Abstract
Regulation of emotions is generally associated exclusively with the brain. However, there is evidence that peripheral systems are also involved in mood, stress vulnerability vs. resilience, and emotion‐related memory encoding. Prevalence of stress and mood disorders such as major depression, bipolar disorder, and post‐traumatic stress disorder is increasing in our modern societies. Unfortunately, 30%–50% of individuals respond poorly to currently available treatments highlighting the need to further investigate emotion‐related biology to gain mechanistic insights that could lead to innovative therapies. Here, we provide an overview of inflammation‐related mechanisms involved in mood regulation and stress responses discovered using animal models. If clinical studies are available, we discuss translational value of these findings including limitations. Neuroimmune mechanisms of depression and maladaptive stress responses have been receiving increasing attention, and thus, the first part is centered on inflammation and dysregulation of brain and circulating cytokines in stress and mood disorders. Next, recent studies supporting a role for inflammation‐driven leakiness of the blood–brain and gut barriers in emotion regulation and mood are highlighted. Stress‐induced exacerbated inflammation fragilizes these barriers which become hyperpermeable through loss of integrity and altered biology. At the gut level, this could be associated with dysbiosis, an imbalance in microbial communities, and alteration of the gut–brain axis which is central to production of mood‐related neurotransmitter serotonin. Novel therapeutic approaches such as anti‐inflammatory drugs, the fast‐acting antidepressant ketamine, and probiotics could directly act on the mechanisms described here improving mood disorder‐associated symptomatology. Discovery of biomarkers has been a challenging quest in psychiatry, and we end by listing promising targets worth further investigation.
Keywords: bipolar, blood‐brain barrier, cytokine, depression, emotion, microbiome, PTSD
Inflammation‐driven brain and gut barrier dysfunction in stress and mood disorders. Mood disorders, such as post‐traumatic stress disorder (PTSD), major depressive disorder (MDD), and bipolar disorder (BD), are associated with high rates of treatment resistance and relapse. Increasing evidence links blood–brain barrier (BBB) and gut barrier leakiness to negative emotional symptoms reported in mood disorders, possibly through stress‐induced inflammation, although specific biological mechanisms remain to be elucidated. Novel therapeutic approaches targeting BBB and gut barrier permeability could contribute to bridging the gap in treatment response.
1. INTRODUCTION
1.1. Current state of stress and mood disorders: Prevalence and treatment
Stress is a psychological state that people experience in their lifetime. It can be good in some situations to give us a needed boost or motivation; however, it can become a chronic condition in absence of coping strategies and may compromise physical as well as mental health. Mood disorders, including major depressive disorder (MDD) and bipolar disorder (BD), and stress disorders, such as post‐traumatic stress disorder (PTSD), are common around the world (see Table 1). Comorbidity between these disorders is frequently observed, as this is the case for PTSD and MDD with a 50% comorbidity rate throughout the lifetime (Elhai et al., 2008; Kessler et al., 1995, 2005).
TABLE 1.
Prevalence and symptomatology of stress and mood disorders
| Disorder | Prevalence | Male/female | Symptomatology | Treatment resistance |
|---|---|---|---|---|
| Anxiety disorders |
Global: 18.1% (Kessler et al., 2005) Canada: 11.6% (Statistics Canada, 2020) United States: 19.1% (Substance Abuse and Mental Health Services Administration, 2019) Europe: 7% (WHO, 2012) |
Female > Male ≈ 2:1 |
Restlessness Irritability Sleep problems Difficulty concentrating Fatigue |
|
| Major depressive disorder (MDD) |
Global: 6.7% (Kessler et al., 2005) Canada: 3.9% (Patten et al., 2015) United States: 7.1% (Substance Abuse and Mental Health Services Administration, 2019) Europe: 7% (WHO, 2012) |
Female > Male ≈ 2:1 |
Decreased energy, fatigue Difficulty concentrating Weight fluctuations Gastrointestinal problems Anhedonia Sadness, anxiety Helplessness Thoughts of death or suicide |
~50% do not respond to currently available antidepressant treatments (Akil et al., 2018). >1/3 of patients are resistant to conventional pharmacologic, psychologic, or somatic treatments (Akil et al., 2018; Dudek et al., 2019). |
| Post‐traumatic stress disorder (PTSD) |
Lifetime: 3.5% (Kessler et al., 2005) Canada: 9.2% a (Van Ameringen et al., 2008) United States: 3.5% (Kessler et al., 2005) Europe: 1.1% (Darves‐Bornoz et al., 2008) |
Female > Male ≈ 2:1 |
Intrusive or recurring memories of the trauma Avoidance behaviors (thoughts, feelings, memories) Negative thinking Anhedonia Irritability Reckless behavior Hypervigilance Decreased concentration Sleep problems |
27.5% of UK veterans do not respond to treatments (Murphy & Smith, 2018). ~20%–40% achieve complete remission with antidepressants and psychotherapy (Madison & Eitan, 2020). |
| Bipolar disorder (BD) |
Lifetime: 2.6% (Kessler et al., 2005) Canada: 0.87% (McDonald et al., 2015) United States: 2.6% (Kessler et al., 2015) Europe: 1% (Pini et al., 2005) |
Female ≈ Male |
Alternating episodes of mania and hypomania Hyperactivity Unrealistically confident Happiness Decreased sleep Reckless behavior |
Treatment resistance is common in BD (Gitlin, 2006) No consensus on specific rates (Hidalgo‐Mazzei et al., 2019) |
Lifetime prevalence. Twelve‐month prevalence data are not available.
Women are twice more susceptible than men to be diagnosed with PTSD or MDD, but the prevalence of BD is similar (Bangasser & Valentino, 2014; Tolin & Foa, 2006). Women experience differences in the symptomatology of stress and mood disorders (Rincón‐Cortés et al., 2019), and sexual dimorphism is reported in treatment responses, including commonly prescribed antidepressants (Kornstein et al., 2000). Nevertheless, there is still an important lack of data regarding sex differences for these conditions. Until the early 2000s, sex was generally not considered as a factor that could affect health and illness, and clinical and preclinical studies mainly used male subjects for homogeneity purposes (Dudek et al., 2019). More recently, sex has been increasingly considered a significant experimental variable (Bangasser & Valentino, 2014; Peña et al., 2019; Rubinow & Schmidt, 2019) which could contribute to the adaptation of diagnostics and treatments.
Treatment resistance represents another challenge in existing therapies, and it is a problem commonly observed for MDD, PTSD, and BD patients (see Table 1). For example, in MDD, approximately half of patients do not or poorly respond to currently available antidepressant treatments (Akil et al., 2018). Similar data are reported in BD patients, with the added obstacle of curating treatments that target both aspects of the disorder in order to keep them in the normal range, or euthymic phase (Gitlin, 2006). Furthermore, a lack of reliable biomarkers complicates the diagnosis and treatment of these disorders. Structural magnetic resonance imaging (MRI) and measurements of neurotransmitters or hormone levels, such as catecholamine and cortisol, revealed differences between PTSD patients and healthy individuals, but none of those are defined as a diagnostic tool of this condition (Pitman et al., 2012). Therefore, there is an urgent need for a deeper understanding of the causal biological mechanisms to promote the discovery of biomarkers to improve diagnosis and development of new therapeutic strategies.
1.2. Animal models of stress and mood disorders: Characteristics and limits
Ample choices of animal models of stress and mood disorders are available, developed according to human etiology (Wang et al., 2017; refer to Box 1 and Table 2 for detailed protocols). However, limitations in the validity, translatability, and efficiency restrict their use and interpretation. As chronic stress is the main environmental risk factor for MDD, BD, and PTSD (Blackburn‐Munro & Blackburn‐Munro, 2001; Negrón‐Oyarzo et al., 2016; Rowland & Marwaha, 2018; Yehuda & Seckl, 2011), it is commonly used in animals to induce anxiety‐ and depressive‐like behaviors (for reviews, see Hodes, Kana, et al., 2015; Menard et al., 2017; Verbitsky et al., 2020). Other models, namely, genetic, surgical, or pharmacological approaches, exist, each providing unique benefits and challenges (refer to Valvassori et al., 2013). Here, we chose to focus on stress models, which have increased in popularity due to their relevance for studying major aspects of human disease which limit treatment efficacy, such as sex differences, individual differences, and underlying factors influencing vulnerability vs. resilience to develop a disorder.
BOX 1. Animal models of stress and mood disorders.
Acute physical stress/fear conditioning
Stressors such as electric shock, restraint stress, and water submersion stress are commonly used, which are the same in some of the chronic models mentioned below. By changing the stress exposure parameters to an acute stress (1 day only), with increased intensity and duration, this paradigm becomes useful for modeling PTSD specifically. In this way, it is less likely to show the symptom comorbidity with MDD and BD as seen in other models. These stressors are often paired with fear conditioning, which is based on coupling a conditioned stimulus of a tone with an unconditioned stress (Verbitsky et al., 2020; Young et al., 2018). Animals are later re‐exposed to the conditioned stress allowing evaluation of learning and memory such as fear memory, retrieval and extinction (Maren et al., 2013). This test can be complexified via multiple parameters and can be combined to many stress models, depending on the fear response evaluated.
Chronic social defeat stress (CSDS)
Stressed mice are exposed to a novel aggressor each day for 10 days, enduring physical stress from the dominant CD1. Thereafter, stressed mice are exposed for the rest of the day to a sensory stress by being in the same cage as their aggressor but separated by a clear and perforated divider (Golden et al., 2011). This paradigm leads to two phenotypes of stressed mice: stress‐susceptible, who present social avoidance, and resilient, which interact with a novel CD1 despite the stress paradigm. These subgroups display distinct behavioral changes reminiscent of depressive symptoms in humans (American Psychological Association [APA], 2013) and is a powerful model for investigating interindividual differences. Witnessed social defeat stress is a variation of this model where the target mouse has no actual physical contact with the dominant mouse, rather is exposed to daily inescapable sensory contact with the rodents undergoing physical social defeat (Warren et al., 2013).
Chronic variable stress (CVS)
A repetitive sequence of three stressors, most often tube restraint, tail suspension, and foot shocks and others. Each stressor endures about 1 hr daily and lasts from 6 days to several weeks (LaPlant et al., 2009; Hodes, Pfau, et al., 2015). Afterwards, a battery of tests is run to assess anxiety and depressive‐like behaviors (Hodes, Kana, et al., 2015). In this paradigm, females and males develop depression‐like symptoms at different time points (Hodes, Kana, et al., 2015) making it a strong model for investigating sex differences.
Repeated social defeat (RSD)
In this paradigm, C57BL/6 resident mice are grouped in cohorts and are introduced to an aggressive intruder CD‐1 mouse for 2 hr/day during 6 consecutive days (McKim et al., 2016). Resident mice usually show submissive behavior as the intruder defeats them which induced anxiety‐like behavior such as social avoidance (Wohleb et al., 2014).
Chronic mild stress (CMS)
Animals are submitted to multiple stressors from a few weeks to months, in different frequencies for each stressor (Willner, 1997). Stress such as food or water deprivation, wet environment, new cage partner, changes in the cage temperature, light during the dark phase, or flashlight, is randomly presented to the animals preventing their habituation (Goshen et al., 2008). This paradigm is quite similar to CVS, although it lasts for a longer period of time and does not have a repeated order of stressors.
TABLE 2.
Mood and anxiety disorders associated to animal models
| Animal model | Mood/anxiety disorder | |||
|---|---|---|---|---|
| MDD | PTSD | Anxiety | BD a | |
| Acute stress | ● | ● | ● | |
| CSDS | ● | ● | ● | |
| CVS | ● | ● | ||
| RSD | ● | |||
| CMS | ● | |||
None of the presented models is used for BD since they don't integrate the manic phase.
As in humans, limited data exist from female rodent models of stress and mood disorders. In chronic stress models, some sex differences have been depicted (Audet et al., 2011; Menard et al., 2017; Takahashi et al., 2017); however, barriers in replicating male behaviors are commonly observed in the female paradigms. Furthermore, due to limitations in the translation of animal models, novel clinical characterization strategies are moving away from symptom‐based categories to domains of observable behaviors and neurobiological measures (Richter‐Levin et al., 2019). Symptoms involving emotional and cognitive processing are currently assessed by clinical interview alone and portraying these complex features in rodents poses a serious risk of anthropomorphizing. Researchers try to translate animal behavior into a human emotional point of view, instead of truly being emotions felt by the animal. Nonetheless, affect is an aspect of the emotional process that exists in all animals, corresponding to the response of an animal toward the valence and arousal of a given situation (Bliss‐Moreau, 2017). Methods have been developed to extrapolate many mood‐related behavioral traits (Stuart et al., 2013; for review, see Malkesman et al., 2009). Still, behaviors such as avoidance, anhedonia, and exaggerated startle response overlap with symptoms of PTSD, MDD, BD, or anxiety (Verbitsky et al., 2020), posing a risk of misattribution biases. Recent studies dissected animal behaviors and attempted to translate them to respective behaviors and symptoms in humans (Deslauriers et al., 2018; Scarpa et al., 2020), although an ongoing caveat is that there are no longitudinal studies in animals as we see in humans. BD models also present a unique challenge to encompass the cyclical nature of the disease phases (Covington et al., 2010). Pharmacological, genetic, and environmental models are currently used which induce behaviors of either the mania phase or the depression phase, but not both (Logan & McClung, 2016; Valvassori et al., 2013). Combined efforts for improving quantification of clinical emotional and cognitive measures (Mukherjee et al., 2020) and novel tracking software techniques in animal research (Fung et al., 2019; Mathis & Mathis, 2020; Murphy et al., 2020; Vouros et al., 2018) bring hope for superior translation of rodent findings to human condition in the future.
1.3. Central and peripheral mechanisms involved in emotion regulation
The complexity of emotion management and how it impacts the brain has been investigated for decades but remains a hot topic. Emotional experience, such as stress, can have a negative or positive valence, for example, if it is associated with fear or reward, respectively. Different brain regions are involved in emotion regulation and are affected under stress conditions (McEwen, 2007). Indeed, alterations are observed in the neurocircuitry of these crucial regions implicated in the modulation of emotions and mood, such as the hippocampus (HIPP), prefrontal cortex (PFC), amygdala (AMY), and nucleus accumbens (NAc) in rodents and humans (Jin & Maren, 2015). Although beyond the scope of this review, other brain regions, namely, the locus coeruleus and the anterior cingulate cortex (ACC), are receiving increasing attention for their roles in modulating arousal, cognition and reward‐related memory (for in‐depth reviews, see Rolls, 2019; Schwarz & Luo, 2015). In MDD patients, changes in limbic neural circuits, for example, by over firing in reward circuits or alterations in synaptic plasticity of neurons (Russo & Nestler, 2013), correlate with symptoms such as anhedonia and sadness (Post & Warden, 2018). Stressful threatening events, via the release of stress hormones, directly modulate brain circuits involved in emotional processing and cognitive functions, for instance, formation of memory and reward making decisions (Joëls et al., 2018) as well as sleep function in human subjects (Drake et al., 2014). Stressors activate the hypothalamo–pituitary–adrenal (HPA) axis and the sympathetic nervous system which among other effects exert immunoregulatory influence. Stress can also have direct effects on activation of the central immune system (Jope et al., 2017) and its specialized cells, namely, microglia, which release inflammatory cytokines, factors that target several systems (Leonard, 2018).
Inflammation is also implicated in peripheral maladaptive responses associated with mood disorders. Indeed, increased circulating cytokines are a hallmark of MDD, and treatment‐resistant patients show high levels of pro‐inflammatory cytokines (Hodes, Kana, et al., 2015; Ménard et al., 2017). In this way, psychological stress induces many of the same inflammatory signals linked to injury and disease such as activation of peripheral immune cells, including monocytes, lymphocytes, and mastocytes (Hodes, Kana, et al., 2015; Kempuraj et al., 2017). Dysregulated peripheral and central innate immune responses are shown in MDD, BD and PTSD patients (Bauer & Teixeira, 2019; Hoge et al., 2009; Hung et al., 2014; Ortiz‐Domínguez et al., 2007). Therefore, investigation of brain‐induced activation of inflammatory pathways is crucial in the context of mood and anxiety disorders. Inflammation processes initiated in the periphery can not only signal to the brain regions implicated in emotional processes (Harrison et al., 2009; Thomson et al., 2014), in fact, administration of certain pro‐inflammatory cytokines or endotoxins is sufficient to induce behavioral symptoms observed in depression, anxiety, or PTSD (Dantzer et al., 2008; Zhao et al., 2019). Thus, identification of cytokine profiles generated and correlated to negative emotion‐associated behaviors in rodents, such as acute or chronic stress, could lead to signatures that may be exploited for diagnosis and treatment of stress and mood disorders in humans.
Increased peripheral inflammation is widely recognized in the pathogenesis of various maladaptive stress responses, yet there is still a lack of mechanistic insights on how circulating inflammatory mediators can affect the brain. The blood–brain barrier (BBB) is a highly selective physical frontier between the central nervous system (CNS) and peripheral circulation, formed by endothelial cells, a basement membrane, astrocytes, and pericytes (Figure 1a). Endothelial cells in the vasculature of the brain are held together by dynamic barrier‐forming proteins, including the tight junctions (TJ) (Lee et al., 2018; see Figure 1b). Specific pro‐inflammatory cytokines have been shown to alter BBB permeability through redistribution of TJ proteins (Małkiewicz et al., 2019; Figure 1c). These cytokines can also alter the expression of BBB transporters, increasing their uptake into the brain while promoting transepithelial migration of immune cells (Langgartner et al., 2019). Unraveling the interactions between peripheral and central inflammation with brain regions affected in these disorders could provide strategies for future targeted therapies (Dantzer et al., 2008; Hodes et al., 2014; Kim et al., 2007; Maes et al., 2002; Muneer, 2016). Therefore, as discussed in the upcoming sections, a proposed mechanism for the precipitation of depressive or anxious behaviors is the concept that stress‐induced increased peripheral inflammation affects BBB permeability, allowing infiltration of circulating inflammatory factors into the parenchyma of mood‐related brain regions, producing damage and altering neural circuits (Beurel et al., 2020; Menard et al., 2017).
FIGURE 1.
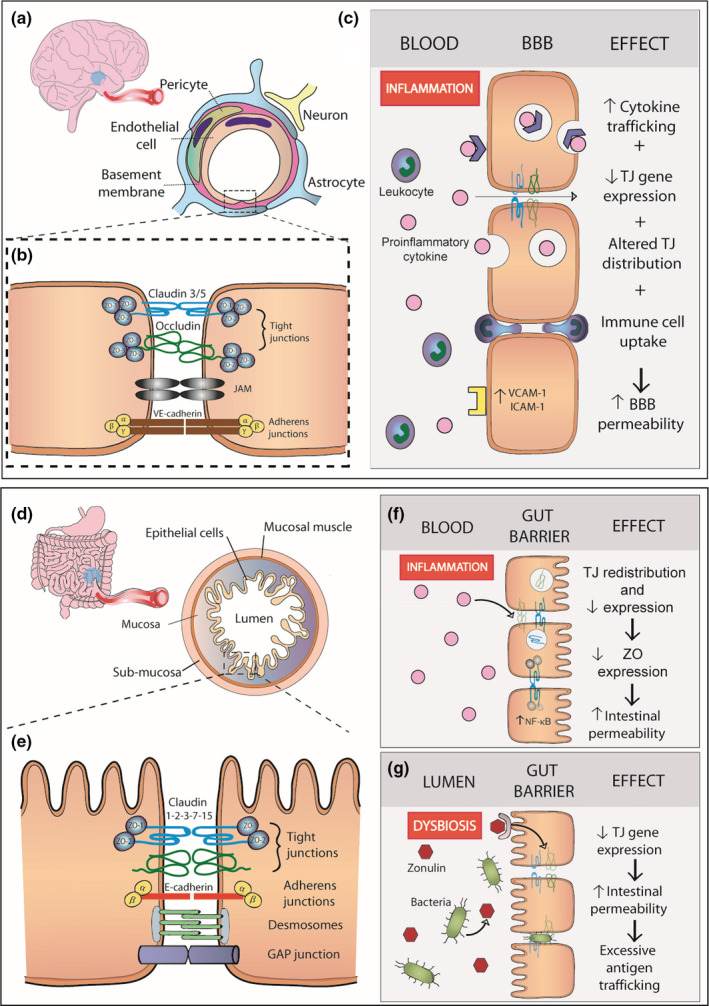
Blood‐brain barrier and intestinal barrier leakiness in stress and mood disorders. The blood‐brain barrier (BBB) is formed by endothelial cells, pericytes and astrocyte end‐feet ensheathing the capillary wall (a). The restricted permeability between endothelial cells of the BBB is maintained by junctional complexes, such as TJs, JAMs and adherens junctions (b). MDD, PTSD, and BD have all been associated with increased levels of circulating pro‐inflammatory cytokines, such as IL‐6, TNF‐α, and IL‐1β. These cytokines are trafficked to the brain through different transport mechanisms. Inflammation‐induced increase in VCAM‐1 and ICAM‐1 expression leads to downregulation of TJ gene expression and altered distribution at the endothelium. Together, these inflammation‐induced adaptations lead to increased BBB permeability (c). The gut barrier is formed by the mucosa, composed of an epithelial cell monolayer, a connective tissue layer, and the mucosal muscle (d). Epithelial cells maintain intestinal integrity through TJ and adherens junction complexes, desmosomes, and GAP junctions (e). Increased peripheral inflammation in mood disorders has been linked to increased TJ downregulation and redistribution, as well as decreased ZO expression through activation of the NF‐kB pathway (f). Moreover, MDD, PTSD, and BD are associated with dysbiosis, linked to increased intestinal permeability. Together, these mechanisms induce excessive bacterial translocation to the bloodstream and increased pro‐inflammatory cytokine production by gut‐associated lymphoïd tissue, exacerbating the dysbiosis‐induced inflammation (g). Abbreviations: JAM, junctional adhesion molecules; NF‐kB, nuclear factor kappa‐B; TJ, tight junctions; ZO, zona occludens
1.4. The gut–brain axis and microbiome in stress and mood disorders
Stress and mood disorders show high comorbidity with inflammatory bowel disease (IBD), Crohn's disease, ulcerative colitis and other inflammatory gastrointestinal (GI) diseases (Abautret‐Daly et al., 2017; Camara et al., 2011; Cole et al., 2006; McIntyre & Calabrese, 2019; McIntyre et al., 2007; O’Donovan, Cohen, et al., 2015) suggesting that inflammation‐driven gut barrier dysfunction may affect emotion regulation and vice versa. In these disorders, elevated levels of pro‐inflammatory cytokines promote permeability in the intestinal tract by suppressing TJ barrier function (Xu et al., 2020; Figure 1d–g) as described above for the BBB (Figure 1a–c). Stress has been linked to the deterioration of the intestinal barrier via gut–brain signaling in GI disorders (Sun et al., 2019; Vancamelbeke & Vermeire, 2017). Furthermore, the microorganisms composing gut bacteria, or the microbiome, play a critical role in maintaining health (Daneman & Rescigno, 2009) and influence the brain through complex bidirectional signaling pathways known as the gut–brain axis (for reviews, see Asai et al., 2013; Cryan & O’Mahony, 2011; Dinan & Cryan, 2017; Grenham et al., 2011; Lach et al., 2018; Mayer et al., 2014). Alternatively, stress, diet, and other environmental factors can disrupt the microbiome homeostasis triggering downstream signaling pathways at the intestinal epithelium to the lamina propria beneath where immune cells elicit a pro‐inflammatory response (Schoultz & Keita, 2020). Accumulating evidence implies a role for dysregulated gut–brain axis signaling in stress and mood disorder pathogenesis (Beurel et al., 2020; Dinan & Cryan, 2017; Mayer et al., 2014; Wang & Kasper, 2014). Through this system, novel roles for peripheral serotonin metabolism are implicated in inflammatory, immune and metabolic signaling pathways (Banskota et al., 2019; Maes et al., 2011). Serotonin does not cross the BBB, albeit many precursors and metabolites can therefore alterations in peripheral serotonin signaling could have direct effects on the brain by altered precursor availability and indirectly by interactions with inflammatory and immune pathways (Fukui et al., 1991; Schwarcz et al., 2012).
In the following sections, we overview how negative emotional valence induced by chronic stress or a traumatic event affects brain–body communication with an emphasis on the immune system and inflammation. Also, we highlight a role of gut barrier and BBB leakiness, a consequence of exacerbated peripheral inflammation in the pathophysiology of mood disorders. Finally, we discuss the potential role of serotonin as a modulator of inflammatory response in the context of mood and stress disorders.
2. INFLAMMATION AND NEGATIVE EMOTIONAL BEHAVIORS
Emotion regulation is a highly complex process involving networks throughout multiple brain areas. Mostly frontal and limbic regions are part of the reward circuit, a key player of emotion regulation which includes different inhibiting and activating neuronal connections from the PFC, NAC, ventral tegmental area (VTA), HIPP, and AMY, among others. Those regions respond to environmental stimuli and react depending on their rewarding or aversive nature, resulting in an emotional answer or behavior (for review, see Russo & Nestler, 2013). Abnormalities in connectivity or neurodegeneration lead to behavioral dysfunction like hyperarousal, attention deficit, or altered emotional processing (Park et al., 2019; Pick et al., 2019). The reward circuitry is affected in stress and mood disorders and functional or structural changes can be investigated by neuroimaging techniques. Cerebral blood flow, an indirect measure of neuronal activity observable by functional MRI (fMRI), is altered in multiple reward‐associated regions such as the PFC and correlated with symptoms in MDD and BD patients (Cantisani et al., 2016; Harrison et al., 2009; Orosz et al., 2012; Pizzagalli, 2011). In mice, MRI reveals neuroanatomic changes in the VTA and HIPP following chronic social stress, adding translational interest in this model (Anacker et al., 2016). Negative emotions were induced by a stressful challenge activate the HPA axis, directly impacting not only the brain but also the immune system, the first defense of our body. The innate immune system quickly recruits several defense leukocyte cells, among others macrophages and monocytes, leading to the release of pro‐inflammatory signals including cytokine interleukin‐1β (IL‐1β), interleukin‐6 (IL‐6), and tumor necrosis factor alpha (TNF‐α) (Ménard et al., 2017; Powell et al., 2013) (for detailed reviews on immune mechanisms of depression please refer to Hodes, Kana, et al., 2015; Miller & Raison, 2016; Nettis & Pariante, 2020). The HPA axis is overactivated in stress and mood disorders, leading to abnormal activation or inhibition of key brain regions involved in the maintenance of adapted response to stressful events (Park et al., 2019). Similarly, increasing evidence suggests that chronic stress promotes sustained peripheral inflammation and high levels of circulating inflammatory mediators at least in subpopulations of patients. As discussed in the next sections, these peripheral pro‐inflammatory signals could directly enter the brain or communicate with microglia, the central primary immune cells, through the neurovascular network. Thus, creating a highly reactive environment which in long term would negatively affect neuronal circuits of mood regulation (Beurel et al., 2020; Menard et al., 2017; Nettis & Pariante, 2020; Schedlowski et al., 2014).
2.1. Dysregulation of cytokines in mood and stress disorders
Emotions are linked with inflammatory cytokine profiles specific to a positive or negative valence (Graham‐Engeland et al., 2018). Fear is an influential challenge to the body; baseline fear and post‐stressor fear are both correlated with an increase in circulating IL‐6 and cortisol levels in humans (Moons et al., 2010). These types of fear refer to the simplest tasks such as doing a mathematical problem or speaking in front of an audience. Traumatic events also change the inflammatory response profile as is the case for individuals with PTSD (Table 3). A recent study showed an increase of plasma TNF‐α and IL‐6 levels compared to healthy controls (Brahmajothi & Abou‐Donia, 2020), but other pro‐inflammatory cytokines are also upregulated in PTSD men and women (Hoge et al., 2009; see Figure 2a). Interestingly, a study conducted in Japanese PTSD women reported that increases in TNF‐α and IL‐6 blood levels were correlated with self‐reported resilience, while C‐reactive protein (CRP) was correlated with quality of life (Imai et al., 2019). Maladaptive responses of the peripheral immune system were also observed in MDD patients (Hodes, Kana, et al., 2015; Wohleb et al., 2016; see Table 3). A recent meta‐analysis regrouping 5,166 patients and 5,083 controls reported that MDD patients are characterized by higher circulating levels of pro‐inflammatory cytokines, including IL‐1β, IL‐6, soluble IL‐6 receptor (sIL‐6R), and decreased level of anti‐inflammatory interleukin‐4 (IL‐4) (Osimo et al., 2020; see Figure 2b). Several studies directed their analysis by considering mood profile, but also subjective symptoms perceived and obtained similar results for peripheral cytokines levels including elevated IL‐6 and TNF‐α peripheral levels but without changes for IL‐1β (Köhler et al., 2017). Correlation was observed between inflammatory response, assessed by measurement of a panel of serum cytokines, and the score of a self‐assessment questionnaire, which reflect the degree of the disorder associated with self‐reported symptoms (Janssen et al., 2014). Peripheral and central CRP levels correlate together and multiple pro‐inflammatory peripheral cytokines are increased, including CRP levels in MDD patients (Felger et al., 2020). In a recent imaging study, increased serum CRP was associated with reduced BBB permeability of the neuroinflammatory indicator 18‐kDa translocator protein (TSPO) radioligand in healthy volunteers and depressed patients (Turkheimer et al., 2021). This counterintuitive finding highlights the complexity of peripheral‐to‐central immune interactions and possible adaptations occurring under prolonged exacerbated peripheral inflammation. There are however limits using TSPO as a biomarker as it may not be specific for local immune cell activation (Al‐Khishman et al., 2020). Finally, pro‐inflammatory cytokine changes were also observed in BD; however, it is important to mention that pro‐inflammatory profiles can vary between the phases (Table 3). In the manic phase, blood levels of interleukin‐2 (IL‐2), IL‐6, and TNF‐α increased in pooled males and females BD patients compared with healthy controls (Kim et al., 2007; Brietzke, Kauer‐Sant’Anna, et al., 2009; see Figure 2c). In these same studies, results report both increases and decreases of IL‐4 levels in the manic phase of BD patients. Additionally, IL‐2 and IL‐6 levels were correlated with mood symptoms such as irritability and aggressive behaviors, based on the patient self‐rating of his symptoms during the previous 2 days (Brietzke, Kauer‐Sant’Anna, et al., 2009; Young et al., 1978). In the depressive phase, patients only had an increase in IL‐6 blood levels (Kim et al., 2007). In another study where BD euthymic patients were compared to healthy control, no significant changes were observed for serum levels of sIL‐6R, IL‐1β and TNF‐α. However, IL‐1β levels of patients were correlated with a low Inventory of Depressive Symptoms (IDS‐30) score measuring the depressive symptoms in BD patients (Vares et al., 2020). The variability of emotional state of BD patients throughout time could be related to different cytokines release and potentially give a clear inflammatory profile that defines the different stages of BD (Tondo et al., 2016; see Figure 2f).
TABLE 3.
Potential circulating cytokine biomarkers of stress and mood disorders
| Marker | Disorder | Changes | Clinical symptoms | References |
|---|---|---|---|---|
| IL‐1β (interleukin‐1) | BD | ↑ | Positive correlation with depressive symptoms (IDS‐30 score) | a Rao et al. (2010) and Vares et al., (2020) |
| — | Euthymic phase | Vares et al., (2020) | ||
| ↓ | Manic Phase | Ortiz‐Domínguez et al., (2007) | ||
| MDD | ↑ | Positive correlation with severity of the symptoms (HAM‐D) | Osimo et al., (2020) and Das et al. (2021) | |
| PTSD | ↑/— | Waheed et al. (2018) | ||
| ↑ |
Negative correlation with HIPP volume Inflammation load (high IL‐1β and IL‐6) correlates with symptom severity (re‐experiencing, arousal, CAPS and MADRS scores) |
Zimmerman et al. (2012) | ||
| IL‐4 (interleukin‐4) | BD | ↑/↓ |
Contradictions on manic phase Increase in euthymic phase |
Kim et al., (2007), Ortiz‐Domínguez et al., (2007), and Brietzke, Kauer‐Sant’Anna, et al., (2009) |
| MDD | ↓ | Decreases in suicide patients | Osimo et al., (2020) and Yuan et al., (2019) | |
| PTSD | ↑ | N/A | Guo et al., (2012) | |
| — | Yuan et al., (2019) | |||
| IL‐6 (interleukin‐6) | BD | ↑ | Manic and depressive phase, correlates with irritability and aggressivity in manic phase | Kim et al., (2007) and Brietzke, Kauer‐Sant’Anna, et al., (2009) |
| ↑ | Acute and remission phases | Pantović‐Stefanović et al., (2018) | ||
| MDD | ↑ | No association with depressive symptoms | Köhler et al., (2017) and Osimo et al., (2020) | |
| Mahajan et al., (2018) | ||||
| Associated with symptom severity (HAM‐D) | Carboni et al., (2019) | |||
| PTSD | ↑ | Brahmajothi and Abou‐Donia (2020) | ||
| ↑ | Anhedonia and avoidance correlation with PFC activation in patients with high levels, correlation with self‐reported resilience | Mehta et al., (2020) | ||
| TNF‐α (tumor necrosis factor alpha) | BD |
↑ — |
Manic phase | Kim et al., (2007), Goldsmith et al. (2016), and Brietzke, Stertz, et al. (2009) |
| — | Euthymic and depressive phase | Goldsmith et al. (2016) and Vares et al., (2020) | ||
| ↓ | Acute and remission phases | Pantović‐Stefanović et al., (2018) | ||
| MDD | ↑ | Köhler et al., (2017) and Osimo et al., (2020) | ||
| Correlation with symptoms severity (HAM‐D) | Das et al. (2021) | |||
| PTSD | ↑ | Correlation with self‐reported resilience | O'Donovan, Chao et al. (2015), Imai et al., (2019), and Brahmajothi and Abou‐Donia (2020) | |
| CRP (C‐reactive protein) | BD | ↑ | Manic phase | Evers et al. (2019) |
| — | No changes in all phases | Balukova et al. (2016) | ||
| MDD | ↑ |
Correlation with depression symptom severity (BDI‐II). Correlates with reduced functional connectivity of AMY and PFC (in comorbid PTSD or anxiety only) |
Powers et al. (2019) and Mehta et al., (2018) | |
| Significant association: baseline CRP and treatment (venlafaxine) response (HAM‐D), ♂ | Carboni et al., (2019) | |||
| PTSD | ↑ |
Negatively correlates with mPFC activation Positively correlates with dissociation symptoms, correlates with PTSD symptoms (CAPS) |
Mehta et al., (2020) and Powers et al. (2019) | |
| Michopoulos et al. (2015) | ||||
| Positive correlation with disease severity (re‐experiencing and arousal) | Farr et al., (2015) |
Abbreviations: BDI‐II, Beck Depression Inventory‐II; CAPS, Clinician‐Administered PTSD Scale;HAM‐D, Hamilton depression rating scale; HC, healthy controls; IDS‐30, Inventory of Depressive Symptoms; MADRS, Montgomery–Asberg Depression Rating Scale.
