Abstract
Background and study aims Duodenoscopes that are contaminated due to inadequate reprocessing are well-documented. However, studies have demonstrated poor reprocessing of other kinds of endoscopes as well, including echoendoscopes, gastroscopes, and colonoscopes. We estimated the contamination rate beyond the elevator of gastrointestinal endoscopes based on available data.
Methods We searched PubMed and Embase from January 1, 2010 to October 10, 2020, for studies investigating contamination rates of reprocessed gastrointestinal endoscopes. A random-effects model was used to calculate the contamination rate of patient-ready gastrointestinal endoscopes. Subgroup analyses were conducted to investigate differences among endoscope types, countries, and colony-forming unit (CFU) thresholds.
Results Twenty studies fulfilled the inclusion criteria, including 1,059 positive cultures from 7,903 samples. The total contamination rate was 19.98 % ± 0.024 (95 % confidence interval [Cl]: 15.29 %–24.68 %; I 2 = 98.6 %). The contamination rates of colonoscope and gastroscope channels were 31.95 % ± 0.084 and 28.22 % ± 0.076, respectively. Duodenoscope channels showed a contamination rate of 14.41 % ± 0.029. The contamination rates among studies conducted in North America and Europe were 6.01 % ± 0.011 and 18.16% ± 0.053 %, respectively. The contamination rate among studies using a CFU threshold > 20 showed contamination of 30.36 % ± 0.094, whereas studies using a CFU threshold < 20 showed a contamination rate of 11 % ± 0.026.
Conclusions On average, 19.98 % of reprocessed gastrointestinal endoscopes may be contaminated when used in patients and varies between different geographies. These findings highlight that the elevator mechanism is not the only obstacle when reprocessing reusable endoscopes; therefore, guidelines should recommend more surveillance of the endoscope channels as well.
Introduction
In recent years, reusable duodenoscopes have become an area of interest because of numerous reports of infection transmission by contaminated duodenoscopes following ERCP 1 2 3 4 . Duodenoscopes are prone to reprocessing errors because of their complex designs, especially around the elevator mechanism. Many studies found that microbes harbor in the instrument channel and other places in the endoscope as well. In addition, the channels of the endoscopes are prone to scratches when tools are inserted, which can create additional areas for the microbes to harbor 5 6 . Microbiological testing is standard at most endoscopy units; however, sampling methods and requirements vary across countries.
Adenosine triphosphate (ATP) testing is an established and inexpensive indicator for washing efficacy 7 . Nevertheless, this test should not replace routine microbiologic methods because of their low sensitivity and specificity 8 . ATP tests poorly correlate with microbiologic standards for assessing endoscope contamination 9 . Visual inspection using a borescope has been suggested as a quality assurance step in reprocessing to detect scratches and other irregularities within endoscope channels. Several studies identified internal defects of instrument channels to be more frequent than anticipated, increasing their microbiological contamination susceptibility 6 10 11 . Inconsistencies in recommended quality measures to detect microbiological debris in endoscope channels may also pose safety risks.
In July 2019, the United States Food and Drug Administration (FDA) was made aware of a hospital in Oklahoma that had used contaminated gastroscopes on almost 1,000 patients. However, no patient-related infections were allegedly reported or detected 12 . Several studies investigating duodenoscope contamination rates sampled both the elevator and the working channel and detected microbiological organisms in both parts 9 13 14 15 . Duodenoscopes with disposable endcaps have been introduced as an attempt to overcome the challenges of duodenoscopes being vectors for patient cross-infections 16 . However, studies showed that even single-use endcap duodenoscopes remain contaminated after reprocessing 17 18 . Bronchoscopes have been implicated in multiple outbreaks and associated with high contamination rates, even without the elevator 18 19 20 21 . The fact that positive microbiological samples have been identified in various non-elevator endoscopes may indicate that contamination issues due to inadequate reprocessing are not limited to duodenoscopes. Previous studies investigated contamination rates in endoscope channels and areas beyond the elevator mechanism; nevertheless, no studies estimated the overall contamination rate associated with patient-ready gastrointestinal endoscopes unrelated to the elevator mechanism. We aimed to assess the contamination rate beyond the elevator of patient-ready gastrointestinal endoscopes based on the data from 2010 to 2020.
Methods
Study selection
We conducted a systematic literature review to identify full-text studies published in English, investigating contamination rates associated with all types of gastrointestinal endoscopes. Studies concerning duodenoscopes and linear echoendoscopes, which are both endoscope types with an elevator, were included if data were available for any channels sampled. The comprehensive literature search is presented in Fig. 1 . The analysis and inclusion criteria were based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Guideline 22 .
Fig. 1.
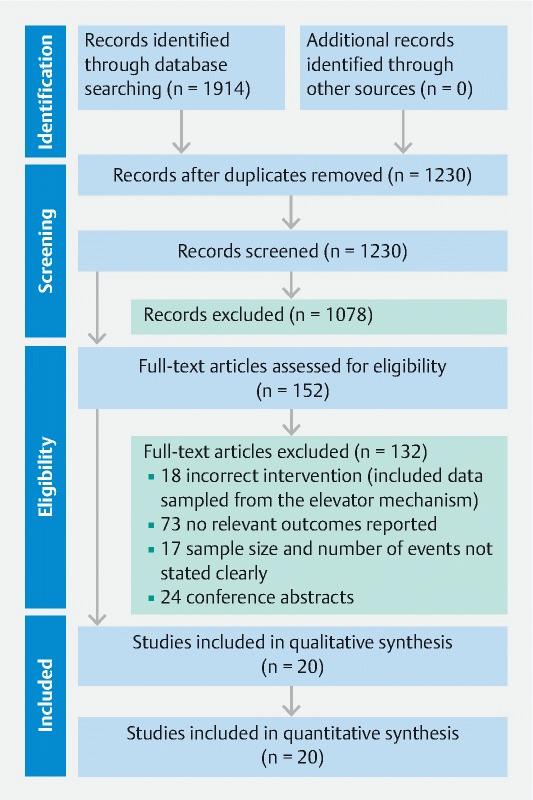
Flowchart illustrating the study process and the selection of included publications. From Moher D, Liberati A, Tetzlaff J et al. The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses; The PRISMA Statement. PLoS Med 6; e1000097
Studies were identified through a systematic literature search from January 1, 2010 until October 10, 2020 in the electronic databases PubMed, Web of Science, and Embase. To identify relevant studies, we conducted the search using the following medical subject headings (MeSH) and keywords: (duodenoscope* [MeSH Terms]) OR (gastroscope* [MeSH Terms])) OR (colonoscope* [MeSH Terms])) OR (endoscope [MeSH Terms])) OR (endoscopic ultrasonography [MeSH Terms])) OR (endoscopic ultrasonographies [MeSH Terms])) OR (cholangiopancreatographies, endoscopic retrograde [MeSH Terms])) OR (double balloon enteroscopies [MeSH Terms])) OR (double balloon enteroscopy [MeSH Terms])) OR (enteroscopies, double balloon [MeSH Terms])) OR (enteroscopy, double balloon [MeSH Terms])) OR (cholangiographies [MeSH Terms])) OR (cholangiography [MeSH Terms])) OR (Spyglass) AND ((contamination, equipment [MeSH Terms]) OR (cross-contamination)) OR (bacterial infections [MeSH Terms])) OR (disinfection [MeSH Terms])) OR (disinfectants [MeSH Terms])) OR (reprocessing)) OR (equipment reusability [MeSH Terms])). Truncation was deployed after some keywords to include different variations of the term and thereby broaden the search.
