Abstract
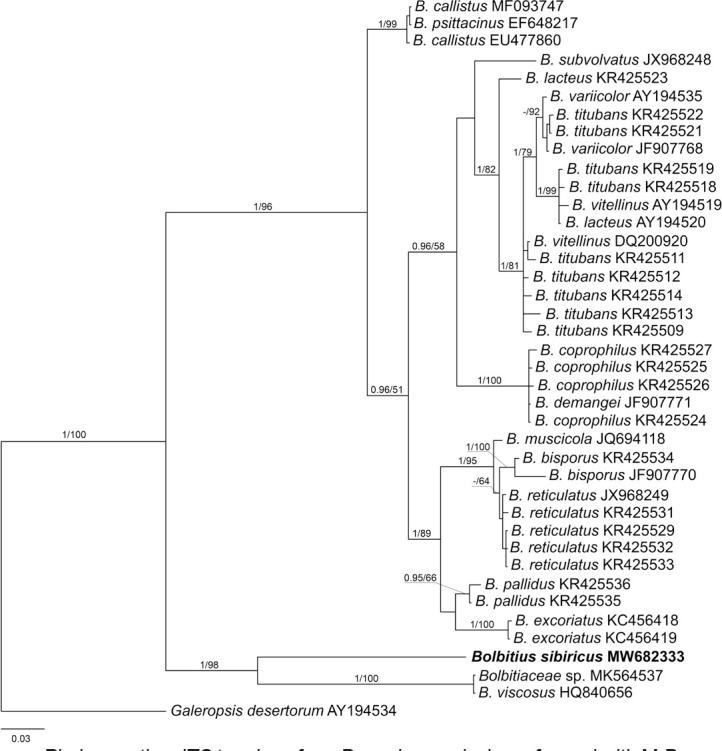
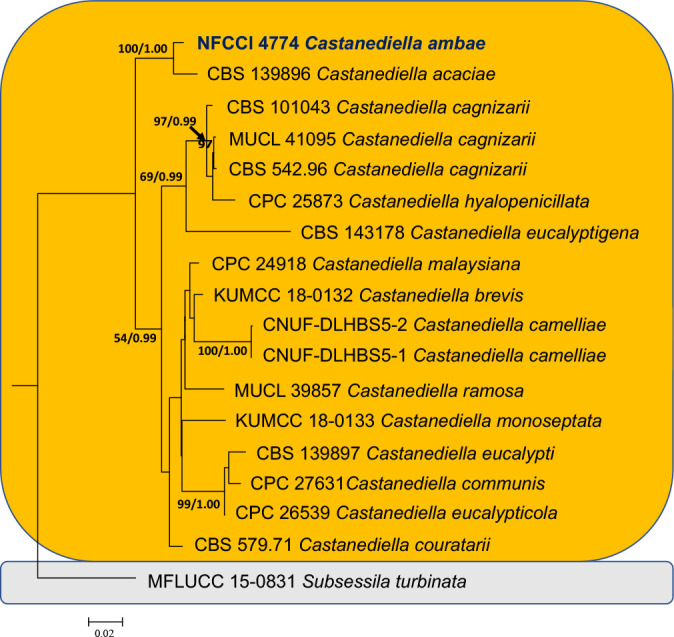
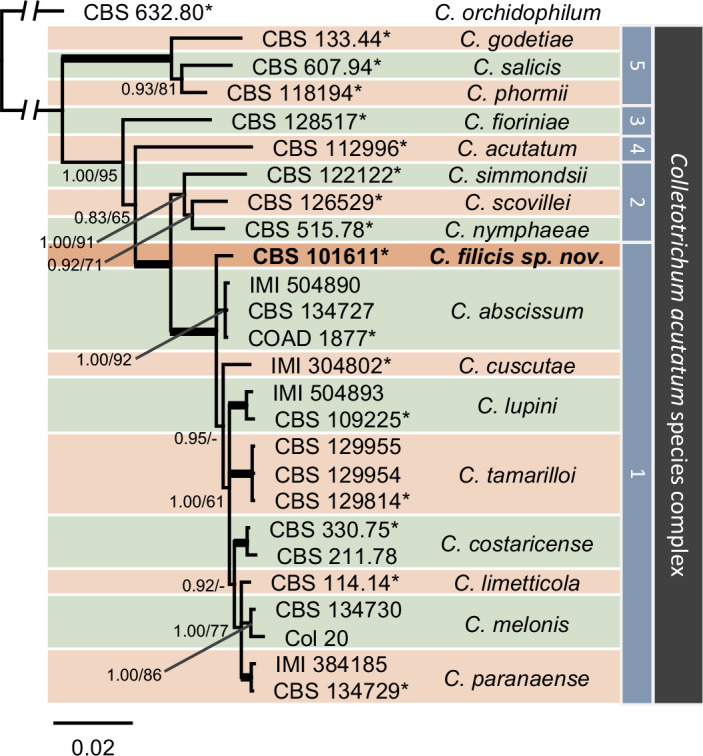
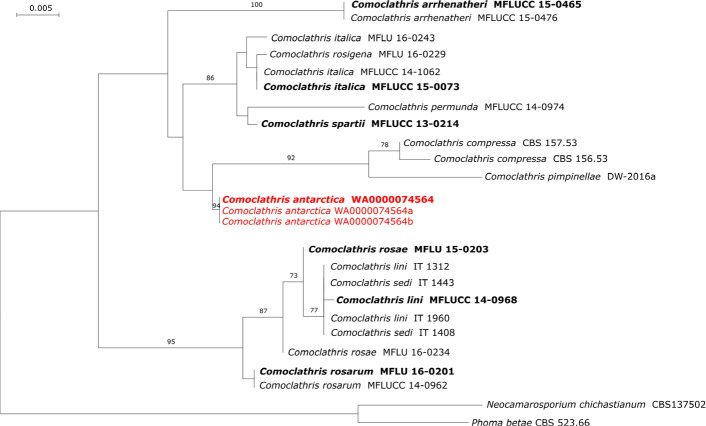
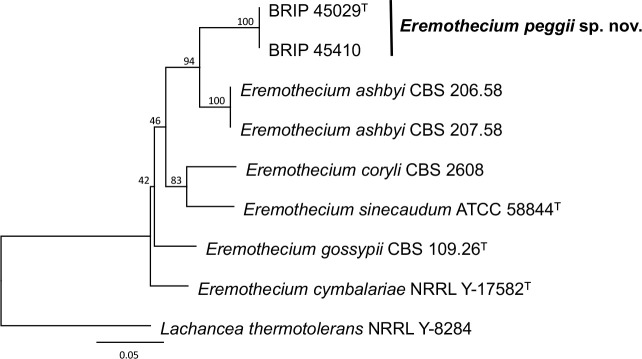
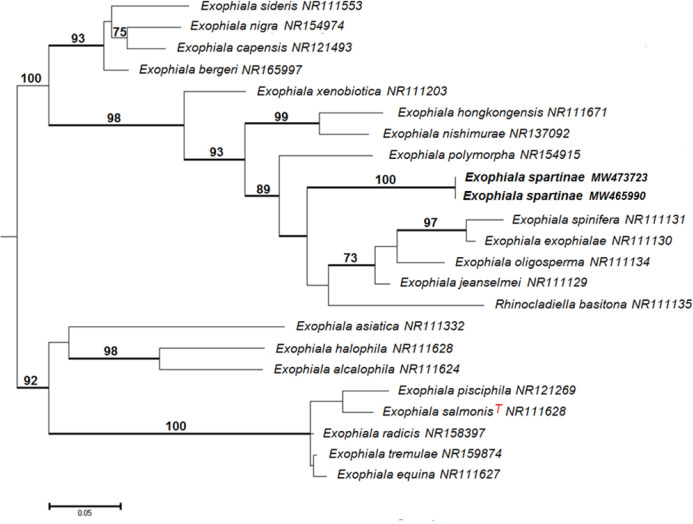
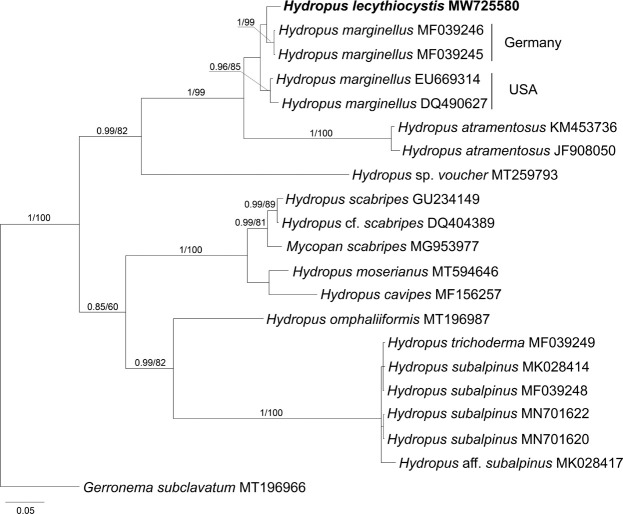
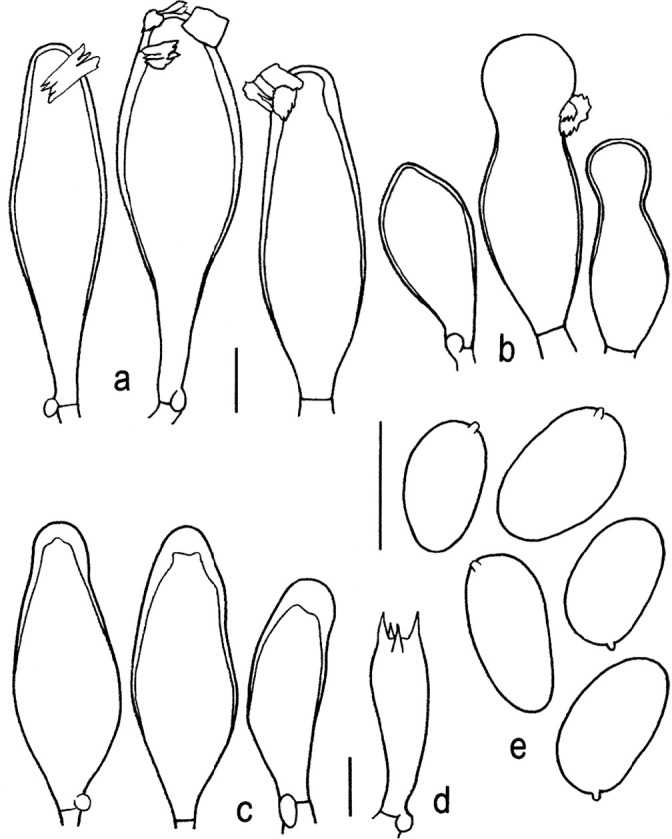
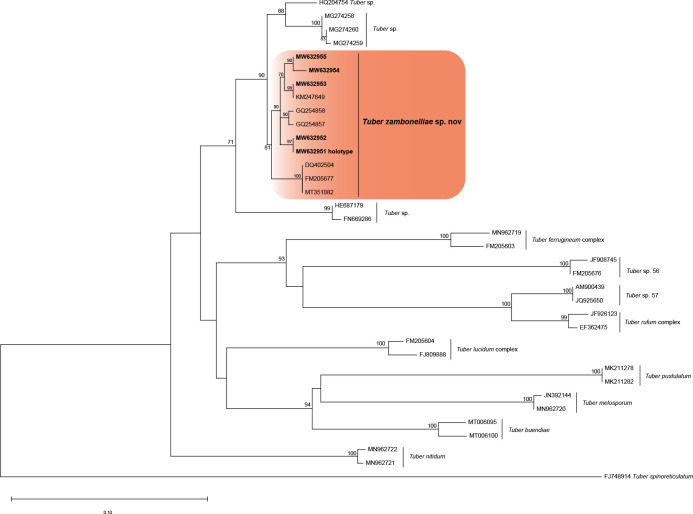
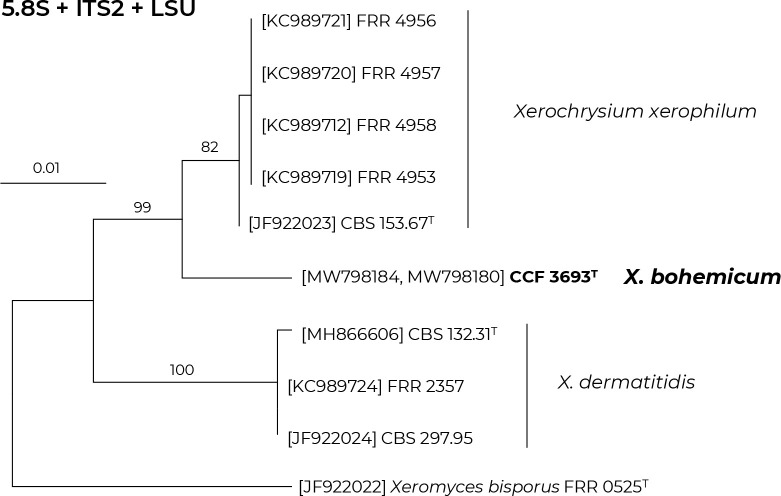
Novel species of fungi described in this study include those from various countries as follows: Algeria, Phaeoacremonium adelophialidum from Vitis vinifera. Antarctica, Comoclathris antarctica from soil. Australia, Coniochaeta salicifolia as endophyte from healthy leaves of Geijera salicifolia, Eremothecium peggii in fruit of Citrus australis, Microdochium ratticaudae from stem of Sporobolus natalensis, Neocelosporium corymbiae on stems of Corymbia variegata, Phytophthora kelmanii from rhizosphere soil of Ptilotus pyramidatus, Pseudosydowia backhousiae on living leaves of Backhousia citriodora, Pseudosydowia indooroopillyensis, Pseudosydowia louisecottisiae and Pseudosydowia queenslandica on living leaves of Eucalyptus sp. Brazil, Absidia montepascoalis from soil. Chile, Ilyonectria zarorii from soil under Maytenus boaria. Costa Rica, Colletotrichum filicis from an unidentified fern. Croatia, Mollisia endogranulata on deteriorated hardwood. Czech Republic, Arcopilus navicularis from tea bag with fruit tea, Neosetophoma buxi as endophyte from Buxus sempervirens, Xerochrysium bohemicum on surface of biscuits with chocolate glaze and filled with jam. France, Entoloma cyaneobasale on basic to calcareous soil, Fusarium aconidiale from Triticum aestivum, Fusarium juglandicola from buds of Juglans regia. Germany, Tetraploa endophytica as endophyte from Microthlaspi perfoliatum roots. India, Castanediella ambae on leaves of Mangifera indica, Lactifluus kanadii on soil under Castanopsis sp., Penicillium uttarakhandense from soil. Italy, Penicillium ferraniaense from compost. Namibia, Bezerromyces gobabebensis on leaves of unidentified succulent, Cladosporium stipagrostidicola on leaves of Stipagrostis sp., Cymostachys euphorbiae on leaves of Euphorbia sp., Deniquelata hypolithi from hypolith under a rock, Hysterobrevium walvisbayicola on leaves of unidentified tree, Knufia hypolithi and Knufia walvisbayicola from hypolith under a rock, Lapidomyces stipagrostidicola on leaves of Stipagrostis sp., Nothophaeotheca mirabibensis (incl. Nothophaeotheca gen. nov.) on persistent inflorescence remains of Blepharis obmitrata, Paramyrothecium salvadorae on twigs of Salvadora persica, Preussia procaviicola on dung of Procavia sp., Sordaria equicola on zebra dung, Volutella salvadorae on stems of Salvadora persica. Netherlands, Entoloma ammophilum on sandy soil, Entoloma pseudocruentatum on nutrient poor (acid) soil, Entoloma pudens on plant debris, amongst grasses. New Zealand, Amorocoelophoma neoregeliae from leaf spots of Neoregelia sp., Aquilomyces metrosideri and Septoriella callistemonis from stem discolouration and leaf spots of Metrosideros sp., Cadophora neoregeliae from leaf spots of Neoregelia sp., Flexuomyces asteliae (incl. Flexuomyces gen. nov.) and Mollisia asteliae from leaf spots of Astelia chathamica, Ophioceras freycinetiae from leaf spots of Freycinetia banksii, Phaeosphaeria caricis-sectae from leaf spots of Carex secta. Norway, Cuphophyllus flavipesoides on soil in semi-natural grassland, Entoloma coracis on soil in calcareous Pinus and Tilia forests, Entoloma cyaneolilacinum on soil semi-natural grasslands, Inocybe norvegica on gravelly soil. Pakistan, Butyriboletus parachinarensis on soil in association with Quercus baloot. Poland, Hyalodendriella bialowiezensis on debris beneath fallen bark of Norway spruce Picea abies. Russia, Bolbitius sibiricus on à moss covered rotting trunk of Populus tremula, Crepidotus wasseri on debris of Populus tremula, Entoloma isborscanum on soil on calcareous grasslands, Entoloma subcoracis on soil in subalpine grasslands, Hydropus lecythiocystis on rotted wood of Betula pendula, Meruliopsis faginea on fallen dead branches of Fagus orientalis, Metschnikowia taurica from fruits of Ziziphus jujube, Suillus praetermissus on soil, Teunia lichenophila as endophyte from Cladonia rangiferina. Slovakia, Hygrocybe fulgens on mowed grassland, Pleuroflammula pannonica from corticated branches of Quercus sp. South Africa, Acrodontium burrowsianum on leaves of unidentified Poaceae, Castanediella senegaliae on dead pods of Senegalia ataxacantha, Cladophialophora behniae on leaves of Behnia sp., Colletotrichum cliviigenum on leaves of Clivia sp., Diatrype dalbergiae on bark of Dalbergia armata, Falcocladium heteropyxidicola on leaves of Heteropyxis canescens, Lapidomyces aloidendricola as epiphyte on brown stem of Aloidendron dichotomum, Lasionectria sansevieriae and Phaeosphaeriopsis sansevieriae on leaves of Sansevieria hyacinthoides, Lylea dalbergiae on Diatrype dalbergiae on bark of Dalbergia armata, Neochaetothyrina syzygii (incl. Neochaetothyrina gen. nov.) on leaves of Syzygium chordatum, Nothophaeomoniella ekebergiae (incl. Nothophaeomoniella gen. nov.) on leaves of Ekebergia pterophylla, Paracymostachys euphorbiae (incl. Paracymostachys gen. nov.) on leaf litter of Euphorbia ingens, Paramycosphaerella pterocarpi on leaves of Pterocarpus angolensis, Paramycosphaerella syzygii on leaf litter of Syzygium chordatum, Parateichospora phoenicicola (incl. Parateichospora gen. nov.) on leaves of Phoenix reclinata, Seiridium syzygii on twigs of Syzygium chordatum, Setophoma syzygii on leaves of Syzygium sp., Starmerella xylocopis from larval feed of an Afrotropical bee Xylocopa caffra, Teratosphaeria combreti on leaf litter of Combretum kraussii, Teratosphaericola leucadendri on leaves of Leucadendron sp., Toxicocladosporium pterocarpi on pods of Pterocarpus angolensis. Spain, Cortinarius bonachei with Quercus ilex in calcareus soils, Cortinarius brunneovolvatus under Quercus ilex subsp. ballota in calcareous soil, Extremopsis radicicola (incl. Extremopsis gen. nov.) from root-associated soil in a wet heathland, Russula quintanensis on acidic soils, Tubaria vulcanica on volcanic lapilii material, Tuber zambonelliae in calcareus soil. Sweden, Elaphomyces borealis on soil under Pinus sylvestris and Betula pubescens. Tanzania, Curvularia tanzanica on inflorescence of Cyperus aromaticus. Thailand, Simplicillium niveum on Ophiocordyceps camponoti-leonardi on underside of unidentified dicotyledonous leaf. USA, Calonectria californiensis on leaves of Umbellularia californica, Exophiala spartinae from surface sterilised roots of Spartina alterniflora, Neophaeococcomyces oklahomaensis from outside wall of alcohol distillery. Vietnam, Fistulinella aurantioflava on soil. Morphological and culture characteristics are supported by DNA barcodes.
