Abstract
Context
Body fat gain associated with menopause has been attributed to estradiol (E2) withdrawal. Hypoestrogenism is unlikely to be the only contributing factor, however.
Objective
Given the links between sleep and metabolic health, we examined the effects of an experimental menopausal model of sleep fragmentation on energy metabolism.
Methods
Twenty premenopausal women (age 21-45 years) underwent a 5-night inpatient study during the mid-to-late follicular phase (estrogenized; n = 20) and the same protocol was repeated in a subset of the participants (n = 9) following leuprolide-induced E2 suppression (hypo-estrogenized). During each 5-night study, there were 2 nights of unfragmented sleep followed by 3 nights of fragmented sleep. Indirect calorimetry was used to assess fasted resting energy expenditure (REE) and substrate oxidation.
Results
Sleep fragmentation in the estrogenized state increased the respiratory exchange ratio (RER) and carbohydrate oxidation while decreasing fat oxidation (all P < 0.01). Similarly, in the hypo-estrogenized state without sleep fragmentation, RER and carbohydrate oxidation increased and fat oxidation decreased (all P < 0.01); addition of sleep fragmentation to the hypo-estrogenized state did not produce further effects beyond that observed for either intervention alone (P < 0.05). There were no effects of either sleep fragmentation or E2 state on REE.
Conclusion
Sleep fragmentation and hypoestrogenism each independently alter fasting substrate oxidation in a manner that may contribute to body fat gain. These findings are important for understanding mechanisms underlying propensity to body fat gain in women across the menopause transition.
Keywords: sleep fragmentation, estradiol, women, menopause, indirect calorimetry, substrate oxidation
Menopause is a reproductive transition that occurs in midlife women and is accompanied by decreases in production, and hence circulating levels, of the primary female sex hormone estradiol (E2), referred to herein as E2 withdrawal. Numerous health problems are associated with E2 withdrawal, including changes in body composition, obesity, and related pathologies such as diabetes and cardiovascular disease (1, 2). E2 withdrawal is hypothesized to contribute to gains in body fat, specifically abdominal visceral adiposity, which occurs in more than 50% of women during the menopausal transition (MT) (3).
E2 is a key regulator of energy metabolism (4, 5) and menopause has been associated with changes in components of energy metabolism including energy expenditure and substrate utilization (6-8). Although E2 withdrawal is likely a key contributor in mediating the metabolic changes associated with menopause, these changes are likely multifactorial in nature given that (i) not all women experience body fat gains (3) despite universal progression to E2 deficiency; and (ii) body fat accumulates even while E2 continues to be produced intermittently during the MT (9, 10).
One factor that may contribute to metabolic changes and body fat gain during menopause is chronological aging, as it is inherently associated with menopause. While observational studies report decreases in resting energy expenditure (REE) and fat oxidation, both risk factors for weight gain (11), in post- compared with premenopausal women (6, 7, 12), it is difficult in such studies to isolate the effects of the concurrently changing endocrine milieu, chronological aging, and other potential contributing factors such as changes in sleep.
Sleep disturbance is reported by ~40% of women during the MT (13). Sleep fragmentation (i.e., awakening during the night), without a reduction in sleep duration, is the most commonly reported sleep disturbance in women during the MT (14). The primary predictor of self-reported sleep disturbance and chronic insomnia in women during the MT is the presence of vasomotor symptoms (VMS) (14, 15), comprising hot flashes and night sweats. In women who experience moderate to severe VMS, 30% to 44% meet diagnostic criteria for chronic insomnia, compared with only 11% of those without VMS (15). Given the importance of sleep in maintaining metabolic health (16-18), VMS-related sleep fragmentation, along with E2 withdrawal and aging, may therefore play an important role in the increase in body fat gain during the MT.
In men, 2 nights of fragmented sleep has been shown to increase the respiratory exchange ratio (RER), a measure of substrate oxidation, without a change in REE (19). Whether an increase in RER, which is associated with weight gain (20), also occurs in response to sleep fragmentation in women has not been investigated. In the current study, therefore, we examined the impact of experimentally induced sleep fragmentation, while preserving total sleep time (TST) (Fig. 1), on substrate oxidation and REE as measured via indirect calorimetry in 20 healthy, young premenopausal women studied during the mid-to-late follicular phase of their menstrual cycle (referred to as “estrogenized state”). Based on changes in calorimetry outcomes following sleep fragmentation in men (20), we hypothesized that sleep fragmentation would increase RER, indicating a shift toward less fat and greater carbohydrate utilization, with no change in REE.
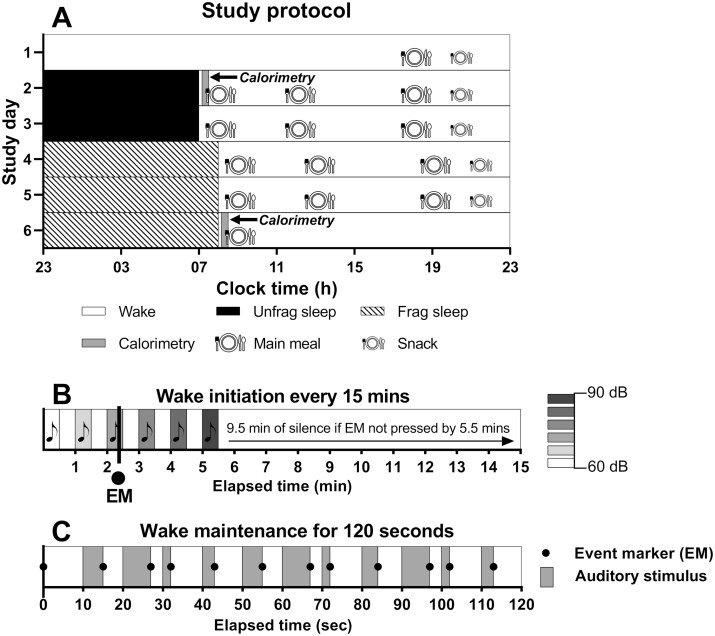
Figure 1.
Study protocol overview and sleep fragmentation procedure details. A) The study protocol consisted of a 6-day inpatient study with 2 nights of unfragmented (“unfrag”) sleep followed by 3 nights of fragmented (“frag”) sleep. Calorimetry (gray bar) was conducted in the fasted state on day 2 and day 6. B) Sleep was fragmented using an auditory stimulus delivered every 15 minutes that increased in intensity (60-90 dB) until wake was initiated, as indicated by the participant pressing an event marker (EM). C) Once initiated, wake was maintained for 120 seconds using an auditory stimulus and confirmed by an EM every 10 seconds.
