Abstract
Autism spectrum disorder (ASD) is acknowledged as a highly heterogeneous, behaviorally defined neurodevelopmental disorder with multiple etiologies. In addition to its high heritability, we have come to recognize a role for maternal immune system dysregulation as a prominent risk factor for the development of ASD in the child. Examples of these risk factors include altered cytokine/chemokine activity and the presence of autoantibodies in mothers that are reactive to proteins in the developing brain. In addition to large clinical studies, the development of pre-clinical models enables the ability to evaluate the cellular and molecular underpinnings of immune-related pathology. For example, the novel animal models of maternal autoantibody-related (MAR) ASD described herein will serve as a preclinical platform for the future testing of targeted therapeutics for one ‘type’ of ASD. Identification of the cellular targets will advance precision medicine efforts toward tailored therapeutics and prevention. This minireview highlights emerging evidence for the role of maternal immune dysregulation as a potential biomarker, as well as a pathologically relevant mechanism for the development of ASD in offspring. Further, we will discuss the current limitations of these models as well as potential avenues for future research.
Keywords: autoantibodies - blood, cytokines, chemokines, neurodevelopment, inflammation, gestational inflammation
Introduction
Autism spectrum disorder (ASD) is acknowledged as a highly heterogeneous, behaviorally defined neurodevelopmental disorder with multiple etiologies. In addition to its high heritability, we have come to recognize a role for maternal immune system dysregulation as a prominent risk factor for the development of ASD in the child. Examples of these risk factors include altered cytokine/chemokine activity and the presence of autoantibodies in mothers that are reactive to proteins in the developing brain. In addition to large clinical studies, the development of pre-clinical models enables the ability to evaluate the cellular and molecular underpinnings of immune-related pathology. For example, the novel animal models of maternal autoantibody-related (MAR) ASD described herein will serve as a preclinical platform for the future testing of targeted therapeutics for one ‘type’ of ASD. Identification of the cellular targets will advance precision medicine efforts toward tailored therapeutics and prevention. This minireview highlights emerging evidence for the role of maternal immune dysregulation as a potential biomarker, as well as a pathologically relevant mechanism for the development of ASD in offspring. Further, we will discuss the current limitations of these models as well as potential avenues for future research.
The Role of the Maternal Immune System in the Development of ASD
In the past two decades, an increasingly large number of studies have provided evidence that maternal immune dysregulation can impact offspring neurodevelopment (1–11). For example, it is now well-understood that the maternal immune system plays a critical role in the healthy development of the fetus. In fact, maternal immunoglobulin G (IgG) can cross the placenta and enter the fetal compartment, thereby providing early immunological protection to the infant during the perinatal stage (12, 13). However, this protective mechanism is not selective and harmful antibodies reactive to “self” proteins can also cross the placenta (14). Once within the fetal compartment, these maternal autoantibodies may bind to their protein targets, potentially impacting protein function. This is especially dangerous during the perinatal period given the permissiveness of the blood-brain barrier (BBB) and vulnerability of the developing central nervous system (CNS) (15). In addition to the production of autoantibodies, other perturbations of the maternal immune system can result in harmful effects on the developing fetus. It has been shown that alterations in the maternal cytokine/chemokine profile can result in changes in the inflammatory state, skewed cellular signaling, and altered neurodevelopment (16, 17).
Cytokines and Chemokines as Potential Biomarkers
In recent years, several researchers have reported on specific cytokines and/or chemokines that are associated with ASD risk both in children with ASD and mothers that have children diagnosed with ASD. Interestingly, several cytokines and chemokines are constitutively expressed throughout the developing CNS (18, 19). Cytokines in the brain perform functions similar to those seen in the periphery, acting as cues in cell development and differentiation, and regulating the types and number of neuronal and non-neuronal cells (20, 21). Together, these complex processes contribute to early neurodevelopment and homeostasis. In the context of maternal-fetal interaction, some cytokines, in particular IL-6, can be transported from the mother to the offspring and access the developing CNS (17). Although the source of abnormal cytokine levels in mothers during gestation may differ, this could alter placental production and overall levels of cytokines and chemokines in the fetal compartment, impacting the cascade of neurodevelopment.
To date, a variety of clinical studies have demonstrated altered cytokine levels in mothers and neonatal blood samples that are strongly associated with ASD (Table 1), increasing the potential for cytokines/chemokines to serve as biomarkers for risk of abnormal neurodevelopment. Most of the reported cytokines associated with ASD induce the BBB to become more permissive (22) increasing the vulnerability and altering the homeostatic levels of cytokines in the developing CNS. Numerous researchers have corroborated the concept that significant changes in maternal cytokine/chemokine homeostasis are correlated with ASD development even in the absence of maternal/fetal infection. Such changes in maternal cytokines and chemokines can not only directly impact the fetus but can also stimulate placental production of pro-inflammatory cytokines within the fetal compartment (17, 23, 24). For example, dysregulation of serum and/or amniotic fluid IL-4, IL-6, and IL-8 have been described in mothers of children with ASD and were associated with altered behavioral outcomes including non-verbal cognitive ability and stereotypical behaviors in children (25–28). The cytokine IL-17, which has been implicated in several neurodevelopmental disorders, including ASD, has been shown to promote inflammation and disrupt the BBB postnatally (29, 30). In addition, IFN-γ, which is involved in neuronal differentiation and synaptic formation, was shown to be higher in mothers of children with ASD during the second trimester (25, 28), potentially suggesting disrupted synaptic connectivity.
