Abstract
Objectives
Water immersion during labour using a birth pool to achieve relaxation and pain relief during the first and possibly part of the second stage of labour is an increasingly popular care option in several countries. It is used particularly by healthy women who experience a straightforward pregnancy, labour spontaneously at term gestation and plan to give birth in a midwifery led care setting. More women are also choosing to give birth in water. There is debate about the safety of intrapartum water immersion, particularly waterbirth. We synthesised the evidence that compared the effect of water immersion during labour or waterbirth on intrapartum interventions and outcomes to standard care with no water immersion. A secondary objective was to synthesise data relating to clinical care practices and birth settings that women experience who immerse in water and women who do not.
Design
Systematic review and meta-analysis.
Data sources
A search was conducted using CINAHL, Medline, Embase, BioMed Central and PsycINFO during March 2020 and was replicated in May 2021.
Eligibility criteria for selecting studies
Primary quantitative studies published in 2000 or later, examining maternal or neonatal interventions and outcomes using the birthing pool for labour and/or birth.
Data extraction and synthesis
Full-text screening was undertaken independently against inclusion/exclusion criteria in two pairs. Risk of bias assessment included review of seven domains based on the Robbins-I Risk of Bias Tool. All outcomes were summarised using an OR and 95% CI. All calculations were conducted in Comprehensive Meta-Analysis V.3, using the inverse variance method. Results of individual studies were converted to log OR and SE for synthesis. Fixed effects models were used when I2 was less than 50%, otherwise random effects models were used. The fail-safe N estimates were calculated to determine the number of studies necessary to change the estimates. Begg’s test and Egger’s regression risk assessed risk of bias across studies. Trim-and-fill analysis was used to estimate the magnitude of effect of the bias. Meta-regression was completed when at least 10 studies provided data for an outcome.
Results
We included 36 studies in the review, (N=157 546 participants). Thirty-one studies were conducted in an obstetric unit setting (n=70 393), four studies were conducted in midwife led settings (n=61 385) and one study was a mixed setting (OU and homebirth) (n=25 768). Midwife led settings included planned home and freestanding midwifery unit (k=1), alongside midwifery units (k=1), planned homebirth (k=1), a freestanding midwifery unit and an alongside midwifery unit (k=1) and an alongside midwifery unit (k=1). For water immersion, 25 studies involved women who planned to have/had a waterbirth (n=151 742), seven involved water immersion for labour only (1901), three studies reported on water immersion during labour and waterbirth (n=3688) and one study was unclear about the timing of water immersion (n=215).
Water immersion significantly reduced use of epidural (k=7, n=10 993; OR 0.17 95% CI 0.05 to 0.56), injected opioids (k=8, n=27 391; OR 0.22 95% CI 0.13 to 0.38), episiotomy (k=15, n=36 558; OR 0.16; 95% CI 0.10 to 0.27), maternal pain (k=8, n=1200; OR 0.24 95% CI 0.12 to 0.51) and postpartum haemorrhage (k=15, n=63 891; OR 0.69 95% CI 0.51 to 0.95). There was an increase in maternal satisfaction (k=6, n=4144; OR 1.95 95% CI 1.28 to 2.96) and odds of an intact perineum (k=17, n=59 070; OR 1.48; 95% CI 1.21 to 1.79) with water immersion. Waterbirth was associated with increased odds of cord avulsion (OR 1.94 95% CI 1.30 to 2.88), although the absolute risk remained low (4.3 per 1000 vs 1.3 per 1000). There were no differences in any other identified neonatal outcomes.
Conclusions
This review endorses previous reviews showing clear benefits resulting from intrapartum water immersion for healthy women and their newborns. While most included studies were conducted in obstetric units, to enable the identification of best practice regarding water immersion, future birthing pool research should integrate factors that are known to influence intrapartum interventions and outcomes. These include maternal parity, the care model, care practices and birth setting.
PROSPERO registration number
CRD42019147001.
Keywords: Pain management, Maternal medicine, PRIMARY CARE
Strengths and limitations of this study.
This study incorporated meta-regression, using covariates identified a priori, to identify sources of heterogeneity in previous studies.
This study included cumulative meta-analysis and fail-safe analysis to provide estimates of the stability of the findings.
Inconsistency of reporting on birth setting, care practices, interventions and outcomes prevented us from achieving our secondary objective to account for intrapartum care variation.
Meta-regression was only possible for three outcomes: intact perineum, episiotomy and postpartum haemorrhage.
Few studies were conducted in midwifery-led settings.
Introduction
Immersion in a birthing pool offers women a non-pharmacological option of pain relief during labour, which also enhances their sense of control. Resting and labouring in water can reduce fear, anxiety and pain perception; it helps optimise the physiology of childbirth through the release of endogenous endorphins and oxytocin. Evidence from randomised controlled trials (RCTs) showed that labouring in water reduces the need for epidural analgesia while identifying no adverse maternal or neonatal effects.1 In the UK, most birthing pool use occurs in midwifery-led birth settings: these include alongside midwifery units (colocated with a maternity hospital setting) and freestanding midwifery units (in the community setting) and home birth.2 The outcomes of birthing pool use may be different in midwifery-led settings compared with an obstetric setting because healthy women experience fewer interventions and operative birth when the birth occurs in a midwifery-led setting compared with an obstetric setting.3
Variations in care between waterbirth services may contribute to the differences in outcomes with water immersion, particularly variations in use of labour augmentation, hands on/off the perineum for the birth, pushing position, use of active management of third stage of labour and placenta birth in the water.3–9 It is likely that women who use water immersion for labour and birth experience different care practices than women who have standard birth care. Though prior evidence has found no increased risk of adverse events for newborns born in water, heterogeneity in outcomes and limited reporting of the clinical guidance used for water immersion make implementation of evidence-based guidelines difficult.10–12 There is a need to understand which clinical practices, when performed as part of water immersion care, result in the optimum outcomes for mother and newborn. It has been argued that an international RCT would be desirable.13 14 However, an RCT proposal is likely to encounter ethical and recruitment challenges due to increasing acknowledgement of the importance of enabling women to take an active part in decision making during labour. Additionally, an unblinded trial and expected uneven crossover carry an inevitable limitation.15
Water immersion in a birth pool during labour and birth can be divided into two distinct but overlapping categories. Water immersion during labour involves using a birth pool to achieve relaxation and pain relief during the first and possibly part of the second stage of labour but exiting the pool for the birth. With this practice, the infant emerges into air to breathe. With waterbirth, the woman remains in the birth pool for the birth of the baby. The infant emerges into the water and is brought to the surface to initiate breathing.
The primary objective of this systematic review was to compare intrapartum interventions and outcomes for water immersion during labour/waterbirth to standard care with no water immersion. The secondary objective was to analyse data reported for clinical care practices and birth settings experienced by women who use water and women who do not.
Review questions
What interventions do women experience with water immersion for labour and birth?
What are the maternal and newborn outcomes following water immersion during labour and waterbirth compared with similar women who labour and/or give birth on land?
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guideline was followed for conducting this work.16
Patient and public involvement
Patients were not involved in the development of the research question, study design or selection of outcome measures.
Eligibility criteria included:
Studies using any primary quantitative study design published in peer-reviewed journal or unpublished thesis.
Studies that examined maternal or neonatal interventions and/or outcomes when using the birthing pool for labour and/or birth.
Studies published in 2000 or later.
Studies conducted in any language if it could be translated into English using Google Translate.
A search was conducted using CINAHL, Medline, Embase, BioMed Central (BMC) and PsycINFO during March 2020. The search was replicated in May 2021. A predesigned search strategy was designed using the PICOT/PEOT framework to develop search terms17:
Population: women in labour and early post partum.
Intervention/Exposure: water immersion during labour and/or birth.
Comparison: no water immersion during labour or birth.
Outcomes: Maternal: artificial rupture of the membranes, need for labour augmentation, epidural analgesia, opioid injection, planned and actual place of birth, reason for transfer to an obstetric setting, mode of birth, perineal trauma, third-stage management, postpartum haemorrhage (PPH)/blood transfusion, infection, breastfeeding initiation. Newborn: APGAR score, resuscitation, admission to a neonatal intensive care unit (NICU), infection, breastfeeding at 6 weeks.
Time: labour and early puerperium.
A tested, sensitive and reproducible search strategy was developed with the specialist healthcare librarian, VF.18 The refined search terms and strategy with Boolean operators are provided in online supplemental file 1. These were adapted for specific database architecture. Additional searches were carried out via referencing, checking all included studies with no further records found. Publication alerts were set up via BMC updates that alerted CF1 to a new publication that met our inclusion/exclusion criteria. A final search to determine if any additional papers were published after analysis was conducted by VF in May 2021.
bmjopen-2021-056517supp001.pdf (271KB, pdf)
Study selection
Records were deduplicated in Zotero and collated into Rayyan systematic review software.19 Initial screening (title/abstract) was carried out blind by HTC, CF1, CF2 against the inclusion/exclusion criteria. Consensus meetings were held to discuss and resolve disagreements. Full-text screening was carried out independently against the inclusion/exclusions criteria and in pairs: JV and CF1, EB and PJH. Disagreements were resolved by consensus meeting. In the case of duplication of a sample across multiple papers, the paper which provided the largest sample for each outcome provided the data for synthesis.
Data collection was completed using pilot tested forms created in REDCap data collection software. Researchers worked in teams of two (JV and EB, JV and PJH) to individually abstract data for each study, identify discrepancies and reach consensus when needed. Data collected included the study type; sample characteristics, care practices for water immersion, if it was a midwifery-led setting; rates of interventions including amniotomy, labour induction, augmentation, fetal monitoring, epidural, injected opioid, episiotomy and active management of third stage; and outcome data including mode of birth, level of pain, maternal satisfaction, intact perineum, obstetric anal sphincter injury (OASI), shoulder dystocia, maternal infection defined by symptoms and positive test, primary PPH, manual removal of the placenta, 5 min APGAR, newborn resuscitation, transient tachypnoea of the newborn, respiratory distress of the newborn, neonatal intensive unit admission within the first 24 hours and lasting for 48 hours, death in neonatal period, newborn infection defined by both symptoms and positive test, cord avulsion and breastfeeding initiation.
Risk of bias assessment
Risk of bias assessment included review of seven domains based on the Robbins-I Risk of Bias Tool.20 The domains included bias due to confounding, bias in selection of participants, bias in measurement of intervention, bias due to departures of intended treatment, bias in measurement of outcomes, bias due to missing data, bias in selection of reported results. Bias due to departure of intended treatment was modified to track studies that did not provide information about water immersion use for the control group. Risk of bias assessment was completed independently by two researchers (JV and EB, JV and PJH). Disagreements were resolved by consensus meeting.
Summary measures and synthesis of results
All outcomes were summarised using an OR and 95% CI. All calculations were conducted in Comprehensive Meta-Analysis V.3, using the inverse variance method.21 Results of individual studies were converted to log OR and SE for synthesis. Fixed effects models were used when I2 was less than 50%, otherwise random effects models were used. This decision was made because (1) the population eligible for water immersion is restricted to women at low risk of birth complications and (2) the goal of the analysis was to determine if variations in care practices result in changes in outcomes. Outcomes without adequate heterogeneity in estimates were considered unlikely to be affected by care practices and so a fixed effects model was appropriate for analysis. When possible, subgroup analysis was conducted to determine effect of the birth setting and parity on the estimate. In addition, analysis limited to studies published within the past 10 years was conducted when possible. Per protocol, we intended to conduct subgroup analysis by maternal age, maternal body mass index (BMI), prior caesarean, and pool type, however, the data did not allow for these analyses. Cumulative meta-analysis was used to identify the stability of the estimates over time.22 The fail-safe N estimates were calculated to determine the number of studies necessary to change the estimates.23 Forest plots were created in RevMan V.5.4.1.24
Additional analyses
Begg’s test and Egger’s Regression Risk assessed risk of bias across studies.25 Trim-and-fill analysis was used to estimate the magnitude of effect of the bias.26 Meta-regression was completed when at least 10 studies provided data for an outcome when I2 >50%.26–28 Tested covariates included the sample characteristics and care practices identified a priori as the structure and process variables likely to be responsible for heterogeneity in the outcomes. Directed acyclic graphs of the covariates and their role are available in online supplemental file 2.29 For continuous covariates, the rate of a covariate (eg, the induction rate in the sample) were used for regression. Categorical covariates were coded as dichotomous (eg, described appropriate birth pool or did not describe the immersion receptacle).
bmjopen-2021-056517supp002.pdf (1.2MB, pdf)
Certainty assessment
The fail-safe N estimates were calculated to determine the number of studies necessary to change the estimates.23 Fail-safe calculates the number of studies needed to change the estimate. Cumulative meta-analysis was used to identify the stability of the estimates over time.22 Assessment of certainty with GRADE criteria was considered inappropriate for this review because the goal of this study was to identify variations between reports of outcomes with water immersion that contribute to inconsistency, imprecision, variations and confounding—three assessments made when considering certainty of evidence. However, the authors recognise the importance of a standardised GRADE assessment for readers. The individual assessments made in this review were prepared in a table outlining scores per standard Grade criteria as online supplemental file 3.
