Abstract
Culex pipiens mosquitoes are the most widely distributed primary vector of the West Nile virus worldwide. Many attempts for investigation of botanical pesticides to avoid the development of pesticide resistance to conventional synthetic pesticides that are recognized as a threat to the diversity of ecosystems. The study aimed to determine the components of three essential oils of Lamiaceae family, lavender (Lavandula angustifolia), peppermint (Mentha piperita L.), and rosemary (Rosmarinus officinalis L.) by gas chromatography-mass spectrometry (GC–MS) analysis. Furthermore, aimed to validate the insecticidal activities of these oils as larvicidal agents against the third instar larvae of Culex pipiens using five different concentrations (62.5, 125, 250, 500, and 1000 ppm) for each oil in five replicates and as an adulticidal agent against approximately three-day-old female adults of Cx. Pipiens using 0.5, 1, 2, 4, and 5% concentrations in three replicates. The results generally showed a dose-related response. At 1000 ppm, rosemary oil showed the highest larvicidal (100%) (LC50, 214.97 ppm), followed by peppermint oil (92.00% mortality and LC50 (269.35 ppm). Lavender oil showed the lowest efficacy with 87.20% mortality and LC50 (301.11 ppm). At 5% oil concentration, the highest knockdown rate at 1 h was recorded for lavender oil (95.55%), followed by peppermint oil (88.89%) and lastly rosemary oil (84.44%). After 24 h, rosemary oil showed the lowest adult mortality rate (88.89%; LC50, 1.44%), while lavender and peppermint oils both showed a 100% mortality rate, with (LC50, 0.81% and 0.91%, respectively). The chemical constituents of the oils consisted of monoterpenes and sesquiterpenes that determined their insecticidal activities against the target insect stage. The study proposed that rosemary essential oil may be useful for the control of Cx. pipiens larvae as part of an integrated water treatment strategy, and lavender and peppermint oils may be used in an integrated plan for adult’s control.
Keywords: Lavender, Peppermint, Rosemary, Essential oils, Culex pipiens, Insecticidal
1. Introduction
The Lamiaceae family called the mint family is characterized by economically important species of aromatic plants including 250 genera and 7825 species (Prakash et al., 2016). Their essential oils are obtained from different aerial parts (seeds, leaves, flowers, shoots, and fruits) or roots. These oils contain characteristic functional groups such as aldehydes, esters, phenols, ketones, alcohols, organic acids, hydrocarbons and terpenoids Essential oils may be preferred in the industrial economic sector due to the development of fewer toxic side products and economic viability (Nazar et al., 2022, Ramos da Silva et al., 2021).
The present study investigated three essential oils from three genera of the family Lamiaceae. The first was lavender oil from the flowering parts of Lavandula angustifolia L. This species showed anti-inflammatory, antibacterial, antiviral, and antifungal properties and anticancer activity as well as to provide mood disturbance relief (Cavanagh and Wilkinson, 2002, Denner, 2009, Rai et al., 2020). The second was peppermint oil from the aerial parts of Mentha × piperita L. which is a hybrid mint (M. aquatica × M. spicata). Peppermint oil is used in the health industry because of its wide spectrum of therapeutic properties. These include analgesic, antispasmodic, and antiemetic action, abdominal pain relief, antioxidant activity, and cytotoxicity against bacteria and fungi as well as insecticidal effects against many pests (da Silva Ramos et al., 2017, Kalemba and Synowiec, 2019, Mahendran and Rahman, 2020). The third was rosemary oil from the flowering tops of the plant Rosmarinus officinalis L. which has been shown to have anti-inflammatory properties with potential applications in inflammatory-related diseases. Furthermore, it exerts anti-depressive, antimicrobial, antioxidant, antiallergic, and smooth muscle relaxant effects, as well as shows antifungal, antimutagenic, and insecticidal activities (Borges et al., 2019, De Oliveira et al., 2019, Isman et al., 2008, Krzyżowski et al., 2020).
Culex pipiens is commonly known as house mosquito and is one of the most widely distributed mosquitoes worldwide and several studies reported the involvement of Cx. pipiens in transmission of the West Nile virus (Al-Mekhlafi et al., 2018, El-Akhal et al., 2015). A previous study suggested that control efforts focused on Cx. pipiens alone may greatly reduce both human exposure, infection, and epidemic distribution (Hamer et al., 2008). The research interest for botanical pesticides containing natural compounds as active ingredients, from which essential oils are considered a part. Where the use of these compounds attempts to avoid the development of pesticide resistance to conventional synthetic pesticides that are also now recognized as a threat to the diversity of ecosystems (Ahmed et al., 2021).
The present study aimed to evaluate the adulticidal and larvicidal potential of lavender, peppermint, and rosemary oils against the common house mosquito, Cx. pipiens. Furthermore, we aimed to determine the oils compounds by gas chromatography-mass spectrometry (GC–MS) analysis.
2. Material and methods
2.1. Mosquito colony rearing and experimental conditions
Mosquitoes (Cx. pipiens) were reared for several generations at the Center for Environmental Research and Studies at Jazan University. Rearing was performed under controlled conditions (27 ± 2 °C, relative humidity at 70% ± 10%, and 12:12 h light:dark regime). Adult mosquitoes were reared in wooden cages and supplied daily by 10% sucrose solution soaked in sponge pieces for 3–4 days post-emergence. Then, females were supplied pigeon blood meal. Plastic oviposition cups containing tap water (without chlorine) were placed in the cages. The resulting egg mass were transferred into plastic pans containing 3 L of tap water for 24 h. The hatching larvae were provided with a diet of fish food daily. The third instar Cx. pipiens larvae were used for the larvicidal examination, and the adults used for the adulticidal examination were glucose-fed female mosquitoes reared under the aforementioned controlled conditions.
2.2. Essential oils
Lavender essential oil, steam-distilled from flowering tops of L. angustifolia, (Lamiales: Lamiaceae; Case No. 8000-28-0), Peppermint essential oil, steam-distilled from the aerial parts of M. piperita L. (Lamiales: Lamiaceae; Case No. 8006-90-4), and Rosemary essential oil, steam-distilled from the tops of R. officinalis L. (Lamiales: Lamiaceae; Case No. 8000-25-7) were purchased from NOW Foods company (Natural Organic and Wholesome Foods), a distributor in Jeddah, Kingdom of Saudi Arabia.
2.3. Identification of the chemical composition using gas chromatography-mass spectrometer
The investigation of the chemical composition of the essential oils was performed by GC–MS using the Trace GC-TSQ mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) with a direct capillary column, TG–5MS (30 m × 0.25 mm × 0.25 m thickness of the film). The operating conditions of the GC were column oven temperature initially maintained at 50 °C, then elevated at a rate of 5 °C/min up to 250 °C, maintained for 2 min, then elevated at a rate of 30 °C/min to 300 °C. The MS transfer line and the injector were adjusted at 270 °C and 260 °C, respectively, and helium was the carrier gas at the rate of 1 mL/min. The solvent retention time was 4 min, and 1 μL of the diluted samples was automatically injected using an AS1300 autosampler and GC split mode. In full scanning mode, electrospray ionization (EI) mass spectra were in the range of 50–650 m/s at an ionization voltage of 70 V. The temperature of the ion source was fixed at 200 °C. The mass spectra of the components were then compared with those in the mass spectral libraries from the NIST 14 and WILEY 09 databases, the selected constituents were identified from the Total Ion Chromatogram (TIC).
