1. Introduction
Persistent musculoskeletal pain is a global health issue [34]. The complexity and heterogeneity of pain presentations make management of musculoskeletal conditions challenging and have led to unhelpful terms such as “non-specific low back pain” and “temporomandibular joint dysfunction”, which cannot guide treatment. Selection of treatment based on identification of the neurobiological mechanisms that maintain an individual’s pain is a plausible approach to improve outcomes [5,10,29,32]. A major challenge is whether pain mechanisms can be accurately identified in clinical practice and research [19]. The International Association for the Study of Pain (IASP) identifies three main pain mechanism categories to explain pain (nociceptive, neuropathic, and nociplastic – Table 1) [13], but there is considerable debate if or how these mechanisms can be identified and differentiated from each other [25,26].
Table 1.
IASP pain mechanism definitions
| Pain mechanism | Definition |
|---|---|
| Nociceptive pain | Pain that arises from actual or threatened damage to non-neural tissue and is due to the activation of nociceptors. |
| Neuropathic pain | Pain caused by a lesion or disease of the somatosensory nervous system. |
| Nociplastic pain | Pain that arises from altered nociception despite no clear evidence of actual or threatened tissue damage causing the activation of peripheral nociceptors or evidence for disease or lesion of the somatosensory system causing the pain. |
Definitions of pain mechanism categories as proposed by the IASP [13].
Operationalisation of a treatment approach for musculoskeletal conditions that targets specific pain mechanisms requires agreement of a feature, or more likely a cluster of features, that can identify the probable underlying mechanisms. This approach could include methods to identify features that are present in one or two, but not all pain mechanism categories. Several methods to discriminate between pain mechanisms have been proposed based on narrative review [4,36], and clinical experts from a single [7] or unknown discipline(s) [23]. There has been a recent rapid expansion of research regarding pain mechanisms, rigorous testing of measurement paradigms, and changes to terminology (e.g., endorsement of “nociplastic pain” by IASP [19]). As a first step towards refinement of a consensus and evidence-based approach to discriminate between pain mechanisms, two studies systematically reviewed the literature regarding the features that characterize the different mechanisms [25], and the methods proposed to discriminate between them [26]. Although some convergence was apparent, the reviews highlighted divergence in opinion.
Three major issues challenge the development of an accepted method to discriminate between pain mechanism categories. First, as no direct in-vivo measures are available to confirm the putative neurobiological mechanisms responsible for pain in many individuals, there is no gold standard method to validate the discrimination between mechanisms [3,32]. Second, many individuals likely present with pain that includes a combination of pain mechanisms, although one might be predominant. Third, interpretation of the literature is hampered by divergence of opinion regarding features that might be unique to a specific pain mechanism category or shared between multiple categories. When evidence is contradictory, divergent, or unavailable, consensus of experts is necessary [14].
The primary aim of this study was to use a Delphi process to reach consensus amongst experts on features that are unique to one pain mechanism category, shared by two major categories, or present in all three, and therefore unhelpful for discrimination. This study built on the recent systematic review of features advocated to discriminate between pain mechanisms [26]. The secondary aim was to develop a ranked list of features based on agreement between experts. Such a list could form the foundation for future development of a consensus-based approach to identify and discriminate between pain mechanism categories in individuals presenting with pain experienced in the musculoskeletal system.
2. Methods
2.1. Study design and overview
An online Delphi design [33,35] was used to evaluate expert opinion on features/assessment findings that have been proposed to guide discrimination between the pain mechanisms that contribute to an individual’s pain experience. This approach involves multiple rounds of questionnaires which include rating of items and/or open-ended responses [33]. This study involved two rounds undertaken and reported as recommended by guidelines for Delphi studies [15,27]. Round 1 involved presentation of a list of features and assessment findings derived from systematic reviews [25,26] that have been proposed to aid discrimination between pain mechanism categories. Participants were asked to indicate which (if any) pain mechanism would be attributed to each, to propose additional features, and to provide comments on terminology/wording. Round 2 involved clarification of outcomes of Round 1 to refine the final ranked list. This study was approved by the institutional Human Research Ethics Committee (#2020002324) at the University of Queensland and participants provided informed consent.
2.2. Steering committee
A steering committee was established to oversee the project, including preparation of the list of features and assessment findings to be evaluated in Round 1, evaluation of the outcome of Round 1, and review of responses to Round 2. The committee involved four members with backgrounds in pain neurobiology. All were physiotherapists, but each with different research expertise and clinical experience (years of experience: MAS – pain, neuroscience – 5 years; MS – pain, clinical research – 37 years; KS – basic neuroscience, translational and clinical pain science – 36 years; PH – pain, neuroscience – 30 years). Two members (MS, KS) had been involved in IASP projects related to definitions of pain and pain mechanisms [19,24] and two members (MAS, PH) had published extensive literature reviews that provided a foundation for this work [25,26].
2.3. Expert selection
Selection of an expert panel is an essential part of the Delphi process [16]. Expert panelists should be committed to the project, credible, and sufficiently heterogeneous to represent the range of experts who have an interest in results [17]. No standard method is available to calculate a panel size to undertake a Delphi process [6]. As this project required diversity of opinion, a minimum sample size of 40 panelists from different countries was defined a priori for each round. Heterogeneity of the sample was critical to ensure a wide spectrum of opinions [21,35]. To meet this goal, it was considered essential to include researchers, academics, clinicians, and individuals with pain with consideration of diversity of discipline, international location, career level, and gender.
The steering committee developed a preliminary list of potential panelists who met at least two of the following criteria: (1) contribution to at least three published works related to pain in the preceding three years; (2) keynote or invited presentations at major meetings related to pain; (3) contribution to major working groups/committees of pain organisations; (4) contribution to organisation of major pain meetings/conferences; (5) contribution to pain textbooks; (6) contribution to clinical practice guidelines/systematic reviews related to pain; (7) membership of any international pain organisation; and (8) postgraduate certification in pain or pain management. The final list was refined based on diversity of expertise, clinical discipline, international distribution, career level, and gender. In addition, the steering committee identified two representatives with lived experience of chronic pain who have had additional training in pain mechanisms. Potential experts were contacted via an email invitation letter and reminders were sent after two weeks if no response was received. Experts who agreed to participate were provided with a link to more detailed information and to provide consent. Demographic data collected included: age, gender, country, discipline, major topic area in the pain field, years working in the pain field, and number of publications related to pain. Although data were presented to the panelists in a de-identified manner, they were informed that data could be re-identified by the steering committee if clarification of responses was required.
2.4. Development of the initial list of features/assessment findings
A list of features/assessment findings that have been proposed to aid the discrimination between pain mechanism categories was derived from two recent systematic reviews [25,26] and categorised under four main method groupings: (1) clinical examination, (2) quantitative sensory testing, (3) imaging and diagnostic testing, and (4) pain-type questionnaires. This initial list was refined during five meetings of the steering committee. The following refinements were made: (1) subjective descriptors of pain were converged under groupings described by the McGill pain questionnaire where applicable [22] (descriptors that could not be grouped in this manner were retained separately); (2) features/assessment findings that had similar meaning were converged (e.g., clinical bedside sensory testing was converged with quantitative sensory testing); and (3) items that did not describe specific assessment findings (e.g., diagnosis of a pain mechanism category by exclusion of another) were excluded. The completed list of features/assessment findings is presented in Supplemental Digital Content 1.
2.5. Delphi process
The Delphi process was undertaken on an online surveying platform (Qualtrics, Seattle, US). Experts participated in two rounds (Figure 1). In the first Delphi round, experts were presented with a description of the purpose of the study, a brief definition of pain mechanism categories (from the IASP [13]), and a list of features/assessment findings. They were asked to nominate the category (nociceptive, neuropathic, nociplastic) in which each feature/assessment finding might be observed. Experts could select; none, one, two, or three pain mechanism categories. If one category was selected the feature/assessment finding was considered to be unique to that category. If two categories were selected it would be considered to aid discrimination between those two categories and the third category, but not between them. If three categories were selected it was considered that this feature, while present in each condition, would not aid the discrimination between them. Experts could also select “unsure”. Contributors had the opportunity to suggest changes to the terminology or wording applied to a feature/assessment finding or nominate others that they believe might aid discrimination between pain mechanism categories.
Figure 1.
