Abstract
BACKGROUND
The obesity paradox states that patients with higher body mass index (BMI) and cardiovascular disease may experience better prognosis. However, this is less clear in patients with coronary heart disease.
METHODS AND RESULTS
The prospective STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) trial included 15 828 patients with stable coronary heart disease with 3 to 5 years’ follow‐up on optimal secondary preventive treatment. BMI was measured at baseline (n=15 785). Associations between BMI and cardiovascular outcomes were evaluated by Cox regression analyses with multivariable adjustments. Mean age was 64±9 years and 19% women. Most risk markers (diabetes, hypertension, inflammatory biomarkers, triglycerides) showed a graded association with higher BMI. The frequency of smoking, levels of high‐density lipoprotein, growth differentiation factor 15, and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) were higher at lower BMI. Low BMI (<20 kg/m2; n=244 [1.5%]) was associated with doubled risk of total death (hazard ratio [HR], 2.27; 95% CI, 1.60–3.22), cardiovascular death (HR, 2.26; 95% CI, 1.46–3.49), and heart failure (HR, 2.51; 95% CI, 1.35–4.68) compared with BMI of 25 to <30 kg/m2 (n=6752 [42.8%]) as reference. Similarly, high BMI of ≥35 kg/m2 (n=1768 [11.2%]) was associated with increased risk of the same outcomes. A BMI between 20 and <25 kg/m2 was associated with increased risk of cardiovascular death (HR, 1.26; 95% CI, 1.03–1.54) and total death (HR, 1.21; 95% CI, 1.03–1.42).
CONCLUSIONS
Patients with stable coronary heart disease showed a graded increase in cardiometabolic and inflammatory risk factors with increasing BMI category >25 kg/m2. All‐cause and cardiovascular mortality were lowest at BMI of 25 to 35 kg/m2. Underweight with BMI of <20 kg/m2 and very high BMI of ≥35 kg/m2 were strong risk markers for poor prognosis.
REGISTRATION
URL: https://clinicaltrials.gov/; Unique identifier NCT00799903.
Keywords: coronary artery disease, obesity, risk factors
Subject Categories: Obesity, Risk Factors, Heart Failure, Chronic Ischemic Heart Disease
Nonstandard Abbreviations and Acronyms
- GDF‐15
growth differentiation factor 15
- STABILITY
Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy
Clinical Perspective
What Is New?
Patients with stable coronary heart disease had a graded increase in cardiometabolic and inflammatory risk factors with increasing body mass index (BMI) category >25 kg/m2.
All‐cause and cardiovascular mortality were lowest at a BMI between 25 and 35 kg/m2, which is somewhat higher than what is currently recommended in prevention guidelines.
Underweight with BMI <20 kg/m2 and very high BMI ≥35 kg/m2 were strong risk markers for poor prognosis.
What Are the Clinical Implications?
We need to better establish the ideal BMI for patients with coronary heart disease and determine if recommendations should be revised.
It is important to identify underweight and extreme overweight as strong markers of poor prognosis.
Overweight is an increasingly common phenomenon in most parts of the world. 1 , 2 Obesity, assessed using body mass index (BMI) >30 kg/m2, is an established risk factor for development of coronary heart disease (CHD) in healthy individuals. 3 Furthermore, obesity is associated with many of the known cardiovascular risk factors, such as hypertension and dyslipidemia. 4 However, in some epidemiological studies, increased obesity has not been associated with a lower risk. Studies have demonstrated that in patients with heart failure, acute coronary syndromes, 5 , 6 , 7 atrial fibrillation, 8 cardiovascular disease with diabetes, 9 or after coronary revascularization 10 there may be an “overweight paradox” phenomenon, with better cardiovascular prognosis among patients with higher BMI. 8 , 11 The association between BMI and cardiovascular outcomes in patients with stable CHD seems inconsistent and complex. In a recent retrospective study of >15 000 patients with stable coronary artery disease with long‐term follow‐up, obesity was independently associated with increased mortality, whereas overweight was not. 12 There is limited and partly conflicting data on the ideal weight and BMI in patients with known CHD in secondary prevention. The aim of the current substudy, a post hoc analysis of the prospective STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) trial was to explore associations between BMI and clinical outcomes in patients with stable CHD, and possibly identify an optimal BMI for the risk of cardiovascular events. In addition, we aimed to better understand potential mechanisms behind the associations.
METHODS
The data underlying this article can be shared on reasonable request from www.clinicalstudydatarequest.com.
Study Design, Population, and Clinical End Points
The prospective STABILITY trial was a randomized placebo‐controlled study evaluating the effects of a lipoprotein‐associated phospholipase A2 inhibitor darapladib and included 15 828 patients with stable CHD with a follow‐up of 3 to 5 (median, 3.7) years on optimal secondary preventive treatment. 13 The study design, baseline characteristics, and main results have been presented previously. 14 , 15 In brief, patients with CHD, as documented by at least 1 of the following: previous myocardial infarction (MI), previous percutaneous coronary intervention or coronary artery bypass grafting, or multivessel coronary artery disease were eligible. In addition, at least 1 specified risk indicator was required. The study was performed in accordance with the Declaration of Helsinki, approved by institutional review boards, and all patients provided written informed consent.
Primary outcomes were the composite of cardiovascular death, MI, and stroke. Secondary outcomes were the individual major adverse cardiovascular event components, urgent coronary revascularization, stroke, all‐cause mortality, hospitalization for heart failure, and new cancer diagnosis. All end points were centrally adjudicated to minimize variability. Details of the end point definitions have been published elsewhere. 14
Analyses
BMI and waist circumference were measured at baseline (n=15 785). BMI was divided into 5 categories, based on standard cutoff values for normal weight (20 to <25 kg/m2), overweight (25 to <30 kg/m2), obesity (30 to <35 kg/m2), extreme obesity (≥35 kg/m2), and underweight (<20 kg/m2). Venous blood samples were obtained at inclusion in the morning after 9 hours of fasting. EDTA plasma aliquots were stored at −80°C until biochemical analyses. Established biochemical assays for NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), high‐sensitivity cardiac troponin T, high‐sensitivity C‐reactive protein, interleukin‐6, growth differentiation factor 15 (GDF‐15), and lipoprotein‐associated phospholipase A2 were centrally performed as previously published. 16 , 17 , 18 , 19 , 20 , 21 Physical activity at baseline was assessed on the basis of a lifestyle questionnaire.
Statistical Analysis
To investigate differences across the 5 BMI groups, categorical variables were presented as count and proportion and continuous variables as mean and SD. Groups were compared with the chi‐square test. Continuous variables were compared with Kruskal‐Wallis nonparametric tests. Associations between BMI and clinical outcomes were evaluated using Cox proportional hazards regression models and expressed with hazard ratios (HRs) and 95% CIs, presented in tables or with forest plots. Survival curves for cardiovascular death, all‐cause death, MI, and hospitalization for heart failure were performed by using the Kaplan‐Meier method (not shown). The underlying proportional hazards assumptions of the Cox proportional hazard models were verified by visual inspection of Kaplan‐Meier graphs and Schoenfeld residual plots. The basic model was adjusted for age, sex, and randomized treatment. In the multivariable model, we adjusted for age, sex, randomized treatment, prior percutaneous coronary intervention/coronary artery bypass grafting, prior MI, renal dysfunction, polyvascular disease, diabetes, smoking, stroke/transient ischemic attack, congestive heart failure, systolic blood pressure, geographic region, chronic obstructive pulmonary disease, cancer diagnosis, and Asian/Japanese origin. Spline plots with 4 knots were constructed to show relations between BMI study outcomes. All analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC). A 2‐sided P value of <0.05 was considered statistically significant. There was no adjustment for multiplicity.