bmjopen-2021-056517supp003.pdf (65.4KB, pdf)
Results
Study selection
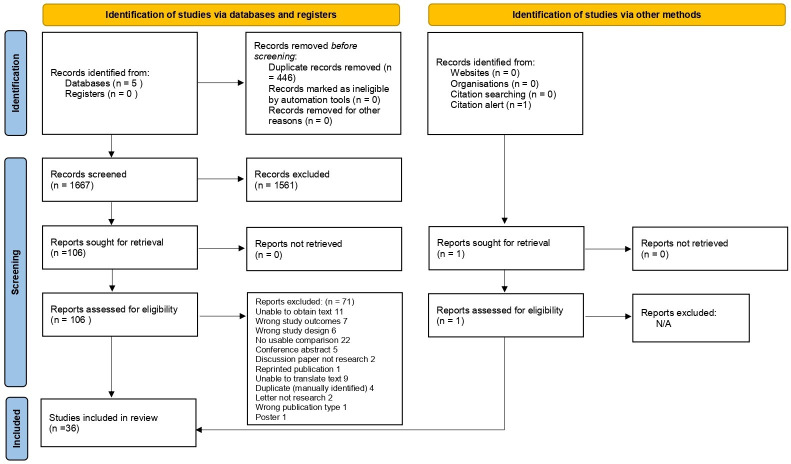
The searches generated 2113 hits, reduced to 1667 after duplicates were removed; n=1561 records were discarded at the initial screening stage. Of 106 records that were full-text screened, n=71 records did not meet the criteria. See online supplemental file 4 for the list of excluded studies and the reasons. One additional study was found via BMC updates, therefore, k=36 papers reporting on outcomes for 157 546 women were included into the review.13 30–64 Figure 1 illustrates the study selection process.16
Figure 1.
PRISMA 2020 flow diagram for new systematic reviews which included searches of databases, registers and other sources. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
bmjopen-2021-056517supp004.pdf (149KB, pdf)
Study description
Most studies (k=31) were conducted in an obstetric setting or did not adequately report the setting, while four studies were conducted in midwife-led settings; two included planned home and birth centre births,33 57 one that involved a birth centre (not explicitly described as freestanding) and an alongside midwifery unit (colocated in an obstetric unit).32 Studies included RCTs (k=6; n=1862), prospective studies (k=13; n=28 226), retrospective studies (k=16; n=127 477), and one pre–post study (n=11). Studies reported on waterbirth (k=25; n= 151 742), water immersion for labour (k=7; n=1901), both (k=3; n=3688
) and one whose timing of immersion could not be determined (n=215). Full information is available in table 1.
Table 1.
Characteristics of included studies; meta-analysis of water immersion for labour and birth
| Author | Study type | Setting | Immersion exposure | Sample Size |
Interventions and outcomes reported |
| Bailey et al30 | RO | Obstetric | Waterbirth | 2422 | 1, 5, 10, 11, 13, 17 |
| Barry et al31 | PO | Obstetric | Both | 380 | 8, 10, 11, 13, 17, 23 |
| Benfield et al32 | Pre-Post | Obstetric | Labour | 11 | 4, 7 |
| Bovbjerg et al33 | RO | Midwifery | Waterbirth | 18 355 | 10, 11, 12, 17, 21 |
| Cluett et al34 | RCT | Obstetric | Labour | 99 | 2, 6, 7, 8, 15, 16 |
| da Silva et al35 | RCT | Obstetric | Labour | 108 | 2, 4, 7, 10, 12, 17 |
| Eckert et al36 | RCT | Obstetric | Labour | 274 | 1, 5, 6, 7, 8, 11, 12, 16, 17, 18 |
| Geisbuehler et al38 | PO | Obstetric | Waterbirth | 5584 | 12, 20 |
| Geissbuehler et al39 | PO | Obstetric | Waterbirth | 9518 | 5, 9, 10, 11, 13, 15, 17 |
| Geissbühler and Eberhard37 | PO | Obstetric | Waterbirth | 7508 | 6, 16 |
| Haslinger et al40 | RO | Obstetric | Waterbirth | 7832 | 11, 12 |
| Henderson et al41 | PO | Obstetric | Both | 3078 | 2, 3, 8, 10, 12, 13, 14, 18 |
| Hodgson et al42 | RO | Mixed | Waterbirth | 25 768 | 4, 11, 17, 18 |
| Jacoby et al43 | RO | Obstetric | Waterbirth | 23 036 | 11, 13, 15, 17, 18, 20, 21, 23 |
| Lathrop et al44 | PO | Obstetric | Waterbirth | 198 | 13, 16 |
| Lim et al45 | RO | Obstetric | Waterbirth | 236 | 4, 9, 10, 12, 13, 14, 17, 19 |
| Liu et al46 | PO | Obstetric | Labour | 108 | 4, 7, 8, 13 |
| Mallen-Perez et al47 | PO | Obstetric | Unclear | 215 | 7 |
| Menakaya et al48 | RO | Obstetric | Waterbirth | 438 | 9, 10, 11, 12, 13, 17, 18 |
| Mollamahmutoglu et al49 | PO | Obstetric | Waterbirth | 602 | 1, 7, 10, 12, 13 |
| Neiman et al50 | RO | Obstetric | Both | 230 | 4, 8, 9, 10, 12, 13, 17, 22, 23 |
| Ohlsson et al51 | RCT | Obstetric | Labour | 1237 | 6, 8, 11, 14, 19, 20 |
| Otigbah et al52 - | RO | Obstetric | Waterbirth | 602 | 1, 4, 5, 9, 10, 11, 12, 13 |
| Pagano et al,13 - | RO | Obstetric | Waterbirth | 220 | 10, 17 |
| Peacock et al,53 - | RO | Obstetric | Waterbirth | 3507 | 17 |
| Preston et al54 - | RO | Midwifery | Waterbirth | 15 734 | 5, 9, 11 |
| Ros55 - | PO | Obstetric | Waterbirth | 54 | 17 |
| Sert et al56 - | RCT | Obstetric | Labour | 64 | 17 |
| Snapp et al57 - | RO | Midwifery | Waterbirth | 26 684 | 9, 10, 13, 17, 21, 23 |
| Thoeni et al58 - | RO | Obstetric | Waterbirth | 1600 | 10, 11, 12 |
| Torkamani et al59 -- | PO | Obstetric | Waterbirth | 100 | 5, 7, 12 |
| Ulfsdottir et al60- | RO | Midwifery | Waterbirth | 612 | 1, 2, 3, 4, 6, 10, 11, 12, 13, 14, 16, 17, 23, 24 |
| Woodward and Kelly61 2004 | RCT | Obstetric | Waterbirth | 80 | 4, 5, 6, 8, 10, 17, 24 |
| Zanetti-Dällenbach et al62 2006 | PO | Obstetric | Waterbirth | 513 | 2, 3, 6, 9, 12 |
| Zanetti-Dallenbach et al63 2007 | PO | Obstetric | Waterbirth | 368 | 4, 5, 10, 11, 13, 14, 17 |
| Ziolkowski et al64 2009 | RO | Obstetric | Waterbirth | 171 | 16, 17 |
Interventions amd Outcomes Key: (1) Labour Induction (2) Amniotomy (3) Augmentation (4) Fetal Monitoring (5) Opioids (6) Epidural (7) Pain (8) Caesarean Delivery (9) Shoulder Dystocia (10) Intact Perineum (11) OASI (12) Episiotomy (13) Postpartum Haemorrhage (14) Manual Removal of Placenta (15) Maternal Infection (16) Maternal Satisfaction (17) 5 min APGAR (18) Newborn Resuscitation (19) Transient Tachypnoea of the Newborn (20) Respiratory Distress of the Newborn (21) Neonatal Death (22) Infection in newborn period (23) Cord Avulsion (24) Breastfeeding Initiation.
No studies provided comparison data for third-stage management.
No studies met the definition used for neonatal intensive care unit admission.
OASI, obstetric anal sphincter injury; PO, prospective observational; RCT, randomised controlled trial; RO, retrospective observational.
Few studies provided sample characteristics beyond parity (see table 2). Eleven studies reported the sample was restricted to persons in spontaneous labour while seven included the rate of labour induction for each group. Two studies excluded participation based on BMI while six provided weight or BMI distributions in the sample characteristics. Most studies (k=19; n=77 180) excluded multiple pregnancies, the rest did not address this characteristic. Prior caesarean was excluded by seven studies (n=2292) and reported as a sample characteristic for five studies (n=22 439).
Table 2.
Reported characteristics of study samples abstracted from inclusion and exclusion criteria or sample descriptions
| Author | Excludes multiparous | Excludes induced labour | Excludes for BMI | Excludes multiples | Excludes prior caesarean |
| Bailey et al30 | No | No | No | Yes | No |
| Barry et al31 | No | Yes | >30 | Yes | n.d. |
| Benfield et al32 | No | n.d. | n.d. | n.d. | n.d. |
| Bovbjerg et al33 | No | n.d. | n.d. | Yes | No |
| Cluett et al34 | Yes | Yes | n.d. | n.d. | n.d. |
| da Silva et al35 | Yes | n.d. | n.d. | Yes | n.d. |
| Eckert et al36 | No | No | n.d. | Yes | n.d. |
| Geisbuehler et al38 | No | n.d. | n.d. | n.d. | n.d. |
| Geisbuehler et al39 | No | n.d. | >40 | n.d. | n.d. |
| Geisbuehler et al37 | No | n.d. | n.d. | n.d. | n.d. |
| Haslinger et al40 | No | n.d. | n.d. | Yes | n.d. |
| Henderson et al41 | No | No | n.d. | n.d. | No |
| Hodgson et al42 | No | n.d. | n.d. | Yes | n.d. |
| Jacoby et al43 | No | Yes | n.d. | Yes | n.d. |
| Lathrop et al44 | No | n.d. | n.d. | Yes | n.d. |
| Lim et al45 | No | n.d. | n.d. | Yes | No |
| Liu et al46 | Yes | n.d. | No | Yes | Yes |
| Mallen-Perez et al47 | n.d. | Yes | No | Yes | n.d. |
| Menakaya et al48 | No | Yes | n.d. | Yes | n.d. |
| Mollamahmutoglu et al49 | No | No | No | n.d. | Yes |
| Neiman et al50 | No | Yes | n.d. | Yes | Yes |
| Ohlsson et al51 | No | n.d. | n.d. | Yes | n.d. |
| Otigbah et al52 | No | No | n.d. | n.d. | n.d. |
| Pagano et al13 | Yes | n.d. | n.d. | n.d. | n.d. |
| Peacock et al53 | No | Yes | n.d. | n.d. | n.d. |
| Preston et al54 | No | Yes | No | n.d. | n.d. |
| Ros et al55 | No | n.d. | n.d. | Yes | Yes |
| Sert et al56 | No | Yes | n.d. | n.d. | Yes |
| Snapp et al57 | No | n.d. | n.d. | n.d. | n.d. |
| Thoeni et al58 | Yes | n.d. | n.d. | Yes | Yes |
| Torkamani et al59 | No | n.d. | n.d. | n.d. | n.d. |
| Ulfsdottir et al60 | No | No | No | n.d. | No |
| Woodward et al61 | No | Yes | n.d. | n.d. | Yes |
| Zanetti-Dallenbach et al62 | No | n.d. | n.d. | Yes | n.d. |
| Zanetti-Dallenbach et al63 | No | n.d. | n.d. | Yes | n.d. |
| Ziolkowski et al64 | n.d. | n.d. | n.d. | n.d. | n.d. |
n.d. This item was not described in the paper; it was neither listed as an inclusion/exclusion criteria nor in the description of the sample.
BMI, body mass index.
Few studies provided descriptions of the care practices used with water immersion and water birth (see table 3). The description of the immersion receptacle used was adequate to determine the woman had freedom of movement in seven studies (n=3273). Method of induction was not reported. Sixteen studies reported a fetal heart monitoring method as either intermittent auscultation (k=10; n=50 846), continuous monitoring (k=5; n=967) or a mix of methods (k=1; n=367). Six studies reported using ‘hands-off’ (k=4; n=5595) or ‘hands-on’ (k=2; n=6463) the perineum. Third-stage management was reported by six studies (n=5595), all indicating that active management was used. Three studies indicated whether the placenta and membranes were delivered in the birth pool (k=1; n=513) or out of the birth pool (k=2; n=1396).
Table 3.