Inclusion and exclusion criteria
The search was conducted to identify relevant randomized controlled trials, surveillance studies, and prospective or retrospective cohort studies investigating contamination rates associated with reprocessed gastrointestinal endoscopes. The search was limited to studies published after 2010, as microbiological surveillance testing in endoscopy and was recommended in both European and US guidelines in this period followed by varies updates in reprocessing guidelines 23 24 25 26 27 because a time horizon of 10 years was considered reasonable due to various updates in endoscope reprocessing guidelines in last 10 years. For inclusion, the total number of microbiological samples (N) and the number of positive cultures (n) needed to be reported. It was imperative that all samples were acquired from a gastrointestinal endoscope excluding samples taken from the elevator mechanism and not from any patients or other medical equipment. Exclusion criteria included all types of studies performed on animals or in vitro models, as well as conference abstracts, editorials, letters, and gray literature that did not report any original findings. We assumed high heterogeneity between studies due to varying study design and definitions of positivity. To account for the heterogeneity, studies with sample size of less than 50 were excluded to avoid bias in the random-effects model 28 .
Titles and abstracts of all identified studies were independently reviewed by two authors (SL and NBL). Studies that did not fulfill the inclusion criteria were excluded, and the full texts of the remaining publications were independently reviewed by three authors (SL, NBL, and SA). Any disagreements related to the inclusion or exclusion of studies were resolved by consensus.
Data extraction
All included studies were assessed for eligibility by three independent reviewers (SL, NBL, and SA). The authors were not blinded to any information within the studies. For each included study, we extracted the following baseline characteristics: First author, year, study design, country, hospital, endoscope type(s), sampled channels/areas, positive cultures, sample size, type of microorganism, reprocessing method, and CFU threshold.
Outcomes
The primary outcome of the meta-analysis was the total weighted contamination rate beyond the elevator, based on the number of positive microbiological sample cultures (n) relative to the number of samples in total (N). Three subgroup analyses were carried out to assess potential significant differences between countries and applied CFU thresholds. The first subgroup analysis was conducted for studies only including samples from gastroscope channels and colonoscope channels both individually and combined. The second subgroup analysis was conducted for studies only, including samples taken from duodenoscope channels and areas beyond the elevator. The third subgroup analysis was conducted for studies that originated in North America, Europe, and the rest of the world (RoW). The fourth subgroup analysis was conducted among studies with a CFU threshold > 20 and those with a CFU threshold < 20.
No patient-specific data were assessed because the analysis only focused on gastrointestinal endoscopes. There were no missing data for any of the data points used to calculate the weighted contamination rates.
Data analysis and statistical methods
A meta-analysis was conducted based on data from studies where contamination rates of gastrointestinal endoscope channels, insertion cord, and all other surface areas beyond the elevator mechanism were assessed. The primary objective of the meta-analysis was to calculate the total contamination rate beyond the elevator of reprocessed patient-ready gastrointestinal endoscopes. Four subgroup analyses were carried out to do the following: 1) investigate the contamination rate among samples from gastroscope and colonoscope channels both separately and combined; 2) investigate the contamination rate among samples from duodenoscope channels; 3) assess the contamination rate in various countries (North America, European countries, and RoW); and 4) assess the contamination rate among studies using a CFU threshold > 20 and studies using a CFU threshold < 20.
We used the meta-package (metafor) in RStudio version 3.6.2 to conduct the statistical analyses. All data were pooled using a random-effects model based on proportions (prop). The random-effects model was applied because we anticipated heterogeneity, predominantly arising from variations in both sample size (N) and outcome (positive samples, n). We used the inconsistency index (I 2 ) test to estimate the level of heterogeneity between the included studies. I 2 indicates the proportion (%) of variation between the studies linked to heterogeneity rather than a coincidence 29 30 . Heterogeneity values below 50 % indicated low to moderate heterogeneity levels 30 . Publication bias was assessed using funnel plots. To avoid drawing any subjective conclusions based solely on the funnel plot, we evaluated the asymmetry of the funnel using Egger’s regression. All study outcomes were presented in forest plots ( Fig. 2 ).
Fig. 2.
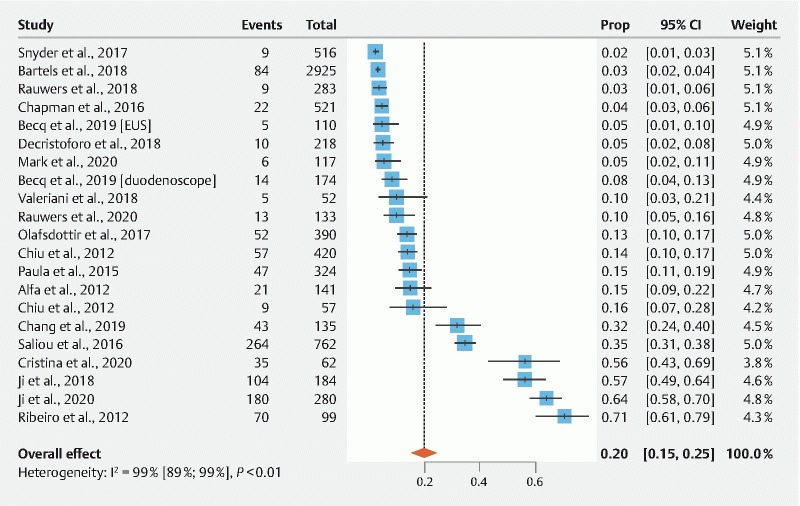
Pooled estimates of contamination rates beyond the elevator. Cl, confidence interval; EUS, endoscopic ultrasound.
Results
Characteristics of included studies
We identified a total of 1,914 peer-reviewed studies. After duplicates were removed, a total of 1,230 studies were screened based on title and abstract. After applying our inclusion and exclusion criteria, the number of studies was narrowed to 152 studies that were assessed in full text for eligibility. After the full-text assessment, 20 studies fulfilled all inclusion criteria and were included in the final meta-analysis. Fig. 1 shows the PRISMA flowchart illustrating the study selection process.