RESULTS
A summary of baseline characteristics and demographics is shown in Table 1. Mean age was 64±9 years, and 18.7% were women. In total, 244 (1.5%) patients had a BMI of <20 kg/m2, 3060 (19.4%) a BMI of 20 kg/m2 to <25 kg/m2, 6752 (42.8%) a BMI of 25 kg/m2 to <30 kg/m2, 3961 (25.1%) a BMI of 30 kg/m2 to <35 kg/m2, and 1768 (11.2%) with BMI of ≥35 kg/m2, respectively. Severe underweight (BMI of <18.5 kg/m2) was observed in 79 patients (0.5%). Several established risk markers, such as diabetes, hypertension, previous revascularization, and levels of both inflammatory biomarkers and triglycerides showed an increased and graded association with higher BMI. In addition, both systolic and diastolic blood pressure were higher with higher BMI category. The prevalence of smoking, and serum levels of high‐density lipoprotein, GDF‐15, and NT‐proBNP were higher at lower BMI. Patients with a high BMI of >30 kg/m2, classified as obese, were slightly younger, more often women, and more often of White race than those classified as having normal weight or overweight. The association between BMI and biomarkers of special interest (interleukin‐6, high‐sensitivity cardiac troponin T, NT‐proBNP and GDF‐15) are shown in Figure 1. Interleukin‐, GDF‐15, and high‐sensitivity cardiac troponin were highest in the low and high end of BMI categories. NT‐proBNP was highest among patients with very low BMI and were lowest among patients with highest BMI. Physical activity assessed as metabolic equivalents of task h/week was lowest among patients with BMI of <25 kg/m2.
Table 1.
Summary of Demographic and Baseline Characteristics by BMI Category
| Baseline characteristics |
<20 kg/m2 N=244 |
20 < 25 kg/m2 N=3060 |
25 < 30 kg/m2 N=6752 |
30 < 35 kg/m2 N=3961 |
≥35 kg/m2 N=1768 |
Total N=15 785 |
P value for overall tests | |
|---|---|---|---|---|---|---|---|---|
| Age at randomization (years) | Median (Q1–Q3) | 68.0 (58.5–75.0) | 66.0 (59.0–72.0) | 65.0 (59.0–71.0) | 64.0 (58.0–70.0) | 63.0 (58.0–69.0) | 65.0 (59.0– 71.0) | <0.0001 |
| Sex, n (%) | Female | 78 (32.0) | 614 (20.1) | 1061 (15.7) | 757 (19.1) | 448 (25.3) | 2958 (18.7) | <0.0001 |
| Race, n (%) | Black | 5 (2.0) | 51 (1.7) | 144 (2.1) | 99 (2.5) | 63 (3.6) | 362 (2.3) | <0.0001 |
| Central/South/Southeast Asian | 92 (37.7) | 479 (15.7) | 485 (7.2) | 108 (2.7) | 25 (1.4) | 1189 (7.5) | <0.0001 | |
| East Asian/Japanese | 49 (20.1) | 686 (22.4) | 674 (10.0) | 103 (2.6) | 10 (0.6) | 1522 (9.6) | <0.0001 | |
| Other | 4 (1.6) | 57 (1.9) | 164 (2.4) | 79 (2.0) | 37 (2.1) | 341 (2.2) | <0.0001 | |
| White | 94 (38.5) | 1787 (58.4) | 5285 (78.3) | 3572 (90.2) | 1633 (92.4) | 12 371 (78.4) | <0.0001 | |
| Geographic region, n (%) | Asia/Pacific | 150 (61.5) | 1204 (39.3) | 1337 (19.8) | 312 (7.9) | 80 (4.5) | 3083 (19.5) | <0.0001 |
| Eastern Europe | 28 (11.5) | 484 (15.8) | 1587 (23.5) | 1061 (26.8) | 370 (20.9) | 3530 (22.4) | <0.0001 | |
| North America | 27 (11.1) | 478 (15.6) | 1471 (21.8) | 1215 (30.7) | 820 (46.4) | 4011 (25.4) | <0.0001 | |
| South America | 10 (4.1) | 206 (6.7) | 575 (8.5) | 304 (7.7) | 101 (5.7) | 1196 (7.6) | <0.0001 | |
| Western Europe | 29 (11.9) | 688 (22.5) | 1782 (26.4) | 1069 (27.0) | 397 (22.5) | 3965 (25.1) | <0.0001 | |
| BMI | Median (Q1–Q3) | 19.0 (18.2–19.5) | 23.5 (22.4–24.3) | 27.5 (26.2–28.7) | 31.9 (30.9–33.2) | 37.6 (36.1–40.3) | 28.3 (25.5–31.7) | <0.0001 |
| Weight, kg | Median (Q1–Q3) | 52.0 (47.0–55.3) | 66.0 (60.0–72.0) | 80.0 (73.1–86.0) | 93.5 (86.0–100.5) | 110.2 (101.0–121.0) | 82.0 (71.6–94.0) | <0.0001 |
| Waist/Hip ratio at randomization, n (%) | Level 1: Males: ≤0.90, Females: ≤0.83 | 118 (48.4) | 861 (28.1) | 717 (10.6) | 190 (4.8) | 71 (4.0) | 1957 (12.4) | <0.0001 |
| Level 2: Men: >0.90 to ≤0.95; Women: >0.83 to ≤0.9 | 63 (25.8) | 987 (32.3) | 1879 (27.8) | 712 (18.0) | 257 (14.5) | 3898 (24.7) | <0.0001 | |
| Level 3: Men: >0.95; Women: >0.90 | 62 (25.4) | 1183 (38.7) | 4085 (60.5) | 3003 (75.8) | 1419 (80.3) | 9752 (61.8) | <0.0001 | |
| Physical activity MET h/wk | Median (Q1–Q3) | 28.0 (14.0–52.0) | 40.0 (18.0–70.0) | 42.0 (20.0–76.0) | 40.0 (20.0–74.0) | 32.0 (14.0–66.0) | 40.0 (18.0–72.0) | <0.0001 |
| Diabetes, n (%) | 58 (23.8) | 848 (27.7) | 2326 (34.4) | 1814 (45.8) | 1078 (61.0) | 6124 (38.8) | <0.0001 | |
| Smoking status, n (%) | Never smoked | 88 (36.1) | 1060 (34.6) | 2104 (31.2) | 1124 (28.4) | 501 (28.3) | 4877 (30.9) | <0.0001 |
| Current smoker | 66 (27.0) | 628 (20.5) | 1255 (18.6) | 650 (16.4) | 255 (14.4) | 2854 (18.1) | <0.0001 | |
| Former smoker | 90 (36.9%) | 1372 (44.8%) | 3392 (50.2%) | 2187 (55.2%) | 1012 (57.2%) | 8053 (51.0%) | <0.0001 | |
| Systolic blood pressure (mm Hg) | Median (Q1–Q3) | 125.5 (112.0–141.0) | 129.0 (117.0–141.0) | 131.0 (121.0–142.0) | 132.0 (122.0–143.0) | 132.0 (122.0–144.0) | 131.0 (120.0–142.0) | <0.0001 |
| Diastolic blood pressure (mm Hg) | Median (Q1–Q3) | 75.0 (66.0–84.0) | 76.0 (69.0–84.0) | 79.0 (72.0–86.0) | 80.0 (73.0–86.0) | 80.0 (73.0–87.0) | 79.0 (72.0–85.0) | <0.0001 |
| Diagnosis of hypertension, n (%) | 132 (54.1) | 1861 (60.8) | 4722 (69.9) | 3052 (77.1) | 1517 (85.8) | 11 284 (71.5) | <0.0001 | |