All markers measured in the blood, with the exception of one measurement from frontal cortex (post‐mortem tissue) (Rao et al., 2010).
FIGURE 2.
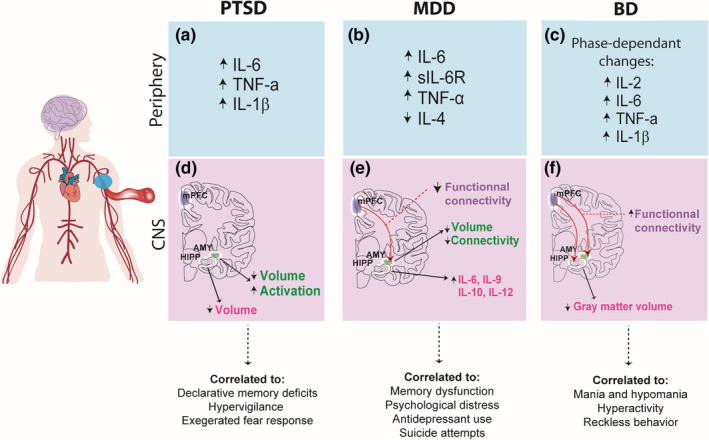
Effects of peripheral and central inflammation on emotion in stress and mood disorders (a–c.). PTSD, MDD, and BD have been associated with a shift toward a pro‐inflammatory profile in the periphery. However, in BD, the specific cytokines profiles have been found to shift between phases of the disease (d). Increased AMY activation concomitant with decreased volume was reported in PTSD patients vs. healthy controls and reduced hippocampal volume correlated to the severity of the symptoms, such as hypervigilance and memory deficits (e). Decreased volume and connectivity of emotion‐regulating brain regions, namely, the HIPP and the AMY, and increased expression of brain IL‐6, IL‐9, IL‐10, and IL‐12 were reported in MDD patients, possibly contributing to the emergence of symptoms (f). Finally, increased functional connectivity has been reported in BD patients between the mPFC‐HIPP and mPFC‐AMY, as well as decreased gray matter volume in the HIPP, suggesting that different immune and neurophysiological profiles could correlate with the different phases of the disease. Abbreviations: AMY, amygdala; BD, bipolar disorder; CNS, central nervous system; HIPP, hippocampus; IL, interleukin; MDD, major depressive disorder; mPFC, medial prefrontal cortex; PTSD, post‐traumatic stress disorder; sIL‐6R, soluble interleukin 6 receptor; TNF‐α, tumor necrosis factor alpha
In addition to having a clear relation between increasing levels of circulating inflammatory cytokines and mood disorders, symptoms of patients also correlate with alterations of central biological inflammatory markers (see Figure 2). IL‐1β, which main source is microglia, is known to be found in the HIPP of depressed patients and to influence neurogenesis, thus impacting memory encoding (Zunszain et al., 2012). Increased expression in genes related to pro‐inflammatory IL‐6, interleukin‐9 (IL‐9), and interleukin‐12 (IL‐12) in the HIPP is observed in MDD patients who committed suicide (Mahajan et al., 2018; see Figure 2d). The increase of another important acute inflammation marker, fibrinogen (Luyendyk et al., 2019), is also associated with self‐reported factors in depressive individuals such as hospitalization, psychological distress described by not‐accomplishing and giving‐up, and use of antidepressant drugs (Wium‐Andersen et al., 2013). Moreover, changes in volume and connectivity for important regions in mood regulation such as HIPP and AMY are observed in MDD patients (Harrison et al., 2009; Frodl et al., 2012; see Figure 2e). In line with the findings discussed above, CRP reduces the functional connectivity of the AMY and mPFC in MDD patients vs. healthy controls (Mehta et al., 2018). In addition to morphological and functional modifications in the HIPP and AMY, blood IL‐6 levels were correlated with the atrophied volume of these regions in female MDD patients (Ironside et al., 2019). As for PTSD, increased symptom severity was accompanied by upregulated TNF‐α receptors and reduction of HIPP volume (O'Donovan, Chao et al., 2015). Similarly, HIPP and AMY volumes are found to be decreased in PTSD patients compared to healthy individuals (Ahmed‐Leitao et al., 2016; see Figure 2d). PTSD symptoms such as hypervigilance and exaggerated fear response are found to be linked to an increased activation of the AMY (Fragkaki et al., 2016) as blood IL‐6 levels are increased following an induced stressor (Muscatell et al., 2015). Moreover, in PTSD Afro‐American women, functional connectivity in the PFC is reported to negatively correlate with IL‐6 and CRP levels. Functional connectivity was also negatively correlated with anhedonia and avoidance in women with high inflammatory response as compared with those having adapted reactions to stress (Mehta et al., 2020). Unfortunately, few studies investigate inflammation impacts on mood‐regulating brain regions in BD. One interesting study noted a positive correlation between sIL‐6R levels and functional connectivity from mPFC to AMY and HIPP (Tu et al., 2017). Another study observed a correlation between neuron activation in Positron emission tomography (PET) imaging and inflammation in the left HIPP (Haarman et al., 2016, 2017). HIPP gray matter volume is negatively correlated with pro‐inflammatory cytokines IL‐1β and soluble tumor necrosis factor receptor‐1 (sTNF‐R1) in older BD patients (Tsai et al., 2019), as observed in MDD patients (see Figure 2f). Brain‐derived neurotrophic factor (BDNF), a neuronal survival and growth factor crucial for learning and memory (Tyler et al., 2002), which expression is decreased in stressed rodents but also MDD and BD patients (Molendijk et al., 2011; Mora et al., 2019), is correlated with cognitive functions in BD patients as IL‐6 and IL‐10 levels are increased (Mora et al., 2019). More investigations are needed to determine the effects of inflammation in BD on regions affected by the disorder, regarding the different stages of the disease.
Overall, these results support the idea that dysregulated activation of the peripheral and central immune systems alters limbic brain regions functioning. In MDD, this would be reflected by memory dysfunction and difficulty to concentrate (DSM‐V), among other alterations. As observed in rodent stress models which will be discussed in the next section, changes in central and peripheral inflammation along with brain connectivity in important limbic structures appear to be involved in mood regulation of MDD patients (Kim & Won, 2017). Similarly, studies including PTSD patients revealed that cytokine changes are related to specific symptoms and the mood perceived by the patients. This connection could be an emergence of cytokine levels constituting a biological marker to complement self‐reported patients' symptoms (Table 3). Many studies in the past did not consider sex or gender as a biological variable, and thus, further investigations are required to better understand the dimorphism observed in prevalence, symptomatology, and treatment of mood disorders and possibly associated those with brain and/or circulating cytokine profiles.
2.2. Peripheral and central immune activation by stress in animals
Subsets of emotional and cognitive symptoms associated with PTSD, MDD, and/or BD are challenging to mimic in animals as discussed previously. For instance, suicidal ideation is a cognitive behavior afflicting many patients (Hällgren et al., 2019; Isometsä, 2014; Tondo et al., 2016) that does not occur in animals. However, trait behaviors such as impulsivity, hopelessness, and impaired decision making (for review, see Gould et al., 2017) among others have been identified as putting individuals with stress or mood disorders at increased risk for suicidal tendencies (Brezo et al., 2006; Bridge et al., 2007). To assess these responses in rodents, behavioral paradigms have been developed (see Box 1) to gain mechanistic insights for potential therapies or promising biological targets for drug development (Stuart et al., 2013; for review, see Malkesman et al., 2009).
Stress paradigms, which are based on inducing behaviors indicative of negative emotional valence, highlight the implication of the peripheral immune system through changes in circulating cytokine levels. These findings are in line with clinical observations obtained in humans, presented in the previous section. As an example, in the fear conditioning test (see Box 1), fear memory is measured by freezing behavior of the animal after previous exposure to a foot shock paired with a tone (auditory stimulus). In these conditions, blood level of IL‐6 and mouse behavior, here retrieval of fear memory through freezing at the tone, are positively correlated (Young et al., 2018). Moreover, a second exposure to the conditioned stimulus, a few days later, is again associated with increased levels of IL‐6 in these animals, revealing an association between peripheral inflammation and fear memory formation. In line with these findings, both IL‐6 knockout (KO) mice and animals intraperitoneally injected with an IL‐6 antagonist, reduction of the freezing behavior is observed, suggesting improvement of the fear extinction (Young et al., 2018). Conversely, injection of IL‐6 in the AMY, a hub region for memory processing, decision making, and emotional responses associated with fear, anxiety and aggression, delays fear extinction. In rats, injection of IL‐6 in the AMY at low doses does not affect freezing during the conditioning and extinction session when the tone is played without the unconditioned stress, when compared with vehicle‐injected controls (Hao et al., 2014). However, higher doses have an impact on conditioning and extinction suggesting that the role of AMY in fear response and encoding is affected by IL‐6 level (Hao et al., 2014). Interestingly, injection of LPS, which is known to induce inflammation including increased IL‐6 levels, in the AMY of young rats impairs fear extinction (Doenni et al., 2017). Inflammation driven by stress thus has a direct impact in the AMY by altering its sensitivity to fear. This is where rodent models of depression can provide valuable clues. Indeed, changes in IL‐6 levels appear to be modulated by negative, stressful events lived by animals, but they could also be causal. Variations of IL‐6 can be correlated not only with one definite aspect but also with multiple behavioral components. These cannot testify directly as how the animal experienced or perceived the event but can give insight via measurable physical changes.
In the CSDS paradigm (see Box 1), stress‐induced changes in IL‐6 serum levels are exacerbated in stress‐susceptible vs. resilient mice despite exposure to the same stressor. Indeed, 20 min after experiencing the first bout of social defeat, IL‐6 levels are higher in mice later classified as susceptible as compared to resilient and unstressed control mice (Hodes et al., 2014; see Box 1). This exacerbated immune response is maintained 48 hr after the last stressor of the classic 10‐day paradigm (Hodes et al., 2014), suggesting that circulating lL‐6 could represent a potential biomarker of social stress vulnerability. No difference is observed prior to stress exposure between future stress‐susceptible vs. resilient mice when compared to unstressed controls (Hodes et al., 2014) indicating that an acute stress is necessary to reveal underlying maladaptive immune responses. It is important to mention the necessity of controlling wounding in CSDS, particularly for immune‐related studies. Cytokine release is not specific to the CSDS model, as an elevation of blood IL‐6 also occurs after the chronic variable stress and witness defeat paradigms (Hodes et al., 2014). Injection of bone marrow hematopoietic progenitor cells harvested from a wild‐type stress‐susceptible donor induces depression‐like behaviors in receiving naïve mice. Conversely, injection of these cells from IL‐6 KO mice promotes stress resilience in naïve mice, confirming causality of increased peripheral IL‐6 in stress responses. It is important to mention that IL‐6 is not the only cytokine affected by chronic social stress. TNF‐α, IL‐1β and interleukin‐10 (IL‐10) are also increased in the periphery after CSDS (Hodes et al., 2014; Zhu et al., 2019) reinforcing the relevance to determine immune profiles or signatures of stress responses.
Recently emerged female models provide sex‐specific novel insights of biological mechanisms to social stress, including the immune system. Differences in blood IL‐6 levels are also observed between unstressed and stressed female mice but not between resilient and susceptible animals following CSDS, unlike their male counterparts. Interestingly, differences between these two latter phenotypes are only observed when the female animals are group‐housed (Takahashi et al., 2017), suggesting environmental context could promote resilience by mediating the increase in blood IL‐6 levels observed when animals are single‐housed at least in females. In contrast, another group using the CSDS model in male mice observed an increase in IL‐6 and other pro‐inflammatory cytokines in the blood of stressed animals as compared to the control group, but no significant differences are observed between resilient and susceptible animals (Elkhatib et al., 2020). Discrepancies might be related to aggression level. While the social interaction test is the golden standard following the CSDS paradigm, other behavioral tests, such as the sucrose preference test which evaluates anhedonia (Zhu et al., 2019), the open field test (Elkhatib et al., 2020), and the light/dark box test (Nasca et al., 2019), are commonly used to measure anxiety in rodents. In parallel with these supplementary behavioral tests, increases in peripheral and brain IL‐6 levels are observed in these animals subjected to the CSDS. In fact, the co‐presence of anxiety, elevated systemic IL‐6, and decreased hippocampal volume was proposed as a multidimensional predictor of susceptibility vs. resilience to social defeat stress (Nasca et al., 2019). Thus, combining performance in behavioral tests and blood biomarkers could represent an interesting avenue to possibly improve the diagnosis of mood disorders.
Stress‐induced changes in central inflammatory cytokines have been correlated with modifications in mood‐regulating regions such as the HIPP and AMY. The HIPP, in addition to being involved in emotional response, is implicated in memory formation, and spatial recognition (Strange et al., 2014), while the AMY is implicated in memory formation with positive or negative impacts, for example in the reward system (Beyeler et al., 2016). Thus, interleukin levels have been assessed not only in the periphery but also these brain regions following CSDS and correlated with behavioral components. TNF‐α and IL‐6 expression is upregulated in the whole brain after CSDS or chronic restraint stress (Zhu et al., 2019). However, no significant difference was observed for IL‐6, TNF‐α, and IL‐1β in HIPP and PFC between stress‐susceptible and resilient mice (Szyszkowicz et al., 2017). Inflammation is mediated by multiple, dynamic events, and stress responses involve various circuits and processes. Discrepancies between studies could be related to different timelines or brain regions studied but also complex interactions between the brain and the periphery. As an example, microglial recruitment of IL‐1β‐producing monocytes to the brain endothelium, favoring an inflamed environment, is implicated in stress‐induced anxiety after chronic social stress exposure (McKim et al., 2018). Social defeat could affect vulnerability to a subsequent aggressive or immune challenge through brain cytokine release priming or dampening from mood‐related structures (Audet et al., 2011). As for the chronic mild stress paradigm (Box 1), it induces an increase in pro‐inflammatory cytokine IL‐1β brain levels along with a decrease of neurogenesis in the HIPP dentate gyrus (Goshen et al., 2008) suggesting that elevated inflammation could impair memory formation. Accordingly, in female mice exposed to repeated social defeat (RSD) (see Box 1), an increase in IL‐1β mRNA is observed in the AMY and HIPP along with neuronal activation (Yin et al., 2019). Furthermore, ionized calcium‐binding adapter molecule 1 (Iba‐1) level is enhanced, supporting microglia activation, and even a significant number of monocytes were detected, specifically in the AMY (Yin et al., 2019). Concomitantly, these animals showed social avoidance and decreased exploratory behaviors in the open field test paired with an increase of plasma IL‐6 that was not detected in unstressed control female mice (Yin et al., 2019). Microglia priming toward a pro‐inflammatory condition following chronic stress exposure has been proposed since microglia from stressed mice overexpress IL‐6, TNF‐α, and IL‐1β after LPS stimulation when compared to unstressed controls, indicating potential recruitment of these cells in future stress responses (Ramirez et al., 2015). The role of microglia activation following chronic stress has been largely reported; thus, it is beyond the scope of our review (Stein et al., 2017). In a transgenic mouse model of IL‐1β overexpression, which promotes spontaneous inflammation, chronic isolation leads to impaired memory cognition and decreased hippocampal neurogenesis (Ben Menachem‐Zidon et al., 2008), supporting a role for stress‐induced central inflammation in HIPP dysfunction. It would be interesting to subject these animals, both males and females, to the different stress paradigms to better define the role of this cytokine in mood. Finally, BDNF is upregulated in astrocytes by TNF‐α (Saha et al., 2006). This factor is also modulated in a chronic stress PTSD rat model along with pro‐inflammatory cytokines IL‐1β and IL‐6 in the AMY and HIPP (Règue et al., 2019). These intriguing findings indicate an influential role of inflammatory response on neurotrophic factors implicated in key structures of cognitive functions and synaptic plasticity underlying mood disorders and antidepressant action (for review, see Duman et al., 2016).
Together, these studies suggest that stress does not only affect the peripheral immune system by modifying circulating components but also impairs neurogenesis and activation in limbic regions which act as the center of emotions and certain types of memory, such as fear, in rodents. This could represent a loop in which the CNS and the immune system would be affected by stress, leading to cognitive dysfunctions (Leonard, 2018). In stress and mood disorders, this brain dysregulation of emotion hubs would be reflected in symptoms such as memory dysfunction and psychological distress including anhedonia, feelings of worthlessness and suicidal attempts (DSM‐V).
2.3. Intestinal dysfunction and inflammation in stress and mood disorders
Approximately 49% of people with IBD suffer from depressive symptoms (Bhandari et al., 2017) and symptomatic IBD patients have the highest depression and anxiety scores, coincident with increased intestinal expression of IL‐1β and IL‐6 and extracellular matrix protein, matrix metalloproteinase‐9 (MMP‐9) (Abautret‐Daly et al., 2017). Additionally, fMRI studies demonstrate aberrant brain function in emotion processing and regulation regions (Icenhour et al., 2019; Tadin Hadjina et al., 2019) reflecting changes seen in patients with MDD (Grandjean et al., 2016; Rzepa & McCabe, 2016; Servaas et al., 2017). Shared profiles of upregulated pro‐inflammatory cytokines in the blood such as IL‐1β, TNF‐α, IL‐6, and IFN‐γ (Abautret‐Daly et al., 2017; Martin‐Subero et al., 2016) occur in GI disorders and mood disorders, which could be related to increased intestinal permeability, through local effects on TJs (Lee et al., 2018; Xu et al., 2020; Figure 1). Permeability of the epithelial layer may increase interaction of antigens with immune cells of the lamina propria, the layer beneath, encouraging robust pro‐inflammatory responses (Figure 1g). Microbial translocation, from the intestinal lumen into the systemic circulation in the absence of acute infection, is proposed as a mechanism behind the chronic inflammation in MDD (Alvarez‐Mon et al., 2019; Maes et al., 2008; Slyepchenko et al., 2016). Indeed, the passage of bacterial products and immune factors as indirect measures of bacterial translocation has been reported in stress and mood disorders (see Table 5).
TABLE 5.
Gut metabolites (serum) in stress and mood disorders
| Marker | Disorder | Changes | Details | References |
|---|---|---|---|---|
| Zonulin (Pre‐haptoglobin‐2) | MDD + Anxiety | ↑ | Stevens et al., (2018) | |
| MDD | ↓ | Specific to recent suicide attempt group | Ohlsson et al., (2019) | |
| BD | — | No alteration between groups, treatment response, or symptoms | Aydın et al. (2020) | |
| FABP2 (Fatty acid‐binding protein 2) | MDD + Anxiety | ↑ | (Even if asymptomatic for gastrointestinal physical distress) | Stevens et al., (2018) |
| MDD | ↑ | Alvarez‐Mon et al., (2019) | ||
| ↑ | Specific to recent suicide attempt group | Ohlsson et al., (2019) | ||
| BD | ↑ | Along with claudin‐5 (BBB) | Kılıç et al., (2020) | |
| Citrulline | MDD | ↓ | Unmedicated | Hess et al., (2017) |
| ↓ | Chrapko et al. (2004) | |||
| PTSD | ↓ | Somvanshi et al., (2019) | ||
| TFF3 (Trefoil factor 3) | MDD/Anxiety | ↑ | ♂ ‐ specific | Ramsey et al. (2016) |
| CD14 | MDD | — | sCD14 | Musil et al., (2011) and Ohlsson et al., (2019) |
| BD | ↑ | Severance et al., (2013) | ||
| LPS | MDD/Anxiety | ↑ | Stevens et al., (2018) | |
| (LPS‐specific Ig) | MDD | ↑ | IgM and IgA | Maes et al. (2007, 2013) |
| MDD, BD | ↑ | Specific to recent suicide group. No difference between disorders. | Dickerson et al. (2017) | |
| LBP (LPS‐binding protein) | MDD | ↑ | High‐LBP coincided with ↑ serum zonulin | Alvarez‐Mon et al., (2019) |
| BD | — | Severance et al., (2013) | ||
| PTSD | ↑ | Bajaj et al., (2019) | ||
| ASCA (Anti‐Saccharomyces cerevisiae antibodies) | BD | ↑ | With and without recent onset of psychosis | Severance et al., (2014) |
| MDD, BD | ↑ | Specific to recent suicide group. No difference between disorders | Dickerson et al. (2017) | |
| GLP‐1 (Glucagon‐like peptide‐1) | BD | ↓ | Rosso et al., (2015) |
In MDD, BD, and PTSD changes in prominent microbiome species occur, some of which correspond to reports in inflammatory GI disorders (Prosberg et al., 2016; Varela et al., 2013). For MDD, distinct signatures within global communities are associated with specific symptoms (Yang et al., 2020; for review, see Li et al., 2019). Reinstating a healthy microbiome with probiotics have improved gut symptoms in IBD (Nikfar et al., 2008; O’Mahony et al., 2005; Whorwell, 2009), while reducing circulating CRP, TNF‐α, and IL‐6 levels and improving depression scores and quality of life scores (Groeger et al., 2013). In healthy humans, abundance of Lactobacillus spp. is directly related to positive self‐judgment (Heym et al., 2019), a cognitive process that is reduced in patients with MDD and related to anhedonic symptoms (Dunn et al., 2009). Hence, treatments for re‐establishing commensal populations could be beneficial for mood symptoms both retroactively or as prophylaxis.
The gut microbiome is also sensitive to stressors (Bharwani et al., 2016), and HPA axis activation can influence population levels (Rios et al., 2017). This stress‐induced dysbiosis upsets colonization resistance, the ability of commensal bacteria to resist the expansion of opportunistic pathogens (Lupp et al., 2007). Overexpansion of pathogenic strains reduces the abundance of healthy species and overall microbiome biodiversity (Livanos et al., 2018; Shen et al., 2012; Wang et al., 2012), which is associated with negative health effects (de la Cuesta‐Zuluaga et al., 2019) including reduced intestinal barrier integrity (Ohlsson et al., 2019; Parker et al., 2019). In MDD, reduced alpha diversity is reported (Evans et al., 2017; Huang et al., 2018; Rong et al., 2019), and additionally, in BD, this reduction correlates negatively with illness duration (Painold et al., 2019). In MDD and BD, reduced commensal Faecalibacterium is described (Evans et al., 2017; Jiang et al., 2015; Naseribafrouei et al., 2014). This species has anti‐inflammatory properties, and reductions are associated with inflammation in GI disorders (Evans et al., 2017; Ferreira‐Halder et al., 2017; Sokol et al., 2008). Other health‐promoting species, such as certain Clostridium, break down carbohydrates to form short‐chain fatty acids (SCFAs), metabolites with local and systemic immunomodulatory effects. SCFAs bolster intestinal epithelial cell integrity by inhibiting enhancing anti‐inflammatory cytokine production (Kalina et al., 2002), modulating the mucous layer (Singh et al., 2010), and activating regulatory T cells (Arpaia et al., 2013). Therefore, stress‐induced dysbiosis may compromise the intestinal barrier, feeding into a loop of exacerbated pro‐inflammatory environment (Huang et al., 2018). Through the gut‐brain axis, commensal microbes can also influence the central immune system, such as modulation of microglia proliferation and function (Erny et al., 2015). Depletion of the microbiome by antibiotics or in germ‐free mice compromises microglia maturation and ability to respond to pathogens such as LPS (Erny et al., 2015). Reversal of this effect could be seen by reinstating a healthy microbiome or by supplementation with the SCFA butyrate (Erny et al., 2015). Gut–brain axis communication to microglia may be occurring by two predominant routes, the vagus nerve and/or signaling molecules such as SCFAs in the peripheral circulation (for review, see Abdel‐Haq et al., 2019; Forsythe et al., 2014). The mechanisms by which the microbiome and gut–brain axis signaling influence microglia phenotypes and activity in the CNS are a complex system that is not well understood. However, this is a promising direction for research unraveling how stress‐induced dysbiosis could promote low‐grade systemic and central inflammation in mood disorders. Future studies on the microbiome, gut barrier integrity, and gut–brain axis signaling are critical for understanding how chronic stress is implicated in these peripheral symptoms of stress and mood disorders. In the next sections, we are proposing that the brain and gut barriers could play a role and be affected by stress‐induced exacerbated inflammation.
2.4. Peripheral serotonin impacts inflammatory pathways and behaviors
Approximately 95% of all serotonin (5‐HT) is found in the gut, produced mostly by enterochromaffin cells and certain microbial species (Banskota et al., 2019; Yang et al., 2020). Tryptophan (TRP) is the sole precursor of 5‐HT; however, more than 90% of dietary TRP is degraded through the predominant kynurenine (KYN) pathway (Brundin et al., 2016; Figure 3a). The KYN pathway is strictly immune‐related and evidence suggests that stress and inflammation increase KYN production by activating TRP‐degrading enzymes, diverting away from 5‐HT synthesis (Hattori et al., 2018; Raison et al., 2010; Sforzini et al., 2019). IBD patients have higher circulating KYN: TRP ratio (Abautret‐Daly et al., 2017), supporting this hypothesis. Increased KYN production causing TRP depletion may be implicated in mood disorder pathogenesis (Sforzini et al., 2019 see Figure 3c), as hyper‐function of the KYN pathway and its downstream metabolites are implicated in other stress‐related disorders (for review, see Maes et al., 2011), but specific roles are still unclear. KYN and other TRP metabolites can activate the aryl hydrocarbon receptor, resulting in immunoregulatory effects such as induction of functional regulatory T cells that suppress inflammation (Mezrich et al., 2010; Zelante et al., 2013). Therefore, KYN upregulation may have a protective function in acute stress, though overextending these pathways, resulting TRP depletion could have negative effects in the context of chronic stress. Many studies have found low circulating TRP in MDD patients with implication in emotional and cognitive symptoms (Doolin et al., 2018; Hughes et al., 2012; Ryan et al., 2020) such as suicidal ideation (Messaoud et al., 2019). KYN pathway metabolites have different neuroactive potentials in the brain, linked to neurotoxic or neuroprotective effects (Maes & Rief, 2012), and this balance can be reflected by metabolite ratios in the blood and thus TRP pathways' activity (Lanser et al., 2020; Figure 3a).
FIGURE 3.
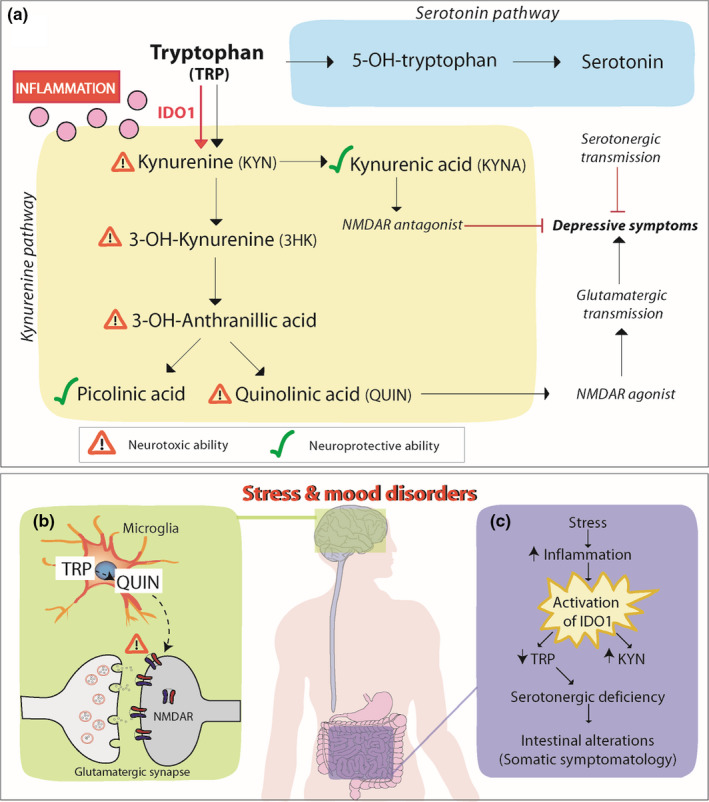
Tryptophan metabolism alterations in stress and mood disorders. TRP is an essential amino acid involved in the metabolic pathways for serotonin and KYN. In the latter pathway, TRP is first metabolized by IDO1 to KYN. Pro‐inflammatory cytokines, such as IL‐6, TNF‐a, and IFN‐y (pink dots) increase IDO1 expression, thus the KYN pathway. Increased production of KYN metabolite KYNA and picolinic acid provides neuroprotective effects, while increased production of 3‐HK, 3‐OH‐anthranilic, acid and QUIN have neurotoxic effects (a). In stress and mood disorders, characterized by increased peripheral and central inflammation, TRP‐KYN pathway is favored. Macrophages in the periphery and microglia in the central nervous system metabolize TRP through the KYN pathway to QUIN, a potent NMDAR agonist, which increases glutamatergic neurotransmission and has been linked to depressive behaviors (b). Similarly, in the gut, increased stress‐induced inflammation promotes the metabolism of TRP in KYN, diverting serotonin production and contributing to TRP depletion. Serotonergic deficiency has been linked to gastrointestinal symptoms in mood disorders. Moreover, peripheral TRP depletion leads to central reduction in TRP and serotonin, an effect that is linked to mood symptoms in the disorders. Abbreviations: 3‐HK, 3‐OH‐kynurenine; IDO1, indoleamine 2,3‐dioxygenase‐1; KYN, kynurenine; KYNA, kynurenic acid; NMDAR, N‐methyl‐D‐aspartate receptor; QUIN: quinolinic acid; TRP, Tryptophan
Low serum TRP levels are related to increased immune activation in patients with inflammatory diseases through Indoleamine 2,3 dioxygenase‐1 (IDO‐1) (Gupta et al., 2012). IDO‐1 is mainly expressed in innate immune cells and is the main rate‐limiting enzyme in the conversion of TRP into KYN and other downstream metabolites (Gupta et al., 2012). Elevated IDO‐1 expression promotes TRP catabolism pathway to favor KYN synthesis over 5‐HT in the periphery and brain (Ryan et al., 2020). In humans, IDO‐1 is highly upregulated in the gut epithelium during inflammation, injury, and infection (Alvarado et al., 2019), induced by pro‐inflammatory factors: IFN‐γ, TNF‐α, IL‐1β, and IL‐6 (Fujigaki et al., 2006) or directly by LPS (O’Connor et al., 2009; Troché et al., 2020). Indeed, clinical IFN‐α therapy in hepatitis C patients significantly decreased blood TRP levels, while increasing KYN in the blood and cerebrospinal fluid (CSF) (Raison et al., 2010), showing evidence of altered IDO‐1 activity. Furthermore, KYN levels increased along with IFN‐α, soluble TNF‐α receptor 2, and monocyte chemoattractant protein‐1 (MCP‐1) in the CSF positively correlated with depressive symptoms (Raison et al., 2010). This evidence highlights the relationship between TRP/KYN metabolites and the immune system in the manifestation of mood symptoms. Indeed, low serum 5‐HT is detected in MDD patients in conjunction with higher IDO‐1 and increased pro‐inflammatory cytokines (Zoga et al., 2014). During depressive episodes, BD patients show a strong interaction between serum IFN‐y and IL‐6 with KYN and TRP (Ameele et al., 2020). Interestingly, selective serotonin reuptake inhibitor (SSRI) treatment, the most commonly prescribed class of antidepressants, reduced concentrations of IDO‐1 which positively correlated with symptom improvement (Zoga et al., 2014). Therefore, IDO‐1 function is proposed as a mechanism, connecting inflammation and TRP depletion in the context of depression (see Figure 3c). However, more work is needed considering that other studies did not find elevated peripheral IDO‐1 expression or KYN pathway activity in MDD (Doolin et al., 2018; Hughes et al., 2012), although TRP levels were still depleted (Hughes et al., 2012), suggesting a missing link behind the TRP decrease in these patients.
