breaks between periods of concerted effort near the end of the session). The results were qualitatively unchanged when the total experimental time (from the start time of the first trial to the end time of the 10-min break that ended the session) was analyzed instead [paired t tests; monkey 1: n = 7 pairs of days, t(6) = −4.2, P = 5.7 × 10−3; monkey 2: n = 5 pairs of days, t(4) = −3.8, P = 0.019].
The time worked was illustrated as the normalized time worked (Fig. 2). To normalize the time worked per monkey, first the mean time that monkey worked across all placebo control days was calculated. Next, each amount of time worked per day was divided by the mean time worked on control days. Thus, Fig. 2 illustrates each amount of time worked as a ratio, in comparison to the mean time worked on control days.
To determine the effect of methylphenidate on performance (Figs. 3 and 4 C and SI Appendix, Figs. S2–S5), the behavioral and/or neuronal datasets were analyzed. For analyses of performance, only the first two blocks collected per experimental session were analyzed (one block with attention cued to the left hemifield stimulus location, one block with attention cued to the right hemifield stimulus location; Fig. 1). Only the first two blocks were analyzed per experimental session to control for potential changes in drug efficacy and motivation levels across the session. Instruction and catch trials were not included in the analyses.
To determine the effect of methylphenidate on neuronal population activity (Fig. 4 and SI Appendix, Fig. S5), the neuronal datasets were analyzed. Recorded units were included in the analyses on a pair-by-pair basis. The same units were analyzed for both days of a pair, based on the responses of the units on the control day of the pair: The analyzed units were the units that passed a mean stimulus-evoked firing rate of at least 10 Hz and a mean stimulus-evoked firing rate that was significantly higher than the mean firing rate during a baseline period in which no stimuli were presented (stimulus analysis period: 60 to 200 ms from stimulus onset to account for V4 response latency; baseline analysis period: 100-ms interval prior to the onset of the first stimulus/trial; included trials: completed orientation-change and catch trials; included stimuli: all stimuli but the first stimulus/trial and any orientation-change stimuli; based on a two-sided Wilcoxon signed-rank test of whether the response ratio of the mean stimulus-evoked firing rate compared with the mean baseline firing rate was different from 1). Results were not qualitatively different when these same criteria were applied on a day-by-day basis (applied to each session individually, regardless of day pairing). The population size of simultaneously recorded units included in the analyses was 26 to 32 units for monkey 2 (mean 30) and 3 to 29 units for monkey 3 (mean 17).
To analyze the firing rates and correlated variability of the V4 neuronal populations in response to stimuli presented at the receptive field location (Fig. 1B), stimuli presented during attended orientation-change, catch, and false alarm trials (the attended condition) were compared with stimuli presented during unattended orientation-change, catch, and false alarm trials (the unattended condition). All stimuli were included except the first stimulus per trial, orientation-change stimuli, and stimulus presentations during which the monkey made a false alarm (a saccade to a stimulus location where no orientation change had occurred). The neuronal responses to a stimulus were calculated during the analysis period of 60 to 260 ms from stimulus onset.
The neuronal population correlated variability was calculated as the mean (across all pairs of units) correlation coefficient between the responses of two units to repeated presentations of the same stimulus. The correlation coefficient per pair of units was calculated per starting orientation and averaged across all starting orientations. Correlation coefficients >0.5 and <−0.1 were excluded from mean calculations.
Supplementary Material
Acknowledgments
A.M.N. received support from NIH Grant 1K99NS118117-01 and a fellowship from the Simons Foundation Collaboration on the Global Brain. M.R.C. received support from NIH Grants 4R00EY020844-03, R01 EY022930, and R01NS121913 and Core Grant P30 EY008098s, a grant from the Whitehall Foundation, a Klingenstein–Simons Fellowship, a Sloan Research Fellowship, a McKnight Scholar Award, and a grant from the Simons Foundation Collaboration on the Global Brain. We thank Nancy Ator for guidance and discussions throughout this study. We thank Lily E. Kramer for assistance with data collection. We thank Karen McCracken for technical assistance. We thank John H. R. Maunsell, Julio C. Martínez-Trujillo, Sébastien Tremblay, and Cheng Xue for comments on previous versions of this manuscript.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2120529119/-/DCSupplemental.
