Abstract
Recently, cardiovascular diseases (CVDs) were identified as the leading cause of mortality, imposing a heavy burden on health care systems and the social economy. Nicotinamide adenine dinucleotide (NAD+), as a pivotal co-substrate for a range of different enzymes, is involved in many signal transduction pathways activated in CVDs. Emerging evidence has shown that NAD+ can exert remediating effects on CVDs by regulating metabolism, maintaining redox homeostasis and modulating the immune response. In fact, NAD+ might delay ageing through sirtuin and non-sirtuin pathways and thus contribute to interventions for age-related diseases such as CVDs. Considering that robust clinical studies of NAD+ are ongoing, we discuss current challenges and the future translational potential of NAD+ based on existing studies and our understanding. Despite some remaining gaps in its clinical application, NAD+ has been shown to have broad prospects and pan-effects, making it a suitable prophylactic drug for CVDs.
Keywords: Ageing, Cardiovascular disease, Metabolism, Nicotinamide adenine dinucleotide, Sirtuin
Introduction
Cardiovascular diseases (CVDs) are heart and circulatory system disorders that collectively are the leading cause of premature mortality and disability in humans worldwide.1 The incidence of CVDs continues to increase worldwide, and it is predicted that by 2030 nearly 24 million individuals will die from a CVD each year.2 CVDs are complex diseases, including coronary artery disease, arrhythmia, hypertension, cardiomyopathy and heart failure. The physiopathology of CVDs is also complex and mainly involves metabolic disorders, oxidative stress and inflammation.3 For example, abnormal glucose and lipid metabolism can accelerate the formation of atherosclerosis.4 Excessive reactive oxygen species (ROS) production can cause mitochondrial dysfunction and aggravate cardiomyocyte injury in ischemic cardiomyopathy.5 Inflammatory signaling networks have also been indicated to contribute to ischaemic cardiovascular disease.6
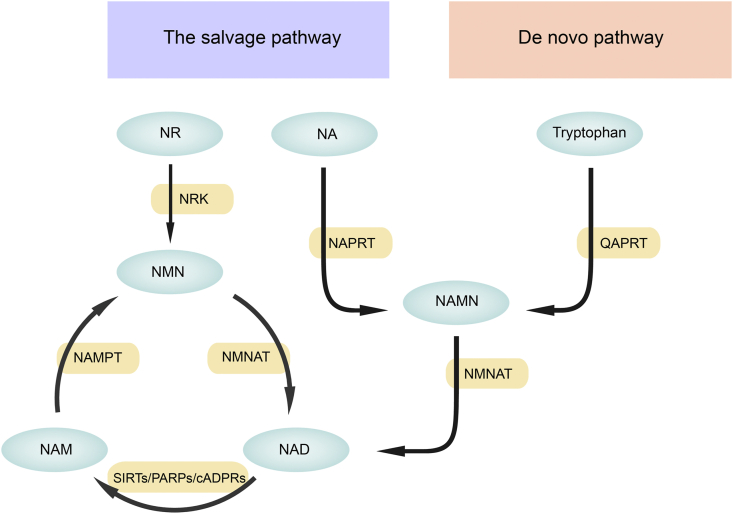
Nicotinamide adenine dinucleotide (NAD+) is a pyridine nucleotide that plays a crucial role in the catabolism of fuel substrates in a variety of cells.7 In mammals, NAD+ is synthesized from a variety of precursors and consumed in many pathways, which have been comprehensively reviewed,8, 9, 10, 11 and are thus only briefly described here. NAD+ can be produced from tryptophan (Trp) in the de novo pathway or from nicotinic acid (NA), nicotinamide (NAM), nicotinamide riboside (NR), or nicotinamide mononucleotide (NMN) in the salvage pathway. NA, NAM and NR, also known as three different forms of vitamin B3, have shown potential as dietary supplements to increase intracellular NAD+ levels.8 The de novo pathway relies on tryptophan obtained from food for the synthesis of quinolinic acid (QA), which is then transformed by QAPRT (quinolinate phosphoribosyltransferase) into nicotinate mononucleotide (NAMN). Nicotinate mononucleotide (NAMN) can also be converted from NA by nicotinate phosphoribosyl transferase (NAPRT). Nicotinate mononucleotide (NAMN) is further catalysed by nicotinamide mononucleotide adenylyltransferase (NMNAT) to produce NAD+. The reaction process in which NA is converted to NAD+ is called the Preiss handler pathway.12 In another reaction in the salvage pathway, NAM is first catalysed by nicotinamide phosphoribosyl transferase (NAMPT) to produce NMN, which is further transformed by nicotinamide mononucleotide adenylyltransferase (NMNAT) to produce NAD+. NMN can also be converted from NR by nicotinamide riboside kinases (NRKs) (Fig. 1). Notably, the salvage pathway is the main source of NAD+ in mammals.11
Figure 1.
Nicotinamide adenine dinucleotide (NAD+) biosynthetic pathways in mammals. There are two major pathways of NAD+ synthesis: the de novo pathway from tryptophan and the salvage pathway from NA, NAM and NR with different catalysing enzymes. NAD+ can also be converted to NAM by three major NAD+-consuming enzymes, SIRTs, PARPs and cADPRs. cADPRs, cyclic ADP- ribose synthases; NA, nicotinic acid; NAD+, nicotinamide adenine dinucleotide; NAM, nicotinamide; NAMN, nicotinic acid mononucleotide; NAMPT, nicotinamide phosphoribosyltransferase; NAPRT, nicotinic acid phosphoribosyltransferase; NMN, nicotinamide mononucleotide; NMNAT, nicotinamide mononucleotide adenylyltransferase; NR, nicotinamide riboside; NRK, nicotinamide ribose kinase; PARPs, poly-ADP-ribose polymerases; QAPRT, quinolinate phosphoribosyltransferase; SIRTs, sirtuin.
The homeostasis of NAD+ in cells depends on the balance of NAD+ biosynthesis and consumption. There are three major NAD+-consuming enzymes: sirtuin family members (SIRTs), poly(ADP-ribose) polymerases (PARPs) and cyclic ADP-ribose synthases (cADPRs). The SIRT family consists of “longevity proteins” with conserved sequences that are ubiquitous in almost all organisms and NAD+-dependent deacetylase activity, which is extensively involved in metabolic regulation, antioxidation and cell fate.13 PARPs constitute a protease family consisting of 17 members. They transfer the ADP-ribose unit from NAD+ to a target protein, which leads to the poly ADP-ribosylation of various proteases and itself, and these proteases are involved in nuclear DNA damage repair and induce mitochondrial signalling.14 cADPRs consist of a pair of extracellular enzymes, CD38 and CD157, called lymphocytic antigens that are involved in the calcium signalling pathway, cell life cycle and insulin secretion by hydrolysing NAD+ to generate the second messenger cADP.15 In this review, we explain our contention that NAD+ is involved in the signalling pathways of CVDs and provide an overview of the benefits and underlying mechanisms of NAD+ replenishment in the cardiovascular system. In addition, we discuss the therapeutic potential of NAD+ in clinical practice (Figure 2, Figure 3, 3).
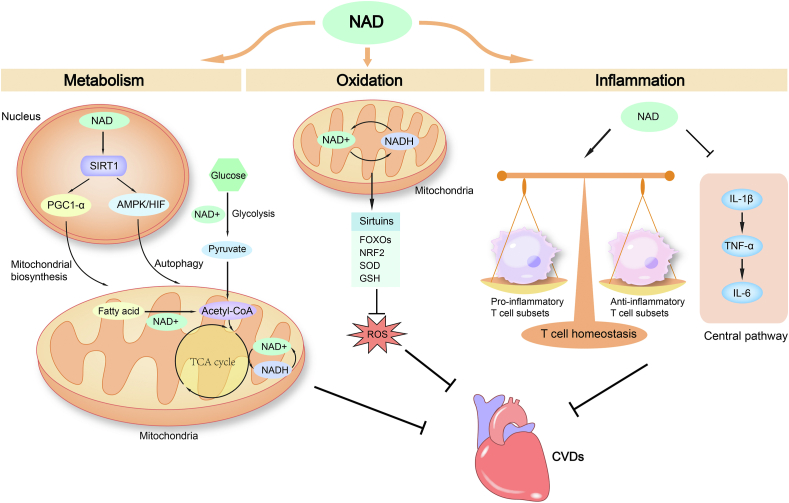
Figure 2.
