Abstract

Beside honey, honeybees (Apis mellifera L.) are able to produce many byproducts, including bee pollen, propolis, bee bread, royal jelly, and beeswax. Even if the medicinal properties of these byproducts have been recognized for thousands of years by the ancient civilizations, in the modern era, they have a limited use, essentially as nutritional supplements or health products. However, these natural products are excellent sources of bioactive compounds, macro- and micronutrients, that, in a synergistic way, confer multiple biological activities to these byproducts, such as, for example, antimicrobial, antioxidant, and anti-inflammatory properties. This work aims to update the chemical and phytochemical composition of bee pollen, propolis, bee bread, royal jelly, and beeswax and to summarize the main effects exerted by these byproducts on human health, from the anticancer and immune-modulatory activities to the antidiabetic, hypolipidemic, hypotensive, and anti-allergic properties.
Keywords: honeybee byproducts, bee pollen, propolis, bee bread, royal jelly, beeswax
1. Introduction
Bees are a large group of social insects belonging to the Apidae family, which includes honey or domestic bees, stingless bees, and other specific groups. In particular, honeybees (western Apis mellifera, distributed mainly in Europe, America, Africa, and Asia, and eastern Apis cerana, native to Southeast Asia) are the two best known domesticated species used in modern beekeeping. These honeybees produce and store in the beehives several products that are potentially beneficial for human health. Undoubtedly, the most famous and widely appreciated honeybee product is honey, a complex mixture of nutrients and bioactive compounds with multiple biological activities.1−7 However, besides honey, bees are also able to make several other types of products, including bee pollen (BP), propolis, bee bread (BB), royal jelly (RJ), and beeswax (BW). These products may derive from pollen grains, nectars, and other plant materials alone or mixed with the bee salivary gland secretions and plant secretions.8 In the last years, they have also attracted the interest of the scientific community worldwide: numerous studies have found beneficial effects exerted by these natural products on human health, highlighting their potential use as active pharmaceutical ingredients.8 In addition, some efforts have also been done to introduce these products in clinical practice, but these attempts have almost failed because of their high chemical, nutritional, and phytochemical variabilities that depend upon several parameters, including, for example, the honeybee varieties, the plant, the geographical area, and the seasons, which makes the medicinal standardization difficult to perform. Despite all of this evidence, currently, these products have few applications, mainly in the nutraceutical and food industries as dietary supplements. With this background, this review aims to present the nutritional and phytochemical contents of BP, propolis, BB, RJ, and beeswax and to summarize the biological properties played by these products, given the urgent need to find new remedies against the most common human pathologies, such as metabolic and cardiovascular diseases.
2. Bee Pollen
Bee pollen (BP) is produced as a result of the collection of pollen grains by bees that are agglutinated using salivary secretions, nectar, and/or honey to form a granule of ∼1.4–4 mm in size, which is stored in the alveoli of the hive until consumption. A colony of bees can collect between 50 and 250 g of pollen per day, totaling 15–40 kg per year. The main function of pollen is to serve as a source of nutrients for the colony, thus guaranteeing its development and maintenance. From a compositional point of view, BP is often a valuable source of proteins, essential amino acids, vitamins, and fatty acids as well as other compounds that, although they lack a nutritional function, exhibit an important functional character, such as pigments (carotenoids) and polyphenols, which can act as potent antioxidants.9,10 This composition is influenced by several factors, for instance, the plant species, geographical region, and season of the year in which the pollen is collected. Thus, these constituents vary in content levels.11 Despite this, pollen remains a valuable source of essential nutrients and non-nutritive compounds, to the point that is often regarded as “the world’s best food product”.12
BP is an excellent source of carbohydrates, its main component. In fact, carbohydrates make up two-thirds of its dry weight13,14 and are incorporated from honey or nectar used for pollen pellet formation.15 Therefore, the type of plant, together with climatic conditions and geographical origin, plays a fundamental role in the carbohydrate content.11 For these reasons, data collected on BP samples of different botanical and geographical origins show a huge variation (Table 1). Monosaccharides are the main sugars present, accounting for about 94% of total sugars,9 with a large amount of reducing sugars, making it distinct from plant pollen.13 Fructose and glucose, with the ratio varying between 1.20 and 1.50, are the most abundant sugars, followed by sucrose, maltose, and other disaccharides, such as sucrose, turanose, erlose, maltose, and trehalose.16 A total of 3–4% of BP is cellulose, which is the main component of the layers of pollen grains, and its presence significantly affects the digestibility of BP.9
Table 1. Bee Pollen Composition from Different Geographical and Botanical Originsa 9−14,17,18.
| proximate | content (min–max) | ||
|---|---|---|---|
| moisture (%) | 1.50–13.80 | ||
| dietary fiber (%) | 0.15–30.00 | ||
| proteins (%) | 2.50–62.00 | ||
| ash (%) | 0.50–6.50 | ||
| lipids (%) | 0.41–24.40 | ||
| carbohydrates (%) | 18.50–82.80 | ||
| sugars (g/100 g) | |||
| glucose | 2.77–28.49 | ||
| fructose | 4.90–33.48 | ||
| sucrose | 0.05–9.02 | ||
| maltose | 0.16–6.03 | ||
| isomaltose | 0.10–0.60 | ||
| raffinose | 0.10–0.20 | ||
| trehalose | 0.10–0.40 | ||
| erlose | 0.10–0.30 | ||
| polyphenols | |||
| total phenolic content (mg of GAE/g) | 0.69–213.00 | ||
| total flavonoid content (mg of QE/g) | 1.82–107.00 | ||
| fatty acids (g/100 g) | |||
| C4:0 butyric acid | traces–0.26 | C18:3 α-linolenic acid | 0.1–56.90 |
| C6:0 caproic acid | traces–4.53 | C18:3 γ-linolenic acid | traces–41.99 |
| C8:0 caprylic acid | traces–6.34 | C20:0 arachidic acid | traces–6.88 |
| C10:0 capric acid | traces–14.98 | C20:1 eicosenoic acid | 0.0073–3.35 |
| C11:0 undecanoic acid | traces–1.70 | C21:1 heneicosenoic acid | traces–3.92 |
| C12:0 lauric acid | traces–8.51 | C20:2 eicosadienoic acid | traces–4.27 |
| C14:0 myristic acid | traces–20.70 | C20:3 dihomo-γ-linolenic acid | traces–54.01 |
| C15:0 pentadecanoic acid | traces–29.97 | C20:3 eicosatrienoic acid | traces–18.36 |
| C16:0 palmitic acid | traces–64.38 | C20:4 arachidonic acid | traces–2.40 |
| C16:1 palmitoleic acid | traces–4.39 | C22:0 behenic acid | traces–17.89 |
| C17:0 heptadecanoic acid | 0.07–25.60 | C22:1 erucic acid | 0.0038–1.40 |
| C17:1 heptadecenoic acid | traces–6.61 | C22:6 docosahexaenoic acid | traces–1.29 |
| C18:0 stearic acid | 0.0027–8.52 | C23:0 tricosanoic acid | 0.09–5.39 |
| C18:1 cis-oleic acid | 1.33–20.61 | C20:5 eicosapentaenoic acid | 0.35–3.36 |
| C18:2 trans-linoelaidic acid | 0.22–12.62 | C24:0 lignoceric acid | traces–1.59 |
| C18:2 cis-linoleic acid | 0.0085–53.92 | C24:1 nervonic acid | traces–0.97 |
| total amino acids (g/100 g) | |||
| essential amino acids | non-essential amino acids | ||
| arginine | 0.03–2.58 | alanine | 0.09–2.33 |
| histidine | 0.07–4.49 | aspartic acid | 0.21–3.23 |
| isoleucine | 0.01–1.60 | cysteine | 0.07–0.30 |
| leucine | 0.06–2.47 | glutamic acid | 0.13–3.03 |
| lysine | 0.03–3.71 | glycine | 0.04–1.84 |
| methionine | 0.01–0.62 | proline | 0.04–19.8 |
| phenylalanine | 0.03–2.72 | serine | 0.23–1.33 |
| threonine | 0.02–5.58 | tyrosine | 0.03–3.76 |
| tryptophan | 0.05–14.80 | asparagine | 0.08–0.57 |
| valine | 0.03–1.57 | glutamine | 0.02–1.42 |
| vitamins (mg/100 g) | |||
| fat soluble | water soluble | ||
| α-carotene | 0.33–32.47 | thiamine (vitamin B1) | 0.20–1.30 |
| β-carotene | 0.08–19.89 | riboflavin (vitamin B2) | 0.40–2.56 |
| γ-carotene | 5.38–12.87 | niacin (vitamin B3) | 1.30–15.34 |
| ξ-carotene | 4.49–11.58 | nicotinamide (vitamin B3) | 0.51–12.10 |
| ε-carotene | 5.80–12.39 | pantothenic acid (vitamin B5) | 0.50–2.00 |
| lutein | 4.45–47.63 | pyridoxine (vitamin B6) | 0.10–3.80 |
| β-cryptoxanthin | 0.13–8.54 | biotin (vitamin B7) | 0.05–0.07 |
| isocryptoxanthin | 3.11–8.05 | folic acid | 0.30–1.00 |
| isozeaxanthin | 3.80–26.54 | vitamin C | 6.03–79.70 |
| lactucaxanthin | 3.17–9.80 | ||
| neoxanthin | 4.45–7.22 | ||
| violaxanthin | 4.83–10.60 | ||
| antheraxanthin | 4.01–9.15 | ||
| astaxanthin | 3.69–9.01 | ||
| canthaxanthin | 4.50–9.01 | ||
| tocopherols (vitamin E) | 0.46–9.57 | ||
| minerals (mg/kg) | |||
| macrominerals | microminerals | ||
| potassium (K) | 3.60–13366.60 | zinc (Zn) | 0.10–340.00 |
| calcium (Ca) | 1.09–5752.19 | iron (Fe) | 2.60–1180.00 |
| phosphorus (P) | 234.40–9687.00 | manganese (Mn) | 0.10–430.00 |
| magnesium (Mg) | 44.0–4680.53 | copper (Cu) | 3.73–42.00 |
| sodium (Na) | 4.95–8350.27 | selenium (Se) | 0.01–4.50 |
GAE, gallic acid equivalent; QE, quercetin equivalent.
Proteins are the second most abundant component in BP. They constitute between 10 and 40% (w/w) of its dry weight. In addition to their nutritional contribution, they influence its taste value.14 The protein content may vary according to the type of plant, indicating a wide variation even between similar plant species from different geographical regions,17,18 and according to the collection method. The protein content in pollen collected by bees and hand-collected pollen is often high, probably related to the bees adding nectar to the pollen.19 Protein levels in bee pollen can also be affected by the harvest season. Pollen collected in spring showed the highest contents of crude protein and total amino acids as well as the highest levels of leucine, glutamic acid, valine, isoleucine, threonine, and glycine. On the other hand, the highest contents of phenylalanine, lysine, tryptophan, threonine, tyrosine, arginine, and cysteine were found in samples collected in winter. The highest contents of histidine, methionine, and serine were observed in BP collected in fall, while the highest levels of aspartic acid, proline, and alanine were identified in samples collected in summer.20 Protein levels in dehydrated BP can vary between 2.5 and 62 g/100 g (Table 1),11 mainly influenced by botanical origin,11 where the main protein fractions consist of albumins (35.4%), globulins (18.9%), glutelins (18.6%), prolamins (21.8%), and other proteins (including enzymes at 5.3%).21 BP also provides a total of 20 essential and non-essential amino acids.10 The amino acid profile and content of pollen do not focus only on nutritional value, because the level of certain amino acids could serve as an indicator of freshness, proper drying, and storage process. In addition, some of the acids have been proposed as possible botanical and geographical markers of bee pollen, mainly on the basis of the fact that botanical origin influences the amino acid profile more from a quantitative point of view than a qualitative point of view.11 Glutamic acid, proline, and aspartic acid have been described among the predominant amino acids in BP from various plant species and different geographical regions.10 However, other amino acids also stand out for their content, including leucine, lysine, threonine, histidine, tyrosine, and especially tryptophan (Table 1).10,11 The total essential amino acids in BP constitute between 12 and 45.02% of the total amino acid content,11 making it an important source of these macronutrients and suggesting their potential use as an innovative dietary supplement, especially for vegetarians and athletes.
