Abstract
Background and Aims
Screen media activity (SMA) may impact neurodevelopment in youth. Cross-sectionally, SMA has been linked to brain structural patterns including cortical thinning in children. However, it remains unclear whether specific brain structural co-variation patterns are related to SMA and other clinically relevant measures such as psychopathology, cognition and sleep in children.
Methods
Adolescent Brain Cognitive Development (ABCD) participants with useable baseline structural imaging (N = 10,691; 5,107 girls) were analyzed. We first used the Joint and Individual Variation Explained (JIVE) approach to identify cortical and subcortical covariation pattern(s) among a set of 221 brain features (i.e., surface area, thickness, or cortical and subcortical gray matter (GM) volumes). Then, the identified structural covariation pattern was used as a predictor in linear mixed-effect models to investigate its associations with SMA, psychopathology, and cognitive and sleep measures.
Results
A thalamus-prefrontal cortex (PFC)-brainstem structural co-variation pattern (circuit) was identified. The pattern suggests brainstem and bilateral thalamus proper GM volumes covary more strongly with GM volume and/or surface area in bilateral superior frontal gyral, rostral middle frontal, inferior parietal, and inferior temporal regions. This covariation pattern highly resembled one previously linked to alcohol use initiation prior to adulthood and was consistent in girls and boys. Subsequent regression analyses showed that this co-variation pattern associated with SMA (β = 0.107, P = 0.002) and externalizing psychopathology (β = 0.117, P = 0.002), respectively.
Discussion and Conclusions
Findings linking SMA-related structural covariation to externalizing psychopathology in youth resonate with prior studies of alcohol-use initiation and suggest a potential neurodevelopmental mechanism underlying addiction vulnerability.
Keywords: externalizing behavior, child, screen media activity, addictive behaviors, cortical thinning
Introduction
Screen media activity (SMA) is a considerable concern for children and adolescents (Potenza, Faust, & Faust, 2020). By 12 years of age, 69% of children have their own smartphone and approximately 85% of children and adolescents engage in recreational screen use (Rideout & Robb, 2019), with estimates of exposure to electronic media totaling 11 h/day among 11–18 year-olds (Bagot et al., 2018; Wade et al., 2021). Understanding whether SMA has negative impacts on brain and behavioral outcomes in youth is particularly important given that brain structure and social and emotional development undergo remarkable changes from childhood to adulthood. There are considerable debates on the effects of SMA. Behaviorally, mixed findings have been reported in terms of how SMA might impact mental and cognitive development. Some studies have found no or weak associations between SMA and negative health outcomes (Ferguson, 2017; Paulich, Ross, Lessem, & Hewitt, 2021), while in other studies SMA has been linked to sleep problems (Spies Shapiro & Margolin, 2014; Canan, Karaca, Toprak, Kuloğlu, & Potenza, 2019; Wong et al., 2020), poor mental health (George, Russell, Piontak, & Odgers, 2018) and cognitive problems in youth (Kirlic et al., 2021). Additionally, there is emerging evidence that high-quantity SMA is associated with substance and behavioral addictions (Christodoulou, Majmundar, Chou, & Pentz, 2020). For example, engaging in greater than 3 h of screen use behaviors (TV watching, video gaming) as compared to 0–2 h day has been associated with higher likelihoods of alcohol and solvent use in children (Armstrong, Bush, & Jones, 2010). Further, spending more than 4 h per day playing online games has been associated with the occurrence or persistence of high risk for internet gaming disorder over a two-year period in children and adolescents (Jeong et al., 2020).
From a brain-based perspective, there are multiple cross-sectional and longitudinal studies examining associations between gaming and social media use and brain structure, with fewer relationships between overall SMA and brain structure reported. The literature demonstrates associations between the amount of internet gaming and increased mean diffusivity (suggestive of poorer white matter integrity) in multiple cortical and subcortical brain regions (Takeuchi et al., 2016). Other data linking use of social media to gray matter (GM) density in the amygdala (Kanai, Bahrami, Roylance, & Rees, 2012; Von Der Heide, Vyas, & Olson, 2014) suggest that popular non-gaming forms of SMA, such as social media use, a possible concern particularly for girls and women (Su, Han, Yu, Wu, & Potenza, 2020), may impact developing brains (Crone & Konijn, 2018). Recently, SMA-related brain structures covariation in children suggested advanced cortical thinning in the visual system (Paulus et al., 2019). More importantly, some SMA-related structural patterns were associated with more severe externalizing problems and lower crystalizing and fluid intelligence scores (Paulus et al., 2019).
Taken together, these prior studies have led to important progress towards understanding relationships between and potential impacts of SMA on brain and behavioral outcomes in youth, although with some mixed findings. To further elucidate relationships between SMA and brain development, as well as SMA's links with mental health and cognitive performance, it is necessary to use data from large-scale cohort studies. The primary goals of this investigation were to understand whether certain brain regions/structures covary together more often than other regions in children and whether such brain structural covariation patterns are related to SMA and SMA-associated behavioral measures including sleep disturbances, psychopathology, and cognitive performance. Based on the structural covariance/maturational coupling hypothesis (Raznahan et al., 2011; Alexander-Bloch, Giedd, & Bullmore, 2013), changes in cortical and subcortical regions are well coordinated during brain development. Indeed, our prior study revealed that brain structural covariation is related to age in children and adolescents (Zhao, Klein, Castellanos, & Milham, 2019). More recently, co-development of brain regions in a thalamus-prefrontal cortex (PFC)-brainstem circuit was linked to age at first full drink prior to 21 years in young adults (Zhao, Constable, Hien, Chung, & Potenza, 2021), consistent with findings indicating an important role of PFC-brainstem circuitry in compulsive alcohol consumption in rodents (Siciliano et al., 2019). Specifically, the ability of activity within the PFC-brainstem circuitry to bidirectionally modulate alcohol intake in mice could be both constitute an important biomarker relating to the initiation of alcohol consumption as well as a driving factor underlying compulsive drinking (Siciliano et al., 2019). Models have been proposed that suggest that compulsive engagement in SMA and substance use may share neural underpinnings (Brand et al., 2019), and early initiation of and heavy engagement in potentially addictive behaviors have been linked to greater severity of addictive problems later in life (Jordan & Andersen, 2017). As such, understanding brain-behavior relationships related to SMA is important. Given the above-listed findings and results from a subsample of the ABCD study (Paulus et al., 2019), we hypothesized that a structural covariation pattern suggestive of co-development of brain regions in a thalamus-PFC-brainstem circuit would associate with total screen time in children. Given links between SMA and sleep disturbances, psychopathology and cognitive impairments, we also explored whether this structural covariation pattern was related to sleep disturbances, psychopathology, and cognitive performance. Given gender-related differences in SMA, including with respect to problematic or addictive levels of engagement (Su, Han, Jin, Yan, & Potenza, 2019; Su et al., 2020), we considered gender in our analyses.