2.4. Larvicidal assay
The larvicidal activity was determined for the lavender, peppermint, and rosemary oils against Cx. pipiens third instar larvae according to guidelines of the World Health Organization (WHO, 2005). The solution of each oil was set as stock by mixing 1 mL oil with distilled water containing 0.2 mL Tween-20. Subsequently, five different concentrations were prepared, (62.5, 125, 250, 500, and 1000 ppm) based on v/v percent of 1% of stock solution. Twenty-five Cx. pipiens larvae were offered to each oil in 250 mL glass beakers containing 150 mL water at the aforementioned controlled conditions. Five replicates per concentration per oil and control were conducted. The larval mortalities were detected after 24 h for estimation of larval lethal concentration (LC50) by probit analysis.
2.5. Adulticidal assay
The adulticidal activity for the oils was analyzed by the adapted CDC bottle protocol (Ilahi et al., 2021, WHO, 2016). Five different concentrations from each oil dissolved in ethanol were prepared (0.5, 1, 2, 4, and 5%). Each prepared concentration for every oil was used to coat the CDC bottles (250 mL Wheaton bottles with screw lids) similarly to the control bottle, which was coated with only ethanol. The solvent was evaporated from the bottles for 1 h at 27 ± 2 °C. Three replicates per concentration per oil and the control were conducted. Adult glucose-fed female mosquitoes (n = 15) aged 3–4 d were selected using an aspirator and gently introduced to each bottle, and the bottles were closed with their lids. If a mosquito was knocked down or unable to move or stand within 60 min of exposure, it was considered as dead. The number was recorded for each bottle after 5, 10, 20, 30 and 60 min for determination of the median knockdown time (KT50) value and KT90 and KT95 values of each concentration through probit analysis. Live mosquitoes were then removed from bottles after 1 h and placed in separate paper cups containing 10% sucrose solution. Subsequently, the adult mortality rate was measured after 24 h within the replicates for determination of the sLC50, LC90 and LC95 by probit analysis.
2.6. Data analysis
The percentage of mortalities were calculated according to Abbott’s formula (Abbott, 1925). The larval control results did not need correction, as the mortality was less than 5%, according to the WHO guidelines (WHO, 2005) (no larval control mortality was recorded throughout the study). The adult data were also not corrected, as the control adults had a mortality of less than 20% (WHO, 2016). Data from all the replicates were statistically analyzed to determine the larval and adult LC50, LC50, and LC95 values and the adult KT50, KT90, and KT95 values as well as chi-square values within confidence limits at 95% (lower confidence limit [LCL] and upper confidence limit [UCL]) by probit analysis using regression between log oil concentration and probit values. Mortality data were subjected to a one-way analysis of variance (ANOVA) (with a least significant difference (LSD) test). Data analysis was performed using SPSS software (IBM SPSS Statistics v22 – 64 bit), and p less than 0.05 was considered significant.
3. Results
3.1. Chemical analysis
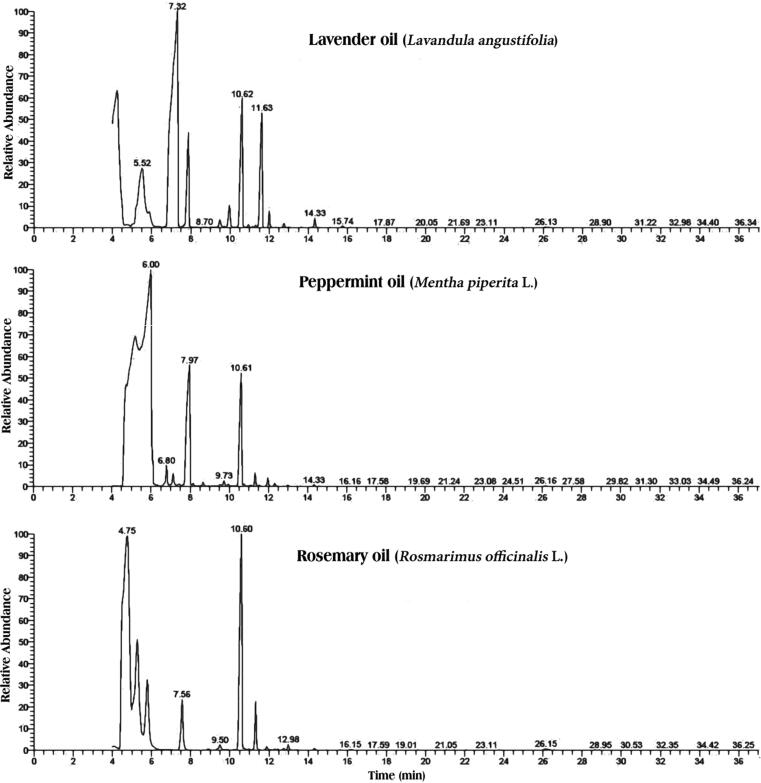
The GC–MS analysis represented that the main compounds of the three essential oils were the monoterpenoids and sesquiterpenes (Table 4; Fig. 4). In lavender (L. angustifolia) essential oil, the main represented area% were monoterpenoids, linalool (23.75%), linalyl anthranilate (21.92%), lavandulyl acetate (11.85%), 4-terpineol (8.90%), and linalyl acetate (6.66%), followed by geranyl acetate (2.99%), L-α-terpineol (0.70%), and bornyl acetate (0.10%). The sesquiterpenoids were β-caryophyllene (16.35%), (E)-β-famesene (14.09%), germacrene D (1.29%), caryophyllene oxide (0.77%), γ-muurolene (0.35%), and transe-α-bergamotene (0.26%). In peppermint (M. piperita L.) oil, the main represented monoterpenoids were menthyl acetate (32.76%), L-menthol (26.41%), and L-menthone (13.57%), followed by pulegone (1.94%), L-alpha-terpineol (1.63%), and piperitone (1.32%). The main sesquiterpenoid was β-caryophyllene (18.48%), followed by humulene (1.41%), germacrene D (0.88%), β-bourbonene (0.58%), elemene isomer (0.46%), and alloaromadendrene (0.34%). In Rosemary (R. officinalis L.) essential oil, the chemical compounds were represented by camphor (56.55%) as the major monoterpenoid, followed by isoborneol (7.16%), α-terpineol (5.40%), and bornyl acetate (3.69%), and the main sesquiterpenoid was β-caryophyllene (23.0%), followed by humulene (3.20%), cadina-1(10),4-diene (0.39%), α-copaene (0.34%), and γ-muurolene (0.26%).
Table 4.
