Abstract
Background:
Studies suggest associations between exposure to individual polybrominated diphenyl ethers (PBDEs) with preterm birth (PTB) and shorter gestational age. Little is known about exposure to PBDE mixtures and these outcomes. We evaluated associations of multiple PBDEs in early pregnancy with gestational age at delivery and PTB.
Methods:
Data were collected from 2046 women without obesity and 396 women with obesity from the NICHD Fetal Growth Studies, who had early pregnancy plasma PBDEs concentrations and gestational age at delivery. PTB was defined as < 37 weeks of gestation at delivery and further categorized into subtypes (late or very early/moderate; spontaneous or medically indicated). We applied (1) generalized linear models (GLM); (2) principal component analysis (PCA); and (3) Bayesian Kernel Machine Regression (BKMR) to evaluate the individual and joint associations of log-transformed PBDE concentrations with gestational age at delivery and PTB, adjusting for potential confounders and evaluating effect modifiers.
Results:
In GLM analyses, a 1-standard deviation (SD) increase in log-PBDE 153 was associated with shorter gestational age at delivery [adjusted β (95% CI) = −0.19 (−0.31, −0.06) weeks] among women without obesity. In PCA analyses, 1-SD increase in the principal component summarizing most of PBDE 153 variability was associated with shorter gestational age at delivery [adjusted β (95% CI) = −0.18 (−0.30, −0.06) weeks], very early/moderate PTB [adjusted OR (95% CI) = 1.91 (1.19, 3.07)], and spontaneous PTB [adjusted OR (95% CI) = 1.34 (1.00, 1.80)] among women without obesity. Associations were stronger among non-Hispanic Black women, women with BMI ranging between 25-30 kg/m2, and women who were ≥ 35 years old among those without obesity. In BKMR analyses, a suggestive inverse association between PBDE 153 and gestational age at delivery, and an inverse U-shaped association between PBDE 154 and gestational age at delivery were observed in women without obesity. No statistically significant association of PBDEs and gestational age or PTB was observed among women with obesity.
Conclusions:
PBDEs, specifically PBDE 153, were associated with shorter gestation and higher risk of certain PTB subtypes among pregnant women without obesity.
Keywords: Polybrominated diphenyl ethers, preterm birth, gestational age, environmental mixtures
INTRODUCTION
Preterm birth (PTB), defined as delivery prior to 37 weeks of gestation, affects 9-10% of pregnancies in the U.S. (Martin and Osterman, 2018), and is a strong predictor of neonatal mortality and morbidity (Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes, 2007). PTB is also associated with an increased risk of maternal morbidity and mortality, as well as adverse health outcomes later in life in both mothers (such as increased risk of type 2 diabetes and cardiovascular disease) (Henderson et al., 2016; Wu et al., 2018) and their offspring (such as developmental disabilities, asthma, and metabolic syndromes) (Parkinson et al., 2013; Saigal and Doyle, 2008; Sonnenschein-van der Voort et al., 2014). In addition, recent studies suggest a gradient of increasing risk of poorer outcomes with decreasing gestational age at delivery instead of a fixed threshold for PTB on perinatal mortality and morbidity (Boyle et al., 2012; Zhang and Kramer, 2009). Although certain factors, such as prior PTB, multiple pregnancy, age, maternal comorbidities (e.g. hypertension, diabetes), psychosocial stress, and tobacco smoking, are established risk factors for PTB (Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes, 2007), there remains a large proportion of cases with unknown etiology (Ferrero et al., 2016). Thus, further identification of contributors to shortened gestational age at delivery is needed, such as exposure to environmental chemicals with endocrine disrupting properties (Ferguson et al., 2013).
Polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyls (PBBs) are environmentally persistent polyhalogenated organic compounds that are ubiquitously distributed due to their use as flame retardants in a variety of consumer products, including electronic equipment, furniture, textiles, and small appliances (Eskenazi et al., 2013). Since PBDEs are semi-volatile and not chemically bound to a substrate, they can leach from products and become environmental contaminants (Darnerud et al., 2001). Exposure to PBDEs in the general population occurs through inhalation of contaminated air, ingestion of contaminated water and food, indoor dust, as well as dermal contact (Johnson-Restrepo and Kannan, 2009; Stapleton et al., 2005). PBDEs can bioaccumulate in the body, with long half-lives ranging from 2 to 12 years (Chevrier et al., 2010). Exposure to PBDEs have been associated with a range of adverse perinatal outcomes such as altered thyroid hormone levels in pregnant women and infants (Herbstman et al., 2008) and low birth weight (Harley et al., 2011; Zhao et al., 2017). Potential mechanisms linking PBDEs to PTB are supported by the results of in vitro studies, where PBDEs were linked with biomarkers indicating altered placental development during mid-gestation (Varshavsky et al., 2020a), with oxidative stress and pro-inflammatory mediators (Park et al., 2014; Peltier et al., 2012), and with altered placental DNA methylation (Zhao et al., 2016), which are on pathophysiological pathways leading to both spontaneous and medically-indicated PTB (Farina and Winkelman, 2005; McElrath et al., 2008; Morgan, 2016).
Previous findings from population-based studies on the associations between PBDEs and PTB or gestational age at delivery have been inconsistent. One study found no association between 4 PBDE congeners (PBDE 47, 99, 100, 153) and gestational age at delivery (Harley et al., 2011), while two other studies found higher maternal serum concentrations of PBDE 47 associated with increased odds of PTB (Peltier et al., 2015) and shorter gestational age at delivery (Eick et al., 2020). However, most studies have focused on a few specific PBDE congeners failing to account for the complex nature of these exposures as a joint exposure to a PBDEs mixture with potential synergistic/antagonistic effects. In addition, previous studies had relatively small sample sizes and under-represent certain racial/ethnic groups, and thus may have inadequate power to detect associations or ascertain racial/ethnic differences, especially given the pronounced racial/ethnic disparities in both PTB (14% in non-Hispanic black compared to 9% in non-Hispanic white women) (Burris et al., 2019, 2011) and exposure levels to PBDEs (e.g. higher median levels of PBDE 47, 99, 100, 153 and 154 found in non-Hispanic black women compared to non-Hispanic white women in a healthy obstetric cohort) (Buck Louis et al., 2018; Nguyen et al., 2020; Varshavsky et al., 2020b) that are disproportionally affecting non-Hispanic black women in the US.
In this study, we aimed to prospectively evaluate the associations of exposures to multiple PBDE congeners during early pregnancy with both gestational age at delivery and PTB within a large, multi-center, multi-racial/ethnic cohort of U.S. pregnant women. We used three different statistical approaches that accommodated individual effects and mixtures effects. As secondary aims, we evaluated whether these associations differed across race/ethnicity, age, or pre-pregnancy BMI, or by categorizing PTB subtypes based on the length of gestation at delivery and indication of delivery.
METHODS
Study population
This study was conducted among women from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Fetal Growth Studies – Singleton Cohort, a multi-center, multi-racial prospective study of pregnant women recruited between July 2009 and January 2013 from 12 U.S. clinical sites (Grewal et al., 2018). Women aged 18-40 years with a viable singleton pregnancy were enrolled between 8 to 13 weeks of gestation and followed through delivery. Specifically, two cohorts of singleton pregnancies were included: (1) a cohort of 2334 women with a low-risk pregnancy and without obesity (i.e. pre-pregnancy BMI < 30 kg/m2) across four self-reported race/ethnicity groups (non-Hispanic white, non-Hispanic Black, Hispanic, or Asian/Pacific Islander); and (2) a cohort of 468 women with a low-risk pregnancy and with obesity (i.e. pre-pregnancy BMI ≥ 30 kg/m2) unselected by race/ethnicity. Both cohorts excluded women with major chronic conditions before pregnancy (autoimmune disease, cancer, chronic hypertension, chronic renal disease, diabetes, HIV/AIDS, or psychiatric disorders). In addition, the non-obese cohort further excluded several other conditions or comorbidities (shown in Supplemental Figure S1). Further details of the study can be found elsewhere (Grewal et al., 2018; Zhang et al., 2018).
For this analysis, we further restricted the study population to women who: (1) remained eligible for study participation after enrollment and consented to having their biospecimen used for research; (2) provided first trimester blood samples with available measurement of 10 brominated chemicals (PBB 153; PBDE 28, 47, 85, 99, 100, 153, 154, 183, and 209); (3) had data on gestational age at delivery; and (4) had a live birth. The final study population consisted of 2046 women without obesity and 396 women with obesity. All analyses were conducted separately in the non-obese and obese cohorts because chemical assays were performed at separate times and locations for each of these cohorts, and the cohorts had different eligibility/exclusion criteria that may reflect different demographic, lifestyle, and health conditions. For all statistical analyses evaluating the associations of PBDEs with gestational age at delivery and PTB, we further excluded observations with PBDEs concentrations greater than 5 standard deviations from the mean values to avoid potential distortion from extreme values (n = 38 in women without obesity; n = 6 in women with obesity). A detailed flow diagram of the inclusion and exclusion criteria is provided in Supplemental Figure S1.
Blood collection and PBDE quantification
Concentrations of 1 polybrominated biphenyl (PBB 153) and 9 PBDEs (PBDE 28, 47, 85, 99, 100, 153, 154, 183, and 209) were measured from blood specimens, which were collected at enrollment (median: 11 weeks of gestation) for both cohorts. Samples were processed immediately after collection and stored at −80 °C until analysis. Analysis was performed at the Wadsworth Center, New York State Department of Health (for samples from the non-obese cohort) and NYU Langone (for samples from the obese cohort) based on an isotope dilution method described previously using a gas chromatograph (GC 7890A, Agilent Technologies, Atlanta, GA) coupled with a high resolution mass spectrometer (MSD 5975 as well as JEOL800D) as previously discussed in detail (Ma et al., 2014). All chemical concentrations were reported as ng/mL plasma. The limits of quantification (LOQs) varied by congener, ranging between 0.0025 to 0.01 ng/mL for both the obese and non-obese cohorts. Machine-observed values were used for all chemicals in the analysis without substitution, including concentrations below the LOQ, as this has been shown to yield the least biased estimates compared to simple imputations (Schisterman et al., 2006). In addition, plasma lipids were measured from non-fasting blood samples collected at enrollment (Bao et al., 2018). Lipids were quantified using commercially available enzymatic methods as described in previous studies (Akins et al., 1989).
Outcome measurement
The two primary study outcomes of interest were: (1) gestational age at delivery (weeks), calculated as the difference between date of delivery and ultrasound validated self-reported date of first day of last menstrual period (LMP); and (2) a binary outcome of PTB, defined as delivery prior to 37 weeks of gestation. Delivery dates were abstracted by trained staff from the medical records. Only those with consistent self-reported LMP dates and ultrasound-estimated dates (i.e. within 5 days for those from 8+0 to 10+6 weeks, within 6 days for those from 11+0 to 12+6 weeks, and within 7 days for those from 13+0 to 13+6 weeks) were enrolled (Skupski et al., 2017).
Secondary study outcomes of interest included: (1) PTB further categorized as “spontaneous/inflammatory” or “medically indicated/placental dysfunction” based on prior classifications (McElrath et al., 2008) and (2) PTB further categorized as very early (< 32 weeks), moderate (32 to < 34 weeks), or late preterm (34 to < 37 weeks) (Ananth et al., 2013) based on gestational age at delivery. Spontaneous/inflammatory PTB was defined by the absence of a maternal or fetal indication/intrauterine growth restriction (IUGR) and included preterm labor, preterm premature rupture of membranes, cervical insufficiency, or placental abruption. Medically indicated/placental dysfunction PTB was characterized by infarcts and increased placental syncytial knots with the relative absence of inflammation and included maternal indications (such as preeclampsia, hypertension, or diabetes), or fetal indications (such as IUGR, non-reassuring fetal testing, or anomalies) (James-Todd et al., 2018).
