Abstract
Background:
Branched-chain amino acids (BCAAs: isoleucine, leucine, and valine) and aromatic amino acids (AAAs: phenylalanine and tyrosine) are hypothesized to influence early-life obesity risk.
Objective:
To assess HM free amino acid (AA) concentrations and infant intakes of HM AAs from women with obesity (OB) compared to those with normal weight (NW) and determine the relationships between HM AA consumption and infant growth.
Methods:
HM samples were collected at 0.5 (n=151), 2 (n=129), and 6 (n=93) months postpartum from mothers with NW (body mass index (BMI) =18.5–24.9 kg/m2) and OB (BMI>30 kg/m2). HM AAs were quantified via mass spectrometry. Infant HM intake, anthropometrics and body composition were assessed. Linear mixed-effects models (LMEM) examined the relationships between maternal BMI and HM AA intakes, and HM AA intake and infant growth over the first 6 months postpartum after adjusting for maternal and infant characteristics.
Results:
Maternal BMI was positively associated with infant intakes of isoleucine, leucine, and AAAs across timepoints. HM AA intakes were positively associated with weight-for-length z-score, fat mass index, and fat free mass index in infants (p<0.05).
Conclusions:
Maternal BMI led to differences in HM AA composition, which was associated with infant body composition.
Keywords: BCAA, aromatic amino acids, breastmilk, essential amino acids, postnatal programming
Introduction
Early-life growth and development are shaped by genetics 1,2 as well as both in-utero 3 and postnatal 4 environmental exposures. Human milk is a complex biological fluid that is considered the gold standard for infant nutrition 5 and is a likely source of postnatal nutritional programing. Varied biological environments affect human milk composition and volume, which may influence the delivery of optimal amounts of nutritive and nonnutritive components to the infant. To help optimize infants’ well-being, it is imperative to define the nutritive and nonnutritive factors that are associated with early-life growth and development that may program life-long patterns in health and disease.
Free amino acids are amongst the most variable metabolites in human milk across all lactation phases 6,7 and can vary significantly between mothers 8,9. Free amino acids, such as branched-chain amino acids (BCAAs: leucine, isoleucine, and valine) and aromatic amino acids (AAA: phenylalanine and tyrosine) are increased in both the plasma and human milk of women with obesity (OB) and are negatively associated with metabolic health10-12. Studies examining the relationship between elevated human milk BCAAs and infant growth and development have not been carried out for term infants; however, a positive association was found between human milk BCAA content and faster growth in preterm infants 13. Furthermore, infant intakes of human milk free BCAAs, AAAs, His, Thr, and Met have been shown to correlate significantly with the respective infant plasma concentrations 14, supporting the potential for a relationship between these free amino acids and infant physiology. Importantly, it remains to be determined whether or not infants fed by mothers with OB also consume a greater amount of these amino acids, contributing to altered growth patterns.
Circulating BCAAs and AAAs are involved in cell signaling 15,16, are hypothesized to take part in obesogenic metabolic programming (the early protein hypothesis 17,18), and some have insulin stimulating properties 19. Although less research exists for the pediatric population, plasma BCAA levels are reportedly associated with adolescent BMI 20 and are linked to insulin resistance during puberty 21. Compared to breastfed infants, plasma BCAAs are increased in formula fed babies 18 which may negatively impact metabolism via impaired beta-oxidation 22 leading to increased growth velocity and adiposity 23. To date, studies testing the effect of early-life protein consumption on metabolic programming have focused on formula protein type or protein content 22,24,25. However, whether specific human milk protein and free amino acid concentrations influence infant metabolism and growth remain largely unknown.
In this study, we first set out to determine the free amino acid concentrations of human milk from women with normal weight (NW) and OB and the subsequent infant intakes of BCAAs and AAAs. Thereafter, we characterized the relationship between human milk free amino acid consumption, infant growth, and body composition in the first 6 months of life. We hypothesized that infants born to OB mothers would consume more BCAAs and AAAs. We further hypothesized that BCAA and AAA intakes would be positively associated with infants’ growth and adiposity 26.
Subjects and Methods
Participants
This was a secondary analysis of healthy breastfeeding participants (169 mother-child pairs, Supplemental Figure 1) with NW (body mass index (BMI) 18.5-24.9 kg/m2) or OB (BMI 30-65 kg/m2) enrolled in two parent longitudinal studies (www.clinicaltrials.gov, ID# NCT01131117 and ID# NCT02125149). Enrollment criteria for NCT01131117 have been previously described in detail elsewhere 27,28. The women in this secondary analysis were recruited prior to pregnancy or during their first trimester and attended their first study visit prior to pregnancy (mean = 10 weeks pre-pregnancy, SD = 12 weeks) or during their first trimester (mean = 10 weeks of gestation, with a range of 4-14 weeks). Exclusion criteria included preexisting or ongoing medical conditions (e.g., diabetes mellitus, hypertension), use of medications during pregnancy that are known to influence fetal growth, smoking, or alcohol consumption. Additional enrollment criteria for NCT02125149 also required participants to be sedentary and cleared for an exercise program by their physician. The study procedures were in accordance with the ethical standards of the Institutional Review Board of the University of Arkansas for Medical Sciences.
Maternal Anthropometrics and Gestational Weight Gain
During the first study visit, maternal weight and height were measured with a standing digital scale (Tanita Corporation, Tokyo, Japan) and a wall-mounted stadiometer (Perspective Enterprises, Portage, Michigan), respectively. BMI was calculated as kg/m2. Gestational weight gain was calculated based upon the differences in measured weights between the participant’s first study visit and week 36 of gestation. We have previously demonstrated that there are very few variations between pre-pregnancy or first trimester measures in categorizing women 27. The 2009 Institute of Medicine (IOM) guidelines for gestational weight gain (GWG) based on BMI 29 were used to evaluate the number of women categorized as having inadequate (less than the recommended weight gain for BMI category), appropriate (within the recommended weight gain for BMI category), and excessive (exceeding the recommended weight gain for BMI category) weight gain.
Infant Body Composition
At each postnatal visit (0.5, 2, and 6 months), infant weight and length were measured using a tared scale (Seca, Hamburg, Germany) and a length board with a sliding foot piece (Perspective Enterprises, Michigan, USA), respectively. Appropriately trained and highly experienced research assistants performed these measures. Of note, the Lin’s concordance coefficient for inter-rater reliability was 0.999 for length measurements presented in this analysis. Infants’ gestational weight-for-age categories (small for gestational age (SGA), appropriate for gestational age (AGA), and large for gestational age (LGA)) were calculated using the updated US-based birth weight for gestational age reference 30. Weight-for-length (WLZ) and weight-for-age (WAZ) Z-scores were calculated based on the WHO Child Growth Standards 31. Infant fat (FM) and fat free (FFM) mass were determined by quantitative nuclear magnetic resonance (EchoMRI-AH, Echo Medical Systems, Houston, Texas) as previously described 32. Fat mass index (FMI) and fat free mass index (FFMI) were calculated as kg/m2.
Self-reported outcomes
Maternal race and age were self-reported. At 0.5 months postpartum, mothers reported their infants’ race, sex, birth weight, and birth length as well as their delivery mode. Gestational age was calculated using the mother’s last menstrual period and the child’s date of birth.
Human Milk Collection
Participants were asked to collect a human milk sample at the second feeding of the day (or before 9 am) by fully expressing one breast (manually or using a pump [electrical or manual] at home or during their study visit) at postnatal age 0.5, 2, and 6 months. All milk samples were stored at −70°C until analysis. At each visit, women reported their frequency of breastfeeding and completed weighed food records (for expressed human milk and additional formula feeding). Once the participant discontinued breastfeeding completely, they could no longer participate in this secondary analysis (see Supplemental Figure 1 for details).
