Significance
Heat waves are becoming more frequent, and we find that high air temperatures under dry conditions are associated with shorter telomere length in wild nestling purple-crowned fairy-wrens. Furthermore, impacts of heat exposure in nestlings may carry over into adulthood, as shorter early-life telomeres are associated with reduced lifetime fitness. Models using telomere data, fitness estimates, and climate change projections suggest that temperature-mediated telomere shortening could lead to population decline. However, the evolution of increased telomere length potentially counteracts these negative effects of warming and maintains population viability. For wildlife, increased early-life heat exposure from a warming climate may affect later life history with implications for population persistence and conservation.
Keywords: early life, telomere, climate change, fitness
Abstract
Climate warming is increasingly exposing wildlife to sublethal high temperatures, which may lead to chronic impacts and reduced fitness. Telomere length (TL) may link heat exposure to fitness, particularly at early-life stages, because developing organisms are especially vulnerable to adverse conditions, adversity can shorten telomeres, and TL predicts fitness. Here, we quantify how climatic and environmental conditions during early life are associated with TL in nestlings of wild purple-crowned fairy-wrens (Malurus coronatus), endangered songbirds of the monsoonal tropics. We found that higher average maximum air temperature (range 31 to 45 °C) during the nestling period was associated with shorter early-life TL. This effect was mitigated by water availability (i.e., during the wet season, with rainfall), but independent of other pertinent environmental conditions, implicating a direct effect of heat exposure. Models incorporating existing information that shorter early-life TL predicts shorter lifespan and reduced fitness showed that shorter TL under projected warming scenarios could lead to population decline across plausible future water availability scenarios. However, if TL is assumed to be an adaptive trait, population viability could be maintained through evolution. These results are concerning because the capacity to change breeding phenology to coincide with increased water availability appears limited, and the evolutionary potential of TL is unknown. Thus, sublethal climate warming effects early in life may have repercussions beyond individual fitness, extending to population persistence. Incorporating the delayed reproductive costs associated with sublethal heat exposure early in life is necessary for understanding future population dynamics with climate change.
Climate warming is increasing environmental temperatures beyond the thermal tolerance of many species, causing species distribution changes, phenological shifts, phenotypic changes, and mass mortality events (1–5). Current climate projections indicate that temperature extremes will increase in frequency, intensity, and duration, leading to more die-offs and a loss of biodiversity (6). High sublethal air temperatures are also likely to negatively affect fitness through tradeoffs between alleviating the immediate organismal impacts of heat exposure to improve short-term survival, at the expense of later survival and reproduction (7–9). Developing animals are particularly vulnerable to such tradeoffs because they are highly sensitive to environmental perturbations, and early-life adversity often leads to lifelong impairment (10). However, in wild populations, long-term consequences of heat exposure of developing young are virtually unexplored. In view of the accelerating pace of global warming, the heightened vulnerability of the early life stages, and the potential for delayed population impacts, there is an urgent need to understand the consequences of early-life heat exposure in the wild. This is particularly true for the tropics because climate-related local extinctions, while already occurring globally, are even more common in tropical animals (11).
Birds are acutely vulnerable to climate warming due to their small body size, high metabolism, and diurnal activity (12–14). To avoid hyperthermia, birds make behavioral (e.g., reducing activity, heat dissipation behaviors) and physiological (e.g., increasing evaporative water loss) adjustments to increase heat loss (15, 16), which can bring about dehydration and a loss of energy reserves (16, 17). Increased body temperatures result in higher resting metabolic rates, reduced enzyme performance, and increased production of heat shock proteins and glucocorticoid stress hormones (18–20). These physiological changes may affect the development and growth of offspring, with potential fitness consequences into adulthood. Nestlings are highly sensitive to heat due to high metabolic rates associated with growth and limited options for physiological and behavioral coping strategies (12, 17). Due to this sensitivity, the fitness effects of heat exposure may be disproportionate in nestling birds because they may trade off growth and survival at the expense of future fitness prospects (10). Such long-term negative effects of early-life disturbances can occur in the absence of any obvious immediate consequences (10). Quantifying physiological or molecular indicators of long-term fitness in response to early-life heat exposure can aid in our understanding of global warming effects on future performance or fitness.
Telomere length (TL) is considered a composite biomarker of cellular renewal, growth, and exposure to adverse environmental conditions (21) that is associated with organismal aging and fitness (22, 23). Telomeres are DNA “caps” on the end of eukaryotic chromosomes consisting of noncoding repetitive DNA sequences (TTAGGG). These caps function to maintain chromosome integrity and protect coding DNA but are eroded over time until a critical limit is reached, causing the cell to become senescent or to initiate apoptosis (24). A wide range of poor environmental conditions during development result in shorter TL (25–29). Specifically, the effects of adversely high temperatures during development—for example, higher metabolic rate, oxidative stress, dehydration, and nutrition reduction—are likely to similarly reduce TL. While there is evidence for such early-life effects of heat exposure in ectotherms (30), no studies have addressed this for endotherms in the wild. However, badger cubs born in colder conditions (in winter, at lower temperature) have shorter TL (28, 31), which is consistent with the negative effect of adverse temperatures. Because shorter early-life TL predicts reduced lifespan and fitness (22, 32, 33), an association between early-life TL and environmental temperature may indicate not only the lifelong impact of thermal conditions during development, but also future fitness consequences.
Here, we examine the effects of ambient air temperature on early-life TL in nestling purple-crowned fairy-wrens (Malurus coronatus coronatus). This small (∼11 g) passerine is insectivorous, breeds cooperatively, and is endangered (according to the EPBC Act 2015). They occur in a tropical monsoon climate where maximum air temperatures frequently rise above 45 °C, with high temperatures and most annual rainfall during the wet season (November to April). Previous research in this species showed that longer early-life TL predicts higher lifetime reproductive success and longer lifespan (22). Therefore, we quantify how environmental and climate factors influence TL to gain insight into potential delayed fitness consequences of climate warming. Our study aimed to examine 1) whether temperature variables projected to increase under climate change are associated with TL during the nestling period, including minimum (Tmin) and maximum air temperatures (Tmax), while 2) controlling for a broad range of environmental and life-history variables that may interact with temperature or individual fitness. Our findings suggest that higher air temperatures and low water conditions during nestling development lead to shorter telomeres and can, thus, have substantial fitness consequences. We, therefore, investigated the effect of a warming climate on TL-related fitness by 3) mathematically exploring the rate of change in mean fitness per generation across two climate change scenarios (intergovernmental panel on climate change [IPCC]: representative concentration pathways [RCP] 4.5 and 8.5) with and without evolution and the subsequent implications for population persistence across water availability scenarios.
Results
Predictors of Early-Life TL.