Table 1.
Maternal cytokines/chemokines and maternal autoantibody targets implicated in ASD.
| Cytokines/chemokines | Sample source | Reference |
|---|---|---|
| IL-1, IL-4, IL-5, IL-8, IL-7A, IFN-γ | Maternal serum collected mid-gestation | (25, 26, 28) |
| IL-4, IL-10 MCP-1 TNF-α, TNF-β |
Amniotic fluid | (27, 32) |
| Target antigen | Protein function | |
| CRMP1, CRMP2, STIP1, YBOX | Microtubule dynamics, protein chaperone, transcription factor | (12–14, 42–44) |
| LDH-A, LDH-B, GDA, NSE | Metabolic enzymes | (14–16, 37–41) |
| CASPR2 | Cell adhesion | (45, 52) |
Studies have also observed altered cytokine and chemokine levels in neonates who are later diagnosed with autism. For example, researchers found that newborns later diagnosed with ASD have higher peripheral levels of IL-1β, which is expressed throughout the CNS particularly during the early neurodevelopmental period (16, 21). In our recent findings, CCL27, also known as CTACK, was significantly lower in newborns later diagnosed with ASD. This decrease was associated with an increase in the risk of both ASD with and without intellectual disability when compared to newborns with developmental delay or those with typical development (31). Elevated levels of monocyte chemoattractant protein-1 (MCP-1) and macrophage-derived chemokine (MDC) were found in the amniotic fluid, plasma, cerebellum, and cerebrospinal fluid of children with ASD (10, 32, 33), implying involvement of these chemokines in neurodevelopment. Increased levels of the chemokines macrophage inflammatory protein-1 (MIP-1) and eotaxin are also often noted in children with ASD, and have been associated with behavioral deficits (10, 34–36).
It is unlikely that one specific cytokine or chemokine plays a role in the development of ASD, but rather a combination of cytokines/chemokines are more likely to contribute to the neuropathology associated with autism. The exact consequences of altered cytokine/chemokine levels during pregnancy that lead to the ASD phenotype is an important area of research. Furthermore, the trigger(s) for changes in maternal cytokine and chemokine levels, and how other aspects of the maternal immune system may also be affected are essential questions to address. Although the number of research studies that include time-specific/longitudinal examination as well as mother-infant pair-matched studies are lacking, it is promising that similar groups of cytokines and chemokines are frequently shown to be altered in both children with ASD and their mothers, increasing the promise of cytokines and chemokines as biomarkers for risk of altered neurodevelopment.
Maternal Autoantibody Targets in Fetal Brain
Several groups have now identified multiple brain antigens that cross-react with maternal IgG and are related to neurodevelopmental impairments in the child, including ASD (37–45). Early studies used fetal brain extracts to probe maternal plasma by western blot (WB) and reported reactivity to bands at ~36 kDa that were present in 10% of the ASD samples vs. only 2% of controls. In addition, they observed increased maternal IgG cross-reactivity to multiple bands at 27, 36, at 73 kD in the ASD-positive mothers (46). Concurrent studies reported similar band patterns of reactivity at 37/73 kDa and 39/73 kDa which were ASD specific and not present in controls (47–50). Several groups, including our own, conducted a series of experiments that lead to the identification of eight maternal autoantibody-related (MAR) ASD antigens and their pathogenic epitopes (51–54). The proteins were identified as collapsin response mediator proteins 1 and 2 (CRMP1, CRMP2), guanine deaminase or cypin (GDA), lactate dehydrogenase A and B (LDHA, LDHB), neuron-specific enolase (NSE), stress-induced phosphoprotein-1 (STIP1), and Y-box binding protein 1 (YBX1) (functions are summarized in Table 1). In this early study, instances of maternal seroreactivity to at least one of the proteins in both the case and control groups was observed, indicating that reactivity to any single antigen is not enough to predict ASD risk. Instead, reactivity to a combination of two or more specific antigens (MAR ASD patterns) increased the risk of a child developing ASD with high accuracy: up to 20% of ASD cases and <1% of the controls; the most relevant MAR ASD pattern found was LDHA+LDHB+CRMP1+STIP1 (23% ASD vs. 1% TD) (51). In a more recent study using machine learning techniques, Ramiz-Celis et al. demonstrated that autoantibody combinations composed of CRMP1+CRMP2, CRMP1+GDA, and NSE+STIP1 predicted up to 20% of ASD cases with 100% accuracy, suggesting a significant potential for these patterns as biomarkers for ASD risk (52).
