Abstract
Perioperative neurocognitive disorders (NCDs) refer to neurocognitive abnormalities detected during the perioperative periods, including preexisting cognitive impairment, preoperative delirium, delirium occurring up to 7 days after surgery, delayed neurocognitive recovery, and postoperative NCD. The Diagnostic and Statistical Manual of Mental Disorders‐5th edition (DSM‐5) is the golden standard for diagnosing perioperative NCDs. Given the impracticality of using the DSM‐5 by non‐psychiatric practitioners, many diagnostic tools have been developed and validated for different clinical scenarios. The etiology of perioperative NCDs is multifactorial and includes predisposing and precipitating factors. Identifying these risk factors is conducive to preoperative risk stratification and perioperative risk reduction. Prevention for perioperative NCDs should include avoiding possible contributors and implementing nonpharmacologic and pharmacological interventions. The former generally includes avoiding benzodiazepines, anticholinergics, prolonged liquid fasting, deep anesthesia, cerebral oxygen desaturation, and intraoperative hypothermia. Nonpharmacologic measures include preoperative cognitive prehabilitation, comprehensive geriatric assessment, implementing fast‐track surgery, combined use of regional block, and sleep promotion. Pharmacological measures including dexmedetomidine, nonsteroidal anti‐inflammatory drugs, and acetaminophen are found to have beneficial effects. Nonpharmacological treatments are the first‐line measures for established perioperative NCDs. Pharmacological treatments are still limited to severely agitated or distressed patients.
Keywords: delirium, neurocognitive disorders, perioperative period, postoperative cognitive complications
Perioperative neurocognitive disorders constitute a great challenge for older patients scheduled for surgery because their occurrence is associated with increased morbidity and mortality as well as enormous medical costs. Preoperative risk stratification and perioperative risk reduction should be adopted for perioperative NCDs prevention and treatment.
![]()
1. INTRODUCTION
Perioperative neurocognitive disorders (NCDs), especially postoperative delirium, delayed neurocognitive recovery, and postoperative NCD, are significant challenges to older patients scheduled for surgery. 1 The resulting cognitive declines can persist for months or years and have a detrimental impact on self‐dependence, quality of life, risk of developing dementia, and even long‐term survival. With the aging population and growing number of surgeries, perioperative NCDs have become a public health problem and attracted worldwide attention. 2 This review aims to discuss recent advances regarding perioperative NCDs, especially the diagnosis, prevention, and treatment.
2. DEFINITION AND CLASSIFICATION OF PERIOPERATIVE NCDS
According to a recent consensus, 1 “perioperative NCDs” are recommended to describe the overall situation identified during the pre‐ and postoperative periods. Perioperative NCDs are further classified into preexisting cognitive impairment or delirium, delirium occurred up to 7 days after surgery, cognitive decline diagnosed up to 30 days after surgery (delayed neurocognitive recovery), and cognitive decline diagnosed thereafter until 12 months (post‐operative NCD) (Figure 1).
FIGURE 1.
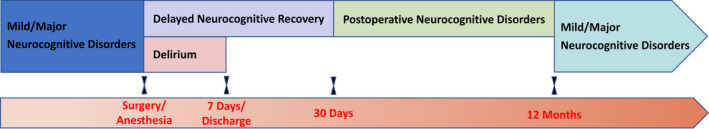
Classification of perioperative neurocognitive disorders
It is suggested that cognitive screening should be performed in high‐risk patients before surgery. 3 Those with preexisting impairment in one or more cognitive domains (complex attention, executive function, learning, memory, language, perceptual‐motor, and/or social cognition) are considered to have baseline NCD, which can be further classified as mild (mild cognitive impairment) or major disorder (dementia) according to the severity of impairment.
Delirium is a syndrome of acutely occurring and fluctuating changes in attention, level of consciousness, and cognitive function. It can occur preoperatively, but most often occurs within 7 days after surgery. Psychomotor disturbances (hyperactive, hypoactive, or mixed), perceptual disturbances (hallucination and delusion), emotional disturbances, and impaired sleep–wake cycle may also occur during delirium, although these features are not required for diagnosis.
Delayed neurocognitive recovery is a newly proposed term indicating a new‐onset cognitive decline within 30 days after surgery and replaces the traditional term “early postoperative cognitive dysfunction (POCD).” Because evidence shows that many and perhaps most patients recover completely from early postoperative cognitive impairment. Thus, the term “delayed neurocognitive recovery” interprets more accurately the meaning of potential recovery.
The term “postoperative NCD” now specifically refers to cognitive decline detected from 30 days after surgery to 12 months of follow‐up. After 12 months, the specifier “postoperative” is no longer attached when diagnosing cognitive decline, as the etiology cannot be reasonably attributed to the effects of prior anesthesia and surgery. Therefore, the term “NCD” is reused for the diagnosis after this timepoint.
The above diagnoses are made according to the Diagnostic and Statistical Manual of Mental Disorders fifth edition (DSM‐5). 1 In most available studies, we note that POCD diagnosis was based on the presence of cognitive decline but not the DSM‐5 criteria. We, therefore, continue to use the term “POCD” rather than “postoperative NCD” in this paper when referring to previous results regarding cognitive decline occurred between 30 days to 12 months after surgery.
3. EPIDEMIOLOGY AND OUTCOMES OF PERIOPERATIVE NCDS
Numerous previous studies investigated the occurrence of postoperative delirium, delayed neurocognitive recovery, and POCD. The reported incidences vary widely due to the differences in patient populations and surgical types, the heterogeneity in the composition of test batteries, the criteria or definition for diagnoses, the timing of assessment, and the nature of studies (retrospective studies often underestimate the incidence when compared with prospective ones).
3.1. Postoperative delirium
In general adult patients, the incidence of postoperative delirium is 2.5%–4.5%. 4 , 5 In patients aged 60 years or above, the incidence of postoperative delirium is increased to 12.0%–23.8%. 6 , 7 The type and complexity of surgery also greatly affect the occurrence of delirium. According to the report from the American College of Surgeons National Surgical Quality Improvement Program, among the 20,212 older patients, the incidence of delirium was 13.7% after cardiothoracic surgery, 13.0% after orthopedic surgery, 13.0% after general surgery, 11.4% after vascular surgery, 8.0% after neurosurgery, 7.1% after plastics and otolaryngology, 6.6% after urology, and 4.7% after gynecology. 7 Other studies also reported high incidence of delirium in patients following cardiovascular surgery (15.3–23.4%), 8 , 9 , 10 hip fracture surgery (16.9%), 11 and emergency surgery (22.7–26%), 12 , 13 and in those who were admitted to intensive care unit after surgery (24.4%). 14
Delirium is an imperative predictor of adverse outcomes. In the early postoperative period, delirium is associated with a prolonged hospital stay, increased institutional discharge, and higher 30‐day readmission; the outcomes of delirium are additive to those of other major complications. 15 , 16 Mounting evidence suggests that delirium is associated with an increased risk of perioperative and long‐term mortality. 16 , 17 , 18 , 19 Among survivors, delirium is significantly linked to cognitive decline, both early (1 month after surgery) and long‐term after surgery 20 , 21 ; delirium is also associated with a decrease in health‐related quality of life 18 and an increased risk of dementia. 16 , 22
3.2. Delayed neurocognitive recovery and POCD
An early multicenter study, the International Study of Post‐Operative Cognitive Dysfunction (ISPOCD1), investigated the occurrence of cognitive decline in 1218 elderly patients after major abdominal and orthopedic surgery. Delayed neurocognitive recovery was diagnosed in 25.8% of patients at 1 week after surgery; POCD was diagnosed in 9.9% of patients at 3 month after surgery. 23 In another prospective study of 1064 adult patients following noncardiac surgery, the incidence of delayed neurocognitive recovery was 34.5% at hospital discharge, and that of POCD was 7.0% at 3‐month follow‐up. 24 In a systematic review involving 24 studies with 8314 patients following noncardiac and non‐neurological surgeries, the pooled incidence of POCD at 3 months was 11.7%. 25
The majority of patients who developed early cognitive decline recover with time, only about 1/6 to 1/2 of them have POCD during the follow‐up period. 23 , 24 , 26 , 27 However, delayed neurocognitive recovery is also associated with adverse outcomes. For example, patients with cognitive decline 1 week after surgery are more likely to leave the labor market and depend on social transfer payment. 28 A recent study reported that those with delayed neurocognitive recovery between 3 and 5 days after surgery had more self‐reported cognitive impairment in memory, attention, action, and perception at 12 month after radical prostatectomy. 29 It seems that patients with POCD have even worse outcomes. For example, studies found that patients with POCD at 3 months after surgery had twice the proportion of new impairment in activities of daily living when compared with those without 30 ; they also had a higher risk of long‐term mortality. 24 , 28 Available data do not find a significant association between delayed neurocognitive recovery or POCD and long‐term dementia 31 ; further studies are required to clarify this issue.
4. RISK FACTORS OF PERIOPERATIVE NCDS
The etiologies of perioperative NCDs are multifactorial 32 and can be classified as predisposing and precipitating factors. The risk factors for delirium, delayed neurocognitive recovery, and POCD are significantly overlapped and are presented in Table 1. 9 , 10 , 14 , 20 , 23 , 24 , 25 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 The development of perioperative NCDs results from the interaction of multiple factors. Many prediction models have been developed to predict the risk of perioperative NCDs. 41 , 91 , 92 , 93 , 94 , 95 , 96 However, none of these models have been externally validated or widely accepted for clinical use.
TABLE 1.
Risk factors of perioperative neurocognitive disorders
| Risk factors | Delirium | Delayed neurocognitive recovery and POCD |
|---|---|---|
| Predisposing factors | Advanced age 9 , 14 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 | Advanced age 23 , 24 , 25 , 38 , 45 , 68 , 69 , 70 , 71 , 72 |
| Lower educational level 44 | Lower educational level 23 , 24 , 25 , 61 , 70 , 73 | |
| Functional status: Cognitive impairment, 9 , 10 , 14 , 35 , 36 , 39 , 41 , 43 , 45 , 46 hearing impairment, 46 frailty 38 , 47 , 48 , 49 | Functional status: cognitive impairment, 69 , 71 , 74 , 75 , 76 frailty 38 , 77 | |
| Comorbidities: depression, 9 , 35 , 37 , 39 , 45 , 50 psychiatric illness, 36 , 39 , 40 cerebrovascular disease, 10 , 36 , 37 , 42 , 45 , 46 parkinsonism, 42 heart failure, 33 , 39 hypertension, 9 , 39 , 44 mitral valve disease, 44 diabetes, 14 , 35 , 37 obstructive sleep apnea, 36 pulmonary diseases, 37 , 39 kidney disease, 10 , 39 number of medications 37 , 51 | Comorbidities: depression, 45 cerebrovascular disease, 24 , 78 heart failure, 69 hypertension, 69 , 79 diabetes, 69 , 72 , 80 , 81 renal failure 72 | |
| Comorbidity scores: higher ASA grade, 10 , 34 , 40 NYHA functional class III or IV, 35 higher EuroSCORE, 45 higher Charlson Comorbidity Index 52 | Comorbidity scores: higher ASA grade, 82 higher EuroSCORE 69 | |
| Alcohol abuse 34 , 39 , 40 | Alcohol abuse 79 , 83 | |
| Nutritional status: malnutrition/low albumin, 14 , 34 , 37 , 40 , 41 , 51 , 53 low preoperative hematocrit, 37 , 42 , 51 vitamin D deficiency 54 , 55 | Nutritional status: anemia, 70 vitamin D deficiency 55 , 84 | |
| Precipitating factors | Preoperative preparation: long‐duration fluid fasting, 56 , 57 preoperative pain 39 , 50 | |
| Perioperative medications: anticholinergic drugs, 58 benzodiazepines, 14 , 59 opioid use, 33 , 37 , 39 use of pethidine 60 | Perioperative medications: anticholinergic drugs, 85 opioid use 86 , 87 | |
| Intraoperative factors/management: deep anesthesia, 61 intraoperative blood loss/blood transfusion, 34 , 36 , 37 , 40 , 51 cerebral oxygen desaturation, 62 , 63 , 64 hypotension, 65 , 66 hypothermia 67 | Intraoperative factors/management: deep anesthesia, 61 , 88 intraoperative bleeding, 79 cerebral oxygen desaturation 64 , 89 , 90 | |
| Surgical management: abdominal/orthopedic surgery or higher Surgical Apgar score, 41 , 42 long‐duration surgery 37 , 51 | Surgical management: major surgery, 71 long‐duration surgery 23 , 69 , 78 | |
| Postoperative management: severe pain, 40 long‐duration mechanical ventilation, 14 , 35 prolonged stay in the intensive care unit 35 , 45 | Postoperative management: severe pain, 79 occurrence of delirium, 20 , 25 , 45 prolonged stay in the intensive care unit 45 |
Abbreviations: ASA, American Society of Anesthesiologist; EuroSCORE, European System for Cardiac Operative Risk Evaluation score; NYHA, New York Heart Association; POCD, postoperative neurocognitive disorder.
5. DIAGNOSIS OF PERIOPERATIVE NCDS
5.1. Diagnosis of delirium
According to the recent consensus, postoperative delirium is considered a particular category consistent with DSM‐5 terminology and occurs in the immediate postoperative period. 1 In other words, postoperative delirium is a new‐onset event that occurs during a hospital stay for up to 7 days after surgery or until discharge and meets the diagnostic criteria of DSM‐5. 1 , 97 Delirium occurring after discharge is no longer specified as “postoperative” unless persistent from the in‐hospital postoperative period. Attention should be paid to differentiating emergence delirium from postoperative delirium when making the diagnosis. Emergence delirium occurs during or immediately after emergence from general anesthesia and usually resolves within minutes or hours, whereas postoperative delirium mainly occurs 24–72 h after surgery and usually resolves within hours to days. 98 During research and clinical practice, emergence delirium is mainly monitored during the stay in the post‐anesthesia care unit, whereas postoperative delirium is mainly monitored from the first postoperative day. 67 , 99
DSM‐5 is the gold standard for diagnosing postoperative delirium. However, the use of DSM‐5 is impractical by non‐psychiatric practitioners. So far, more than 20 diagnostic tools have been introduced and validated to facilitate the diagnosis of delirium. 100 , 101 , 102 Among these, the frequently used screening tools including the Confusion Assessment Method (CAM), 103 the Confusion Assessment Method for the Intensive Care Unit (CAM‐ICU), 104 the Brief Confusion Assessment Method (bCAM), 105 the 3‐Minute Diagnostic Interview for Delirium using the Confusion Assessment Method (3D‐CAM), 106 the Intensive Care Delirium Screening Checklist (ICDSC), 107 and the 4A's Test (4AT). 108 Several instruments have been validated to measure delirium severity. The Memory Delirium Assessment Scale (MDAS), 109 the delirium Rating Scale‐Revised‐98 (DRS‐R‐98), 110 and the Confusion Assessment Method‐Severity (CAM‐S) 111 are the three most commonly used tools for assessing delirium severity. The characteristics of these tools are presented in Table 2. 97 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115
TABLE 2.