Data Availability
Matlab files for the data reported in this article have been deposited in a public GitHub repository, https://github.com/AmyMNi/NiBowesRuffCohen2022.
References
- 1.Mueller A., Hong D. S., Shepard S., Moore T., Linking ADHD to the neural circuitry of attention. Trends Cogn. Sci. 21, 474–488 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visser S. N., et al. , Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003–2011. J. Am. Acad. Child Adolesc. Psychiatry 53, 34–46.e2 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maher B., Poll results: Look who’s doping. Nature 452, 674–675 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Lakhan S. E., Kirchgessner A., Prescription stimulants in individuals with and without attention deficit hyperactivity disorder: Misuse, cognitive impact, and adverse effects. Brain Behav. 2, 661–677 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maunsell J. H. R., Neuronal mechanisms of visual attention. Annu. Rev. Vis. Sci. 1, 373–391 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagot K. S., Kaminer Y., Efficacy of stimulants for cognitive enhancement in non-attention deficit hyperactivity disorder youth: A systematic review. Addiction 109, 547–557 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koelega H. S., Stimulant drugs and vigilance performance: A review. Psychopharmacology (Berl.) 111, 1–16 (1993). [DOI] [PubMed] [Google Scholar]
- 8.McLellan T. M., Caldwell J. A., Lieberman H. R., A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci. Biobehav. Rev. 71, 294–312 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Murray D. W., Treatment of preschoolers with attention-deficit/hyperactivity disorder. Curr. Psychiatry Rep. 12, 374–381 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Pietrzak R. H., Mollica C. M., Maruff P., Snyder P. J., Cognitive effects of immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. Neurosci. Biobehav. Rev. 30, 1225–1245 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Spencer T. J., et al. , Effect of psychostimulants on brain structure and function in ADHD: A qualitative literature review of magnetic resonance imaging-based neuroimaging studies. J. Clin. Psychiatry 74, 902–917 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swanson J., Baler R. D., Volkow N. D., Understanding the effects of stimulant medications on cognition in individuals with attention-deficit hyperactivity disorder: A decade of progress. Neuropsychopharmacology 36, 207–226 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wickens J. R., Hyland B. I., Tripp G., Animal models to guide clinical drug development in ADHD: Lost in translation? Br. J. Pharmacol. 164, 1107–1128 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ni A. M., Ruff D. A., Alberts J. J., Symmonds J., Cohen M. R., Learning and attention reveal a general relationship between population activity and behavior. Science 359, 463–465 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen M. R., Kohn A., Measuring and interpreting neuronal correlations. Nat. Neurosci. 14, 811–819 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnsten A. F., Stimulants: Therapeutic actions in ADHD. Neuropsychopharmacology 31, 2376–2383 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Noudoost B., Moore T., The role of neuromodulators in selective attention. Trends Cogn. Sci. 15, 585–591 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soto P. L., et al. , Long-term exposure to oral methylphenidate or dl-amphetamine mixture in peri-adolescent rhesus monkeys: Effects on physiology, behavior, and dopamine system development. Neuropsychopharmacology 37, 2566–2579 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gamo N. J., Wang M., Arnsten A. F., Methylphenidate and atomoxetine enhance prefrontal function through α2-adrenergic and dopamine D1 receptors. J. Am. Acad. Child Adolesc. Psychiatry 49, 1011–1023 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kodama T., et al. , Oral administration of methylphenidate (Ritalin) affects dopamine release differentially between the prefrontal cortex and striatum: A microdialysis study in the monkey. J. Neurosci. 37, 2387–2394 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oemisch M., Johnston K., Paré M., Methylphenidate does not enhance visual working memory but benefits motivation in macaque monkeys. Neuropharmacology 109, 223–235 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Rajala A. Z., Henriques J. B., Populin L. C., Dissociative effects of methylphenidate in nonhuman primates: Trade-offs between cognitive and behavioral performance. J. Cogn. Neurosci. 