Alongside carbohydrates and proteins, lipids are an important nutritional component of BP. Although the total lipid content in pollen from various plant species was previously reported in a range of 1–13% of dry weight,22 recent studies have revealed higher contents of up to 24.4% (Table 1).17,23−25 A total of nine lipid classes, including phosphatidylcholine (41 species), phosphatidylethanolamine (43 species), phosphatidylglycerol (9 species), phosphatidylserine (10 species), lysophosphatidylcholine (12 species), ceramide (8 species), diglyceride (27 species), triglyceride (137 species), and fatty acids (47 species), were reported in BP.26 In addition, a wide variety of up to 20 types of fatty acids (FAs) have been reported in BP: from C4 to C20, where ω-3 fatty acids are predominant.10 This content and variety are directly influenced by the predominant and secondary pollen botanical origins.27 Among the saturated acids, palmitic and myristic acids stand out for their content levels, followed by stearic and lauric acids in a lower concentration, while α-linolenic, γ-linolenic, and oleic acids constitute the most prominent unsaturated acids (Table 1). According to data collected in more than 100 BP studies from different geographical regions and botanical origin, saturated fatty acids represent between 4.29 and 71.47% of total lipids, while monounsaturated fatty acids range between 1.29 and 53.24%, and polyunsaturated fatty acids range between 4.33 and 75.7%. Meanwhile, ω-3 fatty acids ranged between 8.07 and 44.1%, and ω-6 fatty acids ranged between 1.77 and 38.25%.9,10
Despite the low number of reports available, BP is considered an important source of fat-soluble vitamins (0.1%), such as vitamin A in terms of β-carotene and tocopherols (vitamin E) (Figure S1 of the Supporting Information), as well as several water-soluble vitamins (0.3%) of vitamin B complex and vitamin C (Table 1).11 Dependent upon the season of the year, environmental conditions, geographical regions, and floral species, the content of certain vitamins in pollen varies.28 Among the fat-soluble vitamins, a wide range of α- and β-carotene contents has been reported in BP.14 Additionally, other carotenoids and their isoforms (γ-, ξ-, and ε-carotene) in trans- and cis-geometric shape (Figure S1 of the Supporting Information) have been reported in BP, standing out equally for their content levels,29 while four tocopherols (α-, β-, γ-, and δ-tocopherol) were also reported.28 With regard to the water-soluble vitamins, the main values reported correspond to vitamin B3 (nicotinamide and niacin) from Brazilian pollen17 as well as the vitamin C content, ranging from trace amounts to important contents, like 79.70 mg/100 g.28
BP also contains an important group of macro- and microelements, which vary according to different floral sources and geographical origins, where it has been suggested that soil type is the main cause of the variation.30 Up to 25 different compounds of this type have been reported in BP, where potassium (K), calcium (Ca), phosphorus (P), magnesium (Mg), sodium (Na), zinc (Zn), iron (Fe), manganese (Mn), copper (Cu), and selenium (Se) represent the most common elements reported worldwide (Table 1).10,11 The recommended daily amount of BP consumption for an adult is between 20 and 40 g.9 Nevertheless, considering the content of these elements, a moderate consumption of 25 g of BP could cover all or part of the recommended daily intake for most of the micro- and macroelements presented in Table 1, which highlights bee pollen as a relevant source of these elements. BP has also been proposed as a potential geographical marker.31 Therefore, the profile and mineral content of bee pollen could not only serve as food supplements but also as markers to identify its botanical origin and quality.
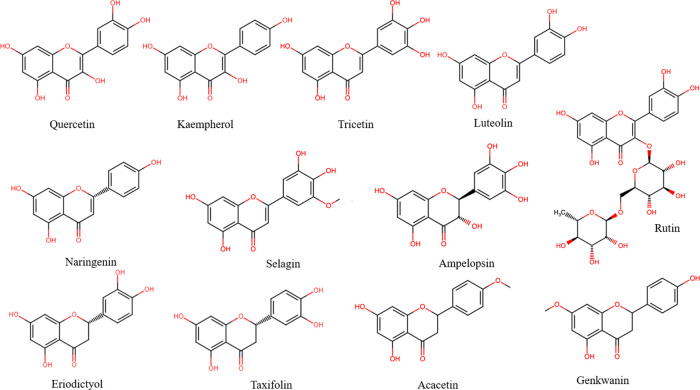
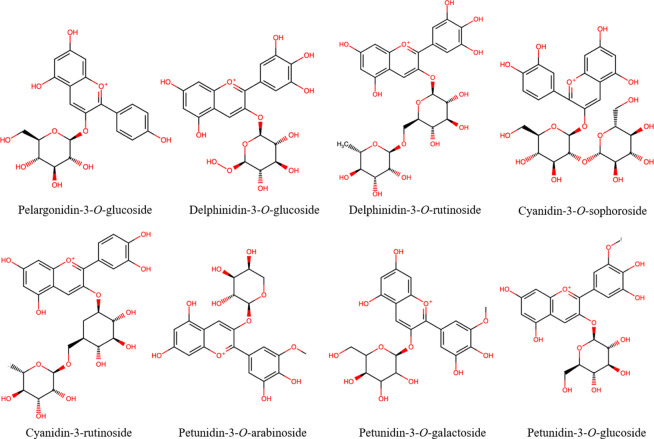
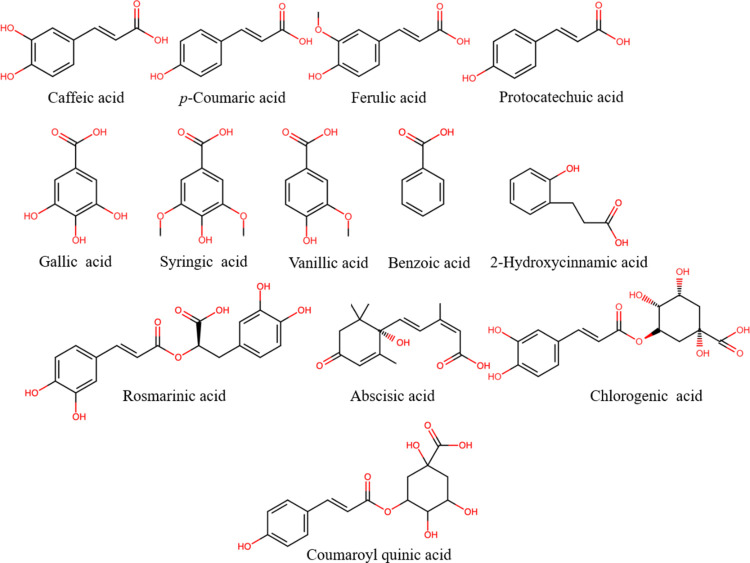
Given its floral origin, BP contains a wide group of polyphenolic compounds (Figures 1–3), mainly flavonoids and their glycosylated forms, along with phenolic acids and their derivates, which come from both the pollen itself and the nectar and honey that bees use to form the pollen granules. Phenolic compounds are responsible for the color of the pollen grain (yellow, brown, red, violet, etc.)32 as well as partly responsible for its most relevant biological activities, e.g., antioxidant activity.33 The phenolic acid and flavonoid contents in bee pollen show great variability (Table S1 of the Supporting Information)9−11,13,14,34 as a result of differing floral and geographical origins.17 Approximately 11 different phenolic acids and their derivatives or glycosylated forms (e.g., hydroxycinnamic acid glycosides) and hydroxycinnamic acid amide derivatives have been identified in BP from different geographical and floral origins,9−11,35,36 where ferulic acid (as a trace amount of 149 μg/g of pollen)37−39 and 2-hydroxycinnamic acid (43–180 μg/g of pollen)39,40 stood out for their content levels. BP also shows a broad flavonoid profile (Table S1 of the Supporting Information). About 19 different non-glycosylated flavonoids have been reported in bee pollen with a predominance of flavonols and flavone, followed by flavanones and, to a lesser extent, flavanonols and flavan-3-ols. Furthermore, a diverse group of glycosylated flavonoids, with a predominance of quercetin glycosides, has been identified, followed by isorhamnetin, myricetin, and kaempferol glycosides and, to a smaller degree, luteolin and apigenin.9,10 According to the concentration reports of these compounds, rutin (trace amount of 956 μg/g of pollen),38−41 and quercetin (trace amount of 530 μg/g of pollen)37,38,40,41 showed the highest concentrations among the non-glycosylated forms, while quercetin-3-O-β-d-glucosyl-(2→l)-β-glucoside (0.65–5108 μg/g of pollen) and kaempferol-3-O-β-d-glucosyl-(2→l)-β-d-glucoside and kaempferol-3,4′-di-O-β-d-glucoside (0.2–4243 μg/g of pollen) had significantly higher contents than the rest of the flavonoids.42,43
Figure 1.
Chemical structures of the most representative flavonoids found in bee pollen.
Figure 3.
Chemical structures of the most representative anthocyanins found in bee pollen.
Figure 2.
Chemical structures of the most representative phenolic acids found in bee pollen.
Along with its valuable nutritional contribution, BP also possesses potential therapeutic properties that are closely determined by its chemical composition. Given the polyphenol content of bee pollen, in vitro and in vivo models have demonstrated its outstanding antioxidant activity.44−46 BP has also been shown to have significant anti-inflammatory effects. In vitro studies have pointed out that this capacity is associated with the ability of BP compounds to suppress the production of pro-inflammatory cytokines, including cyclooxygenase 2, inducible nitric oxide synthase, interleukin (IL) 6, and tumor necrosis factor (TNF) α,47 as well as to downregulate inflammatory-related gene expression and block the activation of mitogen-activated protein kinase (MAPK) and nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) signaling pathways.48 Likewise, in vivo studies in rats demonstrated the anti-inflammatory effects of BP because of its capacity to regulate interferonγ (IFN-γ), noradrenaline, 5-hydroxytryptamine, dopamine, and caspase 3.26
The beneficial effect of BP consumption on the cardiovascular system has been linked to is hypolipidemic activity because it reduces the content of cholesterol, triacylglycerol, and total lipids.49 The regular intake of BP significantly decreases the level of lipids in blood correlated with the content of hormones, for instance, insulin, testosterone, and thyroxine.49 The daily supplementation of 40 g in patients with heart failure caused a reduction in the serum cholesterol level, blood viscosity, and fibrinogen and fibrin concentrations.50 Similarly, in humans supplemented with bee pollen, a decrease in the aggregation capacity of blood platelets and the formation of an atherosclerotic plaque has been confirmed as well as an increase in the activity of the fibrinolytic system, possibly related to the levels of ω-3 fatty acids, such as α-linolenic acid, that act as a prostaglandin 3 precursor and an inhibitor against platelet aggregation.51 Although it has been linked to allergic reactions, BP can be used to improve and even prevent certain allergic conditions, e.g., rhinitis and asthma. This effect is associated with its ability to obstruct the expression of β-hexosaminidase and reduce serum levels of immunoglobulin E (IgE) and immunoglobulin G1 (IgG1) as well as the inhibition of leukocyte migration to bronchoalveolar lavage.52 Moreover, researchers have found that it can also act to reduce degranulation in mast cells by inhibiting tyrosine protein phosphorylation. The lipid-soluble fraction of BP has also been proven to exert anti-allergic activity by hindering the binding of IgE to FcεRI in cutaneous cells.53 Although these results help to elucidate the possible anti-allergic activity of BP, the underlying mechanisms between anti-allergic activity and immune responses should be studied in greater depth.
BP has also been shown to possess relevant antimicrobial activities on several Gram-positive and Gram-negative pathogenic strains (Escherichia coli, Staphylococcus epidermidis, Staphylococcus aureus, Staphylococcus pyogenes, Salmonella enteritidis, Listeria monocytogenes, and Pseudomonas aeruginosa)54 as well as against different strains of fungi and yeasts (Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger, Candida albicans, Candida glabrata, Candida krusei, Candida parapsilosis, Candida tropicalis, Geotrichum candidum, Fusarium culmorum, Penicillium verrucosum, and Rhodotorula mucilaginosa).55,56 Despite this broad spectrum of antimicrobial activity, BP can be a suitable substrate for the growth of mycotoxins, such as aflatoxins, if no adequate drying is done by the beekeeper, so that the strict control of toxins should be considered when considering the quality standards of BP.57
3. Bee Bread
One of the least known and investigated bee products is bee bread (BB). Honeybees cop with their nutritional needs by collecting water, nectar, and pollen. Carbohydrates are mainly provided by nectar but also by pollen. However, the rest of the nutrients, including lipids, proteins, minerals, and vitamins, comes almost exclusively from pollen. BB is produced when bees collect the pollen and mix it with saliva and honey, and this mixture is packed into the cells of the honeycomb. Under the action of temperature (35–36 °C), moisture, activity of different enzymes from glandular secretions, and microorganisms (including bacteria and yeasts), after 2 weeks, BB is formed. During winter and early spring, BB together with honey is the only source of food for the bee colony. Moreover, BB is used to produce royal jelly by young bees and worker bees to fed larvae.9,14
The composition of BB is mediated by its source materials as well as the physical–chemical and microbiological processes that take place during its production.9,14 Thus, BB is produced with considerable variations in its centesimal composition (in both macro- and micronutrients and even non-nutritional bioactive compounds) depending upon the geographical and botanical origin of the pollen as well as the microbiota present in the cell. Other factors affecting BB composition are the climatic conditions, soil type, beekeeper activities, or storage treatments in commercial production.58 Overall, water in BB (Table 2) represents up to 30% of fresh weight. Concerning carbohydrates (Table 2), analyzed studies reported proportions ranging from 13 to 72% of dry weight. Among the observed carbohydrates, different sugars can be found (Table 2), mainly fructose, glucose, sucrose, trehalose, and maltose but also fructofuranose α and β, α glucopyranose, mannitol, β-d-glucopyranose, melezitose, and raffinose. In the process of making BB, the carbohydrate content is increased as a result of the addition of nectar and honey with the fresh pollen. Moreover, the sugar content varies because the action of bee salivary glands. Thus, the action of α- and β-amylase and α-glucosidase results in the breakdown of polysaccharides into simple sugars. Glucose and fructose are required by bacteria to ferment bee pollen into BB that is digested into ethanol, lactic acid, and carbon dioxide by lactic acid bacteria; glucose and fructose can also be transformed into mannitol after mixing with the bee salivary enzymes. All of these processes and probably others are responsible for the final sugar content available in BB.9,14,58
Table 2. Bee Bread (BB) Composition from Different Geographical and Botanical Originsa 58−60,62−65,67,68.