Characteristics of the frequently used delirium screening tools
| Screening tools | Based criteria | No. of items | Target patients | Time taken (min) | Sensitivity (%) | Specificity (%) | Interrater reliability |
|---|---|---|---|---|---|---|---|
| Diagnosis of delirium | |||||||
| DSM‐5 | — | 5 criterion | General medical and surgical | 30 | 100 97 | 100 97 | κ = 0.73 112 |
| CAM | DSM‐3R |
4 core features 9 items |
General medical and surgical | 5 | 43–94 113 | 83–100 113 | κ = 0.88 114 |
| CAM‐ICU | CAM | 4 core features | Critically ill, especially intubated and sedated | 2 | 93–100 104 | 98–100 104 | κ = 0.96 104 |
| bCAM | CAM |
4 core features 7 items |
Emergency | <2 | 78–84 105 | 96–97 105 | κ = 0.88 105 |
| 3D‐CAM | CAM |
4 core features 20 items |
General medical and surgical | 3 | 95 106 | 94 106 | 95% a , 106 |
| ICDSC | DSM‐4 | 8 items | Critically ill | <5 | 99 107 | 64 107 | NR |
| 4AT | DSM‐4 | 4 items | General medical and surgical | <2 | 90 108 | 84 108 | NR |
| Severity of delirium | |||||||
| MDAS | DSM‐4 | 10 items | General medical and surgical | 10–15 |
Correlations among three tools b : MDAS and DRS‐R‐98, r = 0.91; MDAS and CAM‐S LF, r = 0.91; MDAS and CAM‐S SF, r = 0.87; DRS‐R‐98 and CAM‐S LF, r = 0.88; DRS‐R‐98 and CAM‐S SF, r = 0.82; CAM‐S LF and CAM‐SF, r = 0.96. 115 |
ICC = 0.92 109 | |
| DRS‐R‐98 | DSM‐3 | 16 items (3 items for diagnosis and 13 items for severity) | General medical and surgical | 20–30 |
DRS‐R‐98 total, ICC = 0.98; DRS‐R‐98 severity, ICC = 0.99 110 |
||
| CAM‐S | CAM |
SF, 4 core features; LF, 10 core features |
General medical and surgical |
LF, 10; SF, 5 |
LF, ICC = 0.88; SF, ICC = 0.92 111 |
||
Abbreviation: 3D‐CAM, 3‐Minute Diagnostic Interview for Delirium using the Confusion Assessment Method; 4AT, The 4‐item Assessment Test; bCAM, Brief Confusion Assessment Method; CAM, Confusion Assessment Method; CAM‐ICU, Confusion Assessment Method for the Intensive Care Unit; CAM‐S, Confusion Assessment Method‐Severity; CI, confidential interval; DRS‐R‐98, Delirium Rating Scale, Revised Version; DSM, Diagnostic and Statistical Manual; ICC, intraclass correlation coefficient; ICDSC, Intensive Care Delirium Screening Checklist; LF, long‐form; MDAS, Memory Delirium Assessment Scale; NR, not reported; SF, short‐form.
Indicating interrater agreement.
There is no gold standard for assessing the severity of delirium.
Perioperative delirium assessment is usually performed in high‐risk patients or in those who suffer any acute change in mental status (Figure 2).
FIGURE 2.
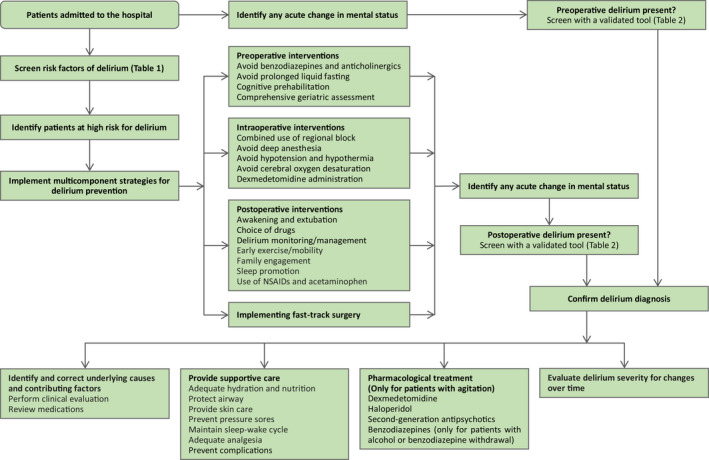
Suggested algorithm for assessment, prevention, and treatment of delirium
5.2. Diagnosis of delayed neurocognitive recovery and postoperative NCD/POCD
The objective evidence for NCD is “modest (mild NCD) or significant (major NCD) cognitive decline from the previous level of performance in one or more cognitive domains (complex attention, executive function, learning, and memory, language, perceptual‐motor, or social cognition).” 97 Traditionally, a battery of neurocognitive tests is used to evaluate the function of different cognitive domains. Among the individual neurocognitive tests, the digit span test (including forward and backward subtests), the trail‐making test part A, and the digit symbol substitution test are the most commonly used. 116 , 117 However, accomplishing a battery of tests is time‐consuming. To simplify the diagnosing process, several brief tools are being used to detect mild cognitive impairment; among them, the Montreal Cognitive Assessment (MoCA), the Addenbrooke's Cognitive Exam (ACE‐III), and the Quick MCI Screen (Qmci) are regarded as promising in the perioperative environment (Table 3). 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129
TABLE 3.
Characteristics of brief tools for detecting mild cognitive impairment
| Screening tools | Domains included | Time taken (min) | Total score | Cut‐off score | Sensitivity (%) | Specificity (%) | Interrater reliability |
|---|---|---|---|---|---|---|---|
| MoCA | Visuospatial/executive, language, memory, and delayed recall | 10–15 min | 30 | 23 | 78–87 119 , 120 , 121 | 73–98 119 , 120 , 121 | 0.96 119 |
| 24 | 80–89 122 , 123 , 124 , 125 | 64–83 122 , 123 , 124 , 125 | |||||
| 26 | 90–96 120 , 124 , 126 , 127 | 58–87 120 , 124 , 126 , 127 | |||||
| ACE‐III | Visuospatial, verbal fluency, language, memory, and attention | 16 min | 100 | 88 | 75–80 125 , 128 , 129 | 86–92 125 , 128 , 129 | 0.996 128 |
| Qmci | Orientation, word registration, clock drawing, delayed recall, verbal fluency, and logical memory | 3–5 min | 100 | 52 | 69 127 | 97 127 | 1.0 127 |
| 62 | 90 124 | 87 124 | |||||
| 65 | 94 124 | 80 124 |
Abbreviation: MoCA, Montreal Cognitive Assessment; ACE, Addenbrooke's Cognitive Exam; Qmci, Quick Mild Cognitive Impairment Screen.
According to the DSM‐5, mild NCD requires a decline of 1 to 2 standard deviations (for test results with normal distribution) or 3rd to 16th percentile (for test results with nonnormal distribution); major NCD requires a decline of >2 standard deviations (normal distribution) or 3rd percentile or below (nonnormal distribution). 1 , 97 Normal or baseline values are required when using the above cut‐points to quantify the extent of cognitive changes. The z‐score calculation is widely accepted when both baseline and control groups are available. 130 For each test, the mean practice effect is subtracted from the difference between preoperative and postoperative test scores; the result is then divided by the control group standard deviation to obtain a z‐score. The z‐scores of all tests in an individual patient are summarized and divided by the standard deviations for this sum of z‐scores in the control subjects, creating a composite z‐score. Cognitive decline is defined when two z‐scores in individual tests or the composite z‐score are −1.96 or less. 130
The diagnosis of POCD is confined to research and requires an only objective decline in cognition. 116 Different from POCD, the diagnosis of NCD requires the presence of cognitive concerns and the evaluation of daily activity in addition to the evidence of cognitive decline. 97 Cognitive concerns can be provided by individuals, informants, or clinicians. History‐taking should be performed to assess cognitive concerns before and after surgery. Daily activity can be assessed with an appropriate tool for subtle changes; it can also be self‐reported or informant‐reported. Mild NCD requires that the daily function is overall maintained, whereas major NCD requires a decline of daily function. 1 Because of pharmacological and surgical interventions, assessing cognitive symptoms and daily activity is problematic during a hospital stay and even after hospital discharge until a full recovery is achieved. Therefore, the Nomenclature Consensus Working Group 1 recommends that the term “delayed neurocognitive recovery” is used to describe the state of “NCD” for up to 30 days after surgery and that the term “postoperative mild/major NCD” is used thereafter until 12 months after surgery. The specifier “postoperative” is no longer used beyond 12 months unless the diagnosis is made within 12 months.
Cognitive function assessment is usually performed in high‐risk patients during preoperative and postoperative periods (Figure 2).
6. PREVENTION FOR PERIOPERATIVE NCDS
As expected, multiple strategies implemented by a multidisciplinary team have yielded substantial benefits. Some single interventions have demonstrated efficacies (Figures 2 and 3).
FIGURE 3.
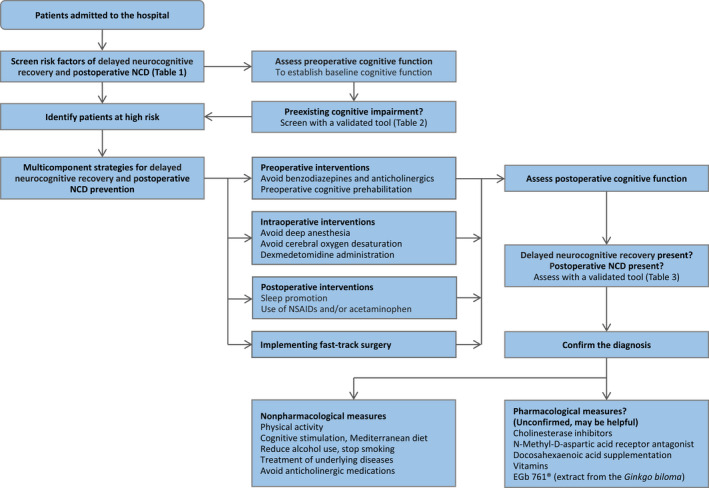
Suggested algorithm for assessment, prevention, and treatment of delayed neurocognitive recovery and postoperative neurocognitive disorders
6.1. Prevention for postoperative delirium
6.1.1. Preoperative measures
6.1.1.1. Avoiding benzodiazepines and anticholinergics
Benzodiazepines produce amnesia, anxiolysis, and sedation and are commonly used to treat anxiety and insomnia. Benzodiazepines also play a vital role in anesthetic practice. However, perioperative exposure to benzodiazepines is associated with delirium. 131 , 132 , 133 , 134 Guidelines recommend avoiding routine premedication with benzodiazepines, except for patients with severe anxiety, to reduce the risk of postoperative delirium. 135 , 136 Dexmedetomidine may be a suitable substitute for benzodiazepines but requires further investigation. 137
Anticholinergics have long been identified as an important risk factor for postoperative delirium and should be avoided. 58 , 138
6.1.1.2. Avoiding prolonged liquid fasting
Prolonged liquid‐fasting results in discomfort feelings such as thirst and anxiety and increases the incidence of postoperative nausea and vomiting. Long duration of preoperative liquid‐fasting is also associated with an increased risk of emergence delirium in both adult 56 and pediatric patients. 57 Shortening the duration of liquid‐fasting to 2 h, as recommended by guidelines, 139 may reduce the incidence of emergence delirium but requires further validation.
6.1.1.3. Cognitive prehabilitation
Low cognitive reserve, as manifested by low educational levels, less participation in cognitive activities (such as reading, writing, playing games, emailing, and singing), or low cognitive test scores, is a crucial risk factor for developing delirium after surgery. 140 , 141 Cognitive training effectively maintains cognitive function in healthy older people and improves cognitive function in patients with dementia. 142 , 143 In a recent trial, 251 older patients undergoing major noncardiac surgery were randomly assigned to receive no cognitive training or 1‐h cognitive exercise for 10 consecutive days before surgery; the results showed that cognitive prehabilitation reduced the incidence of postoperative delirium from 23.0% to 14.4%, although the differences did not reach statistical significance (p = 0.08). 144 More extensive trials are warranted to verify the effects.
6.1.1.4. Comprehensive geriatric assessment
Comprehensive geriatric assessment is a coordinated multidisciplinary assessment of the medical, psychosocial, environmental, and functional concerns in risky older patients. The purpose is to identify risk factors and establish a treatment plan. A recent comment suggested that a combined preoperative assessment for cognition, frailty, and mental disorders should be performed for older patients to identify those at high risk of postoperative neurological complications. 3 Available studies showed that comprehensive geriatric assessment reduces delirium and improves outcomes in patients with hip fracture, 145 , 146 possibly by the multicomponent intervention. 147
6.1.2. Intraoperative measures
6.1.2.1. Neuraxial anesthesia and peripheral nerve block
Neuraxial anesthesia has the advantages of reducing opioid consumption, blunting stress response, and providing better analgesia when compared with general anesthesia alone. Neuraxial anesthesia is recommended over general anesthesia for patients undergoing major surgery to improve postoperative outcomes. 148 , 149
Lower limb orthopedic surgery can be performed with either neuraxial anesthesia or general anesthesia. Early studies regarding the effect of neuraxial anesthesia in hip fracture patients did not reach a conclusion. 150 A population‐based cohort study found that the use of neuraxial anesthesia was associated with less delirium following hip/knee arthroplasties. 5 However, two recent large sample size trials reported neutral results, that is, neuraxial anesthesia compared with general anesthesia did not reduce delirium in patients following hip fracture surgery. 151 , 152 So neuraxial anesthesia is not superior to general anesthesia regarding postoperative delirium in this patient population.
Most major surgeries are performed under general anesthesia. A 2014 meta‐analysis showed that compared with general anesthesia alone, those given combined epidural‐general anesthesia developed less cardiopulmonary and gastrointestinal complications and had lower mortality after surgery. 148 In a recent randomized trial of 1802 patients following major thoracic and abdominal surgeries, those given combined epidural‐general anesthesia had one‐third as much delirium when compared with those given general anesthesia alone. 153 Combined epidural‐general anesthesia should be considered in patients undergoing major surgery and at high risk of postoperative delirium.
Peripheral nerve block is increasingly used as an alternative to neuraxial anesthesia; when compared with neuraxial block, it provides a slightly less analgesic effect but has fewer side effects, including hypotension and urinary retention. 154 , 155 , 156 Studies reported that femoral nerve block reduced the incidence of postoperative delirium in patients following hip fracture surgery 157 or total knee replacement. 158 A recent meta‐analysis of 8 randomized controlled trials showed that regional nerve block reduced perioperative delirium in older hip fracture patients, but the effect was limited to those without preoperative cognitive impairment. 159 The effect of peripheral nerve block on postoperative delirium requires further validation.
6.1.2.2. Choice of anesthetics during general anesthesia
Both intravenous and inhalational anesthetics are commonly used during general anesthesia. A recent meta‐analysis included 12 high‐quality randomized trials with 1440 patients; the results showed that propofol‐based intravenous maintenance compared with inhalational maintenance reduced emergence delirium by a half. 160 Emergence delirium is found to be associated with postoperative delirium. 67 However, the impact of anesthetic choice on postoperative delirium remains unclear. In a 2018 meta‐analysis, only five trials with 321 patients compared the effect of anesthetic maintenance agents on postoperative delirium and found no significant difference. 161 A 2019 meta‐analysis included trials in patients undergoing coronary artery bypass grafting; no difference was found in postoperative delirium between volatile versus intravenous anesthesia. 162
Xenon is a noble gas with anesthetic properties, mainly by inhibiting N‐methyl‐D aspartate receptors in the central nervous system. Xenon anesthesia provides more stable intraoperative hemodynamics and faster emergence from anesthesia than propofol and other volatile agents. 163 In an early pilot trial, patients given xenon anesthesia had less delirium following cardiac surgery. 164 However, a subsequent trial did not find a difference between the xenon and sevoflurane groups. 165 In a phase II randomized trial of 256 elderly patients undergoing hip fracture surgery, xenon anesthesia did not reduce postoperative delirium. 166
Therefore, the impact of anesthetic maintenance agents on postoperative delirium deserves further study.
