Abstract
Coxsackievirus A10 (CVA10) is one of the major causative agents of hand, foot and mouth disease (HFMD). To investigate the epidemiological characteristics as well as genetic features of CVA10 currently circulating in Shanghai, China, we collected a total of 9,952 sporadic HFMD cases from January 2016 to December 2020. In the past five years, CVA10 was the fourth prevalent causatives associated with HFMD in Shanghai and the overall positive rate was 2.78%. The annual distribution experienced significant fluctuations over the past five years. In addition to entire VP1 sequencing, complete genome sequencing and recombination analysis of CVA10 isolates in Shanghai were further performed. A total of 64 near complete genomes and 11 entire VP1 sequences in this study combined with reference sequences publicly available were integrated into phylogenetic analysis. The CVA10 sequences in this study mainly belonged to genogroup C and presented 91%–100% nucleotide identity with other Chinese isolates based on VP1 region. For the first time, our study reported the appearance of CVA10 genogroup D in Chinese mainland, which had led to large-scale outbreaks in Europe previously. The recombination analysis showed the recombination break point located between 5,100 nt and 6,700 nt, which suggesting intertypic recombination with CVA16 genogroup D. To conclusion, CVA10 genogroup C was the predominant genogroup in Shanghai during 2016–2020. CVA10 recombinant genogroup D was firstly reported in circulating in Chinese mainland. Continuous surveillance is needed to better understand the evolution relationships and transmission pathways of CVA10 to help to guide disease control and prevention.
Keywords: Coxsackievirus A10 (CVA10); Genogroup D; Intertypic recombination; Hand, foot and mouth disease (HFMD)
Highlights
-
•
Systematic profiles of genetic features of CVA10 near complete genome.
-
•
First report of the appearance of CVA10 genogroup D in Chinese mainland.
-
•
Genomic comparisons indicate the potential recombinant origin of CVA10 genogroup D.
1. Introduction
Hand, foot and mouth disease (HFMD) is a common infectious disease characterized by fever and vesicular eruptions on hand, foot and in mouth among children ≤ 5-year-old (Xing et al., 2014; Liu et al., 2015). Generally, enterovirus (EV) A71 and coxsackievirus (CV) A16 are considered as the main causative agents involved in HFMD since its first large outbreak in 2008 in Chinese mainland (Tan et al., 2011; Chen et al., 2013; Zhang et al., 2013; Liu et al., 2014; Xing et al., 2014; Zhuang et al., 2015). Since 2013, other enteroviruses such as CVA6 and CVA10 have been detected in more and more provinces in China (He et al., 2013; Tian et al., 2014; Zhang et al., 2015; Wang et al., 2018; Meng et al., 2020). In the year of 2013 and 2015, CVA6 became the leading causative agent of HFMD in some provinces (Fu et al., 2020). However, recent studies also provided strong evidence of CVA10 as an important causative associated with increasing sporadic HFMD cases and outbreaks globally (Blomqvist et al., 2010; Bracho et al., 2011; Davia et al., 2011; Lu et al., 2012; Mirand et al., 2012; He et al., 2013; Tian et al., 2014; Ji et al., 2018; Munivenkatappa et al., 2018). Based on a nation-wide HFMD surveillance network, Ji et al. reported an increasing proportion of CVA10 infected HFMD cases which indicated that CVA10 was gradually becoming one of the most prevalent agents during the epidemic interval of EVA71 and CVA16 (Ji et al., 2018).
CVA10 belongs to species Enterovirus A, genus Enterovirus, family Picornaviridae. The virus genome is a positive-sense single-stranded RNA of approximate 7.4 kilobases (kb) in length, which comprises a single open reading frame (ORF) flanked by 5′ and 3′ untranslated regions (UTR) (Oberste et al., 2004; Hu et al., 2011). The ORF encoding a polyprotein which can be divided into three parts P1, P2 and P3. The P1 region encodes four capsid proteins 1A–1D (VP4, VP2, VP3 and VP1, respectively). The P2 and P3 regions encode non-capsid proteins 2A–2C and 3A–3D, respectively. As the major antigenic and neutralizing protein, complete VP1 sequence has been widely employed in identifying EV serotypes and genogroups (Oberste et al., 1999; Caro et al., 2001). Based on VP1 sequences, CVA10 can be assigned to seven genogroups (A–G) (Dalldorf, 1953; Tian et al., 2014, 2017; Ji et al., 2018). CVA10 genogroup B and genogroup C have been reported persistently in circulating in Chinese mainland. Genogroup B was active during 2004–2009 while genogroup C was the absolute predominant genogroup since 2009 (Ji et al., 2018). However, CVA10 molecular epidemiologic studies were still limited and were mainly based on 5′UTR, VP4 or VP1 sequences (Yang et al., 2011; Chen et al., 2014; Tian et al., 2014, Tian et al., 2017; Guan et al., 2015; Li et al., 2017; Ji et al., 2019; Meng et al., 2020; Xie et al., 2020; Zhao et al., 2020). So far, there has been a lack of systematic research on CVA10 genetic features based on complete genome as well as epidemiological characteristics of CVA10 infection in Shanghai, China for years.
HFMD has been listed as the C-class notifiable disease in China since 2008. Subsequently, Shanghai Municipal Center for Disease Control and Prevention (CDC) built up its own HFMD epidemiological surveillance laboratory network. In the current study, the sporadic HFMD cases were collected from 2016 to 2020. The epidemiological characteristic as well as genetic features of CVA10 based on near complete genomes were investigated. Totally, 2.78% of the HFMD cases were caused by CVA10 infection. CVA10 genogroup C was the dominated genogroup in Shanghai at the last five years. For first time, our study reported the emergence and spread of CVA10 genogroup D in Chinese mainland. Recombination analysis provided evidence of intertypic recombination with CVA16 genogroup D. The findings could help to expand the knowledge of the genetic diversity of CVA10 and prevent and control the potential transmission and outbreaks.
2. Materials and methods
2.1. Sample collection and virus isolation
Clinical specimens were obtained from the routine HFMD surveillance network from 2016 to 2020. Samples including stools and throat swabs were processed based on the official National Guidelines for the Diagnosis and Treatment of HFMD in China (2009 edition) (http://www.gov.cn/gzdt/2009-06/04/content_1332078.htm). All these samples were primarily tested using commercial real-time PCR Kit (BioPerfectus, JC20107, Taizhou, China). CVA10 positive samples were incubated into human rhabdomyosarcoma (RD) cell lines and were cultured at 37 °C for seven days. The infected cells were cultured for at least three passages until cytopathic effect (CPE) was observed. The isolates were then harvested for viral RNA extraction and sequencing.
2.2. Near complete genome and entire VP1 sequencing
Viral RNA was extracted from the virus culture using Roche MagNA Pure LC 2.0 nucleic extraction system (Roche, 3038505001, Basel, Switzerland). Commercial real-time PCR Kits were used for a second test to preliminary evaluate virus concentration. Isolates with cycle threshold (Ct) value less than 26 were used for complete genome sequencing and those with Ct value between 26 and 28 were used for VP1 sequencing.
For VP1 sequencing, PCR was performed using an Invitrogen Onestep RT-PCR kit (ThermoFisher, 12574026, California, USA) and specific primers as described (Ji et al., 2018). The PCR products were purified using a QIAquick® PCR Purification Kit (Qiagen, 28104, Hilden, Germany). All the amplicons were sequenced using an ABI 3730 Genetic Analyzer (ThermoFisher).
Complete genome of CVA10 was amplified using Tarich Virus Target Enrichment System for enterovirus species A (BioGerm, SJ-CX-601-1, Shanghai, China). The PCR products were purified and then quantified by Qubit Fluorometer (ThermoFisher) using Qubit dsDNA high sensitivity assay kit (ThermoFisher, Q32852). The purified PCR products were physically sheared with the M220 Focused-ultrasonicator™ (Covaris, Massachusetts, USA) and were then used to prepare libraries with the Ion Plus Fragment Library Kit (ThermoFisher, 4471269) according to the manufacture’s procedure. The cDNA library of 32 samples was pooled together and normalized to a final concentration of 50 pmol/L. The complete genome sequencing was performed using Ion Torrent S5 platform (ThermoFisher).
Raw sequencing reads were de novo assembled to produce sequence contigs using CLC Genomics Workbench (CLC, Version 20). The assembled contigs were then identified using the Basic Local Alignment Search Tool (BLAST). The reference sequences with highest percent identity were downloaded from National Center of Biotechnology Information (NCBI) GenBank database (www.ncbi.nlm.nih.gov). Reference-based mapping and de novo assembling led to the retrieval of near complete viral genomes.
