Abstract
Osteosarcoma is one of the most common primary tumours of the bone, with a 5‐year survival rate of less than 20% after the development of metastases. Osteosarcoma is highly predisposed in Paget's disease of the bone, and both have common characteristic skeletal features due to rapid bone remodelling. Osteosarcoma prognosis is location dependent, which further emphasizes the likely contribution of the bone microenvironment in its pathogenesis. Mechanobiology describes the processes involved when mechanical cues from the changing physical microenvironment of the bone are transduced to biological pathways through mechanosensitive cellular components. Mechanobiology‐driven therapies have been used to curb tumour progression by direct alteration of the physical microenvironment or inhibition of metastasis‐associated mechanosensitive proteins. This review emphasizes the contribution of mechanobiology to the progression of osteosarcoma and sheds light on current mechanobiology‐based therapies and potential new targets for improving disease management. Additionally, the many different 3D models currently used to study osteosarcoma mechanobiology are summarized.
Keywords: 3D cell culture, bisphosphonates, bone remodelling, bone sarcoma, mechanobiology, mechanotransduction, osteosarcoma, pharmacology, therapy
Abbreviations
- E
Young's modulus
- ECM
extracellular matrix
- FA
focal adhesion
- MSCs
mesenchymal stem cells JNK c‐Jun N‐terminal kinase
- NLK
nemo‐like kinase
- OCL
osteoclast
- OPG
- OS
osteosarcoma
- PCL
polycaprolactone
- PCP
planar cell polarity
- PLA
polylactic acid
1. INTRODUCTION
Every living organism, regardless of complexity, can ultimately be simplified to the fundamental principles of cell biology—which spans cellular pathways, genetics/epigenetics and the cell microenvironment. Understanding the interactions among these three components and the extent of their influence on cell behaviour and function can provide a comprehensive picture of cancer progression and metastasis, with consequent acceleration of new therapeutic approaches. This interdisciplinary review highlights the interactive relationship between osteosarcoma cell biology and the physical microenvironment with an emphasis on mechanobiology‐related pharmacological interventions that have the potential to alter bone mechanotransductive pathways. These mechanobiological insights might facilitate the identification of new targets and perspectives for osteosarcoma therapy.
2. OSTEOSARCOMA
Osteosarcoma (OS) is the most common primary tumour of the bone and affects two distinct patient populations, young growing adolescents and people 50 years or older with pre‐existing bone‐deforming conditions such as Paget's disease (Hansen et al., 2006). While capable of affecting either axial or appendicular skeletal sites, OS typically arises within weight‐bearing long bones with the most common sites being the distal femur and proximal tibia. The observed predilection for OS to develop during conditions of active skeletal growth (adolescence) or dysregulated bone remodelling (Paget's disease), coupled with its highest incidence of development within weight‐bearing bones, underscores the importance of mechanobiology cues that are likely to participate in OS tumorigenesis and progression. Mechanobiology is the term used to describe the effects of physical forces (internal or external) on the structure and function of cells.
The 5‐year relative survival rate of people with localized appendicular tumours is 70%, but in those who present with distant metastasis, survival is dramatically diminished to less than 30% (Howlader et al., 2019). Despite advances in localized disease management, which largely consists of surgical excision of tumours or stereotactic radiotherapy interventions, curbing of metastatic dissemination remains a real problem and the survival rate has not improved over the past two decades (Anderson, 2016). The stagnation of current treatment options to effectively thwart OS metastatic progression is in part due to an incomplete understanding of the metastatic factors and mechanisms that might be influenced by mechanobiological cues operative within the tumour microenvironment.
Although surgery has improved survival rates of OS, it is still associated with morbidities such as phantom pain, locomotor dysfunction and surgical complications. Moreover, surgical resection of primary tumours is not always possible in certain types of axial OS given close association with critical structures, such as the spinal cord. As such, mortality due to axial OS is mainly due to local tumour growth and/or recurrence at the site of surgery, as seen in 66% ‐ 80% of axial OS cases (Dickerson et al., 2001; Heyman et al., 1992). Thus, developing new interventions for curbing primary tumour invasive growth and progression should also be the focus of research, which will be essential for improving survival rates of patients with axial OS. For non‐resectable OS tumours, pharmacological interventions that might constrain OS cells within the immediate bone microenvironment to curb metastases can have the potential to improve long‐term outcomes in patients with localized axial OS.
The presence of microscopic residual disease is thought to underlie the development of local recurrence and pulmonary metastases post‐surgery. Interestingly, not all residual cancer cells are equipotent for disease re‐emergence, but rather cells that adopt cancer stem cell‐like cell (CSC) properties, which seemingly evade chemotherapy and surgery (Brown et al., 2017), have been incriminated as the crucial perpetrators. Additionally, the metastatic rate of axial OS is dependent on location (Cook et al., 2021), which partly could be due to surgical limitations, but it also raises the possibility of certain bone regions having pro‐CSC and pro‐metastatic mechanobiological properties.
The possibility of certain bone regions being more conducive for metastatic niche progression is supported by the observation that metastasis‐free survival of patients afflicted with appendicular OS is also dependent on location, with the proximal humerus showing the worst prognostic outcome in both human and canine OS, despite not being the most common site of occurrence (Boerman et al., 2012). Hence, dissecting the mechanical cues provided by structurally different bones and its influence on OS biology will be a step towards understanding and curbing metastatic progression and offers the opportunity for the rational development of therapeutic interventions.
2.1. OS bone pathology
Bone consists of an outer layer of compact bone called the cortex and an inner area of spongy bone called the trabeculae. The bone growth plate is the metaphysis and is composed mainly of the trabecular bone with a thin cortical layer. OS develops most commonly in the metaphysis of long bones, originating within the intramedullary cavity, and is accompanied by changes in the texture and structural integrity of the bone. OS is characterized by upregulated activity of osteoclasts, leading to increased bone resorption and a reparative and compensatory deposition of the osteoid extracellular matrix (ECM) by reactive osteoblasts.
Modulation of the localized bone microenvironment and associated ECM by OS tumours can be heterogenous, with associated skeletal changes being predominantly osteoblastic, osteolytic or mixed lytic/proliferative, resulting in a patchwork of sclerotic and lytic lesions (Misaghi et al., 2018). Osteoblastic OS is characterized by a solid mass of malignant osteoblasts and associated osteoid matrix, which erode through the bone cortex and endosteum into the surrounding soft tissues. In contrast, osteolytic OS is primarily resorptive in nature with minimal ECM deposition, and pathological osteolysis can assume different patterns of lytic lesions including a moth‐eaten pathology characterized by small holes or a central hollow region of lysis, which can expand and elevate the bone periosteum while destroying the bone boundaries, leading to a sun‐burst appearance (Codman's triangle). Thus, high‐grade, aggressive OS tends to be characterised by accelerated resorption and a more discontinuous bone boundary (Bandyopadhyay et al., 2019).
Although multiple histological subtypes of conventional OS have been identified, based upon their predominant stromal differentiation pattern including osteoblastic, chondroblastic, fibroblastic, telangiectatic and giant‐cell rich, the participation of stromal cells and ECM within the primary tumour microenvironment in shaping the metastatic biology of OS cells requires further exploration. In preclinical models of OS, conflicting data exist for the permissive or protective effects exerted by osteoclasts for perturbing metastatic progression (Endo‐Munoz et al., 2012), and the mechanobiology of stromal elements in orchestrating OS metastatic biology remains largely unexplored. The most common site of OS metastasis is the lungs, and this observed pulmonary organotropism can be explained by the downstream capillary bed with the alveoli capturing the OS cells due to their size and due to the lung being prepared as a pre‐metastatic niche for OS cells by the primary OS tumour (Fan et al., 2020). Recently, using 3D engineered modelling systems, metastasis to the lung parenchyma has been linked to increased expression of VEGF in a study using the LM8 cell line in a 3D collagen gel culture (Tanaka et al., 2013).
2.2. OS molecular pathways
A specific cell of origin for OS has not been defined yet, but it is thought to be a bone‐forming mesenchymal progenitor cell (Ferguson & Turner, 2018). Despite the lack of a unifying single genetic event leading to formation of OS, common molecular defects associated with OS are chromosomal aberrations and mutations in common cell cycle regulating oncogenes such as Ras and tumour suppressors pRB and p53 (Martin et al., 2012). Some main pathways dysregulated in OS that exert activities through mechanobiological mechanisms include the RANKL/RANK, Hippo and Wnt pathways. Table 1 provides a summary of the factors in these pathways and their roles in OS.
TABLE 1.
Important molecular pathways in OS
| Pathway | Downstream mediators and targets | Biological output |
|---|---|---|
| Wnt/β catenin | β‐catenin, LEF1, C‐MYC, CCDN1, Runx2, TGFβ | Cell proliferation, differentiation |
| Wnt/PCP | Rho kinase, Rac1, NLK, JNK | Cytoskeletal organization, cell polarization, LEF1 suppression, Hippo kinase suppression |
| Wnt/Ca+2 | PKC, NFAT, ezrin, Cdc42, PAK ½ | Cell migration, filopodia/lamellipodia formation, cytoskeletal organization, LEF1 suppression |
| Hippo pathway | YAP/TAZ, Smad, p300, BRD4, NF‐kB | Histone acetylation, osteoclast activation |
| PI3K/Akt | IKK, NF‐kB | Osteoclast activation |
| TGFβ | TAK1, TGFβ receptors I and II, NLK, NF‐kB | Cytoskeletal organization, LEF1 suppression, osteoclast activation |
2.2.1. Wnt signalling pathways
Wnt signalling pathways are responsible for cell fate determination, osteogenic differentiation, reorganization of the actin cytoskeleton, cell adhesion and migration, organogenesis and embryogenesis. The Wnt pathways are mediated by a wide variety of Wnt ligands and membrane receptors and co‐receptors (Fsh, Dsh and LRP). Interestingly, there is heterogeneity in the expression of different types of Wnt ligands and receptors across OS cell lines and patient samples (Cai et al., 2014), suggesting the heterogenous roles of the Wnt signalling pathway in OS.
The pathway most widely studied and linked with OS is the canonical Wnt/β‐catenin pathway, and its constitutive activation due to overexpression of Wnt ligands and receptors, epigenetic modifications or inhibition of Wnt antagonists has been observed in OS progression and tumorigenesis (McQueen et al., 2011). Transcriptional targets of Wnt/β‐catenin signalling include genes responsible for cell proliferation (MYC and Cyclin D1), osteogenic differentiation (Runx2) and osteoclast activation ligand (RANKL), representing pathways involved in OS progression. Additionally, Wnt/β‐catenin signalling maintains OS stem cell populations and promotes chemoresistance (Marchandet et al., 2021). Recently, a low MW inhibitor, PRI‐724, has been demonstrated to suppress Wnt/β‐catenin‐mediated transcription and to decrease OS proliferation and invasion in vitro (Fang et al., 2018). In contradiction to a large body of research, there is also evidence of inactivated Wnt/β‐catenin signalling in OS cell lines and tumour biopsies (Du et al., 2014).
2.2.2. The RANK pathway
The RANK pathway is responsible for osteoclastogenesis, and the dysregulation of the triad components being RANK, RANKL and osteoprotegerin (OPG) is fundamental for the upregulated osteoclast activity and increased bone resorption, which is characteristic of osteolytic OS (Mori et al., 2009). RANK ligand (RANKL) present on osteoblasts binds to RANK (receptor activator of NF‐κB) on osteoclast precursors and activates osteoclasts to initiate bone resorption. There are contradictory data on the role of OPG in OS. Whereas most studies suggest it serves as an inhibitor of the RANK pathway, other studies demonstrate the pro‐proliferative effect of OPG on OS cells (and other cancer types) by increased nuclear accumulation of the p65 subunit of NF‐κB (RELA) and activation of receptor tyrosine kinase pathways by the phosphorylation of AK and ERK (Marley et al., 2015; Ren et al., 2019). Biosensors in live cell imaging could provide dynamic information on phosphorylation dynamics (Damayanti et al., 2017) to understand the basic mechanisms of signalling and crosstalk in OS cells and the extent of perturbation due to forces.
A variety of therapies has been developed to target the RANKL/RANK/OPG axis, which include RANKL inhibitors, OPG injections and anti‐RANKL siRNAs, but the only drug currently being used clinically is the RANKL inhibitor denosumab. However, the medically judicious use of denosumab is required because of its observed side effects of inducing an osteopetrosis‐like bone phenotype and its predicted side effects of growth defects in adolescents (Trinidad & González‐Suárez, 2016). Additionally, its combined use with conventional chemotherapeutic drugs for the treatment of OS is controversial because of its inhibitory effect on the in vitro effect of doxorubicin (Punzo et al., 2020), as well as a small increased proportion of second malignancy development in patients with diverse solid tumours being treated with denosumab, compared with zoledronate (Tovazzi et al., 2019).
