Abstract
BACKGROUND:
Optimal outcomes after large-vessel occlusion (LVO) stroke are highly dependent on prompt diagnosis, effective communication, and treatment, making LVO an attractive avenue for the application of artificial intelligence (AI), specifically machine learning (ML). Our objective is to conduct a systematic review to describe existing AI applications for LVO strokes, delineate its effectiveness, and identify areas for future AI applications in stroke treatment and prognostication.
METHODS:
A systematic review was conducted by searching the PubMed, Embase, and Scopus databases. After deduplication, studies were screened by title and abstract. Full-text studies were screened for final inclusion based on prespecified inclusion and exclusion criteria. Relevant data were extracted from each study.
RESULTS:
Of 11,512 resultant articles, 40 were included. Of 30 studies with reported ML algorithms, the most commonly used ML algorithms were convolutional neural networks in 10 (33.3%), support vector machines in 10 (33.0%), and random forests in 9 (30.0%). Studies examining triage favored direct transport to a stroke center and predicted improved outcomes. ML techniques proved vastly accurate in identifying LVO on computed tomography. Applications of AI to patient selection for thrombectomy are lacking, although some studies determine individual patient eligibility for endovascular treatment with high accuracy. ML algorithms have reasonable accuracy in predicting clinical and angiographic outcomes and associated factors.
CONCLUSIONS:
AI has shown promise in the diagnosis and triage of patients with acute stroke. However, the role of AI in the management and prognostication remains limited and warrants further research to help in decision support.
Keywords: Artificial intelligence, Ischemic stroke, Large-vessel occlusion, Machine learning, Stroke
INTRODUCTION
Acute ischemic stroke (AIS) is a common cause of morbidity and mortality globally, and continues to have a high incidence.1–5 Large-vessel occlusion (LVO) comprises 29.3% of AIS cases and has an incidence of 24 per 100,000 people per year, of which most occurs in the anterior circulation.6,7 LVO confers a 4.5-fold increase in mortality compared with other AIS.8–10 Randomized controlled trials have provided evidence that timely mechanical thrombectomy (MT) in patients with LVO is safe and effective in improving functional outcomes, such that MT with stent retriever or direct aspiration has become the standard of care for LVO.11–21 Recanalization is the strongest independent predictor of functional independence.22
Despite these advances, LVO has numerous management challenges. Existing prediction instruments aimed at identifying patients with stroke with LVO for rapid transport to centers offering MT have low sensitivity and specificity.23 At the hospital, management of stroke depends heavily on information from imaging studies.24 Determination of treatment strategy is critical, because MT must be timely, given the adage “time is brain.”25 Recently, artificial intelligence (AI), a broad term referring to the use of computers to perform complex tasks, has been applied to LVO care and research to address these challenges.24,26,27 Machine learning (ML), a subset of AI involving computer problem solving using data-driven algorithms derived from large data sets without explicit human programming, has become particularly prominent because of the potential for rapid automatic evaluation of patients with stroke and selection for MT.24,26–28 However, no systematic review has characterized the role of AI in LVO across the duration of care.
We conducted a systematic review to characterize the scope of the literature regarding the use of AI for LVO. We aimed to 1) describe existing AI applications for LVO triage, diagnosis, patient selection, and outcome prediction, 2) delineate the effectiveness and usefulness of existing LVO applications, and 3) identify areas for future AI applications for LVO stroke. Our findings will inform neurosurgeons regarding recent developments in this burgeoning field, promote awareness regarding the ability of AI to improve numerous facets of stroke care, and catalyze further research in the field and application to patient care.
METHODS
We conducted a systematic review according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines to investigate the use of AI for LVO strokes.29 PubMed MEDLINE (National Library of Medicine), Embase (Elsevier), and Scopus (Elsevier) were searched in November 2020 from inception to the present using a combination of keywords including “artificial intelligence,” “machine learning,” “large vessel occlusion,” and “stroke.” No restrictions on date, language, or article type were applied. Detailed search terms for all databases are shown in Supplementary Table 1.
After completing the search, duplicates were removed. All remaining articles were screened by title and abstract for relevance. Articles progressing to full-text review were screened for final inclusion based on prespecified inclusion and exclusion criteria. Inclusion criteria were articles published in or translated into English, with full text available, population of patients with LVO, and discussing the application of AI techniques. Exclusion criteria were articles not translated into English, conference abstracts, commentaries or letters, case reports, narrative reviews, scoping reviews, systematic reviews, meta-analyses, and not discussing application of AI to patients with LVO. Articles that discussed AIS without specifying LVO in particular or those that did not discuss LVO separately from other subtypes of AIS were excluded. A second reviewer replicated the search strategy. Disagreements were resolved after discussion with a third reviewer.
After we finalized the list of included articles, they were reviewed for bibliographic data, aim, design, duration, participants, intervention, and outcome data. The World Bank income classification was used to determine the income status of the countries of origin for all studies.30 Primary outcomes of interest included area under the curve (AUC), sensitivity, specificity, and accuracy of the AI technique used for LVO. Secondary outcomes included modified Rankin Scale (mRS) score, TICI (thrombolysis in cerebral infarction) score, comparison of the outcomes of AI techniques for LVO, and comparison of AI techniques to human raters. Descriptive statistics for the different types of ML algorithms used were also assessed. These statistics included convolutional neural network (CNN) (a class of deep neural networks used primarily for analyzing visual imagery), support vector machines (SVMs) (algorithms that analyze data for classification and regression analysis), and random forest (RF) (an ensemble learning method for classification and regression).
Critical appraisal of the quality of included studies was performed using the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) framework.31 The GRADE framework incorporates study design, biases, and the rigor of methodology to determine the quality of a study. The risk of bias for and applicability for each included study was assessed using PROBAST (Prediction Model Study Risk of Bias Assessment Tool).32 PROBAST considers the participants, predictors, outcomes, and analyses to determine the risk of bias for a study. Judgments on overall risk of bias and applicability for this systematic review were determined based on considering the risk of bias and applicability of all included studies in aggregate.
RESULTS
Included Studies
Our search resulted in 11,512 articles, 40 of which were included based on the criteria defined previously.33–72 Figure 1 shows the PRISMA flowchart for article selection. Thirty studies (75.0%) were single-country studies, whereas 10 (25.0%) were international collaborations. Of the single-country studies, 13 (43.3%) originated from the United States followed by 3 (7.5%) each from Germany and Switzerland. All articles were published between 2016 and 2020, with 21 (52.5%) in 2020, 14 (35.0%) in 2019, 4 (10.0%) in 2018, and 1 (2.5%) in 2016.
Figure 1.
