Abstract
BACKGROUND & AIMS:
Nonalcoholic fatty liver disease (NAFLD) is associated with sugar-sweetened beverage (SSB) consumption in cross-sectional studies. In a prospective cohort, we examined the association of beverage consumption (SSB and diet soda) with incident NAFLD and changes in hepatic fat in the Framingham Heart Study (FHS).
METHODS:
We conducted a prospective observational study of participants from the FHS Third Generation and Offspring cohorts who participated in computed tomography sub-studies. Participants were classified according to their average SSB or diet soda consumption, which was derived from baseline and follow-up food frequency questionnaires: non-consumers (0-<1/month), occasional consumers (1/month-<1/week), and frequent consumers (≥1/week-≥1/day). Hepatic fat was quantified by the liver fat attenuation measurements on computed tomography scan. The primary dependent variable was incident NAFLD; secondarily, we investigated change in liver fat.
RESULTS:
The cohorts included 691 Offspring (mean age, 62.8 ± 8.2 years; 57.7% women) and 945 Third Generation participants (mean age, 48.4 ± 6.3 years; 46.6% women). In the Offspring cohort, there was a dose-response relationship with SSB consumption and incident NAFLD. Frequent SSB consumers had 2.53 times increased odds of incident NAFLD compared with non-consumers (95% confidence interval, 1.36-4.7) after multivariable analysis. For Offspring cohort participants, occasional and frequent consumers of SSB had a more adverse increase in liver fat compared with non-consumers.
CONCLUSIONS:
Higher average SSB intake is associated with increase in liver fat over 6 years of follow-up and increased odds of incident NAFLD especially among the older cohort, whereas no consistent association was observed for the younger Third Generation cohort.
Keywords: NAFLD, Liver Fat, Hepatic Steatosis, Sugar-Sweetened Beverage, Diet Soda, Fructose
Nonalcoholic fatty liver disease (NAFLD) is a major public health problem worldwide; however, most people with NAFLD are asymptomatic and unaware of their diagnosis.1 Obesity and unhealthy lifestyles are strong modifiable risk factors that contribute to NAFLD development.2 Diets high in glycemic load and sugar-sweetened beverage (SSB) consumption are associated with an increased prevalence of NAFLD.2 SSBs contain sucrose or high fructose corn syrup, and consumption of SSBs contributes to excess calorie consumption.3 SSBs may contribute to the development and progression of NAFLD by increasing visceral adiposity,4 insulin resistance, and inflammation.5 Along with recent increased consumption of SSBs in the United States,6 diet soda consumption has also increased.7 Artificial sweeteners in diet soda may result in gut microbiome dysbiosis, leaky gut syndrome, and the release of proinflammatory mediators that may contribute to NAFLD.8 The extent to which SSB or diet soda intake contributes to the progression and incidence of NAFLD is not known.
Prior studies examining the association between SSB or diet soda intake and risk of NAFLD yield inconsistent results and are limited by cross-sectional designs.9,10 In cross-sectional analysis of data from the Framingham Heart Study (FHS), high consumption of SSB, but not diet soda, was associated with prevalent NAFLD, particularly in overweight and obese individuals11,12; however, another study observed no such association.9 A systematic review demonstrated that high intake of SSB was adversely associated with NAFLD prevalence in a dose-response relationship but was also limited by the inclusion of primarily cross-sectional studies. Thus, the relationship between SSB or diet soda use and NAFLD incidence was not assessed.13
The impact of SSB or diet soda consumption on progression and incidence of NAFLD is unknown. We aimed to investigate the prospective association between intake of SSB or diet soda and changes in liver fat, as measured by computed tomography (CT), over 6 years of follow-up in the FHS.
Methods
Study Sample
The study sample included participants from the FHS’s Offspring and Third Generation cohorts, which have been described in detail.14,15 From 2002 to 2005, 3477 participants underwent abdominal multi-detector CT scans to quantify abdominal fat and coronary calcium.16 Among these participants, 1886 participants underwent repeat abdominal multi-detector CT scanning between 2008 and 2011.17 Participants with high alcohol consumption, defined as >14 drinks weekly for women and >21 drinks weekly for men18 (n = 453), lacked dietary data (n = 95), or other covariates (n = 7) were excluded. Thus, a total of 1636 participants (Offspring n = 691 and Third generation n = 945) were included in the primary analysis. For the incident NAFLD analysis, we excluded 282 participants with prevalent NAFLD (Offspring n = 127 and Third Generation n = 155). The institutional review boards at Boston University Medical Center and Massachusetts General Hospital approved of this study, and all participants provided written informed consent.