Other groups have also looked at maternal IgG reactivity to individual brain antigens as potential ASD predictors. Due to their pivotal roles in neurodevelopment, the two most well-studied candidates are contactin associated protein 2 (CASPR2) (43, 44, 55) and N- methyl-D-aspartate receptor (NMDA receptor) (56). Further, several groups have reported that maternal seroreactivity to these proteins is associated with neurological alterations in the offspring, including ASD (37, 38, 40, 57, 58). For example, Brimberg et al. reported that anti-CASPR2 antibodies were elevated in mothers of a child with autism when compared to the controls (37% ASD vs. 8% TD) (44, 59). However, results from a recent Danish study that examined maternal IgG cross-reactivity against several brain proteins (including CASPR2 and NMDA) and child outcomes concluded that seroreactivity to both proteins was associated with intellectual disability but not with ASD specifically (43, 60). However, experiments using animal models suggests that maternal autoantibodies against CASPR2 and NMDA can disrupt proper neuronal function/development and result in ASD-like manifestations in the offspring (43–45, 56). Given data from the various studies, the pathologic significance of gestational exposure to maternal autoantibodies is clearly complex and future analyses will be needed to determine how their presence leads to specific changes in neurodevelopment.
Maternal Autoantibodies as Potential ASD-Risk Biomarkers
In one study of 450 mothers of children with ASD and 342 mothers of TD children, the presence of select autoantibodies in maternal blood was associated with ~20% of ASD cases compared to <1% of the typically developing (TD) controls, suggesting that these autoantibodies appear to be highly specific in their ability to detect risk of a child getting an ASD diagnosis (51, 52). Therefore, at this point in time, the association of autism with maternal autoantibodies to proteins in developing brain is higher than any single gene mutation described thus far (61, 62). This MAR subtype of ASD is now a focus of intense clinical and pre-clinical research. It is believed that the ASD phenotype observed in children of mothers with these autoantibodies is the result of gestational exposure to these pathologically significant autoantibodies (47, 63–68). While rigorous clinical validation is still necessary, maternal autoantibodies have the potential to be used as a precision medicine tool to evaluate the risk of a child being diagnosed with autism. The etiological relevance of MAR ASD was supported by multiple clinical studies in diverse populations and by experimental rodent and non-human primate animal models [reviewed in (37, 40, 58)], that in the future could lead to the development of MAR ASD prophylactic treatments for women at risk.
Although MAR ASD profiles are promising ASD-risk biomarkers for a subtype of autism, substantial analytical and clinical validation is still needed before they can be introduced into clinical practice. Meanwhile, the use of in vitro and in vivo animal models will allow us to better understand the pathogenic mechanisms of maternal autoantibodies in neurodevelopment. In addition, they will have the potential to facilitate development of prophylactic treatments to mitigate the neurodevelopmental changes associated with MAR ASD.
Animal Models of MAR ASD
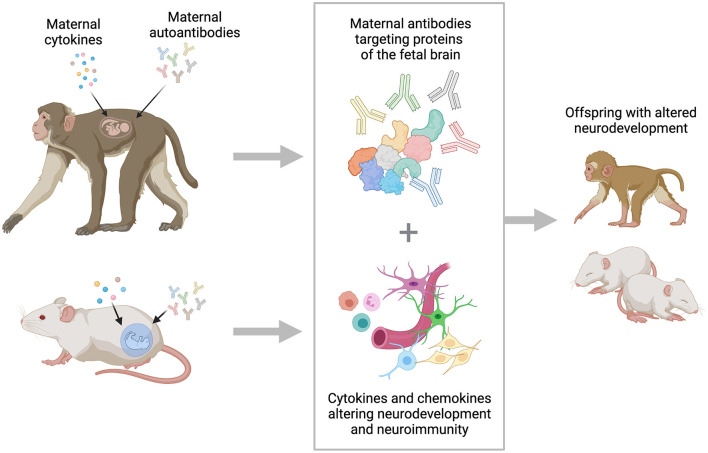
While strong correlations between the presence of maternal autoantibodies and ASD diagnosis have been observed, the pathological role of maternal autoantibodies in the development of ASD is the focus of ongoing research. Multiple preclinical models, including mice, rats, and non-human primates have been utilized to understand the underlying pathology associated with the presence of these maternal autoantibodies (Figure 1). Early animal models of MAR ASD relied on a passive transfer method, utilizing purified antibodies isolated from mothers of children with autism or from mothers of typically developing children that were transferred to an otherwise healthy animal during gestation. First conducted in mice, and subsequently in non-human primates, the passive transfer models yielded two main findings: (1) They confirmed that maternal antibodies do cross the placenta and can be detected in fetal tissues, including the brain, and (2) they confirmed that exposure to maternal autoantibodies results in changes in offspring behavior and brain development. More specifically, the offspring from mouse dams that received IgG from mothers of children with ASD spent more time in self-grooming and marble-burying behavior, reflective of the stereotypic behaviors observed in ASD. They also showed increased levels of anxiety, a comorbidity many children with ASD experience (69, 70). Furthermore, IgG deposition was observed in the brains of offspring from dams treated with IgG from mothers of children with ASD, but not in controls (69, 70). Similar passive transfer models studying antibodies to CASPR2 and NMDA have also yielded important findings. In one study, mouse offspring exposed to anti-CASPR2 antibodies at gestational day 13.5 had differences in sociability, as determined by the amount of time spent with an unfamiliar object vs. an unfamiliar stimulus mouse. In addition, fetuses examined 2 days after exposure to anti-CASPR2 antibodies showed reduced cortical thickness and changes in cell proliferation (44). Exposure to the NR1 subunit of NMDA receptor at gestational days 13 and 17 resulted in offspring who displayed impaired early postnatal reflexes and decreased prepulse inhibition (56).