Materials and methods
Study sample
Cross-sectional analyses of baseline data from the ABCD (Release 3.0) study (Volkow et al., 2018) were performed. The ABCD Study is an ongoing longitudinal cohort study aiming to understand brain and behavior development through adolescence into young adulthood (Karcher & Barch, 2021). The 11,245 participants aged between 9 and 10 years were recruited from 21 sites across the US. The basic demographic information including sex/gender, race/ethnicity, and income levels of the participants are representative of the United States population. In this study, subjects not passing FreeSurfer QC (quality control) criteria were excluded from analyses. Subjects were also excluded if they had missing basic demographic variables (see the statistical analysis section), total screen time, sleep disturbance, psychopathology and cognitive measures. Thus, our final dataset included 9,738 participants with complete baseline data.
Imaging preprocessing
MRI data were collected on one of three 3T scanners with the following key imaging parameters: TR = 2,500 ms (Siemens Prisma and General Electric 750) or 6.31 ms (Philips), TI = 1,060 ms, flip angle = 12°, voxel size = 1mm3, acquisition matrices=256 × 256 (Casey et al., 2018). Raw imaging data were preprocessed by the ABCD Data Acquisition and Integration Core using FreeSurfer v5.3.0 with standardized pipelines (Hagler et al., 2019), and the QC of the processed images was done by the ABCD investigative team. Subjects were excluded from analyses if they had severe problem(s) in one or more of the following QC criteria: motion, intensity inhomogeneity, white-matter underestimation, pial overestimation, and/or magnetic susceptibility artifacts (Hagler et al., 2019). Images were parcellated into 34 regions per hemisphere according to the Desikan-Killiany atlas (Desikan et al., 2006). In this study and as previously (Zhao et al., 2021), we were interested in covariation patterns among four morphological features in cortical and subcortical regions. Specifically, these included surface area, thickness, and GM volume of 68 cortical regions and GM volume of 17 subcortical regions (including bilateral hippocampus, amygdala, caudate, putamen, pallidum, nucleus accumbens, ventral diencephalon, thalamus proper, and brainstem).
Measures
SMA The primary SMA measure was the total number of hours spent on all SMA activities based on youth self-report. Participants reported their engagement in six types of SMA activities including watching TV shows/movies, watching videos, playing video games, texting on an electronic device, visiting social networking sites, and video-chatting. For each SMA activity, participants were given one of the following choices: none, < 30 min, 30 min, 1 h, 2 h, 3 h, and 4+ hours.
Sleep disturbances Based on its characteristics (Barch et al., 2018), the summary score of total sleep problems from the parent-report Sleep Disturbance Scale for Children (SDSC) was used to measure the degree of sleep disturbances in the ABCD study (Bruni et al., 1996). The scale has shown a reasonable test-retest reliability of 0.71 in children and adolescents (Bruni et al., 1996) and has demonstrated links with SMA in the ABCD cohort (Hisler, Twenge, & Krizan, 2020).
Mental and cognitive measures Internalizing and externalizing symptoms from the parent-reported Child Behavior Checklist (CBCL) were used to assess participant's psychopathologies (Achenbach, 2009). Both scores were normalized by gender, age, and race/ethnicity. Age-corrected fluid and crystalized composite scores in the NIH Toolbox Cognitive Battery (NIHTB-CB) were used to assess participant's overall cognitive abilities. Briefly, higher fluid intelligence is linked to better capacities to learn new things in novel situations, while higher crystalized intelligence reflects better capacities for applying knowledge acquired through previously learned experiences (Akshoomoff et al., 2013).
Statistical analyses
Cortical and subcortical structural covariation patterns
JIVE, or Joint and Individual Variance Explained (Lock, Hoadley, Marron, & Nobel, 2013; Feng, Hannig, & Marron, 2015), was used to characterize structural covariation patterns among different morphometric measures (i.e., cortical GM volume, thickness, surface area, and subcortical GM volume). JIVE is a dimension reduction and pattern discovery method for detecting common and distinct covariation patterns among multiple data sources. Briefly, given data matrices for morphological measures, the goal of JIVE is to find joint components denoting shared covariation patterns shared across all morphological measures and as individual components for covariation patterns specific to individual morphological measures. The numbers of joint and individual components are determined via permutation test (Lock et al., 2013). In this study, we were interested in identifying shared subcortical and cortical covariation patterns across multiple morphological measures. Our prior studies (Zhao et al., 2019, 2021) have shown that JIVE has the potential to provide insights at a brain network level into coordinated brain structural relationships.
Generalized Linear Mixed Models (GLMMs) were subsequently used to establish relationships between the JIVE component and behavioral and cognitive measures (i.e., total SMA hours, internalizing and externalizing scores, fluid and crystalized intelligence scores, and total sleep problem scores). The joint component was treated as the predictor in the models. All variables were standardized to allow for cross-measure comparisons. We used R functions in the lme4 package, and the parameters of the mixed model were estimated using maximum likelihood estimation. Site and family were included as random effects. All mixed effects models included age, gender, race (a four-level variable), parental highest education level, family income, marital status, handedness, and the whole brain volume as covariates. Tests were two-sided, and the statistical significance level was controlled at FDR of 0.05 level to account for multiple comparisons.