Nonalcoholic Fatty Liver Disease and Continuous Liver Fat Measures
The protocols for measuring liver fat have been described in detail previously.19 Participants underwent an abdominal scan with an 8-slice multi-detector CT scanner (LightSpeed Ultra; General Electric Health Care, Milwaukee, WI) at baseline (2002–2005) and with a 64-slice scanner at follow-up (2008–2011). The Hounsfield units across 3 separate areas in the liver were averaged to determine the mean liver attenuation. The mean liver attenuation was divided by the attenuation of a “phantom” control and multiplied by 100 to determine the liver phantom ratio (LPR).20 A lower value of the LPR represents more liver fat. Change in liver fat was calculated by subtracting the baseline LPR measure from the follow-up LPR. Increasing liver fat was defined as a decrease in LPR from baseline to end of follow-up. Incident NAFLD was defined as LPR ≤0.33 on the follow-up CT scan in participants without NAFLD at baseline.19
Sugar-Sweetened Beverage and Diet Soda Consumption
SSB and diet soda intakes were assessed using the Harvard semiquantitative food frequency questionnaire, which collects average frequency of intake over the prior year. We used the cumulative average approach to determine average intake of SSB and diet soda using data from the 2 exam cycles in Offspring cohort (7th and 8th) and Third Generation cohort (1st and 2nd), reflecting approximately 6 years of follow-up in each cohort.
Participants from each cohort were categorized according to the frequency of SSB and diet soda consumption: none to <1 serving/month (non-consumers), 1 serving/month to <1 serving/week (occasional consumers), and ≥1 serving/week (frequent consumers). In addition, we classified participants according to the change in SSB or diet soda consumption from baseline to end of follow-up: decreasing beverage consumption intake by ≥0.5 serving/week, no change in beverage consumption (<0.5 to >0.5 servings/week), and increasing beverage consumption intake by ≥0.5 servings/week.
Anthropometry and Covariates Assessment
Standard protocols were used in physical and medical examinations at each visit. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Physical activity level was calculated on the basis of questionnaire-derived time and intensity of activities in a typical day.21 To assess overall diet quality, we calculated the 2010 Alternate Healthy Eating Index (AHEI) without the SSB component (when dietary exposure was SSB) or without the diet soda component (when dietary exposure was diet soda).22
Statistical Analysis
Multivariable adjusted, logistic, and linear regression models were used to determine the odds ratio (OR) for incident NAFLD and least-square means of liver fat change across SSB and diet soda consumption categories. For extreme intakes of diet soda (>10 servings per day, n = 5), data were imputed using the next maximum values. We designated the lowest intake category as the reference category. We examined a predefined list of potential confounders (including current smoking status, physical activity, BMI, multivitamin use, AHEI adherence, and intake of coffee, diet soda, saturated fat, whole grains, fruits, vegetables, alcohol, fish, nuts, multivitamin use) and evaluated the extent to which they altered the age, sex, energy intake (kcal/day), and baseline LPR adjusted parameter estimates by approximately 10% or more. Factors not found to confound the effects of the beverage consumption on liver fat change or incident NAFLD were excluded from final models. Multivariable logistic models were adjusted for sex, change in cigarette smoking status, and averages of the following variables between the 2 exams including age, energy intake, physical activity, coffee intake, and AHEI. Multivariable linear models were adjusted for factors mentioned above and baseline LPR. Because body weight may partly mediate the association between beverage intake and risk of NAFLD, we adjusted the multivariable models for BMI. A test for linear trend across categories of beverage intake was performed on the basis of the category-specific medians of beverage intake. In secondary analyses, we repeated the above analyses using the change in beverage intake between the 2 exams as the independent variable. In these analyses, we adjusted for baseline SSB and diet soda simultaneously.
Cohort-specific analyses were performed to generate ORs that were pooled together using meta-analysis. Fixed and random-effect models produced similar results. However, in some analyses heterogeneity across the cohorts using the I2 statistic was >50%, indicating substantial heterogeneity23; therefore, we presented all results from the random-effects models. We conducted statistical analyses using SAS statistical software (version 9.4; SAS Institute, Cary, NC). All P values were based on 2-sided tests and were considered statistically significant at P <.05.
Results
Baseline Characteristics
Table 1 shows the characteristics at baseline according to SSB intake categories in Offspring and Third Generation participants, respectively. Supplementary Table 1 shows the average characteristics of the study participants over the study period. Overall, participants consuming >1 servings of SSB per week from both cohorts were younger and more likely to be men compared with those consuming <1 serving of SSB per week. More frequent consumers of SSB also had higher caloric and saturated fat intake compared with less frequent SSB consumers. Overall diet quality, as assessed by the AHEI, as well as intake of whole grains was lower in frequent SSB consumers compared with less frequent consumers.
Table 1.
