Abstract
We described behavioral studies to highlight emotional processing deficits in Alzheimer’s disease (AD). The findings suggest prominent deficit in recognizing negative emotions, pronounced effect of positive emotion on enhancing memory, and a critical role of cognitive deficits in manifesting emotional processing dysfunction in AD. We reviewed imaging studies to highlight morphometric and functional markers of hippocampal circuit dysfunction in emotional processing deficits. Despite amygdala reactivity to emotional stimuli, hippocampal dysfunction conduces to deficits in emotional memory. Finally, the reviewed studies implicating major neurotransmitter systems in anxiety and depression in AD supported altered cholinergic and noradrenergic signaling in AD emotional disorders. Overall, the studies showed altered emotions early in the course of illness and suggest the need of multimodal imaging for further investigations. Particularly, longitudinal studies with multiple behavioral paradigms translatable between preclinical and clinical models would provide data to elucidate the time course and underlying neurobiology of emotion processing dysfunction in AD.
Keywords: emotion, hippocampus and Alzheimer’s disease
Significance Statement
• Deficits in emotion recognition and memory manifest early in the course of AD.
• Processing of subtle and negative emotion is more significantly impaired than of intense and positive emotions.
• Despite amygdala reactivity to emotional stimuli, hippocampal dysfunction conduces to the deficits in emotional memory.
• Cholinergic and noradrenergic dysfunction may play a prominent role in emotional memory and the pathophysiology of emotional disorders, including anxiety and apathy.
• Multimodal imaging may help in revealing the neural bases of emotional aging.
Alzheimer’s disease (AD) is the most common neurodegenerative disorder and a major cause of dementia among the elderly. Patients with AD manifest both cognitive and noncognitive symptoms. Cognitive deficit typically starts with subtle memory lapses, which progress through the course of AD. Early AD patients may show impairment in episodic memory with declarative memory relatively spared. Affective symptoms, especially depression and anxiety, may present during the early stage of AD.1,2 As emotional disturbance and difficulty in social interaction worsen, patients can suffer restlessness, delusion, and aggression.3,4 However, perhaps because emotion deficits appear to be less severe or obvious than cognitive deficits, relatively less research has focused specifically on emotional dysfunction and its neural bases in AD.
Atrophy and functional changes of the medial temporal lobe, including the hippocampus, is among the earliest neural markers of mild cognitive impairment (MCI) and AD.5,6 For instance, resting state functional connectivity (rsFC) between the right cornu ammonis CA1 and middle temporal gyrus was diminished in amnestic MCI (aMCI) as compared to controls and correlated with reduced capacity of episodic memory in aMCI subjects longitudinally. 7 Although mostly examined with respect to memory function and dysfunction, 8 the hippocampus along with the amygdala and other regions of the limbic circuit plays a central role in emotional processing.9-13 The hippocampus and amygdala coactivated in response to fear-related behavior. 14 Hippocampal involvement in emotion regulation and memory has also been demonstrated in numerous functional magnetic resonance imaging (fMRI) studies.15-17 Volumetric studies with voxel based morphometry in healthy individuals showed hippocampal gray matter volume (GMV) in positive correlation with the capacity in episodic future thinking that engages emotion and goal-directed processing. 18 Hippocampal volume and hippocampal–insula connectivity were correlated with trait anxiety and risk-taking in episodic future thinking. 19 Patients with posttraumatic stress disorder (PTSD) showed smaller hippocampal volume relative to trauma-exposed group without PTSD and healthy controls. Further, likely reflecting a compensatory process, hippocampal GMV was positively correlated with diminished cognitive-emotional regulation and severity of experiential avoidance in PTSD. 20 Chronic stress exposure was associated with diminished hippocampal–prefrontal cortical (PFC) rsFC, possibly reflecting a reduction of PFC control of hippocampal activity. 21
Thus, degenerative pathology of the hippocampus, amygdala, anterior temporal cortex, ventromedial PFC, and other cortical regions may underpin the increasing severity of emotion dysfunction as AD progresses.22,23 On the other hand, how hippocampal circuit dysfunction contributes to emotional processing deficits in AD remains unclear. This represents a critical gap of knowledge given the involvement of hippocampal pathology early in the course of AD.
This overview aims to provide a summary and synthesis of findings implicating hippocampal dysfunction in emotion processing deficits in AD. We will describe (1) briefly the clinical features and neural biology of AD; (2) behavioral and (3) imaging studies of emotion processing dysfunction in AD, the latter with a focus on volumetric (mostly gray matter) and functional changes; and (4) preclinical (rodent) and human studies implicating neurotransmitter systems in emotional dysfunction, including apathy, and mood disorders in AD. We did not specifically discuss behavioral or imaging studies of apathy or mood disorders, which have been extensively reviewed elsewhere.24-30 Also, we did not attempt a systemic review or meta-analysis of structural or functional imaging studies of emotion processing in AD, because of insufficient sample size, and/or task and clinical heterogeneity across studies.
Clinical Features and the Neural Biology of AD
Alzheimer’s disease is characterized by the presence of neurofibrillary tangles (NFTs), consisting of abnormally phosphorylated protein “tau,” and neuropil threads (NTs), comprising ubiquitin and tau in the brain. 5 Regional accumulation of NFTs and NTs defines AD progression with initial involvement of the entorhinal regions and progression to the hippocampus, paralimbic region, medial–basal temporal cortex, association neocortex, and finally primary sensory and motor cortex, according to Braak staging. 6 Another proteinopathy involves extracellular amyloid-β (Aβ) deposition in the characteristic form of neuritic plaques, consisting of a dense central Aβ core surrounded by dystrophic neurites in the periphery. 31 Multiple studies have suggested a stronger association of tau than Aβ pathology with cognitive impairment.32-34
Current diagnostic criteria distinguish amnestic and nonamnestic AD. 35 Amnestic patients present medial temporal lobe atrophy whereas nonamnestic patients demonstrate more prominent atrophy in lateral temporal, parietal, and frontal lobes with relatively sparse changes in medial temporal regions. Further, amnestic patients are considered “typical AD” with NFT distribution balanced in the hippocampus and associative cortices and “limbic-predominant AD” with NFT observed predominantly in the hippocampus, respectively. The nonamnestic patients are “atypical hippocampal-sparing” with NFT mainly seen in the associative cortices. 35 Imaging studies have demonstrated an association between NFT deposition and regional atrophy with least hippocampal and prominent cortical atrophy in hippocampal-sparing and the opposite in limbic-predominant AD.36,37
Atypical AD is more difficult to diagnose as it features a broader array of symptoms. 38 Further, no population-based study of atypical AD exists to date 39 and the prevalence of the atypical symptoms remains unclear. However, approximately 25% of AD cases do not conform to the typical AD NFT pathology as proposed by Braak. 40 Atypical variants represent approximately one third of early-onset and 6% of late-onset AD. 39
The hippocampus plays important roles in episodic memory, autobiographical memory, as well as emotional experience and regulation. 16 As most of the atypical AD cases are “hippocampal-sparing,” emotion may be differentially affected as compared to typical AD patients. However, most of the studies of emotional dysfunction have not distinguished typical and atypical AD. A review of emotion processing in MCI reported a similar issue and highlighted the importance of differentiating amnestic and nonamnestic emotion processing dysfunction. 41 It is also worth noting that the extent of emotion processing deficit appears to be more severe in patients with behavioral and semantic frontotemporal dementia, vascular dementia, Huntington’s disease, and supranuclear palsy, relative to AD.42,43 Nonetheless, emotion plays an integral role in cognitive processing, social interaction, and quality of life, and it is of critical importance that we understand the neural bases of emotional dysfunction in AD.
Emotion Processing in AD: Behavioral Findings
As discussed earlier, AD is associated with emotion processing dysfunction and emotional disturbance,44,45 likely as a result of pathological changes in the limbic circuits. 9 A meta-analysis summarized the findings from various behavioral paradigms, including identification of facial expression, as well as exposure to emotions through audio, video, and audiovisual presentation, and reported dysfunctional emotion processing independent of the nature of task, emotional stimuli, disease severity, and cognitive state in AD. 46 Here, we briefly discussed AD emotion processing across different behavioral tasks, broadly categorized into facial and social emotion processing. We also included a brief section on emotion–cognition interaction focusing on effects of emotional reactivity on memory. Figure 1 summarizes the main findings across various categories of studies, as details in following sections.
Figure 1.
Summary of emotion processing deficits in Alzheimer’s disease. The description under each category refers to findings in Alzheimer’s disease relative to controls. The arrow pointing up and down each indicates elevation and diminution of the function/effect in Alzheimer’s disease (studies discussed in Sections 2.1–2.3).
Facial Emotion Processing
Pathological changes in the amygdala and ventral hippocampus lead to impaired recognition of faces and facial emotions in AD. 47 The latter review summarized deficient performance in AD patients in approximately 67% of the studies of facial emotion identification, 75% of emotion matching/selection, and 40% of emotion discrimination. The findings remained consistent in 50% of the identification and matching/selection and 40% of discrimination studies after controlling for general cognitive ability. 47
Impairment in facial emotional identification appeared to be more prominent when subtle (low-intensity) emotions were to be identified48-50 and less so when easier (less cognitively demanding) tasks were involved. 51 Other than the intensity of emotion, facial emotion identification also depends on the valence of emotion and deficits in identifying sad and other negative facial emotion are most consistently shown across studies of AD.47,48,52,53 Further, facial emotion processing dysfunction progresses through the course of illness.23,54
Studies have also evaluated emotion recognition and emotional responses using film clips in AD that integrates visual and auditory information. Overall, patients correctly identified the emotions55,56 and showed enhancement of emotions—elevated positive or negative affect that sustained for a measured period of time (e.g., 30 m 57 ). In particular, the enhancement of sad emotions was found comparable to healthy controls.57,58 This is notable, considering impairment in the recognition of sad facial emotions in AD, as discussed earlier. However, the emotion enhancement did not appear to benefit declarative memory and AD patients performed worse than healthy controls on recall tasks.56,58
In another setting, attentional response to emotional facial cues was assessed using emotional Stroop task. In the task, participants were presented faces with, for example, happy/sad expression along with words “happy/sad” written in the middle of the face. Face and word valence are matched and nonmatched during congruent and incongruent trials, respectively. The task was to recognize the emotion as displayed by faces (with words as a distractor) during the “face block” or words (faces as a distractor) during the “word block.” Using the emotion Stroop task, studies have reported less interference in word than in face blocks (i.e., less distracted by face) in young and middle-aged adults.59,60 This was also found true in AD patients, whereas interference was equitable for emotional faces and words in age-matched healthy adults. 61 Though it cannot be ruled out that words are over-learned and better recognized, 61 the findings suggest less efficient face information processing in AD.
Although the right hemisphere may play a more dominant role in emotion processing, 62 investigators have noted the potential influences of right vs left hemispheric neuropathology on facial emotional identification in AD. 63 For instance, difficulty in comprehending and remembering task instruction disrupted performance in “low verbal (left-hemispheric dominance),” whereas visuo-perceptual impairment did so in “low spatial (right-hemispheric dominance)” AD patients. 64 Thus, facial emotion processing dysfunction may vary in severity depending on testing contingencies as well as the neuropathological characteristics of AD.23,51,54
Social Emotion
In the real world, experienced emotions may change dynamically and one needs to regulate behavior accordingly to optimize social interaction. In a dynamic valence tracking task, participants rated the changes in target character’s emotion in a movie clip using a dial pointing to extreme left, extreme right, and middle to signal “very bad” (frowning), “very good” (smiling), and “neutral,” respectively. 65 Patients with AD performed worse than controls on the task, suggesting compromised recognition of social emotion. Further, AD performed worse on the dynamic tracking compared to emotion labeling task where participants watched film clips and identified one of the 11 emotions experienced by a character. In contrast, healthy controls performed better in dynamic tracking vs emotion labeling. 65 Performance was not examined separately for valence of emotion in this study.
Social interaction requires one to modify (suppress/amplify) emotional expressions according to contexts. AD patients appear to experience difficulty in contextual regulation of emotional expressions. 66 In the latter study, the ability to “manipulate” emotion was assessed using amusement film clips in three conditions—spontaneous, suppressed, or amplified expression—interleaved with neutral film clips. Participants were instructed to forcefully suppress or enhance the expressions during the manipulated while mapping the natural expressions during spontaneous conditions. The study also assessed theory of mind (ToM) where participants judged complex emotion (e.g., jealousy) depicted by the pictures. AD patients relative to controls showed significant impairment in amplifying emotional expression but were intact in emotional expression suppression. Expression amplification, but not suppression, correlated with the ToM performance, which in itself was impaired in AD relative to controls, suggesting that expression amplification requires better understanding of others’ interpretation of behavior. Thus, the impairment in emotion expression amplification may affect social interaction in AD. 66 In another study on facial expression regulation, display of positive facial expression seemed compromised in AD patients, as assessed using facial electromyographic recordings of corrugator and zygomatic activity, while presented with affective stimuli. In contrast, electromyographic patterns recorded during exposure to negative stimuli were comparable between AD and healthy controls. 67
Empathy is crucial for social functioning and regulated by affective bottom–up and cognitive top–down processes that involve emotion awareness and regulation.68,69 A recent work summarized findings on different aspects of empathy, including emotion processing/decoding, self-awareness, affective sharing, mental flexibility, and self-regulation, and reported deficits in emotion decoding and elevated affect sharing in AD. 70 The seemingly enhanced ability in affect sharing or “emotional contagion” may result from dysfunctional emotion inhibition in AD, potentially because of degeneration of the hippocampus and parahippocampal gyrus. 12 The general ability of self-other-awareness appears intact, though awareness about their AD-related self (e.g., about dementia) may be impaired in AD. 70 Mental flexibility and self-regulation are preserved in AD as long as the situational cognitive demand is not taxing for the patients. As cognitive capabilities decrease along the course of illness or the complexity of social situations increases, an impairment becomes apparent. Thus, cognition affects empathy processing in a context dependent manner in AD. 70
Interaction of Emotion and Cognitive Processing
Emotion is inherently embedded in cognitive, including language and memory, processes.71,72 In this context, studies have evaluated the emotion enhancement effect (EEE) of memory. EEE is evident in nondemented elderly people as in quicker and more accurate recall of emotional vs nonemotional memory, relative to younger people. 73 Though present for both, EEE favors memory of positive vs negative valence.73,74 EEE is demonstrated in AD by most but not all studies, with overall memory impairment potentially masking the actual effects of emotion. 75 For instance, on both immediate and (20-minute) delayed recall of valenced words, AD patients performed worse than patients with aMCI and healthy controls. Emotional enhancement observed for positive word during delayed recall, as reflected by more correct recall of positive vs neutral words, was also significantly weaker in AD. 76 Increasingly impaired emotion discrimination (e.g., facial emotion discrimination in memory tasks on facial emotional cues) with increased cognitive complexity of task in AD associates diminished EEE with AD pathology. 77 Task repetitions, shorter time delay in recollection, higher emotional load (intensity), and self-reference of the stimuli benefit AD patients in manifesting EEE.
The formation of episodic memory is typically laden with emotions. 78 Associated with amygdala atrophy and hippocampal deafferentation, dysfunctional episodic memory is noted in early stage AD 78 and may lead to over-generality or inability to reach past personal events. 79 A fundamental component of self-awareness, autobiographical memory is impaired 80 as a result of amygdala–hippocampus atrophy in AD. 81 Studies have shown a positive effect of sensorial stimulation by familiar music/odor/taste on episodic autobiographical memory, which in turn may improve self-awareness in AD. 79 As described earlier, for episodic and autobiographical memory, the presence of emotional benefit may depend upon task complexity. 82 Cognitive demand may mask the benefits in AD. For instance, in contrast to simple recall of valenced stimuli, a task that requires association of the emotional context with its destination—an object defined by the task—may be cognitively too demanding and see no emotional benefits in AD. 82
EEE is likewise evident for working memory. 83 A study assessing age-related differences noted memory enhancement in recalling emotional vs neutral words in elderly people. 84 Young adults recalled more correct words than old adults, all recalled more negative than neutral and positive words, and old but not young adults recalled more positive than neutral words. As a result, the EEE was highly pronounced in old adults, particularly for negative words, nullifying age-related differences. 84 Other studies noted positive emotion bias in emotion perception and memory in healthy old adults but not in patients with early-stage AD.55,85
In another setting with the Deese–Roediger–McDermott paradigm, participants were presented with semantically related words (e.g., hill, valley, climb, top, glacier, and steep.) and may erroneously recall a nonpresented theme word (e.g., mountain) during free recall or falsely recognize the lure words in the recognition test.86,87 AD patients demonstrated less true and false recognition compared to controls.88,89 In an emotion variant of the paradigm with four lists of semantically related theme words—depression (loneliness), delusion (betrayal), positive (holidays), or neutral (window)—AD and MCI patients relative to controls showed more false recognition for positively valenced words but comparable performance for other categories. 90 Further, true recognition was comparable in AD while enhanced for emotional than neutral items in MCI and healthy controls, highlighting the absence of emotional memory benefit in AD. However, among the different categories of emotions, positive items were better recognized by AD, in contrast with equitable recognition across all emotions in MCI and positive/delusion-related items in controls. 90 Thus, positively valenced emotional words may not only enhance recognition in AD but also lead to false memories. 90
On the other hand, AD is associated with impairment in attentional shift towards emotional stimuli. 91 The latter study involved pro- and anti-saccades dictated by emotional visual stimuli with positive/negative/neutral valence. AD patients presented similar mean error rates in anti-saccades for both valenced and neutral stimuli, as opposed to controls who demonstrated more errors for emotional than neutral stimuli, indicating impaired attention capture by emotional stimuli in AD. 91 In contrast, AD and controls did not show differences in pro-saccade error rates across all stimuli or with respective to emotional vs neutral stimuli, although AD did not show faster RT to emotional, particularly negative vs neutral stimuli, as observed in controls, again in accord with deficits in attention capture by emotional stimuli. Another study investigated the influences of attention on emotional enhancement of memory (EEM) using a divided vs full attention paradigm with highly emotionally arousing stimuli. 92 A cue was presented with (divided attention) and without (focused attention) distractor, and participants responded whether or not the cue stimulus comprised humans (discrimination) and whether or not the cue was presented earlier than the distractor (recognition). Regardless of the presence of distractor, no EEE was observed for discrimination, whereas valenced vs neutral pictures were better recognized leading to increase in both true and false recognition in AD. Thus, highly arousing stimuli do not recruit attentional resources explicitly, but the “feeling of familiarity” may influence recognition. 92 Such lack of EEE was also evident in a visual target search task where AD patients showed comparable performance for neutral and valenced visual stimuli, contrary to controls who detected negative targets more efficiently. 93 Thus, AD patients do not demonstrate EEE in behavioral tasks that require rapid attentional shift.
Neural Correlates of Emotion Processing Dysfunction in AD
In a broad conceptual scheme, the perception of emotional stimuli is followed by (i) identification and judgment on emotional components, (ii) production of stimulus-specific affective state, and (iii) adjustment of affective state/emotional behavior to be contextually relevant by modulating/inhibiting processes (i) and (ii). 94 These processes can be explained in terms of three models—basic emotion, dimensional feeling, and regulation 95 and two neural systems—the ventral system comprising the insula, amygdala, ventral striatum, and ventral PFC, including the ACC for processes (i) and (ii), and the dorsal system comprising the hippocampus, dorsal prefrontal, and cingulate gyrus for process (iii). 94
Numerous studies have employed fMRI and PET imaging to investigate the neural correlates of these processes. 96 A detailed review of these studies is available elsewhere.97-104 Briefly, the amygdala is involved in processing salient (particularly negative) stimuli, including those that elicit fear and require rapid “on-the-flight” decisions; the subcallosal cingulate cortex is involved in responses to stimuli that evoke negative mood; the insula is involved in recall of emotional memory and emotional experience; and the ventromedial and dorsolateral PFC are engaged during emotional regulation, likely via interaction with the amygdala and insula.102,104,105 These neural hubs along with other regions of the limbic and cognitive motor circuits support the perception, dimensional feeling, and regulatory processes of emotion. Studies have also provided evidence for regional specialization, with the amygdala in response to fear, insula, and globus pallidus to disgust, and lateral OFC to anger. 96 In contrast, the medial PFC, including the ACC, plays a more general role in emotional perception and regulation. 96
The Role of the Hippocampus in Emotion Processing
The hippocampus interacts with the amygdala in responses to emotional stimuli and supports emotional memory.71,106 Studies of amygdala–hippocampal interaction have mainly focused on how the amygdala affects hippocampal processes of episodic memory. 16 On the other hand, recollection of emotional experiences engages the hippocampus to influence amygdala responses. 106
Anatomically, the hippocampus is divided into CA1, CA3, and dentate gyrus (DG) along the transverse axis. 107 The DG forms the input and CA1 the output node of the tri-synaptic loop of hippocampus: DG→CA3→CA1. 108 Projecting to the subiculum and entorhinal cortex, dorsal CA1 is mainly involved in episodic memory and spatial navigation. 107 Projecting to the nucleus accumbens, amygdala, and PFC, the ventral CA1 is positioned to regulate affective and motivated behaviors.107,109,110 Thus, the hippocampus works in synergy with the emotion circuits whenever “emotion needs memory.” 111 Rolls suggests that the emotion system (including amygdala, OFC, and ACC) follows the feed-forward pattern to learn stimulus-reward association, whereby reinforcers are recognized, evaluated for affective value, and translated to influence behavior. The hippocampus, on the other hand, works with recurrent collateral feedbacks, whereby contextual information in the CA3 can be recalled to facilitate recognition and evaluation of environmental stimuli and decision making. 111 Figure 2 illustrates the hippocampal emotion circuit.
Figure 2.