Nicotinamide adenine dinucleotide (NAD+) exerts beneficial effects on CVDs by regulating metabolism, maintaining redox homeostasis and modulating immune responses. The NAD+ coenzyme is reduced to NADH during glycolysis, fatty acid β-oxidation and the tricarboxylic acid (TCA) cycle. NAD+ can regulate mitochondrial biogenesis through the SIRT1/PGC1α pathway and enhance autophagy through the SIRT1/AMPK/HIF pathway. These effects of NAD+ in metabolism contribute to its invention potential for CVDs treatment. The balance of NAD+ and NADH is a key component of the redox state of a cell. NAD+ can exert antioxidant effects by activating SIRTs to regulate FOXOs, NRF2, SOD and GSH, thus countering oxidative stress in CVDs. NAD+ can also suppress the central immune pathway and regulate T-cell homeostasis to exert immunomodulatory effects, which might be beneficial as a treatment of CVDs. AMPK, adenosine monophosphate-activated protein kinase; CVDs, cardiovascular diseases; FOXOs, class O of forkhead box family; GSH, glutathione; HIF, hypoxia inducible factors; NRF2, nuclear factor E2-related factor 2; PGC1α, peroxisome proliferator-activated receptor γ (PPARγ) coactivator 1α; ROS, reactive oxygen species; SOD, superoxide dismutase.
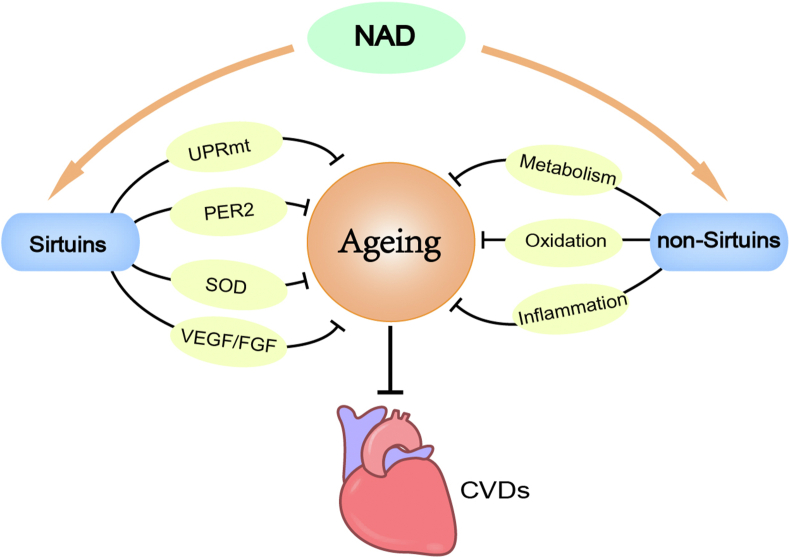
Figure 3.
The role of NAD+in age-related CVDs. Nicotinamide adenine dinucleotide (NAD+) can exert anti-ageing effects by modulating sirtuin activity to regulate UPRmt, PER2, SOD and VEGF/FGF. NAD+ can also counter ageing in non-sirtuin-dependent ways through its beneficial effects on metabolism, oxidation and inflammation. Thus, NAD+ can treat age-related CVDs by delaying ageing. CVDs, cardiovascular diseases; FGF, fibroblast growth factor; PER2, period 2; SOD, superoxide dismutase; UPRmt, mitochondrial unfolded protein response; VEGF, vascular endothelial growth factor.
Mechanism underlying the effects of NAD+ in cardiovascular diseases
NAD+ is an important component in metabolic pathways
Different subcellular NAD+ pools are interconnected through a complex set of redox processes, thus modulating the activity of compartment-specific metabolic pathways such as glycolysis in the cytoplasm and the tricarboxylic acid (TCA) cycle in the mitochondria.10 In the cytoplasm, the synthesis of pyruvate by glycolysis requires two NAD+ molecules per molecule of glucose, following the synthesis of acetyl-CoA (coenzyme A) through pyruvate oxidation. Acetyl-CoA can also be produced by the β-oxidation of fatty acids, which reduces NAD+ to nicotinamide adenine dinucleotide with one hydrogen atom (NADH). Acetyl-CoA then enters the tricarboxylic acid (TCA) cycle in the mitochondrial matrix, which reduces NAD+ molecules to produce multiple NADH molecules. NADH molecules then undergo oxidative phosphorylation, which strips them of their electrons, leading to the oxidized form, NAD+, in the electron transport chain (ETC) located at the mitochondrial membrane.
Metabolic disorders play crucial roles in CVDs, such as atherosclerosis,4 cardiomyopathy16 and heart failure.17 Therefore, NAD+ supplementation may improve these diseases through metabolic pathways. First, NAD+ may influence the metabolic process of nutrients such as glucose and fatty acids. Studies have shown that NR supplementation can stimulate glycolysis and enhance citrate and acetyl-CoA metabolism in heart failure.18 Fang et al observed a distinct metabolic profile (different levels of amino acids and metabolites in the TCA cycle) in ataxia-telangiectasia mutated (ATM)-deficient worms and mice. However, NAD+ can restore most of these metabolites.19 Recent evidence also indicates that NAD+ can redirect glucose metabolic flux through the pentose phosphate pathway, which links glucose homeostasis to redox balance.20 In addition, NAD+ can regulate metabolism by modulating SIRT activity. Since SIRT1 has been reported to function as a low-energy sensor in concert with adenosine monophosphate-activated protein kinase (AMPK) and hypoxia inducible factors (HIFs) to enhance autophagy and maintain mitochondrial homeostasis,21 NAD+ may enhance the metabolic state in heart failure through SIRT1/AMPK/HIF pathways. NAD+ can also regulate mitochondrial biogenesis and function through the SIRT1/PGC1α (peroxisome proliferator-activated receptor γ (PPARγ) coactivator 1α) pathway, which is essential for energy metabolism in cardiomyocytes.22 Interestingly, as the beneficial effects of niacin on dyslipidaemia might be partly explained by increasing NAD+ levels to promote SIRT1 activity,23 NAD+ supplementation may decrease serum triglyceride (TG) and free fatty acid (FFA) levels through a similar pathway. Although its specific role in metabolic control is still debated, NAD+ has been shown to be a node in the metabolic network. As CVDs are closely associated with metabolic disorders and energy deficiency,24 NAD+ may be an attractive target to improve energy metabolism to ultimately improve cardiac performance.
NAD+ is involved in sustaining redox homeostasis
Redox reactions involve the transfer of electrons between species in which atoms oxidation state have changed. Many diseases and disorders have been linked with oxidant- antioxidant imbalance within cells. Indeed, oxidative stress is an important factor involved in CVDs progression.5 An increase in ROS production has been described in several CVDs, such as myocardial fibrosis,25 cardiac hypertrophy,26 myocardial infarction27 and heart failure.28 As a coenzyme, NAD+ mediates redox reactions by carrying electrons from one reactant to another. The NAD+/NADH ratio is a key component in the redox state of a cell.29 An altered cytosolic redox state and energy deficiency have been observed in human heart failure, and their restoration can alleviate heart failure by restoring the NAD+ redox balance and thus suppress mitochondrial protein hyperacetylation, including that of the proteins in the malate aspartate shuttle and regulators of the mitochondrial permeability transition pore (mPTP),30 which suggests a novel therapy for heart failure. More importantly, NAD+ is a cofactor for aldehyde oxidation by aldehyde dehydrogenase 2 (ALDH2).31 Studies have found that imbalanced NAD+/NADH ratios in the heart during ischaemia can prevent the hydroxynonenal (HNE) oxidation catalysed by ALDH2,32 thus increasing oxidative stress and mitochondrial injury. Therefore, increasing NAD+ during ischaemia may be a pharmaceutical approach to confer cardioprotective effects by restoring the HNE reaction.
Additionally, the fact that the deacetylase activity of SIRTs is dependent upon NAD+ lends credence to the idea that NAD+ can regulate cellular antioxidant and redox signalling in a SIRT-dependent manner.33,34 SIRTs have been found to act as critical regulators of the antioxidant response through multiple targets, such as the proteins in the forkhead box O (FOXO) family,35 nuclear factor E2-related factor 2 (NRF2),36 superoxide dismutase (SOD)37 and glutathione (GSH),38 which provides opportunities for NAD+ supplementation to counter oxidative stress in CVDs. Moreover, exogenous NAD+ administration has been reported to alleviate ischaemia/reperfusion-induced oxidative injury in rat hearts via the SIRT5-SDH-succinate pathway.33 NMN, as an intermediate in NAD+ synthesis, can also protect the heart from ischaemia/reperfusion injury through the SIRT1/FOXO1 pathway.39 Therefore, NAD+ has shown great therapeutic potential in CVDs because it can be used to sustain redox homeostasis.