Baseline Characteristicsa of Participants According to Sugar-Sweetened Beverage Consumption Categories in the Offspring and Third Generation Cohorts
| SSB consumption categories (servings) |
||||||
|---|---|---|---|---|---|---|
| Offspring |
Third Generation |
|||||
| Non-consumers (0–<1/month) | Occasional (1/month–<1/week) | Frequent (≥1/week) | Non-consumers (0–<1/month) | Occasional (1/month–<1/week) | Frequent (≥1/week) | |
| N (%) | 197 (28.5) | 202 (29.2) | 292 (42.3) | 225 (23.8) | 269 (28.5) | 451 (47.7) |
|
| ||||||
| Median intake (serv/week) | 0 | 0.5 | 3.2 | 0 | 0.5 | 3.5 |
|
| ||||||
| Age (y) | 60.8 (± 8.1) | 60.2 (± 8.4) | 58.0 (± 9.0) | 47.9 (± 6.2) | 45.6 (± 6.2) | 43.9 (± 6.0) |
|
| ||||||
| Women (%) | 139 (70.6) | 131 (64.9) | 129 (44.2) | 156 (69.3) | 156 (58.0) | 128 (28.4) |
|
| ||||||
| Current smoker (%) | 9 (4.6) | 16 (7.9) | 27 (9.3) | 17 (7.6) | 13 (4.8) | 46 (10.2) |
|
| ||||||
| Physical activity score (METs/day) | 36.4 (7.2) | 37.5 (6.5) | 36.3 (7.4) | 34.2 (7.5) | 35.3 (7.2) | 35.5 (8.9) |
|
| ||||||
| BMI (kg/m2) | 28.3 (± 5.3) | 27.5 (± 4.8) | 28.2 (± 4.8) | 27.7 (± 6.0) | 26.7 (± 5.2) | 27.4 (± 4.8) |
|
| ||||||
| Daily energy intake (kcal/day) | 1635.2 (621.4) | 1682.8 (713.0) | 1962.4 (821.4) | 1729.7 (771.3) | 1831.1 (749.7) | 2142.8 (874.0) |
|
| ||||||
| Saturated fatty acids (% energy) | 18.5 (10.0) | 20.0 (11.8) | 23.3 (13.7) | 20.8 (12.5) | 21.2 (11.0) | 27.0 (13.6) |
|
| ||||||
| Whole grain (g/day) | 16.5 (23.8) | 13.8 (21.1) | 14.8 (20.2) | 25.0 (26.4) | 26.9 (23.2) | 24.5 (22.6) |
|
| ||||||
| Fruits (serv/day) | 2.1 (2.0) | 2.1 (1.4) | 2.1 (1.6) | 2.3 (2.0) | 2.2 (1.6) | 1.8 (1.7) |
|
| ||||||
| Vegetables (serv/day) | 2.2 (1.7) | 2.3 (1.7) | 1.9 (1.7) | 1.6 (1.9) | 1.8 (1.6) | 1.7(1.7) |
|
| ||||||
| Nuts (serv/day) | 0.2 (0.4) | 0.1 (0.4) | 0.2 (0.4) | 0.4 (0.7) | 0.2 (0.4) | 0.2 (0.4) |
|
| ||||||
| Fish (serv/day) | 0.3 (0.2) | 0.3 (0.3) | 0.3 (0.3) | 0.3 (0.2) | 0.3 (0.3) | 0.3 (0.2) |
|
| ||||||
| Diet soda (serv/day) | 0.8 (1.1) | 0.4 (0.8) | 0.4 (1.0) | 0.6 (1.0) | 0.5 (1.0) | 0.4 (0.9) |
|
| ||||||
| Coffee intake (serv/week) | 17.5 (10.5) | 17.5 (10.5) | 17.5 (10.5) | 7.0 (10.5) | 17.5 (10.5) | 17.5 (10.5) |
|
| ||||||
| Alcohol (drinks/week) | 4.0 (5.0) | 4.0 (6.0) | 4.0 (6.0) | 3.7 (6.0) | 3.7 (5.1) | 3.7 (6.7) |
|
| ||||||
| AHEI (no SSB) | 53.5 (± 9.9) | 51.2 (± 10.1) | 48.9 (± 9.8) | 56.9 (± 11.0) | 55.9 (± 10.2) | 50.6 (± 10.3) |
|
| ||||||
| Liver phantom ratio | 0.372 (0.040) | 0.367 (0.039) | 0.372 (0.048) | 0.373 (0.031) | 0.365 (0.040) | 0.369 (0.042) |
AHEI, Alternate Healthy Eating Index; BMI, body mass index; METs, metabolic equivalents; serv, servings; SSB, sugar-sweetened beverage.
Expressed as means (± standard deviation), median (interquartile range), or otherwise stated.
Sugar-Sweetened Beverages and Nonalcoholic Fatty Liver Disease
Table 2 shows the cohort-specific and pooled adjusted ORs for incident NAFLD associated with SSB and diet soda intake. Participants in the Offspring cohort were followed up for 6 years (median with interquartile range of 4.5–7.5), whereas those in the Third Generation had a median follow-up of 6.2 years (interquartile range, 5.5–6.9). Participants in the Offspring cohort who occasionally and frequently consumed SSB had increased odds of 1.58 (95% confidence interval [CI], 0.86–2.89) and 2.14 (95% CI, 1.18–3.87) of developing NAFLD, respectively, compared with those consuming <1 serving of SSB per week in the multivariable adjusted model. After further adjustment for BMI, associations became stronger because occasional and frequent consumption of SSB was associated with increased odds of developing NAFLD (OR, 1.82; 95% CI, 0.97–3.43 and OR, 2.54; 95% CI, 1.36–4.73), respectively. Results in the Third Generation had a similar direction, although they were weaker with no statistically significant trend across consumption categories. In the pooled analyses, frequent consumption of SSB was associated with 77% increased odds of incident NAFLD compared with no consumption (95% CI, 1.11–2.83).
Table 2.