Infant Daily Intake
A single test weigh combined with nursing frequencies was used to estimate daily infant intakes of human milk 33. Infants were fasted for at least 2 hours prior to the test weighing procedure. Infant weights were measured immediately before and after a nursing session to determine the volume of human milk consumed at 0.5, 2, and 6 month visits. Mothers recorded their nursing frequency over 3 days (2 week and 1 weekend day) or weighed the expressed milk fed to their infant prior to the corresponding study visit. Human milk volume was estimated from the average daily nursing frequency, single test weight or bottle weighing for expressed milk to estimate daily human milk intake. Infants were categorized as either exclusively breastfed or mixed fed based upon the infant’s formula intake. If at any visit the infant received more than 100 mL of formula per day, they were then considered a mixed feeder from that visit onwards. Data from visits that lacked dietary intake data were not used in the daily intake analyses, but human milk amino acid concentrations were still included in the study analysis.
Human Milk Free Amino Acid Concentrations Analysis
Concentrations of free amino acids; arginine (Arg), alanine (Ala), asparagine (Asn), aspartic acid (Asp), cysteine (Cys), cystine (C-C), glutamic acid (Glu), glutamine (Gln), glycine (Gly), histidine (His), isoleucine (Ile), leucine (Leu), lysine (Lys), methionine (Met), phenylalanine (Phe), proline (Pro), selenocysteine (Se-C), serine (Ser), threonine (Thr), tryptophan (Trp), tyrosine (Try), and valine (Val) were quantified in human milk samples. Dried extracts were reconstituted in 100 μL of mobile phase (A:B 1:2) just prior to analysis. Briefly, an EZ:faast amino acid analysis kit was purchased from Phenomenex (Torrance, CA). Se-C and Cys standards (1 mg/mL in H2O) were obtained from Sigma Aldrich (St. Louis, MO) and were added to the calibration mixtures at 20, 100, and 200 nmol/mL. All other reagents used were of optima grade from Fisher Scientific (Pittsburgh, PA). Standards and samples (100 μL) were processed in accordance with the manufacturers’ instructions.
Chromatographic separation was performed on an UltiMate 3000 UHPLC system (Thermo Fisher Scientific, Waltham, MA) fitted with the Phenomenex EZ:faast column (250 x 3 mm, 4 μm) kept at 35 °C. A flow rate of 500 μL/min and injection volume of 5 μL was used. Mobile phases consisted of 10 mM ammonium formate in water (A) and 10 μM ammonium formate in methanol (B) with a 20-minute elution gradient as follows: ramp from 68 to 86% B over 13 min, return to 68% B in 0.01 min and hold 68% B for 7 min. Identification was carried out on a SCIEX 4000 QTRAP (Framingham, MA) mass spectrometer with data acquisition and analysis performed using Analyst 1.7 software. Data were acquired by multiple reaction monitoring (MRM) in positive Turbo spray ionization mode. Nitrogen as curtain, CAD, GS1, and GS2 gas was set at 10, medium, 10, and 10 units, respectively. Ion spray voltage and source temperature were at 5500 V and 425 °C. MRM parameters such as declustering potential (DP), entrance potential (EP), collision energy (CE), and collision cell exit potential (CXP) are supplied in supplemental data (Supplemental Table 1). Due to the high-throughput analysis, a mixture of all standard amino acids was injected every 15 samples to monitor the column degeneration. The amino acid intensities of each of the 15 samples were normalized by the standards in the same batch. All samples were assayed in duplicate. The sample coefficient of variances (Supplemental Table 2) were used to determine the validity of each measurement.
Statistical Analysis
Descriptive statistics (mean, standard deviation, counts, and percent) were calculated for demographic data, clinical characteristics (Table 1, Supplemental Table 3), infant feeding patterns (Supplemental Table 4), human milk free amino acid concentrations (Table 2), and daily infant intake of free amino acids (Table 3). T-tests and Pearson’s Chi-squared tests were used to compare values between groups (NW and OB) for continuous and categorical data, respectively. Significance was set at alpha ≤ 0.05. Linear mixed-effects models with Bonferroni-corrected post hoc pairwise comparisons were constructed to investigate any differences between the human milk free amino acid composition (nmol/mL) of NW and OB mothers while adjusting for maternal race, maternal age, and infant sex (Figure 1). Linear mixed-effects models were also constructed to determine: 1) the associations between maternal BMI and normalized free amino acid intakes (μmol/day/kg infant weight) over time (repeated measures at 0.5, 2, and 6 months, Table 4), 2) the associations between human milk free amino acid intake (μmol/day) on infant body composition (FMI and FFMI) after adjusting for infant age, infant birth weight, infant sex, maternal BMI, gestational weight gain, maternal race, delivery mode (vaginal or C-section), and breastfeeding status over time (repeated measures at 0.5, 2, and 6 months, Table 5) and 3) the associations between human milk free amino acid intake (μmol/day) on infant growth (WAZ and WLZ) after adjusting for infant age, infant birth weight, maternal BMI, gestational weight gain, maternal race, delivery mode (vaginal or C-section) and breastfeeding status over time (repeated measures at 0.5, 2, and 6 months). Linear mixed-effects models were fitted with all available data and confounders were chosen a priori, where subject identification number was considered as a random effect. Statistical analyses were conducted in R (version 3.6.0) and IBM SPSS® software version 27.
Table 1. Maternal and Infant Characteristics.
Maternal and infant clinical and demographic characteristics are summarized as N (%) or mean ± SD. Comparisons were made between NW and OB groups where t-tests were performed for continuous data and Chi-squared tests were used for categorical data. NW = normal weight, OB = obese, GWG = gestational weight gain, IOM = Institute of Medicine, LGA = large for gestational age, AGA = appropriate for gestational age, SGA = small for gestational age
| N (%) or mean ± SD | All | NW | OB | P - Value |
|---|---|---|---|---|
| Number of Participants | 169 | 83 | 86 | 0.473 |
| 0.5M, N (%) | 151 (100%) | 74 (49%) | 77 (51%) | |
| 2M, N (%) | 129 (100%) | 66 (51%) | 63 (49%) | |
| 6M, N (%) | 93 (100%) | 53 (57%) | 40 (43%) | |
| Maternal Age at Delivery (years) | 30.1 ± 4.0 | 30.4 ± 3.4 | 29.9 ± 4.5 | 0.376 |
| Race | 0.017 | |||
| Caucasian, N (%) | 132 (78%) | 71 (86%) | 61 (71%) | |
| Non-Caucasian, N (%) | 37 (22%) | 12 (14%) | 25 (29%) | |
| Maternal BMI (kg/m2) | 29.5 ± 8.3 | 22.3 ± 1.7 | 36.4 ± 5.7 | <0.001 |
| GWG (kg) | 10.6 ± 4.6 | 12.6 ± 2.8 | 8.7 ± 5.2 | <0.001 |
| GWG (IOM Category) | <0.001 | |||
| Inadequate, N (%) | 45 (27%) | 26 (31%) | 19 (22%) | |
| Adequate, N (%) | 71 (42%) | 46 (56%) | 25 (29%) | |
| Excessive, N (%) | 48 (28%) | 9 (11%) | 39 (45%) | |
| Unknown, N (%) | 5 (3%) | 2 (2%) | 3 (4%) | |
| Gestational Age at Delivery (weeks) | 39.2 ± 1.5 | 39.3 ± 1.3 | 39.2 ± 1.6 | 0.714 |
| Delivery Method | 0.007 | |||
| Vaginal, N (%) | 112 (66%) | 63 (76%) | 49 (57%) | |
| C-Section, N (%) | 57 (34%) | 20 (24%) | 37 (43%) | |
| Infant Sex | 0.153 | |||
| Female, N (%) | 77 (46%) | 34 (41%) | 43 (40%) | |
| Male, N (%) | 92 (54%) | 49 (59%) | 43 (50%) | |
| Birth Weight (kg) | 3.5 ± 0.55 | 3.5 ± 0.51 | 3.5 ± 0.59 | 0.643 |
| Birth Length (cm) | 50.7 ± 3.0 | 50.8 ± 3.0 | 50.6 ± 2.9 | 0.697 |
| Weight-for-Gestational Age category | 0.478 | |||
| LGA, N (%) | 29 (17%) | 12 (15%) | 17 (20%) | |
| AGA, N (%) | 134 (79%) | 67 (81%) | 67 (78%) | |
| SGA, N (%) | 6 (4%) | 4 (5%) | 2 (2%) |
Table 2. Human Milk Amino Acid Concentrations.