NAD+ participates in anti-inflammation processes
Inflammation plays a crucial role in CVDs, and its involvement in the pathogenesis of heart failure40,41 and ischaemic cardiovascular disease has been recognized.6 However, the outcomes of anti-inflammatory strategies used in cardiovascular system therapy have been disappointing thus far,42 which may explain the limited role of these regimens in the clinic. Interestingly, the administration of canakinumab, a therapeutic monoclonal antibody targeting interleukin-1β, demonstrates beneficial effects in those patients.43 Although the underlying mechanisms are not fully understood, this antibody has shown promise for the treatment of CVDs. The following questions are raised at this stage: Will anti-inflammatory therapies eventually form the cornerstone of cardiovascular risk reduction? Which novel therapeutic targets will be identified among the different pathological phenotypes of inflammation? NAD+, a cofactor for numerous enzymes, may be involved in the modification of systemic immune responses. We hypothesize that NAD+ can suppress the central immune pathway and regulate T-cell homeostasis to exert immunomodulatory effects. Supplementation with NAD+ or its precursors might constitute a promising strategy in anti-inflammatory therapies. Recent studies have revealed that NAD+ can depress the levels of inflammatory cytokines,44,45 which may indicate its potential in targeting the central immune pathway, specifically by linking IL-1β, TNF-α, and IL-6, which are considered upstream inflammatory biomarkers.46 NAD+ has also been reported to regulate T cell homeostasis during inflammation by participating in the signalling pathway of CD4+ T cell differentiation and apoptosis.47,48 Since many acute and chronic inflammatory diseases are accompanied by an imbalance in T cell subsets, NAD+ may be an ideal molecule to treat these diseases because of its homeostatic properties. Notably, NAD+ exerts its effects through immunomodulation, not immunosuppression. It can promote the systemic production of anti-inflammatory cytokines49 and regulate CD4+ T cell fate in the absence of antigen.50 Despite its complicated role in immune responses, its anti-inflammatory potential may further support the clinical application of NAD+ or its precursors for CVDs treatment.
NAD+ exerts anti-ageing effects
Ageing is characterized by a progressive loss of physiological integrity leading to impaired function and increased vulnerability until death.51 Slowing the ageing process is an enduring aim that has generated an exciting field of study. Ageing is also considered an important risk factor for CVDs because it is associated with excessive oxidative stress, chronic low-grade inflammation and the overactivation of the sympathetic nervous system.52 Ageing contributes to many cellular and even structural changes in the cardiovascular system, including myocardial fibrosis,53 amyloidosis in the myocardium,54 cardiac hypertrophy,55 endothelial dysfunction and artery stiffness.56 These changes are associated with an increased risk of coronary artery disease, hypertension, atrial fibrillation, cardiomyopathy and heart failure.57
The coenzyme NAD+ is critical in cellular bioenergetics and adaptive stress responses. Emerging evidence suggests that boosting cellular NAD+ levels alleviates physical deterioration, chronic inflammation, metabolic dysregulation, ROS overproduction and DNA damage, which can delay ageing in four respects.58 NAD+ is widely involved in signal transduction in the ageing process and has shown broad prospects for treating age-related CVDs. Both dietary and injected replenishment of NAD+ have shown beneficial effects on age-associated phenotypes. A recent clinical trial reported that oral NR supplementation in 12 aged men was well tolerated and reduced the levels of circulating cytokines (IL-6, IL-5, IL-2, and TNF-α), with altered energy metabolism at the transcriptional level.44 Other studies have provided evidence showing that NAD+ can improve physical activity,59 metabolic status60 and memory function19 in aged mice. Additionally, studies have found that NAD+ replenishment significantly extends the lifespan of both worms and mice.19,61,62 Notably, supplementation with NAD+ precursors can prevent vascular ageing by enhancing endothelial relaxation, reducing artery stiffness63 and increasing muscular capillary density.59 As shown in Table 1, NAD+ replenishment not only can extend the lifespan but can also improve healthspan. Table 1 also shows that NAD+ can delay the processes implicated in age-related CVDs.
Table 1.
Effects of NAD+ on ageing in humans and animal models.
| Animals & strain | Supplement method & dose | Benefits | Refs |
|---|---|---|---|
| 12 men aged (median age of 75 years) |
NR 1000mg/2 × /d 21 d |
Downregulation of energy metabolism in skeletal muscle depressed circulating levels of IL-6, IL-5, IL-2, TNF-α |
44 |
| DBA/2J (D2) mice | NAM 550 mg/kg/d |
Reduced vulnerability to glaucoma reduced the incidence of optic nerve degeneration |
67 |
| C57BL/6N mice |
NMN 500 mg/kg NR 400 mg/kg/d |
Enhanced nighttime locomotor activity rhythms. | 64 |
| C57BL/6 mice |
NMN 300 mg/kg/d |
Improved carotid artery endothelium-dependent dilation reduced aortic stiffness |
63 |
| C57BL/6J mice |
NMN 400 mg/kg/d |
Increased capillary density and blood flow Increased exercise endurance |
59 |
| C57BL/6N Mice |
NMN 100 mg/kg/d 300 mg/kg/d |
Decreased age-associated body weight gain, enhanced energy metabolism and physical activity, improved insulin sensitivity, plasma lipid profile, eye function and bone density. | 60 |
| C57BL/10SnJ mice |
NR 400 mg/kg/d |
Reduction in cardiac fibrosis, necrosis, and inflammatory cell infiltration | 66 |
| C57BL/6J mice |
NR 400 mg/kg/d |
Improved muscle function expanded lifespan | 61 |
| B6 Atm−/− Mice |
NR 12 mM NMN 12 mM |
Improved behavioral and memory function expanded lifespan |
19 |
| C57BL/6J mice |
NAM 500 mg/kg 1000 mg/kg |
Improved glucose metabolism enhanced physical performance reduced hepatic steatosis |
20 |
| N2(WT) C. elegans |
NR 500uM |
Improved locomotion and cognitive function expanded lifespan | 19 |
| N2(WT) C. elegans |
NR 500uM NAM 200uM |
Improved metabolic state expanded lifespan | 62 |
NA, nicotinic acid; NAM, nicotinamide; NMN, nicotinamide mononucleotide; NR, nicotinamide riboside.
Potential NAD+-mediated mechanisms have been explored. Because SIRTs are longevity-associated deacetylases, NAD+ can be used to counter ageing by modulating SIRT activity. For example, NAD+ can prevent age-associated metabolic decline and extend lifespan through the SIRT1/mitochondrial unfolded protein response (UPRmt)/superoxide dismutase (SOD) signalling pathway.61,62 Additionally, NAD+ has been demonstrated to counteract the age-related decline in circadian rhythms through SIRT1 to deacetylate clock repressor period 2 (PER2).64 Since evidence has indicated that metabolic homeostasis is intimately linked with the circadian clock,65 NAD+ may also have the potential to treat metabolic disorders associated with the ageing process. NMN supplementation has been reported to reverse age-related arterial dysfunction by decreasing oxidative stress in old mice, an outcome associated with the activation of SIRT1 and manganese superoxide dismutase (MnSOD).63 NAD+ can prevent vascular ageing by activating SIRT1 to mediate vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (FGF) secretion from myocytes to influence endothelial cells.59 NR treatment reduced cardiac fibrosis, necrosis, and inflammatory cell infiltration in aged mdx mice (a Duchene's muscular dystrophy model) with cardiomyopathy by activating SIRT1 and enhancing mitochondrial function.66 Therefore, NAD+ has shown promise for treating age-related CVDs in a SIRT-dependent manner.
The exact molecular mechanisms of the anti-ageing effect of NAD+ are not yet understood. Therefore, our team has suggested that a non-sirtuin-dependent mechanism as an emerging new direction, especially for future anti-ageing studies of NAD+. Although studies have shown that NAD+ can delay ageing through its beneficial effects on metabolic regulation,60 redox homeostasis63 and anti-inflammation,44 the role of NAD+ in these studies is still debated, and further investigations are needed to explore the underlying mechanisms.
Clinical evidence and future prospects for NAD+ translation
Research and current problems
Given its multiple properties, including its ability to regulate metabolism, maintain the redox balance, exert anti-inflammatory effects and delay ageing, NAD+ has gained attention for its therapeutic potential. The question remains: Can increasing NAD+ levels are clinically beneficial? As shown in Table 2, the administration of various NAD+ precursors can lead to a dose-dependent increase in blood NAD+ levels and does not appear to have significant adverse effects. Some studies indicate that NAD+ may decrease the risk of certain CVDs, such as hypertension, arterial stiffness and hyperlipidaemia.67, 68, 69 Several other studies also show that NAD+ can improve metabolic and inflammatory profiles,44,70,71 which suggests that NAD+ is involved in related signalling pathways in humans.
Table 2.