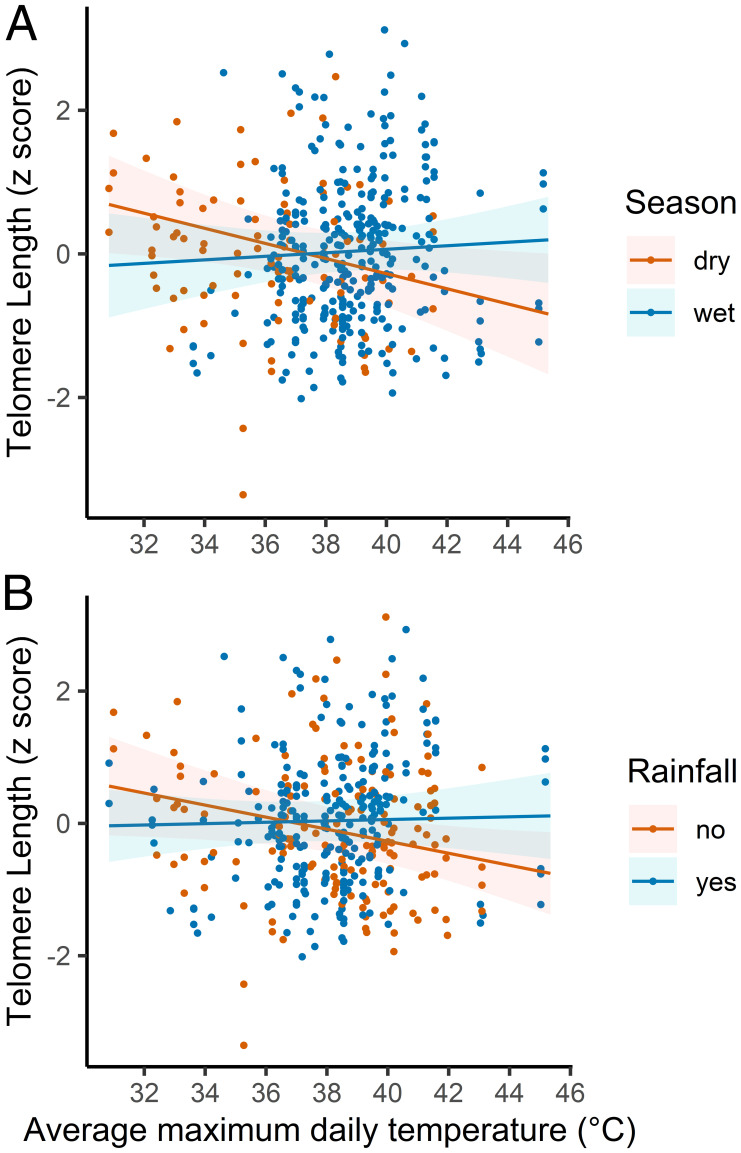
Daily air temperatures ranged between 9 and 46 °C, with an average Tmax over the nestling period (between hatching and TL measurement on day ∼7) of 38.1 ± 2.5 °C (mean ± SD; SI Appendix, Fig. S1), while cumulative rainfall ranged between 0 and 218 mm (SI Appendix, Fig. S2). There was a clear negative association between Tmax and early-life TL (Table 1), while average daily Tmin was nonsignificant (Table 1). Additionally, both interactions of Tmax with water availability (season: dry or wet season; rainfall: rain or no rain) were significant (Table 1), indicating that the effect of Tmax on early-life TL differs according to season (Fig. 1A) and rainfall (Fig. 1B). When there was no rain during the nestling period or during the dry season, the effect of Tmax on nestling TL was negative. However, during the wet season, or if there was rain during the nestling period, the effect of Tmax on TL was close to zero. The overall effect of Tmax when weighting the effect sizes for these four water availability conditions (season × rainfall) by the proportion of nestlings in each condition was negative but small (weighted Tmax β = −0.008; SI Appendix, Table S3). These results show that higher Tmax is negatively associated with TL under low water availability conditions (Fig. 1), but when considering that most nestlings experience high water availability conditions, because population productivity is higher in the wet season, the overall population effect is close to zero.
Table 1.
Environmental determinants of early-life TL (n = 417 individuals)
| Parameter | Estimate (se) | df | t | p |
|---|---|---|---|---|
| Intercept | −0.140 (0.275) | 153.494 | −0.508 | 0.612 |
| Tmax | −0.156 (0.048) | 126.135 | −3.240 | 0.002 |
| Tmin | −0.010 (0.027) | 132.535 | −0.355 | 0.723 |
| Rainfall (rain) | 0.148 (0.159) | 146.331 | 0.931 | 0.354 |
| Season (wet) | 0.109 (0.190) | 124.784 | 0.573 | 0.568 |
| Rainfall (rain) × Tmax | 0.101 (0.047) | 148.023 | 2.168 | 0.032 |
| Season (wet) × Tmax | 0.130 (0.062) | 159.316 | 2.106 | 0.037 |
| Parental provisioning rate | 0.092 (0.048) | 176.522 | 1.932 | 0.055 |
| Prey size | 0.081 (0.175) | 143.359 | 0.464 | 0.644 |
| Territory quality | 0.021 (0.016) | 112.354 | 1.378 | 0.171 |
| Number of helpers: one helper | 0.072 (0.129) | 133.720 | 0.560 | 0.576 |
| two helpers | −0.087 (0.162) | 141.128 | −0.539 | 0.591 |
| three or more helpers | −0.525 (0.186) | 115.972 | −2.830 | 0.006 |
| Sex (male) | 0.204 (0.079) | 344.075 | 2.571 | 0.011 |
| Mass (g) | −0.132 (0.078) | 351.952 | −1.694 | 0.091 |
| Tarsus length (mm) | 0.031 (0.048) | 353.590 | 0.643 | 0.520 |
| Brood size (range 1 to 4) | 0.038 (0.075) | 157.246 | 0.511 | 0.610 |
| Age at sampling (5 to 9 d) | 0.076 (0.067) | 198.481 | 1.132 | 0.259 |
| Blood storage buffer (Longmire’s) | 0.022 (0.233) | 99.179 | 0.095 | 0.925 |
| Variance components (SD) | ||||
| Maternal ID | 0.310 (0.557) | |||
| Nest ID | 0.083 (0.290) | |||
| qPCR run ID | 0.234 (0.484) | |||
| Residual | 0.444 (0.666) | |||
Shown are results from the linear mixed-effects model with TL (z-score transformed) as dependent variable; all continuous explanatory variates were mean centered. Daily maximum (Tmax) and minimum temperature (Tmin) were averaged across the nestling period (from hatching to day seven). Random (intercept only) variance estimates included: maternal ID, nest ID, and qPCR Run ID. Paternal ID was removed from the final model because it explained zero variance. Bold indicates significant predictors (P < 0.05). Model residuals and predictor covariance estimates were examined to confirm that the model assumptions were met. AICc = 1,144.65; including fixed predictors only, R2m = 0.08; including random and fixed predictors R2c = 0.62.
Fig. 1.
Higher air temperatures in conditions of low water availability are associated with shorter early-life TL. Shown are (A) the interaction effect between season and average daily maximum temperature during the nestling period (Tmax) and (B) the interaction effect between rainfall during the nestling period and Tmax, predicting early-life TL. Data represent TL values z-score transformed. The regression lines and confidence intervals are based on model estimates.
To control for methodological and other environmental conditions that might influence TL estimates, we included several covariates in the statistical model (Table 1). Of these, age at sampling, territory quality, brood size, tarsus length (body size), and body mass had no significant effect on TL (Table 1). There was a positive effect of parental provisioning rate (P = 0.055) on early-life TL but no additional effect of prey size (Table 1). This implies that food provided to nestlings, the most likely confounding variable for negative effects of Tmax, appears to have an independent effect on early-life TL (further supported by a nonsignificant interaction between Tmax and provisioning rate; see Materials and Methods). We also controlled for group size, as the number of helpers in the group might influence early-life conditions. We did not find a significant effect of smaller group sizes (one or two helpers), but when group sizes were larger (three or more helpers), there was a negative effect on TL (Table 1) for reasons we do not fully understand; regardless, our analyses ensure that the climate effects we find are independent of social environment. Finally, males had longer early-life TL than females, on average (Table 1), but Tmax effects did not vary with sex. The fact that Tmax remains a predictor of early-life TL after controlling for the preceding covariates and that Tmax strongly predicts nest temperature (SI Appendix, Table S2 and Fig. S3) suggests that the Tmax effect is not due to indirect temperature effects via environmental conditions but a direct effect on early-life TL, which is dependent on environmental water availability.