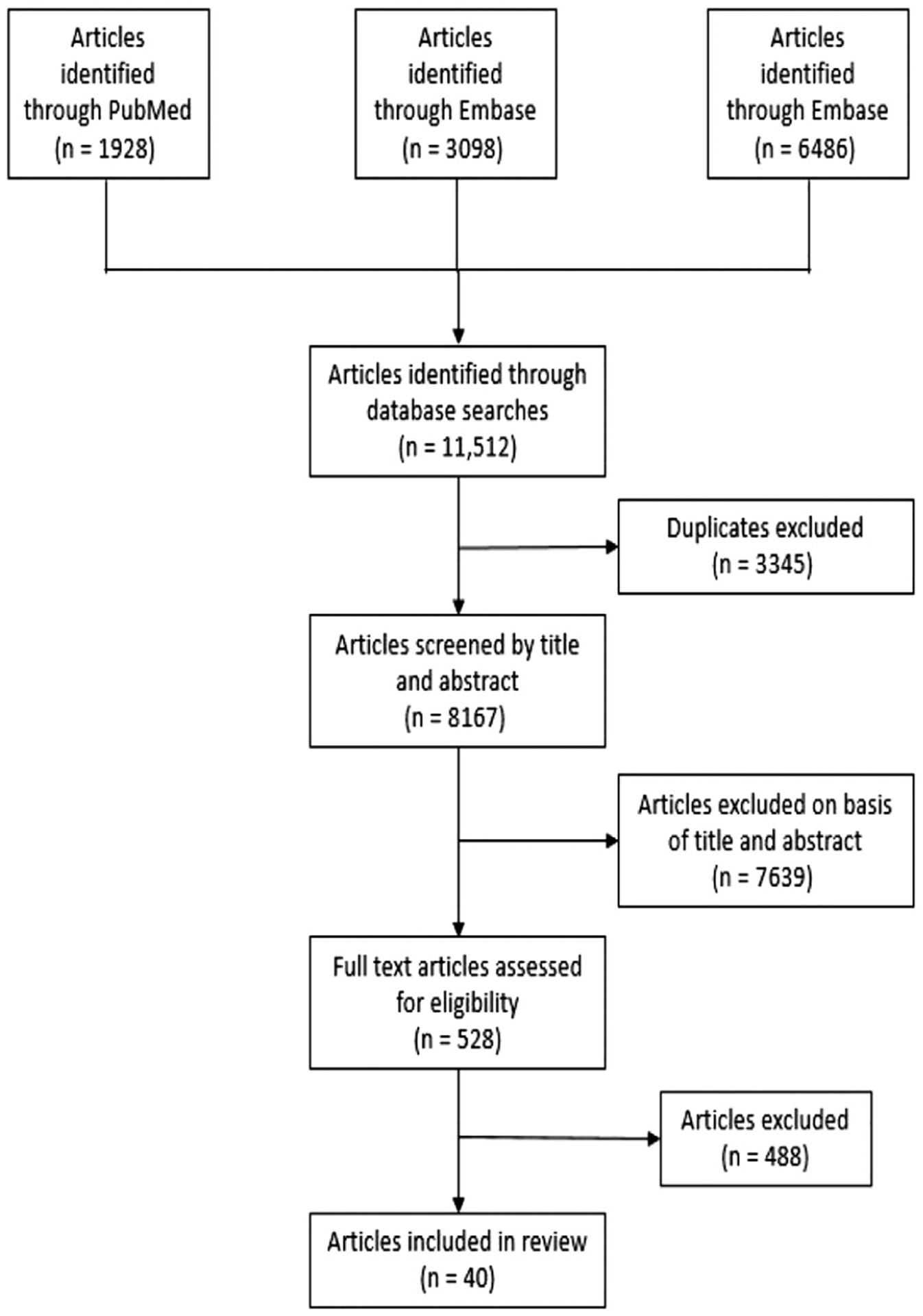
PRISMA flowchart outlining the search and review process used to identify and select articles for inclusion.
Of the 30 studies with reported ML algorithms, the most commonly used ML algorithms were CNNs in 10 (33.3%), SVMs in 10 (33.3%), RFs in 9 (30.0%), artificial neural networks (ANNs) in 5 (16.7%), and decision trees in 4 (13.3%). Study designs included 28 retrospective cohorts (70.0%), 7 prospective cohorts (17.5%), 2 mixed retrospective and prospective cohorts (5.0%), and 3 others (7.5%). All studies were moderate quality based on the GRADE framework. Sample sizes ranged from 25 to 5713. Ten studies (25.0%) used validation, 8 (20.0%) 10-fold cross-validation, 5 (12.5%) 5-fold cross-validation, and 1 (2.5%) each used nested cross-validation, leave-one-out cross-validation, recursive feature elimination with cross-validation, and both validation and 5-fold cross-validation. The remaining 13 studies (32.5%) did not mention any type of validation. Concerns regarding applicability of the models in included studies was low in 37 (92.5%), unclear in 2 (5.0%), and high in (2.5%). Risk of bias was low in 29 (72.5%), unclear in 6 (15.0%), and high in 5 (12.5%), predisposing this review to a low risk of bias overall.
The main topics of included articles are shown in Figure 2. These topics generally fell into triage, diagnosis, patient selection, and outcome prediction, and are reviewed in the following sections.
Figure 2.
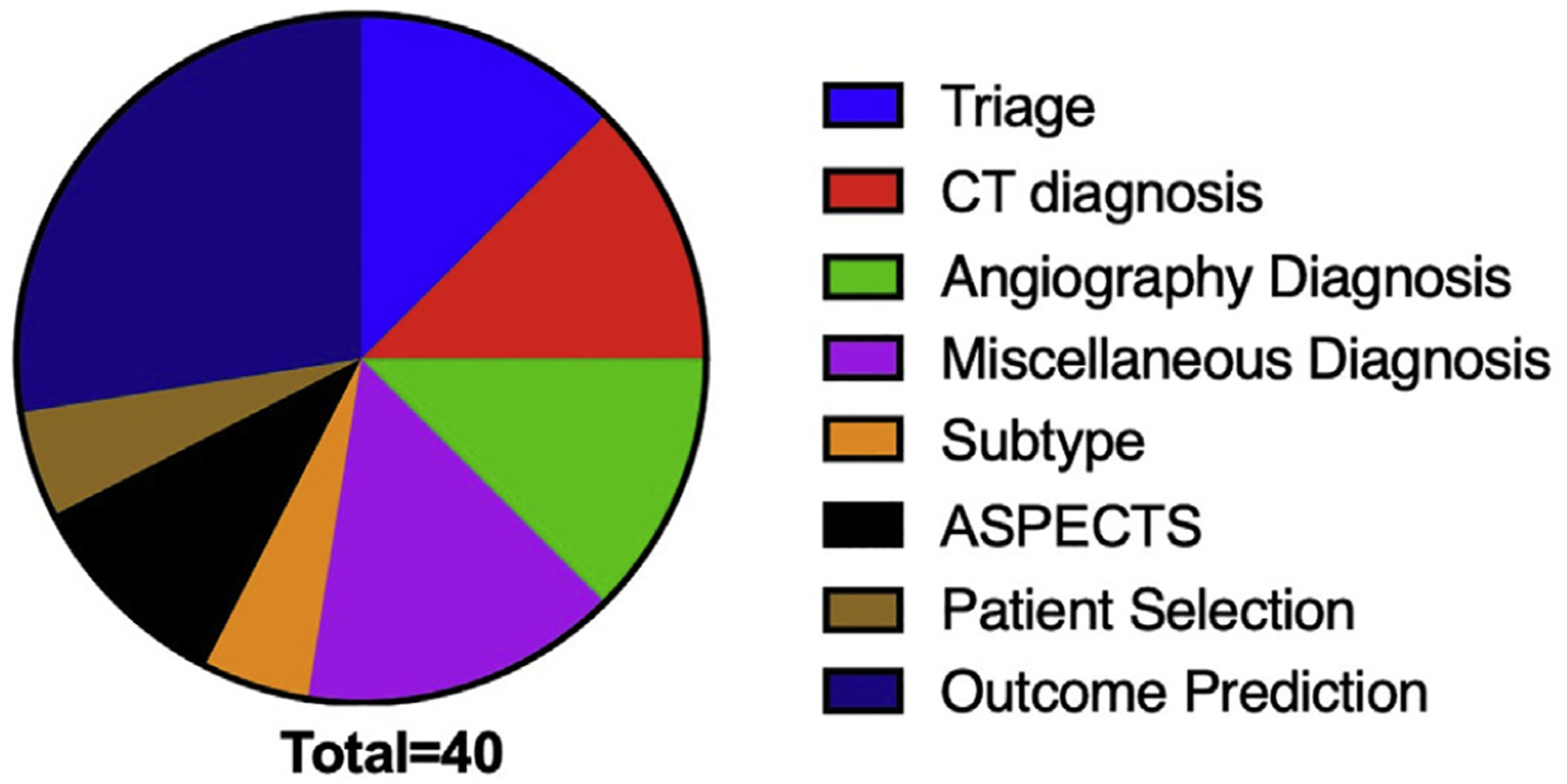
The main topic of included articles. ASPECTS, Alberta Stroke Program Early CT Score; CT, computed tomography.
