Abstract
Megalencephaly-capillary malformation-polymicrogyria syndrome (MCAP) is an uncommon malformation syndrome, characterized by primary megalencephaly, capillary malformations of the midline face and body, or distal limb anomalies such as syndactyly and polymicrogyria. Herein, we report a young male child, who presented with complaints of increasing head size, delay in speech, and one episode of focal seizure with distinctive morphological and neuroradiological manifestations which led to the diagnosis of MCAP. We have also reviewed recently published literature and the various diagnostic criteria proposed by authors to achieve the early clinical diagnosis of these patients in the outpatient department.
Keywords: phosphatidylinositol 3-kinase (pi3k)-akt pathway, megalencephaly, brain vascular malformation, mcap syndrome, megalencephaly-capillary malformation-polymicrogyria syndrome
Introduction
Megalencephaly-capillary malformation-polymicrogyria syndrome (MCAP) is an uncommon genetic syndrome characterized by primary megalencephaly, cutaneous vascular malformations, polymicrogyria, and other anomalies [1]. This condition was first described in 1997 as macrocephaly-cutis marmorata telangiectasia congenita (M-CMTC) by Clayton-Smith et al. [2] and Moore et al. [3]. After diagnosis in 1997, around 300 cases have been reported in the literature [4]. In 2007, this condition was renamed macrocephaly-capillary malformation syndrome (MCM) by Toriello and Mulliken et al. [5] and Conway et al. [6] Finally, in 2012 Mirzaa et al. renamed MCM to megalencephaly-capillary malformation-polymicrogyria syndrome (MCAP) to reflect the abnormally large size of the brain and to highlight the importance of perisylvian polymicrogyria [1]. Current studies have found its genetic cause to be linked with the PI3K-AKT pathway [7]. Herein, we describe a case, that presented to us with both intracranial and cutaneous manifestations of MCAP. Clinicians should have a high degree of suspicion with regular follow-up as the entity has highly dynamic clinical manifestations.
Case presentation
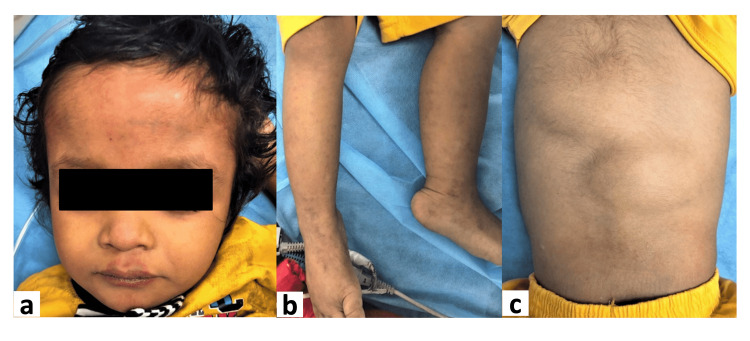
A three-and-a-half-year-old male child presented with complaints of increasing head size [>2 Standard Deviation (2SD)], delay in speech, and one episode of focal seizure at three years of age. His perinatal history was uneventful. There was no family history of similar illnesses in the family. Examination revealed macrocephaly with frontal bossing (Figure 1a) with numerous cutaneous capillary malformations on the face and bilateral lower limbs (Figures 1a, 1b). Multiple thick doughy subcutaneous tissues were also present over the back (Figure 1c), which was confirmed as fibrofatty tissue on USG. No evidence of facial, body asymmetry, syndactyly, or polydactyly was found.
Figure 1. shows features seen on clinical examination of the patient.
Figure 1 shows the features found on general examination of the patient, macrocephaly with frontal bossing with cutaneous capillary malformations above the upper lip of face (1a), multiple cutaneous capillary malformations over bilateral lower limbs(1b), multiple thick doughy subcutaneous tissues over the back (1c).
