Abstract
For centuries, regular exercise has been acknowledged as a potent stimulus to promote, maintain, and restore healthy functioning of nearly every physiological system of the human body. With advancing understanding of the complexity of human physiology, continually evolving methodological possibilities, and an increasingly dire public health situation, the study of exercise as a preventative or therapeutic treatment has never been more interdisciplinary, or more impactful. During the early stages of the NIH Common Fund Molecular Transducers of Physical Activity Consortium (MoTrPAC) Initiative, the field is well-positioned to build substantially upon the existing understanding of the mechanisms underlying benefits associated with exercise. Thus, we present a comprehensive body of the knowledge detailing the current literature basis surrounding the molecular adaptations to exercise in humans to provide a view of the state of the field at this critical juncture, as well as a resource for scientists bringing external expertise to the field of exercise physiology. In reviewing current literature related to molecular and cellular processes underlying exercise-induced benefits and adaptations, we also draw attention to existing knowledge gaps warranting continued research effort.
Introduction
To date, regular physical activity (bodily movement which results in energy expenditure) is one of the most efficacious methods of improving and maintaining human health. Physical activity impacts a wide variety of physiological systems and improves health and health-related quality of life across a wide variety of clinical conditions. In particular, physical exercise [a subset of physical activity that is planned, structured, repetitive, and performed with the intent to improve health or fitness (301)] demonstrates the greatest benefits; these often present in a linear, dose-dependent fashion (1396). The health benefits of physical exercise are wide-ranging, impacting numerous physiological systems and influencing the progression of chronic conditions including (but not limited to) cardiovascular disease (CVD), neurocognitive decline, psychological disorders, musculoskeletal disorders, metabolic syndrome, type 2 diabetes (T2D), some forms of cancer, and all-cause mortality (968, 1342). Thus, it is well-established that exercise is a powerful intervention to improve various aspects of health throughout the lifespan.
Despite the tremendous health value of exercise, several challenges persist in harnessing the full potential of exercise as a therapeutic for the individual and in reducing healthcare-related costs at the societal level. Unfortunately, only a minority of individuals meet minimum guidelines for exercise participation in the United States and in developed countries worldwide (1056, 1333). Compounding this problem, a significant portion of individuals do not even receive basic exercise/physical activity recommendations from their healthcare provider(s) (572, 940), despite increasing evidence that inactivity and physical deconditioning should be considered as a unique risk factor in medical decision making (657, 776). Given these issues, implementation of successes from exercise clinical trials into clinical practice and community programs has remained largely elusive.
In addition to focusing efforts on increasing adherence/implementation, a significant opportunity exists to understand how exercise improves health (74). While it is known that exercise impacts a vast array of physiological functions and pathobiological risks, many questions remain regarding the cellular and molecular mechanisms driving these effects. A pharmaceutical that fully mimics the wide-ranging benefits of exercise across physiological health and other domains of wellness is unlikely to exist (248, 483, 536, 1397). Still, because of its multipotent effects on the body’s organ systems, exercise has been proposed by several investigators as a “polypill” for improving health (406, 1006, 1442). As outlined previously (74), opportunities surrounding an enhanced understanding of the mechanisms underlying the health benefits of exercise may lead to improving exercise prescriptions based on individual characteristics that influence the extent of exercise adaptations (i.e., “exercise responsiveness”), optimization of exercise as a therapy, and development or repurposing of adjuvant pharmaceuticals to enhance exercise tolerance in the presence of a comorbid condition.
In view of these expansive opportunities, the National Institutes of Health (NIH), via the NIH Director’s Common Fund, recently funded a landmark study known as the Molecular Transducers of Physical Activity Consortium (MoTrPAC) (1146). The stated aim of MoTrPAC is to: “catalogue the biological molecules affected by exercise in people, to assemble a comprehensive map of the molecular changes that occur in response to movement and, when possible, relate these changes to the benefits of physical activity. This molecular map will contain the many molecular signals that transmit the health effects of physical activity, and indicate how they are altered by age, sex, body composition, fitness level, and exposure to exercise. The program also aims to develop a user-friendly database that any researcher can access to develop hypotheses regarding the mechanisms whereby physical activity improves or preserves health, facilitating investigator-initiated studies and catalyzing the field of physical activity research (1146).” The MoTrPAC study is expected to generate an abundance of new data related to the fundamental molecular biology of human physiologic responses to exercise in several body tissues. Still, this endeavor is only getting underway; thus meaningful outputs of the study remain years away. The objectives of the present article are thus to assemble a compendium of the current state of knowledge surrounding the biological responses and adaptations to exercise in humans, to provide a comprehensive contextual resource for newcomers to the field, and to outline potential opportunities for advancing the field in the decades to come.
A Brief History of the Field
Exercise as ancient medicine
The history of exercise physiology overlaps considerably with that of human medicine. In a recent review of the contributions of ancient civilizations to the American College of Sports Medicine’s ongoing “Exercise is Medicine” initiative (1312), Charles Tipton (a recognized leader in the field of exercise physiology) stated that physicians in many early civilizations prescribed exercise, believing that it could promote health and avoid diseases. This belief is thought to date back nearly 3000 years BCE. Evidence suggests that “medical gymnastic” breathing exercises were often prescribed in China during this period (1312). In approximately 7th century BCE, ancient Indian physician Susruta declared that exercise “should be taken daily,” depending on the health and state of the individual (1313). Interestingly, even in these early days, excessive exercise was warned against, for fear of exhaustion or even death (1312). Nonetheless, the fact that bodily movement was touted as prevention for disease and promotion of healthy aging across geographies and ethnic groups demonstrates the fundamental human “drive to move.” Thus it is not surprising, as later philosophers famously noted, that disease follows when this drive is resisted or ignored.
Historians seem to agree that the ancient Greek and Roman civilizations contributed most significantly to the foundation of the field. Herodicus (ca. 500 BCE) is often called the “Father of Sports Medicine” for his approach to integrate physical fitness (e.g., Greek gymnasiums) with medicine (116, 455). Hippocrates (460–370 BCE), a contested student of Herodicus, continued this practice and is credited with composing a detailed exercise prescription to aid a diseased patient. Ultimately, Galen (130–210 CE), a Greek physician who lived in the Roman Empire, drew from the teachings of his forebears and created a lasting impact on the world’s culture by imprinting exercise into prescription for numerous diseases (116). He described the “naturals” (healthy bodily processes), “nonnaturals” (external stimuli such as activity and diet that bring peace and health), and the “contra-naturals” that disrupt them. Galen also distinguished exercise from movement in general by its vigorous nature requiring noticeable exertion, a critical distinction relevant to describing and prescribing exercise versus general physical activity guidelines. Galen’s influence is believed to have lasted at least 14 centuries (1312).
The Renaissance
Medical advancements throughout the Renaissance period (sometimes called the Scientific Revolution) greatly enhanced the understanding of human health. In 1543, Andreas Vesalius published the first human anatomy text, De Humani Corpus Fabrica. This text, along with Vesalius’ other contributions, is credited with revolutionizing the medical community’s understanding of cardiovascular, neural, and musculoskeletal anatomy (152, 887, 936). His unique approaches to dissection, comparative anatomy, and pedagogy (1196) earned him the moniker “Father of Modern Anatomy” and are believed to have been pivotal in surgical practice (1349). Shortly thereafter, Santorio Santorio (1561–1636), his name often written as variations on this theme, studied the basics of human metabolism (364). He famously constructed a life-size balance and tracked changes in his body mass with perturbations brought on by daily living (291). Through this work, Santorio laid the foundation for studies of metabolism, nutrition, and dietetics, and he further contributed to exercise physiology through his documentation of perspiration (291). The Scientific Revolution period also saw William Harvey publish insights into the mechanics of the human circulatory system in 1628’s De Motu Cordis (1009, 1170) and Antonie van Leeuwenhoek’s invention of the microscope, allowing observation of fine microstructures [most pertinently to exercise, the sarcolemma and striations of whale skeletal muscle fibers (1087)]; thus new knowledge began to advance the teachings of Galen and the ancient world.
Enormous contributions to the field came from Antoine Lavoisier (1743–1794), a Frenchman recognized as the Father of Modern Chemistry and Combustion. Lavoisier is partially credited with the discovery of oxygen, an element he termed “oxigene,” along with his contemporaries Joseph Priestly and William Scheele (1182, 1183). He also designed the first proper calorimeter in 1784, which he put to good use the next decade in what is considered the first true exercise physiology experiment (672). Tracking consumption of oxygen, Lavoisier demonstrated a sizable (more than tripled) increase in resting respiration when a subject continually pressed a foot pedal (1416). His wife reportedly observed, sketched (Figure 1), and took notes on all experiments, aiding in Lavoisier’s rise to prominence as an innovative scientist (1416). Unfortunately, since his research violated (much less debunked) the prevailing phlogiston theory (based on a concept that toxic gas was expelled rather than life-giving oxygen inhaled), Lavoisier was executed as the French Revolution raged on in 1794.
Figure 1.
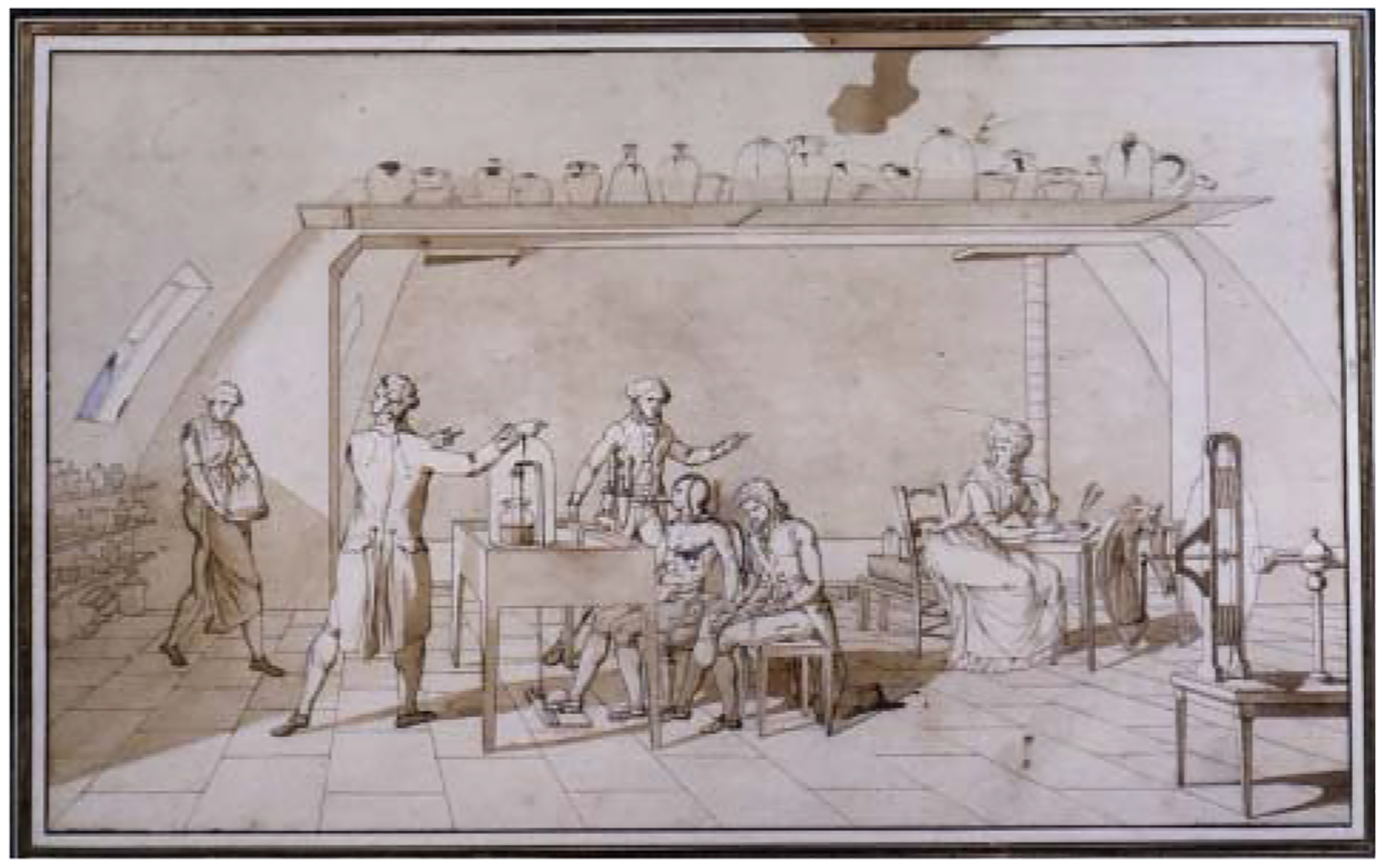
Marie-Ann Pierrette Lavoisier’s sketch depicting her husband Antoine Lavoisier conducting the first exercise physiology experiment and herself taking notes in the right corner. Note the use of the bell-jar calorimeter and the subject pressing a foot pedal below the table. Reused, with permission, from West JB, 2013 (1416).
The golden age of discovery
Spurred on by recently acquired knowledge, researchers in the 1800s continued to progress down the path toward establishing exercise physiology as a distinct discipline, then referred to as physical education, hygiene, or bodily exercise. Physiologist Claude Bernard (1813–1878) led the way, with his discoveries related to digestion, metabolism, circulation, and the nervous system engendering the concept of “le milieu intérieur,” or internal environment that all bodily systems strive to maintain despite constant fluctuations in the external environment (269). This idea eventually developed into the concept of homeostasis, now a central tenet of exercise physiology. Bernard called particular attention to the nervous system as the central regulator of the interior milieu.
Concurrently, knowledge of the workings of the cardiovascular system was improved with Adolph Fick’s application of physic principles to cardiac dynamics, enabling a better understanding of gas exchange and, eventually, the measurement of cardiac output (1186). Fick’s observation that, if the amount and concentration of a substance carried in blood are known, the volume of blood can be calculated (1346) is aptly applied to exercise physiology in an equation linking oxygen uptake to the product of cardiac output and the arteriovenous oxygen difference, that is, = oxygen consumption (Vo2) = cardiac output (Q) ÷ arteriovenous oxygen difference (a–vo2). Further insight came from studies in energy expenditure conducted by chemist Wilbur O. Atwater and physicist Edward B. Rosa. Through his career, Atwater (1844–1907) conducted hundreds of studies related to metabolism (some at rest, others during cycling exercise), famously running a calorimeter continuously for days with lab staff rotating through the role of test subject. Atwater’s work in combustion help determines the caloric value of macronutrients, with clear implications for exercise metabolism and sports nutrition (193). This series of breakthroughs highlight the integrative nature of exercise as an activity involving multiple body systems. Thus it is not surprising that, even today, we continue to identify mechanisms of cross talk between body tissues in response to exercise.
Work physiology in the early 20th century
The turn of the century brought on an era of interest in human performance and resilience, and several notable researchers rose to prominence during this time. For example, John Scott Haldane (1860–1936) examined conditions that challenge the limits of respiratory physiology (e.g., altitude, deep diving, air composition of sewers and mines) (1417). Haldane is likely best known for developing an apparatus to quantify gas exchange (513). Decades later, the device would be optimized by Per Scholander (1164) and others (1187), providing more efficient measurement, greater portability, and overall increased accuracy in assessing metabolic processes.
Great strides were made during this time in the Scandinavian region, with the charge led by August Krogh (1874–1949), sometimes considered the Father of Exercise Physiology (61). Trained in zoology and physics in Denmark, Krogh imbued his work in human physiology with this knowledge, which allowed him a unique perspective on existing and arising scientific problems. This, in combination with his skills in “visual thinking” (1093) enabled him to design and build innovative tools to facilitate research, including a microtonometer (806), a magnetically braked cycle ergometer, and a balance impressively precise enough to detect changes in body mass down to approximately 2 g (1093). During his training and later career, Krogh worked closely with his wife Marie in the study of respiration and oxygen dynamics (605, 1163). Together they published a series of papers entitled the “Seven Little Devils,” so named because they refuted the mechanism of active gas exchange proposed by Krogh’s mentor, Christian Bohr (464). Namely, the Kroghs demonstrated that simple diffusion was responsible for gas exchange by optimizing the measurement of partial pressure of oxygen in the alveolar air and arterial blood. Marie went on to elaborate on the plasticity of this system during periods of high demand (e.g., muscular work and/or low oxygen availability) to earn her doctoral degree in 1914 (1163), while August continued to study the mechanics of oxygen delivery and earned 1920s Nobel Prize in Physiology or Medicine after elegantly demonstrating that muscle capillaries open and close based on tissue needs, that is, capillary recruitment (1093). Krogh himself trained a number of notable individuals that shaped the Scandinavian future of exercise physiology, including a trio of motivated individuals he nicknamed “The Three Musketeers” (1214), which included Erik Hohwü Christensen, Marius Nielsen, and Erling Asmussen. Krogh’s profound influence led to an Institute at the University of Copenhagen dedicated in his name in 1970 (1214).
Exercise physiology as a so-named discipline had still not yet taken shape, but textbooks such as the Physiology of Bodily Exercise and the Physiology of Muscular Work had begun to be published and would receive repeated revisions in their subsequent iterations. A notable contributor during this period was Archibald Vivian (A.V.) Hill (1886–1977), a so-called “giant” in the field of exercise physiology (94). Hill specialized in skeletal muscle thermodynamics, mechanics, and metabolism, laying the groundwork for understanding the various biochemical energy systems and setting into motion a “Revolution in Muscle Physiology” (565). Hill’s collaborative work in muscle heat production with biochemist Otto Meyerhof earned the Nobel Prize in 1922 (94). Hill is also credited with the concept of maximal oxygen consumption (Vo2max) as a plateau in oxygen uptake despite increased workload (95). This measurement went on to be not only a robust correlate of endurance performance but the most powerful independent predictor of frailty and mortality (954), further linking exercise capacity to human health.
Biographers of Hill consistently comment on his light-hearted nature and enjoyable approach to his work. He famously made bold conjectures (731) and even “challenges” to the field (567), appreciating that scientific hypotheses are made to be disproven to lead to breakthroughs (94). Hill himself conceded, when questioned why he bothered studying athletes, exercise, and muscles, that the work was “amusing” (94). Pursuing his amusement, Hill set into motion the field of applied exercise physiology. In this realm, Hill enjoyed studying athletes because he viewed them as exemplars of the limits of human performance and could repeat their performances with consistency. His conjecture that exercise physiology, due to its integrative, complex, and fascinating nature, might recruit bright minds from other disciplines (94, 409) has proven itself time and again as the field enters the era of interdisciplinary team science.
The study of athletes was further expanded by the legendary Harvard Fatigue Laboratory (HFL), which set out to build a fundamental understanding of physiological stresses endured by industrial workers and army soldiers (409). During their two-decade tenure in the basement of Harvard University’s Business School, the HFL team generated over 300 scientific publications (650, 1314), including series entitled “Blood as a Physicochemical System” and “Studies in Muscular Activity”. The HFL was founded in 1927 by Lawrence J. Henderson and led by David Bruce Dill, who served as director of research. Initially assembled to elucidate the mechanisms underlying fatigue in the industrial worker, the HFL team quickly came to appreciate that no population better demonstrated fatigue exposure and resilience than athletes (1157). They took advantage of their location, studying blood gas composition and hemodynamics in Boston marathon runners, such as seven-time victor Clarence DeMar.
As American culture changed and World War II loomed, the HFL focus shifted from industry to military physiology, necessitating examination of various environmental challenges. The facilities enabled the research team to reproduce these with chambers designed to simulate high altitude and extreme cold and heat (409), supplemented by field research projects such as desert walks, altitude exposure, and artic simulations. HFL researchers established the precedent of subjecting themselves to many of their experiments (in fact, with relatively little effort, it is possible to discern which members of the HFL staff participated as subjects, since many publications include individual subject initials in data tables). The advantages of this practice were several (among them convenience of access and demonstration of feasibility/fairness of protocols), but, most importantly, this practice allowed the HFL to create a consistent and robust database for internal validation of repeated measurements and comparisons across conditions (650).
Upon its dissolution in 1947, the HFL propelled its staff into leadership positions across the United States, and they continued to be productive researchers and mentors. Some estimate that most American exercise physiology laboratories can “trace their lineage to the Fatigue Lab in only two or three academic generations” (650). Fittingly, the HFL’s reputation is held in high esteem, its scientific prowess bolstered by legends of treadmill-running laboratory dogs (650) and the infamous 40-40-40 Club, membership in which was reserved for those who could tolerate the extreme challenges of −40 °C, 40,000 ft, and walking 40 miles in 12 h (107, 650).
The modern era
The 1950s brought Watson and Crick’s discovery of the chemical structure of DNA, the establishment of the American College of Sports Medicine, and Roger Bannister’s record-setting mile under four minutes. The field was poised to take advantage of all three, with renewed interest in human health and performance and a scientific direction toward a Molecular Revolution that continues to advance. Skeletal muscle in particular began to receive notable attention (516). Hugh E. Huxley’s sliding filament theory, published in 1954 (616), provided a mechanical perspective on the workings of contractile elements actin and myosin in skeletal muscle (1408). Not long after, Johannes Bergström introduced the muscle biopsy technique to extract muscle tissue from living individuals (112), enabling much of the field’s foundation in mechanisms of exercise adaptations. This contribution allowed our understanding to extend beyond more accessible tissues such as blood and has been optimized (279, 378) and enriched over time with the growing appreciation for the various roles of skeletal muscle in metabolism and signaling.
A frontrunner in the studies of muscle biology was Bengt Saltin (1935–2014), a trainee of one of August Krogh’s Musketeers, Erik Hohwü-Christensen (1156). Throughout his career, Saltin examined human performance, adaptations to training, and maladaptations to unloading in the context of the cardiovascular and skeletal muscle systems. He was notably interested in the mechanisms underlying such phenomena (including, but not limited to, skeletal muscle fiber type composition and selective use of energy pathways), many of which remain relevant in today’s research landscape (658). Saltin served as head of the Copenhagen Muscle Research Center (CMRC), established with funds to encourage Danish leadership in international collaboration and aimed to understand mechanisms of skeletal muscle physiology. Due to funding restrictions in Denmark, it did not last more than a decade (1994–2004), but it has left a lasting impact on the field. Studies during its heyday and in the years that followed truly blended exercise physiology with medicine (660), realizing the ideals of early historians and physicians such as Susruta and Hippocrates. For instance, a team of CMRC alumni, led by Bente Pedersen, coined the term “myokines” after identifying that muscle-derived IL-6 was upregulated and secreted during contractile activity and had notable interactions with other tissues to influence glucose metabolism (1020).
Several visiting scholars from the U.S. collaborated with the CMRC, including David Costill and Phil Gollnick (78, 1156). Costill is credited with popularizing the Bergström muscle biopsy technique in the U.S., applying it to the investigation of metabolism during aerobic exercise (280). The lay articles that accompanied his scientific publications made performance- and health-related research accessible to nonacademicians and helped to endorse the American Running Boom of the early 1970s. Gollnick took a basic science approach to the mechanisms underlying exercise, supplementing human work (471) with research in animal models (48). Contemporary John Holloszy’s contributions to exercise and aging provided a basic understanding of metabolic pathways involved in training and detraining (509), enabling a more complete understanding of carbohydrate bioenergetics that support exercise (583). These researchers directed 20th century exercise physiology in the United States, and the current research landscape continues to be shaped by their direct academic descendants.
A major motivator for increased understanding of skeletal muscle exercise biology was the more widespread use of the muscle biopsy sampling technique in human subjects research. Key molecular mechanisms of muscle adaptation, often first revealed in preclinical studies, have proven instrumental in guiding human research in this direction. Notably, Frank Booth and colleagues investigated the influence of immobilization on muscle contractile protein balance in a rat model, highlighting the complex relationship between transcription and translation of actin (1401). This work in a prolific line of research led by Booth (928, 1304) laid the groundwork for a better understanding of the molecular regulation of synthesis of muscle proteins such as actin and cytochrome C during periods of loading and unloading. Others such as Williams and colleagues (1432) built on this, investigating transcriptional dynamics of muscle mitochondrial proteins.
These discoveries eventually led to the “Transient mRNA Hypothesis,” which states that long-term changes in protein abundance are the result of short-term exercise-induced increases in encoding messenger RNA (833, 986). Since early evidence generated by Neufer and Dohm (970), subsequent studies in both animals and humans have supported this (358, 732, 1033, 1049), forming the basic understanding of how acute responses beget stable adaptations to chronic exercise training. While these studies laid important groundwork for future research into muscle size and strength adaptations characteristic of resistance exercise (730), the dynamics of muscle gene transcription and translation influence all exercise outcomes reliant on production of new proteins, including key adaptations to aerobic exercise training. For example, transiently increased expression of genes encoding factors related to mitochondrial biogenesis (1033), lipoprotein lipase (1176), and other metabolic pathways (564) are critical molecular events in skeletal muscle that may form the basis of adaptations to long-term aerobic exercise.
The Human Genome Project and MoTrPAC
At the turn of the millennium, a battle between government- and industry-based efforts (1219, 1380) to sequence the full human genome resulted in an enormous amount of publicly available data that could be used to guide future hypotheses. In perspective allowed by our 20-year vantage point, this momentous event has become an inflection point in molecular biology research, subsequently leading to an explosion of knowledge based on transcriptomics, proteomics, and epigenetics that continues to grow steadily today. The application of these so-called ‘omics data sets to exercise and physical activity was spearheaded by leaders such as Claude Bouchard. Today, we continue to apply molecular mapping and data modeling to understand fitness (143), responsiveness to exercise training (1308), or genetic proclivity to be physically active (802).
Despite the growing volume of molecular data sets collected from exercise studies, the ability to carry out clinical trials directed toward developing exercise-based treatments for disease continued to be constrained by limited knowledge of its mechanisms of action based on data from appropriately sized human trials. Highlighting this critical information gap, a collaboration of leading researchers in the field, led by Dr. Darrell Neufer, came together to summarize current knowledge and the potential of exercise trials for discovery of actionable targets to promote human health (969). Perhaps motivated by this, Dr. Francis Collins, current NIH Director and a key scientist in the Human Genome Project, leveraged the NIH Invited Exercise Community gathering as a platform to address this need. Under Dr. Collins’ direction, the NIH eventually directed Common Fund resources toward the MoTrPAC initiative. Clearly, this level of support from a scientific giant highlights the need to develop this area of scientific inquiry, as well as its potential impact on public health. Ongoing initiatives such as MoTrPAC and the worldwide Athlome Project (1055) will bolster the available knowledge base in the context of exercise, leading to continued expansion of knowledge in healthy populations and providing a reference map to allow more mechanistic characterization of the influence of exercise training in chronic disease.
Section summary
Exercise physiology continues to permeate new avenues of human health, following our tendency to be fascinated by the infinitely large (e.g., the frontiers of space) and intricately small (e.g., regulation of gene expression by methylation or acetylation). In addition to emerging ‘omics platforms that survey the transducers of exercise, the field is invigorated by examination of different physiological stressors such as unloading (in bedrest or spaceflight), aging, chronic disease, and the continual drive to test the limits of human performance (589). Throughout human history, exercise has always been viewed as medicine, and we continue to understand its mechanisms of action, optimize its prescription, and apply its power. At this prospective inflection point in the trajectory of the field, we acknowledge the historical figures on whose shoulders we stand and review the knowledge amassed in their wake.
Important Considerations in Exercise Research
To provide adequate context for an understanding of molecular adaptations to exercise, we first briefly overview key considerations for conducting and interpreting exercise research. While these may be common understanding for those in the field, we intend to include this information for all readers, regardless of familiarity with these tenets of exercise research. Through this objective, this article may guide new and talented investigators in other areas of expertise toward continuing to develop the breadth of knowledge related to exercise in humans.
Exercise and physical activity
Whereas physical activity (PA) is classically defined as energy expenditure from bodily movement (208), exercise is a subcategory of PA that is planned, structured, repetitive, intentional (208), and typically paired with a goal or desired outcome. Most commonly, exercise is divided into aerobic exercise (AE) and resistance exercise (RE). AE usually involves repeated movement cycles (e.g., running, swimming, cycling) and is defined based on the large contribution of oxidative phosphorylation to bioenergetic metabolism. RE is so named due to movements being performed against a load, ranging from body weight to external weighted equipment (e.g., bars, dumbbells, elastic bands). While the primary outcomes for AE and RE tend to involve cardiorespiratory fitness and muscle mass/strength, respectively, both modes have numerous benefits for multiple physiological systems due to a unique set of challenges imposed by each. While performance-focused individuals typically adopt a training regimen that specifically optimizes their goals (259), combined training in AE and RE provides a range of benefits to maximize overall health, that is, reducing morbidity and mortality risk throughout aging (699). Thus, exercise guidelines of most major public health organizations and governments emphasize combined AE and RE training. For example, the CDC/American College of Sports Medicine (ACSM) recommends 150 min/week of moderate AE or 75 min/week of vigorous AE, combined with 2 days/week of RE. Soberingly, however, less than 5% of American adults actually meet these criteria (1048, 1333).
Regular exercise promotes maintained or enhanced cardiorespiratory fitness (CRF), a strong predictor of health and mortality in adults (954). CRF is measured as maximal or peak oxygen consumption (Vo2max or Vo2peak) and expressed as a rate (mL O2/kg/min or liters O2/min). Briefly, Vo2max refers to the concept introduced by A.V. Hill and reflects a true physiological ceiling in oxygen uptake, which may be attained by different means depending on the individual. Vo2peak refers to the highest measured rate of oxygen consumption given the experimental parameters (e.g., mode of exercise, test protocol used) (495, 1060). In determining CRF, the most important factor is participant effort, which may be assessed based on classical criteria (356) including a true plateau in rate of oxygen uptake with increasing workload, maximum heart rate (HR) above age-predicted target, respiratory exchange ratio >1.1, blood lactate >8 mmol/liter, and maximal perceived exertion. Notably, the appropriate thresholds for these test termination criteria are under continued consideration. Vo2max improvements of 4% to 13% have been reported following as little as two weeks of training (55, 539, 633, 1217, 1424). Remarkably, highly trained athletes may demonstrate Vo2max values 40% to 50% greater than their untrained counterparts (639, 1206). Even more dramatic benefits of continued exercise are apparent in advanced age (196, 499, 1323), as Vo2max undergoes age-related decreases in sedentary adults (197, 1282, 1283). However, Vo2max does not increase indefinitely with continued training, as some have revealed that adaptability in Vo2max is partially genetically constrained (136, 137, 1308).
Accelerometry
For many years, prior to the development of accelerometer technology, objective assessment of physical activity outside the laboratory in free-living settings remained a challenge for the field. Accelerometry aids in describing and quantifying PA in a relatively unbiased and reproducible manner, in terms of its type (e.g., leisure time, occupational), intensity (e.g., light, moderate, high), duration, frequency, and timing. Accelerometers are easy to use (for both researcher and participant) and have high sensitivity for detection of change (911). Despite these benefits, accelerometry data are not represented consistently in the literature. Montoye and colleagues recommend reporting 12 key elements for complete PA assessment (910): accelerometer brand, model, and placement on body, number of days worn, number distributed and mode of distribution, validity considerations such as minimum number of days and min/day necessary to declare validity, criteria for determining accelerometer was not worn, and determining noncompliance, and data metrics such as data epoch length and outcomes of interest derived from raw data. Researchers are encouraged to strive for completeness in data reporting, including how the specific limitations of the chosen device may influence study findings.
Exercise dose
Several variables contribute to the overall amount, or dose, of an exercise stimulus. Factors including intensity, duration, and frequency interact to determine the overall stress of exercise, leading to differential activation of molecular transducers across physiological systems. Chronic exposure to a given exercise dose facilitates long-term adaptation.
Intensity
Intensity is a metric of work or power necessitated by exercise such that higher intensity will require higher energy expenditure. In RE, intensity may be defined based on workload relative to maximum, which is usually measured as the maximum load lifted one time (as one-repetition maximum, or 1RM, Table 1). Intensity dictates the burden on skeletal muscle cells (myofibers). In humans, myofiber subtypes are defined based on the predominant isoform of the contractile protein myosin, including type I (smallest, slow, oxidative), type IIa (larger, faster, more glycolytic), and type IIx (fastest, most fatigable) (1159). These fiber types are innervated by type-specific motor neurons, whereby a motor neuron and all the myofibers it innervates comprise a motor unit. The sequential firing of motor units based on the size of the motor unit (a concept termed the “size principle”) was introduced in the 1960s by Elwood Henneman (554). This enables incremental muscle contraction, with the smallest (type I) motor units possessing the lowest activation threshold and thus typically engaged before the larger, more powerful (type II) motor units. Due to this relationship, tissue-level adaptations are usually specific to the muscle under load or tension and the contraction intensity/demand that defines the proportion of motor units recruited up to maximum (i.e., all motor units activated).
Table 1.
Commonly Used Intensity Ranges
| Intensity classification | Aerobic exercise: %heart rate reserve | Resistance exercise: %1 repetition max |
|---|---|---|
| Low | 40–50 | <50 |
| Moderate | 50–70 | 50–70 |
| High | 70–85 | 70–85 |
| Very high/vigorous | 85–100 | 85–100 |
For AE, using HR as a reference point for intensity assumes that demand on the cardiovascular system reflects the overall physiological stress. This metric can be easily and quickly collected, facilitating exercise prescription and periodization. In addition to representing intensity relative to maximum HR (HRmax), intensity is often expressed as a percentage of HR reserve (HRR, calculated as HRmax − HRresting), a robust reflection of cardiovascular reserve (1275, 1276). To define target exercise HR, a given HRR percentage is added to HRresting. Common training regimens based on intensity include moderate-intensity continuous training and interval training [e.g., high-intensity interval training (HIIT), or sprint interval training] which typically involves short periods of high-intensity work followed by longer periods of low- to moderate-intensity recovery. Physiologically, moderate and HIIT elicit both central (i.e., cardiovascular) and peripheral benefits, and both regimens can lead to similar improvements in Vo2max (550, 931).
Intensity is the primary determinant of energy utilization throughout exercise (1115). Whereas carbohydrate-based stores such as muscle glycogen are preferentially used in high-intensity activities, low- or moderate-intensity exercise permits sufficient time for utilization of fats, which are more energy-rich due to their high carbon bond composition. Elegant studies by Romijn and colleagues using a stable isotope infusion design demonstrated differential contributions to overall energy expenditure in men (1115) and later women (1116) at a range of exercise intensities. Interestingly, at all intensities, the absolute energy derived from plasma sources is identical, whereas the skeletal muscle component (glycogen and intramuscular triglycerides) changes depending on intensity. These and other works (123, 278) highlight the importance of skeletal muscle glycogen for maintenance of exercise intensity. Alternatively, increasing available carbohydrate exogenously (283) may supplement declining muscle glycogen stores in activities performed at a high intensity for a longer duration.
Duration
Duration is the other major determinant of energy utilization during and after exercise. The length of a single exercise session (i.e., duration) may last anywhere from several seconds (e.g., 100 m sprint) to ≥24 h (e.g., ultra-endurance running). When intensity is held constant (i.e., “steady-state” exercise), prolonged duration facilitates a shift toward fatty acid utilization. By manipulating intensity and duration, it is possible to control the total session work output and thus influence the mechanical and metabolic adaptations enforced by the training regimen. For example, by the end of a 2 h cycling bout at 65% of Vo2max, >60% of overall energy expenditure is derived from fat sources (1115). Thus, adaptations in fatty acid cycling and fat oxidation enzymes are typically seen with moderate intensity AE training (238).
Frequency
In RE, frequency is generally described as the number of sessions per body part or per week within the shortest training cycle. Two recent systematic reviews and meta-analyses describe the impact of RE frequency on outcomes such as muscle strength (497) and hypertrophy (1169). Briefly, overall training volume is the primary driver of differential responses (187, 1169, 1303): the frequency of sessions over which this volume is accrued may be less important (985). Conversely, when volume is not matched, an increase in frequency increases overall exercise dose, leading to greater improvements with RE (1168). An important consideration of exercise frequency is recovery time (i.e., time elapsed between exercise sessions), which may differ based on muscle group exercised (497, 1246), biological characteristics [e.g., sex (610, 612), age (387)], and lifestyle factors such as sleep and diet patterns.
In general, patterns in AE frequency mirror RE such that adaptations (196, 489) and health benefits (361) are dependent on overall dose rather than frequency of sessions. Importantly, this dose-response relationship diminishes at very high volumes: thus, some evidence supports an ideal range of 2 to 3 sessions per week (1166). While there is some debate regarding whether higher lifetime AE load actually contributes to negative health outcomes, such as cardiac events [reviewed in (362)] and mortality (1166), these findings may be influenced by factors such as exercise intensity and eccentric loading component. In terms of performance, AE frequency and/or duration may be manipulated to peak for competition in a process known as “tapering” (1215). The taper period is typically designed such that overall intensity is maintained while dose is reduced by approximately 25% to 50% (824). In young healthy males, tapering results in increased type IIa myofiber size and power along with significantly improved performance (824, 947, 1319).
Effects of biological sex
Biological sex, defined by a given sex chromosome complement or sex hormone profile, plays an important role in adaptations or acute responses to exercise. To date, much research has focused on exclusively male populations (1051), whether because of availability of male participants as members of exercise laboratories or perceived complications introduced by hormonal fluctuations throughout the menstrual cycle (275, 655, 848, 1095, 1270, 1428). While these within- and between-sex differences do exist and are biologically meaningful, the general consensus is that it is necessary to continue to study both males and females to best characterize these differences and their potential impacts on performance, health, and disease. A feasible practice may be to recruit participants of both sexes and perform independent downstream statistical analyses. However, it remains important to reconcile any differences revealed to increase our understanding of relevant processes contributing to sex-specific patterns.
In the context of exercise, research has revealed some key differences in physiological properties between the sexes (Figure 2). Briefly, differences in skeletal muscle (611, 612, 1105, 1113, 1413, 1455), cardiovascular (8, 326, 499, 667, 854, 1419), endocrine (218, 491, 713, 1105, 1242, 1415), and metabolic (202, 328, 1287, 1288) phenotypes have been observed. Importantly, many key adaptative outcomes to exercise training appear to be similar between the sexes (4, 103, 593, 612, 849, 1220, 1415, 1464), although the underlying mechanisms may be different (2, 507). Despite these interesting findings, the area of sex-specific responses to exercise generally remains poorly defined and is clearly an area warranting extensive future investigation.
Figure 2.
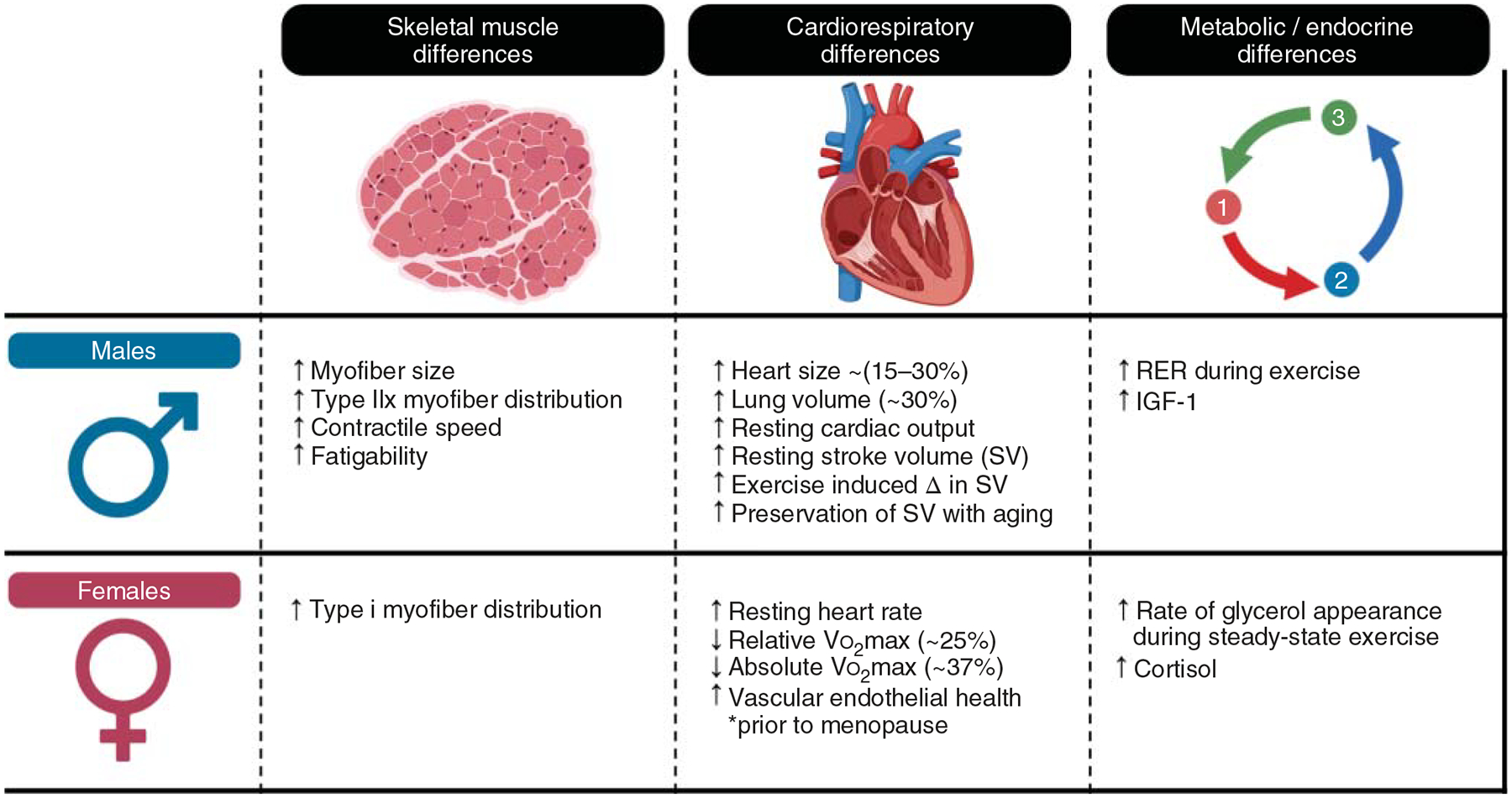
This figure illustrates an overview of sex-specific differences in skeletal muscle, the cardiovascular system, and energy metabolism that may be important considerations relevant to human exercise.
Section summary
A rapidly progressing field has outlined numerous controllable factors that influence the magnitude, direction, and target system/tissue of adaptation to exercise training. It remains necessary to consider carefully the influence of these variables in study findings and to shift attention toward existing knowledge gaps. In particular, a focus on equalizing the imbalance between all-male and sex-matched research is of utmost importance as the field of exercise prescription research migrates toward a more individualized strategy. Furthermore, due to emerging evidence from genetics and genome-wide association studies (GWAS), the potential influences of race and/or ethnicity warrant significantly more attention. These factors should be viewed in light of potential for discovery. For instance, ongoing efforts such as the MoTrPAC initiative will leverage variability in human exercise biology to build a comprehensive and diverse molecular map of acute responses and adaptations to exercise.
Current Evidence of Exercise as Medicine
A continually growing body of evidence strongly supports that exercise has multiple long-term benefits across the entire lifespan, from the womb until late life (Figure 3). Exercise improves general health and fitness, psychological well-being, social interaction, etc., enhancing every dimension of quality of life while reducing the risk of chronic disease and mortality (1034, 1144) through a range of mechanisms. Yet in the midst of overwhelming evidence to suggest that exercise is essential to preserve health, most adults are still inactive (1400), a sobering statistic that costs an estimated $53.8 billion worldwide. Concerningly, prevalence of inactivity is higher among older adults, women, many racial and ethnic minority groups, and individuals with an underlying chronic disease (335, 1400). Regardless of age, sex, race, ethnicity, or fitness level, habitual exercise and an active lifestyle are cornerstones for maintenance of physical independence, health, and well-being (1034, 1048). Furthermore, exercise has shown promising results as a preventative and/or rehabilitative strategy for a wide range of diseases by improving the function of numerous body systems.
Figure 3.
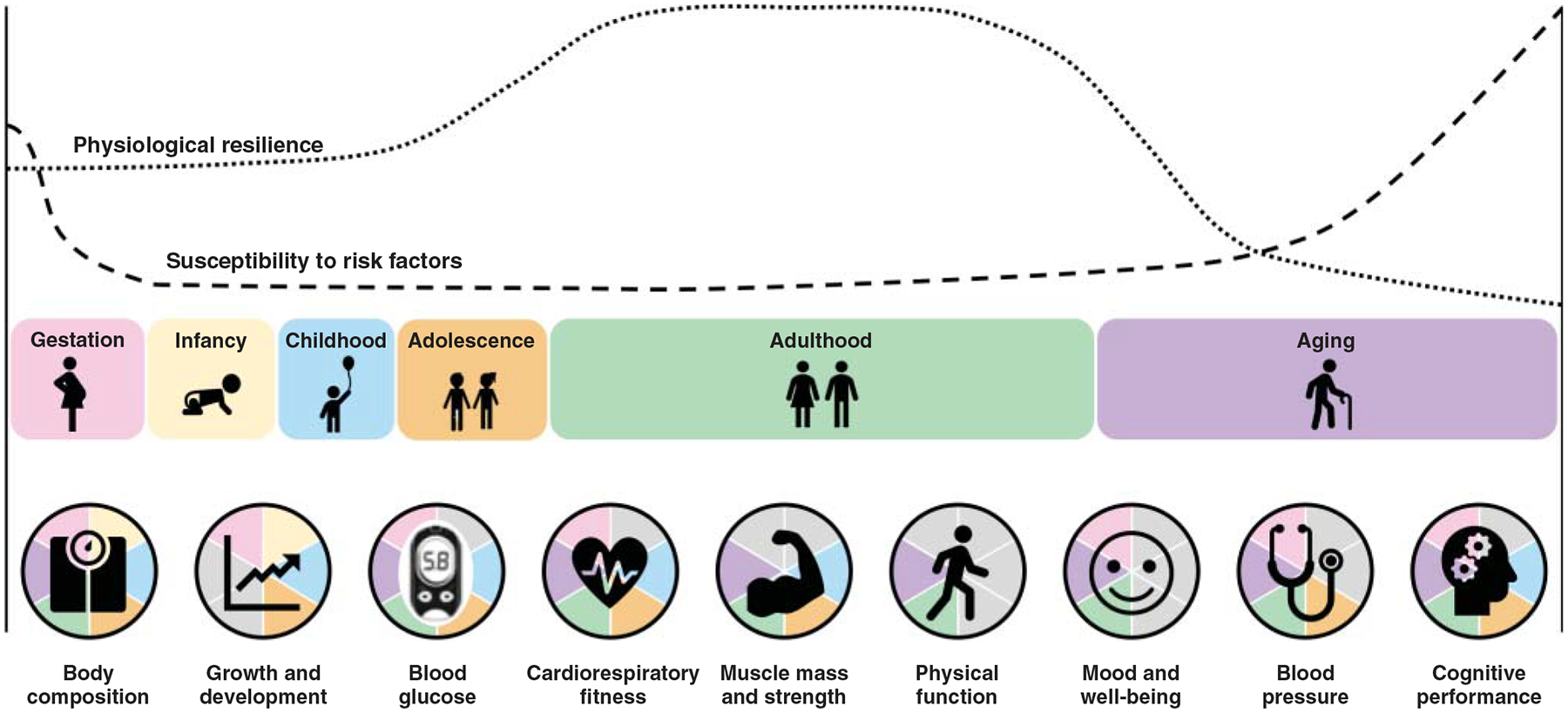
This figure illustrates exercise benefits across the lifespan. Normal age-related changes affect physiological resilience to environmental or genetic stressors, while the susceptibility to metabolic, cardiovascular, and other risk factors increases. Exercise improves multiple functions and physiological indices of wellness. Pie chart slices are greyed out when (i) the physiological index shown is not relevant for a given age (either no longer growing/developing or process has not yet begun to decline) or (ii) the physiological index has not been sufficiently studied in a population at that life stage.
General health maintenance
Gestation and early life
In the absence of obstetric or medical contraindications, exercise is considered safe during pregnancy (303–305, 346, 858, 1027, 1070, 1143) and is recommended for its wide-ranging benefits in maternal and neonatal outcomes (1027, 1144). Exercise reduces the risk of pregnancy complications such as preeclampsia, gestational hypertension, and gestational diabetes (164, 305). Sustained maternal cardiorespiratory fitness and gestational weight management protect against risk of postpartum CVD, T2D (106, 506, 524, 717, 1027), and obesity (1028). In addition to controlling maternal gestational weight (1301) and associated risks (82, 304, 859), exercise during pregnancy reduces the likelihood of high gestational weight (macrosomia) in infants (1388), an outcome associated with numerous health defects throughout life (242, 1388). Evidence is still insufficient regarding the benefits of exercise for cesarean section, labor duration, and high-birth weight fetus delivery (164, 858). Nevertheless, neonates whose mothers exercise during pregnancy demonstrate birth weight within normal range and attain higher scores on the Apgar scale [a gauge of responsivity in neonates (306, 858, 1071, 1143)] than counterparts of sedentary mothers. Furthermore, exercise may reduce the risk of postpartum depression (303, 1070), a common psychiatric disorder affecting approximately 10% to 15% of women during/after pregnancy (101, 167).
Childhood and adolescence
Multiple studies demonstrate exercise-induced improvements in cardiorespiratory fitness, muscle mass, and strength in children and adolescents (102, 245, 381, 488, 769, 770, 952, 1436). A potential countermeasure to combat the dramatic increase in childhood obesity prevalence in recent years (740), exercise is an effective weight management strategy (698, 771, 772, 1202, 1347). Concerningly, obese children have a doubled risk of becoming obese adults (740, 1151) and premature death (765). Exercise, however, can decrease the metabolic burden of obesity independent of weight loss (6, 310, 771, 772). Furthermore, regular exercise alleviates the severity of pulmonary deficits associated with childhood asthma, a common disorder limiting both maximal lung function and exercise tolerance (797, 800, 823, 898, 1036, 1395).
In children and adolescents, HIIT is a time-effective intervention to elicit cardiovascular health effects (189, 268, 353, 1083, 1121, 1418). Further, concurrent training in AE+RE (as encouraged by many organized sports) may have synergistic effects in children and adolescents, improving strength, power, CRF, and sports performance (445). Provided that it is properly designed, RE has positive effects on skeletal mass and bone development during childhood and youth (102, 488, 952), in addition to increasing muscle strength, power, endurance, and neuromuscular control (777). Further evidence suggests RE training may reduce the risk of sports-related injuries in youth (102, 381, 488, 777, 952). Remarkably, children and adolescents that undertake RE show greater gains in strength compared to adults in the initial stages of RE, benefits that are carried into adulthood (419).
A critical component of normal health, neurodevelopment during childhood and adolescence is commonly affected by disorders such as attention deficit/hyperactivity disorder (ADHD) [affecting 8% to 10% of children (972)] and autism spectrum disorder (ASD) [affecting approximately 2% of children (69)]. Notably, moderate-to-vigorous AE may alleviate the severity of characteristics associated with these disorders, for example, response inhibition, response time, cognitive control, attention allocation, cognitive flexibility, processing speed, and vigilance (322, 972). In children with ASD, exercise attenuates deficits in social skills, language and communication, cognition, and attention (393, 651, 1317), in addition to improvements in blood lipid profile, parent-perceived quality of life (1317), and motor control (146). Importantly, these studies demonstrate safety in addition to efficacy, highlighting the potential utility of exercise to treat and manage functioning in neurodevelopmental disorders (393, 1317). In these populations as well as neurotypical children and adolescents, exercise appears effective at improving domains of cognition, metacognition, self-esteem, enhanced self-concept, and increased life skills (26, 322, 527, 561, 592, 1029).
Adulthood
Exercise throughout the lifespan promotes optimal functioning of most (if not all) physiological systems. During adulthood, higher CRF is associated with lower risk of premature mortality and lower incidence of CVD, respiratory disease, and colorectal cancer (1247). Furthermore, a prospective cohort study of >500,000 adults aged 40 to 69 years found that both CRF and grip strength (a physical performance measure of muscle strength) were negatively associated with mortality (699), indicating that both cardiovascular and skeletal muscle function are important indicators of general health in middle-aged adults (941). Certainly, other physiological systems play a role in determining overall health status. For instance, failure to reach peak bone mass is predictive of skeletal fragility and fracture risk in later life (1334), whereas exercise has positive effects on bone mass and morphology (323, 486).
Regularly assessed vital signs (e.g., blood pressure, heart rate variability) can serve as important biomarkers of health, and exercise appears to have a positive influence on these in adults (272, 619, 620, 700, 796, 944). Endocrine indicators of health are also easily accessible in circulation and provide valuable insight into system functioning. Due in part to drastic hormonal changes during perimenopause and eventual menopause, adult females are more likely to undergo a more precipitous rate of muscle and bone mass declines, contributing to heightened risk of falls and fractures (1211), in addition to an array of other chronic conditions. Exercise interventions may forestall the declines in muscle, bone, and metabolic health (211, 510, 1152), positively impacting physical capacity in mid-life. Furthermore, the combination of exercise with adjuvant hormone replacement therapy is under study.
Beyond maintenance of physical health, regular exercise promotes mental wellness in adults (229, 485, 528, 743, 1266). Mood and anxiety disorders are increasingly common in this age range (556). While the estimated prevalence of depression and anxiety are 4.4% and 3.6%, respectively (425), many adults live with both disorders simultaneously (425), and it is estimated that approximately 50% of U.S. adults will experience a mental health disorder at some point during adulthood (689). In a large cross-sectional study of 1.2 million adults, exercise was associated with lower self-reported mental health burden, regardless of age, race, gender, household income, educational level, and exercise type (229). In contrast, cessation of regular exercise is associated with increased depressive symptoms in healthy adults (919). These effects are particularly pronounced in females, a subpopulation generally at higher risk for developing a mental health disorder during adulthood (77, 425, 556).
Healthy aging
Aging is a process shared by all living things and involves a complex series of biological changes that lead to a general decrease in physiological resilience (i.e., ability to tolerate and recover from stressors) and increased vulnerability to adverse events (380, 573). Even in the absence of chronic disease, the generally downward trajectory of aging varies across individuals, likely as an integrated result of genetic, epigenetic, environmental, behavioral, and other factors (39). Nevertheless, there is overwhelming evidence that regular engagement in exercise has potent antiaging effects (173, 1025), protecting the function of most physiological systems, including cardiovascular, respiratory, immune, and musculoskeletal (70, 131, 140, 307, 408, 595, 615, 798, 915, 1293). In fact, skeletal muscle tends to be a tissue of aging and exercise research focus, due to its steady decline in mass and function with age, in combination with its critical roles in movement, metabolic homeostasis, and support of the immune system. Maintenance of skeletal muscle function with aging reduces the risk of falls, which often result in injury, onset of disability, and loss of independence in older adults (179, 336, 421, 442, 515). In a systematic review and meta-analysis in >20,000 older adults, exercise reduced the risk of falling by 21% (1192), a robust effect given the high fall risk with advancing age (49).
Those who age “successfully” enjoy a long health span, or lifespan free of chronic disease (1173); habitual exercise appears to be a critical component of successful aging (39, 129, 416, 735, 1235, 1371). A recent study by Gries et al. in aging men and women who reported engaging in lifelong exercise demonstrated that individuals were biologically nearly 30 years “younger” than their calendar ages, based on a range of maximal CRF parameters (499). A rapidly expanding area of research sampling lifelong exercisers continues to demonstrate preserved cardiovascular health (196, 1193), mitochondrial health (499, 1323), skeletal muscle mass and performance (219, 498), and muscle endocrine function (327, 757) in the face of aging. At this time, much of the research is focused on AE-trained older adults, but continued research is necessary to examine the potential benefits of RE and/or concurrent training throughout the lifespan. Furthermore, the benefits of lifelong training for other domains of health (e.g., cognition, mental health) represent an emerging knowledge gap (447, 448).
Musculoskeletal diseases
Musculoskeletal diseases encompass a collective group of conditions that affect locomotor organs and tissues (muscles, bones, joints, tendons, ligaments, etc.). Musculoskeletal diseases affect 20% to 33% of people globally, and rates increase with advancing age (1444), accounting for approximately $213 billion in annual health care expenses in the US alone (1340). Clinical symptoms include pain and mobility limitations, together contributing to decreased engagement in physical activity and increased likelihood of disability (1340, 1444). Exercise may be used as both primary and secondary prevention to prevent the onset or reduce the clinical burden of this class of diseases. Here, we focus on four prevalent musculoskeletal disorders: sarcopenia, osteoarthritis, rheumatoid arthritis, and osteoporosis.
Sarcopenia
Sarcopenia is a multifactorial neuromuscular disease clinically characterized by age-related declines in skeletal muscle mass and sometimes associated with decrements in strength and function (231, 287, 922, 1032, 1267). Multiple operational definitions of sarcopenia exist, leading to difficulty in accurately assessing its prevalence in the population (171, 288, 1105). After muscle mass peaks around age 30 to 40 years, it naturally declines with advancing age (~10% per decade) (287, 337, 726), and strength losses occur at a faster rate (~2%–4% per year). Lower limbs are usually more dramatically affected (337, 482, 726, 805, 828). Consequences of low muscle mass may be exacerbated by complications including incomplete muscle mass recovery following illness, infection, or hospitalization (289).
Exercise is one of the best-studied and most effective countermeasures for sarcopenia. Despite heterogeneity across studies in age range and exercise mode, consistent improvements are seen in total skeletal muscle mass, strength, and other functional outcomes in sarcopenia (821, 1377). RE, in particular, is a potent stimulus for reversal of sarcopenia, given its positive impact on muscle hypertrophy (163, 289, 337) and muscle protein synthesis (72). While the degree of hypertrophy is highly variable across individuals, all individuals garner multiple positive adaptive responses to training (249). On the other hand, AE can improve exercise tolerance and metabolic function through enhanced oxidative enzyme capacity and heightened insulin sensitivity (581, 749). These adaptations occur through increased mitochondrial biogenesis (1108, 1145), reduced low-grade inflammation (153, 337, 752, 857), and improved skeletal muscle plasticity (752).
Osteoarthritis
Osteoarthritis (OA) is characterized by structural changes in articular cartilage, subchondral bone, ligaments, capsule, synovial membranes, and periarticular muscles (53, 608, 926, 1022) that are often accompanied by chronic pain and mobility impairment. OA affects 10% to 13% of noninstitutionalized adults in the United States (252, 1475) but the prevalence doubles to approximately 25% in individuals ≥65 years (1475). In addition to age, factors such as sex, race, ethnicity, bone density, obesity, joint structure and mechanics, nutritional factors, and genetic predisposition also influence the incidence of osteoarthritis (53, 816, 926, 1254, 1370, 1475). Due to their biomechanical roles as weight-bearing structures, the hip and knee joints are most commonly affected (53, 926). Many individuals with OA elect to undergo joint replacement surgery to alleviate pain, swelling, stiffness, and crepitus of the affected joint (926). OA contributes substantially to emergency hospital costs (1209) and affects >250 million people worldwide (609).
Regular exercise is recommended to prevent, manage, and “prehabilitate” OA (81, 750, 966, 1343). Evidence from multiple systematic reviews suggests that, when exercise is properly prescribed, pain and stiffness are reduced without damage to cartilage or synovial tissue (91, 150, 844, 973, 1034) or accelerated OA progression (91, 844, 973, 1414). In addition to improving general functioning, flexibility, and muscle strength, exercise can enhance mood and quality of life (91, 92, 119, 206, 222, 325, 668, 795, 1307). Furthermore, exercise yields similar or large effect sizes in comparison to pharmacologic treatments such as nonsteroidal antiinflammatory drugs (81, 418). In a network meta-analysis of 9134 patients with knee and hip OA in 103 randomized controlled trials, AE training was the most beneficial intervention for managing pain and improving performance (468), although a range of other modalities have been examined for their effects on muscle strength, walking speed, weight management, and quality of life. For instance, several systematic reviews and meta-analyses indicate that benefits are conferred from activities such as AE, RE (91, 1387), combined flexibility and strength programs (149, 468, 754, 795, 966, 1111, 1470), yoga (754, 1470), Tai Chi Chuan (222, 794), aquatics (90, 822), and proprioceptive training (646, 1225). The optimal prescription likely varies based on an individual’s needs, preferences, and symptoms.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic autoimmune disease that affects approximately 1% of the adult population and is characterized by degenerative arthritis in synovial joints, including proximal (e.g., hands and wrists), intervertebral (cervical, lumbar), and other joints (e.g., hips, knees, ankles, toes, shoulders, etc.) (890, 1430). RA is clinically characterized by inflammation, deformation of the affected joint(s), pain, stiffness, and fatigue that lead to progressive deteriorations in mobility, functional ability, and quality of life (890, 1430), as well as hospitalization and disability in many individuals (227). Concerningly, RA-associated changes in lean and fat body mass are associated with increased CVD risk (57, 890, 892, 894, 1430). A range of pharmacologic and nonpharmacologic RA treatment options are available (1430), but the most intensive involve a combination of conventional synthetic and biological disease-modifying antirheumatic drugs and are costly to patients and health care systems (227).
Exercise interventions have consistently been demonstrated as a cost-effective and sustainable treatment with multiple general systemic benefits and positive impacts on RA symptomology. These include aerobic fitness (540), strength (67, 613), functional ability (67, 613, 1430), cardiovascular health (286, 892), fatigue (298, 681, 1354), and inflammatory burden (60, 891). Furthermore, evidence from prospective observational and experimental studies demonstrates that exercise promotes positive effects on pain (68), cognition (1184) and quality of life in individuals with RA (68). Practically, higher fitness is inversely associated with number of hospital admissions and length of hospitalization (893), and higher physical activity level time is associated with lower 10-year CVD risk (391, 895) in individuals with RA.
Osteoporosis
Osteoporosis is a systemic skeletal disorder characterized by reduced bone mass and disruption of bone microarchitecture, increasing the susceptibility to bone fragility, osteoporotic fractures, and mortality (558, 670, 711, 1147, 1449). Osteoporosis is common among older adults, especially postmenopausal women (1001, 1449); its current prevalence is expected to increase with the growth of the aging population and the concerted action of multiple risk factors that contribute to osteoporosis pathophysiology (746). Existing pharmacotherapies have side effects and transient benefits at best; thus, adherence to pharmacologic regimens is poor (692, 874). Fortunately, exercise boosts bone health (874, 1152) across a range of individuals and exercise prescriptions (479, 1240, 1472–1474).
RE is particularly well-studied as a high-impact, weight-bearing exercise modality to modulate adverse outcomes associated with osteoporosis. Whether performed independently, progressively, or as part of a multimodal intervention, RE improves bone health across a range of ages, including the high-risk postmenopausal female demographic (479, 1240, 1472, 1473). Exercise interventions modulate osteoporosis adverse outcomes through improvements in bone mineral density (479, 1473), fall risk factors (e.g. sway velocity and anterior-posterior sway range)(1405), fear of falling (1240) and fall-related injuries (1472), while improving muscle strength, functional mobility, balance and quality of life (1177, 1240). Exercise volume is associated with positive and stable changes in bone density (479). Exercise may also be used as a primary prevention strategy: it has been suggested that achieving 10% higher peak bone mass in young adulthood can delay the onset of osteoporosis by 13 years and reduce subsequent risk of lifetime fracture risk by 50% (100, 1252). However, the appropriate exercise mode must be carefully considered: non-weight-bearing activities may provide little to no benefit on bone structure (100).
Cardiovascular diseases
CVDs are a class of conditions affecting the health of the heart and vasculature and are the global leading cause of death (1445). General risk factors for CVD include but are not limited to obesity, dyslipidemia, inflammation, and oxidative stress. Most premature deaths attributed to heart attack and stroke could theoretically be prevented with early detection and management and/or effective prevention via preservation of CRF. Conversely, increasing CRF through exercise reduces the risk of CVD and all-cause mortality (718, 845, 1255).
Hypertension
Hypertension, clinically defined by elevated blood pressure, is incredibly common, affecting >40% of the adult population worldwide (785, 916, 1097). Physiologically, high blood pressure is the end-product of disturbances in systemic vascular resistance and/or on cardiac output due to numerous circulating and vasoactive factors, many stemming from chronic hyperactivation of the sympathetic nervous system. Hypertension increases the load on not only the peripheral vasculature but also organs such as the heart, kidneys, and brain (172, 804, 1097). Hypertensive patients have an increased risk of cardiovascular and cerebrovascular morbidity and all-cause mortality (172, 192, 384, 392, 511, 512, 641, 1047, 1097, 1180, 1260, 1420). Most patients use one or a combination of antihypertensive medications, but long-term use of pharmacotherapies increases the likelihood of side effects (502, 1298). In contrast, regular exercise has multiple acute and chronic benefits for management of high blood pressure.
Habitual exercise is associated with reduced side-effects, optimization of pharmacologic treatment, and prevention or postponement of development of hypertension (636, 916, 1047, 1097, 1100, 1420). In addition, physical exercise positively impacts office and ambulatory blood pressure, a continuous measurement of blood pressure over hours (140, 194, 207, 1097). A single bout of AE and RE consistently decreases 24 h ambulatory blood pressure (191, 272) as well as office blood pressure for up to 2 h postexercise, a phenomenon known as postexercise hypotension (194, 207). The magnitude of the blood pressure-lowering response varies with exercise dose, and the optimal strategy to maximize postexercise hypotension is still not identified (1097).
Coronary heart disease
Coronary heart disease (CHD) is a cardiovascular pathological condition characterized by ischemic cardiomyopathy via narrowing or blockage of the coronary arteries, commonly due to atherosclerotic plaque constriction (1022). CHD has a long asymptomatic development phase but frequently leads to major acute cardiovascular events (e.g., myocardial infarction, sudden cardiac death) (766). While prevalence of CHD is higher in males across age ranges, advancing age reduces this sex difference (294, 1185). Primary and secondary preventative strategies include lifestyle changes, optimal medical care, myocardial revascularization, use of antiplatelet agents (415), and regular exercise (417, 459).
The UK Biobank, a large, longitudinal cohort study, showed that strength and fitness were inversely associated with incident CHD and atrial fibrillation in adults genetically predisposed to develop CVD (1306). Similarly, a study in U.S. veterans demonstrated a relationship between higher CRF and lower incidence of major cardiovascular events (718). Given the broad range of cardiovascular benefits across exercise modes, it appears that most types of activity exert a protective effect (417, 475, 580, 1010, 1356). Some have reported clinically relevant cardiovascular preconditioning benefits detectable immediately after a single exercise bout (1011, 1302).
Exercise-based cardiac rehabilitation after an acute ischemic event is the cornerstone for secondary prevention of CHD; this practice reduces cardiovascular (38) and all-cause mortality risks (853) by 26% and 13%, respectively. Despite remarkable benefits, only 62% of patients are referred to cardiac rehabilitation at the time of discharge after an acute event, and an even smaller fraction actually attend one or more sessions (339). Finally, evidence suggests that precaution should be taken when prescribing high-intensity interventions in high-risk CHD patients due to an acutely elevated risk of events (417, 580).
Heart failure
In heart failure (HF), cardiac muscle progressively weakens, resulting in compromised blood delivery throughout the body. HF may be further classified based on its etiology, potentially resulting from either pressure overload associated with hypertension (888) or volume overload associated with valvular defects (e.g., mitral valve regurgitation) (255, 902). Further discrimination includes the anatomical site (e.g., left or right HF) (723) and the discernment of whether ejection fraction is reduced (HF-REF) or preserved (HF-PEF) (706). HF affects >5 million Americans (~12%) (939, 1352), is more common in individuals >70 years (1174, 1352), and requires vigilant monitoring to continually optimize treatments to its progression (1281). HF patients also have poor respiratory muscle strength and endurance, cardiopulmonary perfusion, and skeletal muscle function (255, 1022). Prognosis is grim in that most HF patients do not survive five years after diagnosis (1372), while others become reliant on ventricular assist device implantation or heart transplant (255, 1174, 1372).
Exercise, particularly aerobic, is an effective primary prevention strategy (210, 1433): stratification of high-risk individuals into fitness-based quintiles supports an incrementally protective effect of CRF (1022). In contrast, low fitness is a strong independent predictor of adverse outcomes (957). Additionally, lower CRF in young adulthood is associated with left ventricular dysfunction and higher prevalence of subclinical abnormalities and other complications in late life (115, 1003), suggesting that primary prevention is critical. Nonetheless, exercise-based cardiac rehabilitation has beneficial effects on prognosis, functional capacity, and quality of life in individuals with HF (255, 271, 1002). Even in patients with ventricular assist devices (7, 537, 673) and heart transplant (37), evidence supports that both short- and long-term training interventions are safe and effective.
Metabolic diseases
Metabolism, the cell-level process of converting nutrients into energy, is fundamentally disrupted in the cluster of conditions known as metabolic diseases, a leading cause of death worldwide (19). Lifestyle habits, such as meeting the recommended exercise levels (1048), have numerous effects on prevention and management of metabolic diseases, sometimes demonstrating a stronger impact on metabolic risk factors and mortality than achieved by pharmacotherapies (955).
Obesity
Across the lifespan, overweight and obesity are common conditions, and prevalence across all age ranges has been steadily increasing throughout the last century (698, 771, 772, 1347, 1446). While its primary feature is excessive amount of body fat, obesity results from a range of etiologies including genetic, environmental, and endocrine factors. In both children (740) and adults (265), obesity contributes to a broad range of comorbidities, including (but not limited to) T2D, hypertension, nonalcoholic fatty liver disease, obstructive sleep apnea, and dyslipidemia. Obesity and its network of associated pathologies constitute a substantial economic burden (1446), in addition to impairing quality of life and increasing mortality.
Regardless of age, sex, race, and exercise type, regular physical exercise is an effective weight-loss and weight-management intervention (771, 772). Three months of AE or RE significantly reduces body fat percentage, waist circumference, and visceral, subcutaneous and intrahepatic adipose while simultaneously improving insulin sensitivity and skeletal muscle mass in obese adolescents (771, 772). Even independent of weight loss (310), exercise training promotes meaningful changes in body composition and a favorable metabolic profile. Illustrating this, a prospective study tracked the development of CVD risk factors in >100,000 adults across an approximately 6 year period (855). In comparison to those who decreased physical activity rates over time, the individuals that increased or maintained habitual exercise patterns demonstrated lower rates of hypertension, T2D, and hypercholesterolemia regardless of whether they lost, gained, or maintained weight (855). While weight loss may be a strong and visible motivator for individuals, it may be of clinical import to communicate that not all interventions result in a change in overall body weight to temper expectations appropriately. Changes in other easily measurable parameters (e.g., body fat percentage, waist-to-hip ratio) may be a better reflection of health and may provide incentive to continue an exercise regimen.
Diabetes
Currently, more than 13% of U.S. adults are affected by diabetes, and prevalence has been rising in children and adolescents as well (212). In 2017, diabetes was the seventh leading cause of death in the United States and demanded a total estimated cost of $327 billion (212). The majority of cases are diagnosed as T2D (175), a condition characterized by impaired function of insulin-producing pancreatic beta cells caused by a combination of insulin resistance, relative insulin deficiency, and abnormal fat and protein metabolism (30, 1022). On the other hand, type 1 diabetes results from autoimmune destruction of the beta cells. Both conditions manifest as hyperglycemia (high blood glucose); in T2D, development of hyperglycemia is progressive and insidious, which may cause classic diabetic symptoms to be overlooked before more severe complications arise (637). In comparison with nondiabetics, individuals with T1D or T2D have two to four times higher risk of co-morbidity (e.g., hypertension) and mortality (257, 466, 704, 1022). Furthermore, low CRF is a strong independent predictor of all-cause mortality in both T1D and T2D (138, 257, 803, 1411).
As part of lifestyle change therapy (637), exercise has been shown to improve insulin sensitivity, fasting insulin, and glycated hemoglobin (HbA1c), a classic marker of long-term blood glucose regulation, in children, adults, and older adults (257, 261, 347, 386, 637, 1202). Impressively, metabolic benefits of exercise are brought about quickly (e.g., within a week of vigorous AE training (705)), and a single bout may have effects on whole-body insulin sensitivity that persist for up to 96 h (704). Higher intensity or longer-duration training amplifies the stability of these positive changes (138, 704, 811, 1268, 1434). Meta-analyses including both AE and RE demonstrate positive effects of exercise on glucose sensitivity (704), glycemic control (811), and inflammation and oxidative stress (803). RE may also augment overall physical functioning through increased skeletal muscle mass and bone mineral density (257, 261, 637, 803, 811). Preclinical studies suggest that exercise may improve pancreatic beta cell mass and function (290). Exercise also exerts immunomodulatory benefits on pancreatic beta cells and systemic inflammation in T1D (256, 257).
Dyslipidemia
Dyslipidemia includes a number of lipoprotein metabolism disorders, primarily elevated blood levels of total cholesterol (hypercholesterolemia) and/or triglycerides (hypertriglyceridemia) which are major risk factors for atherosclerotic CVD (724, 1022). More than 13% of U.S. adults ≥20 year have high total cholesterol and 18% have low levels of protective HDL cholesterol (199), an inverse risk factor for metabolic and CVD (19, 785, 1022, 1047). High total cholesterol levels have been associated with obesity, breast cancer (178), diabetes-associated morbidities (984), Alzheimer’s disease (AD) (1457), stroke (882), and CHD (142). Through its influence on rates of cholesterol synthesis, transport, and clearance (351), regular exercise can reduce total cholesterol, triglycerides, and LDL cholesterol and increase protective HDL (724, 939, 1022, 1099, 1258, 1420). These effects reduce reliance on pharmacological treatments: the National Health and Nutrition Estimation Survey estimates that a 15% reduction in LDL cholesterol can reduce the need for antidyslipidemic drugs in 5% to 14% of the population (199). Given that dyslipidemias are a risk factor for many cardiovascular and metabolic diseases, exercise-induced improvements in blood lipid profile positively affect a range of outcomes to reduce overall health risk.
Metabolic syndrome
Metabolic syndrome (MetS) is astoundingly prevalent, affecting one in every three U.S. adults ≥40 years (14). While international criteria differ (19, 962), MetS is generally described as a multifactorial cluster of five key factors: central adiposity, dyslipidemia, insulin resistance, glucose intolerance, and hypertension. MetS is a major contributor to CVD risk (1191), a precursor for other metabolic chronic conditions (312), and an emerging epidemic of its own. Despite being at elevated risk for a coronary event, individuals with MetS do not exhibit symptoms of cardiovascular dysfunction. Thus, primary prevention via exercise is likely the most effective treatment option for MetS (331).
The benefits of exercise for each of the subcomponents of MetS are well-studied; thus it is logical that exercise reduces overall risk of MetS. As recently reviewed (953), increasing exercise and/or higher CRF positively impact MetS incidence and prevalence, while low fitness and/or physical activity is associated with higher incidence of MetS (350, 625, 679, 953, 1262, 1469). Meeting at least the recommended exercise guidelines (1048) can help prevent the development of MetS in later life (71, 312, 953). Through its impact on MetS factors, habitual exercise can also reverse MetS: Katzmarzyk et al. (676) found that >30% participants diagnosed with MetS at baseline were no longer affected after a 20 week AE intervention. The exercise intervention significantly decreased triglycerides, blood pressure, and waist circumference, while improving HDL cholesterol and fasting plasma glucose.
Cancer
Cancer is the second leading cause of mortality in the United States, with approximately 1.8 million new cases and 600,000 deaths expected in 2020 alone (1200). The risk of developing an invasive cancer is dependent on intricate and compounded risk factors such as genetic predisposition, environmental exposure to DNA-damaging agents, and lifestyle factors. It has been estimated that 40% to 60% of cancers can be prevented by modifying factors such as obesity, smoking, and physical inactivity (1230). Given the prevalence and ambiguous, complex origins of cancer, the development and/or combination of effective therapeutic approaches to prevent, treat, and manage it are of great public health importance. The relatively young field of exercise oncology is targeted at leveraging exercise to offset treatment-related side effects and improve quality of life in cancer patients. Early trials focused on AE in breast cancer patients (438, 712, 1263), and promising results in this population spurred exercise interventions in other cancers. Collective evidence supports that AE and RE elicit improvements in symptom-related outcomes such as exercise tolerance, quality of life, fatigue, and overall function (9, 439, 1175).
Many cell survival pathways targeted by chemotherapy are also critical for protecting the heart (221). Exercise may alleviate the cardiotoxic burden of chemotherapy and other cancer drugs, as was first demonstrated in 2006 in a preclinical study using treadmill running in rats (239). In a subsequent breast cancer case study, AE prior to and throughout treatment resulted in reduced fatigue and improved functional capacity (324). In further support, adverse side-effects such as hemodynamic shifts, depression, soreness, and pain were significantly less prevalent in individuals performing vigorous AE before every chemotherapy session than those undergoing usual care (703). Furthermore, exercise may be associated with changes in the tumor microenvironment, including tumor treatment sensitivity, induction of antitumor immunity, reductions in inflammation, and increases in antioxidative capacity (52, 578). While poor vasculature and permeability of solid tumors often pose a challenge for treatment, exercise can expand vessel density and tumor perfusion, improving the efficacy of anticancer agents (52). Ongoing research is needed to consider the range of cancer types, chemotherapeutic agents, and exercise modalities (299).
Cancer patients, including pediatric patients, are now living longer after treatment (901). Unfortunately, long-term side effects of toxic treatments are a growing concern, contributing to a rising burden of cancer treatment-related disease (e.g., CVD, HF) even 30 years after treatment (299). Given its short-term cardioprotective effects in individuals undergoing chemotherapy, long-term exercise could have a role in reducing the risk of treatment-related adverse outcomes. In support, a prospective study in 2973 breast cancer patients found a dose-dependent protective effect of physical activity during recovery on CVD risk (654). Similar findings have been reported in testicular cancer (299). Finally, a recent meta-analysis by McTiernan et al. reported that physical activity was inversely related to both all-cause and cancer-specific mortality in survivors of breast, colorectal, and prostate cancers (878). Despite promising evidence that exercise is necessary before, during, and after cancer treatment, greater insight into molecular mechanisms driving exercise-induced improvements in tumor biology, cardiotoxicity, and long-term survival is needed. This will continue to guide the field of exercise oncology toward developing and optimizing specific exercise prescriptions for individuals with cancer (965).
Neurodegenerative diseases
Neurodegenerative diseases are on the rise in the rapidly aging population (1453), with today’s prevalence rates expected to triple 2050 (158). Associated dementia is a major public health concern, projected to cost $2 trillion globally in the next decade (971). Dementia is highly debilitating, demolishing an individual’s independence and quality of life while placing immense emotional and financial strain on their family and caregivers. The current and projected economic burden of neurodegenerative disease and dementia prompted a call-to-action in 2015 (1453) for research directed at modifying progression of neurodegenerative diseases As there are currently no cures or specific biomarkers for these conditions, the impact of lifestyle factors such as exercise is of great research interest (367, 454, 873). While most exercise research focuses on the most common neurodegenerative disorders, dementia exists in numerous forms with wide variability in pathology, presentation, and progression.
Alzheimer’s disease
AD is the most common neurodegenerative disease: clinical AD affects approximately 4% of older adults worldwide (394) and preclinical AD or mild cognitive impairment (MCI) impacts significantly more at an earlier life stage (158). Postmortem AD brains show neurofibrillary tangles and plaques made of the peptide amyloid-β, but the current best biomarker is a single nucleotide polymorphism in apolipoprotein E4 (ApoE4), shared by over 50% of adults living with AD (1137). ApoE4 genotype may be relevant to exercise adaptation: in a longitudinal study of 1646 older adults, habitual exercise is associated with lower risk of dementia in only noncarriers (389). Conversely, a study by DeMarco et al. (321) found that acute exercise heightened brain function in young female ApoE4 carriers but not in noncarriers, an area warranting further investigation in older populations. Nevertheless, physical activity reportedly reduces the incidence of AD by approximately 40% to 50% (168, 504) and higher levels of regular exercise may be associated with reduced amyloid-β burden (161, 162). In older adults with MCI, short-term RE (154) and AE (1295) training mitigate reductions in volume and plasticity of key brain regions impacted in dementia and AD. Furthermore, important changes are sustained up to one year following the cessation of training (154). In a sophisticated study examining the relationship between regular exercise (reported as step-count) and gene expression in the hippocampal brain region, Berchtold et al. (108) found that physical activity promoted transcriptional patterns of genes primarily associated with mitochondrial health, synaptic plasticity, and neuromuscular communication that were inversely related to cognitive impairment and AD. Thus, exercise may target critical signaling pathways that are dysregulated in AD and dementia, representing a potential mechanistic strategy for preservation of cognitive function.
Parkinson’s disease
The second most prevalent neurodegenerative disorder (affecting ~1% of adults over age 65 year) is Parkinson’s disease (PD), which stems from death of dopaminergic neurons in the substantia nigra of the midbrain. PD is considered a neurodegenerative movement disorder: the first symptoms of PD are not detectable until most of these neurons have died (122) but predominantly manifest as motor function abnormalities (tremor, bradykinesia) in addition to deficits in senses, sleep quality, mood, and cognition. Some cases of PD are accompanied by abnormal accumulation of α-synuclein into Lewy bodies in the brain. Lewy body dementia (LBD), a separate but overlapping type of dementia often manifests in so-called “Parkinsonian” symptoms (47, 1441). Perhaps due to this complexity, exercise training studies in populations with exclusively LBD are lacking (626), highlighting an existing knowledge gap.
A prevailing theory is that motor symptoms of PD take years to manifest because of a physiological tolerance threshold for loss of nigrostriatal dopamine, below which motor function is compromised (122). In a longitudinal study of nearly 200,000 cross-country skiers, Olsson et al. demonstrated that exercisers lived longer before onset of PD, suggesting that AE may fortify this “motor reserve” (991). In populations with moderately advanced PD, RE may restore skeletal muscle size and strength to levels found in healthy adults, in addition to reducing the severity of nonmotor symptoms (29, 682). Furthermore, relative motor unit activation (the degree of neuromuscular “effort” relative to maximal contraction that is required to perform a task) is improved with RE in PD (682, 683). Regular exercise also can improve balance and gait speed (1205), although the lasting effects of such interventions are not presently clear (1386, 1441). Large cohort studies suggest that an exercise regimen started in middle-age may reduce risk of PD (17). Mechanistically, this may be linked to higher insulin sensitivity (861), reduced neuroinflammation (873), or acutely elevated activity of the substantia nigra (685) in individuals who exercise regularly. Ongoing research is investigating whether beginning exercise shortly after diagnosis may delay PD progression or the need for dopaminergic medication (1158). Finally, limited data exist as to the effects of exercise on MCI and dementia in PD, which are highly common in its later stages (552).
Section summary
Overall, strong evidence supports that exercise has beneficial effects for organ function in both health and disease, reducing overall risk of morbidity and mortality. A range of disorders and diseases are becoming increasingly prevalent as our population grows steadily more sedentary (213, 1447). Physical activity, whether recreational or structured exercise, promotes the preservation of multiple physiological systems, including musculoskeletal, cardiovascular, neurological, and metabolic function. Some have referred to exercise as a “polypill,” that can not only mimic but outperform pharmaceutical interventions (955) with little to no side effects and at lower cost. Nevertheless, there is a tendency of clinicians to prioritize pharmacologic treatments over exercise (953). Thus, research efforts should be directed at understanding the molecular mechanisms driving exercise-induced improvements in physiological function to provide evidence-based guidance for clinicians (1146). In the general public, behavioral interventions targeting individuals, groups, and/or communities are absolutely necessary to build exercise habits and facilitate a society in which a physically active lifestyle is commonplace.
Current and Emerging Methodologies for Biospecimen Assessment
Much of our current knowledge surrounding the molecular mechanisms mediating the effects of exercise in humans comes from analysis of biospecimens collected at various time points before, during, and after chronic training (i.e., adaptation) or a single acute bout of exercise (i.e., short-term response/transient effect). While exercise is a robust physiological stimulus affecting nearly every system, this article and MoTrPAC clinical studies focus on tissues most accessible in humans: skeletal muscle, blood, and adipose. MoTrPAC and countless independent investigations utilize animal models to capture molecular activity in inaccessible human tissues such as brain, liver, heart, and so on; these preclinical studies continue to provide mechanistic clarity regarding important pathways underlying exercise adaptation.