2.3. Phylogenetic and recombination analysis of CVA10
Reference sequences of CVA10 complete genome were downloaded from GenBank database as listed in Supplementary Table S1 and were aligned pairwise using the ClustalW program present in MEGA 7.0 (Kumar et al., 2016). Phylogenetic analysis was conducted based on 5′UTR, VP1, P1, P2 and P3 region using neighbor-joining method (Saitou and Nei, 1987). A bootstrap replication of 1000 cycles was performed to assess the robustness. Percent nucleotide difference was deduced using the pairwise distances, within group mean distance and between group mean distance methods in MEGA software. Simplot 3.5.0 software (StuartRay, Johns Hopkins University, Baltimore, MD) (http://sray.med.som.jhmi.edu/SCRoftware/simplot/) was performed using the default parameter settings to identify the potential genetic recombination sites in the viral genome. Bootscan analysis was performed using the neighbor-joining tree model and the Kimura 2-parameter distance algorithm with a window size of 200 nucleotides moving along the alignment in increments of 20 nucleotides with 1000 resampling. The use of the PHYLIP internal code (v3.5) (Joseph Felsenstein) and a 70% parental threshold were selected for notification if a region of recombination was detected. RDP4.1 (Martin et al., 2015) was used to verify natural recombinant strains within complete genome sequence alignments. Seven detection methods including RDP (Martin and Rybicki, 2000), BootScan (Martin et al., 2005), MaxChi (Smith, 1992), Chimaera (Posada and Crandall, 2001), Phylpro (Weiller, 1998), SiScan (Gibbs et al., 2000) and 3Seq (Lam et al., 2018) were conducted. Sequences would be considered as a potential recombinant if at least 3/7 detection methods showed a significant difference (P-value < 0.05).
3. Results
3.1. Epidemiological surveillance of CVA10 related HFMD in Shanghai
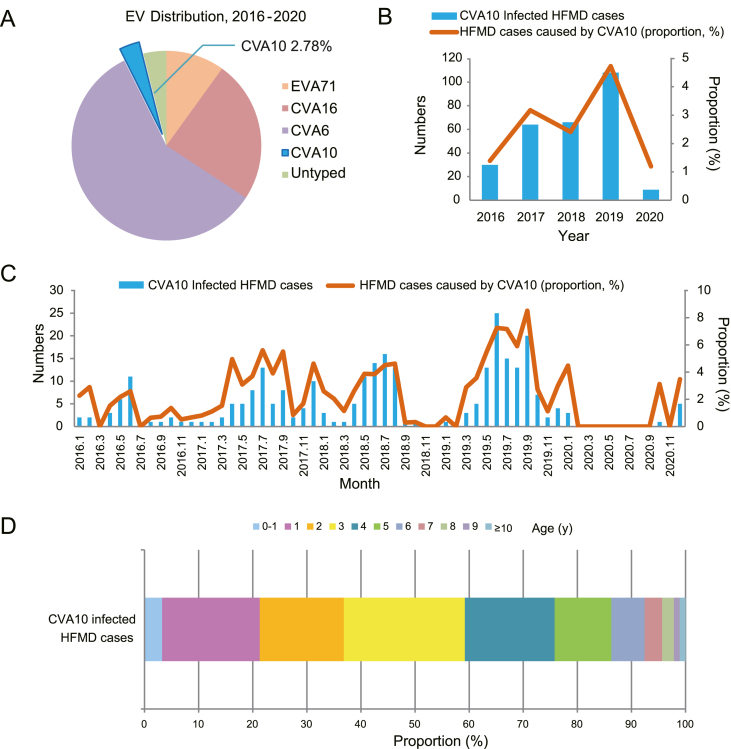
A total of 9,952 sporadic HFMD cases were recruited in Shanghai from January 2016 to December 2020 in the current study. EV infection was identified in 80.90% of patients (8051/9952). CVA6 (4705/9952, 47.28%) was the most prevalent causatives during the five past years (Fig. 1A, Table 1). CVA10 infection accounted for 2.78% of HFMD cases (277/9,952, 2.78%). The annual distribution of CVA10 infected cases were 1.39%, 3.17%, 2.41%, 4.72% and 1.20%, respectively, with significant fluctuations over the past five years (Fig. 1B, Table 1). Around 60% of the HFMD cases (5,987/9,952, 60.16%) distributed in the suburbs while the proportion reached 72.20% (200/277) for CVA10 infection (Supplementary Table S2). The positive rate of CVA10 was 1.94% in urban versus 3.34% in suburbs (Supplementary Table S2). A total of 150 out of 9,952 cases (150/9952, 1.51%) were diagnosed as severe cases (Table 1) with neurological or cardiopulmonary complications according to the National Health and Family Planning Commission of China in 2018 (http://www.nhc.gov.cn/cms-search/xxgk/getManuscriptXxgk.htm?id=5db274d8697a41ea84e88eedd8bf8f63). In contrast, only one severe case was caused by CVA10. Most CVA10 infection (99.64%, 276/277) presented only mild symptoms. Totally, 71.48% (198/277) of CVA10-infected cases were collected during May and September, when was late spring and early autumn in Shanghai. Meanwhile, there was no CVA10 positive HFMD case collected during February to September 2020, which contained the summer peak of HFMD incidence in Shanghai previously (Fig. 1C).
Fig. 1.
Temporal distribution of CVA10 circulating in Shanghai, China, 2016–2020. A Overall percentages of EVA71, CVA16, CVA6, CVA10 and untyped enterovirus detectedin HFMD cases. B Number and proportion of CVA10-infected HFMD cases by year of illness onset. C Number and proportion of CVA10-infected HFMD cases by month of illness onset. D Age distribution of CVA10-infected HFMD cases from 2016 to 2020 in Shanghai, China. The continuous lines in B and C described the proportion of CVA10-infected HFMD cases and the histograms showed the number of CVA10-infected HFMD cases. EV, enterovirus; CV, Coxsackievirus; HFMD, Hand, foot, and mouth disease.
Table 1.
Temporal and EV serotype distribution of HFMD cases in Shanghai, China, 2016–2020.
| Year | Case number | Severe cases | EV positive (%) | EVA71 (%) | CVA16 (%) | CVA6 (%) | CVA10 (%) | Untyped (%) |
|---|---|---|---|---|---|---|---|---|
| 2016 | 2156 | 105 | 1820 (84.42) | 415 (19.25) | 460 (21.34) | 831 (38.54) | 30 (1.39) | 84 (3.90) |
| 2017 | 2018 | 28 | 1618 (80.18) | 347 (17.20) | 277 (13.73) | 850 (42.12) | 64 (3.17) | 80 (3.96) |
| 2018 | 2739 | 11 | 2200 (80.32) | 25 (0.91) | 421 (15.37) | 1598 (58.34) | 66 (2.41) | 90 (3.29) |
| 2019 | 2286 | 6 | 1869 (81.76) | 15 (0.66) | 771 (33.73) | 919 (40.20) | 108 (4.7) | 56 (2.45) |
| 2020 | 753 | 0 | 544 (72.24) | 0 (0.00) | 24 (3.19) | 507 (67.33) | 9 (1.20) | 4 (0.53) |
| Total | 9952 | 150 | 8051 (80.90) | 802 (8.06) | 1953 (19.62) | 4705 (47.28) | 277 (2.78) | 314 (3.16) |
EV, enterovirus; EVA71, enterovirus A71; CVA16, coxsackievirus A16; CVA6, coxsackievirus A6; CVA10, coxsackievirus A10.
The patients enrolled in this study aged from 0.1 to 32 years. The highest incidence rate of CVA10 infection was occurred in 3- to 4-year-old group (Fig. 1D).The average patient age of onset was 3.41 years old and the median age was 3 years old. In details, 86.28% (239/277) of the patients were under 6-year-old. Children under 1-year-old accounted for 3.25% (9/277) cases and those under 4-year-old accounted for 59.21% (164/277) cases. Male-to-female ratio of CVA10 infection reached 1.59 (170 male cases and 107 female cases).