All 20 studies included in the final analysis were published between January 1, 2010, and October 10, 2020. The included studies yielded a sample size of 7,903 cultures sampled from various gastrointestinal endoscope channels and areas beyond the elevator. There were a total of 1,059 positive samples. One study (Becq et al., 2019 31 ) provided complete data for both echoendoscopes and duodenoscopes and, therefore, was included in the analysis twice (i. e., 21 data points were included in the random-effects model).
Baseline characteristics of all included studies (n = 20) in the primary analysis are provided in Table 1 . Of the included studies, six studies (30 %) were conducted in the United States, seven (35 %) were conducted in Europe, including studies from the Netherlands (n = 2), Italy (n = 2), France (n = 1), and Austria (n = 2). Five studies (25 %) were conducted in Asia, including studies from Taiwan (n = 3) and China (n = 2). Finally, one study (5 %) was conducted in Canada, and one study (5 %) was conducted in Brazil. Table 2 shows the total sample size and number of positive samples taken from gastroscopes and colonoscopes separately and combined.
Table 1. Characteristics of included studies.
| First author, year | Study design | Country | Hospital | Endoscopes type(s) | Sampled channels/areas | Positive cultures, n | Sample size, N | Type of microorganism | Reprocessing method | CFU threshold |
| Saliou, 2016 32 | Descriptive study | France | Brest Teaching Hospital | Gastroscopes, colonoscopes, duodenoscopes, echoendoscopes, transnasal gastroscopes, enteroscopes, choledoscope | Working channel, air/water channel, elevator-guidewire channel, waterjet channel | 264 | 762 | Coagulase-negative staphylococci, Bacillus spp., Micrococcus spp., Corynebacterium spp., Enterococcus spp., Actinomyces spp., Brevibacterium spp., Staphylococcus aureus , Streptococcus spp., Streptomyces spp, Pseudomonas spp., Pseudomonas aeruginosa , Stenotrophomonas spp., Klebsiella spp., Rhizobium radiobacter , Acinetobacter spp., Enterobacter spp., Sphingomonas spp., Escherichia coli , Methylobacterium spp., Brevundimonas spp., Citrobacter spp., Moraxella spp., Morganella morganii , Candida spp., Rhodotorula spp., Cladosporium spp., other fungi and yeasts | HLD | > 25 CFU |
| Snyder, 2017 33 | Parallel group randomized study | United States | Beth Israel Deaconess Medical Center | Duodenoscopes | Working channel | 9 | 516 | N/A | HLD, dHLD, HLD/EtO | > 0 CFU |
| Rauwers, 2018 34 | Prospective nationwide cross-sectional study | Netherlands | 67 Dutch ERCP centers | Duodenoscopes | Biopsy channel, suction channel | 9 | 283 | Yeasts, Moraxella spp., Klebsiella pneumoniae, Streptococcus salivarius, Enterobacter cloacae, Moraxella osloensis, Escherichia coli, Streptococcus mitis, Klebsiella oxytoca, Neisseria flavescens, Enterococcus faecium, Rothia spp., Enterococcus faecalis, Streptococcus mutans, Pseudomonas aeruginosa, Streptococcus oralis, Staphylococcus aureus, Streptococcus spp. Bacillus spp., Stenotrophomonas maltophilia, Micrococcus luteus, Acinetobacter spp., Staphylococcus epidermidis, Agrobacterium radiobacter, Kocuria spp., Paracoccus yeeii, Staphylococcus hominis, Achromobacter xylosoxidans, Staphylococcus warneri, Alternaria spp., Kocuria rhizophila, Pseudomonas monteilii, Micrococcus spp., Pseudomonas putida, Staphylococcus auricularis, Sphingomonas paucimobilis, Staphylococcus spp. (CNS), Rhizobium spp. Or Sphingobium spp. | HLD | ≥ 20 CFU |
| Olafsdottir, 2017 9 | Parallel group randomized study | United States | Beth Israel Deaconess Medical Center | Duodenoscopes | Working channel | 52 | 390 | N/A | HLD | > 0 CFU |
| Paula, 2015 35 | Descriptive study | Austria | Vienna University Hospital | Duodenoscopes | Air, water, suction, and biopsy channel | 47 | 412 | Unspecified skin bacteria and aerobe spore-forming bacilli | HLD | > 100 CFU |
| Mark, 2020 13 | Descriptive study | United States | Children’s Hospital Colorado | Duodenoscopes | Working channel | 6 | 117 | Pseudomonas aeruginosa , fungal organisms, Staphylococcus aureus , Coagulase-negative staphylococcus, Streptococcus viridans | HLD | > 10 CFU |
| Alfa, 2012 36 | Descriptive study | Canada | St Boniface General Hospital | Colonoscopes, gastroscopes, duodenoscopes | All channels | 21 | 141 | gram-positive Bacilli, gram-positive Cocci | HLD | N/A |
| Cristina, 2020 15 | Descriptive study | Italy | N/A | Duodenoscopes | Distal end, instrument channel | 35 | 62 | Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter baumannii, Klebsiella oxytoca, Stenotrophomonas maltophilia, Escherichia coli, Citrobacter freundii, Enterobacter spp | HLD | > 10 CFU |
| Ji, 2018 37 | Descriptive study | China | Unspecified, all endoscopy units in Tianjin, China | Colonoscopes, gastroscopes | Biopsy channel | 104 | 184 | Pseudomonas aeruginosa, Escherichia coli, Acinetobacter lwoffii, Stenotrophomonas maltophilia , Malodorous mononeurosis, Enterococcus faecalis, Testicular pseudomonas, Burkholderia cepacia | HLD | > 20 CFU |
| Chang, 2019 38 | Descriptive study | Taiwan | Unspecified, 14 major tertiary care teaching hospitals | Duodenoscopes | Distal end outer surface, distal attachment cap, elevator wire channel, suction biopsy channel | 43 | 135 | N/A | HLD, dHLD, EtO | N/A |
| Chapman, 2016 39 | Descriptive study | United States | N/A | Echoendoscopes | Suction channel | 22 | 521 | Klebsiella pneumoniae, Citrobacter freundii, Escherichia coli, Pseudomonas aeruginosa, Klebsiella oxytoca, Sphingomonas paucimobilis, Acinetobacter baumanii, Hafnia alvei, Enterobacter cloacae, Pseudomonas putida, Stenotrophomonas maltophilia | HLD | N/A |