Triage
Based on our review, 5 studies discussed ML methods for better triage (Table 1).35,44,67,68,71 Most (3/5) examined prehospital transport using decision trees.67,68,71 One of these studies67 determined that direct transport to an intervention center led to improved outcomes when the likelihood of LVO as the cause of AIS was >33%. Direct transportation to an intervention center was beneficial when the risk of LVO was ≥24% in an urban setting and ≥49% in a rural setting.67 A second study71 found that transporting patients directly to a comprehensive stroke center for tissue plasminogen activator and MT (if indicated) rather than to a primary stroke center was favored in most real-world scenarios regardless of formal LVO screening. A third study determined implementation of a modified American Heart Association algorithm for prehospital transport within 4.5 hours after symptom onset led to 1.8%–2.4% increase in favorable outcomes.68
Table 1.
Included Studies on Large-Vessel Occlusion Triage
| Category | Reference | Country (Income Status) | Quality Grade | Risk of Bias | Concern for Applicability | Artificial Intelligence Techniques | Key Findings |
|---|---|---|---|---|---|---|---|
| Prehospital transport | Venema et al., 201967 | Netherlands, United States (high) | Moderate | Low | Low | Decision tree | Direct transportation led to better outcomes when likelihood of LVO as a cause of acute ischemic stroke was >33% Benefit of direct transportation was 0.10 quality-adjusted life-years with a 66% likelihood of LVO |
| Venema et al., 202068 | Netherlands, United States (high) | Moderate | Low | Low | Decision tree | Nationwide implementation of the American Heart Association algorithm increased the number of good outcomes by +1.0% compared with transportation to the nearest center | |
| Xu et al., 201971 | United States (high) | Moderate | Low | Low | Decision tree | Dip-and-ship strategy almost always favored in the absence of formal LVO screening Mothership favored if additional transport time to comprehensive stroke center was <3–23 minutes for patients screened positive for LVO |
|
| Other | Chen et al., 201835 | China (upper middle) | Moderate | Low | Low | Artificial neural network | Mean Youden index, sensitivity, specificity, and accuracy of the artificial neural network model on 10-fold cross-validation analysis were 0.640, 0.807, 0.833 and 0.820, respectively Area under curve, Youden index, and accuracy were higher than National Institutes of Health Stroke Scale and other prehospital prediction scales |
| Hassan et al., 202044 | United States (high) | Moderate | High | Low | Not applicable | Median computed tomography angiography time at primary stroke center to door-in at comprehensive stroke center significantly reduced by 22.5 minutes |
LVO, large-vessel occlusion.
Diagnosis
LVO Detection.
As the primary method to detect stroke, LVO diagnosis on computed tomography (CT) imaging using AI models has primarily involved the prediction of clot signs or infarction volume. Five studies37,54,56,65,72 discussed diagnosis of LVO via CT (Table 2). One study54 aiming to identify clot signs on noncontrast CT found an AUC of 0.87 with a CNN, which increased to 0.91 when National Institutes of Health Stroke Scale (NIHSS) and time from onset were added to the model. A study72 found that an extreme gradient boost model incorporating noncontrast CT data in addition to demographic and clinical data had superior performance in detecting LVO to extreme gradient boost using demographic and clinical data and to RF and SVM models. On the other hand, Qui et al.56 found a model using CNN and RF to detect infarction on noncontrast CT that showed good agreement with stroke volume on diffusion-weighted imaging MRI scans. One study65 also used CT perfusion imaging and found an AUC of 0.90 for right-sided deficits, 0.96 for left-sided deficits, and 0.93 for no deficit.
Table 2.
Included Studies on Diagnosis
| Category | Reference | Country (Income Status) | Quality Grade | Risk of Bias | Concern for Applicability | Artificial Intelligence Technique Used | Key Findings |
|---|---|---|---|---|---|---|---|
| Angiography | Rava et al., 202057 | United States (high) | Moderate | Low | Low | SVM | Most accurate classification of infarct regions was plotting mean transit time versus peak height and mean transmit time versus AUC |
| Reid et al., 201958 | Australia, Canada (high) | Moderate | Low | Low | SVM | mCTA-venous had a large effect on accurately identifying early ischemia when dichotomized for Alberta Stroke Program Early CT Score ≥6 versus <6 compared with the moderate effect of NCCT and mCTA-regional leptomeningeal score SVM identified mCTA-venous as the most important imaging covariate for predicting 24-hour National Institutes of Health Stroke Scale and 90-day modified Rankin Scale score |
|
| Sheth et al., 201959 | United States (high) | Moderate | Low | Low | CNN | CNN autonomously learned to identify the intracerebral vasculature on CTA and detected LVO with AUC 0.88 CNN determined infarct core as defined by CTP-RAPID from the CTA source images with AUC 0.88 for ischemic core ≤30 mL and 0.90 for ischemic core ≤50 mL. CNN corresponded with CTP-RAPID volumes |
|
| Stib et al., 202061 | United States (high) | Moderate | Low | Low | CNN | Single-phase CTA achieved an AUC of 0.74 with sensitivity of 77% and specificity of 71% Phases 1, 2, and 3 achieved greater AUC, sensitivity, and specificity and improved fit compared with single-phase CTA | |
| Su et al., 202062 | Netherlands (high) | Moderate | Low | Low | CNN | Accuracy of AUC of 0.8 and dichotomized collateral score accuracy of 0.9 Error comparable to interobserver variation and results comparable to 2 independent radiologists | |
| Computed Tomography | Fang et al., 202037 | China (upper middle) | Moderate | Unclear | Unclear | AdaBoost, ANN, SVM, RF | RF had the best performance Delay between stroke and randomization to treatment was related to infarct visible on computed tomography |
| Olive-Gadea et al., 202054 | Spain (high) | Moderate | Low | Low | CNN | AUC for the identification of LVO with Methinks LVO was 0.87, and improved to 0.91 with Methinks LVO+ | |
| Qiu et al., 202056 | Canada, Korea, Switzerland (high) | Moderate | Low | Low | CNN, RF | Algorithm-detected lesion volume correlated with the reference standard of expertcontoured lesion volume in acute DWI scans Mean difference between algorithmsegmented volume and the DWI volume was nonsignificant | |
| Vargas et al., 201965 | United States (high) | Moderate | Low | Low | CNN | Best model was able to achieve an accuracy of 85.8% on validation data AUC was 0.90 for right-sided deficits, 0.96 for left-sided deficits, and 0.93 for no deficits |
|
| You et al., 202072 | Hong Kong (high) | Moderate | Low | Low | RF, SVM, XGBoost | XGBoost model at the third level of evaluation achieved the best model performance on testing group Youden index, accuracy, sensitivity, specificity, F1 score, and AUC were 0.638, 0.800, 0.953, 0.684, 0.804, and 0.847, respectively |
|
| Miscellaneous | Erani et al., 202036 | United States (high) | Moderate | Low | Low | ANN | AUC of 0.864 and sensitivity of 76% at 80% specificity |
| Fitzgerald et al., 201939 | Canada, United States (high) | Moderate | High | Low | NA | Proportion of platelet-rich clots and percentage of platelet content higher in the large artery atherosclerosis group compared with the cardioembolic group Large artery atherosclerosis and cryptogenic cases had a similar proportion of platelet-rich clots |
|