6.1.2.3. Monitoring of anesthesia depth
Electroencephalogram‐based anesthetic depth, such as bispectral index (BIS), is increasingly monitored during general anesthesia. Studies suggest that BIS‐guided anesthesia maintenance avoids unnecessary deep anesthesia, decreases anesthetic consumption, and improves emergence from anesthesia after surgery. 167 , 168 , 169 Meta‐analyses showed that BIS‐guided anesthesia compared with no BIS‐guided anesthesia decreased delirium at 1 day after surgery. 170 , 171 Another meta‐analysis of 10 randomized controlled trials also showed that light anesthesia was associated with a decrease in postoperative delirium when compared with deep anesthesia. 61 A recent trial reported similar results, that is, patients given light anesthesia developed less postoperative delirium. 172 Thus, anesthesia depth should be monitored to avoid deep anesthesia in high‐risk patients.
6.1.2.4. Avoiding intraoperative hypotension and hypothermia
Accumulating evidence shows that even short durations of intraoperative hypotension are associated with adverse outcomes, including acute kidney injury, myocardial injury, and death. 173 , 174 Some retrospective cohort studies reported an association between intraoperative hypotension and postoperative delirium. 66 , 175 However, a meta‐analysis of randomized trials did not find a significant association between intraoperative hypotension and the risk of postoperative delirium. 176 Two systematic reviews also conclude that there is no convincing evidence that intraoperative hypotension is a risk factor of postoperative delirium. 177 , 178 Nevertheless, intraoperative hypotension should be avoided considering the harmful effects on other organs.
Hypothermia is common during general anesthesia and surgery. 179 Intraoperative hypothermia is associated with adverse outcomes such as increased bleeding, more wound infection, and increased cardiac events. 180 Furthermore, intraoperative hypothermia prolongs the duration of anesthetic agents and delays postanesthesia recovery. 179 , 181 In a prospective cohort study, intraoperative hypothermia (<36°C) was an independent risk factor of the emergence of delirium. 67 A recent retrospective study found a significant relationship between perioperative hypothermia and postoperative delirium and a complex relationship among perioperative hypothermia, age, ASA class, and postoperative delirium. 182 Avoiding intraoperative hypothermia may help to reduce postoperative delirium but requires further validation.
6.1.2.5. Monitoring of cerebral oxygen saturation
In critically ill patients, dysfunction of cerebral autoregulation is associated with delirium. 183 Indeed, cerebral hypoxia is found to be a potential risk factor for perioperative NCDs. For example, studies reported that patients with low preoperative cerebral oxygen saturation had a higher incidence of postoperative delirium 184 , 185 ; intraoperative cerebral oxygen desaturation was associated with increased postoperative delirium. 62 , 63 , 64 A pairwise and network meta‐analysis included 12 randomized trials with 1626 patients investigating the effect of brain near‐infrared spectroscopy‐based intervention; the results showed that cerebral oxygenation‐guided management reduced delirium after cardiac surgery but not major noncardiac surgery. 186 Cerebral oxygenation‐guided management may be helpful in patients undergoing cardiac surgery. The optimal threshold and the effect in noncardiac surgery patients require further evaluation.
6.1.2.6. Dexmedetomidine
Dexmedetomidine is a highly selective α2‐receptor agonist with anxiolytic, sedative, and analgesic properties. It has been widely used as an adjuvant during anesthesia and for sedation in the ICU. A 2021 meta‐analysis of 13 randomized trials showed that perioperative administration of dexmedetomidine, either intraoperatively or intra‐ and postoperatively, significantly reduced delirium in elderly patients following noncardiac surgery. 187 Studies in cardiac patients remains controversial. A recent meta‐analysis included 30 randomized trials comprising 4090 patients undergoing cardiac surgery. With unselected trials, dexmedetomidine compared with control decreased the incidence of postoperative delirium (RR 0.62, 95% CI 0.44–0.86, p = 0.005); however, the difference was not statistically significant after excluding trials at high risk of bias (RR 0.71 95% CI 0.49–1.03, p = 0.070). 188
6.1.3. Postoperative measures
6.1.3.1. Nonpharmacologic strategies
Nonpharmacologic strategies targeting multiple risk factors are the first‐line measures for preventing postoperative delirium. 189 The relevant interventions usually include reorientation and cognitive stimulation, sleep enhancement, early mobility and exercise, vision and hearing optimization, family engagement and empowerment, and early oral intake or nutrition. A recent meta‐analysis shows that multicomponent nonpharmacological interventions effectively reduce the incidence of delirium; the interventions may also shorten the length of hospital stay. 190 The Awakening and Breathing Coordination, Choice of drugs, Delirium monitoring and management, Early mobility, and Family engagement (ABCDEF) bundle are designed for critically ill patients in the ICU; it effectively reduces delirium and promotes weaning from mechanical ventilation. 191 , 192 A subsequent large sample size study also showed that implementation of the bundle is associated with meaningful improvement in the outcomes, including delirium and survival. 193
6.1.3.2. Sleep promotion
Sleep disturbances frequently occur in patients after surgery. Poor sleep quality is associated with an increased risk of delirium. 194 Sleep promotion is an essential component of delirium prevention strategies. 195 As mentioned above, nonpharmacological interventions are first‐line measures for sleep promotion; these include maintaining a quiet and dim environment, decreasing interruptions from care activities at night, and using overnight eye masks and earplugs. Evidence shows that the use of earplugs and/or eye masks may be beneficial to improve sleep and reduce delirium in ICU patients. 196
As part of a multimodal approach, pharmacotherapy may be necessary to improve sleep. Nonbenzodiazepine agents are preferred for this purpose. 197 Melatonin and melatonin receptor agonists (ramelteon) are now used to consolidate the circadian rhythm. A 2021 meta‐analysis of 14 trials with 1712 participants reported that the use of melatonin or ramelteon is associated with improved sleep quality and reduced delirium in surgical and ICU patients. 198 However, a recent multicenter trial of 847 patients (including 1/4 surgical patients) showed that enteral melatonin initiated within 48 h of ICU admission did not reduce delirium. 199 Suvorexant is a new orexin receptor antagonist approved for the treatment of insomnia. It has been used to improve sleep in hospitalized patients. A systematic review of 6 studies with 645 acutely hospitalized patients (including surgical patients) showed that those given suvorexant for sleep promotion developed less delirium and had a shorter length of hospital stay. 200
Dexmedetomidine exerts sedative effects by activating the endogenous sleep‐promoting pathway 201 and is thus used to promote sleep. In critically ill patients with mechanical ventilation, night‐time infusion of sedative‐dose dexmedetomidine improves sleep by increasing sleep efficacy and stage 2 sleep and maintaining circadian rhythm. 202 Night‐time low‐dose dexmedetomidine infusion in ICU patients without mechanical ventilation also improves sleep architecture and subjective sleep quality. 203 In line with these, night‐time dexmedetomidine reduced delirium in ICU patients. 99 , 204 It is worthy to note that an even lower dose of dexmedetomidine, such as used as an adjuvant in the patient‐controlled analgesia pump, also improves sleep quality after surgery. 205 Dexmedetomidine supplemented analgesia may also reduce delirium but requires further investigation. 206
6.1.3.3. Nonsteroidal anti‐inflammatory drugs and acetaminophen
Severe pain is associated with an increased risk of delirium. Inflammation provoked by surgery plays a vital role in the pathogenesis of postoperative delirium. Nonsteroidal anti‐inflammatory drugs (NSAIDs) and acetaminophen are commonly used adjuvant analgesics; NSAIDs also effectively alleviate the degree of surgery‐related inflammatory response. These drugs are therefore investigated to prevent postoperative delirium. In a randomized trial of elderly patients undergoing hip or knee arthroplasty, parecoxib supplemented morphine analgesia almost halves the incidence of postoperative delirium. 207 In older patients following cardiac surgery, intravenous acetaminophen reduced delirium by 64%. 208 In a small sample size trial, flurbiprofen axetile supplemented analgesia decreased delirium in a subgroup of patients over 70 years after major noncardiac surgery. 209 Thus, NSAIDs and acetaminophen should be considered for delirium prevention in patients without contraindications.
6.1.4. Implementing fast‐track surgery
Fast‐track surgery (or enhanced recovery after surgery) is a multimodal approach to improve patient care by adopting a combination of evidence‐based interventions by a multidisciplinary team; the purpose is to expedite recovery after surgery. Strategies are adopted to reduce the surgical stress response and inflammation, facilitate physical rehabilitation, and enhance early return to normal circadian rhythm, all of which may improve postoperative cognitive functions. Studies reported that fast‐track surgery is associated with a reduced incidence of delirium in patients following hip and knee arthroplasty 210 , 211 and colorectal surgery. 212 In a meta‐analysis, implementing enhanced recovery after surgery programs in hip fracture patients halved the incidence of postoperative delirium. 213 Strategies for fast‐track surgery and enhanced recovery after surgery are recommended for postoperative delirium prevention. 135
6.1.5. Delirium prevention in patients with COVID‐19
Neurological manifestations may occur in COVID‐19 patients. 214 , 215 Encephalopathy, which is defined as global cerebral disturbance and expressed as either subsyndromal delirium, delirium, or coma, predicts adverse outcomes in patients with COVID‐19 infection. 216 Delirium is common but often ignored in ICU patients with COVID‐19. 217 , 218 Identified risk factors include mechanical ventilation, use of restraints, infusion of benzodiazepines, opioids and vasopressors, and administration of antipsychotics; whereas family visitation (in person or online) was associated with less delirium. 218 However, implementation of the ABCDEF bundle is challenging in this patient population due to multiple reasons, such as changes in critical care hierarchy and priorities, shortages of medical staff and personal protective equipment, reduced bedside presence, and increased use of deep sedation and neuromuscular blockade. 219 Strategies to optimize bundle performance have been proposed. 219 , 220 According to recent study results, reducing benzodiazepine sedation and increasing family visit should be especially encouraged for delirium prevention in this patient population. 218
6.2. Prevention for delayed neurocognitive recovery and postoperative NCD/POCD
6.2.1. Preoperative measures
6.2.1.1. Avoiding benzodiazepines and anticholinergics
Studies in the geriatric population showed that the use of benzodiazepine is associated with cognitive decline, dementia and Alzheimer's disease, although mixed findings exist; it seems that longer‐acting drugs, longer‐duration use, and early exposures are more harmful in this aspect. 221 A randomized placebo‐controlled trial in children showed that premedication with low‐dose midazolam provoked significant short‐term impairment of cognitive function and amnesia enduring for 48 h. 222 In a randomized trial of old patients, intraoperative sedation with midazolam increased delayed neurocognitive recovery at 7 days; but no difference in cognition function was found at 1 year. 223 Routine premedication with benzodiazepine is not recommended in high‐risk patients.
Anticholinergics are also associated with an increased risk of cognitive decline after surgery and should be avoided in older patients. 224 , 225
6.2.1.2. Cognitive prehabilitation
Preoperative cognitive training may augment cognitive reserve and improve cognitive recovery after surgery. In a randomized trial of 141 older patients undergoing gastrointestinal surgery, cognitive training for three 1‐h sessions before surgery reduced the incidence of delayed neurocognitive recovery from 36.1% to 15.9% (p < 0.05). 226 The feasibility of perioperative cognitive training via a mobile device was also demonstrated in older adults undergoing cardiac surgery; those who received training reported improved memory and thinking ability. 227 Other trials are under way to further examine the effect of cognitive prehabilitation on postoperative cognition. 228
6.2.2. Intraoperative measures
6.2.2.1. Neuraxial anesthesia and peripheral nerve block
A small sample size trial compared general versus spinal anesthesia in hip fracture patients 229 ; another small sample size trial compared general versus combined epidural‐general anesthesia in older patients undergoing abdominal surgery. 230 Both reported no significant difference in delayed neurocognitive recovery after surgery. 229 , 230 As high‐quality studies are still lacking, conclusions cannot be reached regarding the effect of neuraxial anesthesia on postoperative cognition at the moment.
In a 2021 meta‐analysis of 122 studies on patients undergoing total hip/knee arthroplasty surgery, the use of peripheral nerve block (compared with no use) was associated with lower risks of numerous complications including cognitive dysfunction after surgery. 231 In a small sample trial of patients following esophageal cancer surgery, the combined use of intercostal nerve block was associated with improved cognitive function recovery. 232 Therefore, peripheral nerve block should be considered for patients without contraindications, either alone or in combination with neuraxial or general anesthesia. 233 Further studies are required to confirm these findings.
6.2.2.2. Choice of anesthetics during general anesthesia
In patients undergoing cardiac surgery, two small sample size trials reported that inhalational anesthesia was associated with less delayed neurocognitive recovery than propofol. 234 , 235 However, evidence is limited in this aspect. Subsequent meta‐analysis and a large sample size trial showed that anesthetic (volatile or total intravenous) choice does not change clinical outcomes including 1‐year mortality in cardiac surgery patients. 162 , 236
In patients undergoing non‐cardiac surgery, a 2018 meta‐analysis showed that propofol‐based total intravenous anesthesia may reduce delayed neurocognitive recovery with low‐certainty evidence 161 ; whereas a 2019 meta‐analysis showed that propofol has more adverse effect on postoperative cognitive function in lung cancer patients. 237 Subsequent trials also gave conflicting results, one reported less delayed neurocognitive recovery with intravenous anesthesia 130 ; two others did not find significant differences. 238 , 239 Thus, available evidence does not suggest which anesthetic (propofol or volatile anesthetics) is better regarding postoperative cognitive recovery.
In a small sample size trial, Bronco et al 240 reported that xenon anesthesia was associated with faster emergence and better cognitive recovery at 30 and 60 min after extubation. However, two other trials did not find difference in postoperative cognitive function at 6–12 and 66–72 h after surgery when comparing xenon versus sevoflurane 241 or desflurane anesthesia. 242 Current evidence does not support that xenon is superior to other anesthetics regarding postoperative cognitive function.
6.2.2.3. Monitoring of anesthesia depth
A 2020 meta‐analysis including 8 trials showed that BIS‐guided (or BIS ≥50) anesthesia compared with no BIS‐guided (or BIS <50) anesthesia decreased POCD at 12 weeks after surgery. 171 Two subsequent large sample size trials reported different results. A trial including 1232 older patients found that BIS‐guided anesthesia compared with usual care did not reduce cognitive impairment at 30 days. 243 Another multicenter trial including 655 patients reported that patients given light anesthesia (BIS 50) had better cognitive function than those given deep anesthesia (BIS 35) at 1‐year follow‐up. 172
There are also contrary results. For example, two small sample size trials comparing the effect of deep (BIS 30–45) versus light (BIS 45–65) total intravenous anesthesia reported that patients given deep anesthesia developed less delayed neurocognitive recovery. 244 , 245 The underlying reasons leading to the conflicting results are unclear but may include the difference in anesthetics. We note that most of the available studies were conducted in patients given inhalational or combined inhalational‐intravenous anesthesia; avoiding deep anesthesia may be helpful to improve postoperative cognitive recovery. However, the optimal anesthesia depth during total intravenous anesthesia requires further study.
6.2.2.4. Avoiding intraoperative hypotension
A 2020 comprehensive review included 13 studies with 3017 patients undergoing noncardiac surgery, 177 a 2021 systematic review included 6 studies with 1222 patients undergoing cardiac surgery 178 ; both reviews did not find a significant association between intraoperative hypotension and postoperative cognitive impairment. Two other systematic review/meta‐analysis included studies of patients undergoing either cardiac or noncardiac surgeries also gave similar results. 176 , 246 Thus, available evidence did not find a conclusive association between intraoperative hypotension and postoperative cognitive decline.