Covariates
Based on a priori knowledge, the following variables were selected as potential confounders: maternal age, self-reported race/ethnicity, pre-pregnancy BMI, parity, education level, marital status, family income during last year, plasma cotinine level, plasma total lipids, total activity, sedentary activity, and acculturation status. Information about sociodemographic, lifestyle and reproductive factors were collected from a standardized questionnaire at baseline. Acculturation status in this analysis was classified as U.S.-born, recent immigrant (defined as lived in the U.S. for less than 10 years), or long-term immigrant (defined as lived in the U.S. for ≥ 10 years) based on previous publications (Mitro et al., 2019). Maternal weight and height were measured by trained research staff at enrollment using standardized methods (Grewal et al., 2018). Pre-pregnancy BMI was calculated from self-recalled pre-pregnancy weight divided by measured height squared (Pugh et al., 2017). Total plasma lipids (in ng/mL) were calculated using the following equation described elsewhere: total lipids = (total cholesterol × 2.27) + triglycerides + 62.3 (Bernert et al., 2007; Phillips et al., 1989). Plasma cotinine levels (in ng/mL) were measured in specimens collected at enrollment using an ultraperformance liquid chromatography coupled with an electrospray triple quadrupole tandem mass spectrometry (Buck Louis et al., 2018).
Statistical analysis
Baseline characteristics of the non-obese and obese cohorts were reported in the form of mean ± standard deviation (SD) for continuous variables or number (percentage) for categorical variables, in the overall cohorts as well as stratified by PTB (yes/no). Distributions of each of the 10 chemicals were summarized with number (percentage) above the LOQ and lipid-standardized geometric means (95% confidence intervals) and lipid-standardized medians (interquartile ranges). Molar sum of the congeners was calculated by dividing each chemical concentration by its molecular weight and summing all detectable concentrations. Total PBDEs was calculated using the sum of the detectable concentrations of all PBDE congeners. Chemicals that had quantification rates > 30% in each cohort were included in the main analyses. Correlations between the chemical concentrations were assessed using Spearman correlation coefficients.
For all analytical models measuring the associations between PBDEs and gestational age at delivery (weeks) or PTB (yes/no), we natural log-transformed the machine-observed values of the chemical concentrations to account for skewedness of their distributions, using ln (1 + concentration) transformation for PBDE congeners 28, 100, 153, 154 and 183, and ln (5 + concentration) for PBDE congeners 47 and 99 to ensure positive minimum values. Log-transformed chemicals were then standardized (subtracted the mean and divided by the SD) to generate comparable scales. We did not include lipid-standardized chemical concentrations in the models when analyzing the associations between PBDEs and gestational age at delivery or PTB. Instead, we adjusted for plasma total lipids as a covariate in the model, as a simulation study showed this to provide the less biased results (Schisterman et al., 2005). All models were adjusted for the following covariates: maternal age (years), race/ethnicity (non-Hispanic white, non-Hispanic Black, Hispanic, Asian/Pacific Islander), pre-pregnancy BMI (kg/m2), parity (0, 1, 2+), education level (< college degree, some college/undergraduate, graduate/post-graduate), marital status (married or living with partner, not married), family income during last year (< $30,000, $30,000-$49,999, $50,000-$99,999, ≥ $100,000, not reported), plasma cotinine level (ng/mL), plasma total lipids (ng/mL), total activity (MET hours/week), sedentary activity (MET hours/week), and acculturation (U.S.-born, recent immigrant, long-term immigrant). We utilized three approaches to evaluate the associations between PBDEs and gestational age at delivery (weeks) or PTB (yes/no): (1) generalized linear model regression (GLM) approaches; (2) GLM combined with principal component analysis (PCA) to identify a reduced set of exposure factors; and (3) Bayesian Kernel Machine Regression (BKMR), as described below.
First, we used multivariable linear regression models to identify the association between each individual PBDE congener and gestational age (weeks) at delivery. Beta coefficients (95% CI) for one standard deviation (SD) increase in each log-transformed PBDE congener concentration were calculated in three models: (1) crude; (2) adjusted for the above-mentioned covariates; and (3) mutually adjusted (i.e. further adjusted for all the other PBDE congeners). Likewise, we used multivariable logistic regression models to evaluate the individual associations between PBDEs and odds of PTB. Odds ratios (OR) and 95% CIs of PTB for a one-SD increase in each log-transformed PBDE congener concentration were calculated in the crude, adjusted, and mutually adjusted models.
Second, we used PCA as a dimension reduction approach, an unsupervised method to reduce the number of correlated variables to a smaller number of uncorrelated principal components (PCs) that explain most of the variability of the data (Chiu et al., 2018). In this study, we used PCA with varimax rotation and identified four PCs that cumulatively explained > 90% of variability of the PBDE congeners. We then fitted a multivariable linear regression and a multivariable logistic regression model of gestational age at delivery and PTB as outcomes, respectively, using the selected PCs as exposure measures, adjusted for the above-mentioned covariates. As secondary analyses, we also evaluated the associations between the PCs and PTB further categorized as spontaneous or medically indicated (compared to non-preterm), as well as further categorized as very/moderate preterm or late preterm (compared to non-preterm), using multinomial logistic regression models in the non-obese cohort only (due to insufficient numbers in the obese cohort). In addition, we assessed potential effect measure modification via stratifying the multivariate linear regression models by race/ethnicity (non-Hispanic white, non-Hispanic Black, Hispanic, Asian/Pacific Islander) or maternal age (< 35 vs. ≥ 35 years) in the non-obese and obese cohorts, and by pre-pregnancy BMI (< 25 vs. ≥ 25 kg/m2) in the non-obese cohort only. P-for-interactions were obtained via including interactions terms of the principal components and the proposed modifier(s), and p-for-interactions < 0.10 were conventionally considered as statistically significant.
Third, we applied BKMR to evaluate the associations between PBDE mixtures and gestational age at delivery. BKMR is a flexible approach that utilizes a kernel function that allows for non-linear estimation of individual exposure-outcome relationships for each chemical while taking into account the correlation structure of the multiple chemicals; it also provides intuitive graphical visualization of potential interactions between chemicals (Bobb et al., 2015). We applied the Gaussian kernel and the default tuning parameters for Markov Chain Monte Carlo (MCMC) processes with 50,000 iterations to assess the relative importance of each standardized log-transformed PBDE congener on standardized gestational weeks at delivery based on their posterior inclusion probabilities (PIPs), and qualitatively evaluated potential interactions between the chemicals.
To determine the robustness of the results, we additionally performed several sensitivity/secondary analyses. First, based on a priori knowledge of the potential correlations of certain dietary factors with PBDEs concentrations and PTB (Gete et al., 2020; Schecter et al., 2010), we evaluated the correlations between PBDEs and several dietary factors [including dairy intake, meat from beef/pork/lamb/organ, processed meats, fish/seafood, and a summarized Alternate Healthy Eating Index 2010 (AHEI-2010)] in a subset of the study population who completed a food frequency questionnaire (FFQ) at baseline, and further adjusted for these dietary factors to account for any potential confounding. Second, in the generalized linear models, we examined the associations based on quantiles of the chemical concentrations as predictors of gestational age at delivery to relax the linearity assumptions. Third, given PBDEs were shown to disturb lipid metabolism (Cowens et al., 2015), we conducted a sensitivity analysis without adjusting for plasma lipids to avoid any potential blockage of the causal pathway between PBDEs and gestational age or PTB. Fourth, we conducted survival analyses modelling gestational age at delivery as a time-to-event outcome to evaluate the associations between PBDEs and time to delivery using Cox proportional hazards models. Lastly, we conducted a sensitivity analysis where we included data from the individuals with extreme values of PBDEs (which was excluded in our main analyses).
Data analyses were conducted in SAS version 9.4 (SAS Institute Inc) and R version 4.0.2 (R Foundation for Statistical Computing). P-values < 0.05 were conventionally considered statistically significant unless otherwise specified. R packages used for main analyses included stats (for GLMs and PCA) and bkmr (for BKMR) (Bobb et al., 2018).
RESULTS
Table 1 presents baseline characteristics of the study population in the non-obese (n = 2046) and obese cohorts (n = 396). Study participants in both cohorts predominantly had some college degree or higher, were married or living with a partner, and were U.S.-born. The non-obese cohort consisted of 28% non-Hispanic white, 25% non-Hispanic Black, 28% Hispanic and 19% Asian/Pacific Islander women, while the obese cohort consisted of 31% non-Hispanic white, 36% non-Hispanic Black, 32% Hispanic, and only 1% Asian/Pacific Islander women. The non-obese cohort also had higher percentages of nulliparous women, lower percentages of family income < $30,000, lower mean plasma cotinine levels, as well as lower total and sedentary activities compared to the obese cohort. The percentages of PTB were 6.0% in the non-obese and 6.6% in the obese cohort. The mean gestational ages at delivery and further categorizations of PTB are summarized in Supplemental Table S1.
Table 1.
Main characteristics of the study population among the non-obese and obese cohorts, total and stratified by gestational age at delivery.
| Non-obese cohort (n=2046) |
Obese cohort (n=396) |
|||||
|---|---|---|---|---|---|---|
| Total (n=2046) | Preterm (GA < 37 weeks) (n=122) | Non-preterm (GA ≥ 37 weeks) (n=1924) | Total (n=396) | Preterm (GA < 37 weeks) (n=26) | Non-preterm (GA ≥ 37 weeks) (n=370) | |
| Age (years) | 28.3 ± 5.5 | 28.2 ± 6.0 | 28.3 ± 5.4 | 27.9 ± 5.6 | 29.3 ± 5.3 | 27.8 ± 5.6 |
| Race/ethnicity | ||||||
| Non-Hispanic White | 563 (27.5) | 29 (23.8) | 534 (27.8) | 122 (30.8) | 12 (46.2) | 110 (29.7) |
| Non-Hispanic Black | 517 (25.3) | 48 (39.3) | 469 (24.4) | 142 (35.9) | 7 (26.9) | 135 (36.5) |
| Hispanic | 576 (28.2) | 27 (22.1) | 549 (28.5) | 127 (32.1) | 6 (23.1) | 121 (32.7) |
| Asian/Pacific Islander | 390 (19.1) | 18 (14.8) | 372 (19.3) | 5 (1.3) | 1 (3.9) | 4 (1.1) |
| Pre-pregnancy BMI (kg/m2) | 23.6 ± 3.0 | 24.2 ± 3.1 | 23.6 ± 3.0 | 34.5 ± 3.9 | 35.9 ± 4.1 | 34.4 ± 3.8 |
| Parity | ||||||
| 0 | 1001 (48.9) | 68 (55.7) | 933 (48.5) | 136 (34.3) | 8 (30.8) | 128 (34.6) |
| 1 | 703 (34.4) | 32 (26.2) | 671 (34.9) | 133 (33.6) | 8 (30.8) | 125 (33.8) |
| 2+ | 342 (16.7) | 22 (18.0) | 320 (16.6) | 127 (32.1) | 10 (38.5) | 117 (31.6) |
| Education level | ||||||
| Less than college degree | 561 (27.4) | 32 (26.2) | 529 (27.5) | 151 (38.1) | 6 (23.1) | 145 (39.2) |
| Some college or undergraduate | 1107 (54.1) | 71 (58.2) | 1036 (53.9) | 213 (53.8) | 18 (69.2) | 195 (52.7) |
| Graduate or post-graduate | 378 (18.5) | 19(15.6) | 359 (18.7) | 32 (8.1) | 2 (7.7) | 30 (8.1) |
| Marital status a | ||||||
| Married or living with partner | 1565 (76.6) | 86 (70.5) | 1479 (77.0) | 266 (67.2) | 23 (88.5) | 243 (65.7) |
| Not married | 479 (23.4) | 36 (29.5) | 443 (23.1) | 130 (32.8) | 3 (11.5) | 127 (34.3) |
| Family income during last year | ||||||
| Less than $30,000 | 479 (23.4) | 28 (23.0) | 451 (23.4) | 129 (32.6) | 6 (23.1) | 123 (33.2) |
| $30,000-$49,999 | 294 (14.4) | 23 (18.9) | 271 (14.1) | 88 (22.2) | 7 (26.9) | 81 (21.9) |
| $50,000-$99,999 | 464 (22.7) | 30 (24.6) | 434 (22.6) | 93 (23.5) | 5 (19.2) | 88 (23.8) |
| $100,000 or more | 530 (25.9) | 24 (29.7) | 506 (26.3) | 52 (13.1) | 5 (19.2) | 47 (12.7) |
| Unknown | 279 (13.6) | 17 (13.9) | 262 (13.6) | 34 (8.6) | 3 (11.5) | 31 (8.4) |
| Plasma cotinine (ng/mL) a | 1.1 ± 12.6 | 0.6 ± 3.6 | 1.1 ± 13.0 | 3.4 ± 19.3 | 3.3 ± 14.4 | 3.4 ± 19.6 |
| Plasma total lipids (mg/dL) a | 609.9 ± 99.2 | 613.4 ± 104.8 | 609.7 ± 98.9 | 607.5 ± 101.7 | 636.8 ± 97.3 | 605.5 ± 101.8 |
| Alternative Healthy Eating Index (AHEI-2010) score a | 51.4 ± 9.7 | 50.0 ± 9.5 | 51.5 ± 9.7 | 49.2 ± 8.7 | 47.6 ± 8.3 | 49.3 ± 8.7 |
| Total activity (MET hours per week) a | 323.1 ± 167.9 | 351.9 ± 207.7 | 321.3 ± 165.0 | 330.8 ± 156.3 | 268.1 ± 127.4 | 335.2 ± 157.3 |
| Sedentary activity (MET hours per week) a | 26.1 ± 18.3 | 30.4 ± 19.5 | 25.8 ± 18.2 | 27.2 ± 18.7 | 29.0 ± 16.3 | 27.1 ± 18.9 |
| Acculturation a | ||||||
| U.S.-born | 1353 (66.2) | 89 (73.0) | 1264 (65.8) | 327 (82.6) | 20 (76.9) | 307 (83.0) |
| Recent immigrant (< 10 years) | 305 (14.9) | 12 (9.8) | 293 (15.3) | 24 (6.1) | 2 (7.7) | 22 (6.0) |
| Long-term immigrant (≥ 10 years) | 385 (18.8) | 21 (17.2) | 364 (19.0) | 45 (11.4) | 4 (15.4) | 41 (11.1) |
Means ± SD for continuous variables. N (%) for categorical variables.
