Abstract
Evidence for nusinersen administration in adult 5q spinal muscular atrophy (5q-SMA) patients is scarce and based on real-world observational data. The present systematic review and meta-analysis aimed to explore the efficacy and safety of nusinersen in patients older than 12 years of age with 5q-SMA. We searched MEDLINE, EMBASE, the Cochrane Library, and grey literature through April 2021. Cross-sectional studies, case reports, review articles, and studies with follow-up less than 6 months were excluded. We included 12 records (seven case-series, five cohorts) representing 11 population cohorts and enrolling 428 SMA patients. We observed statistically significant improvements on motor function Hammersmith Functional Motor Scale Expanded (HFMSE) and Revised Upper Limb Module (RULM) scores at the longest follow-up assessments [SMD = 0.17(95% CI 0.01–0.33), SMD = 0.22(95% CI 0.06–0.38), respectively]. HFMSE and RULM significant improvements were also detected at the subgroup analysis during 10 and 14 months. HFMSE and RULM amelioration occurred earlier in patients with SMA type 3 or 4 during short-term analysis (≤ 6 months). 6-min walk tests (6MWT) and pulmonary function tests did not change. Minimal clinically important differences in HFMSE and RULM were observed in 43.3% (95% CI 34.5–52.3) and 38.9% (95% CI 27.7–50.7), respectively. Severe adverse events were reported in 2% (95% CI 0–5.8). Treatment withdrawal rate was 3% (95% CI 0.5–6.6). Despite the low quality of evidence and the unmet need for randomized data to establish the safety and efficacy of nusinersen in adults, our meta-analysis confirms that nusinersen is a valuable treatment option for older patients with longer-disease duration.
Trial registration: PROSPERO database CRD42020223109.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-022-01200-3.
Keywords: Adult spinal muscular atrophy, Meta-analysis, Therapeutics, Antisense oligonucleotides, Nusinersen
Introduction
Spinal muscular atrophy (SMA) is a rare neuromuscular disease occurring in ∼1 in 11,000 births [1]. A single gene mutation, survival motor neuron 1 (SMN1) deletion on chromosome 5q13.2 leading to decreased full-length functional SMN protein and extensive motor neuron degeneration in the spinal cord, has been identified as the most common etiologic factor [2, 3]. The inheritance pattern of 5q-SMA is autosomal recessive. SMA phenotypic variability is explained by the SMN protein additional production by the homologous SMN2 gene [4], while SMN2 copy number has been inversely associated with disease severity [5]. Disease severity is reflected on SMA types that have been classified according to highest acquired motor milestone (sitters, standers, or walkers) and age at onset (infantile-onset type 1 to adulthood type 4) [9].
Nusinersen, an intrathecally delivered antisense oligonucleotide, became available worldwide on 2017 as the first disease modifying treatment for SMA patients of all types and ages [6]. Drug’s interference with SMN2 exon 7 splicing results in augmented fully functional SMN protein production [7]. Nusinersen’s approval and determination of dosing regimen was based on pivotal sham-controlled clinical trials in infants and children [8, 9]. Nevertheless, patients older than 12 years of age represent a significant portion of the overall SMA population considering that SMA type 3 and 4 patients have a normal life-expectancy [10]. Furthermore, more SMA patients are expected to survive into adulthood with the advent of various disease modifying treatments [11].
However, the effect of nusinersen in adult SMA patients with longer disease duration and slower rate of progression, who often are severely affected and may present with severe scoliosis, is still questionable given the lack of targeted clinical trials in this population. Natural history data of adult SMA are scarce, hindering the establishment of treatment benefit. It remains unclear whether stabilization of motor function or muscle strength or pulmonary function should be interpreted as prevention of disease progression and attributed to treatment. Evidence for nusinersen tolerability, safety and efficacy in adults is based on real-world observational studies usually including a small number of patients. Hence, a higher level of evidence is warranted prior to the introduction of recommendations for adult SMA individualized therapeutic management based on sound start and stop criteria in the dawn of different, yet high-cost, therapeutic options [12, 13].
The objective of this study is to systematically review the literature and conduct a meta-analysis to explore the efficacy, tolerability, and safety of nusinersen therapy in adult patients with genetically confirmed 5q-SMA.
Methods
The present systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) 2020 statement [online-only electronic Supplemental Material 1 (ESM 1)] [14]. The study’s protocol was registered in the International Prospective Register of Ongoing Systematic Reviews PROSPERO database (ID: CRD42020223109). A thorough description of methods is presented in ESM 2.
Information Sources, Search, and Eligibility Criteria
Last literature search at MEDLINE (via PubMed), EMBASE (via Ovid), and the Cochrane Library was performed on April 1, 2021. Our search strategy was based on the key words ‘spinal muscular atrophy’, ‘nusinersen’, and ‘outcome assessment’. No search restriction was applied. The complete search strategy for MEDLINE database is presented at ESM 3. Conference proceedings, ClinicalTrials.gov site, and reference lists from both included studies and relevant reviews were examined to identify additional studies.
We searched for any randomized controlled trial (RCT), case–control, cohort (prospective/retrospective) study, or case series reporting outcomes of nusinersen intrathecal administration in adult patients (≥ 12 years) with genetically confirmed 5q-SMA during a follow-up period of at least 6 months. Cross-sectional studies, case reports, and review articles were excluded. We included only studies reporting a comparator, such as data on a natural history group or a comparison between baseline and post treatment outcomes of the same population.
The primary outcome of interest was nusinersen therapeutic efficacy in SMA adults determined by the change from baseline score of any of the following: motor function scales [Hammersmith Functional Motor Scale Expanded (HFMSE), Revised Upper Limb Module (RULM), 6-min or 10-m walk tests (6MWT or 10MWT), ALS Functional Rating Scale (ALSFR-S), SMA Function Rating scale (SMAFRS)] or muscle strength [Medical Research Council (MRC) sum score, dynamometer testing] or pulmonary function tests [Forced Expiratory Volume in 1 s (FEV1), Forced Vital Capacity (FVC), and peak expiratory flow (PEF)].
