ABSTRACT
Fusion between cells of different organisms (i.e., xenogeneic hybrids) can occur, and for humans this may occur in the course of tissue transplantation, animal handling, and food production. Previous work shows that conferred advantages are rare in xenogeneic hybrids, whereas risks of cellular dysregulation are high. Here, we explore the transcriptome of individual xenogeneic hybrids of human mesenchymal stem cells and murine cardiomyocytes soon after fusion and ask whether the process is stochastic or involves conserved pathway activation. Toward this end, single‐cell RNA sequencing was used to analyze the transcriptomes of hybrid cells with respect to the human and mouse genomes. Consistent with previous work, hybrids possessed a unique transcriptome distinct from either fusion partner but were dominated by the cardiomyocyte transcriptome. New in this work is the documentation that a few genes that were latent in both fusion partners were consistently expressed in hybrids. Specifically, human growth hormone 1, murine ribosomal protein S27, and murine ATP synthase H+ transporting, mitochondrial Fo complex subunit C2 were expressed in nearly all hybrids. The consistent activation of latent genes between hybrids suggests conserved signaling mechanisms that either cause or are the consequence of fusion of these 2 cell types and might serve as a target for limiting unwanted xenogeneic fusion in the future.—Yuan, C., Freeman, B. T., McArdle, T. J., Jung, J. P., Ogle, B. M. Conserved pathway activation following xenogeneic, heterotypic fusion. FASEB J. 33, 6767–6777 (2019). www.fasebj.org
Keywords: cell fusion, human growth hormone, mesenchymal stem cell, cardiomyocyte, ribosome
ABBREVIATIONS
- BiFC
bimolecular fluorescence complementation
- DEG
differentially expressed gene
- FPKM
fragments per kilobase per million
- GH1
human growth hormone 1
- HC
hierarchical clustering
- HL1cm
murine HL1 cardiomyocyte
- hMSC
human MSC
- hSLAM
human signaling lymphocytic activation molecule
- LCR
locus control region
- MSC
mesenchymal stem cell
- Pit‐1
pituitary transcription factor 1
- POU1F1
POU domain class 1 transcription factor 1
- scRNA‐Seq
single‐cell RNA sequencing.
Fusion of cells of the same type in a given species (i.e., homotypic fusion) typically confers enhanced biologic function. Homotypic fusion is especially important in development and healing processes. Common examples include the fusion of myoblasts to form multinucleated skeletal muscle fibers (1–6), the fusion of macrophages to form osteoclasts in bone resorption (7, 8), and the fusion of trophoblasts to form syncytiotrophoblasts in the human placenta (9–11). Homotypic fusion typically represents the final steps of cell maturation; and therefore, cells must often reach a certain differentiation state to achieve fusion. For example, it was recently shown that trophoblast fusion requires the assembly of a matrix of annexin A5 at the plasma membrane, a process that only occurs in later stages of maturation, to facilitate cell aggregation as a prerequisite for fusion (12). The resultant syncytiotrophoblast is a tremendous and essential benefit for the placenta because it effectively bars immune cells from the mother from invading the fetus and secretes large quantities of hormones needed to support pregnancy.
Cell fusion between different cell types (i.e., heterotypic fusion) may also confer advantages as evidenced by embryogenesis following sperm‐egg fusion. But heterotypic fusion can also render the cell susceptible to dysregulation as different cytoplasmic and nuclear environments collide. With heterotypic fusion, nuclei might 1) compete for gene regulation, 2) fuse and undergo chromosomal reorganization, or 3) be ejected (13–15). Mesenchymal stem cells (MSCs) in particular are emerging as a frequent heterotypic fusion partner because they have been shown to fuse with cells of various tissues following transplantation to the heart, intestine, brain, liver, and muscle (16–23). In the context of the liver, MSC fusion with hepatocytes has been shown to be beneficial for liver regeneration (16, 24, 25); similarly, MSC fusion with myocytes following transplantation has been shown to be beneficial for muscle regeneration (26, 27). However, in most other scenarios, the benefit is unclear, and there is rising concern about the threat of carcinogenesis and associated metastases with heterotypic fusion involving local or transplanted MSCs (28–33).
Fusion of cells of different types between different species (i.e., heterotypic, xenogeneic fusion) also occurs spontaneously and suffers the same susceptibilities to gene dysregulation as allogeneic or autogeneic, heterotypic hybrids. In addition, xenogeneic hybrids have been shown to promote zoonosis. For example, transmission of the porcine endogenous retroviruses was observed in xenogeneic hybrids following transplantation of human bone marrow—derived cells to fetal pigs (34). In addition, it has been postulated that transmission of active virus might be facilitated by xenogeneic fusion (14). An animal virus might enter a human cell via fusion with an infected animal cell. The virus that emerges from the hybrid could then acquire a “human” viral coat, thus enabling the transmission of viral tropism. Given the implications of xenogeneic fusion, it is important to understand how fusion is accomplished in this setting. This is a very challenging question to address, but technology is emerging that can at least give us first cues. With the advent of single‐cell RNA—sequencing (scRNA‐Seq) techniques, the transcriptome of individual hybrid cells has been revealed. Here the entire transcriptome of xenogeneic, heterotypic fusion products formed between human MSCs (hMSCs) and murine HL1 cardiomyocytes (HL1cms) was probed to identify conserved mechanisms that at least support, and perhaps enable, the hybrid state.
MATERIALS AND METHODS
Cell culture
The hMSCs were cultured as previously described by Trivedi et al. (35). Cells were cultured in flasks treated with 0.1% gelatin (MilliporeSigma, Burlington, MA, USA). The culture medium used was α‐minimum essential medium (MEM) (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (GE Healthcare, Chicago, IL, USA), 0.1 mM non‐essential amino acids (Thermo Fisher Scientific), and 2 mM l‐glutamine (Thermo Fisher Scientific). MSC passage numbers were maintained between 6 and 11.
HL1cm cells were cultured in flasks treated with 0.0125 mg/ml fibronectin in 0.02% gelatin (MilliporeSigma). The culture medium consisted of Claycomb (MilliporeSigma), 10% fetal bovine serum (MilliporeSigma), 100 µg/ml penicillin‐streptomycin (Lonza, Basel, Switzerland), 0.1 mM norepinephrine (MilliporeSigma), and 2 mM l‐glutamine (Thermo Fisher Scientific). The HL1cm culture protocol was used during coculture experiments. All cell cultures were stored in a 37°C, 5% CO2 incubator. HL1cm passage numbers were maintained between 70 and 85.
Induction of cell fusion
Components of a nonreplicative measles virus were used to induce cell fusion, as previously described by Freeman et al. (33). The hMSCs were transfected with both hemagglutinin and fusion proteins. The HL1cms were transfected with the measles virus receptor, human signaling lymphocytic activation molecule (hSLAM). The viral hemagglutinin protein of the hMSC binds to the human viral receptor hSLAM, which triggers the receptor complex to trimerize with the fusion protein, thus inducing fusion (36). The presence of hSLAM was confirmed in the majority of the fusion products, verifying the successful execution of this method.
