Abstract
Turbot (Scophthalmus maximus), commercially important flatfish species, is widely cultivated in Europe and China. With the continuous expansion of the intensive breeding scale, turbot is exposed to various stresses, which greatly impedes the healthy development of turbot industry. Here, we present an improved high-quality chromosome-scale genome assembly of turbot using a combination of PacBio long-read and Illumina short-read sequencing technologies. The genome assembly spans 538.22 Mb comprising 27 contigs with a contig N50 size of 25.76 Mb. Annotation of the genome assembly identified 104.45 Mb repetitive sequences, 22,442 protein-coding genes and 3,345 ncRNAs. Moreover, a total of 345 stress responsive candidate genes were identified by gene co-expression network analysis based on 14 published stress-related RNA-seq datasets consisting of 165 samples. Significantly improved genome assembly and stress-related candidate gene pool will provide valuable resources for further research on turbot functional genome and stress response mechanism, as well as theoretical support for the development of molecular breeding technology for resistant turbot varieties.
Subject terms: Gene expression profiling, Gene expression, Transcriptomics
| Measurement(s) | whole genome sequencing |
| Technology Type(s) | PacBio long-read and Illumina short-read sequencing technologies |
Background & Summary
Scophthalmus maximus (FishBase ID: 1348), as known as turbot, an economically important flatfish (Pleuronectiformes), is native to Northeast Atlantic throughout the Mediterranean and along the European coasts to Arctic Circle1, and now is the most widely cultivated commercial flatfish around the world with the highest annual aquaculture production1,2. Since its firstly introduction into China in 1992, turbot aquaculture industry has made great progress, leading to the rise of the fourth wave of mariculture industry in China2. However, turbot was affected by various biotic and abiotic stresses during the breeding process, which seriously threatened the healthy development of turbot aquaculture industry and caused huge economic losses. Therefore, carrying out research on the resistance of turbot and obtaining genetic resources related to stress resistance will contribute to the research on the resistance molecular mechanism of turbot and provide theoretical support for the subsequent genetic improvement of turbot germplasm.
In recent years, numerous RNA-seq studies have been conducted to explore the stress responsive genes and molecular mechanisms under various stresses, such as pathogens stress (Enteromyxum scophthalmi3,4, Vibrio anguillarum5), heat stress6, oxygen stress7, crowding stress8, salinity stress9, and feeding stress10. All these researches were solely focused on the identification of differentially expressed genes (DEGs), whereas connectivity analysis has not yet been taken into account. Instead of focusing only on DEGs, gene co-expression network (GCN) analysis provides new insight into the identification of co-expressed gene modules, their correlation with specific traits, and the pinpointing of key hub genes11,12, which cannot be detected by standard transcriptome analysis. This powerful approach has been widely applied to detect diverse stresses response in Nibea albiflora13, Oysters14, Scophthalmus maximus6, etc.
In this study, we reported an improved high-quality chromosome-scale genome assembly of turbot combing PacBio single molecule sequencing technique (SMRT) and Illumina short-read sequencing technologies. Based on this improved genome assembly, we re-annotated the protein-coding genes, repetitive sequences and ncRNAs. In addition, we re-analyzed multiple stress-related RNA-seq datasets from National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) database by gene co-expression network analysis, and identified multiple gene modules and candidate genes response to various stresses in turbot. Taken together, these resources will not only serve as key resources for studying genomics and further research into the stress response mechanisms, but will also promote the progress of genetic improvement and comprehensive stress-resistant molecular breeding of turbot.
Methods
Turbot samples and genome sequencing
Genomic DNA was extracted from the muscle samples of a super-female (WW) turbot using Puregene Tissue Core Kit A (Qiagen, USA) according to the manufacturer’s instruction. The quality of the extracted genomic DNA was checked using electrophoresis on 1% agarose gel and the concentration was quantified using a NanoDrop 2000 to ensure the DNA samples met libraries sequencing requirements.
The extracted DNA molecules were firstly used to construct an Illumina pair-end (PE) library with 350 bp insert size using standard protocols provided by Illumina (San Diego, CA, USA). The PE library was then sequenced using the Illumina HiSeq 4000 platform with 150 bp PE mode according to the manufacturer’s instructions. Finally, a total of 51.80 Gb raw reads, accounting ~90X coverage of whole genome, were generated (Table 1).
Table 1.
Data statistics of whole genome sequencing reads of S. maximus.
| Library Type | Sequencing Platform | Insert Size (bp) | Raw data (Gb) | Sequence coverage (X) |
|---|---|---|---|---|
| Illumina | Illumina HiSeq 4000 | 350 | 51.80 | 90 |
| Pacbio | PacBio Sequel II | 20,000 | 150.30 | 265 |
We also constructed a 20 kb PacBio library following the PacBio manufacturing protocols (Pacific Biosciences, CA, USA) and sequenced it using the PacBio Sequel II platform with the continuous long-read (CLR) mode following the manufacturer’s instruction. In total, we obtained 150.30 Gb (~265X) PacBio long reads (Table 1). The average and N50 lengths of the subreads were 14.13 kb and 25.47 kb, respectively.
Genome assembly
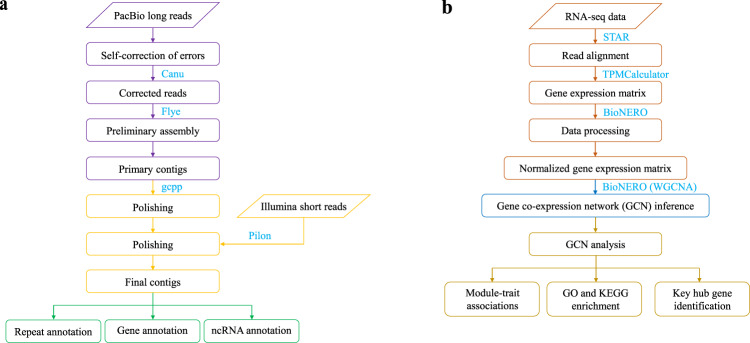
Long reads generated from the PacBio Sequel II platform were firstly processed by a self-correction of errors using Canu15 with default parameters. And then corrected reads were subsequently assembled by Flye (v2.7)16 (--pacbio-corr -- threads 80 --genome-size 568 m). To obtain the final assembly, the draft assembly was removed haplotypic duplication by purge_dups17 and polished by gcpp (https://github.com/PacificBiosciences/gcpp) with default parameters using PacBio data, then Pilon18(--fix bases) was used to further polish the genome using Illumina data (Fig. 1a). Finally, we obtained a new assembled genome of turbot containing 27 contigs with a total length of 538.22 Mb and a contig N50 length of 25.76 Mb, exhibiting higher contiguity and completeness comparable to other published turbot genomes19–21 (Table 2). In addition, GC content of the genome assembly was estimated to be 43.53%.