Citation: Crous PW, Cowan DA, Maggs-Kölling, et al. 2021. Fungal Planet description sheets: 1182–1283. Persoonia 46: 313–528. https://doi.org/10.3767/persoonia.2021.46.11.
Keywords: ITS nrDNA barcodes, LSU, new taxa, systematics
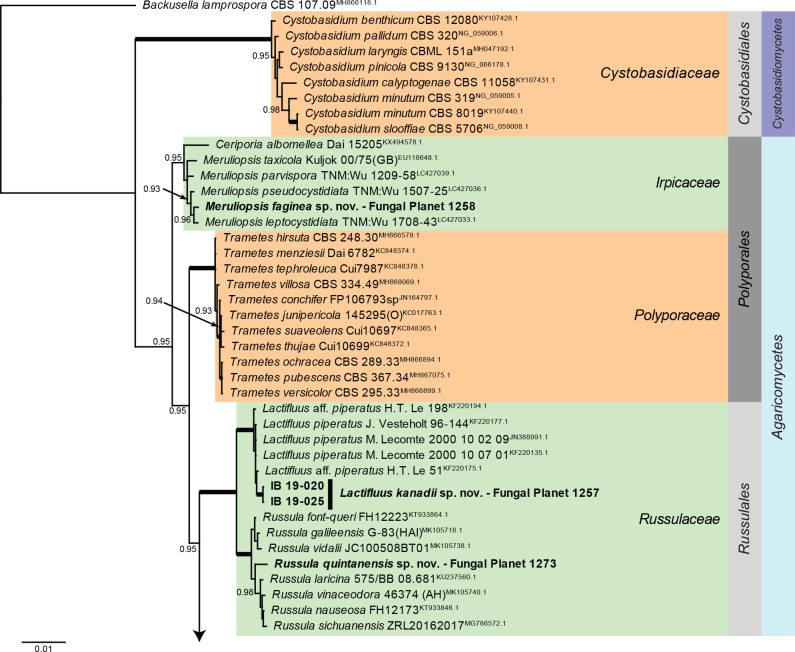
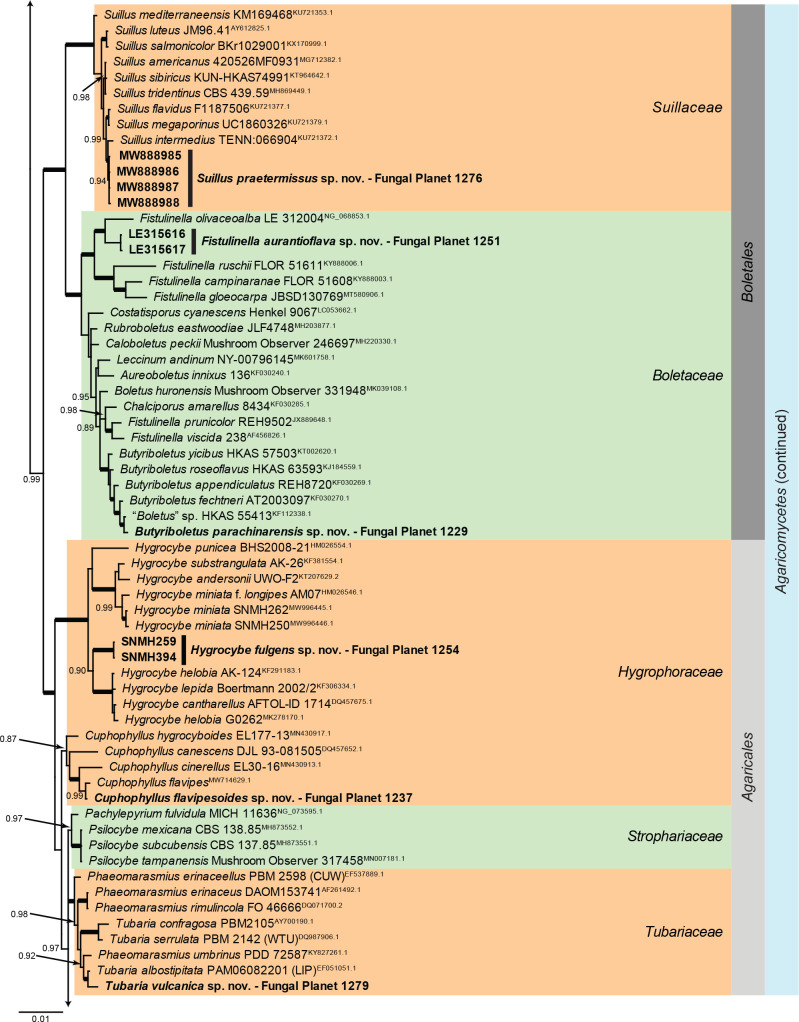
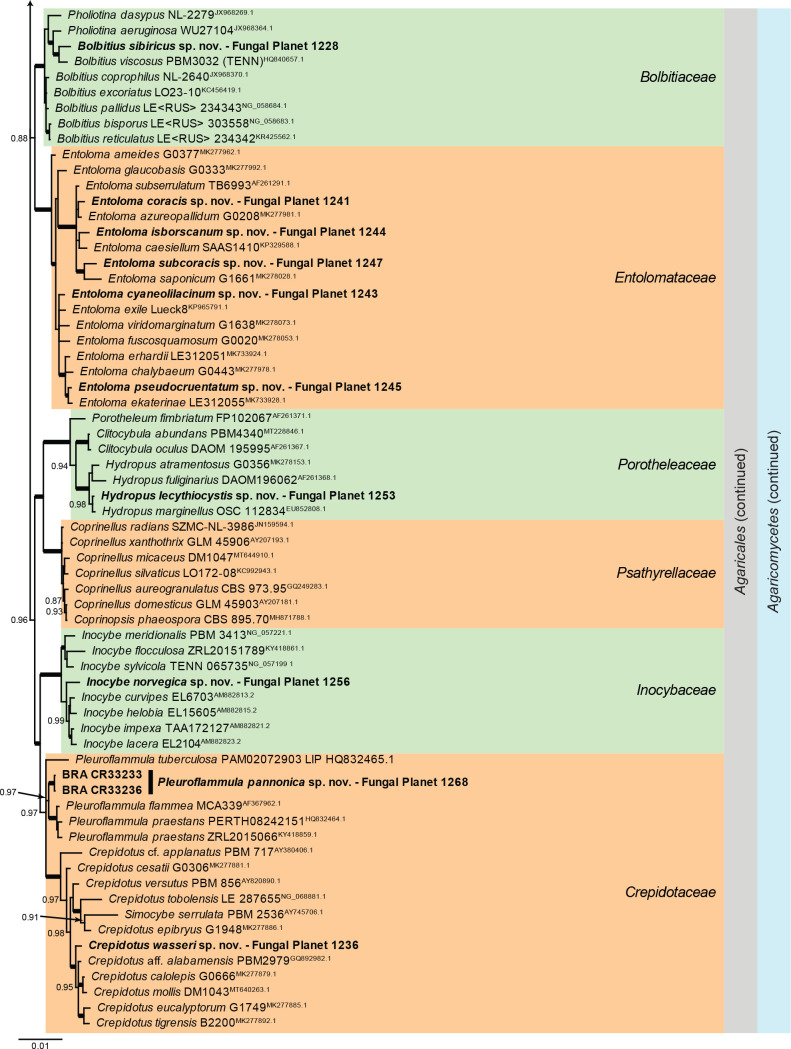
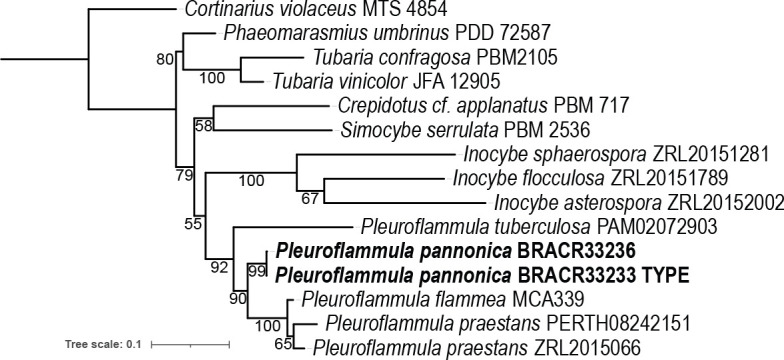
Overview Agaricomycetes phylogeny.
Consensus phylogram (50 % majority rule) of 279 752 trees resulting from a Bayesian analysis of the LSU sequence alignment (170 sequences including outgroup; 948 aligned positions; 553 unique site patterns; 1 865 000 generations with trees sampled every 10 generations) using MrBayes v. 3.2.7a (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families, orders and classes are indicated with coloured blocks to the right of the tree. Culture collection/voucher, GenBank accession (in superscript) and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Backusella lamprospora (GenBank MH866118.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID 28129).
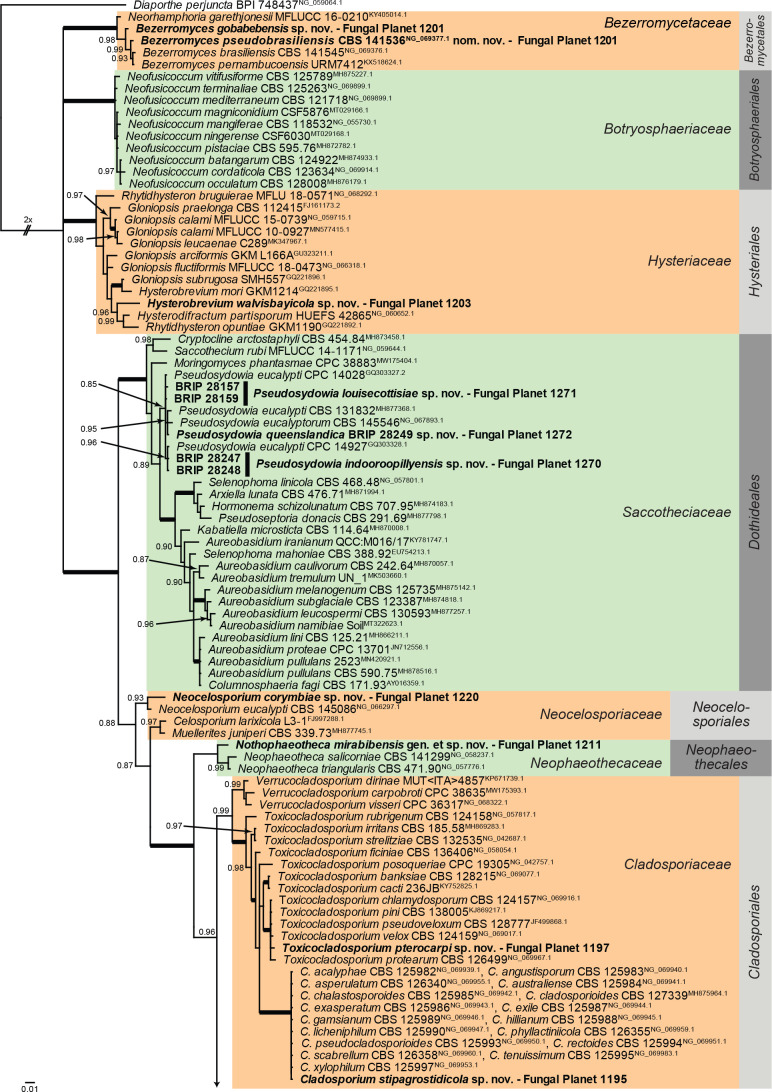
Overview Dothideomycetes (Other orders) phylogeny.
Consensus phylogram (50 % majority rule) of 56 102 trees resulting from a Bayesian analysis of the LSU sequence alignment (179 sequences including outgroup; 832 aligned positions; 378 unique site patterns; 3 740 000 generations with trees sampled every 100 generations) using MrBayes v. 3.2.7a (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. Culture collection/voucher, GenBank accession (in superscript) and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Diaporthe perjuncta (GenBank NG_059064.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The most basal branched was halved in length to facilitate layout. The alignment and tree were deposited in TreeBASE (Submission ID 28129).