2.2.3. The Hippo pathway
The Hippo pathway regulates organ size and maintains normal tissue homeostasis and tissue regeneration. Dysregulation of the Hippo pathway and its major downstream effectors, YAP and TAZ, give cancer cells the unchecked advantage of repair and regeneration, followed with increased “stemness” and chemoresistance (Moya & Halder, 2019). Hippo kinases MST1/2 and LATS1/2 form a complex with their regulatory subunits and consequently inhibit YAP (Yes‐Associated Protein) and its homologue TAZ (transcriptional co‐activator with PDZ‐binding motif) by phosphorylating and tagging them for degradation in the cytosol. Dephosphorylated and active YAP and TAZ translocate to the nucleus and bind with TEAD transcription factors and recruit BRD4 and RNA polymerase II to histones 3 and 4 to promote gene transcription (Zanconato et al., 2018). Interestingly, BRD4 binds to acetylated RELA (p65 subunit of NF‐κB) and keeps it constitutively active, further promoting the osteolytic RANK pathway (Zou et al., 2014). YAP target genes indirectly upregulate the PI3K/Akt pathway and focal adhesion (FA) turnover by inhibiting tumour suppressor PTEN (Tumaneng et al., 2012). YAP/TAZ overexpression in OS is a potential therapeutic target, and low MW inhibitors, verteporfin and CA3, which directly curb YAP‐mediated transcription, have been developed to inhibit primary OS tumour growth (Morice et al., 2020).
Some of the gene targets of the Hippo pathway—MYC and CCND1—are also common with the Wnt pathway. Additionally, PTEN and NF‐kB are negatively correlated in OS, and both are suggested as potential biomarkers for OS prognosis (Gong et al., 2017). Thus, the crosstalk between a range of dysregulated pathways facilitate tumorigenesis in OS.
2.2.4. Role of epigenetic modifications
Acetylation and methylation and related epigenetic enzymes play an important role in OS in disrupting normal molecular pathways. These epigenetic mediators are relevant to OS mechanobiology because their nuclear import changes with alterations in cell shape and cytoskeletal organization in response to substrate stiffness. One of the widely studied methylated epigenetic markers in OS is the trimethylation of the 27th lysine residue of histone 3 (H3K27me3), which is mediated by the methyltransferase EZH2. However, there are contradictory data on the role of EZH2 and H3K27me3 in OS progression. Heterogeneity across OS cell lines was observed, with some OS cases showing a complete loss of EZH2 and H3K27me3 (Feng et al., 2018) and other studies showing EZH2 to be overexpressed in OS and linked to poor prognosis (Sun et al., 2016). In one investigation, silencing of methyltransferase EZH2 and downregulation of H3K27me3 inhibited OS progression and lung metastasis (Lv et al., 2015). However, a recent study demonstrated that downregulation of H3K27me3 leads to increased stemness and decreased chemosensitivity in OS, while upregulated H3K27me3 leads to reduced levels of stem cell maintenance proteins (SOX9 and CD117/c‐kit) and increased sensitivity to the chemotherapy drug cisplatin (He et al., 2019). Interestingly, overexpression of the histone demethylase KDM6B, which is specific to H3K27me3, has been implicated in OS lung metastasis and poor prognosis (Jiang et al., 2021).
Single‐cell studies on epigenetic modifications (Cui et al., 2017; Cui & Irudayaraj, 2015; Liu et al., 2019) along with sequencing have provided both broad and focused perspectives on epigenetic events and mechanisms during OS progression, which might facilitate targeted therapy. A DNA methyltransferase inhibitor, decitabine, improved the chemosensitivity of OS cells to cisplatin when used together (Chaiyawat et al., 2020), as well as providing an opportunity to promote OS differentiation through expression of the oestrogen receptor ER‐α (Osuna et al., 2019). Histone deacetylase inhibitors such as panobinostat, domatinostat, entinostat, vorinostat, trichostatin A and sodium butyrate have been tested alone (Deng et al., 2016; McGuire et al., 2020; Mu et al., 2015; Rao‐Bindal et al., 2013; Torres et al., 2020; Xie et al., 2016) or in combination with doxorubicin/cisplatin (Pettke et al., 2016) or decitabine (Capobianco et al., 2014) and have shown anti‐proliferative and anti‐metastatic effect in OS cells.
3. PHYSICAL MICROENVIRONMENT
Phenotype expressions are the result of both genetic and environmental factors. Bidirectional and reciprocal communications exist between the cell and its surrounding ECM, consisting of physical and biochemical signals. Different types of physical forces existent within the tumour microenvironment and associated ECM, such as rigidity, intercellular spacing, topology and matrix orientation, influence cell behaviour and migration in different ways. Cells show preferential migration to stiffer areas of a substrate and can be explained by an increase in Young's modulus that is accompanied with increased traction forces and spreading area (Lo et al., 2000). A simple scheme of the effects of substrate stiffness on cell behaviour is shown in Figure 1. External physical forces acting on the cell surface are sensed and transduced across the membrane and then converted into biochemical signals, which affect molecular pathways. The resulting biological changes can direct a change in cell shape, movement, and cell forces exerted on the ECM. This study of the crosstalk between mechanical forces and cell biology is mechanobiology.
FIGURE 1.
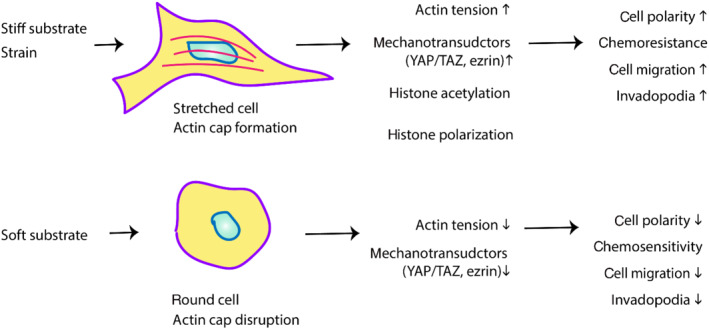
Diagram showing effects of substrate stiffness on cell behaviour. A stiffer substrate has been shown to increase cell spread and invasion; thus, a stiffer ECM and bone microarchitecture might contribute to aggressive cancer behaviour
The importance of biophysical ECM signals in OS is underscored by the fact that p53‐ and Rb‐deficient mesenchymal stem cells (MSCs) are directed towards OS development after being treated with OS cell culture (SAOS‐2) media and receiving physical (calcified substrate) signals from the microenvironment (Rubio et al., 2014). The translational relevance of biophysical cues in naturally occurring skeletal pathologies can be exemplified by mechanical stimuli that dysregulate the balance between bone resorption and formation, leading to abnormal bone remodelling and bone disorders such as Paget's disease and OS. The significant changes in bone structure at macroscopic and microscopic levels are discussed in greater detail to explain their influence on the co‐occurrence and progression of these disorders. Figure 2 shows how the change in bone structure contributes to a cancer niche in the bone.
FIGURE 2.
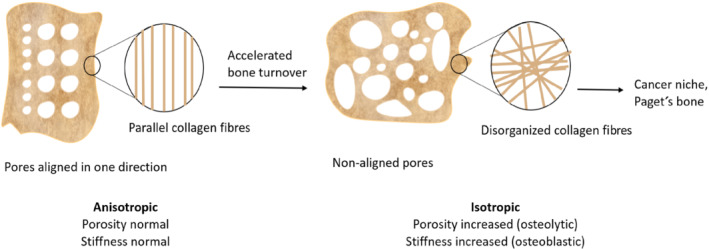
Rapid bone remodelling in Paget's disease and osteosarcoma contributes to a deformed, isotropic bone structure that helps in forming a “cancer niche” in the bone, possibly facilitating bone sarcomas as well as homing metastases from secondary non‐bone tumours. The pores in normal mature bone are aligned in one direction to support the body's weight. Immature bone or woven bone with rapid bone resorption and deposition shows disorganized alignment and increased isotropy in porosity and collagen fibres, leading to decreased bone strength. Depending on how osteolytic or osteoblastic the bone remodelling process is, the porosity and stiffness of the bone deviate from the normal, and bone becomes brittle and prone to fracture. Most importantly, highly isotropic bone is a characteristic feature of cancerous bone
3.1. Bone architecture in OS
When the bone is in a growing or healing phase after mechanical fracture, reparative osteogenesis is initially deposited in an immature form. The immature bone deposition is rapid and is characterized by a woven, loosely packed structure, which is subsequently remodelled into a parallel‐oriented compact lamellar bone as it matures. Collagen type I makes up to 95% of the collagen content and 80% protein content in bones (Viguet‐Carrin et al., 2006). The smallest unit of the collagen fibre is a monomer, which is a triple‐helix structure of 300‐nm length and 1.5‐nm diameter, composed of polypeptide helices α1 and α2. Bone maturation is also accompanied with isomerization within the α1 chain of the collagen molecule, so that there exists an equilibrium of 20% α to 80% β isoform in adult mature bone (Fledelius et al., 1997). Five collagen monomers align in a parallel but staggered manner, to make one microfibril (4‐nm diameter), which can then arrange into a fibril bundle (30–300‐nm diameter). The fibril bundles arrange parallel to each other to form one collagen fibre of 1–2‐μm diameter, stabilized by covalent crosslinks. The orientation of hydroxyapatite crystals found within the fibres is dependent on fibre orientation and become disrupted when the latter is disorganized.
Although altered crosslinks, mineralization, and collagen orientation form a complex relationship affecting bone strength, the mechanical properties of bone and the load it can handle depend largely on collagen fibre orientation relative to the applied force (Viguet‐Carrin et al., 2006). Additionally, osteocyte arrangement in the bone matrix mediated via integrins also changes with collagen orientation, with flat osteocytes aligned along parallel fibres versus rounder osteocytes arranged randomly in woven fibres (Warshaw et al., 2017).
3.1.1. Bone and collagen isotropy
The prevalence of OS in growing bones and Paget's disease of the bone is noteworthy because it highlights the potential contributions of bone structure and microarchitecture in OS pathogenesis. In both OS and Paget's disease, the microarchitecture of the bone deviates from normal anisotropic (aligned in one direction) to abnormal isotropic (non‐aligned) structure due to variation in bone matrix, especially collagen alignment, and such deviation is likely to contribute to disease establishment and progression.
OS is characterized by a disordered woven bone structure and increased porosity induced by accelerated bone turnover. Bone damaged by OS has compromised mechanical properties due to lower anisotropy than normal bone because of this disordered structure, which can be quantified and detected by micro‐computed tomography (μCT) measure of anisotropy (Cole et al., 2014). Anisotropy of normal long bones is in the longitudinal direction, which is why Young's modulus of cortical bone in the longitudinal direction is 40% greater than in the transverse direction (Turner et al., 1999). Therefore, highly isotropic bone is more brittle and has lower load capacity. Osteolytic OS can pathologically resorb through the bone cortex, causing increasing cortical porosity and decreased Young's modulus of the bone, which contributes to bone fragility (Cooper et al., 2016). Vascular pores or “osteonic canals” in normal bone exist in the form of Haversian systems channelling blood vessels and nerves. Notably, the rapid skeletal growth phase in adolescence leads to increased canal numbers, whereas bone loss with advancing age leads to increased canal diameters. The bone alterations occurring at the two distinct age peaks of OS prevalence (adolescence and geriatric) likely contribute to skeletal tumorigenesis and lead to increased bone porosity.
Paget's disease shows irregular and woven alignment of collagen fibres—a hallmark of accelerated bone turnover (Gennari et al., 2019). A large body of research has described Paget's disease bone as poorly mineralized but dense, making it less stiff (14% lower Young's modulus) and more brittle than normal lamellar bone (Singer, 2016; Zimmermann et al., 2015). However, there are also some discrepant data indicating that Paget's disease bone can be highly mineralized and dense (Viguet‐Carrin et al., 2006).
A similar skeletal phenotype of disorganized collagen microarchitecture, abnormal arrangement of osteoblasts and apatite, and increased osteolytic activity has also been described in skeletal metastatic niches, induced by aggressive disseminated prostate (Sekita et al., 2017) and breast cancer cells (Sekita et al., 2016). Another hallmark of accelerated bone remodelling is the imbalance of collagen isomerization represented as decreased β isoform levels, which are present in bone samples from Paget's disease patients (Garnero et al., 1997) and is indicative of bone metastases (Leeming et al., 2006). Collectively, these findings associated with skeletal metastases provide further evidence that the disorganized bone microarchitecture is an important element of cancer niche formation.
3.1.2. Molecular link with Paget's disease
Classical Paget's disease is a late age‐onset localized disorder of bone structure and metabolism (Gennari et al., 2019). The Paget's disease family of pathologies includes other similar disorders as juvenile/early‐onset Paget's disease and familial expansile osteolysis. The genetic components that have been associated with Paget's disease so far include linkage to chromosome regions 2q36, 10p13, 5q35 (Hocking et al., 2001), and 18q23 (Good et al., 2002); OPG gene (TNFRSF11B) (Daroszewska et al., 2004); and SQSTM1 gene (Lucas et al., 2006)—although there is contradictory evidence across genders and pedigrees (Michou & Brown, 2011).
Although Paget's disease is often associated with an increased risk for OS development, a common genetic mutation has not been identified to definitively link Paget's disease and OS pathogenesis (Sparks et al., 2001). A common area of mutation observed in early‐onset familial Paget's disease complicated with OS was the chromosome region 18q21‐22, wherein lies the RANK (TNFRSF11A) gene (McNairn et al., 2001). Although TNFRSF11A mutations have been associated with the Paget's disease family of disorders (Nakatsuka et al., 2003), none have been observed in classical Paget's disease and OS. However, further study is required to elucidate the molecular links between OS and Paget's disease.
3.2. OS mechanobiology
Factors including substrate stiffness (i.e., bone) that increase actin polymerization and actin–myosin interaction and contraction will promote cell movement and lamellipodia (cell protrusion) formation (Charras & Sahai, 2014). The bone cell's preference for a stiffer substrate is made apparent by the fact that Young's modulus (E) of normal bone is in the range of 14 to 23 GPa (Turner et al., 1999). Moreover, E of a single collagen type I fibre is 0.1–0.3 GPa, and that of collagen molecule is 5–7 GPa (Lin et al., 2020).