Skeletal muscle
Skeletal muscle biopsy considerations
The first percutaneous needle was created by Guillaume-Benjamin Duchenne in 1865 allowing the sampling of skeletal muscle, which resulted in the discovery of Duchenne Muscular Dystrophy (226). This sampling method was further adapted by Jonas Bergström, (112) and further modified by Evans et al. in 1982 with the application of suction through the trocar, increasing the yield of biopsy specimens by approximately four-fold (378, 1289). Since then, the Bergström needle has been used to sample primarily the vastus lateralis due to its large size, subcutaneous location, and distance from major neurovascular structures. In addition to the vastus lateralis, the deltoid, anterior tibialis, soleus, gastrocnemius, trapezius, biceps, and triceps have also been sampled (109, 281, 446, 473, 825, 837). While it is advantageous to sample the vastus lateralis muscle, some technical controls should be implemented whenever possible, as the distribution of type I and II myofibers (and thus the molecular characteristics of the sample) may differ depending on participant demographics (sex, age), dominant leg (right or left), location along the muscle, and depth sampled (788–792).
In addition to the widely used Bergström needle, other sampling needles such as the microbiopsy needle, the Well-Blakesley conchotome, the myotome, the Polly-Bickel needle, and others have been introduced (555, 995). The microbiopsy needle has been reported to reduce the invasiveness of the muscle sampling procedure due to its smaller diameter, while negating the need for skin and fascial incisions to gain access to the muscle (538, 606). Anecdotal reflection from a participant perspective indicates a preference for the microbiopsy procedure (606). The microbiopsy needle typically yields a short and wide muscle specimen (~70–90 mg), compared to approximately 50 to 400 mg of long and thin muscle tissue obtained from a standard Bergström needle biopsy. As such, the optimal approach should be carefully considered in light of downstream research objectives that may be particularly reliant on a given arrangement of myofibers within the bundle and total tissue yield required.
Regardless of the instrument chosen for extraction of the biospecimen, tissue processing should be performed according to research objectives. First, it is necessary to remove any blood, fat, fascia, and connective tissue from the biospecimen, as these may contaminate the sample and influence downstream results. Timing is of the utmost importance: dynamic, transient effects such as phosphorylation (182, 451), methylation (89), etc. necessitate preferential allocation of tissue for proteomics and metabolomics (1125). Homogenate analyses (such as these and other ‘omics) actually capture a variety of cell types in addition to skeletal myofibers (1127). In contrast, single-fiber analyses reveal enriched biological signatures that may be diluted by a homogenate approach (1091). In terms of tissue allocation, a longer bundle may be better suited for teasing apart and clipping individual myofibers for fiber type-specific analyses, whereas a wider bundle may provide extensive surface area better suited for histological analyses. Most commonly, fibers are oriented transversely, such that slicing the mounted specimen with a cryostat yields a cross-sectional view of the myofibers within the bundle (945). Alternatively, longitudinal muscle sections may be preferable for visualization of certain subcellular structures at high resolution, such as sarcomeric structures and mitochondria.
Histological staining and imaging
Tissues designated for histology can be preserved immediately by submersion into a fixative such as glutaraldehyde or formaldehyde and subsequently used for methodologies including transmission electron microscopy (TEM) (453). TEM is considered to be a gold standard methodology for skeletal muscle imaging, allowing the detection of changes in microstructures such as mitochondria, including volume, density, number, and morphology (1190). Imaging techniques continue to evolve. Focused ion beam-scanning electron microscopy (FIB-SEM) has allowed detection of myofiber-type specific differences in mitochondrial morphology following 3D reconstruction of human vastus lateralis single myofibers and muscle sections (180, 293).
Obtaining TEM and FIB-SEM images in combination with the analysis process can be very time consuming and costly. The more widely used alternatives include confocal, light, or fluorescence microscopy. Biopsy samples are preserved through a controlled freezing technique to limit the expansion of intracellular water and prevent freeze-fracture artifacts in the tissue (739). Briefly, samples are mounted in a water-soluble embedding medium such as optimal cutting temperature compound mixed with tragacanth gum, submerged in isopentane cooled with either liquid nitrogen or dry ice, and frozen for long-term storage at −80 °C or below.
These commonly used methods can prepare muscle for staining ranging from hematoxylin and eosin to more complex co-staining procedures (e.g., neutral lipid stain, oil red O, and immunohistochemical co-stains) (834, 945, 1190). These techniques can be applied to detect myofiber type-specific phenomena, such as changes in oxidative capacity measured through cytochrome C oxidase expression, and changes in lipid droplet associated proteins before and after sprint interval training (1190). Confocal images of sectioned or single muscle fibers enable in-depth analysis of mitochondrial morphological changes in relation to skeletal muscle intramyocellular lipid stores (733).
Finally, laser capture microdissection (LCM) is an advanced method in which specific myofiber populations are physically excised. While technical barriers exist, a major strength of LCM is the ability to perform both targeted and high-throughput ‘omics analyses on a relatively smaller amount of muscle tissue than required by other applications. Illustrating the efficiency of data collection, Murgia and colleagues reported the detection of >2000 proteins in approximately 2 h of measurement time (949). Combining LCM with downstream gene and protein analysis has enormous potential for characterizing differences between myofiber populations, for example, based on myosin heavy chain isoform (1265) or abundance of another target of interest (949).
Single myofiber physiology
Skeletal muscle fibers may be assessed for parameters such as contractile force, shortening velocity, and power in the absence of nervous system input and physiological fluctuations in calcium availability. Briefly, for this approach, myofiber bundles are permeabilized in a skinning solution, and then individual myofibers are teased out and tied between a force transducer and motor arm. To determine maximal shortening velocity, the myofiber is submitted to a slack test (355): the myofiber is slackened by moving the motor arm, and time to redevelop force (whereby the slack is alleviated) is calculated and averaged at four settings along the length of the myofiber. Another commonly performed assay relies on measurement of the relationship between force and shortening velocity at a series of fiber lengths to calculate theoretical maximal velocity and power (405). Single myofiber physiology experiments have aided in illustrating fundamental differences in contractile properties between myofiber types, such as approximately five-fold higher power in pure type IIa than type I myofibers (437, 1321). These techniques have also enabled detection of intricate changes in the myofiber contractile apparatus that arise from exercise training (405, 1320, 1325, 1328), aging (498, 1321), and unloading (1425, 1426).
Muscle satellite cell culture
Mature skeletal muscle contains satellite cells, a pool of stem-like cells that facilitate remodeling and regeneration in response to stress (534, 1039). This regenerative potential and the mechanistic impact of biochemical and mechanical perturbations on muscle satellite cells can be assessed in vitro using cell culture models. In isolating satellite cells from human skeletal muscle biopsies, the surface marker cluster of differentiation (CD)56 (also known as neural cell adhesion molecule, or NCAM) is often used to differentiate satellite cells from fibroblasts and other cell types in the specimen (12). Alternatively, animal-derived cell lines such as C2C12 (mouse) or L6 (rat) cell lines may be employed. Some evidence suggests variation in the basal expression of metabolic, proliferative, and developmental genes across species (3), potentially complicating the translatability of cell culture experiments.
Throughout a controlled period of growth in a cultured environment, muscle satellite cells differentiate into myoblasts and eventually fuse into multinucleated myotubes (1244). Notably, myotubes can be stimulated to contract, mimicking exercise in the absence of systemic or neural influences with the use of methodologies such as electrical pulse stimulation (EPS) (148, 375), pulsed forskolin and ionomycin (276, 1232), 5-aminoimidazole-4-carboxamide-1-β–ribofuranoside (AICAR), and caffeine (203). These stressors allow recapitulation of some of the intermyofibrillar adaptations to an exercise-like stress, such as accretion of contractile protein (148), bioenergetic adaptations underlying metabolic flexibility (1232), and myokine production and release (435). While administration of these biochemical stressors provides mechanistic insight into key pathways underlying myocellular adaptations, it is important to note that they are only able to partially capture the impact of exercise exposure and often cannot mimic the characteristic multisystem elevation in cellular energy demand.
Blood
Many of the beneficial effects of exercise are the integrated result of multiple physiological systems engaging in cross-tissue communication. In this regard, the circulatory system is important facilitator of tissue cross talk via delivery of signaling molecules, cells, and other structures between tissues. A primarily relevant example of this is myokines such as IL-6, which is secreted from glycogen-depleted skeletal muscle and acts in a paracrine fashion on the liver to stimulate glucose release in support of continued muscle contraction (1026, 1249). Exercise-induced myokine secretion facilitates adaptations including hypertrophy (myostatin, IL-4, -6, -7, and -15), and osteogenesis (IGF-1, FGF-2), fat oxidation, insulin sensitivity, and anti-inflammation (IL-6) (1018). In addition, lipids (e.g., 12,13-dihydroxy-9Z-octadecenoic acid, or 12,13-diHOME) and metabolites (e.g., succinate) can also serve as circulating signaling molecules during and after exercise. Arteriovenous balance studies of the forearm, leg, and splanchnic bed [classically been used to examine fuel metabolism during exercise (15)] allowed the characterization of the temporal release of IL-6 from the leg following acute exercise (1250) and can be a powerful approach to identify other novel factors released from tissues of interest.
A developing area in the context of exercise biology is the production, release, and packaging of proteins and microRNAs (miRNA) into secretory vesicles such as extracellular vesicles (EVs), microvesicles (1018), and exosomes (444, 594, 793, 959). These signaling factors are thought to convey molecular factors underlying a range of systemic adaptations to exercise; thus, much remains to be learned by characterizing their contents and identifying the tissue of origin (751). Many of the standard biochemical workflows used to analyze tissue homogenate and single myofibers (e.g., protein immunoassay, targeted gene expression, other ‘omics) can also be applied to secretory vesicles. For instance, combining EV isolation with mass-spectrometry, Whitham et al. identified 322 proteins that were significantly increased immediately after exercise and 3 at 4 h postexercise time point (1423). Continued research into EVs and related species released in response to exercise may prove to be a powerful resource in designing integrative disease therapies.
Leukocytes, the circulating cells of the immune system, are also involved in mediating the beneficial effects of acute and chronic exercise. Acute exercise induces a temporal shift toward increased circulating natural killer cells and neutrophils (1479). Leukocyte (e.g., neutrophil and monocyte) infiltration into peripheral tissues such as skeletal muscle plays a critical role in repair and regeneration following exercise (843) and long-term hypertrophy (422). Beyond a standard complete blood count with differential, flow cytometry can be used to study leukocyte redistribution, alterations in expression of cell surface markers, and changes in leukocyte function with exercise (1082). Leukocytes can be sorted into specific populations for follow-up analyses using flow cytometry or via incubation and centrifugation in specific collection tubes coated with preservative chemicals.
Adipose
Adipose tissue was classically perceived as a relatively inert organ for storage of triglycerides; however, it is now recognized as an endocrine organ (687) displaying phenotypic diversity across adipocyte subpopulations (e.g., white, beige, brown) with distinct metabolic and functional roles (463). As the body’s major energy reservoir, adipose tissue plays a key part in whole-body metabolism, with dysfunction contributing to pathologies such as obesity, T2D, dyslipidemia, and insulin resistance. Excess accumulation of upper body adipose in particular is linked to increased risk for CVD (413, 481). Dysfunction in adipose tissue is characterized by larger, metabolically inflexible adipocytes, immune cell infiltration and activation, senescence, impaired lipid turnover, and secretion of deleterious factors (315). In turn, exercise plays a key role in maintaining a healthy metabolic phenotype in adipose tissue (314).
Subcutaneous adipose tissue specimens can be extracted from the abdomen or gluteal prominence using a punch biopsy, aspiration with a Bergström or Mercedes liposuction needle, or surgical removal (20, 185, 302, 1008). Depending on the technique, approximately 100 mg to several grams of tissue can be obtained. The sample should be washed with ice cold phosphate-buffered saline and then flash-frozen in liquid nitrogen or prepared for histology by embedding in parafilm. A number of histomorphological methods have been developed to image and quantify adipocyte size and number (121, 230, 852). Furthermore, collagenase digestion of the adipose specimen allows the separation of stromal vascular cells (T-regulatory cells, macrophages, smooth muscle cells, and mesenchymal stem cells) from mature adipocytes, which can then be analyzed via flow cytometry or single-cell ‘omics approaches (54, 130).
High-throughput ‘omics approaches
Transcriptomics
Relatively recent advancements in high-throughput data collection, analysis, and computation have greatly accelerated the discovery of the molecular transducers of biological phenomena in health, disease, and exercise. Prior to the advent of next-generation sequencing tools, studies employed microarray technology to examine transcriptome-wide changes in gene expression as a result of AE (253, 839, 1296, 1308) and RE (253, 1040, 1051, 1299, 1300). Since this time, newer direct sequencing technologies designed to more precisely and deeply define tissue- and cell-level transcriptomes have been applied to the study of exercise (264, 332, 1051, 1053, 1062). RNA-sequencing (RNA-seq) directly sequences cellular RNA and maps reads to a reference genome through bioinformatic analysis to quantify levels of individual transcripts (929). RNA-seq can be applied to measurement of all available forms of RNA or limited to messenger RNA (via pulldown of all polyadenylated RNA species). True to its nature as a discovery tool, RNA-seq can reveal nonprotein coding transcripts (e.g., long noncoding, circular, small, and miRNAs), which are receiving increased attention as regulatory factors in exercise biology (813, 1203).
A comprehensive and powerful approach is to integrate RNA-seq with other ‘omics (264, 532), linking gene expression to variation in the genomic architecture, epigenetic regulation, and other molecular phenotypes. For example, chromatin immunoprecipitation sequencing (ChIP-seq) involves immunoprecipitation of DNA-interacting proteins and subsequent direct sequencing of associated DNA fragments (652). Using antibodies to specific histone modifications, the epigenetic landscape of the sample can be determined. A recent elegant study used epigenetic markers of transcriptional enhancers (demethylation of histone 3 lysine 4 and acetylated histone 3 lysine 27) in combination with RNA-seq in a mouse wheel running model (1088). Integration of the gene expression and epigenetic data revealed activation of transcription factors myocyte enhancer factor 2 (MEF-2) and estrogen-related receptor (ERR) upstream of heightened expression of oxidative metabolism genes (1088). Integrated multi-omics designs such as this, along with ever-evolving molecular mapping technologies (532) may provide mechanistic insight into observed biological relationships (440).
Bulk tissue RNA-seq and related techniques, while powerful, represent composite gene expression in an entire sample. In contrast, recent advancements in cDNA synthesis from single cells allow interrogation of single-cell transcriptomes by RNA-seq (929), providing the ability to profile gene expression in individual cell populations before and after a given intervention. Studies investigating the cellular composition of skeletal muscle tissue samples (319, 461, 1127) have revealed a number of resident cell types (e.g., immune, endothelial, satellite, and fibro-adipogenic progenitor cells), each displaying a unique transcriptomic signature. Due to size limitations of the single-cell isolation, these studies typically filter myofibers out of analysis. Other technologies such as single-nuclei RNA-seq should theoretically enable assessment of all cell types including myofibers (500, 597); however, the multinucleated nature of mature skeletal myofibers may introduce complexity in this approach. The emerging spatial transcriptomic technology, which enables measurement of gene expression on intact histological specimens (913, 1243), may become a viable technique for assessing gene expression in specific regions of muscle tissue.
Proteomics
Our understanding of molecular interactions governing adaptations to exercise is more deeply enriched by evaluation of multiple levels of omics data (899). Similar to high-throughput sequencing technologies, advances in mass spectrometry allow broad and unbiased survey of the proteome (478). Using this approach, several studies have reported training-induced changes in skeletal muscle metabolic proteins encoded by mitochondrial and genetic DNA, a finding consistent with transcriptomic datasets (357, 586, 1109, 1160). The circulating plasma proteome also reflects a positive influence of activity status on mitochondrial proteins, in addition to reductions in inflammatory, immune, and stress response proteins (1149). Single myofiber proteomics has also been developed (948). When applied to humans, this technique reveals differences in glycolytic as well as sarcomere chaperone pathways in slow and fast fibers (950). These approaches will undoubtedly be useful in assessing myofiber type-specific proteomic changes and adaptations to exercise. Furthermore, agnostic bioinformatics pipelines originally developed for gene expression datasets (e.g., Weighted Gene Correlation Network Analysis) can be applied to proteomics and other high-throughput datasets with relative ease (1149).
Measurements of posttranslational modifications such as phosphorylation and acetylation provide an additional layer of information and biological complexity. Phosphorylation is typically reversible and regulates multiple aspects of protein function (e.g., conformational change, enzyme activation, subcellular localization, degradation, stability). A small but seminal study by Hoffman et al. identified changes in the human skeletal muscle phosphoproteome following a high-intensity exercise bout (576): this work outlined a complex network of phosphorylation events mediated by multiple kinase pathways. A follow-up study combined this dataset with phosphoproteomics in mice and rats to identify exercise responsive adenosine monophosphate-activated protein kinase (AMPK)-dependent regulation of store-operated calcium entry (967). Unlike phosphorylation events catalyzed by kinases, acetylation of lysine residues on nonhistone proteins is thought to be mediated by nonenzymatic mass action (1381). Affinity purification of acetylated lysine residues followed by mass spectrometry allows for an unbiased survey of the acetylome (543). Given the role of acetylation in mitochondrial dynamics and metabolism (338, 1264, 1429), assessment of the acetylome will likely be a valuable tool in exercise biology.
Finally, proteins are susceptible to modification by reactive oxygen species and reactive nitrogen species, collectively termed redox modifications. These processes are known to contribute to age-related muscle loss and atrophy (1139). Mass spectrometry-based techniques utilizing differential labeling of free thiol groups on cysteine residues have now been developed, with studies focusing on redox modifications in aging mouse skeletal muscle (867, 1221). Future application of this and other proteomic techniques to human exercise is likely to reveal transducers of acute responses and long-term adaptation.
Metabolomics
Both acute and chronic exercise-induced flux in energy substrate utilization (584) may be reflected in the metabolite pool of systemic circulation or specific tissues. Like proteomics, advances in mass spectrometry allow for both supervised (targeted) and unsupervised (unbiased) surveys of metabolites influenced by exercise. For instance, Lewis et al. observed acute increases in circulating lactate, adenine nucleotide catabolites, and tricarboxylic acid cycle intermediates (e.g., succinate, pyruvate, malate) following a Vo2max test, as well as reductions in gluconeogenic amino acids following marathon running (786). Following long-term (~6 month) AE training, changes in circulating metabolites have been associated with changes in cardiorespiratory fitness (147) and insulin sensitivity (604). Thus, the circulating metabolome may represent an accessible method for assessment of the influence of exercise on health and disease. Naturally, metabolomics may also be applied to tissues of interest, such as skeletal muscle (603).
Lipidomics
Mass spectrometry may also be applied to measurement of lipid species (519), including both structural (phospholipids, sphingolipids, etc.) and signaling (prostaglandins, leukotrienes, etc.) molecules. Most commonly, the technique involves an organic extraction followed by a liquid chromatography (LC) step prior to mass spectrometry. This approach has been used to characterize exercise-induced changes in skeletal muscle lipids in both preclinical models and humans (768, 850, 885, 1178, 1239). Prominently, changes in levels of the mitochondrial structural lipid cardiolipin have been shown to relate to mitochondrial capacity following weight loss and moderate AE training (258, 885). Others have shown an acute rise in the signaling lipid 12,13-diHOME following a single bout of AE (1239). Recently, a more comprehensive lipidomics approach has been developed: removal of the LC step has the potential to identify a range of lipid species missed by traditional methods (520). This method has recently been used to measure lipids in skeletal muscle, including after exercise (1053, 1331).
Section summary
A range of complex approaches to collect, process, and comprehensively profile biospecimens have been developed over the past 50 years (Figure 4). In addition to molecular mapping with transcriptomics, methylomics, proteomics, etc., the influence of internal [e.g., gut microbiome, (1421)] and external environmental factors [e.g., the “exposome” (88)] has yet to be explored as a mediator of inter-individual response to exercise. At this point, our capacity for generating data exceeds our ability to interpret findings. Like genetic data, metabolomic and lipidomic data sets may be limited by the reference against which identified species are matched; thus, continued efforts to expand these resources will be fruitful. As such, continued development of both data and knowledge driven bioinformatics analysis tools will aid in expansion, integration, and interpretation of multi-omics data sets from exercise studies (532).
Figure 4.
This figure illustrates the range of current and emerging tools in analysis of human tissue for exercise research, with a focus on preparation of blood, skeletal muscle, and adipose tissues (most easily accessed in humans) for downstream high-throughput ‘omics analyses.
Skeletal Muscle Adaptations to Exercise
For obvious reasons, skeletal muscle has been a tissue of interest for insight into adaptation to exercise. Early studies led by pioneers such as Bengt Saltin, John Holloszy, David Costill, and others provided insight into key histological and cellular phenotypes affected by long-term training and acute exercise. Since these early works, collective knowledge of the molecular biology of skeletal muscle has been broadly amplified. The plasticity of skeletal muscle to perturbations such as training, overtraining, detraining, bed rest, unloading, and microgravity is astounding. Beyond this, skeletal muscle is no longer seen as merely a machine for movement, but an endocrine organ, an immune reservoir, and an indicator of physiological wellness.
Structure and function of skeletal muscle
Muscle comprises 40% to 50% of total body mass (428). At the system level, muscle is a hierarchically arranged structure divided into fascicles by the presence of connective tissue called perimysium. Fascicles are comprised of myofibers, and myofibers are composed of myofibrils, which house a contractile unit referred to as the sarcomere. At this structure, receipt of an action potential from an innervating neuron is translated into the release of calcium from the sarcoplasmic reticulum, which enables interaction between the primary filaments of the contractile apparatus (24, 484). To summarize an exquisitely described concept introduced by Huxley and Hanson (616), myosin filaments slide over interlocking actin, a process by which the sarcomere is shortened and the muscle “contracts.” During a contraction, the physical interaction between actin and myosin is continually broken and renewed in a process known as cross-bridge cycling. This process requires energy, and the energy system by which a given myosin isoform generates the necessary ATP dictates its metabolic profile (753). For instance, myosin heavy chain (MHC) I preferentially utilizes oxidative phosphorylation, whereas MHC II has greater glycolytic ATPase activity.
In addition to a well-engineered mechanism for contraction and movement, skeletal muscle tissue houses a range of other cell types such as satellite cells, macrophages, endothelial cells, pericytes, and others (1127). Muscle itself is considered a syncytium: each myofiber is multinucleated. As such, muscle nuclei may be able to orchestrate a wide-ranging yet integrated response to a given stimulus, such as exercise or loading. The degree to which muscle secretions, such as myokines (1020) and exosome-like vesicles (1423), influence adaptation continues to be elucidated. Finally, muscle’s extensive protein content makes it a primary amino acid reservoir for an organism (428, 1438); thus, muscle is a common casualty in conditions requiring a substantial, sustained immune response. While the complexity of muscle has presented unique methodological challenges, its relevance for exercise, performance and health, relative ease of access, and rich history of its study have led the field to an appreciation for its adaptability to AE and RE (Figure 5) and an enthusiasm to continue to explore the unknown.
Figure 5.
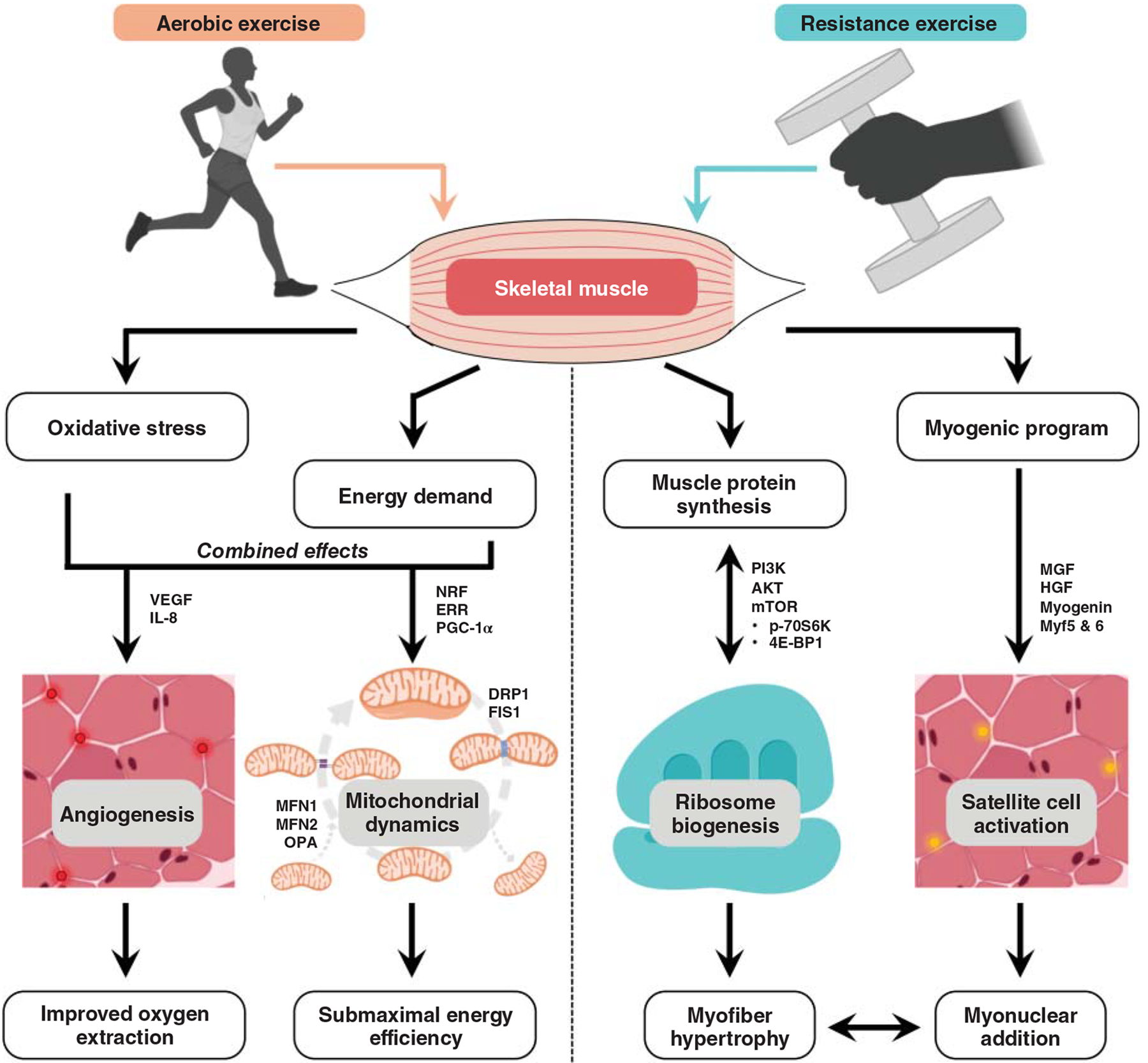
This figure illustrates key skeletal muscle adaptations to aerobic and resistance exercise, highlighting the role of molecular transducers of these effects that are described in the text.
Skeletal muscle adaptations to aerobic exercise
The molecular and histological adaptations to an AE training stimulus have been classically examined in skeletal muscle. AE performance is contingent on both high oxidative capacity and fuel economy, and muscle-level adaptations contribute to both factors. Importantly, the maximal metabolic capacity (33) and blood flow (756) of skeletal muscle exceed what can be physiologically achieved by cardiac output, indicating that the physiological ceiling exists outside of muscle itself in most environments (the obvious exception being low oxygen tension at altitude). Nevertheless, whole-body oxygen consumption is highly correlated with molecular indices of muscle oxidative capacity, including activity of enzymes such as succinate dehydrogenase (277) and citrate synthase (1368), both involved in oxidative phosphorylation. Despite the directional correlation, the magnitude of plasticity in these outcomes varies considerably: Gollnick et al. found that five months of AE training induced a 25% increase in Vo2max but a 95% increase in succinate dehydrogenase activity and a 116% increase in phosphofructokinase activity (469). These adaptations are likely to facilitate submaximal activity common in long-duration AE, improving the efficiency of energy production and utilization.
Myofiber adaptations
In addition to being characteristically smaller (~10%–20% vs. type IIa) and less powerful (~5-fold lower power vs. type IIa), type I myofibers are less fatigable than their fast-twitch counterparts (404). In humans, AE training does not usually induce a II-to-I shift (1322), although this has occasionally been reported (708). Also, hybrid (e.g., IIa/IIx or I/IIa) fibers may transition toward a pure type I myofiber phenotype (1322). Cross-sectional studies that report higher type I myofiber distribution in AE-trained individuals (470) cannot definitively rule out that individuals with differential proportions of slow-versus-fast myofibers self-select for a given exercise mode. In fact, genetic predisposition to athletic success has been an area of academic interest (623). It is unlikely that the observed differences are the effects of long-term training, since lifelong AE-trained older adults do not demonstrate a significantly higher proportion of type I myofibers than age-matched counterparts (498). The phenomenon by which type II myofibers undergo preferential age-related atrophy and apoptosis adds an additional complexity to this comparison (979, 1105). While preclinical studies have identified some of the molecular determinants that lead to an oxidative myofiber phenotype (e.g., MEF-2) (1063, 1452), it is unclear whether these factors play similar roles in adult human muscle adaptations to exercise. Illustrating a potential connection, muscle unloading, a stimulus that induces a slow-to-fast myofiber shift in humans (133, 1324), has been associated with inhibition of MEF-2 (1130).
At the cellular level, AE training induces important adaptations in the structure and function of type I myofibers. Some evidence suggests a reduction in type I myofiber diameter (1322, 1427), which is thought to be beneficial for reducing diffusion distance between oxygen-supplying capillaries and the center of the myofiber. However, this is not a universal finding (469, 522, 1075). Furthermore, the addition of muscle capillaries with AE training is likely to keep pace with or exceed myofiber hypertrophy, such that unavailability of blood supply is unlikely to impose a size limitation (708). Myofiber functions such as shortening velocity (405, 522), power (498), and oxidative capacity (544) are commonly improved or preserved with AE, with the strongest effects usually reported in type I myofibers. The common AE practice of tapering appears to have additional effects in type IIa myofibers that may contribute to athletic performance (523, 824, 1319). Thus, despite the classically overengineered architecture of skeletal muscle (35, 139), long-term training elicits beneficial effects at the level of the muscle cell.
Mitochondrial biogenesis
As the site of ATP production, muscle mitochondria form a dense and active network that supports the bioenergetic needs of skeletal muscle (535). Animal-based research as early as the 1950’s demonstrated a relationship between aerobic capacity and respiratory enzyme content (760, 1012), leading to a host of studies examining the influence of AE on mitochondrial biogenesis in a range of tissues and populations (344, 472, 809, 1132). Increased mitochondrial biogenesis in response to AE may be reflected by changes in copy number of mitochondrial DNA (mtDNA) (884, 1431). Similarly, membrane phospholipid cardiolipin may be used as a marker for mitochondrial content (883). Copy number is elevated by >50% in AE-trained versus untrained individuals (1079) and may increase following short-term training in older adults (883). These effects are thought to be a result of acute “bursts” in transcription of mtDNA following exercise (426, 1033).
Mitochondrial proteins are encoded on both the nuclear and mitochondrial genomes (424). Mechanistically, much regarding the transcription and regulation of these factors have been discovered using animal models and later translated to humans in targeted studies. Transcription factor families such as nuclear respiratory factors (NRF) and estrogen-related receptors (ERRs) are key regulators of transcription of nuclear-encoded mitochondrial genes (465, 1154). Both NRF and ERR have been shown to be increased in response to AE (204, 398). Transcription of the mitochondrially encoded genes is largely regulated by Transcription factor A mitochondrial (Tfam) (465, 1373). Tfam is more highly expressed in skeletal muscle of highly AE-trained adults (1323), but acute AE does not consistently lead to upregulation of Tfam at the mRNA (260, 1061) or protein (490) level.
Co-activators such as peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) interact with these transcription factors to promote increased mitochondrial number, size, and protein content in response to AE (62, 273). Both acute (521, 1050) and chronic (490, 1131, 1323) AE stimulate expression of PGC-1α in human skeletal muscle. PGC-1α may be processed via alternative splicing mechanisms into any of four transcripts (PGC-1α1–4) with distinct but overlapping biological roles (856): PGC-1α1 appears most important for adaptations in aerobic metabolism (273, 801), while PGC-1α4 is believed to play a role in muscle hypertrophy (1126). The family of PGC-1α isoforms also partially controls key metabolic processes in skeletal muscle, including lipid oxidation (600), mitochondrial autophagy (1344), and angiogenesis (43, 240) via upregulation of VEGF.
Mitochondrial dynamics
Along with increased mitochondrial biogenesis, dynamic changes in mitochondrial volume and connectivity accompany muscle adaptations to AE (50, 59, 914). In key processes involving inner and outer mitochondrial membrane proteins, mitochondria can either be joined together (fusion) or cleaved apart (fission), based on cellular energy state (45, 366, 410). In a balanced process, fusion and fission maintain overall mitochondrial function as the cellular mechanism of cycling newly formed mitochondria into the mitochondrial pool while damaged/dysfunctional mitochondria undergo autophagy (45).
Fission is promoted through phosphorylation of dynamin-related protein-1 (DRP1), which then binds to an outer membrane receptor such as mitochondrial fission 1 protein (FIS1) or mitochondrial fission factor (MFF) (998). DRP1 activity is increased in response to acute and chronic AE in human muscle (734). Mitochondrial fusion occurs when the outer membrane GTPases mitofusin 1 and 2 (MFN1 and 2) dimerize and pull two mitochondria together (410). The inner membrane compartments are then joined by optic atrophy-1 (OPA1) (836). Markers of both fission and fusion have been shown to be elevated after AE training (721, 1033). Studies in older adults suggest that long-term AE training appears to promote fusion as the ratio of fusion-to-fission proteins increases. These changes are associated with improved metabolic functions including insulin sensitivity and glucose handling (50, 59, 385, 1456). While these and other (1212, 1213, 1273) mechanistic factors underlying improved oxidative capacity have been identified in animal skeletal muscle, continued research is necessary to fully elucidate how mitochondrial biogenesis and dynamics impact exercise training adaptations in health and disease.
Subcellular adaptations
As a regulator of crossbridge formation, calcium plays a key role in skeletal muscle intracellular signaling. During exercise, action potentials travel along the transverse tubules of skeletal muscle to activate dihydropyridine receptors. This promotes opening of the sarcoplasmic reticulum channel ryanodine receptor 1 (RYR1), leading to an increase in cytosolic calcium (24, 484). Naturally, calcium release, muscle contraction, and re-sequestering of calcium are energetically costly. Thus, improved capacity for oxidative phosphorylation is essential for skeletal muscle to support long periods of repetitive contractions during AE (233, 582, 585). The CaMK family plays roles in calcium-sensing, with downstream effects relevant to AE. In mice, CaMK IV activates PGC-1α and associated downstream pathways leading to mitochondrial biogenesis (1451), whereas CaMK II has been associated with structural and contractile adaptations (363). CaMK II is the primary isoform expressed in human muscle (1119, 1120). Basal CaMK II phosphorylation is elevated following AE training (1061, 1118) in a muscle-specific fashion (1118); acute AE also leads to increased activity of CaMK II (1119).
In 1982, Davies et al. reported a molecular signature associated with tissue-damaging exercise (308). Since this time, reactive oxidative species (ROS) and reactive nitrogen species have received attention for their roles in communication and signal transduction in response to exercise (1067, 1068). For instance, superoxide (O2−) is produced in a variety of skeletal muscle structures such as the sarcoplasmic reticulum, transverse tubules, sarcolemma, and (most often) complexes I and III of the mitochondrial electron transport chain (85). Active superoxide can undergo dismutation into hydrogen peroxide (H2O2) either spontaneously or via enzymatic reaction. In animals, mechanisms that facilitate dismutation are more abundant in type II myofibers (36). Notably, H2O2 is a weak oxidant and may react with many signaling pathways to promote mitochondrial health (383, 628, 629, 1067, 1364). In humans, emission of H2O2 is elevated during inactivity and reduced by AE training (487). Another key signaling molecule is NO, which is converted from the amino acid l-arginine by one of four nitric oxide synthase (NOS) isoforms; NOS1 and NOS3 are the most prevalent isoforms produced in skeletal muscle (457, 938). When NO is increased in skeletal muscle in response to aerobic exercise, it may promote mitochondrial biogenesis through PGC-1α (807). Alternatively, NO may react with molecular oxygen to form the oxidizing agent peroxynitrite, which may result in inflammatory (1272) or cytotoxic (938) stress.
While these factors are known to contribute to mitochondrial turnover and exercise adaptation (1067), the full range of their function in skeletal muscle adaptation is a complex and hotly debated research area (200). Some evidence suggests that skeletal muscle increases endogenous ROS scavenging in response to the heightened oxidative stress of training, indicated by increased expression of superoxide dismutase (SOD), glutathione peroxidase (GPX), and markers of mitochondrial biogenesis (1102). However, it remains incompletely clear whether supplementation with exogenous antioxidants (e.g., vitamins C & E) is detrimental during this early adaptation window. Ristow et al. found that, while four weeks of AE training-induced improvements in glucose and fatty acid metabolism, insulin sensitivity, and antioxidant defense in both trained and untrained men, vitamins C and E blocked these favorable metabolic adaptations (1102). Although several of these factors appear to be more highly expressed at baseline in muscle of trained individuals (Vo2max ~20% higher vs. untrained), this was not statistically evaluated. In conflict, Bente Pedersen and colleagues have performed a series of studies that collectively demonstrate that vitamin C & E supplementation has no effect on training-induced increases in aerobic capacity, mitochondrial adaptations, superoxide dismutase activity, and insulin sensitivity in healthy young men (1460, 1462), although it does alter muscle inflammatory signaling (400, 401, 1461). More recently, it was shown that antioxidants blunt long-term increases in several other cellular adaptations, independently of any negative effects on whole-body or mitochondrial aerobic capacity (927, 1013). Notably, however, many of these studies assess performance based only on Vo2max, whereas metabolic efficiency (i.e., running economy) is an equally important determinant of AE performance (96). Given the unique metabolic stress associated with longer-duration AE (529, 975, 1357), the subcellular effects of exogenous antioxidant supplementation may be differentially manifested in longer events. This area clearly warrants further investigation, particularly given the apparent influence of sex, age, and/or training status (141, 329).
Myokines
The role of skeletal muscle in intracellular communication is illustrated by a class of factors known as myokines, a muscle-specific subcategory of signaling molecules called cytokines (1023). These factors were originally characterized for their roles in coordinating immune cell motility, activation, and function (1385), but knowledge of their wide-ranging behavior has increased exponentially. Furthermore, the cytokine nomenclature has been amended as necessary for application to signaling molecules exchanged by other tissue types (e.g., adipokines, hepatokines), while myokine has since been expanded to include all factors produced and released by muscle, including lipid-derived molecules and other metabolites. IL-6 was the first signaling factor identified as a myokine (997): it is now known to serve a range of functions relevant to skeletal muscle, including promotion and resolution of exercise-induced inflammation (1019), protein balance (508, 1348), and energy metabolism (549, 1440). Acute AE activates skeletal muscle transcription and secretion of IL-6, along with other myokines, including (but certainly not limited to) IL-10, IL-4, and IL-8 (220, 820, 977, 978, 1241).
The time point at which peak myokine activity occurs postexercise appears to vary widely based on the intensity and duration of the stimulus. For example, using a time course series of muscle biopsies, Louis et al. found that muscle IL-6 gene expression was elevated up to 24 h after a 30-min AE bout but peaked at the 8 h time point (820). Steensberg et al. demonstrated that muscle IL-6 increased 30 min into a 3 h knee extension exercise bout and remained significantly elevated at the cessation of exercise (1248). It is also likely that training status plays a role in the myokine response to acute exercise (402). As little as one previous exposure to an identical exercise bout may influence myokine production (330, 602) and release (571, 881), even when spaced up to 4 weeks apart. At the other extreme, males with a lifelong history of AE exhibit a less robust inflammatory response to an unaccustomed loading stimulus than age-matched nonexercisers (757). Continued research is necessary to elucidate what impact this effect has on immune health and/or muscle adaptation.
Research using ‘omics approaches has expanded knowledge of the full range of myokines produced and secreted by skeletal muscle. For example, Pourteymour et al. applied RNA-sequencing to the discovery of the muscle “secretome” (1064) and revealed an important role for macrophage colony-stimulating factor-1 in response to combined AE+RE training. This and other molecules that elicit communication with immune cells (904, 1259) are likely to play a role in muscle adaptations to exercise, given the role of macrophage biology in resolution of inflammation and skeletal muscle regeneration (228, 691). The complex interplay of myokine dynamics, biology of target cells, and phenotypic influences (e.g., age, training status) clearly warrants continued investigation via integrated application of ‘omics platforms.
Skeletal muscle adaptations to resistance exercise
The inherently high degree of plasticity in skeletal muscle tissue is perhaps most apparent in its adaptation to RE, in which muscle contracts against a load. Classically, RE involves progressive overload of the muscle to challenge homeostasis and trigger numerous molecular pathways resulting in structural and physiological adaptations (76, 647, 758, 1107). One of the hallmark adaptations to RE is increased skeletal muscle mass, or hypertrophy (76, 531, 647, 758, 1107). Provided that the amount of skeletal muscle mass is associated with increased healthspan, protection against various diseases (97, 758, 1309), and better survival outcomes following infection, hospitalization, and surgery (274, 666, 773, 1353), research focus has been directed toward identifying and understanding mechanisms of RE-induced muscle hypertrophy. However, other dimensions of skeletal muscle health (e.g., strength, fatigability, fuel economy) are also common RE outcomes.
Histological and cellular adaptations
In addition to increased whole muscle size, myofiber cross-sectional area commonly increases with RE training (1016). Some studies report that this is most notable in type IIa myofibers (1244, 1246). Application of the size principle for motor neuron firing would dictate that higher RE loads necessitate the recruitment of myofibers with a higher firing threshold, that is, type II myofibers (880). Indeed, most studies support that higher loads elicit a higher contribution of energy from type II myofibers (471, 496), yielding a more pronounced molecular response (1091). Emerging research is investigating whether similar effects can be achieved at lower intensities performed to failure (933) or with blood flow restriction (1167, 1201). While a complete slow-to-fast shift is uncommon, RE training induces an increase in type IIa myofiber distribution, often at the expense of hybrid or type IIx myofibers (1244, 1246). Beyond this, RE training enables myofibers to contract in synchronicity due to underlying neural adaptations to improve efficiency of movement (1, 1140). Within the myofiber, physiological parameters such as unloaded shortening velocity and power are increased following RE training in both fiber types (1325, 1328), although these patterns are not well-sustained into the ninth decade of life (1090, 1216), suggesting a potential age-related impairment in muscle plasticity. Thus, while RE training is important for maintenance of muscle mass and function throughout aging (758), there is evidence to suggest that an RE regimen must be initiated at least in middle-age and continued through later life. Future investigation of muscle and other health phenotypes in lifelong RE-trained individuals may shed light on the optimal strategy to preserve lifetime peak muscle mass throughout aging.
Muscle protein balance
Skeletal muscle mass is regulated by the equilibrium between muscle protein synthesis and breakdown. Naturally, a positive muscle protein balance, in which synthesis rates exceed breakdown, yields increased overall muscle protein accretion (297, 309, 872, 1043, 1256). In healthy, disease-free, normal conditions, RE has a more pronounced effect on muscle protein synthesis than on protein breakdown (56, 467, 741). In fact, an acute bout of RE is a potent enough stimulus to increase protein synthesis for up to 48 h (297), favoring a positive net protein balance. Transiently elevated muscle protein synthesis eventually returns to basal levels after acute RE. Thus, to facilitate long-term hypertrophy, it is necessary to perform repeated bouts (i.e., training). Previous studies have corroborated that the magnitude of the hypertrophic response is predicted by changes in acute muscle protein synthesis (157, 296), but others have found otherwise (905). These discrepancies may be related to the method used to quantify protein synthesis (infusion protocols vs. deuterium-enriched water), selected sampling time frame, participant demographics (age, sex, training status) (296), and a mixture of other intrinsic factors (e.g., individual genotype, epigenetic effects, etc.). Despite the wide range of hypertrophic responses to RE, there is a consensus that markers of protein synthesis are at least qualitatively predictive of long-term muscle hypertrophy (157, 296, 297, 905). Therefore, complementing measurement of muscle protein balance with abundance of molecular factors known to influence synthesis and/or breakdown may provide the clearest picture of the effects of RE.
Protein translational efficiency
In addition to contractile proteins actin and myosin, skeletal muscle hypertrophy is dependent on increased availability of proteins that serve a range of muscle functions. To produce functional proteins, muscle must be equipped to translate nascent transcripts (mRNAs) upregulated in response to RE in an efficient manner. So-called “translational efficiency” can be defined as the rate of mRNA translation by skeletal muscle cell (868). The current understanding is that changes in translation efficiency are mainly mediated by a variety of molecular pathways including phosphatidylinositol 3-kinase (PI3K), protein kinase B (Akt), and mammalian target of rapamycin (mTOR). In particular, mTOR targets p70S6 kinase (p70s6k) as well as eukaryotic translation initiation factor binding protein-1 (4E-BP1). Activity of these factors is upregulated following acute (607, 905, 1297) and chronic (530) RE.
Protein translational capacity
In addition to an increased abundance of enzymes and other factors in the translation pipeline, overall capacity to translate mRNAs is a rate-limiting step in protein synthesis. The highly conserved organelle responsible for translation is the ribosome, an amalgamation of ribosomal proteins and single-stranded rRNA into distinct subunits that position mRNAs while facilitating binding of specific tRNAs to yield an amino acid sequence. While the overall muscle RNA pool is composed of rRNA, mRNA, tRNA, and other noncoding RNA species (395), rRNA constitutes almost approximately 80% of the total pool. Thus, assessment of overall RNA content may be used as a proxy for ribosomal content and, indirectly, a reflection of the capacity for regulation of muscle protein synthesis (155, 217, 395).
Increases in the ribosome pool (i.e., ribosome biogenesis) demand a fine and coordinated process of several pathways. Briefly, this involves transcription of ribosomal DNA (rDNA), followed by processing, maturation, and assembly of rRNA and its ribosomal proteins (155, 395). It has been demonstrated that acute bouts of RE can upregulate some of these pathways, facilitating the molecular environment to promote ribosome biogenesis (396, 397). For instance, Figueiredo et al. (397) demonstrated increases in the phosphorylation of rDNA transcription factors such as upstream binding factor (UBF) and c-Myc, as well as the total protein levels of UBF and transcription initiation factor (TIF)-IA; these changes were accompanied by increases in pre-rRNA-45S. With chronic RE exposure, these acute elevations in factors related to ribosome biogenesis yield augmented total RNA concentration and rRNA density within skeletal muscle (157, 396, 517, 1094, 1244), increasing the overall capacity to support hypertrophy. Muscle cell culture models corroborate the importance of ribosomes in muscle hypertrophy in the absence of systemic influences: myotube growth in vitro may be completely blunted by administration of mTORC1 inhibitor rapamycin (956) or inhibition of polymerase I, the DNA polymerase responsible for transcription of rRNA (1244).
Studies in humans have demonstrated a relationship between ribosomal density and the magnitude of RE-induced hypertrophy (396, 517, 908, 1244). For example, using total muscle RNA as a surrogate for ribosomal density, Stec et al. found that individuals that experienced the most robust hypertrophic response (“extreme responders”) presented not only a higher ribosomal density at baseline but also heightened posttraining ribosomal density, indicating ribosome biogenesis, in comparison to individuals that responded poorly to RE (1244). In concert with these results, Mobley et al. (908) demonstrated that only individuals that demonstrated moderate to large gains in muscle mass exhibited increased total RNA concentration in response to an RE intervention. Ribosome biogenesis, or at least translational capacity, is thought to be a contributor to the observed differences in hypertrophic response to RE between young and old individuals: data support that older individuals may present an attenuated hypertrophic response in comparison to younger adults. An underlying reason may be impaired activation of molecular pathways related to ribosome biogenesis (e.g., c-Myc gene expression and total protein levels of c-Myc and TIF-IA) in older individuals (156). Similarly, others have found that only middle-aged adults exhibit increased expression of 45S-preRNA after a single acute bout of resistance exercise, whereas older adults do not (1245). Many other potential mechanisms are thought to be at play in the attenuated age-related response to RE training, including (but not limited to) changes in the hormonal milieu (638), oxidative stress (656), and chronic basal muscle inflammation (757, 886).
Satellite cells and the myogenic program
There is limited evidence in humans that muscle fibers increase in number (hyperplasia) in response to RE. However, progressive overload contributes to a stress that eventually necessitates a key structural change: the addition of nuclei to the mature muscle syncytium. Each myonucleus is thought to be responsible for (and only capable of) coordinating homeostatic processes within a limited volume of cytoplasm, a region known as its “myonuclear domain” (533, 663, 1039). Accordingly, the preexisting number of myonuclei may eventually become a limiting factor as myofiber size increases in response to RE. The addition of new myonuclei into skeletal muscle is reliant on specialized, mononuclear, stem-like cells known as satellite cells (SCs), found between the sarcolemma and the basal lamina (862). In resting conditions, SCs are quiescent but are activated in response to an exercise stimulus, such as mechanical stress imposed by RE. Activated SCs may then proliferate and either fuse with an existing myofiber, donating its nucleus, or return to a quiescent state, rejoining the SC pool (225, 349, 533, 534, 1014, 1227, 1463).
RE promotes an orchestrated increase in the expression of several factors thought to be crucial in regulating the SC cycle, such as mechano-growth factor (MGF), hepatocyte growth factor, and myogenic regulatory factors: for example, increased MyoD, myogenin, myogenic factors (Myf)-5 and -6, decreased myostatin (75, 871, 993, 1227, 1463). In addition to these molecular factors, studies typically examine SC content in histological muscle preparations using immuonostaining for their characteristic markers CD56 or paired-box protein 7 (Pax7) (390). Several studies have demonstrated an expansion of the SC pool in response to both acute (345, 964, 1228) and chronic RE (295, 908, 1039, 1229, 1360). Furthermore, several have shown that long-term hypertrophy is related to expansion of the SC pool (104, 1038, 1039, 1361, 1362), while others have demonstrated that the basal SC pool size is of equal importance (1039). However, still others have not observed significant relationships between SC number and magnitude of RT-related hypertrophic response (908).
Notwithstanding the apparent effects of acute and chronic RE on SC dynamics, the exact threshold at which addition of myonuclei becomes necessary is still not clearly understood (946). While the standing estimate had been that an approximately 25% increase in myofiber size would necessitate myonuclear addition (664), a recent meta-analysis indicated that increases above 10% were sufficient (263). Even within this meta-analysis, the myonuclear addition response appears to track with the cellular demand such that more myonuclei are added at approximately 20% than at 10% myofiber hypertrophy. Furthermore, myofiber type may play a role in this process: some studies have observed that type I myofibers are more likely than type II myofibers to increase the number of myonuclei (104, 924, 1229). This could be explained by a higher myonuclear domain ceiling in type II myofibers or characteristically different architecture between myofiber types; for example, type I myofibers tend to be situated near more muscle capillaries, which may deliver factors to activate SCs more efficiently (648, 963). While the threshold for addition of myonuclei to existing myofibers is not presently clear, skeletal muscle is capable of managing RE-induced perturbations to homeostasis without the immediate addition of myonuclei, indicating that SC fusion is a more stable change or that their heightened activity after RE may serve a different biological purpose (946).
Importantly, the role of SCs in hypertrophic response to RE training has been under increased scrutiny. Animal models provide an avenue for mechanistic manipulation of SCs, such as the use of a tamoxifen-inducible muscle-specific Pax7 knockout mouse. Using this design, McCarthy et al. (866) found that depleting approximately 90% of the muscle SC pool in mature mice does not impair hypertrophic capacity, at least in response to short-term (i.e., 2 week) mechanical overload stimulus. In contrast, Fry et al. (429) demonstrated that longer-term hypertrophy (i.e., 8 week) was attenuated in animals with SC depletion. Thus, perhaps the muscle hypertrophy attained in the former study was not sufficient to surpass the threshold at which addition of new myonuclei via SC fusion was necessary. In addition to continued research into the concept of the myonuclear domain, discovery of other factors that play a role in the myogenic program may prove useful, particularly as potential targets for individuals that are poor responders to RE.
Section summary
Skeletal muscle mass and function are clearly important for movement, but available evidence supports that the molecular environment within the tissue is equally critical for healthy function. While decades of research in human exercise studies have elaborated on the molecular transducers of skeletal muscle adaptations to both AE and RE, there is a rapidly growing understanding of muscle’s roles in physiology that extend beyond movement and contraction. For example, mechanisms by which muscle communicates with other organ systems are still being elucidated. Given the ease of access to skeletal muscle tissue and the insight it may provide into overall health, continued investigation of its molecular profile in response to acute and chronic exercise is likely to provide guidance toward therapeutic targets to improve exercise tolerance and responsiveness across individuals, particularly in the context of morbidity and disease.
Cardiovascular System Adaptations to Exercise
The cardiovascular system is often acknowledged as the primary limitation to exercise performance (35) and is a key determinant of overall aerobic capacity, an indicator of whole-body health (954). Exercise (particularly AE) necessitates sustained, elevated cardiac output, reduces peripheral vascular resistance, and increases venous return of blood to the heart, stressing the cardiovascular system to adapt to heightened mechanical and metabolic demands. Beyond this, assessment of cardiovascular function using electrocardiography, blood pressure, and circulating metabolic markers is relatively well-developed and highly accessible. As such, the cardiovascular system is a frequently investigated and well-understood system in the context of exercise.
Cardiovascular adaptations to aerobic exercise
Ventricular morphology
Adaptations to chronic AE are highly studied and include physiological cardiac hypertrophy (551, 1114), increased myocardial oxygen supply, blood flow, and transport capacity, increased vessel size, and improved endothelial function (551). In particular, the left ventricle (LV), whose action is responsible for pumping oxygen-rich blood into the aorta for delivery to the periphery, is often increased in both size (volumetric hypertrophy) and wall thickness (structural hypertrophy) (1412), driven by increased cardiomyocyte size (545). This process, referred to as cardiac remodeling, is brought about to meet the heightened physiological demands associated with higher tissue oxygen consumption rates during exercise (840, 1359).
Cardiac remodeling is traditionally categorized as either concentric or eccentric (1179, 1359). Under physiological conditions, concentric growth is typically defined by greater increase in LV wall thickness compared to internal diameter, whereas physiological eccentric growth is characterized by increases in both LV internal diameter and wall thickness (1359). The time course of these adaptations is not uniformly linear. For instance, one year of intensive AE marathon training in young, sedentary men and women progressively increases both left and right ventricular mass; however, LV volume does not demonstrate a significant increase until after six months of training (44). Furthermore, adaptations are easily reversed: although sometimes accrued within two to three months of training, they may diminish as early as a few weeks into detraining (360, 686, 851).
Cardiac remodeling is influenced by mechanical stimuli known as hemodynamic forces such as flow, pressure, stretch, strain, and compression (166, 492). The combination and/or contribution of these hemodynamic forces upon the cardiovascular system establish the appropriate parameters for adaptation (1359). Thus, exercise type and modality influence physiological cardiovascular adaptations. During a purely aerobic activity, heart rate, venous return, and contractility are all elevated above resting levels; still, even within this broad category, important distinctions exist. For example, although running and rowing are both AE activities requiring a substantial cardiovascular component, downstream outcomes on cardiac remodeling can vary considerably (Figure 6). Rowing is associated with both volume (isotonic) and pressure (isometric) stress, while running involves primarily isotonic forces on the heart (1399). As such, highly trained individuals in both modalities exhibit larger LV volumes (341), but rowers exhibit greater LV mass (1399).
Figure 6.
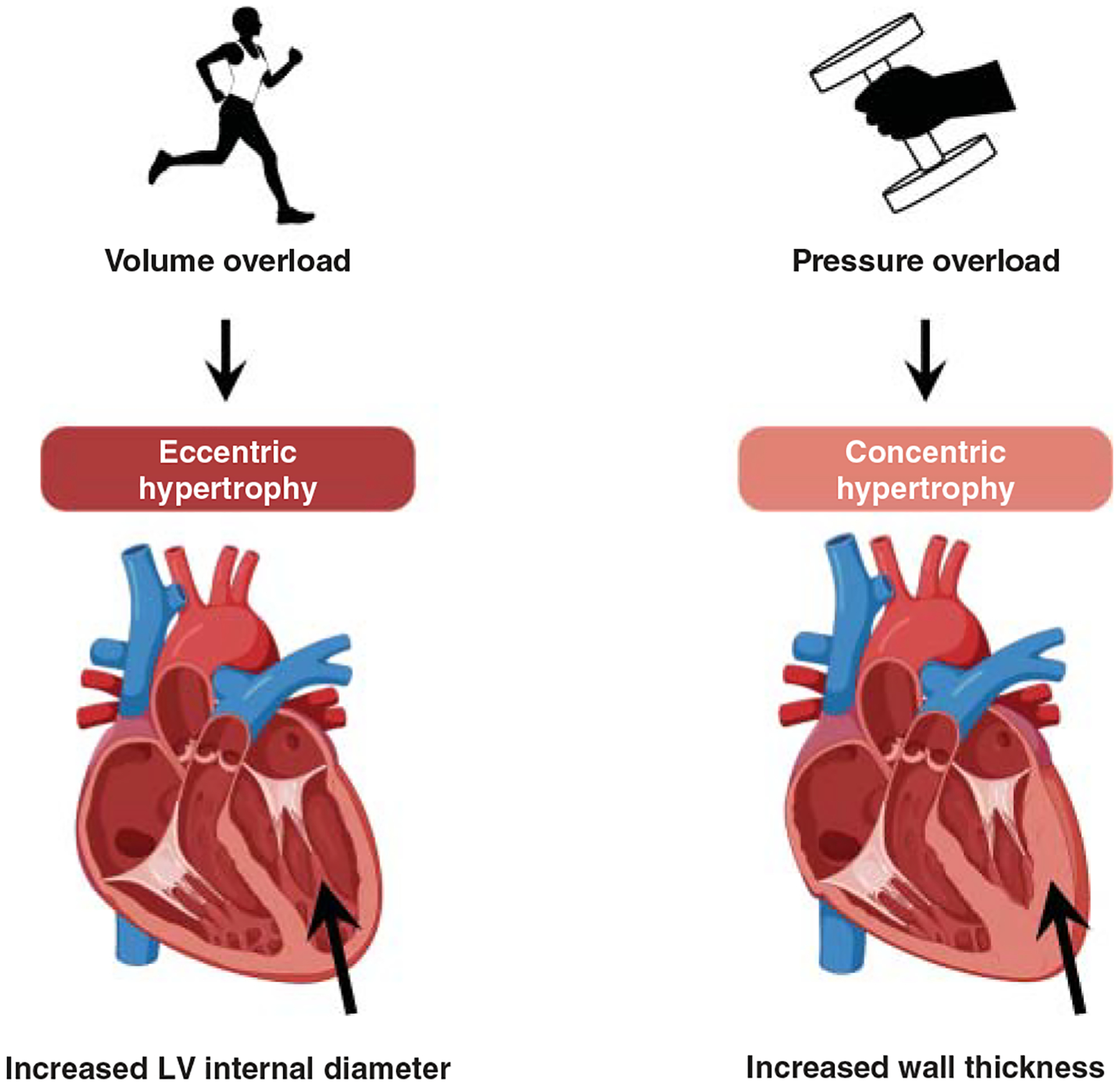
This figure illustrates physiological hypertrophy resulting from exercise training. The long-term cardiovascular adaptive response depends on the type of hemodynamic stress resulting from the modality of exercise training. Most commonly, aerobic exercise imposes volume overload, leading to eccentric hypertrophy, whereas resistance exercise elicits concentric hypertrophy through pressure overload. Relevant molecular and cellular transducers are explored in the text.
Cardiac cell adaptations
Increases in cardiomyocyte size drive LV hypertrophy (1179, 1359) and are considered a hallmark feature of AE training (420, 678, 1066). Like skeletal muscle, cardiomyocytes are terminally differentiated and primarily increase in size rather than number after exercise training. However, AE training in animals has been shown to lead to increases in newly formed cardiomyocytes, angiogenesis, and other parameters of myocardial remodeling through a range of cardiomyocyte growth factors including neuregulin (NRG)-1, bone morphogenic protein-10, and periostin (1398). Examination of cardiomyocyte physiology is naturally limited to preclinical models but provides important insight into potential mechanisms of adaptation. For example, in rats, AE enhances contractility and calcium handling (961, 1393, 1437). Molecular cues that propagate these effects in addition to cardiomyocyte hypertrophy are wide-ranging and likely vary by exercise type (AE vs. RE), hemodynamic stress-induced hypertrophic outcome (concentric vs. eccentric), and other phenotypic variables (840).
Although cardiomyocytes are the primary cause of cardiac hypertrophy in adults (784), there is evidence that other cardiac cell types also adapt to exercise training and may be the basis of other beneficial training effects. For instance, fibroblasts are responsible for production and release of angiogenic factors including VEGF and matrix metalloproteinases (MMPs) (1074). Furthermore, adult endothelial cardiac stem cells can be activated to differentiate toward the cardiomyocyte lineage by increased cardiac workload for smooth muscle cells and endothelial cells (1341). In the periphery, endothelial progenitor cells (EPCs) are affected by both AE and RE (431, 1086) in a manner influenced by exercise duration and intensity (431, 755). Derived from bone marrow stem cells (782), EPCs are involved in the regulation of large vessels and microvasculature expansion. EPCs are involved in vascular repair and vessel formation and have the ability to differentiate into mature endothelial cells when prompted by exercise training (51). In peripheral blood, EPC concentration increases with AE (1086), indicated by heightened presence of the common surface marker CD34 (51). Moreover, EPCs may secrete VEGF and granulocyte-colony factors, leading to neoangiogenesis (51).
Arterial adaptations
Arteries constitute the first branch of peripheral circulation and are responsible for transportation of blood from the heart to other organs. In addition to an approximately five-fold increase from basal cardiac output, exercise necessitates a highly regulated redistribution of blood flow to contracting muscle in lieu of visceral organs, the brain, and so on (431, 1124). To facilitate this redistribution, both conduit and resistance arteries play roles in regulating blood flow (551). Conduit arteries are highly elastic and handle the highest pressure loads from the aorta; thus, exercise-induced adaptation in major conduits has been an area of clinical interest. A hallmark study by Hambrecht et al. investigated the role of hemodynamic forces on vascular function of the coronary artery (514), which supplies blood to the myocardium. They demonstrated that four weeks of exercise in individuals with stable coronary artery disease led to improved endothelium-dependent vasodilatory capacity (514). Others have illustrated that exercise improves coronary artery diameter and blood flow (343, 492).
Additionally, Dinenno et al. (334) found that AE increases femoral arterial remodeling, and Miyachi et al. reported that AE-induced increases in femoral artery size were associated with regional increases in blood flow (907). Furthermore, exercise training may promote increased flow within the carotid artery in healthy populations (810) and women with sarcopenic obesity (1007). These training-induced improvements may facilitate efficient delivery of energy substrates, heightened capacity for clearance of metabolic by-products, and reduced endothelial shear stress, the latter of which is thought to contribute to impaired endothelial cell function (494).
Resistance arteries are largely responsible for directing blood flow (244) and can be assessed in humans using plethysmography (659) or flow-mediated dilation (1310). Hemodynamic forces are critical for vascular adaptation: during acute AE, increased heart rate and blood pressure impose cyclic circumferential stress on the cardiovascular system. In a complex pathway, endothelial cells respond via upregulation of genes including endothelial nitric oxide synthase (eNOS), leading to heightened abundance of ROS such as NO (492). Training may also increase superoxide dismutases 1 and 3 (430, 570, 1069), enzymes that play a role in antioxidant defense (1466). Thus, while exercise acutely increases oxidative and shear stress, adaptive mechanisms are in place to handle repeated bouts (492), concurrently improving the basal health of the system.
Chronic exercise training-induced acute shear stress may prompt arterial remodeling, structurally preparing the vasculature to handle increases in shear stress or other hemodynamic perturbations (493). The mechanisms behind this effect continue to receive attention but appear to involve increased arterial diameter partly mediated by NO (551), which is primarily produced in vasculature through the actions of eNOS (1310). Further supporting that intermittent high shear stress is an adaptive trigger, complete removal of shear stress in rats increases expression of pro-inflammatory genes such as ICAM-1, VCAM-1, E-selectin, and monocyte chemoattractant protein-1 (MCP-1) (643).
Microcirculation
Broadly, the microcirculation is a network of arterioles, capillaries, and venules which distribute, perfuse, and collect blood from tissues. Several clinical trials have investigated adaptations in microcirculation in response to exercise training. Capillaries are in direct contact with skeletal myofibers and can be easily visualized and counted in skeletal muscle biopsy samples. Via angiogenesis, AE commonly leads to proliferation of the capillary network in human skeletal muscle (34, 930). Increased muscle capillarization is one of the later adaptations to AE (35), and newly added capillaries are fairly stable, lasting a few weeks into detraining (708, 943) or even throughout aging if training is continued (499, 1326). Angiogenesis is largely an adaptation to low oxygen stress, as a hypoxia-based training paradigm (four weeks single-leg cycling with ischemia) increases capillary-to-myofiber ratio (376). Increased capillarization, induced by shear and passive stress, is mediated through angiogenic factors such as VEGF (579, 842, 990, 1284). In animals, removal of VEGF blunts exercise-induced increases in muscle capillary density and capillary-to-myofiber ratio (317).
Cardiovascular adaptations to resistance exercise
Although the vast majority of literature focuses on cardiorespiratory adaptations to AE, research into RE adaptations has increased, exposing a knowledge gap related to cellular, molecular, and regulatory mechanisms that may be specific to RE. Generally, RE has been shown to lead to increased ventricular mass, ventricular wall thickness, septum thickness, and peripheral vascular resistance (431, 1257). Short, intense bouts characteristic of RE are associated with increased blood pressure (1359). Thus, this physiological stimulus is more often associated with a pressure than volume load, leading to concentric hypertrophy (1057). Indeed, LV adaptations to RE are uncommon when accounting for LV wall thickness (1233). Others have shown that six months of RE training leads to decreased carotid wall thickness; however, the authors suggest that these changes may be driven by general exercise-induced increments in blood flow and shear stress, rather than a feature unique to RE (1234). It is well-established that RE training elicits pressure overload response in cardiac cells, which leads to intracellular signaling through endocrine cascades (113). Specifically, the renin-angiotensin system (RAS) responds to overload eliciting a mechanical stretch, and angiotensin II type I receptor (AT1) expression is associated with physiological cardiac hypertrophy after RE in a rat model (83). In the periphery, moderate RE can lead to increases in circulating EPCs and angiogenic factors such as MMP-2, MMP-3, and MMP-9 (1122); MMP-9 is a key regulator of vascularization, as its absence in a mouse knockout model reduces the capacity to recruit EPCs and develop blood vessels (598). Studies in humans have demonstrated increased skeletal muscle capillarization following short-term RE in young (587) and older men (1363), and additional evidence supports that capillarization may be highly important in muscle hypertrophy, a fundamental RE outcome (923, 1226). Together, the paucity of literature examining cardiovascular adaptations to RE represents an area ripe for continued study, particularly in the context of mechanistic factors that facilitate adaptation.
Molecular transducers of cardiovascular adaptations
Growth factors
Ligands including IGF-1, VEGF, and thyroid hormones modulate cellular growth, survival, and metabolism necessary for angiogenesis. Released from liver in response to growth hormone, circulating IGF-1 increases in response to both AE (1467) and RE (1482). Both insulin and IGF-1 are necessary for growth and development through intracellular signaling cascades (526). The IGF-1 receptor is a regulator of physiological cardiac hypertrophy (697, 875). In clinical models, VEGF interacts with NO in response to hemodynamic force such as shear stress. Known for regulating both vasculogenesis in development and angiogenesis in a mature organism, VEGF stimulates the production of NO in endothelial cells (551, 1005).
Thyroid hormones
Thyroid hormones can have significant cardioprotective effects on the cardiomyocytes and the vasculature (631). Furthermore, thyroid hormone increases venous return, cardiac output, and systemic vascular resistance (476). Thyroid hormone signaling occurs through the thyroid gland, which secretes thyroxine (T4) and triiodothyronine (T3), the active form of the thyroid hormone; both are associated with physiological cardiac hypertrophy (665). Conflicting findings exist in regard to the effects of a single bout of aerobic treadmill exercise on thyroid hormones (250, 601). Some evidence demonstrates decreased thyroid hormone after acute AE (695), whereas others report an increase (714); these discrepancies may be the result of duration, intensity, or exercise mode. In support, Simsch et al. found a differential effect of AE versus RE on levels of thyroid stimulating hormone (thyrotropin) (1208), a pituitary hormone upstream of the thyroid gland (1004). In this study, well-trained rowers performed three weeks of RE training followed by one week of rest and subsequent three weeks of AE training. Thyroid stimulating hormone decreased after RE training and increased after AE training, an effect that the authors attribute to energy demand associated with high intensity.
Key pathways
PGC-1α plays an important role in physiological cardiac hypertrophy. Exercise training increases circulating catecholamine (e.g., β-adrenergic signaling) which, in turn, upregulates PGC-1α (431, 1359). PGC-1α is mediated via peroxisome proliferator-activated receptor α (PPARα) and appears to play a role in exercise adaptations related to energy metabolism and mitochondrial biogenesis. Downstream targets of PGC-1α include NRFs 1 and 2, as well as the transcription factor ERR, a regulator of mitochondria and fatty acid oxidation. Akt is a central regulator of cell growth and survival (553) and is activated downstream of kinase cascades such as phosphatidylinositol 3-kinase (PI3K). In preclinical models, it is well established that exercise training alters IGF-1 and insulin-induced signaling through PI3K cascade (1179, 1181, 1410). Briefly, binding of ligands to receptor tyrosine kinases (780) leads to activation of the 110 kDa lipid kinase subunit α of PI3K and internal stimulation of adaptor proteins insulin receptor substrate (IRS)-1 and 2 (553, 875). The PI3K pathway has been associated with cell growth, survival, differentiation, and proliferation (188, 224).
Downstream, acute increases in activity of Akt can promote growth, whereas chronic increases may lead to pathological hypertrophy (840). These differential effects appear to depend on the active isoform of Akt and the activity/abundance of downstream targets. For instance, the Akt1 isoform appears to be involved in physiological rather than pathological cardiac hypertrophy (313). Akt can also inhibit glycogen synthase kinase-3 β (GSK3β), which can lead to pathological hypertrophy (40). Another notable target of Akt is mammalian target of rapamycin (mTOR) (188, 1078), a key regulator of protein translation through atypical serine/threonine protein kinases composed of two adaptor proteins. Moreover, mTOR complex 1 (mTORC1) regulates protein synthesis, cell growth, and proliferation (1454), while complex 2 (mTORC2) is involved in cell survival and polarity (1454). Combined, these complexes are necessary for adaptive physiological hypertrophy (1172). In addition, mTORC1 is responsive to growth factors including IGF-1 induction of the P13K/Akt pathway (875).
Akt is also capable of exerting a cardioprotective effect through a pathway involving the growth factor NRG1, ErbB4, and the CCAAT enhancer-binding protein β (C/EBPβ) pathway. In a preclinical model, the activity of this pathway is associated with increased myocardial regeneration (117). Briefly, Akt blocks the activity of C/EBPβ, serine (Ser473)/threonine (Thr308) kinase (847, 1286), an inhibitor of the positive effects of Creb binding protein (CBP)/p300-interacting transactivator with ED-rich carboxyl-terminal domain-4 (CITED4) (840). In animals, downregulation of transcription factor C/EBPβ may drive cardiomyocyte proliferation and increase CITED4 expression in mice after aerobic exercise (135). Continued research into this pathway and its relationship to exercise adaptation (135) could have implications for cardiac remodeling and resistance to HF.
Emerging research into regulators of gene expression has revealed a role for miRNAs in exercise-induced adaptations in the cardiovascular system (1286). Briefly, miRNAs are small, noncoding RNA molecules that influence translation of target messenger RNAs. Found throughout the genome, miRNAs are involved in various cardiac functions, including adaptive processes, contractile force generation, and inflammation (65). Conveniently, miRNA may be sampled from circulation to gain insight into cardiovascular function, adaptation, and remodeling. Several species of miRNA are thought to be either cardiac-specific or to have a particularly high degree of relevance to cardiovascular function. Of note, miRNAs regulating the vascular endothelium (miR-208a and miR-126), cardiac remodeling (miR-222), and inflammatory pathways (miR-146a) have been associated with the heart (65). MiR-126 is acutely increased in circulation following AE in humans (65), and some research proposes an intensity-dependent effect (1382). This endothelial-specific miRNA may be related to exercise-induced cardiac angiogenesis through pathways such as MAPK and PI3K/Akt/eNOS (1204). Targets of miRNA-126 include Sprouty-related protein 1 (Spred-1) and PI3K regulatory subunit 2, negative regulators of angiogenesis through inhibition of the VEGF pathway (918). Moreover, EPC-derived exosomes may promote vascular repair and angiogenesis through miR-126. This regulatory network including Spred-1 and VEGF may represent a mechanism by which exercise protects endothelial cells (830).
A noteworthy mediator of cardiac hypertrophy influenced by exercise is miR-222, which inhibits adverse cardiac remodeling through targets including cyclin-dependent kinase inhibitor 1B (p27), homeobox-containing 1, and homeodomain-interacting protein kinases 1 and 2 (814). In humans, circulating miR-222 is increased after both acute (1165) and chronic HIIT (590). Additionally, free circulating miRNAs (c-miRNAs) may be released by cardiac cells after acute stress such as exercise (65). Preclinical and cell culture-based studies are useful in discovery of c-miRNAs and elucidation of their mechanisms of action; these approaches can then be complemented clinically with targeted assessment in human exercise studies. There appear to be a range of possible effects of acute and chronic exercise on c-miRNAs (64, 976), and it is likely that mode- and intensity-dependent effects also exist.
Section summary
The central role of the cardiovascular system in blood and oxygen supply makes it an extremely adaptive system in response to exercise. Highly responsive to metabolic (energy stress, hypoxia), and mechanical (hemodynamic forces) stressors, the heart and associated vascular increase in size and function to meet peripheral demands of heightened training loads. On the molecular level, signaling cascades initiated by hormones, growth factors, and other regulatory molecules are critical for these adaptations to occur. Certainly, continued investigation in areas of sparse knowledge would be advantageous for the field, for example, a focus on cardiac outcomes in response to RE or combined training.
Adipose Tissue Adaptations to Exercise
Now recognized as highly metabolically active, adipose tissue (AT) actively engages in cross talk with skeletal muscle in response to exercise to positively modulate hormones, energy metabolism, and the resolution of exercise-induced inflammation. In addition, AT secretes regulatory factors that influence multiple physiological systems beyond skeletal muscle. While a significant body of research has traditionally examined the influence of exercise on the quantity of adipose, emerging research supports that adipose quality is of utmost importance for health.
Structure and function of adipose tissue
AT is considered a connective tissue and is found throughout the body in specific sites called depots that derive from different origins. These depots thus play distinct functional roles under the overarching role of AT in metabolism and endocrine signaling. At the cellular level, adipose cells (adipocytes) are classified as white, brown, and beige adipocytes. Beyond the primary adipocyte populations (762), AT is a heterogeneous and complex tissue comprised of fibroblasts, endothelial cells, immune cells, and innervating sympathetic nerves, activation of which is required to mediate breakdown or lipolysis (66, 93).
White adipocytes are well-characterized for their role in storage of excess energy as triglycerides and the release of hormones and adipokines (762). They are often distinguishable by their single large lipid droplet and sparse mitochondrial population (1198). White adipocytes constitute white adipose tissue (WAT), the most abundant and ubiquitous adipose depot, which forms the basis of both subcutaneous and visceral AT (742). Subcutaneous adipose can be easily sampled, whereas visceral adipose is situated within the abdominal cavity, presenting a challenge for direct analysis. Thus, there is generally a better understanding of the mechanistic role of subcutaneous WAT in adaptation to exercise. Given that WAT is found in various specific depots, acts in different metabolic and endocrine capacities, and has been shown to arise in certain patterns during embryogenesis and growth, it has been generally accepted that the origins of WAT vary based on developmental patterning.
During embryogenesis, brown adipose tissue (BAT) develops before WAT (774) and arises from progenitor cells expressing Myf5 (774). In humans, BAT is primarily localized to the intrascapular region (1198) but also exists in select depots in the neck, supraclavicular, axillary, paravertebral, and a few vascular regions in adult humans (778). Originally, BAT was thought to be metabolically active during only embryogenesis and infancy; however, in 2009, Virtanen et al. (1374) reported its measurable metabolic function in adults. Biopsy samples taken from supraclavicular BAT exhibit three-fold higher oxygen consumption versus WAT (1369). BAT is primarily responsible for generation of heat through nonshivering thermogenesis, a process mediated by the actions of uncoupling protein 1 (UCP-1). Briefly, UCP-1 partially alleviates the hydrogen ion gradient established across the mitochondrial membrane during aerobic respiration, reducing its potential to be harvested for energy in favor of heat production (66). Fittingly, brown adipocytes have an extensive mitochondrial network and are comprised of multiple small lipid droplets (1198). While many studies examine BAT via 18F-fluorodeoxyglucose positron emission tomography-computed tomography imaging, molecular studies using biopsies are rare due to the difficulty of access, complicating its examination in the context of human exercise (241).
Recently, it was discovered that mature white adipocytes exhibited considerable plasticity and could transition toward a brown adipocyte phenotype via a process known as “browning” (774). These apparently white adipocytes are referred to as “beige adipocytes,” which are thought to be derived from the vascular smooth muscle niche (21, 114, 818) and thus distinct from fully brown adipocytes. Characteristically, beige adipocytes have a unique dendritic morphology and typically express the markers CD34, PDGF Receptor α, spinocerebellar ataxia type 1, and UCP-1; morphologically, they are similar to brown adipocytes with a large number of mitochondria and multiple lipid droplets (762, 774, 1339). Beige adipocytes tend to be interspersed throughout WAT and are thought to play a role in response to injury and cold stress (1199, 1338). Currently, the process of WAT browning has not been elucidated in humans (1338), perhaps a consequence of small biopsy size. Nevertheless, the study of factors that promote browning or beiging of WAT is targeted at harnessing potential mechanisms of obesity treatment, based on the premise that conversion of energy-rich WAT to the essentially energy-inefficient BAT might represent an intervention to increase basal metabolic rate (1355).
In disuse, disease, and energy surplus, adipose tissue can be mobilized from depots, redistribute throughout the body, and infiltrate other tissues such as skeletal muscle. Intermuscular adipose tissue (IMAT) is considered an ectopic deposition of subcutaneous AT induced by factors such as age, overall adiposity, chronic disease, and inactivity (10, 219, 320, 846). A high degree of IMAT is disruptive to normal muscle function and has been linked to impaired strength and mobility, chronic inflammation, insulin resistance, T2D, CVD, and other chronic conditions (10, 174).
While not a proper AT depot, skeletal muscle stores fat molecules as intramuscular triglyceride molecules (IMTG) that have a high degree of relevance for exercise. IMTG are found in lipid droplets in all fiber types, with higher density in oxidative, type I myofibers (292, 693). Spatially, IMTG lipid droplets are conveniently located in proximity to the endoplasmic reticulum and mitochondria (292). IMTGs, composed of three fatty acids linked to a glycerol backbone, can be catabolized for energy production to support muscle contraction in both AE (614) and RE (377). Free fatty acids released from other adipose depots can also be transported to skeletal muscle via circulating albumin and trafficked into these lipid droplets until oxidation (669). Before ultimately undergoing oxidation, fatty acids are bound to a carnitine molecule and shuttled into muscle mitochondria via the enzyme carnitine palmitoyl acyl-transferase (827). However, supplementation with carnitine has failed to yield ergogenic benefits (87, 983, 1327), indicating that this substrate is not a rate-limiting factor.
Role of adipose tissue in acute exercise
In AT, exercise initiates a cascade of events that leads to lipolysis and recruitment of fatty acids to skeletal muscle. Skeletal muscle further promotes this through release of myokines, key mediators of lipid oxidation (621, 762, 1238). Through the exercise-induced exchange of myokines and adipokines, the two systems interact to influence metabolic function of immune, cardiac, and endocrine systems (1238). This requires a molecular cascade that stimulates lipolysis to respond adequately to heightened energy consumption and metabolic demand during exercise. As such, it is necessary to consider the role of AT in exercise in its context downstream of endocrine events signaling its activation.
Initially, exercise stimulates the hypothalamus (1384) to produce corticotropin-releasing hormone (CRH), which in turn stimulates the anterior pituitary gland to secrete ACTH. ACTH acts upon the adrenal cortex in the kidney to release cortisol, a catabolic glucocorticoid hormone, into the blood-stream (170): cortisol helps maintain blood pressure during exercise and plays a central role in fat metabolism. During exercise, circulating levels of cortisol are monitored by the hypothalamus and anterior pituitary to determine the amount of ACTH that is released (568). Glucocorticoids such as cortisol promote AT lipolysis through increased expression of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) (342) and glucocorticoid receptor-α (GRα) (649). As demonstrated by animal models, both factors are important in the appropriate response to exercise-induced increases in cortisol (184): 11β-HSD1 is capable of activating inactive glucocorticoids, and GRα is a necessary component in lipolysis and lipid secretion. Interestingly, upregulation of GRα has been correlated to the reduction of AT mass (649), leading some to pursue it as a treatment for obesity (649).
White adipose tissue signaling in exercise
Exercise-induced lipolysis in WAT is relatively well-understood due to the size and ease of access of subcutaneous WAT depots. Given the important endocrine role of AT, a great deal about its health can be ascertained by the secretory phenotype (315) at rest or in response to a challenge, such as exercise. While aging negatively influences these parameters, training promotes an oxidative phenotype and increases mitochondria in WAT (1052). WAT is primarily stimulated through sympathetic innervation of WAT or the action of circulating endocrine factors (e.g., norepinephrine, epinephrine, cortisol, and cardiac-derived natriuretic peptides) (21, 93). Following stimulation of a primary receptor on the surface of an adipocyte, such as β-adrenergic receptor, a cAMP/PKA cascade is activated (920), resulting in downstream phosphorylation of various lipase enzymes to initiate lipolysis at the surface of the lipid droplet. These include hormone-sensitive lipase (HSL), adipose triglyceride lipase, and monoacylglycerol lipase, acting in turn until the glycerol backbone is separated from the fatty acids. The resulting single fatty acids are released into circulation, bound to serum albumin, and directed to various tissues, including working muscle, to generate ATP for energy (920).
To further support lipolytic metabolism, exercising muscle releases an array of myokines such as IL-6, a prominent factor in glucose sensing. Often considered a pleiotropic cytokine, IL-6 has numerous beneficial effects on AT when stimulated by exercise. In addition to promoting AT lipolysis through the activation of the AMPK pathway (762), exercise-induced IL-6 has been associated with reduced visceral AT mass in humans (1409) and reduced inflammatory cell infiltration into AT in an animal model (835). Furthermore, myokine β-aminoisobutyric acid (BAIBA) is produced and released by skeletal muscle downstream of PGC-1α activation. BAIBA can lead to the upregulation of UCP-1 and other markers of browning in pluripotent stem cells (1106). BAIBA has been linked to obesity treatment through stimulation of fatty acid oxidation, attenuation of lipogenesis in WAT, and reduction of inflammation and insulin resistance (460, 662). Muscle-derived irisin has also been associated with lower levels of visceral AT and browning of WAT (762, 774), although the extent of its direct effects on browning is still a subject of debate.
An important class of myokine mediators of AT is the peroxisome proliferator-activated receptor (PPAR) isoform family (762, 778). These receptors bind fatty acids to initiate a range of pertinent effects on processes including lipolysis, adipogenesis, and lipid storage (649). Together, PPARs play complementary roles in AT, acting as the “master regulators” of lipolysis and lipid storage balance. For instance, PPARγ is involved in adipogenesis and lipid storage, leading to its study as a target of obesity research (649). On the other hand, PPARα plays a prominent role in lipolysis and has been linked to the role of BAIBA in browning (1106) and increased fatty acid uptake and oxidation in skeletal muscle (177, 591). Similarly, PPARδ also functions to increase fatty acid uptake and oxidation in muscle (177) and can be activated in response to exercise-induced increases in myokine IL-15, a factor inversely correlated to AT mass (762). PPARs initiate downstream effects in both AT and skeletal muscle. For example, angiopoietin-like 4 (ANGPTL4) is both a myokine and an adipokine with roles in multiple processes related to energy metabolism (762) including promoting lipolysis (624), raising insulin sensitivity (251), and increasing circulating fatty acids (1089).