Figure 1.
Schematic of maternal cytokines and autoantibodies affecting fetal brain development as studied using animal models. Dysregulation of maternal immune system is a strong risk factor for the development of ASD. Alterations in the production of maternal cytokines and chemokines can impact the neurodevelopmental process, and the presence of maternal autoantibodies reactive to critical proteins can alter how the developmental trajectory of the brain. Pre-clinical models are used to better understand the underlying mechanisms for both proposed pathways to altered neurodevelopment. The figure was generated by Biorender.
In non-human primate passive transfer studies, the results were similar, with offspring from treated dams displaying increased stereotypic behaviors with both novel and familiar social partners (47). When examining the brains of offspring from treated dams, investigators noted that male offspring had a higher rate of brain growth between 3 and 6 months of age, a finding that has been observed in some clinical ASD cases and may be correlated with atypical connectivity and altered brain maturation (71, 72). Together, these studies were essential in validating the hypothesis that maternal autoantibodies have pathologic significance and the potential to alter neurodevelopment (47, 69–71, 73).
The passive transfer model was an essential first step in advancing the understanding of maternal autoantibody pathogenesis. However, this route of administration does not reflect the true exposure a fetus would experience during pregnancy. Thus, to create a more appropriate mimic of the gestational environment where a fetus would be exposed to maternal autoantibodies throughout pregnancy, we generated an antigen-driven model of endogenous exposure. In contrast to the passive transfer model, the antigen-driven model involves immunizing experimental dams with specific protein combinations prior to pregnancy, to induce selective, endogenous autoantibody production (64). Clinical studies had previously identified specific patterns of antigen reactivity as well as the epitopes recognized by the maternal autoantibodies in human samples (51, 53). This knowledge was then used to generate a mouse model with reliable construct validity to clinical MAR ASD. Described briefly, mouse dams were injected with synthetic peptide epitopes for lactate dehydrogenase A and B (LDH-A and B), collapsin response mediator protein 1 (CRMP1), and stress induced phosphoprotein 1 (STIP1), a protein reactivity pattern identified in clinical samples specific to mothers of children with ASD (53). Once immune tolerance to the self-proteins was bypassed, these dams continuously produced autoantibodies to the protein epitopes of interest in the absence of inflammation and other immune perturbations that are also thought to impact offspring development. This approach resulted in offspring that were exposed to the autoantibodies throughout the gestational period, more closely resembling the exposure noted in clinical MAR ASD (64). Using the antigen-driven mouse model, researchers were able to test the behavioral and neurological impacts of constant gestational exposure to maternal autoantibodies. Offspring from treated dams had reduced social behaviors, including fewer bouts of nose-to-nose sniffing and front approaches. In addition, researchers observed elevated repetitive behaviors, decreased ultrasonic vocalizations, and differences in neuroanatomical development; specifically increased brain volume in the offspring from treated dams (64, 74).
Our current studies seek to build upon this foundation through use of an antigen-driven rat model with an expanded repertoire of maternal antibody combinations recently identified in a large clinical study (52). Recent work identifying the consequences of acute peripheral immune activation of offspring neurodevelopment (75), suggests that the rat model is a promising next step in understanding the pathology of maternal autoantibodies. The antigen-driven rat model will allow for a more thorough identification of the behavioral and social impacts of maternal autoantibody exposure, thanks to their more complex neuroanatomy and social interactions. Additionally, utilizing the specific autoantibody combinations identified in clinical research will allow us to identify the consequences of exposure to select autoantibodies, providing researchers with a better understanding of which autoantibody combinations result in more severe pathology, and which can be used as risk-biomarkers. Since the development of this model yields dams that produce autoantibodies in the absence of inflammation, we can directly test how skewing of one arm of the maternal immune system impacts offspring behavior and neuroanatomy. The rat model will not only be more translationally specific, but it will also provide researchers with a pre-clinical model in which to test future therapeutic interventions. In the future, we hope to be able to generate an antigen-driven non-human primate model to further advance our preclinical model.
Future Directions
Clinical studies of MAR ASD have revealed the critical protein targets for maternal autoantibodies and drawn links between the specific autoantibody combinations and ASD severity. In addition, they have begun to elucidate other immunological factors, such as variances in maternal cytokine/chemokine profile, that can also be used as predictive measures for ASD risk. The use of animal models has helped to confirm the pathologic significance of at least some of the maternal autoantibodies, as well as provided researchers with a means to better understand the downstream molecular consequences and mechanisms involved in these effects. Despite the advances made thus far, many questions remain which, once answered, will aid in the creation of more precise diagnostic tools, preventative treatments, and therapeutics.