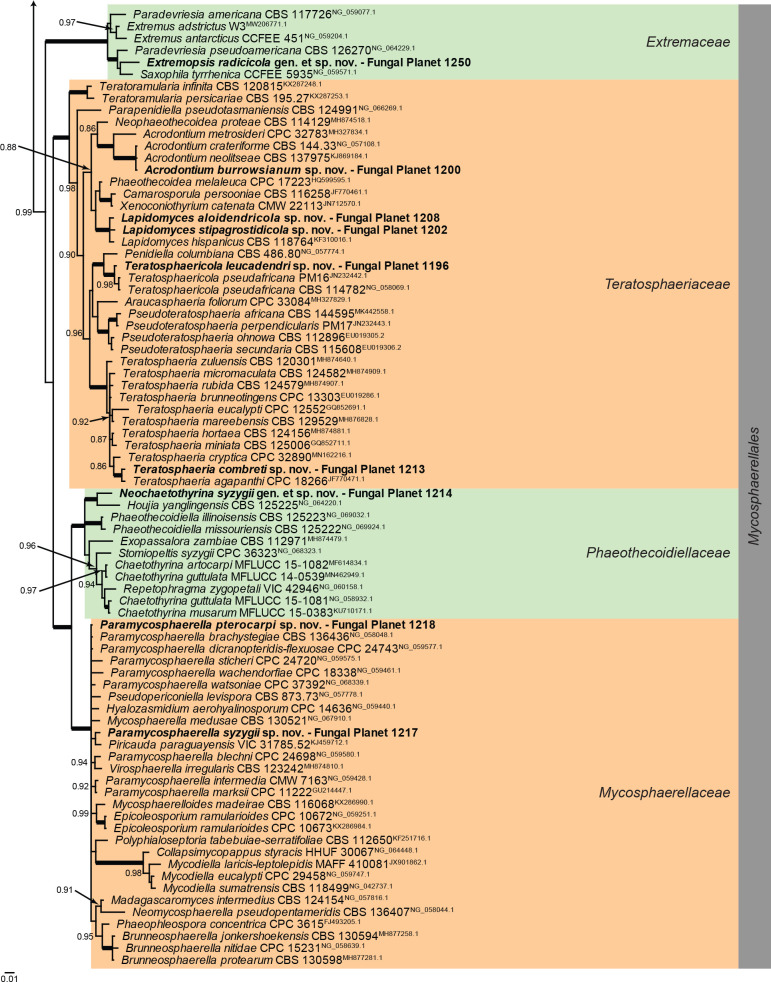
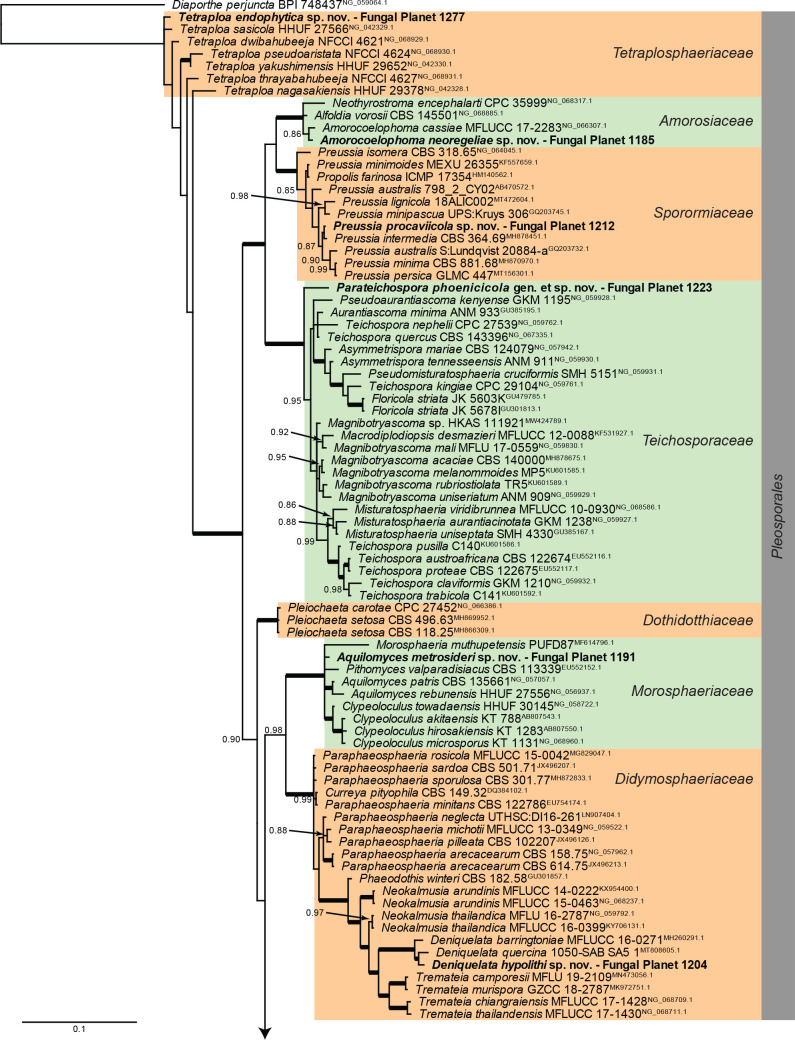
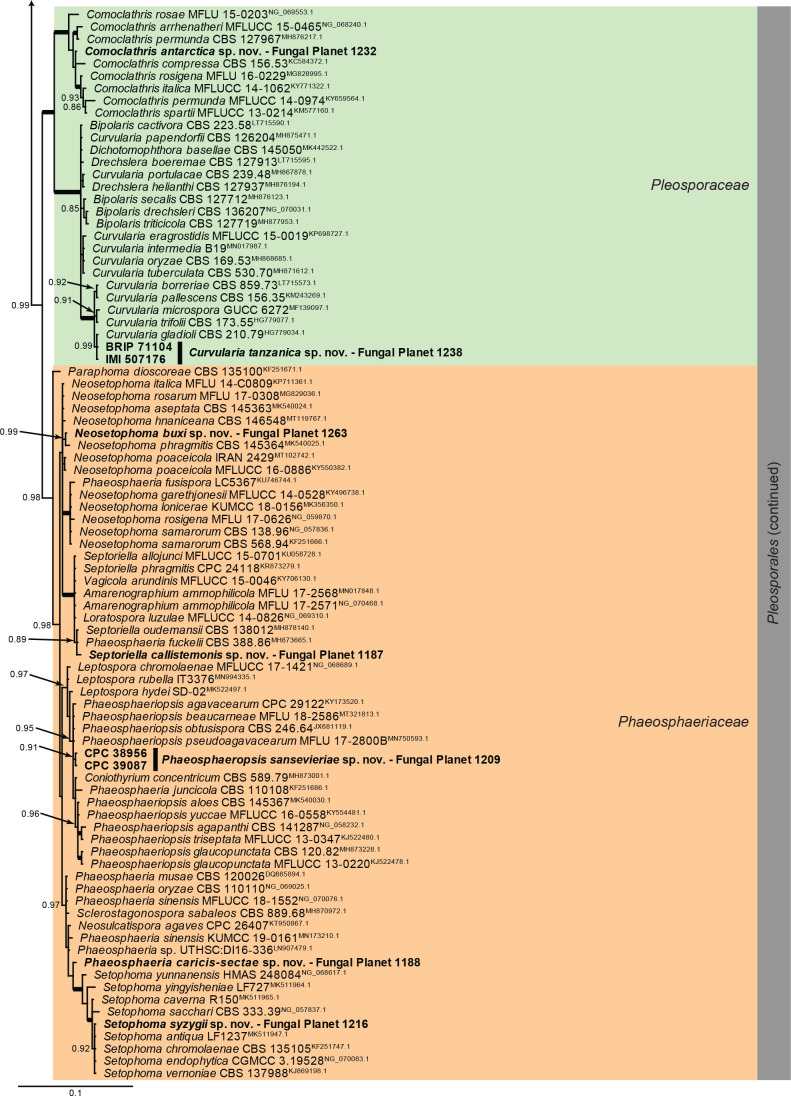
Overview Dothideomycetes (Pleosporales) phylogeny.
Consensus phylogram (50 % majority rule) of 91 128 trees resulting from a Bayesian analysis of the LSU sequence alignment (170 sequences including outgroup; 799 aligned positions; 295 unique site patterns; 6 075 000 generations with trees sampled every 100 generations) using MrBayes v. 3.2.7a (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. Culture collection/voucher, GenBank accession (in superscript) and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Diaporthe perjuncta (GenBank NG_059064.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID 28129).
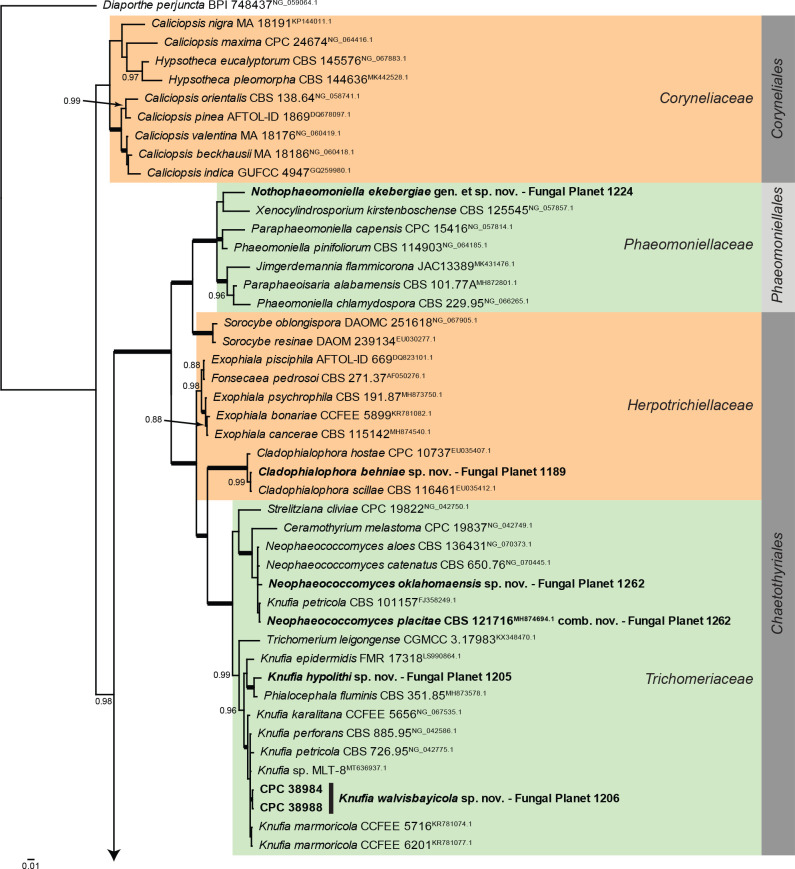
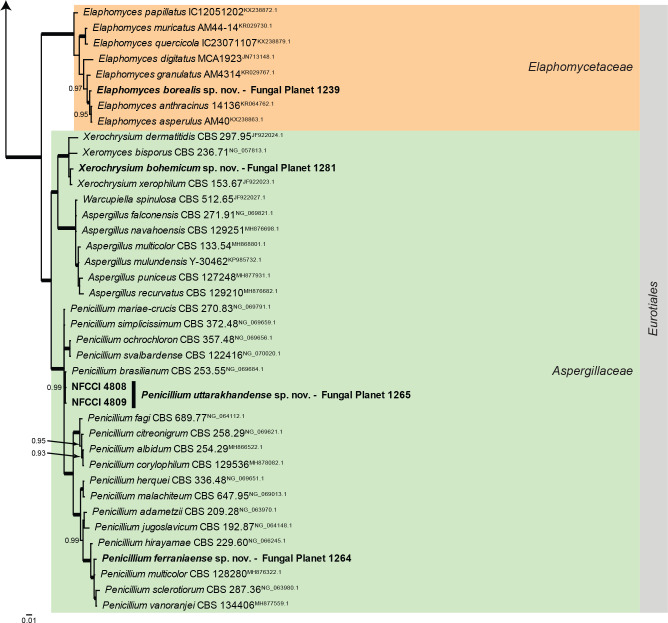
Overview Eurotiomycetes phylogeny.
Consensus phylogram (50 % majority rule) of 146 252 trees resulting from a Bayesian analysis of the LSU sequence alignment (85 sequences including outgroup; 847 aligned positions; 357 unique site patterns; 1 010 000 generations with trees sampled every 10 generations) using MrBayes v. 3.2.7a (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. Culture collection/voucher, GenBank accession (in superscript) and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Diaporthe perjuncta (GenBank NG_059064.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID 28129).
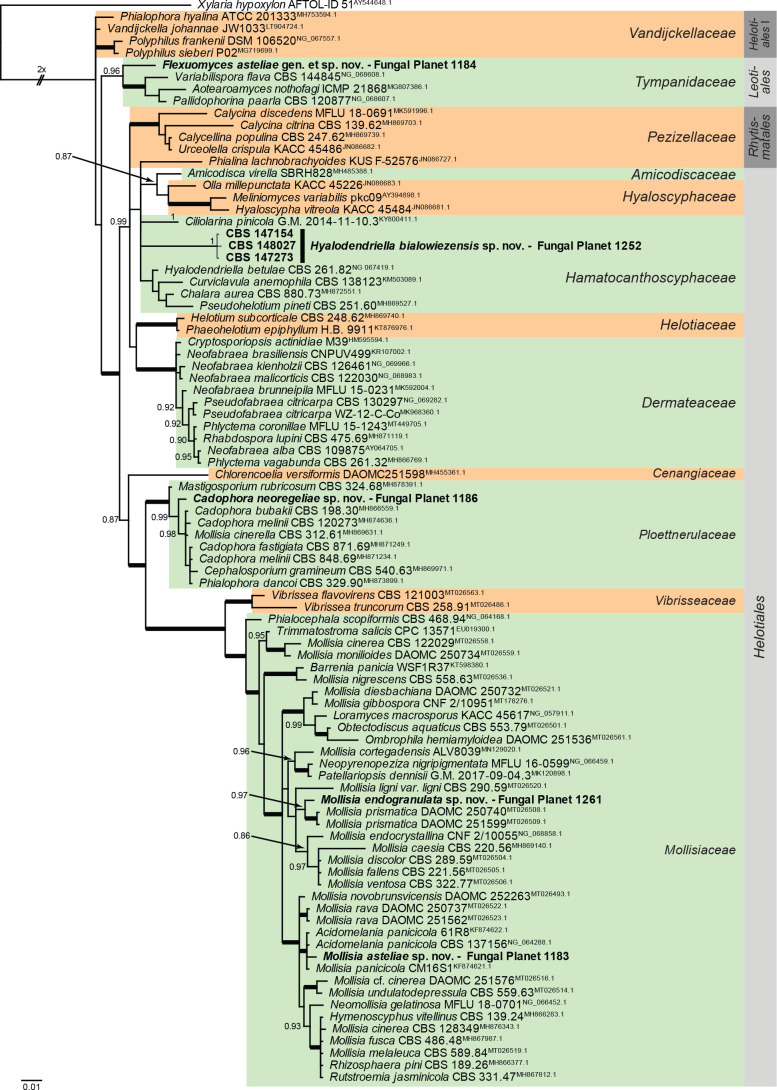
Overview Leotiomycetes phylogeny.
Consensus phylogram (50 % majority rule) of 408 002 trees resulting from a Bayesian analysis of the LSU sequence alignment (90 sequences including outgroup; 826 aligned positions; 283 unique site patterns; 2 720 000 generations with trees sampled every 10 generations) using MrBayes v. 3.2.7a (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. Culture collection/voucher, GenBank accession (in superscript) or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Xylaria hypoxylon (GenBank AY544648.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The most basal branched was halved in length to facilitate layout. The alignment and tree were deposited in TreeBASE (Submission ID 28129).
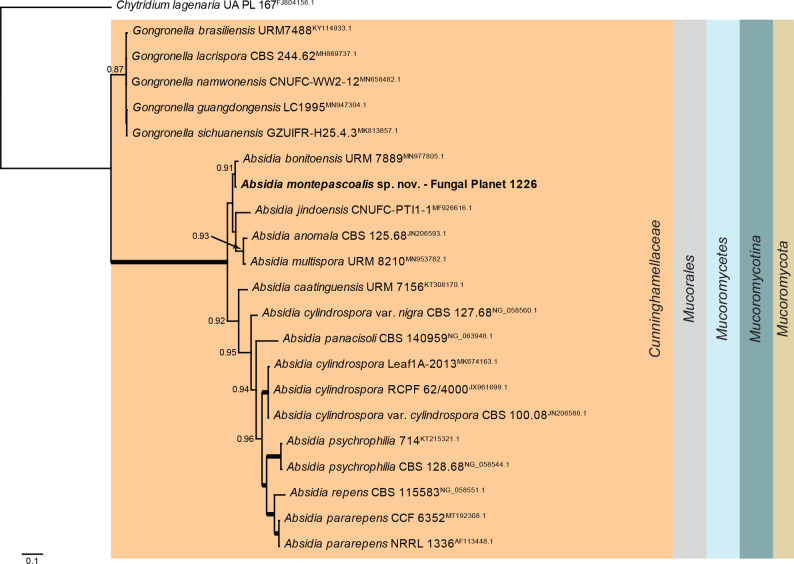
Overview Mucoromycetes phylogeny.
Consensus phylogram (50 % majority rule) of 141 002 trees resulting from a Bayesian analysis of the LSU sequence alignment (22 sequences including outgroup; 660 aligned positions; 319 unique site patterns; 470 000 generations with trees sampled every five generations) using MrBayes v. 3.2.7a (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. The higher taxonomic classification is indicated with coloured blocks to the right of the tree. Culture collection/voucher, GenBank accession (in superscript) or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Chytridium lagenaria (GenBank FJ804156.1) and the taxonomic novelty described in this study for which LSU sequence data were available is indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID 28129).
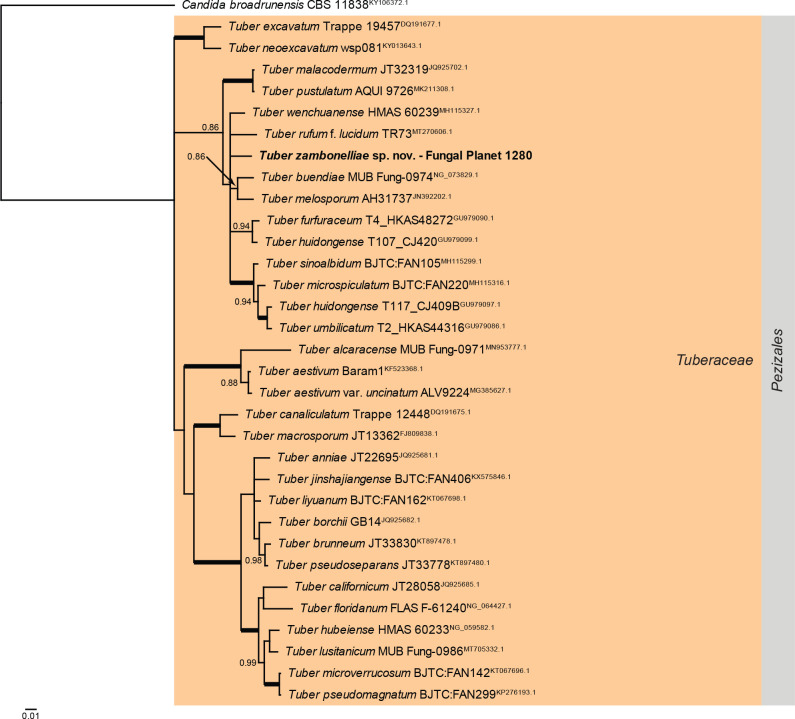
Overview Pezizomycetes phylogeny.