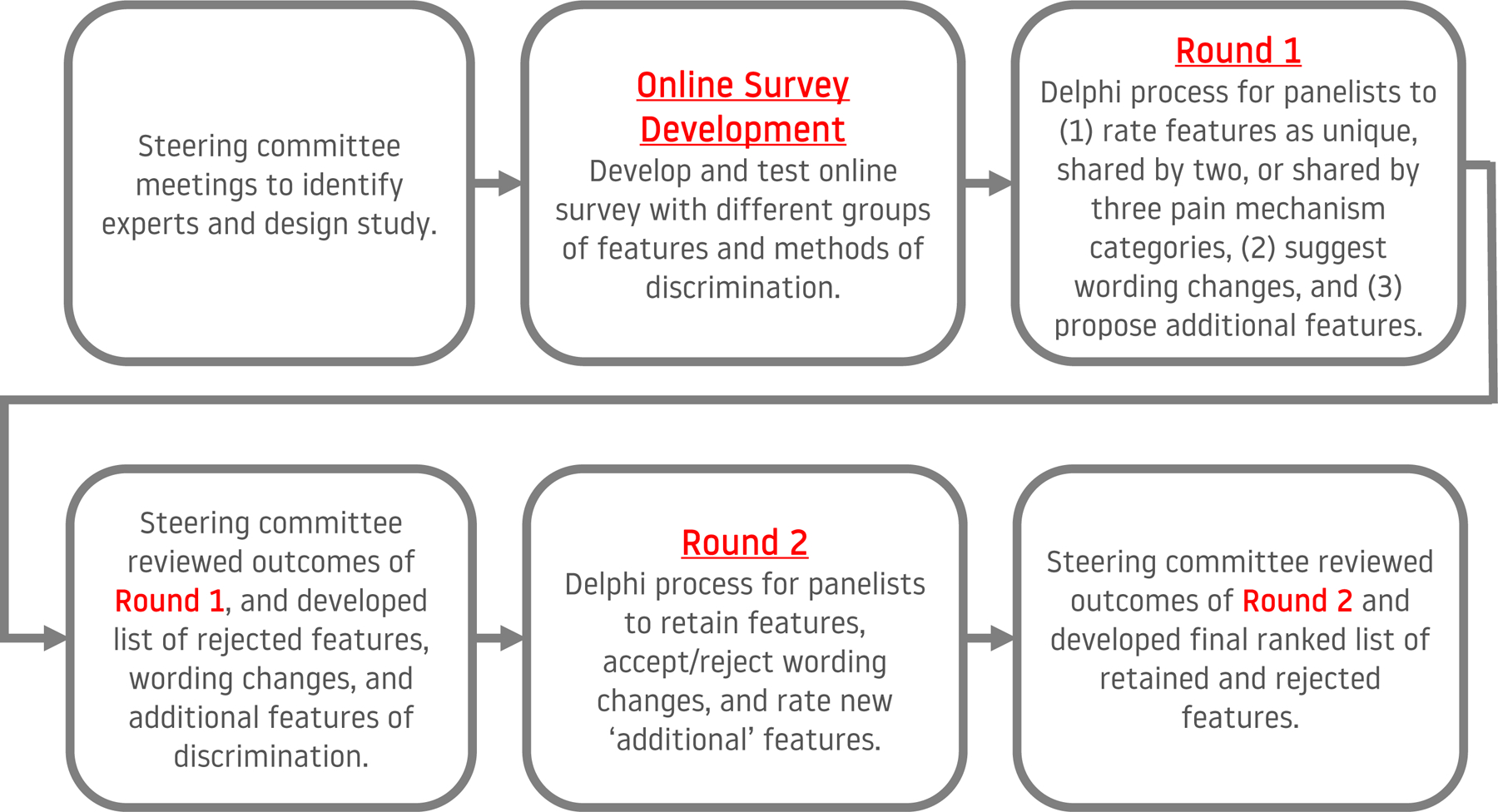
Flow diagram of the process of developing and conducting the Delphi process.
From Round 1, a list of features was generated that experts agreed might aid discrimination between pain mechanism categories that contribute to an individual’s pain experience. Because of the diversity of opinion in the field and the absence of gold standard to address disputes in opinion, and because the features identified in this study would be subjected to additional evaluation prior to reduction to the minimum set of measures, it was decided to use a lenient threshold that is lower than that commonly used in Delphi studies [8]. This was set at 40% agreement to ensure that the process eliminated measures that the group very consistently did not consider helpful [2], but retained features that might be controversial yet have some potential to aid discrimination between pain mechanisms and worthy of further testing. To be retained in this list, features would need to reach agreement as: (1) Unique to one pain mechanism category – if >40% of experts state that the feature/finding is present in only one category (must be the same category identified by each expert); or (2) Shared by two pain mechanism categories – if >40% of experts state that the feature/finding is present in the same two categories. If a feature failed to reach either of these thresholds, but the sum of unique to one category and shared by two categories exceeded 40% the feature was retained and allocated according to the category or combination of categories with the highest score, or both if the score was equal. A ranked list was generated of features/assessment findings using the percentage agreement.
In Round 2, features/assessment findings that did not reach threshold to be retained in the list as being helpful to discriminate between pain mechanism categories (i.e., failed to reach threshold for “unique” or “shared by two”) were represented to panelists who were asked whether any of the pain mechanism categories not meeting threshold should be retained. Panelists were also given an opportunity to provide justification and/or evidence to support their opinion. To be retained, at least 15% of respondents should independently identify that the feature/assessment finding should be retained. Panelists were also presented with a list of the additional feature/assessment findings that were nominated in the first round. These were judged with the same consensus criteria applied in Round 1. Finally, any suggested changes to terminology/wording from Round 1 were presented to panelists to indicate their agreement/disagreement with the suggested changes. Wording changes were accepted if a majority of panelists (>50%) and the steering committee were in agreement. The steering committee also considered additional refinements of wording based upon panelist input. The complete list of additional features/assessment findings added in Round 2 are included in Supplemental Digital Content 1.
3. Results
Seventy-three potential panelists were identified and invited to participate in this consensus study. Fifty-five panelists accepted the invitation and 49 (89%) and 48 (87%) provided responses to Round 1 and 2 of this Delphi process, respectively.
3.1. Contributor demographics
The forty-nine panelists (average [SD] age; 55 [10] years) included 29 males (56 [11] years) and 20 females (54 [9] years) from fifteen countries. Together they represented a total of 1291 years working in the pain field( (average 26 years) and 8388 publications related to pain (average 171, median 102 [range 0–1000] publications related to pain). The country, roles designation, and major discipline/research field related to pain are provided in Table 2.
Table 2.
Panellist country, role designation and expertise
| Country (n, %) | ||||
|---|---|---|---|---|
|
Region of the Americas (19, 39%) |
European Region (17, 35%) |
Western Pacific Region (12, 24%) |
African Region | |
| United States (16, 33%) |
Denmark (5, 10%) |
Germany (2, 4%) |
Australia (9, 18%) |
South Africa (1, 2%) |
| Brazil (2, 4%) |
United Kingdom (4, 8%) |
Netherlands (1, 2%) |
Japan (1, 2%) |
|
| Canada (1, 2%) |
Belgium (2, 4%) |
Ireland (1, 2%) |
New Zealand (1, 2%) |
|
| Sweden (2, 4%) |
Malaysia (1, 2%) |
|||
|
Role designation (n, %)
| ||||
| Clinical scientist / researcher (32, 65%) |
Basic scientist (8, 16%) |
Clinician (7, 14%) |
Consumer (2, 4%) |
|
|
Discipline/major field (n, %)
*
| ||||
| Physiotherapy (14, 29%) |
Basic science (9, 18%) |
Neurology (4, 8%) |
Occupational therapy (3, 6%) |
Nursing (1, 2%) |
| Pain medicine (7, 14%) |
Musculoskeletal (7, 14%) |
Psychology (4, 8%) |
Dentistry (2, 4%) |
Rheumatology (1, 2%) |
| Rehabilitation medicine (5, 10%) |
Neuroscience (5, 10%) |
Anaesthesiology (3, 6%) |
Chiropractic (1, 2%) |
People with lived pain experience (2, 4%) |
|
Neuropharmacology (3, 6%) |
Orthopaedic surgery (3, 6%) |
|||
|
Neurophysiology (2, 4%) |
||||
|
Neuropathic pain research (2, 4%) |
||||
n = number.
The sum of disciplines/major fields exceeds the number of panelists as most nominated more than one.
3.2. Delphi Round 1 – Quantitative results for features/assessment findings of pain mechanism categories
A total of 185 from 277 (67%) features that represented each of the main method groupings were identified as having potential to discriminate between pain mechanism categories (unique to one pain mechanism category or shared between two pain mechanism categories – identified by >40% of contributors). With respect to the methods groupings, 124 features were identified from the clinical examination grouping (75% of 165), 33 features from quantitative sensory testing (83% of 40), 14 features from imaging and diagnostic testing (34% of 41), and 14 features from pain-type questionnaires (47% of 30). A total of 70 features were identified as unique for one category – 17, 33 and 20 for nociceptive, neuropathic, and nociplastic pain, respectively (Table 3). A total of 115 features were identified as being shared between two categories – 26, 19, and 75 for nociceptive + neuropathic, nociceptive + nociplastic, and neuropathic + nociplastic pain, respectively (Table 4).
Table 3.