Fig. 1.
The workflows of genome assembly and gene co-expression network inference used in this study. (a) The genome assembly and annotation pipeline. (b) The gene co-expression network inference and analyses pipeline.
Table 2.
Comparative statistic of the S. maximus genome assembly with old ones.
| Genome assembly | This study | Martínez et al.21 | Xu et al.19 | Figueras et al.20 | |
|---|---|---|---|---|---|
| female | male | ||||
| Scaffold N50 (Mb) | 25.76 | 25.95 | 25.17 | 5.93 | 24.81 |
| Contig N50 (Mb) | 25.76 | 20.47 | 0.028 | 0.045 | 0.054 |
| Total scaffold number | 27 | 127 | 28,256 | 9,724 | 22 |
| Total contig number | 27 | 178 | 65,796 | 36,500 | 21,326 |
| Total length (Mb) | 538.22 | 556.70 | 568.47 | 587.19 | 524.98 |
| GC Content (%) | 43.53 | 43.30 | 43.42 | 43.70 | 43.30 |
Genome annotation
We detected and classified repetitive sequences in the final turbot genome assembly by a combination of homology-based and de novo prediction strategies. In homology-based searching, known repeats were identified using RepeatMasker (V4.1.1)22 based on the RepBase TE library (version 10/26/2018)23. In addition, de novo prediction was conducted using RepeatMasker to further detect novel repeats, which based on the de novo repeats library of the turbot genome constructed with RepeatModeler (http://www.repeatmasker.org/RepeatModeler/) and LTR-FINDER24. Finally, a total of 104.45 Mb of non-redundant repetitive sequences (Combined TEs) were obtained, accounting for 19.41% of the assembled genome (Table 3). Amid predominant repeats, DNA transposons were the most abundant (54.16 Mb), representing 10.06% of the genome, followed by long interspersed elements (LINEs, 3.10%), long terminal repeats (LTRs, 2.95%) and short interspersed nuclear elements (SINEs, 0.51%) (Table 3).
Table 3.
Classified statistics of repeat sequences of S. maximus.
| RepBase TEs | TE Proteins | De novo | Combined TEs | |||||
|---|---|---|---|---|---|---|---|---|
| Length (bp) | % in Genome | Length (bp) | % in Genome | Length (bp) | % in Genome | Length (bp) | % in Genome | |
| DNA | 38,217,303 | 7.10 | 2,321,886 | 0.43 | 23,128,062 | 4.30 | 54,159,141 | 10.06 |
| LINE | 13,026,936 | 2.42 | 6,871,234 | 1.28 | 7,405,321 | 1.38 | 16,693,988 | 3.10 |
| SINE | 2,309,601 | 0.43 | 0 | 0 | 857,212 | 0.16 | 2,740,574 | 0.51 |
| LTR | 11,363,027 | 2.11 | 2,222,887 | 0.41 | 4,790,157 | 0.89 | 15,901,294 | 2.95 |
| Satellite | 2,989,136 | 0.56 | 0 | 0 | 499,041 | 0.09 | 3,462,111 | 0.64 |
| Simple_repeat | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 2,814 | 0 | 135 | 0 | 0 | 0 | 2,949 | 0 |
| Unknown | 537,749 | 0.10 | 13,890 | 0 | 23,176,727 | 4.31 | 23,566,810 | 4.38 |
| Total | 58,685,000 | 10.90 | 11,419,271 | 2.12 | 58,413,629 | 10.85 | 104,452,847 | 19.41 |
Protein-coding gene annotations were then conducted with MAKER (v3.01.03)25 by a combined strategy of homology-based, de novo, and transcriptome-assisted predictions. For homology-based prediction, protein sequences of seven teleost species, Anabas testudineus, Cynoglossus semilaevis, Danio rerio, Gasterosteus aculeatus, Oryzias latipes, Scophthalmus maximux, Takifugu rubripes, were downloaded from Ensembl and NCBI, and mappped to turbot genome using TBLASTN26 (e-value ≤ 1e-5). Exonerate (v2.4.0)27 was used to align homologous protein sequences to turbot genome. Homologous genes were predicted ranging from 35,093 to 48,770 in above species reference sequences (Table 4). For de novo prediction, Augustus28 and Genscan29 were employed to analyze the repeats masked genome, which detected 30,320 and 40,007 genes, respectively (Table 4). For transcriptome-assisted prediction, RNA-seq data (NCBI accession number: SRP261889, SRP273870) were aligned to turbot genome to identify potential gene structures, and 16,356 genes were supported. Finally, we performed MAKER (v3.01.03) to integrate genes generated by above predictions to produce a consensus protein-coding gene set consisting of 22,442 genes with an average gene length of 15,828 bp (Table 4). Comparisons of gene features between turbot and other seven species indicated similar distribution patterns in average length of gene, coding sequence (CDS), exon and intron (Fig. 2).
Table 4.
General statistics of predicted protein-coding genes in S. maximus genome.
| Gene set | Protein coding gene number | Average gene length (bp) | Average CDS length (bp) | Average exon per gene | Average exon length (bp) | Average intron length (bp) | |
|---|---|---|---|---|---|---|---|
| De novo | Genscan | 30,320 | 12,927 | 1,595 | 8.92 | 178.87 | 1,431 |
| AUGUSTUS | 40,007 | 8,114 | 1,220 | 6.53 | 186.85 | 1,246 | |
| Homolog | D.rerio | 38,658 | 12,345 | 1,120 | 6.69 | 167.55 | 1,974 |
| S.maximus | 40,864 | 12,956 | 1,153 | 6.74 | 171.11 | 2,056 | |
| G.aculeatus | 35,093 | 11,413 | 1,114 | 6.89 | 161.56 | 1,748 | |
| A.testudineus | 39,404 | 14,059 | 1,167 | 6.69 | 174.59 | 2,267 | |
| C.semilaevis | 37,758 | 12,151 | 1,163 | 6.84 | 169.92 | 1,880 | |
| O.latipes | 40,717 | 14,894 | 1,149 | 6.36 | 180.75 | 2,566 | |
| T.rubripes | 48,770 | 12,386 | 950.24 | 5.65 | 168.14 | 2,458 | |
| trans.orf/RNAseq | 16,356 | 19,894 | 2,040 | 12.87 | 358.55 | 1,287 | |
| MAKER | 22,442 | 15,828 | 1,703 | 10.51 | 327.83 | 1,302 | |
Fig. 2.