To examine the effects of E2 withdrawal on these metabolic outcomes without the confounding effect of age, a subset of participants (n = 9) who completed the first study visit also completed a second study visit after they had received the gonadotropin-releasing hormone (GnRH) agonist leuprolide, which temporarily suppressed E2 production (referred to as “hypo-estrogenized state”) to mimic E2 withdrawal in the postmenopausal state. Based on studies of postmenopausal women (6, 7, 12), we hypothesized that E2 suppression in premenopausal women, and sleep fragmentation in combination with E2 suppression, would increase RER and also decrease REE, both of which are metabolic changes that promote body fat gain.
Methods
Participants
Twenty women (mean age ± SD = 30.4 ± 6.3 years; race n [%] = 11 [55%] Caucasian; mean body mass index [BMI] ± SD = 25.8 ± 4.0 kg/m2) were studied in a 6-day (5-night) inpatient protocol. Participants were healthy nonpregnant premenopausal women aged 18 to 45 years, as determined by medical, psychiatric and sleep disorder screening, including a physical examination and blood tests. Participants reported and were prospectively confirmed for 2+ menstrual cycles to have regular monthly menses. This study was approved by the Massachusetts General Brigham Institutional Review Board and all participants provided written informed consent prior to the study.
Protocol
Participants completed the inpatient protocols in the Intensive Physiological Monitoring Unit at the Brigham and Women’s Hospital Center for Clinical Investigation. Throughout the inpatient portion of the study, participants maintained a eucaloric diet, engaged in limited physical activity (e.g., walking in their ~23 square-meter suite), and had their sleep monitored each night via polysomnography (PSG). The 5-night inpatient protocol consisted of unfragmented sleep for the first 2 nights followed by experimentally fragmented sleep during the next 3 consecutive nights (Fig. 1A). Indirect calorimetry was measured in bed upon awakening in the fasted state following the first night of unfragmented sleep and repeated following the third night of sleep fragmentation (Fig. 1A). For all 5 sleep episodes, bedtime was scheduled at 23:00 h for all participants. The average bedtime reported in sleep diaries for the week leading up to the inpatient visit was 23:25 ± 00:29, with an average sleep duration on 7.43 ± 0.48 hours per night. During scheduled wake episodes, maximum ambient light intensity was ~190 lux (48 μW/cm2 measured in the horizontal plane at a height of 187 cm), and ambient lighting was turned off (0 lux) during all sleep episodes.
Sleep Fragmentation
Participants had 8 hours of time-in-bed (TIB; 23:00 to 07:00 h) on unfragmented nights and 9 hours TIB (23:00 to 08:00 h) on fragmentation nights, the latter including up to 68 minutes of induced wakefulness distributed across the night (details presented below). The overall goal of the fragmentation protocol was to induce a uniform sleep fragmentation for all participants that maximized wake such that each participant had ~1 hour of wakefulness distributed across the night. TIB was extended on the fragmentation nights to provide the same 8-hour sleep opportunity during all 5 sleep episodes and to minimize potential confounding effects of sleep restriction in this sleep fragmentation paradigm.
Sleep was fragmented using an auditory stimulus delivered every 15 minutes, starting 30 minutes after the beginning of the scheduled sleep episode, for a total of 34 15-minute fragmentation intervals. In the wake-initiation phase, a 30-second tone was played at 60 dB until an event marker, synchronized to the PSQ recording system, was pressed by the participant to indicate wakefulness (Fig. 1B). If the participant did not respond to the tone within the allotted 30 seconds, then the tone was replayed at a higher intensity the next minute following 30 seconds of silence. The intensity of the tone increased each time up to 90 dB If the participant did not respond to the 90 dB tone, then the tone was changed from a ringing tone to an alarm and then siren tone while maintaining the intensity at 90 dB.
Once the event marker was pressed to indicate wakefulness, the wake-maintenance phase began where the same tone at the same intensity that initiated wakefulness was played repeatedly every 10 seconds for 2 consecutive minutes. The participant pressed the event marker each time the tone played within the 120-second interval to indicate maintenance of wakefulness (Fig. 1C). Following the 120-second wake-maintenance phase, participants could resume sleep for the remainder of the 15-min interval. The auditory stimulus was delivered by an upright speaker (Trouper Compact Performance PA system, Alto Professional, Cumberland RI, USA) positioned next to the head of the bed. The intensity of the auditory stimulus was calibrated before each admission and monitored for every stimulus presentation using a digital sound meter (Model 407750, Extech Instruments, Nashua NH, USA). The timing and delivery of each stimulus was automated by customized software.
Estradiol Suppression
Nine participants (mean age ± SD = 27.9 ± 4.3 years; race n [%] = 6 [67%] Caucasian; mean BMI ± SD = 26.5 ± 3.9 kg/m2) chose to continue the study and completed an identical second inpatient visit while hypoestrogenic, induced by an intramuscular 3.75 mg dose of the gonadotrophin-releasing hormone (GnRH) agonist leuprolide acetate (Lupron Depot, AbbVie Inc., North Chicago, IL). This is a standard dose that rapidly achieves a hypo-estrogenized state (20). The GnRH agonist was administered during the mid-luteal phase of the menstrual cycle to healthy volunteers under Investigational New Drug monitoring by the Food and Drug Administration.
Study Diet
Food intake was monitored for 4 days prior to admission to the laboratory. On days −4, −3, and −1 before the admission, participants were provided a nutritionist-designed eucaloric diet at home. Caloric needs were calculated using the Mifflin-St Jeor equation with an activity factor of 1.4 (mean kCal ± SD = 2069 ± 205 kCal), and macronutrient distribution (± 5%) of 15% protein, 50% carbohydrate and 35% fat per day. Two days before admission (−2 days), participants self-selected their meals and logged them using the mobile phone application MealLogger (Wellness Foundry, Inc., Helsinki, Finland) (21). Caloric intake on the self-selected day was 2169 ± 581 kCal with macronutrient distribution (% ± SD) of 16 ± 3% protein, 47 ± 7% carbohydrate and 37 ± 7% fat.
Throughout the inpatient study, participants were provided a nutritionist-designed eucaloric diet consisting of 3 meals (breakfast, lunch, dinner) and snack each day. Caloric needs were calculated using the Mifflin-St Jeor equation with an activity factor of 1.4 (mean kCal ± SD = 2015 ± 188 kCal), and macronutrient distribution (±5%) of 15% protein, 50% carbohydrate and 35% fat per day. Participants received 1 mL/kCal of fluid per day, divided equally across their 4 meals, and were permitted one caffeinated drink (no more than 55 mg caffeine) per day with breakfast (no later than 13 hours before bedtime).
