Abstract
Fear-associated memories and behavior are often expressed in contexts/environments distinctively different from those in which they are created. This generalization process contributes to psychological disorders, particularly PTSD. Stress-related neurocircuits in the basolateral amygdala (BLA) receive inputs from hypothalamic orexin (Orx) neurons, which mediate neuronal activity by targeting orexin 1 (Orx1R) and orexin 2 (Orx2R) receptors via opposing functions. In BLA, inhibition of Orx1R or activation of Orx2R ameliorate stress responsiveness and behavior. We discovered that most Orx1R+ cells also express CamKIIα, while a majority of Orx2R+ cells are colocalized with GAD67. Further, Orx1R gene Hcrtr1 expression was positively correlated, and Orx2R gene Hcrtr2 expression was negatively correlated, with freezing in a phenotype-dependent fashion (Escape vs Stay) in the Stress Alternatives Model (SAM). The SAM consists of 4-days of social interaction between test mice and novel larger aggressors. Exits positioned at opposite ends of the SAM oval arena provide opportunities to actively avoid aggression. By Day 2, mice commit to behavioral phenotypes: Escape or Stay. Pharmacologically manipulating Orx receptor activity in the BLA, before Day 3 of the SAM, was followed with standard tests of anxiety: Open Field (OF) and Elevated Plus Maze (EPM). In Stay mice, freezing in response to social conflict and locomotion during SAM interaction (not home cage locomotion) were generalized to OF, and blocked by intra-BLA Orx1R antagonism, but not Orx2R antagonism. Moreover, patterns of social avoidance for Escape and Stay mice were recapitulated in OF, with generalization mediated by Orx1R and Orx2R antagonism, plus Orx2R stimulation.
Keywords: Anxiety, Defeat, Depression, Fear conditioning, Freezing, Hypocretin, Locomotion, PTSD, Resilience, Susceptibility, Stress-alternatives model, Transference
1. Introduction
Fear learning plays an important role in many psychological disorders, including anxiety, depression, and post-traumatic stress disorder (PTSD). In these disorders, particularly PTSD (and related animal models), fear-associated memories and behavior are often expressed in contexts and/or environments that are distinctively different from those in which they are generated, a learning process known as generalization or transference (Armony et al., 1995, 1997; Baker et al., 2019; Baldi et al., 2004; Kaczkurkin et al., 2017; Thome et al., 2018).
Animal responses in the Stress Alternatives Model (SAM) provide a window onto development of anxious and depressive behavior, and the mechanisms of decision-making that produce resilient and susceptible phenotypes. As such, this model reflects characteristics of PTSD, such as 1. Trauma 2. Primarily generated from a social structure that 3. Produces specific phenotypes, with some 4. Individuals displaying social avoidance and withdrawal, potentially including 5. Behavioral dysfunction, and 6. Generalization of behavior to other contexts as a part of recurrent memory of trauma (Blanchard et al., 2013; Robertson et al., 2015; van der Kolk, 2006; Yehuda and LeDoux, 2007). In an oval SAM arena with apical escape routes, novel larger aggressive individuals interact with smaller adult test subjects (Fig. 1). Test animals self-select one of two phenotypes: Escape or Stay, which exhibit stress-resilient (social engagement) and susceptible (social avoidance) responses to social interaction/preference tests (SIP) and differences in reactive plasma glucocorticoid concentrations (Smith et al., 2016; Staton et al., 2018; Yaeger et al., 2022). Production of two easily identified phenotypes via a simple dichotomous choice, as occurs in the SAM, results in distinctively different behaviors, and neurochemical responses, which develop over a short time course (Robertson et al., 2015; Smith et al., 2014; Yaeger et al., 2022), and are dependent on specific types of learning (Carpenter and Summers, 2009; Smith et al., 2016; Yaeger et al., 2020). Resilient status is confirmed for Escape animals, because anxiolytic drugs (corticotropin releasing factor type 1 receptor [CRF1] antagonist antalarmin, orexin 1 receptor [Orx1R] antagonist SB-674042, and orexin 2 receptor [Orx2R] agonist [Ala11, ᴅ-Leu15]–OrxB) promote Escape behavior in Stay animals (Smith et al., 2016; Staton et al., 2018; Yaeger et al., 2022). Alternatively, anxiogenic drugs (Yohimbine, an α2 adrenoreceptor antagonist, and Orx2R antagonist MK-1064) promote Stay behavior in Escape phenotype mice, clearly suggesting that Stay responses represent stress-susceptible behavior. Behaviors reflecting motivation to escape the SAM are also modified by stress-related neuromodulatory events (Staton et al., 2018; Yaeger et al., 2022). A fear conditioning protocol, during which a tone (conditioned stimulus [CS]) precedes aggressive interaction (unconditioned stimulus [US]), also produces associative learning in the SAM. Although a trace period is used between the CS (cue = tone) and US (aggression), this association yields Pavlovian conditioning, which occurs concomitantly with contextual conditioning. The cued response (CR = freezing) to tone alone in conditioned Stay mice is reduced by intra-basolateral amygdala (intra-BLA) injection of an Orx1R antagonist (Yaeger et al., 2022).
Fig. 1.
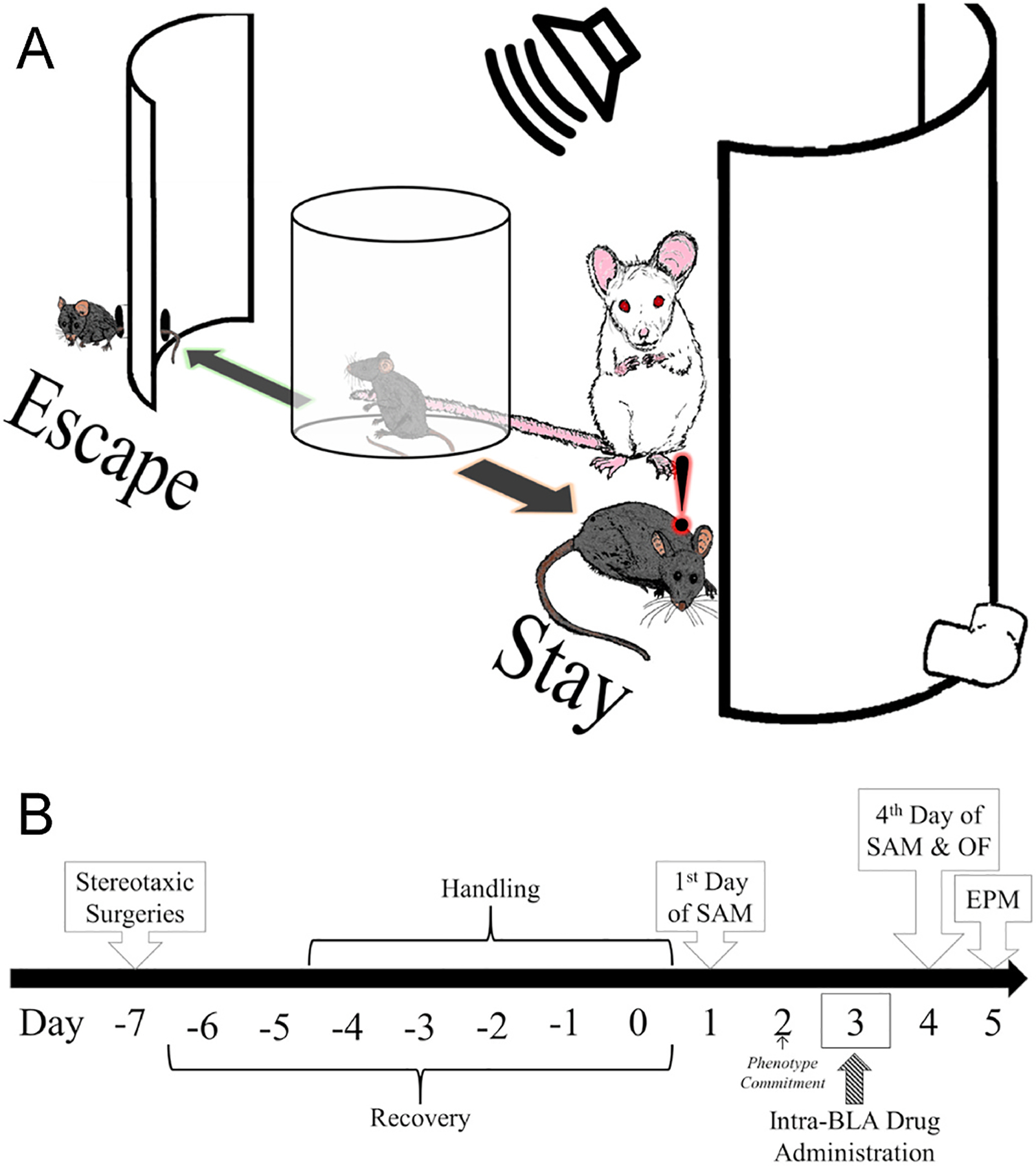
The Stress Alternatives Model (SAM) results in phenotype establishment after two days of social stress. A) The SAM is a 4-day paradigm in which test mice are conditioned to a tone (middle) before an opaque cylinder is lifted and animals must decide whether to Escape from (left) or submit to (Stay, right) a large social aggressor. By the end of Day 2, test mice commit to the Escape or Stay behavioral phenotype. B) Experimental design for behavioral trials include stereotaxic surgeries for cannula implantation followed by a recovery and handling period before the beginning of the SAM. On Day 3, 1 h before SAM exposure, mice were administered Orx receptor targeting drugs into the BLA. Following SAM social interaction on Day 4, mice were exposed to the Open Field (OF) Test and on Day 5 they were introduced to the Elevated Plus Maze (EPM).
Orexins (hypocretins) are comprised of two peptides, OrxA (HCrt1) and OrxB (HCrt2), cleaved from the same pro-peptide, produced in equal proportions in the lateral, dorsomedial hypothalamus perifornical area (LH-DMH/PeF) (Broberger et al., 1998; Nambu et al., 1999). While OrxA has equally high binding affinity for the Gq-linked GPCR Orx1 and Orx2 receptors, the Orx2R receptor binds OrxA and OrxB with similar high affinities, and OrxB binds Orx1R with somewhat reduced affinity (Ammoun et al., 2003; Soya and Sakurai, 2020a). In the BLA, and in other regions such as the paraventricular thalamus, the two receptor types are functionally opposed (Arendt et al., 2013, 2014; Meffre et al., 2019; Yaeger et al., 2020). Our recent work demonstrates significant opposing effects of Orx1R (pro-stress) and Orx2R (anti-stress) on Phenotype expression, as well as on associative learning (cued and contextual conditioning), learned spatiotemporal positioning in the SAM, and behaviors such as latency to escape, motivation to escape, socially-induced freezing (in response to aggression), social approach/avoidance (in the SIP test), startle, and depressive immobility (in forced swim test) (Arendt et al., 2013, 2014; Staton et al., 2018; Summers et al., 2020; Yaeger et al., 2020, 2022). We suspect therefore, that specific receptor binding is defined by cellular localization (Yaeger et al., 2022), such that ultimately, functions of the Orx receptors are determined by the output of the neuronal types in which each primarily exists.