Studies that detected an increase in NAD+ levels.
| Pharmacological Agent | Subjects | Health Outcomes Observed | References |
|---|---|---|---|
| NR 1000 mg/d 6 weeks |
13 men and women overweight (BMI 27–35 kg/m2) |
NAD↑ body fat-free mass↑ sleeping metabolic rate↑ |
70 |
| NR 1000mg/2 × /d 21d |
12 men aged (median age of 75 years) |
energy metabolism in skeletal muscle↑ IL-6, IL-5, IL-2, TNF-α↓ |
44 |
| NR 100 mg/day, 300 mg/day, and 1000 mg/day 8weeks |
140 men and women overweight (BMI 25–30 kg/m2) |
NAD↑ No clinically meaningful changes in vital sign, hematological and biochemical indexes. No adverse events |
72 |
| NR 500 mg, 2 × /day 6 weeks |
30 men and women lean and healthy (average BMI = 24 ± 4 kg/m2) | NAD↑ No effects on haematology, plasma glucose, insulin, TC and TG potential for reducing systolic blood pressure and arterial stiffness |
69 |
| NR 1000 mg/day 1week |
A male Healthy; 52-year-old; 65 kg |
NAD↑ No adverse events |
108 |
| NR 250–1000 mg/d 1 week |
8 participants healthy | NAD↑ No adverse events |
74 |
| NRPT 1X; 250 mg NR + 50 mg PT NRPT 2X 500 mg NR + 100 mg PT 8weeks |
120 participants healthy | NAD↑ No adverse events ALT↓ diastolic blood pressure ↓ mobility↑ lower body strength↑ total cholesterol ↑ LDL-cholesterol↑ |
68 |
| NAM 1080 mg/day 12 weeks |
65 patients hemodialysis | NAD↑ Serum phosphate↓, parathyroid hormone↓ HDL-C↑, LDL-C↓ |
67 |
| Acipimox 250 mg, 3 × /day 2 weeks |
21 patients T2DM |
NAD↑ Mitochondrial unfolded- protein response↑, ATP↑, muscle lipid content↑, mitochondrial respiration↑ |
71 |
NAD, nicotinamide adenine dinucleotide; NAM, nicotinamide; NR, nicotinamide riboside; NRPT, nicotinamide riboside and pterostilbene; PT, pterostilbene; BMI, Body Mass Index; T2DM, type 2 diabetes; TG, triglyceride; TC, total cholesterol; ALT, Alanine aminotransferase; HDL-C, High-density lipoprotein cholesterol; LDL-C, Low-density lipoprotein cholesterol; ATP, Adenosine triphosphate.
However, these studies leave many questions unanswered. On the one hand, none of these studies show a direct benefit to the cardiovascular system, despite the benefits listed in Table 2, Table 3, and many experiments had only neutral outcomes.72, 73, 74, 75, 76 On the other hand, in some studies, NAD+ levels were not determined after supplementation with its precursors, including NA, NR, NAM and NMN, as shown in Table 3. Moreover, NA is not exclusively a NAD+ precursor; it was shown a long time ago to also be a lipid-lowering agent.77 Almost all studies indicate that the treatment of NA or its derivative acipimox can efficiently enhance lipid metabolism and thus exert beneficial effects on CVDs.78, 79, 80, 81, 82, 83, 84, 85, 86 To some extent, it is still not clear whether these effects depend on NAD+ replenishment. Some studies have attributed these beneficial actions of NA to the activation of the putative G protein-coupled receptor GPR109A.87 However, emerging evidence suggests that some of the effects promoted by NA might be achieved through NAD+-induced activation of SIRT1.23,88 Arguing in favour of the latter hypothesis, many of the beneficial effects of NA take place at concentrations much higher than the EC50 for GPR109A (i.e., the concentration to activate 50% of the receptor).89 NA also has some undesirable effects, such as flushing, pruritus and burning pain,90 which largely limits its clinical application. Consequently, whether it is feasible to increase NAD+ levels by supplementing endogenous NA continues to be debated.
Table 3.
Studies in which NAD+ levels were not determined.
| Pharmacological Agent | Subjects | Health Outcomes Observed | References |
|---|---|---|---|
| NA 3000 mg/d 6 years |
3908 patients previous MI |
TG↓, TC↓ incidence of definite, non-fatal MI ↓ |
78 |
| NA 2000 mg/d 7–14 months |
315 Patients CHD |
TG↓, TC↑, HDL-C↑, LDL-C↑ CIMT ↓ |
85 |
| NA 375–1000 mg 4weeks + 1500 mg 6weeks |
24 patients HDL-C < 0.9 mmol/L |
TG↓, TC↓, LDL-C↓, HDL-C↑, Adiponectin↑, resistin↓ |
84 |
| NA 1000 mg 4 × /d + colestipol 10000 mg 3 × /d 2.5 years |
120 men high Apo B levels coronary artery disease a family history of vascular disease |
TG↓, LDL-C↓, HDL-C↑ Atherosclerotic progression↓ incidence of cardiovascular events↓ |
86 |
| NA + colestipol 3000–12000 mg/d 2 years |
162 men previous coronary bypass surgery | TG↓, LDL-C↓, HDL-C↑ Atherosclerotic progression↓ |
79 |
| NA + Simvastatin 1000–4000 mg/d 3 years |
160 patients CHD low HDL-C levels |
LDL-C↓, HDL-C↑ Coronary stenosis↓ occurrence of a first cardiovascular event↓ |
81 |
| NA + statins 1000 mg/d 1 year |
167 patients CHD low HDL-C levels |
HDL-C↑ Atherosclerotic progression↓ |
83 |
| NA 1500–2000 mg/d + Simvastatin 40–80 mg/d + ezetimibe 10 mg/d 3 years |
3414 patients cardiovascular disease |
TG↓, LDL-C↓, HDL-C↑ | 80 |
| NA 2000 mg/d + ezetimibe/simvastatin 10/20 mg/d 24weeks |
1220 patients type IIa or IIb hyperlipidemic | TG↓, LDL-C↓, Apo B↓, lipid/lipoprotein ratio↓, HDL-C↑, Apo A-I↑ well tolerated aside from N-associated flushing |
82 |
| NR 2000 mg/d 12 weeks |
40 men healthy; obese (BMI > 30 kg/m2) | No effects on glucose and lipid homeostasis | 73 |
| NR 2000 mg/d 12 weeks |
40 men obese (BMI 30 kg/m2) | No effects on fasting/postglucose plasma glucose, insulin, C-peptide, glucagon and β-cell function | 109 |
| NAM 1500 mg 2 × /d 24 weeks |
31 patients mild to moderate dementia | no significant effects on the cognitive function No adverse events |
76 |
| NMN (100 mg/d, 250 mg/d, and 500 mg/d) |
10 men healthy | Bilirubin↑, creatinine↓, chloride↓, glucose ↓ No adverse events |
110 |
| Acipimox 1000 mg/d 1week |
25 individuals overweight (BMI 22–30 kg/m2) | FFA↓, insulin sensitivity↑, C-peptide↓, HOMA index↓, systolic BP↓, cardiac function↓ | 75 |
| Acipimox 750 mg/d 4–9 weeks |
30 patients hyperlipoproteinaemia |
TG↓, LDL-C↓, HDL-C↑ | 111 |
| Acipimox 750–1200 mg/d 60 d–9months |
17 patients hyperlipoproteinaemia |
TG↓, plasma TC↓, HDL-C↑ | 112 |
| Acipimox 1500 mg/d 3 d |
8 patients NIDDM |
FFA with rebound↓, TG↓, glucose↓, insulin↓ | 113 |
| Acipimox 1000 mg/d 7 d |
7 patients T2DM |
FFA↓, insulin sensitivity↑, glucose↓, glucose tolerance↑ | 114 |
| Acipimox 1000 mg/d |
8 men hypopituitary | Glucose↓, FFA↓, insulin sensitivity↑ | 115 |
| Acipimox 150 mg/d |
14 volunteers healthy | FFA↓, disposition index during 24-h fasting↑, insulin response↑, insulin sensitivity↑ | 116 |
| Acipimox 750 mg/d |
9 subjects, lean control 13 subjects, obese 10 subjects, obese, impaired glucose tolerance, 11 patients, T2DM |
FFA↓, insulin↓, insulin- stimulated glucose uptake↑, glucose tolerance↑ | 117 |
| Acipimox 750 mg/d |
8 men overwerght (BMI: 30.06 0.7 kg/m2) 8 men, hypopituitary |
FFA↓, GLP-1 ↑, glucose- infusion rate↑ | 118 |
| Acipimox 250 mg/d |
14 male patients T2DM |
FFA during exercise↓, glucose and insulin during recovery from exercise↓, glycaemic control↑ | 119 |
| EH301 1200 mg/d 4 months |
32 people ALS |
Fat mass↓, Pulmonary function↑, muscular strength↑ | 120 |
NA, nicotinic acid; NR, nicotinamide riboside; NAM, nicotinamide; NMN, nicotinamide mononucleotide; BMI, body mass index; T2DM, type 2 diabetes; TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; CHD, coronary heart disease; CIMT, carotid intima-media thickness; ALS, amyotrophic lateral sclerosis; NIDDM, noninsulin-dependent diabetes mellitus; FFA, free fatty acid; GLP-1, glucagon-like peptide 1; HOMA index, homeostasis model assessment; BP, blood pressure; Apo A-I, apolipoprotein A-I; Apo B, apolipoprotein B.