| Prior MI, n (%) | 169 (69.3) | 1823 (59.6) | 4028 (59.7) | 2329 (58.8) | 947 (53.6) | 9296 (58.9) | <0.0001 | |
| Prior PCI or CABG, n (%) | 148 (60.7) | 2272 (74.2) | 5064 (75.0) | 2968 (74.9) | 1380 (78.1) | 11 832 (75.0) | <0.0001 | |
| Multivessel CHD, n (%) | 45 (18.4) | 447 (14.6) | 1028 (15.2) | 582 (14.7) | 276 (15.6) | 2378 (15.1) | 0.4554 | |
| Time from CHD event to randomization, n (%) | Remote | 172 (70.5) | 2172 (71.0) | 5093 (75.4) | 3114 (78.6) | 1393 (78.8) | 11 944 (75.7) | <0.0001 |
| Recent | 72 (29.5) | 879 (28.7) | 1647 (24.4) | 838 (21.2) | 368 (20.8) | 3804 (24.1) | <0.0001 | |
| Family history of premature CHD, n (%) | 35 (14.4) | 615 (20.1) | 1654 (24.6) | 1119 (28.3) | 627 (35.5) | 4050 (25.7) | <0.0001 | |
| Polyvascular disease, n (%) | 35 (14.3) | 444 (14.5) | 946 (14.0) | 651 (16.4) | 289 (16.3) | 2365 (15.0) | 0.0052 | |
| NYHA class, n (%) | Class I | 27 (42.2) | 277 (43.1) | 516 (34.5) | 297 (28.8) | 116 (24.6) | 1233 (33.3) | <0.0001 |
| Class II | 24 (37.5) | 302 (47.0) | 835 (55.8) | 617 (59.8) | 276 (58.6) | 2054 (55.4) | <0.0001 | |
| Class III | 7 (10.9) | 48 (7.5) | 87 (5.8) | 72 (7.0) | 61 (13.0) | 275 (7.4) | <0.0001 | |
| Class IV | 6 (9.4) | 16 (2.5) | 58 (3.9) | 45 (4.4) | 18 (3.8) | 143 (3.9) | <0.0001 | |
| COPD or asthma, n (%) | 26 (10.8) | 258 (8.5) | 566 (8.5) | 410 (10.5) | 276 (15.8) | 1536 (9.8) | <0.0001 | |
| Aspirin at randomization, n (%) | 219 (89.8) | 2824 (92.3) | 6267 (92.8) | 3662 (92.5) | 1615 (91.3) | 14 587 (92.4) | 0.1371 | |
| ACE inhibitor or ARB at randomization, n (%) | 170 (69.7) | 2150 (70.3) | 5148 (76.2) | 3218 (81.2) | 1486 (84.0) | 12 172 (77.1) | <0.0001 | |
| Statin at randomization, n (%) | 235 (96.3) | 2972 (97.1) | 6604 (97.8) | 3836 (96.8) | 1710 (96.7) | 15 357 (97.3) | 0.0102 | |
| Beta blocker at randomization, n (%) | 172 (70.5) | 2230 (72.9) | 5340 (79.1) | 3258 (82.3) | 1479 (83.7) | 12 479 (79.1) | <0.0001 | |
| P2Y12 at randomization, n (%) | 110 (45.1) | 1224 (40.0) | 2244 (33.2) | 1217 (30.7) | 592 (33.5) | 5387 (34.1) | <0.0001 | |
| LDL‐C, mmol/L, n (%) | Median (Q1–Q3) | 2.01 (1.52–2.55) | 2.06 (1.61–2.60) | 2.10 (1.65–2.65) | 2.08 (1.62–2.63) | 1.97 (1.55–2.48) | 2.07 (1.62–2.62) | <0.0001 |
| HDL‐C, mmol/L, n (%) | Median (Q1–Q3) | 1.42 (1.13–1.73) | 1.25 (1.05–1.50) | 1.17 (1.00–1.39) | 1.12 (0.96– 1.31) | 1.09 (0.94–1.25) | 1.15 (1.00–1.38) | <0.0001 |
| Triglycerides, mmol/L, n (%) | Median (Q1–Q3) | 1.11 (0.86–1.42) | 1.29 (0.96–1.78) | 1.49 (1.09–2.06) | 1.68 (1.24–2.34) | 1.80 (1.34–2.52) | 1.52 (1.10–2.13) | <0.0001 |
| eGFR (CKD‐EPI) | Median (Q1–Q3) | 73.7 (59.5–89.0) | 76.0 (62.9–88.5) | 74.3 (61.5–86.2) | 73.9 (61.8–86.5) | 73.4 (59.8–86.7) | 74.4 (61.7–86.7) | <0.0001 |
| Creatinine, µmol/L | Median (Q1–Q3) | 87.0 (73.5–104.5) | 88.0 (78.0–100.0) | 89.0 (80.0–105.0) | 90.0 (80.0–105.0) | 90.0 (80.0–106.0) | 89.0 (80.0–105.0) | <0.0001 |
| Significant renal dysfunction, n (%) | 73 (29.9) | 795 (26.0) | 1959 (29.0) | 1257 (31.7) | 682 (38.6) | 4766 (30.2) | <0.0001 | |
| Hemoglobin, g/L | Median (Q1–Q3) | 135.0 (123.0–146.0) | 141.0 (132.0–150.0) | 144.0 (135.0–152.0) | 145.0 (135.0–153.0) | 142.0 (132.0–151.0) | 144.0 (134.0–152.0) | <0.0001 |
| WBC, GI/L | Median (Q1–Q3) | 6.55 (5.40–7.80) | 6.40 (5.30–7.70) | 6.50 (5.50–7.70) | 6.70 (5.70–7.90) | 6.80 (5.70–8.10) | 6.60 (5.50–7.80) | <0.0001 |
| hsCRP, mg/L | Median (Q1–Q3) | 0.90 (0.40–2.60) | 0.90 (0.40–2.20) | 1.20 (0.60–2.60) | 1.70 (0.80–3.60) | 2.50 (1.20–4.90) | 1.30 (0.60–3.10) | <0.0001 |
| hsTroponin T, ng/L | Median (Q1–Q3) | 9.2 (6.0–15.3) | 8.6 (5.8–13.0) | 9.0 (6.1–13.8) | 9.5 (6.4–14.6) | 10.6 (7.0–16.8) | 9.3 (6.2–14.2) | <0.0001 |
| NT‐proBNP, ng/L | Median (Q1–Q3) | 346 (143–857) | 194 (93–443) | 172 (83–376) | 160 (77–329) | 172 (75–356) | 173 (83–379) | <0.0001 |
| Interleukin ‐6, ng/L | Median (Q1–Q3) | 2.10 (1.30–4.05) | 1.80 (1.20–2.90) | 2.00 (1.40–3.00) | 2.20 (1.50–3.30) | 2.70 (1.90–3.90) | 2.10 (1.40–3.20) | <0.0001 |
| Cystatin C, mg/L | Median (Q1–Q3) | 1.12 (0.94–1.35) | 0.99 (0.86–1.16) | 0.99 (0.86–1.16) | 0.98 (0.86–1.16) | 1.04 (0.90–1.25) | 0.99 (0.86–1.18) | <0.0001 |
| GDF‐15, ng/L | Median (Q1–Q3) | 1573 (1044–2435) |
1271 (935–1847) |
1235 (898–1778) |
1208 (886–1781) |
1359 (982–1966) |
1253 (914–1826) |
<0.0001 |
| LpPLA2 activity, µmol/min per L | Median (Q1–Q3) | 164.3 (131.6–197.0) | 169.8 (137.9–203.5) | 173.4 (145.3–204.5) | 173.5 (145.4–205.8) | 171.3 (142.0–202.4) | 172.5 (143.1–204.3) | <0.0001 |
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafting; CDK‐EPI, Chronic Kidney Disease Epidemiology Collaboration; CHD, coronary heart disease; eGFR, estimated glomerular filtration rate; GDF‐15, growth differentiation factor 15; HDL‐C, high‐density lipoprotein cholesterol; hsCRP, high‐sensitive C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; LpPLA2, lipoprotein‐associated phospholipase A2; MET h, Metabolic Equivalents of Task, hours per week; MI, myocardial infarction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PCI, percutaneous coronary intervention; Q, quartile; and WBC, white blood cell.