Population Projections for Warming and Water Availability Scenarios.
To characterize the consequences of warming-associated TL shortening for population mean fitness, we applied a simple quantitative genetic model (34, 35) to predict the rates and directions of change in TL and fitness (for full details, see Box 1). We parameterized the model with empirical estimates of: 1) the regression of Tmax on TL () for the four water availability conditions (dry season with no rainfall; dry season with rainfall; wet season with no rainfall; and wet season with rainfall; SI Appendix, Table S3) weighted by the proportion of nestlings that experienced each condition in our dataset; 2) contemporary mean fitness of the population (), which is the intercept of the regression between fitness and standardized TL (SI Appendix, Table S4); 3) the regression of standardized TL effect on contemporary fitness (lifetime number of offspring that obtain a breeding position; ) (SI Appendix, Table S4); and 4) the heritability of TL (SI Appendix, Table S5). We included two scenarios of climate change predicted for monsoonal northern Western Australia (36): intermediate warming (IPCC RCP4.5: increase in Tmax 0.026 °C per year) and high warming (IPCC RCP8.5: increase in Tmax 0.054 °C per year). Rainfall probabilities were assumed to be constant over the timescale of our predictions, though uncertainty in the estimated proportions of offspring born in each of the four conditions of season and rainfall (which reflect the current intrinsic variability of the region; ref. 36) was included in the analysis. We calculated rates of change per year, assuming a generation time of 5.065 y, following Saether et al. (37) (SI Appendix, section VII). It was not necessary to incorporate sex differences into the model because males and females do not differ in the relationship between TL and fitness (22), in the effect of Tmax on TL, or in the heritability of TL (SI Appendix, section VII). Models were evaluated using simulated parameter sets that incorporate uncertainty in our parameter estimates, as described in SI Appendix, Fig. S4.
Box 1. Modeling the effects of warming-induced telomere shortening and evolution on population fitness
This model aimed to predict short-term change in population fitness as a function of current climate projections, probable water availability scenarios, and our empirical estimates of telomere evolutionary potential, with the latter taking into account the telomere’s heritability and its covariance with fitness. By taking the set of relevant environmental and evolutionary factors into account, the model below quantifies the relative likelihood of a positive versus a negative change in population fitness in the near future, in scenarios that include or that exclude evolution.
There are four conditions in which offspring may be born: 1): in the dry season with no rainfall (condition 1); 2) in the dry season with rainfall (condition 2); 3), in the wet season with no rainfall (condition 3); and 4) in the wet season with rainfall (condition 4). The following vector represents the probability that an offspring is born in each of those four scenarios:
We modeled TL for 7-d-old juveniles using a linear function:
where x1, x2, x3, x4 ∼ multinomial (1, p); is the maximum daily temperature during early development in condition i; represents the sensitivity of TL to temperature in condition i ( when an increase in temperature decreases TL); G is the breeding value for TL; and E is the residual effect of environment on TL of the individual. We assume that G, E, and ε are independent, normally distributed random variables with G ∼ N(μG, VG), E ∼ N(0, VE), and εi ∼N(με,i, Vε), with the terms of μ and V referring to means and variances, respectively. Consequently, among individuals born in a given year, TL at day 7 (T) will be normally distributed with a mean of
and a variance of VT, which we hereafter scale to unit variance (VT = 1). The expected change in mean TL per year is
where each denotes change in the relevant mean. Assuming that rate of change in the mean maximum temperature over time is the same in each of the four conditions (, with time in years), we have
where is the average effect of temperature on TL. Change in the breeding value is given by
where tgen is the generation time of the population, and is the mean fitness of the population. This follows from the breeder’s equation, with time scaled in units of years (e.g., refs. 34, 82).
We assume that the expected lifetime fitness of individuals born within a given generation is a function of TL at day 7. Also, we assume that fitness with respect to TL follows a Gaussian function:
where is a hypothetical optimum TL, Wmax is the average fitness of individuals expressing the optimal T, and describes the width of the fitness surface. This function is sufficiently general to accommodate directional or stabilizing selection on TL and has the biologically plausible properties that fitness is neither negative nor open ended.
With VT scaled to unit variance, the mean fitness of individuals born in a given generation is
The covariance between TL and fitness is
With unit variance, the covariance corresponds to slope for the linear regression of fitness on TL (i.e., .
The net effect of evolutionary and environmentally induced changes in TL on change in mean fitness is
| [Eq. 1] |
where h2 is TL heritability; the final approximation is accurate to first order in the annual change in mean TL.
Supposing that evolution cannot offset fitness declines induced by environmental change (i.e., if h2 = 0, or if TL is merely correlated with fitness rather than causal), and assuming , , and are constants, then the general solution for after t years is given by
which declines over time when (as our empirical results imply), ultimately leading to subreplacement fertility and population decline.
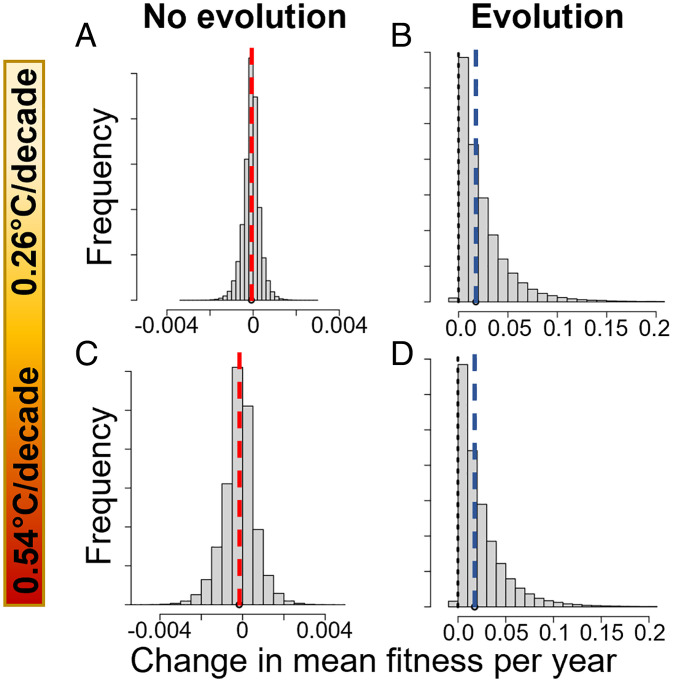
For both IPCC scenarios of climate warming, the models predict that the reduction in early-life TL with increasing air temperature has a weak negative effect on mean population fitness (Fig. 2 A and C). However, due to cumulative uncertainty in the set of parameter estimates, population fitness did not always decline over the short term, with 41% of simulations predicting an increase in mean fitness (Fig. 2 A and C).
Fig. 2.
For both intermediate and high warming scenarios, the change in mean fitness over a generation is negative in most simulations (59%) but overwhelmingly positive when assuming TL evolution. Model and simulations are those described in Box 1. (A and B) An intermediate warming scenario (RCP4.5) and (C and D) a high rate of warming (RCP8.5) are assumed. (A and C) Outcomes of models without adaptive evolution of TL (zero heritability) and (B and D) model outcomes assuming TL is an adaptive trait (based on estimated links to fitness and heritability). Models take into account uncertainty in parameter estimates; dashed lines represent the values predicted using point estimates of the parameters with red for negative values and blue for positive values.