| proximate | content (min–max) | ||
|---|---|---|---|
| moisture (%) | 5.91–30.12 | ||
| protein (%) | 17.11–30.34 | ||
| ashes (%) | 1.93–3.42 | ||
| lipids (%) | 1.95–11.55 | ||
| carbohydrates (%) | 13.02–72.23 | ||
| sugars (g/100 g) | |||
| fructose | 0.994–19.73 | ||
| glucose | 8.82–12.40 | ||
| maltose | 0.945–1.244 | ||
| sucrose | 0.845–1.492 | ||
| trehalose | 0.544–0.921 | ||
| melezitose | 0.97–1.15 | ||
| raffinose | 0.96–1.24 | ||
| polyphenols | |||
| total phenolic content (mg of GAE/g) | 2.53–13.75 | ||
| total flavonoid content (mg of QE/g) | 1.94–4.51 | ||
| TEAC (μmol of Trolox/g) | 46.13–76.38 | ||
| FRAP (μmol of Trolox/g) | 35.03–70.17 | ||
| amino acids (g/100 g) | |||
| essential amino acids | non-essential amino acids | ||
| arginine | 0.11–2.76 | alanine | 0.5–1.7 |
| histidine | 0.82–1.21 | aspartic acid | 1.4–5.2 |
| isoleucine | 0.45–0.94 | glutamic acid | 0.2–1.7 |
| leucine | 0.16–2.13 | glycine | 0.4–1.4 |
| lysine | 0.53–0.89 | proline | 1.5–22.2 |
| methionine | 0.14–0.53 | serine | 0.6–2.2 |
| phenylalanine | 1.89–3.32 | tyrosine | 0.6–1.6 |
| threonine | 0.18–2.01 | asparagine | 2.47–5.89 |
| tryptophan | 0.14–1.42 | glutamine | 0.04–0.34 |
| valine | 0.17–1.20 | ||
| fatty acids (g/100 g) | |||
| C4:0 butyric acid | 0.75–1.29 | C20:1 eicosenoic acid | 0.28–6.28 |
| C6:0 caproic acid | 0.08–0.35 | C21:1 heneicosenoic acid | 0.39–0.43 |
| C8:0 caprylic acid | 0.02–0.34 | C20:2 eicosadienoic acid | 0.08–1.73 |
| C10:0 capric acid | 0.02–0.19 | C20:3 dihomo-γ-linolenic acid | 0.02–2.10 |
| C11:0 undecanoic acid | 0.07–0.90 | C20:3 eicosatrienoic acid | 0.47–0.52 |
| C12:0 lauric acid | 0.05–6.15 | C20:4 arachidonic acid | 0.02–25.12 |
| C14:0 myristic acid | 0.21–1.36 | C22:0 behenic acid | 0.08–2.60 |
| C15:0 pentadecanoic acid | 0.14–0.53 | C22:1 erucic acid | 0.11–5.43 |
| C16:0 palmitic acid | 10.21–38.69 | C22:6 docosahexaenoic acid | 0.04–0.29 |
| C16:1 palmitoleic acid | 0.05–1.14 | C23:0 tricosanoic acid | 0.58–5.61 |
| C17:0 heptadecanoic acid | 0.20–0.91 | C20:5 eicosapentaenoic acid | 0.05–0.64 |
| C17:1 heptadecenoic acid | 0.11–0.49 | C24:0 lignoceric acid | 0.03–1.65 |
| C18:0 stearic acid | 1.31–6.40 | C24:1 nervonic acid | 0.08–1.21 |
| C18:1 cis-oleic acid | 3.90–21.25 | SFA | 23.15–28.68 |
| C18:2 trans-linoelaidic acid | 0.03–0.22 | UFA | 71.03–77.32 |
| C18:2 cis-linoleic acid | 6.26–36.96 | MUFA | 31.11–72.15 |
| C18:3 α-linolenic acid | 0.17–40.02 | PUFA | 45.54–64.70 |
| C20:0 arachidic acid | 0.61–4.7 | ||
| minerals (mg/kg) | |||
| macrominerals | microminerals | ||
| potassium (K) | 3380.21–7551.54 | zinc (Zn) | 44.91–331.03 |
| calcium (Ca) | 1455.21–1980.21 | iron (Fe) | 119.52–273.61 |
| phosphorus (P) | 2510–6577.11 | manganese (Mn) | 37.27–890.20 |
| magnesium (Mg) | 610.21–2000.20 | copper (Cu) | 6.80–7.20 |
| sodium (Na) | 115.70–155.51 | selenium (Se) | 0.18–0.90 |
| vitamins (mg/100 g) | |||
| fat soluble | water soluble | ||
| tocopherols (vitamin E) | 10.01–11.22 | vitamin C | 10.87–11.52 |
GAE, gallic acid equivalent; QE, quercetin equivalent; FRAP, ferric-ion-reducing antioxidant power; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid; UFA, unsaturated fatty acid; and TEAC, Trolox equivalent antioxidant capacity.
Proteins represent between 17 and 30% of dry weight according to the reviewed literature.9 According to Mohammad et al.,59 protein use is higher in BB than in bee pollen (BP), while on other hand, Zuluaga and co-workers60 described that the content of protein in BB can be similar to BP because the biochemical process induced by bees is aimed at degrading the outer layer of the pollen grain, without any damage of the inner content. However, because of the addition of sugar by the bee, BB protein can be unpredictable.61 With regard to the amino acidic profile, the content is higher compared to BP, which has been suggested to happen because of degradation of pollen protein, leading to more peptides and amino acids.9,59 The microbial presence might be responsible for both the increase of amino acids through proteolysis and decrease of amino acids by its use as an energy source. It has been described as an important number of amino acids present in different BB samples with a very variable content.62 A summary with the observed range can be shown in Table 2.
The lipid content in BB may vary from 1.1 to 11.55% of dry weight.63 Particular attention has been focused on the fatty acid profile of BB (Table 2). Up to 37 fatty acids have been described in BB.64 Unsaturated fatty acids (UFAs) predominate over saturated fatty acids (SFAs), with up to 77.32% of total fatty acids with respect to 28.68%, respectively. Among UFAs, there is a good correlation between monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs), with a maximum of 72.15% and a 64.70%, respectively. The presence of some essential PUFAs in BB is important, which is very relevant from the point of view of human health. From the point of view of bees, it has been described that some fatty acids, like oleic and palmitic acids, are important for nutrition. On other hand, myristic, linoleic, and linolenic acids are relevant because they can inhibit the growth of spore-forming bacteria.63
BB is also a moderate source of organic acids, such as gluconic, formic, acetic, propionic, and butyric acids,65 and even probiotics, including lactic acid.66
Ashes represent between 1.93 and 3.42% of dry weight.60,64,65 An important fraction of ashes is formed by minerals (Table 2). Considering the studies in which the most accurate technology has been used to analyze minerals, i.e., inductively coupled plasma mass spectrometry, the most abundant mineral reported for BB is K, followed by P, Ca, and Mg.59,63 As for BP, the principal origin of minerals is pollen and soil, but in BB, also nectar is a relevant source of these chemical elements. Concerning vitamins, it is expected that, because BB is derived from pollen, which is rich in vitamins, BB also has them. However, the literature is scarce on this matter. In fact, despite the scientific literature when describing BB composition, no mention is made on its content in various fat- and water-soluble vitamins; after an exhaustive search for vitamin values, only levels of tocopherol and vitamin C have been documented in recent literature (Table 2).
Because one of its main components is pollen, the presence of phenolic compounds is expected in BB. Table 2 shows the total contents of phenols and total flavonoids according to the literature. Concerning individual compounds, research on the subject indicates the presence of numerous phenolic molecules in BB.14,58,62,63,65 Urcan et al.67 found that the phenolic profile of BB is similar to that of BP (Table 2), despite the transformations that take place in pollen during the manufacture of BB. Rutin is the main component observed, and quercetin is also present in an important concentration.62 Transformations during BB production as a result of, for example, bacterial action could modify the relative proportions of various compounds between BP and BB. Thus, Bayran et al.62 have observed some compounds to be higher in BP (caffeic acid, rutin, ethyl gallate, trans-ferulic acid, and myricetin) and in other cases higher in BB (protocatechuic acid, p-coumaric acid, quercetin, 2,5-dihydroxybenzoic acid, kaempferol, gallic acid, chlorogenic acid, salicylic acid, luteolin, and isorhamnetin).
In the last years, numerous investigations have been conducted to study its biomedical potential that is strictly correlated with the source pollens and, consequently, the nutrient and non-nutrient compounds present in BB.58 Because of the presence of phenolic compounds and other ingredients, the antioxidant capacity has often been analyzed. Table 2 presents typical values for Trolox equivalent antioxidant capacity (TEAC) and ferric-ion-reducing antioxidant power (FRAP) from the literature. It has been described that BB has a similar or higher antioxidant capacity than honey or propolis.63 The antimicrobial effect of BB has been extensively investigated. Bakour et al.63 found that different bacterial strains and fungi were sensitive to a hydromethanolic BB extract. It has been described that BB extracts are more effective against Gram-positive bacteria compared to Gram-negative bacteria.68 Antitumoral activity has also been assayed in vitro in some studies.69 Additionally, some investigations have been conducted regarding anti-inflammatory and immunomodulatory effects, antihypertensive activity, hypolipidemic effect, and hepatoprotective actions.58 More standardized and systematic experimental research is needed to know in depth the biomedical properties of BB beyond its antioxidant and antimicrobial capacity, aspects on which more information is available to date.
4. Royal Jelly
Royal jelly (RJ) is a gelatinous and creamy secretion produced by the hypopharyngeal and mandibular glands of the young honeybee (A. mellifera L.) workers, named nurses.70 It is usually used to feed the larvae of bee workers until the third day of their life (after this period, they are nourished with a mix of honey, RJ, and pollen), while it represents the unique food for the queen bee for her entire life.71−73 The consistency of RJ is not always homogeneous because some undissolved pollen grains of different sizes may persist; it is moderately acidic, with a pH ranging from 3.1 to 3.9 and a density of 1.1 g/mL; its color varies from white to yellow; and its odor and taste are lightly pungent.74−76
With regard to the chemical and nutritional composition, RJ is essentially made up of water (60–70%), carbohydrates, proteins and free amino acids, lipids, minerals, vitamins, and polyphenols.77 Sugar contents range between 7 and 18%, with glucose and fructose the most representative sugars, accounting for about 90% of the total sugars found in RJ; other sugars that may be present in smaller quantities are ribose, sucrose, trehalose, maltose, galactose, erlose, and melibiose.78,79
Protein contents vary between 9 and 18%, of which 80% is represented by the so-called major royal jelly proteins (MRJPs). These proteins are water-soluble and comprise nine members MRJP1–MRJP9, with molecular masses of 49–87 kDa.80,81 From the structural point of view, MRJPs have repetitive pentapeptide zones enriched with nitrogen-rich amino acids or repetitive tripeptide zones enriched with methionine (i.e., MRJP5);81 they exert essential physiological effects in the development of the queen bee.82 Beside MRJPs, other proteins present in small amounts in RJ are aspimin, jelleines, royalisin,78 and small peptides, such as dipeptides (i.e., Lys-Tyr, Arg-Tyr, Ala-Leu, Phe-Lys, Ile-Arg, Lys-Leu, Arg-Tyr, Tyr-Tyr, and Tyr-Tyr) with high antioxidative and antibacterial activities.78,83,84 RJ is also rich in free amino acids, with glycine, lysine, glutamic acid, and proline being the most abundant, followed by alanine, leucine, isoleucine, arginine, phenylalanine, aspartic acid, threonine, serine, methionine, valine, and tyrosine.71,80,85
Fatty acids, waxes, phenols, steroids, and phospholipids constitute the lipid fraction of RJ (3–8%). Specifically, fatty acids contain 8–12 carbons and are typically either dicarboxylic or hydroxyl fatty acids. They include trans-10-hydroxy-2-decenoic acid, gluconic acid, and 10-hydroxydecanoic acid that are the main fatty acids in RJ from the quantitative point of view, followed by 9-hydroxydecanoic, 7- and 8-hydroxyoctanoic, 9-hydroxy-2-decenoic, 3-hydroxydecanoic, 3,10-dihydroxydecanoic, and 10-hydroxydecanoic acids; 2-decene-1,10-dioic and 2-octene-1,8-dioic acids have been also reported in RJ, together with the mono- and diesters of 10-hydroxy-2-trans-decenoic acid and hydroxyl-2-trans-decenoic acid 10-phosphate.72,74,86−88 Finally, 24-methylenecholesterol and Δ5-avenasterol are the main sterols found in RJ.80
With regard to micronutrients, the most abundant minerals in RJ include Ca, Cu, Fe, K, Mg, Mn, Na, and Zn, followed, in trace amounts, by aluminum, antimony, barium, bismuth, cadmium, cobalt, chrome, lead, thallium, and vanadium, among others,75,87 while pantothenic acid is the most abundant vitamin present in RJ, followed in trace amounts by ascorbic acid, biotin, folic acid, niacin, pyridoxal, riboflavin, and thiamine.70,89 RJ may also contain nucleotides, as both free bases, including adenosine, cytidine guanosine, uridine, and phosphates, comprising adenosine mono-, di-, and triphosphate.78