GA = gestational age
Numbers may not add up to total numbers due to missing values. Variables with missing values (missing rate) included: marital status (0.1%), plasma cotinine (1.6%), plasma total lipids (1.1%), AHEI 2010 score (37.4%), total activity (0.2%), sedentary activity (0.2%), acculturation (0.1%).
Table 2 shows the distributions of lipid-adjusted PBDEs concentrations in the non-obese and obese cohorts. Chemicals that had quantification rates > 30% in each cohort (PBDE 28, 47, 99, 100, 153, and 154 among those without obesity; PBDE 47, 99, 100, 153, 154, and 183 among those with obesity) were included in the main analyses. In the non-obese cohort, PBDE congeners were detected in plasma samples in the decreasing order of PBDE 47, 100, 99, 153, 154, and 28. In the obese cohort, PBDE congeners were detected in plasma samples in the decreasing order of PBDE 153, 100, 154, 47, 99, and 183. Among the five congeners that had quantification rates > 30% in both cohorts, women with obesity had higher geometric means and higher quantification rates compared to women without obesity. The mean molar sum of PBDEs and total PBDEs were also higher in women with obesity than women without obesity. PBDEs concentrations were weakly to highly correlated with each other (Supplemental Figure S2). Concentrations for most of the PBDEs were lower than the concentrations from a representative sample of women from the U.S. National Health and Nutrition Examination Survey (NHANES) 2005-2014 (geometric means in NHANES for PBDE 28, 47, 99, 100, and 154 were: 1.2, 23.6, 4.7. 7.9 and 0.4 ng/g lipid, respectively), except for higher geometric mean of PBDE 154 in those with obesity (5.7 ng/g lipid, compared to 0.4 ng/g lipid in NHANES) (Sjödin et al., 2019).
Table 2.
Distributions of lipid-standardized PBDEs concentrations of the study population among the non-obese and obese cohorts.
| Chemicals, ng/g lipid | Non-obese cohort (n = 2046) |
Obese cohort (n=396) |
||||
|---|---|---|---|---|---|---|
| > LOQ n (%) | Geometric mean (95% CI)a | Median (IQR)b | > LOQ n (%) | Geometric mean (95% CI)a | Median (IQR)b | |
| PBB 153 | 25 (1.2) | 1.5 (1.5, 1.5) | 0 (0, 0) | 78 (19.7) | 0.2 (0.2, 0.2) | 0 (0, 0.6) |
| PBDE 28 | 749 (36.6) | 0.3 (0.2, 0.3) | 0 (0, 1.0) | 51 (12.9) | 0.4 (0.4, 0.5) | 0 (0, 0) |
| PBDE 47 | 1895 (92.6) | 5.6 (5.1, 6.1) | 8.7 (3.9, 17.4) | 370 (93.4) | 6.2 (5.4, 7.3) | 7.8 (3.1, 16.1) |
| PBDE 85 | 52 (2.5) | 0.4 (0.4, 0.4) | 0 (0, 0) | 0 (0.0) | 0 (0, 0) | 0 (0, 0) |
| PBDE 99 | 1267 (61.9) | 0.3 (0.3, 0.4) | 2.2 (0, 5.4) | 341 (86.1) | 2.3 (2.0, 2.5) | 2.3 (1.4, 4.5) |
| PBDE 100 | 1466 (71.7) | 0.9 (0.8, 0.9) | 2.2 (0, 4.3) | 392 (99.0) | 4.4 (4.1, 4.7) | 4.7 (3.0, 6.3) |
| PBDE 153 | 846 (41.4) | 1.2 (1.1, 1.3) | 0 (0, 7.0) | 393 (99.2) | 5.3 (4.8, 5.7) | 5.5 (3.0, 8.8) |
| PBDE 154 | 781 (38.2) | 0.1 (0.1, 0.1) | 0.4 (0, 2.8) | 378 (95.5) | 5.7 (5.3, 6.1) | 6.7 (5.0, 8.4) |
| PBDE 183 | 4 (0.2) | 3.6 (3.6, 3.6) | 0 (0, 0) | 319 (80.6) | 2.2 (1.9, 2.6) | 3.3 (0.8, 7.1) |
| PBDE 209 | 2 (0.1) | 947.2 (940.6, 953.9) | 0 (0, 0) | 0 (0.0) | 0 (0, 0) | 0 (0, 0) |
| Molar sum of PBDEs, pmol/g lipidc | / | 37.1 (35.3, 39.0) | 39.4 (18.5, 79.9) | / | 61.0 (57.2, 65.0) | 57.7 (39.8, 85.7) |
| Total PBDEs, ng/g lipidd | / | 19.8 (18.8, 20.8) | 21.2 (9.9, 43.3) | / | 35.6 (33.5, 37.9) | 34.6 (24.2, 49.3) |
Chemicals with >30% detection rate in each cohort (i.e. PBDE 28, 47, 99, 100, 153, and 154 in the non-obese cohort; PBDE 47, 99, 100, 153, 154 and 183 in the obese cohort) were selected for the main analyses, respectively.
Geometric means (95% CI) calculated using all machine-observed values including those below the LOQs, where zero and negative values were assigned the value of (lowest positive value)/2.
Percentiles and median calculated among all machine-observed values including those below the LOQs.
Molar sum of PBDEs (in pmol/g lipid) were calculated by dividing each lipid-standardized chemical concentration by its molecular weight and summing all detectable concentrations.
Total PBDEs (in ng/g lipid) were the sum of the detectable concentrations of all PBDE congeners.
Associations of individual PBDEs with gestational age at delivery and PTB
Table 3 displays the crude, covariate adjusted, and mutually adjusted results from linear regression models for the associations between individual PBDEs and gestational age at delivery. We found that a 1-SD increase in log-PBDE 153 concentration was associated with 0.19-week (~1.3 day) shorter gestational age at delivery in the mutually adjusted model among those without obesity [β (95% CI) = −0.19 (−0.31, −0.06) weeks], while there was no significant association between PBDE 153 and gestational age among those with obesity [β (95% CI) = 0.26 (−0.16, 0.68) weeks]. We did not observe any statistically significant associations of other PBDE congeners or the sum of PBDEs with gestational age at delivery in either cohort.
Table 3.
Association between each standard deviation increment in log transformed plasma PBDE concentrations and gestational age at delivery (weeks) among the non-obese and obese cohorts.
| Plasma PBDEs | Non-obese cohorta |
Obese cohorta |
||||
|---|---|---|---|---|---|---|
| Crude β (95% CI) (n =2008) | Adjustedb β (95% CI) (n =1975) | Mutually adjustedb, c β (95% CI) (n =1975) | Crude β (95% CI) (n =390) | Adjustedb β (95% CI) (n =356) | Mutually adjustedb, c β (95% CI) (n =356) | |
| PBDE 28 | −0.15 (−0.35, 0.05) | −0.12 (−0.33, 0.09) | 0.00 (−0.27, 0.28) | / d | / d | / d |
| PBDE 47 | −0.11 (−0.21, −0.01) | −0.07 (−0.18, 0.03) | −0.05 (−0.22, 0.12) | 0.06 (−0.33, 0.45) | 0.04 (−0.38, 0.45) | 0.07 (−0.80, 0.95) |
| PBDE 99 | −0.09 (−0.26, 0.08) | −0.05 (−0.23, 0.12) | −0.02 (−0.22, 0.17) | 0.03 (−0.59, 0.65) | 0.05 (−0.60, 0.70) | 0.20 (−1.20, 1.60) |
| PBDE 100 | −0.14 (−0.26, −0.02) | −0.11 (−0.23, 0.01) | 0.01 (−0.19, 0.22) | −0.27 (−0.80, 0.26) | 0.01 (−0.55, 0.57) | −0.34 (−1.30, 0.62) |
| PBDE 153 | −0.17 (−0.27, −0.06) | −0.17 (−0.28, −0.06) | −0.19 (−0.31, −0.06) | −0.01 (−0.33, 0.31) | 0.19 (−0.14, 0.52) | 0.26 (−0.16, 0.68) |
| PBDE 154 | 0.05 (−0.04, 0.13) | 0.04 (−0.05, 0.13) | 0.08 (−0.01, 0.17) | −0.12 (−0.33, 0.09) | −0.21 (−0.43, 0.02) | −0.20 (−0.44, 0.03) |
| PBDE 183 | /d | / d | / d | −0.04 (−0.25, 0.19) | 0.01 (−0.22, 0.23) | −0.09 (−0.35, 0.16) |
| Molar sum of PBDEs | −0.11 (−0.27, 0.04) | −0.06 (−0.22, 0.10) | / | −0.01 (−0.69, 0.68) | 0.07 (−0.64, 0.79) | / |
| Total PBDEs | −0.13 (−0.35, 0.08) | −0.06 (−0.28, 0.15) | / | 0.03 (−1.92, 1.87) | 0.38 (−1.63, 2.38) | / |
SD = standard deviation; CI = confidence interval; β estimates represent the mean differences in gestational age at delivery per 1-SD increase in log transformed plasma concentrations of each of the PBDEs.
For all statistical analyses on the associations of PBDEs with gestational age at delivery and preterm birth, we excluded observations with PBDEs concentrations greater than 5 SDs from the mean values to avoid potential distortion of extreme values.