| Chiu, 2012 40 | Prospective surveillance study | Taiwan | Chang Gung Memorial Hospital, Kaohsiung Medical Center | Colonoscopes, gastroscopes | Biopsy channel | 57 | 420 | GNGN bacteria, Klebsiella pneumoniae, Acinetobacter baumanni, Enterococcus spp., Comamonas testosterone, Chryseobacterium indologenes, Sphingomonas paucimobilis, Pseudomonas putida, Viridans Streptococcus, Stomatococcus spp., Prevotella bivia, Escherichia coli, Pseudomonas aeruginosa, Enterococcus faecium, Bacteroides fragilis, Bacteroides vulgatus, Bacteroides distasonis, Clostridium perfringens, Proteus mirabilis, Moraxella osloensis, Candida glabrata | HLD | 10 3 CFU/mL |
| Valeriani, 2018 41 | Descriptive study | Italy | Unspecified, 10 Italian hospitals | Colonoscopes | Unspecified, inner channels | 5 | 52 | B. vulgatus 16S amplicon, B. vulgatus OmpA, Enterococcus faecalis, Escherichia coli, B. fragilis, S. aureus | HLD | N/A |
| Becq, 2019 31 | Prospective single-center study | United States | N/A | Echoendoscopes | Working channel | 5 | 110 | N/A | HLD | > 0 CFU |
| Duodenoscopes | 14 | 174 | ||||||||
| Chiu, 2012 42 | Descriptive study | Taiwan | N/A | Enteroscopes (DBE) | Suction channel | 9 | 57 | Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, E. aerogenes, A. baumannii, Enterococcus sp., Gm ( + ) bacilli glucose-nonfermenting gp., Proteus vulgaris, Staphylococcus epidermidis, Bacteroides caccae, Prevotella melaninogenica | HLD | N/A |
| Decristoforo, 2018 43 | Descriptive study | Austria | Unspecified, 29 endoscopy centers | Colonoscopes, gastroscopes, duodenoscopes | Biopsy/suction channel | 10 | 218 | Sphingomonas parasanguinis, Streptococcus viridans, Moraxella osloensis, Pseudomonas pseudoalcaligenes, Pseudomonas oleovorans, Pseudomonas luteola, Streptococcus mitis, Moraxella osloensis, Staphylococcus aureus | HLD | ≤ 10 CFU |
| Ribeiro, 2012 44 | Descriptive study | Brazil | Unspecified, gastrointestinal endoscopy units in Belo Horizonte | Colonoscopes, gastroscopes | Air/water channel | 70 | 99 | Pseudomonas aeruginosa, Escherichia coli, Acinetobacter baumannii, Klebsiella pneumoniae | HLD | N/A |
| Rauwers, 2020 45 | Prospective nationwide cross-sectional study | Netherlands | 61 Dutch ERCP centers | Duodenoscopes, echoendoscopes 1 | Balloon channel, biopsy channel, suction channel | 13 | 133 | N/A | HLD | ≥ 20 CFU |
| Ji, 2020 46 | Descriptive study | China | Unspecified, 59 Endoscopy units in Tianjin | Colonoscopes, gastroscopes | Biopsy channel | 180 | 280 | N/A | HLD | > 20 CFU |
| Bartles, 2018 47 | Controlled randomized study | United States | Unspecified, four facilities with endoscopy labs | Echoendoscopes and duodenoscopes | Suction and working channel | 84 | 2,925 | Enterococcus spp, Enterobacter cloacae , Aeromonas spp, E. coli (ESBL + ), E. coli (ESBL-), Enterococcus faecium 2 | dHLD, HLD | N/A |
The study was conducted in the main hospitals of different Italian regions (Campania, Emilia Romagna, Lazio, Liguria, Marche, Molise, Tuscany, Veneto, Sardinia, and Sicily) involving ten endoscopy units that reprocess approximately 50–100 endoscopes per business day.
Samples from the original biopsy channels for both duodenoscopes and echoendoscopes.
Only high-concern pathogens were specified in study.
Table 2. Characteristics of studies that included samples from colonoscopes and gastroscopes.
| First author, year | Country | Endoscopes type(s) | Positive cultures, n | Sample size, N |
| Saliou, 2016 32 | France | Gastroscopes | 86 | 274 |
| Colonoscopes | 74 | 190 | ||
| Alfa, 2012 36 | Canada | Gastroscopes | 3 | 29 |
| Colonoscopes | 13 | 69 | ||
| Ji, 2018 37 | China | Gastroscopes | 36 | 72 |
| Colonoscopes | 68 | 112 | ||
| Chiu, 2012 40 | Taiwan | Gastroscopes | 32 | 300 |
| Colonoscopes | 25 | 120 | ||
| Valeriani, 2018 41 | Italy | Colonoscopes | 5 | 52 |
| Decristoforo, 2018 43 | Austria | Gastroscopes | 3 | 107 |
| Colonoscopes | 6 | 95 | ||
| Ribeiro, 2012 44 | Brazil | Gastroscopes | 42 | 60 |
| Colonoscopes | 28 | 39 | ||
| Ji, 2020 46 | China | Gastroscopes & colonoscopes | 180 | 280 |
The majority of the studies (17 of 20, 85 %) reported using high-level disinfection (HLD) as the reprocessing method used to clean the gastrointestinal endoscopes. Two studies (10 %) tested a combination of both HLD, double HLD (dHLD), and ethylene oxide (EtO) sterilization, and one study compared dHLD and HLD (5 %). Thirteen of 20 studies (65 %) reported a CFU threshold, six studies (30 %) reported a CFU threshold >20, and seven studies (35 %) reported a CFU threshold < 20.
Analysis of primary outcomes
Meta-analysis of the included studies demonstrated a pooled contamination rate beyond the elevator of 19.98 % ± 0.024 % (95 % confidence interval [Cl]: 15.29 %–24.68 %; I 2 = 98.6 %; Fig. 2 ). Heterogeneity between the included data points (n = 21) was considered to be high. Funnel plot analysis and Egger’s regression test indicated no significant publication bias ( P = 0.0531).
Subgroup analyses
Meta-analysis of studies only including samples from colonoscopes (n = 7) showed a contamination rate of 31.95 % ± 0.084 (95 % Cl: 15.55 %–48.36 %; I 2 = 95.2 %;) ( Fig. 3 ). Egger’s regression test indicated significant publication bias ( P = 0.0469). Meta-analysis of studies with gastroscope-specific samples (n = 6) showed a contamination rate of 28.22 % ± 0.076 (95 % Cl: 13.35 %–43.10 %; I 2 = 96.4 %) ( Fig. 4 ). Egger’s regression test indicated no significant publication bias ( P = 0.1293). Meta-analysis of studies including samples from both gastroscopes and colonoscopes (n = 8) showed a contamination rate of 33.20 % ± 0.084 (95 % Cl: 16.80 %–49.60 %; I 2 = 98.9 %) ( Fig. 5 ). Egger’s regression test indicated significant publication bias ( P = 0.0434). Meta-analysis of studies with duodenoscope channel-specific samples (n = 8) showed a contamination rate of 14.41 % ± 0.029 % (95 % Cl: 8.70 %–20.13 %; I 2 = 96.4 %) ( Fig. 6 ). Egger’s regression test indicated no significant publication bias ( P = 0.9919).
Fig. 3.