Ethics
IRB approval and informed written consent was obtained from participating ABCD sites during data collection. The current analyses involved deidentified data and were exempted by the Yale IRB, the Yale Human Investigation Committee. Thus, the study is in accordance with the principles of the Declaration of Helsinki.
Results
Demographics and sample characteristics
Table 1 shows the demographics of the sample by gender. Boys and girls were not significantly different in terms of age, race/ethnicity, parental highest education level, family income, parental marital status, or crystalized intelligence score. However, compared to girls, boys on average spent 35 and 52 min more time daily with screens on typical weekday and weekend days, respectively. In addition, boys on average had higher levels of internalizing (Boys: 49.36 ± 10.70 vs. Girls: 47.43 ± 10.48) and externalizing problems (46.47 ± 10.70 vs. 44.87 ± 9.83), higher total sleep problems scores (36.81 ± 8.52 vs. 36.22 ± 7.90), larger whole brain volumes (1258.65 ± 104.88 cm3 vs. 1152.69 ± 94.72 cm3), and lower fluid intelligence scores (95.08 ± 17.43 vs. 96.66 ± 17.13).
Table 1.
Demographics and behavioral characteristics of the ABCD baseline sample
| Variables | Girls (n = 4,661) | Boys (n = 5,077) |
| Age, Months | 118.84 ± 7.47 | 119.17 ± 7.50 |
| Crystalized intelligence | 106.18 ± 18.31 | 106.86 ± 18.19 |
| Fluid intelligence* | 97.13 ± 17.11 | 95.52 ± 17.40 |
| Externalizing score* | 44.84 ± 9.79 | 46.36 ± 10.63 |
| Internalizing score* | 47.51 ± 10.41 | 49.39 ± 10.65 |
| Total sleep problem* | 36.19 ± 7.78 | 36.79 ± 8.38 |
| Typical weekday SMA time (Hours)* | 3.06 ± 2.90 | 3.68 ± 3.13 |
| Typical weekend SMA time (Hours)* | 4.06 ± 3.45 | 4.98 ± 3.63 |
| Whole brain volume (cm3)* | 1155.62 ± 94.79 | 1261.92 ± 104.53 |
| Race/Ethnicity | ||
| White | 3062.00 (65.69%) | 3438.00 (67.72%) |
| Black | 689.00 (14.78%) | 692.00 (13.63%) |
| Other/Mixed | 803.00 (17.23%) | 838.00 (16.51%) |
| Asian | 107.00 (2.30%) | 109.00 (2.15%) |
| Parental Highest Education Level | ||
| Post Graduate Degree | 1704.00 (36.56%) | 1813.00 (35.71%) |
| Bachelor | 1237.00 (26.54%) | 1354.00 (26.67%) |
| Some College | 1168.00 (25.06%) | 1309.00 (25.78%) |
| HS Diploma/GED | 375.00 (8.05%) | 422.00 (8.31%) |
| < HS Diploma | 177.00 (3.80%) | 179.00 (3.53%) |
| Family Income | ||
| [≥100K] | 1984.00 (42.57%) | 2187.00 (43.08%) |
| [≥50K & <100K] | 1337.00 (28.68%) | 1457.00 (28.70%) |
| [<50K] | 1340.00 (28.75%) | 1433.00 (28.23%) |
| Parental Marital Status (Yes) | 3238.00 (69.47%) | 3586.00 (70.63%) |
| Major Depressive Disorder (Present) | 21.00 (0.45%) | 31.00 (0.61%) |
| Social Anxiety Disorder (Present) | 18.00 (0.39%) | 17.00 (0.33%) |
*Indicating significantly different between boys and girls at an FDR of P < 0.05.
1: Mental health conditions considered whether the participant had any of present major depressive disorder, persistent depressive disorder, and/or social anxiety disorder.
Cortical and subcortical covariation pattern
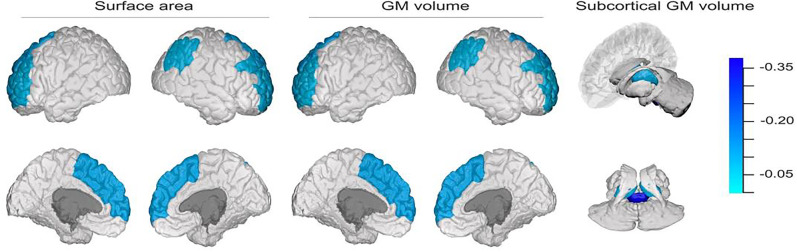
JIVE analyses identified one joint component that explained 33.8% of the total variation across four morphological measures. This joint component was dominated by covariation among subcortical and cortical GM volumes and cortical surface areas. Specifically, the joint component accounted for 38.6% of variation in subcortical GM volume and roughly 50% of variation in cortical GM volume and surface area, respectively. Cortical thickness did not covary with GM volume and surface area, as indicated by the fact that the joint component accounted for only 1.1% of variation in cortical thickness. Figure 1 shows key brain structures with large loading magnitudes in the joint component. These regions include brainstem, thalamus proper, rostral middle frontal gyrus, superior frontal gyrus, and right inferior parietal lobule. The JIVE component identified covariation among regions in our hypothesized thalamus-PFC-brainstem circuitry that included GM volumes of the brain stem and bilateral thalamus proper, as well as GM volumes and/or surface area of the bilateral superior and rostral middle frontal gyri.
Fig. 1.
Plots are shown of brain regions in the joint component with loadings larger than 0.13 (approximately corresponding to the top 5% of regions with the largest loading magnitudes). Interior and exterior views of the brain regions are presented for each morphological measure. This JIVE component was related to both total screen time (β = 0.107, P = 0.002) and externalizing behaviors (β = 0.117, P = 0.002).