Description of care practices reported in included studies
| Author | Appropriate pool described | Induction method | Intermittent auscultation | Perineum method | Third-stage management | Placenta and membranes |
| Bailey et al30 | No | n.d. | n.d. | n.d. | Active | Out of Pool |
| Barry et al31 | Yes | None | Mixed | Hands Off | Active | n.d. |
| Benfield et al32 | No | n.d. | No | n.d. | n.d. | n.d. |
| Bovbjerg et al33 | No | n.d. | n.d. | n.d. | n.d. | n.d. |
| Cluett et al34 | Yes | None | n.d. | n.d. | n.d. | n.d. |
| da Silva et al35 | No | n.d. | No | n.d. | n.d. | n.d. |
| Eckert et al36 | Yes | n.d. | n.d. | n.d. | n.d. | n.d. |
| Geisbuehler et al38 | No | n.d. | Yes | n.d. | n.d. | n.d. |
| Geissbuehler et al39 | No | n.d. | Yes | n.d. | n.d. | n.d. |
| Geissbuhler et al38 | No | n.d. | Yes | n.d. | n.d. | n.d. |
| Haslinger et al40 | No | n.d. | n.d. | Hands On | n.d. | n.d. |
| Henderson et al41 | No | n.d. | n.d. | Hands Off | Active | n.d. |
| Hodgson et al42 | No | n.d. | Yes | n.d. | n.d. | n.d. |
| Jacoby et al43 | No | None | n.d. | n.d. | n.d. | n.d. |
| Lathrop et al44 | No | n.d. | n.d. | n.d. | n.d. | n.d. |
| Lim et al45 | No | n.d. | No | n.d. | n.d. | n.d. |
| Liu et al46 | No | n.d. | Yes | n.d. | n.d. | n.d. |
| Mallen-Perez et al47 | Yes | None | n.d. | n.d. | n.d. | n.d. |
| Menakaya et al48 | Yes | None | n.d. | n.d. | n.d. | n.d. |
| Mollamahmutoglu et al49 | Yes | n.d. | Yes | Hands Off | Active | n.d. |
| Neiman et al50 | No | None | Yes | n.d. | n.d. | n.d. |
| Ohlsson et al51 | No | n.d. | n.d. | n.d. | n.d. | n.d. |
| Otigbah et al52 | Yes | n.d. | Yes | Hands Off | Active | Out of Pool |
| Pagano et al13 | No | n.d. | n.d. | n.d. | n.d. | n.d. |
| Peacock et al53 | No | None | n.d. | n.d. | n.d. | n.d. |
| Preston et al54 | No | None | n.d. | n.d. | n.d. | n.d. |
| Ros et al55 | No | n.d. | n.d. | n.d. | n.d. | n.d. |
| Sert et al,56 | Yes | None | n.d. | n.d. | n.d. | n.d. |
| Snapp et al57 | No | n.d. | n.d. | n.d. | n.d. | n.d. |
| Thoeni et al58 | No | n.d. | n.d. | Hands On | n.d. | n.d. |
| Torkamani et al59 | No | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ulfsdottir et al60 | Yes | None | No | n.d. | n.d. | n.d. |
| Woodward and Kelly61 | No | None | Yes | n.d. | n.d. | n.d. |
| Zanetti-Dällenbach et al62 | No | n.d. | No | n.d. | Active | In Pool |
| Zanetti-Dallenbach et al63 | No | n.d. | No | n.d. | n.d. | n.d. |
| Ziolkowski et al64 | No | n.d. | Yes | n.d. | n.d. | n.d. |
n.d. Care practice not described in the paper in methods or results.
Risk of bias assessment
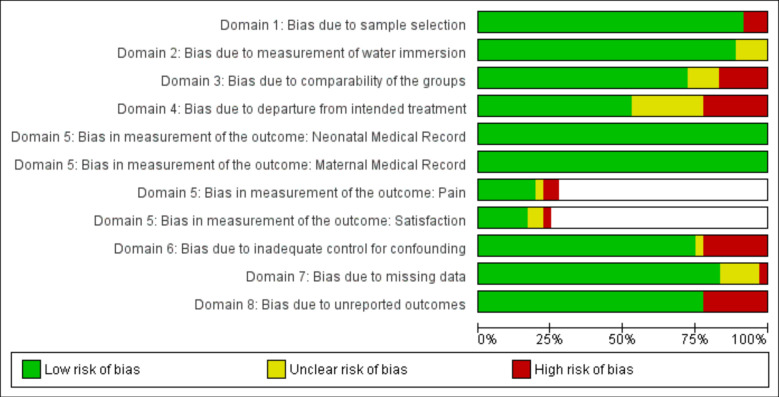
Overall risk of bias is presented in figure 2. Domain 3, bias due to comparability of the groups, was most often identified in retrospective studies that did not provide adequate sample restriction to ensure comparability. Domain 4, bias due to departure from intended treatment, had the highest potential for bias because studies did not provide information about if or why the comparison group included persons who used water in labour but not during birth. Bias in measurement of outcomes was rare because most outcomes were standard medical record items. However, measurement for pain and maternal satisfaction was not consistently described. Individual study results and risk of bias for each outcome are provided in the forest plots found in figures 3–24.
Figure 2.
Risk of bias assessment.
Figure 3.
Forest plot of synthesis of labour induction. IV, inverse variance.
Figure 4.
Forest plot of synthesis of amniotomy. IV, inverse variance.
Figure 5.
Forest plot of synthesis of augmentation of labour. IV, inverse variance.
Figure 6.
Forest plot of synthesis of opioid use. IV, inverse variance.
Figure 7.
Forest plot of synthesis of epidural use. IV, inverse variance.
Figure 8.
Forest plot of synthesis of pain. IV, inverse variance.
Figure 9.
Forest plot of synthesis of caesarean delivery. IV, inverse variance.
Figure 10.
Forest plot of synthesis of shoulder dystocia. IV, inverse variance.
Figure 11.
Forest plot of synthesis of intact perineum. IV, inverse variance.
Figure 12.
Forest plot of synthesis of obstetric anal sphincter injuries. IV, inverse variance.
Figure 13.
Forest plot of synthesis of episiotomy. IV, inverse variance.
Figure 14.
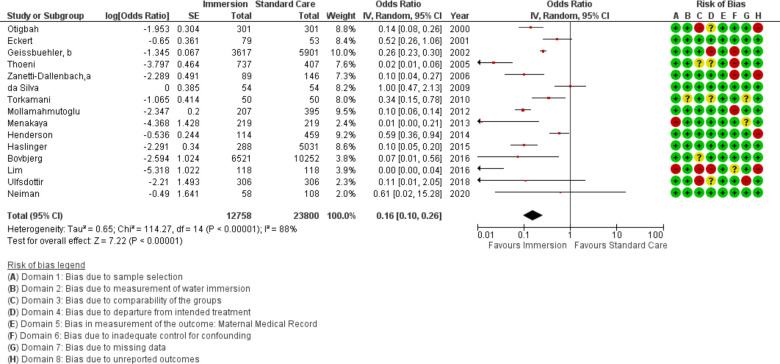
Forest plot of synthesis of postpartum haemorrhage.
Figure 15.
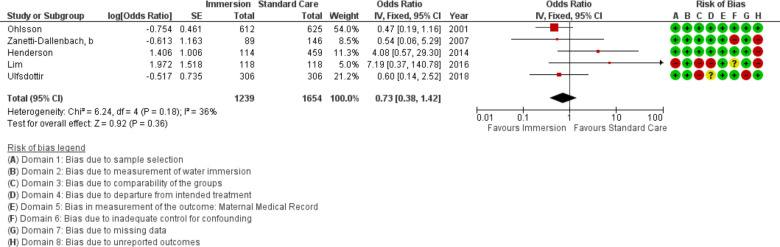
Forest plot of synthesis of manual removal of the placenta. IV, inverse variance.
Figure 16.
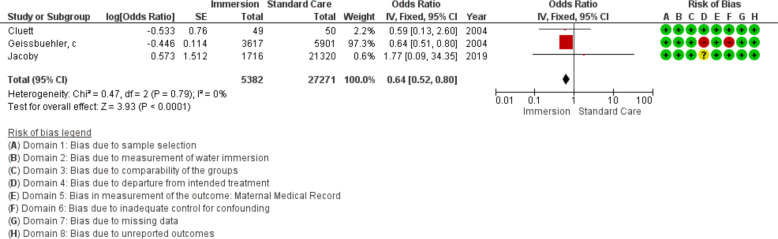
Forest plot of synthesis for maternal infection. IV, inverse variance.
Figure 17.
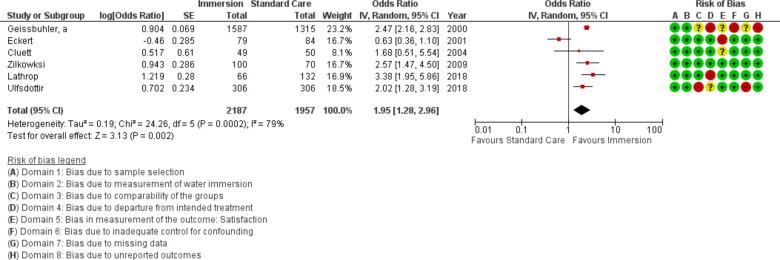
Forest plot of synthesis of maternal satisfaction measures. IV, inverse variance.
Figure 18.
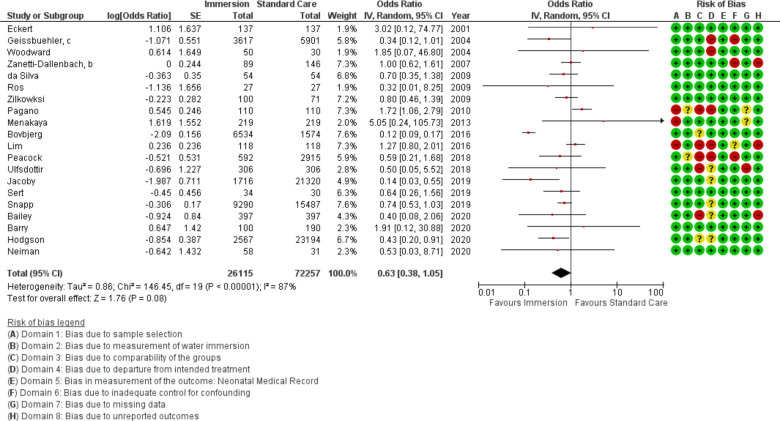
Forest plot of synthesis of 5 min APGAR. IV, inverse variance.
Figure 19.
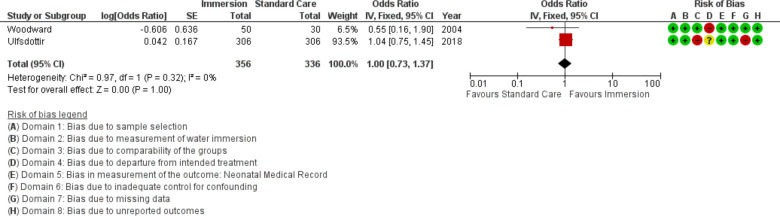
Forest plot of synthesis of neonatal resuscitation. IV, inverse variance.
Figure 20.
Forest plot of synthesis of transient tachypnoea of the newborn. IV, inverse variance.
Figure 21.
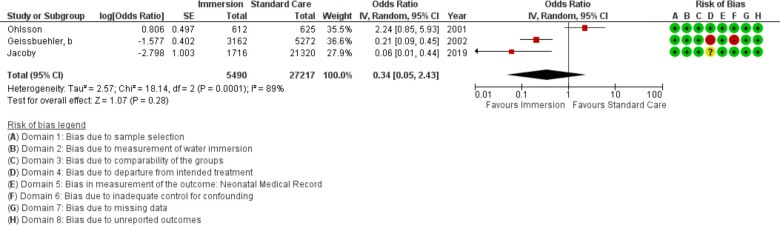
Forest plot of synthesis of respiratory distress. IV, inverse variance.
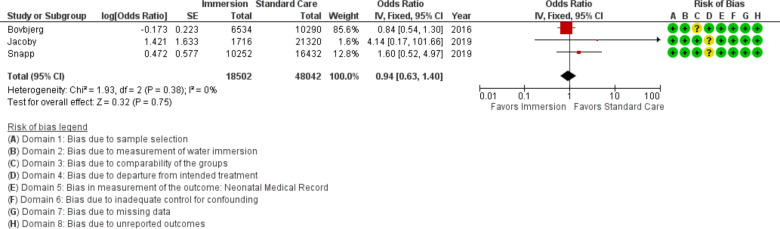
Figure 22.
Forest plot of synthesis of neonatal death. IV, inverse variance.
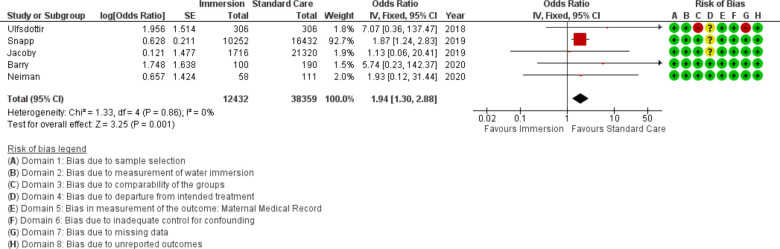
Figure 23.