In order to detect fusion, the bimolecular fluorescence complementation (BiFC) system was used (37). The hMSCs were transfected with the gene encoding the C terminus of the yellow fluorescent protein fused with histone H3.1 (YCH3.1), whereas HL1cms were transfected with the gene encoding the N terminus of venus fluorescent protein fused with histone H3.1 (VNH3.1). After fusion, the proteins can combine and fluoresce, allowing for detection. The association of fluorescence with bona fide fusion has been demonstrated in previous work by Chitwood et al. (38) via FISH for species‐specific chromosomal markers.
The Neon transfection system (Thermo Fisher Scientific) was used for transfection according to the manufacturer's manual. For the HL1cms, a 30 ms, 1300 V pulse was used; for MSCs, a 20 ms, 1500 V pulse. HL1cms were transfected with 2 µg of hSLAM and 2 µg of the venus fluorescent protein fused with histone H3.1 (VNH3.1) plasmid. Similarly, MSCs were transfected with 2 µg of fusion‐hemagglutinin protein and 2 µg of the yellow fluorescent protein fused with histone H3.1 (YCH3.1).1 plasmid and placed directly into the transfected HL1cms.
Cell capture and scRNA‐Seq
After 18 h of coculture, cells were trypsinized and suspended in 1× PBS. BiFC+ fusion products (green fluorescent protein—positive) were sorted using fluorescent‐activated cells sorting (Aria, II; BD Biosciences, San Jose, CA, USA). After sorting, the cells were imaged with both phase‐contrast and fluorescence microscopy in order to confirm the BiFC‐positive signal. After confirming the signal, cells were resuspended in Claycomb media mixture used to culture the HL1cms. These cell suspensions were then loaded into the Fluidigm C1 system for single‐cell capture. Viability of captured cells was determined using Dead Cell Viability Assay (Molecular Probes, Eugene, OR, USA; Thermo Fisher Scientific). Again, captured cells were imaged with phase‐contrast and fluorescent imaging to confirm capture and BiFC signal. If cells were determined to be single, live, and BiFC‐positive, they were used in further analysis. Control HL1cms and MSCs were also captured and imaged in a similar matter. Both were captured after coculture and in a separate Fluidigm C1 system using CellTracker Green and Red (Molecular Probes) to identify the individual parent cells. After capture, the Smarter Ultra Low RNA Kit for the Fluidigm system (Clontech Laboratories, Mountain View, CA, USA) was used to prepare cDNA for each captured cell. The Illumina Nextera XT Preparation Kit (Illumina, San Diego, CA, USA) was used to prepare the mRNA library from the cDNA. Sequencing was performed on the Illumina MiSeq using v.3 chemistry and generating 75 bp paired‐end reads with 18–22 million read depth. The raw FastQ files generated were used for downstream bioinformatics analysis and stored at the University of Minnesota Supercomputing Institute.
Transcript alignment and expression analysis
All basic sequence analyses of the scRNA‐Seq were performed using established software programs using Linux high‐performance computing clusters provided by the Minnesota Supercomputing Institute (Supplemental Fig. S1). First, raw reads passing quality controls were mapped to the mouse (mm10) and human (hg19) genomes using hierarchical indexing for spliced alignment of transcripts (HISAT v.0.1.6; ccb.jhu.edu/software/hisat/). Then, the mapped reads were summarized and normalized using the Cufflinks and Cuffquant software packages (v.2.2.1; cole‐trapnell‐lab.github.io/cujflinks/) to generate the fragments per kilobase per million (FPKM) mapped reads values (Supplemental Tables S1–S3) (39). Default settings were used for both the HISAT and Cufflinks software.
To determine differential gene expression of fusion products compared with HL1cms and MSCs, we performed the Wilcoxon rank‐sum test. The false discovery rate adjustment was then applied to the P values (Supplemental Tables S5 and S6). Differentially expressed genes (DEGs) were defined as false discovery rate‐adjusted values of P < 0.05 and expression fold differences over 1.5. Pathway enrichment analysis of the DEGs was then performed by executing the overrepresentation enrichment test and visualized using the Biological Networks Gene Ontology plugin in Cytoscape (40) corresponding to gene levels significantly increased or decreased relative to parental cells (Supplemental Tables S7–S10). Heatmaps and hierarchical clustering (HC) were generated with the pheatmap package in R statistical language (average linkage clustering method with euclidean distance as similarity metric).
Validation of scRNA‐Seq analysis via quantitative RT‐PCR for select genes
The cDNA was acquired from the single‐cell reaction performed in the Illumina chip. Primer sequences for human glyceraldehyde 3‐phosphate dehydrogenase were: forward 5′‐TTAAAAGCAGCCCTGGTGAC‐3′ and reverse 5′‐CTCTGCTCCTCCTGTTCGAC‐3′. Primers for murine cardiac muscle troponin T2 were purchased (10041595; Bio‐Rad, Hercules, CA, USA). Primer efficiencies were extracted from RealPlex2 software (Eppendorf North America, Hauppauge, NY USA) and verified with melting curves. The comparative Ct method could not be used to determine the relative changes in gene expression because there was not enough product to amplify both a housekeeping gene and gene of interest for each species. Therefore, Ct values were reported relative to Ct values of the positive control parental line.
Detection of human growth hormone protein via ELISA
HL1cms and hMSCs were prepared as for coculture mentioned above, and the presence of fusion products was confirmed via fluorescence microscopy for green fluorescent protein corresponding to inducible BiFC. Supernatants of cocultures, 3 wells of a 6‐well plate, were collected and pooled daily for 3 d after initiation of coculture. The total volume collected was concentrated to 100 µl using vacuum centrifugation and applied directly to the well of an ELISA plate per the manufacturer's instructions (Ultrasensitive Growth Hormone ELISA; Alpco, Salem, NH, USA). Plates were read using a Biotek Plate Reader (BioTek, Winooski, VT, USA) at 450 nm with a reference filter at 620 nm.
RESULTS
Dual transcriptome analysis of xenogeneic MSC‐cardiomyocyte hybrids
To determine the transcriptome of individual, heterotypic, xenogeneic hybrids formed between hMSCs and murine HL1cms, scRNA‐Seq was performed. These 2 cell types were selected because multiple investigators have reported the formation of hybrids of this type following transplantation of hMSCs or bone marrow—derived cells to the heart (17, 23, and 41–46). The hMSCs and HL1cms were cocultured for 18 h, at which point hybrids were identified (Supplemental Fig. S2) and sorted based on inducible fluorescence associated with BiFC of fluorescent fragments linked to histone H3.1, as previously described by Freeman et al. (33). The frequency of hybrid formation was ∼1 in 20,000 cells (Supplemental Fig. S3). Parental controls were obtained from cocultures generated in the same way and for the same time duration (Fig. 1A ). Alignment of the sequence reads with reference genome was nontrivial because human and mouse genomes had to be combined with assurance that mouse reads would not inadvertently be assigned to human genes and vice versa. Given the homology of many human and mouse genes, we first determined the rate of crossmapping between human and mouse genomes using the hMSC and HL1cm samples. To do this, the number of species 1 transcripts that aligned with species 2 genomes with FPKM values above that aligned to species 1 were identified as crossmapping genes and were removed from subsequent analysis. The fraction of genes removed from analysis was quite small because only 0.046 ± 0.019% of human genes crossmapped to the mouse genome, and only 0.092 ± 0.013% of mouse genes crossmapped to the human genome. Thus, the analyses here correspond to at least 99.90% of all genes with 100% certainty of species specificity.