Secondary outcomes of interest included (i) incidence of patients achieving minimal clinically important difference (MCID) at any of the primary outcomes, incidence of overall responders, and incidence of patients with motor function decline under nusinersen and (ii) safety of nusinersen therapy determined by the incidence of severe nusinersen-related adverse events in SMA adults and treatment withdrawal rate.
Based on previous reports, MCID was estimated at an increment of at least 3 points from baseline for HFMSE score, 2 points for RULM, and 30 m in the 6MWT [15–18]. Overall responders were defined as patients presenting with MCID in at least one of the HFMSE, RULM, or 6MWT. Severe adverse events (attributed to nusinersen treatment by the authors of each original record) were determined as those requiring or lengthening hospitalization, being life-threatening or fatal. Treatment withdrawal rate was determined as the proportion of patients who stopped nusinersen according to original records.
Summary Measures and Synthesis of Results
We performed meta-analysis for primary and secondary outcomes whenever data were available by three studies or more using the Comprehensive Meta-analysis software (version 2.0, Biostat Inc.) and OpenMeta[Analyst], respectively.
The primary outcome of interest regarding pre- and post-treatment comparisons of motor function scales, muscle strength, and pulmonary function tests was assessed using a standardized mean difference (SMD) with a 95% confidence interval (CI). We calculated the effect size for each outcome measure using a random-effect model based on follow-up sample size. We applied a conservative value of 0.5 as a correlation between pre- and post-treatment assessments when the correlation was not reported. We pooled together data regarding the same outcome measure assessed either during short-term (≤ 6 months) or long-term (> 6 months) follow-up. Secondary outcomes were assessed in the form of incidence rates using the Freeman-Tukey double arcsine transformation [19]. We quantified the degree of heterogeneity by I2 statistics [20]. We considered an I2 value > 50% as substantial heterogeneity.
Results
Study Selection — Flow Diagram
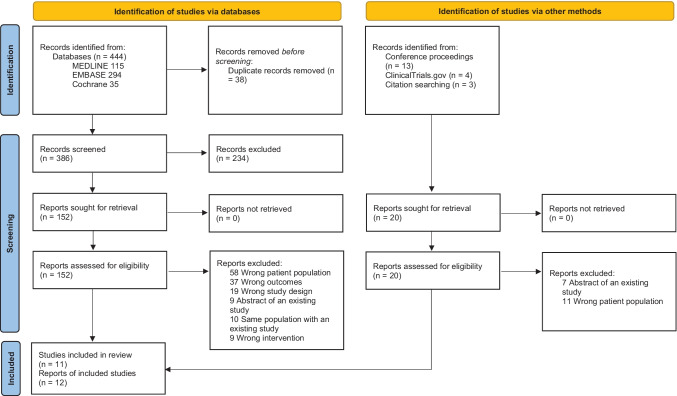
Our search yielded a total of 406 records after deduplication as shown at PRISMA flow diagram (Fig. 1). Twelve reports (seven case series, five cohort studies) representing eleven population cohorts enrolling 428 SMA patients older than 12 years with a follow-up of at least 6 months were included in qualitative and quantitative synthesis.
Fig. 1.
PRISMA flow diagram
Descriptive Characteristics and Risk of Bias Within Studies
We present the characteristics of included studies and risk of bias (RoB) assessment for the primary outcome in Table 1 and ESM 4. A total of 384 SMA patients were treated with nusinersen; most of whom were SMA type 3. De Wel et al. evaluated the effectiveness and safety of nusinersen in 16 SMA adult patients in a prospective cohort study but also reported retrospective natural history data of 48 untreated patients [21]. Moreover, Moshe-Lilie et al. described in a case series 22 SMA adult patients, ten of whom were treated with nusinersen [22]. The remaining reports presented comparisons between pre- and post-treatment evaluations of our primary outcome of interest in the same group of nusinersen-treated SMA patients older than 12 years [23–32]. Hagenacker et al. and Freigang et al. both examined patients of the German multicenter cohort [23, 26]. Hence, these two reports were merged in synthesis of the results.
Table 1.