Consensus phylogram (50 % majority rule) of 87 002 trees resulting from a Bayesian analysis of the LSU sequence alignment (33 sequences including outgroup; 792 aligned positions; 203 unique site patterns; 290 000 generations with trees sampled every five generations) using MrBayes v. 3.2.7a (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. The family and order are indicated with coloured blocks to the right of the tree. Culture collection/voucher, GenBank accession (in superscript) or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Candida broadrunensis (GenBank KY106372.1) and the taxonomic novelty described in this study for which LSU sequence data were available is indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID 28129).
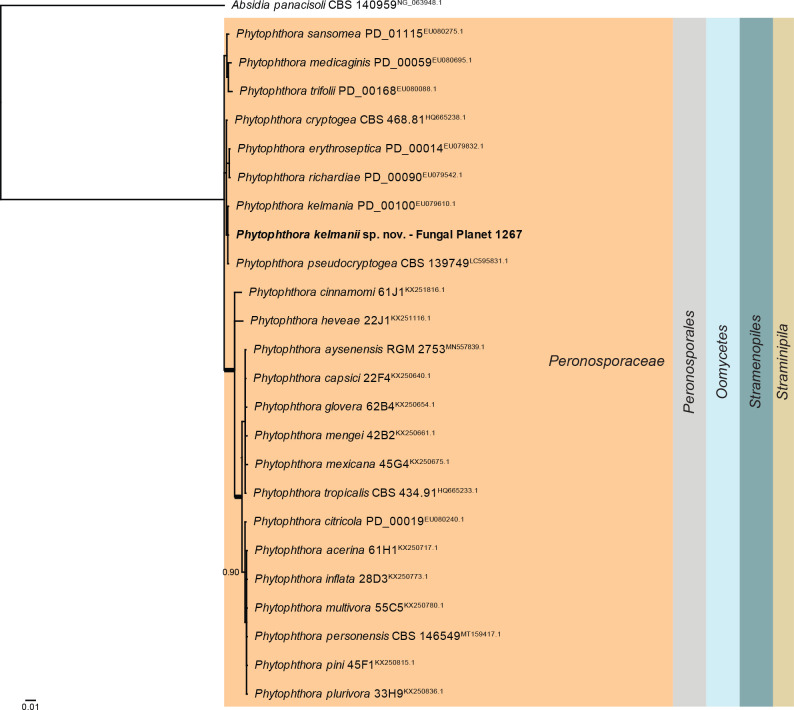
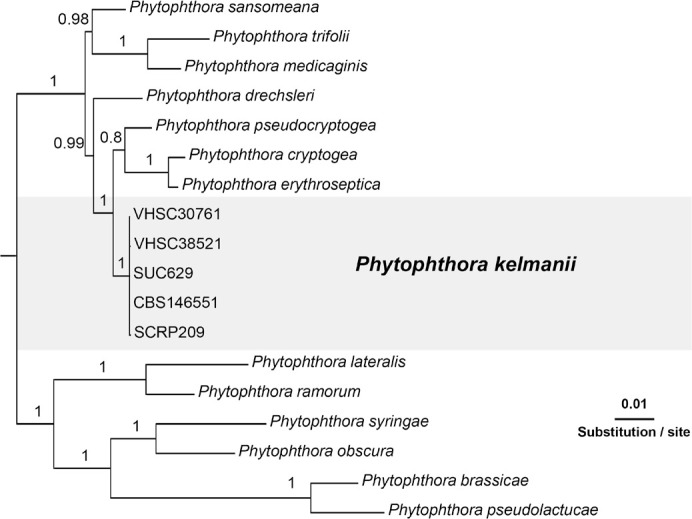
Overview Phytophthora phylogeny.
Consensus phylogram (50 % majority rule) of 64 502 trees resulting from a Bayesian analysis of the LSU sequence alignment (25 sequences including outgroup; 1 110 aligned positions; 68 unique site patterns; 215 000 generations with trees sampled every five generations) using MrBayes v. 3.2.7a (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. The higher taxonomic classification is indicated with coloured blocks to the right of the tree. Culture collection/voucher, GenBank accession (in superscript) or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Absidia panacisoli (GenBank NG_063948.1) and the taxonomic novelty described in this study for which LSU sequence data were available is indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID 28129).
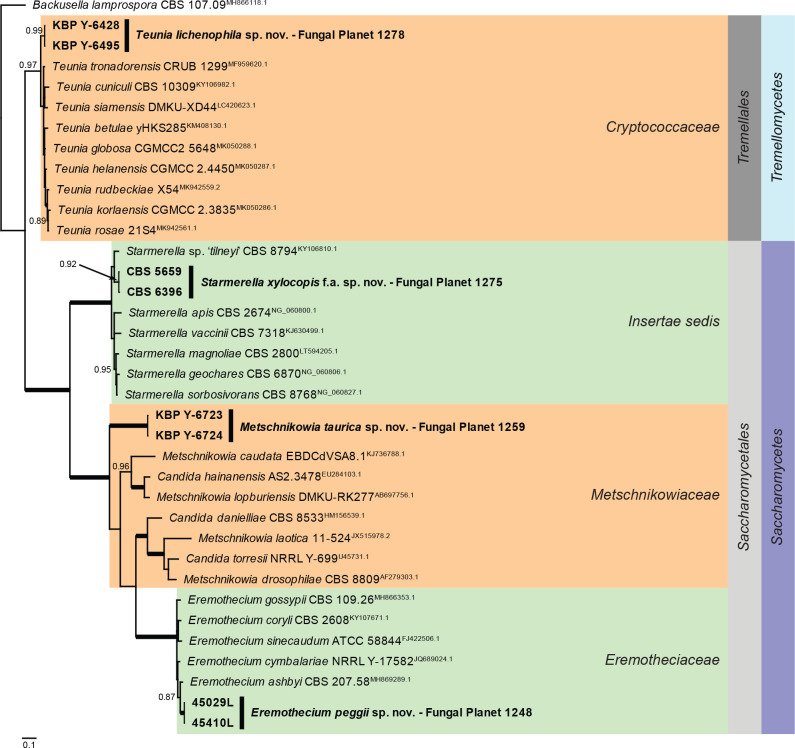
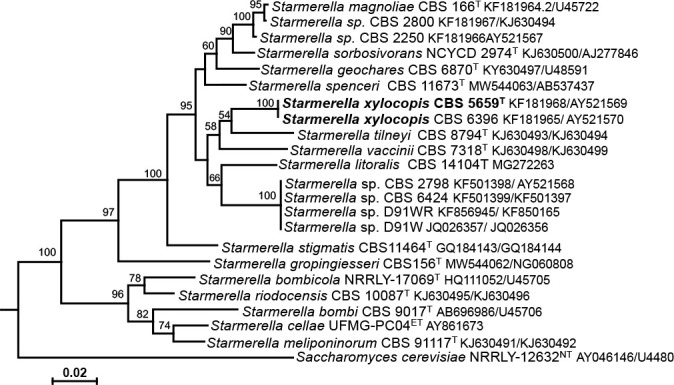
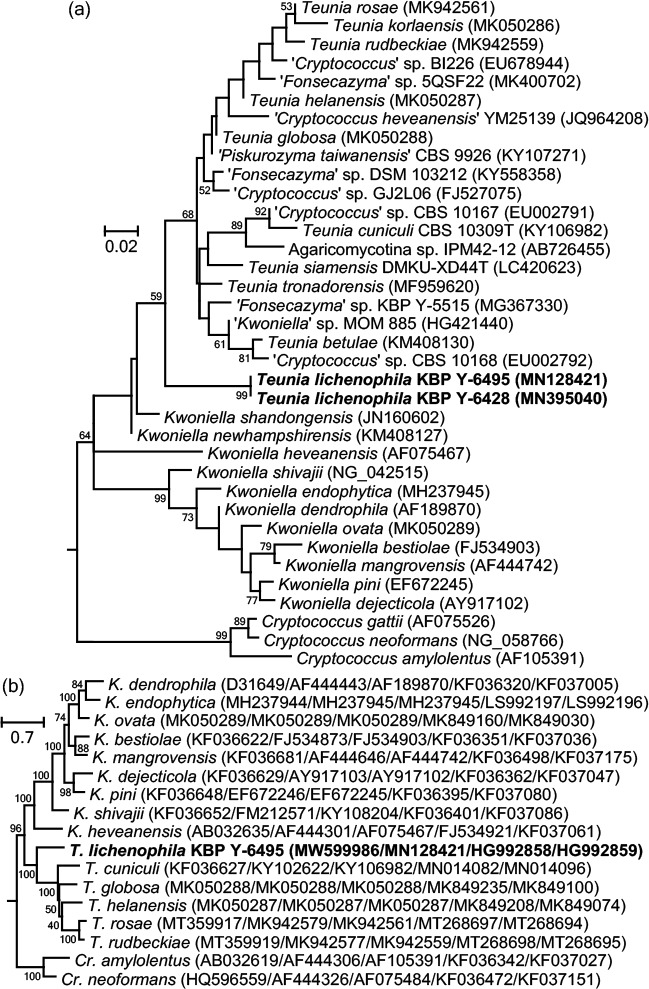
Overview Saccharomycetes and Tremellomycetes phylogeny.
Consensus phylogram (50 % majority rule) of 136 502 trees resulting from a Bayesian analysis of the LSU sequence alignment (36 sequences including outgroup; 667 aligned positions; 432 unique site patterns; 910 000 generations with trees sampled every 10 generations) using MrBayes v. 3.2.7a (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. The families, orders and classes are indicated with coloured blocks to the right of the tree. Culture collection/voucher, GenBank accession (in superscript) and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Backusella lamprospora (GenBank MH866118.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID 28129).
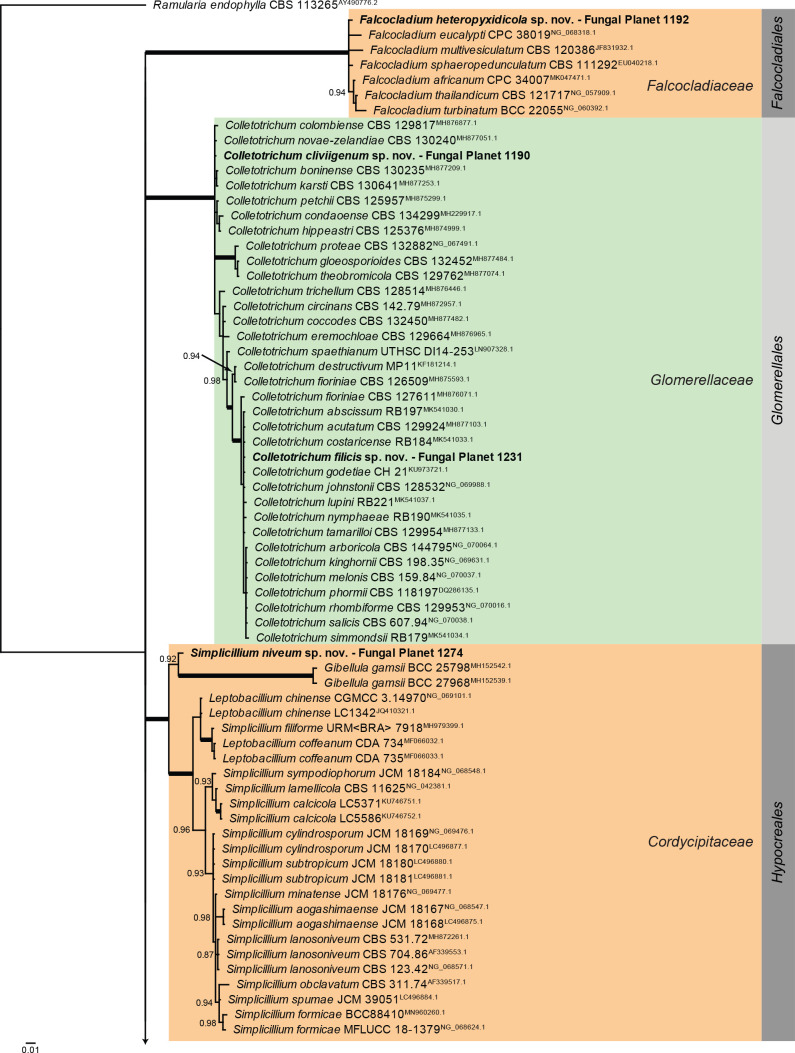
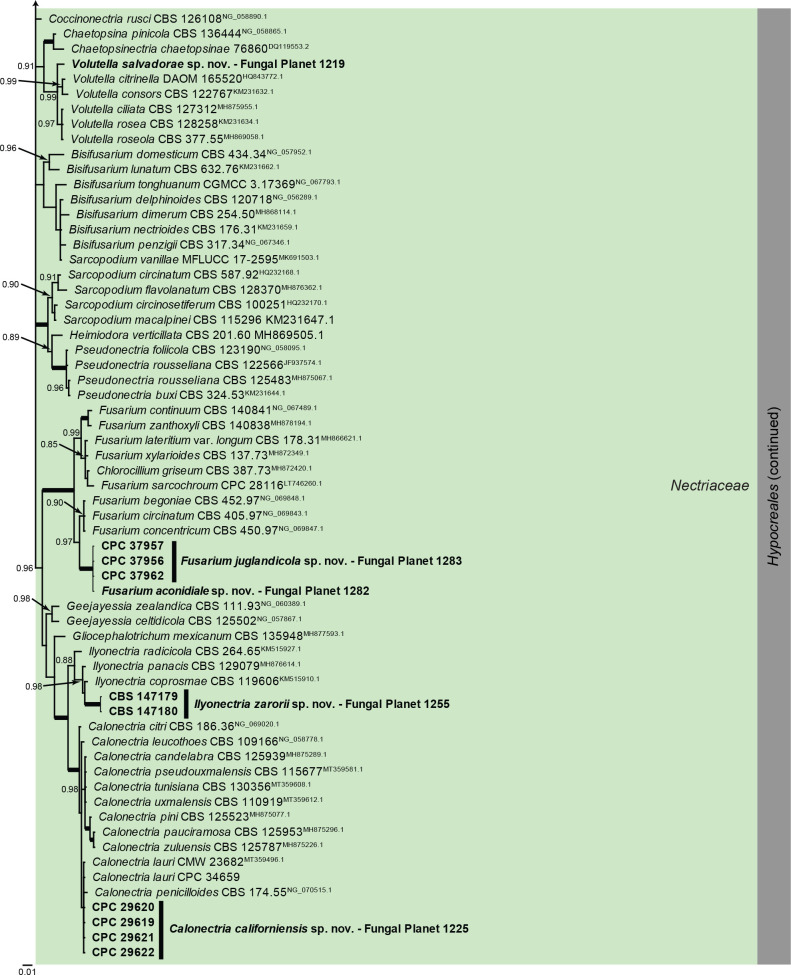
Overview Sordariomycetes (Falcocladiales, Glomerellales and Hypocreales) phylogeny.
Consensus phylogram (50 % majority rule) of 846 978 trees resulting from a Bayesian analysis of the LSU sequence alignment (194 sequences including outgroup; 816 aligned positions; 310 unique site patterns; 56 465 000 generations with trees sampled every 100 generations) using MrBayes v. 3.2.7a (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. Culture collection/voucher, GenBank accession (in superscript) and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Ramularia endophylla (GenBank AY490776.2) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID 28129).