3.2. Phylogenetic analysis of CVA10 genomes
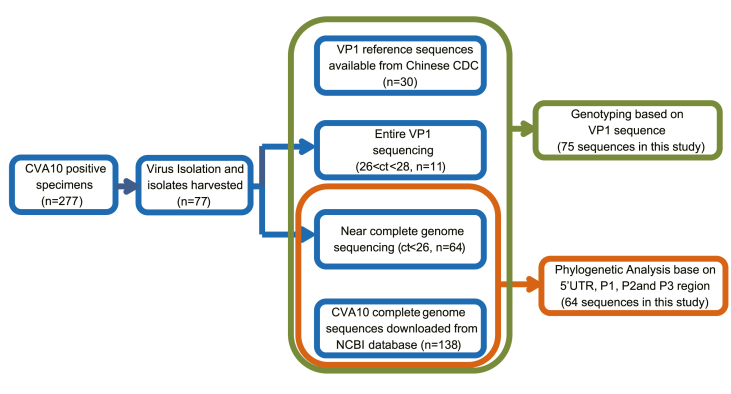
A total of 75 representative virus isolates were used for sequence analyses. Nearly complete genome sequencing was conducted in 64 isolates whereas entire VP1 sequencing was conducted in 11 isolate (Fig. 2). All the sequences were submitted to GenBank database under the accession numbers: MW929238–MW929301 for near complete genome and MW929306–MW929316 for VP1 sequences. “Coxsackievirus A10 complete genome” were used as key words to search the whole genome sequences of CVA10. Before March 22nd, 2021, there were 138 complete genome sequences available from GenBank database (Supplementary Table S1). These sequences were collected from ten countries during 1950 and 2018. Another three sequences (accession no: LT617117, LT617118 and MH118027) which were previously identified as CVA16 but showed high nucleotide identity with sequences in this study were also used as reference sequences (Supplementary Table S1). Thirty entire VP1 reference sequences (Supplementary Table S3) which were available from Ministry of Health Key Laboratory for Medical Virology, Chinese CDC were used for virus genotyping (Tan et al., 2011, Tian et al., 2017; Ji et al., 2018). All these sequences were included to build phylogenetic trees. The prototype EVA71 BrCr strain was used as an out-group.
Fig. 2.
Methodological approach of CVA10 sequencing and phylogenetic analysis. CV, coxsackievirus.
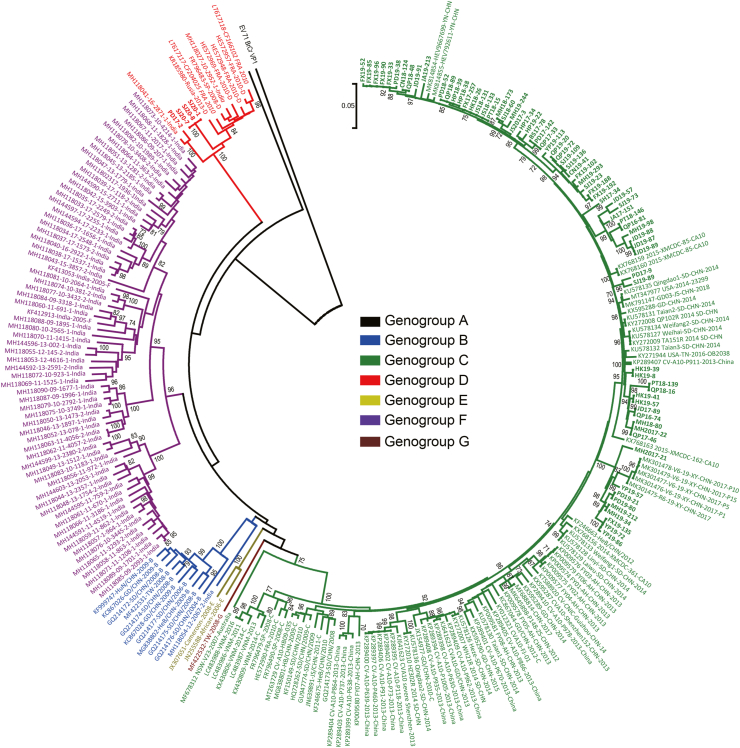
Phylogenetic analysis was conducted based on nucleotide comprising VP1 (Fig. 3), 5′UTR, P1 (VP1–VP4), P2 (2A–2C), P3 (3A–3D) regions and near complete genome sequences (Supplementary Fig. S1, S2). Mean distance of different clusters was determined using MEGA software based on entire VP1 sequences. On the basis of VP1 region (Fig. 3), CVA10 was assigned into seven genogroups, including genogroup A to genogroup G (Dalldorf, 1953; Tian et al., 2014, 2017; Ji et al., 2018). Isolates in this study belonged to two genogroups. Most isolates (71/75, 94.67%) belonged to genogroup C and were closely related to CVA10 isolates circulating in Chinese mainland. The VP1 region of these isolates presented the nucleotide identity of 91%–100%. Another four isolates in this study were assigned to genogroup D. The related cases were collected in Pudong District in 2017 (PD17-2) and in Songjiang District in 2020 (SJ20-1, SJ20-7 and SJ20-8) (Supplementary Table S4). The three Songjiang patients have visited the same hospital. However, all these cases were enrolled as outpatient surveillance cases which had no epidemiological associations available. These patients were below 5 years old (mean age 2.75) and only presented mild HFMD symptoms. The genogroup D in Shanghai isolates showed 25.2% nucleotide difference with genogroup C sequences based on entire VP1 nucleotide sequences, much higher than the 14.97% nucleotide divergence threshold (Ji et al., 2018) between different genogroups. Although these isolates belonged to the same genogroup, they located in different branches of the phylogenetic tree (Fig. 3). PD17-2 was clustered with the Indian isolate and showed 8.4% nucleotide difference with SJ20-1, SJ20-7 and SJ20-8. Moreover, SJ20-1, SJ20-7 and SJ20-8 presented 100% nucleotide identity based on VP1 region and were clustered with European isolates within CVA10 genogroup D. Three reference sequences previously defined as CVA16 (accession no: LT617117, LT617118 and MH118027) were also clustered in CVA10 genogroup D based on VP1 region. Isolates LT617117 and LT617118 were collected in Clermont-Ferrand, France and were defined CVA16 as an organism but CVA10 as a serotype. Isolate MH118027 was collected in Kerala State, India and was defined CVA16 as an organism. All of them were collected in the year of 2010. Nucleotide identity analysis showed that these three reference sequences presented highest homology with CVA10 genogroup D isolates. In details, they exhibited 4.9%, 22.9% and 54.3% nucleotide differences with CVA10 genogroup D isolates, CVA10 prototype Kowalik strain and CVA16 prototype G-10 strain (accession no: U05876), respectively, based on entire VP1 region. The plot of phylogenetic trees also showed that the three reference isolates were clustered with genogroup D isolates and constituted independent lineages based on 5′UTR, P1, P2 and P3 regions as well as near complete genome (Supplementary Fig. S1, S2).
Fig. 3.
Phylogenetic analysis of CVA10 isolates based on complete VP1 sequences. The phylogenetic dendrogram was constructed by the neighbor-joining method and validated with 1000 replicates. Only bootstrap values over 70% are shown. The prototype EVA71 BrCr strain was used as an out-group. Sequences available from GenBank database and Chinese CDC were listed in Supplementary Tables S1 and S3. Sequences of CVA10 genogroup A is indicated in black, genogroup B in blue, genogroup C in green, genogroup D in red, genogroup E in grass green, genogroup F in purple and genogroup G in brown. Sequences in this study are highlighted in bold. The underlying sequences in italic type represent reference sequences previously defined as CVA16 but were redefined as CVA10 genogroup D in this study. CV, Coxsackievirus; EV, enterovirus.