Since these clinical trials have some disappointing outcomes, some points need to be reconsidered. First, the number of participants in some clinical trials was too small to enable the acquisition of significant results. Second, the time of these studies might be too short for NAD+ to show its full beneficial effects. Moreover, there is a gap between the doses administered in vitro and the effective intracellular concentrations. Although NAD+ may exert a long-term benefit on the cardiovascular system in the future, the side effects of NA indicate that we cannot neglect its risk. Further large, well-designed clinical studies are needed to clarify the therapeutic potential and to establish treatment protocols for the use of NAD+ or its precursors.
Boosting NAD+ levels and filling remaining gaps
As mentioned above, emerging evidence has shown that boosting NAD+ levels may have favourable effects, and the best way to increase NAD+ levels for clinical applications remains unclear. Pharmacologically modulating NAD+ levels has emerged as a hot topic for drug development.91 There are three main approaches that researchers and drug developers are exploring to increase NAD+ levels, including supplementation with NAD+ precursors or their biosynthetic intermediates, stimulating NAD+ synthesis and inhibiting NAD+ consumptions.10 In addition, NAD+ levels can be increased during physiological processes, such as caloric restriction92 and exercise,93 while they can be decreased upon intake of high-fat diets (HFDs).94 Maintaining a low-energy lifestyle may be a simpler way to increase NAD+ than taking medicine.
As the oral route is most commonly used for drug administration, we cannot neglect the interactions between the gut microbiome and administered drugs. A recent study has reported that E. coli can enhance the chemotherapeutic toxicity of fluoropyrimidines by converting 5′-fluorodeoxyuridine (FUdR) into 5-fluorouridine monophosphate (FUMP).95 The complexity of drug–microbe–host co-metabolism indicates that NAD+ precursors may interact with the gut microbiome directly or indirectly,96 thus modulating drug efficacy and toxicity.
Given the fact that NAD+ is not evenly distributed in the cell97, 98, 99, 100 and its subcellular distribution is highly variable between tissues,91 we cannot neglect the gap between the doses administered in vitro and the effective concentrations in different subcellular compartments. On the one hand, the permeability of subcellular organelles to NAD+ is different. NAD+ is considered to be easily translocated between the nucleus and cytoplasm, but the mitochondrial free NAD+ level is thought to fluctuate differently than the nuclear and cytoplasmic levels.97 However, a recent study was the first to identify a mammalian mitochondrial NAD+ transporter, SLC25A51,98 which shed light on the mechanism critical for the uptake of NAD+ into mitochondria. Hence, whether it is feasible to replenish the mitochondrial NAD+ pool by increasing the cytoplasmic NAD+ level remains controversial. In addition, the subcellular distribution of NAD+ biosynthetic enzymes differs. Most enzymes important for NAD+ synthesis have been detected only in the cytosol, including nicotinamide riboside kinase 1 (NRK1), nicotinamide riboside kinase 2 (NRK2)99 and nicotinate phosphoribosyl transferase (NAPRT).101 However, nicotinamide mononucleotide adenylyltransferase (NMNAT) has been found in the cytosol, nucleus, mitochondria and even the Golgi apparatus, although as different isoforms.100 Similarly, nicotinamide phosphoribosyl transferase (NAMPT) is ubiquitous in the cytosol, nucleus and mitochondria,102 with NAM considered the common precursor of NAD+. The manner of NAD+ replenishment and the type of NAD precursors supplemented might affect its subcellular distribution.
Perspective
The SIRT family was first characterized in yeast as silent information regulator 2 (Sir2).103 More homologues of Sir2 were found in Caenorhabditis elegans and Drosophila melanogaster.104 In mammals, seven SIRTs (SIRT1-7) have different subcellular locations, enzymatic activities and targets.13 As NAD+ is an essential substrate of SIRTs, this compartmentalized localization of SIRTs might be regulated by the uneven distribution of NAD+ pools and may be adjusted on the basis of the complex metabolic state of the cell. This evolutionary process might be partly due to the compartmentalized distribution of NAD+ pools, which can help organisms adapt to externally changing environments and constant stress. We think the benefits of NAD+ pool homeostasis may be related to this evolutionary process to some extent. Restoring the homeostasis of NAD+ pools may contribute to the maintenance of SIRT activity, which has favourable effects on healthspan. This hypothesis can partly explain why a single SIRT agonist does not show more clinical benefits.105,106 Therefore, we hypothesize that NAD+ may be involved in the evolutionary process of SIRTs and that the homeostasis of NAD+ pools might be more important than the simple activation of SIRTs.
Importantly, the pan-effects of NAD+ have shown broad application prospects for the treatment of CVDs, including atherosclerosis, coronary heart disease, hypertension, arrhythmias and heart failure.107 In the future, applying NAD+ for primary prevention may have profound significance for the management of CVDs. We confirmed that NAD+ can be a prophylactic drug for CVDs, which can decrease health care costs and relieve socioeconomic burdens.
Author contributions
Xiaokai Zhang contributed to literature searching and wrote the manuscript. Yang Zhang initiated and designed the review. Junbo Ge and Aijun Sun supervised the study and revised the manuscript. All authors read and approved the final manuscript.
Conflict of interests
The authors declare that they have no competing interests.
Acknowledgements
We authors thank Dr. Qinfeng Hu's help in this work.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Abbreviations
- cADPRs
cyclic ADP- ribose synthases
- NA
nicotinic acid
- NAD
nicotinamide adenine dinucleotide
- NAM
nicotinamide
- NAMN
nicotinic acid mononucleotide
- NAMPT
nicotinamide phosphoribosyltransferase
- NAPRT
nicotinic acid phosphoribosyltransferase
- NMN
nicotinamide mononucleotide
- NMNAT
nicotinamide mononucleotide adenylyltransferase
- NR
nicotinamide riboside
- NRK
nicotinamide ribose kinase
- PARPs
poly-ADP-ribose polymerases
- QAPRT
quinolinate phosphoribosyltransferase
- SIRTs
sirtuins
- AMPK
adenosine monophosphate-activated protein kinase
- CVDs
cardiovascular diseases
- TCA
tricarboxylic acid
- FOXOs
class O of forkhead box family
- GSH
glutathione
- HIF
hypoxia inducible factors
- NRF2
nuclear factor E2-related factor 2
- PGC1α
peroxisome proliferator-activated receptor γ (PPARγ) coactivator 1α
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- FGF
fibroblast growth factor
- PER2
period 2
- UPRmt
mitochondrial unfolded protein response
- VEGF
vascular endothelial growth factor
- ATM
ataxia-telangiectasia mutated
- TG
triglyceride
- FFA
free fatty acid
- ALDH2
aldehyde dehydrogenase 2
- HNE
hydroxynonenal
- FUdR
5′-fluorodeoxyuridine
- FUMP
5-fluorouridine monophosphate
References
- 1.Francula-Zaninovic S., Nola I.A. Management of measurable variable cardiovascular disease' risk factors. Curr Cardiol Rev. 2018;14(3):153–163. doi: 10.2174/1573403X14666180222102312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afzal M. Recent updates on novel therapeutic targets of cardiovascular diseases. Mol Cell Biochem. 2020;476(1):145–155. doi: 10.1007/s11010-020-03891-8. [DOI] [PubMed] [Google Scholar]
- 3.Zhazykbayeva S., Pabel S., Mügge A., Sossalla S., Hamdani N. The molecular mechanisms associated with the physiological responses to inflammation and oxidative stress in cardiovascular diseases. Biophys Rev. 2020;12(4):947–968. doi: 10.1007/s12551-020-00742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theodorou K., Boon R.A. Endothelial cell metabolism in atherosclerosis. Front Cell Dev Biol. 2018;6 doi: 10.3389/fcell.2018.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubois-Deruy E., Peugnet V., Turkieh A., Pinet F. Oxidative stress in cardiovascular diseases. Antioxidants (Basel) 2020;9(9) doi: 10.3390/antiox9090864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Libby P., Nahrendorf M., Swirski F.K. Leukocytes link local and systemic inflammation in ischemic cardiovascular disease: an expanded "cardiovascular continuum". J Am Coll Cardiol. 2016;67(9):1091–1103. doi: 10.1016/j.jacc.2015.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sultani G., Samsudeen A.F., Osborne B., Turner N. NAD+ : a key metabolic regulator with great therapeutic potential. J Neuroendocrinol. 2017;29(10) doi: 10.1111/jne.12508. [DOI] [PubMed] [Google Scholar]