Adjusted for maternal age (years), race/ethnicity (Non-Hispanic white, Non-Hispanic Black, Hispanic, Asian/Pacific Islander), pre-pregnancy BMI (kg/m2), parity (0, 1, 2+), education level (<college degree, some college/undergraduate, graduate/post-graduate), marital status (married or living with partner, not married), family income during last year (<$30,000, $30,000-$49,999, $50,000-$99,999, $100,000 or more, not reported), plasma cotinine level (ng/mL), plasma total lipids (ng/mL), total activity (MET hours per week), sedentary activity (MET hours per week), and acculturation (US-born, recent immigrant, long-term immigrant). Observations with missing covariates were excluded from the adjusted models.
Additionally adjusted for all the other PBDEs.
Results not available, as only congeners with >30% detection rate in each cohort (i.e. PBDE 28, 47, 99, 100, 153, and 154 in the non-obese cohort; PBDE 47, 99, 100, 153, 154 and 183 in the obese cohort) were selected for the main analyses, respectively.
Results from sensitivity analyses of associations of PBDE concentrations divided into quartiles above the LOQ with gestational age at delivery are presented in Supplemental Table S2. Among those without obesity, we found that the adjusted average gestational age at delivery in the highest quartile of PBDE 153 was 0.34-week shorter than that in the category below the LOQ (p-trend < 0.01). In addition, among those with obesity, we found that the adjusted average gestational age at delivery in the highest quartile of PBDE 183 was 0.91-week shorter than that in the category below the LOQ (p-trend = 0.02).
Table 4 shows the results from logistic regression models for the association between individual PBDEs and the odds of PTB. We did not observe any statistically significant associations of any PBDEs with the odds of PTB in either cohort.
Table 4.
Association between each standard deviation increment in log transformed plasma PBDEs concentrations and odds of PTB among the non-obese and obese cohorts.
| Plasma PBDEs | Non-obese cohorta |
Obese cohorta |
||||
|---|---|---|---|---|---|---|
| Crude OR (95% CI) (n=2008) | Adjustedb OR (95% CI) (n=1975) | Mutually adjustedb, c OR (95% CI) (n=1975) | Crude OR (95% CI) (n=390) | Adjustedb OR (95% CI) (n=356) | Mutually adjustedb, c OR (95% CI) (n=356) | |
| PBDE 28 | 1.20 (0.72, 1.90) | 1.02 (0.58, 1.69) | 0.87 (0.42, 1.73) | / d | / d | / d |
| PBDE 47 | 1.17 (0.92, 1.44) | 1.04 (0.79, 1.32) | 1.01 (0.66, 1.49) | 0.54 (0.14, 1.35) | 0.61 (0.13, 1.79) | 1.05 (0.11, 9.59) |
| PBDE 99 | 1.10 (0.70, 1.64) | 0.98 (0.64, 1.47) | 0.95 (0.59, 1.51) | 0.20 (0.01, 1.27) | 0.16 (0.01, 1.54) | 0.02 (0.00, 1.66) |
| PBDE 100 | 1.23 (0.93, 1.58) | 1.12 (0.83, 1.46) | 1.10 (0.68, 1.74) | 1.49 (0.57, 3.26) | 1.59 (0.42, 4.41) | 7.23 (0.64, 91.1) |
| PBDE 153 | 1.21 (0.95, 1.50) | 1.19 (0.92, 1.50) | 1.20 (0.89, 1.59) | 1.23 (0.68, 1.97) | 1.21 (0.57, 2.24) | 1.05 (0.40, 2.41) |
| PBDE 154 | 0.91 (0.71, 1.14) | 0.89 (0.69, 1.12) | 0.85 (0.66, 1.09) | 1.26 (0.83, 2.02) | 1.34 (0.79, 2.40) | 1.38 (0.78, 2.55) |
| PBDE 183 | /d | /d | /d | 0.85 (0.50, 1.29) | 0.77 (0.40, 1.30) | 1.11 (0.51, 1.17) |
| Molar sum of PBDEs | 1.13 (0.79, 1.46) | 0.98 (0.64, 1.29) | / | 0.49 (0.06, 2.02) | 0.50 (0.03, 2.98) | / |
| Total PBDEs | 1.14 (0.66, 1.58) | 0.97 (0.49, 1.38) | / | 0.24 (0.01, 9.29) | 0.24 (0.00, 25.4) | / |
SD = standard deviation; OR = odds ratio; CI = confidence interval; OR estimates represent the odds ratio of PTB per 1-SD increase in log transformed plasma concentrations of each of the PBDEs.
For all statistical analyses on the associations of PBDEs with gestational age at delivery and preterm birth, we excluded observations with PBDEs concentrations greater than 5 SDs from the mean values to avoid potential distortion of extreme values.
Adjusted for maternal age (years), race/ethnicity (Non-Hispanic white, Non-Hispanic Black, Hispanic, Asian/Pacific Islander), pre-pregnancy BMI (kg/m2), parity (0, 1, 2+), education level (<college degree, some college/undergraduate, graduate/post-graduate), marital status (married or living with partner, not married), family income during last year (<$30,000, $30,000-$49,999, $50,000-$99,999, $100,000 or more, not reported), plasma cotinine level (ng/mL), plasma total lipids (ng/mL), total activity (MET hours per week), sedentary activity (MET hours per week), and acculturation (US-born, recent immigrant, long-term immigrant). Observations with missing covariates were excluded from the adjusted models.
Additionally adjusted for all the other PBDEs.
Results not available (/) for this congener in this cohort, as only congeners with >30% detection rate in each cohort (i.e. PBDE 28, 47, 99, 100, 153, and 154 in the non-obese cohort; PBDE 47, 99, 100, 153, 154 and 183 in the obese cohort) were selected for the main analyses, respectively.
Principal component analysis of PBDEs with gestational age at delivery and PTB
In each cohort, application of varimax-rotated PCA yielded four principal components (PCs) that cumulatively accounted for > 90% of the variability in PBDE concentrations. Loading factors for each chemical in each of the PCs are presented in Supplemental Table S3. The associations of the PCs as predictors of gestational age at delivery (from linear regression models) and the odds of PTB (from logistic regression models) are presented in Figure 1 and Supplemental Table S4. Among those without obesity, we found that a 1-SD increase in PC2 scores (with high loadings for PBDE 153 and 100) were associated with shorter gestational age at delivery [adjusted β (95% CI) = −0.18 (−0.30, −0.06) weeks]. However, none of the four PCs was significantly associated with odds of PTB. Among those with obesity, we did not observe statistically significant association between the PCs with gestational age at delivery or PTB.
Figure 1.
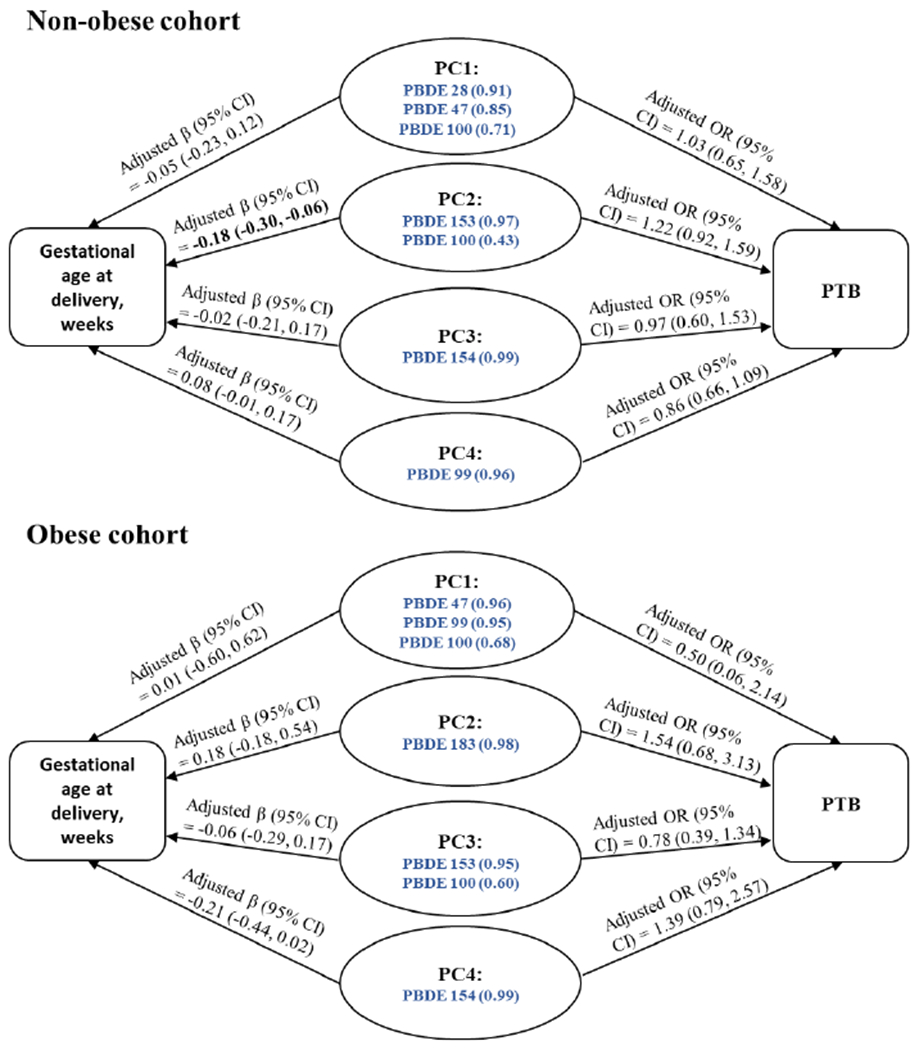
Adjusted associations between each standard deviation increment in principle components scores of PBDEs and gestational age at delivery (weeks) or odds of preterm birth among the non-obese and obese cohorts.
PC = principal component; SD = standard deviation; PC = principle component; PTB = preterm birth; CI = confidence interval; OR = odds ratio; β estimates represent the mean differences in gestational age at delivery per 1-SD increase in each of the PC scores. ORs represent the odds ratio of PTB per 1-SD increase in each of the PC scores. For each PC, blue, bolded PBDE congeners (loadings) with varimax-rotated |loadings| > 0.4 are listed. All estimates (95% CIs) adjusted for maternal age (years), race/ethnicity (Non-Hispanic white, Non-Hispanic Black, Hispanic, Asian/Pacific Islander), pre-pregnancy BMI (kg/m2), parity (0, 1, 2+), education level (<college degree, some college/undergraduate, graduate/post-graduate), marital status (married or living with partner, not married), family income during last year (<$30,000, $30,000-$49,999, $50,000-$99,999, $100,000 or more, not reported), plasma cotinine level (ng/mL), plasma total lipids (ng/mL), total activity (MET hours per week), sedentary activity (MET hours per week), and acculturation (US-born, recent immigrant, long-term immigrant). Observations with missing covariates were excluded from the adjusted models.
PBDE mixtures and gestational age at delivery from BKMR
Based on the estimated PIPs from the BKMR analyses, PBDE 153 was considered as the most important contributor to the overall association between the PBDE mixtures and gestational age at delivery among those without obesity (PIP = 0.90), while PBDE 154 was considered the most important contributor among those with obesity (PIP = 0.44). Figure 2 shows the results from BKMR modelling PBDEs as mixtures, where the covariate-adjusted univariate exposure-response functions and 95% credible intervals for each chemical and standardized gestational age at delivery are presented separately for each cohort, while holding all other PBDEs at their median concentrations. We found that: (1) Among women without obesity, there was a suggestive relationship of higher PBDE 153 concentrations with shorter gestational age at delivery. (2) Among women without obesity, there was a suggestive non-linear relationship between PBDE 154 and gestational age, with a positive association at lower concentrations and an inverse association at higher concentrations. (3) Among women with obesity, there was a suggestive relationship of higher PBDE 154 and 183 concentrations with shorter gestational age at delivery. However, in both cohorts, the 95% credible intervals of the differences in gestational age included zero, when comparing each chemical’s 90th percentile to the 10th percentile while holding all other chemicals at the median (Supplemental Figure S3). In addition, no apparent trend was observed in either cohort when evaluating the overall joint effect of the PBDE mixture on gestational age at delivery (Supplemental Figure S4).