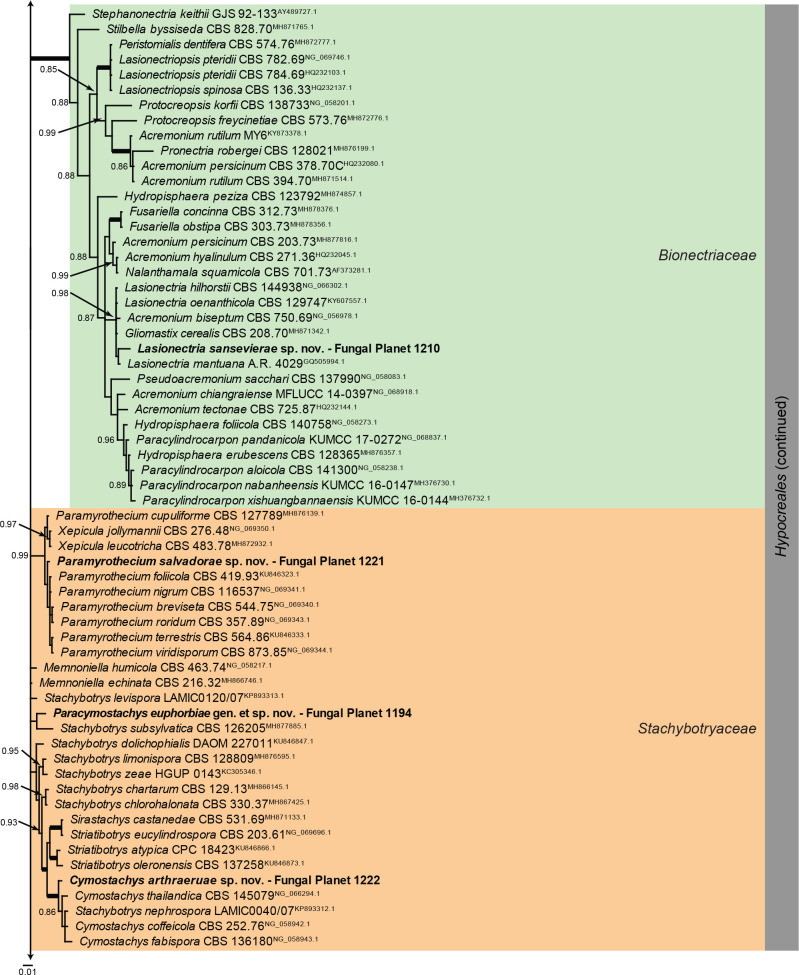
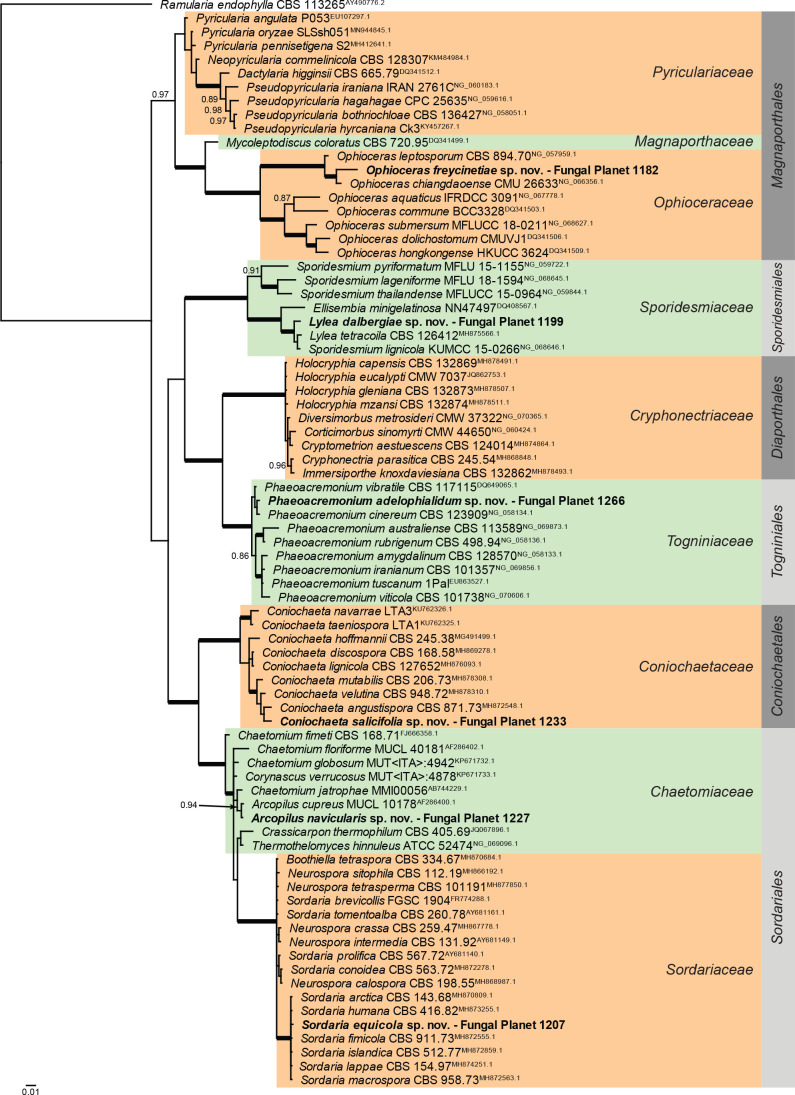
Overview Sordariomycetes (Other orders) phylogeny.
Consensus phylogram (50 % majority rule) of 229 502 trees resulting from a Bayesian analysis of the LSU sequence alignment (79 sequences including outgroup; 813 aligned positions; 293 unique site patterns; 1 530 000 generations with trees sampled every 10 generations) using MrBayes v. 3.2.7a (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. Culture collection/voucher, GenBank accession (in superscript) and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Ramularia endophylla (GenBank AY490776.2) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID 28129).
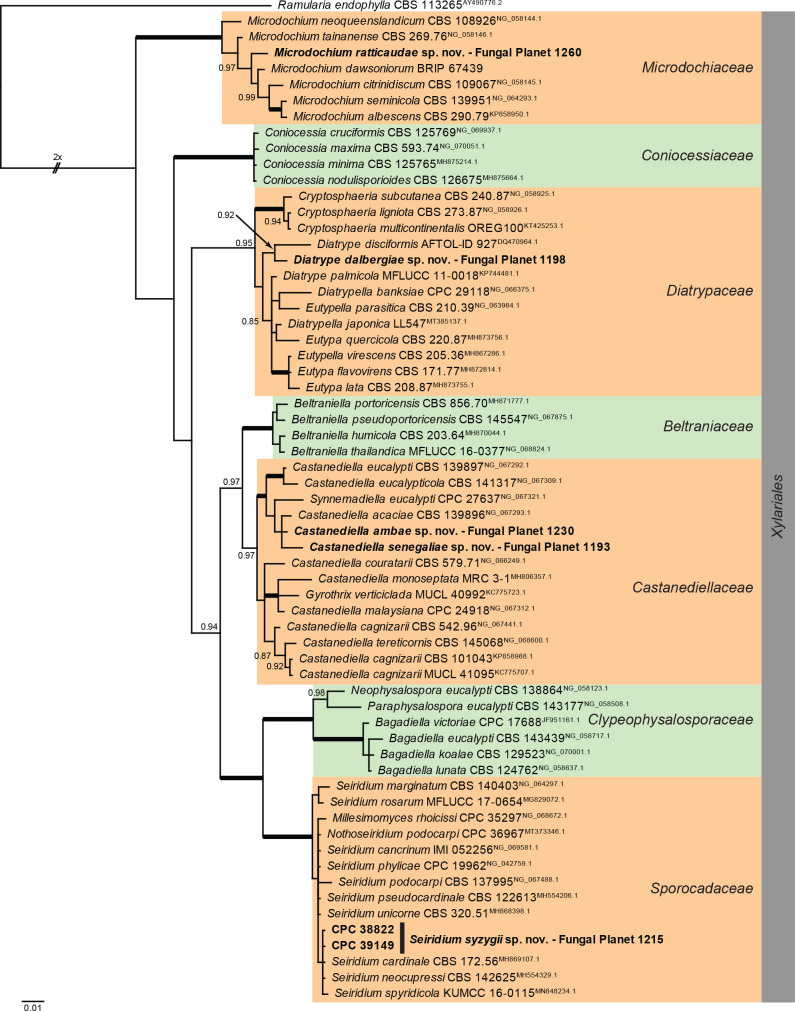
Overview Sordariomycetes (Xylariales) phylogeny.
Consensus phylogram (50 % majority rule) of 118 502 trees resulting from a Bayesian analysis of the LSU sequence alignment (63 sequences including outgroup; 800 aligned positions; 192 unique site patterns; 790 000 generations with trees sampled every 10 generations) using MrBayes v. 3.2.7a (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and the order are indicated with coloured blocks to the right of the tree. Culture collection/voucher, GenBank accession (in superscript) and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Ramularia endophylla (GenBank AY490776.2) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The most basal branched was halved in length to facilitate layout. The alignment and tree were deposited in TreeBASE (Submission ID 28129).
Acknowledgments
Leslie W.S. de Freitas and colleagues express their gratitude to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for scholarships provided to Leslie Freitas and for the research grant provided to André Luiz Santiago; their contribution was financed by the projects ‘Diversity of Mucoromycotina in the different ecosystems of the Atlantic Rainforest of Pernambuco’ (FACEPE–First Projects Program PPP/FACEPE/CNPq–APQ–0842-2.12/14) and ‘Biology of conservation of fungi s.l. in areas of Atlantic Forest of Northeast Brazil’ (CNPq/ICMBio 421241/2017-9) H.B. Lee was supported by the Graduate Program for the Undiscovered Taxa of Korea (NIBR202130202). The study of O.V. Morozova, E.F. Malysheva, V.F. Malysheva, I.V. Zmitrovich, and L.B. Kalinina was carried out within the framework of a research project of the Komarov Botanical Institute RAS (AAAA-A19-119020890079-6) using equipment of its Core Facility Centre ‘Cell and Molecular Technologies in Plant Science’. The work of O. V. Morozova, L.B. Kalinina, T. Yu. Svetasheva, and E.A. Zvyagina was financially supported by Russian Foundation for Basic Research project no. 20-04-00349. E.A. Zvyagina and T.Yu. Svetasheva are grateful to A.V. Alexandrova, A.E. Kovalenko, A.S. Baykalova for the loan of specimens, T.Y. James, E.F. Malysheva and V.F. Malysheva for sequencing. J.D. Reyes acknowledges B. Dima for comparing the holotype sequence of Cortinarius bonachei with the sequences in his database. A. Mateos and J.D. Reyes acknowledge L. Quijada for reviewing the phylogeny and S. de la Peña-Lastra and P. Alvarado for their support and help. Vladimir I. Kapitonov and colleagues are grateful to Brigitta Kiss for help with their molecular studies. This study was conducted under research projects of the Tobolsk Complex Scientific Station of the Ural Branch of the Russian Academy of Sciences (N AAAA-A19-119011190112-5). E. Larsson acknowledges the Swedish Taxonomy Initiative, SLU Artdatabanken, Uppsala (dha.2019.4.3-13). The study of D.B. Raudabaugh and colleagues was supported by the Schmidt Science Fellows, in partnership with the Rhodes Trust. Gregorio Delgado is grateful to Michael Manning and Kamash Pillai (Eurofins EMLab P&K) for provision of laboratory facilities. Jose G. Maciá-Vicente acknowledges support from the German Research Foundation under grant MA7171/1-1, and from the Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz (LOEWE) of the state of Hesse within the framework of the Cluster for Integrative Fungal Research (IPF). Thanks are also due to the authorities of the Cabañeros National Park and Los Alcornocales Natural Park for granting the collection permit and for support during field work. The study of Alina V. Alexandrova was carried out as part of the Scientific Project of the State Order of the Government of Russian Federation to Lomonosov Moscow State University No. 121032300081-7. Michał Gorczak was financially supported by the Ministry of Science and Higher Education through the Faculty of Biology, University of Warsaw intramural grant DSM 0117600-13. M. Gorczak acknowledges M. Klemens for sharing a photo of the Białowieża Forest logging site and M. Senderowicz for help with preparing the illustration. Ivona Kautmanová and D. Szabóová were funded by the Operational Program of Research and Development and co-financed with the European Fund for Regional Development (EFRD). ITMS 26230120004: ‘Building of research and development infrastructure for investigation of genetic biodiversity of organisms and joining IBOL initiative’. Ishika Bera, Aniket Ghosh, Jorinde Nuytinck and Annemieke Verbeken are grateful to the Director, Botanical Survey of India (Kolkata), Head of the Department of Botany & Microbiology & USIC Dept. HNB Garhwal University, Srinagar, Garhwal for providing research facilities. Ishika Bera and Aniket Ghosh acknowledge the staff of the forest department of Arunachal Pradesh for facilitating the macrofungal surveys to the restricted areas. Sergey Volobuev was supported by the Russian Science Foundation (RSF project N 19-77-00085). Aleksey V. Kachalkin and colleagues were supported by the Russian Science Foundation (grant No. 19-74-10002). The study of Anna M. Glushakova was carried out as part of the Scientific Project of the State Order of the Government of Russian Federation to Lomonosov Moscow State University No. 121040800174-6. Tracey V. Steinrucken and colleagues were supported by AgriFutures Australia (Rural Industries Research and Development Corporation), through funding from the Australian Government Department of Agriculture, Water and the Environment, as part of its Rural Research and Development for Profit program (PRJ-010527). Neven Matočec and colleagues thank the Croatian Science Foundation for their financial support under the project grant HRZZ-IP-2018-01-1736 (ForFungiDNA). Ana Pošta thanks the Croatian Science Foundation for their support under the grant HRZZ-2018-09-7081. The research of Milan Spetik and co-authors was supported by Internal Grant of Mendel University in Brno No. IGA-ZF/2021-SI1003. K.C. Rajeshkumar thanks SERB, the Department of Science and Technology, Government of India for providing financial support under the project CRG/2020/000668 and the Director, Agharkar Research Institute for providing research facilities. Nikhil Ashtekar thanks CSIR-HRDG, INDIA, for financial support under the SRF fellowship (09/670(0090)/2020-EMR-I), and acknowledges the support of the DIC Microscopy Facility, established by Dr Karthick Balasubramanian, B&P (Plants) Group, ARI, Pune. The research of Alla Eddine Mahamedi and co-authors was supported by project No. CZ.02.1.01/0.0/0.0/16_017/0002334, Czech Republic. Tereza Tejklová is thanked for providing useful literature. A. Polhorský and colleagues were supported by the Operational Program of Research and Development and co-financed with the European fund for Regional Development (EFRD), ITMS 26230120004: Building of research and development infrastructure for investigation of genetic biodiversity of organisms and joining IBOL initiative. Yu Pei Tan and colleagues thank R. Chen for her technical support. Ernest Lacey thanks the Cooperative Research Centres Projects scheme (CRCPFIVE000119) for its support. Suchada Mongkolsamrit and colleagues were financially supported by the Platform Technology Management Section, National Center for Genetic Engineering and Biotechnology (BIOTEC), Project Grant No. P19-50231. Dilnora Gouliamova and colleagues were supported by a grant from the Bulgarian Science Fund (KP-06-H31/19). The research of Timofey A. Pankratov was supported by the Russian Foundation for Basic Research (grant No. 19-04-00297a). Gabriel Moreno and colleagues wish to express their gratitude to L. Monje and A. Pueblas of the Department of Drawing and Scientific Photography at the University of Alcalá for their help in the digital preparation of the photographs, and to J. Rejos, curator of the AH herbarium, for his assistance with the specimens examined in the present study. Vit Hubka was supported by the Charles University Research Centre program No. 204069. Alena Kubátová was supported by The National Programme on Conservation and Utilization of Microbial Genetic Resources Important for Agriculture (Ministry of Agriculture of the Czech Republic). The Kits van Waveren Foundation (Rijksherbariumfonds Dr E. Kits van Waveren, Leiden, Netherlands) contributed substantially to the costs of sequencing and travelling expenses for M. Noordeloos. The work of B. Dima was supported by the ÚNKP-20-4 New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund, and by the ELTE Thematic Excellence Programme 2020 supported by the National Research, Development and Innovation Office of Hungary (TKP2020-IKA-05). The Norwegian Entoloma studies received funding from the Norwegian Biodiversity Information Centre (NBIC), and the material was partly sequenced through NorBOL. Gunnhild Marthinsen and Katriina Bendiksen (Natural History Museum, University of Oslo, Norway) are acknowledged for performing the main parts of the Entoloma barcoding work. Asunción Morte is grateful to AEI/FEDER, UE (CGL2016-78946-R) and Fundación Séneca - Agencia de Ciencia y Tecnología de la Región de Murcia (20866/PI/18) for financial support. Vladimír Ostrý was supported by the Ministry of Health, Czech Republic - conceptual development of research organization (National Institute of Public Health – NIPH, IN 75010330). Konstanze Bensch (Westerdijk Fungal Biodiversity Institute, Utrecht) is thanked for correcting the spelling of various Latin epithets.