Immediately after exercise, cardiac-derived natriuretic peptides, such as atrial (ANP) and B-type (BNP), are released from the heart (423). These have widespread effects on several tissues within the body, including AT (744). In AT, natriuretic peptides promote lipolysis through a cAMP-independent pathway induced by natriuretic peptide guanylyl cyclase receptor A (NPR-A) (744). In addition, evidence suggests that natriuretic peptides can cause the browning of WAT by inducing a browning program in gene expression (132). However, the magnitude of contribution that natriuretic peptides have in inducing lipolysis is unknown and likely small when compared to the typical induction of lipolysis. Overall, exercise induces a wide variety of molecular responses in WAT beyond those covered here, and continued investigation using ‘omics is likely to reveal important signaling factors beyond the currently understood adipokine-myokine cross talk.
Role of other adipose depots in exercise
Brown adipose tissue
In contrast to WAT, there is a less-developed body of knowledge surrounding molecular effects of exercise on BAT, as BAT depots are difficult to access and study. As such, our understanding of the molecular mechanisms of exercise cross talk with BAT is limited and primarily based on animal models and in vitro human cell studies. Unfortunately, existing evidence in human exercise is not only sparse but contradictory: while some evidence suggests that overall BAT mass decreases and becomes less active in response to exercise (778, 1379), others have demonstrated that BAT activity is higher in young, lean humans and associated with better glucose tolerance in obesity (1422). In an effort to clarify the role of BAT in humans, several animal models of BAT have identified BAT-derived chemokines (so-called “batokines”) that play major roles in metabolism, glucose and lipid homeostasis, and overall have similar endocrine, paracrine and autocrine effects similar to WAT (1422). Theoretically, batokines could be used as biomarkers of BAT activity in human exercise. In reality, detection of batokines in humans has been difficult, likely due to the predominant outpouring of WAT signaling factors induced by exercise. Identification of a circulating factor specific to BAT or BAT metabolism is needed to further explore BAT’s role in acute and chronic exercise.
Intermuscular AT
Despite the physical proximity of the two, the molecular cross talk between skeletal muscle and IMAT has not been well defined to date. It can be hypothesized that due to the secretory nature of AT, IMAT is also a source of regulatory molecules that directly impact skeletal muscle. A recent study found that, at rest, IMAT secretes factors that decrease skeletal muscle insulin sensitivity and increase free fatty acid concentration, potentially promoting muscle insulin resistance (1135). Additionally, myostatin, an inhibitor of myogenesis, has been positively correlated with IMAT mass, suggesting that IMAT may negatively regulate muscle growth (722). However, AE can reduce (722) or prevent (219) the accumulation of IMAT, suggesting that this may be an easily accessible energy reservoir for working muscle. In further support, exercise-induced changes in the quantity of IMAT appear to be site-specific, as a recent study by Chambers et al. found lower age-related IMAT accumulation in the thigh but not the calf muscles of lifelong-trained men and women performing primarily cycling (219). Furthermore, higher intensity training was associated with attenuated deposition of IMAT. Thus, continued research is needed to understand whether the overall abundance of IMAT is a primary determinant of muscle quality or if exercise training positively modulates the composition of IMAT (e.g., via browning or other indices of adipocyte health).
Intramuscular triglycerides
While IMTG content is negatively related to insulin sensitivity in untrained individuals, training reverses this relationship, a phenomenon known as the “athlete’s paradox” (111, 729). IMTG are a substantial source of energy during (693, 1350, 1403) and after (694) moderate-intensity long-duration exercise and chronic AE training increases reliance on IMTG at an absolute workload (110, 614). Interestingly, a sex-specific pattern may exist such that females typically store more IMTG than males (618) and may preferentially utilize IMTG during exercise (1251). During exercise, IMTG are acted upon by HSL and subsequently by perilipin 5 (PLIN5), which is thought to mediate shuttling of liberated fatty acids into the mitochondrion for efficient oxidation (134, 693). Interestingly, HSL can be differentially regulated in skeletal muscle and AT by selective phosphorylation of distinct serine residues, allowing the source of energy substrates for lipolysis to be controlled (1404).
Section summary
While the majority of studies related to exercise training effects on adipose focus on changes in total fat mass, the field is shifting toward a more intricate understanding of adipose tissue health. In the context of human exercise, examining molecular communication between AT and skeletal muscle may reveal factors with key bioenergetic, inflammatory, and endocrine roles that can be leveraged for therapeutic benefits. For instance, exercise-induced adipokines or myokines that promote browning or beiging have potential for treatment of chronic metabolic diseases. Regardless, AT is clearly required for proper endocrine function, response to exercise, and energetic support of the skeletal muscle system; benefits of long-term exercise on adipose health are likely to further promote these critical functions.
Liver Adaptations to Exercise
Metabolic support for exercise is largely regulated by the liver. Through its roles in managing glycogen, lactate, lipids, and other metabolites, the liver is critical to support continued muscle contraction during exercise. In addition, the liver is a filtration system; thus, good insight into overall health, liver function, and inter-organ communication can be assessed from circulating factors, including lipoproteins (e.g., LDL, HDL) and cholesterol. While much is known about the liver in acute exercise (particularly AE), the specific molecular benefits of long-term exercise on liver health are less understood outside of preclinical models. Nevertheless, an understanding of the liver’s actions in supporting acute exercise provides important perspective into overall health and the adaptive effects that support improved exercise performance and metabolic homeostasis.
Structure and function of liver
The liver is the largest gland in the body (~2.5% of total body weight) (951) and its energy expenditure accounts for approximately 20% of basal metabolic rate (432, 1394). This is related to various homeostatic roles, including synthesis of proteins and hormones, extraction and processing of nutrients (e.g., lipids), removal of waste products, antigens, and microbes, and storage of glucose and bile (84, 951, 1189). Briefly, oxygen-rich blood enters the liver through the hepatic artery. Peripheral blood from the gastrointestinal system gathers nutrients, metabolites, and other molecules that may be carried to liver via the portal vein. Within the liver, blood is processed in the liver acini and various substances are synthesized, either for export through the hepatic vein to remain in circulation or conversion into bile for excretion. At the cellular level, liver is comprised of hepatocytes, cholangiocytes, liver sinusoidal endothelial cells, natural killer cells, Kupffer cells, and hepatic stellate cells. Together, these structures orchestrate the functions of liver as the molecular milieu and metabolite flux change.
Role of liver in acute exercise
As a digestive organ, the liver is affected by reduced blood flow to the splanchnic bed at the onset of exercise: while it receives approximately 25% of cardiac output at rest, redistribution of blood flow substantially reduces this (548, 1031) in favor of skeletal muscle (359, 432). However, while working muscle metabolic demand increases, liver is capable of maintaining its functioning to support the metabolite flux of exercise (432, 932), including mobilization of lipids (160) and glucose output (1383). Rodent models demonstrate that acute exercise markedly affects liver expression of genes related to cellular stress (575), perhaps a reflection of the liver’s importance in bearing the metabolic burden of exercise.
Carbohydrate metabolism
Glycogen is the major storage form of carbohydrate (644), a highly branched structure arranged that is easily dismantled to constituent glucose molecules. Liver glycogen stores are highly concentrated but, given the small mass of the liver (~1.5–2 kg), quite limited in comparison to those in skeletal muscle (644), particularly in well-trained individuals (477). In fact, it is estimated that hepatic glycogen utilization alone could not support exercise for longer than ~20 min (865). Thus, muscle glycogen reservoirs are critical to provide carbohydrate during exercise (477), and further advantages are conferred by carbohydrate-sparing adaptations within muscle or exogenous carbohydrate supplementation (477).
Despite its inadequate glycogen storage capacity, the liver plays a major role in coordinating carbohydrate flux to support exercise (11, 300). This role was first illustrated by Carl and Gerty Cori in a sophisticated mechanism for which they were awarded the 1947 Nobel Prize in Physiology or Medicine (1128). Since this time, it has become well appreciated that liver is the central hub of carbohydrate traffic during exercise. For instance, lactate and pyruvate, byproducts of glycolysis, can leave working muscle and travel to the liver to be converted into glucose (11, 456, 477) and resynthesized into glycogen (270), or simply shuttled to inactive tissues (e.g., other muscles) for immediate use as energy substrates (16). During exercise, skeletal muscle preferentially uses its own stored glycogen as a carbohydrate source, especially at higher intensities (1115), but may draw from plasma glucose as resident stores are depleted. As plasma glucose drops, glucagon is released from the pancreas (1103) and stimulates liver glycogenolysis, by which liver glycogen is broken down to fortify circulating glucose (1103) and support continued muscle contraction (1383). Glucose production may also be stimulated in response to exercise-induced increases in skeletal muscle IL-6 (1018) and circulating epinephrine (348, 1103), although the latter effects may only be seen at very high concentrations (707).
The process by which circulating glucose enters skeletal muscle has been of great research interest, particularly in the context of metabolic diseases (e.g., T2D). This occurs in both an insulin-dependent (644, 1285) and insulin-independent fashion (28, 1073). The former is reliant on the upregulation of the membrane-bound insulin-mediated GLUT4 transporter (644). Within the myofiber, insulin may activate glycogen synthase, which is necessary for glycogen production and eventual storage in skeletal muscle (644). Due to high metabolic demands, the actions of insulin in promoting resynthesis and storage of glycogen are limited during exercise but prominent after cessation of an acute bout (645, 1073). Throughout and after exercise, muscle-derived IL-6 may function as a carbohydrate sensor, promoting insulin-mediated glucose uptake through communication with the liver (549, 1018). Preclinical evidence from John Holloszy’s laboratory demonstrated that skeletal muscle glycogen was resynthesized more quickly than liver glycogen 24 h following exhaustive exercise in rats (388), supporting that skeletal muscle is preferentially replenished and equipped for future metabolically demanding activity.
Lipid metabolism
In addition to storing small depots of lipids, liver coordinates overall lipid metabolism (Figure 7) through its interactions with adipose (source) and muscle (sink) (1035, 1189). Under resting conditions, liver metabolizes approximately 40% of free fatty acids (FFAs) circulating throughout the body for disposal or storage. During exercise, however, FFAs are redirected to support muscle energetics (1189). In fact, hepatic lipid metabolism is largely unchanged during exercise (1189), but hepatic lipid concentrations are increased after acute AE (653). In regulating hepatic lipid metabolism during exercise, pancreatic glucagon serves a dual purpose, stimulating fatty acid breakdown in the liver while simultaneously inhibiting lipogenesis (1103). These actions are thought to be primarily driven through activation of AMPK, which is known to affect both glucose and lipid metabolism in the liver (897).
Figure 7.
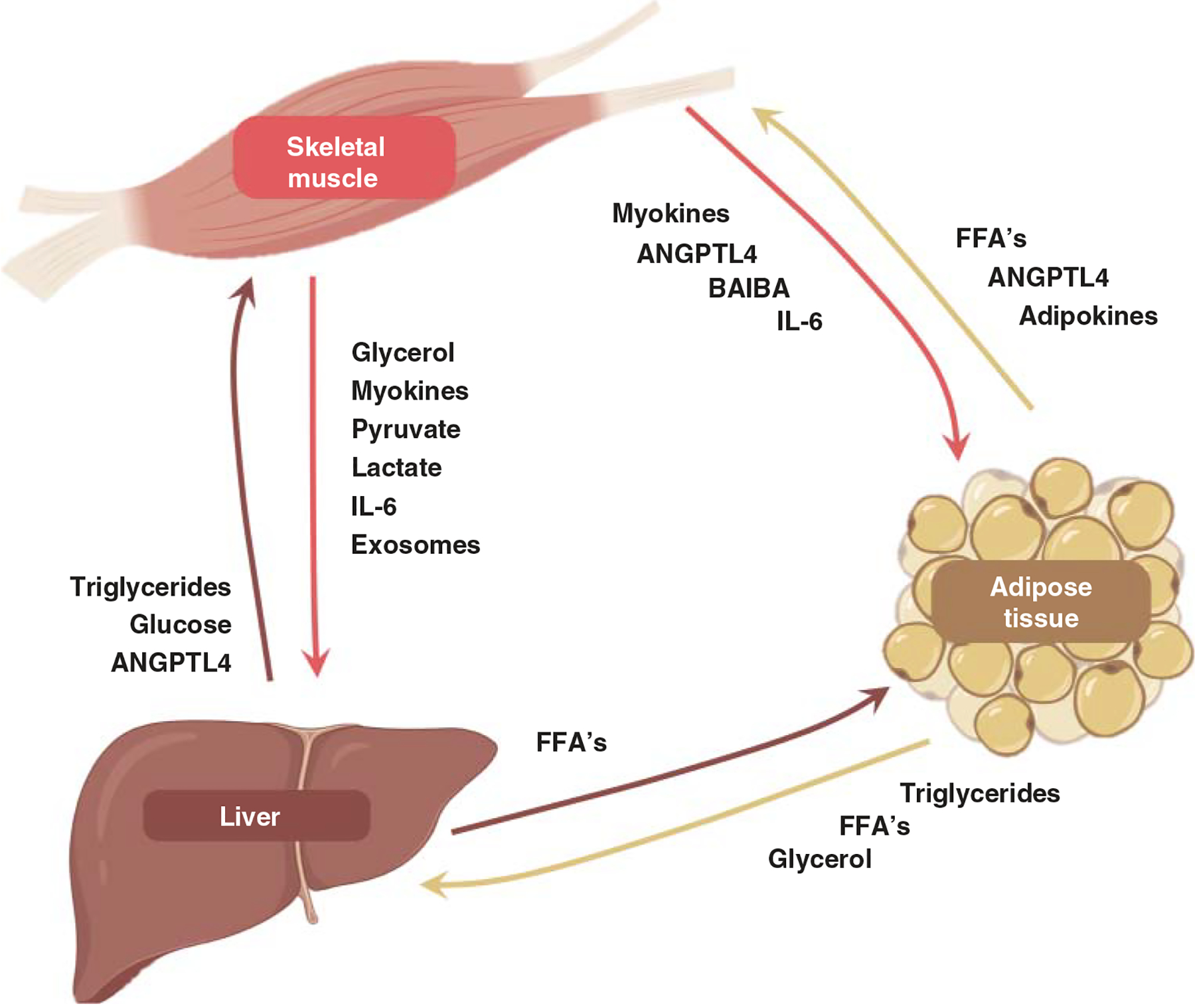
This figure illustrates molecular-level cross talk between skeletal muscle, adipose tissue, and liver to facilitate energetics during exercise. Key myokines, adipokines, and hepatokines are highlighted.
The liver acts as the central mediator of lipolysis and lipogenesis, feeding fatty acids to the body tissues such as skeletal muscle and adipose tissue (999). Glycerol byproducts of lipolysis in AT can be shuttled to the liver, where they are then used as a noncarbohydrate substrate for conversion to glucose via gluconeogenesis (1123). Alternatively, glycerol may also be processed into a triglyceride via reesterification with free fatty acids (958, 1439). This process occurs both in adipocytes and skeletal muscle (354, 917). Throughout the body, the degree of fatty acid cycling that occurs at rest and after exercise is remarkable (1439), and flexibility in the process appears important to transition from resting metabolism to the heightened demands of a perturbation such as exercise.
Molecular transducers of liver roles in exercise
Key pathways
The mechanisms mediating the range of liver functions are incompletely characterized but include several key factors. For instance, 5′-AMPK acts as a central metabolic switch that is particularly sensitive to exercise in both the liver and skeletal muscle (640). In the liver, AMPK activation is involved in glucose and lipid metabolism (348, 897, 1103), along with other processes including catabolism, cell cycle arrest, mitochondrial biogenesis, fatty acid oxidation, glucose uptake, and insulin signaling (942). Specifically, AMPK phosphorylation leads to suppression of fatty acid synthesis and favors fatty acid uptake and oxidation (640). The specific downstream effects of AMPK activation in energy sensing and metabolism appear to depend on differential activities of its subunits. For example, phosphorylation of the α subunit of AMPK facilitates hepatic glucose secretion (640), while the β2 subunit has been described to bind to glycogen and promote in an increase of skeletal muscle glucose uptake (640). Additionally, angiopoietin-like protein-4 (ANGPTL4) is a plasma protein secreted by adipose and liver (688, 696) that reduces the activity of lipoprotein lipase to regulate plasma triglyceride levels (624). In response to exercise, the liver increases ANGPTL4 production through glucagon-induced stimulation of the cAMP/PKA pathway (624). ANGPTL4 also plays roles in lipolysis in adipose and skeletal muscle, angiogenesis, and vascular permeability (577). Continued investigation of the impact of these factors in response to differential energy stress is warranted in the context of human exercise.
Exosomes
The role of exosomes in exercise-mediated tissue cross talk is a developing area of study (1136), but the liver may be particularly relevant to these research efforts, since exosomes released from skeletal muscle have an apparent tendency to localize in the liver (1423). This may represent a mechanism for muscle-liver cross talk or a central role for the liver in filtering and/or redistributing muscle-derived exosomes to other tissues. Whitham et al. recently demonstrated that the vesicular adhesion protein integrin beta 5 (ITGB5) was both released by exercising muscle and taken up by animal liver cells in vitro, indicating its potential importance in directing exosomes to liver (1423). These findings provide direction toward identification of mechanisms underlying exosome and extracellular vesicle trafficking through the liver. Given the wide range of possible downstream effects, continued investigation is necessary.
Liver adaptations to chronic exercise
The effects of chronic exercise training on the liver are poorly understood in healthy human populations. The present understanding of molecular pathways driving liver adaptations to exercise is obtained through a combination of preclinical models and histological observations in human disease (e.g., fatty liver disease, diabetes). Chronic AE leads to lower hepatic fat deposition (432, 1330), and studies in animals have associated these changes with improved coupling of mitochondrial oxidation to the tricarboxylic acid cycle and decreased oxidation of palmitate (1330). As most of these utilize a treadmill running paradigm, is unclear whether other modes of exercise (e.g., RE, HIIT) elicit the same metabolic impact that contributes to these effects. Nevertheless, exercise-induced reductions in liver lipid droplet distribution have potential relevance for nonalcoholic fatty liver disease (84). Impaired PPAR signaling is thought to play a role in the development of characteristic features of this condition, including liver steatosis, dysregulated lipid profile, and inflammation (177, 808).
While the mechanisms are not completely clear, exercise training often leads to changes in levels of circulating lipoproteins produced in the liver, for example, cholesterol, HDL, LDL (1358). However, reductions in cholesterol are not a universal outcome of exercise training studies (725). Some evidence suggests that an intensity threshold exists to modify blood lipid profile (1129); this may be a result of metabolic stress associated with increased carbohydrate utilization at higher intensity. Nevertheless, exercise-induced increases in HDL may contribute to reduced overall systemic burden, promoting hydrolysis of cholesteryl esters in the liver through the actions of sterol carrier protein 2 and fatty acid binding protein 1 (27). Furthermore, HDL facilitates removal of cholesterol released by arterial macrophages (1081), a process upregulated with regular exercise (1129).
As the body’s toxin filtration system, the liver sees inflammatory and oxidizing factors originating from a variety of sources, including free radicals and ammonia derived from active skeletal muscle (1134, 1330). Acute inflammation following exercise is associated with increased production of ROS in liver mitochondria, which may result in liver injury in animal models (84, 403). Some evidence supports that IL-6, a key factor in carbohydrate metabolism during/after exercise, can contribute to liver pathology when chronically elevated (1162). However, chronic exercise training has both anti-inflammatory and antioxidant effects, which may bolster resistance to these acute stresses (84, 1084). In support, moderate- and high-intensity exercise reduces inflammatory cytokines and injury-related markers in the liver (84), including superoxide dismutase, catalase, and reduced glutathione. In consideration of these results, it is important to recognize that combination of exercise with antioxidant supplementation may obscure the natural antioxidant effects of exercise.
Another prime liver-derived indicator of overall health is C-reactive protein (CRP). Liver produces CRP in response to inflammatory signals such as IL-6 and IL-1 (441, 1162). CRP serves as a nonspecific diagnostic marker for the progression of a variety of conditions characterized by an inflammatory component (42), which, incidentally, is a feature of many chronic diseases (433). At the cellular level, CRP is involved with activation of immune functions including phagocytosis (5) and complement activation (1236). While more highly fit, higher-functioning individuals often demonstrate lower circulating CRP than their counterparts (450, 462, 1096, 1280), short-term exercise does not consistently induce changes in circulating CRP (518, 716, 974). It is therefore unclear whether the etiology of an exercise training effect is based in the liver or another tissue, for example, adipose (186, 1367). Nevertheless, exercise likely lessens the overall systemic inflammatory burden, reducing the load on the liver and enabling its optimal functioning.
Section summary
The capacity to maintain normal liver function is important both for resting and exercising metabolism. Continued investigation into the role of the liver in managing metabolites affected by exercise may be supplemented by exercise studies employing metabolomics, lipidomics, and other unsupervised ‘omics platforms. Finally, the health of other splanchnic organs, including the gastrointestinal system, is likely to be important in overall metabolism. Attention to this area via improved understanding of the gut microbiome will be fruitful in our understanding of the integration of physiological systems to maintain homeostasis during acute and chronic exercise.
Nervous System Adaptations to Exercise
A.V. Hill once postulated that exercise performance was likely controlled by a physiological ceiling in work output imposed by the heart or the nervous system (566). Nearly a century later, Timothy Noakes introduced what is known as the Central Governor Theory, maintaining that the brain enforces necessary limitations to exercise performance to protect the organism as a whole (458, 981). While the theory’s underlying mechanisms (1054, 1148) and shortcomings (627, 1188) continue to captivate the field, it is clear that the nervous system is both an integral player in adaptation to exercise and a beneficiary of regular structured exercise.
The central nervous system
Until very recently (374), it was believed that the central nervous system (CNS) was not plastic in adult humans; that is, neurogenesis did not occur in a fully formed adult brain. We now appreciate that exercise training may exert a powerful influence on the architecture and thus the function of the CNS, typically defined as the brain itself and the spinal cord. In an elegant series of animal studies, van Praag et al. have established a direct effect of treadmill running on neurogenesis (1351, 1376). In humans, compelling evidence exists to suggest that elite exercise performance may be attributable to neural plasticity (541, 542, 826). Cross-sectional studies suggest structural differences in the CNS of highly trained athletes (1290) that may confer performance-related advantages (599). Evidence in young (79) and older (80) adults suggests that baseline neural architecture may determine the degree of adaptability to training: individuals with increased separation between neural networks (termed “modularity”) exhibit more favorable changes in learning and cognition with exercise training. Thus, as with many physiological systems, it is likely that a combination of baseline phenotype and extrinsic factors (e.g., exercise mode, frequency, and intensity) combine to influence exercise adaptations in the CNS.
Central nervous system adaptations to aerobic exercise
Research into the effects of exercise on the CNS has largely focused on the hippocampus (267), the learning and memory center of the brain that appears very plastic to exercise training. In adult humans, the left hemisphere of the hippocampus is generally more responsive, although some studies demonstrate additional benefits for the right hippocampus (399). These structures are involved in episodic and spatial memory, respectively (379). These effects are thought to be mediated by induction of long-term potentiation and upregulation of receptors for the neurotransmitters excitatory glutamate (1138) and inhibitory GABA (267). Animal research demonstrates that voluntary wheel running (AE) promotes the proliferation, development, and survival of hippocampal neurons, which are then guided toward the nearby entorhinal cortex, a structure involved in spatiotemporal processing and memory (1375). In adult humans, the entorhinal cortex is activated by walking (371) and combined exercise (819, 1195), including AE, RE, walking, and stretching. Interestingly, animal data also suggest a positive correlation between the magnitude of cardiovascular adaptability to AE and degree of hippocampal neurogenesis (982), suggesting a strong influence of genetics on these connections.
Many studies link neural plasticity (1171), hippocampal volume (215, 372), and cognitive function (1101) to aerobic capacity at various stages of life. However, in mid-life (i.e., outside periods of brain growth or atrophy), fitness correlates best with viscoelasticity, a measure of brain structural organization (1171). This raises the important distinction that exercise does not necessarily increase hippocampal volume during periods of maintenance (e.g., mid-life) but is most effective during development and age-related atrophy (373, 399). During childhood brain development, exercise appears to have a beneficial role in hippocampal volume (215, 562, 1029), translating to better learning (562), memory (215), and academic performance (449). In older adults, AE interventions may slow or reverse age-related hippocampal atrophy in a year or less (373). Evidence in Master’s athletes supports that high-intensity AE may protect structural integrity. This population exhibits increased cortical thickness (1443), preserved integrity of white matter composed primarily of myelinated axons (1337), and attenuated loss of grey matter in brain regions associated with memory and motor control (1336). These patterns are complemented by an 83% reduction in white matter hyperintensities, hotspots that indicate loss of myelination and are correlated with risk of dementia and death (311, 1337).
In addition to promoting improvements in size and function of these brain regions, regular exercise prepares the brain for subsequent bouts of exercise. Anticipatory increases in heart rate and respiration, as well as appropriate redistribution of blood flow, are components of this adaptation mediated by the cortical autonomic network (23). CNS regions including the insular, medial prefrontal, and anterior cingular cortices communicate with peripheral mediators to reduce parasympathetic input, aiding in preparing the body for the onset of exercise. Whereas impaired activity of the cortical autonomic network in aging (1443) and disease (31) may contribute to increased perceived effort during exercise, long-term training enhances the system’s efficiency, improving exercise tolerance. This is supported by examining the alpha:beta ratio, a comprehensive measure of brain efficiency in multiple regions monitored through electroencephalogram (826). During cycling, highly trained cyclists with higher Vo2max demonstrate greater alpha:beta activity ratio, indicating less active cognitive processing during an accustomed exercise bout (826).
Central nervous system adaptations to resistance exercise
Functional domains of neural health are also improved after short-term RE in older adults (209, 560). Progressive RE outperforms computer-based cognition training in older women with MCI (1271), improving both cognitive performance and white matter lesion frequency. In a randomized controlled trial of 100 community-dwelling adults, RE-induced improvements in cognition were better associated with gains in strength than with change in aerobic capacity (863). This may be due to greater absolute dynamic range in skeletal muscle strength gains than in aerobic capacity, which others have shown to be associated with neurocognitive outcomes and structural integrity in the pretrained state (215, 372, 1101, 1171). Data regarding structural adaptations to RE are less conclusive. It has been shown that a year of RE training in older women reduces the rate of total white matter atrophy (118), with benefits persisting up to a year after cessation of supervised training. Hippocampal neurogenesis in animals subjected to weighted ladder climbing, a model of RE, is not consistently elevated (474, 982), but simply the act of learning a motor-based task may improve synapse formation in the rat motor cortex (709). Additional research is needed to understand the impact of RE on prevention of hippocampal atrophy throughout aging.
RE generally receives less attention than AE in studies designed to examine neural adaptations to exercise. This discrepancy might be due to the vast preponderance of mechanistic information from animal research using treadmill running, difficulty in discerning the tissue origin of systemic responses to AE, the lack of a universally accepted RE-based analog to cardiovascular fitness (e.g., muscle size, strength, or power), and/or some other methodological consideration. Further evidence exists to suggest that cardiovascular stress, presumably reflective of greater systemic burden, is necessary to elicit beneficial effects of exercise on memory (1378) and functional connectivity (869). In support, some authors have suggested using blood flow-restricted RE to increase the metabolic load above that of more traditional RE, theoretically enhancing the production of neuroactive substances that impact cognitive outcomes (1318). Clearly, further work using a wider range of intensities and alternative modes of exercise is needed to elaborate on these findings (559).
Molecular transducers of central nervous system adaptations
Growth factors
Several factors believed to influence neural adaptability to exercise have been identified, and it is thought that muscle either directly secretes them or indirectly promotes their secretion (318, 1021). In distinguishing whether a muscle-derived signaling molecule may act in the CNS, it is critical to establish whether the factor has the capacity to cross the blood-brain barrier (BBB). This physical barricade between peripheral circulation and cerebrospinal fluid is selectively permeable and regulates leakage of potentially toxic materials into proximity with the brain. Some evidence suggests that BBB integrity is generally fortified by exercise training (246, 864), whereas its permeability is increased in aging (370, 909) and disease (799, 841).
Studies in humans have linked exercise-induced increases in hippocampal volume to a rise in circulating brain-derived neurotrophic factor, BDNF (373). Binding to tropomyosin receptor kinase (Trk)B receptor (812, 1041), BDNF acts centrally by promoting long-term potentiation and survival of neurons (737). BDNF may also exert peripheral effects through other receptors, such as muscle regeneration in myocytes via neurotrophin receptor p75NTR (262). Numerous studies demonstrate increased concentrations of circulating BDNF during and after exercise (812, 1277). However, it remains unclear whether muscle is the direct source of this increase, with studies showing varying results (860, 1389). Circulating megakaryocytes are likely to produce a substantial fraction of BDNF in humans (214, 860), suggesting an integration of body systems is involved in this response to exercise. Furthermore, successive exercise bouts are believed to have a synergistic effect on circulating BDNF (1277), although conflicting data exist as to the impact of long-term AE on basal BDNF (63, 105, 1435, 1481). The role of BDNF in neural adaptations to RE is also unclear. Animal models often demonstrate hippocampal neurogenesis independent of an increase in BDNF (474, 982). Similarly, BDNF is unchanged after 12-week RE in humans (617), though some data suggest responsiveness to RE may be contingent on variables such as sex (411, 412), intensity (870), or training status (247, 1459).
VEGF is intricately involved in cardiovascular adaptations to exercise (579) but may also be a major player in neural adaptations (671, 1098). In animals, exercise-induced VEGF promotes neurogenesis after injury (1476) and cerebral angiogenesis via hydroxycarboxylic acid receptor (HCAR1) (921). While upregulation of VEGF mRNA is commonly detectable in human skeletal muscle following both AE and RE (128, 285, 1292), there is a broad range of potential sources of exercise-induced VEGF. Concerningly, however, an age-dependent decrease in acute AE-induced muscle VEGF expression is apparent in women (285), and long-term AE may not be fully protective against this (1150). Nevertheless, cerebrospinal fluid concentrations of VEGF are increased after RE in individuals with chronic hydrocephalus (1458), a neural condition contributing to dementia and cognitive impairment. Thus, improved blood flow promoted by VEGF signaling may represent a potential therapeutic mechanism for restoring delivery of blood and oxygen to the brain (1194).
PGC-1α plays a central role in coordinating production/secretion of VEGF (779) and other neuroactive molecules following exercise (318). For example, irisin and kynurenine aminotransferase (KAT) enzymes are upregulated through the transcriptional co-factor activity of PGC-1α. Irisin represents the secreted form of fibronectin III domain-containing protein 5 (FNDC5), which is upregulated postexercise in mouse hippocampal and cortical neurons upstream of an increase in BDNF (1448). In humans, acute exercise increases circulating irisin approximately 15% to 20% (414), although long-term training does not appear to influence basal irisin levels (120). Skeletal muscle FNDC5 shows no increase with acute exercise or chronic training (1024) but is increased in young men following 20 days of twice-daily high volume HIIT (352), suggesting a potential intensity- or frequency-mediated effect. The precise implications of the PGC1α-FNDC5-irisin axis for neural adaptations to exercise in humans are not yet clear, but this pathway is receiving increasing attention as a target for neurodegenerative (1465) and neurological conditions (151).
Neuroactive peptides
KAT facilitates the breakdown of kynurenine (KYN), a byproduct of tryptophan metabolism. Under normal physiological conditions, tryptophan is metabolized into neurotoxic and BBB-transient KYN; however, exercise promotes the breakdown of this substance into kynurenic acid (KYNA). The BBB is impenetrable to KYNA (1021), and this has been associated with neuroprotective, antidepressive, and anxiolytic effects (13, 912). KAT expression in healthy adult skeletal muscle is increased by acute exercise and long-term AE (1161), and acute increases in KYNA are seen after marathon running (786). Furthermore, skeletal muscle expression of genes in the KYN pathway are upregulated following RE training (25), and dysregulated urine concentrations of KYN in chemotherapy patients are normalized by RE (1480). However, depressed individuals undergoing moderate AE or combined AE/RE training do not exhibit changes in circulating KYN or KYNA, despite improvements in depression score and fitness (903). As with BDNF (1277, 1459, 1481) and VEGF (1292), a higher training status may predict a stronger signaling response; thus, longer-term studies may be necessary to elucidate the dynamics of tryptophan metabolite processing in exercise and mood disorders.
Exercise promotes the release of other neuroactive factors that may improve mood and pain tolerance (18, 452). For instance, endorphins interact with opioid receptors (1133), and endocannabinoids such as anandamide bind to cannabinoid receptors (436). Heightened endorphin activity is detectable in the human brain after HIIT (1133) and AE lasting approximately 2 h (124). Increases in circulating endocannabinoids are thought to result from cortisol signaling (569) and may be involved with BDNF (563). Circulating anandamide is increased following 30 min of moderate-intensity AE, but not after high- or low-intensity (1085). Further, anandamide is increased in healthy adults regardless of whether exercise is prescribed or self-selected (144), whereas only the former elicits increases in depressed individuals (896). Given these mechanisms of action, it is enticing to consider that exercise training might help reduce dependence on recreational drugs (145, 829, 1110). However, clinical trials investigating this connection have produced equivocal results (1153, 1332, 1478). More human-based research is clearly needed to expand on the mechanisms of exercise-induced improvements in mood disorders and addiction, including whether long-term exercise reduces lifetime risk.
Furthermore, while regular exercise has numerous benefits for cognitive health throughout the lifespan, it is worth noting that dependence on exercise remains a risk to mental health (266, 906), especially in young individuals and those involved in physique-oriented sports (e.g., gymnastics, bodybuilding, wrestling). Thus, mindfulness of exercise dose, as well as purpose (e.g., competition, wellness, weight loss), is encouraged. On the other hand, abstinence from exercise in an exercise-dependent individual may bring about disordered mood and affect, apparently mediated by dysregulated endorphin and anandamide signaling (41). This fragile balance between wellness and illness further highlights the range of physical activity thresholds that exist across body systems. The field is encouraged to consider this when designing integrated exercise trials to target the health of multiple organ systems.
The peripheral nervous system
The peripheral nervous system (PNS) includes the somatic and autonomic systems, which are under voluntary and involuntary control, respectively. Skeletal muscle is innervated by both systems through physical connections with both motor (169, 1311) and sympathetic (86, 223, 1261) neurons (Figure 8). The neuromuscular junction (NMJ) represents the direct physical interface between the somatic nervous system and skeletal muscle. Sympathetic input typically exists in close proximity to an NMJ (690) and, through the adrenergic receptors (690), may influence a range of skeletal muscle functions and processes (1104) including ion transport, calcium sequestering, and even gene expression (1112). These neural inputs are critical for muscle survival in addition to contraction, locomotion, and exercise.
Figure 8.
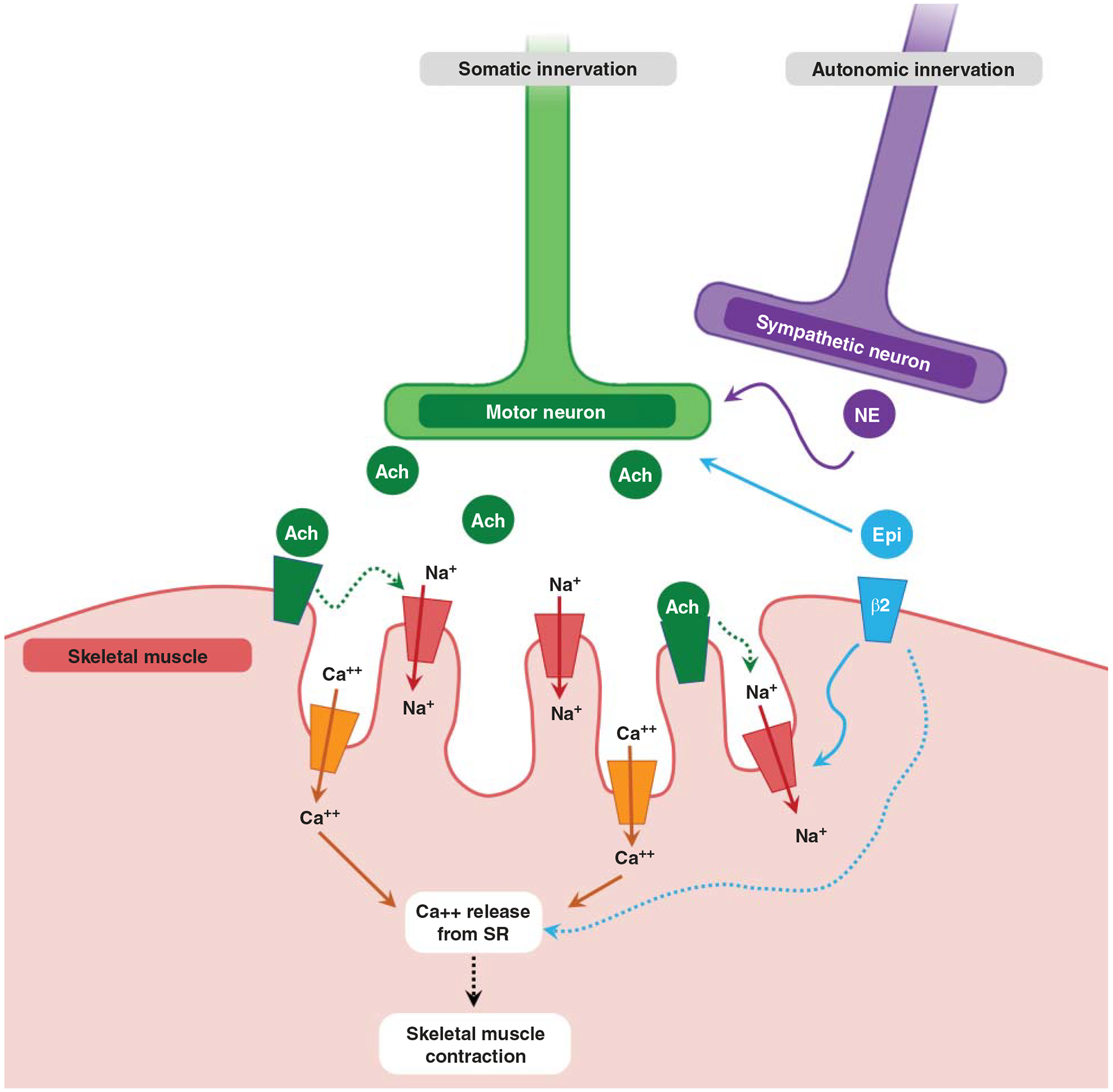
This figure illustrates simultaneous innervation in skeletal muscle by the somatic and the autonomic nervous systems. Briefly (1104), motor neuron release of acetylcholine (Ach) promotes influx of sodium (Na+) and calcium (Ca++) into skeletal muscle cell. This promotes release of calcium from sarcoplasmic reticulum (SR), leading to contraction. The sympathetic neuron innervates muscle in close proximity and may release norepinephrine (NE) to augment Ach release. Circulating epinephrine from the endocrine system supports sympathetic activation by augmenting Ach release in addition to enhancing Na+ influx and calcium-induced calcium release and re-sequestering.
Somatic nervous system
A given motor neuron and all fibers that it innervates are considered one motor unit. According to Henneman’s “size principle” (554), the motor units associated with the smallest neuron bodies (and, usually, the least fatigable myofibers) fire at the lowest threshold. Thus, exercise intensity dictates the demand on the motor unit pool of a given muscle and is likely to explain adaptations to different types of training. For example, regular AE reduces the firing threshold, allowing maintenance of rhythmic muscle contractions with lesser neural current. Rats subjected to treadmill training exhibit hyperpolarized resting potential, specifically in the slow motor neurons most impacted by continuous AE (98). It is suspected that this finding may be driven by trophic factors such as BDNF (98) or adaptative changes in abundance of receptors for neurotransmitters (e.g., GABA and serotonin) and other signals whose integration determines muscular contraction (831).
Motor units can be indirectly assessed in humans using motor unit number estimate (MUNE), calculated by dividing the summed muscle action potential of all motor units by the average motor unit potential measured throughout the muscle (316). While not sensitive to a large range in motor unit potentials across the muscle, this approach is well-correlated with the number of motor units and enables the detection of general dynamic changes in neuromuscular remodeling (557). A salient finding in humans is that primary aging tends to reduce MUNE (876, 1044) while increasing estimates of motor unit size. This observation is most commonly understood as the result of myofiber denervation followed by collateral reinnervation by a neighboring motor neuron’s axon (787, 877). The functional consequences of this are only beginning to be elucidated, but it is generally thought to be preferable to denervation without reinnervation, in which myofibers undergo death and contribute to overall atrophy and impairments in function (1045, 1105).
In older adults, habitual exercise appears to promote reinnervation in a mode-specific manner, as supported by higher MUNE values (1065) in muscles that are regularly activated by the activity of choice (1044). On the other hand, neither AE nor RE has an effect on motor unit potential in young adults (1044), although power training may increase the overall number of motor units. In highly AE-trained older adults, reinnervation may occur to such an extent that type I motor units are remodeled into a “grouped” configuration (934, 935, 1065), due to shifts in myosin heavy chain composition caused by collateral innervation with a type I motor neuron. The underlying molecular mechanisms are not completely clear but may involve signaling to promote cell adhesion, NMJ stability, and myofiber survival (683, 684, 759).
Evidence from animals supports that age-related changes in NMJ structure are mitigated by exercise (1345). In humans, improvements in muscle innervation are reported to occur with short-term training (683, 889), and histological evidence supports that long-term AE may protect against denervation and/or failed reinnervation in old age (934). Denervated skeletal muscle undergoes predictable changes in membrane composition, including disassembly of the Ach receptor (505), and recapitulated expression of factors such as neonatal myosin, neural cell adhesion molecule (340), and developmental ion channels. Presently, insight as to the effects of muscle contraction on these factors comes from diseased populations such as individuals with PD (683) or spinal cord injury (195). The mechanistic effects of long-term training on NMJ stability in healthy populations constitute a considerable knowledge gap. Nevertheless, that a considerable degree of plasticity still exists in chronic disease may suggest that adaptation is possible in healthy older muscle, given the appropriate type and degree of exercise stress.
Animal models have provided considerable insight into mechanisms underlying exercise-induced alterations in neuromuscular junction integrity. For instance, in an animal model of denervation, exercise facilitates more extensive dendrite branching (237), axonal sprouting, and NMJ stability (980). In mice bred for high versus low running performance, factors related to NMJ integrity are differentially responsive to AE initiated in later life (165), suggesting an influence of genetic composition, as is thought to exist in the CNS (79, 80, 982). While the ability to discern whether a given genotype predetermines response to exercise in humans is an attractive area of current research, it is not currently known whether responder status is associated with improvements in NMJ dynamics such as reduced denervation or heightened success of reinnervation throughout aging.
Autonomic nervous system
Downstream of the cortical autonomic network, the other primary PNS pathway includes both the sympathetic and parasympathetic nervous systems. Sympathetic output orchestrates the endocrine responses that accompany exercise, including upregulations in the hormone norepinephrine. Norepinephrine consequently interacts directly with NMJs, the site of chemical exchange underlying muscle contraction (1104), augmenting the effect of Ach exchange. In animals, removal of sympathetic innervation obliterates the catecholamine response, alters neurotransmitter release, reduces membrane Ach receptor density, and contributes to an array of muscle gene expression changes that mirror motor denervation [e.g., upregulated expression of Ach receptor subunit γ and histone deacetylase 4 (HDAC4), upstream of an increase in atrophy signal muscle ring-finger 1 (MuRF-1) (1112)]. Individuals with spinal cord injury impacting autonomic function display a blunted sympathetic response to AE (e.g., wheelchair cycling over a half-marathon distance) (574, 987), but it is presently unclear what the ramifications are for long-term adaptability or recovery. In healthy populations, regular training does not notably impact the sympathetic response to exercise (22, 201), likely due to its importance as a highly conserved integrated response. At rest, parasympathetic tone is elevated in highly trained athletes (201), just as resting sympathetic output may be curtailed in individuals with metabolically stressful conditions such as hypertension (201) or during periods of altered hormonal flux, such as menopause (992). Notably, extremely high training doses are thought to partially reverse these beneficial effects (254, 1278), an area warranting continued investigation.
Additional knowledge gaps facing the field include molecular examination of PNS structures such as muscle spindle fibers and the Golgi tendon organ (832). These proprioceptive mechanisms enable continual awareness of body position (1076) and muscle forces to protect against damage from excessive stretch or ill-timed contraction (1077). Methodological difficulties are likely contributors to the lack of molecular knowledge related to these structures in humans, but researchers are encouraged to consider their roles in exercise adaptations, fatigue, and performance. For example, function of the muscle spindle appears to be linked to skeletal muscle satellite cell abundance (369, 632), with clear implications for adaptability to a training regimen.
Section summary
The nervous system is a master regulator of physiological function and performance, and the full range of its plasticity with exercise training is continuing to unfold. Across the lifespan, exercise confers benefits for neural functions such as learning, memory, and spatial awareness, while facilitating neural adaptations underlying exercise tolerance. Presently, a range of mechanistic factors have been identified, and continued pursuit of their roles in human exercise will be fruitful. Eventually, this knowledge could be applied to the use of exercise as a preventative or adjuvant therapy for neurodegenerative disease, cognitive impairment and dementia, neuromuscular conditions, mental health disorders, and substance dependence.
Skeletal System and Associated Structures in Adaptation to Exercise
Critical to locomotion and load-bearing, the skeletal system (e.g., bone) provides structural support and protection for many organs. Skeletal muscles, directly attached to bones through tendons, contract to create bone movement that forms the basis of exercise. Like most physiological systems, bone is adaptive to structured exercise training. Though most mechanistic work is limited to preclinical studies, system-level exercise-induced improvements in bone, joint, and tendon (Figure 9) illustrate that these structures are active and responsive to acute and chronic exercise.
Figure 9.
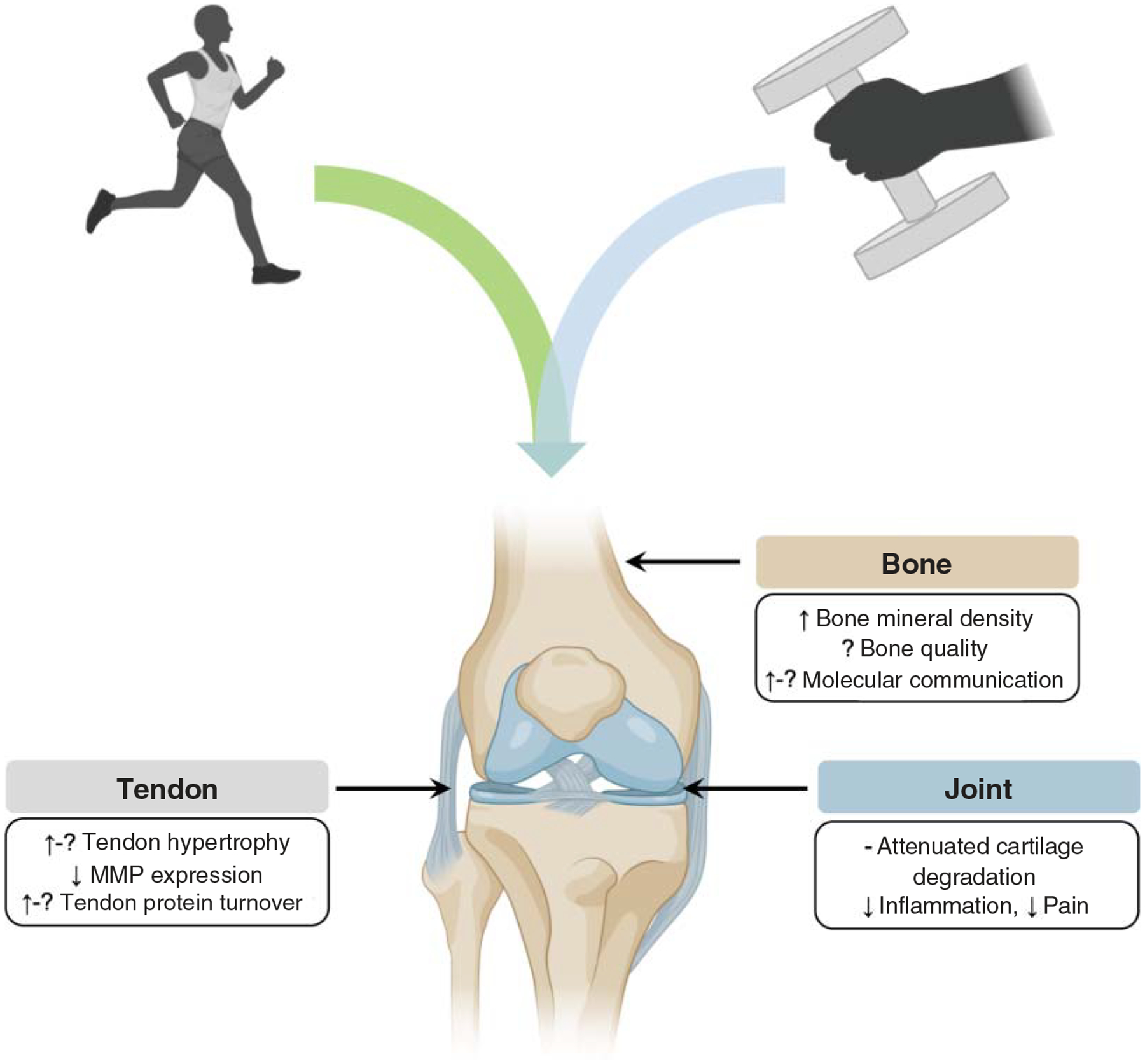
This figure illustrates summary of exercise-induced adaptations in bone, joint, and tendon; existing knowledge gaps in humans are indicated by question marks (?).
Structure and function of bone
Bone is an osseous tissue comprised of four primary cell types: osteocytes, osteoclasts, osteoblasts, and bone lining cells known as the basic multicellular unit (BMU) (407, 501, 1406). BMU cells are responsible for sensing changes occurring within their niche and respond accordingly by reabsorbing or laying down new bone (407). Normally, osteoblasts secrete bone matrix and osteoclasts reabsorb matrix in a coupled fashion to maintain relative homeostasis. However, when this equilibrium is dysregulated (e.g., as in osteoporosis), osteoclasts promote greater demineralization than the osteoblasts restore, resulting in decreased bone mineral density (BMD) (58). Exercise also affects this balance, most typically in a positive manner to promote bone health outcomes (1147), such as increased BMD and strength (1407).
BMD is the most common measure of bone health and is commonly taken at the femoral head, hip, and lumbar spine with the use of dual-energy X-ray absorptiometry (DEXA) (588, 635, 1279). While common, use of DEXA alone provides information on only one facet of bone health and may not capture the intricacy of other important outcomes related to bone strength, such as cortical and trabecular volume (127, 236, 443, 642). Attempts to provide more comprehensive measurements include geometric analysis by peripheral quantitative computer tomography (pQCT) (365, 630, 1334). Use of DEXA in conjunction with pQCT may provide complementary approaches for assessment of exercise-induced adaptations in bone health (815).
Adaptive responses in bone are brought about by metabolic and mechanotransductive forces that initiate signaling cascades specific to a given microenvironment (1147). Briefly, mechanical sheer signals through ion-gated channels and propagates through cell-to-cell contact via integrins and gap junctions (480). These forces upon bone promote anabolism by blunting osteocyte release of receptor activator of nuclear factor- ligand (RANKL) and activating the Wnt/β-catenin pathway (1000). Through the latter mechanism, β-catenin is stabilized and can translocate to the nucleus to promote expression of encoding RUNT-related transcription factor 2 (RUNX2), aiding in progression toward the osteogenic lineage (205).
Bone adaptations to aerobic exercise
Under load or high muscle force typical of exercise, bone is formed (480). The greater the load, the more immense the skeletal strain, and, likewise, the larger the increase in BMD (216). Thus, when considering aerobic exercise prescription for bone health and increased BMD, modalities with greater concussive forces may lead to a more dramatic outcome (715). Specifically, AE such as walking may produce forces from 2.2 to 2.5 times body weight, while descending stairs increases the force absorbed to 2.8 times body weight (1294). Moreover, activities such as sprinting or downhill running increase the load on the tibia up to three-fold that of walking (176). Thus, athletes that participate in sports involving high forces due to ground contact have higher BMD than their counterparts (715). However, increases in BMD appear site-specific and dependent on activity (634).
Acutely, markers of bone turnover in humans do not differ between weight-bearing (running) or weight-assisted (cycling) AE modalities (727). However, in chronically trained female athletes across high-, medium-, and low-impact sports, all three groups exhibit similar markers of resorption, but only the high- and medium-impact groups exhibit greater BMD bone formation (284). This suggests that there is a differential response to chronic loading on BMD and bone formation markers. Sclerostin, a negative regulator of bone formation via attenuation of the Wnt/β-catenin pathway, increases in serum following AE in both males and females (382, 727, 1046). However, the effect of this in humans is not yet clear, as there are no long-term changes in bone turnover markers such as procollagen type I amino-terminal propeptide (PINP) and cross-linked telopeptide of type I collagen (CXTI) (727, 728). In contrast, chronic AE training consisting of walking, running, step-ups, and mobility exercises led to decreased sclerostin levels and higher levels of bone anabolism (46). Thus, multiple exercise bouts may be necessary to propagate intracellular signaling that leads to meaningful changes in BMD.
Bone adaptations to resistance exercise
There appear to be many benefits of RE in relation to bone health (588). Presently, a large fraction of human research into bone adaptations to RE examines postmenopausal women, an at-risk group for osteoporosis and osteopenia (675). These interventions are based on the premise that RE may be leveraged to mitigate the progression of bone health decline (368, 596, 635, 674, 680, 1059, 1253, 1402, 1468, 1474), which may start as early as three years after the onset of menopause (675). Strong evidence in a recent meta-analysis by Zhao et al. suggests that RE is an effective strategy for increasing BMD in these cohorts (1474). Likewise, males exhibit similar increases in BMD following resistance training (126, 738). Less is known about the influence of RE training on bone compartments such as the trabeculae. However, in diseases such as type II diabetes, bone quality is more affected than BMD, suggesting a need to examine this phenotype. Some evidence in literature reviews and meta-analyses describes the ability of exercise to prolong trabecular volume, but discrepancies in study design limit the statistical power to completely investigate this (588, 1059). In a rat model of type II diabetes, RE in the form of percutaneous muscle stimulation promotes BMD and bone quality (622), and human exercise trials are ongoing (73).
Recent studies have provided valuable insight into molecular communication of bone with surrounding tissues beyond the currently understood mechanisms of mechanotransduction. In particular, signaling between bone and the closely associated skeletal muscle has been of research interest (159, 190) with an emphasis on myokine, osteokine, and growth factor cross talk, as reviewed in detail (761). Muscle-bone cross talk has the capacity to regulate each tissue in an anabolic or catabolic fashion, depending upon the stimulus (701, 702). Harry et al. reported that open tibial fractures in mice recovered significantly faster when a flap of skeletal muscle was placed over the wound in comparison to a fasciocutaneous tissue, resulting in 50% greater cortical bone content (525). Thus, muscle secretions may promote regeneration and repair when bone is compromised. Even healthy bone allows molecular penetrants through the periosteum in a size-dependent fashion in mice (745), and a number of myokines and other muscle-derived species (such as IGF-1, IL-15, PGE2, and FGF-2) fall within the size constraint predicted to be able to penetrate through the periosteum (745). Given that muscle has a well-established role as a secretory organ (1017) and myokines are augmented during exercise (767), it appears that exercise-induced adaptations in bone are at least partially mediated by myokine production and cross talk with bone (761).
Despite the benefits of exercise for BMD and other parameters of bone health on earth, there is limited knowledge of the optimal exercise intervention to support the skeletal system without a gravitational vector: that is, in space. While exercise countermeasures are protective for the skeletal muscle and cardiovascular systems in surprisingly small doses, loss of bone integrity in microgravity poses a major barrier to long-duration spaceflight missions (282, 1222, 1224). Furthermore, bone formation upon return to gravity does not appear to fully recover (1366), potentially heightening long-term fracture risk. Existing exercise devices aboard the International Space Station appear unable in isolation to apply sufficient loads to stimulate bone formation over resorption, prompting investigation into nutritional supplementation approaches to promote bone health (764, 1037, 1197, 1222, 1223). Animal models taken aboard space-faring vessels have provided insight into the time course of bone integrity loss and molecular signatures associated with this process (1291, 1450), and earth-based analogs such as bedrest can provide an avenue to conduct microgravity research in a controlled setting (719, 763). Certainly, continuation of this line of research is essential to support long-duration space travel and protect long-term astronaut bone health.
Structure and function of joints
Joints are loosely defined as the point of connection between two bones and are classified into three types in humans (661). Fibrous joints are fixed, and collagen is the primary connective tissue holding these joints in place (661). Cartilaginous joints are subcategorized based on the type of cartilage separating bone as either primary (hyaline cartilage) or secondary (fibrocartilage) (1471). Synovial joints are capable of large movements and are made up of a fibrous capsule, which is coated in synovial fluid to permit movement of the articulating bone with minimal friction (661). Joints are formed through a complex network of molecular signaling that occurs throughout development. The main pathways regulating joint development were recently reviewed in detail by Salva and Merrill (1142) and include Wnt, Hedgehog, Notch, and bone morphogenetic protein.
Joint adaptations to aerobic exercise
In relation to the effect of exercise on joint adaptations, the vast majority of mechanistic research is based on individuals with RA and osteoarthritis OA, both degenerative joint diseases. The primary proinflammatory cytokines believed to drive RA progression [e.g., tumor necrosis factor (TNF)-α, IL-1β)] have been demonstrated to destroy joint integrity (434) and induce pain (1390). Conversely, chronic AE elicits a systemic anti-inflammatory effect (757, 1477), which may be able to mitigate inflammatory pathology characteristic of disease progression. In support, a meta-analysis by Baillet et al. showed AE activities in the range of 50% to 90% maximum heart rate decreased pain and increased radiographically assessed bone sparing and quality of life in patients with RA (1155). Continued investigation into mechanisms underlying this effect may reveal both inflammatory and other molecules that could provide therapeutic benefit in RA.
In addition to cartilage and periarticular degradation, OA is characterized by a robust inflammatory pathology in the affected joint and surrounding tissues, compounding the joint damage via molecular tissue cross talk promoting further inflammation and impaired proteostasis (817). As introduced above, exercise prehabilitation may alleviate long-term pain in individuals with OA (1343) through its effects on hypoalgesia (1058). Though potential mechanisms of diminished pain response are wide-ranging, they may include increased release of endorphins, inhibition of the N-methyl-d-aspartate receptor (NMDA) subunit NR1, and decreased serotonin transporter activity (1218).
Joint adaptations to resistance exercise
Molecular-level joint adaptations to RE in the context of humans are poorly understood: most of the present knowledge is derived from animal models but could point toward future directions for human research. Rat models of OA have shown favorable responses to agents that reduce oxidative stress, as exercise training might accomplish (234). For instance, hydrogen-rich water inhibits cyclooxygenase activity and MMP-3 and -13 (234). Pathologically expressed MMPs have been shown to play a noteworthy role in OA pathogenesis by degrading cartilage ECM within a joint (879). MMP production is driven by inflammatory mediators such as TNF-α (879). The effects of RE on the inflammatory response are context-dependent. For instance, while long-term RE may alleviate low-grade inflammation in humans, reductions in circulating TNF-α are not a universal finding (99, 181, 1042, 1072). However, mechanotransductive forces have been shown to positively alter joint integrity by diminishing inflammatory mediators and MMPs responsible for cartilage breakdown in animal models (783). Specifically, tissue loading decreases IL-1-induced MMP activation and mitigates collagen loss (1316). Thus, available evidence supports that RE may be beneficial for joint health in inflammatory degradative conditions and highlights the need for further study in human trials to investigate mechanistic underpinnings.
Structure and function of tendon
Tendons are composed of a complex array of bundled molecules making up the functional unit that transmits forces from muscle to bone. The tendon is primarily made of the collagen I molecule, but other collagens (III and IV) and proteoglycans contribute to their structure (1391). Tendons exhibit a hierarchical structure beginning with the collagen molecule (1392): these molecules constitute fibrils, many fibrils form the fiber, fibers form the fascicle, and many fascicles comprise the tendon unit (1391). The tendon attaches to the bone at a point known as the enthesis and allows the contraction of muscle to pull on the bone for locomotion (1391). Tendons have received attention in the context of human exercise due to their obvious role in movement as well as reasonably easy sampling access via microbiopsy needle (900, 937, 1329). For these reasons, the effects of acute and chronic exercise on molecular transducers of tendon adaptation are relatively well-characterized in comparison to bone and joint adaptation.
Tendon adaptations to aerobic exercise
In response to exercise, tendons generally adapt through increased collagen turnover, stiffness, and size (cross-sectional area) (427). Based on a recent meta-analysis, exercise that elicits loading of greater magnitude appears to be most beneficial, and chronic (>12 week) loading is preferable to acute (125). Nevertheless, acute AE has been shown to induce collagen I synthesis in humans (546, 748). Moreover, chronic long-distance runners have approximately 23% greater cross-sectional area at the Achilles tendon than their counterparts (1117). This appears to be partially driven by an acute inflammatory cascade involving IL-6 and PGE2 (32, 243), which are detectable in serum after high-intensity AE (996, 1015). Attenuation of PGE2 with nonsteroidal anti-inflammatory drugs has a negative effect on collagen synthesis in young men after acute AE (243). Notably, PGE2 has been linked to IL-6 in skeletal muscle tissue in vitro (1237), and findings suggest that a regulatory relationship between these factors may exist in tendon (503). In response to IL-6, collagen synthesis markers such as procollagen type I NH2-terminal propeptide (PINP) are increased (32), but the effects of AE on this marker are dose- and time-dependent (546, 747, 748). Growth factors such as IGF-1, transforming growth factor-beta (TGF-β), and platelet-derived growth factor BB have been shown to positively influence dynamics of tendon remodeling in vitro (710, 994, 1305). Many of these factors are modulated by exercise. However, data in humans is far more equivocal than results from animal studies. It has been suggested that this is due to clinical studies sampling tendon outside of a period of active growth; conversely, many animals are tested during growth, allowing detection of trends in tendon biology that may be masked in mature human tendon (838).
Tendon adaptations to resistance exercise
RE has been shown to lead to tendon adaptations in a load-dependent fashion (125). The primary adaptation observed following RE training in humans is increased tendon stiffness, or Young’s modulus (736, 1092). Interestingly, hypertrophy has also been observed in the human patellar tendon, although it is not uniform throughout the length of the tendon: cross-sectional area tends to increase at the proximal and distal ends (720). Investigations have examined the response to acute RE in tendinous tissue, revealing a basis for molecular transducers of these adaptations. Following RE (3 sets of 10 repetitions of knee extension exercise), tendon expression of collagen I, III, and MMP-3 were decreased 4 h postexercise but returned to baseline at 24 h (1269). Others have found that acute RE increases connective tissue growth factor (CTGF) and type I collagen; however, this increase was not matched by fractional synthetic rate of collagen I or other regulatory markers such as TGF-β (333). In preclinical models, TGF-β interacts with CTGF and plays a vital role in tendon remodeling (710, 1335). Like AE, the discrepancy of the effect of RE on tendons may be due to the adult human tendon being more stable than actively remodeling or growing animal tendons (547, 838). Even chronic (10 year) RE training in young adults does not lead to appreciable differences in tendon structure or size (781). Given the effects of age on tendon health (198, 1274), it is possible that meaningful exercise-induced changes in tendon physiology might be best captured using longitudinal studies extending into older age or examining lifelong-trained older adults.
Section summary
Health of the skeletal system and associated structures such as joints and tendons is of utmost importance for exercise. It is important to expand our knowledge of exercise-induced molecular transducers underlying promotion of healthy bones, joints, and tendons in children and adolescents and maintain their health into old age or during periods of high-volume training. Given sampling limitations of some of these structures in humans, much remains to be learned with regard to molecular drivers of adaptation in the context of performance, health, and disease.
Conclusions and Future Directions
While available evidence supports that exercise may forestall, alter, or even partially reverse the course of many diseases and disorders (Figure 10), a number of knowledge gaps and practical challenges limit its utility as a formal prescription or a tool for leveraging toward development of novel drug targets. First, individual variability in responsiveness (as exists with pharmaceutical interventions) to exercise presents a potential issue (1231). It is not yet completely understood why individuals respond differently to the same exercise regimen, but it is likely that large molecular-level cohort studies, such as MoTrPAC, will shed light on important factors whose variability impacts exercise responsiveness. In examination of these potential influences, it is critical to strive for inclusivity in study cohorts, including racial/ethnic diversity, sex balance, age, and consideration of other important demographics that may introduce variability into a data set (e.g., socioeconomic status, education level). Continued development of bioinformatics platforms [e.g., surrogate variable analysis, svaSeq (775)] to identify confounding artifacts in data and to integrate ‘omics data from multiple phenotypic levels (532) may aid in discriminating molecular associations that are the result of biological relationships or simply individual heterogeneity (i.e., noise).
Figure 10.
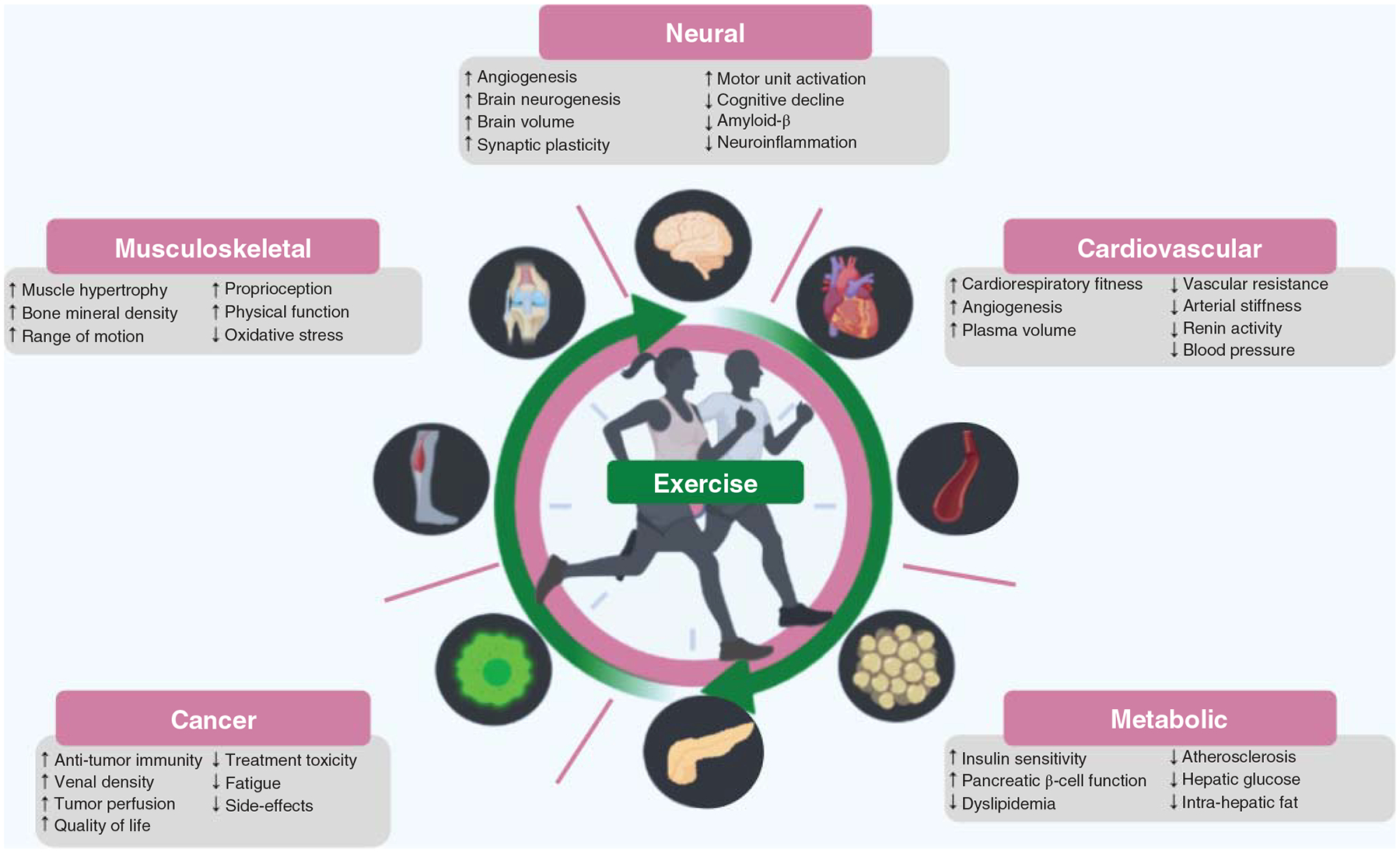
This figure illustrates the range of the impact of exercise on risk factors and pathologies associated with chronic diseases. Exercise is associated with reduced morbidity, lower hospitalization rates, and decreased risk of all-cause mortality and premature death from disease. Overall, higher cardiorespiratory fitness and a regular exercise training decrease the economic burden of multiple diseases. ↑ indicates increase; ↓ indicates decrease.
Secondly, human behavior presents a major challenge to prescription of exercise. While researchers may facilitate a robust, supervised exercise intervention, commitment to long-term exercise is a lifestyle choice. Cross-sectional studies examining the benefits of the exercise lifestyle have provided insight into long-term effects of exercise, but these tend to focus primarily on AE. Attention in this area has revealed which physiological systems are completely, partially, and poorly protected by exercise throughout normal human aging (196, 219, 498, 499, 757, 1030, 1193, 1323). It is likely that examination of lifelong engagement in practices such as RE will reveal differential effects and guide us toward optimization of exercise as an antiaging strategy. Nevertheless, not all individuals may be persuaded to exercise by the promise of a healthier and/or longer life; thus, the challenge is to encourage individuals to discover a preferred modality that is maintainable and enjoyable for its own sake while simultaneously accruing functional benefits. Collaboration with behavioral scientists may guide exercise biologists toward encouraging individuals to build a sustainable habit of physical activity (677, 1141).
Third, the landscape of human health and disease is continually changing. For example, at the time of preparation of this article, the novel coronavirus (COVID-19) pandemic is taking foothold in the United States and much of the world (232, 1210), presenting challenges to both physical (925, 960) and psychological resilience (1315). While it may be impossible to predict such events, strong evidence supports that regular exercise fortifies physiological reserves that may be critical in protection and/or recovery from threats to human health and life. Presently, the impact of regular exercise on strengthening the immune system is of enormous public health relevance (183, 989, 1207). It is probable that the full range of these effects extends beyond our present understanding of skeletal muscle as the primary amino acid reservoir (1080, 1365, 1438). Furthermore, the protective effects of exercise on physiological reserves other than physical [e.g., cognitive, emotional (235, 988)] are likely of importance. While much research focuses on molecular mechanisms underlying physical benefits of exercise, consideration of exercise as a holistically favorable activity will yield insight into important factors influencing mood, affect, and quality of life. Activities blending physical exercise with mindfulness practices (e.g., yoga, Tai Chi) may be of particular interest.
Despite the challenges ahead and a somewhat incomplete picture of its mechanisms of action, exercise is an accessible, affordable, and effective strategy for prolonging healthspan through a range of physiological benefits. A more complete mechanistic knowledge of exercise adaptations may enable greater specificity in prescribing exercise across demographics (e.g., age, sex, race, health status) and heterogeneous molecular profiles (e.g., transcriptomic, epigenetic). Furthermore, it may enable flexibility of physical activity recommendations in response to changes in population structure and public health status.
In closing, we recognize the rich history of the field of exercise research. Although (to our knowledge) this article will be the most comprehensive ever constructed on the molecular adaptations to exercise (Table 2), we recognize that not all pertinent literature may have been addressed, and this is an inherent limitation. Still, despite the wealth of literature in the area, much remains to be elucidated regarding the molecular adaptations that underlie the beneficial health effects of exercise, and we are hopeful that this article will have illustrated such knowledge gaps. Undoubtedly, the anticipated MoTrPAC dataset will provide substantial opportunity in this area, including evaluation of the molecular map of exercise in healthy individuals, investigation of biological interactions across ‘omics, and direction toward understanding variance in human biology. Even with this promise, many questions remain; as such, continued interdisciplinary collaboration is encouraged to expand our collective understanding of the role of exercise as an ultimate protector of human health.
Table 2.
List of Abbreviations
| Abbreviation | Term | Abbreviation | Term |
|---|---|---|---|
| 11β-HSD1 | 11β-Hydroxysteroid dehydrogenase 2 | HDAC4 | Histone deacetylase 4 |
| 12,13-diHOME | 12,13-Dihydroxy-9Z-octadecenoic acid | HF | Heart failure |
| 1RM | One-repetition maximum | HFL | Harvard Fatigue Laboratory |
| 4E-BP1 | Eukaryotic translation initiation factor binding protein 1 | HIIT | High-intensity interval training |
| AD | Alzheimer’s disease | HR | Heart rate |
| ADHD | Attention deficity/Hyperactivity disorder | HRR | Heart rate reserve |
| AE | Aerobic exercise | HSL | Hormone-sensitive lipase |
| AICAR | 5-Aminoimidazole-4-carboxaminde-1-β-d-ribofuranoside | IMAT | Intermuscular adipose tissue |
| Akt | Protein kinase B | IMTG | Intramuscular triglycerides |
| ANGPTL4 | Angiopoietin-like protein-4 | KAT | Kynurenine aminotransferase |
| ANP | Atrial natriuretic peptide | KYNA | Kynurenic acid |
| ApoE4 | Apolipoprotein E4 | LBD | Lewy body dementia |
| ASD | Autism spectrum disorder | LC | Liquid chromatography |
| AT | Adipose tissue | LV | Left ventricle |
| BAIBA | β-Aminoisobutyric acid | MCI | Mild cognitive impairment |
| BAT | Brown adipose tissue | MEF-2 | Myocyte enhancer factor 2 |
| BBB | Blood-brain barrier | MetS | Metabolic Syndrome |
| BMD | Bone mineral density | MFF | Mitochondrial fission factor |
| BMU | Basic multicellular unit | MFN | Mitofusin |
| BNDF | Brain-derived neurotrophic factor | MHC | Myosin heavy chain |
| BNP | Brain natriuretic peptide | miRNA | microRNA |
| C/EBPβ | CCAAT enhancer-binding protein β | MMP | Matrix metalloproteases |
| CD | Cluster of differentiation | MoTrPAC | Molecular Transducers of Physical Activity Consortium |
| CHD | Coronary heart disease | mtDNA | Mitochondrial DNA |
| CITED4 | Creb binding protein (CBP)/p300-interacting transactivator with ED-rich carboxyl-terminal domain-4 | mTOR | Mammalian target of rapamycin; C denotes complex 1 vs. 2 |
| CMRC | Copenhagen muscle research center | MUNE | Motor unit number estimate |
| CNS | Central nervous system | MuRF-1 | Muscle ring finger 1 |
| CRH | Corticotropin-releasing hormone | Myf | Myogenic factor |
| CRF | Cardiorespiratory fitness | NCAM | Neural cell adhesion molecule |
| CRP | C-reactive protein | NMDA | N-methyl-d-aspartate receptor |
| CTGF | Connective tissue growth factor | NMJ | Neuromuscular junction |
| CVD | Cardiovascular disease | NO | Nitric oxide |
| CXTI | Cross-linked telopeptide of type I collagen | NOS | Nitric oxide synthase |
| DEXA | Dual-energy X-ray absorptiometry | NPR-A | Natriuretic peptide guanylyl cyclase receptor A |
| DRP1 | Dynamin-related protein 1 | NRF | Nuclear respiratory factors |
| EPC | Endothelial progenitor cells | NRG | Neuregulin |
| EPS | Electrical pulse stimulation | OA | Osteoarthritis |
| ERR | Estrogen-related receptor | p70s6k | p70s6 kinase |
| EV | Extracellular vesicle | p75NTR | Neurotrophin receptor |
| FFA | Free fatty acid | PA | Physical activity |
| FIB-SEM | Focused ion beam scanning electron microscopy | PD | Parkinson’s disease |
| FIS1 | Mitochondrial fission 1 protein | PGC-1α | Peroxisome proliferator-activated receptor-γ coactivator 1α |
| FNDC5 | Fibronectin III domain-containing protein 5 | PINP | Procollagen type I amino-terminal propeptide |
| GRα | Glucocorticoid receptor-α | PNS | Peripheral nervous system |
| GSK3β | Glycogen synthase kinase-3 β | PPAR | Peroxisome proliferator-activated receptor |
| H2O2 | Hydrogen peroxide | pQCT | Peripheral quantitative computer tomography |
| HCAR1 | Hydroxycarboxylic acid receptor 1 | RA | Rheumatoid arthritis |
| RANKL | Receptor activator of nuclear factor-κ ligand | TGF-β | Transforming growth factor-β |
| RE | Resistance exercise | TIF | Transcription initiation factor |
| ROS | Reactive oxygen species | TNF-α | Tumor necrosis factor α |
| RUNX2 | RUNT-related transcription factor 2 | TrkB | Tropomyosin receptor kinase B |
| RYR1 | Ryanodine receptor 1 | UBF | Upstream binding factor |
| SC | Satellite cell | UCP-1 | Uncoupling protein 1 |
| Spred-1 | Sprouty-related protein 1 | Vo2 | Oxygen consumption |
| T1D/T2D | Type 1 diabetes | Vo2max | Maximal oxygen consumption |
| T2D | Type 2 diabetes | Vo2peak | Peak oxygen consumption |
| TEM | Transmission electron microscopy | WAT | White adipose tissue |
| Tfam | Transcription factor A mitochondrial |
Please see full list of other APS-approved common abbreviations: https://journals.physiology.org/pb-assets/PDFs/abbreviations-1510761719900.pdf.
Didactic Synopsis.
Major teaching points
Regular, structured exercise promotes prevention of disease, as well as a variety of benefits for general health.
Throughout its history, the study of exercise has been a multidisciplinary pursuit.
Basic methodological considerations, analysis techniques, and emerging advancements continue to facilitate collaboration, discovery, and progress.
Key mechanisms underlying exercise-induced adaptations are driven by cellular and molecular cues throughout the body’s physiological systems.
Aerobic and resistance exercises stimulate distinct but overlapping adaptive mechanisms that improve health, human performance, and system functioning. With the Molecular Transducers of Physical Activity Consortium (MoTrPAC) as important infrastructure, critical knowledge gaps present opportunities for future investigation.