Figure 2.
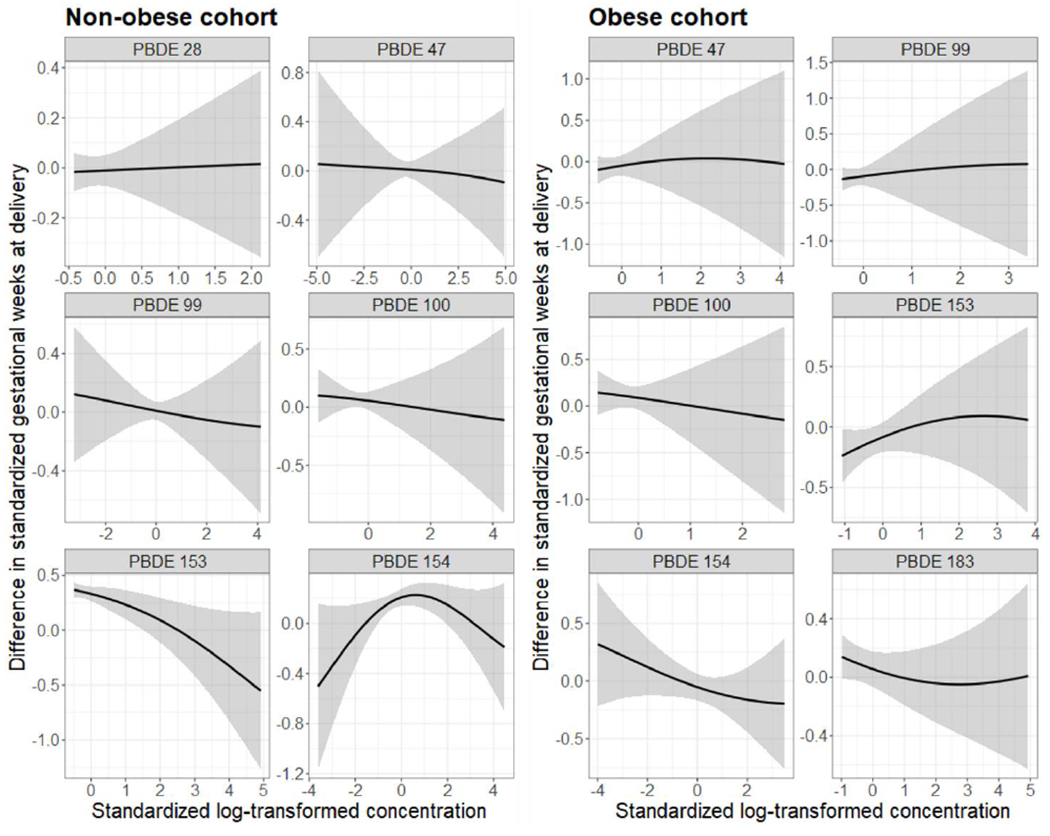
Univariate exposure-response functions (95% credible intervals) between PBDEs concentrations and standardized gestational age at delivery estimated by BKMR, holding all other congeners at the median levels, among the non-obese cohort (left, n = 1975) and the obese cohort (right, n = 356).
All estimates (95% CIs) adjusted for maternal age (years), race/ethnicity (Non-Hispanic white, Non-Hispanic Black, Hispanic, Asian/Pacific Islander), pre-pregnancy BMI (kg/m2), parity (0, 1, 2+), education level (<college degree, some college/undergraduate, graduate/post-graduate), marital status (married or living with partner, not married), family income during last year (< $30,000, $30,000-$49,999, $50,000-$99,999, $100,000 or more, not reported), plasma cotinine level (ng/mL), plasma total lipids (ng/mL), total activity (MET hours per week), sedentary activity (MET hours per week), and acculturation (US-born, recent immigrant, long-term immigrant). Observations with missing covariates were excluded from the adjusted models.
To explore interactions between chemicals, we plotted the exposure-response functions of each chemical with a second chemical fixed at various percentiles (25th, 50th, 75th), holding all other chemicals at the median. We observed a potential interaction between PBDEs 100 and 154 among those without obesity (Figure 3A), where there was a stronger relationship of higher PBDE 100 concentrations with shorter gestational age at delivery when PBDE 154 concentrations were fixed at lower levels. We also observed a potential interaction between PBDEs 154 and 183 among those with obesity (Figure 3B), where the suggestive relationship of higher PBDE 154 concentrations with shorter gestational age at delivery was stronger when the PBDE 183 concentrations were fixed at higher levels.
Figure 3A.
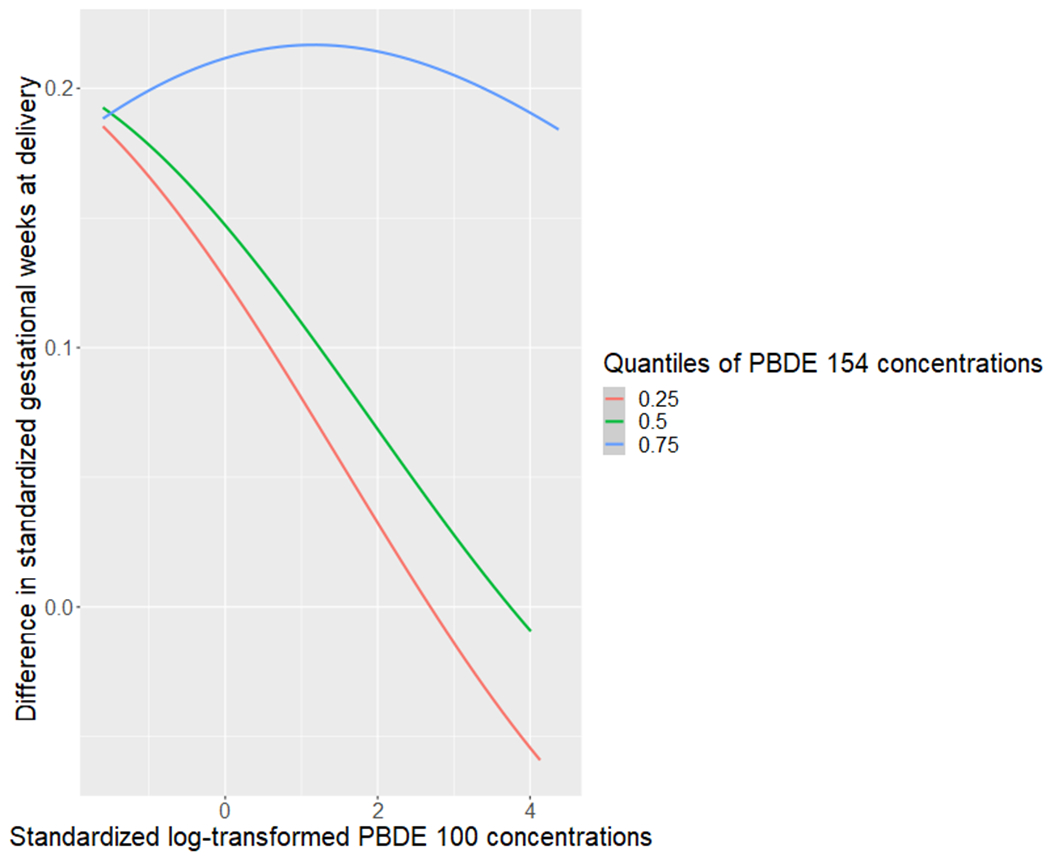
Exposure-response functions between PBDE 100 and gestational age at delivery, when fixing PBDE 154 concentrations at the 25th, 50th, and 75th percentiles, and all other congeners at the median, estimated with BKMR in the non-obese cohort.
Figure 3B.
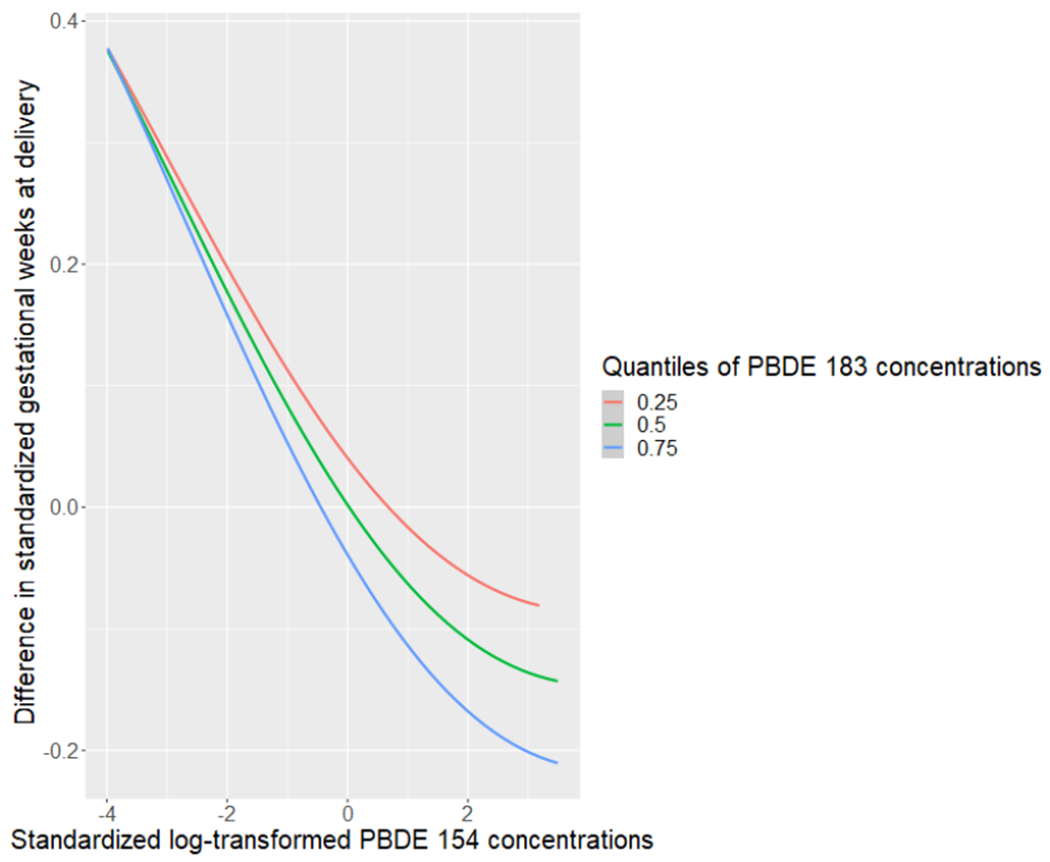
Exposure-response functions between PBDE 154 and gestational age at delivery, when fixing PBDE 183 concentrations at the 25th, 50th, and 75th percentiles, and all other congeners at the median, estimated with BKMR in the obese cohort.
Secondary and sensitivity analyses
The associations between the PCs from PCA and categories of PTB are presented in Supplemental Tables S4 and S5. We found that among those without obesity, the PC2 (predominantly contributed by PBDEs 153 and 100) scores were associated with higher odds of very early/moderate PTB [OR (95% CI) = 1.91 (1.19, 3.07)], but not significantly associated with odds of late PTB (Supplemental Table S5). We also found that the PC2 scores were marginally associated with higher odds of spontaneous PTB [OR (95% CI) = 1.34 (1.00, 1.80)], but not significantly associated with the odds of medically indicated PTB among those without obesity (Supplemental Table S6).