Human milk amino acid concentrations (nmol/mL) from 0.5, 2, and 6 months postpartum are presented as mean ± SEM. Independent samples t-tests were performed where p < 0.05 was considered significant. NW = normal weight, OB = obese, Arg = arginine, Ala = alanine, Asn = asparagine, Asp = aspartic acid, C-C = cystine, Glu = glutamic acid, Gln = glutamine, Gly = glycine, His = histidine, Ile = isoleucine, Leu = leucine, Lys = lysine, Phe = phenylalanine, Pro = proline, Ser = serine, Tyr = tyrosine, and Val = valine.
| 0.5 Months | 2 Months | 6 Months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NW (N = 74) |
OB (N = 77) |
P - Value |
NW (N = 66) |
OB (N = 63) |
P - Value |
NW (N = 53) |
OB (N = 40) |
P - Value |
|
| Branched-chain Amino Acids (nmol/mL) | |||||||||
| Ile | 10.3 ± 1.0 | 16.7 ± 1.2 | <0.001 | 10.4 ± 0.6 | 16.3 ± 1.0 | <0.001 | 10.3 ± 0.5 | 14.6 ± 1.0 | <0.001 |
| Leu | 25.5 ± 2.6 | 36.7 ± 2.7 | 0.003 | 27.5 ± 1.2 | 37.0 ± 2.3 | <0.001 | 29.2 ± 1.1 | 33.5 ± 1.9 | 0.044 |
| Val | 40.7 ± 2.2 | 48.5 ± 2.6 | 0.023 | 47.7 ± 2.2 | 49.6 ± 1.9 | 0.517 | 42.8 ± 2.0 | 44.9 ± 2.5 | 0.502 |
| Aromatic Amino Acids (nmol/mL) | |||||||||
| Phe | 10.1 ± 0.6 | 13.2 ± 0.8 | 0.004 | 11.9 ± 0.7 | 14.3 ± 0.8 | 0.020 | 12.3 ± 0.7 | 16.2 ± 1.1 | 0.004 |
| Tyr | 13.6 ± 0.9 | 20.3 ± 1.4 | <0.001 | 12.9 ± 0.9 | 18.9 ± 1.2 | <0.001 | 13.2 ± 0.9 | 17.3 ± 1.2 | 0.006 |
| Other Amino Acids (nmol/mL) | |||||||||
| Ala | 158.8 ± 8.3 | 165.4 ± 8.3 | 0.556 | 188.6 ± 8.7 | 209.7 ± 10.6 | 0.122 | 211.1 ± 8.2 | 210.9 ± 11.6 | 0.988 |
| Asn | 13.2 ± 1.4 | 11.5 ± 1.4 | 0.397 | 19.0 ± 21.7 | 11.6 ± 1.4 | 0.001 | 23.0 ± 2.2 | 14.2 ± 2.2 | 0.006 |
| Asp | 44.7 ± 3.1 | 52.9 ± 3.3 | 0.070 | 58.2 ± 4.2 | 87.4 ± 5.9 | <0.001 | 81.2 ± 7.0 | 104.3 ± 8.8 | 0.041 |
| C-C | 12.9 ± 0.7 | 10.2 ± 0.8 | 0.013 | 21.6 ± 1.0 | 14.5 ± 0.8 | <0.001 | 20.9 ± 1.1 | 19.7 ± 1.5 | 0.512 |
| Gln | 108.6 ± 11.4 | 102.7 ± 10.9 | 0.711 | 406.7 ± 21.5 | 286.2 ± 18.7 | <0.001 | 535.1 ± 21.8 | 337.9 ± 26.3 | <0.001 |
| Glu | 645.8 ± 25.4 | 606.3 ± 24.5 | 0.265 | 851.5 ± 26.6 | 743.0 ± 23.5 | 0.003 | 866.2 ± 24.8 | 789.9 ± 25.5 | 0.038 |
| Gly | 70.6 ± 3.5 | 66.1 ± 3.2 | 0.348 | 105.9 ± 4.9 | 86.5 ± 4.0 | 0.003 | 126.5 ± 5.0 | 98.5 ± 5.6 | <0.001 |
| His | 32.2 ± 1.7 | 22.1 ± 1.9 | <0.001 | 33.5 ± 1.7 | 22.7 ± 1.6 | <0.001 | 28.9 ± 1.4 | 17.7 ± 1.7 | <0.001 |
| Lys | 33.8 ± 3.2 | 44.1 ± 4.3 | 0.059 | 21.6 ± 2.2 | 24.9 ± 2.7 | 0.348 | 19.8 ± 1.9 | 21.0 ± 2.2 | 0.691 |
| Pro | 44.4 ± 3.1 | 52.1 ± 3.2 | 0.084 | 40.7 ± 1.9 | 42.0 ± 2.3 | 0.672 | 36.0 ± 1.4 | 38.6 ± 1.9 | 0.265 |
| Ser | 94.9 ± 4.7 | 94.9 ± 4.0 | 0.997 | 147.3 ± 6.0 | 125.1 ± 4.7 | 0.004 | 171.8 ± 7.7 | 132.9 ± 7.0 | <0.001 |
Table 3. Daily Infant Intakes of Human Milk Amino Acids, Normalized to Infant Weight.
Daily infant intakes of amino acids (μmol/day) were normalized to infant body weight (kg) and are presented as mean ± SEM. Independent samples t-tests were performed where p < 0.05 was considered significant. NW = normal weight, OB = obese, Arg = arginine, Ala = alanine, Asn = asparagine, Asp = aspartic acid, C-C = cystine, Glu = glutamic acid, Gln = glutamine, Gly = glycine, His = histidine, Ile = isoleucine, Leu = leucine, Lys = lysine, Phe = phenylalanine, Pro = proline, Ser = serine, Tyr = tyrosine, and Val = valine.