REFERENCES
- Abad ZG, Abad JA, Creswell T. 2002. Advances in the integration of morphological and molecular chraraterization in Phytophthora genus: The case of P. kelmania and other putative new species. Phytopathology 92 (6 suppl): S1.18943805 [Google Scholar]
- Abad ZG, Burgess T, Bienapfl JC, et al. 2019. IDphy: Molecular and morphological identification of Phytophthora based on the types. USDA APHIS PPQ S&T Beltsville Lab, USDA APHIS PPQ S&T ITP, Centre for Phytophthora Science and Management, and World Phytophthora Collection. https://idtools.org/id/phytophthora/index.php <different dates in 2020>. [Google Scholar]
- Abdollahzadeh J, Groenewald JZ, Coetzee MPA, et al. 2020. Evolution of lifestyles in Capnodiales. Studies in Mycology 95: 381–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike H. 1974. A new look at the statistical model identification. IEEE Transactions on Automatic Control 19: 716–723. [Google Scholar]
- Alcorn JL. 1982. Ovariicolous bipolaris species on Sporobolus and other grasses. Mycotaxon 15: 20–48. [Google Scholar]
- Ames LM. 1949. New cellulose destroying fungi isolated from military material and equipment. Mycologia 41: 637–648. [Google Scholar]
- Ariyawansa HA, Maharachchikumbura SS, Karunarathne SC, et al. 2013. Deniquelata barringtoniae gen. et sp. nov., associated with leaf spots of Barringtonia asiatica. Phytotaxa 105: 11–20. [Google Scholar]
- Ariyawansa HA, Phookamsak R, Tibpromma S, et al. 2014. A molecular and morphological reassessment of Diademaceae. The Scientific World Journal: 675348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyawansa HA, Thambugala KM, Manamgoda DS, et al. 2015. Towards a natural classification and backbone tree for Pleosporaceae. Fungal Diversity 71: 85–139. [Google Scholar]
- Arora D, Frank JL. 2014. Clarifying the butter Boletes: a new genus, Butyriboletus, is established to accommodate Boletus sect. Appendiculati, and six new species are described. Mycologia 106: 464–480. [DOI] [PubMed] [Google Scholar]
- Badali H, Gueidan C, Najafzadeh MJ, et al. 2008. Biodiversity of the genus Cladophialophora. Studies in Mycology 61: 175–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baiswar P, Ngachan S, Rymbai H, et al. 2014. Simplicillium lanosoniveum, a hyperparasite on Aecidium elaeagni-latifoliae in India. Australasian Plant Disease Notes 9: 144–149. [Google Scholar]
- Ballará J, Cadiñanos-Aguirre JA, Campos JC, et al. 2009. Cortinarius íbero-insulares-2. Fungi non Delineati. Pars XLVIII-XLIX: 33–35. Edizioni Candusso, Alassio (SV). [Google Scholar]
- Ballarà J, Suárez E, Mahiques R, et al. 2017. Cortinarius iunii, una nueva especie de la sección Bovini. The Journal of the Journées européennes du Cortinaire 19: 11–27. [Google Scholar]
- Bandini D, Oertel B, Schüssler C, et al. 2020. Noch mehr Risspilze: Fünfzehn neue und zwei wenig bekannte Arten der Gattung Inocybe. Mycologia Bavarica 20: 13–101. [Google Scholar]
- Baral H-O. 1987. Lugol’s solution / IKI versus Melzer’s reagent: hemiamyloidity, a universal feature of the ascus wall. Mycotaxon 29: 399–450. [Google Scholar]
- Bas C. 1999. Hydropus Kuhner ex Singer. In: Bas C, Kuyper TW, Noordeloos ME, et al. (eds), Flora Agaricina Neerlandica 4: 166–173. Rotterdam, Balkema. [Google Scholar]
- Bensch K, Braun U, Groenewald JZ, et al. 2012. The genus Cladosporium. Studies in Mycology 72: 1–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch K, Groenewald JZ, Braun U, et al. 2015. Common but different: The expanding realm of Cladosporium. Studies in Mycology 82: 23–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch K, Groenewald JZ, Dijksterhuis J, et al. 2010. Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales). Studies in Mycology 67: 1–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch K, Groenewald JZ, Meijer M, et al. 2018. Cladosporium species in indoor environments. Studies in Mycology 89: 177–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berraf-Tebbal A, Bouznad Z, Santos JM, et al. 2011. Phaeoacremonium species associated with Eutypa dieback and esca of grapevines in Algeria. Phytopathologia Mediterranea 50: S86–S97. [Google Scholar]
- Bezerra JDP, Oliveira RJV, Paiva LM, et al. 2017. Bezerromycetales and Wiesneriomycetales ord. nov. (class Dothideomycetes), with two novel genera to accommodate endophytic fungi from Brazilian cactus. Mycological Progress 16: 297–309. [Google Scholar]
- Bidaud A, Carteret X, Eyssartier G, et al. 2002. Atlas des Cortinaires XII. Éditions Fédération mycologique Dauphiné Savoie, Marlioz, France. [Google Scholar]
- Bidaud A, Carteret X, Eyssartier G, et al. 2004. Atlas des Cortinaires XIV. Éditions Fédération mycologique Dauphiné Savoie, Marlioz, France. [Google Scholar]
- Bidaud A, Moënne-Loccoz P, Reumaux P, et al. 2009. Atlas des Cortinaires XVIII. Éditions Fédération mycologique Dauphiné Savoie, Marlioz, France. [Google Scholar]
- Bien S, Damm U. 2020. Arboricolonus simplex gen. et sp. nov. and novelties in Cadophora, Minutiella and Proliferodiscus from Prunus wood in Germany. MycoKeys 2020 63: 119–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bien S, Kraus C, Damm U. 2020. Novel collophorina-like genera and species from Prunus trees and vineyards in Germany. Persoonia 45: 46–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm EWA, Mugambi GK, Miller AN, et al. 2009. A molecular phylogenetic reappraisal of the Hysteriaceae, Mytilinidiaceae and Gloniaceae (Pleosporomycetidae, Dothideomycetes) with keys to world species. Studies in Mycology 64: 49–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boertmann D. 2010. The genus Hygrocybe, 2nd revised edition. Danish Mycological Society, Copenhagen. [Google Scholar]
- Bon M. 1992. Clé monographique des especes Galero-Naucorioides. Documents Mycologiques 84: 1–89. [Google Scholar]
- Bonito GM, Gryganskyi AP, Trappe JM, et al. 2010. A global meta-analysis of Tuber ITS rDNA sequences: species diversity, host associations and long-distance dispersal. Molecular Ecology 19: 4994–5008. [DOI] [PubMed] [Google Scholar]
- Bonthond G, Sandoval-Denis M, Groenewald JZ, et al. 2018. Seiridium (Sporocadaceae): an important genus of plant pathogenic fungi. Persoonia 40: 96–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragança CAD, Damm U, Baroncelli R, et al. 2016. Species of the Colletotrichum acutatum complex associated with anthracnose diseases of fruit in Brazil. Fungal Biology 120: 547–561. [DOI] [PubMed] [Google Scholar]
- Brandrud TE, Dima B, Liimatainen K, et al. 2017. Telamonioid Cortinarius species of the C. puellaris group from calcareous Tilia forests. Sydowia 69: 37–45. [Google Scholar]
- Brandrud TE, Lindström H, Marklund H, et al. 1989. Cortinarius Flora Photographica. Vol. I (Swedish version). Cortinarius HB, Matfors, Sweden. [Google Scholar]
- Brandrud TE, Lindström H, Marklund H, et al. 1992. Cortinarius Flora Photographica. Vol. II (Swedish version). Cortinarius HB, Matfors, Sweden. [Google Scholar]
- Brandrud TE, Lindström H, Marklund H, et al. 1994. Cortinarius Flora Photographica. Vol. III (Swedish version). Cortinarius HB, Matfors, Sweden. [Google Scholar]
- Brandrud TE, Lindström H, Marklund H, et al. 1998. Cortinarius Flora Photographica. Vol. IV (Swedish version). Cortinarius HB, Matfors, Sweden. [Google Scholar]
- Brandrud TE, Lindström H, Marklund H, et al. 2012. Cortinarius Flora Photographica. Vol. V (Swedish version). Cortinarius HB, Matfors, Sweden. [Google Scholar]
- Brayford D, Chapman AU. 1987. Cylindrocladium ilicicola on cuttings of evergreen ornamental shrubs in the UK. Plant Pathology 36: 413–414. [Google Scholar]
- Cabral A, Groenewald JZ, Rego C, et al. 2012. Cylindrocarpon root rot: multi-gene analysis reveals novel species within the Ilyonectria radicicola species complex. Mycological Progress 11: 655–688. [Google Scholar]
- Câmara MP, Ramaley AW, Castlebury LA, et al. 2003. Neophaeosphaeria and Phaeosphaeriopsis, segregates of Paraphaeosphaeria. Mycological Research 107: 516–522. [DOI] [PubMed] [Google Scholar]
- Castresana J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution 17: 540–552. [DOI] [PubMed] [Google Scholar]
- Ceruti A, Fontana A, Nosenzo C. 2003. Le specie europee del genere Tuber: una revisione storica. Vol. 37. Museo Regionale di Scienze Naturali, Turin, Italy. [Google Scholar]
- Chen CC, Chen CY, Lim YW, et al. 2020. Phylogeny and taxonomy of Ceriporia and other related taxa and description of three new species. Mycologia 112: 64–82. [DOI] [PubMed] [Google Scholar]
- Chen WH, Liu C, Han YF, et al. 2019. Three novel insect-associated species of Simplicillium (Cordycipitaceae, Hypocreales) from Southwest China. MycoKeys 58: 83–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements FE. 1909. The genera of fungi. The HW Wilson Company. [Google Scholar]
- Consiglio G, Setti L. 2008. Il genere Crepidotus in Europa. A.M.B. Fondazione Centro Studi Micologici, Vincenza. [Google Scholar]
- Cordeiro TRL, Nguyen TTT, Lima DX, et al. 2020. Two new species of the industrially relevant genus Absidia (Mucorales) from soil of the Brazilian Atlantic Forest. Acta Botanica Brasilica 34: 549–558. [Google Scholar]
- Crous PW, Braun U, Schubert K, et al. 2007a. Delimiting Cladosporium from morphologically similar genera. Studies in Mycology 58: 33–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Carnegie AJ, Wingfield MJ, et al. 2019a. Fungal Planet description sheets: 868–950. Persoonia 42: 291–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Cowan DA, Maggs-Kölling G, et al. 2020a. Fungal Planet description sheets: 1112–1181. Persoonia 45: 251–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Gams W. 2000. Phaeomoniella chlamydospora gen. et comb. nov., a causal organism of Petri grapevine decline and esca. Phytopathologia Mediterranea 39: 112–118. [Google Scholar]
- Crous PW, Groenewald JZ, Himaman W. 2007b. Falcocladium thailandicum. In: Crous PW, Seifert KA, Samson RA, et al. (eds), Fungal Planet – A Global Initiative to promote the Study of Fungal Biodiversity. CBS, Utrecht, Netherlands. Fungal Planet No. 18. [Google Scholar]
- Crous PW, Groenewald JZ, Summerell B. 2007c. Exophiala placitae. In: Crous PW, Seifert KA, Samson RA, et al. (eds), Fungal Planet – A Global Initiative to promote the Study of Fungal Biodiversity. CBS, Utrecht, Netherlands. Fungal Planet No. 17. [Google Scholar]
- Crous PW, Groenewald JZ, Wingfield MJ, et al. 2003. The value of ascospore septation in separating Mycosphaerella from Sphaerulina in the Dothideales: a Saccardoan myth? Sydowia 55: 136–152. [Google Scholar]
- Crous PW, Hernández-Restrepo M, Schumacher RK, et al. 2021. New and interesting fungi. 4. Fungal Systematics and Evolution 7: 255–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Lennox CL, Sutton BC. 1995. Selenophoma eucalypti and Stigmina robbenensis spp. nov. from Eucalyptus leaves on Robben Island. Mycological Research 99: 648–652. [Google Scholar]
- Crous PW, Luangsa-ard JJ, Wingfield MJ, et al. 2018a. Fungal Planet description sheets: 785–867. Persoonia 41: 238–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schubert K, Braun U, et al. 2007d. Opportunistic, human-pathogenic species in the Herpotrichiellaceae are phenotypically similar to saprobic or phytopathogenic species in the Venturiaceae. Studies in Mycology 58: 185–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schumacher RK, Akulov A, et al. 2019b. New and interesting fungi. 2. Fungal Systematics and Evolution 3: 57–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schumacher RK, Wingfield MJ, et al. 2018b. New and interesting fungi. 1. Fungal Systematics and Evolution 1: 169–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Shivas RG, Quaedvlieg W, et al. 2014a. Fungal Planet description sheets: 214–280. Persoonia 32: 184–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Tanaka K, Summerell BA, et al. 2011. Additions to the Mycosphaerella complex. IMA Fungus 2: 49–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, et al. 2016. Fungal Planet description sheets: 469–557. Persoonia 37: 218–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, et al. 2017. Fungal Planet description sheets: 625–715. Persoonia 39: 270–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Cheewangkoon R, et al. 2019c. Foliar pathogens of eucalypts. Studies in Mycology 94: 125–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Groenewald JZ. 2009. Niche sharing reflects a poorly understood biodiversity phenomenon. Persoonia 22: 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Guarro J, et al. 2015a. Fungal Planet description sheets: 320–370. Persoonia 34: 167–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Le Roux JJ, et al. 2015b. Fungal Planet description sheets: 371–399. Persoonia 35: 264–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Lombard L, et al. 2019d. Fungal Planet description sheets: 951–1041. Persoonia 43: 223–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Mansilla JP, et al. 2006. Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. II. Studies in Mycology 55: 99–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Schumacher RK, et al. 2014b. Fungal Planet description sheets: 281–319. Persoonia 33: 212–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Schumacher RK, et al. 2020b. New and interesting fungi. 3. Fungal Systematics and Evolution 6: 157–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Cannon PF, Woudenberg JHC, et al. 2012a. The Colletotrichum acutatum species complex. Studies in Mycology 73: 37–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Cannon PF, Woudenberg JHC, et al. 2012b. The Colletotrichum boninense species complex. Studies in Mycology 73: 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Fourie P, Crous PW. 2010. Coniochaeta (Lecythophora), Collophora gen. nov. and Phaeomoniella species associated with wood necroses of Prunus trees. Persoonia 24: 60–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Sato T, Alizadeh A, et al. 2019. The Colletotrichum dracaenophilum, C. magnum and C. orchidearum species complexes. Studies in Mycology 92: 1–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darriba D, Taboada GL, Doallo R, et al. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K, Sharma JR, Verbeken A. 2003. New species of Lactarius from Kumaon Himalaya, India. Mycotaxon 88: 333–342. [Google Scholar]
- De Almeida DAC, Gusmão LFP, Miller AN. 2016. Taxonomy and molecular phylogeny of Diatrypaceae (Ascomycota, Xylariales) species from the Brazilian semi-arid region, including four new species. Mycological Progress 15: 53. [Google Scholar]
- De Crop E, Nuytinck J, Van de Putte K, et al. 2014. Lactifluus piperatus (Russulales, Basidiomycota) and allied species in Western Europe and a preliminary overview of the group worldwide. Mycological Progress 13: 493–511. [Google Scholar]
- De Gruyter J, Woudenberg JHC, Aveskamp MM, et al. 2010. Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia 102: 1066–1081. [DOI] [PubMed] [Google Scholar]
- De Hoog GS. 1972. The genera Beauveria, Isaria, Tritirachium and Acrodontium gen. nov. Studies in Mycology 1: 1–41. [Google Scholar]
- Dennis RWG. 1950. Karsten’s species of Mollisia. Kew Bulletin 5: 171–187. [Google Scholar]
- Dubrulle G, Pensec F, Picot A, et al. 2020. Phylogenetic diversity and effect of temperature on pathogenicity of Colletotrichum lupini. Plant Disease 104: 938–950. [DOI] [PubMed] [Google Scholar]
- Edler D, Klein J, Antonelli A, et al. 2021. raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods in Ecology and Evolution 12: 373–377. [Google Scholar]
- Egidi E, De Hoog GS, Isola D, et al. 2014. Phylogeny and taxonomy of meristematic rock-inhabiting black fungi in the Dothideomycetes based on multi-locus phylogenies. Fungal Diversity 65: 127–165. [Google Scholar]
- Ekanayaka AH, Hyde KD, Gentekaki E, et al. 2019. Preliminary classification of Leotiomycetes. Mycosphere 10: 310–489. [Google Scholar]
- Ellis MB. 1971. Dematiaceous Hyphomycetes. CABI Publishing, Wallingford. [Google Scholar]
- Erdoğdu M, Özbek MU. 2017. First record of Phaeoseptoria and new species records on Carex for Turkey. Plant Pathology & Quarantine 7: 154–158. [Google Scholar]
- Fassatiová O. 1986. Moulds and filamentous fungi in technical microbiology. Elsevier, Amsterdam. [Google Scholar]
- Garrido-Benavent I, Ballarà J, Mahiques R. 2014. Cortinarius cadiaguirrei, un nou tàxon de la secció Fulvescentes Melot. The Journal of the Journées européennes du Cortinaire 16: 24–34. [Google Scholar]