Baseline characteristics and risk of bias assessment of the 11 studies
| First author | Study type | N | N SMA type 1 | N SMA type 2 | N SMA type 3 | N SMA type 4 | Age, years (range) | Ambulant (%) | Scoliosis* (%) | RoB | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| De Wel 2020 | Cohort | Total | 48 | 0 | 15 | 30 | 3 | 37.1 (20–66) | 20.8 | 41.7 | Low risk |
| Treated group | 16 | 0 | 0 | 14 | 2 | 37.5 (22–66) | 43.7 | 0 | |||
| Faravelli 2019 | Case series | 12 | 0 | 0 | 12 | 0 | 28.5 (15–34.8) | 83.3 | 0 | Fair | |
| German multicenter cohorta | Cohorts | 139 | 2 | 47 | 89 | 1 | 37 (16–65) | 37 | 22 | Low risk | |
| Inan 2020 | Case series | 40 | 0 | 4 | 36 | 0 | 34.4 (19–60) | 42.5 | 15 | High risk | |
| Jochmann 2020 | Case series | 7 | 0 | 4 | 3 | 0 | 45 (20–68) | 14.2 | 42.8 | Low risk | |
| Maggi 2020 | Cohort | 116 | 0 | 13 | 103 | 0 | 34 (18–72) | 34.5 | 13.8 | Low risk | |
| Moshe-Lilie 2020 | Case series | Total | 22 | 0 | 9 | 13 | 0 | 36 (20–71) | 9 | 77 | Low risk |
| Treated group | 10 | 0 | 0 | 33 (20–48) | |||||||
| SHINE study 2020 b | Case series | 7 | 0 | 1 | 6 | 0 | 14.4 (13–16) | 71.4 | na | Fair | |
| Veerapandiyan 2020 | Case series | 12 | 1 | 4 | 7 | 0 | 22 (12–52) | 25 | 66.7 | Low risk | |
| Walter 2019 | Cohort | 19 | 0 | 0 | 19 | 0 | 34 (18–59) | 63 | 0 | Low risk | |
| Yeo 2020 | Case series | 6 | 0 | 0 | 6 | 0 | 29.9 (24.9–56.5) | 67 | 0 | Low risk | |
| Total | 428 | 3 | 97 | 324 | 4 | 12–72 | 36.4% | 24% |
N number of patients, SMA spinal muscular atrophy, na non-applicable, RoB, risk of bias
*Previous surgery for scoliosis and/or severe scoliosis
aHagenacker 2020 and Freigang 2021 reports
bMuntoni 2020 report
Five reports designed as cohorts scored low risk (good quality) at Newcastle–Ottawa scale (SM 3A) [21, 23–26]. RoB assessment for included case series using a tool proposed by Murad et al. is presented at SM 3B. RoB of two case series [31, 32] was deemed fair (satisfying quality). One case series scored high risk (moderate quality) at RoB [29]. The remaining four case series scored low risk (good quality) [22, 27, 28, 30].
Nusinersen Effect on Motor Function, Muscle Strength, and Pulmonary Function Tests
Each of the included reports evaluated at least one of our primary outcomes of interest (change from baseline measurements of motor function scales or muscle strength or pulmonary function tests) as presented at ESM 5. We performed quantitative synthesis of our primary outcome measures HFMSE, RULM, 6MWT, and FVC during short-term and long-term follow-up period using SMD based on data availability. The effect of nusinersen treatment on the remaining outcome measures is presented at qualitative synthesis.
Qualitative Synthesis
Motor Function Scales
ALSFRS-R remained stable over 10 months of nusinersen injections as reported by both Walter and Jochmann et al. [25, 30]. Moreover, Yeo et al. found that SMAFRS which was designed for SMA type 3 patients and 10MWT showed high individual variability in a case series [33]. Veerapandiyan et al. assessed 30-foot walk TFT only in two patients in a case series [28]. Among TFTs examined by Maggi and Bello, rise from chair velocity and 10 m run/walk speed improved significantly during follow-up [24].
Muscle Strength
De Wel et al. showed that muscle strength, measured by both handheld dynamometry measurements and MRC sum scores, improved significantly after 14 months of nusinersen treatment in a cohort of SMA adults type 3 or 4 [21]. Interestingly, Moshe-Lille et al. observed that %MRC improved 2.5% at 12 months and 3.9% at 24 months of follow-up in the treatment group in contrast with the observed stabilization or decline in the untreated group. However, no significant change was reported in handheld dynamometry measurements [22]. Finally, Walter et al. did not show a statistically significant amelioration of a non-classic MRC score in adult SMA type 3 patients during the first 10 months of nusinersen administration [25].
Pulmonary Function Tests
PEF remained stable over 14 months of nusinersen treatment as reported by De Wel et al. [21]. Nevertheless, Walter et al. showed that peak cough flow (PCF) changed statistically significant at the 6-month analysis [25]. Similarly, Maggi et al. proved that FEV1% improved in adult SMA 3 patients after 14 months of treatment.
Quantitative Synthesis
HFMSE
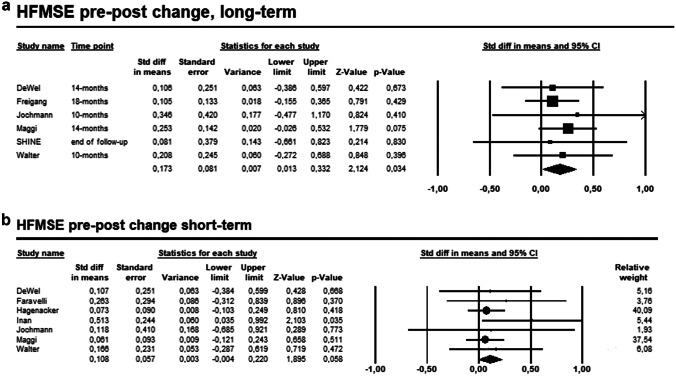
The pooled effect estimate of the six studies on the comparison between pre- and post-treatment at the longest follow-up of adult SMA patients receiving nusinersen showed statistically significant improvement of HFMSE (SMD = 0.173, 95% CI 0.013–0.332, p = 0.034, I2 = 0%), with no heterogeneity (Fig. 2A). Nevertheless, no significant improvement of HFMSE resulted from the comparison between pre- and post-treatment of the seven studies reporting short-term HFMSE assessment (SMD = 0.108, 95% CI − 0.04 to 0.220, p = 0.058, I2 = 0%, Fig. 2B).
Fig. 2.
A Forest plot presenting the pooled analysis of the six studies on the comparison between pre- and post-treatment HFMSE change at the longest follow-up. B Forest plot presenting the pooled analysis of the seven studies on the comparison between pre- and post-treatment HFMSE change during short-term follow-up
We performed a pre-planned subgroup analysis at different follow-up time-points. We observed a statistically significant amelioration of HFMSE on adult SMA patients both at 10 months (SMD = 0.188, 95% CI 0.048–0.328, p = 0.009, I2 = 0%, ESM 6A) and at 14 months (SMD = 0.257, 95% CI 0.078–0.436, p = 0.005, I2 = 0%, ESM 6B) after nusinersen treatment. Moreover, the statistically significant improvement of HFMSE was also observed at the pre-planned subgroup analysis according to SMA types. HFMSE score of adult SMA type 3 or 4 changed significantly during short-term (SMD = 0.239, 95% CI 0.012–0.465, p = 0.039, I2 = 54%, ESM 6C) and longest follow-up (SMD = 0.391, 95% CI 0.020–0.761, p = 0.039, I2 = 70.39%, ESM 6D).