| 0.5 Months | 2 Months | 6 Months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NW (N = 56) |
OB (N = 46) |
P - Value |
NW (N = 54) |
OB (N = 46) |
P - Value |
NW (N = 51) |
OB (N = 27) |
P - Value |
|
| Branched-chain Amino Acid Intake (μmol/kg/day) | |||||||||
| Ile | 1.8 ± 0.2 | 2.5 ± 0.3 | 0.083 | 1.2 ± 0.1 | 1.8 ± 0.2 | 0.002 | 0.9 ± 0.1 | 1.2 ± 0.2 | 0.152 |
| Leu | 4.6 ± 0.5 | 5.1 ± 0.5 | 0.492 | 3.2 ± 0.2 | 4.0 ± 0.3 | 0.049 | 2.5 ± 0.2 | 2.8 ± 0.5 | 0.567 |
| Val | 7.1 ± 0.5 | 7.5 ± 0.8 | 0.685 | 5.8 ± 0.5 | 5.9 ± 0.6 | 0.933 | 3.6 ± 0.6 | 3.7 ± 0.6 | 0.975 |
| Aromatic Amino Acid Intake (μmol/kg/day) | |||||||||
| Phe | 1.8 ± 0.2 | 2.2 ± 0.3 | 0.273 | 1.4 ± 0.1 | 1.7 ± 0.2 | 0.114 | 1.0 ± 0.1 | 1.3 ± 0.2 | 0.213 |
| Tyr | 2.4 ± 0.2 | 3.0 ± 0.3 | 0.134 | 1.5 ± 0.2 | 2.2 ± 0.2 | 0.013 | 1.2 ± 0.1 | 1.4 ± 0.3 | 0.324 |
| Other Amino Acid Intake (μmol/kg/day) | |||||||||
| Ala | 27.2 ± 2.3 | 27.9 ± 2.8 | 0.829 | 23.3 ± 2.0 | 24.9 ± 2.4 | 0.621 | 19.0 ± 1.8 | 20.0 ± 3.5 | 0.763 |
| Asn | 2.4 ± 0.3 | 1.7 ± 0.4 | 0.195 | 2.7 ± 0.4 | 1.3 ± 0.2 | 0.002 | 2.0 ± 0.3 | 1.5 ± 0.5 | 0.295 |
| Asp | 7.2 ± 0.7 | 8.2 ± 1.0 | 0.425 | 7.6 ± 0.9 | 9.8 ± 1.2 | 0.154 | 7.4 ± 0.9 | 7.4 ± 1.0 | 0.961 |
| C-C | 2.3 ± 0.2 | 1.9 ± 0.2 | 0.263 | 2.8 ± 0.3 | 1.9 ± 0.2 | 0.007 | 1.8 ± 0.2 | 1.9 ± 0.4 | 0.680 |
| Gln | 18.8± 2.7 | 20.4 ± 3.2 | 0.698 | 53.4 ± 4.4 | 41.1 ± 4.7 | 0.057 | 47.4 ± 4.2 | 34.8 ± 7.3 | 0.115 |
| Glu | 114.7 ± 8.9 | 108.3 ± 11.3 | 0.655 | 107.4 ± 7.7 | 92.0 ± 8.0 | 0.168 | 74.6 ± 5.9 | 66.2 ± 9.4 | 0.433 |
| Gly | 12.5 ± 1.0 | 11.6 ± 1.5 | 0.613 | 13.7 ± 1.1 | 10.3 ± 0.9 | 0.024 | 10.7 ± 0.9 | 8.8 ± 1.5 | 0.276 |
| His | 5.4 ± 0.5 | 4.1 ± 0.6 | 0.064 | 4.2 ± 0.4 | 2.8 ± 0.4 | 0.009 | 2.4 ± 0.2 | 1.6 ± 0.3 | 0.034 |
| Lys | 6.1 ± 0.7 | 5.8 ± 0.8 | 0.732 | 2.5 ± 0.3 | 2.8 ± 0.5 | 0.536 | 1.7 ± 0.2 | 1.9 ± 0.4 | 0.720 |
| Pro | 7.8 ± 0.7 | 8.2 ± 0.9 | 0.680 | 4.9 ± 0.4 | 4.7 ± 0.4 | 0.735 | 3.0 ± 0.3 | 3.1 ± 0.5 | 0.920 |
| Ser | 16.5 ± 1.3 | 16.0 ± 1.9 | 0.827 | 18.4 ± 1.4 | 15.9 ± 1.5 | 0.225 | 14.8 ± 1.3 | 12.0 ± 2.2 | 0.244 |
Figure 1. The Effect of Maternal Obesity and Time on Human Milk Amino Acid Concentrations.
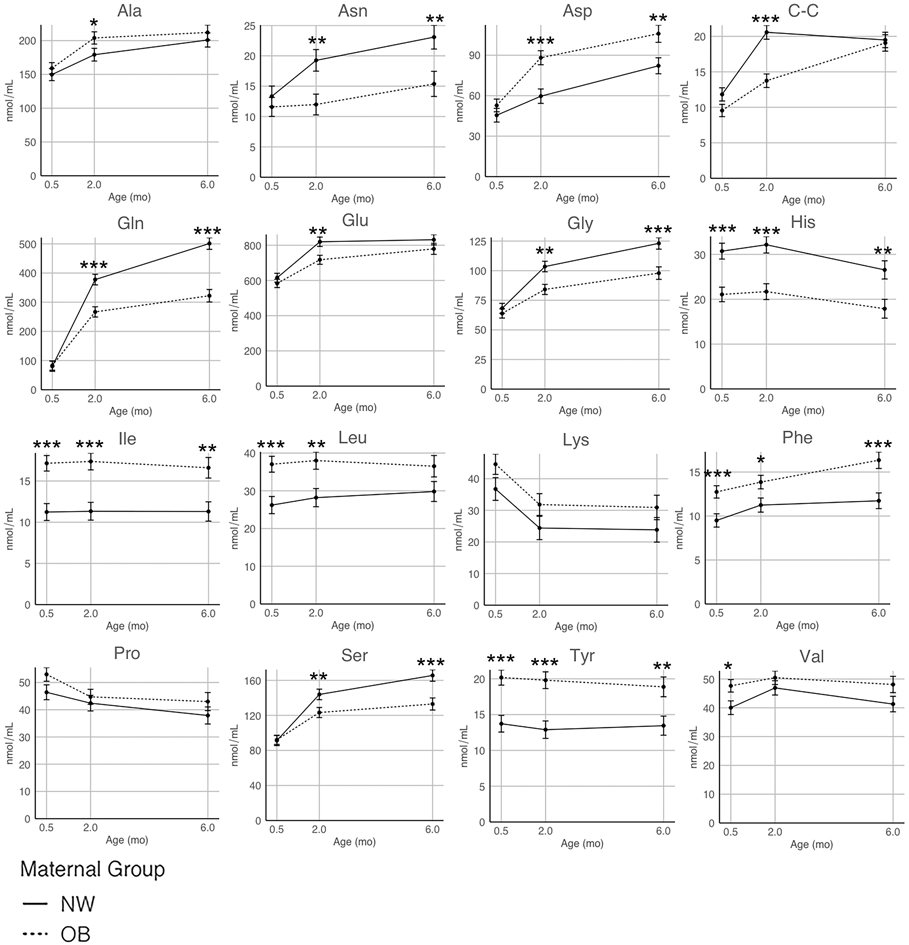
Linear mixed-effects models were constructed to test the associations between maternal BMI group (* p < 0.05, ** p < 0.01, *** p < 0.001) and human milk amino acid concentrations (nmol/mL). Models were adjusted for potential confounding factors: maternal age, maternal race, and infant sex. All data are presented as the model estimated marginal mean amino acid concentrations over three timepoints: 0.5 months (n = 151), 2 months (n = 129), and 6 months (n = 93). Solid line = NW group, dotted line = OB group.
Table 4. Maternal BMI is Associated with Daily Infant Intakes of Human Milk Amino Acids Over the First 6 Months of Life.