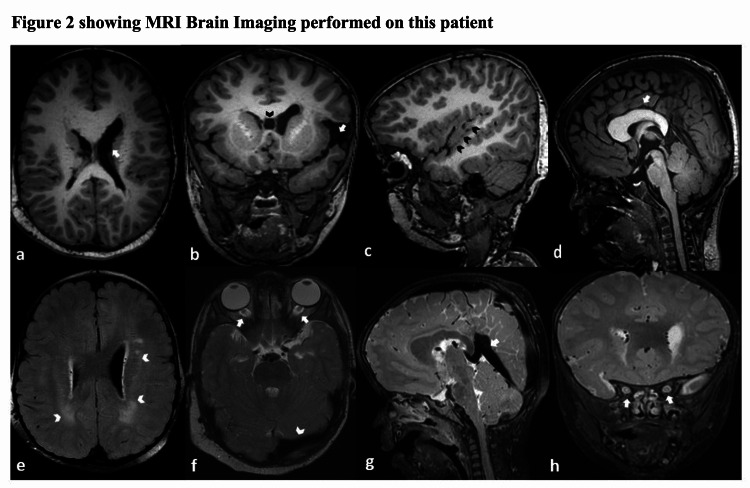
Magnetic resonance imaging (MRI) of the brain showed ventricular asymmetry with prominent left lateral ventricle (Figure 2a), left-sided incomplete opercularization with widened left sylvian fissure and cavum septum pellucidum (Figure 2b), bilateral perisylvian polymicrogyria (Figure 2c), abnormally thickened mega corpus callosum (Figure 2d), multiple foci of T2/FLAIR hyperintensities in bilateral deep and periventricular white matter (Figure 2e), prominent bilateral optic nerve sheaths (Figure 2f, 2h) and enlarged venous sinuses (Figure 2f, 2g).
Figure 2. showing MRI Brain Imaging performed on this patient.
Figure 2 shows MRI Brain imaging performed on this patient. Axial T1W image reveals ventricular asymmetry with prominent left lateral ventricle (white arrow, Fig. 2a). Coronal T1W image reveals left-sided incomplete opercularization with widened left Sylvian fissure (white arrow, Fig. 2b), cavum septum pellucidum with prominent left lateral ventricle is also seen (black arrowhead Fig. 2b).T1W parasagittal image reveals perisylvian polymicrogyria (black arrowheads, Fig. 2c). T1W mid-sagittal image reveals abnormally thickened mega corpus callosum (white arrow, Fig. 2d). Axial FLAIR image demonstrates multiple foci of abnormally increased signals in bilateral deep and periventricular white matter (white arrowheads, Fig. 2e). Axial T2W image reveals prominent bilateral optic nerve sheaths (white arrows, Fig. 2f) and enlarged left transverse sinus flow void (white arrowhead, Fig. 2f). T2W mid-sagittal image reveals an enlarged straight sinus flow void (white arrow, Fig.2g). Coronal T2W image reveals prominent bilateral optic nerve sheaths (white arrows, Fig. 2h).
Based on the clinical and neuroimaging findings, the diagnosis of a megalencephaly-capillary malformation-polymicrogyria syndrome (MCAP) was made as per the proposed criteria by Mirzaa et al. with four core features (megalencephaly, capillary malformations in midline face and body, polymicrogyria and connective tissue dysplasia) and four supportive features (mega corpus callosum, the prominent venous system, frontal bossing and developmental delay) [1]. At present, parents are unwilling to perform a genetic work-up of the patient or his siblings fearing social stigmata, despite adequate assurances of maintaining the confidentiality of the genetic profiling results at our end. The child was managed with antiepileptic medication for control of seizures and provided with rehabilitation therapy for developmental delay, as well as is presently under follow-up. At the last follow-up at eight months, speech difficulty was persistent, however, he was seizure-free on a single antiepileptic. Due to his speech difficulty, his parents have been apprehensive about sending him to school, so they have been advised to enroll him in institutions meant for specially-abled children.
Discussion
MCAP is a rare genetic syndrome characterized by a wide range of abnormalities like primary megalencephaly, cutaneous vascular malformations, prenatal overgrowth, connective tissue dysplasia, digital anomalies, body asymmetry with distinctive brain imaging features like polymicrogyria, asymmetry of the lateral ventricles, hydrocephalus, polymicrogyria, large cerebellum resulting in the crowded posterior fossa, cerebellar tonsillar herniation or ectopia, thick corpus callosum, and other features [1]. Riviere et al. [7] identified the de novo postzygotic or germline mutations in the AKT3, PIK3R2, PIK3CA genes to be associated with MCAP, and also suggested the substantial role of phosphatidylinositol 3-kinase (PI3K)-AKT pathway in the development of the brain, vasculature, and limbs. Familial MCAP is also reported, thereby advocating the germline mosaicism or autosomal recessive inheritance in parents [7].