Forest plot of synthesis of cord avulsion. IV, inverse variance.
Figure 24.
Forest plot of synthesis of breastfeeding initiation. IV, inverse variance.
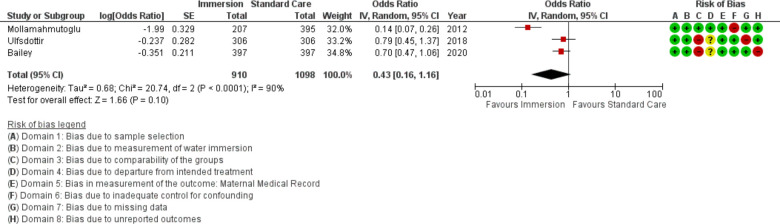
Labour induction
Three studies provided data on labour induction (n=2008), all conducted after 2010. Overall, this analysis found no difference between use of labour induction with water immersion and standard care (OR 0.43; 95% CI 0.16 to 1.16; random effects; Q=20.7 p<0.001; I2=90%). Subgroup analysis of studies reporting in an obstetric setting remained no difference. Results of the subgroup analyses are in table 4. Three studies were too few for cumulative meta-analysis. Two additional studies indicated there was no difference but did not provide data to synthesise.36 52
Table 4.
Results of subgroup analysis of interventions on outcomes of water immersion for labour and waterbirth compared with standard care
| Outcome | Studies | Sample | Effect OR (95% CI) model |
Heterogeneity Q (p) I2% |
| Labour Induction* | ||||
| Obstetric units | 2 | 604 Immersion 792 Standard care |
0.32 (0.06 to 1.58) Random effects |
18 (<0.01) 94 |
| Amniotomy* | ||||
| Obstetric units | 4 | 306 Immersion 709 Standard care |
0.95 (0.62 to 1.46) Random effects |
5 (0.17) 40 |
| 2010 and earlier | 3 | 192 Immersion 250 Standard care |
0.87 (0.46 to 1.64) Random effects |
4 (0.13) 51 |
| 2011 and later | 2 | 420 Immersion 765 Standard care |
0.56 (0.15 to 2.02) Random effects |
14 (<0.01) 93 |
| Augmentation* | ||||
| Obstetric units | 2 | 203 Immersion 605 Standard care |
0.48 (0.16 to 1.51) Random effects |
6 (0.02) 83 |
| 2011 and later | 2 | 420 Immersion 765 Standard care |
0.32 (0.05 to 2.24) Random effects |
19 (<0.01) 95 |
| Opioid use | ||||
| 2010 and earlier | 6 | 4298 Immersion 6565 Standard care |
0.23 (0.08 to 0.70) Random effects |
95 (<0.01) 95 |
| 2011 and later | 2 | 1641 Immersion 14 887 Standard care |
0.17 (0.15 to 0.20) Fixed effects |
0 (0.54) 0 |
| Epidural* | ||||
| Obstetric units | 6 | 4104 Immersion 6889 Standard care |
0.26 (0.08 to 0.83) Random effects |
89 (<0.01) 94 |
| 2010 and earlier | 6 | 4104 Immersion 6889 Standard care |
0.26 (0.08 to 0.83) Random effects |
89 (<0.01) 94 |
| Pain | ||||
| 2010 and earlier | 3 | 182 Immersion 188 Standard care |
0.53 (0.27 to 1.03) Random effects |
6 (0.05) 68 |
| 2011 and later | 5 | 417 Immersion 413 Standard care |
0.15 (0.06 to 0.42) Random effects |
48 (<0.01) 92 |
| Caesarean delivery | ||||
| 2010 and earlier | 4 | 790 Immersion 745 Standard care |
1.05 (0.63 to 1.74) Fixed effects |
3 (0.43) 0 |
| 2011 and later | 4 | 400 Immersion 830 Standard care |
0.84 (0.32 to 2.23) Fixed effects |
6 (0.12) 48 |
| Shoulder dystocia | ||||
| Obstetric units | 6 | 5528 Immersion 21 155 Standard care |
1.06 (0.64 to 1.74) Fixed effects |
4 (0.60) 0 |
| 2010 and earlier | 3 | 4007 Immersion 6335 Standard care |
0.88 (0.42 to 1.83) Fixed effects |
2 (0.39) 0 |
| 2011 and later | 4 | 11 773 Immersion 31 252 Standard care |
0.87 (0.33 to 2.26) Random effects |
11 (0.01) 73 |
| Intact perineum | ||||
| Obstetric units | 14 | 6170 Immersion 8866 Standard care |
1.55 (1.12 to 2.16) Random effects |
147 (<0.01) 91 |
| Midwifery-led units | 3 | 17 079 Immersion 23 249 Standard care |
1.07 (0.91 to 1.26) Random effects |
15 (<0.01) 87 |
| Nulliparas | 5 | 1065 Immersion 894 Standard care |
1.59 (1.01 to 2.50) Random effects |
12 (0.01) 68 |
| Waterbirth versus no water | 8 | 954 Immersion 1696 Standard care |
1.35 (0.67 to 2.72) Random effects |
83 (<0.01) 92 |
| 2010 and earlier | 7 | 4958 Immersion 6949 Standard care |
1.28 (0.90 to 1.82) Random effects |
39 (<0.01) 85 |
| 2011 and later | 10 | 18 292 Immersion 28 871 Standard care |
1.59 (1.22 to 2.07) Random effects |
156 (<0.01) 94 |
| OASI | ||||
| Obstetric units | 13 | 10 720 Immersion 57 870 Standard care |
0.85 (0.57 to 1.30) Random effects |
51 (<0.001) 77 |
| Midwifery-led units | 2 | 6827 Immersion 10 558 Standard care |
0.71 (0.47 to 1.08) Fixed effects |
0 (0.527) 0 |
| Nulliparas | 2 | 870 Immersion 540 Standard care |
1.25 (0.42 to 3.71) Fixed effects |
1 (0.385) 0 |
| Waterbirth versus no water | 3 | 408 Immersion 550 Standard care |
0.57 (0.19 to 1.69) Fixed effects |
1 (0.681) 0 |
| 2010 and earlier | 6 | 5493 Immersion 7517 Standard care |
0.73 (0.58 to 0.91) Fixed effects |
8 (0.16) 37 |
| 2011 and later | 9 | 13 298 Immersion 67 382 Standard care |
0.78 (0.48 to 1.28) Random effects |
42 (<0.01) 81 |
| Episiotomy* | ||||
| Obstetric units | 14 | 6177 Immersion 13 548 Standard care |
0.17 (0.11 to 0.28) Random effects |
109 (<0.001) 88 |
| Nulliparas | 3 | 886 Immersion 582 Standard care |
0.10 (0.02 to 0.60) Random effects |
14 (<0.001) 86 |
| Waterbirth versus no water | 5 | 691 Immersion 1022 Standard care |
0.63 (0.02 to 0.20) Random effects |
14 (0.008) 71% |
| 2010 and earlier | 7 | 4927 Immersion 6912 Standard care |
0.21 (0.11 to 0.41) Random effects |
52 (<0.01) 88 |
| 2011 and later | 8 | 7831 Immersion 16 888 Standard care |
0.09 (0.03 to 0.25) Random effects |
53 (<0.01) 87 |
| Postpartum faemorrhage | ||||
| Obstetric units | 13 | 7040 Immersion 29 555 Standard care |
0.75 (0.60 to 0.94) Random effects |
30 (0.002) 60 |
| Midwifery-led units | 2 | 10 558 Immersion 16 738 Standard care |
0.39 (0.08 to 1.86) Random effects |
56 (<0.001) 98 |
| Waterbirth versus no mater | 5 | 758 Immersion 1177 Standard care |
1.02 (0.76 to 1.36) Fixed effects |
4 (0.439 0 |
| 2010 and earlier | 3 | 4007 Immersion 6348 Standard care |
0.72 (0.59 to 0.88) Random effects |
2 (0.30) 17 |
| 2011 and later | 12 | 13 591 Immersion 39 945 Standard care |
0.76 (0.48 to 1.20) Random effects |
97 (<0.01) 89 |
| Manual removal of placenta | ||||
| Obstetric units | 4 | 1239 Immersion 1654 Standard care |
0.78 (0.37 to 1.64) Fixed effects |
6 (0.105) 51 |
| 2010 and earlier | 2 | 701 Immersion 771 Standard care |
0.48 (0.21 to 1.11) Fixed effects |
0 (0.91) 0 |
| 2011 and later | 3 | 538 Immersion 883 Standard care |
1.48 (0.50 to 4.38) Fixed effects |
4 (0.16) 45 |
| Maternal satisfaction | ||||
| Obstetric units | 5 | 1802 Immersion 1568 Standard care |
2.02 (1.28 to 3.19) Random effects |
24 (<0.01) 83 |
| 2010 and earlier | 4 | 1815 Immersion 1519 Standard care |
1.64 (0.83 to 3.24) Random effects |
22 (<0.01) 86 |
| 2011 and later | 2 | 372 Immersion 438 Standard care |
2.55 (1.54 to 4.23) Random effects |
2 (0.16) 50 |
| APGAR | ||||
| Obstetric units | 18 | 10 286 Immersion 54 361 Standard care |
0.85 (0.66 to 1.08) Random effects |
29 (0.047) 38 |
| Midwifery-led units | 3 | 17 092 Immersion 18,31 Standard care |
0.33 (0.07 to 1.54) Random effects |
57 (<0.001) 96 |
| Waterbirth versus no water | 6 | 614 Immersion 655 Standard care |
1.07 (0.76 to 1.51) Fixed effects |
3 (0.643) 0 |
| 2010 and earlier | 8 | 4184 Immersion 6476 Standard care |
1.00 (0.77 to 1.29) Fixed effects |
7 (0.120) 39 |
| 2011 and later | 12 | 21 931 Immersion 65 781 Standard care |
0.52 (0.25 to 1.05) Random effects |
101 (<0.001) 89 |
| Neonatal death | ||||
| Midwifery-led units | 2 | 16 786 Immersion 26 722 Standard care |
0.91 (0.61 to 1.34) Fixed effects |
1 (0.297) 8 |
| Cord avulsion | ||||
| Obstetric units | 3 | 1874 Immersion 21 621 Standard care |
2.18 (0.34 to 11.97) Fixed effects |
1 (0.757) 0 |
| Midwifery-led units | 2 | 10 649 Immersion 16 829 Standard care |
1.92 (1.28 to 2.89) Fixed effects |
1 (0.386) 0 |
*Random effects models were used for intervention (labour induction, amniotomy, augmentation, epidural, and episiotomy) models because variation in use of these procedures is dependent on practice habits of the provider which are not otherwise controlled.
OASI, obstetric anal sphincter injury.
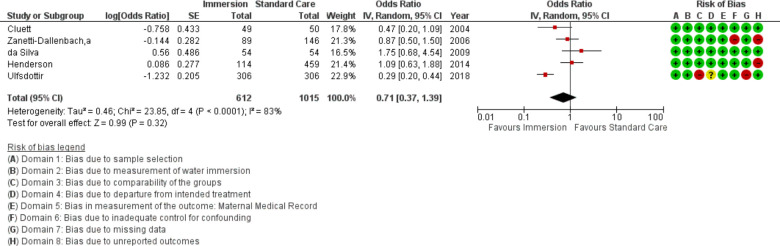
Amniotomy
Five studies provided data on amniotomy (n=1627). Overall, this analysis found no difference (OR 0.71; 95% CI 0.37 to 1.39; random effects; Q=23.9 p<0.001; I2=83%). Cumulative meta-analysis indicated the available evidence has consistently indicated no difference in the rate of amniotomy. Subgroup analysis of studies reporting in an obstetric setting and the most recent studies remained no difference.
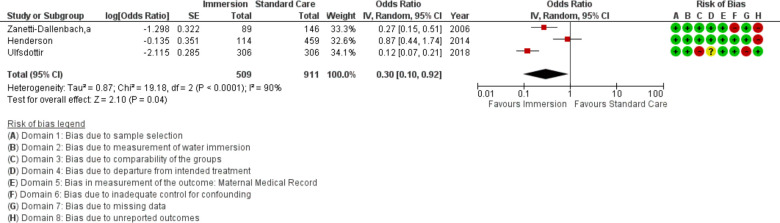
Augmentation
Three studies provided data to compare augmentation of labour (n=1420). This analysis favoured water immersion (OR 0.30; 95% CI 0.10 to 0.92; random effects; Q=19.2 p<0.001; I2=90%). Subgroup analysis of studies reporting in an obstetric setting and the most recent studies remained no difference. Fail-safe analysis estimated 34 additional studies finding no difference would be needed to change the estimate to no difference. Three studies were too few for cumulative meta-analysis.
Fetal monitoring
No studies provided data to compare the use of intermittent or continuous fetal monitoring during immersion to standard care.