Linear mixed-effects models (n = 169 subjects) included human milk amino acid intakes (μmol/kg/day) as the dependent variables and maternal BMI (kg/m2) at first visit as the fixed effect. Model covariates included: infant age, infant sex, maternal race, gestational weight gain, and breastfeeding status (exclusive or mixed). Asn = asparagine, C-C = cystine, His = histidine, Ile = isoleucine, Leu = leucine, Phe = phenylalanine, and Tyr = tyrosine.
| All BMI | ||
|---|---|---|
| Amino Acid Intake (μmol/kg/day) |
β | P Value |
| Ile | 0.04 | 0.001 |
| Leu | 0.05 | 0.08 |
| Phe | 0.03 | 0.01 |
| Tyr | 0.05 | 0.002 |
| Asn | −0.04 | 0.03 |
| C-C | −0.03 | 0.05 |
| His | −0.07 | 0.004 |
Table 5. Daily Infant Intakes of Human Milk Amino Acids are Associated with Infant Body Composition at 0.5 - 6 Months of Age.
Linear mixed-effects models included infant weight-for-length z-score (WLZ), fat mass index (FMI), and fat free mass index (FFMI) over the first 6 months of life (time points 0.5, 2, and 6 months) as the dependent variables and infants' intakes of human milk amino acids (μmol/day) as the fixed effects. Model covariates included: infant age, infant birth weight, infant sex (FMI and FFMI models only), maternal BMI, gestational weight gain, maternal race, delivery mode (vaginal or C-section), and breastfeeding status. Random effects included: participant identification number, to account for repeated measures. Asp = aspartic acid, C-C = cystine, Gln = glutamine, Glu = glutamic acid, His = histidine, and Ser = serine.
| Weight-for-Length Z-score |
Fat Mass Index (kg fat mass / m2) |
Fat Free Mass Index (kg fat free mass / m2) |
||||
|---|---|---|---|---|---|---|
| Amino Acid Intake (μmol/day) |
β | p-value | β | p-value | β | p-value |
| Asp | 0.0024 | 0.093 | 0.0038 | 0.011 | 0.0034 | 0.011 |
| C-C | 0.0103 | 0.088 | 0.0174 | 0.006 | 0.0163 | 0.004 |
| Gln | 0.0004 | 0.15 | 0.0011 | <0.001 | 0.0007 | 0.009 |
| Glu | 0.0004 | 0.042 | 0.0005 | 0.005 | 0.0004 | 0.026 |
| His | 0.0080 | 0.039 | 0.0106 | 0.008 | 0.0088 | 0.015 |
| Ser | 0.0011 | 0.23 | 0.0029 | 0.002 | 0.0014 | 0.088 |
Results
Participant characteristics and infant feeding
Of the 169-breastfeeding mother-infant pairs studied (Table 1), 83 mothers had NW (mean BMI = 22.3 ± 1.7 kg/m2) and 86 mothers had OB (mean BMI = 36.4 ± 5.7 kg/m2). The number of participants who provided human milk at each postnatal visit (0.5, 2, and 6 months) are listed in Supplemental Figure 1 and Table 1. Women were, on average, 30.1 ± 4.0 years of age at the time of delivery (39.2 ± 1.5 weeks gestation). The majority of women were Caucasian (78%) and had vaginal deliveries (66%). However, compared to women with NW, a greater proportion of women with OB were non-Caucasian (29% vs. 14%, p = 0.017) and delivered via C-section (43% vs. 24%, p = 0.007). Women with OB gained significantly less weight during gestation compared to women with NW (8.7 ± 5.2 kg vs. 12.6 ± 2.8 kg, p < 0.001), as recommended by IOM guidelines 29. However, the majority of women with OB (45%) were categorized as gaining an excessive amount of weight during pregnancy based on IOM categorizations 29, while a greater proportion of women with NW gained an inadequate amount of weight as compared to women with OB (31% vs. 22%, p < 0.001).
Similar proportions of male and female infants were included in this study (Table 1). There were no differences in sex, birth weight, birth length, or weight-for-gestational-age category between infants born to women with NW vs. those with OB (Table 1). Infants born to women with OB had 16.6% greater FM (p = 0.001) and 17.8% greater FMI (p < 0.001) at 0.5 months postpartum compared to those born to women with NW (Supplemental Table 3). However, there were no observed differences in adiposity at later time points (2 and 6 months), Supplemental Table 3. The FFMI of infants born to women with OB was slightly, but significantly greater at 0.5 (3.1%, p = 0.023) and 6 months (6.0%, p = 0.001) compared to those born to women with NW (Supplemental Table 3). There were no differences in the total amount of milk consumed (human milk plus formula) or the total amount of human milk consumed by infants born to OB women compared to NW women after normalizing intake to infant body weight (kg) at any timepoint measured in this study (Supplemental Table 4). However, the proportion of women who supplemented their infants’ intake with formula was significantly greater for women with OB than for women with NW at both 0.5 months (21.7% vs. 3.6%, p = 0.005) and 2 months postpartum (15.2% vs. 3.7%, p = 0.048) (Supplemental Table 4).
Human milk free amino acid concentrations are altered by maternal BMI and time
Sixteen of the 22 targeted free amino acid concentrations were detected in human milk at 0.5, 2, and 6 months postpartum (Table 2). BCAAs (Ile (53.5%) and Leu (31.1%)) and AAAs (Phe (27.5%) and Tyr (42.3%)) were significantly increased in human milk from women with OB at all timepoints, while the essential amino acid His was significantly decreased by ~35% compared to human milk from women with NW. At 2 and 6 months postpartum, Asn (39%), Gln (33%), Glu (11%), Gly (20%), and Ser (19%) concentrations were significantly lower, while Asp (39%) was significantly higher in human milk from women with OB vs. NW. Human milk concentrations of C-C at 0.5 and 2 months were, on average, 27.0% significantly lower in human milk from women with OB compared to women with NW.
Linear mixed-effects models were developed to test the effects of maternal OB on free amino acid concentrations in human milk, adjusting for maternal race, maternal age, infant age, and infant sex (Figure 1). The majority of free amino acid concentrations (Ala, Asn, Asp, C-C, Gln, Glu, Gly, Phe, and Ser) significantly increased in human milk over time (0.5 – 6 months). However, Pro and the positively charged free amino acids, His and Lys, decreased significantly as lactation progressed, while Ile, Leu, and Tyr did not change (Figure 1). There was a significant group effect (NW vs. OB) on 13 of the 16 free amino acid concentrations (Asn, Asp, C-C, Gln, Glu, Gly, His, Ile, Leu, Phe, Ser, Tyr, and Val), where women with OB had significantly lower Asn, C-C, Gln, Glu, Gly, and Ser concentrations at later timepoints (2 and/or 6 months) and decreased His levels at each timepoint measured. Higher BCAA (Ile, Leu, and Val), AAA (Phe and Tyr), and Asp concentrations were observed in milk from women with OB compared to women with NW.
Daily infant intakes of human milk free amino acids change over time and are altered by maternal BMI
Actual intakes of human milk free amino acids were estimated at 0.5, 2, and 6 months of age to better understand how differences in human milk free amino acid concentrations translate to infant nutrition and growth (Table 3). Contrary to what was observed for human milk free amino acid concentrations, normalized infant consumption of human milk free amino acids decreased for the majority of free amino acids measured (Ala, Glu, Gly, His, Ile, Leu, Lys, Phe, Pro, Ser, Tyr, and Val) over the first 6 months of life. However, infant intakes of human milk Gln tended to increase over time (Table 3). There were only a few differences detected in infants’ daily human milk free amino acids intakes after normalizing to infant weight (kg) among infants born to women with NW or OB (Table 3). At 0.5 months, the daily intakes of His were numerically lower (~24% lower, p = 0.064) while Ile intakes were numerically higher (~39% higher, p = 0.083) in infants born to women with OB. The daily intakes of Asn (~52%), C-C (~32%), Gln (~23%), Gly (~25%), and His (~33%) were significantly lower whereas Ile (~50%) and Leu (~25%) were significantly higher in infants born to women with OB compared to those with NW at 2 months postpartum. The daily intake of human milk His was the only free amino acid intake that differed at 6 months of age, and was ~33% lower in infants born to women with OB compared to the NW group.