3.3. Genetic features of CVA10 genogroup D
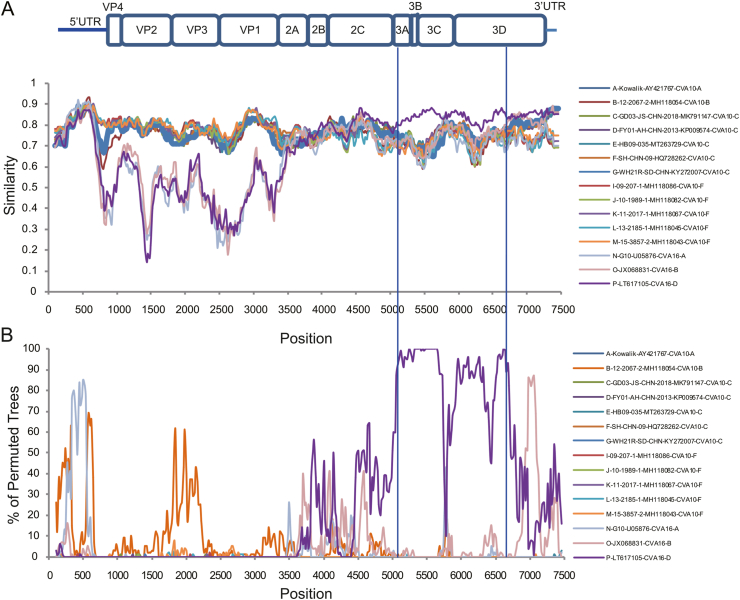
Similarity plots analysis was conducted to investigate the genetic features of CVA10 genogroup D. Bootscan analysis was also performed to identify potential genetic recombination sites in the viral genomes. Near complete genomes of 13 CVA10 isolates (genogroup A, n = 1; genogroup B, n = 1; genogroup C, n = 5; genogroup D, n = 1; genogroup F, n = 5) and three CVA16 isolates (genogroup A, n = 1; genogroup B, n = 1; genogroup D, n = 1) were used as reference sequences. SJ20-1 was used as query when similarity plot analysis and bootscan analysis were conducted, respectively (Fig. 4). The region located between the 3A gene and the 3D gene (at approximate nucleotide positions of 5,100 nt and 6,700 nt) of CVA10 genogroup D genome was more similar to CVA16 genogroup D genomes (Fig. 4A). The bootscan plot showed the recombination fragments located at the P3 region, suggesting intertypic recombination with CVA16 genogroup D (Fig. 4B). Phylogenetic analysis with genomic segments of different region also supported this report (Supplementary Fig. S3). SJ20-1 was clustered with CVA10 isolates in 1–5,100 nt region (Supplementary Fig. S3A). In 5,100–6,700 nt region, SJ20-1 was clustered with CVA16 genogroup D, distinct from other CVA10 isolates (Supplementary Fig. S3B). In 6,700–7,400 nt region, SJ20-1 presented higher homology with CVA16 genogroup B and genogroup D isolates, which was accordance with bootscan analysis (Supplementary Fig. S3C). Similar results were shown when other CVA10 genogroup D isolates in this study were used as query. Recombination analysis of CVA10 genogroup D genome with RDP program provided statistical evidence of a recombination breakpoint at the end of 2C gene (Table 2). Sequences would be considered as a potential recombinant if at least 3/7 detection methods showed a significant difference (P-value < 0.05). At least five out of seven methods supported this event with P-values ranging from 7.51 × 10−10 to 6.71 × 10−21. The putative major and minor parent strains with best recombination score were CVA10 genogroup C isolate WH21R and CVA16 genogroup D isolate CF350028.
Fig. 4.
Similarity plots and bootscan analysis of CVA10 and CVA16 using SimPlot. The plots were obtained with a dataset of 13 CVA10 genomes (genogroup A, n = 1; genogroup B, n = 1; genogroup C, n = 5; genogroup D, n = 1; genogroup F, n = 5) and three CVA16 genomes (genogroup A, n = 1; genogroup B, n = 1; genogroup D, n = 1). SJ20-1 was chosen as query. The similarity was calculated in a sliding window size of 200 nucleotides moving in steps of 20 nucleotides. The bootscan analysis plot was obtained using the neighbor-joining tree model and the Kimura 2-parameter distance algorithm with a window size of 200 nucleotides. A Nucleotide similarity analysis. B Bootscan analysis. CV, coxsackievirus.
Table 2.
Summary of CVA10 recombination events detected by RDP.
| Isolate | Recombination event no. | Breakpoint |
Major Parent | Minor Parent | Detection methods and P-values |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Begin | End | RDP | Bootscan | Maxchi | Chimaera | SiSscan | Phylpro | 3Seq | ||||
| SJ20-1 | 1 | 5035 | 6690 | CV-A10 WH21R/Shandong/China/2014 | CVA16 CF350028_FRA_2011 |
1.58E-18 | 6.71E-21 | 1.54E-14 | 7.51E-10 | NS | 2.22E-16 | NS |
| SJ20-7 | 1 | 5078 | 6690 | CV-A10 WH21R/Shandong/China/2014 | CVA16 CF350028_FRA_2011 |
2.98E-19 | 1.117E-20 | 2.188E-14 | 1.010E-9 | 9.872E-19 | 2.220E-16 | NS |
| SJ20-8 | 1 | 5182 | 6690 | CV-A10 WH21R/Shandong/China/2014 | CVA16 CF350028_FRA_2011 |
2.45E-19 | 8.007E-21 | 1.456E-10 | 6.524E-6 | 1.268E-18 | 1.110E-16 | NS |
NS represented no significant P-value was recorded for recombination event using this method.
Isolate WH21R/Shandong/China/2014 (Genbank accession no: KY272007) and CVA16_C_CF350028_FRA_2011 (Genbank accession no: LT617115) were chosen to be the reference sequences for recombination analysis.
CVA10, Coxsackievirus A10; CVA16, coxsackievirus A16.
Near complete genome of 7 CVA10 genogroup D isolates (isolates in this study, n = 3; isolates publicly available, n = 4) were further analyzed for nucleotide identity analysis. Pairwise distances were computed within genogroup D as well as representatives of CVA10 genogroup A, B, C, F and CVA16 genogroup A, B, D. EVA71 prototype BrCr strain was used as an out-group. Isolates of CVA10 genogroup D displayed high similarities with each other, with nucleotide identities of 91.7%–99.9% along the whole genome sequence. In details, based on VP1, P1, P2 and P3 region, nucleotide of seven CVA10 genogroup D isolates presented identities of 91.2%–100.0%, 90.6%–99.9%, 91.9%–99.9% and 91.5%–99.9%, respectively (Table 3). SJ20-1, SJ20-7 and SJ20-8, the three CVA10 genogroup D isolates in this study, showed 99.9%–100% nucleotide identities on the basis of complete genome sequence. CVA10 genogroup D showed higher sequence identities to genogroup F from 5′ non coding region to the end of P1 region. The P3 region showed higher similarity to CVA16 genogroup D, where the recombination breakpoints were located (Table 3).
Table 3.
Pairwise nucleotide identities within CVA10 genogroup D as well as between genogroup D CVA10 and different genogroups of CVA10 and CVA16 isolates. EVA71 prototype BrCr strain was used as an outgroup.
| Virus | Region | Nucleotide identity (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Within CVA10 Genogroup D | CVA10 prototype (Kowalik) | CVA10 Genogroup B (MH118054) | CVA10 Genogroup C (MT263726) | CVA10 Genogroup F (MH118084) | CVA16 prototype (G10) | CVA16 Genogroup B (J1746661) |
CVA16 Genogroup D (LT617105) |
EVA71 prototype (BrCr) | ||
| CVA10 Genogroup D (7 isolates) | Genome | 91.7–99.9 | 76.63–77.00 | 75.45–76.11 | 76.53–77.08 | 76.79–77.76 | 66.79–67.26 | 68.37–68.88 | 71.32–72.04 | 67.58–68.21 |
| 5′UTR | 94.6–99.9 | 76.52–77.36 | 81.68–83.40 | 79.81–80.46 | 89.59–90.09 | 81.75–82.71 | 77.75–79.38 | 77.87–80.26 | 81.41–81.99 | |
| P1 | 90.6–99.9 | 75.86–76.81 | 76.08–77.51 | 75.49–76.68 | 78.18–79.40 | 50.26–51.38 | 51.77–52.22 | 51.43–51.96 | 48.05–49.06 | |
| VP4 | 86.3–100.0 | 74.34–78.17 | 67.53–75.23 | 74.34–78.17 | 77.52–85.19 | 46.45–50.41 | 47.62–54.87 | 53.21–55.86 | 44.93–51.26 | |
| VP2 | 90.7–99.9 | 75.86–78.06 | 77.86–79.34 | 75.66–77.86 | 78.12–80.47 | 55.09–56.84 | 57.63–59.58 | 53.83–57.66 | 56.23–55.36 | |
| VP3 | 90.7–99.9 | 73.20–76.62 | 76.25–77.69 | 73.20–76.62 | 77.49–79.92 | 51.59–53.63 | 52.63–54.68 | 52.41–53.66 | 50.95–54.58 | |
| VP1 | 91.2–100.0 | 76.21–77.56 | 74.78–76.57 | 75.49–77.71 | 76.06–78.67 | 44.09–46.05 | 43.71–45.55 | 43.47–47.25 | 37.87–41.39 | |
| P2 | 91.9–99.9 | 76.00–77.54 | 75.25–76.21 | 76.18–77.96 | 73.86–76.13 | 73.99–75.44 | 73.57–75.55 | 75.11–76.59 | 75.91–77.55 | |
| 2A | 92.3–99.8 | 74.13–75.79 | 73.12–75.57 | 73.78–76.12 | 73.64–77.08 | 72.88–74.94 | 71.59–74.81 | 69.59–71.74 | 73.29–76.01 | |
| 2B | 92.8–100.0 | 72.18–75.42 | 73.93–76.96 | 71.15–75.42 | 69.41–74.33 | 70.08–72.69 | 70.85–74.59 | 71.52–77.91 | 72.44–76.59 | |
| 2C | 91.5–99.9 | 77.16–79.06 | 75.32–76.98 | 77.91–79.64 | 73.96–77.52 | 74.81–76.45 | 73.78–76.95 | 78.29–79.45 | 78.06–79.56 | |
| P3 | 91.5–99.9 | 75.94–76.83 | 71.36–72.69 | 76.21–76.90 | 72.88–73.67 | 71.67–72.60 | 75.92–76.64 | 83.41–84.60 | 74.20–75.62 | |
| 3A | 88–100.0 | 71.93–74.10 | 68.57–75.05 | 71.93–75.25 | 68.65–73.94 | 70.11–74.10 | 68.08–72.20 | 78.70–83.39 | 67.16–69.14 | |
| 3B | 90.1–100.0 | 67.39–76.65 | 60.13–65.27 | 67.39–76.65 | 55.92–67.39 | 59.85–65.39 | 54.59–62.83 | 84.06–91.77 | 57.53–62.94 | |
| 3C | 91.5–100.0 | 74.67–75.55 | 72.03–75.15 | 74.39–75.22 | 73.11–76.62 | 69.67–73.40 | 72.54–73.91 | 84.05–85.03 | 70.46–72.62 | |
| 3D | 91.5–99.9 | 76.47–78.41 | 70.74–72.66 | 76.81–78.63 | 72.60–74.39 | 72.21–73.65 | 78.59–79.73 | 82.79–84.96 | 76.53–78.55 | |
Bold represented the highest homology to CVA10 genogroup D isolates in different regions.