- Gelardi M, Angelini C, Costanzo F, et al. 2021. Outstanding pinkish brown-spored neotropical Boletes: Austroboletus subflavidus and Fistulinella gloeocarpa (Boletaceae, Boletales) from the Dominican Republic. Mycobiology 49: 24–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierczyk B, Kubiński R. 2019. The first report of Pleuroflammula ragazziana in Poland. Acta Mycologica 54: 1121. [Google Scholar]
- Glynou K, Ali T, Buch A-K, et al. 2016. The local environment determines the assembly of root endophytic fungi at a continental scale. Environmental Microbiology 18: 2418–2434. [DOI] [PubMed] [Google Scholar]
- Gorfer M, Blumhoff M, Klaubauf S, et al. 2011. Community profiling and gene expression of fungal assimilatory nitrate reductases in agricultural soil. The ISME Journal 5: 1771–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräfenhan T, Schroers HJ, Nirenberg HI, et al. 2011. An overview of the taxonomy, phylogeny, and typification of nectriaceous fungi in Cosmospora, Acremonium, Fusarium, Stilbella, and Volutella. Studies in Mycology 68: 79–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramaje D, Mostert L, Groenewald JZ, et al. 2015. Phaeoacremonium: From esca disease to phaeohyphomycosis. Fungal Biology 119: 759–783. [DOI] [PubMed] [Google Scholar]
- Guarro J, Gené J, Stchigel AM, et al. 2012. Atlas of soil ascomycetes. CBS Biodiversity Series no. 10. Westerdijk Fungal Biodiversity Centre, Utrecht, the Netherlands. [Google Scholar]
- Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 55: 696–704. [DOI] [PubMed] [Google Scholar]
- Guindon S, Lethiec F, Duroux P, et al. 2010. PHYML Online – a web server for fast maximum likelihood-based phylogenetic inference. Nucleic Acids Research 33 (Web Server issue): W557–W559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JG, Hosoya T, Sung GH, et al. 2014. Phylogenetic reassessment of Hyaloscyphaceae sensu lato (Helotiales, Leotiomycetes) based on multigene analyses. Fungal Biology 118: 150–167. [DOI] [PubMed] [Google Scholar]
- Hansen EM, Maxwell DP. 1991. Species of the Phytophthora megasperma complex. Mycologia 83: 376–381. [DOI] [PubMed] [Google Scholar]
- Hansen EM, Wilcox WF, Reeser PW, et al. 2009. Phytophthora rosacearum and P. sansomeana, new species segregated from the Phytophthora megasperma ‘complex’. Mycologia 101: 129–135. [DOI] [PubMed] [Google Scholar]
- Harrington AH, Del Olmo-Ruiz M, U’Ren JM, et al. 2019. Coniochaeta endophytica sp. nov., a foliar endophyte associated with healthy photosynthetic tissue of Platycladus orientalis (Cupressaceae). Plant and Fungal Systematics 64: 65–79. [Google Scholar]
- He F, Lin B, Sun J, et al. 2013. Knufia aspidiotus sp. nov., a new black yeast from scale insects. Phytotaxa 153: 39–50. [Google Scholar]
- Heilmann-Clausen J, Verbeken A, Vesterholt J. 1998. The genus Lactarius (Fungi of Northern Europe, Vol. 2). Danish Mycological Society, Copenhagen. [Google Scholar]
- Hennings P. 1901. Beiträge zur Flora von Afrika. XXI. Fungi. camerunenses novi. III. Botanische Jahrbücher für Systematik Pflanzengeschichte und Pflanzengeographie 30: 39–57. [Google Scholar]
- Hernández-Restrepo M, Gené J, Castañeda-Ruiz RF, et al. 2017. Phylogeny of saprobic microfungi from Southern Europe. Studies in Mycology 86: 53–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Restrepo M, Groenewald JZ, Crous PW. 2016. Taxonomic and phylogenetic re-evaluation of Microdochium, Monographella and Idriella. Persoonia 36: 57–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesler LR. 1967. Entoloma in southeastern North America. Beihefte Nova Hedwigia 23: 1–245. Cramer, Germany. [Google Scholar]
- Hesler LR, Smith AH. 1963. North American species of Hygrophorus. University of Tennessee Press, Knoxville, Tennessee. [Google Scholar]
- Hesler LR, Smith AH. 1965. North American species of Crepidotus. Hafner Publishing Company, New York. [Google Scholar]
- Hesler LR, Smith AH. 1979. North American species of Lactarius. Ann Arbor, University of Michigan. [Google Scholar]
- Hesseltine CW, Ellis JJ. 1964. The genus Absidia: Gongronella and cylindrical-spored species of Absidia. Mycologia 56: 568–601. [Google Scholar]
- Hoang DT, Chernomor O, Von Haeseler A, et al. 2018. UFBoot2: Improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35: 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holubová-Jechová V. 1978. Lignicolous hyphomycetes from Czechoslovakia 5. Septonema, Hormiactella, and Lylea. Folia Geobotanica et Phytotaxonomica 13: 421–442. [Google Scholar]
- Hongsanan S, Zhao RL, Hyde KD. 2017. A new species of Chaetothyrina on branches of mango, and introducing Phaeothecoidiellaceae fam. nov. Mycosphere 8: 137–146. [Google Scholar]
- Horak E. 1978. Pleuroflammula. Persoonia 9: 439–451. [Google Scholar]
- Horak E. 1986. Beiträge zur Systematik und Oekologie von Pleuroflammula (Agaricales, Fungi). Veröffentlichungen des Geobotanischen Institutes der Eidgenössische Technische Hochschule 87: 35. [Google Scholar]
- Horak E. 2018. Fungi of New Zealand. Volume 6. Agaricales (Basidiomycota) of New Zealand. 2. Brown spored genera p.p. Crepidotus, Flammulaster, Inocybe, Phaeocollybia, Phaeomarasmius, Pleuroflammula, Pyrrhoglossum, Simocybe, Tubaria and Tympanella. Westerdijk Biodiversity Series 16: 1–205. [Google Scholar]
- Houbraken J, Kocsubé S, Visagie , et al. 2020. Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): an overview of families, genera, subgenera, sections, series and species. Studies in Mycology 95: 5–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubka V, Réblová M, Řehulka J, et al. 2014. Bradymyces gen. nov. (Chaetothyriales, Trichomeriaceae), a new ascomycetous genus accommodating poorly differentiated melanized fungi. Antonie van Leeuwenhoek 106: 979–992. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. 2001. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Isola D, Zucconi L, Onofri S, et al. 2016. Extremotolerant rock inhabiting black fungi from Italian monumental sites. Fungal Diversity 76: 75–96. [Google Scholar]
- Jančovičova S, Adamčik S. 2012. Entoloma jahnii (Fungi, Agaricales) reported from Slovakia and notes on differences with E. byssisedum. Czech Mycology 64: 209–222. [Google Scholar]
- Jayasiri SC, Hyde KD, Jones EBG, et al. 2019. Diversity, morphology and molecular phylogeny of Dothideomycetes on decaying wild seed pods and fruits. Mycosphere 10: 1–186. [Google Scholar]
- Johnston PR, Quijada L, Smith CA, et al. 2019. A multigene phylogeny toward a new phylogenetic classification of Leotiomycetes. IMA Fungus 10: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyaanamoorthy S, Minh BQ, Wong TKF, et al. 2017. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nature Methods 14: 587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Rozewicki J, Yamada KD. 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics 20: 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan Z, Gené J, Ahmad S, et al. 2013. Coniochaeta polymorpha, a new species from endotracheal aspirate of a preterm neonate, and transfer of Lecythophora species to Coniochaeta. Antonie van Leeuwenhoek 104: 243–252. [DOI] [PubMed] [Google Scholar]
- Knapp DG, Kovács GM, Zajta E, et al. 2015. Dark septate endophytic pleosporalean genera from semiarid areas. Persoonia 35: 87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobmoo N, Mongkolsamrit S, Tasanathai K, et al. 2012. Molecular phylogenies reveal host-specific divergence of Ophiocordyceps unilateralis sensu lato following its host ants. Molecular Ecology 21: 3022–3031. [DOI] [PubMed] [Google Scholar]
- Kornerup A, Wanscher JH. 1978. Methuen handbook of colour. 3rd ed. London: Eyre Methuen. [Google Scholar]
- Kozlov AM, Darriba D, Flouri T, et al. 2019. RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 35: 4453–4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus C, Damm U, Bien S, et al. 2020. New species of Phaeomoniellales from a German vineyard and their potential threat to grapevine (Vitis vinifera) health. Fungal Systematics and Evolution 6: 139–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieglsteiner L. 2004. Ascomycetenfunde während des Seminars an der Schwarzwälder Pilzlehrschau vom . bis 27. Juni 2003. Zeitschrift für Mykologie 70: 49–58. [Google Scholar]
- Kubátová A. 2006. Chaetomium in the Czech Republic and notes to three new records. Czech Mycology 58: 155–171. [Google Scholar]
- Kühner R, Romagnesi H. 1953. Flore analytique des champignons superieus (Agarics, Bolets, Chanterelles). Paris. [Google Scholar]
- Kumar AM, Vrinda KB, Pradeep CK. 2018a. Two new species of Crepidotus (Basidiomycota, Agaricales) from peninsular India. Phytotaxa 372: 67–78. [Google Scholar]
- Kumar S, Stecher G, Li M, et al. 2018b. MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35: 1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzman CP. 1995. Relationships among the genera Ashbya, Eremothecium, Holleya and Nematospora determined from rDNA sequence divergence. Journal of Industrial Microbiology 14: 523–530. [DOI] [PubMed] [Google Scholar]
- Kurtzman CP, Fell JW, Boekhout T, et al. 2011. Methods for isolation, phenotypic characterization and maintenance of yeasts. In: Kurtzman CP, Fell JW, Boekhout T. (eds), The yeasts (fifth edition): 87–110. Elsevier. [Google Scholar]
- Kurtzman CP, Robnett CJ. 2003. Phylogenetic relationships among yeasts of the ‘Saccharomyces complex’ determined from multigene sequence analyses. FEMS Yeast Research 3: 417–432. [DOI] [PubMed] [Google Scholar]
- Kurtzman CP, Robnett CJ, Basehoar E, et al. 2018. Four new species of Metschnikowia and the transfer of seven Candida species to Metschnikowia and Clavispora as new combinations. Antonie van Leeuwenhoek 111: 2017–2035. [DOI] [PubMed] [Google Scholar]
- Kušan I, Matočec N, Antonić O, et al. 2014. Biogeographical variability and re-description of an imperfectly known species Hamatocanthoscypha rotundispora (Helotiales, Hyaloscyphaceae). Phytotaxa 170: 1–12. [Google Scholar]
- Kuyper TW. 1986. A revision of the genus Inocybe in Europe. I. Subgenus Inosperma and the smooth-spored species of subgenus Inocybe. Persoonia Supplement 3: 1–247. [Google Scholar]
- Kytövuori I, Niskanen T, Liimatainen T, et al. 2005. Cortinarius sordidemaculatus and two new related species, C. anisatus and C. neofurvolaesus, in Fennoscandia (Basidiomycota, Agaricales). Karstenia 45: 33–49. [Google Scholar]
- Lachance MA. 2011. Starmerella Rosa & Lachance (1998). In: Kurtzman CP, Fell JW, Boekhout T. (eds), The yeasts, a taxonomic study, vol II: 811–815. Elsevier, New York. [Google Scholar]
- Lachance MA. 2016. Metschnikowia: half tetrads, a regicide and the fountain of youth. Yeast 33: 563–574. [DOI] [PubMed] [Google Scholar]
- Læssøe T. 2008. Hydropus Singer. In: Knudsen H, Vesterholt J. (eds), Funga Nordica: Agaricoid, boletoid and cyphelloid genera: 282–285. Nordsvamp, Copenhagen. [Google Scholar]
- Le Gal M, Mangenot MF. 1958. Contribution à l’étude des Mollisioi̋dées. II. (1re série). Revue de Mycologie 23: 28–86. [Google Scholar]
- Le Gal M, Mangenot MF. 1961. Contribution à l’étude des Mollisioi̋dées. IV. (3e série). Revue de Mycologie 26: 263–331. [Google Scholar]
- Lechat C, Crous PW, Groenewald JZ. 2010. The enigma of Calonectria species occurring on leaves of Ilex aquifolium in Europe. IMA Fungus 1: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DM, Chen XR. 2010. A new superficial fungal infection caused by Coniosporium epidermidis. Journal of the American Academy of Dermatology 63: 725–727. [DOI] [PubMed] [Google Scholar]
- Li GJ, Hyde KD, Zhao RL, et al. 2016. Fungal diversity notes 253–366: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 78: 1–237. [Google Scholar]
- Liang J, Li G, Zhao M, et al. 2019. A new leaf blight disease of turfgrasses caused by Microdochium poae, sp. nov., Mycologia 111: 265–273. [DOI] [PubMed] [Google Scholar]
- Liimatainen K, Niskanen T, Dima B. 2020. Mission impossible completed: unlocking the nomenclature of the largest and most complicated subgenus of Cortinarius, Telamonia. Fungal Diversity 104: 291–331. [Google Scholar]
- Lima DX, Cordeiro TR, De Souza CA, et al. 2020. Morphological and molecular evidence for two new species of Absidia from Neotropic soil. Phytotaxa 446: 61–71. [Google Scholar]
- Lin CG, Bhat DJ, Liu JK, et al. 2019. The genus Castanediella. MycoKeys 51: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Cai L. 2012. Morphological and molecular characterization of a novel species of Simplicillium from China. Cryptogamie Mycologie 33: 137–144. [Google Scholar]
- Liu F, Wang J, Li H, et al. 2019. Setophoma spp. on Camellia sinensis. Fungal Systematics and Evolution 4: 43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Li T, Ding Y, et al. 2017. Dark septate endophytes colonizing the roots of ‘non-mycorrhizal’ plants in a mine tailing pond and in a relatively undisturbed environment, Southwest China. Journal of Plant Interactions 12: 264–271. [Google Scholar]
- Liu Q, Li JQ, Wingfield MJ, et al. 2020. Reconsideration of species boundaries and proposed DNA barcodes for Calonectria. Studies in Mycology 97: 100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Padamsee M, Matheny PB, et al. 2014. Molecular phylogeny, morphology, pigment chemistry and ecology in Hygrophoraceae (Agaricales). Fungal Diversity 64: 1–99. [Google Scholar]
- Lombard L, Houbraken J, Decock C, et al. 2016. Generic hyper-diversity in Stachybotriaceae. Persoonia 36: 156–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, Van der Merwe NA, Groenewald JZ, et al. 2015. Generic concepts in Nectriaceae. Studies in Mycology 80: 189–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell ES. 1976. Ovarian infection of Sporobolus poiretii by Bipolaris ravenelii. Phytopathology 66: 260–268. [Google Scholar]
- MacKenzie SJ, Peres NA, Barquero MP, et al. 2009. Host range and genetic relatedness of Colletotrichum acutatum isolates from fruit crops and leatherleaf fern in Florida. Phytopathology 99: 620–631. [DOI] [PubMed] [Google Scholar]
- Magnago AC, Neves MA, Da Silveira BRM. 2017. Fistulinella ruschii, sp. nov., and a new record of Fistulinella campinaranae var. scrobiculata for the Atlantic Forest, Brazil. Mycologia 109: 1003–1013. [DOI] [PubMed] [Google Scholar]
- Mahiques R, Mateos A, Reyes JD, et al. 2013. Algunos Cortinarius de Sierra Mágina y Despeñaperros (Jaén). I. Lactarius 22: 7–49. [Google Scholar]
- Marin-Felix Y, Hernández-Restrepo M, Iturrieta-González I, et al. 2019a. Genera of phytopathogenic fungi: GOPHY 3. Studies in Mycology 94: 1–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Felix Y, Hernández-Restrepo M, Wingfield MJ, et al. 2019b. Genera of phytopathogenic fungi: GOPHY 2. Studies in Mycology 92: 47–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melot J. 1990. Une classification du genre Cortinarius (Pers.) S.F. Gray. Documents Mycologiques 20: 43–59. [Google Scholar]
- Meng W, Damodara B, Li W, et al. 2017. Molecular phylogeny of Neodevriesia, with two new species and several new combinations. Mycologia 109: 965–974. [DOI] [PubMed] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov. 2010, New Orleans: 1–8. [Google Scholar]
- Minh BQ, Nguyen MA, Von Haeseler A. 2013. Ultrafast approximation for phylogenetic bootstrap. Molecular Biology and Evolution 30: 1188–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra B, Thines M. 2014. siMBa – a simple graphical user interface for the Bayesian phylogenetic inference program MrBayes. Mycological Progress 13: 1255–1258. [Google Scholar]
- Molia A, Larsson E, Jeppson M, et al. 2020. Elaphomyces section Elaphomyces (Eurotiales, Ascomycota) – taxonomy and phylogeny of North European taxa, with the introduction of three new species. Fungal Systematics and Evolution 5: 283–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Arroyo B, Llistosella J, De la Osa LR. 2002. Gymnomyces sublevisporus (Russulales), una nueva especie de la región mediterránea. Revista Catalana de Micologia 24: 179–186. [Google Scholar]
- Morgan-Jones G. 1975. Notes on hyphomycetes. VIII. Lylea, a new genus. Mycotaxon 3: 129–132. [Google Scholar]
- Morozova OV, Malysheva EV, Popov ES, et al. 2015. Macromycetes of the Izborsk-Maly Valley, rare and new to the Pskov Region. Novosti Sistematiki Vysshikh i Nizshikh Rastenii 49: 186–203. [Google Scholar]
- Moser M. 1978. Die Röhrlinge und Blätterpilze, 4th edition. In: Gams H. (ed), Kleine Kryptogamenflora IIb/2. Fischer Verlag, Stuttgart. [Google Scholar]
- Moser M. 1983. Die Röhrlinge und Blätterpilze. In: Gams H. (ed), Kleine Kryptogamenflora, Band IIb/2, 5th edn. Fischer Verlag, Stuttgart, Germany. [Google Scholar]
- Moser M. 2001. Rare, debated and new taxa of the genus Cortinarius (Agaricales). Fungi Delineati 15: 1–57. [Google Scholar]