Hippocampal emotion circuit. Primary and secondary reinforcers processed for the “what” component of the stimuli by the insula/frontal operculum/olfactory cortex/primary somatosensory cortex and visual/auditory cortices, respectively. These regions project to the orbitofrontal cortex and amygdala, which in turn project to the entorhinal cortex and (for amygdala additionally) to the CA1/subiculum of the hippocampus (curved dashed arrows in black). Both entorhinal cortex and CA1/subiculum project to the cingulate, hypothalamus, striatum, and medial prefrontal cortical, depending upon stimulus-specific action (solid straight/angled arrows in black). Parietal cortex, temporal cortex, and prefrontal cortical (processing “where” component of the stimulus) project to the entorhinal cortex via parahippocampal/perirhinal gyri (dashed straight arrows in black). Medial PFC and cingulate/hypothalamus also project to the entorhinal cortex (dashed straight/angled arrows in black). Finally, the entorhinal cortex projects back to the amygdala, orbitofrontal cortex, medial prefrontal cortical, striatum, cingulate/hypothalamus, and other prefrontal cortical regions (dashed curved, angled, and straight arrows in yellow). Within the hippocampus, feed-forward and unidirectional projections are shown in green thick curved arrows, direct feed-forward projection from CA1 to entorhinal cortex in green thin curved arrows, CA3 back-projection to dentate gyrus, and recurrent CA3 connections in blue thick curved/circular arrows, back projections from entorhinal cortex to CA1/subiculum in blue thin curved arrows, and noncanonical subiculum back projections in red solid arrows.108,110-112
The hippocampus is connected with basolateral amygdala via (1) lateral entorhinal cortex indirectly, (2) hypothalamus and medial septum indirectly, and (3) CA1/subiculum directly. 113 An fMRI study of adults 60–91 years noted higher hippocampal DG and CA3 activity during correct rejection of lure negative vs neutral images during emotional pattern separation. 114 In contrast, the amygdala showed higher activity during false identification of negative vs neutral images; that is, during over-generalized responses. 114 An extension of this work combining diffusion tensor imaging (DTI) with fMRI showed that the integrity of uncinate fasciculus (connecting the medial temporal regions and OFC) is central to hippocampal DG/CA3 activity and emotional discrimination. 115 In real-time fMRI, neuro-feedback training resulted in enhanced emotion regulation along with elevated hippocampal activation and hippocampus–amygdala connectivity.16,116,117 The hippocampus partakes in down-regulation of negative emotion using humorous reappraisal, likely via a hippocampal–thalamic–frontal pathway, to “re-present” emotions, that is, cognitive restructuring of negative to positive emotions. 118 Hippocampal rsFC is central to EEM. 119 In contrast, focused attention to reduce the emotional impact of distressing memory is associated with reduced hippocampus, amygdala, and anterior parahippocampal activity. 120 Reduced hippocampal activity and connectivity with the PFC and amygdala was also observed in paradigms involving suppression of emotional memory.121,122 Other studies broadly highlighted the role of the hippocampus in facial recognition and music-evoked17,123,124 as well as social125-127 emotions.
Together, these studies support a critical role of the hippocampal circuit in emotional memory and regulation in youth and during healthy aging.
Hippocampal Atrophy and Morphometrics in AD
Hippocampus is one of the brain regions affected earliest in the course of AD dementia. 128 Machine learning showed that hippocampal volume distinguished AD, MCI, and cognitively normal individuals with ∼80% accuracy. 128 Other studies implicated hippocampal texture—the texture descriptor comprising spectral histograms that capture different micro-structural properties, including steep transitions and blobs 129 —as a critical feature in computer-aided differential diagnosis of dementia. 130 A study reported fastest reduction of gray matter density (GMD) in hippocampal CA2, amygdala, and hippocampal–amygdala transition area in AD vs controls over a 2-year period, with GMD changes predicting Mini Mental State Examination (MMSE) scores in AD. 131 A 14-year cohort study of nondemented older adults showed that the annual rate of CA1-3 atrophy correlated significantly with increased risk of developing AD symptoms, independent of age, education, gender, or ApoE4 genotype. 132 Hippocampal atrophy was mirrored by CSF levels of tau protein longitudinally in tau(+)AD.133,134 A recent work refined the hippocampal subareas where the GMV best differentiated AD from controls. 135 Another study associated hippocampal atrophy with depression and anxiety in AD. 136 Pronounced right hippocampal atrophy was observed in AD with psychiatric symptoms relative to those without, even after controlling for frontal lobe volume. 137 Numerous other studies have documented altered hippocampal structure in link with visuospatial, executive, visual association, and verbal memory dysfunction in MCI and/or AD.138-140
Hippocampal atrophy is noted even in atypical AD and logopenic progressive aphasia, which are generally considered “hippocampal sparing” 141 as well as in semantic dementia and dementia with Lewy bodies. 142 Hippocampal atrophy is associated with cognitive impairment during aging independent of AD neuropathologies. 143 Other studies have focused on hippocampal local surface roughness as a predictive marker of progression from MCI to AD, 144 and hippocampal shape and asymmetry in differential diagnosis of AD145,146 and early vs late onset AD, 147 and documented functional, metabolic, and shape changes in the hippocampus.148-152
Hippocampal Atrophy and Emotion Processing Deficits in AD
In this subsection, we summarize the studies of volumetric correlates of emotion processing and highlight the hippocampus specifically. The studies are grouped into those assessing emotional memory, facial emotion processing, and social emotion.
Emotional memory and EEM
With film clips depicting happy and sad emotions presented to the subjects, a study associated amygdala and hippocampal volume with emotion ratings and declarative memory, respectively. 57 Experienced emotion and its persistence were comparable between AD and HC. Hippocampal volume was significantly smaller but amygdala volume was comparable in AD relative to HC. Persistence of happy and negative emotion each showed a positive and negative correlation with amygdala volume; in contrast, hippocampal volume was positively correlated with memory, irrespective of emotion. 57 Another study reported in a pooled sample of AD, MCI, and cognitively normal controls a correlation of left amygdala volume with immediate recall of both positive and negative words and with delayed recall of positive words, right amygdala volume with delayed recall of negative words, left hippocampus volume with immediate recall of positive words, and bilateral hippocampus volumes with delayed recall of negative words. Thus, both the amygdala and hippocampus partake in emotional memory. 76 In another study, AD vs HC demonstrated bilateral amygdala and hippocampal atrophy and deficits in both immediate and delayed recall of emotional pictures, and hippocampal volumes were positively correlated with recall of pictures of positive valence in AD. 153 On the other hand, amygdalar but not hippocampal volumes were positively correlated with memory of real-life emotional events (e.g., Kobe earthquake), even after controlling for age, sex, education, and whole brain atrophy. 154 Amygdala thus appeared to play a more dominant role during recall of self-referencing emotional events.154,155 However, although AD vs controls showed impairment in fear conditioning, the deficits did not appear related to amygdala volume. 156
The amygdala interacts with the hippocampus and PFC to modulate cognition, 75 including EEM. Presented emotionally charged and neutral story followed by recall of details one day later, patients with mild AD showed EEM comparable to controls. Further, EEM was correlated with bilateral hippocampus, parahippocampus, fusiform gyrus, and frontal pole volumes in AD. 157 However, another study of recognition of valenced and neutral pictures reported EEM in HC but not AD. 158 Overall, the EEM correlated with right amygdala and bilateral hippocampus volumes in AD. More specifically, memory enhanced by positive stimuli correlated with right amygdala and hippocampus volume, while EEM of negative stimuli correlated with bilateral hippocampus volume in AD. No such correlations were evident in HC. 158 Perrin and colleagues 159 assessed EEM using cued and free recall of valenced and neutral pictures (content) preceded and followed by an emotional or neutral dialogue in AD. Free-recall EEM was correlated with right amygdala volume for both positive and negative content, whereas left amygdala volume was correlated with negative content only. No volumetric association was observed for cued recalls. Thus, although the hippocampus and amygdala are involved in emotion memory and EEM, the findings were mixed in terms of EEM by positive vs negative stimuli and the potential hemispherity-specific roles of hippocampus vs amygdala.
The volumetric correlates of emotional memory and EEM are summarized in Figure 3.
Figure 3.
Gray matter volumetric correlates of emotional memory and emotional enhancement of memory. Lower volumes of these regions are associated with impairment in emotion memory or EEM in AD. Asterisks indicate the strength of supporting evidence: *** > ** > *. OFC: orbitofrontal cortex (studies included in Supplemental Table S1 and discussed in section 3.3.1).
Identification of facial emotion and intentional emotional facial expression
Impairment in facial emotion processing is evident in AD, and the great majority of studies reported a positive association between regional GMVs and performance (see Ref. 160 for contrasting findings). A study associated the capacity in identifying facial emotions with left middle frontal and superior/middle/inferior temporal gyrus volumes in a population of HC, MCI, AD, and FTD. 161 Recognition of negative emotion (anger, sadness, and fear) was positively correlated with volumes of the right inferior and middle temporal gyri and right anterior inferior temporal gyrus extending into right lateral OFC in HC, AD, MCI, progressive supranuclear palsy (PSP), and frontotemporal lobar degeneration. 162 Right superior temporal gyrus volume correlated with recognition of sadness independent of disgust and anger. 162 Recognition performance for a broad range of positive and negative emotions correlated with GMVs of bilateral caudate, thalamus, inferior orbitofrontal and inferior/middle frontal cortex, left fusiform gyrus, supplementary motor area, posterior insula, right superior frontal gyrus and gyrus rectus in a combined sample of AD, behavioral variant FTD (bvFTD), semantic variant primary progressive aphasia (svPPA), nonfluent variant primary progressive aphasia (nfvPPA), PSP, corticobasal syndrome (CBS), and HC. 65 Other studies associated emotion identification with right-hemispheric anterior temporal cortex, insula, amygdala, and posterior fusiform volumes in a combined sample of HC, SD, and AD 63 and bilateral insula thickness and right hippocampal volumes in AD. 42 On dynamic emotion tracking, lower performance correlated with atrophy of bilateral OFC and right inferior/middle frontal gyri in a sample including HC, AD, PSP, CBS, and frontotemporal lobar degeneration. 163 Specifically, lower performance for positive and negative emotion was associated with tissue loss in right frontopolar cortex (Brodmann area or BA10) and lateral OFC (BA47), respectively. 163 Another study with emotion tracking highlighted the roles of bilateral superior medial frontal gyrus, ACC, supplementary motor area, right middle/inferior temporal gyri, fusiform gyrus, middle temporal pole, amygdala, insula, and inferior OFC in a sample of AD, bvFTD, svPPA, nfvPPA, PSP, CBS, and HC. 65 Performance in dynamic emotion tracking was impaired in AD vs HC in the latter study but was not compared between groups in Ref. 163.
Intentional emotional facial expression is learned through social communication and likely to involve neuroanatomical correlates distinct from those of basic facial expression. 164 Patients with AD but not those with FTD, PSP, and primary progressive aphasia (PPA) were intact in generating facial expressions following verbal command and in imitating the emotions displayed by pictures, relative to controls. 165 Overall (verbal + imitation) deficits were associated with volume loss in bilateral central operculum, frontal operculum, anterior insula, medial OFC, inferior frontal gyrus, dorsal anterior cingulate, precentral gyrus, putamen, thalamus, and medial temporal gyrus, with right-lateralized loss specifically for imitation deficits, across all subjects. 165
In sum, except for dynamic emotion tracking and intentional imitation of facial expressions, which involve most prominently the OFC, frontoparietal cortex, and insula, hippocampal dysfunction in facial emotion recognition/identification were evident in AD. However, most studies have included patients with other types of dementia, likely to increase sample size, and the deficits specific to AD pathology remain to be investigated. These volumetric correlates of facial emotion processing are summarized in Figure 4.
Figure 4.
Gray matter volumetric correlates of emotional face identification. Note that almost all studies evaluated regional volumetric correlates across a sample of multiple forms of dementia. Thus, we do not specifically indicate the strength of evidence here. G: gyrus; AMG: amygdala; HPC: hippocampus; OFC: orbitofrontal cortex; MFG/SFG/IFG: middle/superior/inferior frontal gyrus; SMA: supplementary motor area; STG/MTG/ITG: superior/middle/inferior temporal gyrus; SMG: supramarginal gyrus; FPC: frontopolar cortex; CO: central operculum; FO: frontal operculum (studies included in Supplemental Table S1 and discussed in Section 3.3.2).
Social emotion
The total awareness of social inference test (TASIT) evaluates the ability to recognize dynamic display of emotions and sincerity or sarcasm in videotaped vignettes of everyday social interactions. 166 It has two parts: TASIT 1 assesses recognition of spontaneous emotional expression; TASIT 2 assesses social inference, including for example, comprehension of sincere vs sarcastic exchanges, with speaker’s voice and facial expression indicating the intended meaning of the exchange. Social inference is assessed via questions probing for understanding of the emotions, intentions, and beliefs of the speakers. AD patients relative to controls did worse in the recognition of disgust, sadness, surprise, and happiness but not anger, anxiety, or fear. 42 The deficits correlated with right insula and anterior cingulate cortical thinning as well as bilateral hippocampus, right amygdala, bilateral accumbens, and putamen atrophy in AD. 42 Another study of TASIT reported no deficits in emotion recognition and social inference, after controlling for overall cognitive ability, in AD. 167 Right posterior middle/inferior temporal, right middle temporal/temporo-occipital, left fusiform, right frontal pole, left hippocampus, left insula, right inferior frontal, left postcentral/angular gyrus, and right superior parietal GMVs were positively correlated with performance in emotion recognition in AD after accounting for education and cognitive abilities; in contrast, no volumetric correlates were identified for social inference. 167 Rankin et al 168 likewise found intact comprehension of sarcasm in AD, which was correlated positively with GMVs of right superior temporal pole, bilateral parahippocampal gyrus, right superior frontal gyrus, middle temporal gyrus, and caudate in a combined sample of HC, AD, FTD, SD, progressive non-fluent aphasia (PNFA), corticobasal degeneration (CBD), and PSP.
A study assessed AD, bvFTD, SD, and HC with a social–emotional questionnaire (SEQ) and observed relatively intact social emotion in AD. 169 In the pooled sample, SEQ overall score was correlated with the GMVs of bilateral anterior temporal poles and fusiform gyri, and left frontal pole, OFC, and insula. Across groups, emotion recognition alone was correlated with the GMVs of left OFC, left temporal pole, bilateral hippocampus, and left cerebellum, and “empathy” with right insula, left OFC, left temporal pole, and left cerebellum. No volumetric correlates were identified in AD alone. 169 Another study used the Revised Self-Monitoring Scale—sensitivity to expressive behavioral subscale (RSMS-EX) to evaluate the ability to interpret subtle nonverbal social cues and Interpersonal Reactivity Index–empathic concern (IRI-EC) to evaluate the tendency of emotional empathy. 170 Prosocial motivation (reflected by IRI-EC, after controlling for RSMS-EX score) and affect sharing (vice versa) were evaluated for patients with AD, PSP, PPA, bvFTD, and HC. The results noted comparable empathic concern but diminished prosocial motivation in the patients relative to controls. Further, affect sharing was associated with amygdala and hippocampus and prosocial motivation with frontal cortical and striatal volumes. 170 Another study examined IRI subscales “perspective taking” (cognitive empathy) and “empathetic concern” (emotional empathy) in FTD, semantic dementia (SD), PNFA, AD, CBD, PSP, and HC. 171 AD patients alone vs controls showed worse cognitive but comparable emotional empathy, though no volumetric differences survived the threshold. 171 In the entire sample, emotional empathy was correlated with GMVs of the right temporal pole, right caudate, and right inferior frontal gyrus, and cognitive empathy with right temporal pole, right caudate/subcallosal gyrus, and right fusiform gyrus. A more recent work likewise noted impairment in perspective taking but not empathetic concern in AD relative to HC. 172 Impairment of perspective taking in AD was correlated with atrophy in left inferior/middle/superior temporal cortex, left angular gyrus, left parahippocampal gyrus, left cerebellum, and right middle temporal gyrus. 172 Insight is crucial to emotional awareness. Frontotemporal and subcortical regional volumes were implicated in insight in patients with AD, bvFTD, SD, PNFA, and LPA. 173 Among the different components of insight, social interaction deficit correlated with left OFC, left parahippocampus, right middle temporal gyrus, bilateral insula, and right amygdala atrophy; motivation/organization insight deficit correlated with bilateral orbitofrontal, left anterior cingulate, and right frontopolar atrophy; deficits in emotion insight correlated with bilateral frontopolar, right dorsolateral PFC, SMA, bilateral anterior cingulate, and left amygdala atrophy in all groups combined. No correlation survived on separate analysis for each group. 173 Other studies implicated the hippocampus, temporal cortex, and amygdala in emotional contagion, 12 a physiological and behavioral state that promotes affective stimulation among peers, possibly through the activation of visceromotor mirroring mechanisms.174-176 Emotion contagion may increase in intensity at each stage of AD (HC<MCI<AD) and appear to be correlated with smaller bilateral temporal cortical, temporal pole, hippocampal, and parahippocampal and bilateral amygdala volumes. 12
These volumetric correlates of social emotion processing are summarized in Figure 5. The hippocampus and parahippocampal gyrus seem involved in many different dimensions of social emotional dysfunction. On the other hand, as with facial emotion identification and imitation, many studies involved patients with dementia other than AD; thus, the AD-specific neuroanatomical correlates of social emotion processing remain to be clarified.
Figure 5.
Gray matter volumetric correlates of social emotions. Lower volumes of the brain regions are associated with social emotion deficits. * Findings specific to AD. G: gyrus, HPC: hippocampus, AMG: amygdala, IFG: inferior frontal gyrus, PHG: parahippocampal gyrus, SFG: superior frontal gyrus, OFC: orbitofrontal cortex, STP: superior temporal pole, MTG: middle temporal gyrus, and BG: basal ganglia (studies included in Supplemental Table S1 and discussed in Section 3.3.3).
Hippocampal Circuit Activity and Connectivity and Emotion Processing Deficits in AD
This subsection will summarize fMRI studies of AD, which have commonly assessed reactivity to emotional stimuli and emotional memory of faces and pictures. Connectivity studies on AD emotion are limited but discussed where relevant.
Reactivity to emotional stimuli
Functional magnetic resonance imaging studies on emotion processing are far fewer in AD than in other clinical populations, probably because of the difficulty in engaging patients in such tasks. In the conceptual scheme of emotion processing, as described earlier, emotion perception and reactivity represents the first stage of information processing. AD patients relative to young and old HCs showed greater amygdala activation during exposure to both novel fearful and familiar neutral faces but only early during the experiment, suggesting hyperactivation rather than reduced habituation. 177 Higher right amygdala activation remained significant in AD vs old HC, after adjusting for the amygdala volume, which was significantly reduced in AD vs old HC. 177 In another study, patients with mild AD relative to HC showed reduced responses in left insula/frontal operculum to happy, left MPFC to sad, and left ventral premotor cortex to fearful facial expression during passive viewing. 178 AD patients with vs without apathy showed lower activation in bilateral amygdala and fusiform gyri during passive viewing of sad vs neutral faces but no differences viewing happy vs neutral faces. 179 The latter finding suggests the influence in motivational state on emotional reactivity and amygdala responses in AD.
Reactivity to emotional stimuli influences working memory when the emotional stimuli are not the targets to be encoded. In a visual working memory task with emotionally neutral and negative background pictures as distractors, while both AD/MCI and HC reacted slower in target identification with emotional vs neutral distractors and with increased memory load, AD/MCI were less distracted than HC by emotional pictures. 180 Overall, AD/MCI vs HC showed higher activation in left cerebellum, putamen, fusiform, amygdala, right superior temporal pole, and parahippocampal gyrus, and lower activation in bilateral occipital, insula, and frontal regions. Further, HC showed lower activation of the amygdala with increased memory load, while AD/MCI showed amygdala activation during both low and high load, reflecting dysfunctional inhibition of distractor processing in AD/MCI. Finally, AD/MCI vs HC showed higher left amygdala responses to negative emotion distractors but diminished DLPFC activation during working memory, independent of memory load. 180
These functional neural correlates are summarized in Figure 6. Together, along with the discussion of the impairment in identifying negative emotions, these findings suggest that higher amygdala reactivity to negative emotions likely did not reach awareness to influence cognitive processing or impact hippocampal mechanisms of memory encoding in AD.
Figure 6.
Functional correlates of reactivity to emotional stimuli in AD. The results from a small number of studies suggest higher amygdala and lower cortical responses to negative emotions in AD. Up and down pointing arrows each indicate higher and lower response in AD relative to control. ACC: anterior cingulate cortex; DLPFC: dorsolateral prefrontal cortex; G: gyrus; and MPFC: medial prefrontal cortex (studies included in Supplemental Table S2 and discussed in section 3.4.1).
Emotional memory
In studies of emotional memory, participants are asked to remember emotionally valenced and neutral stimuli and to recall these stimuli after fMRI scan. For instance, investigators observed enhanced recognition of positive emotional scenes in HC but not MCI or AD subjects. 181 Negative emotional stimuli were not studied in the latter report. Recognition performance was worse in AD compared to HC and MCI, while HC and MCI were comparable. AD relative to HC and MCI showed diminished parahippocampal/hippocampal activation for both neutral and emotional vs baseline (i.e., visual fixation) but not between emotional vs neutral stimuli. Middle temporal cortical activation was correlated with recognition performance for emotional pictures across all groups and AD/MCI. The study also noted enhanced hippocampal/parahippocampal activation to both emotional and neutral scenes in MCI relative to HC and attributed the same to compensatory enhancement (though insufficient to impact performance) during early phases of cognitive decline. 181 Music is emotionally arousing and often evokes emotional memory. A study examined how long-known and recently-heard music differentially recruit the emotion circuits in MCI and early AD, and noted activation of bilateral prefrontal, limbic, motor, auditory cortical, and subcortical regions for long-known music. 182 Recently heard vs baseline engaged activation of the inferior frontal gyrus, precentral gyrus, and superior/medial temporal lobe and “deactivation” of the DLPFC and MPFC, including the ACC. Long-known vs baseline and vs recently-heard recruited many of the same regions, as well as the supplementary motor area, precentral gyrus, inferior frontal gyrus, hippocampus, parahippocampus, amygdala, superior/inferior temporal cortex, anterior insula, putamen, pallidum, and anterior thalamus. Thus, long-known music activated regions involved in autobiographical memory and emotion processing in AD. 182 However, no control participants were scanned in the latter study. The functional neural correlates of emotional memory are summarized in Figure 7.