EVA71, enterovirus A71; CVA16, coxsackievirus A16; CVA10, coxsackievirus A10.
4. Discussion
As a common childhood illness, HFMD imposes a severe burden on societies especially in east and Southeast Asia (Xing et al., 2014). EVA71 and CVA16 were the top two EVs responsible for HFMD cases since the year of 2008 (Liu et al., 2015; Yang et al., 2017). However, in recent years, the prevalence of EVA71 decreased sharply while the prevalence of CVA16 was observed increasing stably (Fu et al., 2020). During the same period, a rapid increase in the prevalence of other EVs, especially CVA6, was also observed (He et al., 2013; Yang et al., 2017; Fu et al., 2020). Although CVA16 and CVA6 were reported as major serotypes related to HFMD cases in recent years, CVA10 still aroused increasing concern in recent years (Mirand et al., 2012; He et al., 2013; Tian et al., 2014, Tian et al., 2017; Ji et al., 2018; Munivenkatappa et al., 2018). In the present study, epidemiological features of CVA10 isolates circulating in Shanghai from 2016 to 2020 were described. In accordance with previous studies (He et al., 2013; Xu et al., 2015; Wang et al., 2018; Song et al., 2020), CVA10 was only the fourth most common agent lead to HFMD cases. Except for mild symptoms, CVA10 infection can also lead to severe or fatal cases, not only in HFMD cases (Fuschino et al., 2012; Lu et al., 2012; He et al., 2013; Okada et al., 2013; Xu et al., 2013; Chen et al., 2017; Li et al., 2020). The proportion of CVA10-infected severe HFMD cases also exhibited an increasing trend in Chinese mainland (Ji et al., 2018). However, in our study, CVA10 mainly caused mild symptoms, which presented variance with another study conducted in Shanghai during the corresponding period (Li et al., 2020). As the second most common EV detected in non-polio EV-associated central nervous system infection, CVA10 was reported in causing over 20% viral encephalitis and meningitis in Shanghai in 2016–2018 (Li et al., 2020). This phenomenon indicates that disease-based surveillance system may not fully reflect the prevalence of CVA10. The establishment of pathogen-based surveillance system may be helpful to understand the infectibility and pathogenicity of CVA10 in order to improve virus control and prevention.
Endemic distribution indicated that children in suburbs were at higher risk of CVA10 infection. The regional differences may result in several reasons. Firstly, due to different demographic composition (permanent resident population versus floating population), discrepancies may occur between pathogen spectrum of HFMD in different region. This phenomenon has also been reported in other pathogens. Zeng et al. reported that children of migrant workers were at a higher risk of EVA71 infection (Zeng et al., 2013). Secondly, the Children’s Hospital of Fudan University, the designated hospital of HFMD severe cases in Shanghai, is located in Minhang district, which is defined as suburbs in the current study. Owing to its reputation and geographical location, the Children’s Hospital of Fudan University may admit both mild and severe HFMD cases in Yangtze River Delta region, which may lead to discrepancies between incidences of different pathogens. Consistent with other studies, the peak season of CVA10 infection occurred between May and September, which was late spring and early autumn in Shanghai and the overall seasonal trend overlapped the summer peak of HFMD incidences (Ge et al., 2015; Fu et al., 2016; Li et al., 2018). However there is no CVA10 positive case reported during February to September in 2020. Due to the prevalence of novel coronavirus disease 2019 (COVID-19), interventions with different intensity has been implement in Chinese mainland since 2020 (Zhang et al., 2021). In most provinces except Hubei, schools didn’t reopen until the end of April 2020. Children were quarantined at home, which reduced the risk of infection. Both the number and proportion of CVA10-related HFMD cases in 2020 underwent the lowest value in the past five years. This phenomenon reflects interventions like home quarantine, keeping social distance, wearing face masks and frequent hand-washing may also be helpful to control the spread of EV. With the relaxing on human contact patterns, the proportion of CVA10 infection started to pick up at the end of 2020 (Fig. 1C). Continuous monitoring of CVA10 epidemiological and etiological characteristics is necessary to guide disease control and prevention.
In the current study, genotyping based on entire VP1 region as well as phylogenetic analysis based on near complete genome were performed. Seven genogroups, including genogroup A to genogroup G can be divided based on entire VP1 region with nucleotide divergence of 14.97% (Ji et al., 2018). Genogroup A was the prototype Kowalik strain isolated in 1950 in USA (Dalldorf, 1953). Genogroup B and genogroup C consistently dominated the diffusion of CVA10 in Chinese mainland before and after the year of 2009 (Tian et al., 2014, 2017; Ji et al., 2018). Genogroup D was reported mainly circulating in Europe and involved in multiple outbreaks of HFMD and herpangina (HA) (Blomqvist et al., 2010; Mirand et al., 2012). Genogroup E and genogroup F were mainly consisted of African strains and Indian strains (Munivenkatappa et al., 2018), and a Taiwan strain was assigned to genogroup G. In accordance with Ji’s study (Ji et al., 2018), most isolates in this study belonged to CVA10 genogroup C and were closely related to other Chinese isolates. Different clusters of Shanghai genogroup C isolates also showed high homology with each other. However, our study reported the presence of CVA10 genogroup D in Chinese mainland for the first time. In the current study, four CVA10 genogroup D isolates were identified. Nucleotide identity and phylogenetic analysis based on entire VP1 region provided strong evidence for virus genotyping. Viral genome of Shanghai genogroup D isolates showed high similarity with foreign genogroup D isolates, indicating the presence of these isolates might derive from imported cases. However, the four Shanghai genogroup D isolates may have different sources. The three Songjiang isolates presented almost 100% nucleotide identity based on sequences comprising the 5′UTR, P1, P2 and P3 regions while they presented 8.4%-nucleotide-difference with PD17-2 based on VP1 region. Consistent with nucleotide identity analysis, the three Songjiang isolates were clustered with European isolates and were distantly related to PD17-2 which was clustered with Indian isolates on the phylogenetic tree. Thus, we speculated PD17–2 may be obtained from an independent imported case and the three Songjiang isolates may be obtained from another. Since all these isolates were collected from sporadic cases, CVA10 genogroup D might have been spread on a small scale in Shanghai. Since the origin of importation still remains unclear, continuous genomic surveillance is necessary to better understand virus transmission. Another three reference isolates which were previously defined as CVA16 were redefined as CVA10 genogroup D in this study. Nucleotide identity analysis between the reference sequences and CVA10 genogroup D isolates, CVA10 prototype Kowalik strain as well as CVA16 prototype G-10 strain corroborated virus definition (Table 3). Phylogenetic relationships based on VP1, 5′UTR, P1, P2, P3 region and near complete genome also supported this result (Fig. 3, Supplementary Fig. S1, S2). All of them were previously defined CVA16 as organism, but the two French isolates were also defined CVA10 as serotype. According to the GenBank database, all these isolates were collected in the year of 2010, which was the early phase of the prevalence of CVA10 genogroup D. Further studies are needed to better understand the evolutionary relationship of this genogroup.