Comparisons of gene features among S. maximus, Anabas testudineus, Cynoglossus semilaevis, Danio rerio, Gasterosteus aculeatus, Oryzias latipes, Scophthalmus maximux and Takifugu rubripes. (a) Gene length distributions of the species. (b) CDS length distributions of the species. (c) Exon length distributions of the species. (d) Intron length distributions of the species.
To obtain functional annotation of the predicted protein-coding genes in turbot genome, InterPro30, Pfam31, Swissprot32 and TrEMBL32 databases were respectively used to predict protein function based on the conserved protein domains by InterProScan (v5.46)33. BLASTP (e-value ≤ 1e-5) was used for the homolog search in multiple databases, such as Gene Ontology (GO)34, Kyoto Encyclopedia of Genes and Genomes (KEGG)35, and NCBI non-redundant protein (NR)36 databases. Ultimately, a total of 21,360 genes (95.18% of all predicted genes) could be functionally annotated by at least one of the abovementioned databases (Table 5).
Table 5.
General statistics of gene function annotation of S. maximus.
| Type | Number | Percent (%) | |
|---|---|---|---|
| Total | 22,442 | ||
| Annotated | 21,360 | 95.18 | |
| InterPro | 19,732 | 87.92 | |
| GO | 15,096 | 67.27 | |
| KEGG_ALL | 20,917 | 93.2 | |
| KEGG_KO | 13,810 | 61.54 | |
| Swissprot | 19,137 | 85.27 | |
| TrEMBL | 21,313 | 94.97 | |
| TF | 3,328 | 14.83 | |
| Pfam | 19,126 | 85.22 | |
| NR | 21,065 | 93.86 | |
| KOG | 17,738 | 79.04 | |
| Unannotated | 1,082 | 4.82 | |
For non-coding genes, a total of 1,796 tRNAs were identified using tRNAscan-SE37. Moreover, 538 rRNAs were detected through searching for homology against rRNA sequences of related species using BLASTN. Besides, 430 miRNAs and 581 snRNAs were predicted using INFERNAL38 tool based on Rfam database (Table 6), respectively.
Table 6.
General statistics of non-coding annotation of S. maximus.
| Type | Copy | Average length(bp) | Total length(bp) | % of genome | |
|---|---|---|---|---|---|
| miRNA | 430 | 85 | 36,407 | 0.006764 | |
| tRNA | 1,796 | 75 | 134,264 | 0.024946 | |
| rRNA | rRNA | 538 | 138 | 74,432 | 0.013829 |
| 18 S | 6 | 1,849 | 11,094 | 0.002061 | |
| 28 S | 0 | 0 | 0 | 0 | |
| 5.8 S | 8 | 156 | 1,247 | 0.000232 | |
| 5 S | 524 | 118 | 62,091 | 0.011536 | |
| snRNA | snRNA | 581 | 137 | 79,403 | 0.014753 |
| CD-box | 193 | 121 | 23,313 | 0.004332 | |
| HACA-box | 75 | 151 | 11,302 | 0.002100 | |
| splicing | 306 | 141 | 43,069 | 0.008002 | |
| scaRNA | 7 | 246 | 1,719 | 0.000319 | |
Gene co-expression network inference and module-trait associations analysis
A total of 165 published stress-related RNA-seq samples data from 14 independent SRA studies (Table 7) that surveyed transcriptome profiling in turbot under different stresses (i.e., crowding, feeding, heat, oxygen, pathogens, and salinity) were downloaded from the NCBI SRA database using SRAtoolkit (v2.11.0)39. Following, RNA-seq data in SRA format were converted into FASTQ format using fastq-dump tool of SRAtoolkit. Then, reads were aligned to the latest assembled turbot genome using STAR40 with default parameters. TPMCalculator (-q 1)41 was used to calculate transcripts per million (TPM) values for all genes using sorted bam files obtained from reads alignment. Subsequently, we used BioNERO42 to preprocess the gene expression data according to the following steps: I) Replacing missing values (NAs) with 0 using replace_na function; II) Removing the genes whose average gene expression was less than 1 with remove_nonexp function; III) Removing outlying samples with ZKfiltering function; IV) Adjusting for confounding artifacts with PC_correction function to make every gene follow an approximate normal distribution. After filtering and processing (Fig. 1b), a normalized gene expression matrix consisting of 12,271 genes with medial expression value ≥ 1 from 160 RNA-seq samples were obtained.
Table 7.
Overview of the RNA-seq datasets used in this study.