Acknowledgments
We are grateful to all who contributed to the vast body of literature presently available in the field of exercise biology, including that which we were not able to cite, even in a scoping review such as this. Additional thanks to Scott and Todd Trappe at our MoTrPAC sister site, Ball State University, for support and accumulation of knowledge relevant to this work. Furthermore, we acknowledge BioRender for graphical support in preparation of figures. Additional financial support for the authors was provided by the following funding sources:
KML : NIA F32AG062048
PMC : NIA K01AG044437; NIA R01AG060153; NIA R01AG060542
LCB : NIA R01AG056769
MBB : NICHD T32HD071866
DD : NICHD T32HD071866
SAH : NICHD T32HD071866; AHA 20POST34990005
MEL : Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) process numbers: 2016/22635-6 and 2018/15691-2
JSM : DARPA contract FA8650-19-C-7944
SMO : T32HD071866 SR: NIA K01AG044437; NIA R01AG060153; NIA R01AG060542
LMR : NIGMS IRACDA K12GM088010
RBV : NIA K01AG044437; NIA R01AG060153; NIA R01AG060542
BHG : NIH Common Fund U01AR071133
MMB : NIH Common Fund U01AR071133
TWB : NIA R21AG049974, NIA R01AG054538, NIA R01AG056769, NIA K02AG062498
Footnotes
Related Articles
Cardiovascular Adaptations to Exercise Training
Lack of Exercise Is a Major Cause of Chronic Diseases Molecular Mechanisms of Muscle Plasticity with Exercise Exercise Physiology of Normal Development, Sex Differences, and Aging
Genomics and Genetics in the Biology of Adaptation to Exercise
References
- 1.Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Neural adaptation to resistance training: Changes in evoked v-wave and h-reflex responses. J Appl Physiol (1985) 92: 2309–2318, 2002. DOI: 10.1152/japplphysiol.01185.2001. [DOI] [PubMed] [Google Scholar]
- 2.Abbasi A, de Paula Vieira R, Bischof F, Walter M, Movassaghi M, Berchtold NC, Niess AM, Cotman CW, Northoff H. Sex-specific variation in signaling pathways and gene expression patterns in human leukocytes in response to endotoxin and exercise. J Neuroinflammation 13: 289, 2016. DOI: 10.1186/s12974-016-0758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdelmoez AM, Puig LS, Smith JAB, Gabriel BM, Savikj M, Dollet L, Chibalin AV, Krook A, Zierath JR, Pillon NJ. Comparative profiling of skeletal muscle models reveals heterogeneity of transcriptome and metabolism. Am J Phys Cell Phys 318: C615–C626, 2020. DOI: 10.1152/ajpcell.00540.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abe T, DeHoyos DV, Pollock ML, Garzarella L. Time course for strength and muscle thickness changes following upper and lower body resistance training in men and women. Eur J Appl Physiol 81: 174–180, 2000. DOI: 10.1007/s004210050027. [DOI] [PubMed] [Google Scholar]
- 5.Ablij H, Meinders A. C-reactive protein: History and revival. Eur J Intern Med 13: 412, 2002. DOI: 10.1016/s0953-6205(02)00132-2. [DOI] [PubMed] [Google Scholar]
- 6.Ackel- D’Elia C, Carnier J, Bueno CR, Campos RMS, Sanches PL, Clemente APG, Tufik S, de Mello MT, Dâmaso AR. Effects of different physical exercises on leptin concentration in obese adolescents. Int J Sports Med 35: 164–171, 2014. DOI: 10.1055/s-0033-1345128. [DOI] [PubMed] [Google Scholar]
- 7.Adamopoulos S, Corrà U, Laoutaris ID, Pistono M, Agostoni PG, Coats AJS, Leiro MGC, Cornelis J, Davos CH, Filippatos G, Lund LH, Jaarsma T, Ruschitzka F, Seferovic PM, Schmid J-P, Volterrani M, Piepoli MF. Exercise training in patients with ventricular assist devices: A review of the evidence and practical advice. A position paper from the committee on exercise physiology and training and the committee of advanced heart failure of the heart failure association of the European society of cardiology. Eur J Heart Fail 21: 3–13, 2019. DOI: 10.1002/ejhf.1352. [DOI] [PubMed] [Google Scholar]
- 8.Adams KF, Vincent LM, McAllister SM, El-Ashmawy H, Sheps DS. The influence of age and gender on left ventricular response to supine exercise in asymptomatic normal subjects. Am Heart J 113: 732–742, 1987. DOI: 10.1016/0002-8703(87)90714-9. [DOI] [PubMed] [Google Scholar]
- 9.Adamsen L, Quist M, Andersen C, Møller T, Herrstedt J, Kronborg D, Baadsgaard MT, Vistisen K, Midtgaard J, Christiansen B, Stage M, Kronborg MT, Rørth M. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: Randomised controlled trial. BMJ 339: b3410, 2009. DOI: 10.1136/bmj.b3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Addison O, Marcus RL, Lastayo PC, Ryan AS. Intermuscular fat: A review of the consequences and causes. Int J Endocrinol 2014: 309570, 2014. DOI: 10.1155/2014/309570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adeva-Andany M, López-Ojén M, Funcasta-Calderón R, Ameneiros-Rodríguez E, Donapetry-García C, Vila-Altesor M, Rodríguez-Seijas J. Comprehensive review on lactate metabolism in human health. Mitochondrion 17: 76–100, 2014. DOI: 10.1016/j.mito.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Agley CC, Rowlerson AM, Velloso CP, Lazarus NL, Harridge SDR. Isolation and quantitative immunocytochemical characterization of primary myogenic cells and fibroblasts from human skeletal muscle. J Vis Exp 52049, 2015. DOI: 10.3791/52049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agudelo LZ, Femen’ιa T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, Correia JC, Izadi M, Bhat M, Schuppe-Koistinen I, Pettersson AT, Ferreira DMS, Krook A, Barres R, Zierath JR, Erhardt S, Lindskog M, Ruas JL. Skeletal muscle pgc-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 159: 33–45, 2014. DOI: 10.1016/j.cell.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 14.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the united states, 2003–2012. JAMA 313: 1973–1974, 2015. DOI: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 15.Ahlborg G, Felig P. Lactate and glucose exchange across the forearm, legs, and splanchnic bed during and after prolonged leg exercise. J Clin Invest 69: 45–54, 1982. DOI: 10.1172/jci110440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahlborg G, Hagenfeldt L, Wahren J. Substrate utilization by the inactive leg during one-leg or arm exercise. J Appl Physiol 39: 718–723, 1975. DOI: 10.1152/jappl.1975.39.5.718. [DOI] [PubMed] [Google Scholar]
- 17.Ahlskog JE. Does vigorous exercise have a neuroprotective effect in parkinson disease? Neurology 77: 288–294, 2011. DOI: 10.1212/WNL.0b013e318225ab66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahn AH. Why does increased exercise decrease migraine? Curr Pain Headache Rep 17: 379, 2013. DOI: 10.1007/s11916-013-0379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med 23: 469–480, 2006. DOI: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 20.Alderete TL, Sattler FR, Sheng X, Tucci J, Mittelman SD, Grant EG, Goran MI. A novel biopsy method to increase yield of subcutaneous abdominal adipose tissue. Int J Obes 39: 183–186, 2015. DOI: 10.1038/ijo.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aldiss P, Betts J, Sale C, Pope M, Budge H, Symonds ME. Exercise-induced ‘browning’ of adipose tissues. Metab Clin Exp 81: 63–70, 2018. DOI: 10.1016/j.metabol.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alex C, Lindgren M, Shapiro PA, McKinley PS, Brondolo EN, Myers MM, Zhao Y, Sloan RP. Aerobic exercise and strength training effects on cardiovascular sympathetic function in healthy adults: A randomized controlled trial. Psychosom Med 75: 375–381, 2013. DOI: 10.1097/PSY.0b013e3182906810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Khazraji BK, Shoemaker JK. The human cortical autonomic network and volitional exercise in health and disease. Appl Physiol Nutr Metab 43: 1122–1130, 2018. DOI: 10.1139/apnm-2018-0305. [DOI] [PubMed] [Google Scholar]
- 24.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: Cellular mechanisms. Physiol Rev 88: 287–332, 2008. DOI: 10.1152/phys-rev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 25.Allison DJ, Nederveen JP, Snijders T, Bell KE, Kumbhare D, Phillips SM, Parise G, Heisz JJ. Exercise training impacts skeletal muscle gene expression related to the kynurenine pathway. Am J Phys Cell Phys 316: C444–C448, 2019. DOI: 10.1152/ajpcell.00448.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Álvarez-Bueno C, Pesce C, Cavero-Redondo I, Sánchez-López M, Martínez-Hortelano JA, Martínez-Vizcaíno V. The effect of physical activity interventions on children’s cognition and metacognition: A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry 56: 729–738, 2017. DOI: 10.1016/j.jaac.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Alves-Bezerra M, Cohen DE. Triglyceride metabolism in the liver. Compr Physiol 8: 1–8, 2017. DOI: 10.1002/cphy.c170012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alvim RO, Cheuhen MR, Machado SR, Sousa AGP, Santos PCJL. General aspects of muscle glucose uptake. An Acad Bras Cienc 87: 351–368, 2015. DOI: 10.1590/0001-3765201520140225. [DOI] [PubMed] [Google Scholar]
- 29.Amara AW, Memon AA. Effects of exercise on non-motor symptoms in parkinson’s disease. Clin Ther 40: 8–15, 2018. DOI: 10.1016/j.clinthera.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care 40: S11–S24, 2017. DOI: 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 31.Anazodo UC, Shoemaker JK, Suskin N, Lawrence KSS. An investigation of changes in regional gray matter volume in cardiovascular disease patients, pre and post cardiovascular rehabilitation. NeuroImage Clin 3: 388–395, 2013. DOI: 10.1016/j.nicl.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersen MB, Pingel J, Kjær M, Langberg H. Interleukin-6: A growth factor stimulating collagen synthesis in human tendon. J Appl Physiol (1985) 110: 1549–1554, 2011. DOI: 10.1152/japplphysiol.00037.2010. [DOI] [PubMed] [Google Scholar]
- 33.Andersen P, Adams RP, Sjøgaard G, Thorboe A, Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol (1985) 59: 1647–1653, 1985. DOI: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- 34.Andersen P, Henriksson J. Capillary supply of the quadriceps femoris muscle of man: Adaptive response to exercise. J Physiol 270: 677–690, 1977. DOI: 10.1113/jphysiol.1977.sp011975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol 366: 233–249, 1985. DOI: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson EJ, Neufer PD. Type ii skeletal myofibers possess unique properties that potentiate mitochondrial h(2)O(2) generation. Am J Phys Cell Phys 290: C844–C851, 2006. DOI: 10.1152/ajpcell.00402.2005. [DOI] [PubMed] [Google Scholar]
- 37.Anderson L, Nguyen TT, Dall CH, Burgess L, Bridges C, Taylor RS. Exercise-based cardiac rehabilitation in heart transplant recipients. Cochrane Database Syst Rev 4: CD012264, 2017. DOI: 10.1002/14651858.CD012264.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson L, Thompson DR, Oldridge N, Zwisler A-D, Rees K, Martin N, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev: CD001800, 2016. DOI: 10.1002/14651858.CD001800.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anton SD, Woods AJ, Ashizawa T, Barb D, Buford TW, Carter CS, Clark DJ, Cohen RA, Corbett DB, Cruz-Almeida Y, Dotson V, Ebner N, Efron PA, Fillingim RB, Foster TC, Gundermann DM, Joseph A-M, Karabetian C, Leeuwenburgh C, Manini TM, Marsiske M, Mankowski RT, Mutchie HL, Perri MG, Ranka S, Rashidi P, Sandesara B, Scarpace PJ, Sibille KT, Solberg LM, Someya S, Uphold C, Wohlgemuth S, Wu SS, Pahor M. Successful aging: Advancing the science of physical independence in older adults. Ageing Res Rev 24: 304–327, 2015. DOI: 10.1016/j.arr.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Antos CL, McKinsey TA, Frey N, Kutschke W, McAnally J, Shelton JM, Richardson JA, Hill JA, Olson EN. Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo. Proc Natl Acad Sci U S A 99: 907–912, 2002. DOI: 10.1073/pnas.231619298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antunes HKM, Leite GSF, Lee KS, Barreto AT, Santos RVTD, de S’a Souza H, Tufik S, de Mello MT. Exercise deprivation increases negative mood in exercise-addicted subjects and modifies their biochemical markers. Physiol Behav 156: 182–190, 2016. DOI: 10.1016/j.physbeh.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 42.Anush MM, Ashok VK, Sarma RI, Pillai SK. Role of c-reactive protein as an indicator for determining the outcome of sepsis. Indian J Crit Care Med 23: 11–14, 2019. DOI: 10.5005/jp-journals-10071-23105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arany Z, Foo S-Y, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. HIF-independent regulation of vegf and angiogenesis by the transcriptional coactivator pgc-1alpha. Nature 451: 1008–1012, 2008. DOI: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 44.Arbab-Zadeh A, Perhonen M, Howden E, Peshock RM, Zhang R, Adams-Huet B, Haykowsky MJ, Levine BD. Cardiac remodeling in response to 1 year of intensive endurance training. Circulation 130: 2152–2161, 2014. DOI: 10.1161/CIRCULATIONAHA.114.010775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Archer SL. Mitochondrial dynamics–mitochondrial fission and fusion in human diseases. N Engl J Med 369: 2236–2251, 2013. DOI: 10.1056/NEJMra1215233. [DOI] [PubMed] [Google Scholar]
- 46.Ardawi M-SM, Rouzi AA, Qari MH. Physical activity in relation to serum sclerostin, insulin-like growth factor-1, and bone turnover markers in healthy premenopausal women: A cross-sectional and a longitudinal study. J Clin Endocrinol Metab 97: 3691–3699, 2012. DOI: 10.1210/jc.2011-3361. [DOI] [PubMed] [Google Scholar]
- 47.Armstrong MJ. Lewy body dementias. Continuum 25: 128–146, 2019. DOI: 10.1212/CON.0000000000000685. [DOI] [PubMed] [Google Scholar]
- 48.Armstrong RB, Saubert CW, Sembrowich WL, Shepherd RE, Gollnick PD. Glycogen depletion in rat skeletal muscle fibers at different intensities and durations of excercise. Pflugers Arch 352: 243–256, 1974. DOI: 10.1007/bf00590489. [DOI] [PubMed] [Google Scholar]
- 49.Arnold CM, Sran MM, Harrison EL. Exercise for fall risk reduction in community-dwelling older adults: A systematic review. Physiother Can 60: 358–372, 2008. DOI: 10.3138/physio.60.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arribat Y, Broskey NT, Greggio C, Boutant M, Alonso SC, Kulkarni SS, Lagarrigue S, Carnero EA, Besson C, Canto C, Amati F. Distinct patterns of skeletal muscle mitochondria fusion, fission and mitophagy upon duration of exercise training. Acta Physiol (Oxf) 225: e13179, 2019. DOI: 10.1111/apha.13179. [DOI] [PubMed] [Google Scholar]
- 51.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science (New York, NY) 275: 964–967, 1997. DOI: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 52.Ashcraft KA, Warner AB, Jones LW, Dewhirst MW. Exercise as adjunct therapy in cancer. Semin Radiat Oncol 29: 16–24, 2019. DOI: 10.1016/j.semradonc.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ashford S, Williard J. Osteoarthritis: A review. Nurse Pract 39: 1–8, 2014. DOI: 10.1097/01.NPR.0000445886.71205.c4. [DOI] [PubMed] [Google Scholar]
- 54.Astori G, Vignati F, Bardelli S, Tubio M, Gola M, Albertini V, Bambi F, Scali G, Castelli D, Rasini V, Soldati G, Moccetti T. “In vitro” and multicolor phenotypic characterization of cell subpopulations identified in fresh human adipose tissue stromal vascular fraction and in the derived mesenchymal stem cells. J Transl Med 5: 55, 2007. DOI: 10.1186/1479-5876-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Astorino TA, Allen RP, Roberson DW, Jurancich M. Effect of high-intensity interval training on cardiovascular function, vo2max, and muscular force. J Strength Cond Res 26: 138–145, 2012. DOI: 10.1519/JSC.0b013e318218dd77. [DOI] [PubMed] [Google Scholar]
- 56.Atherton PJ, Smith K. Muscle protein synthesis in response to nutrition and exercise. J Physiol 590: 1049–1057, 2012. DOI: 10.1113/jphysiol.2011.225003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis 71: 1524–1529, 2012. DOI: 10.1136/annrheumdis-2011-200726. [DOI] [PubMed] [Google Scholar]
- 58.Awasthi H, Mani D, Singh D, Gupta A. The underlying pathophysiology and therapeutic approaches for osteoporosis. Med Res Rev 38: 2024–2057, 2018. DOI: 10.1002/med.21504. [DOI] [PubMed] [Google Scholar]
- 59.Axelrod CL, Fealy CE, Mulya A, Kirwan JP. Exercise training remodels human skeletal muscle mitochondrial fission and fusion machinery towards a pro-elongation phenotype. Acta Physiol (Oxf) 225: e13216, 2019. DOI: 10.1111/apha.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Azeez M, Clancy C, O’Dwyer T, Lahiff C, Wilson F, Cunnane G. Benefits of exercise in patients with rheumatoid arthritis: A randomized controlled trial of a patient-specific exercise programme. Clin Rheumatol 39: 1783–1792, 2020. DOI: 10.1007/s10067-020-04937-4. [DOI] [PubMed] [Google Scholar]
- 61.Åstrand PO. Influence of scandinavian scientists in exercise physiology. Scand J Med Sci Sports 1: 3–9, 2007. DOI: 10.1111/j.1600-0838.1991.tb00264.x. [DOI] [Google Scholar]
- 62.Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: Rapid increase in the transcriptional coactivator pgc-1. FASEB J 16: 1879–1886, 2002. DOI: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 63.Babaei P, Damirchi A, Mehdipoor M, Tehrani BS. Long term habitual exercise is associated with lower resting level of serum bdnf. Neurosci Lett 566: 304–308, 2014. DOI: 10.1016/j.neulet.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 64.Baggish AL, Hale A, Weiner RB, Lewis GD, Systrom D, Wang F, Wang TJ, Chan SY. Dynamic regulation of circulating microRNA during acute exhaustive exercise and sustained aerobic exercise training. J Physiol 589: 3983–3994, 2011. DOI: 10.1113/jphysiol.2011.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baggish AL, Park J, Min P-K, Isaacs S, Parker BA, Thompson PD, Troyanos C, D’Hemecourt P, Dyer S, Thiel M, Hale A, Chan SY. Rapid upregulation and clearance of distinct circulating microRNAs after prolonged aerobic exercise. J Appl Physiol (1985) 116: 522–531, 2014. DOI: 10.1152/japplphysiol.01141.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bahler L, Molenaars RJ, Verberne HJ, Holleman F. Role of the autonomic nervous system in activation of human brown adipose tissue: A review of the literature. Diabetes Metab 41: 437–445, 2015. DOI: 10.1016/j.diabet.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 67.Baillet A, Vaillant M, Guinot M, Juvin R, Gaudin P. Efficacy of resistance exercises in rheumatoid arthritis: Meta-analysis of randomized controlled trials. Rheumatology (Oxford) 51: 519–527, 2012. DOI: 10.1093/rheumatology/ker330. [DOI] [PubMed] [Google Scholar]
- 68.Baillet A, Zeboulon N, Gossec L, Combescure C, Bodin L-A, Juvin R, Dougados M, Gaudin P. Efficacy of cardiorespiratory aerobic exercise in rheumatoid arthritis: Meta-analysis of randomized controlled trials. Arthritis Care Res 62: 984–992, 2010. DOI: 10.1002/acr.20146. [DOI] [PubMed] [Google Scholar]
- 69.Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, Kurzius-Spencer M, Zahorodny W, Rosenberg CR, White T, Durkin MS, Imm P, Nikolaou L, Yeargin-Allsopp M, Lee L-C, Harrington R, Lopez M, Fitzgerald RT, Hewitt A, Pettygrove S, Constantino JN, Vehorn A, Shenouda J, Hall-Lande J, Braun KVN, Dowling NF. Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, united states, 2014. Morb Mortal Wkly Rep Surveill Summ 67: 1–23, 2018. DOI: 10.15585/mmwr.ss6706a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baker BS, Stannard MS, Duren DL, Cook JL, Stannard JP. Does blood flow restriction therapy in patients older than age 50 result in muscle hypertrophy, increased strength, or greater physical function? A systematic review. Clin Orthop Relat Res 478: 593–606, 2020. DOI: 10.1097/CORR.0000000000001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bakker EA, Lee D-C, Sui X, Artero EG, Ruiz JR, Eijsvogels TMH, Lavie CJ, Blair SN. Association of resistance exercise, independent of and combined with aerobic exercise, with the incidence of metabolic syndrome. Mayo Clin Proc 92: 1214–1222, 2017. DOI: 10.1016/j.mayocp.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Balagopal P, Schimke JC, Ades P, Adey D, Nair KS. Age effect on transcript levels and synthesis rate of muscle mhc and response to resistance exercise. Am J Phys Endocrinol Metab 280: E203–E208, 2001. DOI: 10.1152/ajpendo.2001.280.2.E203. [DOI] [PubMed] [Google Scholar]
- 73.Balducci S, Conti F, Sacchetti M, Russo CR, Argento G, Haxhi J, Orlando G, Rapisarda G, D’Errico V, Cardelli P, Pugliese L, Laghi A, Vitale M, Bollanti L, Zanuso S, Nicolucci A, Pugliese G. Study to weigh the effect of exercise training on bone quality and strength (sweet bone) in type 2 diabetes: Study protocol for a randomised clinical trial. BMJ Open 9: e027429, 2019. DOI: 10.1136/bmjopen-2018-027429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bamman MM, Cooper DM, Booth FW, Chin ER, Neufer PD, Trappe S, Lightfoot JT, Kraus WE, Joyner MJ. Exercise biology and medicine: Innovative research to improve global health. Mayo Clin Proc 89: 148–153, 2014. DOI: 10.1016/j.mayocp.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bamman MM, Petrella JK, Kim J-s, Mayhew DL, Cross JM. Cluster analysis tests the importance of myogenic gene expression during myofiber hypertrophy in humans. J Appl Physiol (1985) 102: 2232–2239, 2007. DOI: 10.1152/japplphysiol.00024.2007. [DOI] [PubMed] [Google Scholar]
- 76.Bamman MM, Roberts BM, Adams GR. Molecular regulation of exercise-induced muscle fiber hypertrophy. Cold Spring Harb Perspect Med 8, 2018. DOI: 10.1101/cshperspect.a029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bandelow B, Michaelis S. Epidemiology of anxiety disorders in the 21st century [Online]. Dialogues Clin Neurosci 17: 327–335, 2015. http://www.ncbi.nlm.nih.gov/pubmed/26487813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bangsbo J, Kjær M, Hellsten Y. Bengt saltin (1935–2014). J Physiol 592: 5149–5151, 2014. DOI: 10.1113/jphysiol.2014.285411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baniqued PL, Gallen CL, Kranz MB, Kramer AF, D’Esposito M. Brain network modularity predicts cognitive training-related gains in young adults. Neuropsychologia 131: 205–215, 2019. DOI: 10.1016/j.neuropsychologia.2019.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baniqued PL, Gallen CL, Voss MW, Burzynska AZ, Wong CN, Cooke GE, Duffy K, Fanning J, Ehlers DK, Salerno EA, Aguiñaga S, McAuley E, Kramer AF, D’Esposito M. Brain network modularity predicts exercise-related executive function gains in older adults. Front Aging Neurosci 9: 426, 2017. DOI: 10.3389/fnagi.2017.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, Kraus VB, Lohmander LS, Abbott JH, Bhandari M, Blanco FJ, Espinosa R, Haugen IK, Lin J, Mandl LA, Moilanen E, Nakamura N, Snyder-Mackler L, Trojian T, Underwood M, McAlindon TE. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil 27: 1578–1589, 2019. DOI: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 82.Barakat R, Pelaez M, Cordero Y, Perales M, Lopez C, Coteron J, Mottola MF. Exercise during pregnancy protects against hypertension and macrosomia: Randomized clinical trial. Am J Obstet Gynecol 214: 649.e1–8- 10.1016/j.ajog.2015.11.039, 2016. [DOI] [PubMed] [Google Scholar]
- 83.Barauna VG, Magalhaes FC, Krieger JE, Oliveira EM. AT1 receptor participates in the cardiac hypertrophy induced by resistance training in rats. Am J Physiol Regul Integr Comp Physiol 295: R381–R387, 2008. DOI: 10.1152/ajpregu.00933.2007. [DOI] [PubMed] [Google Scholar]
- 84.Barcelos RP, Royes LFF, Gonzalez-Gallego J, Bresciani G. Oxidative stress and inflammation: Liver responses and adaptations to acute and regular exercise. Free Radic Res 51: 222–236, 2017. DOI: 10.1080/10715762.2017.1291942. [DOI] [PubMed] [Google Scholar]
- 85.Barja G Mitochondrial oxygen radical generation and leak: Sites of production in states 4 and 3, organ specificity, and relation to aging and longevity. J Bioenerg Biomembr 31: 347–366, 1999. DOI: 10.1023/a:1005427919188. [DOI] [PubMed] [Google Scholar]
- 86.Barker D, Saito M. Autonomic innervation of receptors and muscle fibres in cat skeletal muscle. Proc R Soc London, Ser B 212: 317–332, 1981. DOI: 10.1098/rspb.1981.0042. [DOI] [PubMed] [Google Scholar]
- 87.Barnett C, Costill DL, Vukovich MD, Cole KJ, Goodpaster BH, Trappe SW, Fink WJ. Effect of l-carnitine supplementation on muscle and blood carnitine content and lactate accumulation during high-intensity sprint cycling. Int J Sport Nutr 4: 280–288, 1994. DOI: 10.1123/ijsn.4.3.280. [DOI] [PubMed] [Google Scholar]
- 88.Barouki R, Audouze K, Coumoul X, Demenais F, Gauguier D. Integration of the human exposome with the human genome to advance medicine. Biochimie 152: 155–158, 2018. DOI: 10.1016/j.biochi.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 89.Barrès R, Yan J, Egan B, Treebak JT, Rasmussen M, Fritz T, Caidahl K, Krook A, O’Gorman DJ, Zierath JR. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab 15: 405–411, 2012. DOI: 10.1016/j.cmet.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 90.Bartels EM, Juhl CB, Christensen R, Hagen KB, Danneskiold-Samsøe B, Dagfinrud H, Lund H. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev 3: CD005523, 2016. DOI: 10.1002/14651858.CD005523.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bartholdy C, Juhl C, Christensen R, Lund H, Zhang W, Henriksen M. The role of muscle strengthening in exercise therapy for knee osteoarthritis: A systematic review and meta-regression analysis of randomized trials. Semin Arthritis Rheum 47: 9–21, 2017. DOI: 10.1016/j.semarthrit.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 92.Bartholdy C, Klokker L, Bandak E, Bliddal H, Henriksen M. A standardized “rescue” exercise program for symptomatic flare-up of knee osteoarthritis: Description and safety considerations. J Orthop Sports Phys Ther 46: 942–946, 2016. DOI: 10.2519/jospt.2016.6908. [DOI] [PubMed] [Google Scholar]
- 93.Bartness TJ, Liu Y, Shrestha YB, Ryu V. Neural innervation of white adipose tissue and the control of lipolysis. Front Neuroendocrinol 35: 473–493, 2014. DOI: 10.1016/j.yfrne.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bassett DR. Scientific contributions of a. V. Hill: Exercise physiology pioneer. J Appl Physiol (1985) 93: 1567–1582, 2002. DOI: 10.1152/japplphysiol.01246.2001. [DOI] [PubMed] [Google Scholar]
- 95.Bassett DR, Howley ET. Maximal oxygen uptake: “Classical” versus “contemporary” viewpoints. Med Sci Sports Exerc 29: 591–603, 1997. DOI: 10.1097/00005768-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 96.Bassett DR, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc 32: 70–84, 2000. DOI: 10.1097/00005768-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 97.Beaudart C, Reginster JY, Petermans J, Gillain S, Quabron A, Locquet M, Slomian J, Buckinx F, Bruyère O. Quality of life and physical components linked to sarcopenia: The sarcophage study. Exp Gerontol 69: 103–110, 2015. DOI: 10.1016/j.exger.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 98.Beaumont E, Gardiner PF. Endurance training alters the biophysical properties of hindlimb motoneurons in rats. Muscle Nerve 27: 228–236, 2003. DOI: 10.1002/mus.10308. [DOI] [PubMed] [Google Scholar]
- 99.Beavers KM, Hsu F-C, Isom S, Kritchevsky SB, Church T, Goodpaster B, Pahor M, Nicklas BJ. Long-term physical activity and inflammatory biomarkers in older adults. Med Sci Sports Exerc 42: 2189–2196, 2010. DOI: 10.1249/MSS.0b013e3181e3ac80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Beck BR, Daly RM, Singh MAF, Taaffe DR. Exercise and sports science australia (essa) position statement on exercise prescription for the prevention and management of osteoporosis. J Sci Med Sport 20: 438–445, 2017. DOI: 10.1016/j.jsams.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 101.Becker M, Weinberger T, Chandy A, Schmukler S. Depression during pregnancy and postpartum. Curr Psychiatry Rep 18: 32, 2016. DOI: 10.1007/s11920-016-0664-7. [DOI] [PubMed] [Google Scholar]
- 102.Behm DG, Young JD, Whitten JHD, Reid JC, Quigley PJ, Low J, Li Y, Lima CD, Hodgson DD, Chaouachi A, Prieske O, Granacher U. Effectiveness of traditional strength vs. Power training on muscle strength, power and speed with youth: A systematic review and meta-analysis. Front Physiol 8: 423, 2017. DOI: 10.3389/fphys.2017.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Belanger AY, McComas AJ. Extent of motor unit activation during effort. J Appl Physiol Respir Environ Exerc Physiol 51: 1131–1135, 1981. DOI: 10.1152/jappl.1981.51.5.1131. [DOI] [PubMed] [Google Scholar]
- 104.Bellamy LM, Joanisse S, Grubb A, Mitchell CJ, McKay BR, Phillips SM, Baker S, Parise G. The acute satellite cell response and skeletal muscle hypertrophy following resistance training. PLoS One 9: e109739, 2014. DOI: 10.1371/journal.pone.0109739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Belviranli M, Okudan N, Kabak B, Erdoǧan M, Karanfilci M. The relationship between brain-derived neurotrophic factor, irisin and cognitive skills of endurance athletes. Phys Sportsmed 44: 290–296, 2016. DOI: 10.1080/00913847.2016.1196125. [DOI] [PubMed] [Google Scholar]
- 106.Ben-Haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med 21: 103–113, 2004. DOI: 10.1046/j.1464-5491.2003.00985.x. [DOI] [PubMed] [Google Scholar]
- 107.Bennett BL. David bruce dill: A man of many seasons and environ-mentsCommitted to life, heat, and altitude. Wilderness Environ Med 17: e10–e13, 2006. DOI: 10.1580/1080-6032(2006)17[e10:dbdamo]2.0.co;2. [DOI] [Google Scholar]
- 108.Berchtold NC, Prieto GA, Phelan M, Gillen DL, Baldi P, Bennett DA, Buchman AS, Cotman CW. Hippocampal gene expression patterns linked to late-life physical activity oppose age and ad-related transcriptional decline. Neurobiol Aging 78: 142–154, 2019. DOI: 10.1016/j.neurobiolaging.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Berg J, Undebakke V, Rasch-Halvorsen Ø, Aakerøy L, Sandbakk Ø, Tjønna AE. Comparison of mitochondrial respiration in m. Triceps brachii and m. Vastus lateralis between elite cross-country skiers and physically active controls. Front Physiol 10: 365, 2019. DOI: 10.3389/fphys.2019.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bergman BC, Butterfield GE, Wolfel EE, Casazza GA, Lopaschuk GD, Brooks GA. Evaluation of exercise and training on muscle lipid metabolism. Am J Phys 276: E106–E117, 1999. DOI: 10.1152/ajpendo.1999.276.1.E106. [DOI] [PubMed] [Google Scholar]
- 111.Bergman BC, Perreault L, Strauss A, Bacon S, Kerege A, Harrison K, Brozinick JT, Hunerdosse DM, Playdon MC, Holmes W, Bui HH, Sanders P, Siddall P, Wei T, Thomas MK, Kuo MS, Eckel RH. Intramuscular triglyceride synthesis: Importance in muscle lipid partitioning in humans. Am J Phys Endocrinol Metab 314: E152–E164, 2018. DOI: 10.1152/ajpendo.00142.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bergstrom J Muscle electrolytes in man determined by neutron activation analysis on needle biopsy specimens [Online]. Scand J Clin Lab Invest Suppl 14, 1962. https://www.osti.gov/servlets/purl/4636890. [Google Scholar]
- 113.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: Experimental findings and therapeutic strategies. Pharmacol Ther 128: 191–227, 2010. DOI: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 114.Berry DC, Jiang Y, Graff JM. Mouse strains to study cold-inducible beige progenitors and beige adipocyte formation and function. Nat Commun 7: 10184, 2016. DOI: 10.1038/ncomms10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Berry JD, Pandey A, Gao A, Leonard D, Farzaneh-Far R, Ayers C, DeFina L, Willis B. Physical fitness and risk for heart failure and coronary artery disease. Circ Heart Fail 6: 627–634, 2013. DOI: 10.1161/CIRCHEARTFAILURE.112.000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Berryman JW. The tradition of the “six things non-natural”: Exercise and medicine from hippocrates through ante-bellum america [Online]. Exerc Sport Sci Rev 17: 515–559, 1989. http://www.ncbi.nlm.nih.gov/pubmed/2676556. [PubMed] [Google Scholar]
- 117.Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/erbb4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 138: 257–270, 2009. DOI: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 118.Best JR, Chiu BK, Hsu CL, Nagamatsu LS, Liu-Ambrose T. Long-term effects of resistance exercise training on cognition and brain volume in older women: Results from a randomized controlled trial. J Int Neuropsychol Soc 21: 745–756, 2015. DOI: 10.1017/S1355617715000673. [DOI] [PubMed] [Google Scholar]
- 119.Bieler T, Siersma V, Magnusson SP, Kjaer M, Christensen HE, Beyer N. In hip osteoarthritis, nordic walking is superior to strength training and home-based exercise for improving function. Scand J Med Sci Sports 27: 873–886, 2017. DOI: 10.1111/sms.12694. [DOI] [PubMed] [Google Scholar]
- 120.Biniaminov N, Bandt S, Roth A, Haertel S, Neumann R, Bub A. Irisin, physical activity and fitness status in healthy humans: No association under resting conditions in a cross-sectional study. PLoS One 13: e0189254, 2018. DOI: 10.1371/journal.pone.0189254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Björnheden T, Jakubowicz B, Levin M, Odén B, Edén S, Sjöström L, Lönn M. Computerized determination of adipocyte size. Obes Res 12: 95–105, 2004. DOI: 10.1038/oby.2004.13. [DOI] [PubMed] [Google Scholar]
- 122.Blesa J, Trigo-Damas I, Dileone M, Rey NL-GD, Hernandez LF, Obeso JA. Compensatory mechanisms in parkinson’s disease: Circuits adaptations and role in disease modification. Exp Neurol 298: 148–161, 2017. DOI: 10.1016/j.expneurol.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 123.Blom PC, Vøllestad NK, Costill DL. Factors affecting changes in muscle glycogen concentration during and after prolonged exercise [Online]. Acta Physiol Scand Suppl 556: 67–74, 1986. http://www.ncbi.nlm.nih.gov/pubmed/3471058. [PubMed] [Google Scholar]
- 124.Boecker H, Sprenger T, Spilker ME, Henriksen G, Koppenhoefer M, Wagner KJ, Valet M, Berthele A, Tolle TR. The runner’s high: Opioidergic mechanisms in the human brain. Cereb Cortex 18: 2523–2531, 2008. DOI: 10.1093/cercor/bhn013. [DOI] [PubMed] [Google Scholar]
- 125.Bohm S, Mersmann F, Arampatzis A. Human tendon adaptation in response to mechanical loading: A systematic review and meta-analysis of exercise intervention studies on healthy adults. Sports Med Open 1: 7, 2015. DOI: 10.1186/s40798-015-0009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bolam KA, van Uffelen JGZ, Taaffe DR. The effect of physical exercise on bone density in middle-aged and older men: A systematic review. Osteoporos Int 24: 2749–2762, 2013. DOI: 10.1007/s00198-013-2346-1. [DOI] [PubMed] [Google Scholar]
- 127.Bolotin HH, Sievänen H. Inaccuracies inherent in dual-energy x-ray absorptiometry in vivo bone mineral density can seriously mislead diagnostic/prognostic interpretations of patient-specific bone fragility. J Bone Miner Res Off J Am Soc Bone Miner Res 16: 799–805, 2001. DOI: 10.1359/jbmr.2001.16.5.799. [DOI] [PubMed] [Google Scholar]
- 128.Bonafiglia JT, Edgett BA, Baechler BL, Nelms MW, Simpson CA, Quadrilatero J, Gurd BJ. Acute upregulation of pgc-1α mRNA correlates with training-induced increases in sdh activity in human skeletal muscle. Appl Physiol Nutr Metab 42: 656–666, 2017. DOI: 10.1139/apnm-2016-0463. [DOI] [PubMed] [Google Scholar]
- 129.Booth FW, Roberts CK, Thyfault JP, Ruegsegger GN, Toedebusch RG. Role of inactivity in chronic diseases: Evolutionary insight and pathophysiological mechanisms. Physiol Rev 97: 1351–1402, 2017. DOI: 10.1152/physrev.00019.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bora P, Majumdar AS. Adipose tissue-derived stromal vascular fraction in regenerative medicine: A brief review on biology and translation. Stem Cell Res Ther 8: 145, 2017. DOI: 10.1186/s13287-017-0598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Borde R, Hortob’agyi T, Granacher U. Dose-response relationships of resistance training in healthy old adults: A systematic review and meta-analysis. Sports Med 45: 1693–1720, 2015. DOI: 10.1007/s40279-015-0385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bordicchia M, Liu D, Amri E-Z, Ailhaud G, Dessì-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 mapk to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest 122: 1022–1036, 2012. DOI: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Borina E, Pellegrino MA, D’Antona G, Bottinelli R. Myosin and actin content of human skeletal muscle fibers following 35 days bed rest. Scand J Med Sci Sports 20: 65–73, 2010. DOI: 10.1111/j.1600-0838.2009.01029.x. [DOI] [PubMed] [Google Scholar]
- 134.Bosma M, Minnaard R, Sparks LM, Schaart G, Losen M, de Baets MH, Duimel H, Kersten S, Bickel PE, Schrauwen P, Hesselink MKC. The lipid droplet coat protein perilipin 5 also localizes to muscle mitochondria. Histochem Cell Biol 137: 205–216, 2012. DOI: 10.1007/s00418-011-0888-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Boström P, Mann N, Wu J, Quintero PA, Plovie ER, Pan’akov’a D, Gupta RK, Xiao C, MacRae CA, Rosenzweig A, Spiegelman BM. C/ebpβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell 143: 1072–1083, 2010. DOI: 10.1016/j.cell.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bouchard C, Lesage R, Lortie G, Simoneau JA, Hamel P, Boulay MR, Pérusse L, Thériault G, Leblanc C. Aerobic performance in brothers, dizygotic and monozygotic twins [Online]. Med Sci Sports Exerc 18: 639–646, 1986. http://www.ncbi.nlm.nih.gov/pubmed/3784876. [PubMed] [Google Scholar]
- 137.Bouchard C, Rankinen T, Chagnon YC, Rice T, Pérusse L, Gagnon J, Borecki I, An P, Leon AS, Skinner JS, Wilmore JH, Province M, Rao DC. Genomic scan for maximal oxygen uptake and its response to training in the heritage family study. J Appl Physiol (1985) 88: 551–559, 2000. DOI: 10.1152/jappl.2000.88.2.551. [DOI] [PubMed] [Google Scholar]
- 138.Boule NG, Kenny GP, Haddad E, Wells GA, Sigal RJ. Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in type 2 diabetes mellitus. Diabetologia 46: 1071–1081, 2003. DOI: 10.1007/s00125-003-1160-2. [DOI] [PubMed] [Google Scholar]
- 139.Boushel R, Saltin B. Ex vivo measures of muscle mitochondrial capacity reveal quantitative limits of oxygen delivery by the circulation during exercise. Int J Biochem Cell Biol 45: 68–75, 2013. DOI: 10.1016/j.biocel.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 140.Boutcher YN, Boutcher SH. Exercise intensity and hypertension: What’s new? J Hum Hypertens 31: 157–164, 2017. DOI: 10.1038/jhh.2016.62. [DOI] [PubMed] [Google Scholar]
- 141.Bouzid MA, Filaire E, Matran R, Robin S, Fabre C. Lifelong voluntary exercise modulates age-related changes in oxidative stress. Int J Sports Med 39: 21–28, 2018. DOI: 10.1055/s-0043-119882. [DOI] [PubMed] [Google Scholar]
- 142.Bozkurt B, Aguilar D, Deswal A, Dunbar SB, Francis GS, Horwich T, Jessup M, Kosiborod M, Pritchett AM, Ramasubbu K, Rosendorff C, Yancy C. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: A scientific statement from the american heart association. Circulation 134: e535–e578, 2016. DOI: 10.1161/CIR.0000000000000450. [DOI] [PubMed] [Google Scholar]
- 143.Bray MS, Hagberg JM, Pérusse L, Rankinen T, Roth SM, Wolfarth B, Bouchard C. The human gene map for performance and health-related fitness phenotypes: The 2006–2007 update. Med Sci Sports Exerc 41: 35–73, 2009. DOI: 10.1249/mss.0b013e3181844179. [DOI] [PubMed] [Google Scholar]
- 144.Brellenthin AG, Crombie KM, Hillard CJ, Koltyn KF. Endocannabinoid and mood responses to exercise in adults with varying activity levels. Med Sci Sports Exerc 49: 1688–1696, 2017. DOI: 10.1249/MSS.0000000000001276. [DOI] [PubMed] [Google Scholar]
- 145.Brellenthin AG, Koltyn KF. Exercise as an adjunctive treatment for cannabis use disorder. Am J Drug Alcohol Abuse 42: 481–489, 2016. DOI: 10.1080/00952990.2016.1185434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bremer E, Cairney J. Adaptive behavior moderates health-related pathways in children with autism spectrum disorder. J Autism Dev Disord 50: 491–499, 2020. DOI: 10.1007/s10803-019-04277-6. [DOI] [PubMed] [Google Scholar]
- 147.Brennan AM, Benson M, Morningstar J, Herzig M, Robbins J, Gerszten RE, Ross R. Plasma metabolite profiles in response to chronic exercise. Med Sci Sports Exerc 50: 1480–1486, 2018. DOI: 10.1249/MSS.0000000000001594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brevet A, Pinto E, Peacock J, Stockdale FE. Myosin synthesis increased by electrical stimulation of skeletal muscle cell cultures. Science (New York, NY) 193: 1152–1154, 1976. DOI: 10.1126/science.959833. [DOI] [PubMed] [Google Scholar]
- 149.Briani RV, Ferreira AS, Pazzinatto MF, Pappas E, Silva DDO, de Azevedo FM. What interventions can improve quality of life or psychosocial factors of individuals with knee osteoarthritis? A systematic review with meta-analysis of primary outcomes from randomised controlled trials. Br J Sports Med 52: 1031–1038, 2018. DOI: 10.1136/bjsports-2017-098099. [DOI] [PubMed] [Google Scholar]
- 150.Bricca A, Juhl CB, Steultjens M, Wirth W, Roos EM. Impact of exercise on articular cartilage in people at risk of, or with established, knee osteoarthritis: A systematic review of randomised controlled trials. Br J Sports Med 53: 940–947, 2019. DOI: 10.1136/bjsports-2017-098661. [DOI] [PubMed] [Google Scholar]
- 151.Briken S, Rosenkranz SC, Keminer O, Patra S, Ketels G, Heesen C, Hellweg R, Pless O, Schulz K-H, Gold SM. Effects of exercise on irisin, bdnf and il-6 serum levels in patients with progressive multiple sclerosis. J Neuroimmunol 299: 53–58, 2016. DOI: 10.1016/j.jneuroim.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 152.Brinkman RJ, Hage JJ. Andreas vesalius’ 500th anniversary: The initiation of hand and forearm myology. J Hand Surg Eur Vol 40: 987–994, 2015. DOI: 10.1177/1753193415594090. [DOI] [PubMed] [Google Scholar]
- 153.Brioche T, Lemoine-Morel S. Oxidative stress, sarcopenia, antioxidant strategies and exercise: Molecular aspects. Curr Pharm Des 22: 2664–2678, 2016. DOI: 10.2174/1381612822666160219120531. [DOI] [PubMed] [Google Scholar]
- 154.Broadhouse KM, Singh MF, Suo C, Gates N, Wen W, Brodaty H, Jain N, Wilson GC, Meiklejohn J, Singh N, Baune BT, Baker M, Foroughi N, Wang Y, Kochan N, Ashton K, Brown M, Li Z, Mavros Y, Sachdev PS, Valenzuela MJ. Hippocampal plasticity underpins long-term cognitive gains from resistance exercise in mci. NeuroImage Clin 25: 102182, 2020. DOI: 10.1016/j.nicl.2020.102182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Brook MS, Wilkinson DJ, Smith K, Atherton PJ. It’s not just about protein turnover: The role of ribosomal biogenesis and satellite cells in the regulation of skeletal muscle hypertrophy. Eur J Sport Sci 19: 952–963, 2019. DOI: 10.1080/17461391.2019.1569726. [DOI] [PubMed] [Google Scholar]
- 156.Brook MS, Wilkinson DJ, Mitchell WK, Lund JN, Phillips BE, Szewczyk NJ, Greenhaff PL, Smith K, Atherton PJ. Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. J Physiol 594: 7399–7417, 2016. DOI: 10.1113/JP272857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Brook MS, Wilkinson DJ, Mitchell WK, Lund JN, Szewczyk NJ, Greenhaff PL, Smith K, Atherton PJ. Skeletal muscle hypertrophy adaptations predominate in the early stages of resistance exercise training, matching deuterium oxide-derived measures of muscle protein synthesis and mechanistic target of rapamycin complex 1 signaling. FASEB J 29: 4485–4496, 2015. DOI: 10.1096/fj.15-273755. [DOI] [PubMed] [Google Scholar]
- 158.Brookmeyer R, Abdalla N, Kawas CH, Corrada MM. Forecasting the prevalence of preclinical and clinical alzheimer’s disease in the united states. Alzheimers Dement 14: 121–129, 2018. DOI: 10.1016/j.jalz.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Brotto M, Bonewald L. Bone and muscle: Interactions beyond mechanical. Bone 80: 109–114, 2015. DOI: 10.1016/j.bone.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brouwers B, Hesselink MKC, Schrauwen P, Schrauwen-Hinderling VB. Effects of exercise training on intrahepatic lipid content in humans. Diabetologia 59: 2068–2079, 2016. DOI: 10.1007/s00125-016-4037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Brown BM, Sohrabi HR, Taddei K, Gardener SL, Rainey-Smith SR, Peiffer JJ, Xiong C, Fagan AM, Benzinger T, Buckles V, Erickson KI, Clarnette R, Shah T, Masters CL, Weiner M, Cairns N, Rossor M, Graff-Radford NR, Salloway S, Vöglein J, Laske C, Noble J, Schofield PR, Bateman RJ, Morris JC, Martins RN. Habitual exercise levels are associated with cerebral amyloid load in presymptomatic autosomal dominant alzheimer’s disease. Alzheimers Dement 13: 1197–1206, 2017. DOI: 10.1016/j.jalz.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Brown BM, Peiffer JJ, Taddei K, Lui JK, Laws SM, Gupta VB, Taddei T, Ward VK, Rodrigues MA, Burnham S, Rainey-Smith SR, Villemagne VL, Bush A, Ellis KA, Masters CL, Ames D, Macaulay SL, Szoeke C, Rowe CC, Martins RN. Physical activity and amyloid-β plasma and brain levels: Results from the australian imaging, biomarkers and lifestyle study of ageing. Mol Psychiatry 18: 875–881, 2013. DOI: 10.1038/mp.2012.107. [DOI] [PubMed] [Google Scholar]
- 163.Brown DM, Goljanek-Whysall K. MicroRNAs: Modulators of the underlying pathophysiology of sarcopenia? Ageing Res Rev 24: 263–273, 2015. DOI: 10.1016/j.arr.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 164.Brown J, Ceysens G, Boulvain M. Exercise for pregnant women with gestational diabetes for improving maternal and fetal outcomes. Cochrane Database Syst Rev 6: CD012202, 2017. DOI: 10.1002/14651858.CD012202.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Brown LA, Judge JL, Macpherson PC, Koch LG, Qi NR, Britton SL, Brooks SV. Denervation and senescence markers data from old rats with intrinsic differences in responsiveness to aerobic training. Data Brief 27: 104570, 2019. DOI: 10.1016/j.dib.2019.104570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Brown MD. Exercise and coronary vascular remodelling in the healthy heart. Exp Physiol 88: 645–658, 2003. DOI: 10.1113/eph8802618. [DOI] [PubMed] [Google Scholar]
- 167.Brummelte S, Galea LAM. Postpartum depression: Etiology, treatment and consequences for maternal care. Horm Behav 77: 153–166, 2016. DOI: 10.1016/j.yhbeh.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 168.Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of ad and cognitive decline in older adults. Neurology 78: 1323–1329, 2012. DOI: 10.1212/WNL.0b013e3182535d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Buchthal F, Schmalbruch H. Motor unit of mammalian muscle. Physiol Rev 60: 90–142, 1980. DOI: 10.1152/physrev.1980.60.1.90. [DOI] [PubMed] [Google Scholar]
- 170.Budde H, Machado S, Ribeiro P, Wegner M. The cortisol response to exercise in young adults. Front Behav Neurosci 9: 13, 2015. DOI: 10.3389/fnbeh.2015.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Buford TW. Sarcopenia: Relocating the forest among the trees. Toxicol Pathol 45: 957–960, 2017. DOI: 10.1177/0192623317723540. [DOI] [PubMed] [Google Scholar]
- 172.Buford TW. Hypertension and aging. Ageing Res Rev 26: 96–111, 2016. DOI: 10.1016/j.arr.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Buford TW, Anton SD, Clark DJ, Higgins TJ, Cooke MB. Optimizing the benefits of exercise on physical function in older adults. PM R 6: 528–543, 2014. DOI: 10.1016/j.pmrj.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Buford TW, Lott DJ, Marzetti E, Wohlgemuth SE, Vandenborne K, Pahor M, Leeuwenburgh C, Manini TM. Age-related differences in lower extremity tissue compartments and associations with physical function in older adults. Exp Gerontol 47: 38–44, 2012. DOI: 10.1016/j.exger.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bullard KM, Cowie CC, Lessem SE, Saydah SH, Menke A, Geiss LS, Orchard TJ, Rolka DB, Imperatore G. Prevalence of diagnosed diabetes in adults by diabetes type—united states, 2016. MMWR Morb Mortal Wkly Rep 67: 359–361, 2018. DOI: 10.15585/mmwr.mm6712a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Burr DB, Milgrom C, Fyhrie D, Forwood M, Nyska M, Finestone A, Hoshaw S, Saiag E, Simkin A. In vivo measurement of human tibial strains during vigorous activity. Bone 18: 405–410, 1996. DOI: 10.1016/8756-3282(96)00028-2. [DOI] [PubMed] [Google Scholar]
- 177.Burri L, Thoresen GH, Berge RK. The role of pparα activation in liver and muscle. PPAR Res 2010, 2010. DOI: 10.1155/2010/542359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Buss LA, Dachs GU. The role of exercise and hyperlipidaemia in breast cancer progression [Online]. Exerc Immunol Rev 24: 10–25, 2018. http://www.ncbi.nlm.nih.gov/pubmed/29461968. [PubMed] [Google Scholar]
- 179.Cadore EL, Rodr’ιguez-Mañas L, Sinclair A, Izquierdo M. Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: A systematic review. Rejuvenation Res 16: 105–114, 2013. DOI: 10.1089/rej.2012.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Caffrey BJ, Maltsev AV, Gonzalez-Freire M, Hartnell LM, Ferrucci L, Subramaniam S. Semi-automated 3D segmentation of human skeletal muscle using focused ion beam-scanning electron microscopic images. J Struct Biol 207: 1–11, 2019. DOI: 10.1016/j.jsb.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Calle MC, Fernandez ML. Effects of resistance training on the inflammatory response. Nutr Res Pract 4: 259–269, 2010. DOI: 10.4162/nrp.2010.4.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Camera DM, Edge J, Short MJ, Hawley JA, Coffey VG. Early time course of akt phosphorylation after endurance and resistance exercise. Med Sci Sports Exerc 42: 1843–1852, 2010. DOI: 10.1249/MSS.0b013e3181d964e4. [DOI] [PubMed] [Google Scholar]
- 183.Campbell JP, Turner JE. Debunking the myth of exercise-induced immune suppression: Redefining the impact of exercise on immunological health across the lifespan. Front Immunol 9: 648, 2018. DOI: 10.3389/fimmu.2018.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Campbell JE, Fediuc S, Hawke TJ, Riddell MC. Endurance exercise training increases adipose tissue glucocorticoid exposure: Adaptations that facilitate lipolysis. Metab Clin Exp 58: 651–660, 2009. DOI: 10.1016/j.metabol.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 185.Campbell KL, Makar KW, Kratz M, Foster-Schubert KE, McTiernan A, Ulrich CM. A pilot study of sampling subcutaneous adipose tissue to examine biomarkers of cancer risk. Cancer Prev Res (Phila) 2: 37–42, 2009. DOI: 10.1158/1940-6207.CAPR-08-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Campbell PT, Campbell KL, Wener MH, Wood BL, Potter JD, McTiernan A, Ulrich CM. A yearlong exercise intervention decreases crp among obese postmenopausal women. Med Sci Sports Exerc 41: 1533–1539, 2009. DOI: 10.1249/MSS.0b013e31819c7feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Candow DG, Burke DG. Effect of short-term equal-volume resistance training with different workout frequency on muscle mass and strength in untrained men and women. J Strength Cond Res 21: 204–207, 2007. DOI: 10.1519/00124278-200702000-00037. [DOI] [PubMed] [Google Scholar]
- 188.Cantley LC. The phosphoinositide 3-kinase pathway. Science (New York, NY) 296: 1655–1657, 2002. DOI: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 189.Cao M, Quan M, Zhuang J. Effect of high-intensity interval training versus moderate-intensity continuous training on cardiorespiratory fitness in children and adolescents: A meta-analysis. Int J Environ Res Public Health 16, 2019. DOI: 10.3390/ijerph16091533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cardozo CP, Graham ZA. Muscle-bone interactions: Movement in the field of mechano-humoral coupling of muscle and bone. Ann N Y Acad Sci 1402: 10–17, 2017. DOI: 10.1111/nyas.13411. [DOI] [PubMed] [Google Scholar]
- 191.Carlson DJ, Dieberg G, Hess NC, Millar PJ, Smart NA. Isometric exercise training for blood pressure management: A systematic review and meta-analysis. Mayo Clin Proc 89: 327–334, 2014. DOI: 10.1016/j.mayocp.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 192.Carnevale D, Perrotta M, Lembo G, Trimarco B. Pathophysiological links among hypertension and alzheimer’s disease. High Blood Press Cardiovasc Prev 23: 3–7, 2016. DOI: 10.1007/s40292-015-0108-1. [DOI] [PubMed] [Google Scholar]
- 193.Carpenter KJ. The life and times of w. O. Atwater (1844–1907). J Nutr 124: 1707S–1714S, 1994. DOI: 10.1093/jn/124.suppl_9.1707s. [DOI] [PubMed] [Google Scholar]
- 194.Carpio-Rivera E, Moncada-Jiménez J, Salazar-Rojas W, Solera-Herrera A. Acute effects of exercise on blood pressure: A meta-analytic investigation. Arq Bras Cardiol 106: 422–433, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Carraro U, Boncompagni S, Gobbo V, Rossini K, Zampieri S, Mosole S, Ravara B, Nori A, Stramare R, Ambrosio F, Piccione F, Masiero S, Vindigni V, Gargiulo P, Protasi F, Kern H, Pond A, Marcante A. Persistent muscle fiber regeneration in long term denervation. Past, present, future. Eur J Transl Myol 25: 4832, 2015. DOI: 10.4081/ejtm.2015.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Carrick-Ranson G, Hastings JL, Bhella PS, Fujimoto N, Shibata S, Palmer MD, Boyd K, Livingston S, Dijk E, Levine BD. The effect of lifelong exercise dose on cardiovascular function during exercise. J Appl Physiol (1985) 116: 736–745, 2014. DOI: 10.1152/japplphysiol.00342.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Carrick-Ranson G, Sloane NM, Howden EJ, Bhella PS, Sarma S, Shibata S, Fujimoto N, Hastings JL, Levine BD. The effect of lifelong endurance exercise on cardiovascular structure and exercise function in women. J Physiol 598: 2589–2605, 2020. DOI: 10.1113/JP278503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Carroll CC, Dickinson JM, Haus JM, Lee GA, Hollon CJ, Aagaard P, Magnusson SP, Trappe TA. Influence of aging on the in vivo properties of human patellar tendon. J Appl Physiol (1985) 105: 1907–1915, 2008. DOI: 10.1152/japplphysiol.00059.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Carroll MD, Fryar CD, Nguyen DT. Total and High-density Lipoprotein Cholesterol in Adults: United States, 2015–2016 [Online]. National Center for Health Statistics. https://www.cdc.gov/nchs/data/databriefs/db290.pdf. [Google Scholar]
- 200.Cartee GD, Hepple RT, Bamman MM, Zierath JR. Exercise promotes healthy aging of skeletal muscle. Cell Metab 23: 1034–1047, 2016. DOI: 10.1016/j.cmet.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Carter JR, Ray CA. Sympathetic neural adaptations to exercise training in humans. Auton Neurosci 188: 36–43, 2015. DOI: 10.1016/j.autneu.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 202.Carter SL, Rennie C, Tarnopolsky MA. Substrate utilization during endurance exercise in men and women after endurance training. Am J Phys Endocrinol Metab 280: E898–E907, 2001. DOI: 10.1152/ajpendo.2001.280.6.E898. [DOI] [PubMed] [Google Scholar]
- 203.Carter S, Solomon TPJ. In vitro experimental models for examining the skeletal muscle cell biology of exercise: The possibilities, challenges and future developments. Pflugers Arch—Eur J Physiol 471: 413–429, 2019. DOI: 10.1007/s00424-018-2210-4. [DOI] [PubMed] [Google Scholar]
- 204.Cartoni R, Léger B, Hock MB, Praz M, Crettenand A, Pich S, Ziltener J-L, Luthi F, Dériaz O, Zorzano A, Gobelet C, Kralli A, Russell AP. Mitofusins 1/2 and erralpha expression are increased in human skeletal muscle after physical exercise. J Physiol 567: 349–358, 2005. DOI: 10.1113/jphysiol.2005.092031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Case N, Rubin J. Beta-catenin–a supporting role in the skeleton. J Cell Biochem 110: 545–553, 2010. DOI: 10.1002/jcb.22574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Casilda-L’opez J, Valenza MC, Cabrera-Martos I, D’ιaz-Pelegrina A, Moreno-Ram’ιrez MP, Valenza-Demet G. Effects of a dance-based aquatic exercise program in obese postmenopausal women with knee osteoarthritis: A randomized controlled trial. Menopause 24: 768–773, 2017. DOI: 10.1097/GME.0000000000000841. [DOI] [PubMed] [Google Scholar]
- 207.Casonatto J, Goessler KF, Cornelissen VA, Cardoso JR, Polito MD. The blood pressure-lowering effect of a single bout of resistance exercise: A systematic review and meta-analysis of randomised controlled trials. Eur J Prev Cardiol 23: 1700–1714, 2016. DOI: 10.1177/2047487316664147. [DOI] [PubMed] [Google Scholar]
- 208.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research [Online]. Public Health Rep 100: 126–131, 1985. http://www.ncbi.nlm.nih.gov/pubmed/3920711. [PMC free article] [PubMed] [Google Scholar]
- 209.Cassilhas RC, Viana VAR, Grassmann V, Santos RT, Santos RF, Tufik S, Mello MT. The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc 39: 1401–1407, 2007. DOI: 10.1249/mss.0b013e318060111f. [DOI] [PubMed] [Google Scholar]
- 210.Cattadori G, Segurini C, Picozzi A, Padeletti L, Anzà C. Exercise and heart failure: An update. ESC Heart Fail 5: 222–232, 2018. DOI: 10.1002/ehf2.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cauley JA, Giangregorio L. Physical activity and skeletal health in adults. Lancet Diabetes Endocrinol 8: 150–162, 2020. DOI: 10.1016/s2213-8587(19)30351-1. [DOI] [PubMed] [Google Scholar]
- 212.CDC. National Diabetes Statistics Report 2020: Estimates of diabetes and its burden in the United States. [Online]. Centers for Disease Control; Prevention. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. [Google Scholar]
- 213.CDC. Adult Physical Inactivity Prevalence Maps by Race/Ethnicity [Online]. Centers for Disease Control and Prevention: 2020. https://www.cdc.gov/physicalactivity/data/inactivity-prevalence-maps/index.html [19 Mar. 2020]. [Google Scholar]
- 214.Chacón-Fernández P, Säuberli K, Colzani M, Moreau T, Ghevaert C, Barde Y-A. Brain-derived neurotrophic factor in megakaryocytes. J Biol Chem 291: 9872–9881, 2016. DOI: 10.1074/jbc.M116.720029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, Vanpatter M, Pontifex MB, Raine LB, Konkel A, Hillman CH, Cohen NJ, Kramer AF. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res 1358: 172–183, 2010. DOI: 10.1016/j.brainres.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chahal J, Lee R, Luo J. Loading dose of physical activity is related to muscle strength and bone density in middle-aged women. Bone 67: 41–45, 2014. DOI: 10.1016/j.bone.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 217.Chaillou T, Kirby TJ, McCarthy JJ. Ribosome biogenesis: Emerging evidence for a central role in the regulation of skeletal muscle mass. J Cell Physiol 229: 1584–1594, 2014. DOI: 10.1002/jcp.24604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chakrabarti S, Morton JS, Davidge ST. Mechanisms of estrogen effects on the endothelium: An overview. Can J Cardiol 30: 705–712, 2014. DOI: 10.1016/j.cjca.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 219.Chambers TL, Burnett TR, Raue U, Lee GA, Finch WH, Graham BM, Trappe TA, Trappe S. Skeletal muscle size, function, and adiposity with lifelong aerobic exercise. J Appl Physiol (1985) 128: 368–378, 2020. DOI: 10.1152/japplphysiol.00426.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chan MHS, Carey AL, Watt MJ, Febbraio MA. Cytokine gene expression in human skeletal muscle during concentric contraction: Evidence that il-8, like il-6, is influenced by glycogen availability. Am J Physiol Regul Integr Comp Physiol 287: R322–R327, 2004. DOI: 10.1152/ajpregu.00030.2004. [DOI] [PubMed] [Google Scholar]
- 221.Chang VY, Wang JJ. Pharmacogenetics of chemotherapy-induced cardiotoxicity. Curr Oncol Rep 20: 52, 2018. DOI: 10.1007/s11912-018-0696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Chang W-D, Chen S, Lee C-L, Lin H-Y, Lai P-T. The effects of tai chi chuan on improving mind-body health for knee osteoarthritis patients: A systematic review and meta-analysis. Evid Based Complement Alternat Med 2016: 1813979, 2016. DOI: 10.1155/2016/1813979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Chan-Palay V, Engel AG, Wu JY, Palay SL. Coexistence in human and primate neuromuscular junctions of enzymes synthesizing acetylcholine, catecholamine, taurine, and gamma-aminobutyric acid. Proc Natl Acad Sci U S A 79: 7027–7030, 1982. DOI: 10.1073/pnas.79.22.7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Chao W, Matsui T, Novikov MS, Tao J, Li L, Liu H, Ahn Y, Rosenzweig A. Strategic advantages of insulin-like growth factor-i expression for cardioprotection. J Gene Med 5: 277–286, 2003. DOI: 10.1002/jgm.347. [DOI] [PubMed] [Google Scholar]
- 225.Charge BPS, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev 84: 209–238, 2004. DOI: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 226.Charriere M, Duchenne DBG. Emporte piece histologique. Bull Acad Natl Med 30: 1050–1051, 1865. [Google Scholar]
- 227.Chaudhari P The impact of rheumatoid arthritis and biologics on employers and payers [Online]. Biotechnol Healthc 5: 37–44, 2008. http://www.ncbi.nlm.nih.gov/pubmed/22478712. [PMC free article] [PubMed] [Google Scholar]
- 228.Chazaud B, Brigitte M, Yacoub-Youssef H, Arnold L, Gherardi R, Sonnet C, Lafuste P, Chretien F. Dual and beneficial roles of macrophages during skeletal muscle regeneration. Exerc Sport Sci Rev 37: 18–22, 2009. DOI: 10.1097/JES.0b013e318190ebdb. [DOI] [PubMed] [Google Scholar]
- 229.Chekroud SR, Gueorguieva R, Zheutlin AB, Paulus M, Krumholz HM, Krystal JH, Chekroud AM. Association between physical exercise and mental health in 1·2 million individuals in the USA between 2011 and 2015: A cross-sectional study. Lancet Psychiatry 5: 739–746, 2018. DOI: 10.1016/s2215-0366(18)30227-x. [DOI] [PubMed] [Google Scholar]
- 230.Chen HC, Farese RV. Determination of adipocyte size by computer image analysis [Online]. J Lipid Res 43: 986–989, 2002. http://www.ncbi.nlm.nih.gov/pubmed/12032175. [PubMed] [Google Scholar]
- 231.Chen L-K, Liu L-K, Woo J, Assantachai P, Auyeung T-W, Bahyah KS, Chou M-Y, Chen L-Y, Hsu P-S, Krairit O, Lee JSW, Lee W-J, Lee Y, Liang C-K, Limpawattana P, Lin C-S, Peng L-N, Satake S, Suzuki T, Won CW, Wu C-H, Wu S-N, Zhang T, Zeng P, Akishita M, Arai H. Sarcopenia in asia: Consensus report of the asian working group for sarcopenia. J Am Med Dir Assoc 15: 95–101, 2014. DOI: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 232.Chen P, Mao L, Nassis GP, Harmer P, Ainsworth BE, Li F. Coronavirus disease (covid-19): The need to maintain regular physical activity while taking precautions. J Sport Health Sci 9: 103–104, 2020. DOI: 10.1016/j.jshs.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Cheng AJ, Place N, Westerblad H. Molecular basis for exercise-induced fatigue: The importance of strictly controlled cellular ca2+ handling. Cold Spring Harb Perspect Med 8, 2018. DOI: 10.1101/cshperspect.a029710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Cheng S, Peng L, Xu B, Chen W, Chen Y, Gu Y. Protective effects of hydrogen-rich water against cartilage damage in a rat model of osteoarthritis by inhibiting oxidative stress, matrix catabolism, and apoptosis. Med Sci Monit 26: e920211, 2020. DOI: 10.12659/MSM.920211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Cheng S-T. Cognitive reserve and the prevention of dementia: The role of physical and cognitive activities. Curr Psychiatry Rep 18: 85, 2016. DOI: 10.1007/s11920-016-0721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Cheung AM, Detsky AS. Osteoporosis and fractures: Missing the bridge? JAMA 299: 1468–1470, 2008. DOI: 10.1001/jama.299.12.1468. [DOI] [PubMed] [Google Scholar]
- 237.Chew C, Sengelaub DR. Neuroprotective effects of exercise on the morphology of somatic motoneurons following the death of neighboring motoneurons. Neurorehabil Neural Repair 33: 656–667, 2019. DOI: 10.1177/1545968319860485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chi MM, Hintz CS, Coyle EF, Martin WH, Ivy JL, Nemeth PM, Holloszy JO, Lowry OH. Effects of detraining on enzymes of energy metabolism in individual human muscle fibers. Am J Phys 244: C276–C287, 1983. DOI: 10.1152/ajpcell.1983.244.3.C276. [DOI] [PubMed] [Google Scholar]
- 239.Chicco AJ, Schneider CM, Hayward R. Exercise training attenuates acute doxorubicin-induced cardiac dysfunction. J Cardiovasc Pharmacol 47: 182–189, 2006. DOI: 10.1097/01.fjc.0000199682.43448.2d. [DOI] [PubMed] [Google Scholar]
- 240.Chinsomboon J, Ruas J, Gupta RK, Thom R, Shoag J, Rowe GC, Sawada N, Raghuram S, Arany Z. The transcriptional coactivator pgc-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proc Natl Acad Sci U S A 106: 21401–21406, 2009. DOI: 10.1073/pnas.0909131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Chondronikola M, Annamalai P, Chao T, Porter C, Saraf MK, Cesani F, Sidossis LS. A percutaneous needle biopsy technique for sampling the supraclavicular brown adipose tissue depot of humans. Int J Obes 39: 1561–1564, 2015. DOI: 10.1038/ijo.2015.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metab Clin Exp 92: 6–10, 2019. DOI: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 243.Christensen B, Dandanell S, Kjaer M, Langberg H. Effect of anti-inflammatory medication on the running-induced rise in patella tendon collagen synthesis in humans. J Appl Physiol (1985) 110: 137–141, 2011. DOI: 10.1152/japplphysiol.00942.2010. [DOI] [PubMed] [Google Scholar]
- 244.Christensen KL, Mulvany MJ. Location of resistance arteries. J Vasc Res 38: 1–12, 2001. DOI: 10.1159/000051024. [DOI] [PubMed] [Google Scholar]
- 245.Christodoulos AD, Douda HT, Tokmakidis SP. Cardiorespiratory fitness, metabolic risk, and inflammation in children. Int J Pediatr 2012: 270515, 2012. DOI: 10.1155/2012/270515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Chupel MU, Minuzzi LG, Furtado G, Santos ML, Hogervorst E, Filaire E, Teixeira AM. Exercise and taurine in inflammation, cognition, and peripheral markers of blood-brain barrier integrity in older women. Appl Physiol Nutr Metab 43: 733–741, 2018. DOI: 10.1139/apnm-2017-0775. [DOI] [PubMed] [Google Scholar]
- 247.Church DD, Hoffman JR, Mangine GT, Jajtner AR, Townsend JR, Beyer KS, Wang R, La Monica MB, Fukuda DH, Stout JR. Comparison of high-intensity vs. High-volume resistance training on the bdnf response to exercise. J Appl Physiol (1985) 121: 123–128, 2016. DOI: 10.1152/japplphysiol.00233.2016. [DOI] [PubMed] [Google Scholar]
- 248.Church TS, Blair SN. When will we treat physical activity as a legitimate medical therapy…even though it does not come in a pill? Br J Sports Med 43: 80–81, 2009. DOI: 10.1136/bjsm.2008.053850. [DOI] [PubMed] [Google Scholar]
- 249.Churchward-Venne TA, Tieland M, Verdijk LB, Leenders M, Dirks ML, de Groot LCPGM, van Loon LJC. There are no nonresponders to resistance-type exercise training in older men and women. J Am Med Dir Assoc 16: 400–411, 2015. DOI: 10.1016/j.jamda.2015.01.071. [DOI] [PubMed] [Google Scholar]
- 250.Ciloglu F, Peker I, Pehlivan A, Karacabey K, Ilhan N, Saygin O, Ozmerdivenli R. Exercise intensity and its effects on thyroid hormones [Online]. Neuro Endocrinol Lett 26: 830–834, 2005. http://www.ncbi.nlm.nih.gov/pubmed/16380698. [PubMed] [Google Scholar]
- 251.Cinkajzlova A, Mraz M, Lacinova Z, Klouckova J, Kavalkova P, Kratochvilova H, Trachta P, Krizova J, Haluzikova D, Skrha J, Papezova H, Haluzik M. Angiopoietin-like protein 3 and 4 in obesity, type 2 diabetes mellitus, and malnutrition: The effect of weight reduction and realimentation. Nutr Diabetes 8: 21, 2018. DOI: 10.1038/s41387-018-0032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Cisternas MG, Murphy L, Sacks JJ, Solomon DH, Pasta DJ, Helmick CG. Alternative methods for defining osteoarthritis and the impact on estimating prevalence in a us population-based survey. Arthritis Care Res 68: 574–580, 2016. DOI: 10.1002/acr.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Clarke K, Ricciardi S, Pearson T, Bharudin I, Davidsen PK, Bonomo M, Brina D, Scagliola A, Simpson DM, Beynon RJ, Khanim F, Ankers J, Sarzynski MA, Ghosh S, Pisconti A, Rozman J, de Angelis MH, Bunce C, Stewart C, Egginton S, Caddick M, Jackson M, Bouchard C, Biffo S, Falciani F. The role of eif6 in skeletal muscle homeostasis revealed by endurance training co-expression networks. Cell Rep 21: 1507–1520, 2017. DOI: 10.1016/j.celrep.2017.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Coates AM, Incognito AV, Seed JD, Doherty CJ, Millar PJ, Burr JF. Three weeks of overload training increases resting muscle sympathetic activity. Med Sci Sports Exerc 50: 928–937, 2018. DOI: 10.1249/MSS.0000000000001514. [DOI] [PubMed] [Google Scholar]
- 255.Coats AJS, Forman DE, Haykowsky M, Kitzman DW, McNeil A, Campbell TS, Arena R. Physical function and exercise training in older patients with heart failure. Nat Rev Cardiol 14: 550–559, 2017. DOI: 10.1038/nrcardio.2017.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Codella R, Adamo M, Maffi P, Piemonti L, Secchi A, Luzi L. Ultra-marathon 100Â km in an islet-transplanted runner. Acta Diabetol 54: 703–706, 2017. DOI: 10.1007/s00592-016-0938-x. [DOI] [PubMed] [Google Scholar]
- 257.Codella R, Terruzzi I, Luzi L. Why should people with type 1 diabetes exercise regularly? Acta Diabetol 54: 615–630, 2017. DOI: 10.1007/s00592-017-0978-x. [DOI] [PubMed] [Google Scholar]
- 258.Coen PM, Menshikova EV, Distefano G, Zheng D, Tanner CJ, Standley RA, Helbling NL, Dubis GS, Ritov VB, Xie H, Desimone ME, Smith SR, Stefanovic-Racic M, Toledo FGS, Houmard JA, Goodpaster BH. Exercise and weight loss improve muscle mitochondrial respiration, lipid partitioning, and insulin sensitivity after gastric bypass surgery. Diabetes 64: 3737–3750, 2015. DOI: 10.2337/db15-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Coffey VG, Hawley JA. Concurrent exercise training: Do opposites distract? J Physiol 595: 2883–2896, 2017. DOI: 10.1113/JP272270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Coffey VG, Jemiolo B, Edge J, Garnham AP, Trappe SW, Hawley JA. Effect of consecutive repeated sprint and resistance exercise bouts on acute adaptive responses in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 297: R1441–R1451, 2009. DOI: 10.1152/ajpregu.00351.2009. [DOI] [PubMed] [Google Scholar]
- 261.Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, Horton ES, Castorino K, Tate DF. Physical activity/exercise and diabetes: A position statement of the american diabetes association. Diabetes Care 39: 2065–2079, 2016. DOI: 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Colombo E, Bedogni F, Lorenzetti I, Landsberger N, Previtali SC, Farina C. Autocrine and immune cell-derived bdnf in human skeletal muscle: Implications for myogenesis and tissue regeneration. J Pathol 231: 190–198, 2013. DOI: 10.1002/path.4228. [DOI] [PubMed] [Google Scholar]
- 263.Conceição MS, Vechin FC, Lixandrão M, Damas F, Libardi CA, Tricoli V, Roschel H, Camera D, Ugrinowitsch C. Muscle fiber hypertrophy and myonuclei addition: A systematic review and meta-analysis. Med Sci Sports Exerc 50: 1385–1393, 2018. DOI: 10.1249/MSS.0000000000001593. [DOI] [PubMed] [Google Scholar]
- 264.Contrepois K, Wu S, Moneghetti KJ, Hornburg D, Ahadi S, Tsai M-S, Metwally AA, Wei E, Lee-McMullen B, Quijada JV, Chen S, Chris-tle JW, Ellenberger M, Balliu B, Taylor S, Durrant MG, Knowles DA, Choudhry H, Ashland M, Bahmani A, Enslen B, Amsallem M, Kobayashi Y, Avina M, Perelman D, Rose SMS-F, Zhou W, Ashley EA, Montgomery SB, Chaib H, Haddad F, Snyder MP. Molecular choreography of acute exercise. Cell 181: 1112–1130.e16, 2020. DOI: 10.1016/j.cell.2020.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Conway B, Rene A. Obesity as a disease: No lightweight matter. Obes Rev 5: 145–151, 2004. DOI: 10.1111/j.1467-789X.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 266.Cook B, Karr TM, Zunker C, Mitchell JE, Thompson R, Sherman R, Erickson A, Cao L, Crosby RD. The influence of exercise identity and social physique anxiety on exercise dependence. J Behav Addict 4: 195–199, 2015. DOI: 10.1556/2006.4.2015.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Cooper C, Moon HY, van Praag H. On the run for hippocampal plasticity. Cold Spring Harb Perspect Med 8, 2018. DOI: 10.1101/cshperspect.a029736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Cooper SB, Dring KJ, Nevill ME. High-intensity intermittent exercise: Effect on young people’s cardiometabolic health and cognition. Curr Sports Med Rep 15: 245–251, 2016. DOI: 10.1249/JSR.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 269.Cooper SJ. From claude bernard to walter cannon. Emergence of the concept of homeostasis. Appetite 51: 419–427, 2008. DOI: 10.1016/j.appet.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 270.Cori CF, Cori GT. Carbohydrate metabolism. Annu Rev Biochem 15: 193–218, 1946. DOI: 10.1146/annurev.bi.15.070146.001205. [DOI] [PubMed] [Google Scholar]
- 271.Cornelis J, Beckers P, Taeymans J, Vrints C, Vissers D. Comparing exercise training modalities in heart failure: A systematic review and meta-analysis. Int J Cardiol 221: 867–876, 2016. DOI: 10.1016/j.ijcard.2016.07.105. [DOI] [PubMed] [Google Scholar]
- 272.Cornelissen VA, Smart NA. Exercise training for blood pressure: A systematic review and meta-analysis. J Am Heart Assoc 2: e004473, 2013. DOI: 10.1161/JAHA.112.004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Correia JC, Ferreira DMS, Ruas JL. Intercellular: Local and systemic actions of skeletal muscle pgc-1s. Trends Endocrinol Metab 26: 305–314, 2015. DOI: 10.1016/j.tem.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 274.Cosquéric G, Sebag A, Ducolombier C, Thomas C, Piette F, Weill-Engerer S. Sarcopenia is predictive of nosocomial infection in care of the elderly. Br J Nutr 96: 895–901, 2006. DOI: 10.1017/bjn20061943. [DOI] [PubMed] [Google Scholar]
- 275.Costello JT, Bieuzen F, Bleakley CM. Where are all the female participants in sports and exercise medicine research? Eur J Sport Sci 14: 847–851, 2014. DOI: 10.1080/17461391.2014.911354. [DOI] [PubMed] [Google Scholar]
- 276.Costford SR, Bajpeyi S, Pasarica M, Albarado DC, Thomas SC, Xie H, Church TS, Jubrias SA, Conley KE, Smith SR. Skeletal muscle nampt is induced by exercise in humans. Am J Phys Endocrinol Metab 298: E117–E126, 2010. DOI: 10.1152/ajpendo.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Costill DL, Daniels J, Evans W, Fink W, Krahenbuhl G, Saltin B. Skeletal muscle enzymes and fiber composition in male and female track athletes. J Appl Physiol 40: 149–154, 1976. DOI: 10.1152/jappl.1976.40.2.149. [DOI] [PubMed] [Google Scholar]
- 278.Costill DL, Gollnick PD, Jansson ED, Saltin B, Stein EM. Glycogen depletion pattern in human muscle fibres during distance running. Acta Physiol Scand 89: 374–383, 1973. DOI: 10.1111/j.1748-1716.1973.tb05532.x. [DOI] [PubMed] [Google Scholar]
- 279.Costill DL, Pearson DR, Fink WJ. Impaired muscle glycogen storage after muscle biopsy. J Appl Physiol (1985) 64: 2245–2248, 1988. DOI: 10.1152/jappl.1988.64.5.2245. [DOI] [PubMed] [Google Scholar]
- 280.Costill DL, Sparks K, Gregor R, Turner C. Muscle glycogen utilization during exhaustive running. J Appl Physiol 31: 353–356, 1971. DOI: 10.1152/jappl.1971.31.3.353. [DOI] [PubMed] [Google Scholar]
- 281.Cotter JA, Yu A, Kreitenberg A, Haddad FH, Baker MJ, Fox JC, Adams GR. Suction-modified needle biopsy technique for the human soleus muscle. Aviat Space Environ Med 84: 1066–1073, 2013. DOI: 10.3357/asem.3632.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Coulombe JC, Senwar B, Ferguson VL. Spaceflight-induced bone tissue changes that affect bone quality and increase fracture risk. Curr Osteoporos Rep 18: 1–12, 2020. DOI: 10.1007/s11914-019-00540-y. [DOI] [PubMed] [Google Scholar]
- 283.Coyle EF, Coggan AR, Hemmert MK, Ivy JL. Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. J Appl Physiol (1985) 61: 165–172, 1986. DOI: 10.1152/jappl.1986.61.1.165. [DOI] [PubMed] [Google Scholar]
- 284.Creighton DL, Morgan AL, Boardley D, Brolinson PG. Weight-bearing exercise and markers of bone turnover in female athletes. J Appl Physiol (1985) 90: 565–570, 2001. DOI: 10.1152/jappl.2001.90.2.565. [DOI] [PubMed] [Google Scholar]
- 285.Croley AN, Zwetsloot KA, Westerkamp LM, Ryan NA, Pendergast AM, Hickner RC, Pofahl WE, Gavin TP. Lower capillarization, vegf protein, and vegf mRNA response to acute exercise in the vastus lateralis muscle of aged vs. Young women. J Appl Physiol (1985) 99: 1872–1879, 2005. DOI: 10.1152/japplphysiol.00498.2005. [DOI] [PubMed] [Google Scholar]
- 286.Crowson CS, Liao KP, Davis JM, Solomon DH, Matteson EL, Knutson KL, Hlatky MA, Gabriel SE. Rheumatoid arthritis and cardiovascular disease. Am Heart J 166: 622–628.e1, 2013. DOI: 10.1016/j.ahj.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel J-P, Rolland Y, Schneider SM, Topinkov’a E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis: Report of the european working group on sarcopenia in older people. Age Ageing 39: 412–423, 2010. DOI: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Cruz-Jentoft AJ, Landi F, Schneider SM, Z’uñiga C, Arai H, Boirie Y, Chen L-K, Fielding RA, Martin FC, Michel J-P, Sieber C, Stout JR, Studenski SA, Vellas B, Woo J, Zamboni M, Cederholm T. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the international sarcopenia initiative (ewgsop and iwgs). Age Ageing 43: 748–759, 2014. DOI: 10.1093/ageing/afu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet 393: 2636–2646, 2019. DOI: 10.1016/s0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 290.Curran M, Drayson MT, Andrews RC, Zoppi C, Barlow JP, Solomon TPJ, Narendran P. The benefits of physical exercise for the health of the pancreatic β-cell: A review of the evidence. Exp Physiol 105: 579–589, 2020. DOI: 10.1113/EP088220. [DOI] [PubMed] [Google Scholar]
- 291.Dacome L Balancing acts: Picturing perspiration in the long eighteenth century. Stud Hist Phil Biol Biomed Sci 43: 379–391, 2012. DOI: 10.1016/j.shpsc.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 292.Daemen S, van Polanen N, Hesselink MKC. The effect of diet and exercise on lipid droplet dynamics in human muscle tissue. J Exp Biol 221, 2018. DOI: 10.1242/jeb.167015. [DOI] [PubMed] [Google Scholar]
- 293.Dahl R, Larsen S, Dohlmann TL, Qvortrup K, Helge JW, Dela F, Prats C. Three-dimensional reconstruction of the human skeletal muscle mitochondrial network as a tool to assess mitochondrial content and structural organization. Acta Physiol (Oxf) 213: 145–155, 2015. DOI: 10.1111/apha.12289. [DOI] [PubMed] [Google Scholar]
- 294.Dalen JE, Alpert JS, Goldberg RJ, Weinstein RS. The epidemic of the 20(th) century: Coronary heart disease. Am J Med 127: 807–812, 2014. DOI: 10.1016/j.amjmed.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 295.Damas F, Libardi CA, Ugrinowitsch C, Vechin FC, Lixandrão ME, Snijders T, Nederveen JP, Bacurau AV, Brum P, Tricoli V, Roschel H, Parise G, Phillips SM. Early- and later-phases satellite cell responses and myonuclear content with resistance training in young men. PLoS One 13: e0191039, 2018. DOI: 10.1371/journal.pone.0191039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Damas F, Phillips SM, Libardi CA, Vechin FC, Lixandrão ME, Jannig PR, Costa LAR, Bacurau AV, Snijders T, Parise G, Tricoli V, Roschel H, Ugrinowitsch C. Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. J Physiol 594: 5209–5222, 2016. DOI: 10.1113/JP272472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Damas F, Phillips S, Vechin FC, Ugrinowitsch C. A review of resistance training-induced changes in skeletal muscle protein synthesis and their contribution to hypertrophy. Sports Med 45: 801–807, 2015. DOI: 10.1007/s40279-015-0320-0. [DOI] [PubMed] [Google Scholar]