Similar to other EVs, different genogroups of CVA10 were reported to have different geographic distribution pattern (Dalldorf, 1953; Blomqvist et al., 2010; Mirand et al., 2012; Ji et al., 2018; Munivenkatappa et al., 2018). However, we found that genogroup D didn’t show much geographic limitation. The first report of CVA10 genogroup D can be traced to Japan in 2003 (accession no: AB163730). Afterwards, European countries like Germany (Roth et al., 2007), Finland (Blomqvist et al., 2010), France (Mirand et al., 2012) and Russia (Lukashev et al., 2014) as well as Asian countries like Japan and India (Munivenkatappa et al., 2018) reported the circulating of CVA10 genogroup D to vary degree. However, CVA10 genogroup D has always been reported to be involved in several large-scale outbreaks in Europe. (Blomqvist et al., 2010) reported the nationwide outbreak of HFMD in Finland in 2008, which was mainly caused by CVA6 and CVA10. CVA10 genogroup D was responsible for 33% EV-positive cases during this outbreak. In 2010, Mirandet et al. (Mirand et al., 2012) reported the third dual outbreak in Europe happened in France and CVA10 genogroup D was identified as the most predominant EV serotype. However, the main infected population and the clinical symptoms they showed were quite different. The outbreak in Finland involved a large proportion of adult patients and considerable cases suffered from onychomadesis (Blomqvist et al., 2010). Meanwhile, patients enrolled in the French outbreak consisted of children with a mean age of two years, presenting typical HFMD/HA clinical symptoms (Mirand et al., 2012). Consistent with the French report, the Shanghai cases comprised children under three years old manifested mild HFMD symptoms. The transformation of infected population and clinical symptoms calls for further studies on virulence and pathogenesis of CVA10 genogroup D. Although CVA10 genogroup D has aroused multiple outbreaks in Europe, molecular epidemiological studies on this genogroup are limited. Before our study, only four complete genome sequences of genogroup D CVA10 were available in GenBank. In accordance with the nucleotide identity analysis, recombination detection indicated intertypic recombinant origin of CVA10 genogroup D, showing higher similarity with CVA16 genogroup D between P3 region. As one of the parent strain, CVA16 genogroup D was a novel identified recombinant genogroup (Hassel et al., 2017), which was putatively resulted from genetic features acquired through recombination. Thus, the recombination analysis may be misidentification because one of the parent strains presented recombinant origin. CVA16 genogroup D was first reported in Peru (Carrion et al., 2016) and was then found circulating in France, where the outbreak of CVA10 genogroup D once occurred. The time origin of genogroup D was assigned in 2004 (2001–2007) (Hassel et al., 2017). Meanwhile, the first CVA10 genogroup D isolate was reported in the same period. Further evolutionary studies are needed to determine the genetic origins of CVA10 genogroup D.
As one of the most prevalent causative agents, CVA10 poses challenges in prevention and control of HFMD and HA. The current study systematically described the molecular epidemiology on the basis of near complete genome of CVA10 circulating in Shanghai in the past five years. Our study firstly reported the appearance and spread of CVA10 genogroup D in Chinese mainland, which probably belonged to two independent imported cases. Further recombination analysis may help to expand the knowledge of genetic features as well as evolutionary origin of CVA10 genogroup D. With the control of circulating of EVA71 by vaccination programs, the prevalence of non-EVA71 EVs could rise further. Continuous and comprehensive surveillance for CVA10 are needed to better understand the evolution relationships and transmission of CVA10 as well as to guide disease control and prevention.
5. Conclusions
In conclusion, this study described the genetic features as well as epidemiological characteristics of CVA10 circulating in Shanghai, China between 2016 and 2020. The result suggested that CVA10 was the fourth prevalent causatives associated with HFMD and genogroup C was the predominant CVA10 genogroup circulating in Shanghai. Meanwhile, the recombinant genogroup D was firstly reported in circulating in Chinese mainland. Further continuous surveillance is helpful for disease control and prevention.
Data availability
All the sequences in this study were submitted to GenBank database (www.ncbi.nlm.nih.gov) under the accession numbers: MW929238–MW929301 for near complete genome and MW929306–MW929316 for VP1 sequences. The detailed case information in this study would remain confidential and would not be shared.
Ethics statement
This study was approved by the Ethics Committees of Shanghai Municipal Center for Disease Control and Prevention (Ethics Number, 2020-89).
Author contributions
Jiayu Wang: funding acquisition, data curation, formal analysis, writing-original draft; Jiajing Liu: data curation, formal analysis, writing-original draft; Fanghao Fang: data curation, methodology, software; Jiajin Wu: investigation, resources; Tianjiao Ji: methodology, validation; Yuying Yang: methodology; Ling Liu: investigation, resources; Chongshan Li: methodology, software, validation; Wanju Zhang: data curation; formal analysis, methodology, writing-reviewing and editing; Xi Zhang: conceptualization, resources, funding acquisition, supervision; Zheng Teng: conceptualization, resources, funding acquisition, supervision.
Conflict of interest
The authors have declared that they have no conflict of interest.
Acknowledgments
This work was supported by Shanghai Sailing Program (Grant no: 19YF1441500), Shanghai Municipal Commission of Health and Family Planning (Grant no: 20184Y0101) and Three-Year Action Plan of Shanghai Public Health System Construction (Grant no: GWV-2, GWV-10.1-XK03). The funders had no role in study design, data extraction and analysis, the decision to publish, or the preparation of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.virs.2022.01.028.
Contributor Information
Wanju Zhang, Email: zhangwanju@scdc.sh.cn.
Xi Zhang, Email: zhangxi@scdc.sh.cn.
Zheng Teng, Email: tengzheng@scdc.sh.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Blomqvist S., Klemola P., Kaijalainen S., Paananen A., Simonen M.L., Vuorinen T., Roivainen M. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J. Clin. Virol. 2010;48:49–54. doi: 10.1016/j.jcv.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Bracho M.A., Gonzalez-Candelas F., Valero A., Cordoba J., Salazar A. Enterovirus co-infections and onychomadesis after hand, foot, and mouth disease, Spain, 2008. Emerg. Infect. Dis. 2011;17:2223–2231. doi: 10.3201/eid1712.110395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro V., Guillot S., Delpeyroux F., Crainic R. Molecular strategy for 'serotyping' of human enteroviruses. J. Gen. Virol. 2001;82:79–91. doi: 10.1099/0022-1317-82-1-79. [DOI] [PubMed] [Google Scholar]
- Carrion G., Huaman J.L., Silva M., Ampuero J.S., Paz I., Ocana V.R., Laguna-Torres V.A., Hontz R.D. Molecular epidemiology of coxsackievirus A16 strains from four sentinel surveillance sites in Peru. Int. J. Infect. Dis. 2016;52:83–85. doi: 10.1016/j.ijid.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Chen J.F., Zhang R.S., Ou X.H., Chen F.M., Sun B.C. The role of enterovirus 71 and coxsackievirus A strains in a large outbreak of hand, foot, and mouth disease in 2012 in Changsha, China. Int. J. Infect. Dis. 2014;28:17–25. doi: 10.1016/j.ijid.2014.07.024. [DOI] [PubMed] [Google Scholar]
- Chen M., He S., Yan Q., Xu X., Wu W., Ge S., Zhang S., Chen M., Xia N. Severe hand, foot and mouth disease associated with Coxsackievirus A10 infections in Xiamen, China in 2015. J. Clin. Virol. 2017;93:20–24. doi: 10.1016/j.jcv.2017.05.011. [DOI] [PubMed] [Google Scholar]