The composition of bioactive compounds in RJ is strictly correlated with the regional and seasonal conditions. The phenolic content varies between 3.1 and 15.4 mg of gallic acid equivalent (GAE)/g and comprises mainly flavonoids, phenolic acids, organic acids, and their esters (Table S2 of the Supporting Information).78,90−92 The main volatile compounds found in RJ are esters, aldehydes, ketones, acids, and alcohols (Table S2 of the Supporting Information), but their presence and concentrations strictly depend upon several factors, such as honeybee species, geographical area, harvest time, storage type, and processing system, making the comparison among different RJ samples difficult to do.90,93,94
The presence and amount of the different macro- and micronutrients and bioactive compounds determine the biological properties of RJ. For example, several RJ samples from different geographical zones have been found to exert important (i) antibacterial activities against both Gram-positive (Staphylococcus aureus, Staphylococcus epidermidis, Micrococcus luteus, Listeria monocytogenes, and Streptomyces griseus) and Gram-negative (Proteus vulgaris, Escherichia coli, Pseudomonas aeruginosa, Enterobacter cloacae, and Klebsiella pneumoniae) bacteria, (ii) anti-inflammatory effects in different in vitro and in vivo models, through the decrease of pro-inflammatory cytokine secretion and the modulation of different molecular pathways, such as TNF-κB, IL-1β, IL-18, and MAPK, and (iii) antioxidant properties in some in vitro and in vivo studies, with the ability of RJ to act as radical scavengers and affect protein expression in a dose-dependent manner.95 Other biological activities exerted by RJ include the immunomodulatory effects,95−97 vasodilative, hypotensive, and antihypercholesterolemic activities,95,98−102 and antitumor properties.103−108 In particular, 10-hydroxy-2-decenoic acid, the main lipid component of RJ, has been associated with several biological effects, including estrogenic, antimicrobial, anti-inflammatory, antitumor, and immunomodulatory activities as well as the increase of the lifespan of Caenorhabditis elegans through dietary restriction and target of rapamycin signaling, inhibition of the vascular endothelial growth factor, activation of TRPA1 and TRPV1 receptors, induction of neurogenesis, suppression of skin pigmentation, and protective effect against ultraviolet B in the human skin.109
5. Propolis
Propolis, also named bee glue, is a sticky resinous substance that honeybees (Apis spp.) collect from living plants. It is a complex mixture, made essentially by plant resins (50%), waxes (30%), aromatic and essential oils (10%), pollens (5%), and other organic compounds (5%);110 according to the botanical source, its color may range from green to reddish and brown.111 Propolis is usually used by honeybees to maintain a stable temperature and moisture inside the hive as well as to smooth walls and seal cracks. Moreover, it is also used by bees to mummify dead invader insects that are too heavy to be removed from the colony.112
Before commercialization, it must be purified through the use of different methods, including, for example, shaking, soaking, Soxhlet extraction or reflux, and different solvents, such as absolute ethanol or aqueous ethanol at 70–95%, pure water, hexane, acetone, methanol, chloroform, etc., to remove the undesired material; the type of extraction and the solvents used strongly affect the yields of bioactive compounds that remain in propolis.110
With regard to the chemical and nutritional composition of propolis, the main group of compounds is represented by phenolic components, mainly flavonoids, phenolic acids, lignans, stilbenes, and coumarins (Table S3 of the Supporting Information); other minor components are beeswax, lipid–wax substances, terpenes, resins, balms, and sugars as well as mono- (glucose and fructose) and disaccharides (sucrose), proteins, amino acids, minerals (Ca, Cu, K, Mg, Mn, Na, and Zn), and vitamins (B1, B2, B6, C, and E) that account for a negligible part of propolis composition.110,113−117
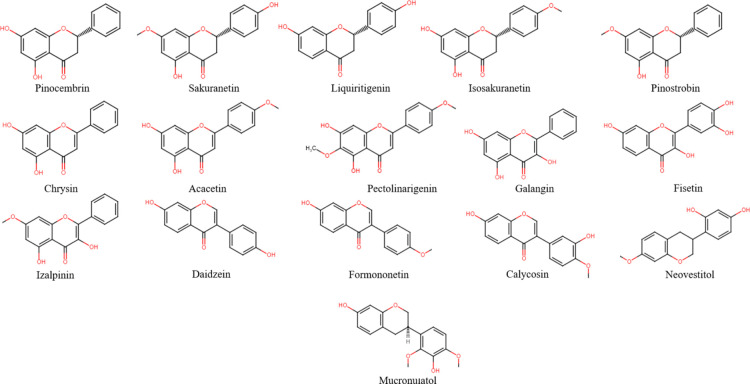
The phenolic profile of propolis is strongly influenced by the geographical area and the climatic conditions where plants grow; for example, in Europe, North America, and Asia (temperate zone), phenolic patterns are characterized by high levels of flavonoids (mainly flavones and flavanones) and low levels of phenolic acids, while in tropical areas, propolis has more complex composition, with prenylated flavonoids, prenylated p-coumaric acids, and lignans, among others.118 The total phenolic content usually ranges from 127 to 142 mg of GAE/g, while the total flavonoid concentration varies between 33 and 53 mg of quercetin equivalents/g.119 The total flavonoid content represents an index of quality of raw propolis: if it is less than 11%, crude propolis is considered of low quality; between 11–14%, acceptable; between 14–17%, good; and >17%, high quality.120 Until now, more than 150 flavonoids have been identified in propolis, including flavones, flavanones, flavanonols, flavonols, isoflavones, isodihydroflavone, flavan, and isoflavan, according to the plant source.110,118 Generally, these compounds are in the form of aglycone, because β-glucosidase secreted by bees usually removes sugar residues from most of the flavonoids present in the plants,118 even if it could be ineffective in hydrolyzing some glycosides, such as C-glycosides or β-diglycosides; this could explain, for example, the presence of quercetin 3-O-rutinoside, isorhamnetin 3-O-rutinoside, kaempferol rutinoside, flavone C-glycosides, naringenin, and luteolin glucosides in propolis from different countries.118 The most representative flavonoids found in propolis are illustrated in Figure 4.
Figure 4.
Chemical structures of the most representative flavonoids found in propolis.
Hydroxybenzoic and hydroxycinnamic acids are the main phenolic acids of propolis collected in different parts of the world, from Brazil to Australia: the first comprises gallic, gentisic, protocatechuic, salicylic, and vanillic acids, and the latter includes p-coumaric, caffeic, and ferulic acids. Moreover, besides the free forms, propolis can also contain the conjugated forms of phenolic acids, such as cinnamyl, benzyl, phenylethyl, and methyl butenyl esters. For example, in propolis collected in the temperate zone, caffeic acid phenethyl ester is the main component, while hydroxycinnamic acid derivatives are common in Australian propolis; in addition, according to the plant source, in Brazilian samples, chlorogenic acids and prenylated phenylpropanoids, such as artepillin C or chromene, were also shown to be very common.118
Lignans are present in few samples collected especially in tropical zones, mainly Brazil,121 and the Canary Islands,122 especially as syringaresinol and pinoresinol, while stilbenes have been reported in samples from Kenya, the Solomon Islands, and Australian Kangaroo Island, specifically in the form of geranylstilbenes (scheweinfurthin A and B), 5-farnesyl-3′-hydroxyresveratrol, pterostilbene, pinosylvin, O-prenylated tetrahydroxystilbenes, and C-prenylated tetrahydroxystilbenes;123 some coumarins have also been identified in a few samples124 (Table S3 of the Supporting Information).
Finally, another class of chemical constituents present in propolis is represented by volatiles.125 Even if they are minority compounds, they are important because they confer to propolis, from different origins, the typical aroma. The type and amount of volatiles in propolis depend upon several factors, including the type of bee, the geographical area, the plant, and also the methods used for their extraction and analysis. The main volatiles found in propolis are present in Table S3 of the Supporting Information.
Even if the use of propolis in medicine dates back to ancient times, only recently, its use for preventive and therapeutic purposes has been re-evaluated in a more scientific way. Several studies have indeed demonstrated the multiple biological properties of propolis, including the antioxidant, anti-inflammatory, immune-stimulating, antimicrobial, and even anticancer activities, highlighting the strong relationship between these effects and the chemical composition of propolis. For example, the antioxidant capacity can be ascribed to the presence of phenolic compounds of propolis that has been found to decrease oxidative stress in different in vitro and in vivo experimental models, including neuronal cell cultures and fibroblasts stressed with hydrogen peroxide or in mice treated with 1,1-diphenyl-2-picrylhydrazyl (DPPH).126
With regard to anti-inflammatory and immune-modulatory effects, propolis seems to play a key role, by inhibiting, for example, the release of TNF-α and IFN-γ and the degradation of tryptophan in stimulated peripheral blood mononuclear cells,127 upregulating the expression of TLR-4 and CD80 expression and downregulating the production of TNF-α and IL-10 in human monocytes, increasing the levels of anti-inflammatory cytokines IL-4 and IL-10 in mice blood,128 or increasing the expression of TLR-2 and TLR-4 and the production of IL-1β and IL-6 in the spleen cells of mice.129,130
The most studied and recognized property of propolis is the antimicrobial activity that mainly depends upon the synergistic effects of its several antimicrobial components (especially flavonoids and phenolic acids) rather than individual components. Specifically, propolis is active against several aerobic and anaerobic Gram-positive (Bacillus cereus, Enterococcus faecalis, Micrococcus luteus, Nocardia asteroides, Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus haemolyticus, Streptococcus faecalis, Streptococcus pneumioniae, Streptococcus pyogenes, Streptococcus haemolyticus, Streptococcus mutans, Actinomyces naeslundii, Lactobacillus acidophilus, and Peptostreptococcus micros) and Gram-negative (Aeromonas hydrophila, Brucella abortus, Corynebacterium pseudotuberculosis, Escherichia coli, Helicobacter pylori, Klebsiella pneumoniae, Salmonella enteritidis, Salmonella typhi, Salmonella Typhimurium, Pseudomonas aeruginosa, Proteus mirabilis, Proteus vulgaris, Shigella dysenteriae, Porphyromonas gingivalis, Fusobacterium nucleatum, and Prevotella oralis) bacteria, among others; it is also effective against different viruses, including adenovirus, influenza viruses, herpes simplex virus, poliovirus, rotavirus, coronavirus, and vesicular stomatitis virus, protozoa, such as Plasmodium falciparum, Plasmodium malariae, Trichomonas vaginalis, Trypanosoma brucei, Trypanosoma evansi, Trypanosoma cruzi, Giardia duodenalis, Giardia lambia, and Entamoeba histolytica, helminths, comprising Taenia saginata, Fasciola gigantica, and Toxocara vitulorum, and fungi, especially different strains of Candida, as well as Microsporum gypseum, Trichophyton rubrum, and Trichophyton mentagrophytes.126
Thanks to its antioxidant, anti-inflammatory, and antimicrobial activities, propolis is effective in (i) wound treatment, including surgical wounds, infected wounds, gastric ulcers, and diabetic ulcers, (ii) diabetic conditions, by decreasing the levels of blood glucose, glycated hemoglobin, and oxidative stress, enhancing the antioxidant systems, and improving the lipid profile in animal models of diabetes and atherosclerosis, and (iii) proliferative disorders, including colon, breast, prostate, brain, lung, and cervical cancer, both in vitro and in animal studies.126
6. Beeswax
Beeswax (BW) is the product of the wax glands of bees that use it to build their combs.131 BW is obtained by melting the combs of the hives after the honey has been removed from them. The combs are melted using steam or boiling water. The wax obtained after pressure filtration is called yellow beeswax. During the process, activated carbon or diatomaceous earth can be used to remove certain impurities. A more aggressive procedure on yellow beeswax, such as bleaching the natural pigments by exposure to sunlight, activated carbon, diatomaceous earth, and other earth or peroxides, leads to the production of white beeswax.131 According to the European Food Safety Authority (EFSA), “beeswax is a complex mixture of saturated and unsaturated linear and complex monoesters, hydrocarbons, free fatty acids, free fatty alcohols and other minor substances produced by the worker honeybee”.132 In 1980, Tulloch reported that more than 300 individual components are present in BW.133 Additionally, around 50 aroma components have been identified in this wax.134 For the same species, BW composition is quite stable, with small changes in proportions. More relevant differences are present between species.131 Main components are shown in Table 3, together with the detailed composition in some groups of molecules, including fatty acid monoesters, which represent, from a quantitative point of view, the most important compounds in BW. Also, the content in aliphatic hydrocarbons, total fatty acids, total alcohols, and chemical elements can be found in Table 3.