6.2.2.5. Monitoring of cerebral oxygen saturation
Accumulating evidence suggests that intraoperative cerebral oxygen desaturation is associated with an increased risk of delayed neurocognitive recovery. 27 , 64 , 89 , 90 In a 2018 meta‐analysis of 15 randomized trials comprising 1822 adult patients, management according to perioperative cerebral oxygenation monitoring reduced the occurrence of delayed neurocognitive recovery; however, the quality of evidence was low. 247 In a recent meta‐analysis of 12 randomized trials with 1868 cardiac surgical patients, cerebral oxygenation‐guided intervention is associated with less delayed neurocognitive recovery. 248 Monitoring of cerebral oxygen saturation may be especially helpful for the management of patients with dysfunctional cerebral autoregulation. 183 A recent consensus recommends that cerebral oxygenation should be monitored in patients at risk of acute cerebral hypoperfusion. 249
6.2.2.6. Dexmedetomidine
Perioperative dexmedetomidine administration exerts beneficial effects on postoperative cognitive recovery. A meta‐analysis of 26 randomized trials confirmed that patients given perioperative dexmedetomidine had reduced incidence of delayed neurocognitive recovery within 7 days after surgery; the effect was partially attributed to the blunted inflammatory response. 250 In a long‐term follow‐up study of 700 older patients admitted to ICU after surgery, those given dexmedetomidine had better cognitive function at 3 years. 251 The effects of perioperative dexmedetomidine on early and long‐term neurocognitive outcomes deserve further investigation.
6.2.3. Postoperative measures
6.2.3.1. Nonpharmacological measures
In a recent randomized trial of 609 older patients scheduled for cardiovascular surgery, nonpharmacological measures for delirium prevention including reorientation, sleep aids and early mobilization did not reduce delayed neurocognitive recovery at 1 week and POCD at three and 12 months after surgery. 252
6.2.3.2. Sleep promotion
Sleep disturbance is associated with an increased risk of cognitive impairment after surgery, especially in older patients. 253 , 254 The effect of pharmacological sleep promotion on postoperative cognitive function remains insufficiently investigated. In a small sample trial with 54 patients following breast cancer surgery, use of melatonin for sleep promotion increased sleep efficiency and total sleep time but did not affect cognitive function. 255 In a randomized trial of 139 older patients undergoing hip arthroplasty, supplemental melatonin from 1 day before until 5 days after surgery improved subjective sleep quality and cognitive function after surgery. 256 Non‐sedative dose dexmedetomidine seems also effective for this purpose. 99 , 203 Three‐year follow‐up of a randomized trial found that low‐dose dexmedetomidine improved long‐term cognitive function and quality of life. 251 Sleep promotion may be effective to improve postoperative cognitive recovery but deserves further validation.
6.2.3.3. Nonsteroidal anti‐inflammatory drugs
Peripheral inflammation provoked by anesthetic exposure and surgery can induce blood–brain barrier disruption 257 ; the resulting neuroinflammation is found to be an important underlying mechanism of perioperative NCDs. 258 Therefore, intervention targeting perioperative inflammation may be effective in the prevention. 259 A 2020 meta‐analysis included 8 randomized trials with 1106 patients preparing for orthopedic surgery; the results showed that perioperative parecoxib administration decreased the incidence of delayed neurocognitive recovery and improved Mini‐Mental Examination score early after surgery. 260 Parecoxib should be considered in high‐risk patients without contraindications. Further studies are required to validate the effect of other nonsteroidal anti‐inflammatory drugs.
6.2.4. Implementing fast‐track surgery
Implementing strategies of fast‐track surgery (or enhanced recovery after surgery) are associated with improved outcomes including reduced complications and shortened length‐of‐stay. 261 , 262 Observational studies showed that delayed neurocognitive recovery and POCD were rare after fast‐track hip‐ and knee‐ arthroplasty. 86 , 263 Whether a fast‐track surgery strategy can improve postoperative cognitive function needs to be verified by well‐designed randomized controlled trials.
7. TREATMENT FOR PERIOPERATIVE NCDS
7.1. Treatment for postoperative delirium
Diagnosed delirium can be classified into hypoactive, hyperactive, and mixed subtypes. No matter what subtype is, the basic treatment measures include correcting the underlying causes, providing supportive care, and controlling delirious symptoms. 100 , 101 , 264 The underlying causes are usually multiple and should be identified and promptly addressed. The purposes of supportive care are to meet patients' daily needs and prevent complications. Multicomponent nonpharmacological measures for delirium prevention are also suggested for treatment purposes, 265 but the effectiveness has not been verified. 266 , 267
Pharmacological treatment is only reserved for patients with severe agitation, that is, when delirium symptoms may threaten patients' safety or the safety of others. 101 , 264 Antipsychotics including haloperidol and second‐generation antipsychotics are traditionally used to control symptoms. However, recent meta‐analyses do not find differences in delirium duration, delirium severity, and other clinical outcomes when comparing antipsychotics versus placebo. 268 , 269 , 270 The potential adverse effects of antipsychotics also cause concerns. For example, in a randomized trial investigating the treatment effect of antipsychotics in palliative care settings, patients given risperidone or haloperidol had higher delirium symptom scores and more extrapyramidal effects; patients given haloperidol also had worse overall survival. 271 A meta‐analysis reported that antipsychotic therapy in dementia and general mental health care is associated with increased mortality. 272 Therefore, antipsychotics should not be routinely used for delirium treatment. 270
Studies regarding the treatment effect of dexmedetomidine for delirium are relatively rare. In a 2019 meta‐analysis of randomized trials investigating the effects of pharmacological treatment of delirium in critically ill adults, dexmedetomidine is the only drug that shortens delirium duration; it also shortens the duration of mechanical ventilation. 273 However, the results were based on a single study. 273 A 2021 meta‐analysis of 10 randomized trials and 5 observational studies evaluated the efficacy of dexmedetomidine in treating delirium; in one trial, dexmedetomidine shortened the duration of delirium; in 6 studies, it was associated with a lower point‐prevalence of delirium and a shorter time to resolution of delirium; in 4 trials, it was superior to haloperidol in shortening the time to delirium resolution. 274 Dexmedetomidine is a promising agent for delirium treatment. On the contrary, benzodiazepines should not be used to treat agitated delirious patients unless their use is specifically indicated such as for the treatment of alcohol or benzodiazepine withdrawal. 275
Treatment for postoperative delirium is summarized in Figure 2.
7.2. Treatment for delayed neurocognitive recovery and postoperative NCD
For established delayed neurocognitive recovery, many and perhaps most patients will recover completely from early postoperative cognitive impairment; only a fraction of patients evolve into postoperative NCD. It is necessary to inform patients of the occurrence of this event and track the changes in cognitive function.
There are no specific treatments for delayed neurocognitive recovery or postoperative NCD. Measures for cognitive decline, including mild cognitive impairment or dementia in the general population, are proposed for treatment purposes. 276 Among these, nonpharmacological measures mainly target modifiable risk factors and include physical activity, 277 , 278 cognitive stimulation, 279 , 280 Mediterranean diet, 281 reducing alcohol use, 282 stop smoking, 283 treatment of underlying diseases, 284 and avoiding anticholinergic medications. 285
Several pharmacological agents are approved for the treatment of Alzheimer's Disease and dementia with modest benefits, including cholinesterase inhibitors (donepezil, galantamine, and rivastigmine) and an N‐Methyl‐D‐aspartic acid receptor antagonist (memantine). 286 , 287 Currently, there are no pharmacological therapies approved to treat mild cognitive impairment. Donepezil does not show benefit in this patient population. 288 Vitamins and docosahexaenoic acid supplementation may be helpful but requires further confirmation. 289 , 290 Multidomain intervention may be more beneficial. 291 Many studies are still ongoing. EGb 761®, a special extract from the Ginkgo biloba, is suggested in the treatment of mild cognitive impairment and dementia. 292 , 293 However, more high‐quality evidences are needed to support the efficacy. It should be noted that available studies are rarely performed in perioperative patients; therefore, the effects of these interventions on postoperative cognitive recovery remain to be determined.
Potential treatment for delayed neurocognitive recovery and postoperative NCD is summarized in Figure 3.
8. SUMMARY
Perioperative NCDs are common and distressing conditions in older patients following surgery and are associated with increased morbidity and mortality and enormous medical costs. Advances in diagnosis have improved assessment and risk stratification. Identifying high‐risk patients and avoiding precipitating factors during the perioperative period is pivotal for prevention. Effective measures are emerging but require further validation. Ongoing efforts are needed on preventive and treatment strategies to optimize patients' outcomes.
9. AUTHORS’ CONTRIBUTIONS
HK participated in literature search, figure drawing, and initial writing and revision of the manuscript; L‐MX participated in literature search, figure drawing, and initial writing of the manuscript; D‐XW participated in literature search, critical revision, and approval of the final version. We confirmed that the manuscript had been read and approved by all named authors.
11. CONFLICT OF INTEREST
The authors declare no conflict of interest.
12.
10. ACKNOWLEDGMENT
None.
Kong H, Xu L‐M, Wang D‐X. Perioperative neurocognitive disorders: A narrative review focusing on diagnosis, prevention, and treatment. CNS Neurosci Ther. 2022;28:1147‐1167. doi: 10.1111/cns.13873
Funding information
Supported by National Key R&D Program of China (Beijing, China) grant No. 2018YFC2001800.
12.1. DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Evered L, Silbert B, Knopman DS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery‐2018. Br J Anaesth. 2018;121(5):1005‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khachaturian AS, Hayden KM, Devlin JW, et al. International drive to illuminate delirium: A developing public health blueprint for action. Alzheimers Dement. 2020;16(5):711‐725. [DOI] [PubMed] [Google Scholar]
- 3. Alam A, Ma D. Is it time to assess neurological status before surgery to improve postoperative outcomes? Ann Surg. 2022;275(4):644‐645. [DOI] [PubMed] [Google Scholar]
- 4. Winter A, Steurer MP, Dullenkopf A. Postoperative delirium assessed by post anesthesia care unit staff utilizing the Nursing Delirium Screening Scale: a prospective observational study of 1000 patients in a single Swiss institution. BMC Anesthesiol. 2015;15:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Memtsoudis S, Cozowicz C, Zubizarreta N, et al. Risk factors for postoperative delirium in patients undergoing lower extremity joint arthroplasty: a retrospective population‐based cohort study. Reg Anesth Pain Med. 2019;44(10):934‐943. [DOI] [PubMed] [Google Scholar]
- 6. Silva AR, Regueira P, Albuquerque E, et al. Estimates of geriatric delirium frequency in noncardiac surgeries and its evaluation across the years: a systematic review and meta‐analysis. J Am Med Dir Assoc. 2021;22(3):613‐620. [DOI] [PubMed] [Google Scholar]
- 7. Berian JR, Zhou L, Russell MM, et al. Postoperative delirium as a target for surgical quality improvement. Ann Surg. 2018;268(1):93‐99. [DOI] [PubMed] [Google Scholar]
- 8. Gosselt AN, Slooter AJ, Boere PR, Zaal IJ. Risk factors for delirium after on‐pump cardiac surgery: a systematic review. Crit Care. 2015;19(1):346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aitken SJ, Blyth FM, Naganathan V. Incidence, prognostic factors and impact of postoperative delirium after major vascular surgery: a meta‐analysis and systematic review. Vasc Med. 2017;22(5):387‐397. [DOI] [PubMed] [Google Scholar]
- 10. Oldroyd C, Scholz AFM, Hinchliffe RJ, McCarthy K, Hewitt J, Quinn TJ. A systematic review and meta‐analysis of factors for delirium in vascular surgical patients. J Vasc Surg. 2017;66(4):1269‐1279. [DOI] [PubMed] [Google Scholar]
- 11. Wu J, Yin Y, Jin M, Li B. The risk factors for postoperative delirium in adult patients after hip fracture surgery: a systematic review and meta‐analysis. Int J Geriatr Psychiatry. 2021;36(1):3‐14. [DOI] [PubMed] [Google Scholar]
- 12. Saljuqi AT, Hanna K, Asmar S, et al. Prospective evaluation of delirium in geriatric patients undergoing emergency general surgery. J Am Coll Surg. 2020;230(5):758‐765. [DOI] [PubMed] [Google Scholar]
- 13. Saravana‐Bawan B, Warkentin LM, Rucker D, Carr F, Churchill TA, Khadaroo RG. Incidence and predictors of postoperative delirium in the older acute care surgery population: a prospective study. Can J Surg. 2019;62(1):33‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chaiwat O, Chanidnuan M, Pancharoen W, et al. Postoperative delirium in critically ill surgical patients: incidence, risk factors, and predictive scores. BMC Anesthesiol. 2019;19(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gleason LJ, Schmitt EM, Kosar CM, et al. Effect of delirium and other major complications on outcomes after elective surgery in older adults. JAMA Surg. 2015;150(12):1134‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304(4):443‐451. [DOI] [PubMed] [Google Scholar]
- 17. Hamilton GM, Wheeler K, Di Michele J, Lalu MM, McIsaac DI. A systematic review and meta‐analysis examining the impact of incident postoperative delirium on mortality. Anesthesiology. 2017;127(1):78‐88. [DOI] [PubMed] [Google Scholar]
- 18. Crocker E, Beggs T, Hassan A, et al. Long‐term effects of postoperative delirium in patients undergoing cardiac operation: a systematic review. Ann Thorac Surg. 2016;102(4):1391‐1399. [DOI] [PubMed] [Google Scholar]
- 19. Bai J, Liang Y, Zhang P, et al. Association between postoperative delirium and mortality in elderly patients undergoing hip fractures surgery: a meta‐analysis. Osteoporos Int. 2020;31(2):317‐326. [DOI] [PubMed] [Google Scholar]
- 20. Goldberg TE, Chen C, Wang Y, et al. Association of delirium with long‐term cognitive decline: a meta‐analysis. JAMA Neurol. 2020;77(11):1373‐1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang H, Li H, Zhang X, et al. Association of postoperative delirium with cognitive outcomes: a meta‐analysis. J Clin Anesth. 2021;75:110496. [DOI] [PubMed] [Google Scholar]
- 22. Pereira JV, Aung Thein MZ, Nitchingham A, Caplan GA. Delirium in older adults is associated with development of new dementia: a systematic review and meta‐analysis. Int J Geriatr Psychiatry. 2021;36(7):993‐1003. [DOI] [PubMed] [Google Scholar]
- 23. Moller JT, Cluitmans P, Rasmussen LS, et al. Long‐term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post‐Operative Cognitive Dysfunction. Lancet. 1998;351(9106):857‐861. [DOI] [PubMed] [Google Scholar]