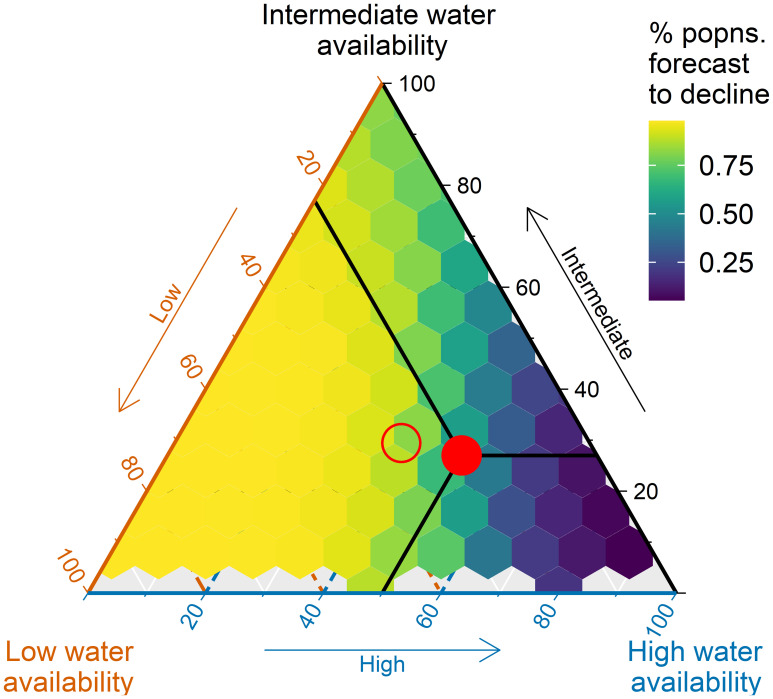
Because the effect of temperature on TL varies with water availability (season × rainfall), we also modeled changes in mean fitness for scenarios in which nestlings grow up in relatively arid conditions and in conditions where rainfall is abundant. The proportions of offspring experiencing different conditions of seasonality and rainfall can potentially change through a shift in breeding time or future changes in rainfall patterns. To explore the full range of possibilities, we randomly generated the proportion of nestlings experiencing each of the four water availability conditions and estimated (for each sample of proportions) the fraction of simulated populations with declines in mean fitness, as above. The fraction of populations with declining fitness is negatively skewed with a long tail toward zero (SI Appendix, Fig. S5), indicating a strong tendency for simulated populations to decline. Scenarios with low water availability (dry season without rainfall) resulted in a greater proportion of simulated populations with declining mean population fitness (Fig. 3), while the highest water availability conditions (wet season with rainfall) can nullify the effect of rising temperatures on TL and fitness. Increasing the number of nestlings experiencing intermediate conditions (dry season with rain and wet season without rain) can somewhat increase the proportion of populations with increasing mean fitness over time (Fig. 3). Nevertheless, these intermediate conditions generally resulted in fitness declines over time (approximately >75% of simulated populations).
Fig. 3.
Modeling the effect of climate warming across the full range of potential water availability conditions reveals a bias toward population decline. Simulations were based on predicted changes in mean fitness under any rate of warming and without evolution. For presentation purposes, the four environmental conditions (500,000 random parameter sets for p1, p2, p3, and p4) were combined into three categories: low water availability = dry season nestlings without rainfall (p1); high water availability = wet season nestlings with rainfall (p4); and intermediate water availability = wet season nestlings without rainfall and dry season with rainfall (p2 + p3). The scale represents the percentage of simulated populations that declined (0% dark blue, 100% yellow). The red-filled circle shows the prediction based on the current water availability conditions, which corresponds to declines for 59% of simulated populations (Fig. 2 A and C). The open red circle represents the most likely future water availability conditions that nestlings will encounter based on rainfall projections for the region (36) (SI Appendix, Table S3).
Population Projections When Including Adaptive TL Evolution.
To determine if population viability could be maintained through the evolution of TL, we included estimates of heritability (SI Appendix, Table S5) and selection for TL (the latter is a function of the covariance between TL and fitness; SI Appendix, Table S4) in the model. These models show that evolution of TL could, theoretically, compensate for the negative effects of telomere shortening induced by climate warming (Fig. 2 B and D). This is true even for the most severe scenario of highest warming and lowest water availability (i.e., 100% nestlings in the dry season without rain and high warming rates), for which the estimated change in mean fitness is overwhelmingly negative in the absence of TL evolution (e.g., 98% of simulated populations decline; SI Appendix, Fig. S6 A–D). Thus, while warming-induced telomere shortening is expected to reduce the fitness components of individual fairy-wrens, the population should be sufficiently fecund and evolutionarily labile to bear such costs and remain viable, provided TL is an adaptive trait that evolves in the manner we assume in our model.
Discussion
Temperature-Dependent TL.
In wild purple-crowned fairy-wrens, we demonstrate that higher air temperatures during the nestling period are associated with shorter nestling TL, but this relationship is dependent on water availability (seasonal or in the short term). In low water conditions (dry season or without rainfall), the association between maximum temperature and TL is negative, but it is neutral when water availability is high (wet season or with rainfall). Interestingly, a similar benign effect of spring rainfall in interaction with air temperature was observed in a temperate mammal (Meles meles; ref. 28), although the effect of temperature on early-life TL was positive here, most likely owing to the much cooler temperature range. In our study, there was no evidence for a nonlinear effect of maximum temperature or a suggestion that minimum temperature or diurnal temperature variability influenced early-life TL. This is, perhaps, not surprising in this tropical species that is already exposed to extremely high temperatures, near avian heat tolerance limits (38). The mechanisms by which sublethal temperatures influence TL are unknown but may relate to a species thermoregulatory capacity, physiological response, and tolerance, as suggested in ectotherms where TL can be shorter if environmental temperatures deviate from the optimal body temperature for the species (30, 39, 40). Taken together, ours and these studies highlight how effects of ambient temperature on early-life TL can vary with environmental context and reflect which conditions represent a greater challenge to the organism.
We controlled for several nonclimate environmental variables that are also likely to affect early-life TL (41) and might confound or mask the effect of air temperature if they exacerbate or compensate for temperature effects. For instance, nestling parental care and food provisioning is often reduced in warmer conditions as parents avoid heat exposure (16, 42). Therefore, we quantified and controlled for the amount of food provided to nestlings, which tended to be positively related to nestling TL, but it did not influence the effect of maximum temperature. Similarly, in purple-crowned fairy-wrens, additional group members (helpers) contribute to offspring care, which can increase breeding success (43) and potentially compensate for harsh thermal conditions. However, nestling TL was shorter in groups with three or more helpers (Table 1), which might suggest density dependence or a cost to living in larger groups. Within-nest competition (brood size) and habitat quality (territory quality) had no clear effect on early-life TL, which was unexpected, as experimental evidence shows that larger broods (e.g., refs. 25, 44) and poor habitats can shorten early-life telomeres in other bird species (e.g., refs. 29, 45, 46). Possibly, variation in brood size (most broods contained three nestlings) and habitat quality (most of the study area is in a protected reserve) was insufficient to cause a detectable effect. Black bulbs placed in nests showed that nest maximum temperature is, on average, cooler than air maximum temperature and that nests vary in their deviance from air temperature (SI Appendix, Fig. S3). This suggests that cooler microsites created by Pandanus vegetation might be important in this species and that other environmental factors may account for additional microclimate variation (e.g., thermal buffering of extreme temperatures due to emergent trees, nest height, or proximity to water). The most parsimonious explanation of the association between hot and arid conditions and shorter early-life TL might be a direct adverse physiological and cellular effect of heat exposure (38). However, we acknowledge the relationship could be due to unknown variables that are correlated with temperature and that experimental studies may provide further insight into the mechanisms underpinning these results.