Odds Ratios for the Association Between Beverage Intake and Odds of Incident NAFLD in the Offspring and Third Generation Cohorts
| Cohort and categories of beverage intake (servings) | Median intake (servings/week) | No. of cases/N | Multivariable adjusted OR (95% CI)a | Multivariablea and BMI adjusted OR (95% CI) |
|---|---|---|---|---|
| SSB intake | ||||
| Offspring | ||||
| Non-consumers (<1/mo) | 0 | 22/165 | 1 (Reference) | 1 (Reference) |
| Occasional (1/mo–<1/wk) | 0.5 | 32/166 | 1.58 (0.86–2.89) | 1.82 (0.97–3.43) |
| Frequent (≥1/wk) | 3.2 | 59/233 | 2.14 (1.18–3.87) | 2.54 (1.36–4.73) |
| P trend | .02 | .01 | ||
| Third Generation | ||||
| Non-consumers (<1/mo) | 0 | 33/190 | 1 (Reference) | 1 (Reference) |
| Occasional (1/mo–<1/wk) | 0.5 | 38/225 | 1.12 (0.66–1.95) | 1.19 (0.66–2.14) |
| Frequent (≥1/wk) | 3.5 | 67/375 | 1.12 (0.65–1.92) | 1.29 (0.73–2.30) |
| P trend | .79 | .46 | ||
| Pooled | ||||
| Non-consumers (<1/mo) | 1 (Reference) | 1 (Reference) | ||
| Occasional (1/mo–<1/wk) | 1.31 (0.87–1.96) | 1.45 (0.94–2.23) | ||
| Frequent (≥1/wk) | 1.51 (0.97–2.36) | 1.77 (1.11–2.83) | ||
| P trend | .23 | .09 | ||
|
| ||||
| Diet soda intake | ||||
| Offspring | ||||
| Non-consumers (<1/mo) | 0 | 36/199 | 1 (Reference) | 1 (Reference) |
| Occasional (1/mo–<1/wk) | 0.5 | 21/109 | 1.21 (0.66–2.24) | 1.13 (0.60–2.14) |
| Frequent (≥1/wk) | 5 | 56/256 | 1.24 (0.77–1.97) | 0.97 (0.59–1.60) |
| P trend | .47 | .76 | ||
| Third Generation | ||||
| Non-consumers (<1/mo) | 0 | 46/318 | 1 (Reference) | 1 (Reference) |
| Occasional (1/mo–<1/wk) | 0.5 | 19/158 | 0.83 (0.46–1.48) | 0.72 (0.39–1.33) |
| Frequent (≥1/wk) | 3 | 73/314 | 1.70 (1.11–2.59) | 1.25 (0.79–1.97) |
| P trend | .003 | .15 | ||
| Pooled | ||||
| Non-consumers (<1/mo) | 1 (Reference) | 1 (Reference) | ||
| Occasional (1/mo–<1/wk) | 0.99 (0.65–1.51) | 0.89 (0.57–1.39) | ||
| Frequent (≥1/wk) | 1.48 (1.08–2.03) | 1.11 (0.80–1.56) | ||
| P trend | .10 | .53 | ||
AHEI, Alternate Healthy Eating Index; BMI, body mass index; CI, confidence interval; OR, odds ratio; SSB, sugar-sweetened beverage.
The model was adjusted for sex, averages of age, physical activity level, energy intake, coffee intake, AHEI (without SSB), intake of diet soda or SSB, and change in current cigarette smoking status.
Diet Soda and Nonalcoholic Fatty Liver Disease
Associations between diet soda intakes and NAFLD risk were weaker than those observed for SSB. For Third Generation participants, we observed a statistically significant increased odds of incident NAFLD for frequent consumption of diet soda compared with none (OR, 1.70; 95% CI, 1.11–2.59); however, the association became weak after also accounting for BMI (Table 2). Similar results were observed in the pooled analyses.
Secondary Analyses
Overall, participants from both cohorts had an increase in liver fat from baseline to follow-up. Figure 1 represents adjusted mean liver fat changes associated with categories of SSB (A) and diet soda (B) intakes in the Framingham Offspring and the Third Generation. Offspring participants who were frequent consumers of SSB significantly increased their liver fat compared with non-consumers (LPR: −0.016 ± 0.003 vs −0.004 ± 0.003; Figure 1A, Supplementary Table 2). There was no consistent association with increasing diet soda intake and liver fat changes (Figure 1B). Associations between SSB and diet soda in the Third Generation were inconsistent (Figure 1, Supplementary Table 2).
Figure 1.
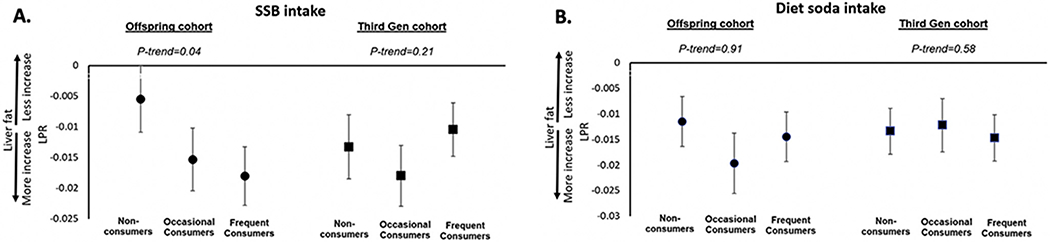
Multivariable adjusted means of change in liver phantom ratio associated with SSB and diet soda intake categories in Offspring cohort (A) and Third Generation (B). The models were adjusted for sex, averages of age, physical activity level, energy intake, coffee intake, AHEI (without SSB), BMI, intake of diet soda or SSB, change in current cigarette smoking status, and baseline LPR. AHEI, Alternate Healthy Eating Index; BMI, body mass index; LPR, liver phantom ratio; SSB, sugar-sweetened beverage.
