Abstract
Aim
To assess the relationship between neonatal brain development and injury with early motor outcomes in infants with critical congenital heart disease (CCHD).
Method
Neonatal brain magnetic resonance imaging was performed after open‐heart surgery with cardiopulmonary bypass. Cortical grey matter (CGM), unmyelinated white matter, and cerebellar volumes, as well as white matter motor tract fractional anisotropy and mean diffusivity were assessed. White matter injury (WMI) and arterial ischaemic stroke (AIS) with corticospinal tract (CST) involvement were scored. Associations with motor outcomes at 3, 9, and 18 months were corrected for repeated cardiac surgery.
Results
Fifty‐one infants (31 males, 20 females) were included prospectively. Median age at neonatal surgery and postoperative brain magnetic resonance imaging was 7 days (interquartile range [IQR] 5–11d) and 15 days (IQR 12–21d) respectively. Smaller CGM and cerebellar volumes were associated with lower fine motor scores at 9 months (CGM regression coefficient=0.51, 95% confidence interval [CI]=0.15–0.86; cerebellum regression coefficient=3.08, 95% CI=1.07–5.09) and 18 months (cerebellum regression coefficient=2.08, 95% CI=0.47–5.12). The fractional anisotropy and mean diffusivity of white matter motor tracts were not related with motor scores. WMI was related to lower gross motor scores at 9 months (mean difference −0.8SD, 95% CI=−1.5 to −0.2). AIS with CST involvement increased the risk of gross motor problems and muscle tone abnormalities. Cerebral palsy (n=3) was preceded by severe ischaemic brain injury.
Interpretation
Neonatal brain development and injury are associated with fewer favourable early motor outcomes in infants with CCHD.
Abbreviations
- AIS
Arterial ischaemic stroke
- Bayley‐III‐NL
Bayley Scales of Infant and Toddler Development, Third Edition (Dutch)
- CCHD
Critical congenital heart disease
- CGM
Cortical grey matter
- CST
Corticospinal tract
- DTI
Diffusion tensor imaging
- UMW
Unmyelinated white matter
- WMI
White matter injury
What this paper adds.
Neonatal brain development and injury were associated with fewer favourable early motor outcomes in infants with critical congenital heart disease (CCHD).
Smaller cortical and cerebellar volumes were related to poorer fine motor skills.
Ischaemic brain injury with corticospinal tract involvement negatively affected muscle tone and gross motor outcomes.
Cerebral palsy was preceded by severe ischaemic brain injury.
Neonatal brain magnetic resonance imaging is a useful biomarker for early motor outcomes in infants with CCHD.
Neurodevelopmental disabilities are common in infants with critical congenital heart disease (CCHD) who undergo neonatal open‐heart surgery with cardiopulmonary bypass. 1 Delayed neonatal brain development and injury might contribute to motor skill delays (30–60%), which are already present in the early phases of development. 2 , 3 , 4
Delayed volumetric brain development occurs already in utero, persists neonatally, and is thought to be the result of reduced brain oxygenation and perfusion in individuals with CCHD. 4 Smaller brain volumes after neonatal cardiac surgery are associated with worse cognitive and language outcomes at 1 year. 5 In infants born preterm, cortical grey matter (CGM), unmyelinated white matter (UMW), and cerebellar volumes at term‐equivalent age are associated with neurodevelopmental outcome through early school age. 6
Delayed microstructural brain development, expressed by white matter fractional anisotropy and mean diffusivity, differs preoperatively between neonates with transposition of the great arteries, single‐ventricle physiology, and left ventricular outflow tract with aortic arch obstruction, which might be explained by differences in brain oxygenation and perfusion. 2 In infants born preterm, fractional anisotropy of the posterior limb of the internal capsule and white matter tracts are associated with adverse motor outcomes at 15 and 18 months respectively. 7 , 8 , 9 In infants and adolescents with transposition of the great arteries and single‐ventricle physiology, delayed microstructural white matter development contributes to lower cognitive performance. 10 , 11
Ischaemic brain injury is commonly observed in neonates with CCHD and is most likely caused by fluctuations in brain perfusion and oxygenation and thromboembolic events. 12 Neonatal white matter injury (WMI) and posterior limb internal capsule involvement are associated with lower IQ, as well as more attention and motor problems in school‐age infants with CCHD. 3 Likewise, neonatal WMI severity is related to cerebral palsy (CP) and neurodevelopmental deficits at 24 months in infants born preterm. 13
The impact on early motor skills by delayed neonatal brain development and neonatal brain injury have been rarely reported in infants with CCHD. It is clinically important to adequately predict motor deficits to intervene at an early stage and prevent complications later in life. 14 In infants with CCHD, we hypothesized that (1) the neonatal volumes of the CGM, UMW, and cerebellum are positively associated with motor performance, (2) higher neonatal fractional anisotropy and lower neonatal mean diffusivity values of white matter motor tracts, representing increased microstructural development, are associated with improved early motor outcomes, and (3) neonates with postoperative WMI and arterial ischaemic stroke (AIS) with corticospinal tract (CST) involvement have fewer favourable motor outcomes.