Features identified as unique to one pain mechanism category
| Contributor responses (%) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feature /assessment finding | Method Category (Subcategory) | Nociceptive | Neuropathic | Nociplastic | Nociceptive + Neuropathic | Nociceptive + Nociplastic | Neuropathic + Nociplastic | ALL Nociceptive + Neuropathic + Nociplastic |
None | Unsure | |||
| Nociceptive… | Generally responsive to anti-inflammatory drugs (NSAIDs). | Response to drugs | W | 71% | 8% | 12% | 2% | 2% | 4% | ||||
| Signs of inflammation (redness, heat/warmth, tenderness, swelling). | Associated signs and symptoms | 67% | 2% | 10% | 10% | 4% | 6% | ||||||
| Pain recovery or healing time predictable based on expected time of tissue recovery. | Recovery/healing period | W | 65% | 20% | 2% | 2% | 8% | 2% | |||||
| Proportional and direct relationship with aggravating factors (e.g. provocative movements). | Aggravating factors | W | 57% | 2% | 12% | 4% | 14% | 8% | 2% | ||||
| Consistent pain provocation by testing of specific movements (e.g., specific movement tests for the shoulder, tests of tempromandibular joint motion) | Movement, joint, and functional testing | W | 53% | 2% | 22% | 4% | 6% | 2% | 10% | ||||
| Localised distribution of pain. | Pain location | 51% | 39% | 2% | 6% | 2% | |||||||
| NO generalised hypersensitivity. | Pain location | W | 51% | 2% | 27% | 6% | 6% | 8% | |||||
| Findings from imaging of body regions of potential relevance to the pain experience. | Imaging/radiography | W | 51% | 22% | 8% | 10% | 8% | ||||||
| Mechanical testing shows a clear, consistent, and proportional pattern of pain or symptom provocation. | Movement, joint, and functional testing | 49% | 2% | 22% | 4% | 4% | 8% | 10% | |||||
| Generally responsive to tissue-based treatments (e.g. manual therapy, massage, acupuncture, heat/cold, removal of tissue pathology, occlusal splints). | Response to physical treatments | W | 47% | 12% | 12% | 2% | 22% | 4% | |||||
| Consistently provoked by specific postures. | Aggravating factors | 45% | 4% | 27% | 6% | 8% | 2% | 8% | |||||
| Generally NOT responsive to anticonvulsants. | Response to drugs | W | 45% | 16% | 8% | 6% | 24% | ||||||
| Joint testing consistently demonstrates painful response. | Movement, joint, and functional testing | 45% | 2% | 4% | 16% | 8% | 6% | 18% | |||||
| ABSENCE of autonomic symptoms and/or signs. | Associated signs and symptoms | 43% | 2% | 2% | 4% | 4% | 8% | 10% | 27% | ||||
| Consistently provoked by specific movements. | Aggravating factors | 41% | 29% | 10% | 20% | ||||||||
| Generally NOT responsive to antidepressants. | Response to drugs | W | 41% | 2% | 4% | 8% | 16% | 29% | |||||
| Below cut-off (≤ 12). | Neuropathic questionnaires (Modified PainDETECT) | 41% | 2% | 10% | 2% | 10% | 35% | ||||||
|
| |||||||||||||
| Neuropathic… | Dermatomal or peripheral nerve distribution of pain. | Neurological testing | 2% | 86% | 4% | 2% | 6% | ||||||
| Demonstrate evidence of lesion or disease of nervous system. | Imaging/radiography | 86% | 2% | 4% | 8% | ||||||||
| Dermatomal or peripheral nerve distribution of pain. | Pain location | 84% | 8% | 4% | 2% | 2% | |||||||
| Sensory deficits (e.g. numbness) in dermatomal pattern. | Associated signs and symptoms | A | 79% | 4% | 6% | 2% | 2% | 6% | |||||
| Evidence of damage/disease to the nervous system. | Neuroimaging / electrophysiological testing (Electroneuromyography) | 78% | 2% | 2% | 4% | 14% | |||||||
| Demonstrates evidence of lesion or disease of nervous system. | Neuroimaging / electrophysiological testing (Neuroimaging (e.g. CT, MRI)) | 73% | 10% | 4% | 2% | 4% | 6% | ||||||
| Hypoaesthesia. | Associated signs and symptoms | 69% | 20% | 4% | 6% | ||||||||
| Abnormal nerve conduction velocity. | Neuroimaging / electrophysiological testing (Electroneuromyography) | 69% | 4% | 8% | 4% | 14% | |||||||
| Electric shock-like, lightning. | Subjective descriptors | 67% | 2% | 20% | 2% | 2% | 6% | ||||||
| Negative symptoms (e.g. numbness, hypoalgesia). | Neurological testing | 67% | 2% | 18% | 4% | 2% | 6% | ||||||
| Sensory deficits (e.g. numbness) relevant to territory of innervation of injured peripheral nerve or central somatosensory projection area relevant to lesion or disease of CNS. | Associated signs and symptoms | W | 61% | 2% | 24% | 8% | 4% | ||||||
| Altered or absent deep tendon reflexes. | Neurological testing | 2% | 61% | 4% | 2% | 31% | |||||||
| Pins and needles. | Associated signs and symptoms | 57% | 33% | 4% | 6% | ||||||||
| Prickling. | Associated signs and symptoms | 57% | 2% | 31% | 2% | 2% | 6% | ||||||
| Itchy. | Associated signs and symptoms | 2% | 57% | 2% | 4% | 10% | 6% | 6% | 12% | ||||
| Provoked by movements that load or compress neural tissue. | Aggravating factors | 4% | 55% | 12% | 8% | 12% | 2% | 6% | |||||
| Formication (sensation that resembles that of small insects crawling on or under the skin when there is nothing there). | Subjective descriptors | 55% | 8% | 12% | 2% | 8% | 14% | ||||||
| Hypoalgesia. | Associated signs and symptoms | 55% | 6% | 2% | 6% | 4% | 8% | 18% | |||||
| Altered deep tendon reflexes. | Associated signs and symptoms | 55% | 6% | 2% | 10% | 2% | 24% | ||||||
| Tingling. | Associated signs and symptoms | 53% | 35% | 6% | 2% | 4% | |||||||
| Increased threshold/hypoalgesia. | Mechanical testing (LOCAL mechanical/pressure pain threshold OR NOXIOUS touch/pressure) | 53% | 2% | 4% | 4% | 20% | 16% | ||||||
| Increased threshold/hypoaesthesia. | Mechanical testing (LOCAL mechanical detection threshold OR NON-NOXIOUS touch/pressure) | 53% | 2% | 8% | 6% | 18% | 12% | ||||||
| Decreased deep tendon reflexes. | Neurological testing | A | 52% | 4% | 4% | 2% | 8% | 29% | |||||
| Fire-like. | Subjective descriptors | 49% | 2% | 4% | 20% | 6% | 4% | 14% | |||||
| Cool, cold, freezing. | Subjective descriptors | 49% | 2% | 2% | 20% | 4% | 4% | 18% | |||||
| Crawling. | Associated signs and symptoms | 2% | 49% | 8% | 16% | 4% | 2% | 18% | |||||
| Positive findings (e.g. pain provocation, Tinel’s sign - pins and needles). | Nerve provocation testing (palpation/tapping) | 49% | 4% | 18% | 10% | 2% | 16% | ||||||
| Myotomal muscle atrophy. | Associated signs and symptoms | A | 2% | 48% | 6% | 4% | 6% | 33% | |||||
| Shooting, jumping, flashing. | Subjective descriptors | 2% | 47% | 6% | 20% | 8% | 4% | 12% | |||||
| Phantom pain. | Other pain qualities | 47% | 16% | 2% | 14% | 4% | 2% | 14% | |||||
| Above cut-off (≥ 4). | Neuropathic questionnaires (Douleur Neuropathique 4 (DN4)) | 43% | 20% | 2% | 35% | ||||||||
| Above cut-off (≥ 0). | Neuropathic questionnaires (Neuropathic Pain Questionnaire (NPQ)) | 43% | 22% | 2% | 33% | ||||||||
| Tingling, itchy, smarting, stinging. | Subjective descriptors | 2% | 41% | 4% | 2% | 20% | 12% | 6% | 12% | ||||
| Hot, burning, scalding, searing. | Subjective descriptors | 41% | 2% | 4% | 27% | 14% | 4% | 8% | |||||
| Muscle spasticity. | Associated signs and symptoms | 4% | 41% | 4% | 2% | 8% | 4% | 20% | 16% | ||||
| Skin biopsy demonstrates reduced intraepidermal nerve fiber density. | Other diagnostic tests | 41% | 18% | 8% | 33% | ||||||||
| Motor deficits (e.g. weakness) in a neuroanatomically plausible distribution. | Neurological testing | R, W | 15% | 4% | 20% | 10% | 10% | 41% | 2% | 12% | |||
|
| |||||||||||||
| Nociplastic… | Diffuse, widespread, or poorly localised distribution of pain. | Pain location | 82% | 6% | 10% | 2% | |||||||
| Generalised hypersensitivity. | Pain location | W | 2% | 71% | 12% | 10% | 4% | ||||||
| Multiple somatic symptoms (e.g. fatigue, memory difficulties, concentration difficulties, sleep disturbances, mood disturbances). | Associated signs and symptoms | 65% | 12% | 12% | 6% | 4% | |||||||
| Varying distribution of pain. | Pain location | 59% | 4% | 18% | 8% | 2% | 8% | ||||||
| Presence of hypersensitivity to stimuli (e.g. pressure, temperature, sound, odor, taste, and light). | Associated signs and symptoms | W | 2% | 57% | 2% | 22% | 8% | 4% | 4% | ||||
| Generally NOT responsive to local anaesthetics. | Response to drugs | W | 55% | 2% | 14% | 2% | 12% | 14% | |||||
| Variability or no consistency in descriptors. | Subjective descriptors | 2% | 55% | 8% | 10% | 8% | 16% | ||||||
| Generally NOT responsive to surgery. | Response to physical treatments | W | 53% | 2% | 20% | 8% | 2% | 14% | |||||
| Inconsistent, confusing, and ambiguous responses and findings to clinical tests which vary over sessions. | Other pain qualities | 53% | 22% | 4% | 4% | 16% | |||||||
| NO findings from imaging of body regions of potential relevance to the pain experience. | Imaging/radiography | W | 53% | 12% | 4% | 18% | 6% | 6% | |||||
| Positive findings (no cut-off proposed). | Other questionnaires (Fibromyalgia Criteria and Severity Scales (FCSS)) | 53% | 2% | 4% | 2% | 4% | 35% | ||||||
| Generally NOT responsive to peripheral nerve block (where relevant). | Response to drugs | W | 4% | 51% | 10% | 10% | 4% | 12% | 8% | ||||
| Positive findings (no cut-off proposed). | Other questionnaires (Revised Fibromyalgia Impact Questionnaire (RFIQ)) | 51% | 2% | 2% | 2% | 4% | 39% | ||||||
| Mechanical testing shows a disproportionate, inconsistent, non-mechanical pattern of pain or symptom provocation. | Movement, joint, and functional testing | 49% | 24% | 14% | 2% | 10% | |||||||
| Above cut-off (≥ 40). | Central sensitisation questionnaires (Central Sensitization Inventory (CSI)) | 49% | 18% | 2% | 8% | 22% | |||||||
| Multi-site pain (3 or more regions) | Pain location | A | 46% | 13% | 13% | 25% | 4% | ||||||
| Nonspecific neurological findings or ABSENCE of clear findings. | Neurological testing | 45% | 16% | 6% | 6% | 6% | 20% | ||||||
| Pain experienced in a non-dermatomal or non-neuroanatomic distribution in a body region. | Pain location | W | 2% | 43% | 18% | 14% | 16% | 6% | |||||
| More concern for bodily function. | Associated signs and symptoms | 4% | 43% | 2% | 4% | 8% | 6% | 33% | |||||
| Spread of pain over time to new body sites/areas. | Pain location | A | 42% | 4% | 23% | 25% | 6% | ||||||
| History of failed, variable, or unpredictable response to interventions. | Recovery/healing period | 41% | 33% | 16% | 2% | 8% | |||||||
| Disproportionate or abnormal reaction during and after the patient’s assessment and/or treatment. | Other pain qualities | 2% | 41% | 31% | 14% | 2% | 10% | ||||||
Note:
Features are ranked by % of contributors in descending order.