Table 3. Beeswax Composition from Different Geographical and Botanical Origins50,132,133,138,139.
| main component (%) | content (min–max) | ||
|---|---|---|---|
| monoesters | 27–40 | ||
| hydroxymonoesters | 9–23 | ||
| diesters | 7–16 | ||
| hydroxydiesters | 3–9 | ||
| hydrocarbons | 11–28 | ||
| free fatty acids | 1–18 | ||
| free fatty alcohols | 0–0.3 | ||
| others | 4–8 | ||
| monoesters (%) | |||
| C40 | 10.13–10.27 | C46:1 | 0.02–0.22 |
| C42 | 5.80–5.99 | C48 | 8.16–8.31 |
| C42:1 | 0.22–0.25 | C48:1 | 0.31–0.35 |
| C44 | 6.27–6.60 | C50 | 0.56–0.83 |
| C44:1 | 0.19–0.23 | C50:1 | 0.54–0.92 |
| C46 | 13.75–16.49 | ||
| aliphatic hydrocarbons (%) | |||
| H19 | 0.14–0.22 | H33:1 | 1.77–4.31 |
| H20 | 0.05–0.39 | H33 | 0.39–0.74 |
| H21 | 0.10–0.43 | H34 | 0.11–0.12 |
| H22 | 0.03–0.15 | H35:1 | 0.11–0.23 |
| H23 | 0.71–3.08 | H35 | 0.42–0.46 |
| H24 | 0.07–0.19 | H36 | 0.00–0.16 |
| H25 | 1.79–5.51 | H37 | 0.05–0.11 |
| H26 | 0.20–0.39 | H38 | 0.00–0.03 |
| H27 | 3.17–15.96 | H39 | 0.02–0.09 |
| H28 | 0.18–0.41 | H40 | 0.09–0.11 |
| H29 | 1.99–9.53 | H41 | 0.01–0.09 |
| H30 | 0.13–0.32 | H42 | 0.02–0.04 |
| H31:1 | 0.82–2.79 | H43 | 0.01–0.06 |
| H31 | 2.27–7.47 | H44 | 0.00–0.01 |
| H32 | 0.09–0.37 | ||
| total fatty acids | |||
| C14 | 0.09–0.11 | C24 | 4.21–5.11 |
| C16 | 16.28–19.22 | C26 | 1.50–1.95 |
| C18:2 | 0.03–0.11 | C28 | 1.43–2.11 |
| C18:1 | 2.13–2.34 | C30 | 1.55–1.98 |
| C18 | 0.51–0.67 | C32 | 1.50–1.85 |
| C20 | 0.03–0.11 | C34 | 1.43–1.90 |
| C22 | 0.43–0.65 | C36 | 0.24–0.65 |
| total alcohols (%) | |||
| C22-OH | 0.05–0.09 | C32:1-OH | 0.25–0.83 |
| C24-OH | 5.45–7.12 | C32-OH | 8.25–10.87 |
| C26-OH | 4.45–6.25 | C34:1-OH | 0.08–0.41 |
| C28-OH | 4.85–6.12 | C34-OH | 1.12–1.87 |
| C30-OH | 9.18–11.25 | ||
| chemical elements (mg/kg) | |||
| Mg | 17.02–27.50 | Co | 0.00–0.01 |
| Al | 7.55–14.31 | Zn | 9.70–11.42 |
| Si | 0.00–3.30 | As | 0.01–0.02 |
| P | 45.08–50.62 | Se | 0.00–0.03 |
| K | 78.65–99.56 | Y | 0.01–0.02 |
| Ca | 76.05–302.43 | Cd | 0.00–0.04 |
| V | 0.01–0.03 | Au | 0.00–3.51 |
| Mn | 0.26–0.84 | Hg | 0.20–0.72 |
| Fe | 16.90–24.76 | Pb | 0.00–4.48 |
Apart from the original use of beeswax by bees for foundation, this type of wax has been traditionally used for the preparation of cosmetics, pharmaceutical products, candles, and other purposes.131 Concerning the nutraceutical, pharmacological, and food-processing uses of beeswax, it must be known that there are pharmacopeia standards for BW.134 This bee product has been used as a glazing agent on confectionery. Thus, many products of fine bakery are coated with BW. Also, it has been used for the treatment of some types of fruits. Its use is also allowed as a color carrier in food supplements.135 In medicine, dated use since the second century BC, BW has been used to coat pills, facilitating ingestion but retarding dissolution. Prepared in a mix with some drugs, it can function as a time-release mechanism.131 Additionally, its use has been documented in the treatment of burns, abscesses, wounds, and dental problems.136,137
7. Conclusion and Future Directions
Bee products (pollen, propolis, bee bread, royal jelly, and beeswax) have been shown to be an important source of bioactive compounds with relevant biological effects. Recently, several studies have started to evaluate their biological activities, such as antimicrobial, anti-inflammatory, antioxidant, anticancer, immune-modulatory, antidiabetic, hypolipidemic, hypotensive, and anti-allergic properties. These pharmacological properties can be undoubtedly ascribed to the multiplicity of the active components that are contained in these complex matrices and that make them emblematic examples of functional foods and sources of bioactive molecules. Despite this, these byproducts are still not considered in the healthcare system because of several factors, including the lack of standardization of their composition, which strongly depends upon several parameters (i.e., geographical area, climatic conditions, plants, methods used for the extraction and analysis, etc.); the scarcity of data regarding the safety, allergy, and toxicity correlated to their use or their therapeutic effectiveness from clinical studies.
Therefore, further studies are strongly needed to (i) standardize the protocols for the extraction and analysis of these food matrices, (ii) assess their content of nutrients and bioactive compounds, (iii) evaluate their bioaccessibility, bioavailability, and metabolism and the influence of the gut microbiota, (iv) measure their safe and toxic dosages, (v) determine their effects in preclinical and clinical studies, (vi) investigate in a deeper way the molecular mechanisms and targets involved in their beneficial effects, and (vii) investigate the possible synergistic/antagonist effects with synthetic drugs. A deeper knowledge and understanding of these honeybee byproducts could be of crucial importance to both promote their use in the general population, for the prevention of the most common pathologies, and discover new pharmaceutical natural products for the treatment of several diseases, in combination with classic therapies.
Glossary
Abbreviations Used
- BB
bee bread
- BP
bee pollen
- BW
beeswax
- GAE
gallic acid equivalent
- IFN-γ
interferon γ
- IgE
immunoglobulin E
- IgG1
immunoglobulin G1
- IL
interleukin
- MAPK
mitogen-activated protein kinase
- MRJP
major royal jelly protein
- MUFA
monounsaturated fatty acid
- NF-κB
nuclear factor κ-light-chain-enhancer of activated B cells
- PUFA
polyunsaturated fatty acid
- RJ
royal jelly
- SFA
saturated fatty acid
- TNF-α
tumor necrosis factor α
- UFA
unsaturated fatty acid
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jafc.1c05822.
Main fat-soluble vitamins (Figure S1) and main polyphenols and other relevant compounds present in bee pollen (Table S1), royal jelly (Table S2), and propolis (Table S3) (PDF)
Author Contributions
† Francesca Giampieri and Jose Luis Quiles contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Battino M.; Giampieri F.; Cianciosi D.; Ansary J.; Chen X.; Zhang D.; Gil E.; Forbes-Hernández T. The Roles of Strawberry and Honey Phytochemicals on Human Health: A Possible Clue on the Molecular Mechanisms Involved in the Prevention of Oxidative Stress and Inflammation. Phytomedicine 2021, 86, 153170. 10.1016/j.phymed.2020.153170. [DOI] [PubMed] [Google Scholar]
- Cianciosi D.; Forbes-Hernández T.; Afrin S.; Gasparrini M.; Reboredo-Rodriguez P.; Manna P.; Zhang J.; Bravo Lamas L.; Martínez Flórez S.; Agudo Toyos P.; Quiles J.; Giampieri F.; Battino M. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. 10.3390/molecules23092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Suarez J. M.; Giampieri F.; Cordero M.; Gasparrini M.; Forbes-Hernández T. Y.; Mazzoni L.; Afrin S.; Beltrán-Ayala P.; González-Paramás A. M.; Santos-Buelga C.; Varela-Lopez A.; Quiles J. L.; Battino M. Activation of AMPK/Nrf2 Signalling by Manuka Honey Protects Human Dermal Fibroblasts against Oxidative Damage by Improving Antioxidant Response and Mitochondrial Function Promoting Wound Healing. J. Funct. Foods 2016, 25, 38–49. 10.1016/j.jff.2016.05.008. [DOI] [Google Scholar]
- Amessis-Ouchemoukh N.; Maouche N.; Otmani A.; Terrab A.; Madani K.; Ouchemoukh S. Evaluation of Algerian’s Honey in Terms of Quality and Authenticity Based on the Melissopalynology and Physicochemical Analysis and Their Antioxidant Powers. Med. J. Nutr. Metab. 2021, 14, 305–324. 10.3233/MNM-210561. [DOI] [Google Scholar]
- Afrin S.; Giampieri F.; Cianciosi D.; Pistollato F.; Ansary J.; Pacetti M.; Amici A.; Reboredo-Rodríguez P.; Simal-Gandara J.; Quiles J. L.; Forbes-Hernández T. Y.; Battino M. Strawberry Tree Honey as a New Potential Functional Food. Part 1: Strawberry Tree Honey Reduces Colon Cancer Cell Proliferation and Colony Formation Ability, Inhibits Cell Cycle and Promotes Apoptosis by Regulating EGFR and MAPKs Signaling Pathways. J. Funct. Foods 2019, 57, 439–452. 10.1016/j.jff.2019.04.035. [DOI] [Google Scholar]
- Afrin S.; Forbes-Hernández T. Y.; Cianciosi D.; Pistollato F.; Zhang J.; Pacetti M.; Amici A.; Reboredo-Rodríguez P.; Simal-Gandara J.; Bompadre S.; Quiles J. L.; Giampieri F.; Battino M. Strawberry Tree Honey as a New Potential Functional Food. Part 2: Strawberry Tree Honey Increases ROS Generation by Suppressing Nrf2-ARE and NF-κB Signaling Pathways and Decreases Metabolic Phenotypes and Metastatic Activity in Colon Cancer Cells. J. Funct. Foods 2019, 57, 477–487. 10.1016/j.jff.2019.04.037. [DOI] [Google Scholar]
- Osés S. M.; Nieto S.; Rodrigo S.; Pérez S.; Rojo S.; Sancho M. T.; Fernández-Muiño M. Á. Authentication of Strawberry Tree (Arbutus unedo L.) Honeys from Southern Europe Based on Compositional Parameters and Biological Activities. Food Biosci. 2020, 38, 100768. 10.1016/j.fbio.2020.100768. [DOI] [Google Scholar]
- Bee Products—Chemical and Biological Properties; Alvarez-Suarez J. M., Ed.; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Kieliszek M.; Piwowarek K.; Kot A. M.; Błażejak S.; Chlebowska-Śmigiel A.; Wolska I. Pollen and Bee Bread as New Health-Oriented Products: A Review. Trends Food Sci. Technol. 2018, 71, 170–180. 10.1016/j.tifs.2017.10.021. [DOI] [Google Scholar]
- Thakur M.; Nanda V. Composition and Functionality of Bee Pollen: A Review. Trends Food Sci. Technol. 2020, 98, 82–106. 10.1016/j.tifs.2020.02.001. [DOI] [Google Scholar]
- De-Melo A. A. M.; de Almeida-Muradian L. B.. Chemical Composition of Bee Pollen. In Bee Products—Chemical and Biological Properties; Alvarez-Suarez J. M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp 221–259. [Google Scholar]
- Bobiş O.; Al Mǎrghitaş L.; Dezmirean D.; Morar O.; Bonta V.; Chirilǎ F. Quality Parameters and Nutritional Value of Different Commercial Bee Products. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca, Anim. Sci. Biotechnol. 2010, 67, 91–96. 10.15835/buasvmcn-asb:67:1-2:5254. [DOI] [Google Scholar]
- Li Q. Q.; Wang K.; Marcucci M. C.; Sawaya A. C. H. F.; Hu L.; Xue X. F.; Wu L. M.; Hu F. L. Nutrient-Rich Bee Pollen: A Treasure Trove of Active Natural Metabolites. J. Funct. Foods 2018, 49, 472–484. 10.1016/j.jff.2018.09.008. [DOI] [Google Scholar]
- Aylanc V.; Falcão S. I.; Ertosun S.; Vilas-Boas M. From the Hive to the Table: Nutrition Value, Digestibility and Bioavailability of the Dietary Phytochemicals Present in the Bee Pollen and Bee Bread. Trends Food Sci. Technol. 2021, 109, 464–481. 10.1016/j.tifs.2021.01.042. [DOI] [Google Scholar]
- Fuenmayor B C.; Zuluaga D C.; Díaz M C.; Quicazán de C M.; Cosio M.; Mannino S. Evaluation of the Physicochemical and Functional Properties of Colombian Bee Pollen. Rev. MVZ. Córdoba 2014, 4003–4014. 10.21897/rmvz.120. [DOI] [Google Scholar]
- Bobis O.; Moise A. R.; Ballesteros I.; Reyes E. S.; Durán S. S.; Sánchez-Sánchez J.; Cruz-Quintana S.; Giampieri F.; Battino M.; Alvarez-Suarez J. M. Eucalyptus Honey: Quality Parameters, Chemical Composition and Health-Promoting Properties. Food Chem. 2020, 325, 126870. 10.1016/j.foodchem.2020.126870. [DOI] [PubMed] [Google Scholar]
- De-Melo A. A. M.; Estevinho L. M.; Moreira M. M.; Delerue-Matos C.; de Freitas A. da S.; Barth O. M.; de Almeida-Muradian L. B. A Multivariate Approach Based on Physicochemical Parameters and Biological Potential for the Botanical and Geographical Discrimination of Brazilian Bee Pollen. Food Biosci. 2018, 25, 91–110. 10.1016/j.fbio.2018.08.001. [DOI] [Google Scholar]
- Liolios V.; Tananaki C.; Dimou M.; Kanelis D.; Goras G.; Karazafiris E.; Thrasyvoulou A. Ranking Pollen from Bee Plants According to Their Protein Contribution to Honey Bees. J. Apic. Res. 2015, 54, 582–592. 10.1080/00218839.2016.1173353. [DOI] [Google Scholar]
- Vanderplanck M.; Leroy B.; Wathelet B.; Wattiez R.; Michez D. Standardized Protocol to Evaluate Pollen Polypeptides as Bee Food Source. Apidologie 2014, 45, 192–204. 10.1007/s13592-013-0239-0. [DOI] [Google Scholar]
- Al-Kahtani S. N.; Taha E.-K.; Khan K. A.; Ansari M. J.; Farag S. A.; Shawer D. M. B.; Elnabawy E.-S. M. Effect of Harvest Season on the Nutritional Value of Bee Pollen Protein. PLoS One 2020, 15, e0241393. 10.1371/journal.pone.0241393. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kędzia B. Chemical Composition and Adaptogenic Activity of Honeybee-Collected Pollen. Part One. Chemical Composition. Postep. Fitoter. 2008, 1, 47–58. [Google Scholar]
- Campos M. G. R.; Bogdanov S.; de Almeida-Muradian L. B.; Szczesna T.; Mancebo Y.; Frigerio C.; Ferreira F. Pollen Composition and Standardisation of Analytical Methods. J. Apic. Res. 2008, 47, 154–161. 10.1080/00218839.2008.11101443. [DOI] [Google Scholar]
- Martins M. C. T.; Morgano M. A.; Vicente E.; Baggio S. R.; Rodriguez-Amaya D. B. Physicochemical Composition of Bee Pollen from Eleven Brazilian States. J. Apic. Sci. 2011, 55, 107–116. [Google Scholar]
- Odoux J. F.; Feuillet D.; Aupinel P.; Loublier Y.; Tasei J. N.; Mateescu C. Territorial Biodiversity and Consequences on Physico-Chemical Characteristics of Pollen Collected by Honey Bee Colonies. Apidologie 2012, 43, 561–575. 10.1007/s13592-012-0125-1. [DOI] [Google Scholar]
- Liolios V.; Tananaki C.; Kanelis D.; Rodopoulou M. A.; Dimou M.; Argena N.. The Determination of Fat Content and Fatty Acid Composition of Bee Pollen Based on Its Botanical Origin. Proceedings of the 5th International Symposium on Bee Products; Malta, May 7–10, 2019.