RULM
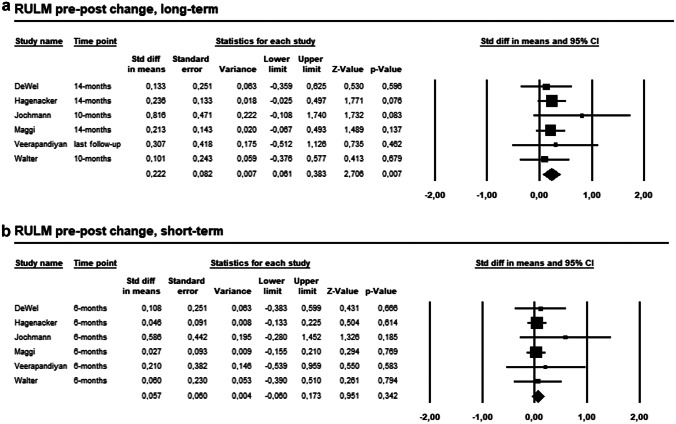
Six studies examined the effect of nusinersen treatment at RULM score of adult SMA patients during long-term follow-up. The pooled analysis on the comparison between pre- and post-treatment at the longest follow-up showed a statistically significant improvement of RULM (SMD = 0.222, 95% CI 0.061–0.383, p = 0.007, I2 = 0%, Fig. 3A). However, no statistically significant change of RULM score was revealed at the short-term analysis including SMA patients of any type (SMD = 0.057, 95% CI − 0.060 to 0.173, p = 0.342, I2 = 0%, Fig. 3B).
Fig. 3.
A Forest plot presenting the pooled analysis of the six studies on the comparison between pre- and post-treatment RULM change at the longest follow-up. B Forest plot presenting the pooled analysis of the six studies on the comparison between pre- and post-treatment RULM change during short-term follow-up
At the pre-planned subgroup analysis, we observed a statistically significant change of RULM on adult SMA patients both at 10 months (SMD = 0.172, 95% CI 0.032–0.312, p = 0.016, I2 = 0%, ESM 7A) and at 14 months (SMD = 0.213, 95% CI 0.035–0.301, p = 0.019, I2 = 0%, ESM 7B) after nusinersen treatment. Likewise, in SMA type 3 or 4, RULM increased significantly from baseline both during short-term (SMD = 0.156, 95% CI 0.021–0.292, p = 0.024, I2 = 0%, ESM 7C) and long-term (SMD = 0.317, 95% CI 0.130–0.504, p = 0.001, I2 = 0%, ESM 7D) follow-up.
6MWT
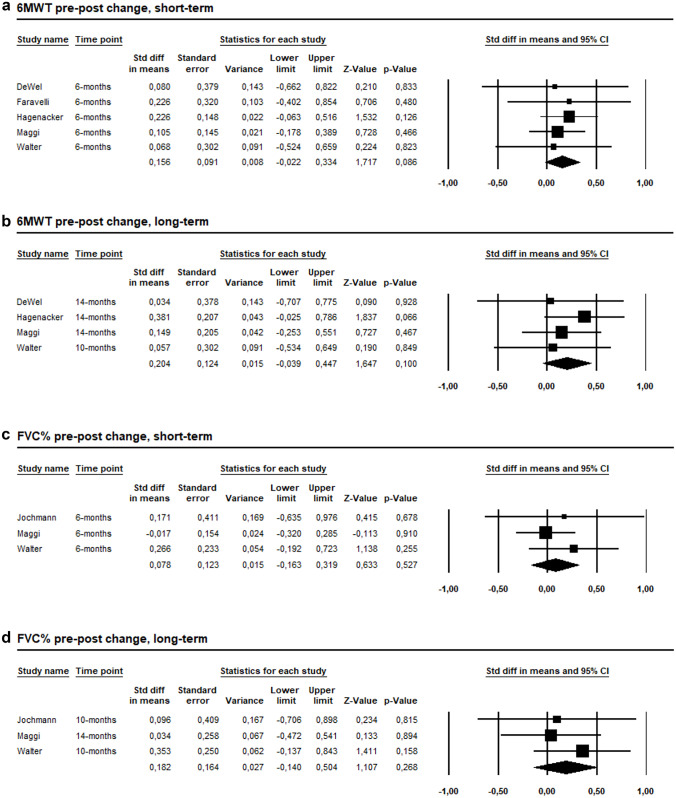
The pooled effect estimates showed no significant difference between pre- and post- nusinersen treatment on 6MWT during short-term (SMD = 0.156, 95% CI −0.022 to 0.334, p = 0.086, I2 = 0%, Fig. 4A) and long-term (SMD = 0.204, 95% CI −0.039 to 0.447, p = 0.1, I2 = 0%, Fig. 4B) follow-up. Similarly, no significant difference on 6MWT was observed at 10 months (SMD = 0.123, 95% CI −0.093 to 0.340, p = 0.263, I2 = 0%, ESM 8A) or at 14 months (SMD = 0.234, 95% CI −0.033 to 0.501, p = 0.085, I2 = 0%, ESM 8B) of treatment.
Fig. 4.