- 298.Dartel SAAR-v, Repping-Wuts H, Flendrie M, Bleijenberg G, Metsios GS, van den Hout WB, van den Ende CHM, Neuberger G, Reid A, van Riel PLCM, Fransen J. Effect of aerobic exercise training on fatigue in rheumatoid arthritis: A meta-analysis. Arthritis Care Res 67: 1054–1062, 2015. DOI: 10.1002/acr.22561. [DOI] [PubMed] [Google Scholar]
- 299.D’Ascenzi F, Anselmi F, Fiorentini C, Mannucci R, Bonifazi M, Mondillo S. The benefits of exercise in cancer patients and the criteria for exercise prescription in cardio-oncology. Eur J Prev Cardiol 28 (7): 725–735. [DOI] [PubMed] [Google Scholar]
- 300.Dashty M A quick look at biochemistry: Carbohydrate metabolism. Clin Biochem 46: 1339–1352, 2013. DOI: 10.1016/j.clinbiochem.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 301.Dasso NA. How is exercise different from physical activity? A concept analysis. Nurs Forum 54: 45–52, 2019. DOI: 10.1111/nuf.12296. [DOI] [PubMed] [Google Scholar]
- 302.Daum SM, Knittle J, Roseman K, Rom WN, Holstein EC. A simple technique for fat biopsy of pbb-exposed individuals. Environ Health Perspect 23: 183–185, 1978. DOI: 10.1289/ehp.7823183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Davenport MH, McCurdy AP, Mottola MF, Skow RJ, Meah VL, Poitras VJ, Garcia AJ, Gray CE, Barrowman N, Riske L, Sobierajski F, James M, Nagpal T, Marchand A-A, Nuspl M, Slater LG, Barakat R, Adamo KB, Davies GA, Ruchat S-M. Impact of prenatal exercise on both prenatal and postnatal anxiety and depressive symptoms: A systematic review and meta-analysis. Br J Sports Med 52: 1376–1385, 2018. DOI: 10.1136/bjsports-2018-099697. [DOI] [PubMed] [Google Scholar]
- 304.Davenport MH, Meah VL, Ruchat S-M, Davies GA, Skow RJ, Barrowman N, Adamo KB, Poitras VJ, Gray CE, Garcia AJ, Sobierajski F, Riske L, James M, Kathol AJ, Nuspl M, Marchand A-A, Nagpal TS, Slater LG, Weeks A, Barakat R, Mottola MF. Impact of prenatal exercise on neonatal and childhood outcomes: A systematic review and meta-analysis. Br J Sports Med 52: 1386–1396, 2018. DOI: 10.1136/bjsports-2018-099836. [DOI] [PubMed] [Google Scholar]
- 305.Davenport MH, Ruchat S-M, Poitras VJ, Garcia AJ, Gray CE, Barrowman N, Skow RJ, Meah VL, Riske L, Sobierajski F, James M, Kathol AJ, Nuspl M, Marchand A-A, Nagpal TS, Slater LG, Weeks A, Adamo KB, Davies GA, Barakat R, Mottola MF. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: A systematic review and meta-analysis. Br J Sports Med 52: 1367–1375, 2018. DOI: 10.1136/bjsports-2018-099355. [DOI] [PubMed] [Google Scholar]
- 306.Davenport MH, Ruchat S-M, Sobierajski F, Poitras VJ, Gray CE, Yoo C, Skow RJ, Garcia AJ, Barrowman N, Meah VL, Nagpal TS, Riske L, James M, Nuspl M, Weeks A, Marchand A-A, Slater LG, Adamo KB, Davies GA, Barakat R, Mottola MF. Impact of prenatal exercise on maternal harms, labour and delivery outcomes: A systematic review and meta-analysis. Br J Sports Med 53: 99–107, 2019. DOI: 10.1136/bjsports-2018-099821. [DOI] [PubMed] [Google Scholar]
- 307.Davies EJ, Moxham T, Rees K, Singh S, Coats AJS, Ebrahim S, Lough F, Taylor RS. Exercise training for systolic heart failure: Cochrane systematic review and meta-analysis. Eur J Heart Fail 12: 706–715, 2010. DOI: 10.1093/eurjhf/hfq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun 107: 1198–1205, 1982. DOI: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 309.Davies RW, Bass JJ, Carson BP, Norton C, Kozior M, Amigo-Benavent M, Wilkinson DJ, Brook MS, Atherton PJ, Smith K, Jakeman PM. Differential stimulation of post-exercise myofibrillar protein synthesis in humans following isonitrogenous, isocaloric pre-exercise feeding. Nutrients 11, 2019. DOI: 10.3390/nu11071657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Dâmaso AR, da Silveira Campos RM, Caranti DA, de Piano A, Fisberg M, Foschini D, de Lima Sanches P, Tock L, Lederman HM, Tufik S, de Mello MT. Aerobic plus resistance training was more effective in improving the visceral adiposity, metabolic profile and inflammatory markers than aerobic training in obese adolescents. J Sports Sci 32: 1435–1445, 2014. DOI: 10.1080/02640414.2014.900692. [DOI] [PubMed] [Google Scholar]
- 311.Debette S, Markus HS. The clinical importance of white matter hyper-intensities on brain magnetic resonance imaging: Systematic review and meta-analysis. BMJ 341: c3666, 2010. DOI: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.DeBoer MD, Filipp SL, Gurka MJ. Use of a metabolic syndrome severity z score to track risk during treatment of prediabetes: An analysis of the diabetes prevention program. Diabetes Care 41: 2421–2430, 2018. DOI: 10.2337/dc18-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation 113: 2097–2104, 2006. DOI: 10.1161/CIRCULATION-AHA.105.595231. [DOI] [PubMed] [Google Scholar]
- 314.De Carvalho FG, Sparks LM. Targeting white adipose tissue with exercise or bariatric surgery as therapeutic strategies in obesity. Biology 8, 2019. DOI: 10.3390/biology8010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.De Carvalho FG, Justice JN, de Freitas EC, Kershaw EE, Sparks LM. Adipose tissue quality in aging: How structural and functional aspects of adipose tissue impact skeletal muscle quality. Nutrients 11, 2019. DOI: 10.3390/nu11112553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.de Carvalho M, Barkhaus PE, Nandedkar SD, Swash M. Motor unit number estimation (mune): Where are we now? Clin Neurophysiol 129: 1507–1516, 2018. DOI: 10.1016/j.clinph.2018.04.748. [DOI] [PubMed] [Google Scholar]
- 317.Delavar H, Nogueira L, Wagner PD, Hogan MC, Metzger D, Breen EC. Skeletal myofiber vegf is essential for the exercise training response in adult mice. Am J Physiol Regul Integr Comp Physiol 306: R586–R595, 2014. DOI: 10.1152/ajpregu.00522.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Delezie J, Handschin C. Endocrine crosstalk between skeletal muscle and the brain. Front Neurol 9: 698, 2018. DOI: 10.3389/fneur.2018.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Dell’Orso S, Juan AH, Ko K-D, Naz F, Perovanovic J, Gutierrez-Cruz G, Feng X, Sartorelli V. Single cell analysis of adult mouse skeletal muscle stem cells in homeostatic and regenerative conditions. Development 146, 2019. DOI: 10.1242/dev.174177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, Goodpaster BH. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr 90: 1579–1585, 2009. DOI: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.De Marco M, Clough PJ, Dyer CE, Vince RV, Waby JS, Midgley AW, Venneri A. Apolipoprotein e îμ4 allele modulates the immediate impact of acute exercise on prefrontal function. Behav Genet 45: 106–116, 2015. DOI: 10.1007/s10519-014-9675-5. [DOI] [PubMed] [Google Scholar]
- 322.Den Heijer AE, Groen Y, Tucha L, Fuermaier ABM, Koerts J, Lange KW, Thome J, Tucha O. Sweat it out? The effects of physical exercise on cognition and behavior in children and adults with adhd: A systematic literature review. J Neural Transm Suppl 124: 3–26, 2017. DOI: 10.1007/s00702-016-1593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.de Oliveira PA, Blasczyk JC, Junior GS, Lagoa KF, Soares M, de Oliveira RJ, Filho PJBG, Carregaro RL, Martins WR. Effects of elastic resistance exercise on muscle strength and functional performance in healthy adults: A systematic review and meta-analysis. J Phys Act Health 14: 317–327, 2017. DOI: 10.1123/jpah.2016-0415. [DOI] [PubMed] [Google Scholar]
- 324.de Paleville DT, Topp RV, Swank AM. Effects of aerobic training prior to and during chemotherapy in a breast cancer patient: A case study. J Strength Cond Res 21: 635–637, 2007. DOI: 10.1519/R-19735.1. [DOI] [PubMed] [Google Scholar]
- 325.de Rooij M, van der Leeden M, Cheung J, van der Esch M, Häkkinen A, Haverkamp D, Roorda LD, Twisk J, Vollebregt J, Lems WF, Dekker J. Efficacy of tailored exercise therapy on physical functioning in patients with knee osteoarthritis and comorbidity: A randomized controlled trial. Arthritis Care Res 69: 807–816, 2017. DOI: 10.1002/acr.23013. [DOI] [PubMed] [Google Scholar]
- 326.de Simone G, Devereux RB, Daniels SR, Meyer RA. Gender differences in left ventricular growth. Hypertension 26: 979–983, 1995. DOI: 10.1161/01.hyp.26.6.979. [DOI] [PubMed] [Google Scholar]
- 327.Dethlefsen MM, Halling JF, Møller HD, Plomgaard P, Regenberg B, Ringholm S, Pilegaard H. Regulation of apoptosis and autophagy in mouse and human skeletal muscle with aging and lifelong exercise training. Exp Gerontol 111: 141–153, 2018. DOI: 10.1016/j.exger.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 328.Devries MC, Hamadeh MJ, Phillips SM, Tarnopolsky MA. Menstrual cycle phase and sex influence muscle glycogen utilization and glucose turnover during moderate-intensity endurance exercise. Am J Physiol Regul Integr Comp Physiol 291: R1120–R1128, 2006. DOI: 10.1152/ajpregu.00700.2005. [DOI] [PubMed] [Google Scholar]
- 329.Devrim-Lanpir A, Bilgic P, Kocahan T, Deliceoǧlu G, Rosemann T, Knechtle B. Total dietary antioxidant intake including polyphenol content: Is it capable to fight against increased oxidants within the body of ultra-endurance athletes? Nutrients 12, 2020. DOI: 10.3390/nu12061877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Deyhle MR, Gier AM, Evans KC, Eggett DL, Nelson WB, Parcell AC, Hyldahl RD. Skeletal muscle inflammation following repeated bouts of lengthening contractions in humans. Front Physiol 6: 424, 2015. DOI: 10.3389/fphys.2015.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Diabetes Prevention Program Research Group. The diabetes prevention program (dpp): Description of lifestyle intervention. Diabetes Care 25: 2165–2171, 2002. DOI: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Dickinson JM, D’Lugos AC, Naymik MA, Siniard AL, Wolfe AJ, Curtis DR, Huentelman MJ, Carroll CC. Transcriptome response of human skeletal muscle to divergent exercise stimuli. J Appl Physiol (1985) 124: 1529–1540, 2018. DOI: 10.1152/japplphysiol.00014.2018. [DOI] [PubMed] [Google Scholar]
- 333.Dideriksen K, Sindby AKR, Krogsgaard M, Schjerling P, Holm L, Langberg H. Effect of acute exercise on patella tendon protein synthesis and gene expression. Springerplus 2: 109, 2013. DOI: 10.1186/2193-1801-2-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Dinenno FA, Tanaka H, Monahan KD, Clevenger CM, Eskurza I, DeSouza CA, Seals DR. Regular endurance exercise induces expansive arterial remodelling in the trained limbs of healthy men. J Physiol 534: 287–295, 2001. DOI: 10.1111/j.1469-7793.2001.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Ding D, Lawson KD, Kolbe-Alexander TL, Finkelstein EA, Katzmarzyk PT, van Mechelen W, Pratt M. The economic burden of physical inactivity: A global analysis of major non-communicable diseases. Lancet 388: 1311–1324, 2016. DOI: 10.1016/s0140-6736(16)30383-x. [DOI] [PubMed] [Google Scholar]
- 336.Dionyssiotis Y Analyzing the problem of falls among older people. Int J Gen Med 5: 805–813, 2012. DOI: 10.2147/IJGM.S32651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Distefano G, Goodpaster BH. Effects of exercise and aging on skeletal muscle. Cold Spring Harb Perspect Med 8, 2018. DOI: 10.1101/csh-perspect.a029785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Dittenhafer-Reed KE, Richards AL, Fan J, Smallegan MJ, Siahpirani AF, Kemmerer ZA, Prolla TA, Roy S, Coon JJ, Denu JM. SIRT3 mediates multi-tissue coupling for metabolic fuel switching. Cell Metab 21: 637–646, 2015. DOI: 10.1016/j.cmet.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Doll JA, Hellkamp A, Ho PM, Kontos MC, Whooley MA, Peterson ED, Wang TY. Participation in cardiac rehabilitation programs among older patients after acute myocardial infarction. JAMA Intern Med 175: 1700–1702, 2015. DOI: 10.1001/jamainternmed.2015.3819. [DOI] [PubMed] [Google Scholar]
- 340.Doppler K, Mittelbronn M, Bornemann A. Myogenesis in human denervated muscle biopsies. Muscle Nerve 37: 79–83, 2008. DOI: 10.1002/mus.20902. [DOI] [PubMed] [Google Scholar]
- 341.Dores H, Freitas A, Malhotra A, Mendes M, Sharma S. The hearts of competitive athletes: An up-to-date overview of exercise-induced cardiac adaptations. Rev Port Cardiol 34: 51–64, 2015. DOI: 10.1016/j.repc.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 342.Dovio A, Roveda E, Sciolla C, Montaruli A, Raffaelli A, Saba A, Calogiuri G, De Francia S, Borrione P, Salvadori P, Carandente F, Angeli A. Intense physical exercise increases systemic 11beta-hydroxysteroid dehydrogenase type 1 activity in healthy adult subjects. Eur J Appl Physiol 108: 681–687, 2010. DOI: 10.1007/s00421-009-1265-5. [DOI] [PubMed] [Google Scholar]
- 343.Dozio E, Passeri E, Cardani R, Benedini S, Aresta C, Valaperta R, Romanelli MC, Meola G, Sansone V, Corbetta S. Circulating irisin is reduced in male patients with type 1 and type 2 myotonic dystrophies. Front Endocrinol 8: 320, 2017. DOI: 10.3389/fendo.2017.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Drake JC, Wilson RJ, Yan Z. Molecular mechanisms for mitochondrial adaptation to exercise training in skeletal muscle. FASEB J 30: 13–22, 2016. DOI: 10.1096/fj.15-276337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Dreyer HC, Blanco CE, Sattler FR, Schroeder ET, Wiswell RA. Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve 33: 242–253, 2006. DOI: 10.1002/mus.20461. [DOI] [PubMed] [Google Scholar]
- 346.Du M-C, Ouyang Y-Q, Nie X-F, Huang Y, Redding SR. Effects of physical exercise during pregnancy on maternal and infant outcomes in overweight and obese pregnant women: A meta-analysis. Birth 46: 211–221, 2019. DOI: 10.1111/birt.12396. [DOI] [PubMed] [Google Scholar]
- 347.Dube JJ, Allison KF, Rousson V, Goodpaster BH, Amati F. Exercise dose and insulin sensitivity: Relevance for diabetes prevention. Med Sci Sports Exerc 44: 793–799, 2012. DOI: 10.1249/MSS.0b013e31823f679f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Dufour S, Lebon V, Shulman GI, Petersen KF. Regulation of net hepatic glycogenolysis and gluconeogenesis by epinephrine in humans. Am J Phys Endocrinol Metab 297: E231–E235, 2009. DOI: 10.1152/ajpendo.00222.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Dumont NA, Bentzinger CF, Sincennes M-C, Rudnicki MA. Satellite cells and skeletal muscle regeneration. Compr Physiol 5: 1027–1059, 2015. DOI: 10.1002/cphy.c140068. [DOI] [PubMed] [Google Scholar]
- 350.Duncan GE. Exercise, fitness, and cardiovascular disease risk in type 2 diabetes and the metabolic syndrome. Curr Diab Rep 6: 29–35, 2006. DOI: 10.1007/s11892-006-0048-1. [DOI] [PubMed] [Google Scholar]
- 351.Durstine JL, Haskell WL. Effects of exercise training on plasma lipids and lipoproteins [Online]. Exerc Sport Sci Rev 22: 477–521, 1994. http://www.ncbi.nlm.nih.gov/pubmed/7925552. [PubMed] [Google Scholar]
- 352.Eaton M, Granata C, Barry J, Safdar A, Bishop D, Little JP. Impact of a single bout of high-intensity interval exercise and short-term interval training on interleukin-6, fndc5, and metrnl mRNA expression in human skeletal muscle. J Sport Health Sci 7: 191–196, 2018. DOI: 10.1016/j.jshs.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Eddolls WTB, McNarry MA, Stratton G, Winn CON, Mackintosh KA. High-intensity interval training interventions in children and adolescents: A systematic review. Sports Med 47: 2363–2374, 2017. DOI: 10.1007/s40279-017-0753-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Edens NK, Leibel RL, Hirsch J. Mechanism of free fatty acid reesterification in human adipocytes in vitro [Online]. J Lipid Res 31: 1423–1431, 1990. http://www.ncbi.nlm.nih.gov/pubmed/2280183. [PubMed] [Google Scholar]
- 355.Edman KA. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol 291: 143–159, 1979. DOI: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Edvardsen E, Hem E, Anderssen SA. End criteria for reaching maximal oxygen uptake must be strict and adjusted to sex and age: A cross-sectional study. PLoS One 9: e85276, 2014. DOI: 10.1371/journal.pone.0085276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Egan B, Dowling P, O’Connor PL, Henry M, Meleady P, Zierath JR, O’Gorman DJ. 2-d dige analysis of the mitochondrial proteome from human skeletal muscle reveals time course-dependent remodelling in response to 14 consecutive days of endurance exercise training. Proteomics 11: 1413–1428, 2011. DOI: 10.1002/pmic.201000597. [DOI] [PubMed] [Google Scholar]
- 358.Egan B, O’Connor PL, Zierath JR, O’Gorman DJ. Time course analysis reveals gene-specific transcript and protein kinetics of adaptation to short-term aerobic exercise training in human skeletal muscle. PLoS One 8: e74098, 2013. DOI: 10.1371/journal.pone.0074098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17: 162–184, 2013. DOI: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 360.Ehsani AA, Hagberg JM, Hickson RC. Rapid changes in left ventricular dimensions and mass in response to physical conditioning and deconditioning. Am J Cardiol 42: 52–56, 1978. DOI: 10.1016/0002-9149(78)90984-0. [DOI] [PubMed] [Google Scholar]
- 361.Eijsvogels TMH, George KP, Thompson PD. Cardiovascular benefits and risks across the physical activity continuum. Curr Opin Cardiol 31: 566–571, 2016. DOI: 10.1097/HCO.0000000000000321. [DOI] [PubMed] [Google Scholar]
- 362.Eijsvogels TMH, Molossi S, Lee D-C, Emery MS, Thompson PD. Exercise at the extremes: The amount of exercise to reduce cardiovascular events. J Am Coll Cardiol 67: 316–329, 2016. DOI: 10.1016/j.jacc.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 363.Eilers W, Jaspers RT, de Haan A, Ferrié C, Valdivieso P, Flück M. CaMKII content affects contractile, but not mitochondrial, characteristics in regenerating skeletal muscle. BMC Physiol 14: 7, 2014. DOI: 10.1186/s12899-014-0007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Eknoyan G Santorio sanctorius (1561–1636)—founding father of metabolic balance studies. Am J Nephrol 19: 226–233, 1999. DOI: 10.1159/000013455. [DOI] [PubMed] [Google Scholar]
- 365.Eleftheriou KI, Rawal JS, Kehoe A, James LE, Payne JR, Skip-worth JR, Puthucheary ZA, Drenos F, Pennell DJ, Loosemore M, World M, Humphries SE, Haddad FS, Montgomery HE. The lichfield bone study: The skeletal response to exercise in healthy young men. J Appl Physiol (1985) 112: 615–626, 2012. DOI: 10.1152/japplphysiol.00788.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Elgass K, Pakay J, Ryan MT, Palmer CS. Recent advances into the understanding of mitochondrial fission. Biochim Biophys Acta 1833: 150–161, 2013. DOI: 10.1016/j.bbamcr.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 367.Ellis T, Rochester L. Mobilizing parkinson’s disease: The future of exercise. J Parkinsons Dis 8: S95–S100, 2018. DOI: 10.3233/JPD-181489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Engelke K, Kemmler W, Lauber D, Beeskow C, Pintag R, Kalender WA. Exercise maintains bone density at spine and hip efops: A 3-year longitudinal study in early postmenopausal women. Osteoporos Int 17: 133–142, 2006. DOI: 10.1007/s00198-005-1938-9. [DOI] [PubMed] [Google Scholar]
- 369.Englund DA, Murach KA, Dungan CM, Figueiredo VC, Vechetti IJ, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Depletion of resident muscle stem cells negatively impacts running volume, physical function, and muscle fiber hypertrophy in response to lifelong physical activity. Am J Phys Cell Phys 318: C1178–C1188, 2020. DOI: 10.1152/ajpcell.00090.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Erdő F, Denes L, de Lange E. Age-associated physiological and pathological changes at the blood-brain barrier: A review. J Cereb Blood Flow Metab 37: 4–24, 2017. DOI: 10.1177/0271678X16679420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Erickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, Newman AB, Gach HM, Thompson PM, Ho AJ, Kuller LH. Physical activity predicts gray matter volume in late adulthood: The cardiovascular health study. Neurology 75: 1415–1422, 2010. DOI: 10.1212/WNL.0b013e3181f88359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, W’ojcicki TR, McAuley E, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus 19: 1030–1039, 2009. DOI: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A 108: 3017–3022, 2011. DOI: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med 4: 1313–1317, 1998. DOI: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 375.Erlich AT, Brownlee DM, Beyfuss K, Hood DA. Exercise induces tfeb expression and activity in skeletal muscle in a pgc-1α-dependent manner. Am J Phys Cell Phys 314: C62–C72, 2018. DOI: 10.1152/ajp-cell.00162.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Esbjörnsson M, Jansson E, Sundberg CJ, Sylvén C, Eiken O, Nygren A, Kaijser L. Muscle fibre types and enzyme activities after training with local leg ischaemia in man. Acta Physiol Scand 148: 233–241, 1993. DOI: 10.1111/j.1748-1716.1993.tb09554.x. [DOI] [PubMed] [Google Scholar]
- 377.Essén-Gustavsson B, Tesch PA. Glycogen and triglyceride utilization in relation to muscle metabolic characteristics in men performing heavy-resistance exercise. Eur J Appl Physiol Occup Physiol 61: 5–10, 1990. DOI: 10.1007/BF00236686. [DOI] [PubMed] [Google Scholar]
- 378.Evans WJ, Phinney SD, Young VR. Suction applied to a muscle biopsy maximizes sample size [Online]. Med Sci Sports Exerc 14: 101–102, 1982. http://www.ncbi.nlm.nih.gov/pubmed/7070249. [PubMed] [Google Scholar]
- 379.Ezzati A, Katz MJ, Zammit AR, Lipton ML, Zimmerman ME, Sliwinski MJ, Lipton RB. Differential association of left and right hippocampal volumes with verbal episodic and spatial memory in older adults. Neuropsychologia 93: 380–385, 2016. DOI: 10.1016/j.neuropsychologia.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Fabbri E, Zoli M, Gonzalez-Freire M, Salive ME, Studenski SA, Ferrucci L. Aging and multimorbidity: New tasks, priorities, and frontiers for integrated gerontological and clinical research. J Am Med Dir Assoc 16: 640–647, 2015. DOI: 10.1016/j.jamda.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Faigenbaum AD. Youth resistance training: The good, the bad, and the ugly-the year that was 2017. Pediatr Exerc Sci 30: 19–24, 2018. DOI: 10.1123/pes.2017-0290. [DOI] [PubMed] [Google Scholar]
- 382.Falk B, Haddad F, Klentrou P, Ward W, Kish K, Mezil Y, Radom-Aizik S. Differential sclerostin and parathyroid hormone response to exercise in boys and men. Osteoporos Int 27: 1245–1249, 2016. DOI: 10.1007/s00198-015-3310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Fan X, Hussien R, Brooks GA. H2O2-induced mitochondrial fragmentation in c2c12 myocytes. Free Radic Biol Med 49: 1646–1654, 2010. DOI: 10.1016/j.freeradbiomed.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Faraco G, Iadecola C. Hypertension: A harbinger of stroke and dementia. Hypertension 62: 810–817, 2013. DOI: 10.1161/HYPERTENSIONAHA.113.01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Fealy CE, Mulya A, Lai N, Kirwan JP. Exercise training decreases activation of the mitochondrial fission protein dynamin-related protein-1 in insulin-resistant human skeletal muscle. J Appl Physiol (1985) 117: 239–245, 2014. DOI: 10.1152/japplphysiol.01064.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Fedewa MV, Gist NH, Evans EM, Dishman RK. Exercise and insulin resistance in youth: A meta-analysis. Pediatrics 133: e163–e174, 2014. DOI: 10.1542/peds.2013-2718. [DOI] [PubMed] [Google Scholar]
- 387.Fell J, Williams D. The effect of aging on skeletal-muscle recovery from exercise: Possible implications for aging athletes. J Aging Phys Act 16: 97–115, 2008. DOI: 10.1123/japa.16.1.97. [DOI] [PubMed] [Google Scholar]
- 388.Fell RD, McLane JA, Winder WW, Holloszy JO. Preferential resynthesis of muscle glycogen in fasting rats after exhausting exercise. Am J Phys 238: R328–R332, 1980. DOI: 10.1152/ajpregu.1980.238.5.R328. [DOI] [PubMed] [Google Scholar]
- 389.Fenesi B, Fang H, Kovacevic A, Oremus M, Raina P, Heisz JJ. Physical exercise moderates the relationship of apolipoprotein e (apoe) genotype and dementia risk: A population-based study. J Alzheimers Dis 56: 297–303, 2017. DOI: 10.3233/JAD-160424. [DOI] [PubMed] [Google Scholar]
- 390.Feng X, Naz F, Juan AH, Dell’Orso S, Sartorelli V. Identification of skeletal muscle satellite cells by immunofluorescence with pax7 and laminin antibodies. J Vis Exp, 2018. DOI: 10.3791/57212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Fenton SAM, van Zanten JJCSV, Kitas GD, Duda JL, Rouse PC, Yu C-A, Metsios GS. Sedentary behaviour is associated with increased long-term cardiovascular risk in patients with rheumatoid arthritis independently of moderate-to-vigorous physical activity. BMC Musculoskelet Disord 18: 131, 2017. DOI: 10.1186/s12891-017-1473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Ferdinand KC, Nasser SA. Management of essential hypertension. Cardiol Clin 35: 231–246, 2017. DOI: 10.1016/j.ccl.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 393.Ferreira JP, Ghiarone T, J’unior CRC, Furtado GE, Carvalho HM, Rodrigues AM, Toscano CVA. Effects of physical exercise on the stereotyped behavior of children with autism spectrum disorders. Medicina (Kaunas) 55, 2019. DOI: 10.3390/medicina55100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Fiest KM, Roberts JI, Maxwell CJ, Hogan DB, Smith EE, Frolkis A, Cohen A, Kirk A, Pearson D, Pringsheim T, Venegas-Torres A, Jetté N. The prevalence and incidence of dementia due to alzheimer’s disease: A systematic review and meta-analysis. Can J Neurol Sci 43 (Suppl 1): S51–S82, 2016. DOI: 10.1017/cjn.2016.36. [DOI] [PubMed] [Google Scholar]
- 395.Figueiredo VC, McCarthy JJ. Regulation of ribosome biogenesis in skeletal muscle hypertrophy. Physiology (Bethesda) 34: 30–42, 2019. DOI: 10.1152/physiol.00034.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Figueiredo VC, Caldow MK, Massie V, Markworth JF, Cameron-Smith D, Blazevich AJ. Ribosome biogenesis adaptation in resistance training-induced human skeletal muscle hypertrophy. Am J Phys Endocrinol Metab 309: E72–E83, 2015. DOI: 10.1152/ajpendo.00050.2015. [DOI] [PubMed] [Google Scholar]
- 397.Figueiredo VC, Roberts LA, Markworth JF, Barnett MPG, Coombes JS, Raastad T, Peake JM, Cameron-Smith D. Impact of resistance exercise on ribosome biogenesis is acutely regulated by post-exercise recovery strategies. Physiol Rep 4, 2016. DOI: 10.14814/phy2.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Fiorenza M, Gunnarsson TP, Hostrup M, Iaia FM, Schena F, Pilegaard H, Bangsbo J. Metabolic stress-dependent regulation of the mitochondrial biogenic molecular response to high-intensity exercise in human skeletal muscle. J Physiol 596: 2823–2840, 2018. DOI: 10.1113/JP275972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Firth J, Stubbs B, Vancampfort D, Schuch F, Lagopoulos J, Rosenbaum S, Ward PB. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. NeuroImage 166: 230–238, 2018. DOI: 10.1016/j.neuroimage.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 400.Fischer CP, Hiscock NJ, Basu S, Vessby B, Kallner A, Sjöberg L-B, Febbraio MA, Pedersen BK. Vitamin e isoform-specific inhibition of the exercise-induced heat shock protein 72 expression in humans. J Appl Physiol (1985) 100: 1679–1687, 2006. DOI: 10.1152/japplphysiol.00421.2005. [DOI] [PubMed] [Google Scholar]
- 401.Fischer CP, Hiscock NJ, Penkowa M, Basu S, Vessby B, Kallner A, Sjöberg L-B, Pedersen BK. Supplementation with vitamins c and e inhibits the release of interleukin-6 from contracting human skeletal muscle. J Physiol 558: 633–645, 2004. DOI: 10.1113/jphysiol.2004.066779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Fischer CP, Plomgaard P, Hansen AK, Pilegaard H, Saltin B, Pedersen BK. Endurance training reduces the contraction-induced interleukin-6 mRNA expression in human skeletal muscle. Am J Phys Endocrinol Metab 287: E1189–E1194, 2004. DOI: 10.1152/ajpendo.00206.2004. [DOI] [PubMed] [Google Scholar]
- 403.Fisher-Wellman K, Bloomer RJ. Acute exercise and oxidative stress: A 30 year history. Dynamic Med 8: 1, 2009. DOI: 10.1186/1476-5918-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev 74: 49–94, 1994. DOI: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- 405.Fitts RH, Costill DL, Gardetto PR. Effect of swim exercise training on human muscle fiber function. J Appl Physiol (1985) 66: 465–475, 1989. DOI: 10.1152/jappl.1989.66.1.465. [DOI] [PubMed] [Google Scholar]
- 406.Fiuza-Luces C, Garatachea N, Berger NA, Lucia A. Exercise is the real polypill. Physiology (Bethesda) 28: 330–358, 2013. DOI: 10.1152/physiol.00019.2013. [DOI] [PubMed] [Google Scholar]
- 407.Florencio-Silva R, da Silva Sasso GR, Sasso-Cerri E, Simões MJ, Cerri PS. Biology of bone tissue: Structure, function, and factors that influence bone cells. Biomed Res Int 2015: 421746, 2015. DOI: 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Foley TR, Armstrong EJ, Waldo SW. Contemporary evaluation and management of lower extremity peripheral artery disease. Heart 102: 1436–1441, 2016. DOI: 10.1136/heartjnl-2015-309076. [DOI] [PubMed] [Google Scholar]
- 409.Folk GE. The harvard fatigue laboratory: Contributions to world war ii. Adv Physiol Educ 34: 119–127, 2010. DOI: 10.1152/advan.00041.2010. [DOI] [PubMed] [Google Scholar]
- 410.Formosa LE, Ryan MT. Mitochondrial fusion: Reaching the end of mitofusin’s tether. J Cell Biol 215: 597–598, 2016. DOI: 10.1083/jcb.201611048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Forti LN, Van Roie E, Njemini R, Coudyzer W, Beyer I, Delecluse C, Bautmans I. High versus low load resistance training: The effect of 24 weeks detraining on serum brain derived-neurotrophic factor (bdnf) in older adults. J Frailty Aging 6: 53–58, 2017. DOI: 10.14283/jfa.2017.2. [DOI] [PubMed] [Google Scholar]
- 412.Forti LN, Van Roie E, Njemini R, Coudyzer W, Beyer I, Delecluse C, Bautmans I. Dose-and gender-specific effects of resistance training on circulating levels of brain derived neurotrophic factor (bdnf) in community-dwelling older adults. Exp Gerontol 70: 144–149, 2015. DOI: 10.1016/j.exger.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 413.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu C-Y, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D’Agostino RB, O’Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the framingham heart study. Circulation 116: 39–48, 2007. DOI: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 414.Fox J, Rioux BV, Goulet EDB, Johanssen NM, Swift DL, Bouchard DR, Loewen H, Sénéchal M. Effect of an acute exercise bout on immediate post-exercise irisin concentration in adults: A meta-analysis. Scand J Med Sci Sports 28: 16–28, 2018. DOI: 10.1111/sms.12904. [DOI] [PubMed] [Google Scholar]
- 415.Fox KAA, Metra M, Morais J, Atar D. The myth of ‘stable’ coronary artery disease. Nat Rev Cardiol 17: 9–21, 2020. DOI: 10.1038/s41569-019-0233-y. [DOI] [PubMed] [Google Scholar]
- 416.Fragala MS, Cadore EL, Dorgo S, Izquierdo M, Kraemer WJ, Peterson MD, Ryan ED. Resistance training for older adults: Position statement from the national strength and conditioning association. J Strength Cond Res 33: 2019–2052, 2019. DOI: 10.1519/JSC.0000000000003230. [DOI] [PubMed] [Google Scholar]
- 417.Franklin BA, Thompson PD, Al-Zaiti SS, Albert CM, Hivert M-F, Levine BD, Lobelo F, Madan K, Sharrief AZ, Eijsvogels TMH. Exercise-related acute cardiovascular events and potential deleterious adaptations following long-term exercise training: Placing the risks into perspective-an update: A scientific statement from the american heart association. Circulation 141: e705–e736, 2020. DOI: 10.1161/CIR.0000000000000749. [DOI] [PubMed] [Google Scholar]
- 418.Fransen M, McConnell S. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev: CD004376, 2008. DOI: 10.1002/14651858.CD004376.pub2. [DOI] [PubMed] [Google Scholar]
- 419.Fraser BJ, Schmidt MD, Huynh QL, Dwyer T, Venn AJ, Magnussen CG. Tracking of muscular strength and power from youth to young adulthood: Longitudinal findings from the childhood determinants of adult health study. J Sci Med Sport 20: 927–931, 2017. DOI: 10.1016/j.jsams.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 420.Frasier CR, Moore RL, Brown DA. Exercise-induced cardiac preconditioning: How exercise protects your achy-breaky heart. J Appl Physiol (1985) 111: 905–915, 2011. DOI: 10.1152/japplphysiol.00004.2011. [DOI] [PubMed] [Google Scholar]
- 421.Freiberger E, Häberle L, Spirduso WW, Zijlstra GAR. Long-term effects of three multicomponent exercise interventions on physical performance and fall-related psychological outcomes in community-dwelling older adults: A randomized controlled trial. J Am Geriatr Soc 60: 437–446, 2012. DOI: 10.1111/j.1532-5415.2011.03859.x. [DOI] [PubMed] [Google Scholar]
- 422.Freidenreich DJ, Volek JS. Immune responses to resistance exercise [Online]. Exerc Immunol Rev 18: 8–41, 2012. http://www.ncbi.nlm.nih.gov/pubmed/22876721. [PubMed] [Google Scholar]
- 423.Freund BJ, Wade CE, Claybaugh JR. Effects of exercise on atrial natriuretic factor. Release mechanisms and implications for fluid homeostasis. Sports Med 6: 364–377, 1988. DOI: 10.2165/00007256-198806060-00003. [DOI] [PubMed] [Google Scholar]
- 424.Freyssenet D, Berthon P, Denis C. Mitochondrial biogenesis in skeletal muscle in response to endurance exercises. Arch Physiol Biochem 104: 129–141, 1996. DOI: 10.1076/apab.104.2.129.12878. [DOI] [PubMed] [Google Scholar]
- 425.Friedrich MJ. Depression is the leading cause of disability around the world. JAMA 317: 1517, 2017. DOI: 10.1001/jama.2017.3826. [DOI] [PubMed] [Google Scholar]
- 426.Fritzen AM, Thøgersen FB, Thybo K, Vissing CR, Krag TO, Ruiz-Ruiz C, Risom L, Wibrand F, Høeg LD, Kiens B, Duno M, Vissing J, Jeppesen TD. Adaptations in mitochondrial enzymatic activity occurs independent of genomic dosage in response to aerobic exercise training and deconditioning in human skeletal muscle. Cell 8, 2019. DOI: 10.3390/cells8030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Frizziero A, Salamanna F, Bella ED, Vittadini F, Gasparre G, Aldini NN, Masiero S, Fini M. The role of detraining in tendon mechanobiology. Front Aging Neurosci 8: 43, 2016. DOI: 10.3389/fnagi.2016.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Frontera WR, Ochala J. Skeletal muscle: A brief review of structure and function. Calcif Tissue Int 96: 183–195, 2015. DOI: 10.1007/s00223-014-9915-y. [DOI] [PubMed] [Google Scholar]
- 429.Fry CS, Lee JD, Jackson JR, Kirby TJ, Stasko SA, Liu H, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J 28: 1654–1665, 2014. DOI: 10.1096/fj.13-239426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Fukai T, Siegfried MR, Ushio-Fukai M, Cheng Y, Kojda G, Harrison DG. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest 105: 1631–1639, 2000. DOI: 10.1172/JCI9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Fulghum K, Hill BG. Metabolic mechanisms of exercise-induced cardiac remodeling. Front Cardiovasc Med 5: 127, 2018. DOI: 10.3389/fcvm.2018.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Fuller SE, Huang T-Y, Simon J, Batdorf HM, Essajee NM, Scott MC, Waskom CM, Brown JM, Burke SJ, Collier JJ, Noland RC. Low-intensity exercise induces acute shifts in liver and skeletal muscle substrate metabolism but not chronic adaptations in tissue oxidative capacity. J Appl Physiol (1985) 127: 143–156, 2019. DOI: 10.1152/japplphysiol.00820.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM. Chronic inflammation in the etiology of disease across the life span. Nat Med 25: 1822–1832, 2019. DOI: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Furst DE, Emery P. Rheumatoid arthritis pathophysiology: Update on emerging cytokine and cytokine-associated cell targets. Rheumatology (Oxford) 53: 1560–1569, 2014. DOI: 10.1093/rheumatology/ket414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Furuichi Y, Manabe Y, Takagi M, Aoki M, Fujii NL. Evidence for acute contraction-induced myokine secretion by c2c12 myotubes. PLoS One 13: e0206146, 2018. DOI: 10.1371/journal.pone.0206146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Fuss J, Steinle J, Bindila L, Auer MK, Kirchherr H, Lutz B, Gass P. A runner’s high depends on cannabinoid receptors in mice. Proc Natl Acad Sci U S A 112: 13105–13108, 2015. DOI: 10.1073/pnas.1514996112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Galpin AJ, Raue U, Jemiolo B, Trappe TA, Harber MP, Minchev K, Trappe S. Human skeletal muscle fiber type specific protein content. Anal Biochem 425: 175–182, 2012. DOI: 10.1016/j.ab.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Galvão DA, Newton RU. Review of exercise intervention studies in cancer patients. J Clin Oncol 23: 899–909, 2005. DOI: 10.1200/JCO.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 439.Galvão DA, Nosaka K, Taaffe DR, Spry N, Kristjanson LJ, McGuigan MR, Suzuki K, Yamaya K, Newton RU. Resistance training and reduction of treatment side effects in prostate cancer patients. Med Sci Sports Exerc 38: 2045–2052, 2006. DOI: 10.1249/01.mss.0000233803.48691.8b. [DOI] [PubMed] [Google Scholar]
- 440.Gan Z, Fu T, Kelly DP, Vega RB. Skeletal muscle mitochondrial remodeling in exercise and diseases. Cell Res 28: 969–980, 2018. DOI: 10.1038/s41422-018-0078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Ganter U, Arcone R, Toniatti C, Morrone G, Ciliberto G. Dual control of c-reactive protein gene expression by interleukin-1 and interleukin-6 [Online]. EMBO J 8: 3773–3779, 1989. http://www.ncbi.nlm.nih.gov/pubmed/2555173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Garc’ιa-Hermoso A, Ramirez-Vélez R, de Asteasu MLS, Mart’ιnez-Velilla N, Zambom-Ferraresi F, Valenzuela PL, Lucia A, Izquierdo M. Safety and effectiveness of long-term exercise interventions in older adults: A systematic review and meta-analysis of randomized controlled trials. Sports Med 50: 1095–1106, 2020. DOI: 10.1007/s40279-020-01259-y. [DOI] [PubMed] [Google Scholar]
- 443.Garg MK, Kharb S. Dual energy x-ray absorptiometry: Pitfalls in measurement and interpretation of bone mineral density. Indian J Endocrinol Metab 17: 203–210, 2013. DOI: 10.4103/2230-8210.109659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Garner RT, Solfest JS, Nie Y, Kuang S, Stout J, Gavin TP. Multi-vesicular body and exosome pathway responses to acute exercise. Exp Physiol 105: 511–521, 2020. DOI: 10.1113/EP088017. [DOI] [PubMed] [Google Scholar]
- 445.Gäbler M, Prieske O, Hortob’agyi T, Granacher U. The effects of concurrent strength and endurance training on physical fitness and athletic performance in youth: A systematic review and meta-analysis. Front Physiol 9: 1057, 2018. DOI: 10.3389/fphys.2018.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Gea JG, Pasto M, Carmona MA, Orozco-Levi M, Palomeque J, Broquetas J. Metabolic characteristics of the deltoid muscle in patients with chronic obstructive pulmonary disease. Eur Respir J 17: 939–945, 2001. DOI: 10.1183/09031936.01.17509390. [DOI] [PubMed] [Google Scholar]
- 447.Geard D, Reaburn PRJ, Rebar AL, Dionigi RA. Masters athletes: Exemplars of successful aging? J Aging Phys Act 25: 490–500, 2017. DOI: 10.1123/japa.2016-0050. [DOI] [PubMed] [Google Scholar]
- 448.Geard D, Rebar AL, Reaburn P, Dionigi RA. Testing a model of successful aging in a cohort of masters swimmers. J Aging Phys Act 26: 183–193, 2018. DOI: 10.1123/japa.2016-0357. [DOI] [PubMed] [Google Scholar]
- 449.Geertsen SS, Thomas R, Larsen MN, Dahn IM, Andersen JN, Krause-Jensen M, Korup V, Nielsen CM, Wienecke J, Ritz C, Krustrup P, Lundbye-Jensen J. Motor skills and exercise capacity are associated with objective measures of cognitive functions and academic performance in preadolescent children. PLoS One 11: e0161960, 2016. DOI: 10.1371/journal.pone.0161960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, Tracy RP. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol 153: 242–250, 2001. DOI: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- 451.Gehlert S, Bungartz G, Willkomm L, Korkmaz Y, Pfannkuche K, Schiffer T, Bloch W, Suhr F. Intense resistance exercise induces early and transient increases in ryanodine receptor 1 phosphorylation in human skeletal muscle. PLoS One 7: e49326, 2012. DOI: 10.1371/journal.pone.0049326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Geisler M, Eichelkraut L, Miltner WHR, Weiss T. Expectation of exercise in trained athletes results in a reduction of central processing to nociceptive stimulation. Behav Brain Res 356: 314–321, 2019. DOI: 10.1016/j.bbr.2018.08.036. [DOI] [PubMed] [Google Scholar]
- 453.Gejl KD, Ørtenblad N, Andersson E, Plomgaard P, Holmberg H-C, Nielsen J. Local depletion of glycogen with supramaximal exercise in human skeletal muscle fibres. J Physiol 595: 2809–2821, 2017. DOI: 10.1113/JP273109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.George EK, Reddy PH. Can healthy diets, regular exercise, and better lifestyle delay the progression of dementia in elderly individuals? J Alzheimers Dis 72: S37–S58, 2019. DOI: 10.3233/JAD-190232. [DOI] [PubMed] [Google Scholar]
- 455.Georgoulis AD, Kiapidou I-S, Velogianni L, Stergiou N, Boland A. Herodicus, the father of sports medicine. Knee Surg Sports Traumatol Arthrosc 15: 315–318, 2007. DOI: 10.1007/s00167-006-0149-z. [DOI] [PubMed] [Google Scholar]
- 456.Gerich JE, Meyer C, Woerle HJ, Stumvoll M. Renal gluconeogenesis: Its importance in human glucose homeostasis. Diabetes Care 24: 382–391, 2001. DOI: 10.2337/diacare.24.2.382. [DOI] [PubMed] [Google Scholar]
- 457.Ghafourifar P, Cadenas E. Mitochondrial nitric oxide synthase. Trends Pharmacol Sci 26: 190–195, 2005. DOI: 10.1016/j.tips.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 458.Gibson ASC, Schabort EJ, Noakes TD. Reduced neuromuscular activity and force generation during prolonged cycling. Am J Physiol Regul Integr Comp Physiol 281: R187–R196, 2001. DOI: 10.1152/ajpregu.2001.281.1.R187. [DOI] [PubMed] [Google Scholar]
- 459.Gielen S, Laughlin MH, O’Conner C, Duncker DJ. Exercise training in patients with heart disease: Review of beneficial effects and clinical recommendations. Prog Cardiovasc Dis 57: 347–355, 2014. DOI: 10.1016/j.pcad.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 460.Ginter E, Simko V. Recent data on obesity research: B-aminoisobutyric acid. Bratisl Lek Listy 115: 492–493, 2014. DOI: 10.4149/bll_2014_095. [DOI] [PubMed] [Google Scholar]
- 461.Giordani L, He GJ, Negroni E, Sakai H, Law JYC, Siu MM, Wan R, Corneau A, Tajbakhsh S, Cheung TH, Le Grand F. High-dimensional single-cell cartography reveals novel skeletal muscle-resident cell populations. Mol Cell 74: 609–621.e6, 2019. DOI: 10.1016/j.molcel.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 462.Giovannini S, Onder G, Liperoti R, Russo A, Carter C, Capoluongo E, Pahor M, Bernabei R, Landi F. Interleukin-6, c-reactive protein, and tumor necrosis factor-alpha as predictors of mortality in frail, community-living elderly individuals. J Am Geriatr Soc 59: 1679–1685, 2011. DOI: 10.1111/j.1532-5415.2011.03570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Giralt M, Villarroya F. White, brown, beige/brite: Different adipose cells for different functions? Endocrinology 154: 2992–3000, 2013. DOI: 10.1210/en.2013-1403. [DOI] [PubMed] [Google Scholar]
- 464.Gjedde A Diffusive insights: On the disagreement of christian bohr and august krogh at the centennial of the seven little devils. Adv Physiol Educ 34: 174–185, 2010. DOI: 10.1152/advan.00092.2010. [DOI] [PubMed] [Google Scholar]
- 465.Gleyzer N, Vercauteren K, Scarpulla RC. Control of mitochondrial transcription specificity factors (tfb1m and tfb2m) by nuclear respiratory factors (nrf-1 and nrf-2) and pgc-1 family coactivators. Mol Cell Biol 25: 1354–1366, 2005. DOI: 10.1128/MCB.25.4.1354-1366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Glovaci D, Fan W, Wong ND. Epidemiology of diabetes mellitus and cardiovascular disease. Curr Cardiol Rep 21: 21, 2019. DOI: 10.1007/s11886-019-1107-y. [DOI] [PubMed] [Google Scholar]
- 467.Glynn EL, Fry CS, Drummond MJ, Dreyer HC, Dhanani S, Volpi E, Rasmussen BB. Muscle protein breakdown has a minor role in the protein anabolic response to essential amino acid and carbohydrate intake following resistance exercise. Am J Physiol Regul Integr Comp Physiol 299: R533–R540, 2010. DOI: 10.1152/ajpregu.00077.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Goh S-L, Persson MSM, Stocks J, Hou Y, Welton NJ, Lin J, Hall MC, Doherty M, Zhang W. Relative efficacy of different exercises for pain, function, performance and quality of life in knee and hip osteoarthritis: Systematic review and network meta-analysis. Sports Med 49: 743–761, 2019. DOI: 10.1007/s40279-019-01082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Gollnick PD, Armstrong RB, Saltin B, Saubert CW, Sembrowich WL, Shepherd RE. Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol 34: 107–111, 1973. DOI: 10.1152/jappl.1973.34.1.107. [DOI] [PubMed] [Google Scholar]
- 470.Gollnick PD, Armstrong RB, Saubert CW, Piehl K, Saltin B. Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. J Appl Physiol 33: 312–319, 1972. DOI: 10.1152/jappl.1972.33.3.312. [DOI] [PubMed] [Google Scholar]
- 471.Gollnick PD, Armstrong RB, Sembrowich WL, Shepherd RE, Saltin B. Glycogen depletion pattern in human skeletal muscle fibers after heavy exercise. J Appl Physiol 34: 615–618, 1973. DOI: 10.1152/jappl.1973.34.5.615. [DOI] [PubMed] [Google Scholar]
- 472.Gollnick PD, King DW. Effect of exercise and training on mitochondria of rat skeletal muscle. Am J Phys 216: 1502–1509, 1969. DOI: 10.1152/ajplegacy.1969.216.6.1502. [DOI] [PubMed] [Google Scholar]
- 473.Gollnick PD, Sjödin B, Karlsson J, Jansson E, Saltin B. Human soleus muscle: A comparison of fiber composition and enzyme activities with other leg muscles. Pflugers Arch—Eur J Physiol 348: 247–255, 1974. DOI: 10.1007/BF00587415. [DOI] [PubMed] [Google Scholar]
- 474.Gomes FGN, Fernandes J, Campos DV, Cassilhas RC, Viana GM, D’Almeida V, de Moraes Rêgo MK, Buainain PI, Cavalheiro EA, Arida RM. The beneficial effects of strength exercise on hippocampal cell proliferation and apoptotic signaling is impaired by anabolic androgenic steroids. Psychoneuroendocrinology 50: 106–117, 2014. DOI: 10.1016/j.psyneuen.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 475.Gomes-Neto M, Durães AR, Reis HFCD, Neves VR, Martinez BP, Carvalho VO. High-intensity interval training versus moderate-intensity continuous training on exercise capacity and quality of life in patients with coronary artery disease: A systematic review and meta-analysis. Eur J Prev Cardiol 24: 1696–1707, 2017. DOI: 10.1177/2047487317728370. [DOI] [PubMed] [Google Scholar]
- 476.Gonçalves A, Resende ES, Fernandes MLMP, da Costa AM. Effect of thyroid hormones on cardiovascular and muscle systems and on exercise tolerance: A brief review. Arq Bras Cardiol 87: e45–e47, 2006. DOI: 10.1590/s0066-782x2006001600033. [DOI] [PubMed] [Google Scholar]
- 477.Gonzalez JT, Fuchs CJ, Betts JA, van Loon LJC. Liver glycogen metabolism during and after prolonged endurance-type exercise. Am J Phys Endocrinol Metab 311: E543–E553, 2016. DOI: 10.1152/ajpendo.00232.2016. [DOI] [PubMed] [Google Scholar]
- 478.Gonzalez-Freire M, Semba RD, Ubaida-Mohien C, Fabbri E, Scalzo P, Højlund K, Dufresne C, Lyashkov A, Ferrucci L. The human skeletal muscle proteome project: A reappraisal of the current literature. J Cachexia Sarcopenia Muscle 8: 5–18, 2017. DOI: 10.1002/jcsm.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Gonzalo-Encabo P, McNeil J, Boyne DJ, Courneya KS, Friedenreich CM. Dose-response effects of exercise on bone mineral density and content in post-menopausal women. Scand J Med Sci Sports 29: 1121–1129, 2019. DOI: 10.1111/sms.13443. [DOI] [PubMed] [Google Scholar]
- 480.Goodman CA, Hornberger TA, Robling AG. Bone and skeletal muscle: Key players in mechanotransduction and potential overlapping mechanisms. Bone 80: 24–36, 2015. DOI: 10.1016/j.bone.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 46: 1579–1585, 1997. DOI: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 482.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61: 1059–1064, 2006. DOI: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 483.Goodyear LJ. The exercise pill–too good to be true? N Engl J Med 359: 1842–1844, 2008. DOI: 10.1056/NEJMcibr0806723. [DOI] [PubMed] [Google Scholar]
- 484.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev 80: 853–924, 2000. DOI: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 485.Gordon BR, McDowell CP, Lyons M, Herring MP. The effects of resistance exercise training on anxiety: A meta-analysis and meta-regression analysis of randomized controlled trials. Sports Med 47: 2521–2532, 2017. DOI: 10.1007/s40279-017-0769-0. [DOI] [PubMed] [Google Scholar]
- 486.Gordt K, Gerhardy T, Najafi B, Schwenk M. Effects of wearable sensor-based balance and gait training on balance, gait, and functional performance in healthy and patient populations: A systematic review and meta-analysis of randomized controlled trials. Gerontology 64: 74–89, 2018. DOI: 10.1159/000481454. [DOI] [PubMed] [Google Scholar]
- 487.Gram M, Vigelsø A, Yokota T, Helge JW, Dela F, Hey-Mogensen M. Skeletal muscle mitochondrial h2 o2 emission increases with immobilization and decreases after aerobic training in young and older men. J Physiol 593: 4011–4027, 2015. DOI: 10.1113/JP270211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Granacher U, Lesinski M, Büsch D, Muehlbauer T, Prieske O, Puta C, Gollhofer A, Behm DG. Effects of resistance training in youth athletes on muscular fitness and athletic performance: A conceptual model for long-term athlete development. Front Physiol 7: 164, 2016. DOI: 10.3389/fphys.2016.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Granata C, Oliveira RSF, Little JP, Renner K, Bishop DJ. Mitochondrial adaptations to high-volume exercise training are rapidly reversed after a reduction in training volume in human skeletal muscle. FASEB J 30: 3413–3423, 2016. DOI: 10.1096/fj.201500100R. [DOI] [PubMed] [Google Scholar]
- 490.Granata C, Oliveira RSF, Little JP, Renner K, Bishop DJ. Training intensity modulates changes in pgc-1α and p53 protein content and mitochondrial respiration, but not markers of mitochondrial content in human skeletal muscle. FASEB J 30: 959–970, 2016. DOI: 10.1096/fj.15-276907. [DOI] [PubMed] [Google Scholar]
- 491.Green DJ, Hopkins ND, Jones H, Thijssen DHJ, Eijsvogels TMH, Yeap BB. Sex differences in vascular endothelial function and health in humans: Impacts of exercise. Exp Physiol 101: 230–242, 2016. DOI: 10.1113/EP085367. [DOI] [PubMed] [Google Scholar]
- 492.Green DJ, Hopman MTE, Padilla J, Laughlin MH, Thijssen DHJ. Vascular adaptation to exercise in humans: Role of hemodynamic stimuli. Physiol Rev 97: 495–528, 2017. DOI: 10.1152/physrev.00014.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol 561: 1–25, 2004. DOI: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Green DJ, Smith KJ. Effects of exercise on vascular function, structure, and health in humans. Cold Spring Harb Perspect Med 8, 2018. DOI: 10.1101/cshperspect.a029819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Green S, Askew C. Vo2peak is an acceptable estimate of cardiorespiratory fitness but not vo2max. J Appl Physiol (1985) 125: 229–232, 2018. DOI: 10.1152/japplphysiol.00850.2017. [DOI] [PubMed] [Google Scholar]
- 496.Grgic J, Schoenfeld BJ. Are the hypertrophic adaptations to high and low-load resistance training muscle fiber type specific? Front Physiol 9: 402, 2018. DOI: 10.3389/fphys.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Grgic J, Schoenfeld BJ, Davies TB, Lazinica B, Krieger JW, Pedisic Z. Effect of resistance training frequency on gains in muscular strength: A systematic review and meta-analysis. Sports Med 48: 1207–1220, 2018. DOI: 10.1007/s40279-018-0872-x. [DOI] [PubMed] [Google Scholar]
- 498.Gries KJ, Minchev K, Raue U, Grosicki GJ, Begue G, Finch WH, Graham B, Trappe TA, Trappe S. Single-muscle fiber contractile properties in lifelong aerobic exercising women. J Appl Physiol (1985) 127: 1710–1719, 2019. DOI: 10.1152/japplphysiol.00459.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Gries KJ, Raue U, Perkins RK, Lavin KM, Overstreet BS, D’Acquisto LJ, Graham B, Finch WH, Kaminsky LA, Trappe TA, Trappe S. Cardiovascular and skeletal muscle health with lifelong exercise. J Appl Physiol (1985) 125: 1636–1645, 2018. DOI: 10.1152/japplphysiol.00174.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Grindberg RV, Yee-Greenbaum JL, McConnell MJ, Novotny M, O’Shaughnessy AL, Lambert GM, Ara’uzo-Bravo MJ, Lee J, Fishman M, Robbins GE, Lin X, Venepally P, Badger JH, Galbraith DW, Gage FH, Lasken RS. RNA-sequencing from single nuclei. Proc Natl Acad Sci U S A 110: 19802–19807, 2013. DOI: 10.1073/pnas.1319700110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Gruber R. Molecular and cellular basis of bone resorption. Wien Med Wochenschr 165: 48–53, 2015. DOI: 10.1007/s10354-014-0310-0. [DOI] [PubMed] [Google Scholar]
- 502.Gudsoorkar PS, Tobe SW. Changing concepts in hypertension management. J Hum Hypertens 31: 763–767, 2017. DOI: 10.1038/jhh.2017.57. [DOI] [PubMed] [Google Scholar]
- 503.Gump BS, McMullan DR, Cauthon DJ, Whitt JA, Mundo JDD, Letham T, Kim PJ, Friedlander GN, Pingel J, Langberg H, Carroll CC. Short-term acetaminophen consumption enhances the exercise-induced increase in achilles peritendinous il-6 in humans. J Appl Physiol (1985) 115: 929–936, 2013. DOI: 10.1152/japplphysiol.00219.2013. [DOI] [PubMed] [Google Scholar]
- 504.Guure CB, Ibrahim NA, Adam MB, Said SM. Impact of physical activity on cognitive decline, dementia, and its subtypes: Meta-analysis of prospective studies. Biomed Res Int 2017: 9016924, 2017. DOI: 10.1155/2017/9016924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Guzzini M, Raffa S, Geuna S, Nicolino S, Torrisi MR, Tos P, Battiston B, Grassi F, Ferretti A. Denervation-related changes in acetylcholine receptor density and distribution in the rat flexor digitorum sublimis muscle [Online]. Ital J Anat Embryol 113: 209–216, 2009. http://www.ncbi.nlm.nih.gov/pubmed/19507461. [PubMed] [Google Scholar]
- 506.Haakstad LAH, Kissel I, Bø K. Long-term effects of participation in a prenatal exercise intervention on body weight, body mass index, and physical activity level: A 6-year follow-up study of a randomized controlled trial. J Matern Fetal Neonatal Med 34 (9): 1347–1355. [DOI] [PubMed] [Google Scholar]
- 507.Hackney AC, Kallman AL, Aǧgön E. Female sex hormones and the recovery from exercise: Menstrual cycle phase affects responses. Biomed Hum Kinet 11: 87–89, 2019. DOI: 10.2478/bhk-2019-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol (1985) 98: 911–917, 2005. DOI: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- 509.Hagberg JM, Coyle EF, Baldwin KM, Cartee GD, Fontana L, Joyner MJ, Kirwan JP, Seals DR, Weiss EP. The historical context and scientific legacy of john o. Holloszy. J Appl Physiol (1985) 127: 277–305, 2019. DOI: 10.1152/japplphysiol.00669.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Hagstrom AD, Marshall PW, Halaki M, Hackett DA. The effect of resistance training in women on dynamic strength and muscular hypertrophy: A systematic review with meta-analysis. Sports Med 50: 1075–1093, 2020. DOI: 10.1007/s40279-019-01247-x. [DOI] [PubMed] [Google Scholar]
- 511.Hajjar I, Lackland DT, Cupples LA, Lipsitz LA. Association between concurrent and remote blood pressure and disability in older adults. Hypertension 50: 1026–1032, 2007. DOI: 10.1161/HYPERTENSIONAHA.107.097667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Hajjar I, Wharton W, Mack WJ, Levey AI, Goldstein FC. Racial disparity in cognitive and functional disability in hypertension and all-cause mortality. Am J Hypertens 29: 185–193, 2016. DOI: 10.1093/ajh/hpv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Haldane J A new form of apparatus for measuring the respiratory exchange of animals. J Physiol 13: 419–430, 1892. DOI: 10.1113/jphysiol.1892.sp000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Hambrecht R, Adams V, Erbs S, Linke A, Kränkel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 107: 3152–3158, 2003. DOI: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- 515.Hamed A, Bohm S, Mersmann F, Arampatzis A. Follow-up efficacy of physical exercise interventions on fall incidence and fall risk in healthy older adults: A systematic review and meta-analysis. Sports Med Open 4: 56, 2018. DOI: 10.1186/s40798-018-0170-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Hamilton MT, Booth FW. Skeletal muscle adaptation to exercise: A century of progress. J Appl Physiol (1985) 88: 327–331, 2000. DOI: 10.1152/jappl.2000.88.1.327. [DOI] [PubMed] [Google Scholar]
- 517.Hammarström D, Øfsteng S, Koll L, Hanestadhaugen M, Hollan I, Apr’o W, Whist JE, Blomstrand E, Rønnestad BR, Ellefsen S. Benefits of higher resistance-training volume are related to ribosome biogenesis. J Physiol 598: 543–565, 2020. DOI: 10.1113/JP278455. [DOI] [PubMed] [Google Scholar]
- 518.Hammett CJK, Oxenham HC, Baldi JC, Doughty RN, Ameratunga R, French JK, White HD, Stewart RAH. Effect of six months’ exercise training on c-reactive protein levels in healthy elderly subjects. J Am Coll Cardiol 44: 2411–2413, 2004. DOI: 10.1016/j.jacc.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 519.Han X, Gross RW. Global analyses of cellular lipidomes directly from crude extracts of biological samples by esi mass spectrometry: A bridge to lipidomics. J Lipid Res 44: 1071–1079, 2003. DOI: 10.1194/jlr.R300004-JLR200. [DOI] [PubMed] [Google Scholar]
- 520.Han X, Yang K, Gross RW. Multi-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analyses. Mass Spectrom Rev 31: 134–178, 2011. DOI: 10.1002/mas.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Harber MP, Crane JD, Dickinson JM, Jemiolo B, Raue U, Trappe TA, Trappe SW. Protein synthesis and the expression of growth-related genes are altered by running in human vastus lateralis and soleus muscles. Am J Physiol Regul Integr Comp Physiol 296: R708–R714, 2009. DOI: 10.1152/ajpregu.90906.2008. [DOI] [PubMed] [Google Scholar]
- 522.Harber M, Trappe S. Single muscle fiber contractile properties of young competitive distance runners. J Appl Physiol 105: 629–636, 2008. DOI: 10.1152/japplphysiol.00995.2007. [DOI] [PubMed] [Google Scholar]
- 523.Harber MP, Gallagher PM, Creer AR, Minchev KM, Trappe SW. Single muscle fiber contractile properties during a competitive season in male runners. Am J Physiol Regul Integr Comp Physiol 287: R1124–R1131, 2004. DOI: 10.1152/ajpregu.00686.2003. [DOI] [PubMed] [Google Scholar]
- 524.Harrison AL, Shields N, Taylor NF, Frawley HC. Exercise improves glycaemic control in women diagnosed with gestational diabetes mellitus: A systematic review. J Physiother 62: 188–196, 2016. DOI: 10.1016/j.jphys.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 525.Harry LE, Sandison A, Paleolog EM, Hansen U, Pearse MF, Nanchahal J. Comparison of the healing of open tibial fractures covered with either muscle or fasciocutaneous tissue in a murine model. J Orthop Res 26: 1238–1244, 2008. DOI: 10.1002/jor.20649. [DOI] [PubMed] [Google Scholar]
- 526.Harston RK, Kuppuswamy D. Integrins are the necessary links to hypertrophic growth in cardiomyocytes. J Signal Transduct 2011: 521742, 2011. DOI: 10.1155/2011/521742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Harveson AT, Hannon JC, Brusseau TA, Podlog L, Papadopoulos C, Hall MS, Celeste E. Acute exercise and academic achievement in middle school students. Int J Environ Res Public Health 16, 2019. DOI: 10.3390/ijerph16193527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Harvey SB, Øverland S, Hatch SL, Wessely S, Mykletun A, Hotopf M. Exercise and the prevention of depression: Results of the hunt cohort study. Am J Psychiatry 175: 28–36, 2018. DOI: 10.1176/appi.ajp.2017.16111223. [DOI] [PubMed] [Google Scholar]
- 529.Hattori N, Hayashi T, Nakachi K, Ichikawa H, Goto C, Tokudome Y, Kuriki K, Hoshino H, Shibata K, Yamada N, Tokudome M, Suzuki S, Nagaya T, Kobayashi M, Tokudome S. Changes of ros during a twoday ultra-marathon race. Int J Sports Med 30: 426–429, 2009. DOI: 10.1055/s-0028-1112144. [DOI] [PubMed] [Google Scholar]
- 530.Haun CT, Vann CG, Mobley CB, Osburn SC, Mumford PW, Roberson PA, Romero MA, Fox CD, Parry HA, Kavazis AN, Moon JR, Young KC, Roberts MD. Pre-training skeletal muscle fiber size and predominant fiber type best predict hypertrophic responses to 6 weeks of resistance training in previously trained young men. Front Physiol 10: 297, 2019. DOI: 10.3389/fphys.2019.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Haun CT, Vann CG, Roberts BM, Vigotsky AD, Schoenfeld BJ, Roberts MD. A critical evaluation of the biological construct skeletal muscle hypertrophy: Size matters but so does the measurement. Front Physiol 10: 247, 2019. DOI: 10.3389/fphys.2019.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Hawe JS, Theis FJ, Heinig M. Inferring interaction networks from multi-omics data. Front Genet 10: 535, 2019. DOI: 10.3389/fgene.2019.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Hawke TJ. Muscle stem cells and exercise training. Exerc Sport Sci Rev 33: 63–68, 2005. DOI: 10.1097/00003677-200504000-00002. [DOI] [PubMed] [Google Scholar]
- 534.Hawke TJ, Garry DJ. Myogenic satellite cells: Physiology to molecular biology. J Appl Physiol (1985) 91: 534–551, 2001. DOI: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- 535.Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. Integrative biology of exercise. Cell 159: 738–749, 2014. DOI: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 536.Hawley JA, Joyner MJ, Green DJ. Mimicking exercise: What matters most and where to next? J Physiol 599: 791–802, 2021. DOI: 10.1113/JP278761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Hayes K, Leet AS, Bradley SJ, Holland AE. Effects of exercise training on exercise capacity and quality of life in patients with a left ventricular assist device: A preliminary randomized controlled trial. J Heart Lung Transplant 31: 729–734, 2012. DOI: 10.1016/j.healun.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 538.Hayot M, Michaud A, Koechlin C, Caron M-A, Leblanc P, Préfaut C, Maltais F. Skeletal muscle microbiopsy: A validation study of a minimally invasive technique. Eur Respir J 25: 431–440, 2005. DOI: 10.1183/09031936.05.00053404. [DOI] [PubMed] [Google Scholar]
- 539.Hazell TJ, Macpherson REK, Gravelle BMR, Lemon PWR. 10 or 30-s sprint interval training bouts enhance both aerobic and anaerobic performance. Eur J Appl Physiol 110: 153–160, 2010. DOI: 10.1007/s00421-010-1474-y. [DOI] [PubMed] [Google Scholar]
- 540.Häkkinen A, Pakarinen A, Hannonen P, Kautiainen H, Nyman K, Kraemer WJ, Häkkinen K. Effects of prolonged combined strength and endurance training on physical fitness, body composition and serum hormones in women with rheumatoid arthritis and in healthy controls [Online]. Clin Exp Rheumatol 23: 505–512, 2005. http://www.ncbi.nlm.nih.gov/pubmed/16095120. [PubMed] [Google Scholar]
- 541.Hänggi J, Koeneke S, Bezzola L, Jäncke L. Structural neuroplasticity in the sensorimotor network of professional female ballet dancers. Hum Brain Mapp 31: 1196–1206, 2010. DOI: 10.1002/hbm.20928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Hänggi J, Langer N, Lutz K, Birrer K, Mérillat S, Jäncke L. Structural brain correlates associated with professional handball playing. PLoS One 10: e0124222, 2015. DOI: 10.1371/journal.pone.0124222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Hebert AS, Dittenhafer-Reed KE, Yu W, Bailey DJ, Selen ES, Boersma MD, Carson JJ, Tonelli M, Balloon AJ, Higbee AJ, West-phall MS, Pagliarini DJ, Prolla TA, Assadi-Porter F, Roy S, Denu JM, Coon JJ. Calorie restriction and sirt3 trigger global reprogramming of the mitochondrial protein acetylome. Mol Cell 49: 186–199, 2013. DOI: 10.1016/j.molcel.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Heden TD, Ryan TE, Ferrara PJ, Hickner RC, Brophy PM, Neufer PD, McClung JM, Funai K. Greater oxidative capacity in primary myotubes from endurance-trained women. Med Sci Sports Exerc 49: 2151–2157, 2017. DOI: 10.1249/MSS.0000000000001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7: 589–600, 2006. DOI: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 546.Heinemeier K, Langberg H, Olesen JL, Kjaer M. Role of tgf-beta1 in relation to exercise-induced type i collagen synthesis in human tendinous tissue. J Appl Physiol (1985) 95: 2390–2397, 2003. DOI: 10.1152/japplphysiol.00403.2003. [DOI] [PubMed] [Google Scholar]
- 547.Heinemeier KM, Schjerling P, Heinemeier J, Magnusson SP, Kjaer M. Lack of tissue renewal in human adult achilles tendon is revealed by nuclear bomb (14)C. FASEB J 27: 2074–2079, 2013. DOI: 10.1096/fj.12-225599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Heinonen I, Kalliokoski KK, Hannukainen JC, Duncker DJ, Nuutila P, Knuuti J. Organ-specific physiological responses to acute physical exercise and long-term training in humans. Physiology (Bethesda) 29: 421–436, 2014. DOI: 10.1152/physiol.00067.2013. [DOI] [PubMed] [Google Scholar]
- 549.Helge JW, Stallknecht B, Pedersen BK, Galbo H, Kiens B, Richter EA. The effect of graded exercise on il-6 release and glucose uptake in human skeletal muscle. J Physiol 546: 299–305, 2003. DOI: 10.1113/jphysiol.2002.030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Helgerud J, Høydal K, Wang E, Karlsen T, Berg P, Bjerkaas M, Simonsen T, Helgesen C, Hjorth N, Bach R, Hoff J. Aerobic high-intensity intervals improve vo2max more than moderate training. Med Sci Sports Exerc 39: 665–671, 2007. DOI: 10.1249/mss.0b013e3180304570. [DOI] [PubMed] [Google Scholar]
- 551.Hellsten Y, Nyberg M. Cardiovascular adaptations to exercise training. Compr Physiol 6: 1–32, 2015. DOI: 10.1002/cphy.c140080. [DOI] [PubMed] [Google Scholar]
- 552.Hely MA, Reid WGJ, Adena MA, Halliday GM, Morris JGL. The sydney multicenter study of parkinson’s disease: The inevitability of dementia at 20 years. Mov Disord 23: 837–844, 2008. DOI: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 553.Hemmings BA, Restuccia DF. PI3K-pkb/akt pathway. Cold Spring Harb Perspect Biol 4: a011189, 2012. DOI: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Henneman E, Somjen G, Carpenter DO. Excitability and inhibitability of motoneurons of different sizes. J Neurophysiol 28: 599–620, 1965. DOI: 10.1152/jn.1965.28.3.599. [DOI] [PubMed] [Google Scholar]
- 555.Henriksson KG. “Semi-open” muscle biopsy technique. A simple outpatient procedure [Online]. Acta Neurol Scand 59: 317–323, 1979. http://www.ncbi.nlm.nih.gov/pubmed/484204. [PubMed] [Google Scholar]
- 556.Heo M, Murphy CF, Fontaine KR, Bruce ML, Alexopoulos GS. Population projection of us adults with lifetime experience of depressive disorder by age and sex from year 2005 to 2050. Int J Geriatr Psychiatry 23: 1266–1270, 2008. DOI: 10.1002/gps.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Hepple RT, Rice CL. Innervation and neuromuscular control in ageing skeletal muscle. J Physiol 594: 1965–1978, 2016. DOI: 10.1113/JP270561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA. Osteoporosis in the european union: Medical management, epidemiology and economic burden. A report prepared in collaboration with the international osteoporosis foundation (iof) and the european federation of pharmaceutical industry associations (efpia). Arch Osteoporos 8: 136, 2013. DOI: 10.1007/S11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Herold F, Müller P, Gronwald T, Müller NG. Dose-response matters!—a perspective on the exercise prescription in exercise-cognition research. Front Psychol 10: 2338, 2019. DOI: 10.3389/fpsyg.2019.02338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Herold F, Törpel A, Schega L, Müller NG. Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements—a systematic review. Eur Rev Aging Phys Act 16: 10, 2019. DOI: 10.1186/s11556-019-0217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Herting MM, Chu X. Exercise, cognition, and the adolescent brain. Birth Defects Res 109: 1672–1679, 2017. DOI: 10.1002/bdr2.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Herting MM, Nagel BJ. Aerobic fitness relates to learning on a virtual morris water task and hippocampal volume in adolescents. Behav Brain Res 233: 517–525, 2012. DOI: 10.1016/j.bbr.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Heyman E, Gamelin F-X, Goekint M, Piscitelli F, Roelands B, Leclair E, Marzo VD, Meeusen R. Intense exercise increases circulating endocannabinoid and bdnf levels in humans–possible implications for reward and depression. Psychoneuroendocrinology 37: 844–851, 2012. DOI: 10.1016/j.psyneuen.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 564.Hildebrandt AL, Pilegaard H, Neufer PD. Differential transcriptional activation of select metabolic genes in response to variations in exercise intensity and duration. Am J Phys Endocrinol Metab 285: E1021–E1027, 2003. DOI: 10.1152/ajpendo.00234.2003. [DOI] [PubMed] [Google Scholar]
- 565.Hill AV. The revolution in muscle physiology. Physiol Rev 12: 56–67, 1932. [Google Scholar]
- 566.Hill AV, Long CNH, Lupton H. Muscular exercise, lactic acid and the supply and utilisation of oxygen.– Parts VII–VIII. Proc R Soc Lond B Biol Sci 97: 155–176, 1924. DOI: 10.1098/rspb.1924.0048. [DOI] [Google Scholar]
- 567.Hill AV. A challenge to biochemists. Biochim Biophys Acta 4: 4–11, 1950. DOI: 10.1016/0006-3002(50)90003-5. [DOI] [PubMed] [Google Scholar]
- 568.Hill EE, Zack E, Battaglini C, Viru M, Viru A, Hackney AC. Exercise and circulating cortisol levels: The intensity threshold effect. J Endocrinol Investig 31: 587–591, 2008. DOI: 10.1007/BF03345606. [DOI] [PubMed] [Google Scholar]
- 569.Hillard CJ. Circulating endocannabinoids: From whence do they come and where are they going? Neuropsychopharmacology 43: 155–172, 2018. DOI: 10.1038/npp.2017.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Hinkley JM, Konopka AR, Suer MK, Harber MP. Short-term intense exercise training reduces stress markers and alters the transcriptional response to exercise in skeletal muscle. Am J Physiol Regul Integr Comp Physiol 312: R426–R433, 2017. DOI: 10.1152/ajpregu.00356.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Hirose L, Nosaka K, Newton M, Laveder A, Kano M, Peake J, Suzuki K. Changes in inflammatory mediators following eccentric exercise of the elbow flexors [Online]. Exerc Immunol Rev 10: 75–90, 2004. http://www.ncbi.nlm.nih.gov/pubmed/15633588. [PubMed] [Google Scholar]
- 572.Hivert M-F, Arena R, Forman DE, Kris-Etherton PM, McBride PE, Pate RR, Spring B, Trilk J, Van Horn LV, Kraus WE. Medical training to achieve competency in lifestyle counseling: An essential foundation for prevention and treatment of cardiovascular diseases and other chronic medical conditions: A scientific statement from the american heart association. Circulation 134: e308–e327, 2016. DOI: 10.1161/CIR.0000000000000442. [DOI] [PubMed] [Google Scholar]
- 573.Hodes RJ, Sierra F, Austad SN, Epel E, Neigh GN, Erlandson KM, Schafer MJ, LeBrasseur NK, Wiley C, Campisi J, Sehl ME, Scalia R, Eguchi S, Kasinath BS, Halter JB, Cohen HJ, Demark-Wahnefried W, Ahles TA, Barzilai N, Hurria A, Hunt PW. Disease drivers of aging. Ann N Y Acad Sci 1386: 45–68, 2016. DOI: 10.1111/nyas.13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Hoekstra SP, Leicht CA, Kamijo Y-I, Kinoshita T, Stephenson BT, Goosey-Tolfrey VL, Bishop NC, Tajima F. The inflammatory response to a wheelchair half-marathon in people with a spinal cord injury—the role of autonomic function. J Sports Sci 37: 1717–1724, 2019. DOI: 10.1080/02640414.2019.1586296. [DOI] [PubMed] [Google Scholar]
- 575.Hoene M, Weigert C. The stress response of the liver to physical exercise [Online]. Exerc Immunol Rev 16: 163–183, 2010. http://www.ncbi.nlm.nih.gov/pubmed/20839498. [PubMed] [Google Scholar]
- 576.Hoffman NJ, Parker BL, Chaudhuri R, Fisher-Wellman KH, Kleinert M, Humphrey SJ, Yang P, Holliday M, Trefely S, Fazakerley DJ, Stöckli J, Burchfield JG, Jensen TE, Jothi R, Kiens B, Wojtaszewski JFP, Richter EA, James DE. Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise-regulated kinases and ampk substrates. Cell Metab 22: 922–935, 2015. DOI: 10.1016/j.cmet.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Hoffmann C, Weigert C. Skeletal muscle as an endocrine organ: The role of myokines in exercise adaptations. Cold Spring Harb Perspect Med 7, 2017. DOI: 10.1101/cshperspect.a029793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Hofmann P. Cancer and exercise: Warburg hypothesis, tumour metabolism and high-intensity anaerobic exercise. Sports 6, 2018. DOI: 10.3390/sports6010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Hoier B, Hellsten Y. Exercise-induced capillary growth in human skeletal muscle and the dynamics of vegf. Microcirculation 21: 301–314, 2014. DOI: 10.1111/micc.12117. [DOI] [PubMed] [Google Scholar]
- 580.Hollings M, Mavros Y, Freeston J, Singh MF. The effect of progressive resistance training on aerobic fitness and strength in adults with coronary heart disease: A systematic review and meta-analysis of randomised controlled trials. Eur J Prev Cardiol 24: 1242–1259, 2017. DOI: 10.1177/2047487317713329. [DOI] [PubMed] [Google Scholar]
- 581.Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle [Online]. J Biol Chem 242: 2278–2282, 1967. http://www.ncbi.nlm.nih.gov/pubmed/4290225. [PubMed] [Google Scholar]
- 582.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol Respir Environ Exerc Physiol 56: 831–838, 1984. DOI: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- 583.Holloszy JO. A forty-year memoir of research on the regulation of glucose transport into muscle. Am J Phys Endocrinol Metab 284: E453–E467, 2003. DOI: 10.1152/ajpendo.00463.2002. [DOI] [PubMed] [Google Scholar]
- 584.Holloszy JO, Kohrt WM, Hansen PA. The regulation of carbohydrate and fat metabolism during and after exercise. Front Biosci 3: D1011–D1027, 1998. DOI: 10.2741/a342. [DOI] [PubMed] [Google Scholar]
- 585.Holloszy JO, Oscai LB, Don IJ, Molé PA. Mitochondrial citric acid cycle and related enzymes: Adaptive response to exercise. Biochem Biophys Res Commun 40: 1368–1373, 1970. DOI: 10.1016/0006-291x(70)90017-3. [DOI] [PubMed] [Google Scholar]
- 586.Holloway KV, O’Gorman M, Woods P, Morton JP, Evans L, Cable NT, Goldspink DF, Burniston JG. Proteomic investigation of changes in human vastus lateralis muscle in response to interval-exercise training. Proteomics 9: 5155–5174, 2009. DOI: 10.1002/pmic.200900068. [DOI] [PubMed] [Google Scholar]
- 587.Holloway TM, Snijders T, Kranenburg JV, Loon LJCV, Verdijk LB. Temporal response of angiogenesis and hypertrophy to resistance training in young men. Med Sci Sports Exerc 50: 36–45, 2018. DOI: 10.1249/MSS.0000000000001409. [DOI] [PubMed] [Google Scholar]
- 588.Hong AR, Kim SW. Effects of resistance exercise on bone health. Endocrinol Metab 33: 435–444, 2018. DOI: 10.3803/EnM.2018.33.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Hoogkamer W, Kram R, Arellano CJ. How biomechanical improvements in running economy could break the 2-hour marathon barrier. Sports Med 47: 1739–1750, 2017. DOI: 10.1007/s40279-017-0708-0. [DOI] [PubMed] [Google Scholar]
- 590.Horak M, Zlamal F, Iliev R, Kucera J, Cacek J, Svobodova L, Hlavonova Z, Kalina T, Slaby O, Bienertova-Vasku J. Exercise-induced circulating microRNA changes in athletes in various training scenarios. PLoS One 13: e0191060, 2018. DOI: 10.1371/journal.pone.0191060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Horowitz JF, Leone TC, Feng W, Kelly DP, Klein S. Effect of endurance training on lipid metabolism in women: A potential role for pparalpha in the metabolic response to training. Am J Phys Endocrinol Metab 279: E348–E355, 2000. DOI: 10.1152/ajpendo.2000.279.2.E348. [DOI] [PubMed] [Google Scholar]
- 592.Hosker DK, Elkins RM, Potter MP. Promoting mental health and wellness in youth through physical activity, nutrition, and sleep. Child Adolesc Psychiatr Clin N Am 28: 171–193, 2019. DOI: 10.1016/j.chc.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 593.Hostler D, Crill MT, Hagerman FC, Staron RS. The effectiveness of 0.5-lb increments in progressive resistance exercise [Online]. J Strength Cond Res 15: 86–91, 2001. http://www.ncbi.nlm.nih.gov/pubmed/11708713. [PubMed] [Google Scholar]
- 594.Hou Z, Qin X, Hu Y, Zhang X, Li G, Wu J, Li J, Sha J, Chen J, Xia J, Wang L, Gao F. Longterm exercise-derived exosomal miR-342–5p: A novel exerkine for cardioprotection. Circ Res 124: 1386–1400, 2019. DOI: 10.1161/CIRCRESAHA.118.314635. [DOI] [PubMed] [Google Scholar]
- 595.Howard VJ, McDonnell MN. Physical activity in primary stroke prevention: Just do it! Stroke 46: 1735–1739, 2015. DOI: 10.1161/STROKEAHA.115.006317. [DOI] [PubMed] [Google Scholar]
- 596.Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, Harbour RT, Caldwell LM, Creed G. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev: CD000333, 2011. DOI: 10.1002/14651858.CD000333.pub2. [DOI] [PubMed] [Google Scholar]
- 597.Hu P, Fabyanic E, Kwon DY, Tang S, Zhou Z, Wu H. Dissecting cell-type composition and activity-dependent transcriptional state in mammalian brains by massively parallel single-nucleus rna-seq. Mol Cell 68: 1006–1015.e7, 2017. DOI: 10.1016/j.molcel.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Huang P-H, Chen Y-H, Wang C-H, Chen J-S, Tsai H-Y, Lin F-Y, Lo W-Y, Wu T-C, Sata M, Chen J-W, Lin S-J. Matrix metalloproteinase-9 is essential for ischemia-induced neovascularization by modulating bone marrow-derived endothelial progenitor cells. Arterioscler Thromb Vasc Biol 29: 1179–1184, 2009. DOI: 10.1161/ATVBAHA.109.189175. [DOI] [PubMed] [Google Scholar]
- 599.Huang R, Lu M, Song Z, Wang J. Long-term intensive training induced brain structural changes in world class gymnasts. Brain Struct Funct 220: 625–644, 2015. DOI: 10.1007/s00429-013-0677-5. [DOI] [PubMed] [Google Scholar]
- 600.Huang T-Y, Zheng D, Houmard JA, Brault JJ, Hickner RC, Cortright RN. Overexpression of pgc-1α increases peroxisomal activity and mitochondrial fatty acid oxidation in human primary myotubes. Am J Phys Endocrinol Metab 312: E253–E263, 2017. DOI: 10.1152/ajpendo.00331.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Huang W-S, Yu M-D, Lee M-S, Cheng C-Y, Yang S-P, Chin H-ML, Wu S-Y. Effect of treadmill exercise on circulating thyroid hormone measurements. Med Princ Pract 13: 15–19, 2003. DOI: 10.1159/000074045. [DOI] [PubMed] [Google Scholar]
- 602.Hubal MJ, Chen TC, Thompson PD, Clarkson PM. Inflammatory gene changes associated with the repeated-bout effect. Am J Physiol Regul Integr Comp Physiol 294: R1628–R1637, 2008. DOI: 10.1152/ajpregu.00853.2007. [DOI] [PubMed] [Google Scholar]