METHOD
Study design and population
This prospective study is part of the CHD LifeSpan program at the Wilhelmina Children’s Hospital, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands. 2 , 15 Neonates with CCHD who underwent cardiac surgery with cardiopulmonary bypass within the first month after birth between 2016 and 2018 were included. Neonates born preterm or infants with a genetic disorder were excluded. Neonatal brain magnetic resonance imaging (MRI) scans were performed as part of clinical care at 5 to 10 days after surgery. This study included neonatal postoperative MRI since this was the last imaging modality before developmental testing. The neonatal MRI/diffusion tensor imaging (DTI) protocol is presented in the Appendix S1 (online supporting information). Motor assessment was performed at the ages of 3, 9, and 18 months at the outpatient clinic as part of routine care. The medical ethical committee approved the study and written parental informed consent for the use of clinical data for research purposes (no. 16‐093) was obtained.
Neonatal volumetric brain development
Coronal T2‐weighted images were automatically segmented in CGM, UMW, myelinated white matter, basal ganglia and thalamus, cerebellum, brainstem, and ventricular and extracerebral cerebrospinal fluid using a developed convolutional neural network method. 4 , 16 Tissue segmentations were checked manually and included if quality was reliable. The volumes of the CGM, UMW, and cerebellum were used since these were previously related to neurodevelopmental outcome through early school age in infants born preterm. 6 Neonates with brain injury were included in the volumetric analysis when the quality of segmentations was not affected (manually evaluated).
Neonatal microstructural brain development
DTI data were analysed according to the DTI toolbox ExploreDTI (PROVIDI Lab, Utrecht; http://www.exploredti.com/). 17 Fractional anisotropy and mean diffusivity values were calculated for different anatomical brain regions using a multicontrast human neonatal brain atlas. 18 Data were visually checked and included if the quality of tensor estimation, motion correction, and template registration was reliable. 2 Fractional anisotropy (expected to increase in white matter with increasing age) and mean diffusivity (expected to decrease in all brain regions with increasing age) of projection white matter fibres were chosen since this was based on our hypothesis. The following motor tracts were included: anterior, superior, and posterior corona radiata; posterior limb internal capsule; and cerebral peduncle (in addition to anterior and retrolenticular internal capsule, posterior thalamic radiation, and sagittal striatum). 2 Neonates with brain injury were included in the DTI analysis when the quality of the DTI data was not affected (manually evaluated).
Neonatal brain injury
MRI scans were assessed by experienced neonatal neurologists (LdV, MB) to score WMI (including WMI volume) and AIS (specific arterial distribution, involving CGM and/or central grey matter), including CST involvement. WMI was classified as none, mild (1–3 foci ≤2mm), moderate (>3–6 foci ≤2mm or 1–2 foci of 2–4mm), or severe (>6 foci ≤2mm or >2 foci of 2–4mm or any foci >4mm). 3 , 15 The volumes of WMI lesions were manually segmented on T1‐weighted images and calculated using 3D Slicer (https://www.slicer.org/). 19
Early motor assessment
Infants with CCHD underwent age‐appropriate neurological and motor developmental assessments by an experienced neonatologist and paediatric physical therapist. Neurological examination included testing of the cranial nerves (eye contact/tracking movements, facial motor functioning), muscle tone (overall, axial, extremities), strength (arms/legs), reflexes, and signs of asymmetry. The quality of general movements, absence of fidgety movements, Amiel‐Tison abnormal muscle tone (hypertonia/hypotonia axial/extremities), high risk/development of CP, and Bayley Scales of Infant and Toddler Development, Third Edition (Dutch) (Bayley‐III‐NL) scaled fine/gross motor scores were assessed. 20 , 21 , 22 The quality of general movements was scored as normal (normal‐optimal, normal‐suboptimal) and abnormal (mildly abnormal, definitely abnormal). 20 Bayley‐III‐NL scaled fine and gross motor scores (mean=10, SD=3) and cut‐off points of 7 or lower (≤1SD, mild/moderate impairment) and 4 or lower (≤2SD, severe impairment) were used; z‐scores were calculated based on individual Bayley‐III‐NL outcomes using the normative group average and SDs. 21 Maternal educational level was classified as low, middle, or high (https://www.cbs.nl/).