| Keenan et al., 202046 | United States (high) | Moderate | Low | Low | NA | Cranial accelerometry was 65% sensitive and 87% specific. Adding asymmetric arm weakness increased specificity to 91% Exploratory analysis requiring asymmetric arm weakness before cranial accelerometry mode improved sensitivity to 91% and specificity to 93% and minimized false-positive and false-negative results | |
| Smith et al., 202060 | United States (high) | Moderate | Unclear | Unclear | NA | In most patients with LVO, head pulses showed little cardiac contraction correlation Using biometric data only, properly classified 15/19 patients with LVOs and 20/23 patients with non-LVO, with AUC of 0.79, sensitivity of 73%, and specificity of 87% |
|
| Thorpe et al., 202063 | United States (high) | Moderate | High | High | NA | Observed flow types provide the foundation for objective methods of real-time automated flow type classification | |
| Meier et al., 201949 | Switzerland (high) | Moderate | Low | Low | CNN | Strong correlations of lesion volumes and good spatial overlap of respective lesion segmentations between the CNN method and reference output CNN underestimated smaller lesion volumes, leading to disagreement between the CNN and reference method in 9% of patients |
|
| Subtype | Garg et al., 201940 | United States (high) | Moderate | Low | Low | Natural language processing with k-nearest neighbors, gradient boosting, RF, SVM, extra random trees, and XGBoost | Best machine-based classification achieved a k of 0.25 using radiology reports alone, 0.57 using progress notes alone, and 0.57 using combined data Machine-based classification agreed with rater classification in 40 of 50 cases (κ = 0.72) |
| Wu et al., 201970 | Multiple (high) | Moderate | Low | Low | CNN | Ensemble consisting of a mixture of large database and single-center CNNs performed best Automated and manual lesion determination correlated well |
SVM, support vector machine; AUC, area under the curve; mCTA, multiphase computed tomography angiography; NCCT, noncontrast computed tomography; CNN, convolutional neural network; CTA, computed tomography angiography; LVO, large-vessel occlusion; ANN, artificial neural network; RF, random forest; DWI, diffusion-weighted imaging; XGBoost, extreme gradient boost; NA, not applicable.
In addition to CT imaging, several other studies used ML techniques to assist in LVO detection on angiographic imaging. Five studies57–59,61,62 discussed identification of LVO on angiography (Table 2). Four of those 5 discussed CT angiography (CTA).58,59,61,62 Sheth et al.59 found that a CNN detected LVO with an AUC of 0.88 and determined the infarct core with an AUC of 0.88 for ischemic core ≤30 mL and 0.90 for ischemic core ≤50 mL. An additional study using a CNN62 found an accuracy of 0.80 for automatic collateral scoring from three-dimensional CTA images. Rava et al. determined that angiographic parameter imaging based on digital subtraction angiography provided accurate localization of the ischemic core in patients with LVO using an SVM.57 Besides CT and digital subtraction angiography, 6 other studies36,39,46,49,60,63 investigated an array of miscellaneous aspects ranging from atherosclerotic features of platelet-rich clot to cranial electroencephalography in detecting LVO (Table 2).
Diagnosis
Stroke Cause and Severity.
In addition to detecting LVO presence, other studies have used ML to diagnose the type and severity of stroke. Two studies40,70 described strategies to determine the subtype of stroke via the TOAST (Trial of Org 10172 in Acute Stroke Treatment) classification (Table 2). One study70 used CNN to segment lesion volumes from diffusion-weighted MRI, followed by regression to combine the segmented lesion with other clinical factors to predict stroke phenotype. This study reported that an ensemble consisting of a mixture of large databases and single-center CNNs performed best in determining the subtype of AIS with a precision of 0.83, and results from automatic and manual lesion volumes correlated well.70 In addition to these studies, 4 others41,42,50,51 used ML to predict the ASPECTS (Alberta Stroke Program Early CT Score) for LVO, as summarized in Table 3. Overall e-ASPECTS was correlated with both CTA collateral score as estimated by radiologists and e-CTA in identifying laterality of ischemia.41
Table 3.
Included Studies Regarding Alberta Stroke Program Early CT Score and Patient Selection
| Category | Reference | Country (Income Status) | Quality Grade | Risk of Bias | Concern for Applicability | Artificial Intelligence Techniques | Key Findings |
|---|---|---|---|---|---|---|---|
| ASPECTS | Grunwald et al., 201941 | Germany, United Kingdom (high) | Moderate | Unclear | Low | NA | Addition of e-CTA improved intraclass correlation coefficient among neuroradiologists Automated e-CTA, without neuroradiologist input, agreed with consensus score in 90% of scans with remaining 10% within 1 point of the consensus Sensitivity of 0.99 and specificity of 0.94 for identifying favorable collateral flow (collateral score 2–3) |
| Guberina et al., 201842 | Germany (high) | Moderate | Unclear | Low | NA | Higher interrater correlation coefficient of 0.71–0.80 with definite infarct core, compared with 0.59 for ASPECTS in the acute ischemic stroke setting | |
| Nagel et al., 201650 | Canada, Germany, United Kingdom, United States (high) | Moderate | Unclear | Low | NA | Two e-ASPECTS operating points were noninferior to all 3 neuroradiologists Matthews correlation coefficients for e-ASPECTS were higher than those of all neuroradiologists | |
| Neuhaus et al., 202051 | United States, United Kingdom (high) | Moderate | Low | Low | NA | Median ASPECTS was 9 for manual scoring and 8.5 for e-ASPECTS with κ of 0.248 When corrected for the low number of infarcts, κ ranged from 0.483 (insula) to 0.888 (M3), with greater agreement for cortical areas Intraclass correlation coefficients ranged from 0.09 (M1) to 0.556 (lentiform) | |
| Patient Selection | Alawieh et al., 201933 | Lebanon, United States (multiple) | Moderate | Low | Low | Regression tree | Sensitivity of 89.4% and specificity of 89.6% with AUC of 0.95 Negative predictive value was >95% Patients who were not selected by algorithm higher rates of symptomatic intracerebral hemorrhage after mechanical thrombectomy |
| Wang et al., 202069 | United States (high) | Moderate | Low | Low | Convolutional neural network, random forest, support vector machine | Voxel-wise AUC of 0.958, whereas other machine learning algorithms ranged from 0.897 to 0.933 Accuracy of 92%, with a sensitivity of 0.89 and specificity of 0.95, for retrospective determination for subject-level endovascular treatment eligibility Voxel-wise AUC of 0.94 and a subject-level accuracy of 92% for endovascular treatment eligibility |
NA, not applicable; CTA, computed tomography angiography; ASPECTS, Alberta Stroke Program Early CT Score; AUC, area under the curve.
Patient Selection
Two studies33,69 discussed the use of ML tools in the selection of patients for MT (Table 3). One study69 used a CNN with arterial spin labeling data and found an accuracy of 92% for retrospective determination for individual patient eligibility for endovascular treatment. Another study33 using a regression tree with a retrospective cohort of patients who underwent MT for LVO found that the regression tree performed better than the NIHSS, ASPECTS, or baseline deficits for selecting elderly patients for MT. Patients who were not selected for MT by the regression tree had higher rates of symptomatic intracerebral hemorrhage.33 Another study49 (previously mentioned) found that dynamic susceptibility perfusion imaging feasibly assessed eligibility for late-window reperfusion treatment.