- Li Q.; Liang X.; Zhao L.; Zhang Z.; Xue X.; Wang K.; Wu L. UPLC-Q-Exactive Orbitrap/MS-Based Lipidomics Approach to Characterize Lipid Extracts from Bee Pollen and Their in Vitro Anti-Inflammatory Properties. J. Agric. Food Chem. 2017, 65, 6848–6860. 10.1021/acs.jafc.7b02285. [DOI] [PubMed] [Google Scholar]
- Mǎrgǎoan R.; Mǎrghitaş L. A.; Dezmirean D. S.; Dulf F. V.; Bunea A.; Socaci S. A.; Bobiş O. Predominant and Secondary Pollen Botanical Origins Influence the Carotenoid and Fatty Acid Profile in Fresh Honeybee-Collected Pollen. J. Agric. Food Chem. 2014, 62, 6306–6316. 10.1021/jf5020318. [DOI] [PubMed] [Google Scholar]
- Sattler J. A. G.; de Melo I. L. P.; Granato D.; Araújo E.; da Silva de Freitas A.; Barth O. M.; Sattler A.; de Almeida-Muradian L. B. Impact of Origin on Bioactive Compounds and Nutritional Composition of Bee Pollen from Southern Brazil: A Screening Study. Food Res. Int. 2015, 77, 82–91. 10.1016/j.foodres.2015.09.013. [DOI] [Google Scholar]
- Abd Alla A.; Salem R. Impact of Storage Period on Different Types of Bee Pollen Pigments. J. Plant Prot. Pathol. 2020, 11, 9–13. 10.21608/jppp.2020.68178. [DOI] [Google Scholar]
- Kastrati G.; Paçarizi M.; Sopaj F.; Tašev K.; Stafilov T.; Mustafa M. K. Investigation of Concentration and Distribution of Elements in Three Environmental Compartments in the Region of Mitrovica, Kosovo: Soil, Honey and Bee Pollen. Int. J. Environ. Res. Public Health 2021, 18, 2269. 10.3390/ijerph18052269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha E.-K. A. Chemical Composition and Amounts of Mineral Elements in Honeybee-Collected Pollen in Relation to Botanical Origin. J. Apic. Sci. 2015, 59, 75–81. 10.1515/jas-2015-0008. [DOI] [Google Scholar]
- Zuluaga C.; Martínez A.; Fernández J.; López-Baldó J.; Quiles A.; Rodrigo D. Effect of High Pressure Processing on Carotenoid and Phenolic Compounds, Antioxidant Capacity, and Microbial Counts of Bee-Pollen Paste and Bee-Pollen-Based Beverage. Innovative Food Sci. Emerging Technol. 2016, 37, 10–17. 10.1016/j.ifset.2016.07.023. [DOI] [Google Scholar]
- Rzepecka-Stojko A.; Stojko J.; Kurek-Górecka A.; Górecki M.; Kabała-Dzik A.; Kubina R.; Moździerz A.; Buszman E. Polyphenols from Bee Pollen: Structure, Absorption, Metabolism and Biological Activity. Molecules 2015, 20, 21732–21749. 10.3390/molecules201219800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur M.; Nanda V. Screening of Indian Bee Pollen Based on Antioxidant Properties and Polyphenolic Composition Using UHPLC-DAD-MS/MS: A Multivariate Analysis and ANN Based Approach. Food Res. Int. 2021, 140, 110041. 10.1016/j.foodres.2020.110041. [DOI] [PubMed] [Google Scholar]
- Negri G.; Teixeira E. W.; Alves M. L. T. M. F.; de Camargo Carmello Moreti A. C.; Otsuk I. P.; Borguini R. G.; Salatino A. Hydroxycinnamic Acid Amide Derivatives, Phenolic Compounds and Antioxidant Activities of Extracts of Pollen Samples from Southeast Brazil. J. Agric. Food Chem. 2011, 59, 5516–5522. 10.1021/jf200602k. [DOI] [PubMed] [Google Scholar]
- Alimoglu G.; Guzelmeric E.; Yuksel P. I.; Celik C.; Deniz I.; Yesilada E. Monofloral and Polyfloral Bee Pollens: Comparative Evaluation of Their Phenolics and Bioactivity Profiles. LWT- Food Sci. Technol. 2021, 142, 110973. 10.1016/j.lwt.2021.110973. [DOI] [Google Scholar]
- Fanali C.; Dugo L.; Rocco A. Nano-Liquid Chromatography in Nutraceutical Analysis: Determination of Polyphenols in Bee Pollen. J. Chromatogr. A 2013, 1313, 270–274. 10.1016/j.chroma.2013.06.055. [DOI] [PubMed] [Google Scholar]
- Ulusoy E.; Kolayli S. Phenolic Composition and Antioxidant Properties of Anzer Bee Pollen. J. Food Biochem. 2014, 38, 73–82. 10.1111/jfbc.12027. [DOI] [Google Scholar]
- Kaškonienė V.; Ruočkuvienė G.; Kaškonas P.; Akuneca I.; Maruška A. Chemometric Analysis of Bee Pollen Based on Volatile and Phenolic Compound Compositions and Antioxidant Properties. Food Anal. Methods 2015, 8, 1150–1163. 10.1007/s12161-014-9996-2. [DOI] [Google Scholar]
- Mohdaly A. A. A.; Mahmoud A. A.; Roby M. H. H.; Smetanska I.; Ramadan M. F. Phenolic Extract from Propolis and Bee Pollen: Composition, Antioxidant and Antibacterial Activities. J. Food Biochem. 2015, 39, 538–547. 10.1111/jfbc.12160. [DOI] [Google Scholar]
- Lv H.; Wang X.; He Y.; Wang H.; Suo Y. Identification and Quantification of Flavonoid Aglycones in Rape Bee Pollen from Qinghai-Tibetan Plateau by HPLC-DAD-APCI/MS. J. Food Compos. Anal. 2015, 38, 49–54. 10.1016/j.jfca.2014.10.011. [DOI] [Google Scholar]
- Zhou J.; Qi Y.; Ritho J.; Zhang Y.; Zheng X.; Wu L.; Li Y.; Sun L. Flavonoid Glycosides as Floral Origin Markers to Discriminate of Unifloral Bee Pollen by LC–MS/MS. Food Control 2015, 57, 54–61. 10.1016/j.foodcont.2015.03.035. [DOI] [Google Scholar]
- Li Y.; Qi Y.; Ritho J.; Zhang Y.; Zheng X.; Zhou J.; Sun L. Characterization of Flavonoid Glycosides from Rapeseed Bee Pollen Using a Combination of Chromatography, Spectrometry and Nuclear Magnetic Resonance with a Step-Wise Separation Strategy. Nat. Prod. Res. 2016, 30, 228–231. 10.1080/14786419.2015.1041942. [DOI] [PubMed] [Google Scholar]
- Soares de Arruda V. A.; Vieria dos Santos A.; Figueiredo Sampaio D.; da Silva Araújo E.; de Castro Peixoto A. L.; Estevinho L. M.; de Almeida-Muradian L. B. Brazilian Bee Pollen: Phenolic Content, Antioxidant Properties and Antimicrobial Activity. J. Apic. Res. 2021, 60, 775–783. 10.1080/00218839.2020.1840854. [DOI] [Google Scholar]
- Shen Z.; Geng Q.; Huang H.; Yao H.; Du T.; Chen L.; Wu Z.; Miao X.; Shi P. Antioxidative and Cardioprotective Effects of Schisandra Chinensis Bee Pollen Extract on Isoprenaline-Induced Myocardial Infarction in Rats. Molecules 2019, 24, 1090. 10.3390/molecules24061090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyarzún J. E.; Andia M. E.; Uribe S.; Núñez Pizarro P.; Núñez G.; Montenegro G.; Bridi R. Honeybee Pollen Extracts Reduce Oxidative Stress and Steatosis in Hepatic Cells. Molecules 2021, 26, 6. 10.3390/molecules26010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saisavoey T.; Sangtanoo P.; Chanchao C.; Reamtong O.; Karnchanatat A. Identification of Novel Anti-Inflammatory Peptides from Bee Pollen (Apis Mellifera) Hydrolysate in Lipopolysaccharide-Stimulated RAW264.7 Macrophages. J. Apic. Res. 2021, 60, 280–289. 10.1080/00218839.2020.1745434. [DOI] [Google Scholar]
- Li Q.; Sun M.; Wan Z.; Liang J.; Betti M.; Hrynets Y.; Xue X.; Wu L.; Wang K. Bee Pollen Extracts Modulate Serum Metabolism in Lipopolysaccharide-Induced Acute Lung Injury Mice with Anti-Inflammatory Effects. J. Agric. Food Chem. 2019, 67, 7855–7868. 10.1021/acs.jafc.9b03082. [DOI] [PubMed] [Google Scholar]
- Komosinska-Vassev K.; Olczyk P.; Kaźmierczak J.; Mencner L.; Olczyk K. Bee Pollen: Chemical Composition and Therapeutic Application. Evidence-Based Complement. Altern. Med. 2015, 2015, 297425. 10.1155/2015/297425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos M. G. R.; Frigerio C.; Lopes J.; Bogdanov S. What Is the Future of Bee-Pollen?. J. ApiProduct ApiMedical Sci. 2010, 2 (4), 131–144. 10.3896/IBRA.4.02.4.01. [DOI] [Google Scholar]
- Pascoal A.; Rodrigues S.; Teixeira A.; Feás X.; Estevinho L. M. Biological Activities of Commercial Bee Pollens: Antimicrobial, Antimutagenic, Antioxidant and Anti-Inflammatory. Food Chem. Toxicol. 2014, 63, 233–239. 10.1016/j.fct.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Medeiros K. C. P.; Figueiredo C. A. V.; Figueredo T. B.; Freire K. R. L.; Santos F. A. R.; Alcantara-Neves N. M.; Silva T. M. S.; Piuvezam M. R. Anti-Allergic Effect of Bee Pollen Phenolic Extract and Myricetin in Ovalbumin-Sensitized Mice. J. Ethnopharmacol. 2008, 119, 41–46. 10.1016/j.jep.2008.05.036. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y.; Tokura T.; Ushio H.; Niyonsaba F.; Yamamoto Y.; Tadokoro T.; Ogawa H.; Okumura K. Lipid-Soluble Components of Honeybee-Collected Pollen Exert Antiallergic Effect by Inhibiting IgE-Mediated Mast Cell Activation in Vivo. Phytother. Res. 2009, 23, 1581–1586. 10.1002/ptr.2824. [DOI] [PubMed] [Google Scholar]
- Didaras N. A.; Karatasou K.; Dimitriou T. G.; Amoutzias G. D.; Mossialos D. Antimicrobial Activity of Bee-Collected Pollen and Beebread: State of the Art and Future Perspectives. Antibiotics 2020, 9, 811. 10.3390/antibiotics9110811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacániová M.; Vukovic N.; Chlebo R.; Hascík P.; Rovná K.; Cubon J.; Dzugan M.; Pasternakiewicz A. The Antimicrobial Activity of Honey, Bee Pollen Loads and Beeswax from Slovakia. Arch. Biol. Sci. 2012, 64, 927–934. 10.2298/ABS1203927K. [DOI] [Google Scholar]
- Kyselka J.; Bleha R.; Dragoun M.; Bialasová K.; Horáčková Š.; Schätz M.; Sluková M.; Filip V.; Synytsya A. Antifungal Polyamides of Hydroxycinnamic Acids from Sunflower Bee Pollen. J. Agric. Food Chem. 2018, 66, 11018–11026. 10.1021/acs.jafc.8b03976. [DOI] [PubMed] [Google Scholar]
- Garcia-Villanova R. J.; Cordón C.; González Paramás A. M.; Aparicio P.; Garcia Rosales M. E. Simultaneous Immunoaffinity Column Cleanup and HPLC Analysis of Aflatoxins and Ochratoxin A in Spanish Bee Pollen. J. Agric. Food Chem. 2004, 52, 7235–7239. 10.1021/jf048882z. [DOI] [PubMed] [Google Scholar]
- Khalifa S. A. M.; Elashal M.; Kieliszek M.; Ghazala N. E.; Farag M. A.; Saeed A.; Xiao J.; Zou X.; Khatib A.; Göransson U.; El-Seedi H. R. Recent Insights into Chemical and Pharmacological Studies of Bee Bread. Trends Food Sci. Technol. 2020, 97, 300–316. 10.1016/j.tifs.2019.08.021. [DOI] [Google Scholar]
- Mohammad S. M.; Mahmud-Ab-Rashid N.-K.; Zawawi N. Botanical Origin and Nutritional Values of Bee Bread of Stingless Bee (Heterotrigona itama) from Malaysia. J. Food Qual. 2020, 2020, 1–12. 10.1155/2020/2845757. [DOI] [Google Scholar]
- Zuluaga C. M.; Serrato J. C.; Quicazan M. C. Chemical, Nutritional and Bioactive Characterization of Colombian Bee-Bread. Chem. Eng. Trans. 2015, 43, 175–180. 10.3303/CET1543030. [DOI] [Google Scholar]
- Conti I.; Medrzycki P.; Argenti C.; Meloni M.; Vecchione V.; Boi M.; Mariotti M. G. Sugar and Protein Content in Different Monofloral Pollens—Building a Database. Bull. Insectol. 2016, 69, 318–320. [Google Scholar]