Figure 7.
Functional correlates of emotional memory in AD. The regions with upward/downward arrows depict increased/decreased BOLD activity in AD compared to HC. The regions without arrows depict BOLD correlates. G: gyrus, STP: superior temporal pole, PHG: parahippocampal gyrus, MFG: middle frontal gyrus, PFC: prefrontal cortex, IFG: inferior frontal gyrus, STG: superior temporal gyrus, MTG: middle temporal gyrus, SMA: supplementary motor area, and ITG: inferior temporal gyrus (studies included in Supplemental Table S2 and discussed in section 3.4.2).
Resting-state functional connectivity/co-activation of the hippocampal circuit
Whereas few fMRI studies examined emotional dysfunction by engaging participants in a behavioral task, resting-state studies have demonstrated altered hippocampal and amygdala functional connectivity in AD, though most have not related imaging to clinical or behavioral findings.152,183,184 AD showed higher and lower bilateral hippocampal connectivity with the precuneus and superior temporal gyri, respectively, 152 and a reduction of amygdala connectivity with the hippocampus, temporal, and frontoparietal areas that progressed in severity, with probable implications for emotional processing dysfunction. 184
Another study noted significant bilateral hippocampal/entorhinal cortex coactivation with the default mode network (DMN) in both AD and HC and lower resting-state low frequency activity in the hippocampus and posterior cingulate in AD relative to HC. 185 In prodromal AD, the hippocampus decoupled functionally from the posterior DMN during resting state. 186 DMN-hippocampal coupling has been implicated during different phases of memory processing. 187 An earlier review posits a role of the DMN in representing discrete emotions by abstraction. 188 In AD, altered DMN-hippocampus FC may impair emotion processing.
A recent work associated altered FC of the frontal pole, temporal, and insular cortex with impaired emotion detection and cognitive empathy in AD. 189 The study assessed social cognition using TASIT-Emotion Evaluation Test (TASIT-EET), RSMS, IRI, and Social Norms Questionnaire in AD, PD, FTD, and HC. All three patient groups scored worse than HC on perspective-taking (IRI-PT), and across all subjects, IRI-PT score was positively correlated with left inferior temporal cortical connectivity with bilateral frontal pole and posterior cingulate gyrus, and with right inferior parietal connectivity with right frontal pole and temporal gyri. 189 Another study associated higher socioemotional sensitivity with mean FC of the salience network (SN) across dementia patients and controls, although the patients did not differ from HC in socioemotional sensitivity (as assessed with RSMS) and showed lower connectivity of the DMN and sensorimotor but not the SN, relative to HC. 190 Of the SN, right anterior insula connectivity with the ACC was positively correlated with RSMS score, in support of SN’s role in socioemotional functions, 190 as also demonstrated in other studies.191,192 AD and aMCI patients each showed 13.2% and 5.9% lower SN GMV, along with reduced intra-SN FC in AD, as compared to controls. 193 A recent meta-analysis supported altered SN connectivity as an imaging marker of MCI, 194 though Ng et al, 2021 195 noted intact graph-theoretic metrics of the SN in AD. The roles of the SN in emotional processing dysfunction remain to be clarified.
A longitudinal study evaluated affective symptoms in healthy older adults with the self-report version of the revised NEO (Neuroticism, Extraversion, and Openness) Personality Inventory, followed by amyloid-PET scan and rsfMRI. 196 Emotional reactivity, as indexed by a composite score of anxiety, depression, and emotional vulnerability, increased with age in subjects later found to be amyloid-positive (PiB+) but not in PiB− subjects, whereas all showed decline in interpersonal warmth, indexed by a composite score of extraversion. PiB+ relative to PiB− subjects showed significantly higher global connectivity within the right insula and superior temporal sulcus, left hippocampus and entorhinal cortex, bilateral midbrain and midline pons, as well as the cerebellum. Higher superior temporal sulcus connectivity was correlated with lower interpersonal warmth and higher emotional reactivity, regardless of PiB status. Thus, temporal and insular cortical connectivity may influence affective processing even during the preclinical stage of AD. 196
Structural connectivity of the hippocampus and parahippocampal gyrus
A study combining DTI and PET imaging showed higher diffusivity of the anterior hippocampus in AD patients vs controls and in negative correlation with FDG uptake in the hippocampus, parahippocampus, and posterior cingulate cortex in AD patients. 197 Delayed verbal recall score correlated negatively with anterior hippocampal diffusivity and positively with FDG uptake in posterior cingulate cortex and bilateral hippocampus and parahippocampus. 197 The uncinate fasciculus (UF), connecting the PFC to anteromedial temporal lobe, and cingulate-parahippocampal fasciculus (CHG) are two major white matter tracks in the middle temporal lobe. Higher diffusivity of the UF and CHG at baseline predicted decline in episodic memory over a 3-year period in healthy elderly at higher risk of AD, including those with a family history of dementia and carriers of apolipoprotein E ε4 allele. 198 Another study reported reduced fractional anisotropy (FA) and elevated radial diffusivity in bilateral UF, CHG, and fornix in MCI relative to HC and a significant positive correlation between left UF FA and episodic memory in MCI alone. 199
A recent work evaluated the integrity of UF, inferior longitudinal fasciculus (ILF, connecting occipital cortex with anterior temporal lobe and amygdala), and inferior fronto-occipital fasciculus (IFOF, connecting occipital cortex to OFC through temporal cortex dorsal to uncinate fasciculus) along with the facial emotion selection test in AD. 200 Patients performed significantly worse in identifying negative but not positive or neutral facial emotions relative to HC. No differences in FA and mean diffusivity were observed between groups, though left UF but not ILF or IFOF diffusivity was negatively correlated with negative emotion score in patients. Thus, in AD patients, disruption of UF may affect the top-down PFC regulation of the amygdala during evaluation and recognition of negative facial emotions. 200 Notably, functional imaging studies of emotional reactivity as discussed in Section 4.4.1 suggest higher amygdala and lower cortical responses to negative emotions in AD. Thus, higher amygdala activity to negative facial emotional stimuli, whether reflecting a pathophysiological change or a compensatory process, did not appear to capture attention or facilitate the identification of emotional stimuli. It is highly likely that the disruption of amygdala cortical and hippocampal connectivity prevents emotional signals from being processed further in AD.
Progression of Emotion Processing Impairment in AD and Potential Neural Bases
It is difficult to characterize systematically how emotion processing dysfunction progresses through the course of AD. First, most of the studies included early-stage AD patients; second, mostly the studies are cross-sectional rather than longitudinal; and third, the studies were highly heterogeneous in the behavioral tasks employed to assess emotion processing. These limitations are also noted in a recent review. 201 The findings of impairment in facial emotion processing in early stage AD appear to be robust after controlling for cognitive impairment.47-50,52 A study targeting MCI and early stage AD observed impaired facial emotion recognition, but only for negative emotion (sadness). 53 A review on emotion recognition reported compromised identification of facial expression in MCI subjects. 41 However, another study observed that basic facial emotion processing is preserved in mild as well as severe AD. 51 On dynamic emotion recognition using film clips, recognition was preserved during early AD.55-58 In prospective assessments, emotion processing was worse during follow-up compared to baseline.23,56 Additionally, emotional memory appears to be impaired during early stage AD.57,58,78 Cognitive and attention dysfunctions may influence emotion processing, including labeling, recognition, and discrimination,48,49 but the great majority of studies suggest that AD may manifest emotional processing deficits independent of cognitive dysfunction even during the early stages.202,203 To fully understand how emotion processing dysfunction manifests during MCI and progresses through the course of AD would require longitudinal assessment of multiple domains of emotional function.
Both amygdala and hippocampus undergo atrophy during early stage AD, 153 with hippocampal atrophy associated with emotional memory dysfunction consistently across volumetric studies57,76,153,157,158 and amygdala atrophy associated with deficits in both emotion perception 57 and memory.76,153,154,157-159 These differences are also reflected in fMRI, with studies reporting amygdala hyperactivity during emotion perception, especially of negative emotion; 177 hippocampal hypoactivity during emotional memory 181 ; and hippocampal along with frontoparietal cortical hypoactivity during complex emotional memory (e.g., autobiographical memory). 182 However, it was noted in a study that the amygdala but not hippocampus was involved in self-referential negative memory. 154 Emotion processing implicates a number of other cortical and subcortical regions, including the prefrontal cortex, pre-/postcentral gyrus, cingulate, insula, and basal ganglia,42,63,65,161-163 which may be differentially involved in the neuropathology through the course of AD. The nature and extent of emotional processing deficits may depend on which specific brain regions and/or circuits are compromised across the stages of AD, a topic clearly warranting further investigation.
Studies assessing social emotion likewise utilized different tasks, targeted different functions, and reported mixed findings. Social emotion appears to be intact after accounting for cognitive ability167,168 in some studies but impaired in others42,170 in early AD. Atrophy of the insula, inferior frontal cortex, cingulate, hippocampus, amygdala, accumbens, and putamen correlated with social emotion processing dysfunction in AD. 42 Emotional empathy appeared to be intact, while cognitive empathy was impaired in early AD.171,172 Temporal cortical atrophy correlated with the severity of both emotional and cognitive empathy.171,172
Thus, although the findings are likely task-specific, emotion recognition, and memory appear to be impaired at the early stage and processing through the course of AD. Further, although emotion processing impairment may be present independent of cognitive dysfunction, cognitive demand of the emotional tasks and atrophy of brain regions required of the behavioral tasks may determine the overall severity.
Neurotransmitter Systems and Emotion Processing Dysfunction in AD
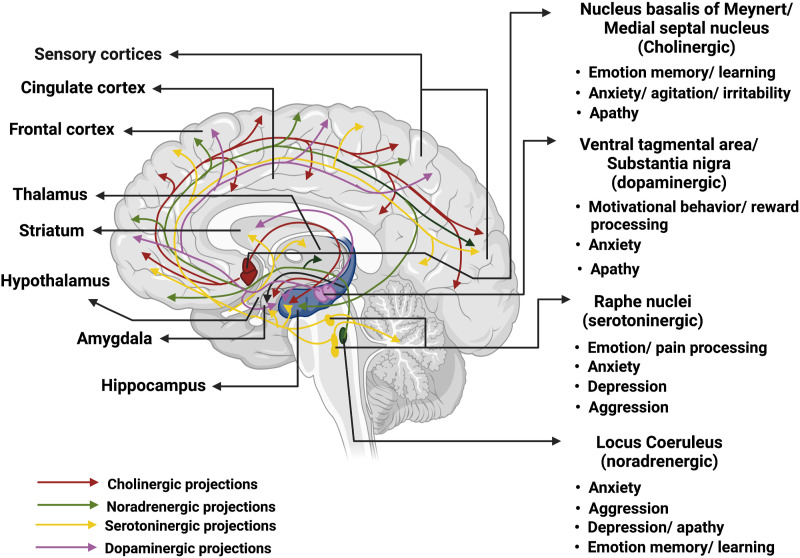
We review preclinical (AD rodent model) and clinical studies of the cholinergic, noradrenergic, serotoninergic, dopaminergic, and glutamatergic systems in emotional processing dysfunction in AD. Figure 8 provides a summary of the main findings as detailed in the following subsections.
Figure 8.
Implications of key neurotransmitter systems in AD emotion dysfunction. The major neurotransmitter systems are noted on the right, with colored arrows indicating the projections to cortical and subcortical targets. The hippocampus is highlighted in dark blue. Produced in almost all central nervous system cells (including neurons and glia) with receptors broadly available in the brain, glutamate interacts with many neurotransmitter pathways in all brain regions; thus, we did not depict the glutamatergic system in the figure.
Cholinergic System
Acetylcholine (ACh) is central to cognitive functioning; altered cholinergic transmission has been reported in various dementia syndromes, including AD. 204 In AD, cholinergic deficiency in the hippocampus and other cortico–limbic regions may contribute to the behavioral and psychological symptoms of dementia (BPSD), including apathy, agitation/irritability, and aggression. 205 The nucleus basalis of Meynert (nbM) along with the medial septum provides the primary cholinergic innervations to the cerebral cortex and hippocampus, and supports memory, attention, aversive learning, and executive functions.206,207 The nbM cholinergic neurons undergo approximately 5-fold decrease in number during advanced AD as compared to healthy aging. 208 Pathological changes of the nbM disrupt limbic ACh inputs to the neocortex and emotion processing, possibly leading to apathy, in AD. 209
Preclinical studies
Preclinical studies have provided ample evidence implicating the cholinergic system in the manifestation of AD symptoms. Immunohistochemistry demonstrated dystrophy of cholinergic neurites in AD mice model with amyloid pathology, as quantified by cholinergic presynaptic bouton density in the cortex. 210 Studies of transgenic mouse models showed cholinergic vulnerability to amyloid pathology, 211 up-regulated acetylcholinesterase (AChE) activity in the hippocampus, amygdala as well as lateral PFC, thalamus, and insula, 212 and anxiety-like symptoms along with a reduction in basal forebrain and amygdala cholinergic neurons as well as hippocampal and cortical levels of ACh. 213 Further, AChE inhibitors led to improvement in fear memory 214 and in anxiety-like behavioral responses 215 and memory in AD mouse models. 216 In accord, time-dependent changes in basal forebrain AChE expression coincided with anxiety-like behavior in aged mice. 217 Chronic ventricular infusion of Aβ fragments produced AD-like symptoms in rats, with the severity of anxiety (passive avoidance) correlated with decreases in ACh release in the frontal cortex and hippocampus. 218 Dietary choline supplements partially prevented these changes. 213 In mice exposed to chronic restraint stress, AChE inhibitors remediated loss of motivation and emotional responsiveness. 219 N-methyl-d-aspartate (NMDA)-induced neuronal loss in the amygdala led to reduced fear-freezing behavior and transplantation of neural stem cells overexpressing choline acetyltransferase gene successfully restored fear-freezing. 220
Clinical studies
Very few studies have specifically investigated the cholinergic system in humans. By examining the cytoarchitectonics of postmortem human brains, Zaborszky and colleagues constructed stereotaxic probabilistic maps in Montreal Neurological Institute coordinates of the basal forebrain areas, including the nbM. 221 Both the right and left nbM showed significantly smaller volumes with increasing age. 221 Building on this brain template, an earlier work described whole-brain functional connectivity (FC) of the nbM, showing that nbM FC with the visual and somatomotor cortices decreases while FC with subcortical structures including the midbrain, thalamus, and pallidum increases with age. 222
Pharmacological trials provide evidence of cholinergic dysfunction in AD. Administration of selective AChE and cholinesterase (ChE) inhibitors is known to improve AD symptoms.205,223-226 In a longitudinal study, rivastigmine improved anxiety, apathy, and agitation by more than 55% from the baseline. 227 Galantamine showed efficacy in ameliorating the deterioration in Neuropsychiatric Inventory (NPI) score, including agitation/aggression, anxiety, apathy, and irritability.228,229 Donepezil may improve the symptoms in moderately severe noninstitutionalized AD but showed no efficacy in mild to moderate noninstitutionalized AD or severe institutionalized AD patients, 230 suggesting a limited window of therapeutic efficacy. Other studies showed that ChE231,232 and AChE inhibitors 233 may improve apathy (see, however Ref. 234 for contrasting findings). These clinical trials involved patients at different stages of AD, which could account for the less than consistent findings. 235 A trial of donepezil with choline alphoscerate (choline-containing phospholipid) showed significant benefit in alleviating apathy, depression, and anxiety, as compared to donepezil alone. 236 The efficacy of most of ChE drugs reached a plateau after one to three years 237 and a switch in medications may improve the condition.238,239
Thus, preclinical and clinical studies support the roles of the cholinergic circuits in emotion processing dysfunction, most consistently with respect to anxiety-related behavior, in AD. Cholinergic circuit function likely undergoes dynamic changes through the course of illness, raising challenges to the efficacy of treatment with cholinergic agents.
Noradrenergic System
The cerebral cortex receives noradrenergic (NA) inputs primarily from the locus coeruleus (LC), a small nucleus located adjacent to the fourth ventricle in the midbrain with each LC comprising ∼1500 neurons in rodents 240 and ∼50 000 neurons in humans. 241 LC neurons project widely across cortical and subcortical regions. Projections to hypothalamus regulate hypothalamus–pituitary–adrenal circuit response to stress. 242 Animal work has focused on the role of the LC circuit in supporting arousal, attention, stress-related responses, and adaptive regulation of behavior.243-246 In particular, studies have accumulated to suggest that the NA circuits may contribute significantly to effort and motivated behavior. 247 These studies suggest a potentially critical role of LC circuit dysfunction in the pathogenesis of apathy in AD. There are very few imaging studies of the LC in humans, likely because of its small size, limited spatial resolution of MRI, and susceptibility of the MR signals in this region to physiological artifacts. Earlier studies controlled for physiological signals and demonstrated whole-brain FC of the LC in humans.248,249 On the other hand, combining neuromelanin imaging and fMRI offers a promising venue in delineating LC circuit function and dysfunction in humans. 250
Preclinical studies
Animal work implicates altered LC-NA signaling in depression. Loss of LC NA neurons by administration of 6-hydroxydopamine led depression-like behavior, as shown in increased immobility during a forced swim test (FST), in adult C57BL/6 mice. 251 The number of surviving LC neurons was positively correlated with FST immobility times, highlighting an ill-adaptive compensatory process leading to depressive behavior. 251 Chronic stress along with treatment with N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride, a selective LC neurotoxin, induced AD-like despair (measured using FST) in C57BL/6 mice. 252 A study quantified aminergic neurotransmission using micro-dialysis followed by high performance liquid chromatography and reported more frequent depressive behavior in 3 × Tg-AD mice along with reduced basal and K+ stimulated extracellular release of monoamines in frontal cortex and ventral hippocampus, compared to wild-type littermates. 253
Indeed, hippocampal NA signaling plays pivotal roles in contextual fear conditioning, where norepinephrine (NE) generally enhances hippocampal synaptic efficacy. However, NE beyond the optimal levels may compromise hippocampal long-term potentiation (LTP) and memory formation. 254 Propranolol (β-adrenergic receptor antagonist) treatment induced impairment in contextual fear conditioning.255,256 Clonidine (α2-adrenoreceptor agonist) administration blocked reconsolidation of fear memory, probably by altering hippocampal circuit activity. 257 Age-related decrease in NE levels affected hippocampal LTP, leading to impaired fear conditioning, a deficit reversible by administration of NE. 258 During fear conditioning, infusion of NE into hippocampal CA1 enhanced consolidation of extinction, an effect that could be blocked by treatment with propranolol. 259 Injection of low-dose of propranolol bilaterally to the hippocampus immediately after contextual fear conditioning induced generalized fear to safe cues, and the PTSD-like memory was associated with elevated hippocampal NE levels. 260 Another study showed that NA activation of basolateral amygdala may enhance hippocampus-dependent long-term emotional memory. 261 Thus, hippocampal NA signaling is involved in the consolidation and extinction of emotional memory via a number of cellular and molecular mechanisms.262-264
Clinical studies
Noradrenergic (NA) signaling modulates emotional memory and NA dysregulation may influence emotional memory in AD. 258 A 7-Tesla fMRI study observed LC activity in conjunction with amygdala and hippocampal activation each during memory encoding and consolidation, along with elevated heart rate and saliva α amylase levels. 265 A review of NA modulation of fear conditioning and extinction noted that amygdala-LC interaction may be governed by inverted-U effects of LC-NA inputs to the medial PFC (MPFC). 254 That is, under stress and other high-arousal conditions, LC enhances amygdala activity, leading to encoding/cued fear learning with concurrent blunting of MPFC activity. In contrast, during low-arousal conditions, LC enhances MPFC activity that in turn leads to inhibition of amygdala activity and extinction of fear memory. 254
AD presents with pathological changes in the LC during early stages of the illness, followed by widespread loss of NA neurons as the disease processes. 5 Studies reported a 12–66% reduction in tissue NE levels in the hippocampus, frontal and temporal cortex, thalamus, and amygdala. 266 Dysregulation of LC-NA system was associated with depression, anxiety, and agitation.267,268 LC neuronal loss was greater in AD patients with depression than those without, 209 in contrast with an earlier report. 269 The reduction in NE levels in both neocortex and allocortex was associated with higher rates of depressive AD symptoms. 270 In a recent study, bupropion (a NE reuptake inhibitor) vs placebo showed a positive effect on improving Neuropsychiatric Inventory (NPI) total score, distress, depression, and quality of life in AD patients but not improving apathy in nondepressed AD patients with apathy. 271
A positron emission tomography (PET) imaging study noted lower NE concentration in the mid-temporal and orbitofrontal cortex (OFC) in AD than controls and lower cell count in rostral LC in correlation with aggression in AD. 272 Higher cerebellar NA nerve fibers and α2 receptor densities 209 as well as cerebellar but not frontal cortical or hypothalamic α2, β1, and β2 adrenoreceptor density were noted in AD patients with aggression compared to those without aggression and healthy controls. 273 In addition, the CSF levels of 3-methoxy-4-hydroxyphenylethyleneglycol—a marker of NE metabolism, with a higher level indicating reduced NE levels 274 —was positively correlated with NPI score in a combined sample of patients with subjective cognitive decline, MCI, and probable AD. 267 An α1 adrenergic receptor blocker, prazosin improved NPI, and Brief Psychiatric Rating Scale scores with a profile of adverse effects comparable to placebo. 275 Propranolol also improved total NPI and Clinical Global Impression of Change scores, especially agitation/aggression and anxiety. 276 A study showed efficacy of low dose (10–80 mg/day) propranolol in alleviating agitation by 67% without prominent side effects over a period of 14 months. 277
Thus, ample direct and indirect evidence implicates the LC-NA systems in affective dysfunction in AD.