The potential for heat exposure–related telomere shortening to be buffered by rainfall may indicate the direct benefits of available water for evaporative cooling or a lower risk of dehydration (47). Evaporative heat loss is more challenging when humidity is high, but this could be temporary, whereas the benefits of water availability may persist (also suggested by the positive effect of season). Access to water is considered important for riparian species such as purple-crowned fairy-wrens because they are generally prone to high rates of evaporative water loss (48). Insectivore nestlings rely on the water content of food to maintain hydration, and in wetter conditions, the arthropod prey may have higher water content and, thereby, lower risk of dehydration when evaporative cooling is required. In low water conditions, heat exposure impacts may be unavoidable and directly shorten telomeres. Multiple studies have shown that higher ambient temperatures can increase metabolism, oxidative damage, glucocorticoid stress, and heat shock protein levels, each of which has been found to relate to telomere dynamics (e.g., refs. 49–51), and TL tends to be shorter when growth is inhibited by suboptimal conditions (25, 44, 52–55). Thus, in purple-crowned fairy-wren nestlings, the physiological benefits of wetter conditions may exceed or neutralize any negative effects of hot conditions, whereas in drier conditions, organismal or cellular effects of heat exposure may alter growth and physiology with negative effects on TL.
Individual Fitness Costs.
Because nestling TL is a biomarker of lifespan and lifetime reproductive success (22), our results imply that early-life heat exposure can have lasting consequences for fitness. The effect of hot and arid conditions on TL implies that nestlings subjected to those conditions may be required to trade off investment in self-maintenance at the expense of lifespan (56). However, the proximal mechanism explaining the link between early-life TL and lifespan is unresolved (56, 57). One potential mechanistic link involves a direct role of TL in the aging process. Telomeres shorten until a threshold is reached and the cell enters a nonreplicative state or initiates apoptosis. The buildup of cells in this senescent state is thought to contribute to organismal aging (58). Alternatively, TL dynamics could indirectly relate to lifespan because they are correlated with other physiological factors involved in mortality. Indeed, we have observed that juvenile purple-crowned fairy-wrens with faster rates of TL shortening have shorter lifespans (59). Irrespective of the mechanism, the relationship between TL and lifespan could have implications beyond the individual level.
Since purple-crowned fairy-wrens with longer early-life TL have higher lifetime reproductive success (22), and since hotter, drier conditions during early life predicts shorter telomeres, climate warming may result in progressive telomere shortening and thereby depress population productivity, which—if left unchecked—may eventually result in population decline. As the population declines, the number of large groups that produce nestlings with shorter TL may also decline, potentially countering the reduction in TL. However, given the rarity of such large groups (<10% with three or more helpers) and the weak covariation between group size and population size (SI Appendix, section II), this effect is likely to be small. One hypothesis to counter warming-induced TL shortening would be an adaptive evolutionary response to warming temperatures through selection for longer TL or coadapted traits reflected by TL, which could mitigate the negative effects of a warmer climate on fitness. There is at least some capacity for TL to evolve in purple-crowned fairy-wrens because of its link with fitness (22) and heritability (h2 = 0.36 to 0.38; SI Appendix, Table S5). An alternative population response to environmental change—geographic range shift—is unlikely because the population is severely fragmented, and subpopulations are isolated by surrounding unsuitable habitat (60). This suggests that purple-crowned fairy-wren populations may have to cope with changing local environmental conditions in their current range, either with or without adaptive evolution. Given the alternative outcomes of ongoing climate warming on future fitness, we quantified several future climate scenarios and the effect of evolutionary adaptation in simple models.
Consequences of Climate-Induced Early-Life Telomere Shortening for Population Viability.
Our model predicts short-term changes in population fitness as a function of predicted climate change in the near future. We estimated the effect of increasing Tmax on early-life TL related fitness by taking into account uncertainty in our estimates and assuming all potential other variables that are important for survival and reproduction are constant. Under both intermediate and high climate warming scenarios, increasing temperatures are projected to reduce mean population fitness (Fig. 2 A and C). The reduction in mean fitness is subtle due to the short period (one generation) over which it is modeled. However, such reductions could, if left unchecked, substantially reduce population viability over time under sustained climate warming. Incorporating changes in water availability, we revealed a clear tendency for population decline in all but the wettest conditions (Fig. 3). Future populations could respond to warming by shifting toward breeding strategies that maximize the number of offspring experiencing high water availability conditions. However, given that all breeding purple-crowned fairy-wrens attempt to raise offspring in the wet season and in response to unseasonal rainfall events (61), this species may already be maximizing the chance that nestlings experience optimal conditions. Additionally, it is predicted that rainfall patterns will likely become more erratic and individual rainfall events more extreme (36). Thus, if low water conditions were to become more common than our modeling assumes, the likelihood of the population declining rapidly increases. This environmental uncertainty precludes precise long-term predictions of population dynamics. Nonetheless, the simple models imply that climate change effects early in life, independent of other population perturbations, could conceivably reduce population viability and, given enough time, elevate extinction risk.
Incorporating adaptive evolution into the model (Fig. 2 B and D) shifts the change in mean fitness from negative to positive, even under the most extreme climate warming scenarios (e.g., high rates of warming and low water availability; SI Appendix, Fig. S6 B and D). Therefore, selection for longer early-life TL can potentially fully compensate for the climate warming effects on early-life TL. Currently, whether TL is an adaptive trait under selection is debated because it is unclear whether early-life TL causally relates to fitness with the capacity to adapt as we model it here, or whether it reflects other physiological damage that may or may not have the same adaptive capacity. Nonetheless, early-life TL is heritable in our dataset, and TL appears to have a genetic basis that is associated with lifespan in some species (62, 63), so the evolutionary assumptions in our models are plausible. To determine whether evolutionary changes in early-life TL can act as a safeguard against climate change, long-term and experimental evolutionary studies of early-life TL in response to increasing temperatures are needed.
Conclusions.
While TL has previously been reported to indicate population extinction risk due to climate change (64), our study also shows how heat exposure during the developmental stage alone could increase telomere shortening and reduce population persistence. Physiological biomarkers that reflect impacts of high temperatures and that relate to fitness, such as telomeres, may identify sublethal effects of climate warming that would otherwise be overlooked. As such, current extinction estimates based on direct extreme temperature mortality events, range shifts, or life-history changes may underestimate the negative impact of climate change on biodiversity (65). It is, therefore, necessary to incorporate the delayed lifetime costs associated with sublethal heat exposure early in life for understanding future population dynamics with climate change.
Materials and Methods
Study Site, Species, and Data Collection.
Sampling took place at the Australian Wildlife Conservancy’s Mornington Wildlife Sanctuary (S17.516° E126.10°) along a 15-km stretch of Annie Creek and the Adcock River. Most (>95%) dome-shaped nests are built deep into the crowns of Pandanus aquaticus (66), a palm-like monocot. Nest height is limited by vegetation height and varied between 0.25 and 6 m, with a median of 1.4 m. Purple-crowned fairy-wrens form monogamous breeding pairs that defend year-round exclusive territories with up to nine nonbreeding subordinate helpers (67, 68). Offspring provisioning and predator defense is shared between group members (66). Since 2005, all individuals in the population have been uniquely marked and their social status recorded (69). Breeding can occur throughout the year, but peaks during the wet season (December to March) (70). For this study, nest building, incubation (1 to 4 eggs per nest), hatching (13 or 14 d until fledging), and fledging were monitored between 2007 and 2011 and between 2016 and 2018. After approximately 7 d from hatching (mean = 7.1 ± 0.9 SD, range = 4 to 9 d), morphometric measurements (mass and tarsus length) and a blood sample for TL measurement were collected and identification bands fitted. Nestling sex was determined by PCR (71) and validated in adults by sexually dimorphic plumage (72).