- Bayram N. E.; Gercek Y. C.; Çelik S.; Mayda N.; Kostić A. Ž.; Dramićanin A. M.; Özkök A. Phenolic and Free Amino Acid Profiles of Bee Bread and Bee Pollen with the Same Botanical Origin – Similarities and Differences. Arabian J. Chem. 2021, 14, 103004. 10.1016/j.arabjc.2021.103004. [DOI] [Google Scholar]
- Bakour M.; Fernandes Â.; Barros L.; Sokovic M.; Ferreira I. C. F. R.; Lyoussi B. Bee Bread as a Functional Product: Chemical Composition and Bioactive Properties. LWT-Food Sci. Technol. 2019, 109, 276–282. 10.1016/j.lwt.2019.02.008. [DOI] [Google Scholar]
- Kaplan M.; Karaoglu Ö.; Eroglu N.; Silici S. Fatty Acids and Proximate Composition of Beebread. Food Technol. Biotechnol. 2016, 54, 497–504. 10.17113/ftb.54.04.16.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranca F.; Ursachi F.; Oroian M. Bee Bread: Physicochemical Characterization and Phenolic Content Extraction Optimization. Foods 2020, 9, 1358. 10.3390/foods9101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vásquez A.; Olofsson T. C. The Lactic Acid Bacteria Involved in the Production of Bee Pollen and Bee Bread. J. Apic. Res. 2009, 48, 189–195. 10.3896/IBRA.1.48.3.07. [DOI] [Google Scholar]
- Urcan A. C.; Marghitas L. Al; Dezmirean D. S.; Bobis O.; Bonta V.; Muresan C. I.; Margaoan R. Chemical Composition and Biological Activities of Beebread—Review. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca, Anim. Sci. Biotechnol. 2017, 74, 6. 10.15835/buasvmcn-asb:12646. [DOI] [Google Scholar]
- Pełka K.; Otłowska O.; Worobo R. W.; Szweda P. Bee Bread Exhibits Higher Antimicrobial Potential Compared to Bee Pollen. Antibiotics 2021, 10, 125. 10.3390/antibiotics10020125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewicz-Żukowska R.; Naliwajko S. K.; Bartosiuk E.; Moskwa J.; Isidorov V.; Soroczyńska J.; Borawska M. H. Chemical Composition and Antioxidant Activity of Beebread, and Its Influence on the Glioblastoma Cell Line (U87MG). J. Apic. Sci. 2013, 57, 147–157. 10.2478/jas-2013-0025. [DOI] [Google Scholar]
- Xue X.; Wu L.; Wang K.. Chemical Composition of Royal Jelly. In Bee Products—Chemical and Biological Properties; Springer International Publishing: Cham, Switzerland, 2017; pp 181–190. [Google Scholar]
- Boselli E.; Caboni M. F.; Sabatini A. G.; Marcazzan G. L.; Lercker G. Determination and Changes of Free Amino Acids in Royal Jelly during Storage. Apidologie 2003, 34, 129–137. 10.1051/apido:2003011. [DOI] [Google Scholar]
- Ferioli F.; Armaforte E.; Caboni M. F. Comparison of the Lipid Content, Fatty Acid Profile and Sterol Composition in Local Italian and Commercial Royal Jelly Samples. J. Am. Oil Chem. Soc. 2014, 91, 875–884. 10.1007/s11746-014-2446-x. [DOI] [Google Scholar]
- Wang Y.; Ma L.; Zhang W.; Cui X.; Wang H.; Xu B. Comparison of the Nutrient Composition of Royal Jelly and Worker Jelly of Honey Bees (Apis mellifera). Apidologie 2016, 47, 48–56. 10.1007/s13592-015-0374-x. [DOI] [Google Scholar]
- Ramadan M. F.; Al-Ghamdi A. Bioactive Compounds and Health-Promoting Properties of Royal Jelly: A Review. J. Funct. Foods 2012, 4, 39–52. 10.1016/j.jff.2011.12.007. [DOI] [Google Scholar]
- Nabas Z.; Haddadin M.; Haddadin J.; Nazer I. Chemical Composition of Royal Jelly and Effects of Synbiotic with Two Different Locally Isolated Probiotic Strains on Antioxidant Activities. Pol. J. Food Nutr. Sci. 2014, 64, 171–180. 10.2478/pjfns-2013-0015. [DOI] [Google Scholar]
- Muresan C. I.; Mǎrghitaş L. A.; Dezmirean D. S.; Bobiş O.; Bonta V.; Zacharias I.; Mǎrgǎoan R.; Paşca C. Quality Parameters for Commercial Royal Jelly. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca, Anim. Sci. Biotechnol. 2016, 73, 1–8. 10.15835/buasvmcn-asb:11630. [DOI] [Google Scholar]
- Balkanska R.; Karadjova I.; Ignatova M. Comparative Analyses of Chemical Composition of Royal Jelly and Drone Brood. Bulg. Chem. Commun. 2014, 46, 412–416. [Google Scholar]
- Kunugi H.; Ali A. M. Royal Jelly and Its Components Promote Healthy Aging and Longevity: From Animal Models to Humans. Int. J. Mol. Sci. 2019, 20, 4662. 10.3390/ijms20194662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wytrychowski M.; Chenavas S.; Daniele G.; Casabianca H.; Batteau M.; Guibert S.; Brion B. Physicochemical Characterisation of French Royal Jelly: Comparison with Commercial Royal Jellies and Royal Jellies Produced through Artificial Bee-Feeding. J. Food Compos. Anal. 2013, 29, 126–133. 10.1016/j.jfca.2012.12.002. [DOI] [Google Scholar]
- Bǎrnuţiu L. I.; Mǎrghitaş L. A.; Dezmirean D. S.; Mihai C. M.; Bobiş O. Chemical Composition and Antimicrobial Activity of Royal Jelly—Review. Sci. Pap. Anim. Sci. Biotechnol. 2011, 44, 67–72. [Google Scholar]
- Drapeau M. D.; Albert S.; Kucharski R.; Prusko C.; Maleszka R. Evolution of the Yellow/Major Royal Jelly Protein Family and the Emergence of Social Behavior in Honey Bees. Genome Res. 2006, 16, 1385–1394. 10.1101/gr.5012006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki R.; Tamura S.; Ito A.; Moriyama T.; Yamaguchi K.; Kono T. A Rapid Method to Isolate Soluble Royal Jelly Proteins. Food Chem. 2012, 134, 2332–2337. 10.1016/j.foodchem.2012.03.106. [DOI] [PubMed] [Google Scholar]
- Stanoeva J. P.; Stefova M.; Andonovska K. B.; Stafilov T. LC/DAD/MSn and ICP-AES Assay and Correlations between Phenolic Compounds and Toxic Metals in Endemic Thymus alsarensis from the Thallium Enriched Allchar Locality. Nat. Prod. Commun. 2017, 12, 1934578X1701200. 10.1177/1934578X1701200206. [DOI] [PubMed] [Google Scholar]
- Bílikova K.; Huang S.-C.; Lin I.-P.; Šimuth J.; Peng C.-C. Structure and Antimicrobial Activity Relationship of Royalisin, an Antimicrobial Peptide from Royal Jelly of Apis mellifera. Peptides 2015, 68, 190–196. 10.1016/j.peptides.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Liming W.; Jinhui Z.; Xiaofeng X.; Yi L.; Jing Z. Fast Determination of 26 Amino Acids and Their Content Changes in Royal Jelly during Storage Using Ultra-Performance Liquid Chromatography. J. Food Compos. Anal. 2009, 22, 242–249. 10.1016/j.jfca.2008.10.022. [DOI] [Google Scholar]
- Noda N.; Umebayashi K.; Nakatani T.; Miyahara K.; Ishiyama K. Isolation and Characterization of Some Hydroxy Fatty and Phosphoric Acid Esters of 10-Hydroxy-2-decenoic Acid from the Royal Jelly of Honeybees (Apis mellifera). Lipids 2005, 40, 833–838. 10.1007/s11745-005-1445-6. [DOI] [PubMed] [Google Scholar]
- Sabatini A. G. Quality and Standardisation of Royal Jelly. J. ApiProduct ApiMedical Sci. 2009, 1, 16–21. 10.3896/IBRA.4.01.1.04. [DOI] [Google Scholar]
- Terada Y.; Narukawa M.; Watanabe T. Specific Hydroxy Fatty Acids in Royal Jelly Activate TRPA1. J. Agric. Food Chem. 2011, 59, 2627–2635. 10.1021/jf1041646. [DOI] [PubMed] [Google Scholar]
- Ciulu M.; Floris I.; Nurchi V. M.; Panzanelli A.; Pilo M. I.; Spano N.; Sanna G. HPLC Determination of Pantothenic Acid in Royal Jelly. Anal. Methods 2013, 5, 6682. 10.1039/c3ay41284a. [DOI] [Google Scholar]
- Isidorov V. A.; Bakier S.; Grzech I. Gas Chromatographic–Mass Spectrometric Investigation of Volatile and Extractable Compounds of Crude Royal Jelly. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2012, 885–886, 109–116. 10.1016/j.jchromb.2011.12.025. [DOI] [PubMed] [Google Scholar]
- López-Gutiérrez N.; Aguilera-Luiz M. M.; Romero-González R.; Vidal J. L. M.; Garrido Frenich A. Fast Analysis of Polyphenols in Royal Jelly Products Using Automated TurboFlowTM-Liquid Chromatography–Orbitrap High Resolution Mass Spectrometry. J. Chromatogr. B 2014, 973, 17–28. 10.1016/j.jchromb.2014.09.038. [DOI] [PubMed] [Google Scholar]
- El-Guendouz S.; Machado A. M.; Aazza S.; Lyoussi B.; Miguel M. G.; Mateus M. C.; Figueiredo A. C. Chemical Characterization and Biological Properties of Royal Jelly Samples From the Mediterranean Area. Nat. Prod. Commun. 2020, 15, 1934578X2090808. 10.1177/1934578X20908080. [DOI] [Google Scholar]
- Zhao Y.; Li Z.; Tian W.; Fang X.; Su S.; Peng W. Differential Volatile Organic Compounds in Royal Jelly Associated with Different Nectar Plants. J. Integr. Agric. 2016, 15, 1157–1165. 10.1016/S2095-3119(15)61274-6. [DOI] [Google Scholar]
- Miguel M. G.; El-Guendouz S.. Volatile Compounds of Royal Jelly. In Bee Products—Chemical and Biological Properties; Springer International Publishing: Cham, Switzerland, 2017; pp 191–197. [Google Scholar]
- Viuda-Martos M.; Pérez-Alvarez J. A.; Fernández-López J.. Royal Jelly: Health Benefits and Uses in Medicine. In Bee Products—Chemical and Biological Properties; Springer International Publishing: Cham, Switzerland, 2017; pp 199–218. [Google Scholar]
- Dzopalic T.; Vucevic D.; Tomic S.; Djokic J.; Chinou I.; Colic M. 3,10-Dihydroxy-Decanoic Acid, Isolated from Royal Jelly, Stimulates Th1 Polarising Capability of Human Monocyte-Derived Dendritic Cells. Food Chem. 2011, 126, 1211–1217. 10.1016/j.foodchem.2010.12.004. [DOI] [Google Scholar]
- Vucevic D.; Melliou E.; Vasilijic S.; Gasic S.; Ivanovski P.; Chinou I.; Colic M. Fatty Acids Isolated from Royal Jelly Modulate Dendritic Cell-Mediated Immune Response in Vitro. Int. Immunopharmacol. 2007, 7, 1211–1220. 10.1016/j.intimp.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Tokunaga K.; Yoshida C.; Suzuki K.; Maruyama H.; Futamura Y.; Araki Y.; Mishima S. Antihypertensive Effect of Peptides from Royal Jelly in Spontaneously Hypertensive Rats. Biol. Pharm. Bull. 2004, 27, 189–192. 10.1248/bpb.27.189. [DOI] [PubMed] [Google Scholar]
- Guo H.; Saiga A.; Sato M.; Miyazawa I.; Shibata M.; Takahata Y.; Morimatsu F. Royal Jelly Supplementation Improves Lipoprotein Metabolism in Humans. J. Nutr. Sci. Vitaminol. 2007, 53, 345–348. 10.3177/jnsv.53.345. [DOI] [PubMed] [Google Scholar]
- Fan P.; Han B.; Feng M.; Fang Y.; Zhang L.; Hu H.; Hao Y.; Qi Y.; Zhang X.; Li J. Functional and Proteomic Investigations Reveal Major Royal Jelly Protein 1 Associated with Anti-Hypertension Activity in Mouse Vascular Smooth Muscle Cells. Sci. Rep. 2016, 6, 30230. 10.1038/srep30230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azab K.; Bashandy M.; Salem M.; Ahmed O.; Tawfik Z.; Helal H. Royal Jelly Modulates Oxidative Stress and Tissue Injury in Gamma Irradiated Male Wister Albino Rats. N. Am. J. Med. Sci. 2011, 268–276. 10.4297/najms.2011.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrinoudaki I.; Augoulea A.; Rizos D.; Politi M.; Tsoltos N.; Moros M.; Chinou I.; Graikou K.; Kouskouni E.; Kambani S.; Panoulis K.; Moutsatsou P. Greek-Origin Royal Jelly Improves the Lipid Profile of Postmenopausal Women. Gynecol. Endocrinol. 2016, 32, 835–839. 10.1080/09513590.2016.1188281. [DOI] [PubMed] [Google Scholar]