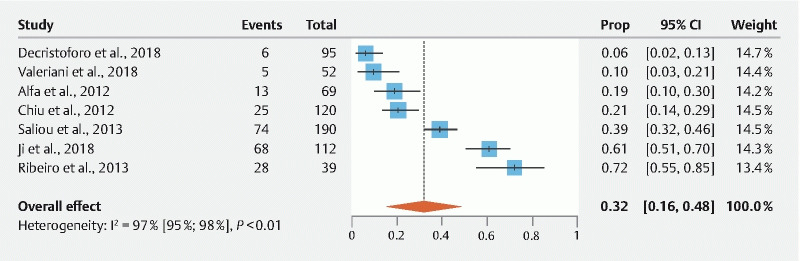
Pooled estimates of contamination rates for studies that included samples only from colonoscopes. Cl, confidence interval; prop, proportion.
Fig. 4.
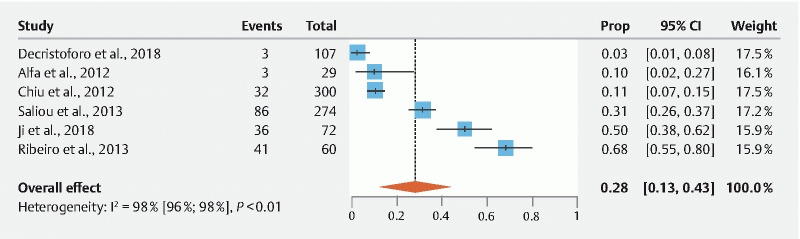
Pooled estimates of contamination rates for studies that included samples only from gastroscopes. Cl, confidence interval; prop, proportion.
Fig. 5.
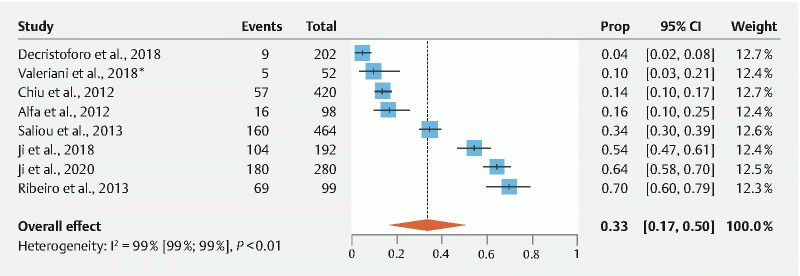
Pooled estimates of contamination rates for studies that included samples from both gastroscopes and colonoscopes. Cl, confidence interval; prop, proportion.
Fig. 6.
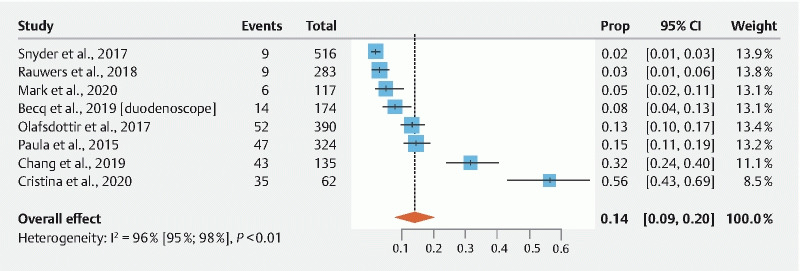
Pooled estimates of contamination rates beyond the elevator for studies that included only samples from duodenoscopes. Cl, confidence interval; prop, proportion.
Meta-analysis of studies conducted in North America (USA and Canada) (n = 7) showed a pooled contamination rate of 6.01 % ± 0.011 % (95 % Cl: 3.88 %–8.15 %; I 2 = 89.3 %; Supplementary Fig. 1 ). The pooled contamination rate among studies conducted in European countries (n = 7) was 18.16 % ± 0.053 % (95 % Cl: 7.75 %–28.57 %; I 2 = 98.1 %; Supplementary Fig. 2 ). Studies defined as RoW (n = 6) demonstrated a contamination rate of 42.10 % ± 0.011 % (95 % Cl: 19.78 %–64.41 %; I 2 = 98.7 %; Supplementary Fig. 3 ). Egger’s regression test indicated significant publication bias ( P = 0.0025) for studies conducted in Europe. Egger’s regression test did not indicate significant publication bias for studies conducted in North America and RoW ( P = 0.0655 and P = 0.2231). Finally. meta-analysis of studies using a CFU threshold > 20 (n = 6) showed a pooled contamination rate beyond the elevator of 30.36 % ± 0.094 % (95 % Cl: 11.96%–48.75 %; I 2 = 99.3 %), whereas studies using a CFU threshold < 20 (n = 8) showed a contamination rate of 11 % ± 0.026 % (95 % Cl: 5.94 %–16.06 %; I 2 = 95.3 %) ( Supplementary Fig. 4 and Supplementary Fig. 5 ). Egger’s regression test only indicated significant publication bias for studies using a CFU threshold > 20 ( P = 0.026). Heterogeneity was considered high for all subgroup analyses.
Discussion
We performed a meta-analysis to estimate contamination rates unrelated to the elevator mechanism among patient-ready gastrointestinal endoscopes. Our findings suggest that the overall reported contamination rate beyond the elevator of patient-ready gastrointestinal endoscopes is 19.98 %. Subgroup analyses found different contamination rates depending on the type of endoscope. Studies only including samples from colonoscopes showed a contamination rate of 31.95 % ± 0.084 % compared to studies only including samples from gastroscopes where the contamination rate was 28.22 % ± 0.076 %. The endoscope type with the lowest contamination rate was duodenoscopes (14.41 % ± 0.01 %). Additionally, subgroup analyses found different contamination rates across countries, with the highest contamination rate among studies conducted in what was defined as “RoW,” including studies from China, Taiwan, and Brazil (42.10 % ± 0.011 %). The contamination rates in studies originating from Europe and North America were 18.16 % ± 0.053 % and 6.01 % ± 0.011 %, respectively. Finally, studies using a CFU threshold > 20 revealed contamination rates of 30.36 % ± 0.094 %. In contrast to these findings, studies using a CFU threshold < 20 showed a significantly lower contamination rate of 11 % ± 0.026 %. However, we should also note that these conclusions could also be impacted by differences in study design, definitions of positivity, and apparent neglect to categorize any sample with a pathogen as a positive, high-risk finding.