Indirect Calorimetry
Indirect calorimetry (VMAX Encore Metabolic Cart, CareFusion, CA) was used to estimate steady state resting energy expenditure (REE; kCal/day) and respiratory exchange ratio (RER). Each calorimetry session was performed prior to the participant getting out of bed in the fasted state for 20 minutes, starting 10 minutes after waking on day 2 (following 1 night of unfragmented sleep) and day 6 (following 3 nights of fragmented sleep) of the study (Fig. 1). Participants remained in a semi-supine posture in quiet wakefulness leading up to and for the duration of the 20-minute recording. Oxygen uptake (VO2) and carbon dioxide (VCO2) production were recorded on a minute-by-minute basis throughout the session; the first 5 minutes of data were discarded from analyses. Only data points with an associated fraction of exhaled carbon dioxide (FECO2) between 0.6% and 0.9% were included in the analysis. The remaining VO2 and VCO2 data were used to calculate the RER (VCO2/VO2), and carbohydrate and lipid oxidation (g/min) using Frayn’s formulae (22), assuming negligible protein oxidation.
Estradiol Measurement
E2 was assayed from a fasting blood sample collected on day 3 and day 6 of each inpatient study and assayed using liquid chromatography-mass spectroscopy (LC-MS, Brigham Research Assay Core, Brigham and Women’s Hospital, Boston, MA). Intra- and interassay coefficients of variation (% CV) were 5% and 12%, respectively.
Polysomnographic Sleep Assessment
PSG recordings were collected during all sleep episodes using a digital polysomnographic recorder (Vitaport-3 digital recorder, TEMEC Technologies B.V., Heerlen, The Netherlands). Recordings included electroencephalogram (EEG), electrooculogram (EOG), chin electromyogram (EMG) and a two-lead electrocardiogram (ECG). Electrodes were positioned according to the International 10-20 system, with linked mastoid references (Fz, C3, Cz, C4, Pz, Oz). Electrode impedances (<10 kΩ) were checked (OhmMate impedance meter, TEMEC Instruments B.V., Kerkade, The Netherlands) at the beginning and end of the sleep recordings. All EEG signals were high-pass filtered (EEG/EOG: 0.159-30 Hz; EMG/ECG: 1.061-70 Hz), digitized (sampling rate—EEG/EOG/EMG/ECG: 256 Hz) and stored on a Flash RAM card. PSG recordings were visually scored according to the American Academy of Sleep Medicine Scoring Manual (Version 2.6) (23), by a registered polysomnographic technologist.
Measurement of Vasomotor Symptoms
VMS frequency was reported in a diary for 1 week prior to and 1 week following the inpatient study. Participants completed a standard diary before bed and upon awakening to record daytime and nighttime VMS, respectively. The average number of VMS per 24 hours, including both daytime and nighttime VMS, was calculated for each participant.
Data Analysis
Data are expressed as mean ± SEM unless otherwise specified. Calorimetry data for 1 participant in one of the 4 conditions (hypo-estrogenized state following sleep fragmentation) was excluded as it was > 3 times the interquartile range below the 25th percentile for RER and carbohydrate oxidation, and above the 75th percentile for fat oxidation (24). All other data for this participant remained in analyses.
To assess the effectiveness of the sleep fragmentation protocol in achieving the fragmentation goal of ~1 hour of wakefulness, sleep stages (N1, N2, N3, and REM), total sleep time (TST), sleep onset latency (SOL) and wake after sleep onset (WASO) in minutes were compared between sleep on night 1 (unfragmented) and sleep on night 5 (fragmented) in the estrogenized state using paired-samples t tests or Wilcoxon signed-rank test as appropriate. To assess the effectiveness of the GnRH agonist in suppressing E2, the average E2 levels measured on study days 2 and 6 was compared between the estrogenized and hypo-estrogenized state using a paired-samples t test.
Linear mixed models were used to analyze the influence of sleep fragmentation on calorimetry outcomes in the estrogenized state (n = 20). Sleep condition (unfragmented vs fragmented) was modeled as a fixed effect with participant as a random effect. To jointly assess the effect of estradiol suppression and sleep fragmentation (n = 9), sleep condition, estradiol state (estrogenized vs hypo-estrogenized), and their interaction were modeled as fixed effects and participant was modeled as a random effect. In sensitivity analyses, BMI (kg/m2) was included as a covariate in the analysis of the effect of sleep fragmentation (n = 21), and BMI and VMS per 24 hours were included as covariates in the analysis of data in women who received the GnRH agonist (n = 9), as both of these variables have been associated with changes in calorimetry outcomes (25, 26). All analyses and graphical representations were conducted in SAS 9.4 (SAS Inc., Cary, NC, USA) and GraphPad Prism 9.0.0. (GraphPad Software La Jolla, CA, USA).
Results
Effect of Sleep Fragmentation on Calorimetry Outcomes
As designed, the sleep fragmentation protocol increased wake after sleep onset (WASO) by 56.1 ± 8.7 minutes (P < 0.001), without reducing TST (unfragmented: 421.9 ± 9.4 minutes; fragmented: 420.2 ± 7.7; Fig. 2A). The sleep fragmentation protocol also altered sleep architecture compared to nights with unfragmented sleep, including increased non–rapid eye movement (NREM) stage N1 sleep (+20.2 ± 5.4 minutes; P < 0.01) and decreased stage N3 sleep (26.5 ± 4.6 minutes; P < 0.001), but no change in stage N2 or rapid eye movement (REM) sleep (Fig. 2A).
Figure 2.
Effects of study interventions: sleep fragmentation and GnRH agonist–induced E2 suppression. A) Polysomnographically recorded sleep stages (non–rapid eye movement stages N1, N2, and N3, and rapid eye movement [REM]), sleep onset latency (SOL), wake after sleep onset (WASO), total sleep time (TST), and time-in-bed (TIB) during unfragmented (“unfrag”) and fragmented (“frag”) sleep. B) Box plot showing median, interquartile range, and extreme values for serum E2 concentration in mid/late follicular phase of the menstrual cycle prior to and ~4 weeks post-administration of a GnRH agonist to rapidly suppress E2 levels to the postmenopausal range. Significance is denoted by ** P < 0.01; *** P < 0.001; n.s., nonsignificant (P > 0.05).