Daily Tmin and Tmax (°C) were extrapolated from a regression (Tmin R2 = 0.93 and Tmax R2 = 0.76, n = 574 d) between the air temperature variables from two nearby Australian Bureau of Meteorology weather stations (Mornington station [site number 002076, S17.51° E126.11°] is located on the field site, while Fitzroy Crossing Aero Station [site number 003093, S18.18° E125.56°] is located approximately 93 km from the field site) because of incomplete temperature data at Mornington station. Rainfall data were obtained from Mornington station only. Daily Tmin and Tmax were each averaged for the period between hatch date and when the individuals were sampled at approximately 7 d old (nestling period) and were moderately correlated (r = 0.49). Following suggestions from Cunningham et al. (73), we also calculated a measure of heatwave intensity (count of the number of days above average Tmax) to investigate if this predicted TL. However, this measure was excluded from further analysis because it was highly correlated with Tmax (r > 0.99), preventing any independent assessment of heatwave effects. In addition, diurnal temperature range (Tmax − Tmin) has been proposed to mask or confound effects of Tmax (8). Even though diurnal temperature was only weakly correlated with nest Tmax (Pearson’s r = 0.2), it was highly correlated with Tmin (Pearson’s r = -0.75), precluding its inclusion in the model. Similarly, Tmax during the incubation period was not included, as it was highly correlated with nestling period Tmax (Pearson’s r = 0.71, P < 0.001), and we had no a priori expectation of a clear effect because egg temperature is highly regulated and there is less growth (therefore, lower TL attrition) compared to the nestling stage. Tmax distribution was similar for nests with and without rain (with rain: mean = 38.15, SD = 2.41, n = 93; no rain: mean = 38.09, SD = 2.54, n = 82; t = 0.17, degrees of freedom [df] = 173, P = 0.87; SI Appendix, Fig. S1A). Air temperature profiles were relatively similar in each season (Tmax dry: mean = 36.1, SD = 2.7, n = 45; Tmax wet: mean = 38.8, SD = 1.9, n = 130; SI Appendix, Fig. S1B), although dry season nests were cooler (t test: t = 7.41, df = 173, P < 0.001). Average air temperature (Tmax and Tmin) strongly predicts average in-nest temperature (estimated by black bulb thermometers placed in empty nests for 6 to 8 d). Moreover, the effect of air temperature on in-nest temperature does not vary with environmental conditions (rain, season; SI Appendix, section III, Table S2, and Fig. S3).
Nutrition and hydration during the nestling period may vary with temperature or may be associated with parental care. Hence, we quantified parental provisioning rate and average prey size as previously described (66). To control for habitat quality, we quantified the amount of Pandanus aquaticus vegetation, which is critical for this species (70, 74) (SI Appendix, section II).
Telomere Measurement.
To extract DNA from Longmire’s buffer and ethanol, we used a modified QIAamp DNA kit (Qiagen) and automated the process using a QIAcube HT instrument. DNA purity and concentration was assessed using a NanoDrop (ND-1000), and DNA integrity was assessed on an agarose gel (75). A qPCR method based on Criscuolo et al. (76), and validated for use in M. coronatus by Eastwood et al. (75), was used to measure TL relative to the glyceraldehyde-3-phoshate dehydrogenase control gene. Efficiencies and intra- and interassay repeatabilities were high (75). qPCR quality control and the calculation of relative TL followed Eastwood et al. (22) (SI Appendix, section I).
Statistical Analysis.
The sample size was n = 417 individuals from n = 175 nests. Statistical analyses were conducted in R version 3.5.1 (77) and graphically presented using the packages ggplot2 and ggtern (78, 79). To test for air temperature effects on early-life TL, we conducted linear mixed-effects models with restricted maximum likelihood estimates using lme4 package, version 1.1-19 (80). TL was converted to z-scores with a mean of zero and a SD of one (81). To best estimate the effect of the variable Tmax, we controlled for potential biological, ecological, or methodological predictors of early-life TL (see SI Appendix, section II for detailed justifications). The controlling random variables included qPCR plate ID, nest ID, and maternal ID. Paternal ID was excluded because it explained <1% of the variance. The fixed covariates included age at sampling, mass, tarsus length, and brood size. Factors included sex, number of helpers (zero, one, two, and three or more), and blood storage buffer. To control for habitat, parental care, and nutrition, we included the covariates territory quality, parental provisioning rate, and average prey size (SI Appendix, section II). In addition, rainfall was included as a fixed factor (rain or no rain) and in an interaction with Tmax. Season (wet = November to April; dry = May to October) was also included in the model with its interaction with Tmax. The interactions are necessary because higher temperatures may be more damaging under arid conditions—as increased water availability can increase evaporative cooling capacity or, alternatively, reduce it under humid conditions—and adult purple-crowned fairy-wren immunity varies with temperature in interaction with rainfall (74). We did not include interactions among Tmin, season, and rainfall because we had no a priori predictions. We also fitted a series of models as a post hoc exploration of alternative covariates and interactions. Cumulative rainfall was included as a covariate instead of the binary rainfall factor to account for rain volume (SI Appendix, Fig. S2) but was removed from further analysis because it was not significant (P > 0.05). In addition, we investigated nonlinear temperature effects by including the temperature variable squared with its nonsquared equivalent because cooler conditions may also affect TL. Model fit was not improved by including Tmax as a quadratic term (ΔAICc = +9.27). The two-way interactions between temperature and either sex, number of helpers, brood size, territory quality, or parental provisioning rate were explored because temperature-driven effects may be exacerbated or ameliorated by those predictors (SI Appendix, section IV). Each interaction was inserted into the model individually because we lack the statistical power to include temperature with seven interactions simultaneously. However, they were all nonsignificant (P > 0.26). Finally, we included maternal and paternal TL in the year of conception of each nestling in the final model to ensure that seasonal effects were not driven by differences in the timing of breeding of parents with shorter or longer telomeres; their inclusion did not alter any conclusions, and parental TLs did not significantly predict nestling TL (SI Appendix, section IV and Table S1). All continuous variables were mean centered. In all analyses, we examined if model assumptions were satisfied and assessed variable collinearity (none identified), and model fit was assessed using AICc and R2.
Supplementary Material
Acknowledgments
We thank the Australian Wildlife Conservancy’s Mornington Wildlife Sanctuary for support and all field volunteers who helped collect data. Also, we are grateful for the constructive feedback from two anonymous reviewers, which greatly improved the manuscript. Research was approved by the Australian Bird and Bat Banding Scheme, the Western Australian Department of Parks and Wildlife, the Australian Wildlife Conservancy, the Animal Ethics Committees of the Max Planck Society, and the School of Biological Sciences of Monash University. Funding was provided by the Max Planck Society Minerva Program, the Australian Research Council (FT10100505, DP150103595, and DP180100058), and Monash University.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2122944119/-/DCSupplemental.
Data Availability
Data used in this study are available in Dataset S1.