- 24. Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108(1):18‐30. [DOI] [PubMed] [Google Scholar]
- 25. Paredes S, Cortínez L, Contreras V, Silbert B. Post‐operative cognitive dysfunction at 3 months in adults after non‐cardiac surgery: a qualitative systematic review. Acta Anaesthesiol Scand. 2016;60(8):1043‐1058. [DOI] [PubMed] [Google Scholar]
- 26. Evered L, Scott DA, Silbert B, Maruff P. Postoperative cognitive dysfunction is independent of type of surgery and anesthetic. Anesth Analg. 2011;112(5):1179‐1185. [DOI] [PubMed] [Google Scholar]
- 27. Holmgaard F, Vedel AG, Rasmussen LS, Paulson OB, Nilsson JC, Ravn HB. The association between postoperative cognitive dysfunction and cerebral oximetry during cardiac surgery: a secondary analysis of a randomised trial. Br J Anaesth. 2019;123(2):196‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS. Long‐term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110(3):548‐555. [DOI] [PubMed] [Google Scholar]
- 29. Kahl U, Callsen S, Beck S, et al. Health‐related quality of life and self‐reported cognitive function in patients with delayed neurocognitive recovery after radical prostatectomy: a prospective follow‐up study. Health Qual Life Outcomes. 2021;19(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deiner S, Liu X, Lin HM, et al. Does postoperative cognitive decline result in new disability after surgery? Ann Surg. 2021;274(6):e1108‐e1114. [DOI] [PubMed] [Google Scholar]
- 31. Steinmetz J, Siersma V, Kessing LV, Rasmussen LS. Is postoperative cognitive dysfunction a risk factor for dementia? A cohort follow‐up study. Br J Anaesth. 2013;110(Suppl 1):i92‐i97. [DOI] [PubMed] [Google Scholar]
- 32. Liu B, Huang D, Guo Y, et al. Recent advances and perspectives of postoperative neurological disorders in the elderly surgical patients. CNS Neurosci Ther. 2022;28(4):470‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang Y, Zhao X, Dong T, Yang Z, Zhang Q, Zhang Y. Risk factors for postoperative delirium following hip fracture repair in elderly patients: a systematic review and meta‐analysis. Aging Clin Exp Res. 2017;29(2):115‐126. [DOI] [PubMed] [Google Scholar]
- 34. Scholz AF, Oldroyd C, McCarthy K, Quinn TJ, Hewitt J. Systematic review and meta‐analysis of risk factors for postoperative delirium among older patients undergoing gastrointestinal surgery. Br J Surg. 2016;103(2):e21‐e28. [DOI] [PubMed] [Google Scholar]
- 35. Chen H, Mo L, Hu H, Ou Y, Luo J. Risk factors of postoperative delirium after cardiac surgery: a meta‐analysis. J Cardiothorac Surg. 2021;16(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rong X, Ding ZC, Yu HD, Yao SY, Zhou ZK. Risk factors of postoperative delirium in the knee and hip replacement patients: a systematic review and meta‐analysis. J Orthop Surg Res. 2021;16(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhu C, Wang B, Yin J, et al. Risk factors for postoperative delirium after spinal surgery: a systematic review and meta‐analysis. Aging Clin Exp Res. 2020;32(8):1417‐1434. [DOI] [PubMed] [Google Scholar]
- 38. Chen Y, Qin J. Modified frailty index independently predicts postoperative delirium and delayed neurocognitive recovery after elective total joint arthroplasty. J Arthroplasty. 2021;36(2):449‐453. [DOI] [PubMed] [Google Scholar]
- 39. Wu X, Sun W, Tan M. Incidence and risk factors for postoperative delirium in patients undergoing spine surgery: a systematic review and meta‐analysis. Biomed Res Int. 2019;2019:2139834‐2139820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang Z, Wang XF, Yang LF, Fang C, Gu XK, Guo HW. Prevalence and risk factors for postoperative delirium in patients with colorectal carcinoma: a systematic review and meta‐analysis. Int J Colorectal Dis. 2020;35(3):547‐557. [DOI] [PubMed] [Google Scholar]
- 41. Li GH, Zhao L, Lu Y, et al. Development and validation of a risk score for predicting postoperative delirium after major abdominal surgery by incorporating preoperative risk factors and surgical Apgar score. J Clin Anesth. 2021;75:110408. [DOI] [PubMed] [Google Scholar]
- 42. Kang SY, Seo SW, Kim JY. Comprehensive risk factor evaluation of postoperative delirium following major surgery: clinical data warehouse analysis. Neurol Sci. 2019;40(4):793‐800. [DOI] [PubMed] [Google Scholar]
- 43. Guan HL, Liu H, Hu XY, et al. Urinary albumin creatinine ratio associated with postoperative delirium in elderly patients undergoing elective non‐cardiac surgery: A prospective observational study. CNS Neurosci Ther. 2022;28(4):521‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oliveira FR, Oliveira VH, Oliveira ÍM, et al. Hypertension, mitral valve disease, atrial fibrillation and low education level predict delirium and worst outcome after cardiac surgery in older adults. BMC Anesthesiol. 2018;18(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Greaves D, Psaltis PJ, Davis DHJ, et al. Risk factors for delirium and cognitive decline following coronary artery bypass grafting surgery: a systematic review and meta‐analysis. J Am Heart Assoc. 2020;9(22):e017275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oliveira JESL, Berning MJ, Stanich JA, et al. Risk factors for delirium in older adults in the emergency department: a systematic review and meta‐analysis. Ann Emerg Med. 2021;78(4):549‐565. [DOI] [PubMed] [Google Scholar]
- 47. Persico I, Cesari M, Morandi A, et al. Frailty and delirium in older adults: a systematic review and meta‐analysis of the literature. J Am Geriatr Soc. 2018;66(10):2022‐2030. [DOI] [PubMed] [Google Scholar]
- 48. Gracie TJ, Caufield‐Noll C, Wang NY, Sieber FE. The association of preoperative frailty and postoperative delirium: a meta‐analysis. Anesth Analg. 2021;133(2):314‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang XM, Jiao J, Xie XH, Wu XJ. The association between frailty and delirium among hospitalized patients: an updated meta‐analysis. J Am Med Dir Assoc. 2021;22(3):527‐534. [DOI] [PubMed] [Google Scholar]
- 50. Kosar CM, Tabloski PA, Travison TG, et al. Effect of preoperative pain and depressive symptoms on the development of postoperative delirium. Lancet Psychiatry. 2014;1(6):431‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shi C, Yang C, Gao R, Yuan W. Risk factors for delirium after spinal surgery: a meta‐analysis. World Neurosurg. 2015;84(5):1466‐1472. [DOI] [PubMed] [Google Scholar]
- 52. Korc‐Grodzicki B, Sun SW, Zhou Q, et al. Geriatric assessment as a predictor of delirium and other outcomes in elderly patients with cancer. Ann Surg. 2015;261(6):1085‐1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mazzola P, Ward L, Zazzetta S, et al. Association between preoperative malnutrition and postoperative delirium after hip fracture surgery in older adults. J Am Geriatr Soc. 2017;65(6):1222‐1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Qiu Y, Sessler DI, Chen L, et al. Preoperative vitamin D deficiency is associated with postoperative delirium in critically ill patients. J Intensive Care Med. 2022;37(5):655‐662. [DOI] [PubMed] [Google Scholar]
- 55. Hung KC, Wang LK, Lin YT, et al. Association of preoperative vitamin D deficiency with the risk of postoperative delirium and cognitive dysfunction: A meta‐analysis. J Clin Anesth. 2022;79:110681. [DOI] [PubMed] [Google Scholar]
- 56. Radtke FM, Franck M, MacGuill M, et al. Duration of fluid fasting and choice of analgesic are modifiable factors for early postoperative delirium. Eur J Anaesthesiol. 2010;27(5):411‐416. [DOI] [PubMed] [Google Scholar]
- 57. Khanna P, Saini K, Sinha R, Nisa N, Kumar S, Maitra S. Correlation between duration of preoperative fasting and emergence delirium in pediatric patients undergoing ophthalmic examination under anesthesia: a prospective observational study. Paediatr Anaesth. 2018;28(6):547‐551. [DOI] [PubMed] [Google Scholar]
- 58. Egberts A, Moreno‐Gonzalez R, Alan H, Ziere G, Mattace‐Raso FUS. Anticholinergic drug burden and delirium: a systematic review. J Am Med Dir Assoc. 2021;22(1):65‐73. [DOI] [PubMed] [Google Scholar]
- 59. Kok L, Slooter AJ, Hillegers MH, van Dijk D, Veldhuijzen DS. Benzodiazepine use and neuropsychiatric outcomes in the ICU: a systematic review. Crit Care Med. 2018;46(10):1673‐1680. [DOI] [PubMed] [Google Scholar]
- 60. Swart LM, van der Zanden V, Spies PE, de Rooij SE, van Munster BC. The Comparative Risk of Delirium with Different Opioids: A Systematic Review. Drugs Aging. 2017;34(6):437‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li Y, Zhang B. Effects of anesthesia depth on postoperative cognitive function and inflammation: a systematic review and meta‐analysis. Minerva Anestesiol. 2020;86(9):965‐973. [DOI] [PubMed] [Google Scholar]
- 62. Cui F, Zhao W, Mu DL, et al. Association between cerebral desaturation and postoperative delirium in thoracotomy with one‐lung ventilation: a prospective cohort study. Anesth Analg. 2021;133(1):176‐186. [DOI] [PubMed] [Google Scholar]
- 63. Lim L, Nam K, Lee S, et al. The relationship between intraoperative cerebral oximetry and postoperative delirium in patients undergoing off‐pump coronary artery bypass graft surgery: a retrospective study. BMC Anesthesiol. 2020;20(1):285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Roberts ML, Lin HM, Tinuoye E, et al. The association of cerebral desaturation during one‐lung ventilation and postoperative recovery: a prospective observational cohort study. J Cardiothorac Vasc Anesth. 2021;35(2):542‐550. [DOI] [PubMed] [Google Scholar]
- 65. Hirsch J, DePalma G, Tsai TT, Sands LP, Leung JM. Impact of intraoperative hypotension and blood pressure fluctuations on early postoperative delirium after non‐cardiac surgery. Br J Anaesth. 2015;115(3):418‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Maheshwari K, Ahuja S, Khanna AK, et al. Association between perioperative hypotension and delirium in postoperative critically ill patients: a retrospective cohort analysis. Anesth Analg. 2020;130(3):636‐643. [DOI] [PubMed] [Google Scholar]
- 67. Zhang Y, He ST, Nie B, Li XY, Wang DX. Emergence delirium is associated with increased postoperative delirium in elderly: a prospective observational study. J Anesth. 2020;34(5):675‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Xu T, Bo L, Wang J, et al. Risk factors for early postoperative cognitive dysfunction after non‐coronary bypass surgery in Chinese population. J Cardiothorac Surg. 2013;8:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Florido‐Santiago M, Pérez‐Belmonte LM, Osuna‐Sánchez J, et al. Assessment of long‐term cognitive dysfunction in older patients who undergo heart surgery. Neurologia. 2021;2021:30443‐30446. [DOI] [PubMed] [Google Scholar]
- 70. Huang H, Lin F, Cen L, Jing R, Pan L. Cancer‐related anemia is a risk factor for medium‐term postoperative cognitive dysfunction in laparoscopic surgery patients: an observational prospective study. Neural Plast. 2020;2020:4847520‐4847527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Plas M, Rotteveel E, Izaks GJ, et al. Cognitive decline after major oncological surgery in the elderly. Eur J Cancer. 2017;86:394‐402. [DOI] [PubMed] [Google Scholar]
- 72. Kadoi Y, Goto F. Factors associated with postoperative cognitive dysfunction in patients undergoing cardiac surgery. Surg Today. 2006;36(12):1053‐1057. [DOI] [PubMed] [Google Scholar]
- 73. Feinkohl I, Winterer G, Spies CD, Pischon T. Cognitive reserve and the risk of postoperative cognitive dysfunction. Dtsch Arztebl Int. 2017;114(7):110‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ghezzi ES, Ross TJ, Davis D, Psaltis PJ, Loetscher T, Keage HAD. Meta‐analysis of prevalence and risk factors for cognitive decline and improvement after transcatheter aortic valve implantation. Am J Cardiol. 2020;127:105‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bekker A, Lee C, de Santi S, et al. Does mild cognitive impairment increase the risk of developing postoperative cognitive dysfunction? Am J Surg. 2010;199(6):782‐788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Silbert B, Evered L, Scott DA, et al. Preexisting cognitive impairment is associated with postoperative cognitive dysfunction after hip joint replacement surgery. Anesthesiology. 2015;122(6):1224‐1234. [DOI] [PubMed] [Google Scholar]
- 77. Evered LA, Vitug S, Scott DA, Silbert B. Preoperative frailty predicts postoperative neurocognitive disorders after total hip joint replacement surgery. Anesth Analg. 2020;131(5):1582‐1588. [DOI] [PubMed] [Google Scholar]
- 78. Otomo S, Maekawa K, Baba T, Goto T, Yamamoto T. Evaluation of the risk factors for neurological and neurocognitive impairment after selective cerebral perfusion in thoracic aortic surgery. J Anesth. 2020;34(4):527‐536. [DOI] [PubMed] [Google Scholar]
- 79. Li YL, Huang HF, Le Y. Risk factors and predictive value of perioperative neurocognitive disorders in elderly patients with gastrointestinal tumors. BMC Anesthesiol. 2021;21(1):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nötzold A, Michel K, Khattab AA, Sievers HH, Hüppe M. Diabetes mellitus increases adverse neurocognitive outcome after coronary artery bypass grafting surgery. Thorac Cardiovasc Surg. 2006;54(5):307‐312. [DOI] [PubMed] [Google Scholar]
- 81. Feinkohl I, Winterer G, Pischon T. Diabetes is associated with risk of postoperative cognitive dysfunction: A meta‐analysis. Diabetes Metab Res Rev. 2017;33(5):e2884. [DOI] [PubMed] [Google Scholar]
- 82. Uzoigwe CE, O'Leary L, Nduka J, et al. Factors associated with delirium and cognitive decline following hip fracture surgery. Bone Joint J. 2020;102‐B(12):1675‐1681. [DOI] [PubMed] [Google Scholar]
- 83. Hudetz JA, Patterson KM, Byrne AJ, et al. A history of alcohol dependence increases the incidence and severity of postoperative cognitive dysfunction in cardiac surgical patients. Int J Environ Res Public Health. 2009;6(11):2725‐2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhang Y, Shan GJ, Zhang YX, et al. Preoperative vitamin D deficiency increases the risk of postoperative cognitive dysfunction: a predefined exploratory sub‐analysis. Acta Anaesthesiol Scand. 2018;62(7):924‐935. [DOI] [PubMed] [Google Scholar]
- 85. Rossi A, Burkhart C, Dell‐Kuster S, et al. Serum anticholinergic activity and postoperative cognitive dysfunction in elderly patients. Anesth Analg. 2014;119(4):947‐955. [DOI] [PubMed] [Google Scholar]
- 86. Awada HN, Luna IE, Kehlet H, Wede HR, Hoevsgaard SJ, Aasvang EK. Postoperative cognitive dysfunction is rare after fast‐track hip‐ and knee arthroplasty ‐ But potentially related to opioid use. J Clin Anesth. 2019;57:80‐86. [DOI] [PubMed] [Google Scholar]
- 87. Kristek G, Radoš I, Kristek D, et al. Influence of postoperative analgesia on systemic inflammatory response and postoperative cognitive dysfunction after femoral fractures surgery: a randomized controlled trial. Reg Anesth Pain Med. 2019;44(1):59‐68. [DOI] [PubMed] [Google Scholar]
- 88. Hou R, Wang H, Chen L, Qiu Y, Li S. POCD in patients receiving total knee replacement under deep vs light anesthesia: a randomized controlled trial. Brain Behav. 2018;8(2):e00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Momeni M, Meyer S, Docquier MA, et al. Predicting postoperative delirium and postoperative cognitive decline with combined intraoperative electroencephalogram monitoring and cerebral near‐infrared spectroscopy in patients undergoing cardiac interventions. J Clin Monit Comput. 2019;33(6):999‐1009. [DOI] [PubMed] [Google Scholar]