- 603.Huffman KM, Koves TR, Hubal MJ, Abouassi H, Beri N, Bateman LA, Stevens RD, Ilkayeva OR, Hoffman EP, Muoio DM, Kraus WE. Metabolite signatures of exercise training in human skeletal muscle relate to mitochondrial remodelling and cardiometabolic fitness. Diabetologia 57: 2282–2295, 2014. DOI: 10.1007/s00125-014-3343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Huffman KM, Slentz CA, Bateman LA, Thompson D, Muehlbauer MJ, Bain JR, Stevens RD, Wenner BR, Kraus VB, Newgard CB, Kraus WE. Exercise-induced changes in metabolic intermediates, hormones, and inflammatory markers associated with improvements in insulin sensitivity. Diabetes Care 34: 174–176, 2011. DOI: 10.2337/dc10-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Hughes JMB, Bates DV. Historical review: The carbon monoxide diffusing capacity (dlco) and its membrane (dm) and red cell (theta.vc) components. Respir Physiol Neurobiol 138: 115–142, 2003. DOI: 10.1016/j.resp.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 606.Hughes MC, Ramos SV, Turnbull PC, Nejatbakhsh A, Baechler BL, Tahmasebi H, Laham R, Gurd BJ, Quadrilatero J, Kane DA, Perry CGR. Mitochondrial bioenergetics and fiber type assessments in microbiopsy vs. Bergstrom percutaneous sampling of human skeletal muscle. Front Physiol 6: 360, 2015. DOI: 10.3389/fphys.2015.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Hulston CJ, Woods RM, Dewhurst-Trigg R, Parry SA, Gagnon S, Baker L, James LJ, Markey O, Martin NRW, Ferguson RA, van Hall G. Resistance exercise stimulates mixed muscle protein synthesis in lean and obese young adults. Physiol Rep 6: e13799, 2018. DOI: 10.14814/phy2.13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet 393: 1745–1759, 2019. DOI: 10.1016/s0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 609.Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol 10: 437–441, 2014. DOI: 10.1038/nrrheum.2014.44. [DOI] [PubMed] [Google Scholar]
- 610.Hunter SK. Sex differences in fatigability of dynamic contractions. Exp Physiol 101: 250–255, 2016. DOI: 10.1113/EP085370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Hunter SK, Butler JE, Todd G, Gandevia SC, Taylor JL. Supraspinal fatigue does not explain the sex difference in muscle fatigue of maximal contractions. J Appl Physiol (1985) 101: 1036–1044, 2006. DOI: 10.1152/japplphysiol.00103.2006. [DOI] [PubMed] [Google Scholar]
- 612.Hunter SK. Sex differences in human fatigability: Mechanisms and insight to physiological responses. Acta Physiol (Oxf) 210: 768–789, 2014. DOI: 10.1111/apha.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Hurkmans E, van der Giesen FJ, Vlieland TPV, Schoones J, Van den Ende ECHM. Dynamic exercise programs (aerobic capacity and/or muscle strength training) in patients with rheumatoid arthritis. Cochrane Database Syst Rev: CD006853, 2009. DOI: 10.1002/14651858.CD006853.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Hurley BF, Nemeth PM, Martin WH, Hagberg JM, Dalsky GP, Holloszy JO. Muscle triglyceride utilization during exercise: Effect of training. J Appl Physiol (1985) 60: 562–567, 1986. DOI: 10.1152/jappl.1986.60.2.562. [DOI] [PubMed] [Google Scholar]
- 615.Hussain SR, Macaluso A, Pearson SJ. High-intensity interval training versus moderate-intensity continuous training in the prevention/management of cardiovascular disease. Cardiol Rev 24: 273–281, 2016. DOI: 10.1097/CRD.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 616.Huxley H, Hanson J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature 173: 973–976, 1954. DOI: 10.1038/173973a0. [DOI] [PubMed] [Google Scholar]
- 617.Hvid LG, Nielsen MKF, Simonsen C, Andersen M, Caserotti P. Brain-derived neurotrophic factor (bdnf) serum basal levels is not affected by power training in mobility-limited older adults—a randomized controlled trial. Exp Gerontol 93: 29–35, 2017. DOI: 10.1016/j.exger.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 618.Høeg L, Roepstorff C, Thiele M, Richter EA, Wojtaszewski JFP, Kiens B. Higher intramuscular triacylglycerol in women does not impair insulin sensitivity and proximal insulin signaling. J Appl Physiol (1985) 107: 824–831, 2009. DOI: 10.1152/japplphysiol.91382.2008. [DOI] [PubMed] [Google Scholar]
- 619.Igarashi Y, Nogami Y. Running to lower resting blood pressure: A systematic review and meta-analysis. Sports Med 50: 531–541, 2020. DOI: 10.1007/s40279-019-01209-3. [DOI] [PubMed] [Google Scholar]
- 620.Igarashi Y, Nogami Y. The effect of regular aquatic exercise on blood pressure: A meta-analysis of randomized controlled trials. Eur J Prev Cardiol 25: 190–199, 2018. DOI: 10.1177/2047487317731164. [DOI] [PubMed] [Google Scholar]
- 621.Iizuka K, Machida T, Hirafuji M. Skeletal muscle is an endocrine organ. J Pharmacol Sci 125: 125–131, 2014. DOI: 10.1254/jphs.14r02cp. [DOI] [PubMed] [Google Scholar]
- 622.Ikedo A, Kido K, Ato S, Sato K, Lee J-W, Fujita S, Imai Y. The effects of resistance training on bone mineral density and bone quality in type 2 diabetic rats. Physiol Rep 7: e14046, 2019. DOI: 10.14814/phy2.14046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Ingalls CP. Nature vs. Nurture: Can exercise really alter fiber type composition in human skeletal muscle? J Appl Physiol (1985) 97: 1591–1592, 2004. DOI: 10.1152/classicessays.00010.2004. [DOI] [PubMed] [Google Scholar]
- 624.Ingerslev B, Hansen JS, Hoffmann C, Clemmesen JO, Secher NH, Scheler M, de Angelis MH, Häring HU, Pedersen BK, Weigert C, Plomgaard P. Angiopoietin-like protein 4 is an exercise-induced hepatokine in humans, regulated by glucagon and cAMP. Mol Metab 6: 1286–1295, 2017. DOI: 10.1016/j.molmet.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Ingle L, Mellis M, Brodie D, Sandercock GR. Associations between cardiorespiratory fitness and the metabolic syndrome in british men. Heart 103: 524–528, 2017. DOI: 10.1136/heartjnl-2016-310142. [DOI] [PubMed] [Google Scholar]
- 626.Inskip M, Mavros Y, Sachdev PS, Singh MAF. Exercise for individuals with lewy body dementia: A systematic review. PLoS One 11: e0156520, 2016. DOI: 10.1371/journal.pone.0156520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Inzlicht M, Marcora SM. The central governor model of exercise regulation teaches us precious little about the nature of mental fatigue and self-control failure. Front Psychol 7: 656, 2016. DOI: 10.3389/fpsyg.2016.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Iqbal S, Hood DA. Oxidative stress-induced mitochondrial fragmentation and movement in skeletal muscle myoblasts. Am J Phys Cell Phys 306: C1176–C1183, 2014. DOI: 10.1152/ajpcell.00017.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Irrcher I, Ljubicic V, Hood DA. Interactions between ros and amp kinase activity in the regulation of pgc-1alpha transcription in skeletal muscle cells. Am J Phys Cell Phys 296: C116–C123, 2009. DOI: 10.1152/ajpcell.00267.2007. [DOI] [PubMed] [Google Scholar]
- 630.Izard RM, Fraser WD, Negus C, Sale C, Greeves JP. Increased density and periosteal expansion of the tibia in young adult men following short-term arduous training. Bone 88: 13–19, 2016. DOI: 10.1016/j.bone.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 631.Jabbar A, Pingitore A, Pearce SHS, Zaman A, Iervasi G, Razvi S. Thyroid hormones and cardiovascular disease. Nat Rev Cardiol 14: 39–55, 2017. DOI: 10.1038/nrcardio.2016.174. [DOI] [PubMed] [Google Scholar]
- 632.Jackson JR, Kirby TJ, Fry CS, Cooper RL, McCarthy JJ, Peterson CA, Dupont-Versteegden EE. Reduced voluntary running performance is associated with impaired coordination as a result of muscle satellite cell depletion in adult mice. Skelet Muscle 5: 41, 2015. DOI: 10.1186/s13395-015-0065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Jacobs RA, Flück D, Bonne TC, Bürgi S, Christensen PM, Toigo M, Lundby C. Improvements in exercise performance with high-intensity interval training coincide with an increase in skeletal muscle mitochondrial content and function. J Appl Physiol (1985) 115: 785–793, 2013. DOI: 10.1152/japplphysiol.00445.2013. [DOI] [PubMed] [Google Scholar]
- 634.James MM-S, Carroll S. Effects of different impact exercise modalities on bone mineral density in premenopausal women: A meta-analysis. J Bone Miner Metab 28: 251–267, 2010. DOI: 10.1007/s00774-009-0139-6. [DOI] [PubMed] [Google Scholar]
- 635.James MM-S, Carroll S. High-intensity resistance training and post-menopausal bone loss: A meta-analysis. Osteoporos Int 17: 1225–1240, 2006. DOI: 10.1007/s00198-006-0083-4. [DOI] [PubMed] [Google Scholar]
- 636.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth joint national committee (jnc 8). JAMA 311: 507–520, 2014. DOI: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 637.Janez A, Guja C, Mitrakou A, Lalic N, Tankova T, Czupryniak L, Tabak AG, Prazny M, Martinka E, Smircic-Duvnjak L. Insulin therapy in adults with type 1 diabetes mellitus: A narrative review. Diabetes Ther 11: 387–409, 2020. DOI: 10.1007/s13300-019-00743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Janssen JAMJL. Impact of physical exercise on endocrine aging. Front Horm Res 47: 68–81, 2016. DOI: 10.1159/000445158. [DOI] [PubMed] [Google Scholar]
- 639.Jansson E, Kaijser L. Substrate utilization and enzymes in skeletal muscle of extremely endurance-trained men. J Appl Physiol (1985) 62: 999–1005, 1987. DOI: 10.1152/jappl.1987.62.3.999. [DOI] [PubMed] [Google Scholar]
- 640.Janzen NR, Whitfield J, Hoffman NJ. Interactive roles for ampk and glycogen from cellular energy sensing to exercise metabolism. Int J Mol Sci 19, 2018. DOI: 10.3390/ijms19113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Javanshiri K, Waldö ML, Friberg N, Sjövall F, Wickerström K, Haglund M, Englund E. Atherosclerosis, hypertension, and diabetes in alzheimer’s disease, vascular dementia, and mixed dementia: Prevalence and presentation. J Alzheimers Dis 65: 1247–1258, 2018. DOI: 10.3233/JAD-180644. [DOI] [PubMed] [Google Scholar]
- 642.Järvinen TL, Kannus P, Sievänen H. Have the dxa-based exercise studies seriously underestimated the effects of mechanical loading on bone? J Bone Miner Res Off J Am Soc Bone Miner Res 14: 1634–1635, 1999. DOI: 10.1359/jbmr.1999.14.9.1634. [DOI] [PubMed] [Google Scholar]
- 643.Jenkins NT, Martin JS, Laughlin MH, Padilla J. Exercise-induced signals for vascular endothelial adaptations: Implications for cardiovascular disease. Curr Cardiovasc Risk Rep 6: 331–346, 2012. DOI: 10.1007/s12170-012-0241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Jensen J, Rustad PI, Kolnes AJ, Lai Y-C. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front Physiol 2: 112, 2011. DOI: 10.3389/fphys.2011.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Jensen TE, Richter EA. Regulation of glucose and glycogen metabolism during and after exercise. J Physiol 590: 1069–1076, 2012. DOI: 10.1113/jphysiol.2011.224972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Jeong HS, Lee S-C, Jee H, Song JB, Chang HS, Lee SY. Proprioceptive training and outcomes of patients with knee osteoarthritis: A meta-analysis of randomized controlled trials. J Athl Train 54: 418–428, 2019. DOI: 10.4085/1062-6050-329-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Joanisse S, Lim C, McKendry J, Mcleod JC, Stokes T, Phillips SM. Recent advances in understanding resistance exercise training-induced skeletal muscle hypertrophy in humans. F1000Research 9, 2020. DOI: 10.12688/f1000research.21588.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Joanisse S, Nederveen JP, Snijders T, McKay BR, Parise G. Skeletal muscle regeneration, repair and remodelling in aging: The importance of muscle stem cells and vascularization. Gerontology 63: 91–100, 2017. DOI: 10.1159/000450922. [DOI] [PubMed] [Google Scholar]
- 649.John K, Marino JS, Sanchez ER, Hinds TD. The glucocorticoid receptor: Cause of or cure for obesity? Am J Phys Endocrinol Metab 310: E249–E257, 2016. DOI: 10.1152/ajpendo.00478.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Johnson A “They sweat for science”: The harvard fatigue laboratory and self-experimentation in american exercise physiology. J Hist Biol 48: 425–454, 2015. DOI: 10.1007/s10739-014-9387-y. [DOI] [PubMed] [Google Scholar]
- 651.Johnson CP, Myers SM. Identification and evaluation of children with autism spectrum disorders. Pediatrics 120: 1183–1215, 2007. DOI: 10.1542/peds.2007-2361. [DOI] [PubMed] [Google Scholar]
- 652.Johnson DS, Mortazavi A, Myers RM, Wold B. Genome-wide mapping of in vivo protein-dna interactions. Science (New York, NY) 316: 1497–1502, 2007. DOI: 10.1126/science.1141319. [DOI] [PubMed] [Google Scholar]
- 653.Johnson NA, van Overbeek D, Chapman PG, Thompson MW, Sachinwalla T, George J. Effect of prolonged exercise and pre-exercise dietary manipulation on hepatic triglycerides in trained men. Eur J Appl Physiol 112: 1817–1825, 2012. DOI: 10.1007/s00421-011-2158-y. [DOI] [PubMed] [Google Scholar]
- 654.Jones LW, Habel LA, Weltzien E, Castillo A, Gupta D, Kroenke CH, Kwan ML, Quesenberry CP, Scott J, Sternfeld B, Yu A, Kushi LH, Caan BJ. Exercise and risk of cardiovascular events in women with nonmetastatic breast cancer. J Clin Oncol 34: 2743–2749, 2016. DOI: 10.1200/JCO.2015.65.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Jonge XJD, Thompson B, Han A. Methodological recommendations for menstrual cycle research in sports and exercise. Med Sci Sports Exerc 51: 2610–2617, 2019. DOI: 10.1249/MSS.0000000000002073. [DOI] [PubMed] [Google Scholar]
- 656.Joseph A-M, Adhihetty PJ, Leeuwenburgh C. Beneficial effects of exercise on age-related mitochondrial dysfunction and oxidative stress in skeletal muscle. J Physiol 594: 5105–5123, 2016. DOI: 10.1113/JP270659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Joyner MJ. Standing up for exercise: Should deconditioning be medicalized? J Physiol 590: 3413–3414, 2012. DOI: 10.1113/jphysiol.2012.238550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Joyner MJ. Bengt saltin and exercise physiology: A perspective. Appl Physiol Nutr Metab 42: 101–103, 2017. DOI: 10.1139/apnm-2016-0314. [DOI] [PubMed] [Google Scholar]
- 659.Joyner MJ, Dietz NM, Shepherd JT. From belfast to mayo and beyond: The use and future of plethysmography to study blood flow in human limbs. J Appl Physiol (1985) 91: 2431–2441, 2001. DOI: 10.1152/jappl.2001.91.6.2431. [DOI] [PubMed] [Google Scholar]
- 660.Joyner M, Kjaer M, Larsen PO. The copenhagen muscle research centre (cmrc) 1994–2004. Scand J Med Sci Sports 25 (Suppl 4): 22–28, 2015. DOI: 10.1111/sms.12598. [DOI] [PubMed] [Google Scholar]
- 661.Juneja P, Munjal A, Hubbard JB. Anatomy, joints [Online]. In: StatPearls. StatPearls Publishing, 2020. http://www.ncbi.nlm.nih.gov/books/NBK507893/ [1 Oct. 2020]. [PubMed] [Google Scholar]
- 662.Jung TW, Park HS, Choi GH, Kim D, Lee T. B-aminoisobutyric acid attenuates lps-induced inflammation and insulin resistance in adipocytes through ampk-mediated pathway. J Biomed Sci 25: 27, 2018. DOI: 10.1186/s12929-018-0431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Kadi F, Charifi N, Denis C, Lexell J, Andersen JL, Schjerling P, Olsen S, Kjaer M. The behaviour of satellite cells in response to exercise: What have we learned from human studies? Pflugers Arch—Eur J Physiol 451: 319–327, 2005. DOI: 10.1007/s00424-005-1406-6. [DOI] [PubMed] [Google Scholar]
- 664.Kadi F, Schjerling P, Andersen LL, Charifi N, Madsen JL, Christensen LR, Andersen JL. The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol 558: 1005–1012, 2004. DOI: 10.1113/jphysiol.2004.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.Kadir FH, Moore GR. Bacterial ferritin contains 24 haem groups. FEBS Lett 271: 141–143, 1990. DOI: 10.1016/0014-5793(90)80391-u. [DOI] [PubMed] [Google Scholar]
- 666.Kallwitz ER. Sarcopenia and liver transplant: The relevance of too little muscle mass. World J Gastroenterol 21: 10982–10993, 2015. DOI: 10.3748/wjg.v21.i39.10982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667.Kaminsky LA, Arena R, Myers J. Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing: Data from the fitness registry and the importance of exercise national database. Mayo Clin Proc 90: 1515–1523, 2015. DOI: 10.1016/j.mayocp.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Kan L, Zhang J, Yang Y, Wang P. The effects of yoga on pain, mobility, and quality of life in patients with knee osteoarthritis: A systematic review. Evid Based Complement Alternat Med 2016: 6016532, 2016. DOI: 10.1155/2016/6016532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Kanaley JA, Shadid S, Sheehan MT, Guo Z, Jensen MD. Relationship between plasma free fatty acid, intramyocellular triglycerides and long-chain acylcarnitines in resting humans. J Physiol 587: 5939–5950, 2009. DOI: 10.1113/jphysiol.2009.180695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Kanis JA, Cooper C, Rizzoli R, Reginster J-Y. Review of the guideline of the american college of physicians on the treatment of osteoporosis. Osteoporos Int 29: 1505–1510, 2018. DOI: 10.1007/s00198-018-4504-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Kannangara TS, Vani MA. The muscles’ grip on neurogenesis: Contributions of skeletal muscle-derived vascular endothelial growth factor to running-induced stem cell proliferation. J Physiol 595: 6821–6822, 2017. DOI: 10.1113/JP275251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672.Karamanou M, Androutsos G. Antoine-laurent de lavoisier (1743–1794) and the birth of respiratory physiology. Thorax 68: 978–979, 2013. DOI: 10.1136/thoraxjnl-2013-203840. [DOI] [PubMed] [Google Scholar]
- 673.Karapolat H, Engin C, Eroglu M, Yagdi T, Zoghi M, Nalbantgil S, Durmaz B, Kirazlι Y, Ozbaran M. Efficacy of the cardiac rehabilitation program in patients with end-stage heart failure, heart transplant patients, and left ventricular assist device recipients. Transplant Proc 45: 3381–3385, 2013. DOI: 10.1016/j.transproceed.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 674.Karinkanta S, Heinonen A, Sievänen H, Uusi-Rasi K, Pasanen M, Ojala K, Fogelholm M, Kannus P. A multi-component exercise regimen to prevent functional decline and bone fragility in home-dwelling elderly women: Randomized, controlled trial. Osteoporos Int 18: 453–462, 2007. DOI: 10.1007/s00198-006-0256-1. [DOI] [PubMed] [Google Scholar]
- 675.Karlamangla AS, Burnett-Bowie S-AM, Crandall CJ. Bone health during the menopause transition and beyond. Obstet Gynecol Clin N Am 45: 695–708, 2018. DOI: 10.1016/j.ogc.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.Katzmarzyk PT, Leon AS, Wilmore JH, Skinner JS, Rao DC, Rankinen T, Bouchard C. Targeting the metabolic syndrome with exercise: Evidence from the heritage family study. Med Sci Sports Exerc 35: 1703–1709, 2003. DOI: 10.1249/01.MSS.0000089337.73244.9B. [DOI] [PubMed] [Google Scholar]
- 677.Kaushal N, Rhodes RE, Meldrum JT, Spence JC. The role of habit in different phases of exercise. Br J Health Psychol 22: 429–448, 2017. DOI: 10.1111/bjhp.12237. [DOI] [PubMed] [Google Scholar]
- 678.Kavazis AN. Exercise preconditioning of the myocardium. Sports Med 39: 923–935, 2009. DOI: 10.2165/11317870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 679.Kelley E, Imboden MT, Harber MP, Finch H, Kaminsky LA, Whaley MH. Cardiorespiratory fitness is inversely associated with clustering of metabolic syndrome risk factors: The ball state adult fitness program longitudinal lifestyle study. Mayo Clin Proc Innov Qual Outcomes 2: 155–164, 2018. DOI: 10.1016/j.mayocpiqo.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.Kelley GA, Kelley KS, Tran ZV. Resistance training and bone mineral density in women: A meta-analysis of controlled trials. Am J Phys Med Rehabil 80: 65–77, 2001. DOI: 10.1097/00002060-200101000-00017. [DOI] [PubMed] [Google Scholar]
- 681.Kelley GA, Kelley KS, Callahan LF. Aerobic exercise and fatigue in rheumatoid arthritis participants: A meta-analysis using the minimal important difference approach. Arthritis Care Res 70: 1735–1739, 2018. DOI: 10.1002/acr.23570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682.Kelly NA, Ford MP, Standaert DG, Watts RL, Bickel CS, Moellering DR, Tuggle SC, Williams JY, Lieb L, Windham ST, Bamman MM. Novel, high-intensity exercise prescription improves muscle mass, mitochondrial function, and physical capacity in individuals with parkinson’s disease. J Appl Physiol (1985) 116: 582–592, 2014. DOI: 10.1152/japplphysiol.01277.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683.Kelly NA, Hammond KG, Bickel CS, Windham ST, Tuggle SC, Bamman MM. Effects of aging and parkinson’s disease on motor unit remodeling: Influence of resistance exercise training. J Appl Physiol (1985) 124: 888–898, 2018. DOI: 10.1152/japplphysiol.00563.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Kelly NA, Hammond KG, Stec MJ, Bickel CS, Windham ST, Tuggle SC, Bamman MM. Quantification and characterization of grouped type i myofibers in human aging. Muscle Nerve 57: E52–E59, 2018. DOI: 10.1002/mus.25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685.Kelly NA, Wood KH, Allendorfer JB, Ford MP, Bickel CS, Marstrander J, Amara AW, Anthony T, Bamman MM, Skidmore FM. High-intensity exercise acutely increases substantia nigra and prefrontal brain activity in parkinson’s disease. Med Sci Monit 23: 6064–6071, 2017. DOI: 10.12659/msm.906179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686.Kemi OJ, Haram PM, Wisløff U, Ellingsen Ø. Aerobic fitness is associated with cardiomyocyte contractile capacity and endothelial function in exercise training and detraining. Circulation 109: 2897–2904, 2004. DOI: 10.1161/01.CIR.0000129308.04757.72. [DOI] [PubMed] [Google Scholar]
- 687.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89: 2548–2556, 2004. DOI: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 688.Kersten S, Mandard S, Tan NS, Escher P, Metzger D, Chambon P, Gonzalez FJ, Desvergne B, Wahli W. Characterization of the fasting-induced adipose factor fiaf, a novel peroxisome proliferator-activated receptor target gene. J Biol Chem 275: 28488–28493, 2000. DOI: 10.1074/jbc.M004029200. [DOI] [PubMed] [Google Scholar]
- 689.Kessler RC, Angermeyer M, Anthony JC, Graaf RD, Demyttenaere K, Gasquet I, Girolamo GD, Gluzman S, Gureje O, Haro JM, Kawakami N, Karam A, Levinson D, Mora MEM, Browne MAO, Posada-Villa J, Stein DJ, Tsang CHA, Aguilar-Gaxiola S, Alonso J, Lee S, Heeringa S, Pennell B-E, Berglund P, Gruber MJ, Petukhova M, Chatterji S, Ustün TB. Lifetime prevalence and age-of-onset distributions of mental disorders in the world health organization’s world mental health survey initiative [Online]. World Psychiatry 6: 168–176, 2007. http://www.ncbi.nlm.nih.gov/pubmed/18188442. [PMC free article] [PubMed] [Google Scholar]
- 690.Khan MM, Lustrino D, Silveira WA, Wild F, Straka T, Issop Y, O’Connor E, Cox D, Reischl M, Marquardt T, Labeit D, Labeit S, Benoit E, Molg’o J, Lochmüller H, Witzemann V, Kettelhut IC, Navegantes LCC, Pozzan T, Rudolf R. Sympathetic innervation controls homeostasis of neuromuscular junctions in health and disease. Proc Natl Acad Sci U S A 113: 746–750, 2016. DOI: 10.1073/pnas.1524272113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691.Kharraz Y, Guerra J, Mann CJ, Serrano AL, Muñoz-C’anoves P. Macrophage plasticity and the role of inflammation in skeletal muscle repair. Mediat Inflamm 2013: 491497, 2013. DOI: 10.1155/2013/491497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Khosla S, Shane E. A crisis in the treatment of osteoporosis. J Bone Miner Res Off J Am Soc Bone Miner Res 31: 1485–1487, 2016. DOI: 10.1002/jbmr.2888. [DOI] [PubMed] [Google Scholar]
- 693.Kiens B, Alsted TJ, Jeppesen J. Factors regulating fat oxidation in human skeletal muscle. Obes Rev 12: 852–858, 2011. DOI: 10.1111/j.1467-789X.2011.00898.x. [DOI] [PubMed] [Google Scholar]
- 694.Kiens B, Richter EA. Utilization of skeletal muscle triacylglycerol during postexercise recovery in humans. Am J Phys 275: E332–E337, 1998. DOI: 10.1152/ajpendo.1998.275.2.E332. [DOI] [PubMed] [Google Scholar]
- 695.Kilic M Effect of fatiguing bicycle exercise on thyroid hormone and testosterone levels in sedentary males supplemented with oral zinc [Online]. Neuro Endocrinol Lett 28: 681–685, 2007. http://www.ncbi.nlm.nih.gov/pubmed/17984944. [PubMed] [Google Scholar]
- 696.Kim I, Kim HG, Kim H, Kim HH, Park SK, Uhm CS, Lee ZH, Koh GY. Hepatic expression, synthesis and secretion of a novel fibrinogen/angiopoietin-related protein that prevents endothelial-cell apoptosis [Online]. Biochem J 346 (Pt 3): 603–610, 2000. http://www.ncbi.nlm.nih.gov/pubmed/10698685. [PMC free article] [PubMed] [Google Scholar]
- 697.Kim J, Wende AR, Sena S, Theobald HA, Soto J, Sloan C, Wayment BE, Litwin SE, Holzenberger M, LeRoith D, Abel ED. Insulin-like growth factor i receptor signaling is required for exercise-induced cardiac hypertrophy. Mol Endocrinol 22: 2531–2543, 2008. DOI: 10.1210/me.2008-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698.Kim J, Son W-M Iii RJH, Pekas EJ, Noble JM, Park S-Y. The effects of a 12-week jump rope exercise program on body composition, insulin sensitivity, and academic self-efficacy in obese adolescent girls. J Pediatr Endocrinol Metab 33: 129–137, 2020. DOI: 10.1515/jpem-2019-0327. [DOI] [PubMed] [Google Scholar]
- 699.Kim Y, White T, Wijndaele K, Westgate K, Sharp SJ, Helge JW, Wareham NJ, Brage S. The combination of cardiorespiratory fitness and muscle strength, and mortality risk. Eur J Epidemiol 33: 953–964, 2018. DOI: 10.1007/s10654-018-0384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700.Kingsley JD, Figueroa A. Acute and training effects of resistance exercise on heart rate variability. Clin Physiol Funct Imaging 36: 179–187, 2016. DOI: 10.1111/cpf.12223. [DOI] [PubMed] [Google Scholar]
- 701.Kirk B, Feehan J, Lombardi G, Duque G. Muscle, bone, and fat crosstalk: The biological role of myokines, osteokines, and adipokines. Curr Osteoporos Rep 18: 388–400, 2020. DOI: 10.1007/s11914-020-00599-y. [DOI] [PubMed] [Google Scholar]
- 702.Kirk B, Saedi AA, Duque G. Osteosarcopenia: A case of geroscience. Aging Med 2: 147–156, 2019. DOI: 10.1002/agm2.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703.Kirkham AA, Eves ND, Shave RE, Bland KA, Bovard J, Gelmon KA, Virani SA, McKenzie DC, Stöhr EJ, Waburton DER, Campbell KL. The effect of an aerobic exercise bout 24 h prior to each doxorubicin treatment for breast cancer on markers of cardiotoxicity and treatment symptoms: A rct. Breast Cancer Res Treat 167: 719–729, 2018. DOI: 10.1007/s10549-017-4554-4. [DOI] [PubMed] [Google Scholar]
- 704.Kirwan JP, Sacks J, Nieuwoudt S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin J Med 84: S15–S21, 2017. DOI: 10.3949/ccjm.84.s1.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Kirwan JP, Solomon TPJ, Wojta DM, Staten MA, Holloszy JO. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Phys Endocrinol Metab 297: E151–E156, 2009. DOI: 10.1152/ajpendo.00210.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 706.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA 288: 2144–2150, 2002. DOI: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 707.Kjaer M, Engfred K, Fernandes A, Secher NH, Galbo H. Regulation of hepatic glucose production during exercise in humans: Role of sympathoadrenergic activity. Am J Phys 265: E275–E283, 1993. DOI: 10.1152/ajpendo.1993.265.2.E275. [DOI] [PubMed] [Google Scholar]
- 708.Klausen K, Andersen LB, Pelle I. Adaptive changes in work capacity, skeletal muscle capillarization and enzyme levels during training and detraining. Acta Physiol Scand 113: 9–16, 1981. DOI: 10.1111/j.1748-1716.1981.tb06854.x. [DOI] [PubMed] [Google Scholar]
- 709.Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT. Synaptogenesis and fos expression in the motor cortex of the adult rat after motor skill learning [Online]. J Neurosci 16: 4529–4535, 1996. http://www.ncbi.nlm.nih.gov/pubmed/8699262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Klein MB, Yalamanchi N, Pham H, Longaker MT, Chang J. Flexor tendon healing in vitro: Effects of tgf-beta on tendon cell collagen production. J Hand Surg Am 27: 615–620, 2002. DOI: 10.1053/jhsu.2002.34004. [DOI] [PubMed] [Google Scholar]
- 711.Klop C, van Staa TP, Cooper C, Harvey NC, de Vries F. The epidemiology of mortality after fracture in england: Variation by age, sex, time, geographic location, and ethnicity. Osteoporos Int 28: 161–168, 2017. DOI: 10.1007/s00198-016-3787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 712.Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: A systematic review of randomized and controlled clinical trials. J Clin Oncol 23: 3830–3842, 2005. DOI: 10.1200/JCO.2005.02.148. [DOI] [PubMed] [Google Scholar]
- 713.Knowlton AA, Lee AR. Estrogen and the cardiovascular system. Pharmacol Ther 135: 54–70, 2012. DOI: 10.1016/j.pharmthera.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 714.Kocahan S, Dundar A. Effects of different exercise loads on the thyroid hormone levels and serum lipid profile in swimmers. Horm Mol Biol Clin Invest 38, 2018. DOI: 10.1515/hmbci-2018-0025. [DOI] [PubMed] [Google Scholar]
- 715.Kohrt WM, Barry DW, Schwartz RS. Muscle forces or gravity: What predominates mechanical loading on bone? Med Sci Sports Exerc 41: 2050–2055, 2009. DOI: 10.1249/MSS.0b013e3181a8c717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 716.Kohut ML, McCann DA, Russell DW, Konopka DN, Cunnick JE, Franke WD, Castillo MC, Reighard AE, Vanderah E. Aerobic exercise, but not flexibility/resistance exercise, reduces serum il-18, crp, and il-6 independent of beta-blockers, bmi, and psychosocial factors in older adults. Brain Behav Immun 20: 201–209, 2006. DOI: 10.1016/j.bbi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 717.Kokic IS, Ivanisevic M, Biolo G, Simunic B, Kokic T, Pisot R. Combination of a structured aerobic and resistance exercise improves glycaemic control in pregnant women diagnosed with gestational diabetes mellitus. A randomised controlled trial. Women Birth 31: e232–e238, 2018. DOI: 10.1016/j.wombi.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 718.Kokkinos PF, Faselis C, Myers J, Narayan P, Sui X, Zhang J, Lavie CJ, Moore H, Karasik P, Fletcher R. Cardiorespiratory fitness and incidence of major adverse cardiovascular events in us veterans: A cohort study. Mayo Clin Proc 92: 39–48, 2017. DOI: 10.1016/j.mayocp.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 719.Konda NN, Karri RS, Winnard A, Nasser M, Evetts S, Boudreau E, Caplan N, Gradwell D, Velho RM. A comparison of exercise interventions from bed rest studies for the prevention of musculoskeletal loss. NPJ Microgravity 5: 12, 2019. DOI: 10.1038/s41526-019-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720.Kongsgaard M, Reitelseder S, Pedersen TG, Holm L, Aagaard P, Kjaer M, Magnusson SP. Region specific patellar tendon hypertrophy in humans following resistance training. Acta Physiol (Oxf) 191: 111–121, 2007. DOI: 10.1111/j.1748-1716.2007.01714.x. [DOI] [PubMed] [Google Scholar]
- 721.Konopka AR, Suer MK, Wolff CA, Harber MP. Markers of human skeletal muscle mitochondrial biogenesis and quality control: Effects of age and aerobic exercise training. J Gerontol A Biol Sci Med Sci 69: 371–378, 2014. DOI: 10.1093/gerona/glt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722.Konopka AR, Wolff CA, Suer MK, Harber MP. Relationship between intermuscular adipose tissue infiltration and myostatin before and after aerobic exercise training. Am J Physiol Regul Integr Comp Physiol 315: R461–R468, 2018. DOI: 10.1152/ajpregu.00030.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723.Konstam MA, Kiernan MS, Bernstein D, Bozkurt B, Jacob M, Kapur NK, Kociol RD, Lewis EF, Mehra MR, Pagani FD, Raval AN, Ward C. Evaluation and management of right-sided heart failure: A scientific statement from the american heart association. Circulation 137: e578–e622, 2018. DOI: 10.1161/CIR.0000000000000560. [DOI] [PubMed] [Google Scholar]
- 724.Kopin L, Lowenstein C. Dyslipidemia. Ann Intern Med 167: ITC81–ITC96, 2017. DOI: 10.7326/AITC201712050. [DOI] [PubMed] [Google Scholar]
- 725.Kosaki K, Kamijo-Ikemori A, Sugaya T, Tanahashi K, Sawano Y, Akazawa N, Ra S-G, Kimura K, Shibagaki Y, Maeda S. Effect of habitual exercise on urinary liver-type fatty acid-binding protein levels in middle-aged and older adults. Scand J Med Sci Sports 28: 152–160, 2018. DOI: 10.1111/sms.12867. [DOI] [PubMed] [Google Scholar]
- 726.Koster A, Ding J, Stenholm S, Caserotti P, Houston DK, Nicklas BJ, You T, Lee JS, Visser M, Newman AB, Schwartz AV, Cauley JA, Tylavsky FA, Goodpaster BH, Kritchevsky SB, Harris TB. Does the amount of fat mass predict age-related loss of lean mass, muscle strength, and muscle quality in older adults? J Gerontol A Biol Sci Med Sci 66: 888–895, 2011. DOI: 10.1093/gerona/glr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727.Kouvelioti R, Kurgan N, Falk B, Ward WE, Josse AR, Klentrou P. Response of sclerostin and bone turnover markers to high intensity interval exercise in young women: Does impact matter? Biomed Res Int 2018: 4864952, 2018. DOI: 10.1155/2018/4864952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 728.Kouvelioti R, LeBlanc P, Falk B, Ward WE, Josse AR, Klentrou P. Effects of high-intensity interval running versus cycling on sclerostin, and markers of bone turnover and oxidative stress in young men. Calcif Tissue Int 104: 582–590, 2019. DOI: 10.1007/s00223-019-00524-1. [DOI] [PubMed] [Google Scholar]
- 729.Koves TR, Sparks LM, Kovalik JP, Mosedale M, Arumugam R, DeBalsi KL, Everingham K, Thorne L, Phielix E, Meex RC, Kien CL, Hesselink MKC, Schrauwen P, Muoio DM. PPARÎ3 coactivator-1α contributes to exercise-induced regulation of intramuscular lipid droplet programming in mice and humans. J Lipid Res 54: 522–534, 2013. DOI: 10.1194/jlr.P028910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 730.Kraemer WJ, Ratamess NA, Flanagan SD, Shurley JP, Todd JS, Todd TC. Understanding the science of resistance training: An evolutionary perspective. Sports Med 47: 2415–2435, 2017. DOI: 10.1007/s40279-017-0779-y. [DOI] [PubMed] [Google Scholar]
- 731.Kram R, Roberts TJ. A. V. Hill sticks his neck out. J Exp Biol 219: 468–469, 2016. DOI: 10.1242/jeb.123372. [DOI] [PubMed] [Google Scholar]
- 732.Kraniou GN, Cameron-Smith D, Hargreaves M. Effect of short-term training on glut-4 mRNA and protein expression in human skeletal muscle. Exp Physiol 89: 559–563, 2004. DOI: 10.1113/expphysiol.2004.027409. [DOI] [PubMed] [Google Scholar]
- 733.Kristensen MD, Petersen SM, Møller KE, Lund MT, Hansen M, Hansen CN, Courraud J, Helge JW, Dela F, Prats C. Obesity leads to impairments in the morphology and organization of human skeletal muscle lipid droplets and mitochondrial networks, which are resolved with gastric bypass surgery-induced improvements in insulin sensitivity. Acta Physiol (Oxf) 224: e13100, 2018. DOI: 10.1111/apha.13100. [DOI] [PubMed] [Google Scholar]
- 734.Kruse R, Pedersen AJT, Kristensen JM, Petersson SJ, Wojtaszewski JFP, Højlund K. Intact initiation of autophagy and mitochondrial fission by acute exercise in skeletal muscle of patients with typeâ 2 diabetes. Clin Sci (Lond) 131: 37–47, 2017. DOI: 10.1042/CS20160736. [DOI] [PubMed] [Google Scholar]
- 735.Ku P-W, Fox KR, Gardiner PA, Chen L-J. Late-life exercise and difficulty with activities of daily living: An 8-year nationwide follow-up study in taiwan. Ann Behav Med 50: 237–246, 2016. DOI: 10.1007/s12160-015-9749-5. [DOI] [PubMed] [Google Scholar]
- 736.Kubo K, Kanehisa H, Fukunaga T. Effects of resistance and stretching training programmes on the viscoelastic properties of human tendon structures in vivo. J Physiol 538: 219–226, 2002. DOI: 10.1113/jphysiol.2001.012703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 737.Kuipers SD, Bramham CR. Brain-derived neurotrophic factor mechanisms and function in adult synaptic plasticity: New insights and implications for therapy [Online]. Curr Opin Drug Discov Devel 9: 580–586, 2006. http://www.ncbi.nlm.nih.gov/pubmed/17002218. [PubMed] [Google Scholar]
- 738.Kukuljan S, Nowson CA, Bass SL, Sanders K, Nicholson GC, Seibel MJ, Salmon J, Daly RM. Effects of a multi-component exercise program and calcium-vitamin-d3-fortified milk on bone mineral density in older men: A randomised controlled trial. Osteoporos Int 20: 1241–1251, 2009. DOI: 10.1007/s00198-008-0776-y. [DOI] [PubMed] [Google Scholar]
- 739.Kumar A, Accorsi A, Rhee Y, Girgenrath M. Do’s and don’ts in the preparation of muscle cryosections for histological analysis. J Vis Exp: e52793, 2015. DOI: 10.3791/52793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740.Kumar S, Kelly AS. Review of childhood obesity: From epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin Proc 92: 251–265, 2017. DOI: 10.1016/j.mayocp.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 741.Kumar V, Atherton P, Smith K, Rennie MJ. Human muscle protein synthesis and breakdown during and after exercise. J Appl Physiol (1985) 106: 2026–2039, 2009. DOI: 10.1152/japplphysiol.91481.2008. [DOI] [PubMed] [Google Scholar]
- 742.Kusminski CM, Bickel PE, Scherer PE. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat Rev Drug Discov 15: 639–660, 2016. DOI: 10.1038/nrd.2016.75. [DOI] [PubMed] [Google Scholar]
- 743.Kvam S, Kleppe CL, Nordhus IH, Hovland A. Exercise as a treatment for depression: A meta-analysis. J Affect Disord 202: 67–86, 2016. DOI: 10.1016/j.jad.2016.03.063. [DOI] [PubMed] [Google Scholar]
- 744.Lafontan M, Moro C, Berlan M, Crampes F, Sengenes C, Galitzky J. Control of lipolysis by natriuretic peptides and cyclic gmp. Trends Endocrinol Metab 19: 130–137, 2008. DOI: 10.1016/j.tem.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 745.Lai X, Price C, Lu XL, Wang L. Imaging and quantifying solute transport across periosteum: Implications for muscle-bone crosstalk. Bone 66: 82–89, 2014. DOI: 10.1016/j.bone.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 746.Lane NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol 194: S3–S11, 2006. DOI: 10.1016/j.ajog.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 747.Langberg H, Skovgaard D, Asp S, Kjaer M. Time pattern of exercise-induced changes in type i collagen turnover after prolonged endurance exercise in humans. Calcif Tissue Int 67: 41–44, 2000. DOI: 10.1007/s00223001094. [DOI] [PubMed] [Google Scholar]
- 748.Langberg H, Skovgaard D, Petersen LJ, Bulow J, Kjaer M. Type i collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J Physiol 521 (Pt 1): 299–306, 1999. DOI: 10.1111/j.1469-7793.1999.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 749.Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS. Endurance exercise as a countermeasure for aging. Diabetes 57: 2933–2942, 2008. DOI: 10.2337/db08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 750.Larmer PJ, Reay ND, Aubert ER, Kersten P. Systematic review of guidelines for the physical management of osteoarthritis. Arch Phys Med Rehabil 95: 375–389, 2014. DOI: 10.1016/j.apmr.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 751.Larssen P, Wik L, Czarnewski P, Eldh M, Löf L, Ronquist KG, Dubois L, Freyhult E, Gallant CJ, Oelrich J, Larsson A, Ronquist G, Villablanca EJ, Landegren U, Gabrielsson S, Kamali-Moghaddam M. Tracing cellular origin of human exosomes using multiplex proximity extension assays. Mol Cell Proteomics 16: 502–511, 2017. DOI: 10.1074/mcp.M116.064725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 752.Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, Kirkland JL, Sandri M. Sarcopenia: Aging-related loss of muscle mass and function. Physiol Rev 99: 427–511, 2019. DOI: 10.1152/physrev.00061.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 753.Larsson L, Moss RL. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol 472: 595–614, 1993. DOI: 10.1113/jphysiol.1993.sp019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 754.Lauche R, Hunter DJ, Adams J, Cramer H. Yoga for osteoarthritis: A systematic review and meta-analysis. Curr Rheumatol Rep 21: 47, 2019. DOI: 10.1007/s11926-019-0846-5. [DOI] [PubMed] [Google Scholar]
- 755.Laufs U, Urhausen A, Werner N, Scharhag J, Heitz A, Kissner G, Böhm M, Kindermann W, Nickenig G. Running exercise of different duration and intensity: Effect on endothelial progenitor cells in healthy subjects. Eur J Cardiovasc Prev Rehabil 12: 407–414, 2005. DOI: 10.1097/01.hjr.0000174823.87269.2e. [DOI] [PubMed] [Google Scholar]
- 756.Laughlin MH, Armstrong RB. Muscle blood flow during locomotory exercise [Online]. Exerc Sport Sci Rev 13: 95–136, 1985. http://www.ncbi.nlm.nih.gov/pubmed/3891377. [PubMed] [Google Scholar]
- 757.Lavin KM, Perkins RK, Jemiolo B, Raue U, Trappe SW, Trappe TA. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation. J Appl Physiol (1985) 128: 87–99, 2020. DOI: 10.1152/japplphysiol.00495.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 758.Lavin KM, Roberts BM, Fry CS, Moro T, Rasmussen BB, Bamman MM. The importance of resistance exercise training to combat neuromuscular aging. Physiology (Bethesda) 34: 112–122, 2019. DOI: 10.1152/physiol.00044.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 759.Lavin KM, Sealfon SC, McDonald M-LN, Roberts BM, Wilk K, Nair VD, Ge Y, Kumar PL, Windham ST, Bamman MM. Skeletal muscle transcriptional networks linked to type i myofiber grouping in parkinson’s disease. J Appl Physiol (1985) 128: 229–240, 2020. DOI: 10.1152/japplphysiol.00702.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 760.Lawrie RA. The activity of the cytochrome system in muscle and its relation to myoglobin. Biochem J 55: 298–305, 1953. DOI: 10.1042/bj0550298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 761.Leal DV, Ferreira A, Watson EL, Wilund KR, Viana JL. Muscle-bone crosstalk in chronic kidney disease: The potential modulatory effects of exercise. Calcif Tissue Int 108: 461–475, 2021. [DOI] [PubMed] [Google Scholar]
- 762.Leal LG, Lopes MA, Batista ML. Physical exercise-induced myokines and muscle-adipose tissue crosstalk: A review of current knowledge and the implications for health and metabolic diseases. Front Physiol 9: 1307, 2018. DOI: 10.3389/fphys.2018.01307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 763.LeBlanc AD, Spector ER, Evans HJ, Sibonga JD. Skeletal responses to space flight and the bed rest analog: A review [Online]. J Musculoskelet Neuronal Interact 7: 33–47, 2007. http://www.ncbi.nlm.nih.gov/pubmed/17396004. [PubMed] [Google Scholar]
- 764.Leblanc A, Matsumoto T, Jones J, Shapiro J, Lang T, Shackelford L, Smith SM, Evans H, Spector E, Ploutz-Snyder R, Sibonga J, Keyak J, Nakamura T, Kohri K, Ohshima H. Bisphosphonates as a supplement to exercise to protect bone during long-duration spaceflight. Osteoporos Int 24: 2105–2114, 2013. DOI: 10.1007/s00198-012-2243-z. [DOI] [PubMed] [Google Scholar]
- 765.Lee EY, Yoon K-H. Epidemic obesity in children and adolescents: Risk factors and prevention. Front Med 12: 658–666, 2018. DOI: 10.1007/s11684-018-0640-1. [DOI] [PubMed] [Google Scholar]
- 766.Lee I-M, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 380: 219–229, 2012. DOI: 10.1016/s0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 767.Lee JH, Jun H-S. Role of myokines in regulating skeletal muscle mass and function. Front Physiol 10: 42, 2019. DOI: 10.3389/fphys.2019.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 768.Lee S, Norheim F, Gulseth HL, Langleite TM, Aker A, Gundersen TE, Holen T, Birkeland KI, Drevon CA. Skeletal muscle phosphatidylcholine and phosphatidylethanolamine respond to exercise and influence insulin sensitivity in men. Sci Rep 8: 6531, 2018. DOI: 10.1038/s41598-018-24976-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 769.Lee S, Arslanian S. Body composition and cardiorespiratory fitness between metabolically healthy versus metabolically unhealthy obese black and white adolescents. J Adolesc Health 64: 327–332, 2019. DOI: 10.1016/j.jadohealth.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 770.Lee S, Bacha F, Gungor N, Arslanian SA. Cardiorespiratory fitness in youth: Relationship to insulin sensitivity and beta-cell function. Obesity 14: 1579–1585, 2006. DOI: 10.1038/oby.2006.182. [DOI] [PubMed] [Google Scholar]
- 771.Lee S, Bacha F, Hannon T, Kuk JL, Boesch C, Arslanian S. Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys: A randomized, controlled trial. Diabetes 61: 2787–2795, 2012. DOI: 10.2337/db12-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 772.Lee S, Deldin AR, White D, Kim Y, Libman I, Rivera-Vega M, Kuk JL, Sandoval S, Boesch C, Arslanian S. Aerobic exercise but not resistance exercise reduces intrahepatic lipid content and visceral fat and improves insulin sensitivity in obese adolescent girls: A randomized controlled trial. Am J Phys Endocrinol Metab 305: E1222–E1229, 2013. DOI: 10.1152/ajpendo.00285.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 773.Lee Y, Park HK, Kim WY, Kim MC, Jung W, Ko BS. Muscle mass depletion associated with poor outcome of sepsis in the emergency department. Ann Nutr Metab 72: 336–344, 2018. DOI: 10.1159/000488994. [DOI] [PubMed] [Google Scholar]
- 774.Lee Y-H, Mottillo EP, Granneman JG. Adipose tissue plasticity from wat to bat and in between. Biochim Biophys Acta 1842: 358–369, 2014. DOI: 10.1016/j.bbadis.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 775.Leek JT. Svaseq: Removing batch effects and other unwanted noise from sequencing data. Nucleic Acids Res 42, 2014. DOI: 10.1093/nar/gku864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 776.Lees SJ, Booth FW. Physical inactivity is a disease. World Rev Nutr Diet 95: 73–79, 2005. DOI: 10.1159/000088274. [DOI] [PubMed] [Google Scholar]
- 777.Legerlotz K, Marzilger R, Bohm S, Arampatzis A. Physiological adaptations following resistance training in youth athletes-a narrative review. Pediatr Exerc Sci 28: 501–520, 2016. DOI: 10.1123/pes.2016-0023. [DOI] [PubMed] [Google Scholar]
- 778.Lehnig AC, Stanford KI. Exercise-induced adaptations to white and brown adipose tissue. J Exp Biol 221, 2018. DOI: 10.1242/jeb.161570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 779.Leick L, Hellsten Y, Fentz J, Lyngby SS, Wojtaszewski JFP, Hidalgo J, Pilegaard H. PGC-1alpha mediates exercise-induced skeletal muscle vegf expression in mice. Am J Phys Endocrinol Metab 297: E92–E103, 2009. DOI: 10.1152/ajpendo.00076.2009. [DOI] [PubMed] [Google Scholar]
- 780.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 141: 1117–1134, 2010. DOI: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 781.Lemoine JK, Lee JD, Trappe TA. Impact of sex and chronic resistance training on human patellar tendon dry mass, collagen content, and collagen cross-linking. Am J Physiol Regul Integr Comp Physiol 296: R119–R124, 2009. DOI: 10.1152/ajpregu.90607.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 782.Leone AM, Valgimigli M, Giannico MB, Zaccone V, Perfetti M, D’Amario D, Rebuzzi AG, Crea F. From bone marrow to the arterial wall: The ongoing tale of endothelial progenitor cells. Eur Heart J 30: 890–899, 2009. DOI: 10.1093/eurheartj/ehp078. [DOI] [PubMed] [Google Scholar]
- 783.Leong DJ, Hardin JA, Cobelli NJ, Sun HB. Mechanotransduction and cartilage integrity. Ann N Y Acad Sci 1240: 32–37, 2011. DOI: 10.1111/j.1749-6632.2011.06301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 784.Lerchenmüller C, Rosenzweig A. Mechanisms of exercise-induced cardiac growth. Drug Discov Today 19: 1003–1009, 2014. DOI: 10.1016/j.drudis.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 785.Leung AA, Daskalopoulou SS, Dasgupta K, McBrien K, Butalia S, Zarnke KB, Nerenberg K, Harris KC, Nakhla M, Cloutier L, Gelfer M, Lamarre-Cliche M, Milot A, Bolli P, Tremblay G, McLean D, Tran KC, Tobe SW, Ruzicka M, Burns KD, Vallée M, Prasad GVR, Gryn SE, Feldman RD, Selby P, Pipe A, Schiffrin EL, McFarlane PA, Oh P, Hegele RA, Khara M, Wilson TW, Penner SB, Burgess E, Sivapalan P, Herman RJ, Bacon SL, Rabkin SW, Gilbert RE, Campbell TS, Grover S, Honos G, Lindsay P, Hill MD, Coutts SB, Gubitz G, Campbell NRC, Moe GW, Howlett JG, Boulanger J-M, Prebtani A, Kline G, Leiter LA, Jones C, Côté A-M, Woo V, Kaczorowski J, Trudeau L, Tsuyuki RT, Hiremath S, Drouin D, Lavoie KL, Hamet P, Grégoire JC, Lewanczuk R, Dresser GK, Sharma M, Reid D, Lear SA, Moullec G, Gupta M, Magee LA, Logan AG, Dionne J, Fournier A, Benoit G, Feber J, Poirier L, Padwal RS, Rabi DM. Hypertension canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol 33: 557–576, 2017. DOI: 10.1016/j.cjca.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 786.Lewis GD, Farrell L, Wood MJ, Martinovic M, Arany Z, Rowe GC, Souza A, Cheng S, McCabe EL, Yang E, Shi X, Deo R, Roth FP, Asnani A, Rhee EP, Systrom DM, Semigran MJ, Vasan RS, Carr SA, Wang TJ, Sabatine MS, Clish CB, Gerszten RE. Metabolic signatures of exercise in human plasma. Sci Transl Med 2: 33ra37, 2010. DOI: 10.1126/scitranslmed.3001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 787.Lexell J, Downham DY. The occurrence of fibre-type grouping in healthy human muscle: A quantitative study of cross-sections of whole vastus lateralis from men between 15 and 83 years. Acta Neuropathol 81: 377–381, 1991. DOI: 10.1007/BF00293457. [DOI] [PubMed] [Google Scholar]
- 788.Lexell J, Henriksson-Larsén K, Sjöström M. Distribution of different fibre types in human skeletal muscles. 2. A study of cross-sections of whole m. Vastus lateralis. Acta Physiol Scand 117: 115–122, 1983. DOI: 10.1111/j.1748-1716.1983.tb07185.x. [DOI] [PubMed] [Google Scholar]
- 789.Lexell J, Taylor C. Fiber density: A fast and accurate way to estimate human muscle fiber areas [Online]. Muscle Nerve 14: 476–477, 1991. http://www.ncbi.nlm.nih.gov/pubmed/1870638. [PubMed] [Google Scholar]
- 790.Lexell J, Taylor CC. A morphometrical comparison of right and left whole human vastus lateralis muscle: How to reduce sampling errors in biopsy techniques. Clin Physiol 11: 271–276, 1991. DOI: 10.1111/j.1475-097x.1991.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 791.Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84: 275–294, 1988. DOI: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 792.Lexell J, Taylor C, Sjöström M. Analysis of sampling errors in biopsy techniques using data from whole muscle cross sections. J Appl Physiol (1985) 59: 1228–1235, 1985. DOI: 10.1152/jappl.1985.59.4.1228. [DOI] [PubMed] [Google Scholar]
- 793.Li G, Liu H, Ma C, Chen Y, Wang J, Yang Y. Exosomes are the novel players involved in the beneficial effects of exercise on type 2 diabetes. J Cell Physiol 234 (9): 14896–14905. [DOI] [PubMed] [Google Scholar]
- 794.Li L, Cheng S, Wang G, Duan G, Zhang Y. Tai chi chuan exercises improve functional outcomes and quality of life in patients with primary total knee arthroplasty due to knee osteoarthritis. Complement Ther Clin Pract 35: 121–125, 2019. DOI: 10.1016/j.ctcp.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 795.Li Y, Su Y, Chen S, Zhang Y, Zhang Z, Liu C, Lu M, Liu F, Li S, He Z, Wang Y, Sheng L, Wang W, Zhan Z, Wang X, Zheng N. The effects of resistance exercise in patients with knee osteoarthritis: A systematic review and meta-analysis. Clin Rehabil 30: 947–959, 2016. DOI: 10.1177/0269215515610039. [DOI] [PubMed] [Google Scholar]
- 796.Li Y, Hanssen H, Cordes M, Rossmeissl A, Endes S, Schmidt-Trucksäss A. Aerobic, resistance and combined exercise training on arterial stiffness in normotensive and hypertensive adults: A review. Eur J Sport Sci 15: 443–457, 2015. DOI: 10.1080/17461391.2014.955129. [DOI] [PubMed] [Google Scholar]
- 797.Liao P-C, Lin H-H, Chiang B-L, Lee J-H, Yu H-H, Lin Y-T, Yang YH, Li P-Y, Wang L-C, Sun W-Z. Tai chi chuan exercise improves lung function and asthma control through immune regulation in childhood asthma. Evid Based Complement Alternat Med 2019: 9146827, 2019. DOI: 10.1155/2019/9146827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 798.Liberman K, Forti LN, Beyer I, Bautmans I. The effects of exercise on muscle strength, body composition, physical functioning and the inflammatory profile of older adults: A systematic review. Curr Opin Clin Nutr Metab Care 20: 30–53, 2017. DOI: 10.1097/MCO.0000000000000335. [DOI] [PubMed] [Google Scholar]
- 799.Liebner S, Dijkhuizen RM, Reiss Y, Plate KH, Agalliu D, Constantin G. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol 135: 311–336, 2018. DOI: 10.1007/s00401-018-1815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 800.Lin H-C, Lin H-P, Yu H-H, Wang L-C, Lee J-H, Lin Y-T, Yang Y-H, Li P-Y, Sun W-Z, Chiang B-L. Tai-chi-chuan exercise improves pulmonary function and decreases exhaled nitric oxide level in both asthmatic and nonasthmatic children and improves quality of life in children with asthma. Evid Based Complement Alternat Med 2017: 6287642, 2017. DOI: 10.1155/2017/6287642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 801.Lin J, Wu H, Tarr PT, Zhang C-Y, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator pgc-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418: 797–801, 2002. DOI: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 802.Lin X, Eaton CB, Manson JE, Liu S. The genetics of physical activity. Curr Cardiol Rep 19: 119, 2017. DOI: 10.1007/s11886-017-0938-7. [DOI] [PubMed] [Google Scholar]
- 803.Lin X, Zhang X, Guo J, Roberts CK, McKenzie S, Wu W-C, Liu S, Song Y. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc 4, 2015. DOI: 10.1161/JAHA.115.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 804.Lin Y-Y, Lee S-D. Cardiovascular benefits of exercise training in postmenopausal hypertension. Int J Mol Sci 19, 2018. DOI: 10.3390/ijms19092523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 805.Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, Roy TA, Hurley BF. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol (1985) 83: 1581–1587, 1997. DOI: 10.1152/jappl.1997.83.5.1581. [DOI] [PubMed] [Google Scholar]
- 806.Lindsten J Schack August Steenberg Krogh – A versatile genius [Online]. The Nobel Prize, 2001. https://www.nobelprize.org/prizes/medicine/1920/krogh/article/. [Google Scholar]
- 807.Lira VA, Brown DL, Lira AK, Kavazis AN, Soltow QA, Zeanah EH, Criswell DS. Nitric oxide and ampk cooperatively regulate pgc-1 in skeletal muscle cells. J Physiol 588: 3551–3566, 2010. DOI: 10.1113/jphysiol.2010.194035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 808.Liss KHH, Finck BN. PPARs and nonalcoholic fatty liver disease. Biochimie 136: 65–74, 2017. DOI: 10.1016/j.biochi.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 809.Little JP, Safdar A, Benton CR, Wright DC. Skeletal muscle and beyond: The role of exercise as a mediator of systemic mitochondrial biogenesis. Appl Physiol Nutr Metab 36: 598–607, 2011. DOI: 10.1139/h11-076. [DOI] [PubMed] [Google Scholar]
- 810.Liu H-B, Yuan W-X, Wang Q-Y, Wang Y-X, Cao H-W, Xu J, Qin K-R. Carotid arterial stiffness and hemodynamic responses to acute cycling intervention at different times during 12-week supervised exercise training period. Biomed Res Int 2018: 2907548, 2018. DOI: 10.1155/2018/2907548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 811.Liu J-X, Zhu L, Li P-J, Li N, Xu Y-B. Effectiveness of high-intensity interval training on glycemic control and cardiorespiratory fitness in patients with type 2 diabetes: A systematic review and meta-analysis. Aging Clin Exp Res 31: 575–593, 2019. DOI: 10.1007/s40520-018-1012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 812.Liu PZ, Nusslock R. Exercise-mediated neurogenesis in the hippocampus via bdnf. Front Neurosci 12: 52, 2018. DOI: 10.3389/fnins.2018.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 813.Liu S, Zheng F, Cai Y, Zhang W, Dun Y. Effect of long-term exercise training on lncRNAs expression in the vascular injury of insulin resistance. J Cardiovasc Transl Res 11: 459–469, 2018. DOI: 10.1007/s12265-018-9830-0. [DOI] [PubMed] [Google Scholar]
- 814.Liu X, Xiao J, Zhu H, Wei X, Platt C, Damilano F, Xiao C, Bezzerides V, Boström P, Che L, Zhang C, Spiegelman BM, Rosenzweig A. MiR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab 21: 584–595, 2015. DOI: 10.1016/j.cmet.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 815.Liu-Ambrose TYL, Khan KM, Eng JJ, Heinonen A, McKay HA. Both resistance and agility training increase cortical bone density in 75- to 85-year-old women with low bone mass: A 6-month randomized controlled trial. J Clin Densitom 7: 390–398, 2004. DOI: 10.1385/jcd:7:4:390. [DOI] [PubMed] [Google Scholar]
- 816.Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol 12: 412–420, 2016. DOI: 10.1038/nrrheum.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 817.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum 64: 1697–1707, 2012. DOI: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 818.Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, Rao RR, Lou J, Lokurkar I, Baur W, Castellot JJ, Rosen ED, Spiegelman BM. A smooth muscle-like origin for beige adipocytes. Cell Metab 19: 810–820, 2014. DOI: 10.1016/j.cmet.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 819.Loprinzi PD. The effects of physical exercise on parahippocampal function. Physiol Int 106: 114–127, 2019. DOI: 10.1556/2060.106.2019.10. [DOI] [PubMed] [Google Scholar]
- 820.Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol (1985) 103: 1744–1751, 2007. DOI: 10.1152/japplphysiol.00679.2007. [DOI] [PubMed] [Google Scholar]
- 821.Lozano-Montoya I, Correa-Pérez A, Abraha I, Soiza RL, Cherubini A, O’Mahony D, Cruz-Jentoft AJ. Nonpharmacological interventions to treat physical frailty and sarcopenia in older patients: A systematic overview—the senator project ontop series. Clin Interv Aging 12: 721–740, 2017. DOI: 10.2147/CIA.S132496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 822.Lu M, Su Y, Zhang Y, Zhang Z, Wang W, He Z, Liu F, Li Y, Liu C, Wang Y, Sheng L, Zhan Z, Wang X, Zheng N. Effectiveness of aquatic exercise for treatment of knee osteoarthritis: Systematic review and meta-analysis. Z Rheumatol 74: 543–552, 2015. DOI: 10.1007/s00393-014-1559-9. [DOI] [PubMed] [Google Scholar]
- 823.Lucas JA, Moonie S, Hogan MB, Evans WN. Efficacy of an exercise intervention among children with comorbid asthma and obesity. Public Health 159: 123–128, 2018. DOI: 10.1016/j.puhe.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 824.Luden N, Hayes E, Galpin A, Minchev K, Jemiolo B, Raue U, Trappe TA, Harber MP, Bowers T, Trappe S. Myocellular basis for tapering in competitive distance runners. J Appl Physiol (1985) 108: 1501–1509, 2010. DOI: 10.1152/japplphysiol.00045.2010. [DOI] [PubMed] [Google Scholar]
- 825.Luden N, Minchev K, Hayes E, Louis E, Trappe T, Trappe S. Human vastus lateralis and soleus muscles display divergent cellular contractile properties. Am J Physiol Regul Integr Comp Physiol 295: R1593–R1598, 2008. DOI: 10.1152/ajpregu.90564.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 826.Ludyga S, Gronwald T, Hottenrott K. The athlete’s brain: Cross-sectional evidence for neural efficiency during cycling exercise. Neural Plast 2016: 4583674, 2016. DOI: 10.1155/2016/4583674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 827.Lundsgaard A-M, Fritzen AM, Kiens B. Molecular regulation of fatty acid oxidation in skeletal muscle during aerobic exercise. Trends Endocrinol Metab 29: 18–30, 2018. DOI: 10.1016/j.tem.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 828.Lynch NA, Metter EJ, Lindle RS, Fozard JL, Tobin JD, Roy TA, Fleg JL, Hurley BF. Muscle quality. I. Age-associated differences between arm and leg muscle groups. J Appl Physiol (1985) 86: 188–194, 1999. DOI: 10.1152/jappl.1999.86.1.188. [DOI] [PubMed] [Google Scholar]
- 829.Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a novel treatment for drug addiction: A neurobiological and stage-dependent hypothesis. Neurosci Biobehav Rev 37: 1622–1644, 2013. DOI: 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 830.Ma C, Wang J, Liu H, Chen Y, Ma X, Chen S, Chen Y, Bihl JI, Yang YI. Moderate exercise enhances endothelial progenitor cell exosomes release and function. Med Sci Sports Exerc 50: 2024–2032, 2018. DOI: 10.1249/MSS.0000000000001672. [DOI] [PubMed] [Google Scholar]
- 831.MacDonell CW, Gardiner PF. Mechanisms and functional implications of motoneuron adaptations to increased physical activity. Appl Physiol Nutr Metab 43: 1186–1193, 2018. DOI: 10.1139/apnm-2018-0185. [DOI] [PubMed] [Google Scholar]
- 832.Macefield VG, Knellwolf TP. Functional properties of human muscle spindles. J Neurophysiol 120: 452–467, 2018. DOI: 10.1152/jn.00071.2018. [DOI] [PubMed] [Google Scholar]
- 833.MacLean PS, Zheng D, Dohm GL. Muscle glucose transporter (glut 4) gene expression during exercise [Online]. Exerc Sport Sci Rev 28: 148–152, 2000. http://www.ncbi.nlm.nih.gov/pubmed/11064847. [PubMed] [Google Scholar]
- 834.MacPherson REK, Herbst EAF, Reynolds EJ, Vandenboom R, Roy BD, Peters SJ. Subcellular localization of skeletal muscle lipid droplets and plin family proteins oxpat and adrp at rest and following contraction in rat soleus muscle. Am J Physiol Regul Integr Comp Physiol 302: R29–R36, 2012. DOI: 10.1152/ajpregu.00163.2011. [DOI] [PubMed] [Google Scholar]
- 835.Macpherson REK, Huber JS, Frendo-Cumbo S, Simpson JA, Wright DC. Adipose tissue insulin action and il-6 signaling after exercise in obese mice. Med Sci Sports Exerc 47: 2034–2042, 2015. DOI: 10.1249/MSS.0000000000000660. [DOI] [PubMed] [Google Scholar]
- 836.MacVicar T, Langer T. OPA1 processing in cell death and disease—the long and short of it. J Cell Sci 129: 2297–2306, 2016. DOI: 10.1242/jcs.159186. [DOI] [PubMed] [Google Scholar]
- 837.Magistris MR, Kohler A, Pizzolato G, Morris MA, Baroffio A, Bernheim L, Bader CR. Needle muscle biopsy in the investigation of neuromuscular disorders. Muscle Nerve 21: 194–200, 1998. DOI: . [DOI] [PubMed] [Google Scholar]
- 838.Magnusson SP, Kjaer M. The impact of loading, unloading, ageing and injury on the human tendon. J Physiol 597: 1283–1298, 2019. DOI: 10.1113/JP275450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 839.Mahoney DJ, Parise G, Melov S, Safdar A, Tarnopolsky MA. Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J 19: 1498–1500, 2005. DOI: 10.1096/fj.04-3149fje. [DOI] [PubMed] [Google Scholar]
- 840.Maillet M, van Berlo JH, Molkentin JD. Molecular basis of physiological heart growth: Fundamental concepts and new players. Nat Rev Mol Cell Biol 14: 38–48, 2013. DOI: 10.1038/nrm3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 841.Maiuolo J, Gliozzi M, Musolino V, Scicchitano M, Carresi C, Scarano F, Bosco F, Nucera S, Ruga S, Zito MC, Mollace R, Palma E, Fini M, Muscoli C, Mollace V. The “frail” brain blood barrier in neurodegenerative diseases: Role of early disruption of endothelial cell-to-cell connections. Int J Mol Sci 19, 2018. DOI: 10.3390/ijms19092693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 842.Malek MH, Olfert IM, Esposito F. Detraining losses of skeletal muscle capillarization are associated with vascular endothelial growth factor protein expression in rats. Exp Physiol 95: 359–368, 2010. DOI: 10.1113/expphysiol.2009.050369. [DOI] [PubMed] [Google Scholar]
- 843.Malm C, Nyberg P, Engstrom M, Sjodin B, Lenkei R, Ekblom B, Lundberg I. Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J Physiol 529 (Pt 1): 243–262, 2000. DOI: 10.1111/j.1469-7793.2000.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 844.Maly MR, Marriott KA, Chopp-Hurley JN. Osteoarthritis year in review 2019: Rehabilitation and outcomes. Osteoarthr Cartil 28: 249–266, 2020. DOI: 10.1016/j.joca.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 845.Mandsager K, Harb S, Cremer P, Phelan D, Nissen SE, Jaber W. Association of cardiorespiratory fitness with long-term mortality among adults undergoing exercise treadmill testing. JAMA Netw Open 1: e183605, 2018. DOI: 10.1001/jamanetworkopen.2018.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 846.Manini TM, Clark BC, Nalls MA, Goodpaster BH, Ploutz-Snyder LL, Harris TB. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr 85: 377–384, 2007. DOI: 10.1093/ajcn/85.2.377. [DOI] [PubMed] [Google Scholar]
- 847.Marini M, Lapalombella R, Margonato V, Ronchi R, Samaja M, Scapin C, Gorza L, Maraldi T, Carinci P, Ventura C, Veicsteinas A. Mild exercise training, cardioprotection and stress genes profile. Eur J Appl Physiol 99: 503–510, 2007. DOI: 10.1007/s00421-006-0369-4. [DOI] [PubMed] [Google Scholar]
- 848.Markofski MM, Braun WA. Influence of menstrual cycle on indices of contraction-induced muscle damage. J Strength Cond Res 28: 2649–2656, 2014. DOI: 10.1519/JSC.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 849.Markofski MM, Volpi E. Protein metabolism in women and men: Similarities and disparities. Curr Opin Clin Nutr Metab Care 14: 93–97, 2011. DOI: 10.1097/MCO.0b013e3283412343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 850.Markworth JF, Vella L, Lingard BS, Tull DL, Rupasinghe TW, Sinclair AJ, Maddipati KR, Cameron-Smith D. Human inflammatory and resolving lipid mediator responses to resistance exercise and ibuprofen treatment. Am J Physiol Regul Integr Comp Physiol 305: R1281–R1296, 2013. DOI: 10.1152/ajpregu.00128.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 851.Maron BJ, Pelliccia A, Spataro A, Granata M. Reduction in left ventricular wall thickness after deconditioning in highly trained olympic athletes. Br Heart J 69: 125–128, 1993. DOI: 10.1136/hrt.69.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 852.Maroni BJ, Haesemeyer R, Wilson LK, DiGirolamo M. Electronic determination of size and number in isolated unfixed adipocyte populations [Online]. J Lipid Res 31: 1703–1709, 1990. http://www.ncbi.nlm.nih.gov/pubmed/2246619. [PubMed] [Google Scholar]
- 853.Martin B-J, Arena R, Haykowsky M, Hauer T, Austford LD, Knudtson M, Aggarwal S, Stone JA. Cardiovascular fitness and mortality after contemporary cardiac rehabilitation. Mayo Clin Proc 88: 455–463, 2013. DOI: 10.1016/j.mayocp.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 854.Martin TR, Castile RG, Fredberg JJ, Wohl ME, Mead J. Airway size is related to sex but not lung size in normal adults. J Appl Physiol (1985) 63: 2042–2047, 1987. DOI: 10.1152/jappl.1987.63.5.2042. [DOI] [PubMed] [Google Scholar]
- 855.Martinez-Gomez D, Lavie CJ, Hamer M, Cabanas-Sanchez V, Garcia-Esquinas E, Pareja-Galeano H, Struijk E, Sadarangani KP, Ortega FB, Rodr’ιguez-Artalejo F. Physical activity without weight loss reduces the development of cardiovascular disease risk factors—a prospective cohort study of more than one hundred thousand adults. Prog Cardiovasc Dis 62: 522–530, 2019. DOI: 10.1016/j.pcad.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 856.Mart’ιnez-Redondo V, Pettersson AT, Ruas JL. The hitchhiker’s guide to pgc-1α isoform structure and biological functions. Diabetologia 58: 1969–1977, 2015. DOI: 10.1007/s00125-015-3671-z. [DOI] [PubMed] [Google Scholar]
- 857.Marzetti E, Calvani R, Tosato M, Cesari M, Bari MD, Cherubini A, Broccatelli M, Savera G, D’Elia M, Pahor M, Bernabei R, Landi F. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin Exp Res 29: 35–42, 2017. DOI: 10.1007/s40520-016-0705-4. [DOI] [PubMed] [Google Scholar]
- 858.Mascio DD, Magro-Malosso ER, Saccone G, Marhefka GD, Berghella V. Exercise during pregnancy in normal-weight women and risk of preterm birth: A systematic review and meta-analysis of randomized controlled trials. Am J Obstet Gynecol 215: 561–571, 2016. DOI: 10.1016/j.ajog.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 859.Matsuda N, Kitagaki K, Perrein E, Tsuboi Y, Ebina A, Kondo Y, Murata S, Isa T, Okumura M, Kawaharada R, Horibe K, Ono R. Association between excessive weight gain during pregnancy and persistent low back and pelvic pain after delivery. Spine 45: 319–324, 2020. DOI: 10.1097/BRS.0000000000003271. [DOI] [PubMed] [Google Scholar]
- 860.Matthews VB, Aström M-B, Chan MHS, Bruce CR, Krabbe KS, Prelovsek O, Akerström T, Yfanti C, Broholm C, Mortensen OH, Penkowa M, Hojman P, Zankari A, Watt MJ, Bruunsgaard H, Pedersen BK, Febbraio MA. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of amp-activated protein kinase. Diabetologia 52: 1409–1418, 2009. DOI: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- 861.Mattson MP. Interventions that improve body and brain bioenergetics for parkinson’s disease risk reduction and therapy. J Parkinsons Dis 4: 1–13, 2014. DOI: 10.3233/JPD-130335. [DOI] [PubMed] [Google Scholar]
- 862.Mauro A Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9: 493–495, 1961. DOI: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 863.Mavros Y, Gates N, Wilson GC, Jain N, Meiklejohn J, Brodaty H, Wen W, Singh N, Baune BT, Suo C, Baker MK, Foroughi N, Wang Y, Sachdev PS, Valenzuela M, Singh MAF. Mediation of cognitive function improvements by strength gains after resistance training in older adults with mild cognitive impairment: Outcomes of the study of mental and resistance training. J Am Geriatr Soc 65: 550–559, 2017. DOI: 10.1111/jgs.14542. [DOI] [PubMed] [Google Scholar]
- 864.Małkiewicz MA, Szarmach A, Sabisz A, Cubała WJ, Szurowska E, Winklewski PJ. Blood-brain barrier permeability and physical exercise. J Neuroinflammation 16: 15, 2019. DOI: 10.1186/s12974-019-1403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 865.McArdle WD, Katch FI, Katch VL. Exercise Physiology: Nutrition, Energy, and Human Performance. Lippincott Williams & Wilkins, 2010. [Google Scholar]
- 866.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138: 3657–3666, 2011. DOI: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 867.McDonagh B, Sakellariou GK, Smith NT, Brownridge P, Jackson MJ. Differential cysteine labeling and global label-free proteomics reveals an altered metabolic state in skeletal muscle aging. J Proteome Res 13: 5008–5021, 2014. DOI: 10.1021/pr5006394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 868.McGlory C, Devries MC, Phillips SM. Skeletal muscle and resistance exercise training; the role of protein synthesis in recovery and remodeling. J Appl Physiol (1985) 122: 541–548, 2017. DOI: 10.1152/japplphysiol.00613.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 869.McGregor KM, Crosson B, Krishnamurthy LC, Krishnamurthy V, Hortman K, Gopinath K, Mammino KM, Omar J, Nocera JR. Effects of a 12-week aerobic spin intervention on resting state networks in previously sedentary older adults. Front Psychol 9: 2376, 2018. DOI: 10.3389/fpsyg.2018.02376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 870.McKay BR, Nederveen JP, Fortino SA, Snijders T, Joanisse S, Kumbhare DA, Parise G. Brain-derived neurotrophic factor is associated with human muscle satellite cell differentiation in response to muscle-damaging exercise. Appl Physiol Nutr Metab 45: 581–590, 2020. DOI: 10.1139/apnm-2019-0501. [DOI] [PubMed] [Google Scholar]
- 871.McKay BR, O’Reilly CE, Phillips SM, Tarnopolsky MA, Parise G. Co-expression of igf-1 family members with myogenic regulatory factors following acute damaging muscle-lengthening contractions in humans. J Physiol 586: 5549–5560, 2008. DOI: 10.1113/jphysiol.2008.160176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 872.McKendry J, Shad BJ, Smeuninx B, Oikawa SY, Wallis G, Greig C, Phillips SM, Breen L. Comparable rates of integrated myofibrillar protein synthesis between endurance-trained master athletes and untrained older individuals. Front Physiol 10: 1084, 2019. DOI: 10.3389/fphys.2019.01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 873.McKenzie JA, Spielman LJ, Pointer CB, Lowry JR, Bajwa E, Lee CW, Klegeris A. Neuroinflammation as a common mechanism associated with the modifiable risk factors for alzheimer’s and parkinson’s diseases. Curr Aging Sci 10: 158–176, 2017. DOI: 10.2174/1874609810666170315113244. [DOI] [PubMed] [Google Scholar]
- 874.McMillan LB, Zengin A, Ebeling PR, Scott D. Prescribing physical activity for the prevention and treatment of osteoporosis in older adults. Healthcare 5, 2017. DOI: 10.3390/healthcare5040085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 875.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci U S A 100: 12355–12360, 2003. DOI: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 876.McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve 31: 461–467, 2005. DOI: 10.1002/mus.20276. [DOI] [PubMed] [Google Scholar]
- 877.McNeil CJ, Rice CL. Neuromuscular adaptations to healthy aging. Appl Physiol Nutr Metab 43: 1158–1165, 2018. DOI: 10.1139/apnm-2018-0327. [DOI] [PubMed] [Google Scholar]
- 878.McTiernan A, Friedenreich CM, Katzmarzyk PT, Powell KE, Macko R, Buchner D, Pescatello LS, Bloodgood B, Tennant B, Vaux-Bjerke A, George SM, Troiano RP, Piercy KL. Physical activity in cancer prevention and survival: A systematic review. Med Sci Sports Exerc 51: 1252–1261, 2019. DOI: 10.1249/MSS.0000000000001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 879.Mehana E-SE, Khafaga AF, El-Blehi SS. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci 234: 116786, 2019. DOI: 10.1016/j.lfs.2019.116786. [DOI] [PubMed] [Google Scholar]
- 880.Mendell LM. The size principle: A rule describing the recruitment of motoneurons. J Neurophysiol 93: 3024–3026, 2005. DOI: 10.1152/classicessays.00025.2005. [DOI] [PubMed] [Google Scholar]
- 881.Meneghel AJ, Verlengia R, Crisp AH, Aoki MS, Nosaka K, da Mota GR, Lopes CR. Muscle damage of resistance-trained men after two bouts of eccentric bench press exercise. J Strength Cond Res 28: 2961–2966, 2014. DOI: 10.1519/JSC.0000000000000494. [DOI] [PubMed] [Google Scholar]
- 882.Menet R, Bernard M, ElAli A. Hyperlipidemia in stroke pathobiology and therapy: Insights and perspectives. Front Physiol 9: 488, 2018. DOI: 10.3389/fphys.2018.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 883.Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci 61: 534–540, 2006. DOI: 10.1093/gerona/61.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 884.Menshikova EV, Ritov VB, Ferrell RE, Azuma K, Goodpaster BH, Kelley DE. Characteristics of skeletal muscle mitochondrial biogenesis induced by moderate-intensity exercise and weight loss in obesity. J Appl Physiol (1985) 103: 21–27, 2007. DOI: 10.1152/japplphysiol.01228.2006. [DOI] [PubMed] [Google Scholar]
- 885.Menshikova EV, Ritov VB, Dube JJ, Amati F, Stefanovic-Racic M, Toledo FGS, Coen PM, Goodpaster BH. Calorie restriction-induced weight loss and exercise have differential effects on skeletal muscle mitochondria despite similar effects on insulin sensitivity. J Gerontol A Biol Sci Med Sci 73: 81–87, 2017. DOI: 10.1093/gerona/glw328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 886.Merritt EK, Stec MJ, Thalacker-Mercer A, Windham ST, Cross JM, Shelley DP, Tuggle SC, Kosek DJ, Kim J-S, Bamman MM. Heightened muscle inflammation susceptibility may impair regenerative capacity in aging humans. J Appl Physiol (1985) 115: 937–948, 2013. DOI: 10.1152/japplphysiol.00019.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 887.Mesquita ET, de Souza Júnior CV, Ferreira TR. Andreas vesalius 500 years–a renaissance that revolutionized cardiovascular knowledge. Rev Bras Cir Cardiovasc 30: 260–265, 2015. DOI: 10.5935/1678-9741.20150024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 888.Messerli FH, Rimoldi SF, Bangalore S. The transition from hypertension to heartâ failure: Contemporary update. JACC Heart Fail 5: 543–551, 2017. DOI: 10.1016/j.jchf.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 889.Messi ML, Li T, Wang Z-M, Marsh AP, Nicklas B, Delbono O. Resistance training enhances skeletal muscle innervation without modifying the number of satellite cells or their myofiber association in obese older adults. J Gerontol A Biol Sci Med Sci 71: 1273–1280, 2016. DOI: 10.1093/gerona/glv176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 890.Metsios GS, Kitas GD. Physical activity, exercise and rheumatoid arthritis: Effectiveness, mechanisms and implementation. Best Pract Res Clin Rheumatol 32: 669–682, 2018. DOI: 10.1016/j.berh.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 891.Metsios GS, Koutedakis Y, van Zanten JJCSV, Stavropoulos-Kalinoglou A, Vitalis P, Duda JL, Ntoumanis N, Rouse PC, Kitas GD. Cardiorespiratory fitness levels and their association with cardiovascular profile in patients with rheumatoid arthritis: A cross-sectional study. Rheumatology (Oxford) 54: 2215–2220, 2015. DOI: 10.1093/rheumatology/kev035. [DOI] [PubMed] [Google Scholar]
- 892.Metsios GS, Moe RH, van der Esch M, van Zanten JJCSV, Fenton SAM, Koutedakis Y, Vitalis P, Kennedy N, Brodin N, Bostrom C, Swinnen TW, Tzika K, Niedermann K, Nikiphorou E, Fragoulis GE, Vlieland TPVM, Van den Ende CHM, Kitas GD. The effects of exercise on cardiovascular disease risk factors and cardiovascular physiology in rheumatoid arthritis. Rheumatol Int 40: 347–357, 2020. DOI: 10.1007/s00296-019-04483-6. [DOI] [PubMed] [Google Scholar]
- 893.Metsios GS, Stavropoulos-Kalinoglou A, Treharne GJ, Nevill AM, Sandoo A, Panoulas VF, Toms TE, Koutedakis Y, Kitas GD. Disease activity and low physical activity associate with number of hospital admissions and length of hospitalisation in patients with rheumatoid arthritis. Arthritis Res Ther 13: R108, 2011. DOI: 10.1186/ar3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 894.Metsios GS, Stavropoulos-Kalinoglou A, Kitas GD. The role of exercise in the management of rheumatoid arthritis. Expert Rev Clin Immunol 11: 1121–1130, 2015. DOI: 10.1586/1744666X.2015.1067606. [DOI] [PubMed] [Google Scholar]
- 895.Metsios GS, Stavropoulos-Kalinoglou A, Panoulas VF, Wilson M, Nevill AM, Koutedakis Y, Kitas GD. Association of physical inactivity with increased cardiovascular risk in patients with rheumatoid arthritis. Eur J Cardiovasc Prev Rehabil 16: 188–194, 2009. DOI: 10.1097/HJR.0b013e3283271ceb. [DOI] [PubMed] [Google Scholar]
- 896.Meyer JD, Crombie KM, Cook DB, Hillard CJ, Koltyn KF. Serum endocannabinoid and mood changes after exercise in major depressive disorder. Med Sci Sports Exerc 51: 1909–1917, 2019. DOI: 10.1249/MSS.0000000000002006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 897.Mihaylova MM, Shaw RJ. The ampk signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 13: 1016–1023, 2011. DOI: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 898.Milanese M, Giudice EMD, Peroni DG. Asthma, exercise and metabolic dysregulation in paediatrics. Allergol Immunopathol 47: 289–294, 2018. DOI: 10.1016/j.aller.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 899.Miller BF, Konopka AR, Hamilton KL. The rigorous study of exercise adaptations: Why mRNA might not be enough. J Appl Physiol (1985) 121: 594, 6, 2016. DOI: 10.1152/japplphysiol.00137.2016. [DOI] [PubMed] [Google Scholar]