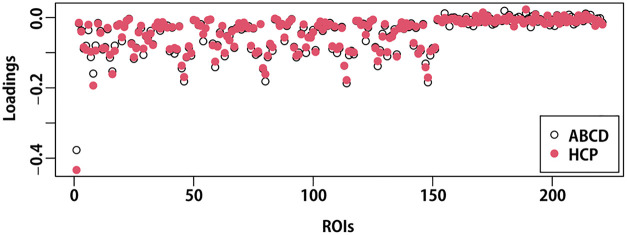
Of note, the joint component is highly reproducible and developmentally stable. The joint component structure was almost identical (with Pearson correlation coefficient of 0.985) to the one identified in a recent Human Connectome Project study (Zhao et al., 2021) involving young adults (age range: 22–36 years) that associated the joint component with early initiation of alcohol use (Fig. 2).
Fig. 2.
Overlayed scatter plot of the loadings for 221 ROI (region of interest) structural features in the joint components derived from the ABCD (Adolescent Brain Cognitive Development) baseline data and the HCP (Human Connectome Project) subsample.
Joint component statistically predicts total screen time and externalizing behaviors
Controlling for potentially confounding factors listed in the statistical analysis section, linear mixed model analyses showed that the joint component was associated with total screen time ( ) and externalizing behaviors ( ), but not with total sleep problems ( ), crystalized intelligence ( ), or internalizing problems ( ). Specifically, individuals with a larger joint component score as indicated by smaller GM volumes and/or smaller surface areas of regions implicated in the thalamus-PFC-brainstem circuitry, exhibited more total screen time and more externalizing behaviors. In addition, linear mixed model analysis showed that gender moderated the relationship between the joint component and fluid intelligence ( = 0.004). However, subgroup analyses revealed that the joint component was not significantly associated with fluid intelligence score in either boys ( ) or girls ( ).
Despite significant associations, the effect sizes as measured by variance explained by the joint component were small for both total screen time (R 2 = 0.104%) and externalizing behavior (R 2 = 0.10%). Of note, our exploratory analyses indicated that basic demographic variables such as age, gender, race/ethnicity, parental highest education level, family income, marital status, and the whole brain volume were all significantly related to total screen time (Appendix Table 1). Further, the model including these factors as fixed effects explained 13.24% of variance in total screen time.
Discussion
This investigation used the ABCD data to investigate associations between structural covariation networks in the brain and SMA, psychopathology, cognition, and sleep. There were three main results. First, the JIVE analyses identified one joint component that was characterized by a covariation pattern common across surface areas and GM volumes in key brain regions comprising a previously described thalamus-PFC-brainstem circuit. Second, the identified joint component was associated with total screen time and externalizing behaviors but not with total sleep problems, internalizing behaviors, and crystalized intelligence. Third, gender moderated the relationship between the joint component and fluid intelligence, suggesting the strength of the relationship differs in girls and boys. However, subgroup analyses did not show a significant relationship between the joint component and fluid intelligence in either boys or girls. Further, the joint component was highly similar to one linked to early onset of alcohol use in young adults. Taken together, the finding that similar brain structural covariations are associated with both high-quantity SMA and early alcohol use suggest a common neural mechanism underpinning risk for substance and behavioral addictions. Further, the link between the joint component and externalizing behaviors in children further resonates with the literature on externalizing processes and addiction risk, further highlighting the potential clinical and developmental relevance of the structural covariation pattern.
A recent study using a smaller subsample from the ABCD study (4,277 subjects as compared to the 9,738 subjects used in the current analyses) identified several SMA-related latent factors linking to SMA-related covariation among cortical features (Paulus et al., 2019). The current study differs from the prior one in that we used a larger sample and that we aimed to use an unsupervised approach to characterize cortical and subcortical structural covariation patterns across multiple morphological measures within a hypothesized circuit previously implicated in underage initiation of alcohol use and supported by preclinical data in rodents. Childhood is a critical period for brain development with differential maturation rates between cortical and subcortical regions. We speculate that differences in maturational coupling between cortical (mainly the PFC) and subcortical structures may explain individual differences in SMA and other potentially risky and addictive behaviors. An unsupervised approach (i.e., SMA information was not included in the structural covariation pattern discovery process) was used here as we believed that it would allow us to detect circuit level covariation patterns important to multiple psychiatric conditions or corresponding risk factors.
Brain structural covariance (Mechelli, Friston, Frackowiak, & Price, 2005; Alexander-Bloch et al., 2013) or maturational coupling (Raznahan et al., 2011) has attracted recent attention. A central concept of structural covariance is that brain features often covary across regions with similar functions, and these covariation patterns may reflect coordinated brain development across different brain regions (Alexander-Bloch et al., 2013). Emerging evidence supports this concept. For example, age-related changes in structural covariation patterns were observed in regions within the default-mode network and other networks associated with language-related tasks and executive control (Mechelli et al., 2005; Li et al., 2013). Our analyses showed the structural covariation among regions in the thalamus-PFC-brainstem circuit was remarkably consistent across different developmental stages and in females and males (Zhao et al., 2021). Similarly, relatively modest gender-related differences were observed relative to the relationships between the identified structural covariation pattern and the explored behavioral measures. These findings resonate thematically with substantial gender-related differences in types of SMA use and related problems and the relatively similar psychological factors linked to various forms of problematic use of the internet reported previously (Rumpf et al., 2015). It is likely that this identified covariation pattern is both genetically determined (Alexander-Bloch et al., 2013) and determined as a result of brain plasticity induced by the environment experienced during life functioning (Markham & Greenough, 2004), consistent with preclinical findings (Siciliano et al., 2019). The structural covariation identified in the current analysis may be shaped by or related to the coordinated development of white-matter structural connectivity, resting-state and/or task-based functional connectivity, or other factors. More research is needed to better understand the biological mechanisms of the identified structural covariance and its potential clinical value with respect to developing or implementing interventions.