Figure 1.
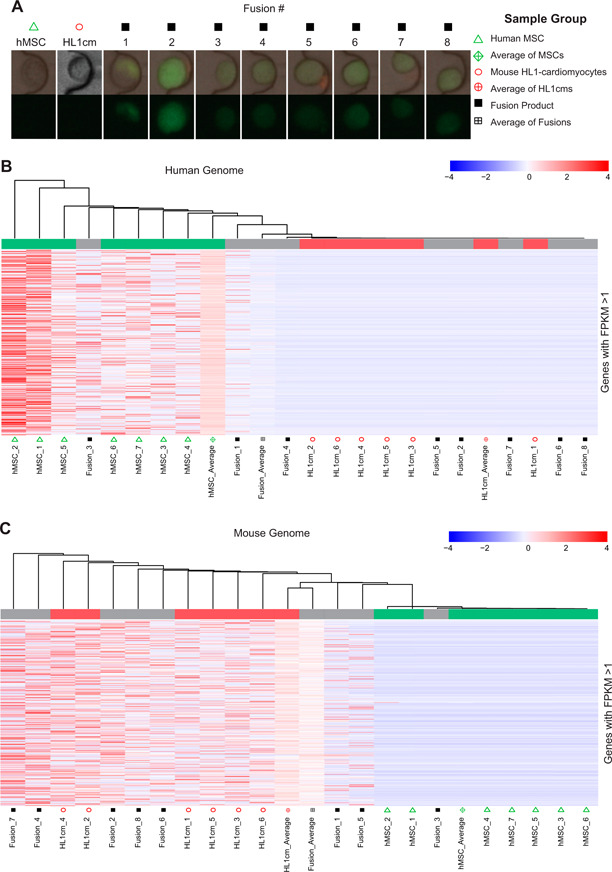
Global comparison of hybrids relative to parental controls. A) Images of single cells captured via the Fluidigm system and poised for transcript isolation and sequencing. Green fluorescence indicates BiFC only accomplished as a consequence of cell‐cell fusion. B) HC of all genes with FPKM >1 for any sample relative to the human genome. C) HC of all genes with FPKM >1 for any sample relative to the mouse genome.
HC analysis was then utilized to provide a global view of the expression profiles of hybrids relative to parental controls. A total of 8 independent fusion products, 7 hMSC controls, and 6 HL1cm controls were analyzed. HC included all genes with FPKMs above unity for any sample, and the results were displayed with respect to the human genome (Fig. 1B ) and the mouse genome (Fig. 1C ). As shown, the human and mouse parental cells cluster by species, whereas the hybrids are somewhat scattered between the 2 species. Also included in the HC analysis were averages of the fusion products and the 2 parental controls. When FPKM values of hybrids were combined to an overall mean (as would occur with population‐based RNA‐sequencing), the hybrids appear to cluster with the HL1cm. This is misleading because, although hybrids were more apt to cluster with the HL1cm, some cluster with hMSCs. In particular, of the 8 hybrids, 2 hybrids (Fusion_1, Fusion_5) cluster with both the hMSC and HL1cm controls, 1 (Fusion_3) clusters with only the hMSC controls, and the remaining 5 fusion products cluster with the HL1cm controls.
Dissecting heterogeneity of hybrid cells
To further probe the extent to which the hybrid cells sustain the phenotype of either parental cell type, a set of genes was selected representing essential functions or robust biomarkers of each parental cell type (Supplemental Table S4). Similar methods have been previously used by Palermo et al. (15) to determine whether and to which extent the functional capacity of 1 parental cell type is more or less preserved in a given fusion product. Here, the 2 gene sets represent murine cardiac and human MSC function and were used to compare individual fusion hybrids to the mean transcript expression of each parent cell for each gene (Fig. 2A, B ). Much like in the HC analysis, most (6) hybrids more closely align with the cardiomyocyte phenotype than with the hMSC phenotype. Importantly, expression levels of mouse cardiomyocyte genes are quite variable between hybrids and overall lower in the hybrids on average relative to the cardiomyocyte mean. One hybrid (Fusion_3) shares no similarities to the mouse cardiac gene set but highly expresses some of the human MSC genes. Another hybrid (Fusion_1) shares similarities with both the mouse cardiac gene set and the human MSC gene set. Importantly, none of the fusion products share the same overall expression patterns of the selected parent genes, nor do any of the hybrids exhibit high expression levels of all of the selected parent genes.
Figure 2.
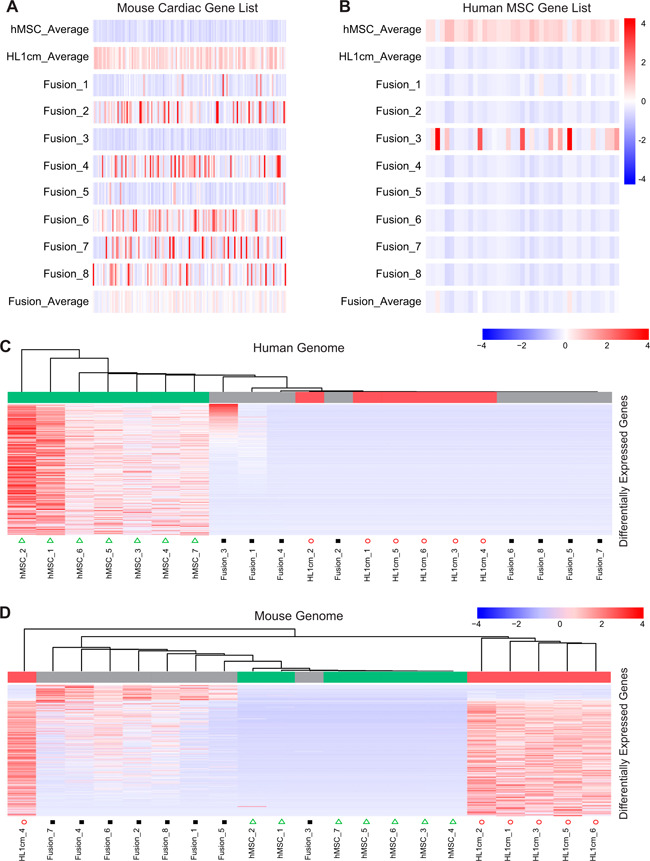
Extent of bidirectional reprogramming of hybrids to parental controls via lists of functional genes of parental controls and differential gene expression. A) Comparison of hybrids (Fusion_1—Fusion_8) to list of genes associated with mouse cardiomyocyte function. B) Comparison of hybrids (Fusion_1—Fusion_8) to list of genes associated with hMSC function. C, D) Summary of DEGs in hybrids relative to hMSC and HL1cm in reference to the human (C) and mouse (D) genome.