Chemical constituents of lavender, peppermint, and rosemary essential oils.
| Oil | Molecular formula | Chemical name, (synonym) | Area (%) | RT | Nature of compound |
|---|---|---|---|---|---|
| Lavender (Lavandula angustifolia) | C10H18O |
Linalool, 3,7-Dimethylocta-1,6-dien-3-ol |
23.75 | 4.26 4.50 |
Monoterpenoid |
| C10H18O |
4-terpineol,3-Cyclohexen-1-ol, 4-methyl-1-(1-methylethyl)-, (R) - |
8.90 | 5.19 5.52 |
Monoterpenoid | |
| C10H18O |
L-alpha-Terpineol,3-Cyclohexene-1-methanol, à,à,4-trimethyl-,(S) - |
0.70 | 5.89 | Monoterpene alcohol | |
| C12H20O2 |
Linalyl acetate, 1,6-Octadien-3-ol, 3,7-dimethyl-, acetate |
6.66 | 6.88 7.12 |
Acyclic monoterpenoid | |
| C17H223NO2 |
Linalyl anthranilate, 1,5-Dimethyl-1-vinyl-4-hexen-1-yl o-aminobenzoate, |
21.92 | 7.33 | Aromatic monoterpenoid | |
| C12H20O2 | Bornyl acetate, Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, acetate, endo- | 0.10 | 7.66 | Bicyclic monoterpenoid | |
| C12H20O2 |
Lavandulyl acetate, 4-Hexen-1-ol,5-methyl-2-(1-methylethenyl) -, Acetate |
11.85 | 7.89 | Monoterpenoid Acetic acid lavandulylester |
|
| C12H20O2 |
Geranyl acetate, 2,6-Octadien-1-ol, 3,7-dimethyl-,acetate, (Z) - |
2.99 | 9.48 9.97 |
Monoterpenoid | |
| C15H24 | β-Caryophyllene, Bicyclo[7.2.0]undec-4-ene, 4,11,11-trimethyl-8-methylene-,[1R-(1R*,4Z,9S*)]- | 16.35 | 10.63 | Sesquiterpenoid | |
| C15H24 |
transe-Alpha-Bergamotene, Bicyclo[3.1.1]hept-2-ene,2,6-dimethyl-6-(4-methyl-3-pentenyl) - |
0.26 | 10.95 | Sesquiterpenoid | |
| C15H24 | (E)-beta-Famesene, 1,6,10-Dodecatriene, 7,11-dimethyl-3-methylene-, (E)- | 14.09 | 11.63 | Sesquiterpenoid | |
| C15H24 | Germacrene D, (S,1Z,6Z)-8-Isopropyl-1-methyl-5-methylenecyclodeca-1,6-diene | 1.29 | 12.01 | Sesquiterpenoid | |
| C15H24 | γ-Muurolene, Naphthalene, 1,2,3,4,4a,5,6,8a-octahydro-7-methyl-4-methylene-1-(1-methylethyl)-, (1α,4aβ,8aα)- | 0.35 | 12.76 | Sesquiterpenoid | |
| C15H24O | Caryophyllene oxide, 5-Oxatricyclo[8.2.0.0(4,6)-]dodecane, 4,12,12-trimethyl-9-methylene-, [1R-(1R*,4R*,6R*,10S*)]- | 0.77 | 14.33 | Sesquiterpenoid | |
| Peppermint (Mentha piperita L.) | C10H18O | L-Menthone, 2-Isopropyl-5-methylcyclohexanone | 13.57 | 4.68 4.88 5.19 |
Monoterpenoid |
| C10H20O |
L-Menthol,Cyclohexanol, 5-methyl-2-(1-methylethyl)-, [1R-(1à,2á,5à) ]- |
26.41 | 6.01 | Monoterpenoid | |
| C10H18O |
L-alpha-Terpineol,3-Cyclohexene-1-methanol, à,à,4-trimethyl-, (S) - |
1.63 | 6.09 | Monoterpenoid | |
| C10H16O |
Pulegone, Cyclohexanone,5-methyl-2-(1-methylethylidene) - |
1.94 | 6.80 | Monoterpenoid | |
| C10H16O | Piperitone, 6-isopropyl-3-methylcyclohex-2-enone | 1.32 | 7.13 | Monoterpenoid | |
| C12H22O2 | Menthyl acetate, Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate, (1à,2á,5à)- | 32.76 | 7.98 | Monoterpenoid | |
| C15H24 |
Elemene isomer, Cyclohexane,1-ethenyl-1-methyl-2-(1-methylethenyl)-4-(1-methylethylidene) - |
0.46 | 8.67 | Sesquiterpenoid | |
| C15H24 |
Beta-Bourbonene,Cyclobuta(1,2:3,4) dicyclopentene, 1,2,3,3a,3bbeta,4,5,6,6abeta,6balpha-decahydro-1alpha-isopropyl-3aalpha-methyl-6-methylene |
0.58 | 9.73 | Sesquiterpenoid | |
| C15H24 | β-Caryophyllene, Bicyclo[7.2.0]undec-4-ene, 4,11,11-trimethyl-8-methylene-,[1R-(1R*,4Z,9S*)]- | 18.48 | 10.62 | Sesquiterpenoid | |
| C15H24 |
Humulene,1,4,8-Cycloundecatriene, 2,6,6,9-tetramethyl-, (E,E,E) - |
1.41 | 11.32 | Sesquiterpenoid | |
| C15H24 |
Germacrene D,1-Methyl-5-methylene-8-(1-methylethyl) -1,6-cyclodecadiene |
0.88 | 11.97 | Sesquiterpenoid | |
| C15H24 | Alloaromadendrene | 0.34 | 12.33 | Sesquiterpenoid | |
| Rosemary (Rosmarinus officinalis L) | C10H16O | Camphor, Bicyclo[2.2.1]heptan-2-one, 1,7,7-trimethyl-, (1R)- | 56.55 | 4.74 5.02 |
Bicyclic monoterpenoid |
| C10H18O |
Isoborneol, Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, exo- |
7.16 | 5.27 | Bicyclic monoterpenoid | |
| C10H18O |
alpha-Terpineol,3-Cyclohexene-1-methanol, à,à,4-trimethyl-, (S) - |
5.40 | 5.79 | Monoterpene alcohol | |
| C12H20O2 | Bornyl acetate, Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, acetate, (1S-endo)- | 3.69 | 7.57 | Bicyclic monoterpenoid | |
| C15H24 |
alpha-Copaene,Tricyclo[4.4.0.0(2,7)]dec-3-ene, 1,3-dimethyl-8-(1-methylethyl) -, st |
0.34 | 9.50 | Sesquiterpenes | |
| C15H24 | β-Caryophyllene, Bicyclo[7.2.0]undec-4-ene, 4,11,11-trimethyl-8-methylene-, [1R-(1R*,4E,9S*)]- | 23.00 | 10.60 | Sesquiterpenes | |
| C15H24 | Humulene, alpha-Caryophyllene, 1,4,8-Cycloundecatriene, 2,6,6,9-tetramethyl-, (E,E,E)- | 3.20 | 11.32 | Sesquiterpenes | |
| C15H24 | γ-Muurolene, Naphthalene, 1,2,3,4,4a,5,6,8a-octahydro-7-methyl-4-methylene-1-(1-methylethyl)-, (1α,4aβ,8aα)- | 0.26 | 11.88 | Sesquiterpenes | |
| C15H24 | Cadina-1(10),4-diene, delta-Cadinene, Naphthalene, 1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-, (1S-cis)- | 0.39 | 12.98 | Sesquiterpenes |
Fig. 4.
The TIC chromatograms of lavender, peppermint and rosemary essential oils chemical constituents detected by GC–MS.