Statistical analysis
Statistical analyses were performed with SPSS v25.0 (IBM Corp., Armonk, NY, USA). Continuous data were assessed for normality with histograms, Q‐Q plots, and Kolmogorov–Smirnov and Shapiro–Wilk tests. CGM, UMW, cerebellar volumes, and fractional anisotropy/mean diffusivity values of white matter motor tracts were corrected for postmenstrual age at scan by calculating their linear growth during the time period from 38.1 to 46.6 weeks for the whole cohort. Multiple regression was performed to analyse the association between fine or gross motor scores (dependent variable) and CGM, UMW, cerebellar volumes, or fractional anisotropy/mean diffusivity values of white matter motor tracts with correction for repeated cardiac surgery. The χ 2 test was performed to test the difference in categorical early motor outcomes between infants with and without WMI, AIS, and CST involvement. A two‐sided independent t‐test (for normally distributed data) or Mann–Whitney U test (for non‐normally distributed data) were used to test uncorrected differences (with 95% confidence intervals [CIs]) in motor scores between infants with and without WMI, AIS, and CST involvement. A general linear model including repeated cardiac surgery (covariate) was conducted to calculate the corrected difference (with 95% CI) in scaled fine or gross motor scores (dependent variable) between infants with and without WMI, AIS, and CST involvement (independent variable). Regarding multiple regression and the general linear model, the conditions for inference were met. Significance was set at p<0.05. Since the association between motor outcome and brain structure might be similar for several brain volumes and fractional anisotropy and mean diffusivity values, we decided not to adjust our p‐values.
RESULTS
Sixty‐three infants with CCHD fulfilled the inclusion criteria. Infants without postoperative MRI (n=1), additional non‐cardiac neonatal interventions/surgeries (n=2), transferred to another center (n=2), and without neurodevelopmental follow‐up at 9 and 18 months (n=7) were excluded. MRI was performed in 51 neonates at a median age of 7 days after cardiac surgery (Table 1). Early motor outcomes including loss to follow‐up at 3, 9, and 18 months are presented in Table S1 (online supporting information).
Table 1.
Baseline characteristics of the study cohort
| Characteristic | n=51 |
|---|---|
| Male, n (%) | 31 (61) |
| Gestational age, wks | 39.6±1.4 |
| Birthweight, g | 3411±594 |
| Prenatal diagnosis, n (%) | 37 (73) |
| Congenital heart disease, n (%) | |
| Transposition of the great arteries | 24 (47) |
| Single‐ventricle physiology | 15 (29) |
| Left ventricular outflow tract with aortic arch obstruction | 8 (16) |
| Tetralogy of Fallot | 1 (2) |
| Total anomalous pulmonary venous connection | 3 (6) |
| Balloon atrioseptostomy, n (%) | 15 (29) |
| Age at surgery, d | 7 (5–11) |
| Primary surgical procedure, n (%) | |
| Arterial switch | 24 (47) |
| Norwood/aortopulmonary shunt | 16 (31) |
| Aortic arch reconstruction | 7 (14) |
| Ross–Konno procedure | 1 (2) |
| Correction of total anomalous pulmonary venous connection | 3 (6) |
| Age at postoperative MRI, d | 15 (12–21) |
| Postmenstrual age postoperative MRI, wks | 42.1±1.8 |
| Age between surgery and postoperative MRI, d | 7 (6–9) |
| Repeated cardiac surgery, n (%) | 17 (33) |
| Maternal educational level, n (%) | |
| Low | 3 (6) |
| Middle | 18 (36) |
| High | 30 (58) |
Data are presented as the mean±SD (normally distributed) or median (interquartile range) (non‐normally distributed) or as n (%). MRI, magnetic resonance imaging.
Neonatal volumetric brain development and early motor outcomes
Infants with abnormal general movements and absent fidgety movements showed smaller CGM volume compared to infants with normal general movements at 3 months (p=0.03). Higher CGM and cerebellar volumes were associated with more favourable scaled fine motor scores at 9 months (CGM unstandardized regression coefficient=0.51, 95% CI=0.15–0.86; cerebellum unstandardized regression coefficient=3.08, 95% CI=1.07–5.09) and 18 months (cerebellum unstandardized regression coefficient=2.80, 95% CI=0.47–5.12). No associations were found between UMW volume and early motor outcome (Table 2, Fig. 1). When excluding infants with repeated cardiac surgery, similar associations were found between neonatal brain volumes and motor scores.
Table 2.