The identified thalamus-PFC-brainstem connections might be developmentally sensitive structural covariation networks that are important for many behaviors related to addictions. First, it has been well-recognized that the epoch from late childhood to adolescence is a critical period with rapid and differential brain development reflecting imbalanced maturation of cortical (mainly PFC) and subcortical structures that mature earlier than the PFC (Casey, Getz, & Galvan, 2008; Somerville, Jones, & Casey, 2010; Kuss, Pontes, & Griffiths, 2018). The joint component was dominated by structural covariance of the brainstem, thalamus proper, and several PFC regions. This structural organization pattern is the basis for dual systems or maturational imbalance models that have been proposed to explain adolescent risk-taking (Casey et al., 2008; Casey, Jones, & Somerville, 2011; Shulman et al., 2016). A recent murine optogenetic study demonstrated the critical role of medial PFC-brainstem circuit in compulsive drinking (Siciliano et al., 2019). Interestingly, our recent finding using the same JIVE approach found that the thalamus-PFC-brainstem circuit was related to early initiation of alcohol use (Zhao et al., 2021). The observations that the joint component was also significantly associated with total screen time and externalizing behavior may suggest that risk or propensity for engaging in high-quantity SMA may share neural mechanisms with risk or propensity for engaging in alcohol use (or, given links with externalizing behaviors, other substance use), resonating with prior studies (Weinstein, Livny, & Weizman, 2017; Kuss et al., 2018; Turel, He, Brevers, & Bechara, 2018). Indeed, activity in the brainstem and other regions has been implicated in internet gaming disorder (Zhang et al., 2016), and brainstem communication with other brain regions is important for controlling sleep-wake transitions (Gompf & Anaclet, 2020). Given that the brainstem and thalamus serve as key information relay stations and that the PFC is critical for executive functioning, the identified thalamus-PFC-brainstem covariation pattern may reflect a circuit that communicates and processes sensory information between the body and PFC in relation to high-quantity SMA and substance use behaviors. Given the links with externalizing behaviors, the structural covariation pattern may link to poor control over behaviors. However, as these brain regions and related circuits have also been linked to other aspects of human behavior and that in other species (e.g., in impulsive and compulsive behaviors) (Fineberg et al., 2010, 2014; Figee et al., 2016), these currently speculative notions warrant further direct examination.
Limitations
This investigation is based on cross-sectional data. Therefore, no causal inferences can be made in terms of whether SMA impacts the brain development or vice versa. It is possible that the identified structural covariation pattern represents a risk factor for the development of problems with SMA as well as problematic drinking and externalizing behaviors. However, longitudinal data are needed to investigate this possibility directly, as may be facilitated by future releases of the ABCD study that will allow us to investigate the directionality of relationships. The structural covariation pattern appears linked to multiple measures, although these measures may be considered relatively blunt. For example, although it has been hypothesized that high/frequent engagement in different types of SMA may be associated with different neural mechanisms, the joint component here is associated with the total amount of SMA. However, positive correlations that exist across all types of SMA and our focus on structural covariation common across multiple morphological measures limited our ability to identify neural mechanisms specific to individual types of SMA. Nonetheless, future studies should consider including direct measurement of types and patterns of SMA. Similarly, future studies should consider direct measurements of sleep activity and performance on cognitive tasks.
Conclusions
Our study identified a structural covariation pattern that appears highly reproducible across different developmental stages and associated with high-quantity SMA and externalizing behaviors. Even though the corresponding effect sizes were small, a model that incorporated demographics and whole brain volume accounted for 13.24% of the variance in total SMA. Further, the findings resonate with prior studies linking SMA-related structural covariation to externalizing psychopathology in youth and suggest a potential contributory mechanism underlying substance and behavioral addictions.
Funding sources
This study was supported by grants from Children and Screens (CSDMB001) and the NIH (RF1 MH128614, R01 AA029611). The views presented in this manuscript represent those of the authors and not necessarily those of the funding agencies.
Author’s contribution
Y.Z. and M.N.P designed the study. Y.Z. conducted the statistical analysis and wrote the first draft of the manuscript. All authors revised, contributed to, and approved the final manuscript. Data collection was conducted by the ABCD consortium, and the present study used publicly available data (https://nda.nih.gov/abcd/).
Conflict of interest
The authors declare no conflicts of interest. Dr. Potenza has consulted for Opiant Therapeutics, Game Day Data, Baria-Tek, the Addiction Policy Forum, AXA and Idorsia Pharmaceuticals; has been involved in a patent application with Yale University and Novartis; has received research support from Mohegan Sun Casino and the National Center for Responsible Gaming; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse-control disorders or other health topics; has consulted for and/or advised gambling and legal entities on issues related to impulse-control/addictive disorders; has provided clinical care in a problem gambling services program; has performed grant reviews for research-funding agencies; has edited journals and journal sections; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts. Dr. Potenza is an associate editor of the Journal of Behavioral Addictions. The other authors do not report disclosures.
Acknowledgement
The data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), which are stored in the NIMH Data Archive (NDA). The ABCD Study is a multisite, longitudinal study designed to recruit more than 10,000 children aged 9–10 years and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, U24DA041147, U01DA041093, and U01DA041025. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/scientists/workgroups/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in the analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from https://doi.org/10.15154/1503209. We would like to thank all individuals who participated in this study.
Appendix
Table A1.