A newer classification was proposed by Mirzaa et al, in 2012, which is commonly used for diagnosis [1]. Associated neuroimaging features reported in this disease include cerebral asymmetry, increased white matter signal, cavum septum pellucidum, ‘‘hydropic’’ appearing optic nerve sheaths, cortical dysgenesis/dysplasia, dilated perivascular spaces of the cortical veins, and venous sinus thrombosis [6]. The condition is associated with dynamic changes like - ventriculomegaly which may progress to hydrocephalus, cerebellar tonsillar ectopia, and mega corpus callosum because of which follows up with MRI is required [1]. In our case, there was the absence of supportive features like ventriculomegaly, and cerebellar tonsillar ectopia. Presently, no specific treatment for MCAP is available, but ARQ 092, an allosteric AKT inhibitor showed an antiproliferative effect on overgrowth syndromes including MCAP due to inhibition of the PI3K/AKT pathway [8]. Supportive management includes medical therapy for control of seizures and other symptoms, physiotherapy, speech, occupational and physical therapy to improve daily functioning. Surgical management includes shunt placement for hydrocephalus and posterior fossa decompression for symptomatic cerebellar tonsillar ectopia, especially with features of brainstem compression or syringomyelia [1].
Few similar MCAP cases were registered in the Johns Hopkins maintained Online Medillian Inheritance of Man (OMIM) with varied clinical and imaging manifestations. Initially, 13 unrelated cases were described in 1997 by Moore et al., with abnormalities in somatic growth, face, brain, vasculature, and connective tissue disorder and combined classified as megalencephaly-cutis marmorata telangiectasia congenita (MCMTC) [3]. Subsequently, nine additional patients were reported by Clayton-Smith et al. in 1997, [2] and Carcao et al. in 1998 where the latter supported the hypothesis that CNS and vascular dysgenesis leads to MCMTC [9]. Four additional cases were reported by Vogels et al. in 1998 [10], three cases by Yano and Watanabe et al. in 2001 [11], six patients by Lapunzina et al. in 2004, [12] seven patients by Giuliano et al. in 2004 [13], 10 patients by Garavelli et al. in 2005, [14] 17 patients by Conway et al. in 2007, [6] one patient by Canham and Holder in 2008, [15] three patients by Gripp et al. in 2009 [16]. As there were overlapping features between megalencephaly, polymicrogyria-polydactyly hydrocephalus syndrome, and capillary malformation, so Gripp et al., proposed the term megalencephaly-polydactyly-polymicrogyria-hydrocephalus capillary malformation (MPPH-CM) to this phenotypic spectrum [16]. Approximately 21 cases MPPH cases were also reported in the OMIM literature with the highest number of cases by Mirzaa et al. in 2014 [17] and there was significant phenotypic overlap and have a common genetic basis between these two by Nakamura et al. in 2014 [18].
Other simulating brain overgrowth syndrome includes megalencephaly-polydactyly-polymicrogyria-hydrocephalus (MPPH) syndrome, where megalencephaly is seen associated with distal limb anomalies like postaxial polydactyly and hydrocephalus [1]. Other diseases associated with megalencephaly and skin manifestations are congenital lipomatous overgrowth, vascular malformations, epidermal nevi (CLOVE) syndrome, and Bannayan-Riley-Ruvalcaba syndrome (BRRS) [19].
Even though imaging features like hydrocephalus and cerebellar tonsillar ectopia were absent at present in the present case, he is planned for regular follow-up with interval imaging at one year.
Conclusions
Being a rare condition, MCAP requires careful clinical evaluation and neuroimaging for its diagnosis. Moreover, clinicians should be aware of its dynamic nature and so follow-up with MRI is required for cerebellar tonsillar ectopia and brainstem compression which may be life-threatening.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Not applicable issued approval Not applicable. The nature of this report does not require an ethics approval.