A = Additional features proposed by contributors.
W = Wording changes proposed by contributors.
R = Retained features where >15% of contributes indicated that the feature should be retained with generally consistent rationales/reasoning. The corresponding % of contributors is included.
Table 4.
Features identified as shared between two pain mechanism categories
| Contributor responses (%) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feature /assessment finding | Method Category (Subcategory) | Nociceptive | Neuropathic | Nociplastic | Nociceptive + Neuropathic | Nociceptive + Nociplastic | Neuropathic + Nociplastic | ALL Nociceptive + Neuropathic + Nociplastic |
None | Unsure | ||
| Nociceptive + Neuropathic | Generally responsive to surgery. | Response to physical treatments | W | 35% | 2% | 39% (76%) | 4% | 8% | 12% | |||
| Movements decompressing structure provide pain relief. | Movement, joint, and functional testing | 12% | 22% | 27% (61%) | 2% | 6% | 4% | 27% | ||||
| Normal threshold/absence of hyperalgesia in areas remote to the area of primary pain. | Mechanical testing (REMOTE mechanical/pressure pain threshold OR NOXIOUS touch/pressure) | W | 37% | 2% | 18% (57%) | 2% | 2% | 2% | 20% | 16% | ||
| Positive response. | Diagnostic anaesthetic injections/blocks (Sensory/motor spinal blocks) | 8% | 14% | 33% (55%) | 12% | 6% | 27% | |||||
| Localised muscle atrophy. | Associated signs and symptoms | A | 4% | 23% | 27% (54%) | 2% | 4% | 21% | 4% | 15% | ||
| Intermittent or transient pain. | Other pain qualities | 20% | 8% | 2% | 24% (53%) | 2% | 10% | 24% | 2% | 6% | ||
| Generally response to opioid analgesics (e.g. fentanyl). | Response to drugs | W | 27% | 2% | 2% | 22% (51%) | 6% | 4% | 27% | 2% | 8% | |
| Generally responsive to peripheral nerve block (where relevant). | Response to drugs | W | 10% | 22% | 49% | 12% | 6% | |||||
| Sharp, cutting, lacerating. | Subjective descriptors | 14% | 27% | 2% | 8% (49%) | 6% | 6% | 22% | 4% | 10% | ||
| Knife-like. | Subjective descriptors | 6% | 29% | 14% (49%) | 4% | 10% | 20% | 4% | 12% | |||
| Efficient conditioned pain modulation (increased pain threshold). | Pain modulation testing (Conditioned pain modulation (e.g. pressure cuff, cold pressor test)) | 39% | 2% | 2% | 8% (49%) | 2% | 8% | 22% | 16% | |||
| Generally responsive to local anaesthetics (e.g. lidocaine). | Response to drugs | W | 29% | 6% | 47% | 2% | 14% | 2% | ||||
| Unilateral distribution of pain. | Pain location | 10% | 6% | 4% | 31% (47%) | 2% | 2% | 27% | 4% | 14% | ||
| Minimal or absent psychological features, disturbances, or behaviours related to the pain experience or having bearing on the pain experience. | Psychological assessment | W | 24% | 2% | 20% (47%) | 14% | 27% | 12% | ||||
| Primary hyperalgesia. | Other pain qualities | 16% | 18% | 12% (47%) | 2% | 20% | 24% | 2% | 4% | |||
| Normal threshold/absence of heat hyperalgesia in areas remote to the area of primary pain. | Heat testing (REMOTE heat pain threshold OR NOXIOUS heat application) | W | 33% | 2% | 14% (47%) | 6% | 10% | 12% | 22% | |||
| Non-enhanced temporal summation. | Pain modulation testing (Temporal summation (e.g. repetitive mechanical/heat/cold/electrical stimuli via monofilaments, thermode, or electrodes)) | 39% | 4% | 2% | 2% (45%) | 4% | 6% | 31% | 12% | |||
| Below cut-off (< 40). | Central sensitisation questionnaires (Central Sensitization Inventory (CSI)) | 24% | 20% (45%) | 2% | 4% | 18% | 31% | |||||
| Stimulus-dependent or evoked pain. | Other pain qualities | 29% | 16% (45%) | 2% | 6% | 39% | 8% | |||||
| Normal threshold/absence of cold hyperalgesia in areas remote to the area of primary pain. | Cold testing (REMOTE cold pain threshold OR NOXIOUS cold application) | W | 29% | 4% | 2% | 12% (45%) | 2% | 4% | 8% | 4% | 35% | |
| Referred pain or distal pain radiation. | Pain location | 2% | 27% | 4% | 14% (43%) | 8% | 12% (43%) | 29% | 4% | |||
| Stretching. | Subjective descriptors | 29% | 8% | 6% (43%) | 8% | 12% | 10% | 27% | ||||
| Normal TPD threshold (normal tactile acuity). | Higher sensory function testing (Two-point discrimnation testing) | 35% | 4% | 4% (43%) | 8% (43%) | 12% | 14% | 22% | ||||
| Throbbing, pulsing/pulsating, pounding, beating, flickering quivering. | Subjective descriptors | 18% | 16% | 4% | 6% (41%) | 10% | 6% | 24% | 4% | 10% | ||
| Jabbing. | Subjective descriptors | 12% | 20% | 2% | 8% (41%) | 6% | 6% | 14% | 6% | 24% | ||
| Muscle atrophy. | Associated signs and symptoms | 6% | 12% | 2% | 22% (41%) | 10% | 22% | 6% | 18% | |||
| Positive findings (e.g. pain provocation/muscle spasm with decreased range of movement). | Neurodynamic testing | 10% | 24% | 2% | 6% (41%) | 10% | 18% | 2% | 27% | |||
|
| ||||||||||||
| Nociceptive + Nociplastic | Arthralgic (joint) and/or myalgic (muscle) pain. | Subjective descriptors | W | 35% | 6% | 31% (71%) | 2% | 6% | 20% | |||
| Normal deep tendon reflexes. | Neurological testing | 20% | 4% | 37% (61%) | 10% | 4% | 24% | |||||
| Consistently provoked by localised pressure (e.g. palpation). | Aggravating factors | 37% | 6% | 18% | 16% (59%) | 2% | 20% | |||||
| Sensory abnormalities in localized non-dermatomal distribution. | Neurological testing | 16% | 18% | 27% | 4% | 14% (57%) | 2% | 4% | 2% | 12% | ||
| ABSENCE of negative symptoms. | Neurological testing | 31% | 4% | 20% (55%) | 8% | 22% | 14% | |||||
| Myofascial trigger points. | Mechanical testing (LOCAL mechanical/pressure pain threshold OR NOXIOUS touch/pressure) | 16% | 16% | 22% (55%) | 4% | 12% | 8% | 20% | ||||
| Below cut-off (< 19). | Neuropathic questionnaires (PainDETECT) | 39% | 2% | 16% (55%) | 2% | 10% | 31% | |||||
| Negative findings (absence of abnormal findings). | Nerve provocation testing (palpation/tapping) | 35% | 2% | 2% | 16% (53%) | 4% | 8% | 33% | ||||
| Dull, sore, hurting, aching, heavy. | Subjective descriptors | 16% | 4% | 4% | 33% (53%) | 2% | 29% | 4% | 8% | |||
| Reduced joint range of motion due to stiffness. | Associated signs and symptoms | 39% | 2% | 14% (53%) | 29% | 4% | 12% | |||||
| ABSENCE of neurological findings. | Neurological testing | A | 4% | 4% | 40% (48%) | 13% | 4% | 35% | ||||
| Negative findings (absence of abnormal findings). | Neurodynamic testing | 18% | 14% | 14% (47%) | 8% | 14% | 31% | |||||
| Provoked by all activity/movements. | Aggravating factors | 8% | 29% | 10% (47%) | 12% | 14% | 18% | 8% | ||||
| Aggravated by fatigue or overexertion. | Aggravating factors | 31% | 14% (45%) | 10% | 39% | 2% | 4% | |||||
| Predisposed by prior experiences including emotional and/or physical trauma. | Aggravating factors | 37% | 8% (45%) | 8% (45%) | 39% | 4% | 4% | |||||
| Tender, taut, rasping, splitting. | Subjective descriptors | 22% | 4% | 4% | 6% | 16% (43%) | 4% | 16% | 6% | 20% | ||
| ABSENCE of positive symptoms. | Neurological testing | 29% | 2% | 4% | 12% (43%) | 8% | 31% | 14% | ||||
| High Waddell score. | Psychological assessment | 4% | 35% | 2% (41%) | 6% (41%) | 12% | 4% | 37% | ||||
| Inconsistency between structural changes and pain. | Imaging/radiography | 2% | 29% | 4% | 10% (41%) | 12% (41%) | 29% | 8% | 6% | |||
|
| ||||||||||||
| Neuropathic + Nociplastic | NOT consistently provoked by specific movements, activity, or changes in position or posture (pain independent of these factors). | Aggravating factors | 2% | 14% | 39% | 27% (80%) | 4% | 8% | 6% | |||
| Signs and/or symptoms of autonomic dysfunction or vasomotor instability (trophic abnormalities e.g. shiny atrophic skin, cracking or excess growth of nails, bone atrophy, hair loss; sudomotor abnormalities e.g. sweating, swelling, edema; vasomotor abnormalities e.g. skin color or temperature changes). | Associated signs and symptoms | W | 37% | 14% | 2% | 29% (80%) | 4% | 2% | 12% | |||
| Decreased threshold/allodynia in areas remote to the area of primary pain. | Mechanical testing (REMOTE mechanical detection threshold OR NON-NOXIOUS touch/pressure) | W | 10% | 39% | 31% (80%) | 6% | 14% | |||||
| Pain and/or symptoms are disproportionate or in excess to the nature and extent of the pathological changes or inciting injury. | Aggravating factors | 2% | 39% | 2% | 37% (78%) | 16% | 2% | 2% | ||||