- 8.Rajman L., Chwalek K., Sinclair D.A. Therapeutic potential of NAD-boosting molecules: the in vivo evidence. Cell Metabol. 2018;27(3):529–547. doi: 10.1016/j.cmet.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonkowski M.S., Sinclair D.A. Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nat Rev Mol Cell Biol. 2016;17(11):679–690. doi: 10.1038/nrm.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canto C., Menzies K.J., Auwerx J. NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metabol. 2015;22(1):31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang E.F., Lautrup S., Hou Y., et al. NAD+ in aging: molecular mechanisms and translational implications. Trends Mol Med. 2017;23(10):899–916. doi: 10.1016/j.molmed.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lautrup S., Sinclair D.A., Mattson M.P., Fang E.F. NAD+ in brain aging and neurodegenerative disorders. Cell Metabol. 2019;30(4):630–655. doi: 10.1016/j.cmet.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finkel T., Deng C.X., Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460(7255):587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang E.F., Scheibye-Knudsen M., Chua K.F., Mattson M.P., Croteau D.L., Bohr V.A. Nuclear DNA damage signalling to mitochondria in ageing. Nat Rev Mol Cell Biol. 2016;17(5):308–321. doi: 10.1038/nrm.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aksoy P., White T.A., Thompson M., Chini E.N. Regulation of intracellular levels of NAD: a novel role for CD38. Biochem Biophys Res Commun. 2006;345(4):1386–1392. doi: 10.1016/j.bbrc.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 16.Greenwell A.A., Gopal K., Ussher J.R. Myocardial energy metabolism in non-ischemic cardiomyopathy. Front Physiol. 2020;11 doi: 10.3389/fphys.2020.570421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noordali H., Loudon B.L., Frenneaux M.P., Madhani M. Cardiac metabolism - a promising therapeutic target for heart failure. Pharmacol Ther. 2018;182:95–114. doi: 10.1016/j.pharmthera.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Diguet N., Trammell S.A.J., Tannous C., et al. Nicotinamide riboside preserves cardiac function in a mouse model of dilated cardiomyopathy. Circulation. 2018;137(21):2256–2273. doi: 10.1161/CIRCULATIONAHA.116.026099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang E.F., Kassahun H., Croteau D.L., et al. NAD+ replenishment improves lifespan and healthspan in ataxia telangiectasia models via mitophagy and DNA repair. Cell Metabol. 2016;24(4):566–581. doi: 10.1016/j.cmet.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell S.J., Bernier M., Aon M.A., et al. Nicotinamide improves aspects of healthspan, but not lifespan, in mice. Cell Metabol. 2018;27(3):667–676.e4. doi: 10.1016/j.cmet.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Packer M. Role of deranged energy deprivation signaling in the pathogenesis of cardiac and renal disease in states of perceived nutrient overabundance. Circulation. 2020;141(25):2095–2105. doi: 10.1161/CIRCULATIONAHA.119.045561. [DOI] [PubMed] [Google Scholar]
- 22.Lehman J.J., Barger P.M., Kovacs A., Saffitz J.E., Medeiros D.M., Kelly D.P. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106(7):847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romani M., Hofer D.C., Katsyuba E., Auwerx J. Niacin: an old lipid drug in a new NAD+ dress. J Lipid Res. 2019;60(4):741–746. doi: 10.1194/jlr.S092007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karwi Q.G., Uddin G.M., Ho K.L., Lopaschuk G.D. Loss of metabolic flexibility in the failing heart. Front Cardiovasc Med. 2018;5 doi: 10.3389/fcvm.2018.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermida N., Michel L., Esfahani H., et al. Cardiac myocyte β3-adrenergic receptors prevent myocardial fibrosis by modulating oxidant stress-dependent paracrine signaling. Eur Heart J. 2018;39(10):888–898. doi: 10.1093/eurheartj/ehx366. [DOI] [PubMed] [Google Scholar]
- 26.Dai D.F., Johnson S.C., Villarin J.J., et al. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ Res. 2011;108(7):837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ide T., Tsutsui H., Hayashidani S., et al. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res. 2001;88(5):529–535. doi: 10.1161/01.res.88.5.529. [DOI] [PubMed] [Google Scholar]
- 28.Dai D.F., Hsieh E.J., Liu Y., et al. Mitochondrial proteome remodelling in pressure overload-induced heart failure: the role of mitochondrial oxidative stress. Cardiovasc Res. 2012;93(1):79–88. doi: 10.1093/cvr/cvr274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh C.K., Chhabra G., Ndiaye M.A., Garcia-Peterson L.M., Mack N.J., Ahmad N. The role of sirtuins in antioxidant and redox signaling. Antioxidants Redox Signal. 2018;28(8):643–661. doi: 10.1089/ars.2017.7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee C.F., Chavez J.D., Garcia-Menendez L., et al. Normalization of NAD+ redox balance as a therapy for heart failure. Circulation. 2016;134(12):883–894. doi: 10.1161/CIRCULATIONAHA.116.022495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gross E., Goodnough C. Precision medicine considerations for the management of heart disease and stroke in East Asians. Cardiology Plus. 2020;5(3):101–108. doi: 10.4103/cp.cp_17_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill B.G., Awe S.O., Vladykovskaya E., et al. Myocardial ischaemia inhibits mitochondrial metabolism of 4-hydroxy-trans-2-nonenal. Biochem J. 2009;417(2):513–524. doi: 10.1042/BJ20081615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L., Wang Q., Zhao B., Wu Q., Wang P. Exogenous nicotinamide adenine dinucleotide administration alleviates ischemia/reperfusion-induced oxidative injury in isolated rat hearts via Sirt5-SDH-succinate pathway. Eur J Pharmacol. 2019;858 doi: 10.1016/j.ejphar.2019.172520. [DOI] [PubMed] [Google Scholar]
- 34.Wu X., Hu F., Zeng J., et al. NMNAT2-mediated NAD+ generation is essential for quality control of aged oocytes. Aging Cell. 2019;18(3) doi: 10.1111/acel.12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunet A., Sweeney L.B., Sturgill J.F., et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303(5666):2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 36.Yang X., Park S.H., Chang H.C., et al. Sirtuin 2 regulates cellular iron homeostasis via deacetylation of transcription factor NRF2. J Clin Invest. 2017;127(4):1505–1516. doi: 10.1172/JCI88574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tao R., Coleman M.C., Pennington J.D., et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40(6):893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou L., Wang F., Sun R., et al. SIRT5 promotes IDH2 desuccinylation and G6PD deglutarylation to enhance cellular antioxidant defense. EMBO Rep. 2016;17(6):811–822. doi: 10.15252/embr.201541643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto T., Byun J., Zhai P., Ikeda Y., Oka S., Sadoshima J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PloS One. 2014;9(6) doi: 10.1371/journal.pone.0098972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westermann D., Lindner D., Kasner M., et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2011;4(1):44–52. doi: 10.1161/CIRCHEARTFAILURE.109.931451. [DOI] [PubMed] [Google Scholar]
- 41.Dick S.A., Epelman S. Chronic heart failure and inflammation: what do we really know? Circ Res. 2016;119(1):159–176. doi: 10.1161/CIRCRESAHA.116.308030. [DOI] [PubMed] [Google Scholar]
- 42.Van Linthout S., Tschöpe C. Inflammation - cause or consequence of heart failure or both? Curr Heart Fail Rep. 2017;14(4):251–265. doi: 10.1007/s11897-017-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ridker P.M., Everett B.M., Thuren T., et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 44.Elhassan Y.S., Kluckova K., Fletcher R.S., et al. Nicotinamide riboside augments the aged human skeletal muscle NAD+ metabolome and induces transcriptomic and anti-inflammatory signatures. Cell Rep. 2019;28(7):1717–1728.e6. doi: 10.1016/j.celrep.2019.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Desdín-Micó G., Soto-Heredero G., Aranda J.F., et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science. 2020;368(6497):1371–1376. doi: 10.1126/science.aax0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ridker P.M., Lüscher T.F. Anti-inflammatory therapies for cardiovascular disease. Eur Heart J. 2014;35(27):1782–1791. doi: 10.1093/eurheartj/ehu203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tullius S.G., Biefer H.R., Li S., et al. NAD+ protects against EAE by regulating CD4+ T-cell differentiation. Nat Commun. 2014;5 doi: 10.1038/ncomms6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J., Zhao C., Kong P., et al. Treatment with NAD(+) inhibited experimental autoimmune encephalomyelitis by activating AMPK/SIRT1 signaling pathway and modulating Th1/Th17 immune responses in mice. Int Immunopharm. 2016;39:287–294. doi: 10.1016/j.intimp.2016.07.036. [DOI] [PubMed] [Google Scholar]