To discern genes of hybrids with expression levels in greatest opposition to parental controls, we determined DEGs among all 3 cell types with reference to either the human or mouse genome (Fig. 2C, D ). From this type of analysis, one can identify genes that critically drive association with 1 parental cell or the other. Based on results of our previous work (33), we predicted gene driving clustering tendencies would vary for each hybrid. This was true for the majority of genes analyzed (Supplemental Tables S5 and S6). However, a small subset of genes was identified that consistently turned on in hybrids relative to parental controls in which the genes were latent. Thus, hybrid cells exhibit a heterogenous and unique transcriptome with respect to parental cells, although they tend toward a cardiomyocyte phenotype; importantly, they develop de novo expression of a gene set consistent among the fusion products.
Functional analysis of hybrid gene expression
To analyze DEGs of hybrids relative to parental cells with functional gene groups, we performed the pathway overrepresentation test. DEGs were analyzed through the Biological Networks Gene Ontology (BINGO) plugin in Cytoscape with respect to each parental cell, and according to up‐ or down‐regulation relative to each parental cell (Supplemental Figs. S4–S7 and Supplemental Tables S7–S10). The most significant trends in functional gene groups were procured and displayed in Fig. 3 . We report decreased FPKMs for the purposes of completeness but focus on increased FPKMs because decreased FPKMs may simply reflect a dilution of transcripts in the hybrid and not a true cell‐regulatory event. Even so, the gene pathways of hybrids that decrease relative to parental cells do provide a nice validation of sorts because pathways associated with MSC function (e.g., adhesion, blood vessel morphogenesis, and development) are decreased in hybrids, as are pathways associated with HL1cm function (e.g., cellular metabolic processes, muscle structure development, sarcomere organization, and ion transport). In addition, the hybrid cells were found to consistently lose transcripts of genes associated with biologic regulation (i.e., all aspects of cellular scale regulation including migration, apoptosis, cell shape, and gene expression) and response to stress (Fig. 3), which is perhaps not surprising given the circumstances imposed by the merging of cytoplasmic and nuclear material. In terms of pathways that showed increases in FPKM relative to parental controls, we see increased mitochondrial activity in hybrids relative to the MSC parent. This could reflect a higher demand for energy with the imposition of sarcomeres from the HL1cm parent. Interestingly, the fusion products also substantially up‐regulate ribosomal proteins relative to the translationally limited cardiomyocyte parent. The high degree of increase of ribosomal protein transcript is a trend that has been previously observed by Freeman et al. (33) in fusion products of this type, but the biologic implications are not yet known.
Figure 3.
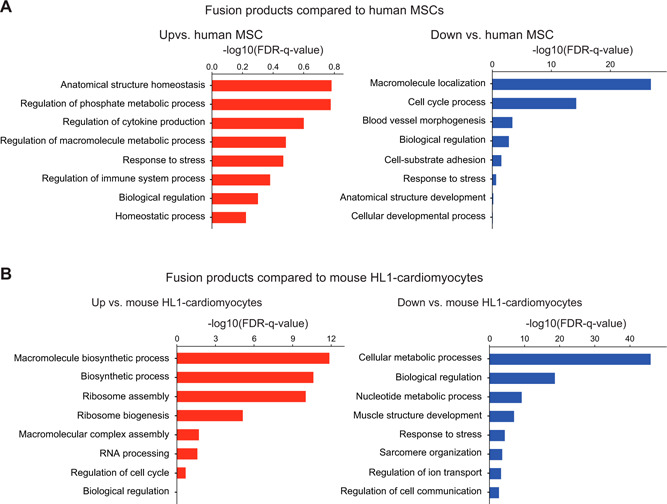
Gene Ontology of hybrids relative to hMSC (A) and HL1cm (B). Blue columns indicate a decrease in FPKM of hybrid relative to parental control, and red columns indicate an increase in FPKM of hybrid relative to parental control. FDR, false discovery rate; HL1, HL‐1 cardiomyocyte.
High‐resolution transcriptome analysis and protein‐level confirmation
To carefully delineate de novo expression of gene sets unique and consistent among the fusion products, actual FPKM levels are shown for individual hybrids relative to mean parental cell FPKM levels. Figure 1 shows de novo gene expression of nearly all hybrids and not of parental cells; outcomes of select genes were verified using quantitative RT‐PCR (Supplemental Fig. S8). To further compel the validity of the data, we show levels of human histone H3.1 gene. This gene is expressed in human cells, as expected, and also in hybrids because forced expression of human histone H3.1 gene in cardiomyocytes and hMSC was used to enable BiFC‐inducible fluorescence with fusion. All hybrids and not HL1cm parental controls express this gene, further supporting the validity of cells designated as fusion products. Similarly, SLAM family member 1 (SLAMF1) was expressed in nearly all hybrids and not in either parental control. Expression of this protein was also forced in HL1cm fusion partners as a means to facilitate fusion with the viral fusion proteins expressed on the hMSC fusion partner. Next, human housekeeping gene glyceraldehyde 3‐phosphate dehydrogenase was assessed, as were randomly selected human MSC marker genes human endoglin and ecto‐5′‐nucleotidase. Results are consistent with the heterogeneity of previously documented hybrids because only a portion of them express the human‐specific genes. However, human growth hormone 1 (GH1) is expressed in nearly all hybrids and, importantly, is not expressed in either the mean hMSC comparator (Fig. 4A ) or in any individual hMSC cells analyzed (Supplemental Table S2). Growth hormone is a protein synthesized primarily in the anterior pituitary. Only in the past 15 yr has it been discovered that growth hormone is expressed in extrapituitary sites throughout the body (47, 48). Given this, and the fact that none of the fusion products express pituitary transcription factor 1 (Pit‐1), the transcription factor thought responsible for GH1 expression, it is extremely intriguing but unusual that the fusion products show such high levels of GH1 expression. To further confirm this finding, we probed the supernatant of cocultures containing hMSC‐HL1cm fusion products and detected GH1 protein in the range of 170 ng/µl (Supplemental Fig. 9).
Figure 4.