Association between volumetric brain development and early motor outcomes
| Bayley‐III‐NL | Volumetric brain development | Microstructural brain development: white matter motor tracts | |||
|---|---|---|---|---|---|
| CGM (×10=mm3) | UWM (×10=mm3) | Cerebellum (×10=mm3) | Fractional anisotropy/10 | Mean diffusivity/1000 | |
| 3 months | n=41 | n=40 | n=33 | n=43 | n=42 |
| Scaled fine motor score | 0.58 (−0.11 to 1.27) | 0.44 (−0.05 to 0.93) | 0.47 (−3.72 to 4.66) | −5.84 (−11.84 to 0.87) | −5.20 (−25.48 to 15.08) |
| Scaled gross motor score | 0.02 (−0.50 to 0.54) | −0.15 (−0.51 to 0.22) | −0.24 (−3.01 to 2.53) | −2.85 (−7.87 to 2.18) | −11.93 (−27.26 to 3.40) |
| 9 months | n=43 | n=43 | n=36 | n=45 | n=44 |
| Scaled fine motor score | 0.51 (0.15–0.86)a | 0.15 (−0.12 to 0.42) | 3.08 (1.07–5.09)a | −1.97 (−5.39 to 1.45) | −1.85 (−13.12 to 9.42) |
| Scaled gross motor score | 0.31 (−0.55 to 1.17) | −0.15 (−0.77 to 0.46) | 2.58 (−2.18 to 7.34) | 1.74 (−5.26 to 8.73) | −13.90 (−36.37 to 8.57) |
| 18 months | n=41 | n=41 | n=33 | n=45 | n=44 |
| Scaled fine motor score | 0.43 (−0.04 to 0.90) | 0.17 (−0.20 to 0.54) | 2.80 (0.47–5.12)b | 1.66 (−2.39 to 5.71) | −5.81 (−18.80 to 7.17) |
| Scaled gross motor score | 0.11 (−0.56 to 0.77) | −0.24 (−0.73 to 0.25) | 2.57 (−0.74 to 5.89) | −0.71 (−6.40 to 4.97) | −11.79 (−29.68 to 6.10) |
Mean volume, fractional anisotropy, and mean diffusivity values were corrected for postmenstrual age at the time of the scan. Multiple regression was performed to analyse the association between mean brain volume, fractional anisotropy and mean diffusivity values, and Bayley Scales of Infant and Toddler Development, Third Edition, Dutch (Bayley‐III‐NL) scaled fine and gross motor scores at 3, 9, and 18 months. Motor outcomes were corrected for repeated cardiac surgery at 9 and 18 months. Unstandardized regression coefficients (with 95% confidence intervals) are presented; significant associations are presented as a p<0.01 and b p<0.05. The regression coefficient represents the mean increase in scaled fine or gross motor score for every additional unit of cortical grey matter (CGM), unmyelinated white matter (UWM), cerebellum, and white matter motor tract fractional anisotropy or mean diffusivity.
Figure 1.
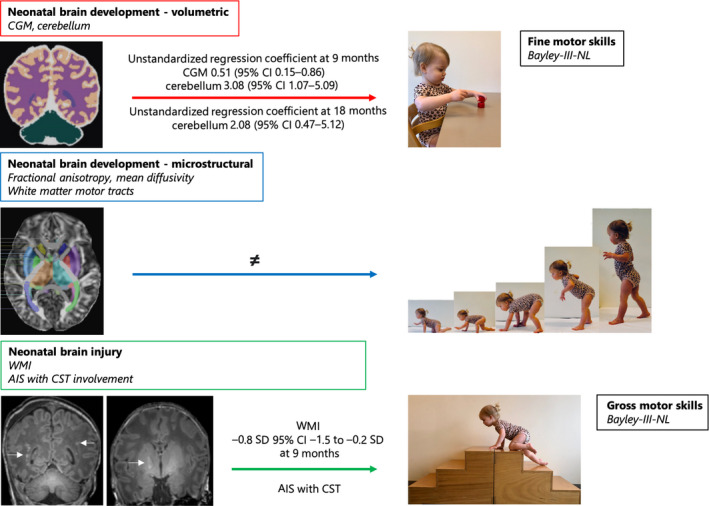
Overview of the associations between neonatal postoperative magnetic resonance imaging and early fine and gross motor skills. The neonatal volumetric brain development of the cortical grey matter (CGM) and cerebellum is associated with fine motor skills (red arrow). Neonatal microstructural brain development of white matter tracts is not related to motor skills (blue arrow). Neonatal brain injury, white matter injury (WMI), or arterial ischaemic stroke (AIS) with corticospinal tract (CST) involvement are associated with lower gross motor scores (green arrow). CI, confidence interval; Bayley‐III‐NL, Bayley Scales of Infant and Toddler Development, Third Edition (Dutch).
Neonatal microstructural brain development and motor outcomes
The microstructural development of white matter motor tracts did not differ between infants with abnormal general movements/absent fidgety movements and normal general movements at 3 months. Fractional anisotropy and mean diffusivity of white matter motor tracts were not associated with motor scores (Table 2, Fig. 1), even after exclusion of infants with repeated cardiac surgery.
Neonatal WMI and early motor outcomes
Sixty‐one per cent (n=31 out of 51) of infants showed mild (n=8), moderate (n=6), or severe (n=17) neonatal WMI with a median volume of 88mm3 (interquartile range [IQR]=26–173). At the age of 3 months, no differences in motor outcomes were seen between infants with or without neonatal WMI. However, at 9 months, lower mean Bayley‐III‐NL scaled gross motor scores (−2.4, 95% CI=−4.3 to −0.5) were seen for infants with neonatal WMI compared to infants without neonatal WMI. Infants with neonatal WMI more often showed scaled gross motor scores less than minus 1SD at 9 months (48% vs 17%, p=0.03) and 18 months (24% vs 11%, p=0.29) than infants without WMI (Figs 1 and 2, Table S2, online supporting information). The percentage of infants with abnormal muscle tone at 3, 9, and 18 months was not different between those with and without neonatal WMI (Table S2). Increased neonatal WMI volume was associated with lower Bayley‐III‐NL scaled fine and gross motor scores, although not significantly. CGM, UMW, cerebellar volume, and white matter motor tract fractional anisotropy and mean diffusivity did not differ between neonates with and without WMI.