| Stress | SRA Study | SRA-Experiments | Number of individuals | Platform (Illumina) | Size (GB) | References | |
|---|---|---|---|---|---|---|---|
| Crowding | — | SRP129900 | 12 | 500 | HiSeq 4000 | 68.20 | 8 |
| Feeding | myo-inositol | SRP188583 | 15 | 300 | HiSeq 4000 | 115.45 | 10 |
| fish meal, soybean meal | SRP074811 | 2 | 360 | NextSeq 500 | 42.56 | 87 | |
| sodium butyrate, soybean meal | SRP275545 | 6 | 270 | HiSeq 2000 | 50.23 | 88 | |
| Heat | — | SRP152627 | 10 | — | HiSeq 4000 | 88.99 | 6 |
| Oxygen | — | SRP167318 | 9 | 9 | HiSeq 2500 | 58.99 | 7 |
| Pathogens | Enteromyxum scophthalmi | SRP308109 | 49 | 280 | HiSeq 4000 | 381.62 | 3 |
| SRP255305 | 10 | 120 | HiSeq 4000 | 17.55 | 89 | ||
| SRP065375 | 12 | — | HiSeq 2000 | 31.48 | 4 | ||
| SRP050607 | 12 | 120 | HiSeq 2000 | 36.02 | 90 | ||
| Vibrio anguillarum | SRP191266 | 4 | 90 | HiSeq 2500 | 53.34 | 5 | |
| Salinity | — | SRP277001 | 6 | 360 | HiSeq 4000 | 49.35 | 91 |
| SRP238143 | 9 | 180 | HiSeq 2000 | 70.48 | 92 | ||
| SRP153594 | 9 | — | HiSeq 4000 | 70.86 | 9 | ||
| Total | — | — | 165 | — | 1135.12 | ||
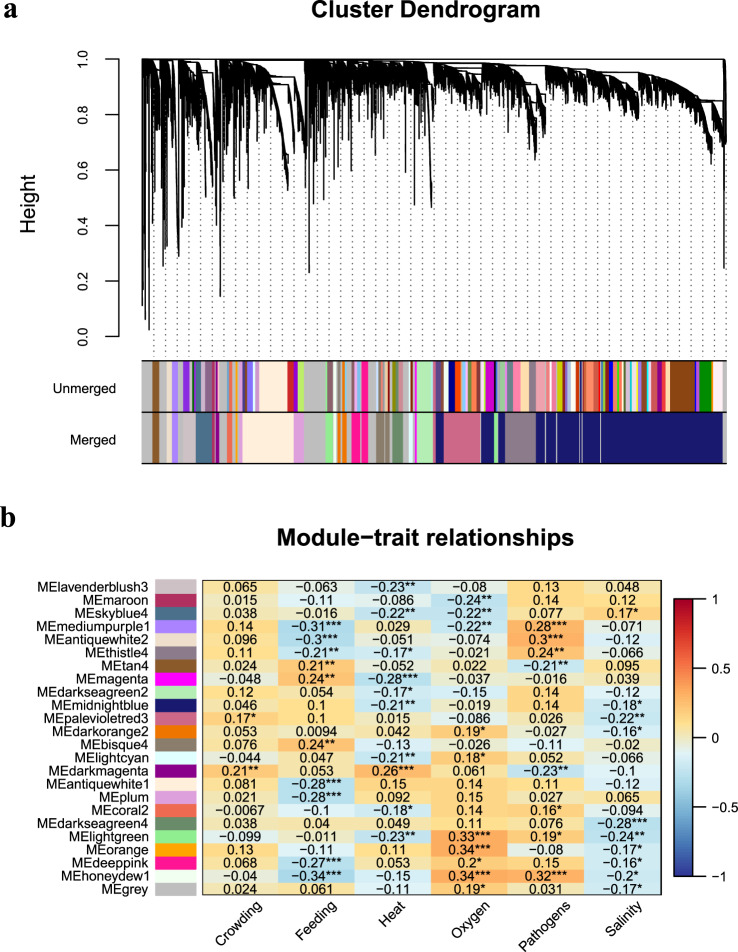
After we filtered and normalized the expression data, BioNERO42 was used to construct a gene co-expression network (GCN) (Fig. 1b). First of all, we identified the most optimal β power to make the network satisfy the scale-free topology with the function SFT_fit. According to the result, the optimal power is 11, for which the scale-free topology fit index (R2) reaches 0.8 and mean connectivity tends to 0. Next, we used the exp2gcn function to infer the GCN with power 11. As a result, a total of 24 co-expression modules were eventually identified (Fig. 3a), with the number of genes per module ranging from 34 (magenta) to 4,396 (midnightblue).
Fig. 3.
Gene co-expression network analysis of different stresses. (a) Cluster Dendrogram of genes and modules. The branches and color bands represent the assigned module. The tips of the branches represent genes. (b) Correlation between modules and stresses. The value in the box is the correlation coefficients. Correlation coefficients with ** or *** represent extremely significant correlation and significant correlation with *.
We then identified modules that were extremely significant (p-value < 0.01) positively or negatively correlated with particular traits (stresses) by calculating module-trait spearman correlation coefficients using the module_trait_cor function from BioNERO. As shown in Fig. 3b, significantly related modules could be found for each trait, which provides rich resources for the study of turbot stress resistance mechanism. To detect the functionality of the modules that extremely significant correlated with each stress, for each module, GO and KEGG enrichment analyses were performed on all genes in the module using TBtools43 (corrected p-value (BH method) < 0.5).
Identification of key hub genes
To identify candidate key hub genes related to every stress, we firstly constructed hub genes set. Hub genes, defined as the top 10% genes with highest degree (i.e., sum of connection weights of a gene to all other genes in the module) that have module membership (MM) (i.e., correlation of a gene to its module eigengene) > 0.8, were identified using the function get_hubs_gcn. Then, hub genes belonging to modules that were extremely significant associated with same stress were merged as hub genes set for this stress. Following, we set up the differentially expressed genes (DEGs) set. Firstly, we used featureCounts44 software program in Subread45 package to construct reads count matrixes. Then, edgeR46 was used to identify DEGs with false discovery rate (FDR) < 0.05 and |log2FC| > 1. DEGs, related to the same stress, were merged as DEGs set for this stress. Finally, genes, included in both hub genes set and DEGs set corresponding to the same stress, were defined as the candidate key hub genes for this stress. Candidate key hub genes related to crowding, feeding, heat, oxygen, pathogens and salinity stress were 0, 128, 40, 7, 90 and 80, respectively.
Heat-related modules enrichment analysis and identification of key hub genes
To heat stress, GO enrichment analyses illustrated that metabolic process, cellular process, catabolic process, catalytic activity, hydrolase activity, oxidoreductase activity, cellular response to stress, biosynthetic process, and binding were the significantly enriched terms (GO enrichment.xlsx47) in modules that were extremely significant correlated with heat stress. Meanwhile, KEGG enrichment analyses were employed on the same modules, and the results manifested that metabolism, lipid metabolism, carbohydrate metabolism, glycolysis/gluconeogenesis, PPAR signaling pathway, proteasome, digestive system, fat digestion and absorption, peroxisome, cell growth and death, transport and catabolism, cellular processes, and protein kinases were the significantly enriched pathways (KEGG enrichment.xlsx47). Finally, we identified 40 candidate heat-related key hub genes, of which 7 genes including ABI148, CD4449, CCDC15350, G2e351, PATJ52, HYKK53 and occludin54 has been verified to contribute to heat stress. For instance, G2e3 was one of the candidate genes in the liver of heat-treated large yellow croaker51. Exposure to heat stress (39 °C or 41 °C) resulted in increased expression of occludin protein in Caco-2 cells54.