3.2. Larvicidal activity
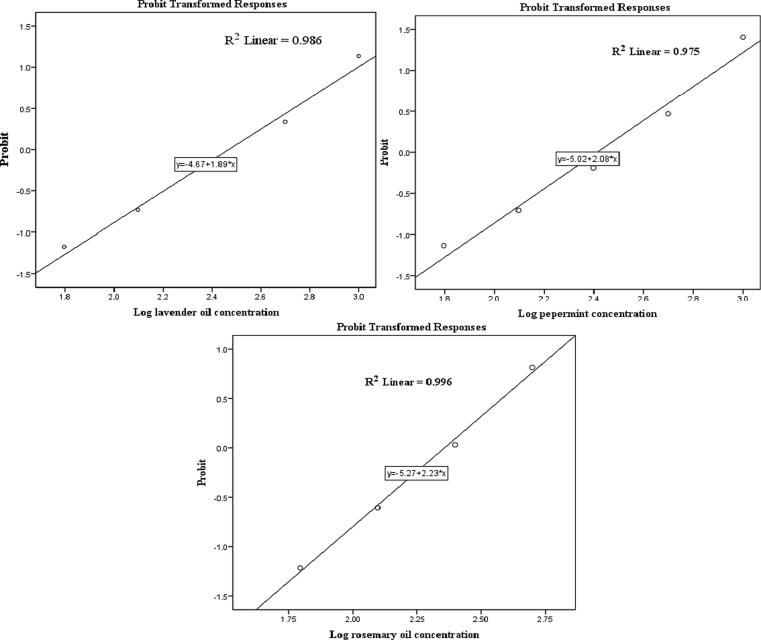
The data on the larvicidal activity of the tested essential oils against the third instar larvae of Cx. pipiens are represented in Table 1. The probit regression responses of the essential oils tested revealed that the larvicidal activities after 24 h ranged from 87.20 to 100% mortality at 1000 ppm. Rosemary essential oil showed the highest efficacy by inducing 100% mortality (LC50, 214.97, LC90, 671.77, and LC95, 927.90 ppm), followed by peppermint oil by 92.00% mortality (LC50, 269.35, LC90, 1137.74, and LC95, 1711.70 ppm). Lavender oil was the least effective, inducing 87.20% mortality (LC50, 301.11, LC90, 1437.63, and LC95, 2239.31 ppm) (Fig. 1).
Table 1.
The larvicidal effects of essential oils against the third instar larvae of Culex pipiens at 24 h post-treatment.
| Oil | Conc. ppm | Mortality% (Mean ± SE) |
LC50 (LCL - UCL.) |
LC90 (LCL - UCL.) |
LC95 (LCL - UCL.) |
Chi (Sig) |
|---|---|---|---|---|---|---|
|
Lavender Lavandula angustifolia |
0.0 | 0.00 ± 0.0a | 301.11 (259.12-352.37) |
1437.63 (1087.06-2096.14) |
2239.31 (1600.19-3544.70) |
2.175 (0.537a) |
| 62.5 | 12.00 ± 1.26b | |||||
| 125 | 23.20 ± 1.50c | |||||
| 250 | 40.80 ± 1.96d | |||||
| 500 | 63.20 ± 4.27e | |||||
| 1000 | 87.20 ± 3.44f | |||||
|
Peppermint Mentha piperita L. |
0.0 | 0.00 ± 0.0a | 269.35 (233.37-311.12) |
1137.74 (890.87-1571.50) |
1711.70 (1276.39-2536.21) |
4.148 (0.246a) |
| 62.5 | 12.80 ± 0.80b | |||||
| 125 | 24.00 ± 1.79c | |||||
| 250 | 42.40 ± 1.60d | |||||
| 500 | 68.00 ± 2.83e | |||||
| 1000 | 92.00 ± 1.79f | |||||
|
Rosemary Rosmarinus officinalis L |
0.0 | 0.00 ± 0.0a | 214.97 (190.54-242.28) |
671.77 (560.35-845.93) |
927.90 (748.20-1226.12) |
7.742 (0.052a) |
| 62.5 | 11.20 ± 1.50b | |||||
| 125 | 27.20 ± 1.50c | |||||
| 250 | 51.20 ± 2.94d | |||||
| 500 | 79.20 ± 0.80e | |||||
| 1000 | 100.00 ± 0.00f |
Significance at 0.05 level between different superscripts. (a) In Chi-Square Tests, no heterogeneity factor was used in the calculation of confidence limits because the significance level was greater than 0.050.
Fig. 1.
Probit regression responses of lavender, peppermint and rosemary essential oils against Culex pipiens larval mortality.
3.3. Adulticidal activity
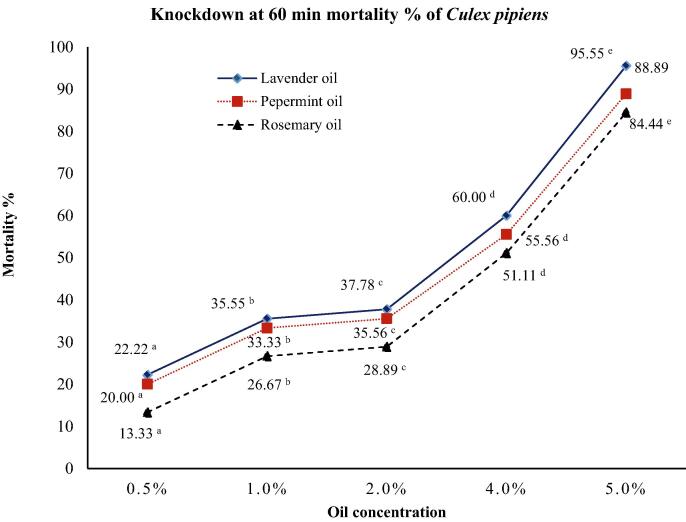
The effect of the test concentrations of the three essentials oils on Cx. pipiens females were evaluated after 60 min of exposure in terms of LC50, which is shown in Fig. 2, KT50, KT90, and KT95, which are listed in Table 2. According to the analysis, the highest knockdown rate was recorded for lavender oil (95.55%) followed by that for peppermint oil (88.89%) and lastly rosemary oil (84.44%) at the highest tested concentration of the oils (5%).
Fig. 2.
Knockdown rate (mortality %) of lavender, peppermint and rosemary essential oils against Culex pipiens female adults. Significant differences at 0.05 level between different superscript letters to means of the same oil.
Table 2.