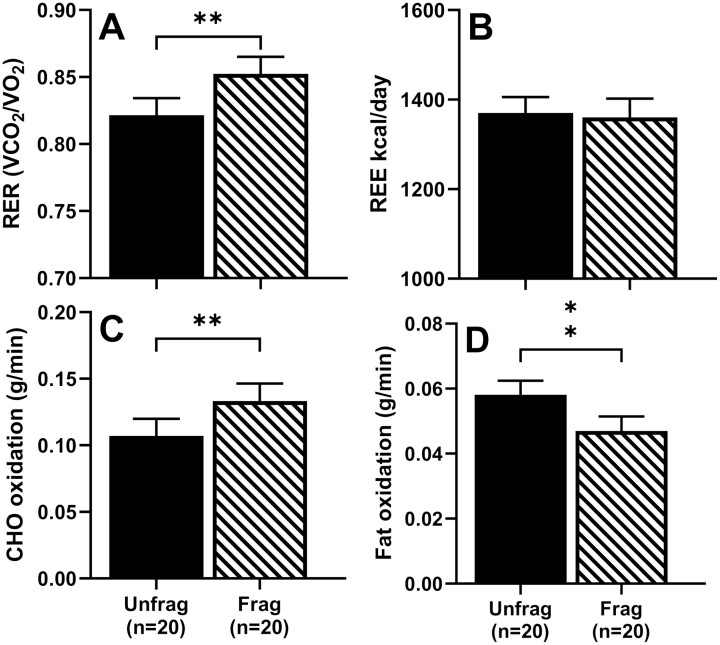
In the estrogenized states, there was a significant effect of sleep fragmentation on RER (F1,19 = 8.99, P < 0.01), carbohydrate oxidation (F1,19 = 9.35, P < 0.01) and fat oxidation (F1,19 = 8.24, P < 0.01), but not on REE (F1,19 = 0.17, P = 0.68). Compared with unfragmented sleep, average RER and carbohydrate oxidation were higher by 3.8% and 24.4%, respectively, while fat oxidation was lower by 19.1% following 3 nights of sleep fragmentation (Fig. 3). Adjustment for BMI did not alter the significant effect of sleep fragmentation on RER or substrate oxidation (all P < 0.01).
Figure 3.
Effect of sleep fragmentation on calorimetry outcomes. Mean ± SEM of A) respiratory exchange ratio (RER), B) resting energy expenditure (REE), C) carbohydrate (CHO) oxidation rate, and D) fat oxidation rate following unfragmented (“unfrag” in solid fill) and fragmented (“frag” in hatched fill) sleep. Significance is denoted by ** P < 0.01.
Effect of E2 Suppression With or Without Sleep Fragmentation on Calorimetry Outcomes
The effect of sleep fragmentation on sleep continuity and architecture was similar in both the estrogenized and hypo-estrogenized state for the subset of participants (n = 9) who completed both study conditions (see Supplemental Figure S1 (27)). Following administration of leuprolide, serum E2 levels decreased by 78.5 ± 6.5% (P < 0.01) from mid-to-late follicular phase levels, to levels comparable to those in postmenopausal women (28) (Fig. 2B). In the women who received leuprolide, 78% (7/9) developed VMS, with an average of 1.3 ± 0.6 hot flashes reported per 24 hours.
In analyses of the subsample (n = 9) who completed study visits in both the estrogenized and hypo-estrogenized states, there was a significant main effect of E2 state on fat oxidation (F1,21.9 = 4.9, P < 0.05) such that fat oxidation was lower in the hypo-estrogenized than in the estrogenized state. Additionally, there was a trend toward increased RER (P = 0.06) and carbohydrate oxidation (P = 0.06) in the hypo-estrogenized compared to estrogenized state. There was no effect of E2 suppression on REE. In this subsample, there was no main effect of sleep fragmentation on any metabolic outcome; however, there was a significant interaction between sleep condition and E2 state for RER (F1,22.1 = 4.47, P < 0.05), carbohydrate (F1,22.7 = 4.3, P < 0.05) and fat oxidation (F1,22.2 = 6.10, P < 0.05), but not REE (trend: F1,23.1 = 3.07, P = 0.09), indicating that the effect of sleep fragmentation differed by E2 state (Fig. 3).
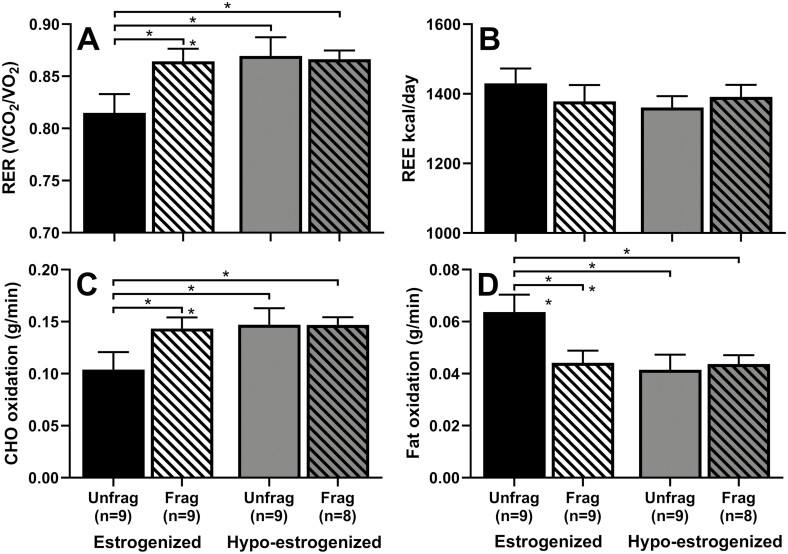
To further evaluate the interaction between sleep condition (i.e., unfragmented and fragmented) and E2 state, post hoc comparisons were conducted for the analysis of the n = 9 subsample. These showed, in response to sleep fragmentation in the estrogenized state (Fig. 4), a 6.1% and 38.0% increase in average RER (t21.9 = −2.67, P < 0.05) and carbohydrate oxidation (t22.6 = −2.67, P < 0.05), respectively, and a 30.7% decrease in fat oxidation (t21.7 = 2.94, P < 0.01), findings consistent with the result in the larger sample reported above.
Figure 4.
Effect of E2 suppression with or without sleep fragmentation on calorimetry outcomes. Mean ± SEM of A) respiratory exchange ratio (RER), B) resting energy expenditure (REE), C) carbohydrate (CHO) oxidation rate, and D) fat oxidation rate following unfragmented (“unfrag”, in solid fill) and fragmented (“frag”, in hatched fill) sleep in an estrogenized (black) and hypo-estrogenized (gray) state. Significance is denoted by * P < 0.05; ** P < 0.01.
E2 suppression showed independent effects in the absence of sleep fragmentation. There was an effect of E2 state on average RER (t21.9 = -2.96, P < 0.01), and on carbohydrate (t22.6 = −2.91, P < 0.01) and fat oxidation (t21.7 = 3.35, P < 0.01) (Fig. 4), such that hypoestrogenism led to a 6.7% and 41.3% increase in RER and carbohydrate oxidation, respectively, and a 34.9% decrease in fat oxidation compared to the estrogenized state. There was also a trend toward a decrease in REE in response to E2 suppression (−4.8%; t23.1 = 2.02, P = 0.055). In the presence of sleep fragmentation, there was no effect of E2 state on RER, substrate oxidation, or REE.