OS cells showed the highest cell area, spread morphology, and increased survival when grown on stiff collagen I‐coated substrates with E = 55 kPa, versus more apoptotic cells on soft substrates with E = 1–7 kPa (Mylona et al., 2008). Aggressive OS is usually accompanied with fibroblastic, stretched cells with high cytoskeletal tension and reduced FAs (Müller & Silvan, 2019). Similarly, OS cells grown on stiff substrates with E = 50 kPa showed optimum tumour‐sphere growth and increased expression of stem cell markers (Jabbari et al., 2015). ECM components bind with cells via integrins, leading to changes in cytoskeletal arrangement and localization of membrane linker proteins, which have extensive downstream cell signalling effects. Studies exploring the application of uniaxial stretching and cyclic strain on OS cells and MSCs have demonstrated the effects of physical forces on altering cell shape, FAK activation and expression of proteins ERK 1/2, p38 and Smad 2/3 (Chen et al., 2013; Grier et al., 2017). The intricacies of mechanobiological crosstalk between cytoskeletal components, actin‐binding proteins and the Wnt and Hippo pathways are a promising avenue of research to understand OS progression in response to ECM microarchitecture. A simplified overview of the complex crosstalk between external forces and signalling pathways has been summarized in Figure 3.
FIGURE 3.
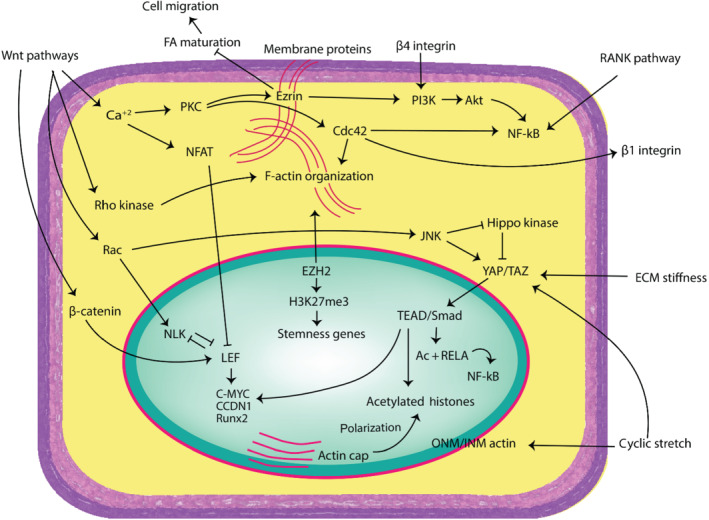
An overview of the crosstalk of molecular pathways with cytoskeletal agents, mechanosensors and external forces. Some of these relationships present exciting new targets for cancer research and therapy and need to be explored more fully in osteosarcoma cells in the context of mechanotransduction. ECM, extracellular matrix; FA, focal adhesions; ONM/INM, outer nuclear membrane/inner nuclear membrane; AC + RELA, acetylated RELA
3.2.1. Cytoskeleton and nucleoskeleton
Physical forces altering actin organization across the cytoplasm and the nucleus can induce changes in gene expression patterns. To dissect the mechanobiological processes in cancer, an understanding of the actin cytoskeleton and related mechanosensing nuclear components is a prerequisite, and a brief overview of the relationship among ECM forces, actin and gene expressions is provided here.
Actin is involved in gene expression by being a part of RNA polymerase complexes and binding to transcriptional regulators (Wei et al., 2020). Actin is depolymerized and complexed with cofilin and imported into the nucleus by the nuclear transport receptor importin‐9, where it is complexed with profilin and exported into the cytoplasm by another nuclear transport receptor, exportin‐6 (Misu et al., 2017). Cofilin has been directly linked to the RNA transcription rate, and its role as a mechanotransductive factor has been established by its increased nuclear presence due to its sensitivity to substrate stiffness (Domingues et al., 2020). Nuclear actin is responsible for transcriptional regulation and causes osteogenesis in MSCs through the expression of Runx2, osteocalcin and osterix (Sen et al., 2015).
When studying mechanobiology, nuclear architecture should be given as much consideration as cytoskeletal changes. Cytoskeletal actin is linked to nuclear lamins present at the inner nuclear membrane through LINC proteins (linker of nucleoskeleton and cytoskeleton). Any change in actin–nucleoskeleton linkage causes change in genetic expression because the lamina is linked to the heterochromatin via centromeric regions (Holaska & Wilson, 2006). The nuclear actin cap responds to substrate stiffness, and these nucleoskeletal changes lead to variation in histone distribution and its access to epigenetic enzymes and transcriptional machinery (Kim & Wirtz, 2015). The actin cap is disrupted in many cancers including the OS cell line U2OS, although the cells still featured actin arcs and stress fibres (Kim et al., 2012).
The actin cap exerts a mechanical force on the nucleus and changes the distribution of lamins and some acetylated histones (Kim & Wirtz, 2015). Reactively, A‐type lamins are polarized and localize preferentially to the apical nuclear surface to absorb this force, which restricts access to deacetylases, resulting in the apical localization of acetylated histones H4K12ac and H4K5ac. Disruption of the actin cap leads to isotropic distribution of A‐type lamins and uniform nucleoplasm distribution of H4K12ac and H4K5ac. Notably, B‐type lamins, unacetylated H4 and H4K16ac are not polarized by the presence or absence of the actin cap. Additional studies on the absence of the actin cap and its consequences in OS progress are needed. H4K12ac already shows promise as a therapeutic target in breast cancer when combined with conventional hormone therapy (Liu et al., 2019), and its relevance in OS therapy could be an exciting avenue of future research.
Another interesting interaction between external forces and actin re‐arrangement is the formation of a cytoplasmic perinuclear actin rim due to the application of force on cell edges (Shao et al., 2015). The actin polymerization at the outer nuclear membrane, caused by mechanical strain, reduced nuclear actin levels and increased H3K27me3 levels (Le et al., 2016). This mechanical stimulus also caused transcriptional repression of differentiation‐related and cell cycle regulation genes (CDKN2A). In OS, the methylation and repression of the CDKN2A gene promoter caused p53 degradation (Oh et al., 2006), which might explain OS proliferation and aggression in mechanically stimulated ECM.
Accumulating knowledge on mechanobiology has shown that mechanical cues, actin re‐arrangement and epigenetics play a synergistic role in gene expression regulation but how it influences the structure–function during OS metastasis is still unclear, providing an exciting opportunity for future studies.
3.2.2. Mechanosensors and transductors
There is a reciprocally influential relationship between cytoskeletal and nucleoskeletal components and certain cellular pathway proteins, making them valuable therapeutic targets. An understanding of the mechanisms through which these pathway proteins regulate each other and alter the expression of differentiation‐ and metastasis‐related genes in response to physical stimuli is essential for manipulating OS mechanobiology.
Role of YAP/TAZ
YAP and TAZ are mechanosensitive proteins downstream of the Hippo pathway; they promote cell migration by inhibiting the maturation of FAs and evading cell contact inhibition (Mason et al., 2019). ECM stiffness and F‐actin cytoskeletal arrangement affect YAP/TAZ nuclear import and activation (Panciera et al., 2017). A stiff substrate and mechanical stress on the nucleus increased the nuclear import of YAP/TAZ by flattening the nucleus and increasing nuclear pore size (Elosegui‐Artola et al., 2017). YAP/TAZ is inactive in round cells and is activated in cells spread on stiff substrates. This was shown by higher YAP/TAZ expression in cells grown on optimum matrix stiffness, which was E = 50 kPa for OS cells (Jabbari et al., 2015). Moreover, increased YAP/TAZ‐mediated differentiation was accompanied with upregulated Wnt/β‐catenin signalling in stiffer, mineralized bone regenerative scaffolds (Zhou et al., 2021).
YAP inhibits Runx2 expression, suggesting a possible role of increased nuclear YAP and undifferentiated phenotype and stemness in OS. An antagonistic relationship exists between YAP/TAZ and nuclear actin, which gives insight into a possible link between the G‐/F‐actin ratio and YAP/TAZ activation. Accumulated nuclear actin exports YAP from the nucleus and allows osteogenesis via Runx2 (Sen et al., 2015).
Non‐canonical Wnt pathways
The non‐canonical Wnt pathways deserve special attention in the context of cell movement and the physical ECM because of their direct involvement with actin polymerization and membrane–cytoskeletal linker proteins. In addition to the complex crosstalk between Wnt pathways, the activation of a specific Wnt pathway depends on the Wnt ligand and the corresponding membrane receptor domain (Komiya & Habas, 2008). Exploring the interaction between Wnt pathways could provide valuable insight into OS stemness, migration and angiogenesis relative to cytoskeletal organization.
The Wnt/Ca+2 pathway is important in cell movement and mechanobiology because it regulates actin organization and endothelial invasion through protein kinase C (PKC) and Rho GTPase‐Cdc42 activation and by regulation of β1‐integrin cell–ECM adhesion. The NF‐kB gene and membrane linker protein ezrin are downstream targets of the Wnt/Ca+2 pathway, and both have been implicated in OS metastasis and poor prognosis (Li et al., 2019). The Wnt/Ca+2 pathway could potentially be targeted to manipulate their expression levels.
The Wnt/planar cell polarity (PCP) pathway regulates G‐ to F‐actin polymerization and its binding with profilin, by activating JNK and Rho GTPases (Habas & Dawid, 2005). JNK expression in response to cyclic stretching of cells caused increased nuclear YAP and inhibition of the Hippo pathway, leading to increased proliferation (Codelia et al., 2014). JNK expression in stretched osteoblasts led to the upregulation of connective tissue growth factor (CTGF), which has been shown to facilitate OS metastasis and angiogenesis by activating αvβ3 integrin and by increasing VCAM‐1 and NF‐κB expression (Hou et al., 2018; Huang et al., 2016; Xiao et al., 2011). Interestingly, endothelial cells promote angiogenesis by first inhibiting the Wnt/β‐catenin pathway and then activating the PCP pathway (Danieau et al., 2019). Regulating the PCP pathway and JNK expression could help elucidate angiogenic processes underlying OS metastasis.
The upregulation of non‐canonical pathways seems to be accompanied with the suppression of the canonical Wnt/β‐catenin pathway. A common downstream effector of non‐canonical pathways is nemo‐like kinase (NLK), which phosphorylates and inactivates the transcription factors TCF/LEF and downregulates the Wnt/β‐catenin signalling, while also increasing matrix metalloproteinase 13 (MMP‐13) expression and OS invasiveness (Cai et al., 2014; Ishitani et al., 1999). This complex interplay between Wnt pathways suggests the possibility that there might be a delicate switching between pathways when cells shift from proliferative to invasive stage. This switch presents a possible avenue for targeted therapy to inhibit invasiveness and metastasis.
Role of ezrin
The ERM family of proteins consists of the members ezrin, radixin, moesin, and merlin; they link the cytoskeleton to the cell surface and to transmembrane proteins. Although there is a certain degree of redundancy in their function due to their highly similar amino acid sequences, ezrin plays significant roles in certain processes, which cannot be replaced by other ERM proteins. Ezrin is necessary for OS metastatic progression while other ERM proteins are dispensable (Ren et al., 2009). Primary OS tumours and circulating tumour cells with higher ezrin content showed increased metastatic potential (Di Cristofano et al., 2010). Ezrin regulates FA and invadopodia turnover, which is why overexpressed ezrin leads to decreased cell adherence and increased migration, characteristic of invasive fronts of metastatic tumours, and it is also necessary for initial seeding of metastatic tumour cells within the lung and for maintaining long‐term growth at distant organs (Hoskin et al., 2015).
The subcellular localization of ezrin is dependent on cell density, site of phosphorylation and stage of metastatic progression (Di Cristofano et al., 2010; Ren et al., 2009). Ezrin phosphorylated at C‐terminal threonine567 bridges F‐actin and cell membrane components such as integrins, CD43, CD44, ICAM‐1 and ICAM‐2, and its regulation has been strongly linked to PKC activation (Ren et al., 2009), a downstream target of the Wnt/Ca+2 pathway. Phospho‐Thr567 ezrin is complexed with β1 integrin in invadopodia and triggers invadopodia‐mediated proteolytic degradation of the ECM in metastatic cancers (Greco et al., 2021). Ezrin interaction with β4 integrin is needed to maintain β4 integrin overexpression in aggressive OS lines and is necessary for pulmonary metastasis, as this integrin activates the PI3K/Akt pathway, leading to increased cell proliferation (Wan et al., 2009). However, it is still unclear how ezrin influences β4 integrin transcription. Interestingly, ezrin phosphorylated at its tyrosine353 residue also activates PI3K/Akt independently by binding to the regulatory subunit p85 of PI3K, and this phosphorylation state leads to its nuclear import in OS (Di Cristofano et al., 2010; Gautreau et al., 1999).
Due to its connection with F‐actin and its localization in both the nucleus and cell membrane, ezrin is likely to play a mechanotransductor role and to interact with nuclear factors. This highlights ezrin as an exciting target to elucidate the metastatic mechanisms in OS in the context of physical forces.