Linear mixed-effects models (Table 4) revealed that as maternal BMI increases (1 kg/m2 BMI), the daily infant human milk intakes (μmol/kg/day) of Asn (β = −0.04, p =0.03), C-C (β = −0.03, p = 0.05), and His (β = −0.07, p = 0.004) decreases after adjusting for infant age, infant sex, maternal race, gestational weight gain, and breastfeeding status (exclusive or mixed). On the other hand, daily infant human milk intakes (μmol/kg/day) of BCAAs, Ile (β = 0.04, p = 0.001) and Leu (β = 0.05, p = 0.08), and AAAs, Phe (β = 0.03, p = 0.01) and Tyr (β = 0.05, p = 0.002), were associated with a significant increase as maternal BMI increased (per 1 kg/m2 BMI) after controlling for the same covariates.
Normalized daily infant free amino acid intakes are associated with infant FMI and FFMI over the first 6 months of age
To test the possible associations between infant intakes of free amino acids and infant growth (Table 5), linear mixed-effects models were constructed after adjusting for infant age, infant sex (FMI and FFMI models only), infant birth weight, maternal race, gestational weight gain, and breastfeeding status and considering the trajectories of the repeated measurements. The individual daily intakes for each free amino acid were modeled to associate with infant WLZ, WAZ, FMI, and FFMI over the first 6 months of life (repeated measures at 0.5, 2, and 6 month visits). Infant intakes of free amino acids were not associated with WAZ. For every μmol/day increase of free Asp (trend, p = 0.093), C-C (trend, p = 0.088), Glu (p = 0.042), and His (p = 0.039) consumed, child WLZ increases by 0.0024 kg/m2, 0.0103 kg/m2, 0.0004 kg/m2, and 0.0080 kg/m2, respectively. For every μmol/day increase of free Asp (p = 0.011), C-C (p = 0.006), Gln (p < 0.001), Glu (p = 0.005), His (p = 0.008), and Ser (p = 0.002) consumed, child FMI increases by 0.0011 kg/m2, 0.0038 kg/m2, 0.0174 kg/m2, 0.0011 kg/m2, 0.0005 kg/m2, 0.0106 kg/m2, 0.0185 kg/m2, and 0.0029 kg/m2, respectively.. On the other hand, free Asp (β = 0.0034 kg/m2, p = 0.011), C-C (β = 0.0163 kg/m2, p = 0.004), Gln (β = 0.0007 kg/m2, p = 0.009), Glu (β = 0.0004 kg/m2, p = 0.026), His (β = 0.0088 kg/m2, p = 0.015), and Ser (β = 0.0014 kg/m2, p = 0.088) intakes were positively associated with infant FFMI. Together, these data suggest that differences in the daily consumption of several human milk free amino acids is associated with infant body composition.
Discussion
As increased numbers of breastfeeding mothers develop overweight or obesity, it is important to define the impact of maternal BMI on human milk composition and on infant intakes of various human milk constituents. From this study, three key observations were made. 1) Women with obesity produce human milk that differs significantly from women of normal weight in terms of free amino acid composition. Of interest, changes in human milk free amino acid content does not directly translate to altered infant free amino acid consumptions, suggesting that accurate estimation of infant intakes is crucial in understanding nutritional exposures during infancy. 2) Free BCAAs and AAAs concentration were significantly higher in human milk from women with obesity and maternal BMI was positively associated with Ile, Leu, Phe, and Tyr concentrations over the first 6 months postpartum. However, infants born to women with obesity only consumed greater quantities of these free amino acids, relative to infant body weight, at 2 months of age. 3) The daily intakes of several free amino acids were positively associated with FMI and FFMI over the first 6 months of life. None of the BCAA or AAA intakes were significantly associated with infant growth or infant body composition. These results suggest that specific human milk free amino acid consumption positively associates with infant body composition.
The concept of early-life metabolic programming has been widely demonstrated in both clinical and preclinical studies. Specific to the postnatal period, faster weight gain during infancy is linked to obesity risk later in life 34 and augmented consumption of amino acids can increase weight gain velocity in formula fed infants 17,35, supporting the early protein hypothesis 18. Accordingly, the early protein hypothesis is well adopted in formula fed infants and predicts that the risk for obesity increases with augmented protein consumption in early life via an elevation in circulating BCAAs and subsequent activation of insulin and insulin-like growth factor 1 pathways 22,36. In a randomized clinical trial examining the effects of feeding infant formula containing low (1.7 g/100 kcal) vs. high protein (2.9 g/100 kcal) compared to exclusively breastfed infants on growth, Koletzko et. al. found that the 24-month WAZ of infants fed the lower protein milk formula was significantly lower than that of the higher protein group, but not different than that of the breastfed reference group 35. These findings suggest that lower protein infant formulas may normalize weight gain velocity in formula fed infants to that of breastfed infants, decreasing their potential for future obesity risk. Interestingly, the potential impact of variations in human milk protein compositions on infant growth and metabolism are still relatively unknown. In our previously published work 28, we observed that lactating women with overweight and obesity have a greater concentration of total protein in their milk at 5 months postpartum and that the daily average infants’ intake of total protein is significantly greater at both 1 and 6 months of age compared to infants born to women with normal weight. Furthermore, daily protein intake was positively associated with an increase in length-for-age z score and WAZ after adjusting for infant feeding mode and infant sex, but negatively associated with both FMI and FFMI 28. Conversely, Young et. al. found decreased total protein concentrations in human milk from women with overweight and obesity at 2 weeks after giving birth 37, suggesting that the timing of human milk sampling is an important consideration.
Excess circulating BCAAs can promote growth through activation of the insulin and insulin-like growth factor 1 pathways that are upstream of the mammalian target of rapamycin (mTOR) signaling network 38,39. Amino acids also directly stimulate mTOR independent of insulin, and adequate amounts of amino acids are essential for muscle growth during the neonatal period 40,41. Conversely, through the insulin pathways of mTOR activation, adipogenic signaling is thought to be activated 39 leading to increased susceptibility to obesity 23. We therefore hypothesized that with increased consumption of free BCAAs, infants born to women with obesity would have increased growth and/or adiposity. Our data indicate that there is not an association between the intake of free BCAAs and infant growth over the first 6 months of age.
It is possible that because human milk free amino acids provide only around 3-5% of the amino acids required to perform metabolic activities in the infant 36, that total human milk amino acid intakes (free amino acids + amino acids in proteins) may provide a more comprehensive perspective on infant growth. We recently published that the protein concentration in HM from women with overweight was higher than in women with NW only at 5 and 6 months postpartum, and the offspring’s protein intake was negatively associated with both FMI and FFMI 28. Further, free amino acids are rapidly absorbed in the intestine, and circulating and intracellular essential amino acid availabilities are primary regulators of muscle protein synthesis 42,43. This absorption and regulation highlights the significance of determining the association between HM free amino acid consumption and infant growth. Of interest, HM concentrations of several essential amino acids were elevated in the OB vs. NW group (Table 2; BCAAs, Phe), but this did not always translate to higher daily infant intakes of these amino acids when normalized to infant weight. On the other hand, the essential amino acid His was significantly lower in OB vs. NW HM, leading to a lower daily normalized infant intake (Table 3). Of interest, intake of the conditionally essential amino acids, Glu, Tyr and C-C, were all positively related to FFMI, together with His and Val, highlighting their importance in growth.