Compared with unfragmented sleep in the estrogenized state, the combination of E2 suppression and sleep fragmentation increased RER (t22.3 = −2.48, P < 0.05) and carbohydrate oxidation (t22.8 = −2.49, P < 0.05) by 5.9% and 36.9%, and decreased fat oxidation (t22.1 = −2.68, P < 0.05) by 29.0%; however, these effects were not greater than either sleep fragmentation or E2 suppression alone (Fig. 4). Adjustment for BMI, and for whether the participant experienced VMS, did not alter the significant effect of sleep fragmentation, E2 suppression, or their combined effect, on REE, RER, or substrate oxidation.
Discussion
This study examined the effects of sleep fragmentation and GnRH agonist–induced E2 suppression on energy expenditure and substrate oxidation measured by indirect calorimetry. We found that sleep fragmentation, E2 suppression, and their combined effect, led to changes in substrate oxidation that may promote body fat gain. There was no effect on REE. Given the association between substrate oxidation and subsequent increases in body weight and fat mass, independent of REE (29-31), our results suggest that both menopause-related sleep fragmentation and hypoestrogenism may each contribute to body fat gain and associated health complications such as metabolic syndrome (32).
Experimental sleep fragmentation, recapitulating menopause-related sleep fragmentation, was found to increase RER and carbohydrate oxidation rate while decreasing fat oxidation rate. The effects of sleep fragmentation while maintaining sleep duration have not been previously examined in women; our results are consistent with the effect of sleep fragmentation observed in men (19), in whom TST was also reduced (6.5 hours). When subjected to 2 nights of sleep fragmentation induced in the laboratory, men exhibited elevated RER, increased carbohydrate oxidation, decreased fat oxidation but no change in REE. These changes in energy metabolism following sleep fragmentation in both men and women differ from those reported following sleep restriction without fragmentation (i.e., 4-6 hours of consolidated sleep per 24 hours) or total sleep deprivation (i.e., no sleep for > 24 hours). For example, when sleep is either restricted to 4 to 5 hours per night for 3 to 4 nights or totally deprived for 1 night, 24-hour energy expenditure increases with no change in RER (33-35). Differences in the metabolic effects of sleep fragmentation compared to sleep restriction and deprivation may be a result of the effects of these different sleep manipulations on the duration of time spent awake within the 24-hour day, in addition to the changes that they induce in sleep architecture.
Since the amount of energy expended during wakefulness is greater than that during sleep (33), an increase in energy expenditure may occur when there is an increase in the proportion of the 24-hour day spent awake, a consequence of experimentally restricted or entirely deprived sleep. Conversely, when sleep is fragmented, but the duration of sleep and therefore the proportion of wakefulness across 24 hours is conserved, altered energy expenditure attributed to changes in sleep duration would not be expected. Whether these effects would be maintained over repeated exposure to sleep restriction or fragmentation remains unclear, however, as restricting sleep over longer durations (~1-3 weeks) has been shown to reduce fasting REE (36, 37) and increase RER (38), different to the effects reported following short-duration exposures (39, 40).
Changes in sleep architecture, including the reduction in stage N3/slow wave sleep (SWS) resulting from sleep fragmentation, may play a role in the changes observed in substrate oxidation reported in men (19) and shown for the first time here in women. Secreted at higher levels during SWS (41), growth hormone (GH) stimulates metabolism of fat (42), such that suppression of SWS may reduce GH secretion, thereby decreasing fat oxidation while increasing carbohydrate oxidation and therefore RER. Consistent with this hypothesis, RER was increased in our study of women and also in men following sleep fragmentation that reduced SWS (19), whereas fasting RER was unchanged following short-term sleep restriction in women (39), during which SWS was likely conserved even with sleep opportunities as little as 3 or 4 hours per night (43, 44). Furthermore, although we did not measure GH in the current study, we did observe a significant positive relationship between fat oxidation and the percentage of stage N3 sleep (R2 = 0.25; Supplemental Figure S2 (27)), such that less SWS was associated with lower fat oxidation. Future studies will be required to examine how sleep fragmentation affects GH to systematically investigate a possible mechanistic role of changes in SWS and GH mediating the effect of sleep fragmentation on substate oxidation (45).
Similar to the effects of sleep fragmentation, leuprolide-induced E2 suppression increased fasting RER and carbohydrate oxidation and decreased fat oxidation. Consistent with our findings, a longitudinal study of midlife women showed that 24-hour fat oxidation decreased significantly at 4-year follow-up only in those women who transitioned from pre- to postmenopausal status (8). Our finding is also consistent with changes reported in fat oxidation during exercise in pre- and postmenopausal women (6), where fat oxidation is lower in post- compared with premenopausal women. While our findings for fat oxidation are consistent with other studies, the changes that we observed in carbohydrate oxidation rate and RER have not been observed in other studies during exercise (6), in the fasted state (7), or across 24 hours (8), which report no change in RER.
The current experimental study of induced hypoestrogenism in young premenopausal women supports a role for sex steroid–driven changes in fat oxidation independent of age. The effect of sex hormones on substrate oxidation that we observed is consistent with changes in fat and carbohydrate oxidation during exercise reported in different menstrual cycle phases in premenopausal women (46, 47). Precisely which sex hormones are contributing to the effect cannot be discerned from the current study, however, given that leuprolide also suppresses progesterone in addition to E2. Results from studies in ovariectomized rodents, however, support a role for E2 in regulating substrate oxidation. Compared to placebo add-back and to combined E2 and progestin add-back, estrogen only add-back increased fat oxidation and decreased carbohydrate oxidation rates during exercise (48). Similarly, exogenous E2 administration in men (49) and in postmenopausal women also increased fat oxidation, although in women the method of E2 administration modulates the effect (50). Specifically, transdermal E2 administration increases fat oxidation (51), while oral administration decreases fat oxidation (52, 53), a paradoxical effect potentially due to hepatic metabolism of oral E2 (52). The current study of young premenopausal women suggests that reduction of sex hormone production influences substrate oxidation, and together with previous findings (46-52), suggests that E2 may be the primary sex steroid driving this adverse effect.
Whereas we only observed a trend toward a decrease in REE following E2 suppression, several studies have reported a significant reduction in energy expenditure during menopause (6-8, 12) and in response to a GnRH antagonist in premenopausal women (54, 55). Since we observed a 65 kcal/day change in REE, similar in magnitude to that reported in other premenopausal women in whom an E2 deficient state was induced (71 kcal/day (54); 54 kcal/day (55)), the lack of a statistically significant change in REE may be due to the small sample size in the current study. Despite this, however, the magnitude of the change in REE that we observed in response to E2 suppression was approximately half of the mean difference reported between pre- and postmenopausal women in previous studies (142 kcal/day (7, 8, 12)), suggesting that chronological aging or other changes associated with menopause may also be contributing to the decrease in energy expenditure associated with menopause. Although a recent study (56) reported that age-related changes in energy expenditure begin almost a decade after the average age of onset of menopause in the US (57), our hypothesis that chronological aging contributes to changes in REE associated with menopause is supported by the results of a longitudinal study (8) in which there was a decline in REE at 4-year follow-up not only in postmenopausal women but also in women who remained premenopausal. Therefore, the relationship between reduced REE and menopause may in part be driven by chronological aging across this reproductive transition.