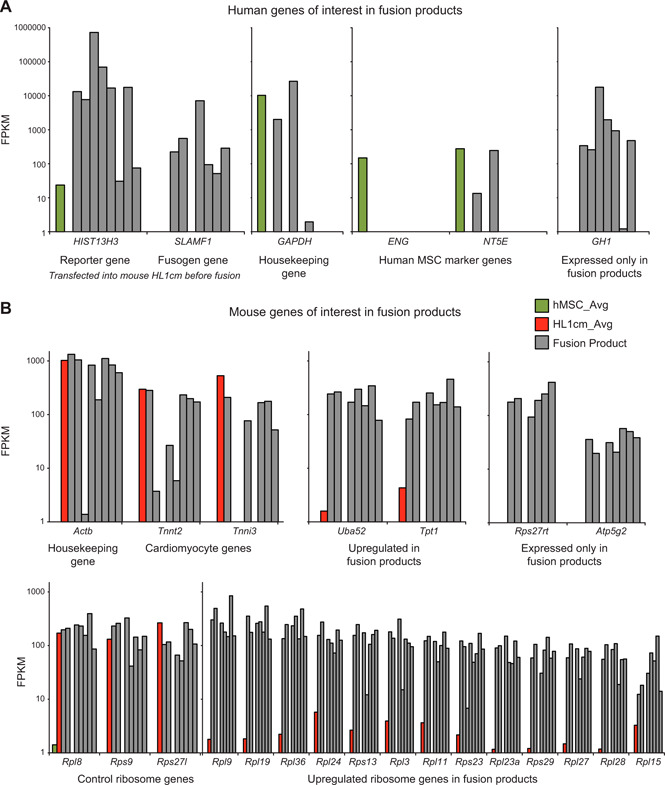
Consistent de novo expression of select genes of hybrids. A) Relevant human genes of hybrids. Forced expression of reporter and fusogenic genes were confirmed in hybrids. In addition, a housekeeping gene and randomly selected genes corresponding to the hMSC phenotype were displayed as a representative contrast to expression of GH1 expressed in hybrids but neither parental cell type. B) Relevant mouse genes of hybrids. A housekeeping gene and randomly selected genes corresponding to the HL1cm phenotype were displayed as representative contrast to expression of ribosomal protein S27 (Rps27rt) and mitochondrial ATPase, Atp5g2, expressed in hybrids but neither parental cell type. In addition, a profile of ribosomal genes all highly expressed in hybrids and either highly expressed or lowly expressed in HL1cm.
A closer look at mouse housekeeping β‐actin gene Actb and randomly selected cardiomyocyte markers cardiac muscle troponin T2 (Tnnt2) and cardiac muscle troponin I shows more widespread expression in fusion products, again supporting a trend that has been previously observed in single‐species experiments by Freeman et al. (33) (Fig. 4B ). Also, seen in the mouse genome is the up‐regulation of the ubiquitin gene ubiquitin A‐52 residue ribosomal protein fusion product 1 (Uba52), which acts in part as a regulator of the ribosomal protein complex (49) and histamine‐releasing factor translationally controlled tumor protein 1, which also acts to stabilize microtubules (47). Both genes have been shown to be involved in the stress response of cells and therefore may play a critical role in protein reorganization that may be necessary in hybrids in response to the chaos after fusion. De novo expression of mouse genes was also observed in the ribosomal protein family, including ribosomal protein S27 retrogene (Rps27rt), and the mitochondrial ATPase, Atp5g2 (Fig. 4B ), which helps provide explanation for the increased levels of ribosomal activity and mitochondrial activity observed in the pathway overrepresentation analysis.
The pathway overrepresentation analysis showed that up‐regulation of ribosomal proteins was among the most prominent upward trends seen in the fusion products relative to the HL1cm (Fig. 3). Thus, the expression of various mouse ribosomal proteins in the fusion products was explored (Fig. 4). To determine whether up‐regulation of ribosomal proteins represented a universal up‐regulation of ribosomal proteins, several other ribosomal protein genes were selected and displayed. For some selected ribosomal genes (Rpl8, Rps9, and Rps27), most fusion products expressed levels similar to the mouse parent cells, HL1cm. However, the majority of ribosomal genes were up‐regulated relative to HL1cm. Even more interesting is the fact that the up‐regulation of ribosomal pathways is seen in the mouse genome contributed by the cardiomyocytes, despite the fact that the human partner (hMSC) is more transcriptionally active than the mouse partner in this particular case.
DISCUSSION
scRNA‐Seq permitted a detailed look into the transcriptional diversification and novel gene activity arising in heterotypic, xenogeneic hybrids. On a population level, one could expect the fusion products to display an intermediate phenotype relative to the parental cells. The MSCs, being master manipulators of extracellular matrix proteins, are cells that generally produce massive amounts of proteins, assist in wound healing, and secrete various enzymes, enabling them to invade and migrate throughout their microenvironment. On the other hand, cardiomyocytes are nonmigratory and secrete little protein while expending large amounts of cellular energy in order to drive cellular contractions. And indeed, fusion products at the low resolution of the population (Fig. 1B, C ) do represent approximate intermediates of these phenotypes. Relative to the hMSCs, the hybrids have increased levels of metabolic activity, a trait most likely inherited from the energetically active cardiomyocytes. On the other hand, relative to the cardiomyocytes, the fusion products have increased levels of gene expression related to ribosome structure, a trait reminiscent of the protein‐secreting MSC parents. Looking at the down‐regulated gene activity, this population‐wide trend is further exemplified. The fusion products have decreased expression of genes related to cell adhesion and tissue development, which are traits of the MSC phenotype. Similarly, the fusion products had decreased levels of genes related to energy generation, contractile fibers, actin binding, and muscle development indicative of the mouse cardiomyocyte. These trends seen in the small population of fusion products are important in that they highlight the fact that fusion products possess phenotypes distinct from either parent cells, and, as a whole, can be seen as an intermediate cell type. However, these population trends mask the extensive heterogeneity within the hybrid population and the trends unique to fusion products irrespective of parent cell transcriptome.
Analysis of the transcriptome of hybrids at the single‐cell level allowed an appreciation for the diversity of a given population and revealed subtle changes not apparent at the population scale. Arguably the most intriguing finding confirmed only via single‐cell analysis is the de novo expression of GH1 in 7 of the 8 fusion products. As such, we focus much of the discussion on this finding. As previously stated, growth hormone 1 is a protein traditionally found in somatotrope cells of the anterior pituitary and has been shown to be expressed in extrapituitary tissues only within the last 15 yr (47, 48). GH1 has been shown to be under the control of a locus control region (LCR) 14–32 kb upstream of the GH1 promoter. The major determinant of LCR activation is the DNase I hypersensitive site of the LCR, which contains 3 binding arrays for the POU domain class 1 transcription factor 1 (POU1F1). In somatotrope cells, it has been shown that the initiation of GH1 expression, as well as its continual maintenance, is dependent upon activation of these 3 binding arrays. Deletion of the Pit‐1 binding arrays in embryos causes a lack of pituitary GH1 expression, and, in adult somatrope cells, the extinction of GH1 expression (50). Given that GH1 expression, at least in somatotrope cells, is completely dependent on the presence of Pit‐1, it would be expected that the fusion products exhibit at least modest levels of POU1F1. However, this is not the case. None of the fusion products show any POU1F1 expression, nor do they show expression of Pit‐1 enhancer PROP paired‐like homeobox 1 (PROP1 or PIFX1) (51). It is impressive that the fusion products not only express GH1 without any of the necessary trans regulatory elements, but the expression levels of GH1 are very large, with FPKMs ranging from 100 to 10,000 in 6 of fusion products and production of GH1 at levels detectable with ELISA.