- 90. Colak Z, Borojevic M, Bogovic A, Ivancan V, Biocina B, Majeric‐Kogler V. Influence of intraoperative cerebral oximetry monitoring on neurocognitive function after coronary artery bypass surgery: a randomized, prospective study. Eur J Cardiothorac Surg. 2015;47(3):447‐454. [DOI] [PubMed] [Google Scholar]
- 91. de la Varga‐Martínez O, Gómez‐Pesquera E, Muñoz‐Moreno MF, et al. Development and validation of a delirium risk prediction preoperative model for cardiac surgery patients (DELIPRECAS): an observational multicentre study. J Clin Anesth. 2021;69:110158. [DOI] [PubMed] [Google Scholar]
- 92. Wang J, Ji Y, Wang N, et al. Establishment and validation of a delirium prediction model for neurosurgery patients in intensive care. Int J Nurs Pract. 2020;26(4):e12818. [DOI] [PubMed] [Google Scholar]
- 93. Lin N, Liu K, Feng J, et al. Development and validation of a postoperative delirium prediction model for pediatric patients: a prospective, observational, single‐center study. Medicine. 2021;100(20):e25894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Xing H, Zhou W, Fan Y, Wen T, Wang X, Chang G. Development and validation of a postoperative delirium prediction model for patients admitted to an intensive care unit in China: a prospective study. BMJ Open. 2019;9(11):e030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Li Y, Zhao L, Wang Y, et al. Development and validation of prediction models for neurocognitive disorders in adult patients admitted to the ICU with sleep disturbance. CNS Neurosci Ther. 2022;28(4):554‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hu XY, Liu H, Zhao X, et al. Automated machine learning‐based model predicts postoperative delirium using readily extractable perioperative collected electronic data. CNS Neurosci Ther. 2022;28(4):608‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. APA . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; 2013. [Google Scholar]
- 98. Silverstein JH, Timberger M, Reich DL, Uysal S. Central nervous system dysfunction after noncardiac surgery and anesthesia in the elderly. Anesthesiology. 2007;106(3):622‐628. [DOI] [PubMed] [Google Scholar]
- 99. Su X, Meng ZT, Wu XH, et al. Dexmedetomidine for prevention of delirium in elderly patients after non‐cardiac surgery: a randomised, double‐blind, placebo‐controlled trial. Lancet. 2016;388(10054):1893‐1902. [DOI] [PubMed] [Google Scholar]
- 100. Hayhurst CJ, Pandharipande PP, Hughes CG. Intensive care unit delirium: a review of diagnosis, prevention, and treatment. Anesthesiology. 2016;125(6):1229‐1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in older persons: advances in diagnosis and treatment. JAMA. 2017;318(12):1161‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456‐1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The confusion assessment method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM‐ICU). JAMA. 2001;286(21):2703‐2710. [DOI] [PubMed] [Google Scholar]
- 105. Han JH, Wilson A, Vasilevskis EE, et al. Diagnosing delirium in older emergency department patients: validity and reliability of the delirium triage screen and the brief confusion assessment method. Ann Emerg Med. 2013;62(5):457‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Marcantonio ER, Ngo LH, O'Connor M, et al. 3D‐CAM: derivation and validation of a 3‐minute diagnostic interview for CAM‐defined delirium: a cross‐sectional diagnostic test study. Ann Intern Med. 2014;161(8):554‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27(5):859‐864. [DOI] [PubMed] [Google Scholar]
- 108. Bellelli G, Morandi A, Davis DH, et al. Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing. 2014;43(4):496‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Breitbart W, Rosenfeld B, Roth A, Smith MJ, Cohen K, Passik S. The memorial delirium assessment scale. J Pain Symptom Manage. 1997;13(3):128‐137. [DOI] [PubMed] [Google Scholar]
- 110. Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale‐revised‐98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229‐242. [DOI] [PubMed] [Google Scholar]
- 111. Inouye SK, Kosar CM, Tommet D, et al. The CAM‐S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med. 2014;160(8):526‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sepulveda E, Franco JG, Trzepacz PT, et al. Delirium diagnosis defined by cluster analysis of symptoms versus diagnosis by DSM and ICD criteria: diagnostic accuracy study. BMC Psychiatry. 2016;16:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Shi Q, Warren L, Saposnik G, Macdermid JC. Confusion assessment method: a systematic review and meta‐analysis of diagnostic accuracy. Neuropsychiatr Dis Treat. 2013;9:1359‐1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Maybrier HR, Mickle AM, Escallier KE, et al. Reliability and accuracy of delirium assessments among investigators at multiple international centres. BMJ Open. 2018;8(11):e023137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Gross AL, Tommet D, D'Aquila M, et al. Harmonization of delirium severity instruments: a comparison of the DRS‐R‐98, MDAS, and CAM‐S using item response theory. BMC Med Res Methodol. 2018;18(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. van Sinderen K, Schwarte LA, Schober P. Diagnostic criteria of postoperative cognitive dysfunction: a focused systematic review. Anesthesiol Res Pract. 2020;2020:7384394‐7384313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Liu J, Huang K, Zhu B, et al. Neuropsychological tests in post‐operative cognitive dysfunction: methods and applications. Front Psychol. 2021;12:684307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Needham MJ, Webb CE, Bryden DC. Postoperative cognitive dysfunction and dementia: what we need to know and do. Br J Anaesth. 2017;119(suppl_1):i115‐i125. [DOI] [PubMed] [Google Scholar]
- 119. Chu LW, Ng KH, Law AC, Lee AM, Kwan F. Validity of the cantonese Chinese montreal cognitive assessment in Southern Chinese. Geriatr Gerontol Int. 2015;15(1):96‐103. [DOI] [PubMed] [Google Scholar]
- 120. Carson N, Leach L, Murphy KJ. A re‐examination of Montreal Cognitive Assessment (MoCA) cutoff scores. Int J Geriatr Psychiatry. 2018;33(2):379‐388. [DOI] [PubMed] [Google Scholar]
- 121. Wang BR, Zheng HF, Xu C, Sun Y, Zhang YD, Shi JQ. Comparative diagnostic accuracy of ACE‐III and MoCA for detecting mild cognitive impairment. Neuropsychiatr Dis Treat. 2019;15:2647‐2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tsai JC, Chen CW, Chu H, et al. Comparing the sensitivity, specificity, and predictive values of the montreal cognitive assessment and mini‐mental state examination when screening people for mild cognitive impairment and dementia in Chinese population. Arch Psychiatr Nurs. 2016;30(4):486‐491. [DOI] [PubMed] [Google Scholar]
- 123. Ciesielska N, Sokołowski R, Mazur E, Podhorecka M, Polak‐Szabela A, Kędziora‐Kornatowska K. Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini‐Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta‐analysis. Psychiatr Pol. 2016;50(5):1039‐1052. [DOI] [PubMed] [Google Scholar]
- 124. O'Caoimh R, Timmons S, Molloy DW. Screening for mild cognitive impairment: comparison of "MCI Specific" screening instruments. J Alzheimers Dis. 2016;51(2):619‐629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Senda M, Terada S, Takenoshita S, et al. Diagnostic utility of the Addenbrooke's Cognitive Examination ‐ III (ACE‐III), Mini‐ACE, Mini‐Mental State Examination, Montreal Cognitive Assessment, and Hasegawa Dementia Scale‐Revised for detecting mild cognitive impairment and dementia. Psychogeriatrics. 2020;20(2):156‐162. [DOI] [PubMed] [Google Scholar]
- 126. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695‐699. [DOI] [PubMed] [Google Scholar]
- 127. Lee MT, Chang WY, Jang Y. Psychometric and diagnostic properties of the Taiwan version of the quick mild cognitive impairment screen. PLoS ONE. 2018;13(12):e0207851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Takenoshita S, Terada S, Yoshida H, et al. Validation of Addenbrooke's cognitive examination III for detecting mild cognitive impairment and dementia in Japan. BMC Geriatr. 2019;19(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Li X, Yang L, Yin J, Yu N, Ye F. Validation study of the chinese version of addenbrooke's cognitive examination iii for diagnosing mild cognitive impairment and mild dementia. J Clin Neurol. 2019;15(3):313‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Zhang Y, Shan GJ, Zhang YX, et al. Propofol compared with sevoflurane general anaesthesia is associated with decreased delayed neurocognitive recovery in older adults. Br J Anaesth. 2018;121(3):595‐604. [DOI] [PubMed] [Google Scholar]
- 131. Nandi S, Harvey WF, Saillant J, Kazakin A, Talmo C, Bono J. Pharmacologic risk factors for post‐operative delirium in total joint arthroplasty patients: a case‐control study. J Arthroplasty. 2014;29(2):268‐271. [DOI] [PubMed] [Google Scholar]
- 132. Davies EA, O'Mahony MS. Adverse drug reactions in special populations ‐ the elderly. Br J Clin Pharmacol. 2015;80(4):796‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Panitchote A, Tangvoraphonkchai K, Suebsoh N, et al. Under‐recognition of delirium in older adults by nurses in the intensive care unit setting. Aging Clin Exp Res. 2015;27(5):735‐740. [DOI] [PubMed] [Google Scholar]
- 134. Duprey MS, Devlin JW, Griffith JL, et al. Association between perioperative medication use and postoperative delirium and cognition in older adults undergoing elective noncardiac surgery. Anesth Analg. 2022;134(6):1154‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hughes CG, Boncyk CS, Culley DJ, et al. American society for enhanced recovery and perioperative quality initiative joint consensus statement on postoperative delirium prevention. Anesth Analg. 2020;130(6):1572‐1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence‐based and consensus‐based guideline on postoperative delirium. Eur J Anaesthesiol. 2017;34(4):192‐214. [DOI] [PubMed] [Google Scholar]
- 137. Lang B, Zhang L, Zhang W, Lin Y, Fu Y, Chen S. A comparative evaluation of dexmedetomidine and midazolam in pediatric sedation: a meta‐analysis of randomized controlled trials with trial sequential analysis. CNS Neurosci Ther. 2020;26(8):862‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Mueller A, Spies CD, Eckardt R, et al. Anticholinergic burden of long‐term medication is an independent risk factor for the development of postoperative delirium: a clinical trial. J Clin Anesth. 2020;61:109632. [DOI] [PubMed] [Google Scholar]
- 139. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the american society of anesthesiologists task force on preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration. Anesthesiology. 2017;126(3):376‐393. [DOI] [PubMed] [Google Scholar]
- 140. Tow A, Holtzer R, Wang C, et al. Cognitive reserve and postoperative delirium in older adults. J Am Geriatr Soc. 2016;64(6):1341‐1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Yang Y, Zhao X, Gao L, Wang Y, Wang J. Incidence and associated factors of delirium after orthopedic surgery in elderly patients: a systematic review and meta‐analysis. Aging Clin Exp Res. 2021;33(6):1493‐1506. [DOI] [PubMed] [Google Scholar]
- 142. Bahar‐Fuchs A, Martyr A, Goh AM, Sabates J, Clare L. Cognitive training for people with mild to moderate dementia. Cochrane Database Syst Rev. 2019;3(3):CD013069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Gates NJ, Rutjes AW, Di Nisio M, et al. Computerised cognitive training for 12 or more weeks for maintaining cognitive function in cognitively healthy people in late life. Cochrane Database Syst Rev. 2020;2(2):CD012277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Humeidan ML, Reyes JC, Mavarez‐Martinez A, et al. Effect of cognitive prehabilitation on the incidence of postoperative delirium among older adults undergoing major noncardiac surgery: the neurobics randomized clinical trial. JAMA Surg. 2021;156(2):148‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Eamer G, Taheri A, Chen SS, et al. Comprehensive geriatric assessment for older people admitted to a surgical service. Cochrane Database Syst Rev. 2018;1(1):CD012485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Shields L, Henderson V, Caslake R. Comprehensive geriatric assessment for prevention of delirium after hip fracture: a systematic review of randomized controlled trials. J Am Geriatr Soc. 2017;65(7):1559‐1565. [DOI] [PubMed] [Google Scholar]
- 147. Oberai T, Laver K, Crotty M, Killington M, Jaarsma R. Effectiveness of multicomponent interventions on incidence of delirium in hospitalized older patients with hip fracture: a systematic review. Int Psychogeriatr. 2018;30(4):481‐492. [DOI] [PubMed] [Google Scholar]
- 148. Pöpping DM, Elia N, Van Aken HK, et al. Impact of epidural analgesia on mortality and morbidity after surgery: systematic review and meta‐analysis of randomized controlled trials. Ann Surg. 2014;259(6):1056‐1067. [DOI] [PubMed] [Google Scholar]
- 149. Memtsoudis SG, Cozowicz C, Bekeris J, et al. Anaesthetic care of patients undergoing primary hip and knee arthroplasty: consensus recommendations from the International Consensus on Anaesthesia‐Related Outcomes after Surgery group (ICAROS) based on a systematic review and meta‐analysis. Br J Anaesth. 2019;123(3):269‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. O'Donnell CM, McLoughlin L, Patterson CC, et al. Perioperative outcomes in the context of mode of anaesthesia for patients undergoing hip fracture surgery: systematic review and meta‐analysis. Br J Anaesth. 2018;120(1):37‐50. [DOI] [PubMed] [Google Scholar]
- 151. Neuman MD, Feng R, Carson JL, et al. Spinal anesthesia or general anesthesia for hip surgery in older adults. N Engl J Med. 2021;385(22):2025‐2035. [DOI] [PubMed] [Google Scholar]
- 152. Li T, Li J, Yuan L, et al. Effect of regional vs general anesthesia on incidence of postoperative delirium in older patients undergoing hip fracture surgery: The RAGA randomized trial. JAMA. 2022;327(1):50‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Li YW, Li HJ, Li HJ, et al. Delirium in older patients after combined epidural‐general anesthesia or general anesthesia for major surgery: a randomized trial. Anesthesiology. 2021;135(2):218‐232. [DOI] [PubMed] [Google Scholar]
- 154. Yeung JH, Gates S, Naidu BV, Wilson MJ, Gao SF. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev. 2016;2(2):CD009121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Desai N, El‐Boghdadly K, Albrecht E. Epidural vs. transversus abdominis plane block for abdominal surgery ‐ a systematic review, meta‐analysis and trial sequential analysis. Anaesthesia. 2021;76(1):101‐117. [DOI] [PubMed] [Google Scholar]
- 156. Gerrard AD, Brooks B, Asaad P, Hajibandeh S, Hajibandeh S. Meta‐analysis of epidural analgesia versus peripheral nerve blockade after total knee joint replacement. Eur J Orthop Surg Traumatol. 2017;27(1):61‐72. [DOI] [PubMed] [Google Scholar]
- 157. Lim EJ, Koh WU, Kim H, Kim HJ, Shon HC, Kim JW. Regional nerve block decreases the incidence of postoperative delirium in elderly hip fracture. J Clin Med. 2021;10(16):3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kinjo S, Lim E, Sands LP, Bozic KJ, Leung JM. Does using a femoral nerve block for total knee replacement decrease postoperative delirium? BMC Anesthesiol. 2012;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Kim CH, Yang JY, Min CH, Shon HC, Kim JW, Lim EJ. The effect of regional nerve block on perioperative delirium in hip fracture surgery for the elderly: a systematic review and meta‐analysis of randomized controlled trials. Orthop Traumatol Surg Res. 2022;108(1):103151. [DOI] [PubMed] [Google Scholar]