While sleep fragmentation and E2 suppression each had an independent effect on substrate oxidation, the combined effect of fragmentation and hypoestrogenism was no greater than either intervention alone. The lack of an additive effect may be due to RER and substrate oxidation rates reaching a floor/ceiling for the physiological conditions under which they were measured: limited physical activity and a diet with fixed macronutrient intake prior to measurement in the fasted resting state. Given the small sample size, however, the lack of an additive effect warrants confirmation in a larger cohort.
This study has several limitations. First, the sample size for the analysis of the effects of E2 suppression and also the combined effect of sleep fragmentation and E2 suppression was small, as not all participants continued with the second part of the study. Despite this, there was a high degree of consistency in the direction of change in RER, fat, and carbohydrate oxidation between participants, suggesting robust effects (Supplementary Figure S3 [28]). Second, while our results suggest that changes in substrate oxidation may lead to body fat gain over time, we only conducted measurements in the fasted state and additional data are required to determine whether these changes persist across 24 hours, including under postprandial and exercise conditions and after chronic exposure.
Our findings suggest that experimental sleep fragmentation and hormone changes mimicking those associated with menopause can each alter substrate oxidation in a way that may promote body fat gain. Given the high prevalence of vasomotor symptom-related sleep fragmentation and the universal withdrawal of E2 in menopause, these findings have implications for understanding the potential mechanisms underlying body fat gain in this population.
Acknowledgments
We thank the technical, dietary, and laboratory staff, nurses, and physicians at the BWH Center for Clinical Investigation, Joseph M. Ronda for designing and implementing the sleep fragmentation software, and the study participants.
Glossary
Abbreviations
- BMI
body mass index
- E2
estradiol
- ECG
electrocardiography
- EEG
electroencephalography
- EMG
electromyography
- EOG
electrooculography
- GH
growth hormone
- GnRH
gonadotropin-releasing hormone
- MT
menopausal transition
- PSG
polysomnography
- REE
resting energy expenditure
- RER
respiratory exchange ratio
- SWS
slow wave sleep
- TIB
time in bed
- TST
total sleep time
- VMS
vasomotor symptoms
Contributor Information
Leilah K Grant, Division of Sleep and Circadian Disorders, Departments of Medicine and Neurology, Brigham and Women’s Hospital, Boston, MA 02115, USA; Division of Sleep Medicine, Harvard Medical School, Boston, MA 02115, USA; Mary Horrigan Connors Center for Women’s Health and Gender Biology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Jamie E Coborn, Mary Horrigan Connors Center for Women’s Health and Gender Biology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA; Women’s Hormones and Aging Research Program, Department of Psychiatry, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Aviva Cohn, Women’s Hormones and Aging Research Program, Department of Psychiatry, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA; Division of Endocrinology, Diabetes and Hypertension, Department of Medicine, Brigham and Women’s Hospital, Boston, MA 02115, USA.
Margo D Nathan, Women’s Hormones and Aging Research Program, Department of Psychiatry, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Frank A J L Scheer, Division of Sleep and Circadian Disorders, Departments of Medicine and Neurology, Brigham and Women’s Hospital, Boston, MA 02115, USA; Division of Sleep Medicine, Harvard Medical School, Boston, MA 02115, USA.
Elizabeth B Klerman, Division of Sleep and Circadian Disorders, Departments of Medicine and Neurology, Brigham and Women’s Hospital, Boston, MA 02115, USA; Division of Sleep Medicine, Harvard Medical School, Boston, MA 02115, USA; Department of Neurology, Massachusetts General Hospital, Boston, MA 02114, USA.
Ursula B Kaiser, Division of Endocrinology, Diabetes and Hypertension, Department of Medicine, Brigham and Women’s Hospital, Boston, MA 02115, USA.
Jessica Harder, Women’s Hormones and Aging Research Program, Department of Psychiatry, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Mathena Abramson, Women’s Hormones and Aging Research Program, Department of Psychiatry, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Elkhansaa Elguenaoui, Women’s Hormones and Aging Research Program, Department of Psychiatry, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Julia A Russell, Women’s Hormones and Aging Research Program, Department of Psychiatry, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Aleta Wiley, Mary Horrigan Connors Center for Women’s Health and Gender Biology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA; Women’s Hormones and Aging Research Program, Department of Psychiatry, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Shadab A Rahman, Division of Sleep and Circadian Disorders, Departments of Medicine and Neurology, Brigham and Women’s Hospital, Boston, MA 02115, USA; Division of Sleep Medicine, Harvard Medical School, Boston, MA 02115, USA; Mary Horrigan Connors Center for Women’s Health and Gender Biology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Hadine Joffe, Division of Sleep Medicine, Harvard Medical School, Boston, MA 02115, USA; Mary Horrigan Connors Center for Women’s Health and Gender Biology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA; Women’s Hormones and Aging Research Program, Department of Psychiatry, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Financial Support
The project described was supported by the National Institute of Aging R01AG053838 (PI: HJ), Clinical Translational Science Award UL1RR025758 to Harvard University and Brigham and Women’s Hospital from the National Center for Research Resources. Co-authors were also supported in part by R01HL140574 (FAJLS), K24HL105664 (EBK), R37HD0199138 (UBK), R01HL159207 (SAR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Author Contributions
All authors have contributed to and approved this manuscript. F.A.J.L.S., E.B.K., U.B.K., and H.J. contributed to the initial concept and design of the study. A.C., M.A., E.E., J.A.R., A.W., and M.D.N. contributed to recruitment and screening on study participants. L.K.G., J.E.C., M.A., E.E., J.A.R., A.W., S.A.R., and H.J. contributed to, or oversaw, the collection of the data. L.K.G., J.E.C., S.A.R., and H.J. contributed to the analysis of the data. All authors contributed to the interpretation of the data and drafting of the manuscript.