| Paroxysmal episodes or sudden pain attacks. | Other pain qualities | 31% | 8% | 2% | 35% (73%) | 8% | 2% | 14% | ||||
| Above cut-off (≥ 19). | Neuropathic questionnaires (PainDETECT) | 39% | 35% (73%) | 2% | 24% | |||||||
| Mind of its own, bizarre, indescribable, ineffable. | Subjective descriptors | 10% | 37% | 24% (71%) | 6% | 8% | 14% | |||||
| Secondary allodynia (adjacent to primary area of pain). | Other pain qualities | W | 2% | 20% | 20% | 8% | 29% (69%) | 12% | 8% | |||
| Exhibits a non-linear relationship between nociception and pain intensity (or stimulus and response). | Other pain qualities | 2% | 6% | 29% | 2% | 35% (69%) | 12% | 4% | 10% | |||
| Latent or persistent pain following stimulus. | Other pain qualities | 4% | 10% | 18% | 2% | 37% (65%) | 20% | 2% | 6% | |||
| Decreased threshold/ heat hyperalgesia in areas remote to the area of primary pain. | Heat testing (REMOTE heat pain threshold OR NOXIOUS heat application) | W | 8% | 31% | 27% (65%) | 10% | 24% | |||||
| Decreased acuity, mislocalisation of stimuli and/or sensory neglect. | Higher sensory function testing (Tactile acuity test) | 18% | 20% | 27% (65%) | 4% | 6% | 24% | |||||
| Above cut-off (≥ 12). | Neuropathic questionnaires (Leeds Assessment of Neuropathic Symptoms and Signs (LANSS)) | 37% | 29% (65%) | 2% | 33% | |||||||
| Generally NOT responsive to tissue-based treatments. | Response to physical treatments | W | 2% | 16% | 20% | 2% | 27% (63%) | 4% | 22% | 6% | ||
| Pricking, lancinating, stabbing, drilling, boring. | Subjective descriptors | 2% | 37% | 2% | 4% | 24% (63%) | 14% | 4% | 12% | |||
| Hyperaesthesia. | Associated signs and symptoms | 27% | 8% | 29% (63%) | 24% | 2% | 10% | |||||
| Positive symptoms (e.g. burning, paraesthesias, hyperalgesia, allodynia). | Neurological testing | 24% | 2% | 39% (63%) | 31% | 4% | ||||||
| Decreased threshold/hyperalgesia in areas remote to the area of primary pain. | Mechanical testing (REMOTE mechanical/pressure pain threshold OR NOXIOUS touch/pressure) | W | 2% | 2% | 35% | 4% | 27% (63%) | 12% | 18% | |||
| Above cut-off (> 12). | Neuropathic questionnaires (Modified PainDETECT) | 29% | 2% | 35% (63%) | 2% | 2% | 31% | |||||
| Positive findings (no cut-off proposed). | Neuropathic questionnaires (Neuropathic Pain Scale (NPS)) | 39% | 24% (63%) | 2% | 35% | |||||||
| Secondary hyperalgesia (adjacent to primary area of pain). | Other pain qualities | W | 2% | 12% | 20% | 4% | 29% (61%) | 22% | 2% | 8% | ||
| Decreased threshold/heat allodynia in areas remote to the area of primary pain. | Heat testing (REMOTE heat detection threshold OR NON-NOXIOUS heat application) | W | 6% | 29% | 2% | 2% | 27% (61%) | 4% | 12% | 18% | ||
| Above cut-off (≥ 12). | Neuropathic questionnaires (Self-Administered LANSS (s-LANSS)) | 35% | 27% (61%) | 2% | 2% | 35% | ||||||
| Punishing, gruelling, cruel, vicious, killing. | Subjective descriptors | 2% | 33% | 24% (59%) | 20% | 6% | 14% | |||||
| Primary allodynia. | Other pain qualities | 10% | 33% | 4% | 10% | 2% | 22% (59%) | 10% | 8% | |||
| Inefficient conditioned pain modulation (unchanged or decreased pain threshold). | Pain modulation testing (Conditioned pain modulation (e.g. pressure cuff, cold pressor test)) | 4% | 2% | 24% | 6% | 33% (59%) | 14% | 2% | 14% | |||
| Positive findings (no cut-off proposed). | Neuropathic questionnaires (Neuropathic Pain Symptom Inventory (NPSI)) | 33% | 27% (59%) | 2% | 39% | |||||||
| Increased TPD threshold (decreased tactile acuity or hypoaesthesia). | Higher sensory function testing (Two-point discrimnation testing) | 22% | 8% | 2% | 27% (57%) | 8% | 10% | 22% | ||||
| Generally responsive to anticonvulsants (e.g. gabapentin, pregabalin). | Response to drugs | W | 35% | 2% | 55% | 6% | 2% | |||||
| Decreased threshold/hyperaesthesia. | Mechanical testing (LOCAL mechanical detection threshold OR NON-NOXIOUS touch/pressure) | 2% | 18% | 6% | 2% | 2% | 31% (55%) | 27% | 4% | 8% | ||
| Stimulus-independent or spontaneous pain. | Other pain qualities | 2% | 4% | 10% | 2% | 2% | 53% | 20% | 6% | |||
| Decreased threshold/vibrational allodynia. | Vibration testing (LOCAL vibration detection threshold OR tuning fork application) | 20% | 14% | 2% | 18% (53%) | 4% | 20% | 20% | ||||
| Increased threshold/cold allodynia in areas remote to the area of primary pain. | Cold testing (REMOTE cold detection threshold OR NON-NOXIOUS cold application) | W | 2% | 10% | 33% | 2% | 10% (53%) | 8% | 35% | |||
| Altered body perception. | Higher sensory function testing (Left-right discrimination task) | 8% | 29% | 16% (53%) | 4% | 8% | 35% | |||||
| Demonstrates increased or altered activity in pain-processing brain regions in response to non-noxious stimuli. | Neuroimaging / electrophysiological testing (fMRI) | 24% | 29% (53%) | 27% | 4% | 16% | ||||||
| Abnormal somatosensory evoked potentials (SEPs). | Neuroimaging / electrophysiological testing (Electroencephalography (EEG)) | 22% | 6% | 2% | 24% (53%) | 8% | 2% | 35% | ||||
| Sensory deficits (e.g. numbness) in non-dermatomal pattern. | Associated signs and symptoms | A | 10% | 33% | 13% | 8% (52%) | 8% | 13% | 15% | |||
| Pain persists beyond expected tissue healing period or pathology recovery times. | Recovery/healing period | 33% | 51% | 14% | 2% | |||||||
| Decreased threshold/allodynia localised to the area of primary pain. | Mechanical testing (LOCAL mechanical detection threshold OR NON-NOXIOUS touch/pressure) | W | 4% | 16% | 4% | 4% | 2% | 31% (51%) | 31% | 2% | 6% | |
| Decreased threshold/heat allodynia localised to the area of primary pain. | Heat testing (LOCAL heat detection threshold OR NON-NOXIOUS heat application) | W | 2% | 14% | 10% | 2% | 2% | 27% (51%) | 14% | 8% | 20% | |
| Greater pain sensitvity to cold/cold hyperalgesia localised to the area of primary pain. | Cold testing (LOCAL cold pain threshold OR NOXIOUS cold application) | W | 2% | 18% | 6% | 2% | 2% | 27% (51%) | 12% | 6% | 24% | |
| Increased threshold/cold hyperalgesia in areas remote to the area of primary pain. | Cold testing (REMOTE cold pain threshold OR NOXIOUS cold application) | W | 2% | 8% | 27% | 4% | 2% | 16% (51%) | 10% | 31% | ||
| Altered body perception. | Higher sensory function testing (Graphesthesia tests) | 8% | 22% | 2% | 2% | 20% (51%) | 6% | 39% | ||||
| Greater sensitvity to cold/cold allodynia localised to the area of primary pain. | Cold testing (LOCAL cold detection threshold OR NON-NOXIOUS cold application) | W | 16% | 10% | 4% | 2% | 24% (51%) | 8% | 6% | 29% | ||
| Temporal summation facilitation at remote areas | Pain modulation testing (Temporal summation (e.g. repetitive mechanical/heat/cold/electrical stimuli via monofilaments, thermode, or electrodes)) | A | 4% | 31% | 4% | 15% (50%) | 15% | 2% | 29% | |||
| Generally NOT responsive to anti-inflammatory drugs (NSAIDs). | Response to drugs | W | 18% | 16% | 49% | 6% | 6% | 4% | ||||
| Generally responsive to antidepressants (e.g. duloxetine, amitriptyline). | Response to drugs | W | 4% | 14% | 49% | 29% | 4% | |||||
| Generally NOT responsive to opioid analgesics. | Response to drugs | W | 2% | 6% | 24% | 18% (49%) | 10% | 18% | 20% | |||
| Symmetrical or bilateral distribution of pain. | Pain location | 8% | 18% | 12% | 6% | 22% (49%) | 12% | 4% | 16% | |||
| Spreading, radiating, penetrating, piercing. | Subjective descriptors | 24% | 4% | 4% | 4% | 20% (49%) | 31% | 4% | 8% | |||
| Intolerable sensations. | Subjective descriptors | 14% | 14% | 20% (49%) | 33% | 8% | 10% | |||||
| Associated with high levels of functional disability. | Associated signs and symptoms | 2% | 27% | 2% | 22% (49%) | 37% | 10% | |||||
| Abnormal laser evoked potentials (LEPs). | Neuroimaging / electrophysiological testing (Electroencephalography (EEG)) | 16% | 6% | 2% | 27% (49%) | 8% | 2% | 39% | ||||
| Abnormal changes, heightened response, or expanded receptive field. | Neuroimaging / electrophysiological testing (Nociceptive withdrawal reflex) | 2% | 6% | 20% | 4% | 2% | 22% (49%) | 18% | 2% | 22% | ||
| Nagging, nauseating, agonizing, dreadful, torturing. | Subjective descriptors | 2% | 27% | 18% (47%) | 33% | 6% | 14% | |||||
| Temporal summation or wind-up/hyperpathia (abnormally painful reaction to a stimulus, especially a repetitive stimulus, as well as an increased threshold). | Other pain qualities | 10% | 20% | 47% | 20% | 2% | ||||||