- Chen X., Tan X., Li J., Jin Y., Gong L., Hong M., Shi Y., Zhu S., Zhang B., Zhang S., Zhang Y., Mao N., Xu W. Molecular epidemiology of coxsackievirus A16: intratype and prevalent intertype recombination identified. PLoS One. 2013;8 doi: 10.1371/journal.pone.0082861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalldorf G. The coxsackie virus group. Ann. N. Y. Acad. Sci. 1953;56:583–586. doi: 10.1111/j.1749-6632.1953.tb30251.x. [DOI] [PubMed] [Google Scholar]
- Davia J.L., Bel P.H., Ninet V.Z., Bracho M.A., Gonzalez-Candelas F., Salazar A., Gobernado M., Bosch I.F. Onychomadesis outbreak in Valencia, Spain associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr. Dermatol. 2011;28:1–5. doi: 10.1111/j.1525-1470.2010.01161.x. [DOI] [PubMed] [Google Scholar]
- Fu X., Wan Z., Li Y., Hu Y., Jin X., Zhang C. National epidemiology and evolutionary history of four hand, foot and mouth disease-related enteroviruses in China from 2008 to 2016. Virol. Sin. 2020;35:21–33. doi: 10.1007/s12250-019-00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Sun Q., Liu B., Xu H., Wang Y., Zhu W., Pan L., Zhu L. Epidemiological characteristics and pathogens attributable to hand, foot, and mouth disease in Shanghai, 2008-2013. J. Infect Dev. Ctries. 2016;10:612–618. doi: 10.3855/jidc.6118. [DOI] [PubMed] [Google Scholar]
- Fuschino M.E., Lamson D.M., Rush K., Carbone L.S., Taff M.L., Hua Z., Landi K., George K.S. Detection of coxsackievirus A10 in multiple tissues of a fatal infant sepsis case. J. Clin. Virol. 2012;53:259–261. doi: 10.1016/j.jcv.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Ge Y., Zheng Y., Pan H., Mao S., Li Y., Xia A., Zhu Q., Hu J., Zeng M. [Epidemiological surveillance of hand, foot and mouth disease in Shanghai, 2010-2014] Zhonghua Er Ke Za Zhi. 2015;53:676–683. [PubMed] [Google Scholar]
- Gibbs M.J., Armstrong J.S., Gibbs A.J. Sister-scanning: a Monte Carlo procedure for assessing signals in recombinant sequences. Bioinformatics. 2000;16:573–582. doi: 10.1093/bioinformatics/16.7.573. [DOI] [PubMed] [Google Scholar]
- Guan H., Wang J., Wang C., Yang M., Liu L., Yang G., Ma X. Etiology of multiple non-EV71 and non-CVA16 enteroviruses associated with hand, foot and mouth disease in Jinan, China, 2009-June 2013. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassel C., Mirand A., Farkas A., Diedrich S., Huemer H.P., Peigue-Lafeuille H., Archimbaud C., Henquell C., Bailly J.L., Network H.F.S. Phylogeography of coxsackievirus A16 reveals global transmission pathways and recent emergence and spread of a recombinant genogroup. J. Virol. 2017;91:e00630–17. doi: 10.1128/JVI.00630-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y.Q., Chen L., Xu W.B., Yang H., Wang H.Z., Zong W.P., Xian H.X., Chen H.L., Yao X.J., Hu Z.L., Luo M., Zhang H.L., Ma H.W., Cheng J.Q., Feng Q.J., Zhao D.J. Emergence, circulation, and spatiotemporal phylogenetic analysis of coxsackievirus a6- and coxsackievirus a10-associated hand, foot, and mouth disease infections from 2008 to 2012 in Shenzhen, China. J. Clin. Microbiol. 2013;51:3560–3566. doi: 10.1128/JCM.01231-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.F., Yang F., Du J., Dong J., Zhang T., Wu Z.Q., Xue Y., Jin Q. Complete genome analysis of coxsackievirus A2, A4, A5, and A10 strains isolated from hand, foot, and mouth disease patients in China revealing frequent recombination of human enterovirus A. J. Clin. Microbiol. 2011;49:2426–2434. doi: 10.1128/JCM.00007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H., Fan H., Lu P.X., Zhang X.F., Ai J., Shi C., Huo X., Bao C.J., Shan J., Jin Y. Surveillance for severe hand, foot, and mouth disease from 2009 to 2015 in Jiangsu province: epidemiology, etiology, and disease burden. BMC Infect. Dis. 2019;19:79. doi: 10.1186/s12879-018-3659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji T., Guo Y., Huang W., Shi Y., Xu Y., Tong W., Yao W., Tan Z., Zeng H., Ma J., Zhao H., Han T., Zhang Y., Yan D., Yang Q., Zhu S., Zhang Y., Xu W. The emerging sub-genotype C2 of CoxsackievirusA10 associated with hand, foot and mouth disease extensively circulating in mainland of China. Sci. Rep. 2018;8:13357. doi: 10.1038/s41598-018-31616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam H.M., Ratmann O., Boni M.F. Improved algorithmic complexity for the 3SEQ recombination detection algorithm. Mol. Biol. Evol. 2018;35:247–251. doi: 10.1093/molbev/msx263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Wang X., Cai J., Ge Y., Wang C., Qiu Y., Xia A., Zeng M. Non-polio enterovirus infections in children with central nervous system disorders in Shanghai, 2016-2018: serotypes and clinical characteristics. J. Clin. Virol. 2020;129:104516. doi: 10.1016/j.jcv.2020.104516. [DOI] [PubMed] [Google Scholar]
- Li Y., Bao H., Zhang X., Zhai M., Bao X., Wang D., Zhang S. Epidemiological and genetic analysis concerning the non-enterovirus 71 and non-coxsackievirus A16 causative agents related to hand, foot and mouth disease in Anyang city, Henan Province, China, from 2011 to 2015. J. Med. Virol. 2017;89:1749–1758. doi: 10.1002/jmv.24847. [DOI] [PubMed] [Google Scholar]
- Li Y., Chang Z., Wu P., Liao Q., Liu F., Zheng Y., Luo L., Zhou Y., Chen Q., Yu S., Guo C., Chen Z., Long L., Zhao S., Yang B., Yu H., Cowling B.J. Emerging enteroviruses causing hand, foot and mouth disease, China, 2010-2016. Emerg. Infect. Dis. 2018;24:1902–1906. doi: 10.3201/eid2410.171953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.L., Pan H., Liu P., Amer S., Chan T.C., Zhan J., Huo X., Liu Y., Teng Z., Wang L., Zhuang H. Comparative epidemiology and virology of fatal and nonfatal cases of hand, foot and mouth disease in mainland China from 2008 to 2014. Rev. Med. Virol. 2015;25:115–128. doi: 10.1002/rmv.1827. [DOI] [PubMed] [Google Scholar]
- Liu W., Wu S., Xiong Y., Li T., Wen Z., Yan M., Qin K., Liu Y., Wu J. Co-circulation and genomic recombination of coxsackievirus A16 and enterovirus 71 during a large outbreak of hand, foot, and mouth disease in Central China. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q.B., Zhang X.A., Wo Y., Xu H.M., Li X.J., Wang X.J., Ding S.J., Chen X.D., He C., Liu L.J., Li H., Yang H., Li T.Y., Liu W., Cao W.C. Circulation of Coxsackievirus A10 and A6 in hand-foot-mouth disease in China, 2009-2011. PLoS One. 2012;7 doi: 10.1371/journal.pone.0052073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukashev A.N., Shumilina E.Y., Belalov I.S., Ivanova O.E., Eremeeva T.P., Reznik V.I., Trotsenko O.E., Drexler J.F., Drosten C. Recombination strategies and evolutionary dynamics of the Human enterovirus A global gene pool. J. Gen. Virol. 2014;95:868–873. doi: 10.1099/vir.0.060004-0. [DOI] [PubMed] [Google Scholar]
- Martin D., Rybicki E. RDP: detection of recombination amongst aligned sequences. Bioinformatics. 2000;16:562–563. doi: 10.1093/bioinformatics/16.6.562. [DOI] [PubMed] [Google Scholar]
- Martin D.P., Murrell B., Golden M., Khoosal A., Muhire B. RDP4: detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015;1:vev003. doi: 10.1093/ve/vev003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D.P., Posada D., Crandall K.A., Williamson C. A modified bootscan algorithm for automated identification of recombinant sequences and recombination breakpoints. AIDS Res. Hum. Retrovir. 2005;21:98–102. doi: 10.1089/aid.2005.21.98. [DOI] [PubMed] [Google Scholar]
- Meng X.D., Tong Y., Wei Z.N., Wang L., Mai J.Y., Wu Y., Luo Z.Y., Li S., Li M., Wang S., Wei S., Gong W., Zhang W., Hu X., Huang J., Shi J., Yang G., Meng S., Wang Z., Guan X., Shen S. Epidemical and etiological study on hand, foot and mouth disease following EV-A71 vaccination in Xiangyang, China. Sci. Rep. 2020;10:20909. doi: 10.1038/s41598-020-77768-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirand A., Henquell C., Archimbaud C., Ughetto S., Antona D., Bailly J.L., Peigue-Lafeuille H. Outbreak of hand, foot and mouth disease/herpangina associated with coxsackievirus A6 and A10 infections in 2010, France: a large citywide, prospective observational study. Clin. Microbiol. Infect. 2012;18:E110–E118. doi: 10.1111/j.1469-0691.2012.03789.x. [DOI] [PubMed] [Google Scholar]