- 49.Elkhal A., Rodriguez Cetina Biefer H., Heinbokel T., et al. NAD(+) regulates Treg cell fate and promotes allograft survival via a systemic IL-10 production that is CD4(+) CD25(+) Foxp3(+) T cells independent. Sci Rep. 2016;6 doi: 10.1038/srep22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez Cetina Biefer H., Heinbokel T., Uehara H., et al. Mast cells regulate CD4+ T-cell differentiation in the absence of antigen presentation. J Allergy Clin Immunol. 2018;142(6):1894–1908.e7. doi: 10.1016/j.jaci.2018.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Triposkiadis F., Xanthopoulos A., Butler J. Cardiovascular aging and heart failure: JACC review topic of the week. J Am Coll Cardiol. 2019;74(6):804–813. doi: 10.1016/j.jacc.2019.06.053. [DOI] [PubMed] [Google Scholar]
- 53.Gazoti Debessa C.R., Mesiano Maifrino L.B., Rodrigues de Souza R. Age related changes of the collagen network of the human heart. Mech Ageing Dev. 2001;122(10):1049–1058. doi: 10.1016/s0047-6374(01)00238-x. [DOI] [PubMed] [Google Scholar]
- 54.Tanskanen M., Peuralinna T., Polvikoski T., et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study. Ann Med. 2008;40(3):232–239. doi: 10.1080/07853890701842988. [DOI] [PubMed] [Google Scholar]
- 55.Cheng S., Fernandes V.R., Bluemke D.A., McClelland R.L., Kronmal R.A., Lima J.A. Age-related left ventricular remodeling and associated risk for cardiovascular outcomes: the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imag. 2009;2(3):191–198. doi: 10.1161/CIRCIMAGING.108.819938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gavish B., Izzo J.L., Jr. Arterial stiffness: going a step beyond. Am J Hypertens. 2016;29(11):1223–1233. doi: 10.1093/ajh/hpw061. [DOI] [PubMed] [Google Scholar]
- 57.Fleg J.L., Strait J. Age-associated changes in cardiovascular structure and function: a fertile milieu for future disease. Heart Fail Rev. 2012;17(4–5):545–554. doi: 10.1007/s10741-011-9270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang R., Chen H.Z., Liu D.P. The four layers of aging. Cell Syst. 2015;1(3):180–186. doi: 10.1016/j.cels.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Das A., Huang G.X., Bonkowski M.S., et al. Impairment of an endothelial NAD+-H2S signaling network is a reversible cause of vascular aging. Cell. 2018;173(1):74–89.e20. doi: 10.1016/j.cell.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mills K.F., Yoshida S., Stein L.R., et al. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metabol. 2016;24(6):795–806. doi: 10.1016/j.cmet.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang H., Ryu D., Wu Y., et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352(6292):1436–1443. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- 62.Mouchiroud L., Houtkooper R.H., Moullan N., et al. The NAD(+)/Sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell. 2013;154(2):430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Picciotto N.E., Gano L.B., Johnson L.C., et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15(3):522–530. doi: 10.1111/acel.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levine D.C., Hong H., Weidemann B.J., et al. NAD+ controls circadian reprogramming through PER2 nuclear translocation to counter aging. Mol Cell. 2020;78(5):835–849.e7. doi: 10.1016/j.molcel.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sato S., Solanas G., Peixoto F.O., et al. Circadian reprogramming in the liver identifies metabolic pathways of aging. Cell. 2017;170(4):664–677.e11. doi: 10.1016/j.cell.2017.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ryu D., Zhang H., Ropelle E.R., et al. NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Sci Transl Med. 2016;8(361) doi: 10.1126/scitranslmed.aaf5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takahashi Y., Tanaka A., Nakamura T., et al. Nicotinamide suppresses hyperphosphatemia in hemodialysis patients. Kidney Int. 2004;65(3):1099–1104. doi: 10.1111/j.1523-1755.2004.00482.x. [DOI] [PubMed] [Google Scholar]
- 68.Dellinger R.W., Santos S.R., Morris M., et al. Repeat dose NRPT (nicotinamide riboside and pterostilbene) increases NAD+ levels in humans safely and sustainably: a randomized, double-blind, placebo-controlled study. NPJ Aging Mech Dis. 2017;3 doi: 10.1038/s41514-017-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martens C.R., Denman B.A., Mazzo M.R., et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat Commun. 2018;9(1) doi: 10.1038/s41467-018-03421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Remie C.M.E., Roumans K.H.M., Moonen M.P.B., et al. Nicotinamide riboside supplementation alters body composition and skeletal muscle acetylcarnitine concentrations in healthy obese humans. Am J Clin Nutr. 2020;112(2):413–426. doi: 10.1093/ajcn/nqaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van de Weijer T., Phielix E., Bilet L., et al. Evidence for a direct effect of the NAD+ precursor acipimox on muscle mitochondrial function in humans. Diabetes. 2015;64(4):1193–1201. doi: 10.2337/db14-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Conze D., Brenner C., Kruger C.L. Safety and metabolism of long-term administration of NIAGEN (nicotinamide riboside chloride) in a randomized, double-blind, placebo-controlled clinical trial of healthy overweight Adults. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-46120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dollerup O.L., Christensen B., Svart M., et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects. Am J Clin Nutr. 2018;108(2):343–353. doi: 10.1093/ajcn/nqy132. [DOI] [PubMed] [Google Scholar]
- 74.Airhart S.E., Shireman L.M., Risler L.J., et al. An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS One. 2017;12(12) doi: 10.1371/journal.pone.0186459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lehto H.R., Pärkkä J., Borra R., et al. Effects of acute and one-week fatty acid lowering on cardiac function and insulin sensitivity in relation with myocardial and muscle fat and adiponectin levels. J Clin Endocrinol Metab. 2012;97(9):3277–3284. doi: 10.1210/jc.2012-1219. [DOI] [PubMed] [Google Scholar]
- 76.Phelan M.J. Phase II clinical trial of nicotinamide for the treatment of mild to moderate Alzheimer’s disease. J Geriatr Med Gerontol. 2017;3(1) [Google Scholar]
- 77.Altschul R., Hoffer A., Stephen J.D. Influence of nicotinic acid on serum cholesterol in man. Arch Biochem Biophys. 1955;54(2):558–559. doi: 10.1016/0003-9861(55)90070-9. [DOI] [PubMed] [Google Scholar]
- 78.Berge K.G., Canner P.L. Coronary drug project: experience with niacin. Coronary drug project research group. Eur J Clin Pharmacol. 1991;40(Suppl 1):S49–S51. [PubMed] [Google Scholar]
- 79.Blankenhorn D.H., Nessim S.A., Johnson R.L., Sanmarco M.E., Azen S.P., Cashinhemphill L. Beneficial-effects of combined colestipol-niacin therapy on coronary atherosclerosis and coronary venous bypass grafts. JAMA. 1987;257(23):3233–3240. [PubMed] [Google Scholar]
- 80.Boden W.E., Probstfield J.L., Anderson T., et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365(24):2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 81.Brown B.G., Zhao X.Q., Chait A., et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345(22):1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 82.Guyton J.R., Brown B.G., Fazio S., Polis A., Tomassini J.E., Tershakovec A.M. Lipid-altering efficacy and safety of ezetimibe/simvastatin coadministered with extended-release niacin in patients with type IIa or type IIb hyperlipidemia. J Am Coll Cardiol. 2008;51(16):1564–1572. doi: 10.1016/j.jacc.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 83.Taylor A.J., Sullenberger L.E., Lee H.J., Lee J.K., Grace K.A. Arterial biology for the investigation of the treatment effects of reducing cholesterol (ARBITER) 2 - a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004;110(23):3512–3517. doi: 10.1161/01.CIR.0000148955.19792.8D. [DOI] [PubMed] [Google Scholar]