- 160. Yang Y, Feng L, Ji C, Lu K, Chen Y, Chen B. Inhalational versus propofol‐based intravenous maintenance of anesthesia for emergence delirium in adults: a meta‐analysis and trial sequential analysis. J Neurosurg Anesthesiol. 2022. doi: 10.1097/ANA.0000000000000830 [DOI] [PubMed] [Google Scholar]
- 161. Miller D, Lewis SR, Pritchard MW, et al. Intravenous versus inhalational maintenance of anaesthesia for postoperative cognitive outcomes in elderly people undergoing non‐cardiac surgery. Cochrane Database Syst Rev. 2018;8(8):CD012317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Jiao XF, Lin XM, Ni XF, et al. Volatile anesthetics versus total intravenous anesthesia in patients undergoing coronary artery bypass grafting: an updated meta‐analysis and trial sequential analysis of randomized controlled trials. PLoS ONE. 2019;14(10):e0224562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Law LS, Lo EA, Gan TJ. Xenon anesthesia: a systematic review and meta‐analysis of randomized controlled trials. Anesth Analg. 2016;122(3):678‐697. [DOI] [PubMed] [Google Scholar]
- 164. Al Tmimi L, Van Hemelrijck J, Van de Velde M, et al. Xenon anaesthesia for patients undergoing off‐pump coronary artery bypass graft surgery: a prospective randomized controlled pilot trial. Br J Anaesth. 2015;115(4):550‐559. [DOI] [PubMed] [Google Scholar]
- 165. Al Tmimi L, Verbrugghe P, Van de Velde M, et al. Intraoperative xenon for prevention of delirium after on‐pump cardiac surgery: a randomised, observer‐blind, controlled clinical trial. Br J Anaesth. 2020;124:454‐462. [DOI] [PubMed] [Google Scholar]
- 166. Coburn M, Sanders RD, Maze M, et al. The hip fracture surgery in elderly patients (HIPELD) study to evaluate xenon anaesthesia for the prevention of postoperative delirium: a multicentre, randomized clinical trial. Br J Anaesth. 2018;120(1):127‐137. [DOI] [PubMed] [Google Scholar]
- 167. Chan MT, Cheng BC, Lee TM, Gin T. BIS‐guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25(1):33‐42. [DOI] [PubMed] [Google Scholar]
- 168. Punjasawadwong Y, Phongchiewboon A, Bunchungmongkol N. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database Syst Rev. 2014;2014(6):CD003843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Lewis SR, Pritchard MW, Fawcett LJ, Punjasawadwong Y. Bispectral index for improving intraoperative awareness and early postoperative recovery in adults. Cochrane Database Syst Rev. 2019;9(9):Cd003843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Luo C, Zou W. Cerebral monitoring of anaesthesia on reducing cognitive dysfunction and postoperative delirium: a systematic review. J Int Med Res. 2018;46(10):4100‐4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Bocskai T, Kovács M, Szakács Z, et al. Is the bispectral index monitoring protective against postoperative cognitive decline? a systematic review with meta‐analysis. PLoS ONE. 2020;15(2):e0229018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Evered LA, Chan MTV, Han R, et al. Anaesthetic depth and delirium after major surgery: a randomised clinical trial. Br J Anaesth. 2021;127(5):704‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Wesselink EM, Kappen TH, Torn HM, Slooter AJC, van Klei WA. Intraoperative hypotension and the risk of postoperative adverse outcomes: a systematic review. Br J Anaesth. 2018;121(4):706‐721. [DOI] [PubMed] [Google Scholar]
- 174. Sessler DI, Bloomstone JA, Aronson S, et al. Perioperative quality initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122(5):563‐574. [DOI] [PubMed] [Google Scholar]
- 175. Wachtendorf LJ, Azimaraghi O, Santer P, et al. Association between intraoperative arterial hypotension and postoperative delirium after noncardiac surgery: a retrospective multicenter cohort study. Anesth Analg. 2021;134:822‐833. [DOI] [PubMed] [Google Scholar]
- 176. Feng X, Hu J, Hua F, Zhang J, Zhang L, Xu G. The correlation of intraoperative hypotension and postoperative cognitive impairment: a meta‐analysis of randomized controlled trials. BMC Anesthesiol. 2020;20(1):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Krzych ŁJ, Pluta MP, Putowski Z, Czok M. Investigating association between intraoperative hypotension and postoperative neurocognitive disorders in non‐cardiac surgery: a comprehensive review. J Clin Med. 2020;9(10):3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Czok M, Pluta MP, Putowski Z, Krzych ŁJ. Postoperative neurocognitive disorders in cardiac surgery: investigating the role of intraoperative hypotension. a systematic review. Int J Environ Res Public Health. 2021;18(2):786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Yi J, Lei Y, Xu S, et al. Intraoperative hypothermia and its clinical outcomes in patients undergoing general anesthesia: National study in China. PLoS ONE. 2017;12(6):e0177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Horosz B, Malec‐Milewska M. Inadvertent intraoperative hypothermia. Anaesthesiol Intensive Ther. 2013;45(1):38‐43. [DOI] [PubMed] [Google Scholar]
- 181. Reynolds L, Beckmann J, Kurz A. Perioperative complications of hypothermia. Best Pract Res Clin Anaesthesiol. 2008;22(4):645‐657. [DOI] [PubMed] [Google Scholar]
- 182. Wagner D, Hooper V, Bankieris K, Johnson A. The relationship of postoperative delirium and unplanned perioperative hypothermia in surgical patients. J Perianesth Nurs. 2021;36(1):41‐46. [DOI] [PubMed] [Google Scholar]
- 183. Lee KF, Wood MD, Maslove DM, Muscedere JG, Boyd JG. Dysfunctional cerebral autoregulation is associated with delirium in critically ill adults. J Cereb Blood Flow Metab. 2019;39(12):2512‐2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Lei L, Katznelson R, Fedorko L, et al. Cerebral oximetry and postoperative delirium after cardiac surgery: a randomised, controlled trial. Anaesthesia. 2017;72(12):1456‐1466. [DOI] [PubMed] [Google Scholar]
- 185. Susano MJ, Dias M, Seixas FS, et al. Association among preoperative cognitive performance, regional cerebral oxygen saturation, and postoperative delirium in older portuguese patients. Anesth Analg. 2021;132(3):846‐855. [DOI] [PubMed] [Google Scholar]
- 186. Ortega‐Loubon C, Herrera‐Gómez F, Bernuy‐Guevara C, et al. Near‐infrared spectroscopy monitoring in cardiac and noncardiac surgery: pairwise and network meta‐analyses. J Clin Med. 2019;8(12):2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Qin C, Jiang Y, Lin C, Li A, Liu J. Perioperative dexmedetomidine administration to prevent delirium in adults after non‐cardiac surgery: a systematic review and meta‐analysis. J Clin Anesth. 2021;73:110308. [DOI] [PubMed] [Google Scholar]
- 188. Patel M, Onwochei DN, Desai N. Influence of perioperative dexmedetomidine on the incidence of postoperative delirium in adult patients undergoing cardiac surgery. Br J Anaesth. 2022. doi: 10.1016/j.jia.2021.11.041 [DOI] [PubMed] [Google Scholar]
- 189. Jin Z, Hu J, Ma D. Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth. 2020;125(4):492‐504. [DOI] [PubMed] [Google Scholar]
- 190. Burton JK, Craig LE, Yong SQ, et al. Non‐pharmacological interventions for preventing delirium in hospitalised non‐ICU patients. Cochrane Database Syst Rev. 2021;7(7):Cd013307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Balas MC, Vasilevskis EE, Olsen KM, et al. Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle. Crit Care Med. 2014;42(5):1024‐1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Barnes‐Daly MA, Phillips G, Ely EW. Improving hospital survival and reducing brain dysfunction at seven california community hospitals: implementing pad guidelines via the ABCDEF bundle in 6,064 patients. Crit Care Med. 2017;45(2):171‐178. [DOI] [PubMed] [Google Scholar]
- 193. Pun BT, Balas MC, Barnes‐Daly MA, et al. Caring for critically ill patients with the ABCDEF bundle: Results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med. 2019;47(1):3‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Cho MR, Song SK, Ryu CH. Sleep disturbance strongly related to the development of postoperative delirium in proximal femoral fracture patients aged 60 or older. Hip Pelvis. 2020;32(2):93‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Kamdar BB, King LM, Collop NA, et al. The effect of a quality improvement intervention on perceived sleep quality and cognition in a medical ICU. Crit Care Med. 2013;41(3):800‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Litton E, Carnegie V, Elliott R, Webb SA. The efficacy of earplugs as a sleep hygiene strategy for reducing delirium in the ICU: a systematic review and meta‐analysis. Crit Care Med. 2016;44(5):992‐999. [DOI] [PubMed] [Google Scholar]
- 197. Fontaine GV, Der Nigoghossian C, Hamilton LA. Melatonin, ramelteon, suvorexant, and dexmedetomidine to promote sleep and prevent delirium in critically ill patients: a narrative review with practical applications. Crit Care Nurs Q. 2020;43(2):232‐250. [DOI] [PubMed] [Google Scholar]
- 198. Khaing K, Nair BR. Melatonin for delirium prevention in hospitalized patients: a systematic review and meta‐analysis. J Psychiatr Res. 2021;133:181‐190. [DOI] [PubMed] [Google Scholar]
- 199. Wibrow B, Martinez FE, Myers E, et al. Prophylactic melatonin for delirium in intensive care (Pro‐MEDIC): a randomized controlled trial. Intensive Care Med. 2022;48(4):414‐425. [DOI] [PubMed] [Google Scholar]
- 200. Adams AD, Pepin MJ, Brown JN. The role of suvorexant in the prevention of delirium during acute hospitalization: A systematic review. J Crit Care. 2020;59:1‐5. [DOI] [PubMed] [Google Scholar]
- 201. Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2‐adrenoceptor agonist dexmedetomidine converges on an endogenous sleep‐promoting pathway to exert its sedative effects. Anesthesiology. 2003;98(2):428‐436. [DOI] [PubMed] [Google Scholar]
- 202. Alexopoulou C, Kondili E, Diamantaki E, et al. Effects of dexmedetomidine on sleep quality in critically ill patients: a pilot study. Anesthesiology. 2014;121(4):801‐807. [DOI] [PubMed] [Google Scholar]
- 203. Wu XH, Cui F, Zhang C, et al. Low‐dose dexmedetomidine improves sleep quality pattern in elderly patients after noncardiac surgery in the intensive care unit: a pilot randomized controlled trial. Anesthesiology. 2016;125(5):979‐991. [DOI] [PubMed] [Google Scholar]
- 204. Skrobik Y, Duprey MS, Hill NS, Devlin JW. Low‐dose nocturnal dexmedetomidine prevents icu delirium. a randomized, placebo‐controlled trial. Am J Respir Crit Care Med. 2018;197(9):1147‐1156. [DOI] [PubMed] [Google Scholar]
- 205. Chen Z, Tang R, Zhang R, Jiang Y, Liu Y. Effects of dexmedetomidine administered for postoperative analgesia on sleep quality in patients undergoing abdominal hysterectomy. J Clin Anesth. 2017;36:118‐122. [DOI] [PubMed] [Google Scholar]
- 206. Hong H, Zhang DZ, Li M, et al. Impact of dexmedetomidine supplemented analgesia on delirium in patients recovering from orthopedic surgery: a randomized controlled trial. BMC Anesthesiol. 2021;21(1):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Mu DL, Zhang DZ, Wang DX, et al. Parecoxib supplementation to morphine analgesia decreases incidence of delirium in elderly patients after hip or knee replacement surgery: a randomized controlled trial. Anesth Analg. 2017;124(6):1992‐2000. [DOI] [PubMed] [Google Scholar]
- 208. Subramaniam B, Shankar P, Shaefi S, et al. Effect of intravenous acetaminophen vs placebo combined with propofol or dexmedetomidine on postoperative delirium among older patients following cardiac surgery: The DEXACET randomized clinical trial. JAMA. 2019;321(7):686‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Wang X, Wang Y, Hu Y, et al. Effect of flurbiprofen axetil on postoperative delirium for elderly patients. Brain Behav. 2019;9(6):e01290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Krenk L, Rasmussen LS, Hansen TB, Bogø S, Søballe K, Kehlet H. Delirium after fast‐track hip and knee arthroplasty. Br J Anaesth. 2012;108(4):607‐611. [DOI] [PubMed] [Google Scholar]
- 211. Petersen PB, Jørgensen CC, Kehlet H. Delirium after fast‐track hip and knee arthroplasty ‐ a cohort study of 6331 elderly patients. Acta Anaesthesiol Scand. 2017;61(7):767‐772. [DOI] [PubMed] [Google Scholar]
- 212. Jia Y, Jin G, Guo S, et al. Fast‐track surgery decreases the incidence of postoperative delirium and other complications in elderly patients with colorectal carcinoma. Langenbecks Arch Surg. 2014;399(1):77‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Liu SY, Li C, Zhang PX. Enhanced recovery after surgery for hip fractures: a systematic review and meta‐analysis. Perioper Med. 2021;10(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Baig AM. Neurological manifestations in COVID‐19 caused by SARS‐CoV‐2. CNS Neurosci Ther. 2020;26(5):499‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Swain O, Romano SK, Miryala R, Tsai J, Parikh V, Umanah GKE. SARS‐CoV‐2 neuronal invasion and complications: potential mechanisms and therapeutic approaches. J Neurosci. 2021;41(25):5338‐5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Tang L, Liu S, Xiao Y, et al. Encephalopathy at admission predicts adverse outcomes in patients with SARS‐CoV‐2 infection. CNS Neurosci Ther. 2021;27(10):1127‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. O'Hanlon S, Inouye SK. Delirium: a missing piece in the COVID‐19 pandemic puzzle. Age Ageing. 2020;49(4):497‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Pun BT, Badenes R, Heras La Calle G, et al. Prevalence and risk factors for delirium in critically ill patients with COVID‐19 (COVID‐D): a multicentre cohort study. Lancet Respir Med. 2021;9(3):239‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Devlin JW, O'Neal HR Jr, Thomas C, et al. Strategies to optimize ICU Liberation (A to F) bundle performance in critically ill adults with coronavirus disease 2019. Crit Care Explor. 2020;2(6):e0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Donato M, Carini FC, Meschini MJ, et al. Consensus for the management of analgesia, sedation and delirium in adults with COVID‐19‐associated acute respiratory distress syndrome. Rev Bras Ter Intensiva. 2021;33(1):48‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Picton JD, Marino AB, Nealy KL. Benzodiazepine use and cognitive decline in the elderly. Am J Health Syst Pharm. 2018;75(1):e6‐e12. [DOI] [PubMed] [Google Scholar]
- 222. Millar K, Asbury AJ, Bowman AW, et al. A randomised placebo‐controlled trial of the effects of midazolam premedication on children's postoperative cognition. Anaesthesia. 2007;62(9):923‐930. [DOI] [PubMed] [Google Scholar]
- 223. Li WX, Luo RY, Chen C, et al. Effects of propofol, dexmedetomidine, and midazolam on postoperative cognitive dysfunction in elderly patients: a randomized controlled preliminary trial. Chin Med J. 2019;132(4):437‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Cartailler J, Loyer C, Vanderlynden E, et al. Determinants of post‐operative cognitive decline in elderly people. J Prev Alzheimers Dis. 2021;8(3):322‐228. [DOI] [PubMed] [Google Scholar]
- 225. Taylor‐Rowan M, Edwards S, Noel‐Storr AH, et al. Anticholinergic burden (prognostic factor) for prediction of dementia or cognitive decline in older adults with no known cognitive syndrome. Cochrane Database Syst Rev. 2021;5(5):CD013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Saleh AJ, Tang GX, Hadi SM, et al. Preoperative cognitive intervention reduces cognitive dysfunction in elderly patients after gastrointestinal surgery: a randomized controlled trial. Med Sci Monit. 2015;21:798‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. O'Gara BP, Mueller A, Gasangwa DVI, et al. Prevention of early postoperative decline: a randomized, controlled feasibility trial of perioperative cognitive training. Anesth Analg. 2020;130(3):586‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Greaves D, Psaltis PJ, Lampit A, et al. Computerised cognitive training to improve cognition including delirium following coronary artery bypass grafting surgery: protocol for a blinded randomised controlled trial. BMJ Open. 2020;10(2):e034551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Tzimas P, Samara E, Petrou A, Korompilias A, Chalkias A, Papadopoulos G. The influence of anesthetic techniques on postoperative cognitive function in elderly patients undergoing hip fracture surgery: General vs spinal anesthesia. Injury. 2018;49(12):2221‐2226. [DOI] [PubMed] [Google Scholar]