During experiments examining the anti-stress properties of Orx1R antagonism in the BLA, it became clear that the classical fear conditioning we expected for stress-susceptible animals alone (Carpenter and Summers, 2009; Smith et al., 2016) was more complex than originally hypothesized (Yaeger et al., 2022). Instead of classical fear conditioning being limited to susceptible Stay mice, both Stay and Escape mice exhibit cued CRs. Moreover, Stay mice also exhibit enhanced contextual conditioning (freezing prior to tone in opaque divider) during the same SAM protocol (Yaeger et al., 2022). Additionally, intra-BLA injection of an Orx1R antagonist and intracerebroventricular (icv) delivery of an Orx2R agonist reduce cued fear conditioning (Staton et al., 2018; Yaeger et al., 2022). Although individuals that Escape experience significantly reduced stress-related neural, endocrine, and behavioral reactivity to stress, compared to Stay animals (Carpenter and Summers, 2009; Smith et al., 2016; Staton et al., 2018), they also undergo OrxR mediated classical (cued, tone) conditioning. Thus, Stay mice produce enhanced freezing under 3 conditions: 1. In response to cued conditioning (greater response than Escape mice), 2. In response to contextual conditioning (not evident in Escape mice), and 3. In responses to social aggression in the SAM (also greater than Escape mice). Thus, while the phenotypes are fundamentally different in the magnitude of responses (such as freezing), fear learning develops in both vulnerable (Stay) and resilient (Escape) animals (Yaeger et al., 2022).
In addition to the Orx1R antagonism reducing the plasma stress hormone corticosterone in both vulnerable (Stay) and resilient (Escape) mice, specific alterations of signaling-related gene expression occurred (Yaeger et al., 2022). Specifically, inhibition of Orx1R in BLA promoted increased Orx2R (Hcrtr2) gene expression in non-glutamatergic, (and because most BLA neurons not expressing CamKII express GAD1) presumably GABAergic neurons (Yaeger et al., 2022). In BLA, following Orx1R inhibition, likely in pyramidal neurons, there was also a significant increase in ERK1 (Mapk3) and Brain-Derived Neurotrophic Factor (Bdnf) transcripts. We posit that these gene expression changes occur in cells containing Orx2R, because Orx2R remain stimulated by native OrxA/OrxB even while Orx1R are blocked. Additionally, we presume cells not containing OrxR, are not directly affected by Orx related events. This significant potential cross-neuron stimulation of Orx2R, ERK1, and BDNF mRNA production was only evident in vulnerable Stay mice following intra-BLA administration of an Orx1R antagonist. The data suggest the Orx system not only modifies behavioral activity through actions in the BLA, it also acts to shift the signaling systems that underlie those behaviors (Yaeger et al., 2022). We surmised that if behavior and signaling systems were both altered following intra-BLA Orx1R antagonism, then the learning and memory systems that allow and support those behaviors might also be changed.
As inhibition of Orx1R in the BLA corresponded with transcriptional changes in Orx receptors and conditioned fear responses (Yaeger et al., 2022), we first predicted that mRNA levels of Orx receptors in the BLA would be related to phenotype-dependent socially induced freezing behavior in the SAM arena. Additionally, we noticed adaptive adjustment of specific self-positioning strategies, during avoidance of the aggressor, appear during SAM trials (which always follows contextual isolation and CS treatment). That is to say, resilient Escape mice seemed to make more use of SAM arena edge space on the way to using an escape tunnel at the apical edge, even though the CD1 aggressors primarily patrol there. Not surprisingly, susceptible Stay mice make greater use of center areas. Those observations led us to examine if the development of unique Escape and Stay coping strategies were being translated into equivalent behavior in subsequent trials using alternative testing models post-treatment. As the Open Field (OF) and Elevated Plus Maze (EPM) tests have uniquely defined movement strategies that have been associated with anxious responses (Haller et al., 2013; Prut and Belzung, 2003; Walf and Frye, 2007), our surprising results suggest that typically reduced time spent in center (OF), or open arms (EPM), in stress-susceptible animals may be dramatically shifted by generalization of learned adaptive responses in a social setting (SAM). There appear to be similarities with PTSD, because SAM generalizations occur after social interaction stress, and involve trauma avoidance with persistent behavioral dysfunction (Kaczkurkin et al., 2017; van der Kolk, 2006; Yehuda and LeDoux, 2007). Complementary to these findings, we hypothesized that social stress-induced fear freezing behavior will be generalized to a novel OF Test environment, and that Orx1R inhibition or Orx2R stimulation would mute this learning response. As intra-BLA Orx receptors are important for generalization of fearful responses, we also hypothesized that specific learning of environmental self-positioning strategies in the SAM, such as avoiding/frequenting the primary larger mouse patrolling lane along the edges (learned by receiving aggression when wandering into that lane by stress-vulnerable Stay mice, or by using the edge as a landmark to find the tunnel by stress-resilient Escape mice), may be transferred/generalized to the non-social OF Test following pharmacological manipulation of Orx receptor activity. Finally, we proposed that measures of anxious behavior in the classical EPM after social stress and behavioral testing may be unreliable and inconsistent with results from preceding SAM and OF Test learning trials.
2. Methods
2.1. Subjects and housing
Adult male C57BL/6NHsd mice (6–8 weeks old) weighing ~22–28 g were obtained from Envigo (Indianapolis, IN; N = 194) and acclimated for a 5-day period in groups of five, after which animals were singly housed in rooms held at 22 °C and 35% relative humidity for the remainder of the experiments. Food and water were provided ad libitum. For studies involving pharmacological manipulations (N = 109), bilateral stereotaxic surgeries were performed where guide cannula (26 ga cut to 4.0 mm) were directed at the basolateral amygdala (intra-BLA). A separate set of retired male breeder Hsd:ICR mice (CD1, N = 30) weighing ~50 g (Envigo) were individually housed, and used to initiate aggression in the Stress Alternatives Model (SAM; Fig. 1A).
Mice were subjected to a 12:12 light-dark cycle (lights off at 6 p.m.), and behavioral experiments were performed during the animals’ active phase (scotophase). Two days (48 h) after surgeries, test subjects (C57BL/6NHsd mice) were handled daily for 5 days before SAM exposure and behavioral testing the five proceeding days (Fig. 1B). All procedures (surgery and behavioral testing) were performed in a manner that minimized suffering. The number of animals used was in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) and approved by the Institutional Animal Care and Use Committee of the University of South Dakota.
2.2. Stereotaxic surgeries
Mice were anesthetized using isoflurane (2% at 1.0 L/min flow rate) before bilateral intra-BLA guide cannula (PlasticsOne, Roanoke, VA; 26 ga cut to 4.0 mm) implantation. Following surgery, mice were provided a recovery period (7 days) before behavioral testing. Cannula placement was performed using the following stereotaxic coordinates: 1.35 mm AP, ± 3.30 ML, and 4.90 mm DV. During surgery and for ~45 min post-surgery, mice were kept on a warming pad to maintain core body temperature. Immediately following surgery and 24 h after surgical procedures, mice were provided pain relief in the form of subcutaneous injections of the analgesic ketorolac (5 mg/kg).
2.3. Drugs & drug administration
As several drugs were used to activate or inhibit intra-BLA Orx receptors, we broke the assessments into two broad categories: Orx receptor antagonist groups and Orx receptor stimulation groups. The Orx receptor antagonist groups consisted of mice treated with the Orx1R antagonist SB-674042 (N = 20; IC50 = 3.76 nM for Orx1R; MedChemExpress, Monmouth Junction, NJ) and the Orx2R antagonist MK-1064 (N = 17; IC50 = 0.5 nM for Orx2R; MedChemExpress). For Orx receptor stimulation groups, mice were administered OrxA (N = 13; EC50 = 20 nM for Orx1R & Orx2R; ToCris, Minneapolis, MN), a concoction of OrxA & MK-1064 (N = 19; for biased Orx1R activation), or the Orx2R agonist YNT-185 (N = 12; EC50 = 28 nM for Orx2R; Wako Chemicals, Richmond, VA). Drug effects were compared to vehicle-treated (N = 28; artificial cerebrospinal fluid; aCSF + 25% DMSO) control animals that underwent cannula implantation surgeries and were exposed to the same testing conditions and procedures as drug-treated mice. On Day 3 of the behavioral design (Fig. 1B), mice were infused bilaterally in the BLA (300 nL/side) with their designated treatment an hour before social interaction in the SAM.
All drug treatments were diluted using a 3:1 ratio of aCSF to dimethylsulfoxide (DMSO); and all treatments, excluding SB-674042 and YNT-185, were brought to a 0.1 nmol/0.3 μL concentration. The dose for the Orx2R antagonist, MK-1064, was 3x lower than previously used concentrations that produced anxiogenic effects when administered to the whole brain (intracerebroventricularly; icv) (Staton et al., 2018). Similarly, the intra-BLA dose for OrxA was selected and adjusted based on icv administrations that produced anxious behaviors in mice (Suzuki et al., 2005). As the Orx1R antagonist SB-674042 and the Orx2R agonist YNT-185 have lower binding affinities compared to the Orx2R antagonist (MK-1064), we chose a slightly higher doses (0.3 nmol/0.3 μL for SB-674042 and 10 nmol/0.3 μL for YNT-185) in order to compensate for these differences.
Artificial cerebrospinal fluid (aCSF; 8.59 g NaCl, 0.201 g KCl, 0.279 g, CaCl2, 0.16 MgCl2, 0.124 g NaH2PO4, 0.199 g Na2HPO4/L H2O) was mixed and brought to a physiological pH (~7.33) using NaOH before being filtered, degassed, and stored at 4 °C. Drugs were infused using injector cannulae (33 ga cut to 4.9 mm, extending 0.9 mm below each guide cannula) placed into implanted guide cannulae, and injecting with a 1.0 μL digital syringe (Model 7101 Zero Dead Volume, Knurled Hub 2.75”, 22 GA Needle; Hamilton Company, Reno, NV) at a rate of 0.5 μL/min. After drug administration, the injector and syringe were left in place for 90 s. Home cage mobility was measured briefly (~3 min) after SAM interaction on Day 3 in order to note changes in locomotion that resulted, not from social stress, but instead from drug interactions.
2.4. Social stress and decision-making paradigm
In the SAM paradigm (Fig. 1A), social conflict between a larger novel CD1 mouse and smaller C57BL/6NHsd male mouse takes place for 5 min each day, over four days, during which test animals may shorten interaction with the aggressor by escaping through size-limited tunnels at the ends of an oval open field arena. Prior to the social interaction, a tone given as a conditioned stimulus (CS) during isolation in the SAM apparatus allows for Pavlovian Conditioning of test subjects to the upcoming social interaction (unconditioned stimulus, US). As distinct and stable phenotypes (active avoidance (Escape) and accepting confrontation (Stay); determined over 45 experiments with 98% reliability (Robertson et al., 2015; Yaeger et al., 2022)) are established on Day 2, drug manipulation on Day 3 allows for within-sample and between group behavioral comparisons for phenotypes and drug controls (vehicle) during the SAM (Days 3 & 4) (Robertson et al., 2015; Smith et al., 2014, 2016; Staton et al., 2018; Yaeger et al., 2022) and in tests of anxiety that follow SAM exposure (Open Field and Elevated Plus Maze) (Yaeger et al., 2022). In this model unique behavioral patterns and phenotypes develop over time. The design allows for within sample before and after treatment comparisons, which allow for identification of pre- and post-traumatic neuromodulatory plasticity. These qualities of the SAM present development of specific phenotypes, one (Stay) of which is associated with failure to recover from trauma, as with PTSD (Yehuda and LeDoux, 2007). All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) and approved by the USD Institutional Animal Care and Use Committee.