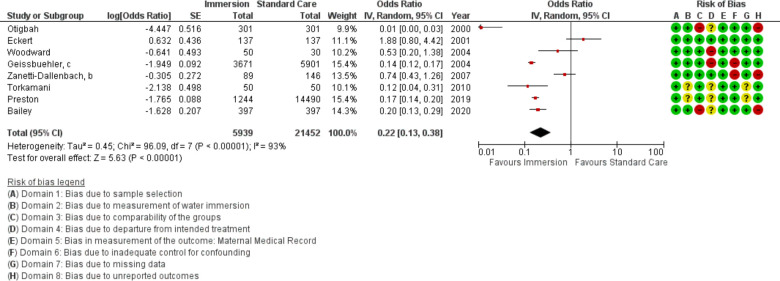
Opioid use
Eight studies provided data on opioid use (n=27 391), all were conducted in an obstetric setting. Overall, this analysis found reduced use of opioids with water immersion (OR 0.22 95% CI 0.13 to 0.38; random effects; Q=96.1 p<0.001; I2=93%). Subgroup analysis of the most recent studies remained no difference. Cumulative meta-analysis indicated the available evidence consistently favoured water immersion. Fail-safe analysis estimated 972 additional studies would be needed to change the estimate to no difference.
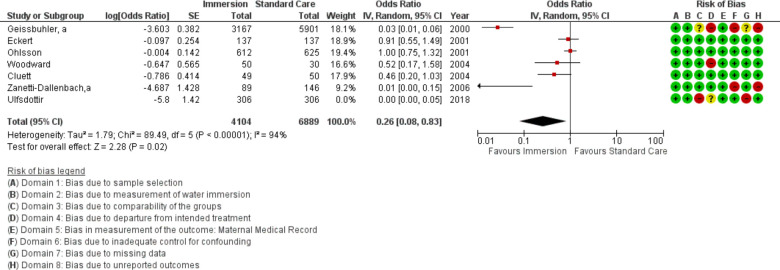
Epidural use
Seven studies provided data on epidural use (n=10 993). Overall, this analysis favoured water immersion (OR 0.26 95% CI 0.08 to 0.83; random effects; Q=89.5 p<0.001; I2=94%). Cumulative meta-analysis revealed the estimate moved from no difference to favour water immersion in 2007. Fail-safe analysis indicated 100 additional studies would be needed to change the estimate to no difference. Subgroup analysis revealed the use of epidural was reduced with water immersion in an obstetric setting.
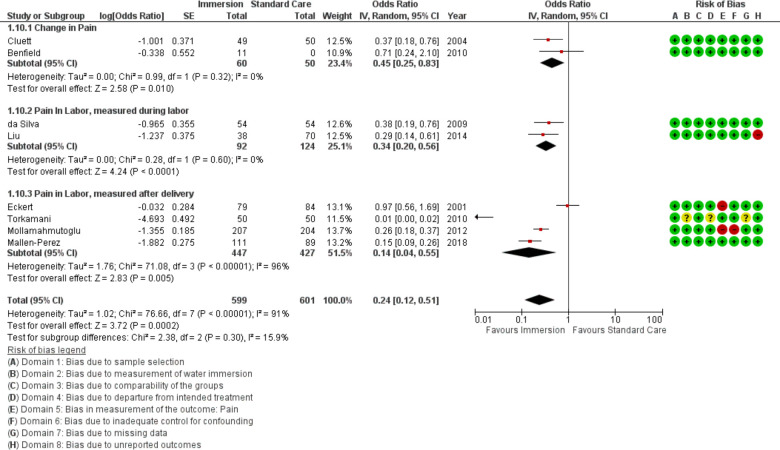
Pain
Eight studies provided data for analysis of pain (n=1200), all were conducted in an obstetric setting. Because these studies varied in their measurement timing and scale, they were combined with a random effects model for an overall score and the results were stratified by timing of measurement in the forest plot. Overall, the results indicated reduced pain with water immersion (OR 0.24 95% CI 0.12 to 0.51; random effects; Q=76.7 p<0.001; I2=91%). One additional study reported in favour of water immersion but did not provide the data in a way that allowed synthesis.31 Subgroup analysis of the most recent studies indicated reduced reports of pain with water immersion. Cumulative meta-analysis indicated the available evidence moved from no difference to favour water immersion in 2009 and has been stable since. Fail-safe analysis estimated 279 studies finding no difference would be necessary to change the estimate from favouring water to no difference.
Caesarean birth
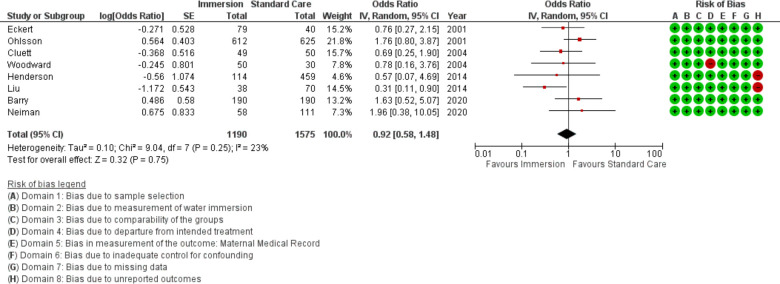
Eight studies provided data on mode of birth comparing water immersion (n=1190) vs standard care (n=1575), all were conducted in an obstetric setting. All but one study reported on the difference in caesarean with water immersion during labour; the final study was an RCT that analysed using intention to treat. The meta-analysis indicated no difference between water immersion and standard care for caesarean birth (OR 0.92 95% CI 0.58 to 1.48; fixed effects; Q=9.0 p=0.249; I2=23%). Subgroup analysis of studies reporting by year of publication remained no difference. Cumulative meta-analysis indicated this result has been stable at no difference since the first time the outcome was reported in 2001.
Shoulder dystocia
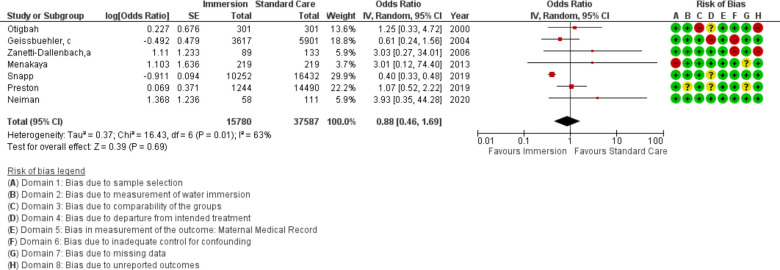
Seven studies provided data that could be synthesised for shoulder dystocia (n=53 367). One additional study reported zero events in the sample and could not be included in the synthesis.16 There was no difference between water immersion and standard care (OR 0.88 95% CI 0.46 to 1.69; random effects; Q=16 p=0.012; I2=63%). The subgroup analysis of studies in an obstetric setting and the most recent studies remained no difference. Cumulative meta-analysis indicated there has consistently been no difference.
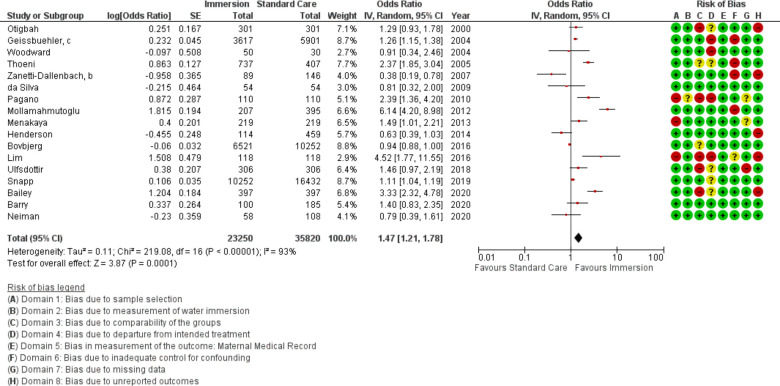
Intact perineum
Seventeen studies provided data on intact perineum (n=59 070). This analysis favoured water immersion (OR 1.47; 95% CI 1.21 to 1.78; random effects; Q=219.1 p<0.001; I2=93%). Note the direction of effect for figure 11 reflects that intact perineum is a positive outcome. Subgroup analysis revealed no difference in odds of intact perineum in midwifery-led settings, in studies that compare waterbirth to no immersion. Subgroup analysis revealed higher odds of intact perineum with water immersion in an obstetric setting and in the most recent studies. Cumulative meta-analysis indicated the available evidence has consistently indicated no difference or favoured water immersion, with evidence stable at favouring water immersion since 2016. Fail-safe analysis estimated 358 additional studies finding no difference would be necessary to change the estimate from favouring water to no difference. Subgroup analysis revealed no difference in odds of intact perineum in midwifery-led settings and in favour of water immersion in an obstetric setting.
Meta-regression identified the episiotomy rate (p<0.001) and the proportion of nulliparas in the sample (p=0.001) accounted for the variation in odds of an intact perineum (R2=1.00). Though only six studies provided the necessary data to test this association, the statistically significant result indicated the analysis was adequately powered to find this association. After accounting for these variables, the result was in favour of water immersion (OR 3.03 95% CI 1.52 to 6.04; random effects; Q=2 p=0.504 I2=0%).
Obstetric anal sphincter injury
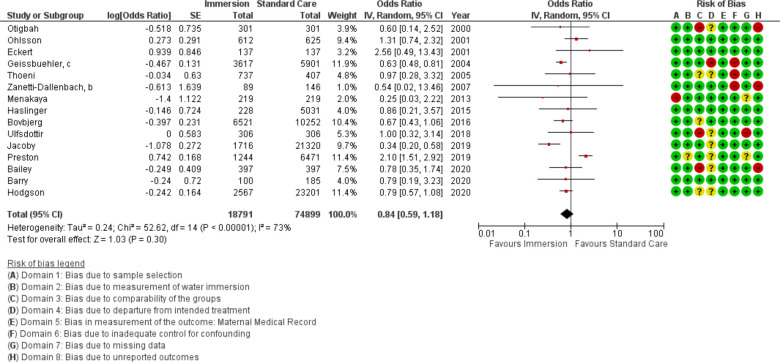
Fifteen studies provided data on OASI (n=93 690). This analysis found no difference (OR 0.84 95% CI 0.59 to 1.18; random effects; Q=52.6 p<0.001; I2=73%). Cumulative meta-analysis indicated the estimate has moved between no difference and favouring water, with the most recent change to no difference occurring in 2019. Analysis of subgroups by setting found consistent results of no difference in both settings. Meta-regression of the studies with the a priori selected control variables was not able to reduce the heterogeneity.
Episiotomy
Fifteen studies provided data on use of episiotomy (n=36 558). This analysis found reduced use of episiotomy with water immersion (OR 0.16; 95% CI 0.10 to 0.26; random effects; Q=114.3 p<0.001; I2=88%). Subgroup analysis revealed a reduction with water immersion in an obstetric setting, for nulliparas, and in the most recent studies. Cumulative meta-analysis indicated the available evidence has consistently favoured water immersion. Fail-safe analysis estimated 1525 additional studies finding no difference would be necessary to change the estimate from favouring water to no difference.
Meta-regression of the studies in an obstetric setting indicated the proportion of nulliparas in the sample accounted for some of the variance (R2=0.76; p=0.001; seven studies). Though this analysis was limited to seven studies, the finding of an association indicates the analysis had adequate power to identify the association. After accounting for the variation in proportion of nulliparas, the result remained in favour of water immersion (OR 0.04 95% CI 0.01 to 0.13; random effects; Q=12 p=0.038; I2=57%).
Third-stage management
No studies provided comparison data for third-stage management.
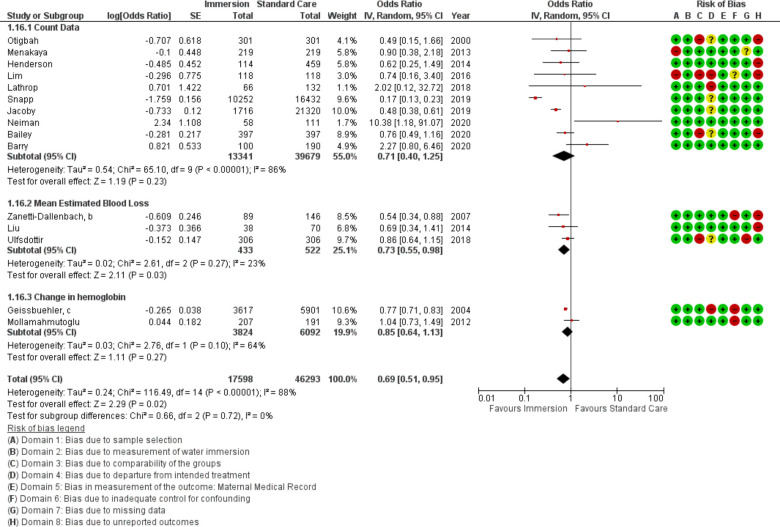
Postpartum haemorrhage
Fifteen studies provided data about PPH (n=63 891) using three different measures: count of PPH defined as >500 mL blood loss, mean estimated blood loss, and change in haemoglobin. Overall, this analysis favoured water immersion (OR 0.69 95% CI 0.51 to 0.95; random effects; Q=116.5 p<0.001; I2=88%). Subgroup analysis revealed no difference in odds of PPH in midwife-led settings, in studies comparing waterbirth to no water use, and the most recent studies. Subgroup analysis revealed a reduction with water immersion in an obstetric setting. Cumulative meta-analysis of the random effects model found the available evidence has consistently indicated no difference. Fail-safe analysis estimated 198 additional studies finding no difference would be necessary to change the estimate from favouring water to no difference.