References
- 1.Megalencephaly-capillary malformation (MCAP) and megalencephaly-polydactyly-polymicrogyria-hydrocephalus (MPPH) syndromes: two closely related disorders of brain overgrowth and abnormal brain and body morphogenesis. Mirzaa GM, Conway RL, Gripp KW, et al. Am J Med Genet A. 2012;158A:269–291. doi: 10.1002/ajmg.a.34402. [DOI] [PubMed] [Google Scholar]
- 2.Macrocephaly with cutis marmorata, haemangioma and syndactyly--a distinctive overgrowth syndrome. Clayton-Smith J, Kerr B, Brunner H, et al. Clin Dysmorphol. 1997;6:291–302. doi: 10.1097/00019605-199710000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Macrocephaly-cutis marmorata telangiectatica congenita: a distinct disorder with developmental delay and connective tissue abnormalities. Moore CA, Toriello HV, Abuelo DN, et al. https://pubmed.ncbi.nlm.nih.gov/9129744/ Am J Med Genet. 1997;70:67–73. [PubMed] [Google Scholar]
- 4.Syndrome description: overview. [ Apr; 2022 ];https://www.m-cm.net/description/overview. 2022
- 5.Accurately renaming macrocephaly-cutis marmorata telangiectatica congenita (M-CMTC) as macrocephaly-capillary malformation (M-CM) Toriello HV, Mulliken JB. Am J Med Genet A. 2007;143A:3009. doi: 10.1002/ajmg.a.31971. [DOI] [PubMed] [Google Scholar]
- 6.Neuroimaging findings in macrocephaly-capillary malformation: a longitudinal study of 17 patients. Conway RL, Pressman BD, Dobyns WB, et al. Am J Med Genet A. 2007;143A:2981–3008. doi: 10.1002/ajmg.a.32040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Rivière JB, Mirzaa GM, O'Roak BJ, et al. Nat Genet. 2012;44:934–940. doi: 10.1038/ng.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.In vitro efficacy of ARQ 092, an allosteric AKT inhibitor, on primary fibroblast cells derived from patients with PIK3CA-related overgrowth spectrum (PROS) Ranieri C, Di Tommaso S, Loconte DC, et al. Neurogenetics. 2018;19:77–91. doi: 10.1007/s10048-018-0540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MRI findings in macrocephaly-cutis marmorata telangiectatica congenita. Carcao M, Blaser SI, Grant RM, Weksberg R, Siegel-Bartelt J. https://pubmed.ncbi.nlm.nih.gov/9511980/ Am J Med Genet. 1998;76:165–167. [PubMed] [Google Scholar]
- 10.The macrocephaly-cutis marmorata telangiectatica congenita syndrome. Long-term follow-up data in 4 children and adolescents. Vogels A, Devriendt K, Legius E, Decock P, Marien J, Hendrickx G, Fryns JP. https://pubmed.ncbi.nlm.nih.gov/9894160/ Genet Couns. 1998;9:245–253. [PubMed] [Google Scholar]
- 11.Association of arrhythmia and sudden death in macrocephaly-cutis marmorata telangiectatica congenita syndrome. Yano S, Watanabe Y. Am J Med Genet. 2001;102:149–152. doi: 10.1002/ajmg.1428. [DOI] [PubMed] [Google Scholar]
- 12.Macrocephaly-cutis marmorata telangiectatica congenita: report of six new patients and a review. Lapunzina P, Gairí A, Delicado A, et al. Am J Med Genet A. 2004;130A:45–51. doi: 10.1002/ajmg.a.30235. [DOI] [PubMed] [Google Scholar]
- 13.Macrocephaly-cutis marmorata telangiectatica congenita: seven cases including two with unusual cerebral manifestations. Giuliano F, David A, Edery P, Sigaudy S, Bonneau D, Cormier-Daire V, Philip N. Am J Med Genet A. 2004;126A:99–103. doi: 10.1002/ajmg.a.20551. [DOI] [PubMed] [Google Scholar]
- 14.MRI and neurological findings in macrocephaly-cutis marmorata telangiectatica congenita syndrome: report of ten cases and review of the literature. Garavelli L, Leask K, Zanacca C, et al. https://europepmc.org/article/med/16080291. Genet Couns Geneva Switz. 2005;16:117–128. [PubMed] [Google Scholar]
- 15.Macrocephaly-cutis marmorata telangiectatica congenita: a report on the natural history of a mild case. Canham NL, Holder SE. Clin Dysmorphol. 2008;17:279–281. doi: 10.1097/MCD.0b013e3283136948. [DOI] [PubMed] [Google Scholar]
- 16.Significant overlap and possible identity of macrocephaly capillary malformation and megalencephaly polymicrogyria-polydactyly hydrocephalus syndromes. Gripp KW, Hopkins E, Vinkler C, Lev D, Malinger G, Lerman-Sagie T, Dobyns WB. Am J Med Genet A. 2009;149A:868–876. doi: 10.1002/ajmg.a.32732. [DOI] [PubMed] [Google Scholar]
- 17.De novo CCND2 mutations leading to stabilization of cyclin D2 cause megalencephaly-polymicrogyria-polydactyly-hydrocephalus syndrome. Mirzaa G, Parry DA, Fry AE, et al. Nat Genet. 2014;46:510–515. doi: 10.1038/ng.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AKT3 and PIK3R2 mutations in two patients with megalencephaly-related syndromes: MCAP and MPPH. Nakamura K, Kato M, Tohyama J, et al. Clin Genet. 2014;85:396–398. doi: 10.1111/cge.12188. [DOI] [PubMed] [Google Scholar]
- 19.A clinical review on megalencephaly: A large brain as a possible sign of cerebral impairment. Pavone P, Praticò AD, Rizzo R, Corsello G, Ruggieri M, Parano E, Falsaperla R. Medicine (Baltimore) 2017;96:0. doi: 10.1097/MD.0000000000006814. [DOI] [PMC free article] [PubMed] [Google Scholar]