There are several questions we are unable to answer using the animal model, such as: What is the triggering event for maternal autoantibody production in humans? What are the longstanding immunological impacts on exposed children? How does previous history of autoimmune disease influence ASD risk through the MAR mechanism? How do autoantibody and cytokine/chemokine levels prior to pregnancy compare to those present during and after pregnancy? To develop targeted therapeutics, it will also be important to further identify when offspring are most vulnerable to autoantibody and/or cytokine/chemokine exposure. To answer these questions, continued clinical studies and use of our pre-clinical models will be necessary. The development of a more sophisticated model using non-human primates would increase the translational ability to the more complex behaviors seen in autism. Although some non-human primate studies have been completed, an antigen-driven model is currently under development, and we remain hopeful that this will soon be another model for researchers to utilize. Future research will continue to elucidate the mechanisms by which maternal autoantibodies impact their target proteins, and the optimal intervention strategies to mitigate damage. In addition, we will aim to identify connections between maternal cytokine/chemokine changes and autoantibody production.
Conclusions
Autism is an incredibly complex disorder with a wide range of behavioral and cognitive phenotypes. Despite multiple known etiologies, a preventative strategy for the disorder remains to be discovered. Furthermore, testing for specific phenotypic subsets of ASD are also lacking. MAR ASD is one subtype of autism in which the maternal immune system plays a critical role. We now know that specific risk factors exist for immune mediated ASD, including maternal autoantibody production, and skewed maternal and fetal chemokine/cytokine profile. While much is left to be discovered, we continue to use clinical studies and refined animal models to tease apart the mechanisms by which MAR ASD develops. Further, studies are underway to understand how neonatal cytokines and chemokines influence symptom severity as well as the relationship between maternal immune dysregulation during gestation and changes in the brain of affected offspring. We hope this knowledge will yield future preventatives and treatments.
Author Contributions
This mini-review contains review of research performed by each of the authors. JV is the senior author and oversaw all aspects of this mini-review. JM is the first author and compiled all of the information from the other authors as well as wrote the animal model section. DK wrote the section on maternal immune activation. MB developed the animal models and edited the entire review. AR-C wrote the section on maternal autoantibodies. All authors contributed to the article and approved the submitted version.
Funding
NICHD funded IDDRC P50 (P50HD103526).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. (2010) 40:1423. 10.1007/s10803-010-1006-y [DOI] [PubMed] [Google Scholar]
- 2.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring[[/i]]. J Neurosci. (2003) 23:297. 10.1523/JNEUROSCI.23-01-00297.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stubbs EG, Crawford ML. Depressed lymphocyte responsiveness in autistic children. J Autism Child Schizophr. (1977) 7:49. 10.1007/BF01531114 [DOI] [PubMed] [Google Scholar]
- 4.Plioplys AV, Greaves A, Kazemi K, Silverman E. Lymphocyte function in autism and Rett syndrome. Neuropsychobiology. (1994) 29:12. 10.1159/000119056 [DOI] [PubMed] [Google Scholar]
- 5.Warren RP, Foster A, Margaretten NC. [[i]]Reduced natural killer cell activity in autism[[/i]]. Journal for the American Academy of Child & Adolescent Psychiatry. (1987) 26:333. 10.1097/00004583-198705000-00008 [DOI] [PubMed] [Google Scholar]
- 6.Sweeten TL, Posey DJ, McDougle CJ. High blood monocyte counts and neopterin levels in children with autistic disorder. Am J Psychiatry. (2003) 160:1691. 10.1176/appi.ajp.160.9.1691 [DOI] [PubMed] [Google Scholar]
- 7.Mead J, Ashwood P. Evidence supporting an altered immune response in ASD. Immunol Lett. (2015) 163:49. 10.1016/j.imlet.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 8.Abdallah MW, Larsen N, Grove J, Bonefeld-Jørgensen EC, Nørgaard-Pedersen B, Hougaard DM, et al. Neonatal chemokine levels and risk of autism spectrum disorders: findings from a Danish historic birth cohort follow-up study. Cytokine. (2013) 61:370. 10.1016/j.cyto.2012.11.015 [DOI] [PubMed] [Google Scholar]
- 9.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. (2011) 25:40. 10.1016/j.bbi.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J Neuroimmunol. (2011) 232:196. 10.1016/j.jneuroim.2010.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zerbo O, Yoshida C, Grether JK, Van de Water J, Ashwood P, Delorenze GN, et al. Neonatal cytokines and chemokines and risk of Autism Spectrum Disorder: the Early Markers for Autism (EMA) study: a case-control study. J Neuroinflammation. (2014) 11:113. 10.1186/1742-2094-11-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garty BZ, Ludomirsky A, Danon YL, Peter JB, Douglas SD. Placental transfer of immunoglobulin G subclasses. Clin Diagn Lab Immunol. (1994) 1:667. 10.1128/cdli.1.6.667-669.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. (2007) 7:715. 10.1038/nri2155 [DOI] [PubMed] [Google Scholar]