It remains to be determined if other mechanisms of metabolic programming are associated with this greater exposure to human milk free BCAAs. Hellmuth et al. found a significant positive association between human milk protein content and infant lyso-phosphatidylcholine levels 44, a compound that has been linked to increased risk of childhood obesity 45. Although these data do not speak specifically to the effects of human milk BCAAs on metabolic programming, they do suggest that human milk proteins may play a role in nonhereditary nutritional programming. Future studies should focus on molecular markers of metabolic risk in the infants and longer-term growth outcomes.
Human milk free amino acid content changes dynamically over lactation, where some amino acids increase while others decrease or stay relatively unchanged (recently reviewed here 46) and are in-line with those observed in the current study. Interestingly, the plasma pools of BCAAs, AAAs, as well as basic and neutral amino acids in lactating women were found to be 1- to 15-fold higher than those found in human milk 47, suggesting that there is a selectivity in amino acid transport at the level of the mammary epithelium during lactation 46. The mammary gland expresses BCAA aminotransferase and the branched-chain α-ketoacid dehydrogenase complex that are responsible for the two-step breakdown of BCAAs 48. Although only demonstrated in species other than human, the lactating mammary gland is known to take up copious amounts of BCAAs and extensively degrades them for the synthesis of other non-essential amino acids, such as Glu 48,49. Throughout the body, catabolism of BCAAs may be an important means of dealing with a surplus in supply 38. Accordingly, in non-lactating individuals with metabolic disease, BCAA plasma concentrations increase as a function of impaired BCAA catabolism 38. We theorize that the dysregulation of BCAA catabolic enzymes in mammary epithelial cells from women with obesity may therefore contribute to elevations in human milk BCAA content and lower Glu levels associated with maternal obesity.
Herein, we have presented a longitudinal assessment of human milk free amino acid concentrations using state of the art technologies in one of the largest, well-characterized cohorts of mother-infant dyads representing both women with normal weight and with obesity to date. Furthermore, we have presented infant human milk intakes of free amino acids, estimated with validated methods, which were then used to assess the relationship between infant exposure to human milk free amino acids and infant growth. However, it is imperative to interpret these data within the scope of their limitations. While one-feed test weighing is a validated method for measuring human milk intake, it is not as reliable as 24 hour test-weighing 33. However, this method, compared to 24 hour test-weighing, provides a significantly decreased burden on participants and is financially favorable for a large population such as the one presented here. To date, the nutritive and non-nutritive importance of human milk free amino acids has not been completely established. Although our data suggest that there are associations between infant intake of human milk free amino acids and infant body composition, we cannot conclude that this association results from a direct cause-and-effect relationship. However, infant intake of human milk free BCAAs, AAAs, His, Thr, and Met have been shown to correlate significantly with the respective infant plasma concentrations 14, supporting the potential for a more direct relationship between human milk amino acid intake and the impact on infant physiology. Additionally, although we observed clear differences in various free amino acid concentrations in human milk from women with normal weight compared to those with obesity, these data are observational, and we do not have maternal dietary intake to rule out the potential influence of dietary amino acids on human milk composition. Therefore, conclusions regarding the mechanisms driving these differences cannot be made.
In summary, we have demonstrated a strong relationship between maternal BMI and human milk free amino acid composition over the first 6 months postpartum. Additionally, daily infant intake of human milk free amino acids did not always mimic the corresponding differences in concentrations, indicating the importance for estimating infant intakes to accurately determine infant nutritional exposure. Finally, although our data indicate increased consumptions of BCAAs and AAAs in infants born to women with obesity, BCAA and AAA intakes were not associated with infant growth. However, infant intakes of human milk Asp, C-C, Gln, Glu, His, and Ser showed associations with infant body composition.
Supplementary Material
Acknowledgements:
AA designed the research; CRS, JLS, LP, and RL conducted the research; CRS and JLS analyzed the data; JLS, CRS, LP, RL, EB, and AA wrote and edited the manuscript; AA has primary responsibility for the final content. We thank the participants and the clinical research team at ACNC for their dedication and hard work in producing and collecting the samples and data presented in this manuscript.
Sources of Support:
USDA ARS Project # 6026-51000-010-05S and 6026-51000-012-06S. AA, EB and CRS are partially supported by NIH R01 DK107516.
Footnotes
Conflict of Interest: The authors have no conflict of interest.
Clinical Trial Registry Numbers: www.clinicaltrials.gov, ID# NCT01131117 and ID# NCT02125149
References
- 1.Czerwinski SA, Lee M, Choh AC, et al. Genetic factors in physical growth and development and their relationship to subsequent health outcomes. Am J Hum Biol. 2007;19(5):684–691. [DOI] [PubMed] [Google Scholar]
- 2.Cameron N. The biology of growth. Nestle Nutr Workshop Ser Pediatr Program. 2008;61:1–19. [DOI] [PubMed] [Google Scholar]
- 3.Ollikainen M, Smith KR, Joo EJ, et al. DNA methylation analysis of multiple tissues from newborn twins reveals both genetic and intrauterine components to variation in the human neonatal epigenome. Hum Mol Genet. 2010;19(21):4176–4188. [DOI] [PubMed] [Google Scholar]
- 4.Haschke F, Binder C, Huber-Dangl M, Haiden N. Early-Life Nutrition, Growth Trajectories, and Long-Term Outcome. Nestle Nutr Inst Workshop Ser. 2019;90:107–120. [DOI] [PubMed] [Google Scholar]
- 5.Casavale KO, Ahuja JKC, Wu X, et al. NIH workshop on human milk composition: summary and visions. Am J Clin Nutr. 2019;110(3):769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spevacek AR, Smilowitz JT, Chin EL, Underwood MA, German JB, Slupsky CM. Infant Maturity at Birth Reveals Minor Differences in the Maternal Milk Metabolome in the First Month of Lactation. J Nutr. 2015;145(8):1698–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundekilde UK, Downey E, O'Mahony JA, et al. The Effect of Gestational and Lactational Age on the Human Milk Metabolome. Nutrients. 2016;8(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agostoni C, Carratu B, Boniglia C, Lammardo AM, Riva E, Sanzini E. Free glutamine and glutamic acid increase in human milk through a three-month lactation period. J Pediatr Gastroenterol Nutr. 2000;31(5):508–512. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Adelman AS, Rai D, Boettcher J, Lonnerdal B. Amino acid profiles in term and preterm human milk through lactation: a systematic review. Nutrients. 2013;5(12):4800–4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bloomgarden Z. Diabetes and branched-chain amino acids: What is the link? J Diabetes. 2018;10(5):350–352. [DOI] [PubMed] [Google Scholar]
- 11.Libert DM, Nowacki AS, Natowicz MR. Metabolomic analysis of obesity, metabolic syndrome, and type 2 diabetes: amino acid and acylcarnitine levels change along a spectrum of metabolic wellness. PeerJ. 2018;6:e5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Luca A, Hankard R, Alexandre-Gouabau MC, Ferchaud-Roucher V, Darmaun D, Boquien CY. Higher concentrations of branched-chain amino acids in breast milk of obese mothers. Nutrition. 2016;32(11-12):1295–1298. [DOI] [PubMed] [Google Scholar]