Percentages calculated with patients with data as denominator. P value calculated for difference between groups.
Figure 1. Levels of biomarkers (interleukin ‐6, GDF‐15, Troponin T and NT‐proBNP) in relation to categories of BMI.
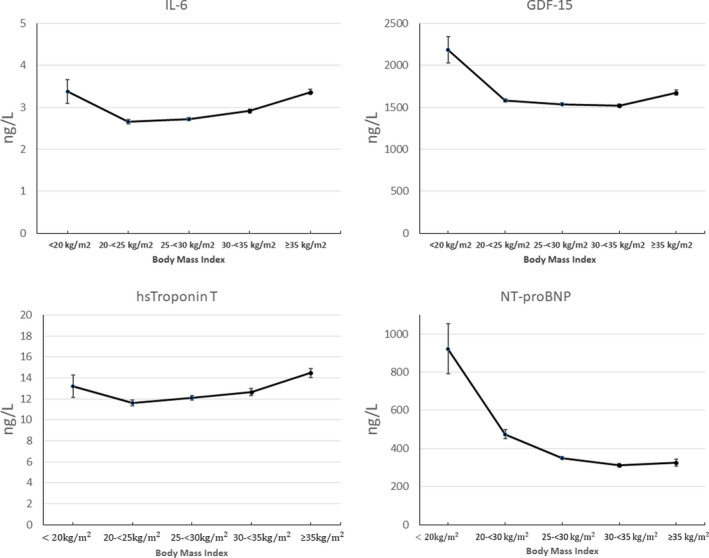
BMI indicates body mass index; GDF‐15, growth differentiation factor 15; hsTroponin T, high‐sensitivity cardiac troponin T; IL‐6, interleukin 6; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
BMI and Association With Clinical Outcomes
The associations between BMI and risk of major adverse cardiovascular event, total death, cardiovascular death, and heart failure hospitalization are presented in Table 2 and Figure 2. For cardiovascular and total mortality and heart failure, the event rates (Table 2) were lowest in the BMI category of 25 to <30 kg/m2. Spline plots for clinical outcomes (cardiovascular and total mortality, heart failure, MI, and stroke) showed the lowest risk at a BMI of around 27 kg/m2 (Figure 2A). Spline plots were also created for the Asian and non‐Asian populations, separately (Figures 2B and 2C). The U‐shaped curve was more pronounced in the Asian population than in the non‐Asian population. The HR and 95% CI for total and cardiovascular death and for hospitalization for heart failure are presented in Figure S1A and S1B and Figure 2A and 2B), in the respective populations. In the Asian population, the risk of cardiovascular death was significantly higher among those with the lowest BMI (<20 kg/m2; HR, 2.87; 95% CI, 1.56–5.26), and with a BMI of >35 kg/m2 (HR, 3.35; 95% CI, 1.36–8.26). The corresponding risks for heart failure hospitalization were HR of 3.02 (95% CI, 1.33–6.85) and HR of 3.23 (95% CI, 1.13–9.24), respectively.
Table 2.
Clinical Outcomes During Follow‐up by BMI Category
| Clinical outcome, n (%) |
<20 kg/m2 N=244 |
20 to <25 kg/m2 N=3060 |
25 to <30 kg/m2 N=6752 |
30 to <35 kg/m2 N=3961 |
≥35 kg/m2 N=1768 |
Total N=15 785 |
P value |
|---|---|---|---|---|---|---|---|
| MACE (cardiovascular death, MI and stroke) | 34 (13.9) | 298 (9.7) | 658 (9.7) | 386 (9.7) | 205 (11.6) | 1581 (10.0) | 0.0375 |
| All‐cause death | 37 (15.2) | 242 (7.9) | 465 (6.9) | 260 (6.6) | 146 (8.3) | 1150 (7.3) | <0.0001 |
| Cardiovascular death | 24 (9.8) | 157 (5.1) | 287 (4.3) | 171 (4.3) | 90 (5.1) | 729 (4.6) | 0.0004 |
| Hospitalization for heart failure | 12 (4.9) | 45 (1.5) | 135 (2.0) | 91 (2.3) | 64 (3.6) | 347 (2.2) | <0.0001 |
| MI | 11 (4.5) | 127 (4.2) | 310 (4.6) | 210 (5.3) | 105 (5.9) | 763 (4.8) | 0.0314 |
| Major coronary event | 32 (13.1) | 273 (8.9) | 635 (9.4) | 405 (10.2) | 200 (11.3) | 1545 (9.8) | 0.0153 |
| Stroke | 5 (2.0) | 58 (1.9) | 145 (2.1) | 61 (1.5) | 35 (2.0) | 304 (1.9) | 0.2935 |
| Cancer during the study | 11 (4.5) | 189 (6.2) | 491 (7.3) | 272 (6.9) | 143 (8.1) | 1106 (7.0) | 0.0478 |
BMI indicates body mass index; MACE, major cardiovascular event; and MI, myocardial infarction.
P value calculated for difference between groups.
Figure 2. Spline plot shows rates of major cardiovascular event (MACE), cardiovascular (CV) death, myocardial infarction (MI), stroke, total death, hospitalization for heart failure by body mass index (BMI).