A Forest plot presenting the pooled analysis of the five studies on the comparison between pre- and post-treatment 6MWT change during short-term follow-up. B Forest plot presenting the pooled analysis of the four studies on the comparison between pre- and post-treatment 6MWT change at the longest follow-up. C Forest plot presenting the pooled analysis of the three studies on the comparison between pre- and post-treatment FVC% change during short-term follow-up. D Forest plot presenting the pooled analysis of the three studies on the comparison between pre- and post-treatment FVC% change at the longest follow-up
FVC
FVC% remained unchanged both during short-term (SMD = 0.078, 95% CI − 0.163 to 0.319, p = 0.527, I2 = 0%, Fig. 4C), at 10 months (SMD = 0.211, 95% CI − 0.089 to 0.511, p = 0.167, I2 = 0%, ESM 9), and long-term (SMD = 0.182, 95% CI − 0.14 to 0.504, p = 0.268, I2 = 0%, Fig. 4D) follow-up.
Responder Rates
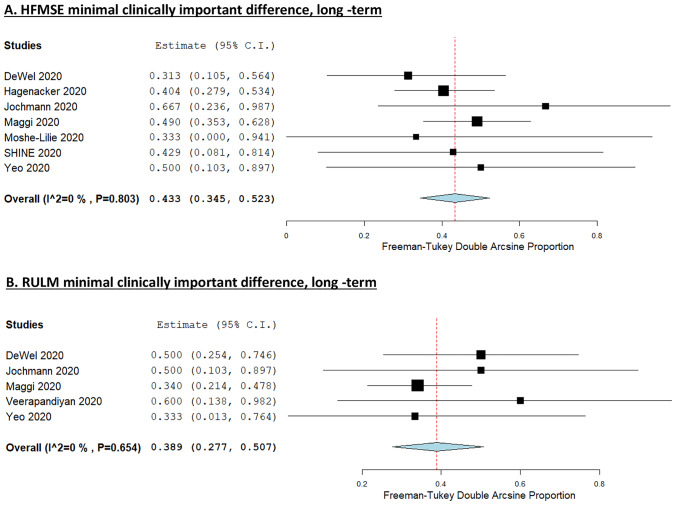
MCID in HFMSE was observed in 35.3% (95% CI 22.1–49.7, I2 = 77.2%, ESM 10) of adult SMA patients receiving nusinersen during short-term and 43.3% (95% CI 34.5–52.3, I2 = 0%, Fig. 5A) during long-term follow-up. Likewise, 38.9% (95% CI 27.7–50.7, I2 = 0%, Fig. 5B) of adult SMA patients achieved a MCID in RULM score during long-term evaluation. Overall responder rate was examined only by Maggi et al. representing 53%, 63%, and 69% of treated patients at 6, 10, and 14 months after initial administration [24]. We did not perform a pooled analysis for the secondary outcome of motor function decline under treatment because of studies’ heterogeneity on outcome measures reported and time-points assessed. Hence, we present the proportion of SMA patients with motor function decline under nusinersen as reported by each study in Table 2.
Fig. 5.
A Forest plot presenting the pooled incidence of the seven studies reporting on HFMSE MCID during long-term follow-up. B Forest plot presenting the pooled incidence of the five studies reporting on RULM MCID during long-term follow-up
Table 2.
Data regarding motor function decline, severe adverse events, and treatment withdrawal as defined and reported in each study
| First author | N treated | Treatment duration (months) | Motor function decline under treatment (%N) | Severe adverse events (N) | Severe adverse events description | Treatment withdrawal (N) |
Reasons of treatment withdrawal |
|---|---|---|---|---|---|---|---|
| De Wel 2020 | 16 | 6–14 | 18.8 | 0 | na | 0 | na |
| Faravelli 2019 | 12 | 6 | na | 0 | na | 0 | na |
| German multicenter cohorta | 139 | 6–14 | 10 | 0 | na | 4 | Post-LP syndrome (n = 2), patient’s wish (n = 2) |
| Inan 2020 | 40 | 3–9 | na | 0 | na | 2 | LP intolerance (n = 2) |
| Jochmann 2020 | 7 | 2–10 | 14.3 | 0 | na | 1 | Post-LP syndrome (n = 1) |
| Maggi 2020 | 116 | 6–14 | na | 6 | Post-LP syndrome (n = 5), renal colic (n = 1) | 2 | LP intolerance (n = 2) |
| Moshe-Lilie 2020 | 10 | 6–24 | 0 | 3 | LUTS (n = 1), bacterial meningitis (n = 1), deathc (n = 1) | 3 | Lack of improvement (n = 1), pneumonia (n = 1), proteinuria (n = 1) |
| SHINE study 2020b | 7 | 64–82 | 28.5 | 3 | Post-LP syndrome (n = 1), proteinuria (n = 1), LUTS/pyelonephritis (n = 1) | 0 | na |
| Veerapandiyan 2020 | 12 | 4–26 | 0 | 2 | Post-LP syndrome (n = 1), generalized tonic clonic seizure (n = 1) | 0 | na |
| Walter 2019 | 19 | 2–10 | 29.4 | 0 | na | 2 | Patient’s wish (n = 2) |
| Yeo 2020 | 6 | 14–21 | 16.7 | 2 | Fall with sacral compression fractures (n = 1), leg cellulitis (n = 1) | 0 | 0 |
na non-applicable, N number of patients, LP lumbar puncture, LUTS lower urinary tract symptoms
aHagenacker 2020 and Freigang 2021 reports
bMuntoni 2020 report
cBy respiratory failure in the setting of pneumonia
Adverse Events
We also intended to examine the adverse events following nusinersen treatment. We present in Table 2 a narrative description of severe adverse events and reasons of treatment withdrawal as extracted from each study. Severe adverse events were reported in 5.8% (95% CI 0.9–13.2, I2 = 69.51%, ESM 11A). In a subgroup analysis according to number of patients included in each study (N > 10), we found that 2% (95% CI 0–5.8, I2 = 47.52%, SM 11B) of patients experienced a severe adverse event. Furthermore, treatment withdrawal rate was 3% (95% CI 0.5–6.6, I2 = 26.93%, ESM 12). The most frequent non-severe adverse events were related to the administration procedure. Post lumbar puncture headache occurred in 43.9% (95% CI 33.3–54.7, I2 = 52.77%, ESM 13A) and back pain in 28.9% (95% CI 12.8–48, I2 = 85.9%, ESM 13B) of SMA adults following intrathecal nusinersen injections.