- 900.Miller BF, Olesen JL, Hansen M, Døssing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol 567: 1021–1033, 2005. DOI: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 901.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 66: 271–289, 2016. DOI: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 902.Miller WL. Fluid volume overload and congestion in heart failure: Time to reconsider pathophysiology and how volume is assessed. Circ Heart Fail 9: e002922, 2016. DOI: 10.1161/CIRCHEART-FAILURE.115.002922. [DOI] [PubMed] [Google Scholar]
- 903.Millischer V, Erhardt S, Ekblom Ö, Forsell Y, Lavebratt C. Twelve-week physical exercise does not have a long-lasting effect on kynurenines in plasma of depressed patients. Neuropsychiatr Dis Treat 13: 967–972, 2017. DOI: 10.2147/NDT.S131746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 904.Mills CD, Thomas AC, Lenz LL, Munder M. Macrophage: SHIP of immunity. Front Immunol 5: 620, 2014. DOI: 10.3389/fimmu.2014.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 905.Mitchell CJ, Churchward-Venne TA, Parise G, Bellamy L, Baker SK, Smith K, Atherton PJ, Phillips SM. Acute post-exercise myofibrillar protein synthesis is not correlated with resistance training-induced muscle hypertrophy in young men. PLoS One 9: e89431, 2014. DOI: 10.1371/journal.pone.0089431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 906.Mitchell L, Murray SB, Cobley S, Hackett D, Gifford J, Capling L, O’Connor H. Muscle dysmorphia symptomatology and associated psychological features in bodybuilders and non-bodybuilder resistance trainers: A systematic review and meta-analysis. Sports Med 47: 233–259, 2017. DOI: 10.1007/s40279-016-0564-3. [DOI] [PubMed] [Google Scholar]
- 907.Miyachi M, Tanaka H, Yamamoto K, Yoshioka A, Takahashi K, Onodera S. Effects of one-legged endurance training on femoral arterial and venous size in healthy humans. J Appl Physiol (1985) 90: 2439–2444, 2001. DOI: 10.1152/jappl.2001.90.6.2439. [DOI] [PubMed] [Google Scholar]
- 908.Mobley CB, Haun CT, Roberson PA, Mumford PW, Kephart WC, Romero MA, Osburn SC, Vann CG, Young KC, Beck DT, Martin JS, Lockwood CM, Roberts MD. Biomarkers associated with low, moderate, and high vastus lateralis muscle hypertrophy following 12 weeks of resistance training. PLoS One 13: e0195203, 2018. DOI: 10.1371/journal.pone.0195203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 909.Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85: 296–302, 2015. DOI: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 910.Montoye AHK, Moore RW, Bowles HR, Korycinski R, Pfeiffer KA. Reporting accelerometer methods in physical activity intervention studies: A systematic review and recommendations for authors. Br J Sports Med 52: 1507–1516, 2018. DOI: 10.1136/bjsports-2015-095947. [DOI] [PubMed] [Google Scholar]
- 911.Montoye AH, Pfeiffer KA, Suton D, Trost SG. Evaluating the responsiveness of accelerometry to detect change in physical activity. Meas Phys Educ Exerc Sci 18: 273–285, 2014. DOI: 10.1080/1091367X.2014.942454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 912.Moon HY, van Praag H. Muscle over mind. Cell Metab 20: 560–562, 2014. DOI: 10.1016/j.cmet.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 913.Moor AE, Itzkovitz S. Spatial transcriptomics: Paving the way for tissue-level systems biology. Curr Opin Biotechnol 46: 126–133, 2017. DOI: 10.1016/j.copbio.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 914.Moore TM, Zhou Z, Cohn W, Norheim F, Lin AJ, Kalajian N, Strumwasser AR, Cory K, Whitney K, Ho T, Ho T, Lee JL, Rucker DH, Shirihai O, van der Bliek AM, Whitelegge JP, Seldin MM, Lusis AJ, Lee S, Drevon CA, Mahata SK, Turcotte LP, Hevener AL. The impact of exercise on mitochondrial dynamics and the role of drp1 in exercise performance and training adaptations in skeletal muscle. Mol Metab 21: 51–67, 2019. DOI: 10.1016/j.molmet.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 915.Mora JC, Valencia WM. Exercise and older adults. Clin Geriatr Med 34: 145–162, 2018. DOI: 10.1016/j.cger.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 916.Moraes-Silva IC, Mostarda CT, Silva-Filho AC, Irigoyen MC. Hypertension and exercise training: Evidence from clinical studies. Adv Exp Med Biol 1000: 65–84, 2017. DOI: 10.1007/978-981-10-4304-8_5. [DOI] [PubMed] [Google Scholar]
- 917.Morales PE, Bucarey JL, Espinosa A. Muscle lipid metabolism: Role of lipid droplets and perilipins. J Diabetes Res 2017: 1789395, 2017. DOI: 10.1155/2017/1789395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 918.Moreira IS, Fernandes PA, Ramos MJ. Vascular endothelial growth factor (vegf) inhibition–a critical review. Anti Cancer Agents Med Chem 7: 223–245, 2007. DOI: 10.2174/187152007780058687. [DOI] [PubMed] [Google Scholar]
- 919.Morgan JA, Olagunju AT, Corrigan F, Baune BT. Does ceasing exercise induce depressive symptoms? A systematic review of experimental trials including immunological and neurogenic markers. J Affect Disord 234: 180–192, 2018. DOI: 10.1016/j.jad.2018.02.058. [DOI] [PubMed] [Google Scholar]
- 920.Morigny P, Houssier M, Mouisel E, Langin D. Adipocyte lipolysis and insulin resistance. Biochimie 125: 259–266, 2016. DOI: 10.1016/j.biochi.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 921.Morland C, Andersson KA, Haugen P, Hadzic A, Kleppa L, Gille A, Rinholm JE, Palibrk V, Diget EH, Kennedy LH, Stølen T, Hennestad E, Moldestad O, Cai Y, Puchades M, Offermanns S, Vervaeke K, Bjørås M, Wisløff U, Storm-Mathisen J, Bergersen LH. Exercise induces cerebral vegf and angiogenesis via the lactate receptor hcar1. Nat Commun 8: 15557, 2017. DOI: 10.1038/ncomms15557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 922.Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, Cederholm T, Coats AJS, Cummings SR, Evans WJ, Fearon K, Ferrucci L, Fielding RA, Guralnik JM, Harris TB, Inui A, Kalantar-Zadeh K, Kirwan B-A, Mantovani G, Muscaritoli M, Newman AB, Rossi-Fanelli F, Rosano GMC, Roubenoff R, Schambelan M, Sokol GH, Storer TW, Vellas B, von Haehling S, Yeh S-S, Anker SD. Sarcopenia with limited mobility: An international consensus. J Am Med Dir Assoc 12: 403–409, 2011. DOI: 10.1016/j.jamda.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 923.Moro T, Brightwell CR, Phalen DE, McKenna CF, Lane SJ, Porter C, Volpi E, Rasmussen BB, Fry CS. Low skeletal muscle capillarization limits muscle adaptation to resistance exercise training in older adults. Exp Gerontol 127: 110723, 2019. DOI: 10.1016/j.exger.2019.110723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 924.Moro T, Brightwell CR, Volpi E, Rasmussen BB, Fry CS. Resistance exercise training promotes fiber type-specific myonuclear adaptations in older adults. J Appl Physiol (1985) 128: 795–804, 2020. DOI: 10.1152/japplphysiol.00723.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 925.Moro T, Paoli A. When covid-19 affects muscle: Effects of quarantine in older adults. Eur J Transl Myol 30: 9069, 2020. DOI: 10.4081/ejtm.2019.9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 926.Morouço P, Fernandes C, Santos-Rocha R. Osteoarthritis, exercise, and tissue engineering: A stimulating triad for health professionals. J Aging Res 2019: 1935806, 2019. DOI: 10.1155/2019/1935806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 927.Morrison D, Hughes J, Gatta PAD, Mason S, Lamon S, Russell AP, Wadley GD. Vitamin c and e supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic Biol Med 89: 852–862, 2015. DOI: 10.1016/j.freeradbiomed.2015.10.412. [DOI] [PubMed] [Google Scholar]
- 928.Morrison PR, Biggs RB, Booth FW. Daily running for 2 wk and mRNAs for cytochrome c and alpha-actin in rat skeletal muscle. Am J Phys 257: C936–C939, 1989. DOI: 10.1152/ajpcell.1989.257.5.C936. [DOI] [PubMed] [Google Scholar]
- 929.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by rna-seq. Nat Methods 5: 621–628, 2008. DOI: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 930.Mortensen SP, Saltin B. Regulation of the skeletal muscle blood flow in humans. Exp Physiol 99: 1552–1558, 2014. DOI: 10.1113/expphysiol.2014.081620. [DOI] [PubMed] [Google Scholar]
- 931.Morton JP, Croft L, Bartlett JD, Maclaren DPM, Reilly T, Evans L, McArdle A, Drust B. Reduced carbohydrate availability does not modulate training-induced heat shock protein adaptations but does upregulate oxidative enzyme activity in human skeletal muscle. J Appl Physiol (1985) 106: 1513–1521, 2009. DOI: 10.1152/japplphysiol.00003.2009. [DOI] [PubMed] [Google Scholar]
- 932.Morton N Despite intensive efforts, egg-related salmonellosis outbreaks continue [Online]. J Ark Med Soc 90: 116–118, 1993. http://www.ncbi.nlm.nih.gov/pubmed/8226573. [PubMed] [Google Scholar]
- 933.Morton RW, Sonne MW, Zuniga AF, Mohammad IYZ, Jones A, McGlory C, Keir PJ, Potvin JR, Phillips SM. Muscle fibre activation is unaffected by load and repetition duration when resistance exercise is performed to task failure. J Physiol 597: 4601–4613, 2019. DOI: 10.1113/JP278056. [DOI] [PubMed] [Google Scholar]
- 934.Mosole S, Carraro U, Kern H, Loefler S, Fruhmann H, Vogelauer M, Burggraf S, Mayr W, Krenn M, Paternostro-Sluga T, Hamar D, Cvecka J, Sedliak M, Tirpakova V, Sarabon N, Musaró A, Sandri M, Protasi F, Nori A, Pond A, Zampieri S. Long-term high-level exercise promotes muscle reinnervation with age. J Neuropathol Exp Neurol 73: 284–294, 2014. DOI: 10.1097/NEN.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 935.Mosole S, Carraro U, Kern H, Loefler S, Zampieri S. Use it or lose it: Tonic activity of slow motoneurons promotes their survival and preferentially increases slow fiber-type groupings in muscles of old lifelong recreational sportsmen. Eur J Transl Myol 26: 5972, 2016. DOI: 10.4081/ejtm.2016.5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 936.da Mota Gomes M, Moscovici M, Engelhardt E. Andreas vesalius as a renaissance innovative neuroanatomist: His 5th centenary of birth. Arq Neuropsiquiatr 73: 155–158, 2015. DOI: 10.1590/0004-282X20140201. [DOI] [PubMed] [Google Scholar]
- 937.Movin T Tendon tissue sampling. Scand J Med Sci Sports 10: 368–371, 2000. DOI: 10.1034/j.1600-0838.2000.010006368.x. [DOI] [PubMed] [Google Scholar]
- 938.Moylan JS, Reid MB. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve 35: 411–429, 2007. DOI: 10.1002/mus.20743. [DOI] [PubMed] [Google Scholar]
- 939.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després J-P, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics–2015 update: A report from the american heart association. Circulation 131: e29–e322, 2015. DOI: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 940.Mu L, Cohen AJ, Mukamal KJ. Prevalence and predictors of resistance and aerobic exercise among hypertensive adults in the united states. J Hum Hypertens 29: 394–395, 2015. DOI: 10.1038/jhh.2014.104. [DOI] [PubMed] [Google Scholar]
- 941.Muehlbauer T, Gollhofer A, Granacher U. Associations between measures of balance and lower-extremity muscle strength/power in healthy individuals across the lifespan: A systematic review and meta-analysis. Sports Med 45: 1671–1692, 2015. DOI: 10.1007/s40279-015-0390-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 942.Muise ES, Guan H-P, Liu J, Nawrocki AR, Yang X, Wang C, Rodríguez CG, Zhou D, Gorski JN, Kurtz MM, Feng D, Leavitt KJ, Wei L, Wilkening RR, Apgar JM, Xu S, Lu K, Feng W, Li Y, He H, Previs SF, Shen X, van Heek M, Souza SC, Rosenbach MJ, Biftu T, Erion MD, Kelley DE, Kemp DM, Myers RW, Sebhat IK. Pharmacological ampk activation induces transcriptional responses congruent to exercise in skeletal and cardiac muscle, adipose tissues and liver. PLoS One 14: e0211568, 2019. DOI: 10.1371/journal.pone.0211568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 943.Mujika I, Padilla S. Detraining: Loss of training-induced physiological and performance adaptations. Part i: Short term insufficient training stimulus. Sports Med 30: 79–87, 2000. DOI: 10.2165/00007256-200030020-00002. [DOI] [PubMed] [Google Scholar]
- 944.Muñoz-Durango N, Fuentes CA, Castillo AE, Gonz’alez-G’omez LM, Vecchiola A, Fardella CE, Kalergis AM. Role of the reninangiotensin-aldosterone system beyond blood pressure regulation: Molecular and cellular mechanisms involved in end-organ damage during arterial hypertension. Int J Mol Sci 17, 2016. DOI: 10.3390/ijms17070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 945.Murach KA, Dungan CM, Kosmac K, Voigt TB, Tourville TW, Miller MS, Bamman MM, Peterson CA, Toth MJ. Fiber typing human skeletal muscle with fluorescent immunohistochemistry. J Appl Physiol (1985) 127: 1632–1639, 2019. DOI: 10.1152/japplphysiol.00624.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 946.Murach KA, Englund DA, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Myonuclear domain flexibility challenges rigid assumptions on satellite cell contribution to skeletal muscle fiber hypertrophy. Front Physiol 9: 635, 2018. DOI: 10.3389/fphys.2018.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 947.Murach K, Raue U, Wilkerson B, Minchev K, Jemiolo B, Bagley J, Luden N, Trappe S. Single muscle fiber gene expression with run taper. PLoS One 9: e108547, 2014. DOI: 10.1371/journal.pone.0108547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 948.Murgia M, Nagaraj N, Deshmukh AS, Zeiler M, Cancellara P, Moretti I, Reggiani C, Schiaffino S, Mann M. Single muscle fiber proteomics reveals unexpected mitochondrial specialization. EMBO Rep 16: 387–395, 2015. DOI: 10.15252/embr.201439757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 949.Murgia M, Tan J, Geyer PE, Doll S, Mann M, Klopstock T. Proteomics of cytochrome c oxidase-negative versus -positive muscle fiber sections in mitochondrial myopathy. Cell Rep 29: 3825–3834.e4, 2019. DOI: 10.1016/j.celrep.2019.11.055. [DOI] [PubMed] [Google Scholar]
- 950.Murgia M, Toniolo L, Nagaraj N, Ciciliot S, Vindigni V, Schiaffino S, Reggiani C, Mann M. Single muscle fiber proteomics reveals fiber-type-specific features of human muscle aging. Cell Rep 19: 2396–2409, 2017. DOI: 10.1016/j.celrep.2017.05.054. [DOI] [PubMed] [Google Scholar]
- 951.Muriel P. Liver Pathophysiology: Therapies and Antioxidants. Academic Press, 2017. [Google Scholar]
- 952.Myers AM, Beam NW, Fakhoury JD. Resistance training for children and adolescents. Transl Pediatr 6: 137–143, 2017. DOI: 10.21037/tp.2017.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 953.Myers J, Kokkinos P, Nyelin E. Physical activity, cardiorespiratory fitness, and the metabolic syndrome. Nutrients 11, 2019. DOI: 10.3390/nu11071652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 954.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 346: 793–801, 2002. DOI: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 955.Naci H, Ioannidis JPA. Comparative effectiveness of exercise and drug interventions on mortality outcomes: Metaepidemiological study. BMJ 347: f5577, 2013. DOI: 10.1136/bmj.f5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 956.Nader GA, McLoughlin TJ, Esser KA. MTOR function in skeletal muscle hypertrophy: Increased ribosomal rna via cell cycle regulators. Am J Phys Cell Phys 289: C1457–C1465, 2005. DOI: 10.1152/ajp-cell.00165.2005. [DOI] [PubMed] [Google Scholar]
- 957.Nadruz W, West E, Sengeløv M, Santos M, Groarke JD, Forman DE, Claggett B, Skali H, Shah AM. Prognostic value of cardiopulmonary exercise testing in heart failure with reduced, midrange, and preserved ejection fraction. J Am Heart Assoc 6, 2017. DOI: 10.1161/JAHA.117.006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 958.Nagle CA, Klett EL, Coleman RA. Hepatic triacylglycerol accumulation and insulin resistance. J Lipid Res 50 (Suppl): S74–S79, 2009. DOI: 10.1194/jlr.R800053-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 959.Nair VD, Ge Y, Li S, Pincas H, Jain N, Seenarine N, Amper MAS, Goodpaster BH, Walsh MJ, Coen PM, Sealfon SC. Sedentary and trained older men have distinct circulating exosomal microRNA profiles at baseline and in response to acute exercise. Front Physiol 11: 605, 2020. DOI: 10.3389/fphys.2020.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 960.Narici M, De Vito G, Franchi M, Paoli A, Moro T, Marcolin G, Grassi B, Baldassarre G, Zuccarelli L, Biolo G, di Girolamo FG, Fiotti N, Dela F, Greenhaff P, Maganaris C. Impact of sedentarism due to the covid-19 home confinement on neuromuscular, cardiovascular and metabolic health: Physiological and pathophysiological implications and recommendations for physical and nutritional countermeasures. Eur J Sport Sci 21 (4): 614–635, 2021. [DOI] [PubMed] [Google Scholar]
- 961.Natali AJ, Wilson LA, Peckham M, Turner DL, Harrison SM, White E. Different regional effects of voluntary exercise on the mechanical and electrical properties of rat ventricular myocytes. J Physiol 541: 863–875, 2002. DOI: 10.1113/jphysiol.2001.013415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 962.National Cholesterol Education Program Expert Panel on Detection and Evaluation and Treatment of High Blood Cholesterol in Adults. Third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii) final report [Online]. Circulation 106: 3143–3421, 2002. http://www.ncbi.nlm.nih.gov/pubmed/12485966. [PubMed] [Google Scholar]
- 963.Nederveen JP, Joanisse S, Snijders T, Thomas ACQ, Kumbhare D, Parise G. The influence of capillarization on satellite cell pool expansion and activation following exercise-induced muscle damage in healthy young men. J Physiol 596: 1063–1078, 2018. DOI: 10.1113/JP275155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 964.Nederveen JP, Joanisse S, Séguin CML, Bell KE, Baker SK, Phillips SM, Parise G. The effect of exercise mode on the acute response of satellite cells in old men. Acta Physiol (Oxf) 215: 177–190, 2015. DOI: 10.1111/apha.12601. [DOI] [PubMed] [Google Scholar]
- 965.Neil-Sztramko SE, Winters-Stone KM, Bland KA, Campbell KL. Updated systematic review of exercise studies in breast cancer survivors: Attention to the principles of exercise training. Br J Sports Med 53: 504–512, 2019. DOI: 10.1136/bjsports-2017-098389. [DOI] [PubMed] [Google Scholar]
- 966.Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: The chronic osteoarthritis management initiative of the u.s. Bone and joint initiative. Semin Arthritis Rheum 43: 701–712, 2014. DOI: 10.1016/j.semarthrit.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 967.Nelson ME, Parker BL, Burchfield JG, Hoffman NJ, Needham EJ, Cooke KC, Naim T, Sylow L, Ling NX, Francis D, Norris DM, Chaudhuri R, Oakhill JS, Richter EA, Lynch GS, Stöckli J, James DE. Phosphoproteomics reveals conserved exercise-stimulated signaling and ampk regulation of store-operated calcium entry. EMBO J 38: e102578, 2019. DOI: 10.15252/embj.2019102578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 968.Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Macera CA, Castaneda-Sceppa C. Physical activity and public health in older adults: Recommendation from the american college of sports medicine and the american heart association. Circulation 116: 1094–1105, 2007. DOI: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- 969.Neufer PD, Bamman MM, Muoio DM, Bouchard C, Cooper DM, Goodpaster BH, Booth FW, Kohrt WM, Gerszten RE, Mattson MP, Hepple RT, Kraus WE, Reid MB, Bodine SC, Jakicic JM, Fleg JL, Williams JP, Joseph L, Evans M, Maruvada P, Rodgers M, Roary M, Boyce AT, Drugan JK, Koenig JI, Ingraham RH, Krotoski D, Garcia-Cazarin M, McGowan JA, Laughlin MR. Understanding the cellular and molecular mechanisms of physical activity-induced health benefits. Cell Metab 22: 4–11, 2015. DOI: 10.1016/j.cmet.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 970.Neufer PD, Dohm GL. Exercise induces a transient increase in transcription of the glut-4 gene in skeletal muscle. Am J Phys 265: C1597–C1603, 1993. DOI: 10.1152/ajpcell.1993.265.6.C1597. [DOI] [PubMed] [Google Scholar]
- 971.Neurology TL. Response to the growing dementia burden must be faster. Lancet Neurol 17: 651, 2018. DOI: 10.1016/S1474-4422(18)30256-4. [DOI] [PubMed] [Google Scholar]
- 972.Ng QX, Ho CYX, Chan HW, Yong BZJ, Yeo W-S. Managing childhood and adolescent attention-deficit/hyperactivity disorder (adhd) with exercise: A systematic review. Complement Ther Med 34: 123–128, 2017. DOI: 10.1016/j.ctim.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 973.Nguyen C, Lefèvre-Colau M-M, Poiraudeau S, Rannou F. Rehabilitation (exercise and strength training) and osteoarthritis: A critical narrative review. Ann Phys Rehabil Med 59: 190–195, 2016. DOI: 10.1016/j.rehab.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 974.Nicklas BJ, Hsu F-C, Brinkley TJ, Church T, Goodpaster BH, Kritchevsky SB, Pahor M. Exercise training and plasma c-reactive protein and interleukin-6 in elderly people. J Am Geriatr Soc 56: 2045–2052, 2008. DOI: 10.1111/j.1532-5415.2008.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 975.Nielsen HG, Hagberg IA, Lyberg T. Marathon running leads to partial exhaustion of ros-generating capacity in leukocytes. Med Sci Sports Exerc 36: 68–73, 2004. DOI: 10.1249/01.MSS.0000106168.12113.95. [DOI] [PubMed] [Google Scholar]
- 976.Nielsen S, Åkerström T, Rinnov A, Yfanti C, Scheele C, Pedersen BK, Laye MJ. The miRNA plasma signature in response to acute aerobic exercise and endurance training. PLoS One 9: e87308, 2014. DOI: 10.1371/journal.pone.0087308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 977.Nieman DC, Henson DA, Davis JM, Murphy EA, Jenkins DP, Gross SJ, Carmichael MD, Quindry JC, Dumke CL, Utter AC, McAnulty SR, McAnulty LS, Triplett NT, Mayer EP. Quercetin’s influence on exercise-induced changes in plasma cytokines and muscle and leukocyte cytokine mRNA. J Appl Physiol (1985) 103: 1728–1735, 2007. DOI: 10.1152/japplphysiol.00707.2007. [DOI] [PubMed] [Google Scholar]
- 978.Nieman DC, Davis JM, Henson DA, Walberg-Rankin J, Shute M, Dumke CL, Utter AC, Vinci DM, Carson JA, Brown A, Lee WJ, McAnulty SR, McAnulty LS. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J Appl Physiol (1985) 94: 1917–1925, 2003. DOI: 10.1152/japplphysiol.01130.2002. [DOI] [PubMed] [Google Scholar]
- 979.Nilwik R, Snijders T, Leenders M, Groen BBL, van Kranenburg J, Verdijk LB, van Loon LJC. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type ii muscle fiber size. Exp Gerontol 48: 492–498, 2013. DOI: 10.1016/j.exger.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 980.Nishimune H, Stanford JA, Mori Y. Role of exercise in maintaining the integrity of the neuromuscular junction. Muscle Nerve 49: 315–324, 2014. DOI: 10.1002/mus.24095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 981.Noakes TD, Peltonen JE, Rusko HK. Evidence that a central governor regulates exercise performance during acute hypoxia and hyperoxia [Online]. J Exp Biol 204: 3225–3234, 2001. http://www.ncbi.nlm.nih.gov/pubmed/11581338. [DOI] [PubMed] [Google Scholar]
- 982.Nokia MS, Lensu S, Ahtiainen JP, Johansson PP, Koch LG, Britton SL, Kainulainen H. Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J Physiol 594: 1855–1873, 2016. DOI: 10.1113/JP271552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 983.Novakova K, Kummer O, Bouitbir J, Stoffel SD, Hoerler-Koerner U, Bodmer M, Roberts P, Urwyler A, Ehrsam R, Krähenbühl S. Effect of l-carnitine supplementation on the body carnitine pool, skeletal muscle energy metabolism and physical performance in male vegetarians. Eur J Nutr 55: 207–217, 2016. DOI: 10.1007/s00394-015-0838-9. [DOI] [PubMed] [Google Scholar]
- 984.O’Brien T, Nguyen TT, Zimmerman BR. Hyperlipidemia and diabetes mellitus. Mayo Clin Proc 73: 969–976, 1998. DOI: 10.4065/73.10.969. [DOI] [PubMed] [Google Scholar]
- 985.Ochi E, Maruo M, Tsuchiya Y, Ishii N, Miura K, Sasaki K. Higher training frequency is important for gaining muscular strength under volume-matched training. Front Physiol 9: 744, 2018. DOI: 10.3389/fphys.2018.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 986.O’Doherty RM, Bracy DP, Osawa H, Wasserman DH, Granner DK. Rat skeletal muscle hexokinase ii mRNA and activity are increased by a single bout of acute exercise. Am J Phys 266: E171–E178, 1994. DOI: 10.1152/ajpendo.1994.266.2.E171. [DOI] [PubMed] [Google Scholar]
- 987.Ogawa T, Nakamura T, Banno M, Sasaki Y, Umemoto Y, Kouda K, Kawasaki T, Tajima F. Elevation of interleukin-6 and attenuation of tumor necrosis factor-α during wheelchair half marathon in athletes with cervical spinal cord injuries. Spinal Cord 52: 601–605, 2014. DOI: 10.1038/sc.2014.88. [DOI] [PubMed] [Google Scholar]
- 988.Oldenburg C, Lundin A, Edman G, Deboussard CN, Bartfai A. Emotional reserve and prolonged post-concussive symptoms and disability: A swedish prospective 1-year mild traumatic brain injury cohort study. BMJ Open 8: e020884, 2018. DOI: 10.1136/bmjopen-2017-020884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 989.Olesen J, Biensø RS, Meinertz S, van Hauen L, Rasmussen SM, Gliemann L, Plomgaard P, Pilegaard H. Impact of training status on lps-induced acute inflammation in humans. J Appl Physiol (1985) 118: 818–829, 2015. DOI: 10.1152/japplphysiol.00725.2014. [DOI] [PubMed] [Google Scholar]
- 990.Olfert IM, Howlett RA, Wagner PD, Breen EC. Myocyte vascular endothelial growth factor is required for exercise-induced skeletal muscle angiogenesis. Am J Physiol Regul Integr Comp Physiol 299: R1059–R1067, 2010. DOI: 10.1152/ajpregu.00347.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 991.Olsson TT, Svensson M, Hållmarker U, James S, Deierborg T. Delayed clinical manifestation of parkinson’s disease among physically active: Do participants in a long-distance ski race have a motor reserve? J Parkinsons Dis 10: 267–274, 2020. DOI: 10.3233/JPD-191762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 992.Oneda B, Cardoso CG, Forjaz CLM, Ara’ujo TG, Bernardo FR, de Gusmão JL, Pinto LG, Labes E, Abrahão SB, Mion D, Fonseca AM, Tinucci T. Effects of estrogen therapy and aerobic training on sympathetic activity and hemodynamics in healthy postmenopausal women: A double-blind randomized trial. Menopause 21: 369–375, 2014. DOI: 10.1097/GME.0b013e31829d2a00. [DOI] [PubMed] [Google Scholar]
- 993.O’Reilly C, McKay B, Phillips S, Tarnopolsky M, Parise G. Hepatocyte growth factor (hgf) and the satellite cell response following muscle lengthening contractions in humans. Muscle Nerve 38: 1434–1442, 2008. DOI: 10.1002/mus.21146. [DOI] [PubMed] [Google Scholar]
- 994.Orfei CP, Vigano M, Pearson JR, Colombini A, De Luca P, Ragni E, Santos-Ruiz L, de Girolamo L. In vitro induction of tendon-specific markers in tendon cells, adipose- and bone marrow-derived stem cells is dependent on tgfβ3, bmp-12 and ascorbic acid stimulation. Int J Mol Sci 20, 2019. DOI: 10.3390/ijms20010149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 995.O’Rourke KS, Blaivas M, Ike RW. Utility of needle muscle biopsy in a university rheumatology practice [Online]. J Rheumatol 21: 413–424, 1994. http://www.ncbi.nlm.nih.gov/pubmed/8006885. [PubMed] [Google Scholar]
- 996.Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK. Proand anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol 515 (Pt 1): 287–291, 1999. DOI: 10.1111/j.1469-7793.1999.287ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 997.Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol 508 (Pt 3): 949–953, 1998. DOI: 10.1111/j.1469-7793.1998.949bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 998.Otera H, Wang C, Cleland MM, Setoguchi K, Yokota S, Youle RJ, Mihara K. Mff is an essential factor for mitochondrial recruitment of drp1 during mitochondrial fission in mammalian cells. J Cell Biol 191: 1141–1158, 2010. DOI: 10.1083/jcb.201007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 999.Ozougwu JC. Physiology of the liver. Int J Res Pharm Biosci 4: 13–24, 2017. [Google Scholar]
- 1000.Pagnotti GM, Styner M, Uzer G, Patel VS, Wright LE, Ness KK, Guise TA, Rubin J, Rubin CT. Combating osteoporosis and obesity with exercise: Leveraging cell mechanosensitivity. Nat Rev Endocrinol 15: 339–355, 2019. DOI: 10.1038/s41574-019-0170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1001.Palazzo C, Ravaud J-F, Papelard A, Ravaud P, Poiraudeau S. The burden of musculoskeletal conditions. PLoS One 9: e90633, 2014. DOI: 10.1371/journal.pone.0090633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1002.Palmer K, Bowles K-A, Paton M, Jepson M, Lane R. Chronic heart failure and exercise rehabilitation: A systematic review and meta-analysis. Arch Phys Med Rehabil 99: 2570–2582, 2018. DOI: 10.1016/j.apmr.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 1003.Pandey A, Allen NB, Ayers C, Reis JP, Moreira HT, Sidney S, Rana JS, Jacobs DR, Chow LS, de Lemos JA, Carnethon M, Berry JD. Fitness in young adulthood and long-term cardiac structure and function: The cardia study. JACC Heart Fail 5: 347–355, 2017. DOI: 10.1016/j.jchf.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1004.Papaleontiou M, Cappola AR. Thyroid-stimulating hormone in the evaluation of subclinical hypothyroidism. JAMA 316: 1592–1593, 2016. DOI: 10.1001/jama.2016.9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1005.Papapetropoulos A, Garc’ιa-Cardeña G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest 100: 3131–3139, 1997. DOI: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1006.Pareja-Galeano H, Garatachea N, Lucia A. Exercise as a polypill for chronic diseases. Prog Mol Biol Transl Sci 135: 497–526, 2015. DOI: 10.1016/bs.pmbts.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 1007.Park J, Kwon Y, Park H. Effects of 24-week aerobic and resistance training on carotid artery intima-media thickness and flow velocity in elderly women with sarcopenic obesity. J Atheroscler Thromb 24: 1117–1124, 2017. DOI: 10.5551/jat.39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1008.Pasarica M, Gowronska-Kozak B, Burk D, Remedios I, Hymel D, Gimble J, Ravussin E, Bray GA, Smith SR. Adipose tissue collagen vi in obesity. J Clin Endocrinol Metab 94: 5155–5162, 2009. DOI: 10.1210/jc.2009-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1009.Pasipoularides A Greek underpinnings to his methodology in unraveling de motu cordis and what harvey has to teach us still today. Int J Cardiol 168: 3173–3182, 2013. DOI: 10.1016/j.ijcard.2013.07.253. [DOI] [PubMed] [Google Scholar]
- 1010.Pattyn N, Beulque R, Cornelissen V. Aerobic interval vs. Continuous training in patients with coronary artery disease or heart failure: An updated systematic review and meta-analysis with a focus on secondary outcomes. Sports Med 48: 1189–1205, 2018. DOI: 10.1007/s40279-018-0885-5. [DOI] [PubMed] [Google Scholar]
- 1011.Pattyn N, Vanhees L, Cornelissen VA, Coeckelberghs E, De Maeyer C, Goetschalckx K, Possemiers N, Wuyts K, Van Craenenbroeck EM, Beckers PJ. The long-term effects of a randomized trial comparing aerobic interval versus continuous training in coronary artery disease patients: 1-year data from the saintex-cad study. Eur J Prev Cardiol 23: 1154–1164, 2016. DOI: 10.1177/2047487316631200. [DOI] [PubMed] [Google Scholar]
- 1012.Paul MH, Sperling E. Cyclophorase system. XXIII. Correlation of cyclophorase activity and mitochondrial density in striated muscle. Proc Soc Exp Biol Me 79: 352–354, 1952. DOI: 10.3181/00379727-79-19375. [DOI] [PubMed] [Google Scholar]
- 1013.Paulsen G, Cumming KT, Holden G, Hallén J, Rønnestad BR, Sveen O, Skaug A, Paur I, Bastani NE, Østgaard HN, Buer C, Midttun M, Freuchen F, Wiig H, Ulseth ET, Garthe I, Blomhoff R, Benestad HB, Raastad T. Vitamin c and e supplementation hampers cellular adaptation to endurance training in humans: A double-blind, randomised, controlled trial. J Physiol 592: 1887–1901, 2014. DOI: 10.1113/jphysiol.2013.267419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1014.Paulsen G, Mikkelsen UR, Raastad T, Peake JM. Leucocytes, cytokines and satellite cells: What role do they play in muscle damage and regeneration following eccentric exercise? [Online]. Exerc Immunol Rev 18: 42–97, 2012. http://www.ncbi.nlm.nih.gov/pubmed/22876722. [PubMed] [Google Scholar]
- 1015.Peake JM, Suzuki K, Hordern M, Wilson G, Nosaka K, Coombes JS. Plasma cytokine changes in relation to exercise intensity and muscle damage. Eur J Appl Physiol 95: 514–521, 2005. DOI: 10.1007/s00421-005-0035-2. [DOI] [PubMed] [Google Scholar]
- 1016.Pearson AM. Muscle growth and exercise. Crit Rev Food Sci Nutr 29: 167–196, 1990. DOI: 10.1080/10408399009527522. [DOI] [PubMed] [Google Scholar]
- 1017.Pedersen BK. Muscle as a secretory organ. Compr Physiol 3: 1337–1362, 2013. DOI: 10.1002/cphy.c120033. [DOI] [PubMed] [Google Scholar]
- 1018.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat Rev Endocrinol 8: 457–465, 2012. DOI: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 1019.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol Rev 88: 1379–1406, 2008. DOI: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 1020.Pedersen BK. Muscles and their myokines. J Exp Biol 214: 337–346, 2011. DOI: 10.1242/jeb.048074. [DOI] [PubMed] [Google Scholar]
- 1021.Pedersen BK. Physical activity and muscle-brain crosstalk. Nat Rev Endocrinol 15: 383–392, 2019. DOI: 10.1038/s41574-019-0174-x. [DOI] [PubMed] [Google Scholar]
- 1022.Pedersen BK, Saltin B. Exercise as medicine—evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports 25 (Suppl 3): 1–72, 2015. DOI: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 1023.Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, Febbraio M, Saltin B. Searching for the exercise factor: Is il-6 a candidate? J Muscle Res Cell Motil 24: 113–119, 2003. DOI: 10.1023/a:1026070911202. [DOI] [PubMed] [Google Scholar]
- 1024.Pekkala S, Wiklund PK, Hulmi JJ, Ahtiainen JP, Horttanainen M, Pollanen E, Makela KA, Kainulainen H, Hakkinen K, Nyman K, Alen M, Herzig K-H, Cheng S. Are skeletal muscle fndc5 gene expression and irisin release regulated by exercise and related to health? J Physiol 591: 5393–5400, 2013. DOI: 10.1113/jphysiol.2013.263707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1025.Penninx BW, Messier SP, Rejeski WJ, Williamson JD, DiBari M, Cavazzini C, Applegate WB, Pahor M. Physical exercise and the prevention of disability in activities of daily living in older persons with osteoarthritis. Arch Intern Med 161: 2309–2316, 2001. DOI: 10.1001/archinte.161.19.2309. [DOI] [PubMed] [Google Scholar]
- 1026.Peper E Problems in biofeedback training: An experimential analogy-urination. Perspect Biol Med 19: 404–412, 1976. DOI: 10.1353/pbm.1976.0018. [DOI] [PubMed] [Google Scholar]
- 1027.Perales M, Santos-Lozano A, Ruiz JR, Lucia A, Barakat R. Benefits of aerobic or resistance training during pregnancy on maternal health and perinatal outcomes: A systematic review. Early Hum Dev 94: 43–48, 2016. DOI: 10.1016/j.earlhumdev.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 1028.Perales M, Valenzuela PL, Barakat R, Cordero Y, Pel’aez M, L’opez C, Ruilope LM, Santos-Lozano A, Lucia A. Gestational exercise and maternal and child health: Effects until delivery and at post-natal follow-up. J Clin Med 9, 2020. DOI: 10.3390/jcm9020379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1029.Perez EC, Bravo DR, Rodgers SP, Khan AR, Leasure JL. Shaping the adult brain with exercise during development: Emerging evidence and knowledge gaps. Int J Dev Neurosci 78: 147–155, 2019. DOI: 10.1016/j.ijdevneu.2019.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1030.Perkins RK, Lavin KM, Raue U, Jemiolo B, Trappe SW, Trappe TA. Effects of aging and lifelong aerobic exercise on expression of innate immune components in human skeletal muscle. J Appl Physiol (1985) 129: 1483–1492, 2020. DOI: 10.1152/japplphysiol.00615.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1031.Perko MJ, Nielsen HB, Skak C, Clemmesen JO, Schroeder TV, Secher NH. Mesenteric, coeliac and splanchnic blood flow in humans during exercise. J Physiol 513 (Pt 3): 907–913, 1998. DOI: 10.1111/j.1469-7793.1998.907ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1032.Perreault C, Bélanger R, Bonny Y, Gyger M, Roy DC. Critical issues in bone marrow transplantation immunology [Online]. Bone Marrow Transplant 7 (Suppl 1): 24–25, 1991. http://www.ncbi.nlm.nih.gov/pubmed/2043881. [PubMed] [Google Scholar]
- 1033.Perry CGR, Lally J, Holloway GP, Heigenhauser GJF, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol 588: 4795–4810, 2010. DOI: 10.1113/jphysiol.2010.199448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1034.Pescatello LS, Riebe D, Thompson PD. ACSM’s Guidelines for Exercise Testing and Prescription. Lippincott Williams & Wilkins, 2014. [DOI] [PubMed] [Google Scholar]
- 1035.Petäjä EM, Yki-Järvinen H. Definitions of normal liver fat and the association of insulin sensitivity with acquired and genetic nafld-a systematic review. Int J Mol Sci 17, 2016. DOI: 10.3390/ijms17050633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1036.Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol 141: 1169–1179, 2018. DOI: 10.1016/j.jaci.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1037.Petersen N, Jaekel P, Rosenberger A, Weber T, Scott J, Castrucci F, Lambrecht G, Ploutz-Snyder L, Damann V, Kozlovskaya I, Mester J. Exercise in space: The european space agency approach to in-flight exercise countermeasures for long-duration missions on iss. Extreme Physiol Med 5: 9, 2016. DOI: 10.1186/s13728-016-0050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1038.Petrella JK, Kim J-S, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Phys Endocrinol Metab 291: E937–E946, 2006. DOI: 10.1152/ajpendo.00190.2006. [DOI] [PubMed] [Google Scholar]
- 1039.Petrella JK, Kim J-S, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: A cluster analysis. J Appl Physiol (1985) 104: 1736–1742, 2008. DOI: 10.1152/japplphysiol.01215.2007. [DOI] [PubMed] [Google Scholar]
- 1040.Phillips BE, Williams JP, Gustafsson T, Bouchard C, Rankinen T, Knudsen S, Smith K, Timmons JA, Atherton PJ. Molecular networks of human muscle adaptation to exercise and age. PLoS Genet 9: e1003389, 2013. DOI: 10.1371/journal.pgen.1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1041.Phillips C, Baktir MA, Srivatsan M, Salehi A. Neuroprotective effects of physical activity on the brain: A closer look at trophic factor signaling. Front Cell Neurosci 8: 170, 2014. DOI: 10.3389/fncel.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1042.Phillips MD, Patrizi RM, Cheek DJ, Wooten JS, Barbee JJ, Mitchell JB. Resistance training reduces subclinical inflammation in obese, postmenopausal women. Med Sci Sports Exerc 44: 2099–2110, 2012. DOI: 10.1249/MSS.0b013e3182644984. [DOI] [PubMed] [Google Scholar]
- 1043.Phillips SM, Parise G, Roy BD, Tipton KD, Wolfe RR, Tamopolsky MA. Resistance-training-induced adaptations in skeletal muscle protein turnover in the fed state. Can J Physiol Pharmacol 80: 1045–1053, 2002. DOI: 10.1139/y02-134. [DOI] [PubMed] [Google Scholar]
- 1044.Piasecki M, Ireland A, Piasecki J, Degens H, Stashuk DW, Swiecicka A, Rutter MK, Jones DA, McPhee JS. Long-term endurance and power training may facilitate motor unit size expansion to compensate for declining motor unit numbers in older age. Front Physiol 10: 449, 2019. DOI: 10.3389/fphys.2019.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1045.Piasecki M, Ireland A, Piasecki J, Stashuk DW, Swiecicka A, Rutter MK, Jones DA, McPhee JS. Failure to expand the motor unit size to compensate for declining motor unit numbers distinguishes sarcopenic from non-sarcopenic older men. J Physiol 596: 1627–1637, 2018. DOI: 10.1113/JP275520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1046.Pickering M-E, Simon M, Sornay-Rendu E, Chikh K, Carlier M-C, Raby A-L, Szulc P, Confavreux CB. Serum sclerostin increases after acute physical activity. Calcif Tissue Int 101: 170–173, 2017. DOI: 10.1007/s00223-017-0272-5. [DOI] [PubMed] [Google Scholar]
- 1047.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney M-T, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, FDR H, Løchen M-L, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, WMM V, Binno S. 2016 european guidelines on cardiovascular disease prevention in clinical practice: The sixth joint task force of the european society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the european association for cardiovascular prevention & rehabilitation (eacpr). Eur Heart J 37: 2315–2381, 2016. DOI: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1048.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for americans. JAMA 320: 2020–2028, 2018. DOI: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1049.Pilegaard H, Keller C, Steensberg A, Helge JW, Pedersen BK, Saltin B, Neufer PD. Influence of pre-exercise muscle glycogen content on exercise-induced transcriptional regulation of metabolic genes. J Physiol 541: 261–271, 2002. DOI: 10.1113/jphysiol.2002.016832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1050.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the pgc-1alpha gene in human skeletal muscle. J Physiol 546: 851–858, 2003. DOI: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1051.Pillon NJ, Gabriel BM, Dollet L, Smith JAB, Puig LS, Botella J, Bishop DJ, Krook A, Zierath JR. Transcriptomic profiling of skeletal muscle adaptations to exercise and inactivity. Nat Commun 11: 470, 2020. DOI: 10.1038/s41467-019-13869-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1052.Pino MF, Parsons SA, Smith SR, Sparks LM. Active individuals have high mitochondrial content and oxidative markers in their abdominal subcutaneous adipose tissue. Obesity 24: 2467–2470, 2016. DOI: 10.1002/oby.21669. [DOI] [PubMed] [Google Scholar]
- 1053.Pino MF, Stephens NA, Eroshkin AM, Yi F, Hodges A, Cornnell HH, Pratley RE, Smith SR, Wang M, Han X, Coen PM, Goodpaster BH, Sparks LM. Endurance training remodels skeletal muscle phospholipid composition and increases intrinsic mitochondrial respiration in men with type 2 diabetes. Physiol Genomics 51: 586–595, 2019. DOI: 10.1152/physiolgenomics.00014.2019. [DOI] [PubMed] [Google Scholar]
- 1054.Pires FO, Anjos CASD, Covolan RJM, Pinheiro FA, Gibson ASC, Noakes TD, Magalhães FH, Ugrinowitsch C. Cerebral regulation in different maximal aerobic exercise modes. Front Physiol 7: 253, 2016. DOI: 10.3389/fphys.2016.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1055.Pitsiladis YP, Tanaka M, Eynon N, Bouchard C, North KN, Williams AG, Collins M, Moran CN, Britton SL, Fuku N, Ashley EA, Klissouras V, Lucia A, Ahmetov II, de Geus E, Alsayrafi M. Athlome project consortium: A concerted effort to discover genomic and other “omic” markers of athletic performance. Physiol Genomics 48: 183–190, 2016. DOI: 10.1152/physiolgenomics.00105.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1056.Pleis JR, Ward BW, Lucas JW. Summary health statistics for U.S. Adults: National health interview survey, 2009 [Online]. Vital Health Stat 10: 1–207, 2010. http://www.ncbi.nlm.nih.gov/pubmed/21905346. [PubMed] [Google Scholar]
- 1057.Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE. The athlete’s heart. A meta-analysis of cardiac structure and function. Circulation 101: 336–344, 2000. DOI: 10.1161/01.cir.101.3.336. [DOI] [PubMed] [Google Scholar]
- 1058.Polaski AM, Phelps AL, Kostek MC, Szucs KA, Kolber BJ. Exercise-induced hypoalgesia: A meta-analysis of exercise dosing for the treatment of chronic pain. PLoS One 14: e0210418, 2019. DOI: 10.1371/journal.pone.0210418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1059.Polidoulis I, Beyene J, Cheung AM. The effect of exercise on pQCT parameters of bone structure and strength in post-menopausal women–a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int 23: 39–51, 2012. DOI: 10.1007/s00198-011-1734-7. [DOI] [PubMed] [Google Scholar]
- 1060.Poole DC, Jones AM. Measurement of the maximum oxygen uptake vo2max: VO2peak is no longer acceptable. J Appl Physiol (1985) 122: 997–1002, 2017. DOI: 10.1152/japplphysiol.01063.2016. [DOI] [PubMed] [Google Scholar]
- 1061.Popov DV, Lysenko EA, Bokov RO, Volodina MA, Kurochkina NS, Makhnovskii PA, Vyssokikh MY, Vinogradova OL. Effect of aerobic training on baseline expression of signaling and respiratory proteins in human skeletal muscle. Physiol Rep 6: e13868, 2018. DOI: 10.14814/phy2.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1062.Popov DV, Makhnovskii PA, Shagimardanova EI, Gazizova GR, Lysenko EA, Gusev OA, Vinogradova OL. Contractile activity-specific transcriptome response to acute endurance exercise and training in human skeletal muscle. Am J Phys Endocrinol Metab 316: E605–E614, 2019. DOI: 10.1152/ajpendo.00449.2018. [DOI] [PubMed] [Google Scholar]
- 1063.Potthoff MJ, Wu H, Arnold MA, Shelton JM, Backs J, McAnally J, Richardson JA, Bassel-Duby R, Olson EN. Histone deacetylase degradation and mef2 activation promote the formation of slow-twitch myofibers. J Clin Invest 117: 2459–2467, 2007. DOI: 10.1172/JCI31960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1064.Pourteymour S, Eckardt K, Holen T, Langleite T, Lee S, Jensen J, Birkeland KI, Drevon CA, Hjorth M. Global mRNA sequencing of human skeletal muscle: Search for novel exercise-regulated myokines. Mol Metab 6: 352–365, 2017. DOI: 10.1016/j.molmet.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1065.Power GA, Allen MD, Gilmore KJ, Stashuk DW, Doherty TJ, Hepple RT, Taivassalo T, Rice CL. Motor unit number and transmission stability in octogenarian world class athletes: Can age-related deficits be outrun? J Appl Physiol (1985) 121: 1013–1020, 2016. DOI: 10.1152/japplphysiol.00149.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1066.Powers SK. Exercise: Teaching myocytes new tricks. J Appl Physiol (1985) 123: 460–472, 2017. DOI: 10.1152/japplphysiol.00418.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1067.Powers SK, Duarte J, Kavazis AN, Talbert EE. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp Physiol 95: 1–9, 2010. DOI: 10.1113/expphysiol.2009.050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1068.Powers SK, Jackson MJ. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol Rev 88: 1243–1276, 2008. DOI: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1069.Powers SK, Ji LL, Leeuwenburgh C. Exercise training-induced alterations in skeletal muscle antioxidant capacity: A brief review. Med Sci Sports Exerc 31: 987–997, 1999. DOI: 10.1097/00005768-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 1070.Poyatos-León R, García-Hermoso A, Sanabria-Martínez G, Álvarez-Bueno C, Cavero-Redondo I, Mart’ιnez-Vizca’ιno V. Effects of exercise-based interventions on postpartum depression: A meta-analysis of randomized controlled trials. Birth 44: 200–208, 2017. DOI: 10.1111/birt.12294. [DOI] [PubMed] [Google Scholar]
- 1071.Poyatos-León R, García-Hermoso A, Sanabria-Martínez G, Álvarez-Bueno C, Sánchez-López M, Martínez-Vizcaíno V. Effects of exercise during pregnancy on mode of delivery: A meta-analysis. Acta Obstet Gynecol Scand 94: 1039–1047, 2015. DOI: 10.1111/aogs.12675. [DOI] [PubMed] [Google Scholar]
- 1072.Prestes J, Shiguemoto G, Botero JP, Frollini A, Dias R, Leite R, Pereira G, Magosso R, Baldissera V, Cavaglieri C, Perez S. Effects of resistance training on resistin, leptin, cytokines, and muscle force in elderly post-menopausal women. J Sports Sci 27: 1607–1615, 2009. DOI: 10.1080/02640410903352923. [DOI] [PubMed] [Google Scholar]
- 1073.Price TB, Rothman DL, Taylor R, Avison MJ, Shulman GI, Shulman RG. Human muscle glycogen resynthesis after exercise: Insulin-dependent and -independent phases. J Appl Physiol (1985) 76: 104–111, 1994. DOI: 10.1152/jappl.1994.76.1.104. [DOI] [PubMed] [Google Scholar]
- 1074.Prior BM, Yang HT, Terjung RL. What makes vessels grow with exercise training? J Appl Physiol (1985) 97: 1119–1128, 2004. DOI: 10.1152/japplphysiol.00035.2004. [DOI] [PubMed] [Google Scholar]
- 1075.Proctor DN, Sinning WE, Walro JM, Sieck GC, Lemon PW. Oxidative capacity of human muscle fiber types: Effects of age and training status. J Appl Physiol (1985) 78: 2033–2038, 1995. DOI: 10.1152/jappl.1995.78.6.2033. [DOI] [PubMed] [Google Scholar]
- 1076.Proske U. Exercise, fatigue and proprioception: A retrospective. Exp Brain Res 237: 2447–2459, 2019. DOI: 10.1007/s00221-019-05634-8. [DOI] [PubMed] [Google Scholar]
- 1077.Proske U, Allen T. The neural basis of the senses of effort, force and heaviness. Exp Brain Res 237: 589–599, 2019. DOI: 10.1007/s00221-018-5460-7. [DOI] [PubMed] [Google Scholar]
- 1078.Proud CG. Ras, pi3-kinase and mTOR signaling in cardiac hypertrophy. Cardiovasc Res 63: 403–413, 2004. DOI: 10.1016/j.cardiores.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 1079.Puntschart A, Claassen H, Jostarndt K, Hoppeler H, Billeter R. MRNAs of enzymes involved in energy metabolism and mtDNA are increased in endurance-trained athletes. Am J Phys 269: C619–C625, 1995. DOI: 10.1152/ajpcell.1995.269.3.C619. [DOI] [PubMed] [Google Scholar]
- 1080.Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, Hopkinson NS, Phadke R, Padhke R, Dew T, Sidhu PS, Velloso C, Seymour J, Agley CC, Selby A, Limb M, Edwards LM, Smith K, Rowlerson A, Rennie MJ, Moxham J, Harridge SDR, Hart N, Montgomery HE. Acute skeletal muscle wasting in critical illness. JAMA 310: 1591–1600, 2013. DOI: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 1081.Qiu C, Zhao X, Zhou Q, Zhang Z. High-density lipoprotein cholesterol efflux capacity is inversely associated with cardiovascular risk: A systematic review and meta-analysis. Lipids Health Dis 16: 212, 2017. DOI: 10.1186/s12944-017-0604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1082.Qu C, Wang J, Pu C, Xing Y, Shang K, Dong N, Li X. Efficiency of leukocyte differential using flow cytometry with cytodiff in different workflows. Clin Lab 63: 659–668, 2017. DOI: 10.7754/Clin.Lab.2017.161221. [DOI] [PubMed] [Google Scholar]
- 1083.Racil G, Coquart JB, Elmontassar W, Haddad M, Goebel R, Chaouachi A, Amri M, Chamari K. Greater effects of high- compared with moderate-intensity interval training on cardio-metabolic variables, blood leptin concentration and ratings of perceived exertion in obese adolescent females. Biol Sport 33: 145–152, 2016. DOI: 10.5604/20831862.1198633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1084.Radak Z, Chung HY, Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic Biol Med 44: 153–159, 2008. DOI: 10.1016/j.freeradbiomed.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 1085.Raichlen DA, Foster AD, Seillier A, Giuffrida A, Gerdeman GL. Exercise-induced endocannabinoid signaling is modulated by intensity. Eur J Appl Physiol 113: 869–875, 2013. DOI: 10.1007/s00421-012-2495-5. [DOI] [PubMed] [Google Scholar]
- 1086.Rakobowchuk M, Harris E, Taylor A, Baliga V, Cubbon RM, Rossiter HB, Birch KM. Heavy and moderate interval exercise training alters low-flow-mediated constriction but does not increase circulating progenitor cells in healthy humans. Exp Physiol 97: 375–385, 2012. DOI: 10.1113/expphysiol.2011.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1087.Rall JA. What makes skeletal muscle striated? Discoveries in the endosarcomeric and exosarcomeric cytoskeleton. Adv Physiol Educ 42: 672–684, 2018. DOI: 10.1152/advan.00152.2018. [DOI] [PubMed] [Google Scholar]
- 1088.Ramachandran K, Senagolage MD, Sommars MA, Futtner CR, Omura Y, Allred AL, Barish GD. Dynamic enhancers control skeletal muscle identity and reprogramming. PLoS Biol 17: e3000467, 2019. DOI: 10.1371/journal.pbio.3000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1089.Raschke S, Eckel J. Adipo-myokines: Two sides of the same coin–mediators of inflammation and mediators of exercise. Mediat Inflamm 2013: 320724, 2013. DOI: 10.1155/2013/320724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1090.Raue U, Slivka D, Minchev K, Trappe S. Improvements in whole muscle and myocellular function are limited with high-intensity resistance training in octogenarian women. J Appl Physiol (1985) 106: 1611–1617, 2009. DOI: 10.1152/japplphysiol.91587.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1091.Raue U, Trappe TA, Estrem ST, Qian H-R, Helvering LM, Smith RC, Trappe S. Transcriptome signature of resistance exercise adaptations: Mixed muscle and fiber type specific profiles in young and old adults. J Appl Physiol (1985) 112: 1625–1636, 2012. DOI: 10.1152/japplphysiol.00435.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1092.Reeves ND, Maganaris CN, Narici MV. Effect of strength training on human patella tendon mechanical properties of older individuals. J Physiol 548: 971–981, 2003. DOI: 10.1113/jphysiol.2002.035576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1093.Rehberg PB. August krogh, november 15, 1874-september 13, 1949 [Online]. Yale J Biol Med 24: 83–102, 1951. http://www.ncbi.nlm.nih.gov/pubmed/14901880. [PMC free article] [PubMed] [Google Scholar]
- 1094.Reidy PT, Fry CS, Dickinson JM, Drummond MJ, Rasmussen BB. Postexercise essential amino acid supplementation amplifies skeletal muscle satellite cell proliferation in older men 24 hours postexercise. Physiol Rep 5, 2017. DOI: 10.14814/phy2.13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1095.Reis E, Frick U, Schmidtbleicher D. Frequency variations of strength training sessions triggered by the phases of the menstrual cycle. Int J Sports Med 16: 545–550, 1995. DOI: 10.1055/s-2007-973052. [DOI] [PubMed] [Google Scholar]
- 1096.Reuben DB, Judd-Hamilton L, Harris TB, Seeman TE. The associations between physical activity and inflammatory markers in high-functioning older persons: MacArthur studies of successful aging. J Am Geriatr Soc 51: 1125–1130, 2003. DOI: 10.1046/j.1532-5415.2003.51380.x. [DOI] [PubMed] [Google Scholar]
- 1097.Rêgo ML, Cabral DA, Costa EC, Fontes EB. Physical exercise for individuals with hypertension: It is time to emphasize its benefits on the brain and cognition. Clin Med Insights Cardiol 13: 1179546819839411, 2019. DOI: 10.1177/1179546819839411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1098.Rich B, Scadeng M, Yamaguchi M, Wagner PD, Breen EC. Skeletal myofiber vascular endothelial growth factor is required for the exercise training-induced increase in dentate gyrus neuronal precursor cells. J Physiol 595: 5931–5943, 2017. DOI: 10.1113/JP273994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1099.Rich MW, Chyun DA, Skolnick AH, Alexander KP, Forman DE, Kitzman DW, Maurer MS, McClurken JB, Resnick BM, Shen WK, Tirschwell DL. Knowledge gaps in cardiovascular care of the older adult population: A scientific statement from the american heart association, american college of cardiology, and american geriatrics society. J Am Coll Cardiol 67: 2419–2440, 2016. DOI: 10.1016/j.jacc.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1100.Riebe D, Ehrman JK, Ligouri G, Magal M, of Sports Medicine AC. ACSM’s Guidelines for Exercise Testing and Prescription (10th ed). Lippincott williams & wilkins, 2017. [Google Scholar]
- 1101.Rigdon B, Loprinzi PD. The association of cardiorespiratory fitness on memory function: Systematic review. Medicina (Kaunas) 55, 2019. DOI: 10.3390/medicina55050127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1102.Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A 106: 8665–8670, 2009. DOI: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1103.Rix I, Nexøe-Larsen C, Bergmann NC, Lund A, Knop FK. Glucagon physiology [Online]. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dungan K, Grossman A, Hershman JM, Hofland HJ, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Purnell J, Singer F, Stratakis CA, Trence DL, Wilson DP, editors. Endotext. MDText.com, Inc, 2020. http://www.ncbi.nlm.nih.gov/books/NBK279127/ [1 Oct. 2020]. [Google Scholar]
- 1104.Roatta S, Farina D. Sympathetic actions on the skeletal muscle. Exerc Sport Sci Rev 38: 31–35, 2010. DOI: 10.1097/JES.0b013e3181c5cde7. [DOI] [PubMed] [Google Scholar]
- 1105.Roberts BM, Lavin KM, Many GM, Thalacker-Mercer A, Merritt EK, Bickel CS, Mayhew DL, Tuggle SC, Cross JM, Kosek DJ, Petrella JK, Brown CJ, Hunter GR, Windham ST, Allman RM, Bamman MM. Human neuromuscular aging: Sex differences revealed at the myocellular level. Exp Gerontol 106: 116–124, 2018. DOI: 10.1016/j.exger.2018.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1106.Roberts LD, Boström P, O’Sullivan JF, Schinzel RT, Lewis GD, Dejam A, Lee Y-K, Palma MJ, Calhoun S, Georgiadi A, Chen M-H, Ramachandran VS, Larson MG, Bouchard C, Rankinen T, Souza AL, Clish CB, Wang TJ, Estall JL, Soukas AA, Cowan CA, Spiegelman BM, Gerszten RE. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab 19: 96–108, 2014. DOI: 10.1016/j.cmet.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1107.Roberts MD, Haun CT, Mobley CB, Mumford PW, Romero MA, Roberson PA, Vann CG, McCarthy JJ. Physiological differences between low versus high skeletal muscle hypertrophic responders to resistance exercise training: Current perspectives and future research directions. Front Physiol 9: 834, 2018. DOI: 10.3389/fphys.2018.00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1108.Robinson MM, Dasari S, Konopka AR, Johnson ML, Manjunatha S, Esponda RR, Carter RE, Lanza IR, Nair KS. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab 25: 581–592, 2017. DOI: 10.1016/j.cmet.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1109.Robinson MM, Dasari S, Konopka AR, Johnson ML, Manjunatha S, Esponda RR, Carter RE, Lanza IR, Nair KS. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab 25: 581–592, 2017. DOI: 10.1016/j.cmet.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1110.Robison LS, Swenson S, Hamilton J, Thanos PK. Exercise reduces dopamine d1r and increases d2r in rats: Implications for addiction. Med Sci Sports Exerc 50: 1596–1602, 2018. DOI: 10.1249/MSS.0000000000001627. [DOI] [PubMed] [Google Scholar]
- 1111.Roddy E, Zhang W, Doherty M. Aerobic walking or strengthening exercise for osteoarthritis of the knee? A systematic review. Ann Rheum Dis 64: 544–548, 2005. DOI: 10.1136/ard.2004.028746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1112.Rodrigues ACZ, Messi ML, Wang Z-M, Abba MC, Pereyra A, Birbrair A, Zhang T, O’Meara M, Kwan P, Lopez EIS, Willis MS, Mintz A, Files DC, Furdui C, Oppenheim RW, Delbono O. The sympathetic nervous system regulates skeletal muscle motor innervation and acetylcholine receptor stability. Acta Physiol (Oxf) 225: e13195, 2019. DOI: 10.1111/apha.13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1113.Roepstorff C, Thiele M, Hillig T, Pilegaard H, Richter EA, Wojtaszewski JFP, Kiens B. Higher skeletal muscle alpha2AMPK activation and lower energy charge and fat oxidation in men than in women during submaximal exercise. J Physiol 574: 125–138, 2006. DOI: 10.1113/jphysiol.2006.108720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1114.Romero SA, Minson CT, Halliwill JR. The cardiovascular system after exercise. J Appl Physiol (1985) 122: 925–932, 2017. DOI: 10.1152/japplphysiol.00802.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1115.Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Phys 265: E380–E391, 1993. DOI: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- 1116.Romijn JA, Coyle EF, Sidossis LS, Rosenblatt J, Wolfe RR. Substrate metabolism during different exercise intensities in endurance-trained women. J Appl Physiol (1985) 88: 1707–1714, 2000. DOI: 10.1152/jappl.2000.88.5.1707. [DOI] [PubMed] [Google Scholar]
- 1117.Rosager S, Aagaard P, Dyhre-Poulsen P, Neergaard K, Kjaer M, Magnusson SP. Load-displacement properties of the human triceps surae aponeurosis and tendon in runners and non-runners. Scand J Med Sci Sports 12: 90–98, 2002. DOI: 10.1034/j.1600-0838.2002.120205.x. [DOI] [PubMed] [Google Scholar]
- 1118.Rose AJ, Frøsig C, Kiens B, Wojtaszewski JFP, Richter EA. Effect of endurance exercise training on ca2+ calmodulin-dependent protein kinase ii expression and signalling in skeletal muscle of humans. J Physiol 583: 785–795, 2007. DOI: 10.1113/jphysiol.2007.138529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1119.Rose AJ, Hargreaves M. Exercise increases ca2+-calmodulin-dependent protein kinase ii activity in human skeletal muscle. J Physiol 553: 303–309, 2003. DOI: 10.1113/jphysiol.2003.054171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1120.Rose AJ, Kiens B, Richter EA. Ca2+-calmodulin-dependent protein kinase expression and signalling in skeletal muscle during exercise. J Physiol 574: 889–903, 2006. DOI: 10.1113/jphysiol.2006.111757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1121.Rosenkranz SK, Rosenkranz RR, Hastmann TJ, Harms CA. High-intensity training improves airway responsiveness in inactive nonasthmatic children: Evidence from a randomized controlled trial. J Appl Physiol (1985) 112: 1174–1183, 2012. DOI: 10.1152/japplphysiol.00663.2011. [DOI] [PubMed] [Google Scholar]
- 1122.Ross MD, Wekesa AL, Phelan JP, Harrison M. Resistance exercise increases endothelial progenitor cells and angiogenic factors. Med Sci Sports Exerc 46: 16–23, 2014. DOI: 10.1249/MSS.0b013e3182a142da. [DOI] [PubMed] [Google Scholar]
- 1123.Rotondo F, Ho-Palma AC, Remesar X, Fernández-López JA, Romero MDM, Alemany M. Glycerol is synthesized and secreted by adipocytes to dispose of excess glucose, via glycerogenesis and increased acyl-glycerol turnover. Sci Rep 7: 8983, 2017. DOI: 10.1038/s41598-017-09450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1124.Rowell LB, Rowell BLB. Human Cardiovascular Control. Oxford University Press, 1993. [Google Scholar]
- 1125.Römisch-Margl W, Prehn C, Bogumil R, Röhring C, Suhre K, Adamski J. Procedure for tissue sample preparation and metabolite extraction for high-throughput targeted metabolomics. Metabolomics 8: 133–142, 2011. DOI: 10.1007/s11306-011-0293-4. [DOI] [Google Scholar]
- 1126.Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, Greene NP, Wu J, Estall JL, Irving BA, Lanza IR, Rasbach KA, Okutsu M, Nair KS, Yan Z, Leinwand LA, Spiegelman BM. A pgc-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 151: 1319–1331, 2012. DOI: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1127.Rubenstein AB, Smith GR, Raue U, Begue G, Minchev K, Ruf-Zamojski F, Nair VD, Wang X, Zhou L, Zaslavsky E, Trappe TA, Trappe S, Sealfon SC. Single-cell transcriptional profiles in human skeletal muscle. Sci Rep 10: 229, 2020. DOI: 10.1038/s41598-019-57110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1128.Rubin RP. Carl and Gerty Cori: A collaboration that changed the face of biochemistry. J Med Biogr 29: 143–148, 2019. DOI: 10.1177/0967772019866954. [DOI] [PubMed] [Google Scholar]
- 1129.Ruiz-Ramie JJ, Barber JL, Sarzynski MA. Effects of exercise on hdl functionality. Curr Opin Lipidol 30: 16–23, 2019. DOI: 10.1097/MOL.0000000000000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1130.Rullman E, Fernandez-Gonzalo R, Mekjavi’c IB, Gustafsson T, Eiken O. MEF2 as upstream regulator of the transcriptome signature in human skeletal muscle during unloading. Am J Physiol Regul Integr Comp Physiol 315: R799–R809, 2018. DOI: 10.1152/ajpregu.00452.2017. [DOI] [PubMed] [Google Scholar]
- 1131.Russell AP, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C, Meier CA, Bell DR, Kralli A, Giacobino J-P, Dériaz O. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes 52: 2874–2881, 2003. DOI: 10.2337/diabetes.52.12.2874. [DOI] [PubMed] [Google Scholar]
- 1132.Russell AP, Foletta VC, Snow RJ, Wadley GD. Skeletal muscle mitochondria: A major player in exercise, health and disease. Biochim Biophys Acta 1840: 1276–1284, 2014. DOI: 10.1016/j.bbagen.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 1133.Saanijoki T, Tuominen L, Tuulari JJ, Nummenmaa L, Arponen E, Kalliokoski K, Hirvonen J. Opioid release after high-intensity interval training in healthy human subjects. Neuropsychopharmacology 43: 246–254, 2018. DOI: 10.1038/npp.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1134.Sachdev S, Davies KJA. Production, detection, and adaptive responses to free radicals in exercise. Free Radic Biol Med 44: 215–223, 2008. DOI: 10.1016/j.freeradbiomed.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 1135.Sachs S, Zarini S, Kahn DE, Harrison KA, Perreault L, Phang T, Newsom SA, Strauss A, Kerege A, Schoen JA, Bessesen DH, Schwarzmayr T, Graf E, Lutter D, Krumsiek J, Hofmann SM, Bergman BC. Intermuscular adipose tissue directly modulates skeletal muscle insulin sensitivity in humans. Am J Phys Endocrinol Metab 316: E866–E879, 2019. DOI: 10.1152/ajpendo.00243.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1136.Safdar A, Tarnopolsky MA. Exosomes as mediators of the systemic adaptations to endurance exercise. Cold Spring Harb Perspect Med 8, 2018. DOI: 10.1101/cshperspect.a029827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1137.Safieh M, Korczyn AD, Michaelson DM. ApoE4: An emerging therapeutic target for alzheimer’s disease. BMC Med 17: 64, 2019. DOI: 10.1186/s12916-019-1299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1138.Sah N, Peterson BD, Lubejko ST, Vivar C, van Praag H. Running reorganizes the circuitry of one-week-old adult-born hippocampal neurons. Sci Rep 7: 10903, 2017. DOI: 10.1038/s41598-017-11268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1139.Sakellariou GK, McDonagh B. Redox homeostasis in age-related muscle atrophy. Adv Exp Med Biol 1088: 281–306, 2018. DOI: 10.1007/978-981-13-1435-3_13. [DOI] [PubMed] [Google Scholar]
- 1140.Sale DG. Neural adaptation to resistance training. Med Sci Sports Exerc 20: S135–S145, 1988. DOI: 10.1249/00005768-198810001-00009. [DOI] [PubMed] [Google Scholar]
- 1141.Sallis R, Franklin B, Joy L, Ross R, Sabgir D, Stone J. Strategies for promoting physical activity in clinical practice. Prog Cardiovasc Dis 57: 375–386, 2014. DOI: 10.1016/j.pcad.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 1142.Salva JE, Merrill AE. Signaling networks in joint development. Dev Dyn 246: 262–274, 2017. DOI: 10.1002/dvdy.24472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1143.Sanabria-Mart’ιnez G, Garc’ιa-Hermoso A, Poyatos-Le’on R, Gonz’alez-Garc’ιa A, S’anchez-L’opez M, Mart’ιnez-Vizca’ιno V. Effects of exercise-based interventions on neonatal outcomes: A meta-analysis of randomized controlled trials. Am J Health Promot 30: 214–223, 2016. DOI: 10.1177/0890117116639569. [DOI] [PubMed] [Google Scholar]
- 1144.Sanabria-Martínez G, Poyatos-León R, Notario-Pacheco B, Álvarez-Bueno C, Cavero-Redondo I, Martinez-Vizcaino V. Effects of physical exercise during pregnancy on mothers’ and neonates’ health: A protocol for an umbrella review of systematic reviews and meta-analysis of randomised controlled trials. BMJ Open 9: e030162, 2019. DOI: 10.1136/bmjopen-2019-030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1145.Sandri M, Barberi L, Bijlsma AY, Blaauw B, Dyar KA, Milan G, Mammucari C, Meskers CGM, Pallafacchina G, Paoli A, Pion D, Roceri M, Romanello V, Serrano AL, Toniolo L, Larsson L, Maier AB, Munoz-Canoves P, Musaro A, Pende M, Reggiani C, Rizzuto R, Schiaffino S. Signalling pathways regulating muscle mass in ageing skeletal muscle: The role of the igf1-akt-mTOR-foxo pathway. Biogerontology 14: 303–323, 2013. DOI: 10.1007/s10522-013-9432-9. [DOI] [PubMed] [Google Scholar]
- 1146.Sanford JA, Nogiec CD, Lindholm ME, Adkins JN, Amar D, Dasari S, Drugan JK, Fern’andez FM, Radom-Aizik S, Schenk S, Snyder MP, Tracy RP, Vanderboom P, Trappe S, Walsh MJ. Molecular transducers of physical activity consortium (motrpac): Mapping the dynamic responses to exercise. Cell 181: 1464–1474, 2020. DOI: 10.1016/j.cell.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1147.Santos L, Elliott-Sale KJ, Sale C. Exercise and bone health across the lifespan. Biogerontology 18: 931–946, 2017. DOI: 10.1007/s10522-017-9732-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1148.Santos-Concejero J, Billaut F, Grobler L, Oliv’an J, Noakes TD, Tucker R. Brain oxygenation declines in elite kenyan runners during a maximal interval training session. Eur J Appl Physiol 117: 1017–1024, 2017. DOI: 10.1007/s00421-017-3590-4. [DOI] [PubMed] [Google Scholar]
- 1149.Santos-Parker JR, Santos-Parker KS, McQueen MB, Martens CR, Seals DR. Habitual aerobic exercise and circulating proteomic patterns in healthy adults: Relation to indicators of healthspan. J Appl Physiol (1985) 125: 1646–1659, 2018. DOI: 10.1152/japplphysiol.00458.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1150.Santos-Parker JR, Strahler TR, Vorwald VM, Pierce GL, Seals DR. Habitual aerobic exercise does not protect against micro- or macrovascular endothelial dysfunction in healthy estrogen-deficient postmenopausal women. J Appl Physiol (1985) 122: 11–19, 2017. DOI: 10.1152/japplphysiol.00732.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1151.Sanyaolu A, Okorie C, Qi X, Locke J, Rehman S. Childhood and adolescent obesity in the united states: A public health concern. Glob Pediatr Health 6: 2333794X19891305, 2019. DOI: 10.1177/2333794X19891305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1152.Sañudo B, de Hoyo M, Pozo-Cruz JD, Carrasco L, Pozo-Cruz BD, Tejero S, Firth E. A systematic review of the exercise effect on bone health: The importance of assessing mechanical loading in perimenopausal and postmenopausal women. Menopause 24: 1208–1216, 2017. DOI: 10.1097/GME.0000000000000872. [DOI] [PubMed] [Google Scholar]
- 1153.Sari S, Bilberg R, Nielsen AS, Roessler KK. The effect of exercise as adjunctive treatment on quality of life for individuals with alcohol use disorders: A randomized controlled trial. BMC Public Health 19: 727, 2019. DOI: 10.1186/s12889-019-7083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1154.Scarpulla RC. Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim Biophys Acta 1576: 1–14, 2002. DOI: 10.1016/s0167-4781(02)00343-3. [DOI] [PubMed] [Google Scholar]
- 1155.Scarvell J, Elkins MR. Aerobic exercise is beneficial for people with rheumatoid arthritis. Br J Sports Med 45: 1008–1009, 2011. DOI: 10.1136/bjsports-2011-090388. [DOI] [PubMed] [Google Scholar]
- 1156.Schantz P Along paths converging to bengt saltin’s early contributions in exercise physiology. Scand J Med Sci Sports 25 (Suppl 4): 7–15, 2015. DOI: 10.1111/sms.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1157.Scheffler RW. The power of exercise and the exercise of power: The harvard fatigue laboratory, distance running, and the disappearance of work, 1919–1947. J Hist Biol 48: 391–423, 2015. DOI: 10.1007/s10739-014-9392-1. [DOI] [PubMed] [Google Scholar]
- 1158.Schenkman M, Moore CG, Kohrt WM, Hall DA, Delitto A, Comella CL, Josbeno DA, Christiansen CL, Berman BD, Kluger BM, Melanson EL, Jain S, Robichaud JA, Poon C, Corcos DM. Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo parkinson disease: A phase 2 randomized clinical trial. JAMA Neurol 75: 219–226, 2018. DOI: 10.1001/jamaneurol.2017.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1159.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev 91: 1447–1531, 2011. DOI: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 1160.Schild M, Ruhs A, Beiter T, Zügel M, Hudemann J, Reimer A, Krumholz-Wagner I, Wagner C, Keller J, Eder K, Krüger K, Krüger M, Braun T, Nieß A, Steinacker J, Mooren FC. Basal and exercise induced label-free quantitative protein profiling of m. Vastus lateralis in trained and untrained individuals. J Proteome 122: 119–132, 2015. DOI: 10.1016/j.jprot.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 1161.Schlittler M, Goiny M, Agudelo LZ, Venckunas T, Brazaitis M, Skurvydas A, Kamandulis S, Ruas JL, Erhardt S, Westerblad H, Andersson DC. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am J Phys Cell Phys 310: C836–C840, 2016. DOI: 10.1152/ajpcell.00053.2016. [DOI] [PubMed] [Google Scholar]
- 1162.Schmidt-Arras D, Rose-John S. IL-6 pathway in the liver: From physiopathology to therapy. J Hepatol 64: 1403–1415, 2016. DOI: 10.1016/j.jhep.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 1163.Schmidt-Nielsen B. August and marie krogh and respiratory physiology. J Appl Physiol Respir Environ Exerc Physiol 57: 293–303, 1984. DOI: 10.1152/jappl.1984.57.2.293. [DOI] [PubMed] [Google Scholar]
- 1164.Schmidt-Nielsen K Per fredrik thorkelsson scholander: November 29, 1905-june 13, 1980 [Online]. Biogr Mem Natl Acad Sci 56: 387–412, 1986. http://www.ncbi.nlm.nih.gov/pubmed/11621210. [PubMed] [Google Scholar]
- 1165.Schmitz B, Rolfes F, Schelleckes K, Mewes M, Thorwesten L, Krüger M, Klose A, Brand S-M. Longer work/rest intervals during high-intensity interval training (hiit) lead to elevated levels of miR-222 and miR-29c. Front Physiol 9: 395, 2018. DOI: 10.3389/fphys.2018.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1166.Schnohr P, O’Keefe JH, Marott JL, Lange P, Jensen GB. Dose of jogging and long-term mortality: The copenhagen city heart study. J Am Coll Cardiol 65: 411–419, 2015. DOI: 10.1016/j.jacc.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 1167.Schoenfeld BJ. Is there a minimum intensity threshold for resistance training-induced hypertrophic adaptations? Sports Med 43: 1279–1288, 2013. DOI: 10.1007/s40279-013-0088-z. [DOI] [PubMed] [Google Scholar]
- 1168.Schoenfeld BJ, Ogborn D, Krieger JW. Dose-response relationship between weekly resistance training volume and increases in muscle mass: A systematic review and meta-analysis. J Sports Sci 35: 1073–1082, 2017. DOI: 10.1080/02640414.2016.1210197. [DOI] [PubMed] [Google Scholar]
- 1169.Schoenfeld BJ, Grgic J, Krieger J. How many times per week should a muscle be trained to maximize muscle hypertrophy? A systematic review and meta-analysis of studies examining the effects of resistance training frequency. J Sports Sci 37: 1286–1295, 2019. DOI: 10.1080/02640414.2018.1555906. [DOI] [PubMed] [Google Scholar]
- 1170.Schultz SG. William harvey and the circulation of the blood: The birth of a scientific revolution and modern physiology. News Physiol Sci 17: 175–180, 2002. DOI: 10.1152/nips.01391.2002. [DOI] [PubMed] [Google Scholar]
- 1171.Schwarb H, Johnson CL, Daugherty AM, Hillman CH, Kramer AF, Cohen NJ, Barbey AK. Aerobic fitness, hippocampal viscoelasticity, and relational memory performance. NeuroImage 153: 179–188, 2017. DOI: 10.1016/j.neuroimage.2017.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1172.Sciarretta S, Volpe M, Sadoshima J. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ Res 114: 549–564, 2014. DOI: 10.1161/CIRCRESAHA.114.302022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1173.Seals DR, Justice JN, LaRocca TJ. Physiological geroscience: Targeting function to increase healthspan and achieve optimal longevity. J Physiol 594: 2001–2024, 2016. DOI: 10.1113/jphysiol.2014.282665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1174.Seferovic PM, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JGF, de Boer RA, Drexel H, Gal TB, Hill L, Jaarsma T, Jankowska EA, Anker MS, Lainscak M, Lewis BS, McDonagh T, Metra M, Milicic D, Mullens W, Piepoli MF, Rosano G, Ruschitzka F, Volterrani M, Voors AA, Filippatos G, Coats AJS. Clinical practice update on heart failure 2019: Pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the heart failure association of the european society of cardiology. Eur J Heart Fail 21: 1169, 2019–1186. DOI: 10.1002/ejhf.1531. [DOI] [PubMed] [Google Scholar]
- 1175.Segal RJ, Reid RD, Courneya KS, Sigal RJ, Kenny GP, Prud’Homme DG, Malone SC, Wells GA, Scott CG, D’Angelo MES. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol 27: 344–351, 2009. DOI: 10.1200/JCO.2007.15.4963. [DOI] [PubMed] [Google Scholar]
- 1176.Seip RL, Mair K, Cole TG, Semenkovich CF. Induction of human skeletal muscle lipoprotein lipase gene expression by short-term exercise is transient. Am J Phys 272: E255–E261, 1997. DOI: 10.1152/ajpendo.1997.272.2.E255. [DOI] [PubMed] [Google Scholar]
- 1177.Sen EI, Esmaeilzadeh S, Eskiyurt N. Effects of whole-body vibration and high impact exercises on the bone metabolism and functional mobility in postmenopausal women. J Bone Miner Metab 38: 392–404, 2020. DOI: 10.1007/s00774-019-01072-2. [DOI] [PubMed] [Google Scholar]
- 1178.Senoo N, Miyoshi N, Goto-Inoue N, Minami K, Yoshimura R, Morita A, Sawada N, Matsuda J, Ogawa Y, Setou M, Kamei Y, Miura S. PGC-1α-mediated changes in phospholipid profiles of exercise-trained skeletal muscle. J Lipid Res 56: 2286–2296, 2015. DOI: 10.1194/jlr.M060533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1179.Seo DY, Kwak H-B, Kim AH, Park SH, Heo JW, Kim HK, Ko JR, Lee SJ, Bang HS, Sim JW, Kim M, Han J. Cardiac adaptation to exercise training in health and disease. Pflugers Arch—Eur J Physiol 472: 155–168, 2020. DOI: 10.1007/s00424-019-02266-3. [DOI] [PubMed] [Google Scholar]
- 1180.Seravalle G, Grassi G. Obesity and hypertension. Pharmacol Res 122: 1–7, 2017. DOI: 10.1016/j.phrs.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 1181.Serneri GGN, Boddi M, Modesti PA, Cecioni I, Coppo M, Padeletti L, Michelucci A, Colella A, Galanti G. Increased cardiac sympathetic activity and insulin-like growth factor-i formation are associated with physiological hypertrophy in athletes. Circ Res 89: 977–982, 2001. DOI: 10.1161/hh2301.100982. [DOI] [PubMed] [Google Scholar]
- 1182.Severinghaus JW. The most important discovery of science. Adv Exp Med Biol 876: 1–16, 2016. DOI: 10.1007/978-1-4939-3023-4_1. [DOI] [PubMed] [Google Scholar]
- 1183.Severinghaus JW. Fire-air and dephlogistication. Revisionisms of oxygen’s discovery [Online]. Adv Exp Med Biol 543: 7–19, 2003. http://www.ncbi.nlm.nih.gov/pubmed/14713111. [PubMed] [Google Scholar]
- 1184.Shadick NA, Katz P, Iannaccone CI, Maica G, Coblyn J, Weinblatt ME, Cui J. The impact of exercise, lifestyle, and clinical factors on perceived cognitive function in patients with rheumatoid arthritis: Results from a prospective cohort study. ACR Open Rheumatol 1: 620–626, 2019. DOI: 10.1002/acr2.11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1185.Shah T, Palaskas N, Ahmed A. An update on gender disparities in coronary heart disease care. Curr Atheroscler Rep 18: 28, 2016. DOI: 10.1007/s11883-016-0574-5. [DOI] [PubMed] [Google Scholar]
- 1186.Shapiro E Adolf fickForgotten genius of cardiology. Am J Cardiol 30: 662–665, 1972. DOI: 10.1016/0002-9149(72)90606-6. [DOI] [PubMed] [Google Scholar]
- 1187.Shephard RJ. Open-circuit respirometry: A brief historical review of the use of douglas bags and chemical analyzers. Eur J Appl Physiol 117: 381–387, 2017. DOI: 10.1007/s00421-017-3556-6. [DOI] [PubMed] [Google Scholar]
- 1188.Shephard RJ. Is it time to retire the ‘central governor’? Sports Med 39: 709–721, 2009. DOI: 10.2165/11315130-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 1189.Shephard RJ, Johnson N. Effects of physical activity upon the liver. Eur J Appl Physiol 115: 1–46, 2015. DOI: 10.1007/s00421-014-3031-6. [DOI] [PubMed] [Google Scholar]
- 1190.Shepherd SO, Cocks M, Meikle PJ, Mellett NA, Ranasinghe AM, Barker TA, Wagenmakers AJM, Shaw CS. Lipid droplet remodelling and reduced muscle ceramides following sprint interval and moderate-intensity continuous exercise training in obese males. Int J Obes 41: 1745–1754, 2017. DOI: 10.1038/ijo.2017.170. [DOI] [PubMed] [Google Scholar]
- 1191.Sherling DH, Perumareddi P, Hennekens CH. Metabolic syndrome. J Cardiovasc Pharmacol Ther 22: 365–367, 2017. DOI: 10.1177/1074248416686187. [DOI] [PubMed] [Google Scholar]