Table 3 shows the cohort-specific and pooled adjusted ORs for incident NAFLD associated with change in SSB and diet soda intake from baseline to end of follow-up. The proportion of participants in the Offspring cohort who decreased, maintained, or increased their SSB consumption by a half serving per week were 38.6%, 38.6%, and 22.8%, respectively. Those who increased their SSB intake by a half serving per week had ~ 2-fold increase in NAFLD odds (OR, 2.20; 95% CI, 1.18–4.09) compared with those with stable SSB intake over a 6.2-year median follow-up after adjusting for multiple confounders and BMI. Similar results were observed in the Third Generation, although they were not statistically significant. Proportions in the Third Generation cohort for decreasing, maintaining, and increasing SSB consumption were 42.3%, 31.9%, and 25.8%, respectively. In the pooled analyses adjusting for multiple confounders and BMI, increased SSB intake was strongly and adversely associated with NAFLD odds (OR, 1.70; 95% CI, 1.12–2.56). Finally, there were no consistent adverse effects of increasing or decreasing diet soda consumption on incident NAFLD odds in the individual cohorts and pooled analyses after adjusting for multiple confounders and BMI.
Table 3.
Odds Ratios for the Association Between Change in Beverage Intake (SSB and Diet Soda) From Baseline to End of Follow-up and Risk of NAFLD in the Offspring and Third Generation Cohorts
| Cohort and categories of change in beverage intake | Median intake change (servings/week) | No. of cases/N | Multivariable adjusted OR (95% CI)1 | P value | Multivariablea and BMI adjusted OR (95% CI) | P value |
|---|---|---|---|---|---|---|
| SSB intake | ||||||
| Offspring | ||||||
| Decrease by ½ serv/week | −2 | 53/205 | 1.53 (0.88–2.65) | .13 | 1.51 (0.85–2.68) | .16 |
| Stable | 0 | 30/205 | 1 (Reference) | 1 (Reference) | ||
| Increase by ½ serv/week | 1.5 | 30/121 | 1.87 (1.04–3.39) | .038 | 2.20 (1.18–4.09) | .01 |
| Third Generation | ||||||
| Decrease by ½ serv/week | −4 | 58/334 | 1.12 (0.69–1.08) | .65 | 1.15 (0.69–1.94) | .59 |
| Stable | 0 | 41/252 | 1 (Reference) | 1 (Reference) | ||
| Increase by ½ serv/week | 3 | 39/204 | 1.32 (0.79–2.23) | .29 | 1.38 (0.79–2.40) | .26 |
| Pooled | ||||||
| Decrease by ½ serv/week | 1.12 (1.08–1.16) | <.001 | 1.10 (0.77–1.57) | .59 | ||
| Stable | 1 (Reference) | 1 (Reference) | ||||
| Increase by ½ serv/week | 1.54 (1.04–2.28) | .03 | 1.70 (1.12–2.56) | .01 | ||
|
| ||||||
| Diet soda intake | ||||||
| Offspring | ||||||
| Decrease by ½ serv/week | −3 | 32/158 | 1.06 (0.64–1.73) | .83 | 0.83 (0.49–1.39) | .47 |
| Stable | 0 | 55/294 | 1 (Reference) | 1 (Reference) | ||
| Increase by ½ serv/week | 3.5 | 26/112 | 1.26 (0.73–2.16) | .41 | 1.05 (0.60–1.83) | .87 |
| Third Generation | ||||||
| Decrease by ½ serv/week | −3.5 | 43/207 | 1.48 (0.95–2.30) | .08 | 1.22 (0.76–1.96) | .41 |
| Stable | 0 | 64/449 | 1 (Reference) | 1 (Reference) | ||
| Increase by ½ serv/week | 3 | 31/134 | 1.86 (1.13–3.07) | .02 | 1.32 (0.77–2.30) | .32 |
| Pooled | ||||||
| Decrease by ½ serv/week | 1.27 (0.92–1.77) | .15 | 1.02 (0.72–1.45) | .90 | ||
| Stable | 1 (Reference) | 1 (Reference) | ||||
| Increase by ½ serv/week | 1.55 (1.08–2.24) | .02 | 1.18 (0.79–1.74) | .42 | ||
AHEI, Alternate Healthy Eating Index; BMI, body mass index; CI, confidence interval;; OR, odds ratio; SSB, sugar-sweetened beverage.
The model was adjusted for sex, averages of age, physical activity level, energy intake, coffee intake, AHEI (without SSB), intake of diet soda or SSB, and change in current cigarette smoking status.