Analysis of IDO1 gene KO mice, show a significant decrease in brain 5‐HT concentrations, highlighting the relevancy of IDO1 in the central serotonergic transmission deficit implicated in anxiety and depression (Too et al., 2016). CSDS depletes 5‐HT in the blood and increases KYN pathway activity peripherally and in the brain (Xie et al., 2020; Box 1), accentuating potential roles of IDO‐1. Reflecting this effect, peripheral l‐kynurenine administration induced depressive‐like behavior in a dose‐dependent manner (O'Connor et al., 2009). Stress‐induced metabolite changes could be ablated by IDO‐1 inhibition (Fuertig et al., 2016) with subsequent reduction of immune activation and depressive‐like behaviors (Sublette et al., 2011). In rats, chronic social stress exposure increases plasma and hippocampal IDO‐1 with a correlation to anhedonia behaviors in the sucrose preference test. Further, specific IDO‐1 KO in the HIPP attenuates depressive‐like behaviors (Kim et al., 2012). Combined, these clinical and animal studies highlight the imperative roles TRP plays in the balance between immune tolerance and inflammation in the context of stress and mood disorders.
3. A ROLE FOR INFLAMMATION‐DRIVEN LEAKINESS OF BARRIERS
Structural similarities exist between the intestinal barrier and the BBB (Kelly et al., 2015; Figure 1); therefore, common mechanisms behind barrier disruption have been receiving increasing attention. Stress activates inflammatory and immune pathways, as well as other aspects of the gut–brain axis such as peripheral nerve neurotransmitters and direct neural innervation influencing the gut in the context of stress and mood disorders. This may drive dysbiosis and lead to greater systemic inflammation. Contrarily, gut dysbiosis could be a source of inflammation that then impacts the brain of individuals at risk for psychiatric illnesses. The origin and directionality of these dysregulated systems are still debated; however, the clinical relevance remains promising. In the following sections, we provide an overview of recent preclinical and clinical findings as well as mechanistic insights raising the intriguing possibility of a direct implication of BBB and/or the intestinal barrier in the development of maladaptive stress responses and neuropsychiatric disorders.
3.1. BBB leakiness in mood disorders and animal models of stress
PTSD, MDD, and BD are associated with the concept of BBB hyperpermeability, supported by many reports of increased circulating markers in the blood (Jergović et al., 2015; Niklasson & Agren, 1984; Sumner et al., 2018) (summarized in Table 4). Indeed, in MDD patients, region‐specific changes in barrier and transporter proteins reflecting increased permeability occur in symptom‐related brain structures. Claudin‐5 (CLDN5), an important TJ which is predominantly expressed by the BBB endothelial cells (Castro Dias et al., 2019), is decreased in post‐mortem human samples from depressed individuals, specifically in the NAc (Menard et al., 2017), a region associated with reward and motivation (Dudek et al., 2020; Menard et al., 2017). As well, CLDN5 mRNA expression was reduced in the HIPP of patients diagnosed with depression (Greene et al., 2020). Interestingly, CLDN5 is increased in the occipital cortex and cerebellum of BD patients, but not in MDD patients (Greene et al., 2020). While occludin (OCLN) is significantly increased in the MDD occipital cortex, no significant changes were detected in other TJs such as CLDN12, tight junction protein 1 (ZO‐1), ZO‐2, and Platelet And Endothelial Cell Adhesion Molecule 1 (PECAM1) (Greene et al., 2020). Transcriptomic studies have looked at the PFC of PTSD patients to discover sex‐specific genomic signatures, but no work seems to include TJ genes specifically (Wang et al., 2021).
TABLE 4.
Potential circulating blood‐brain barrier metabolites markers of stress and mood disorders
| Marker | Disorder | Changes | Details/clinical symptoms | References |
|---|---|---|---|---|
| VCAM‐1 (Vascular cell adhesion molecule 1) | PTSD | ↑ | Sumner et al., (2018) | |
| MDD | ↑/↓ | Sex‐specific effects (↓♀, ↑ ♂) | Ramsey et al. (2016) | |
| sVCAM‐1 | BD | ↑ | First manic episode | Turan et al. (2014) |
| ↓ | Acute and remission phases | Pantović‐Stefanović et al., (2018) | ||
| ICAM‐1 (Intercellular cell adhesion molecule 1) | PTSD | ↑ | Sumner et al., (2018) | |
| — | Jergović et al., (2015) | |||
| MDD | ↑ | Lespérance et al. (2004) and Dimopoulos et al. (2006) | ||
| ↑ | Pre‐treatment levels associated with treatment response | Chan et al., (2016) | ||
| sICAM‐1 | PTSD | ↑ | Positive correlation with higher adversity and PTSD scores (UCLA PTSD scales) | Farr et al., (2015) |
| MDD | ↑ | Late‐life depression | Van Agtmaal et al. (2017) | |
| ↑ | 3‐days post antidepressant washout | Baghai et al. (2018) | ||
| BD | ↑ | First manic episode | Turan et al. (2014) | |
| ↑ | Acute and remission phases | Pantović‐Stefanović et al., (2018) | ||
| ↑ | Subthreshold hypomanic symptoms and depressive symptoms | Reininghaus et al., (2016) | ||
| Claudin‐5 | BD | ↑ | Kılıç et al., (2020) | |
| PAI‐1 (plasminogen activator inhibitor 1) | PTSD | ↑ | Positive correlation with symptoms of disease severity (re‐experiencing and arousal) | Farr et al., (2015) |
| MDD | ↑ | No association with symptom severity (HAM‐D, HAM‐A) or disease duration | Eskandari et al., (2005) | |
| Linked to severity of symptoms (CES‐D) | Lahlou‐Laforet et al., (2006) | |||
| ♀, Increase linked to venlafaxine treatment response (HAM‐D) | Carboni et al., (2019) | |||
| — | Linked to treatment response (HAM‐D, IDS) | Chan et al., (2016) | ||
| CCL11/eotaxin‐1 | MDD | ↑ | Related to suicidal ideation | Simon et al. (2008) and Grassi‐Oliveira et al., (2012) |
| — | Leighton et al. (2018) | |||
| BD | — | Euthymic state | Brietzke, Kauer‐Sant’Anna, et al., (2009) | |
| ↑ | Euthymic patients, late‐stage only | Panizzutti et al. (2015) | ||
| MCP1/CCL2 (Monocyte chemoattractant protein 1) | PTSD | ↑ | Toft et al. (2018) | |
| MDD | ↑ | Piletz et al. (2009) | ||
| — | Possibly related to metabolic effects | Bai et al. (2014) | ||
| MMP2 (Matrix metalloproteinase 2) | PTSD | ↑ | Brahmajothi and Abou‐Donia (2020) | |
| MDD | ↓ | Associated with response to ECT | Shibasaki et al. (2016) and Shibasaki et al. (2018) | |
| ↑ | Bobińska et al. (2016) | |||
| BD | ↓ | Shibasaki et al. (2018) | ||
| MMP3 | BD | ↓ | Haenisch et al. (2016) | |
| MMP7 | MDD | ↑ | Bobińska et al. (2016) | |
| BD | ↑ | Haenisch et al. (2016) | ||
| MMP9 | PTSD | ↑ | Brahmajothi and Abou‐Donia (2020) | |
| MDD | ↑ | ♀, associated with treatment response | Domenici et al. (2010), Bobińska et al. (2016), Shibasaki et al. (2018), and Carboni et al., (2019) | |
| — |
Associated with severity of symptoms (depression, quality of life scores, and social function scores) ↓ in non‐relapsing patients after ECT |
Yoshida et al. (2012) and Shibasaki et al. (2016) | ||
| BD | ↑ | Haenisch et al. (2016) | ||
| ↑ | Euthymic stage, correlated negatively with subthreshold hypomanic symptoms (YMRS), negative association with d2 Test of Attention | Reininghaus et al., (2016), Young et al., (1978) | ||
| S100β (S100 calcium binding protein B) | PTSD | — | (Brahmajothi & Abou‐Donia, 2020) | |
| MDD | ↑ | Associated with symptom severity and treatment response (HAM‐D) | Schroeter et al. (2002) | |
| In remission | Arolt et al. (2003), Dietrich et al. (2004), Hetzel et al. (2005), and Schroeter et al. (2008) | |||
| No correlation with clinical severity of the patients (BDI‐II and HAM‐D) | Arora et al. (2019) | |||
| ♀, higher in remitting disorder | Yang et al. (2008) and Arora et al. (2019) | |||
| High baseline levels associated with treatment response (HAM‐D) | Ambrée et al. (2015) | |||
|
Associated with treatment response (escitalopram) High baseline levels associated with smaller reductions in anhedonia (IDS‐C) |
Jha et al. (2019) | |||
| Associated with memory processes | Zhang et al. (2009) | |||
| — | Remission, correlated with lower cognitive performance | Ottesen et al. (2020) | ||
| BD | ↑ |
Manic phase Manic and depressed phase |
Machado‐Vieira et al. (2002) and Andreazza et al. (2007) | |
| — | ↓ after treatment | Tsai and Huang (2017) | ||
| NSE (Neuron‐specific enolase) | BD | ↓ | Manic phase | Machado‐Vieira et al. (2007) and Wiener et al. (2013) |
| Chronic patients, not first episode | Akcan et al. (2018) | |||
| — | Manic phase | Tsai & Huang, (2017) |
Abbreviations: BDI‐II, Beck Depression Inventory‐II; CES‐D, Center of Epidemiologic Studies Depression Scale; HAM‐A, Hamilton anxiety rating scale; HAM‐D, Hamilton depression rating scale; HC, healthy controls; IDS, Inventory of Depressive Symptoms; IDS‐C, 30‐item Inventory of Depressive Symptomatology Clinician‐Rated; YMRS, Young Mania Rating Scale.
In vitro BBB models using human cerebral microvascular endothelial cells show that TNF‐α downregulates CLDN5 and reduces barrier resistance (Förster et al., 2008). Similarly, following application of either IL‐1β, IFN‐γ, and TNF‐α, a notable induction of soluble adhesion proteins such as intercellular adhesion molecule‐1 (ICAM‐1) and vascular cell adhesion protein 1 (VCAM‐1), that are implicated in immune cell uptake into the BBB, is observed (Wong et al., 1999; Banks, 2005; O’Carroll et al., 2015; see Figure 1c). Increased ICAM‐1 levels are described in late‐life depression patients in the dorsolateral prefrontal cortex (dlPFC), but not the ACC (Thomas et al., 2000, 2003). However, other studies either reported a decrease or no significant difference in post‐mortem tissue from MDD patients in dlPFC (Miguel‐Hidalgo et al., 2011; Thomas et al., 2004), suggesting potential age‐related impacts, but the evidence is contradictory. In BD, significantly higher levels of ICAM‐1 immunoreactivity is observed in the ACC, and not the dlPFC (Thomas et al., 2004). Pro‐inflammatory cytokines cause the shedding of adhesion proteins from the endothelium, and one mechanism suggested in this process is the activation of MMP‐9 which induces sICAM‐1 shedding (Fiore et al., 2002). Endothelial VCAM‐1 as a soluble form (sVCAM‐1) binds to very late antigen‐4 (VLA‐4) on peripheral blood mononuclear cells and promotes their extravasation into the brain (Haarmann et al., 2015). As well, sVCAM‐1 can interact in an autocrine manner, binding to integrin α‐4 on the endothelial cells and increasing the permeability through downstream mechanisms (Haarmann et al., 2015). Elevated levels of sVCAM‐1 occur in the serum and CSF of patients with multiple sclerosis, correlating with enhanced lesions and BBB permeability visualized with MRI (Rieckmann et al., 1997). Therefore, the increased peripheral levels of these markers that are reported in stress and mood disorders could reflect BBB damage.
In BD patients, the mean serum levels of CLDN5 levels were significantly higher than in healthy controls; however, no differences occurred in levels between patients during manic episodes compared to patients in remission (Kılıç et al., 2020). Though these types of studies have yet to be conducted in patients with MDD or PTSD, similar investigations have been done recently in obsessive compulsive disorder (Işık et al., 2020), schizophrenia (Maes et al., 2019; Usta et al., 2020), and attention deficit hyperactivity disorder (Aydoğan Avşar et al., 2021) patients, with significant changes noted in each between patients and controls. In MDD, BD, and PTSD, changes in circulating soluble VCAM‐1 and ICAM‐1 are reported as summarized in Table 4. Most studies consistently display increased levels across all the disorders; however, some contradict those findings (Jergović et al., 2015; Pantović‐Stefanović et al., 2018). Regardless, it highlights the potential of evaluating circulating BBB markers, including adhesion molecules, in the context of stress and mood disorders.
Reflecting post‐mortem changes seen in the brain of MDD patients, in mouse models of depression, loss of TJ protein CLDN5 compromises BBB permeability and is linked to the manifestation of depression‐like behaviors (Dudek et al., 2020; Menard et al., 2017). Using a viral approach, artificial opening of the BBB through downregulation of Cldn5 expression in a region‐specific manner was sufficient to induce depression‐like behaviors in male mice (Menard et al., 2017). Furthermore, this effect was reversible by rescuing Cldn5 expression confirming the importance of BBB hyperpermeability in depression physiopathology. Loss of BBB integrity provoked by chronic social stress was shown to allow passage of circulating pro‐inflammatory cytokine IL‐6 in the brain parenchyma (Menard et al., 2017), supporting the hypothesis that inflammatory factors accessing the brain are implicated in mood disorder pathogenesis. In rats, during acute restraint stress, 1 day of stress was sufficient to significantly reduce CLDN5 in the HIPP (Sántha et al., 2016). Electron microscopy confirmed that 1‐day acute stress induced morphological changes indicating capillary endothelial cell damage in the PFC and HIPP that had progressed by 21 days (Sántha et al., 2016), suggesting that stress‐induced changes in BBB integrity could be long‐lasting or even cumulative.
In PTSD, repeated acute stress and hyperactivity of the sympathetic nervous system diminishes glucocorticoid activity over time, potentially dysregulating immune signaling (Gill et al., 2009; Pitman et al., 2012). Supporting this theory, in mice, chronic but not acute social defeat stress reduces morning corticosterone levels in stress‐susceptible mice (Verbitsky et al., 2020). During acute stress, specific adaptive immune responses are suppressed to preserve energy for a crisis. Cortisol plays a role by reducing adaptive immune function which defends against infection (Segerstrom & Miller, 2004), shifting toward humoral immune activities (Gill et al., 2009). However, during excessive or prolonged stress, downstream effects of these changes can be increased inflammation (Gill et al., 2009). IL‐1β, TNF‐α, and IL‐6 can all cross the BBB via specific transporters (Langgartner et al., 2019), and murine IL‐1β and IL‐1α are transported into the mouse brain after a peripheral injection (Banks et al., 1991). However, saturable transport limits indicate that their peripheral upregulation alone would not be pathogenic except if paired with a more permeable BBB. RSD stress induces increased vascular mRNA and protein expression of VCAM‐1 and ICAM‐1 in the cortex, hypothalamus, and AMY in an exposure‐dependent manner (Sawicki et al., 2015). This effect was mediated by pro‐inflammatory cytokines in circulation and in the brain, providing evidence supporting increased immune trafficking into brain regions associated with threat appraisal. RSD stress induces macrophage trafficking into the brain in stressed mice, with subsequent anxiety‐like behavior, persisting for 8 days (Wohleb et al., 2014). Stressed mice later re‐exposed to an acute stressor, re‐established anxiety‐like behavior and immune activation, showing effects of stress sensitization (Wohleb et al., 2014) which could be relevant to PTSD flashbacks triggering episodes of psychological stress.
Another theory of acute stress enhanced trafficking of T lymphocytes to the brain via ICAM‐1 (Lewitus et al., 2008; Figure 1c) introduced a protective mechanistic standpoint on monocyte recruitment into the brain. Here, enhanced T‐cell recruitment is associated with recovery of BDNF levels and increased adaptation to stress (Lewitus et al., 2008), contrary to previous findings suggesting monocyte recruitment to the brain is pivotal to RSD‐induced anxiety‐like behaviors (Wohleb et al., 2011, 2013) and involved in CNS autoimmune disorders (Oukka & Bettelli, 2018; Reboldi et al., 2009). Importantly, it seems T‐cell recruitment in CNS immune response is not always detrimental to the brain (Beurel et al., 2020). During a spatial learning and memory task, IL‐4–producing T‐cell accumulation in meningeal spaces was crucial to cognitive function (Derecki et al., 2010). Inhibiting the recruitment of T cells by blocking VCAM‐1 impaired learning and memory in mice during this task (Derecki et al., 2010). In conjunction with the previous study (Lewitus et al., 2008), BDNF expression is suggested as a possible mechanism underlying the beneficial role of T‐cell response in the brain. Furthermore, blocking T‐cell recruitment through the BBB skewed pro‐inflammatory myeloid phenotype, increasing TNF and IL‐12 expression (Derecki et al., 2010), underscoring the complicated dynamics involved in these systems. A better understanding of inflammatory phenotypes of mood disorders and their processes is essential before considering novel therapeutics such as immunosuppressive agents or anti‐inflammatory treatments for these disorders.
3.2. The gut barrier in stress and mood disorders
The microbiome is critical in maintaining health through various functions including the facilitation of nutrient absorption, mediating immune functions and providing a barrier to prevent pathogens from entering into circulation (Daneman & Rescigno, 2009). A main function of the gut microbiota is the development and maintenance of the intestinal barrier across the lifespan, and generally, a diverse microbiome is associated with integrity of the epithelial barrier (Ohlsson et al., 2019) and maintenance of intestinal metabolic and immune homeostasis (Parker et al., 2019). In mouse models, chronic stressors have been shown to alter microbiome diversity (Szyszkowicz et al., 2017) and promote intestinal permeability (Wei et al., 2019). Furthermore, in mice, imbalances of healthy gut microbes are also associated with increased permeability of the BBB (Braniste et al., 2014) indicating that perhaps, inflammatory gut responses can even trigger BBB permeability, promoting depression behaviors.
A wide range of claudins existxs, which exert different functions and tissue expression (Günzel & Yu, 2013). CLDNs 2, 3, 7, and 15 are the most highly expressed in the intestine (Lu et al., 2013) and altered expression of many intestinal claudins have been observed in GI disorders (Garcia‐Hernandez et al., 2017). Similar to the effects of the BBB, upregulated pro‐inflammatory cytokines in the blood could be implicated in increased intestinal permeability (Lee et al., 2018), through local effects on TJs. Pro‐inflammatory cytokines such as TNF‐α and IL‐1β downregulate ZO‐1 through activation of the NF‐κB pathway and subsequently increases intestinal paracellular permeability (Ma et al., 2004; Al‐Sadi & Ma, 2007, see Figure 1f). IL‐6, IFNγ and TNF‐α have been shown to regulate CLDN2 expression (Krishnan & McCole, 2017; Suzuki et al., 2011) whereas the two latter can decrease CLDN3 and redistribute CLDN4 (Mankertz et al., 2000; Prasad et al., 2005), CLDN1, ZO‐1, and OCLN (Mankertz et al., 2000) with marked increase in the gut paracellular permeability (see Figure 1). Zonulin (pre‐haptoglobin‐2) is a protein that regulates endothelial and epithelial permeability (Stevens et al., 2018; Sturgeon & Fasano, 2016; Wang et al., 2000) by dissociating ZO‐1 and OCLN from the junctional complex (Sturgeon & Fasano, 2016; Wang, Llorente, et al., 2015). Zonulin regulates permeability of both the BBB and the intestinal barrier permeability (Usta et al., 2020). Serum zonulin levels are significantly higher in BD patients compared to healthy controls (Kılıç et al., 2020) and also elevated in patients with MDD and anxiety (Stevens et al., 2018; refer to Table 4). Some of these barrier proteins are expressed in both the BBB and the gut barrier, such as zonulin, ZO‐1, OCLN, and potentially CLDN1 and CLDN3, though the presence of these in the BBB are still debated (Berndt et al., 2019; Castro Dias et al., 2019; Milatz et al., 2010; Steinemann et al., 2016). Therefore, shared mechanisms could occur related to their increased permeability in the context of stress and mood disorders. To our knowledge, no study has yet been conducted in vivo simultaneously investigating the BBB and gut barrier in the same animals (in disease models for IBD or in chronic stress models); therefore, this could be an interesting avenue in the future.
3.3. Microbial antigens and BBB disruption in the pathophysiology of mood disorders
LPS is a product of the cell wall of gram‐negative bacteria and known to play an essential role in inflammatory pathogenesis in IBD (Guo et al., 2015). In a microbiome investigation of MDD patients, KEGG (Kyoto Encyclopedia of Genes and Genomes) analysis showed enriched pathways related to these pathogenic enterotoxins (Huang et al., 2018). For instance, LPS biosynthesis, cellular antigen production, and lipoic acid metabolism, functions implicated in degradation of mucus layer of the epithelial layer. Bacterial toxins from species such as Vibrio cholerae and Escherichia coli gain access to cells and disrupt ion flux (Schnaar & Taniguchi, 2017). Indeed, Clostridium perfringens enterotoxin, a pathogen involved in food‐poisoning and necrotizing enteritis, binds to CLDN3 and 4 causing reorganization which disrupts ion flux and compromises the paracellular barrier (Barmeyer et al., 2015). LPS from members of Gammaproteobacteria are also known to be immunogenic (Maes et al., 2008; Dinan & Cryan, 2012) with implications for gut barrier permeability through the zonulin pathway. The zonulin analog, zonula occludens toxin (Zot) is an enterotoxin produced by V. cholerae (Fasano et al., 1991), which can also activate these pathways, ultimately dissociating TJ‐associated proteins, ZO‐1 and OCLN, from CLDN1 in intestinal epithelial cells (Goldblum et al., 2011). Altered microbiome populations of V. cholerae specifically have not been reported; however, other bacteria within its class, Gammaproteobacteria, are associated with MDD (Jiang et al., 2015). Other enteric pathogens, including E. coli and Salmonella typhi have also been shown to release zonulin from the intestine (Asmar et al., 2002). Considering zonulin is also expressed in the BBB, circulating levels of these toxins could influence permeability there as well. To our knowledge, no researchers have looked into possible bacterial population variations in PTSD and BD patients, giving more importance to continue digging in how these intestinal changes could play a role in these disorders.
Clinical trials have found higher serum levels of LPS in patients with MDD (Ishimura et al., 2013) and in women with post‐partum depression (Zhou et al., 2018). High levels of circulating IgM and IgA of certain pathogenic enterobacteria levels are related to symptoms of fatigue, autonomic, and GI symptoms (Maes et al., 2008). Circulating LPS may impact the brain, as PET imaging study shows healthy humans injected with LPS have increased uptake of TSPO, an inflammatory marker (Woodcock et al., 2020); however, other studies show contradictory evidence (Sandiego et al., 2015). PET imaging on MDD patients showed elevations of TSPO volume in PFC, correlating with depression severity and duration (Setiawan et al., 2015, 2016). Using a different TSPO ligand, Hannestad et al. did not observe this elevation; however, a small sample size and patients classified as having lower clinical severity of the disorder may explain this discrepancy (Hannestad et al., 2013). Finally, intravenous or intramuscular injection of LPS alters mood and produces related behavioral symptoms in humans (Schedlowski et al., 2014).
LPS treatment causes expression of certain depression‐like symptoms in rodents (Yirmiya, 1996) and is clinically relevant to human depression (Alvarez‐Mon et al., 2019). Administration of LPS is frequently used to study neuroinflammation‐associated diseases in mice (Zhao et al., 2019); ergo this model enables a focus on the inflammatory‐related pathways in depression (Dantzer et al., 2008; Mousavi et al., 2019; Remus & Dantzer, 2016; Walker et al., 2019). Systemic LPS can trigger a pro‐inflammatory response and microbicidal responses through engagement of pattern recognition receptors such as Toll‐like receptors on immune cells (Amarante‐Mendes et al., 2018). Binding initiates downstream pathway activation of transcription factor NFkB, upregulating production of TN‐Fα and IL‐1β, as well as VCAM‐1 and ICAM‐1 (Zhang & Ghosh, 2001). Indeed, in mice, LPS administration significantly increases pro‐inflammatory cytokines and chemokines, including IL‐1α, IL‐1β, IL‐9 and MCP‐1 in the blood and the PFC, HIPP, and striatum (Banks et al., 2015). A single injection of LPS induces symptoms of anorexia, reduction of locomotion, and decreased social interaction, paralleling effects in chronic stress models (Zhao et al., 2019). Similarly, intraperitoneal injection of LPS increases TNF‐α, IL‐1β, and IL‐6 in stress‐related structures such as the AMY and the mPFC (Saavedra et al., 2011) linked to subsequent cognitive impairments (Zhao et al., 2019), in memory (Iwai et al., 2014) and fear extinction training (Quiñones et al., 2016). Furthermore, post‐LPS administration, a strong positive correlation exists between BBB disruption, demonstrated by brain uptake tracer molecule 99mTc‐DTPA, and levels of blood and brain pro‐inflammatory cytokines associated with innate immune cell trafficking (Banks et al., 2015; Erickson et al., 2014, 2018). However, the BBB is relatively resistant to effects of LPS on permeability, with some brain regions being more vulnerable than others. Only high doses (>3 mg/kg) increase BBB disruption (Banks et al., 2015) as measured by permeability of small molecule 14C‐sucrose (340 Da) and three peripheral injections of 3 mg/kg LPS significantly and consistently disrupts the BBB to large molecule albumin as well (Erickson et al., 2018). LPS can also be transported through extracellular vesicles, intercellular communication systems which are released from many cell types (Banks et al., 2020). These vesicles can cross the BBB and stimulate trafficking of immune cells into the CNS (Banks et al., 2020). Increased systemic LPS‐positive extracellular vesicles have been detected in the serum of IBD patients (Tulkens et al., 2020). LPS treatment in mice stimulates the trafficking of immune cell extracellular vesicles across the BBB (Yuan et al., 2017) and differentially modulates extracellular vesicles uptake into different regions of the brain (Matsumoto et al., 2017). Little is known about the precise function and mechanisms behind these vesicles; nevertheless, they could be highly valuable to investigate for roles in mood disorders and potential treatments.
Bacterial translocation may impact BBB integrity directly or even be able to migrate into the parenchyma itself (Bested et al., 2013). Mice peripherally injected with pathogenic S. aureus exhibit evidence of BBB uptake, with inflammation and significant levels of brain bacterial counts (Sheen et al., 2010). As well, the microbial product peptidoglycan can translocate from the gut into the blood and ultimately cross the BBB (Arentsen et al., 2017). Peptidoglycan can trigger a pro‐inflammatory response through activation of engagement of pattern recognition receptors. In mice, manipulation of the microbiome shows corresponding changes of peptidoglycan‐recognition proteins in the brain (Arentsen et al., 2017). Interestingly, KO of these receptors in mice causes alterations in social behavior (Arentsen et al., 2017). How peptidoglycan may be transported into the brain is unclear; however, this is a novel concept of a pathway by which the microbiome could directly influence the brain. Further studies to ascertain a potential role in the context of mood disorders could provide valuable insight into implications in social behaviors in these diseases.
3.4. Mechanisms of peripheral serotonin signaling and inflammation of barriers
Enhanced KYN/TRP ratio in the blood of MDD patients correlates with anxiety and cognitive deficit symptoms specifically (Capuron et al., 2003; Maes et al., 2002). Shifts in serum KYN metabolite levels are associated with volume changes in the PFC, HIPP, and striatum in MDD, with implications for altered neuroplasticity, cognitive dysfunction and abnormal reward response (Doolin et al., 2018; Savitz, Dantzer, et al., 2015). KYN metabolite quinolinic acid (QUIN) is an N‐methyl‐D‐aspartate (NMDA) receptor agonist and overproduction can lead to its accumulation with excitotoxic effects (Lanser et al., 2020; see Figure 3b). It is proposed that this effect of increased central QUIN production contributes to MDD pathogenesis. In fact, the density of microglial cells producing QUIN is increased in the ACC of post‐mortem tissue from depressed suicidal patients (Steiner et al., 2011), a region implicated in depression etiology and specifically, inflammation‐associated mood deterioration (Harrison et al., 2009). MRI shows decreased cortical thickness in MDD patients in regions coinciding with increased QUIN ratios, indicative of deleterious effects (Sforzini et al., 2019). As QUIN can be released from monocytes/macrophages in the periphery and microglia in the brain during neuroinflammatory states (Maes & Rief, 2012), changes in this and other metabolites could be a mechanism linking chronic inflammatory pathology to affective symptoms in these disorders. Increased peripheral QUIN is seen in MDD (Savitz, Drevets, et al., 2015); however, it is still unclear if peripheral metabolite levels reflect CNS concentrations (Jacobs et al., 2019), and further studies are needed in this regard.
Increased brain KYN, 3‐hydroxykynurenine (3‐HK), anthranilic acid, and QUIN levels are linked to neurotoxic effects (Maes et al., 2011; Wichers et al., 2005). In rats, intracerebroventricular infusion of QUIN increased the permeability of the BBB indicated by increased extravasation of plasma albumin. Significantly higher tissue levels of plasma albumin occurred in regions of the HIPP, which also exhibited the highest neuronal loss (Št'astný et al., 2000). QUIN cannot translocate to the brain; however, central synthesis by microglia follows uptake of precursors (Doolin et al., 2018; Guillemin et al., 2005; Török et al., 2020; Figure 3b), such as TRP, KYN and 3‐HK which can all cross the BBB (Fukui et al., 1991; Schwarcz et al., 2012; Walker et al., 2019). Most cerebral KYN is suspected to be brought in from the periphery (Savitz, 2020), though it can also be centrally synthesized. In inflammatory conditions, active transport of KYN across the BBB increases, mediated by the large neutral amino acid transporter (Fukui et al., 1991). Therefore, inflammation‐driven increase in peripheral KYN production and subsequent transport into the brain could lead to heightened microglia production of QUIN with neurotoxic potential. Furthermore, activated macrophages can produce 32‐fold higher amounts of QUIN compared to microglia (Espey et al., 1997), and as previously described in animal models of depression, stress induces recruitment of circulating bone marrow‐derived monocytes into the brain (McKim et al., 2018; Wohleb et al., 2013; Yin et al., 2019). Though it seems that this effect has not yet been directly demonstrated in stress and mood disorders, there have been hints toward this concept in depressed patients. Indeed, increased IBA1, CD45, and MCP‐1 gene expression were increased in the ACC white matter in depressed patients who died by suicide (Torres‐Platas et al., 2014), markers which are reflective of activated microglia, monocyte recruitment and perivascular macrophages. An encephalitis model in vitro showed that downregulation of CLDN5 and OCLN enhanced monocyte passage across the BBB (Persidsky et al., 2006). Therefore, downregulation of these TJs seen in depression could be mediating this effect as well. Despite evidence in vitro and in vivo of monocyte recruitment through the endothelium by way adhesion molecules, further evidence is needed to understand if there is a precise role in the context of stress and mood disorders. Indeed, several studies demonstrated that QUIN may be linked to mood disorders with implicated to impact on the BBB integrity and monocyte recruitment into the brain could be a significant link in this relationship.
4. FUTURE DIRECTIONS
4.1. Therapeutic approaches and strategies
Alterations in pro‐inflammatory cytokines in MDD, BD, and PTSD have been extensively studied and appear to be impacted by the negative emotional valence of these disorders. However, the underlying mechanisms linking activation of the inflammatory system with stress and mood disorders are still poorly defined. Clinical trials with anti‐inflammatory treatments have been conducted on groups of depressed patients (for review, see Ménard et al., 2017). These novel therapeutic approaches seemed to have a positive effect on depressive symptoms, particularly in treatment‐resistant patients characterized by elevated circulating pro‐inflammatory cytokine levels, despite the heterogeneous results between the studies (Köhler et al., 2014). Potential beneficial effects of celecoxib, a nonsteroidal anti‐inflammatory drug, have been assessed, alone or in combination with other anti‐inflammatory drugs (Köhler et al., 2014). It improved the state of BD adolescents with acute manic episodes (Mousavi et al., 2017) and depressive symptoms of MDD patients with high inflammation when combined with antidepressants (Köhler et al., 2014). However, another recent study revealed that celecoxib and minocycline, which targets activated microglia, do not improve symptoms and state of BD patients (Husain et al., 2020). Although minocycline alters microglia as well as gut microbiome and flora (Schmidtner et al., 2019), it can represent a challenge to target central immune cells. Even if they are overactivated by chronic stress and might be implicated in the establishment of depressive behaviors, they do play crucial roles in healthy brain processes (Stein et al., 2017). Older patients respond differently to anti‐inflammatory drugs like celecoxib (Fields et al., 2012), explained by the effects of aging on multiple factors including immune responses, gut microbiome, and memory encoding (Martinez‐Canabal et al., 2013). Cytokine neutralizing drugs, like Infliximab which targets TNF‐α, were shown to reduce depression and anxiety‐like behaviors in rats exposed to chronic mild stress (see Box 1) (Karson et al., 2013). Infliximab has also improved depressive symptoms in an MDD clinical trial (Raison et al., 2013). Sirukumab, an anti‐IL‐6 monoclonal antibody, reduces CRP and IL‐6 levels in depressed patients with rheumatoid arthritis (Ménard et al., 2017; Smolen et al., 2014; Zhou et al., 2017). These possible treatments have not yet been reported in PTSD patients to our knowledge (Hori & Kim, 2019). Potential of anti‐inflammatory treatments is still controversial with inconsistent reports, but efficacy and benefits seem apparent in subpopulations of individuals more affected by inflammation (Miller & Pariante, 2020).