24, 1371–1381 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajala A. Z., Jenison R. L., Populin L. C., Decision making: Effects of methylphenidate on temporal discounting in nonhuman primates. J. Neurophysiol. 114, 70–79 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajala A. Z., Populin L. C., Jenison R. L., Methylphenidate affects task-switching and neural signaling in non-human primates. Psychopharmacology (Berl.) 237, 1533–1543 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soto P. L., Dallery J., Ator N. A., Katz B. R., A critical examination of best dose analysis for determining cognitive-enhancing potential of drugs: Studies with rhesus monkeys and computer simulations. Psychopharmacology (Berl.) 228, 611–622 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen M. R., Maunsell J. H. R., Attention improves performance primarily by reducing interneuronal correlations. Nat. Neurosci. 12, 1594–1600 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Posner M. I., Orienting of attention. Q. J. Exp. Psychol. 32, 3–25 (1980). [DOI] [PubMed] [Google Scholar]
- 28.Luo T. Z., Maunsell J. H. R., Neuronal modulations in visual cortex are associated with only one of multiple components of attention. Neuron 86, 1182–1188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berridge C. W., Arnsten A. F., Catecholamine mechanisms in the prefrontal cortex: Proven strategies for enhancing higher cognitive function. Curr. Opin. Behav. Sci. 4, 33–40 (2015). [Google Scholar]
- 30.Clatworthy P. L., et al. , Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J. Neurosci. 29, 4690–4696 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Devilbiss D. M., Berridge C. W., Cognition-enhancing doses of methylphenidate preferentially increase prefrontal cortex neuronal responsiveness. Biol. Psychiatry 64, 626–635 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dinse H. R., Ragert P., Pleger B., Schwenkreis P., Tegenthoff M., Pharmacological modulation of perceptual learning and associated cortical reorganization. Science 301, 91–94 (2003). [DOI] [PubMed] [Google Scholar]
- 33.Dodds C. M., et al. , Methylphenidate has differential effects on blood oxygenation level-dependent signal related to cognitive subprocesses of reversal learning. J. Neurosci. 28, 5976–5982 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garrett D. D., et al. , Amphetamine modulates brain signal variability and working memory in younger and older adults. Proc. Natl. Acad. Sci. U.S.A. 112, 7593–7598 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta M. A., et al. , Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J. Neurosci. 20, RC65 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bain J. N., et al. , Enhanced attention in rhesus monkeys as a common factor for the cognitive effects of drugs with abuse potential. Psychopharmacology (Berl.) 169, 150–160 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Prendergast M. A., et al. , Age-related differences in distractibility and response to methylphenidate in monkeys. Cereb. Cortex 8, 164–172 (1998). [DOI] [PubMed] [Google Scholar]
- 38.Tomasi D., et al. , Methylphenidate enhances brain activation and deactivation responses to visual attention and working memory tasks in healthy controls. Neuroimage 54, 3101–3110 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tremblay S., Pieper F., Sachs A., Joober R., Martinez-Trujillo J., The effects of methylphenidate (Ritalin) on the neurophysiology of the monkey caudal prefrontal cortex. eNeuro 6, ENEURO.0371-18.2018 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sprague R. L., Sleator E. K., Methylphenidate in hyperkinetic children: Differences in dose effects on learning and social behavior. Science 198, 1274–1276 (1977). [DOI] [PubMed] [Google Scholar]