Oxygen-related modules enrichment analysis and identification of key hub genes
To oxygen stress, GO enrichment results showed that aerobic respiration, aerobic electron transport chain, metabolic process, oxidoreductase activity, mitochondrial inner membrane, mitochondrial respirasome, respiratory chain complex, oxidative phosphorylation, oxidoreductase complex, catabolic process, catalytic activity, response to stress, response to external stimulus were the significantly enriched terms (GO enrichment.xlsx47) in modules that were extremely significant correlated with oxygen stress. Furthermore, we employed KEGG enrichment analyses on the same modules, and the results demonstrated that metabolism, oxidative phosphorylation, environmental adaptation, energy metabolism, and peroxisome were significantly enriched pathways (KEGG enrichment.xlsx47). In addition, we obtained 7 candidate oxygen-related hub genes, among which AMBP55 and CNN156 had been confirmed to conduce to heat stress. Such as, the gene for A1M is denoted AMBP, which has a physiological role as a protective antioxidant55. Five percent oxygen concentration significantly increased the expression levels of CNN1 in adipose-derived stem cell cultures after 2 weeks of induction56.
Pathogens-related modules enrichment analysis and identification of key hub genes
To pathogens stress, GO enrichment results showed that immune response, immune system process, response to wound healing, blood coagulation, hemostasis, response to external stimulus, response to stress, biosynthetic process, catalytic activity, metabolic process, and cellular process were the significantly enriched terms (GO enrichment.xlsx47) in modules that were extremely significant related with pathogens stress. Meanwhile, KEGG enrichment analyses were employed on the same modules, and the results manifested that immune system, human diseases, complement and coagulation cascades, CD molecules, lysosome, phagosome, B cell receptor signaling pathway, hematopoietic cell lineage metabolism, glycosaminoglycan binding proteins, exosome, neutrophil extracellular trap formation, were the significantly enriched pathways (KEGG enrichment.xlsx47). Ultimately, we determined 90 candidate pathogens-relevant hub genes, thereinto, 18 genes, such as CMKLR157, CSF3R58, SIGLEC1059, RAP1GAP260, Cd300lf61, NPTN62, MRC163, LILRA664, BLNK65, CXCL1266, PIGR67, SIGLEC1568, GULP169, MARCO70, NLRP1271, CRP72, FGG73, and lysozyme74, had been proved to be conducive to pathogens stress. For example, LILRA6 is essential for macrophage-mediated immune responses and it has the potential to complement the innate and adaptive immune system against pathogens64. Siglec-15 probably plays a conserved, regulatory role in the immune system of vertebrates68. In addition to its direct antimicrobial role, more recent evidence has shown that lysozyme modulates the host immune response to infection74.
Salinity-related modules enrichment analysis and identification of key hub genes
To salinity stress, according to GO enrichment analyses, terms (GO enrichment.xlsx47), such as, ion binding, small molecule binding, anion binding, proteasome complex, proteasome-activating activity, catabolic process, metabolic process, cellular process, cellular response to stress, binding, and ATP binding were significantly enriched in modules that were extremely significant related with salinity stress. Simultaneously, we employed KEGG enrichment analyses on the same modules, and the results indicated that proteasome pathway, protein kinases were the significantly enriched pathways (KEGG enrichment.xlsx47). After taking the intersection of hub genes set and DEGs set, 80 genes were defined as candidate salinity-associated hub genes, and six genes (NDUFV175, EMSY76, RBBP677, ATF278, Map3k779 and PSMC280) had been certified to be related to salinity stress. For example, RBBP6 was one of the identified candidate genes for freshwater vs. marine adaptation in threespine stickleback77. MAP3K7, also known as TAK1, could be highly activated by osmotic stress79.
Data Records
The raw data, including Illumina and PacBio sequencing data of the whole genome, was submitted to the NCBI SRA with accession number SRP35261081. The final genome assembly and annotation gff file are available at National Genomics Data Center with accession number GWHBHEA00000000.182. The final genome assembly is also available through NCBI with accession number GCA_022379125.183. The functional annotation of protein-coding genes, gene expression matrix used for gene co-expression network inference, and gene co-expression network analysis results including genes per module, hub genes set, DEGs set, GO and KEGG enrichment, key hub genes, are available at Figshare47.
Technical Validation
Quality and completeness assessment of genome assembly
The quality and completeness of the new assembly were evaluated through three independent approaches. Firstly, the base-level accuracy and completeness were estimated using Merqury84 by comparing k-mers in the assembly to those found in the high-accuracy Illumina reads. The results revealed that per-base accuracy rates for turbot assembly was 0.999994 and completeness value was 99.38%. Secondly, the completeness of the final assembled genome was also assessed using Benchmarking Universal Single-Copy Orthologs (BUSCO v4.1.6)85 with 4,584 single-copy orthologs from actinopterygii_odb9 database. BUSCO analysis revealed that 97.4% (4,465) complete BUSCOs (94.9% single-copy and 2.5% duplicated BUSCOs) and 1.1% (49) fragmented BUSCOs were identified in the assembled genome of turbot. Thirdly, we further evaluated the assembly quality using Inspector86 by aligning PacBio long reads to the assembled contigs for generating read-to-contig alignment and performing downstream assembly evaluation. As a result, read-to-contig mapping rate and quality value (QV) were 91.93% and 45.41, respectively. All these indicators suggested a high-quality and complete genome assembly for the further research in genetics and genomics of turbot.
Acknowledgements
This project was funded by Special Scientific Research Funds for Central Non-profit Institutes, Chinese Academy of Fisheries Science (2020TD20), Taishan Scholar Climbing Project of Shandong Province, China. the Key R&D Project of Shandong Province (2019GHY112023), National Natural Science Foundation of China (31402284), Key Research and Development Project of Shandong Province (2021LZGC028).
Author contributions
S.C. and X.X. applied, designed and supervised the project. X.X., W.Z. analyzed the data. X.X., Z.M., W.X. prepared the samples for whole genome sequencing and conducted the experiments. X.X., W.Z., S.C., W.X. and Y.L. wrote and revised the manuscript. All authors read and approved the final manuscript.
Code availability
The data analysis methods, software and associated parameters used in this study are described in the Methods section. Default parameters were applied if no parameter was described. No custom scripts were generated in this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xi-wen Xu, Weiwei Zheng.
References
- 1.Bjørndal, T. & Øiestad, V. J. W. P. The development of a new farmed species: production technology and markets for turbot. Working Paper (2010).