Mixed effects model with study site and family as random intercepts showed that multiple basic demographic variables are associated with total screen media activity.
| Variable | Estimate | StdError | df | T-value | P-value |
| Intercept | −0.1131 | 0.1914 | 5672.147 | −0.5910 | 0.5546 |
| Gender (M) | 0.2585 | 0.0210 | 9587.588 | 12.3383 | <0.0001 |
| Whole brain volume | 0.0000 | 0.0000 | 9447.010 | −2.8873 | 0.0039 |
| Race/Ethnicity | |||||
| Black | 0.3955 | 0.0323 | 8122.383 | 12.2405 | <0.0001 |
| Asian | −0.1395 | 0.0646 | 8960.879 | −2.1578 | 0.0310 |
| Other/Mixed | 0.0236 | 0.0267 | 8278.754 | 0.8848 | 0.3763 |
| Age | 0.0041 | 0.0012 | 9301.023 | 3.4075 | 0.0007 |
| Parental Education | |||||
| HS Diploma/GED | 0.1562 | 0.0590 | 8479.466 | 2.6477 | 0.0081 |
| Some College | 0.1611 | 0.0537 | 8435.787 | 3.0013 | 0.0027 |
| Bachelor | −0.0285 | 0.0568 | 8381.003 | −0.5013 | 0.6162 |
| Post Graduate Degree | −0.2197 | 0.0575 | 8366.119 | −3.8184 | 0.0001 |
| Family Income | |||||
| [≥50K & <100K] | −0.0973 | 0.0294 | 8162.802 | −3.3089 | 0.0009 |
| [≥100K] | −0.1850 | 0.0331 | 8060.059 | −5.5947 | <0.0001 |
| Parental Marital Status (Yes) | −0.1316 | 0.0248 | 8002.610 | −5.3109 | <0.0001 |
Contributor Information
Yihong Zhao, Email: yz2135@caa.columbia.edu.
Martin Paulus, Email: mpaulus@laureateinstitute.org.
Kara S. Bagot, Email: kara.bagot@mssm.edu.
R. Todd Constable, Email: todd.constable@yale.edu.
H. Klar Yaggi, Email: henry.yaggi@yale.edu.
Nancy S. Redeker, Email: nancy.redeker@yale.edu.
Marc N. Potenza, Email: marc.potenza@yale.edu.
References
- Achenbach, T. M. (2009). The achenbach system of empirically based assessment (ASEBA): Development, findings, theory, and applications . . Burlington, VT: University of Vermont Research Center for Children, Youth, & Families. [Google Scholar]
- Akshoomoff, N. , Beaumont, J. L. , Bauer, P. J. , Dikmen, S. S. , Gershon, R. C. , Mungas, D. , & Heaton, R. K. (2013). VIII. NIH Toolbox cognition Battery (CB): Composite scores of crystallized, fluid, and overall cognition. Monographs of the Society for Research in Child Development , 78(4), 119–132. 10.1111/mono.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Bloch, A. , Giedd, J. N. , & Bullmore, E. (2013). Imaging structural co-variance between human brain regions. Nature Reviews. Neuroscience , 14(5), 322–336. 10.1038/nrn3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, K. E. , Bush, H. M. , & Jones, J. (2010). Television and video game viewing and its association with substance use by Kentucky elementary school students, 2006. Public Health Reports , 125(3), 433–440. 10.1177/003335491012500312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot, K. S. , Matthews, S. A. , Mason, M. , Squeglia, L. M. , Fowler, J. , Gray, K. , … Patrick, K. (2018). Current, future and potential use of mobile and wearable technologies and social media data in the ABCD study to increase understanding of contributors to child health. Developmental Cognitive Neuroscience , 32, 121–129. 10.1016/j.dcn.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch, D. M. , Albaugh, M. D. , Avenevoli, S. , Chang, L. , Clark, D. B. , Glantz, M. D. , … Sher, K. J. (2018). Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description. Developmental Cognitive Neuroscience , 32, 55–66. 10.1016/j.dcn.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand, M. , Wegmann, E. , Stark, R. , Muller, A. , Wolfling, K. , Robbins, T. W. , & Potenza, M. N. (2019). The Interaction of Person-Affect-Cognition-Execution (I-PACE) model for addictive behaviors: Update, generalization to addictive behaviors beyond internet-use disorders, and specification of the process character of addictive behaviors. Neuroscience and Biobehavioral Reviews , 104, 1–10. 10.1016/j.neubiorev.2019.06.032. [DOI] [PubMed] [Google Scholar]
- Bruni, O. , Ottaviano, S. , Guidetti, V. , Romoli, M. , Innocenzi, M. , Cortesi, F. , & Giannotti, F. (1996). The Sleep Disturbance Scale for Children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. Journal of Sleep Research , 5(4), 251–261. 10.1111/j.1365-2869.1996.00251.x. [DOI] [PubMed] [Google Scholar]
- Canan, F. , Karaca, S. , Toprak, M. , Kuloğlu, M. , & Potenza, M. (2019). Gender-related differences in the relationship between problematic and pathological Internet use and self-reported sleep-wake habits among university students. Archives of Behavioral Addictions , http://www.aba-journal.com/gender-related-differences-in-the-relationship-between-problematic-and-pathological-internet-use-and-self-reported-sleep-wake-habits-among-university-students/. [Google Scholar]
- Casey, B. J. , Cannonier, T. , Conley, M. I. , Cohen, A. O. , Barch, D. M. , Heitzeg, M. M. , … Workgroup, A. I. A. (2018). The adolescent brain cognitive development (ABCD) study: Imaging acquisition across 21 sites. Developmental Cognitive Neuroscience , 32, 43–54. 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey, B. J. , Getz, S. , & Galvan, A. (2008). The adolescent brain. Dev Rev , 28(1), 62–77. 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey, B. J. , Jones, R. M. , & Somerville, L. H. (2011). Braking and accelerating of the adolescent brain. J Res Adolesc , 21(1), 21–33. 10.1111/j.1532-7795.2010.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulou, G. , Majmundar, A. , Chou, C. P. , & Pentz, M. A. (2020). Anhedonia, screen time, and substance use in early adolescents: A longitudinal mediation analysis. J Adolesc , 78, 24–32. 10.1016/j.adolescence.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone, E. A. , & Konijn, E. A. (2018). Media use and brain development during adolescence. Nature Communications , 9(1), 588. 10.1038/s41467-018-03126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan, R. S. , Segonne, F. , Fischl, B. , Quinn, B. T. , Dickerson, B. C. , Blacker, D. , … Killiany, R. J. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage , 31(3), 968–980. 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Feng, Q. , Hannig, J. , & Marron, J. S. (2015). Non-iterative joint and individual variation explained. arXiv:1512 .04060. [Google Scholar]