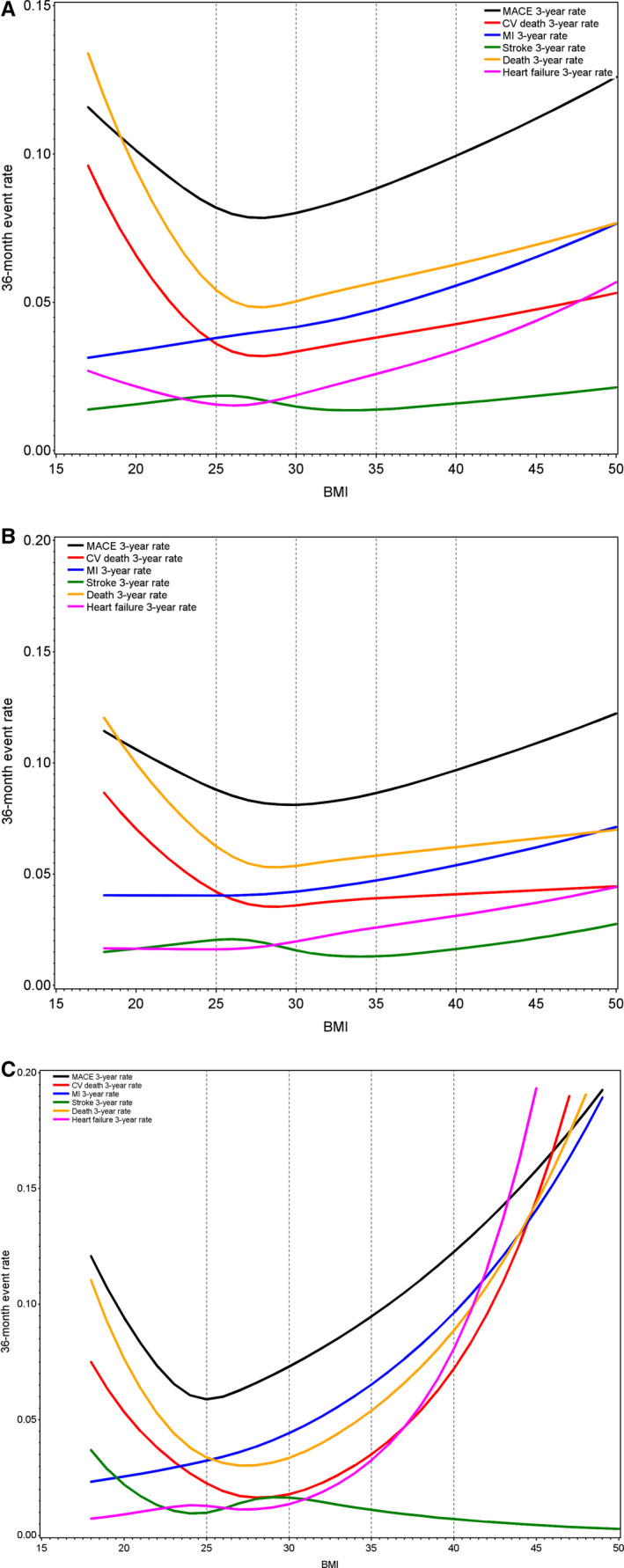
(A) Total population. (B) Non‐Asian population. (C) Asian population.
The associations between BMI and clinical outcomes in all patients (basic adjustment model) are shown in Figure 3A. After multivariable adjustments, as shown in Figure 3B, there remained a significantly increased risk of cardiovascular death in patients with a BMI of 20 to <25 kg/m2 (HR, 1.26; 95% CI, 1.03–1.54) with a BMI of 25 to <30 kg/m2 as reference, and the risk was more than doubled in those with very low BMI (<20 kg/m2; HR, 2.26; 95% CI, 1.46–3.49). However, the risk of cardiovascular death in patients with a BMI of 30 to <35 kg/m2 and ≥35 kg/m2, respectively, was numerically higher but not statistically significant.
Figure 3. The association of body mass index (BMI) with clinical outcomes.
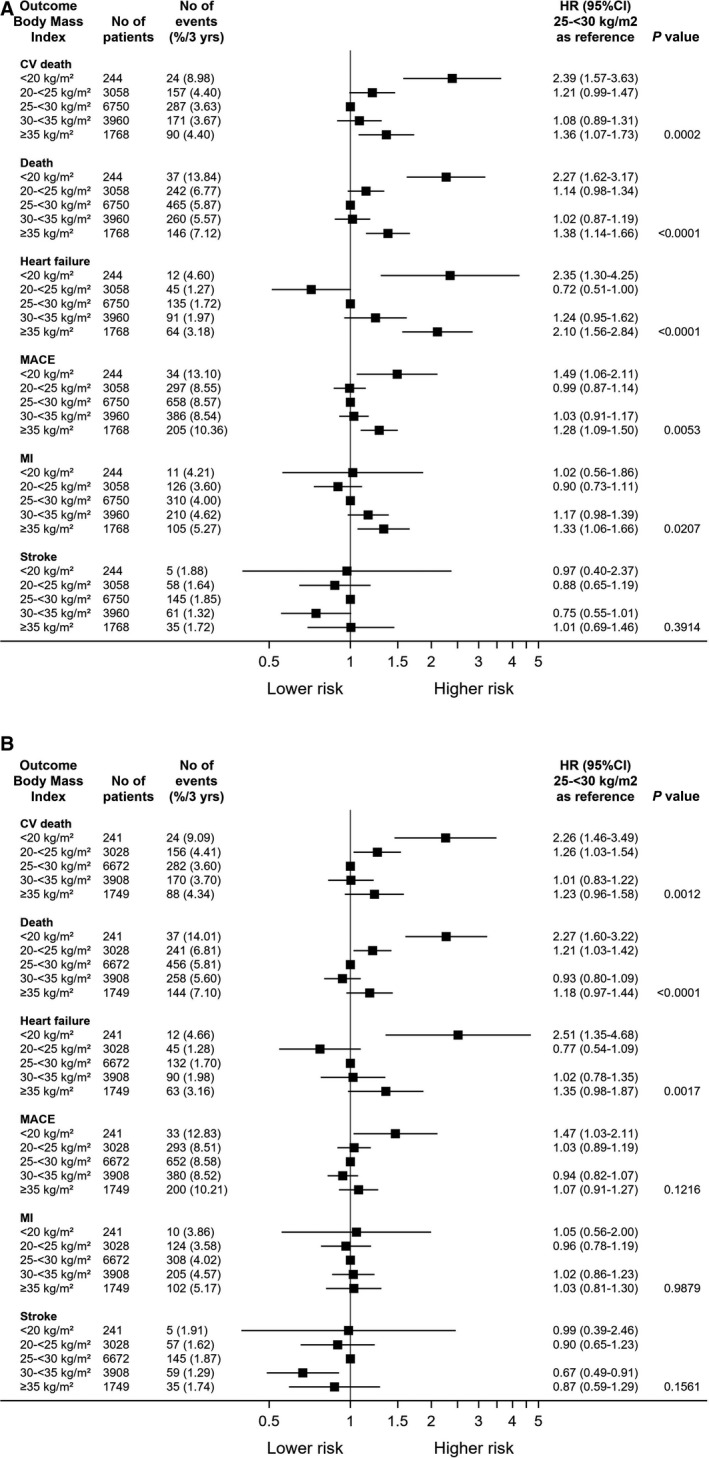
(A) Basic adjustment model. (B) Fully adjusted. (A) BMI 25 < 30 kg/m2 as reference. Adjusted for age, sex, and randomized treatment. P value denotes difference between groups. (B) BMI of 25 < 30 kg/m2 as reference. Adjusted for age, sex, and randomized treatment, prior percutaneous coronary intervention (PCI)/coronary artery bypass grafting (CABG), prior MI, renal dysfunction, polyvascular disease, diabetes, smoking, stroke/transient ischemic attack (TIA), congestive heart failure, systolic blood pressure, geographic region, chronic obstructive pulmonary disease (COPD), cancer diagnosis and Asian/Japanese origin. P value denotes difference between groups. CV indicates cardiovascular; MACE, major adverse cardiovascular event; and MI, myocardial infarction.