Ketamine, a non‐competitive NMDA receptor antagonist, is another promising recent treatment for stress and mood disorders. Enthusiasm is mainly due to the rapid‐acting antidepressant effect, vs. several weeks for commonly prescribed drugs, making it an ideal candidate for relief of suicidal ideation. Briefly, it promotes glutamatergic receptor activation, leading to an increase of BDNF growth factor, synaptogenesis, and neurogenesis in mood‐related brain regions such as the PFC and HIPP (Abdallah et al., 2015; Duman et al., 2016). In mice, ketamine reverses depressive‐like symptoms (Brachman et al., 2016; Iñiguez et al., 2018), induces a small increase in hippocampal volume, and decreases pro‐inflammatory cytokines levels (Wang, Yu, et al., 2015; Zhou et al., 2020). In a clinical trial that included treatment‐resistant depressive patients, ketamine administration led to a small decrease of circulating IL‐6 and IL‐1α (Kiraly et al., 2017). In addition, ketamine could have an impact on the KYN pathway via its effects on NMDA receptors, as investigated in the context of depression (Allen et al., 2018; Miller, 2013; Steiner et al., 2012). In BD patients, ketamine has rapid effects on KYN and neuroprotective kynurenic acid (KYNA) levels altogether with beneficial changes of cytokine levels (Kadriu et al., 2019). Ketamine should be considered in more clinical experiments to assess its influence on depressive symptoms and its long‐term effects on them and the disturbed inflammation profile of these patients.
As for PTSD, SSRIs are widely used for treatment (Kim et al., 2019), but they are not specific for this disorder and its symptoms as they are commonly used in mood disorders as antidepressants (Ravindran & Stein, 2010). Gałecki et al., (2018) performed a meta‐analysis about the response of different vascular and pro‐inflammatory factors following SSRI uptake, such as IL‐1β and IL‐6. In animal model of PTSD, Sertraline, which belongs to the SSRI family, successfully reduces expression of pro‐inflammatory cytokines in the brain and increased the anti‐inflammatory cytokines in the PFC and HIPP of rats after a PTSD stress involving a predator threat (Wilson et al., 2014). Obviously, more research needs to be done in terms of the anti‐inflammatory effects of SSRIs and the possibility to combine them with treatments targeting other systems like the gut or immune system. Mechanistic insights for specific symptoms could help refine diagnosis but also treatment approaches.
Modulation of the microbiome with probiotics could complement psychotherapies and/or pharmacological treatments possibly improving efficiency, especially in the context of reducing inflammation. Reinstating a healthy microbiome with probiotics in IBD has shown positive results on gut symptoms, peripheral inflammation (O’Mahony et al., 2005; Nikfar et al., 2008; Whorwell, 2009) while also improving depression scores and quality of life scores (Groeger et al., 2013). Among MDD patients treated with probiotics, no changes were seen in IL‐6 or TNFα expression levels; however, IL‐22 and TRANCE were significantly upregulated in treatment responders specifically (Okubo et al., 2019). These two cytokines play an important role in the gut barrier function, therefore improving the permeability in the gut may contribute to an improved mental state. Probiotic supplementation is associated with a lower rate of rehospitalization in BD patients (Dickerson et al., 2018), and some have shown a reduction of manic symptoms (Reininghaus et al., 2020), while others found no significant changes (Shahrbabaki et al., 2020). This variability could be explained by factors not limited to different combinations of microbial species in the supplementation or using alternate BD psychiatric questionnaires. In MDD patients, probiotic supplementation has had beneficial effects on Beck Depression Inventory scores and serum hs‐CRP compared with placebo or prebiotic (Akkasheh et al., 2016; Kazemi et al., 2019). Circulating BDNF levels increased significantly in the probiotic group only, correlating inversely with depression severity. Since peripheral BDNF levels are linked with antidepressant response in MDD (Björkholm & Monteggia, 2016; Carboni et al., 2019), this could be pertinent for understanding the neuroregulatory potential of microbiome changes in these disorders. PTSD microbiome and probiotic studies are limited (Brenner et al., 2017); however, a recent pilot study achieved results indicating safety of probiotic supplementation and supporting further investigations (Brenner et al., 2020). Additionally, an fMRI analysis showed that probiotic treatments with certain species of Lactobacillus spp. and Bifidobacterium can dampen brain responses to negative emotional stimuli in the AMY and in the frontal and temporal cortices of IBD patients (Pinto‐Sanchez et al., 2017), which could be relevant for PTSD patients. These preliminary reports highlight the future possibilities for the use of probiotics in mood disorders.
4.2. Biomarkers
Cytokines that have been studied in mood and anxiety disorders have the exciting perspective to be used as biomarkers and give a profile beyond symptoms based on self‐reported questionnaires (Table 3). For example, IL‐6 is overexpressed in the blood of all disorders studied in this review and correlated with patients' psychological state, although does not indicate a specific modulation for a single mood (Hodes et al., 2016). Therefore, it is relevant to say that this cytokine plays a strong role in inflammation in the context of mental health and negative stressful emotion, for humans and in animal models (Wiener et al., 2019). Many other inflammation markers have been investigated in each disorder; some have been seen to be modulated for a unique disorder, while others are up or downregulated for multiple disorders (Yuan et al., 2019). Extended stress and mood disorders than the ones discussed in his review have been compared in this study, but investigating these markers according to symptoms in these patients could generate a signature for specific symptoms. The need for markers specific to symptoms is also relevant and actual with the high comorbidity rates of MDD and PTSD with many other mental disorders, which have an impact on the precision of diagnosis based on different tests (Regier et al., 2013). Furthermore, measuring pro‐inflammatory cytokines in the blood could represent promising biomarkers of mood disorders for older people since studies have shown specific peripheral immune changes in these patients (Gaarden et al., 2018). Among mood disorders, elderly depression prevalence is quite comparable to the general population, since 4.4% of women and 2.7% of men in the United States are currently depressed, with a lifetime prevalence of 16,5% (Aziz & Steffens, 2013). Some genes signature in regions of interest for MDD have been looked at in both sexes and could as well be considered for representative markers specific for men and women (Fatma & Labonte, 2019). More studies are needed to identify specific biomarkers or signatures as a tool to diagnose and give information about specific mental states associated with MDD, BD, and PTSD.
BBB markers could also hold great promise as putative biomarkers of mood disorders as they are upregulated in many of them, revealing the leakiness of this important protective structure (Table 4). BBB disruption correlates with many psychiatric symptoms, and having indications of this dysfunction in the blood could reflect increased interactions of inflammatory cytokines and other toxic molecules with the brain, particularly in mood regulation brain regions (Dudek et al., 2020; Menard et al., 2017). BBB integrity, mostly taken over by TJ CLDN5, is compromised in multiple brain regions in depressive and BD patients, considering its use as a hallmark of mood disorder (Kealy et al., 2020). These studies provide compelling evidence to support the investigation of certain novel BBB‐related biomarkers for stress and mood disorders. Markers, such as S100β, ICAM‐1, and VCAM‐1 are not specific to the BBB and are expressed in cells throughout the body. Still, their expression can be an indication of increased pro‐inflammatory environment, trafficking of inflammatory cells, microbial pathogenesis, and antigen presentation, which are relevant to demonstrate the increased peripheral inflammation in these patients (Hubbard & Rothlein, 2000; Kadry et al., 2020). Studies have highlighted a potential role of the soluble form of these two markers: sICAM and sVCAM as measurable in the periphery in mood disorders as levels increase in the blood of BD and MDD patients respectively (Lopez‐Vilchez et al., 2016; Schaefer et al., 2016). Potentially treatment of peripheral symptoms related to disease comorbidities such as therapies aiming at vascular dysfunction could improve the cognitive and emotional symptoms for treatment‐resistant patients. Plasminogen Activator Inhibitor 1 (PAI‐1), indicative of impaired fibrinolytic function in cardiovascular diseases, is elevated in PTSD and MDD (Eskandari et al., 2005; Farr et al., 2015; Han et al., 2019; Hou et al., 2009; Lahlou‐Laforet et al., 2006) and linked to treatment response in MDD patients (Carboni et al., 2019; Chan et al., 2016). Other markers such as MMP‐9 and soluble cell adhesion molecules are not only related to cardiovascular disease but also indicative of inflammatory BBB disturbances (Reininghaus et al., 2016). Conversely, these blood biomarkers could help provide individualized selection of pharmacotherapies or for monitoring treatment response. Indeed, ICAM‐1, PAI‐1, and thrombopoietin are all associated with treatment response, and specific panels of immune‐endocrine proteins are significantly associated with treatment response to specific types of antidepressants (Chan et al., 2016). Patients with higher baseline ICAM‐1 levels responded better to mixed antidepressants and had poorer responses to Venlafaxine treatment (Chan et al., 2016). ICAM‐1 and VCAM‐1 also have potential as vulnerability markers, as a compelling study assessing healthy individuals linked affective temperaments and mood symptoms characteristic of MDD and BD to these markers. Using MDD and BD diagnostic tests (Ivković et al., 2017) showed a high negative correlation between depressive and irritable temperament scores with sVCAM‐1 levels and sICAM‐1 was related to the state severity of manic symptoms. These findings align correlations between sICAM‐1 and subthreshold depressive/hypomanic symptoms in BD patients even during the euthymic stage (Reininghaus et al., 2016). Since affective temperaments are related to the development of mood disorders, these molecules show potential as predictive markers for vulnerability. Investigation of the BBB in the context of anxiety and mood disorders could enhance opportunities for finding BBB markers specific to MDD, PTSD, and BD patients and target them to prevent unwanted infiltration of inflammatory molecules into the brain. Recently, Kamintsky et al., (2020) performed dynamic contrast‐enhanced (DCE‐)MRI to assess BBB dysfunction in BD patients. A subset of BD patients showed significantly increased BBB dysfunction corresponding to a worse disease outcome. Comparable to controls, the other BD group showed “normal” amounts of BBB leakage and scored lower on measures of disease severity (Kamintsky et al., 2020). Since there is a lack of clinical imaging studies investigating BBB permeability in mood disorders, hopefully this breakthrough study will lead the way for future DCE‐MRI investigations for PTSD and MDD as well. Also, comparing BBB imaging reports with peripheral inflammatory factors in the various disorders could help to uncover pertinent biomarkers.
Markers of gut dysfunction have also been noted in mood disorders (MDD, Musil et al., 2011; Maes et al., 2013; Hess et al., 2017; Stevens et al., 2018; Alvarez‐Mon et al., 2019; Ohlsson et al., 2019; PTSD, Bajaj et al., 2019; Somvanshi et al., 2019; BD, Severance et al., 2013, 2014; Rosso et al., 2015; Kılıç et al., 2020), as summarized in Table 5. Serum detection of specific microbes by testing for pathogen‐associated molecular patterns has proven insightful in GI disorders (Fukui, 2016; Sitaraman et al., 2005; Ziegler et al., 2008); however, high false‐positive rates do occur in LPS detection (Vancamelbeke & Vermeire, 2017). Alternatively, endogenous immune responses to microbes can be tested (Fröhlich et al., 2015; Fukui, 2016; Stevens et al., 2018), indicating immune activation secondary to microbial translocation (Landmann et al., 1996). However, some proteins, such as CD14 can be released by immune cells via non‐LPS dependent mechanisms. Otherwise, serum levels of intestinal cell proteins or intestinal TJs can be markers of gut damage (Grootjans, 2010; Vancamelbeke & Vermeire, 2017), like CLDN3 and zonulin, which have potential as valuable markers. However, questions of the tissue and protein specificity of current tests limit the application of these measurements at this time (Ajamian et al., 2019; Volynets et al., 2016). Still, it will be interesting to compare in future studies circulating TJ and cytokine profiles along with brain circuitry functional assessments to possibly reveal commonalities between gut and mood disorders.
Investigating the microbiome has become increasingly popular, especially in the context of psychiatric disorders. However, the use of different sequencing technologies, for instance, 16S rRNA gene‐sequencing compared to the more sensitive metagenomic sequencing, introduces subtle differences in result interpretation. Even modern high‐throughput sequencing of DNA has limitations, highlighted recently using a mock human gut microbiome for assessment of current databases (Dias et al., 2020). Lack of specific microbiota signatures of mood disorders is also an issue as many disagree on even broad level changes in MDD patients (Chung et al., 2019; Huang et al., 2018; Jiang et al., 2015; Liu et al., 2016; Parker et al., 2019). Developing a cohesive database with improved specificity of microbiome sequencing will be invaluable for future targeted therapeutic approaches, for monitoring treatment response, or assessing predisposition to a disorder.
Combination of treatment with SSRI and probiotics improves cognitive performance and decreases KYN concentration in MDD patients (Rudzki et al., 2019). Recent studies suggest that TRP, KYN, or TRP/KYN ratios and downstream metabolites could be implicated in the expression of specific symptoms (Brundin et al., 2016; Doolin et al., 2018; Ryan et al., 2020). Decreased KYN metabolite, picolinic acid, and picolinic acid/QUIN ratio in the blood and CSF are associated with suicidal behavior across groups of psychiatric patients (Brundin et al., 2016). Indeed, one study found TRP pathways unchanged in depressed individuals; however, aberrations were observed for MDD patients with symptoms of somatization (Maes & Rief, 2012), such as aches and pain, muscular tension, fatigue, irritable bowel, and headache. Identifying TRP metabolite pathways related to specific symptoms may help improve targeted therapies. Therapeutic intervention with ketamine in MDD patients showed relative elevation in levels of KYN, KYNA, and TRP after treatment, correlating with depression symptom improvement (Zhou et al., 2020). Therefore, monitoring of relevant mood‐related TRP metabolites could be a promising strategy to improve treatment efficiency.
5. CONCLUSION
In this review, we have compiled studies identifying the impact of prolonged stress and negative emotions on inflammation, BBB, and gut barrier environment and structure. We also reported literature about the serotonin pathways linking both the immune system and mood disorders. As stress and mood disorders affect many people each year (Kessler et al., 2005), the need of finding innovative diagnostic tools and effective treatments is crucial. Many non‐CNS systems are modulated by stress in mood disorders and resulting in unappropriated functions. The peripheral immune system undergoes changes, leading to effects on the brain such as dysregulated cognitive functions, BBB permeability increase, and infiltration of undesired molecules. As well, effects stemming from the gut such as barrier dysregulation and dysbiosis could lead to unwanted circulating molecules in the blood, weakening the BBB and altering brain circuits. Finally, the serotonin system and pathway which links both CNS and the gut is modulated, resulting in downregulated serotonin levels, immune alterations, and correlation with cognitive dysfunctions. Animal models are crucial to understanding all the mechanisms involved: chronic stress models allow us to reproduce as best as possible depressive and anxiety‐like behaviors as seen in patients as stress is an important cause of mood and anxiety disorders (Verbitsky et al., 2020). It would be unethical to expose humans deliberately to a chronic stressful environment, and the technical approaches such as MRI, blood analysis, and post‐mortem studies are limited for looking at molecular and structural levels. Moreover, animal models are valuable for promoting inflammatory body responses such as LPS injection (Erickson et al., 2018). Sex differences are also a major concern that needs to be considered not only because of the higher prevalence of MDD, PTSD, and anxiety disorders in women but also because of the differences in biological mechanisms between men and women. Hopefully, this could lead to representative treatments and specific biomarkers for each sex, improving our understanding and way of treating current, and also future mood and anxiety patients.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
AUTHOR CONTRIBUTIONS
ED and AC contributed equally to the manuscript. ED and AC wrote the manuscript with support of CM. LDA made the figures and tables. ML edited the manuscript. All authors commented and edited the final version of the manuscript, figures, and tables.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ejn.15239.
ACKNOWLEDGEMENTS
This work was supported by the Natural Sciences and Engineering Research Council of Canada (Discovery Grant to C.M.), New Frontiers in Research Fund (Exploration Grant to C.M.), Sentinel North Initiative funded by Canada First Research Excellence Fund (Research Chair on the Neurobiology of Stress and Resilience to C.M.), Fonds de recherche du Quebec – Health (junior 1 salary award to C.M.), and the Canadian Institutes for Health Research (Project Grant to C.M., MSc scholarship to LDA).
Doney E, Cadoret A, Dion‐Albert L, Lebel M, Menard C. Inflammation‐driven brain and gut barrier dysfunction in stress and mood disorders. Eur J Neurosci. 2022;55(9):2851–2894. 10.1111/ejn.15239
Ellen Doney and Alice Cadoret contributed equally.
REFERENCES
- Abautret‐Daly, Á. , Dempsey, E. , Riestra, S. , de Francisco‐García, R. , Parra‐Blanco, A. , Rodrigo, L. , Medina, C. , Connor, T. J. , & Harkin, A. (2017). Association between psychological measures with inflammatory anddisease‐related markers of inflammatory bowel disease. International Journal of Psychiatry in Clinical Practice, 21, 221–230. [DOI] [PubMed] [Google Scholar]
- Abdallah, C. G. , Sanacora, G. , Duman, R. S. , & Krystal, J. H. (2015). Ketamine and rapid‐acting antidepressants: A window into a new neurobiology for mood disorder therapeutics. Annual Review of Medicine, 66, 509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel‐Haq, R. , Schlachetzki, J. C. M. , Glass, C. K. , & Mazmanian, S. K. (2019). Microbiome–microglia connections via the gut–brain axis. Journal of Experimental Medicine, 216, 41–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed‐Leitao, F. , Spies, G. , van den Heuvel, L. , & Seedat, S. (2016). Hippocampal and amygdala volumes in adults with posttraumatic stress disorder secondary to childhood abuse or maltreatment: A systematic review. Psychiatry Research: Neuroimaging, 256, 33–43. [DOI] [PubMed] [Google Scholar]
- Ajamian, M. , Steer, D. , Rosella, G. , & Gibson, P. R. (2019). Serum zonulin as a marker of intestinal mucosal barrier function: May not be what it seems. PLoS One, 14, e0210728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akcan, U. , Karabulut, S. , Ismail Küçükali, C. , Çakir, S. , & Tüzün, E. (2018). Bipolar disorder patients display reduced serum complement levels and elevated peripheral blood complement expression levels. Acta Neuropsychiatrica, 30, 70–78. [DOI] [PubMed] [Google Scholar]
- Akil, H. , Gordon, J. , Hen, R. , Javitch, J. , Mayberg, H. , McEwen, B. , Meaney, M. J. , & Nestler, E. J. (2018). Treatment resistant depression: A multi‐scale, systems biology approach. Neuroscience and Biobehavioral Reviews, 84, 272–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkasheh, G. , Kashani‐Poor, Z. , Tajabadi‐Ebrahimi, M. , Jafari, P. , Akbari, H. , Taghizadeh, M. , Reza Memarzadeh, M. , Asemi, Z. , & Esmaillzadeh, A. (2016). Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double‐blind, placebo‐controlled trial. Nutrition, 32, 315–320. [DOI] [PubMed] [Google Scholar]
- Al‐Khishman, N. U. , Qi, Q. , Roseborough, A. D. , Levit, A. , Allman, B. L. , Anazodo, U. C. , Fox, M. S. , Whitehead, S. N. , & Thiessen, J. D. (2020). TSPO PET detects acute neuroinflammation but not diffuse chronically activated MHCII microglia in the rat. EJNMMI Research, 10, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, A. P. , Naughton, M. , Dowling, J. , Walsh, A. , O’Shea, R. , Shorten, G. , Scott, L. , McLoughlin, D. M. , Cryan, J. F. , Clarke, G. , & Dinan, T. G. (2018). Kynurenine pathway metabolism and the neurobiology of treatment‐resistant depression: Comparison of multiple ketamine infusions and electroconvulsive therapy. Journal of Psychiatric Research, 100, 24–32. [DOI] [PubMed] [Google Scholar]
- Al‐Sadi, R. M. , & Ma, T. Y. (2007). IL‐1β causes an increase in intestinal epithelial tight junction permeability. The Journal of Immunology, 177, 2310–2322. [Google Scholar]
- Alvarado, D. M. , Chen, B. , Iticovici, M. , Thaker, A. I. , Dai, N. , VanDussen, K. L. , Shaikh, N. , Lim, C. K. , Guillemin, G. J. , Tarr, P. I. , & Ciorba, M. A. (2019). Epithelial indoleamine 2,3‐dioxygenase 1 modulates aryl hydrocarbon receptor and Notch signaling to increase differentiation of secretory cells and alter mucus‐associated microbiota. Gastroenterology, 157, 1093–1108.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez‐Mon, M. A. , Gómez, A. M. , Orozco, A. , Lahera, G. , Sosa, M. D. , Diaz, D. , Auba, E. , Albillos, A. , Monserrat, J. , & Alvarez‐Mon, M. (2019). Abnormal distribution and function of circulating monocytes and enhanced bacterial translocation in major depressive disorder. Frontiers in Psychiatry, 10, 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarante‐Mendes, G. P. , Adjemian, S. , Branco, L. M. , Zanetti, L. C. , Weinlich, R. , & Bortoluci, K. R. (2018). Pattern recognition receptors and the host cell death molecular machinery. Frontiers in Immunology, 107, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrée, O. , Bergink, V. , Grosse, L. , Alferink, J. , Drexhage, H. A. , Rothermundt, M. , Arolt, V. , & Birkenhäger, T. K. (2015). S100B serum levels predict treatment response in patients with melancholic depression. International Journal of Neuropsychopharmacology, 19(3), pyv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameele, S. , Nuijs, A. L. , Lai, F. Y. , Schuermans, J. , Verkerk, R. , Diermen, L. , Coppens, V. , Fransen, E. , Boer, P. , Timmers, M. , Sabbe, B. , & Morrens, M. (2020). A mood state‐specific interaction between kynurenine metabolism and inflammation is present in bipolar disorder. Bipolar Disorders, 22, 59–69. [DOI] [PubMed] [Google Scholar]
- Anacker, C. , Scholz, J. , O’Donnell, K. J. , Allemang‐Grand, R. , Diorio, J. , Bagot, R. C. , Nestler, E. J. , Hen, R. , Lerch, J. P. , & Meaney, M. J. (2016). Neuroanatomic differences associated with stress susceptibility and resilience. Biological Psychiatry, 79, 840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreazza, A. C. , Cassini, C. , Rosa, A. R. , Leite, M. C. , de Almeida, L. M. V. , Nardin, P. , Cunha, A. B. N. , Ceresér, K. M. , Santin, A. , Gottfried, C. , Salvador, M. , Kapczinski, F. , & Gonçalves, C. A. (2007). Serum S100B and antioxidant enzymes in bipolar patients. Journal of Psychiatric Research, 41(6), 523–529. 10.1016/j.jpsychires.2006.07.013 [DOI] [PubMed] [Google Scholar]
- Arentsen, T. , Qian, Y. , Gkotzis, S. , Femenia, T. , Wang, T. , Udekwu, K. , Forssberg, H. , & Diaz Heijtz, R. (2017). The bacterial peptidoglycan‐sensing molecule Pglyrp2 modulates brain development and behavior. Molecular Psychiatry, 22, 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arolt, V. , Peters, M. , Erfurth, A. , Wiesmann, M. , Missler, U. , Rudolf, S. , Kirchner, H. , & Rothermundt, M. (2003). S100B and response to treatment in major depression: a pilot study. European Neuropsychopharmacology, 13(4), 235–239. 10.1016/s0924-977x(03)00016-6 [DOI] [PubMed] [Google Scholar]
- Arora, P. , Sagar, R. , Mehta, M. , Pallavi, P. , Sharma, S. , & Mukhopadhyay, A. K. (2019). Serum S100B levels in patients with depression. Indian Journal of Psychiatry, 61(1), 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaia, N. , Campbell, C. , Fan, X. , Dikiy, S. , Van Der Veeken, J. , Deroos, P. , Liu, H. , Cross, J. R. , Pfeffer, K. , Coffer, P. J. , & Rudensky, A. Y. (2013). Metabolites produced by commensal bacteria promote peripheral regulatory T‐cell generation. Nature, 504, 451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai, M. , Ramachandrappa, S. , Joachim, M. , Shen, Y. , Zhang, R. , Nuthalapati, N. , Ramanathan, V. , Strochlic, D. E. , Ferket, P. , Linhart, K. , Ho, C. , Novoselova, T. V. , Garg, S. , Ridderstråle, M. , Marcus, C. , Hirschhorn, J. N. , Keogh, J. M. , O’Rahilly, S. , Chan, L. F. , … Majzoub, J. A. (2013). Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science, 341, 275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmar, R. E. , Panigrahi, P. , Bamford, P. , Berti, I. , Not, T. , Coppa, G. V. , Catassi, C. , & Fasano, A. (2002). Host‐dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology, 123, 1607–1615. [DOI] [PubMed] [Google Scholar]
- Audet, M. C. , Jacobson‐Pick, S. , Wann, B. P. , & Anisman, H. (2011). Social defeat promotes specific cytokine variations within the prefrontal cortex upon subsequent aggressive or endotoxin challenges. Brain, Behavior, and Immunity, 25, 1197–1205. [DOI] [PubMed] [Google Scholar]
- Aydoğan Avşar, P. , Işık, Ü. , Aktepe, E. , Kılıç, F. , Doğuç, D. K. , & Büyükbayram, H. İ. (2021). Serum zonulin and claudin‐5 levels in children with attention‐deficit/hyperactivity disorder. International Journal of Psychiatry in Clinical Practice, 25(1), 49–55. [DOI] [PubMed] [Google Scholar]
- Aydın, O. , Kocabaş, T. , Sarandöl, A. , Taştan, İ. , Onur, E. , Aydemir, Ö. , & Esen‐Danacı, A. (2020). Examination of plasma zonulin levels in bipolar I disorder: a case–control study with follow‐up. Journal of Neural Transmission, 127, 1419–1426. [DOI] [PubMed] [Google Scholar]
- Aziz, R. , & Steffens, D. C. (2013). What are the causes of late‐life depression? Psychiatric Clinics of North America, 36, 497–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghai, T. , Varallo‐Bedarida, G. , Born, C. , Häfner, S. , Schüle, C. , Eser, D. , Zill, P. , Manook, A. , Weigl, J. , Jooyandeh, S. , Nothdurfter, C. , von Schacky, C. , Bondy, B. , & Rupprecht, R. (2018). Classical risk factors and inflammatory biomarkers: One of the missing biological links between cardiovascular disease and major depressive disorder. International Journal of Molecular Sciences, 19(6), 1740– 10.3390/ijms19061740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, Y.‐M. , Chiou, W.‐F. , Su, T.‐P. , Li, C.‐T. , & Chen, M.‐H. (2014). Pro‐inflammatory cytokine associated with somatic and pain symptoms in depression. Journal of Affective Disorders, 155, 28–34. 10.1016/j.jad.2013.10.019 [DOI] [PubMed] [Google Scholar]
- Bajaj, J. S. , Sikaroodi, M. , Fagan, A. , Heuman, D. , Gilles, H. , Gavis, E. A. , Fuchs, M. , Gonzalez‐Maeso, J. , Nizam, S. , Gillevet, P. M. , & Wade, J. B. (2019). Posttraumatic stress disorder is associated with altered gut microbiota that modulates cognitive performance in veterans with cirrhosis. The American Journal of Physiology‐Gastrointestinal and Liver Physiology, 317, G661–G669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balukova, S. M. , Haarman, B. C. M. , Riemersma‐van der Lek, R. F. , & Schoevers, R. A. (2016). Does CRP predict outcome in bipolar disorder in regular outpatient care? International Journal of Bipolar Disorders, 4, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser, D. A. , & Valentino, R. J. (2014). Sex differences in stress‐related psychiatric disorders: Neurobiological perspectives. Frontiers in Neuroendocrinology, 35, 303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks, W. (2005). Blood‐brain barrier transport of cytokines: A mechanism for neuropathology. Current Pharmaceutical Design, 11, 973–984. [DOI] [PubMed] [Google Scholar]
- Banks, W. A. , Gray, A. M. , Erickson, M. A. , Salameh, T. S. , Damodarasamy, M. , Sheibani, N. , Meabon, J. S. , Wing, E. E. , Morofuji, Y. , Cook, D. G. , & Reed, M. J. (2015). Lipopolysaccharide‐induced blood‐brain barrier disruption: Roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. Journal of Neuroinflammation, 12, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks, W. A. , Ortiz, L. , Plotkin, S. R. , & Kastin, A. J. (1991). Human interleukin (IL) 1 alpha, murine IL‐1 alpha and murine IL‐1 beta are transported from blood to brain in the mouse by a shared saturable mechanism. Journal of Pharmacology and Experimental Therapeutics, 259, 988–996. [PubMed] [Google Scholar]
- Banks, W. A. , Sharma, P. , Bullock, K. M. , Hansen, K. M. , Ludwig, N. , & Whiteside, T. L. (2020). Transport of extracellular vesicles across the blood‐brain barrier: Brain pharmacokinetics and effects of inflammation. International Journal of Molecular Sciences, 21, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banskota, S. , Ghia, J. E. , & Khan, W. I. (2019). Serotonin in the gut: Blessing or a curse. Biochimie, 161, 56–64. [DOI] [PubMed] [Google Scholar]
- Barmeyer, C. , Schulzke, J. D. , & Fromm, M. (2015). Claudin‐related intestinal diseases. Seminars in Cell & Developmental Biology, 42, 30–38. [DOI] [PubMed] [Google Scholar]
- Bauer, M. E. , & Teixeira, A. L. (2019). Inflammation in psychiatric disorders: What comes first? Annals of the New York Academy of Sciences, 1437, 57–67. [DOI] [PubMed] [Google Scholar]
- Ben Menachem‐Zidon, O. , Goshen, I. , Kreisel, T. , Ben Menahem, Y. , Reinhartz, E. , Ben Hur, T. , & Yirmiya, R. (2008). Intrahippocampal transplantation of transgenic neural precursor cells overexpressing interleukin‐1 receptor antagonist blocks chronic isolation‐induced impairment in memory and neurogenesis. Neuropsychopharmacology, 33, 2251–2262. [DOI] [PubMed] [Google Scholar]