4. CURRENT MODELS FOR STUDYING OS MECHANOTRANSDUCTION
The ideal in vitro model would mimic the tumour microenvironment as closely as possible. A 2D culture does not mimic in vivo tumour characteristics because it does not reproduce a tumour niche as it lacks microenvironmental elements including vasculature, stromal cells and macrophages and does not allow the tumour to grow into a polymorphic 3D mass with regions of hypoxia, invasive margins and cancer stem cells. The 2D culture substratum is usually made of glass or plastic and its stiffness, porosity and topology are very different from bone collagen ECM. Given how pivotal the stiffness and mechanical properties of bone ECM are in differentiating normal bone from abnormal bone, the general 2D culture conditions are not satisfactory for studying bone ECM effects. Thus, cells cultured in 3D models show significant change in cell behaviour and epigenetics from cells in standard 2D cultures.
A diverse range of 3D OS models exists with complexity ranging from simple collagen gels and fibre scaffolds to artificial bone models. The optimal model for studying OS would mimic the natural OS cell–ECM interaction while being highly reproducible and simple enough to provide control over ECM components. Some of the models include cancer cells grown on in vitro crosshatched fibres, which induce metabolic upregulation and transcriptional changes in genes related to metabolism and post‐transcriptional modification (Jana et al., 2019). Additionally, cells grown on printed PDMS stamps of different shapes can alter nuclear import of methyltransferases such as tose encoded by SETDB1 and SMYD3 due to change in cell shape and integrity of actin filaments (Pereira et al., 2020).
As discussed earlier, collagen orientation is a crucial feature of cancerous and diseased bone; thus, the replication of disorganized bone microarchitecture is the basic feature that should be a part of any OS or PDB model system. Fibre scaffolds are one of the simplest forms of 3D OS models that can mimic and give exemplary control over ECM orientation. Cellular biochemical processes and cell movement changes depend on the cell substrate composition. As supported by previous studies highlighting the effects of collagen, OS cells grown on collagen I substrates had an enhanced activation of MMP‐2 and MMP‐9 and increased migratory behaviour as compared with OS cells grown on non‐coated collagen‐deficient substrates (Poudel et al., 2014).
Although collagen‐only gels or fibres provide biological cues for bone cell differentiation and proliferation, they are not as stable as synthetic scaffolds and do not recapitulate the mechanical stiffness of bone matrix. More sophisticated scaffolds that more closely mimic bone would be composed of a mix of synthetic polymers (such as polycaprolactone [PCL] and polylactic acid [PLA]) or inorganic bone mineral composite (hydroxyapatite) coated with biomaterials (collagen and chitosan). Polymeric fibres or mineral scaffold would provide structural stability and stiffness, whereas collagen would provide hydrophilicity and the necessary biological cues. Such blended scaffolds have been used successfully in many tissue engineering applications using MSCs, osteoblasts, chondrocytes and fibroblasts (Dong & Lv, 2016).
Hydrogels are highly tunable models that can mimic ECM structure and are used to study mechanical cues. Hydrogels can be made of natural polymers (collagen, silk and alginate) or synthetic polymers (PEG, poly‐aspartic acid [PASP] and PCL) (Yue et al., 2020). As with other types of model systems, synthetic hydrogels have enhanced mechanical strength over natural material‐based hydrogel that comes with the price of compromised bioactivity (Bai et al., 2018). PASP is an amino acid polymer with high structural diversity and easy synthesis. PASP hydrogels are biodegradable and biocompatible scaffolds that have been used to culture MG63 OS cells (Juriga et al., 2016). Some disadvantages of PASP hydrogels are its autofluorescence properties and its easy degradation by cell culture chemicals (trypsin–EDTA) and enzymes (collagenase I). While PASP hydrogels are suboptimal for studying mechanotransductive cellular biology given their inherent degradability and consequent diminished mechanical properties, hydrogels are well suited as therapeutic carriers for bone regeneration applications. Their ability to serve as a dissolvable scaffold with controlled release of drugs and growth factors in vivo to promote new tissue growth is highly relevant for developing OS model systems for screening applications.
Biomimetic Matrigel is a reconstituted basement membrane, that is, a collagen and solubilized basement membrane preparation extracted from the EHS mouse sarcoma. It contains growth factors and ECM proteins and is used in both 2D and 3D hydrogel models. A Matrigel–collagen model is considered more useful than Matrigel alone. As it is created from natural components, Matrigel is biocompatible with bioactive factors. However, Matrigel has poor mechanical properties, low tunability, and low reproducibility. Additionally, it is difficult to control protein and growth factor content in ECM–protein containing hydrogels, and these factors might confound the effective measurement of applied treatment or cellular function to be observed (Tibbitt & Anseth, 2009). Improvements to Matrigel models have been achieved through gelatin/ferulic acid hydrogel models, which are used for studying early metastasis and hypoxia by controlling oxygenation levels and analysing hypoxia‐induced cues responsible for tumour cell movement, collagen deposition and matrix remodelling during invasion towards oxygen‐rich gradient (Lewis et al., 2016). Additionally, OS cell arrangement in Matrigel and gelatin hydrogels also affects its invasive behaviour and chemoresistance, with spheroids showing increased aggressiveness compared with non‐spheroid cell arrangement in hydrogels (Monteiro et al., 2020).
Bone vasculature is necessary in providing nutrients for tumour growth and escape routes for invading cells; thus, an ideal model would replicate the skeletal vasculature along with the mechanical and biochemical cues in tumour microenvironment by including flow perfusion. Sophisticated vascular mimicry models have been created, combining vasculature with 3D spheroid of OS MG63 cells by depositing and culturing the spheroids on an endothelial cell monolayer (Chaddad et al., 2017), and successfully resulted in the expression of angiogenic factors (VEGF and CXCR4), markers CD31 and collagen IV, and a vascular tubule mimicking network.
Artificial bone scaffolds have been a major focus of regenerative medicine. Improvement in bone scaffolds for in vivo transplant has provided significant insight into bone formation and repair (Ren et al., 2019), as well as providing a valuable in vitro model for OS biology exploration. Improvements have led to the production of mineralized collagen scaffolds to provide better mechanical properties and microarchitecture conducive of osteogenic growth. Collagenous bone scaffolds are highly tunable with regard to collagen content, stiffness and porosity and can retain growth factors such as BMP2 and VEGF to improve vascularization and osteogenesis in vivo (Tiffany et al., 2020). Type I collagen‐based scaffolds have been mineralized with a form of calcium phosphate such as hydroxyapatite (Yu et al., 2020) or brushite (Tiffany et al., 2019) to mimic bone composition and improve osteogenesis. The mechanical strength of mineralized collagen scaffolds has been enhanced with the incorporation of zinc (Tiffany et al., 2019) or polymer PLA (Liao et al., 2004), reaching an E of 1.4 and 47.3 MPa, respectively. However, one shortcoming of mineralized scaffolds can be reduction in the elastic modulus of less than 5 kPa after treatment and hydration (Tiffany et al., 2020). Further fine tuning of scaffolds has been done by incorporating spatially graded PLA fibres in scaffolds and creating regions of varying stiffness (Mozdzen et al., 2017).
The main difference between using a model for studying mechanobiology and using it as a regenerative implant is its biodegradability and mechanical strength. A robust study model would preferably maintain stiffness and a stable microarchitecture across time and treatment. Biodegradability and malleability are desired in implants that are required to dissolve after prompting native bone growth at sites of trauma. However, for in vitro studies, this property makes the scaffold an unsuitable model of bone ECM, as it collapses with extended hydration and media treatment, precluding the ability to discern scaffold dissolution due to treatment versus protease degradation by OS. More importantly, these soft scaffolds have an extremely low modulus of elasticity and are not ideal for studies related to the effects of increased mechanical stiffness on OS aggression and might even negatively affect the growth of OS cells, which prefer a stiffer substrate.
5. POTENTIAL MECHANISTIC THERAPIES FOR OS TARGETING MECHANOBIOLOGY
Therapies for modifying mechanobiology may act at a physical or biochemical level by either manipulating the mechanics and/or structure of bone directly or modulating the activity of mechanosensitive proteins.
5.1. Therapies targeting mechanotransductors
Therapies targeting mechanotransductors and cytoskeletal elements work by disrupting the communication between the physical ECM and cell and are mainly categorized as low MW inhibitors. As many details of mechanobiological pathways and their relevance in OS have yet to be explored as therapeutic targets, this is a rich area for future clinical study.
The compounds NSC305787 and NSC668394 reduced cell invasion and pulmonary metastasis of OS by directly binding and inhibiting ezrin in mouse models and in vitro studies (Bulut et al., 2012), by preventing either ezrin phosphorylation or ezrin–protein interactions. NSC305787 demonstrated greater effectivity against pulmonary metastasis and upregulated stress response genes but was ineffective against primary tumour growth and proved to be toxic in long‐term treatment studies (Çelik et al., 2016).
Integrins mediate cell–ECM communication by relaying signals from the physical microenvironment downstream to trigger cell processes and invadopodia formation. Integrin overexpression contributes to OS invasion and angiogenesis by interacting with mechanotransductors ezrin and cofilin and influencing actin arrangement. A combination treatment of αVβ3 integrin inhibitor Cyclo (RGDyK) and Timosaponin AIII inhibited OS metastasis by disrupting F‐actin organization, decreasing integrin levels and deactivating cofilin (Hsieh et al., 2021). Deactivating cofilin would inhibit actin nuclear import and osteogenesis, which might explain the decreased proliferation and spread of OS cells.
YAP/TAZ have been considered as therapeutic targets in breast cancer and many solid tumours particularly due to their contribution to chemoresistance and stemness (Pobbati & Hong, 2020). Anti‐YAP/TAZ therapies work by affecting their upstream receptors, inhibiting YAP/TAZ nuclear localization or disrupting their interaction with TEAD and BRD4 (Rothzerg et al., 2021). Some inhibitors targeting YAP/TAZ directly or indirectly in OS include verteporfin, agave extract, dasatinib, and pazopanib (Luu & Viloria‐Petit, 2020). Verteporfin is the only low MW inhibitor which binds directly to YAP/TAZ and decrease cell migration, FAK levels in OS and β1 integrin levels in MSCs (Husari et al., 2019).
5.2. Therapies targeting bone structure
The concept of attenuating disease conditions by tuning bone structure and mechanics is currently being utilized by a range of therapies against bone metastases, sarcomas and benign skeletal diseases. Broadly, these can be grouped into two classes of therapies—those promoting bone formation or attenuating bone resorption.
Anti‐resorptive drugs comprising bisphosphonates and aminobisphosphonates have been used to increase bone density in patients with osteoporosis and Paget's disease (Drake et al., 2008). Based upon chemical structure, specifically phosphate and hydroxyl groups, bisphosphonates/aminobisphosphonates avidly bind to mineralized bone matrix. Zoledronic acid is an aminobisphosphonate that has been shown to be effective against OS by directly decreasing OS‐generated RANKL and CCL2 levels and consequently reducing osteoclastogenesis, osteolysis and tumour size (Ohba et al., 2014). However, there is contrasting evidence of the role of the aminobisphosphonates as “osteocyte‐protective” agents (Plotkin et al., 2005) as they can stimulate osteoblast apoptosis, which they are shown to mediate using the same intracellular pathways as osteoclast apoptosis (Idris et al., 2008). Other studies have shown that zoledronic acid‐induced loss of osteoclast (OCL) and reduced osteolysis in primary tumour promote OS pulmonary metastasis (Endo‐Munoz et al., 2010). The effect of altered bone structure on OS biology, as well as interactions with hormones in young adolescents, needs to be further evaluated to understand the discrepancies in aminobisphosphonate activities described in different preclinical mouse models.
A Phase III clinical trial published in 2016 compared outcomes of combining chemotherapy and ZOL treatment and showed a slightly higher incidence of primary tumour recurrence and metastases in the group receiving zoledronic acid (Piperno‐Neumann et al., 2016), which contrasted with previously published animal model and clinical studies. However, two of the recent studies that conjugated zoledronic acid with nanoparticles carrying chemotherapy drugs emcitabine and epirubicin or pamidronate‐coated nanoparticles containing doxorubicin showed improved drug uptake due to aminobisphosphonate conjugation and improved tumouricidal action (Yin et al., 2016; Yuan et al., 2020).
Considering the significance of the RANKL/RANK axis in osteoclastogenesis and osteolysis, therapies consisting of RANKL inhibitors and OPG injections have been developed to block the RANKL–RANK interaction. Denosumab is a RANKL‐inhibiting monoclonal antibody and it improved bone mineral density in mouse models and clinical studies of osteoporosis and bone metastases (Body et al., 2006). Denosumab and aminobisphosphonate treatments led to similar survival rates and similar side effects of hypocalcaemia and osteonecrosis of the jaw. However, anti‐RANKL inhibitors had more sustainable anti‐resorptive effects without renal toxicity (Heymann, 2012). Unfortunately, the anti‐osteoclast action of denosumab could also tilt the bone remodelling process towards an osteopetrosis phenotype (Trinidad & González‐Suárez, 2016). An orally administered anti‐RANKL inhibitor AS2676293 showed promising anti‐osteoclastogenesis action and decreased bone metastasis of breast cancer and melanoma cells, and its effectiveness in OS is worth evaluating (Nakai et al., 2019). Another emerging approach against RANKL is the use of small interfering RNA (siRNA) in combination with chemotherapy, which has been shown to reduce tumour growth in mice models and have a protective effect on bone microarchitecture (Rousseau et al., 2011).