Similarly, total death was increased among patients with very low BMI (<20 kg/m2) (HR, 2.27; 95% CI, 1.60–3.22) but not for those with higher BMI categories. For heart failure hospitalization, the risk among patients with very low BMI was increased with HR of 2.51 (95% CI, 1.35–4.68) but not significantly increased with higher BMI categories. For patients with very high BMI (≥35 kg/m2), the adjusted risk of heart failure hospitalization did not reach statistical significance (HR, 1.35; 95% CI, 0.98–1.87). The risk of the composite major adverse cardiovascular event (cardiovascular death, MI, and stroke) was not significantly associated (P=0.11) with BMI, indicating that the driver of major adverse cardiovascular events was mainly cardiovascular death both in the low and high ranges of BMI. For the risk of MI, there was no significant association with BMI. Consistently, there was no association with BMI with the risk of stroke.
Subgroup and Sensitivity Analyses
Several prespecified subgroups, such as age, sex, diabetes, and type of MI were analyzed. Similar findings with a U‐shaped curve were found also in these subgroups (data not shown). The curves were consistent with the main study results, and there was no significant interaction with age, sex, diabetes, or type of MI (type 1 versus types 2–5). A sensitivity analysis of patients after exclusion of patients with low BMI (<20 kg/m2) showed consistent results with a U‐shaped risk curve as with the main analysis. Figure 2A and 2B show that the association between BMI and outcomes were more pronounced in both the low and high end of BMI in the Asian subgroup and the risk curves among non‐Asians were flatter in the higher end of BMI range. The HR and 95% CI are shown in Figure S1A and S1B.
DISCUSSION
In this contemporary study of patients with stable CHD, high levels of standard of care and a long‐term follow‐up of 3.7 years, we report the associations between BMI and clinical cardiovascular outcomes. The main findings from these analyses were that (1) almost 75% of the patients were overweight or obese and (2) the risk curve for cardiovascular death, total death, and hospitalization for heart failure was U‐shaped, with the highest risk in the low and high ends of BMI. The lowest risk was observed in patients with a BMI of 27 kg/m2. Of note, this is considered as overweight in most secondary prevention guidelines. 22 In contrast, for patients with underweight (<20 kg/m2), the risk of cardiovascular and total death was more than doubled. In addition, we could not confirm the appearance of an overweight paradox in our study, with lower risk in the higher BMI levels, as has been shown for patients with other cardiovascular conditions. 5 , 7 , 23 , 24 , 25 Of note, international guidelines on prevention 22 recommend a target BMI of 20 to <25 kg/m2. Our results do not provide firm support for this interval as being optimal, but suggests that a slightly higher BMI may be optimal with respect to risk of clinical outcomes. Although speculative, a slightly higher BMI than recommended might indicate greater reserves when developing CHD, which may be beneficial. The ideal BMI in terms of risk of cardiovascular events in patients with stable CHD cannot be established on the basis of our study. However, our data could be interpreted as maintaining a BMI in the range of what is considered as slightly overweight appears to be optimal for cardiovascular prognosis.
Of note, BMI was not associated with the risk of either MI or stroke, after multivariable adjustments, but rather to the risk of mortality and heart failure hospitalization. The reasons behind this lack of association are unknown and cannot be explained from this study. Our findings are corroborated by a recent study in patients with diabetes and prediabetes, in which similar associations were reported. 26 In contrast, in the INTERHEART study, 3 a cross‐sectional case‐control study, obesity assessed using BMI was associated with the risk of MI.
Many of the established risk markers for cardiovascular death were associated with increased BMI, as could be expected. In contrast, the levels of several biomarkers of special interest in terms of risk, 27 often related to mortality in other studies, such as interleukin‐6, cystatin C, and GDF‐15 showed a U‐shaped association with BMI. However, NT‐proBNP levels were highest in those with the lowest BMI and then increased, indicating that underlying explanations for the associations may differ in the low and high BMI range, respectively.
U‐Shaped Curve Across Different Events
The U‐shaped association between BMI and long‐term risk of death and heart failure hospitalization is in contrast to others that have not confirmed such a relationship. 3 , 6 In a recent study 28 in elderly patients >80 years undergoing percutaneous coronary intervention, mortality was highest in the lowest BMI tertile in acute coronary syndromes, but this association was not confirmed in patients with stable CHD. Our study evaluated BMI with clinical outcomes, both as a continuous marker and based on clinically established categories, and clearly show an increased risk in patients with a BMI of 30 to <35 kg/m2. We performed several subgroup analyses but could not confirm any interaction for either sex, age, diabetes, or type of MI. The results were consistent across all prespecified subgroups. We saw a different risk pattern when analyzing the Asian and non‐Asian population separately. Asians have a different body composition and different risk levels of obesity for developing diabetes compared with other ethnicities. Other BMI cutoffs for obesity have thus been proposed by the World Health Organization. 29 Applying the lower BMI cutoff values for overweight would lead to a higher proportion of Asians considered overweight in our study. The spline curves were clearly steeper and more pronounced in the Asian population in both the low and high end of BMI. In contrast, for non‐Asians, the risk of death and heart failure hospitalization increased similarly in the low BMI region but increased only marginally in the high BMI categories.
Patients who are underweight and have very low BMI is an important group that deserves special focus. They often have comorbidities, such as current smoking, severe heart failure, chronic obstructive pulmonary disease, malignancy, chronic kidney disease, or other severe diseases. Indeed, there were more smokers among the underweight group who also are at higher risk of smoking‐related diseases such as chronic obstructive pulmonary disease. Thus, a possibility of collider bias may exist. Levels of NT‐proBNP were higher in those who were underweight, indicating that heart failure with cachexia was more prevalent. Thus, a BMI of <20 kg/m2 may be considered a proxy for other concomitant diseases and not seldom poor long‐term prognosis. The results remained consistent despite adjustments for all known such factors, although hidden or unmeasured confounding may still partly explain some of the elevated risk in these patients. Our results stress the importance of a thorough diagnosing and treatment of patients who are underweight.
We chose to study the associations between BMI and clinical outcomes as a prespecified substudy. This information was easily available in this large global study. However, measures other than BMI may be considered more accurate, such as detailed measures of body composition, that is, waist‐to‐height ratio suggested by others 30 or truncal adiposity. 31 Also, biomarkers such as triglycerides to high‐density lipoprotein‐cholesterol ratio 32 and hypertriglyceridemic waist phenotype 33 has been suggested as a marker of increased atherosclerosis related risk. We also assessed waist circumference, which was strongly correlated with BMI and the associations to outcome were consistent with what was observed for BMI.