Serotoninergic System
Serotonin or 5-hydroxytryptamine (5-HT) is central to the regulation of sleep–wake cycle, emotion, pain, as well as memory and learning.278,279 The raphe nuclei project widely across the central nervous system (CNS), to the cortex, thalamus, hypothalamus, basal ganglia, brain stem, and spinal cord, highlighting potential roles of the serotoninergic circuits in cognitive and emotion processing. 280
Preclinical studies
Serotonin receptors colocalize on cholinergic, glutamatergic as well as GABAergic neurons 281 and serotonergic dysfunction may lead to AD pathology and symptomatology.278,282,283 A study highlighted the interaction of serotonergic, glutamatergic, and cholinergic systems in the formation of emotion memory and contextual freezing. 284 Other studies showed that serotonin-selective reuptake inhibitor paroxetine ameliorated anxiety-like behavior in AD mice.285,286 Fluoxetine treatment reversed depressive behavior elicited by intraventricular injection of Aβ-oligomers in mice, 287 likely via mechanisms related to Toll-like receptor 4-dependent microglial activity. 288 Preclinical studies have also associated serotoninergic system with aggression in AD models.280,286,289
Clinical studies
In postmortem, studies decreases in cortical tissue 5-HT levels were evident in agitated vs nonagitated AD and CSF levels of 5-HT metabolites were correlated with the severity of anxiety and fear in AD. 290 AD vs controls showed no significant differences in 5-HT(1A) receptor affinity and density in postmortem neocortical samples; however, within the AD group, 5-HT(1A) receptor density was correlated negatively with aggression. 291 Another postmortem study reported reduced temporal cortical 5-HT transporter (5-HTT) density in AD patients without anxiety but not those with anxiety, relative to controls. 292 The study also observed that polymorphism in 5-HTT promoter region in the homozygous condition led to enhanced incidence of anxiety in AD. 292 A review supports this finding and suggests that the anti-aggressive effects of 5-HT(1A/1B) receptor agonist are due to reduction rather than enhancement of serotonergic transmission during aggressive/combative social situations. 293 5-HT(1A/1B) receptor agonists, when administered systemically, decreased 5-HT release via their inhibitory actions at somatodendritic and terminal autoreceptors. 293 AD patients with involuntary emotional expression—prevalent in 15–39% of AD and characterized by episodes of uncontainable crying/laughing, irritation, anger, and frustration 294 —demonstrated significantly lower platelet 5-HT concentration compared to AD patients with aggression and controls. 295
Serotonin reuptake inhibitors (SRIs) form one of the therapeutic regimens for AD behavioral symptoms. 296 SRIs reduced irritability, 297 agitation,298,299 anxiety, and depression 209 in patients with AD and other forms of dementia. On the other hand, a meta-analysis showed no benefits of SRIs on cognition, mood, agitation, and daily activities in patients with dementia. 300 Combination of agonists and antagonists targeting different serotonin receptors may represent one therapeutic avenue in managing behavioral and psychological symptoms of dementia. 280
Dopaminergic System
The ventral tegmental area (VTA) is the origin of dopaminergic (DA/TH+) axons that project to the hippocampus, nucleus accumbens, PFC, olfactory bulb, and amygdala. 301 This mesocorticolimbic DA pathway supports reward processing and motived behavior, 302 and degeneration of the VTA may manifest in behavioral symptoms in AD. 301 Aging is associated with decrease in dopamine release in the caudate, putamen, hippocampus, and frontal cortex 303 ; however, the exact roles of DA dysfunction in the pathogenesis of AD remain unclear. 304 Recent studies demonstrated anti-amyloidogenic activity of dopamine and its derivatives in ameliorating inflammation and oxidative stress, suggesting a potential pathway of DA dysfunction in the disease process of AD. 305
Pre-clinical studies
AD animal models showed early degeneration of VTA in the preplaque phase,306,307 resulting in reduced DA outflow and altered neuronal excitability and synaptic plasticity in the hippocampus and nucleus accumbens.306,308 Reduction of VTA DA inputs to the nucleus accumbens shell was associated with impairment in food conditioned place preference (CPP), highlighting the contribution of dopamine to age-related changes in reward processing and learning. 306 Neurochemical studies have aimed to delineate the functional roles of DA receptor systems in learning. Pre-training infusion of SCH-23390 (D1 receptor antagonist) in the MPFC led to impaired fear memory retrieval in rats.309,310 SCH-23390 infusion into the hippocampus and basolateral amygdala led to impaired contextual fear conditioning. 311 In addition, systemic administration of D1 receptor agonist enhanced fear conditioning 312 and extinction. 313 A food reward study implicated D1/D5 and D2 receptors each in context-dependent and context-independent extinction. 314 D2 antagonist and agonist enhanced fear response and reduced fear conditioning, respectively. 315 In rats engaged in an effort-based decision-making task, D1 antagonist SCH-23390 and D2 antagonist haloperidol reduced the effort expended to obtain reward. 316 DA agonist d-amphetamine reversed these effects and rats were driven to more frequent reward-seeking behavior. In contrast, treatment with D3 receptor antagonist U99194 and agonist 7-OH-DPAT showed no effect. 316 Stress elevated DA signals in the MPFC 317 and administration of dopamine D3 receptor antagonist-YQA14A and SB-277011A reduced stress-like behavior in rats. 318 Thus, preclinical studies point to dopamine’s complex roles in emotional and motivational behavior. How DA system and behavior supported by the DA system are impacted by aging and AD models associating changes in DA functions to emotional behavior remain to be investigated.
Clinical studies
Studies in healthy individuals have documented the roles of DA transmission in reward and emotion processing.315,319,320 Other studies noted stress-induced enhancement of dopamine release in the PFC in healthy adults, suggesting a complex and likely region-specific relationship between DA signaling and stress/anxiety.321,322 Other PET and fMRI studies have implicated the DA system in response inhibition, 323 reward learning 324 as well as reward and loss processing, 325 which are known to alter during aging.326-332
Studies have proposed a DA basis of apathy in AD, considering dopamine’s role in reward processing and motivated behavior. A PET study associated reduced levels of dopamine transporter (DAT) or loss of putaminal DA neurons with apathy in AD. 333 Another study noted a correlation between the reduction in bilateral caudate dopamine receptors and apathy in AD. 334
In recent years, a number of studies have suggested modest efficacy of methylphenidate (a DAT inhibitor) in alleviating apathy in AD.335-337 However, methylphenidate also blocks norepinephrine (NE) transporter (NET), and thus, the benefit cannot be conclusively attributed to DA signaling.268,338 Rodent studies have suggested that methylphenidate produces an even greater increase in NE than DA in medial PFC. 339 Moreover, therapeutic doses of methylphenidate in humans have yielded receptor occupancy that is at least as great for NET 340 as for DAT, 341 consistent with the higher in vitro affinity of methylphenidate for NET than DAT 342 and suggesting the potential relevance of NET inhibition in the therapeutic effects of methylphenidate. In contrast, a recent study of the combined DAT/NET inhibitor bupropion showed no benefit in ameliorating apathy in non-depressed AD patients. 271 Dextroamphetamine, modafinil, amantadine, and bromocriptine are some other dopamine modulating drugs that have been studied for the treatment of AD apathy, 343 although some of these have noradrenergic effects as well.
In AD, neuropsychiatric symptoms (NPS) including agitation, irritability, and disinhibition correlated with VTA-parahippocampal gyrus and cerebellum connectivity. 344 In addition, DA receptor agonists and antagonists promote and attenuate reward-seeking behavior, respectively, in AD. 343
Thus, DA neurotransmission may contribute to behavioral pathophysiology of AD, but the area is relatively new and much more needs to be studied.
Glutamatergic System
A major excitatory neurotransmitter glutamate at high synaptic concentrations may cause excitotoxicity and neuronal death. In AD, dysregulated activation of glutamate receptors, mainly the N-methyl-D-aspartate receptor (NMDAR), may instigate excitotoxicity and affect learning and memory.283,345 In contrast, hypo-NMDAR activity induced by NMDAR antagonists may also cause neurodegeneration. 346 Frontal presynaptic glutamatergic bouton density was upregulated in MCI but depleted in mild and severe AD. 347 Findings of CSF glutamate levels were inconsistent. 348 It is possible that glutamatergic activity is enhanced during the initial stages, leading to neuronal loss and under-active glutamatergic signaling during later stages 349 of AD. Notably, the glutamatergic systems support both long- and short-range neural connections across the brain 350 and glutamatergic dysfunction is likely to have broad impacts on cognition and emotion in pathological aging.
Pre-clinical studies
The protein structure, binding specificity, signaling mechanism, and anatomical distribution of glutamate receptors vary and thus their actions are specific and may even be functionally antagonistic. 350 Sustained suppression of group-II metabotropic glutamate receptors promoted hippocampal neurogenesis and improved anxiety-like behavior in AD animal model. 351 Another study showed reduced expression of hippocampal metabotropic receptor-7 in association with anxiety-like behavior in AD mice model. 352 Further, overexpression of hippocampal EphB2, a regulator of synaptic localization of NMDA receptors, improved anxiety, and depression-like behavior in AD mice model. 353 Hippocampal NMDA receptor blockade improved anxiety and depression-like symptoms induced by streptozotocin in rat models of sporadic AD. 354 Guanosine modulated glutamatergic signaling and prevented anhedonia in AD-like mouse model possibly via restoring the hippocampal glutamate uptake. 355 In AD mouse model with aggression and impaired fear memory, hippocampal GluN1 expression was increased and GluA2 was decreased, potentially affecting the formation of stable synapses. 356 Further, paroxetine, which improved anxiety- and depression-like behavior, may functionally restore PFC glutamate receptors in an AD mice model. 285 Thus, preclinical studies implicate the glutamatergic system in emotion processing dysfunction and the pathophysiology of anxiety and depression.
Clinical studies
Dysfunctional glutamatergic system is also implicated in the pathogenesis of anxiety, depression, and agitation in humans. Both enhancement and attenuation of glutamate signaling have benefited behavioral symptoms, suggesting a complex role of glutamatergic signaling in AD. 346 For instance, an NMDAR partial antagonist, memantine could alleviate agitation by normalizing frontal cortical and cingulate glutamatergic dysfunction or by reducing tau phosphorylation leading to reduced NFT formation. 357 Further, patients with probable AD and major depression showed higher CSF glutamate and glutamine levels relative to healthy controls. 348 Orbitofrontal cortical density of NMDAR subunit NR2A was reduced in high vs low anxiety AD patients. 358 In addition, AD patients with high vs low anxiety showed elevated NMDAR glycine recognition site (GlyRS) binding affinity for GlyRS antagonist in association with reduction of NR2A density and the severity of anxiety. 358 Thus, the literature suggests a complex, receptor, and/or receptor subunit-specific alterations in glutamate functions in association with AD behavioral and psychological symptoms.
Interaction Between Neurotransmitter Systems
Although discussed separately, the neurotransmitter systems may interact to influence cognition and emotion in AD. For instance, Aβ oligomers bind with α7 nicotinic acetylcholine receptors (nAChR) with high affinity, 304 which may lead to a reduction of DA signaling304,359 and apathy and depression in addition to cognitive dysfunction in AD. 204 LC may enhance the release of ACh via activating α2-adrenoceptors on the cholinergic terminals of the nbM during healthy aging, a mechanism that when going awry may lead to AD.360,361 In addition, a dopamine D2-like receptor agonist, rotigotine induced cortical excitability and restored central cholinergic transmission in AD. 362 In the prefrontal cortex, glutamate release gets attenuated by acetylcholine receptor AChR antagonist. 349 Further, a postmortem study showed that an imbalance in serotonergic and cholinergic signaling may underpin the behavioral symptoms in AD. 363 However, a detailed review is beyond the scope of the current work.
Summary of emotion Processing Dysfunction in AD
We reviewed the neural bases of emotional dysfunction in AD with two foci: (1) clinical psychological and imaging studies of AD and (2) clinical and preclinical studies implicating the major neurotransmitter systems in emotion processing in AD as well as anxiety- and depression-related behavior in animal models of AD. Studies broadly support multiple chemical circuits in emotion processing dysfunction in AD, although the evidence appears the strongest and most direct for a role of the cholinergic and noradrenergic system each in emotion memory and anxiety. The basal nucleus of Meynert undergoes significant loss of neurons and may lead to disconnection with the cortico–limbic circuits during emotion processing. Likewise, early degeneration of the locus coeruleus disrupts noradrenergic signaling in the hippocampal circuits and contributes to emotion processing dysfunction and emotional disorders in AD. Psychological studies have employed different paradigms to address emotional dysfunction in AD and the findings vary significantly with respect to the experimental tasks, particularly in terms of the demand on cognitive capacity. Nonetheless, the studies most consistently reported deficits in processing negative emotions (sadness and fear) in AD. Notably, environmental stimuli that elicit negative emotions tend to be more salient and demand attention and arousal in fight or flight decisions, in broad accord with cholinergic and noradrenergic circuit dysfunction in AD. Further, although imaging studies have not differentiated the neural bases of positive and negative emotions, negative emotions more significantly engage the left insula, amygdala, and anterior hippocampus, 364 consistent with behavioral findings and evidence of hippocampal circuit dysfunction early in the course of AD. Emotional enhancement of memory appears to be relatively preserved, and the effects seem most prominent for positive emotions; in contrast, emotional stimuli do not capture attention, despite evidence of emotion reactivity in AD. In social emotions, AD subjects exhibit contagious emotional behavior and emotional empathy, although the extent of social emotion deficits varies across studies. Imaging studies support elevated amygdala reactivity to emotional stimuli; however, structural and functional cortical and hippocampal connectivity are disrupted in AD. Further, the hippocampus demonstrates structural (volumetric and connectivity) and functional changes that impact emotion processing in AD. It is possible that the amygdala responds to emotional stimuli but, as a result of cortical and hippocampal circuit dysfunction, the stimuli were not properly registered, perceived, and/or evaluated in AD. Overall, the studies suggest altered experience of emotions early in the course of illness and a central role of the cholinergic and noradrenergic signaling in the hippocampal circuits in manifesting emotional deficits in AD.
A number of research directions may be fruitful in understanding emotional dysfunction in AD. First, studies can systematically distinguish the neural processes of emotional reactivity and emotional memory both during healthy aging and early in the course of AD. In addition to subjective report of emotional experience and assessment of the accuracy of emotion memory, investigators can quantify arousal by recording galvanic skin response and pupil size to fully understand the physiological impact of the emotional stimuli. Further, by employing neuromelanin imaging investigators will be able to localize the locus coeruleus and characterize the roles of noradrenergic as well as cholinergic circuits in supporting emotional reactivity and memory. Second, emotional experience depends significantly on how one regulates emotions and successful emotion regulation conduces to well-being during aging. 365 Research in experimental psychology has described the neural processes of emotion regulation via reappraisal and distancing as well as age-related changes in the psychological processes of emotion regulation. For instance, older people seem able to suppress emotion without experiencing higher anxiety as observed in younger individuals. 366 However, it remains unclear whether aging contributes to positive emotional experience because of more efficient regulation or because of diminished registration of negative emotions? No studies to our knowledge have distinguished these mechanisms or investigated these issues in AD. Finally, the hippocampus plays important roles in the encoding of episodic and autobiographical memory as well as in emotional experience and regulation. 16 As most of the atypical AD cases are “hippocampal-sparing,” emotion may be differentially affected as compared to typical AD patients. Yet, most of the studies of emotional dysfunction have not distinguished typical and atypical AD. Research focusing on both limbic predominant and hippocampal-sparing AD would provide an opportunity to specify the roles of the hippocampal circuit dysfunction in emotion processing deficits in AD.
Supplemental Material
Supplemental Material, sj-pdf-1-aja-10.1177_15333175221082834 for Emotion Processing Dysfunction in Alzheimer’s Disease: An Overview of Behavioral Findings, Systems Neural Correlates, and Underlying Neural Biology by Shefali Chaudhary, Simon Zhornitsky, Herta H. Chao, Christopher H. van Dyck and Chiang-Shan R. Li in American Journal of Alzheimer's Disease & Other Dementias
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The NIH and VA are otherwise not responsible for the design of the study or data analyses and interpretation or in the decision to publish this review. Authors have no conflicts of interest in the current study.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The current study is supported by NIH grants R21AG067024 (Li), R01AG072893 (Li), R01CA218501 (Chao), and P30AG066508 (van Dyck) as well as a VA Merit Award CX001301 (Chao).