2.5. Experimental design (see also supplemental information)
For these experiments, Orx receptor-targeting drugs (Orx1R antagonist: SB-674042, Orx2R antagonist: MK-1064, OrxA, concoction of OrxA & MK-1064 [for biased stimulation of Orx1R], and Orx2R agonist: YNT-185) were directed at the BLA 1h prior (based on previous and preliminary experimentation (Yaeger et al., 2020; Yaeger et al., 2022) to SAM interaction on Day 3 (Fig. 1B). After SAM interaction on Day 4, mice were exposed to the OF Test (Day 4) and EPM (Day 5). Behavioral measurements were taken during the active phase (dark cycle), and include freezing (socially induced, which is usually associated with aggressive conflict [SAM] and generalized [OF Test]), locomotion (SAM, OF Test, and home cage), time spent in center area (SAM and OF Test), and standard EPM measurements (time in open/closed arms and intersection zone). Brains were collected and used for visual representations of mRNA (using RNAscope) or relative changes in gene expression (rt-qPCR) of Orx1R (Hcrtr1) and Orx2R (Hcrtr2) receptors, Ca++/Calmodulin Kinase type 2 alpha (CamkIIα), Glutamate Decarboxylase (Gad1), and parvalbumin (Pvalb).
2.6. Quantitative reverse transcription PCR (RT-qPCR)
Purchased assays (Thermo Fisher Scientific, Waltham, MA) for the PCR analyses included Hcrtr1 (4351370, Mm01185776_m1), Hcrtr2 (4351370, Mm01179312_m1), and Gapdh (4453320, Mm99999915_g1) as the housekeeping gene. A master mix for each PCR target was created using a one-step RT-qPCR kit (Cat. No. 4392653) before being mixed with RNA samples from BLA tissue in individual PCR tubes (MIDSCI, Valley Park, MO; Pryme Ergonomic PCR Tubes; Cat. No. B77201). The PCR tubes were then loaded into Applied Biosystems QuantStudio 3 No. B77201 thermal cycler (Thermo Fisher Scientific, Waltham, MA; Cat. No. A28131) and, as per Taqman Assay vendor recommendations, were run through 40 cycles at the following conditions: reverse transcription (48 °C, 15 min), DNA polymerase activation (95 °C, 10 min), denaturation (95 °C, 15 s), and annealing & extension (60 °C, 1 min).
No enzyme and no template control PCR sample tubes were created to rule out the possibility of contamination during PCR runs. Individual samples from non-stressed cage control mice (N = 6), Escape mice (N = 10), and Stay mice (N = 14) were used for PCR analysis. Duplicates for each sample were run and the average Ct value was subtracted from the average housekeeping gene (Gapdh) Ct to give the ΔCt for analysis. Determination of relative gene expression levels was made using the 2−ΔΔCt method (Livak and Schmittgen, 2001), which was then compared to the average ΔCt of the non-stressed cage controls. Regression curves were made for these data where average fold change is correlated to SAM freezing behavior. The calculated fold change values are based on the experimental design, in which the mean ΔCt value (gene of interest Ct-housekeeping gene [GAPDH] gene) of the cage control group, and this value is used to derive the ΔΔCt for experimental groups (experimental ΔCt – control ΔCt) as well as for the cage controls. As each cage control animal ΔΔCt is based on the average ΔCt for the control group, the ΔΔCt value for each individual of the control group is a non-zero value. Therefore, when calculating the 2−ΔΔCt value, the mean fold change value for the control group is always close but never precisely one.
2.6.1. In situ hybridization (RNAscope)
Fresh frozen brains (N = 12) of C57BL/6NHsd mice (9–10 weeks old) not exposed to social stress or behavioral testing were sectioned into 20 μm coronal sections and positioned on slides (Fisher Scientific, Pittsburgh, PA; Superfrost Plus, Cat. No. 12-550-15). Tissue that incorporated the BLA from AP −1.50 to −1.80 relative to bregma was incubated in cold (4 °C) 10% formalin for 20 min and then washed (2x for 1 min) in 1x phosphate buffer solution (PBS). Dehydration of tissue was performed by sequentially washing the sections in ethanol (50%, 70%, and 100%; 5 min each) followed by a final ethanol (100%) wash overnight in a −20 °C freezer.
Proteins were digested in the tissue sections the next day with a protease treatment before being rinsed in distilled H2O. Bathing of tissue in RNAscope (Advanced Cell Diagnostics, Newark, CA) probes (Hcrtr1, Cat. No. 466631; Hcrtr2, Cat. No. 581631; Gad1, Cat No. 400951; CamkIIα, Cat. No. 445231; Pvalb, Cat. No. 421931) took place at 40 °C for 2 h in a specially designed hybridization oven (ACD HybEZ II oven, Cat. No. 321711). Next, sequential washes (RNAscope Wash Buffer Re-agents [310091]: Wash Buffer 50x diluted to 1x) and bathing with amplification buffers (RNAscope Fluorescent Multiplex Detection Re-agents [320851]: AMP1 [320852], AMP2 [320853], AMP3 [320854], AMP4 ALT A [320855], AMP4 ALT B [320856]) was performed to bind fluorophores and enhance the signaling of target mRNA. Lastly, the sections were stained with DAPI (20 s) and placed using a mounting medium (Fisher Scientific; Prolong Gold Antifade Mountant, Cat. No. P10144) before being coverslipped and stored at 4 °C in the dark until imaging.
Section visualization was performed using a Nikon A1 (10 × /0.30 Plan Fluor or 20x/0.75 Plan Apo VC Nikon objectives) confocal microscope system with NIS Elements software for image acquisition. Areas of interest were selected from images and analyzed and counted for fluorescence using ImageJ software. The colocalization of fluorescence-tagged mRNA were identified as overlap of signal or as puncta of different fluorescence clustering on the same DAPI signaling, which would suggest that the mRNA expression is in a single cell.
2.7. Statistical analyses
Experimental designs and statistical analyses and were based on a priori hypotheses, for the purpose of avoiding combinatorial exponential expansion of error from multiple tests (Veazie, 2006). This statistical pre-planning allows for a wider range of multiple comparison analyses across hypothetical designs. Analysis made use of two-way ANOVA for Orx drug × Phenotype (Stay × Escape) designs, one-way ANOVA drug-dependent home cage locomotor activity, and regression for correlations between gene expression (Hcrtr1 & Hcrtr2), SAM socially induced freezing, SAM-dependent behavioral responses (Day 4), or OF Test behaviors (Day 4). Comparisons between two treatments (Vehicle, Orx1R Ant., Orx2R Ant., OrxA, Orx1R Stim, or Orx2R Stim within a given phenotype (Escape or Stay) were investigated by Student’s t-tests. The results are reported without α adjustment (Feise, 2002; Jennions and Moller, 2003; Moran, 2003; Nakagawa, 2004; Perneger, 1998; Rothman, 1990) based on a priori hypothesis driven exclusion from combinatorial effects (Veazie, 2006). Significant effects between groups for one-way analyses were examined with Student–Newman–Keuls post hoc analyses (to minimize Type I error) and Duncan’s Multiple Range Test (to minimize Type II error).
3. Results
3.1. Social stress-induced freezing linked to specific Orx receptors in specific neurons
Freezing in response to social conflict is common, differentiated by Stay or Escape phenotype, and most pronounced on Day 4 of the SAM paradigm (Fig. 2A; t56 = 3.825, p < 0.001). This freezing exhibits phenotype specific positive (Escape mice) and negative (Stay) regression relationships with Hcrtr1 (Escape; Fig. 2B; F1,8 = 7.8, p ≤ 0.0233) and Hcrtr2 (Stay; Fig. 2C; F1,12 = 9.7416, p ≤ 0.0088) respectively. The Orx1R and Orx2R are found in a minority of BLA neurons (Fig. 2D; F3,44 = 134.0, p < 0.001), suggesting that the strong functional relationship to freezing is determined by specific neurocircuits, presumably including CamKII-positive glutamatergic pyramidal cells for Orx1R effects on freezing in Escape mice (Fig. 2E–G; Interaction Effect: F2,30 = 37.4, p < 0.001), and likely GAD67-positive GABAergic neurons for Orx2R relationship with freezing in Stay mice (Fig. 2H–J; Expression Effect: F1,24 =322.9, p < 0.001; Interaction Effect: F2,24 = 73.3, p < 0.001).
Fig. 2.

Stress-induced and phenotype-dependent freezing behavior is bidirectionally correlated with Orx1R (Hcrtr1), expressed predominantly in glutamatergic neurons, and Orx2R (Hcrtr2), more prominent in GABAergic neurons, gene expression in the BLA. A) Phenotype (Escape & Stay) distinctions in SAM-derived socially induced freezing are significant and most pronounced on Day 4 (t56 = 3.8, *p < 0.001). B) In Escape mice, intra-BLA Orx1R (Hcrtr1) transcription is positively associated with socially induced freezing (social stress-related freezing) in the SAM (F1,8 = 7.8, R2 = 0.4946, p ≤ 0.0233), but this regression in stay mice is not significant (Fig. S2A). C) Conversely, socially induced freezing behavior in the SAM is negatively related to Orx2R (Hcrtr2) mRNA levels in the BLA of Stay mice (F1,12 = 9.7, R2 = 0.4481, p ≤ 0.0088), and not significant in Escape mice (Fig. S2B). While Orx receptors are associated to phenotype and freezing behavior, D) only a small percentage of the total number of BLA cells contain Orx1R, Orx2R, or both receptor subtypes (F3,44 = 134.0, p < 0.001; significance is by unique symbol, e.g. A is significantly different from B, C, & D). E & F) In the BLA, Orx1R (green), but not Orx2R (white), are highly co-expressed with the glutamatergic cell marker CamKIIα (red; some of the observed colocalizations are indicated with solid green arrows = Orx1R+ + CamKIIα+, solid white arrow = Orx2R+ +CamKIIα+, and unfilled white arrows = Orx2R+ + CamKIIα−). (G) The number of BLA Orx1R+ cells expressing CamKIIα is over 60% while Orx2R+ cells co-express the glutamatergic marker ~30% of the time, and about 50% of the small proportion of BLA cells that express both Orx1R and Orx2R also express CamKIIα. H & I) Expression of Orx2R (white) overlaps with GAD67 (red) more than Orx2R (green) in the BLA (a few observed colocalizations are identified with unfilled green arrows = Orx1R+ + GAD67−, solid white arrow = Orx2R+ + GAD67+, and unfilled white arrows = Orx2R+ + GAD67−). J) Analyses reveal Orx2R are expressed in GABA neurons in a greater proportion than Orx1R or cells that co-express Orx1R & Orx2R. −p ≤ 0.05 for comparisons to CamKIIα− /GAD67− cells in the same receptor (Orx1R+ or Orx2R+) group; +p ≤ 0.05 for comparisons to Orx1R+ of the same CamKIIα+/GAD67+ profile; #p ≤ 0.05 for comparisons to Orx2R+ of the same CamKIIα+/GAD67+ profile. CeA = central amygdala; ITC = intercalated cells of the amygdala.
3.2. Orx receptor-dependent generalization of freezing and locomotion are phenotype specific
Importantly, freezing in response to social conflict in the SAM is generalizable to the OF test for vehicle-treated Stay mice (Fig. 3A and B; SAM: Drug Effect, F5,70 = 9.6, p < 0.001; Phenotype Effect, F1,70 = 17.6, p < 0.001; OF: Drug Effect, F5,70 = 3.3, p ≤ 0.011; Phenotype Effect, F1,70 = 21.4, p < 0.001). These Stay mice exhibit significantly more socially induced freezing than Escape mice (Fig. 3A; t19 = 2.67, p ≤ 0.015). The distinctive phenotype difference in behavior is eliminated by Orx1R antagonist treatment, but not by Orx2R antagonist (Fig. 3A; t11 = 3.8, p < 0.001 following two-way ANOVA above). Further, phenotype differences are abolished with OrxA stimulation as a result of Escape mice displaying more freezing, and after Orx2R agonist as both phenotypes experience a reduction in freezing (Fig. 3A; t17 = 3.3, p ≤ 0.004).