Probit analysis of knockdown time and mortality rates of Culex pipiens females after oil exposure for 60 min.
| Oil | Conc. (%) |
KT50 | KT90 | KT95 | R2 | Equation | Chi (Sig) |
|---|---|---|---|---|---|---|---|
|
Lavender Lavandula angustifolia |
0.5 | 508.20 | 17769.11 | 48670.18 | 0.982 | 0.84*x-2.26 | 0.90 (0.962a) |
| 1.0 | 125.63 | 1559.97 | 3186.07 | 0.990 | 1.19*x-2.49 | 0.329(0.954a) | |
| 2.0 | 122.96 | 2743.34 | 6615.54 | 0.996 | 0.96*x-2 | 0.111 (0.990a) | |
| 4.0 | 47.70 | 441.56 | 829.79 | 0.960 | 1.29*x-2.18 | 2.759 (0.430a) | |
| 5.0 | 17.43 | 57.51 | 80.68 | 0.958 | 2.57*x-3.13 | 6.900 (0.075a) | |
|
Peppermint Mentha piperita L. |
0.5 | 871.38 | 48153.74 | 150169.81 | 0.973 | 0.75*x-2.18 | 0.330 (0.954a) |
| 1.0 | 152.41 | 2260.18 | 4854.49 | 0.980 | 1.12*x-2.43 | 0.657 (0.883a) | |
| 2.0 | 152.49 | 4339.66 | 11212.58 | 0.995 | 0.89*x-1.94 | 0.108 (0.991a) | |
| 4.0 | 70.59 | 633.83 | 1263.49 | 0.967 | 1.19*x-2.08 | 1.965 (0.580a) | |
| 5.0 | 22.81 | 95.54 | 143.40 | 0.926 | 2.08*x-2.77 | 11.017 (0.012a) | |
|
Rosemary Rosmarinus officinalis L |
0.5 | 1803.86 | 79,399 | 232145.78 | 0.957 | 0.77*x-2.53 | 0.495 (0.920a) |
| 1.0 | 256.06 | 6176.80 | 15228.61 | 0.971 | 0.9*x-2.19 | 0.617 (0.893a) | |
| 2.0 | 186.45 | 1677.43 | 3126.90 | 0.990 | 1.28*x-2.96 | 0.360 (0.948a) | |
| 4.0 | 77.40 | 772.27 | 1482.45 | 0.899 | 1.19*x-2.3 | 5.688 (0.128a) | |
| 5.0 | 26.64 | 102.56 | 150.29 | 0.951 | 2.13*x-3.01 | 7.743 (0.52a) |
Data represent the mean knockdown time. (a) In Chi-Square Tests, no heterogeneity factor was used in the calculation of confidence limits because the significance level was greater than 0.050.
When considering knockdown times, data revealed that at a concentration of 5%, the knockdown times were the lowest for lavender oil compared with those recorded for the other two oils. Lavender oil had a KT50 of 17.43 min, KT90 of 57.51 min, and KT95 of 80.68 min. Peppermint oil showed a KT50 of 22.81 min, KT90 of 95.54 min, and KT95 of 143.40 min. Furthermore, rosemary oil revealed a KT50 of 26.64 min, KT90 of 102.56 min, and KT95 of 150.29 min.
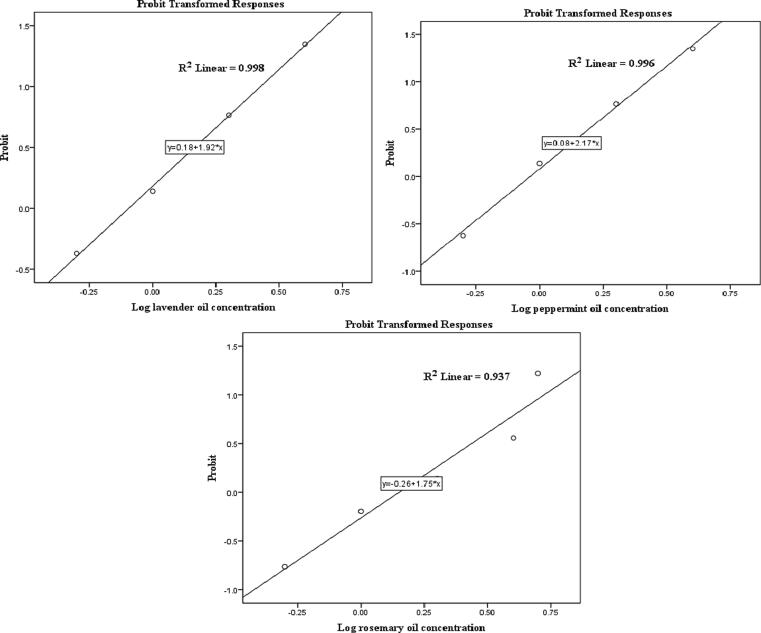
Adult toxicity of the three tested oils after 24 h was also assessed (Table 3), and probit regression results are shown in Fig. 3. Data showed contrary results to those observed for the larvicidal activity of the tested oils. Here, rosemary essential oil showed the least adulticidal efficiency (88.89% mortality rate) and an LC50 of 1.44% after 24 h at the highest tested concentration (5%). Lavender and peppermint oils at 5% concentration both showed a mortality rate of 100%, with an LC50 of 0.81% and 0.91%, respectively, after 24 h. It was noted that the lowest median adulticidal concentration was that of lavender oil which was in line with knockdown assay results.
Table 3.
The adulticidal effects of essential oils against female adults of Culex pipiens at 24 h post-treatment.
| Oil | Conc. % | Mortality% (Mean ± SE) |
LC50 (LCL - UCL.) |
LC90 (LCL - UCL.) |
LC95 (LCL - UCL.) |
Chi (Sig) |
|---|---|---|---|---|---|---|
|
Lavender Lavandula angustifolia |
0.0 | 2.47 ± 0.63a | 0.81 (0.67-0.94) |
3.09 (2.57-3.93) |
4.52 (3.60-6.15) |
6.642 (0.084a) |
| 0.5 | 35.56 ± 2.22b | |||||
| 1.0 | 55.56 ± 2.22c | |||||
| 2.0 | 77.78 ± 2.22d | |||||
| 4.0 | 91.11 ± 2.22e | |||||
| 5.0 | 100.00 ± 0.00f | |||||
|
Peppermint Mentha piperita L. |
0.0 | 2.47 ± 0.63a | 0.91 (0.78-1.04) |
3.02 (2.55-3.74) |
4.24 (3.46-5.55) |
5.891 (0.117a) |
| 0.5 | 26.67 ± 3.85b | |||||
| 1.0 | 55.56 ± 2.22c | |||||
| 2.0 | 77.78 ± 2.22d | |||||
| 4.0 | 91.11 ± 2.22e | |||||
| 5.0 | 100.00 ± 0.00f | |||||
|
Rosemary Rosmarinus officinalis L |
0.0 | 2.47 ± 0.63a | 1.44 (1.20-1.69) |
8.24 (6.14-12.51) |
13.52 (9.38-22.95) |
6.449 (0.092a) |
| 0.5 | 22.22 ± 2.22b | |||||
| 1.0 | 42.22 ± 2.22c | |||||
| 2.0 | 55.56 ± 2.22d | |||||
| 4.0 | 71.11 ± 2.22e | |||||
| 5.0 | 88.89 ± 5.88f |
(a) In Chi-Square Tests, no heterogeneity factor was used in the calculation of confidence limits because the significance level was greater than 0.050.
Fig. 3.
Probit regression responses of lavender, peppermint and rosemary essential oils against Culex pipiens adult mortality.
4. Discussion
The present study tested the larvicidal and adulticidal efficacy of lavender, peppermint, and rosemary essential oils against Cx. pipiens mosquitos. These effects were expected owing to the active constituents of these oils. The major components present in lavender essential oil are as determined by GC–MS in this study corresponded with the findings of previous studies (El-Akhal et al., 2021, Karamaouna et al., 2013), as did the components present in the peppermint essential oil (Ebadollahi et al., 2017, Mackled et al., 2019, Samarasekera et al., 2008) and the rosemary essential oil (Jafari-sales and Pashazadeh, 2020, Zeghib et al., 2020). Generally, the tested three essential oils recorded chemical constituents and their insecticidal activities in accordance with the methods of several previous studies reviewed by Ebadollahi et al (2020).