- Moser M, Horak E. 1975. Cortinarius Fr. und nahe verwandte Gattungen in Südamerika. Beihefte Nova Hedwigia 52: 1–628. [Google Scholar]
- Moser M, McKnight KH, Ammirati JF. 1995. Studies on North American Cortinarii I. New and interesting taxa from the greater Yellowstone area. Mycotaxon 55: 301–346. [Google Scholar]
- Mostert L, Groenewald JZ, Summerbell RC, et al. 2006. Taxonomy and pathology of Togninia (Diaporthales) and its Phaeoacremonium anamorphs. Studies in Mycology 54: 1–115. [Google Scholar]
- Munsell Soil Color Charts. 1954 edition. Munsell Color, Baltimore, Maryland, USA. [Google Scholar]
- Nasr S, Bien S, Soudi MR, et al. 2018. Novel Collophorina and Coniochaeta species from Euphorbia polycaulis, an endemic plant in Iran. Mycological Progress 17: 755–771. [Google Scholar]
- Nguyen L-T, Schmidt HA, Von Haeseler A, et al. 2015. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution 32: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen NH, Vellinga EC, Bruns TD, et al. 2016. Phylogenetic assessment of global Suillus ITS sequences supports morphologically defined species and reveals synonymous and undescribed taxa. Mycologia 108: 1216–1228. [DOI] [PubMed] [Google Scholar]
- Nirenberg HI, Feiler U, Hagedorn G. 2002. Description of Colletotrichum lupini comb. nov. in modern terms. Mycologia 94: 307–320. [PubMed] [Google Scholar]
- Niskanen T, Kytövuori I, Liimatainen K. 2009. Cortinarius sect. Brunnei (Basidiomycota, Agaricales) in North Europe. Mycological Research 113: 182–206. [DOI] [PubMed] [Google Scholar]
- Niskanen T, Kytövuori I, Liimatainen K, et al. 2013. Cortinarius section Bovini (Agaricales, Basidiomycota) in northern Europe, conifer associated species. Mycologia 105: 977–993. [DOI] [PubMed] [Google Scholar]
- Nonaka K, Kaifuchi S, Omura S, et al. 2013. Five new Simplicillium species (Cordycipitaceae) from soils in Tokyo, Japan. Mycoscience 54: 42–53. [Google Scholar]
- Noordeloos ME. 1984. Studies in Entoloma 10–13. Persoonia 12: 193–122. [Google Scholar]
- Noordeloos ME. 1987. Entoloma (Agaricales) in Europe. Synopsis and keys to all species and a monograph of the subgenera Trichopilus, Inocephalus, Alboleptonia, Leptonia, Paraleptonia, and Omphaliopsis. Beihefte zur Nova Hedwigia 91: 1–419. [Google Scholar]
- Noordeloos ME. 1988. Entoloma in North America. The species described by L.R. Hesler, A.H. Smith & S.J. Mazzer: type-species and comments. Cryptogamic Studies, Vol. 2. Gustav Fisher Verlag, Stuttgart, Germany. [Google Scholar]
- Noordeloos ME. 1992. Entoloma s.l. Fungi Europaei, vol. 5. Giovanna Biella, Saronno, Italy. [Google Scholar]
- Noordeloos ME. 2004. Entoloma s.l. Fungi Europaei, vol. 5a. Edizione Candusso, Italy. [Google Scholar]
- Orton PD. 1960. New checklist of British Agarics and Boleti. Part 3: Notes on genera and species in the list. Transactions of the British Mycolological Society Supplement 43: 159–439. [Google Scholar]
- Parmelee JA. 1956. The identification of the Curvularia parasite of Gladiolus. Mycologia 48: 558–567. [Google Scholar]
- Paz A, Bellanger JM, Lavoise C, et al. 2017. The genus Elaphomyces (Ascomycota, Eurotiales): a ribosomal DNA-based phylogeny and revised systematics of European ‘deer truffles’. Persoonia 38: 197–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pethybridge GH. 1913. On the rotting of potato tubers by a new species of Phytophthora having a method of sexual reproduction hitherto undescribed. Scientific Proceedings of the Royal Dublin Society 13: 529–565. [Google Scholar]
- Pethybridge GH, Lafferty HA. 1919. A disease of tomato and other plants caused by a new species of Phytophthora. Scientific Proceedings of the Royal Dublin Society 15: 487–503. [Google Scholar]
- Phukhamsakda C, McKenzie EHC, Phillips AJL, et al. 2020. Microfungi associated with Clematis (Ranunculaceae) with an integrated approach to delimiting species boundaries. Fungal Diversity 102: 1–203. [Google Scholar]
- Pitt JI, Lantz H, Pettersson OV, et al. 2013. Xerochrysium gen. nov. and Bettsia, genera encompassing xerophilic species of Chrysosporium. IMA Fungus 4: 229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W, Binder M, Groenewald JZ, et al. 2014. Introducing the Consolidated Species Concept to resolve species in the Teratosphaeriaceae. Persoonia 33: 1–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W, Verkley GJM, Shin H-D, et al. 2013. Sizing up Septoria. Studies in Mycology 75: 307–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quélet L. 1886. Les Champignons du Jura et des Vosges. C. r. Ass. Franc. Av. Sci. (Grenoble, 1885): 446. [Google Scholar]
- Ramírez C, Martínez AT. 1981. Seven new species of Penicillium and a new variety of Penicillium novae-caledoniae Smith. Mycopathologia 74: 35–49. [Google Scholar]
- Rashmi M, Kushveer JS, Sarma VV. 2019. A worldwide list of endophytic fungi with notes on ecology and diversity. Mycosphere 10: 798–1079. [Google Scholar]
- Rayner RW. 1970. A mycological colour chart. Commonwealth Mycological Institute, Kew and British Mycological Society. [Google Scholar]
- Raza M, Zhang Z-F, Hyde KD, et al. 2019. Culturable plant pathogenic fungi associated with sugarcane in southern China. Fungal Diversity 99: 1–104. [Google Scholar]
- Ridgway R. 1912. Color standards and color nomenclature. Ridgway, Washington, DC. [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, Van der Mark P, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaiefarahani B, Mostowfizadeh-Ghalamfarsa RSTJ, Hardy GSJ, et al. 2015. Re-evaluation of the Phytophthora cryptogea species complex and the description of a new species, Phytophthora pseudocryptogea sp. nov. Mycological Progress 14: 108. [Google Scholar]
- Salom JC, Esteve-Raventós F. 2011. Phaeomarasmius siquierii (Agaricoid clade, Tubariaceae), a new Mediterranean resupinate species found in Formentera (Balearic Islands, Spain). Micologia e Vegetazione Mediterranea 26: 29–36. [Google Scholar]
- Samarakoon BC, Wanasinghe DN, Phookamsak R, et al. 2021. Stachybotrys musae sp. nov., S. microsporus, and Memnoniella levispora (Stachybotryaceae, Hypocreales) Found on Bananas in China and Thailand. Life 11: 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels GJ. 1977. Nectria consors and its Volutella conidial state. Mycologia 69: 255–262. [Google Scholar]
- Sandoval-Denis M, Gené J, Sutton DA, et al. 2016. New species of Cladosporium associated with human and animal infections. Persoonia 36: 281–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval-Denis M, Guarnaccia V, Polizzi G, et al. 2018. Symptomatic Citrus trees reveal a new pathogenix lineage in Fusarium and two new Neocosmospora species. Persoonia 40: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller M, Lübeck M, Sundelin T, et al. 2006. Two subpopulations of Colletotrichum acutatum are responsible for anthracnose in strawberry and leatherleaf fern in Costa Rica. European Journal of Plant Pathology 116: 107–118. [Google Scholar]
- Segeth MP, Bonnefoy A, Broenstrup M, et al. 2003. Coniosetin, a novel tetramic acid antibiotic from Coniochaeta ellipsoidea DSM 13856. The Journal of Antibiotics 56: 114–122. [DOI] [PubMed] [Google Scholar]
- Séguy E. 1936. Encyclopedie Pratique du Naturaliste, 30. Paul Lechevalier, Paris. [Google Scholar]
- Shang QJ, Phookamsak R, Camporesi E, et al. 2018. The holomorph of Fusarium celtidicola sp. nov. from Celtis australis. Phytotaxa 361: 251–265. [Google Scholar]
- Shivas RG, Smith MW, Marney TS, et al. 2005. First record of Nematospora coryli in Australia and its association with dry rot of Citrus. Australasian Plant Pathology 34: 99–101. [Google Scholar]
- Shoemaker RA, Babcock CE. 1989. Phaeosphaeria. Canadian Journal of Botany 67: 1500–1599. [Google Scholar]
- Shoemaker RA, Babcock CE. 1992. Applanodictyosporous Pleosporales: Clathrospora, Comoclathris, GraphyIlium, Macrospora, and Platysporoides. Canadian Journal of Botany 70: 1617–1658. [Google Scholar]
- Singer R. 1947. ‘The Boletoideae of Florida with notes on extralimital species III’. American Midland Naturalist 37: 1–135. [Google Scholar]
- Smith AH, Hesler LR. 1968. The North American species of Pholiota. Hafner Publishing Co., New York, USA. [Google Scholar]
- Smith AH, Thiers HD. 1964. A contribution toward a monograph of the North American species of Suillus (Boletaceae). Lubrecht & Cramer, Ann Arbor, Michigan. [Google Scholar]
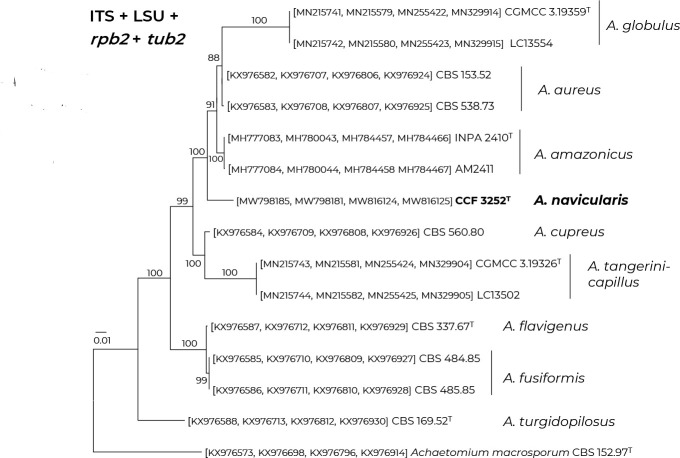
- Sousa TF, Dos Santos AO, Da Silva FMA, et al. 2020. Arcopilus amazonicus (Chaetomiaceae), a new fungal species from the Amazon rainforest native plant Paullinia cupana. Phytotaxa 456: 145–156. [Google Scholar]
- Spies CFJ, Moyo P, Halleen F, et al. 2018. Phaeoacremonium species diversity on woody hosts in the Western Cape Province of South Africa. Persoonia 40: 26–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: A toll for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Su L, Yang S, et al. 2020. Unveiling the hidden diversity of rock-inhabiting fungi: Chaetothyriales from China. Journal of Fungi 6: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svrček M. 1987. New or less known Discomycetes. XVI. Česká Mykologie 41: 88–96. [Google Scholar]
- Swofford DL. 2003. PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods). Version 4. Sinauer Associates. Sunderland, MA. [Google Scholar]
- Tamura K, Nei M. 1993. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution 10: 512–526. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, et al. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YP, Crous PW, Shivas RG. 2018. Cryptic species of Curvularia in the culture collection of the Queensland Plant Pathology Herbarium. MycoKeys 35: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Hirayama K, Yonezawa H, et al. 2009. Molecular taxonomy of bambusicolous fungi: Tetraplosphaeriaceae, a new pleosporalean family with Tetraploa-like anamorphs. Studies in Mycology 64: 175–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Hirayama K, Yonezawa H, et al. 2015. Revision of the Massarineae (Pleosporales, Dothideomycetes). Studies in Mycology 82: 75–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanney JB, Seifert KA. 2020. Mollisiaceae: An overlooked lineage of diverse endophytes. Studies in Mycology 95: 293–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennakoon DS, Thambugala KM, Wanasinghe DN, et al. 2020. Additions to Phaeosphaeriaceae (Pleosporales): Elongaticollum gen. nov., Ophiosphaerella taiwanensis sp. nov., Phaeosphaeriopsis beaucarneae sp. nov. and a new host record of Neosetophoma poaceicola from Musaceae. MycoKeys 70: 59–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thambugala KM, Ariyawansa HA, Li Y, et al. 2014. Dothideales. Fungal Diversity 68: 105–158. [Google Scholar]
- Trifinopoulos J, Nguyen L-T, Von Haeseler A, et al. 2016. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research: 44 (W1): W232–W235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui CKM, Leung YM, Hyde KD, et al. 2001. Three new Ophioceras species (Ascomycetes) from the tropics. Mycoscience 42: 321–326. [Google Scholar]
- Tsuneda A, Currah RS. 2005. Pleomorphic conidiogenesis among strains of Knufia cryptophialidica. Canadian Journal of Botany 83: 510–517. [Google Scholar]
- Tsuneda A, Hambleton S, Currah RS. 2011. The anamorph genus Knufia and its phylogenetically allied species in Coniosporium, Sarcinomyces, and Phaeococcomyces. Botany 89: 523–536. [Google Scholar]
- Tucker CM. 1931. Taxonomy of the genus Phytophthora de Bary. Research Bulletin of the Missouri Agricultural Experiment Station 153: 207. [Google Scholar]
- Tulasne LR, Tulasne C. 1851. Fungi Hypogaei, Histoire et Monographie des Champignons Hypogés. Klincksieck F. (ed.), Paris, France. [Google Scholar]
- Untereiner WA, Gueidan C, Orr MJ, et al. 2011. The phylogenetic position of the lichenicolous ascomycete Capronia peltigerae. Fungal Diversity 49: 225–233. [Google Scholar]
- Vasco-Palacios AM, Lopez-Quintero CA, Franco-Molano AE, et al. 2014. Austroboletus amazonicus sp. nov. and Fistulinella campinaranae var. scrobiculata, two commonly occurring boletes from a forest dominated by Pseudomonotes tropenbosii (Dipterocarpaceae) in Colombian Amazonia. Mycologia 106: 1004–1014. [DOI] [PubMed] [Google Scholar]
- Verbeken A, Van de Putte K, De Crop E. 2012. New combinations in Lactifluus, 3: L. subgenera Lactifluus and Piperati. Mycotaxon 120: 443–450. [Google Scholar]
- Verwoerd L, Du Plessis SJ. 1931. Descriptions of some new species of South African fungi and species not previously recorded from South Africa. III. South African Journal of Science 28: 290–297. [Google Scholar]
- Vidal JM, Alvarado P, Loizides M, et al. 2019. A phylogenetic and taxonomic revision of sequestrate Russulaceae in Mediterranean and temperate Europe. Persoonia 42: 127–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira SIR, Groenewald JZ, Braun U, et al. 2016. All that glitters is not Ramularia. Studies in Mycology 83: 49–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira SIR, Groenewald JZ, Nakashima C, et al. 2017. Mycosphaerellaceae – chaos or clarity? Studies in Mycology 87: 257–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizzini A. 2008. Novitates. Tubariaceae fam. nov. Rivista di Micologia 51: 174. [Google Scholar]
- Vizzini A, Consiglio G, Marchetti M. 2019. Mythicomycetaceae fam. nov. (Agaricineae, Agaricales) for accommodating the genera Mythicomyces and Stagnicola, and Simocybe parvispora reconsidered. Fungal Systematics and Evolution 3: 41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voitk A, Saar I, Lodge J, et al. 2020. New species and reports of Cuphophyllus from northern North America compared with related Eurasian species. Mycologia 112: 438–452. [DOI] [PubMed] [Google Scholar]
- Von Arx JA, Guarro J, Figueras MJ. 1986. The ascomycete genus Chaetomium. Beihefte zur Nova Hedwigia 84: 1–162. [Google Scholar]
- Walsh E, Luo J, Zhang N. 2014. Acidomelania panicicola gen. et sp. nov. from switchgrass roots in acidic New Jersey pine barrens. Mycologia 106: 856–864. [DOI] [PubMed] [Google Scholar]
- Wanasinghe DN, Phukhamsakda C, Hyde KD, et al. 2018. Fungal diversity notes 709–839: taxonomic and phylogenetic contributions to fungal taxa with an emphasis on fungi on Rosaceae. Fungal Diversity 89: 1–236. [Google Scholar]
- Wang GS, Zhou Y, Xue L, et al. 2020. Teunia rosae sp. nov. and Teunia rudbeckiae sp. nov. (Cryptococcaceae, Tremellales), two novel basidiomycetous yeast species isolated from flowers. International Journal of Systematics and Evolutionary Microbiology 70: 5394–5400. [DOI] [PubMed] [Google Scholar]
- Wang H-J, Gloer JB, Scott JA, et al. 1995. Coniochaetones A and B: new antifungal benzopyranones from the coprophilous fungus Coniochaeta saccardoi. Tetrahedron Letters 36: 5847–5850. [Google Scholar]
- Wang XW, Houbraken J, Groenewald JZ, et al. 2016. Diversity and taxonomy of Chaetomium and chaetomium-like fungi from indoor environments. Studies in Mycology 84: 145–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watling R. 1975. Observations on the Bolbitiaceae 11: A species of Bolbitius with ornamented basidiospores. Notes from the Royal Botanic Garden. Edinburgh 34: 241–244. [Google Scholar]
- Watling R. 1987. Observations on the Bolbitiaceae – 30. Agaricus callistus Peck. Mycologia 79: 310–313. [Google Scholar]
- Wei DP, Wanasinghe DN, Hyde KD, et al. 2019. The genus Simplicillium. MycoKeys 60: 69–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton SR, McKenzie EHC, Hyde KD. 2012. Anamorphic Fungi associated with Pandanaceae. In: Whitton SR, McKenzie EHC, Hyde KD. (eds), Fungi associated with Pandanaceae: 125–353. Springer, Dordrecht. [Google Scholar]
- Xia J-W, Ma Y-R, Zhang X-G. 2014. New species of Corynesporopsis and Lylea from China. Sydowia 66: 241–248. [Google Scholar]
- Xie J, Strobel GA, Feng T, et al. 2015. An endophytic Coniochaeta velutina producing broad spectrum antimycotics. Journal of Microbiology 53: 390–397. [DOI] [PubMed] [Google Scholar]
- Yang X, Tyler BM, Hong C. 2017. An expanded phylogeny for the genus Phytophthora. IMA Fungus 8: 355–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare R, Gams W. 2001. A revision of Verticillium section Prostrata. IV. The genera Lecanicillium and Simplicillium gen. nov. Nova Hedwigia 73: 1–50. [Google Scholar]
- Zhang D, Gao F, Jakovlić I, et al. 2020. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Molecular Ecology Resources 20: 348–355. [DOI] [PubMed] [Google Scholar]
- Zhang ZF, Liu F, Zhou X, et al. 2017. Culturable mycobiota from Karst caves in China, with descriptions of 20 new species. Persoonia 39: 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]