Fig. 3.
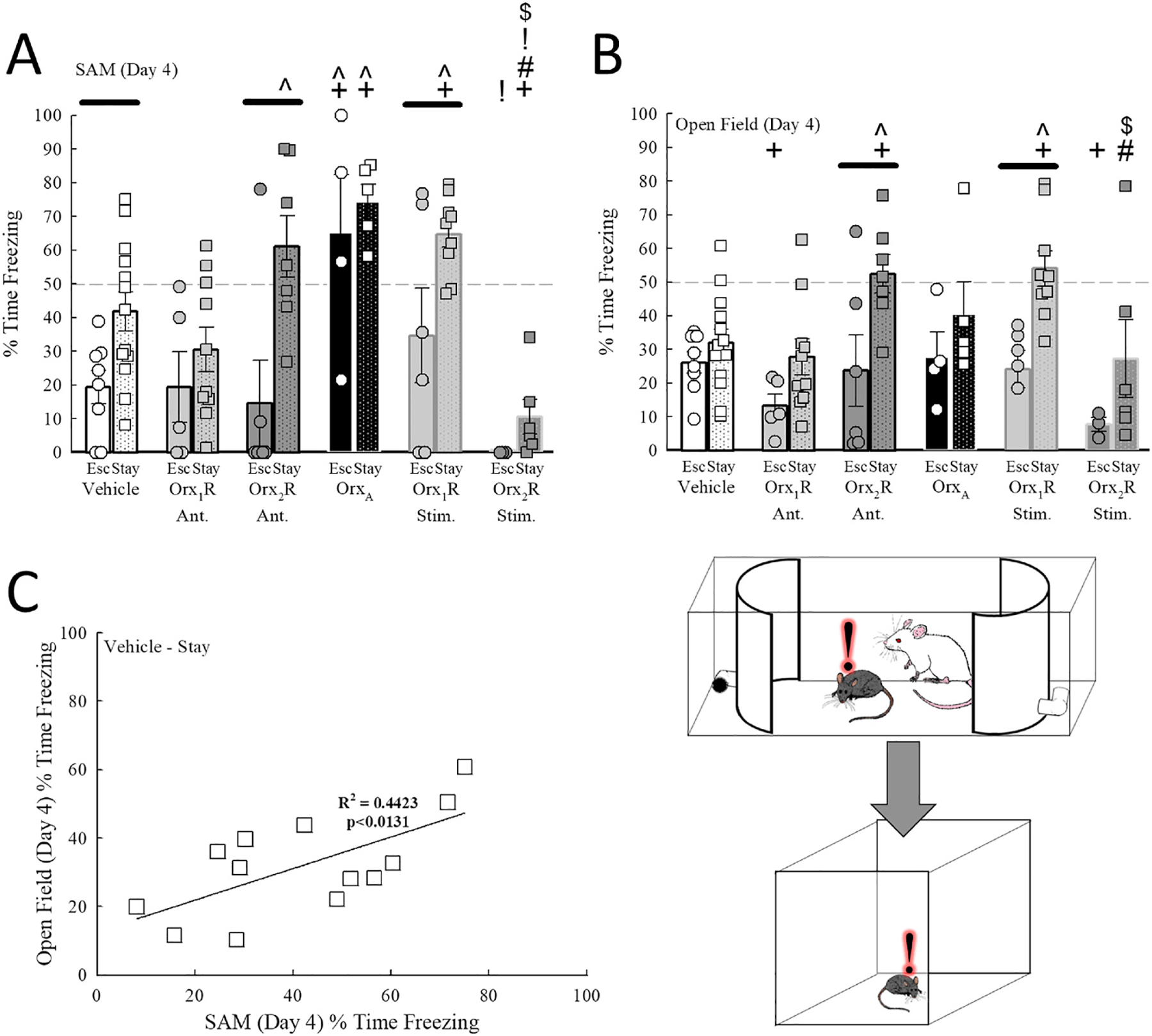
Socially induced freezing behavior in the SAM is transferred to the non-social OF Test in Stay mice. A) Stay mice treated with intra-BLA infusion of an Orx2R antagonist, but not an Orx1R antagonist, experience enhanced freezing in the SAM (Drug Effect, F5,70 = 9.6, p < 0.001; Phenotype Effect, F1,70 = 17.6, p < 0.001). Further, mice in OrxA and Orx1R stimulation groups exhibit enhanced freezing, while animals treated with an Orx2R agonist demonstrate significantly reduced freezing in the SAM. B) Antagonism of Orx1R receptors in the BLA reduced generalized OF Test freezing in Escape mice only, while Orx2R antagonist treatment increased OF freezing in Stay animals (Drug Effect, F5,70 = 3.3, p ≤ 0.011; Phenotype Effect, F1,70 = 21.4, p < 0.001). Additionally, freezing in the OF Test was increased in Orx1R stimulation group mice, while intra-BLA agonism of Orx2R reduced freezing in both phenotypes. C) In vehicle-treated control Stay mice, socially induced freezing in the SAM is positively correlated to OF Test freezing (F1,11 = 8.7, R2 = 0.4423, p ≤ 0.0131). −p ≤ 0.05 for comparisons between phenotypes in the same treatment group; +p ≤ 0.006 for comparisons to Vehicle-treated mice of the same phenotype; ^p ≤ 0.006 for comparisons to Orx1R Ant. group of the same phenotype; #p ≤ 0.006 for comparisons to Orx2R Ant. group of the same phenotype; !p ≤ 0.006 for comparisons to OrxA treatment animals of the same phenotype; $p ≤ 0.006 for comparisons to mice in the Orx1R stimulation group of the same phenotype.
Stimulation of Orx2R in the BLA on day 3 also reduced day 4 OF Test freezing in Stay mice compared to mice in the Orx2R Antagonist (t11 = 2.7, p ≤ 0.004) or Orx1R Stimulation (t13 = 2.4, p ≤ 0.034) groups (Fig. 3B). As predicted, intra-BLA Orx2R inhibition elevated OF Test freezing behavior relative to Escape mice that underwent the same treatment (Fig. 3C; t11 = 2.5, p ≤ 0.029). Further, these Stay mice that were administered an Orx2R antagonist experienced increased freezing in the OF Test compared to vehicle- (t19 = 3.0, p ≤ 0.008) and Orx1R Ant.-treated mice (Fig. 3B; t15 = 3.1, p ≤ 0.008). This elevated OF Test freezing in Stay mice was also observed in those mice in the Orx1R Stimulation group (Fig. 3B; Vehicle vs Orx1R Stim.: t21 = 3.4, p ≤ 0.003; Orx1R Ant. vs Orx1R Stim.: t17 = 3.4, p < 0.001). Interestingly, a significant and positive association between SAM and OF Test freezing was observed in vehicle-treated Stay animals (Fig. 3C; F1,11 = 8.7, p ≤ 0.0131).
Generalization of locomotion is also transferable from SAM to OF for Escape mice (Fig. 4; Drug Effect, F5,70 = 2.6, p ≤ 0.033; Phenotype Effect, F1,70 = 11.9, p < 0.001; OF: Phenotype Effect, F1,70 = 12.6, p < 0.001), with significant positive regressions between OF and SAM locomotion in mice treated with Orx1R antagonist (F2,3 = 15.9, p ≤ 0.028), but also for OrxA (F2,2 = 49.4, p ≤ 0.02) and Orx2R agonist treated mice (Fig. 4C–F; F2,1 = 351.5, p ≤ 0.034). Escape mice (vehicle-treated) exhibit significantly more locomotion in the SAM (t19 = 2.2, p ≤ 0.034) and OF (Fig. 4A and B; t19 = 2.2, p ≤ 0.032), but not in the home cage (Fig. S7). This phenotypic distinction is prevented by Orx1R (in both SAM and OF) but not Orx2R antagonism (in SAM only), and not by Orx1R stimulation (in SAM only; Fig. 4A and B; SAM: t13 = 2.3, p ≤ 0.025; OF: t13 = 2.3, p ≤ 0.026).
Fig. 4.
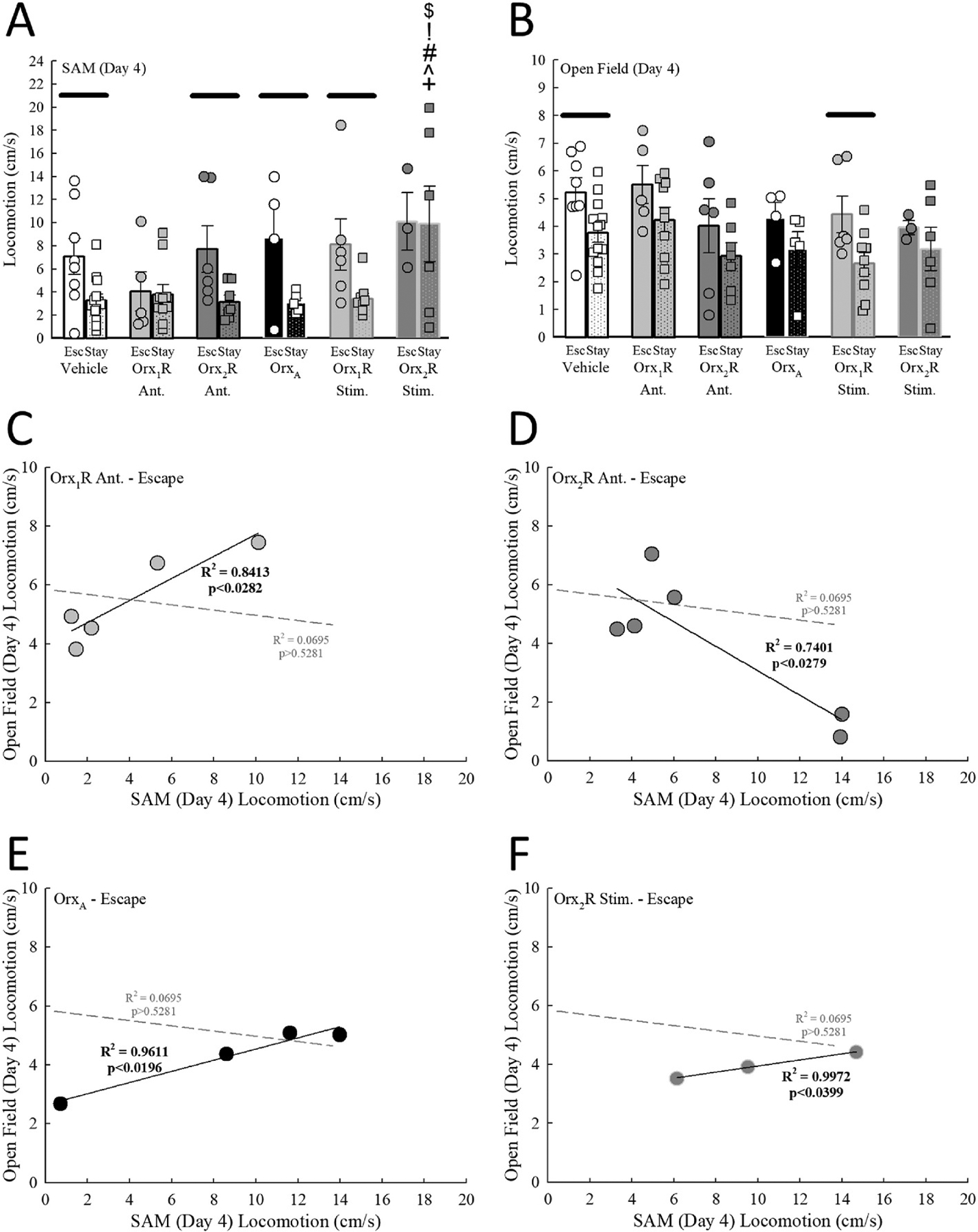
Social stress-induced locomotion in SAM is generalized/transferred to a non-social OF Test environment after intra-BLA manipulation of Orx receptor activity in Escape animals. A) Escape mice express higher locomotor activity compared to Stay animals during social stress in the SAM, but this phenotype difference is not observed after intra-BLA Orx1R antagonism (Drug Effect, F5,70 = 2.6, p ≤ 0.033; Phenotype Effect, F1,70 = 11.9, p < 0.001). Also, infusion in the BLA of an Orx2R agonist enhances locomotion in the SAM in Stay mice. B) While Escape animals in the vehicle control group display higher locomotion compared to Stay mice in the OF Test, this divergent phenotype response is not observed after intra-BLA Orx1R or Orx2R antagonism (Drug Effect, F5,70 = 2.1, p ≥ 0.071; Phenotype Effect, F1,70 = 12.6, p < 0.001). Similarly, OrxA and Orx2R stimulation eliminates the difference in locomotion between Escape and Stay mice in the OF Test. C) Unlike vehicle controls (gray dotted line represents vehicle control linear regression line, F1,12 = , R2 = 0.0695, p ≥ 0.5281), a significant positive relationship between SAM and OF Test locomotion is observed in Escape mice treated with an Orx1R antagonist (F2,3 = 15.9, R2 = 0.8413, p ≤ 0.0282). D) A significant negative correlation is revealed between SAM and OF Test locomotion in Escape mice treated with an Orx2R antagonist (F1,4 = 11.4, R2 = 0.7401, p ≤ 0.0279; dotted gray line represents vehicle control regression line). Like Orx2R antagonism, significant positive associations between locomotor activity in the SAM and OF Test for Escape mice treated with E) OrxA (F2,2 = 49.4, R2 = 0.9611, p ≤ 0.02; dotted gray line represents vehicle control regression line) or F) an Orx2R agonist (F2,1 = 351.5, R2 = 0.9972, p ≤ 0.034; dotted gray line represents vehicle control regression line). −p ≤ 0.05 for comparisons between phenotypes in the same treatment group; +p ≤ 0.005 for comparisons to Vehicle-treated mice of the same phenotype; ^p ≤ 0.005 for comparisons to Orx1R Ant. group of the same phenotype; #p ≤ 0.005 for comparisons to Orx2R Ant. group of the same phenotype; !p ≤ 0.005 for comparisons to OrxA treatment animals of the same phenotype; $p ≤ 0.005 for comparisons to mice in the Orx1R stimulation group of the same phenotype.