Supplemental Table S7 presents the associations between the PCs and gestational age at delivery, stratified by race/ethnicity. There was statistically significant effect modification (p-for-interaction = 0.01) by race/ethnicity in the non-obese cohort for the relationship between PC4 (predominantly contributed by PBDE 99) and gestational age, where there was a positive association between PC4 and gestational age only among non-Hispanic Black women [β (95% CI) = 0.33 (0.09, 0.58)]. We also observed a significant inverse association between the PC2 scores and gestational age only among non-Hispanic Black women in the non-obese cohort [β (95% CI) = −0.40 (−0.67, −0.12)]. There was statistically significant effect modification by maternal age (p-for-interaction <0.01) for the associations between higher PC2 scores and shorter gestational age in the non-obese cohort, where the associations appeared stronger among those aged ≥ 35 years compared to those aged < 35 years (Supplemental Table S8). In addition, the associations between the PC2 scores and gestational age were also modified by BMI (p-for-interaction = 0.04) in the non-obese cohort, where the associations appeared stronger among those with BMI ≥ 25 kg/m2 compared to those with BMI < 25 kg/m2 (Supplemental Table S9).
In sensitivity analyses, we identified a few dietary factors that were weakly correlated with PBDE concentrations among a subset of women who completed a baseline FFQ (Supplemental Figure S5). Further adjusting for the Alternative Healthy Eating Index (AHEI-2010) score and these dietary factors resulted in stronger inverse associations of PBDE 153 with gestational age among those without obesity (Supplemental Table S10). In addition, not adjusting for plasma total lipids had very little impact on the effect estimates in either cohort (data not shown). In the survival analysis evaluating gestational age at delivery as a time-to-event outcome, we found similar associations between PBDE 153 and time-to-delivery [adjusted hazard ratio (95% CI) = 1.10 (1.02, 1.19)] in the non-obese cohort. Lastly, including data from individuals with extreme values of PBDEs yielded results in similar directions and magnitudes (data not shown).
DISCUSSION
In this study of pregnant U.S. women from a large, multi-center, multi-racial/ethnic cohort, we observed associations of higher concentrations of plasma PBDE 153 in early pregnancy with shorter gestational age at delivery among women without obesity, via different statistical approaches that evaluated individual and mixture effects of PBDEs. We also observed associations of higher levels PBDE 153 and 100 (represented by PC2 from PCA) with higher odds of very early/moderate PTB and spontaneous PTB among women without obesity. There was also a suggestive non-linear dose-response relationship between PBDE 154 concentrations and gestational age at delivery among women without obesity. On the other hand, in the smaller sized obese cohort, there was no statistically significant association among women with obesity, although suggestive inverse dose-response relationships of PBDE 154, 183 and their interactions with gestational age at delivery were observed in BKMR analyses.
Previous epidemiological studies that evaluated certain PBDEs and PTB or gestational age at delivery have reported inconsistent results. One longitudinal cohort study of low income and predominantly Hispanic women recruited during 1999-2000 (n = 286) evaluated four serum PBDE congeners (PBDE 47, 99, 100, 153, and their molar sum) in the second trimester (mean: 26.1 weeks), and found no association with gestational age at delivery (Harley et al., 2011). In contrast, another cohort study during 2014-2018 (n = 506) that evaluated serum PBDE 47 and 99 in the second trimester (range: 12-28 weeks) found an inverse association between PBDE 47 and gestational age at delivery (Eick et al., 2020). A smaller sized case-control study (n = 138) during 2008-2011 that specifically measured PBDE 47 (collected at delivery) found higher concentrations associated with a higher odds of PTB (Peltier et al., 2015). In addition, a study conducted at a brominated flame retardant production area in China during 2010-2012 (n = 207) showed a positive association between PTB and serum PBDE 153 (collected at delivery), but not with other PBDE congeners (Gao et al., 2016). However, these studies were limited to evaluating the individual effects of certain PBDE congeners, or only used an additive summary measure of multiple chemicals, without taking into account the potential correlations and interactions between these chemicals. Moreover, most of the existing studies had relatively small sample sizes and might not have sufficient power to detect any racial/ethnic disparities. Our study addresses these limitations via evaluating the effect of PBDEs, both individually and as a mixture, on gestational age and PTB in a larger, multi-center, multi-racial/ethnic cohort of U.S. pregnant women.
In our study, we utilized three different statistical approaches to evaluate the individual and joint associations of PBDEs with gestational age and PTB. We found statistically significant or suggestive evidence of associations of plasma PBDE 153 concentrations with shorter gestational age at delivery among women without obesity from all three methods. Despite the fact that a 0.19-week reduction in gestational age at delivery might be of less clinical relevance, we also found significant evidence of PBDE 153 and 100 (represented by PC2) associated with higher odds of very early/moderate PTB and spontaneous PTB, which carries important clinical implications. These findings suggest a potential adverse role of PBDE 153 on gestation length/PTB. PTB is a complex phenotype that involves multiple etiologic factors acting through multiple pathophysiological pathways. One pathway is through inflammation, where higher levels of cytokines promotes labor through stimulating the production of prostaglandin that leads to cervical dilation, stimulating the expression of oxytocin receptors, as well as inhibiting adhesions in fibroblasts that lead to premature membrane rupture (Farina and Winkelman, 2005). Another pathway involves placental dysfunction, where abnormal uterine spiral artery remodeling contributes to several adverse pregnancy outcomes, including PTB (Morgan, 2016). PBDE 153 has been shown to play a role in both pathways. For example, PBDE 153 has been shown to increase the production of reactive oxygen species (Huang et al., 2010), contributing to the inflammatory pathway that may result in spontaneous PTB, which is consistent with our findings. In addition, PBDE 153 has been found to alter DNA methylation of the IGF2 gene that plays a role in placental development and fetal growth (Zhao et al., 2016), potentially contributing to the progression of medically indicated PTB. However, we did not any observe significant association between PBDE 153 and medically indicated PTB. Additional mechanistic studies are needed to further explore and validate these findings.
We also found a suggestive inverse U-shaped dose-response relationship between PBDE 154 and gestational age among women without obesity from BKMR analyses, with a similar non-linear pattern observed in the sensitivity analysis categorizing PBDE 154 into quantiles. The scientific literature on the toxicity of PBDE 154 is lacking, with only one study that showed PBDE 154 was less toxic than PBDE 47 in inducing apoptotic cell death in liver cells (Souza et al., 2016). To date, no studies have evaluated the toxicity of PBDE 154 in the placenta or other tissues. On the other hand, we did not find any significant association between PBDE 47 and gestational age or PTB, despite PBDE 47 having the highest detection rate among those without obesity. A case-control study found PBDE 47 associated with higher odds of PTB (Peltier et al., 2015). However, this study had a small sample size (n = 138) and collection of PBDE 47 was cross-sectional (at delivery). One study conducted in rats demonstrated that for CYP1A1, an oxidative stress-related gene that was found to be associated with PTB risk (Sheikh et al., 2016), its expression level was positively correlated with increased bromine content of the three PBDE congeners (PBDE 47, 99, or 153) (Sanders et al., 2005), pointing to potentially stronger adverse effects on PTB comparing PBDE 153 to PBDE 47. However, further research in comparing the toxicity of PBDE congeners in human cell lines or tissues relevant to pregnancy outcomes is warranted to complement epidemiological findings.
Unlike the associations among women without obesity, we did not observe statistically significant associations of PBDE 153 with gestational age at delivery or PTB among women with obesity. The analyses in the obese cohort were limited by lower power due to a much smaller sample size. However, we did find effect modification by BMI in the non-obese cohort, suggesting some modification potentially related with adiposity. In addition, a suggestive yet consistent dose-response relationship of PBDE 154 and gestational age at delivery were observed in the obese cohort, across all three statistical approaches. Within women with obesity, each 1 SD increase in log PBDE 154 corresponded to a 0.20-week decrease in gestational age at delivery; this finding was similar to the 0.21-week decrease in gestational age at delivery observed for PC4 (which consisted almost entirely of PBDE 154) from the PCA, and similar linear patterns were suggested by the BKMR analyses. One possible explanation for the differences observed between the obese and non-obese cohorts might be that the obese cohort had higher detections and mean concentrations of PBDEs (particularly PBDE 153 and 154) than the non-obese cohort, and therefore might be capturing different patterns of the dose-response relationships between these chemicals and gestational age at delivery. In addition, the two cohorts had different eligibility criteria where the non-obese cohort excluded more medical conditions, some of which might be risk factors for adverse pregnancy outcomes including PTB. Furthermore, PBDEs have been shown to alter adipocyte metabolism, a hallmark feature of metabolic obesity (Hoppe and Carey, 2007). Therefore, it is possible that PBDEs interact with unmeasured metabolic features related to adiposity or lifestyle factors that lead to different effects on gestational length in individuals with and without obesity. Studies in rodents also have shown that different PBDE congeners may have different disposition patterns (e.g. more PBDE 47 distributed to adipose tissue, while more PBDE 153 accumulated in the liver) (U.S. EPA., 2008). Thus, future studies beyond plasma measurements of PBDEs, such as measurements in adipose tissue, might be helpful to further investigate their potential effects in women with obesity.
We observed effect modification by race/ethnicity for the association of PBDEs with gestational age at delivery among women without obesity. A positive association between PBDE 99 (presented by PC4) and gestational age at delivery was found only among non-Hispanic Black women. We also observed a significant inverse association between the PC2 (predominantly contributed by PBDEs 153 and 100) scores and gestational age only among non-Hispanic Black women in the non-obese cohort. Potential explanations may include higher exposure levels to these chemicals (Nguyen et al., 2020) among non-Hispanic black women, as well as potential interactions of higher PBDE concentrations with factors such as diet/micronutrients (e.g. vitamin D deficiency was shown to be associated with PTB in some observational studies, and vitamin D deficiency is more common among black women) (Burris et al., 2019), higher psychosocial stress levels (Forrester et al., 2019; Gump et al., 2014) and higher exposures to other pollutants (Muller et al., 2018; Stieb et al., 2012) that might jointly impact non-Hispanic Black women to disproportionately increase the risk of adverse pregnancy outcomes (Grobman et al., 2018). We also observed significant effect modification by age, suggesting that women with older age at pregnancy might be more susceptible to the potential adverse effect of PBDE 153 on shorter gestation length.
Our study had some limitations. First, chemical concentrations were measured only once during the first trimester, which may have misclassified exposure for some women at biologically relevant windows. Nevertheless, PBDEs are persistent chemicals that are expected to be stable over time with long half-lives (Chevrier et al., 2010). Second, this study population constitutes low-risk pregnancies only; thus, the results may not be generalizable to higher-risk populations. Third, potential batch effects may exist for comparing chemical concentrations in the non-obese and obese cohorts given that chemical assays were performed at different locations multiple years apart. However, inter-lab variability is less likely given the assay was performed by the same laboratory scientist with the same QA/QC standards. Fourth, type 1 errors might occur from multiple testing, but our findings were consistent across three different statistical approaches. Fifth, the arbitrary cutoff of 30% above the LOQ for selection of PBDE congeners to be included in the main analyses was lower compared to other studies. Furthermore, the obese cohort consisted of a much smaller sample size compared to the non-obese cohort. We therefore have less power to detect any potential associations in the obese cohort even if they did exist (e.g. the suggestive yet non-significant relations between PBDE 154 and gestational age at delivery among those with obesity could be due to insufficient power). Lastly, selection bias may occur from restricting to live births, since PBDEs were shown to increase the risk of pregnancy loss (Choi et al., 2019), and unmeasured or residual confounding may exist.
Despite these limitations, our study has several strengths. First, the relatively large sample size of pregnant U.S. women allowed us to apply mixtures analyses to estimate the association of multiple PBDE congeners with gestational age at delivery and PTB in addition to evaluating individual effects. Second, this study population consists of four relatively evenly distributed racial/ethnic groups, which allowed for sufficient power to detect effect measure modification by race/ethnicity in the non-obese cohort. The large sample size also allowed us to evaluate potential effect modification by other factors, such as age and BMI. Third, we were able to evaluate clinically confirmed endpoints classified by PTB subtypes as secondary outcomes. Lastly, compared to other studies that measured PBDEs concentrations during the second trimester or upon delivery, the prospective design of this cohort minimized the possibility of reverse causation, and the comprehensive questionnaire and biomarker data allowed for proper control of confounding.