Figure 2.
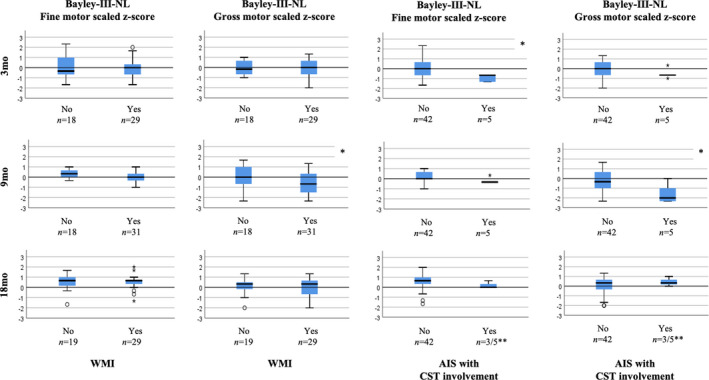
Neonatal postoperative brain injury and early motor outcomes. The graphs represent Bayley Scales of Infant and Toddler Development, Third Edition (Dutch) (Bayley‐III‐NL) scaled fine and gross motor z‐scores at the ages of 3, 9, and 18 months in infants with and without neonatal postoperative brain injury, including white matter injury (WMI) or arterial ischaemic stroke (AIS) with corticospinal tract (CST) involvement. *p<0.05 (statistically significant difference). **Two infants with critical congenital heart disease with neonatal postoperative AIS and CST involvement were lost to follow‐up at 18 months. Both infants had hemiparesis and were unable to visit the outpatient clinic because of poor clinical condition and receiving rehabilitation.
Neonatal AIS with CST involvement and early motor outcomes
Twenty‐six per cent (n=13 out of 51) of infants had AIS, including 10 perforator branch (in the basal ganglia and thalamic region) and three middle cerebral artery branches (one main, one middle, one cortical). No differences in motor scores were found between infants with and without AIS. CST involvement was observed in 38% of infants with AIS (n=5 out of 13). At 3 months, infants with neonatal AIS and CST involvement (n=5) often had abnormal general movements and absent fidgety movements (60%) and scored lower than average on the Bayley‐III‐NL scaled fine motor scores (8, IQR=6–8). Likewise, at 9 months, infants with neonatal AIS and CST involvement had lower than average Bayley‐III‐NL scaled gross motor scores (4, IQR=3–8.5) and often had scaled gross motor scores of minus 1SD or less (80%) and minus 2SD or less (60%). At 18 months, two infants with AIS and CST involvement were unable to visit the outpatient clinic because of poor clinical condition and receiving therapy at the rehabilitation center. The other three infants with AIS and CST involvement had normal Bayley‐III‐NL motor scores at 18 months (Figs 1 and 2, Table S3, online supporting information). Infants with neonatal AIS and CST involvement often showed abnormalities in muscle tone at 3 months (80%), 9 months (75%), and 18 months (67%) (Table S3).
CP and neonatal brain injury
Three infants (6%) developed CP. One child with transposition of the great arteries was diagnosed with severe neonatal WMI (lesion volume=158mm3) and developed CP at 18 months. A second infant with hypoplastic left heart syndrome had severe neonatal WMI (lesion volume=235mm3), main branch middle cerebral artery stroke with CST involvement, and already showed a high risk of CP from 3 months. A third infant with tricuspid atresia showed a perforator stroke with CST involvement on neonatal MRI and had additional brain injury after repeated cardiac surgery; high risk of CP was observed at 9 months. All three infants had abnormal general movements, absent fidgety movements, abnormal muscle tone, signs of asymmetry, and Bayley‐III‐NL motor scaled scores of minus 1SD or less or minus 2SD or less.
Repeated cardiac surgery and early motor outcome
Infants with repeated cardiac surgery had lower gross (mean difference=−2.5, 95% CI=−4.0 to −0.9) and fine (−1.8, 95% CI=−2.9 to −0.6) motor scores at 18 months compared to infants who underwent only neonatal cardiac surgery.
DISCUSSION
This study highlights the impact of delayed neonatal brain development and acquired neonatal brain injury on early motor outcomes in infants with CCHD. Smaller CGM volumes were associated with abnormal general and absent fidgety movements at 3 months and smaller cortical and cerebellar volumes with lower fine motor scores at 9 and 18 months. Delayed microstructural development of white matter motor tracts was not associated with motor skills. Ischaemic brain injury with CST involvement was related to less favourable gross motor outcomes, muscle tone abnormalities, and development of CP. Infants with repeated cardiac surgery had worse motor outcomes.