- Ferguson, C. J. (2017). Everything in moderation: Moderate use of screens unassociated with child behavior problems. The Psychiatric Quarterly , 88(4), 797–805. 10.1007/s11126-016-9486-3. [DOI] [PubMed] [Google Scholar]
- Figee, M. , Pattij, T. , Willuhn, I. , Luigjes, J. , van den Brink, W. , Goudriaan, A. , … Denys, D. (2016). Compulsivity in obsessive-compulsive disorder and addictions. European Neuropsychopharmacology , 26(5), 856–868. 10.1016/j.euroneuro.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Fineberg, N. A. , Chamberlain, S. R. , Goudriaan, A. E. , Stein, D. J. , Vanderschuren, L. J. , Gillan, C. M. , … Potenza, M. N. (2014). New developments in human neurocognition: Clinical, genetic, and brain imaging correlates of impulsivity and compulsivity. CNS Spectrums , 19(1), 69–89. 10.1017/S1092852913000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg, N. A. , Potenza, M. N. , Chamberlain, S. R. , Berlin, H. A. , Menzies, L. , Bechara, A. , … Hollander, E. (2010). Probing compulsive and impulsive behaviors, from animal models to endophenotypes: A narrative review. Neuropsychopharmacology , 35(3), 591–604. 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George, M. J. , Russell, M. A. , Piontak, J. R. , & Odgers, C. L. (2018). Concurrent and subsequent associations between daily digital technology use and high-risk adolescents' mental health symptoms. Child Development , 89(1), 78–88. 10.1111/cdev.12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompf, H. S. , & Anaclet, C. (2020). The neuroanatomy and neurochemistry of sleep-wake control. Curr Opin Physiol , 15, 143–151. 10.1016/j.cophys.2019.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler, D. J., Jr. , Hatton, S. , Cornejo, M. D. , Makowski, C. , Fair, D. A. , Dick, A. S. , … Dale, A. M. (2019). Image processing and analysis methods for the adolescent brain cognitive development study. Neuroimage , 202, 116091. 10.1016/j.neuroimage.2019.116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisler, G. , Twenge, J. M. , & Krizan, Z. (2020). Associations between screen time and short sleep duration among adolescents varies by media type: Evidence from a cohort study. Sleep Medicine , 66, 92–102. 10.1016/j.sleep.2019.08.007. [DOI] [PubMed] [Google Scholar]
- Jeong, H. , Yim, H. W. , Lee, S. Y. , Lee, H. K. , Potenza, M. N. , & Lee, H. (2020). Factors associated with severity, incidence or persistence of internet gaming disorder in children and adolescents: A 2-year longitudinal study. Addiction . 10.1111/add.15366. [DOI] [PubMed] [Google Scholar]
- Jordan, C. J. , & Andersen, S. L. (2017). Sensitive periods of substance abuse: Early risk for the transition to dependence. Developmental Cognitive Neuroscience , 25, 29–44. 10.1016/j.dcn.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai, R. , Bahrami, B. , Roylance, R. , & Rees, G. (2012). Online social network size is reflected in human brain structure. Proc Biol Sci , 279(1732), 1327–1334. 10.1098/rspb.2011.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcher, N. R. , & Barch, D. M. (2021). The ABCD study: Understanding the development of risk for mental and physical health outcomes. Neuropsychopharmacology , 46(1), 131–142. 10.1038/s41386-020-0736-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirlic, N. , Colaizzi, J. M. , Cosgrove, K. T. , Cohen, Z. P. , Yeh, H. W. , Breslin, F. , … Paulus, M. P. (2021). Extracurricular activities, screen media activity, and sleep may Be modifiable factors related to children's cognitive functioning: Evidence from the ABCD study((R)). Child Development , 92(5), 2035–2052. 10.1111/cdev.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuss, D. J. , Pontes, H. M. , & Griffiths, M. D. (2018). Neurobiological correlates in internet gaming disorder: A systematic literature review. Front Psychiatry , 9, 166. 10.3389/fpsyt.2018.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Pu, F. , Fan, Y. , Niu, H. , Li, S. , & Li, D. (2013). Age-related changes in brain structural covariance networks. Front Hum Neurosci , 7, 98. 10.3389/fnhum.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock, E. F. , Hoadley, K. A. , Marron, J. S. , & Nobel, A. B. (2013). Joint and individual variation explained (Jive) for integrated analysis of multiple data types. Ann Appl Stat , 7(1), 523–542. 10.1214/12-AOAS597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham, J. A. , & Greenough, W. T. (2004). Experience-driven brain plasticity: Beyond the synapse. Neuron Glia Biology , 1(4), 351–363. 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli, A. , Friston, K. J. , Frackowiak, R. S. , & Price, C. J. (2005). Structural covariance in the human cortex. The Journal of Neuroscience , 25(36), 8303–8310. 10.1523/JNEUROSCI.0357-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulich, K. N. , Ross, J. M. , Lessem, J. M. , & Hewitt, J. K. (2021). Screen time and early adolescent mental health, academic, and social outcomes in 9- and 10- year old children: Utilizing the Adolescent Brain Cognitive Development (ABCD) Study. Plos One , 16(9), e0256591. 10.1371/journal.pone.0256591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus, M. P. , Squeglia, L. M. , Bagot, K. , Jacobus, J. , Kuplicki, R. , Breslin, F. J. , … Tapert, S. F. (2019). Screen media activity and brain structure in youth: Evidence for diverse structural correlation networks from the ABCD study. Neuroimage , 185, 140–153. 10.1016/j.neuroimage.2018.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza, M. N. , Faust, K. , & Faust, D. (2020). The Oxford Handbook of Digital technologies and mental health . New York, NY, USA: Oxford University Press. [Google Scholar]