- 14.Pakhathirathien P, Janjindamai W, Dissaneevate S, Thatrimontrichai A, Maneenil G. Neonatal outcomes in pregnant women with systemic lupus erythematosus: a 13-year experience in Southern Thailand. J Trop Pediatr. (2021) 67:58. 10.1093/tropej/fmab058 [DOI] [PubMed] [Google Scholar]
- 15.Saunders NR, Liddelow SA, Dziegielewska KM. Barrier mechanisms in the developing brain. Front Pharmacol. (2012) 3:46. 10.3389/fphar.2012.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krakowiak P, Goines PE, Tancredi DJ, Ashwood P, Hansen RL, Hertz-Picciotto I, et al. Neonatal cytokine profiles associated with autism spectrum disorder. Biol Psychiatry. (2017) 81:442. 10.1016/j.biopsych.2015.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. (1997) 42:1. 10.1203/00006450-199707000-00001 [DOI] [PubMed] [Google Scholar]
- 18.Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. (2009) 64:61. 10.1016/j.neuron.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 19.Mousa A, Bakhiet M. Role of cytokine signaling during nervous system development. Int J Mol Sci. (2013) 14:13931. 10.3390/ijms140713931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramesh G, MacLean AG, Philipp MT. Cytokines and chemokines at the crossroads of neuro-inflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm. (2013) 2013:480739. 10.1155/2013/480739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goines PE, Ashwood P. Cytokine dysregulation in autism spectrum disorders (ASD): possible role of the environment. Neurotoxicol Teratol. (2013) 36:67. 10.1016/j.ntt.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang C, Hawkins KE, Dore S, Candelario-Jalil E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am J Physiol Cell Physiol. (2019) 316:C135. 10.1152/ajpcell.00136.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hauguel-de Mouzon S, Guerre-Millo M. The placenta cytokine network and inflammatory signals. Placenta. (2006) 27:794–8. 10.1016/j.placenta.2005.08.009 [DOI] [PubMed] [Google Scholar]
- 24.Siwetz M, Blaschitz A, El-Heliebi A, Hiden U, Desoye G, Huppertz B, et al. TNF-alpha alters the inflammatory secretion profile of human first trimester placenta. Lab Invest. (2016) 96:428. 10.1038/labinvest.2015.159 [DOI] [PubMed] [Google Scholar]
- 25.Goines PE, Croen LA, Braunschweig D, Yoshida CK, Grether J, Hansen R, et al. Increased midgestational IFN-gamma, IL-4 and IL-5 in women bearing a child with autism: a case-control study. Mol Autism. (2011) 2:13. 10.1186/2040-2392-2-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carter M, Casey S, O'Keeffe GW, Gibson L, Murray DM. Mid-gestation cytokine profiles in mothers of children affected by autism spectrum disorder: a case-control study. Sci Rep. (2021) 11:22315. 10.1038/s41598-021-01662-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghassabian A, Albert PS, Hornig M, Yeung E, Cherkerzian S, Goldstein RB, et al. Gestational cytokine concentrations and neurocognitive development at 7 years. Transl Psychiatry. (2018) 8:64. 10.1038/s41398-018-0112-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones KL, Croen LA, Yoshida CK, Heuer L, Hansen R, Zerbo O, et al. Autism with intellectual disability is associated with increased levels of maternal cytokines and chemokines during gestation. Mol Psychiatry. (2017) 22:273. 10.1038/mp.2016.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cipollini V, Anrather J, Orzi F, Iadecola C. Th17 and cognitive impairment: possible mechanisms of action. Front Neuroanat. (2019) 13:95. 10.3389/fnana.2019.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong H, Hoeffer C. Maternal IL-17A in autism. Exp Neurol. (2018) 299(Pt A):228–40. 10.1016/j.expneurol.2017.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hj Kim D, Krakowiak P, Meltzer A, Hertz-Picciotto I, Van de Water J. Neonatal chemokine markers predict subsequent diagnosis of autism spectrum disorder and delayed development. Brain Behav Immun. (2021) 100:121–33. 10.1016/j.bbi.2021.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdallah MW, Larsen N, Grove J, Norgaard-Pedersen B, Thorsen P, Mortensen EL, et al. Amniotic fluid chemokines and autism spectrum disorders: an exploratory study utilizing a Danish historic birth cohort. Brain Behav Immun. (2012) 26:170. 10.1016/j.bbi.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 33.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. (2005) 57:67–81. 10.1002/ana.20315 [DOI] [PubMed] [Google Scholar]
- 34.Masi A, Breen EJ, Alvares GA, Glozier N, Hickie IB, Hunt A, et al. Cytokine levels and associations with symptom severity in male and female children with autism spectrum disorder. Mol Autism. (2017) 8:63. 10.1186/s13229-017-0176-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen Y, Ou J, Liu M, Shi L, Li Y, Xiao L, et al. Altered plasma levels of chemokines in autism and their association with social behaviors. Psychiatry Res. (2016) 244:300. 10.1016/j.psychres.2016.07.057 [DOI] [PubMed] [Google Scholar]