- 41.Luo T. Z., Maunsell J. H. R., Attentional changes in either criterion or sensitivity are associated with robust modulations in lateral prefrontal cortex. Neuron 97, 1382–1393.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sridharan D., Steinmetz N. A., Moore T., Knudsen E. I., Does the superior colliculus control perceptual sensitivity or choice bias during attention? Evidence from a multialternative decision framework. J. Neurosci. 37, 480–511 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelly S. P., Gomez-Ramirez M., Montesi J. L., Foxe J. J., l-theanine and caffeine in combination affect human cognition as evidenced by oscillatory alpha-band activity and attention task performance. J. Nutr. 138, 1572S–1577S (2008). [DOI] [PubMed] [Google Scholar]
- 44.Koek W., Gerak L. R., France C. P., Effects of amphetamine, morphine, and CP 55, 940 on go/no-go task performance in rhesus monkeys. Behav. Pharmacol. 26, 481–484 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heal D. J., Smith S. L., Gosden J., Nutt D. J., Amphetamine, past and present—A pharmacological and clinical perspective. J. Psychopharmacol. 27, 479–496 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noudoost B., Moore T., Control of visual cortical signals by prefrontal dopamine. Nature 474, 372–375 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deco G., Thiele A., Attention: Oscillations and neuropharmacology. Eur. J. Neurosci. 30, 347–354 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmitz T. W., Duncan J., Normalization and the cholinergic microcircuit: A unified basis for attention. Trends Cogn. Sci. 22, 422–437 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Engelmann J. B., Damaraju E., Padmala S., Pessoa L., Combined effects of attention and motivation on visual task performance: Transient and sustained motivational effects. Front. Hum. Neurosci. 3, 4 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Padmala S., Pessoa L., Reward reduces conflict by enhancing attentional control and biasing visual cortical processing. J. Cogn. Neurosci. 23, 3419–3432 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Botvinick M., Braver T., Motivation and cognitive control: From behavior to neural mechanism. Annu. Rev. Psychol. 66, 83–113 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Corbetta M., Shulman G. L., Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3, 201–215 (2002). [DOI] [PubMed] [Google Scholar]
- 53.Kastner S., Ungerleider L. G., Mechanisms of visual attention in the human cortex. Annu. Rev. Neurosci. 23, 315–341 (2000). [DOI] [PubMed] [Google Scholar]
- 54.Moore T., Zirnsak M., Neural mechanisms of selective visual attention. Annu. Rev. Psychol. 68, 47–72 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Ruff D. A., Ni A. M., Cohen M. R., Cognition as a window into neuronal population space. Annu. Rev. Neurosci. 41, 77–97 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doerge D. R., Fogle C. M., Paule M. G., McCullagh M., Bajic S., Analysis of methylphenidate and its metabolite ritalinic acid in monkey plasma by liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 14, 619–623 (2000). [DOI] [PubMed] [Google Scholar]
- 57.Czoty P. W., Martelle S. E., Gould R. W., Nader M. A., Effects of chronic methylphenidate on cocaine self-administration under a progressive-ratio schedule of reinforcement in rhesus monkeys. J. Pharmacol. Exp. Ther. 345, 374–382 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calabrese E. J., Baldwin L. A., U-shaped dose-responses in biology, toxicology, and public health. Annu. Rev. Public Health 22, 15–33 (2001). [DOI] [PubMed] [Google Scholar]
- 59.Borota D., et al. , Post-study caffeine administration enhances memory consolidation in humans. Nat. Neurosci. 17, 201–203 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martelle S. E., Porrino L. J., Nader M. A., Effects of chronic methylphenidate in adolescence on later methylphenidate self-administration in rhesus monkeys. Behav. Pharmacol. 24, 478–481 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fredholm B. B., Bättig K., Holmén J., Nehlig A., Zvartau E. E., Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 51, 83–133 (1999). [PubMed] [Google Scholar]
- 62.Brainard D. H., The Psychophysics Toolbox. Spat. Vis. 10, 433–436 (1997). [PubMed] [Google Scholar]
- 63.Pelli D. G., The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spat. Vis. 10, 437–442 (1997). [PubMed] [Google Scholar]
- 64.Trautmann E. M., et al. , Accurate estimation of neural population dynamics without spike sorting. Neuron 103, 292–308.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Matlab files for the data reported in this article have been deposited in a public GitHub repository, https://github.com/AmyMNi/NiBowesRuffCohen2022.