- Munivenkatappa A., Yadav P.D., Nyayanit D.A., Majumdar T.D., Sangal L., Jain S., Sinha D.P., Shrivastava A., Mourya D.T. Molecular diversity of Coxsackievirus A10 circulating in the southern and northern region of India [2009-17] Infect. Genet. Evol. 2018;66:101–110. doi: 10.1016/j.meegid.2018.09.004. [DOI] [PubMed] [Google Scholar]
- Oberste M.S., Maher K., Kilpatrick D.R., Pallansch M.A. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J. Virol. 1999;73:1941–1948. doi: 10.1128/jvi.73.3.1941-1948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste M.S., Penaranda S., Maher K., Pallansch M.A. Complete genome sequences of all members of the species Human enterovirus A. J. Gen. Virol. 2004;85:1597–1607. doi: 10.1099/vir.0.79789-0. [DOI] [PubMed] [Google Scholar]
- Okada H., Wada M., Sato H., Yamaguchi Y., Tanji H., Kurokawa K., Kawanami T., Takahashi T., Kato T. Neuromyelitis optica preceded by hyperCKemia and a possible association with coxsackie virus group A10 infection. Intern. Med. 2013;52:2665–2668. doi: 10.2169/internalmedicine.52.1042. [DOI] [PubMed] [Google Scholar]
- Posada D., Crandall K.A. Evaluation of methods for detecting recombination from DNA sequences: computer simulations. Proc. Natl. Acad. Sci. U. S. A. 2001;98:13757–13762. doi: 10.1073/pnas.241370698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth B., Enders M., Arents A., Pfitzner A., Terletskaia-Ladwig E. Epidemiologic aspects and laboratory features of enterovirus infections in Western Germany, 2000-2005. J. Med. Virol. 2007;79:956–962. doi: 10.1002/jmv.20917. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Smith J.M. Analyzing the mosaic structure of genes. J. Mol. Evol. 1992;34:126–129. doi: 10.1007/BF00182389. [DOI] [PubMed] [Google Scholar]
- Song C., Li Y., Zhou Y., Liang L., Turtle L., Wang F., Wu P., Qiu Q., Yang J., Wang K., Cui P., Cheng Y., Zhang T., Guo C., Zeng M., Long L., Peiris M., Zhou C., Cowling B.J., Yu H. Enterovirus genomic load and disease severity among children hospitalised with hand, foot and mouth disease. EBioMedicine. 2020;62:103078. doi: 10.1016/j.ebiom.2020.103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X., Huang X., Zhu S., Chen H., Yu Q., Wang H., Huo X., Zhou J., Wu Y., Yan D., Zhang Y., Wang D., Cui A., An H., Xu W. The persistent circulation of enterovirus 71 in People's Republic of China: causing emerging nationwide epidemics since 2008. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H., Zhang Y., Shi Y., Li X., Sun Q., Liu L., Zhao D., Xu B. Epidemiological and aetiological characteristics of hand, foot, and mouth disease in Shijiazhuang City, Hebei province, China, 2009-2012. PLoS One. 2017;12 doi: 10.1371/journal.pone.0176604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H., Zhang Y., Sun Q., Zhu S., Li X., Pan Z., Xu W., Xu B. Prevalence of multiple enteroviruses associated with hand, foot, and mouth disease in Shijiazhuang City, Hebei province, China: outbreaks of coxsackieviruses a10 and b3. PLoS One. 2014;9 doi: 10.1371/journal.pone.0084233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Teng Z., Cui X., Li C., Pan H., Zheng Y., Mao S., Yang Y., Wu L., Guo X., Zhang X., Zhu Y. Epidemiological and serological surveillance of hand-foot-and-mouth disease in Shanghai, China, 2012-2016. Emerg. Microb. Infect. 2018;7:8. doi: 10.1038/s41426-017-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiller G.F. Phylogenetic profiles: a graphical method for detecting genetic recombinations in homologous sequences. Mol. Biol. Evol. 1998;15:326–335. doi: 10.1093/oxfordjournals.molbev.a025929. [DOI] [PubMed] [Google Scholar]
- Xie J., Yang X.H., Hu S.Q., Zhan W.L., Zhang C.B., Liu H., Zhao H.Y., Chai H.Y., Chen K.Y., Du Q.Y., Liu P., Yin A.H., Luo M.Y. Co-circulation of coxsackieviruses A-6, A-10, and A-16 causes hand, foot, and mouth disease in Guangzhou city, China. BMC Infect. Dis. 2020;20:271. doi: 10.1186/s12879-020-04992-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing W., Liao Q., Viboud C., Zhang J., Sun J., Wu J.T., Chang Z., Liu F., Fang V.J., Zheng Y., Cowling B.J., Varma J.K., Farrar J.J., Leung G.M., Yu H. Hand, foot, and mouth disease in China, 2008-12: an epidemiological study. Lancet Infect. Dis. 2014;14:308–318. doi: 10.1016/S1473-3099(13)70342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Su L., Cao L., Zhong H., Dong N., Dong Z., Xu J. Genotypes of the enterovirus causing hand foot and mouth disease in Shanghai, China, 2012-2013. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Su L., Cao L., Zhong H., Dong N., Xu J. Enterovirus genotypes causing hand foot and mouth disease in Shanghai, China: a molecular epidemiological analysis. BMC Infect. Dis. 2013;13:489. doi: 10.1186/1471-2334-13-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Liu F., Liao Q., Wu P., Chang Z., Huang J., Long L., Luo L., Li Y., Leung G.M., Cowling B.J., Yu H. Epidemiology of hand, foot and mouth disease in China, 2008 to 2015 prior to the introduction of EV-A71 vaccine. Euro Surveill. 2017;22:16–00824. doi: 10.2807/1560-7917.ES.2017.22.50.16-00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Zhang T., Hu Y., Wang X., Du J., Li Y., Sun S., Sun X., Li Z., Jin Q. Survey of enterovirus infections from hand, foot and mouth disease outbreak in China, 2009. Virol. J. 2011;8:508. doi: 10.1186/1743-422X-8-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng M., Pu D., Mo X., Zhu C., Gong S., Xu Y., Lin G., Wu B., He S., Jiao X., Wang X., Wang X., Zhu Q., Altmeyer R. Children of rural-to-urban migrant workers in China are at a higher risk of contracting severe hand, foot and mouth disease and EV71 infection: a hospital-based study. Emerg. Microb. Infect. 2013;2:e72. doi: 10.1038/emi.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Zhu R., Yang Y., Chi Y., Yin J., Tang X., Yu L., Zhang C., Huang Z., Zhou D. Phylogenetic analysis of the major causative agents of hand, foot and mouth disease in Suzhou City, Jiangsu province, China, in 2012-2013. Emerg. Microb. Infect. 2015;4:e12. doi: 10.1038/emi.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Litvinova M., Liang Y., Zheng W., Shi H., Vespignani A., Viboud C., Ajelli M., Yu H. The impact of relaxing interventions on human contact patterns and SARS-CoV-2 transmission in China. Sci. Adv. 2021;7:eabe2584. doi: 10.1126/sciadv.abe2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Tan X., Cui A., Mao N., Xu S., Zhu Z., Zhou J., Shi J., Zhao Y., Wang X., Huang X., Zhu S., Zhang Y., Tang W., Ling H., Xu W. Complete genome analysis of the C4 subgenotype strains of enterovirus 71: predominant recombination C4 viruses persistently circulating in China for 14 years. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T.S., Du J., Li H.J., Cui Y., Liu Y., Yang Y., Cui F., Lu Q.B. Molecular epidemiology and clinical characteristics of herpangina children in Beijing, China: a surveillance study. PeerJ. 2020;8 doi: 10.7717/peerj.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Z.C., Kou Z.Q., Bai Y.J., Cong X., Wang L.H., Li C., Zhao L., Yu X.J., Wang Z.Y., Wen H.L. Epidemiological research on hand, foot, and mouth disease in mainland China. Viruses. 2015;7:6400–6411. doi: 10.3390/v7122947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the sequences in this study were submitted to GenBank database (www.ncbi.nlm.nih.gov) under the accession numbers: MW929238–MW929301 for near complete genome and MW929306–MW929316 for VP1 sequences. The detailed case information in this study would remain confidential and would not be shared.