Disclosures
LKG, AC, MDN, FAJLS, UBK, JH, MA, EE, JAR and AW have nothing to declare. In the interest of full disclosure, the following authors reports declarations of interest for the previous 36 months: JEC is now an employee of Novo Nordisk Inc.. EBK reports travel support from Gordon Research Conference, Sleep Research Society, Santa Fe institute, DGSM (German Sleep Society); consultancy for American Academy of Sleep Medicine, Circadian Therapeutics, National Sleep Foundation, Puerto Rico Science Technology Trust, Sanofi-Genzyme, and Yale University Press; partner owns Chronsulting. SAR holds patents for (1) Prevention of Circadian Rhythm Disruption by Using Optical Filters, and (2) Improving sleep performance in subject exposed to light at night; SAR owns equity in Melcort Inc.; has provided paid consulting services to Sultan & Knight Limited, Bambu Vault LLC, Lucidity Lighting Inc.; and has received honoraria as an invited speaker and travel funds from Starry Skies Lake Superior, University of Minnesota Medical School, PennWell Corp., and Seoul Semiconductor Co. Ltd. SAR has received grant/research support from Seoul Semiconductor Co. Ltd., Biological Innovation and Optimization Systems, LLC, Merck & Co., Inc., Pfizer Inc., Vanda Pharmaceuticals Inc., Lighting Science Group, NIH, and NASA. These interests were reviewed and managed by Brigham and Women’s Hospital and Mass General Brigham in accordance with their conflict of interest policies. HJ reports receiving active grant support from NIH, Merck, and Pfizer, and recently concluded grant support from Que-Oncology and NeRRe/KaNDy. She also reports consultant and advisory fees from Eisai, Jazz, and Bayer. Joffe reports her spouse is an employee of Arsenal Biosciences and has an equity stake in Merck Research Labs.
Data Availability
The datasets and code supporting the current study have not been deposited in a public repository to preserve patient confidentiality but are available from the corresponding author on reasonable request. Execution of a Materials Transfer Agreement is required if the data will be used in research supported by a for-profit company, per Mass General Brigham Institutional Review Board policy.
References
- 1. Lovejoy JC. The menopause and obesity. Prim Care. 2003;30(2):317-325. [DOI] [PubMed] [Google Scholar]
- 2. Stefanska A, Bergmann K, Sypniewska G. Metabolic syndrome and menopause: pathophysiology, clinical and diagnostic significance. Adv Clin Chem. 2015;72:1-75. [DOI] [PubMed] [Google Scholar]
- 3. Thurston RC, Sowers MR, Sternfeld B, et al. Gains in body fat and vasomotor symptom reporting over the menopausal transition: the study of women’s health across the nation. Am J Epidemiol. 2009;170(6):766-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34(3):309-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu Y, López M. Central regulation of energy metabolism by estrogens. Mol Metab. 2018;15:104-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abildgaard J, Pedersen AT, Green CJ, et al. Menopause is associated with decreased whole body fat oxidation during exercise. Am J Physiol Endocrinol Metab. 2013;304(11):E1227-E1236. [DOI] [PubMed] [Google Scholar]
- 7. Hodson L, Harnden K, Banerjee R, et al. Lower resting and total energy expenditure in postmenopausal compared with premenopausal women matched for abdominal obesity. J Nutr Sci. 2014;3:e3. doi:10.1017/jns.2013.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond). 2008;32(6):949-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davis SR, Castelo-Branco C, Chedraui P, et al. ; Writing Group of the International Menopause Society for World Menopause Day 2012. Understanding weight gain at menopause. Climacteric. 2012;15(5):419-429. [DOI] [PubMed] [Google Scholar]
- 10. Gibson CJ, Thurston RC, El Khoudary SR, Sutton-Tyrrell K, Matthews KA. Body mass index following natural menopause and hysterectomy with and without bilateral oophorectomy. Int J Obes (Lond). 2013;37(6):809-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ravussin E, Lillioja S, Knowler WC, et al. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med. 1988;318(8):467-472. [DOI] [PubMed] [Google Scholar]
- 12. Schattinger CM, Leonard JT, Pappas CL, Ormsbee MJ, Panton LB. The effects of pre-sleep consumption of casein protein on next-morning measures of RMR and appetite compared between sedentary pre- and postmenopausal women. Br J Nutr. 2021;125(2):121-128. [DOI] [PubMed] [Google Scholar]
- 13. Nelson HD. Menopause. Lancet. 2008;371(9614):760-770. [DOI] [PubMed] [Google Scholar]
- 14. Kravitz HM, Joffe H. Sleep during the perimenopause: a SWAN story. Obstet Gynecol Clin North Am. 2011;38(3):567-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohayon MM. Severe hot flashes are associated with chronic insomnia. Arch Intern Med. 2006;166(12):1262-1268. [DOI] [PubMed] [Google Scholar]
- 16. Nedeltcheva AV, Scheer FA. Metabolic effects of sleep disruption, links to obesity and diabetes. Curr Opin Endocrinol Diabetes Obes. 2014;21(4):293-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reutrakul S, Van Cauter E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism. 2018;84:56-66. [DOI] [PubMed] [Google Scholar]
- 18. Schmid SM, Hallschmid M, Schultes B. The metabolic burden of sleep loss. Lancet Diabetes Endocrinol. 2015;3(1):52-62. [DOI] [PubMed] [Google Scholar]
- 19. Hursel R, Rutters F, Gonnissen HK, Martens EA, Westerterp-Plantenga MS. Effects of sleep fragmentation in healthy men on energy expenditure, substrate oxidation, physical activity, and exhaustion measured over 48 h in a respiratory chamber. Am J Clin Nutr. 2011;94(3):804-808. [DOI] [PubMed] [Google Scholar]
- 20. Joffe H, White DP, Crawford SL, et al. Adverse effects of induced hot flashes on objectively recorded and subjectively reported sleep: Results of a gonadotropin-releasing hormone agonist experimental protocol. Menopause. 2013;20(9):905-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McHill AW, Phillips AJ, Czeisler CA, et al. Later circadian timing of food intake is associated with increased body fat. Am J Clin Nutr. 2017;106(5):1213-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol 1983;55(2):628-634. [DOI] [PubMed] [Google Scholar]
- 23. Berry RB, Quan SF, Abreu AR, et al. ; for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.6. Darien, IL: American Academy of Sleep Medicine; 2020. [Google Scholar]
- 24. Bakker M, Wicherts JM. Outlier removal, sum scores, and the inflation of the Type I error rate in independent samples t tests: the power of alternatives and recommendations. Psychol Methods. 2014;19(3):409-427. [DOI] [PubMed] [Google Scholar]
- 25. Carneiro IP, Elliott SA, Siervo M, et al. Is Obesity Associated with Altered Energy Expenditure? Adv Nutr. 2016;7(3):476-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carpenter JS, Gilchrist JM, Chen K, Gautam S, Freedman RR. Hot flashes, core body temperature, and metabolic parameters in breast cancer survivors. Menopause 2004;11(4):375-381. [DOI] [PubMed] [Google Scholar]