3.3. Generalization of phenotypic behavior patterns are modulated by Orx receptors
As freezing and locomotion in response to social conflict are generalizable from SAM apparatus to OF, we sought to understand whether the basic patterns of phenotypic response (Stay and Escape), would be reflected in standard tests of anxious responsiveness, such as OF or EPM. There was a generalization effect of phenotypic behavior in OF (Phenotype Effect, F1,43 = 15.0, p < 0.001), however, this transference was strictly dependent for drug treated mice, on movement patterns learned during 4 days in the SAM. Escape mice, which used the edges of the SAM to locate apical escape routes located on the edge, also favored edges in the OF (Fig. 5D top). In contrast, Stay mice frequented the center of the SAM apparatus to avoid patrolling CD1 aggressors (Fig. 5A, D bottom), and maintained that pattern in OF (Fig. 5D bottom) when treated with Orx1R antagonist (F1,8 = 16.8, p ≤ 0.003; Fig. 5A, B, C), Orx2R antagonists (F1,5 = 13.5, p ≤ 0.014; Fig. 5B, D, E), or an Orx2R agonist (F1,4 = 40.2, p ≤ 0.003; Fig. 5A, D, F).
Fig. 5.
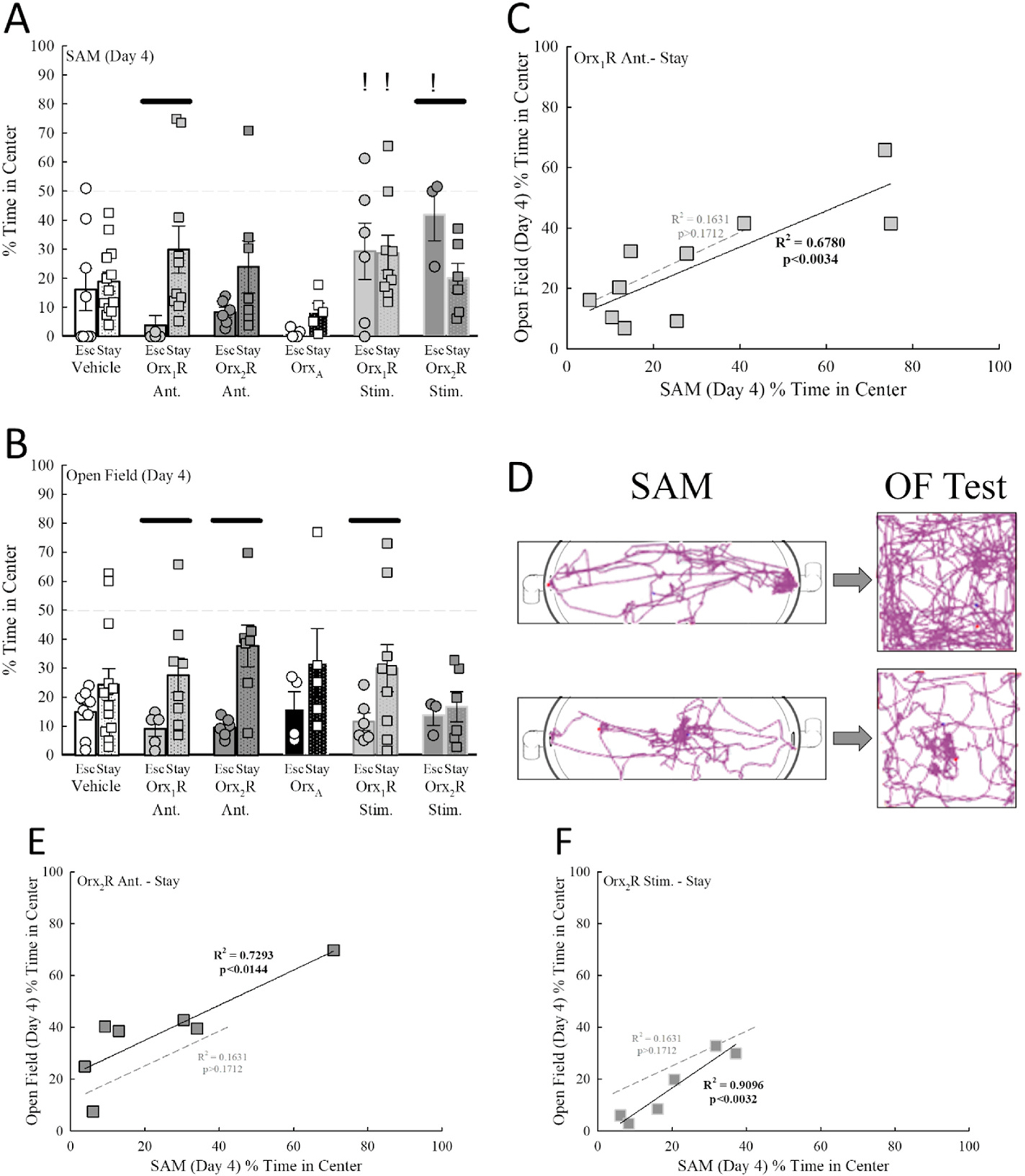
Treatments targeting Orx receptors in the BLA promote transfer learning from the SAM to the OF Test in Stay mice. A) In the SAM, the amount of time spent in the center of the arena is not different between Escape and Stay animals in the vehicle control group, but phenotype divergence occurs after intra-BLA Orx1R antagonism with Stay mice spending more time in the center. Further, Escape mice treated with an Orx2R agonist display increased time in the center of the SAM arena. B) While Escape and Stay vehicle-treated mice did not show differences in the amount of time spent in the center of the OF Test, intra-BLA Orx1R antagonism, Orx2R antagonism, and Orx1R Stimulation prompted phenotype separation with Stay animals spending more time in the center of the OF (Phenotype Effect, F1,43 = 15.0, p < 0.001). C) Regression analysis revealed a significant and positive relationship (F1,8 = 16.8, R2 = 0.6780, p ≤ 0.0034) between time spent in the center of the SAM and time spent in the center of the OF Test after intra-BLA Orx1R antagonism in Stay animals, but not in vehicle-treated Stay mice (dotted gray line represents vehicle control regression line, F1,12 = 2.1, R2 = 0.1631, p ≥ 0.1712). D) Examples of tracking software maps for Orx1R antagonist-treated Stay mice that prefer the edges of the SAM and OF Test (top) and Stay that are biased toward the center regions (bottom). E) Significant and positive correlations exist for time spent in the center of the SAM and time spent in the center of the OF Test for Stay mice treated with an Orx2R antagonist (F1,5 = 13.5, R2 = 0.7293, p ≤ 0.0144; dotted gray line represents vehicle control regression line) or F) an Orx2R agonist (F1,4 = 40.2, R2 = 0.9096, p ≤ 0.0032; dotted gray line represents vehicle control regression line). −p ≤ 0.05 for comparisons between phenotypes in the same treatment group; !p ≤ 0.05 for comparisons to OrxA-treated mice of the same phenotype.
The EPM results following 4 days of SAM interaction did not produce a phenotypic distinction between Escape and Stay mice for open arm, closed arm, or interaction zone times (Fig. S8). Surprisingly, anxious, stress-vulnerable Stay mice (Smith et al., 2016; Yaeger et al., 2020), exhibited significantly more time in open arms following Orx1R stimulation (Fig. S8A); opposite of the expected finding relative to anxiety.
4. Discussion
The process of phenotype development in the SAM requires numerous learning phases, as does phenotype development in PTSD (Allen et al., 2019; Carpenter and Summers, 2009; Lissek and van Meurs, 2015; Yehuda and LeDoux, 2007). Decision-making for stress-vulnerable individuals (Stay) in the SAM paradigm shifts to resilient (Escape) responses after anxiolytic drugs or behavioral modifications (such as exercise) are administered (Smith et al., 2014, 2016). This is also true after intra-BLA Orx1R inhibition or icv Orx2R stimulation (Staton et al., 2018; Yaeger et al., 2022). Conversely, decisions in the SAM switch from stress resilient responses to stress susceptible responses in Escape phenotype animals following anxiogenic treatments that include Orx2R antagonism (Smith et al., 2016; Yaeger et al., 2022). What is more, social aggression-based contextual and cued fear conditioning are reduced by intra-BLA Orx1R antagonism and by icv Orx2R stimulation (Staton et al., 2018; Yaeger et al., 2022), which suggested to us that amygdalar Orx receptors modify associative learning related to fear behavior. Activation of the Orx system amends performance in novel object recognition, while reducing social interaction following defeat (Eacret et al., 2019), further suggesting that Orx and stress-related behavior together modify the conditions for learning. Emotionally salient information activates Orx neurons, potentiating freezing behavior, as well as Orx1R activity in locus coeruleus (LC) and BLA (Soya et al., 2017). Together they modulate cue-dependent fear memory and retrieval (Soya et al., 2013; Yaeger et al., 2022), suggesting that these systems are likely to be involved in PTSD symptomology.