- 1192.Sherrington C, Michaleff ZA, Fairhall N, Paul SS, Tiedemann A, Whitney J, Cumming RG, Herbert RD, Close JCT, Lord SR. Exercise to prevent falls in older adults: An updated systematic review and meta-analysis. Br J Sports Med 51: 1750–1758, 2017. DOI: 10.1136/bjsports-2016-096547. [DOI] [PubMed] [Google Scholar]
- 1193.Shibata S, Fujimoto N, Hastings JL, Carrick-Ranson G, Bhella PS, Hearon CM, Levine BD. The effect of lifelong exercise frequency on arterial stiffness. J Physiol 596: 2783–2795, 2018. DOI: 10.1113/JP275301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1194.Shim JW, Madsen JR. VEGF signaling in neurological disorders. Int J Mol Sci 19, 2018. DOI: 10.3390/ijms19010275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1195.Shimada H, Ishii K, Makizako H, Ishiwata K, Oda K, Suzukawa M. Effects of exercise on brain activity during walking in older adults: A randomized controlled trial. J Neuroeng Rehabil 14: 50, 2017. DOI: 10.1186/s12984-017-0263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1196.Shotwell RA. Animals, pictures, and skeletons: Andreas vesalius’s reinvention of the public anatomy lesson. J Hist Med Allied Sci 71: 1–18, 2016. DOI: 10.1093/jhmas/jrv001. [DOI] [PubMed] [Google Scholar]
- 1197.Sibonga J, Matsumoto T, Jones J, Shapiro J, Lang T, Shackelford L, Smith SM, Young M, Keyak J, Kohri K, Ohshima H, Spector E, LeBlanc A. Resistive exercise in astronauts on prolonged spaceflights provides partial protection against spaceflight-induced bone loss. Bone 128: 112037, 2019. DOI: 10.1016/j.bone.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 1198.Sidossis L, Kajimura S. Brown and beige fat in humans: Thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest 125: 478–486, 2015. DOI: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1199.Sidossis LS, Porter C, Saraf MK, Børsheim E, Radhakrishnan RS, Chao T, Ali A, Chondronikola M, Mlcak R, Finnerty CC, Hawkins HK, Toliver-Kinsky T, Herndon DN. Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab 22: 219–227, 2015. DOI: 10.1016/j.cmet.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1200.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 70: 7–30, 2020. DOI: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 1201.Sieljacks P, Wang J, Groennebaek T, Rindom E, Jakobsgaard JE, Herskind J, Gravholt A, Møller AB, Musci RV, de Paoli FV, Hamilton KL, Miller BF, Vissing K. Six weeks of low-load blood flow restricted and high-load resistance exercise training produce similar increases in cumulative myofibrillar protein synthesis and ribosomal biogenesis in healthy males. Front Physiol 10: 649, 2019. DOI: 10.3389/fphys.2019.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1202.Sigal RJ, Alberga AS, Goldfield GS, Prud’homme D, Hadjiyannakis S, Gougeon R, Phillips P, Tulloch H, Malcolm J, Doucette S, Wells GA, Ma J, Kenny GP. Effects of aerobic training, resistance training, or both on percentage body fat and cardiometabolic risk markers in obese adolescents: The healthy eating aerobic and resistance training in youth randomized clinical trial. JAMA Pediatr 168: 1006–1014, 2014. DOI: 10.1001/jamapediatrics.2014.1392. [DOI] [PubMed] [Google Scholar]
- 1203.Silva GJJ, Bye A, Azzouzi HE, Wisløff U. MicroRNAs as important regulators of exercise adaptation. Prog Cardiovasc Dis 60: 130–151, 2017. DOI: 10.1016/j.pcad.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 1204.Silva NDD, Fernandes T, Soci UPR, Monteiro AWA, Phillips MI, Oliveira EMD. Swimming training in rats increases cardiac microrna-126 expression and angiogenesis. Med Sci Sports Exerc 44: 1453–1462, 2012. DOI: 10.1249/MSS.0b013e31824e8a36. [DOI] [PubMed] [Google Scholar]
- 1205.Silva-Batista C, Corcos DM, Roschel H, Kanegusuku H, Gobbi LTB, Piemonte MEP, Mattos ECT, Mello MTD, Forjaz CLM, Tricoli V, Ugrinowitsch C. Resistance training with instability for patients with parkinson’s disease. Med Sci Sports Exerc 48: 1678–1687, 2016. DOI: 10.1249/MSS.0000000000000945. [DOI] [PubMed] [Google Scholar]
- 1206.Simon J, Young JL, Blood DK, Segal KR, Case RB, Gutin B. Plasma lactate and ventilation thresholds in trained and untrained cyclists. J Appl Physiol (1985) 60: 777–781, 1986. DOI: 10.1152/jappl.1986.60.3.777. [DOI] [PubMed] [Google Scholar]
- 1207.Simpson RJ, Lowder TW, Spielmann G, Bigley AB, LaVoy EC, Kunz H. Exercise and the aging immune system. Ageing Res Rev 11: 404–420, 2012. DOI: 10.1016/j.arr.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 1208.Simsch C, Lormes W, Petersen KG, Baur S, Liu Y, Hackney AC, Lehmann M, Steinacker JM. Training intensity influences leptin and thyroid hormones in highly trained rowers. Int J Sports Med 23: 422–427, 2002. DOI: 10.1055/s-2002-33738. [DOI] [PubMed] [Google Scholar]
- 1209.Singh JA, Yu S. Septic arthritis in emergency departments in the us: A national study of health care utilization and time trends. Arthritis Care Res 70: 320–326, 2018. DOI: 10.1002/acr.23270. [DOI] [PubMed] [Google Scholar]
- 1210.Singhal T A review of coronavirus disease-2019 (covid-19). Indian J Pediatr 87: 281–286, 2020. DOI: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1211.Sipilä S, Törmäkangas T, Sillanpää E, Aukee P, Kujala UM, Kovanen V, Laakkonen EK. Muscle and bone mass in middle-aged women: Role of menopausal status and physical activity. J Cachexia Sarcopenia Muscle 11: 698–709, 2020. DOI: 10.1002/jcsm.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1212.Siu PM, Donley DA, Bryner RW, Alway SE. Citrate synthase expression and enzyme activity after endurance training in cardiac and skeletal muscles. J Appl Physiol (1985) 94: 555–560, 2003. DOI: 10.1152/japplphysiol.00821.2002. [DOI] [PubMed] [Google Scholar]
- 1213.Siu PM, Donley DA, Bryner RW, Alway SE. Myogenin and oxidative enzyme gene expression levels are elevated in rat soleus muscles after endurance training. J Appl Physiol (1985) 97: 277–285, 2004. DOI: 10.1152/japplphysiol.00534.2004. [DOI] [PubMed] [Google Scholar]
- 1214.Sjøgaard G The august krogh institute: Capillaries and beyond. Scand J Med Sci Sports 25 (Suppl 4): 16–21, 2015. DOI: 10.1111/sms.12552. [DOI] [PubMed] [Google Scholar]
- 1215.Skovgaard C, Almquist NW, Kvorning T, Christensen PM, Bangsbo J. Effect of tapering after a period of high-volume sprint interval training on running performance and muscular adaptations in moderately trained runners. J Appl Physiol (1985) 124: 259–267, 2018. DOI: 10.1152/japplphysiol.00472.2017. [DOI] [PubMed] [Google Scholar]
- 1216.Slivka D, Raue U, Hollon C, Minchev K, Trappe S. Single muscle fiber adaptations to resistance training in old (>80 yr) men: Evidence for limited skeletal muscle plasticity. Am J Physiol Regul Integr Comp Physiol 295: R273–R280, 2008. DOI: 10.1152/ajpregu.00093.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1217.Sloth M, Sloth D, Overgaard K, Dalgas U. Effects of sprint interval training on vo2max and aerobic exercise performance: A systematic review and meta-analysis. Scand J Med Sci Sports 23: e341–e352, 2013. DOI: 10.1111/sms.12092. [DOI] [PubMed] [Google Scholar]
- 1218.Sluka KA, Frey-Law L, Bement MH. Exercise-induced pain and analgesia? Underlying mechanisms and clinical translation. Pain 159 (Suppl 1): S91–S97, 2018. DOI: 10.1097/j.pain.0000000000001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1219.Smaglik P, Butler D. Celera turns to public genome data to speed up endgame. Nature 403: 119, 2000. DOI: 10.1038/35003269. [DOI] [PubMed] [Google Scholar]
- 1220.Smith GI, Atherton P, Reeds DN, Mohammed BS, Jaffery H, Rankin D, Rennie MJ, Mittendorfer B. No major sex differences in muscle protein synthesis rates in the postabsorptive state and during hyperinsulinemia-hyperaminoacidemia in middle-aged adults. J Appl Physiol (1985) 107: 1308–1315, 2009. DOI: 10.1152/japplphysiol.00348.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1221.Smith NT, Soriano-Arroquia A, Goljanek-Whysall K, Jackson MJ, McDonagh B. Redox responses are preserved across muscle fibres with differential susceptibility to aging. J Proteome 177: 112–123, 2018. DOI: 10.1016/j.jprot.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1222.Smith SM, Heer MA, Shackelford LC, Sibonga JD, Ploutz-Snyder L, Zwart SR. Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: Evidence from biochemistry and densitometry. J Bone Miner Res Off J Am Soc Bone Miner Res 27: 1896–1906, 2012. DOI: 10.1002/jbmr.1647. [DOI] [PubMed] [Google Scholar]
- 1223.Smith SM, Heer M, Shackelford LC, Sibonga JD, Spatz J, Pietrzyk RA, Hudson EK, Zwart SR. Bone metabolism and renal stone risk during international space station missions. Bone 81: 712–720, 2015. DOI: 10.1016/j.bone.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 1224.Smith SM, Abrams SA, Davis-Street JE, Heer M, O’Brien KO, Wastney ME, Zwart SR. Fifty years of human space travel: Implications for bone and calcium research. Annu Rev Nutr 34: 377–400, 2014. DOI: 10.1146/annurev-nutr-071813-105440. [DOI] [PubMed] [Google Scholar]
- 1225.Smith TO, King JJ, Hing CB. The effectiveness of proprioceptive-based exercise for osteoarthritis of the knee: A systematic review and meta-analysis. Rheumatol Int 32: 3339–3351, 2012. DOI: 10.1007/s00296-012-2480-7. [DOI] [PubMed] [Google Scholar]
- 1226.Snijders T, Nederveen JP, Joanisse S, Leenders M, Verdijk LB, van Loon LJC, Parise G. Muscle fibre capillarization is a critical factor in muscle fibre hypertrophy during resistance exercise training in older men. J Cachexia Sarcopenia Muscle 8: 267–276, 2017. DOI: 10.1002/jcsm.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1227.Snijders T, Nederveen JP, McKay BR, Joanisse S, Verdijk LB, van Loon LJC, Parise G. Satellite cells in human skeletal muscle plasticity. Front Physiol 6: 283, 2015. DOI: 10.3389/fphys.2015.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1228.Snijders T, Verdijk LB, Smeets JSJ, McKay BR, Senden JMG, Hartgens F, Parise G, Greenhaff P, van Loon LJC. The skeletal muscle satellite cell response to a single bout of resistance-type exercise is delayed with aging in men. Age (Dordr) 36: 9699, 2014. DOI: 10.1007/s11357-014-9699-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1229.Snijders T, Smeets JSJ, van Kranenburg J, Kies AK, van Loon LJC, Verdijk LB. Changes in myonuclear domain size do not precede muscle hypertrophy during prolonged resistance-type exercise training. Acta Physiol (Oxf) 216: 231–239, 2016. DOI: 10.1111/apha.12609. [DOI] [PubMed] [Google Scholar]
- 1230.Song M, Giovannucci E. Preventable incidence of carcinoma associated with adiposity, alcohol and physical inactivity according to smoking status in the united states. Int J Cancer 146: 2960–2967, 2020. DOI: 10.1002/ijc.32602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1231.Sparks LM. Exercise training response heterogeneity: Physiological and molecular insights. Diabetologia 60: 2329–2336, 2017. DOI: 10.1007/s00125-017-4461-6. [DOI] [PubMed] [Google Scholar]
- 1232.Sparks LM, Moro C, Ukropcova B, Bajpeyi S, Civitarese AE, Hulver MW, Thoresen GH, Rustan AC, Smith SR. Remodeling lipid metabolism and improving insulin responsiveness in human primary myotubes. PLoS One 6: e21068, 2011. DOI: 10.1371/journal.pone.0021068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1233.Spence AL, Carter HH, Murray CP, Oxborough D, Naylor LH, George KP, Green DJ. Magnetic resonance imaging-derived right ventricular adaptations to endurance versus resistance training. Med Sci Sports Exerc 45: 534–541, 2013. DOI: 10.1249/MSS.0b013e3182780b0e. [DOI] [PubMed] [Google Scholar]
- 1234.Spence AL, Carter HH, Naylor LH, Green DJ. A prospective randomized longitudinal study involving 6 months of endurance or resistance exercise. Conduit artery adaptation in humans. J Physiol 591: 1265–1275, 2013. DOI: 10.1113/jphysiol.2012.247387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1235.Spirduso WW, Cronin DL. Exercise dose-response effects on quality of life and independent living in older adults. Med Sci Sports Exerc 33: S598–608; discussion S609–10, 2001. DOI: 10.1097/00005768-200106001-00028. [DOI] [PubMed] [Google Scholar]
- 1236.Sproston NR, Ashworth JJ. Role of c-reactive protein at sites of inflammation and infection. Front Immunol 9: 754, 2018. DOI: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1237.Standley RA, Liu SZ, Jemiolo B, Trappe SW, Trappe TA. Prostaglandin e2 induces transcription of skeletal muscle mass regulators interleukin-6 and muscle ring finger-1 in humans. Prostaglandins Leukot Essent Fatty Acids 88: 361–364, 2013. DOI: 10.1016/j.plefa.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1238.Stanford KI, Goodyear LJ. Muscle-adipose tissue cross talk. Cold Spring Harb Perspect Med 8, 2018. DOI: 10.1101/cshperspect.a029801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1239.Stanford KI, Lynes MD, Takahashi H, Baer LA, Arts PJ, May FJ, Lehnig AC, Middelbeek RJW, Richard JJ, So K, Chen EY, Gao F, Narain NR, Distefano G, Shettigar VK, Hirshman MF, Ziolo MT, Kiebish MA, Tseng Y-H, Coen PM, Goodyear LJ. 12,13-diHOME: An exercise-induced lipokine that increases skeletal muscle fatty acid uptake. Cell Metab 27: 1111–1120.e3, 2018. DOI: 10.1016/j.cmet.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1240.Stanghelle B, Bentzen H, Giangregorio L, Pripp AH, Skelton DA, Bergland A. Effects of a resistance and balance exercise programme on physical fitness, health-related quality of life and fear of falling in older women with osteoporosis and vertebral fracture: A randomized controlled trial. Osteoporos Int 31: 1069–1078, 2020. DOI: 10.1007/s00198-019-05256-4. [DOI] [PubMed] [Google Scholar]
- 1241.Starkie RL, Arkinstall MJ, Koukoulas I, Hawley JA, Febbraio MA. Carbohydrate ingestion attenuates the increase in plasma interleukin-6, but not skeletal muscle interleukin-6 mRNA, during exercise in humans. J Physiol 533: 585–591, 2001. DOI: 10.1111/j.1469-7793.2001.0585a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1242.Staron RS, Karapondo DL, Kraemer WJ, Fry AC, Gordon SE, Falkel JE, Hagerman FC, Hikida RS. Skeletal muscle adaptations during early phase of heavy-resistance training in men and women. J Appl Physiol (1985) 76: 1247–1255, 1994. DOI: 10.1152/jappl.1994.76.3.1247. [DOI] [PubMed] [Google Scholar]
- 1243.Ståhl PL, Salmén F, Vickovic S, Lundmark A, Navarro JF, Magnusson J, Giacomello S, Asp M, Westholm JO, Huss M, Mollbrink A, Linnarsson S, Codeluppi S, Borg Å, Pontén F, Costea PI, Sahlén P, Mulder J, Bergmann O, Lundeberg J, Frisén J. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science (New York, NY) 353: 78–82, 2016. DOI: 10.1126/science.aaf2403. [DOI] [PubMed] [Google Scholar]
- 1244.Stec MJ, Kelly NA, Many GM, Windham ST, Tuggle SC, Bamman MM. Ribosome biogenesis may augment resistance training-induced myofiber hypertrophy and is required for myotube growth in vitro. Am J Phys Endocrinol Metab 310: E652–E661, 2016. DOI: 10.1152/ajpendo.00486.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1245.Stec MJ, Mayhew DL, Bamman MM. The effects of age and resistance loading on skeletal muscle ribosome biogenesis. J Appl Physiol (1985) 119: 851–857, 2015. DOI: 10.1152/japplphysiol.00489.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1246.Stec MJ, Thalacker-Mercer A, Mayhew DL, Kelly NA, Tuggle SC, Merritt EK, Brown CJ, Windham ST, Dell’Italia LJ, Bickel CS, Roberts BM, Vaughn KM, Isakova-Donahue I, Many GM, Bamman MM. Randomized, four-arm, dose-response clinical trial to optimize resistance exercise training for older adults with age-related muscle atrophy. Exp Gerontol 99: 98–109, 2017. DOI: 10.1016/j.exger.2017.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1247.Steell L, Ho FK, Sillars A, Petermann-Rocha F, Li H, Lyall DM, Iliodromiti S, Welsh P, Anderson J, MacKay DF, Pell JP, Sattar N, Gill JM, Gray SR, Celis-Morales CA. Dose-response associations of cardiorespiratory fitness with all-cause mortality and incidence and mortality of cancer and cardiovascular and respiratory diseases: The uk biobank cohort study. Br J Sports Med 53: 1371–1378, 2019. DOI: 10.1136/bjsports-2018-099093. [DOI] [PubMed] [Google Scholar]
- 1248.Steensberg A, Keller C, Starkie RL, Osada T, Febbraio MA, Pedersen BK. IL-6 and tnf-alpha expression in, and release from, contracting human skeletal muscle. Am J Phys Endocrinol Metab 283: E1272–E1278, 2002. DOI: 10.1152/ajpendo.00255.2002. [DOI] [PubMed] [Google Scholar]
- 1249.Steensberg A, Febbraio MA, Osada T, Schjerling P, van Hall G, Saltin B, Pedersen BK. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J Physiol 537: 633–639, 2001. DOI: 10.1111/j.1469-7793.2001.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1250.Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Pedersen BK. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol 529 (Pt 1): 237–242, 2000. DOI: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1251.Steffensen CH, Roepstorff C, Madsen M, Kiens B. Myocellular triacylglycerol breakdown in females but not in males during exercise. Am J Phys Endocrinol Metab 282: E634–E642, 2002. DOI: 10.1152/ajpendo.00078.2001. [DOI] [PubMed] [Google Scholar]
- 1252.Steib S, Schoene D, Pfeifer K. Dose-response relationship of resistance training in older adults: A meta-analysis. Med Sci Sports Exerc 42: 902–914, 2010. DOI: 10.1249/MSS.0b013e3181c34465. [DOI] [PubMed] [Google Scholar]
- 1253.Stengel SV, Kemmler W, Pintag R, Beeskow C, Weineck J, Lauber D, Kalender WA, Engelke K. Power training is more effective than strength training for maintaining bone mineral density in postmenopausal women. J Appl Physiol (1985) 99: 181–188, 2005. DOI: 10.1152/japplphysiol.01260.2004. [DOI] [PubMed] [Google Scholar]
- 1254.Stevens-Lapsley JE, Kohrt WM. Osteoarthritis in women: Effects of estrogen, obesity and physical activity. Women’s Health (Lond Engl) 6: 601–615, 2010. DOI: 10.2217/whe.10.38. [DOI] [PubMed] [Google Scholar]
- 1255.Stewart RAH, Held C, Hadziosmanovic N, Armstrong PW, Cannon CP, Granger CB, Hagström E, Hochman JS, Koenig W, Lonn E, Nicolau JC, Steg PG, Vedin O, Wallentin L, White HD. Physical activity and mortality in patients with stable coronary heart disease. J Am Coll Cardiol 70: 1689–1700, 2017. DOI: 10.1016/j.jacc.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 1256.Stokes T, Hector AJ, Morton RW, McGlory C, Phillips SM. Recent perspectives regarding the role of dietary protein for the promotion of muscle hypertrophy with resistance exercise training. Nutrients 10, 2018. DOI: 10.3390/nu10020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1257.Stone MH, Fleck SJ, Triplett NT, Kraemer WJ. Health- and performance-related potential of resistance training. Sports Med 11: 210–231, 1991. DOI: 10.2165/00007256-199111040-00002. [DOI] [PubMed] [Google Scholar]
- 1258.Stone NJ, Robinson JG, Lichtenstein AH, Goff DC, Lloyd-Jones DM, Smith SC, Blum C, Schwartz JS. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: Synopsis of the 2013 american college of cardiology/american heart association cholesterol guideline. Ann Intern Med 160: 339–343, 2014. DOI: 10.7326/M14-0126. [DOI] [PubMed] [Google Scholar]
- 1259.Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol 175: 342–349, 2005. DOI: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 1260.Strain WD, Pald’anius PM. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol 17: 57, 2018. DOI: 10.1186/s12933-018-0703-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1261.Straka T, Vita V, Prokshi K, Hörner SJ, Khan MM, Pirazzini M, Williams MPI, Hafner M, Zaglia T, Rudolf R. Postnatal development and distribution of sympathetic innervation in mouse skeletal muscle. Int J Mol Sci 19, 2018. DOI: 10.3390/ijms19071935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1262.Strasser B Physical activity in obesity and metabolic syndrome. Ann N Y Acad Sci 1281: 141–159, 2013. DOI: 10.1111/j.1749-6632.2012.06785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1263.Strasser B, Steindorf K, Wiskemann J, Ulrich CM. Impact of resistance training in cancer survivors: A meta-analysis. Med Sci Sports Exerc 45: 2080–2090, 2013. DOI: 10.1249/MSS.0b013e31829a3b63. [DOI] [PubMed] [Google Scholar]
- 1264.Strating A, Bunn TO, Goff MT, Phillips CE. Efficacy of inactivated tissue culture rabies vaccine in dogs [Online]. J Am Vet Med Assoc 167: 809–812, 1975. http://www.ncbi.nlm.nih.gov/pubmed/1237485. [PubMed] [Google Scholar]
- 1265.Stuart CA, Stone WL, Howell MEA, Brannon MF, Hall HK, Gibson AL, Stone MH. Myosin content of individual human muscle fibers isolated by laser capture microdissection. Am J Phys Cell Phys 310: C381–C389, 2016. DOI: 10.1152/ajpcell.00317.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1266.Stubbs B, Vancampfort D, Rosenbaum S, Firth J, Cosco T, Veronese N, Salum GA, Schuch FB. An examination of the anxiolytic effects of exercise for people with anxiety and stress-related disorders: A meta-analysis. Psychiatry Res 249: 102–108, 2017. DOI: 10.1016/j.psychres.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 1267.Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, Ferrucci L, Guralnik JM, Fragala MS, Kenny AM, Kiel DP, Kritchevsky SB, Shardell MD, Dam T-TL, Vassileva MT. The fnih sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 69: 547–558, 2014. DOI: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1268.Støa EM, Meling S, Nyhus L-K, Strømstad KMG, Mangerud J, Helgerud S, Bratland-Sanda S, Støren EM. High-intensity aerobic interval training improves aerobic fitness and hba1c among persons diagnosed with type 2 diabetes. Eur J Appl Physiol 117: 455–467, 2017. DOI: 10.1007/s00421-017-3540-1. [DOI] [PubMed] [Google Scholar]
- 1269.Sullivan BE, Carroll CC, Jemiolo B, Trappe SW, Magnusson SP, Døssing S, Kjaer M, Trappe TA. Effect of acute resistance exercise and sex on human patellar tendon structural and regulatory mRNA expression. J Appl Physiol (1985) 106: 468–475, 2009. DOI: 10.1152/japplphysiol.91341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1270.Sung E, Han A, Hinrichs T, Vorgerd M, Manchado C, Platen P. Effects of follicular versus luteal phase-based strength training in young women. Springerplus 3: 668, 2014. DOI: 10.1186/2193-1801-3-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1271.Suo C, Singh MF, Gates N, Wen W, Sachdev P, Brodaty H, Saigal N, Wilson GC, Meiklejohn J, Singh N, Baune BT, Baker M, Foroughi N, Wang Y, Mavros Y, Lampit A, Leung I, Valenzuela MJ. Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Mol Psychiatry 21: 1633–1642, 2016. DOI: 10.1038/mp.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1272.Supinski GS, Callahan LA. Free radical-mediated skeletal muscle dysfunction in inflammatory conditions. J Appl Physiol (1985) 102: 2056–2063, 2007. DOI: 10.1152/japplphysiol.01138.2006. [DOI] [PubMed] [Google Scholar]
- 1273.Suzuki J Endurance performance is enhanced by intermittent hyper-baric exposure via up-regulation of proteins involved in mitochondrial biogenesis in mice. Physiol Rep 5, 2017. DOI: 10.14814/phy2.13349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1274.Svensson RB, Heinemeier KM, Couppé C, Kjaer M, Magnusson SP. Effect of aging and exercise on the tendon. J Appl Physiol (1985) 121: 1237–1246, 2016. DOI: 10.1152/japplphysiol.00328.2016. [DOI] [PubMed] [Google Scholar]
- 1275.Swain DP, Leutholtz BC. Heart rate reserve is equivalent to. Med Sci Sports Exerc 29: 410–414, 1997. DOI: 10.1097/00005768-199703000-00018. [DOI] [PubMed] [Google Scholar]
- 1276.Swain DP, Leutholtz BC, King ME, Haas LA, Branch JD. Relationship between. Med Sci Sports Exerc 30: 318–321, 1998. DOI: 10.1097/00005768-199802000-00022. [DOI] [PubMed] [Google Scholar]
- 1277.Szuhany KL, Bugatti M, Otto MW. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J Psychiatr Res 60: 56–64, 2015. DOI: 10.1016/j.jpsychires.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1278.Słomko W, Słomko J, Kowalik T, Klawe JJ, Tafil-Klawe M, Cudnoch-Jędrzejewska A, Newton JL, Zalewski P. Long-term high intensity sport practice modulates adaptative changes in athletes’ heart and in the autonomic nervous system profile. J Sports Med Phys Fitness 58: 1146–1152, 2017. DOI: 10.23736/S0022-4707.17.07230-9. [DOI] [PubMed] [Google Scholar]
- 1279.Taaffe DR, Duret C, Wheeler S, Marcus R. Once-weekly resistance exercise improves muscle strength and neuromuscular performance in older adults. J Am Geriatr Soc 47: 1208–1214, 1999. DOI: 10.1111/j.1532-5415.1999.tb05201.x. [DOI] [PubMed] [Google Scholar]
- 1280.Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and c-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci 55: M709–M715, 2000. DOI: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- 1281.Tanai E, Frantz S. Pathophysiology of heart failure. Compr Physiol 6: 187–214, 2015. DOI: 10.1002/cphy.c140055. [DOI] [PubMed] [Google Scholar]
- 1282.Tanaka H, Desouza CA, Jones PP, Stevenson ET, Davy KP, Seals DR. Greater rate of decline in maximal aerobic capacity with age in physically active vs. Sedentary healthy women. J Appl Physiol (1985) 83: 1947–1953, 1997. DOI: 10.1152/jappl.1997.83.6.1947. [DOI] [PubMed] [Google Scholar]
- 1283.Tanaka H, Seals DR. Invited review: Dynamic exercise performance in masters athletes: Insight into the effects of primary human aging on physiological functional capacity. J Appl Physiol (1985) 95: 2152–2162, 2003. DOI: 10.1152/japplphysiol.00320.2003. [DOI] [PubMed] [Google Scholar]
- 1284.Tang K, Breen EC, Gerber H-P, Ferrara NMA, Wagner PD. Capillary regression in vascular endothelial growth factor-deficient skeletal muscle. Physiol Genomics 18: 63–69, 2004. DOI: 10.1152/physiolgenomics.00023.2004. [DOI] [PubMed] [Google Scholar]
- 1285.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: Insights into insulin action. Nat Rev Mol Cell Biol 7: 85–96, 2006. DOI: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 1286.Tao L, Bei Y, Zhang H, Xiao J, Li X. Exercise for the heart: Signaling pathways. Oncotarget 6: 20773–20784, 2015. DOI: 10.18632/onco-target.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1287.Tarnopolsky LJ, MacDougall JD, Atkinson SA, Tarnopolsky MA, Sutton JR. Gender differences in substrate for endurance exercise. J Appl Physiol (1985) 68: 302–308, 1990. DOI: 10.1152/jappl.1990.68.1.302. [DOI] [PubMed] [Google Scholar]
- 1288.Tarnopolsky MA, Atkinson SA, Phillips SM, MacDougall JD. Carbohydrate loading and metabolism during exercise in men and women. J Appl Physiol (1985) 78: 1360–1368, 1995. DOI: 10.1152/jappl.1995.78.4.1360. [DOI] [PubMed] [Google Scholar]
- 1289.Tarnopolsky MA, Pearce E, Smith K, Lach B. Suction-modified bergstrã¶m muscle biopsy technique: Experience with 13,500 procedures. Muscle Nerve 43: 717–725, 2011. DOI: 10.1002/mus.21945. [DOI] [PubMed] [Google Scholar]
- 1290.Taubert M, Wenzel U, Draganski B, Kiebel SJ, Ragert P, Krug J, Villringer A. Investigating neuroanatomical features in top athletes at the single subject level. PLoS One 10: e0129508, 2015. DOI: 10.1371/journal.pone.0129508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1291.Tavella S, Ruggiu A, Giuliani A, Brun F, Canciani B, Manescu A, Marozzi K, Cilli M, Costa D, Liu Y, Piccardi F, Tasso R, Tromba G, Rustichelli F, Cancedda R. Bone turnover in wild type and pleiotrophin-transgenic mice housed for three months in the international space station (iss). PLoS One 7: e33179, 2012. DOI: 10.1371/journal.pone.0033179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1292.Taylor CW, Ingham SA, Hunt JEA, Martin NRW, Pringle JSM, Ferguson RA. Exercise duration-matched interval and continuous sprint cycling induce similar increases in ampk phosphorylation, pgc-1α and vegf mRNA expression in trained individuals. Eur J Appl Physiol 116: 1445–1454, 2016. DOI: 10.1007/s00421-016-3402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1293.Taylor RS, Sagar VA, Davies EJ, Briscoe S, Coats AJS, Dalal H, Lough F, Rees K, Singh S. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev: CD003331, 2014. DOI: 10.1002/14651858.CD003331.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1294.Taylor SJ, Walker PS, Perry JS, Cannon SR, Woledge R. The forces in the distal femur and the knee during walking and other activities measured by telemetry. J Arthroplast 13: 428–437, 1998. DOI: 10.1016/s0883-5403(98)90009-2. [DOI] [PubMed] [Google Scholar]
- 1295.ten Brinke LF, Bolandzadeh N, Nagamatsu LS, Hsu CL, Davis JC, Miran-Khan K, Liu-Ambrose T. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: A 6-month randomised controlled trial. Br J Sports Med 49: 248–254, 2015. DOI: 10.1136/bjsports-2013-093184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1296.Teran-Garcia M, Rankinen T, Koza RA, Rao DC, Bouchard C. Endurance training-induced changes in insulin sensitivity and gene expression. Am J Phys Endocrinol Metab 288: E1168–E1178, 2005. DOI: 10.1152/ajpendo.00467.2004. [DOI] [PubMed] [Google Scholar]
- 1297.Terzis G, Georgiadis G, Stratakos G, Vogiatzis I, Kavouras S, Manta P, Mascher H, Blomstrand E. Resistance exercise-induced increase in muscle mass correlates with p70S6 kinase phosphorylation in human subjects. Eur J Appl Physiol 102: 145–152, 2008. DOI: 10.1007/s00421-007-0564-y. [DOI] [PubMed] [Google Scholar]
- 1298.Testa G, Ceccofiglio A, Mussi C, Bellelli G, Nicosia F, Bo M, Riccio D, Curcio F, Martone AM, Noro G, Landi F, Ungar A, Abete P. Hypotensive drugs and syncope due to orthostatic hypotension in older adults with dementia (syncope and dementia study). J Am Geriatr Soc 66: 1532–1537, 2018. DOI: 10.1111/jgs.15421. [DOI] [PubMed] [Google Scholar]
- 1299.Thalacker-Mercer AE, Dell’Italia LJ, Cui X, Cross JM, Bamman MM. Differential genomic responses in old vs. Young humans despite similar levels of modest muscle damage after resistance loading. Physiol Genomics 40: 141–149, 2010. DOI: 10.1152/physiolgenomics.00151.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1300.Thalacker-Mercer A, Stec M, Cui X, Cross J, Windham S, Bamman M. Cluster analysis reveals differential transcript profiles associated with resistance training-induced human skeletal muscle hypertrophy. Physiol Genomics 45: 499–507, 2013. DOI: 10.1152/physiolgenomics.00167.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1301.Thapa M, Paneru R. Gestational weight gain and its relation with birth weight of the newborn [Online]. JNMA J Nepal Med Assoc 56: 309–313, 2017. http://www.ncbi.nlm.nih.gov/pubmed/29255311. [PubMed] [Google Scholar]
- 1302.Thijssen DHJ, Redington A, George KP, Hopman MTE, Jones H. Association of exercise preconditioning with immediate cardioprotection: A review. JAMA Cardiol 3: 169–176, 2018. DOI: 10.1001/jamacardio.2017.4495. [DOI] [PubMed] [Google Scholar]
- 1303.Thomas MH, Burns SP. Increasing lean mass and strength: A comparison of high frequency strength training to lower frequency strength training [Online]. Int J Exerc Sci 9: 159–167, 2016. http://www.ncbi.nlm.nih.gov/pubmed/27182422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1304.Thomason DB, Biggs RB, Booth FW. Protein metabolism and beta-myosin heavy-chain mRNA in unweighted soleus muscle. Am J Phys 257: R300–R305, 1989. DOI: 10.1152/ajpregu.1989.257.2.R300. [DOI] [PubMed] [Google Scholar]
- 1305.Thomopoulos S, Das R, Sakiyama-Elbert S, Silva MJ, Charlton N, Gelberman RH. BFGF and pdgf-bb for tendon repair: Controlled release and biologic activity by tendon fibroblasts in vitro. Ann Biomed Eng 38: 225–234, 2010. DOI: 10.1007/s10439-009-9844-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1306.Tikkanen E, Gustafsson S, Ingelsson E. Associations of fitness, physical activity, strength, and genetic risk with cardiovascular disease: Longitudinal analyses in the uk biobank study. Circulation 137: 2583–2591, 2018. DOI: 10.1161/CIRCULATIONAHA.117.032432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1307.Timmins KA, Leech RD, Batt ME, Edwards KL. Running and knee osteoarthritis: A systematic review and meta-analysis. Am J Sports Med 45: 1447–1457, 2017. DOI: 10.1177/0363546516657531. [DOI] [PubMed] [Google Scholar]
- 1308.Timmons JA, Knudsen S, Rankinen T, Koch LG, Sarzynski M, Jensen T, Keller P, Scheele C, Vollaard NBJ, Nielsen S, Akerström T, MacDougald OA, Jansson E, Greenhaff PL, Tarnopolsky MA, van Loon LJC, Pedersen BK, Sundberg CJ, Wahlestedt C, Britton SL, Bouchard C. Using molecular classification to predict gains in maximal aerobic capacity following endurance exercise training in humans. J Appl Physiol (1985) 108: 1487–1496, 2010. DOI: 10.1152/japplphysiol.01295.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1309.Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med 337: 1279–1284, 1997. DOI: 10.1056/NEJM199710303371806. [DOI] [PubMed] [Google Scholar]
- 1310.Tinken TM, Thijssen DHJ, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension 55: 312–318, 2010. DOI: 10.1161/HYPERTENSIONAHA.109.146282. [DOI] [PubMed] [Google Scholar]
- 1311.Tintignac LA, Brenner H-R, Rüegg MA. Mechanisms regulating neuromuscular junction development and function and causes of muscle wasting. Physiol Rev 95: 809–852, 2015. DOI: 10.1152/physrev.00033.2014. [DOI] [PubMed] [Google Scholar]
- 1312.Tipton CM. The history of “exercise is medicine” in ancient civilizations. Adv Physiol Educ 38: 109–117, 2014. DOI: 10.1152/advan.00136.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1313.Tipton CM. Susruta of india, an unrecognized contributor to the history of exercise physiology. J Appl Physiol (1985) 104: 1553–1556, 2a008. DOI: 10.1152/japplphysiol.00925.2007. [DOI] [PubMed] [Google Scholar]
- 1314.Tipton CM. Contemporary exercise physiology: Fifty years after the closure of harvard fatigue laboratory [Online]. Exerc Sport Sci Rev 26: 315–339, 1998. http://www.ncbi.nlm.nih.gov/pubmed/9696994. [PubMed] [Google Scholar]
- 1315.Torales J, O’Higgins M, Castaldelli-Maia JM, Ventriglio A. The outbreak of covid-19 coronavirus and its impact on global mental health. Int J Soc Psychiatry 66: 317–320, 2020. DOI: 10.1177/0020764020915212. [DOI] [PubMed] [Google Scholar]
- 1316.Torzilli PA, Bhargava M, Park S, Chen CTC. Mechanical load inhibits il-1 induced matrix degradation in articular cartilage. Osteoarthr Cartil 18: 97–105, 2010. DOI: 10.1016/j.joca.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1317.Toscano CVA, Carvalho HM, Ferreira JP. Exercise effects for children with autism spectrum disorder: Metabolic health, autistic traits, and quality of life. Percept Mot Skills 125: 126–146, 2018. DOI: 10.1177/0031512517743823. [DOI] [PubMed] [Google Scholar]
- 1318.Törpel A, Herold F, Hamacher D, Müller NG, Schega L. Strengthening the brain-is resistance training with blood flow restriction an effective strategy for cognitive improvement? J Clin Med 7, 2018. DOI: 10.3390/jcm7100337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1319.Trappe S, Costill D, Thomas R. Effect of swim taper on whole muscle and single muscle fiber contractile properties [Online]. Med Sci Sports Exerc 33: 48–56, 2001. http://www.ncbi.nlm.nih.gov/pubmed/11194111. [PubMed] [Google Scholar]
- 1320.Trappe S, Costill D, Thomas R. Effect of swim taper on whole muscle and single muscle fiber contractile properties [Online]. Med Sci Sports Exerc 32: 48–56, 2000. http://www.ncbi.nlm.nih.gov/pubmed/11224794. [PubMed] [Google Scholar]
- 1321.Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol 552: 47–58, 2003. DOI: 10.1113/jphysiol.2003.044966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1322.Trappe S, Harber M, Creer A, Gallagher P, Slivka D, Minchev K, Whitsett D. Single muscle fiber adaptations with marathon training. J Appl Physiol (1985) 101: 721, 6, 2006. DOI: 10.1152/japplphysiol.01595.2005. [DOI] [PubMed] [Google Scholar]
- 1323.Trappe S, Hayes E, Galpin A, Kaminsky L, Jemiolo B, Fink W, Trappe T, Jansson A, Gustafsson T, Tesch P. New records in aerobic power among octogenarian lifelong endurance athletes. J Appl Physiol (1985) 114: 3–10, 2013. DOI: 10.1152/japplphysiol.01107.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1324.Trappe S, Trappe T, Gallagher P, Harber M, Alkner B, Tesch P. Human single muscle fibre function with 84 day bed-rest and resistance exercise. J Physiol 557: 501–513, 2004. DOI: 10.1113/jphysiol.2004.062166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1325.Trappe S, Godard M, Gallagher P, Carroll C, Rowden G, Porter D. Resistance training improves single muscle fiber contractile function in older women. Am J Phys Cell Phys 281: C398–C406, 2001. DOI: 10.1152/ajpcell.2001.281.2.C398. [DOI] [PubMed] [Google Scholar]
- 1326.Trappe SW, Costill DL, Fink WJ, Pearson DR. Skeletal muscle characteristics among distance runners: A 20-yr follow-up study. J Appl Physiol (1985) 78: 823–829, 1995. DOI: 10.1152/jappl.1995.78.3.823. [DOI] [PubMed] [Google Scholar]
- 1327.Trappe SW, Costill DL, Goodpaster B, Vukovich MD, Fink WJ. The effects of l-carnitine supplementation on performance during interval swimming. Int J Sports Med 15: 181–185, 1994. DOI: 10.1055/s-2007-1021044. [DOI] [PubMed] [Google Scholar]
- 1328.Trappe S, Williamson D, Godard M, Porter D, Rowden G, Costill D. Effect of resistance training on single muscle fiber contractile function in older men. J Appl Physiol (1985) 89: 143–152, 2000. DOI: 10.1152/jappl.2000.89.1.143. [DOI] [PubMed] [Google Scholar]
- 1329.Trappe TA, Carroll CC, Jemiolo B, Trappe SW, Døssing S, Kjaer M, Magnusson SP. Cyclooxygenase mRNA expression in human patellar tendon at rest and after exercise. Am J Physiol Regul Integr Comp Physiol 294: R192–R199, 2008. DOI: 10.1152/ajpregu.00669.2007. [DOI] [PubMed] [Google Scholar]
- 1330.Trefts E, Williams AS, Wasserman DH. Exercise and the regulation of hepatic metabolism. Prog Mol Biol Transl Sci 135: 203–225, 2015. DOI: 10.1016/bs.pmbts.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1331.Trevino MB, Zhang X, Standley RA, Wang M, Han X, Reis FCG, Periasamy M, Yu G, Kelly DP, Goodpaster BH, Vega RB, Coen PM. Loss of mitochondrial energetics is associated with poor recovery of muscle function but not mass following disuse atrophy. Am J Phys Endocrinol Metab 317: E899–E910, 2019. DOI: 10.1152/ajpendo.00161.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1332.Trivedi MH, Greer TL, Rethorst CD, Carmody T, Grannemann BD, Walker R, Warden D, Shores-Wilson K, Stoutenberg M, Oden N, Silverstein M, Hodgkins C, Love L, Seamans C, Stotts A, Causey T, Szucs-Reed RP, Rinaldi P, Myrick H, Straus M, Liu D, Lindblad R, Church T, Blair SN, Nunes EV. Randomized controlled trial comparing exercise to health education for stimulant use disorder: Results from the ctn-0037 stimulant reduction intervention using dosed exercise (stride) study. J Clin Psychiatry 78: 1075–1082, 2015. DOI: 10.4088/JCP.15m10591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1333.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the united states measured by accelerometer. Med Sci Sports Exerc 40: 181–188, 2008. DOI: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 1334.Troy KL, Mancuso ME, Butler TA, Johnson JE. Exercise early and often: Effects of physical activity and exercise on women’s bone health. Int J Environ Res Public Health 15, 2018. DOI: 10.3390/ijerph15050878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1335.Tsai C-C, Wu S-B, Kau H-C, Wei Y-H. Essential role of connective tissue growth factor (ctgf) in transforming growth factor-β1 (tgf-β1)-induced myofibroblast transdifferentiation from graves’ orbital fibroblasts. Sci Rep 8: 7276, 2018. DOI: 10.1038/s41598-018-25370-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1336.Tseng BY, Uh J, Rossetti HC, Cullum CM, Diaz-Arrastia RF, Levine BD, Lu H, Zhang R. Masters athletes exhibit larger regional brain volume and better cognitive performance than sedentary older adults. J Magn Reson Imaging 38: 1169–1176, 2013. DOI: 10.1002/jmri.24085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1337.Tseng BY, Gundapuneedi T, Khan MA, Diaz-Arrastia R, Levine BD, Lu H, Huang H, Zhang R. White matter integrity in physically fit older adults. NeuroImage 82: 510–516, 2013. DOI: 10.1016/j.neuroimage.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1338.Tsiloulis T, Carey AL, Bayliss J, Canny B, Meex RCR, Watt MJ. No evidence of white adipocyte browning after endurance exercise training in obese men. Int J Obes 42: 721–727, 2018. DOI: 10.1038/ijo.2017.295. [DOI] [PubMed] [Google Scholar]
- 1339.Tsoli M, Moore M, Burg D, Painter A, Taylor R, Lockie SH, Turner N, Warren A, Cooney G, Oldfield B, Clarke S, Robertson G. Activation of thermogenesis in brown adipose tissue and dysregulated lipid metabolism associated with cancer cachexia in mice. Cancer Res 72: 4372–4382, 2012. DOI: 10.1158/0008-5472.CAN-11-3536. [DOI] [PubMed] [Google Scholar]
- 1340.United States Bone and Joint Initiative. Musculoskeletal Diseases [Online]. The Burden of Musculoskeletal Diseases in the United States (BMUS), Fourth Edition: [date unknown]. https://www.boneandjointburden.org/ [15 Mar. 2020]. [Google Scholar]
- 1341.Urbanek K, Quaini F, Tasca G, Torella D, Castaldo C, Nadal-Ginard B, Leri A, Kajstura J, Quaini E, Anversa P. Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc Natl Acad Sci U S A 100: 10440–10445, 2003. DOI: 10.1073/pnas.1832855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1342.US Dept of Health and Human Services. 2008 Physical Activity Guidelines for Americans [Online]. U.S. Department of Health; Human Services: 2008. https://health.gov/sites/default/files/2019-09/paguide.pdf. [Google Scholar]
- 1343.Uthman OA, van der Windt DA, Jordan JL, Dziedzic KS, Healey EL, Peat GM, Foster NE. Exercise for lower limb osteoarthritis: Systematic review incorporating trial sequential analysis and network meta-analysis. Br J Sports Med 48: 1579, 2014. DOI: 10.1136/bjsports-2014-5555rep. [DOI] [PubMed] [Google Scholar]
- 1344.Vainshtein A, Tryon LD, Pauly M, Hood DA. Role of pgc-1α during acute exercise-induced autophagy and mitophagy in skeletal muscle. Am J Phys Cell Phys 308: C710–C719, 2015. DOI: 10.1152/ajpcell.00380.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1345.Valdez G, Tapia JC, Kang H, Clemenson GD, Gage FH, Lichtman JW, Sanes JR. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci U S A 107: 14863–14868, 2010. DOI: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1346.Vandam LD, Fox JA. Adolf fick (1829–1901), physiologist: A heritage for anesthesiology and critical care medicine. Anesthesiology 88: 514–518, 1998. DOI: 10.1097/00000542-199802000-00030. [DOI] [PubMed] [Google Scholar]
- 1347.Van Der Heijden G-J, Wang ZJ, Chu Z, Toffolo G, Manesso E, Sauer PJJ, Sunehag AL. Strength exercise improves muscle mass and hepatic insulin sensitivity in obese youth. Med Sci Sports Exerc 42: 1973–1980, 2010. DOI: 10.1249/MSS.0b013e3181df16d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1348.van Hall G, Steensberg A, Fischer C, Keller C, Moller K, Moseley P, Pedersen BK. Interleukin-6 markedly decreases skeletal muscle protein turnover and increases nonmuscle amino acid utilization in healthy individuals. J Clin Endocrinol Metab 93: 2851–2858, 2008. DOI: 10.1210/jc.2007-2223. [DOI] [PubMed] [Google Scholar]
- 1349.Van Hee R Andreas vesalius: His surgical activities and influence on modern surgery. Acta Chir Belg 116: 62–68, 2016. DOI: 10.1080/00015458.2016.1140958. [DOI] [PubMed] [Google Scholar]
- 1350.van Loon LJC, Schrauwen-Hinderling VB, Koopman R, Wagenmakers AJM, Hesselink MKC, Schaart G, Kooi ME, Saris WHM. Influence of prolonged endurance cycling and recovery diet on intramuscular triglyceride content in trained males. Am J Phys Endocrinol Metab 285: E804–E811, 2003. DOI: 10.1152/ajpendo.00112.2003. [DOI] [PubMed] [Google Scholar]
- 1351.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2: 266–270, 1999. DOI: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 1352.van Riet EES, Hoes AW, Wagenaar KP, Limburg A, Landman MAJ, Rutten FH. Epidemiology of heart failure: The prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail 18: 242–252, 2016. DOI: 10.1002/ejhf.483. [DOI] [PubMed] [Google Scholar]
- 1353.van Vugt JLA, Buettner S, Alferink LJM, Bossche N, de Bruin RWF, Murad SD, Polak WG, Metselaar HJ, IJzermans JNM. Low skeletal muscle mass is associated with increased hospital costs in patients with cirrhosis listed for liver transplantation-a retrospective study. Transpl Int 31: 165–174, 2018. DOI: 10.1111/tri.13048. [DOI] [PubMed] [Google Scholar]
- 1354.van Zanten JJCSV, Rouse PC, Hale ED, Ntoumanis N, Metsios GS, Duda JL, Kitas GD. Perceived barriers, facilitators and benefits for regular physical activity and exercise in patients with rheumatoid arthritis: A review of the literature. Sports Med 45: 1401–1412, 2015. DOI: 10.1007/s40279-015-0363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1355.Vargas-Castillo A, Fuentes-Romero R, Rodriguez-Lopez LA, Torres N, Tovar AR. Understanding the biology of thermogenic fat: Is browning a new approach to the treatment of obesity? Arch Med Res 48: 401–413, 2017. DOI: 10.1016/j.arcmed.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 1356.Varghese T, Schultz WM, McCue AA, Lambert CT, Sandesara PB, Eapen DJ, Gordon NF, Franklin BA, Sperling LS. Physical activity in the prevention of coronary heart disease: Implications for the clinician. Heart 102: 904–909, 2016. DOI: 10.1136/heartjnl-2015-308773. [DOI] [PubMed] [Google Scholar]
- 1357.Vassalle C, Turco SD, Sabatino L, Basta G, Maltinti M, Sbrana F, Ndreu R, Mastorci F, Pingitore A. New inflammatory and oxidative stress-based biomarker changes in response to a half-marathon in recreational athletes. J Sports Med Phys Fitness 60: 1390–1395, 2020. DOI: 10.23736/S0022-4707.20.10738-2. [DOI] [PubMed] [Google Scholar]
- 1358.Välimäki IA, Vuorimaa T, Ahotupa M, Vasankari TJ. Strenuous physical exercise accelerates the lipid peroxide clearing transport by hdl. Eur J Appl Physiol 116: 1683–1691, 2016. DOI: 10.1007/s00421-016-3422-y. [DOI] [PubMed] [Google Scholar]
- 1359.Vega RB, Konhilas JP, Kelly DP, Leinwand LA. Molecular mechanisms underlying cardiac adaptation to exercise. Cell Metab 25: 1012–1026, 2017. DOI: 10.1016/j.cmet.2017.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1360.Verdijk LB, Gleeson BG, Jonkers RAM, Meijer K, Savelberg HHCM, Dendale P, van Loon LJC. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci 64: 332–339, 2009. DOI: 10.1093/gerona/gln050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1361.Verdijk LB, Snijders T, Beelen M, Savelberg HHCM, Meijer K, Kuipers H, Van Loon LJC. Characteristics of muscle fiber type are predictive of skeletal muscle mass and strength in elderly men. J Am Geriatr Soc 58: 2069–2075, 2010. DOI: 10.1111/j.1532-5415.2010.03150.x. [DOI] [PubMed] [Google Scholar]
- 1362.Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, van Loon LJC. Satellite cells in human skeletal muscle; from birth to old age. Age (Dordr) 36: 545–547, 2014. DOI: 10.1007/s11357-013-9583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1363.Verdijk LB, Snijders T, Holloway TM, Kranenburg JV, Loon LJCV. Resistance training increases skeletal muscle capillarization in healthy older men. Med Sci Sports Exerc 48: 2157–2164, 2016. DOI: 10.1249/MSS.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 1364.Vernay A, Marchetti A, Sabra A, Jauslin TN, Rosselin M, Scherer PE, Demaurex N, Orci L, Cosson P. MitoNEET-dependent formation of intermitochondrial junctions. Proc Natl Acad Sci U S A 114: 8277–8282, 2017. DOI: 10.1073/pnas.1706643114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1365.Vesali RF, Klaude M, Rooyackers O, Wernerman J. Amino acid metabolism in leg muscle after an endotoxin injection in healthy volunteers. Am J Phys Endocrinol Metab 288: E360–E364, 2005. DOI: 10.1152/ajpendo.00248.2004. [DOI] [PubMed] [Google Scholar]
- 1366.Vico L, van Rietbergen B, Vilayphiou N, Linossier M-T, Locrelle H, Normand M, Zouch M, Gerbaix M, Bonnet N, Novikov V, Thomas T, Vassilieva G. Cortical and trabecular bone microstructure did not recover at weight-bearing skeletal sites and progressively deteriorated at non-weight-bearing sites during the year following international space station missions. J Bone Miner Res Off J Am Soc Bone Miner Res 32: 2010–2021, 2017. DOI: 10.1002/jbmr.3188. [DOI] [PubMed] [Google Scholar]
- 1367.Vieira VJ, Hu L, Valentine RJ, McAuley E, Evans EM, Baynard T, Woods JA. Reduction in trunk fat predicts cardiovascular exercise training-related reductions in c-reactive protein. Brain Behav Immun 23: 485–491, 2009. DOI: 10.1016/j.bbi.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 1368.Vigelso A The relationship between skeletal muscle mitochondrial citrate synthase activity and whole body oxygen uptake adaptations in response to exercise training [Online]. Int J Physiol Pathophysiol Pharmacol 6: 84–101, 2014. http://www.ncbi.nlm.nih.gov/pubmed/25057335. [PMC free article] [PubMed] [Google Scholar]
- 1369.Vijgen GHEJ, Sparks LM, Bouvy ND, Schaart G, Hoeks J, van Marken Lichtenbelt WD, Schrauwen P. Increased oxygen consumption in human adipose tissue from the “brown adipose tissue” region. J Clin Endocrinol Metab 98: E1230–E1234, 2013. DOI: 10.1210/jc.2013-1348. [DOI] [PubMed] [Google Scholar]
- 1370.Vina ER, Kwoh CK. Epidemiology of osteoarthritis: Literature update. Curr Opin Rheumatol 30: 160–167, 2018. DOI: 10.1097/BOR.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1371.Viña J, Rodriguez-Mañas L, Salvador-Pascual A, Tarazona-Santabalbina FJ, Gomez-Cabrera MC. Exercise: The lifelong supplement for healthy ageing and slowing down the onset of frailty. J Physiol 594: 1989–1999, 2016. DOI: 10.1113/JP270536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1372.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW. Heart disease and stroke statistics-2020 update: A report from the american heart association. Circulation 141: e139–e596, 2020. DOI: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 1373.Virbasius JV, Scarpulla RC. Activation of the human mitochondrial transcription factor a gene by nuclear respiratory factors: A potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc Natl Acad Sci U S A 91: 1309–1313, 1994. DOI: 10.1073/pnas.91.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1374.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto N-J, Enerbäck S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med 360: 1518–1525, 2009. DOI: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 1375.Vivar C, Peterson BD, van Praag H. Running rewires the neuronal network of adult-born dentate granule cells. NeuroImage 131: 29–41, 2016. DOI: 10.1016/j.neuroimage.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1376.Vivar C, van Praag H. Running changes the brain: The long and the short of it. Physiology (Bethesda) 32: 410–424, 2017. DOI: 10.1152/physiol.00017.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1377.Vlietstra L, Hendrickx W, Waters DL. Exercise interventions in healthy older adults with sarcopenia: A systematic review and meta-analysis. Australas J Ageing 37: 169–183, 2018. DOI: 10.1111/ajag.12521. [DOI] [PubMed] [Google Scholar]
- 1378.Voss MW, Weng TB, Narayana-Kumanan K, Cole RC, Wharff C, Reist L, Dubose L, Sigurdsson G, Mills JA, Long JD, Magnotta VA, Pierce GL. Acute exercise effects predict training change in cognition and connectivity. Med Sci Sports Exerc 52: 131–140, 2020. DOI: 10.1249/MSS.0000000000002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1379.Vosselman MJ, Hoeks J, Brans B, Pallubinsky H, Nascimento EBM, van der Lans AAJJ, Broeders EPM, Mottaghy FM, Schrauwen P, van Marken Lichtenbelt WD. Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Int J Obes 39: 1696–1702, 2015. DOI: 10.1038/ijo.2015.130. [DOI] [PubMed] [Google Scholar]
- 1380.Wadman M Company aims to beat nih human genome efforts. Nature 393: 101, 1998. DOI: 10.1038/30057. [DOI] [PubMed] [Google Scholar]
- 1381.Wagner GR, Hirschey MD. Nonenzymatic protein acylation as a carbon stress regulated by sirtuin deacylases. Mol Cell 54: 5–16, 2014. DOI: 10.1016/j.molcel.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1382.Wahl P, Wehmeier UF, Jansen FJ, Kilian Y, Bloch W, Werner N, Mester J, Hilberg T. Acute effects of different exercise protocols on the circulating vascular microRNAs −16, −21, and −126 in trained subjects. Front Physiol 7: 643, 2016. DOI: 10.3389/fphys.2016.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1383.Wahren J, Ekberg K. Splanchnic regulation of glucose production. Annu Rev Nutr 27: 329–345, 2007. DOI: 10.1146/annurev.nutr.27.061406.093806. [DOI] [PubMed] [Google Scholar]
- 1384.Waldrop TG, Stremel RW. Muscular contraction stimulates posterior hypothalamic neurons. Am J Phys 256: R348–R356, 1989. DOI: 10.1152/ajpregu.1989.256.2.R348. [DOI] [PubMed] [Google Scholar]
- 1385.Wallach D, Kang T-B, Kovalenko A. Concepts of tissue injury and cell death in inflammation: A historical perspective. Nat Rev Immunol 14: 51–59, 2014. DOI: 10.1038/nri3561. [DOI] [PubMed] [Google Scholar]
- 1386.Wallén MB, Hagströmer M, Conradsson D, Sorjonen K, Franzén E. Long-term effects of highly challenging balance training in parkinson’s disease-a randomized controlled trial. Clin Rehabil 32: 1520–1529, 2018. DOI: 10.1177/0269215518784338. [DOI] [PubMed] [Google Scholar]
- 1387.Waller B, Munukka M, Rantalainen T, Lammentausta E, Nieminen MT, Kiviranta I, Kautiainen H, Häkkinen A, Kujala UM, Heinonen A. Effects of high intensity resistance aquatic training on body composition and walking speed in women with mild knee osteoarthritis: A 4-month rct with 12-month follow-up. Osteoarthr Cartil 25: 1238–1246, 2017. DOI: 10.1016/j.joca.2017.02.800. [DOI] [PubMed] [Google Scholar]
- 1388.Walsh JM, McAuliffe FM. Prediction and prevention of the macro-somic fetus. Eur J Obstet Gynecol Reprod Biol 162: 125–130, 2012. DOI: 10.1016/j.ejogrb.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 1389.Walsh JJ, Edgett BA, Tschakovsky ME, Gurd BJ. Fasting and exercise differentially regulate bdnf mRNA expression in human skeletal muscle. Appl Physiol Nutr Metab 40: 96–98, 2015. DOI: 10.1139/apnm-2014-0290. [DOI] [PubMed] [Google Scholar]
- 1390.Walsmith J, Roubenoff R. Cachexia in rheumatoid arthritis. Int J Cardiol 85: 89–99, 2002. DOI: 10.1016/s0167-5273(02)00237-1. [DOI] [PubMed] [Google Scholar]
- 1391.Wang JH-C. Mechanobiology of tendon. J Biomech 39: 1563–1582, 2006. DOI: 10.1016/j.jbiomech.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 1392.Wang JH-C, Guo Q, Li B. Tendon biomechanics and mechanobiology–a minireview of basic concepts and recent advancements. J Hand Ther 25: 133–140; quiz 141, 2011. DOI: 10.1016/j.jht.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1393.Wang X, Fitts RH. Effects of regular exercise on ventricular myocyte biomechanics and katp channel function. Am J Phys Heart Circ Phys 315: H885–H896, 2018. DOI: 10.1152/ajpheart.00130.2018. [DOI] [PubMed] [Google Scholar]
- 1394.Wang Z, Heshka S, Gallagher D, Boozer CN, Kotler DP, Heymsfield SB. Resting energy expenditure-fat-free mass relationship: New insights provided by body composition modeling. Am J Phys Endocrinol Metab 279: E539–E545, 2000. DOI: 10.1152/ajpendo.2000.279.3.E539. [DOI] [PubMed] [Google Scholar]
- 1395.Wanrooij VHM, Willeboordse M, Dompeling E, van de Kant KDG. Exercise training in children with asthma: A systematic review. Br J Sports Med 48: 1024–1031, 2014. DOI: 10.1136/bjsports-2012-091347. [DOI] [PubMed] [Google Scholar]
- 1396.Warburton DER, Nicol CW, Bredin SSD. Health benefits of physical activity: The evidence. Can Med Assoc J 174: 801–809, 2006. DOI: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1397.Warden SJ, Fuchs RK. Are “exercise pills” the answer to the growing problem of physical inactivity? Br J Sports Med 42: 862–863, 2008. DOI: 10.1136/bjsm.2008.053512. [DOI] [PubMed] [Google Scholar]
- 1398.Waring CD, Vicinanza C, Papalamprou A, Smith AJ, Purushothaman S, Goldspink DF, Nadal-Ginard B, Torella D, Ellison GM. The adult heart responds to increased workload with physiologic hypertrophy, cardiac stem cell activation, and new myocyte formation. Eur Heart J 35: 2722–2731, 2014. DOI: 10.1093/eurheartj/ehs338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1399.Wasfy MM, Weiner RB, Wang F, Berkstresser B, Lewis GD, DeLuca JR, Hutter AM, Picard MH, Baggish AL. Endurance exercise-induced cardiac remodeling: Not all sports are created equal. J Am Soc Echocardiogr 28: 1434–1440, 2015. DOI: 10.1016/j.echo.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 1400.Watson KB, Carlson SA, Gunn JP, Galuska DA, O’Connor A, Green-lund KJ, Fulton JE. Physical inactivity among adults aged 50 years and older—united states, 2014. MMWR Morb Mortal Wkly Rep 65: 954–958, 2016. DOI: 10.15585/mmwr.mm6536a3. [DOI] [PubMed] [Google Scholar]
- 1401.Watson PA, Stein JP, Booth FW. Changes in actin synthesis and alpha-actin-mRNA content in rat muscle during immobilization. Am J Phys 247: C39–C44, 1984. DOI: 10.1152/ajpcell.1984.247.1.C39. [DOI] [PubMed] [Google Scholar]
- 1402.Watson SL, Weeks BK, Weis LJ, Harding AT, Horan SA, Beck BR. High-intensity resistance and impact training improves bone mineral density and physical function in postmenopausal women with osteopenia and osteoporosis: The liftmor randomized controlled trial. J Bone Miner Res Off J Am Soc Bone Miner Res 33: 211–220, 2018. DOI: 10.1002/jbmr.3284. [DOI] [PubMed] [Google Scholar]
- 1403.Watt MJ, Heigenhauser GJF, Spriet LL. Intramuscular triacylglycerol utilization in human skeletal muscle during exercise: Is there a controversy? J Appl Physiol (1985) 93: 1185–1195, 2002. DOI: 10.1152/japplphysiol.00197.2002. [DOI] [PubMed] [Google Scholar]
- 1404.Watt MJ, Holmes AG, Pinnamaneni SK, Garnham AP, Steinberg GR, Kemp BE, Febbraio MA. Regulation of hsl serine phosphorylation in skeletal muscle and adipose tissue. Am J Phys Endocrinol Metab 290: E500–E508, 2006. DOI: 10.1152/ajpendo.00361.2005. [DOI] [PubMed] [Google Scholar]
- 1405.Wayne PM, Kiel DP, Buring JE, Connors EM, Bonato P, Yeh GY, Cohen CJ, Mancinelli C, Davis RB. Impact of tai chi exercise on multiple fracture-related risk factors in post-menopausal osteopenic women: A pilot pragmatic, randomized trial. BMC Complement Altern Med 12: 7, 2012. DOI: 10.1186/1472-6882-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1406.Weatherholt AM, Fuchs RK, Warden SJ. Specialized connective tissue: Bone, the structural framework of the upper extremity. J Hand Ther 25: 123–131; quiz 132, 2011. DOI: 10.1016/j.jht.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1407.Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, O’Karma M, Wallace TC, Zemel BS. The national osteoporosis foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos Int 27: 1281–1386, 2016. DOI: 10.1007/s00198-015-3440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1408.Weber A, Franzini-Armstrong C, Hugh E. Huxley: Birth of the filament sliding model of muscle contraction. Trends Cell Biol 12: 243–245, 2002. DOI: 10.1016/s0962-8924(02)02270-5. [DOI] [PubMed] [Google Scholar]
- 1409.Wedell-Neergaard A-S, Lehrskov LL, Christensen RH, Legaard GE, Dorph E, Larsen MK, Launbo N, Fagerlind SR, Seide SK, Nymand S, Ball M, Vinum N, Dahl CN, Henneberg M, Ried-Larsen M, Nybing JD, Christensen R, Rosenmeier JB, Karstoft K, Pedersen BK, Ellingsgaard H, Krogh-Madsen R. Exercise-induced changes in visceral adipose tissue mass are regulated by il-6 signaling: A randomized controlled trial. Cell Metab 29: 844–855.e3, 2019. DOI: 10.1016/j.cmet.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 1410.Weeks KL, Bernardo BC, Ooi JYY, Patterson NL, McMullen JR. The igf1-pi3k-akt signaling pathway in mediating exercise-induced cardiac hypertrophy and protection. Adv Exp Med Biol 1000: 187–210, 2017. DOI: 10.1007/978-981-10-4304-8_12. [DOI] [PubMed] [Google Scholar]
- 1411.Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med 132: 605–611, 2000. DOI: 10.7326/0003-4819-132-8-200004180-00002. [DOI] [PubMed] [Google Scholar]
- 1412.Weiner RB, Baggish AL. Exercise-induced cardiac remodeling. Prog Cardiovasc Dis 54: 380–386, 2012. DOI: 10.1016/j.pcad.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 1413.Welle S, Tawil R, Thornton CA. Sex-related differences in gene expression in human skeletal muscle. PLoS One 3: e1385, 2008. DOI: 10.1371/journal.pone.0001385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1414.Wellsandt E, Golightly Y. Exercise in the management of knee and hip osteoarthritis. Curr Opin Rheumatol 30: 151–159, 2018. DOI: 10.1097/BOR.0000000000000478. [DOI] [PubMed] [Google Scholar]
- 1415.West DWD, Burd NA, Churchward-Venne TA, Camera DM, Mitchell CJ, Baker SK, Hawley JA, Coffey VG, Phillips SM. Sex-based comparisons of myofibrillar protein synthesis after resistance exercise in the fed state. J Appl Physiol (1985) 112: 1805–1813, 2012. DOI: 10.1152/japplphysiol.00170.2012. [DOI] [PubMed] [Google Scholar]
- 1416.West JB. The collaboration of antoine and marie-anne lavoisier and the first measurements of human oxygen consumption. Am J Physiol Lung Cell Mol Physiol 305: L775–L785, 2013. DOI: 10.1152/ajplung.00228.2013. [DOI] [PubMed] [Google Scholar]
- 1417.West JB. J.S. Haldane and some of his contributions to physiology. Adv Exp Med Biol 605: 9–15, 2008. DOI: 10.1007/978-0-387-73693-8_2. [DOI] [PubMed] [Google Scholar]
- 1418.Weston KL, Azevedo LB, Bock S, Weston M, George KP, Batter-ham AM. Effect of novel, school-based high-intensity interval training (hit) on cardiometabolic health in adolescents: Project ffab (fun fast activity blasts)—an exploratory controlled before-and-after trial. PLoS One 11: e0159116, 2016. DOI: 10.1371/journal.pone.0159116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1419.Wheatley CM, Snyder EM, Johnson BD, Olson TP. Sex differences in cardiovascular function during submaximal exercise in humans. Springerplus 3: 445, 2014. DOI: 10.1186/2193-1801-3-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1420.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, De Palma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT. acc/aha/aapa/abc/acpm/ags/apha/ash/aspc/nma/pcna guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation 138: e426–e483, 2017, 2018. DOI: 10.1161/CIR.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 1421.White AK, Smith RJ, Bigler CR, Brooke WF, Schauer PR. Head and neck manifestations of neurofibromatosis. Laryngoscope 96: 732–737, 1986. DOI: 10.1288/00005537-198607000-00005. [DOI] [PubMed] [Google Scholar]
- 1422.White JD, Dewal RS, Stanford KI. The beneficial effects of brown adipose tissue transplantation. Mol Asp Med 68: 74–81, 2019. DOI: 10.1016/j.mam.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1423.Whitham M, Parker BL, Friedrichsen M, Hingst JR, Hjorth M, Hughes WE, Egan CL, Cron L, Watt KI, Kuchel RP, Jayasooriah N, Estevez E, Petzold T, Suter CM, Gregorevic P, Kiens B, Richter EA, James DE, Wojtaszewski JFP, Febbraio MA. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab 27: 237–251.e4, 2018. DOI: 10.1016/j.cmet.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 1424.Whyte LJ, Gill JMR, Cathcart AJ. Effect of 2 weeks of sprint interval training on health-related outcomes in sedentary over-weight/obese men. Metab Clin Exp 59: 1421–1428, 2010. DOI: 10.1016/j.metabol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 1425.Widrick JJ, Knuth ST, Norenberg KM, Romatowski JG, Bain JL, Riley DA, Karhanek M, Trappe SW, Trappe TA, Costill DL, Fitts RH. Effect of a 17 day spaceflight on contractile properties of human soleus muscle fibres. J Physiol 516 (Pt 3): 915–930, 1999. DOI: 10.1111/j.1469-7793.1999.0915u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1426.Widrick JJ, Romatowski JG, Norenberg KM, Knuth ST, Bain JL, Riley DA, Trappe SW, Trappe TA, Costill DL, Fitts RH. Functional properties of slow and fast gastrocnemius muscle fibers after a 17-day spaceflight. J Appl Physiol (1985) 90: 2203–2211, 2001. DOI: 10.1152/jappl.2001.90.6.2203. [DOI] [PubMed] [Google Scholar]
- 1427.Widrick JJ, Trappe SW, Blaser CA, Costill DL, Fitts RH. Isometric force and maximal shortening velocity of single muscle fibers from elite master runners. Am J Phys 271: C666–C675, 1996. DOI: 10.1152/ajpcell.1996.271.2.C666. [DOI] [PubMed] [Google Scholar]
- 1428.Wikström-Frisén L, Boraxbekk CJ, Henriksson-Larsén K. Effects on power, strength and lean body mass of menstrual/oral contraceptive cycle based resistance training. J Sports Med Phys Fitness 57: 43–52, 2015. DOI: 10.23736/S0022-4707.16.05848-5. [DOI] [PubMed] [Google Scholar]
- 1429.Williams AS, Koves TR, Davidson MT, Crown SB, Fisher-Wellman KH, Torres MJ, Draper JA, Narowski TM, Slentz DH, Lantier L, Wasserman DH, Grimsrud PA, Muoio DM. Disruption of acetyllysine turnover in muscle mitochondria promotes insulin resistance and redox stress without overt respiratory dysfunction. Cell Metab 31: 131–147.e11, 2020. DOI: 10.1016/j.cmet.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1430.Williams MA, Srikesavan C, Heine PJ, Bruce J, Brosseau L, Hoxey-Thomas N, Lamb SE. Exercise for rheumatoid arthritis of the hand. Cochrane Database Syst Rev 7: CD003832, 2018. DOI: 10.1002/14651858.CD003832.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1431.Williams RS. Mitochondrial gene expression in mammalian striated muscle. Evidence that variation in gene dosage is the major regulatory event [Online]. J Biol Chem 261: 12390–12394, 1986. http://www.ncbi.nlm.nih.gov/pubmed/3745193. [PubMed] [Google Scholar]
- 1432.Williams RS, Garcia-Moll M, Mellor J, Salmons S, Harlan W. Adaptation of skeletal muscle to increased contractile activity. Expression nuclear genes encoding mitochondrial proteins [Online]. J Biol Chem 262: 2764–2767, 1987. http://www.ncbi.nlm.nih.gov/pubmed/2880844. [PubMed] [Google Scholar]
- 1433.Wilson MG, Ellison GM, Cable NT. Basic science behind the cardiovascular benefits of exercise. Heart 101: 758–765, 2015. DOI: 10.1136/heartjnl-2014-306596. [DOI] [PubMed] [Google Scholar]
- 1434.Winding KM, Munch GW, Iepsen UW, Van Hall G, Pedersen BK, Mortensen SP. The effect on glycaemic control of low-volume high-intensity interval training versus endurance training in individuals with type 2 diabetes. Diabetes Obes Metab 20: 1131–1139, 2018. DOI: 10.1111/dom.13198. [DOI] [PubMed] [Google Scholar]
- 1435.Winker R, Lukas I, Perkmann T, Haslacher H, Ponocny E, Lehrner J, Tscholakoff D, Dal-Bianco P. Cognitive function in elderly marathon runners: Cross-sectional data from the marathon trial (apsoem). Wien Klin Wochenschr 122: 704–716, 2010. DOI: 10.1007/s00508-010-1485-z. [DOI] [PubMed] [Google Scholar]
- 1436.Winsley RJ, Armstrong N, Middlebrooke AR, Ramos-Ibanez N, Williams CA. Aerobic fitness and visceral adipose tissue in children. Acta Paediatr 95: 1435–1438, 2006. DOI: 10.1080/08035250600643244. [DOI] [PubMed] [Google Scholar]
- 1437.Wisløff U, Loennechen JP, Falck G, Beisvag V, Currie S, Smith G, Ellingsen O. Increased contractility and calcium sensitivity in cardiac myocytes isolated from endurance trained rats. Cardiovasc Res 50: 495–508, 2001. DOI: 10.1016/s0008-6363(01)00210-3. [DOI] [PubMed] [Google Scholar]
- 1438.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr 84: 475–482, 2006. DOI: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 1439.Wolfe RR, Klein S, Carraro F, Weber JM. Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. Am J Phys 258: E382–E389, 1990. DOI: 10.1152/ajpendo.1990.258.2.E382. [DOI] [PubMed] [Google Scholar]
- 1440.Wolsk E, Mygind H, Grøndahl TS, Pedersen BK, van Hall G. IL-6 selectively stimulates fat metabolism in human skeletal muscle. Am J Phys Endocrinol Metab 299: E832–E840, 2010. DOI: 10.1152/ajpendo.00328.2010. [DOI] [PubMed] [Google Scholar]
- 1441.Wong-Yu ISK, Mak MKY. Multi-dimensional balance training programme improves balance and gait performance in people with parkinson’s disease: A pragmatic randomized controlled trial with 12-month follow-up. Parkinsonism Relat Disord 21: 615–621, 2015. DOI: 10.1016/j.parkreldis.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 1442.Wood F, Salam A, Singh K, Day S, Jan S, Prabhakaran D, Rodgers A, Patel A, Thom S, Ward H. Process evaluation of the impact and acceptability of a polypill for prevention of cardiovascular disease. BMJ Open 5: e008018, 2015. DOI: 10.1136/bmjopen-2015-008018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1443.Wood KN, Luchyshyn TA, Shoemaker JK. High cardiorespiratory fitness in early to late middle age preserves the cortical circuitry associated with brain-heart integration during volitional exercise. J Neurophysiol 117: 1831–1840, 2017. DOI: 10.1152/jn.00592.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1444.World Health Organization. Muscuoskeletal Conditions [Online]. World Health Organization: [date unknown]. https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions [15 Mar. 2020].
- 1445.World Health Organization. Cardio Vascular Disease [Online]. World Health Organization: [date unknown]. https://www.who.int/cardiovascular_diseases/about_cvd/en/ [5 Mar. 2020].
- 1446.World Health Organization. Obesity and Overweight [Online]. World Health Organization: [date unknown]. World Health Organization. Obesity and overweight. Available at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight [15 Mar. 2020]. [Google Scholar]
- 1447.World Health Organization. Prevalence of Insufficient Physical Activity [Online]. World Health Organization: [date unknown]. https://www.who.int/gho/ncd/risk_factors/physical_activity_text/ [5 Mar. 2020].
- 1448.Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM. Exercise induces hippocampal bdnf through a pgc-1α/fndc5 pathway. Cell Metab 18: 649–659, 2013. DOI: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1449.Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B. The recent prevalence of osteoporosis and low bone mass in the united states based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res Off J Am Soc Bone Miner Res 29: 2520–2526, 2014. DOI: 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1450.Wronski TJ, Morey ER. Alterations in calcium homeostasis and bone during actual and simulated space flight [Online]. Med Sci Sports Exerc 15: 410–414, 1983. http://www.ncbi.nlm.nih.gov/pubmed/6645871. [PubMed] [Google Scholar]
- 1451.Wu H, Kanatous SB, Thurmond FA, Gallardo T, Isotani E, Bassel-Duby R, Williams RS. Regulation of mitochondrial biogenesis in skeletal muscle by camk. Science (New York, NY) 296: 349–352, 2002. DOI: 10.1126/science.1071163. [DOI] [PubMed] [Google Scholar]
- 1452.Wu H, Naya FJ, McKinsey TA, Mercer B, Shelton JM, Chin ER, Simard AR, Michel RN, Bassel-Duby R, Olson EN, Williams RS. MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type. EMBO J 19: 1963–1973, 2000. DOI: 10.1093/emboj/19.9.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1453.Wu Y-T, Beiser AS, Breteler MMB, Fratiglioni L, Helmer C, Hendrie HC, Honda H, Ikram MA, Langa KM, Lobo A, Matthews FE, Ohara T, Pérés K, Qiu C, Seshadri S, Sjölund B-M, Skoog I, Brayne C. The changing prevalence and incidence of dementia over time—current evidence. Nat Rev Neurol 13: 327–339, 2017. DOI: 10.1038/nrneurol.2017.63. [DOI] [PubMed] [Google Scholar]
- 1454.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 124: 471–484, 2006. DOI: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 1455.Wüst RCI, Morse CI, de Haan A, Jones DA, Degens H. Sex differences in contractile properties and fatigue resistance of human skeletal muscle. Exp Physiol 93: 843–850, 2008. DOI: 10.1113/expphysiol.2007.041764. [DOI] [PubMed] [Google Scholar]
- 1456.Wyckelsma VL, Levinger I, McKenna MJ, Formosa LE, Ryan MT, Petersen AC, Anderson MJ, Murphy RM. Preservation of skeletal muscle mitochondrial content in older adults: Relationship between mitochondria, fibre type and high-intensity exercise training. J Physiol 595: 3345–3359, 2017. DOI: 10.1113/JP273950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1457.Xue-Shan Z, Juan P, Qi W, Zhong R, Li-Hong P, Zhi-Han T, Zhi-Sheng J, Gui-Xue W, Lu-Shan L. Imbalanced cholesterol metabolism in alzheimer’s disease. Clin Chim Acta 456: 107–114, 2016. DOI: 10.1016/j.cca.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 1458.Yang J, Shanahan KJ, Shriver LP, Luciano MG. Exercise-induced changes of cerebrospinal fluid vascular endothelial growth factor in adult chronic hydrocephalus patients. J Clin Neurosci 24: 52–56, 2016. DOI: 10.1016/j.jocn.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 1459.Yarrow JF, White LJ, McCoy SC, Borst SE. Training augments resistance exercise induced elevation of circulating brain derived neurotrophic factor (bdnf). Neurosci Lett 479: 161–165, 2010. DOI: 10.1016/j.neulet.2010.05.058. [DOI] [PubMed] [Google Scholar]
- 1460.Yfanti C, Akerström T, Nielsen S, Nielsen AR, Mounier R, Mortensen OH, Lykkesfeldt J, Rose AJ, Fischer CP, Pedersen BK. Antioxidant supplementation does not alter endurance training adaptation. Med Sci Sports Exerc 42: 1388–1395, 2010. DOI: 10.1249/MSS.0b013e3181cd76be. [DOI] [PubMed] [Google Scholar]
- 1461.Yfanti C, Fischer CP, Nielsen S, Akerström T, Nielsen AR, Veskoukis AS, Kouretas D, Lykkesfeldt J, Pilegaard H, Pedersen BK. Role of vitamin c and e supplementation on il-6 in response to training. J Appl Physiol (1985) 112: 990–1000, 2012. DOI: 10.1152/japplphysiol.01027.2010. [DOI] [PubMed] [Google Scholar]
- 1462.Yfanti C, Nielsen AR, Akerström T, Nielsen S, Rose AJ, Richter EA, Lykkesfeldt J, Fischer CP, Pedersen BK. Effect of antioxidant supplementation on insulin sensitivity in response to endurance exercise training. Am J Phys Endocrinol Metab 300: E761–E770, 2011. DOI: 10.1152/ajpendo.00207.2010. [DOI] [PubMed] [Google Scholar]
- 1463.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev 93: 23–67, 2013. DOI: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1464.Young A The relative isometric strength of type i and type ii muscle fibres in the human quadriceps. Clin Physiol 4: 23–32, 1984. DOI: 10.1111/j.1475-097x.1984.tb00641.x. [DOI] [PubMed] [Google Scholar]
- 1465.Young MF, Valaris S, Wrann CD. A role for fndc5/irisin in the beneficial effects of exercise on the brain and in neurodegenerative diseases. Prog Cardiovasc Dis 62: 172–178, 2019. DOI: 10.1016/j.pcad.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1466.Younus H Therapeutic potentials of superoxide dismutase [Online]. Int J Health Sci 12: 88–93, 2018. http://www.ncbi.nlm.nih.gov/pubmed/29896077. [PMC free article] [PubMed] [Google Scholar]
- 1467.Zebrowska A, Gąsior Z, Langfort J. Serum igf-i and hormonal responses to incremental exercise in athletes with and without left ventricular hypertrophy [Online]. J Sports Sci Med 8: 67–76, 2009. http://www.ncbi.nlm.nih.gov/pubmed/24150558. [PMC free article] [PubMed] [Google Scholar]
- 1468.Zehnacker CH, Bemis-Dougherty A. Effect of weighted exercises on bone mineral density in post menopausal women. A systematic review. J Geriatr Phys Ther 30: 79–88, 2007. DOI: 10.1519/00139143-200708000-00007. [DOI] [PubMed] [Google Scholar]
- 1469.Zhang D, Liu X, Liu Y, Sun X, Wang B, Ren Y, Zhao Y, Zhou J, Han C, Yin L, Zhao J, Shi Y, Zhang M, Hu D. Leisure-time physical activity and incident metabolic syndrome: A systematic review and dose-response meta-analysis of cohort studies. Metab Clin Exp 75: 36–44, 2017. DOI: 10.1016/j.metabol.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 1470.Zhang Q, Young L, Li F. Network meta-analysis of various nonpharmacological interventions on pain relief in older adults with osteoarthritis. Am J Phys Med Rehabil 98: 469–478, 2019. DOI: 10.1097/PHM.0000000000001130. [DOI] [PubMed] [Google Scholar]
- 1471.Zhang X, Blalock D, Wang J. Classifications and definitions of normal joints. In: Chen Q, editor. Osteoarthritis—Progress in Basic Research and Treatment. InTechOpen, 2015. [Google Scholar]
- 1472.Zhao R, Bu W, Chen X. The efficacy and safety of exercise for prevention of fall-related injuries in older people with different health conditions, and differing intervention protocols: A meta-analysis of randomized controlled trials. BMC Geriatr 19: 341, 2019. DOI: 10.1186/s12877-019-1359-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1473.Zhao R, Zhao M, Zhang L. Efficiency of jumping exercise in improving bone mineral density among premenopausal women: A meta-analysis. Sports Med 44: 1393–1402, 2014. DOI: 10.1007/s40279-014-0220-8. [DOI] [PubMed] [Google Scholar]
- 1474.Zhao R, Zhao M, Xu Z. The effects of differing resistance training modes on the preservation of bone mineral density in postmenopausal women: A meta-analysis. Osteoporos Int 26: 1605–1618, 2015. DOI: 10.1007/s00198-015-3034-0. [DOI] [PubMed] [Google Scholar]
- 1475.Zhao X, Shah D, Gandhi K, Wei W, Dwibedi N, Webster L, Sambamoorthi U. Clinical, humanistic, and economic burden of osteoarthritis among noninstitutionalized adults in the united states. Osteoarthr Cartil 27: 1618–1626, 2019. DOI: 10.1016/j.joca.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 1476.Zhao Y, Pang Q, Liu M, Pan J, Xiang B, Huang T, Tu F, Liu C, Chen X. Treadmill exercise promotes neurogenesis in ischemic rat brains via caveolin-1/vegf signaling pathways. Neurochem Res 42: 389–397, 2017. DOI: 10.1007/s11064-016-2081-z. [DOI] [PubMed] [Google Scholar]
- 1477.Zheng G, Qiu P, Xia R, Lin H, Ye B, Tao J, Chen L. Effect of aerobic exercise on inflammatory markers in healthy middle-aged and older adults: A systematic review and meta-analysis of randomized controlled trials. Front Aging Neurosci 11: 98, 2019. DOI: 10.3389/fnagi.2019.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1478.Zhou Y, Zhao M, Zhou C, Li R. Sex differences in drug addiction and response to exercise intervention: From human to animal studies. Front Neuroendocrinol 40: 24–41, 2016. DOI: 10.1016/j.yfrne.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1479.Zimmer P, Schenk A, Kieven M, Holthaus M, Lehmann J, Lövenich L, Bloch W. Exercise induced alterations in nk-cell cytotoxicity—methodological issues and future perspectives [Online]. Exerc Immunol Rev 23: 66–81, 2017. http://www.ncbi.nlm.nih.gov/pubmed/28230531. [PubMed] [Google Scholar]
- 1480.Zimmer P, Schmidt ME, Prentzell MT, Berdel B, Wiskemann J, Kellner KH, Debus J, Ulrich C, Opitz CA, Steindorf K. Resistance exercise reduces kynurenine pathway metabolites in breast cancer patients undergoing radiotherapy. Front Oncol 9: 962, 2019. DOI: 10.3389/fonc.2019.00962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1481.Zoladz JA, Pilc A, Majerczak J, Grandys M, Zapart-Bukowska J, Duda K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men [Online]. J Physiol Pharmacol 59 (Suppl 7): 119–132, 2008. http://www.ncbi.nlm.nih.gov/pubmed/19258661. [PubMed] [Google Scholar]
- 1482.Żebrowska A, Wa’skiewicz Z, Zając A, Gąsior Z, Galbo H, Langfort J. IGF-1 response to arm exercise with eccentric and concentric muscle contractions in resistance-trained athletes with left ventricular hypertrophy. Int J Sports Med 34: 116–122, 2013. DOI: 10.1055/s-0032-1321720. [DOI] [PubMed] [Google Scholar]