| Decreased threshold/cold hypoaesthesia. | Cold testing (LOCAL cold detection threshold OR NON-NOXIOUS cold application) | 35% | 4% | 2% | 8% (47%) | 4% | 10% | 37% | ||||
| Decreased threshold/vibrational hyperaesthesia. | Vibration testing (LOCAL vibration detection threshold OR tuning fork application) | 20% | 14% | 2% | 12% (47%) | 8% | 18% | 24% | ||||
| Increased threshold/vibrational hypoaesthesia. | Vibration testing (LOCAL vibration detection threshold OR tuning fork application) | 39% | 2% | 8% (47%) | 6% | 14% | 31% | |||||
| Decreased threshold/vibrational allodynia in areas remote to the area of primary pain. | Vibration testing (REMOTE vibration detection threshold OR tuning fork application) | W | 6% | 27% | 12% (45%) | 22% | 33% | |||||
| After-sensations or sensory after-effects. | Other pain qualities | 18% | 4% | 2% | 45% | 14% | 2% | 14% | ||||
| Generally responsive to NMDA antagonists (e.g. ketamine, memantine). | Response to drugs | W | 4% | 14% | 8% | 27% (45%) | 12% | 8% | 27% | |||
| Fearful, frightful, terrifying. | Subjective descriptors | 4% | 27% | 2% | 14% (45%) | 31% | 6% | 16% | ||||
| Increased threshold/heat hypoaesthesia. | Heat testing (LOCAL heat detection threshold OR NON-NOXIOUS heat application) | 37% | 4% | 8% (45%) | 2% | 18% | 31% | |||||
| Sickening, suffocating. | Subjective descriptors | 2% | 4% | 24% | 2% | 16% (45%) | 22% | 8% | 20% | |||
| Disproportionate, unpredictable pattern of pain provocation in response to multiple aggravating factors. | Aggravating factors | 2% | 43% | 43% | 6% | 6% | ||||||
| Increased deep tendon reflexes. | Neurological testing | A | 31% | 6% | 2% | 4% (42%) | 2% | 19% | 35% | |||
| Enhanced temporal summation (wind-up). | Pain modulation testing (Temporal summation (e.g. repetitive mechanical/heat/cold/electrical stimuli via monofilaments, thermode, or electrodes)) | 4% | 18% | 2% | 2% | 41% | 20% | 4% | 8% | |||
| Constant, continuous, or persisting pain even at rest. | Other pain qualities | 2% | 6% | 6% | 4% | 4% | 41% | 29% | 2% | 6% | ||
| Presence of pain or worse pain during the night or disrupted sleep. | Aggravating factors | 4% | 8% | 8% | 4% | 8% | 24% (41%) | 27% | 2% | 14% | ||
| Tight, numb, drawing, squeezing, tearing. | Subjective descriptors | 4% | 18% | 8% | 6% | 4% | 14% (41%) | 22% | 4% | 18% | ||
| Wretched, blinding. | Subjective descriptors | 4% | 24% | 12% (41%) | 14% | 12% | 33% | |||||
| Decreased threshold/vibrational hyperaesthesia in areas remote to the area of primary pain. | Vibration testing (REMOTE vibration detection threshold OR tuning fork application) | W | 6% | 22% | 12% (41%) | 24% | 35% | |||||
| Demonstrates increases in cortical event-related potential amplitudes (e.g. increases in cortical pin prick-related potential amplitudes). | Neuroimaging / electrophysiological testing (Electroencephalography (EEG)) | 2% | 12% | 2% | 27% (41%) | 16% | 6% | 35% | ||||
Note:
Features are ranked by % of contributors in descending order.
When a featue failed to reach the cut-off for ‘Unique’ (present in 1 PMC) or ‘Shared by 2’, the combination of ‘Unique’ and ‘Shared by 2 PMC’ were summed and are shown in brackets.
If two pairs of ‘Shared by 2’ or the combination of ‘Unique’ and ‘Shared by 2’achieved the cut-off >40%, only the greater percentage is reported in brackets. If the values are equal, then both are reported in brackets.
A = Additional features proposed by contributors.
W = Wording changes proposed by contributors.
The remaining features (n=92) did not meet any of the consensus criteria for inclusion. (Supplemental Digital Content 1).
Three steering committee meetings were held to discuss the panelists’ feedback and suggestions. This generated a list of 18 wording changes to original features (changes to 56 features) and 15 additional features (for complete list see Supplemental Digital Content 1) to be included in Round 2.
3.3. Delphi Round 2 – Features/assessment findings to retain, wording changes, and additional features/assessment findings
From a total of 92 features/assessment findings that were not recommended for retention (failed to reach threshold agreement), only one feature was nominated to be retained by at least 15% of panelists. This feature was considered to be present in one pain mechanism category (neuropathic) after a change to wording based on panelist’s feedback/comments, and after gained consensus amongst the steering committee (Table 3). All wording changes were supported by >50% of panelists with some minor modifications suggested by contributors which were approved by consensus amongst the steering committee. From the 15 additional features that were proposed in Round 1, 10 were retained as features with potential to discriminate between pain mechanism categories – 5 unique to one category and 5 shared between two categories (Tables 3 – 4), and 5 features did not meet any of the cut-off criteria (Supplemental Digital Content 1).
With the refinements and additions from Round 1, a final list was generated that included a total of 196 features to be retained. Of these features, 76 were unique to one pain mechanism category (17, 37, and 22 for nociceptive, neuropathic, and nociplastic pain, respectively), 120 were shared between two pain mechanism categories (27, 20, and 78 for nociceptive + neuropathic, nociceptive + nociplastic, and neuropathic + nociplastic pain, respectively, in five cases agreement was similar to for two pairs of categories). When the retained features were categorized based on methods, clinical examination contained 134 features (74% of 180, unique - 61), quantitative sensory testing contained 34 features (83% of 41, unique - 2), imaging and diagnostic testing contained 14 features (34% of 41, unique - 7), and pain-type questionnaires contained 14 features (47% of 30, unique - 6).
3.3.1. Conflicting views on wording changes
Although the majority of panelists (>50%) in Round 2 agreed to the wording changes that had been suggested in Round 1, conflicting views were expressed for some. Of note, there were differing opinions regarding the terms ‘primary/local’ and ‘secondary/remote’ hyperalgesia/allodynia in the quantitative sensory testing category. Some suggested that only the terms “local” and “remote” should be used, whereas others recommended that “secondary” should be described separately from “remote” as it specifically relates to areas “adjacent to the primary area of pain”. The steering committee resolved to use the terms local and remote with a note that remote excludes areas adjacent to the primary area of pain.
With respect to the feature ‘proportional and direct relationship with aggravating factors’, some suggest the use of the term ‘direct’ is redundant as only a direct relationship can be ‘proportional’. Conflicting comments arose for the feature ‘generalized pain hypersensitivity’ which was reworded to ‘generalized hypersensitivity’. Some panelists suggested that it is not possible to be sensitive to pain as it is a “response”, and not a “stimulus”. Other panelists suggested that hypersensitivity is restricted to nociceptive modalities and NOT to other modalities or precepts. Relating to pain location, the feature of ‘a non-dermatomal or non-neuroanatomic distribution’ of pain was challenged as the term ‘non-neuroanatomic’ was considered by some to be unclear, whereas others suggested using not ‘neuroanatomically plausible’ to be consistent with the NeuPSIG guideline’s definition and criteria of neuropathic pain [9,11,31]. The final wording was finalised by the steering committee based on input from the panelists.
Finally, there was disagreement regarding the description of psychological features. Some suggested such terms are unclear because it is difficult to define whether features differ from a normal psychological response to “the pain experience”. There were some conflicting comments whether the psychological features have to be “related to the pain experience” or just “have bearing on the pain experience” (e.g., post-traumatic stress disorder may not be related to the pain experience but might impact the pain experience). Both alternatives were included in the final wording.