- 13.Alexandre-Gouabau MC, Moyon T, David-Sochard A, et al. Comprehensive Preterm Breast Milk Metabotype Associated with Optimal Infant Early Growth Pattern. Nutrients. 2019;11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janas LM, Picciano MF, Hatch TF. Indices of protein metabolism in term infants fed human milk, whey-predominant formula, or cow's milk formula. Pediatrics. 1985;75(4):775–784. [PubMed] [Google Scholar]
- 15.Cummings NE, Williams EM, Kasza I, et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J Physiol. 2018;596(4):623–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10(12):723–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Socha P, Grote V, Gruszfeld D, et al. Milk protein intake, the metabolic-endocrine response, and growth in infancy: data from a randomized clinical trial. Am J Clin Nutr. 2011;94(6 Suppl):1776S–1784S. [DOI] [PubMed] [Google Scholar]
- 18.Heinig MJ, Nommsen LA, Peerson JM, Lonnerdal B, Dewey KG. Energy and protein intakes of breast-fed and formula-fed infants during the first year of life and their association with growth velocity: the DARLING Study. Am J Clin Nutr. 1993;58(2):152–161. [DOI] [PubMed] [Google Scholar]
- 19.Fleddermann M, Demmelmair H, Grote V, et al. Role of selected amino acids on plasma IGF-I concentration in infants. Eur J Nutr. 2017;56(2):613–620. [DOI] [PubMed] [Google Scholar]
- 20.Hirschel J, Vogel M, Baber R, et al. Relation of Whole Blood Amino Acid and Acylcarnitine Metabolome to Age, Sex, BMI, Puberty, and Metabolic Markers in Children and Adolescents. Metabolites. 2020;10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Ojanen X, Zhuang H, Wu N, Cheng S, Wiklund P. Branched-Chain and Aromatic Amino Acids Are Associated With Insulin Resistance During Pubertal Development in Girls. J Adolesc Health. 2019;65(3):337–343. [DOI] [PubMed] [Google Scholar]
- 22.Kirchberg FF, Harder U, Weber M, et al. Dietary protein intake affects amino acid and acylcarnitine metabolism in infants aged 6 months. J Clin Endocrinol Metab. 2015;100(1):149–158. [DOI] [PubMed] [Google Scholar]
- 23.Socha P, Hellmuth C, Gruszfeld D, et al. Endocrine and Metabolic Biomarkers Predicting Early Childhood Obesity Risk. Nestle Nutr Inst Workshop Ser. 2016;85:81–88. [DOI] [PubMed] [Google Scholar]
- 24.Karlsland Akeson PM, Axelsson IE, Raiha NC. Protein and amino acid metabolism in three- to twelve-month-old infants fed human milk or formulas with varying protein concentrations. J Pediatr Gastroenterol Nutr. 1998;26(3):297–304. [DOI] [PubMed] [Google Scholar]
- 25.Gunther AL, Remer T, Kroke A, Buyken AE. Early protein intake and later obesity risk: which protein sources at which time points throughout infancy and childhood are important for body mass index and body fat percentage at 7 y of age? Am J Clin Nutr. 2007;86(6):1765–1772. [DOI] [PubMed] [Google Scholar]
- 26.Saben JS, Clark; Pack, Lindsay; Lan Renny; Andres Aline. Infant Intakes of Human Milk Amino Acids Are Associated With Maternal Obesity and Infant Growth. Current Developments in Nutrition. 2021;Volume 5(Issue Supplement_2):Page 810. [Google Scholar]
- 27.Krukowski RA, West DS, DiCarlo M, et al. Are early first trimester weights valid proxies for preconception weight? BMC Pregnancy Childbirth. 2016;16(1):357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sims CR, Lipsmeyer ME, Turner DE, Andres A. Human milk composition differs by maternal BMI in the first 9 months postpartum. Am J Clin Nutr. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Institute of Medicine. Institute of Medicine and National Reserach Council Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines. In: Rasmussen KM, Yaktine AL, eds. Washington (DC). USA: National Academies Press; 2009. [PubMed] [Google Scholar]
- 30.Talge NM, Mudd LM, Sikorskii A, Basso O. United States birth weight reference corrected for implausible gestational age estimates. Pediatrics. 2014;133(5):844–853. [DOI] [PubMed] [Google Scholar]
- 31.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization. 2006. [Google Scholar]
- 32.Andres A, Gomez-Acevedo H, Badger TM. Quantitative nuclear magnetic resonance to measure fat mass in infants and children. Obesity (Silver Spring). 2011;19(10):2089–2095. [DOI] [PubMed] [Google Scholar]
- 33.Neville MC, Keller R. Accuracy of single- and two-feed test weighing in assessing 24 h breast milk production. Early Hum Dev. 1984;9(3):275–281. [DOI] [PubMed] [Google Scholar]
- 34.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. Bmj. 2005;331(7522):929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koletzko B, von Kries R, Closa R, et al. Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr. 2009;89(6):1836–1845. [DOI] [PubMed] [Google Scholar]
- 36.Axelsson I. Effects of high protein intakes. Nestle Nutrition workshop series Paediatric programme. 2006;58:121–129; discussion 129-131. [DOI] [PubMed] [Google Scholar]
- 37.Young BE, Levek C, Reynolds RM, et al. Bioactive components in human milk are differentially associated with rates of lean and fat mass deposition in infants of mothers with normal vs. elevated BMI. Pediatr Obes. 2018;13(10):598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siddik MAB, Shin AC. Recent Progress on Branched-Chain Amino Acids in Obesity, Diabetes, and Beyond. Endocrinol Metab (Seoul). 2019;34(3):234–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koletzko B, Beyer J, Brands B, et al. Early influences of nutrition on postnatal growth. Nestle Nutr Inst Workshop Ser. 2013;71:11–27. [DOI] [PubMed] [Google Scholar]
- 40.Kimball SR. The role of nutrition in stimulating muscle protein accretion at the molecular level. Biochem Soc Trans. 2007;35(Pt 5):1298–1301. [DOI] [PubMed] [Google Scholar]
- 41.Suryawan A, Davis TA. Regulation of protein synthesis by amino acids in muscle of neonates. Front Biosci (Landmark Ed). 2011;16:1445–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dickinson JM, Rasmussen BB. Essential amino acid sensing, signaling, and transport in the regulation of human muscle protein metabolism. Curr Opin Clin Nutr Metab Care. 2011;14(1):83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Columbus DA, Fiorotto ML, Davis TA. Leucine is a major regulator of muscle protein synthesis in neonates. Amino Acids. 2015;47(2):259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hellmuth C, Uhl O, Demmelmair H, et al. The impact of human breast milk components on the infant metabolism. PLoS One. 2018;13(6):e0197713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rzehak P, Hellmuth C, Uhl O, et al. Rapid growth and childhood obesity are strongly associated with lysoPC(14:0). Ann Nutr Metab. 2014;64(3-4):294–303. [DOI] [PubMed] [Google Scholar]
- 46.van Sadelhoff JHJ, Wiertsema SP, Garssen J, Hogenkamp A. Free Amino Acids in Human Milk: A Potential Role for Glutamine and Glutamate in the Protection Against Neonatal Allergies and Infections. Front Immunol. 2020;11:1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramirez I, DeSantiago S, Tovar AR, Torres N. Amino acid intake during lactation and amino acids of plasma and human milk. Adv Exp Med Biol. 2001;501:415–421. [DOI] [PubMed] [Google Scholar]
- 48.Matsumoto T, Nakamura E, Nakamura H, et al. Production of free glutamate in milk requires the leucine transporter LAT1. Am J Physiol Cell Physiol. 2013;305(6):C623–631. [DOI] [PubMed] [Google Scholar]
- 49.Li P, Knabe DA, Kim SW, Lynch CJ, Hutson SM, Wu G. Lactating porcine mammary tissue catabolizes branched-chain amino acids for glutamine and aspartate synthesis. J Nutr. 2009;139(8):1502–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