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Shefali Chaudhary https://orcid.org/0000-0003-4505-0506
Chiang-Shan R. Li https://orcid.org/0000-0002-9393-1212
References
- 1.Kuca K, Maresova P, Klimova B, Valis M, Hort J. Alzheimer& rsquo;s disease and language impairments: social intervention and medical treatment. Clin Interv Aging. 2015;10:1401. doi: 10.2147/CIA.S89714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X-L, Hu N, Tan M-S, Yu J-T, Tan L. Behavioral and psychological symptoms in Alzheimer’s disease. BioMed Res Int. 2014;2014:1-9. doi: 10.1155/2014/927804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Förstl H, Kurz A. Clinical features of Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci. 1999;249(6):288-290. doi: 10.1007/s004060050101. [DOI] [PubMed] [Google Scholar]
- 4.López OL, Dekosky ST. Clinical symptoms in Alzheimer’s disease. Handb Clin Neurol. 2008;89:207-216. doi: 10.1016/S0072-9752(07)01219-5. [DOI] [PubMed] [Google Scholar]
- 5.Peterson AC, Li C-sR. Noradrenergic dysfunction in Alzheimer’s and Parkinson’s diseases-an overview of imaging studies. Front Aging Neurosci. 2018;10:127. doi: 10.3389/fnagi.2018.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239-259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Jia X, Qi Z, et al. Disrupted functional connectivity of cornu ammonis subregions in amnestic mild cognitive impairment: a longitudinal resting-state fMRI study. Front Hum Neurosci. 2018;12:413. doi: 10.3389/fnhum.2018.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maruszak A, Thuret S. Why looking at the whole hippocampus is not enough—a critical role for anteroposterior axis, subfield and activation analyses to enhance predictive value of hippocampal changes for Alzheimer’s disease diagnosis. Front Cell Neurosci. 2014;8:95. doi: 10.3389/fncel.2014.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. Behav Brain Sci. 2012;35(3):121-143. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindquist DH. Emotion in motion: a three-stage model of aversive classical conditioning. Neurosci Biobehav Rev. 2020;115:363-377. doi: 10.1016/j.neubiorev.2020.04.025. [DOI] [PubMed] [Google Scholar]
- 11.Rolls ET. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct Funct. 2019;224(9):3001-3018. doi: 10.1007/s00429-019-01945-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sturm VE, Yokoyama JS, Seeley WW, Kramer JH, Miller BL, Rankin KP. Heightened emotional contagion in mild cognitive impairment and Alzheimer’s disease is associated with temporal lobe degeneration. Proc Natl Acad Sci. 2013;110(24):9944-9949. doi: 10.1073/pnas.1301119110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogt BA. Cingulate cortex in the three limbic subsystems. Handb Clin Neurol. 2019;166:39-51. doi: 10.1016/B978-0-444-64196-0.00003-0. [DOI] [PubMed] [Google Scholar]
- 14.Girardeau G, Inema I, Buzsáki G. Reactivations of emotional memory in the hippocampus–amygdala system during sleep. Nat Neurosci. 2017;20(11):1634-1642. doi: 10.1038/nn.4637. [DOI] [PubMed] [Google Scholar]
- 15.Immordino-Yang MH, Singh V. Hippocampal contributions to the processing of social emotions. Hum Brain Mapp. 2013;34(4):945-955. doi: 10.1002/hbm.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Y, Gao H, Tong L, et al. Emotion regulation of hippocampus using real-time fMRI neurofeedback in healthy human. Front Hum Neurosci. 2019;13:242. doi: 10.3389/fnhum.2019.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellace M, Williams JM, Mohamed FB, Faro SH. An fMRI study of the activation of the hippocampus by emotional memory. Int J Neurosci. 2012;123(2):121-127. doi: 10.3109/00207454.2012.742894. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Chen Z, Zhang R, Xu T, Feng T. Neural substrates underlying episodic future thinking: a voxel-based morphometry study. Neuropsychologia. 2020;138:107255. doi: 10.1016/j.neuropsychologia.2019.107255. [DOI] [PubMed] [Google Scholar]
- 19.Huo H, Zhang R, Seger CA, Feng T, Chen Q. The effect of trait anxiety on risk-taking: Functional coupling between right hippocampus and left insula. Psychophysiology. 2020;57(10):e13629. doi: 10.1111/psyp.13629. [DOI] [PubMed] [Google Scholar]
- 20.Yun J-Y, Jin MJ, Kim S, Lee S-H. Stress-related cognitive style is related to volumetric change of the hippocampus and FK506 binding protein 5 polymorphism in post-traumatic stress disorder. Psychol Med. 2020;7:1-12. doi: 10.1017/S0033291720002949. [DOI] [PubMed] [Google Scholar]
- 21.Dark HE, Harnett NG, Goodman AM, et al. Violence exposure, affective style, and stress-induced changes in resting state functional connectivity. Cogn Affect Behav Neurosci. 2020;20(6):1261-1277. doi: 10.3758/s13415-020-00833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bediou B, Brunelin J, d’Amato T, et al. A comparison of facial emotion processing in neurological and psychiatric conditions. Front Psychol. 2012;3:98. doi: 10.3389/fpsyg.2012.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavenu I, Pasquier F. Perception of emotion on faces in frontotemporal dementia and Alzheimer’s disease: a longitudinal study. Dement Geriatr Cogn Disord. 2005;19(1):37-41. doi: 10.1159/000080969. [DOI] [PubMed] [Google Scholar]
- 24.Alves G, Carvalho A, Carvalho L, et al. Neuroimaging findings related to behavioral disturbances in Alzheimer's disease: a systematic review. Curr Alzheimer Res. 2016;14(1):61-75. doi: 10.2174/1567205013666160603010203. [DOI] [PubMed] [Google Scholar]
- 25.Boublay N, Schott AM, Krolak‐Salmon P. Neuroimaging correlates of neuropsychiatric symptoms in Alzheimer’s disease: a review of 20 years of research. Eur J Neurol. 2016;23(10):1500-1509. doi: 10.1111/ene.13076. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Dang M, Zhang Z. Brain mechanisms underlying neuropsychiatric symptoms in Alzheimer’s disease: a systematic review of symptom-general and -specific lesion patterns. Mol Neurodegener. 2021;16(1):38. doi: 10.1186/s13024-021-00456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberg PB, Nowrangi MA, Lyketsos CG. Neuropsychiatric symptoms in Alzheimer’s disease: What might be associated brain circuits? Mol Aspects Med. 2015;43-44:25-37. doi: 10.1016/j.mam.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theleritis C, Politis A, Siarkos K, Lyketsos CG. A review of neuroimaging findings of apathy in Alzheimer’s disease. Int psychogeriatrics. 2014;26(2):195-207. doi: 10.1017/S1041610213001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Victoroff J, Lin FV, Coburn KL, Shillcutt SD, Voon V, Ducharme S. Noncognitive behavioral changes associated with Alzheimer’s disease: implications of neuroimaging findings. J Neuropsychiatry Clin Neurosci. 2018;30(1):14-21. doi: 10.1176/appi.neuropsych.16080155. [DOI] [PubMed] [Google Scholar]
- 30.Wenzler S, Knochel C, Balaban C, et al. Integrated biomarkers for depression in Alzheimer’s disease: a critical review. Curr Alzheimer Res. 2017;14(4):441-452. doi: 10.2174/1567205013666160603011256. [DOI] [PubMed] [Google Scholar]
- 31.Jack CR. Alzheimer disease: new concepts on its neurobiology and the clinical role imaging will play. Radiology. 2012;263(2):344-361. doi: 10.1148/radiol.12110433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gómez-Isla T, Hollister R, West H, et al. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol. 1997;41(1):17-24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- 33.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61(3):378-384. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- 34.Ingelsson M, Fukumoto H, Newell KL, et al. Early Abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology. 2004;62(6):925-931. doi: 10.1212/01.wnl.0000115115.98960.37. [DOI] [PubMed] [Google Scholar]
- 35.Ferreira D, Verhagen C, Hernández-Cabrera JA, et al. Distinct subtypes of Alzheimer’s disease based on patterns of brain atrophy: longitudinal trajectories and clinical applications. Sci Rep. 2017;7(1):46263. doi: 10.1038/srep46263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitwell JL, Dickson DW, Murray ME, et al. Neuroimaging correlates of pathologically defined subtypes of Alzheimer’s disease: a case-control study. Lancet Neurol. 2012;11(10):868-877. doi: 10.1016/S1474-4422(12)70200-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitwell JL, Josephs KA, Murray ME, et al. MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology. 2008;71(10):743-749. doi: 10.1212/01.wnl.0000324924.91351.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dickerson BC, McGinnis SM, Xia C, et al. Approach to atypical Alzheimer’s disease and case studies of the major subtypes. CNS Spectr. 2017;22(6):439-449. doi: 10.1017/S109285291600047X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graff-Radford J, Yong KXX, Apostolova LG, et al. New insights into atypical Alzheimer's disease in the era of biomarkers. Lancet Neurol. 2021;20(3):222-234. doi: 10.1016/S1474-4422(20)30440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer's disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011;10(9):785-796. doi: 10.1016/S1474-4422(11)70156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCade D, Savage G, Naismith SL. Review of emotion recognition in mild cognitive impairment. Dement Geriatr Cognit Disord. 2011;32(4):257-266. doi: 10.1159/000335009. [DOI] [PubMed] [Google Scholar]
- 42.Kumfor F, Sapey-Triomphe L-A, Leyton CE, Burrell JR, Hodges JR, Piguet O. Degradation of emotion processing ability in corticobasal syndrome and Alzheimer's disease. Brain. 2014;137(Pt 11):3061-3072. doi: 10.1093/brain/awu246. [DOI] [PubMed] [Google Scholar]
- 43.Shimokawa A, Yatomi N, Anamizu S, et al. Comprehension of emotions: comparison between Alzheimer type and vascular type dementias. Dement Geriatr Cognit Disord. 2000;11(5):268-274. doi: 10.1159/000017249. [DOI] [PubMed] [Google Scholar]
- 44.Derouesné C, Piquard A, Thibault S, Baudouin-Madec V, Lacomblez L. [Noncognitive symptoms in Alzheimer's disease. A study of 150 community-dwelling patients using a questionnaire completed by the caregiver]. Revue neurologique. 2001;157(2):162-177. http://www.ncbi.nlm.nih.gov/pubmed/11283463. [PubMed] [Google Scholar]
- 45.Fernández M, Gobartt AL, Gobartt AL, Balañá M, COOPERA Study Group . Behavioural symptoms in patients with Alzheimer's disease and their association with cognitive impairment. BMC Neurol. 2010;10:87. doi: 10.1186/1471-2377-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein-Koerkamp Y, Beaudoin M, Baciu M, Hot P. Emotional decoding abilities in Alzheimer's disease: a meta-analysis. J Alzheim Dis. 2012;32(1):109-125. doi: 10.3233/JAD-2012-120553. [DOI] [PubMed] [Google Scholar]
- 47.McLellan T, Johnston L, Dalrymple-Alford J, Porter R. The recognition of facial expressions of emotion in Alzheimer's disease: a review of findings. Acta Neuropsychiatr. 2008;20(5):236-250. doi: 10.1111/j.1601-5215.2008.00315.x. [DOI] [PubMed] [Google Scholar]
- 48.Phillips LH, Scott C, Henry JD, Mowat D, Bell JS. Emotion perception in Alzheimer's disease and mood disorder in old age. Psychol Aging. 2010;25(1):38-47. doi: 10.1037/a0017369. [DOI] [PubMed] [Google Scholar]
- 49.Torres B, Santos RL, Sousa MFBd, et al. Facial expression recognition in Alzheimer's disease: a longitudinal study. Arq Neuro Psiquiatr. 2015;73(5):383-389. doi: 10.1590/0004-282X20150009. [DOI] [PubMed] [Google Scholar]
- 50.Jiskoot LC, Poos JM, Vollebergh ME, et al. Emotion recognition of morphed facial expressions in presymptomatic and symptomatic frontotemporal dementia, and Alzheimer's dementia. J Neurol. 2021;268(1):102-113. doi: 10.1007/s00415-020-10096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luzzi S, Piccirilli M, Provinciali L. Perception of emotions on happy/sad chimeric faces in Alzheimer disease: relationship with cognitive functions. Alzheimer Dis Assoc Disord. 2007;21(2):130-135. doi: 10.1097/WAD.0b013e318064f445. [DOI] [PubMed] [Google Scholar]
- 52.Kohler CG, Anselmo-Gallagher G, Bilker W, Karlawish J, Gur RE, Clark CM. Emotion-discrimination deficits in mild Alzheimer disease. Am J Geriatr Psychiatr. 2005;13(11):926-933. doi: 10.1176/appi.ajgp.13.11.926. [DOI] [PubMed] [Google Scholar]
- 53.Chuang Y-C, Chiu M-J, Chen T-F, et al. An exploration of the own-age effect on facial emotion recognition in normal elderly people and individuals with the preclinical and demented Alzheimer's disease. J Alzheim Dis. 2021;80:259-269. doi: 10.3233/JAD-200916. [DOI] [PubMed] [Google Scholar]
- 54.Vogel A, Jørgensen K, Larsen IU. Normative data for Emotion Hexagon test and frequency of impairment in behavioral variant frontotemporal dementia, Alzheimer's disease and Huntington's disease. Appl Neuropsychol: Adult. 2020;29:127-132. doi: 10.1080/23279095.2020.1720686. [DOI] [PubMed] [Google Scholar]
- 55.Goodkind MS, Sturm VE, Ascher EA, et al. Emotion recognition in frontotemporal dementia and Alzheimer's disease: a new film-based assessment. Emotion. 2015;15(4):416-427. doi: 10.1037/a0039261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Satler C, Uribe C, Conde C, Da-Silva SL, Tomaz C. Emotion processing for arousal and neutral content in Alzheimer's disease. Int J Alzheimer's Dis. 2009;2009:1-6. doi: 10.4061/2009/278615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guzmán-Vélez E, Warren DE, Feinstein JS, Bruss J, Tranel D. Dissociable contributions of amygdala and hippocampus to emotion and memory in patients with Alzheimer's disease. Hippocampus. 2016;26(6):727-738. doi: 10.1002/hipo.22554. [DOI] [PubMed] [Google Scholar]
- 58.Guzmán-Vélez E, Feinstein JS, Tranel D. Feelings without memory in Alzheimer disease. Cogn Behav Neurol. 2014;27(3):117-129. doi: 10.1097/WNN.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beall PM, Herbert AM. The face wins: stronger automatic processing of affect in facial expressions than words in a modified Stroop task. Cognit Emot. 2008;22(8):1613-1642. doi: 10.1080/02699930801940370. [DOI] [Google Scholar]
- 60.Ovaysikia S, Tahir KA, Chan JL, DeSouza JFX. Word wins over face: emotional stroop effect activates the frontal cortical network. Front Hum Neurosci. 2011;4:234. doi: 10.3389/fnhum.2010.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meléndez JC, Satorres E, Oliva I. Comparing the effect of interference on an emotional stroop task in older adults with and without Alzheimer's disease. J Alzheim Dis. 2020;73(4):1445-1453. doi: 10.3233/JAD-190989. [DOI] [PubMed] [Google Scholar]
- 62.Gainotti G. The role of the right hemisphere in emotional and behavioral disorders of patients with frontotemporal lobar degeneration: an updated review. Front Aging Neurosci. 2019;11:55. doi: 10.3389/fnagi.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsieh S, Hornberger M, Piguet O, Hodges JR. Brain correlates of musical and facial emotion recognition: evidence from the dementias. Neuropsychologia. 2012;50(8):1814-1822. doi: 10.1016/j.neuropsychologia.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 64.Cadieux NL, Greve KW. Emotion processing in Alzheimer's disease. J Int Neuropsychol Soc. 1997;3(5):411-419. http://www.ncbi.nlm.nih.gov/pubmed/9322399. [PubMed] [Google Scholar]
- 65.Brown CL, Hua AY, De Coster L, et al. Comparing two facets of emotion perception across multiple neurodegenerative diseases. Soc Cognit Affect Neurosci. 2020;15(5):511-522. doi: 10.1093/scan/nsaa060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Henry JD, Rendell PG, Scicluna A, Jackson M, Phillips LH. Emotion experience, expression, and regulation in Alzheimer's disease. Psychol Aging. 2009;24(1):252-257. doi: 10.1037/a0014001. [DOI] [PubMed] [Google Scholar]
- 67.Burton KW, Kaszniak AW. Emotional experience and facial expression in Alzheimer's disease. Aging Neuropsychol Cognit. 2006;13(3-4):636-651. doi: 10.1080/13825580600735085. [DOI] [PubMed] [Google Scholar]
- 68.Decety J, Meyer M. From emotion resonance to empathic understanding: a social developmental neuroscience account. Dev Psychopathol. 2008;20(4):1053-1080. doi: 10.1017/S0954579408000503. [DOI] [PubMed] [Google Scholar]
- 69.Decety J. Dissecting the neural mechanisms mediating empathy. Emotion Review. 2011;3(1):92-108. doi: 10.1177/1754073910374662. [DOI] [Google Scholar]
- 70.Fischer A, Landeira-Fernandez J, Sollero de Campos F, Mograbi DC. Empathy in Alzheimer's Disease: Review of Findings and Proposed Model. J Alzheim Dis. 2019;69(4):921-933. doi: 10.3233/JAD-180730. [DOI] [PubMed] [Google Scholar]
- 71.Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9(2):148-158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- 72.Okon-Singer H, Hendler T, Pessoa L, Shackman AJ. The neurobiology of emotionâ€"cognition interactions: fundamental questions and strategies for future research. Front Hum Neurosci. 2015;9:58. doi: 10.3389/fnhum.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Broster LS, Blonder LX, Jiang Y. Does emotional memory enhancement assist the memory-impaired? Front Aging Neurosci. 2012;4:2. doi: 10.3389/fnagi.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nashiro K, Sakaki M, Mather M. Age differences in brain activity during emotion processing: reflections of age-related decline or increased emotion regulation? Gerontology. 2012;58(2):156-163. doi: 10.1159/000328465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klein-Koerkamp Y, Baciu M, Hot P. Preserved and impaired emotional memory in Alzheimer's disease. Front Psychol. 2012;3:331. doi: 10.3389/fpsyg.2012.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mistridis P, Taylor KI, Kissler JM, Monsch AU, Kressig RW, Kivisaari SL. Distinct neural systems underlying reduced emotional enhancement for positive and negative stimuli in early Alzheimer's disease. Front Hum Neurosci. 2013;7:939. doi: 10.3389/fnhum.2013.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cárdenas J, Blanca MJ, Carvajal F, Rubio S, Pedraza C. Emotional processing in healthy ageing, mild cognitive impairment, and Alzheimer's disease. Int J Environ Res Publ Health. 2021;18(5):2770. doi: 10.3390/ijerph18052770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dere E, Pause BM, Pietrowsky R. Emotion and episodic memory in neuropsychiatric disorders. Behav Brain Res. 2010;215(2):162-171. doi: 10.1016/j.bbr.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 79.Arroyo-Anlló EM, Sánchez JC, Gil R. Could self-consciousness be enhanced in Alzheimer's disease? An approach from emotional sensorial stimulation. J Alzheim Dis. 2020;77(2):505-521. doi: 10.3233/JAD-200408. [DOI] [PubMed] [Google Scholar]
- 80.Irish M, Lawlor BA, O'Mara SM, Coen RF. Impaired capacity for autonoetic reliving during autobiographical event recall in mild Alzheimer's disease. Cortex. 2011;47(2):236-249. doi: 10.1016/j.cortex.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 81.Philippi N, Botzung A, Noblet V, et al. Impaired emotional autobiographical memory associated with right amygdalar-hippocampal atrophy in Alzheimer's disease patients. Front Aging Neurosci. 2015;7:21. doi: 10.3389/fnagi.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.El Haj M, Raffard S, Antoine P, Gely-Nargeot M-C. Emotion and destination memory in Alzheimer's Disease. Curr Alzheimer Res. 2015;12(8):796-801. doi: 10.2174/1567205012666150710112802. [DOI] [PubMed] [Google Scholar]
- 83.Berger N, Richards A, Davelaar EJ. When emotions matter: focusing on emotion improves working memory updating in older adults. Front Psychol. 2017;8:1565. doi: 10.3389/fpsyg.2017.01565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mammarella N, Borella E, Carretti B, Leonardi G, Fairfield B. Examining an emotion enhancement effect in working memory: evidence from age-related differences. Neuropsychol Rehabil. 2013;23(3):416-428. doi: 10.1080/09602011.2013.775065. [DOI] [PubMed] [Google Scholar]
- 85.Zhang F, Ho Y, Fung H. Learning from normal aging:preserved emotional functioning facilitates adaptation among early Alzheimer's disease patient. Aging Dis. 2015;6(3):208-215. doi: 10.14336/AD.2014.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pardilla-Delgado E, Payne JD. The Deese-Roediger-McDermott (DRM) task: a simple cognitive paradigm to investigate false memories in the laboratory. JoVE. 2017;119. doi: 10.3791/54793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roediger HL, McDermott KB. Creating false memories: remembering words not presented in lists. J Exp Psychol Learn Mem Cognit. 1995;21(4):803-814. doi: 10.1037/0278-7393.21.4.803. [DOI] [Google Scholar]
- 88.Balota DA, Cortese MJ, Duchek JM, et al. Veridical and false memories in healthy older adults and in dementia of the Alzheimer's type. Cogn Neuropsychol. 1999;16(3-5):361-384. doi: 10.1080/026432999380834. [DOI] [Google Scholar]
- 89.Budson AE, Daffner KR, Desikan R, Schacter DL. When false recognition is unopposed by true recognition: gist-based memory distortion in Alzheimer's disease. Neuropsychology. 2000;14(2):277-287. doi: 10.1037//0894-4105.14.2.277. [DOI] [PubMed] [Google Scholar]
- 90.Brueckner K, Moritz S. Emotional valence and semantic relatedness differentially influence false recognition in mild cognitive impairment, Alzheimer's disease, and healthy elderly. J Int Neuropsychol Soc. 2009;15(2):268-276. doi: 10.1017/S135561770909047X. [DOI] [PubMed] [Google Scholar]
- 91.Bourgin J, Guyader N, Chauvin A, et al. Early emotional attention is impacted in Alzheimer's disease: an eye-tracking study. J Alzheim Dis. 2018;63(4):1445-1458. doi: 10.3233/JAD-180170. [DOI] [PubMed] [Google Scholar]
- 92.Sava A-A, Paquet C, Dumurgier J, Hugon J, Chainay H. The role of attention in emotional memory enhancement in pathological and healthy aging. J Clin Exp Neuropsychol. 2016;38(4):434-454. doi: 10.1080/13803395.2015.1123225. [DOI] [PubMed] [Google Scholar]
- 93.Bourgin J, Silvert L, Borg C, et al. Impact of emotionally negative information on attentional processes in normal aging and Alzheimer's disease. Brain Cognit. 2020;145:105624. doi: 10.1016/j.bandc.2020.105624. [DOI] [PubMed] [Google Scholar]