- 2.Lei JL, Liu XF, Guan CT. Turbot culture in China for two decades: achievements and prospect. Progress in Fishery Sciences. 2012;33:123–130. [Google Scholar]
- 3.Ronza P, et al. Blood transcriptomics of turbot Scophthalmus maximus: A tool for health monitoring and disease studies. Animals. 2021;11:1296. doi: 10.3390/ani11051296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ronza P, et al. RNA-seq analysis of early enteromyxosis in turbot (Scophthalmus maximus): new insights into parasite invasion and immune evasion strategies. Int J Parasitol. 2016;46:507–517. doi: 10.1016/j.ijpara.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Gao C, et al. Comparative analysis of the miRNA-mRNA regulation networks in turbot (Scophthalmus maximus L.) following Vibrio anguillarum infection. Developmental and Comparative Immunology. 2021;124:104164. doi: 10.1016/j.dci.2021.104164. [DOI] [PubMed] [Google Scholar]
- 6.Huang Z, et al. Transcriptome analysis and weighted gene co-expression network reveals potential genes responses to heat stress in turbot Scophthalmus maximus. Comp Biochem Physiol Part D Genomics Proteomics. 2020;33:100632. doi: 10.1016/j.cbd.2019.100632. [DOI] [PubMed] [Google Scholar]
- 7.Nie X, et al. Characterizing transcriptome changes in gill tissue of turbot (Scophthalmus maximus) for waterless preservation. Aquaculture. 2020;518:734830. doi: 10.1016/j.aquaculture.2019.734830. [DOI] [Google Scholar]
- 8.Huo H, et al. Transcriptomic profiling of the immune response to crowding stress in juvenile turbot (Scophthalmus maximus) Journal of Ocean University of China. 2020;19:911–922. doi: 10.1007/s11802-020-4242-6. [DOI] [Google Scholar]
- 9.Cui W, et al. Transcriptomic analysis reveals putative osmoregulation mechanisms in the kidney of euryhaline turbot Scophthalmus maximus responded to hypo-saline seawater. Journal of Oceanology and Limnology. 2019;38:467–479. doi: 10.1007/s00343-019-9056-2. [DOI] [Google Scholar]
- 10.Cui W, et al. myo-inositol facilitates salinity tolerance by modulating multiple physiological functions in the turbot Scophthalmus maximus. Aquaculture. 2020;527:735451. doi: 10.1016/j.aquaculture.2020.735451. [DOI] [Google Scholar]
- 11.Panahi B, Hejazi MA. Weighted gene co-expression network analysis of the salt-responsive transcriptomes reveals novel hub genes in green halophytic microalgae Dunaliella salina. Sci Rep. 2021;11:1607. doi: 10.1038/s41598-020-80945-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu M, et al. WGCNA analysis of salt-responsive core transcriptome identifies novel hub genes in rice. Genes. 2019;10:719. doi: 10.3390/genes10090719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao X, Sun Z, Xu H, Song N, Gao T. Transcriptome and co-expression network analyses reveal the regulatory pathways and key genes associated with temperature adaptability in the yellow drum (Nibea albiflora) J Therm Biol. 2021;100:103071. doi: 10.1016/j.jtherbio.2021.103071. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, et al. Network analysis of oyster transcriptome revealed a cascade of cellular responses during recovery after heat shock. Plos One. 2012;7:e35484. doi: 10.1371/journal.pone.0035484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koren S, et al. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Research. 2017;27:722–736. doi: 10.1101/gr.215087.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolmogorov M, Yuan J, Lin Y, Pevzner PA. Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol. 2019;37:540–546. doi: 10.1038/s41587-019-0072-8. [DOI] [PubMed] [Google Scholar]
- 17.Guan D, et al. Identifying and removing haplotypic duplication in primary genome assemblies. Bioinformatics. 2020;36:2896–2898. doi: 10.1093/bioinformatics/btaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker BJ, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One. 2014;9:e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu XW, et al. Draft genomes of female and male turbot Scophthalmus maximus. Sci Data. 2020;7:90. doi: 10.1038/s41597-020-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Figueras A, et al. Whole genome sequencing of turbot (Scophthalmus maximus; Pleuronectiformes): a fish adapted to demersal life. DNA Res. 2016;23:181–192. doi: 10.1093/dnares/dsw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez P, et al. A genome-wide association study, supported by a new chromosome-level genome assembly, suggests sox2 as a main driver of the undifferentiatiated ZZ/ZW sex determination of turbot (Scophthalmus maximus) Genomics. 2021;113:1705–1718. doi: 10.1016/j.ygeno.2021.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Tarailo-Graovac M, Chen N. Using RepeatMasker to identify repetitive elements in genomic sequences. Curr. Protoc. Bioinformatics. 2009;25:4.10.11–14.10.14. doi: 10.1002/0471250953.bi0410s25. [DOI] [PubMed] [Google Scholar]
- 23.Bao W, Kojima KK, Kohany O. Repbase update, a database of repetitive elements in eukaryotic genomes. Mob. DNA. 2015;6:11. doi: 10.1186/s13100-015-0041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Z, Wang H. LTR_FINDER: an efficient tool for the prediction of full-length LTR retrotransposons. Nucleic Acids Research. 2007;35:W265–W268. doi: 10.1093/nar/gkm286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holt C, Yandell M. MAKER2: an annotation pipeline and genome-database management tool for second-generation genome projects. Bmc Bioinformatics. 2011;12:491. doi: 10.1186/1471-2105-12-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gertz EM, Yu Y-K, Agarwala R, Schaffer AA, Altschul SF. Composition-based statistics and translated nucleotide searches: Improving the TBLASTN module of BLAST. Bmc Biology. 2006;4:41. doi: 10.1186/1741-7007-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slater GS, Birney E. Automated generation of heuristics for biological sequence comparison. BMC Bioinformatics. 2005;6:31. doi: 10.1186/1471-2105-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanke M, et al. AUGUSTUS: ab initio prediction of alternative transcripts. Nucleic Acids Research. 2006;34:W435–W439. doi: 10.1093/nar/gkl200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burge C, Karlin S. Prediction of complete gene structures in human genomic DNA. Journal of molecular biology. 1997;268:78–94. doi: 10.1006/jmbi.1997.0951. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell AL, et al. InterPro in 2019: improving coverage, classification and access to protein sequence annotations. Nucleic Acids Research. 2019;47:D351–D360. doi: 10.1093/nar/gky1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mistry J, et al. Pfam: The protein families database in 2021. Nucleic Acids Research. 2020;49:D412–D419. doi: 10.1093/nar/gkaa913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bairoch A, Apweiler R. The SWISS-PROT protein sequence database and its supplement TrEMBL in 2000. Nucleic Acids Research. 2000;28:45–48. doi: 10.1093/nar/28.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones P, et al. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30:1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashburner M, et al. Gene Ontology: tool for the unification of biology. Nature Genetics. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Research. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pruitt KD, Tatusova T, Maglott DR. NCBI reference sequences (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Research. 2007;35:D61–D65. doi: 10.1093/nar/gkl842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan PP, Lowe TMJO. tRNAscan-SE: Searching for tRNA genes in genomic sequences. Methods in Molecular Biology. 2019;1962:1–14. doi: 10.1007/978-1-4939-9173-0_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nawrocki EP, Eddy SR. Infernal 1.1: 100-fold faster RNA homology searches. Bioinformatics. 2013;29:2933–2935. doi: 10.1093/bioinformatics/btt509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Team, S. T. D. SRAtoolkit version 2.11.0. (2021).