The study was coverage the larvicidal activity of the tested oils against the third instar larvae of Cx. pipiens. The study evaluated the concentration-related mortalities for each of the oils, and graded the mortalities at the highest used concentration (1000 ppm), where rosemary oil acquired the most potent larvicidal activity (100% mortality) followed by peppermint oil (92%) and lastly lavender oil (87%).
In line with the present results about larvicidal LC50 value, Radwan et al. (2008), recorded an LC50 of 216.10 ppm for rosemary essential oil and lower larvicidal efficacy of peppermint oil (>500 ppm) against Cx. pipiens. The study presented that the saturated monoterpenoid alcohol menthol, which is the major component in peppermint oil, exhibited lower larvicidal activity against Cx. pipiens. Zeghib et al. (2020) evaluated larvicidal activity against the fourth instar larvae of Cx. pipiens of rosemary essential oil extracted from the aerial parts of R. officinalis. They recorded 100% mortality at 99.34 ppm of the oil concentration and evaluated camphor as one of the major components. A further component that had a role in toxicity was β-caryophyllene, which was the second most abundant component detected in rosemary oil (23%) and the third most abundant in lavender (16.35%) and peppermint oils (18.48%) and displayed effective larvicidal activity as an individual compound against Anopheles subpictus, Aedes albopictus, and Cx. tritaeniorhynchus (Govindarajan et al., 2016). A sesquiterpene compound, α-humulene, was present in a higher percentage in rosemary oil (3.20%) than in peppermint oil (1.41%) and not detected in lavender oil. This compound exhibited larvicidal activity at low dosages against A. subpictus, Ae. albopictus, and Cx. tritaeniorhynchus third instar larvae (Govindarajan and Benelli, 2016). Linalool, the main constituent in lavender oil, had larvicidal activity against third instar larvae of Ae. aegypti, and morphological alterations detected in larvae exposed to sublethal doses of linalool included abdomen elongation and curving and gut content partial extrusion involving the peritrophic matrix (Fujiwara et al., 2017). The essential oil extracted from M. piperita leaves exhibited larvicidal and repellent efficacy against the early fourth instar larvae of Ae. aegypti (Kumar et al., 2011). Another study showed the toxicity effects of peppermint and lavender essential oils against M. domestica larvae in conjunction with larval morphological abnormalities (Bosly, 2013). Moreover, studies reported that minor compounds found such as bornyl acetate (present in rosemary oil) exhibited strong larvicidal activity against Cx. pipiens larvae (Zeghib et al., 2020). Other compounds present in high concentrations, similarly to our study, such as linalool and terpineol (in lavender oil) and menthone (in peppermint oil) individually exhibited low larvicidal activities with recorded LD50 of 193,194, and 156 mg/L, respectively, against Cx. pipiens larvae (Radwan et al., 2008, Traboulsi et al., 2002).
Contrary to the larvicidal results obtained in this study, lavender, peppermint, and rosemary essential oils at 1000 ppm concentration resulted in larval mortality of 68, 100 and 80%, respectively, after 24 h against Cx. quinquefasciatus, revealing the potent larvicidal effect of peppermint oil (Manimaran et al., 2012). El-Akhal et al (2021) showed that lavender oil at 800 ppm resulted in 100% larval mortality with an LC50 of 140 ppm against Cx. pipiens larvae. Zhu and Tian (2013) tested the larvicidal activities of camphor, eucalyptol, terpine-4-ol, germacrene D, caryophyllene oxide, and caryophyllene against A. anthropophagus and found that the most potent were caryophyllene oxide and germacrene D, followed by terpine-4-ol and camphor, and the least potent was caryophyllene. In addition, Dias and Moraes (2014) reported that the majority of essential oils showed potent larvicidal activity derived from species of particular families, such as Laminaceae that are rich in sesquiterpenes and monoterpene hydrocarbons, and their individual pure compounds showed against mosquito larvae high activity.
Herein, the tested plant oils are not toxic to vertebrates and are identified to be eco-friendly as well as used as medicinal plants. However, their varied related toxicity potential with insect stage. Rosemary oil displayed the strongest larvicidal activity, while lavender and peppermint oils exhibited the strongest adulticidal activity. The adulticidal and knockdown activities of the tested oils recorded 100% mortalities for lavender and peppermint oils with KT50 of 17.43 and 22.81 min, respectively, while rosemary oil showed only 88.89% mortality with a KT50 of 26.64 min.
Structural variations of peppermint constituents, menthol, menthyl acetate, menthone, α-terpineol, pulegone, and β-caryophyllene, which were previously shown to be adulticidal and have knockdown efficacy against 3-day-old females of Cx. Quinquefasciatus, could contribute towards retaining or enhancing the oil mosquitocidal activity (Samarasekera et al., 2008). Pulegone was the most effective adulticidal among different tested terpenes with LC50 values lower than 0.1 mg/L against Ae. aegypti adults (Flores Guillermo et al., 2020). Linalool had an LC50 value of 14.87 μg/mL against adult Cx. pipiens mosquitoes (Tabari et al., 2017). Additionally, the repellent properties of peppermint essential oil were established against adults Ae. aegypti (Kumar et al., 2011, Manh and Tuyet, 2020). The effective repellent activity was also evaluated for lavender and rosemary essential oils against adult mosquitoes of Cx. pipiens pallens (Choi et al., 2002).
The toxicity of rosemary essential oil towards adults of Cx. pipiens was previously evaluated and proposed be attributed to its major monoterpenes constituents and recorded strong fumigant toxicity against adults, meanwhile, showed weak toxicity against larvae (Zahran et al., 2017).
The study confirmed the oil constituent’s role in toxicity, where interaction between the oil constituents is very important for the development of its insecticidal formulation and adjustment of the content active substances for formulation to ensure the biological efficacy as previously confirmed (Pavela, 2015, Sharma et al., 2022).
5. Conclusion
The present study reviled that rosemary oil acquired the most potent larvicidal activity (100% mortality) followed by peppermint oil (92%) and lastly lavender oil (87%). The adulticidal and knockdown activities of the tested oils recorded 100% mortalities for lavender and peppermint oils with KT50 of 17.43 and 22.81 min, respectively, while rosemary oil showed only 88.89% mortality with a KT50 of 26.64 min. The present study suggests that the synergistic effect of compounds present in each essential oil may elevate its biological activity against the target insect stage and proposed rosemary essential oil may be useful for control of Cx. pipiens larvae as a water treatment product and lavender and peppermint for application in control of adult mosquitoes.
Funding
None.