- 94.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatr. 2003;54(5):504-514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 95.Fossati P. Neural correlates of emotion processing: from emotional to social brain. Eur Neuropsychopharmacol. 2012;22(suppl 3):S487-S491. doi: 10.1016/j.euroneuro.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 96.Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cognit Affect Behav Neurosci. 2003;3(3):207-233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- 97.Silvers JA, Guassi Moreira JF. Capacity and tendency: a neuroscientific framework for the study of emotion regulation. Neurosci Lett. 2019;693:35-39. doi: 10.1016/j.neulet.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 98.Gu S, Wang F, Cao C, Wu E, Tang Y-Y, Huang JH. An integrative way for studying neural basis of basic emotions with fMRI. Front Neurosci. 2019;13:628. doi: 10.3389/fnins.2019.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Celeghin A, Diano M, Bagnis A, Viola M, Tamietto M. Basic emotions in human neuroscience: neuroimaging and beyond. Front Psychol. 2017;8:1432. doi: 10.3389/fpsyg.2017.01432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kirby LAJ, Robinson JL. Affective mapping: an activation likelihood estimation (ALE) meta-analysis. Brain Cognit. 2017;118:137-148. doi: 10.1016/j.bandc.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 101.Kragel PA, LaBar KS. Decoding the nature of emotion in the brain. Trends Cognit Sci. 2016;20(6):444-455. doi: 10.1016/j.tics.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251(1):E1-E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vytal K, Hamann S. Neuroimaging support for discrete neural correlates of basic emotions: a voxel-based meta-analysis. J Cognit Neurosci. 2010;22(12):2864-2885. doi: 10.1162/jocn.2009.21366. [DOI] [PubMed] [Google Scholar]
- 104.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331-348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 105.Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19(3):513-531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- 106.Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14(2):198-202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 107.Yang Y, Wang J-Z. From structure to behavior in basolateral amygdala-hippocampus circuits. Front Neural Circ. 2017;11:86. doi: 10.3389/fncir.2017.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xu X, Sun Y, Holmes TC, López AJ. Noncanonical connections between the subiculum and hippocampal CA1. J Comp Neurol. 2016;524(17):3666-3673. doi: 10.1002/cne.24024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S. Ventral CA1 neurons store social memory. Science. 2016;353(6307):1536-1541. doi: 10.1126/science.aaf7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ciocchi S, Passecker J, Malagon-Vina H, Mikus N, Klausberger T. Selective information routing by ventral hippocampal CA1 projection neurons. Science. 2015;348(6234):560-563. doi: 10.1126/science.aaa3245. [DOI] [PubMed] [Google Scholar]
- 111.Rolls ET. Limbic systems for emotion and for memory, but no single limbic system. Cortex. 2015;62:119-157. doi: 10.1016/j.cortex.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 112.Zhou S-S, Gao X, Hu Y-J, Zhu Y-M, Tian Y-H, Wang K. Selective impairment of musical emotion recognition in patients with amnesic mild cognitive impairment and mild to moderate Alzheimer disease. Chinese Med J. 2019;132(19):2308-2314. doi: 10.1097/CM9.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev. 2001;38(1-2):247-289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- 114.Leal SL, Noche JA, Murray EA, Yassa MA. Age-related individual variability in memory performance is associated with amygdala-hippocampal circuit function and emotional pattern separation. Neurobiol Aging. 2017;49:9-19. doi: 10.1016/j.neurobiolaging.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Granger SJ, Leal SL, Larson MS, et al. Integrity of the uncinate fasciculus is associated with emotional pattern separation-related fMRI signals in the hippocampal dentate and CA3. Neurobiol Learn Mem. 2021;177:107359. doi: 10.1016/j.nlm.2020.107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Herwig U, Lutz J, Scherpiet S, et al. Training emotion regulation through real-time fMRI neurofeedback of amygdala activity. Neuroimage. 2019;184:687-696. doi: 10.1016/j.neuroimage.2018.09.068. [DOI] [PubMed] [Google Scholar]
- 117.Dehghani A, Soltanian-Zadeh H, Hossein-Zadeh G-A. Global data-driven analysis of brain connectivity during emotion regulation by electroencephalography neurofeedback. Brain Connect. 2020;10(6):302-315. doi: 10.1089/brain.2019.0734. [DOI] [PubMed] [Google Scholar]
- 118.Wu X, Guo T, Zhang C, et al. From "Aha!" to "Haha!" using humor to cope with negative stimuli. Cerebr Cortex. 2020;31:2238-2250. Published online December 1, 2020. doi: 10.1093/cercor/bhaa357 [DOI] [PubMed] [Google Scholar]
- 119.Li X, Li X, Chen S, et al. Effect of emotional enhancement of memory on recollection process in young adults: the influence factors and neural mechanisms. Brain Imaging and Behav. 2020;14(1):119-129. doi: 10.1007/s11682-018-9975-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dolcos F, Katsumi Y, Bogdan PC, et al. The impact of focused attention on subsequent emotional recollection: a functional MRI investigation. Neuropsychologia. 2020;138:107338. doi: 10.1016/j.neuropsychologia.2020.107338. [DOI] [PubMed] [Google Scholar]
- 121.Katsumi Y, Dolcos S. Suppress to feel and remember less: neural correlates of explicit and implicit emotional suppression on perception and memory. Neuropsychologia. 2020;145:106683. doi: 10.1016/j.neuropsychologia.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 122.Katsumi Y, Dolcos S, Dixon RA, Fabiani M, Stine-Morrow EAL, Dolcos F. Immediate and long-term effects of emotional suppression in aging: a functional magnetic resonance imaging investigation. Psychol Aging. 2020;35(5):676-696. doi: 10.1037/pag0000437. [DOI] [PubMed] [Google Scholar]
- 123.Iidaka T, Terashima S, Yamashita K, Okada T, Sadato N, Yonekura Y. Dissociable neural responses in the hippocampus to the retrieval of facial identity and emotion: an event-related fMRI study. Hippocampus. 2003;13(4):429-436. doi: 10.1002/hipo.10059. [DOI] [PubMed] [Google Scholar]
- 124.Koelsch S, Fritz T, V Cramon DY, Müller K, Friederici AD. Investigating emotion with music: an fMRI study. Hum Brain Mapp. 2006;27(3):239-250. doi: 10.1002/hbm.20180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cooper RA, Ritchey M. Progression from feature-specific brain activity to hippocampal binding during episodic encoding. J Neurosci. 2020;40(8):1701-1709. doi: 10.1523/JNEUROSCI.1971-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dahlgren K, Ferris C, Hamann S. Neural correlates of successful emotional episodic encoding and retrieval: an SDM meta-analysis of neuroimaging studies. Neuropsychologia. 2020;143:107495. doi: 10.1016/j.neuropsychologia.2020.107495. [DOI] [PubMed] [Google Scholar]
- 127.Sambuco N, Bradley MM, Herring DR, Lang PJ. Common circuit or paradigm shift? The functional brain in emotional scene perception and emotional imagery. Psychophysiology. 2020;57(4):e13522. doi: 10.1111/psyp.13522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Uysal G, Ozturk M. Hippocampal atrophy based Alzheimer's disease diagnosis via machine learning methods. J Neurosci Methods. 2020;337:108669. doi: 10.1016/j.jneumeth.2020.108669. [DOI] [PubMed] [Google Scholar]
- 129.Sorensen L, Nielsen M, Pechin Lo P, Ashraf H, Pedersen JH, de Bruijne M. Texture-based analysis of COPD: a data-driven approach. IEEE Trans Med Imag. 2012;31(1):70-78. doi: 10.1109/TMI.2011.2164931. [DOI] [PubMed] [Google Scholar]
- 130.Sørensen L, Igel C, Pai A, et al. Differential diagnosis of mild cognitive impairment and Alzheimer's disease using structural MRI cortical thickness, hippocampal shape, hippocampal texture, and volumetry. Neuroimage: Clin. 2017;13:470-482. doi: 10.1016/j.nicl.2016.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Blair JC, Lasiecka ZM, Patrie J, Barrett MJ, Druzgal TJ. Cytoarchitectonic mapping of MRI detects rapid changes in Alzheimer's disease. Front Neurol. 2020;11:241. doi: 10.3389/fneur.2020.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nadal L, Coupé P, Helmer C, et al. Differential annualized rates of hippocampal subfields atrophy in aging and future Alzheimer's clinical syndrome. Neurobiol Aging. 2020;90:75-83. doi: 10.1016/j.neurobiolaging.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 133.Xie L, Wisse LEM, Das SR, et al. Longitudinal atrophy in early Braak regions in preclinical Alzheimer's disease. Hum Brain Mapp. 2020;41(16):4704-4717. doi: 10.1002/hbm.25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Josephs KA, Martin PR, Weigand SD, et al. Protein contributions to brain atrophy acceleration in Alzheimer's disease and primary age-related tauopathy. Brain. 2020;143(11):3463-3476. doi: 10.1093/brain/awaa299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zach P, Bartoš A, Lagutina A, et al. Easy identification of optimal coronal slice on brain magnetic resonance imaging to measure hippocampal area in Alzheimer's disease patients. BioMed Res Int. 2020;2020:1-6. doi: 10.1155/2020/5894021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mohamed Nour AeA, Jiao Y, Teng G-J, Alzheimer’s Disease Neuroimaging Initiative . Neuroanatomical associations of depression, anxiety and apathy neuropsychiatric symptoms in patients with Alzheimer's disease. Acta Neurol Belg. 2020;121:1469-1480. Published online April 21. doi: 10.1007/s13760-020-01349-8. [DOI] [PubMed] [Google Scholar]
- 137.Lee K, Lee YM, Park JM, et al. Right hippocampus atrophy is independently associated with Alzheimer's disease with psychosis. Psychogeriatrics. 2019;19(2):105-110. doi: 10.1111/psyg.12369. [DOI] [PubMed] [Google Scholar]
- 138.Hirjak D, Sambataro F, Remmele B, et al. The relevance of hippocampal subfield integrity and clock drawing test performance for the diagnosis of Alzheimer's disease and mild cognitive impairment. World J Biol Psychiatr. 2019;20(3):197-208. doi: 10.1080/15622975.2017.1355474. [DOI] [PubMed] [Google Scholar]
- 139.Huang K-L, Hsiao I-T, Kuo H-C, et al. Correlation between visual association memory test and structural changes in patients with Alzheimer's disease and amnestic mild cognitive impairment. J Formos Med Assoc. 2019;118(9):1325-1332. doi: 10.1016/j.jfma.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 140.Putcha D, Brickhouse M, Wolk DA, Dickerson BC. Fractionating the rey auditory verbal learning test: distinct roles of large-scale cortical networks in prodromal Alzheimer's disease. Neuropsychologia. 2019;129:83-92. doi: 10.1016/j.neuropsychologia.2019.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gabere M, Thu Pham NT, Graff-Radford J, et al. Automated hippocampal subfield volumetric analyses in atypical alzheimer's disease. J Alzheim Dis. 2020;78(3):927-937. doi: 10.3233/JAD-200625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huang L, Chen K, Hu X, Guo Q. Differential atrophy in the hippocampal subfield volumes in four types of mild dementia. Front Neurosci. 2020;14:699. doi: 10.3389/fnins.2020.00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dawe RJ, Yu L, Arfanakis K, Schneider JA, Bennett DA, Boyle PA. Late-life cognitive decline is associated with hippocampal volume, above and beyond its associations with traditional neuropathologic indices. Alzheimer's Dementia. 2020;16(1):209-218. doi: 10.1002/alz.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Platero C, López ME, Carmen Tobar Md, Yus M, Maestu F. Discriminating Alzheimer's disease progression using a new hippocampal marker from T1-weighted MRI: The local surface roughness. Hum Brain Mapp. 2019;40(5):1666-1676. doi: 10.1002/hbm.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li A, Li F, Elahifasaee F, Liu M, Zhang L. Hippocampal shape and asymmetry analysis by cascaded convolutional neural networks for Alzheimer's disease diagnosis. Brain Imaging Behav. 2021;15:2330-2339. Published online January. doi: 10.1007/s11682-020-00427-y. [DOI] [PubMed] [Google Scholar]
- 146.Liu M, Li F, Yan H, et al. A multi-model deep convolutional neural network for automatic hippocampus segmentation and classification in Alzheimer's disease. Neuroimage. 2020;208:116459. doi: 10.1016/j.neuroimage.2019.116459. [DOI] [PubMed] [Google Scholar]
- 147.Lee E-C, Kang JM, Seo S, et al. Association of subcortical structural shapes with tau, amyloid, and cortical atrophy in early-onset and late-onset Alzheimer's disease. Front Aging Neurosci. 2020;12:563559. doi: 10.3389/fnagi.2020.563559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chapleau M, Bedetti C, Devenyi GA, et al. Deformation-based shape analysis of the hippocampus in the semantic variant of primary progressive aphasia and Alzheimer's disease. Neuroimage: Clin. 2020;27:102305. doi: 10.1016/j.nicl.2020.102305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bai H, Zeng H-M, Han H-B, Zhang Q-F. Application of modern neuroimaging technology in the diagnosis and study of Alzheimer's disease. Neural Regen Res. 2021;16(1):73-79. doi: 10.4103/1673-5374.286957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Filippi M, Basaia S, Canu E, et al. Changes in functional and structural brain connectome along the Alzheimer's disease continuum. Mol Psychiatr. 2020;25(1):230-239. doi: 10.1038/s41380-018-0067-8. [DOI] [PubMed] [Google Scholar]
- 151.Schwab S, Afyouni S, Chen Y, et al. Functional connectivity alterations of the temporal lobe and hippocampus in semantic dementia and Alzheimer's disease. J Alzheim Dis. 2020;76(4):1461-1475. doi: 10.3233/JAD-191113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Xue J, Guo H, Gao Y, et al. Altered directed functional connectivity of the hippocampus in mild cognitive impairment and Alzheimer's disease: a resting-state fMRI study. Front Aging Neurosci. 2019;11:326. doi: 10.3389/fnagi.2019.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schultz RR, de Castro CC, Bertolucci PHF. Memory with emotional content, brain amygdala and Alzheimer's disease. Acta Neurol Scand. 2009;120(2):101-110. doi: 10.1111/j.1600-0404.2008.01132.x. [DOI] [PubMed] [Google Scholar]
- 154.Mori E, Ikeda M, Hirono N, Kitagaki H, Imamura T, Shimomura T. Amygdalar volume and emotional memory in Alzheimer's disease. Am J Psychiatr. 1999;156(2):216-222. doi: 10.1176/ajp.156.2.216. [DOI] [PubMed] [Google Scholar]
- 155.Hamann S, Monarch ES, Goldstein FC. Impaired fear conditioning in Alzheimer's disease. Neuropsychologia. 2002;40(8):1187-1195. doi: 10.1016/s0028-3932(01)00223-8. [DOI] [PubMed] [Google Scholar]
- 156.Hoefer M, Allison SC, Schauer GF, et al. Fear conditioning in frontotemporal lobar degeneration and Alzheimer's disease. Brain. 2008;131(Pt 6):1646-1657. doi: 10.1093/brain/awn082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kumfor F, Irish M, Hodges JR, Piguet O. Frontal and temporal lobe contributions to emotional enhancement of memory in behavioral-variant frontotemporal dementia and Alzheimer's disease. Front Behav Neurosci. 2014;8:225. doi: 10.3389/fnbeh.2014.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Landré L, Sava A-A, Krainik A, Lamalle L, Krolak-Salmon P, Chainay H. Effects of emotionally-rated material on visual memory in Alzheimer's disease in relation to medial temporal atrophy. J Alzheim Dis. 2013;36(3):535-544. doi: 10.3233/JAD-130170. [DOI] [PubMed] [Google Scholar]
- 159.Perrin M, Henaff M-A, Padovan C, Faillenot I, Merville A, Krolak-Salmon P. Influence of emotional content and context on memory in mild Alzheimer's disease. J Alzheim Dis. 2012;29(4):817-826. doi: 10.3233/JAD-2012-111490. [DOI] [PubMed] [Google Scholar]
- 160.Sapey-Triomphe L-A, Heckemann RA, Boublay N, et al. Neuroanatomical correlates of recognizing face expressions in mild stages of Alzheimer's disease. PLoS One. 2015;10(12):e0143586. doi: 10.1371/journal.pone.0143586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Park S, Kim T, Shin SA, et al. Behavioral and neuroimaging evidence for facial emotion recognition in elderly korean adults with mild cognitive impairment, Alzheimer's disease, and frontotemporal dementia. Front Aging Neurosci. 2017;9:389. doi: 10.3389/fnagi.2017.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rosen HJ, Wilson MR, Schauer GF, et al. Neuroanatomical correlates of impaired recognition of emotion in dementia. Neuropsychologia. 2006;44(3):365-373. doi: 10.1016/j.neuropsychologia.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 163.Goodkind MS, Sollberger M, Gyurak A, et al. Tracking emotional valence: the role of the orbitofrontal cortex. Hum Brain Mapp. 2012;33(4):753-762. doi: 10.1002/hbm.21251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Niedenthal PM, Brauer M. Social functionality of human emotion. Annu Rev Psychol. 2012;63:259-285. doi: 10.1146/annurev.psych.121208.131605. [DOI] [PubMed] [Google Scholar]
- 165.Gola KA, Shany-Ur T, Pressman P, et al. A neural network underlying intentional emotional facial expression in neurodegenerative disease. Neuroimage: Clin. 2017;14:672-678. doi: 10.1016/j.nicl.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.McDonald S, Flanagan S, Rollins J, Kinch J. Tasit. J Head Trauma Rehabil. 2003;18(3):219-238. doi: 10.1097/00001199-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 167.Kumfor F, Honan C, McDonald S, Hazelton JL, Hodges JR, Piguet O. Assessing the "social brain" in dementia: applying TASIT-S. Cortex. 2017;93:166-177. doi: 10.1016/j.cortex.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 168.Rankin KP, Salazar A, Gorno-Tempini ML, et al. Detecting sarcasm from paralinguistic cues: anatomic and cognitive correlates in neurodegenerative disease. Neuroimage. 2009;47(4):2005-2015. doi: 10.1016/j.neuroimage.2009.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hutchings R, Hodges JR, Piguet O, Kumfor F, Boutoleau-Bretonniére C. Why should I care? Dimensions of socio-emotional cognition in younger-onset dementia. J Alzheim Dis. 2015;48(1):135-147. doi: 10.3233/JAD-150245. [DOI] [PubMed] [Google Scholar]
- 170.Shdo SM, Ranasinghe KG, Gola KA, et al. Deconstructing empathy: Neuroanatomical dissociations between affect sharing and prosocial motivation using a patient lesion model. Neuropsychologia. 2018;116(Pt A):126-135. doi: 10.1016/j.neuropsychologia.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rankin KP, Gorno-Tempini ML, Allison SC, et al. Structural anatomy of empathy in neurodegenerative disease. Brain. 2006;129(Pt 11):2945-2956. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dermody N, Wong S, Ahmed R, Piguet O, Hodges JR, Irish M. Uncovering the neural bases of cognitive and affective empathy deficits in Alzheimer's disease and the behavioral-variant of frontotemporal dementia. J Alzheim Dis. 2016;53(3):801-816. doi: 10.3233/JAD-160175. [DOI] [PubMed] [Google Scholar]
- 173.Hornberger M, Yew B, Gilardoni S, et al. Ventromedial-frontopolar prefrontal cortex atrophy correlates with insight loss in frontotemporal dementia and Alzheimer's disease. Hum Brain Mapp. 2014;35(2):616-626. doi: 10.1002/hbm.22200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Decety J, Jackson PL. The functional architecture of human empathy. Behav Cognit Neurosci Rev. 2004;3(2):71-100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- 175.Hatfield E, Cacioppo JT, Rapson RL. Emotional contagion. Curr Dir Psychol Sci. 1993;2(3):96-100. doi: 10.1111/1467-8721.ep10770953. [DOI] [Google Scholar]
- 176.de Waal FBM. Putting the altruism back into altruism: the evolution of empathy. Annu Rev Psychol. 2008;59(1):279-300. doi: 10.1146/annurev.psych.59.103006.093625. [DOI] [PubMed] [Google Scholar]
- 177.Wright CI, Dickerson BC, Feczko E, Negeira A, Williams D. A functional magnetic resonance imaging study of amygdala responses to human faces in aging and mild Alzheimer's disease. Biol Psychiatr. 2007;62(12):1388-1395. doi: 10.1016/j.biopsych.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 178.Lee TMC, Sun D, Leung M-K, Chu L-W, Keysers C. Neural activities during affective processing in people with Alzheimer's disease. Neurobiol Aging. 2013;34(3):706-715. doi: 10.1016/j.neurobiolaging.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 179.Zhao H, Tang W, Xu X, Zhao Z, Huang L. Functional magnetic resonance imaging study of apathy in Alzheimer's disease. J Neuropsychiatry Clin Neurosci. 2014;26(4):134-141. doi: 10.1176/appi.neuropsych.12110261. [DOI] [PubMed] [Google Scholar]
- 180.Berger C, Erbe A-K, Ehlers I, Marx I, Hauenstein K, Teipel S. Effects of task-irrelevant emotional stimuli on working memory processes in mild cognitive impairment. J Alzheim Dis. 2015;44(2):439-453. doi: 10.3233/JAD-141848. [DOI] [PubMed] [Google Scholar]
- 181.Parra MA, Pattan V, Wong D, et al. Medial temporal lobe function during emotional memory in early Alzheimer's disease, mild cognitive impairment and healthy ageing: an fMRI study. BMC Psychiatr. 2013;13:76. doi: 10.1186/1471-244X-13-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Thaut MH, Fischer CE, Leggieri M, et al. Neural basis of long-term musical memory in cognitively impaired older persons. Alzheimer Dis Assoc Disord. 2020;34(3):267-271. doi: 10.1097/WAD.0000000000000382. [DOI] [PubMed] [Google Scholar]
- 183.Allen G, Barnard H, McColl R, et al. Reduced hippocampal functional connectivity in Alzheimer disease. Arch Neurol. 2007;64(10):1482-1487. doi: 10.1001/archneur.64.10.1482. [DOI] [PubMed] [Google Scholar]
- 184.Ortner M, Pasquini L, Barat M, et al. Progressively disrupted intrinsic functional connectivity of basolateral amygdala in very early Alzheimer's disease. Front Neurol. 2016;7:132. doi: 10.3389/fneur.2016.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci Unit States Am. 2004;101(13):4637-4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dillen KNH, Jacobs HIL, Kukolja J, et al. Functional disintegration of the default mode network in prodromal Alzheimer's disease. J Alzheim Dis. 2017;59(1):169-187. doi: 10.3233/JAD-161120. [DOI] [PubMed] [Google Scholar]