- Berndt, P. , Winkler, L. , Cording, J. , Breitkreuz‐Korff, O. , Rex, A. , Dithmer, S. , Rausch, V. , Blasig, R. , Richter, M. , Sporbert, A. , Wolburg, H. , Blasig, I. E. , & Haseloff, R. F. (2019). Tight junction proteins at the blood–brain barrier: Far more than claudin‐5. Cellular and Molecular Life Sciences, 76, 1987–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bested, A. C. , Logan, A. C. , & Selhub, E. M. (2013). Intestinal microbiota, probiotics and mental health: From Metchnikoff to modern advances: Part II – Contemporary contextual research. Gut Pathogens, 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel, E. , Toups, M. , & Nemeroff, C. B. (2020). The bidirectional relationship of depression and inflammation: Double trouble. Neuron, 107, 234–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyeler, A. , Namburi, P. , Glober, G. F. , Simonnet, C. , Calhoon, G. G. , Conyers, G. F. , Luck, R. , Wildes, C. P. , & Tye, K. M. (2016). Divergent routing of positive and negative information from the amygdala during memory retrieval. Neuron, 90, 348–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari, S. , Larson, M. E. , Kumar, N. , & Stein, D. (2017). Association of inflammatory bowel disease (IBD) with depressive symptoms in the United States population and independent predictors of depressive symptoms in an IBD population: A NHANES Study. Gut and Liver, 11(4), 512–519. 10.5009/gnl16347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharwani, A. , Mian, M. F. , Foster, J. A. , Surette, M. G. , Bienenstock, J. , & Forsythe, P. (2016). Structural and functional consequences of chronic psychosocial stress on the microbiome and host. Psychoneuroendocrinology, 63, 217–227. [DOI] [PubMed] [Google Scholar]
- Björkholm, C. , & Monteggia, L. M. (2016). BDNF – a key transducer of antidepressant effects. Neuropharmacology, 102, 72–79. 10.1016/j.neuropharm.2015.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn‐Munro, G. , & Blackburn‐Munro, R. E. (2001). Chronic pain, chronic stress and depression: Coincidence or consequence? Journal of Neuroendocrinology, 13, 1009–1023. [DOI] [PubMed] [Google Scholar]
- Bliss‐Moreau, E. (2017). Constructing nonhuman animal emotion. Current Opinion in Psychology, 17, 184–188. [DOI] [PubMed] [Google Scholar]
- Bobińska, K. , Szemraj, J. , Czarny, P. , & Gałecki, P. (2016). Expression and activity of metalloproteinases in depression. Medical Science Monitor, 22, 1334–1341. 10.12659/msm.895978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachman, R. A. , McGowan, J. C. , Perusini, J. N. , Lim, S. C. , Pham, T. H. , Faye, C. , Gardier, A. M. , Mendez‐David, I. , David, D. J. , Hen, R. , & Denny, C. A. (2016). Ketamine as a prophylactic against stress‐induced depressive‐like behavior. Biological Psychiatry, 79, 776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmajothi, M. V. , & Abou‐Donia, M. B. (2020). PTSD susceptibility and challenges: Pathophysiological consequences of behavioral symptoms. Military Medicine, 185, 279–285. [DOI] [PubMed] [Google Scholar]
- Braniste, V. , Al‐Asmakh, M. , Kowal, C. , Anuar, F. , Abbaspour, A. , Toth, M. , Korecka, A. , Bakocevic, N. , Ng, L. G. , Kundu, P. , Gulyas, B. , Halldin, C. , Hultenby, K. , Nilsson, H. , Hebert, H. , Volpe, B. T. , Diamond, B. , & Pettersson, S. (2014). The gut microbiota influences blood‐brain barrier permeability in mice. Science Translational Medicine, 6, 263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, L. A. , Forster, J. E. , Stearns‐Yoder, K. A. , Stamper, C. E. , Hoisington, A. J. , Brostow, D. P. , Mealer, M. , Wortzel, H. S. , Postolache, T. T. , & Lowry, C. A. (2020). Evaluation of an immunomodulatory probiotic intervention for veterans with co‐occurring mild traumatic brain injury and posttraumatic stress disorder: A pilot study. Frontiers in Neurology, 11, 1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, L. A. , Stearns‐Yoder, K. A. , Hoffberg, A. S. , Penzenik, M. E. , Starosta, A. J. , Hernández, T. D. , Hadidi, D. A. , & Lowry, C. A. (2017). Growing literature but limited evidence: A systematic review regarding prebiotic and probiotic interventions for those with traumatic brain injury and/or posttraumatic stress disorder. Brain, Behavior, and Immunity, 65, 57–67. [DOI] [PubMed] [Google Scholar]
- Brezo, J. , Paris, J. , Tremblay, R. , Vitaro, F. , Zoccolillo, M. , Hébert, M. , & Turecki, G. (2006). Personality traits as correlates of suicide attempts and suicidal ideation in young adults. Psychological Medicine, 36, 191–202. [DOI] [PubMed] [Google Scholar]
- Bridge, J. A. , Iyengar, S. , Salary, C. B. , Barbe, R. P. , Birmaher, B. , Pincus, H. A. , Ren, L. , & Brent, D. A. (2007). Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: A meta‐analysis of randomized controlled trials. Journal of the American Medical Association, 297, 1683–1696. [DOI] [PubMed] [Google Scholar]
- Brietzke, E. , Kauer‐Sant’Anna, M. , Teixeira, A. L. , & Kapczinski, F. (2009). Abnormalities in serum chemokine levels in euthymic patients with bipolar disorder. Brain, Behavior, and Immunity, 23, 1079–1082. [DOI] [PubMed] [Google Scholar]
- Brietzke, E. , Stertz, L. , Fernandes, B. S. , Kauer‐Sant’Anna, M. , Mascarenhas, M. , Escosteguy Vargas, A. , Chies, J. A. , & Kapczinski, F. (2009). Comparison of cytokine levels in depressed, manic and euthymic patients with bipolar disorder. Journal of Affective Disorders, 116, 214–217. [DOI] [PubMed] [Google Scholar]
- Brundin, L. , Sellgren, C. M. , Lim, C. K. , Grit, J. , Pålsson, E. , Landén, M. , Samuelsson, M. , Lundgren, K. , Brundin, P. , Fuchs, D. , Postolache, T. T. , Traskman‐Bendz, L. , Guillemin, G. J. , & Erhardt, S. (2016). An enzyme in the kynurenine pathway that governs vulnerability to suicidal behavior by regulating excitotoxicity and neuroinflammation. Translational Psychiatry, 6, e865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara, R. J. A. , Gander, M.‐L. , Begre, S. , & von Kanel, R. (2011). Post‐traumatic stress in Crohn’s disease and its association with disease activity. Frontline Gastroenterology, 2, 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantisani, A. , Stegmayer, K. , Bracht, T. , Federspiel, A. , Wiest, R. , Horn, H. , Müller, T. J. , Schneider, C. , Höfle, O. , Strik, W. , & Walther, S. (2016). Distinct resting‐state perfusion patterns underlie psychomotor retardation in unipolar vs. bipolar depression. Acta Psychiatrica Scandinavica, 134, 329–338. [DOI] [PubMed] [Google Scholar]
- Capuron, L. , Neurauter, G. , Musselman, D. L. , Lawson, D. H. , Nemeroff, C. B. , Fuchs, D. , & Miller, A. H. (2003). Interferon‐alpha‐induced changes in tryptophan metabolism: Relationship to depression and paroxetine treatment. Biological Psychiatry, 54, 906–914. [DOI] [PubMed] [Google Scholar]
- Carboni, L. , McCarthy, D. J. , Delafont, B. , Filosi, M. , Ivanchenko, E. , Ratti, E. , Learned, S. M. , Alexander, R. , & Domenici, E. (2019). Biomarkers for response in major depression: Comparing paroxetine and venlafaxine from two randomised placebo‐controlled clinical studies. Translational Psychiatry, 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro Dias, M. , Coisne, C. , Lazarevic, I. , Baden, P. , Hata, M. , Iwamoto, N. , Francisco, D. M. F. , Vanlandewijck, M. , He, L. , Baier, F. A. , Stroka, D. , Bruggmann, R. , Lyck, R. , Enzmann, G. , Deutsch, U. , Betsholtz, C. , Furuse, M. , Tsukita, S. , & Engelhardt, B. (2019). Claudin‐3‐deficient C57BL/6J mice display intact brain barriers. Scientific Reports, 9, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, M. K. , Cooper, J. D. , Bot, M. , Birkenhager, T. K. , Bergink, V. , Drexhage, H. A. , Steiner, J. , Rothermundt, M. , Penninx, B. W. J. H. , & Bahn, S. (2016). Blood‐based immune‐endocrine biomarkers of treatment response in depression. Journal of Psychiatric Research, 83, 249–259. [DOI] [PubMed] [Google Scholar]
- Chrapko, W. E. , Jurasz, P. , Radomski, M. W. , Lara, N. , Archer, S. L. , & Le Mellédo, J.‐M. (2004). Decreased platelet nitric oxide synthase activity and plasma nitric oxide metabolites in major depressive disorder. Biological Psychiatry, 56(2), 129–134. 10.1016/j.biopsych.2004.03.003 [DOI] [PubMed] [Google Scholar]
- Chung, Y. C. E. , Chen, H. C. , Chou, H. C. L. , Chen, I. M. , Lee, M. S. , Chuang, L. C. , Liu, Y. W. , Lu, M. L. , Chen, C. H. , Wu, C. H. , Huang, M. C. , Liao, S. C. , Ni, Y. H. , Lai, M. S. , Shih, W. L. , & Kuo, P. H. (2019). Exploration of microbiota targets for major depressive disorder and mood related traits. Journal of Psychiatric Research, 111, 74–82. [DOI] [PubMed] [Google Scholar]
- Cole, J. A. , Rothman, K. J. , Cabral, H. J. , Zhang, Y. , & Farraye, F. A. (2006). Migraine, fibromyalgia, and depression among people with IBS: A prevalence study. BMC Gastroenterology, 6, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington, H. E. , Lobo, M. K. , Maze, I. , Vialou, V. , Hyman, J. M. , Zaman, S. , LaPlant, Q. , Mouzon, E. , Ghose, S. , Tamminga, C. A. , Neve, R. L. , Deisseroth, K. , & Nestler, E. J. (2010). Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. Journal of Neuroscience, 30, 16082–16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan, J. F. , & O’Mahony, S. M. (2011). The microbiome‐gut‐brain axis: From bowel to behavior. Neurogastroenterology and Motility, 23, 187–192. [DOI] [PubMed] [Google Scholar]
- Daneman, R. , & Rescigno, M. (2009). The gut immune barrier and the blood‐brain barrier: Are they so different? Immunity, 31, 722–735. [DOI] [PubMed] [Google Scholar]
- Dantzer, R. , O’Connor, J. C. , Freund, G. G. , Johnson, R. W. , & Kelley, K. W. (2008). From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience, 9, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darves‐Bornoz, J.‐M. , Alonso, J. , de Girolamo, G. , Graaf, R. D. , Haro, J.‐M. , Kovess‐Masfety, V. , Lepine, J.‐P. , Nachbaur, G. , Negre‐Pages, L. , Vilagut, G. , & Gasquet, I. (2008). Main traumatic events in Europe: PTSD in the European study of the epidemiology of mental disorders survey. Journal of Traumatic Stress, 21(5), 455–462. 10.1002/jts.20357 [DOI] [PubMed] [Google Scholar]
- Das, R. , Emon, M. P. Z. , Shahriar, M. , Nahar, Z. , Islam, S. M. A. , Bhuiyan, M. A. , Islam, S. N. , & Islam, M. R. (2021). Higher levels of serum IL‐1β and TNF‐α are associated with an increased probability of major depressive disorder. Psychiatry Research, 295, 113568. [DOI] [PubMed] [Google Scholar]
- de la Cuesta‐Zuluaga, J. , Kelley, S. T. , Chen, Y. , Escobar, J. S. , Mueller, N. T. , Ley, R. E. , McDonald, D. , Huang, S. , Swafford, A. D. , Knight, R. , & Thackray, V. G. (2019). Age‐ and sex‐dependent patterns of gut microbial diversity in human adults. mSystems, 4, e00261–e319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki, N. C. , Cardani, A. N. , Yang, C. H. , Quinnies, K. M. , Crihfield, A. , Lynch, K. R. , & Kipnis, J. (2010). Regulation of learning and memory by meningeal immunity: A key role for IL‐4. Journal of Experimental Medicine, 207, 1067–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslauriers, J. , Toth, M. , Der‐Avakian, A. , & Risbrough, V. B. (2018). Current status of animal models of posttraumatic stress disorder: Behavioral and biological phenotypes, and future challenges in improving translation. Biological Psychiatry, 83(10), 895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias, C. K. , Starke, R. , Pylro, V. S. , & Morais, D. K. (2020). Database limitations for studying the human gut microbiome. PeerJ Computer Science, 6, e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson, F. , Adamos, M. , Katsafanas, E. , Khushalani, S. , Origoni, A. , Savage, C. , Schweinfurth, L. , Stallings, C. , Sweeney, K. , Alaedini, A. , Uhde, M. , Severance, E. , Wilcox, H. C. , & Yolken, R. (2017). The association between immune markers and recent suicide attempts in patients with serious mental illness: A pilot study. Psychiatry Research, 255, 8–12. 10.1016/j.psychres.2017.05.005 [DOI] [PubMed] [Google Scholar]
- Dickerson, F. , Adamos, M. , Katsafanas, E. , Khushalani, S. , Origoni, A. , Savage, C. , Schweinfurth, L. , Stallings, C. , Sweeney, K. , Goga, J. , & Yolken, R. H. (2018). Adjunctive probiotic microorganisms to prevent rehospitalization in patients with acute mania: A randomized controlled trial. Bipolar Disorders, 20, 614–621. [DOI] [PubMed] [Google Scholar]
- Dietrich, D. E. , Ute, H. , Marion, P. , Yuanyuan, Z. , Martin, W. , Mark, H. , Sebastian, R. , Oliver, J. , Holger, K. , Münte, T. F. , Volker, A. , Emrich, H. M. , Sönke, J. , & Matthias, R. (2004). Target evaluation processing and serum levels of nerve tissue protein S100B in patients with remitted major depression. Neuroscience Letters, 354(1), 69–73. 10.1016/j.neulet.2003.09.062 [DOI] [PubMed] [Google Scholar]
- Dimopoulos, N. , Piperi, C. , Salonicioti, A. , Mitsonis, C. , Liappas, I. , Lea, R. W. , & Kalofoutis, A. (2006). Elevation of plasma concentration of adhesion molecules in late‐life depression. International Journal of Geriatric Psychiatry, 21(10), 965–971. 10.1002/gps.1592 [DOI] [PubMed] [Google Scholar]
- Dinan, T. G. , & Cryan, J. F. (2012). Regulation of the stress response by the gut microbiota: Implications for psychoneuroendocrinology. Psychoneuroendocrinology, 37, 1369–1378. [DOI] [PubMed] [Google Scholar]
- Dinan, T. G. , & Cryan, J. F. (2017). Microbes immunity and behavior: Psychoneuroimmunology meets the microbiome. Neuropsychopharmacology, 42, 178–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doenni, V. M. , Song, C. M. , Hill, M. N. , & Pittman, Q. J. (2017). Early‐life inflammation with LPS delays fear extinction in adult rodents. Brain, Behavior, and Immunity, 63, 176–185. [DOI] [PubMed] [Google Scholar]
- Domenici, E. , Willé, D. R. , Tozzi, F. , Prokopenko, I. , Miller, S. , McKeown, A. , Brittain, C. , Rujescu, D. , Giegling, I. , Turck, C. W. , Holsboer, F. , Bullmore, E. T. , Middleton, L. , Merlo‐Pich, E. , Alexander, R. C. , & Muglia, P. (2010). Plasma protein biomarkers for depression and schizophrenia by multi analyte profiling of case‐control collections. PLoS One, 5(2), e9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolin, K. , Allers, K. A. , Pleiner, S. , Liesener, A. , Farrell, C. , Tozzi, L. , O’Hanlon, E. , Roddy, D. , Frodl, T. , Harkin, A. , & O’Keane, V. (2018). Altered tryptophan catabolite concentrations in major depressive disorder and associated changes in hippocampal subfield volumes. Psychoneuroendocrinology, 95, 8–17. [DOI] [PubMed] [Google Scholar]
- Drake, C. L. , Pillai, V. , & Roth, T. (2014). Stress and sleep reactivity: A prospective investigation of the stress‐diathesis model of insomnia. Sleep, 37, 1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek, K. A. , Dion‐Albert, L. , Kaufmann, F. N. , Tuck, E. , Lebel, M. , & Menard, C. (2019). Neurobiology of resilience in depression: Immune and vascular insights from human and animal studies. European Journal of Neuroscience, 00, 1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek, K. A. , Dion‐Albert, L. , Lebel, M. , LeClair, K. , Labrecque, S. , Tuck, E. , Perez, C. F. , Golden, S. A. , Tamminga, C. , Turecki, G. , Mechawar, N. , Russo, S. J. , & Menard, C. (2020). Molecular adaptations of the blood–brain barrier promote stress resilience vs. Depression. Proceedings of the National Academy of Sciences of the United States of America, 117, 3326–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman, R. S. , Aghajanian, G. K. , Sanacora, G. , & Krystal, J. H. (2016). Synaptic plasticity and depression: New insights from stress and rapid‐acting antidepressants. Nature Medicine, 22, 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn, B. D. , Stefanovitch, I. , Buchan, K. , Lawrence, A. D. , & Dalgleish, T. (2009). A reduction in positive self‐judgment bias is uniquely related to the anhedonic symptoms of depression. Behavior Research and Therapy, 47, 374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhai, J. D. , Grubaugh, A. L. , Kashdan, T. B. , & Frueh, B. C. (2008). Empirical examination of a proposed refinement to DSM‐IV posttraumatic stress disorder symptom criteria using the national comorbidity survey replication data. Journal of Clinical Psychiatry, 69, 597–602. [DOI] [PubMed] [Google Scholar]
- Elkhatib, S. K. , Moshfegh, C. M. , Watson, G. F. , & Case, A. J. (2020). Peripheral inflammation is strongly linked to elevated zero maze behavior in repeated social defeat stress. Brain, Behavior, and Immunity, 90, 279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson, M. A. , Morofuji, Y. , Owen, J. B. , & Banks, W. A. (2014). Rapid transport of CCL11 across the blood‐brain barrier: Regional variation and importance of blood cells. Journal of Pharmacology and Experimental Therapeutics, 349, 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson, M. A. , Sandy Liang, W. , Fernandez, E. G. , Bullock, K. M. , Thysell, J. A. , & Banks, W. A. (2018). Genetics and sex influence peripheral and central innate immune responses and bloodbrain barrier integrity. PLoS One, 13, e0205769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erny, D. , De Angelis, A. L. H. , Jaitin, D. , Wieghofer, P. , Staszewski, O. , David, E. , Keren‐Shaul, H. , Mahlakoiv, T. , Jakobshagen, K. , Buch, T. , Schwierzeck, V. , Utermöhlen, O. , Chun, E. , Garrett, W. S. , Mccoy, K. D. , Diefenbach, A. , Staeheli, P. , Stecher, B. , Amit, I. , & Prinz, M. (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nature Neuroscience, 18, 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandari, F. , Mistry, S. , Martinez, P. E. , Torvik, S. , Kotila, C. , Sebring, N. , Drinkard, B. E. , Levy, C. , Reynolds, J. C. , Csako, G. , Gold, P. W. , Horne, M. , & Cizza, G. (2005). Younger, premenopausal women with major depressive disorder have more abdominal fat and increased serum levels of prothrombotic factors: Implications for greater cardiovascular risk. Metabolism, 54, 918–924. [DOI] [PubMed] [Google Scholar]
- Espey, M. G. , Chernyshev, O. N. , Reinhard, J. F. , Namboodiri, M. A. A. , & Colton, C. A. (1997). Activated human microglia produce the excitotoxin quinolinic acid. NeuroReport, 8, 431–434. [DOI] [PubMed] [Google Scholar]
- Evans, S. J. , Bassis, C. M. , Hein, R. , Assari, S. , Flowers, S. A. , Kelly, M. B. , Young, V. B. , Ellingrod, V. E. , & McInnis, M. G. (2017). The gut microbiome composition associates with bipolar disorder and illness severity. Journal of Psychiatric Research, 87, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers, A. K. , Veeh, J. , McNeill, R. , Reif, A. , & Kittel‐Schneider, S. (2019). C‐reactive protein concentration in bipolar disorder: association with genetic variants. International Journal of Bipolar Disorders, 7, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr, O. M. , Ko, B. J. , Joung, K. E. , Zaichenko, L. , Usher, N. , Tsoukas, M. , Thakkar, B. , Davis, C. R. , Crowell, J. A. , & Mantzoros, C. S. (2015). Posttraumatic stress disorder, alone or additively with early life adversity, is associated with obesity and cardiometabolic risk. Nutrition, Metabolism and Cardiovascular Diseases, 25, 479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano, A. , Baudry, B. , Pumplin, D. W. , Wasserman, S. S. , Tall, B. D. , Ketley, J. M. , & Kaper, J. B. (1991). Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proceedings of the National Academy of Sciences of the United States of America, 88, 5242–5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatma, M. , & Labonte, B. (2019). Molecular programs underlying differences in the expression of mood disorders in males and females. Brain Research, 1719, 89–103. [DOI] [PubMed] [Google Scholar]
- Felger, J. C. , Haroon, E. , Patel, T. A. , Goldsmith, D. R. , Wommack, E. C. , Woolwine, B. J. , Le, N. A. , Feinberg, R. , Tansey, M. G. , & Miller, A. H. (2020). What does plasma CRP tell us about peripheral and central inflammation in depression? Molecular Psychiatry, 25, 1301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira‐Halder, C. V. , de Sousa Faria, A. V. , & Andrade, S.S. (2017). Action and function of Faecalibacterium prausnitzii in health and disease. Best Practice & Research Clinical Gastroenterology, 31, 643–648. [DOI] [PubMed] [Google Scholar]
- Fields, C. , Drye, L. , Vaidya, V. , & Lyketsos, C. (2012). Celecoxib or naproxen treatment does not benefit depressive symptoms in persons age 70 and older: Findings from a randomized controlled trial. American Journal of Geriatric Psychiatry, 20, 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore, E. , Fusco, C. , Romero, P. , & Stamenkovic, I. (2002). Matrix metalloproteinase 9 (MMP‐9/gelatinase B) proteolytically cleaves ICAM‐1 and participates in tumor cell resistance to natural killer cell‐mediated cytotoxicity. Oncogene, 21, 5213–5223. [DOI] [PubMed] [Google Scholar]
- Förster, C. , Burek, M. , Romero, I. A. , Weksler, B. , Couraud, P. O. , & Drenckhahn, D. (2008). Differential effects of hydrocortisone and TNFα on tight junction proteins in an in vitro model of the human blood‐brain barrier. Journal of Physiology, 586, 1937–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe, P. , Bienenstock, J. , & Kunze, W. A. (2014). Vagal pathways for microbiome‐brain‐gut axis communication. Advances in Experimental Medicine and Biology, 817, 115–133. [DOI] [PubMed] [Google Scholar]
- Fragkaki, I. , Thomaes, K. , & Sijbrandij, M. (2016). Posttraumatic stress disorder under ongoing threat: a review of neurobiological and neuroendocrine findings. European Journal of Psychotraumatology, 7, 30915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl, T. , Carballedo, A. , Hughes, M. M. , Saleh, K. , Fagan, A. , Skokauskas, N. , McLoughlin, D. M. , Meaney, J. , O’Keane, V. , & Connor, T. J. (2012). Reduced expression of glucocorticoid‐inducible genes GILZ and SGK‐1: High IL‐6 levels are associated with reduced hippocampal volumes in major depressive disorder. Translational Psychiatry, 2, e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich, E. , Mayerhofer, R. , & Holzer, P. (2015). Reevaluating the hype: Four bacterial metabolites under scrutiny. European Journal of Microbiology and Immunology, 5, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertig, R. , Azzinnari, D. , Bergamini, G. , Cathomas, F. , Sigrist, H. , Seifritz, E. , Vavassori, S. , Luippold, A. , Hengerer, B. , Ceci, A. , & Pryce, C. R. (2016). Mouse chronic social stress increases blood and brain kynurenine pathway activity and fear behaviour: Both effects are reversed by inhibition of indoleamine 2,3‐dioxygenase. Brain, Behavior, and Immunity, 54, 59–72. 10.1016/j.bbi.2015.12.020 [DOI] [PubMed] [Google Scholar]
- Fujigaki, H. , Saito, K. , Fujigaki, S. , Takemura, M. , Sudo, K. , Ishiguro, H. , & Seishima, M. (2006). The signal transducer and activator of transcription 1α and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3‐dioxygenase by lipopolysaccharide: Involvement of p38 mitogen‐activated protein kinase and nuclear factor‐κB. Journal of Biochemistry, 139, 655–662. [DOI] [PubMed] [Google Scholar]
- Fukui, H. (2016). Endotoxin and other microbial translocation markers in the blood: A clue to understand leaky gut syndrome. Cellular & Molecular Medicine: Open Access, 02(03), 10.21767/2573-5365.100023 [DOI] [Google Scholar]
- Fukui, S. , Schwarcz, R. , Rapoport, S. I. , Takada, Y. , & Smith, Q. R. (1991). Blood‐brain barrier transport of kynurenines: Implications for brain synthesis and metabolism. Journal of Neurochemistry, 56(6), 2007–2017. 10.1111/j.1471-4159.1991.tb03460.x [DOI] [PubMed] [Google Scholar]
- Fung, B. J. , Qi, S. , Hassabis, D. , Daw, N. , & Mobbs, D. (2019). Slow escape decisions are swayed by trait anxiety. Nature Human Behaviour, 3, 702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaarden, T. L. , Engedal, K. , Benth, J. Š. , Larsen, M. , Lorentzen, B. , Mollnes, T. E. , Bjølseth, T. M. , & Castellheim, A. (2018). Exploration of 27 plasma immune markers: A cross‐sectional comparison of 64 old psychiatric inpatients having unipolar major depression and 18 non‐depressed old persons. BMC Geriatrics, 18, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gałecki, P. , Mossakowska‐Wójcik, J. , & Talarowska, M. (2018). The anti‐inflammatory mechanism of antidepressants – SSRIs, SNRIs. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 80, 291–294. [DOI] [PubMed] [Google Scholar]
- Garcia‐Hernandez, V. , Quiros, M. , & Nusrat, A. (2017). Intestinal epithelial claudins: Expression and regulation in homeostasis and inflammation. Annals of the New York Academy of Sciences, 1397, 66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill, J. M. , Saligan, L. , Woods, S. , & Page, G. (2009). PTSD is associated with an excess of inflammatory immune activities. Perspectives in Psychiatric Care, 45, 262–277. [DOI] [PubMed] [Google Scholar]
- Gitlin, M. (2006). Treatment‐resistant bipolar disorder. Molecular Psychiatry, 11, 227–240. [DOI] [PubMed] [Google Scholar]
- Goldblum, S. E. , Rai, U. , Tripathi, A. , Thakar, M. , De Leo, L. , Di Toro, N. , Not, T. , Ramachandran, R. , Puche, A. C. , Hollenberg, M. D. , & Fasano, A. (2011). The active Zot domain (aa 288–293) increases ZO‐1 and myosin 1C serine/threonine phosphorylation, alters interaction between ZO‐1 and its binding partners, and induces tight junction disassembly through proteinase activated receptor 2 activation. The FASEB Journal, 25, 144–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden, S. A. , Covington, H. E. , Olivier, B. , & Russo, S. J. (2011). A standardized protocol for repeated social defeat stress in mice. Nature Protocols, 6(8), 1183–1191. 10.1038/nprot.2011.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith, D. R. , Rapaport, M. H. , & Miller, B. J. (2016). A meta‐analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Molecular Psychiatry, 21(12), 1696–1709. 10.1038/mp.2016.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen, I. , Kreisel, T. , Ben‐Menachem‐Zidon, O. , Licht, T. , Weidenfeld, J. , Ben‐Hur, T. , & Yirmiya, R. (2008). Brain interleukin‐1 mediates chronic stress‐induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Molecular Psychiatry, 13, 717–728. [DOI] [PubMed] [Google Scholar]
- Gould, T. D. , Georgiou, P. , Brenner, L. A. , Brundin, L. , Can, A. , Courtet, P. , Donaldson, Z. R. , Dwivedi, Y. , Guillaume, S. , Gottesman, I. I. , Kanekar, S. , Lowry, C. A. , Renshaw, P. F. , Rujescu, D. , Smith, E. G. , Turecki, G. , Zanos, P. , Zarate, C. A. , Zunszain, P. A. , & Postolache, T. T. (2017). Animal models to improve our understanding and treatment of suicidal behavior. Translational Psychiatry, 7, e1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham‐Engeland, J. E. , Sin, N. L. , Smyth, J. M. , Jones, D. R. , Knight, E. L. , Sliwinski, M. J. , Almeida, D. M. , Katz, M. J. , Lipton, R. B. , & Engeland, C. G. (2018). Negative and positive affect as predictors of inflammation: Timing matters. Brain, Behavior, and Immunity, 74, 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean, J. , Azzinnari, D. , Seuwen, A. , Sigrist, H. , Seifritz, E. , Pryce, C. R. , & Rudin, M. (2016). Chronic psychosocial stress in mice leads to changes in brain functional connectivity and metabolite levels comparable to human depression. NeuroImage, 142, 544–552. 10.1016/j.neuroimage.2016.08.013 [DOI] [PubMed] [Google Scholar]
- Grassi‐Oliveira, R. , Brieztke, E. , Teixeira, A. , Pezzi, J. C. , Zanini, M. , Lopes, R. P. , & Bauer, M. E. (2012). Níveis periféricos de quimiocina em mulheres com depressão maior com ideaç̃o suicida. The Revista Brasileira De Psiquiatria, 34, 71–75. [DOI] [PubMed] [Google Scholar]
- Greene, C. , Hanley, N. , & Campbell, M. (2020). Blood‐brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Translational Psychiatry, 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenham, S. , Clarke, G. , Cryan, J. F. , & Dinan, T. G. (2011). Brain‐gut‐microbe communication in health and disease. Frontiers in Physiology, 2, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeger, D. , O’Mahony, L. , Murphy, E. F. , Bourke, J. F. , Dinan, T. G. , Kiely, B. , Shanahan, F. , & Quigley, E. M. M. (2013). Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes, 4, 325–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootjans, J. (2010). Non‐invasive assessment of barrier integrity and function of the human gut. World Journal of Gastrointestinal Surgery, 2, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin, G. J. , Smythe, G. , Takikawa, O. , & Brew, B. J. (2005). Expression of indoleamine 2,3‐dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia, 49, 15–23. [DOI] [PubMed] [Google Scholar]
- Günzel, D. , & Yu, A. S. L. (2013). Claudins and the modulation of tight junction permeability. Physiological Reviews, 93, 525–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, M. , Li, T. , Guo, J. C. , Jiang, X. L. , Chen, F. , & Gao, Y. S. (2012). Study on serum cytokine levels in posttraumatic stress disorder patients. Asian Pacific Journal of Tropical Medicine, 5, 323–325. [DOI] [PubMed] [Google Scholar]
- Guo, S. , Nighot, M. , Al‐Sadi, R. , Alhmoud, T. , Nighot, P. , & Ma, T. Y. (2015). Lipopolysaccharide regulation of intestinal tight junction permeability is mediated by TLR4 signal transduction pathway activation of FAK and MyD88. The Journal of Immunology, 195, 4999–5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, N. K. , Thaker, A. I. , Kanuri, N. , Riehl, T. E. , Rowley, C. W. , Stenson, W. F. , & Ciorba, M. A. (2012). Serum analysis of tryptophan catabolism pathway: Correlation with Crohn’s disease activity. Inflammatory Bowel Diseases, 18, 1214–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarman (‘Benno’), B. C. M. , Burger, H. , Doorduin, J. , Renken, R. J. , Sibeijn‐Kuiper, A. J. , Marsman, J. B. C. , de Vries, E. F. J. , de Groot, J. C. , Drexhage, H. A. , Mendes, R. , Nolen, W. A. , & Riemersma‐Van der Lek, R. F. (2017). Corrigendum to “Volume, metabolites and neuroinflammation of the hippocampus in bipolar disorder – A combined magnetic resonance imaging and positron emission tomography study”. Brain, Behavior, and Immunity, 61, 387–388. [DOI] [PubMed] [Google Scholar]
- Haarman, B. C. M. B. , Burger, H. , Doorduin, J. , Renken, R. J. , Sibeijn‐Kuiper, A. J. , Marsman, J. B. C. , de Vries, E. F. J. , de Groot, J. C. , Drexhage, H. A. , Mendes, R. , Nolen, W. A. , & Riemersma‐Van der Lek, R. F. (2016). Volume, metabolites and neuroinflammation of the hippocampus in bipolar disorder – A combined magnetic resonance imaging and positron emission tomography study. Brain, Behavior, and Immunity, 56, 21–33. [DOI] [PubMed] [Google Scholar]
- Haarmann, A. , Nowak, E. , Deiß, A. , van der Pol, S. , Monoranu, C. M. , Kooij, G. , Müller, N. , van der Valk, P. , Stoll, G. , de Vries, H. E. , Berberich‐Siebelt, F. , & Buttmann, M. (2015). Soluble VCAM‐1 impairs human brain endothelial barrier integrity via integrin α‐4‐transduced outside‐in signalling. Acta Neuropathologica, 129, 639–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenisch, F. , Cooper, J. D. , Reif, A. , Kittel‐Schneider, S. , Steiner, J. , Leweke, F. G. , Rothermundt, M. , van Beveren, N. J. M. , Crespo‐Facorro, B. , Niebuhr, D. W. , Cowan, D. N. , Weber, N. S. , Yolken, R. H. , Penninx, B. W. J. H. , & Bahn, S. (2016). Towards a blood‐based diagnostic panel for bipolar disorder. Brain, Behavior, and Immunity, 52, 49–57. 10.1016/j.bbi.2015.10.001 [DOI] [PubMed] [Google Scholar]
- Hällgren, J. , Ösby, U. , Westman, J. , & Gissler, M. (2019). Mortality trends in external causes of death in people with mental health disorders in Sweden, 1987–2010. Scandinavian Journal of Public Health, 47, 121–126. [DOI] [PubMed] [Google Scholar]
- Han, W. , Dang, R. , Xu, P. , Li, G. , Zhou, X. , Chen, L. , Guo, Y. , Yang, M. , Chen, D. , & Jiang, P. (2019). Altered fibrinolytic system in rat models of depression and patients with first‐episode depression. Neurobiology of Stress, 11, 100188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad, J. , DellaGioia, N. , Gallezot, J. D. , Lim, K. , Nabulsi, N. , Esterlis, I. , Pittman, B. , Lee, J. Y. , O’Connor, K. C. , Pelletier, D. , & Carson, R. E. (2013). The neuroinflammation marker translocator protein is not elevated in individuals with mild‐to‐moderate depression: A [11C]PBR28 PET study. Brain, Behavior, and Immunity, 33, 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao, Y. , Jing, H. , Bi, Q. , Zhang, J. , Qin, L. , & Yang, P. (2014). Intra‐amygdala microinfusion of IL‐6 impairs the auditory fear conditioning of rats via JAK/STAT activation. Behavioral Brain Research, 275, 88–95. [DOI] [PubMed] [Google Scholar]