Meta-regression of the studies in an obstetric setting identified no association with induction rate (R2=0; p=0.777; nine studies). Too few studies provided the data necessary to determine the effect of active management of third stage or the birth of the placenta and membranes into the water.
Manual removal of the placenta
Five studies provided data to assess risk for manual removal of the placenta (n=2893). This analysis indicated no difference (OR 0.73 95% CI 0.38 to 1.42; fixed effects; Q=6.2 p=0.181; I2=36%). Cumulative meta-analysis indicated there has consistently been no difference in manual removal of the placenta. Subgroup analysis revealed no difference in an obstetric setting and in the most recent studies.
Maternal infection
Three studies provided data about maternal infection (n=32 653), all were conducted in an obstetric setting. This analysis favoured water immersion (OR 0.64 95% CI 0.52 to 0.80; fixed effects; Q=0.5 p=0.792; I2=0%), however, one study carried 97% of the weight for this synthesis. Fail-safe analysis estimated two additional studies finding no difference would be necessary to change the estimate from favouring water to no difference. Three studies were too few for cumulative meta-analysis.
Maternal satisfaction
Six studies provided data on a measure of maternal satisfaction (n=4144). Due to heterogeneity in measurement tool, this analysis used random effects modelling and results were stratified by measurement tool in the forest plot. This analysis indicated increased satisfaction with water immersion (OR 1.95 95% CI 1.28 to 2.96; random effects; Q=24.3 p<0.001; I2=33%). Note the direction of effect for figure 17 reflects that maternal satisfaction is a positive outcome. Subgroup analysis revealed increased satisfaction with water immersion in an obstetric setting and in the most recent studies. Cumulative meta-analysis indicated the available evidence moved from no difference to favoured water immersion in 2018. Fail-safe analysis estimated 133 additional studies finding no difference would be necessary to change the estimate from favouring water to no difference.
Five min APGAR
Twenty-one studies provided data for 5 min APGAR (n=98 372). This analysis found no difference (OR 0.63 95% CI 0.38 to 1.05; random effects; Q=146.5 p<0.001; I2=87%). Three additional studies reported on 5 min APGAR but did not provide data in a usable format; two found no difference47 51 and one reported in favour of water immersion.59 Analysis of subgroups found consistent results of no difference. Cumulative meta-analysis indicated the available evidence has consistently demonstrated no difference.
Meta-regression indicated that study setting accounted for some between-study variance (R2=0.85; p=0.001; nine studies). After accounting for setting the analysis favoured water immersion (OR 0.14 95% CI 0.06 to 0.36; random effects; Q=20 p=0.034; I2=50%).
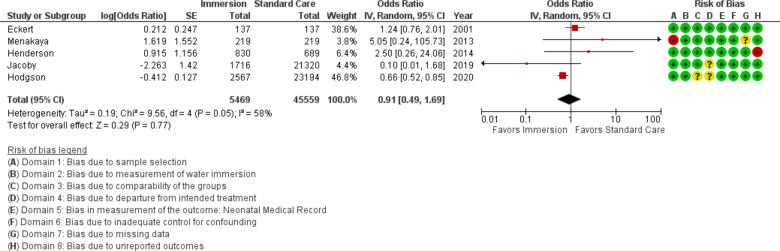
Newborn resuscitation
Five studies provided data on newborn resuscitation (n=51 028), all were conducted in an obstetric setting. This analysis found no difference (OR 0.91; 95% CI 0.49 to 1.69; random effects; Q=9.6 p=0.048; I2=58%. Cumulative meta-analysis indicated this outcome has been stable at no difference since first reported.
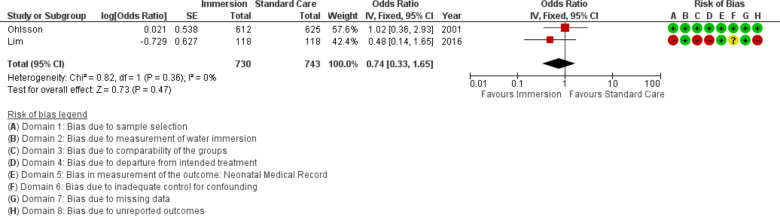
Transient tachypnoea of the newborn
Two studies provided data on transient tachypnoea of the newborn (n=1473), both were conducted in an obstetric setting. This analysis found no difference (OR 0.74; 95% CI 0.33 to 1.65; fixed effects; Q=0.8 p=0.364; I2=0%). Too few studies were available to conduct cumulative meta-analysis and subgroup analysis.
Respiratory distress of the newborn
Three studies provided data on respiratory distress of the newborn (n=32 707), all were conducted in an obstetric setting. This analysis indicated no difference (OR 0.34; 95% CI 0.05 to 2.43; random effects; Q=18.1 p<0.001; I2=89%). Three studies were too few for cumulative meta-analysis.
NICU admission
No studies met the definition for NICU admission.
Neonatal death
Three studies provided data on neonatal death (n=66 544), all were published after 2010. This analysis indicated no difference (OR 0.94; 95% CI 0.63 to 1.40; fixed effects; Q=1.9 p=0.381; I2=0%). Subgroup analysis by setting revealed no difference in midwifery-led settings. Three studies were too few for cumulative meta-analysis.
Infection in newborn period
Only one study met the definition for reporting newborn infection; it reported no difference.
Cord avulsion
Five studies provided data on cord avulsion (n=50 791), all were published after 2010. This analysis favoured standard care (OR 1.94 95% CI 1.30 to 2.88; fixed effects; Q=1.3 p=0.856; I2=0%). One study was responsible for 92.7% of the weight of this analysis, when that study was removed the result became no difference (OR 2.92 95% CI 0.67 to 12.77). Subgroup analysis by setting found no difference in an obstetric setting, but increased odds of cord avulsion in midwifery-led settings. Cumulative meta-analysis indicated the estimate moved from no difference to favour standard care in 2019. Fail-safe analysis estimated five additional studies would be needed to change the estimate to no difference.
Breastfeeding initiation
Two studies provided data on breastfeeding initiation (n=692). This analysis found no difference (OR 1.00 95% CI 0.73 to 1.37; fixed effects; Q=1.0 p=0.325; I2=0%). Note the direction of effect for figure 24 reflects that breastfeeding initiation is a positive outcome. Two studies were too few for cumulative meta-analysis and subgroup analysis.
Risk of bias across studies
Risk of bias analysis results are available in table 5. Begg’s test has moderate power with 25 studies, so is underpowered to find publication bias for this review. Egger’s regression identified risk for publication bias in three outcomes: epidural, intact perineum and shoulder dystocia. In each case, trim-and-fill estimates of the magnitude of bias indicate the magnitude was too small to affect the results.
Table 5.
Analysis of risk of bias across studies comparing water immersion for labour and waterbirth to standard care
| Outcome | K | Begg’s test rank correlation S-statistic (P) |
Egger’s regression Intercept (P) |
Trim-and-fill direction of bias* OR (95% CI) |
| Amniotomy | 5 | 4 (0.164) | 5.04 (0.129) | Standard care 0.43 (0.34 to 0.53) |
| Induction | 3 | −3 (0.059) | −10 (0.238) | – |
| Augmentation | 3 | 3 (0.59) | 28.96 (0.057) | Standard care 0.12 (0.09 to 0.16) |
| Opioid | 8 | −2 (0.402) | 2.13 (0.197) | Standard care 0.17 (0.15 to 0.19) |
| Epidural | 7 | −9 (0.088) | −4.51 (0.039) | Immersion 0.67 (0.54 to 0.83) |
| Caesarean | 8 | −2 (0.402) | −0.74 (0.327) | – |
| Pain | 8 | 0 (0.500) | −1.67 (0.339) | Standard care 0.16 (0.07 to 0.37) |
| Satisfaction | 6 | −5 (0.174) | −1.26 (0.216) | Immersion 1.73 (1.13 to 2.64) |
| Intact perineum | 14 | −10 (0.340) | 2.13 (0.045) | Standard care 1.71 (1.40 to 2.10) |
| Episiotomy | 13 | −11 (0.274) | −1.27 (0.121) | Immersion 0.20 (0.13 to 0.32) |
| OASI | 14 | 3 (0.435) | 0.40 (0.234) | Standard care 0.64 (0.50 to 0.82) |
| Shoulder dystocia | 7 | 5 (0.226) | 1.85 (0.001) | Standard care 0.68 (0.38 to 1.21) |
| Maternal infection | 3 | – | 0.34 (0.290) | – |
| Postpartum haemorrhage | 13 | 9 (0.328) | −0.23 (0.412) | Standard care 0.52 (0.39 to 0.71) |
| Retained placenta | 5 | 6 (0.071) | 2.11 (0.068) | Standard care 0.76 (0.29 to 2.03) |
| APGAR | 16 | −34 (0.179) | 0.86 (0.209) | Standard care 0.59 (0.36 to 0.96) |
| Neonatal resuscitation | 5 | 2 (0.312) | 0.69 (0.282) | – |
| Transient tachypnoea | 2 | – | – | – |
| Respiratory distress | 3 | 1 (0.301) | −1.77 (0.426) | – |
| Neonatal death | 3 | 1 (0.301) | 1.34 (0.078) | Standard care 0.84 (0.53 to 1.33) |
| Cord avulsion | 5 | 6 (0.071) | 0.36 (0.182) | Standard care 1.86 (1.26 to 2.75) |
| Breastfeeding initiation | 2 | – | – | – |
*Trim-and-fill analysis conducted with random effects model and indicates ORs and 95% CI estimate if bias were corrected.
OASI, obstetric anal sphincter injury.
Discussion
The main findings of this systematic review and meta-analysis are that labouring and/or giving birth in water has clear benefits to women in the obstetric setting. These findings are interesting because, in general, healthy women are more likely to experience interventions and adverse outcomes in this setting compared with midwifery-led settings and this has been reported for women who labour and/or give birth in water.3 65–67 Given that globally, most births take place in the obstetric setting, this review shows that water immersion can significantly increase the likelihood of an intact perineum and reduce episiotomy; an intervention which offers no perineal or fetal benefit, can increase postnatal pain, anxiety and impact negatively on a woman’s birth experience.68 69 Furthermore, labouring and/or giving birth in water does not increase the likelihood of OASI, which corroborates previous waterbirth research.7 70 71 A significant PPH reduction was another important finding, which is also supported in the literature.72
In this study, there was no difference in caesarean birth rate between those who used water and those who did not. Interestingly, the caesarean rate in these studies was 3.6%, with all but two studies reporting a caesarean birth rate of less than 10% for the study participants. Given the low caesarean rates reported by most studies, these results should not be generalised to settings with a caesarean rate higher than 10% for women considered low risk. The study with a caesarean rate of 19% is not generalisable to settings with a low-risk caesarean birth rate higher than 10% because it compared the use of water immersion to medical augmentation for women with a stalled labour.34 One study with a caesarean rate of 26% is generalisable to settings with a higher low-risk caesarean birth rate.46
Our results for newborns mirror those reported in three substantial newborn specific systematic reviews.10–12 Additionally, this study improved on prior research, which was limited by variations in definition for reporting newborn infection and NICU admission. The more rigorous definitions used for this study reveals limited reporting of serious complications. Given the lack of association with poor newborn outcomes between this study and prior analyses, it is unlikely that differences in prevalence of serious complications between water immersion and standard care exist.
More cord avulsions were reported for waterbirths and may relate to possible undue traction on the umbilical cord as the newborn is brought up out of the water.3 73 The incidence of cord avulsion was 4.3 per 1000 births in water compared with 1.3 per 1000 births with standard care. Interestingly, the incidence of cord avulsion varied from 0.2 per 1000 to 11.8 per 1000 in the five studies that reported this outcome, suggesting individual practice characteristics are more relevant to the incidence of cord avulsion than whether the birth occurs in water. A review of case reports of poor newborn outcomes found that when reported, cord avulsion was easily managed by the midwife with no consequences for the newborn.74
Our results show that water immersion has the potential to make a meaningful contribution to the global agenda towards promoting physiological birth.75–79 Labouring and/or giving birth in water can reduce maternal pain with no increased risk of an adverse event, and without the risk introduced by epidural and opioids.80–83 Differences between birth settings in intact perineum and PPH suggest water immersion in an obstetric setting may result in outcomes similar to those achieved in midwifery-led settings. This interpretation is supported by the results of subgroup analysis of studies in an obstetric setting that episiotomy is reduced with water immersion, and maternal satisfaction is increased. Given these results, water immersion for labour and waterbirth is an intervention that can be used to achieve physiological birth and improve the quality of care in the obstetric setting.