On the other hand, synthetic RANKL administration has been used to change the phenotype of abnormally stiff and inflexible bone as seen in osteopetrosis by restoring OCL function, leading to recovered bone trabecular architecture and osteoclastogenesis (Sobacchi et al., 2013). Such therapies could be considered while targeting osteoblastic and sclerotic tumour regions. However, due to the lack of mechanobiological studies, it is difficult to clearly determine how other biochemical pathways in OS could be influenced by dose‐dependent changes.
While promising, the anti‐metastatic potential of anti‐resorptive strategies for OS management remains undetermined; it should be recognized that the OS microenvironment is heterogeneous with areas of lysis and production within the same tumour. As such, broad application of untargeted anti‐resorptive strategies might result in unpredictable alterations to the local mechanotransductive factors and consequent changes in tumour biology. As such, a strong clinical impetus to continue research in skeletal pathologies that intensely focuses on understanding the interactive connectivity between mechanotransductive signalling and cancer is needed to develop effective therapies for OS.
6. CONCLUSIONS
The lack of understanding of the molecular pathogenesis of OS is exemplified by the absence of new therapies, low efficacy of current chemotherapies and poor survival rate of metastatic cancer. The co‐incidence of Paget's disease with OS and the location‐dependent prognosis of OS signify the influence of physical ECM and collagen microarchitecture on the niche formation needed for OS cell survival and aggression. The fact that a clear molecular and mechanistic link has yet to be defined between bone phenotype, co‐occurrence and poor prognosis highlights opportunities for new therapy development. The significant change in the physiology and mechanical properties of the bone at macroscopic and microscopic levels caused by OS or Paget's disease can be used to explore and exploit mechanobiology to gain deeper insights of neoplastic and pre‐neoplastic skeletal pathologies. In this review, we have summarized and highlighted current therapies targeting mechanobiology elements in OS and relevant mechanotransductive targets, which could be exploited in the future.
Although pharmacological intervention against mechanobiology is still a recent approach, different classes of therapies directly targeting bone remodelling or mechanotransductor proteins are being currently explored in OS models. These “mechano‐therapies” are a promising approach when they work synergistically with chemotherapy and improve chemosensitivity. Currently, there are many “missing links” in the pathway connecting the drastically changing physical microenvironment, subcellular actin organization and protein expression. Filling in these gaps by dissecting the biomechanical signatures of OS cells relative to different bone regions could help in identifying and targeting the bone niches, which are more conducive for OS occurrence and progression to develop effective interventions.
6.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY (http://www.guidetopharmacology.org) and are permanently archived in the Concise Guide to PHARMACOLOGY 2021/22 (Alexander et al., 2021a, b).
Shoaib, Z. , Fan, T. M. , & Irudayaraj, J. M. K. (2022). Osteosarcoma mechanobiology and therapeutic targets. British Journal of Pharmacology, 179(2), 201–217. 10.1111/bph.15713
Contributor Information
Timothy M. Fan, Email: t-fan@illinois.edu.
Joseph M. K. Irudayaraj, Email: jirudaya@illinois.edu.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article because no new data were created or analysed in this study.
References
- Alexander, S. P. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Beuve, A. , Brouckaert, P. , Bryant, C. , Burnett, J. C. , Farndale, R. W. , Friebe, A. , Garthwaite, J. , … Waldman, S. A. (2021a). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Catalytic receptors. British Journal of Pharmacology, 178(S1), S264–S312. 10.1111/bph.15541 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Boison, D. , Burns, K. E. , Dessauer, C. , Gertsch, J. , Helsby, N. A. , Izzo, A. A. , Koesling, D. , … Wong, S. S. (2021b). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Enzymes. British Journal of Pharmacology, 178(S1), S313–S411. 10.1111/bph.15542 [DOI] [PubMed] [Google Scholar]
- Anderson, M. E. (2016). Update on survival in osteosarcoma. Orthopedic Clinics, 47(1), 283–292. 10.1016/j.ocl.2015.08.022 [DOI] [PubMed] [Google Scholar]
- Bai, X. , Gao, M. , Syed, S. , Zhuang, J. , Xu, X. , & Zhang, X. Q. (2018). Bioactive hydrogels for bone regeneration. Bioactive Materials, 3(4), 401–417. 10.1016/j.bioactmat.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay, O. , Biswas, A. , & Bhattacharya, B. B. (2019). Bone‐cancer assessment and destruction pattern analysis in long‐bone X‐ray image. Journal of Digital Imaging, 32(2), 300–313. 10.1007/s10278-018-0145-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Body, J. J. , Facon, T. , Coleman, R. E. , Lipton, A. , Geurs, F. , Fan, M. , Holloway, D. , Peterson, M. C. , & Bekker, P. J. (2006). A study of the biological receptor activator of nuclear factor‐kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clinical Cancer Research, 12(4), 1221–1228. 10.1158/1078-0432.CCR-05-1933 [DOI] [PubMed] [Google Scholar]
- Boerman, I. , Selvarajah, G. T. , Nielen, M. , & Kirpensteijn, J. (2012). Prognostic factors in canine appendicular osteosarcoma—A meta‐analysis. BMC Veterinary Research, 8(1), 1–12. 10.1186/1746-6148-8-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, H. K. , Tellez‐Gabriel, M. , & Heymann, D. (2017). Cancer stem cells in osteosarcoma. Cancer Letters, 386, 189–195. 10.1016/j.canlet.2016.11.019 [DOI] [PubMed] [Google Scholar]
- Bulut, G. , Hong, S. H. , Chen, K. , Beauchamp, E. M. , Rahim, S. , Kosturko, G. W. , Glasgow, E. , Dakshanamurthy, S. , Lee, H. S. , Daar, I. , Toretsky, J. A. , Khanna, C. , & Üren, A. (2012). Small molecule inhibitors of ezrin inhibit the invasive phenotype of osteosarcoma cells. Oncogene, 31(3), 269–281. 10.1038/onc.2011.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, Y. , Cai, T. , & Chen, Y. (2014). Wnt pathway in osteosarcoma, from oncogenic to therapeutic. Journal of Cellular Biochemistry, 115(4), 625–631. 10.1002/jcb.24708 [DOI] [PubMed] [Google Scholar]
- Capobianco, E. , Mora, A. , La Sala, D. , Roberti, A. , Zaki, N. , Badidi, E. , Taranta, M. , & Cinti, C. (2014). Separate and combined effects of DNMT and HDAC inhibitors in treating human multi‐drug resistant osteosarcoma HosDXR150 cell line. PLoS ONE, 9(4), e95596. 10.1371/journal.pone.0095596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çelik, H. , Bulut, G. , Han, J. , Graham, G. T. , Minas, T. Z. , Conn, E. J. , Hong, S.‐H. , Pauly, G. T. , Hayran, M. , Li, X. , Özdemirli, M. , Ayhan, A. , Rudek, M. A. , Toretsky, J. A. , & Üren, A. (2016). Ezrin inhibition up‐regulates stress response gene expression. Journal of Biological Chemistry, 291(25), 13257–13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddad, H. , Kuchler‐Bopp, S. , Fuhrmann, G. , Gegout, H. , Ubeaud‐Sequier, G. , Schwinté, P. , Bornert, F. , Benkirane‐Jessel, N. , & Idoux‐Gillet, Y. (2017). Combining 2D angiogenesis and 3D osteosarcoma microtissues to improve vascularization. Experimental Cell Research, 360(2), 138–145. 10.1016/j.yexcr.2017.08.035 [DOI] [PubMed] [Google Scholar]
- Chaiyawat, P. , Sirikaew, N. , Budprom, P. , Klangjorhor, J. , Phanphaisarn, A. , Teeyakasem, P. , Settakorn, J. , & Pruksakorn, D. (2020). Expression profiling of DNA methyl transferase I (DNMT1) and efficacy of a DNA‐hypomethylating agent (decitabine) in combination with chemotherapy in osteosarcoma. Journal of Bone Oncology, 25, 100321. 10.1016/j.jbo.2020.100321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charras, G. , & Sahai, E. (2014). Physical influences of the extracellular environment on cell migration. Nature Reviews Molecular Cell Biology, 15(12), 813–824. 10.1038/nrm3897 [DOI] [PubMed] [Google Scholar]
- Chen, Y. , Pasapera, A. M. , Koretsky, A. P. , & Waterman, C. M. (2013). Orientation‐specific responses to sustained uniaxial stretching in focal adhesion growth and turnover. Proceedings of the National Academy of Sciences, 110(26), E2352–E2361. 10.1073/pnas.1221637110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codelia, V. A. , Sun, G. , & Irvine, K. D. (2014). Regulation of YAP by mechanical strain through Jnk and hippo signaling. Current Biology, 24(17), 2012–2017. 10.1016/j.cub.2014.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, H. A. , Ohba, T. , Ichikawa, J. , Nyman, J. S. , Cates, J. M. , Haro, H. , Schwartz, H. S. , & Schoenecker, J. G. (2014). Micro‐computed tomography derived anisotropy detects tumor provoked deviations in bone in an orthotopic osteosarcoma murine model. PLoS ONE, 9(6), e97381. 10.1371/journal.pone.0097381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, M. R. , Lorbach, J. , Husbands, B. D. , Kisseberth, W. C. , Samuels, S. , Silveira, C. , Wustefeld‐Janssens, B. G. , Wouda, R. , Keepman, S. , Oblak, M. L. , & Selmic, L. E. (2021). A retrospective analysis of 12 dogs with surface osteosarcoma. Veterinary and Comparative Oncology. 10.1111/vco.12741 [DOI] [PubMed] [Google Scholar]
- Cooper, D. M. L. , Kawalilak, C. E. , Harrison, K. , Johnston, B. D. , & Johnston, J. D. (2016). Cortical bone porosity: What is it, why is it important, and how can we detect it? Current Osteoporosis Reports, 14(5), 187–198. 10.1007/s11914-016-0319-y [DOI] [PubMed] [Google Scholar]
- Cui, Y. , & Irudayaraj, J. (2015). Dissecting the behavior and function of MBD3 in DNA methylation homeostasis by single‐molecule spectroscopy and microscopy. Nucleic Acids Research, 43(6), 3046–3055. 10.1093/nar/gkv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, Y. , Liu, J. , & Irudayaraj, J. (2017). Beyond quantification: In situ analysis of transcriptome and pre‐mRNA alternative splicing at the nanoscale. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 9(4), e1443. [DOI] [PubMed] [Google Scholar]
- Damayanti, N. P. , Buno, K. , Cui, Y. , Voytik‐Harbin, S. L. , Pili, R. , Freeman, J. , & Irudayaraj, J. M. (2017). Real‐time multiplex kinase phosphorylation sensors in living cells. ACS Sensors, 2(8), 1225–1230. 10.1021/acssensors.7b00359 [DOI] [PubMed] [Google Scholar]
- Danieau, G. , Morice, S. , Rédini, F. , Verrecchia, F. , & Royer, B. L. (2019). New insights about the Wnt/β‐catenin signaling pathway in primary bone tumors and their microenvironment: A promising target to develop therapeutic strategies? International Journal of Molecular Sciences, 20(15), 3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daroszewska, A. , Hocking, L. J. , McGuigan, F. E. , Langdahl, B. , Stone, M. D. , Cundy, T. , Nicholson, G. C. , Fraser, W. D. , & Ralston, S. H. (2004). Susceptibility to Paget's disease of bone is influenced by a common polymorphic variant of osteoprotegerin. Journal of Bone and Mineral Research, 19(9), 1506–1511. 10.1359/JBMR.040602 [DOI] [PubMed] [Google Scholar]
- Deng, Z. , Liu, X. , Jin, J. , Xu, H. , Gao, Q. , Wang, Y. , & Zhao, J. (2016). Histone deacetylase inhibitor trichostatin a promotes the apoptosis of osteosarcoma cells through p53 signaling pathway activation. International Journal of Biological Sciences, 12(11), 1298–1308. 10.7150/ijbs.16569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristofano, C. , Leopizzi, M. , Miraglia, A. , Sardella, B. , Moretti, V. , Ferrara, A. , Petrozza, V. , & Della Rocca, C. (2010). Phosphorylated ezrin is located in the nucleus of the osteosarcoma cell. Modern Pathology, 23(7), 1012–1020. 10.1038/modpathol.2010.77 [DOI] [PubMed] [Google Scholar]