- 27. Grant LK, Coborn JE, Cohn A, Nathan MD, Scheer FAJL, Klerman EB, Kaiser UB, Harder J, Abramson M, Elguenaoui E, Russell JA, Wiley A, Rahman SA, Joffe H. Data from: sleep fragmentation and estradiol suppression decrease fat oxidation in pre-menopausal women. Figshare. Deposited May 25, 2022. https://figshare.com/s/34a7c8521313f50cb060 [DOI] [PMC free article] [PubMed]
- 28. Tepper PG, Randolph JF Jr, McConnell DS, et al. Trajectory clustering of estradiol and follicle-stimulating hormone during the menopausal transition among women in the Study of Women’s Health across the Nation (SWAN). J Clin Endocrinol Metab. 2012;97(8):2872-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ellis AC, Hyatt TC, Hunter GR, Gower BA. Respiratory quotient predicts fat mass gain in premenopausal women. Obesity. 2010;18(12):2255-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shook RP, Hand GA, Paluch AE, et al. High respiratory quotient is associated with increases in body weight and fat mass in young adults. Eur J Clin Nutr. 2016;70(10):1197-1202. [DOI] [PubMed] [Google Scholar]
- 31. Zurlo F, Lillioja S, Esposito-Del Puente A, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol. 1990;259(5 Pt 1):E650-E657. [DOI] [PubMed] [Google Scholar]
- 32. Pujia A, Mazza E, Ferro Y, et al. Lipid oxidation assessed by indirect calorimetry predicts metabolic syndrome and type 2 diabetes. Front Endocrinol. 2019;9:806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol. 2011;589(Pt 1):235-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Markwald RR, Melanson EL, Smith MR, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci USA. 2013;110(14):5695-5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shechter A, Rising R, Albu JB, St-Onge MP. Experimental sleep curtailment causes wake-dependent increases in 24-h energy expenditure as measured by whole-room indirect calorimetry. Am J Clin Nutr. 2013;98(6):1433-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Spaeth AM, Dinges DF, Goel N. Resting metabolic rate varies by race and by sleep duration. Obesity (Silver Spring). 2015;23(12):2349-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buxton OM, Cain SW, O’Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4(129):129ra-12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153(7):435-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shechter A, Rising R, Wolfe S, Albu JB, St-Onge MP. Postprandial thermogenesis and substrate oxidation are unaffected by sleep restriction. Int J Obes (Lond). 2014;38(9):1153-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. St-Onge MP, Roberts AL, Chen J, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94(2):410-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sassin JF, Parker DC, Mace JW, Gotlin RW, Johnson LC, Rossman LG. Human growth hormone release: relation to slow-wave sleep and sleep-walking cycles. Science 1969;165(3892):513-515. [DOI] [PubMed] [Google Scholar]
- 42. Møller N, Jørgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30(2):152-177. [DOI] [PubMed] [Google Scholar]
- 43. St Hilaire MA, Rüger M, Fratelli F, Hull JT, Phillips AJ, Lockley SW. Modeling neurocognitive decline and recovery during repeated cycles of extended sleep and chronic sleep deficiency. Sleep. 2017;40(1):zsw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117-126. [DOI] [PubMed] [Google Scholar]
- 45. Van Cauter E, Latta F, Nedeltcheva A, et al. Reciprocal interactions between the GH axis and sleep. Growth Horm IGF Res. 2004;14(Suppl A):S10-S17. [DOI] [PubMed] [Google Scholar]
- 46. Hackney AC, McCracken-Compton MA, Ainsworth B. Substrate responses to submaximal exercise in the midfollicular and midluteal phases of the menstrual cycle. Int J Sport Nutr. 1994;4(3):299-308. [DOI] [PubMed] [Google Scholar]
- 47. Zderic TW, Coggan AR, Ruby BC. Glucose kinetics and substrate oxidation during exercise in the follicular and luteal phases. J Appl Physiol. 2001;90(2):447-453. [DOI] [PubMed] [Google Scholar]
- 48. Hatta H, Atomi Y, Shinohara S, Yamamoto Y, Yamada S. The effects of ovarian hormones on glucose and fatty acid oxidation during exercise in female ovariectomized rats. Horm Metab Res. 1988;20(10):609-611. [DOI] [PubMed] [Google Scholar]
- 49. Hamadeh MJ, Devries MC, Tarnopolsky MA. Estrogen supplementation reduces whole body leucine and carbohydrate oxidation and increases lipid oxidation in men during endurance exercise. J Clin Endocrinol Metab. 2005;90(6):3592-3599. [DOI] [PubMed] [Google Scholar]
- 50. O’Sullivan AJ, Crampton LJ, Freund J, Ho KK. The route of estrogen replacement therapy confers divergent effects on substrate oxidation and body composition in postmenopausal women. J Clin Invest. 1998;102(5):1035-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. dos Reis CM, de Melo NR, Meirelles ES, Vezozzo DP, Halpern A. Body composition, visceral fat distribution and fat oxidation in postmenopausal women using oral or transdermal oestrogen. Maturitas 2003;46(1):59-68. [DOI] [PubMed] [Google Scholar]
- 52. Lwin R, Darnell B, Oster R, et al. Effect of oral estrogen on substrate utilization in postmenopausal women. Fertil Steril. 2008;90(4):1275-1278. [DOI] [PubMed] [Google Scholar]
- 53. O’Sullivan AJ, Hoffman DM, Ho KK. Estrogen, lipid oxidation, and body fat. N Engl J Med. 1995;333(10):669-670. [DOI] [PubMed] [Google Scholar]
- 54. Day DS, Gozansky WS, Van Pelt RE, Schwartz RS, Kohrt WM. Sex hormone suppression reduces resting energy expenditure and {beta}-adrenergic support of resting energy expenditure. J Clin Endocrinol Metab. 2005;90(6):3312-3317. [DOI] [PubMed] [Google Scholar]
- 55. Melanson EL, Gavin KM, Shea KL, et al. Regulation of energy expenditure by estradiol in premenopausal women. J Appl Physiol (1985). 2015;119(9):975-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pontzer H, Yamada Y, Sagayama H, Ainslie PN, Andersen LF, Speakman JR. IAEA DLW Database Consortium. Daily energy expenditure through the human life course. Science. 2021;373(6556):808-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. The North American Menopause Society. Menopause 101: A Primer for the Perimenopausal. The North American Menopause Society, NAMS. Accessed May 25, 2022. Retrieved from https://www.menopause.org/for-women/menopauseflashes/menopause-symptoms-and-treatments/menopause-101-a-primer-for-the-perimenopausal. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets and code supporting the current study have not been deposited in a public repository to preserve patient confidentiality but are available from the corresponding author on reasonable request. Execution of a Materials Transfer Agreement is required if the data will be used in research supported by a for-profit company, per Mass General Brigham Institutional Review Board policy.