- 187.Huijbers W, Pennartz CMA, Cabeza R, Daselaar SM. The hippocampus is coupled with the default network during memory retrieval but not during memory encoding. PLoS One. 2011;6(4):e17463. doi: 10.1371/journal.pone.0017463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Satpute AB, Lindquist KA. The default mode network's role in discrete emotion. Trends Cognit Sci. 2019;23(10):851-864. doi: 10.1016/j.tics.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Multani N, Taghdiri F, Anor CJ, et al. Association between social cognition changes and resting state functional connectivity in frontotemporal dementia, Alzheimer's disease, parkinson's disease, and healthy controls. Front Neurosci. 2019;13:1259. doi: 10.3389/fnins.2019.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Toller G, Brown J, Sollberger M, et al. Individual differences in socioemotional sensitivity are an index of salience network function. Cortex. 2018;103:211-223. doi: 10.1016/j.cortex.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cisler JM, Esbensen K, Sellnow K, et al. Differential roles of the salience network during prediction error encoding and facial emotion processing among female adolescent assault victims. Biol Psychiatr: Cogn Neurosci Neuroimaging. 2019;4(4):371-380. doi: 10.1016/j.bpsc.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Qi D, Lam CLM, Wong JJ, Chang DHF, Lee TMC. Positive affect is inversely related to the salience and emotion network's connectivity. Brain Imaging Behav. 2021;15(4):2031-2039. doi: 10.1007/s11682-020-00397-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.He X, Qin W, Liu Y, et al. Abnormal salience network in normal aging and in amnestic mild cognitive impairment and Alzheimer's disease. Hum Brain Mapp. 2014;35(7):3446-3464. doi: 10.1002/hbm.22414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Song Y, Xu W, Chen S, et al. Functional MRI-specific alterations in salience network in mild cognitive impairment: an ALE meta-analysis. Front Aging Neurosci. 2021;13:695210. doi: 10.3389/fnagi.2021.695210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ng ASL, Wang J, Ng KK, et al. Distinct network topology in Alzheimer's disease and behavioral variant frontotemporal dementia. Alzheimer's Res Ther. 2021;13(1):13. doi: 10.1186/s13195-020-00752-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fredericks CA, Sturm VE, Brown JA, et al. Early affective changes and increased connectivity in preclinical Alzheimer's disease. Alzheimer's Dementia: Diagnosis, Assessment & Disease Monitoring. 2018;10:471-479. doi: 10.1016/j.dadm.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yakushev I, Schreckenberger M, Müller MJ, et al. Functional implications of hippocampal degeneration in early Alzheimer's disease: a combined DTI and PET study. Eur J Nucl Med Mol Imag. 2011;38(12):2219-2227. doi: 10.1007/s00259-011-1882-1. [DOI] [PubMed] [Google Scholar]
- 198.Lancaster MA, Seidenberg M, Smith JC, et al. Diffusion tensor imaging predictors of episodic memory decline in healthy elders at genetic risk for Alzheimer's disease. J Int Neuropsychol Soc. 2016;22(10):1005-1015. doi: 10.1017/S1355617716000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rémy F, Vayssière N, Saint-Aubert L, Barbeau E, Pariente J. White matter disruption at the prodromal stage of Alzheimer's disease: relationships with hippocampal atrophy and episodic memory performance. Neuroimage: Clin. 2015;7:482-492. doi: 10.1016/j.nicl.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Takahashi M, Kitamura S, Matsuoka K, et al. Uncinate fasciculus disruption relates to poor recognition of negative facial emotions in Alzheimer's disease: a cross‐sectional diffusion tensor imaging study. Psychogeriatrics. 2020;20(3):296-303. doi: 10.1111/psyg.12498. [DOI] [PubMed] [Google Scholar]
- 201.Silva RCRd, Carvalho RLSd, Dourado MCN. Deficits in emotion processing in Alzheimer's disease: a systematic review. Dement Neuropsychol. 2021;15(3):314-330. doi: 10.1590/1980-57642021dn15-030003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Miller LA, Hsieh S, Lah S, Savage S, Hodges JR, Piguet O. One size does not fit all: face emotion processing impairments in semantic dementia, behavioural-variant frontotemporal dementia and Alzheimer's disease are mediated by distinct cognitive deficits. Behav Neurol. 2012;25(1):53-60. doi: 10.3233/BEN-2012-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hargrave R, Maddock RJ, Stone V. Impaired recognition of facial expressions of emotion in Alzheimer's disease. J Neuropsychiatry Clin Neurosci. 2002;14(1):64-71. doi: 10.1176/jnp.14.1.64. [DOI] [PubMed] [Google Scholar]
- 204.Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM. Alzheimer's disease: targeting the cholinergic system. Curr Neuropharmacol. 2016;14(1):101-115. doi: 10.2174/1570159x13666150716165726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Grossberg GT. The ABC of Alzheimer's disease: behavioral symptoms and their treatment. Int Psychogeriatr. 2002;14(suppl 1):27-49. doi: 10.1017/s1041610203008652. [DOI] [PubMed] [Google Scholar]
- 206.Mufson EJ, Counts SE, Perez SE, Ginsberg SD. Cholinergic system during the progression of Alzheimer's disease: therapeutic implications. Expert Rev Neurother. 2008;8(11):1703-1718. doi: 10.1586/14737175.8.11.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mega MS. The cholinergic deficit in Alzheimer's disease: impact on cognition, behaviour and function. Int J Neuropsychopharmacol. 2000;3(7):3-12. doi: 10.1017/S1461145700001942. [DOI] [PubMed] [Google Scholar]
- 208.Schliebs R, Arendt T. The significance of the cholinergic system in the brain during aging and in Alzheimer's disease. J Neural Transm. 2006;113(11):1625-1644. doi: 10.1007/s00702-006-0579-2. [DOI] [PubMed] [Google Scholar]
- 209.Lanari A, Amenta F, Silvestrelli G, Tomassoni D, Parnetti L. Neurotransmitter deficits in behavioural and psychological symptoms of Alzheimer's disease. Mech Ageing Dev. 2006;127(2):158-165. doi: 10.1016/j.mad.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 210.Bell KFS, de Kort GJL, Steggerda S, Shigemoto R, Ribeiro-da-Silva A, Cuello AC. Structural involvement of the glutamatergic presynaptic boutons in a transgenic mouse model expressing early onset amyloid pathology. Neurosci Lett. 2003;353(2):143-147. doi: 10.1016/j.neulet.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 211.Bell KFS, Ducatenzeiler A, Ribeiro-da-Silva A, Duff K, Bennett DA, Claudio Cuello A. The amyloid pathology progresses in a neurotransmitter-specific manner. Neurobiol Aging. 2006;27(11):1644-1657. doi: 10.1016/j.neurobiolaging.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 212.Jazi R, Lalonde R, Qian S, Strazielle C. Regional brain evaluation of acetylcholinesterase activity in PS1/A246E transgenic mice. Neurosci Res. 2009;63(2):106-114. doi: 10.1016/j.neures.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 213.Wang Y, Guan X, Chen X, et al. Choline supplementation ameliorates behavioral deficits and Alzheimer's disease‐like pathology in transgenic APP/PS1 mice. Mol Nutr Food Res. 2019;63(18):1801407. doi: 10.1002/mnfr.201801407. [DOI] [PubMed] [Google Scholar]
- 214.Dong H, Csernansky CA, Martin MV, Bertchume A, Vallera D, Csernansky JG. Acetylcholinesterase inhibitors ameliorate behavioral deficits in the Tg2576 mouse model of Alzheimer's disease. Psychopharmacology. 2005;181(1):145-152. doi: 10.1007/s00213-005-2230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ratia M, Giménez-Llort L, Camps P, Muñoz-Torrero D, Clos M, Badia A. Behavioural effects and regulation of PKCα and MAPK by huprine X in middle aged mice. Pharmacol Biochem Behav. 2010;95(4):485-493. doi: 10.1016/j.pbb.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 216.Giménez-Llort L, Ratia M, Pérez B, et al. Behavioural effects of novel multitarget anticholinesterasic derivatives in Alzheimer's disease. Behav Pharmacol. 2017;28(23-Spec Issue):124-131. doi: 10.1097/FBP.0000000000000292. and [DOI] [PubMed] [Google Scholar]
- 217.Bedrosian TA, Herring KL, Weil ZM, Nelson RJ. Altered temporal patterns of anxiety in aged and amyloid precursor protein (APP) transgenic mice. Proc Natl Acad Sci Unit States Am. 2011;108(28):11686-11691. doi: 10.1073/pnas.1103098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Olariu A, Tran MH, Yamada K, Mizuno M, Hefco V, Nabeshima T. Memory deficits and increased emotionality induced by β-amyloid (25-35) are correlated with the reduced acetylcholine release and altered phorbol dibutyrate binding in the hippocampus. J Neural Transm. 2001;108(8-9):1065-1079. doi: 10.1007/s007020170025. [DOI] [PubMed] [Google Scholar]
- 219.Martinowich K, Cardinale KM, Schloesser RJ, Hsu M, Greig NH, Manji HK. Acetylcholinesterase inhibition ameliorates deficits in motivational drive. Behav Brain Funct. 2012;8:15. doi: 10.1186/1744-9081-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Shin K, Cha Y, Kim KS, et al. Human neural stem cells overexpressing choline acetyltransferase restore unconditioned fear in rats with amygdala injury. Behav Neurol. 2016;2016:1-9. doi: 10.1155/2016/8521297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zaborszky L, Hoemke L, Mohlberg H, Schleicher A, Amunts K, Zilles K. Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. Neuroimage. 2008;42(3):1127-1141. doi: 10.1016/j.neuroimage.2008.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Li C-sR, Ide JS, Zhang S, Hu S, Chao HH, Zaborszky L. Resting state functional connectivity of the basal nucleus of Meynert in humans: in comparison to the ventral striatum and the effects of age. Neuroimage. 2014;97:321-332. doi: 10.1016/j.neuroimage.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rösler M. The efficacy of cholinesterase inhibitors in treating the behavioural symptoms of dementia. Int J Clin Pract Suppl. 2002(127):20-36. http://www.ncbi.nlm.nih.gov/pubmed/12139365. [PubMed] [Google Scholar]
- 224.Spiegel R. [Rivastigmine: a review of its clinical effectiveness]. Rev Neurol. 2002;35(9):859-869. http://www.ncbi.nlm.nih.gov/pubmed/12436385. [PubMed] [Google Scholar]
- 225.Grossberg GT. Effect of rivastigmine in the treatment of behavioral disturbances associated with dementia: review of neuropsychiatric impairment in Alzheimer's disease. Curr Med Res Opin. 2005;21(10):1631-1639. doi: 10.1185/030079905X65402. [DOI] [PubMed] [Google Scholar]
- 226.Grossberg GT. Impact of rivastigmine on caregiver burden associated with Alzheimer's disease in both informal care and nursing home settings. Drugs Aging. 2008;25(7):573-584. doi: 10.2165/00002512-200825070-00004. [DOI] [PubMed] [Google Scholar]
- 227.Gauthier S, Juby A, Rehel B, Schecter R. EXACT: rivastigmine improves the high prevalence of attention deficits and mood and behaviour symptoms in Alzheimer's disease*. Int J Clin Pract. 2007;61(6):886-895. doi: 10.1111/j.1742-1241.2007.01387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kavanagh S, Gaudig M, Van Baelen B, et al. Galantamine and behavior in Alzheimer disease: analysis of four trials. Acta Neurol Scand. 2011;124(5):302-308. doi: 10.1111/j.1600-0404.2011.01525.x. [DOI] [PubMed] [Google Scholar]
- 229.Cummings JL, Schneider L, Tariot PN, Kershaw PR, Yuan W. Reduction of behavioral disturbances and caregiver distress by galantamine in patients with Alzheimer's disease. Am J Psychiatr. 2004;161(3):532-538. doi: 10.1176/appi.ajp.161.3.532. [DOI] [PubMed] [Google Scholar]
- 230.Robert P. Understanding and managing behavioural symptoms in Alzheimer's disease and related dementias: focus on rivastigmine. Curr Med Res Opin. 2002;18(3):156-171. doi: 10.1185/030079902125000561. [DOI] [PubMed] [Google Scholar]
- 231.Ruthirakuhan MT, Herrmann N, Abraham EH, Chan S, Lanctôt KL. Pharmacological interventions for apathy in Alzheimer's disease. Cochrane Database Syst Rev. 2018;2018:CD012197. doi: 10.1002/14651858.CD012197.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Daiello LA, Ott BR, Lapane KL, Reinert SE, Machan JT, Dore DD. Effect of discontinuing cholinesterase inhibitor therapy on behavioral and mood symptoms in nursing home patients with dementia. Am J Geriatr Pharmacother. 2009;7(2):74-83. doi: 10.1016/j.amjopharm.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 233.Pancrazi M-P, Metais P. Maladie d'Alzheimer, traitement des troubles psychologiques et comportementaux. Presse Med. 2005;34(9):667-672. doi: 10.1016/s0755-4982(05)84011-4. [DOI] [PubMed] [Google Scholar]
- 234.Theleritis C, Siarkos K, Katirtzoglou E, Politis A. Pharmacological and nonpharmacological treatment for apathy in Alzheimer disease. J Geriatr Psychiatr Neurol. 2017;30(1):26-49. doi: 10.1177/0891988716678684. [DOI] [PubMed] [Google Scholar]
- 235.Sepehry AA, Sarai M, Hsiung G-YR. Pharmacological therapy for apathy in Alzheimer's disease: a systematic review and meta-analysis. Can J Neurol Sci/J Can Sci Neurol. 2017;44(3):267-275. doi: 10.1017/cjn.2016.426. [DOI] [PubMed] [Google Scholar]
- 236.Carotenuto A, Rea R, Traini E, et al. The effect of the association between Donepezil and Choline Alphoscerate on behavioral disturbances in Alzheimer's disease: interim results of the ASCOMALVA trial. J Alzheim Dis. 2017;56(2):805-815. doi: 10.3233/JAD-160675. [DOI] [PubMed] [Google Scholar]
- 237.Panza F, Solfrizzi V, Frisardi V, et al. Disease-modifying approach to the treatment of Alzheimerʼs disease. Drugs Aging. 2009;26(7):537-555. doi: 10.2165/11315770-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 238.Spalletta G, Caltagirone C, Padovani A, et al. Cognitive and affective changes in mild to moderate Alzheimer's disease patients undergoing switch of cholinesterase inhibitors: A 6-month observational study. PLoS One. 2014;9(2):e89216. doi: 10.1371/journal.pone.0089216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Oka M, Nakaaki S, Negi A, et al. Predicting the neural effect of switching from donepezil to galantamine based on single-photon emission computed tomography findings in patients with Alzheimer's disease. Psychogeriatrics. 2016;16(2):121-134. doi: 10.1111/psyg.12132. [DOI] [PubMed] [Google Scholar]
- 240.Swanson LW. The locus coeruleus: a cytoarchitectonic, Golgi and immunohistochemical study in the albino rat. Brain Res. 1976;110(1):39-56. doi: 10.1016/0006-8993(76)90207-9. [DOI] [PubMed] [Google Scholar]
- 241.Baker KG, Tork I, Hornung J-P, Halasz P. The human locus coeruleus complex: an immunohistochemical and three dimensional reconstruction study. Exp Brain Res. 1989;77(2):257-270. doi: 10.1007/BF00274983. [DOI] [PubMed] [Google Scholar]
- 242.Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113-168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- 243.Aston-Jones G, Waterhouse B. Locus coeruleus: From global projection system to adaptive regulation of behavior. Brain Res. 2016;1645:75-78. doi: 10.1016/j.brainres.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Borodovitsyna O, Flamini M, Chandler D. Noradrenergic modulation of cognition in health and disease. Neural Plast. 2017;2017:1-14. doi: 10.1155/2017/6031478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Borsook D, Linnman C, Faria V, Strassman AM, Becerra L, Elman I. Reward deficiency and anti-reward in pain chronification. Neurosci Biobehav Rev. 2016;68:282-297. doi: 10.1016/j.neubiorev.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 246.Flavin SA, Winder DG. Noradrenergic control of the bed nucleus of the stria terminalis in stress and reward. Neuropharmacology. 2013;70:324-330. doi: 10.1016/j.neuropharm.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Li GY, Ueki H, Kawashima T, Sugataka K, Muraoka T, Yamada S. Involvement of the noradrenergic system in performance on a continuous task requiring effortful attention. Neuropsychobiology. 2004;50(4):336-340. doi: 10.1159/000080962. [DOI] [PubMed] [Google Scholar]
- 248.Zhang S, Hu S, Chao HH, Li C-SR. Resting-state functional connectivity of the Locus Coeruleus in humans: in comparison with the ventral tegmental Area/Substantia Nigra Pars compacta and the effects of age. Cerebr Cortex. 2016;26(8):3413-3427. doi: 10.1093/cercor/bhv172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kline RL, Zhang S, Farr OM, et al. The effects of methylphenidate on resting-state functional connectivity of the basal nucleus of Meynert, Locus Coeruleus, and Ventral Tegmental Area in healthy adults. Front Hum Neurosci. 2016;10:149. doi: 10.3389/fnhum.2016.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wang W, Zhornitsky S, Zhang S, Li C-sR. Noradrenergic correlates of chronic cocaine craving: neuromelanin and functional brain imaging. Neuropsychopharmacology. 2021;46(4):851-859. doi: 10.1038/s41386-020-00937-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Szot P, Franklin A, Miguelez C, et al. Depressive-like behavior observed with a minimal loss of locus coeruleus (LC) neurons following administration of 6-hydroxydopamine is associated with electrophysiological changes and reversed with precursors of norepinephrine. Neuropharmacology. 2016;101:76-86. doi: 10.1016/j.neuropharm.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Choudhary P, Pacholko AG, Palaschuk J, Bekar LK. The locus coeruleus neurotoxin, DSP4, and/or a high sugar diet induce behavioral and biochemical alterations in wild-type mice consistent with Alzheimers related pathology. Metab Brain Dis. 2018;33(5):1563-1571. doi: 10.1007/s11011-018-0263-x. [DOI] [PubMed] [Google Scholar]
- 253.Romano A, Pace L, Tempesta B, et al. Depressive-like behavior is paired to monoaminergic alteration in a murine model of Alzheimer's disease. Int J Neuropsychopharmacol. 2014;18(4):pyu020. doi: 10.1093/ijnp/pyu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Giustino TF, Maren S. Noradrenergic modulation of fear conditioning and extinction. Front Behav Neurosci. 2018;12:43. doi: 10.3389/fnbeh.2018.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Taherian F, Vafaei AA, Vaezi GH, Eskandarian S, Kashef A, Rashidy-Pour A. Propranolol-induced impairment of contextual fear memory reconsolidation in rats: a similar effect on weak and strong recent and remote memories. Basic Clin Neurosci. 2014;5(3):231-239. http://www.ncbi.nlm.nih.gov/pubmed/25337385. [PMC free article] [PubMed] [Google Scholar]
- 256.Muravieva EV, Alberini CM. Limited efficacy of propranolol on the reconsolidation of fear memories. Learn Mem. 2010;17(6):306-313. doi: 10.1101/lm.1794710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Gannon M, Che P, Chen Y, Jiao K, Roberson ED, Wang Q. Noradrenergic dysfunction in Alzheimer's disease. Front Neurosci. 2015;9:220. doi: 10.3389/fnins.2015.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Luo Y, Zhou J, Li MX, et al. Reversal of aging‐related emotional memory deficits by norepinephrine via regulating the stability of surface AMPA receptors. Aging Cell. 2015;14(2):170-179. doi: 10.1111/acel.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Chai N, Liu J-F, Xue Y-X, et al. Delayed noradrenergic activation in the dorsal hippocampus promotes the long-term persistence of extinguished fear. Neuropsychopharmacology. 2014;39(8):1933-1945. doi: 10.1038/npp.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Zhu R-T, Liu X-H, Shi Y-W, Wang X-G, Xue L, Zhao H. Propranolol can induce PTSD-like memory impairments in rats. Brain Behav. 2018;8(2):e00905. doi: 10.1002/brb3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Atucha E, Vukojevic V, Fornari RV, et al. Noradrenergic activation of the basolateral amygdala maintains hippocampus-dependent accuracy of remote memory. Proc Natl Acad Sci Unit States Am. 2017;114(34):9176-9181. doi: 10.1073/pnas.1710819114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.van Stegeren AH, Roozendaal B, Kindt M, Wolf OT, Joëls M. Interacting noradrenergic and corticosteroid systems shift human brain activation patterns during encoding. Neurobiol Learn Mem. 2010;93(1):56-65. doi: 10.1016/j.nlm.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 263.Myskiw JC, Izquierdo I, Furini CRG. Modulation of the extinction of fear learning. Brain Res Bull. 2014;105:61-69. doi: 10.1016/j.brainresbull.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 264.McGaugh JL, Cahill L, Roozendaal B. Involvement of the amygdala in memory storage: interaction with other brain systems. Proc Natl Acad Sci Unit States Am. 1996;93(24):13508-13514. doi: 10.1073/pnas.93.24.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Jacobs HI, Priovoulos N, Poser BA, et al. Dynamic behavior of the locus coeruleus during arousal-related memory processing in a multi-modal 7T fMRI paradigm. Elife. 2020;9:e52059. doi: 10.7554/eLife.52059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Gannon M, Wang Q. Complex noradrenergic dysfunction in Alzheimer's disease: low norepinephrine input is not always to blame. Brain Res. 2019;1702:12-16. doi: 10.1016/j.brainres.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Jacobs HIL, Riphagen JM, Ramakers IHGB, Verhey FRJ. Alzheimer's disease pathology: pathways between central norepinephrine activity, memory, and neuropsychiatric symptoms. Mol Psychiatr. 2019;26:897-906. Published online May 28. doi: 10.1038/s41380-019-0437-x. [DOI] [PubMed] [Google Scholar]
- 268.Herrmann N, Lanctôt KL, Khan LR. The role of norepinephrine in the behavioral and psychological symptoms of dementia. J Neuropsychiatry Clin Neurosci. 2004;16(3):261-276. doi: 10.1176/jnp.16.3.261. [DOI] [PubMed] [Google Scholar]
- 269.Hoogendijk WJG, Sommer IEC, Pool CW, et al. Lack of association between depression and loss of neurons in the locus coeruleus in Alzheimer disease. Arch Gen Psychiatr. 1999;56(1):45-51. doi: 10.1001/archpsyc.56.1.45. [DOI] [PubMed] [Google Scholar]
- 270.Parnetti L, Amici S, Lanari A, Gallai V. Pharmacological treatment of non-cognitive disturbances in dementia disorders. Mech Ageing Dev. 2001;122(16):2063-2069. doi: 10.1016/s0047-6374(01)00316-5. [DOI] [PubMed] [Google Scholar]
- 271.Maier F, Spottke A, Bach J-P, et al. Bupropion for the treatment of apathy in alzheimer disease. JAMA Netw Open. 2020;3(5):e206027. doi: 10.1001/jamanetworkopen.2020.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Matthews KL, Chen CPL-H, Esiri MM, Keene J, Minger SL, Francis PT. Noradrenergic changes, aggressive behavior, and cognition in patients with dementia. Biol Psychiatr. 2002;51(5):407-416. doi: 10.1016/s0006-3223(01)01235-5. [DOI] [PubMed] [Google Scholar]
- 273.Russo-Neustadt A, Cotman CW. Adrenergic receptors in Alzheimer's disease brain: selective increases in the cerebella of aggressive patients. J Neurosci. 1997;17(14):5573-5580. http://www.ncbi.nlm.nih.gov/pubmed/9204938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Bylund DB, Bylund KC. Norepinephrine. Encyclopedia of the Neurological Sciences. Oxford: Academic Press; 2014:614-616, 10.1016/B978-0-12-385157-4.00047-6. [DOI] [Google Scholar]
- 275.Wang LY, Shofer JB, Rohde K, et al. Prazosin for the treatment of behavioral symptoms in patients with Alzheimer disease with agitation and aggression. Am J Geriatr Psychiatr. 2009;17(9):744-751. doi: 10.1097/JGP.0b013e3181ab8c61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Peskind ER, Tsuang DW, Bonner LT, et al. Propranolol for disruptive behaviors in nursing home residents with probable or possible Alzheimer disease. Alzheimer Dis Assoc Disord. 2005;19(1):23-28. doi: 10.1097/01.wad.0000155067.16313.5e. [DOI] [PubMed] [Google Scholar]