Limitations/Strengths
The strengths of the current study are that it is a large, prospective, randomized trial from 39 countries on a homogeneous cohort of patients with stable CHD with high‐quality long‐term follow‐up of centrally adjudicated end points. There are some limitations. Despite the general recommendation to all patients to be physically active and reduce weight, our results are based on a single BMI baseline assessment, and we cannot provide data on variation over time, which has been shown by others to be associated with increased mortality and cardiovascular events. 34 However, patients were on a high standard of a modern secondary prevention regimen during follow‐up including medication, smoking cessation, and advice on weight loss and regular physical exercise were provided. Despite performing multivariable adjustments in the analyses, unmeasured confounding cannot be fully excluded. Our results are observational and the associations are based on nonrandomized data. Results may thus not be used to claim any causal relationship. Since the follow‐up time in this study was relatively short, the possibility of reverse causation cannot be excluded. Longer follow‐up studies may be preferable 35 and more optimal, as has been shown in studies on a general population.
In conclusion, our study of a large prospective cohort of patients with stable CHD did not confirm an overweight paradox for cardiovascular clinical outcomes. After multivariable adjustments, the association between BMI and the risk of cardiovascular and total death as well as for hospitalization for heart failure had a U‐shaped curve. Of note, the lowest risk was seen in patients with a BMI of around 27 kg/m2, somewhat higher and in contrast to what is currently recommended in prevention guidelines. The highest risk was found among patients with very low BMI, indicating a specific high‐risk group of note, as well as in patients with very high BMI. It is recommended to put special focus on these extreme groups to determine the underlying pathology and optimize treatment. Explanatory factors driving these differences may differ.
Sources of Funding
The STABILITY trial was supported by GlaxoSmithKline.
Disclosures
CH reports institutional research grants from GlaxoSmithKline. Honoraria and research grants from Pfizer; consultant and advisory board fees from AstraZeneca, Bayer, Boehringer Ingelheim, Coala Life. NH reports institutional research grant from GlaxoSmithKline. PEA reports research grants from GlaxoSmithKline; and research grants, honoraria, and lecture fees from AstraZeneca, Sanofi, Amgen, CSL Behring, Boehringer Ingelheim, and Bayer. EH reports speaker fees and limited research grants from Sanofi and Amgen; expert committee fees from NovoNordisk. JSH reports a grant from the National Heart, Lung, and Blood Institute; support for drug distribution from AstraZeneca Pharmaceuticals LLC and Arbor Pharmaceuticals LLC; in‐kind donations for participating sites from Abbott Vascular, Medtronic Inc, St. Jude Medical Inc, Volcano Corp, Merck Sharp & Dohme Corp, Omron Healthcare Inc, and Amgen Inc. RAHS reports research grants from GlaxoSmithKline. HDW reports grants from GlaxoSmithKline; grants and steering committee fees from Eli Lilly and Company, Omthera Pharmaceuticals, Eisai Inc., Dalcor Pharma UK, American Regent; grants and steering committee and advisory board fees from CSL Behring LLC; grants, steering committee, and personal fees from Sanofi‐Aventis Australia Pty Ltd, Esperion Therapeutics Inc., Sanofi‐Aventis; advisory board fees from Genentech, Inc.; and personal fees from AstraZeneca. LW reports institutional research grants from AstraZeneca, Bristol‐Myers Squibb/Pfizer, GlaxoSmithKline, Merck & Co, Roche Diagnostics, Boehringer Ingelheim; and holds 2 patents involving GDF‐15, both licensed to Roche Diagnostics.
Supporting information
Figure S1
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023667
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Kushner RF, Kahan S. Introduction: the state of obesity in 2017. Med Clin North Am. 2018;102:1–11. doi: 10.1016/j.mcna.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 2. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case‐control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5 [DOI] [PubMed] [Google Scholar]
- 4. Pi‐Sunyer FX. The obesity epidemic: pathophysiology and consequences of obesity. Obes Res. 2002;10(Suppl 2):97S–104S. doi: 10.1038/oby.2002.202 [DOI] [PubMed] [Google Scholar]
- 5. Dhoot J, Tariq S, Erande A, Amin A, Patel P, Malik S. Effect of morbid obesity on in‐hospital mortality and coronary revascularization outcomes after acute myocardial infarction in the United States. Am J Cardiol. 2013;111:1104–1110. doi: 10.1016/j.amjcard.2012.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diercks DB, Roe MT, Mulgund J, Pollack CV Jr, Kirk JD, Gibler WB, Ohman EM, Smith SC Jr, Boden WE, Peterson ED. The obesity paradox in non‐ST‐segment elevation acute coronary syndromes: results from the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the American College of Cardiology/American Heart Association Guidelines Quality Improvement Initiative. Am Heart J. 2006;152:140–148. doi: 10.1016/j.ahj.2005.09.024 [DOI] [PubMed] [Google Scholar]
- 7. Wienbergen H, Gitt AK, Juenger C, Schiele R, Heer T, Towae F, Gohlke H, Senges J. MITRA PLUS study group. Impact of the body mass index on occurrence and outcome of acute ST‐elevation myocardial infarction. Clin Res Cardiol. 2008;97:83–88. doi: 10.1007/s00392-007-0585-x [DOI] [PubMed] [Google Scholar]
- 8. Sandhu RK, Ezekowitz J, Andersson U, Alexander JH, Granger CB, Halvorsen S, Hanna M, Hijazi Z, Jansky P, Lopes RD, et al. The “obesity paradox” in atrial fibrillation: observations from the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial. Eur Heart J. 2016;37:2869–2878. doi: 10.1093/eurheartj/ehw124 [DOI] [PubMed] [Google Scholar]
- 9. Pagidipati NJ, Zheng Y, Green JB, McGuire DK, Mentz RJ, Shah S, Aschner P, Delibasi T, Rodbard HW, Westerhout CM, et al. Association of obesity with cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease: Insights from TECOS. Am Heart J. 2020;219:47–57. doi: 10.1016/j.ahj.2019.09.016 [DOI] [PubMed] [Google Scholar]
- 10. Gruberg L, Weissman NJ, Waksman R, Fuchs S, Deible R, Pinnow EE, Ahmed LM, Kent KM, Pichard AD, Suddath WO, et al. The impact of obesity on the short‐term and long‐term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol. 2002;39:578–584. doi: 10.1016/S0735-1097(01)01802-2 [DOI] [PubMed] [Google Scholar]
- 11. Lavie CJ, De Schutter A, Parto P, Jahangir E, Kokkinos P, Ortega FB, Arena R, Milani RV. Obesity and prevalence of cardiovascular diseases and prognosis‐the obesity paradox updated. Prog Cardiovasc Dis. 2016;58:537–547. doi: 10.1016/j.pcad.2016.01.008 [DOI] [PubMed] [Google Scholar]
- 12. Younis A, Younis A, Goldkorn R, Goldenberg I, Peled Y, Tzur B, Klempfner R. The association of body mass index and 20‐year all‐cause mortality among patients with stable coronary artery disease. Heart Lung Circ. 2019;28:719–726. doi: 10.1016/j.hlc.2018.02.015 [DOI] [PubMed] [Google Scholar]