4. Discussion
This Delphi study involved international experts from diverse disciplines and two people with lived pain experience, to reach consensus on a list of features and assessment findings that could aid in discrimination between mechanisms that underlie pain experienced in the musculoskeletal system. From an original 277 features identified from systematic reviews and 15 suggested by panelists, 196 reached the a priori defined threshold for agreement. Pain maintained by neuropathic mechanisms comprised the greatest number of unique features (n=37), followed by nociplastic (n=22), and nociceptive (n=17) mechanisms. The greatest number of features shared between two (but not three) categories were identified between neuropathic and nociplastic mechanisms (n=78), followed by nociceptive and neuropathic (n=27), whereas nociceptive and nociplastic shared the fewest (n=17). Overall, the findings highlight that overlap of features presents an inherent challenge for discrimination between pain mechanism categories. Although neuropathic pain may be identified by a higher number of unique features, it shares a greater number of features with nociplastic pain. This reinforces that discrimination between pain mechanisms will depend on consideration of a combination of features.
Features that achieved top consensus for discrimination between pain mechanism categories
This study identified, for each pain mechanism category, the features that are most agreed upon by panelists. Of note, this does not indicate that these features are prevalent amongst individuals with a specific pain mechanism. Instead, it indicates if certain features were present, most panelists would identify a ‘most likely’ pain mechanism category.
Unique features achieving greatest consensus for nociceptive pain were: ‘responsiveness to NSAIDs’–71%, ‘signs of inflammation’–67%, and ‘predictable pain recovery based on expected time of tissue recovery’–65%. These features are unsurprising as they likely present in an acute injury, with clear relevance of nociceptive mechanisms [1]. Similar features have been endorsed in other consensus studies [28]. Although the rationale that these features indicate a nociceptive mechanism is clear, they might be specific to the acute phase, and not beyond with ongoing nociceptive input. Absence of these features is unlikely to exclude nociceptive pain.
For neuropathic pain, unique features achieving greatest consensus related to nerve damage (e.g., neurologically plausible distribution of pain, characteristic signs/symptoms like ‘numbness’, diagnostic tests confirming nerve damage). These findings are unsurprising and consistent with the NeuPSIG criteria [9,11] and definition [31] of neuropathic pain. When present, these findings can support the identification of neuropathic pain; however, if these cardinal signs are less definitive or absent, this would not exclude neuropathic pain, as it presents variably [31].
For nociplastic pain, the most agreed unique features were: ‘diffuse, widespread, or poorly localised pain’–82%, ‘generalised hypersensitivity’–71%, and ‘multiple somatic symptoms (e.g., fatigue, memory/concentration/sleep disturbances)’–65%. These features align with presentations that could be explained by sensitisation of central pain mechanisms and thus consistent with altered nociception/abnormal processing (e.g., hypersensitivity and widespread pain [20]), which is included in the IASP definition [19] and the recently developed criteria/grading system for nociplastic pain [18].
Unique or shared features of pain mechanism categories
Many features were identified as shared by two pain mechanism categories. Whether a feature is unique to one category or shared by two was the most common divergence of opinion amongst panelists. Of note, although a feature shared between two categories could not provide definitive identification of a likely category, it could aid differentiation of those two categories from the third.
The potential for multiple mechanisms to co-exist within an individual is likely to explain some divergence in opinion, particularly regarding features that are unique to, or shared by, neuropathic and nociplastic pain. It was common for features, typically considered to reflect sensitisation of central mechanisms (e.g., remote/widespread mechanical hyperalgesia, paroxysmal pain attacks [28]), to be identified as shared by nociplastic and neuropathic pain. Whether these features primarily manifest from neuropathic pathology or reflect the addition of nociplastic mechanisms on the foundation of a neuropathic condition, is likely to explain this divergence in opinion. It is plausible, if not universal, that conditions that begin as a consequence of neuropathic or nociceptive mechanisms, with some potential sensitisation of central mechanisms, progress to a greater contribution from nociplastic mechanisms. The prevalence of mixed mechanisms [12,30] is likely to explain why many experts consider some features to indicate both neuropathic and nociceptive pain. Any tool to discriminate between pain mechanism categories should aim to identify which category(-ies) contribute most to an individual’s current presentation, rather than expecting to identify only one.
Comparison with previous methods to discriminate between pain mechanism categories
Most features identified as unique to a pain mechanism category in this study agree with previous consensus studies [23,28]. For reasons outlined in the preceding section, some discrepancies primarily relate to features considered to be shared by two categories rather than unique to one. Some features were supported by some panelists, but not sufficient to reach the agreement threshold.
Some specific divergences require additional consideration. One issue relates to the criterion defined by Nijs et al. [23], in their mechanism-based classification of lower back pain that discriminates nociplastic from nociceptive mechanisms based upon pain that is ‘disproportionate to the nature and extent of injury or pathology’. This is in general agreement with views expressed by panelists in the present study, who agreed (53%) that ‘no findings from imaging of body regions of potential relevance to the pain experience’ was a feature of nociplastic pain. However, Nijs et al. [23] argued that “it is necessary to assess the patient’s amount of injury, pathology, and objective dysfunctions capable of generating nociceptive input” using imaging and clinical examination. Whether identification of a nociceptive source is necessary for confirmation of a nociceptive pain mechanism category is not yet clear.
Subtle distinction between presence and absence of a feature is relevant for comparison with the classification proposed by Smart et al. [28], which emphasises a strong association between maladaptive psychological factors in the presentation of sensitised central pain mechanisms (i.e., nociplastic). Agreement amongst panelists was not sufficient for the presence of psychological factors to be considered a unique feature of nociplastic pain. Rather, the absence of significant psychological features (i.e., ‘minimal or absent psychological features…’) was agreed to suggest the presence of nociceptive or neuropathic mechanisms. Further, in Smart et al. [28] consensus project, ‘night pain/disrupted sleep’ was considered characteristic of nociplastic pain and ‘pain of moderate to high severity’ was considered to discriminate neuropathic pain. However, these features were considered by panelists in this study to be shared by all three pain mechanism categories.
Consideration of findings for the development of a tool to differentiate pain mechanism categories
A goal of this work is to set a foundation for developing a multi-dimensional tool to aid in the discrimination between pain mechanism categories in clinical practice and research. Several issues will be critical to consider. First, no single feature will be sufficient to discriminate between pain mechanism categories because many features are shared between two categories, and those that could provide more definitive identification of mechanisms, are not present in all individuals with a specific category. Second, features may depend on the time-course of the condition as the relative contribution of mechanisms are likely to change [1]. Third, the wording used in this study to describe some features will potentially require additional explanation to aid interpretation and utility. Fourth, challenges arise for features for which there are not well-defined methods or biomarkers (e.g., genetics, biologsical markers, brain imaging). Fifth, feasibility, accessibility, reliability, and validity of the methods to discriminate between pain mechanisms will be important to consider. Encouragingly, the domains with the greatest number of features were ‘clinical examination’ (n=134, 74%) and ‘quantitative sensory testing’ (n=34, 83%). The next step towards development of a tool(s) to differentiate between pain mechanism categories in individuals with musculoskeletal pain will be to seek expert opinion on the minimum set of features that are likely to lead to accurate interpretation.
Study strengths and limitations
The strengths of this study are the comprehensive process used to select candidate features/assessment findings, diversity of the expert group, a priori definitions for consensus, and clarification of outcomes with a second Delphi round. Limitations include the majority of panelists being physiotherapists (29%) and from English speaking countries (67%), although WHO regions were covered well (North America, Europe, Asia Pacific). Also, panelists may have interpreted the process of allocating the pain mechanism categories for each feature differently (i.e., feature characteristic of or simply present in a category) which may have influenced allocation and outcomes. Further, the threshold for retention of features was lenient and arbitrary which led to retention of a large number of features.
Conclusion
This study aimed to identify consensus on features and assessment findings that could aid in discrimination between pain mechanism categories. The outcome is an agreed list of potential candidate measures that mostly involve clinical examination or quantitative sensory testing. This list of features that experts agree are unique to one category or shared between two categories provides a strong foundation to develop tools to aid evaluation of individuals experiencing pain in the musculoskeletal system.
Supplementary Material
Acknowledgements
We would like to thank all the expert panelists for their valuable time and expertise they provided to this project: Lars Arendt-Nielsen, Charles Argoff, Karl S. Bagraith, Kirsty Bannister, Ralf Baron, Joletta Belton, Helena Brisby, Mary S. Cardosa, Daniel B. Carr, Ruth L. Chimenti, Daniel J. Clauw, Milton Cohen, Carol A. Courtney, Michele Curatolo, Beth D. Darnall, Antoon De Laat, Anthony Dickenson, Roger B. Fillingim, Nanna Brix Finnerup, Maria Fitzgerald, Jon J. Ford, Simon French, Debra B Gordon, Thomas Graven-Nielsen, Sandra Hilton, Troels S Jensen, Melissa C. Kolski, Eva Kosek, Richard E. Liebano, Shannon L. Merkle, Michael Nicholas, Jo Nijs, Tonya M Palermo, Romy Parker, Felipe J. J. Reis, Andrew S.C. Rice, Keith Smart, Rob J. E. M. Smeets, Steven P. Stanos, Peter Svensson, Bronwyn L. Thompson, Rolf-Detlef Treede, Takahiro Ushida, Owen D. Williamson, Mary Wing.
Funding
1. This study was supported by a Program Grant (APP1091302) from the National Health and Medical Research Council (NHMRC) of Australia.
2. MAS was supported by a post-graduate scholarship from the University of Queensland.
3. PH was supported by a Fellowship (APP1102905) from the NHMRC.
4. MS was supported by an unrestricted grant from the Motor Accident Insurance Commission of Queensland.
5. KS was supported by National Institutes of Health Grants R01 AR073187, U24 NS112873, and UH3 AR07638.
6. LAN and TGN are part of Center for Neuroplasticity and Pain (CNAP) which is supported by the Danish National Research Foundation (DNRF121).