In the SAM, following the CS cue for fear conditioning, socially induced freezing in response to aggressive contact also takes time to learn, and appears most consistently after 4 days of training (Fig. 2A). Importantly, SAM-induced behavioral changes, like freezing, after acute pharmacological intervention (Day 3) is often manifest later (Days 4 & 5) (Smith et al., 2016; Staton et al., 2018; Yaeger et al., 2022). Social aggression-induced, phenotype-dependent freezing behavior on Day 4 is bidirectionally correlated, positively for Escape with Hcrtr1 and negatively for Stay with Hcrtr2 gene expression in the BLA (Fig. 2B and C). The locations of these receptors are distinctively organized primarily (>60%) in glutamatergic pyramidal (Hcrtr1; Fig. 2D–F, S5D, F) and GABAergic neurons (for the majority of Hcrtr2; Fig. 2G–I, S5E, F), suggesting separation of cellular function in the stress circuits of the BLA that are dependent on learning. Simply put, the data suggest that in Escape mice as Orx1R mRNA in BLA (mostly pyramidal neurons) increases, freezing also increases. Additionally, the data suggest that as Orx2R mRNA increases in Stay BLA (mostly GABA neurons), freezing also decreases. Pyramidal Orx1R-containing neurons in the BLA are located in a larger pro-stress circuitry, and activate anxiogenic and pro-depressive behaviors and conditioned fear learning (Kim et al., 2016; Yaeger et al., 2022). This circuitry is also innervated by noradrenergic neurons of the LC, which are modulated by Orx1R and mediate cue-dependent fear memories (Soya and Sakurai, 2020b; Soya et al., 2013, 2017). In the BLA, Orx2R-containing GABA neurons inhibit the pro-stress circuitry, but also result in anxiolytic and anti-depressive behaviors and reduce conditioned-fear learning (Staton et al., 2018; Yaeger et al., 2020, 2022). The data suggest that learned responses are not only tuned to the Orx receptor type and particular neurocircuitry element in which they exist, but also reflected by the gene expression changes that occur over the 4 days of training. Surprisingly, the coping strategies learned in the SAM are transferred to some behavioral tests for anxiety, such as the open field (OF; Figs. 3–5), but not to others, such as the elevated plus maze (EPM; Fig. S8).
We have previously noted that Escape and Stay behavior both require learning social behavioral patterns and associative cues, to efficiently minimize vulnerability from attack while using the escape hole or remaining in the SAM arena (Carpenter and Summers, 2009; Summers et al., 2017). Escaping mice utilize one of the two tunnels for egress with progressively reduced latency, while Stay mice display socially induced freezing with progressively increased duration (Fig. 2A). This suggests that both Stay and Escape animals utilize coping strategies that include learning how to minimize vulnerability from aggression more efficiently with each trial. This means it is necessary to monitor the patrolling patterns of the dominant aggressive male, to avoid those spaces while freezing (for Stay animals), or to develop ballistic or secretive escape movements to safely accomplish Escape. As such, our model demonstrates more than one learned and adaptive reaction is possible in response to an unconditioned fearful stimulus in a fear conditioning paradigm. Social defeat is replete with contextually rich stimuli, including the elements of social rank dynamics, which in natural settings allows for more than one appropriate behavioral response (Blanchard et al., 1995; Blanchard and Blanchard, 1989; Pellman and Kim, 2016; Pellman et al., 2017; Robertson et al., 2015). Other labs have demonstrated that Orx activation during social defeat reduces both social interaction and recognition learning in defeated mice (Eacret et al., 2019), modifying appropriate behavioral responses. Thus, the generalization or transference of responses (freezing, locomotion, center preference in Stay vs. edge preference in Escape mice) from the SAM to the OF arena, suggests behavioral plasticity in coping strategies is linked to specific stress phenotypes. The only thing similar between the SAM and OF arenas is that they are essentially open, and as such, we do not believe that the generalization of behavioral attributes transferred from SAM to OF are due to similarities of environment. The shape (oval vs square), contours (elongate vs equidistant), area (1.44 m2 vs 1.6 m2), and structural materials (smooth Plexiglas and PVC vs textured plastic) are all different between these behavioral testing environments.
Regardless of the eventual phenotype, Escape and Stay mice must learn the patrolling routines of the novel, larger, aggressive (CD1) mice. These dominant mice patrol the edges of the SAM, because it is ecologically safer, but also because they can easily block escape this way, since the tunnels are located on the apical edge. If the open field were presented prior to SAM social interactions, we suspect that, as has been found in numerous other experiments, that more anxious mice would adhere to the periphery. Those anxious mice however, when placed in the SAM social arena, would find that the large dominant CD1 mouse would patrol the edge spaces where they previously preferred (in OF) to frequent. This larger aggressive mouse is a very persuasive motivational force, and we believe that these anxious mice would be driven to frequent the center of the SAM arena. We do not believe that anxious mice would escape more, because they would not frequent the sites of the escape tunnels.
Stay mice are understood to be stress-vulnerable, because Stay behavior can be converted to Escape by means of anxiolytic drugs (antalarmin, diazepam, YNT185, [Ala11, d-Leu15]–OrxB, and SB-674042), and Stay mice have the highest corticosterone concentrations, as well as demonstrate reduced social interaction (Eacret et al., 2019) in the SIP test (Staton et al., 2018; Summers et al., 2020). Not surprisingly, susceptible Stay mice learn to frequent the center of the SAM arena, to avoid the aggressor. After 4 days of SAM training, these anxious, stress-vulnerable Stay mice also frequent the center of the OF arena, which was initially surprising. Conversely, Escape mice are proposed to be stress-resilient, because anxiogenic drugs (yohimbine, MK-1064 and/or OrxA) limit escape, while anxiolytic treatment (exercise, YNT185, antalarmin) makes Escape faster (Smith et al., 2016; Yaeger et al., 2020, 2022). They have only moderately elevated corticosterone (less than Stay) and they exhibit enhanced social interaction in the SIP test. Escape mice seek egress by following the edge of the arena to the tunnel, and thus in the OF, these stress-resilient (Escape) mice also keep close to the edges. Thus, stress-susceptible and -resilient mice exhibit surprising open-vs-edge behavior in the OF, when it follows the SAM. Finally, although results of testing prior to the SAM do not predict Escape or Stay behavior in the SAM (Smith et al., 2014), the duration of socially induced freezing in the OF is correlated with prior freezing in the SAM, reflecting the learning that occurred there (Fig. 3C).
The behavioral transference or generalization learning observed here likely results from distinctive Orx1R or Orx2R signaling within decision-making and anxiety/fear neurocircuitries that are inextricably tied to learning systems (Soya and Sakurai, 2020b; Soya et al., 2013, 2017; Yaeger et al., 2022). As generalization of trauma to other contexts is a critical component of PTSD (Kaczkurkin et al., 2017; Thome et al., 2018), it may be that Orx receptors play an important role. When decision-making coincides with stress, recruitment of neural networks that define executive function, including the dorsolateral prefrontal, anterior cingulate, and orbitofrontal cortices, utilize connections with the emotion processing system of the amygdala (De Visser et al., 2011a, 2011b), which can be modified by other brain regions, such as the LC (Soya and Sakurai, 2020b; Soya et al., 2013, 2017). Likewise, learning events prompted by fear are mediated through potentially distinctive circuits involving hippocampus, lateral hypothalamus, LC, and amygdala (Soya and Sakurai, 2020b; Soya et al., 2013, 2017). In this way, the gating of stress-induced learning behavior, like those associated with transference and generalization, requires amygdalar engagement, also a likely contributor to PTSD fear memory mechanisms (Debiec et al., 2011; Debiec and LeDoux, 2006).
Importantly, we demonstrate transference or generalization is strongly modified by Orx receptor actions in the BLA. Antagonism of Orx receptors in the BLA impacts spatial memory, specifically during the consolidation/re-consolidation phase (Ardeshiri et al., 2019); however, we report a caveat, that Orx influence over BLA-gated learning events may depend on the anxious state of the individual. For example, in stress-susceptible and anxious Stay mice (Smith et al., 2016), vehicle-treated individuals trend toward time in the center of the SAM due to the aggressive CD1 patrolling the edges; though this trend is not statistically significant. Following intra-BLA Orx1R antagonism there is statistical evidence for increased time in the center of the SAM arena, suggesting that this treatment promotes learning of habitual routines of the CD1 aggressor (patrolling the edges), and adapting behaviorally to accommodate them and take advantage of movement toward the center (Fig. 5A, B, C, D). These Orx1R reside predominantly in glutamatergic pyramidal cells of the BLA (Fig. 2A–F, S5F). Interestingly, while Orx2R inhibition promotes activity in the center of the OF, and is suggestive of Stay learning of center aggression avoidance in the SAM (Fig. 5A, B, E), typically BLA Orx1R and Orx2R inhibition have opposite effects on anxious behaviors in the SAM (Yaeger et al., 2022).
Stress-induced generalization learning requires integration of anxiety elements of neurocircuitry (Asok et al., 2019; Dunsmoor and Paz, 2015), which are also involved in generating PTSD (Bennett et al., 2016; Sabban et al., 2018; Zhu et al., 2017). Stress-susceptible (Stay) mice exhibit enhanced socially induced freezing behavior in the SAM (Fig. 3A), which is carried over (generalized or possibly over-generalized) to the non-social OF Test arena (Fig. 3C). Uniquely, our behavioral design incorporates conditioning over four days to a naturalistic fear in the form of social aggression (US+). Our model captures fear generalization when mice are introduced to a new testing context (i. e. OF Test), where the absence of the unconditioned stimulus (social aggressor) should be more immediately distinguishable than the exclusion of a shock, as in less ethologically relevant stress paradigms. While it is true that timing and layout of experimental design may modify the intensity of the generalized behavior (Huckleberry et al., 2016), the transferred fear response observed in our study is tied to additional learned coping strategies (i.e. time in center and locomotion) when mice are moved from the SAM to the OF Test. Thus, we posit that stress-induced generalization learning requires integration of both learning and anxiety elements of neurocircuitry (Asok et al., 2019; Dunsmoor and Paz, 2015), similar to the circuits involved in PTSD (Bennett et al., 2016; Sabban et al., 2018; Zhu et al., 2017).