- Harrison, N. A. , Brydon, L. , Walker, C. , Gray, M. A. , Steptoe, A. , & Critchley, H. D. (2009). Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biological Psychiatry, 66, 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori, S. , Takao, K. , Funakoshi, H. , & Miyakawa, T. (2018). Comprehensive behavioral analysis of tryptophan 2,3‐dioxygenase (Tdo2) knockout mice. Neuropsychopharmacology Reports, 38, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess, S. , Baker, G. , Gyenes, G. , Tsuyuki, R. , Newman, S. , & Le Melledo, J. M. (2017). Decreased serum L‐arginine and L‐citrulline levels in major depression. Psychopharmacology (Berl), 234, 3241–3247. [DOI] [PubMed] [Google Scholar]
- Hetzel, G. , Moeller, O. , Evers, S. , Erfurth, A. , Ponath, G. , Arolt, V. , & Rothermundt, M. (2005). The astroglial protein S100B and visually evoked event‐related potentials before and after antidepressant treatment. Psychopharmacology (Berl), 178(2–3), 161–166. 10.1007/s00213-004-1999-z [DOI] [PubMed] [Google Scholar]
- Heym, N. , Heasman, B. C. , Hunter, K. , Blanco, S. R. , Wang, G. Y. , Siegert, R. , Cleare, A. , Gibson, G. R. , Kumari, V. , & Sumich, A. L. (2019). The role of microbiota and inflammation in self‐judgement and empathy: Implications for understanding the brain‐gut‐microbiome axis in depression. Psychopharmacology (Berl), 236, 1459–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo‐Mazzei, D. , Berk, M. , Cipriani, A. , Cleare, A. J. , Florio, A. D. , Dietch, D. , Geddes, J. R. , Goodwin, G. M. , Grunze, H. , Hayes, J. F. , Jones, I. , Kasper, S. , Macritchie, K. , McAllister‐Williams, R. H. , Morriss, R. , Nayrouz, S. , Pappa, S. , Soares, J. C. , Smith, D. J. , … Stokes, P. R. A. (2019). Treatment‐resistant and multi‐therapy‐resistant criteria for bipolar depression: Consensus definition. British Journal of Psychiatry, 214, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes, G. E. , Kana, V. , Menard, C. , Merad, M. , & Russo, S. J. (2015). Neuroimmune mechanisms of depression. Nature Neuroscience, 18, 1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes, G. E. , Ménard, C. , & Russo, S. J. (2016). Integrating Interleukin‐6 into depression diagnosis and treatment. Neurobiology of Stress, 4, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes, G. E. , Pfau, M. L. , Leboeuf, M. , Golden, S. A. , Christoffel, D. J. , Bregman, D. , Rebusi, N. , Heshmati, M. , Aleyasin, H. , Warren, B. L. , Lebonté, B. , Horn, S. , Lapidus, K. A. , Stelzhammer, V. , Wong, E. H. F. , Bahn, S. , Krishnan, V. , Bolaños‐Guzman, C. A. , Murrough, J. W. , … Russo, S. J. (2014). Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proceedings of the National Academy of Sciences of the United States of America, 111, 16136–16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes, G. E. , Pfau, M. L. , Purushothaman, I. , Ahn, H. F. , Golden, S. A. , Christoffel, D. J. , Magida, J. , Brancato, A. , Takahashi, A. , Flanigan, M. E. , Ménard, C. , Aleyasin, H. , Koo, H. W. , Lorsch, Z. S. , Feng, J. , Heshmati, M. , Wang, M. , Turecki, G. , Neve, R. , … Russo, S. J. (2015). Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. The Journal of Neuroscience, 35, 16362–16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge, E. A. , Brandstetter, K. , Moshier, S. , Pollack, M. H. , Wong, K. K. , & Simon, N. M. (2009). Broad spectrum of cytokine abnormalities in Panic disorder and Posttraumatic stress disorder. Depression and Anxiety, 26, 447–455. [DOI] [PubMed] [Google Scholar]
- Hori, H. , & Kim, Y. (2019). Inflammation and post‐traumatic stress disorder. Psychiatry and Clinical Neurosciences, 73, 143–153. [DOI] [PubMed] [Google Scholar]
- Hou, S. J. , Yen, F. C. , & Tsai, S. J. (2009). Is dysfunction of the tissue plasminogen activator (tPA)‐plasmin pathway a link between major depression and cardiovascular disease? Medical Hypotheses, 72, 166–168. [DOI] [PubMed] [Google Scholar]
- Huang, Y. , Shi, X. , Li, Z. , Shen, Y. , Shi, X. , Wang, L. , Li, G. , Yuan, Y. , Wang, J. , Zhang, Y. , Zhao, L. , Zhang, M. , Kang, Y. , & Liang, Y. (2018). Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatric Disease and Treatment, 14, 3329–3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard, A. K. , & Rothlein, R. (2000). Intercellular adhesion molecule‐1 (ICAM‐1) expression and cell signaling cascades. Free Radical Biology and Medicine, 28, 1379–1386. [DOI] [PubMed] [Google Scholar]
- Hughes, M. M. , Carballedo, A. , McLoughlin, D. M. , Amico, F. , Harkin, A. , Frodl, T. , & Connor, T. J. (2012). Tryptophan depletion in depressed patients occurs independent of kynurenine pathway activation. Brain, Behavior, and Immunity, 26, 979–987. [DOI] [PubMed] [Google Scholar]
- Hung, Y. Y. , Kang, H. Y. , Huang, K. W. , & Huang, T. L. (2014). Association between toll‐like receptors expression and major depressive disorder. Psychiatry Research, 220, 283–286. [DOI] [PubMed] [Google Scholar]
- Husain, M. I. , Chaudhry, I. B. , Khoso, A. B. , Husain, M. O. , Hodsoll, J. , Ansari, M. A. , Naqvi, H. A. , Minhas, F. A. , Carvalho, A. F. , Meyer, J. H. , Deakin, B. , Mulsant, B. H. , Husain, N. , & Young, A. H. (2020). Minocycline and celecoxib as adjunctive treatments for bipolar depression: A multicentre, factorial design randomised controlled trial. The Lancet Psychiatry, 7, 515–527. [DOI] [PubMed] [Google Scholar]
- Icenhour, A. , Tapper, S. , Bednarska, O. , Witt, S. T. , Tisell, A. , Lundberg, P. , Elsenbruch, S. , & Walter, S. (2019). Elucidating the putative link between prefrontal neurotransmission, functional connectivity, and affective symptoms in irritable bowel syndrome. Scientific Reports, 9(1), 1–11. 10.1038/s41598-019-50024-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai, R. , Hori, H. , Itoh, M. , Lin, M. , Niwa, M. , Ino, K. , Ogawa, S. , Sekiguchi, A. , Kunugi, H. , Akechi, T. , Kamo, T. , & Kim, Y. (2019). Relationships of blood proinflammatory markers with psychological resilience and quality of life in civilian women with posttraumatic stress disorder. Scientific Reports, 9(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez, S. D. , Flores‐Ramirez, F. J. , Riggs, L. M. , Alipio, J. B. , Garcia‐Carachure, I. , Hernandez, M. A. , Sanchez, D. O. , Lobo, M. K. , Serrano, P. A. , Braren, S. H. , & Castillo, S. A. (2018). Vicarious social defeat stress induces depression‐related outcomes in female mice. Biological Psychiatry, 83, 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ironside, M. , Admon, R. , Maddox, S. A. , Mehta, M. , Douglas, S. , Olson, D. P. , & Pizzagalli, D. A. (2019). Inflammation and depressive phenotypes: Evidence from medical records from over 12 000 patients and brain morphology. Psychological Medicine, 50(16), 2790–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimura, S. , Furuhashi, M. , Watanabe, Y. , Hoshina, K. , Fuseya, T. , Mita, T. , Okazaki, Y. , Koyama, M. , Tanaka, M. , Akasaka, H. , Ohnishi, H. , Yoshida, H. , Saitoh, S. , & Miura, T. (2013). Circulating levels of fatty acid‐binding protein family and metabolic phenotype in the general population. PLoS One, 8, e81318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Işık, Ü. , Aydoğan Avşar, P. , Aktepe, E. , Doğuç, D. K. , Kılıç, F. , & Büyükbayram, H. İ. (2020). Serum zonulin and claudin‐5 levels in children with obsessive–compulsive disorder. Nordic Journal of Psychiatry, 74, 346–351. [DOI] [PubMed] [Google Scholar]
- Isometsä, E. (2014). Suicidal behaviour in mood disorders‐Who, When, and Why? Canadian Journal of Psychiatry, 59, 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivković, M. , Pantović‐Stefanović, M. , Petronijević, N. , Dunjić‐Kostić, N. , Velimirović, M. , Nikolić, T. , Jurišić, V. , Lačković, M. , Totić‐Poznanović, S. , Jovanović, A. A. , & Damjanović, A. (2017). Predictive value of sICAM‐1 and sVCAM‐1 as biomarkers of affective temperaments in healthy young adults. Journal of Affective Disorders, 207, 47–52. 10.1016/j.jad.2016.09.017 [DOI] [PubMed] [Google Scholar]
- Iwai, T. , Sawabe, T. , Tanimitsu, K. , Suzuki, M. , Sasaki‐Hamada, S. , & Oka, J. I. (2014). Glucagon‐like peptide‐1 protects synaptic and learning functions from neuroinflammation in rodents. Journal of Neuroscience Research, 92, 446–454. [DOI] [PubMed] [Google Scholar]
- Jacobs, K. R. , Lim, C. K. , Blennow, K. , Zetterberg, H. , Chatterjee, P. , Martins, R. N. , Brew, B. J. , Guillemin, G. J. , & Lovejoy, D. B. (2019). Correlation between plasma and CSF concentrations of kynurenine pathway metabolites in Alzheimer’s disease and relationship to amyloid‐β and tau. Neurobiology of Aging, 80, 11–20. [DOI] [PubMed] [Google Scholar]
- Janssen, D. J. A. , Müllerova, H. , Agusti, A. , Yates, J. C. , Tal‐Singer, R. , Rennard, S. I. , Vestbo, J. , & Wouters, E. F. M. (2014). Persistent systemic inflammation and symptoms of depression among patients with COPD in the ECLIPSE cohort. Respiratory Medicine, 108, 1647–1654. [DOI] [PubMed] [Google Scholar]
- Jergović, M. , Bendelja, K. , Mlakar, A. S. , Vojvoda, V. , Aberle, N. , Jovanovic, T. , Rabatić, S. , Sabioncello, A. , & Vidović, A. (2015). Circulating levels of hormones, lipids, and immune mediators in posttraumatic stress disorder – a three‐month follow‐up study. Frontiers in Psychiatry, 6, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha, M. K. , Abu, M. , Gadad, B. S. , Cherise, C. F. , & Trivedi, M. H. (2019). Higher S100B levels predict persistently elevated anhedonia with escitalopram monotherapy versus antidepressant combinations: Findings from CO‐MED trial. Pharmaceuticals, 12(4), 184. 10.3390/ph12040184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, H. , Ling, Z. , Zhang, Y. , Mao, H. , Ma, Z. , Yin, Y. , Wang, W. , Tang, W. , Tan, Z. , Shi, J. , Li, L. , & Ruan, B. (2015). Altered fecal microbiota composition in patients with major depressive disorder. Brain, Behavior, and Immunity, 48, 186–194. [DOI] [PubMed] [Google Scholar]
- Jin, J. , & Maren, S. (2015). Prefrontal‐hippocampal interactions in memory and emotion. Frontiers in Systems Neuroscience, 9, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls, M. , Karst, H. , & Sarabdjitsingh, R. A. (2018). The stressed brain of humans and rodents. Acta Physiologica, 223, e13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope, R. S. , Cheng, Y. , Lowell, J. A. , Worthen, R. J. , Sitbon, Y. H. , & Beurel, E. (2017). Stressed and inflamed, can GSK3 be blamed? Trends in Biochemical Sciences, 42, 180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadriu, B. , Farmer, C. A. , Yuan, P. , Park, L. T. , Deng, Z. D. , Moaddel, R. , Henter, I. D. , Shovestul, B. , Ballard, E. D. , Kraus, C. , Gold, P. W. , Machado‐Vieira, R. , & Zarate, C. A. (2019). The kynurenine pathway and bipolar disorder: Intersection of the monoaminergic and glutamatergic systems and immune response. Molecular Psychiatry, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadry, H. , Noorani, B. , & Cucullo, L. (2020). A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids and Barriers of the CNS, 17, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalina, U. , Koyama, N. , Hosoda, T. , Nuernberger, H. , Sato, K. , Hoelzer, D. , Herweck, F. , Manigold, T. , Singer, M. V. , Rossol, S. , & Böcker, U. (2002). Enhanced production of IL‐18 in butyrate‐treated intestinal epithelium by stimulation of the proximal promoter region. European Journal of Immunology, 32, 2635–2643. [DOI] [PubMed] [Google Scholar]
- Kamintsky, L. , Cairns, K. A. , Veksler, R. , Bowen, C. , Beyea, S. D. , Friedman, A. , & Calkin, C. (2020). Blood‐brain barrier imaging as a potential biomarker for bipolar disorder progression. NeuroImage Clinical, 26, 102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karson, A. , Demirtaş, T. , Bayramgürler, D. , Balci, F. , & Utkan, T. (2013). Chronic administration of infliximab (TNF‐α inhibitor) decreases depression and anxiety‐like behaviour in rat model of chronic mild stress. Basic & Clinical Pharmacology & Toxicology, 112, 335–340. [DOI] [PubMed] [Google Scholar]
- Kazemi, A. , Ali Noorbala, A. , Azam, K. , Hadi Eskandari, M. , & Djafarian, K. (2019). Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clinical Nutrition, 38, 522–528. [DOI] [PubMed] [Google Scholar]
- Kealy, J. , Greene, C. , & Campbell, M. (2020). Blood‐brain barrier regulation in psychiatric disorders. Neuroscience Letters, 726: 133664. [DOI] [PubMed] [Google Scholar]
- Kempuraj, D. , Selvakumar, G. P. , Thangavel, R. , Ahmed, M. E. , Zaheer, S. , Raikwar, S. P. , Iyer, S. S. , Bhagavan, S. M. , Beladakere‐Ramaswamy, S. , & Zaheer, A. (2017). Mast cell activation in brain injury, stress, and post‐traumatic stress disorder and Alzheimer's disease pathogenesis. Frontiers in Neuroscience, 11, 703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, J. R. , Kennedy, P. J. , Cryan, J. F. , Dinan, T. G. , Gerard, C. , & Hyland, N. P. (2015). Breaking down the barriers: the gut microbiome, intestinal permeability and stress‐related psychiatric disorders. Frontiers in Cellular Neuroscience, 9, 392. 10.3389/fncel.2015.00392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler, R. C. , Sampson, N. A. , Berglund, P. , Gruber, M. J. , Al‐Hamzawi, A. , Andrade, L. , Bunting, B. , Demyttenaere, K. , Florescu, S. , de Girolamo, G. , Gureje, O. , He, Y. , Hu, C. , Huang, Y. , Karam, E. , Kovess‐Masfety, V. , Lee, S. , Levinson, D. , Medina Mora, M. E. , … Wilcox, M. A. (2015). Anxious and non‐anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiology and Psychiatric Sciences, 24(3), 210–226. 10.1017/s2045796015000189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler, R. C. , Sonnega, A. , Bromet, E. , Hughes, M. , & Nelson, C. B. (1995). Posttraumatic stress disorder in the national comorbidity survey. Archives of General Psychiatry, 52, 1048–1060. [DOI] [PubMed] [Google Scholar]
- Kessler, R. C. , Wai, T. C. , Demler, O. , & Walters, E. E. (2005). Prevalence, severity, and comorbidity of 12‐month DSM‐IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62, 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kılıç, F. , Işık, Ü. , Demirdaş, A. , Doğuç, D. K. , & Bozkurt, M. (2020). Serum zonulin and claudin‐5 levels in patients with bipolar disorder. Journal of Affective Disorders, 266, 37–42. [DOI] [PubMed] [Google Scholar]
- Kim, H. , Chen, L. , Lim, G. , Sung, B. , Wang, S. , McCabe, M. F. , Rusanescu, G. , Yang, L. , Tian, Y. , & Mao, J. (2012). Brain indoleamine 2,3‐dioxygenase contributes to the comorbidity of pain and depression. Journal of Clinical Investigation, 122, 2940–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y. K. , Amidfar, M. , & Won, E. (2019). A review on inflammatory cytokine‐induced alterations of the brain as potential neural biomarkers in post‐traumatic stress disorder. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 91, 103–112. [DOI] [PubMed] [Google Scholar]
- Kim, Y.‐K. , Jung, H.‐G. , Myint, A.‐M. , Kim, H. , & Park, S.‐H. (2007). Imbalance between pro‐inflammatory and anti‐inflammatory cytokines in bipolar disorder. Journal of Affective Disorders, 104, 91–95. [DOI] [PubMed] [Google Scholar]
- Kim, Y. K. , & Won, E. (2017). The influence of stress on neuroinflammation and alterations in brain structure and function in major depressive disorder. Behavioral Brain Research, 329, 6–11. [DOI] [PubMed] [Google Scholar]
- Kiraly, D. D. , Horn, S. R. , Van Dam, N. T. , Costi, S. , Schwartz, J. , Kim‐Schulze, S. , Patel, M. , Hodes, G. E. , Russo, S. J. , Merad, M. , Iosifescu, D. V. , Charney, D. S. , & Murrough, J. W. (2017). Altered peripheral immune profiles in treatment‐resistant depression: Response to ketamine and prediction of treatment outcome. Translational Psychiatry, 7(3), e1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler, C. A. , Freitas, T. H. , Maes, M. , de Andrade, N. Q. , Liu, C. S. , Fernandes, B. S. , Stubbs, B. , Solmi, M. , Veronese, N. , Herrmann, N. , Raison, C. L. , Miller, B. J. , Lanctôt, K. L. , & Carvalho, A. F. (2017). Peripheral cytokine and chemokine alterations in depression: A meta‐analysis of 82 studies. Acta Psychiatrica Scandinavica, 135, 373–387. [DOI] [PubMed] [Google Scholar]
- Köhler, O. , Benros, M. E. , Nordentoft, M. , Farkouh, M. E. , Iyengar, R. L. , Mors, O. , & Krogh, J. (2014). Effect of anti‐inflammatory treatment on depression, depressive symptoms, and adverse effects a systematic review and meta‐analysis of randomized clinical trials. JAMA Psychiatry, 71, 1381–1391. [DOI] [PubMed] [Google Scholar]
- Kornstein, S. G. , Schatzberg, A. F. , Thase, M. E. , Yonkers, K. A. , McCullough, J. P. , Keitner, G. I. , Gelenberg, A. J. , Davis, S. M. , Harrison, W. M. , & Keller, M. B. (2000). Gender differences in treatment response to sertraline versus imipramine in chronic depression. American Journal of Psychiatry, 157, 1445–1452. [DOI] [PubMed] [Google Scholar]
- Krishnan, M. , & McCole, D. F. (2017). T cell protein tyrosine phosphatase prevents STAT1 induction of claudin‐2 expression in intestinal epithelial cells. Annals of the New York Academy of Sciences, 1405, 116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lach, G. , Schellekens, H. , Dinan, T. G. , & Cryan, J. F. (2018). Anxiety, depression, and the microbiome: A role for gut peptides. Neurotherapeutics: the Journal of the American Society for Experimental NeuroTherapeutics, 15, 36–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahlou‐Laforet, K. , Alhenc‐Gelas, M. , Pornin, M. , Bydlowski, S. , Seigneur, E. , Benetos, A. , Kierzin, J. M. , Scarabin, P. Y. , Ducimetiere, P. , Aiach, M. , Guize, L. , & Consoli, S. M. (2006). Relation of depressive mood to plasminogen activator inhibitor, tissue plasminogen activator, and fibrinogen levels in patients with versus without coronary heart disease. American Journal of Cardiology, 97, 1287–1291. [DOI] [PubMed] [Google Scholar]
- Landmann, R. , Knopf, H. P. , Link, S. , Sansano, S. , Schumann, R. , & Zimmerli, W. (1996). Human monocyte CD14 is upregulated by lipopolysaccharide. Infection and Immunity, 64, 1762–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langgartner, D. , Lowry, C. A. , & Reber, S. O. (2019). Old Friends, immunoregulation, and stress resilience. Pflügers Archiv‐European Journal of Physiology, 471, 237–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanser, L. , Kink, P. , Egger, E. M. , Willenbacher, W. , Fuchs, D. , Weiss, G. , & Kurz, K. (2020). Inflammation‐induced tryptophan breakdown is related with anemia, fatigue, and depression in cancer. Frontiers in Immunology, 11, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlant, Q. , Chakravarty, S. , Vialou, V. , Mukherjee, S. , Koo, J. W. , Kalahasti, G. , Bradbury, K. R. , Taylor, S. V. , Maze, I. , Kumar, A. , Graham, A. , Birnbaum, S. G. , Krishnan, V. , Truong, H. T. , Neve, R. L. , Nestler, E. J. , & Russo, S. J. (2009). Role of nuclear factor κB in ovarian hormone‐mediated stress hypersensitivity in female mice. Biological Psychiatry, 65, 874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, B. , Moon, K. M. , & Kim, C. Y. (2018). Tight junction in the intestinal epithelium: Its association with diseases and regulation by phytochemicals. Journal of Immunology Research, 2018, 2645465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton, S. P. , Nerurkar, L. , Krishnadas, R. , Johnman, C. , Graham, G. J. , & Cavanagh, J. (2018). Chemokines in depression in health and in inflammatory illness: A systematic review and meta‐analysis. Molecular Psychiatry, 23(1), 48–58. 10.1038/mp.2017.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard, B. E. (2018). Inflammation and depression: A causal or coincidental link to the pathophysiology? Acta Neuropsychiatrica, 30, 1–16. [DOI] [PubMed] [Google Scholar]
- Lespérance, F. , Frasure‐Smith, N. , Théroux, P. , & Irwin, M. (2004). The association between major depression and levels of soluble intercellular adhesion molecule 1, interleukin‐6, and C‐reactive protein in patients with recent acute coronary syndromes. American Journal of Psychiatry, 161(2), 271–277. 10.1176/appi.ajp.161.2.271 [DOI] [PubMed] [Google Scholar]
- Lewitus, G. M. , Cohen, H. , & Schwartz, M. (2008). Reducing post‐traumatic anxiety by immunization. Brain, Behavior, and Immunity, 22, 1108–1114. [DOI] [PubMed] [Google Scholar]
- Li, S. , Hua, D. , Wang, Q. , Yang, L. , Wang, X. , Luo, A. , & Yang, C. (2019). The role of bacteria and its derived metabolites in chronic pain and depression: Recent findings and research progress. International Journal of Neuropsychopharmacology, 23, 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Zhang, L. , Wang, X. , Wang, Z. , Zhang, J. , Jiang, R. , Wang, X. , Wang, K. , Liu, Z. , Xia, Z. , Xu, Z. , Nie, Y. , Lv, X. , Wu, X. , Zhu, H. , & Duan, L. (2016). Similar fecal microbiota signatures in patients with diarrhea‐predominant irritable bowel syndrome and patients with depression. Clinical Gastroenterology and Hepatology, 14, 1602–1611.e5. [DOI] [PubMed] [Google Scholar]
- Livanos, A. E. , Snider, E. J. , Whittier, S. , Chong, D. H. , Wang, T. C. , Abrams, J. A. , & Freedberg, D. E. (2018). Rapid gastrointestinal loss of Clostridial Clusters IV and XIVa in the ICU associates with an expansion of gut pathogens. PLoS One, 13, e0200322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan, R. W. , & McClung, C. A. (2016). Animal models of bipolar mania: The past, present and future. Neuroscience, 321, 163–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Vilchez, I. , Diaz‐Ricart, M. , Navarro, V. , Torramade, S. , Zamorano‐Leon, J. , Lopez‐Farre, A. , Galan, A. M. , Gasto, C. , & Escolar, G. (2016). Endothelial damage in major depression patients is modulated by SSRI treatment, as demonstrated by circulating biomarkers and an in vitro cell model. Translational Psychiatry, 6, e886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Z. , Ding, L. , Lu, Q. , & Chen, Y.‐H. (2013). Claudins in intestines: Distribution and functional significance in health and diseases. Tissue Barriers, 1, e24978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupp, C. , Robertson, M. L. , Wickham, M. E. , Sekirov, I. , Champion, O. L. , Gaynor, E. C. , & Finlay, B. B. (2007). Host‐mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host & Microbe, 2, 119–129. [DOI] [PubMed] [Google Scholar]
- Luyendyk, J. P. , Schoenecker, J. G. , & Flick, M. J. (2019). The multifaceted role of fibrinogen in tissue injury and inflammation. Blood, 133, 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, T. Y. , Iwamoto, G. K. , Hoa, N. T. , Akotia, V. , Pedram, A. , Boivin, M. A. , & Said, H. M. (2004). TNF‐α‐induced increase in intestinal epithelial tight junction permeability requires NF‐κB activation. American Journal of Physiology‐Gastrointestinal and Liver Physiology, 286(3), G367–G376. [DOI] [PubMed] [Google Scholar]
- Machado‐Vieira, R. , Andreazza, A. C. , Viale, C. I. , Zanatto, V. , Cereser, V. , Vargas, R. D. S. , Kapczinski, F. , Portela, L. V. , Souza, D. O. , Salvador, M. , & Gentil, V. (2007). Oxidative stress parameters in unmedicated and treated bipolar subjects during initial manic episode: A possible role for lithium antioxidant effects. Neuroscience Letters, 421(1), 33–36. 10.1016/j.neulet.2007.05.016 [DOI] [PubMed] [Google Scholar]
- Machado‐Vieira, R. , Lara, D. R. , Portela, L. V. C. , Gonçalves, C. A. , Soares, J. C. , Kapczinski, F. , & Souza, D. O. (2002). Elevated serum S100B protein in drug‐free bipolar patients during first manic episode: a pilot study. European Neuropsychopharmacology, 12(3), 269–272. 10.1016/s0924-977x(02)00029-9 [DOI] [PubMed] [Google Scholar]
- Madison, C. A. , & Eitan, S. (2020). Buprenorphine: Prospective novel therapy for depression and PTSD. Psychological Medicine, 50, 881–893. [DOI] [PubMed] [Google Scholar]
- Maes, M. , Kubera, M. , & Leunis, J. C. (2008). The gut‐brain barrier in major depression: Intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuroendocrinology Letters, 29, 117–124. [PubMed] [Google Scholar]
- Maes, M. , Kubera, M. , Leunis, J.‐C. , Berk, M. , Geffard, M. , & Bosmans, E. (2013). In depression, bacterial translocation may drive inflammatory responses, oxidative and nitrosative stress (O&NS), and autoimmune responses directed against O&NS‐damaged neoepitopes. Acta Psychiatrica Scandinavica, 127(5), 344–354. 10.1111/j.1600-0447.2012.01908.x [DOI] [PubMed] [Google Scholar]
- Maes, M. , Leonard, B. E. , Myint, A. M. , Kubera, M. , & Verkerk, R. (2011). The new “5‐HT” hypothesis of depression: Cell‐mediated immune activation induces indoleamine 2,3‐dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to th. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 35, 702–721. [DOI] [PubMed] [Google Scholar]
- Maes, M. , Mihaylova, I. , & Leunis, J. C. (2007). Increased serum IgM antibodies directed against phosphatidyl inositol (Pi) in chronic fatigue syndrome (CFS) and major depression: evidence that an IgM‐mediated immune response against Pi is one factor underpinning the comorbidity between both CFS and depression. Neuro Endocrinology Letters, 28(6), 861–867. [PubMed] [Google Scholar]
- Maes, M. , & Rief, W. (2012). Diagnostic classifications in depression and somatization should include biomarkers, such as disorders in the tryptophan catabolite (TRYCAT) pathway. Psychiatry Research, 196, 243–249. [DOI] [PubMed] [Google Scholar]
- Maes, M. , Sirivichayakul, S. , Kanchanatawan, B. , & Vodjani, A. (2019). Upregulation of the intestinal paracellular pathway with breakdown of tight and adherens junctions in deficit schizophrenia. Molecular Neurobiology, 56, 7056–7073. [DOI] [PubMed] [Google Scholar]
- Maes, M. , Verkerk, R. , Bonaccorso, S. , Ombelet, W. , Bosmans, E. , & Scharpé, S. (2002). Depressive and anxiety symptoms in the early puerperium are related to increased degradation of tryptophan into kynurenine, a phenomenon which is related to immune activation. Life Sciences, 1, 1837–1848. [DOI] [PubMed] [Google Scholar]
- Mahajan, G. J. , Vallender, E. J. , Garrett, M. R. , Challagundla, L. , Overholser, J. C. , Jurjus, G. , Dieter, L. , Syed, M. , Romero, D. G. , Benghuzzi, H. , & Stockmeier, C. A. (2018). Altered neuro‐inflammatory gene expression in hippocampus in major depressive disorder. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 82, 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkesman, O. , Pine, D. S. , Tragon, T. , Austin, D. R. , Henter, I. D. , Chen, G. , & Manji, H. K. (2009). Animal models of suicide‐trait‐related behaviors. Trends in Pharmacological Sciences, 30, 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Małkiewicz, M. A. , Szarmach, A. , Sabisz, A. , Cubała, W. J. , Szurowska, E. , & Winklewski, P. J. (2019). Blood‐brain barrier permeability and physical exercise. Journal of Neuroinflammation, 16, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankertz, J. , Tavalali, S. , Schmitz, H. , Mankertz, A. , Riecken, E. O. , Fromm, M. , & Schulzke, J. D. (2000). Expression from the human occludin promoter is affected by tumor necrosis factor α and interferon γ. Journal of Cell Science, 113, 2085–2090. [DOI] [PubMed] [Google Scholar]
- Maren, S. , Phan, K. L. , & Liberzon, I. (2013). The contextual brain: Implications for fear conditioning, extinction and psychopathology. Nature Reviews Neuroscience, 14(6), 417–428. 10.1038/nrn3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez‐Canabal, A. , Akers, K. G. , Josselyn, S. A. , & Frankland, P. W. (2013). Age‐dependent effects of hippocampal neurogenesis suppression on spatial learning. Hippocampus, 23, 66–74. [DOI] [PubMed] [Google Scholar]
- Martin‐Subero, M. , Anderson, G. , Kanchanatawan, B. , Berk, M. , & Maes, M. (2016). Comorbidity between depression and inflammatory bowel disease explained by immune‐inflammatory, oxidative, and nitrosative stress; Tryptophan catabolite; And gut‐brain pathways. CNS Spectrums, 21, 184–198. [DOI] [PubMed] [Google Scholar]
- Mathis, M. W. , & Mathis, A. (2020). Deep learning tools for the measurement of animal behavior in neuroscience. Current Opinion in Neurobiology, 60, 1–11. [DOI] [PubMed] [Google Scholar]
- Matsumoto, J. , Stewart, T. , Sheng, L. , Li, N. , Bullock, K. , Song, N. , Shi, M. , Banks, W. A. , & Zhang, J. (2017). Transmission of α‐synuclein‐containing erythrocyte‐derived extracellular vesicles across the blood‐brain barrier via adsorptive mediated transcytosis: Another mechanism for initiation and progression of Parkinson’s disease? Acta Neuropathologica Communications, 5, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, E. A. , Knight, R. , Mazmanian, S. K. , Cryan, J. F. , & Tillisch, K. (2014). Gut microbes and the brain: Paradigm shift in neuroscience. Journal of Neuroscience, 34, 15490–15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, K. C. , Bulloch, A. G. M. , Anne, D. , Lauren, B. , Williams, J. V. A. , Lavorato, D. H. , & Patten, S. B. (2015). Prevalence of bipolar I and II disorder in Canada. The Canadian Journal of Psychiatry, 60(3), 151–156. 10.1177/070674371506000310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen, B. S. (2007). Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews, 87, 873–904. [DOI] [PubMed] [Google Scholar]
- McIntyre, R. S. , & Calabrese, J. R. (2019). Bipolar depression: The clinical characteristics and unmet needs of a complex disorder. Current Medical Research and Opinion, 35, 1993–2005. [DOI] [PubMed] [Google Scholar]
- McIntyre, R. S. , Soczynska, J. K. , Beyer, J. L. , Woldeyohannes, H. O. , Law, C. W. Y. , Miranda, A. , Konarski, J. Z. , & Kennedy, S. H. (2007). Medical comorbidity in bipolar disorder: Reprioritizing unmet needs. Current Opinion in Psychiatry, 20, 406–416. [DOI] [PubMed] [Google Scholar]
- Mckim, D. B. , Patterson, J. M. , Wohleb, E. S. , Jarrett, B. L. , Reader, B. F. , Godbout, J. P. , & Sheridan, J. F. (2016). Sympathetic release of splenic monocytes promotes recurring anxiety following repeated social defeat. Biological Psychiatry, 79, 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim, D. B. , Weber, M. D. , Niraula, A. , Sawicki, C. M. , Liu, X. , Jarrett, B. L. , Ramirez‐Chan, K. , Wang, Y. , Roeth, R. M. , Sucaldito, A. D. , Sobol, C. G. , Quan, N. , Sheridan, J. F. , & Godbout, J. P. (2018). Microglial recruitment of IL‐1β‐producing monocytes to brain endothelium causes stress‐induced anxiety. Molecular Psychiatry, 23, 1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, N. D. , Haroon, E. , Xu, X. , Woolwine, B. J. , Li, Z. , & Felger, J. C. (2018). Inflammation negatively correlates with amygdala‐ventromedial prefrontal functional connectivity in association with anxiety in patients with depression: Preliminary results. Brain, Behavior, and Immunity, 73, 725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, N. D. , Stevens, J. S. , Li, Z. , Gillespie, C. F. , Fani, N. , Michopoulos, V. , & Felger, J. C. (2020). Inflammation, reward circuitry and symptoms of anhedonia and PTSD in trauma‐exposed women. Social Cognitive and Affective Neuroscience, 15, 1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard, C. , Pfau, M. L. , Hodes, G. E. , Kana, V. , Wang, V. X. , Bouchard, S. , Takahashi, A. , Flanigan, M. E. , Aleyasin, H. , LeClair, K. B. , Janssen, W. G. , Labonté, B. , Parise, E. M. , Lorsch, Z. S. , Golden, S. A. , Heshmati, M. , Tamminga, C. , Turecki, G. , Campbell, M. , … Russo, S. J. (2017). Social stress induces neurovascular pathology promoting depression. Nature Neuroscience, 20, 1752–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard, C. , Pfau, M. L. , Hodes, G. E. , & Russo, S. J. (2017). Immune and neuroendocrine mechanisms of stress vulnerability and resilience. Neuropsychopharmacology, 42, 62–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoud, A. , Mensi, R. , Douki, W. , Neffati, F. , Najjar, M. F. , Gobbi, G. , Valtorta, F. , Gaha, L. , & Comai, S. (2019). Reduced peripheral availability of tryptophan and increased activation of the kynurenine pathway and cortisol correlate with major depression and suicide. World Journal of Biological Psychiatry, 20, 703–711. [DOI] [PubMed] [Google Scholar]