Risk of Bias Across Studies and Publication Bias
We did not detect publication bias for our primary outcome at visual inspection of funnel plots nor when we applied Egger’s linear regression test (ESM 14). We assessed quality of evidence of our primary outcome estimates which was low applying the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool based on observational nature of included studies (ESM 15) [34].
Discussion
Summary of Evidence
The present study is the first systematic review and meta-analysis that explored the efficacy and safety of nusinersen administration in 384 treated adult patients with genetically confirmed 5q-SMA. We documented a statistically significant improvement on motor function HFMSE and RULM scores at the longest follow-up assessments. The favorable outcomes on HFMSE and RULM were also found at the subgroup analysis during 10 and 14 months after initiation of nusinersen. Interestingly, we observed that the statistically significant amelioration of HFMSE and RULM occurred earlier in patients with SMA type 3 or 4 during short-term analysis in a subgroup analysis. Moreover, we found a stabilization on 6MWT and pulmonary function tests. The estimated incidence of adult SMA patients achieving MCID in HFMSE and RULM was 43.3% and 38.9% during long-term follow-up assessments, respectively. The most frequently reported adverse events were post lumbar puncture headache and back pain occurring in 43.9% and 28.9% of SMA adults following intrathecal nusinersen administration. Nevertheless, severe adverse events and treatment withdrawal rates were 2% and 3%, respectively. Overall, our results provide evidence that nusinersen has a favorable outcome on motor and pulmonary function tests and is well-tolerated in adult SMA patients with longer disease duration.
Limitations of the Evidence Included in the Review and the Review Processes Used
The main limitation of evidence included in this meta-analysis is the design of studies with pre- versus post-treatment comparisons along with the lack of an untreated control group. The observational nature of included studies downgraded the overall quality of evidence of this meta-analysis. The approval of nusinersen, an orphan drug, in SMA patients of all ages and types hindered the implementation of sham-controlled trials in adults. Thus, new ethical dilemmas were raised. The possibility of a placebo effect could not be eliminated by studies lacking a control group especially in this group of patients in which the natural history of the disease has not been studied thoroughly [35]. The stabilization of a neurodegenerative disease previously lacking a disease specific treatment may be desirable for patients and their treating physicians. Nevertheless, in certain SMA patients with longer disease duration and milder phenotypes, this stabilization in certain time periods may reflect natural disease course [36]. MCID cut-offs used in the included studies and adopted in this meta-analysis should be interpreted with caution in adult SMA considering that the methods used for their determination remain questionable [37]. Moreover, it should be highlighted that the motor function scales used as primary outcome measures in the included studies may not be sensitive enough to monitor therapeutic efficacy in SMA adults with different SMA types as ceiling and floor effects have been previously reported [38]. Finally, the small sample size, the variability of SMA types examined in each cohort, and the heterogeneity of population cohorts among the studies included in this meta-analysis are still important limiting factors. SMA is a rare disease with a unique phenotypic pattern. Patients with SMA type 2 do not gain the ability to walk independently; yet some SMA type 3 patients lose this ability during disease course, and some may be still ambulatory. It should be pointed out that although our study focused on adult SMA patients, we also included subjects aged 12 to 18 years on the basis of previously published cohorts suggesting that disease trajectory is similar in adolescents and adults [39]. The variability in disease severity may also reflect a variability in therapeutic response. Thus, we observed minor statistically significant improvements on motor function scores changes following nusinersen treatment despite the moderate treatment response as measured with MCID. However, the effect of nusinersen in each SMA type could not be reliably determined in subgroup analyses given the small number of patients included in these cohorts.
The strength of this study is the comprehensive search of all published and grey literature resources according to our study protocol leading to the enrollment of 428 SMA patients in this qualitative and quantitative synthesis. Nevertheless, none of the included studies reported the correlation between pre- and post-treatment assessments. We applied a conservative correlation value of 0.5. Thus, we may have underestimated the effect of nusinersen treatment. Finally, we did not conduct a meta-analysis to assess nusinersen effect on SMA adults muscle strength due to data heterogeneity and insufficiency.
Implications of the Results for Practice and Future Perspectives
Our meta-analysis indicates that nusinersen is a generally safe and promising treatment even in older SMA patients with longer disease duration, although the evidence is based on real-world data with small sample size and without extended follow-up. Given the paucity of randomized data, a meta-analysis of observational studies provides a higher level of evidence for the use of nusinersen in clinical practice and identifies research gaps. DEVOTE is an ongoing double-blind randomized, controlled dose-escalating trial which will enroll SMA patients of all ages and will elucidate the effect of higher regimen dosing on adult SMA [40]. However, several questions remain to be answered to provide superior quality of evidence and justify these costly treatments. Future studies should explore whether there is a wider therapeutic benefit on adult SMA patients that we currently cannot measure due to the inaccuracy of the established outcome measures. Moreover, it should be clarified whether there is a subgroup of SMA adults who could benefit the most from nusinersen administration. Finally, we should determine whether there is a timepoint in which physicians should stop nusinersen treatment because of a possible wearing-off phenomenon.
To that end, future randomized clinical trials should focus on the stratification of SMA participants based on prognostic biomarkers, the introduction of start and stop criteria, and the exploration of additional outcome measures and standardized patient-reported outcomes to monitor therapeutic response.