- 36.Heuer LS, Croen LA, Jones KL, Yoshida CK, Hansen RL, Yolken R, et al. An exploratory examination of neonatal cytokines and chemokines as predictors of autism risk: the early markers for autism study. Biol Psychiatry. (2019) 86:255. 10.1016/j.biopsych.2019.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gata-Garcia A, Diamond B. Maternal antibody and ASD: clinical data and animal models. Front Immunol. (2019) 10:1129. 10.3389/fimmu.2019.01129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones KL, Van de Water J. Maternal autoantibody related autism: mechanisms and pathways. Mol Psychiatry. (2018) 24:252–65. 10.1038/s41380-018-0099-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matelski L, Van de Water J. Risk factors in autism: thinking outside the brain. J Autoimmun. (2016) 67:1. 10.1016/j.jaut.2015.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meltzer A, Van de Water J. The role of the immune system in autism spectrum disorder. Neuropsychopharmacol: off Pub Am College Neuropsychopharmacol. (2017) 42:284. 10.1038/npp.2016.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braunschweig D, Van de Water J. Maternal autoantibodies in autism. Arch Neurol. (2012) 69:693. 10.1001/archneurol.2011.2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fox-Edmiston E, Van de Water J. Maternal anti-fetal brain IgG autoantibodies and autism spectrum disorder: current knowledge and its implications for potential therapeutics. CNS Drugs. (2015) 29:715. 10.1007/s40263-015-0279-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coutinho E, Menassa D, Jacobson L, West S, Domingos J, Moloney T, et al. Maternal CASPR2 antibodies and neurodevelopmental disorders in the offspring: epidemiological findings and an animal model. Lancet. (2017) 389:S18. 10.1016/S0140-6736(17)30414-2 [DOI] [Google Scholar]
- 44.Brimberg L, Mader S, Jeganathan V, Berlin R, Coleman TR, Gregersen PK, et al. Caspr2-reactive antibody cloned from a mother of an ASD child mediates an ASD-like phenotype in mice. Mol Psychiatry. (2016) 21:1663. 10.1038/mp.2016.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JY, Huerta PT, Zhang J, Kowal C, Bertini E, Volpe BT, et al. Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus. Nat Med. (2009) 15:91. 10.1038/nm.1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singer HS, Morris CM, Gause CD, Gillin PK, Crawford S, Zimmerman AW. Antibodies against fetal brain in sera of mothers with autistic children. J Neuroimmunol. (2008) 194:165. 10.1016/j.jneuroim.2007.11.004 [DOI] [PubMed] [Google Scholar]
- 47.Martin LA, Ashwood P, Braunschweig D, Cabanlit M, Van de Water J, Amaral DG. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav Immun. (2008) 22:806. 10.1016/j.bbi.2007.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Croen LA, Goines P, Braunschweig D, Yolken R, Yoshida CK, Grether JK, et al. Brain-derived neurotrophic factor and autism: maternal and infant peripheral blood levels in the early markers for autism (EMA) study. Autism Res. (2008) 1:130. 10.1002/aur.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rossi CC, Fuentes J, Van de Water J, Amaral DG. Brief report: antibodies reacting to brain tissue in basque spanish children with autism spectrum disorder and their mothers. J Autism Dev Disord. (2013). 10.1007/s10803-013-1859-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piras IS, Haapanen L, Napolioni V, Sacco R, Van de Water J, Persico AM. Anti-brain antibodies are associated with more severe cognitive and behavioral profiles in Italian children with autism spectrum disorder. Brain Behav Immun. (2014) 38:91. 10.1016/j.bbi.2013.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braunschweig D, Krakowiak P, Duncanson P, Boyce R, Hansen RL, Ashwood P, et al. Autism-specific maternal autoantibodies recognize critical proteins in developing brain. Transl Psychiatry. (2013) 3:e277. 10.1038/tp.2013.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramirez-Celis A, Becker M, Nuno M, Schauer J, Aghaeepour N, Van de Water J. Risk assessment analysis for maternal autoantibody-related autism (MAR-ASD): a subtype of autism. Mol Psychiatry. (2021) 26:1551. 10.1038/s41380-020-00998-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edmiston E, Jones KL, Vu T, Ashwood P, Van de Water J. Identification of the antigenic epitopes of maternal autoantibodies in autism spectrum disorders. Brain Behav Immun. (2018) 69:399. 10.1016/j.bbi.2017.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramirez-Celis A, Edmiston E, Schauer J, Vu T, Van de Water J. Peptides of neuron specific enolase as potential ASD biomarkers: From discovery to epitope mapping. Brain Behav Immun. (2020) 84:200. 10.1016/j.bbi.2019.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bagnall-Moreau C, Huerta PT, Comoletti D, La-Bella A, Berlin R, Zhao C, et al. In utero exposure to endogenous maternal polyclonal anti-Caspr2 antibody leads to behavioral abnormalities resembling autism spectrum disorder in male mice. Sci Rep. (2020) 10:14446. 10.1038/s41598-020-71201-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jurek B, Chayka M, Kreye J, Lang K, Kraus L, Fidzinski P, et al. Human gestational N-methyl-d-aspartate receptor autoantibodies impair neonatal murine brain function. Ann Neurol. (2019) 86:656. 10.1002/ana.25552 [DOI] [PubMed] [Google Scholar]