One major issue that hindered the potential of this review was that only four studies were conducted in midwifery-led settings. None of the included studies described the care model in operation where the study participants laboured. Healthy women who give birth in a midwifery-led setting are more likely to experience fewer interventions and adverse outcomes compared with those who give birth in an obstetric setting, particularly nullipara.2 3 There is strong evidence showing that the relational element of care matters to service users, and continuity of carer/care is linked to fewer interventions and adverse outcomes when compared with fragmented care models.83 This is important because birth pool use is most prevalent in midwifery-led settings.3 Evidence-based practice of water immersion requires research that reflects the context of care provision.
Few studies provided information generally considered to be relevant to the outcomes reported or controlled for potential confounders. Just over half the studies (k=20, 55%) included some description of the birth pool(s), resulting in uncertainty about whether all participants could move around and adopt different positions with ease. Furthermore, studies did not specify the type of fetal monitoring. Since intermittent auscultation does not inhibit mobility, and continuous electronic fetal monitoring typically does, this could present a confounder. Few studies stratified for parity, even when the outcomes reported occur at higher rates among nullipara. Only six studies (17%) mentioned inclusion of induction of labour while five studies included women with a prior caesarean. Only eight studies (22%) provided birth pool eligibility criteria regarding raised BMI. These studies did not include BMI as a characteristic in their analysis for interventions or outcomes. However, their inclusion in the study populations suggest that water immersion is not considered to be harmful for women who have raised BMI but are otherwise healthy. No studies provided data for the management of the third stage of labour in the studies, to enable examination for any associations between active or physiological management and PPH. Improvements in reporting standards would enable expansion of populations considered appropriate for water immersion and identify best practice for birth pool use.
Strengths and limitations of this work
This was the first substantial systematic review to attempt to include birth setting as an analytic variable. A broad search strategy was developed and all review processes were conducted by at least two reviewers. This study incorporated meta-regression, using covariates identified a priori, to reduce the effect of sources of heterogeneity. The inclusion of analyses of the stability of the results, cumulative meta-analysis and fail-safe, add value to the synthesis by identifying which outcomes may be considered sufficiently researched. The results are further strengthened by use of a trim-and-fill analysis to identify the direction of any potential publication bias.
This review was limited to studies published during or after 2000 or later because earlier studies may not be generalisable to current water immersion practices. This review did not include grey literature, and was limited by language; the search was conducted in English using English-language indices. This analysis was limited to a priori variables for meta-regression. Additional variables, not tested in this study, may contribute to heterogeneity. Inconsistency of reporting on birth setting, care practices, interventions and outcomes prevented us from achieving our secondary objective to account for intrapartum care variation. Meta-regression was only possible for three outcomes: intact perineum, episiotomy and PPH.
Clinical implications
Water immersion provides benefits for the mother and newborn when used in the obstetric setting, making water immersion a low-tech intervention for improving quality and satisfaction with care. In addition, water immersion during labour and waterbirth alter clinical practice resulting in less augmentation, episiotomy and requirements for pharmacological analgesia. Water immersion is an effective method to reduce pain in labour, without increasing risk. Clinicians should be mindful to avoid putting undue traction on the umbilical cord when bringing the newborn to the surface of the water.
Research implications
Water immersion during labour and birth is a low-tech yet complex, nuanced intervention. We suggest that studies incorporate the following fundamentals to advance the evidence: birth pool description, clearly described maternal and obstetric characteristics, the birth setting, the care model and use of standardised definitions. Studies should report potential confounders such as hands-on or hands-off the perineum and third-stage management. When appropriate for the outcome, results should be stratified by maternal parity. The study population should reflect all those now using a birth pool, not just the healthy women who experience an uncomplicated pregnancy. There is a need for additional research conducted in midwifery-led settings to establish best practice.
Conclusion
Water immersion during labour and birth, while low-tech, is a complex, nuanced intervention. Importantly it has clear benefits for healthy women and their newborns when in the obstetric unit setting where the majority of women give birth, and may have benefits for populations previously excluded from water immersion. To enable the identification of best practice regarding water immersion, future birthing pool research should integrate factors that are known to influence intrapartum interventions and outcomes. These include maternal parity, the care model, care practices, birth setting and a clear description of the water immersion receptacle.
Supplementary Material
Footnotes
Twitter: @Ethel_Burns_07
Scientific Advisors: VF: Methodology, validation & Investigation Vicki Farmilo, Health care librarian, Oxford Brookes University, Oxford, UK RM: Methodology, Review. Dr Reem Malouf, Neurologist, Specialist in Internal Medicine, National Perinatal Epidemiology Units, Oxford UK Cindy Farley (CF2): Methodology, Investigation, Review Associate Professor, Georgetown University, Washington DC, USA HC: Validation, Review Dr Harriet Thorn-Cole, Scientific ColLabourator, University of Health Sciences of Western Switzerland, Lausanne, Switzerland. CR: Methodology, Review Dr Charles Roehr, National Perinatal Epidemiology Unit Clinical Trials Unit, Oxford UK. Academic Consultant Neonatologist, Newborn Services, Southmead Hospital, North Bristol Trust, Bristol.
Contributors: EB: conceptualisation, protocol writing, investigation, methodology, writing-original draft, writing-review and editing, project administration, funding acquisition for Open Access publication. EB, Senior Midwifery Lecturer, Midwifery research Lead, Oxford Brookes University, Oxford UKCF1: methodology, protocol writing, validation, writing-original draft, writing- review and editing, visualisation. CF, Midwife Researcher, Associate Lecturer, Oxford Brookes University, Oxford UK. PJH: conceptualisation, investigation, writing - original draft, writing—review and editing. PJH, midwife researcher and senior instructor, Emory University in the Nell Hodgson Woodruff School of Nursing, Atlanta, Georgia USA JV: conceptualisation, methodology, investigation, data curation, formal analysis, writing—original draft, writing—review and editing, visualisation. JV, University of Nevada, Las Vegas School of Nursing, Nevada USA. EB and JV are the guarantors of the article.
Funding: This work was supported by Oxford Brookes University.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Institutional review board approval was not sought as meta-analyses are not human subjects research.
References
- 1.Cluett ER, Burns E, Cuthbert A. Immersion in water during labour and birth. Cochrane Database Syst Rev 2018;5:CD000111. 10.1002/14651858.CD000111.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birthplace in England Collaborative Group, Brocklehurst P, Hardy P, et al. Perinatal and maternal outcomes by planned place of birth for healthy women with low risk pregnancies: the birthplace in England national prospective cohort study. BMJ 2011;343:d7400. 10.1136/bmj.d7400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns EE, Boulton MG, Cluett E, et al. Characteristics, interventions, and outcomes of women who used a birthing pool: a prospective observational study. Birth 2012;39:192–202. 10.1111/j.1523-536X.2012.00548.x [DOI] [PubMed] [Google Scholar]
- 4.Prins M, Boxem J, Lucas C, et al. Effect of spontaneous pushing versus Valsalva pushing in the second stage of labour on mother and fetus: a systematic review of randomised trials. BJOG 2011;118:662–70. 10.1111/j.1471-0528.2011.02910.x [DOI] [PubMed] [Google Scholar]
- 5.Edqvist M, Blix E, Hegaard HK, et al. Perineal injuries and birth positions among 2992 women with a low risk pregnancy who opted for a homebirth. BMC Pregnancy Childbirth 2016;16:196. 10.1186/s12884-016-0990-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta JK, Sood A, Hofmeyr GJ, et al. Position in the second stage of labour for women without epidural anaesthesia. Cochrane Database Syst Rev 2017;5:CD002006. 10.1002/14651858.CD002006.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aasheim V, Nilsen ABV, Lukasse M, et al. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev 2011:CD006672. 10.1002/14651858.CD006672.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulchandani S, Watts E, Sucharitha A, et al. Manual perineal support at the time of childbirth: a systematic review and meta-analysis. BJOG 2015;122:1157–65. 10.1111/1471-0528.13431 [DOI] [PubMed] [Google Scholar]
- 9.Begley CM, Gyte GM, Devane D. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev;2019:CD007412. 10.1002/14651858.CD007412.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies R, Davis D, Pearce M, et al. The effect of waterbirth on neonatal mortality and morbidity: a systematic review and meta-analysis. JBI Database System Rev Implement Rep 2015;13:180–231. 10.11124/jbisrir-2015-2105 [DOI] [PubMed] [Google Scholar]
- 11.Taylor H, Kleine I, Bewley S, et al. Neonatal outcomes of waterbirth: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 2016;101:F357–65. 10.1136/archdischild-2015-309600 [DOI] [PubMed] [Google Scholar]
- 12.Vanderlaan J, Hall PJ, Lewitt M. Neonatal outcomes with water birth: a systematic review and meta-analysis. Midwifery 2018;59:27–38. 10.1016/j.midw.2017.12.023 [DOI] [PubMed] [Google Scholar]
- 13.Pagano E, De Rota B, Ferrando A, et al. An economic evaluation of water birth: the cost-effectiveness of mother well-being. J Eval Clin Pract 2010;16:916–9. 10.1111/j.1365-2753.2009.01220.x [DOI] [PubMed] [Google Scholar]
- 14.Davies MW. Water births and the research required to assess the benefits versus the harms. J Paediatr Child Health 2012;48:726–9. 10.1111/j.1440-1754.2010.01781.x [DOI] [PubMed] [Google Scholar]
- 15.Bovbjerg ML. Opposition to Waterbirth is not evidence based. J Womens Health 2021;30:625–7. 10.1089/jwh.2020.8790 [DOI] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ;2021:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Squires JE, Valentine JC, Grimshaw JM. Systematic reviews of complex interventions: framing the review question. J Clin Epidemiol 2013;66:1215–22. 10.1016/j.jclinepi.2013.05.013 [DOI] [PubMed] [Google Scholar]
- 18.JPT H, Green S. Cochrane Handbook for systematic reviews of interventions. Version 5.1.0. The Cochrane Collaboration, 2011. https://handbook-5-1.cochrane.org/ [Google Scholar]
- 19.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile APP for systematic reviews. Syst Rev 2016;5:210. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biostat Inc . Comprehensive meta-analysis V.3, 2017. [Google Scholar]
- 22.Leimu R, Koricheva J. Cumulative meta-analysis: a new tool for detection of temporal trends and publication bias in ecology. Proc Biol Sci 2004;271:1961–6. 10.1098/rspb.2004.2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carson KP, Schriesheim CA, Kinicki AJ. The Usefulness of the "Fail-Safe" Statistic in Meta-Analysis. Educ Psychol Meas 1990;50:233–43. 10.1177/0013164490502001 [DOI] [Google Scholar]
- 24.The Nordic Cochrane Center TCC . Review Manger (RevMan) 5.4.1. Available: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman
- 25.Murad MH, Chu H, Lin L, et al. The effect of publication bias magnitude and direction on the certainty in evidence. BMJ Evid Based Med 2018;23:84–6. 10.1136/bmjebm-2018-110891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. 10.1111/j.0006-341x.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 27.Baker WL, White CM, Cappelleri JC, et al. Understanding heterogeneity in meta-analysis: the role of meta-regression. Int J Clin Pract 2009;63:1426–34. 10.1111/j.1742-1241.2009.02168.x [DOI] [PubMed] [Google Scholar]