- Raznahan, A. , Lerch, J. P. , Lee, N. , Greenstein, D. , Wallace, G. L. , Stockman, M. , … Giedd, J. N. (2011). Patterns of coordinated anatomical change in human cortical development: A longitudinal neuroimaging study of maturational coupling. Neuron , 72(5), 873–884. 10.1016/j.neuron.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout, V. , & Robb, M. B. (2019). The common sense census: Media use by tweens and teens . Common Sense Media. Retrieved from https://www.commonsensemedia.org/sites/default/files/uploads/research/2019-census-8-to-18-full-report-updated.pdf. [Google Scholar]
- Rumpf, H. , Bischof, G. , Bischof, A. , Besser, B. , Meyer, C. , & John, U. (2015). Applying DSM-5 criteria for Internet gaming disorder for the broader concept of Internet addiction. J Behav Addict , 4, 34–35. [Google Scholar]
- Shulman, E. P. , Smith, A. R. , Silva, K. , Icenogle, G. , Duell, N. , Chein, J. , & Steinberg, L. (2016). The dual systems model: Review, reappraisal, and reaffirmation. Developmental Cognitive Neuroscience , 17, 103–117. 10.1016/j.dcn.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano, C. A. , Noamany, H. , Chang, C. J. , Brown, A. R. , Chen, X. , Leible, D. , … Tye, K. M. (2019). A cortical-brainstem circuit predicts and governs compulsive alcohol drinking. Science , 366(6468), 1008–1012. 10.1126/science.aay1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville, L. H. , Jones, R. M. , & Casey, B. J. (2010). A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition , 72(1), 124–133. 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies Shapiro, L. A. , & Margolin, G. (2014). Growing up wired: Social networking sites and adolescent psychosocial development. Clinical Child and Family Psychology Review , 17(1), 1–18. 10.1007/s10567-013-0135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, W. , Han, X. , Jin, C. , Yan, Y. , & Potenza, M. N. (2019). Are males more likely to be addicted to the internet than females? A meta-analysis involving 34 global jurisdictions. Computers in Human Behavior , 99, 86–100. 10.1016/j.chb.2019.04.021. [DOI] [Google Scholar]
- Su, W. , Han, X. , Yu, H. , Wu, Y. , & Potenza, M. N. (2020). Do men become addicted to internet gaming and women to social media? A meta-analysis examining gender-related differences in specific internet addiction. Computers in Human Behavior , 113, 106480. 10.1016/j.chb.2020.106480. [DOI] [Google Scholar]
- Takeuchi, H. , Taki, Y. , Hashizume, H. , Asano, K. , Asano, M. , Sassa, Y. , … Kawashima, R. (2016). Impact of videogame play on the brain's microstructural properties: Cross-sectional and longitudinal analyses. Molecular Psychiatry , 21(12), 1781–1789. 10.1038/mp.2015.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turel, O. , He, Q. , Brevers, D. , & Bechara, A. (2018). Delay discounting mediates the association between posterior insular cortex volume and social media addiction symptoms. Cognitive, Affective & Behavioral Neuroscience , 18(4), 694–704. 10.3758/s13415-018-0597-1. [DOI] [PubMed] [Google Scholar]
- Volkow, N. D. , Koob, G. F. , Croyle, R. T. , Bianchi, D. W. , Gordon, J. A. , Koroshetz, W. J. , … Weiss, S. R. B. (2018). The conception of the ABCD study: From substance use to a broad NIH collaboration. Developmental Cognitive Neuroscience , 32, 4–7. 10.1016/j.dcn.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Der Heide, R. , Vyas, G. , & Olson, I. R. (2014). The social network-network: Size is predicted by brain structure and function in the amygdala and paralimbic regions. Social Cognitive and Affective Neuroscience , 9(12), 1962–1972. 10.1093/scan/nsu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade, N. E. , Ortigara, J. M. , Sullivan, R. M. , Tomko, R. L. , Breslin, F. J. , Baker, F. C. , … Workgroup, A. N. T. (2021). Passive sensing of preteens' smartphone use: An adolescent brain cognitive development (ABCD) cohort substudy. JMIR Ment Health , 8(10), e29426. 10.2196/29426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein, A. , Livny, A. , & Weizman, A. (2017). New developments in brain research of internet and gaming disorder. Neuroscience and Biobehavioral Reviews , 75, 314–330. 10.1016/j.neubiorev.2017.01.040. [DOI] [PubMed] [Google Scholar]
- Wong, H. Y. , Mo, H. Y. , Potenza, M. N. , Chan, M. N. M. , Lau, W. M. , Chui, T. K. , … Lin, C. Y. (2020). Relationships between severity of internet gaming disorder, severity of problematic social media use, sleep quality and psychological distress. Int J Environ Res Public Health , 17(6). 10.3390/ijerph17061879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. T. , Yao, Y. W. , Potenza, M. N. , Xia, C. C. , Lan, J. , Liu, L. , … Fang, X. Y. (2016). Effects of craving behavioral intervention on neural substrates of cue-induced craving in Internet gaming disorder. Neuroimage Clin , 12, 591–599. 10.1016/j.nicl.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Constable, R. T. , Hien, D. , Chung, T. , & Potenza, M. N. (2021). Brain anatomical covariation patterns linked to binge drinking and age at first full drink. Neuroimage Clin , 29, 102529. 10.1016/j.nicl.2020.102529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Klein, A. , Castellanos, F. X. , & Milham, M. P. (2019). Brain age prediction: Cortical and subcortical shape covariation in the developing human brain. Neuroimage , 202, 116149. 10.1016/j.neuroimage.2019.116149. [DOI] [PMC free article] [PubMed] [Google Scholar]