CONCLUSIONS
In conclusion, using different statistical approaches that evaluated individual and mixture effects of PBDEs, we found evidence of associations of higher early pregnancy plasma PBDE 153 concentrations with shorter gestational age at delivery and higher odds of very early/moderate PTB and spontaneous PTB among women without obesity in a cohort of low-risk U.S. pregnant women. These associations were stronger among non-Hispanic Black women, women with BMI ranging between 25-30 kg/m2, and women aged ≥ 35 years. Suggestive inverse relationships of PBDE 154 with gestational age at delivery were observed among women with obesity. Given the ubiquitous presence of PBDEs and the increasing disparities in PTB, further research is needed to understand the effects of exposures to PBDEs on gestation length, especially in high exposure and high outcome risk subgroups.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge the research teams at all participating clinical centers in the NICHD Fetal Growth Studies, including Christina Care Health Systems, University of California, Irvine, Long Beach Memorial Medical Center, Northwestern University, Medical University of South Carolina, Columbia University, New York Presbyterian Queens, Queens, St. Peters’ University Hospital, University of Alabama at Birmingham, Women and Infants Hospital of Rhode Island, Fountain Valley Regional Hospital and Medical Center, and Tufts University. The authors also acknowledge C-TASC and The EMMES Corporations in providing data and imaging support for this multi-site study.
FUNDING
This work is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Intramural Research Program (contract numbers HHSN275200800013C, HHSN275200800002I, HHSN27500006, HHSN275200800003IC, HHSN275200800014C, HHSN275200800012C, HHSN275200800028C, HHSN275201000009C, HHSN275291199991I and HHSN275201000001Z), the National Institute of Environmental Health Sciences (contract number P30ES000002), and the Office of the Director, National Institutes of Health under Award Number UG3OD023316. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health. Blair J. Wylie is supported by NIEHS R01 ES028688.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF COMPETING INTEREST
None.
REFERENCES
- Akins JR, Waldrep K, Bernert JT, 1989. The estimation of total serum lipids by a completely enzymatic “summation” method. Clin. Chim. Acta Int. J. Clin. Chem 184, 219–226. 10.1016/0009-8981(89)90054-5 [DOI] [PubMed] [Google Scholar]
- Ananth CV, Friedman AM, Gyamfi-Bannerman C, 2013. Epidemiology of Moderate Preterm, Late Preterm and Early Term Delivery. Clin. Perinatol 40, 601–610. 10.1016/j.clp.2013.07.001 [DOI] [PubMed] [Google Scholar]
- Bao W, Dar S, Zhu Y, Wu J, Rawal S, Li S, Weir NL, Tsai MY, Zhang C, 2018. Plasma concentrations of lipids during pregnancy and the risk of gestational diabetes mellitus: A longitudinal study. J. Diabetes 10, 487–495. 10.1111/1753-0407.12563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernert JT, Turner WE, Patterson DG, Needham LL, 2007. Calculation of serum “total lipid” concentrations for the adjustment of persistent organohalogen toxicant measurements in human samples. Chemosphere 68, 824–831. 10.1016/j.chemosphere.2007.02.043 [DOI] [PubMed] [Google Scholar]
- Bobb JF, Claus Henn B, Valeri L, Coull BA, 2018. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health 17, 67. 10.1186/s12940-018-0413-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, Godleski JJ, Coull BA, 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostat. Oxf. Engl 16, 493–508. 10.1093/biostatistics/kxu058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle EM, Poulsen G, Field DJ, Kurinczuk JJ, Wolke D, Alfirevic Z, Quigley MA, 2012. Effects of gestational age at birth on health outcomes at 3 and 5 years of age: population based cohort study. BMJ 344, e896. 10.1136/bmj.e896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Zhai S, Smarr MM, Grewal J, Zhang C, Grantz KL, Hinkle SN, Sundaram R, Lee S, Honda M, Oh J, Kannan K, 2018. Endocrine disruptors and neonatal anthropometry, NICHD Fetal Growth Studies - Singletons. Environ. Int 119, 515–526. 10.1016/j.envint.2018.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris HH, Collins JW, Wright RO, 2011. Racial/ethnic disparities in preterm birth: clues from environmental exposures. Curr. Opin. Pediatr 23, 227–232. 10.1097/MOP.0b013e328344568f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris HH, Lorch SA, Kirpalani H, Pursley DM, Elovitz MA, Clougherty JE, 2019. Racial disparities in preterm birth in USA: a biosensor of physical and social environmental exposures. Arch. Dis. Child 104, 931–935. 10.1136/archdischild-2018-316486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier J, Harley KG, Bradman A, Gharbi M, Sjödin A, Eskenazi B, 2010. Polybrominated Diphenyl Ether (PBDE) Flame Retardants and Thyroid Hormone during Pregnancy. Environ. Health Perspect 118, 1444–1449. 10.1289/ehp.1001905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y-H, Bellavia A, James-Todd T, Correia KF, Valeri L, Messerlian C, Ford JB, Mínguez-Alarcón L, Calafat AM, Hauser R, Williams PL, 2018. Evaluating effects of prenatal exposure to phthalate mixtures on birth weight: A comparison of three statistical approaches. Environ. Int 113, 231–239. 10.1016/j.envint.2018.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G, Wang Y-B, Sundaram R, Chen Z, Barr DB, Buck Louis GM, Smarr MM, 2019. Polybrominated diphenyl ethers and incident pregnancy loss: The LIFE Study. Environ. Res 168, 375–381. 10.1016/j.envres.2018.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowens KR, Simpson S, Thomas WK, Carey GB, 2015. Polybrominated Diphenyl Ether (PBDE)-Induced Suppression of Phosphoenolpyruvate Carboxykinase (PEPCK) Decreases Hepatic Glyceroneogenesis and Disrupts Hepatic Lipid Homeostasis. J. Toxicol. Environ. Health A 78, 1437–1449. 10.1080/15287394.2015.1098580 [DOI] [PubMed] [Google Scholar]
- Darnerud PO, Eriksen GS, Jóhannesson T, Larsen PB, Viluksela M, 2001. Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ. Health Perspect 109, 49–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick SM, Hom Thepaksorn EK, Izano MA, Cushing LJ, Wang Y, Smith SC, Gao S, Park J-S, Padula AM, DeMicco E, Valeri L, Woodruff TJ, Morello-Frosch R, 2020. Associations between prenatal maternal exposure to per- and polyfluoroalkyl substances (PFAS) and polybrominated diphenyl ethers (PBDEs) and birth outcomes among pregnant women in San Francisco. Environ. Health 19, 100. 10.1186/s12940-020-00654-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C, Trujillo C, Sjödin A, Bradman A, 2013. In Utero and Childhood Polybrominated Diphenyl Ether (PBDE) Exposures and Neurodevelopment in the CHAMACOS Study. Environ. Health Perspect 121, 257–262. 10.1289/ehp.1205597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina L, Winkelman C, 2005. A review of the role of proinflammatory cytokines in labor and noninfectious preterm labor. Biol. Res. Nurs 6, 230–238. 10.1177/1099800404271900 [DOI] [PubMed] [Google Scholar]
- Ferguson KK, O’Neill MS, Meeker JD, 2013. Environmental contaminant exposures and preterm birth: A comprehensive review. J. Toxicol. Environ. Health B Crit. Rev 16, 69–113. 10.1080/10937404.2013.775048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero DM, Larson J, Jacobsson B, Di Renzo GC, Norman JE, Martin JN, D’Alton M, Castelazo E, Howson CP, Sengpiel V, Bottai M, Mayo JA, Shaw GM, Verdenik I, Tul N, Velebil P, Cairns-Smith S, Rushwan H, Arulkumaran S, Howse JL, Simpson JL, 2016. Cross-Country Individual Participant Analysis of 4.1 Million Singleton Births in 5 Countries with Very High Human Development Index Confirms Known Associations but Provides No Biologic Explanation for 2/3 of All Preterm Births. PLoS ONE 11, e0162506. 10.1371/journal.pone.0162506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester S, Jacobs D, Zmora R, Schreiner P, Roger V, Kiefe CI, 2019. Racial differences in weathering and its associations with psychosocial stress: The CARDIA study. SSM - Popul. Health 7, 100319. 10.1016/j.ssmph.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Chen L, Wang C, Zhou Y, Wang Y, Zhang Y, Hu Y, Ji L, Shi R, Cui C, Ding G, Jin J, Tian Y, 2016. Exposure to polybrominated diphenyl ethers and female reproductive function: A study in the production area of Shandong, China. Sci. Total Environ 572, 9–15. 10.1016/j.scitotenv.2016.07.181 [DOI] [PubMed] [Google Scholar]
- Gete DG, Waller M, Mishra GD, 2020. Effects of maternal diets on preterm birth and low birth weight: a systematic review. Br. J. Nutr 123, 446–461. 10.1017/S0007114519002897 [DOI] [PubMed] [Google Scholar]
- Grewal J, Grantz KL, Zhang C, Sciscione A, Wing DA, Grobman WA, Newman RB, Wapner R, D’Alton ME, Skupski D, Nageotte MP, Ranzini AC, Owen J, Chien EK, Craigo S, Albert PS, Kim S, Hediger ML, Buck Louis GM, 2018. Cohort Profile: NICHD Fetal Growth Studies–Singletons and Twins. Int. J. Epidemiol 47, 25–251. 10.1093/ije/dyx161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobman WA, Parker CB, Willinger M, Wing DA, Silver RM, Wapner RJ, Simhan HN, Parry S, Mercer BM, Haas DM, Peaceman AM, Hunter S, Wadhwa P, Elovitz MA, Foroud T, Saade G, Reddy UM, 2018. Racial Disparities in Adverse Pregnancy Outcomes and Psychosocial Stress. Obstet. Gynecol 131, 328–335. 10.1097/AOG.0000000000002441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gump BB, Yun S, Kannan K, 2014. Polybrominated Diphenyl Ether (PBDE) Exposure in Children: Possible Associations with Cardiovascular and Psychological Functions. Environ. Res 132, 244–250. 10.1016/j.envres.2014.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley KG, Chevrier J, Aguilar Schall R, Sjödin A, Bradman A, Eskenazi B, 2011. Association of prenatal exposure to polybrominated diphenyl ethers and infant birth weight. Am. J. Epidemiol 174, 885–892. 10.1093/aje/kwr212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson J, Carson C, Redshaw M, 2016. Impact of preterm birth on maternal well-being and women’s perceptions of their baby: a population-based survey. BMJ Open 6. 10.1136/bmjopen-2016-012676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbstman JB, Sjödin A, Apelberg BJ, Witter FR, Halden RU, Patterson DG, Panny SR, Needham LL, Goldman LR, 2008. Birth delivery mode modifies the associations between prenatal polychlorinated biphenyl (PCB) and polybrominated diphenyl ether (PBDE) and neonatal thyroid hormone levels. Environ. Health Perspect 116, 1376–1382. 10.1289/ehp.11379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe AA, Carey GB, 2007. Polybrominated Diphenyl Ethers as Endocrine Disruptors of Adipocyte Metabolism. Obesity 15, 2942–2950. 10.1038/oby.2007.351 [DOI] [PubMed] [Google Scholar]