- 40.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vera Alvarez R, Pongor LS, Marino-Ramirez L, Landsman D. TPMCalculator: one-step software to quantify mRNA abundance of genomic features. Bioinformatics. 2019;35:1960–1962. doi: 10.1093/bioinformatics/bty896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almeida-Silva F, Venancio TM. BioNERO: an all-in-one R/Bioconductor package for comprehensive and easy biological network reconstruction. Functional & Integrative Genomics. 2022;22:131–136. doi: 10.1007/s10142-021-00821-9. [DOI] [PubMed] [Google Scholar]
- 43.Chen C, et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Molecular Plant. 2020;13:1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 44.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 45.Liao Y, Smyth GK, Shi W. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Research. 2013;41:e108. doi: 10.1093/nar/gkt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu X, Zheng W. 2021. An improved high quality genome assembly of turbot (Scophthalmus maximus) figshare. [DOI]
- 48.Li Y, Li Y, Liu Y, Wu Y, Xie Q. The sHSP22 heat shock protein requires the ABI1 protein phosphatase to modulate polar auxin transport and downstream responses. Plant Physiol. 2018;176:2406–2425. doi: 10.1104/pp.17.01206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan L, et al. Sublethal heat treatment of hepatocellular carcinoma promotes intrahepatic metastasis and stemness in a VEGFR1-dependent manner. Cancer Lett. 2019;460:29–40. doi: 10.1016/j.canlet.2019.05.041. [DOI] [PubMed] [Google Scholar]
- 50.Reed KM, et al. Response of turkey muscle satellite cells to thermal challenge. I. transcriptome effects in proliferating cells. BMC Genomics. 2017;18:352. doi: 10.1186/s12864-017-3740-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu Y, et al. GWAS identified candidate variants and genes associated with acute heat tolerance of large yellow croaker. Aquaculture. 2021;540:736696. doi: 10.1016/j.aquaculture.2021.736696. [DOI] [Google Scholar]
- 52.Tabler TW, et al. Intestinal barrier integrity in heat-stressed modern broilers and their ancestor wild jungle fowl. Front Vet Sci. 2020;7:249. doi: 10.3389/fvets.2020.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suring W, Marien J, Broekman R, van Straalen NM, Roelofs D. Biochemical pathways supporting beta-lactam biosynthesis in the springtail Folsomia candida. Biol Open. 2016;5:1784–1789. doi: 10.1242/bio.019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dokladny K, Ye D, Kennedy JC, Moseley PL, Ma TY. Cellular and molecular mechanisms of heat stress-induced up-regulation of occludin protein expression: regulatory role of heat shock factor-1. Am J Pathol. 2008;172:659–670. doi: 10.2353/ajpath.2008.070522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akerstrom B, Gram M. A1M, an extravascular tissue cleaning and housekeeping protein. Free Radic Biol Med. 2014;74:274–282. doi: 10.1016/j.freeradbiomed.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 56.Wang F, et al. Hypoxia enhances differentiation of adipose tissue-derived stem cells toward the smooth muscle phenotype. Int J Mol Sci. 2018;19:517. doi: 10.3390/ijms19020517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vermi W, et al. Role of ChemR23 in directing the migration of myeloid and plasmacytoid dendritic cells to lymphoid organs and inflamed skin. Journal of Experimental Medicine. 2005;201:509–515. doi: 10.1084/jem.20041310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dong F, et al. Identification of a nonsense mutation in the granulocyte-colony-stimulating factor receptor in severe congenital neutropenia. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:4480–4484. doi: 10.1073/pnas.91.10.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang P, et al. Siglec-10 is associated with survival and natural killer cell dysfunction in hepatocellular carcinoma. Journal of Surgical Research. 2015;194:107–113. doi: 10.1016/j.jss.2014.09.035. [DOI] [PubMed] [Google Scholar]
- 60.Schultess J, Danielewski O, Smolenski AP. Rap1GAP2 is a new GTPase-activating protein of Rap1 expressed in human platelets. Blood. 2005;105:3185–3192. doi: 10.1182/blood-2004-09-3605. [DOI] [PubMed] [Google Scholar]
- 61.Moshkovits I, et al. CD300f associates with IL-4 receptor alpha and amplifies IL-4-induced immune cell responses. Proc Natl Acad Sci USA. 2015;112:8708–8713. doi: 10.1073/pnas.1507625112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Korthals M, et al. A complex of Neuroplastin and Plasma Membrane Ca2+ ATPase controls T cell activation. Scientific Reports. 2017;7:8385. doi: 10.1038/s41598-017-08519-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harris N, Super M, Rits M, Chang G, Ezekowitz RA. Characterization of the murine macrophage mannose receptor: demonstration that the downregulation of receptor expression mediated by interferon-gamma occurs at the level of transcription. Blood. 1992;80:2363–2373. doi: 10.1182/blood.V80.9.2363.bloodjournal8092363. [DOI] [PubMed] [Google Scholar]
- 64.Truong AD, et al. Leukocyte immunoglobulin-like receptors A2 and A6 are expressed in avian macrophages and modulate cytokine production by activating multiple signaling pathways. Int J Mol Sci. 2018;19:2710. doi: 10.3390/ijms19092710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fu C, Turck CW, Kurosaki T, Chan AC. BLNK: a central linker protein in B cell activation. Immunity. 1998;9:93–103. doi: 10.1016/S1074-7613(00)80591-9. [DOI] [PubMed] [Google Scholar]
- 66.Poznansky MC, et al. Active movement of T cells away from a chemokine. Nature Medicine. 2000;6:543–548. doi: 10.1038/75022. [DOI] [PubMed] [Google Scholar]
- 67.Xu G, et al. Characteristics of the polymeric immunoglobulin receptor (pIgR) of commercial grass carp and the immune response of pIgR and immunoglobulin to Flavobacterium columnare. Fisheries Science. 2018;85:101–112. doi: 10.1007/s12562-018-1268-4. [DOI] [Google Scholar]