- 57.Hughes HK, Mills Ko E, Rose D, Ashwood P. Immune dysfunction and autoimmunity as pathological mechanisms in autism spectrum disorders. Front Cell Neurosci. (2018) 12:405. 10.3389/fncel.2018.00405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marks K, Vincent A, Coutinho E. Maternal-autoantibody-related (MAR) autism: identifying neuronal antigens and approaching prospects for intervention. J Clin Med. (2020) 9:82564. 10.3390/jcm9082564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brimberg L, Sadiq A, Gregersen PK, Diamond B. [[i]]Brain-reactive IgG correlates with autoimmunity in mothers of a child with an autism spectrum disorder[[/i]]. Mol Psychiatry. (2013) 18:1171. 10.1038/mp.2013.101 [DOI] [PubMed] [Google Scholar]
- 60.Coutinho E, Jacobson L, Pedersen MG, Benros ME, Norgaard-Pedersen B, Mortensen PB, et al. CASPR2 autoantibodies are raised during pregnancy in mothers of children with mental retardation and disorders of psychological development but not autism. J Neurol Neurosurg Psychiatry. (2017) 88:718. 10.1136/jnnp-2016-315251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miles JH. Autism spectrum disorders–a genetics review. Genet Med. (2011) 13:278. 10.1097/GIM.0b013e3181ff67ba [DOI] [PubMed] [Google Scholar]
- 62.Beversdorf DQ, Missouri Autism Summit C. Phenotyping, etiological factors, and biomarkers: toward precision medicine in autism spectrum disorders. J Dev Behav Pediatr. (2016) 37:659. 10.1097/DBP.0000000000000351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martinez-Cerdeno V, Camacho J, Fox E, Miller E, Ariza J, Kienzle D, et al. Prenatal exposure to autism-specific maternal autoantibodies alters proliferation of cortical neural precursor cells, enlarges brain, and increases neuronal size in adult animals. Cereb Cortex. (2016) 26:374. 10.1093/cercor/bhu291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jones KL, Pride MC, Edmiston E, Yang M, Silverman JL, Crawley JN, et al. Autism-specific maternal autoantibodies produce behavioral abnormalities in an endogenous antigen-driven mouse model of autism. Mol Psychiatry. (2018). 10.1038/s41380-018-0126-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simister NE. Placental transport of immunoglobulin G. Vaccine. (2003) 21:3365. 10.1016/S0264-410X(03)00334-7 [DOI] [PubMed] [Google Scholar]
- 66.Diamond B, Huerta PT, Mina-Osorio P, Kowal C, Volpe BT. Losing your nerves? Maybe itB, Huerta PT, Min. Nat Rev Immunol. (2009) 9:449. 10.1038/nri2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. (2012) 2012:985646. 10.1155/2012/985646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diamond B, Honig G, Mader S, Brimberg L, Volpe BT. Brain-reactive antibodies and disease. Annu Rev Immunol. (2013) 31:345. 10.1146/annurev-immunol-020711-075041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singer HS, Morris C, Gause C, Pollard M, Zimmerman AW, Pletnikov M. Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: a pregnant dam mouse model. J Neuroimmunol. (2009) 211:39. 10.1016/j.jneuroim.2009.03.011 [DOI] [PubMed] [Google Scholar]
- 70.Braunschweig D, Golub MS, Koenig CM Qi L, Pessah IN, Van de Water J, et al. Maternal autism-associated IgG antibodies delay development and produce anxiety in a mouse gestational transfer model. J Neuroimmunol. (2012) 252:56. 10.1016/j.jneuroim.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bauman MD, Iosif AM, Ashwood P, Braunschweig D, Lee A, Schumann CM, et al. Maternal antibodies from mothers of children with autism alter brain growth and social behavior development in the rhesus monkey. Transl Psychiatry. (2013) 3:e278. 10.1038/tp.2013.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ha S, Sohn IJ, Kim N, Sim HJ, Cheon KA. Characteristics of brains in autism spectrum disorder: structure, function and connectivity across the lifespan. Exp Neurobiol. (2015) 24:273. 10.5607/en.2015.24.4.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Camacho J, Jones KL, Miller E, Ariza J, Noctor S, Van de Water J, et al. Embryonic intraventricular exposure to autism-specific maternal autoantibodies produces alterations in autistic-like stereotypical behaviors in offspring mice. Behav Brain Res. (2014) 266:46. 10.1016/j.bbr.2014.02.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bruce MR, Jones KL, Vernon AC, Silverman JL, Crawley JN, Ellegood J, et al. Sexually dimorphic neuroanatomical differences relate to ASD-relevant behavioral outcomes in a maternal autoantibody mouse model. Mol Psychiatry. (2021). 10.1038/s41380-021-01215-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bruce M, Streifel KM, Boosalis CA, Heuer L, González EA Li S, et al. Acute peripheral immune activation alters cytokine expression and glial activation in the early postnatal rat brain. J Neuroinflammation. (2019) 16:200. 10.1186/s12974-019-1569-2 [DOI] [PMC free article] [PubMed] [Google Scholar]