- Huang SC, Giordano G, Costa LG, 2010. Comparative Cytotoxicity and Intracellular Accumulation of Five Polybrominated Diphenyl Ether Congeners in Mouse Cerebellar Granule Neurons. Toxicol. Sci 114, 124–132. 10.1093/toxsci/kfp296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes, 2007. Preterm Birth: Causes, Consequences, and Prevention, The National Academies Collection: Reports funded by National Institutes of Health. National Academies Press (US), Washington (DC). [PubMed] [Google Scholar]
- James-Todd T, March MI, Seiglie J, Gupta M, Brown FM, Majzoub JA, 2018. Racial Differences in Neonatal Hypoglycemia among Very Early Preterm Births. J. Perinatol. Off. J. Calif. Perinat. Assoc 38, 258–263. 10.1038/s41372-017-0003-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Restrepo B, Kannan K, 2009. An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States. Chemosphere 76, 542–548. 10.1016/j.chemosphere.2009.02.068 [DOI] [PubMed] [Google Scholar]
- Ma W-L, Gao C, Bell EM, Druschel CM, Caggana M, Aldous KM, Louis GMB, Kannan K, 2014. Analysis of polychlorinated biphenyls and organochlorine pesticides in archived dried blood spots and its application to track temporal trends of environmental chemicals in newborns. Environ. Res 133, 204–210. 10.1016/j.envres.2014.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Osterman MJK, 2018. Describing the Increase in Preterm Births in the United States, 2014-2016. NCHS Data Brief 1–8. [PubMed] [Google Scholar]
- McElrath TF, Hecht JL, Dammann O, Boggess K, Onderdonk A, Markenson G, Harper M, Delpapa E, Allred EN, Leviton A, 2008. Pregnancy Disorders that Lead to Delivery Before the 28th Week of Gestation: An Epidemiologic Approach to Classification. Am. J. Epidemiol 168, 980–989. 10.1093/aje/kwn202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitro SD, Chu MT, Dodson RE, Adamkiewicz G, Chie L, Brown FM, James-Todd TM, 2019. Phthalate metabolite exposures among immigrants living in the United States: findings from NHANES, 1999-2014. J. Expo. Sci. Environ. Epidemiol 29, 71–82. 10.1038/s41370-018-0029-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan TK, 2016. Role of the Placenta in Preterm Birth: A Review. Am. J. Perinatol 33, 258–266. 10.1055/s-0035-1570379 [DOI] [PubMed] [Google Scholar]
- Muller C, Sampson RJ, Winter AS, 2018. Environmental Inequality: The Social Causes and Consequences of Lead Exposure. Annu. Rev. Sociol 44, 263–282. 10.1146/annurev-soc-073117-041222 [DOI] [Google Scholar]
- Nguyen VK, Kahana A, Heidt J, Polemi K, Kvasnicka J, Jolliet O, Colacino JA, 2020. A comprehensive analysis of racial disparities in chemical biomarker concentrations in United States women, 1999-2014. Environ. Int 137, 105496. 10.1016/j.envint.2020.105496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H-R, Kamau PW, Loch-Caruso R, 2014. Involvement of Reactive Oxygen Species in Brominated Diphenyl Ether-47-induced Inflammatory Cytokine Release from Human Extravillous Trophoblasts in vitro. Toxicol. Appl. Pharmacol 274, 283–292. 10.1016/j.taap.2013.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JRC, Hyde MJ, Gale C, Santhakumaran S, Modi N, 2013. Preterm birth and the metabolic syndrome in adult life: a systematic review and meta-analysis. Pediatrics 131, e1240–1263. 10.1542/peds.2012-2177 [DOI] [PubMed] [Google Scholar]
- Peltier MR, Klimova NG, Arita Y, Gurzenda EM, Murthy A, Chawala K, Lerner V, Richardson J, Hanna N, 2012. Polybrominated diphenyl ethers enhance the production of proinflammatory cytokines by the placenta. Placenta 33, 745–749. 10.1016/j.placenta.2012.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier MR, Koo H-C, Getahun D, Menon R, 2015. Does exposure to flame retardants increase the risk for preterm birth? J. Reprod. Immunol 107, 20–25. 10.1016/j.jri.2014.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DL, Pirkle JL, Burse VW, Bernert JT, Henderson LO, Needham LL, 1989. Chlorinated hydrocarbon levels in human serum: Effects of fasting and feeding. Arch. Environ. Contam. Toxicol 18, 495–500. 10.1007/BF01055015 [DOI] [PubMed] [Google Scholar]
- Pugh SJ, Albert PS, Kim S, Grobman W, Hinkle SN, Newman RB, Wing DA, Grantz KL, 2017. Patterns of gestational weight gain and birth weight outcomes in the NICHD Fetal Growth Study – Singletons: A prospective study. Am. J. Obstet. Gynecol 217, 346.e1–346.e11. 10.1016/j.ajog.2017.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigal S, Doyle LW, 2008. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet Lond. Engl 371, 261–269. 10.1016/S0140-6736(08)60136-1 [DOI] [PubMed] [Google Scholar]
- Sanders JM, Burka LT, Smith CS, Black W, James R, Cunningham ML, 2005. Differential expression of CYP1A, 2B, and 3A genes in the F344 rat following exposure to a polybrominated diphenyl ether mixture or individual components. Toxicol. Sci. Off. J. Soc. Toxicol 88, 127–133. 10.1093/toxsci/kfi288 [DOI] [PubMed] [Google Scholar]
- Schecter A, Haffner D, Colacino J, Patel K, Päpke O, Opel M, Birnbaum L, 2010. Polybrominated diphenyl ethers (PBDEs) and hexabromocyclodecane (HBCD) in composite U.S. food samples. Environ. Health Perspect 118, 357–362. 10.1289/ehp.0901345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Vexler A, Whitcomb BW, Liu A, 2006. The Limitations due to Exposure Detection Limits for Regression Models. Am. J. Epidemiol 163, 374–383. 10.1093/aje/kwj039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Whitcomb BW, Buck Louis GM, Louis TA, 2005. Lipid Adjustment in the Analysis of Environmental Contaminants and Human Health Risks. Environ. Health Perspect 113, 853–857. 10.1289/ehp.7640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh IA, Ahmad E, Jamal MS, Rehan M, Assidi M, Tayubi IA, AlBasri SF, Bajouh OS, Turki RF, Abuzenadah AM, Damanhouri GA, Beg MA, Al-Qahtani M, 2016. Spontaneous preterm birth and single nucleotide gene polymorphisms: a recent update. BMC Genomics 17, 759. 10.1186/s12864-016-3089-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjödin A, Jones RS, Wong L-Y, Caudill SP, Calafat AM, 2019. Polybrominated Diphenyl Ethers and Biphenyl in Serum: Time Trend Study from the National Health and Nutrition Examination Survey for Years 2005/06 through 2013/14. Environ. Sci. Technol 53, 6018–6024. 10.1021/acs.est.9b00471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skupski DW, Owen J, Kim S, Fuchs KM, Albert PS, Grantz KL, 2017. Estimating Gestational Age from Ultrasound Fetal Biometrics. Obstet. Gynecol 130, 433–441. 10.1097/AOG.0000000000002137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenschein-van der Voort AMM, Arends LR, de Jongste JC, Annesi-Maesano I, Arshad SH, Barros H, Basterrechea M, Bisgaard H, Chatzi L, Corpeleijn E, Correia S, Craig LC, Devereux G, Dogaru C, Dostal M, Duchen K, Eggesbø M, van der Ent CK, Fantini MP, Forastiere F, Frey U, Gehring U, Gori D, van der Gugten AC, Hanke W, Henderson AJ, Heude B, Iñiguez C, Inskip HM, Keil T, Kelleher CC, Kogevinas M, Kreiner-Møller E, Kuehni CE, Küpers LK, Lancz K, Larsen PS, Lau S, Ludvigsson J, Mommers M, Nybo Andersen A-M, Palkovicova L, Pike KC, Pizzi C, Polanska K, Porta D, Richiardi L, Roberts G, Schmidt A, Sram RJ, Sunyer J, Thijs C, Torrent M, Viljoen K, Wijga AH, Vrijheid M, Jaddoe VWV, Duijts L, 2014. Preterm birth, infant weight gain, and childhood asthma risk: a meta-analysis of 147,000 European children. J. Allergy Clin. Immunol 133, 1317–1329. 10.1016/j.jaci.2013.12.1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza AO, Tasso MJ, Oliveira AMC, Pereira LC, Duarte FV, Oliveira DP, Palmeira CM, Dorta DJ, 2016. Evaluation of Polybrominated Diphenyl Ether Toxicity on HepG2 Cells - Hexabrominated Congener (BDE-154) Is Less Toxic than Tetrabrominated Congener (BDE-47). Basic Clin. Pharmacol. Toxicol 119, 485–497. 10.1111/bcpt.12598 [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Dodder NG, Offenberg JH, Schantz MM, Wise SA, 2005. Polybrominated diphenyl ethers in house dust and clothes dryer lint. Environ. Sci. Technol 39, 925–931. 10.1021/es0486824 [DOI] [PubMed] [Google Scholar]
- Stieb DM, Chen L, Eshoul M, Judek S, 2012. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ. Res 117, 100–111. 10.1016/j.envres.2012.05.007 [DOI] [PubMed] [Google Scholar]
- U.S. EPA., 2008. IRIS Health Assessment Of 2,2’,4,4’-Tetrabromodiphenyl Ether (BDE-47) CASRN 5436-43-12,2’,4,4’,5-Pentabromodiphenyl Ether (BDE-99) CASRN 60348-60-92,2’,4,4’,5,5’-Hexabromodiphenyl Ether (BDE-153) CASRN 68631-49-22,2’,3,3’,4,4’,5,5’,6,6’-Decabromodiphenyl Ether (BDE-209) CASRN 1163-19-5. U.S. Environmental Protection Agency, Washington, DC. [Google Scholar]
- Varshavsky JR, Robinson JF, Zhou Y, Puckett KA, Kwan E, Buarpung S, Aburajab R, Gaw SL, Sen S, Smith SC, Frankenfield J, Park J-S, Fisher SJ, Woodruff TJ, 2020a. Association of polybrominated diphenyl ether (PBDE) levels with biomarkers of placental development and disease during mid-gestation. Environ. Health 19, 61. 10.1186/s12940-020-00617-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky JR, Sen S, Robinson JF, Smith SC, Frankenfield J, Wang Y, Yeh G, Park J-S, Fisher SJ, Woodruff TJ, 2020b. Racial/ethnic and geographic differences in polybrominated diphenyl ether (PBDE) levels across maternal, placental, and fetal tissues during mid-gestation. Sci. Rep 10, 12247. 10.1038/s41598-020-69067-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Gulati M, Kwok CS, Wong CW, Narain A, O’Brien S, Chew-Graham CA, Verma G, Kadam UT, Mamas MA, 2018. Preterm Delivery and Future Risk of Maternal Cardiovascular Disease: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc 7. 10.1161/JAHA.117.007809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Hediger ML, Albert PS, Grewal J, Sciscione A, Grobman WA, Wing DA, Newman RB, Wapner R, D’Alton ME, Skupski D, Nageotte MP, Ranzini AC, Owen J, Chien EK, Craigo S, Kim S, Grantz KL, Louis GMB, 2018. Association of Maternal Obesity with Longitudinal Ultrasonographic Measures of Fetal Growth. JAMA Pediatr. 172, 24–31. 10.1001/jamapediatrics.2017.3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Kramer MS, 2009. Variations in mortality and morbidity by gestational age among infants born at term. J. Pediatr 154, 358–362, 362.e1. 10.1016/j.jpeds.2008.09.013 [DOI] [PubMed] [Google Scholar]
- Zhao X, Peng S, Xiang Y, Yang Y, Li J, Shan Z, Teng W, 2017. Correlation between Prenatal Exposure to Polybrominated Diphenyl Ethers (PBDEs) and Infant Birth Outcomes: A Meta-Analysis and an Experimental Study. Int. J. Environ. Res. Public. Health 14. 10.3390/ijerph14030268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Liu P, Wang J, Xiao X, Meng X, Zhang Y, 2016. Umbilical cord blood PBDEs concentrations are associated with placental DNA methylation. Environ. Int 97, 1–6. 10.1016/j.envint.2016.10.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