- Bincoletto C.; Eberlin S.; Figueiredo C. A. V.; Luengo M. B.; Queiroz M. L. S. Effects Produced by Royal Jelly on Haematopoiesis: Relation with Host Resistance against Ehrlich Ascites Tumour Challenge. Int. Immunopharmacol. 2005, 5, 679–688. 10.1016/j.intimp.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y.; Kohno K.; Inoue S.; Koya-Miyata S.; Okamoto I.; Arai N.; Iwaki K.; Ikeda M.; Kurimoto M. Oral Administration of Royal Jelly Inhibits the Development of Atopic Dermatitis-like Skin Lesions in NC/Nga Mice. Int. Immunopharmacol. 2003, 3, 1313–1324. 10.1016/S1567-5769(03)00132-2. [DOI] [PubMed] [Google Scholar]
- Nakaya M.; Onda H.; Sasaki K.; Yukiyoshi A.; Tachibana H.; Yamada K. Effect of Royal Jelly on Bisphenol A-Induced Proliferation of Human Breast Cancer Cells. Biosci., Biotechnol., Biochem. 2007, 71, 253–255. 10.1271/bbb.60453. [DOI] [PubMed] [Google Scholar]
- Çavuşoǧlu K.; Yapar K.; Yalçin E. Royal Jelly (Honey Bee) Is a Potential Antioxidant Against Cadmium-Induced Genotoxicity and Oxidative Stress in Albino Mice. J. Med. Food 2009, 12, 1286–1292. 10.1089/jmf.2008.0203. [DOI] [PubMed] [Google Scholar]
- Han S. M.; Yeo J. H.; Cho Y. H.; Pak S. C. Royal Jelly Reduces Melanin Synthesis Through Down-Regulation of Tyrosinase Expression. Am. J. Chin. Med. 2011, 39, 1253–1260. 10.1142/S0192415X11009536. [DOI] [PubMed] [Google Scholar]
- Han S. M.; Kim J. M.; Hong I. P.; Woo S. O.; Kim S. G.; Jang H. R.; Park K. K.; Pak S. C. Whitening Effect of Watersoluble Royal Jelly from South Korea. Korean J. Food Sci. Anim. Resour. 2015, 35, 707–713. 10.5851/kosfa.2015.35.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C.-C.; Sun H.-T.; Lin I.-P.; Kuo P.-C.; Li J.-C. The Functional Property of Royal Jelly 10-Hydroxy-2-Decenoic Acid as a Melanogenesis Inhibitor. BMC Complement. Altern. Med. 2017, 17, 392. 10.1186/s12906-017-1888-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.; Zhang C.-P.; Wang K.; Li G.; Hu F.-L. Recent Advances in the Chemical Composition of Propolis. Molecules 2014, 19, 19610–19632. 10.3390/molecules191219610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuropatnicki A. K.; Szliszka E.; Krol W. Historical Aspects of Propolis Research in Modern Times. J. Evidence-Based Complement. Altern. Med. 2013, 2013, 1–11. 10.1155/2013/964149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagh V. D. Propolis: A Wonder Bees Product and Its Pharmacological Potentials. Adv. Pharmacol. Sci. 2013, 2013, 1–11. 10.1155/2013/308249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava P.; Mahanta D.; Kaul A.; Ishida Y.; Terao K.; Wadhwa R.; Kaul S. C. Experimental Evidence for Therapeutic Potentials of Propolis. Nutrients 2021, 13, 2528. 10.3390/nu13082528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurek-Górecka A.; Rzepecka-Stojko A.; Górecki M.; Stojko J.; Sosada M.; Świerczek-Zięba G. Structure and Antioxidant Activity of Polyphenols Derived from Propolis. Molecules 2014, 19, 78–101. 10.3390/molecules19010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonamigo T.; Campos J. F.; Alfredo T. M.; Balestieri J. B. P.; Cardoso C. A. L.; Paredes-Gamero E. J.; de Picoli Souza K.; dos Santos E. L. Antioxidant, Cytotoxic, and Toxic Activities of Propolis from Two Native Bees in Brazil: Scaptotrigona Depilis and Melipona Quadrifasciata Anthidioides. Oxid. Med. Cell. Longevity 2017, 2017, 1–12. 10.1155/2017/1038153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargouri W.; Osés S. M.; Fernández-Muiño M. A.; Sancho M. T.; Kechaou N. Evaluation of Bioactive Compounds and Biological Activities of Tunisian Propolis. LWT- Food Sci. Technol. 2019, 111, 328–336. 10.1016/j.lwt.2019.05.044. [DOI] [Google Scholar]
- Osés S. M.; Marcos P.; Azofra P.; de Pablo A.; Fernández-Muíño M. Á.; Sancho M. T. Phenolic Profile, Antioxidant Capacities and Enzymatic Inhibitory Activities of Propolis from Different Geographical Areas: Needs for Analytical Harmonization. Antioxidants 2020, 9, 75. 10.3390/antiox9010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Buelga C.; González-Paramás A. M.. Phenolic Composition of Propolis. In Bee Products—Chemical and Biological Properties; Springer International Publishing: Cham, Switzerland, 2017; pp 99–111. [Google Scholar]
- Wang X.; Sankarapandian K.; Cheng Y.; Woo S. O.; Kwon H. W.; Perumalsamy H.; Ahn Y.-J. Relationship between Total Phenolic Contents and Biological Properties of Propolis from 20 Different Regions in South Korea. BMC Complement. Altern. Med. 2016, 16, 65. 10.1186/s12906-016-1043-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardana C.; Scaglianti M.; Pietta P.; Simonetti P. Analysis of the Polyphenolic Fraction of Propolis from Different Sources by Liquid Chromatography–Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2007, 45, 390–399. 10.1016/j.jpba.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Li F.; Awale S.; Tezuka Y.; Kadota S. Cytotoxic Constituents from Brazilian Red Propolis and Their Structure–Activity Relationship. Bioorg. Med. Chem. 2008, 16, 5434–5440. 10.1016/j.bmc.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Bankova V. S.; Christov R.; Tejera A. D. Lignans and Other Constituents of Propolis from the Canary Islands. Phytochemistry 1998, 49, 1411–1415. 10.1016/S0031-9422(98)00108-3. [DOI] [Google Scholar]
- Inui S.; Shimamura Y.; Masuda S.; Shirafuji K.; Moli R. T.; Kumazawa S. A New Prenylflavonoid Isolated from Propolis Collected in the Solomon Islands. Biosci., Biotechnol., Biochem. 2012, 76, 1038–1040. 10.1271/bbb.120021. [DOI] [PubMed] [Google Scholar]
- Trusheva B.; Todorov I.; Ninova M.; Najdenski H.; Daneshmand A.; Bankova V. Antibacterial Mono- and Sesquiterpene Esters of Benzoic Acids from Iranian Propolis. Chem. Cent. J. 2010, 4, 8. 10.1186/1752-153X-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel M. G.; Figueiredo A. C.. Propolis and Geopropolis Volatiles. In Bee Products—Chemical and Biological Properties; Springer International Publishing: Cham, Switzerland, 2017; pp 113–136. [Google Scholar]
- Siheri W.; Alenezi S.; Tusiimire J.; Watson D. G.. The Chemical and Biological Properties of Propolis. In Bee Products—Chemical and Biological Properties; Springer International Publishing: Cham, Switzerland, 2017; pp 137–178. [Google Scholar]
- Girgin G.; Baydar T.; Ledochowski M.; Schennach H.; Bolukbasi D. N.; Sorkun K.; Salih B.; Sahin G.; Fuchs D. Immunomodulatory Effects of Turkish Propolis: Changes in Neopterin Release and Tryptophan Degradation. Immunobiology 2009, 214, 129–134. 10.1016/j.imbio.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Liberio S. A.; Pereira A. L. A.; Dutra R. P.; Reis A. S.; Araújo M. J. A.; Mattar N. S.; Silva L. A.; Ribeiro M. N. S.; Nascimento F. R. F.; Guerra R. N.; Monteiro-Neto V. Antimicrobial Activity against Oral Pathogens and Immunomodulatory Effects and Toxicity of Geopropolis Produced by the Stingless Bee Melipona fasciculata Smith. BMC Complement. Altern. Med. 2011, 11, 108. 10.1186/1472-6882-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsatti C. L.; Sforcin J. M. Propolis Immunomodulatory Activity on TLR-2 and TLR-4 Expression by Chronically Stressed Mice. Nat. Prod. Res. 2012, 26, 446–453. 10.1080/14786419.2010.482049. [DOI] [PubMed] [Google Scholar]
- Orsatti C. L.; Missima F.; Pagliarone A. C.; Bachiega T. F.; Búfalo M. C.; Araújo J. P.; Sforcin J. M. Propolis Immunomodulatory Action in Vivo on Toll-like Receptors 2 and 4 Expression and on pro-Inflammatory Cytokines Production in Mice. Phytother. Res. 2010, 24, 1141–1146. 10.1002/ptr.3086. [DOI] [PubMed] [Google Scholar]
- Bogdanov S. Beeswax: Quality Issues Today. Bee World 2004, 85, 46–50. 10.1080/0005772X.2004.11099623. [DOI] [Google Scholar]
- Beeswax (E 901) as a Glazing Agent and as Carrier for Flavours—Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC). EFSA J. 2007, 5, 615. 10.2903/j.efsa.2007.615. [DOI] [Google Scholar]
- Tulloch A. P. Beeswax—Composition and Analysis. Bee World 1980, 61, 47–62. 10.1080/0005772X.1980.11097776. [DOI] [Google Scholar]
- Ferber C. E. M.; Nursten H. E. The Aroma of Beeswax. J. Sci. Food Agric. 1977, 28, 511–518. 10.1002/jsfa.2740280608. [DOI] [Google Scholar]
- Coppock R. W.Bee Products as Nutraceuticals to Nutraceuticals for Bees. In Nutraceuticals: Efficacy, Safety and Toxicity, 2nd ed.; Elsevier: Amsterdam, Netherlands, 2021; Chapter 47, pp 813–833. [Google Scholar]
- Formiga A. S.; Pinsetta J. S.; Pereira E. M.; Cordeiro I. N. F.; Mattiuz B.-H. Use of Edible Coatings Based on Hydroxypropyl Methylcellulose and Beeswax in the Conservation of Red Guava ‘Pedro Sato.’. Food Chem. 2019, 290, 144–151. 10.1016/j.foodchem.2019.03.142. [DOI] [PubMed] [Google Scholar]
- Fratini F.; Cilia G.; Turchi B.; Felicioli A. Beeswax: A Minireview of Its Antimicrobial Activity and Its Application in Medicine. Asian Pac. J. Trop. Med. 2016, 9, 839–843. 10.1016/j.apjtm.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Jiménez J. J.; Bernal J. L.; del Nozal M. J.; Martín M. T.; Bernal J. Sample Preparation Methods for Beeswax Characterization by Gas Chromatography with Flame Ionization Detection. J. Chromatogr. A 2006, 1129, 262–272. 10.1016/j.chroma.2006.06.098. [DOI] [PubMed] [Google Scholar]
- Navarro-Hortal M. D.; Orantes-Bermejo F. J.; Sánchez-González C.; Varela-López A.; Giampieri F.; Torres Fernández-Piñar C.; Serra-Bonvehí J.; Forbes-Hernández T. Y.; Reboredo-Rodríguez P.; Llopis J.; Aranda P.; Battino M.; Quiles J. L. Industrial-Scale Decontamination Procedure Effects on the Content of Acaricides, Heavy Metals and Antioxidant Capacity of Beeswax. Molecules 2019, 24, 1518. 10.3390/molecules24081518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.