Overall, both cohorts had an increase in BMI and weight over the follow-up period. Offspring participants had an average BMI increase of 0.3 ± 2.5 kg/m2 and an average weight change of 0.1 ± 6.9 kg. Third Generation participants had an average BMI increase of 1.0 ± 2.4 kg/m2 and an average weight change of 2.2 ± 6.9 kg. Incidence of diabetes was 3.8% in the Offspring cohort and 3.2% in the Third Generation cohort. Adjusting for diabetes status in the multivariable model did not change the results (results not shown).
Discussion
In this community-based prospective study of adults, we observed an adverse association between higher SSB consumption and incident NAFLD odds. Frequent consumers of SSB had the highest odds of developing NAFLD compared with non-consumers. Findings were stronger in the Offspring cohort of the FHS including a clear dose-response relationship. Furthermore, participants who increased their SSB intake by a half serving per day compared with those who had constant SSB intake over the follow-up period had a 1.7-fold increase in NAFLD odds after adjusting for multiple confounders including BMI. Conversely, decreasing SSB consumption was not associated with decreased liver fat, suggesting that reversing liver fat accumulation may require additional dietary changes apart from lowering SSB intake alone. We observed no association between diet soda consumption and liver fat or incidence of NAFLD.
To date, few studies have evaluated the prospective association between beverage consumption (SSB and diet soda) and risk or progression of NAFLD. Our results are consistent with previous cross-sectional studies. A prior cross-sectional analysis from the FHS including 2634 Offspring and Third Generation participants showed that daily consumption of SSB (vs no consumption) was associated with increased odds of NAFLD prevalence (OR, 1.61; 95% CI, 1.04–2.50). However, higher consumption of SSB was inversely and strongly associated with LPR values in overweight and obese participants, whereas no statistically significant trend was seen in participants with normal BMI.24 Although the study was consistent with our results, it is cross-sectional and lacks longitudinal follow-up, whereas the present study benefits from prospectively updated SSB intake data and repeated CT scans. In addition, a small randomized controlled trial revealed that the consumption of 1 L of sucrose-sweetened soft drink per day could lead to a significant 1.4-fold increase in liver fat compared with isocaloric milk, diet soda, and water over a 6-month period.25 Although this study shows an adverse relationship between heavy SSB consumption and liver fat, the study does not mirror casual beverage consumption patterns for most individuals,26 whereas our study is reflective of the average dietary habits over a significantly longer time period. Finally, our findings of a dose-response relationship between SSB intake and odds of incident NAFLD, especially in the older generation of FHS participants, are also similar with the findings in a meta-analysis where consumption of low (<1 cups/week), moderate (1–6 cups/week), and high (≥7 cups/week) intakes of SSB increased the relative risk of NAFLD by 14%, 26%, and 53%, respectively.13
Although the adverse association between SSB and NAFLD incidence in the Offspring cohort was strong, we did not observe similar findings in the younger Third Generation cohort. It is possible that Third Generation cohort participants, with a mean age of 45 years, included a higher proportion of premenopausal women who may have a decreased risk of incident NAFLD from higher estradiol, which may confer hepatoprotective benefits.27 In addition, the older Offspring participants may reflect a longer history of SSB consumption and be associated with other risk factors before the start of follow-up for this study, which may be relevant for the NAFLD induction period. Additional studies are needed to determine whether SSB is associated with incident NAFLD in both younger and older individuals.
The potential mechanisms underpinning the adverse effects of higher consumption of SSB on the pathogenesis of NAFLD are multifactorial. Fructose, the major ingredient of SSB, may induce de novo lipogenesis by activating sterol regulatory element-binding proteins and carbohydrate-responsive element-binding protein, transcription factors that regulate lipogenesis.28,29 The activation of carbohydrate-responsive element-binding protein has been demonstrated to increase hepatic lipogenic genes such as stearoyl-CoA desaturase, lipogenic carboxylase, and fatty acid synthetase.30 In rodent models, mice fed with high-fructose diets induced endoplasmic reticulum stress, triggering sterol regulatory element-binding protein 1c activation to further exacerbate de novo lipogenesis.31 Fructose is also capable of bypassing phosphofructokinase, a key rate-limiting step in glycolysis, providing substrates for fatty acid synthesis without allosteric inhibition in the presence of excess fructose.32 In contrast, diet soda contains non-metabolizable artificial sweeteners such as sucralose, aspartame, saccharin, or neotame. A human study on the effect of sucralose on small intestinal glucose absorption did not yield significant results in raising blood glucose, plasma glucagon-like peptide-1, or serum 3-O-methylglucose,33 suggesting that artificial sweeteners may not have a metabolic impact. In support of this, a recent mouse study showed that rebaudioside, an artificial sweetener, may have hepatoprotective effects in nonalcoholic steatohepatitis by improving endoplasmic reticulum stress-related gene expressions.34
Strengths of our study include its longitudinal and community-based nature within a relatively large sample. Comprehensive longitudinal data were collected over 6 years, and liver fat was quantified by CT scan. Limitations may include variations in intra- and inter-reader reproducibility, although our prior work shows high reproducibility of 0.99 and 0.99, respectively.19 Furthermore, CT is insensitive to hepatic fibrosis; however, the prevalence of hepatic fibrosis in our community-based sample is likely low. Accuracy in self-reported dietary habits, especially in longitudinal studies, may be limited and subject to recall bias. A review article postulated that food items with a negative health image, ie, SSBs, are more likely to be under-reported compared with more positive health foods such as fruits and vegetables.35 Underreporting (occasional or frequent consumers reporting no consumption) may attenuate results and explain why no association was observed among the younger cohort, especially if they had better knowledge of the harms associated with SSB consumption. Another limitation is the difficulty in interpreting the LPR because this measure is not used clinically. Changes in liver fat may be small, and the clinical significance is not known. There are emerging techniques for converting CT measures of liver fat to magnetic resonance imaging–derived proton density fat fraction, although these methods require external validation.36 Although we investigated the prospective association between changes in SSB intake and liver fat, we lacked data on history of SSB intake and other risk factors before the start of follow-up in the younger cohort, which may be relevant for the development and progression of NAFLD. Finally, the FHS consists of primarily white, non-Hispanic individuals; however, to our knowledge, there are no data to support why the biological processes leading to NAFLD would differ in more racially and ethnically diverse populations.