Conclusions
Despite the relatively low quality of evidence and the unmet need for randomized data to establish the safety and efficacy of nusinersen in adults, our meta-analysis confirms that nusinersen is a valuable treatment option for adult patients with longer-disease duration. Reliable and objective biomarkers should be employed to stratify adult SMA patients in future multicentre trials and monitor therapeutic benefit during a longer follow-up towards a personalized medical approach in the era of newly introduced high-cost disease modifying treatments.
Supplementary Information
Below is the link to the electronic supplementary material.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author Contribution
MG and MM screened the studies and retrieved full texts in mutual agreement. KN and EC extracted data from eligible studies. MG performed the data collection, performed analyses, and wrote the first draft of the manuscript. MG, VP, SP, MA, and VKK collaborated for the design of the study. VKK critically appraised the paper and made the final suggestions. All authors read and approved the final manuscript.
Availability of Data and Material
All data underlying this study are available in this article and in its online supplementary material.
Declarations
Ethics Approval and Consent to Participate
The manuscript presents a systematic review and meta-analysis and does not contain original patient data.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lunn MR, Wang CH. Spinal muscular atrophy. Lancet. 2008;371(9630):2120–2133. doi: 10.1016/S0140-6736(08)60921-6. [DOI] [PubMed] [Google Scholar]
- 2.Sugarman EA, Nagan N, Zhu H, Akmaev VR, Zhou Z, Rohlfs EM, et al. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: clinical laboratory analysis of >72,400 specimens. Eur J Hum Genet. 2012;20(1):27–32. doi: 10.1038/ejhg.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mercuri E, Finkel RS, Muntoni F, Wirth B, Montes J, Main M, et al. Diagnosis and management of spinal muscular atrophy: part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018;28(2):103–115. doi: 10.1016/j.nmd.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Markowitz JA, Singh P, Darras BT. Spinal muscular atrophy: a clinical and research update. Pediatr Neurol. 2012;46(1):1–12. doi: 10.1016/j.pediatrneurol.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Feldkotter M, Schwarzer V, Wirth R, Wienker TF, Wirth B. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am J Hum Genet. 2002;70(2):358–368. doi: 10.1086/338627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finkel RS, Chiriboga CA, Vajsar J, Day JW, Montes J, De Vivo DC, et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet (London, England) 2016;388(10063):3017–3026. doi: 10.1016/S0140-6736(16)31408-8. [DOI] [PubMed] [Google Scholar]
- 7.Singh NK, Singh NN, Androphy EJ, Singh RN. Splicing of a critical exon of human survival motor neuron is regulated by a unique silencer element located in the last intron. Mol Cell Biol. 2006;26(4):1333–1346. doi: 10.1128/MCB.26.4.1333-1346.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mercuri E, Darras BT, Chiriboga CA, Day JW, Campbell C, Connolly AM, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378(7):625–635. doi: 10.1056/NEJMoa1710504. [DOI] [PubMed] [Google Scholar]
- 9.Finkel RS, Mercuri E, Darras BT, Connolly AM, Kuntz NL, Kirschner J, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377(18):1723–1732. doi: 10.1056/NEJMoa1702752. [DOI] [PubMed] [Google Scholar]
- 10.Wadman RI, Wijngaarde CA, Stam M, Bartels B, Otto LAM, Lemmink HH, et al. Muscle strength and motor function throughout life in a cross-sectional cohort of 180 patients with spinal muscular atrophy types 1c–4. Eur J Neurol. 2018;25(3):512–518. doi: 10.1111/ene.13534. [DOI] [PubMed] [Google Scholar]
- 11.Pane M, Coratti G, Sansone VA, Messina S, Catteruccia M, Bruno C, et al. Type I SMA “new natural history”: long-term data in nusinersen-treated patients. Ann Clin Transl Neurol. 2021;8(3):548–557. doi: 10.1002/acn3.51276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dangouloff T, Botty C, Beaudart C, Servais L, Hiligsmann M. Systematic literature review of the economic burden of spinal muscular atrophy and economic evaluations of treatments. Orphanet J Rare Dis. 2021;16(1):47. doi: 10.1186/s13023-021-01695-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gavriilaki M, Kimiskidis VK, Gavriilaki E. Precision medicine in neurology: the inspirational paradigm of complement therapeutics. Pharmaceuticals. 2020;13(11). [DOI] [PMC free article] [PubMed]
- 14.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pera MC, Coratti G, Mazzone ES, Montes J, Scoto M, De Sanctis R, et al. Revised upper limb module for spinal muscular atrophy: 12 month changes. Muscle Nerve. 2019;59(4):426–430. doi: 10.1002/mus.26419. [DOI] [PubMed] [Google Scholar]
- 16.Pera MC, Coratti G, Forcina N, Mazzone ES, Scoto M, Montes J, et al. Content validity and clinical meaningfulness of the HFMSE in spinal muscular atrophy. BMC Neurol. 2017;17(1):39. doi: 10.1186/s12883-017-0790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swoboda KJ, Scott CB, Crawford TO, Simard LR, Reyna SP, Krosschell KJ, et al. SMA CARNI-VAL trial part I: double-blind, randomized, placebo-controlled trial of L-carnitine and valproic acid in spinal muscular atrophy. PLoS ONE. 2010;5(8):e12140. doi: 10.1371/journal.pone.0012140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunaway Young S, Montes J, Kramer SS, Marra J, Salazar R, Cruz R, et al. Six-minute walk test is reliable and valid in spinal muscular atrophy. Muscle Nerve. 2016;54(5):836–842. doi: 10.1002/mus.25120. [DOI] [PubMed] [Google Scholar]
- 19.Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. 1950;21(4):607–611. doi: 10.1214/aoms/1177729756. [DOI] [Google Scholar]
- 20.Higgins J, Green S. Identifying and measuring heterogeneity. Cochrane handbook for systematic reviews of interventions version. 2011;510.