- 68.Angata T, Tabuchi Y, Nakamura K, Nakamura M. Siglec-15: an immune system Siglec conserved throughout vertebrate evolution. Glycobiology. 2007;17:838–846. doi: 10.1093/glycob/cwm049. [DOI] [PubMed] [Google Scholar]
- 69.Song G, et al. Gulp1 is associated with the pharmacokinetics of PEGylated liposomal doxorubicin (PLD) in inbred mouse strains. Nanomedicine. 2016;12:2007–2017. doi: 10.1016/j.nano.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 70.Jing J, et al. Role of macrophage receptor with collagenous structure in innate immune tolerance. J Immunol. 2013;190:6360–6367. doi: 10.4049/jimmunol.1202942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tuladhar S, Kanneganti TD. NLRP12 in innate immunity and inflammation. Molecular Aspects of Medicine. 2020;76:100887. doi: 10.1016/j.mam.2020.100887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baldo B, Fletcher T. C-reactive protein-like precipitins in plaice. Nature. 1973;246:145–146. doi: 10.1038/246145a0. [DOI] [PubMed] [Google Scholar]
- 73.Vo AH, Swaroop A, Liu Y, Norris ZG, Shavit JA. Loss of fibrinogen in zebrafish results in symptoms consistent with human hypofibrinogenemia. Plos One. 2013;8:e74682. doi: 10.1371/journal.pone.0074682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ragland SA, Criss AK. From bacterial killing to immune modulation: recent insights into the functions of lysozyme. PLoS Pathog. 2017;13:e1006512. doi: 10.1371/journal.ppat.1006512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen X, et al. Gill transcriptome analysis revealed the difference in gene expression between freshwater and seawater acclimated guppy (Poecilia reticulata) Mar Biotechnol (NY) 2021;23:615–627. doi: 10.1007/s10126-021-10053-4. [DOI] [PubMed] [Google Scholar]
- 76.De Vos S, et al. Identification of salt stress response genes using the Artemia transcriptome. Aquaculture. 2019;500:305–314. doi: 10.1016/j.aquaculture.2018.09.067. [DOI] [Google Scholar]
- 77.Ferchaud AL, et al. A low-density SNP array for analyzing differential selection in freshwater and marine populations of threespine stickleback (Gasterosteus aculeatus) BMC Genomics. 2014;15:867. doi: 10.1186/1471-2164-15-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang L, Payton R, Dai W, Lu L. Hyperosmotic stress-induced ATF-2 activation through Polo-like kinase 3 in human corneal epithelial cells. Journal of Biological Chemistry. 2011;286:1951–1958. doi: 10.1074/jbc.M110.166009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu W, et al. Perfluorinated compounds disrupted osmoregulation in Oryzias melastigma during acclimation to hypoosmotic environment. Ecotoxicol Environ Saf. 2021;223:112613. doi: 10.1016/j.ecoenv.2021.112613. [DOI] [PubMed] [Google Scholar]
- 80.Dowd WW, Harris BN, Cech JJ, Jr., Kultz D. Proteomic and physiological responses of leopard sharks (Triakis semifasciata) to salinity change. J Exp Biol. 2010;213:210–224. doi: 10.1242/jeb.031781. [DOI] [PubMed] [Google Scholar]
- 81.2021. NCBI Sequence Read Archive. SRP352610
- 82.2022. National Genomics Data Center. https://ngdc.cncb.ac.cn/search/?dbId=gwh&q=GWHBHEA00000000.1
- 83.2022. NCBI Assembly. GCA_022379125.1
- 84.Rhie A, Walenz BP, Koren S, Phillippy AM. Merqury: reference-free quality, completeness, and phasing assessment for genome assemblies. Genome Biol. 2020;21:245. doi: 10.1186/s13059-020-02134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Manni M, Berkeley MR, Seppey M, Simão FA, Zdobnov EM. BUSCO update: novel and streamlined workflows along with broader and deeper phylogenetic coverage for scoring of eukaryotic, prokaryotic, and viral genomes. Molecular Biology and Evolution. 2021;38:4647–4654. doi: 10.1093/molbev/msab199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen Y, Zhang Y, Wang AY, Gao M, Chong Z. Accurate long-read de novo assembly evaluation with Inspector. Genome Biol. 2021;22:312. doi: 10.1186/s13059-021-02527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gu M, Bai N, Zhang Y, Krogdahl Å. Soybean meal induces enteritis in turbot Scophthalmus maximus at high supplementation levels. Aquaculture. 2016;464:286–295. doi: 10.1016/j.aquaculture.2016.06.035. [DOI] [Google Scholar]
- 88.Liu Y, et al. Sodium butyrate supplementation in high-soybean meal diets for turbot (Scophthalmus maximus L.): effects on inflammatory status, mucosal barriers and microbiota in the intestine. Fish Shellfish Immunol. 2019;88:65–75. doi: 10.1016/j.fsi.2019.02.064. [DOI] [PubMed] [Google Scholar]
- 89.Ronza P, et al. The teleost thymus in health and disease: new insights from transcriptomic and histopathological analyses of turbot, Scophthalmus maximus. Biology (Basel) 2020;9:221. doi: 10.3390/biology9080221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robledo D, et al. RNA-seq analysis reveals significant transcriptome changes in turbot (Scophthalmus maximus) suffering severe enteromyxosis. Bmc Genomics. 2014;15:1149. doi: 10.1186/1471-2164-15-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu Z, et al. Transcriptome analysis of liver lipid metabolism disorders of the turbot Scophthalmus maximus in response to low salinity stress. Aquaculture. 2021;534:736273. doi: 10.1016/j.aquaculture.2020.736273. [DOI] [Google Scholar]
- 92.Cui W, Ma A, Wang X. Response of the PI3K‐AKT signalling pathway to low salinity and the effect of its inhibition mediated by wortmannin on ion channels in turbot Scophthalmus maximus. Aquaculture Research. 2020;51:2676–2686. doi: 10.1111/are.14607. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Xu X, Zheng W. 2021. An improved high quality genome assembly of turbot (Scophthalmus maximus) figshare. [DOI]
- 2021. NCBI Sequence Read Archive. SRP352610
- 2022. National Genomics Data Center. https://ngdc.cncb.ac.cn/search/?dbId=gwh&q=GWHBHEA00000000.1
- 2022. NCBI Assembly. GCA_022379125.1
Data Availability Statement
The data analysis methods, software and associated parameters used in this study are described in the Methods section. Default parameters were applied if no parameter was described. No custom scripts were generated in this work.