Like learning how to move (Fig. 4) and identifying safe areas in the social context of the SAM (Fig. 5), generalization of freezing behavior is influenced by Orx receptor activity in the BLA (Fig. 3). Activity from distinct neuronal populations within the lateral amygdala (LA) support the expression of generalized fear (Ghosh and Chattarji, 2015), and likely contribute to the observed transference of freezing behavior reported here. While a relationship of the Orx system and contextual fear response has been identified through indirect noradrenergic connections to the LA from the LC (Soya et al., 2017), we provide evidence for a more direct influence of Orx in the amygdala on fear generalization. In support of the relationship revealed between Orx2R expression in the BLA and socially induced freezing in the SAM (Fig. 2C), Stay mice display enhanced freezing behavior in both the SAM and OF Test environments after Orx2R antagonism, and reduced freezing with Orx2R stimulation (Fig. 3A and B). Curiously, OrxA treatment, which activates both Orx1R and Orx2R, elevated SAM freezing behavior in both Escape and Stay mice (Fig. 3A). As Orx1R are expressed at higher levels in the BLA compared to Orx2R (Fig. 2D), it is sensible that OrxA treatment would disproportionally activate Orx1R over Orx2R. Further, although OF freezing is roughly equivalent in vehicle-treated Escape and Stay mice, Orx1R antagonism reduced freezing in the OF Test in Escape animals (Fig. 3B), perhaps due to this drug’s effect on Hcrtr1 and Hcrtr2 balance in BLA, though it had little effect on socially induced freezing in the SAM, beyond eliminating the phenotypic difference (Fig. 3A). Biased activation of Orx1R enhanced freezing in both SAM and OF Test contexts in Stay animals (Fig. 3A and B). While generalization of freezing behavior was apparent in vehicle-treated Stay mice (Fig. 3C), manipulation of intra-BLA Orx receptor activity disrupted this behavior. What is clear, however, is that Orx receptors in the BLA mitigate freezing behavior as learned in a social environment and carried over to a non-social context (Fig. 3), and these receptors appear to do so in a phenotype-dependent way (Fig. 2B and C).
The EPM results testing for anxiety relationships in vehicle-treated animals do not show socially induced phenotypic separation (Fig. S8). This was surprising at first, because both SAM and SIP results suggest a strong correlation between Escape and resilience, as well as Stay animals having high stress vulnerability. In the EPM, both Escape and Stay mice spend most of their time in the closed arms, with significant excursions into the open arms, which were not affected by either Orx1R or Orx2R antagonists (Figs. S8A and B), an observation consistent with previous studies (Rodgers et al., 2013; Staples and Cornish, 2014). Similarly, animals tested on the EPM before SAM trials, where Escape and Stay phenotypes develop, also do not exhibit differences in open or closed arm times (Smith et al., 2014). It may simply be that social and environmental stressors provide radically dissimilar results. However, with application of Orx1R stimulation, phenotypic differences are again revealed (Figs. S8A, B, C). In the OF, stimulation of Orx1R (OrxA and Orx1R stimulation) prompted Stay animals to spend more time in the open (and less in the closed) arms (Figs. S8A and B). Again, the results seem to have been modified by previous experience in the SAM, which calls into question the value of both the OF and EPM tests. If the results of the tests can be dramatically skewed, or reversed, by previous experience in the SAM, they may also be slanted by additional, perhaps not obvious, environmental or social stresses in other experimental paradigms, or by other life experiences before experimentation. However, several classical tests for anxiety and/or depression (such as Elevated Plus Maze [EPM] or Open Field [OF] Test) in rodents have failed to faithfully translate to successful clinical trials (Haller and Alicki, 2012; Haller et al., 2013). We suggest that more efficacious models, should carefully include ecologically and ethologically designed applications (Blanchard et al., 2013; Robertson et al., 2015) that specifically consider learning in the production of behavioral outcomes and decision-making (Smith et al., 2014). The clinical translatability of these, and other, commonly used tests has previously been called into question (Blanchard et al., 2013; Haller and Alicki, 2012; Haller et al., 2013), and our results add reason to question their validity. We urge caution for all those planning to use EPM or OF, and to consider alternative tests/-models (such as Social Interaction/Preference test (SIP), Sucrose Preference test, Novelty-induced Hypophagia (NIH), Light/Dark Conditioned Place Preference, Fear Conditioning (Social Fear Conditioning being most relevant, and the SAM).
5. Conclusions
The Orx system interacts with BLA neurons to regulate fear learning and generalization during social stress, and we suspect that it is playing an important similar role in PTSD. Additionally, neurons that synthesize Orx1R and Orx2R in the BLA are mostly distinct. While Orx1R are located primarily in glutamatergic neurons, a smaller majority of Orx2R are found in GABAergic interneurons. Although learning strategies are influenced by anxious state and behavioral phenotype, our results suggest that within the BLA, Orx receptors modulate learning outcomes and generalization, while concomitantly modifying stress-related behavior. The intra-BLA Orx receptors bidirectionally balance these learning states with Orx1R inhibition and, alternatively, Orx2R stimulation contributing to behavioral transference and a reduction in fear-reactivity. While orexin’s effect over learning extends beyond the BLA, including targets like the LC and hippocampus, we demonstrate an important role for intra-BLA Orx receptors to influence learning in a receptor- and anxious state-dependent manner.
Supplementary Material
Acknowledgments
We would like to thank Gregory D. Yaeger for providing the artwork used in Figs. 1, 3, & S11. We also thank R. Parrish Waters and Wayne J. Korzan for comments on the manuscript. In addition, we would like to acknowledge Kelly Graber for her help with confocal image acquisition. We also recognize Trent L. Greschke for his assistance in developing the pie chart used in Fig. S5. Further, we acknowledge and commend efforts in the scientific community that stand up against discrimination and social injustices. Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health, USA, under Award Numbers R15 MH125306 and R15 MH104485, through support (for JDWY, KTK) by the National Science Foundation (NSF) Research Training Program, USD-N3 Grant DGE-1633213, by a USD Center for Brain and Behavior Research (CBBRe) pilot grant, the Nolop Endowment via the USDFoundation, and the Sanford Histology and Imaging Core which is supported by the Center for Cancer Biology Research COBRE (NIGMS COBRE P20GM103548). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, NSF, the Department of Veterans Affairs or the United States Government.
Abbreviations
- acsf
artificial cerebrospinal fluid
- [Ala11, d-Leu15]–OrxB
a modified OrxB, peptide used as an Orx2 receptor agonist
- AP
anterior-posterior
- BDNF
brain-derived neurotrophic factor
- Bdnf
brain-derived neurotrophic factor gene
- BLA
basolateral amygdala
- C57BL/6NHsd
a strain of black mice used for stress testing
- CAMKIIα
calcium-calmodulin kinase two-alpha
- CamkIIα
Ca++/Calmodulin Kinase type 2 alpha gene
- CD1 Hsd
ICR retired breeder mice used as aggressors
- CeA
central amygdala
- CR
conditioned response
- CRF1
corticotropin releasing factor 1 receptors
- CS
conditioned stimulus
- DMSO
dimethylsulfoxide;
- DV
dorsal-ventral
- EC50
half-maximal effective concentration
- ERK1
extracellular signal-regulated kinase 1 also mitogen-activated protein kinase 3
- EPM
elevated plus maze, Escape, mice that respond to social stress by leaving
- g
gram(s)
- ga
gauge
- GABA or GABAergic
γ-aminobutyric acid
- GAD1
glutamate decarboxylase 1
- Gad1
glutamate decarboxylase 1 gene
- GPCR
G-protein-coupled receptor
- Gq
G-protein associated with the phospholipase C 2nd messenger system
- Hcrt
orexin/hypocretin
- Hcrt1
orexin A/hypocretin 1
- Hcrt2
orexin B/hypocretin 2
- Hcrtr1
orexin 1 receptor gene
- Hcrtr2
orexin 2 receptor gene
- IC50
half-maximal inhibitory concentration
- icv
intracerebroventricular
- ItC
intercalated region of the amygdala
- L
liter
- LH-DMH/PeF
the perifornical area of the lateral, dorsomedial hypothalamus
- Mapk3
extracellular signal-regulated kinase 1 gene
- μL
microliter
- mg/kg
milligrams per kilogram
- min
minute(s)
- MK-1064
5′′-chloro-N-[(5,6-dimethoxy-2-pyridinyl)methyl] [2,2’:5′,3′′-terpyridine]-3′-carboxamide - an Orx2 antagonist
- ML
medial-lateral
- mm
millimeter
- NIH
National Institutes of Health
- NIMH
National Institute of Mental Health
- OF
open field test
- Orx
orexin/hypocretin
- OrxA
orexin A/hypocretin 1
- OrxB
orexin B/hypocretin 2
- Orx1R
orexin 1 receptors
- Orx2R
orexin 2 receptors
- Pvalb
parvalbumin gene
- PTSD
post-traumatic stress disorder
- s
seconds
- SAM
Stress Alternatives Model
- SB-674042
orexin 1 receptor [Orx1R] antagonist
- SIP
social interaction/preference test
- Stay
socially defeated submissive mice;
- US
unconditioned stimulus
- YNT-185
an Orx2R agonist
Chemical compounds studied in this article:
- MK-1064
-
5″-chloro-N-[(5,6-Dimethoxy-2-pyridinyl) methyl]-[2,2’:5′,3″-terpyridine]-3′-carboxamide
An Orx2 antagonist
- Orexin A
-
PYR-PRO-LEU-PRO-ASP-CYS-CYS-ARG-GLN-LYS-THR-CYS-SER-CYS-ARG-LEU-TYR-GLU-LEU-LEU–HIS–GLY-ALA-GLY-ASN–HIS–ALA-ALA-GLY-ILE-LEU-THR-LEU-NH2
An Orx1R plus Orx2R agonist
- OrxA & MK-1064
-
Targeted Orx1R stimulation
SB-674042 [5-(2-Fluorophenyl)-2-methyl-4-thiazolyl][2(S)-2-[(5-phenyl-1,3,4-oxadiazol-2-yl)methyl-1-pyrrolidinyl]methanone
An orexin 1 receptor [Orx1R] antagonist
- YNT-185
-
3’-[[[3-[[2-[[2-(Dimethylamino)benzoyl]amino]ethyl]amino]phenyl]amino]sulfonyl]-4′-methoxy-N,N-dimethyl-[1,1′-biphenyl]-3-carboxamide dihydrochloride
An Orx2
Footnotes
CRediT authorship contribution statement
Jazmine D.W. Yaeger: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Visualization. Kevin T. Krupp: Validation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Visualization. Tangi R. Summers: Conceptualization, Validation, Formal analysis, Writing – original draft, Writing – review & editing, Visualization. Cliff H. Summers: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition, All authors approved the final version for publication.
Declaration of competing interest
The authors have nothing to disclose.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuropharm.2022.109168.