References
- 1.Lane J. E., Kruuk L. E. B., Charmantier A., Murie J. O., Dobson F. S., Delayed phenology and reduced fitness associated with climate change in a wild hibernator. Nature 489, 554–557 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Møller A. P., Rubolini D., Lehikoinen E., Populations of migratory bird species that did not show a phenological response to climate change are declining. Proc. Natl. Acad. Sci. U.S.A. 105, 16195–16200 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozgul A., et al. , The dynamics of phenotypic change and the shrinking sheep of St. Kilda. Science 325, 464–467 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walther G.-R., et al. , Ecological responses to recent climate change. Nature 416, 389–395 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Welbergen J. A., Klose S. M., Markus N., Eby P., Climate change and the effects of temperature extremes on Australian flying-foxes. Proc. Biol. Sci. 275, 419–425 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellard C., Bertelsmeier C., Leadley P., Thuiller W., Courchamp F., Impacts of climate change on the future of biodiversity. Ecol. Lett. 15, 365–377 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez E., Porreca A. P., Colombo R. E., Menze M. A., Tradeoffs of warm adaptation in aquatic ectotherms: Live fast, die young? Comp. Biochem. Physiol. A Mol. Integr. Physiol. 191, 209–215 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Briga M., Verhulst S., Large diurnal temperature range increases bird sensitivity to climate change. Sci. Rep. 5, 16600 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pörtner H. O., et al. , Trade-offs in thermal adaptation: The need for a molecular to ecological integration. Physiol. Biochem. Zool. 79, 295–313 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Metcalfe N. B., Monaghan P., Compensation for a bad start: Grow now, pay later? Trends Ecol. Evol. 16, 254–260 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Wiens J. J., Climate-related local extinctions are already widespread among plant and animal species. PLoS Biol. 14, e2001104 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKechnie A. E., Wolf B. O., Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol. Lett. 6, 253–256 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jetz W., Wilcove D. S., Dobson A. P., Projected impacts of climate and land-use change on the global diversity of birds. PLoS Biol. 5, e157 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sekercioglu C. H., Schneider S. H., Fay J. P., Loarie S. R., Climate change, elevational range shifts, and bird extinctions. Conserv. Biol. 22, 140–150 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Cunningham S. J., Martin R. O., Hockey P. A., Can behaviour buffer the impacts of climate change on an arid-zone bird? Ostrich 86, 119–126 (2015). [Google Scholar]
- 16.du Plessis K. L., Martin R. O., Hockey P. A. R., Cunningham S. J., Ridley A. R., The costs of keeping cool in a warming world: Implications of high temperatures for foraging, thermoregulation and body condition of an arid-zone bird. Glob. Change Biol. 18, 3063–3070 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Albright T. P., et al. , Mapping evaporative water loss in desert passerines reveals an expanding threat of lethal dehydration. Proc. Natl. Acad. Sci. U.S.A. 114, 2283–2288 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wingfield J. C., et al. , How birds cope physiologically and behaviourally with extreme climatic events. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372, 20160140 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wada H., Coutts V., Detrimental or beneficial? Untangling the literature on developmental stress studies in birds. J. Exp. Biol. 224, jeb227363 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Moagi L. L., et al. , Hot days are associated with short-term adrenocortical responses in a southern African arid-zone passerine bird. J. Exp. Biol. 224, jeb242535 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Monaghan P., Ozanne S. E., Somatic growth and telomere dynamics in vertebrates: Relationships, mechanisms and consequences. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373, 20160446 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eastwood J. R., et al. , Early-life telomere length predicts lifespan and lifetime reproductive success in a wild bird. Mol. Ecol. 28, 1127–1137 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Sahin E., Depinho R. A., Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 464, 520–528 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Deursen J. M., The role of senescent cells in ageing. Nature 509, 439–446 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boonekamp J. J., Mulder G. A., Salomons H. M., Dijkstra C., Verhulst S., Nestling telomere shortening, but not telomere length, reflects developmental stress and predicts survival in wild birds. Proc. Biol. Sci. 281, 20133287 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cram D. L., Monaghan P., Gillespie R., Clutton-Brock T., Effects of early-life competition and maternal nutrition on telomere lengths in wild meerkats. Proc. Biol. Sci. 284, 20171383 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haussmann M. F., Longenecker A. S., Marchetto N. M., Juliano S. A., Bowden R. M., Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc. Biol. Sci. 279, 1447–1456 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Lieshout S. H. J., et al. , Early-life seasonal, weather and social effects on telomere length in a wild mammal. Mol. Ecol., 10.1111/mec.16014 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Chatelain M., Drobniak S. M., Szulkin M., The association between stressors and telomeres in non-human vertebrates: A meta-analysis. Ecol. Lett. 23, 381–398 (2020). [DOI] [PubMed] [Google Scholar]
- 30.McLennan D., et al. , Telomere elongation during early development is independent of environmental temperatures in Atlantic salmon. J. Exp. Biol. 221, jeb178616 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Axelsson J., Wapstra E., Miller E., Rollings N., Olsson M., Contrasting seasonal patterns of telomere dynamics in response to environmental conditions in the ectothermic sand lizard, Lacerta agilis. Sci. Rep. 10, 182 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heidinger B. J., et al. , Telomere length in early life predicts lifespan. Proc. Natl. Acad. Sci. U.S.A. 109, 1743–1748 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heidinger B. J., Kucera A. C., Kittilson J. D., Westneat D. F., Longer telomeres during early life predict higher lifetime reproductive success in females but not males. Proc. Biol. Sci. 288, 20210560 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chevin L.-M., Lande R., Mace G. M., Adaptation, plasticity, and extinction in a changing environment: Towards a predictive theory. PLoS Biol. 8, e1000357 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walsh B., Lynch M., Evolution and Selection of Quantitative Traits (Oxford University Press, 1997). [Google Scholar]
- 36.Moise A., et al. , “Monsoonal north cluster report” in Climate Change in Australia Projections for Australia’s Natural Resource Management Regions: Cluster Reports, Ekström M. et al., Eds. (CSIRO and Bureau of Meteorology, Australia, 2015), pp. 1–51. [Google Scholar]
- 37.Saether B.-E., et al. , Generation time and temporal scaling of bird population dynamics. Nature 436, 99–102 (2005). [DOI] [PubMed] [Google Scholar]
- 38.McKechnie A. E., Wolf B. O., The physiology of heat tolerance in small endotherms. Physiology (Bethesda) 34, 302–313 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Friesen C. R., Wapstra E., Olsson M., Of telomeres and temperature: Measuring thermal effects on telomeres in ectothermic animals. Mol. Ecol., 10.1111/mec.16154 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Debes P. V., Visse M., Panda B., Ilmonen P., Vasemägi A., Is telomere length a molecular marker of past thermal stress in wild fish? Mol. Ecol. 25, 5412–5424 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Dugdale H. L., Richardson D. S., Heritability of telomere variation: It is all about the environment! Philos. Trans. R. Soc. Lond. B Biol. Sci. 373, 20160450 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cunningham S. J., Gardner J. L., Martin R. O., Opportunity costs and the response of birds and mammals to climate warming. Front. Ecol. Environ. 19, 300–307 (2021). [Google Scholar]