- 230. Orhun G, Sungur Z, Koltka K, et al. Comparison of epidural analgesia combined with general anesthesia and general anesthesia for postoperative cognitive dysfunction in elderly patients. Ulus Travma Acil Cerrahi Derg. 2020;26(1):30‐36. [DOI] [PubMed] [Google Scholar]
- 231. Memtsoudis SG, Cozowicz C, Bekeris J, et al. Peripheral nerve block anesthesia/analgesia for patients undergoing primary hip and knee arthroplasty: recommendations from the International Consensus on Anesthesia‐Related Outcomes after Surgery (ICAROS) group based on a systematic review and meta‐analysis of current literature. Reg Anesth Pain Med. 2021;46(11):971‐985. [DOI] [PubMed] [Google Scholar]
- 232. Wang Y, Cheng J, Yang L, Wang J, Liu H, Lv Z. Ropivacaine for intercostal nerve block improves early postoperative cognitive dysfunction in patients following thoracotomy for esophageal cancer. Med Sci Monit. 2019;25:460‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. YaDeau JT, Fields KG, Kahn RL, et al. Readiness for discharge after foot and ankle surgery using peripheral nerve blocks: a randomized controlled trial comparing spinal and general anesthesia as supplements to nerve blocks. Anesth Analg. 2018;127(3):759‐766. [DOI] [PubMed] [Google Scholar]
- 234. Schoen J, Husemann L, Tiemeyer C, et al. Cognitive function after sevoflurane‐ vs propofol‐based anaesthesia for on‐pump cardiac surgery: a randomized controlled trial. Br J Anaesth. 2011;106(6):840‐850. [DOI] [PubMed] [Google Scholar]
- 235. Royse CF, Andrews DT, Newman SN, et al. The influence of propofol or desflurane on postoperative cognitive dysfunction in patients undergoing coronary artery bypass surgery. Anaesthesia. 2011;66(6):455‐464. [DOI] [PubMed] [Google Scholar]
- 236. Landoni G, Lomivorotov VV, Nigro Neto C, et al. Volatile anesthetics versus total intravenous anesthesia for cardiac surgery. N Engl J Med. 2019;380(13):1214‐1225. [DOI] [PubMed] [Google Scholar]
- 237. Sun H, Zhang G, Ai B, et al. A systematic review: comparative analysis of the effects of propofol and sevoflurane on postoperative cognitive function in elderly patients with lung cancer. BMC Cancer. 2019;19(1):1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Guo L, Lin F, Dai H, et al. Impact of sevoflurane versus propofol anesthesia on post‐operative cognitive dysfunction in elderly cancer patients: a double‐blinded randomized controlled trial. Med Sci Monit. 2020;26:e919293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Li Y, Chen D, Wang H, et al. Intravenous versus volatile anesthetic effects on postoperative cognition in elderly patients undergoing laparoscopic abdominal surgery. Anesthesiology. 2021;134(3):381‐394. [DOI] [PubMed] [Google Scholar]
- 240. Bronco A, Ingelmo PM, Aprigliano M, et al. Xenon anaesthesia produces better early postoperative cognitive recovery than sevoflurane anaesthesia. Eur J Anaesthesiol. 2010;27(10):912‐916. [DOI] [PubMed] [Google Scholar]
- 241. Cremer J, Stoppe C, Fahlenkamp AV, et al. Early cognitive function, recovery and well‐being after sevoflurane and xenon anaesthesia in the elderly: a double‐blinded randomized controlled trial. Med Gas Res. 2011;1(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Coburn M, Baumert JH, Roertgen D, et al. Emergence and early cognitive function in the elderly after xenon or desflurane anaesthesia: a double‐blinded randomized controlled trial. Br J Anaesth. 2007;98(6):756‐762. [DOI] [PubMed] [Google Scholar]
- 243. Wildes TS, Mickle AM, Ben Abdallah A, et al. Effect of electroencephalography‐guided anesthetic administration on postoperative delirium among older adults undergoing major surgery: The ENGAGES randomized clinical trial. JAMA. 2019;321(5):473‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. An J, Fang Q, Huang C, et al. Deeper total intravenous anesthesia reduced the incidence of early postoperative cognitive dysfunction after microvascular decompression for facial spasm. J Neurosurg Anesthesiol. 2011;23(1):12‐17. [DOI] [PubMed] [Google Scholar]
- 245. Quan C, Chen J, Luo Y, et al. BIS‐guided deep anesthesia decreases short‐term postoperative cognitive dysfunction and peripheral inflammation in elderly patients undergoing abdominal surgery. Brain Behav. 2019;9(4):e01238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. van Zuylen ML, Gribnau A, Admiraal M, et al. The role of intraoperative hypotension on the development of postoperative cognitive dysfunction: a systematic review. J Clin Anesth. 2021;72:110310. [DOI] [PubMed] [Google Scholar]
- 247. Yu Y, Zhang K, Zhang L, Zong H, Meng L, Han R. Cerebral near‐infrared spectroscopy (NIRS) for perioperative monitoring of brain oxygenation in children and adults. Cochrane Database Syst Rev. 2018;1(1):CD010947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Tian LJ, Yuan S, Zhou CH, Yan FX. The effect of intraoperative cerebral oximetry monitoring on postoperative cognitive dysfunction and ICU stay in adult patients undergoing cardiac surgery: an updated systematic review and meta‐analysis. Front Cardiovasc Med. 2022;8:814313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Thiele RH, Shaw AD, Bartels K, et al. American society for enhanced recovery and perioperative quality initiative joint consensus statement on the role of neuromonitoring in perioperative outcomes: cerebral near‐infrared spectroscopy. Anesth Analg. 2020;131(5):1444‐1455. [DOI] [PubMed] [Google Scholar]
- 250. Yang W, Kong LS, Zhu XX, Wang RX, Liu Y, Chen LR. Effect of dexmedetomidine on postoperative cognitive dysfunction and inflammation in patients after general anaesthesia: A PRISMA‐compliant systematic review and meta‐analysis. Medicine. 2019;98(18):e15383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Zhang DF, Su X, Meng ZT, et al. Impact of dexmedetomidine on long‐term outcomes after noncardiac surgery in elderly: 3‐year follow‐up of a randomized controlled trial. Ann Surg. 2019;270(2):356‐363. [DOI] [PubMed] [Google Scholar]
- 252. Olotu C, Ascone L, Wiede J, et al. The effect of delirium preventive measures on the occurrence of postoperative cognitive dysfunction in older adults undergoing cardiovascular surgery. The DelPOCD randomised controlled trial. J Clin Anesth. 2022;78:110686. [DOI] [PubMed] [Google Scholar]
- 253. Honn KA, Hinson JM, Whitney P, Van Dongen HPA. Cognitive flexibility: a distinct element of performance impairment due to sleep deprivation. Accid Anal Prev. 2019;126:191‐197. [DOI] [PubMed] [Google Scholar]
- 254. Wang X, Hua D, Tang X, et al. The role of perioperative sleep disturbance in postoperative neurocognitive disorders. Nat Sci Sleep. 2021;13:1395‐1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Hansen MV, Madsen MT, Andersen LT, et al. Effect of melatonin on cognitive function and sleep in relation to breast cancer surgery: a randomized, double‐blind, placebo‐controlled trial. Int J Breast Cancer. 2014;2014:416531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Fan Y, Yuan L, Ji M, Yang J, Gao D. The effect of melatonin on early postoperative cognitive decline in elderly patients undergoing hip arthroplasty: a randomized controlled trial. J Clin Anesth. 2017;39:77‐81. [DOI] [PubMed] [Google Scholar]
- 257. Huang X, Hussain B, Chang J. Peripheral inflammation and blood‐brain barrier disruption: effects and mechanisms. CNS Neurosci Ther. 2021;27(1):36‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Liu Y, Fu H, Wang T. Neuroinflammation in perioperative neurocognitive disorders: From bench to the bedside. CNS Neurosci Ther. 2022;28(4):484‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Guo Y, Li P. Recent highlights in periopeative neurological disorders, from bench to bedside. CNS Neurosci Ther. 2022;28(4):467‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Huang JM, Lv ZT, Zhang B, Jiang WX, Nie MB. Intravenous parecoxib for early postoperative cognitive dysfunction in elderly patients: evidence from a meta‐analysis. Expert Rev Clin Pharmacol. 2020;13(4):451‐460. [DOI] [PubMed] [Google Scholar]
- 261. Wu SJ, Xiong XZ, Lu J, et al. Fast‐track programs for liver surgery: a meta‐analysis. J Gastrointest Surg. 2015;19(9):1640‐1652. [DOI] [PubMed] [Google Scholar]
- 262. Zhu S, Qian W, Jiang C, Ye C, Chen X. Enhanced recovery after surgery for hip and knee arthroplasty: a systematic review and meta‐analysis. Postgrad Med J. 2017;93(1106):736‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Krenk L, Kehlet H, Bæk Hansen T, Solgaard S, Soballe K, Rasmussen LS. Cognitive dysfunction after fast‐track hip and knee replacement. Anesth Analg. 2014;118(5):1034‐1040. [DOI] [PubMed] [Google Scholar]
- 264. Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157‐1165. [DOI] [PubMed] [Google Scholar]
- 265. Abraha I, Rimland JM, Trotta F, et al. Non‐pharmacological interventions to prevent or treat delirium in older patients: clinical practice recommendations The SENATOR‐ONTOP Series. J Nutr Health Aging. 2016;20(9):927‐936. [DOI] [PubMed] [Google Scholar]
- 266. Abraha I, Trotta F, Rimland JM, et al. Efficacy of non‐pharmacological interventions to prevent and treat delirium in older patients: a systematic overview. The SENATOR project ONTOP Series. PLoS ONE. 2015;10(6):e0123090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Kolanowski A, Fick D, Litaker M, et al. Effect of cognitively stimulating activities on symptom management of delirium superimposed on dementia: a randomized controlled trial. J Am Geriatr Soc. 2016;64(12):2424‐2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalised non‐ICU patients. Cochrane Database Syst Rev. 2018;6(6):CD005594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Kim MS, Rhim HC, Park A, et al. Comparative efficacy and acceptability of pharmacological interventions for the treatment and prevention of delirium: a systematic review and network meta‐analysis. J Psychiatr Res. 2020;125:164‐176. [DOI] [PubMed] [Google Scholar]
- 270. Nikooie R, Neufeld KJ, Oh ES, et al. Antipsychotics for treating delirium in hospitalized adults: a systematic review. Ann Intern Med. 2019;171(7):485‐495. [DOI] [PubMed] [Google Scholar]
- 271. Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med. 2017;177(1):34‐42. [DOI] [PubMed] [Google Scholar]
- 272. Ralph SJ, Espinet AJ. Increased all‐cause mortality by antipsychotic drugs: updated review and meta‐analysis in dementia and general mental health care. J Alzheimers Dis Rep. 2018;2(1):1‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Burry L, Hutton B, Williamson DR, et al. Pharmacological interventions for the treatment of delirium in critically ill adults. Cochrane Database Syst Rev. 2019;9(9):CD011749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Liu X, Xiong J, Tang Y, Gong CC, Wang DF. Role of dexmedetomidine in the treatment of delirium in critically ill patients: a systematic review and meta‐analysis. Minerva Anestesiol. 2021;87(1):65‐76. [DOI] [PubMed] [Google Scholar]
- 275. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc. 2015;63(1):142‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Evered LA, Silbert BS. Postoperative cognitive dysfunction and noncardiac surgery. Anesth Analg. 2018;127(2):496‐505. [DOI] [PubMed] [Google Scholar]
- 277. Du Z, Li Y, Li J, Zhou C, Li F, Yang X. Physical activity can improve cognition in patients with Alzheimer's disease: a systematic review and meta‐analysis of randomized controlled trials. Clin Interv Aging. 2018;13:1593‐1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Jia RX, Liang JH, Xu Y, Wang YQ. Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: a meta‐analysis. BMC Geriatr. 2019;19(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Liang JH, Shen WT, Li JY, et al. The optimal treatment for improving cognitive function in elder people with mild cognitive impairment incorporating Bayesian network meta‐analysis and systematic review. Ageing Res Rev. 2019;51:85‐96. [DOI] [PubMed] [Google Scholar]
- 280. Chen J, Duan Y, Li H, Lu L, Liu J, Tang C. Different durations of cognitive stimulation therapy for Alzheimer's disease: a systematic review and meta‐analysis. Clin Interv Aging. 2019;14:1243‐1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: a systematic review of the evidence. Adv Nutr. 2016;7(5):889‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Rehm J, Hasan OSM, Black SE, Shield KD, Schwarzinger M. Alcohol use and dementia: a systematic scoping review. Alzheimers Res Ther. 2019;11(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Campos MW, Serebrisky D, Castaldelli‐Maia JM. Smoking and cognition. Curr Drug Abuse Rev. 2016;9(2):76‐79. [DOI] [PubMed] [Google Scholar]
- 284. Hussenoeder FS, Riedel‐Heller SG. Primary prevention of dementia: from modifiable risk factors to a public brain health agenda? Soc Psychiatry Psychiatr Epidemiol. 2018;53(12):1289‐1301. [DOI] [PubMed] [Google Scholar]
- 285. Ruxton K, Woodman RJ, Mangoni AA. Drugs with anticholinergic effects and cognitive impairment, falls and all‐cause mortality in older adults: a systematic review and meta‐analysis. Br J Clin Pharmacol. 2015;80(2):209‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Arvanitakis Z, Shah RC, Bennett DA. Diagnosis and management of dementia: review. JAMA. 2019;322(16):1589‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Adlimoghaddam A, Neuendorff M, Roy B, Albensi BC. A review of clinical treatment considerations of donepezil in severe Alzheimer's disease. CNS Neurosci Ther. 2018;24(10):876‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Doody RS, Ferris SH, Salloway S, et al. Donepezil treatment of patients with MCI: a 48‐week randomized, placebo‐controlled trial. Neurology. 2009;72(18):1555‐1561. [DOI] [PubMed] [Google Scholar]
- 289. Yang T, Wang H, Xiong Y, et al. Vitamin D supplementation improves cognitive function through reducing oxidative stress regulated by telomere length in older adults with mild cognitive impairment: a 12‐month randomized controlled trial. J Alzheimers Dis. 2020;78(4):1509‐1518. [DOI] [PubMed] [Google Scholar]
- 290. Bai D, Fan J, Li M, et al. Effects of folic acid combined with DHA supplementation on cognitive function and amyloid‐β‐related biomarkers in older adults with mild cognitive impairment by a randomized, double blind, placebo‐controlled trial. J Alzheimers Dis. 2021;81(1):155‐167. [DOI] [PubMed] [Google Scholar]
- 291. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255‐2263. [DOI] [PubMed] [Google Scholar]
- 292. Kandiah N, Ong PA, Yuda T, et al. Treatment of dementia and mild cognitive impairment with or without cerebrovascular disease: Expert consensus on the use of Ginkgo biloba extract, EGb 761®. CNS Neurosci Ther. 2019;25(2):288‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Kandiah N, Chan YF, Chen C, et al. Strategies for the use of Ginkgo biloba extract, EGb 761®, in the treatment and management of mild cognitive impairment in Asia: Expert consensus. CNS Neurosci Ther. 2021;27(2):149‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