- Mezrich, J. D. , Fechner, J. H. , Xiaoji, Z. , Johnson, B. P. , Burlingham, W. J. , & Bradfield, C. A. (2010). An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. The Journal of Immunology, 185(6), 3190–3198. 10.4049/jimmunol.0903670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos, V. , Rothbaum, A. O. , Jovanovic, T. , Almli, L. M. , Bradley, B. , Rothbaum, B. O. , Gillespie, C. F. , & Ressler, K. J. (2015). Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. American Journal of Psychiatry, 172, 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel‐Hidalgo, J. J. , Overholser, J. C. , Jurjus, G. J. , Meltzer, H. Y. , Dieter, L. , Konick, L. , Stockmeier, C. A. , & Rajkowska, G. (2011). Vascular and extravascular immunoreactivity for intercellular adhesion molecule 1 in the orbitofrontal cortex of subjects with major depression: Age‐dependent changes. Journal of Affective Disorders, 132, 422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milatz, S. , Krug, S. M. , Rosenthal, R. , Günzel, D. , Müller, D. , Schulzke, J. D. , Amasheh, S. , & Fromm, M. (2010). Claudin‐3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochimica Et Biophysica Acta (BBA) ‐ Biomembranes, 1798, 2048–2057. [DOI] [PubMed] [Google Scholar]
- Miller, A. H. (2013). Conceptual confluence: The kynurenine pathway as a common target for ketamine and the convergence of the inflammation and glutamate hypotheses of depression. Neuropsychopharmacology, 38, 1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, A. H. , & Pariante, C. M. (2020). Trial failures of anti‐inflammatory drugs in depression. The Lancet Psychiatry, 7, 837. [DOI] [PubMed] [Google Scholar]
- Miller, A. H. , & Raison, C. L. (2016). The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nature Reviews Immunology, 16, 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molendijk, M. L. , Bus, B. A. A. , Spinhoven, P. , Penninx, B. W. J. H. , Kenis, G. , Prickaerts, J. , Voshaar, R. C. O. , & Elzinga, B. M. (2011). Serum levels of brain‐derived neurotrophic factor in major depressive disorder: State‐trait issues, clinical features and pharmacological treatment. Molecular Psychiatry, 16, 1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moons, W. G. , Eisenberger, N. I. , & Taylor, S. E. (2010). Anger and fear responses to stress have different biological profiles. Brain, Behavior, and Immunity, 24, 215–219. [DOI] [PubMed] [Google Scholar]
- Mora, E. , Portella, M. J. , Piñol‐Ripoll, G. , López, R. , Cuadras, D. , Forcada, I. , Teres, M. , Vieta, E. , & Mur, M. (2019). High BDNF serum levels are associated to good cognitive functioning in bipolar disorder. European Psychiatry, 60, 97–107. [DOI] [PubMed] [Google Scholar]
- Mousavi, S. Y. , Khezri, R. , Karkhaneh‐Yousefi, M. A. , Mohammadinejad, P. , Gholamian, F. , Mohammadi, M. R. , Zeinoddini, A. , & Akhondzadeh, S. (2017). A randomized, double‐blind placebo‐controlled trial on effectiveness and safety of celecoxib adjunctive therapy in adolescents with acute bipolar mania. Journal of Child and Adolescent Psychopharmacology, 27, 494–500. [DOI] [PubMed] [Google Scholar]
- Mousavi, S. E. , Saberi, P. , Ghasemkhani, N. , Fakhraei, N. , Mokhtari, R. , & Dehpour, A. R. (2019). Licofelone Attenuates LPS‐induced depressive‐like behavior in mice: A possible role for nitric oxide. Journal of Pharmacy and Pharmaceutical Sciences, 21, 184–194. [DOI] [PubMed] [Google Scholar]
- Mukherjee, D. , Lee, S. , Kazinka, R. , Satterthwaite, D. T. , & Kable, J. W. (2020). Multiple facets of value‐based decision making in major depressive disorder. Scientific Reports, 10, 3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muneer, A. (2016). Bipolar disorder: Role of inflammation and the development of disease biomarkers. Psychiatry Investigation, 13, 18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, T. H. , Michelson, N. J. , Boyd, J. D. , Fong, T. , Bolaños, L. A. , Bierbrauer, D. , Siu, T. , Balbi, M. , Bolaños, F. , Vanni, M. , & Ledue, J. M. (2020). Automated task training and longitudinal monitoring of mouse mesoscale cortical circuits using home cages. Elife, 9, 1–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, D. , & Smith, K. V. (2018). Treatment efficacy for veterans with posttraumatic stress disorder: Latent class trajectories of treatment response and their predictors. Journal of Traumatic Stress, 31, 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell, K. A. , Dedovic, K. , Slavich, G. M. , Jarcho, M. R. , Breen, E. C. , Bower, J. E. , Irwin, M. R. , & Eisenberger, N. I. (2015). Greater amygdala activity and dorsomedial prefrontal‐amygdala coupling are associated with enhanced inflammatory responses to stress. Brain, Behavior, and Immunity, 43, 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil, R. , Schwarz, M. J. , Riedel, M. , Dehning, S. , Cerovecki, A. , Spellmann, I. , Arolt, V. , & Müller, N. (2011). Elevated macrophage migration inhibitory factor and decreased transforming growth factor‐beta levels in major depression ‐ No influence of celecoxib treatment. Journal of Affective Disorders, 134, 217–225. [DOI] [PubMed] [Google Scholar]
- Nasca, C. , Menard, C. , Hodes, G. , Bigio, B. , Pena, C. , Lorsch, Z. , Zelli, D. , Ferris, A. , Kana, V. , Purushothaman, I. , Dobbin, J. , Nassim, M. , DeAngelis, P. , Merad, M. , Rasgon, N. , Meaney, M. , Nestler, E. J. , McEwen, B. S. , & Russo, S. J. (2019). Multidimensional predictors of susceptibility and resilience to social defeat stress. Biological Psychiatry, 86, 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseribafrouei, A. , Hestad, K. , Avershina, E. , Sekelja, M. , Linløkken, A. , Wilson, R. , & Rudi, K. (2014). Correlation between the human fecal microbiota and depression. Neurogastroenterology and Motility, 26, 1155–1162. [DOI] [PubMed] [Google Scholar]
- Negrón‐Oyarzo, I. , Aboitiz, F. , & Fuentealba, P. (2016). Impaired functional connectivity in the prefrontal cortex: A mechanism for chronic stress‐induced neuropsychiatric disorders. Neural Plasticity, 2016, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettis, M. A. , & Pariante, C. M. (2020). Is there neuroinflammation in depression? Understanding the link between the brain and the peripheral immune system in depression. International Review of Neurobiology, 152, 23–40. [DOI] [PubMed] [Google Scholar]
- Nikfar, S. , Rahimi, R. , Rahimi, F. , Derakhshani, S. , & Abdollahi, M. (2008). Efficacy of probiotics in irritable bowel syndrome: A meta‐analysis of randomized, controlled trials. Diseases of the Colon and Rectum, 51, 1775–1780. [DOI] [PubMed] [Google Scholar]
- Niklasson, F. , & Agren, H. (1984). Brain energy metabolism and blood‐brain barrier permeability in depressive patients: Analyses of creatine, creatinine, urate, and albumin in CSF and blood. Biological Psychiatry, 19, 1183–1206. [PubMed] [Google Scholar]
- O’Carroll, S. J. , Kho, D. T. , Wiltshire, R. , Nelson, V. , Rotimi, O. , Johnson, R. , Angel, C. E. , & Graham, E. S. (2015). Pro‐inflammatory TNFα and IL‐1β differentially regulate the inflammatory phenotype of brain microvascular endothelial cells. Journal of Neuroinflammation, 12, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor, J. C. , Lawson, M. A. , André, C. , Moreau, M. , Lestage, J. , Castanon, N. , Kelley, K. W. , & Dantzer, R. (2009). Lipopolysaccharide‐induced depressive‐like behavior is mediated by indoleamine 2,3‐dioxygenase activation in mice. Molecular Psychiatry, 14, 511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan, A. , Chao, L. L. , Paulson, J. , Samuelson, K. W. , Shigenaga, J. K. , Grunfeld, C. , Weiner, M. W. , & Neylan, T. C. (2015). Altered inflammatory activity associated with reduced hippocampal volume and more severe posttraumatic stress symptoms in Gulf War veterans. Psychoneuroendocrinology, 51, 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan, A. , Cohen, B. E. , Seal, K. H. , Bertenthal, D. , Margaretten, M. , Nishimi, K. , & Neylan, T. C. (2015). Elevated risk for autoimmune disorders in iraq and afghanistan veterans with posttraumatic stress disorder. Biological Psychiatry, 77, 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahony, L. , Mccarthy, J. , Kelly, P. , Hurley, G. , Luo, F. , Chen, K. , O’Sullivan, G. C. , Kiely, B. , Collins, J. K. , Shanahan, F. , & Quigley, E. M. M. (2005). Lactobacillus and Bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology, 128, 541–551. [DOI] [PubMed] [Google Scholar]
- Ohlsson, L. , Gustafsson, A. , Lavant, E. , Suneson, K. , Brundin, L. , Westrin, Å. , Ljunggren, L. , & Lindqvist, D. (2019). Leaky gut biomarkers in depression and suicidal behavior. Acta Psychiatrica Scandinavica, 139, 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo, R. , Koga, M. , Katsumata, N. , Odamaki, T. , Matsuyama, S. , Oka, M. , Narita, H. , Hashimoto, N. , Kusumi, I. , Xiao, J. , & Matsuoka, Y. J. (2019). Effect of bifidobacterium breve A‐1 on anxiety and depressive symptoms in schizophrenia: A proof‐of‐concept study. Journal of Affective Disorders, 245, 377–385. [DOI] [PubMed] [Google Scholar]
- Orosz, A. , Jann, K. , Federspiel, A. , Horn, H. , Höfle, O. , Dierks, T. , Wiest, R. , Strik, W. , Müller, T. , & Walther, S. (2012). Reduced cerebral blood flow within the default‐mode network and within total gray matter in major depression. Brain Connectivity, 2, 303–310. [DOI] [PubMed] [Google Scholar]
- Ortiz‐Domínguez, A. , Hernández, M. E. , Berlanga, C. , Gutiérrez‐Mora, D. , Moreno, J. , Heinze, G. , & Pavón, L. (2007). Immune variations in bipolar disorder: Phasic differences. Bipolar Disorders, 9, 596–602. [DOI] [PubMed] [Google Scholar]
- Osimo, E. F. , Pillinger, T. , Rodriguez, I. M. , Khandaker, G. M. , Pariante, C. M. , & Howes, O. D. (2020). Inflammatory markers in depression: A meta‐analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain, Behavior, and Immunity, 87, 901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottesen, N. M. , Meluken, I. , Frikke‐Schmidt, R. , Plomgaard, P. , Scheike, T. , Kessing, L. V. , Miskowiak, K. , & Vinberg, M. (2020). S100B and brain derived neurotrophic factor in monozygotic twins with, at risk of and without affective disorders. Journal of Affective Disorders, 274, 726–732. 10.1016/j.jad.2020.05.015 [DOI] [PubMed] [Google Scholar]
- Oukka, M. , & Bettelli, E. (2018). Regulation of lymphocyte trafficking in central nervous system autoimmunity. Current Opinion in Immunology, 55, 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painold, A. , Mörkl, S. , Kashofer, K. , Halwachs, B. , Dalkner, N. , Bengesser, S. , Birner, A. , Birner, F. , Platzer, M. , Queissner, R. , Schütze, G. , Schwarz, M. J. , Moll, N. , Holzer, P. , Holl, A. K. , Kapfhammer, H.‐P. , Gorkiewicz, G. , & Reininghaus, E. Z. (2019). A step ahead: Exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disorders, 21(1), 40–49. 10.1111/bdi.12682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzutti, B. , Gubert, C. , Schuh, A. L. , Ferrari, P. , Bristot, G. , Fries, G. R. , Massuda, R. , Walz, J. , Rocha, N. P. , Berk, M. , Teixeira, A. L. , & Gama, C. S. (2015). Increased serum levels of eotaxin/CCL11 in late‐stage patients with bipolar disorder: An accelerated aging biomarker? Journal of Affective Disorders, 182, 64–69. 10.1016/j.jad.2014.12.010 [DOI] [PubMed] [Google Scholar]
- Pantović‐Stefanović, M. , Petronijević, N. , Dunjić‐Kostić, B. , Velimirović, M. , Nikolić, T. , Jurišić, V. , Lačković, M. , Damjanović, A. , Totić‐Poznanović, S. , Jovanović, A. A. , & Ivković, M. (2018). sVCAM‐1, sICAM‐1, TNF‐α and IL‐6 levels in bipolar disorder type I: Acute, longitudinal and therapeutic implications. The World Journal of Biological Psychiatry, 19, S41–S51. [DOI] [PubMed] [Google Scholar]
- Park, C. , Rosenblat, J. D. , Lee, Y. , Pan, Z. , Cao, B. , Iacobucci, M. , & McIntyre, R. S. (2019). The neural systems of emotion regulation and abnormalities in major depressive disorder. Behavioral Brain Research, 367, 181–188. [DOI] [PubMed] [Google Scholar]
- Parker, A. , Fonseca, S. , & Carding, S. R. (2019). Gut microbes and metabolites as modulators of blood‐brain barrier integrity and brain health. Gut Microbes, 11, 135–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten, S. B. , Williams, J. V. , Lavorato, D. H. , Wang, J. L. , McDonald, K. , & Bulloch, A. G. (2015). Descriptive epidemiology of major depressive disorder in Canada in 2012. The Canadian Journal of Psychiatry, 60, 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña, C. J. , Smith, M. , Ramakrishnan, A. , Cates, H. M. , Bagot, R. C. , Kronman, H. G. , Patel, B. , Chang, A. B. , Purushothaman, I. , Dudley, J. , Morishita, H. , Shen, L. , & Nestler, E. J. (2019). Early life stress alters transcriptomic patterning across reward circuitry in male and female mice. Nature Communications, 10(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky, Y. , Heilman, D. , Haorah, J. , Zelivyanskaya, M. , Persidsky, R. , Weber, G. A. , Shimokawa, H. , Kaibuchi, K. , & Ikezu, T. (2006). Rho‐mediated regulation of tight junctions during monocyte migration across the blood‐brain barrier in HIV‐1 encephalitis (HIVE). Blood, 107, 4770–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick, S. , Goldstein, L. H. , Perez, D. L. , & Nicholson, T. R. (2019). Emotional processing in functional neurological disorder: A review, biopsychosocial model and research agenda. Journal of Neurology, Neurosurgery and Psychiatry, 90, 704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piletz, J. E. , Angelos, H. , Omer, I. , Debra, H. , Jawed, F. , He, Z. , James, S. , & Lindsay, D. V. C. (2009). Pro‐inflammatory biomakers in depression: Treatment with venlafaxine. The World Journal of Biological Psychiatry, 10(4), 313–323. 10.3109/15622970802573246 [DOI] [PubMed] [Google Scholar]
- Pini, S. , de Queiroz, V. , Pagnin, D. , Pezawas, L. , Angst, J. , Cassano, G. B. , & Wittchen, H.‐U. (2005). Prevalence and burden of bipolar disorders in European countries. European Neuropsychopharmacology, 15(4), 425–434. 10.1016/j.euroneuro.2005.04.011 [DOI] [PubMed] [Google Scholar]
- Pinto‐Sanchez, M. I. , Hall, G. B. , Ghajar, K. , Nardelli, A. , Bolino, C. , Lau, J. T. , Martin, F. P. , Cominetti, O. , Welsh, C. , Rieder, A. , Traynor, J. , Gregory, C. , De Palma, G. , Pigrau, M. , Ford, A. C. , Macri, J. , Berger, B. , Bergonzelli, G. , Surette, M. G. , … Bercik, P. (2017). Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: A pilot study in patients with irritable bowel syndrome. Gastroenterology, 153, 448–459. [DOI] [PubMed] [Google Scholar]
- Pitman, R. K. , Rasmusson, A. M. , Koenen, K. C. , Shin, L. M. , Orr, S. P. , Gilbertson, M. W. , Milad, M. R. , & Liberzon, I. (2012). Biological studies of post‐traumatic stress disorder. Nature Reviews Neuroscience, 13, 769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli, D. A. (2011). Frontocingulate dysfunction in depression: Toward biomarkers of treatment response. Neuropsychopharmacology, 36, 183–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post, R. J. , & Warden, M. R. (2018). Melancholy, anhedonia, apathy: The search for separable behaviors and neural circuits in depression. Current Opinion in Neurobiology, 49, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell, N. D. , Sloan, E. K. , Bailey, M. T. , Arevalo, J. M. G. , Miller, G. E. , Chen, E. , Kobor, M. S. , Reader, B. F. , Sheridan, J. F. , & Cole, S. W. (2013). Social stress up‐regulates inflammatory gene expression in the leukocyte transcriptome via β‐adrenergic induction of myelopoiesis. Proceedings of the National Academy of Sciences of the United States of America, 110, 16574–16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers, A. , Dixon, H. D. , Conneely, K. , Gluck, R. , Munoz, A. , Rochat, C. , Mendoza, H. , Hartzell, G. , Ressler, K. J. , Bradley, B. , Pace, T. W. W. , Umpierrez, G. E. , Schwartz, A. C. , Michopoulos, V. , & Gillespie, C. F. (2019). The differential effects of PTSD, MDD, and dissociation on CRP in trauma‐exposed women. Comprehensive Psychiatry, 93, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad, S. , Mingrino, R. , Kaukinen, K. , Hayes, K. L. , Powell, R. M. , MacDonald, T. T. , & Collins, J. E. (2005). Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Laboratory Investigation, 85, 1139–1162. [DOI] [PubMed] [Google Scholar]
- Prosberg, M. , Bendtsen, F. , Vind, I. , Petersen, A. M. , & Gluud, L. L. (2016). The association between the gut microbiota and the inflammatory bowel disease activity: A systematic review and meta‐analysis. Scandinavian Journal of Gastroenterology, 51, 1407–1415. [DOI] [PubMed] [Google Scholar]
- Quiñones, M. M. , Maldonado, L. , Velazquez, B. , & Porter, J. T. (2016). Candesartan ameliorates impaired fear extinction induced by innate immune activation. Brain, Behavior, and Immunity, 52, 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison, C. L. , Dantzer, R. , Kelley, K. W. , Lawson, M. A. , Woolwine, B. J. , Vogt, G. , Spivey, J. R. , Saito, K. , & Miller, A. H. (2010). CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN‐α: Relationship to CNS immune responses and depression. Molecular Psychiatry, 15, 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison, C. L. , Rutherford, R. E. , Woolwine, B. J. , Shuo, C. , Schettler, P. , Drake, D. F. , Haroon, E. , & Miller, A. H. (2013). A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment‐resistant depression: The role of baseline inflammatory biomarkers. Archives of General Psychiatry, 70, 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez, K. , Shea, D. T. , McKim, D. B. , Reader, B. F. , & Sheridan, J. F. (2015). Imipramine attenuates neuroinflammatory signaling and reverses stress‐induced social avoidance. Brain, Behavior, and Immunity, 46, 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey, J. M. , Cooper, J. D. , Bot, M. , Guest, P. C. , Lamers, F. , Weickert, C. S. , Penninx, B. W. J. H. , & Bahn, S. (2016). Sex differences in serum markers of major depressive disorder in the netherlands study of depression and anxiety (NESDA). PLoS One, 11(5), e0156624. 10.1371/journal.pone.0156624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, J. S. , Harry, G. J. , Rapoport, S. I. , & Kim, H. W. (2010). Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Molecular Psychiatry, 15(4), 384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran, L. N. , & Stein, M. B. (2010). Pharmacotherapy of post‐traumatic stress disorder. Current Topics in Behavioral Neurosciences, 2, 505–525. [DOI] [PubMed] [Google Scholar]
- Reboldi, A. , Coisne, C. , Baumjohann, D. , Benvenuto, F. , Bottinelli, D. , Lira, S. , Uccelli, A. , Lanzavecchia, A. , Engelhardt, B. , & Sallusto, F. (2009). C‐C chemokine receptor 6‐regulated entry of TH‐17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nature Immunology, 10, 514–523. [DOI] [PubMed] [Google Scholar]
- Regier, D. A. , Narrow, W. E. , Clarke, D. E. , Kraemer, H. C. , Kuramoto, S. J. , Kuhl, E. A. , & Kupfer, D. J. (2013). DSM‐5 field trials in the United States and Canada, part II: Test‐retest reliability of selected categorical diagnoses. American Journal of Psychiatry, 170, 59–70. [DOI] [PubMed] [Google Scholar]
- Règue, M. , Poilbout, C. , Martin, V. , Franc, B. , Lanfumey, L. , & Mongeau, R. (2019). Increased 5‐HT2C receptor editing predisposes to PTSD‐like behaviors and alters BDNF and cytokines signaling. Translational Psychiatry, 9, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reininghaus, E. Z. , Lackner, N. , Birner, A. , Bengesser, S. , Fellendorf, F. T. , Platzer, M. , Rieger, A. , Queissner, R. , Kainzbauer, N. , Reininghaus, B. , McIntyre, R. S. , Mangge, H. , Zelzer, S. , Fuchs, D. , Dejonge, S. , & Müller, N. (2016). Extracellular matrix proteins matrix metallopeptidase 9 (MMP9) and soluble intercellular adhesion molecule 1 (sICAM‐1) and correlations with clinical staging in euthymic bipolar disorder. Bipolar Disorders, 18, 155–163. [DOI] [PubMed] [Google Scholar]
- Reininghaus, E. Z. , Wetzlmair, L. C. , Fellendorf, F. T. , Platzer, M. , Queissner, R. , Birner, A. , Pilz, R. , Hamm, C. , Maget, A. , Rieger, A. , Prettenhofer, A. , Wurm, W. , Mörkl, S. , & Dalkner, N. (2020). Probiotic treatment in individuals with euthymic bipolar disorder: A pilot‐study on clinical changes and compliance. Neuropsychobiology, 79, 71–79. [DOI] [PubMed] [Google Scholar]
- Remus, J. L. , & Dantzer, R. (2016). Inflammation models of depression in rodents: Relevance to psychotropic drug discovery. International Journal of Neuropsychopharmacology, 19, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter‐Levin, G. , Stork, O. , & Schmidt, M. V. (2019). Animal models of PTSD: A challenge to be met. Molecular Psychiatry, 24, 1135–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann, P. , Altenhofen, B. , Riegel, A. , Baudewig, J. , & Felgenhauer, K. (1997). Soluble adhesion molecules (sVCAM‐1 and sICAM‐1) in cerebrospinal fluid and serum correlate with MRI activity in multiple sclerosis. Annals of Neurology, 41, 326–333. [DOI] [PubMed] [Google Scholar]
- Rincón‐Cortés, M. , Herman, J. P. , Lupien, S. , Maguire, J. , & Shansky, R. M. (2019). Stress: Influence of sex, reproductive status and gender. Neurobiol. Stress, 10, 100–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios, A. C. , Maurya, P. K. , Pedrini, M. , Zeni‐Graiff, M. , Asevedo, E. , Mansur, R. B. , Wieck, A. , Grassi‐Oliveira, R. , McIntyre, R. S. , Hayashi, M. A. F. , & Brietzke, E. (2017). Microbiota abnormalities and the therapeutic potential of probiotics in the treatment of mood disorders. Reviews in the Neurosciences, 28, 739–749. [DOI] [PubMed] [Google Scholar]
- Rolls, E. T. (2019). The cingulate cortex and limbic systems for emotion, action, and memory. Brain Structure and Function, 224, 3001–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong, H. , Xie, X.‐H. , Zhao, J. , Lai, W.‐T. , Wang, M.‐B. , Xu, D. , Liu, Y.‐H. , Guo, Y.‐Y. , Xu, S.‐X. , Deng, W.‐F. , Yang, Q.‐F. , Xiao, L. I. , Zhang, Y.‐L. , He, F.‐S. , Wang, S. , & Liu, T.‐B. (2019). Similarly in depression, nuances of gut microbiota: Evidences from a shotgun metagenomics sequencing study on major depressive disorder versus bipolar disorder with current major depressive episode patients. Journal of Psychiatric Research, 113, 90–99. 10.1016/j.jpsychires.2019.03.017 [DOI] [PubMed] [Google Scholar]
- Rosso, G. , Cattaneo, A. , Zanardini, R. , Gennarelli, M. , Maina, G. , & Bocchio‐Chiavetto, L. (2015). Glucose metabolism alterations in patients with bipolar disorder. Journal of Affective Disorders, 184, 293–298. [DOI] [PubMed] [Google Scholar]
- Rowland, T. A. , & Marwaha, S. (2018). Epidemiology and risk factors for bipolar disorder. Therapeutic Advances in Psychopharmacology, 8, 251–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow, D. R. , & Schmidt, P. J. (2019). Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology, 44, 111–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudzki, L. , Ostrowska, L. , Pawlak, D. , Małus, A. , Pawlak, K. , Waszkiewicz, N. , & Szulc, A. (2019). Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double‐blind, randomized, placebo controlled study. Psychoneuroendocrinology, 100, 213–222. [DOI] [PubMed] [Google Scholar]
- Russo, S. J. , & Nestler, E. J. (2013). The brain reward circuitry in mood disorders. Nature Reviews Neuroscience, 14, 609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, K. M. , Allers, K. A. , McLoughlin, D. M. , & Harkin, A. (2020). Tryptophan metabolite concentrations in depressed patients before and after electroconvulsive therapy. Brain, Behavior, and Immunity, 83, 153–162. [DOI] [PubMed] [Google Scholar]
- Rzepa, E. , & McCabe, C. (2016). Decreased anticipated pleasure correlates with increased salience network resting state functional connectivity in adolescents with depressive symptomatology. Journal of Psychiatric Research, 82, 40–47. 10.1016/j.jpsychires.2016.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra, J. M. , Sánchez‐Lemus, E. , & Benicky, J. (2011). Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: Therapeutic implications. Psychoneuroendocrinology, 36, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha, R. N. , Liu, X. , & Pahan, K. (2006). Up‐regulation of BDNF in astrocytes by TNF‐α: A case for the neuroprotective role of cytokine. Journal of Neuroimmune Pharmacology: the Official Journal of the Society on NeuroImmune Pharmacology, 1, 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandiego, C. M. , Gallezot, J. D. , Pittman, B. , Nabulsi, N. , Lim, K. , Lin, S. F. , Matuskey, D. , Lee, J. Y. , O’Connor, K. C. , Huang, Y. , Carson, R. E. , Hannestad, J. , Cosgrove, K. P. , & Fowler, J. S. (2015). Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proceedings of the National Academy of Sciences of the United States of America, 112, 12468–12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sántha, P. , Veszelka, S. , Hoyk, Z. , Mészáros, M. , Walter, F. R. , Tóth, A. E. , Kiss, L. , Kincses, A. , Oláh, Z. , Seprényi, G. , Rákhely, G. , Dér, A. , Pákáski, M. , Kálmán, J. , Kittel, Á. , & Deli, M. A. (2016). Restraint stress‐induced morphological changes at the blood‐brain barrier in adult rats. Frontiers in Molecular Neuroscience, 8, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz, J. (2020). The kynurenine pathway: A finger in every pie. Molecular Psychiatry, 25, 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz, J. , Dantzer, R. , Meier, T. B. , Wurfel, B. E. , Victor, T. A. , McIntosh, S. A. , Ford, B. N. , Morris, H. M. , Bodurka, J. , Teague, T. K. , & Drevets, W. C. (2015). Activation of the kynurenine pathway is associated with striatal volume in major depressive disorder. Psychoneuroendocrinology, 62, 54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz, J. , Drevets, W. C. , Wurfel, B. E. , Ford, B. N. , Bellgowan, P. S. F. , Victor, T. A. , Bodurka, J. , Teague, T. K. , & Dantzer, R. (2015). Reduction of kynurenic acid to quinolinic acid ratio in both the depressed and remitted phases of major depressive disorder. Brain, Behavior, and Immunity, 46, 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki, C. M. , McKim, D. B. , Wohleb, E. S. , Jarrett, B. L. , Reader, B. F. , Norden, D. M. , Godbout, J. P. , & Sheridan, J. F. (2015). Social defeat promotes a reactive endothelium in a brain region‐dependent manner with increased expression of key adhesion molecules, selectins and chemokines associated with the recruitment of myeloid cells to the brain. Neuroscience, 302, 151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa, J. R. , Fatma, M. , Loh, Y. E. , Traore, S. R. , Stefan, T. , Chen, T. H. , Nestler, E. J. , & Labonté, B. (2020). Shared transcriptional signatures in major depressive disorder and mouse chronic stress models. Biological Psychiatry, 88, 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer, M. , Sarkar, S. , Schwarz, M. , & Friebe, A. (2016). Soluble intracellular adhesion molecule‐1 in patients with unipolar or bipolar affective disorders: Results from a pilot trial. Neuropsychobiology, 74, 8–14. [DOI] [PubMed] [Google Scholar]
- Schedlowski, M. , Engler, H. , & Grigoleit, J. S. (2014). Endotoxin‐induced experimental systemic inflammation in humans: A model to disentangle immune‐to‐brain communication. Brain, Behavior, and Immunity, 35, 1–8. [DOI] [PubMed] [Google Scholar]
- Schmidtner, A. K. , Slattery, D. A. , Gläsner, J. , Hiergeist, A. , Gryksa, K. , Malik, V. A. , Hellmann‐Regen, J. , Heuser, I. , Baghai, T. C. , Gessner, A. , Rupprecht, R. , Di Benedetto, B. , & Neumann, I. D. (2019). Minocycline alters behavior, microglia and the gut microbiome in a trait‐anxiety‐dependent manner. Translational Psychiatry, 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaar, R. L. , & Taniguchi, N. (2017). Glycosphingolipids. New Comprehensive Biochemistry, 10, 1–99. [Google Scholar]
- Schoultz, I. , & Keita, Å. V. (2020). The intestinal barrier and current techniques for the assessment of gut permeability. Cells, 9(8), 1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter, M. L. , Abdul‐Khaliq, H. , Krebs, M. , Diefenbacher, A. , & Blasig, I. E. (2008). Serum markers support disease‐specific glial pathology in major depression. Journal of Affective Disorders, 111(2–3), 271–280. [DOI] [PubMed] [Google Scholar]
- Schroeter, M. L. , Hashim, A.‐K. , Albert, D. , & Blasig, I. E. (2002). S100B is increased in mood disorders and may be reduced by antidepressive treatment. NeuroReport, 13(13), 1675–1678. 10.1097/00001756-200209160-00021 [DOI] [PubMed] [Google Scholar]
- Schwarcz, R. , Bruno, J. P. , Muchowski, P. J. , & Wu, H. Q. (2012). Kynurenines in the mammalian brain: When physiology meets pathology. Nature Reviews Neuroscience, 13, 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, L. A. , & Luo, L. (2015). Organization of the locus coeruleus‐norepinephrine system. Current Biology, 25, R1051–R1056. [DOI] [PubMed] [Google Scholar]
- Segerstrom, S. C. , & Miller, G. E. (2004). Psychological stress and the human immune system: A meta‐analytic study of 30 years of inquiry. Psychological Bulletin, 130, 1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaas, M. N. , Riese, H. , Renken, R. J. , Wichers, M. , Bastiaansen, J. A. , Figueroa, C. A. , Geugies, H. , Mocking, R. J. T. , Geerligs, L. , Marsman, J.‐B. C. , Aleman, A. , Schene, A. H. , Schoevers, R. A. , & Ruhé, H. G. (2017). Associations between daily affective instability and connectomics in functional subnetworks in remitted patients with recurrent major depressive disorder. Neuropsychopharmacology, 42(13), 2583–2592. 10.1038/npp.2017.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan, E. , Wilson, A. A. , Mizrahi, R. , Rusjan, P. M. , Miler, L. , Rajkowska, G. , Suridjan, I. , Kennedy, J. L. , Rekkas, P. V. , Houle, S. , & Meyer, J. H. (2015). Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry, 72, 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan, E. , Wilson, A. A. , Mizrahi, R. , Rusjan, P. M. , Miler, L. , Rajkowska, G. , Suridjan, I. , Kennedy, J. L. , Rekkas, V. , Houle, S. , & Meyer, J. H. (2016). Increased translocator protein distribution volume, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry, 72, 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance, E. G. , Gressitt, K. L. , Stallings, C. R. , Origoni, A. E. , Khushalani, S. , Leweke, F. M. , Dickerson, F. B. , & Yolken, R. H. (2013). Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophrenia Research, 148, 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance, E. G. , Gressitt, K. L. , Yang, S. , Stallings, C. R. , Origoni, A. E. , Vaughan, C. , Khushalani, S. , Alaedini, A. , Dickerson, F. B. , & Yolken, R. H. (2014). Seroreactive marker for inflammatory bowel disease and associations with antibodies to dietary proteins in bipolar disorder. Bipolar Disorders, 16, 230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sforzini, L. , Nettis, M. A. , Mondelli, V. , & Pariante, C. M. (2019). Inflammation in cancer and depression: A starring role for the kynurenine pathway. Psychopharmacology (Berl), 236, 2997–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrbabaki, M. , Sabouri, S. , Sabahi, A. , Barfeh, D. , Divsalar, P. , Esmailzadeh, M. , & Ahmadi, A. (2020). The efficacy of probiotics for treatment of bipolar disorder‐type 1: A randomized, double‐blind, placebo controlled trial, Iran. The American Journal of Psychiatry, 15, 10–16. [PMC free article] [PubMed] [Google Scholar]