- Dickerson, M. E. , Page, R. L. , LaDue, T. A. , Hauck, M. L. , Thrall, D. E. , Stebbins, M. E. , & Price, G. S. (2001). Retrospective analysis of axial skeleton osteosarcoma in 22 large‐breed dogs. Journal of Veterinary Internal Medicine, 15(2), 120–124. [DOI] [PubMed] [Google Scholar]
- Domingues, C. , Geraldo, A. M. , Anjo, S. I. , Matos, A. , Almeida, C. , Caramelo, I. , Lopes‐da‐Silva, J. A. , Paiva, A. , Carvalho, J. , das Neves, R. P. , Manadas, B. , & Grãos, M. (2020). Cofilin‐1 is a mechanosensitive regulator of transcription. Frontiers in Cell and Development Biology, 8, 678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, C. , & Lv, Y. (2016). Application of collagen scaffold in tissue engineering: Recent advances and new perspectives. Polymers, 8(2), 42. 10.3390/polym8020042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake, M. T. , Clarke, B. L. , & Khosla, S. (2008). Bisphosphonates: Mechanism of action and role in clinical practice. In Mayo Clinic proceedings (Vol. 83) (pp. 1032–1045). Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, X. , Yang, J. , Yang, D. , Tian, W. , & Zhu, Z. (2014). The genetic basis for inactivation of Wnt pathway in human osteosarcoma. BMC Cancer, 14(1), 450. 10.1186/1471-2407-14-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elosegui‐Artola, A. , Andreu, I. , Beedle, A. E. , Lezamiz, A. , Uroz, M. , Kosmalska, A. J. , Oria, R. , Kechagia, J. Z. , Rico‐Lastres, P. , Le Roux, A. L. , & Shanahan, C. M. (2017). Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell, 171(6), 1397–1410. 10.1016/j.cell.2017.10.008 [DOI] [PubMed] [Google Scholar]
- Endo‐Munoz, L. , Cumming, A. , Rickwood, D. , Wilson, D. , Cueva, C. , Ng, C. , Strutton, G. , Cassady, A. I. , Evdokiou, A. , Sommerville, S. , Dickinson, I. , Guminski, A. , & Saunders, N. A. (2010). Loss of osteoclasts contributes to development of osteosarcoma pulmonary metastases. Cancer Research, 70(18), 7063–7072. 10.1158/0008-5472.CAN-09-4291 [DOI] [PubMed] [Google Scholar]
- Endo‐Munoz, L. , Evdokiou, A. , & Saunders, N. A. (2012). The role of osteoclasts and tumour‐associated macrophages in osteosarcoma metastasis. Biochimica et Biophysica Acta (BBA)‐Reviews on Cancer, 1826(2), 434–442. 10.1016/j.bbcan.2012.07.003 [DOI] [PubMed] [Google Scholar]
- Fan, T. M. , Roberts, R. D. , & Lizardo, M. M. (2020). Understanding and modeling metastasis biology to improve therapeutic strategies for combating osteosarcoma progression. Frontiers in Oncology, 10, 13. 10.3389/fonc.2020.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, F. , VanCleave, A. , Helmuth, R. , Torres, H. , Rickel, K. , Wollenzien, H. , Sun, H. , Zeng, E. , Zhao, J. , & Tao, J. (2018). Targeting the Wnt/β‐catenin pathway in human osteosarcoma cells. Oncotarget, 9(95), 36780–36792. 10.18632/oncotarget.26377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, H. , Tillman, H. , Wu, G. , Davidoff, A. M. , & Yang, J. (2018). Frequent epigenetic alterations in polycomb repressive complex 2 in osteosarcoma cell lines. Oncotarget, 9(43), 27087–27091. 10.18632/oncotarget.25484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, J. L. , & Turner, S. P. (2018). Bone cancer: Diagnosis and treatment principles. American Family Physician, 98(4), 205–213. [PubMed] [Google Scholar]
- Fledelius, C. , Johnsen, A. H. , Cloos, P. A. , Bonde, M. , & Qvist, P. (1997). Characterization of urinary degradation products derived from type I collagen identification of a β‐isomerized ASP‐GLY sequence within the C‐terminal telopeptide (α1) region. Journal of Biological Chemistry, 272(15), 9755–9763. 10.1074/jbc.272.15.9755 [DOI] [PubMed] [Google Scholar]
- Garnero, P. , Fledelius, C. , Gineyts, E. , Serre, C. M. , Vignot, E. , & Delmas, P. D. (1997). Decreased β‐isomerization of the C‐terminal telopeptide of type I collagen α1 chain in Paget's disease of bone. Journal of Bone and Mineral Research, 12(9), 1407–1415. 10.1359/jbmr.1997.12.9.1407 [DOI] [PubMed] [Google Scholar]
- Gautreau, A. , Poullet, P. , Louvard, D. , & Arpin, M. (1999). Ezrin, a plasma membrane–microfilament linker, signals cell survival through the phosphatidylinositol 3‐kinase/Akt pathway. Proceedings of the National Academy of Sciences, 96(13), 7300–7305. 10.1073/pnas.96.13.7300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennari, L. , Rendina, D. , Falchetti, A. , & Merlotti, D. (2019). Paget's disease of bone. Calcified Tissue International, 1–18. [DOI] [PubMed] [Google Scholar]
- Gong, T. , Su, X. , Xia, Q. , Wang, J. , & Kan, S. (2017). Expression of NF‐κB and PTEN in osteosarcoma and its clinical significance. Oncology Letters, 14(6), 6744–6748. 10.3892/ol.2017.6972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good, D. A. , Busfield, F. , Fletcher, B. H. , Duffy, D. L. , Kesting, J. B. , Andersen, J. , & Shaw, J. T. (2002). Linkage of Paget disease of bone to a novel region on human chromosome 18q23. The American Journal of Human Genetics, 70(2), 517–525. 10.1086/338658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco, M. R. , Moro, L. , Forciniti, S. , Alfarouk, K. , Cannone, S. , Cardone, R. A. , & Reshkin, S. J. (2021). Integrin‐linked kinase links integrin activation to invadopodia function and invasion via the p (T567)‐ezrin/NHERF1/NHE1 pathway. International Journal of Molecular Sciences, 22(4), 2162. 10.3390/ijms22042162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grier, W. K. , Moy, A. S. , & Harley, B. A. C. (2017). Cyclic tensile strain enhances human mesenchymal stem cell Smad 2/3 activation and tenogenic differentiation in anisotropic collagen‐glycosaminoglycan scaffolds. European Cells & Materials, 33, 227–239. 10.22203/eCM.v033a17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas, R. , & Dawid, I. B. (2005). Dishevelled and Wnt signaling: Is the nucleus the final frontier?. Journal of Biology, 4(1), 1–4. 10.1186/jbiol22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, M. F. , Seton, M. , & Merchant, A. (2006). Osteosarcoma in Paget's disease of bone. Journal of Bone and Mineral Research, 21(S2), P58–P63. [DOI] [PubMed] [Google Scholar]
- He, C. , Sun, J. , Liu, C. , Jiang, Y. , & Hao, Y. (2019). Elevated H3K27me3 levels sensitize osteosarcoma to cisplatin. Clinical Epigenetics, 11(1), 8. 10.1186/s13148-018-0605-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman, S. J. , Diefenderfer, D. L. , Goldschmidt, M. H. , & Newton, C. D. (1992). Canine axial skeletal osteosarcoma a retrospective study of 116 cases (1986 to 1989). Veterinary Surgery, 21(4), 304–310. 10.1111/j.1532-950X.1992.tb00069.x [DOI] [PubMed] [Google Scholar]
- Heymann, D. (2012). Anti‐RANKL therapy for bone tumours: Basic, pre‐clinical and clinical evidences. Journal of Bone Oncology, 1(1), 2–11. 10.1016/j.jbo.2012.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking, L. J. , Herbert, C. A. , Nicholls, R. K. , Williams, F. , Bennett, S. T. , Cundy, T. , Nicholson, G. C. , Wuyts, W. , van Hul, W. , & Ralston, S. H. (2001). Genomewide search in familial Paget disease of bone shows evidence of genetic heterogeneity with candidate loci on chromosomes 2q36, 10p13, and 5q35. The American Journal of Human Genetics, 69(5), 1055–1061. 10.1086/323798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaska, J. M. , & Wilson, K. L. (2006). Multiple roles for emerin: Implications for Emery–Dreifuss muscular dystrophy. The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology: An Official Publication of the American Association of Anatomists, 288(7), 676–680. 10.1002/ar.a.20334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskin, V. , Szeto, A. , Ghaffari, A. , Greer, P. A. , Côté, G. P. , & Elliott, B. E. (2015). Ezrin regulates focal adhesion and invadopodia dynamics by altering calpain activity to promote breast cancer cell invasion. Molecular Biology of the Cell, 26(19), 3464–3479. 10.1091/mbc.E14-12-1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, C. H. , Yang, R. S. , & Tsao, Y. T. (2018). Connective tissue growth factor stimulates osteosarcoma cell migration and induces osteosarcoma metastasis by upregulating VCAM‐1 expression. Biochemical Pharmacology, 155, 71–81. 10.1016/j.bcp.2018.06.015 [DOI] [PubMed] [Google Scholar]
- Howlader, N. N. A. M. , Noone, A. M. , Krapcho, M. E. , Miller, D. , Brest, A. , Yu, M. , Ruhl, J. , Tatalovich, Z. , Mariotto, A. , Lewis, D. R. , & Chen, H. S. (2019). SEER cancer statistics review, 1975–2016 (pp. 1423–1437). Bethesda, MD: National Cancer Institute. https://seer.cancer.gov/csr/1975_2016/ [Google Scholar]
- Hsieh, Y. H. , Hsu, W. H. , Yang, S. F. , Liu, C. J. , Lu, K. H. , Wang, P. H. , & Lin, R. C. (2021). Potential antimetastatic effect of timosaponin AIII against human osteosarcoma cells through regulating the integrin/FAK/cofilin axis. Pharmaceuticals, 14(3), 260. 10.3390/ph14030260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y. , Zhao, S. , Zhang, C. , & Li, X. (2016). Downregulation of connective tissue growth factor reduces migration and invasiveness of osteosarcoma cells. Molecular Medicine Reports, 13(2), 1888–1894. 10.3892/mmr.2015.4701 [DOI] [PubMed] [Google Scholar]
- Husari, A. , Steinberg, T. , Dieterle, M. P. , Prucker, O. , Rühe, J. , Jung, B. , & Tomakidi, P. (2019). On the relationship of YAP and FAK in hMSCs and osteosarcoma cells: Discrimination of FAK modulation by nuclear YAP depletion or YAP silencing. Cellular Signalling, 63, 109382. 10.1016/j.cellsig.2019.109382 [DOI] [PubMed] [Google Scholar]
- Idris, A. I. , Rojas, J. , Greig, I. R. , van't Hof, R. J. , & Ralston, S. H. (2008). Aminobisphosphonates cause osteoblast apoptosis and inhibit bone nodule formation in vitro. Calcified Tissue International, 82(3), 191–201. 10.1007/s00223-008-9104-y [DOI] [PubMed] [Google Scholar]
- Ishitani, T. , Ninomiya‐Tsuji, J. , Nagai, S. I. , Nishita, M. , Meneghini, M. , Barker, N. , Waterman, M. , Bowerman, B. , Clevers, H. , Shibuya, H. , & Matsumoto, K. (1999). The TAK1–NLK–MAPK‐related pathway antagonizes signaling between β‐catenin and transcription factor TCF. Nature, 399(6738), 798–802. 10.1038/21674 [DOI] [PubMed] [Google Scholar]
- Jabbari, E. , Sarvestani, S. K. , Daneshian, L. , & Moeinzadeh, S. (2015). Optimum 3D matrix stiffness for maintenance of cancer stem cells is dependent on tissue origin of cancer cells. PLoS ONE, 10(7), e0132377. 10.1371/journal.pone.0132377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana, A. , Nookaew, I. , Singh, J. , Behkam, B. , Franco, A. T. , & Nain, A. S. (2019). Crosshatch nanofiber networks of tunable interfiber spacing induce plasticity in cell migration and cytoskeletal response. The FASEB Journal, 33(10), 10618–10632. 10.1096/fj.201900131R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y. , Li, F. , Gao, B. , Ma, M. , Chen, M. , Wu, Y. , Zhang, W. , Sun, Y. , Liu, S. , & Shen, H. (2021). KDM6B‐mediated histone demethylation of LDHA promotes lung metastasis of osteosarcoma. Theranostics, 11(8), 3868–3881. 10.7150/thno.53347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juriga, D. , Nagy, K. , Jedlovszky‐Hajdú, A. , Perczel‐Kovách, K. , Chen, Y. M. , Varga, G. , & Zrínyi, M. (2016). Biodegradation and osteosarcoma cell cultivation on poly (aspartic acid) based hydrogels. ACS Applied Materials & Interfaces, 8(36), 23463–23476. 10.1021/acsami.6b06489 [DOI] [PubMed] [Google Scholar]
- Kim, D. H. , Khatau, S. B. , Feng, Y. , Walcott, S. , Sun, S. X. , Longmore, G. D. , & Wirtz, D. (2012). Actin cap associated focal adhesions and their distinct role in cellular mechanosensing. Scientific Reports, 2, 555. 10.1038/srep00555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D. H. , & Wirtz, D. (2015). Cytoskeletal tension induces the polarized architecture of the nucleus. Biomaterials, 48, 161–172. 10.1016/j.biomaterials.2015.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya, Y. , & Habas, R. (2008). Wnt signal transduction pathways. Organogenesis, 4(2), 68–75. 10.4161/org.4.2.5851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le, H. Q. , Ghatak, S. , Yeung, C. Y. C. , Tellkamp, F. , Günschmann, C. , Dieterich, C. , Yeroslaviz, A. , Habermann, B. , Pombo, A. , Niessen, C. M. , & Wickström, S. A. (2016). Mechanical regulation of transcription controls polycomb‐mediated gene silencing during lineage commitment. Nature Cell Biology, 18(8), 864–875. 10.1038/ncb3387 [DOI] [PubMed] [Google Scholar]