- 21.De Wel B, Goosens V, Sobota A, Van Camp E, Geukens E, Van Kerschaver G, et al. Nusinersen treatment significantly improves hand grip strength, hand motor function and MRC sum scores in adult patients with spinal muscular atrophy types 3 and 4. 2020. [DOI] [PubMed]
- 22.Moshe-Lilie O, Visser A, Chahin N, Ragole T, Dimitrova D, Karam C. Nusinersen in adult patients with spinal muscular atrophy: observations from a single center. Neurology. 2020;95(4):e413–e416. doi: 10.1212/WNL.0000000000009914. [DOI] [PubMed] [Google Scholar]
- 23.Hagenacker T, Wurster CD, Günther R, Schreiber-Katz O, Osmanovic A, Petri S, et al. Nusinersen in adults with 5q spinal muscular atrophy: a non-interventional, multicentre, observational cohort study. Lancet Neurol. 2020;19(4):317–325. doi: 10.1016/S1474-4422(20)30037-5. [DOI] [PubMed] [Google Scholar]
- 24.Maggi L, Bello L. Nusinersen safety and effects on motor function in adult spinal muscular atrophy type 2 and 3. 2020. [DOI] [PubMed]
- 25.Walter MC, Wenninger S, Thiele S, Stauber J, Hiebeler M, Greckl E, et al. Safety and treatment effects of nusinersen in longstanding adult 5q-SMA type 3 - a prospective observational study. J Neuromuscul Dis. 2019;6(4):453–465. doi: 10.3233/JND-190416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freigang M, Wurster CD, Hagenacker T, Stolte B, Weiler M, Kamm C, et al. Serum creatine kinase and creatinine in adult spinal muscular atrophy under nusinersen treatment. Ann Clin Transl Neurol. 2021. [DOI] [PMC free article] [PubMed]
- 27.Yeo CJJ, Simeone SD, Townsend EL, Zhang RZ, Swoboda KJ. Prospective cohort study of nusinersen treatment in adults with spinal muscular atrophy. J Neuromuscul Dis. 2020;7(3):257–268. doi: 10.3233/JND-190453. [DOI] [PubMed] [Google Scholar]
- 28.Veerapandiyan A, Eichinger K, Guntrum D, Kwon J, Baker L, Collins E, et al. Nusinersen for older patients with spinal muscular atrophy: a real-world clinical setting experience. Muscle Nerve. 2020;61(2):222–226. doi: 10.1002/mus.26769. [DOI] [PubMed] [Google Scholar]
- 29.Inan B, Bekircan-Kurt CE, Kilinc M, Erdem-Ozdamar S, Tan E. The effect and challenges of nusinersen treatment in adult spinal muscular atrophy patients-preliminary results. Eur J Neurol. 2020;27:625. [Google Scholar]
- 30.Jochmann E, Steinbach R, Jochmann T, Chung HY, Rödiger A, Neumann R, et al. Experiences from treating seven adult 5q spinal muscular atrophy patients with nusinersen. Ther Adv Neurol Disord. 2020;13. [DOI] [PMC free article] [PubMed]
- 31.Faravelli I, Meneri M, Saccomanno D, Velardo D, Abati E, Gagliardi D, et al. Nusinersen treatment and cerebrospinal fluid neurofilaments: an explorative study on spinal muscular atrophy type 3 patients. J Cell Mol Med. 2020;24(5):3034–3039. doi: 10.1111/jcmm.14939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muntoni F, Darras BT, Finkel RS, Ryan MM, Mercuri E, Tulinius M, et al. Longer-term experience with nusinersen in teenagers and young adults with spinal muscular atrophy: phosphorylated neurofilament heavy chain (pNF-H) and efficacy results from the CS2-12/SHINE studies. Eur J Neurol. 2020;27:948–949. [Google Scholar]
- 33.Yeo CJJ, Simeone S, Zhang RZ, Trautman K, Damron B, Nwe P, et al. Outcome measures for nusinersen efficacy in adults with spinal muscular atrophy. Neurology. 2019;92(15).
- 34.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vazquez-Costa JF. Natural history data in adults with SMA. Lancet Neurol. 2020;19(7):564–565. doi: 10.1016/S1474-4422(20)30183-6. [DOI] [PubMed] [Google Scholar]
- 36.Gavriilaki M, Moschou M, Papaliagkas V, Notas K, Chatzikyriakou E, Zafeiridou G, et al. Biomarkers of disease progression in adolescents and adults with 5q spinal muscular atrophy: a systematic review and meta-analysis. Neuromuscul Disord. 2022. [DOI] [PubMed]
- 37.Vázquez-Costa JF, Hervás D. Minimal detectable change and minimal clinically important difference in spinal muscular atrophy patients. Eur J Neurol. 2021. [DOI] [PubMed]
- 38.Wijngaarde CA, Stam M, Otto LAM, Bartels B, Asselman FL, van Eijk RPA, et al. Muscle strength and motor function in adolescents and adults with spinal muscular atrophy. Neurology. 2020;95(14):e1988–e1998. doi: 10.1212/WNL.0000000000010540. [DOI] [PubMed] [Google Scholar]
- 39.Coratti G, Messina S, Lucibello S, Pera MC, Montes J, Pasternak A, et al. Clinical variability in spinal muscular atrophy type III. Ann Neurol. 2020;88(6):1109–1117. doi: 10.1002/ana.25900. [DOI] [PubMed] [Google Scholar]
- 40.Biogen. Study of nusinersen (BIIB058) in participants with spinal muscular atrophy (DEVOTE) Clinicaltrials.gov [cited 2021 13/5/2021]. NCT04089566]. 2019. Available from: https://clinicaltrials.gov/ct2/show/NCT04089566.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data underlying this study are available in this article and in its online supplementary material.