Outcome Prediction
For LVO outcome prediction, several studies used ML techniques to predict both clinical and angiographic outcomes on imaging. Eleven studies34,38,43,45,47,48,52,53,55,64,66 used ML to predict clinical outcomes after MT (Table 4). Seven of these studies34,38,43,47,52,53,64 used mRS, a widely accepted functional independence score scale after a stroke, as an outcome variable. One study using ANN and RF43 found that a small infarct core was associated with mRS score of 0–2, and this outcome prediction only slightly improved when imaging data were added. Another study34 using a gradient boosting algorithm found that the most important parameters for predicting mRS score at 90 days were NIHSS score after 24 hours, premorbid mRS score, and final infarction volume on postinterventional CT after 18–36 hours. The 3 remaining studies compared ML algorithms with other methods. Two found that ML models were superior to conventional statistical, other pretreatment models, and ischemic core volumes in predicting 90-day mRS score 0–2, whereas the remaining study found no difference between the best performing ML algorithm and best performing logistic regression model.52,53,64
Table 4.
Included Studies on Outcome Prediction
| Reference | Country (Income Status) | Study Design | Quality Grade | Risk of Bias | Concern for Applicability | Artificial Intelligence Techniques | Key Findings |
|---|---|---|---|---|---|---|---|
| Brugnara et al., 202034 | Germany (high) | Retrospective cohort | Moderate | High | Low | GB | Baseline clinical and conventional imaging characteristics predicted mRS at 90 days with AUC of 0.740 and accuracy of 0.711 Most important parameters for predicting mRS at 90 days were National Institutes of Health Stroke Scale after 24 hours, premorbid mRS score, and final infarction volume on postinterventional computed tomography after 18–36 hours |
| Fiehler et al., 201838 | Canada, Germany (high) | Prospective cohort | Moderate | High | Low | GB | Infarct volume smaller than predicted by intravenous tissue plasminogen activator therapy model, with a median volume of saved tissue of 50 mL mRS score 0–2 at 90 days was observed in 70% TICI2b/3 recanalization rate was 95% |
| Hamann et al., 202043 | Switzerland (high) | Retrospective cohort | Moderate | Low | Low | ANN, RF | Rate of successful recanalization was 78%, with 54% patients having a mRS score 0–2 Small infarct core associated with mRS score 0–2 Outcome prediction improved only slightly when imaging was added |
| Hofmeister et al., 202045 | Switzerland (high) | Mixed | Moderate | Low | Low | SVM | Small subset of radiomic features (n = 9) predicted first-attempt recanalization with thromboaspiration with AUC of 0.88 |
| Kral et al., 202047 | Czechia (high) | Prospective cohort | Moderate | Unclear | Low | Not applicable | Median NCCT ischemic lesion volume was 23 mL and median DWI ischemic lesion volume was 11.5 mL in the TICI2b-3 group NCCT and DWI ischemic lesion volumes lower in patients with mRS score 0–2 High correlation and accuracy in detection of follow-up ischemic changes in particular ASPECTS regions |
| Nishi et al., 201952 | Japan (high) | Retrospective cohort | Moderate | Low | Low | RF, SVM | AUC of RF, which was the worst among the machine learning algorithms, was higher than standard statistical model and best model among the previously reported pretreatment scoring methods |
| Nishi et al., 202053 | Japan (high) | Retrospective cohort | Moderate | Low | Low | CNN | CNN model showed the highest AUC: 0.81 ± 0.06 compared with 0.63 ± 0.05 for ASPECTS and 0.64 ± 0.05 for ischemic core volume model AUC for CNN was significantly superior to other 2 models |
| Qiu et al., 201955 | Canada (high) | Retrospective cohort | Moderate | Low | Low | SVM | Thrombus radiomics features predict of early recanalization with IV alteplase Combination of radiomics features from NCCT, computed tomography angiography, and radiomics changes is best associated with early recanalization with IV alteplase and better than a single feature |
| Van Os et al., 201864 | Netherlands (high) | Prospective cohort | Moderate | Low | Low | ANN, RF, super learner, SVM | All models performed poorly in predicting good reperfusion and moderately in predicting 3 months functional independence using baseline variables All models performed well in predicting 3 months functional independence using both baseline and treatment variables |
| Velasco Gonzalez et al., 202066 | Germany (high) | Retrospective cohort | Moderate | Low | Low | Decision tree | Regression models and decision tree results concurred: carotid tortuosity was the main moderator of efficacy, reducing likelihood of first-pass mTICI 3%–30% Balloon guide catheters located in the distal ICA had a 70% probability of complete recanalization after one pass and 43% if located in the proximal internal carotid artery |
| Martha et al., 201948 | United States (high) | Prospective cohort | Moderate | Low | Low | RF | Genes and subject factors CCR4, IFNA2, IL-9, CXCL3, Age, T2DM, IL-7, CCL4, BMI, IL-5, CCR3, TNFα, and IL-27 predicted infarct volume Genes and subject factor IFNA2, IL-5, CCL11, IL-17C, CCR4, IL-9, IL-7, CCR3, IL-27, T2DM, and CSF2 predicted edema volume Overlap of genes CCR4, IFNA2, IL-9, IL-7, IL-5, CCR3, and IL-27 with T2DM predicted both infarct and edema volumes |
GB, gradient boosting; mRS, modified Rankin Scale; AUC, area under curve; TICI, thrombolysis in cerebral infarction; ANN, artificial neural network; RF, random forest; SVM, support vector machine; NCCT, noncontrast computed tomography; DWI, diffusion-weighted imaging; CNN, convolutional neural network; ASPECTS, Alberta Stroke Program Early CT Score; IV, intravenous.
In addition to clinical outcomes, prediction of vessel recanalization and angiographic outcomes after an MT have also been investigated using ML tools.38,43,45,47,55,66 Three studies38,43,47 found rates of TICI2b/3 recanalization ranging from 71% to 95%. An additional study66 found that both decision trees and regression models indicated that carotid tortuosity was the main moderator of efficacy of MT. One study55 found that a combination of radiomic features from noncontrast CT, CTA, and differences between CT and CTA predicted early recanalization better than any single feature. One radiomic study45 using SVM found 9 features that predicted first-pass recanalization and the overall number of passes needed for successful recanalization.
DISCUSSION
LVO represents a particularly important subset of AIS for AI applications because of the high morbidity and mortality compared with other types of AIS.8–10 ML algorithms and deep learning can improve the timely triage, diagnosis, patient selection, and prognostication in patients with LVO stroke. In this review, we evaluate current evidence and present a systematic review regarding AI applications for LVO in triage, diagnosis, patient selection, and outcome prediction.