In infants with CCHD, motor delays are already present in the early phases of development. 1 In our study, most infants showed mild‐to‐moderate motor impairment rather than severe impairment or CP. The number of infants with a severely impaired gross motor score of minus 2SD or less at 9 months (16%) and 18 months (6%) was much higher compared to the typically developing population (2.3%). 23 Motor difficulties in early childhood can have long‐term consequences for participation in sports, social inclusion, physical activity, exercise capacity, cardiovascular health, and quality of life. 24 Long‐term longitudinal motor developmental assessments are warranted in infants with CCHD since outcomes are not stable over time. 24 Even infants with mild‐to‐moderate gross motor impairments might experience problems at school age due to more complex challenges (growing into their deficits). 1 , 25 Predicting early motor outcomes to intervene at an early stage with specific guidance by paediatric physiotherapists is important for infants with CCHD. 14 , 26
Our study is the first to describe the relationship between smaller neonatal regional brain volumes and less favourable fine motor scores until 18 months in infants with CCHD. The results of our study suggest that specific brain regions such as the CGM and cerebellum affect fine motor development. Previous research showed that the cortico‐cerebellar loop is involved in motor planning. 27 Differences in regional brain development between infants with CCHD and those born preterm might reflect differences in neurodevelopmental impairments. 6 In infants with CCHD, brain volumes are already reduced in utero because of disturbances in cerebral blood flow and oxygenation and are additionally affected by a critical neonatal period including open‐heart surgery. 4 , 5 , 12 , 28
Delayed neonatal microstructural development of white matter motor tracts was not associated with motor outcomes in infants with CCHD. During development, white matter integrity becomes more organized by an increase in myelination and decrease in brain water content corresponding to an increase in fractional anisotropy and a decrease in mean diffusivity respectively. Reduced cerebral oxygenation and perfusion might contribute to white matter microstructural immaturity in neonates with CCHD since premyelinating oligodendrocytes are vulnerable to hypoxia‐ischaemia. 2 , 29 Previously, lower fractional anisotropy was associated with worse cognitive performance in infants and adolescents with transposition of the great arteries or single‐ventricle physiology. 10 , 11 Roze et al. 7 reported that early MRI/DTI of the posterior limb of the internal capsule was associated with development of unilateral spastic CP at 15 months in infants with periventricular haemorrhagic infarction born preterm. Chau et al. 8 showed that white matter fractional anisotropy was lower in infants born preterm with motor delay at 18 months. Tusor et al. 9 observed that punctate WMI was related to abnormalities in white matter microstructure and predictive of unfavourable motor outcome at 20 months. However, our study suggests that delayed microstructural brain development of white matter motor tracts has no impact on early motor outcomes in infants with CCHD.
Many infants with CCHD showed neonatal WMI (61%), which is in the range with previous clinically scanned cohorts. 15 Infants with neonatal WMI had fewer favourable gross motor outcomes, especially at 9 months. Higher neonatal WMI volume showed a trend towards lower motor scores at 18 months, although not significantly. This is in contrast with a previous report showing worse motor outcome at 30 months in infants with higher WMI volume after surgery. 30 In infants born preterm, the severity of WMI on neonatal MRI is associated with the development of CP at 24 months. 13 Our study shows that WMI has adverse consequences for early gross motor outcomes.
Neonatal AIS, mainly consisting of focal perforator strokes, was not related to early motor scores in our study. However, motor outcomes at 3 and 9 months were significantly affected if the CST was involved. At 18 months, motor outcomes in infants with AIS and CST involvement were within normal ranges. This should be interpreted with caution because two infants with CCHD with neonatal AIS and CST involvement developed CP and were unable to undergo motor developmental testing at 18 months.
In our study, three infants with CCHD developed CP and all had severe WMI or AIS with CST involvement on neonatal MRI. In addition, in a previous study from our group investigating a different cohort, motor problems were found in 83% of those with CST injury even at school age. 3 These findings support the evidence for less favourable motor outcomes after neonatal CST injury.
This study has several limitations. First, the small sample size holds back subgroup analysis by cardiac defect. Second, potential other clinical factors that influence motor outcome between neonatal postoperative MRI and outcome assessments may have been missed. Nevertheless, we took repeated cardiac surgery into account in the analyses and indirectly corrected for single versus biventricular physiology and paediatric intensive care admissions. Third, the clinical relevance of our statistically significant results are unclear due to the wide CIs of the regression coefficients and SDs.
CONCLUSIONS
Less favourable early motor outcomes in infants with CCHD are related to delayed neonatal brain development and acquired neonatal brain injury. Delayed CGM and cerebellar development are related to poorer fine motor skills. WMI and AIS with CST involvement increase the risk of adverse motor development and CP. This study supports the usefulness of neonatal brain MRI as a bridging biomarker for early motor outcomes and thus intervening at an early stage to prevent later motor problems. The added value of brain MRI around repeated cardiac surgery should be evaluated further.