- Leeming, D. J. , Delling, G. , Koizumi, M. , Henriksen, K. , Karsdal, M. A. , Li, B. , Qvist, P. , Tankó, L. B. , & Byrjalsen, I. (2006). Alpha CTX as a biomarker of skeletal invasion of breast cancer: Immunolocalization and the load dependency of urinary excretion. Cancer Epidemiology and Prevention Biomarkers, 15(7), 1392–1395. 10.1158/1055-9965.EPI-05-0909 [DOI] [PubMed] [Google Scholar]
- Lewis, D. M. , Park, K. M. , Tang, V. , Xu, Y. , Pak, K. , Eisinger‐Mathason, T. K. , Simon, M. C. , & Gerecht, S. (2016). Intratumoral oxygen gradients mediate sarcoma cell invasion. Proceedings of the National Academy of Sciences, 113(33), 9292–9297. 10.1073/pnas.1605317113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R. , Shi, Y. , Zhao, S. , Shi, T. , & Zhang, G. (2019). NF‐κB signaling and integrin‐β1 inhibition attenuates osteosarcoma metastasis via increased cell apoptosis. International Journal of Biological Macromolecules, 123, 1035–1043. 10.1016/j.ijbiomac.2018.11.003 [DOI] [PubMed] [Google Scholar]
- Liao, S. S. , Cui, F. Z. , Zhang, W. , & Feng, Q. L. (2004). Hierarchically biomimetic bone scaffold materials: Nano‐HA/collagen/PLA composite. Journal of Biomedical Materials Research Part B: Applied Biomaterials: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials, 69(2), 158–165. 10.1002/jbm.b.20035 [DOI] [PubMed] [Google Scholar]
- Lin, J. , Shi, Y. , Men, Y. , Wang, X. , Ye, J. , & Zhang, C. (2020). Mechanical roles in formation of oriented collagen fibers. Tissue Engineering Part B: Reviews, 26(2), 116–128. 10.1089/ten.TEB.2019.0243 [DOI] [PubMed] [Google Scholar]
- Liu, W. , Cui, Y. , Ren, W. , & Irudayaraj, J. (2019). Epigenetic biomarker screening by FLIM‐FRET for combination therapy in ER+ breast cancer. Clinical Epigenetics, 11(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo, C. M. , Wang, H. B. , Dembo, M. , & Wang, Y. L. (2000). Cell movement is guided by the rigidity of the substrate. Biophysical Journal, 79(1), 144–152. 10.1016/S0006-3495(00)76279-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas, G. J. , Daroszewska, A. , & Ralston, S. H. (2006). Contribution of genetic factors to the pathogenesis of Paget's disease of bone and related disorders. Journal of Bone and Mineral Research, 21(S2), P31–P37. 10.1359/jbmr.06s206 [DOI] [PubMed] [Google Scholar]
- Luu, A. K. , & Viloria‐Petit, A. M. (2020). Targeting mechanotransduction in osteosarcoma: A comparative oncology perspective. International Journal of Molecular Sciences, 21(20), 7595. 10.3390/ijms21207595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv, Y. F. , Yan, G. N. , Meng, G. , Zhang, X. , & Guo, Q. N. (2015). Enhancer of zeste homolog 2 silencing inhibits tumor growth and lung metastasis in osteosarcoma. Scientific Reports, 5, 12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchandet, L. , Lallier, M. , Charrier, C. , Baud'huin, M. , Ory, B. , & Lamoureux, F. (2021). Mechanisms of resistance to conventional therapies for osteosarcoma. Cancers, 13(4), 683. 10.3390/cancers13040683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marley, K. , Bracha, S. , & Seguin, B. (2015). Osteoprotegerin activates osteosarcoma cells that co‐express RANK and RANKL. Experimental Cell Research, 338(1), 32–38. [DOI] [PubMed] [Google Scholar]
- Martin, J. W. , Squire, J. A. , & Zielenska, M. (2012). The genetics of osteosarcoma. Sarcoma, 2012, 1–11. 10.1155/2012/627254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, D. E. , Collins, J. M. , Dawahare, J. H. , Nguyen, T. D. , Lin, Y. , Voytik‐Harbin, S. L. , Zorlutuna, P. , Yoder, M. C. , & Boerckel, J. D. (2019). YAP and TAZ limit cytoskeletal and focal adhesion maturation to enable persistent cell motility. Journal of Cell Biology, 218(4), 1369–1389. 10.1083/jcb.201806065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire, J. J. , Nerlakanti, N. , Lo, C. H. , Tauro, M. , Utset‐Ward, T. J. , Reed, D. R. , & Lynch, C. C. (2020). Histone deacetylase inhibition prevents the growth of primary and metastatic osteosarcoma. International Journal of Cancer, 147(10), 2811–2823. 10.1002/ijc.33046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNairn, J. D. , Damron, T. A. , Landas, S. K. , Ambrose, J. L. , & Shrimpton, A. E. (2001). Inheritance of osteosarcoma and Paget's disease of bone: A familial loss of heterozygosity study. The Journal of Molecular Diagnostics, 3(4), 171–177. 10.1016/S1525-1578(10)60669-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen, P. , Ghaffar, S. , Guo, Y. , Rubin, E. M. , Zi, X. , & Hoang, B. H. (2011). The Wnt signaling pathway: Implications for therapy in osteosarcoma. Expert Review of Anticancer Therapy, 11(8), 1223–1232. 10.1586/era.11.94 [DOI] [PubMed] [Google Scholar]
- Michou, L. , & Brown, J. P. (2011). Genetics of bone diseases: Paget's disease, fibrous dysplasia, osteopetrosis, and osteogenesis imperfecta. Joint, Bone, Spine, 78(3), 252–258. 10.1016/j.jbspin.2010.07.010 [DOI] [PubMed] [Google Scholar]
- Misaghi, A. , Goldin, A. , Awad, M. , & Kulidjian, A. A. (2018). Osteosarcoma: A comprehensive review. SICOT‐J, 4, 12. 10.1051/sicotj/2017028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misu, S. , Takebayashi, M. , & Miyamoto, K. (2017). Nuclear actin in development and transcriptional reprogramming. Frontiers in Genetics, 8, 27. 10.3389/fgene.2017.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro, M. V. , Gaspar, V. M. , Ferreira, L. P. , & Mano, J. F. (2020). Hydrogel 3D in vitro tumor models for screening cell aggregation mediated drug response. Biomaterials Science, 8(7), 1855–1864. 10.1039/C9BM02075F [DOI] [PubMed] [Google Scholar]
- Mori, K. , Ando, K. , Heymann, D. , & Rédini, F. (2009). Receptor activator of nuclear factor‐kappa B ligand (RANKL) stimulates bone‐associated tumors through functional RANK expressed on bone‐associated cancer cells?. Histology and Histopathology, 24(2), 235–242. 10.14670/HH-24.235 [DOI] [PubMed] [Google Scholar]
- Mori, K. , Ando, K. , Heymann, D. & Rédini, F. (2009). Receptor activator of nuclear factor‐kappa B ligand (RANKL) stimulates bone‐associated tumors through functional RANK expressed on bone‐associated cancer cells? [DOI] [PubMed]
- Morice, S. , Mullard, M. , Brion, R. , Dupuy, M. , Renault, S. , Tesfaye, R. , Royer, B. L. , Ory, B. , Redini, F. , & Verrecchia, F. (2020). The YAP/TEAD axis as a new therapeutic target in osteosarcoma: Effect of verteporfin and CA3 on primary tumor growth. Cancers, 12(12), 3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya, I. M. , & Halder, G. (2019). Hippo–YAP/TAZ signalling in organ regeneration and regenerative medicine. Nature Reviews Molecular Cell Biology, 20(4), 211–226. 10.1038/s41580-018-0086-y [DOI] [PubMed] [Google Scholar]
- Mozdzen, L. C. , Vucetic, A. , & Harley, B. A. (2017). Modifying the strength and strain concentration profile within collagen scaffolds using customizable arrays of poly‐lactic acid fibers. Journal of the Mechanical Behavior of Biomedical Materials, 66, 28–36. 10.1016/j.jmbbm.2016.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu, X. , Brynien, D. , & Weiss, K. R. (2015). The HDAC inhibitor vorinostat diminishes the in vitro metastatic behavior of osteosarcoma cells. BioMed Research International, 2015, 290368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, D. A. , & Silvan, U. (2019). On the biomechanical properties of osteosarcoma cells and their environment. The International Journal of Developmental Biology, 63(1–2), 1–8. 10.1387/ijdb.190019us [DOI] [PubMed] [Google Scholar]
- Mylona, E. , Dailiana, Z. H. , Trepat, X. , & Lagoudakis, M. G. (2008). Substrate rigidity dictates phenotype, survival, and mechanics of primary human osteosarcoma cells. European Symposium on Biomedical Engineering, 1(4). [Google Scholar]
- Nakai, Y. , Okamoto, K. , Terashima, A. , Ehata, S. , Nishida, J. , Imamura, T. , Ono, T. , & Takayanagi, H. (2019). Efficacy of an orally active small‐molecule inhibitor of RANKL in bone metastasis. Bone Research, 7(1), 1–10. 10.1038/s41413-018-0036-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka, K. , Nishizawa, Y. , & Ralston, S. H. (2003). Phenotypic characterization of early onset paget's disease of bone caused by a 27‐bp duplication in the TNFRSF11A gene. Journal of Bone and Mineral Research, 18(8), 1381–1385. 10.1359/jbmr.2003.18.8.1381 [DOI] [PubMed] [Google Scholar]
- Oh, J. H. , Kim, H. S. , Kim, H. H. , Kim, W. H. , & Lee, S. H. (2006). Aberrant methylation of p14ARF gene correlates with poor survival in osteosarcoma. Clinical Orthopaedics and Related Research (1976–2007), 442, 216–222. 10.1097/01.blo.0000188063.56091.69 [DOI] [PubMed] [Google Scholar]
- Ohba, T. , Cole, H. A. , Cates, J. M. , Slosky, D. A. , Haro, H. , Ando, T. , Schwartz, H. S. , & Schoenecker, J. G. (2014). Bisphosphonates inhibit osteosarcoma‐mediated osteolysis via attenuation of tumor expression of MCP‐1 and RANKL. Journal of Bone and Mineral Research, 29(6), 1431–1445. 10.1002/jbmr.2182 [DOI] [PubMed] [Google Scholar]
- Osuna, M. A. L. , Garcia‐Lopez, J. , El Ayachi, I. , Fatima, I. , Khalid, A. B. , Kumpati, J. , Slayden, A. V. , Seagroves, T. N. , Miranda‐Carboni, G. A. , & Krum, S. A. (2019). Activation of estrogen receptor alpha by decitabine inhibits osteosarcoma growth and metastasis. Cancer Research, 79(6), 1054–1068. 10.1158/0008-5472.CAN-18-1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panciera, T. , Azzolin, L. , Cordenonsi, M. , & Piccolo, S. (2017). Mechanobiology of YAP and TAZ in physiology and disease. Nature Reviews Molecular Cell Biology, 18(12), 758–770. 10.1038/nrm.2017.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, D. , Richert, A. , Medjkane, S. , Hénon, S. , & Weitzman, J. (2020). The impact of cell geometry and the cytoskeleton on the nucleo‐cytoplasmic localisation of the SMYD3 methyltransferase suggests that the epigenetic machinery is mechanosensitive. bioRxiv. [DOI] [PMC free article] [PubMed]
- Pettke, A. , Hotfilder, M. , Clemens, D. , Klco‐Brosius, S. , Schaefer, C. , Potratz, J. , & Dirksen, U. (2016). Suberanilohydroxamic acid (vorinostat) synergistically enhances the cytotoxicity of doxorubicin and cisplatin in osteosarcoma cell lines. Anti‐Cancer Drugs, 27(10), 1001–1010. 10.1097/CAD.0000000000000418 [DOI] [PubMed] [Google Scholar]
- Piperno‐Neumann, S. , Le Deley, M. C. , Rédini, F. , Pacquement, H. , Marec‐Bérard, P. , Petit, P. , Brisse, H. , Lervat, C. , Gentet, J. C. , Entz‐Werlé, N. , Italiano, A. , Corradini, N. , Bompas, E. , Penel, N. , Tabone, M. D. , Gomez‐Brouchet, A. , Guinebretière, J. M. , Mascard, E. , Gouin, F. , … Sarcoma Group of UNICANCER, French Society of Pediatric Oncology (SFCE), French Sarcoma Group (GSF‐GETO) . (2016). Zoledronate in combination with chemotherapy and surgery to treat osteosarcoma (OS2006): A randomised, multicentre, open‐label, phase 3 trial. The Lancet Oncology, 17(8), 1070–1080. [DOI] [PubMed] [Google Scholar]
- Plotkin, L. I. , Aguirre, J. I. , Kousteni, S. , Manolagas, S. C. , & Bellido, T. (2005). Bisphosphonates and estrogens inhibit osteocyte apoptosis via distinct molecular mechanisms downstream of extracellular signal‐regulated kinase activation. Journal of Biological Chemistry, 280(8), 7317–7325. 10.1074/jbc.M412817200 [DOI] [PubMed] [Google Scholar]