Triage and Diagnosis
Advantages of AI are best seen in its applicability in terms of triage and diagnosis of patients with LVO. One of the most important factors in predicting good patient outcome is the timely arrival and triage of probable patients with stroke. It is for this reason that most triage AI applications, as shown in our review, have focused mainly on prehospital transport, given the prognostic importance of timely arrival.67,68,71 Whether a patient is transported to a primary stroke center first or directly to a comprehensive stroke center relies on the risk of LVO and transport time.67,68,71 A study reported by Hassan et al.44 showed the application of an AI-based program called Viz.ai designed to help triage patients with LVO stroke and reduce transfer times. Patients were divided into 2 cohorts: with and without AI program implementation. After implementation of this AI program, the median CTA to door-in time was significantly reduced by an average of 22.5 minutes (132.5 minutes vs. 110 minutes; P = 0.0470). AI-based programs such as Viz.ai serve as excellent real-world examples of AI application that is not only helpful to surgeons and interventionists in their daily practice but also significantly affects the sequence of events and outcomes after an LVO. These programs may further improve triage by synchronizing stroke care in the future.44
In terms of diagnosis, AI has primarily been used to accurately identify LVO on CT using ML that identifies clot sign or infarction volume, although the input of additional information such as demographics and clinical factors improves accuracy.54,56,72 Several studies58,59,61 have also shown that ML techniques can also accurately detect LVO on CTA. All these ML models serve as vital tools in the diagnosis of LVO stroke, although existing methods of diagnosis are highly accurate.73
Patient Selection
Use of AI in patient selection and prognostication by predicting clinical and angiographic outcomes of MT for LVO is one of the most important applications of ML. A recent study reported by Ding et al.26 provides an excellent example of AI applicability in terms of outcome prediction whereby 6 variables from Acute Stroke Registry data were used to train a deep neural network model to accurately predict 3-month mRS score better than the traditional Acute Stroke Registry and Analysis of Lausanne score (AUC, 0.888 vs. 0.839; P < 0.001). Several other studies in our review34,38,43,47,52,53,64 show that ML algorithms predict favorable functional outcomes (mRS score 0–2) after MT and identify associated factors including a small infarct core, NIHSS score after 24 hours, premorbid mRS score, and infarction volume on postinterventional CT. All the formerly mentioned metrics can serve a critical role in not only patient selection but also predicting how well the patients are going to do after an intervention. A combination of radiomic features from various imaging modalities also provides useful prediction of recanalization.45,55,66 The Eric Acute Stroke Recanalization study used a predictive analytics end point, the volume of saved tissue, potentially setting the stage for future studies on LVO using predictive analytics end points as the applicability of ML continues to be investigated.38
Future Direction
The role of AI in LVO stroke is expanding; however, several strides regarding critical decision making and patient selection need to be made. For example, superior applications and ML algorithms are required to identify which patients are better candidates for stent retriever thrombectomy versus aspiration versus both stent retriever and aspiration approach (SOLUMBRA). Another area of interest would be to identify which patients are at higher risk of symptomatic intracerebral hemorrhage after a thrombectomy. Hence, the goal is to focus on aspects related to optimization of treatment such as patient selection and outcome prediction and to be able to select an ideal set of patients who would not only fit the inclusion criteria for an intervention but also have the least number and severity of complications.
As we march toward an era in which clinical decision making continues to be more reliant on proven ML algorithms, it is important to be aware of the potential drawbacks and unintended consequences of these models. Cabitza et al.74 recently highlighted some of the unintended consequences that may occur as ML expands its footprint into all areas of medicine. One of the potential shortcomings highlighted lies in the intrinsic uncertain nature of medicine. Medical interpretations and observations that are an essential part of the input to optimize ML models are not usually considered for interoperator variability.74 The quality and variety of input data can substantially affect the performance of an ML model based on the population intrinsic metrics; this factor could mean that 1 ML model that may be perfect for 1 population may not be so ideal in a different cohort. Hence, it is important that the quality of any ML model, and its subsequent adoption in medical practice, be grounded in not only its performance metrics but also proof of clinical improvement and good outcomes.
Limitations
Our study has limitations. Studies included in this review used a variety of methods and ML algorithms, preventing determination of the optimal ML algorithm, specifically for patients with LVO. No meta-analysis was conducted because of the heterogeneity of study designs, ML models, and outcomes.
CONCLUSIONS
Although AI is useful for the triage, diagnosis, patient selection, and outcome prediction for LVO, applications remain largely at the investigational stage. Most literature has focused on the diagnosis, showing reasonably high accuracy. Priorities for future applications of AI should shift beyond diagnosis to optimization of treatment via more nuanced selection of patients for MT and associated prediction of clinical and angiographic outcomes. Above all, applications of AI to LVO must be clinically translatable.
Supplementary material
Abbreviations and Acronyms
- AI
Artificial intelligence
- AIS
Acute ischemic stroke
- ANN
Artificial neural network
- ASPECTS
Alberta Stroke Program Early CT Score
- AUC
Area under the curve
- CNN
Convolutional neural network
- CT
Computed tomography
- CTA
Computed tomography angiography
- GB
Gradient boosting
- LVO
Large-vessel occlusion
- mCTA
multiphase computed tomography angiography
- mRS
modified Rankin Scale
- MT
Mechanical thrombectomy
- NIHSS
National Institutes of Health Stroke Scale
- RF
Random forest
- SVM
Support vector machine
Footnotes
Conflict of interest statement: competing interests: disclosure of relationships/potential conflicts of interest: N.A.S., M.W., T.R.P., R.H.D., M.W., J.M.C., A.A.B., and V.M.T.: none. E.I.L. has shareholder/ownership interests in NeXtGen Biologics, RAPID Medical, Claret Medical, Cognition Medical, Imperative Care (formerly the Stroke Project), Rebound Therapeutics, StimMed, and Three Rivers Medical; serves as National Principal Investigator or on Steering Committees for Medtronic (merged with Covidien Neurovascular) SWIFT Prime and SWIFT Direct Trials; receives honoraria from Medtronic (training and lectures); is a consultant for Claret Medical, GLG Consulting, Guidepoint Global, Imperative Care, Medtronic, Rebound, and StimMed; serves on the advisory board for Stryker (AIS Clinical Advisory Board), NeXtGen Biologics, MEDX, Cognition Medical, and Endostream Medical; and is Site Principal Investigator for the CONFIDENCE study (MicroVention), STRATIS Study–Sub I (Medtronic). A.H.S. has financial interest/investor/stock options/ownership in Adona Medical, Inc, Amnis Therapeutics (purchased by Boston Scientific, October 2017), Blink TBI Inc., Buffalo Technology Partners Inc., Cerebrotech Medical Systems, Inc., Cognition Medical, Endostream Medical Ltd., Imperative Care, International Medical Distribution Partners, Neurovascular Diagnostics Inc., Q’Apel Medical Inc, Rebound Therapeutics Corp. (purchased in 2019 by Integra Lifesciences Corp.), Rist Neurovascular Inc., Sense Diagnostics, Inc., Serenity Medical Inc., Silk Road Medical, Spinnaker Medical, Inc., StimMed, Synchron, Three Rivers Medical Inc., Vastrax, LLC, VICIS, Inc., ViseonInc; serves as a consultant/advisory board member for Amnis Therapeutics, Boston Scientific, Canon Medical Systems USA Inc., Cerebrotech Medical Systems Inc., Cerenovus, Corindus Inc., Endostream Medical Ltd., Imperative Care, Inc. Integra LifeSciences Corp., Medtronic, MicroVention, MinnetronixNeuro, Inc., Northwest University–DSMB Chair for HEAT Trial, Penumbra, Q’Apel Medical Inc., Rapid Medical, Rebound Therapeutics Corp. (purchased by Integra LifeSciences Corp.), Serenity Medical Inc., Silk Road Medical, StimMed, Stryker, Three Rivers Medical, Inc., VasSol, and W.L. Gore & Associates; and is principal investigator/steering committee member for the following trials: Cerenovus NAPA and ARISE II; Medtronic SWIFT PRIME and SWIFT DIRECT; MicroVention FRED & CONFIDENCE; MUSC POSITIVE; and Penumbra 3D Separator, COMPASS, INVEST, TIGER.