Our subgroup analysis indicated the lowest contamination rate among studies carried out in North America. These findings might reflect the increasing awareness of the risk of contaminated endoscopes and development of FDA guidelines leading to stricter adherence to reprocessing guidelines. However, most of the communications related to endoscope reprocessing has concerned duodenoscopes with a special focus on the elevator, which does not explain why the contamination rate beyond the elevator channel was lower than that of other countries as well. We found the contamination rate beyond the elevator was 18.16 % in Europe, significantly higher than the contamination rate in North America. Despite very limited communications regarding contaminated endoscopes and reprocessing in European countries, these findings may indicate that contamination issues are not limited to the United States. Our previous study on duodenoscope contamination rates found an overall contamination rate of 15.25 %, whereas only four studies were conducted in European countries 32 . Rauwers et al. invited 74 Dutch endoscopy centers to sample duodenoscopes and linear echoendoscopes and found that ~15 % of the endoscopes were contaminated 33 . Our findings suggest a higher contamination rate for colonoscopes and gastroscopes compared to duodenoscopes. This might be due to the fact that most of the samples included in these analyses originated from “RoW” where an overall higher contamination rate was found compared to North America and Europe. These studies may have skewed the data toward higher contamination rates for both colonoscopes and gastroscopes. We also would like to stress on the impact of various culture methods on microbial growth. It is important to note that most studies conducted prior to 2018 did not utilize a neutralizer to counteract the effect of residual reprocessing chemicals on microbial growth, and most of the earlier studies incubated samples for only 48 hours. However, the study by Saliou et al. notes the importance of longer incubation times to grow viable slow-growing microbes. Therefore, the positivity rate in their study was far higher than almost any of the other included studies (35 %). Later in 2018, the US FDA/CDC released new guidance recommending that flush-brush-flush sampling methods be used to harvest samples; neutralizers be used to counteract reprocessing chemicals; and samples be incubated for at least 72 hours.
Very limited evidence exists on the attributable infection risk associated with contaminated gastroscopes and colonoscopes. Wang et al. estimated the post-endoscopic infection per 1,000 procedures within seven days for colonoscopy (screening and non-screening) and gastroscopy. The infection risk for screening colonoscopy was 1.1/1,000, and for non-screening colonoscopy, it was 1.6/1,000. The infection risk for gastroscopy was 3/1,000, which was almost twice as high as that of colonoscopy 34 . Lin et al. compared the incidence of infection within 30 days after colonoscopy and sigmoidoscopy. Following colonoscopy, the overall infection risk was 0.37 %, which was significantly higher than that of the contro group (0.04 %; P < 0.001) 35 . Few cases of gastroscope-associated cross-infections have been published 36 37 38 39 . Naas et al. reported an outbreak where two patients developed carbapenem- and colistin-resistant Klebsiella pneumoniae due to a contaminated gastroscope 37 . The bacteria mutated to 17 different isolates over 4.5 years in one of the infected patients, and the patient died due to sepsis with intestinal bacteria, including the original carbapenem- and colistin-resistant Klebsiella pneumoniae 39 .
However, the lack of evidence linking contaminated gastrointestinal endoscopes other than duodenoscopes to infections could indicate a smaller risk associated with non-endoscopic retrograde cholangiopancreatography procedures. Nevertheless, the discrepancy could be due to lesser degrees of awareness about infection risk from the endoscope parts beyond the elevator mechanism.
In recent years, contaminated duodenoscopes have gained much attention due to their complex design 2 16 . However, duodenoscopes are not the only types of endoscope with complex designs; linear echoendoscopes also have similar designs. Sun et al. stated that there is a significant overlap between the indications for endoscopic ultrasound (EUS) and ERCP, and recommended that similar reprocessing FDA recommendations should be applied for all endoscopes with elevator mechanisms. 40 Despite similarities between duodenoscopes and echoendoscopes, few studies report contamination data or infection related to EUS. Chapman et al. found that 21 of 521 cultures (4.1 %) obtained from echoendoscopes were positive following HLD. 41 Rauwers et al. investigated contamination rates of both duodenoscopes and echoendoscopes and found that 13 of 133 samples (9.8 %) taken from the balloon, biopsy, and suction channels were positive for microbiological growth 33 . This suggests that the elevator may not be the only obstacle when reprocessing elevator-containing endoscopes. Additionally, Olympus recently issued an ‘urgent field safety notice’ concerning the use of EUS endoscopes. Olympus has revised instructions for use for various EUS endoscopes after an investigation indicated a potential risk of infection due to residue in the air/water channel. To further mitigate this risk, Olympus has updated the instructions for use for 23 affected EUS endoscope models by adding an inspection step before reprocessing 42 .
Gastrointestinal endoscope channels are prone to scratches and their long, narrow channels make them difficult to properly investigate for microbiological debris 5 41 43 . Our analysis casts doubt on the suggestion that disposable endcaps are the answer to contamination issues; several studies reported high contamination rates in the channels and areas beyond the elevator. Ridtitid et al. compared bacterial contamination and organic residue using rapid ATP testing and cultures from duodenoscopes with detachable versus fixed distal caps after HLD. The authors found that, after HLD, the proportion of bacterial contamination and the organic residue was significantly lower in the group with detachable end caps than in the group of duodenoscopes with fixed end caps (37.0 % vs. 75.9 %; P < 0.001; relative risk 0.49, 95 % Cl 0.33–0.71). However, even with a significant reduction in the contamination levels, the duodenoscopes were still not completely free of bacterial residues. Our subgroup analysis demonstrated a 14.41 % contamination rate among studies only including samples from duodenoscope channels.
Contaminated endoscopes remain a challenge, and until the potential harmful effects of this are fully investigated, these issues should be taken seriously. The US Centers for Disease Control and Prevention recommends surveillance culture for bacterial contamination from both the elevator and the working channel. Nevertheless, one should keep in mind that stricter recommendations related to meticulous surveillance sampling and microbiological culturing have both practical and financial impacts 17 . However, contamination rates have been shown to drop following the implementation of microbiological surveillance 15 . Microbiologic testing of endoscopes is costly and requires 72 hours for culture; it may be difficult for some endoscopy facilities to achieve this if their budgets are limited 17 44 . On the other hand, endoscope-related infections caused by contaminated endoscopes are also costly to treat, especially as most endoscope-related infections are caused by multidrug-resistant organisms 45 46 47 . Regardless, we should strive for best patient care while keeping the rate of infection transmission as low as possible.
We believe that the findings of this study are informative and relevant for decision-making in infection control and future clinical guidelines. Nevertheless, when concluding on these results important limitations must be considered. One of the main limitations is the high heterogeneity among included studies. The high heterogeneity could indicate that there is no “real” true effect behind the data included in the analysis because there is no consensus regarding the outcomes from the included studies 48 . On the other hand, despite being widely used, I 2 is not always an adequate measure for heterogeneity because it is exquisitely dependent on precision of the included studies and because I 2 tends to be 100 % if the single studies have substantial sample sizes 29 48 . Another limitation is the inconsistency regarding how each study tested the level of contamination and the choice of CFU threshold. Some studies did not state which CFU threshold they applied to determine whether the endoscopes were considered contaminated. A third limitation is the indication of publication bias that may be resulted from the lack of published negative results. Finally, limitations exist with respect to the channels and areas sampled beyond the elevator mechanism and samples pooled from different institutions. Data were derived from studies not directly investigating the contamination rate of a specific area in the endoscope and potentially with varying methodology between institutes, which would increase the risk of confounding factors affecting the findings.