Conclusion
Our study provides important evidence that higher consumption of SSBs may increase the odds of NAFLD development, independent of weight changes, among middle-aged individuals in the United States. We found no association with increasing diet soda intake. Further population-based studies are needed to identify effective intervention strategies to lower SSB consumption for this at-risk population and to confirm the adverse effects of SSB on NAFLD in younger populations.
Supplementary Material
What You Need to Know.
Background
Prior cross-sectional studies examining sugar-sweetened beverages (SSBs) and NAFLD show a positive association. We investigate the prospective association between SSB consumption and incident NAFLD.
Findings
Older adults who drink SSBs are at a higher risk of developing NAFLD, whereas no association was seen in the younger generation. Diet soda was not associated with worsening liver fat.
Implications for patient care
Sugar-sweetened beverages may be a risk factor to the development of NAFLD that is independent to weight changes, especially in older adults. Diet soda may be a safer alternative compared with SSBs in decreasing risk of NAFLD.
Funding
The Framingham Heart Study is supported in part by the National Heart, Lung, and Blood Institute contracts N01-HC-25195, HHSN268201500001, and 75N92019D00031. Dr Long is supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases K23 DK113252, the Doris Duke Charitable Foundation Grant #2019085, Gilead Sciences Research Scholars Award, the Boston University School of Medicine Department of Medicine Career Investment Award, and the Boston University Clinical Translational Science Institute UL1 TR001430.
Abbreviations used in this paper:
- AHEI
Alternate Healthy Eating Index
- BMI
body mass index
- CI
confidence interval
- CT
computed tomography
- FHS
Framingham Heart Study
- LPR
liver phantom ratio
- NAFLD
nonalcoholic fatty liver disease
- OR
odds ratio
- SSB
sugar-sweetened beverage.
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2021.11.001.
Conflicts of interest
The authors disclose no conflicts.
CRediT Authorship Contributions
William Park, MD (Conceptualization: Lead; Formal analysis: Supporting; Methodology: Equal; Writing – original draft: Lead)
Ioanna Yiannakou (Formal analysis: Lead; Methodology: Equal; Writing – review & editing: Equal)
Julie Petersen (Writing – review & editing: Supporting)
Udo Hoffmann (Data curation: Supporting)
Jiantao Ma (Conceptualization: Supporting; Methodology: Supporting)
Michelle T. Long (Conceptualization: Lead; Data curation: Supporting; Formal analysis: Supporting; Funding acquisition: Lead; Writing – review & editing: Supporting)
References
- 1.Younossi Z, Tacke F, Arrese M, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2019;69:2672–2682. [DOI] [PubMed] [Google Scholar]
- 2.Asrih M, Jornayvaz FR. Diets and nonalcoholic fatty liver disease: the good and the bad. Clin Nutr 2014;33:186–190. [DOI] [PubMed] [Google Scholar]
- 3.Patterson ME, Yee JK, Wahjudi P, et al. Acute metabolic responses to high fructose corn syrup ingestion in adolescents with overweight/obesity and diabetes. J Nutr Intermed Metab 2018;14:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma J, McKeown NM, Hwang S-J, et al. Sugar-sweetened beverage consumption is associated with change of visceral adipose tissue over 6 years of follow-up. Circulation 2016;133:370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelley GL, Allan G, Azhar S. High dietary fructose induces a hepatic stress response resulting in cholesterol and lipid dysregulation. Endocrinology 2004;145:548–555. [DOI] [PubMed] [Google Scholar]
- 6.Miller PE, McKinnon RA, Krebs-Smith SM, et al. Sugar-sweetened beverage consumption in the U.S.: novel assessment methodology. Am J Prev Med 2013;45:416–421. [DOI] [PubMed] [Google Scholar]
- 7.Toews I, Lohner S, de Gaudry KD, et al. Association between intake of non-sugar sweeteners and health outcomes: systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. BMJ 2019;364:k4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emamat H, Ghalandari H, Tangestani H, et al. Artificial sweeteners are related to non-alcoholic fatty liver disease: microbiota dysbiosis as a novel potential mechanism. EXCLI J 2020;19:620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofmeister C, Haenle MM, Akinli AS, et al. Is there a connection between the consumption of sugary soft drinks (softdrinks) and hepatic steatosis (fatty liver)? Ernährungs Umschau 2013. [Google Scholar]