7. RLC was supported by the National Institute of Arthritis Musculoskeletal and Skin Disease of the National Institutes of Health (NIH) under award number R00AR071517.
8. BDD was supported by National Institute on Drug Abuse (NIDA) K24 DA053564–01.
9. RDT received support from Deutsche Forschungsgemeinschaft for collaborative research center (SFB 1158).
10. SLM: The opinions or assertions contained herein are the private views of the author(s) and are not to be construed as official or as reflecting the views of the United States Army or the Department of Defense.
Footnotes
Conflicts of interest
There are no conflicts of interest.
Supplemental Digital Content 1. Complete list of features and methods for discrimination between pain mechanisms, retained features, rejected features, additional features, and wording changes. xlsx
References
- [1].Arendt-Nielsen L, Fernández-de-Las-Peñas C, Graven-Nielsen T. Basic aspects of musculoskeletal pain: from acute to chronic pain. The Journal of manual & manipulative therapy 2011;19(4):186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chan AY, Ford JJ, McMeeken JM, Wilde VE. Preliminary evidence for the features of non-reducible discogenic low back pain: survey of an international physiotherapy expert panel with the Delphi technique. Physiotherapy 2013;99(3):212–220. [DOI] [PubMed] [Google Scholar]
- [3].Chimenti RL, Frey-Law LA, Sluka KA. A Mechanism-Based Approach to Physical Therapist Management of Pain. Phys Ther 2018;98(5):302–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Clauw DJ. Diagnosing and treating chronic musculoskeletal pain based on the underlying mechanism(s). Best practice & research Clinical rheumatology 2015;29(1):6–19. [DOI] [PubMed] [Google Scholar]
- [5].Costigan M, Scholz J, Woolf CJ. Neuropathic pain: A maladaptive response of the nervous system to damage. Annu Rev Neurosci 2009;32:1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].de Villiers MR, de Villiers PJ, Kent AP. The Delphi technique in health sciences education research. Med Teach 2005;27(7):639–643. [DOI] [PubMed] [Google Scholar]
- [7].Dewitte V, De Pauw R, De Meulemeester K, Peersman W, Danneels L, Bouche K, Roets A, Cagnie B. Clinical classification criteria for nonspecific low back pain: A Delphi-survey of clinical experts. Musculoskeletal science & practice 2018;34:66–76. [DOI] [PubMed] [Google Scholar]
- [8].Diamond IR, Grant RC, Feldman BM, Pencharz PB, Ling SC, Moore AM, Wales PW. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol 2014;67(4):401–409. [DOI] [PubMed] [Google Scholar]
- [9].Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DL, Bouhassira D, Cruccu G, Freeman R, Hansson P, Nurmikko T, Raja SN, Rice AS, Serra J, Smith BH, Treede RD, Jensen TS. Neuropathic pain: an updated grading system for research and clinical practice. Pain 2016;157(8):1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gifford LS, Butler DS. The integration of pain sciences into clinical practice. Journal of hand therapy : official journal of the American Society of Hand Therapists 1997;10(2):86–95. [DOI] [PubMed] [Google Scholar]
- [11].Haanpaa M, Attal N, Backonja M, Baron R, Bennett M, Bouhassira D, Cruccu G, Hansson P, Haythornthwaite JA, Iannetti GD, Jensen TS, Kauppila T, Nurmikko TJ, Rice AS, Rowbotham M, Serra J, Sommer C, Smith BH, Treede RD. NeuPSIG guidelines on neuropathic pain assessment. Pain 2011;152(1):14–27. [DOI] [PubMed] [Google Scholar]
- [12].Ibor PJ, Sanchez-Magro I, Villoria J, Leal A, Esquivias A. Mixed Pain Can Be Discerned in the Primary Care and Orthopedics Settings in Spain: A Large Cross-Sectional Study. Clin J Pain 2017;33(12):1100–1108. [DOI] [PubMed] [Google Scholar]
- [13].International Association for the Study of Pain. Task Force on Taxonomy. IASP Terminology Updated from “Part III: Pain Terms, A Current List with Definitions and Notes on Usage” (pp 209–214), Classification of Chronic Pain, 2nd Edition. Seattle: IASP, 2017. p. IASP. [Google Scholar]
- [14].Jones J, Hunter D. Qualitative Research: Consensus methods for medical and health services research. Bmj 1995;311(7001):376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jünger S, Payne SA, Brine J, Radbruch L, Brearley SG. Guidance on Conducting and REporting DElphi Studies (CREDES) in palliative care: Recommendations based on a methodological systematic review. Palliat Med 2017;31(8):684–706. [DOI] [PubMed] [Google Scholar]
- [16].Keeney S, Hasson F, McKenna H. Debates, Criticisms and Limitations of the Delphi. The Delphi Technique in Nursing and Health Research Chichester, West Sussex: Wiley-Blackwell, 2010. pp. 18–31. [Google Scholar]
- [17].Keeney S, Hasson F, McKenna H. The Delphi Technique. The Delphi Technique in Nursing and Health Research Chichester, West Sussex: Wiley-Blackwell, 2010. pp. 1–17. [Google Scholar]
- [18].Kosek E, Clauw D, Nijs J, Baron R, Gilron I, Harris RE, Mico JA, Rice AS, Sterling M. Chronic nociplastic pain affecting the musculoskeletal system: clinical criteria and grading system. Pain 2021;162(11):2629–2634. [DOI] [PubMed] [Google Scholar]
- [19].Kosek E, Cohen M, Baron R, Gebhart GF, Mico JA, Rice AS, Rief W, Sluka AK. Do we need a third mechanistic descriptor for chronic pain states? Pain 2016;157(7):1382–1386. [DOI] [PubMed] [Google Scholar]
- [20].Lluch E, Nijs J, Courtney CA, Rebbeck T, Wylde V, Baert I, Wideman TH, Howells N, Skou ST. Clinical descriptors for the recognition of central sensitization pain in patients with knee osteoarthritis. Disability and rehabilitation 2017;40(23):2836–2845. [DOI] [PubMed] [Google Scholar]
- [21].McMillan SS, King M, Tully MP. How to use the nominal group and Delphi techniques. Int J Clin Pharm 2016;38(3):655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Melzack R The McGill Pain Questionnaire: major properties and scoring methods. Pain 1975;1(3):277–299. [DOI] [PubMed] [Google Scholar]
- [23].Nijs J, Apeldoorn A, Hallegraeff H, Clark J, Smeets R, Malfliet A, Girbes EL, De Kooning M, Ickmans K. Low back pain: guidelines for the clinical classification of predominant neuropathic, nociceptive, or central sensitization pain. Pain physician 2015;18(3):E333–346. [PubMed] [Google Scholar]
- [24].Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, Keefe FJ, Mogil JS, Ringkamp M, Sluka KA, Song X-J, Stevens B, Sullivan MD, Tutelman PR, Ushida T, Vader K. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain 2020;161(9):1976–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shraim MA, Massé-Alarie H, Hall LM, Hodges PW. Systematic Review and Synthesis of Mechanism-based Classification Systems for Pain Experienced in the Musculoskeletal System. Clin J Pain 2020;36(10):793–812. [DOI] [PubMed] [Google Scholar]
- [26].Shraim MA, Massé-Alarie H, Hodges PW. Methods to discriminate between mechanism-based categories of pain experienced in the musculoskeletal system: a systematic review. Pain 2021;162(4):1007–1037. [DOI] [PubMed] [Google Scholar]
- [27].Sinha IP, Smyth RL, Williamson PR. Using the Delphi technique to determine which outcomes to measure in clinical trials: recommendations for the future based on a systematic review of existing studies. PLoS Med 2011;8(1):e1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Smart KM, Blake C, Staines A, Doody C. Clinical indicators of ‘nociceptive’, ‘peripheral neuropathic’ and ‘central’ mechanisms of musculoskeletal pain. A Delphi survey of expert clinicians. Manual therapy 2010;15(1):80–87. [DOI] [PubMed] [Google Scholar]
- [29].Smart KM, O’Connell NE, Doody C. Towards a mechanisms-based classification of pain in musculoskeletal physiotherapy? Physical Therapy Reviews 2008;13(1):1–10. [Google Scholar]
- [30].Stanos S, Brodsky M, Argoff C, Clauw DJ, D’Arcy Y, Donevan S, Gebke KB, Jensen MP, Lewis Clark E, McCarberg B, Park PW, Turk DC, Watt S. Rethinking chronic pain in a primary care setting. Postgraduate medicine 2016;128(5):502–515. [DOI] [PubMed] [Google Scholar]
- [31].Treede R, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, Hansson P, Hughes R, Nurmikko T, Serra J. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 2008;70(18):1630–1635. [DOI] [PubMed] [Google Scholar]
- [32].Vardeh D, Mannion RJ, Woolf CJ. Toward a Mechanism-Based Approach to Pain Diagnosis. The journal of pain : official journal of the American Pain Society 2016;17(9 Suppl):T50–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].von der Gracht HA. Consensus measurement in Delphi studies: Review and implications for future quality assurance. Technological Forecasting and Social Change 2012;79(8):1525–1536. [Google Scholar]
- [34].Vos T, GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390(10100):1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Waggoner J, Carline JD, Durning SJ. Is There a Consensus on Consensus Methodology? Descriptions and Recommendations for Future Consensus Research. Acad Med 2016;91(5):663–668. [DOI] [PubMed] [Google Scholar]
- [36].Woolf CJ. Pain: Moving from Symptom Control toward Mechanism-Specific Pharmacologic Management. Annals of Internal Medicine 2004;140(6):441–451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