How expression of GH1 originates in hybrids is yet to be determined. It is possible that the massive chromatin reorganization that must occur with nuclear fusion allows for the translocation of the LCR to the GH1 promoter independent of Pit‐1 binding. Similarly, GH1‐expressing cells have been seen to express GH1 only when the promoter for the gene is located within a specific region of the nuclear periphery (50). The gene expression noted in the hybrids may follow a similar chromatin structural pathway as seen in somatotrope cells, or it may be activated via some novel pathway yet to be discovered. How or whether growth hormone is affecting the fusion products is also yet to be revealed. GH1 has been shown to cause reorganization of the actin cytoskeleton (52), and perhaps its function within the hybrid cells is to assist in the fusion of the membranes and reorganization of the fused cell. Alternatively, GH1 can be expressed in several different types of cancer cells, and is thought to enhance oncogenic properties (53). This could be true in the context of a tissue, but none of the fusion products expressed growth hormone receptor and so autocrine function is unlikely. No matter the pathway fusion opens for GH1 expression, or the role it may serve in the fusion products, it is an excellent example of the novel changes that can occur in cells following cell fusion. It also suggests a level of control and associated sophistication of the processes that may allow fusion or support survival of hybrids.
Growth hormone expression is seemingly an isolated event that only appeared through careful examination of all transcripts of individual hybrids. Contrary to this, the up‐regulation of mRNA‐encoded ribosomal proteins was a large sweeping effect that can be seen quite dramatically in the pathway overrepresentation analysis of Fig. 3 and Supplemental Fig. S6. It could be inferred that the hybrid cells would possess an increased level of ribosomal activity when compared with the relatively energetically inactive MSC parent. However, the magnitude of the increase in protein synthesis machinery is far too great to be considered as a simple intermediate to the 2 parent cells. This large up‐regulation in ribosome protein expression is something that has been previously observed by Freeman et al. (33) in fusion of MSCs and cardiomyocytes. Given the magnitude and uniformity of this trend in the current and previous study, it suggests that ribosomal up‐regulation is a conserved mechanism within MSC and cardiomyocyte fusion. As seen in Fig. 4, almost all of the fusion products have an increase in 13 individual ribosomal proteins, many of which were not expressed in either parent, by nearly 2 orders of magnitude. It could be hypothesized that this increase is largely an increase initiated by the mouse genome in order to increase protein production needed for reorganization and increased cell size. However, if this were the case, it might be expected that the fusion products would experience an increase in all mouse ribosomal proteins, which is not the case. Three of the ribosomal genes that were differentially expressed in the mouse parent cells remain at the same expression levels in the hybrids relative to the parent line. Thus, the increase in specific ribosomal proteins could be a result of more regulated protein production necessary for cell fusion, or perhaps there is a human regulatory protein that selectively activates these 13 proteins. With more mouse proteins being produced, perhaps the fusion products adapt more readily to the mouse genome. The profound change in ribosome assembly seen across the population of hybrids could be a result of cell fusion itself. However, it could be that such phenomena are unique to fusion of MSC and cardiomyocyte cell types specifically, as opposed to fusion events as a whole. Indeed, the MSCs themselves confer a certain level of heterogeneity (54), and therefore, fusion partners with a more homogeneous transcriptional profile may give rise to less heterogeneous hybrids. Whether the conserved pathways observed here are observed in those scenarios is yet to be seen. In addition, the transcriptional profile of hybrids likely changes with time, and so the point in time at which collection of hybrids occurs might also impact the transcriptional profile. Finally, it is possible that the viral fusogens used here to induce fusion contribute to the expression profiles observed. Thus, further studies will be needed to examine the transcriptome of fusion products of different cell types and of different species at multiple time points to confirm the universality of the hybrid expression profiles described here. Even so, forward progress has been made to understand the transcript‐level outcomes of intentional or inadvertent xenogeneic fusion.
AUTHOR CONTRIBUTIONS
B. T. Freeman and B. M. Ogle designed the research; B. T. Freeman and K. P. Jung performed the research; C. Yuan, B. T. Freeman, and T. J. McArdle analyzed the data; and C. Yuan, B. T. Freeman, T. J. McArdle, and B. M. Ogle wrote the manuscript.
Supporting information
Supplementary Material 1
Supplementary Material 2
Supplementary Material 3
Supplementary Material 4
Supplementary Material 5
Supplementary Material 6
Supplementary Material 7
Supplementary Material 8
Supplementary Material 9
Supplementary Material 10
ACKNOWLEDGMENTS
The authors thank Dr. Peiman Hematti (University of Wisconsin—Madison) for the kind donation of pluripotent stem cell—derived mesenchymal stem cells. The authors also thank Joshua Bailer and John Garbe (University of Minnesota Super‐computing Institute) for assistance in setting up single‐cell RNA sequencing workflow analyses for multiple genomes. This study was funded by the U.S. National Institutes of Health, National Heart, Lung, and Blood Institute (Grant HL137204); University of Minnesota Genomics Center New Investigator Award (NSF‐GRFP, 2010105691); Minnesota's Discover, Research, and Innovation Economy, University of Minnesota Informatics Institute Graduate Fellowship; and the Minnesota Stem Cell Institute. The authors declare no conflicts of interest.
Yuan, C. , Freeman, B. T. , McArdle, T. J. , Jung, J. P. , Ogle, B. M. Conserved pathway activation following xenogeneic, heterotypic fusion. FASEB J. 33, 6767–6777 (2019). www.fasebj.org