- 13. White HD, Stewart RAH, Dalby AJ, Stebbins A, Cannon CP, Budaj A, Linhart A, Pais P, Diaz R, Steg PG, et al. In patients with stable coronary heart disease, low‐density lipoprotein‐cholesterol levels < 70 mg/dL and glycosylated hemoglobin A1c <7% are associated with lower major cardiovascular events. Am Heart J. 2020;225:97–107. doi: 10.1016/j.ahj.2020.04.004 [DOI] [PubMed] [Google Scholar]
- 14. Investigators STABILITY , White HD, Held C, Stewart R, Tarka E, Brown R, Davies RY, Budaj A, Harrington RA, Steg PG, et al. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med. 2014;370:1702–1711. doi: 10.1056/NEJMoa1315878 [DOI] [PubMed] [Google Scholar]
- 15. Vedin O, Hagström E, Stewart R, Brown R, Krug‐Gourley S, Davies R, Wallentin L, White H, Held C. Secondary prevention and risk factor target achievement in a global, high‐risk population with established coronary heart disease: baseline results from the STABILITY study. Eur J Prev Cardiol. 2013;20:678–685. doi: 10.1177/2047487312444995 [DOI] [PubMed] [Google Scholar]
- 16. Hagström E, Held C, Stewart RAH, Aylward PE, Budaj A, Cannon CP, Koenig W, Krug‐Gourley S, Mohler ER, Steg PG, et al. Growth differentiation factor 15 predicts all‐cause morbidity and mortality in stable coronary heart disease. Clin Chem. 2017;63:325–333. doi: 10.1373/clinchem.2016.260570 [DOI] [PubMed] [Google Scholar]
- 17. Lindholm D, Lindbäck J, Armstrong PW, Budaj A, Cannon CP, Granger CB, Hagström E, Held C, Koenig W, Östlund O, et al. Biomarker‐based risk model to predict cardiovascular mortality in patients with stable coronary disease. J Am Coll Cardiol. 2017;70:813–826. doi: 10.1016/j.jacc.2017.06.030 [DOI] [PubMed] [Google Scholar]
- 18. Wallentin L, Held C, Armstrong PW, Cannon CP, Davies RY, Granger CB, Hagström E, Harrington RA, Hochman JS, Koenig W, et al. Lipoprotein‐associated phospholipase A2 activity is a marker of risk but not a useful target for treatment in patients with stable coronary heart disease. J Am Heart Assoc. 2016;5:e003407. doi: 10.1161/JAHA.116.003407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. White H, Held C, Stewart R, Watson D, Harrington R, Budaj A, Steg PG, Cannon CP, Krug‐Gourley S, Wittes J, et al. Study design and rationale for the clinical outcomes of the STABILITY Trial (STabilization of Atherosclerotic plaque By Initiation of darapLadIb TherapY) comparing darapladib versus placebo in patients with coronary heart disease. Am Heart J. 2010;160:655–661. doi: 10.1016/j.ahj.2010.07.006 [DOI] [PubMed] [Google Scholar]
- 20. White HD, Held C, Stewart R, Tarka E, Brown R, Davies RY, Budaj A, Harrington RA, Steg PG, Ardissino D, et al. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med. 2014;370:1702–1711. doi: 10.1056/NEJMoa1315878 [DOI] [PubMed] [Google Scholar]
- 21. Held C, White HD, Stewart RAH, Budaj A, Cannon CP, Hochman JS, Koenig W, Siegbahn A, Steg PG, Soffer J, et al. Inflammatory biomarkers interleukin‐6 and C‐reactive protein and outcomes in stable coronary heart disease: experiences from the STABILITY (Stabilization of atherosclerotic plaque by initiation of Darapladib Therapy) trial. J Am Heart Assoc. 2017;6:e005077. doi: 10.1161/JAHA.116.005077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney M‐T, Corrà U, Cosyns B, Deaton C, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Romero‐Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, Mookadam F, Lopez‐Jimenez F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–678. doi: 10.1016/S0140-6736(06)69251-9 [DOI] [PubMed] [Google Scholar]
- 24. Minutello RM, Chou ET, Hong MK, Bergman G, Parikh M, Iacovone F, Wong SC. Impact of body mass index on in‐hospital outcomes following percutaneous coronary intervention (report from the New York State Angioplasty Registry). Am J Cardiol. 2004;93:1229–1232. doi: 10.1016/j.amjcard.2004.01.065 [DOI] [PubMed] [Google Scholar]
- 25. Timóteo AT, Ramos R, Toste A, Oliveira JA, Ferreira ML, Ferreira RC. Impact of body mass index in the results after primary angioplasty in patients with ST segment elevation acute myocardial infarction. Acute Card Care. 2011;13:123–128. doi: 10.3109/17482941.2011.606469 [DOI] [PubMed] [Google Scholar]
- 26. Doehner W, Gerstein HC, Ried J, Jung H, Asbrand C, Hess S, Anker SD. Obesity and weight loss are inversely related to mortality and cardiovascular outcome in prediabetes and type 2 diabetes: data from the ORIGIN trial. Eur Heart J. 2020;41:2668–2677. doi: 10.1093/eurheartj/ehaa293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wallentin L, Eriksson N, Olszowka M, Grammer TB, Hagström E, Held C, Kleber ME, Koenig W, März W, Stewart RAH, et al. Plasma proteins associated with cardiovascular death in patients with chronic coronary heart disease: a retrospective study. PLoS Medicine. 2021;18:e1003513. doi: 10.1371/journal.pmed.1003513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leistner DM, Bazara S, Münch C, Steiner J, Erbay A, Siegrist PT, Skurk C, Lauten A, Müller‐Werdan U, Landmesser U, et al. Association of the body mass index with outcomes in elderly patients (≥80 years) undergoing percutaneous coronary intervention. Int J Cardiol. 2019;292:73–77. doi: 10.1016/j.ijcard.2019.06.044 [DOI] [PubMed] [Google Scholar]
- 29. WHO Expert Consulation . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. [DOI] [PubMed] [Google Scholar]
- 30. Ashwell M, Gunn P, Gibson S. Waist‐to‐height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta‐analysis. Obes Rev. 2012;13:275–286. doi: 10.1111/j.1467-789X.2011.00952.x [DOI] [PubMed] [Google Scholar]
- 31. Gepner Y, Shelef I, Schwarzfuchs D, Zelicha H, Tene L, Yaskolka Meir A, Tsaban G, Cohen N, Bril N, Rein M, et al. Effect of distinct lifestyle interventions on mobilization of fat storage pools: CENTRAL magnetic resonance imaging randomized controlled trial. Circulation. 2018;137:1143–1157. doi: 10.1161/CIRCULATIONAHA.117.030501 [DOI] [PubMed] [Google Scholar]
- 32. Lechner K, Halle M. Are atherogenic lipoprotein phenotype and inflammation indicative of plaque phenotype and clinical stability in coronary artery disease? JAMA Cardiol. 2019;4:950–951. doi: 10.1001/jamacardio.2019.2261 [DOI] [PubMed] [Google Scholar]
- 33. LeBlanc S, Coulombe F, Bertrand OF, Bibeau K, Pibarot P, Marette A, Alméras N, Lemieux I, Després JP, Larose E. Hypertriglyceridemic waist: a simple marker of high‐risk atherosclerosis features associated with excess visceral adiposity/ectopic fat. J Am Heart Assoc. 2018;7:e008139. doi: 10.1161/JAHA.117.008139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bangalore S, Fayyad R, Laskey R, DeMicco DA, Messerli FH, Waters DD. Body‐weight fluctuations and outcomes in coronary disease. N Engl J Med. 2017;376:1332–1340. doi: 10.1056/NEJMoa1606148 [DOI] [PubMed] [Google Scholar]
- 35. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body‐mass index and cause‐specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