Conclusions
Despite the abovementioned limitations, we believe that the findings of this study are highly important and may help overcome issues related to contaminated endoscopes, not only related to the elevator mechanism and duodenoscopes.
Our findings support the notion that contamination issues due to inadequate reprocessing are not only limited to duodenoscopes and the elevator mechanism. We found a 19.98 % contamination rate unrelated to the elevator in several gastrointestinal endoscopes. Meta-analyses found variations in contamination rates among countries, with the highest pooled contamination rate among studies conducted in Asia and Brazil (42.10 %) and in Europe (18.16 %). The lowest pooled contamination rate was found among studies conducted in North America (6.01 %).
Footnotes
Competing interests Hemant Goyal serves as a consultant for Aimloxy LLC.Sara Larsen, Lotte Klinten Ockert, and Dr. Sven Adamsen are employed by Ambu A/S. Dr. Tharian is a consultant and speaker for Boston Scientific and Medtronic. Dr. Thosani is a consultant for Boston Scientific, a consultant for and receives research support from Pentax America, a speaker for Abbvie, an advisory board member at Colubris Rx, and receives royalties from UpToDate.
Supplementary material :
References
- 1.Beg S, Ragunath K, Wyman A et al. Quality standards in upper gastrointestinal endoscopy: a position statement of the British Society of Gastroenterology (BSG) and Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland (AUgastrointestinalS) Gut. 2017;66:1886–1899. doi: 10.1136/gutjnl-2017-314109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubin Z A, Kim S, Thaker A M et al. Safely reprocessing duodenoscopes: current evidence and future directions. Lancet Gastroenterol Hepatol. 2018;3:499–508. doi: 10.1016/S2468-1253(18)30122-5. [DOI] [PubMed] [Google Scholar]
- 3.Humphries R M, Yang S, Kim S et al. Duodenoscope-related outbreak of a carbapenem-resistant klebsiella pneumoniae identified using advanced molecular diagnostics. Clin Infect Dis. 2017;65:1159–1166. doi: 10.1093/cid/cix527. [DOI] [PubMed] [Google Scholar]
- 4.Ross A S, Tombs D, Verma P et al. Culture and quarantine following high level disinfection of duodenoscopes: Results of ongoing surveillance. Gastrointest Endosc. 2016;83:AB531. [Google Scholar]
- 5.Nerandzic M, Antloga K, Litto C et al. Efficacy of flexible endoscope drying using novel endoscope test articles that allow direct visualization of the internal channel systems. Am J Infect Control. 2021;49:614–621. doi: 10.1016/j.ajic.2020.08.034. [DOI] [PubMed] [Google Scholar]
- 6.Thaker A M, Kim S, Sedarat A et al. Inspection of endoscope instrument channels after reprocessing using a prototype borescope. Gastrointest Endosc. 2018;88:612–619. doi: 10.1016/j.gie.2018.04.2366. [DOI] [PubMed] [Google Scholar]
- 7.Petersen B T. Current state and future of infection prevention in endoscopy. Gastrointest Endosc Clin N Am. 2021;31:625–640. doi: 10.1016/j.giec.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Hansen D, Benner D, Hilgenhöner M et al. ATP measurement as method to monitor the quality of reprocessing flexible endoscopes. Ger Med Sci. 2004;2:Doc04. [PMC free article] [PubMed] [Google Scholar]
- 9.Olafsdottir L B, Wright S B, Smithey A et al. Adenosine triphosphate quantification correlates poorly with microbial contamination of duodenoscopes. Infect Control Hosp Epidemiol. 2017;38:678–684. doi: 10.1017/ice.2017.58. [DOI] [PubMed] [Google Scholar]
- 10.Ofstead C L, Wetzler H P, Heymann O L et al. Longitudinal assessment of reprocessing effectiveness for colonoscopes and gastroscopes: Results of visual inspections, biochemical markers, and microbial cultures. Am J Infect Control. 2017;45:e26–e33. doi: 10.1016/j.ajic.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Liu T-C, Peng C-L, Wang H-P et al. SpyGlass application for duodenoscope working channel inspection: Impact on the microbiological surveillance. World J Gastroenterol. 2020;26:3767–3779. doi: 10.3748/wjg.v26.i26.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MAUDE Adverse Event Report. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/detail.cfm?mdrfoi__id=8811666&pc=FDS https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/detail.cfm?mdrfoi__id=8811666&pc=FDS
- 13.Mark J, Underberg K, Kramer R. Results of duodenoscope culture and quarantine after manufacturer-recommended cleaning process. Gastrointest Endosc. 2020;91:1328–1333. doi: 10.1016/j.gie.2019.12.050. [DOI] [PubMed] [Google Scholar]
- 14.Snyder G, Wright S, Mizrahi M et al. Sa1023 DISINFECTS Study: Prospective randomized trial comparing three duodenoscope high-level disinfection and sterilization procedures. Gastrointest Endosc. 2016;83:AB207–AB208. [Google Scholar]
- 15.Cristina M L, Sartini M, Schinca E. Is Post-reprocessing microbiological surveillance of duodenoscopes effective in reducing the potential risk in transmitting pathogens? Int J Environ Res Public Health. 2019;17:140. doi: 10.3390/ijerph17010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration . https://www.fda.gov/medical-devices/safety-communications/fda-recommending-transition-duodenoscopes-innovative-designs-enhance-safety-fda-safety-communication https://www.fda.gov/medical-devices/safety-communications/fda-recommending-transition-duodenoscopes-innovative-designs-enhance-safety-fda-safety-communication
- 17.Ridtitid W, Pakvisal P, Chatsuwan T et al. A newly designed duodenoscope with detachable distal cap significantly reduces organic residue contamination after reprocessing. Endoscopy. 2020;52:754–760. doi: 10.1055/a-1145-3562. [DOI] [PubMed] [Google Scholar]