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Hamoud, N. , Tran, V. , Croteau, L. P. , Kania, A. , and Côté, J. F. (2014) G-protein coupled receptor BAI3 promotes myoblast fusion in vertebrates. Proc. Natl. Acad. Sci. USA 111, 3745–3750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deng, S. , Bothe, I. , and Baylies, M. K. (2015) The formin diaphanous regulates myoblast fusion through actin polymerization and Arp2/3 regulation. PLoS Genet. 11, e1005381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim, J. H. , Jin, P. , Duan, R. , and Chen, E. H. (2015) Mechanisms of myoblast fusion during muscle development. Curr Opin. Genet. Dev. 32, 162–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Millay, D. P. , Carnage, D. G. , Quinn, M. E. , Min, Y. L. , Mitani, Y. , Bassel-Duby, R. , and Olson, E. N. (2016) Structure-function analysis of myomaker domains required for myoblast fusion. Proc. Natl. Acad. Sci. USA 113, 2116–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park, S. Y. , Yun, Y. , Lim, J. S. , Kim, M. J. , Kim, S. Y. , Kim, J. E. , and Kim, I. S. (2016) Stabilin-2 modulates the efficiency of myoblast fusion during myogenic differentiation and muscle regeneration. Nat. Commun. 7, 10871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pizza, F. X. , Martin, R. A. , Springer, E. M. , Leffler, M. S. , Woelmer, B. R. , Recker, I. J. , and Leaman, D. W. (2017) Intercellular adhesion molecule-1 augments myoblast adhesion and fusion through homophilic trans-interactions. Sci. Rep. 7, 5094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vignery, A. (2005) Macrophage fusion: the making of osteoclasts and giant cells. J. Exp. Med. 202, 337–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Verma, S. K. , Leikina, E. , Melikov, K. , and Chernomordik, L. V. (2014) Late stages of the synchronized macrophage fusion in osteoclast formation depend on dynamin. Biochem. J. 464, 293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Toufaily, C. , Lokossou, A. G. , Vargas, A. , Rassart, E. , and Barbeau, B. (2015) A CRE/AP-1-like motif is essential for induced syncytin-2 expression and fusion in human trophoblast-like model. PLoS One 10, e0121468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huppertz, B. , Bartz, C. , and Kokozidou, M. (2006) Trophoblast fusion: fusogenic proteins, syncytins and ADAMs, and other prerequisites for syncytial fusion. Micron 37, 509–517 [DOI] [PubMed] [Google Scholar]
- 11. Gauster, M. , Moser, G. , Orendi, K. , and Huppertz, B. (2009) Factors involved in regulating trophoblast fusion: potential role in the development of preeclampsia. Placenta 30 (Suppl A), S49–S54 [DOI] [PubMed] [Google Scholar]
- 12. Degrelle, S. A. , Gerbaud, P. , Leconte, L. , Ferreira, F. , and Pidoux, G. (2017) Annexin-A5 organized in 2D-network at the plasmalemma eases human trophoblast fusion. Sci. Rep. 7, 42173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ogle, B. M. , and Platt, J. L. (2004) The Biology of Cell Fusion: Cells of different types and from different species can fuse, potentially transferring disease, repairing tissues and taking part in development. Am. Sci. 92, 420–427 [Google Scholar]
- 14. Ogle, B. M. , Cascalho, M. , and Platt, J. L. (2005) Biological implications of cell fusion. Nat. Rev. Mol. Cell Biol. 6, 567–575 [DOI] [PubMed] [Google Scholar]
- 15. Palermo, A. , Doyonnas, R. , Bhutani, N. , Pomerantz, J. , Alkan, O. , and Blau, H. M. (2009) Nuclear reprogramming in heterokaryons is rapid, extensive, and bidirectional. FASEB J. 23, 1431–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vassilopoulos, G. , Wang, P. R. , and Russell, D. W. (2003) Transplanted bone marrow regenerates liver by cell fusion. Nature 422, 901–904 [DOI] [PubMed] [Google Scholar]
- 17. Noiseux, N. , Gnecchi, M. , Lopez-Ilasaca, M. , Zhang, L. , Solomon, S. D. , Deb, A. , Dzau, V. J. , and Pratt, R. E. (2006) Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol. Ther. 14, 840–850 [DOI] [PubMed] [Google Scholar]
- 18. Kemp, K. , Gordon, D. , Wraith, D. C. , Mallam, E. , Hartfield, E. , Uney, J. , Wilkins, A. , and Scolding, N. (2011) Fusion between human mesenchymal stem cells and rodent cerebellar Purkinje cells. Neuropathol. Appl. Neurobiol. 37, 166–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huda, F. , Fan, Y. , Suzuki, M. , Konno, A. , Matsuzaki, Y. , Takahashi, N. , Chan, J. K. , and Hirai, H. (2016) Fusion of human fetal mesenchymal stem cells with “degenerating” cerebellar neurons in spinocerebellar ataxia type 1 model mice. PLoS One 11, e0164202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gonçalves, M. A. , de Vries, A. A. , Holkers, M. , van de Watering, M. J. , van der Velde, I. , van Nierop, G. P. , Valerio, D. , and Knaän-Shanzer, S. (2006) Human mesenchymal stem cells ectopically expressing full-length dystrophin can complement Duchenne muscular dystrophy myotubes by cell fusion. Hum. Mol. Genet. 15, 213–221 [DOI] [PubMed] [Google Scholar]
- 21. Freeman, B. T. , Kouris, N. A. , and Ogle, B. M. (2015) Tracking fusion of human mesenchymal stem cells after transplantation to the heart. Stem Cells Transl. Med. 4, 685–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ferrand, J. , Noël, D. , Lehours, P. , Prochazkova-Carlotti, M. , Chambonnier, L. , Ménard, A. , Mégraud, F. , and Varon, C. (2011) Human bone marrow-derived stem cells acquire epithelial characteristics through fusion with gastrointestinal epithelial cells. PLoS One 6, e19569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Freeman, B. T. , and Ogle, B. M. (2016) Viral-mediated fusion of mesenchymal stem cells with cells of the infarcted heart hinders healing via decreased vascularization and immune modulation. Sci. Rep. 6, 20283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang, X. , Willenbring, H. , Akkari, Y. , Torimaru, Y. , Foster, M. , Al-Dhalimy, M. , Lagasse, E. , Finegold, M. , Olson, S. , and Grompe, M. (2003) Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 422, 897–901 [DOI] [PubMed] [Google Scholar]
- 25. Willenbring, H. , and Grompe, M. (2004) Delineating the hepatocyte's hematopoietic fusion partner. Cell Cycle 3, 1489–1491 [DOI] [PubMed] [Google Scholar]
- 26. De la Garza-Rodea, A. S. , van der Velde-van Dijke, I. , Boersma, H. , Gonçalves, M. A. , van Bekkum, D. W. , de Vries, A. A. , and Knaän-Shanzer, S. (2012) Myogenic properties of human mesenchymal stem cells derived from three different sources. Cell Transplant. 21, 153–173 [DOI] [PubMed] [Google Scholar]
- 27. Ding, Z. , Liu, X. , Ren, X. , Zhang, Q. , Zhang, T. , Qian, Q. , Liu, W. , and Jiang, C. (2016) Galectin-1-induced skeletal muscle cell differentiation of mesenchymal stem cells seeded on an acellular dermal matrix improves injured anal sphincter. Discov. Med. 21, 331–340 [PubMed] [Google Scholar]
- 28. Pawelek, J. M. (2014) Fusion of bone marrow-derived cells with cancer cells: metastasis as a secondary disease in cancer. Chin. J. Cancer 33, 133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li, H. , Feng, Z. , Tsang, T. C. , Tang, T. , Jia, X. , He, X. , Pennington, M. E. , Badowski, M. S. , Liu, A. K. , Chen, D. , Harris, D. T. , Martinez, J. , and Meade-Tollin, L. C. (2014) Fusion of HepG2 cells with mesenchymal stem cells increases cancer-associated and malignant properties: an in vivo metastasis model. Oncol. Rep. 32, 539–547 [DOI] [PubMed] [Google Scholar]