- 43.Kingma S. A., Hall M. L., Arriero E., Peters A., Multiple benefits of cooperative breeding in purple-crowned fairy-wrens: A consequence of fidelity? J. Anim. Ecol. 79, 757–768 (2010). [DOI] [PubMed] [Google Scholar]
- 44.Nettle D., et al. , Brood size moderates associations between relative size, telomere length, and immune development in European starling nestlings. Ecol. Evol. 6, 8138–8148 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salmón P., Nilsson J. F., Nord A., Bensch S., Isaksson C., Urban environment shortens telomere length in nestling great tits, Parus major. Biol. Lett. 12, 20160155 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watson H., Bolton M., Monaghan P., Variation in early-life telomere dynamics in a long-lived bird: Links to environmental conditions and survival. J. Exp. Biol. 218, 668–674 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bourne A. R., Ridley A. R., McKechnie A. E., Spottiswoode C. N., Cunningham S. J., Dehydration risk is associated with reduced nest attendance and hatching success in a cooperatively breeding bird, the southern pied babbler Turdoides bicolor. Conserv. Physiol. 9, coab043 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song S., Beissinger S. R., Environmental and ecological correlates of avian field metabolic rate and water flux. Funct. Ecol. 34, 811–821 (2020). [Google Scholar]
- 49.Maeda T., Guan J.-Z., Koyanagi M., Makino N., Altered expression of genes associated with telomere maintenance and cell function of human vascular endothelial cell at elevated temperature. Mol. Cell. Biochem. 397, 305–312 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Angelier F., Costantini D., Blévin P., Chastel O., Do glucocorticoids mediate the link between environmental conditions and telomere dynamics in wild vertebrates? A review. Gen. Comp. Endocrinol. 256, 99–111 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Reichert S., Stier A., Does oxidative stress shorten telomeres in vivo? A review. Biol. Lett. 13, 20170463 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vedder O., Verhulst S., Zuidersma E., Bouwhuis S., Embryonic growth rate affects telomere attrition: An experiment in a wild bird. J. Exp. Biol. 221, jeb181586 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Stier A., Metcalfe N. B., Monaghan P., Pace and stability of embryonic development affect telomere dynamics: An experimental study in a precocial bird model. Proc. Biol. Sci. 287, 20201378 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Young R. C., et al. , Effects of developmental conditions on growth, stress and telomeres in black-legged kittiwake chicks. Mol. Ecol. 26, 3572–3584 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Geiger S., et al. , Catching-up but telomere loss: Half-opening the black box of growth and ageing trade-off in wild king penguin chicks. Mol. Ecol. 21, 1500–1510 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Monaghan P., Haussmann M. F., Do telomere dynamics link lifestyle and lifespan? Trends Ecol. Evol. 21, 47–53 (2006). [DOI] [PubMed] [Google Scholar]
- 57.Young A. J., The role of telomeres in the mechanisms and evolution of life-history trade-offs and ageing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373, 20160452 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aubert G., Lansdorp P. M., Telomeres and aging. Physiol. Rev. 88, 557–579 (2008). [DOI] [PubMed] [Google Scholar]
- 59.Sheldon E., et al. , Telomere dynamics in the first year of life, but not later in life, predict lifespan in a wild bird. Mol. Ecol., 10.1111/mec.16296 (2021). [DOI] [PubMed] [Google Scholar]
- 60.Skroblin A., et al. , “Western purple-crowned Fairy-wren Malurus coronatus coronatus” in The Action Plan for Australian Birds 2020, Garnett S. T., Baker G. B., Eds. (CSIRO Publishing, 2021), pp. 501–504. [Google Scholar]
- 61.Hidalgo Aranzamendi N., Hall M. L., Kingma S. A., van de Pol M., Peters A., Rapid plastic breeding response to rain matches peak prey abundance in a tropical savanna bird. J. Anim. Ecol. 88, 1799–1811 (2019). [DOI] [PubMed] [Google Scholar]
- 62.Froy H., et al. , Heritable variation in telomere length predicts mortality in Soay sheep. Proc. Natl. Acad. Sci. U.S.A. 118, e2020563118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vedder O., et al. , Telomere length is heritable and genetically correlated with lifespan in a wild bird. Mol. Ecol., 10.1111/mec.15807 (2021). [DOI] [PubMed] [Google Scholar]
- 64.Dupoué A., et al. , Shorter telomeres precede population extinction in wild lizards. Sci. Rep. 7, 16976 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McLean N. M., van der Jeugd H. P., van Turnhout C. A. M., Lefcheck J. S., van de Pol M., Reduced avian body condition due to global warming has little reproductive or population consequences. Oikos 129, 714–730 (2020). [Google Scholar]
- 66.Teunissen N., Kingma S. A., Peters A., Nest defence and offspring provisioning in a cooperative bird: Individual subordinates vary in total contribution, but no division of tasks among breeders and subordinates. Behav. Ecol. Sociobiol. 74, 94 (2020). [Google Scholar]
- 67.Kingma S. A., Hall M. L., Segelbacher G., Peters A., Radical loss of an extreme extra-pair mating system. BMC Ecol. 9, 15 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hall M. L., Peters A., Do male paternity guards ensure female fidelity in a duetting fairy-wren? Behav. Ecol. 20, 222–228 (2009). [Google Scholar]
- 69.Hall M. L., Peters A., Coordination between the sexes for territorial defence in a duetting fairy-wren. Anim. Behav. 76, 65–73 (2008). [Google Scholar]
- 70.Hidalgo Aranzamendi N., Hall M. L., Kingma S. A., Sunnucks P., Peters A., Incest avoidance, extrapair paternity, and territory quality drive divorce in a year-round territorial bird. Behav. Ecol. 27, 1808–1819 (2016). [Google Scholar]
- 71.Kingma S. A., Hall M. L., Peters A., No evidence for offspring sex-ratio adjustment to social or environmental conditions in cooperatively breeding purple-crowned fairy-wrens. Behav. Ecol. Sociobiol. 65, 1203–1213 (2010). [Google Scholar]
- 72.Nolazco S., Hall M. L., Kingma S. A., Delhey K., Peters A., No evidence for an adaptive role of early molt into breeding plumage in a female fairy wren. Behav. Ecol. 31, 411–420 (2020). [Google Scholar]
- 73.Cunningham S. J., Kruger A. C., Nxumalo M. P., Hockey P. A. R., Identifying biologically meaningful hot-weather events using threshold temperatures that affect life-history. PLoS One 8, e82492 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roast M. J., et al. , Short-term climate variation drives baseline innate immune function and stress in a tropical bird: A reactive scope perspective. Physiol. Biochem. Zool. 92, 140–151 (2019). [DOI] [PubMed] [Google Scholar]
- 75.Eastwood J. R., Mulder E., Verhulst S., Peters A., Increasing the accuracy and precision of relative telomere length estimates by RT qPCR. Mol. Ecol. Resour. 18, 68–78 (2018). [DOI] [PubMed] [Google Scholar]
- 76.Criscuolo F., et al. , Real-time quantitative PCR assay for measurement of avian telomeres. J. Avian Biol. 40, 342–347 (2009). [Google Scholar]
- 77.R Core Team, R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2016). [Google Scholar]
- 78.Hamilton N. E., Ferry M., ggtern: Ternary diagrams using ggplot2. J. Stat. Softw. 87, 1–17 (2018). [Google Scholar]
- 79.Wickham H., ggplot2: Elegant Graphics for Data Analysis (Springer-Verlag New York, 2016). [Google Scholar]
- 80.Bates D., Mächler M., Bolker B., Walker S., Fitting linear mixed-effects models using lme4. J. Stat. Softw. 1, 1–48 (2015). [Google Scholar]
- 81.Verhulst S., Improving comparability between qPCR-based telomere studies. Mol. Ecol. Resour. 20, 11–13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoffmann A. A., Sgrò C. M., Climate change and evolutionary adaptation. Nature 470, 479–485 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this study are available in Dataset S1.