- 28.Borenstein M, Hedges LV, Higgins JPT, et al. Regression in meta-analysis, 2017. [Google Scholar]
- 29.Piccininni M, Konigorski S, Rohmann JL, et al. Directed acyclic graphs and causal thinking in clinical risk prediction modeling. BMC Med Res Methodol 2020;20:179. 10.1186/s12874-020-01058-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bailey JM, Zielinski RE, Emeis CL, et al. A retrospective comparison of waterbirth outcomes in two United States hospital settings. Birth 2020;47:98–104. 10.1111/birt.12473 [DOI] [PubMed] [Google Scholar]
- 31.Barry PL, McMahon LE, Banks RA, et al. Prospective cohort study of water immersion for labour and birth compared with standard care in an Irish maternity setting. BMJ Open 2020;10:e038080. 10.1136/bmjopen-2020-038080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benfield RD, Hortobágyi T, Tanner CJ, et al. The effects of hydrotherapy on anxiety, pain, neuroendocrine responses, and contraction dynamics during labor. Biol Res Nurs 2010;12:28–36. 10.1177/1099800410361535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bovbjerg ML, Cheyney M, Everson C. Maternal and newborn outcomes following Waterbirth: the midwives alliance of North America statistics project, 2004 to 2009 cohort. J Midwifery Womens Health 2016;61:11–20. 10.1111/jmwh.12394 [DOI] [PubMed] [Google Scholar]
- 34.Cluett ER, Pickering RM, Getliffe K, et al. Randomised controlled trial of labouring in water compared with standard of augmentation for management of dystocia in first stage of labour. BMJ 2004;328:314. 10.1136/bmj.37963.606412.EE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.da Silva FMB, de Oliveira SMJV, Nobre MRC. A randomised controlled trial evaluating the effect of immersion bath on labour pain. Midwifery 2009;25:286–94. 10.1016/j.midw.2007.04.006 [DOI] [PubMed] [Google Scholar]
- 36.Eckert K, Turnbull D, MacLennan A. Immersion in water in the first stage of labor: a randomized controlled trial. Birth 2001;28:84–93. 10.1046/j.1523-536x.2001.00084.x [DOI] [PubMed] [Google Scholar]
- 37.Geissbühler V, Eberhard J. Waterbirths: a comparative study. A prospective study on more than 2,000 waterbirths. Fetal Diagn Ther 2000;15:291–300. 10.1159/000021024 [DOI] [PubMed] [Google Scholar]
- 38.Geissbuehler V, Eberhard J, Lebrecht A. Waterbirth: water temperature and bathing time--mother knows best! J Perinat Med 2002;30:371–8. 10.1515/JPM.2002.058 [DOI] [PubMed] [Google Scholar]
- 39.Geissbuehler V, Stein S, Eberhard J. Waterbirths compared with landbirths: an observational study of nine years. J Perinat Med 2004;32:308–14. 10.1515/JPM.2004.057 [DOI] [PubMed] [Google Scholar]
- 40.Haslinger C, Burkhardt T, Stoiber B, et al. Position at birth as an important factor for the occurrence of anal sphincter tears: a retrospective cohort study. J Perinat Med 2015;43:715–20. 10.1515/jpm-2014-0172 [DOI] [PubMed] [Google Scholar]
- 41.Henderson J, Burns EE, Regalia AL, et al. Labouring women who used a birthing pool in obstetric units in Italy: prospective observational study. BMC Pregnancy Childbirth 2014;14:17. 10.1186/1471-2393-14-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hodgson ZG, Comfort LR, Albert AAY. Water birth and perinatal outcomes in British Columbia: a retrospective cohort study. J Obstet Gynaecol Can 2020;42:150–5. 10.1016/j.jogc.2019.07.007 [DOI] [PubMed] [Google Scholar]
- 43.Jacoby S, Becker G, Crawford S, et al. Water birth maternal and neonatal outcomes among midwifery clients in Alberta, Canada, from 2014 to 2017: a retrospective study. J Obstet Gynaecol Can 2019;41:805–12. 10.1016/j.jogc.2018.12.014 [DOI] [PubMed] [Google Scholar]
- 44.Lathrop A, Bonsack CF, Haas DM. Women's experiences with water birth: a matched groups prospective study. Birth 2018;45:416–23. 10.1111/birt.12362 [DOI] [PubMed] [Google Scholar]
- 45.Lim KMX, Tong PSY, Chong Y-S. A comparative study between the pioneer cohort of waterbirths and conventional vaginal deliveries in an obstetrician-led unit in Singapore. Taiwan J Obstet Gynecol 2016;55:363–7. 10.1016/j.tjog.2016.04.012 [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Liu Y, Huang X, et al. A comparison of maternal and neonatal outcomes between water immersion during labor and conventional labor and delivery. BMC Pregnancy Childbirth 2014;14:160. 10.1186/1471-2393-14-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mallen-Perez L, Roé-Justiniano MT, Colomé Ochoa N, et al. Use of hydrotherapy during labour: assessment of pain, use of analgesia and neonatal safety. Enferm Clin 2018;28:309–15. 10.1016/j.enfcli.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 48.Menakaya U, Albayati S, Vella E, et al. A retrospective comparison of water birth and conventional vaginal birth among women deemed to be low risk in a secondary level hospital in Australia. Women Birth 2013;26:114–8. 10.1016/j.wombi.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 49.Mollamahmutoğlu L, Moraloğlu O, Ozyer S, et al. The effects of immersion in water on labor, birth and newborn and comparison with epidural analgesia and conventional vaginal delivery. J Turk Ger Gynecol Assoc 2012;13:45–9. 10.5152/jtgga.2012.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neiman E, Austin E, Tan A, et al. Outcomes of Waterbirth in a US Hospital‐Based midwifery practice: a retrospective cohort study of water immersion during labor and birth. J Midwifery Womens Health 2020;65:216–23. 10.1111/jmwh.13033 [DOI] [PubMed] [Google Scholar]
- 51.Ohlsson G, Buchhave P, Leandersson U, et al. Warm tub bathing during labor: maternal and neonatal effects. Acta Obstet Gynecol Scand 2001;80:311–4. 10.1034/j.1600-0412.2001.080004311.x [DOI] [PubMed] [Google Scholar]
- 52.Otigbah CM, Dhanjal MK, Harmsworth G, et al. A retrospective comparison of water births and conventional vaginal deliveries. Eur J Obstet Gynecol Reprod Biol 2000;91:15–20. 10.1016/s0301-2115(99)00238-9 [DOI] [PubMed] [Google Scholar]
- 53.Peacock PJ, Zengeya ST, Cochrane L, et al. Neonatal outcomes following delivery in water: evaluation of safety in a district general Hospital. Cureus 2018;10:e2208. 10.7759/cureus.2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Preston HL, Alfirevic Z, Fowler GE, et al. Does water birth affect the risk of obstetric anal sphincter injury? development of a prognostic model. Int Urogynecol J 2019;30:909–15. 10.1007/s00192-019-03879-z [DOI] [PubMed] [Google Scholar]
- 55.Ros HB. Effect, of waterbirths and traditional bedbirths on outcomes for neonates. In: Curationis. 32, 2009. 10.4102/curationis.v32i2.934 [DOI] [Google Scholar]
- 56.Sert UY, Ozel S, Neselioglu S, et al. Water immersion during the labour and effects on oxidative stress. Fetal Pediatr Pathol 2020;39:185–93. 10.1080/15513815.2019.1651801 [DOI] [PubMed] [Google Scholar]
- 57.Snapp C, Stapleton SR, Wright J, et al. The experience of land and water birth within the American association of birth centers perinatal data registry, 2012-2017. J Perinat Neonatal Nurs 2020;34:16–25. 10.1097/JPN.0000000000000450 [DOI] [PubMed] [Google Scholar]
- 58.Thoeni A, Zech N, Moroder L, et al. Review of 1600 water births. does water birth increase the risk of neonatal infection? J Matern Fetal Neonatal Med 2005;17:357–61. 10.1080/14767050500140388 [DOI] [PubMed] [Google Scholar]
- 59.Torkamani SA, Kangani F, Janani F. The effects of delivery in water on duration of delivery and pain compared with normal delivery. Pakistan Journal of Medical Sciences 20102010;26:551–5. [Google Scholar]
- 60.Ulfsdottir H, Saltvedt S, Georgsson S. Waterbirth in Sweden - a comparative study. Acta Obstet Gynecol Scand 2018;97:341–8. 10.1111/aogs.13286 [DOI] [PubMed] [Google Scholar]
- 61.Woodward J, Kelly SM. A pilot study for a randomised controlled trial of waterbirth versus land birth. BJOG 2004;111:537–45. 10.1111/j.1471-0528.2004.00132.x [DOI] [PubMed] [Google Scholar]
- 62.Zanetti-Dällenbach R, Lapaire O, Maertens A, et al. Water birth, more than a trendy alternative: a prospective, observational study. Arch Gynecol Obstet 2006;274:355–65. 10.1007/s00404-006-0208-1 [DOI] [PubMed] [Google Scholar]
- 63.Zanetti-Daellenbach RA, Tschudin S, Zhong XY, et al. Maternal and neonatal infections and obstetrical outcome in water birth. Eur J Obstet Gynecol Reprod Biol 2007;134:37–43. 10.1016/j.ejogrb.2006.09.012 [DOI] [PubMed] [Google Scholar]
- 64.Ziolkowski R, Dabrus D, Czerniawski W, et al. An assessment of water births based on the author’s own research. Ginekologia i Poloznictwo 2009;14:57–65. [Google Scholar]
- 65.Alliman J, Stapleton SR, Wright J, et al. Strong start in birth centers: socio-demographic characteristics, care processes, and outcomes for mothers and newborns. Birth 2019;46:234–43. 10.1111/birt.12433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koto PS, Fahey J, Meier D, et al. Relative effectiveness and cost-effectiveness of the midwifery-led care in Nova Scotia, Canada: a retrospective, cohort study. Midwifery 2019;77:144–54. 10.1016/j.midw.2019.07.008 [DOI] [PubMed] [Google Scholar]
- 67.Scarf VL, Rossiter C, Vedam S, et al. Maternal and perinatal outcomes by planned place of birth among women with low-risk pregnancies in high-income countries: a systematic review and meta-analysis. Midwifery 2018;62:240–55. 10.1016/j.midw.2018.03.024 [DOI] [PubMed] [Google Scholar]
- 68.He S, Jiang H, Qian X, et al. Women’s experience of episiotomy: a qualitative study from China. BMJ Open 2020;10:e033354. 10.1136/bmjopen-2019-033354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang H, Qian X, Carroli G, et al. Selective versus routine use of episiotomy for vaginal birth. Cochrane Database Syst Rev 2017;2:CD000081. 10.1002/14651858.CD000081.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burns E, Price L, Carpenter J, et al. Predictors of obstetric anal sphincter injury during waterbirth: a secondary analysis of a prospective observational study. Int Urogynecol J 2020;31:651–6. 10.1007/s00192-019-04167-6 [DOI] [PubMed] [Google Scholar]
- 71.Dahlen HG, Dowling H, Tracy M, et al. Maternal and perinatal outcomes amongst low risk women giving birth in water compared to six birth positions on land. A descriptive cross sectional study in a birth centre over 12 years. Midwifery 2013;29:759–64. 10.1016/j.midw.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 72.Aughey H, Jardine J, Moitt N, et al. Waterbirth: a national retrospective cohort study of factors associated with its use among women in England. BMC Pregnancy Childbirth 2021;21:256. 10.1186/s12884-021-03724-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cro S, Preston J. Cord snapping at waterbirth delivery. Br J Midwifery 2002;10:494–7. 10.12968/bjom.2002.10.8.10597 [DOI] [Google Scholar]
- 74.Vanderlaan J, Hall P. Systematic review of case reports of poor neonatal outcomes with water immersion during labor and birth. J Perinat Neonatal Nurs 2020;34:311–23. 10.1097/JPN.0000000000000515 [DOI] [PubMed] [Google Scholar]
- 75.World Health Organization . WHO recommendations: intrapartum care for a positive childbirth experience, 2018. Available: https://www.who.int/publications/i/item/9789241550215 [PubMed]
- 76.Prosser SJ, Barnett AG, Miller YD. Factors promoting or inhibiting normal birth. BMC Pregnancy Childbirth 2018;18:241. 10.1186/s12884-018-1871-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Supporting healthy and normal physiologic childbirth: a consensus statement by ACNM, manA, and NACPM. J Perinat Educ 2013;22:14–18. 10.1891/1058-1243.22.1.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.National Childbirth Trust . Normal birth as a measure of the quality of care, 2010. Available: https://www.nct.org.uk/sites/default/files/related_documents/NormalbirthasameasureofthequalityofcareV3.pdf
- 79.International Confederation of Midwives . Keeping birth normal, 2014. Available: https://www.internationalmidwives.org/assets/files/statement-files/2018/04/keeping-birth-normal-eng.pdf
- 80.Smith LA, Burns E, Cuthbert A. Parenteral opioids for maternal pain management in labour. Cochrane Database Syst Rev 2018;6:CD007396. 10.1002/14651858.CD007396.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moran VH, Thomson G, Cook J, et al. Qualitative exploration of women's experiences of intramuscular pethidine or remifentanil patient-controlled analgesia for labour pain. BMJ Open 2019;9:e032203. 10.1136/bmjopen-2019-032203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fleet J-A, Jones M, Belan I. The influence of intrapartum opioid use on breastfeeding experience at 6 weeks post partum: a secondary analysis. Midwifery 2017;50:106–9. 10.1016/j.midw.2017.03.024 [DOI] [PubMed] [Google Scholar]
- 83.Penuela I, Isasi-Nebreda P, Almeida H, et al. Epidural analgesia and its implications in the maternal health in a low parity comunity. BMC Pregnancy Childbirth 2019;19:52. 10.1186/s12884-019-2191-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-056517supp001.pdf (271KB, pdf)
bmjopen-2021-056517supp002.pdf (1.2MB, pdf)
bmjopen-2021-056517supp003.pdf (65.4KB, pdf)
bmjopen-2021-056517supp004.pdf (149KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.