- 30. Lazova, R. , Laberge, G. S. , Duvall, E. , Spoelstra, N. , Klump, V. , Sznol, M. , Cooper, D. , Spritz, R. A. , Chang, J. T. , and Pawelek, J. M. (2013) A melanoma brain metastasis with a donor-patient hybrid genome following bone marrow transplantation: first evidence for fusion in human cancer. PLoS One 8, e66731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun, C. , Zhao, D. , Dai, X. , Chen, J. , Rong, X. , Wang, H. , Wang, A. , Li, M. , Dong, J. , Huang, Q. , and Lan, Q. (2015) Fusion of cancer stem cells and mesenchymal stem cells contributes to glioma neovascularization. Oncol. Rep. 34, 2022–2030 [DOI] [PubMed] [Google Scholar]
- 32. Luo, F. , Liu, T. , Wang, J. , Li, J. , Ma, P. , Ding, H. , Feng, G. , Lin, D. , Xu, Y. , and Yang, K. (2016) Bone marrow mesenchymal stem cells participate in prostate carcinogenesis and promote growth of prostate cancer by cell fusion in vivo. Oncotarget 7, 30924–30934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Freeman, B. T. , Jung, J. P. , and Ogle, B. M. (2016) Single-cell RNA-seq reveals activation of unique gene groups as a consequence of stem cell-parenchymal cell fusion. Sci. Rep. 6, 23270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ogle, B. M. , Butters, K. A. , Plummer, T. B. , Ring, K. R. , Knudsen, B. E. , Litzow, M. R. , Cascalho, M. , and Platt, J. L. (2004) Spontaneous fusion of cells between species yields transdifferentiation and retroviral transfer in vivo. FASEB J. 18, 548–550 [DOI] [PubMed] [Google Scholar]
- 35. Trivedi, P. , and Hematti, P. (2008) Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Exp. Hematol. 36, 350–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang, P. , Li, L. , Hu, C. , Xu, Q. , Liu, X. , and Qi, Y. (2005) Interactions among measles virus hemagglutinin, fusion protein and cell receptor signaling lymphocyte activation molecule (SLAM) indicating a new fusion-trimer model. J. Biochem. Mol. Biol. 38, 373–380 [DOI] [PubMed] [Google Scholar]
- 37. Kruglyakov, P. V. , Sokolova, I. B. , Zin'kova, N. N. , Viide, S. K. , Aleksandrov, G. V. , Petrov, N. S. , and Polyntsev, D. G. (2006) In vitro and in vivo differentiation of mesenchymal stem cells in the cardiomyocyte direction. Bull. Exp. Biol. Med. 142, 503–506 [DOI] [PubMed] [Google Scholar]
- 38. Chitwood, C. A. , Dietzsch, C. , Jacobs, G. , McArdle, T. , Freeman, B. T. , Banga, A. , Noubissi, F. K. , and Ogle, B. M. (2018) Breast tumor cell hybrids form spontaneously in vivo and contribute to breast tumor metastases. APL Bioengineering 2, 031907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trapnell, C. , Roberts, A. , Goff, L. , Pertea, G. , Kim, D. , Kelley, D. R. , Pimentel, H. , Salzberg, S. L. , Rinn, J. L. , and Pachter, L. (2012) Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578; erratum: 9, 2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maere, S. , Heymans, K. , and Kuiper, M. (2005) BiNGO: a Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics 21, 3448–3449 [DOI] [PubMed] [Google Scholar]
- 41. Oh, H. , Bradfute, S. B. , Gallardo, T. D. , Nakamura, T. , Gaussin, V. , Mishina, Y. , Pocius, J. , Michael, L. H. , Behringer, R. R. , Garry, D. J. , Entman, M. L. , and Schneider, M. D. (2003) Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc. Natl. Acad. Sci. USA 100, 12313–12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kouris, N. A. , Schaefer, J. A. , Hatta, M. , Freeman, B. T. , Kamp, T. J. , Kawaoka, Y. , and Ogle, B. M. (2012) Directed fusion of mesenchymal stem cells with cardiomyocytes via VSV-G facilitates stem cell programming. Stem Cells Int. 2012, 414038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ishikawa, F. , Shimazu, H. , Shultz, L. D. , Fukata, M. , Nakamura, R. , Lyons, B. , Shimoda, K. , Shimoda, S. , Kanemaru, T. , Nakamura, K. , Ito, H. , Kaji, Y. , Perry, A. C. , and Harada, M. (2006) Purified human hematopoietic stem cells contribute to the generation of cardiomyocytes through cell fusion. FASEB J. 20, 950–952 [DOI] [PubMed] [Google Scholar]
- 44. Dedja, A. , Zaglia, T. , Dall'Olmo, L. , Chioato, T. , Thiene, G. , Fabris, L. , Ancona, E. , Schiaffino, S. , Ausoni, S. , and Cozzi, E. (2006) Hybrid cardiomyocytes derived by cell fusion in heterotopic cardiac xenografts. FASEB J. 20, 2534–2536 [DOI] [PubMed] [Google Scholar]
- 45. Alvarez-Dolado, M. , Pardal, R. , Garcia-Verdugo, J. M. , Fike J. R., Lee, H. O. , Pfeffer, K. , Lois, C. , Morrison, S. J. , and Alvarez-Buylla, A. (2003) Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 425, 968–973 [DOI] [PubMed] [Google Scholar]
- 46. Nygren, J. M. , Jovinge, S. , Breitbach, M. , Säwén, P. , Röll, W. , Hescheler, J. , Taneera, J. , Fleischmann, B. K. , and Jacobsen, S. E. (2004) Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat. Med. 10, 494–501 [DOI] [PubMed] [Google Scholar]
- 47. Pérez-Ibave, D. C. , Rodríguez-Sánchez, I. P. , Garza-Rodríguez, M. L. , and Barrera-Saldaña, H. A. (2014) Extrapituitary growth hormone synthesis in humans. Growth Horm. IGF Res. 24, 47–53 [DOI] [PubMed] [Google Scholar]
- 48. Kopchick, J. J. , and Andry, J. M. (2000) Growth hormone (GH), GH receptor, and signal transduction. Mol. Genet. Metab. 71, 293–314 [DOI] [PubMed] [Google Scholar]
- 49. Kobayashi, M. , Oshima, S. , Maeyashiki, C. , Nibe, Y. , Otsubo, K. , Matsuzawa, Y. , Nemo to, Y. , Nagaishi, T. , Okamoto, R. , Tsuchiya, K. , Nakamura, T. , and Watanabe, M. (2016) The ubiquitin hybrid gene UBA52 regulates ubiquitination of ribosome and sustains embryonic development. Sci. Rep. 6, 36780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ho, Y. , Shewchuk, B. M. , Liebhaber, S. A. , and Cooke, N. E. (2013) Distinct chromatin configurations regulate the initiation and the maintenance of hGH gene expression. Mol. Cell. Biol. 33, 1723–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhu, X. , Gleiberman, A. S. , and Rosenfeld, M. G. (2007) Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol. Rev. 87, 933–963 [DOI] [PubMed] [Google Scholar]
- 52. Goh, E. L. , Pircher, T. J. , Wood, T. J. , Norstedt, G. , Graichen, R. , and Lobie, P. E. (1997) Growth hormone-induced reorganization of the actin cytoskeleton is not required for STAT5 (signal transducer and activator of transcription-5)-mediated transcription. Endocrinology 138, 3207–3215 [DOI] [PubMed] [Google Scholar]
- 53. Harvey, S. (2010) Extrapituitary growth hormone. Endocrine 38, 335–359 [DOI] [PubMed] [Google Scholar]
- 54. Freeman, B. T. , Jung, J. P. , and Ogle, B. M. (2015) Single-cell RNA-Seq of bone marrow-derived mesenchymal stem cells reveals unique profiles of lineage priming. PLoS One 10, e0136199 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1
Supplementary Material 2
Supplementary Material 3
Supplementary Material 4
Supplementary Material 5
Supplementary Material 6
Supplementary Material 7
Supplementary Material 8
Supplementary Material 9
Supplementary Material 10