Ethics approval and consent to participate
Not applicable.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The author wishes to appreciate the Center for Environmental Research and Studies at Jazan University for the technical support.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2022.103350.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Abbott W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925;18(2):265–267. doi: 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- Ahmed, N., Alam, M., Saeed, M., Ullah, H., Iqbal, T., Al-Mutairi, K.A., Shahjeer, K., Ullah, R., Ahmed, S., Ahmed, N.A.A.H., Khater, H.F., Salman, M., 2021. Botanical Insecticides Are a Non-Toxic Alternative to Conventional Pesticides in the Control of Insects and Pests. In: Global Decline of Insects. IntechOpen. 10.5772/intechopen.100416. [DOI]
- Al-Mekhlafi F.A., Abutaha N., Farooq M., Al-Wadaan M. Insecticidal effect of Solenostemma argel extracts against Culex pipiens. J. Am. Mosq. Control Assoc. 2018;34(3):217–223. doi: 10.2987/17-6725.1. [DOI] [PubMed] [Google Scholar]
- Borges R.S., Ortiz B.L.S., Pereira A.C.M., Keita H., Carvalho J.C.T. Rosmarinus officinalis essential oil: A review of its phytochemistry, anti-inflammatory activity, and mechanisms of action involved. J. Ethnopharmacol. 2019;229:29–45. doi: 10.1016/j.jep.2018.09.038. [DOI] [PubMed] [Google Scholar]
- Bosly B. Evaluation of insecticidal activities of Mentha piperita and Lavandula angustifolia essential oils against house fly, Musca domestica L. (Diptera: Muscidae). J. Entomol. Nematol. 2013;5(4):50–54. [Google Scholar]
- Cavanagh H.M.A., Wilkinson J.M. Biological activities of lavender essential oil. Phyther. Res. 2002;16(4):301–308. doi: 10.1002/ptr.1103. [DOI] [PubMed] [Google Scholar]
- Choi W.-S., Park B.-S., Ku S.-K., Lee S.-E. Repellent activities of essential oils and monoterpenes against Culex pipiens pallens. J. Am. Mosq. Control Assoc. 2002;18(4):348–351. doi: 10.1016/j.fitote.2006.05.028. [DOI] [PubMed] [Google Scholar]
- da Silva Ramos, R., Rodrigues, A.B.L., Farias, A.L.F., Simões, R.C., Pinheiro, M.T., Ferreira, R.M. dos A., Costa Barbosa, L.M., Picanço Souto, R.N., Fernandes, J.B., Santos, L. da S., 2017. Chemical composition and in vitro antioxidant, cytotoxic, antimicrobial, and larvicidal activities of the essential oil of Mentha piperita L.(Lamiaceae). Sci. World J. 2017. 10.1155/2017/4927214. [DOI] [PMC free article] [PubMed]
- De Oliveira J.R., Camargo S.E.A., De Oliveira L.D. Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. J. Biomed. Sci. 2019;26:1–22. doi: 10.1186/s12929-019-0499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denner S.S. Lavandula angustifolia miller: english lavender. Holist. Nurs. Pract. 2009;23(1):57–64. doi: 10.1097/01.HNP.0000343210.56710.fc. [DOI] [PubMed] [Google Scholar]
- Dias C.N., Moraes D.F.C. Essential oils and their compounds as Aedes aegypti L. (Diptera: Culicidae) larvicides. Parasitol. Res. 2014;113:565–592. doi: 10.1007/s00436-013-3687-6. [DOI] [PubMed] [Google Scholar]
- Ebadollahi A., Davari M., Razmjou J., Naseri B. Separate and combined effects of Mentha piperata and Mentha pulegium essential oils and a pathogenic fungus Lecanicillium muscarium against Aphis gossypii (Hemiptera: Aphididae) J. Econ. Entomol. 2017;110(3):1025–1030. doi: 10.1093/jee/tox065. [DOI] [PubMed] [Google Scholar]
- Ebadollahi A., Ziaee M., Palla F. Essential oils extracted from different species of the Lamiaceae plant family as prospective bioagents against several detrimental pests. Molecules. 2020;25(7):1556. doi: 10.3390/molecules25071556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Akhal F., Guemmouh R., Ez Zoubi Y., El Ouali Lalami A. Larvicidal activity of Nerium oleander against larvae West Nile vector mosquito Culex pipiens (Diptera: Culicidae) J. Parasitol. Res. 2015;2015:1–5. doi: 10.1155/2015/943060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Akhal F., Ramzi A., Farah A., Ez Zoubi Y., Benboubker M., Taghzouti K., El Ouali Lalami A., Galvão C. Chemical Composition and Larvicidal Activity of Lavandula angustifolia Subsp. angustifolia and Lavandula dentata Spp. dentata Essential Oils against Culex pipiens Larvae, Vector of West Nile Virus. Psyche: A J. Entomol. 2021;2021:8872139. doi: 10.1155/2021/8872139. [DOI] [Google Scholar]
- Fujiwara G.M., Annies V., de Oliveira C.F., Lara R.A., Gabriel M.M., Betim F.C.M., Nadal J.M., Farago P.V., Dias J.F.G., Miguel O.G., Miguel M.D., Marques F.A., Zanin S.M.W. Evaluation of larvicidal activity and ecotoxicity of linalool, methyl cinnamate and methyl cinnamate/linalool in combination against Aedes aegypti. Ecotoxicol. Environ. Saf. 2017;139:238–244. doi: 10.1016/j.ecoenv.2017.01.046. [DOI] [PubMed] [Google Scholar]
- Govindarajan M., Benelli G. α-Humulene and β-elemene from Syzygium zeylanicum (Myrtaceae) essential oil: highly effective and eco-friendly larvicides against Anopheles subpictus, Aedes albopictus, and Culex tritaeniorhynchus (Diptera: Culicidae) Parasitol. Res. 2016;115(7):2771–2778. doi: 10.1007/s00436-016-5025-2. [DOI] [PubMed] [Google Scholar]
- Govindarajan M., Rajeswary M., Hoti S.L., Bhattacharyya A., Benelli G. Eugenol, α-pinene and β-caryophyllene from Plectranthus barbatus essential oil as eco-friendly larvicides against malaria, dengue and Japanese encephalitis mosquito vectors. Parasitol. Res. 2016;115(2):807–815. doi: 10.1007/s00436-015-4809-0. [DOI] [PubMed] [Google Scholar]
- Flores Guillermo A.M., Nahuel F., María T.D., Andrés M.V., Sara M.P. Adulticidal effect of seven terpenes and a binary combination against Aedes aegypti. J. Vector Borne Dis. 2020;57(4):356. doi: 10.4103/0972-9062.313975. [DOI] [PubMed] [Google Scholar]
- Hamer G.L., Kitron U.D., Brawn J.D., Loss S.R., Ruiz M.O., Goldberg T.L., Walker E.D. Culex pipiens (Diptera: Culicidae): a bridge vector of West Nile virus to humans. J. Med. Entomol. 2008;45(1):125–128. doi: 10.1603/0022-2585(2008)45[125:cpdcab]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ilahi I., Yousafzai A.M., Attaullah M., Haq T.U., Rahim A., Khan W., Khan A.A., Ullah S., Jan T., Khan M.M., Rahim G., Zaman N. Mosquitocidal activities of Chenopodium botrys whole plant n-hexane extract against Culex quinquefasciatus. Brazilian J. Biol. 2021;83 doi: 10.1590/1519-6984.240842. [DOI] [PubMed] [Google Scholar]
- Isman M.B., Wilson J.A., Bradbury R. Insecticidal Activities of Commercial Rosemary Oils (Rosmarinus officinalis) against larvae of Pseudaletia unipuncta and Trichoplusia ni in Relation to Their Chemical Compositions. Pharm. Biol. 2008;46(1-2):82–87. doi: 10.1080/13880200701734661. [DOI] [Google Scholar]