- 10.Assy N, Nasser G, Kamayse I, et al. Soft drink consumption linked with fatty liver in the absence of traditional risk factors. Can J Gastroenterol 2008;22:811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma J, Sloan M, Fox CS, et al. Sugar-sweetened beverage consumption is associated with abdominal fat partitioning in healthy adults. J Nutr 2014;144:1823–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arenaza L, Medrano M, Oses M, et al. Dietary determinants of hepatic fat content and insulin resistance in overweight/obese children: a cross-sectional analysis of the Prevention of Diabetes in Kids (PREDIKID) study. Br J Nutr 2019;121:1158–1165. [DOI] [PubMed] [Google Scholar]
- 13.Chen H, Wang J, Zheng L, et al. Consumption of sugar-sweetened beverages has a dose-dependent effect on the risk of non-alcoholic fatty liver disease: an updated systematic review and dose-response meta-analysis. Int J Environ Res Public Health 2019;16:2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Splansky GL, Corey D, Yang Q, et al. The Third Generation cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol 2007;165:1328–1335. [DOI] [PubMed] [Google Scholar]
- 15.Kannel WB, Feinleib M, McNamara PM, et al. An investigation of coronary heart disease in families: the Framingham offspring study. Am J Epidemiol 1979;110:281–290. [DOI] [PubMed] [Google Scholar]
- 16.Fox CS, Massaro JM, Schlett CL, et al. Periaortic fat deposition is associated with peripheral arterial disease. Circulation: Cardiovascular Imaging 2010;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roseman DA, Hwang S-J, Manders ES, et al. Renal artery calcium, cardiovascular risk factors, and indexes of renal function. Am J Cardiol 2014;113:156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary guidelines for Americans, 2020-2025. 9th ed. December 2020. [Google Scholar]
- 19.Speliotes EK, Massaro JM, Hoffmann U, et al. Liver fat is reproducibly measured using computed tomography in the Framingham Heart Study. J Gastroenterol Hepatol 2008;23:894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Speliotes EK, Massaro JM, Hoffmann U, et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology 2010;5:1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kannel WB, Belanger A, D’Agostino R, et al. Physical activity and physical demand on the job and risk of cardiovascular disease and death: the Framingham Study. Am Heart J 1986 1986;112:820–825. [DOI] [PubMed] [Google Scholar]
- 22.Chiuve SE, Fung TT, Rimm ER, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma J, Fox CS, Jacques PF, et al. Sugar-sweetened beverage, diet soda, and fatty liver disease in the Framingham Heart Study cohorts. J Hepatol 2015;63:462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maersk M, Belza A, Stodkilde-Jorgensen H, et al. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr 2012;95:283–289. [DOI] [PubMed] [Google Scholar]
- 26.Kumar GS, Pan L, Park S, et al. Sugar-sweetened beverage consumption among adults: 18 states, 2012. Morbidity and Mortality Weekly Report 2014;63:686–690. [PMC free article] [PubMed] [Google Scholar]
- 27.DiStefano JK. NAFLD and NASH in postmenopausal women: implications for diagnosis and treatment. Endocrinology 2020;161:bqaa134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herman MA, Samuel VT. The sweet path to metabolic demise: fructose and lipid synthesis. Trends Endocrinol Metab 2016;27:719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ortega-Prieto P, Postic C. Carbohydrate sensing through the transcription factor ChREBP. Front Genet 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodríguez-Calvo R, Barroso E, Serrano L, et al. Atorvastatin prevents carbohydrate response element binding protein activation in the fructose-fed rat by activating protein kinase A. Hepatology 2009;49:106–115. [DOI] [PubMed] [Google Scholar]
- 31.Ren L-P, Chan SMH, Zeng X-Y, et al. Differing endoplasmic reticulum stress response to excess lipogenesis versus lipid oversupply in relation to hepatic steatosis and insulin resistance. PLoS ONE 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DiNicolantonio JJ, Subramonian AM, O’Keefe JH. Added fructose as a principal driver of non-alcoholic fatty liver disease: a public health crisis. Open Heart 2017;4:e000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma J, Chang J, Checklin HL, et al. Effect of the artificial sweetener, sucralose, on small intestinal glucose absorption in healthy human subjects. Br J Nutr 2010;104:803–806. [DOI] [PubMed] [Google Scholar]
- 34.Xi D, Bhattacharjee J, Salazar-Gonzalez R-M, et al. Rebaudioside affords hepatoprotection ameliorating sugar sweetened beverage-induced nonalcoholic steatohepatitis. Scientific Reports 2020;10:6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macdiarmid J, Blundell J. Assessing dietary intake: who, what and why of under-reporting. Nutr Res Rev 1998;11:231–253. [DOI] [PubMed] [Google Scholar]
- 36.Pickhardt PJ, Graffy PM, Reeder SB, et al. Quantification of liver fat content with unenhanced MDCT: phantom and clinical correlation with MRI proton density fat fraction. Am J Roentgenol 2018;211:W151–W157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