CONFLICT OF INTEREST
The authors have stated that they had no interests which might be perceived as posing a conflict or bias.
Supporting information
Appendix S1: MRI/DTI scanning protocol.
Table S1: Early motor outcomes.
Table S2: Neonatal white matter injury and early motor outcomes.
Table S3: Neonatal arterial ischaemic stroke with corticospinal tract involvement and early motor outcomes.
Acknowledgements
Collaborators of the CHD LifeSpan Study Group Utrecht are as follows: Mona C Toet, Jeroen Dudink, Charlotte M A van de Gronden, Wiebe R Flamman, Sophie A van der Zwart, Martijn G Slieker, Joppe Nijman, Roelie M Wösten‐van Asperen, Renske Schappin, Monica M A Uniken Venema‐van Uden, and Roel de Heus. We thank the CHD LifeSpan Study group, which includes staff from the Departments of Obstetrics, Neonatology, Paediatric Cardiology, Paediatric Intensive Care, Congenital Cardiothoracic Surgery, Anaesthesiology, Radiology, Child Development Exercise and Physical Literacy, Medical Psychology and Social Work. The study was funded by Stichting Hartekind and Vrienden UMC Utrecht Wilhelmina Kinderziekenhuis.
Contributor Information
Manon J N L Benders, Email: M.Benders@umcutrecht.nl.
CHD LifeSpan Study Group Utrecht:
Mona C Toet, Jeroen Dudink, Charlotte M A van de Gronden, Wiebe R Flamman, Sophie A van der Zwart, Martijn G Slieker, Joppe Nijman, Roelie M Wösten‐van Asperen, Renske Schappin, Monica M A Uniken Venema‐van Uden, and Roel de Heus
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in (the supplementary material of) this article.
REFERENCES
- 1. Latal B. Neurodevelopmental outcomes of the child with congenital heart disease. Clin Perinatol 2016; 43: 173–85. [DOI] [PubMed] [Google Scholar]
- 2. Claessens NHP, Breur JMPJ, Groenendaal F, Wösten‐van Asperen RM, Stegeman R, Haas F, et al. Brain microstructural development in neonates with critical congenital heart disease: an atlas‐based diffusion tensor imaging study. Neuroimage Clin 2019; 21: 101672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Claessens NHP, Algra SO, Ouwehand TL, Jansen NJG, Schappin R, Haas F, et al. Perioperative neonatal brain injury is associated with worse school‐age neurodevelopment in children with critical congenital heart disease. Dev Med Child Neurol 2018; 60: 1052–8. [DOI] [PubMed] [Google Scholar]
- 4. Claessens NHP, Khalili N, Isgum I, ter Heide H, Steenhuis TJ, Turk E, et al. Brain and CSF volumes in fetuses and neonates with antenatal diagnosis of critical congenital heart disease: a longitudinal MRI study. AJNR Am J Neuroradiol 2019; 40: 885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meuwly E, Feldmann M, Knirsch W, von Rhein M, Payette K, Dave H, et al. Postoperative brain volumes are associated with one‐year neurodevelopmental outcome in children with severe congenital heart disease. Sci Rep 2019; 9: 10885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keunen K, Išgum I, van Kooij BJM, Anbeek P, van Haastert IC, Koopman‐Esseboom C, et al. Brain volumes at term‐equivalent age in preterm infants: imaging biomarkers for neurodevelopmental outcome through early school age. J Pediatr 2016; 172: 88–95. [DOI] [PubMed] [Google Scholar]
- 7. Roze E, Benders MJ, Kersbergen KJ, van der Aa NE, Groenendaal F, van Haastert IC, et al. Neonatal DTI early after birth predicts motor outcome in preterm infants with periventricular hemorrhagic infarction. Pediatr Res 2015; 78: 298–303. [DOI] [PubMed] [Google Scholar]
- 8. Chau V, Synnes A, Grunau RE, Poskitt KJ, Brant R, Miller SP. Abnormal brain maturation in preterm neonates associated with adverse developmental outcomes. Neurology 2013; 81: 2082–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tusor N, Benders MJ, Counsell SJ, Nongena P, Ederies MA, Falconer S, et al. Punctate white matter lesions associated with altered brain development and adverse motor outcome in preterm infants. Sci Rep 2017; 7: 13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Watson CG, Stopp C, Wypij D, Bellinger DC, Newburger JW, Rivkin MJ. Altered white matter microstructure correlates with IQ and processing speed in children and adolescents post‐Fontan. J Pediatr 2018; 200: 140–9.e4. [DOI] [PubMed] [Google Scholar]
- 11. Panigrahy A, Schmithorst VJ, Wisnowski JL, Watson CG, Bellinger DC, Newburger JW, Rivkin MJ. Relationship of white matter network topology and cognitive outcome in adolescents with d‐transposition of the great arteries. Neuroimage Clin 2015; 7: 438–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Claessens NHP, Kelly CJ, Counsell SJ, Benders MJNL. Neuroimaging, cardiovascular physiology, and functional outcomes in infants with congenital heart disease. Dev Med Child Neurol 2017; 59: 894–902. [DOI] [PubMed] [Google Scholar]