- 277.Shankle WR, Nielson KA, Cotman CW. Low-dose propranolol reduces aggression and agitation resembling that associated with orbitofrontal dysfunction in elderly demented patients. Alzheimer Dis Assoc Disord. 1995;9(4):233-237. http://www.ncbi.nlm.nih.gov/pubmed/8749613. [PubMed] [Google Scholar]
- 278.Rodríguez JJ, Noristani HN, Verkhratsky A. The serotonergic system in ageing and Alzheimer's disease. Prog Neurobiol. 2012;99(1):15-41. doi: 10.1016/j.pneurobio.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 279.Cifariello A, Pompili A, Gasbarri A. 5-HT7 receptors in the modulation of cognitive processes. Behav Brain Res. 2008;195(1):171-179. doi: 10.1016/j.bbr.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 280.Chakraborty S, Lennon JC, Malkaram SA, Zeng Y, Fisher DW, Dong H. Serotonergic system, cognition, and BPSD in Alzheimer's disease. Neurosci Lett. 2019;704:36-44. doi: 10.1016/j.neulet.2019.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.King M, Marsden C, Fone K. A role for the 5-HT1A, 5-HT4 and 5-HT6 receptors in learning and memory. Trends Pharmacol Sci. 2008;29(9):482-492. doi: 10.1016/j.tips.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 282.Kaur S, DasGupta G, Singh S. Altered neurochemistry in Alzheimer's disease: targeting neurotransmitter receptor mechanisms and therapeutic strategy. Neurophysiology. 2019;51(4):293-309. doi: 10.1007/s11062-019-09823-7. [DOI] [Google Scholar]
- 283.Kandimalla R, Reddy PH. Therapeutics of neurotransmitters in Alzheimer's disease. J Alzheim Dis. 2017;57(4):1049-1069. doi: 10.3233/JAD-161118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Woods S, Clarke N, Layfield R, Fone K. 5-HT6 receptor agonists and antagonists enhance learning and memory in a conditioned emotion response paradigm by modulation of cholinergic and glutamatergic mechanisms. Br J Pharmacol. 2012;167(2):436-449. doi: 10.1111/j.1476-5381.2012.02022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ai P-H, Chen S, Liu X-D, et al. Paroxetine ameliorates prodromal emotional dysfunction and late-onset memory deficit in Alzheimer's disease mice. Transl Neurodegener. 2020;9(1):18. doi: 10.1186/s40035-020-00194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Olesen LØ, Bouzinova EV, Severino M, et al. Behavioural phenotyping of APPswe/PS1δE9 mice: age-rrelated changes and effect of long-term paroxetine treatment. PLoS One. 2016;11(11):e0165144. doi: 10.1371/journal.pone.0165144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Ledo JH, Azevedo EP, Clarke JR, et al. Amyloid-β oligomers link depressive-like behavior and cognitive deficits in mice. Mol Psychiatr. 2013;18(10):1053-1054. doi: 10.1038/mp.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Ledo JH, Azevedo EP, Beckman D, et al. Cross talk between brain innate immunity and serotonin signaling underlies depressive-like behavior induced by Alzheimer's amyloid- oligomers in mice. J Neurosci. 2016;36(48):12106-12116. doi: 10.1523/JNEUROSCI.1269-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Moechars D, Gilis M, Kuipéri C, Laenen I, Van Leuven F. Aggressive behaviour in transgenic mice expressing APP is alleviated by serotonergic drugs. Neuroreport. 1998;9(16):3560-3564. doi: 10.1097/00001756-199811160-00004. [DOI] [PubMed] [Google Scholar]
- 290.Lanctôt KL, Herrmann N, Mazzotta P. Role of serotonin in the behavioral and psychological symptoms of dementia. J Neuropsychiatry Clin Neurosci. 2001;13(1):5-21. doi: 10.1176/jnp.13.1.5. [DOI] [PubMed] [Google Scholar]
- 291.Lai MK, Tsang SW, Francis PT, et al. Reduced serotonin 5-HT1A receptor binding in the temporal cortex correlates with aggressive behavior in Alzheimer disease. Brain Research. 2003;974(1-2):82-87. doi: 10.1016/s0006-8993(03)02554-x. [DOI] [PubMed] [Google Scholar]
- 292.Tsang SWY, Lai MKP, Francis PT, et al. Serotonin transporters are preserved in the neocortex of anxious Alzheimer's disease patients. Neuroreport. 2003;14(10):1297-1300. doi: 10.1097/01.wnr.0000077546.91466.a8. [DOI] [PubMed] [Google Scholar]
- 293.de Boer SF, Koolhaas JM. 5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol. 2005;526(1-3):125-139. doi: 10.1016/j.ejphar.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 294.Mimica N, Drmić S, Presecki P. Involuntary emotional expression disorder in Alzheimer's disease - psychopharmacotherapy aspects. Psychiatr Danub. 2009;21(3):425-428. http://www.ncbi.nlm.nih.gov/pubmed/19794369. [PubMed] [Google Scholar]
- 295.Prokšelj T, Jerin A, Muck-Seler D, Kogoj A. Decreased platelet serotonin concentration in Alzheimer's disease with involuntary emotional expression disorder. Neurosci Lett. 2014;578:71-74. doi: 10.1016/j.neulet.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 296.Gottfries CG, Nyth AL. Effect of citalopram, a Selective 5-HT reuptake blocker, in emotionally disturbed patients with dementiaa. Ann N Y Acad Sci. 1991;640:276-279. doi: 10.1111/j.1749-6632.1991.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 297.Siddique H, Hynan LS, Weiner MF. Effect of a serotonin reuptake inhibitor on irritability, apathy, and psychotic symptoms in patients with Alzheimer's disease. J Clin Psychiatr. 2009;70(6):915-918. doi: 10.4088/JCP.08m04828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer disease. JAMA. 2014;311(7):682-691. doi: 10.1001/jama.2014.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Porsteinsson AP, Keltz MA, Smith JS. Role of citalopram in the treatment of agitation in Alzheimer's disease. Neurodegener Dis Manag. 2014;4(5):345-349. doi: 10.2217/nmt.14.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Jones HE, Joshi A, Shenkin S, Mead GE. The effect of treatment with selective serotonin reuptake inhibitors in comparison to placebo in the progression of dementia: a systematic review and meta-analysis. Age Ageing. 2016;45(4):448-456. doi: 10.1093/ageing/afw053. [DOI] [PubMed] [Google Scholar]
- 301.Krashia P, Nobili A, D'Amelio M. Unifying hypothesis of dopamine neuron loss in neurodegenerative diseases: focusing on Alzheimer's disease. Front Mol Neurosci. 2019;12:123. doi: 10.3389/fnmol.2019.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14(9):609-625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Hsu T-W, Fuh J-L, Wang D-W, et al. Disrupted metabolic connectivity in dopaminergic and cholinergic networks at different stages of dementia from 18F-FDG PET brain persistent homology network. Sci Rep. 2021;11(1):5396. doi: 10.1038/s41598-021-84722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Martorana A, Koch G. Is dopamine involved in Alzheimer's disease? Front Aging Neurosci. 2014;6:252. doi: 10.3389/fnagi.2014.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Nam E, Derrick JS, Lee S, et al. Regulatory activities of dopamine and its derivatives toward metal-free and metal-induced amyloid-β aggregation, oxidative stress, and inflammation in Alzheimer's disease. ACS Chem Neurosci. 2018;9(11):2655-2666. doi: 10.1021/acschemneuro.8b00122. [DOI] [PubMed] [Google Scholar]
- 306.Nobili A, Latagliata EC, Viscomi MT, et al. Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer's disease. Nat Commun. 2017;8:14727. doi: 10.1038/ncomms14727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.D’Amelio M, Nisticò R. Unlocking the secrets of dopamine in Alzheimer's Disease. Pharmacol Res. 2018;128:399. doi: 10.1016/j.phrs.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 308.Cordella A, Krashia P, Nobili A, et al. Dopamine loss alters the hippocampus-nucleus accumbens synaptic transmission in the Tg2576 mouse model of Alzheimer's disease. Neurobiol Dis. 2018;116:142-154. doi: 10.1016/j.nbd.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 309.Stubbendorff C, Hale E, Cassaday HJ, Bast T, Stevenson CW. Dopamine D1-like receptors in the dorsomedial prefrontal cortex regulate contextual fear conditioning. Psychopharmacology. 2019;236(6):1771-1782. doi: 10.1007/s00213-018-5162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Runyan J, Dash PK. Intra-medial prefrontal administration of SCH-23390 attenuates ERK phosphorylation and long-term memory for trace fear conditioning in rats. Neurobiol Learn Mem. 2004;82(2):65-70. doi: 10.1016/j.nlm.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 311.Heath FC, Jurkus R, Bast T, et al. Dopamine D1-like receptor signalling in the hippocampus and amygdala modulates the acquisition of contextual fear conditioning. Psychopharmacology. 2015;232(14):2619-2629. doi: 10.1007/s00213-015-3897-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Borowski TB, Kokkinidis L. The effects of cocaine, amphetamine, and the dopamine D₁ receptor agonist SKF 38393 on fear extinction as measured with potentiated startle: Implications for psychomotor stimulant psychosis. Behav Neurosci. 1998;112(4):952-965. doi: 10.1037//0735-7044.112.4.952. [DOI] [PubMed] [Google Scholar]
- 313.Abraham AD, Neve KA, Lattal KM. Activation of D1/5 dopamine receptors: a common mechanism for enhancing extinction of fear and reward-seeking behaviors. Neuropsychopharmacology. 2016;41(8):2072-2081. doi: 10.1038/npp.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.André MAE, Manahan-Vaughan D. Involvement of dopamine D1/D5 and D2 receptors in context-dependent extinction learning and memory reinstatement. Front Behav Neurosci. 2015;9:372. doi: 10.3389/fnbeh.2015.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Badgaiyan RD, Fischman AJ, Alpert NM. Dopamine release during human emotional processing. Neuroimage. 2009;47(4):2041-2045. doi: 10.1016/j.neuroimage.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Bardgett ME, Depenbrock M, Downs N, Points M, Green L. Dopamine modulates effort-based decision making in rats. Behav Neurosci. 2009;123(2):242-251. doi: 10.1037/a0014625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Yoshioka M, Matsumoto M, Togashi H, Saito H. Effect of conditioned fear stress on dopamine release in the rat prefrontal cortex. Neurosci Lett. 1996;209(3):201-203. doi: 10.1016/0304-3940(96)12631-8. [DOI] [PubMed] [Google Scholar]
- 318.Rice OV, Ashby CR, Dixon C, et al. Selective dopamine D3 receptor antagonism significantly attenuates stress-induced immobility in a rat model of post-traumatic stress disorder. Synapse. 2018;72(8):e22035. doi: 10.1002/syn.22035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Takahashi H, Yahata N, Koeda M, et al. Effects of dopaminergic and serotonergic manipulation on emotional processing: a pharmacological fMRI study. Neuroimage. 2005;27(4):991-1001. doi: 10.1016/j.neuroimage.2005.05.039. [DOI] [PubMed] [Google Scholar]
- 320.Berry AS, White RL, Furman DJ, et al. Dopaminergic mechanisms underlying normal variation in trait anxiety. J Neurosci. 2019;39(14):2735-2744. doi: 10.1523/JNEUROSCI.2382-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Lataster J, Collip D, Ceccarini J, et al. Psychosocial stress is associated with in vivo dopamine release in human ventromedial prefrontal cortex: a positron emission tomography study using [18F]fallypride. Neuroimage. 2011;58(4):1081-1089. doi: 10.1016/j.neuroimage.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 322.Nagano-Saito A, Dagher A, Booij L, et al. Stress-induced dopamine release in human medial prefrontal cortex-18F-Fallypride/PET study in healthy volunteers. Synapse. 2013;67(12):821-830. doi: 10.1002/syn.21700. [DOI] [PubMed] [Google Scholar]
- 323.Albrecht DS, Kareken DA, Christian BT, Dzemidzic M, Yoder KK. Cortical dopamine release during a behavioral response inhibition task. Synapse. 2014;68(6):266-274. doi: 10.1002/syn.21736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Vrieze E, Ceccarini J, Pizzagalli DA, et al. Measuring extrastriatal dopamine release during a reward learning task. Hum Brain Mapp. 2011;34(3):575-586. doi: 10.1002/hbm.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.da Silva Alves F, Schmitz N, Figee M, et al. Dopaminergic modulation of the human reward system: a placebo-controlled dopamine depletion fMRI study. J Psychopharmacol. 2011;25(4):538-549. doi: 10.1177/0269881110367731. [DOI] [PubMed] [Google Scholar]
- 326.Hu S, Li C-sR. Age-related structural and functional changes of the hippocampus and the relationship with inhibitory control. Brain Sci. 2020;10(12):1013. doi: 10.3390/brainsci10121013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Le TM, Chao H, Levy I, Li C-SR. Age-related changes in the neural processes of reward-directed action and inhibition of action. Front Psychol. 2020;11:1121. doi: 10.3389/fpsyg.2020.01121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Dhingra I, Zhang S, Zhornitsky S, et al. The effects of age on reward magnitude processing in the monetary incentive delay task. Neuroimage. 2020;207:116368. doi: 10.1016/j.neuroimage.2019.116368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Wang W, Zhornitsky S, Chao HH, Levy I, Joormann J, Li C-sR. The effects of age on cerebral responses to self-initiated actions during social interactions: An exploratory study. Behav Brain Res. 2020;378:112301. doi: 10.1016/j.bbr.2019.112301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Hu S, Job M, Jenks SK, Chao HH, Li C-sR. Imaging the effects of age on proactive control in healthy adults. Brain Imaging Behav. 2019;13(6):1526-1537. doi: 10.1007/s11682-019-00103-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Hu S, Ide JS, Chao HH, et al. Structural and functional cerebral bases of diminished inhibitory control during healthy aging. Hum Brain Mapp. 2018;39(12):5085-5096. doi: 10.1002/hbm.24347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Hu S, Chao HH-A, Winkler AD, Li C-sR. The effects of age on cerebral activations: internally versus externally driven processes. Front Aging Neurosci. 2012;4:4. doi: 10.3389/fnagi.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.David R, Koulibaly M, Benoit M, et al. Striatal dopamine transporter levels correlate with apathy in neurodegenerative diseases. Clin Neurol Neurosurg. 2008;110(1):19-24. doi: 10.1016/j.clineuro.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 334.Udo N, Hashimoto N, Toyonaga T, et al. Apathy in Alzheimer's disease correlates with the dopamine transporter level in the caudate nuclei. Dementia and Geriatric Cognitive Disorders Extra. 2020;10(2):86-93. doi: 10.1159/000509278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Padala PR, Padala KP, Lensing SY, et al. Methylphenidate for apathy in community-dwelling older veterans with mild Alzheimer's disease: a double-blind, randomized, placebo-controlled trial. Am J Psychiatr. 2018;175(2):159-168. doi: 10.1176/appi.ajp.2017.17030316. [DOI] [PubMed] [Google Scholar]
- 336.Rosenberg PB, Lanctôt KL, Drye LT, et al. Safety and efficacy of methylphenidate for apathy in Alzheimer's disease. J Clin Psychiatr. 2013;74(8):810-816. doi: 10.4088/JCP.12m08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Mintzer J, Lanctôt KL, Scherer RW, et al. Effect of methylphenidate on apathy in patients with Alzheimer disease. JAMA Neurol. 2021;78(11):1324-1332. doi: 10.1001/jamaneurol.2021.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.van Dyck CH, Arnsten AFT, Padala PR, et al. Neurobiologic rationale for treatment of apathy in Alzheimer's disease with methylphenidate. Am J Geriatr Psychiatr. 2021;29(1):51-62. doi: 10.1016/j.jagp.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Berridge CW, Devilbiss DM, Andrzejewski ME, et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatr. 2006;60(10):1111-1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 340.Hannestad J, Gallezot J-D, Planeta-Wilson B, et al. Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol Psychiatr. 2010;68(9):854-860. doi: 10.1016/j.biopsych.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Volkow ND, Wang G-J, Fowler JS, et al. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatr. 1998;155(10):1325-1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- 342.Eshleman AJ, Carmolli M, Cumbay M, Martens CR, Neve KA, Janowsky A. Characteristics of drug interactions with recombinant biogenic amine transporters expressed in the same cell type. J Pharmacol Exp Therapeut. 1999;289(2):877-885. [PubMed] [Google Scholar]
- 343.Mitchell RA, Herrmann N, Lanctôt KL. The role of dopamine in symptoms and treatment of apathy in Alzheimer's disease. CNS Neurosci Ther. 2011;17(5):411-427. doi: 10.1111/j.1755-5949.2010.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Serra L, D'Amelio M, Di Domenico C, et al. In vivo mapping of brainstem nuclei functional connectivity disruption in Alzheimer's disease. Neurobiol Aging. 2018;72:72-82. doi: 10.1016/j.neurobiolaging.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 345.Liu J, Chang L, Song Y, Li H, Wu Y. The role of NMDA receptors in Alzheimer's disease. Front Neurosci. 2019;13:43. doi: 10.3389/fnins.2019.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Lin C-H, Lane H-Y. The role of N-Methyl-D-aspartate receptor neurotransmission and precision medicine in behavioral and psychological symptoms of dementia. Front Pharmacol. 2019;10:540. doi: 10.3389/fphar.2019.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Bell KFS, Bennett DA, Cuello AC. Paradoxical upregulation of glutamatergic presynaptic boutons during mild cognitive impairment. J Neurosci. 2007;27(40):10810-10817. doi: 10.1523/JNEUROSCI.3269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Madeira C, Vargas-Lopes C, Brandão CO, et al. Elevated glutamate and glutamine levels in the cerebrospinal fluid of patients with probable Alzheimer's disease and depression. Front Psychiatr. 2018;9:561. doi: 10.3389/fpsyt.2018.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Findley CA, Bartke A, Hascup KN, Hascup ER. Amyloid beta-related alterations to glutamate signaling dynamics during Alzheimer's disease progression. ASN Neuro. 2019;11:175909141985554. doi: 10.1177/1759091419855541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Niciu MJ, Kelmendi B, Sanacora G. Overview of glutamatergic neurotransmission in the nervous system. Pharmacol Biochem Behav. 2012;100(4):656-664. doi: 10.1016/j.pbb.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Kim SH, Steele JW, Lee SW, et al. Proneurogenic group II mGluR antagonist improves learning and reduces anxiety in Alzheimer Aβ oligomer mouse. Mol Psychiatr. 2014;19(11):1235-1242. doi: 10.1038/mp.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Zhang YL, Xing RZ, Luo XB, et al. Anxiety-like behavior and dysregulation of miR-34a in triple transgenic mice of Alzheimer's disease. Eur Rev Med Pharmacol Sci. 2016;20(13):2853-2862. [PubMed] [Google Scholar]
- 353.Hu R, Wei P, Jin L, et al. Overexpression of EphB2 in hippocampus rescues impaired NMDA receptors trafficking and cognitive dysfunction in Alzheimer model. Cell Death Dis. 2017;8(3):e2717. doi: 10.1038/cddis.2017.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Barkus C, McHugh SB, Sprengel R, Seeburg PH, Rawlins JNP, Bannerman DM. Hippocampal NMDA receptors and anxiety: at the interface between cognition and emotion. Eur J Pharmacol. 2010;626(1):49-56. doi: 10.1016/j.ejphar.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Lanznaster D, Mack JM, Coelho V. Guanosine prevents anhedonic-like behavior and impairment in hippocampal glutamate transport following amyloid-β1-40 administration in mice. Mol Neurobiol. 2017;54(7):5482-5496. doi: 10.1007/s12035-016-0082-1. [DOI] [PubMed] [Google Scholar]
- 356.Steele JW, Brautigam H, Short JA, et al. Early fear memory defects are associated with altered synaptic plasticity and molecular architecture in the TgCRND8 Alzheimer's disease mouse model. J Comp Neurol. 2014;522(10):2319-2335. doi: 10.1002/cne.23536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Francis P. Altered glutamate neurotransmission and behaviour in dementia: evidence from studies of memantine. Curr Mol Pharmacol. 2009;2(1):77-82. doi: 10.2174/1874467210902010077. [DOI] [PubMed] [Google Scholar]
- 358.Tsang SWY, Vinters HV, Cummings JL, Wong PT-H, Chen CPL-H, Lai MKP. Alterations in NMDA receptor subunit densities and ligand binding to glycine recognition sites are associated with chronic anxiety in Alzheimer's disease. Neurobiol Aging. 2008;29(10):1524-1532. doi: 10.1016/j.neurobiolaging.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Partridge JG, Apparsundaram S, Gerhardt GA, Ronesi J, Lovinger DM. Nicotinic acetylcholine receptors interact with dopamine in induction of striatal long-term depression. J Neurosci. 2002;22(7):2541-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Marien MR, Colpaert FC, Rosenquist AC. Noradrenergic mechanisms in neurodegenerative diseases: a theory. Brain Res Rev. 2004;45(1):38-78. doi: 10.1016/j.brainresrev.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 361.Samuels E, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part II: physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr Neuropharmacol. 2008;6(3):254-285. doi: 10.2174/157015908785777193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Martorana A, Di Lorenzo F, Esposito Z, et al. Dopamine D2-agonist Rotigotine effects on cortical excitability and central cholinergic transmission in Alzheimer's disease patients. Neuropharmacology. 2013;64:108-113. doi: 10.1016/j.neuropharm.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 363.Garcia-Alloza M, Gil-Bea FJ, Diez-Ariza M, et al. Cholinergic-serotonergic imbalance contributes to cognitive and behavioral symptoms in Alzheimer's disease. Neuropsychologia. 2005;43(3):442-449. doi: 10.1016/j.neuropsychologia.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 364.Lindquist KA, Satpute AB, Wager TD, Weber J, Barrett LF. The brain basis of positive and negative affect: evidence from a meta-analysis of the human neuroimaging literature. Cerebr Cortex. 2016;26(5):1910-1922. doi: 10.1093/cercor/bhv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Suri G, Gross JJ. Emotion regulation and successful aging. Trends Cognit Sci. 2012;16(8):409-410. doi: 10.1016/j.tics.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 366.Urry HL, Gross JJ. Emotion regulation in older age. Curr Dir Psychol Sci. 2010;19(6):352-357. doi: 10.1177/0963721410388395. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-aja-10.1177_15333175221082834 for Emotion Processing Dysfunction in Alzheimer’s Disease: An Overview of Behavioral Findings, Systems Neural Correlates, and Underlying Neural Biology by Shefali Chaudhary, Simon Zhornitsky, Herta H. Chao, Christopher H. van Dyck and Chiang-Shan R. Li in American Journal of Alzheimer's Disease & Other Dementias