REFERENCES
- 1.Brott T, Bogousslavsky J. Treatment of acute ischemic stroke. N Engl J Med. 2000;343:710–722. [DOI] [PubMed] [Google Scholar]
- 2.Virani SS, Alonso A, Aparicio HJ, et al. Heart Disease and Stroke Statistics–2021 Update: A Report From the American Heart Association. Circulation. 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 3.Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaartjes I, O’Flaherty M, Capewell S, Kappelle J, Bots M. Remarkable decline in ischemic stroke mortality is not matched by changes in incidence. Stroke. 2013;44:591–597. [DOI] [PubMed] [Google Scholar]
- 5.Koton S, Schneider AL, Rosamond WD, et al. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA. 2014;312: 259–268. [DOI] [PubMed] [Google Scholar]
- 6.Lakomkin N, Dhamoon M, Carroll K, et al. Prevalence of large vessel occlusion in patients presenting with acute ischemic stroke: a 10-year systematic review of the literature. J Neurointerv Surg. 2019;11:241–245. [DOI] [PubMed] [Google Scholar]
- 7.Rai AT, Seldon AE, Boo S, et al. A population-based incidence of acute large vessel occlusions and thrombectomy eligible patients indicates significant potential for growth of endovascular stroke therapy in the USA. J Neurointerv Surg. 2017; 9:722–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu W, Churilov L, Campbell BCV, et al. Does large vessel occlusion affect clinical outcome in stroke with mild neurologic deficits after intravenous thrombolysis? J Stroke Cerebrovasc Dis. 2014;23: 2888–2893. [DOI] [PubMed] [Google Scholar]
- 9.Smith WS, Tsao JW, Billings ME, et al. Prognostic significance of angiographically confirmed large vessel intracranial occlusion in patients presenting with acute brain ischemia. Neurocrit Care. 2006; 4:14–17. [DOI] [PubMed] [Google Scholar]
- 10.Smith WS, Lev MH, English JD, et al. Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke. 2009;40: 3834–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372: 11–20. [DOI] [PubMed] [Google Scholar]
- 12.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 13.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. [DOI] [PubMed] [Google Scholar]
- 14.Bracard S, Ducrocq X, Mas JL, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15: 1138–1147. [DOI] [PubMed] [Google Scholar]
- 15.Mocco J, Zaidat OO, von Kummer R, et al. Aspiration thrombectomy after intravenous alteplase versus intravenous alteplase alone. Stroke. 2016;47: 2331–2338. [DOI] [PubMed] [Google Scholar]
- 16.Muir KW, Ford GA, Messow CM, et al. Endovascular therapy for acute ischaemic stroke: the Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE) randomised, controlled trial. J Neurol Neurosurg Psychiatry. 2017;88:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell BCV, Donnan GA, Lees KR, et al. Endovascular stent thrombectomy: the new standard of care for large vessel ischaemic stroke. Lancet Neurol. 2015;14:846–854. [DOI] [PubMed] [Google Scholar]
- 18.Palaniswami M, Yan B. Mechanical thrombectomy is now the gold standard for acute ischemic stroke: implications for routine clinical practice. Interv Neurol. 2015;4:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turk AS 3rd, Siddiqui A, Fifi JT, et al. Aspiration thrombectomy versus stent retriever thrombectomy as first-line approach for large vessel occlusion (COMPASS): a multicentre, randomised, open label, blinded outcome, non-inferiority trial. Lancet. 2019;393:998–1008. [DOI] [PubMed] [Google Scholar]
- 20.Waqas M, Kuo CC, Dossani RH, et al. Mechanical thrombectomy versus intravenous thrombolysis for distal large-vessel occlusion: a systematic review and meta-analysis of observational studies. Neurosurg Focus. 2021;51:E5. [DOI] [PubMed] [Google Scholar]
- 21.Monteiro A, Cortez GM, Waqas M, et al. Comparison of effectiveness and outcomes among different thrombectomy techniques in acute basilar artery occlusion: a dual-center experience. Neurosurg Focus. 2021;51:E8. [DOI] [PubMed] [Google Scholar]
- 22.Lin MP, Tsivgoulis G, Alexandrov AV, Chang JJ. Factors affecting clinical outcome in large-vessel occlusive ischemic strokes. Int J Stroke. 2015;10: 479–484. [DOI] [PubMed] [Google Scholar]
- 23.Smith EE, Kent DM, Bulsara KR, et al. Accuracy of prediction instruments for diagnosing large vessel occlusion in individuals with suspected stroke: a systematic review for the 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke. Stroke. 2018;49:e111–e122. [DOI] [PubMed] [Google Scholar]
- 24.Mouridsen K, Thurner P, Zaharchuk G. Artificial intelligence applications in stroke. Stroke. 2020;51: 2573–2579. [DOI] [PubMed] [Google Scholar]
- 25.Saver JL. Time is brain–quantified. Stroke. 2006;37:263–266. [DOI] [PubMed] [Google Scholar]
- 26.Ding L, Liu C, Li Z, Wang Y. Incorporating artificial intelligence into stroke care and research. Stroke. 2020;51:e351–e354. [DOI] [PubMed] [Google Scholar]
- 27.Bivard A, Churilov L, Parsons M. Artificial intelligence for decision support in acute stroke–current roles and potential. Nat Rev Neurol. 2020; 16:575–585. [DOI] [PubMed] [Google Scholar]
- 28.Leslie-Mazwi TM, Lev MH. Towards artificial intelligence for clinical stroke care. Nat Rev Neurol. 2020;16:5–6. [DOI] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Bank Country and Lending Groups. The World Bank Group. Available at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed December 22, 2021. [Google Scholar]
- 31.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolff RF, Moons KGM, Riley RD, et al. PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med. 2019;170:51–58. [DOI] [PubMed] [Google Scholar]
- 33.Alawieh A, Zaraket F, Alawieh MB, Chatterjee AR, Spiotta A. Using machine learning to optimize selection of elderly patients for endovascular thrombectomy. J Neurointerv Surg. 2019;11:847–851. [DOI] [PubMed] [Google Scholar]
- 34.Brugnara G, Neuberger U, Mahmutoglu MA, et al. Multimodal predictive modeling of endovascular treatment outcome for acute ischemic stroke using machine-learning. Stroke. 2020;51:3541–3551. [DOI] [PubMed] [Google Scholar]
- 35.Chen Z, Zhang R, Xu F, et al. Novel prehospital prediction model of large vessel occlusion using artificial neural network. Front Aging Neurosci. 2018; 10:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erani F, Zolotova N, Vanderschelden B, et al. Electroencephalography might improve diagnosis of acute stroke and large vessel occlusion. Stroke. 2020;51:3361–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang G, Liu W, Wang L. A machine learning approach to select features important to stroke prognosis. Comput Biol Chem. 2020;88:107316. [DOI] [PubMed] [Google Scholar]
- 38.Fiehler J, Thomalla G, Bernhardt M, et al. ERASER . Stroke. 2019;50:1275–1278. [DOI] [PubMed] [Google Scholar]
- 39.Fitzgerald S, Dai D, Wang S, et al. Platelet-rich emboli in cerebral large vessel occlusion are associated with a large artery atherosclerosis source. Stroke. 2019;50:1907–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garg R, Oh E, Naidech A, Kording K, Prabhakaran S. Automating ischemic stroke subtype classification using machine learning and natural language processing. J Stroke Cerebrovasc Dis. 2019;28:2045–2051. [DOI] [PubMed] [Google Scholar]