- 18.Mouritsen J M, Ehlers L, Kovaleva J et al. A systematic review and cost effectiveness analysis of reusable vs. single-use flexible bronchoscopes. Anaesthesia. 2020;75:529–540. doi: 10.1111/anae.14891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovaleva J. Infectious complications in gastrointestinal endoscopy and their prevention. Best Pract Res Clin Gastroenterol. 2016;30:689–704. doi: 10.1016/j.bpg.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Kovaleva J, Peters F TM, van der Mei H C et al. Transmission of infection by flexible gastrointestinal endoscopy and bronchoscopy. Clin Microbiol Rev. 2013;26:231–254. doi: 10.1128/CMR.00085-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Troiano G, Lo Nostro A, Calonico C et al. Microbiological surveillance of flexible bronchoscopes after a high-level disinfection with peracetic acid: preliminary results from an Italian teaching hospital. Ann Ig. 2019;31:13–20. doi: 10.7416/ai.2019.2254. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med. 2009;151:264. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 23.Guideline for use of high-level disinfectants and sterilants for reprocessing flexible gastrointestinal endoscopes. Gastroenterol Nurs. 2015;38:70–80. doi: 10.1097/SGA.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 24.Calderwood A H, Chapman F J, Cohen J et al. Guidelines for safety in the gastrointestinal endoscopy unit. Gastrointest Endosc. 2014;79:363–372. doi: 10.1016/j.gie.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beilenhoff U, Bieing H, Blum R et al. Reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA) – Up. Endoscopy. 2018;50:1205–1234. doi: 10.1055/a-0759-1629. [DOI] [PubMed] [Google Scholar]
- 26.Petersen B T, Chennat J, Cohen J et al. Multisociety Guideline on Reprocessing Flexible gastrointestinal Endoscopes: 2011. Infect Control Hosp Epidemiol. 2011;32:527–537. doi: 10.1086/660676. [DOI] [PubMed] [Google Scholar]
- 27.Beilenhoff U, Neumann C S, Rey J F et al. ESGE-ESGENA guideline for quality assurance in reprocessing: Microbiological surveillance testing in endoscopy. Endoscopy. 2007;39:175–181. doi: 10.1055/s-2006-945181. [DOI] [PubMed] [Google Scholar]
- 28.Lin L. Bias caused by sampling error in meta-analysis with small sample sizes. PLoS One. 2018;13:1–19. doi: 10.1371/journal.pone.0204056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins J PT, Thompson S G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 30.Higgins J PT, Thompson S G, Deeks J J et al. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becq A, Snyder G M, Heroux R et al. Prospective assessment of the effectiveness of standard high-level disinfection for echoendoscopes. Gastrointest Endosc. 2019;89:984–989. doi: 10.1016/j.gie.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 32.Larsen S, Russell R V, Ockert L K et al. Rate and impact of duodenoscope contamination: A systematic review and meta-analysis. E Clin Med. 2020;25:100451. doi: 10.1016/j.eclinm.2020.100451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rauwers A W, Voor in 't Holt A F, Buijs J G et al. Nationwide risk analysis of duodenoscope and linear echoendoscope contamination. Gastrointest Endosc. 2020;92:681–6910. doi: 10.1016/j.gie.2020.05.030. [DOI] [PubMed] [Google Scholar]
- 34.Wang P, Xu T, Ngamruengphong S et al. Rates of infection after colonoscopy and osophagogastroduodenoscopy in ambulatory surgery centres in the USA. Gut. 2018;67:1626–1636. doi: 10.1136/gutjnl-2017-315308. [DOI] [PubMed] [Google Scholar]
- 35.Lin J N, Wang C B, Yang C H et al. Risk of infection following colonoscopy and sigmoidoscopy in symptomatic patients. Endoscopy. 2017;49:754–764. doi: 10.1055/s-0043-107777. [DOI] [PubMed] [Google Scholar]
- 36.Sundermann A J, Chen J, Miller J K et al. Outbreak of Pseudomonas aeruginosa infections from a contaminated gastroscope detected by whole genome sequencing surveillance. Clin Infect Dis. 2021;73:e638–e642. doi: 10.1093/cid/ciaa1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naas T, Cuzon G, Babics A et al. Endoscopy-associated transmission of carbapenem-resistant Klebsiella pneumoniae producing KPC-2-lactamase. J Antimicrob Chemother. 2010;65:1305–1306. doi: 10.1093/jac/dkq117. [DOI] [PubMed] [Google Scholar]
- 38.Bajolet O, Ciocan D, Vallet C et al. Gastroscopy-associated transmission of extended-spectrum beta-lactamase-producing Pseudomonas aeruginosa. J Hosp Infect. 2013;83:341–343. doi: 10.1016/j.jhin.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 39.Jousset A B, Bonnin R A, Rosinski-Chupin I et al. A 4.5-Year within-patient evolution of a colistin-resistant Klebsiella pneumoniae carbapenemase-producing K. pneumoniae sequence type 258. Clin Infect Dis. 2018;67:1388–1394. doi: 10.1093/cid/ciy293. [DOI] [PubMed] [Google Scholar]
- 40.Sun S, Wang C, Wang S. Remember, interventional EUS is performed using an elevator-containing scope as well. Endosc Ultrasound. 2018;7:73–75. doi: 10.4103/eus.eus_14_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chapman C G, Siddiqui U D, Konda V J et al. Risk of infection transmission in curvilinear array echoendoscopes: Results of a prospective reprocessing and culture registry. Gastrointest Endosc. 2016;83:AB128. doi: 10.1016/j.gie.2016.07.049. [DOI] [PubMed] [Google Scholar]
- 42.Olympus Corporation . Urgent field safety notice re: updated instructions for use for several Olympus ultrasound endoscopes Attention: Operating Room Manager, Risk Management Department and Reprocessing Units. 2020. https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwi96_-glt32AhXNTd8KHdjJBHoQFnoECAYQAQ&url=https%3A%2F%2Fncmdr.sfda.gov.sa%2FFileDownLoad.ashx%3Ff%3Dca%26fid%3D9007&usg=AOvVaw2ZbAfTt_yrHSwTJ9KqvbpJ https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwi96_-glt32AhXNTd8KHdjJBHoQFnoECAYQAQ&url=https%3A%2F%2Fncmdr.sfda.gov.sa%2FFileDownLoad.ashx%3Ff%3Dca%26fid%3D9007&usg=AOvVaw2ZbAfTt_yrHSwTJ9KqvbpJ
- 43.Thaker A M, Kim S, Sedarat A et al. Inspection of endoscope instrument channels after reprocessing using a prototype borescope. Gastrointest Endosc. 2018;88:612–619. doi: 10.1016/j.gie.2018.04.2366. [DOI] [PubMed] [Google Scholar]
- 44.Gavaldà L, Olmo A R, Hernández R et al. Microbiological monitoring of flexible bronchoscopes after high-level disinfection and flushing channels with alcohol: Results and costs. Respir Med. 2015;109:1079–1085. doi: 10.1016/j.rmed.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 45.Larsen S, Kalloo A, Hutfless S. The hidden cost of colonoscopy including cost of reprocessing and infection rate: The implications for disposable colonoscopes. Gut. 2019;1:1–4. doi: 10.1136/gutjnl-2019-319108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Travis H S, Ehlers L H, Thornton J. The total cost of reusable duodenoscopes-are single-use duodenoscopes the future of ERCP? Pharmacoeconom Open. 2020;5:3–5. [Google Scholar]
- 47.Bang J Y, Sutton B, Hawes R et al. Concept of disposable duodenoscope: at what cost? Gut. 2019 doi: 10.1136/gutjnl-2019-318227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borenstein M, Hedges L V, Higgins J PT. Wiley Online; 2009. Introduction to Meta-Analysis.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