References
- Allen MT, Myers CE, Beck KD, Pang KCH, Servatius RJ, 2019. Inhibited personality temperaments translated through enhanced avoidance and associative learning increase vulnerability for PTSD. Front. Psychol 10, 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammoun, S, Holmqvist T, Shariatmadari R, Oonk HB, Detheux M, Parmentier M, Åkerman KEO, Kukkonen JP, 2003. Distinct recognition of OX1 and OX2 receptors by orexin peptides. J. Pharmacol. Exp. Therapeut 305, 507. [DOI] [PubMed] [Google Scholar]
- Ardeshiri MR, Hosseinmardi N, Akbari E, 2019. The basolateral amygdala orexin 1 and 2 receptors’ involvement in modulating spatial reference memory. Brain Res 1704, 16–25. [DOI] [PubMed] [Google Scholar]
- Arendt DH, Hassell J, Li H, Achua JK, Guarnieri DJ, DiLeone RJ, Ronan PJ, Summers CH, 2014. Anxiolytic function of the orexin 2/hypocretin A receptor in the basolateral amygdala. Psychoneuroendocrinology 40, 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt DH, Ronan PJ, Oliver KD, Callahan LB, Summers TR, Summers CH, 2013. Depressive behavior and activation of the orexin/hypocretin system. Behav. Neurosci 127, 86–94. [DOI] [PubMed] [Google Scholar]
- Armony JL, Servan-Schreiber D, Cohen JD, LeDoux JE, 1995. An anatomically constrained neural network model of fear conditioning. Behav. Neurosci 109, 246. [DOI] [PubMed] [Google Scholar]
- Armony JL, Servan-Schreiber D, Romanski LM, Cohen JD, LeDoux JE, 1997. Stimulus generalization of fear responses: effects of auditory cortex lesions in a computational model and in rats. Cerebr. Cortex 7, 157–165. New York, NY: 1991. [DOI] [PubMed] [Google Scholar]
- Asok A, Kandel ER, Rayman JB, 2019. The neurobiology of fear generalization. Front. Behav. Neurosci 12, 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker HM, Barry TJ, Kumari V, Pandey R, Shanta N, Lau JY, 2019. Problematic attention processing and fear learning in adolescent anxiety: testing a combined cognitive and learning processes model. J. Behav. Ther. Exp. Psychiatr 62, 146–153. [DOI] [PubMed] [Google Scholar]
- Baldi E, Lorenzini CA, Bucherelli C, 2004. Footshock intensity and generalization in contextual and auditory-cued fear conditioning in the rat. Neurobiol. Learn. Mem 81, 162–166. [DOI] [PubMed] [Google Scholar]
- Bennett MR, Hatton SN, Lagopoulos J, 2016. Stress, trauma and PTSD: translational insights into the core synaptic circuitry and its modulation. Brain Struct. Funct 221, 2401–2426. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR, 1995. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology 20, 117–134. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Summers CH, Blanchard RJ, 2013. The role of behavior in translational models for psychopathology: functionality and dysfunctional behaviors. Neurosci. Biobehav. Rev 37, 1567–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, 1989. Antipredator defensive behaviors in a visible burrow system. J. Comp. Psychol 103, 70–82. [DOI] [PubMed] [Google Scholar]
- Broberger C, De Lecea L, Sutcliffe J, Hökfelt T, 1998. Hypocretin/orexin-and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene-related protein systems. J. Comp. Neurol 402, 460–474. [PubMed] [Google Scholar]
- Carpenter RE, Summers CH, 2009. Learning strategies during fear conditioning. Neurobiol. Learn. Mem 91, 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Visser L, Baars A, Lavrijsen M, Van der Weerd C, Van Den Bos R, 2011a. Decision-making performance is related to levels of anxiety and differential recruitment of frontostriatal areas in male rats. Neuroscience 184, 97–106. [DOI] [PubMed] [Google Scholar]
- De Visser L, Baars A, van’t Klooster J, van den Bos R, 2011b. Transient inactivation of the medial prefrontal cortex affects both anxiety and decision-making in male wistar rats. Front. Neurosci 5, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, Bush DE, LeDoux JE, 2011. Noradrenergic enhancement of reconsolidation in the amygdala impairs extinction of conditioned fear in rats–a possible mechanism for the persistence of traumatic memories in PTSD. Depress. Anxiety 28, 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE, 2006. Noradrenergic signaling in the amygdala contributes to the reconsolidation of fear memory: treatment implications for PTSD. Ann. N. Y. Acad. Sci 1071, 521–524. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Paz R, 2015. Fear generalization and anxiety: behavioral and neural mechanisms. Biol. Psychiatr 78, 336–343. [DOI] [PubMed] [Google Scholar]
- Eacret D, Grafe LA, Dobkin J, Gotter AL, Rengerb JJ, Winrow CJ, Bhatnagar S, 2019. Orexin signaling during social defeat stress influences subsequent social interaction behaviour and recognition memory. Behav. Brain Res 356, 444–452. 10.1016/j.bbr.2018.05.032. [DOI] [PubMed] [Google Scholar]
- Feise RJ, 2002. Do multiple outcome measures require p-value adjustment? BMC Med. Res. Methodol 2, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Chattarji S, 2015. Neuronal encoding of the switch from specific to generalized fear. Nat. Neurosci 18, 112–120. [DOI] [PubMed] [Google Scholar]
- Haller J, Alicki M, 2012. Current animal models of anxiety, anxiety disorders, and anxiolytic drugs. Curr. Opin. Psychiatr 25, 59–64. [DOI] [PubMed] [Google Scholar]
- Haller J, Aliczki M, Gyimesine Pelczer K, 2013. Classical and novel approaches to the preclinical testing of anxiolytics: a critical evaluation. Neurosci. Biobehav. Rev 37, 2318–2330. [DOI] [PubMed] [Google Scholar]
- Huckleberry KA, Ferguson LB, Drew MR, 2016. Behavioral mechanisms of context fear generalization in mice. Learn. Mem 23, 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennions MD, Moller AP, 2003. A survey of the statistical power of research in behavioral ecology and animal behavior. Behav. Ecol 14, 438–445. [Google Scholar]
- Kaczkurkin AN, Burton PC, Chazin SM, Manbeck AB, Espensen-Sturges T, Cooper SE, Sponheim SR, Lissek S, 2017. Neural substrates of overgeneralized conditioned fear in PTSD. Am. J. Psychiatr 174, 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Pignatelli M, Xu S, Itohara S, Tonegawa S, 2016. Antagonistic negative and positive neurons of the basolateral amygdala. Nat. Neurosci 19, 1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, van Meurs B, 2015. Learning models of PTSD: theoretical accounts and psychobiological evidence. Int. J. Psychophysiol 98, 594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Meffre J, Sicre M, Diarra M, Marchessaux F, Paleressompoulle D, Ambroggi F, 2019. Orexin in the posterior paraventricular thalamus mediates hunger-related signals in the nucleus accumbens core. Curr. Biol 29, 3298–3306 e3294. [DOI] [PubMed] [Google Scholar]
- Moran MD, 2003. Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos 100, 403–405. [Google Scholar]
- Nakagawa S, 2004. A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav. Ecol 15, 1044–1045. [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K, 1999. Distribution of orexin neurons in the adult rat brain. Brain Res 827, 243–260. [DOI] [PubMed] [Google Scholar]
- Pellman BA, Kim JJ, 2016. What can ethobehavioral studies tell us about the brain’s fear system? Trends Neurosci 39, 420–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellman BA, Schuessler BP, Tellakat M, Kim JJ, 2017. Sexually dimorphic risk mitigation strategies in rats. eNeuro 4, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perneger TV, 1998. What’s wrong with Bonferroni adjustments. BMJ 316, 1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, Belzung C, 2003. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur. J. Pharmacol 463, 3–33. [DOI] [PubMed] [Google Scholar]
- Robertson JM, Prince MA, Achua JK, Carpenter RE, Arendt DH, Smith JP, Summers TL, Summers TR, Summers CH, 2015. Nuance and behavioral cogency: how the visible burrow system inspired the stress-alternatives model and conceptualization of the continuum of anxiety. Physiol. Behav 146, 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Wright FL, Snow NF, Taylor LJ, 2013. Orexin-1 receptor antagonism fails to reduce anxiety-like behaviour in either plus-maze-naïve or plus-maze-experienced mice. Behav. Brain Res 243, 213–219. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, 1990. No adjustments are needed for multiple comparisons. Epidemiology 1, 43–46. [PubMed] [Google Scholar]
- Sabban EL, Serova LI, Newman E, Aisenberg N, Akirav I, 2018. Changes in gene expression in the locus coeruleus-amygdala circuitry in inhibitory avoidance PTSD model. Cell. Mol. Neurobiol 38, 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JP, Achua JK, Summers TR, Ronan PJ, Summers CH, 2014. Neuropeptide S and BDNF gene expression in the amygdala are influenced by social decision-making under stress. Front. Behav. Neurosci 8, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JP, Prince MA, Achua JK, Robertson JM, Anderson RT, Ronan PJ, Summers CH, 2016. Intensity of anxiety is modified via complex integrative stress circuitries. Psychoneuroendocrinology 63, 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soya S, Sakurai T, 2020a. Evolution of orexin neuropeptide system: structure and function. Front. Neurosci 14, 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soya S, Sakurai T, 2020b. Orexin as a modulator of fear-related behavior: hypothalamic control of noradrenaline circuit. Brain Res 1731, 146037. [DOI] [PubMed] [Google Scholar]
- Soya S, Shoji H, Hasegawa E, Hondo M, Miyakawa T, Yanagisawa M, Mieda M, Sakurai T, 2013. Orexin receptor-1 in the locus coeruleus plays an important role in cue-dependent fear memory consolidation. J. Neurosci 33, 14549–14557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soya S, Takahashi TM, McHugh TJ, Maejima T, Herlitze S, Abe M, Sakimura K, Sakurai T, 2017. Orexin modulates behavioral fear expression through the locus coeruleus. Nat. Commun 8, 1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples LG, Cornish JL, 2014. The orexin-1 receptor antagonist SB-334867 attenuates anxiety in rats exposed to cat odor but not the elevated plus maze: an investigation of Trial 1 and Trial 2 effects. Horm. Behav 65, 294–300. [DOI] [PubMed] [Google Scholar]
- Staton CD, Yaeger JDW, Khalid D, Haroun F, Fernandez BS, Fernandez JS, Summers BK, Summers TR, Sathyanesan M, Newton SS, Summers CH, 2018. Orexin 2 receptor stimulation enhances resilience, while orexin 2 inhibition promotes susceptibility, to social stress, anxiety and depression. Neuropharmacology 143, 79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers CH, Yaeger JDW, Staton CD, Arendt DH, Summers TR, 2020. Orexin/hypocretin receptor modulation of anxiolytic and antidepressive responses during social stress and decision-making: potential for therapy. Brain Res 1731, 146085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers TR, Summers TL, Carpenter RE, Smith JP, Young SL, Meyerink B, Orr TZ, Arendt DH, Summers CH, 2017. Learning and CRF-induced indecision during escape and submission in rainbow trout during socially aggressive interactions in the stress-alternatives model. Front. Neurosci 11, 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Beuckmann CT, Shikata K, Ogura H, Sawai T, 2005. Orexin-A (hypocretin-1) is possibly involved in generation of anxiety-like behavior. Brain Res 1044, 116–121. [DOI] [PubMed] [Google Scholar]
- Thome J, Hauschild S, Koppe G, Liebke L, Rausch S, Herzog JI, Muller-Engelmann M, Steil R, Priebe K, Hermans D, Schmahl C, Bohus M, Lis S, 2018. Generalisation of fear in PTSD related to prolonged childhood maltreatment: an experimental study. Psychol. Med 48, 2223–2234. [DOI] [PubMed] [Google Scholar]
- van der Kolk BA, 2006. Clinical implications of neuroscience research in PTSD. Ann. N. Y. Acad. Sci 1071, 277–293. [DOI] [PubMed] [Google Scholar]
- Veazie PJ, 2006. When to combine hypotheses and adjust for multiple tests. Health Serv. Res 41, 804–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA, 2007. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc 2, 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaeger JDW, Krupp KT, Gale JJ, Summers CH, 2020. Counterbalanced microcircuits for Orx1 and Orx2 regulation of stress reactivity. Medicine in Drug Discovery, 100059, 1–20. [Google Scholar]
- Yaeger JDW, Krupp KT, Jacobs BM, Onserio BO, Meyerink BL, Cain JT, Ronan PJ, Renner KJ, DiLeone RJ, Summers CH, 2022. Orexin 1 receptor antagonism in the basolateral amygdala shifts the balance from pro- to antistress signaling and behavior. Biol. Psychiatr 91, 841–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J, 2007. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron 56, 19–32. [DOI] [PubMed] [Google Scholar]
- Zhu X, Helpman L, Papini S, Schneier F, Markowitz JC, Van Meter PE, Lindquist MA, Wager TD, Neria Y, 2017. Altered resting state functional connectivity of fear and reward circuitry in comorbid PTSD and major depression. Depress. Anxiety 34, 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