- 84.Westphal S., Borucki K., Taneva E., Makarova R., Luley C. Adipokines and treatment with niacin. Metabolism. 2006;55(10):1283–1285. doi: 10.1016/j.metabol.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 85.Villines T.C., Stanek E.J., Devine P.J., et al. The ARBITER 6-HALTS trial (arterial biology for the investigation of the treatment effects of reducing cholesterol 6-HDL and LDL treatment strategies in atherosclerosis): final results and the impact of medication adherence, dose, and treatment duration. J Am Coll Cardiol. 2010;55(24):2721–2726. doi: 10.1016/j.jacc.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 86.Delbufalo A.G.A. Regression of coronary-artery disease as a result of intensive lipid-lowering therapy. N Engl J Med. 1991;324(16) doi: 10.1056/NEJM199104183241612. 1133-1133. [DOI] [PubMed] [Google Scholar]
- 87.Tunaru S., Kero J., Schaub A., et al. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med. 2003;9(3):352–355. doi: 10.1038/nm824. [DOI] [PubMed] [Google Scholar]
- 88.Cantó C., Auwerx J. Targeting sirtuin 1 to improve metabolism: all you need is NAD(+)? Pharmacol Rev. 2012;64(1):166–187. doi: 10.1124/pr.110.003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kirkland J.B. Niacin status, NAD distribution and ADP-ribose metabolism. Curr Pharmaceut Des. 2009;15(1):3–11. doi: 10.2174/138161209787185823. [DOI] [PubMed] [Google Scholar]
- 90.Capuzzi D.M., Morgan J.M., Brusco O.A., Jr., Intenzo C.M. Niacin dosing: relationship to benefits and adverse effects. Curr Atherosclerosis Rep. 2000;2(1):64–71. doi: 10.1007/s11883-000-0096-y. [DOI] [PubMed] [Google Scholar]
- 91.Katsyuba E., Romani M., Hofer D., Auwerx J. NAD+ homeostasis in health and disease. Nat Metab. 2020;2(1):9–31. doi: 10.1038/s42255-019-0161-5. [DOI] [PubMed] [Google Scholar]
- 92.Chen D., Bruno J., Easlon E., et al. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008;22(13):1753–1757. doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cantó C., Gerhart-Hines Z., Feige J.N., et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458(7241):1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kraus D., Yang Q., Kong D., et al. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature. 2014;508(7495):258–262. doi: 10.1038/nature13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ke W., Saba J.A., Yao C.H., et al. Dietary serine-microbiota interaction enhances chemotherapeutic toxicity without altering drug conversion. Nat Commun. 2020;11(1) doi: 10.1038/s41467-020-16220-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Celis A.I., Relman D.A. Competitors versus collaborators: micronutrient processing by pathogenic and commensal human-associated gut bacteria. Mol Cell. 2020;78(4):570–576. doi: 10.1016/j.molcel.2020.03.032. [DOI] [PubMed] [Google Scholar]
- 97.Cambronne X.A., Stewart M.L., Kim D., et al. Biosensor reveals multiple sources for mitochondrial NAD+ Science. 2016;352(6292):1474–1477. doi: 10.1126/science.aad5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luongo T.S., Eller J.M., Lu M.J., et al. SLC25A51 is a mammalian mitochondrial NAD+ transporter. Nature. 2020;588(7836):174–179. doi: 10.1038/s41586-020-2741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nikiforov A., Dölle C., Niere M., Ziegler M. Pathways and subcellular compartmentation of NAD biosynthesis in human cells: from entry of extracellular precursors to mitochondrial NAD generation. J Biol Chem. 2011;286(24):21767–21778. doi: 10.1074/jbc.M110.213298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Berger F., Lau C., Dahlmann M., Ziegler M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J Biol Chem. 2005;280(43):36334–36341. doi: 10.1074/jbc.M508660200. [DOI] [PubMed] [Google Scholar]
- 101.Hara N., Yamada K., Shibata T., Osago H., Hashimoto T., Tsuchiya M. Elevation of cellular NAD levels by nicotinic acid and involvement of nicotinic acid phosphoribosyltransferase in human cells. J Biol Chem. 2007;282(34):24574–24582. doi: 10.1074/jbc.M610357200. [DOI] [PubMed] [Google Scholar]
- 102.Kitani T., Okuno S., Fujisawa H. Growth phase-dependent changes in the subcellular localization of pre-B-cell colony-enhancing factor. FEBS Lett. 2003;544(1–3):74–78. doi: 10.1016/s0014-5793(03)00476-9. [DOI] [PubMed] [Google Scholar]
- 103.Shore D., Squire M., Nasmyth K.A. Characterization of two genes required for the position-effect control of yeast mating-type genes. EMBO J. 1984;3(12):2817–2823. doi: 10.1002/j.1460-2075.1984.tb02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Newman B.L., Lundblad J.R., Chen Y., Smolik S.M. A Drosophila homologue of Sir2 modifies position-effect variegation but does not affect life span. Genetics. 2002;162(4):1675–1685. doi: 10.1093/genetics/162.4.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Howells L.M., Berry D.P., Elliott P.J., et al. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases–safety, pharmacokinetics, and pharmacodynamics. Cancer Prev Res (Phila). 2011;4(9):1419–1425. doi: 10.1158/1940-6207.CAPR-11-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Popat R., Plesner T., Davies F., et al. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br J Haematol. 2013;160(5):714–717. doi: 10.1111/bjh.12154. [DOI] [PubMed] [Google Scholar]
- 107.Kane A.E., Sinclair D.A. Sirtuins and NAD+ in the development and treatment of metabolic and cardiovascular diseases. Circ Res. 2018;123(7):868–885. doi: 10.1161/CIRCRESAHA.118.312498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Trammell S.A., Schmidt M.S., Weidemann B.J., et al. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. 2016;7 doi: 10.1038/ncomms12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dollerup O.L., Trammell S.A.J., Hartmann B., et al. Effects of nicotinamide riboside on endocrine pancreatic function and incretin hormones in nondiabetic men with obesity. J Clin Endocrinol Metab. 2019;104(11):5703–5714. doi: 10.1210/jc.2019-01081. [DOI] [PubMed] [Google Scholar]
- 110.Irie J., Inagaki E., Fujita M., et al. Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese men. Endocr J. 2020;67(2):153–160. doi: 10.1507/endocrj.EJ19-0313. [DOI] [PubMed] [Google Scholar]
- 111.Sirtori C.R., Gianfranceschi G., Sirtori M., et al. Reduced triglyceridemia and increased high density lipoprotein cholesterol levels after treatment with acipimox, a new inhibitor of lipolysis. Atherosclerosis. 1981;38(3–4):267–271. doi: 10.1016/0021-9150(81)90042-3. [DOI] [PubMed] [Google Scholar]
- 112.Taskinen M.R., Nikkilä E.A. Effects of acipimox on serum lipids, lipoproteins and lipolytic enzymes in hypertriglyceridemia. Atherosclerosis. 1988;69(2–3):249–255. doi: 10.1016/0021-9150(88)90021-4. [DOI] [PubMed] [Google Scholar]
- 113.Worm D., Henriksen J.E., Vaag A., Thye-Rønn P., Melander A., Beck-Nielsen H. Pronounced blood glucose-lowering effect of the antilipolytic drug acipimox in noninsulin-dependent diabetes mellitus patients during a 3-day intensified treatment period. J Clin Endocrinol Metab. 1994;78(3):717–721. doi: 10.1210/jcem.78.3.8126147. [DOI] [PubMed] [Google Scholar]
- 114.Bajaj M., Suraamornkul S., Romanelli A., et al. Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty Acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes. 2005;54(11):3148–3153. doi: 10.2337/diabetes.54.11.3148. [DOI] [PubMed] [Google Scholar]
- 115.Vestergaard E.T., Cichosz S.L., Møller N., Jørgensen J.O.L., Fleischer J. Short-term acipimox treatment is associated with decreased cardiac parasympathetic modulation. Br J Clin Pharmacol. 2017;83(12):2671–2677. doi: 10.1111/bcp.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Salgin B., Marcovecchio M.L., Humphreys S.M., et al. Effects of prolonged fasting and sustained lipolysis on insulin secretion and insulin sensitivity in normal subjects. Am J Physiol Endocrinol Metab. 2009;296(3):E454–E461. doi: 10.1152/ajpendo.90613.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Santomauro A.T., Boden G., Silva M.E., et al. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes. 1999;48(9):1836–1841. doi: 10.2337/diabetes.48.9.1836. [DOI] [PubMed] [Google Scholar]
- 118.Vestergaard E.T., Hjelholt A.J., Kuhre R.E., et al. Acipimox acutely increases GLP-1 concentrations in overweight subjects and hypopituitary patients. J Clin Endocrinol Metab. 2019;104(7):2581–2592. doi: 10.1210/jc.2018-02503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hansen D., Verboven K., van Dijk J.W., et al. Adipose tissue lipolytic inhibition enhances the glucoregulatory properties of exercise in type 2 diabetes patients. Eur J Sport Sci. 2018;18(9):1245–1254. doi: 10.1080/17461391.2018.1483428. [DOI] [PubMed] [Google Scholar]
- 120.de la Rubia J.E., Drehmer E., Platero J.L., et al. Efficacy and tolerability of EH301 for amyotrophic lateral sclerosis: a randomized, double-blind, placebo-controlled human pilot study. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(1–2):115–122. doi: 10.1080/21678421.2018.1536152. [DOI] [PubMed] [Google Scholar]