- Pobbati, A. V. , & Hong, W. (2020). A combat with the YAP/TAZ‐TEAD oncoproteins for cancer therapy. Theranostics, 10(8), 3622–3635. 10.7150/thno.40889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poudel, B. , Kim, D. K. , Ki, H. H. , Kwon, Y. B. , Lee, Y. M. , & Kim, D. K. (2014). Downregulation of ERK signaling impairs U2OS osteosarcoma cell migration in collagen matrix by suppressing MMP9 production. Oncology Letters, 7(1), 215–218. 10.3892/ol.2013.1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punzo, F. , Tortora, C. , Argenziano, M. , Di Pinto, D. , Pota, E. , Di Martino, M. , Di Paola, A. , & Rossi, F. (2020). Can denosumab be used in combination with doxorubicin in osteosarcoma? Oncotarget, 11(28), 2763–2773. 10.18632/oncotarget.27669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao‐Bindal, K. , Koshkina, N. V. , Stewart, J. , & Kleinerman, E. S. (2013). The histone deacetylase inhibitor, MS‐275 (entinostat), downregulates c‐FLIP, sensitizes osteosarcoma cells to FasL, and induces the regression of osteosarcoma lung metastases. Current Cancer Drug Targets, 13(4), 411–422. 10.2174/1568009611313040005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, L. , Hong, S. H. , Cassavaugh, J. , Osborne, T. , Chou, A. J. , Kim, S. Y. , Gorlick, R. , Hewitt, S. M. , & Khanna, C. (2009). The actin‐cytoskeleton linker protein ezrin is regulated during osteosarcoma metastasis by PKC. Oncogene, 28(6), 792–802. 10.1038/onc.2008.437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, X. , Zhou, Q. , Foulad, D. , Tiffany, A. S. , Dewey, M. J. , Bischoff, D. , Miller, T. A. , Reid, R. R. , He, T.‐c. , Yamaguchi, D. T. , Harley, B. A. C. , & Lee, J. C. (2019). Osteoprotegerin reduces osteoclast resorption activity without affecting osteogenesis on nanoparticulate mineralized collagen scaffolds. Science Advances, 5(6), eaaw4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothzerg, E. , Ingley, E. , Mullin, B. , Xue, W. , Wood, D. , & Xu, J. (2021). The Hippo in the room: Targeting the Hippo signalling pathway for osteosarcoma therapies. Journal of Cellular Physiology, 236(3), 1606–1615. 10.1002/jcp.29967 [DOI] [PubMed] [Google Scholar]
- Rousseau, J. , Escriou, V. , Lamoureux, F. , Brion, R. , Chesneau, J. , Battaglia, S. , Amiaud, J. , Scherman, D. , Heymann, D. , Rédini, F. , & Trichet, V. (2011). Formulated siRNAs targeting Rankl prevent osteolysis and enhance chemotherapeutic response in osteosarcoma models. Journal of Bone and Mineral Research, 26(10), 2452–2462. 10.1002/jbmr.455 [DOI] [PubMed] [Google Scholar]
- Rubio, R. , Abarrategi, A. , Garcia‐Castro, J. , Martinez‐Cruzado, L. , Suarez, C. , Tornin, J. , Santos, L. , Astudillo, A. , Colmenero, I. , Mulero, F. , & Rosu‐Myles, M. (2014). Bone environment is essential for osteosarcoma development from transformed mesenchymal stem cells. Stem Cells, 32(5), 1136–1148. 10.1002/stem.1647 [DOI] [PubMed] [Google Scholar]
- Sekita, A. , Matsugaki, A. , & Nakano, T. (2016). Disruption of collagen matrix alignment in osteolytic bone metastasis induced by breast cancer. Materials Transactions, 57(12), 2077–2082. 10.2320/matertrans.M2016341 [DOI] [Google Scholar]
- Sekita, A. , Matsugaki, A. , & Nakano, T. (2017). Disruption of collagen/apatite alignment impairs bone mechanical function in osteoblastic metastasis induced by prostate cancer. Bone, 97, 83–93. 10.1016/j.bone.2017.01.004 [DOI] [PubMed] [Google Scholar]
- Sen, B. , Xie, Z. , Uzer, G. , Thompson, W. R. , Styner, M. , Wu, X. , & Rubin, J. (2015). Intranuclear actin regulates osteogenesis. Stem Cells, 33(10), 3065–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, X. , Li, Q. , Mogilner, A. , Bershadsky, A. D. , & Shivashankar, G. V. (2015). Mechanical stimulation induces formin‐dependent assembly of a perinuclear actin rim. Proceedings of the National Academy of Sciences, 112(20), E2595–E2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer, F. R. (2016). Bone quality in Paget's disease of bone. Current Osteoporosis Reports, 14(2), 39–42. 10.1007/s11914-016-0303-6 [DOI] [PubMed] [Google Scholar]
- Sobacchi, C. , Schulz, A. , Coxon, F. P. , Villa, A. , & Helfrich, M. H. (2013). Osteopetrosis: Genetics, treatment and new insights into osteoclast function. Nature Reviews Endocrinology, 9(9), 522–536. 10.1038/nrendo.2013.137 [DOI] [PubMed] [Google Scholar]
- Sparks, A. B. , Peterson, S. N. , Bell, C. , Loftus, B. J. , Hocking, L. , Cahill, D. P. , Frassica, F. J. , Streeten, E. A. , Levine, M. A. , Fraser, C. M. , Adams, M. D. , Broder, S. , Venter, J. C. , Kinzler, K. W. , Vogelstein, B. , & Ralston, S. H. (2001). Mutation screening of the TNFRSF11A gene encoding receptor activator of NFkB (RANK) in familial and sporadic paget's disease of bone and osteosarcoma. Calcified Tissue International, 68(3), 151–155. 10.1007/s002230001211 [DOI] [PubMed] [Google Scholar]
- Sun, R. , Shen, J. , Gao, Y. , Zhou, Y. , Yu, Z. , Hornicek, F. , Kan, Q. , & Duan, Z. (2016). Overexpression of EZH2 is associated with the poor prognosis in osteosarcoma and function analysis indicates a therapeutic potential. Oncotarget, 7(25), 38333–38346. 10.18632/oncotarget.9518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, T. , Yui, Y. , Naka, N. , Wakamatsu, T. , Yoshioka, K. , Araki, N. , Yoshikawa, H. , & Itoh, K. (2013). Dynamic analysis of lung metastasis by mouse osteosarcoma LM8: VEGF is a candidate for anti‐metastasis therapy. Clinical & Experimental Metastasis, 30(4), 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbitt, M. W. , & Anseth, K. S. (2009). Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnology and Bioengineering, 103(4), 655–663. 10.1002/bit.22361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany, A. S. , Dewey, M. J. , & Harley, B. A. (2020). Sequential sequestrations increase the incorporation and retention of multiple growth factors in mineralized collagen scaffolds. RSC Advances, 10(45), 26982–26996. 10.1039/D0RA03872E [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany, A. S. , Gray, D. L. , Woods, T. J. , Subedi, K. , & Harley, B. A. (2019). The inclusion of zinc into mineralized collagen scaffolds for craniofacial bone repair applications. Acta Biomaterialia, 93, 86–96. 10.1016/j.actbio.2019.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres, H. M. , Van Cleave, A. , Palmer, M. , Callahan, D. , Smithback, A. , May, D. , May, D. , Roux, K. , & Tao, J. (2020). Antitumor effects of an epigenetic inhibitor, 4SC‐202, on human osteosarcoma cells. [abstract]. In: Proceedings of the Annual Meeting of the AACR 2020, Philadelphia (PA): AACR; Cancer Res 2020;80(16 Suppl):Abstract no. 2917.
- Tovazzi, V. , Dalla Volta, A. , Pedersini, R. , Amoroso, V. , & Berruti, A. (2019). Excess of second tumors in denosumab‐treated patients: A metabolic hypothesis. Future Oncology, 15(20), 2319–2321. 10.2217/fon-2019-0170 [DOI] [PubMed] [Google Scholar]
- Trinidad, E. M. , & González‐Suárez, E. (2016). RANKL inhibitors for osteosarcoma treatment: Hope and caution. Annals of Translational Medicine, 4(24), 534. 10.21037/atm.2016.12.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumaneng, K. , Schlegelmilch, K. , Russell, R. C. , Yimlamai, D. , Basnet, H. , Mahadevan, N. , Fitamant, J. , Bardeesy, N. , Camargo, F. D. , & Guan, K. L. (2012). YAP mediates crosstalk between the Hippo and PI(3)K–TOR pathways by suppressing PTEN via miR‐29. Nature Cell Biology, 14(12), 1322–1329. 10.1038/ncb2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, C. H. , Rho, J. , Takano, Y. , Tsui, T. Y. , & Pharr, G. M. (1999). The elastic properties of trabecular and cortical bone tissues are similar: Results from two microscopic measurement techniques. Journal of Biomechanics, 32(4), 437–441. 10.1016/S0021-9290(98)00177-8 [DOI] [PubMed] [Google Scholar]
- Viguet‐Carrin, S. , Garnero, P. , & Delmas, P. D. (2006). The role of collagen in bone strength. Osteoporosis International, 17(3), 319–336. 10.1007/s00198-005-2035-9 [DOI] [PubMed] [Google Scholar]
- Wan, X. , Kim, S. Y. , Guenther, L. M. , Mendoza, A. , Briggs, J. , Yeung, C. , Currier, D. , Zhang, H. , Mackall, C. , Li, W. J. , & Tuan, R. S. (2009). Beta4 integrin promotes osteosarcoma metastasis and interacts with ezrin. Oncogene, 28(38), 3401–3411. 10.1038/onc.2009.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshaw, J. , Bromage, T. G. , Terranova, C. J. , & Enlow, D. H. (2017). Collagen fiber orientation in primate long bones. The Anatomical Record, 300(7), 1189–1207. 10.1002/ar.23571 [DOI] [PubMed] [Google Scholar]
- Wei, M. , Fan, X. , Ding, M. , Li, R. , Shao, S. , Hou, Y. , Meng, S. , Tang, F. , Li, C. , & Sun, Y. (2020). Nuclear actin regulates inducible transcription by enhancing RNA polymerase II clustering. Science Advances, 6(16), eaay6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, L. W. , Yang, M. , Dong, J. , Xie, H. , Sui, G. L. , He, Y. L. , Lei, J. X. , Liao, E. Y. , & Yuan, X. (2011). Stretch‐inducible expression of connective tissue growth factor (CTGF) in human osteoblasts‐like cells is mediated by PI3K‐JNK pathway. Cellular Physiology and Biochemistry, 28(2), 297–304. 10.1159/000331743 [DOI] [PubMed] [Google Scholar]
- Xie, C. , Wu, B. , Chen, B. , Shi, Q. , Guo, J. , Fan, Z. , & Huang, Y. (2016). Histone deacetylase inhibitor sodium butyrate suppresses proliferation and promotes apoptosis in osteosarcoma cells by regulation of the MDM2–p53 signaling. Oncotargets and Therapy, 9, 4005–4013. 10.2147/OTT.S105418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, Q. , Tang, L. , Cai, K. , Tong, R. , Sternberg, R. , Yang, X. , Dobrucki, L. W. , Borst, L. B. , Kamstock, D. , Song, Z. , Helferich, W. G. , Cheng, J. , & Fan, T. M. (2016). Pamidronate functionalized nanoconjugates for targeted therapy of focal skeletal malignant osteolysis. Proceedings of the National Academy of Sciences, 113(32), E4601–E4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, L. , Rowe, D. W. , Perera, I. P. , Zhang, J. , Suib, S. L. , Xin, X. , & Wei, M. (2020). Intrafibrillar mineralized collagen–hydroxyapatite‐based scaffolds for bone regeneration. ACS Applied Materials & Interfaces, 12(16), 18235–18249. 10.1021/acsami.0c00275 [DOI] [PubMed] [Google Scholar]
- Yuan, Y. , Song, J. X. , Zhang, M. N. , & Yuan, B. S. (2020). A multiple drug loaded, functionalized pH‐sensitive nanocarrier as therapeutic and epigenetic modulator for osteosarcoma. Scientific Reports, 10(1), 1–11. 10.1038/s41598-020-72552-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue, S. , He, H. , Li, B. , & Hou, T. (2020). Hydrogel as a biomaterial for bone tissue engineering: A review. Nanomaterials, 10(8), 1511. 10.3390/nano10081511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanconato, F. , Battilana, G. , Forcato, M. , Filippi, L. , Azzolin, L. , Manfrin, A. , Quaranta, E. , Di Biagio, D. , Sigismondo, G. , Guzzardo, V. , & Lejeune, P. (2018). Transcriptional addiction in cancer cells is mediated by YAP/TAZ through BRD4. Nature Medicine, 24(10), 1599–1610. 10.1038/s41591-018-0158-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Q. , Lyu, S. , Bertrand, A. A. , Hu, A. C. , Chan, C. H. , Ren, X. , Dewey, M. J. , Tiffany, A. S. , Harley, B. A. C. , & Lee, J. C. (2021). Stiffness of nanoparticulate mineralized collagen scaffolds triggers osteogenesis via mechanotransduction and canonical Wnt signaling. Macromolecular Bioscience, 21(3), 2000370. 10.1002/mabi.202000370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann, E. A. , Köhne, T. , Bale, H. A. , Panganiban, B. , Gludovatz, B. , Zustin, J. , Hahn, M. , Amling, M. , Ritchie, R. O. , & Busse, B. (2015). Modifications to nano‐and microstructural quality and the effects on mechanical integrity in Paget's disease of bone. Journal of Bone and Mineral Research, 30(2), 264–273. 10.1002/jbmr.2340 [DOI] [PubMed] [Google Scholar]
- Zou, Z. , Huang, B. , Wu, X. , Zhang, H. , Qi, J. , Bradner, J. , Nair, S. , & Chen, L. F. (2014). Brd4 maintains constitutively active nf‐κb in cancer cells by binding to acetylated rela. Oncogene, 33(18), 2395–2404. 10.1038/onc.2013.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no new data were created or analysed in this study.