- 41.Grunwald IQ, Kulikovski J, Reith W, et al. Collateral automation for triage in stroke: evaluating automated scoring of collaterals in acute stroke on computed tomography scans. Cerebrovasc Dis. 2019;47:217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guberina N, Dietrich U, Radbruch A, et al. Detection of early infarction signs with machine learning-based diagnosis by means of the Alberta Stroke Program Early CT score (ASPECTS) in the clinical routine. Neuroradiology. 2018;60:889–901. [DOI] [PubMed] [Google Scholar]
- 43.Hamann J, Herzog L, Wehrli C, et al. Machine-learning-based outcome prediction in stroke patients with middle cerebral artery-M1 occlusions and early thrombectomy. Eur J Neurol. 2021;28: 1234–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hassan AE, Ringheanu VM, Rabah RR, Preston L, Tekle WG, Qureshi AI. Early experience utilizing artificial intelligence shows significant reduction in transfer times and length of stay in a hub and spoke model. Interv Neuroradiol. 2020;26:615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hofmeister J, Bernava G, Rosi A, et al. Clot-based radiomics predict a mechanical thrombectomy strategy for successful recanalization in acute ischemic stroke. Stroke. 2020;51:2488–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keenan KJ, Lovoi PA, Smith WS. The neurological examination improves cranial accelerometry large vessel occlusion prediction accuracy. Neurocrit Care. 2021;35:103–112. [DOI] [PubMed] [Google Scholar]
- 47.Kral J, Cabal M, Kasickova L, et al. Machine learning volumetry of ischemic brain lesions on CT after thrombectomy-prospective diagnostic accuracy study in ischemic stroke patients. Neuroradiology. 2020;62:1239–1245. [DOI] [PubMed] [Google Scholar]
- 48.Martha SR, Cheng Q, Fraser JF, et al. Expression of cytokines and chemokines as predictors of stroke outcomes in acute ischemic stroke. Front Neurol. 2019;10:1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meier R, Lux P, Med B, et al. Neural network-derived perfusion maps for the assessment of lesions in patients with acute ischemic stroke. Radiol Artif Intell. 2019;1:e190019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagel S, Sinha D, Day D, et al. e-ASPECTS software is non-inferior to neuroradiologists in applying the ASPECT score to computed tomography scans of acute ischemic stroke patients. Int J Stroke. 2017;12:615–622. [DOI] [PubMed] [Google Scholar]
- 51.Neuhaus A, Seyedsaadat SM, Mihal D, et al. Region-specific agreement in ASPECTS estimation between neuroradiologists and e-ASPECTS software. J Neurointerv Surg. 2020;12:720–723. [DOI] [PubMed] [Google Scholar]
- 52.Nishi H, Oishi N, Ishii A, et al. Predicting clinical outcomes of large vessel occlusion before mechanical thrombectomy using machine learning. Stroke. 2019;50:2379–2388. [DOI] [PubMed] [Google Scholar]
- 53.Nishi H, Oishi N, Ishii A, et al. Deep learning-derived high-level neuroimaging features predict clinical outcomes for large vessel occlusion. Stroke. 2020;51:1484–1492. [DOI] [PubMed] [Google Scholar]
- 54.Olive-Gadea M, Crespo C, Granes C, et al. Deep learning based software to identify large vessel occlusion on noncontrast computed tomography. Stroke. 2020;51:3133–3137. [DOI] [PubMed] [Google Scholar]
- 55.Qiu W, Kuang H, Nair J, et al. Radiomics-based intracranial thrombus features on CT and CTA predict recanalization with intravenous alteplase in patients with acute ischemic stroke. AJNR Am J Neuroradiol. 2019;40:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiu W, Kuang H, Teleg E, et al. Machine learning for detecting early infarction in acute stroke with non-contrast-enhanced CT. Radiology. 2020;294: 638–644. [DOI] [PubMed] [Google Scholar]
- 57.Rava RA, Mokin M, Snyder KV, et al. Performance of angiographic parametric imaging in locating infarct core in large vessel occlusion acute ischemic stroke patients. J Med Imaging (Bellingham). 2020;7:016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reid M, Famuyide AO, Forkert ND, et al. Accuracy and reliability of multiphase CTA perfusion for identifying ischemic core. Clin Neuroradiol. 2019;29: 543–552. [DOI] [PubMed] [Google Scholar]
- 59.Sheth SA, Lopez-Rivera V, Barman A, et al. Machine learning-enabled automated determination of acute ischemic core from computed tomography angiography. Stroke. 2019;50:3093–3100. [DOI] [PubMed] [Google Scholar]
- 60.Smith WS, Keenan KJ, Lovoi PA. A unique signature of cardiac-induced cranial forces during acute large vessel stroke and development of a predictive model. Neurocrit Care. 2020;33:58–63. [DOI] [PubMed] [Google Scholar]
- 61.Stib MT, Vasquez J, Dong MP, et al. Detecting large vessel occlusion at multiphase CT angiography by using a deep convolutional neural network. Radiology. 2020;297:640–649. [DOI] [PubMed] [Google Scholar]
- 62.Su J, Wolff L, van Es A, et al. Automatic collateral scoring from 3D CTA images. IEEE Trans Med Imaging. 2020;39:2190–2200. [DOI] [PubMed] [Google Scholar]
- 63.Thorpe SG, Thibeault CM, Canac N, et al. Toward automated classification of pathological transcranial Doppler waveform morphology via spectral clustering. PLoS One. 2020;15:e0228642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Os HJA, Ramos LA, Hilbert A, et al. Predicting outcome of endovascular treatment for acute ischemic stroke: potential value of machine learning algorithms. Front Neurol. 2018;9:784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vargas J, Spiotta A, Chatterjee AR. Initial experiences with artificial neural networks in the detection of computed tomography perfusion deficits. World Neurosurg. 2018. 10.1016/j.wneu.2018.10.084. Accessed October 24, 2018. [DOI] [PubMed] [Google Scholar]
- 66.Velasco Gonzalez A, Görlich D, Buerke B, et al. Predictors of successful first-pass thrombectomy with a balloon guide catheter: results of a decision tree analysis. Transl Stroke Res. 2020;11:900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Venema E, Lingsma HF, Chalos V, et al. Personalized prehospital triage in acute ischemic stroke. Stroke. 2019;50:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Venema E, Burke JF, Roozenbeek B, et al. Prehospital triage strategies for the transportation of suspected stroke patients in the United States. Stroke. 2020;51:3310–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang K, Shou Q, Ma SJ, et al. Deep learning detection of penumbral tissue on arterial spin labeling in stroke. Stroke. 2020;51:489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu O, Winzeck S, Giese AK, et al. Big data approaches to phenotyping acute ischemic stroke using automated lesion segmentation of multi-center magnetic resonance imaging data. Stroke. 2019;50:1734–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu Y, Parikh NS, Jiao B, Willey JZ, Boehme AK, Elkind MSV. Decision analysis model for pre-hospital triage of patients with acute stroke. Stroke. 2019;50:970–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.You J, Tsang ACO, Yu PLH, et al. Automated hierarchy evaluation system of large vessel occlusion in acute ischemia stroke. Front Neuroinform. 2020; 14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lev MH, Farkas J, Rodriguez VR, et al. CT angiography in the rapid triage of patients with hyperacute stroke to intraarterial thrombolysis: accuracy in the detection of large vessel thrombus. J Comput Assist Tomogr. 2001;25:520–528. [DOI] [PubMed] [Google Scholar]
- 74.Cabitza F, Rasoini R, Gensini GF. Unintended consequences of machine learning in medicine. JAMA. 2017;318:517–518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