- Sheen, T. R. , Ebrahimi, C. M. , Hiemstra, I. H. , Barlow, S. B. , Peschel, A. , & Doran, K. S. (2010). Penetration of the blood‐brain barrier by staphylococcus aureus: Contribution of membrane‐anchored lipoteichoic acid. Journal of Molecular Medicine, 88, 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, Q. , Zhao, L. , & Tuohy, K. M. (2012). High‐level dietary fibre up‐regulates colonic fermentation and relative abundance of saccharolytic bacteria within the human faecal microbiota in vitro. European Journal of Nutrition, 51, 693–705. [DOI] [PubMed] [Google Scholar]
- Shibasaki, C. , Itagaki, K. , Abe, H. , Kajitani, N. , Okada‐Tsuchioka, M. , & Takebayashi, M. (2018). Possible association between serum matrix metalloproteinase‐9 (MMP‐9) levels and relapse in depressed patients following electroconvulsive therapy (ECT). International Journal of Neuropsychopharmacology, 21(3), 236–241. 10.1093/ijnp/pyx086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki, C. , Takebayashi, M. , Itagaki, K. , Abe, H. , Kajitani, N. , Okada‐Tsuchioka, M. , & Yamawaki, S. (2016). Altered serum levels of matrix metalloproteinase‐2, ‐9 in response to electroconvulsive therapy for mood disorders. International Journal of Neuropsychopharmacology, 19(9), pyw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, N. M. , McNamara, K. , Chow, C. W. , Maser, R. S. , Papakostas, G. I. , Pollack, M. H. , Nierenberg, A. A. , Fava, M. , & Wong, K. K. (2008). A detailed examination of cytokine abnormalities in major depressive disorder. European Neuropsychopharmacology, 18(3), 230–233. 10.1016/j.euroneuro.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, N. , Thangaraju, M. , Prasad, P. D. , Martin, P. M. , Lambert, N. A. , Boettger, T. , Offermanns, S. , & Ganapathy, V. (2010). Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)‐dependent inhibition of histone deacetylases. Journal of Biological Chemistry, 285, 27601–27608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaraman, S. V. , Klapproth, J. M. , Moore, D. A. , Landers, C. , Targan, S. , Williams, I. R. , & Gewirtz, A. T. (2005). Elevated flagellin‐specific immunoglobulins in Crohn’s disease. The American Journal of Physiology‐Gastrointestinal and Liver Physiology, 288, G403–G406. [DOI] [PubMed] [Google Scholar]
- Slyepchenko, A. , Maes, M. , Jacka, F. N. , Köhler, C. A. , Barichello, T. , McIntyre, R. S. , Berk, M. , Grande, I. , Foster, J. A. , Vieta, E. , & Carvalho, A. F. (2016). Gut microbiota, bacterial translocation, and interactions with diet: Pathophysiological links between major depressive disorder and non‐communicable medical comorbidities. Psychotherapy and Psychosomatics, 86, 31–46. [DOI] [PubMed] [Google Scholar]
- Smolen, J. S. , Weinblatt, M. E. , Sheng, S. , Zhuang, Y. , & Hsu, B. (2014). Sirukumab, a human anti‐interleukin‐6 monoclonal antibody: A randomised, 2‐part (proof‐of‐concept and dose‐finding), phase II study in patients with active rheumatoid arthritis despite methotrexate therapy. Annals of the Rheumatic Diseases, 73, 1616–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol, H. , Pigneur, B. , Watterlot, L. , Lakhdari, O. , Bermúdez‐Humarán, L. G. , Gratadoux, J. J. , Blugeon, S. , Bridonneau, C. , Furet, J. P. , Corthier, G. , Grangette, C. , Vasquez, N. , Pochart, P. , Trugnan, G. , Thomas, G. , Blottière, H. M. , Doré, J. , Marteau, P. , Seksik, P. , & Langella, P. (2008). Faecalibacterium prausnitzii is an anti‐inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proceedings of the National Academy of Sciences of the United States of America, 105, 16731–16736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somvanshi, P. R. , Mellon, S. H. , Flory, J. D. , Abu‐Amara, D. , Wolkowitz, O. M. , Yehuda, R. , Jett, M. , Hood, L. , Marmar, C. , & Doyle, F. J. (2019). Mechanistic inferences on metabolic dysfunction in posttraumatic stress disorder from an integrated model and multiomic analysis: Role of glucocorticoid receptor sensitivity. American Journal of Physiology. Endocrinology and Metabolism, 317, E879–E898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Št’astný, F. , Škultétyová, I. , Pliss, L. , & Ježová, D. (2000). Quinolinic acid enhances permeability of rat brain microvessels to plasma albumin. Brain Research Bulletin, 53, 415–420. [DOI] [PubMed] [Google Scholar]
- Statistics Canada . (2020). Canadian Community Health Survey – Annual component (CCHS). Retrieved from https://www.statcan.gc.ca/eng/survey/household/3226
- Stein, D. J. , Vasconcelos, M. F. , Albrechet‐Souza, L. , Ceresér, K. M. M. , & de Almeida, R. M. M. (2017). Microglial over‐activation by social defeat stress contributes to anxiety‐ and depressive‐like behaviors. Frontiers in Behavioural Neurosciences, 11, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinemann, A. , Galm, I. , Chip, S. , Nitsch, C. , & Maly, I. P. (2016). Claudin‐1, ‐2 and ‐3 are selectively expressed in the epithelia of the choroid plexus of the mouse from early development and into adulthood while Claudin‐5 is restricted to endothelial cells. Frontiers in Neuroanatomy, 10, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner, J. , Bogerts, B. , Sarnyai, Z. , Walter, M. , Gos, T. , Bernstein, H. G. , & Myint, A. M. (2012). Bridging the gap between the immune and glutamate hypotheses of schizophrenia and major depression: Potential role of glial NMDA receptor modulators and impaired bloodbrain barrier integrity. The World Journal of Biological Psychiatry, 13, 482–492. [DOI] [PubMed] [Google Scholar]
- Steiner, J. , Walter, M. , Gos, T. , Guillemin, G. J. , Bernstein, H. G. , Sarnyai, Z. , Mawrin, C. , Brisch, R. , Bielau, H. , zu Schwabedissen, L. , Bogerts, B. , & Myint, A.‐M. (2011). Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: Evidence for an immune‐modulated glutamatergic neurotransmission? Journal of Neuroinflammation, 8(1), 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, B. R. , Goel, R. , Seungbum, K. , Richards, E. M. , Holbert, R. C. , Pepine, C. J. , & Raizada, M. K. (2018). Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut, 67, 1555–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange, B. A. , Witter, M. P. , Lein, E. S. , & Moser, E. I. (2014). Functional organization of the hippocampal longitudinal axis. Nature Reviews Neuroscience, 15, 655–669. [DOI] [PubMed] [Google Scholar]
- Stuart, S. A. , Butler, P. , Munafò, M. R. , Nutt, D. J. , & Robinson, E. S. J. (2013). A translational rodent assay of affective biases in depression and antidepressant therapy. Neuropsychopharmacology, 38, 1625–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgeon, C. , & Fasano, A. (2016). Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers, 4, e1251384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sublette, M. E. , Galfalvy, H. C. , Fuchs, D. , Lapidus, M. , Grunebaum, M. F. , Oquendo, M. A. , John Mann, J. , & Postolache, T. T. (2011). Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain, Behavior, and Immunity, 25(6), 1272–1278. 10.1016/j.bbi.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . (2019). 2018 National Survey on Drug Use and Health: Methodological summary and definitions. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov/data/ [Google Scholar]
- Sumner, J. A. , Chen, Q. , Roberts, A. L. , Winning, A. , Rimm, E. B. , Gilsanz, P. , Glymour, M. M. , Tworoger, S. S. , Koenen, K. C. , & Kubzansky, L. D. (2018). Posttraumatic stress disorder onset and inflammatory and endothelial function biomarkers in women. Brain, Behavior, and Immunity, 69, 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y. , Li, L. , Xie, R. , Wang, B. , Jiang, K. , & Cao, H. (2019). Stress triggers flare of inflammatory bowel disease in children and adults. Frontiers in Pediatrics, 7, 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, T. , Yoshinaga, N. , & Tanabe, S. (2011). Interleukin‐6 (IL‐6) regulates claudin‐2 expression and tight junction permeability in intestinal epithelium. Journal of Biological Chemistry, 286, 31263–31271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyszkowicz, J. K. , Wong, A. , Anisman, H. , Merali, Z. , & Audet, M.‐C. (2017). Implications of the gut microbiota in vulnerability to the social avoidance effects of chronic social defeat in male mice. Brain, Behavior, and Immunity, 66, 45–55. [DOI] [PubMed] [Google Scholar]
- Tadin Hadjina, I. , Zivkovic, P. M. , Matetic, A. , Rusic, D. , Vilovic, M. , Bajo, D. , Puljiz, Z. , Tonkic, A. , & Bozic, J. (2019). Impaired neurocognitive and psychomotor performance in patients with inflammatory bowel disease. Scientific Reports, 9(1), 1–13. 10.1038/s41598-019-50192-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, A. , Chung, J. R. , Zhang, S. , Zhang, H. , Grossman, Y. , Aleyasin, H. , Flanigan, M. E. , Pfau, M. L. , Menard, C. , Dumitriu, D. , Hodes, G. E. , McEwen, B. S. , Nestler, E. J. , Han, M. H. , & Russo, S. J. (2017). Establishment of a repeated social defeat stress model in female mice. Scientific Reports, 7, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer, F. E. , Althubaity, N. , Schubert, J. , Nettis, M. A. , Cousins, O. , Dima, D. , Mondelli, V. , Bullmore, E. T. , Pariante, C. , & Veronese, M. (2021). Increased serum peripheral C‐reactive protein is associated with reduced brain barriers permeability of TSPO radioligands in healthy volunteers and depressed patients: implications for inflammation and depression. Brain, Behavior, and Immunity, 91, 487–497. [DOI] [PubMed] [Google Scholar]
- Thomas, A. J. , Davis, S. , Ferrier, I. N. , Kalaria, R. N. , & O’Brien, J. T. (2004). Elevation of cell adhesion molecule immunoreactivity in the anterior cingulate cortex in bipolar disorder. Biological Psychiatry, 55, 652–655. [DOI] [PubMed] [Google Scholar]
- Thomas, A. J. , Ferrier, I. N. , Kalaria, R. N. , Woodward, S. A. , Ballard, C. , Oakley, A. , Perry, R. H. , & O’Brien, J. T. (2000). Elevation in late‐life depression of intercellular adhesion molecule‐1 expression in the dorsolateral prefrontal cortex. American Journal of Psychiatry, 157, 1682–1684. [DOI] [PubMed] [Google Scholar]
- Thomas, A. J. , Perry, R. , Kalaria, R. N. , Oakley, A. , McMeekin, W. , & O’Brien, J. T. (2003). Neuropathological evidence for ischemia in the white matter of the dorsolateral prefrontal cortex in late‐life depression. International Journal of Geriatric Psychiatry, 18, 7–13. [DOI] [PubMed] [Google Scholar]
- Thomson, C. A. , McColl, A. , Cavanagh, J. , & Graham, G. J. (2014). Peripheral inflammation is associated with remote global gene expression changes in the brain. Journal of Neuroinflammation, 11, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft, H. , Bramness, J. G. , Lien, L. , Abebe, D. S. , Wampold, B. E. , Tilden, T. , Hestad, K. , & Neupane, S. P. (2018). PTSD patients show increasing cytokine levels during treatment despite reduced psychological distress. Neuropsychiatric Disease and Treatment, 14, 2367–2378. 10.2147/ndt.s173659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin, D. F. , & Foa, E. B. (2006). Sex differences in trauma and posttraumatic stress disorder: A quantitative review of 25 years of research. Psychological Bulletin, 132, 959–992. [DOI] [PubMed] [Google Scholar]
- Tondo, L. , Pompili, M. , Forte, A. , & Baldessarini, R. J. (2016). Suicide attempts in bipolar disorders: Comprehensive review of 101 reports. Acta Psychiatrica Scandinavica, 133, 174–186. [DOI] [PubMed] [Google Scholar]
- Tondo, L. , Vazquez, G. , & Baldessarini, R. (2016). Depression and mania in bipolar disorder. Current Neuropharmacology, 15, 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Too, L. K. , Li, K. M. , Suarna, C. , Maghzal, G. J. , Stocker, R. , McGregor, I. S. , & Hunt, N. H. (2016). Deletion of TDO2, IDO‐1 and IDO‐2 differentially affects mouse behavior and cognitive function. Behavioral Brain Research, 312, 102–117. [DOI] [PubMed] [Google Scholar]
- Török, N. , Tanaka, M. , & Vécsei, L. (2020). Searching for peripheral biomarkers in neurodegenerative diseases: The tryptophan‐kynurenine metabolic pathway. International Journal of Molecular Sciences, 21, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres‐Platas, S. G. , Cruceanu, C. , Chen, G. G. , Turecki, G. , & Mechawar, N. (2014). Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain, Behavior, and Immunity, 42, 50–59. [DOI] [PubMed] [Google Scholar]
- Troché, G. , Henry‐Lagarrigue, M. , Soppelsa, F. , Legriel, S. , Yehia, A. , Bruneel, F. , Bédos, J. P. , & Spreux‐Varoquaux, O. (2020). Tryptophan pathway catabolites (serotonin, 5‐hydroxyindolacetic acid, kynurenine) and enzymes (monoamine oxidase and indole amine 2,3 dioxygenase) in patients with septic shock: A prospective observational study versus healthy controls. Medicine (Baltimore), 99, e19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, S. Y. , Gildengers, A. G. , Hsu, J. L. , Chung, K. H. , Chen, P. H. , & Huang, Y. J. (2019). Inflammation associated with volume reduction in the gray matter and hippocampus of older patients with bipolar disorder. Journal of Affective Disorders, 244, 60–66. [DOI] [PubMed] [Google Scholar]
- Tsai, M.‐C. , & Huang, T.‐L. (2017). Decreased S100B serum levels after treatment in bipolar patients in a manic phase. Comprehensive Psychiatry, 74, 27–34. 10.1016/j.comppsych.2016.12.008 [DOI] [PubMed] [Google Scholar]
- Tu, P. C. , Li, C. T. , Lin, W. C. , Chen, M. H. , Su, T. P. , & Bai, Y. M. (2017). Structural and functional correlates of serum soluble IL‐6 receptor level in patients with bipolar disorder. Journal of Affective Disorders, 219, 172–177. [DOI] [PubMed] [Google Scholar]
- Tulkens, J. , Vergauwen, G. , Van Deun, J. , Geeurickx, E. , Dhondt, B. , Lippens, L. , De Scheerder, M. A. , Miinalainen, I. , Rappu, P. , De Geest, B. G. , Vandecasteele, K. , Laukens, D. , Vandekerckhove, L. , Denys, H. , Vandesompele, J. , De Wever, O. , & Hendrix, A. (2020). Increased levels of systemic LPS‐positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction. Gut, 69, 191–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan, C. , Kesebir, S. , & Süner, Ö. (2014). Are ICAM, VCAM and E‐selectin levels different in first manic episode and subsequent remission? Journal of Affective Disorders, 163, 76–80. 10.1016/j.jad.2014.03.052 [DOI] [PubMed] [Google Scholar]
- Tyler, W. J. , Alonso, M. , Bramham, C. R. , & Pozzo‐Miller, L. D. (2002). From acquisition to consolidation: On the role of brain‐derived neurotrophic factor signaling in hippocampal‐dependent learning. Learning & Memory, 9(5), 224–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usta, A. , Kılıç, F. , Demirdaş, A. , Işık, Ü. , Doğuç, D. K. , & Bozkurt, M. (2020). Serum zonulin and claudin‐5 levels in patients with schizophrenia. European Archives of Psychiatry and Clinical Neuroscience, 1, 3. [DOI] [PubMed] [Google Scholar]
- Valvassori, S. S. , Varela, R. B. , & Quevedo, J. (2013). Animal models of mood disorders: Focus on bipolar disorder and depression. In Conn P. M. (Ed.), Animal models for the study of human disease (2nd edn , pp. 991–1001). Cambridge, Massachusetts, United States: Academic press. [Google Scholar]
- Van Agtmaal, M. J. M. , Houben, A. J. H. M. , Pouwer, F. , Stehouwer, C. D. A. , & Schram, M. T. (2017). Association of microvascular dysfunction with late‐life depression. JAMA Psychiatry, 74(7), 729. 10.1001/jamapsychiatry.2017.0984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ameringen, M. , Mancini, C. , Patterson, B. , & Boyle, M. H. (2008). Post‐traumatic stress disorder in Canada. CNS Neuroscience & Therapeutics, 14(3), 171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancamelbeke, M. , & Vermeire, S. (2017). The intestinal barrier: A fundamental role in health and disease. Expert Review of Gastroenterology & Hepatology, 11, 821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela, E. , Manichanh, C. , Gallart, M. , Torrejón, A. , Borruel, N. , Casellas, F. , Guarner, F. , & Antolin, M. (2013). Colonisation by Faecalibacterium prausnitzii and maintenance of clinical remission in patients with ulcerative colitis. Alimentary Pharmacology & Therapeutics, 38, 151–161. [DOI] [PubMed] [Google Scholar]
- Vares, E. A. , Lehmann, S. , Sauer, C. , Pariante, C. , Wieland, F. , Soltmann, B. , Bauer, M. , & Ritter, P. (2020). Association of pro‐inflammatory cytokines with clinical features in euthymic patients with Bipolar‐I‐Disorder. Journal of Affective Disorders, 277, 450–455. [DOI] [PubMed] [Google Scholar]
- Verbitsky, A. , Dopfel, D. , & Zhang, N. (2020). Rodent models of post‐traumatic stress disorder: Behavioral assessment. Translational Psychiatry, 10, 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volynets, V. , Rings, A. , Bárdos, G. , Ostaff, M. J. , Wehkamp, J. , & Bischoff, S. C. (2016). Intestinal barrier analysis by assessment of mucins, tight junctions, and α‐defensins in healthy C57BL/6J and BALB/cJ mice. Tissue Barriers, 4, e1208468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouros, A. , Gehring, T. V. , Szydlowska, K. , Janusz, A. , Tu, Z. , Croucher, M. , Lukasiuk, K. , Konopka, W. , Sandi, C. , & Vasilaki, E. (2018). A generalised framework for detailed classification of swimming paths inside the Morris Water Maze. Scientific Reports, 8(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed, A. , Dalton, B. , Wesemann, U. , Ibrahim, M. A. , & Himmerich, H. (2018). A systematic review of interleukin‐1β in post‐traumatic stress disorder: Evidence from human and animal studies. Journal of Interferon & Cytokine Research, 38(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Walker, A. K. , Wing, E. E. , Banks, W. A. , & Dantzer, R. (2019). Leucine competes with kynurenine for blood‐to‐brain transport and prevents lipopolysaccharide‐induced depression‐like behavior in mice. Molecular Psychiatry, 24, 1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. B. , Wang, P. Y. , Wang, X. , Wan, Y. L. , & Liu, Y. C. (2012). Butyrate enhances intestinal epithelial barrier function via up‐regulation of tight junction protein claudin‐1 transcription. Digestive Diseases and Sciences, 57, 3126–3135. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Zhao, H. , & Girgenti, M. J. (2021). Posttraumatic stress disorder brain transcriptomics: Convergent genomic signatures across biological sex. Biological Psychiatry, 10.1016/j.biopsych.2021.02.012 [DOI] [PubMed] [Google Scholar]
- Wang, L. , Llorente, C. , Hartmann, P. , Yang, A.‐M. , Chen, P. , & Schnabl, B. (2015). Methods to determine intestinal permeability and bacterial translocation during liver disease. Journal of Immunological Methods, 421, 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, N. , Yu, H. Y. , Shen, X. F. , Gao, Z. Q. , Yang, C. , Yang, J. J. , & Zhang, G. F. (2015). The rapid antidepressant effect of ketamine in rats is associated with down‐regulation of pro‐inflammatory cytokines in the hippocampus. Upsala Journal of Medical Sciences, 120, 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. , Timberlake, M. A. , Prall, K. , & Dwivedi, Y. (2017). The recent progress in animal models of depression. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 77, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. , Uzzau, S. , Goldblum, S. E. , & Fasano, A. (2000). Human zonulin, a potential modulator of intestinal tight junctions. Journal of Cell Science, 113, 4435–4440. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , & Kasper, L. H. (2014). The role of microbiome in central nervous system disorders. Brain, Behavior, and Immunity, 38, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, B. L. , Vialou, V. F. , Iñiguez, S. D. , Alcantara, L. F. , Wright, K. N. , Jiang, F. , Kennedy, P. J. , Quincey, L. P. , Li, S. , Nestler, E. J. , & Bolaños‐Guzmán, C. A. (2013). Neurobiological sequelae of witnessing stressful events in adult mice. Biological Psychiatry, 73(1), 7–14. 10.1016/j.biopsych.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, L. , Li, Y. , Tang, W. , Sun, Q. , Chen, L. , Wang, X. , Liu, Q. , Yu, S. , Yu, S. , Liu, C. , & Ma, X. (2019). Chronic unpredictable mild stress in rats induces colonic inflammation. Frontiers in Physiology, 10, 1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . (2012). Depression in Europe. https://www.euro.who.int/en/health‐topics/noncommunicablediseases/mental‐health/news/news/2012/10/depression‐in‐europe
- Whorwell, P. J. (2009). Review: Do probiotics improve symptoms in patients with irritable bowel syndrome? Therapeutic Advances in Gastroenterology, 2, 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers, M. C. , Koek, G. H. , Robaeys, G. , Verkerk, R. , Scharpé, S. , & Maes, M. (2005). IDO and interferon‐α‐induced depressive symptoms: A shift in hypothesis from tryptophan depletion to neurotoxicity. Molecular Psychiatry, 10, 538–544. [DOI] [PubMed] [Google Scholar]
- Wiener, C. D. , Jansen, K. , Ghisleni, G. , Kaster, M. P. , de Mattos Souza, L. D. , Lara, D. R. , Portela, L. V. , Da Silva, R. A. , & Oses, J. P. (2013). Reduced serum levels of neuron specific enolase (NSE) in drug‐naïve subjects with major depression and bipolar disorder. Neurochemical Research, 38(7), 1394–1398. 10.1007/s11064-013-1036-x [DOI] [PubMed] [Google Scholar]
- Wiener, C. D. , Moreira, F. P. , Portela, L. V. , Strogulski, N. R. , Lara, D. R. , da Silva, R. A. , de Mattos Souza, L. D. , Jansen, K. , & Oses, J. P. (2019). Interleukin‐6 and interleukin‐10 in mood disorders: A population‐based study. Psychiatry Research, 273, 685–689. [DOI] [PubMed] [Google Scholar]
- Willner, P. (1997). Validity, reliability and utility of the chronic mild stress model of depression: A 10‐year review and evaluation. Psychopharmacology (Berl), 134, 319–329. [DOI] [PubMed] [Google Scholar]
- Wilson, B. C. , McLaughlin, L. D. , Ebenezer, P. J. , Nair, A. R. , Dange, R. , Harre, J. G. , Shaak, T. L. , Diamond, D. M. , & Francis, J. (2014). Differential effects of sertraline in a predator exposure animal model of post‐traumatic stress disorder. Frontiers in Behavioural Neurosciences, 8, 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wium‐Andersen, M. K. , Ørsted, D. D. , & Nordestgaard, B. G. (2013). Elevated plasma fibrinogen, psychological distress, antidepressant use, and hospitalization with depression: Two large population‐based studies. Psychoneuroendocrinology, 38, 638–647. [DOI] [PubMed] [Google Scholar]
- Wohleb, E. S. , Franklin, T. , Iwata, M. , & Duman, R. S. (2016). Integrating neuroimmune systems in the neurobiology of depression. Nature Reviews Neuroscience, 17, 497–511. [DOI] [PubMed] [Google Scholar]
- Wohleb, E. S. , Hanke, M. L. , Corona, A. W. , Powell, N. D. , Stiner, L. M. , Bailey, M. T. , Nelson, R. J. , Godbout, J. P. , & Sheridan, J. F. (2011). β‐Adrenergic receptor antagonism prevents anxiety‐like behavior and microglial reactivity induced by repeated social defeat. Journal of Neuroscience, 31, 6277–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb, E. S. , McKim, D. B. , Shea, D. T. , Powell, N. D. , Tarr, A. J. , Sheridan, J. F. , & Godbout, J. P. (2014). Re‐establishment of anxiety in stress‐sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biological Psychiatry, 75, 970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb, E. S. , Powell, N. D. , Godbout, J. P. , & Sheridan, J. F. (2013). Stress‐induced recruitment of bone marrow‐derived monocytes to the brain promotes anxiety‐like behavior. Journal of Neuroscience, 33, 13820–13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, D. , Prameya, R. , & Dorovini‐Zis, K. (1999). In vitro adhesion and migration of t lymphocytes across monolayers of human brain microvessel endothelial cells: Regulation by ICAM‐1, VCAM‐1, E‐selectin and PECAM‐1. Journal of Neuropathology and Experimental Neurology, 58, 138–152. [DOI] [PubMed] [Google Scholar]
- Woodcock, E. A. , Schain, M. , Cosgrove, K. P. , & Hillmer, A. T. (2020). Quantification of [11C]PBR28 data after systemic lipopolysaccharide challenge. EJNMMI Research, 10(1), 10.1186/s13550-020-0605-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, R. , Jiang, P. , Lin, L. I. , Jiang, J. , Yu, B. , Rao, J. , Liu, H. , Wei, W. , & Qiao, Y. I. (2020). Oral treatment with Lactobacillus reuteri attenuates depressive‐like behaviors and serotonin metabolism alterations induced by chronic social defeat stress. Journal of Psychiatric Research, 122, 70–78. 10.1016/j.jpsychires.2019.12.013 [DOI] [PubMed] [Google Scholar]
- Xu, C. , Lee, S. K. , Zhang, D. , & Frenette, P. S. (2020). The gut microbiome regulates psychological‐stress‐induced inflammation. Immunity, 53, 417–428.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J. , Zheng, P. , Li, Y. , Wu, J. , Tan, X. , Zhou, J. , Sun, Z. , Chen, X. , Zhang, G. , Zhang, H. , Huang, Y. , Chai, T. , Duan, J. , Liang, W. , Yin, B. , Lai, J. , Huang, T. , Du, Y. , Zhang, P. , … Xie, P. (2020). Landscapes of bacterial and metabolic signatures and their interaction in major depressive disorders. Science Advances, 6(49), eaba8555. 10.1126/sciadv.aba8555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, K. , Xie, G.‐R. , Hu, Y.‐Q. , Mao, F.‐Q. , & Su, L.‐Y. (2008). The effects of gender and numbers of depressive episodes on serum S100B levels in patients with major depression. Journal of Neural Transmission, 115(12), 1687–1694. 10.1007/s00702-008-0130-8 [DOI] [PubMed] [Google Scholar]
- Yehuda, R. , & Seckl, J. (2011). Minireview: Stress‐related psychiatric disorders with low cortisol levels: A metabolic hypothesis. Endocrinology, 152, 4496–4503. [DOI] [PubMed] [Google Scholar]
- Yin, W. , Gallagher, N. R. , Sawicki, C. M. , McKim, D. B. , Godbout, J. P. , & Sheridan, J. F. (2019). Repeated social defeat in female mice induces anxiety‐like behavior associated with enhanced myelopoiesis and increased monocyte accumulation in the brain. Brain, Behavior, and Immunity, 78, 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya, R. (1996). Endotoxin produces a depressive‐like episode in rats. Brain Research, 711, 163–174. [DOI] [PubMed] [Google Scholar]
- Yoshida, T. , Ishikawa, M. , Niitsu, T. , Nakazato, M. , Watanabe, H. , Shiraishi, T. , Shiina, A. , Hashimoto, T. , Kanahara, N. , Hasegawa, T. , Enohara, M. , Kimura, A. , Iyo, M. , & Hashimoto, K. (2012). Decreased serum levels of mature brain‐derived neurotrophic factor (BDNF), but not its precursor proBDNF, in patients with major depressive disorder. PLoS One, 7(8), e42676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, M. B. , Howell, L. L. , Hopkins, L. , Moshfegh, C. , Yu, Z. , Clubb, L. , Seidenberg, J. , Park, J. , Swiercz, A. P. , & Marvar, P. J. (2018). A peripheral immune response to remembering trauma contributes to the maintenance of fear memory in mice. Psychoneuroendocrinology, 94, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, R. C. , Biggs, J. T. , Ziegler, V. E. , & Meyer, D. A. (1978). A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry, 133, 429–435. [DOI] [PubMed] [Google Scholar]
- Yuan, D. , Zhao, Y. , Banks, W. A. , Bullock, K. M. , Haney, M. , Batrakova, E. , & Kabanov, A. V. (2017). Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials, 142, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, N. , Chen, Y. , Xia, Y. , Dai, J. , & Liu, C. (2019). Inflammation‐related biomarkers in major psychiatric disorders: A cross‐disorder assessment of reproducibility and specificity in 43 meta‐analyses. Translational Psychiatry, 9, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelante, T. , Iannitti, R. G. , Cunha, C. , De Luca, A. , Giovannini, G. , Pieraccini, G. , Zecchi, R. , D’Angelo, C. , Massi‐Benedetti, C. , Fallarino, F. , Carvalho, A. , Puccetti, P. , & Romani, R. (2013). Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin‐22. Immunity, 39(2), 372–385. 10.1016/j.immuni.2013.08.003 [DOI] [PubMed] [Google Scholar]
- Zhang, G. , & Ghosh, S. (2001). Toll‐like receptor‐mediated NF‐κB activation: A phylogenetically conserved paradigm in innate immunity. Journal of Clinical Investigation, 107, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Rothermundt, M. , Peters, M. , Wiesmann, M. , Hoy, L. , Arolt, V. , Emrich, H. M. , & Dietrich, D. E. (2009). S100B serum levels and word memory processing in remitted major depression as reflected by brain potentials. Neuropsychobiology, 59(3), 172–177. 10.1159/000219304 [DOI] [PubMed] [Google Scholar]
- Zhao, J. , Bi, W. , Xiao, S. , Lan, X. , Cheng, X. , Zhang, J. , Lu, D. , Wei, W. , Wang, Y. , Li, H. , Fu, Y. , & Zhu, L. (2019). Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Scientific Reports, 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, A. J. , Lee, Y. , Salvadore, G. , Hsu, B. , Fonseka, T. M. , Kennedy, S. H. , & McIntyre, R. S. (2017). Sirukumab: A potential treatment for mood disorders? Advances in Therapy, 34, 78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. L. , Wu, F. C. , Wang, C. Y. , Zheng, W. , Lan, X. F. , Deng, X. R. , & Ning, Y. P. (2020). Relationship between hippocampal volume and inflammatory markers following six infusions of ketamine in major depressive disorder. Journal of Affective Disorders, 276, 608–615. [DOI] [PubMed] [Google Scholar]
- Zhou, Z. , Guille, C. , Ogunrinde, E. , Liu, R. , Luo, Z. , Powell, A. , & Jiang, W. (2018). Increased systemic microbial translocation is associated with depression during early pregnancy. Journal of Psychiatric Research, 97, 54–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y. , Klomparens, E. A. , Guo, S. , & Geng, X. (2019). Neuroinflammation caused by mental stress: The effect of chronic restraint stress and acute repeated social defeat stress in mice. Neurological Research, 42, 762–769. [DOI] [PubMed] [Google Scholar]
- Ziegler, T. R. , Luo, M. , Estívariz, C. F. , Moore, D. A. , Sitaraman, S. V. , Hao, L. , Bazargan, N. , Klapproth, J. M. , Tian, J. , Galloway, J. R. , Leader, L. M. , Jones, D. P. , & Gewirtz, A. T. (2008). Detectable serum flagellin and lipopolysaccharide and upregulated anti‐flagellin and lipopolysaccharide immunoglobulins in human short bowel syndrome. The American Journal of Physiology ‐ Regulatory, Integrative and Comparative Physiology, 294, R402–R410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman, G. , Shaltiel, G. , Barbash, S. , Cohen, J. , Gasho, C. J. , Shenhar‐Tsarfaty, S. , Shalev, H. , Berliner, S. A. , Shelef, I. , Shoham, S. , Friedman, A. , Cohen, H. , & Soreq, H. (2012). Post‐traumatic anxiety associates with failure of the innate immune receptor TLR9 to evade the pro‐inflammatory NFκB pathway. Translational. Psychiatry, 2(2), e78–e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoga, M. , Oulis, P. , Chatzipanagiotou, S. , Masdrakis, V. G. , Pliatsika, P. , Boufidou, F. , Foteli, S. , Soldatos, C. R. , Nikolaou, C. , & Papageorgiou, C. (2014). Indoleamine 2,3‐dioxygenase and immune changes under antidepressive treatment in major depression in females. Vivo (Brooklyn), 28, 633–638. [PubMed] [Google Scholar]
- Zunszain, P. A. , Anacker, C. , Cattaneo, A. , Choudhury, S. , Musaelyan, K. , Myint, A. M. , Thuret, S. , Price, J. , & Pariante, C. M. (2012). Interleukin‐1β: A new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology, 37, 939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]