- Jafari-sales A., Pashazadeh M. Study of chemical composition and antimicrobial properties of Rosemary (Rosmarinus officinalis) essential oil on Staphylococcus aureus and Escherichia coli in vitro. Int. J. Life Sci. Biotechnol. 2020;3:62–69. doi: 10.38001/ijlsb.693371. [DOI] [Google Scholar]
- Kalemba D., Synowiec A. Agrobiological Interactions of Essential Oils of Two Menthol Mints: Mentha piperita and Mentha arvensis. Molecules. 2019;25(1):59. doi: 10.3390/molecules25010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamaouna F., Kimbaris A., Michaelakis Α., Papachristos D., Polissiou M., Papatsakona P., Τsora Ε., Miller T. Insecticidal activity of plant essential oils against the vine mealybug. Planococcus ficus. J. Insect Sci. 2013;13:142. doi: 10.1673/031.013.14201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyżowski M., Baran B., Łozowski B., Francikowski J. The effect of Rosmarinus officinalis essential oil fumigation on biochemical, behavioral, and physiological parameters of Callosobruchus maculatus. Insects. 2020;11(6):344. doi: 10.3390/insects11060344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Wahab N., Warikoo R. Bioefficacy of Mentha piperita essential oil against dengue fever mosquito Aedes aegypti L. Asian Pac. J. Trop. Biomed. 2011;1:85–88. doi: 10.1016/S2221-1691(11)60001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackled M.I., EL-Hefny M., Bin-Jumah M., Wahba T.F., Allam A.A. Assessment of the toxicity of natural oils from Mentha piperita, Pinus roxburghii, and Rosa spp. against three stored product insects. Processes. 2019;7(11):861. doi: 10.3390/pr7110861. [DOI] [Google Scholar]
- Mahendran G., Rahman L.-U. Ethnomedicinal, phytochemical and pharmacological updates on Peppermint (Mentha× piperita L.)—A review. Phyther. Res. 2020;34(9):2088–2139. doi: 10.1002/ptr.6664. [DOI] [PubMed] [Google Scholar]
- Manh H.D., Tuyet O.T. Larvicidal and Repellent Activity of Mentha arvensis L. Essential Oil against Aedes aegypti. Insects. 2020;11(3):198. doi: 10.3390/insects11030198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manimaran A., Cruz M.M.J.J., Muthu C., Vincent S., Ignacimuthu S. Larvicidal and knockdown effects of some essential oils against Culex quinquefasciatus Say, Aedes aegypti (L.) and Anopheles stephensi (Liston) Adv. Biosci. Biotechnol. 2012;03(07):855–862. doi: 10.4236/abb.2012.37106. [DOI] [Google Scholar]
- Nazar N., Howard C., Slater A., Sgamma T. Challenges in Medicinal and Aromatic Plants DNA Barcoding—Lessons from the Lamiaceae. Plants. 2022;11(1):137. doi: 10.3390/plants11010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavela R. Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against Culex quinquefasciatus Say larvae. Parasitol. Res. 2015;114(10):3835–3853. doi: 10.1007/s00436-015-4614-9. [DOI] [PubMed] [Google Scholar]
- Prakash, O.M., Chandra, M., Pant, A.K., Rawat, D.S., 2016. Mint (Mentha spicata L.) oils, in: Essential Oils in Food Preservation, Flavor and Safety. Elsevier, pp. 561–572. ISBN 9780124166448.
- Radwan M.A., El-Zemity S.R., Mohamed S.A., Sherby S.M. Larvicidal activity of some essential oils, monoterpenoids and their corresponding N-methyl carbamate derivatives against Culex pipiens (Diptera: Culicidae) Int. J. Trop. Insect Sci. 2008;28(2):61–68. doi: 10.1017/s1742758408962366. [DOI] [Google Scholar]
- Rai V.K., Sinha P., Yadav K.S., Shukla A., Saxena A., Bawankule D.U., Tandon S., Khan F., Chanotiya C.S., Yadav N.P. Anti-psoriatic effect of Lavandula angustifolia essential oil and its major components linalool and linalyl acetate. J. Ethnopharmacol. 2020;261:113127. doi: 10.1016/j.jep.2020.113127. [DOI] [PubMed] [Google Scholar]
- Ramos da Silva L.R., Ferreira O.O., Cruz J.N., de Jesus Pereira Franco C., Oliveira dos Anjos T., Cascaes M.M., Almeida da Costa W., Helena de Aguiar Andrade E., Santana de Oliveira M., Luís Â. Lamiaceae Essential Oils, Phytochemical Profile, Antioxidant, and Biological Activities. Evidence-Based Complem. Alternative Med. 2021;2021:1–18. doi: 10.1155/2021/6748052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarasekera R., Weerasinghe I.S., Hemalal KD.P. Insecticidal activity of menthol derivatives against mosquitoes. Pest. Manag. Sci. 2008;64(3):290–295. doi: 10.1002/ps.1516. [DOI] [PubMed] [Google Scholar]
- Sharma S., Loach N., Gupta S., Mohan L. Evaluation of larval toxicity, mode of action and chemical composition of citrus essential oils against Anopheles stephensi and Culex quinquefasciatus. Biocatal. Agric. Biotechnol. 2022;39:102284. doi: 10.1016/j.bcab.2022.102284. [DOI] [Google Scholar]
- Tabari M.A., Youssefi M.R., Esfandiari A., Benelli G. Toxicity of β-citronellol, geraniol and linalool from Pelargonium roseum essential oil against the West Nile and filariasis vector Culex pipiens (Diptera: Culicidae) Res. Vet. Sci. 2017;114:36–40. doi: 10.1016/j.rvsc.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Traboulsi A.F., Taoubi K., El-Haj S., Bessiere J?M, Rammal S. Insecticidal properties of essential plant oils against the mosquito Culex pipiens molestus (Diptera: Culicidae) Pest. Manag. Sci. 2002;58(5):491–495. doi: 10.1002/ps.486. [DOI] [PubMed] [Google Scholar]
- WHO, 2005. Guidelines for laboratory and field testing of mosquito larvicides. World Health Organization, 1–41. Retrieved from http://whqlibdoc.who.int/hq/2005/WHO_CDS_WHOPES_GCDPP_2005.13.pdf?ua=1.
- WHO. 2016. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes, (Second edition) (Updated June 2018). pp. 1–48. https://apps.who.int/iris/bitstream/handle/10665/250677/9789241511575-eng.pdf.
- Zahran H.-E.-D.-M., Abou-Taleb H.K., Abdelgaleil S.A.M. Adulticidal, larvicidal and biochemical properties of essential oils against Culex pipiens L. J. Asia. Pac. Entomol. 2017;20(1):133–139. doi: 10.1016/j.aspen.2016.12.006. [DOI] [Google Scholar]
- Zeghib F., Tine-Djebbar F., Zeghib A., Bachari K., Sifi K., Soltani N. Chemical Composition and Larvicidal Activity of Rosmarinus officinalis Essential Oil Against West Nile Vector Mosquito Culex pipiens (L.) J. Essent. Oil Bear. Plants. 2020;23(6):1463–1474. doi: 10.1080/0972060X.2020.1860138. [DOI] [Google Scholar]
- Zhu L., Tian Y. Chemical composition and larvicidal activity of essential oil of Artemisia gilvescens against Anopheles anthropophagus. Parasitol. Res. 2013;112(3):1137–1142. doi: 10.1007/s00436-012-3243-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.