- 13. Martinez‐Biarge M, Groenendaal F, Kersbergen KJ, Benders MJNL, Foti F, van Haastert IC, et al. Neurodevelopmental outcomes in preterm infants with white matter injury using a new MRI classification. Neonatology 2019; 116: 227–35. [DOI] [PubMed] [Google Scholar]
- 14. Haseba S, Sakakima H, Nakao S, Ohira M, Yanagi S, Imoto Y, et al. Early postoperative physical therapy for improving short‐term gross motor outcome in infants with cyanotic and acyanotic congenital heart disease. Disabil Rehabil 2018; 40: 1694–701. [DOI] [PubMed] [Google Scholar]
- 15. Claessens NHP, Chau V, de Vries LS, Jansen NJ, Au‐Young SH, Stegeman R, et al. Brain injury in infants with critical congenital heart disease: insights from two clinical cohorts with different practice approaches. J Pediatr 2019; 215: 75–82.e2. [DOI] [PubMed] [Google Scholar]
- 16. Moeskops P, Viergever MA, Mendrik AM, de Vries LS, Benders MJNL, Išgum I. Automatic segmentation of MR brain images with a convolutional neural network. IEEE Trans Med Imaging 2016; 35: 1252–61. [DOI] [PubMed] [Google Scholar]
- 17. Leemans A, Jeurissen B, Sijbers J, Jones DK. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. Proceedings of the 17th Annual Meeting of the International Society for Magnetic Resonance Medicine, 18–24 April 2009; 3537; Honolulu, Hawaii.
- 18. Oishi K, Mori S, Donohue PK, Ernst T, Anderson L, Buchthal S, et al. Multi‐contrast human neonatal brain atlas: application to normal neonate development analysis. Neuroimage 2011; 56: 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fedorov A, Beichel R, Kalpathy‐Cramer J, Finet J, Fillion‐Robin J‐C, Pujol S, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging 2012; 30: 1323–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hadders‐Algra M, Mavinkurve‐Groothuis AMC, Groen SE, Stremmelaar EF, Martijn A, Butcher PR. Quality of general movements and the development of minor neurological dysfunction at toddler and school age. Clin Rehabil 2004; 18: 287–99. [DOI] [PubMed] [Google Scholar]
- 21. Albers CA, Grieve AJ. Bayley, N. (2006). Bayley Scales of Infant and Toddler Development–Third Edition. San Antonio, TX: Harcourt Assessment. J Psychoeduc Assess 2007; 25: 180–98. [Google Scholar]
- 22. Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl 2007; 109: 8–14. [PubMed] [Google Scholar]
- 23. Boreham C, Riddoch C. The physical activity, fitness and health of children. J Sports Sci 2001; 19: 915–29. [DOI] [PubMed] [Google Scholar]
- 24. Naef N, Wehrle F, Rousson V, Latal B. Cohort and individual neurodevelopmental stability between 1 and 6 years of age in children with congenital heart disease. J Pediatr 2019; 215: 83–9.e2. [DOI] [PubMed] [Google Scholar]
- 25. Brosig CL, Bear L, Allen S, Simpson P, Zhang L, Frommelt M, Mussatto KA. Neurodevelopmental outcomes at 2 and 4 years in children with congenital heart disease. Congenit Heart Dis 2018; 13: 700–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gallagher A, Dagenais L, Doussau A, Décarie J‐C, Materassi M, Gagnon K, et al. Significant motor improvement in an infant with congenital heart disease and a rolandic stroke: the impact of early intervention. Dev Neurorehabil 2017; 20: 165–8. [DOI] [PubMed] [Google Scholar]
- 27. Gao Z, Davis C, Thomas AM, Economo MN, Abrego AM, Svoboda K, et al. A cortico‐cerebellar loop for motor planning. Nature 2018; 563: 113–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. von Rhein M, Buchmann A, Hagmann C, Dave H, Bernet V, Scheer I, et al. Severe congenital heart defects are associated with global reduction of neonatal brain volumes. J Pediatr 2015; 167: 1259–63.e1. [DOI] [PubMed] [Google Scholar]
- 29. Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med 2007; 357: 1928–38. [DOI] [PubMed] [Google Scholar]
- 30. Peyvandi S, Chau V, Guo T, Xu D, Glass HC, Synnes A, et al. Neonatal brain injury and timing of neurodevelopmental assessment in patients with congenital heart disease. J Am Coll Cardiol 2018; 71: 1986–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: MRI/DTI scanning protocol.
Table S1: Early motor outcomes.
Table S2: Neonatal white matter injury and early motor outcomes.
Table S3: Neonatal arterial ischaemic stroke with corticospinal tract involvement and early motor outcomes.
Data Availability Statement
The data that supports the findings of this study are available in (the supplementary material of) this article.


